Abstract
Background
Dysmenorrhoea is a common gynaecological problem consisting of painful cramps accompanying menstruation, which in the absence of any underlying abnormality is known as primary dysmenorrhoea. Research has shown that women with dysmenorrhoea have high levels of prostaglandins, hormones known to cause cramping abdominal pain. Nonsteroidal anti‐inflammatory drugs (NSAIDs) are drugs that act by blocking prostaglandin production. They inhibit the action of cyclooxygenase (COX), an enzyme responsible for the formation of prostaglandins. The COX enzyme exists in two forms, COX‐1 and COX‐2. Traditional NSAIDs are considered 'non‐selective' because they inhibit both COX‐1 and COX‐2 enzymes. More selective NSAIDs that solely target COX‐2 enzymes (COX‐2‐specific inhibitors) were launched in 1999 with the aim of reducing side effects commonly reported in association with NSAIDs, such as indigestion, headaches and drowsiness.
Objectives
To determine the effectiveness and safety of NSAIDs in the treatment of primary dysmenorrhoea.
Search methods
We searched the following databases in January 2015: Cochrane Menstrual Disorders and Subfertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL, November 2014 issue), MEDLINE, EMBASE and Web of Science. We also searched clinical trials registers (ClinicalTrials.gov and ICTRP). We checked the abstracts of major scientific meetings and the reference lists of relevant articles.
Selection criteria
All randomised controlled trial (RCT) comparisons of NSAIDs versus placebo, other NSAIDs or paracetamol, when used to treat primary dysmenorrhoea.
Data collection and analysis
Two review authors independently selected the studies, assessed their risk of bias and extracted data, calculating odds ratios (ORs) for dichotomous outcomes and mean differences for continuous outcomes, with 95% confidence intervals (CIs). We used inverse variance methods to combine data. We assessed the overall quality of the evidence using GRADE methods.
Main results
We included 80 randomised controlled trials (5820 women). They compared 20 different NSAIDs (18 non‐selective and two COX‐2‐specific) versus placebo, paracetamol or each other.
NSAIDs versus placebo
Among women with primary dysmenorrhoea, NSAIDs were more effective for pain relief than placebo (OR 4.37, 95% CI 3.76 to 5.09; 35 RCTs, I2 = 53%, low quality evidence). This suggests that if 18% of women taking placebo achieve moderate or excellent pain relief, between 45% and 53% taking NSAIDs will do so.
However, NSAIDs were associated with more adverse effects (overall adverse effects: OR 1.29, 95% CI 1.11 to 1.51, 25 RCTs, I2 = 0%, low quality evidence; gastrointestinal adverse effects: OR 1.58, 95% CI 1.12 to 2.23, 14 RCTs, I2 = 30%; neurological adverse effects: OR 2.74, 95% CI 1.66 to 4.53, seven RCTs, I2 = 0%, low quality evidence). The evidence suggests that if 10% of women taking placebo experience side effects, between 11% and 14% of women taking NSAIDs will do so.
NSAIDs versus other NSAIDs
When NSAIDs were compared with each other there was little evidence of the superiority of any individual NSAID for either pain relief or safety. However, the available evidence had little power to detect such differences, as most individual comparisons were based on very few small trials.
Non‐selective NSAIDs versus COX‐2‐specific selectors
Only two of the included studies utilised COX‐2‐specific inhibitors (etoricoxib and celecoxib). There was no evidence that COX‐2‐specific inhibitors were more effective or tolerable for the treatment of dysmenorrhoea than traditional NSAIDs; however data were very scanty.
NSAIDs versus paracetamol
NSAIDs appeared to be more effective for pain relief than paracetamol (OR 1.89, 95% CI 1.05 to 3.43, three RCTs, I2 = 0%, low quality evidence). There was no evidence of a difference with regard to adverse effects, though data were very scanty.
Most of the studies were commercially funded (59%); a further 31% failed to state their source of funding.
Authors' conclusions
NSAIDs appear to be a very effective treatment for dysmenorrhoea, though women using them need to be aware of the substantial risk of adverse effects. There is insufficient evidence to determine which (if any) individual NSAID is the safest and most effective for the treatment of dysmenorrhoea. We rated the quality of the evidence as low for most comparisons, mainly due to poor reporting of study methods.
Keywords: Female; Humans; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/adverse effects; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Cyclooxygenase Inhibitors; Cyclooxygenase Inhibitors/adverse effects; Cyclooxygenase Inhibitors/therapeutic use; Dysmenorrhea; Dysmenorrhea/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Nonsteroidal anti‐inflammatory drugs for dysmenorrhoea
Review question
Are nonsteroidal anti‐inflammatory drugs (NSAIDs) safe and effective for relief of period pain (dysmenorrhoea) and how do they compare with each other and with paracetamol?
Background
Nearly three‐quarters of women suffer from period pain or menstrual cramps (dysmenorrhoea). Research has shown that women with severe period pain have high levels of prostaglandins, hormones known to cause cramping abdominal pain. NSAIDs are drugs which act by blocking prostaglandin production. NSAIDs include the common painkillers aspirin, naproxen, ibuprofen and mefenamic acid. Researchers in The Cochrane Collaboration reviewed the evidence about the safety and effectiveness of NSAIDs for period pain. The evidence is current to January 2015.
Study characteristics
We found 80 randomised controlled trials (RCTs), which included a total of 5820 women and compared 20 different types of NSAIDs with placebo (an inactive pill), paracetamol or each other. Most of the studies were commercially funded (59%), and a further 31% did not state their source of funding.
Key results
The review found that NSAIDs appear to be very effective in relieving period pain. The evidence suggests that if 18% of women taking placebo achieve moderate or excellent pain relief, between 45% and 53% taking NSAIDs will do so. NSAIDs appear to work better than paracetamol, but it is unclear whether any one NSAID is safer or more effective than others.
NSAIDs commonly cause adverse effects (side effects), including indigestion, headaches and drowsiness. The evidence suggests that if 10% of women taking placebo experience side effects, between 11% and 14% of women taking NSAIDs will do so.
Based on two studies that made head‐to‐head comparisons, there was no evidence that newer types of NSAID (known as COX‐2‐specific inhibitors) are more effective for the treatment of dysmenorrhoea than traditional NSAIDs (known as non‐selective inhibitors), nor that there is a difference between them with regard to adverse effects.
Quality of the evidence
We rated the quality of the evidence as low for most comparisons, mainly due to poor reporting of study methods.
Summary of findings
Summary of findings for the main comparison. NSAIDs compared to placebo for dysmenorrhoea.
| NSAIDs compared to placebo for dysmenorrhoea | ||||||
|
Population: women with primary dysmenorrhoea Setting: Outpatient Intervention: NSAIDs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of studies | Quality of the evidence (GRADE) | Comments | |
| Assumed risk4 | Corresponding risk | |||||
| Placebo | NSAIDs | |||||
| Pain relief dichotomous data | 180 per 1000 | 490 per 1000 (452 to 528) | OR 4.37 (3.76 to 5.09) | 35 studies | ⊕⊕⊝⊝ low1,2,3 | — |
| All adverse effects | 100 per 1000 | 125 per 1000 (110 to 144) | OR 1.29 (1.11 to 1.51) | 25 studies | ⊕⊕⊝⊝ low1,3 | — |
| *The basis for the assumed risk is provided in a footnote. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drug; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Very poor reporting of study methods by over 75% of studies; high risk of attrition bias in several studies; over 60% of studies commercially sponsored. 2Substantial heterogeneity (I² = 53%) but direction of effect consistent. 3Some suggestion of publication bias, favouring small studies with positive findings for NSAIDs. 4The control group risks are calculated from median values in 31 studies of pain relief and 19 of adverse effects in a previous version of this review.
Summary of findings 2. NSAIDs compared to paracetamol for dysmenorrhoea.
| NSAIDs compared to paracetamol for dysmenorrhoea | ||||||
|
Population: women with primary dysmenorrhoea Setting: Outpatient Intervention: NSAIDs Comparison: paracetamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of studies | Quality of the evidence (GRADE) | Comments | |
| Assumed risk3 | Corresponding risk | |||||
| Paracetamol | NSAIDs | |||||
| Pain relief dichotomous data | 630 per 1000 | 763 per 1000 (641 to 854) | OR 1.89 (1.05 to 3.43) | 3 studies | ⊕⊕⊝⊝ low1 | — |
| All adverse effects ‐ ibuprofen versus paracetamol | 130 per 1000 | 113 per 1000 (44 to 259) | OR 0.85 (0.31 to 2.34) | 1 study | ⊕⊝⊝⊝ very low1,2 | — |
| *The basis for the assumed risk is provided in a footnote. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAID: nonsteroidal anti‐inflammatory drug; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Poor reporting of study methods in two of the studies; high risk of attrition bias in one study; two of the studies commercially funded. 2One small study, findings compatible with benefit/harm from either intervention, or with no difference between the interventions. 3The control group risk is calculated from the median value in the included studies.
Background
Description of the condition
Dysmenorrhoea refers to the occurrence of painful menstrual cramps of uterine origin, usually developing within hours of the start of menstruation and peaking as the flow becomes heaviest during the first day or two of the cycle. Pain is usually centred in the suprapubic area but may radiate to the back of the legs or lower back, and may be accompanied by other symptoms such as nausea, diarrhoea, headache and lightheadedness (Coco 1999). Dysmenorrhoea is a common gynaecological complaint, though prevalence estimates vary widely. It was reported by 72% of Australian women of reproductive age in a recent nationally representative sample (Pitts 2008), and caused severe pain in 15% of cases. Other representative samples report rates ranging from 17% to 81% (Latthe 2006). In addition to the distress associated with dysmenorrhoea, surveys have shown significant socio‐economic repercussions: over 35% of female high school students report missing school due to menstrual pain (Banikarim 2000; Hillen 1999), and 15% of working Hungarian women of reproductive age reported that painful menstruation limited daily activity (Laszlo 2008).
Dysmenorrhoea is commonly defined within two subcategories. When menstrual pelvic pain is associated with an identifiable pathological condition, such as endometriosis or ovarian cysts, it is termed secondary dysmenorrhoea, while menstrual pain without organic pathology is termed primary dysmenorrhoea (Lichten 1987). The initial onset of primary dysmenorrhoea is usually with the first occurrence of menstruation (menarche), when ovulatory cycles are established, or within the following six to 12 months. The duration of pain is commonly 48 to 72 hours and accompanies menstrual flow or precedes it by only a few hours. In contrast, secondary dysmenorrhoea is more likely to occur years after the onset of menarche and pain can occur both before and during menstruation (Dawood 1984).
The aetiology of primary dysmenorrhoea has been the source of considerable debate. Current understanding is that it is caused by an excessive or imbalanced amount of prostanoids (hormone‐like substances including prostaglandin) released from the endometrium during menstruation. These cause the uterus to contract frequently and dysrhythmically, with reduced local blood flow and hyper sensitisation of the peripheral nerves (Dawood 2006; Dawood 2007). Although most women with dysmenorrhoea have higher levels of prostaglandins F2 alpha and E2 than non‐dysmenorrhoeic women (Pickles 1979), some women with severe dysmenorrhoea and normal laparoscopic findings do not have elevated menstrual prostaglandin to account for the symptoms (Chan 1978). The prevalence of such cases is unknown. It has been suggested that the antidiuretic hormone vasopressin may also be involved in the aetiology of primary dysmenorrhoea, but its role remains controversial (Dawood 2006).
Description of the intervention
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are non‐narcotic analgesics. The first drug of this type was aspirin (acetylsalicylic acid), which was introduced in 1899. The term NSAID was first used in the 1950s when phenylbutazone was developed (Hart 1984). Since then NSAIDs have proliferated and many different types are available. NSAIDs inhibit the action of cyclooxygenase (COX), an enzyme responsible for the formation of prostaglandin (and other prostanoids). The COX enzyme exists in two forms, COX‐1 and COX‐2. Traditional NSAIDs are considered 'non‐selective' because they inhibit both COX‐1 and COX‐2 enzymes. The anti‐inflammatory and pain‐relieving effects of NSAIDs are thought to be mainly due to inhibition of COX‐2 enzymes, whereas the side effects (commonly gastrointestinal) appear to be related to the inhibition of COX‐1 enzymes. With the aim of improving the tolerability of NSAIDs, highly selective COX‐2‐specific inhibitors (coxibs) were developed and first launched in 1999. Since then there have been concerns regarding the risk of cardiovascular and/or dermatological adverse events associated with the long‐term use of some coxibs, and some have been withdrawn by manufacturers. There is growing evidence that NSAIDs as a class are associated with some degree of cardiovascular risk when used long‐term, as in the management of chronic pain in the elderly (Shi 2008).
Several other interventions for dysmenorrhoea have been assessed in Cochrane systematic reviews, as follows:
surgical interruption of pelvic nerve pathways (Proctor 2005);
herbal and dietary therapies (Proctor 2001);
spinal manipulation (Proctor 2006);
beta2‐adrenoceptor agonists (Fedorowicz 2012);
Chinese herbal medicine (Zhu 2008);
oral contraceptive pill (Wong 2009);
transcutaneous electrical nerve stimulation (Proctor 2002);
exercise (Brown 2010);
behavioural interventions (Proctor 2007);
acupuncture (Smith 2011).
How the intervention might work
It is thought that NSAIDs relieve primary dysmenorrhoea mainly by suppressing the production of endometrial prostaglandins, thus alleviating cramps and restoring normal uterine activity. In addition there may be direct analgesic action on the central nervous system (Dawood 2006).
Why it is important to do this review
There is a large body of randomised controlled trials evaluating the short‐term use of NSAIDs for treatment of dysmenorrhoea. A previous systematic review of NSAIDs for dysmenorrhoea considered the four most commonly used types: aspirin, ibuprofen, mefenamic acid and naproxen (Zhang 1998). The purpose of the current review is to compare all nonsteroidal anti‐inflammatory drugs used in the treatment of primary dysmenorrhoea with placebo, with paracetamol and with each other to evaluate their effectiveness and safety.
Objectives
To determine the effectiveness and safety of NSAIDs in the treatment of primary dysmenorrhoea.
Methods
Criteria for considering studies for this review
Types of studies
Included
Published and unpublished randomised, controlled, double‐blinded trials using either a parallel‐group or cross‐over design.
Excluded
Trials that failed to include in analysis at least 80% of the women initially randomised, with respect to at least one of the primary outcomes of this review.
Unblinded or single‐blinded trials.
Types of participants
Included
Women of reproductive age with primary dysmenorrhoea.
We included trials where the diagnosis of dysmenorrhoea was not formally assessed with a physical or gynaecological examination provided no clinical indications of pelvic pathology were reported.
Excluded
Studies that reported the inclusion of:
women with secondary dysmenorrhoea (with identified pathology from a physical examination);
women with irregular/infrequent menstrual cycles (outside of the typical range of a 21‐ to 35‐day cycle);
women using an intrauterine contraceptive device (IUCD);
pregnant or breastfeeding women.
Types of interventions
Included comparisons
NSAIDs versus placebo
NSAIDs versus NSAIDs (i.e. comparing one type of NSAID against another type of NSAID)
NSAIDs versus paracetamol
We considered differing doses and routes of administration of NSAIDs (oral and suppository).
We categorised NSAIDs as non‐selective or as COX‐2‐specific inhibitors based on US Food and Drug Administration categories (FDA 2015).
Types of outcome measures
Primary outcomes
Pain relief ‐ measured with a visual analogue scale (VAS) (i.e. a measure of the amount of pain relief on a 1 to 10 scale) or as dichotomous data (i.e. at least moderate pain relief versus no pain relief).
If other scales or labels were used, we collapsed these (if possible) into dichotomous data, based on the authors' descriptions of the scale, so that women experiencing 'at least moderate' pain relief were reported as having pain relief, whereas women with only mild pain relief were reported as having no pain relief. If pain intensity was reported rather than pain relief we also considered this and recorded it as a separate outcome. We reported continuous data if dichotomous data could not be extracted.
-
Adverse effects:
Total number of adverse effects ('all')
Gastrointestinal adverse effects (for example, nausea, vomiting)
Neurological (nervous system) adverse effects (for example, headache, fatigue, dizziness).
Secondary outcomes
Requirement for additional medication
Interference with daily activities
Absence from work or school
Search methods for identification of studies
We searched for all randomised controlled trials of NSAIDs used to treat dysmenorrhoea, using the search strategy described below and in consultation with the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator. There was no restriction by language or publication status. It is the intention of the review authors that a new search for RCTs be performed every two years and the review be updated accordingly.
Electronic searches
We searched the following electronic databases, trial registers and websites from inception to 7 January 2015:
Cochrane Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of controlled trials;
Cochrane Central Register of Controlled Trials (CENTRAL, November 2014);
MEDLINE;
EMBASE;
PsycINFO;
CINAHL.
We combined the MEDLINE search with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0 chapter 6, 6.4.11 (Higgins 2011)). We combined the EMBASE, PsycINFO and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (http://www.sign.ac.uk/methodology/filters.html#random).
Other electronic sources of trials included:
-
trial registers for ongoing and registered trials:
http://www.clinicaltrials.gov (a service of the US National Institutes of Health);
http://www.who.int/trialsearch/Default.aspx (the World Health Organization International Clinical Trials Registry Platform (ICTRP) search portal);
DARE (Database of Abstracts of Reviews of Effects) in The Cochrane Library at http://onlinelibrary.wiley.com/o/cochrane/cochrane_cldare_articles_fs.html (for reference lists from relevant non‐Cochrane reviews);
Web of Science (another source of trials and conference abstracts) to cross‐link citations of relevant articles;
OpenGrey (http://www.opengrey.eu/) for unpublished literature from Europe;
LILACS database (http://regional.bvsalud.org/php/index.php?lang=en) for trials from the Portuguese and Spanish‐speaking world;
PubMed; and
Google (for recent trials not yet indexed in MEDLINE).
Searching other resources
Wee also searched reference lists of relevant publications, review articles, abstracts of major scientific meetings and included studies.
Data collection and analysis
Selection of studies
One review author scanned the titles and abstracts of articles retrieved by the search and removed those that were very clearly irrelevant. We retrieved the full text of all potentially eligible studies. Two review authors independently examined the full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We attempted to contact study investigators as required, to clarify study eligibility (for example, with respect to randomisation). We resolved disagreements as to study eligibility by consensus. We planned to consult a third review author (CF) if there was any ongoing disagreement; however this did not prove necessary.
We documented the selection process with a PRISMA flow chart (Figure 1).
1.
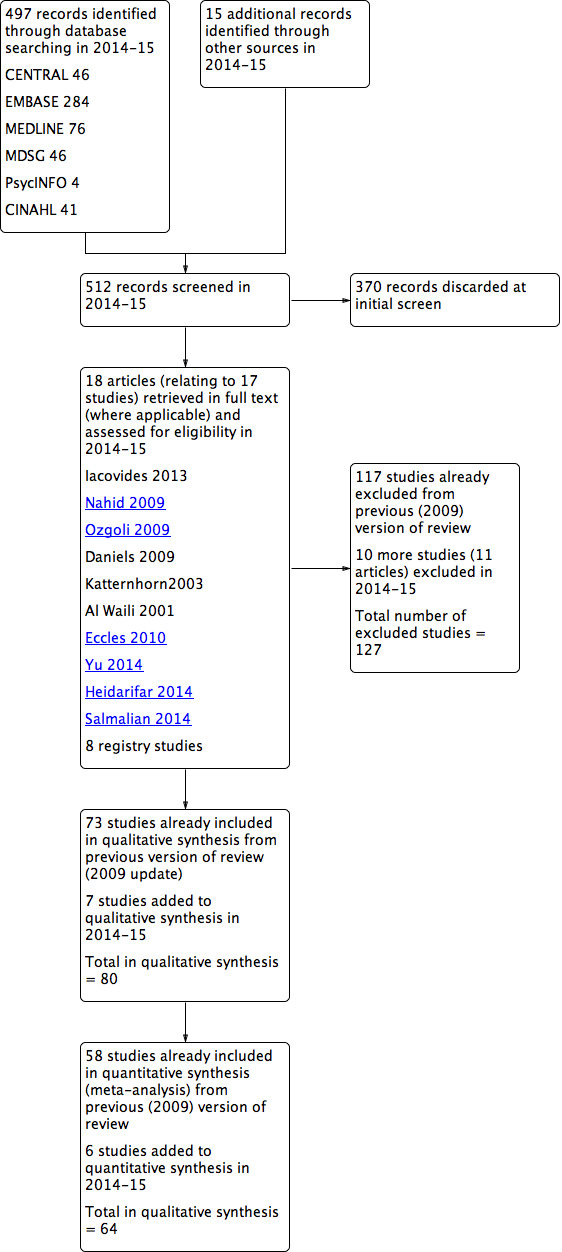
Study flow diagram.
Data extraction and management
Two review authors (JM and either MP or RD) independently extracted data using a standardised form designed by the authors (Figure 2). We resolved discrepancies by discussion. For each study, we extracted data on study design, participants, interventions and outcome measures: these are presented in the Characteristics of included studies table. We also extracted data on study findings: these are presented in the Results and the Data and analyses sections.
2.
Data extraction form
For the first version of this review, we made attempts to contact the authors of 29 trials published since 1985 in order to clarify aspects of methodology or obtain missing data. We received replies from eight authors or co‐authors of these trials. We did not make attempts to contact authors of studies published before 1985 or where no recent address for any of the authors could be found. Where studies had multiple publications, we used the most recent report.
Where studies had multiple publications, we used the main trial report as the reference and derived additional details from secondary papers. The review authors collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review: such studies are grouped under a single study ID with multiple references.
Assessment of risk of bias in included studies
For this review update, two review authors (CF and JM) independently conducted assessment of risk of bias, using the Cochrane 'Risk of bias' assessment tool to evaluate all included studies for the following: adequacy of sequence generation and allocation concealment; adequacy of blinding of women, providers and outcome assessors; completeness of outcome data; risk of selective outcome reporting and risk of other potential sources of bias (Higgins 2011).
Sequence generation
We considered the following methods of random sequence generation adequate:
referring to a random number table;
using a computer random number generator;
coin tossing;
shuffling cards or envelopes;
throwing dice;
drawing of lots.
We deemed the risk of bias low if one of these methods was described. We deemed the risk of bias unclear if the study was described as randomised but the sequence generation method was not described.
Allocation concealment
We considered the following methods of allocation concealment adequate:
central allocation, including telephone, web‐based and pharmacy‐controlled randomisation;
sequentially numbered drug containers of identical appearance;
sequentially numbered, opaque, sealed envelopes.
We deemed the risk of bias low if one of these methods was described. We deemed the risk of bias unclear if the study was described as randomised but the method used for allocation concealment was not described.
Blinding
Blinding refers to whether participants and study personnel knew which women were receiving active treatment and which were receiving placebo. We considered blinding adequate if any of the following were described:
blinding of women and (specified) key study personnel, provided it appeared unlikely that the blinding could have been broken;
use of identical placebo;
unblinding of study personnel at the end of the study.
We deemed the risk of bias low if one of these methods was described. We deemed the risk of bias unclear if the study was described as blinded but no further details were reported. As noted above, we excluded studies that were clearly not blinded.
Attrition bias
We considered outcome data as complete if either of the following applied:
all women randomised were analysed;
data were imputed for those missing.
We deemed the risk of bias low if over 95% of randomised women were included in analysis, unclear if 90% to 95% of randomised women were included in analysis and high if less than 90% of randomised women were included in analysis. As noted above, we excluded studies that clearly analysed less than 80% of randomised women for at least one of the primary outcomes.
Selective reporting
We assessed a study as being free of the risk of selective outcome reporting if both the following applied:
the published report included all expected outcomes;
outcomes were reported systematically for all comparison groups, based on prospectively collected data.
We deemed the risk of bias low if both of the criteria were met, unclear if these criteria were not met and high if there was evidence that data had been collected on outcomes of interest but were not reported in the study publication.
Potential bias related to study funding
We assessed a study as being at unclear risk of bias related to study funding if it was commercially sponsored or the source of funding was not reported
We resolved disagreements by consensus. The results of the assessment of risk of bias are presented in the Characteristics of included studies and in a summary table (Figure 3). We incorporated these results into the interpretation of review findings by means of sensitivity analyses.
3.
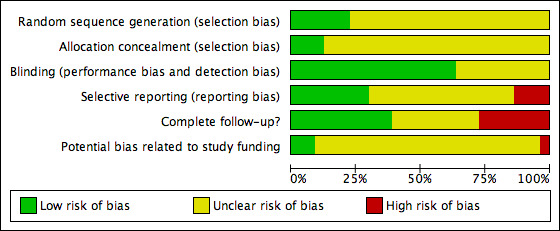
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
For dichotomous data (e.g. numbers reporting relief of pain), we calculated log odds ratios and their standard errors, and entered these in tables using the generic inverse variance option in RevMan (RevMan 2014), where they were displayed as odds ratios and 95% confidence intervals.
For continuous data (e.g. pain scores), we calculated mean differences and their standard errors and entered these in tables using the generic inverse variance option, where they were displayed as mean differences with 95% confidence intervals.
Unit of analysis issues
Denominator
We only included data reported 'per woman' in meta‐analyses. Where studies reported data only 'per menstrual cycle' we briefly summarised results in an additional table. Where trials compared two NSAIDs against placebo, if possible we evenly divided the placebo group between the two trials to avoid double‐counting in the meta‐analysis. Where the placebo group contained an uneven number of women, we entered the placebo group for both comparisons and performed a sensitivity analysis to examine the effect on pooled findings.
Cross‐over trials
For the 2009 update of this review (and subsequent updates) we made an a priori decision to include data from all phases of cross‐over trials, wherever possible. The strength of a cross‐over design is that variation in repeated responses between women is usually less than that between different women and hence the trials can give more precise results. To exploit this correlation, cross‐over trials should be analysed using a method of analysis specific to paired data. Methods are now available for meta‐analysing cross‐over trials and for combining the summary effect measures of parallel and cross‐over trials. However, to date the reporting of cross‐over trials has been very variable and the data required to include a paired analysis in a meta‐analysis are frequently unreported so that there is insufficient information to apply any one synthesis method consistently (Elbourne 2002).
In this review, where cross‐over trials were analysed using methods suitable for paired data and reported an overall measure of effect and standard error (or where this was calculable), we extracted these data and displayed them alongside data from parallel trials. Where cross‐over trials reported dichotomous data or continuous data analysed using non‐paired methods, we extracted these data as if they derived from parallel trials (i.e. as if they had twice as many women). This method of analysis permits the use of more of the available data but is likely to widen confidence intervals, with the possible consequence of disguising clinically important heterogeneity (differences between the studies). Nevertheless, this incorrect analysis is conservative, in that studies are under‐weighted rather than over‐weighted. We explored the effect of this choice of analysis in sensitivity analyses.
Dealing with missing data
We only included analyses reported in the primary studies that included at least 80% of women in the review.
We analysed data on an intention‐to‐treat basis as far as possible. Where data were missing, we made attempts to obtain them from the original investigators. Where they were unobtainable, we only analysed the available data, based on the numerator and denominator reported in study results or calculable from reported percentages. We explored the effect of excluding studies with more than 10% of data missing in sensitivity analyses.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a meaningful summary. Where pooling was conducted, we examined heterogeneity between the results of different studies by inspecting the scatter in the data points and the overlap in their confidence intervals and more formally by checking the results of the Chi2 tests and I2 statistic. We took a P value of less than 0.1 for the Chi2 test to indicate significant heterogeneity and if this was detected, we used the I2 statistic to estimate the percentage of the variability in effect estimates due to heterogeneity rather than sampling error. We took an I2 value greater than 50% to indicate substantial heterogeneity (Higgins 2003; Higgins 2011).
