Abstract
There has been concerns related to the risk of bacterial contamination from raw pet food to humans, but research is still scarce. The purpose of this cross-sectional study was to use a worldwide internet survey-based data to evaluate the impact of raw pet foods on human health from the owners’ experience. From 16 475 households, 0.2 per cent (n=39) reported having had a transmission of pathogen from the raw pet food to a human family member during the time that raw feeding had been used in the household. Only in three of those households the same pathogen that was found in the human sample was analysed and confirmed also in the raw pet food (0.02 per cent of all data). Moreover, 0.1 per cent (n=24) reported suspecting that a disease could have been transmitted to a human from the pet food. Feeding salmon and turkey, using more than 50 per cent of the diet as raw foods and preparing the raw food in the same place and utensils as the family foods all had negative association with infections. Having 2 to 6 year-old children living in the household was associated with more infections, although adults were the most frequently infected.
Keywords: zoonoses, surveys, raw pet food
Introduction
The interest in feeding pet dogs and cats with raw diet is increasing worldwide.1 2 Raw pet diets can contain raw muscle meat, organ meats, fat, cartilage and bones. It can be home-prepared or commercially available complete or complementary food.3 4 In the Netherlands, 60.5 per cent of the dog owners feed their dogs at least some amount of raw dog food.5 Approximately 25 per cent of the agility dog owners in North America feed their dogs with raw/home-prepared or freeze-dried diets.6 From the dog owners in the USA and Australia, 16.2 per cent provide bones or raw meat as a part of the main meal,7 and in the USA and Canada one-third of dog breeders use raw diets or bones.8 In a survey of over 10 000 dog owner respondents in Finland, 26.5 per cent reported that they feed or have been feeding their dogs with bones and other raw foods (A. Hielm-Björkman, personal communication). One reason for the increasing popularity of raw feeding might be the anecdotal evidence of the benefits of raw diet to pets’ health.9 10 On the other hand, the benefits of a raw diet in dogs in calcium oxalate urolithiasis,11 in food digestibility12 13 and in intestinal integrity14 have been reported, among others.
There has also been concerns related to possible transmission of foodborne pathogens to humans from the raw pet foods.15–17 There can be zoonotic pathogens present in the raw pet foods, for example, Escherichia coli, Salmonella species, Clostridium species, Listeria monocytogenes, Yersinia species and Campylobacter.18–22 Also, raw meat fed dogs can shed Salmonella and E coli in their faeces.15 23 24 Nevertheless, there are no studies that have concluded the raw pet food as a health risk for pets or owners,25 and more research in this area is needed.
The purpose of this study was to use, for the first time, an internet-based data to evaluate the significance of raw pet foods on human health. It was done using a survey distributed worldwide to evaluate the possible differences in raw foodborne infections in humans between different countries. The effect of different household structures, pet food handling habits, the amount of raw food fed and the animal proteins used was also determined.
Materials and methods
The raw feeding and pathogen transmission questionnaire for the study was developed and conducted by veterinarians, microbiologists and canine nutritionists. It was planned as a regression study with three dependent groups: confirmed transmission of pathogens (CTP), suspected transmission of pathogens (STP) and non-transmission of pathogens (NTP). To avoid presumption biasness, no control group consisting of owners that served their dogs only with heat-treated dog foods was used. Heat-treated dog foods are usually considered safe by the general public and health professionals, whereas raw feeds are considered a health risk. For this reason, the authors wanted to avoid the situation where people would have gotten sick, but no one would have suspected the risk of transmission from the dog feed because of the animal eating a ‘safe’ food. The survey was open in the internet from July 14, 2017 to February 28, 2018 (228 days) to be able to reach at least 10,000 respondents. It was targeted to dog and cat owners who feed their pets with raw animal products (see online supplementary appendix 1). The questionnaire was translated into English, Finnish, Swedish, Spanish and Portuguese by native speakers and tested extensively before being sent out. The link to the five different language surveys were shared on the research group’s Facebook page and people were free to share the link in any type of Facebook groups as well as in other social medias or by email. To get a maximum number of respondents, all researchers in the group were asked to send out emails about spreading the questionnaire to their private, academic and industrial associates in their respective countries.
vetrec-2018-105122supp001.pdf (1.5MB, pdf)
The questionnaire was validated by sending a request by email to 195 respondents as a convenience sample, six months after the first one, to answer the survey a second time (test–retest repeatability). This sample also included different nationalities. Four questions were chosen to test the test–retest repeatability using Cohen’s kappa: ‘Gender (female/male)’, ‘The animal that is/has been fed a raw diet (dog(s), cat(s), both dog(s) and cat(s))’, ‘Does the animal drink water from trenches/puddles (yes/no)’ and ‘Does the animal eat other animals’ faeces (yes/no)’.
Statistical methods
The data were divided first into two groups according to the outcome measure: “Are there/have there been people in your household that have become sick from handling raw pet food or that have become sick from contact with a raw food eating pet?” (yes/no). Those that answered ‘no’ to that question were named ‘NTP households’. Those that answered ‘yes’ to that question were divided into two groups according to the ability to address the foodborne pathogen in question. Those that were able to address the pathogen were named ‘CTP households’, and those that had answered “I don’t remember” or “I don’t know” to the pathogen question were named ‘STP households’. To get a larger number of positive cases and therefore to be able to use a regression modelling technique, the two transmission groups (CTP+STP) were combined and the NTP was kept as the negative cases. A decision tree analyses (see online (online supplementary appendix 2) including all the covariates showed that the amount of raw meats/organs/bones that the owners had evaluated that they had fed to their animals (<20 per cent, 20 per cent–49 per cent, 50 per cent–89 per cent and 90 per cent–100 per cent), divided the data at 50 per cent. Therefore, this variable was changed into a dichotomous variable: if they had fed less than 50 per cent or ≥50 per cent of the food as raw. The two questions on keeping the raw food in the refrigerator or in room temperature could not be used as the respondents had answered very illogically. There were 3797 missing values from two of the variables: the respondents’ age (n=108 missing) and the time there had been a raw food fed pet in the family (n=3689 missing). Therefore, the models were done with and without them. Collinearity was tested and the following covariates were included in a Forward Stepwise Conditional logistic regression model: which was the raw food fed animal (for groups, see table 1), the people living in the raw pet food using households (for groups, see table 1), from where the raw pet food was obtained (for groups, see table 1), the package size, the amount of raw meat/organs/bones in the diet (for groups, see table 1), the size of packages used (for groups, see table 1), all animal species fed (see table 2), other items eaten (see figure 1) and the way the raw food was handled in the household (see figure 2). Two final models are presented in the results where all resting variables have a significant association (P<0.050) with the dependent variable that either is the sum of the CTP and STP, or only the CTP ones, both versus the NTP. An OR less than 1 is indicative of being a protective factor, whereas an OR greater than 1 is indicative of being a risk factor. The goodness of fit of the final models was determined by the following criteria: P<0.05 in the Omnibus test of model coefficients, P>0.05 in the Hosmer and Lemeshow test and from the Nagelkerke’s R2 test, which shows how many per cent of the variance can be explained by the model. All separate data are also presented as descriptive data. All variables and how they were divided between the three transmission household groups were also analysed using Fisher’s exact two-sided test from a cross-table setting. Significance was set at P<0.05. Missing cases were excluded from analyses. All analyses were done using the SPSS software (V.24, IBM SPSS Statistics, Chicago, Illinois, USA).
