Abstract
Background
Comorbid depression and substance use disorders are common and have poorer outcomes than either disorder alone. While effective psychological treatments for depression or substance use disorders are available, relatively few randomised controlled trials (RCTs) have examined the efficacy of these treatments in people with these comorbid disorders.
Objectives
To assess the efficacy of psychological interventions delivered alone or in combination with pharmacotherapy for people diagnosed with comorbid depression and substance use disorders.
Search methods
We searched the following databases up to February 2019: Cochrane Central Register of Controlled Trials, PubMed, Embase, CINAHL, Google Scholar and clinical trials registers. All systematic reviews identified, were handsearched for relevant articles.
Selection criteria
The review includes data from RCTs of psychological treatments for people diagnosed with comorbid depression and substance use disorders, using structured clinical interviews. Studies were included if some of the sample were experiencing another mental health disorder (e.g. anxiety); however, studies which required a third disorder as part of their inclusion criteria were not included. Studies were included if psychological interventions (with or without pharmacotherapy) were compared with no treatment, delayed treatment, treatment as usual or other psychological treatments.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
Seven RCTs of psychological treatments with a total of 608 participants met inclusion criteria. All studies were published in the USA and predominately consisted of Caucasian samples. All studies compared different types of psychological treatments. Two studies compared Integrated Cognitive Behavioural Therapy (ICBT) with Twelve Step Facilitation (TSF), another two studies compared Interpersonal Psychotherapy for Depression (IPT‐D) with other treatment (Brief Supportive Therapy (BST) or Psychoeducation). The other three studies compared different types or combinations of psychological treatments. No studies compared psychological interventions with no treatment or treatment as usual control conditions. The studies included a diverse range of participants (e.g. veterans, prisoners, community adults and adolescents).
All studies were at high risk of performance bias, other main sources were selection, outcome detection and attrition bias. Due to heterogeneity between studies only two meta‐analyses were conducted. The first meta‐analysis focused on two studies (296 participants) comparing ICBT to TSF. Very low‐quality evidence revealed that while the TSF group had lower depression scores than the ICBT group at post‐treatment (mean difference (MD) 4.05, 95% confidence interval (CI) 1.43 to 6.66; 212 participants), there was no difference between groups in depression symptoms (MD 1.53, 95% CI ‐1.73 to 4.79; 181 participants) at six‐ to 12‐month follow‐up. At post‐treatment there was no difference between groups in proportion of days abstinent (MD ‐2.84, 95% CI ‐8.04 to 2.35; 220 participants), however, the ICBT group had a greater proportion of days abstinent than the TSF group at the six‐ to 12‐month follow‐up (MD 10.76, 95% CI 3.10 to 18.42; 189 participants). There were no differences between the groups in treatment attendance (MD ‐1.27, 95% CI ‐6.10 to 3.56; 270 participants) or treatment retention (RR 0.95, 95% CI 0.72 to 1.25; 296 participants).
The second meta‐analysis was conducted with two studies (64 participants) comparing IPT‐D with other treatment (Brief Supportive Psychotherapy/Psychoeducation). Very low‐quality evidence indicated IPT‐D resulted in significantly lower depressive symptoms at post‐treatment (MD ‐0.54, 95% CI ‐1.04 to ‐0.04; 64 participants), but this effect was not maintained at three‐month follow‐up (MD 3.80, 95% CI ‐3.83 to 11.43) in the one study reporting follow‐up outcomes (38 participants; IPT‐D versus Psychoeducation). Substance use was examined separately in each study, due to heterogeneity in outcomes. Both studies found very low‐quality evidence of no significant differences in substance use outcomes at post‐treatment (percentage of days abstinent, IPD versus Brief Supportive Psychotherapy; MD ‐2.70, 95% CI ‐28.74 to 23.34; 26 participants) or at three‐month follow‐up (relative risk of relapse, IPT‐D versus Psychoeducation; RR 0.67, 95% CI 0.30 to 1.50; 38 participants). There was also very low‐quality evidence for no significant differences between groups in treatment retention (RR 1.00, 95% CI 0.81 to 1.23; 64 participants). No adverse events were reported in any study.
Authors' conclusions
The conclusions of this review are limited due to the low number and very poor quality of included studies. No conclusions can be made about the efficacy of psychological interventions (delivered alone or in combination with pharmacotherapy) for the treatment of comorbid depression and substance use disorders, as they are yet to be compared with no treatment or treatment as usual in this population. In terms of differences between psychotherapies, although some significant effects were found, the effects were too inconsistent and small, and the evidence of too poor quality, to be of relevance to practice.
Plain language summary
Do psychological interventions work for people with both depression and substance use disorders?
What is the aim of this review?
The aim of this Cochrane Review was to find out if psychological interventions (delivered with or without pharmacotherapy) are effective for the treatment of comorbid depression and substance use disorders. Cochrane researchers collected and analysed all relevant studies to answer this question.
Key messages
No conclusions about the effectiveness of psychological interventions for the treatment of comorbid depression and substance use disorders can be made, due to the low number of studies found and very low quality of the evidence. More high‐quality studies comparing psychological interventions versus no treatment, delayed treatment, treatment as usual and other psychological interventions are needed.
What was studied in the review?
Comorbidity occurs for people experiencing mental disorders when the same person has two or more mental disorders. People diagnosed with depression are more likely to have substance use disorders, and vice versa. Comorbid disorders are associated with poorer clinical, social and vocational outcomes than either disorder alone. Psychological treatments for comorbid depression and substance use disorders are available, but relatively few have been tested. These treatments target psychological (thoughts, feelings, behaviours), social (family and personal relationships), and environmental risk factors (access to drugs) for depression and substance use.
What are the main results of the review?
The review authors searched for studies and found seven randomised controlled trials involving 608 people with comorbid depression and substance use disorders published between 2003 and 2014. All seven studies were published in the USA and predominately consisted of individuals from Caucasian backgrounds. No conclusions about the effectiveness of psychological interventions delivered with or without pharmacotherapy could be made, as no studies comparing these interventions with no treatment, delayed treatment or treatment as usual were found. All seven studies compared different types or combinations of psychological treatments. Few consistent differences in depression or substance use treatment outcomes were found. No conclusions about which type of psychological intervention was most effective could be made, due to the low number of studies found and very low quality of the evidence . None of the studies reported any harms related to receiving psychological treatment for depression and substance use disorders. All studies were funded by university and government research grants in the USA.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to February 2019.
Summary of findings
Background
Description of the condition
Comorbidity occurs for people experiencing mental disorders when the same person is diagnosed with two or more mental disorders using Diagnostic and Statistical Manual (DSM)/International Statistical Classification of Diseases and Related Health Problems (ICD) criteria (American Psychiatric Association 2000; World Health Organization 1992). People diagnosed with substance use disorders are more likely to have a depression disorder, and vice versa. High rates of comorbid substance use and depression disorders have consistently been reported in epidemiological surveys (Australian Bureau of Statistics 2007; Degenhardt 2001; EMCDDA 2013; Farrell 2001; Grant 2004; Jane‐Llopis 2006; Kessler 2003). The United States National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) found that individuals with alcohol or drug dependence were four and nine times more likely to experience major depression, respectively than individuals with no substance dependence (Grant 2004). Similarly in Australia, the National Survey of Mental Health and Wellbeing found that individuals with an alcohol or drug use disorder were 3 to 4 times more likely to have experienced depression, or another affective disorder, in the past 12 months compared with the general population (Teesson 2009; Teesson 2010). Within treatment settings, rates of comorbid substance use and affective disorders are even higher, with 30% to 50% of patients meeting criteria for concurrent major depression (Baker 2007a; Bulkstein 1992; Grella 2001; Hall 2009; Lejoyeux 2011; Lubman 2007; Teesson 2005). Such high rates of comorbid disorders are problematic, as they have been linked to treatment non‐compliance, a more severe and chronic illness course, and an increased risk of relapse in both substance use and co‐occurring mental disorders, as well as greater social and vocational impairment, poor physical health, higher risk of suicidal behaviour and greater use of health services (Australian Bureau of Statistics 2007; Davis 2008; Hasin 2002; Sullivan 2005; Šprah 2017).
Description of the intervention
Psychological interventions are theory‐based, manualised approaches to the treatment of depression, substance use and other mental disorders. Common approaches include the following.
Cognitive behavior therapy (CBT), a family of interventions targeting cognitions, behaviours, emotions and environmental factors ,which may predispose, precipitate or perpetuate comorbid depression and substance use disorders (Beck 2011). CBT approaches include cognitive therapy (CT), behaviour therapy (BT), traditional CBT approaches and ‘third wave’ approaches including acceptance and commitment therapy, dialectical behaviour therapy and mindfulness‐based interventions (Hofmann 2012b; Wells 2016).
Motivational interviewing is a psychotherapeutic approach that aims to elicit behaviour change by first exploring and resolving ambivalence about making a change (Miller 2013). A number of strategies are then utilised to enhance commitment to making the change. Motivational interviewing is commonly delivered in combination with other psychotherapies including CBT. Only studies combining motivational interviewing with CBT were included in this review.
Interpersonal psychotherapy (IPT) is most commonly used to treat depression (Frank 2011). It focuses on the role of difficulties in everyday interactions with others on depressive symptoms by targeting the individual's emotional responses to life stressors, role disputes and role transitions (Frank 2011; Stuart 2012).
Contingency management is most‐widely used in the treatment of substance use disorders. Based on the principles of operant conditioning, it provides incentives or rewards to encourage behaviour change (Petry 2012). Typical reward systems include the use of abstinence‐based voucher programs, which increase in value with each consecutive negative drug test (Petry 2012).
How the intervention might work
Psychological interventions aim to modify individual, family, social and environmental factors that may increase risk of depression and substance use disorders (Baker 2007b). Psychological approaches vary depending on the theoretical models underpinning them, but typically target thoughts, feelings, behaviours, interpersonal relationships, social and environmental variables that may predispose, precipitate or perpetuate depression and substance use (Baker 2007b; NCCMH 2009). They can be delivered face‐to‐face, online, via telephone or bibliotherapy (e.g. self‐help books; NCCMH 2009).
Why it is important to do this review
There have been numerous studies investigating the efficacy of psychological interventions for people experiencing depression or substance use disorders. Manualised psychological interventions, including CBT (Cuijpers 2013; Hofmann 2012a), motivational interviewing (Smedslund 2011), IPT (Cuijpers 2011) and contingency management (Benishek 2014; Prendergast 2006), have shown some promise in individually reducing symptoms related to each of these disorders. However, many of the trials excluded comorbid disorders making it difficult to ascertain the efficacy of these interventions for those experiencing both disorders. While there have been some trials examining the efficacy of these treatments within comorbid populations, the few existing meta‐analyses or systematic reviews on comorbid substance use and depression have either mainly focused on pharmacological interventions (e.g. Agabio 2018; Foulds 2015; Pani 2010; Zhou 2015), have included subclinical populations for either depression and/or substance use (Babowitch 2016; Baker 2012; Hesse 2009), or did not exclusively examine randomised controlled trials (Riper 2014). There have been no systematic reviews conducted to determine which psychological intervention is most efficacious among individuals with comorbid depression and substance use disorders.
Objectives
To assess the efficacy of psychological interventions delivered alone or in combination with pharmacotherapy for people diagnosed with comorbid depression and any substance use disorder (excluding nicotine).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Types of participants
Individuals (adults and adolescents) with co‐occurring Diagnostic and Statistical Manual (DSM) or International Classification of Diseases (ICD) depression and substance use disorder (excluding nicotine) derived using a structured clinical interview were included. Where possible, those with psychosis, bipolar disorder and intellectual disability were excluded as these individuals form distinct clinical groups with specific needs. Studies were included if some of the sample were experiencing another mental health disorder (e.g. anxiety); however, studies which required a third disorder as part of their inclusion criteria were not included in the review.
Types of interventions
Experimental conditions (+/‐ pharmacotherapy)
CBT with and without motivational interviewing
Cognitive therapy
Behaviour therapy
Contingency management
Acceptance and commitment therapy
Dialectical behaviour therapy
Interpersonal psychotherapy (IPT)
Control conditions (+/‐ pharmacotherapy)
No, minimal or delayed treatment
Other psychological interventions (including studies comparing those listed above)
Treatment as usual (defined according to study setting but typically consists of case management)
Types of outcome measures
Primary outcomes
Depression: changes in symptom severity on a standardised questionnaire (e.g. Beck Depression Inventory (BDI) or Hamilton Depression Rating Scale (HDRS)) or presence of DSM/ICD disorder on a structured clinical interview (e.g. the Structured Clinical Interview for DSM‐IV (SCID‐IV)).
Substance use: changes in the frequency (including abstinence), quantity, severity of use measured by calendar‐based methods such as Timeline Follow Back (TLFB) and self‐report instruments such as the Alcohol Use Disorders Identification Test (AUDIT) or presence of DSM/ICD substance use disorders.
Treatment retention as measured by the number of participants still in treatment at the end of the study, and treatment attendance as assessed by the average number of sessions attended.
Secondary outcomes
Functioning: changes in social, occupational/educational functioning as measured by changes on standardised measures of quality of life (e.g., World Health Organization‐Quality of Life Scale) or daily functioning (e.g. Global Assessment of Functioning (GAF) scale.
Anxiety: changes in symptom severity on a standardised questionnaire (e.g. Beck Anxiety Inventory (BAI)) or presence of DSM/ICD disorder on a structured clinical interviews (e.g. SCID‐IV).
Global clinical severity of mental health disorders: as measured by changes on standardised instruments such as the Clinical Global Impression Scale (CGI) scale.
Adverse effects linked to treatments delivered.
Search methods for identification of studies
Electronic searches
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
We searched the following databases up to 04 February 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL; (2019, Issue 1), in the Cochrane Library using the strategy in Appendix 1;
PubMed (from 1966 to 04 February 2019) using the strategy in Appendix 2;
Embase (from 1980 to 04 February 2019) using the strategy in Appendix 3;
CINAHL (from 1982 to 04 February 2019) using the strategy in Appendix 4;
Google Scholar, scholar.google.com (searched on 04 February 2019).
We searched the following trials registries on 25 February 2019:
the ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We handsearched relevant articles, systematic or meta‐analytic reviews. We also searched grey literature including internal reports and conference proceedings to identify unpublished studies and we contacted the authors of these studies to obtain relevant information.
Data collection and analysis
Selection of studies
We merged all search results (including records identified from electronic searches and other resources) into Endnote and deleted duplicate records.
Two review authors independently examined titles and abstracts and deleted obviously irrelevant records
Two review authors independently assessed full‐text articles of the potentially relevant records identified for inclusion in the review and linked multiple reports of the same study.
In the event of a disagreement by the independent review authors, resolution followed a step‐wise process. Initially the review authors discussed the disagreement to establish whether there had been an error by one of the extractors that could easily be resolved. If the disagreement remained unresolved, the next step was to contact the study authors directly and any issues that persisted would be reported explicitly in the review. At all stages of this process the presence and resolution of disagreements were recorded and coding techniques were used to differentiate consensus data from extracted data across both review authors.
Data extraction and management
Two review authors independently extracted data using a standardised data collection checklist. We resolved any disagreements via consultation with a third review author.
The following data were extracted.
Address for correspondence.
Methods: study design, study length/number of follow‐up points, setting, country of origin.
Participants: major depressive disorder/substance use disorder inclusion criteria/measure, other defining characteristics (sample size, age mean (standard deviation (SD)), sex (% male), ethnicity, other inclusion and exclusion criteria), study dates/duration (months).
Interventions: number of treatment groups, intervention type and details (content, duration in sessions/weeks), format, intervention target (depression, substance use, both), allocation (number randomised/group), number of sessions attended, fidelity, adjunctive therapy or pharmacotherapy.
Methods for: sequence generation, allocation sequence concealment and blinding.
Outcomes: depression and substance use primary outcomes (definition/measure/unit of measurement, scale (range, interpretation); treatment retention (number of participants still in treatment at the end of the study), treatment attendance (average number of sessions attended).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion. The recommended approach for assessing risk of bias in studies included in Cochrane Reviews is a two‐part tool, addressing seven specific domains, including sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other source of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high or unclear risk. To make these judgments, we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions adapted to the addiction field.
The domains of sequence generation and allocation concealment (avoidance of selection bias) was addressed in the tool by a single entry for each study.
We considered blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) separately for objective outcomes (e.g. treatment retention, attrition, participants engaged in further treatments) and self‐report and interviewer‐rated subjective outcomes (e.g. depression, substance use) separately. Given that participants and personnel cannot be blinded to the type of psychological intervention being delivered, subjective outcomes were always judged at high risk of performance bias.
We assessed treatment fidelity and contamination under the 'Other bias' category, given their importance in ensuring the delivery of high‐quality psychological interventions in RCT's, as well as the potential influence of concurrent out‐of‐study psychotherapy and pharmacotherapy treatment on study outcomes (de Bruin 2015).
See Appendix 5 for details.
Measures of treatment effect
We analysed dichotomous outcomes by calculating the risk ratio (RR) for each trial with the uncertainty in each result being expressed with 95% confidence interval (CI). We analysed continuous outcomes by calculating the mean difference (MD) with 95% CI and standard deviation (SD) when the studies used the same instrument for assessing the outcome. We used the standardised mean difference (SMD) and SD when the studies used different instruments. If the number and range of studies allowed it, we planned to calculate the numbers needed to treat for an additional beneficial outcome (NNTH) or number needed to treat for an additional harmful outcome (NNTH), where data were homogeneous.
Unit of analysis issues
For multi‐arm studies included in the meta‐analyses, when one arm was considered more than once in same comparisons (e.g. two different experimental treatments compared with the same control group), we planned to combine all the relevant experimental groups into a single group and compare it with the control to avoid double‐counting participants in the control groups.
If any cluster‐RCTs were identified, we intended to include them in the analyses along with individual RCTs, planning to synthesise the results unless there was non‐negligible heterogeneity between the trial designs. For cluster‐RCTs that did not adjust for clustering, we intended to adjust the sample sizes using reported or estimated intraclass correlations (ICCs) in line with the recommendations by Higgins 2011.
Dealing with missing data
Whenever possible, we contacted the original investigators to request missing data. We made the assumptions of any methods used to cope with missing data explicit when possible, including whether the data were assumed to be missing at random, or missing values were assumed to have a particular value. The potential impact of missing data on the findings of the review are addressed in the Discussion section.
Assessment of heterogeneity
The presence of clinical, methodological and statistical heterogeneity between the included studies was assessed including: the country of origin, sample characteristics (inclusion and exclusion criteria), settings, types of treatment comparisons (including sample size, content, length of treatment), outcomes reported, measures used and length of follow‐up. We analysed heterogeneity by means of the I2 statistic and the Chi2 test. We regarded heterogeneity as substantial if the I² was greater than 50% or a P value was lower than < 0.10 for the Chi² test for heterogeneity. Following the guidance in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), we distinguished the following values to denote no important, moderate, substantial, and considerable heterogeneity, respectively: 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100%.
Assessment of reporting biases
If meta‐analyses were conducted and number of studies allowed, we planned to use funnel plots (plots of the effect estimate from each study against the standard error (SE)) to assess the potential for bias related to the size of the trials, which could indicate possible publication bias.
Data synthesis
We combined the outcomes from the individual trials through meta‐analysis where possible (comparability of intervention and outcomes between trials), using a random‐effects model, because we expected a certain degree of heterogeneity between trials. If the clinical or statistical heterogeneity between trials was too high (i.e. 75% to 100%), we considered not pooling the data
Subgroup analysis and investigation of heterogeneity
If the number and range of studies allowed it, we planned to conduct subgroup analyses for type of CBT, adults, young people, sex and type of substance used.
Sensitivity analysis
We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in the assumptions about missing data (Higgins 2011).
'Summary of findings' tables
We assessed the overall quality of the evidence for the primary outcome variables using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system. The GRADE Working Group developed a system for grading the quality of evidence (Guyatt 2008; Guyatt 2011; Oxman 2004), which takes into account issues not only related to internal validity, but also to external validity, such as directness of results. The Table 1 presents the main findings of the review in a transparent and simple tabular format. In particular, it provides key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
Summary of findings for the main comparison. Integrated CBT compared with Twelve Step Facilitation for co‐occurring depression and substance use disorders.
| Integrated CBT compared with Twelve Step Facilitation for co‐occurring depression and substance use disorders | |||||||
| Patient or population: co‐occurring depression and substance use disorders Setting: Intervention: Integrated CBT Comparison: Twelve Step Facilitation | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with Twelve Step Facilitation | Risk with Integrated CBT | ||||||
| Depression score Assessed with: Hamilton Depression Rating Scale (HDRS) ‐ Structured clinical interview (21 items) Scale from: 0 to 54 (higher score worse) Follow‐up: end of treatment | The mean depression score ranged from 21.0 to 23.2 | MD 4.05 higher (1.43 higher to 6.66 higher) | ‐ | 212 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| Depression score Assessed with: Hamilton Depression Rating Scale (HDRS) ‐ Structured clinical interview (21 items) Scale from: 0 to 54 (higher score worse) Follow‐up: 6 months to 12 months | The mean depression score ranged from 21.0 to 27.9 | MD 1.53 higher (1.73 lower to 4.79 higher) | ‐ | 181 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| Percent of days abstinent Assessed with: The calendar‐assisted structured interview ‐ Time‐Line Follow‐Back (TLFB) for past 3‐month substance use Scale from: 0 to 100 (lower score better) Follow‐up: end of treatment | The mean proportion of days abstinent ranged from 93 to 90 | MD 2.84 lower (8.04 lower to 2.35 higher) | ‐ | 220 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| Percent of days abstinent Assessed with: TLFB for past 3‐month substance use Scale from: 0 to 100 (lower score better) Follow‐up: 6 months to 12 months | The mean proportion of days abstinent ranged from 72 to 75 | MD 10.76 higher (3.10 higher to 18.42 higher) | ‐ | 189 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 4 | ||
| Treatment retention Assessed with: dropped out of treatment after attending an average of 1.2 sessions | Moderate | RR 0.95 (0.72 to 1.25) | 296 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | |||
| 785 per 1,000 | 745 per 1,000 (565 to 981) | ||||||
| Number of treatment sessions attended Scale from: 0 to 36 | The mean number of Treatment Sessions Attended ranged from 19.4‐22.1 | MD 1.27 lower (6.10 lower to 3.56 higher) | ‐ | 270 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 3 5 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded two levels due to very serious risk of bias: high levels of performance bias, attrition bias and uneven medication use between groups. One study also had high risk of selection bias and unclear risk for selective reporting.
2 Downgraded one level due to Imprecision: small number of trials/participants
3 Downgraded one level due to indirectness: population of predominately Caucasian male veterans
4 Downgraded two levels due to very serious risk of bias: high levels of selection bias, performance bias, attrition bias, unclear risk for selective reporting and uneven attendance between groups at 12‐step Community Meetings
5 Downgraded two levels due to very serious risk of bias: mean attendance was based on a reduced sample, not those originally randomised into the study. Also high risk of selection bias, performance bias and attrition bias
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
Grading is decreased for the following reasons.
Serious (−1) or very serious (−2) study limitation for risk of bias.
Serious (−1) or very serious (−2) inconsistency between study results.
Some (−1) or major (−2) uncertainty about directness (the correspondence between the population, the intervention, or the outcomes measured in the studies actually found and those under consideration in our systematic review).
Serious (−1) or very serious (−2) Imprecision of the pooled estimate.
Strong suspicion of publication bias (−1).
We used GRADEpro GDT 2015 to import data from Review Manager 2014 for the main outcomes of depression score, substance use (percentage of days abstinent, proportion of days abstinent, relapse, percentage of daily use) and treatment retention. Due to the wide variation of treatments compared, we produced one summary table per treatment comparison (Table 1; Table 2).