Assessment of reporting biases
In view of the difficulty in detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. We used a funnel plot to assess the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies) for the primary review outcomes. We cautiously considered visible asymmetry in the funnel plot as a possible indication of publication bias.
Data synthesis
We synthesised (combined) the data from primary studies if they were sufficiently homogeneous. We stratified studies by the type of NSAID and comparator used.
For the 2009 update of the review (and subsequent updates including this one in 2015) we made an a priori decision to pool both cross‐over and parallel data using the inverse variance method. We calculated mean differences (MDs) for continuous data and pooled odds ratios for dichotomous data, with 95% confidence intervals. We used both fixed‐effect and random‐effects statistical models. Fixed‐effect models are displayed in the review where data are homogeneous. An increase in the odds of a particular outcome, which may be beneficial (for example, pain relief) or detrimental (for example, an adverse effect), is displayed graphically in the meta‐analyses to the right of the centre‐line and a decrease in the odds of an outcome to the left of the centre‐line.
If it was not possible to extract from a trial report either dichotomous or continuous data suitable for the calculation of ORs or MDs then we reported statistical data in additional tables. Where trial results were presented only as graphs, we described the findings in the text.
We translated the key results into assumed and comparative risks expressed as a percentage. We estimated control group risks for the main comparison from median values in the placebo group in 31 studies of pain relief and 19 of adverse effects in a previous version of this review, and we estimated the corresponding intervention group risk using the formula suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Section 11.5.5).
Subgroup analysis and investigation of heterogeneity
We planned to subgroup studies by the type of NSAID used (non‐selective or COX‐2‐specific inhibitors) if there were sufficient studies in each group that reported the same outcome (for example, three or more studies in each group). However, this was not done as we only included two studies of COX‐2‐selective inhibitors in the review.
Where a visual scan of the forest plots or the results of statistical tests indicated substantial heterogeneity, we explored possible explanations in sensitivity analyses and/or in the text, and we tested the effect of using a random‐effects model.
We planned to conduct subgroup analyses for primary outcomes only.
Sensitivity analysis
We planned sensitivity analyses for the primary review outcomes to determine whether the results were robust to decisions made during the review process.
These analyses excluded the following studies:
studies that did not clearly describe adequate procedures for allocation concealment and blinding;
studies with more than 10% of data missing or imputed for the primary outcomes;
studies with a unit of analysis error (such as those in which cross‐over data were analysed as if they derived from parallel studies);
studies that contributed twice to a pooled analysis: this occurred occasionally where a study contributed more than one comparison to a pooled analysis and either the numerator or the denominator in the placebo group were odd numbers. Where this occurred it was reported in the results for the relevant analysis.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using the Guideline Development Tool software. This table evaluates the overall quality of the body of evidence for the primary review outcomes (pain relief and adverse effects), using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We incorporated judgements about evidence quality (high, moderate or low) into the reporting of results for each primary outcome.
Results
Description of studies
Results of the search
The search completed in January 2015 retrieved 497 records, of which we discarded 370 as clearly ineligible. We retrieved 18 articles for further assessment regarding their eligibility, 10 from databases (for which we obtained the full text) and eight from trial registers. JM and RA independently checked these 18 articles for eligibility.
Out of these 18 articles, we newly included seven studies in the current (2015) update and we newly excluded 10 studies (11 articles). This gives a total of 80 included studies (seven newly included in 2015, plus 73 from the previous version of the review) and 127 excluded (10 newly excluded in 2015, plus 117 from the previous version of the review). See Figure 1.
Included studies
Trial design and setting
The review includes 80 RCTs, 24 of parallel design and 56 of cross‐over design. They randomised a total of 5820 women, 2372 in parallel studies and 3448 in cross‐over studies. Sample size in the parallel trials ranged from 17 to 410; seven randomised over 100 women. Sample size in the cross‐over trials ranged from 11 to 198.
The studies were conducted in the USA (n = 26 trials), Sweden (n = 9), Italy (n = 6), the UK (n = 5), Brazil, Finland, Mexico (n = 4 each), Iran, Norway, South Africa (n = 3 each), Canada, Nigeria, Spain (n = 2 each), Argentina, China, Colombia, Denmark, France, Germany and Iraq (n = 1 each). The majority were published in English, although five were in Spanish, four in Portuguese and one each in French, Italian and Norwegian. Trials were translated as required by members of The Cochrane Collaboration.
Participants
The inclusion and exclusion criteria for the majority of included studies were quite explicit. All but three of the trials stated clearly either that they included only women with primary dysmenorrhoea, or that women with secondary dysmenorrhoea were excluded. The other three studies had less specific inclusion criteria that did not define dysmenorrhoea (Akerlund 1989; Pauls 1978), or included both primary and secondary dysmenorrhoea but reported results separately (Sahin 2003). The diagnosis of primary dysmenorrhoea was confirmed by a physical or gynaecological examination in 40 of the included studies. Oral contraceptive use was an exclusion criterion in most of the studies, and other common exclusion criteria were pelvic disease, intrauterine device (IUD) use, irregular menstrual cycles, renal or hepatic disorders, contraindications to nonsteroidal anti‐inflammatory drugs, pregnancy, planned pregnancy and use of hormonal preparations, analgesics or other medications that could interfere with the proposed comparisons.
Most studies detailed the demographic characteristics of the women. Their mean age ranged from 15.8 to 32.2 years (where stated).
Interventions
Included comparisons eligible for the review were as follows:
NSAID versus placebo: 56 trials;
NSAID versus NSAID: 17 trials;
NSAID versus NSAID versus placebo: four trials;
NSAID versus paracetamol: one trial;
NSAID versus paracetamol versus placebo: two trials.
Eighteen different types of non‐selective NSAIDs were evaluated in the included studies: aceclofenac, aspirin, dexketoprofen, diclofenac, etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, indomethacin, ketoprofen, lysine clonixinate, mefenamic acid, meloxicam, naproxen, niflumic acid, nimesulide and piroxicam.
Only two types of COX‐2‐specific NSAIDs were evaluated: celecoxib and etoricoxib. Several of the included studies reported data on comparison arms receiving interventions not relevant to this review (e.g. NSAIDs that have been withdrawn by the manufacturers, mild opiate analgesics as a comparison, herbal interventions); we excluded such data from analysis.
Doses of NSAIDs varied, but fell within commonly recommended parameters. Average doses for non‐selective NSAIDs were as follows: aceclofenac (100 mg daily), aspirin (650 mg; four‐hourly), dexketoprofen (12.5 mg to 25 mg; six‐hourly), diclofenac (up to 200 mg daily in divided doses, orally or by suppository), etodolac (200 mg to 300 mg twice daily), fenoprofen (100 mg to 200 mg; four‐hourly), fentiazac (100 mg; twice daily), flufenamic acid (200 mg; eight‐hourly), flurbiprofen (100 mg; twice daily), ibuprofen (400 mg; three, four or six times daily), indomethacin (25 mg tablets or 100 mg suppositories; three times daily), ketoprofen (25 mg to 50 mg; six‐hourly, with or without a loading dose of 25 mg to 70 mg), lysine clonixinate (125 mg; six‐hourly); meclofenamate sodium (100 mg; eight‐hourly), mefenamic acid (250 mg; eight‐hourly), meloxicam (7.5 mg to 15 mg; daily), daily naproxen/naproxen sodium (250 mg to 275 mg; four to eight‐hourly, sometimes with a loading dose of 500 mg to 550 mg), niflumic acid (250 mg; three times daily), nimesulide (50 mg to 100 mg twice daily), piroxicam (20 to 40 mg daily, by tablet or suppository) and tolfenamic acid (200 mg; eight‐hourly). Doses of COX‐2‐specific inhibitors used were: celecoxib: 400 mg then 200 mg 12‐hourly and etoricoxib 120 mg daily.
The duration of treatment in the included studies varied from one cycle (per treatment) to five. For details of the drug regimes used in individual studies, see the Characteristics of included studies table.
Outcomes
Outcomes measures varied. Most studies measured pain relief by asking women to keep a daily record during their menstrual period, rating their degree of pain relief on an ordinal scale, either categorical (e.g. from poor to excellent) or numerical (e.g. 1 to 5), while others used a dichotomous measure (e.g. complete relief/ongoing pain). Some women were asked to rate their pain intensity on various types of continuous numerical scale: few studies used a visual analogue scale. In most cases pain relief was reported as the proportion of women experiencing relief, though some trials instead used the number of menstrual cycles as the denominator. Interference with daily activities and absence from work/school were generally measured as the proportion of women reporting any degree of interference with their normal routine or any need for days off. About a quarter of the trials clearly reported that they measured adverse effects by prospective self report, using a questionnaire, record card or diary in which the women noted any symptoms daily during their menstrual period. Others assessed this outcome retrospectively at follow‐up appointments, by either specific or non‐specific questioning or simply by recording information volunteered by the participant. Many trials did not specify how they measured adverse effects.
Excluded studies
In total we excluded 127 trials from the review, for the following reasons:
35 trials did not mention randomisation, included non‐randomised women in analysis, or their design was unclear and attempts to contact authors for clarification were unsuccessful;
14 trials were randomised but had only single blinding or no blinding at all;
12 trials included NSAIDs that are currently discontinued (for the treatment of dysmenorrhoea) and did not report data on any other relevant comparison;
19 trials included women who had secondary dysmenorrhoea (including IUCD‐related dysmenorrhoea), menorrhagia or eumenorrhoea;
three trials measured uterine pressure or contractibility rather than pain relief;
13 trials did not include a comparison of interest;
five trials were dose‐finding trials of a single NSAID;
26 trials had participant withdrawal rates of 20% or more.
See Characteristics of excluded studies for more information.
Risk of bias in included studies
The quality of the included studies is summarised in Figure 4.
4.
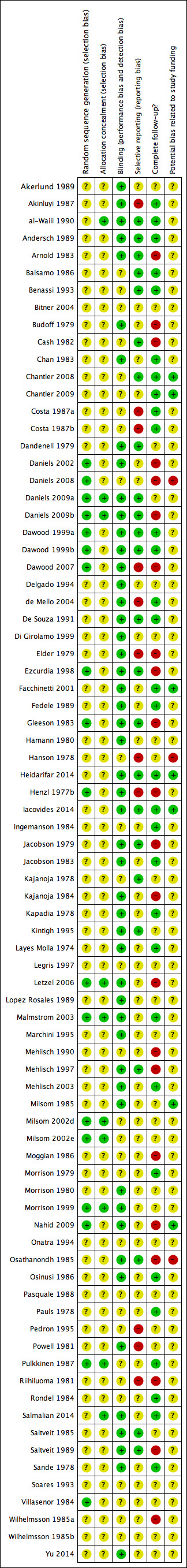
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All studies stated that they were randomised, but only 23% (18/80) described in detail their method of generating a random allocation sequence. We rated these studies as at low risk of bias, while we rated all the other studies as at unclear risk.
Less than 12% of studies (9/80) described an adequate method of allocation concealment. We rated these studies as at low risk of bias, while we rated all the other studies as at unclear risk.
Blinding
All studies were described as double‐blinded, and 50 studies (50/80: 63%) provided details of who was blinded or stated explicitly that the placebo was identical to the active treatment. Given the subjective nature of the pain‐related outcomes assessed in this review, inadequate blinding has a high potential to bias results. We rated the other 30 studies as at unclear risk of bias.
Incomplete outcome data
None of the included studies clearly analysed fewer than 80% of women randomised.
Thirty‐one of the studies (39%) included over 95% of women randomised in analysis for one of our primary outcomes. We rated these as at low risk of attrition bias. Twenty‐seven studies (34%) included 90% to 95% of women in analysis and we rated them as at unclear risk of bias, while the other 22 studies included fewer than 90% of women in analysis, and we rated them as at high risk of bias.
The main reasons for incomplete outcome data (where stated) were as follows: failure to attend follow‐up appointments, poor compliance with the study criteria, and withdrawal from treatment due to adverse effects, pregnancy, lack of efficacy, or wish to use contraceptives such as the oral contraceptive pill (OCP) or IUCD that were excluded by the trial criteria. Losses to follow‐up are likely to be associated with treatment inefficacy or adverse effects, and so have a high potential to bias results.
Selective reporting
Only 24/80 studies (30%) clearly appeared to be free of selective reporting. In most studies (44/80; 55%) it was unclear whether data on adverse effects were collected prospectively. We rated nine studies as at high risk of selective reporting bias because adverse events were not reported as an outcome or it was clear that they were reported selectively. The impact of selective reporting of harms on the pooled result is not obvious, as selective emphasis of those adverse events where analyses were statistically significant might overstate those harms, and selective omission might attenuate the estimated effect (see Characteristics of included studies).
Potential bias related to study funding
Seven studies (7/80; 9%) reported a non‐commercial source of funding and we rated them as at low risk of potential bias related to study funding. We rated the other studies as at unclear risk of such bias: 47/80 studies (59%) were co‐authored or funded by pharmaceutical companies and 25/80(31%) did not mention their source of funding.
Glossary
Please refer to the Cochrane glossary for explanation of unfamiliar terms: http://community.cochrane.org/glossary.
Effects of interventions
Pain relief
1) Nonsteroidal anti‐inflammatory drugs (NSAIDs) versus placebo
There were 47 trials comparing NSAIDs versus placebo from which data on pain relief could be extracted, which were suitable for meta‐analysis. They compared the following NSAIDs versus placebo: aspirin (one study), celecoxib (two studies), diclofenac (three studies), etodolac (one study), etoricoxib (one study), fenoprofen (two studies), flufenamic acid (one study), ibuprofen (six studies), indomethacin (three studies), ketoprofen (two studies), lysine clonixinate (one study), mefenamic acid (four studies), meloxicam (one study), naproxen (21 studies), niflumic acid (one study) and nimesulide (two studies); some trials included more than one comparison. The studies analysed a total of 2602 women, 2006 women in cross‐over trials and 596 women in parallel trials.
When we pooled dichotomous data from 35 studies comparing all NSAIDs versus placebo, NSAIDs were more effective than placebo at producing moderate or excellent pain relief (odds ratio (OR) 4.37, 95% confidence interval (CI) 3.76 to 5.09; I2 = 53%) (Analysis 1.1; Figure 5). Effect sizes varied, with few studies and wide confidence intervals for most comparisons. The most precise finding was for naproxen (OR 3.67, 95% CI 2.94 to 4.58; 16 studies, I2 = 52%). The placebo groups in three studies contributed twice to the pooled analysis of all NSAIDs, but sensitivity analyses excluding these studies did not materially affect the results (Di Girolamo 1999; Marchini 1995; Mehlisch 1990). Heterogeneity in these analyses is discussed below.
1.1. Analysis.
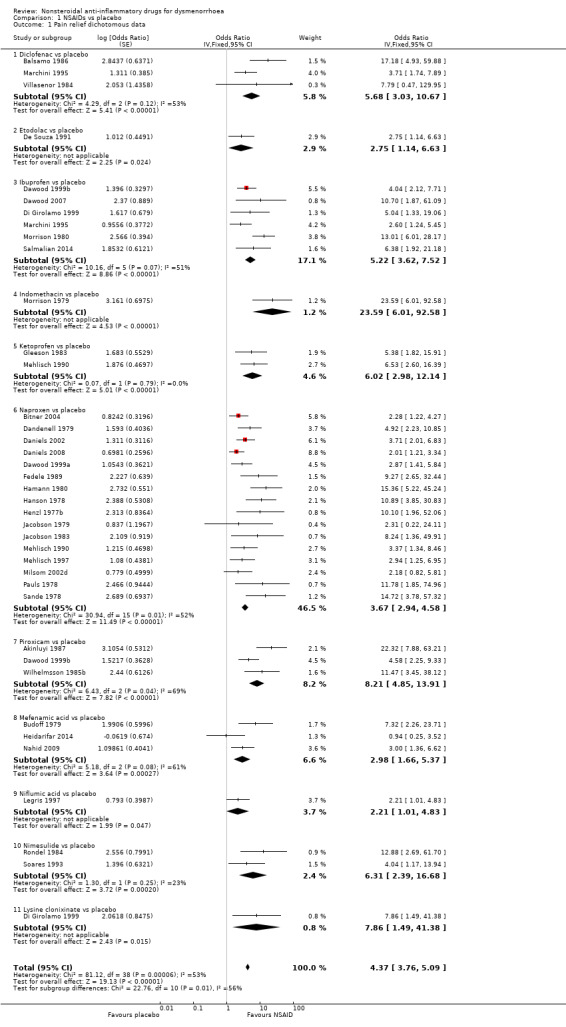
Comparison 1 NSAIDs vs placebo, Outcome 1 Pain relief dichotomous data.
5.
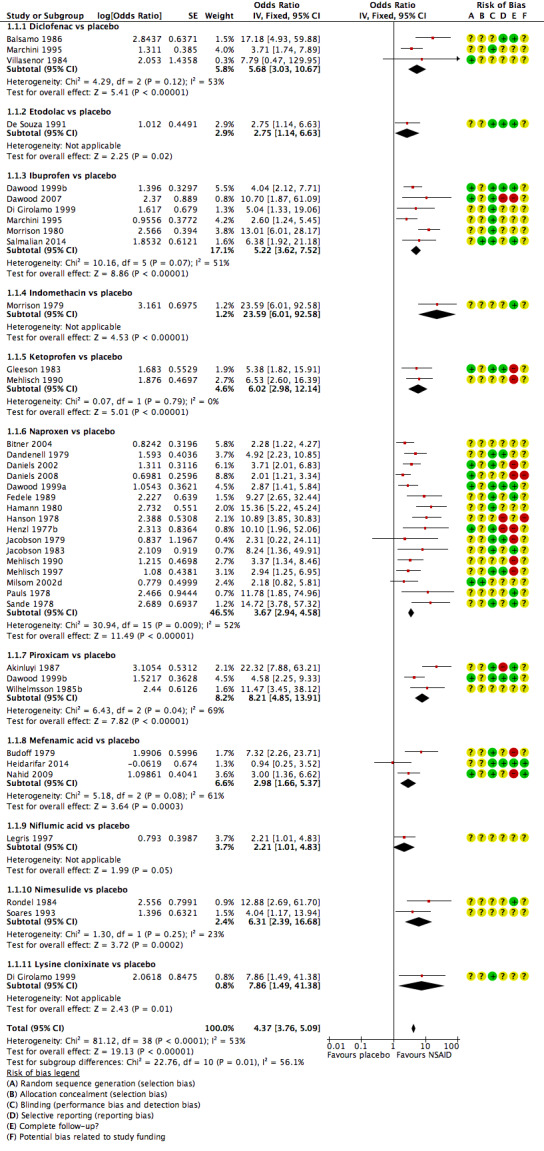
Forest plot of comparison: 1 NSAIDs vs placebo, outcome: 1.1 Pain relief dichotomous data.
Among 12 studies reporting continuous data for this outcome, only two used visual analogue scales (VAS). The other 10 studies compared seven different NSAIDs versus placebo, using five different pain scales. We combined the studies that used common scales, as an attempt to pool scales (and calculate the standardised mean difference (SMD)) resulted in high levels of heterogeneity. In most analyses NSAIDs were more effective than placebo in producing moderate/excellent pain relief and/or in reducing pain scores. The only NSAIDs without clear indication of benefit were aspirin and fenoprofen, which were tested in a single study each (Analysis 1.6).
1.6. Analysis.
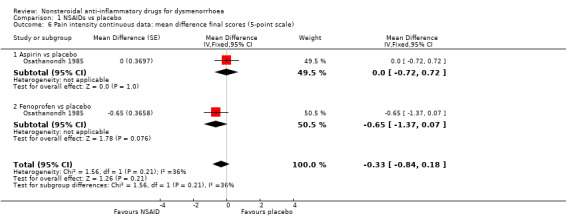
Comparison 1 NSAIDs vs placebo, Outcome 6 Pain intensity continuous data: mean difference final scores (5‐point scale).
Effect estimates for continuous outcomes of effectiveness were as follows:
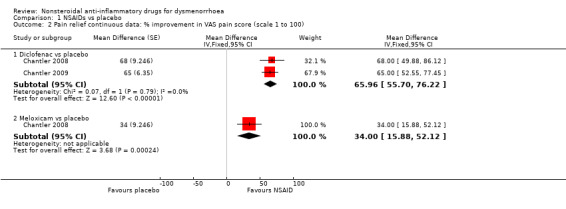
Diclofenac versus placebo (difference in improvement on a 0 to 100 VAS): mean difference (MD) 65.96, 95% CI 55.70 to 76.22, two studies, I2 = 0% (Analysis 1.2).
Meloxicam versus placebo (difference in improvement on a 0 to 100 VAS): MD 34, 95% CI 15.88 to 52.12, one study (Analysis 1.2).
Celecoxib, etoricoxib or naproxen versus placebo (mean difference in total pain relief using a time‐weighted scale (TOPAR)): MD 6.24, 95% CI 4.69 to 7.78, four studies, I2 = 0% (Analysis 1.3).
Flufenamic acid or indomethacin versus placebo (difference in final score on a repeated 0 to 3 scale): MD 4.83, 95% CI 3.61 to 6.06, two studies, I2 = 0% (Analysis 1.4).
Indomethacin versus placebo (difference in final score on a 0 to 18 scale): MD 11.20, 95% CI 7.24 to 15.16, one study (Analysis 1.5).
Naproxen versus placebo (difference in final score on a 0 to 40 scale): MD 15.30, 95% CI 5.64 to 24.96, one study (Analysis 1.5).
Aspirin or fenoprofen versus placebo (difference in pain intensity on a 0 to 4‐point scale): MD ‐0.33, 95% CI ‐0.84 to 0.18, two studies, I2 = 36% (Analysis 1.6).
Mefenamic acid versus placebo (difference in pain intensity on a 1 to 4 scale): MD ‐1.70, 95% CI ‐3.37 to ‐0.03 (Analysis 1.7).
Naproxen versus placebo (difference in final score on a 0 to 40 scale): MD 15.30, 95% CI 5.64 to 24.96, one study (Analysis 1.5).
Aspirin or fenoprofen versus placebo (difference in pain intensity on a 0 to 4‐point scale): MD ‐0.33, 95% CI ‐0.84 to 0.18, two studies, I2 = 36% (Analysis 1.6).
Mefenamic acid versus placebo (difference in pain intensity on a 1 to 4 scale): MD ‐1.70, 95% CI ‐3.37 to ‐0.03 (Analysis 1.7).
1.2. Analysis.
Comparison 1 NSAIDs vs placebo, Outcome 2 Pain relief continuous data: % improvement in VAS pain score (scale 1 to 100).
1.3. Analysis.
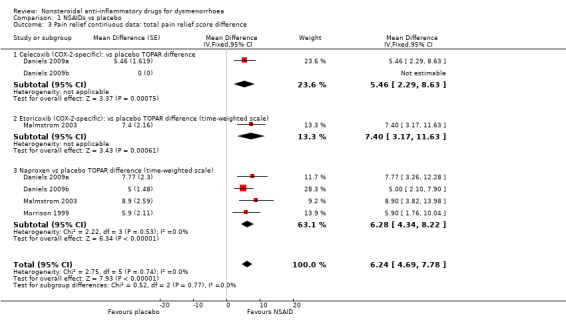
Comparison 1 NSAIDs vs placebo, Outcome 3 Pain relief continuous data: total pain relief score difference.
1.4. Analysis.
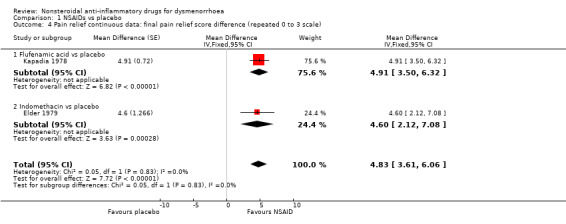
Comparison 1 NSAIDs vs placebo, Outcome 4 Pain relief continuous data: final pain relief score difference (repeated 0 to 3 scale).
1.5. Analysis.
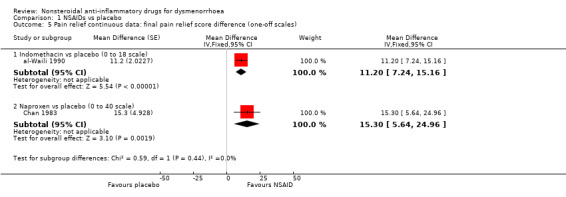
Comparison 1 NSAIDs vs placebo, Outcome 5 Pain relief continuous data: final pain relief score difference (one‐off scales).
1.7. Analysis.
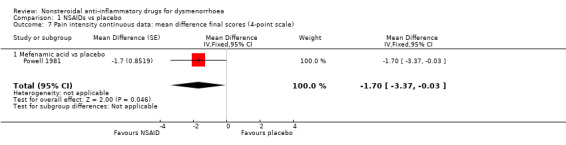
Comparison 1 NSAIDs vs placebo, Outcome 7 Pain intensity continuous data: mean difference final scores (4‐point scale).
A further 16 trials reported results on this outcome in a form from which no data suitable for meta‐analysis could be extracted, such as graphs (Arnold 1983; Cash 1982; Costa 1987a; Iacovides 2014; Kintigh 1995; Letzel 2006), as continuous data without standard deviations (Moggian 1986; Pasquale 1988; Saltveit 1985), without denominators for each group (Ezcurdia 1998; Osinusi 1986), or as per‐cycle data (Kajanoja 1978; Mehlisch 2003; Pulkkinen 1987; Riihiluoma 1981; see Table 3) or as medians (Nahid 2009; see Table 4). They compared the following NSAIDs versus placebo: aspirin, diclofenac, fenoprofen, ibuprofen, indomethacin, mefenamic acid, naproxen, nimesulide and piroxicam. All NSAIDs were more effective than placebo, apart from aspirin, for which there was no evidence of a difference from placebo (Kajanoja 1978).