Table 1.
Characteristics of the households and people as well as feeding habits in the households from the survey to raw feeding dog and cat owners (n=16,475)
| In per cent of the households | |
| Raw food fed animal* | |
| Dog(s) | 73.6 |
| Cat(s) | 6.9 |
| Both dog(s) and cat(s) | 19.5 |
| Does the cat go out in the households that have cat?* | |
| Never | 51.6 |
| Sometimes | 13.6 |
| Almost daily/daily | 34.8 |
| People living in the raw pet food using households† | |
| Under 2 years old | 8.8 |
| 2–6 years old | 11.8 |
| Over 6 but under 18 years old | 23.9 |
| 18–65 years old | 88.5 |
| Over 65 years old | 13.4 |
| Immunocompromised people | 9.9 |
| People living in the raw pet food using households† | |
| Pet food shop | 50.2 |
| Supermarket | 48.5 |
| Wholesale | 27.1 |
| Internet | 23.3 |
| Farm/hunter/fisherman | 30.3 |
| Owner hunts/fishes/butchers | 9.3 |
| The amount of raw meat/organs/bones in the diet* | |
| 50 per cent and over | 90.7 |
| Under 50per cent | 9.3 |
| The size of packages used† | |
| 500 g/1.1 lbs /17.6 oz or under | 44.7 |
| Over 500 g/1.1 lbs/17.6 oz to 1 kg/2.2 lbs/35.3 oz | 42.4 |
| Over 1 kg/2.2 lbs/35.3 oz | 47.3 |
*Could choose one answer.
†Could choose multiple answers.
Table 2.
Raw animal products used in all three household types reported in a survey to raw feeding dog and cat owners (n=16,475)
| Raw animal products used | In per cent of CTP household (n) | In per cent of STP households (n) | In per cent of NTP households (n) | P value† |
| Beef | 89.7 (35) | 83.3 (20) | 91.3 (14,989) | 0.260 |
| Pork | 48.7 (19) | 54.2 (13) | 56.6 (9282) | 0.593 |
| Lamb | 59.0 (23) | 66.7 (16) | 66.0 (10,838) | 0.617 |
| Goat | 5.1 (2) | 16.7 (4) | 20.4 (3347) | 0.040* |
| Broiler/chicken | 84.6 (33) | 75.0 (18) | 82.3 (13,511) | 0.607 |
| Turkey | 53.8 (21) | 58.3 (14) | 76.2 (12,503) | 0.001*** |
| Duck | 33.3 (13) | 45.8 (11) | 54.5 (8946) | 0.020* |
| Reindeer | 5.1 (2) | 4.2 (1) | 7.4 (1218) | 1.000 |
| Moose | 2.6 (1) | 4.2 (1) | 8.7 (1421) | 0.444 |
| Deer | 17.9 (7) | 20.8 (5) | 34.0 (5583) | 0.042* |
| Horse | 10.3 (4) | 8.3 (2) | 13.4 (2203) | 0.801 |
| Bison | 15.4 (6) | 12.5 (3) | 15.6 (2557) | 1.000 |
| Egg | 61.5 (24) | 66.7 (16) | 74.5 (12,220) | 0.113 |
| Salmon | 30.8 (12) | 41.7 (10) | 54.3 (8906) | 0.006** |
| Vendace | 0.0 (0) | 0.0 (0) | 1.7 (280) | 1.000 |
| Herring | 17.9 (7) | 8.3 (2) | 17.8 (2923) | 0.549 |
| Some other fish | 35.9 (14) | 54.2 (13) | 50.2 (8233) | 0.186 |
| Birds | 10.3 (4) | 16.7 (4) | 15.3 (2504) | 0.698 |
| Rabbit | 35.9 (14) | 29.2 (7) | 46.0 (7545) | 0.123 |
*P<0.05, **P<0.01, ***P<0.001.
†Fisher’s exact two-sided test was used.
CTP, confirmed transmission of pathogen; NTP, no transmission of pathogen; STP, suspected transmission of pathogen.
Figure 1.
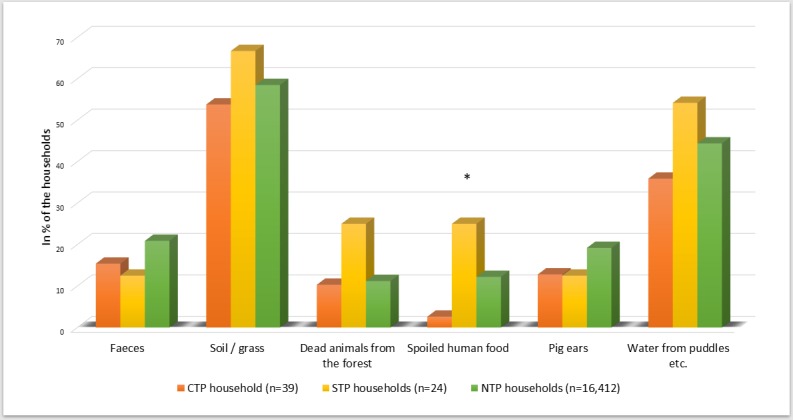
Items other than pet food eaten by the dogs in all three household types (n=16,475). *P<0.05. CTP, confirmed transmission of pathogen; NTP, no transmission of pathogen; STP, suspected transmission of pathogen.