Summary of findings 2. Interpersonal Psychotherapy for Depression (IPT‐D) compared with Other Psychological Interventions for co‐occurring depression and substance use disorders.
| Interpersonal Psychotherapy for Depression (IPT‐D) compared with Other Psychological Interventions for co‐occurring depression and substance use disorders | ||||||
| Patient or population: Individuals experiencing co‐occurring depression and substance use disorders Setting: any setting Intervention: Interpersonal Psychotherapy for Depression (IPT‐D) Comparison: Other Psychological Interventions | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Other Theraputic Interventions | Risk with Interpersonal Psychotherapy for Depression (IPT‐D) | |||||
| Depression score Assessed with: Hamilton Depression Rating Scale (HDRS) ‐ Structured clinical interview (17 and 24 items) Scale from: 0 to 54 (higher score worse) Follow‐up: end of treatment | SMD 0.54 SD lower (1.04 lower to 0.04 lower) | ‐ | 64 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| Depression score Assessed with: Hamilton Depression Rating Scale (HDRS) ‐ Structured clinical interview (17 items) Scale from: 0 to 54 (higher score worse) Follow‐up: 3 months | The mean depression score was 15.8 | MD 3.80 higher (3.83 lower to 11.43 higher) | ‐ | 38 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 5 | |
| Percentage of days abstinent Assessed with: the calendar‐assisted structured interview ‐ Time‐Line Follow‐Back (TLFB) for past month of alcohol use Scale from: 0 to 100 (better) Follow‐up: end of treatment | The mean percentage of days abstinent was 49.7 | MD 2.70 lower (28.74 lower to 23.34 higher) | ‐ | 26 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 6 7 | |
| Substance use ‐ relapse Assessed with: self‐reported heavy drinking (4+ drinks) or drug use on at least 10% of non‐incarcerated days or positive urine test Follow‐up: 3‐months | Study population | RR 0.67 (0.30 to 1.50) | 38 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 4 5 | ||
| 316 per 1,000 | 212 per 1,000 (95 to 474) | |||||
| Treatment retention | Study population | RR 1.00 (0.81 to 1.23) | 64 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 774 per 1,000 | 744 per 1,000 (627 to 952) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; RR: Risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels due to serious risk of bias: high levels of performance bias due to difficulties with blinding participants and personnel, one of the studies also had high attrition bias and reported group differences in use of antidepressants and adjunctive mental health counselling
2 Downgraded one level due to indirectness: one of the study was based on a female prison population, the other were recruited through a medical college, neither sample is likely to be representative of broader population of individuals experience comorbid substance use and depressive disorders
3 Downgraded two levels due to very small sample size
4 Downgraded two levels due to serious risk of bias: high levels of performance bias due to difficulties with blinding participants and personnel and reported group differences in use of antidepressants and adjunctive mental health counselling
5 Downgraded one level due to indirectness: Female prison population unlikely to be representative of broader population of individuals experience comorbid substance use and depressive disorders
6 Downgraded two levels due to serious risk of bias: high levels of performance bias due to difficulties with blinding participants and personnel
7 Downgraded one level due to indirectness: sample recruited through a medical college, predominately White male, unlikely to be representative of broader population of individuals experience comorbid substance use and depressive disorders
Results
Description of studies
Results of the search
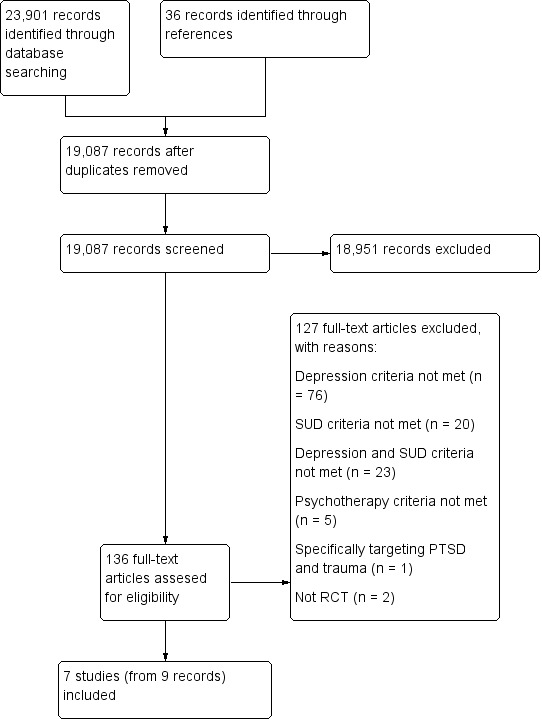
We identified 23,901 records through database searching and 36 from other sources. After removing duplicates, we were left with 19,087 unique references for analysis. We excluded 18,951 on the basis of title and abstract. We retrieved 136 articles in full text for more detailed evaluation, 127 of which were excluded for not meeting the inclusion criteria.
We included seven studies (from nine articles) that satisfied all criteria required for inclusion in the review. See Figure 1.
1.
Study flow diagram.
For substantive descriptions of studies see the Characteristics of included studies and Characteristics of excluded studies tables
Included studies
Seven studies enrolling a total of 608 participants were included (Beutler 2003; Brown 2006; Carpenter 2008; Johnson 2012; Lydecker 2010; Markowitz 2008; Rohde 2014).
Country: all included studies were conducted in the USA.
Participant characteristics: the studies were conducted with a variety of patient groups including veterans (Brown 2006; Lydecker 2010); adults (Beutler 2003; Carpenter 2008;Markowitz 2008); adolescents (Rohde 2014); and incarcerated females (Johnson 2012). The average age ranged from 16.4 to 48.8 years. All studies had samples with a majority of Caucasians (61% to 81%). The proportion of males ranged from 57% to 92%. One study enrolled females only (Johnson 2012).
While all study participants had comorbid DSM‐IV depression and substance use disorders there was considerable variability in the disorder types included. Several studies included participants with major depression disorders only (Brown 2006; Johnson 2012), two included participants with major depressive disorder/dysthymia only (Carpenter 2008; Lydecker 2010), one included dysthymia only (Markowitz 2008), and the others included any depression disorder (Beutler 2003; Rohde 2014).
Two studies included participants with any substance abuse or dependence (Johnson 2012; Rohde 2014), the two veterans studies included individuals with alcohol, cannabis and/or stimulant dependence (Brown 2006; Lydecker 2010), and the three remaining studies included alcohol abuse or dependence only (Markowitz 2008), stimulant (cocaine or methamphetamine) dependence only (Beutler 2003), or opiate dependence only (Carpenter 2008).
Settings: six studies were conducted in outpatient settings, including two in dual diagnosis treatment services for veterans (Brown 2006; Lydecker 2010). One study was conducted among incarcerated females (Johnson 2012).
Types of comparisons: no studies that compared psychological interventions (alone or combined with pharmacotherapy) with delayed treatment, treatment as usual or no treatment control conditions met inclusion criteria; nor did any studies comparing combined psychological and pharmacological interventions with other psychological interventions. All seven studies included for review compared different types of psychological treatments.
Four studies provided group therapy (Brown 2006; Carpenter 2008; Lydecker 2010;Rohde 2014), two individual therapy (Beutler 2003; Markowitz 2008), and one study provided group therapy followed by individual therapy (Johnson 2012).
Five studies compared CBT with other psychological interventions for comorbid depression and substance use disorders.
Two studies compared 24 weeks (36 sessions) of group‐based integrated CBT (ICBT) versus Twelve Step Facilitation (TSF) in veterans accessing a dual diagnosis outpatient clinic with a DSM‐IV major depressive disorder and co‐occurring alcohol, cannabis and/or stimulant dependence (n = 90 Brown 2006; n = 206 Lydecker 2010).
One study compared 24 weeks (24 sessions) of group‐based Behavioral Therapy for Depression in Drug Dependence (BTDD) versus 24 weeks of a group‐based structured Relaxation Intervention (REL) among 38 treatment‐seeking outpatients with DSM‐IV opiate dependence and major depressive disorder/dysthymia (Carpenter 2008).
One study compared 1418 weeks (16‐20 sessions) of Cognitive Therapy (CT) with Narrative Therapy (NT) and Prescriptive Therapy (PT) among 40 patients seeking outpatient care with a DSM‐IV depression disorder and stimulant dependence (Beutler 2003).
One study compared three methods for integrating CBT and Functional Family Therapy (FFT) in 170 adolescents with a comorbid DSM‐IV depression and substance use disorder seeking substance use treatment (Rohde 2014). Participants received 24 weeks (24 sessions) of either the adolescent Coping With Depression (CWD) followed by FFT (CWD/FFT), FFT followed by CWD (FFT/WD) or integrated CWD and FFT care (CWD+FFT).
Two studies compared IPT for depression (IPT‐D) with other psychological interventions.
A pilot study compared 16 weeks (16‐18 sessions) of individual IPT‐D versus Brief Supportive Therapy (BST) in 26 adults seeking outpatient care with DSM‐IV dysthymia and secondary alcohol use disorder (Markowitz 2008).
A second study compared eight weeks (24 sessions) of group IPT‐D with a psychoeducation group for co‐occurring mental health and substance use disorders (PSYCHOED) among 40 incarcerated females with DSM major depressive disorder and substance abuse or dependence. Both treatment groups also received three individual sessions at pre, mid and post the group intervention, as well as treatment as usual for substance use and mental health problems (Johnson 2012).
Outcomes
The depression and substance use outcomes reported differed across studies, as detailed in the Characteristics of included studies table. All but one study used a version of the Hamilton Depression Rating Scale (HDRS, Hamilton 1960), a clinician‐rated measure of the frequency of depression symptoms in the past week. The original version (Hamilton 1960) contained 17 items, but current versions range from 17 to 29 items (Hamilton 1980; Williams 1988). Items are scored on a 3‐or 5‐point scale. Total scores for depression are usually derived from the original 17 items on a 3‐point scale and range from zero to 51 points, with scores from 7 to 17 indicating mild depression, from 18 to 24 indicating moderate depression, and above 24 indicating severe depression.
Three studies also included the Beck Depression Inventory (BDI) (Beck 1961; Beck 1978; Beck 1996), a self‐report measure of the frequency of depression symptoms in the previous two weeks. Rohde 2014 used the Child Depression Rating Scale‐Revised (CDRS‐R, Poznanski 1995), a 17‐item clinician‐rated measure of depressive symptomatology in six‐ to 12‐year olds, which is also widely used in adolescents.
All studies used the Timeline Followback method (TLFB; Miller 1994; Sobell 1980; Sobell 1992) to assess substance use outcomes. This calendar‐based method assesses the frequency and quantity of substance use, including the number of days abstinent over a specified timeframe. Both the timeframe (30 to 90 days) and definition of substance use outcome measures (mean percentage days abstinent, proportion of days abstinent, square root of percentage of daily substance use) varied across studies. One study used the alcohol and drug index score of the Addiction Severity Index (ASI; Beutler 2003).
Definitions of treatment retention varied across studies. Four studies reported the mean number of treatment sessions attended (Brown 2006; Carpenter 2008; Lydecker 2010; Rohde 2014). Three studies reported the percentage of participants who dropped out of treatment but the definitions of dropout varied. Beutler 2003 defined treatment as missing three or more consecutive sessions out of the 20 sessions. Markowitz 2008 reported the percentage of participants who failed to complete treatment, but it is unclear how this was defined. Johnson 2012 defined treatment dropout as missing more than two out of 24 sessions by choice.
No studies reported information on any of the secondary outcome variables specified in the original Cochrane Review research protocol, including functioning, quality of life, anxiety symptoms or disorders or the global clinical severity of mental health disorders. No adverse effects linked to treatments delivered were reported.
Follow‐up
All studies reported post‐treatment outcomes. Three studies conducted three‐month follow‐ups (Brown 2006; Johnson 2012; Lydecker 2010), three conducted six‐month follow‐ups (Beutler 2003; Brown 2006; Lydecker 2010; Rohde 2014), and two conducted 12‐month follow‐ups (Lydecker 2010, Rohde 2014).
Excluded studies
We excluded 127 articles from 124 studies for not meeting the inclusion criteria. Overall 76 articles (from 75 studies) were excluded because they did not meet the depression criteria, 20 articles (from 19 studies) were excluded because they did not meet the substance use criteria, 23 articles (from 22 studies) were excluded because they did not meet both the depression and substance use criteria, two studies were excluded because they were not RCTs, one study was excluded because it focused depression and substance use in the context of Posttraumatic Stress Disorder and Trauma, and five studies were excluded because they did not meet psychotherapy criteria (three of these studies focused on the use of technology as an adjunct to therapy, not actual differences between therapies; and the remaining two studies randomised pharmacological treatments, without randomising psychological therapies) See Figure 1 for further details.
Risk of bias in included studies
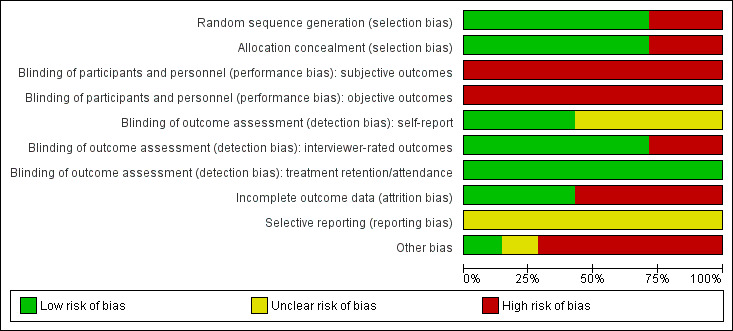
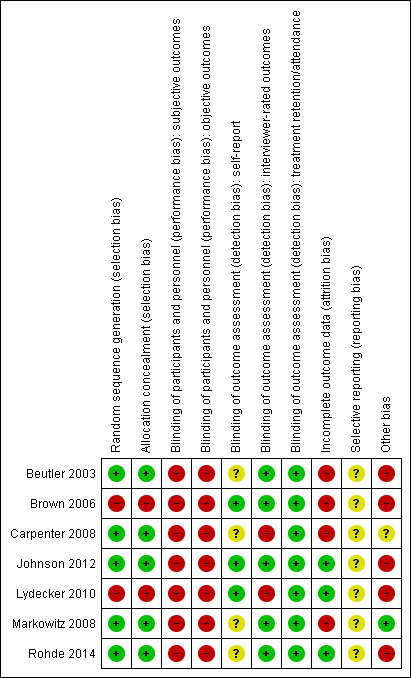
The seven included studies were evaluated by the review author's using Cochrane's 'Risk of bias' tool (see Appendix 5). The results are summarised in Figure 2 and Figure 3.
2.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation: all of the included studies were described as RCTs. Five studies were judged at low risk of selection bias: one study used concealed random draws (Beutler 2003) and four used independent computer‐based randomisation to generate the sequence allocation (Carpenter 2008; Johnson 2012; Markowitz 2008; Rohde 2014). Two studies used sequential allocation of consecutively‐admitted patients (Brown 2006; Lydecker 2010) and were judged to be at high risk of selection bias due to the use of a non‐random sequence generation process. All but one study (Beutler 2003), checked the success of randomisation by comparing the group allocations on baseline variables.
Allocation concealment: five studies were judged at low risk of bias ( Beutler 2003;Carpenter 2008; ,Johnson 2012; Markowitz 2008; Rohde 2014). Two studies were judged at high risk of bias (Brown 2006, Lydecker 2010) because a consecutive allocation was used.
Blinding
Performance bias: no study blinded therapists to the treatment allocation, as this is not possible in psychological trials. Only one study attempted to blind participants to their group allocation, and checked but did not report the success of this blinding process (Beutler 2003). Thus, all studies were rated at high risk of performance bias, due to the subjective nature of the depression and substance use outcomes. These studies were also rated at high risk for performance bias for the treatment retention/attendance variable, as despite the objective nature of this outcome, knowledge of the treatment allocation may influence participant's treatment engagement, participation and attendance.
Detection bias: five of the seven studies were at low risk of detection bias for interviewer‐rated outcomes, due to the use of outcome assessors blind to treatment allocation. Two studies which did not blind the outcome assessors were judged to be at high risk of detection bias (Carpenter 2008; Lydecker 2010). All four studies that reported self‐report outcomes were rated at unclear risk of detection bias (Beutler 2003; Carpenter 2008; Markowitz 2008; Rohde 2014). While participants were not blind to treatment allocation and self‐report measures may be impacted by self‐presentation bias or client insight, it is unlikely that any such risk of bias will vary by treatment condition. There was insufficient information to determine whether the individuals who collected information on treatment attendance/completion were blind to treatment allocation in all studies. However, this objective outcome measure is unlikely to be influenced by lack of blinding, and all seven studies were judged to be at low risk of detection bias on this outcome variable.
Incomplete outcome data
Treatment retention/attendance: less than 50% of participants completed treatment or attended half of the allocated treatment sessions (in at least one group) in four studies (Beutler 2003; Brown 2006; Carpenter 2008; Lydecker 2010). Between‐group differences in the number of sessions participants completed were reported in two studies (Beutler 2003; Rohde 2014).
Outcome assessments: high risk of attrition bias (defined as ≥50% in at least one group) was found in three studies at post‐treatment (Beutler 2003; Brown 2006; Carpenter 2008) and two studies at follow‐up (Beutler 2003; Brown 2006). One study in which one of the treatment groups had double the attrition rate at post‐treatment was considered high‐risk (Markowitz 2008). Attrition rates were typically between 20% and 30% in two other studies (Lydecker 2010; Rohde 2014) and one study among incarcerated females reported zero attrition (Johnson 2012).
Missing data/Intent‐to‐treat (ITT) analysis: three studies used the last observation carried forward (LOCF) method to manage missing data (Beutler 2003; Carpenter 2008; Markowitz 2008) and one imputed missing data, using Markov Chain Monte Carlo multiple imputation (Rohde 2014). All but one study usedITT analyses (Brown 2006). This study also excluded treatment dropouts (attended < 8 treatment sessions) and participants who missed two or more follow‐up assessments from the analyses and was judged to be at very high risk of attrition bias.
Selective reporting
Risk of selective reporting was unclear in all seven studies, as none of them had a published study protocol available. However, the results of all planned analyses using the outcome listed in the methods section are reported in the manuscripts. Two studies did not conduct or report the results of endpoint analyses at all follow‐up points. Trajectory, but not endpoint analyses were conducted (three‐month follow‐up (Brown 2006; Lydecker 2010) or nine‐month follow‐up (Lydecker 2010).
Other potential sources of bias
Fidelity ratings
All seven trials conducted fidelity ratings of the interventions, but only two were performed by independent raters (Johnson 2012; Markowitz 2008). Two studies controlled for therapist allegiance and contamination effects by using separate teams of therapists to deliver the different interventions (Beutler 2003; Markowitz 2008), but it was unclear if either study tested for contamination effects. One study routinely monitored therapist belief in and satisfaction with the treatment model selected and found this was comparable across the treatment groups (Beutler 2003). Risk of bias due to poor treatment fidelity was unclear in the other four studies, although Brown 2006 tested for therapist effects between and within treatment groups and reported it had no impact on the primary depression and substance use outcome variables. While all studies reported information on the number of treatment dropouts or treatment sessions attended, only one controlled for this variable in the analyses (Lydecker 2010).
Concurrent treatment
Only one study (Markowitz 2008) had concurrent pharmacotherapy as an exclusion criterion. None of the remaining studies reported on medication dose or adherence. For those that reported on pharmacotherapy, the main medication prescribed was antidepressants, with 22% to 98% of participants being prescribed an antidepressant during treatment (Brown 2006; Johnson 2012; Lydecker 2010; Rohde 2014), compared with only 1.1% to 2.7% of participants using medication to treat substance use (Johnson 2012; Lydecker 2010). The exception to this is the one study that included people with methadone‐maintained opiate dependence (Carpenter 2008). Few studies reported on the type or number of antidepressants prescribed, though when these were listed selective serotonin reuptake inhibitors (SSRls) or atypical antidepressants (Brown 2006) and lithium, aripiprazole, or quetiapine (Johnson 2012) were described as the most commonly used antidepressants. Only one study stratified the randomisation based on antidepressant use (Carpenter 2008). Five studies compared differences in antidepressant use between the treatment groups or controlled for antidepressant use in their analyses (Brown 2006; Carpenter 2008; Johnson 2012; Lydecker 2010; Rohde 2014), with two studies reporting significant differences between groups in use of antidepressants (Johnson 2012; Lydecker 2010).
One study had concurrent non‐study psychotherapy as an exclusion criterion (Markowitz 2008). A further two studies excluded people attending other psychotherapy beyond 12‐Step Community Addiction Meetings (Brown 2006; Lydecker 2010). It was unclear whether participants were permitted to attend adjunctive psychotherapy in two studies (Beutler 2003; Carpenter 2008), and the risk of other bias was high in the remaining two studies, as participants attended adjunctive therapy and attendance at this therapy was not consistent between the treatment arms (Johnson 2012; Rohde 2014).
Effects of interventions
Due to the heterogeneity of outcomes, only two small meta‐analyses could be conducted, followed by a narrative review of the remaining studies. See Table 1 for Integrated Cognitive Behavioural Therapy (ICBT) versus Twelve Step Facilitation (TSF); Table 2 for Interpersonal Psychotherapy for Depression (IPT‐D) versus other psychological treatments.
Primary outcomes reported include depression score, substance use and treatment retention/adherence. No studies reported information on any of the secondary outcome variables specified in the original Cochrane Review research protocol (Hides 2011), including functioning, quality of life, anxiety symptoms or disorders or the global clinical severity of mental health disorders. No adverse effects linked to treatments delivered were reported.
1. Integrated Cognitive Behavioural Therapy (ICBT) versus Twelve Step Facilitation (TSF)
Two studies with 296 participants compared ICBT to TSF (Brown 2006; Lydecker 2010). Both studies recruited veterans experiencing alcohol, cannabinol and/or stimulant dependence and a major depressive diagnosis. Participants were predominately white males with a mean age of 48.3 (standard deviation (SD) = 7.8). Assessments were completed at post‐treatment and at three months, and six months later. Only one of the two studies assessed outcomes at 12 months post‐treatment (Lydecker 2010).
See: Table 1.
Depression
See: Analysis 1.1 ;Analysis 1.2.
1.1. Analysis.
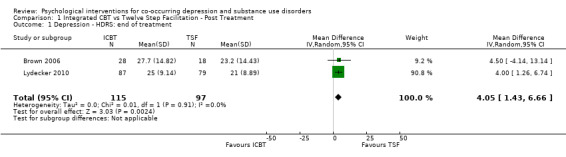
Comparison 1 Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment, Outcome 1 Depression ‐ HDRS: end of treatment.
1.2. Analysis.
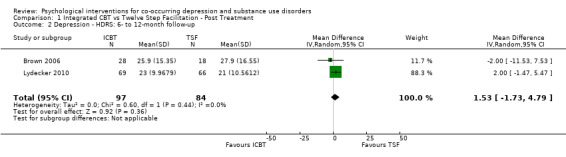
Comparison 1 Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment, Outcome 2 Depression ‐ HDRS: 6‐ to 12‐month follow‐up.
The meta‐analysis examining depression score at post‐treatment and at follow‐up consisted of 296 randomised participants.The meta‐analysis suggested that the ICBT group had higher depression scores, compared with the TSF group, at post‐treatment (mean difference (MD) 4.05, 95% confidence interval (CI) 1.43 to 6.66; 2 studies, 212 participants; Analysis 1.1), however, there did not appear to be any difference between the groups at six‐ to12‐month follow‐up (MD 1.53, 95% CI ‐1.73 to 4.79; 2 studies, 181 participants; Analysis 1.2; Figure 4) follow‐up. Heterogeneity was of no importance (I² = 0%, P = 0.91; I² = 0%, P = 0.44), respectively.
4.
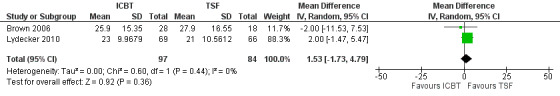
Comparison 1 Integrated CBT vs Twelve Step Facilitation, Outcome: Depression at 6‐12 months
Substance use
See: Analysis 1.3; Analysis 1.4.