1. Pain relief: NSAIDs versus placebo (per cycle data).
| Comparison | Study ID | No of women | Outcome measure | NSAID | Placebo | Significance |
| Aspirin versus placebo | Kajanoja 1978 | 47 | No of cycles where treatment gave moderate/good relief | 13/89 | 9/90 | Not statistically significant |
| Indomethacin versus placebo | Kajanoja 1978 | 37 | No of cycles when women reported moderate/good relief | 42/90 | 9/90 | P value < 0.001 |
| Nimesulide versus placebo | Pulkkinen 1987 | 14 | No of cycles where women rated therapy good/very effective | 22/28 | 9/27 | P value < 0.01 |
| Diclofenac versus placebo | Riihiluoma 1981 | 35 | No of cycles when pain much improved | 14/58 | 3/57 | P value < 0.05 |
NSAID = nonsteroidal anti‐inflammatory drug
2. Pain relief: NSAIDs versus placebo: median data.
| Comparison | Study | No of women | Outcome measure | NSAID group | Placebo group | P value | Finding |
| Mefenamic acid versus placebo | Nahid 2009 | 120 (106 analysed) | Pain score: median (range) on 1 to 10 VAS |
n = 55 At 2 months: 3.6 (2 to 6) At 3 months: 2.4 (1 to 5) |
n = 51 At 2 months: 5 (2 to 6) At 3 months: 6 (4 to 7) |
P value < 0.1) | Favours NSAID |
NSAID = nonsteroidal anti‐inflammatory drug
2) NSAIDs versus NSAIDs
There were 18 studies comparing NSAIDs head‐to‐head from which data suitable for meta‐analysis could be extracted, only two of which compared the same two NSAIDs (Daniels 2009a; Daniels 2009b). They made the following comparisons: aspirin versus fenoprofen (Analysis 2.1); diclofenac versus the following: meloxicam (Analysis 6.2), ibuprofen and nimesulide (Analysis 6.1); ibuprofen versus the following: piroxicam, etoricoxib and lysine clonixinate (Analysis 4.1), mefenamic acid versus the following: meloxicam (Analysis 5.1) and tolfenamic acid; and naproxen versus the following: celecoxib (two studies), diclofenac, ketoprofen, etoricoxib, flurbiprofen, ibuprofen and piroxicam (Analysis 7.1 to Analysis 7.5).
2.1. Analysis.
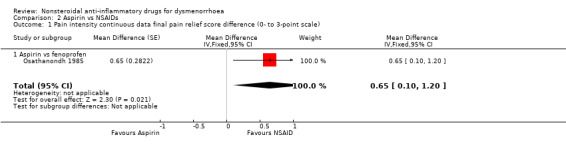
Comparison 2 Aspirin vs NSAIDs, Outcome 1 Pain intensity continuous data final pain relief score difference (0‐ to 3‐point scale).
6.2. Analysis.
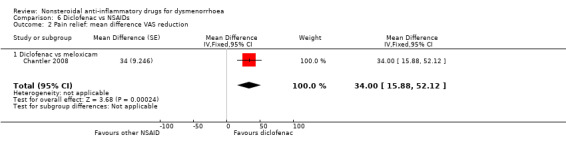
Comparison 6 Diclofenac vs NSAIDs, Outcome 2 Pain relief: mean difference VAS reduction.
6.1. Analysis.
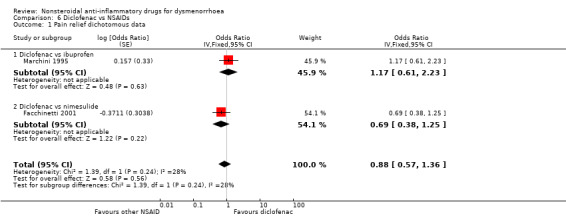
Comparison 6 Diclofenac vs NSAIDs, Outcome 1 Pain relief dichotomous data.
4.1. Analysis.
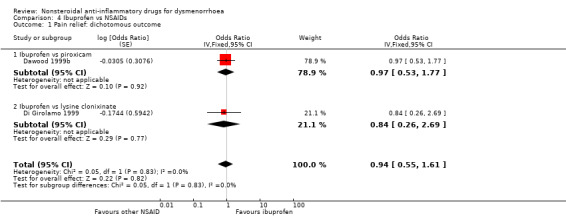
Comparison 4 Ibuprofen vs NSAIDs, Outcome 1 Pain relief: dichotomous outcome.
5.1. Analysis.

Comparison 5 Mefenamic acid vs NSAIDs, Outcome 1 Pain relief: dichotomous data.
7.1. Analysis.
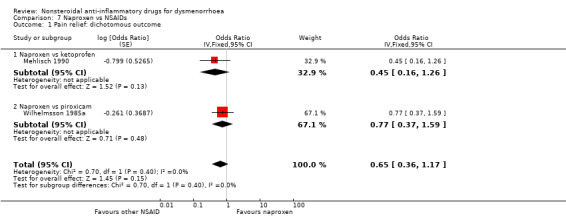
Comparison 7 Naproxen vs NSAIDs, Outcome 1 Pain relief: dichotomous outcome.
7.5. Analysis.
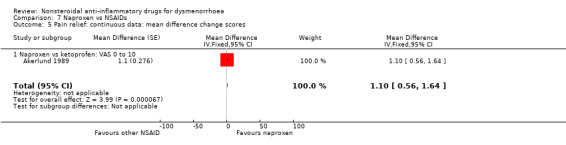
Comparison 7 Naproxen vs NSAIDs, Outcome 5 Pain relief: continuous data: mean difference change scores.
In single studies, diclofenac reduced pain on a visual analogue 100‐point scale more than meloxicam (Analysis 6.2), fenoprofen reduced pain intensity more than aspirin (Analysis 2.1) and etoricoxib was more likely to achieve pain relief than ibuprofen (Analysis 4.2). Naproxen reduced pain scores more than ibuprofen or celecoxib (Analysis 7.3) and was more likely to achieve effective pain relief than ketoprofen (Analysis 7.5). Other head‐to‐head comparisons between NSAIDs showed no evidence of a difference between them.
4.2. Analysis.
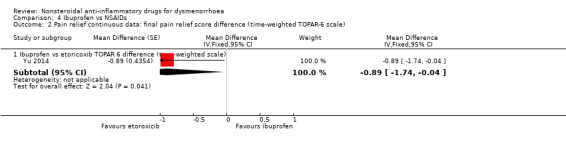
Comparison 4 Ibuprofen vs NSAIDs, Outcome 2 Pain relief continuous data: final pain relief score difference (time‐weighted TOPAR‐6 scale).
7.3. Analysis.
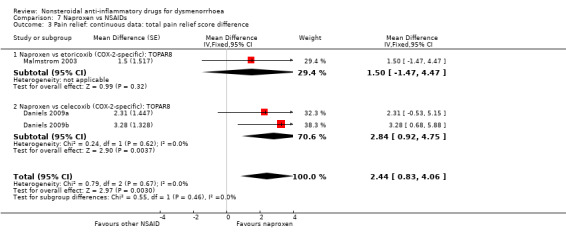
Comparison 7 Naproxen vs NSAIDs, Outcome 3 Pain relief: continuous data: total pain relief score difference.
Effect estimates for all these comparisons were as follows:
Aspirin versus fenoprofen (difference in pain intensity on a 0 to 3‐point scale): MD 0.65, 95% CI 0.10 to 1.20, one study (Analysis 2.1).
Ibuprofen versus piroxicam or lysine clonixinate (rate of pain relief): OR 0.94, 95% CI 0.55 to 1.61 (Analysis 4.1).
Ibuprofen versus etoricoxib (TOPAR 6): MD ‐0.89, 95% CI ‐1.74 to ‐0.04, one study (Analysis 4.2).
Mefenamic acid versus meloxicam (rate of pain relief): OR 0.68, 95% CI 0.32 to 1.44, one study (Analysis 5.1).
Mefenamic acid versus tolfenamic acid (10‐point VAS): MD 0.23, 95% CI ‐0.69 to 1.15, one study (Analysis 5.2).
Diclofenac versus ibuprofen or nimesulide (rate of pain relief): OR 0.88, 95% CI 0.57 to 1.36, two studies, I2 = 28% (Analysis 6.1).
Diclofenac versus meloxicam (reduction on 100‐point VAS): MD 34, 95% CI 15.88 to 52.12 (Analysis 6.2).
Naproxen versus ketoprofen or piroxicam (rate of pain relief): OR 0.65, 95% CI 0.36 to 1.17, two studies, I2 = 0% (Analysis 7.1).
Naproxen versus flurbiprofen (sum of pain intensity difference over time: SPID): MD 0.06, 95% CI ‐0.28 to 0.40, one study (Analysis 7.2).
Naproxen versus etoricoxib or celecoxib (mean difference on total pain relief using a time‐weighted scale (TOPAR8)): MD 2.44, 95% CI 0.83 to 4.06, two studies, I2 = 0% (Analysis 7.3).
Naproxen versus ibuprofen or diclofenac (mean difference final score on a 1 to 5 scale): MD ‐0.17, 95% CI ‐0.39 to 0.06, two studies, I2 = 51% (Analysis 7.4).
Naproxen versus ketoprofen (difference in change scores on a 10‐point VAS): MD 1.10, 95% CI 0.56 to 1.64, one study (Analysis 7.5).
5.2. Analysis.

Comparison 5 Mefenamic acid vs NSAIDs, Outcome 2 Pain relief (VAS).
7.2. Analysis.
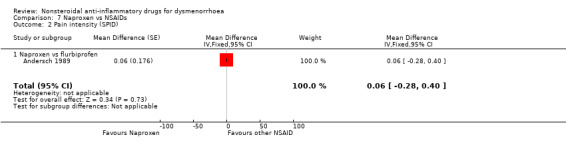
Comparison 7 Naproxen vs NSAIDs, Outcome 2 Pain intensity (SPID).
7.4. Analysis.
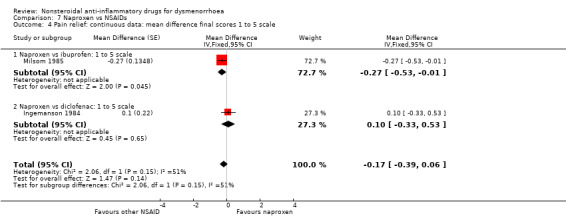
Comparison 7 Naproxen vs NSAIDs, Outcome 4 Pain relief: continuous data: mean difference final scores 1 to 5 scale.
Two additional studies reported only per‐cycle data. One found indomethacin more effective than aspirin (Kajanoja 1978), and one found no evidence of a difference between naproxen and diflunisal (Kajanoja 1984) (Table 5). Twelve trials reported results on this outcome in such a way that no numerical data could be extracted. Some presented graphs (Arnold 1983; Benassi 1993; Costa 1987a; Costa 1987b; Kintigh 1995; Pedron 1995), or continuous data without standard deviations (Pasquale 1988; Saltveit 1989), while one did not provide denominators for each group (Onatra 1994). Only three of these trials reported differences between different NSAIDs: one trial found meclofenamate sodium more effective than naproxen (Benassi 1993), and two trials found piroxicam more effective than naproxen (Costa 1987a; Costa 1987b). However, these trials were very small (with 30, 12 and 14 women respectively) and much larger studies comparing piroxicam with naproxen found no evidence of a difference between them (Saltveit 1989; Wilhelmsson 1985a).
3. Pain relief: NSAIDs versus NSAIDs (per cycle data).
| NSAID 1 | NSAID 2 | Study ID | No of women | Outcome measure | NSAID 1 | NSAID 2 | Significance |
| Aspirin | Indomethacin | Kajanoja 1978 | 47 | No of cycles where treatment gave moderate/good relief | 13/89 | 42/90 | P value < 0.001 |
| Naproxen | Diflunisal | Kajanoja 1984 | 22 (19 analysed) | No of cycles where treatment achieved moderate/good relief | 34/38 | 28/38 cycles | Not statistically significant |
NSAID = nonsteroidal anti‐inflammatory drug
3) NSAIDs versus paracetamol
Two studies compared ibuprofen versus paracetamol and one compared naproxen versus paracetamol. Pooling of these three studies resulted in a difference in the proportion of women reporting good, excellent or complete pain relief, favouring NSAIDs over paracetamol (OR 1.89, 95% CI 1.05 to 3.43) (Analysis 8.1).
8.1. Analysis.
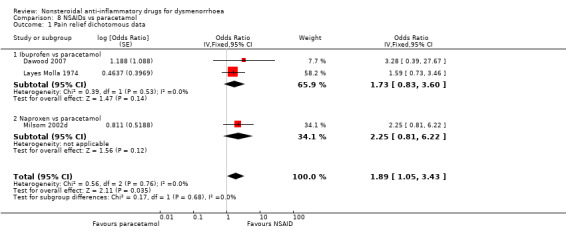
Comparison 8 NSAIDs vs paracetamol, Outcome 1 Pain relief dichotomous data.
Adverse effects
1) NSAIDs versus placebo
All adverse effects
Twenty‐five studies were suitable for meta‐analysis for this outcome. They analysed 2133 women, 1272 in cross‐over studies and 861 in parallel‐group studies. They compared the following NSAIDs versus placebo: naproxen (10 studies), piroxicam (five studies), diclofenac, ibuprofen, ketoprofen (three studies each), celecoxib, fenoprofen (two studies each), aceclofenac, aspirin, dexketoprofen, etodolac, etoricoxib and niflumic acid and nimesulide (one study each).
Although there was no evidence of a difference between any individual NSAID and placebo for this outcome, when we pooled results NSAIDs overall were more likely to cause an adverse effect of any kind than placebo (OR 1.29, 95% CI 1.11 to 1.51, 25 studies, I2 = 0%) (Analysis 1.9). The most commonly reported adverse effects were mild neurological and gastrointestinal symptoms. The placebo groups in two studies contributed twice to the pooled analysis of all NSAIDs, but exclusion of these studies did not materially affect the results (Daniels 2009a; Daniels 2009b).
1.9. Analysis.
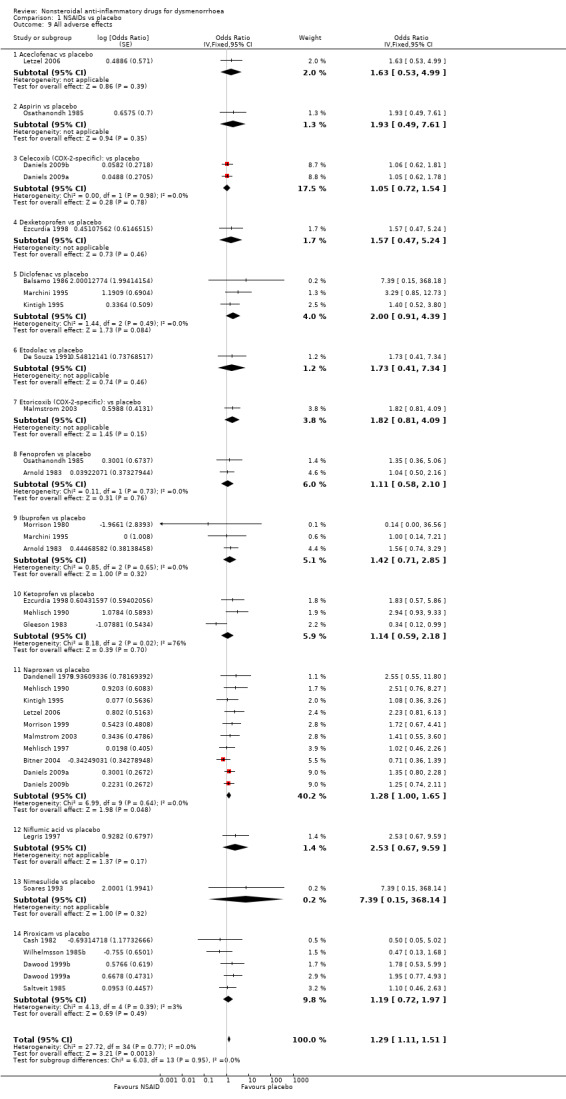
Comparison 1 NSAIDs vs placebo, Outcome 9 All adverse effects.
Two additional cross‐over studies measured this outcome. One stated that no adverse events were reported in association with either diclofenac or placebo (Iacovides 2014); the other reported that no serious side effects occurred in association with either piroxicam or placebo (Osinusi 1986).
Gastrointestinal adverse effects
Fourteen studies were suitable for meta‐analysis for this outcome, which included adverse effects such as nausea and indigestion. They analysed a total of 702 women, 548 in cross‐over studies and 154 in parallel‐group studies, and compared the following NSAIDs versus placebo: naproxen (four studies), indomethacin, piroxicam (three studies), aspirin, mefenamic acid (two studies each), dexketoprofen, fenoprofen and ketoprofen (one study each). When we pooled all studies, gastrointestinal events were more common in the NSAIDs group (OR 1.58, 95% CI 1.12 to 2.23) (Analysis 1.10). A higher incidence of gastrointestinal side effects was associated with two individual NSAIDs: naproxen (OR 2.30, 95% CI 1.02 to 5.19, four studies, I2 = 1%) and dexketoprofen (OR 8.06, 95% CI 0.50 to 130.48). One additional study reported no events in either the piroxicam or the placebo arm (Costa 1987a).
1.10. Analysis.
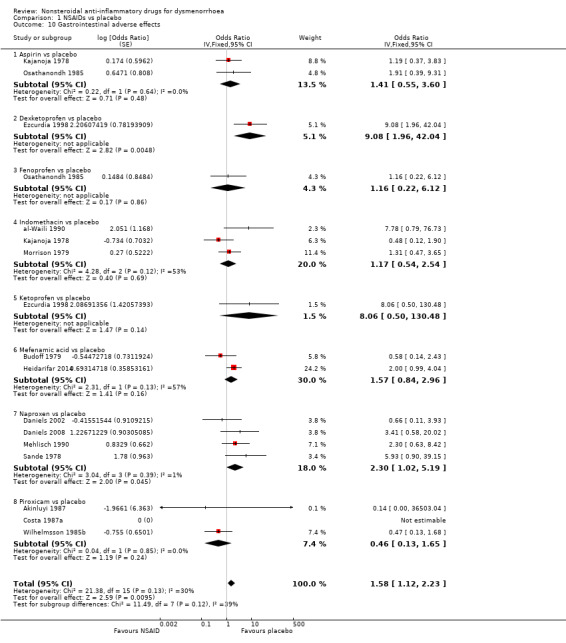
Comparison 1 NSAIDs vs placebo, Outcome 10 Gastrointestinal adverse effects.
Neurological adverse effects
Seven studies were suitable for meta‐analysis for this outcome, which included adverse effects such as headache, drowsiness, dizziness and dryness of the mouth. They analysed a total of 498 women, 381 in cross‐over studies and 117 in parallel‐group studies, and compared the following NSAIDs versus placebo: naproxen (three studies), indomethacin (two studies) aspirin and fenoprofen (one study each). When we pooled studies NSAIDs were more likely than placebo to cause neurological adverse effects (OR 2.74, 95% CI 1.66 to 4.53, seven studies, I2 = 0%) (Analysis 1.11). Two individual NSAIDs were associated with a higher incidence of events than placebo: naproxen (OR 2.20, 95% CI 1.11 to 4.35, three studies, I2 = 0%) and indomethacin (4.96, 95% CI 1.87 to 13.11, two studies, I2 = 0%).
1.11. Analysis.
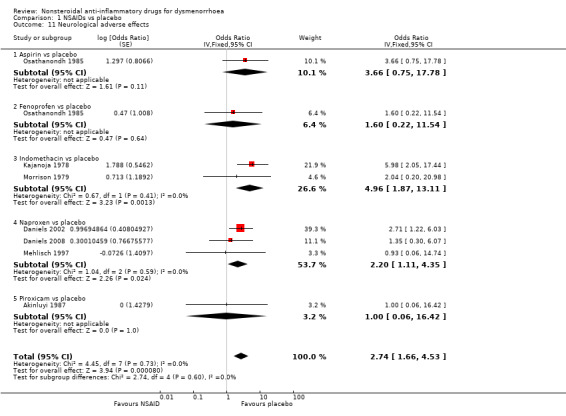
Comparison 1 NSAIDs vs placebo, Outcome 11 Neurological adverse effects.
2) NSAIDs versus NSAIDs
All adverse effects
Fifteen studies reported data suitable for meta‐analysis comparing NSAIDs head‐to‐head for this outcome. Only two compared the same two NSAIDs (Daniels 2009a; Daniels 2009b). They analysed data for 1762 women, 959 in cross‐over studies and 803 in parallel studies. They made the following comparisons: aspirin versus fenoprofen, diclofenac versus ibuprofen, etodolac versus piroxicam, ibuprofen versus fenoprofen, ibuprofen versus etoricoxib, mefenamic acid versus tolfenamic acid, and naproxen versus the following: aceclofenac, celecoxib (two studies), diclofenac, etoricoxib, ketoprofen, meclofenamate and piroxicam. When we pooled data for the six studies comparing naproxen versus other NSAIDs we found no evidence of a difference between the groups (Analysis 7.6). Nor did we find any evidence of a difference between the groups in any individual study comparing any NSAIDs head‐to‐head. Two studies not included in meta‐analysis also reported this outcome: one found no evidence of a difference between naproxen and flurbiprofen for the incidence of any adverse effect. The second, comparing diclofenac versus meloxicam, reported no adverse effects in either group (Chantler 2008).
7.6. Analysis.
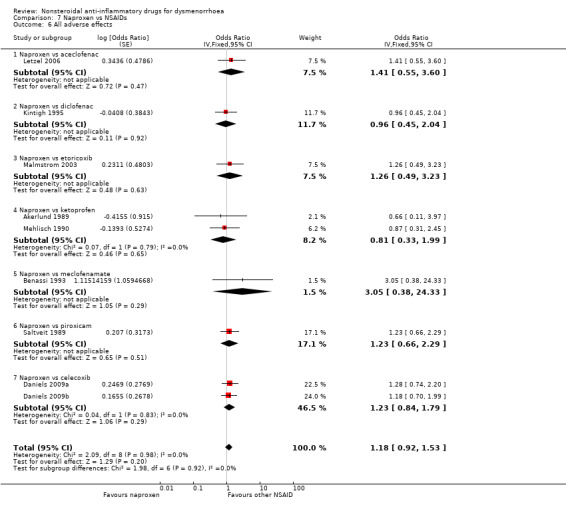
Comparison 7 Naproxen vs NSAIDs, Outcome 6 All adverse effects.
Effect estimates were as follows
Aspirin versus fenoprofen: OR 1.46, 95% CI 0.52 to 4.08, one study (Analysis 2.2).
Etodolac versus piroxicam: OR 1.00, 95% CI 0.06 to 16.70, one study (Analysis 3.1).
Ibuprofen versus fenoprofen or etoricoxib: OR 1.38, 95% CI 0.68 to 2.80, two studies, I2 = 0% (Analysis 4.3).
Mefenamic acid versus tolfenamic acid: OR 1.26, 95% CI 0.54 to 2.96, one study (Analysis 5.3).
Diclofenac versus ibuprofen: OR 3.83, 95% CI 0.76 to 19.28, one study (Analysis 6.3).
Naproxen versus aceclofenac, diclofenac, etoricoxib, ketoprofen, meclofenamate, piroxicam or celecoxib: OR 1.18, 95% CI 0.92 to 1.53, nine studies, I2 = 0% (Analysis 7.6).
2.2. Analysis.
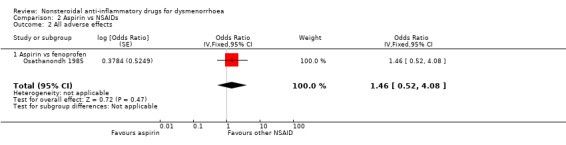
Comparison 2 Aspirin vs NSAIDs, Outcome 2 All adverse effects.
3.1. Analysis.
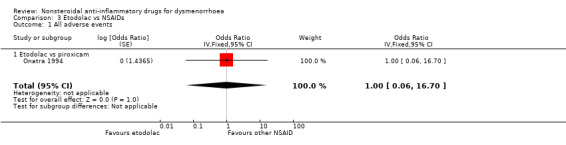
Comparison 3 Etodolac vs NSAIDs, Outcome 1 All adverse events.
4.3. Analysis.
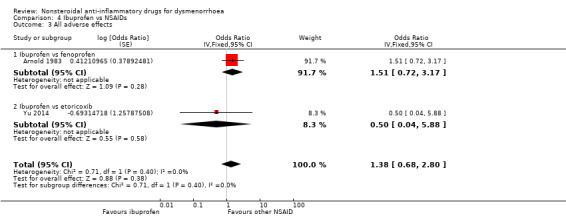
Comparison 4 Ibuprofen vs NSAIDs, Outcome 3 All adverse effects.
5.3. Analysis.
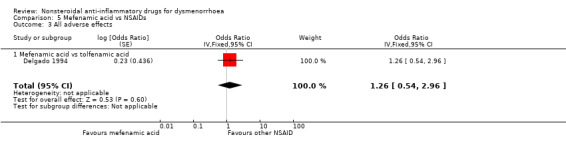
Comparison 5 Mefenamic acid vs NSAIDs, Outcome 3 All adverse effects.
6.3. Analysis.
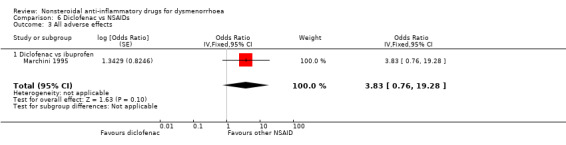
Comparison 6 Diclofenac vs NSAIDs, Outcome 3 All adverse effects.
Gastrointestinal adverse effects
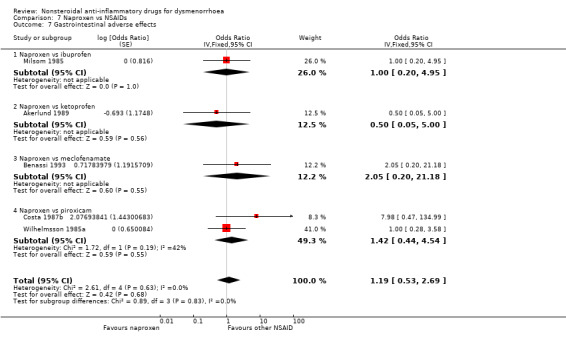
Eight studies reported data suitable for meta‐analysis comparing NSAIDs head‐to‐head for gastrointestinal adverse effects such as nausea and indigestion. Two studies compared the same two NSAIDs but the rest compared different NSAIDs. These studies analysed data for 595 women, 176 in cross‐over studies and 419 in parallel‐group studies. They made the following comparisons: aspirin versus fenoprofen, diclofenac versus nimesulide, and naproxen versus the following: ibuprofen, ketoprofen, meclofenamate (each one study) and piroxicam (two studies). When we pooled data for the four studies comparing naproxen with other NSAIDs there was no evidence of a difference between the groups (Analysis 7.7). Nor did any individual study comparing any NSAIDs head‐to‐head find evidence of a difference between the groups for this outcome.
7.7. Analysis.
Comparison 7 Naproxen vs NSAIDs, Outcome 7 Gastrointestinal adverse effects.
Effect estimates were as follows:
Aspirin versus fenoprofen: OR 2.05, 95% CI 0.84 to 4.96, one study (Analysis 2.3).
Diclofenac versus nimesulide: OR 2.34, 95% CI 0.93 to 5.87, one study (Analysis 6.4).
Naproxen versus ibuprofen, ketoprofen, meclofenamate or piroxicam: OR 1.19, 95% CI 0.53 to 2.69, five studies, I2 = 0% (Analysis 7.7).
2.3. Analysis.
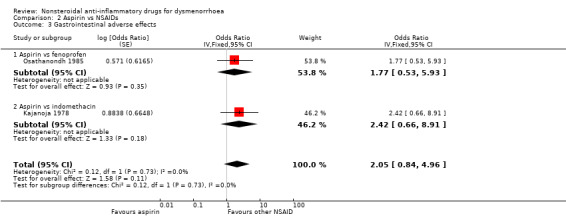
Comparison 2 Aspirin vs NSAIDs, Outcome 3 Gastrointestinal adverse effects.