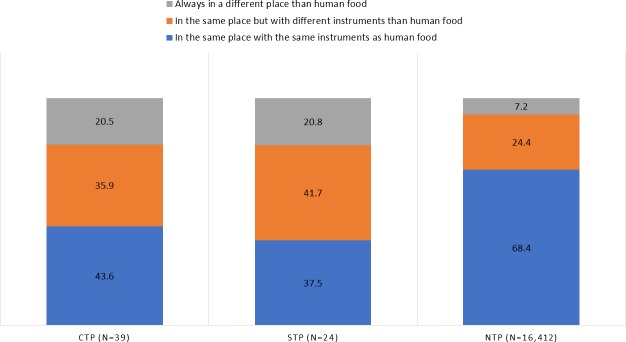
Figure 2.
The raw food handling practices in the three household types (n=16,745). CTP, confirmed transmission of pathogen; NTP, no transmission of pathogen; STP, suspected transmission of pathogen.
Results
Fifty households completed the survey for validation. ‘Gender’, ‘The animal that is/has been fed raw diet’, ‘Does the animal drink water from trenches/puddles’ and ‘Does the animal eat other animals’ faeces’ questions used for the test–retest validation had Cohen’s kappa of 0.88, 0.84, 0.80 and 0.56, respectively. Cohen’s kappa values showed mainly a very good level of agreement, indicating a stable questionnaire.
Altogether, 16,475 completed surveys were obtained. In 73.6 per cent of the households, the raw food fed pet was a dog, in 6.9 per cent a cat and in 19.5 per cent both cat and dog were fed with raw diet (table 1). There were respondents from 81 different countries in the data, and 1–4950 respondents from each country (see online supplementary appendix 3). The median age of the respondents was 43 years (from 13 to 88 years), and 92.4 per cent of them were females. The median time for feeding the animal(s) with raw diet was 3.0 years (ranging from 0.1 to 65 years).
From those 16,475 respondents, 16,412 households reported no transmission of pathogen (NTP households) during the time that raw feeding had been practised in the household. Furthermore, 39 reported a confirmed transmission (CTP households) from the raw pet food to humans (0.2 per cent), meaning that the pathogen was confirmed from a human sample by a laboratory (table 3). Of these, 14 stated that they were sure about the source of the pathogen, but only three of them reported that the same pathogen was confirmed from both the pet food and the sick human by a laboratory (all E coli; one from beef, one from a Big County Raw product, where the type of meat was not specified, and one from a product bought from an abattoir, but the animal species was not mentioned). In addition, 16 of the 39 stated that they were pretty sure about the source of the pathogen (not confirmed by any tests), and 9 households were not able to name any product as a source of transmission. Also, three households reported more than one pathogen (table 4).
Table 3.
Number of respondents in all countries in a survey to raw feeding dog and cat owners that reported a confirmed transmission or suspected having a transmission of pathogen
| Country | No transmission of pathogen (NTP) | Confirmed transmission of pathogen (CTP) | Suspected transmission of pathogen (STP) | Total | Percentage of CTP households within the country |
| Australia | 798 | 2 | 1 | 801 | 0.2 |
| Canada | 2574 | 11 | 1 | 2586 | 0.4 |
| Finland | 1117 | 2 | 0 | 1119 | 0.2 |
| France | 121 | 1 | 0 | 122 | 0.8 |
| Germany | 313 | 2 | 2 | 317 | 0.6 |
| Malta | 27 | 1 | 1 | 29 | 3.4 |
| Mexico | 88 | 0 | 1 | 89 | 0.0 |
| Norway | 82 | 1 | 2 | 85 | 1.2 |
| Sweden | 741 | 2 | 0 | 743 | 0.3 |
| UK | 3133 | 9 | 9 | 3151 | 0.3 |
| USA | 4935 | 8 | 7 | 4950 | 0.2 |
| Others | 2483 | 0 | 0 | 2483 | 0.0 |
| Total | 16 412 | 39 | 24 | 16 475 | 0.2 |
Table 4.
Number of pathogens in the infected people in different countries reported in a survey to raw feeding dog and cat owners (n=39)
| AUS | CAN | FIN | GER | Malta | FRA | NOR | SWE | UK | USA | Total | |
| Salmonella | 5 | 1* | 3† | 3 | 12 | ||||||
| Campylobacter | 1 | 4‡ | 1 | 2 | 1 | 6† | 2 | 17 | |||
| Yersinia | 1 | 1 | |||||||||
| Escherichia coli | 1 | 3‡ | 1 | 1 | 2 | 8 | |||||
| Clostridium | 1‡ | 1* | 1 | 3 | |||||||
| Toxoplasma | 1 | 1 | 2 |
*One Salmonella and one Clostridium reported by the same household.
†One Salmonella and one Campylobacter reported by the same household.
‡One Campylobacter, one E coli and one Clostridium reported by the same household.
AUS, Australia; CAN, Canada; FIN, Finland; GER, Germany; FRA, France; NOR, Norway; SWE, Sweden.
In addition to the 39 respondents, 24 suspected having had a transmission of a pathogen from the raw pet food (STP households), but they, nor the pet food, had not been tested for any pathogen in a laboratory, so they did not report any pathogen in the questionnaire (0.1per cent of all data) (table 3). Two of them reported that they did not remember the pathogen and 22 that they do not know the pathogen.
In total, respondents from 11 countries reported a transmission or suspected transmission of a pathogen (13.6per cent of all countries) (table 3). There were significantly more NTP households in the data than CTP or STP households (chi-squared test, P<0.001). Of all different pathogens reported in the survey, the most frequent ones were Campylobacter and Salmonella (table 4).
Adults were the most frequently infected people in the CTP households (table 5) as well as in the STP households. The median age reported in people that became sick was 40.1 years. Only in 10.3per cent (n=4) of the CTP households the infected person was a child between two and six years old (table 5), even though there were significantly more children between the age of two to six years living in the CTP households than in the STP or NTP households (in 25.6per cent of CTP, in 12.5per cent of STP and in 11.8per cent of NTP households, P=0.027). In the regression model having more children between the age of two and six years was the only factor having a positive association with a transmission of pathogen (OR: 2.20; P=0.012; 95per cent CI: 1.19 to 4.07), even though the adults were more frequently infected.
Table 5.
The pathogens and the number of households that reported the infected person as an adult and/or as a child, if he/she was immunocompromised, and if family pets had clinical signs at the same time (n=39 CTP households)
| Adult | Child | Immunocompromised person | Disease | Number of households where the pet had clinical signs | Animal that was fed with raw food | |||
| Dog | Cat | Both | ||||||
| Salmonella | 10* | 2 | 0 | N/A | 4 | 9 | 2 | 1 |
| Campylobacter | 17*,† | 0 | 0 | N/A | 4‡ | 12 | 1 | 4 |
| Yersinia | 1 | 0 | 0 | N/A | 0 | 1 | 0 | 0 |
| Escherichia coli | 6†,§¶ | 3§ | 1 | Cancer | 4‡ | 6 | 1 | 1 |
| Clostridium | 3*,† | 0 | 1 | Crohn’s disease | 2‡ | 3 | 0 | 0 |
| Toxoplasma | 2 | 0 | 0 | N/A | 0 | 2 | 0 | 0 |
*Including the same adult from a household that reported both bacteria.