1.3. Analysis.
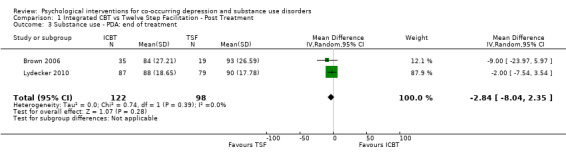
Comparison 1 Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment, Outcome 3 Substance use ‐ PDA: end of treatment.
1.4. Analysis.
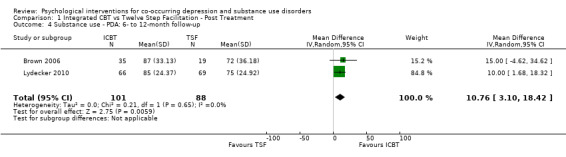
Comparison 1 Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment, Outcome 4 Substance use ‐ PDA: 6‐ to 12‐month follow‐up.
The meta‐analysis consisting of two studies (296 participants) that compared ICBT to TSF, suggested no substantial difference between the groups in proportion of days abstinent in the past three months, as assessed by the Timeline Follow Back (TLFB), at immediately post‐treatment (MD ‐2.84, 95% CI ‐8.04 to 2.35; 2 studies, 220 participants; Analysis 1.3). At six‐to 12‐month follow‐up, the ICBT group experienced on average 10.76% more days abstinent (95% CI 3.10 to 18.42; 2 studies, 189 participants; Analysis 1.4) compared with the TSF group (Figure 5). Heterogeneity was of no importance in either analysis (I² = 0%; P = 0.39; I² = 0%;P = .65), respectively.
5.
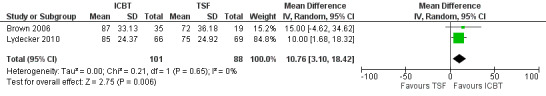
Comparison 1 Integrated CBT vs Twelve Step Facilitation, Outcome: Percentage of Days Abstinent at 6 to 12 months.
Treatment attendance and retention
See: Analysis 1.5; Analysis 1.6
1.5. Analysis.
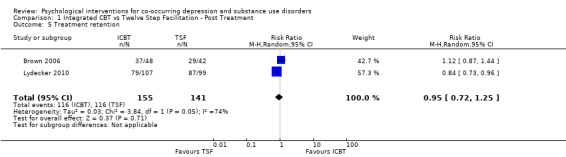
Comparison 1 Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment, Outcome 5 Treatment retention.
1.6. Analysis.
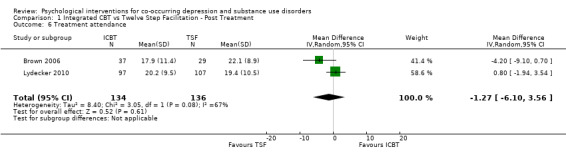
Comparison 1 Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment, Outcome 6 Treatment attendance.
The meta‐analysis consisting of two studies (296 participants) that compared ICBT to TSF, revealed no substantial difference between the groups in treatment retention (RR 0.95, 95% CI 0.72 to 1.25; 2 studies, 270 participants; Analysis 1.5) or attendance (MD ‐1.27, 95% CI ‐6.10 to 3.56; 2 studies, 296 participants; Analysis 1.6). Heterogeneity was substantial for both analyses (I² = 74%, P = 0.05; I2 = 67%, P = 0.08).
2. Interpersonal Psychotherapy for Depression (IPT‐D) versus other psychological treatments
Two studies with 64 participants compared IPT‐D with other psychological treatments, one examining Brief Supportive Psychotherapy, with a sample of patients experiencing major depression/dysthymia and secondary alcohol abuse/dependence drawn from a medical college (n = 26; Markowitz 2008); and the other examining psychoeducation, with a sample of participants experiencing major depression and a substance use disorder (including alcohol, cocaine, opiate, marijuana and sedatives/hypnotics) drawn from a female prison (n = 38; Johnson 2012). Both samples were predominantly female (63%‐ to ‐100%), mostly white, with an average age of 36.4 (SD = 9.9).
See: Table 2.
Depression
See: Analysis 2.1; Analysis 2.2.
2.1. Analysis.
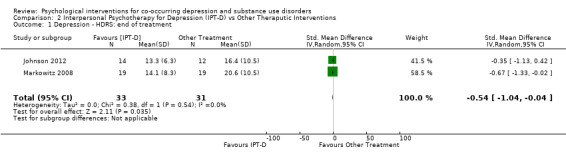
Comparison 2 Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions, Outcome 1 Depression ‐ HDRS: end of treatment.
2.2. Analysis.

Comparison 2 Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions, Outcome 2 Depression ‐ HDRS ‐ 3‐month follow‐up (IPT‐D vs Psychoed).
Two studies contributed 64 participants to end‐of‐treatment analyses (eight weeks/16 weeks), and one study (Johnson 2012) with 38 participants assessed three‐month post‐treatment effects. The common outcome measure used in both studies was the clinician‐rated Hamilton Rating Scale for Depression (HDRS) (17‐item and 24‐item scales were used). The end of treatment results, showed no important heterogeneity (I² = 0%; P = 0.54), and suggested participants receiving IPT‐D had lower depressive symptoms at post‐treatment than other treatment (Brief Supportive Psychotherapy/Psychoeducation; standardised mean difference (SMD) ‐0.54, 95% CI ‐1.04 to ‐0.04; I = 0%; 2 studies, 64 participants; Figure 6); however, there was no evidence that this effect was maintained at follow‐up (IPT‐D versus Psychoeducation; MD 3.80, 95% CI ‐3.83 to 11.43; 1 study, 38 participants).
6.
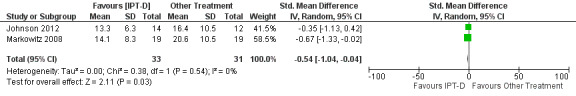
Comparison 2 Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions, Outcome: Depression at end of treatment.
Substance use
See: Analysis 2.3; Analysis 2.4.
2.3. Analysis.
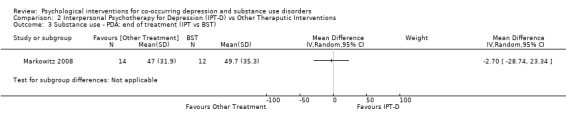
Comparison 2 Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions, Outcome 3 Substance use ‐ PDA: end of treatment (IPT vs BST).
2.4. Analysis.
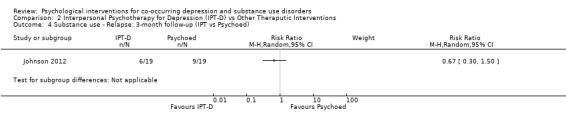
Comparison 2 Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions, Outcome 4 Substance use ‐ Relapse: 3‐month follow‐up (IPT vs Psychoed).
Although both studies used the Timeline Follow‐Back (TLFB) (Sobell 1992;Sobell 1980), the method of reporting this substance use outcome differed between studies, with one study examining percentage of days abstinent in the month prior to the end of treatment (Markowitz 2008), and the other examining relapse at three months post‐treatment (defined as heavy drinking (4+ drinks) or drug use on at least 10% of non‐incarcerated days or positive urine test). Due to this heterogeneity, substance use outcomes were examined separately for each study. There was no significant difference in the percentage of days abstinent in the month prior to the end of treatment when comparing IPT‐D to Brief Supportive Psychotherapy (MD ‐2.70, 95% CI ‐28.74 to 23.34; 26 participants), and no significant difference in relapse at three months post‐treatment when comparing IPT‐D to Psychoeducation (RR 0.67, 95% CI 0.30 to 1.50;38 participants).
Treatment retention
See: Analysis 2.5.
2.5. Analysis.
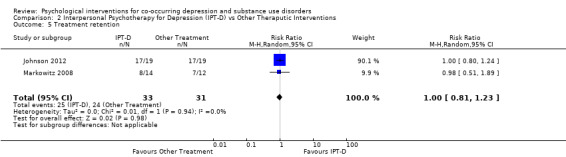
Comparison 2 Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions, Outcome 5 Treatment retention.
For the meta‐analysis comparing IPT‐D with other treatment (Brief supportive psychotherapy/psychoeducation), there was no significant difference between the groups in treatment retention (RR 1.00, 95% CI 0.81 to 1.23; I2 = 0%; 2 studies 64 participants).
3. Integrated Functional Family Therapy (FFT) andCoping With Depression (CWD) versus sequenced FFT‐CWD; Integrated FFT and CWD versus sequenced CWD‐FFT
One study with 170 adolescents compared integrated FFT and CWD to sequential FFT followed by CWD, or CWD followed by FFT (Rohde 2014). Adolescents were aged 13 to 18 years, were predominately white (61%) males (88%) experiencing a depressive disorder (54% major depression) and comorbid substance use disorder, which was predominately a cannabis use disorder (94%), comorbid with alcohol use disorder (65%). For ease of comparison the combined treatment was compared with the sequential treatment on primary outcomes. The first row in each analysis compares integrated FFT and CWD versus sequenced FFT‐CWD; and the second row compares integrated FFT and CWD versus sequenced CWD‐FFT. Assessments were completed at post‐treatment (20 weeks) and six and 12 months later.
Depression
See: Analysis 3.1; Analysis 3.2.
3.1. Analysis.
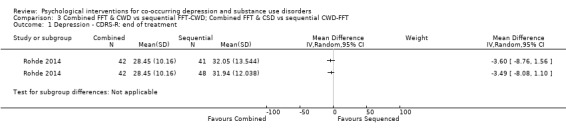
Comparison 3 Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT, Outcome 1 Depression ‐ CDRS‐R: end of treatment.
3.2. Analysis.
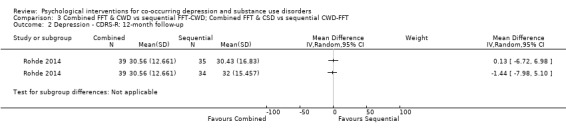
Comparison 3 Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT, Outcome 2 Depression ‐ CDRS‐R: 12‐month follow‐up.
The Children’s Depression Rating Scale‐Revised (CDRS‐R), a clinician‐rated depression tool for adolescents, adapted from the HDRS, was used for all comparisons (Poznanski 1995). The analyses reported in the article were separated based on whether or not an adolescent experienced Major Depressive Disorder. The authors were contacted, and scores were obtained and are reported for the total sample. There was no difference in depression scores between the integrated treatment and either of the sequential treatments (CWD‐FFT or FFT‐CWD) immediately post‐treatment (MD ‐3.60, 95% CI ‐8.76 to 1.56; MD ‐3.49, 95% CI ‐8.08 to 1.10) or at 12‐month follow‐up (MD ‐1.44, 95% CI ‐7.98 to 5.10; MD 0.13, 95% CI ‐6.72 to 6.98).
Substance use
See: Analysis 3.3; Analysis 3.4.
3.3. Analysis.
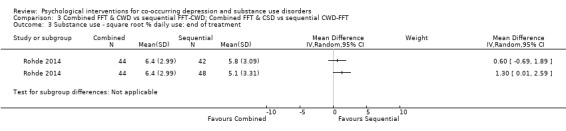
Comparison 3 Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT, Outcome 3 Substance use ‐ square root % daily use: end of treatment.
3.4. Analysis.
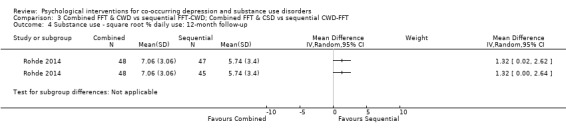
Comparison 3 Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT, Outcome 4 Substance use ‐ square root % daily use: 12‐month follow‐up.
The TLFB interview was used (Miller 1994), with the square root of the percentage of past 90‐day daily use reported at all assessment points. The integrated group had higher daily substance use at post‐treatment when compared with the FFT‐CWD (MD 1.30, 95% CI 0.01 to 2.59) but not the CWD‐FFT (MD 0.60, 95% CI ‐0.69 to 1.89). At 12‐month follow‐up the integrated treatment had higher daily substance use than both the FFT‐CWD (MD 1.32, 95% CI 0.00 to 2.64) and the CWD‐FFT (MD 1.32, 95% CI 0.02 to 2.62) sequential groups.
Treatment retention
See: Analysis 3.5; Analysis 3.6.
3.5. Analysis.
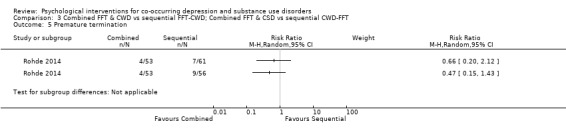
Comparison 3 Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT, Outcome 5 Premature termination.
3.6. Analysis.
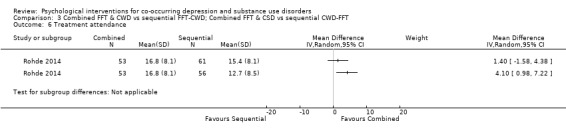
Comparison 3 Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT, Outcome 6 Treatment attendance.
Treatment adherence was assessed through premature termination (attending < 2 sessions) and mean total session attendance (maximum sessions 24). There was no difference between integrated or sequential treatment for treatment retention (RR 0.47, 95% CI 0.15 to 1.43; RR 0.66, 95% CI 0.20 to 2.12); however, integrated treatment had higher mean treatment attendance than CWD‐FFT (MD 4.10, 95% CI 0.98 to 7.22) but not FFT‐CWD (MD 1.40, 95% CI ‐1.58 to 4.38).
4. Behavioral Therapy for Depression in Drug Dependence (BTDD) versus Relaxation Intervention (REL)
One study with 38 participants on methadone maintenance therapy for opiate dependence, who met criteria for a DSM‐IV depressive disorder, compared BTDD with REL (Carpenter 2008). Participants were predominately white (58%) males (82%) with a mean age of 40.1 years (SD = 10.7). Assessments were completed immediately post‐treatment (24 weeks).
Depression
See: Analysis 4.1; Analysis 4.2.
4.1. Analysis.
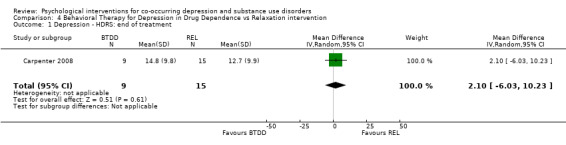
Comparison 4 Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention, Outcome 1 Depression ‐ HDRS: end of treatment.
4.2. Analysis.
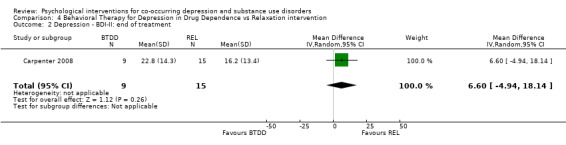
Comparison 4 Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention, Outcome 2 Depression ‐ BDI‐II: end of treatment.
The the HDRS clinician interview and Beck Depression Inventory (BDI‐II) self‐report measure (Beck 1996) were used to assess post‐treatment depression outcomes. Evidence suggested no difference in depression scores for participants receiving BTDD compared with REL, for either the clinician‐rated HDRS (MD 2.10, 95% CI ‐6.03 to 10.23; 24 participants) or the self‐reported BDI‐II (MD 6.60, 95% CI ‐4.94 to 18.14; 24 participants).
Substance use
See: Analysis 4.3; Analysis 4.4; Analysis 4.5.
4.3. Analysis.
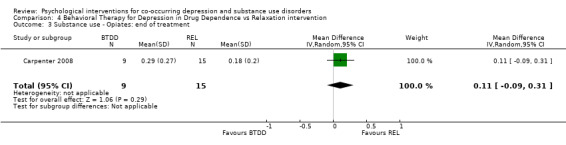
Comparison 4 Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention, Outcome 3 Substance use ‐ Opiates: end of treatment.
4.4. Analysis.
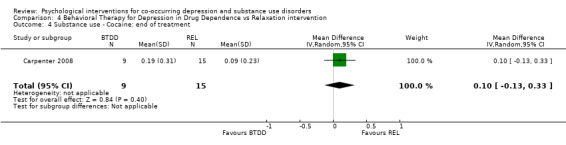
Comparison 4 Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention, Outcome 4 Substance use ‐ Cocaine: end of treatment.
4.5. Analysis.
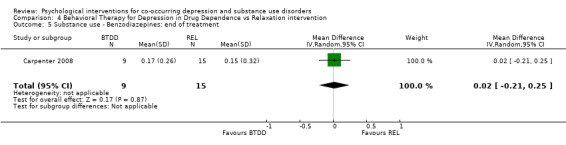
Comparison 4 Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention, Outcome 5 Substance use ‐ Benzodiazepines: end of treatment.
The Substance Use Weekly Inventory (SUI), a modification of the TLFB (Sobell 1980), was used at the beginning of each treatment session to assess the past week number of substance‐using days, with substance use confirmed through a urine test. In the study results, proportion of weeks substances were used was only reported for opiates, cocaine and benzodiazepines. There were no significant differences in the proportion of weeks opiates (MD 0.11, 95% CI ‐0.09 to 0.31; 24 participants), cocaine (MD 0.10, 95% CI ‐0.13 to 0.33; 24 participants), and benzodiazepines (MD 0.02, 95% CI ‐0.21 to 0.25; 24 participants) were used throughout treatment.
Treatment retention
See: Analysis 4.6
4.6. Analysis.
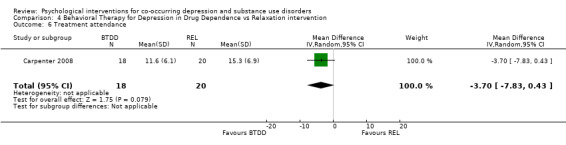
Comparison 4 Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention, Outcome 6 Treatment attendance.
Treatment adherence was examined through the average total session attendance (maximum sessions 24), with no significant difference between the two groups in treatment attendance (MD ‐3.70, 95% CI ‐7.83 to 0.43; 38 participants).
5. Cognitive Therapy (CT) versus Narrative Therapy (NT) versus Prescriptive Therapy (PT)
One study with 40 patients experiencing comorbid DSM‐IV depression disorder and stimulant (cocaine or methamphetamine) dependence, compared CT, NT and PT (Beutler 2003). Participants were predominately white (75%) males (57%) with a mean age of 33.1 years (SD = 1.83). Assessments were completed immediately post‐treatment (20 weeks) and six months post‐treatment. Given the very small sample size, and the multiple comparisons that would be required to be conducted between treatment conditions, a narrative review of findings is presented below.
Depression
Depression scores were assessed through the Beck Depression Inventory (BDI; Beck 1961) and the HDRS. No differences between treatments were reported on either of these measures.
Substance use
A modified TLFB (Sobell 1992) was used to assess past 30‐day mean days per week of reported substance use, the Addiction Severity Index (ASI; McLellan 1980) was used to create alcohol and drug index scores and urine samples were also conducted to determine abstinence of participants. There was no reported difference between treatment conditions for clean urine samples or reported abstinence, or on the TLFB or ASI.
Treatment retention
Dropout was assessed as failure to attend three consecutive appointments. The study reported that the NT condition had lower dropout rates than with PT or CT, but no other information was provided.
Discussion
Summary of main results
This review assessed the efficacy of psychological interventions alone or in combination with pharmacotherapy for comorbid depression and substance use disorders. Seven RCTs with a total of 608 participants were included. All studies compared different types of psychological interventions; no studies combining psychological interventions with pharmacotherapy; or comparing psychological interventions with no treatment, treatment as usual or delayed treatment control conditions were found.
Two studies compared Integrated Cognitive Behavioural Therapy (ICBT( with Twelve Step Facilitation (TSF), another two studies compared Interpersonal Psychotherapy for Depression (IPT‐D) with other treatment (Brief Supportive Therapy or Psychoeducation), a fifth study compared the integrated delivery of Functional Family Therapy (FFT) and Coping With Depression (CWD) with two sequenced methods (CWD then FFT; FFT then CWD), a sixth study compared Behavioral Therapy for Depression in Drug Dependence (BTDD) with a relaxation intervention, and the final study compared cognitive therapy (CT), narrative therapy (NT) and prescriptive therapy (PT).
Due to heterogeneity in outcomes only two meta‐analyses were conducted. The first meta‐analysis focused on the two studies comparing ICBT versus TSF (see Table 1). Very low‐quality evidence indicated there was no difference in depression symptoms, treatment attendance or retention outcomes at post‐treatment or follow‐up, but a significant improvement in substance use outcomes in favour of the ICBT was found at six‐ to 12‐month follow‐up.
The second meta‐analysis, conducted with two studies, compared IPT‐D with other treatment (Brief Supportive Psychotherapy/Psychoeducation) (see Table 2), and found very low‐quality evidence that IPT‐D resulted in lower depressive symptoms at post‐treatment than other treatment, but this effect was not maintained at three‐‐month follow‐up. No significant differences between the groups in treatment retention were found. Substance use was examined separately in each study due to heterogeneity in outcomes. No significant differences in these outcomes (percentage of days abstinent, risk of relapse) were found.
The two other studies, which compared different cognitive behavioural therapy (CBT) interventions (BTDD; CT) with other psychological treatments (Relaxation; NT or PT) found no significant differences on depression, substance use or treatment retention outcomes. The seventh study found integrated FFT and CWD had higher treatment attendance rates than the sequenced treatments but worse substance use outcomes but than the CWD‐FFT group at post‐treatment and both the CWD‐FFT and FFT‐CWD groups at 12‐month follow‐up.
Overall completeness and applicability of evidence
The seven studies identified are insufficient to address the objectives of this review. All included studies were conducted in the USA, which limits their applicability to other contexts. Moreover, the studies that were included in the meta‐analyses were from quite specific populations (i.e. veterans, incarcerated females), with a lack of variation in country source (i.e. all from the USA) or participant demographics (i.e. predominately Caucasian), which also questions the wider application of the findings.
It was difficult to compare the results of studies, as even though five studies compared CBT and two compared IPT‐D with other psychological interventions, there was wide variability in the type of CBT (e.g. cognitive therapy, behaviour therapy) and other psychological treatments compared, as well as the treatment target (depression, substance use, depression and substance use), content, intensity/length and mode of delivery (e.g. group, individual, family). There was also wide variability in the definitions and measures of depression, substance use outcomes use and treatment dropout/retention used across the seven studies. The treatment population also varied considerably ranging from veterans and female prisoners to community adults and adolescents potentially limiting the applicability of results to these particular populations. While requiring all study participants to have comorbid depression and substance use disorders strengthened the internal validity of the review, clinicians in real‐world settings treat only individuals with substance use and depression problems of varying severity, not only people with fully established disorders. Clinicians are also likely to treat individuals with other comorbid presentations (e.g. substance use, depression and anxiety problems). This limits the external validity of the review.
Quality of the evidence
All seven studies were at high risk of performance bias. Two of six studies were at high risk of selection bias due to inadequate random sequence generation (Brown 2006; Lydecker 2010). Two studies were at high risk for detection bias due to the use of non‐blinded interview raters (Carpenter 2008; Lydecker 2010). All four studies that reported self‐report outcomes were at unclear risk of detection bias (Beutler 2003; Carpenter 2008; Markowitz 2008; Rohde 2014) and four of the seven studies were at high risk of attrition bias (Beutler 2003; Brown 2006; Carpenter 2008; Markowitz 2008). Risk of selective reporting was unclear in all seven studies, as none had published research protocols. Due to heterogeneity in outcomes only two meta‐analyses were performed, each comprised of two studies.
The quality of evidence of the meta‐analysis comparing IPT‐D studies with other treatment (Brief Supportive Psychotherapy /Psychoeducation) was very low for assessing its impact on depression and for the substance use and treatment retention outcomes. The meta‐analysis comparing ICBT to TSF also provided very low‐quality evidence for all outcomes. Moreover, other methodological flaws in the seven studies included the use of small sample sizes (Brown 2006; Carpenter 2008; Johnson 2012; Markowitz 2008), the reporting of only post‐treatment outcomes (Beutler 2003; Carpenter 2008; Markowitz 2008) or short‐term outcomes (Johnson 2012), and the uneven group distribution of adjunctive treatment (both pharmacological and psychosocial). While all studies conducted treatment fidelity ratings, risk of bias was unclear in all but the two studies in which the fidelity ratings were performed by independent raters, and no studies tested for possible contamination effects in the control conditions.