6.4. Analysis.
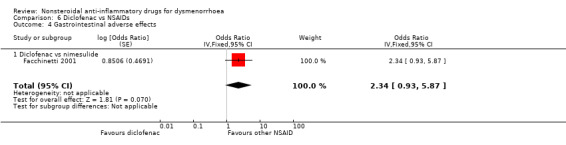
Comparison 6 Diclofenac vs NSAIDs, Outcome 4 Gastrointestinal adverse effects.
Neurological adverse effects
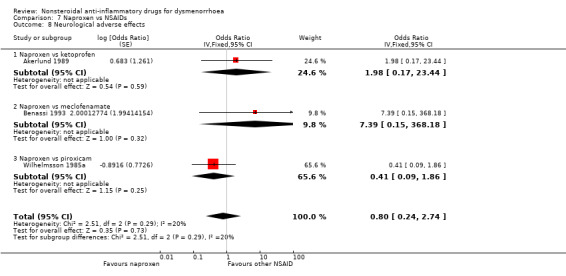
Five studies reported data suitable for meta‐analysis comparing NSAIDs head‐to‐head for neurological adverse effects such as headache, drowsiness and dizziness. No studies compared the same two NSAIDs. These studies analysed data for 527 women, 108 in cross‐over studies and 419 in parallel‐group studies. They made the following comparisons: aspirin versus fenoprofen, diclofenac versus nimesulide, and naproxen versus ketoprofen, meclofenamate and piroxicam. When we pooled data for the three studies comparing naproxen versus other NSAIDs there was no evidence of a difference between the groups (Analysis 7.8). Nor did any individual study comparing any NSAIDs head‐to‐head find evidence of a difference between the groups for this outcome.
7.8. Analysis.
Comparison 7 Naproxen vs NSAIDs, Outcome 8 Neurological adverse effects.
Effect estimates were as follows:
Aspirin versus fenoprofen: OR 3.20, 95% CI 0.92 to 11.11, one study (Analysis 2.4).
Diclofenac versus nimesulide: OR 0.24, 95% CI 0.03 to 2.02, one study (Analysis 6.5).
Naproxen versus ketoprofen, meclofenamate or piroxicam: OR 0.80, 95% CI 0.24 to 2.74, three studies, I2 = 20% (Analysis 7.8).
2.4. Analysis.
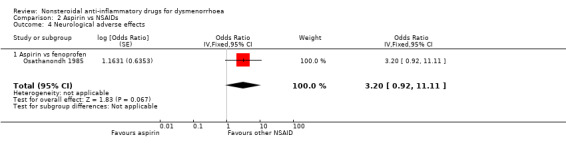
Comparison 2 Aspirin vs NSAIDs, Outcome 4 Neurological adverse effects.
6.5. Analysis.
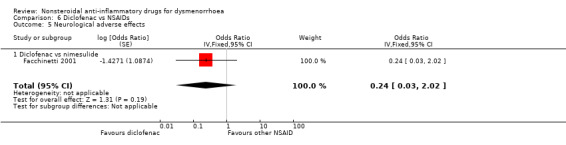
Comparison 6 Diclofenac vs NSAIDs, Outcome 5 Neurological adverse effects.
3) NSAIDs versus paracetamol
Only three studies reported data suitable for meta‐analysis for this outcome (Analysis 8.2 to Analysis 8.4). No evidence of a difference was found between NSAIDs versus paracetamol in the risk of all adverse effects, or gastrointestinal or neurological adverse effects. However, there was only one study for each comparison.
8.2. Analysis.
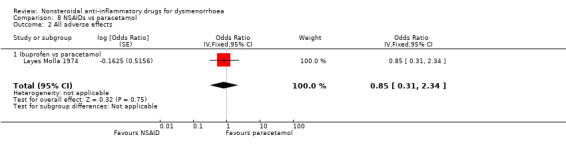
Comparison 8 NSAIDs vs paracetamol, Outcome 2 All adverse effects.
8.4. Analysis.
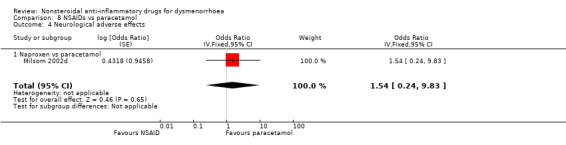
Comparison 8 NSAIDs vs paracetamol, Outcome 4 Neurological adverse effects.
Effect estimates were as follows:
All adverse effects: ibuprofen versus paracetamol: OR 0.85, 95% CI 0.31 to 2.34, one study (Analysis 8.2).
Gastrointestinal adverse effects: naproxen versus paracetamol: OR 1.00, 95% CI 0.06 to 16.62, one study (Analysis 8.3).
Neurological adverse effects: naproxen versus paracetamol: OR 1.54, 95% CI 0.24 to 9.83, one study (Analysis 8.4).
8.3. Analysis.
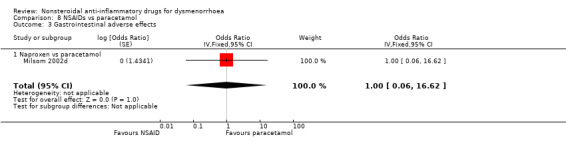
Comparison 8 NSAIDs vs paracetamol, Outcome 3 Gastrointestinal adverse effects.
Requirement for additional medication
1. NSAIDs versus placebo
Eighteen studies were suitable for meta‐analysis for this outcome. These studies analysed data for 1283 women, 702 in cross‐over studies and 581 in parallel‐group studies.They compared the following NSAIDs versus placebo: naproxen (11 studies), ibuprofen (three studies), fenoprofen, celecoxib (two studies each), aspirin, diclofenac, piroxicam and mefenamic acid (one study each). Among individual NSAIDs, there was evidence (versus placebo) favouring naproxen (OR 0.37, 95% CI 0.29 to 0.45, 11 studies, I2 = 48%,), ibuprofen (OR 0.21, 95% CI 0.11 to 0.40, three studies, I2 = 75%), celecoxib (OR 0.67, 95% CI 0.47 to 0.95, two studies, I2 = 21%), mefenamic acid (OR 0.48, 95% CI 0.25 to 0.92, two studies, I2 = 0%) and diclofenac (OR 0.06, 95% CI 0.05 to 0.08, one study, 24 women) (Analysis 1.12). There was no evidence of a difference between other individual NSAIDs and placebo.
1.12. Analysis.
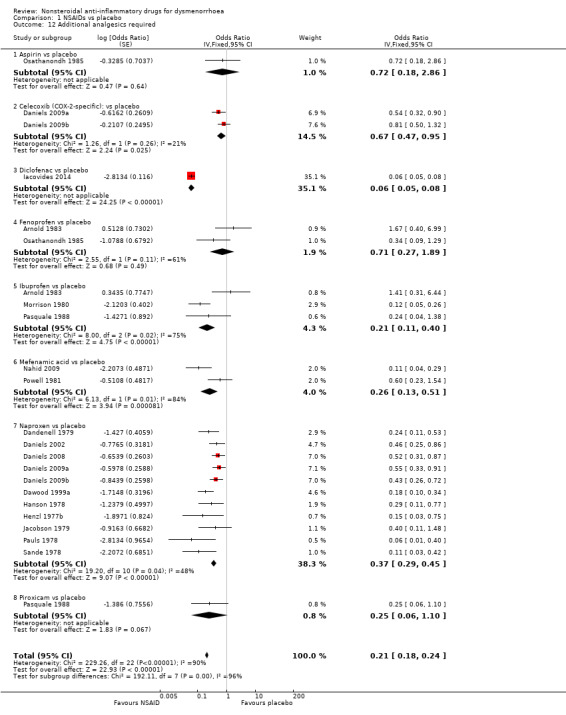
Comparison 1 NSAIDs vs placebo, Outcome 12 Additional analgesics required.
When we pooled data for this outcome, there was high heterogeneity (I2 = 98%). This appeared to relate mainly to a small study in which there were no events in the NSAID (diclofenac) arm (Iacovides 2014). When we omitted this study from analysis, pooling of the data showed a lower rate of requirement for additional medication in the women in the NSAIDs group, and heterogeneity was reduced (OR 0.42, 95% CI 0.36 to 0.50, 17 studies, I2 = 55%). The placebo groups in two cross‐over studies contributed twice to the pooled analysis but exclusion of these studies did not materially affect the results (Daniels 2009a; Daniels 2009b).
2) NSAIDs versus NSAIDs
Seven studies reported data suitable for meta‐analysis for this outcome. Only two compared the same two NSAIDs (Daniels 2009a; Daniels 2009b). They analysed data for 805 women, 458 in cross‐over studies and 347 in parallel‐group studies. They made the following comparisons: aspirin versus fenoprofen, ibuprofen versus piroxicam, ibuprofen versus fenoprofen, ibuprofen verus etoricoxib, naproxen versus celecoxib (two studies) and naproxen versus flurbiprofen. There was no evidence of a difference between any of the NSAIDs compared (Analysis 2.5; Analysis 4.4; Analysis 7.9).
2.5. Analysis.
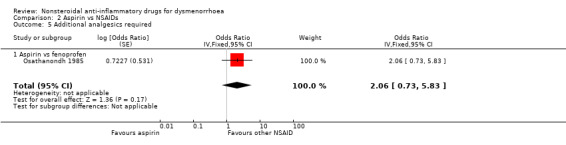
Comparison 2 Aspirin vs NSAIDs, Outcome 5 Additional analgesics required.
4.4. Analysis.
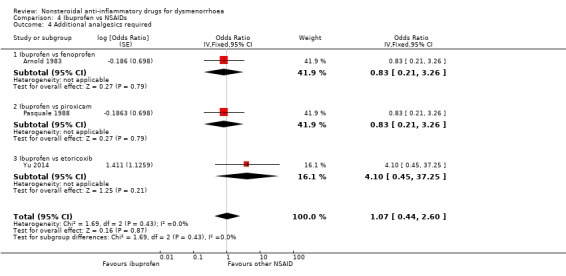
Comparison 4 Ibuprofen vs NSAIDs, Outcome 4 Additional analgesics required.
7.9. Analysis.
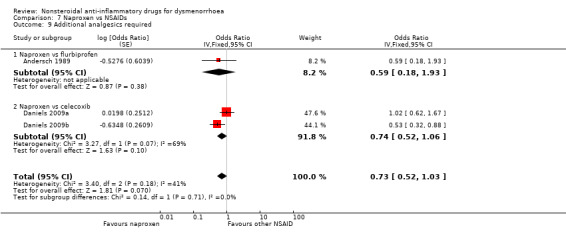
Comparison 7 Naproxen vs NSAIDs, Outcome 9 Additional analgesics required.
3) NSAIDs versus paracetamol
No data were available.
Interference with daily activities
1) NSAIDs versus placebo
Five studies were suitable for meta‐analysis for this outcome. These studies analysed data for 306 women, 90 in cross‐over studies and 216 in parallel‐group studies. They compared the following NSAIDs versus placebo: naproxen (three studies), aspirin, fenoprofen and ibuprofen (one study each). Among individual NSAIDs, there was a difference favouring the following NSAIDs over placebo: naproxen (OR 0.45, 95% CI 0.26 to 0.79, three studies, I2 = 0%), fenoprofen (OR 0.21, 95% CI 0.05 to 0.90) and ibuprofen (OR 0.13, 95% CI 0.05 to 0.32). No evidence of a difference was found between aspirin and placebo. When we pooled all data women in the NSAIDs group were less likely to report interference with daily activities than women in the placebo group (OR 0.32, 95% CI 0.21 to 0.49, five studies, I2 = 27%) (Analysis 1.13).
1.13. Analysis.
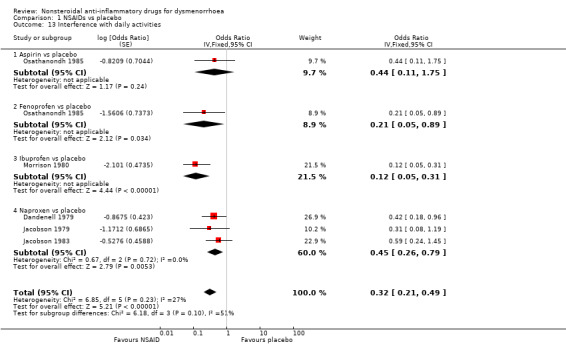
Comparison 1 NSAIDs vs placebo, Outcome 13 Interference with daily activities.
2) NSAIDs versus NSAIDs
Four studies were suitable for meta‐analysis for this outcome. These studies analysed data for 272 women, 187 in cross‐over studies and 85 in parallel‐group studies. They compared the following NSAIDs: naproxen versus flurbiprofen and ibuprofen, aspirin versus fenoprofen, and mefenamic acid versus tolfenamic acid. Women were less likely to report interference with daily activities when taking naproxen than when taking flurbiprofen (OR 0.33, 95% CI 0.12 to 0.91). No evidence of a difference was found between other individual NSAIDs for this outcome. (Analysis 2.6; Analysis 5.4; Analysis 7.10)
2.6. Analysis.
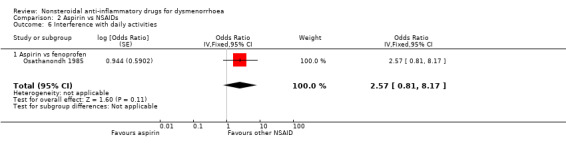
Comparison 2 Aspirin vs NSAIDs, Outcome 6 Interference with daily activities.
5.4. Analysis.
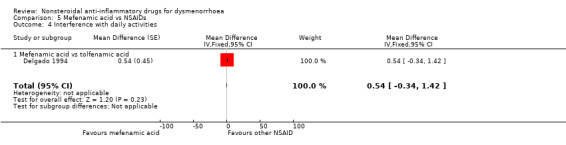
Comparison 5 Mefenamic acid vs NSAIDs, Outcome 4 Interference with daily activities.
7.10. Analysis.
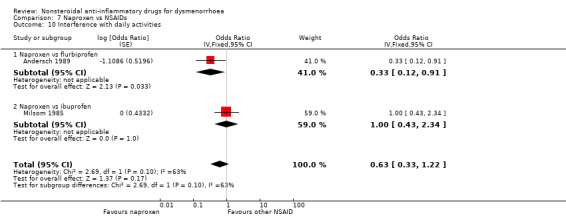
Comparison 7 Naproxen vs NSAIDs, Outcome 10 Interference with daily activities.
3) NSAIDs versus paracetamol
No data were available for this comparison.
Absence from work or school
1) NSAIDs versus placebo (five studies)
Four studies, all parallel‐group, were suitable for meta‐analysis for this outcome. These studies analysed data for 235 women. One compared diclofenac versus placebo and the other three compared naproxen versus placebo. There was less absenteeism from work or school among women were taking diclofenac (OR 0.07, 95% CI 0.01 to 0.40) or naproxen (OR 0.20, 95% CI 0.11 to 0.38, three studies, I2 = 36%) than in the placebo groups. When we pooled the results for the two comparisons, absenteeism was less likely in the NSAIDs group (OR 0.18, 95% CI 0.10 to 0.32, four studies, I2 = 32%) (Analysis 1.14). One cross‐over trial provided data on this outcome, comparing indomethacin versus placebo. The results favoured indomethacin, but the statistical significance of this finding was not reported (see Table 6).
1.14. Analysis.
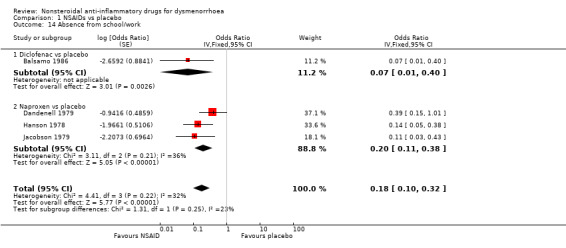
Comparison 1 NSAIDs vs placebo, Outcome 14 Absence from school/work.
4. Absence from work/school: NSAIDs versus placebo (per cycle data).
| Comparison | Study ID | No of women | Outcome measure | NSAID | Placebo | Significance |
| Piroxicam versus placebo | Akinluyi 1987 | 60 | No of cycles in which women needed days off work | 6/80 | 54/80 | Not reported |
NSAID = nonsteroidal anti‐inflammatory drug
2) NSAIDs versus NSAIDs (two studies)
Two studies, both cross‐over, were suitable for meta‐analysis for this outcome. These studies analysed data for 114 women. They compared naproxen versus flurbiprofen and versus ibuprofen. No evidence of a difference was found between naproxen and individual NSAIDs for this outcome, nor was there any evidence of a difference between naproxen versus the other NSAIDs when we pooled the data. (Analysis 7.11)
7.11. Analysis.
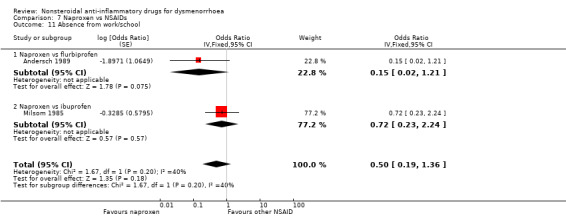
Comparison 7 Naproxen vs NSAIDs, Outcome 11 Absence from work/school.
3) NSAIDs versus paracetamol
No data were available for this comparison.
Heterogeneity
Although the direction of effect in the included studies consistently favoured NSAIDs, there was substantial heterogeneity (P value < 0.1, I2 > 50%) for some of the analyses, notably when the following NSAIDs were compared with placebo for dichotomous measures of pain relief: piroxicam (I2 = 69%), mefenamic acid (I2 = 61%), ibuprofen (I2 = 60%) and naproxen (I2 = 52%). The exclusion of two studies that reported no or negligible placebo effect, Akinluyi 1987 and Morrison 1980, resulted in marked decreases in heterogeneity and lower effect measures, as follows: piroxicam (OR 5.81, 95% CI 3.15 to 10.72, two studies, I2 = 40%), ibuprofen (OR 3.76, 95% CI 2.42 to 5.86, four studies, I2 = 0%). When we combined all NSAIDs for this outcome the I2 value was 53%.
There was also substantial heterogeneity for the outcome of additional analgesics required, in comparisons of fenoprofen and ibuprofen versus placebo (I2 = 61%, I2 = 75% respectively), and in comparisons of naproxen versus celecoxib (I2 = 69%). In the former case heterogeneity was attributable to a single study in which the requirement for additional analgesics was lower in the placebo group (Arnold 1983), but there was no obvious explanation for the heterogeneity in the second instance, and both studies used a similar protocol.
Use of a random‐effects model did not materially affect the results.
Most other analyses were relatively homogeneous.
Funnel plot
Visual scanning of a funnel plot (Figure 6) for the outcome with most data (NSAIDs versus placebo, dichotomous data) suggested a mild trend towards publication bias, with smaller negative studies less likely to be included in the review.
6.
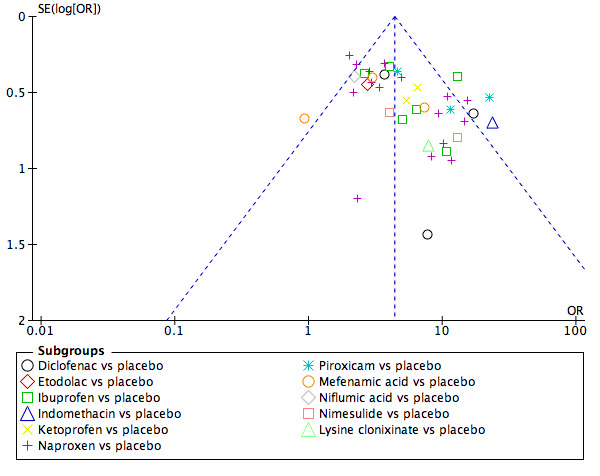
Funnel plot of comparison: 1 NSAIDs vs placebo, outcome: 1.1 Pain relief dichotomous data.
Sensitivity analyses
We carried out the pre‐specified sensitivity analyses as follows:
1. Exclusion of studies that did not clearly describe adequate procedures for allocation concealment and blinding
There were insufficient studies to conduct this planned sensitivity analysis, as only nine studies in the review clearly described adequate procedures for allocation concealment, of which only six adequately described double‐blinding, and these studies reported the same comparison in only two cases.
2. Exclusion of studies with more than 10% of data missing for primary outcomes
We excluded from Analysis 1.1 nine studies that did not include at least 90% of randomised women in analysis (Budoff 1979; Daniels 2002; Daniels 2008; Dawood 2007; Gleeson 1983; Hanson 1978; Henzl 1977b; Jacobson 1979; Mehlisch 1990; Nahid 2009). This resulted in an odds ratio for pain relief comparing all NSAIDs versus placebo of 4.91 (95% CI 4.10 to 5.87, 26 studies, I2 = 52%).
3. Exclusion of studies in which cross‐over data were analysed as if they derived from parallel‐group studies
We excluded from Analysis 1.1 20 studies in which cross‐over data were analysed in the review as if they derived from parallel‐group studies (Akinluyi 1987; Bitner 2004; Budoff 1979; Daniels 2002; Daniels 2008; Dawood 1999a; Dawood 1999b; De Souza 1991; Di Girolamo 1999; Gleeson 1983; Hamann 1980; Jacobson 1983; Legris 1997; Marchini 1995; Mehlisch 1990; Mehlisch 1997; Milsom 2002d; Morrison 1980; Rondel 1984; Wilhelmsson 1985b). This resulted in an odds ratio for pain relief (all NSAIDs versus placebo) of 5.73 (95% CI 4.45 to 7.38, 19 studies, I2 = 18%). For the outcome of all adverse events (all NSAIDs versus placebo), exclusion of studies in which cross‐over data were analysed as if they derived from parallel‐group studies resulted in an OR of 1.41 (0.97 to 2.05, six studies).
Discussion
Summary of main results
Efficacy of NSAIDs
The trials included in this review suggested that nonsteroidal anti‐inflammatory drugs (NSAIDs) are very effective in providing pain relief from dysmenorrhoea. Almost all measures of effectiveness confirmed the overall superiority over placebo of all NSAIDs tested (with the exception of aspirin, about which the volume of evidence was very small). The evidence suggests that if 18% of women taking placebo achieve moderate or excellent pain relief, between 45% and 53% taking NSAIDs will do so.
When NSAIDs were compared with each other, most studies found no evidence of a difference between them. Despite the large number of included trials, only two reported data suitable for meta‐analysis comparing the same two NSAIDs, and sample sizes were generally small. Thus the review was unable to determine which NSAIDs are most effective for dysmenorrhoea nor to determine whether individual NSAIDs have similar efficacy.
Three studies compared NSAIDs with paracetamol. Pooling of these studies found NSAIDs to be more effective for pain relief than paracetamol.
Tolerability and safety of NSAIDs
As might be anticipated, NSAIDs appeared overall more likely than placebo to cause adverse effects. Mild neurological adverse effects (such as headache, drowsiness, dizziness and dryness) and gastrointestinal adverse effects (such as nausea and indigestion) were both more common in the NSAIDs group. It is important for women taking NSAIDs for dysmenorrhoea to be aware of the need to take the medications with food, even though they are administered only for short‐term use.
Among individual NSAIDs, indomethacin was more likely to cause neurological side effects than placebo, dexketoprofen was more likely to cause gastrointestinal side effects and naproxen was more likely to cause both. The findings for naproxen probably relate to the large number of studies on naproxen, compared to other NSAIDs. When NSAIDs were compared with each other, there was no evidence of any difference between them with respect to adverse effects.
The large number of NSAIDs involved in these comparisons reflects the abundance of NSAIDs available. Others have been withdrawn from the world market after doubts emerged about their safety. All the NSAIDs included in comparisons in this review are currently available, to the best of our knowledge, in various parts of the world, but at least one (nimesulide) has been the subject of a risk/benefit review after safety concerns were raised (EMEA 2002).
Traditional NSAIDs versus COX‐2‐specific inhibitors
Although we retrieved a number of studies of COX‐2 inhibitors in the search for the update of this review, we excluded several as they involved NSAIDs that have been withdrawn by the manufacturers due to safety concerns. We ultimately included only two COX‐2‐specific inhibitors in the review: celecoxib and etoricoxib. Celecoxib was less effective for pain relief than naproxen and there was no evidence of any difference between etoricoxib and naproxen. There was no evidence of any difference in tolerability between non‐selective and COX‐2‐specific inhibitors, though data were very scarce.
Overall completeness and applicability of evidence
As noted above, due to the lack of studies comparing NSAIDs head‐to‐head we were unable to determine which specific NSAID is most effective or whether individual NSAIDs have similar efficacy.
It would be useful to know whether it is possible to maintain the benefits of NSAIDs but reduce adverse effects by combining lower doses of NSAIDs with paracetamol, codeine, transcutaneous electrical nerve stimulation (TENS) or acupuncture. It would also be useful to know whether dysmenorrhoea in intrauterine contraceptive device (IUCD) users can be treated in a similar way to primary dysmenorrhoea. However, these questions lie outside the scope of the present review.
Quality of the evidence
With respect to risk of bias, most studies considered for this review were of poor calibre. We excluded nearly 50 for unclear design, lack of double‐blinding or very large numbers of withdrawals, while among the included studies very few clearly described their methods of randomisation and allocation concealment. Recently published studies appeared to be no more likely than older studies to adequately describe their methods (e.g. allocation concealment). Nearly 60% of the studies were co‐authored or financially supported by pharmaceutical company associates and it was unclear how most of the others were funded. Moreover the funnel plot suggested a mild trend towards publication bias, with smaller negative studies less likely to be included in the review.
Reporting of pain relief using a dichotomous measure or visual analogue scale (VAS) provides clinically meaningful results and facilitates meta‐analysis. The included studies used a wide variety of continuous pain scales as their primary or sole measure of effectiveness. We tried combining different continuous measures of final pain score and calculating the standardised mean difference, but findings were highly heterogeneous (I2 = 77%), suggesting that the different scales may not have been measuring the same concept. We recommend the use of standard, validated scales for pain, such as VAS.
The measurement and reporting of adverse effects was generally poor, even taking into account the challenge of distinguishing between dysmenorrhoeic symptoms and medication effects. Only 30% of the studies described the use of prospective self report forms or diaries. The rest assessed adverse effects retrospectively at follow‐up appointments, were vague about their methods or failed to systematically report adverse effects. It is important that studies report numerical results for all outcomes and not just for significant or selected findings. The available evidence suggests that if 10% of women taking placebo experience side effects, between 11% and 14% of women taking NSAIDs will do so.
As there were insufficient studies to conduct a sensitivity analysis to explore the effect of adequate allocation concealment and double‐blinding, the extent to which bias affected estimates of effect is unclear. Moreover the funnel plot suggests the possibility of publication bias, which may have inflated the positive findings for NSAIDs in this review.
Potential biases in the review process
The cross‐over design requires that each study participant receives two or more treatments in a random order, each participant thus acting as her own control. Cross‐over trials are suitable for evaluating interventions with a temporary effect in the treatment of stable, chronic conditions (Higgins 2011). This design was used by most of the included trials, and seems appropriate for exploring the use of NSAIDs for dysmenorrhoea, since dysmenorrhoea is a chronic, recurring problem and the half life of NSAIDs varies widely but is under 60 hours (Brooks 1999). In order for cross‐over trials to be given adequate weight in meta‐analysis, study authors need to report an explicit statement that they used paired statistical analysis, plus a summary effect measure and an estimate of variability for each outcome. As very few cross‐over trials in the review provided this information, most were analysed as if they used a parallel design. As a result their findings may have been under‐weighted in analysis, resulting in wider confidence intervals than would otherwise be the case. Sensitivity analysis excluding cross‐over studies that did not meet these criteria resulted in a much higher estimate of analgesic effectiveness (odds ratio (OR) 7.04 versus OR 4.44). This was probably partly due to confounding by study age, since many of the studies included in the analysis were older parallel‐group studies, which overall tended to report higher effect estimates than the more recent studies.