†Including the same adult from a household that reported all three bacteria.
‡Including one same pet from a household that reported all three bacteria.
§Including one household with both an adult and a child infected.
CTP, confirmed transmission of pathogen (confirmed from human sample); N/A, not available.
Two people (5.1 per cent) among the people that got sick in the CTP household were immunocompromised (table 5), although there were immunocompromised people living in 15per cent of CTP households (n=6) (cancer=one, Crohn’s disease=two, multiple sclerosis=one, Ehlers-Danlos syndrome=one and no spleen=one). In addition, two people (8.3 per cent) among the people that got sick in the STP households were immunocompromised, one having HIV and the other systemic lupus erythematosus, comprising all the STP households that had immunocompromised people (n=2). There were immunocompromised people living in 9.9per cent (n=1617) of the NTP households.
In 31per cent of the CTP households (n=12), the pet had also clinical signs at the same time (table 5), and in the STP households the percentage was 13per cent (n=3). Signs reported in pets were diarrhoea (n=10), vomiting (n=9), fatigue/lethargy (n=3), mucus in the faeces (n=2), loose stools (n=1), bloody diarrhoea (n=1), gas (n=1), dehydration (n=1) and digestive upset (n=1). The outdoor activities of the household cat (options in the question were: going out ‘never’, ‘sometimes’ or ‘almost daily’), showed no difference between the three pathogen transmission household types (P=0.641). The household cat went out almost daily in 34.8per cent of NTP and CTP households, and in 37.5per cent of STP households.
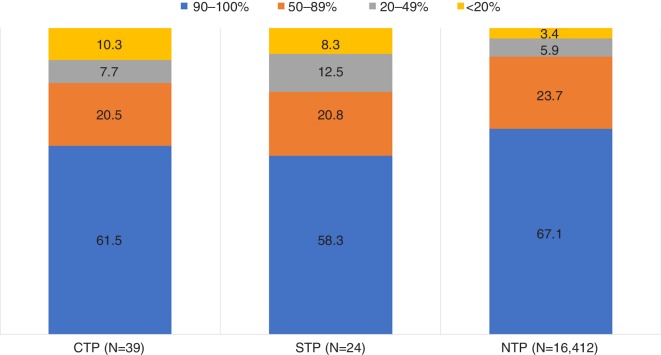
In the CTP households, goat, turkey, duck, deer and salmon were used significantly less than in NTP households (table 2). In the final regression model, salmon and turkey had a negative association with infections (OR: 0.54; P=0.025; 95per cent CI: 0.32 to 0.93 and OR: 0.51; P=0.011; 95per cent CI: 0.30 to 0.86, respectively). None of the meat products was significantly more used in the CTP households. The pets in the CTP and STP households were fed with less than 50per cent raw meat/organs/bones more frequently than in the NTP households (P=0.014) (in figure 3 all four percentage groups are presented). Eating more raw foods (≥50per cent of the diet) had a negative association with transmitting a pathogen in the final regression model (OR: 0.51; P=0.037; 95per cent CI: 0.27 to 0.96). The most frequent items eaten, other than pet food, were soil/grass and water from puddles (figure 1). Pets were fed with spoiled human food in 25per cent of the STP households, which was significantly more than in the other two household types (P=0.024). In addition, in 25.0per cent of the STP households, pets scavenged dead animals (P=0.108), compared with 10.3per cent and 11.2per cent in the CTP and NTP households, respectively.
Figure 3.
The percentage of raw food fed to the pet in different household types (n=16,475). CTP, confirmed transmission of pathogen; NTP, no transmission of pathogen; STP, suspected transmission of pathogen.
Raw pet food was handled in the same place with the same utensils as the household’s human food more frequently in the NTP households (P<0.0001) (figure 2). In the CTP and STP households, the raw pet food was more frequently handled always in a different place than the household’s human food (P=0.001) or in a same place but with different utensils than the human food (P=0.035) (figure 2). The same was shown in the final regression model since handling the raw pet food in the same place and with the same utensils had a negative association with infections (OR: 0.32; P<0.0001; 95per cent CI: 0.20 to 0.54).
Only the results of the combined groups (CTP and STP) versus NTP from the regression model were presented above but the results were nearly identical for the CTP versus NTP comparison; in that final model the ≥50 percentage of raw food with a negative association was replaced by the variable ‘The owner using package sizes of 500 g /1.1 lbs/17.6 oz or under’ (OR: 2.00; P=0.035; 95per cent CI 1.05 to 3.83). The small 95per cent OR CIs, the low Omnibus test values (P<0.0001), the high Hosmer and Lemeshow test values (P=0.603) ensured a good fit of the models, whereas the Nagelkerke R2 only explained 5.1per cent and 6.6per cent of the variation of the final models for the combined transmission and only for CTP versus NTP analyses.
Discussion
This study is the first to evaluate a possible owner-reported transmission of pathogens to humans from raw pet food using survey-based data. The results indicate that foodborne pathogens are seldom transmitted to humans through raw pet food. Only three households were able to confirm that the same pathogen was found in the human sample and in the pet food (0.02per cent of all data). Altogether, 39 households that reported a transmission of pathogen were able to name the pathogen (0.2per cent of all data), but no medical papers were asked to keep the survey anonymous. It should be noted that some of these households might have been using raw food for decades, so this percentage does not refer to infections per year and can therefore not be compared with yearly statistics. It describes the owners’ reported experience of pathogen transmissions in their households during the time of raw food feeding to pets, rather than gives any risk evaluation. In the CTP households, the pets were more often fed with less than 20per cent of raw meat/organs/bones, meaning that over 80per cent of their diet consisted of other than unprocessed raw meat. This highlights the fact that in the remaining 36 households, where the raw pet food was not tested for the pathogen, the infection might have been due to something else than the raw pet food. In addition, 0.1per cent (n=24) of the respondents suspected having had a transmission of pathogens from the raw pet food or from the pet itself but were not able to name the pathogen in question. The pets living in these households were fed with spoiled human food more often than in the two other household types, and 25per cent of these pets also scavenged dead animals. These two factors might also have had an influence on possible gastrointestinal symptoms that the owner thought was related to raw pet food. In addition, since the regression model showed that having two to six years old children in the household had a positive association with infections, there is a possibility that those pathogens spread into the household with children from the day care centre or outside and were transmitted to family adults without causing symptoms in the children. At least one study reports that after first or second year in the day care environment children’s immunity turns protective against symptomatic acute gastroenteritis.26 Nevertheless, there was no question asking if the children in the households spend their days in a day care or not, so the possibility can only be hypothesised.