Potential biases in the review process
There was low risk of publication bias, as a comprehensive search of published and unpublished studies without any language restrictions was conducted. Nevertheless, there is a chance some trials were missed during the search including unpublished studies, particularly those with negative results; as well as some studies in non‐English languages. A funnel plot to assess publication bias was not constructed due to the small number of studies. The authors of studies that did not report the data required to complete the review were contacted. All responded and provided the relevant data.
Agreements and disagreements with other studies or reviews
The findings of this review are consistent with other meta‐analyses and narrative systematic reviews of the literature, which have found insufficient empirical support to inform psychological treatments for comorbid substance use disorders and depression (Babowitch 2016; Baker 2012; Hesse 2009; Hobden 2018; Riper 2014). Previous reviews have focused on adolescent (Babowitch 2016) and adult (Baker 2012; Hesse 2009; Hobden 2018) populations, and on a wide variety of psychological treatments including CBT, FFT, IPT‐D and TSF. Most of the previous reviews found CBT to be the most popular therapy investigated, and all four of the reviews included studies with integrated motivational interviewing/CBT treatments, with one study only investigating the efficacy of motivational interviewing/CBT in treating comorbid alcohol use disorders and depression (Riper 2014).
Previous reviews have highlighted the promise of integrated approaches (Hesse 2009), particularly those that integrate motivational interviewing and CBT (Babowitch 2016; Baker 2012; Hobden 2018; Riper 2014). Small but significant effect sizes have been found for integrated motivational interviewing/CBT in both adolescent (Babowitch 2016) and adult (Baker 2012; Riper 2014) populations, with longer interventions shown to have greater improvements in mood and alcohol use outcomes (Baker 2012). Unfortunately, many of these studies only focused on alcohol misuse (Babowitch 2016; Baker 2012), examined comorbid depression and/or anxiety (Baker 2012; Hesse 2009), or included non‐randomised controlled trials (Hobden 2018; Riper 2014). No integrated motivational interviewing/CBT studies that meet the strict inclusion criteria of this review (i.e. randomised controlled trial, with diagnostic interviews to confirm presence of a substance use and depression disorder at study entry). Therefore, comments on the efficacy of integrated motivational interviewing/CBT treatments for comorbid substance use and depression disorders could not be made in this review.
Authors' conclusions
Implications for practice.
The conclusions of this review are limited due to the low number and poor quality of included studies. No conclusions can be made about the efficacy of psychological interventions (delivered alone or in combination with pharmacotherapy) for the treatment of comorbid depression and substance use disorders, as they are yet to be compared with no treatment, treatment as usual or delayed interventions in this population. All seven studies included in this review compared psychological interventions. However, no conclusions can be made about which psychological treatment is most effective, as although some significant effects were found, the effects were too small and inconsistent, and the evidence too poor quality to be of relevance to practice.
Implications for research.
More research on the psychological treatment of comorbid depression and substance use disorders using well‐designed randomised controlled trials (RCTs) based on accepted guidelines (CONSORT (Consolidated Standards of Reporting Trials), Cochrane, SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials)) are required (Boutron 2008; Chan 2013). This should include RCTs comparing the efficacy of psychological interventions delivered alone or in combination with pharmacotherapy, and comparing them with delayed or no treatment control conditions. A number of studies were excluded from this review because they did not use diagnostic criteria for depression and substance use disorder. Consensus definitions and measures of depression and substance use outcome variables, as well as consistent scale cut‐offs to accurately identify individuals with comorbid depression and substance use problems are needed to improve the quality of evidence. Future research needs to clearly specify the qualifications, training and supervision of therapists and ensure fidelity checks are conducted regularly by independent raters to ensure high‐quality psychological treatments are delivered in RCTs. However, acknowledgement is also required that this need for qualification, training and supervision, and the higher rigor required for RCTs is expensive, and may preclude the conduct of these high‐quality trials, particularly in countries beyond the USA. Hence, consideration of how to adequately fund these trials is also needed. It is imperative that information on the number and length of sessions and the delivered treatment components is recorded and compared with other active and non‐active treatments to avoid contamination effects. The treatment dose within and across intervention arms and the impact of this on depression and substance use outcomes also needs to be examined. Larger sample sizes and more ethnically diverse samples would enable subgroup analyses to control for heterogeneity in the inclusion/exclusion criteria used, as well as the length, intensity, target (depression, substance use, depression and substance use), content and modality of treatment provided. The impact of assessments, treatment as usual and regression to the mean among treatment‐seeking populations also requires consideration when comparing psychological treatments.
Acknowledgements
The authors would like to thank Associate Professor Linda Gowing for her invaluable advice on the revisions of this review and Dr Dawn Proctor for her contribution to the protocol paper for this review. Thank you to Dr Ed Day and Dr Ayorkor Gaba for peer reviewing the manuscript.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Mood Disorders] explode all trees
#2 affective or depression or "depressive disorder" or "mood disorder" or anxiety or dysthymic:ti,ab,kw (Word variations have been searched)
#3 #1 or #2
#4 MeSH descriptor: [Substance‐Related Disorders] explode all trees
#5 drug or substance or alcohol or marijuana or cannabis or meth‐amphetamine or dextro‐ or amphetamine or MDMA or heroin or narcotic or opiate or opioid or opium or ecstasy or methadone or cocaine or psychostimulant* or inhalant* or solvent*:ti,ab,kw and abus* or use* or misus* or usin* or utilis* or depend* or addict* or illegal* or illicit* or habit* or withdraw* or behavi* or abstinence* or abstain* or intoxica* or addict * or disorder*:ti,ab,kw (Word variations have been searched)
#6 #4 or #5
#7 MeSH descriptor: [Psychotherapy] explode all trees
#8 psychotherapy or counselling or behavior* or contigenc* or supportive or reinforcement or motivation* or incentive or "cognitive therapy" (Word variations have been searched)
#9 #7 or #8
#10 #3 and #6 and #9
Appendix 2. PubMed search strategy
mood disorders [mh]
affective[tw] OR depression[tw] OR "depressive disorder" [tw] OR "mood disorder"[tw] OR anxiety[tw] OR dysthymic[tw]
#1 OR #2
Substance‐related disorders [mh]
(( drug OR substance OR alcohol OR marijuana OR cannabis OR meth/dextro‐amphetamine OR amphetamine OR MDMA OR heroin OR narcotic OR opiate OR opioid or opium OR ecstasy OR methadone OR cocaine or psychostimulant* or inhalant* OR solvent* ) AND (abus* OR use* OR misus* OR usin* OR utilis* OR depend* OR addict* OR illegal* OR illicit* OR habit* OR withdraw* OR behavi* OR abstinence* OR abstain* OR intoxica* OR addict * or disorder*))
#5 OR #6
Psychotherapy [mh]
psychotherapy[tw] OR counselling[tw] OR behavior*[tw] OR contigenc*[tw] OR supportive[tw] OR reinforcement[tw] OR motivation*[tw] OR incentive[tw] OR "cognitive therapy"[tw]
#7 OR #8
randomized controlled trial [pt]
controlled clinical trial [pt]
randomized [tw]
placebo [tw]
clinical trials as topic [mesh: noexp]
randomly [tw]
trial [tw]
#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
animals [mh] NOT humans [mh]
#17 NOT #18
#3 AND #6 AND #9 AND #19
[pt] denotes a Publication Type term;
[tiab] denotes a word in the title or abstract;
[sh] denotes a subheading;
[mh] denotes a Medical Subject Heading (MeSH) term (‘exploded’);
[mesh: noexp] denotes a Medical Subject Heading (MeSH) term (not ‘exploded’);
[ti] denotes a word in the title;
[tw] denote text words across the record included in the title, abstract, MeSH, Publication Types or Substance Names
Appendix 3. Embase search strategy
Single syntax:
'mood disorders'/exp/mj OR affective OR depression OR 'depressive disorder' OR 'mood disorder' OR anxiety OR dysthymic AND 'substance‐related disorders'/exp/mj OR 'drug':ab,ti OR 'substance':ab,ti OR 'alcohol':ab,ti OR 'marijuana':ab,ti OR 'cannabis':ab,ti OR 'meth‐amphetamine':ab,ti OR 'dextro':ab,ti OR 'amphetamine':ab,ti OR 'mdma':ab,ti OR 'heroin':ab,ti OR 'narcotic':ab,ti OR 'opiate':ab,ti OR 'opioid':ab,ti OR 'opium':ab,ti OR 'ecstasy':ab,ti OR 'methadone':ab,ti OR 'cocaine':ab,ti OR 'psychostimulant*':ab,ti OR 'inhalant*':ab,ti OR 'solvent*':ab,ti AND ('abus*':ab,ti OR 'use*':ab,ti OR 'misus*':ab,ti OR 'usin*':ab,ti OR 'utilis*':ab,ti OR 'depend*':ab,ti OR 'illegal*':ab,ti OR 'illicit*':ab,ti OR 'habit*':ab,ti OR 'withdraw*':ab,ti OR 'behavi*':ab,ti OR 'abstinence*':ab,ti OR 'abstain*':ab,ti OR 'intoxica*':ab,ti OR 'addict*':ab,ti OR 'disorder*':ab,ti) AND 'psychotherapy'/exp/mj OR psychotherapy OR counselling OR behavior* OR contigenc* OR supportive OR reinforcement OR motivation* OR incentive OR 'cognitive therapy' AND randomized OR placebo OR randomly OR trial NOT animals NOT human AND ([controlled clinical trial]/lim OR [randomized controlled trial]/lim)
Appendix 4. CINAHL Search Strategy
| S20 | S3 AND S6 AND S9 AND S19 |
| S19 | S17 NOT S18 |
| S18 | TX animal* NOT TX human* |
| S17 | S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 |
| S16 | TX trial |
| S15 | TX randomly |
| S14 | SU clinical trial |
| S13 | TX placebo |
| S12 | TX randomized |
| S11 | PT clinical trial |
| S10 | PT randomized controlled trial |
| S9 | (TX psychotherapy OR counselling OR behavior* OR contigenc* OR supportive OR reinforcement OR motivation* OR incentive OR "cognitive therapy") AND (S7 OR S8) |
| S8 | TX psychotherapy OR counselling OR behavior* OR contigenc* OR supportive OR reinforcement OR motivation* OR incentive OR "cognitive therapy" |
| S7 | (MH "Psychotherapy+") OR (MH "Psychotherapy, Brief") OR (MH "Psychotherapy, Psychodynamic") OR (MH "Psychotherapy, Group+") OR (MH "Cognitive Therapy+") OR (MH "Equine‐Assisted Therapy") |
| S6 | (TX ( drug OR substance OR alcohol OR marijuana OR cannabis OR meth/dextro‐amphetamine OR amphetamine OR MDMA OR heroin OR narcotic OR opiate OR opioid or opium OR ecstasy OR methadone OR cocaine or psychostimulant* or inhalant* OR solvent* ) AND TX ( abus* OR use* OR misus* OR usin* OR utilis* OR depend* OR addict* OR illegal* OR illicit* OR habit* OR withdraw* OR behavi* OR abstinence* OR abstain* OR intoxica* OR addict* or disorder* )) AND (S4 OR S5) |
| S5 | TX ( drug OR substance OR alcohol OR marijuana OR cannabis OR meth/dextro‐amphetamine OR amphetamine OR MDMA OR heroin OR narcotic OR opiate OR opioid or opium OR ecstasy OR methadone OR cocaine or psychostimulant* or inhalant* OR solvent* ) AND TX ( abus* OR use* OR misus* OR usin* OR utilis* OR depend* OR addict* OR illegal* OR illicit* OR habit* OR withdraw* OR behavi* OR abstinence* OR abstain* OR intoxica* OR addict* or disorder* ) |
| S4 | (MH "Substance Use Disorders+") OR (MH "Organic Mental Disorders, Substance‐Induced+") OR (MH "Alcohol‐Related Disorders+") |
| S3 | (TX affective OR depression OR "depressive disorder" OR "mood disorder" OR anxiety OR dysthymic) AND (S1 OR S2) |
| S2 | TX affective OR depression OR "depressive disorder" OR "mood disorder" OR anxiety OR dysthymic |
| S1 | (MH "Affective Disorders+") |
Appendix 5. Criteria for 'Risk of bias' assessment
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk. | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| high risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement. | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes |
low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes |
Low risk | Blinding of participants and providers ensured and unlikely that the blinding could have been broken. |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk | No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding. Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 6.Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| high risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data have been imputed using appropriate methods. All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat). |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group). | |
| 8. Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported. One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified. One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| 9. Other bias (treatment fidelity, completeness and contamination adequately addressed) | Low risk | The number of sessions and treatment components delivered were reported; Treatment fidelity was assessed by an independent rater; The content of separate treatments was compared for cross‐contamination effects. Separate therapists delivered different treatments to avoid cross‐contamination. |
| High risk | Treatment completeness, fidelity and contamination not assessed. | |
| Unclear risk | Insufficient information to permit judgement. This is usually the case if treatment fidelity was assessed by non‐independent raters or if treatment completeness, fidelity and contamination were assessed but not described or not described in sufficient detail to allow a definite judgement. |
Data and analyses
Comparison 1. Integrated CBT vs Twelve Step Facilitation ‐ Post Treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression ‐ HDRS: end of treatment | 2 | 212 | Mean Difference (IV, Random, 95% CI) | 4.05 [1.43, 6.66] |
| 2 Depression ‐ HDRS: 6‐ to 12‐month follow‐up | 2 | 181 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐1.73, 4.79] |
| 3 Substance use ‐ PDA: end of treatment | 2 | 220 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐8.04, 2.35] |
| 4 Substance use ‐ PDA: 6‐ to 12‐month follow‐up | 2 | 189 | Mean Difference (IV, Random, 95% CI) | 10.76 [3.10, 18.42] |
| 5 Treatment retention | 2 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.25] |
| 6 Treatment attendance | 2 | 270 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐6.10, 3.56] |
Comparison 2. Interpersonal Psychotherapy for Depression (IPT‐D) vs Other Theraputic Interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression ‐ HDRS: end of treatment | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.04, ‐0.04] |
| 2 Depression ‐ HDRS ‐ 3‐month follow‐up (IPT‐D vs Psychoed) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Substance use ‐ PDA: end of treatment (IPT vs BST) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Substance use ‐ Relapse: 3‐month follow‐up (IPT vs Psychoed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Treatment retention | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
Comparison 3. Combined FFT & CWD vs sequential FFT‐CWD; Combined FFT & CSD vs sequential CWD‐FFT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression ‐ CDRS‐R: end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Depression ‐ CDRS‐R: 12‐month follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Substance use ‐ square root % daily use: end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Substance use ‐ square root % daily use: 12‐month follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Premature termination | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6 Treatment attendance | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 4. Behavioral Therapy for Depression in Drug Dependence vs Relaxation intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression ‐ HDRS: end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐6.03, 10.23] |
| 2 Depression ‐ BDI‐II: end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 6.60 [‐4.94, 18.14] |
| 3 Substance use ‐ Opiates: end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.09, 0.31] |
| 4 Substance use ‐ Cocaine: end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.1 [‐0.13, 0.33] |
| 5 Substance use ‐ Benzodiazepines: end of treatment | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.21, 0.25] |
| 6 Treatment attendance | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐7.83, 0.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beutler 2003.
| Methods |
Design: randomised controlled trial Follow‐up: 20 weeks post‐treatment and 6 months post‐treatment follow‐up. Setting: outpatients recruited from community and advertising Country: USA |
|
| Participants |
Participants: 40 patients with comorbid DSM‐IV depression disorder and stimulant (cocaine or methamphetamine) dependence on the Structured Clinical Interview (SCID) Mean age (years): 33.06 (SD = 8.67) Sex: 57% male Ethnicity: 25% minority group |
|
| Interventions |
Description: Cognitive Therapy (CT) for drug abuse (Beck 1993), Narrative Therapy (NT; Scogin 1987) versus Prescriptive Therapy (PT). CT was therapist‐guided, symptom‐focused and emotionally supportive. NT was patient‐led, non‐confrontational and insight‐focused; outside self‐help groups (e.g. AA, NA) were recommended as part of this treatment. PT targeted the patient‐treatment fit between four patient qualities (functional impairment, coping style, resistance traits, subjective distress) and four treatment variables (intensity, focus, directiveness, affective regulation). Format:individual therapy Duration: CT: 14‐18 weeks (16‐20 sessions); NT: 14‐18 weeks (patient‐selected frequency; weekly recommended); PT: 14‐18 weeks (16‐20 sessions). Allocation: CT n = 15; NT n = 12; PT n = 13 |
|
| Outcomes |
Treatment retention: dropout defined as missing 3 consecutive sessions; recoded as treatment retention for review Self‐report: BDI mean (SD) total score (past 7 days); TLFB mean days/week (past 30 days; modified self‐report version) Interviewer‐rated: HDRS mean (SD) total score (past 7 days), ASI mean (SD) alcohol and drug index scores (past 30 days) Attrition: proportion with missing data at post‐treatment and 6‐month follow‐up HDRS data (overall attrition rate of each treatment group not reported) |
|
| Notes | Therapists carefully selected and trained Funding: National Institute of Drug Abuse (NIDA) RO1DA09294 (Beutler) Conflict of interest: no statement provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (via author email): "Assignment one of the three groups was accomplished by drawing poker chips representing the three groups from a hat. The drawing for therapists within groups was accomplished by poker chips on which were written a therapist number that had been pre‐assigned." Comment: participants were randomly assigned to a treatment group and therapist. |
| Allocation concealment (selection bias) | Low risk | Quote (via author email): "Each participants group assignment was logged into a computer file and stored within a password protected file." Comment: investigators enrolling participants could not foresee assignment because group assignment was concealed in a password‐protected file. |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote (via author email): "Participants ‐ We cautioned therapists and staff to avoid telling patients which group to which they were assigned. Groups were referred to by numbers indicating group and therapist (e.g., group #2, therapist #5). At the end of treatment we checked to see if patient's knew which group they had been assigned to." Comment: blinding of study participants was attempted, the success of this was checked, but not reported and blinding could have been broken and influenced outcomes. Three separate sets of therapists were used to deliver each treatment to reduce treatment allegiance/contamination effects. Quote: "Therapist belief in and satisfaction with the treatment model selected was routinely and formally assessed (p. 72); all had high levels of therapist commitment; PT therapists thought it would more acceptable to their peers. Nevertheless, the authors were unable to blind therapists to the psychological treatment they were delivering, which could have influenced outcomes." |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and providers knowledge of group allocation may influence treatment engagement/participation/ attendance |
| Blinding of outcome assessment (detection bias) self‐report | Unclear risk | Blinding of study participants was attempted, but success of blinding was not reported. Comment: The authors judge risk of bias to be unclear. While participants were not blind to treatment allocation and self‐report measures may be impacted by self‐presentation bias or client insight, it is unlikely that any such risk of bias will vary by treatment condition. |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | Low risk | Quote (via author email): "Blind ratings were made by clinicians who were unfamiliar with the particular patients." |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Treatment retention: 48% of participants; Quote: "Treatments differed significantly in rate of drop out (Chi2 = 9.70; P < 0.05), favouring lower dropout in the NT group (p. 77) Comment: low treatment retention rate (< 50%), between group differences in treatment dropout rate Outcome assessment: attrition rate in each treatment group using HDRS data Attrition post‐treatment: Total 50%; CT 47%; NT 42%; PT 62% Attrition at the 6 month follow‐up: Total 53%; CT 47%; NT 58%; PT 54% No reasons for missing data reported. Comment: high level of attrition (> 50%) at post‐treatment and 6‐month follow‐up unlikely to be related to true outcome, even though the attrition rates across the three treatment groups were similar. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "While the study was constructed as a RCT in which the three groups were compared, the usual methods of analysing RCT designs using end‐point or intent‐to‐treat analysis are inappropriate for detecting multiple contributors to change. (p. 70) ....The current study afforded the opportunity of comparing the relative yield of traditional intent‐to‐treat comparisons with the more flexible and inclusive Hierarchical Liner Methods (p. 70)" Comment: risk is unclear as no study protocol is available. All expected outcomes specified in the method are published in the paper, but end‐point analysis results are only reported at post‐treatment, and not at 6‐month follow‐up. |
| Other bias | High risk |
Fidelity: fidelity ratings of videotapes of early and late sessions conducted by supervisors not independent raters. Other treatments: unclear whether participants were able to receive concurrent out‐of‐study psychotherapy. Reported that antidepressants were prescribed by project psychiatrist; however, the type of medication, dose, and consistency of medication adherence between group conditions is not reported. |
Brown 2006.
| Methods |
Design: randomised controlled trial Follow‐up: 12 weeks (mid‐treatment), 24 weeks (post‐treatment), 3 and 6 months post‐treatment follow‐up Setting: dual diagnosis outpatient clinic Country: USA |
|
| Participants |
Participants: 90 veterans with DSM‐IV Major Depressive Disorder and co‐occurring alcohol, cannabis and/or stimulant dependence on the Comprehensive International Diagnostic Interview (CIDI) Mean age (years): 48.8 (SD = 7.9) Sex: 92% male Ethnicity: 74% Caucasian |
|
| Interventions |
Description: Integrated manualised Cognitive Behavior Therapy (ICBT) versus Twelve Step Facilitation (TSF). ICBT was based on two empirically‐based interventions: Cognitive‐Behavioural Depression Treatment (Muñoz 1993) and Cognitive‐Behavioural Coping Skills Training of Addiction (Kadden 1994). It included three models (thoughts, activities and people) followed by relapse prevention and focus on core skills learned. TSF consisted of the National Institute on Alcohol Abuse and Alcoholism Project MATCH TSF intervention (Nowinski 1994), covering four core topics (e.g. acceptance, surrender) and six electives (e.g. enabling, and persons, places, or things) to support the goal of abstinence. Format: group therapy Duration: 24 weeks (36 sessions); Phase 1 60 minutes biweekly for 12 weeks + Phase 2 60 minutes weekly for 12 weeks + standard pharmacotherapy Allocation: ICBT n = 48; TSF n = 42 |
|
| Outcomes |
Treatment retention: attended at least 8 of the 36 treatment sessions. Treatment attendance: mean number of sessions attended Self‐report: not included Interviewer‐rated: HDRS mean (SD) total score (past 7 days); TLFB mean (SD) proportion of days abstinent (past 90 days) Attrition: treatment dropout (attended < 8 treatment sessions; were excluded from post‐treatment, 3‐ and 6‐month follow‐up) plus those who missed > 2 follow‐up points |
|
| Notes | Matched for therapist contact; both groups running at all times with staggered start times (every 2 weeks) to allow rolling admissions; therapists were rotated every 6 months so that at least one therapist per group was changed to avoid therapist effects; ongoing weekly supervision; TSF continued in the community. Fidelity: all sessions videotaped, a random sample of 25% of both treatments reviewed by supervisors for integrity of implementation, adherence to protocol and avoid contamination. Funding: Veteran Affairs (VA) Medical Research Merit Review Grant (Brown), VA Associate Investigator Awards (Glasner, Tate). Conflict of interest: no statement provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Consecutive admissions ..meeting inclusion criteria were sequentially allocated by cohorts into either into TSF group intervention or ICBT (p. 451). No significant difference between treatment completer groups on baseline demographic, depression or substance use variables; but non‐treatment completers were excluded from this analysis." Comment: high risk due to non‐random component (sequential allocation by cohorts) in the sequence generation process |
| Allocation concealment (selection bias) | High risk | Quote (via author email): "Therapists were verbally informed of participant assignment to condition by the research assistant 1 to 3 days before initial group participation. Participants were similarly informed by the research assistant of their assignment to condition the week prior to starting group attendance." Comment: investigators enrolling participants could possibly foresee assignments due to use of sequential cohort randomisation |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Participants and personnel were not blind to treatment allocation (same procedures as Lydecker 2010, p. 456) |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and personnel knowledge of group allocation may influence treatment engagement/participation/ attendance |
| Blinding of outcome assessment (detection bias) self‐report | Low risk | This study did not include self‐report measures |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | Low risk | Quote (via author email): "Outcome assessors were blind to treatment allocation" |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Treatment retention: ICBT n = 37, 77%; TSF n = 29, 69% (excluded participants who attended < 8 sessions) Treatment attendance: ICBT 17.9 (SD = 11.4); TSF 22.1 (SD = 8.9) (excluded participants who attended < 8 sessions) Comment: Differences in between‐group treatment retention/attendance likely Outcome assessment: Treatment dropout ICBT n = 11; TSF n = 13 plus missed > 2 follow‐ups: ICBT n = 9; TSF n = 11; based on HDRS data) Total attrition: ICBT n = 20, 42%; TSF n = 24, 57% No ITT conducted Comment: reason for missing outcome data likely to be related to true outcome, with imbalance in numbers missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Risk is unclear as no study protocol is available. An endpoint analysis was reported for the 6‐month but not the 3‐month follow‐up, but the results of all planned analyses using the outcome listed in the methods are reported in the manuscript. |
| Other bias | High risk |
Fidelity: assessment of treatment fidelity was conducted but not performed by an independent rater. There was no significant impact of therapists/therapists within treatment groups on primary outcomes. Other treatments: an initial medication evaluation was conducted and 30‐minute monthly appointments were made available with the treating psychiatrist, with 97% of participants prescribed an antidepressant medication (predominately selective serotonin reuptake inhibitors (SSRls) or atypical antidepressants). There were no reported groups differences in the proportion of clients receiving antidepressant medication or in attending medication management appointments. The medication dose and medication adherence for each group was not reported. Groups did not partake in other treatment beyond Community 12‐step involvement, which was higher for the TSF than ICBT group. |
Carpenter 2008.