Agreements and disagreements with other studies or reviews
Our findings are in accordance with a systematic review of NSAIDs and hormonal contraceptives for dysmenorrhoea published in 2010 (Zahradnik 2010), which was based on a search of a single database (PubMed). It included 10 randomised controlled trials (RCTs) of NSAIDs and concluded that NSAIDs are suitable as a first‐line therapy for treating dysmenorrhoea in women without wish for contraception. This review went on to suggest that combined oral contraceptives are a more suitable option for women requiring contraception. As noted above, the use of oral contraceptives for dysmenorrhoea is the topic of another Cochrane review (Wong 2009).
Authors' conclusions
Implications for practice.
Nonsteroidal anti‐inflammatory drugs (NSAIDs) appear to be a very effective treatment for dysmenorrhoea, though women using them need to be aware of the substantial risk that they may cause adverse effects such as indigestion or drowsiness. There is insufficient evidence to indicate whether any individual NSAID is more effective than others, but it appears that NSAIDs are more effective than paracetamol. Based on only two studies that made head‐to‐head comparisons, there was no evidence that COX‐2‐specific inhibitors are more effective or more tolerable than non‐selective NSAIDs, for the treatment of dysmenorrhoea.
Implications for research.
Large numbers of women are needed for comparison in order to achieve sufficient statistical power to reveal any meaningful differences in efficacy and safety between NSAIDs for dysmenorrhoea. This can most easily be achieved by meta‐analysis. In order to facilitate this process, trial publications need to provide a detailed account of statistical methods used and present full results with summary effect measures and measures of variance. Attention to adequate reporting of trial methodology in line with the CONSORT statement, CONSORT 2001, remains a fundamental issue.
If pain is measured as a continuous outcome, use of a validated scale such as a visual analogue scale (VAS) is recommended.
Outcomes of combination therapies in comparison with NSAIDs alone would be a useful topic for a further systematic review.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2015 | New search has been performed | We included the following studies: Daniels 2009a; Daniels 2009b; Heidarifar 2014; Iacovides 2014; Nahid 2009; Salmalian 2014; Yu 2014. We also added Summary of findings tables. |
| 7 January 2015 | New citation required and conclusions have changed | There is no evidence to suggest that COX‐2 inhibitors are safer or more effective than COX‐1. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 13 August 2009 | New citation required and conclusions have changed | New finding re. comparative efficacy of NSAIDs and paracetamol. |
| 13 August 2009 | New search has been performed | Updated. We included nine additional studies (Bitner 2004; Chantler 2008; Chantler 2009; Daniels 2002; Daniels 2008; Dawood 2007; de Mello 2004; Mehlisch 2003; Morrison 1999), converted statistical analysis to inverse variance, updated the format and changed some findings. |
| 22 May 2008 | Amended | Converted to new review format. |
| 20 August 2003 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
For the 2015 update, we would like to thank Jack Wilkinson, who peer reviewed the update and provided invaluable statistical advice to facilitate interpretation of effect estimates. Many thanks to Valeria Ivanova and Owen Sinclair, who advised on the early stages, to all the people who kindly translated trials and to Will Stones, who commented on the final draft of the original review. For the 2009 update, to José GB Derraik, who kindly translated a trial, to Andy Vail for statistical advice and to Roos Derks, who checked data extraction and extracted data on the characteristics of newly included studies.
Thanks also to Marian Showell (Trials Search Co‐ordinator) for designing and running multiple searches for the 2009 and 2015 updates.
Appendices
Appendix 1. MDSG Specialised Register search strategy
Keywords CONTAINS "dysmenorrhea" or "Dysmenorrhea‐Symptoms" or "dysmenorrhoea" or "pelvic pain" or "menstrual cramps" or "menstrual pain" or "Menstruation Disorders" or "pain‐dysmenorrhea" or "pain‐pelvic" or "primary dysmenorrhea" or Title CONTAINS "dysmenorrhea" or "Dysmenorrhea‐Symptoms" or "dysmenorrhoea" or "pelvic pain" or "menstrual cramps" or "menstrual pain" or "Menstruation Disorders" or "pain‐dysmenorrhea" or "pain‐pelvic" or "primary dysmenorrhea"
AND
Keywords CONTAINS "non steroidal" or "non steroidal cytochrome inhibitor" or "NSAID" or "NSAIDs" or "naproxen" or "Naproxen Sodium" or "Ibuprofen" or "Flurbiprofen" or "Meclofenamic Acid" or "Meclofenamate"or "diclofenac"or "acetylsalicylic" or "acetly salicylic acid"or "aspirin"or "indomethacin"or "indometacin"or "Ketoprofen"or "Piroxicam"or "Flufenamic Acid"or "nimesulide"or "COX‐2 inhibitors"or "cyclooxygenase"or "etoricoxib"or "lumiracoxib"or "parecoxib sodium"or "rofecoxib"or "valdecoxib" or Title CONTAINS"non steroidal" or "non steroidal cytochrome inhibitor" or "NSAID" or "NSAIDs" or "naproxen" or "Naproxen Sodium" or "Ibuprofen" or "Flurbiprofen" or "Meclofenamic Acid" or "Meclofenamate"or "diclofenac"or "acetylsalicylic" or "acetly salicylic acid"or "aspirin"or "indomethacin"or "indometacin"or "Ketoprofen"or "Piroxicam"or "Flufenamic Acid"or "nimesulide"or "COX‐2 inhibitors"or "cyclooxygenase"or "etoricoxib"or "lumiracoxib"or "parecoxib sodium"or "rofecoxib"or "valdecoxib"
Appendix 2. CENTRAL search strategy
Searches conducted 26 November 2013, 7 January 2015 (November 2014 issue)
1 exp Dysmenorrhea/ (351) 2 (Dysmenorrh$ or primary dymenorrh$).tw. (767) 3 (menstrual adj5 pain).tw. (198) 4 (painful adj5 mens$).tw. (20) 5 pelvic pain.tw. (507) 6 (menstrual adj5 cramp$).tw. (24) 7 or/1‐6 (1286) 8 exp anti‐inflammatory agents, non‐steroidal/ or exp cyclooxygenase inhibitors/ (13419) 9 (non‐steroidal adj5 anti‐inflammator$).tw. (1310) 10 (non$steroidal adj5 anti$inflammator$).tw. (546) 11 nsaid$.tw. (2242) 12 exp Cyclooxygenase 2/ (291) 13 cyclooxygenase$.tw. (1090) 14 Cox 2.tw. (709) 15 (rofecoxib$ or valdecoxib$).tw. (451) 16 sulphonanilide$.tw. (0) 17 (etoricoxib$ or lumiracoxib$ or parecoxib$).tw. (386) 18 (flufenamic or nimesulide).tw. (315) 19 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine or naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin or cyclooxygenase inhibitors).tw. (17662) 20 or/8‐19 (23406) 21 7 and 20 (325)
Appendix 3. MEDLINE search strategy
Searches conducted 26 November 2013, 7 January 2015
1 exp Dysmenorrhea/ (3208) 2 (Dysmenorrh$ or primary dymenorrh$).tw. (4340) 3 (menstrual adj5 pain).tw. (885) 4 (painful adj5 mens$).tw. (195) 5 pelvic pain.tw. (6256) 6 (menstrual adj5 cramp$).tw. (149) 7 or/1‐6 (11474) 8 exp anti‐inflammatory agents, non‐steroidal/ or exp cyclooxygenase inhibitors/ (163716) 9 (non‐steroidal adj5 anti‐inflammator$).tw. (12194) 10 (non$steroidal adj5 anti$inflammator$).tw. (4132) 11 nsaid$.tw. (19222) 12 exp Cyclooxygenase 2/ (19058) 13 cyclooxygenase$.tw. (35828) 14 Cox 2.tw. (23270) 15 (rofecoxib$ or valdecoxib$).tw. (2319) 16 sulphonanilide$.tw. (5) 17 (etoricoxib$ or lumiracoxib$ or parecoxib$).tw. (1008) 18 (flufenamic or nimesulide).tw. (2342) 19 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine or naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin or cyclooxygenase inhibitors).tw. (121908) 20 or/8‐19 (235466) 21 randomized controlled trial.pt. (406074) 22 controlled clinical trial.pt. (91187) 23 randomized.ab. (325284) 24 randomised.ab. (65317) 25 placebo.tw. (170486) 26 clinical trials as topic.sh. (177593) 27 randomly.ab. (232476) 28 trial.ti. (141619) 29 (crossover or cross‐over or cross over).tw. (64694) 30 or/21‐29 (1020237) 31 exp animals/ not humans.sh. (4120563) 32 30 not 31 (941701) 33 7 and 20 and 32 (342)
Appendix 4. EMBASE search strategy
Searches conducted 26 November 2013, 7 January 2015
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp Dysmenorrhea/ (8163) 2 Dysmenorrh$.mp. or primary dymenorrh$.tw. (9226) 3 (menstrual adj5 pain).tw. (1085) 4 (painful adj5 mens$).tw. (207) 5 pelvic pain.tw. (9046) 6 (menstrual adj5 cramp$).tw. (168) 7 or/1‐6 (17645) 8 exp anti‐inflammatory agents, non‐steroidal/ or exp cyclooxygenase inhibitors/ (455220) 9 (non‐steroidal adj5 anti‐inflammator$).tw. (15296) 10 (non$steroidal adj5 anti$inflammator$).tw. (4822) 11 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine or naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin).mp. or cyclooxygenase inhibitors.tw. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (264823) 12 flufenamic.mp. or nimesulide.tw. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] (4293) 13 nsaid$.tw. (28723) 14 exp Cyclooxygenase 2/ (29849) 15 exp cyclooxygenase 2 inhibitor/ or exp celecoxib/ or exp cimicoxib/ or exp deracoxib/ or exp etoricoxib/ or exp flosulide/ or exp lumiracoxib/ or exp meloxicam/ or exp nimesulide/ or exp parecoxib/ or exp rofecoxib/ or exp tilmacoxib/ or exp valdecoxib/ (40102) 16 cyclooxygenase$.tw. (38938) 17 Cox 2.tw. (28211) 18 sulphonanilide$.tw. (7) 19 (celecoxib$ or cimicoxib$ or deracoxib$ or etoricoxib$ or flosulide$ or lumiracoxib$ or meloxicam$ or nimesulide$ or parecoxib$ or rofecoxib$ or tilmacoxib$ or valdecoxib$).tw. (12299) 20 or/8‐19 (530699) 21 7 and 20 (2266) 22 Controlled study/ or randomized controlled trial/ (4536136) 23 double blind procedure/ (116757) 24 single blind procedure/ (19214) 25 crossover procedure/ (40920) 26 drug comparison/ (81319) 27 placebo/ (249638) 28 random$.ti,ab,hw,tn,mf. (1059364) 29 latin square.ti,ab,hw,tn,mf. (3592) 30 crossover.ti,ab,hw,tn,mf. (66186) 31 cross‐over.ti,ab,hw,tn,mf. (21801) 32 placebo$.ti,ab,hw,tn,mf. (327561) 33 ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf. (209236) 34 (comparative adj5 trial$).ti,ab,hw,tn,mf. (55495) 35 (clinical adj5 trial$).ti,ab,hw,tn,mf. (1108542) 36 or/22‐35 (5724634) 37 nonhuman/ (4421684) 38 animal/ not (human/ and animal/) (1195728) 39 or/37‐38 (5603117) 40 36 not 39 (3555211) 41 21 and 40 (964)
Appendix 5. PsycINFO search strategy
Searches conducted 26 November 2013, 7 January 2015
1 exp Dysmenorrhea/ (168) 2 Dysmenorrh?ea.tw. (303) 3 (menstrual adj5 pain).tw. (163) 4 (painful adj5 mens$).tw. (27) 5 (menstrual adj5 cramp$).tw. (20) 6 or/1‐5 (465) 7 exp Anti Inflammatory Drugs/ (4111) 8 (nonsteroidal adj5 anti‐inflammator$).tw. (501) 9 (non steroidal adj5 anti‐inflammator$).tw. (360) 10 (ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac or diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid or ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid or mesalamine or naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin or cyclooxygenase inhibitors).tw. (2380) 11 nsaid$.tw. (621) 12 or/7‐11 (6251) 13 6 and 12 (15)
Appendix 6. CINAHL search strategy
Database: CINAHL ‐ Cumulative Index to Nursing & Allied Health Literature <1982 to January Week 1 2015>
CINAHL search strategy JM522 14.01.14
| # | Query | Results |
| S33 | S18 AND S32 | 88 |
| S32 | S19 OR S20 or S21 or S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 | Display |
| S31 | TX allocat* random* | Display |
| S30 | (MH "Quantitative Studies") | Display |
| S29 | (MH "Placebos") | Display |
| S28 | TX placebo* | Display |
| S27 | TX random* allocat* | Display |
| S26 | (MH "Random Assignment") | Display |
| S25 | TX randomi* control* trial* | Display |
| S24 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | Display |
| S23 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | Display |
| S22 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | Display |
| S21 | TX clinic* n1 trial* | Display |
| S20 | PT Clinical trial | Display |
| S19 | (MH "Clinical Trials+") | Display |
| S18 | S5 AND S17 | 155 |
| S17 | S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 | 25,032 |
| S16 | TX flufenamic or nimesulide | 84 |
| S15 | TX mesalamine or naproxen or niflumic acid or oxyphenbutazone or pentosan sulfuric polyester or phenylbutazone or piroxicam or prenazone or salicylates or sodium salicylate or sulfasalazine or sulindac or suprofen or tolmetin | 2,063 |
| S14 | TX ibuprofen or indomethacin or ketoprofen or ketorolac or ketorolac tromethamine or meclofenamic acid or mefenamic acid | 3,581 |
| S13 | TX diflunisal or dipyrone or epirizole or etodolac or fenoprofen or flurbiprofen or glycyrrhizic acid | 218 |
| S12 | TX ampyrone or antipyrine or apazone or aspirin or bufexamac or clofazimine or clonixin or curcumin or dapsone or diclofenac | 10,971 |
| S11 | TX nsaid* | 3,036 |
| S10 | TX non‐steroidal anti‐inflammator* | 913 |
| S9 | TX Cox‐2 Inhibitor* | 3,179 |
| S8 | TX cyclooxygenase inhibitor* | 719 |
| S7 | (MM "Cox‐2 Inhibitors") | 1,734 |
| S6 | (MH "Antiinflammatory Agents, Non‐Steroidal+") | 19,979 |
| S5 | S1 OR S2 OR S3 OR S4 | 1,215 |
| S4 | TX menstrual cramp* | 59 |
| S3 | TX menstrua* pain* | 292 |
| S2 | TX Dysmenorrh* | 1,045 |
| S1 | (MM "Dysmenorrhea") | 459 |
Appendix 7. Data extraction form
Methods Allocation Randomisation Blinding Design Number randomised Number analysed Number withdrew and reasons ITT Funding Notes Women Country No of centres Location Participant source Age Sex Inclusion criteria Exclusion criteria
Interventions Treatment Control Duration
Outcomes Primary Secondary Notes
Data and analyses
Comparison 1. NSAIDs vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief dichotomous data | 35 | Odds Ratio (Fixed, 95% CI) | 4.37 [3.76, 5.09] | |
| 1.1 Diclofenac vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 5.68 [3.03, 10.67] | |
| 1.2 Etodolac vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 2.75 [1.14, 6.63] | |
| 1.3 Ibuprofen vs placebo | 6 | Odds Ratio (Fixed, 95% CI) | 5.22 [3.62, 7.52] | |
| 1.4 Indomethacin vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 23.59 [6.01, 92.58] | |
| 1.5 Ketoprofen vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 6.02 [2.98, 12.14] | |
| 1.6 Naproxen vs placebo | 16 | Odds Ratio (Fixed, 95% CI) | 3.67 [2.94, 4.58] | |
| 1.7 Piroxicam vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 8.21 [4.85, 13.91] | |
| 1.8 Mefenamic acid vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 2.98 [1.66, 5.37] | |
| 1.9 Niflumic acid vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 2.21 [1.01, 4.83] | |
| 1.10 Nimesulide vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 6.31 [2.39, 16.68] | |
| 1.11 Lysine clonixinate vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 7.86 [1.49, 41.38] | |
| 2 Pain relief continuous data: % improvement in VAS pain score (scale 1 to 100) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac vs placebo | 2 | Mean Difference (Fixed, 95% CI) | 65.96 [55.70, 76.22] | |
| 2.2 Meloxicam vs placebo | 1 | Mean Difference (Fixed, 95% CI) | 34.0 [15.88, 52.12] | |
| 3 Pain relief continuous data: total pain relief score difference | 4 | Mean Difference (Fixed, 95% CI) | 6.24 [4.69, 7.78] | |
| 3.1 Celecoxib (COX‐2‐specific): vs placebo TOPAR difference | 2 | Mean Difference (Fixed, 95% CI) | 5.46 [2.29, 8.63] | |
| 3.2 Etoricoxib (COX‐2‐specific): vs placebo TOPAR difference (time‐weighted scale) | 1 | Mean Difference (Fixed, 95% CI) | 7.4 [3.17, 11.63] | |
| 3.3 Naproxen vs placebo TOPAR difference (time‐weighted scale) | 4 | Mean Difference (Fixed, 95% CI) | 6.28 [4.34, 8.22] | |
| 4 Pain relief continuous data: final pain relief score difference (repeated 0 to 3 scale) | 2 | Mean Difference (Fixed, 95% CI) | 4.83 [3.61, 6.06] | |
| 4.1 Flufenamic acid vs placebo | 1 | Mean Difference (Fixed, 95% CI) | 4.91 [3.50, 6.32] | |
| 4.2 Indomethacin vs placebo | 1 | Mean Difference (Fixed, 95% CI) | 4.6 [2.12, 7.08] | |
| 5 Pain relief continuous data: final pain relief score difference (one‐off scales) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 Indomethacin vs placebo (0 to 18 scale) | 1 | Mean Difference (Fixed, 95% CI) | 11.2 [7.24, 15.16] | |
| 5.2 Naproxen vs placebo (0 to 40 scale) | 1 | Mean Difference (Fixed, 95% CI) | 15.30 [5.64, 24.96] | |
| 6 Pain intensity continuous data: mean difference final scores (5‐point scale) | 1 | Mean Difference (Fixed, 95% CI) | ‐0.33 [‐0.84, 0.18] | |
| 6.1 Aspirin vs placebo | 1 | Mean Difference (Fixed, 95% CI) | 0.0 [‐0.72, 0.72] | |
| 6.2 Fenoprofen vs placebo | 1 | Mean Difference (Fixed, 95% CI) | ‐0.65 [‐1.37, 0.07] | |
| 7 Pain intensity continuous data: mean difference final scores (4‐point scale) | 1 | Mean Difference (Fixed, 95% CI) | ‐1.7 [‐3.37, ‐0.03] | |
| 7.1 Mefenamic acid vs placebo | 1 | Mean Difference (Fixed, 95% CI) | ‐1.7 [‐3.37, ‐0.03] | |
| 8 Pain relief descriptive data | Other data | No numeric data | ||
| 8.2 Naproxen vs placebo | Other data | No numeric data | ||
| 9 All adverse effects | 25 | Odds Ratio (Fixed, 95% CI) | 1.29 [1.11, 1.51] | |
| 9.1 Aceclofenac vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.63 [0.53, 4.99] | |
| 9.2 Aspirin vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.93 [0.49, 7.61] | |
| 9.3 Celecoxib (COX‐2‐specific): vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 1.05 [0.72, 1.54] | |
| 9.4 Dexketoprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.57 [0.47, 5.24] | |
| 9.5 Diclofenac vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 2.00 [0.91, 4.39] | |
| 9.6 Etodolac vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.73 [0.41, 7.34] | |
| 9.7 Etoricoxib (COX‐2‐specific): vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.82 [0.81, 4.09] | |
| 9.8 Fenoprofen vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 1.11 [0.58, 2.10] | |
| 9.9 Ibuprofen vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 1.42 [0.71, 2.85] | |
| 9.10 Ketoprofen vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 1.14 [0.59, 2.18] | |
| 9.11 Naproxen vs placebo | 10 | Odds Ratio (Fixed, 95% CI) | 1.28 [1.00, 1.65] | |
| 9.12 Niflumic acid vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 2.53 [0.67, 9.59] | |
| 9.13 Nimesulide vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 7.39 [0.15, 368.14] | |
| 9.14 Piroxicam vs placebo | 5 | Odds Ratio (Fixed, 95% CI) | 1.19 [0.72, 1.97] | |
| 10 Gastrointestinal adverse effects | 14 | Odds Ratio (Fixed, 95% CI) | 1.58 [1.12, 2.23] | |
| 10.1 Aspirin vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 1.41 [0.55, 3.60] | |
| 10.2 Dexketoprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 9.08 [1.96, 42.04] | |
| 10.3 Fenoprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.16 [0.22, 6.12] | |
| 10.4 Indomethacin vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 1.17 [0.54, 2.54] | |
| 10.5 Ketoprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 8.06 [0.50, 130.48] | |
| 10.6 Mefenamic acid vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 1.57 [0.84, 2.96] | |
| 10.7 Naproxen vs placebo | 4 | Odds Ratio (Fixed, 95% CI) | 2.30 [1.02, 5.19] | |
| 10.8 Piroxicam vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 0.46 [0.13, 1.65] | |
| 11 Neurological adverse effects | 7 | Odds Ratio (Fixed, 95% CI) | 2.74 [1.66, 4.53] | |
| 11.1 Aspirin vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 3.66 [0.75, 17.78] | |
| 11.2 Fenoprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.60 [0.22, 11.54] | |
| 11.3 Indomethacin vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 4.96 [1.87, 13.11] | |
| 11.4 Naproxen vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 2.20 [1.11, 4.35] | |
| 11.5 Piroxicam vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.06, 16.42] | |
| 12 Additional analgesics required | 18 | Odds Ratio (Fixed, 95% CI) | 0.21 [0.18, 0.24] | |
| 12.1 Aspirin vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.72 [0.18, 2.86] | |
| 12.2 Celecoxib (COX‐2‐specific): vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 0.67 [0.47, 0.95] | |
| 12.3 Diclofenac vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.06 [0.05, 0.08] | |
| 12.4 Fenoprofen vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 0.71 [0.27, 1.89] | |
| 12.5 Ibuprofen vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 0.21 [0.11, 0.40] | |
| 12.6 Mefenamic acid vs placebo | 2 | Odds Ratio (Fixed, 95% CI) | 0.26 [0.13, 0.51] | |
| 12.7 Naproxen vs placebo | 11 | Odds Ratio (Fixed, 95% CI) | 0.37 [0.29, 0.45] | |
| 12.8 Piroxicam vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.25 [0.06, 1.10] | |
| 13 Interference with daily activities | 5 | Odds Ratio (Fixed, 95% CI) | 0.32 [0.21, 0.49] | |
| 13.1 Aspirin vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.44 [0.11, 1.75] | |
| 13.2 Fenoprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.21 [0.05, 0.89] | |
| 13.3 Ibuprofen vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.12 [0.05, 0.31] | |
| 13.4 Naproxen vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 0.45 [0.26, 0.79] | |
| 14 Absence from school/work | 4 | Odds Ratio (Fixed, 95% CI) | 0.18 [0.10, 0.32] | |
| 14.1 Diclofenac vs placebo | 1 | Odds Ratio (Fixed, 95% CI) | 0.07 [0.01, 0.40] | |
| 14.2 Naproxen vs placebo | 3 | Odds Ratio (Fixed, 95% CI) | 0.20 [0.11, 0.38] |
1.8. Analysis.
Comparison 1 NSAIDs vs placebo, Outcome 8 Pain relief descriptive data.