There were respondents from 10 countries among the CTP households. The percentage of transmissions seemed to be above the dataset average in Malta (3.4 per cent), Norway (1.2 per cent), France (0.8 per cent), Germany (0.6 per cent), Canada (0.4 per cent), Sweden (0.3 per cent) and the UK (0.3 per cent). Nevertheless, the number of respondents was small at least in Malta, Norway and France, which might make the results biased. Due to this, it is not possible to say if the risk for contamination is more relevant in some countries compared with others.
Previous studies have reported that there are bacteria present in raw pet foods.18–22 Also, dogs fed raw meat are in some studies shown to shed more Salmonella and E coli in their faeces.15 23 24 In a study by Iennarella-Servantez,14 from 16 samples, taken from 4 different raw meat diets, 2 samples (from a beef meat diet) were positive for E coli, indicating contamination of microbes from faecal sources. In the same study, 2 faecal samples (out of 36 samples) tested positive for Salmonella species (pork meat fed dogs), and 3 saliva samples out of 36 samples tested positive for E coli (two dogs fed pork meat and one fed horse meat); nevertheless, the sample size of that study was somewhat small. Interestingly, in a study by Joffe and Schlesinger,27 Salmonella species were cultured from 80per cent of the raw meat diets but found only in 30per cent of the faecal samples from the dogs fed those diets.
Salmonella has also been found in dry pet foods and treats, including some antibiotic-resistant strains.20 28–33 In addition, Listeria greyii has been found in a dry pet food.20 One study reported no difference in the carriage of Campylobacter species in dogs fed either a raw or a dry diet,34 and one study found Salmonella species in three dogs that had not been given raw food of any kind and found no Salmonella species in dogs fed raw foods (n=24).35 In addition to Salmonella, different kinds of mycotoxins36–39 have been found in heat-treated pet foods, causing health problems to the pets themselves. These studies show that there is not enough research done to establish if a raw pet food is a bigger health risk than other, often heat treated, pet foods.
In the present study, 12 and 17 households reported Salmonella and Campylobacter infections, respectively. These two bacterial pathogens are the most frequently reported causes for human enteritis in Europe,40 indicating that the questionnaire answers were valid. Human salmonellosis and campylobacteriosis are typically associated with poultry and products thereof. In this study, over 80per cent of all the households fed broiler/chicken to their pets. The households that reported infections did not use more poultry than others, although poultry is often regarded as unsafe to feed raw. Beef, which is commonly associated with human Salmonella infections, was also frequently (about 90per cent) included in the raw pet diets. However, there may also be other transmission routes such as from infected humans or from farm and wild animals. Campylobacter particularly, may be transmitted through water contaminated with faeces of wild birds.41
To the authors’ knowledge, there are no reported outbreaks of human salmonellosis from commercial raw pet foods, but association with commercial dry pet foods and treats have been reported previously.29 33 42–44 A study by Lambertini and others45 showed that Salmonella can survive 19 months in dry dog food. In the present study, in 33per cent of the households that reported a Salmonella infection, the family pet was fed with dry pet food in addition to raw food. This makes it impossible to say if the Salmonella was transmitted from the raw or dry pet food, since the source was not confirmed.
In addition to cooked food, people also eat a lot of raw foods, like vegetables, fruits and berries, which can be contaminated with L monocytogenes,46 47 E coli,48–50 Salmonella 49–53 and Campylobacter,54 and compared with pet food recalls, recalls in human food are much more frequent.55 Salmonella and Campylobacter can also be isolated from the hands, contact surfaces and cloths in the households where contaminated chicken for human consumption have been handled.56 In the current study, 92per cent (n=36) of the CTP households did not confirm the source of the pathogen, and for this reason there is a possibility that the people in those households might have been infected by other sources.
Human yersiniosis is mainly associated with raw or undercooked pork.57 The most important infection sources for toxoplasmosis are raw pork or small ruminant meat.57 58 Infection can also be obtained from a contaminated environment, directly from cat faeces and from vegetables and/or fruits contaminated with water containing oocysts.59 Pork was included in the pet food in around 50per cent of the all households in this study but human Yersinia and Toxoplasma infections were reported in only one and two households, respectively. One of the two households with reported Toxoplasma infection did not use pork to feed the pet, and in the other case the source of infection was reported to be beef meat. A human Toxoplasma infection acquired from the pet is possible only if the pet is a cat, and in both households with reported Toxoplasma infection, the pet fed with raw food was a dog (table 5). E coli and Clostridium were isolated from humans in eight and three households, respectively. These bacteria are normally found in faeces of healthy humans and animals and are used as indicators of faeces contamination.60 The owners did not report if they would have been Shiga toxin-producing E coli (STEC) or not.
Although there has been some concern, especially with immunocompromised pet owners and zoonotic diseases,61 in the present study 9.9per cent (n=1617) of the raw feeders from the NPT households reported that there were immunocompromised people living in the household. Among both CTP and STP households (n=63), four (6.3 per cent) reported the infected person to be immunocompromised. In addition, there were significantly more children between the age of two and six living in the CTP households than in the STP or NTP households, and still most of the infected people were adults, probably the ones handling the pet food. This might indicate that in these households the pet food is handled in a way that small children or immunocompromised do not get in contact with it since they are considered as risk groups for infections.62–64
Many times, the infectious intestinal disease is contracted at home,65 66 which could be prevented with good hygiene practices. Poor hygiene of hands, and in toilet, bathroom or kitchen, increases the risk of foodborne infections as well as infection between family members and pets.66 As always when handling raw meat products, safe food handling should be followed, and pet owners should be advised with safe food handling and safe clean-up strategies.9 At least in the UK and Finland, governmental bodies have issued fact sheets to dog and cat owners on responsible raw feeding.67 68 On the other hand, there is also plenty of anecdotal evidence of raw feeding households that store and handle their pets’ food in the same place as their own, and still report that neither animals nor humans get sick in their households. In the present study, only 7.2per cent of the NTP households reported handling the raw pet food in a different place than the human food and a majority (68.4 per cent) used the same place and the same utensils that they used with human food. Also, the regression model showed a negative association with both, feeding ≥50 per cent of the diet as raw food and handling it in the same place and with the same utensils as the human food was handled. Since there were not more detailed questions asked about the hygiene practices related to raw meat handling in the present questionnaire, more research is needed to confirm the safest way to handle raw pet food. The fact that one-fifth of the CTP and STP households handled the raw pet food always in a different place than human food, might also reflect a change in the food handling practices as more cautious after an infection, and might not be the usual habits of those households.