| Methods |
Design: randomised controlled trial Follow‐up: 24 weeks post‐treatment only Setting: two university‐affiliated, outpatient community‐based treatment programs Country: USA |
|
| Participants |
Participants: 38 methadone‐maintained DSM‐IV opiate‐dependent patients with major depressive disorder/dysthymia on the Structured Clinical Interview‐Substance Abuse Comorbidity (SCID‐SAC) Mean age (years): BTDD 38.8 (SD = 10.4); REL 41.2 (SD = 10.9) Sex: 58% male Ethnicity: 82% Caucasian |
|
| Interventions |
Description: Behavioral Therapy for Depression in Drug Dependence (BTDD) versus Structured Relaxation Intervention (REL). BTDD incorporated aspects of three operant‐based treatment programs: Changing Reinforcing Events (Lewinsohn 1980), the Community Reinforcement Approach (Meyers 1995), and Treatment Plan Contingency Management (points were earned for participating in the therapy session (3 points) and completing out‐of‐session assignments (up to 10 points); maximum of 208 points (1 point = 1 dollar) could be earned for 100% attendance (72 points) and completion of out‐of‐session assignments (136 points). Points were exchangeable for goods and services consistent with the treatment plan (Iguchi 1997). REL was based on a structured manual (Brown 1997) focusing on three relaxation strategies: 1) progressive muscle relaxation, 2) autogenic relaxation exercises, and 3) visual imagery based on idiographic scenarios of relaxation or tranquility. Format: group therapy; plus community‐based methadone treatment programs Duration: 24 weekly sessions Allocation: BTDD n = 18 ; REL n = 20 |
|
| Outcomes |
Treatment attendance: mean number of sessions attended Self‐report: BDI mean (SD) total score (past 7 days) Interviewer‐rated: HDRS (SD) mean total score (past 7 days); Substance Use Weekly Inventory (SUI modification of TLFB) ‐ percentage of weeks cocaine, opiate and benzodiazepine use during treatment (past 30 days; data collected weekly for 24 weeks). Attrition: proportion of participants with missing 24 weeks post‐treatment data |
|
| Notes |
Fidelity: all therapists completed a BTDD or Relaxation Therapy Checklist following each treatment session. Checklists outlined key components of each respective treatment and provided a means to assess adherence to each therapy condition. Session audiotapes were reviewed by senior therapists to monitor adherence to the treatment structure. Funding: NIDA grants R01 DA13118, K02 DA00288, K24 DA021850 (Nunes), K23 DA021850 (Carpenter). Conflict of interest: no statement provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (via author email): "The randomisation scheme (blocks) were generated by an independent person (based on a random number process)" Quote: "The treatment groups did not differ significantly on most baseline measures. Although stratification procedures balanced the proportion of participants using illicit substances in the week before treatment between the two treatment groups, the proportion of days that opiates were used in the month prior to treatment was greater among those in BTDD. (p. 647)" |
| Allocation concealment (selection bias) | Low risk | Quote (via author email): "The randomisation grid was maintained in a private password account held by the independent person." Comment: investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Participants and therapists were not blinded to treatment allocation. |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and personnel knowledge of group allocation may influence treatment engagement/participation/ attendance. |
| Blinding of outcome assessment (detection bias) self‐report | Unclear risk | Quote (vie author email): "The self‐report assessments were to be given by the research assistant ‐ who may or may not have been blind to therapy condition." Comment: The authors judge risk of bias to be unclear. While participants were not blind to treatment allocation and self‐report measures may be impacted by self‐presentation bias or client insight, it is unlikely that any such risk of bias will vary by treatment condition. The research assistant administering the self‐report measures is unlikely to have influenced the results. |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | High risk | Quote (via author email): "It could not be guaranteed they were blind to condition." |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Treatment attendance: BTDD n = 11.6 (SD=6.1); REL n = 15.3 (SD = 6.9); (P = 0.10) Comment: the number of treatment sessions completed was similar and did not differ significantly between groups Outcome assessment: attrition total n = 38, 37%; BTDD n = 9, 50%; REL n = 15, 25%; (P = 0 .05) Quote: "No significant differences on baseline measures between those participants completing the study and those who dropped out. (p. 647)." Comment: BTDD group had double the attrition rate of the REL group; LOCF was used for missing data and ITT was not used for the endpoint analysis. |
| Selective reporting (reporting bias) | Unclear risk | Risk is unclear as no study protocol is available. The results of all outcomes included in the method section of the paper are reported. |
| Other bias | Unclear risk |
Fidelity: therapist completed checklists for each session. Recordings were reviewed by senior therapists but no independent fidelity ratings were conducted. Other treatments: all participants were receiving at least 60 mg of methadone, it is unclear whether dosage was consistent between groups. Randomisation was stratified by antidepressant medication, and antidepressant medication was controlled in outcome analyses with a null effect reported for depression outcomes. It is unclear whether participants were able to receive other psychotherapy, in addition to that provided through the study. |
Johnson 2012.
| Methods |
Design: randomised controlled trial Follow‐up: 8 weeks post‐treatment; 3‐months post‐treatment follow‐up (post release) Setting: prison‐based substance use treatment program Country: USA |
|
| Participants |
Participants: 38 volunteer incarcerated females with a current DSM‐IV major depressive disorder (1 month after abstinence + HDRS >17) and substance abuse or dependence disorder (1 month prior to incarceration) diagnosed on the Structured Clinical Interview (SCID); all within 14‐28 weeks of release Mean age (years): 35.0 (SD = 9.2) Sex: 100% female Ethnicity: 18% Hispanic; 18% African American |
|
| Interventions |
Description: IPT‐D (Wilfey 2000) versus psychoeducation for co‐occurring mental health and substance use disorders (PSYCHOED). For the IPT‐D group, the interpersonal deficits focus for MDD among female prisoners was adapted to address current problematic relationship patterns (including isolation) that resulted from interpersonal trauma. PSYCHOED focused on the meaning of dual diagnosis, women’s experience with dual diagnosis, mood, anxiety, personality, psychotic and eating disorders, as well as self‐care. Both groups also received: TAU (16‐30 hours per week for SUD and mental health ‐ drug education and coping skills groups + weekly drug counselling) + 6 weekly post‐release individual sessions. Format: group plus individual therapy; plus prison treatment as usual Duration: group 8 weeks (24 sessions) plus 3 individual sessions (pre‐, mid‐ and post‐group) Allocation: IPT‐D: n = 19; PSYCHOED: n = 19 |
|
| Outcomes |
Treatment retention: missed < 2 sessions by choice ‐ including sessions missed due to medical or legal appointments or early release from prison Treatment attendance: mean number of sessions attended Self report: not included Interviewer‐rated: HDRS mean total score (7 days); TLFB percentage substance use relapse heavy drinking (4+ standard drinks) or drug use on at least 10% of days or any positive breath test/urine drug screen at follow‐up (90 days) Attition: percentage that completed each follow‐up assessment |
|
| Notes | Unequal distribution between groups on ethnicity and comorbidity. One‐month abstinence was a requirement at baseline. Funding: United States National Institute of Drug Abuse (NIDA; K23DA021159, Johnson). Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent individual generated the randomisation sequence and concealed the assignment of each wave before the study started. (p. 1176). The study used wave randomisation with at least an 8 week hiatus between then end of one group and the beginning of the next group to avoid contamination. (p. 1176)" Quote (via author email): "Random number generator (used to generate randomisation)" No significant between‐group differences on baseline demographic, depression or substance use variables (Table 1, p. 1179). |
| Allocation concealment (selection bias) | Low risk | Quote (via author email): "Group allocation concealed via sealed envelopes labelled with stratification variables and order until each group started." |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Participants and therapists not blind to treatment allocation |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and therapists knowledge of group allocation may influence treatment engagement/participation/ attendance. |
| Blinding of outcome assessment (detection bias) self‐report | Low risk | This study did not include self‐report measures |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | Low risk | Quote: "RAs, who conducted follow‐up assessments (after the in‐prison portion of the treatment and at 3 months after prison release), were kept blind to condition assignment. At study completion, blinded study RAs matched participants to conditions with chance (50%) accuracy. (p. 1176)." |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Treatment retention/attendance: IPT 98%; PSYCHOED 89% Treatment attendance: IPT 16 sessions; PSYCHOED 18 sessions Comment: the number of treatment sessions completed across groups was similar Outcome assessment: 100% follow‐up at post‐treatment and 3‐month follow‐up; ITT performed |
| Selective reporting (reporting bias) | Unclear risk | Risk is unclear as no study protocol is available. The results of all outcomes included in the method section of the paper are reported. |
| Other bias | High risk |
Fidelity (via author email): both IPT and PSYCHOED adherence, competence and contamination were independently rated; Quote: "the two conditions were actually cleanly different ‐ much more than for most therapy trials; Therapists trained in and ran both groups but allegiance effects were not assessed." Other treatments: the majority of participants were taking antidepressants, with many taking multiple anti‐depressants (average 2.1; e.g. lithium, aripiprazole, or quetiapine). There were differences between the treatment groups in proportion taking antidepressants (79% PSYCHOED versus 47% IPT). Clients were also able to receive non‐study prison mental health treatment, with uneven distribution of treatment received between groups (PSYCHOED: 63% versus21% IPT). Comment: hIgh risk due to the differences in other treatment received between PSYCHOED and IPT |
Lydecker 2010.
| Methods |
Design: randomised controlled trial Follow‐up: 12‐weeks (mid‐treatment), 24‐weeks (post‐treatment), 3‐, 6‐, 9‐ and 12‐months post‐treatment follow‐up Setting: Dual diagnosis outpatient clinic Country: USA |
|
| Participants |
Participants: 206 Veterans with DSM‐IV Major Depressive Disorder and co‐occurring alcohol, cannabis and/or stimulant dependence on Comprehensive Diagnostic Interview (CIDI) + HDRS > 20 and recent substance use (past 90 days); Mean age (years): 48.2 (SD = 7.7) Sex: 92% male Ethnicity: 71% Caucasian |
|
| Interventions |
Description: Identical to Brown 2006 above. Format: group therapy Duration: 24 weeks (36 sessions); Phase 1: 60 minutes biweekly for 12‐weeks + Phase 2: 60‐minutes weekly for 12‐weeks + standard pharmacotherapy Allocation: ICBT: n = 107; TSF: n = 99 |
|
| Outcomes |
Treatment completion: mean number of sessions completed Self‐report: not included Interviewer‐rated: HDRS mean (SD) total score (past 7 days); TLFB mean percentage days abstinent (past 90 days). Attriton: proportion not completed research follow‐up assessment |
|
| Notes | Abstinence was a requirement at baseline Each therapy session was videotaped, and a random sample (25%) of sessions were reviewed by a condition‐specific clinical supervisor to ensure fidelity to the manual and avoid contamination Funding: Veteran Affairs (VA) Medical Research Merit Review Grant (Brown), VA Merit Review Entry Program Grant (Tate). Conflict of interest: No statement provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote:" Consecutive admissions to the study were sequentially allocated by cohorts into ICBT or TSF. Participants were sequentially assigned to the group with the next entry date. (p. 456) No significant differences between treatment groups on baseline demographic, depression or substance use variables." Comment: high risk due to non‐random component in the sequence generation process |
| Allocation concealment (selection bias) | High risk | Allocation not concealed because participants were consecutively assigned into cohorts Comment: Investigators enrolling participants could possibly foresee assignments |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Quote: "Given the nature of this study in a clinical context, participants, administrators, and interviewers were not blinded to patients’ treatment assignment." (p. 456) |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and personnel knowledge of group allocation may influence treatment engagement/participation/ attendance. |
| Blinding of outcome assessment (detection bias) self‐report | Low risk | This study did not include self‐report measures |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | High risk | Quote:" Given the nature of this study in a clinical context, participants, administrators, and interviewers were not blinded to patients’ treatment assignment." (p. 456) |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Treatment attendance: ICBT: 20.2 (SD = 9.5); TSF: 19.4 (SD = 10.5) Comment: the number of treatment sessions completed across groups was similar Outcome assessment: Attrition mid post 3 month, 6 month, 9 month, 12 month TSF 17% 25% 26% 30% 31% 33% ICBT 14% 19% 22% 22% 28% 36% Comments: the two groups had similar attrition rates; statistical analyses used were robust to missing data; ITT analyses performed |
| Selective reporting (reporting bias) | Unclear risk | Risk is unclear as no study protocol is available. Trajectory analyses and not endpoint analyses at 3‐ and 9‐month follow‐up were performed, but the results of all planned analyses using the outcome listed in the methods are reported in the manuscript. |
| Other bias | High risk |
Fidelity: treatment fidelity assessed but not performed by independent rater; groups had entry times every 4 weeks, unclear if group contamination effects were controlled for. Other treatments: an initial medication evaluation was conducted and 30‐minute monthly appointments were made available with the treating psychiatrist, with 2.7% of participants were prescribed a substance use medication and between 92% and 98% prescribed an antidepressant medication. Differences between the groups were reported for antidepressant prescription (higher for TSF than ICBT) at follow‐up. Reported that participants agreed not to partake in other psychotherapy beyond involvement in community 12‐Step addiction meetings. The TSF group reported greater involvement in 12‐Step community meetings. |
Markowitz 2008.
| Methods |
Design: randomised controlled trial Follow‐up: 16‐week (post‐treatment) Setting: outpatients at Cornell University Medical College Country:USA |
|
| Participants |
Participants: 26 adults with DSM‐IV dysthymia and secondary alcohol abuse or dependence diagnosed on the Structured Clinical Interview for DSM‐IV; HDRS > 13 Mean age (years): 38.4 (SD = 10.5) Sex: 69% male Ethnicity: 69% White |
|
| Interventions |
Description: Interpersonal Psychotherapy Treatment for Depression (IPT‐D) versus Brief Supportive Therapy (BST). IPT‐D employs a medical model of illness that excuses the patient from blame, and focuses on their emotional responses to and coping strategies to resolve life crises. IPT‐D encourages patients to change their life situations so as to improve their mood disorder. BST employs elicitation of affect and reflective listening but provides no explicit theoretical formulation to the patient and does not focus as directly on exploring pragmatic options for changing the environment. Participants in both conditions were encouraged to attend AA meetings. Format: individual therapy Duration: 16‐18 sessions over 16 weeks Allocation: IPT‐D = 14 , BST = 12 |
|
| Outcomes |
Treatment retention: failed to complete treatment but re‐coded into treatment completion for this review Self‐report: BDI mean total score (7 days) Interviewer‐rated: HDRS mean (SD) total score (past 7 days), TLFB mean (SD) percentage of days abstinent (past 30 days) Attrition: proportion not completed research follow‐up assessment |
|
| Notes | A relatively high percentage of participants who met study entry criteria also reported alcohol abstinence in the month before treatment. Funding: National Institute of Mental Health (NIHM; MH‐ 49635), thee Weill Cornell Department of Psychiatry, MINT: Mental Health Initiative. Conflict of interest: no statement provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible subjects signed separate informed consent for study entry and were assigned to IPT‐Dor BSP in a 1:1 distribution using a computer‐generated random number program. (p. 469)." Quote (via author email): "The study statistician, otherwise not involved in the clinical procedure of the trial, computer‐generated a random number sequence. computer‐generated a random number sequence." Quote:" The 2 treatment groups did not differ on demographic or clinical variables." (p. 470) |
| Allocation concealment (selection bias) | Low risk | Quote (via author email): "Patient assignments were sealed in numbered, otherwise blank, opaque, sealed envelopes, which I as Principal Investigator opened in the patient's presence after the patient had signed informed consent." |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Participants, therapists and the principal investigator was not blinded to treatment allocation. |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and therapist knowledge of group allocation may influence treatment engagement/participation/ attendance. |
| Blinding of outcome assessment (detection bias) self‐report | Unclear risk | Comment: The authors judge risk of bias to be unclear. While participants were not blind to treatment allocation and self‐report measures may be impacted by self‐presentation bias or client insight, it is unlikely that any such risk of bias will vary by treatment condition. |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | Low risk | Quote:" Independent evaluators blinded to clinical assignment rated subjects." (p. 470) |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Treatment retention: IPT‐D = 57%; BST = 58% Comment: the number of treatment completers was similar Outcome assessment: IPT‐D=42.9% , BST=16.7%; LOCF and ITT was used Comment: The IPT‐D group had double the attrition rate of the BST group; LOCF was used for missing data |
| Selective reporting (reporting bias) | Unclear risk | Risk is unclear as no study protocol is available, but the results of all planned analyses using the outcome listed in the methods are reported in the manuscript. |
| Other bias | Low risk |
Fidelity: all sessions audio‐taped and random sessions rated by an independent rater. Separate therapists delivered the two treatments to avoid therapist allegiance/contamination effects but the success of this was not formally assessed. Other treatments: concurrent pharmacotherapy or psychotherapy was an exclusion criteria |
Rohde 2014.