| Pain relief descriptive data | ||||
|---|---|---|---|---|
| Study | Comparison | Outcome measure | Design (number analysed) | Result |
| Naproxen vs placebo | ||||
| Mehlisch 1997 | Naproxen 550 mg vs placebo | Global assessment | Cross‐over (n = 57) | Naproxen superior P value = 0.001 |
Comparison 2. Aspirin vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain intensity continuous data final pain relief score difference (0‐ to 3‐point scale) | 1 | Mean Difference (Fixed, 95% CI) | 0.65 [0.10, 1.20] | |
| 1.1 Aspirin vs fenoprofen | 1 | Mean Difference (Fixed, 95% CI) | 0.65 [0.10, 1.20] | |
| 2 All adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 1.46 [0.52, 4.08] | |
| 2.1 Aspirin vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.46 [0.52, 4.08] | |
| 3 Gastrointestinal adverse effects | 2 | Odds Ratio (Fixed, 95% CI) | 2.05 [0.84, 4.96] | |
| 3.1 Aspirin vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.77 [0.53, 5.93] | |
| 3.2 Aspirin vs indomethacin | 1 | Odds Ratio (Fixed, 95% CI) | 2.42 [0.66, 8.91] | |
| 4 Neurological adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 3.20 [0.92, 11.11] | |
| 4.1 Aspirin vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 3.20 [0.92, 11.11] | |
| 5 Additional analgesics required | 1 | Odds Ratio (Fixed, 95% CI) | 2.06 [0.73, 5.83] | |
| 5.1 Aspirin vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 2.06 [0.73, 5.83] | |
| 6 Interference with daily activities | 1 | Odds Ratio (Fixed, 95% CI) | 2.57 [0.81, 8.17] | |
| 6.1 Aspirin vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 2.57 [0.81, 8.17] |
Comparison 3. Etodolac vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All adverse events | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.06, 16.70] | |
| 1.1 Etodolac vs piroxicam | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.06, 16.70] |
Comparison 4. Ibuprofen vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief: dichotomous outcome | 2 | Odds Ratio (Fixed, 95% CI) | 0.94 [0.55, 1.61] | |
| 1.1 Ibuprofen vs piroxicam | 1 | Odds Ratio (Fixed, 95% CI) | 0.97 [0.53, 1.77] | |
| 1.2 Ibuprofen vs lysine clonixinate | 1 | Odds Ratio (Fixed, 95% CI) | 0.84 [0.26, 2.69] | |
| 2 Pain relief continuous data: final pain relief score difference (time‐weighted TOPAR‐6 scale) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 Ibuprofen vs etoricoxib TOPAR 6 difference (time‐weighted scale) | 1 | Mean Difference (Fixed, 95% CI) | ‐0.89 [‐1.74, ‐0.04] | |
| 3 All adverse effects | 2 | Odds Ratio (Fixed, 95% CI) | 1.38 [0.68, 2.80] | |
| 3.1 Ibuprofen vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.51 [0.72, 3.17] | |
| 3.2 Ibuprofen vs etoricoxib | 1 | Odds Ratio (Fixed, 95% CI) | 0.50 [0.04, 5.88] | |
| 4 Additional analgesics required | 3 | Odds Ratio (Fixed, 95% CI) | 1.07 [0.44, 2.60] | |
| 4.1 Ibuprofen vs fenoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.83 [0.21, 3.26] | |
| 4.2 Ibuprofen vs piroxicam | 1 | Odds Ratio (Fixed, 95% CI) | 0.83 [0.21, 3.26] | |
| 4.3 Ibuprofen vs etoricoxib | 1 | Odds Ratio (Fixed, 95% CI) | 4.10 [0.45, 37.25] |
Comparison 5. Mefenamic acid vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief: dichotomous data | 1 | Odds Ratio (Fixed, 95% CI) | 0.68 [0.32, 1.44] | |
| 1.1 Mefenamic acid vs meloxicam | 1 | Odds Ratio (Fixed, 95% CI) | 0.68 [0.32, 1.44] | |
| 2 Pain relief (VAS) | 1 | Mean Difference (Fixed, 95% CI) | 0.23 [‐0.69, 1.15] | |
| 2.1 Mefenamic acid vs tolfenamic acid | 1 | Mean Difference (Fixed, 95% CI) | 0.23 [‐0.69, 1.15] | |
| 3 All adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 1.26 [0.54, 2.96] | |
| 3.1 Mefenamic acid vs tolfenamic acid | 1 | Odds Ratio (Fixed, 95% CI) | 1.26 [0.54, 2.96] | |
| 4 Interference with daily activities | 1 | Mean Difference (Fixed, 95% CI) | 0.54 [‐0.34, 1.42] | |
| 4.1 Mefenamic acid vs tolfenamic acid | 1 | Mean Difference (Fixed, 95% CI) | 0.54 [‐0.34, 1.42] |
Comparison 6. Diclofenac vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief dichotomous data | 2 | Odds Ratio (Fixed, 95% CI) | 0.88 [0.57, 1.36] | |
| 1.1 Diclofenac vs ibuprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.17 [0.61, 2.23] | |
| 1.2 Diclofenac vs nimesulide | 1 | Odds Ratio (Fixed, 95% CI) | 0.69 [0.38, 1.25] | |
| 2 Pain relief: mean difference VAS reduction | 1 | Mean Difference (Fixed, 95% CI) | 34.0 [15.88, 52.12] | |
| 2.1 Diclofenac vs meloxicam | 1 | Mean Difference (Fixed, 95% CI) | 34.0 [15.88, 52.12] | |
| 3 All adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 3.83 [0.76, 19.28] | |
| 3.1 Diclofenac vs ibuprofen | 1 | Odds Ratio (Fixed, 95% CI) | 3.83 [0.76, 19.28] | |
| 4 Gastrointestinal adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 2.34 [0.93, 5.87] | |
| 4.1 Diclofenac vs nimesulide | 1 | Odds Ratio (Fixed, 95% CI) | 2.34 [0.93, 5.87] | |
| 5 Neurological adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 0.24 [0.03, 2.02] | |
| 5.1 Diclofenac vs nimesulide | 1 | Odds Ratio (Fixed, 95% CI) | 0.24 [0.03, 2.02] |
Comparison 7. Naproxen vs NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief: dichotomous outcome | 2 | Odds Ratio (Fixed, 95% CI) | 0.65 [0.36, 1.17] | |
| 1.1 Naproxen vs ketoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.45 [0.16, 1.26] | |
| 1.2 Naproxen vs piroxicam | 1 | Odds Ratio (Fixed, 95% CI) | 0.77 [0.37, 1.59] | |
| 2 Pain intensity (SPID) | 1 | Mean Difference (Fixed, 95% CI) | 0.06 [‐0.28, 0.40] | |
| 2.1 Naproxen vs flurbiprofen | 1 | Mean Difference (Fixed, 95% CI) | 0.06 [‐0.28, 0.40] | |
| 3 Pain relief: continuous data: total pain relief score difference | 3 | Mean Difference (Fixed, 95% CI) | 2.44 [0.83, 4.06] | |
| 3.1 Naproxen vs etoricoxib (COX‐2‐specific): TOPAR8 | 1 | Mean Difference (Fixed, 95% CI) | 1.5 [‐1.47, 4.47] | |
| 3.2 Naproxen vs celecoxib (COX‐2‐specific): TOPAR8 | 2 | Mean Difference (Fixed, 95% CI) | 2.84 [0.92, 4.75] | |
| 4 Pain relief: continuous data: mean difference final scores 1 to 5 scale | 2 | Mean Difference (Fixed, 95% CI) | ‐0.17 [‐0.39, 0.06] | |
| 4.1 Naproxen vs ibuprofen: 1 to 5 scale | 1 | Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.53, ‐0.01] | |
| 4.2 Naproxen vs diclofenac: 1 to 5 scale | 1 | Mean Difference (Fixed, 95% CI) | 0.1 [‐0.33, 0.53] | |
| 5 Pain relief: continuous data: mean difference change scores | 1 | Mean Difference (Fixed, 95% CI) | 1.1 [0.56, 1.64] | |
| 5.1 Naproxen vs ketoprofen: VAS 0 to 10 | 1 | Mean Difference (Fixed, 95% CI) | 1.1 [0.56, 1.64] | |
| 6 All adverse effects | 9 | Odds Ratio (Fixed, 95% CI) | 1.18 [0.92, 1.53] | |
| 6.1 Naproxen vs aceclofenac | 1 | Odds Ratio (Fixed, 95% CI) | 1.41 [0.55, 3.60] | |
| 6.2 Naproxen vs diclofenac | 1 | Odds Ratio (Fixed, 95% CI) | 0.96 [0.45, 2.04] | |
| 6.3 Naproxen vs etoricoxib | 1 | Odds Ratio (Fixed, 95% CI) | 1.26 [0.49, 3.23] | |
| 6.4 Naproxen vs ketoprofen | 2 | Odds Ratio (Fixed, 95% CI) | 0.81 [0.33, 1.99] | |
| 6.5 Naproxen vs meclofenamate | 1 | Odds Ratio (Fixed, 95% CI) | 3.05 [0.38, 24.33] | |
| 6.6 Naproxen vs piroxicam | 1 | Odds Ratio (Fixed, 95% CI) | 1.23 [0.66, 2.29] | |
| 6.7 Naproxen vs celecoxib | 2 | Odds Ratio (Fixed, 95% CI) | 1.23 [0.84, 1.79] | |
| 7 Gastrointestinal adverse effects | 5 | Odds Ratio (Fixed, 95% CI) | 1.19 [0.53, 2.69] | |
| 7.1 Naproxen vs ibuprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.20, 4.95] | |
| 7.2 Naproxen vs ketoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.50 [0.05, 5.00] | |
| 7.3 Naproxen vs meclofenamate | 1 | Odds Ratio (Fixed, 95% CI) | 2.05 [0.20, 21.18] | |
| 7.4 Naproxen vs piroxicam | 2 | Odds Ratio (Fixed, 95% CI) | 1.42 [0.44, 4.54] | |
| 8 Neurological adverse effects | 3 | Odds Ratio (Fixed, 95% CI) | 0.80 [0.24, 2.74] | |
| 8.1 Naproxen vs ketoprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.98 [0.17, 23.44] | |
| 8.2 Naproxen vs meclofenamate | 1 | Odds Ratio (Fixed, 95% CI) | 7.39 [0.15, 368.18] | |
| 8.3 Naproxen vs piroxicam | 1 | Odds Ratio (Fixed, 95% CI) | 0.41 [0.09, 1.86] | |
| 9 Additional analgesics required | 3 | Odds Ratio (Fixed, 95% CI) | 0.73 [0.52, 1.03] | |
| 9.1 Naproxen vs flurbiprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.59 [0.18, 1.93] | |
| 9.2 Naproxen vs celecoxib | 2 | Odds Ratio (Fixed, 95% CI) | 0.74 [0.52, 1.06] | |
| 10 Interference with daily activities | 2 | Odds Ratio (Fixed, 95% CI) | 0.63 [0.33, 1.22] | |
| 10.1 Naproxen vs flurbiprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.33 [0.12, 0.91] | |
| 10.2 Naproxen vs ibuprofen | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.43, 2.34] | |
| 11 Absence from work/school | 2 | Odds Ratio (Fixed, 95% CI) | 0.50 [0.19, 1.36] | |
| 11.1 Naproxen vs flurbiprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.15 [0.02, 1.21] | |
| 11.2 Naproxen vs ibuprofen | 1 | Odds Ratio (Fixed, 95% CI) | 0.72 [0.23, 2.24] |
Comparison 8. NSAIDs vs paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief dichotomous data | 3 | Odds Ratio (Fixed, 95% CI) | 1.89 [1.05, 3.43] | |
| 1.1 Ibuprofen vs paracetamol | 2 | Odds Ratio (Fixed, 95% CI) | 1.73 [0.83, 3.60] | |
| 1.2 Naproxen vs paracetamol | 1 | Odds Ratio (Fixed, 95% CI) | 2.25 [0.81, 6.22] | |
| 2 All adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 0.85 [0.31, 2.34] | |
| 2.1 Ibuprofen vs paracetamol | 1 | Odds Ratio (Fixed, 95% CI) | 0.85 [0.31, 2.34] | |
| 3 Gastrointestinal adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.06, 16.62] | |
| 3.1 Naproxen vs paracetamol | 1 | Odds Ratio (Fixed, 95% CI) | 1.0 [0.06, 16.62] | |
| 4 Neurological adverse effects | 1 | Odds Ratio (Fixed, 95% CI) | 1.54 [0.24, 9.83] | |
| 4.1 Naproxen vs paracetamol | 1 | Odds Ratio (Fixed, 95% CI) | 1.54 [0.24, 9.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akerlund 1989.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 42 women randomised, 39 analysed (3 withdrawals: 1 woman did not meet protocol criteria for the trial, 2 did not return after 1st interview) Method of assessing adverse effects: authors state only "side effects were noted" | |
| Participants | Inclusion: regularly occurring menstrual pain requiring medication Exclusion: lactation; history or sign of severe generalised allergic or gastrointestinal disease; use of analgesics Age: range 17 to 45, median 26 Location: Sweden | |
| Interventions | Ketoprofen (100 mg) Naproxen (500 mg) Single dose Duration: 2 cycles, 1 treatment per cycle | |
| Outcomes | Pain severity 100 mm VAS Activity level permitted 1 to 7 scale Pain reduction 1 to 7 scale Adverse effects | |
| Notes | Differences in baseline pain levels were reported ‐ therefore calculations of outcomes were transformed to difference from base value. The ketoprofen group initially had higher pain levels Data on adverse effects reports number of symptoms but not number of women affected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, code broken at end of study |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether information on side effects actively solicited |
| Complete follow‐up? | Unclear risk | 3/42 patients were not analysed (93% analysed) |
| Potential bias related to study funding | Unclear risk | Not stated |
Akinluyi 1987.
| Methods | Randomisation/allocation method unclear Double‐blind (participant and assessor), cross‐over trial 60 women randomised and analysed Method of assessing adverse effects: unclear ‐ authors state only "women complained of..." | |
| Participants | Inclusion: primary dysmenorrhoea Exclusion: menstrual irregularities; history peptic ulcer, renal or blood disorders; pregnant; use of other medicines for condition Age: 17 to 33, mean 18.4 Parity: 46 nulliparous (8 with previous pregnancy), 14 parous Source: volunteer student nurses/midwives and women from local gynaecological clinic Location: Nigeria | |
| Interventions | Piroxicam (20 mg), placebo 2 tablets per day for 2 days, then 1 tablet per day until symptoms or menses subsided Duration: 4 cycles per participant, 240 cycles in total | |
| Outcomes | Pain ‐ abdominal cramps yes/no Pain scale (very severe, severe, moderate, slight) Adverse effects | |
| Notes | No mention of baseline or cross‐over analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | High risk | Adverse effects data collected prospectively but not reported in detail |
| Complete follow‐up? | Low risk | 60/60 analysed |
| Potential bias related to study funding | Unclear risk | Pharma |
al‐Waili 1990.
| Methods | Random allocation, pharmacy‐coded drugs Cross‐over, double‐blind trial 40 women randomised and analysed Method of assessing adverse effects: reported daily on a questionnaire at trial office (which they visited daily) | |
| Participants | Inclusion: primary dysmenorrhoea; diagnosed by results of questionnaire for self assessment, physical and gynaecological exams; regular cycles 24 to 31 days Exclusion: clinical pathology of genital tract; lactating or contemplating pregnancy; allergies to NSAIDs; use of OCP or other long‐term drug therapy Age: 18 to 40, mean 24 Source: volunteers Location: Iraq | |
| Interventions | Indomethacin suppositories (100 mg, 1 to 3 times daily, at onset of symptoms, max. 5 days) Placebo suppositories Duration: 2 cycles per treatment/4 cycles in total No additional medication allowed during study | |
| Outcomes | Pain relief 0 to 18 scale Dysmenorrhoeic symptoms Side effects ‐ only counted if not experienced in previous menses | |
| Notes | Women visited clinic every day to ensure compliance Baseline assessment performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | Adequate, "coded by pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, codes broken at end of study. "All the patients were provided with identical suppositories" |
| Selective reporting (reporting bias) | Low risk | Adverse effects quantified |
| Complete follow‐up? | Low risk | All women completed the study |
| Potential bias related to study funding | Unclear risk | Not stated |
Andersch 1989.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 60 women randomised, 57 analysed Withdrawals, 1 pregnancy, 2 failed to attend follow‐up Method of assessing adverse effects: self reported prospectively on form | |
| Participants | Inclusion: women with primary dysmenorrhoea; no history or evidence of pelvic pathology as judged by clinical and gynaecological examinations Exclusion: pelvic pathology; IUD; history of peptic ulcer or dyspepsia; asthma; breastfeeding Age: 16 to 44, mean (SD) 29.3 (9.3) Parity: 37 nulliparous, 20 parous Contraception: 17 used OCP, the rest barrier or none Location: Sweden | |
| Interventions | Flurbiprofen (100 mg twice daily as needed) Naproxen sodium (500 mg twice daily) Treatment for a total of 5 days Duration: 4 cycles, 2 per treatment | |
| Outcomes | Pain relief: 5‐point scale Reported mean scores for each individual Interference with everyday life Days off work Additional analgesics No serious side effects reported | |
| Notes | Pain scores etc compared between groups at baseline and at each phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, code not broken until data processing, women and investigators blind. "The capsules supplied were identical to all outward appearance" |
| Selective reporting (reporting bias) | Low risk | Adverse effects data collected prospectively by patient and reported in detail |
| Complete follow‐up? | Low risk | 57/60 analysed (95%) |
| Potential bias related to study funding | Unclear risk | Not stated |
Arnold 1983.
| Methods | Randomisation method ‐ random numbers. Allocation method unclear. Parallel, double‐blind trial 166 women analysed, 562 cycles 25 women inadmissible for various listed reasons Method of assessing adverse effects: listed on form prospectively | |
| Participants | Inclusion: primary dysmenorrhoea, could communicate in English, physical exam performed Exclusion: pelvic pathology, serious medical history, hypersensitivity to any drug, history of drug abuse, use of OCP, analgesics or anti‐inflammatories Location: USA | |
| Interventions | Fenoprofen calcium (200 mg, twice every 4 hours) Ibuprofen (400 mg, twice every 4 hours) Placebo Duration: 4 cycles Rescue analgesia Empirin No.3 was allowed, but if taken all subsequent hourly pain scores recorded as severe | |
| Outcomes | SPID scores Pain intensity Adverse reports Pain scores in ridits (based on frequency distribution) and in graphical form | |
| Notes | No difference between groups at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random numbers were available at each institution prior to the enrolment of patients" |
| Allocation concealment (selection bias) | Unclear risk | "The patients were assigned consecutively to each number as they were chronologically enrolled" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical appearing capsules" |
| Selective reporting (reporting bias) | Low risk | "Patients prospectively asked to list any adverse experiences she had noticed" |
| Complete follow‐up? | High risk | 145/166 analysed (87%) |
| Potential bias related to study funding | Unclear risk | Eli Lilly were sponsors |
Balsamo 1986.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel trial 40 women randomised, 40 analysed Method of assessing adverse effects: unclear ‐ authors state only "tolerability was evaluated" | |
| Participants | Inclusion: primary dysmenorrhoea for at least 3 months; normal gynaecological exam Exclusion: secondary dysmenorrhoea; use of OCP or IUD; use or oral anticoagulants; cardiac, hepatic or renal disease; gastric ulcers; intolerance to NSAIDs Age: range 17 to 30 | |
| Interventions | Diclofenac sodium 75 mg Placebo suppositories Administered for 3 days, dose 2 per day Additional medication was not allowed during the study | |
| Outcomes | Pain relief Absence from work Adverse effects | |
| Notes | No numerical data reported for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Alocados aleatonamente em um dos dois groupos" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Low risk | Adverse effects data collected prospectively |
| Complete follow‐up? | Low risk | 40/40 analysed |
| Potential bias related to study funding | Unclear risk | Ciba‐Geigy |
Benassi 1993.
| Methods | Randomisation/allocation method unclear. Double‐blind, parallel trial 30 women randomised and analysed Method of assessing adverse effects: self reported prospectively on chart | |
| Participants | Inclusion: primary dysmenorrhoea for at least 6 months of medium‐high gravity; regular menstrual cycles; nulliparous Exclusion: light menstrual upsets or irregularities, organic dysmenorrhoea, IUD or OCP use, allergies to NSAIDs, gastrointestinal problems Age: 15 to 25, mean 23.8 (2.6) Location: Italy | |
| Interventions | Meclofenamate sodium 100 mg, naproxen sodium 275 mg Taken at first sign of menses, then every 8 hours Duration: 1 control cycle, then 5 treatment cycles | |
| Outcomes | Pain assessment ‐ VAS (graph) Dysmenorrhoea symptoms | |
| Notes | No mention of baseline comparison. No numerical data reported for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Low risk | Women prospectively asked to record adverse effects |
| Complete follow‐up? | Low risk | 30/30 women analysed |
| Potential bias related to study funding | Unclear risk | Not stated |
Bitner 2004.
| Methods | Randomised, multicentre, double‐blind, placebo‐ and active‐controlled cross‐over design 109 women randomised, 88 analysed. 34 women discontinued of whom 10 never took study medication | |
| Participants | Inclusion: primary dysmenorrhoea, negative pregnancy test, practising an acceptable form of birth control Exclusion: hypersensitivity to paracetamol, aspirin or study drugs, a history of GI disease or peptic ulcer, a severe or uncontrolled medical condition, the use of rifampicin, methotrexate or warfarin was not permitted Location: USA | |
| Interventions | Naproxen 500 mg bid at the onset of moderate‐to‐severe menstrual pain, max. 3 days Placebo Duration: 2 cycles, 1 cycle for each treatment | |
| Outcomes | Pain relief: SPID8 Adverse effects | |
| Notes | This trial also included lumiracoxib (since withdrawn). The trial publication describes a second trial, using lumiracoxib and rofecoxib (also since withdrawn) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | "All adverse events a... recorded and assessed in terms of their ... possible relationship to the study drug" ‐ unclear whether data collected prospectively by patient |
| Complete follow‐up? | Unclear risk | 99/109 analysed (91%) |
| Potential bias related to study funding | Unclear risk | Novartis authors |
Budoff 1979.
| Methods | Randomisation/allocation method unclear Cross‐over, double‐blind trial 46 women randomised, 44 analysed 2 excluded from analysis, 1 due to not completing 3 cycles, 1 only took 3 capsules of medication Method of assessing adverse effects: unclear ‐ authors state that "adverse reactions recorded" at follow‐up visit | |
| Participants | Inclusion: primary spasmodic dysmenorrhoea, pain in recurrent cyclic fashion as diagnosed using Menstrual Distress Questionnaire, regular menstrual cycles, good physical health, emotionally stable, physical and pelvic exam performed Exclusion: IUD or OCP use, congestive dysmenorrhoea, organic pelvic disease, intolerance to fenamates Location: USA | |
| Interventions | Mefenamic acid (250 mg, 4 times daily at onset of menses, max. 3 days, dose could be reduced if needed) Placebo Duration: 3 cycles per treatment phase/6 cycles in total Additional analgesia: 32.4 mg codeine allowed if necessary, no aspirin or paracetamol‐based products, or over the counter analgesia allowed during study | |
| Outcomes | Proportion with decrease in pain, weakness/dizziness/nausea and diarrhoea Adverse effects | |
| Notes | Groups compared at baseline and trial also reported mean differences in pain from entry score for end of first phase. No numerical data reported for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical‐appearing placebo capsules" |
| Selective reporting (reporting bias) | Unclear risk | Data on adverse reactions not solicited prospectively |
| Complete follow‐up? | High risk | 37/46 analysed (80%) |
| Potential bias related to study funding | Unclear risk | Drug supplied by Parke Davis |
Cash 1982.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 25 women randomised, 23 analysed (1 dropped out for personal reasons, 1 due to pulmonary disease, and 1 had a history of gastric upset and was only included in some analyses) Method of assessing adverse effects: recorded at follow‐up visit if volunteered by participant | |
| Participants | Inclusion: regular severe dysmenorrhoea; regular cycles; general physical and gynaecological exams to show no organic cause of dysmenorrhoea; negative pregnancy test Exclusion: organic disease; IUD use; irregular cycles; history of peptic ulcer or dyspepsia following NSAID use Location: UK | |
| Interventions | Piroxicam (20 mg) Placebo Administered as 2 x 10 mg tablets taken each morning at start of dysmenorrhoea until the end of menstruation Duration: 4 cycles, 1 per treatment | |
| Outcomes | Pain relief Overall relief Adverse effects Paracetamol consumption | |
| Notes | Data in graphical form | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Low risk | Data on adverse events prospectively solicited |
| Complete follow‐up? | High risk | 22/25 analysed (88%) |
| Potential bias related to study funding | Unclear risk | Pfizer sponsorship |
Chan 1983.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 12 women randomised and analysed Method of assessing adverse effects: no mention of adverse effects | |
| Participants | Inclusion: history of primary dysmenorrhoea within 1 year of menarche; pelvic and physical examinations Exclusion: use of OCP or IUD; history of allergies or gastrointestinal disorders Age: 16 to 35 Location: USA | |
| Interventions | Naproxen sodium (275 mg) Placebo 2 tablets administered as a loading dose at start of pain then 1 tablet taken 4 x daily for 3 days Duration: 3 cycles, 1 control then randomised to a treatment for the 2nd cycle, and crossed over to the alternate treatment for the 3rd cycle | |
| Outcomes | Relief of dysmenorrhoea | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not prospectively solicited |
| Complete follow‐up? | Low risk | No dropouts |
| Potential bias related to study funding | Unclear risk | Grant in aid from Syntex |
Chantler 2008.
| Methods | Randomisation/allocation method unclear
Double‐blind, cross‐over design 11 randomised 11 analysed |
|
| Participants | Inclusion: history of primary dysmenorrhoea, otherwise healthy Exclusion: chronic medication or hormonal contraception in the previous 6 months, secondary dysmenorrhoea Age: 24 years (standard deviation 4 years) Source: university students Location: South Africa |
|
| Interventions | Diclofenac (50 mg) Meloxicam (7.5 mg) Placebo Single capsule orally as required for pain, no more than 2 capsules daily 3 cycles |
|
| Outcomes | % decrease in VAS pain scale | |
| Notes | Trial included rofecoxib also (now withdrawn) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, identical placebo. "The agents were disguised in identical opaque gelatine capsules" |
| Selective reporting (reporting bias) | Low risk | Patients asked to prospectively record adverse effects |
| Complete follow‐up? | Low risk | No losses to follow‐up |
| Potential bias related to study funding | Low risk | "No funding sought from manufacturer" |
Chantler 2009.
| Methods | Randomisation/allocation method unclear
Double‐blind, cross‐over study 12 women randomised and analysed |
|
| Participants | Inclusion: history of moderate to severe primary dysmenorrhoea, otherwise healthy Exclusion: chronic medication or hormonal contraception in the previous 12 months, secondary dysmenorrhoea Age: 20 years Source: university students Location: South Africa |
|
| Interventions | Diclofenac (100 mg) Placebo 1 dose of each before exercise on first or second day of menstruation while experiencing worst menstrual pain |
|
| Outcomes | Pain on VAS | |
| Notes | Study includes exercise‐related interventions and outcomes also | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, using identical‐looking placebo capsule |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse effects prospectively solicited |
| Complete follow‐up? | Low risk | Yes |
| Potential bias related to study funding | Low risk | Academic funding only |
Costa 1987a.
| Methods | Randomisation/allocation method unclear. 12 women randomised and analysed Double‐blind, cross‐over study | |
| Participants | Inclusion: primary dysmenorrhoea; medical and gynaecological exams to confirm lack of pathology Exclusion: secondary dysmenorrhoea; pregnancy; gastric or duodenal ulcers, ulcerative colitis, liver or kidney disease, asthma, rhinitis or allergy to NSAIDs; OCP in month prior to study Age: means 28 to 30, ranges 18 to 38 Source: outpatients Location: Italy | |
| Interventions | Piroxicam beta‐cyclodextrin 20 mg versus placebo Taken as sachets | |
| Outcomes | Pain intensity Adverse effects Use of additional medication | |
| Notes | Day 1 data in graphical form only. No numerical data reported for adverse effects in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear, not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo described but does not state that it was identical |
| Selective reporting (reporting bias) | High risk | Only GI adverse effects recorded as such (other adverse effects classified as dysmenorrhoea symptoms) |
| Complete follow‐up? | Low risk | No losses to follow‐up |
| Potential bias related to study funding | Unclear risk | Not stated |
Costa 1987b.
| Methods | Randomisation/allocation method unclear. 14 women randomised and analysed Double‐blind, cross‐over study Method of assessing adverse effects: women instructed to self record prospectively | |
| Participants | Inclusion: primary dysmenorrhoea; medical and gynaecological exams to confirm lack of pathology Exclusion:secondary dysmenorrhoea; pregnancy; gastric or duodenal ulcers, ulcerative colitis, liver or kidney disease, asthma, rhinitis or allergy to NSAIDs; OCP in month prior to study Age: means 28 to 30, ranges 18 to 38 Source: outpatients Location: Italy | |
| Interventions | Piroxicam beta‐cyclodextrin 20 mg versus naproxen sodium 550 mg Taken as suppositories All medication taken at onset of symptoms for as long as needed, dosage: once a day in the morning Additional medication was allowed if treatment medication was ineffective after 3 hours | |
| Outcomes | Pain intensity Adverse effects Use of additional medication | |
| Notes | Day 1 data in graphical form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo described but does not state that it was identical |
| Selective reporting (reporting bias) | High risk | Only GI adverse effects recorded as such (other adverse effects classified as dysmenorrhoea symptoms) |
| Complete follow‐up? | Low risk | No losses to follow‐up |
| Potential bias related to study funding | Unclear risk | Not stated |
Dandenell 1979.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel, multicentre study 108 women randomised, 97 analysed (experimental n = 48, placebo n = 48) 11 dropouts, 1 pregnancy, 2 did not have painful menstruation during the study, 1 due to lack of efficacy (placebo group), 1 insufficient data, 6 did not attend follow‐up or start treatment Method of assessing adverse effects: women instructed to self report prospectively on forms | |
| Participants | Inclusion: women with severe primary dysmenorrhoea, physical and pelvic exam Exclusion: women with major cycle irregularities, taking hormonal contraceptives, organic causes of dysmenorrhoea, women with gastrointestinal disorders or allergies to acetylsalicylates Age: 18 to 40, experimental mean 25 (1.1), control mean 26.1 (1.2) Source: gynaecological clinics Location: Sweden | |
| Interventions | Naproxen (250 mg as needed, max. daily dose 1250 mg) Placebo Taken at first sign of menstrual distress Duration: 2 cycles Additional analgesia was allowed if adequate relief was not experienced | |
| Outcomes | Pain relief: 5‐point scale, reported as overall mean scores and graph Supplementary medicine needed Restriction to daily life Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "placebo tables of identical appearance" |
| Selective reporting (reporting bias) | Low risk | Adverse effects prospectively solicited |
| Complete follow‐up? | Unclear risk | 97/108 were analysed (90%) |
| Potential bias related to study funding | Unclear risk | Some authors were Astra‐Syntax affiliated |
Daniels 2002.