There are limitations in survey studies, nevertheless. Those ‘against’ raw feeding might give false positive answers about the transmission of the pathogens and on the other hand, those ‘in favour’ of raw feeding can give false negative answers. Nevertheless, the risk for false answers is equally present in both situations. Pet owners that have been sensitised by the fact that they themselves or their pets have been infected with a pathogen might be more motivated to answer to the survey. In addition, data may be biased as people have evaluated the source of contamination by themselves, introducing some false positives into the data. Only three households were able to confirm that the same pathogen was found in the raw pet food and in the infected human (in two households also the family pet had clinical signs). Also, some mild symptoms might have been overlooked in some households, introducing false negatives into the data. Since the study was conducted worldwide, differences in the diagnostic access and protocols might also exist between countries. The lack of pet owners that feed their pets dry food as a control group could be seen as one limitation in this study. This group was not included in the study since it was not considered as a reliable control group as the owners and medical doctors would have not considered dry pet food as a possible transmission route for pathogens in the same way that raw meat is considered.
As a conclusion, this large study population from all over the world shows that the transmission of zoonotic pathogens might happen, but it seems to be sporadic. It is clear that the precise source of the pathogen is often challenging to find, which makes the interpretation of the result difficult. However, studies using different kind of approaches should be conducted in the future to be able to get a better understanding of the true risks or possible health benefits of feeding raw food diets to pets. This way the true pros and cons can be accurately analysed, before asking pet owners not to feed their pets with a nutritionally balanced raw diet.
Acknowledgments
The authors would like to thank Maria Fredriksson-Ahomaa for her valuable comments and input. The authors would also like to thank statistician Vesa Niskanen for his help in the revised manuscript.
Footnotes
Funding: SZ-L was supported by a postdoctoral contract co-funded by the XXI University of Cordoba Intramural Research Program and the European Regional Development Funds (FEDER). SMB-M was supported by the Brazilian National Council for Research (CNPq-PQ-Proc. 307813/2018-5). Svenska Kulturfonden in Finland contributed to the funding.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Michel KE, Willoughby KN, Abood SK, et al. . Attitudes of PET owners toward PET foods and feeding management of cats and dogs. J Am Vet Med Assoc 2008;233:1699–703. 10.2460/javma.233.11.1699 [DOI] [PubMed] [Google Scholar]
- 2. Wall T. Raw PET food sales growing despite health warnings. PET food industry, 2018. Available: http://www.petfoodindustry-digital.com/201803/index.php#/1 [Accessed 8 Mar 2018].
- 3. Michel KE. Unconventional diets for dogs and cats. Vet Clin North Am Small Anim Pract 2006;36:1269–81. 10.1016/j.cvsm.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 4. Berschneider HM. Alternative diets. Clin Tech Small Anim Pract 2002;17:1–5. 10.1053/svms.2002.27782 [DOI] [PubMed] [Google Scholar]
- 5. Corbee RJ, Breed RD, Hazewinkel HAW. Feeding practice of dog owners active on Internet forums. poster session presented at: 17th European Society of veterinary and comparative nutrition Congress. Ghent, Belgium, 2013. [Google Scholar]
- 6. Dinallo GK, Poplarski JA, Van Deventer GM, et al. . A survey of feeding, activity, supplement use and energy consumption in North American agility dogs. J Nutr Sci 2017;6:e45 10.1017/jns.2017.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laflamme DP, Abood SK, Fascetti AJ, et al. . Pet feeding practices of dog and cat owners in the United States and Australia. J Am Vet Med Assoc 2008;232:687–94. 10.2460/javma.232.5.687 [DOI] [PubMed] [Google Scholar]
- 8. Connolly KM, Heinze CR, Freeman LM. Feeding practices of dog breeders in the United States and Canada. J Am Vet Med Assoc 2014;245:669–76. 10.2460/javma.245.6.669 [DOI] [PubMed] [Google Scholar]
- 9. Stogdale L, Diehl G. In support of bones and RAW food diets. Can Vet J 2003;44:783–4. [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman LM, Chandler ML, Hamper BA, et al. . Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J Am Vet Med Assoc 2013;243:1549–58. 10.2460/javma.243.11.1549 [DOI] [PubMed] [Google Scholar]
- 11. Dijcker JC, Hagen-Plantinga EA, Everts H, et al. . Dietary and animal-related factors associated with the rate of urinary oxalate and calcium excretion in dogs and cats. Vet Rec 2012;171 10.1136/vr.100293 [DOI] [PubMed] [Google Scholar]
- 12. Sandri M, Dal Monego S, Conte G, et al. . Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet Res 2016;13:65 10.1186/s12917-017-0981-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Algya KM, Cross TL, Leuck KN, et al. . Apparent total tract macronutrient digestibility, serum chemistry, urinalysis, and fecal characteristics, metabolites and microbiota of adult dogs fed extruded, mildly cooked, and RAW diets. Journal of animal science. Jun 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iennarella-Servantez CA. Evaluation of raw meat diets on macronutrient digestibility, fecal output, microbial presence, and general health status in domestic dogs. graduate thesis and dissertations. Iowa State University 2017. [Google Scholar]
- 15. Lefebvre SL, Reid-Smith R, Boerlin P, et al. . Evaluation of the risks of shedding Salmonellae and other potential pathogens by therapy dogs fed raw diets in Ontario and Alberta. Zoonoses Public Health 2008;55:470–80. 10.1111/j.1863-2378.2008.01145.x [DOI] [PubMed] [Google Scholar]
- 16. Cima G. Raw food policy draws debate: AVMA advises against feeding dogs, cats RAW animal proteins. J Am Vet Med Assoc 2012;241:679–80. [PubMed] [Google Scholar]