| Methods |
Design: randomised controlled trial; Follow‐up: 20‐weeks (post‐treatment), 6‐ and 12‐months follow‐up Setting: oupatient substance use treatment services in Portland, Oregon, Alburquerque, New Mexico Country:USA |
|
| Participants |
Participants: 170 adolescents with a current DSM‐IV depression disorder (MDD, dysthymia, adjustment disorder with depressed mood; depression NOS) and non‐nicotine substance use disorder on the Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Present and Lifetime Version (K‐SADS‐PL); Drug use within the last 90 days (TLFB) Mean age (years): 13‐18 years Sex: > 25% female Ethnicity: 61% non‐Hispanic white |
|
| Interventions |
Description: (i) Functional Family Therapy (FFT) followed by modified adolescent Coping With Depression course (CWD) (FFT/CWD): CWD provided cognitive and behavioral strategies to address adolescent depression (Clarke 1990). The communication and problem‐solving skills were included in FFT and a points system was added to reward participation. FFT is a behaviourally‐based model of family therapy (Alexander 1982) that targets addictive behaviours by altering family systems using five treatment phases (engagement, motivation, relational assessment, behaviour change, generalisation). (ii) CWD followed by FFT (CWD/FFT) (iii) Integrated FFT & CWD treatment (CWD+FFT): Family sessions followed FFT. Group treatment consisted of CWD augmented to provide skills aimed at reducing substance use (Waldron 2004). Format: group and family therapy Duration: FFT/CWD: 12 x 90min group sessions in 10 weeks + 12 family sessions in 10 weeks (24 sessions in 20 weeks); CWD/FFT: 12 family sessions in 10 weeks + 12 x 90min group sessions in 10 weeks (24 sessions in 20 weeks); CWD+FFT: 4 family sessions prior to 10 group sessions over 10 weeks + 2 additional sessions (24 sessions over 20 weeks) Allocation: FFT/CWD = 61; CWD/FFT = 56; CWD+FFT = 53 |
|
| Outcomes |
Treatment attendance: number of sessions completed Self‐report: CDRS‐R mean (SD) total score (past 7 days) Interviewer‐rated: TLFB mean (SD) percentage of days of all drug use (past 90 days) Attrition: proportion not completed research follow‐up assessment |
|
| Notes | No nesting effects for cohorts or therapists; site effects non‐significant Funding: National Institute on Drug Abuse Research Grant (DA21357). Conflict of interest: no statement provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (via author email). "Adolescents were recruited until a cohort could be formed composed of 4‐9 families. Each cohort (total = 27) was randomly assigned to one of the three sequences. Assignment of cohorts to conditions occurred in blocks of three at each site so that all conditions were completed before any was repeated. We adopted the cohort strategy so that we could coordinate the formation of group CWD sessions across all three treatment sequences. Once cohorts had been randomised, caseload and scheduling factors were used to assign therapists (i.e., therapists were not randomly assigned)." Quote (via author email): "The analyst used a computer‐based randomisation procedure" No baseline differences between treatments groups except for age (CWD+FFT younger; P < 0 .05) |
| Allocation concealment (selection bias) | Low risk | Quote (via author email) "The randomisation sequence was known by the project coordinator in Eugene, Oregon (a separate site) and she told the therapists at each site which condition would be provided next – we needed to do this so that FFT and CWD‐A therapists could be available to provide treatment when it would occur. Therapists did not do the assessments (they were conducted by separate research assistants who remained masked to condition throughout assessments). Once enough families had enrolled and been found to be eligible, they were told which condition they would receive." |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Participants and personnel were not blind to treatment allocation. |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Participant and personnel knowledge of group allocation may influence treatment engagement/participation/ attendance |
| Blinding of outcome assessment (detection bias) self‐report | Unclear risk | Comment: The authors judge risk of bias to be unclear. While participants were not blind to treatment allocation and self‐report measures may be impacted by self‐presentation bias or client insight, it is unlikely that any such risk of bias will vary by treatment condition. |
| Blinding of outcome assessment (detection bias) interviewer‐rated outcomes | Low risk | Quote (via author email):" Follow up assessors were blind to treatment allocation. The interview data were collected by trained research assistants who were not the treating clinician." |
| Blinding of outcome assessment (detection bias) treatment retention/attendance | Low risk | Blinding unclear, but outcome measure is unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Treatment attendance: FFT/CWD = 15.4, CWD/FFT = 12.7 , CWD+FFT = 16.8; Significantly lower in CWD/FFT than CWD+FFT (P < 0.03), neither differed from FFT/CWD. Comment: the number of treatment sessions completed was similar, one significant difference found Outcome assessment: Attrition post‐treatmen: FFT/CWD 21%; CWD/FFT 25%; CWD+FFT 17% Attrition 6‐month: FFT/CWD 39%; CWD/FFT 27%; CWD+FFT 25% Attrition 12‐month:FFT/CWD 26%; CWD/FFT 16%; CWD+FFT 9% Comment: similar attrition across groups missing data imputed (10 imputed data sets to run analyses); ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Risk is unclear as no study protocol is available, but the results of all planned analyses using the outcome listed in the methods are reported in the manuscript. |
| Other bias | High risk |
Fidelity: weekly supervision; videotaped sessions rated for adherence but unclear if rater was independent. Therapists adhered to the treatments with no differences across treatment sequences. Other treatment: participants were able to attend adjunctive treatment or use anxiety/depression medication. A difference between treatment groups is reported for concurrent adjunctive therapy during therapy (FFT/CWD = 16%; CWD/FFT = 49%; CT = 20%), but not at follow‐up. No difference in medication use is reported. Receiving adjunctive therapy was found to positively impact outcome results. |
AA: Alcoholics Anonymous; ASI: Addiction Severity Index; AUD: alcohol use disorder; BDI: Beck Depression Inventory; BTDD: Behavioral Therapy for Depression in Drug Dependence; CT: cognitive therapy; DSM 1V: Diagnostic and Statistical Manual 4th edition; HDRS: Hamilton Rating Scale for Depression; ICBT: Integrated Cognitive Behavioural Therapy; IPT‐D: Interpersonal Psychotherapy for Depression; ITT: intention‐to‐treat; LOCF: last observation carried forward; MDD: major depressive disorder; NT: narrative therapy; pt: prescriptive therapy; SD: standard deviation; SUD: substance use disorder; TAU: treatment as usual; TLFB: Timeline Follow Back
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghataher 2014 | Depression criteria not met |
| Agyapong 2012a | Psychotherapy criteria not met ‐ text messaging ‐ not specified psychotherapy |
| Agyapong 2012b | Psychotherapy criteria not met ‐ text messaging ‐ not specified psychotherapy |
| Agyapong 2013 | Psychotherapy criteria not met ‐ text messaging ‐ not specified psychotherapy |
| Allsop 2014 | Depression criteria not met |
| Arean 2008 | Depression criteria not met |
| Aschbrenner 2015 | Depression and SUD criteria not met |
| Baker 2010 | SUD criteria not met |
| Baker 2013 | SUD criteria not met |
| Baker 2014 | SUD criteria not met |
| Banducci 2015 | Depression criteria not met |
| Barlow 2015 | Depression and SUD criteria not met |
| Battersby 2013 | Depression criteria not met |
| Becker 2015 | Depression criteria not met |
| Bellack 2006 | Depression criteria not met |
| Berman 2015 | Depression criteria not met |
| Bernard 2015 | Depression criteria not met |
| Bluth 2016 | Depression and SUD criteria not met |
| Bolton 2014 | SUD criteria not met |
| Bowman 1996 | Depression criteria not met |
| Bricker 2007 | SUD criteria not met |
| Briere 2014 | Depression criteria not met |
| Brody 2012 | Depression and SUD criteria not met |
| Brown 1997 | Depression criteria not met |
| Brown 2007 | Depression criteria not met |
| Capron 2014 | Depression criteria not met |
| Carroll 1995 | Depression criteria not met |
| Clair‐Michaud 2016 | Depression criteria not met |
| Collins 2015 | Depression and SUD criteria not met |
| Cornelius 2011 | Not RCT |
| Cucciare 2013 | Depression criteria not met |
| Delgadillo 2015 | SUD criteria not met |
| Detweiler 2015 | Depression criteria not met |
| Duffy 2006 | Depression and SUD criteria not met |
| Essex 2014 | Depression and SUD criteria not met |
| Forray 2014 | Depression criteria not met |
| Garcia‐Fernandez 2013 | Depression criteria not met |
| Gardner 2016 | Depression and SUD criteria not met |
| Geisner 2015 | Depression criteria not met |
| Giovancarli 2016 | Depression criteria not met |
| Glasner 2012 | Depression criteria not met |
| Gonzalez‐Menendez 2014 | Depression criteria not met |
| Gottheil 2002 | Depression criteria not met |
| Grothues 2008 | Depression criteria not met |
| Grothues 2008a | Depression criteria not met |
| Guo 2014 | Depression criteria not met |
| Hall 1994 | Depression criteria not met |
| Hall 1996 | Depression criteria not met |
| Hall 2006 | SUD criteria not met |
| Haller 2016 | Depression criteria not met ‐ specifically targeting PTSD and trauma |
| Hallgren 2014 | Depression criteria not met |
| Hickman 2015 | Depression and SUD criteria not met |
| Hides 2010 | SUD criteria not met |
| Hides 2011 | Depression criteria not met |
| Horigian 2013 | Depression criteria not met |
| Hosseinzadeh 2014 | SUD criteria not met |
| Johnson 2017 | Depression criteria not met |
| Jones 2015 | Depression and SUD criteria not met |
| Kahler 2002 | Depression criteria not met |
| Kahler 2015 | Depression criteria not met |
| Kalapatapu 2014 | SUD criteria not met |
| Kapson 2010 | Depression criteria not met |
| Katz 2008 | Depression and SUD criteria not met |
| Kavanagh 2006 | Depression criteria not met |
| Kay‐Lambkin 2009 | SUD criteria not met |
| Kay‐Lambkin 2011a | SUD criteria not met |
| Kay‐Lambkin 2011b | Depression criteria not met |
| Kay‐Lambkin 2017 | SUD criteria not met |
| Lanza 2014 | Depression criteria not met |
| Lehman 1993 | Depression criteria not met |
| McClanahan 2001 | Depression criteria not met |
| McDevitt‐Murphy 2014 | Depression criteria not met |
| McDonell 2013 | Depression criteria not met |
| McHugh 2010 | Depression criteria not met |
| McKay 2002 | Depression criteria not met |
| Menchetti 2014 | SUD criteria not met |
| Milby 2015 | Depression criteria not met |
| Montag 2015 | Depression and SUD criteria not met |
| Morley 2016 | Depression and SUD criteria not met |
| Mujika 2014 | Depression and SUD criteria not met |
| Muller 2015 | SUD criteria not met |
| Myers 2016 | Depression criteria not met |
| Neumann 2016 | Depression criteria not met |
| Oesterle 2015 | Depression and SUD criteria not met |
| Oslin 2003 | Depression and SUD criteria not met |
| Oslin 2004 | Depression criteria not met |
| Ostergaard 2018 | Depression criteria not met |
| Pachankis 2015 | SUD criteria not met |
| Palfai 2014 | Depression criteria not met |
| Patten 1998 | Depression criteria not met |
| Patten 2002 | Depression criteria not met |
| Peck 2005 | Depression criteria not met |
| Peckham 2015 | Depression criteria not met |
| Pedrelli 2015 | Depression and SUD criteria not met |
| Petersen 2009 | Depression criteria not met |
| Polcin 2015 | Depression criteria not met |
| Ponizovsky 2015 | Depression criteria not met |
| Rawson 2015 | Depression criteria not met |
| Reid 2016 | Depression criteria not met |
| Rohde 2014a | Depression and SUD criteria not met |
| Rose 2015 | Depression and SUD criteria not met |
| Ruscio 2016 | Depression criteria not met |
| Ryb 2011 | Depression criteria not met |
| Saedy 2015 | Depression criteria not met |
| Safren 2012 | Depression criteria not met |
| Satre 2013 | SUD criteria not met |
| Satre 2016 | Depression and SUD criteria not met |
| Seghatoleslam 2014 | SUD criteria not met |
| Seitz‐Brown 2015 | Depression and SUD criteria not met |
| Shoptaw 2006 | Depression criteria not met |
| Singla 2019 | Depression criteria not met |
| Stein 2011 | SUD criteria not met |
| Stockings 2014 | Depression and SUD criteria not met |
| Sun 2007 | Depression criteria not met |
| Tapert 2003 | Depression criteria not met |
| Tempersta 1998 | Psychotherapy criteria not met ‐ pharmacological treatment within a therapeutic context ‐ not randomised to psychotherapy, only to pharmacological treatment |
| Tempesta 2000 | Psychotherapy criteria not met ‐ pharmacological treatment within a therapeutic context ‐ not randomised to psychotherapy, only to pharmacological treatment |
| Tiburcio 2016 | Depression criteria not met |
| Vinci 2014 | Depression criteria not met |
| Wilks 2018 | Depression criteria not met |
| Wilson 2014 | Depression criteria not met |
| Womack 2004 | Not RCT |
| Wusthoff 2014 | Depression criteria not met |
| Zemestani 2016 | Depression criteria not met |
PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; SUD: substance use disorder
Differences between protocol and review
The inclusion criteria in the protocol stated all studies including individuals with a current depressive disorder with comorbid substance use problem according to DSM‐IV, ICD‐10 criteria applied by a clinician, via structured clinical interview or via clinical cut‐offs on a psychometric measure of substance misuse would be included. This criteria was revised to only include studies which included individuals with a current comorbid DSM/ICD depression and substance use disorder derived using structured clinical interviews, due to the considerable heterogeneity in the cut‐offs used on psychometric instruments to identify individuals with depression and substance use. This change resulted in the exclusion of four studies that used screening tools to identify individuals with depression and/or substance use disorders (Brown 1997; Johnson 2017; Geisner 2015; Kalapatapu 2014) and seven studies that used various definitions of hazardous or harmful substance use as an inclusion criteria for substance use (Baker 2010; Baker 2013; Baker 2014; Kay‐Lambkin 2009; Kay‐Lambkin 2011a; Satre 2013; Satre 2016).
Planned subgroup and sensitivity analyses could not be conducted due to the small number of included studies and their clinical heterogeneity limiting the possibility to pool data.
Contributions of authors
LH wrote the protocol and the initial version of the review. LH, CQ and SS undertook the searches and screening, LH and CQ extracted the data and appraised the quality of papers. AB and DK provided advice on the screening, quality appraisal and assisted with the interpretation of data. All authors assisted with the writing and editing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Health and Medical Research Council (NHMRC) Senior Research Fellowship (APP1119098; 2017‐2021) awarded to Leanne Hides, Australia.
Salary
Australian Research Council (ARC) Future Fellowship (FT120100780; 2012‐2016) awarded to Leanne Hides, Australia.
Declarations of interest
No authors have any conflict of interests to declare.
New
References
References to studies included in this review
Beutler 2003 {published and unpublished data}
- Beutler LE, Moleiro C, Marlik M, Harwood TM, Romanelli R, Gallagher‐Thompson D, et al. A comparison of the dodo, EST, and ATI factors among comorbid stimulant‐dependent, depressed patients. Clinical Psychology & Psychotherapy 2003;10(2):69‐85. [DOI: 10.1002/cpp.354] [DOI] [Google Scholar]
Brown 2006 {published and unpublished data}
- Brown SA, Glasner‐Edwards SV, Tate SR, McQuaid JR, Chalekian J, Granholm E. Integrated cognitive behavioral therapy versus twelve‐step facilitation therapy for substance‐dependent adults with depressive disorders. Journal of Psychoactive Drugs 2006;38(4):449‐60. [PUBMED: 17373561] [DOI] [PubMed] [Google Scholar]
Carpenter 2008 {published and unpublished data}
- Carpenter KM, Smith JL, Aharonovich E, Nunes EV. Developing therapies for depression in drug dependence: results of a stage 1 therapy study. American Journal of Drug and Alcohol Abuse 2008;34(5):642‐52. [PUBMED: 18821458] [DOI] [PubMed] [Google Scholar]
Johnson 2012 {published and unpublished data}
- Johnson JE, Zlotnick C. Pilot study of treatment for major depression among women prisoners with substance use disorder. Journal of Psychiatric Research 2012;46(9):1174‐83. [PUBMED: 22694906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lydecker 2010 {published and unpublished data}
- Lydecker KP, Tate SR, Cummins KM, McQuaid J, Granholm E, Brown SA. Clinical outcomes of an integrated treatment for depression and substance use disorders. Psychology of Addictive Behaviors 2010;24(3):453‐65. [PUBMED: 20853931] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Tate SR, McQuaid JR, Granholm EL, Brown SA. 12‐step affiliation and attendance following treatment for comorbid substance dependence and depression: a latent growth curve mediation model. Substance Abuse 2013;34(1):43‐50. [PUBMED: 23327503] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Trim RS, Roesch SC, Mrnak‐Meyer J, Tate SR, Brown SA. Comorbid depression and substance use disorder: longitudinal associations between symptoms in a controlled trial. Journal of Substance Abuse Treatment 2012;43(3):291‐302. [PUBMED: 22406052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Markowitz 2008 {published and unpublished data}
- Markowitz JC, Kocsus JH, Christos P, Bleiberg K, Carlin A. Pilot study of interpersonal psychotherapy versus supportive psychotherapy for dysthymic patients with secondary alcohol abuse or dependence. Journal of Nervous and Mental Disease 2008;196(6):468‐74. [PUBMED: 18552624] [DOI] [PubMed] [Google Scholar]
Rohde 2014 {published and unpublished data}
- Rohde P, Waldron HB, Turner CW, Brody J, Jorgensen J. Sequenced versus coordinated treatment for adolescents with comorbid depressive and substance use disorders. Journal of Consulting and Clinical Psychology 2014;82(2):342‐8. [PUBMED: 24491069] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aghataher 2014 {published data only}
- Aghataher A, Mahani KN. The effect of Rational Emotive Behavior Group Therapy on self‐concept and depression of self‐introduced drug abusers referred to Ofogh Addiction Treatment Center in Zarand (Kerman, Iran). Biomedical and Pharmacology Journal 2014;7(1):317‐23. [DOI: 10.13005/bpj/493] [DOI] [Google Scholar]
Agyapong 2012a {published data only}
- Agyapong VI, Ahern S, McLoughlin DM, Farren CK. Supportive text messaging for depression and comorbid alcohol use disorder: single‐blind randomised trial. Journal of Affective Disorders 2012;141(2‐3):168‐76. [PUBMED: 22464008] [DOI] [PubMed] [Google Scholar]
Agyapong 2012b {published data only}
- Agyapong VI, McLoughlin DM, Farren CK. Usefulness of supportive text messages to patients with alcohol use disorder and comorbid depression ‐ a single‐blind randomised trial. Abstracts of the 20th European Congress of Psychiatry. 2012; Vol. 27, issue 1:1. [DOI: 10.1016/S0924-9338(12)74103-2] [DOI]
Agyapong 2013 {published data only}
- Agyapong VI, McLoughlin DM, Farren CK. Six‐months outcomes of a randomised trial of supportive text messaging for depression and comorbid alcohol use disorder. Journal of Affective Disorders 2013;151(1):100‐4. [PUBMED: 23800443] [DOI] [PubMed] [Google Scholar]
Allsop 2014 {published data only}
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 2014;71(3):281‐91. [PUBMED: 24430917] [DOI] [PubMed] [Google Scholar]
Arean 2008 {published data only}
- Arean PA, Ayalon L, Jin C, McCulloch CE, Linkins K, Chen H, et al. Integrated specialty mental health care among older minorities improves access but not outcomes: results of the PRISMe study. International Journal of Geriatric Psychiatry 2008;23(10):1086‐92. [PUBMED: 18727133] [DOI] [PubMed] [Google Scholar]
Aschbrenner 2015 {published data only}
- Aschbrenner KA, Ferron JC, Mueser KT, Bartels SJ, Brunette MF. Social predictors of cessation treatment use among smokers with serious mental illness. Addictive Behaviors 2015;41:169‐74. [PUBMED: 25452062] [DOI] [PubMed] [Google Scholar]
Baker 2010 {published data only}
- Baker AL, Kavanagh DJ, Kay‐Lambkin FJ, Hunt SA, Lewin TJ, Carr VJ, et al. Randomized controlled trial of cognitive‐behavioural therapy for coexisting depression and alcohol problems: short‐term outcome. Addiction (Abingdon, England) 2010;105(1):87‐99. [PUBMED: 19919594] [DOI] [PubMed] [Google Scholar]
- Connolly JM, Kavanagh DJ, Baker AL, Kay‐Lambkin FJ, Lewin TJ, Davis PJ, et al. Craving as a predictor of treatment outcomes in heavy drinkers with comorbid depressed mood. Addictive Behaviors 2013;38(2):1585‐92. [PUBMED: 22727783] [DOI] [PubMed] [Google Scholar]
Baker 2013 {published data only}
- Baker AL, Kay‐Lambkin FJ, Gilligan C, Kavanagh DJ, Baker F, Lewin TJ. When does change begin following screening and brief intervention among depressed problem drinkers?. Journal of Substance Abuse Treatment 2013;44(3):264‐70. [PUBMED: 22981698] [DOI] [PubMed] [Google Scholar]
Baker 2014 {published data only}
- Baker AL, Kavanagh DJ, Kay‐Lambkin FJ, Hunt SA, Lewin TJ, Carr VJ, et al. Randomized controlled trial of MICBT for co‐existing alcohol misuse and depression: outcomes to 36‐months. Journal of Substance Abuse Treatment 2014;46(3):281‐90. [PUBMED: 24210534] [DOI] [PubMed] [Google Scholar]
Banducci 2015 {published data only}
- Banducci AN, Lejuez CW, MacPherson L. Evaluation of a brief smoking cessation intervention for inpatient substance users with elevated depressive symptoms. Drug and Alcohol Dependence 2015;146:e216. [DOI: 10.1016/j.drugalcdep.2014.09.056] [DOI] [Google Scholar]
Barlow 2015 {published data only}
- Barlow A, Mullany B, Neault N, Goklish N, Billy T, Hastings R, et al. Paraprofessional‐delivered home‐visiting intervention for American Indian teen mothers and children: 3‐year outcomes from a randomized controlled trial. American Journal of Psychiatry 2015;172(2):154‐62. [PUBMED: 25321149] [DOI] [PubMed] [Google Scholar]
Battersby 2013 {published data only}
- Battersby MW, Beattie J, Pols RG, Smith DP, Condon J, Blunden S. A randomised controlled trial of the Flinders Program of chronic condition management in Vietnam veterans with co‐morbid alcohol misuse, and psychiatric and medical conditions. Australian and New Zealand Journal of Psychiatry 2013;47(5):451‐62. [PUBMED: 23307806] [DOI] [PubMed] [Google Scholar]
Becker 2015 {published data only}
- Becker J, Haug S, Kraemer T, Schaub MP. Feasibility of a group cessation program for co‐smokers of cannabis and tobacco. Drug and Alcohol Review 2015;34(4):418‐26. [PUBMED: 25676414] [DOI] [PubMed] [Google Scholar]
Bellack 2006 {published data only}
- Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Archives of General Psychiatry 2006;63(4):426‐32. [PUBMED: 16585472] [DOI] [PubMed] [Google Scholar]
Berman 2015 {published data only}
- Berman AH, Wennberg P, Sinadinovic K. Changes in mental and physical well‐being among problematic alcohol and drug users in 12‐month Internet‐based intervention trials. Psychology of Addictive Behaviors 2015;29(1):97‐105. [PUBMED: 25664387] [DOI] [PubMed] [Google Scholar]
Bernard 2015 {published data only}
- Bernard P, Ninot G, Cyprien F, Courtet P, Guillaume S, Georgescu V, et al. Exercise and counseling for smoking cessation in smokers with depressive symptoms: a randomized controlled pilot trial. Journal of Dual Diagnosis 2015;11(3‐4):205‐16. [PUBMED: 26683252] [DOI] [PubMed] [Google Scholar]
Bluth 2016 {published data only}
- Bluth K, Campo RA, Pruteanu‐Malinici S, Reams A, Mullarkey M, Broderick PC. A school‐based mindfulness pilot study for ethnically diverse at‐risk adolescents. Mindfulness 2016;7(1):90‐104. [PUBMED: 27034729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2014 {published data only}
- Bolton P, Lee C, Haroz EE, Murray L, Dorsey S, Robinson C, et al. A transdiagnostic community‐based mental health treatment for comorbid disorders: development and outcomes of a randomized controlled trial among Burmese refugees in Thailand. PLOS Medicine 2014;11(11):e1001757. [PUBMED: 25386945] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowman 1996 {published data only}
- Bowman V, Ward LC, Bowman D, Scogin F. Self‐examination therapy as an adjunct treatment for depressive symptoms in substance abusing patients. Addictive Behaviors 1996;21(1):129‐33. [PUBMED: 8729714] [DOI] [PubMed] [Google Scholar]
Bricker 2007 {published data only}
- Bricker JB, Russo J, Stein MB, Sherbourne C, Craske M, Schraufnagel TJ, et al. Does occasional cannabis use impact anxiety and depression treatment outcomes?: results from a randomized effectiveness trial. Depression and Anxiety 2007;24(6):392‐8. [PUBMED: 17096386] [DOI] [PubMed] [Google Scholar]
Briere 2014 {published data only}
- Briere FN, Rohde P, Shaw H, Stice E. Moderators of two indicated cognitive‐behavioral depression prevention approaches for adolescents in a school‐based effectiveness trial. Behaviour Research and Therapy 2014;53:55‐62. [PUBMED: 24418653] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brody 2012 {published data only}
- Brody GH, Chen YF, Kogan SM, Yu T, Molgaard VK, DiClemente RJ, et al. Family‐centered program deters substance use, conduct problems, and depressive symptoms in black adolescents. Pediatrics 2012;129(1):108‐15. [PUBMED: 22157131] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown RA, Evans DM, Miller IW, Burgess ES, Mueller TI. Cognitive‐behavioral treatment for depression in alcoholism. Journal of Consulting and Clinical Psychology 1997;65(5):715‐26. [PUBMED: 9337490] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown RA, Niaura R, Lloyd‐Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive‐behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco 2007;9(7):721‐30. [PUBMED: 17577801] [DOI] [PMC free article] [PubMed] [Google Scholar]
Capron 2014 {published data only}
- Capron DW, Norr AM, Zvolensky MJ, Schmidt NB. Prospective evaluation of the effect of an anxiety sensitivity intervention on suicidality among smokers. Cognitive Behaviour Therapy 2014;43(1):72‐82. [PUBMED: 23767786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 1995 {published data only}
- Carroll KM, Nich C, Rounsaville BJ. Differential symptom reduction in depressed cocaine abusers treated with psychotherapy and pharmacotherapy. Journal of Nervous and Mental Disease 1995;183(4):251‐9. [PUBMED: 7714514] [DOI] [PubMed] [Google Scholar]
Clair‐Michaud 2016 {published data only}
- Clair‐Michaud M, Martin RA, Stein LA, Bassett S, Lebeau R, Golembeske C. The impact of motivational interviewing on delinquent behaviors in incarcerated adolescents. Journal of Substance Abuse Treatment 2016;65:13‐9. [PUBMED: 26517954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2015 {published data only}
- Collins BN, Nair US, Hovell MF, DiSantis KI, Jaffe K, Tolley NM, et al. Reducing underserved children's exposure to tobacco smoke: a randomized counseling trial with maternal smokers. American Journal of Preventive Medicine 2015;49(4):534‐44. [PUBMED: 26028355] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornelius 2011 {published data only}
- Cornelius JR, Douaihy A, Bukstein OG, Daley DC, Wood SD, Kelly TM, et al. Evaluation of cognitive behavioral therapy/motivational enhancement therapy (CBT/MET) in a treatment trial of comorbid MDD/AUD adolescents. Addictive Behavior 2011;36(8):843‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucciare 2013 {published data only}
- Cucciare MA, Boden MT, Weingardt KR. Brief alcohol counseling improves mental health functioning in veterans with alcohol misuse: results from a randomized trial. Journal of Affective Disorders 2013;147(1‐3):312‐7. [PUBMED: 23218847] [DOI] [PubMed] [Google Scholar]
Delgadillo 2015 {published data only}
- Delgadillo J, Gore S, Ali S, Ekers D, Gilbody S, Gilchrist G, et al. Feasibility randomized controlled trial of cognitive and behavioral interventions for depression symptoms in patients accessing drug and alcohol treatment. Journal of Substance Abuse Treatment 2015;55:6‐14. [PUBMED: 25819701] [DOI] [PubMed] [Google Scholar]
Detweiler 2015 {published data only}
- Detweiler MB, Self JA, Lane S, Spencer L, Lutgens B, Kim DY, et al. Horticultural therapy: a pilot study on modulating cortisol levels and indices of substance craving, posttraumatic stress disorder, depression, and quality of life in veterans. Alternative Therapies in Health and Medicine 2015;21(4):36‐41. [PUBMED: 26030115] [PubMed] [Google Scholar]
Duffy 2006 {published data only}
- Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology 2006;15(11):2203‐8. [PUBMED: 17119047] [DOI] [PubMed] [Google Scholar]
Essex 2014 {published data only}
- Essex HN, White IR, Khadjesari Z, Linke S, McCambridge J, Murray E, et al. Quality of life among hazardous and harmful drinkers: EQ‐5D over a 1‐year follow‐up period. Quality of Life Research 2014;23(2):733‐43. [PUBMED: 24026632] [DOI] [PubMed] [Google Scholar]
Forray 2014 {published data only}
- Forray A, Gotman N, Kershaw T, Yonkers KA. Perinatal smoking and depression in women with concurrent substance use. Addictive Behaviors 2014;39(4):749‐56. [PUBMED: 24447885] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia‐Fernandez 2013 {published data only}
- Garcia‐Fernandez G, Secades‐Villa R, Garcia‐Rodriguez O, Pena‐Suarez E, Sanchez‐Hervas E. Contingency management improves outcomes in cocaine‐dependent outpatients with depressive symptoms. Experimental and Clinical Psychopharmacology 2013;21(6):482‐9. [PUBMED: 24080020] [DOI] [PubMed] [Google Scholar]
Gardner 2016 {published data only}
- Gardner LI, Marks G, Shahani L, Giordano TP, Wilson TE, Drainoni ML, et al. Assessing efficacy of a retention‐in‐care intervention among HIV patients with depression, anxiety, heavy alcohol consumption and illicit drug use. AIDS (London, England) 2016;30(7):1111‐9. [PUBMED: 26760454] [DOI] [PubMed] [Google Scholar]
Geisner 2015 {published data only}
- Geisner IM, Varvil‐Weld L, Mittmann AJ, Mallett K, Turrisi R. Brief web‐based intervention for college students with comorbid risky alcohol use and depressed mood: does it work and for whom?. Addictive Behaviors 2015;42:36‐43. [PUBMED: 25462652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giovancarli 2016 {published data only}
- Giovancarli C, Malbos E, Baumstarck K, Parola N, Pelissier MF, Lancon C, et al. Virtual reality cue exposure for the relapse prevention of tobacco consumption: a study protocol for a randomized controlled trial. Trials 2016;17:96. [PUBMED: 26892001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasner 2012 {unpublished data only}
- Glasner S, Brown SA, Rawson RA. Integrated CBT and motivational therapy versus community 12‐step participation as continuing care for substance dependent adults with major depression. Paper presented at the 46th annual meeting of the Association of Behavior and Cognitive Therapy (National Harbor, MD) November, 2012.