| Methods | Randomisation/allocation method: computer‐generated remotely prepared schedule. Allocation concealment unclear
Double‐blind, cross‐over study
118 women randomised, 96 analysed 22 not analysed: 10 not dosed, 9 non‐compliant, 2 ineligible, 1 lost to follow‐up |
|
| Participants | Inclusion: women aged 18 to 35 years with primary dysmenorrhoea for previous 4 to 6 cycles, moderate to severe cramping routinely treated with oral medication. No other history of pelvic pathology. Not pregnant, using contraception. In good health, as determined by physical examination and medical history Exclusion: pelvic pathology, history of vomiting during menses, IUD or contraceptive implant within previous 3 months, active peptic ulcer or gastrointestinal disease with significant blood loss Age: mean 23 years Location: USA | |
| Interventions | Naproxen sodium 550 mg Placebo Twice daily as needed for up to 3 days for 4 cycles |
|
| Outcomes | Pain intensity difference at 8 and 12 hours after first dose, adverse events | |
| Notes | Also had valdecoxib intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, double dummy "two tablets from bottle A and two capsules from bottle B, the content depended on the assigned treatment" |
| Selective reporting (reporting bias) | Unclear risk | "Adverse events were monitored throughout the study" |
| Complete follow‐up? | High risk | 96/118 analysed (81%) |
| Potential bias related to study funding | Unclear risk | Pharmacia and Pfizer sponsored |
Daniels 2008.
| Methods | Allocation concealment unclear, computer‐generated randomisation sequences, double‐blind, cross‐over design 124/144 analysed |
|
| Participants | Included: women with primary dysmenorrhoea, healthy, non‐lactating, pregnancy test negative, using birth control Excluded: women with secondary dysmenorrhoea, hypersensitivity to NSAIDs, bowel disease or ulcers |
|
| Interventions | Naproxen 500 mg Placebo Medicate twice daily |
|
| Outcomes | Change in pain intensity (SPID score) Adverse effects |
|
| Notes | Study also included lumiracoxib (since withdrawn) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | "Adverse events were recorded throughout the study" |
| Complete follow‐up? | High risk | 124/144 analysed (86%) |
| Potential bias related to study funding | High risk | Novartis sponsored |
Daniels 2009a.
| Methods | Double‐blind, placebo controlled, cross‐over study set in USA 1999‐2000 6‐sequence cross‐over design: all women had 1 cycle of each drug, randomised to 1 of 6 sequences |
|
| Participants | 149 women aged 18 to 44 with primary dysmenorrhoea, onset within 5 years of menarche. Having moderate or severe cramping pain requiring analgesic medication for at least 4 of the 6 menstrual cycles prior to enrollment | |
| Interventions | 1. Celecoxib 400 mg, then 200 mg 12‐hourly prn
2. Naproxen sodium 550 mg 12‐hourly
3. Placebo Over 3 menstrual cycles The study could be extended for up to 5 consecutive cycles if the patient did not medicate for a maximum of 2 nonconsecutive cycles |
|
| Outcomes | Total pain relief 8 hours after first dose using summed hourly pain relief scores on a 5‐point categorical scale (TOTPAR) Pain intensity after 8 hours using summed hourly pain severity scores on a 4‐point categorical scale (SPID) Tolerability: including self report of AEs |
|
| Notes | Same publication reports a second study (Daniels 2009b) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy with matching placebos |
| Selective reporting (reporting bias) | Low risk | Adverse events prospectively solicited from patient |
| Complete follow‐up? | Unclear risk | 136/149 included in analysis (91%); dropouts due to more than 2 consecutive non‐dosing cycles, non‐compliance, protocol violations, pregnancy or loss to follow‐up |
| Potential bias related to study funding | Unclear risk | Funded by Pfizer |
Daniels 2009b.
| Methods | Double‐blind, placebo‐controlled, cross‐over study set in USA 1999‐2000 | |
| Participants | 154 women aged 18 to 44 with primary dysmenorrhoea, onset within 5 years of menarche. Having moderate or severe cramping pain requiring analgesic medication for at least 4 of the 6 menstrual cycles prior to enrollment | |
| Interventions | 1. Celecoxib 400 mg, then 200 mg 12‐hourly prn
2. Naproxen sodium 550 mg 12‐hourly
3. Placebo Over 3 menstrual cycles The study could be extended for up to 5 consecutive cycles if the patient did not medicate for a maximum of 2 nonconsecutive cycles |
|
| Outcomes | Total pain relief 8 hours after first dose using summed hourly pain relief scores on a 5‐point categorical scale (TOTPAR) Pain intensity after 8 hours using summed hourly pain severity scores on a 4‐point categorical scale (SPID) Tolerability Need for extra medication |
|
| Notes | Same publication reports a second study (Daniels 2009a) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy with matching placebos |
| Selective reporting (reporting bias) | Low risk | Adverse events prospectively solicited from patient |
| Complete follow‐up? | High risk | 135/154 included in analysis (88%); dropouts due to more than 2 consecutive non‐dosing cycles, non‐compliance, protocol violations, non‐compliance or loss to follow‐up |
| Potential bias related to study funding | Unclear risk | Funded by Pfizer |
Dawood 1999a.
| Methods | Computer‐generated randomisation schedule. Allocation method not described Double‐blind, cross‐over trial 97 women randomised, 93 analysed Method of assessing adverse effects: women instructed to self record prospectively in diary | |
| Participants | Inclusion: moderate to severe abdominal pain associated with primary dysmenorrhoea during a minimum of 4 of the last 6 menstrual cycles; good health with regular menses every 25 to 35 days; using an effective method of birth control or using OCP for at least 6 months; willing to abstain from alcohol during treatment phase of trial; pelvic and physical exam Exclusion: breastfeeding; IUD use; implant or ingestible contraceptive (Norplant, Depo Provera); history of hypersensitivity or adverse reactions to NSAIDs; history of chronic analgesic use; known cardiovascular, pulmonary, hepatic, gastrointestinal, renal, neurological, musculoskeletal, endocrine or metabolic disorders Age: over 15 Source: outpatients Location: multicentred, USA | |
| Interventions | Piroxicam 20 mg Piroxicam 40 mg Naproxen sodium 275 mg (with loading dose of 550 mg) Placebo Taken as a single dose, started when abdominal cramping became moderate in intensity, taken for 3 days. Additional dosing every 4 hours as needed, max. 4 doses per day Duration: 4 cycles, one of each treatment | |
| Outcomes | Global evaluation Pain intensity Adverse effects | |
| Notes | Ibuprofen 200 mg used as rescue medication; once used participant was not allowed to take any additional study medication This review has used the 40 mg dose of piroxicam for the purpose of comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | Low risk | Adverse events prospectively solicited from patient |
| Complete follow‐up? | Low risk | 93/97 (96%) |
| Potential bias related to study funding | Unclear risk | Chiesi Pharmaceuticals provided trial data and supported the study |
Dawood 1999b.
| Methods | Computer‐generated, randomised allocation schedule Double‐blind, cross‐over trial 96 women randomised, 93 analysed Method of assessing adverse effects: women instructed to self record prospectively | |
| Participants | Inclusion: moderate to severe abdominal pain associated with primary dysmenorrhoea during a minimum of 4 of the last 6 menstrual cycles; good health with regular menses every 25 to 35 days; using an effective method of birth control or using OCP for at least 6 months; willing to abstain from alcohol during treatment phase of trial; pelvic and physical exam Exclusion: breastfeeding; IUD use; implant or ingestible contraceptive (Norplant, Depo Provera); history of hypersensitivity or adverse reactions to NSAIDs; history of chronic analgesic use; known cardiovascular, pulmonary, hepatic, gastrointestinal, renal, neurological, musculoskeletal, endocrine or metabolic disorders Age: over 15 Source: outpatients Location: multicentred, USA | |
| Interventions | Piroxicam 20 mg Piroxicam 40 mg Ibuprofen 400 mg Placebo | |
| Outcomes | Global evaluation Pain intensity Adverse effects | |
| Notes | Ibuprofen 200 mg used as rescue medication, once used participant was not allowed to take any additional study medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | Low risk | Adverse events prospectively solicited from patient |
| Complete follow‐up? | Low risk | 93/96 (97%) |
| Potential bias related to study funding | Unclear risk | Chiesi Pharmaceuticals provided trial data and supported the study |
Dawood 2007.
| Methods | Allocation concealment method unclear Computer‐generated, randomisation schedule Double‐blind, cross‐over trial 10/12 analysed 2 withdrew: 1 protocol violation, 1 discontinued |
|
| Participants | Included: women with primary dysmenorrhoea, using contraception other than oral contraceptive, normal PAP smear and pelvic examination Excluded: women with secondary dysmenorrhoea, peptic ulcer, NSAID allergies, pelvic inflammatory disease, pregnancy Age: 31 (range 22 to 35) Location: USA |
|
| Interventions | Ibuprofen 400 mg Acetaminophen 1000 mg Placebo Medicate with 2 tablets/caplets at pain onset; refrain from further medication for 6 hours |
|
| Outcomes | Pain rating | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, double dummy "matching placebo" |
| Selective reporting (reporting bias) | High risk | Adverse effects not reported |
| Complete follow‐up? | High risk | 10/12 analysed (83%) |
| Potential bias related to study funding | Unclear risk | Ortho‐McNeil were sponsors |
de Mello 2004.
| Methods | Allocation concealment: unclear Method of randomisation: unclear Double‐blinded parallel design 337 patients randomised and 337 analysed |
|
| Participants | Included: women with primary dysmenorrhoea for previous 3 cycles, aged 18 to 10 Excluded: use of oral contraceptives or intrauterine contraception within previous 3 months, secondary dysmenorrhoea, concomitant use of analgesics, other medical conditions (listed in study) Age: mean 28 (range 17 to 40) years Location: Mexico and Brazil |
|
| Interventions | Meloxicam 7.5 or 15 mg Mefenamic acid 500 mg Medicate 3 times daily over 3 to 5 days 3 cycles |
|
| Outcomes | Ratings for pain and tolerability | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "matching" placebo |
| Selective reporting (reporting bias) | High risk | Adverse effects data not solicited prospectively, "no primary endpoints with regard to safety were defined" |
| Complete follow‐up? | Low risk | 337/337 in safety analyses |
| Potential bias related to study funding | Unclear risk | Boehringer‐Ingelheim sponsored and conducted |
De Souza 1991.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 40 women randomised Method of assessing adverse effects: unclear ‐ evaluated retrospectively at follow‐up | |
| Participants | Inclusion: primary dysmenorrhoea of at least moderate intensity; regular cycles for at least 1 year; clinical exam Exclusion: pregnant; lactating; secondary dysmenorrhoea; hypersensitivity to NSAIDs or aspirin; peptic ulcers or any gastrointestinal bleeding; hepatic, cardiac or renal disease; asthma; previous PID; endometriosis, fibroids; IUD use; use of OCP within 4 months of study; use of hormonal preparation, corticosteroids, analgesics, antispasmodics, vitamin B6 Location: Brazil | |
| Interventions | Etodolac 200 mg Placebo Taken every 12 hours, for max. 5 days Use of 500 mg of paracetamol as an additional analgesic if necessary Duration: 2 cycles | |
| Outcomes | Pain relief | |
| Notes | Portuguese ‐ partially translated using altavista Babelfish website Groups comparable at baseline for demographics and pain, menstrual, sexual and obstetric histories Cross‐over analysis performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | Low risk | Adverse effects data solicited prospectively |
| Complete follow‐up? | Low risk | 40/40 analysed |
| Potential bias related to study funding | Unclear risk | Novartis sponsored |
Delgado 1994.
| Methods | Randomisation controlled by pharmaceutical company. Double‐blind, cross‐over trial 80 women randomised, 73 analysed, 7 women did not complete treatment and were excluded Method of assessing adverse effects: not stated | |
| Participants | Inclusion: primary dysmenorrhoea requiring medical treatment, menstrual cycle < 35 days Exclusion: proven secondary dysmenorrhoea, history of duodenal or gastric ulcer, OCP or IUD use, treatment with any other drugs for dysmenorrhoea unless ceased 10 days before entering trial Age: 15 to 39, mean 25.2 (6.1) Parity: 54 nulliparous, 19 parous Location: Mexico | |
| Interventions | Tolfenamic acid 200 mg Mefenamic acid 500 mg Taken 3 times a day for 3 days Duration: 6 cycles/3 per treatment | |
| Outcomes | Mean pain relief (reported for phase 1 and 2) 10‐point VAS Other dysmenorrhoeic symptoms Interference with daily activities Adverse effects | |
| Notes | Baseline and phase data analysed separately for each group, no significant difference at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical capsules" |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data solicited prospectively |
| Complete follow‐up? | Unclear risk | 73/80 analysed (91%) |
| Potential bias related to study funding | Unclear risk | Not stated |
Di Girolamo 1999.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 24 women randomised, 24 analysed Method of assessing adverse effects: reported retrospectively at follow‐up | |
| Participants | Inclusion: primary dysmenorrhoea for over 1 year, regular menstrual cycles, good emotional and physical health, ability to communicate pain intensity during study Exclusion: abnormal gynaecological pathology, gastrointestinal or osteoarticular abnormality, use of OCP within 1 month, bronchial asthma, urticaria or other allergic reaction to NSAIDs, renal or hepatic disease, concurrent medication with NSAIDs or corticosteroids Age: at least 18 Location: Argentina | |
| Interventions | Lysine clonixinate Ibuprofen Placebo Duration ‐ 3 cycles, 1 per treatment | |
| Outcomes | Proportion reporting pain relief Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse effects not prospectively solicited |
| Complete follow‐up? | Unclear risk | Not stated |
| Potential bias related to study funding | Unclear risk | Not stated |
Elder 1979.
| Methods | Randomisation/allocation method unclear Cross‐over, double‐blind trial 38 women randomised, 32 analysed Method of assessing adverse effects: not stated | |
| Participants | Inclusion: primary dysmenorrhoea Exclusion: history of dyspepsia, pelvic abnormality, use of combined OCP Age: 12 to 41, mean 24 All women parous Location: UK | |
| Interventions | Indomethacin (25 mg, 3 times daily, from start of menses until participant thought necessary) Placebo Duration: 3 cycles each treatment/6 in total | |
| Outcomes | Pain relief (4‐point scale) | |
| Notes | First‐phase data presented as mean pain relief in graph form. No numerical data reported for adverse effects in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "the placebo drugs were identical to those containing the active drug and neither patient nor doctor knew which was being taken" |
| Selective reporting (reporting bias) | High risk | Adverse effects not clearly reported |
| Complete follow‐up? | High risk | 32/38 were analysed (84%) |
| Potential bias related to study funding | Unclear risk | Merck Sharpe and Dohme provided drug and placebo |
Ezcurdia 1998.
| Methods | Computer‐generated randomisation. Allocation concealment not described Double‐blind, cross‐over trial 52 women randomised, 44 analysed (3 lost to follow‐up, 1 dropped out due to inefficacy of treatment, 2 used rescue medication in the hour after treatment dose, 2 excluded due to non‐compliance) Method of assessing adverse effects: evaluated retrospectively by "spontaneous reports and non‐suggestive questioning" | |
| Participants | Inclusion: women aged 18 to 40 with minimum 4‐month history of dysmenorrhoea; regular cycles; gynaecological exam and/or ultrasound to exclude organic causes; moderate to severe pain that requires analgesia 75% of the time Exclusion: secondary dysmenorrhoea; OCP use in last 2 months; IUD use; concomitant confounding medication; GI disease; asthma; psychiatric or physical illness; pregnant Age: mean 24.6 years, range 18 to 38 Location: Spain | |
| Interventions | Dexketoprofen 12.5 mg Dexketoprofen 25 mg Racaemic ketoprofen 50 mg Placebo Taken at the start of pain every 6 hours, max. 4 per day for max. 3 days Duration: 4 cycles, 1 cycle of each treatment | |
| Outcomes | Pain intensity (100 mm VAS) Pain relief Ability to perform daily activities Remedication | |
| Notes | Rescue medication was naproxen 500 mg. This review has used the 25 mg dose of dexketoprofen for the purpose of comparison. No denominators reported for pain relief data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "neither patient or doctor was aware of which preparation the patient was taking" |
| Selective reporting (reporting bias) | Low risk | Adverse event data solicited prospectively |
| Complete follow‐up? | High risk | 44/52 were analysed (85%) |
| Potential bias related to study funding | Unclear risk | Not stated |
Facchinetti 2001.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel‐group trial 308 women randomised 304 women analysed 4 women withdrew (1 due to pregnancy, 1 due to side effects, 2 for unknown reasons) Method of assessing adverse effects: recorded retrospectively at follow‐up | |
| Participants | Inclusion: healthy women who required analgesia in the last 6 months because of menstrual cramps, regular menstrual cycles Exclusion: other gynaecological disorders, malignancy, renal, cardiac, haematological or gastrointestinal disease, use of sedatives or muscle relaxants within 48 hours of expected menstrual period, pregnancy (all had pregnancy test) Age: nimesulide group 29.2 +/‐ 6.7 years, diclofenac group 30 +/‐ 3.2 years Source: women attending gynaecology clinics at 4 hospitals in Italy | |
| Interventions | Nimesulide 100 mg Diclofenac 50 mg Up to 3 tablets per day according to need, for the first 3 days of menstrual cycle Duration: 2 cycles "Double dummy" technique: active drug accompanied by placebo resembling alternative treatment | |
| Outcomes | Abdominal pain severity: 100 mm VAS Headache, back pain: 1 to 3 Likert scale Ability to function Adverse effects Global evaluation by woman and clinician | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, placebo‐matched active comparator |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects "reported at each evaluation visit" |
| Complete follow‐up? | Low risk | 304/308 analysed (99%) |
| Potential bias related to study funding | Low risk | Helinski Healthcare supported this study |
Fedele 1989.
| Methods | Randomisation/allocation method unclear
Double‐blind, parallel trial
152 women given placebo in initial pretreatment cycle, of whom 55 responded
45 women randomised to comparison of interest: an additional 10 randomised to pirprofen (drug withdrawn) Placebo response based on 31 Primary outcome data available for all women (14 experimental, 31 placebo) |
|
| Participants | Women with moderately severe primary dysmenorrhoea who responded to initial cycle of placebo, gynaecological exam, sonography of pelvis, clinical history to confirm no secondary cause for symptoms | |
| Interventions | Naproxen 250 mg twice a day for 3 days Identical placebo | |
| Outcomes | Pain relief Absenteeism Adverse effects | |
| Notes | Results for most outcomes; pooled NSAIDs (naproxen and pirprofen) versus placebo: primary purpose of study was to explore placebo effect. Adverse effect data pooled for both active treatments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical placebo" |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects not reported separately for all groups |
| Complete follow‐up? | Low risk | All patients completed the first cycle |
| Potential bias related to study funding | Unclear risk | Not stated |
Gleeson 1983.
| Methods | Randomisation used a random numbers table Cross‐over, double‐blind trial 31 women randomised, 27 analysed Withdrawals: 1 pregnancy, 3 wished to start OCP Method of assessing adverse effects: self reported retrospectively after each menstrual period | |
| Participants | Inclusion: regular cycles, severe primary dysmenorrhoea, good physical health Exclusion: use of IUD or OCP, asthma, hepatic or renal disease Age: 16 to 31, mean 21.7 Source: GPs Location: Canada | |
| Interventions | Ketoprofen (dose not mentioned, every 4 to 6 hours, no more than 4 per day, max. 3 days) Placebo Taken at onset of menstruation or onset of dysmenorrhoea. Duration: 3 cycles each treatment/6 cycles in total | |
| Outcomes | Pain severity scores Adverse effects | |
| Notes | Analyses to check if treatment order affected results, no difference in groups found | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Low risk | Adverse effects data solicited prospectively |
| Complete follow‐up? | High risk | 27/31 analysed (87%) |
| Potential bias related to study funding | Unclear risk | Not stated |
Hamann 1980.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over study 30 women, 26 analysed, 2 women became pregnant, 1 developed ovarian cysts, 1 did not comply with study rules Method of assessing adverse effects: recorded retrospectively at follow‐up | |
| Participants | Inclusion: severe primary dysmenorrhoea confirmed by normal gynaecological exams, women with considerable intake of analgesics and/or days of sick leave due to dysmenorrhoea Exclusion: contraindications to NSAIDs, hepatic or renal disease, gastrointestinal ulcers, treatment with sex hormones, use of OCP in previous month Age: 15 to 45, mean 25.9 Parity: 19 never been pregnant, 2 pregnant but no children, 9 at least 1 birth Location: Denmark | |
| Interventions | Naproxen (500 mg initially then 250 mg as needed, max. daily dose 1250 mg) Placebo Taken at first sign of menstrual distress, for no more than 4 days Duration: 2 cycles per treatment, 4 in total Additional analgesia was allowed if no pain relief was achieved 3 to 4 hours after first treatment dose; women were allowed to take whichever analgesic they had used prior to the study | |
| Outcomes | List of symptoms and number of women experiencing them before and after treatment Adverse effects | |
| Notes | No denominator reported for adverse effect data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | "Side effects noted at follow up visits" |
| Complete follow‐up? | Unclear risk | 27/30 analysed (90%) |
| Potential bias related to study funding | Unclear risk | Not stated |
Hanson 1978.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel trial 69 women randomised, 64 analysed (experimental n = 29, control n = 35) Withdrawals: 4 lost to follow‐up, 1 adverse effects Method of assessing adverse effects: not stated | |
| Participants | Inclusion: women with primary dysmenorrhoea, complete physical and pelvic exams Exclusion: organic causes for dysmenorrhoea, cyclical irregularities Age: 17 to 38, experimental group mean 24.2, control group mean 23.3 Source: referrals to outpatient clinic Location: USA | |
| Interventions | Naproxen sodium (550 mg initially, then 275 mg every 6 hours as needed, max. 5 days) Placebo Taken at first sign of menstrual distress Duration: 3 cycles If the test medication did not provide pain relief women could taken additional analgesics or their next treatment dose sooner | |
| Outcomes | Pain relief: 6‐point scale (reported in graph form as each woman's score, and also numbers with moderate relief etc) Interference with daily activities: 6‐point scale Requirement for additional analgesia Adverse effects | |
| Notes | The 2 treatment groups were comparable at baseline. No numerical data reported for adverse effects in placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | High risk | Adverse effects not reported for both groups |
| Complete follow‐up? | Unclear risk | 64/69 analysed (93%) |
| Potential bias related to study funding | High risk | Syntex supported study and were part of authorship group |
Heidarifar 2014.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group treatment trial 75 women randomised and analysed of whom 50 received mefenamic acid or placebo (third group received Dill); 47 included in analysis |
|
| Participants | Female university nursing students with primary dysmenorrhoea aged 18 to 28 Included: women with primary dysmenorrhoea Excluded: women with mild or secondary dysmenorrhoea, pelvic, organic or systemic disorder, menstrual irregularity, drug sensitivity, taking any medication |
|
| Interventions |
From 2 days before menstruation for 5 days |
|
| Outcomes | Rate of satisfaction with pain relief after treatment AEs ‐ total AEs not calculable so only GI AEs included |
|
| Notes | Emailed authors in Iran asking whether results have been published or are available (6 March 2014) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Not specific ‐ "the researchers and the participants were uninformed of allocating manner of each group" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, researchers and outcome assessment blinded |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Complete follow‐up? | Low risk | 47/50 (96%) of randomised women included in analysis |
| Potential bias related to study funding | Low risk | Funded by Qom University |
Henzl 1977b.
| Methods | Double‐blind, parallel trials Randomisation using a table of random numbers, sequentially assigned to a number as entered study 27 women, 23 analysed (experimental n = 12, placebo n = 11) Method of assessing adverse effects: not stated | |
| Participants | Inclusion: women seeking relief from dysmenorrhoea Exclusion: organic causes of dysmenorrhoea, cycle irregularities, concomitant gastrointestinal, hepatic and renal disorders, women on oral contraceptives or IUDs Age: experimental mean 24.4 (5.2), control mean 24.2 (6.8) Parity: 2 previously pregnant Location: USA | |
| Interventions | Naproxen‐Na (550 mg at first sign of distress then 275 mg every 6 hours for a minimum of 3 days, a max. 5 days, maximum daily dose 1650 mg for first day, 1375 mg for subsequent days) Placebo Duration: 4 cycles If first dose not effective within 2 hours women could take their second dose then, if still no relief after 2 hours (4 hours after 1st dose) then additional analgesics were allowed | |
| Outcomes | Pain relief: 6‐point scale Individual relief scores reported for every cycle and treatment Additional analgesia required Adverse effects | |
| Notes | Women comparable at baseline within each study. No numerical data reported on adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identically appearing" placebo |
| Selective reporting (reporting bias) | High risk | Adverse effect data not systematically reported |
| Complete follow‐up? | High risk | 23/27 (85%) |
| Potential bias related to study funding | Unclear risk | Syntex |
Iacovides 2014.
| Methods | Randomisation based on Latin square design, methods of allocation and allocation concealment not described Double‐blind, cross‐over trial |
|
| Participants | Female university students with a history of primary dysmenorrhoea, starting shortly after menarche, who were nulliparous and not taking chronic medication (including oral contraceptives) for at least 6 months before the study. In addition, the 30‐item version of the General Health Questionnaire was used for psychological screening, and only women who scored less than 6 (indicating normal psychological status) were included in the study | |
| Interventions | Diclofenac potassium 50 mg 3 times a day Placebo 3 times a day Participants had 1 cycle of each drug |
|
| Outcomes | Menstrual pain severity (on VAS 1 to 10), adverse events, rescue medications ‐ recorded in diary | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs disguised in identical gelatine capsules |
| Selective reporting (reporting bias) | Low risk | Adverse effects data prospectively solicited |
| Complete follow‐up? | Low risk | All randomised women included in analysis |
| Potential bias related to study funding | Low risk | Funded by academic institution |
Ingemanson 1984.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 28 women analysed and randomised Method of assessing adverse effects: not stated | |
| Participants | Inclusion: moderate to severe dysmenorrhoea; gynaecological and physical exams Exclusion: secondary dysmenorrhoea; ulcers Age: mean (SD) 31 (8.5) Location: Sweden | |
| Interventions | Diclofenac sodium 50 to 150 mg Naproxen 250 to 1250 mg Duration: 2 cycles, 1 of each treatment | |
| Outcomes | Pain relief (5‐point scale) Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, double dummy: placebo not further described |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively collected |
| Complete follow‐up? | Low risk | No losses, 28/28 analysed |
| Potential bias related to study funding | Unclear risk | Ciba‐Geigy |
Jacobson 1979.