- 17. Burns, J Raw feeding and the risks to people. veterinary records. 181, 2017: 149. [DOI] [PubMed] [Google Scholar]
- 18. Weese JS, Rousseau J, Arroyo L. Bacteriological evaluation of commercial canine and feline RAW diets. Can Vet J 2005;46:513–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Finley R, Reid-Smith R, Ribble C, et al. . The occurrence and antimicrobial susceptibility of salmonellae isolated from commercially available canine raw food diets in three Canadian cities. Zoonoses Public Health 2008;55:462–9. 10.1111/j.1863-2378.2008.01147.x [DOI] [PubMed] [Google Scholar]
- 20. Nemser SM, Doran T, Grabenstein M, et al. . Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various PET foods. Foodborne Pathog Dis 2014;11:706–9. 10.1089/fpd.2014.1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fredriksson-Ahomaa M, Heikkilä T, Pernu N, et al. . Raw meat-based diets in dogs and cats. Vet Sci 2017;4. doi: 10.3390/vetsci4030033. [Epub ahead of print: 28 Jun 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Bree FPJ, Bokken GCAM, Mineur R, et al. . Zoonotic bacteria and parasites found in RAW meat-based diets for cats and dogs. Veterinary Record 2018;182 10.1136/vr.104535 [DOI] [PubMed] [Google Scholar]
- 23. Chengappa MM, Staats J, Oberst RD, et al. . Prevalence of Salmonella in RAW meat used in diets of racing greyhounds. J Vet Diagn Invest 1993;5:372–7. 10.1177/104063879300500312 [DOI] [PubMed] [Google Scholar]
- 24. Morley PS, Strohmeyer RA, Tankson JD, et al. . Evaluation of the association between feeding raw meat and Salmonella enterica infections at a Greyhound breeding facility. J Am Vet Med Assoc 2006;228:1524–32. 10.2460/javma.228.10.1524 [DOI] [PubMed] [Google Scholar]
- 25. Schlesinger DP, Joffe DJ. Raw food diets in companion animals: a critical review. Can Vet J 2011;52:50–4. [PMC free article] [PubMed] [Google Scholar]
- 26. Hullegie S, Bruijning-Verhagen P, Uiterwaal CSPM, et al. . First-Year daycare and incidence of acute gastroenteritis. Pediatrics 2016;137:e20153356 10.1542/peds.2015-3356 [DOI] [PubMed] [Google Scholar]
- 27. Joffe DJ, Schlesinger DP. Preliminary assessment of the risk of Salmonella infection in dogs fed raw chicken diets. Can Vet J 2002;43:441–2. [PMC free article] [PubMed] [Google Scholar]
- 28. Pitout JDD, Reisbig MD, Mulvey M, et al. . Association between handling of PET treats and infection with Salmonella enterica serotype newport expressing the AmpC beta-lactamase, CMY-2. J Clin Microbiol 2003;41:4578–82. 10.1128/JCM.41.10.4578-4582.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Behravesh CB, Ferraro A, Deasy M, et al. . Human Salmonella infections linked to contaminated dry dog and cat food, 2006-2008. Pediatrics 2010;126:477–83. 3rd 10.1542/peds.2009-3273 [DOI] [PubMed] [Google Scholar]
- 30. Selmi M, Stefanelli S, Bilei S, et al. . Contaminated commercial dehydrated food as source of multiple Salmonella serotypes outbreak in a municipal kennel in Tuscany. Vet Ital 2011;47:183–90. [PubMed] [Google Scholar]
- 31. Li X, Bethune LA, Jia Y, et al. . Surveillance of Salmonella prevalence in animal feeds and characterization of the Salmonella isolates by serotyping and antimicrobial susceptibility. Foodborne Pathog Dis 2012;9:692–8. 10.1089/fpd.2011.1083 [DOI] [PubMed] [Google Scholar]
- 32. Bird P, Flannery J, Crowley E, et al. . Evaluation of the 3M™ Petrifilm™ Evaluation of the 3m™ petrifilm™ Salmonella express system. J AOAC Int 2014;97:1563–75. [DOI] [PubMed] [Google Scholar]
- 33. Imanishi M, Rotstein DS, Reimschuessel R, et al. . Outbreak of Salmonella enterica serotype Infantis infection in humans linked to dry dog food in the United States and Canada, 2012. J Am Vet Med Assoc 2014;244:545–53. 10.2460/javma.244.5.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olkkola S, Kovanen S, Roine J, et al. . Population genetics and antimicrobial susceptibility of canine Campylobacter isolates collected before and after a raw feeding experiment. PLoS One 2015;10:e0132660 10.1371/journal.pone.0132660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lefebvre SL, Waltner-Toews D, Peregrine AS, et al. . Prevalence of zoonotic agents in dogs visiting hospitalized people in Ontario: implications for infection control. J Hospital Infection 2006;62:458–66. 10.1016/j.jhin.2005.09.025 [DOI] [PubMed] [Google Scholar]
- 36. Boermans HJ, Leung MCK. Mycotoxins and the PET food industry: toxicological evidence and risk assessment. Int J Food Microbiol 2007;119:95–102. 10.1016/j.ijfoodmicro.2007.07.063 [DOI] [PubMed] [Google Scholar]
- 37. Błajet-Kosicka A, Kosicki R, Twarużek M, et al. . Determination of moulds and mycotoxins in dry dog and cat food using liquid chromatography with mass spectrometry and fluorescence detection. Food Addit Contam Part B Surveill 2014;7:302–8. 10.1080/19393210.2014.933269 [DOI] [PubMed] [Google Scholar]
- 38. Singh SD, Chuturgoon AA. A comparative analysis of mycotoxin contamination of supermarket and premium brand pelleted dog food in Durban, South Africa. J S Afr Vet Assoc 2017;88:e1–6. 10.4102/jsava.v88i0.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh SD, Baijnath S, Chuturgoon AA. A comparison of mycotoxin contamination of premium and grocery brands of pelleted cat food in South Africa. J S Afr Vet Assoc 2017;88:e1–4. 10.4102/jsava.v88i0.1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. EFSA & ECDC The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016. EFSA J 2016;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Griekspoor P, Engvall EO, Åkerlind B, et al. . Genetic diversity and host associations in Campylobacter jejuni from human cases and broilers in 2000 and 2008. Vet Microbiol 2015;178:94–8. 10.1016/j.vetmic.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 42. Centers for Disease Control and Prevention (CDC) Human salmonellosis associated with animal-derived PET treats—United states and Canada, 2005. Morbidity and Morb Mortal Rep 2006;55:702–5. [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention (CDC) Update: recall of dry dog and cat food products associated with human Salmonella Schwarzengrund infections-United states, 2008. MMWR Morb Mortal Wkly Rep 2008;57:1200–2. [PubMed] [Google Scholar]