Gonzalez‐Menendez 2014 {published data only}
- González‐Menéndez A, Fernández P, Rodríguez F, Villagrá P. Long‐term outcomes of Acceptance and Commitment Therapy in drug‐dependent female inmates: a randomized controlled trial. International Journal of Clinical and Health Psychology 2014;14(1):18‐27. [DOI: 10.1016/S1697-2600(14)70033-X] [DOI] [Google Scholar]
Gottheil 2002 {published data only}
- Gottheil E, Thornton C, Weinstein S. Effectiveness of high versus low structure individual counseling for substance abuse. American Journal on Addictions 2002;11(4):279‐90. [PUBMED: 12584871] [DOI] [PubMed] [Google Scholar]
Grothues 2008 {published data only}
- Grothues JM, Bischof G, Reinhardt S, Meyer C, John U, Rumpf HJ. Differences in help seeking rates after brief intervention for alcohol use disorders in general practice patients with and without comorbid anxiety or depressive disorders. International Journal of Methods in Psychiatric Research 2008;17(S1):S74‐7. [PUBMED: 18543367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grothues 2008a {published data only}
- Grothues JM, Bischof G, Reinhardt S, Meyer C, John U, Rumpf HJ. Effectiveness of brief alcohol interventions for general practice patients with problematic drinking behavior and comorbid anxiety or depressive disorders. Drug and Alcohol Dependence 2008;94(1‐3):214‐20. [PUBMED: 18207336] [DOI] [PubMed] [Google Scholar]
Guo 2014 {published data only}
- Guo X, Slesnick N, Feng X. Reductions in depressive symptoms among substance‐abusing runaway adolescents and their primary caretakers: a randomized clinical trial. Journal of Family Psychology 2014;28(1):98‐105. [PUBMED: 24364361] [DOI] [PubMed] [Google Scholar]
Hall 1994 {published data only}
- Hall SM, Munoz RF, Reus VI. Cognitive‐behavioral intervention increases abstinence rates for depressive‐history smokers. Journal of Consulting and Clinical Psychology 1994;62(1):141‐6. [PUBMED: 8034816] [DOI] [PubMed] [Google Scholar]
Hall 1996 {published data only}
- Hall SM, Munoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment: a therapeutic contact and placebo‐controlled study. Journal of Consulting and Clinical Psychology 1996;64(5):1003‐9. [PUBMED: 8916629] [DOI] [PubMed] [Google Scholar]
Hall 2006 {published data only}
- Hall SM, Tsoh JY, Prochaska JJ, Eisendrath S, Rossi JS, Redding CA, et al. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. American Journal of Public Health 2006;96(10):1808‐14. [PUBMED: 17008577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haller 2016 {published data only}
- Haller M, Norman SB, Cummins K, Trim RS, Xu X, Cui R, et al. Integrated cognitive behavioral therapy versus cognitive processing therapy for adults with depression, substance use disorder, and trauma. Journal of Substance Abuse Treatment 2016;62:38‐48. [PUBMED: 26718130] [DOI] [PubMed] [Google Scholar]
Hallgren 2014 {published data only}
- Hallgren M, Romberg K, Bakshi AS, Andreasson S. Yoga as an adjunct treatment for alcohol dependence: a pilot study. Complementary Therapies in Medicine 2014;22(3):441‐5. [PUBMED: 24906582] [DOI] [PubMed] [Google Scholar]
Hickman 2015 {published data only}
- Hickman NJ 3rd, Delucchi KL, Prochaska JJ. Treating tobacco dependence at the intersection of diversity, poverty, and mental illness: a randomized feasibility and replication trial. Nicotine & Tobacco Research 2015;17(8):1012‐21. [PUBMED: 26180227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hides 2010 {published data only}
- Hides L, Carroll S, Catania L, Cotton SM, Baker A, Scaffidi A, et al. Outcomes of an integrated cognitive behaviour therapy (CBT) treatment program for co‐occurring depression and substance misuse in young people. Journal of Affective Disorders 2010;121(1‐2):169‐74. [PUBMED: 19604584] [DOI] [PubMed] [Google Scholar]
Hides 2011 {published data only}
- Hides LM, Elkins KS, Scaffidi A, Cotton SM, Carroll S, Lubman DI. Does the addition of integrated cognitive behaviour therapy and motivational interviewing improve the outcomes of standard care for young people with comorbid depression and substance misuse?. Medical Journal of Australia 2011;195(3):S31‐7. [PUBMED: 21806516] [DOI] [PubMed] [Google Scholar]
Horigian 2013 {published data only}
- Horigian VE, Weems CF, Robbins MS, Feaster DJ, Ucha J, Miller M, et al. Reductions in anxiety and depression symptoms in youth receiving substance use treatment. American Journal on Addictions 2013;22(4):329‐37. [PUBMED: 23795871] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosseinzadeh 2014 {published data only}
- Hosseinzadeh Asl N, Barahmand U. Effectiveness of mindfulness‐based cognitive therapy for co‐morbid depression in drug‐dependent males. Archives of Psychiatric Nursing 2014;28(5):314‐8. [PUBMED: 25439972] [DOI] [PubMed] [Google Scholar]
Johnson 2017 {published and unpublished data}
- Daughters SB, Braun AR, Sargeant MN, Reynolds EK, Hopko DR, Blanco C, et al. Effectiveness of a brief behavioral treatment for inner‐city illicit drug users with elevated depressive symptoms: the life enhancement treatment for substance use (LETS Act!). Journal of Clinical Psychiatry 2008;69(1):122‐9. [PUBMED: 18312046] [DOI] [PubMed] [Google Scholar]
- Johnson KA, Seitz‐Brown C, Anderson K, Degeorge D, Blevins E, Daughters SB. 1‐Year post treatment outcomes from a RCT of a behavioral activation treatment for substance use and depression. Drug and Alcohol Dependence 2017;171:e96. [Google Scholar]
Jones 2015 {published data only}
- Jones HA, Heffner JL, Mercer L, Wyszynski CM, Vilardaga R, Bricker JB. Web‐based acceptance and commitment therapy smoking cessation treatment for smokers with depressive symptoms. Journal of Dual Diagnosis 2015;11(1):56‐62. [PUBMED: 25671683] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahler 2002 {published data only}
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology 2002;111(4):670‐5. [PUBMED: 12428781] [DOI] [PubMed] [Google Scholar]
Kahler 2015 {published data only}
- Kahler CW, Spillane NS, Day AM, Cioe PA, Parks A, Leventhal AM, et al. Positive psychotherapy for smoking cessation: a pilot randomized controlled trial. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco 2015;17(11):1385‐92. [PUBMED: 25646352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalapatapu 2014 {published data only}
- Kalapatapu RK, Ho J, Cai X, Vinogradov S, Batki SL, Mohr DC. Cognitive‐behavioral therapy in depressed primary care patients with co‐occurring problematic alcohol use: effect of telephone‐administered vs. face‐to‐face treatment‐a secondary analysis. Journal of Psychoactive Drugs 2014;46(2):85‐92. [PUBMED: 25052784] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapson 2010 {published data only}
- Kapson HS, Haaga DA. Depression vulnerability moderates the effects of cognitive behavior therapy in a randomized controlled trial for smoking cessation. Behavior Therapy 2010;41(4):447‐60. [PUBMED: 21035610] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2008 {published data only}
- Katz KS, Blake SM, Milligan RA, Sharps PW, White DB, Rodan MF, et al. The design, implementation and acceptability of an integrated intervention to address multiple behavioral and psychosocial risk factors among pregnant African American women. BMC Pregnancy and Childbirth 2008;8:22. [PUBMED: 18578875] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006 {published data only}
- Kavanagh DJ, Sitharthan G, Young RM, Sitharthan T, Saunders JB, Shockley N, et al. Addition of cue exposure to cognitive‐behaviour therapy for alcohol misuse: a randomized trial with dysphoric drinkers. Addiction (Abingdon, England) 2006;101(8):1106‐16. [PUBMED: 16869840] [DOI] [PubMed] [Google Scholar]
Kay‐Lambkin 2009 {published data only}
- Kay‐Lambkin FJ, Baker AL, Lewin TJ, Carr VJ. Computer‐based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction (Abingdon, England) 2009;104(3):378‐88. [PUBMED: 19207345] [DOI] [PubMed] [Google Scholar]
Kay‐Lambkin 2011a {published data only}
- Kay‐Lambkin FJ, Baker AL, Kelly B, Lewin TJ. Clinician‐assisted computerised versus therapist‐delivered treatment for depressive and addictive disorders: a randomised controlled trial. Medical Journal of Australia 2011;195(3):S44‐50. [PUBMED: 21806518] [DOI] [PubMed] [Google Scholar]
Kay‐Lambkin 2011b {published data only}
- Kay‐Lambkin FJ, Baker AL, Lee NM, Jenner L, Lewin TJ. The influence of depression on treatment for methamphetamine use. Medical Journal of Australia 2011;195(3):S38‐43. [PUBMED: 21806517] [DOI] [PubMed] [Google Scholar]
Kay‐Lambkin 2017 {published data only}
- Kay‐Lambkin FJ, Baker AL, Palazzi K, Lewin TJ, Kelly BJ. Therapeutic alliance, client need for approval, and perfectionism as differential moderators of response to eHealth and traditionally delivered treatments for comorbid depression and substance use problems. International Journal of Behavioral Medicine 2017;24(5):728‐39. [DOI] [PubMed] [Google Scholar]
Lanza 2014 {published data only}
- Lanza PV, Garcia PF, Lamelas FR, Gonzalez‐Menendez A. Acceptance and commitment therapy versus cognitive behavioral therapy in the treatment of substance use disorder with incarcerated women. Journal of Clinical Psychology 2014;70(7):644‐57. [PUBMED: 24449031] [DOI] [PubMed] [Google Scholar]
Lehman 1993 {published data only}
- Lehman AF, Herron JD, Schwartz RP, Myers CP. Rehabilitation for adults with severe mental illness and substance use disorders.aA clinical trial. Journal of Nervous and Mental Disease 1993;181(2):86‐90. [PUBMED: 8426176] [DOI] [PubMed] [Google Scholar]
McClanahan 2001 {published data only}
- McClanahan TM. A comparative evaluation of cognitive‐behavioral therapy and insight‐oriented psychotherapy in the treatment of comorbid substance abuse, anxiety, and depression in substance abusing females. Dissertation Abstracts International. ProQuest Dissertations Publishing, 2001; Vol. 62, issue 03B:3009689‐3009689.
McDevitt‐Murphy 2014 {published data only}
- McDevitt‐Murphy ME, Murphy JG, Williams JL, Monahan CJ, Bracken‐Minor KL, Fields JA. Randomized controlled trial of two brief alcohol interventions for OEF/OIF veterans. Journal of Consulting and Clinical Psychology 2014;82(4):562‐8. [PUBMED: 24773573] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonell 2013 {published data only}
- McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. Randomized controlled trial of contingency management for stimulant use in community mental health patients with serious mental illness. American Journal of Psychiatry 2013;170(1):94‐101. [PUBMED: 23138961] [DOI] [PMC free article] [PubMed] [Google Scholar]
McHugh 2010 {published data only}
- McHugh RK, Greenfield SF. Psychiatric symptom improvement in women following group substance abuse treatment: results from the Women's Recovery Group Study. Journal of Cognitive Psychotherapy 2010;24(1):26‐36. [PUBMED: 20625473] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2002 {published data only}
- McKay JR, Pettinati HM, Morrison R, Feeley M, Mulvaney FD, Gallop R. Relation of depression diagnoses to 2‐year outcomes in cocaine‐dependent patients in a randomised continuing care study. Psychology of Addictive Behaviors 2002;16(3):225‐35. [PUBMED: 12236457] [PubMed] [Google Scholar]
Menchetti 2014 {published data only}
- Menchetti M, Rucci P, Bortolotti B, Bombi A, Scocco P, Kraemer HC, et al. Moderators of remission with interpersonal counselling or drug treatment in primary care patients with depression: randomised controlled trial. British Journal of Psychiatry 2014;204(2):144‐50. [PUBMED: 24311553] [DOI] [PubMed] [Google Scholar]
Milby 2015 {published data only}
- Milby JB, Conti K, Wallace D, Mennemeyer S, Mrug S, Schumacher JE. Comorbidity effects on cocaine dependence treatment and examination of reciprocal relationships between abstinence and depression. Journal of Consulting and Clinical Psychology 2015;83(1):45‐55. [PUBMED: 25329491] [DOI] [PubMed] [Google Scholar]
Montag 2015 {published data only}
- Montag A, Dusek M, Mazzetti A, Nelson L, Ortega M, Campillo A, Calac D, et al. Effect of depression on risky drinking and response to a screening, brief intervention, and referral for treatment (SBIRT) intervention.. Alcoholism: Clinical and Experimental Research 2014;38:257a. [PUBMED: 26066915]26066915 [Google Scholar]
- Montag AC, Brodine SK, Alcaraz JE, Clapp JD, Allison MA, Calac DJ, et al. Effect of depression on risky drinking and response to a screening, brief intervention, and referral to treatment intervention. American Journal of Public Health 2015;105(8):1572‐6. [PUBMED: 26066915] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morley 2016 {published data only}
- Morley KC, Baillie A, Leung S, Sannibale C, Teesson M, Haber PS. Is specialized integrated treatment for comorbid anxiety, depression and alcohol dependence better than treatment as usual in a public hospital setting?. Alcohol and Alcoholism 2016;51(4):402‐9. [PUBMED: 26672793] [DOI] [PubMed] [Google Scholar]
Mujika 2014 {published data only}
- Mujika A, Forbes A, Canga N, Irala J, Serrano I, Gasco P, et al. Motivational interviewing as a smoking cessation strategy with nurses: an exploratory randomised controlled trial. International Journal of Nursing Studies 2014;51(8):1074‐82. [PUBMED: 24433609] [DOI] [PubMed] [Google Scholar]
Muller 2015 {published data only}
- Muller S, Rohde P, Gau JM, Stice E. Moderators of the effects of indicated group and bibliotherapy cognitive behavioral depression prevention programs on adolescents' depressive symptoms and depressive disorder onset. Behaviour Research and Therapy 2015;75:1‐10. [PUBMED: 26480199] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2016 {published data only}
- Myers B, Westhuizen C, Naledi T, Stein DJ, Sorsdahl K. Readiness to change is a predictor of reduced substance use involvement: findings from a randomised controlled trial of patients attending South African emergency departments. BMC Psychiatry 2016;16:35. [PUBMED: 26897614] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2016 {published and unpublished data}
- Neumann CL, Reichenberg JL, Graves HR, Nestor BA, Gomez J, Wolff JC, et al. The differential effects of alcohol and cannabis on mood states in a sample of youth with co‐occurring mental health and substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2016;55(10):S146‐S147. [Google Scholar]
Oesterle 2015 {published data only}
- Oesterle S, Hawkins JD, Kuklinski MR, Fagan AA, Fleming C, Rhew IC, et al. Effects of communities that care on males' and females' drug use and delinquency 9 years after baseline in a community‐randomised trial. American Journal of Community Psychology 2015;56(3‐4):217‐28. [PUBMED: 26377418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oslin 2003 {published data only}
- Oslin DW, Sayers S, Ross J, Kane V, Have T, Conigliaro J, et al. Disease management for depression and at‐risk drinking via telephone in an older population of veterans. Psychosomatic Medicine 2003;65(6):931‐7. [PUBMED: 14645769] [DOI] [PubMed] [Google Scholar]
Oslin 2004 {published data only}
- Oslin DW, Thompson R, Kallan MJ, TenHave T, Blow FC, Bastani R, et al. Treatment effects from UPBEAT: a randomised trial of care management for behavioral health problems in hospitalised elderly patients. Journal of Geriatric Psychiatry and Neurology 2004;17(2):99‐106. [PUBMED: 15157351] [DOI] [PubMed] [Google Scholar]
Ostergaard 2018 {published data only}
- Ostergaard M, Jatzkowski L, Seitz R, Speidel S, Weber T, Labke N, et al. Integrated treatment at the first stage: Increasing motivation for alcohol patients with comorbid disorders during inpatient detoxification. Alcohol and Alcoholism 2018;53(6):719‐27. [DOI: 10.1093/alcalc/agy066] [DOI] [PubMed] [Google Scholar]
Pachankis 2015 {published data only}
- Pachankis JE, Hatzenbuehler ML, Rendina HJ, Safren SA, Parsons JT. LGB‐affirmative cognitive‐behavioral therapy for young adult gay and bisexual men: a randomised controlled trial of a transdiagnostic minority stress approach. Journal of Consulting and Clinical Psychology 2015;83(5):875‐89. [PUBMED: 26147563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palfai 2014 {published data only}
- Palfai TP, Cheng DM, Coleman SM, Bridden C, Krupitsky E, Samet JH. The influence of depressive symptoms on alcohol use among HIV‐infected Russian drinkers. Drug and Alcohol Dependence 2014;134:85‐91. [PUBMED: 24120857] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patten 1998 {published data only}
- Patten CA, Martin JE, Myers MG, Calfas KJ, Williams CD. Effectiveness of cognitive‐behavioral therapy for smokers with histories of alcohol dependence and depression. Journal of Studies on Alcohol 1998;59(3):327‐35. [PUBMED: 9598714] [DOI] [PubMed] [Google Scholar]
Patten 2002 {published data only}
- Patten CA, Drews AA, Myers MG, Martin JE, Wolter TD. Effect of depressive symptoms on smoking abstinence and treatment adherence among smokers with a history of alcohol dependence. Psychology of Addictive Behaviors 2002;16(2):135‐42. [PUBMED: 12079252] [DOI] [PubMed] [Google Scholar]
Peck 2005 {published data only}
- Peck JA, Reback CJ, Yang X, Rotheram‐Fuller E, Shoptaw S. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine‐dependent gay and bisexual men. Journal of Urban Health 2005;82(1):i100‐8. [PUBMED: 15738315] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peckham 2015 {published data only}
- Peckham E, Man MS, Mitchell N, Li J, Becque T, Knowles S, et al. Smoking Cessation Intervention for Severe Mental Ill Health Trial (SCIMITAR): a pilot randomised control trial of the clinical effectiveness and cost‐effectiveness of a bespoke smoking cessation service. Health Technology Assessment (Winchester, England) 2015;19(25):1‐148, v‐vi. [PUBMED: 25827850] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedrelli 2015 {published data only}
- Pedrelli P, Borsari B. Depressive symptoms and heavy drinking in college students: how should we treat them?. Alcoholism ‐ Clinical and Experimental Research 2015;39:269A. [Google Scholar]
Petersen 2009 {published data only}
- Petersen CL, Zettle ZD. Treating inpatients with comorbid depression and alcohol use disorders: a comparison of acceptance and commitment therapy versus treatment as usual. Psychological Record 2009;59(4):521‐36. [Google Scholar]
Polcin 2015 {published data only}
- Polcin DL, Korcha RA, Nayak M, Bond J. Methamphetamine dependence and intensive motivational interviewing. Drug and Alcohol Dependence 2015;146:e72. [DOI: 10.1016/j.drugalcdep.2014.09.564] [DOI] [Google Scholar]
Ponizovsky 2015 {published data only}
- Ponizovsky AM, Rosca P, Aronovich E, Weizman A, Grinshpoon A. Baclofen as add‐on to standard psychosocial treatment for alcohol dependence: a randomised, double‐blind, placebo‐controlled trial with 1 year follow‐up. Journal of Substance Abuse Treatment 2015;52:24‐30. [PUBMED: 25572706] [DOI] [PubMed] [Google Scholar]
Rawson 2015 {published data only}
- Rawson RA, Chudzynski J, Gonzales R, Mooney L, Dickerson D, Ang A, et al. The impact of exercise on depression and anxiety symptoms among abstinent methamphetamine‐dependent individuals in a residential treatment setting. Journal of Substance Abuse Treatment 2015;57:36‐40. [PUBMED: 25934458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reid 2016 {published data only}
- Reid HH, Ledgerwood DM. Depressive symptoms affect changes in nicotine withdrawal and smoking urges throughout smoking cessation treatment: preliminary results. Addiction Research & Theory 2016;24(1):48‐53. [PUBMED: 27547173] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohde 2014a {published data only}
- Rohde P, Stice E, Shaw H, Gau JM. Cognitive‐behavioral group depression prevention compared to bibliotherapy and brochure control: non‐significant effects in pilot effectiveness trial with college students. Behaviour Research and Therapy 2014;55:48‐53. [PUBMED: 24655464] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2015 {published data only}
- Rose GL, Guth SE, Badger GJ, Plante DA, Fazzino TL, Helzer JE. Brief intervention for heavy drinking in primary care: role of patient initiation. Journal of Addiction Medicine 2015;9(5):368‐75. [PUBMED: 26083959] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruscio 2016 {published data only}
- Ruscio AC, Muench C, Brede E, Waters AJ. Effect of brief mindfulness practice on self‐reported affect, craving, and smoking: a pilot randomised controlled trial using ecological momentary assessment. Nicotine & Tobacco Research 2016;18(1):64‐73. [PUBMED: 25863520] [DOI] [PubMed] [Google Scholar]
Ryb 2011 {published data only}
- Ryb GE, Dischinger PC, Diclemente C, Auman KM, Kufera JA, Soderstrom CA. Impulsive or depressive personality traits do not impede behavioral change after brief alcohol interventions. Journal of Addictive Diseases 2011;30(1):54‐62. [PUBMED: 21218311] [DOI] [PubMed] [Google Scholar]
Saedy 2015 {published data only}
- Saedy M, Kooshki S, Jamali Firouzabadi M, Emamipour S, Rezaei Ardani A. Effectiveness of acceptance‐commitment therapy on anxiety and depression among patients on methadone treatment: a pilot study. Iranian Journal of Psychiatry and Behavioral Sciences 2015;9(1):e222. [PUBMED: 26251660] [DOI] [PMC free article] [PubMed] [Google Scholar]
Safren 2012 {published data only}
- Safren SA, O'Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT‐AD) in HIV‐infected injection drug users: a randomised controlled trial. Journal of Consulting and Clinical Psychology 2012;80(3):404‐15. [PUBMED: 22545737] [DOI] [PMC free article] [PubMed] [Google Scholar]
Satre 2013 {published data only}
- Satre DD, Delucchi K, Lichtmacher J, Sterling SA, Weisner C. Motivational interviewing to reduce hazardous drinking and drug use among depression patients. Journal of Substance Abuse Treatment 2013;44(3):323‐9. [PUBMED: 22999815] [DOI] [PMC free article] [PubMed] [Google Scholar]
Satre 2016 {published data only}
- Satre DD, Leibowitz A, Sterling SA, Lu Y, Travis A, Weisner C. A randomised clinical trial of Motivational Interviewing to reduce alcohol and drug use among patients with depression. Journal of Consulting and Clinical Psychology 2016;84(7):571‐9. [PUBMED: 26985728] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seghatoleslam 2014 {published data only}
- Seghatoleslam T, West R, Habil H, Zahiroddin A. A pilot study of managing depression and controlling smoking. International Medical Journal 2014;21(1):14‐7. [Google Scholar]
Seitz‐Brown 2015 {published data only}
- Seitz‐Brown C, DeGeorge D, Blevins E, Williams J, Lejuez CW, Daughters SB. A brief behavioral activation treatment for substance use associated with lower rates of recidivism at a one‐year follow‐up. Drug & Alcohol Dependence 2015;146:e93. [DOI: 10.1016/j.drugalcdep.2014.09.621] [DOI] [Google Scholar]
Shoptaw 2006 {published data only}
- Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, et al. Randomized, placebo‐controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug and Alcohol Dependence 2006;85(1):12‐8. [DOI] [PubMed] [Google Scholar]
Singla 2019 {published data only}
- Singla DR, Hollon SD, Velleman R, Weobong B, Nadkarni A, Fairburn CG, et al. Temporal pathways of change in two randomized controlled trials for depression and harmful drinking in Goa, India. Psychological Medicine 2019:1‐9. [PUBMED: 30616698] [DOI] [PMC free article] [PubMed]
Stein 2011 {published data only}
- Stein LA, Lebeau R, Colby SM, Barnett NP, Golembeske C, Monti PM. Motivational interviewing for incarcerated adolescents: effects of depressive symptoms on reducing alcohol and marijuana use after release. Journal of Studies on Alcohol and Drugs 2011;72(3):497‐506. [PUBMED: 21513687] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockings 2014 {published data only}
- Stockings EA, Bowman JA, Baker AL, Terry M, Clancy R, Wye PM, et al. Impact of a postdischarge smoking cessation intervention for smokers admitted to an inpatient psychiatric facility: a randomised controlled trial. Nicotine & Tobacco Research 2014;16(11):1417‐28. [PUBMED: 24939916] [DOI] [PubMed] [Google Scholar]
Sun 2007 {published data only}
- Sun P, Unger JB, Guo Q, Gong J, Ma H, Palmer PH, et al. Comorbidity between depression and smoking moderates the effect of a smoking prevention program among boys in China. Nicotine & Tobacco Research 2007;9(4):S599‐609. [PUBMED: 18067035] [DOI] [PubMed] [Google Scholar]
Tapert 2003 {published data only}
- Tapert SF, Colby SM, Barnett NP, Spirito A, Rohsenow DJ, Myers MG, et al. Depressed mood, gender, and problem drinking in youth. Journal of Child & Adolescent Substance Abuse 2003;12(4):55‐68. [DOI: 10.1300/J029v12n04_04] [DOI] [Google Scholar]
Tempersta 1998 {published data only}
- Tempersta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate in alcohol dependence: a placebo‐controlled study in a comprehensive post‐detoxification program. European Psychiatry 1998;13:234s. [DOI: 10.1093/alcalc/35.2.202] [DOI] [Google Scholar]
Tempesta 2000 {published data only}
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: a placebo‐controlled study. Alcohol and Alcoholism 2000;35(2):202‐9. [PUBMED: 10787398] [DOI] [PubMed] [Google Scholar]
Tiburcio 2016 {published data only}
- Tiburcio M, Lara M, Abrego A, Fernandez M, Valez M, Sanchez A. Web‐based intervention to reduce substance abuse and depressive symptoms in Mexico: development and usability test. Journal Medical Internet Research Mental Health 2016;3(3):e47. [DOI: 10.2196/mental.6001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinci 2014 {published data only}
- Vinci C, Peltier MR, Shah S, Kinsaul J, Waldo K, McVay MA, et al. Effects of a brief mindfulness intervention on negative affect and urge to drink among college student drinkers. Behaviour Research and Therapy 2014;59:82‐93. [PUBMED: 24972492] [DOI] [PubMed] [Google Scholar]
Wilks 2018 {unpublished data only}
- Wilks CR. Internet‐delivered Dialectical Behavior Therapy skills training for suicidal and heavy drinkers. Doctoral dissertation 2018. [DOI] [PMC free article] [PubMed]
Wilson 2014 {published data only}
- Wilson GB, Wray C, McGovern R, Newbury‐Birch D, McColl E, Crosland A, et al. Intervention to reduce excessive alcohol consumption and improve comorbidity outcomes in hypertensive or depressed primary care patients: two parallel cluster randomised feasibility trials. Trials 2014;15:235. [PUBMED: 24947447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Womack 2004 {published data only}
- Womack S, Compton WM, Dennis M, McCormick S, Fraser J, Horton JC, et al. Improving treatment services for substance abusers with comorbid depression. American Journal on Addictions 2004;13(3):295‐304. [PUBMED: 15370949] [DOI] [PubMed] [Google Scholar]
Wusthoff 2014 {published data only}
- Wusthoff LE, Waal H, Grawe RW. The effectiveness of integrated treatment in patients with substance use disorders co‐occurring with anxiety and/or depression‐‐a group randomised trial. BMC Psychiatry 2014;14:67. [PUBMED: 24597469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemestani 2016 {published data only}
- Zemestani M, Ottaviani C. Effectiveness of Mindfulness‐Based Relapse Prevention for co‐occurring substance use and depression disorders. Mindfulness 2016;7(6):1347‐55. [Google Scholar]
Additional references
Agabio 2018
- Agabio R, Trogu E, Pani PP. Antidepressants for the treatment of people with co‐occurring depression and alcohol dependence. Cochrane Database of Systematic Reviews 2018, Issue 4. [DOI: 10.1002/14651858.CD008581.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 1982
- Alexander J, Parsons BV. Functional Family Therapy: Principles and Procedures. Carmel, CA.: Brooks/Cole, 1982. [Google Scholar]
American Psychiatric Association 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV Text Revision (DSM‐IV‐TR). IV‐TR. Washington: American Psychiatric Association, 2000. [Google Scholar]
Australian Bureau of Statistics 2007
- Australian Bureau of Statistics. National Survey of Mental Health and Wellbeing: Summary of Results. Canberra: Australian Bureau of Statistics, 2007. [Google Scholar]
Babowitch 2016
- Babowitch JD, Antshel KM. Adolescent treatment outcomes for comorbid depression and substance misuse: a systematic review and synthesis of the literature. Journal of Affective Disorders 2016;201:25‐33. [PUBMED: 27156096] [DOI] [PubMed] [Google Scholar]
Baker 2007a
- Baker A, Lubeman DI, Cosgrave EM, Killackey E, Yuen H, Hides L, et al. The impact of co‐occurring substance use on six‐month outcomes for young people seeking mental health treatment. Australian & New Zealand Journal of Psychiatry 2007;41:896‐902. [PUBMED: 17924242] [DOI] [PubMed] [Google Scholar]
Baker 2007b
- Baker A, Velleman R. Clinical Handbook of Co‐existing Mental Health and Drug and Alcohol Problems. Clinical Handbook of Co‐existing Mental Health and Drug and Alcohol Problems. East Sussex: Routledge, 2007. [Google Scholar]
Baker 2012
- Baker AL, Thornton LK, Hiles S, Hides L, Lubman DI. Psychological interventions for alcohol misuse among people with co‐occurring depression or anxiety disorders: a systematic review. Journal of Affective Disorders 2012;139(3):217‐29. [DOI: 10.1016/j.jad.2011.08.004] [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐9. [PUBMED: 13688369] [DOI] [PubMed] [Google Scholar]
Beck 1978
- Beck AT. Depression Inventory. Philadelphia: Center for CognitiveTherapy, 1978. [Google Scholar]
Beck 1993
- Beck AT, Wright FD, Newman CF, Liese BS. Cognitive Therapy of Substance Abuse. New York: Guilford Press, 1993. [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory‐Second Edition Manual. San Antonio: The Psychological Corporation, 1996. [Google Scholar]
Beck 2011
- Beck JS. Cognitive Behavior Therapy: Basics and Beyond. New York City: Guilford Press, 2011. [Google Scholar]
Benishek 2014
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, et al. Prize‐based contingency management for the treatment of substance abusers: a meta‐analysis. Addiction (Abingdon, England) 2014;109(9):1426‐36. [PUBMED: 24750232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman D, Schulz K, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine 2008;148(4):295‐309. [PUBMED: 18283207] [DOI] [PubMed] [Google Scholar]
Bulkstein 1992
- Bukstein OG, Glancy LG, Kaminer Y. Patterns of affective comorbidity in a clinical population of dually diagnosed adolescent substance users. Journal of the American Academy of Child & Adolescent Psychiatry 1992;31:1041‐5. [PUBMED: 1429402] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [PUBMED: 23295957] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 1990
- Clarke GN, Lewinsohn PM, Hops H. Adolescent Coping with Depression Course. Castalia Publishing Company, 1990. [Google Scholar]
Cuijpers 2011
- Cuijpers P, Geraedts AS, Oppen P, Andersson G, Markowitz JC, Straten A. Interpersonal psychotherapy for depression: a meta‐analysis. American Journal of Psychiatry 2011;168(6):581‐92. [PUBMED: 21362740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuijpers 2013
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta‐analysis of cognitive‐behavioural therapy for adult depression, alone and in comparison with other treatments. Canadian Journal of Psychiatry: Revue Canadienne de Psychiatrie 2013;58(7):376‐85. [PUBMED: 23870719] [DOI] [PubMed] [Google Scholar]
Davis 2008
- Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Current Opinion in Psychiatry 2008;21(1):14‐8. [PUBMED: 18281835] [DOI] [PubMed] [Google Scholar]
de Bruin 2015
- Bruin M, McCambridge J, Prins JM. Reducing the risk of bias in health behaviour change trials: Improving trial design, reporting or bias assessment criteria? A review and case study. Psychology & Health 2015;30(1):8‐34. [DOI] [PubMed] [Google Scholar]
Degenhardt 2001
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction 2001;96(11):1603‐14. [PUBMED: 11784457] [DOI] [PubMed] [Google Scholar]
EMCDDA 2013
- European Monitoring Centre for Drugs and Drug Addiction. Co‐morbid substance use and mental disorders in Europe: A review of the data. EMCDDA Papers. Lisbon: The European Monitoring Centre for Drugs and Drug Addiction, 2013:1‐12.
Farrell 2001
- Farrell M, Howes S, Bebbington P, Brugha T, Jenkins R, Lewis G, et al. Nicotine, alcohol and drug dependence and psychiatric comorbidity: results of a national household survey. British Journal of Psychiatry 2001;179(5):432‐7. [PUBMED: 11689401] [DOI] [PubMed] [Google Scholar]
Foulds 2015
- Foulds JA, Adamson SJ, Boden JM, Williman JA, Mulder RT. Depression in patients with alcohol use disorders: systematic review and meta‐analysis of outcomes for independent and substance‐induced disorders. Journal of Affective Disorders 2015;185:47‐59. [PUBMED: 26143404] [DOI] [PubMed] [Google Scholar]
Frank 2011
- Frank E, Levenson JC. Interpersonal Psychotherapy. Washington DC: American Psychological Association, 2011. [Google Scholar]
Grant 2004
- Grant BF, Stinson FS, Dawson DA, Chou P, Dufour MC, Compton W, et al. Prevalence and co‐occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry 2004;61:807‐16. [DOI: ] [DOI] [PubMed] [Google Scholar]
Grella 2001
- Grella CE, Hser YI, Joshi V, Rounds‐Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. Journal of Nervous and Mental Disease 2001;189:384‐92. [PUBMED: 11434639] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations.. BMJ 2008;336(7560):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines 1. Introduction‐GRADE evidence profiles and summary of findings tables.. Journal of Clinical Epidemiology 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
Hall 2009
- Hall W, Degenhardt L, Teesson M. Reprint of "Understanding comorbidity between substance use, anxiety and affective disorders: broadening the research base". Addictive Behaviors 2009;34(10):795‐9. [PUBMED: 19604645] [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56‐62. [PUBMED: 14399272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1980
- Hamilton, M. Rating depressive patients. Journal of Clinical Psychiatry 1980;41:21‐4. [PubMed] [Google Scholar]
Hasin 2002
- Hasin DS, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Archives of General Psychiatry 2002;59:375‐80. [DOI: 10.1001/archpsyc.59.4.375] [DOI] [PubMed] [Google Scholar]
Hesse 2009
- Hesse M. Integrated psychological treatment for substance use and co‐morbid anxiety or depression vs. treatment for substance use alone. A systematic review of the published literature. BMC Psychiatry 2009;9:6. [PUBMED: 19232121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hobden 2018
- Hobden B, Bryant J, Carey M, Baker AL, Farrell M. Oldmeadow C, et al. Finding the optimal treatment model: A systematic review of treatment for co‐occurring alcohol misuse and depression. Australian & New Zealand Journal of Psychiatry 2018;52(8):737‐50. [DOI: 10.1177/0004867418758922] [DOI] [PubMed] [Google Scholar]
Hofmann 2012a
- Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of Cognitive Behavioral Therapy: a review of meta‐analyses. Cognitive Therapy and Research 2012a;36(5):427‐40. [PUBMED: 23459093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2012b
- Hofman, SG. An Introduction to Modern CBT: Psychological Solutions to Mental Health Problems. West Sussex: Wiley‐Blackwell, 2012. [Google Scholar]
Iguchi 1997
- Iguchi MY, Belding MA, Morral AR, Lamb RJ, Husband SD. Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. Journal of Consulting and Clinical Psychology 1997;65(3):421‐8. [PUBMED: 9170765] [DOI] [PubMed] [Google Scholar]
Jane‐Llopis 2006
- Jane‐Llopis E, Matytsina I. Mental health and alcohol, drugs and tobacco: a review of the comorbidity between mental disorders and the use of alcohol, tobacco and illicit drugs. Drug and Alcohol Review 2006;25(6):515‐36. [PUBMED: 17132571] [DOI] [PubMed] [Google Scholar]
Kadden 1994
- Kadden R, Carroll K, Donovan D, Cooney N, Monti, P Abrams, D, et al. Cognitive‐behavioral Coping Skills Therapy Manual: a Clinical Research Guide for Treating Individuals with Alcohol Abuse and Dependence. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism, 1994. [Google Scholar]
Kessler 2003
- Kessler RC, Berglund P, Demler OV, Jin R, Koretz D, Merikangas KE, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS‐R). JAMA 2003;289:3095‐3105. [PUBMED: 12813115] [DOI] [PubMed] [Google Scholar]
Lejoyeux 2011
- Lejoyeux M, Lehert P. Alcohol‐use disorders and depression: results from individual patient data meta‐analysis of the acamprosate‐controlled studies. Alcohol and Alcoholism 2011;46(1):61‐7. [PUBMED: 21118900] [DOI] [PubMed] [Google Scholar]
Lewinsohn 1980
- Lewinsohn PM, Sullivan JM, Grosscup SJ. Changing reinforcing events: an approach to the treatment of depression. Psychotherapy Theory Research & Practice 1980;17(3):322. [DOI: 10.1037/h0085929] [DOI] [Google Scholar]
Lubman 2007
- Lubman DI, Allen NB, Rogers N, Cementon E, Bonomo Y. The impact of co‐occurring mood and anxiety disorders among substance‐abusing youth. Journal of Affective Disorders 2007;103:105‐12. [PUBMED: 17291589] [DOI] [PubMed] [Google Scholar]
McLellan 1980
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic instrument for substance abuse patients: The Addiction Severity Index.. Journal of Nervous and Mental Disease 1980;168:26‐33. [DOI] [PubMed] [Google Scholar]
Meyers 1995
- Meyers RJ, Smith JE. Clinical Guide to Alcohol Treatment: The Community Reinforcement Approach. New York: Guilford Press, 1995. [Google Scholar]
Miller 1994
- Miller WR, Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies on Alcohol. Supplement 1994;12:112‐8. [PUBMED: 7722987] [DOI] [PubMed] [Google Scholar]
Miller 2013
- Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. New York City: Guilford Press, 2013. [Google Scholar]
Muñoz 1993
- Muñoz RF, Ying Y, Pérez‐Stable E, Miranda J. The Prevention of Depression :Rresearch and Practice. Baltimore: Johns Hopkins University Press, 1993. [Bookmark: http://trove.nla.gov.au/version/26924052] [Google Scholar]
NCCMH 2009
- National Collaborating Centre for Mental Health. Depression: The Treatment and Management of Depression in Adults. NICE Clinical Guideline 90. London: National Institute for Health and Clinical Excellence, 2009. [Google Scholar]
Nowinski 1994
- Nowinski J, Baker S, Carroll K. Twelve step facilitation therapy manual. In: Mattson ME editor(s). Project Match Monograph Series. Vol. 1, Rockville: US Department of Health and Human Services, 1994. [Google Scholar]
Oxman 2004
- Oxman AD, GRADE Working Group. Grading quality of evidence and strength of recommendations.. BMJ 2004;328(19):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pani 2010
- Pani PP, Vacca R, Trogu E, Amato L, Davoli M. Pharmacological treatment for depression during opioid agonist treatment for opioid dependence. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008373] [DOI] [PubMed] [Google Scholar]
Petry 2012
- Petry NM. Contingency Management for Substance Abuse Treatment: a Guide to Implementing this Evidence‐Based Practice. New York City: Routledge, 2012. [Google Scholar]
Poznanski 1995
- Poznanski EO, Hartmut BM. Children's Depression Rating Scale, Revised (CDRS‐R). Los Angeles: Western Psychological Services, 1995. [Google Scholar]
Prendergast 2006
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta‐analysis. Addiction (Abingdon, England) 2006;101(11):1546‐60. [PUBMED: 17034434] [DOI] [PubMed] [Google Scholar]
Riper 2014
- Riper H, Andersson G, Hunter SB, Wit J, Berking M, Cuijpers P. Treatment of comorbid alcohol use disorders and depression with cognitive‐behavioural therapy and motivational interviewing: a meta‐analysis. Addiction (Abingdon, England) 2014;109(3):394‐406. [PUBMED: 24304463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scogin 1987
- Scogin F, Hamblin D, Beutler L. Bibliotherapy for depressed older adults: a self‐help alternative. Gerontologist 1987;27(3):383‐7. [PUBMED: 2886403] [DOI] [PubMed] [Google Scholar]
Smedslund 2011
- Smedslund G, Berg RC, Hammerstrom KT, Steiro A, Leiknes KA, Dahl HM, et al. Motivational interviewing for substance abuse. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008063.pub2; PUBMED: 21563163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobell 1980
- Sobell MB, Maisto SA, Sobell LC, Cooper AM, Cooper T, Sanders B. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Sobell MB, Ward E editor(s). Evaluating Alcohol and Drug Abuse Treatment Effectiveness: Recent Advances. New York: Pergamon Press, 1980:129‐50. [Google Scholar]
Sobell 1992
- Sobell LC, Sobell MB. Timeline followback: a technique for assessing self‐reported ethanol consumption. In: J Allen, RZ Litten editor(s). Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa (NJ): Humana Press, 1992:41‐72. [Google Scholar]
Stuart 2012
- Stuart S, Robertson MD. Interpersonal Psychotherapy: a Clinician's Guide. London: Hodder Arnold, 2012. [Google Scholar]
Sullivan 2005
- Sullivan LE, Fiellin DA, O'Connor P. The prevalence and impact of alcohol problem in major depression: a systematic review. American Journal of Medicine 2005;118:330‐41. [DOI: 10.1016/j.amjmed.2005.01.007] [DOI] [PubMed] [Google Scholar]
Teesson 2005
- Teesson M, Havard A, Fairbairn S, Ross J, Lynskey M, Darke S. Depression among entrants to treatment for heroin dependence in the Australian Treatment Outcome Study (ATOS): prevalence, correlates and treatment seeking. Drug and Alcohol Dependence 2005;78:309‐15. [PUBMED: 15893162] [DOI] [PubMed] [Google Scholar]
Teesson 2009
- Teesson M, Slade T, Mills K. Comorbidity in Australia: findings of the 2007 National Survey of Mental Health and Wellbeing. Australian and New Zealand Journal of Psychiatry 2009;43(7):606‐14. [PUBMED: 19530017] [DOI] [PubMed] [Google Scholar]
Teesson 2010
- Teesson M, Hall W, Slade T, Mills K, Grove R, Mewton L, et al. Prevalence and correlates of DSM‐IV alcohol abuse and dependence in Australia: findings of the 2007 National Survey of Mental Health and Wellbeing. Addiction (Abingdon, England) 2010;105(12):2085‐94. [PUBMED: 20707771] [DOI] [PubMed] [Google Scholar]
Waldron 2004
- Waldron HB, Kaminer Y. On the learning curve: the emerging evidence supporting cognitive‐behavioral therapies for adolescent substance abuse. Addiction (Abingdon, England) 2004;99 Suppl 2:93‐105. [PUBMED: 15488108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2016
- Wells A, Fisher P. Treating Depression: MCT, CBT and Third Wave Therapies. West Sussex: John Wiley & Sons Inc, 2016. [Google Scholar]
Wilfey 2000
- Wilfey DE, Mackenzie KR, Welch RR, Ayres VE, Weissman MM. Interpersonal Psychotherapy for Groups. New York: Basic Books, 2000. [Google Scholar]
Williams 1988
- Williams JB, Link MJ, Rosenthal NE, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGHSAD). New York: New York Psychiatric Institute, 1988. [Google Scholar]
World Health Organization 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders : Clinical Descriptions and Diagnostic Guidelines. 10. Geneva: World Health Organiztion, 1992. [Google Scholar]
Zhou 2015
- Zhou X, Qin B, Giovane C, Pan J, Gentile S, Liu Y, et al. Efficacy and tolerability of antidepressants in the treatment of adolescents and young adults with depression and substance use disorders: a systematic review and meta‐analysis. Addiction (Abingdon, England) 2015;110(1):38‐48. [PUBMED: 25098732] [DOI] [PubMed] [Google Scholar]
Šprah 2017
- Šprah L, Dernovšek MZ, Wahlbeck K, Haaramo P. Psychiatric readmissions and their association with physical comorbidity: a systematic literature review. BMC Psychiatry 2017;17(1):1172‐3. [DOI: 10.1186/s12888-016-1172-3; PUBMED: 28049441] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hides 2011
- Hides L, Baker A, Kavanagh D, Proctor D. Psychological interventions for co‐occurring depression and substance misuse. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009501] [DOI] [PMC free article] [PubMed] [Google Scholar]