- 44. Centers for Disease Control and Prevention (CDC) Notes from the field: human Salmonella infantis infections linked to dry dog food-United states and Canada. Morb Mortal Wkly Rep 2012;61. [PubMed] [Google Scholar]
- 45. Lambertini E, Mishra A, Guo M, et al. . Modeling the long-term kinetics of Salmonella survival on dry pet food. Food Microbiol 2016;58:1–6. 10.1016/j.fm.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 46. Concha-Meyer A, Eifert J, Williams R, et al. . Survival of Listeria monocytogenes on fresh blueberries (Vaccinium corymbosum) stored under controlled atmosphere and ozone. J Food Prot 2014;77:832–6. 10.4315/0362-028X.JFP-13-441 [DOI] [PubMed] [Google Scholar]
- 47. Hadjilouka A, Andritsos ND, Paramithiotis S, et al. . Listeria monocytogenes serotype prevalence and biodiversity in diverse food products. J Food Prot 2014;77:2115–20. 10.4315/0362-028X.JFP-14-072 [DOI] [PubMed] [Google Scholar]
- 48. Arthur L, Jones S, Fabri M, et al. . Microbial survey of selected Ontario-grown fresh fruits and vegetables. J Food Prot 2007;70:2864–7. 10.4315/0362-028X-70.12.2864 [DOI] [PubMed] [Google Scholar]
- 49. Wijnands LM, Delfgou-van Asch EHM, Beerepoot-Mensink ME, et al. . Prevalence and concentration of bacterial pathogens in RAW produce and minimally processed packaged salads produced in and for the Netherlands. J Food Prot 2014;77:388–94. 10.4315/0362-028X.JFP-13-135 [DOI] [PubMed] [Google Scholar]
- 50. Kłapeć T, Wójcik-Fatla A, Cholewa A, et al. . Microbiological characterization of vegetables and their rhizosphere soil in eastern Poland. Ann Agric Environ Med 2016;23:559–65. 10.5604/12321966.1226846 [DOI] [PubMed] [Google Scholar]
- 51. Quiroz-Santiago C, Rodas-Suárez OR, Carlos R V, et al. . Prevalence of Salmonella in vegetables from Mexico. J Food Prot 2009;72:1279–82. 10.4315/0362-028X-72.6.1279 [DOI] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention (CDC) Multistate outbreaks of Salmonella infections associated with raw tomatoes eaten in restaurants—United States, 2005–2006. Morb Mortal Wkly Rep 2007;56:909–11. [PubMed] [Google Scholar]
- 53. Greene SK, Daly ER, Talbot EA, et al. . Recurrent multistate outbreak of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol Infect 2008;136:157–65. 10.1017/S095026880700859X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Verhoeff-Bakkenes L, Jansen HAPM, in 't Veld PH, et al. . Consumption of raw vegetables and fruits: a risk factor for Campylobacter infections. Int J Food Microbiol 2011;144:406–12. 10.1016/j.ijfoodmicro.2010.10.027 [DOI] [PubMed] [Google Scholar]
- 55. FDA Recalls, market withdrawals, & safety alerts, 2018. Available: https://www.fda.gov/Safety/Recalls/default.htm#additional-info [Accessed Mar 2018].
- 56. Cogan TA, Bloomfield SF, Humphrey TJ. The effectiveness of hygiene procedures for prevention of cross-contamination from chicken carcases in the domestic kitchen. Lett Appl Microbiol 1999;29:354–8. 10.1046/j.1472-765X.1999.00656.x [DOI] [PubMed] [Google Scholar]
- 57. Felin E, Jukola E, Raulo S, et al. . Meat juice serology and improved food chain information as control tools for Pork-Related public health hazards. Zoonoses Public Health 2015;62:456–64. 10.1111/zph.12174 [DOI] [PubMed] [Google Scholar]
- 58. Opsteegh M, Maas M, on behalf of the consortium . EFSA external scientific report. Relationship between seroprevalence in the main livestock species and presence of Toxoplasma gondii in meat (GP/EFSA/BIOHAZ/2013/01), 2016. An extensive literature review. Final report. Available at Available: www.efsa.europa.eu/publications
- 59. EFSA, European Food Safety Authority . Surveillance and monitoring of Toxoplasma in humans, food and animals - Scientific Opinion of the Panel on Biological Hazards. EFSA J 2007;583:1–64. [Google Scholar]
- 60. Jørgensen F, Sadler-Reeves L, Shore J, et al. . An assessment of the microbiological quality of lightly cooked food (including sous-vide) at the point of consumption in England. Epidemiol Infect 2017;145:1500–9. 10.1017/S0950268817000048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mani I, Maguire JH. Small animal zoonoses and Immuncompromised PET owners. Top Companion Anim Med 2009;24:164–74. 10.1053/j.tcam.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 62. Trevejo RT, Barr MC, Robinson RA. Important emerging bacterial zoonotic infections affecting the immunocompromised. Vet Res 2005;36:493–506. 10.1051/vetres:2005011 [DOI] [PubMed] [Google Scholar]
- 63. Schielke A, Rosner BM, Stark K. Epidemiology of campylobacteriosis in Germany - insights from 10 years of surveillance. BMC Infect Dis 2014;14:30 10.1186/1471-2334-14-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boqvist S, Pettersson H, Svensson A, et al. . Sources of sporadic Yersinia enterocolitica infection in children in Sweden, 2004: a case-control study. Epidemiol Infect 2009;137:897–905. 10.1017/S0950268808001209 [DOI] [PubMed] [Google Scholar]
- 65. Scuderi G, Fantasia M, Filetici E, et al. . Foodborne outbreaks caused by Salmonella in Italy, 1991-4. Epidemiol Infect 1996;116:257–65. 10.1017/S0950268800052559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bloomfield SF. Home hygiene: a risk approach. Int J Hyg Environ Health 2003;206:1–8. 10.1078/1438-4639-00193 [DOI] [PubMed] [Google Scholar]
- 67. EVIRA, Finnish food safety authority Koirien JA kissojen eläinperäinen ruoka, 2018. Only in Finnish Available: https://www.evira.fi/globalassets/elaimet/rehut/ohjeet/koirien-ja-kissojen-raakaruoka-12834.pdf [Accessed 20 Jun 2018].
- 68. PFMA, Pet food manufacturers’ association Responsible RAW feeding for cats and dogs, 2017. Available: https://www.pfma.org.uk/_assets/docs/fact-sheet/PFMA-fact-sheet-raw-feeding.pdf [Accessed 15 Jun 2018].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
vetrec-2018-105122supp001.pdf (1.5MB, pdf)