| Methods | Randomisation/allocation method unclear. Double‐blind, parallel trial 40 women randomised, 34 analysed (experimental n = 16, placebo n = 18) No info on dropouts Method of assessing adverse effects: self reported prospectively "on specially printed cards" | |
| Participants | Inclusion: primary dysmenorrhoea, medical, gynaecological and physical exams Exclusion: cycle irregularities, use of hormonal contraceptives, pelvic pathology, history of gastrointestinal disorders, hepatic and renal disease Age: 15 to 40 Location: Sweden | |
| Interventions | Naproxen (loading dose 250 mg to 500 mg then 250 mg every 4 to 6 hours as needed, max. daily dose 1500 mg) Placebo Duration: 2 cycles If treatment drug ineffective women could use additional analgesics | |
| Outcomes | Pain relief: 5‐point scale Adverse effects | |
| Notes | 2 groups comparable at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Low risk | Adverse effects data prospectively solicited |
| Complete follow‐up? | High risk | 34/40 analysed (85%) |
| Potential bias related to study funding | Unclear risk | Astra Syntex authors |
Jacobson 1983.
| Methods | Randomisation/allocation method unclear. Double‐blind, cross‐over study 39 women randomised and analysed Method of assessing adverse effects: self reported prospectively "on specially printed cards" | |
| Participants | Inclusion: primary dysmenorrhoea, women on treatment with oral contraceptives but not receiving relief, full medical and gynaecological exam Exclusion: women with organic causes of dysmenorrhoea, women with contraindications for taking prostaglandin synthetase inhibitors Age: 16 to 40 Location: Sweden | |
| Interventions | Naproxen (500 mg at onset then 250 mg every 4 to 6 hours as needed, max. daily dose 1250 mg) Placebo Duration: 2 cycles each treatment, 4 cycles in total If the test drug did not alleviate pain within 4 hours the women were allowed supplementary analgesics | |
| Outcomes | Pain relief (5‐point scale) Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects data prospectively solicited but not reported for both groups |
| Complete follow‐up? | Low risk | No losses |
| Potential bias related to study funding | Unclear risk | Astra Syntex authors |
Kajanoja 1978.
| Methods | Randomisation/allocation method unclear Cross‐over, double‐blind trial 47 women randomised, 269 cycles analysed, 90 indomethacin, 89 aspirin, 90 placebo Method of assessing adverse effects: self reported prospectively on report cards | |
| Participants | Inclusion: primary dysmenorrhoea, nulliparous, unsatisfactory relief from analgesics Exclusion: use of OCP, specific aetiology of dysmenorrhoea, or symptoms suggesting specific aetiology such as endometriosis Age: 17 to 28, mean 22.8 Location: Finland | |
| Interventions | Indomethacin (25 mg, 3 times daily at first sign of distress for at least 2 days) Aspirin (500 mg, taken as above) Placebo Duration: 2 cycles per treatment/6 cycles in total | |
| Outcomes | Degree of pain Overall effect Adverse effects | |
| Notes | Outcomes recorded per cycle rather than per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Low risk | Adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | Unclear: analysed as cycles |
| Potential bias related to study funding | Unclear risk | Dumex supplied drug |
Kajanoja 1984.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 22 women randomised, 19 analysed 2 women moved out of area, 1 failed to attend follow‐up Method of assessing adverse effects: self reported prospectively on report cards | |
| Participants | Inclusion: severe primary dysmenorrhoea Age: 19 to 31, mean 23.4 Location: Finland | |
| Interventions | Diflunisal 250 mg Naproxen 250 mg Taken 4 times a day as needed, start at first sign of distress and continue as needed Duration: 4 cycles, each cycle randomised | |
| Outcomes | Relief of dysmenorrhoeic symptoms Adverse effects | |
| Notes | Outcomes recorded per cycle rather than per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects data prospectively solicited but reported by cycles |
| Complete follow‐up? | High risk | 19/22 were analysed (86%) |
| Potential bias related to study funding | Unclear risk | Dumex supplied drug |
Kapadia 1978.
| Methods | Randomisation/allocation method unclear Cross‐over, double‐blind trial 44 women randomised and analysed Method of assessing adverse effects: not stated | |
| Participants | Inclusion: primary dysmenorrhoea, medical and gynaecological exam Exclusion: pelvic abnormality, history of dyspepsia or peptic ulceration, use of OCP Age: 15 to 42, mean 22.6 Location: UK | |
| Interventions | Flufenamic acid (200 mg 3 times daily while dysmenorrhoea persisted, encouraged to start medication a few hours prior to menses) Placebo Duration: 3 cycles per treatment/6 in total | |
| Outcomes | Pain relief (4‐point scale) Adverse effects | |
| Notes | First‐phase data shown on graph as mean pain relief | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Data on adverse effects not systematically collected and/or reported |
| Complete follow‐up? | Low risk | 40/40 women analysed |
| Potential bias related to study funding | Unclear risk | Parke‐Davis supplied drug and placebo |
Kintigh 1995.
| Methods | Randomisation/allocation method unclear A multicentre, double‐blind, parallel trial 410 women randomised 383 analysed Method of assessing adverse effects: self reported prospectively in diary | |
| Participants | Inclusion: primary dysmenorrhoea (moderate to severe pain for at least 3 months), pain in at least 80% menses, regular menses, previous response to NSAIDs, physical and pelvic exam Exclusion: any disease that could interfere with the evaluation of efficacy, use of other NSAIDs or analgesics in previous 24 hours, use of OCP or IUD, active ulcer disease, renal or hepatic impairment, congestive heart failure Age: 16 to 42 Location: USA | |
| Interventions | Diclofenac potassium (50 mg 3 times a day with 50 mg loading dose) Diclofenac potassium (50 mg 3 times a day) Naproxen sodium (275 mg 3 times a day with 275 mg loading dose) Placebo Taken at the onset of moderate to severe menstrual pain for 3 days Duration: 2 cycles Additional analgesics were allowed if no pain relief achieved at least 1 hour after dose was taken | |
| Outcomes | Pain relief ‐ 5‐point scale assessed at 15 minutes, 30 minutes then hourly for 8 hours following dose Mean pain relief scores reported for each group in graph form Adverse effects listed as percentages | |
| Notes | No statistically significant demographic or baseline differences between treatment groups, except the placebo group had the smallest percentage of women with severe baseline pain when compared with active treatment groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "matching placebo" |
| Selective reporting (reporting bias) | Low risk | Adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 383/410 analysed (93%) |
| Potential bias related to study funding | Unclear risk | Unclear |
Layes Molla 1974.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 67 women randomised and analysed 7 of these only completed 1 cycle (1 found tablet hard to swallow, 1 dropped out for unspecified reasons, 2 lack of drug effect (1 on each treatment), 3 due to side effects (2 ibuprofen, 1 paracetamol) Method of assessing adverse effects: assessed retrospectively by physician at follow‐up | |
| Participants | Inclusion: primary dysmenorrhoea, women aged 18 to 26 Exclusion: known gynaecological disease or abnormalities; history of peptic ulceration; gastrointestinal, haemorrhage, kidney or liver dysfunction; irregular cycles; IUD or OCP use; wish to get pregnant Location: UK | |
| Interventions | Ibuprofen 200 mg Paracetamol 500 mg 2 capsules, taken 3 times a day, 24 hours prior to pain for a total of 4 days Duration: 2 cycles, 1 per treatment | |
| Outcomes | Global pain assessment (worse, no change, better, much better) Degree of pain relief Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Low risk | No losses |
| Potential bias related to study funding | Unclear risk | Unclear |
Legris 1997.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 69 women randomised, 62 analysed 3 dropouts before end of first cycle, 1 receiving niflumic acid had amenorrhoea, 2 for personal reasons 4 additional women left prior to completing the 2nd treatment, 1 for personal reasons, 1 hospitalised for depression, 1 pregnancy, 1 lost to follow‐up. In addition a woman initially randomised to group 1 (niflumic/placebo) was transferred to the other group and evaluated accordingly Method of assessing adverse effects: self reported prospectively | |
| Participants | Inclusion: primary or essential dysmenorrhoea for more than 6 months; pain with a equal or greater severity than 50 mm on a 100 mm VAS; regular cycles Exclusion: organic origin of dysmenorrhoea; OCP use; IUD use; pregnant; contraindication to NSAIDs; concomitant illness; chronic alcoholism or drug addition Age: group 1: mean 30.6 (8.0), group 2 mean 29.1 (6.4) Source: outpatients Location: France | |
| Interventions | Niflumic acid 750 mg per day in 3 divided doses Placebo Taken for 3 days Duration: 2 cycles, 1 per treatment | |
| Outcomes | Pain relief (efficacy on 4‐point scale) Treatment efficacy evaluated by investigator and participant Pain severity Effect on daily activities Adverse effects | |
| Notes | Groups compared at baseline. French with an English abstract, translated by Richmal Oates‐Whitehead | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse events data prospectively solicited |
| Complete follow‐up? | Unclear risk | 62/69 analysed (90%) |
| Potential bias related to study funding | Unclear risk | Author affiliations with Laboratories UPSA |
Letzel 2006.
| Methods | Randomisation/allocation concealment: computer‐generated sequence; opaque, sequentially numbered envelopes Double‐blind, 3‐way cross‐over design 127 women randomised, 89/127 analysed for efficacy, 99/127 for safety 28 not analysed for efficacy (9 dropped out, 19 did not have data for at least 1 evaluable cycle) |
|
| Participants | Included: women with primary dysmenorrhoea in at least 4 of previous 6 cycles, aged 18 to 45, regularly menstruating Excluded: women with other pelvic pathology, gastric problems, pregnant, lactating, not using suitable contraception, drug sensitivities, serious illness, use of intrauterine device or oral contraceptives within past 6 months |
|
| Interventions | Aceclofenac 100 mg Naproxen 500 mg Placebo Take when pain > 60 on VAS, for 2 cycles each. Rescue medication (paracetamol) could be taken after 2 hours if necessary |
|
| Outcomes | Pain intensity on VAS, adverse events | |
| Notes | Efficacy data not included, < 80% analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque, sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | High risk | 89/127 analysed for efficacy (70%), 99/118 for safety (84%) |
| Potential bias related to study funding | Unclear risk | Funded by Almirall Prodesfarma |
Lopez Rosales 1989.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel trial 40 women randomised and analysed (plus additional 20 women on drug now withdrawn) Method of assessing adverse effects: assessed retrospectively at follow‐up | |
| Participants | Inclusion: primary or incapacitating dysmenorrhoea for at least 6 months, clinically and generally healthy women, pain susceptible to pharmacological treatment Exclusion: pelvic‐genital pathology, secondary dysmenorrhoea, IUD use, irregular menstrual cycles, OCP use, anticoagulants, gastric or peptic disease, hypersensitivity to anti‐inflammatories or steroids Age: 18 to 36, mean 28.5 (4.7) Location: Mexico | |
| Interventions | Nimesulide (100 mg every 12 hours) Fentiazac (100 mg every 12 hours) Mefenamic acid (500 mg every 8 hours) Medication taken 3 times a day with placebo tablets added to ensure blinding was maintained, treatment for 5 days starting day prior to menses Duration: 3 months No use of analgesics or anti‐inflammatories during study period | |
| Outcomes | Pain intensity 0 to 10 scale | |
| Notes | Spanish ‐ translated by Monica C Davis. Outcomes recorded per cycle rather than per participant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, identical placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited. Data reported per cycle not per woman |
| Complete follow‐up? | Unclear risk | Unclear |
| Potential bias related to study funding | Unclear risk | Unclear |
Malmstrom 2003.
| Methods | Computer‐generated randomisation sequence Allocation schedule concealed from investigator, study women and sponsor Double‐blind, cross‐over design 73/73 analysed 13 discontinued treatment |
|
| Participants | Included: women with primary dysmenorrhoea of self reported moderate or severe intensity during at least 4 of previous 6 cycles, aged at least 18 years, no allergies to NSAIDs, negative serum beta‐hCG test, no intrauterine device, no abnormalities of reproductive organs Excluded: women with secondary dysmenorrhoea, history of bleeding disorders, drug or alcohol abuse, pregnancy, other medical conditions (listed in paper) Age: mean 31 years (range 19 to 45) Location: USA |
|
| Interventions | Etoricoxib 120 mg Naproxen 550 mg Placebo Medicate with 1 dose at onset of moderate to severe pain 3 cycles |
|
| Outcomes | Pain relief score (TOTPAR) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Allocation schedule concealed from investigator, study women and sponsor |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "matching" placebo |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Low risk | 73/73 analysed, 13 discontinued treatment |
| Potential bias related to study funding | Unclear risk | Merck authors |
Marchini 1995.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 60 women randomised Method of assessing adverse effects: not stated | |
| Participants | Inclusion: primary dysmenorrhoea for at least 3 months duration; regular cycles; moderate to severe pain in the majority of cycles and in 3 cycles prior to study; medical and gynaecological exam performed Exclusion: secondary dysmenorrhoea; nursing mothers; pregnant women; sexually active women not using reliable contraception; OCP or IUD use; any contraindications to NSAIDs Age: 16 to 40 Source: outpatients Location: Italy | |
| Interventions | Diclofenac 50 mg Ibuprofen 400 mg Placebo Taken 4 x day for a max. of 3 days Duration: 3 cycles, one per treatment | |
| Outcomes | Pain relief Pain intensity Rescue medication Global assessment | |
| Notes | Double‐dummy used to maintain blinding as diclofenac and ibuprofen are different sizes etc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, matching double dummy |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 54/60 analysed (90%) |
| Potential bias related to study funding | Unclear risk | Ciba‐Geigy authors |
Mehlisch 1990.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 70 women randomised, 60 analysed Withdrawals: 7 women did not take any study medication, 3 women did not have acceptable efficacy data for 2 or more cycles Method of assessing adverse effects: evaluated retrospectively by "spontaneous reports and non‐suggestive questioning" | |
| Participants | Inclusion: primary dysmenorrhoea with moderate to severe pain for at least 4 consecutive months Exclusion: any other conditions associated with recurrent pelvic or lower abdominal pain; hypersensitivity to aspirin or NSAIDs; serious illness; history of substance abuse; IUD use; pregnant or nursing Age: 18 to 39 Location: USA | |
| Interventions | Ketoprofen (3 groups: loading dose of 25 mg, 50 mg or 75 mg then 25 mg doses) Naproxen (500 mg loading dose then 250 mg doses) Placebo Taken 4 times a day for 3 days starting when pain moderate to severe Duration: 3 cycles; 1 of each treatment | |
| Outcomes | Pain relief Remedication | |
| Notes | This review has considered the 75 mg loading dose of ketoprofen, for the purpose of comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | High risk | 60/70 women analysed (86%) |
| Potential bias related to study funding | Unclear risk | Wyeth‐Ayerst sponsors |
Mehlisch 1997.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 57 women randomised, 51 to 54 analysed (51 completed all cycles, 54 at least 1 cycle) Method of assessing adverse effects: women were asked "non‐specific questions" at follow‐up | |
| Participants | Inclusion: primary dysmenorrhoea for at least 4 consecutive cycles with moderate to severe pain; medical, gynaecological and general exams Exclusion: dysmenorrhoea onset more than 3 years after menarche; secondary dysmenorrhoea; pregnant, breastfeeding or planning pregnancy; history of hypersensitivity to drugs; history of drug abuse; previous gynaecological surgery; use of OCP or IUD Age: mean (SD) 32.2 (7.3), range 18 to 45 Location: USA | |
| Interventions | Bromfenac sodium (10 mg or 50 mg) Naproxen sodium (550 mg loading dose then 275 mg doses) Placebo Taken up to 4 times a day, starting at onset of moderate pain Duration: 4 cycles, 1 of each treatment | |
| Outcomes | Global pain assessment (worse, no change, better, much better) Adverse effects | |
| Notes | Bromfenac withdrawn from market, not included in comparisons. Global assessment scores (categorical) reported as continuous scores. Entered as additional data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Low risk | Adverse events data prospectively solicited |
| Complete follow‐up? | High risk | 51/57 analysed (89%) |
| Potential bias related to study funding | Unclear risk | Unclear |
Mehlisch 2003.
| Methods | Allocation concealment unclear Randomisation sequences as described by "Ratkowsky" Double‐blind with matching placebo, cross‐over design 104/104 analysed 21/104 did not complete the study |
|
| Participants | Included: women with primary dysmenorrhoea, in good health, no gastric disease, not using oral contraception Excluded: women with secondary dysmenorrhoea, taking concomitant drugs Location: USA |
|
| Interventions | Ibuprofen 200 or 400 mg Placebo Medicate with single dose at pain onset 5 cycles |
|
| Outcomes | Pain intensity rating | |
| Notes | Data unsuitable for meta‐analysis ‐ reported per cycle | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised sequences as described by "Ratkowsky" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "matching" placebo |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited, reported per cycle |
| Complete follow‐up? | Low risk | 21/104 did not complete the study; 104/104 analysed |
| Potential bias related to study funding | Unclear risk | Scirex Corporation |
Milsom 1985.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over study 60 women randomised, 57 analysed Withdrawals: 1 pregnancy, 2 failed to attend 2nd assessment Method of assessing adverse effects: unclear ‐ "information was collected from all women" | |
| Participants | Inclusion: women with primary dysmenorrhoea, clinical and gynaecological exams Exclusion: IUD use, history of pelvic pathology, peptic ulcer, severe dyspepsia or asthma Age: 15 to 45, mean 26.1 Parity: 39 nulliparous, 18 parous Contraceptives: 12 used OCP, remainder used barrier or no contraception Location: Sweden | |
| Interventions | Ibuprofen (400 mg 3 times a day, for 5 days) Naproxen sodium (250 mg, 2 times a day, 1 placebo to match other treatment) Duration: 4 cycles/2 each treatment Additional analgesics allowed | |
| Outcomes | Pain relief (efficacy on 4‐point scale) Treatment efficacy evaluated by investigator and participant Pain severity Effect on daily activities Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" drugs |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 57/60 randomised (95%) |
| Potential bias related to study funding | Low risk | Study sponsored by University of Goteborg |
Milsom 2002d.
| Methods | Randomisation: computer‐generated. Medications coded and numbered sequentially Allocation method: women assigned numbered study medication in increasing order as they enrolled Double‐blind, cross‐over trial 117 women randomised 98 analysed for efficacy 117 analysed for safety Withdrawals: 19/117 (16%) for efficacy Method of assessing adverse effects: unclear ‐ "information was collected from all women" | |
| Participants | Inclusion: at least 4 painful cycles in past 6 months with at least moderate pain. Aged at least 16, 21‐ to 36‐day cycle. Onset of cramps at least 4 hours before menstruation Exclusion: pregnancy, breastfeeding, idiosyncratic response to any of trial medications, co‐existing illnesses, previous use of narcotics for dysmenorrhoea, use of concomitant medications that could interfere with trial treatment, use of OTC medications at higher than recommended doses, any contraception except condoms | |
| Interventions | Naproxen sodium at OTC doses: i.e. 1 to 2 x 220 mg tabs, repeat up to max. daily dose of 660 mg Paracetamol at OTC doses: i.e. 1 to 2 x 500 mg tabs, repeat up to max. daily dose of 4000 mg Placebo No alcohol or illegal drugs for 4 to 12 hours before menstrual flow or for next 3 days | |
| Outcomes | Pain relief Adverse effects | |
| Notes | Adverse effects data pooled with results from other studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation: computer‐generated. Medications coded and numbered sequentially |
| Allocation concealment (selection bias) | Low risk | Adequate allocation method: women assigned numbered study medication in increasing order as they enrolled |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 117 women randomised 98 analysed for efficacy (84%) 117 analysed for safety (100%) |
| Potential bias related to study funding | Unclear risk | Study sponsored by Roche and authors had affiliations with Roche |
Milsom 2002e.
| Methods | Randomisation: computer‐generated. Medications coded and numbered sequentially Allocation method: women assigned numbered study medication in increasing order as they enrolled Double‐blind, cross‐over trial 87 women randomised 81 analysed for efficacy 82 for safety Withdrawals: 6 (7%) for efficacy (1 improperly enrolled, 1 lost to follow‐up, 1 disallowed medication, 3 no explanation) Method of assessing adverse effects: unclear ‐ "information was collected from all women" | |
| Participants | Inclusion: at least 4 painful cycles in past 6 months with at least moderate pain. Aged at least 16, 21‐ to 36‐day cycle. Onset of cramps at least 4 hours before menstruation Exclusion: pregnancy, breastfeeding, idiosyncratic response to any of trial medications, co‐existing illnesses, previous use of narcotics for dysmenorrhoea, use of concomitant medications that could interfere with trial treatment, use of OTC medications at higher than recommended doses, any contraception except condoms | |
| Interventions | Naproxen sodium at OTC doses: i.e. 1 to 2 x 220 mg tabs, repeat up to max. daily dose of 660 mg Ibuprofen at OTC doses: i.e. 1 to 2 200 mg tabs, up to 1200 mg daily Back‐up medication allowed, but to try study medication first | |
| Outcomes | Pain Need for remedication Adverse effects | |
| Notes | Adverse effects data pooled with results from other studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation: computer‐generated. Medications coded and numbered sequentially |
| Allocation concealment (selection bias) | Low risk | Adequate allocation method: women assigned numbered study medication in increasing order as they enrolled |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 87 women randomised 81 analysed for efficacy (93%) 82 for safety (94%) |
| Potential bias related to study funding | Unclear risk | Study sponsored by Roche and authors had affiliations with Roche |
Moggian 1986.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 67 women randomised, 55 analysed Method of assessing adverse effects: unclear | |
| Participants | Inclusion: primary dysmenorrhoea for at least 6 months, nulliparous Exclusion: gastric or duodenal ulcers, history of intolerance to NSAIDs Age: 14 to 33 years Location: Italy | |
| Interventions | Nimesulide, 50% of women received a 50 mg tablet twice a day, the other 50% received 100 mg twice a day Placebo 3 cycles randomised to active treatment‐placebo‐active treatment (A‐P‐A) or the opposite (P‐A‐P) Treatment taken for 7 days, 4 days prior to menses and 3 days during menses | |
| Outcomes | Abdominal pain Back pain Other symptoms | |
| Notes | Italian, partially translated using altavista Babelfish website. No numerical data on adverse effects reported for placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | High risk | 67 women randomised, 55 analysed (82%) |
| Potential bias related to study funding | Unclear risk | Unclear |
Morrison 1979.
| Methods | Randomisation/allocation method unclear Parallel, double‐blind trial 32 women randomised and analysed Method of assessing adverse effects: unclear ‐ authors state "side effects were described" | |
| Participants | Inclusion: primary dysmenorrhoea Exclusion: secondary dysmenorrhoea, contraindications to indomethacin therapy Age: average 21, range 16 to 23 Parity: mostly nulligravidae Location: USA | |
| Interventions | Indomethacin (25 mg, 3 times daily, from 2 days prior to menses to 1 day after symptoms usually end) Placebo Duration: 2 cycles control (establish a baseline), 4 cycles treatment | |
| Outcomes | Change in pain Adverse effects | |
| Notes | Groups same at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | No evidence that adverse effects data prospectively solicited |
| Complete follow‐up? | Low risk | No losses |
| Potential bias related to study funding | Unclear risk | Unclear |
Morrison 1980.
| Methods | Randomisation/allocation method unclear Triple‐blind, cross‐over trial 55 women randomised, 51 analysed (4 did not complete all 3 cycles) Method of assessing adverse effects: by prospective daily self report | |
| Participants | Inclusion: primary dysmenorrhoea requiring analgesics; pelvic exam to rule out organic causes Exclusion: secondary dysmenorrhoea Location: USA | |
| Interventions | Ibuprofen 200 mg Propoxyphene hydrochloride 64 mg Placebo 2 capsules taken every 4 hours Duration: 3 cycles, 1 of each treatment | |
| Outcomes | Global pain assessment (worse, no change, better, much better) Degree of pain relief Adverse effects | |
| Notes | Authors state that no "relevant" adverse effects were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 55 women randomised, 51 analysed (94%) |
| Potential bias related to study funding | Unclear risk | Upjohn sponsored and affiliated with authors |
Morrison 1999.
| Methods | Masked allocation, computer‐generated randomisation Double‐blind, cross‐over design 118/127 completed the protocol 13 discontinued due to protocol violation or withdrawal of consent |
|
| Participants | Included: women with self reported moderate or severe primary dysmenorrhoea aged over 18 years, otherwise healthy no evidence of other causes of dysmenorrhoea on gynaecological examination within previous year, negative beta‐hCG test, no allergies to NSAIDs Excluded: breastfeeding mothers, women with alcohol or drug abuse, women taking other medications (listed in paper) Age: 31 (range 18 to 44) years |
|
| Interventions | Naproxen 550 mg Placebo Medicate every 12 hours or as needed for up to 3 days 4 cycles |
|
| Outcomes | Pain relief score (TOPAR) | |
| Notes | Study also includes rofecoxib (since withdrawn) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | "Masked allocation schedule" concealed from all involved with the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 114/127 completed the protocol, 118/127 analysed (93%) |
| Potential bias related to study funding | Unclear risk | Merck affiliated with the authors |
Nahid 2009.
| Methods | Randomised, parallel‐group, double‐blind, placebo‐controlled pilot study | |
| Participants | Included 180 female students at Isfahan University, aged 18 to 27 with self reported primary dysmenorrhoea; 120 relevant to current review "In the mefenamic acid group, 5/60 (8%) students were excluded from the study analysis: two students were lost to follow‐up and three were excluded because of discontinuation of medication or use of other pain relief drugs. In the placebo group, 9/60 (15%) participants were excluded: four with severe pain, discontinuation of medication, and use of other sedation, and five due to loss of follow‐up" |
|
| Interventions | 1. Mefenamic acid (n = 60) 2. Placebo (n = 60) [3. Herbal drug (n = 60): this arm excluded] 2 to 3‐month follow‐up |
|
| Outcomes | Pain severity score on 1 to 10 VAS: reports median and ranges only, with P values Pain intensity after treatment: severe, moderate, mild, no pain. Dichotomised for this review as severe or moderate versus mild or none Requirement for additional medication At 2 and 3 months |
|
| Notes | Conducted in Iran, fully funded by Isfahan Medical University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Both drugs and as well as placebo were packed in similar capsules (blue capsule) and packaged in similar wrappings". Reported as double‐blinded |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | High risk | 106/120 analysed (88%) |
| Potential bias related to study funding | Low risk | Fully funded by Isfahan university |
Onatra 1994.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 31 women randomised and analysed Method of assessing adverse effects: by prospective self report on report card | |
| Participants | Inclusion: moderate to severe dysmenorrhoea; normal gynaecological exam and pelvic ultrasound; regular menstrual cycles with a minimum of 1 year duration Exclusion: pregnancy; lactation; abnormal bleeding; renal or hepatic dysfunction; use of OCP or IUD; use of anticoagulants, corticosteroids, analgesics or other NSAIDs Age: mean 18, range 13 to 20 Source: teenagers at high school Location: Colombia | |
| Interventions | Etodolac 300 mg (taken every 12 hours) Piroxicam 20 mg (taken every 12 hours, first dose real, second placebo to maintain blinding) Duration: 4 cycles, 1 cycle of each treatment | |
| Outcomes | Pain intensity, scale 1 to 3 Pain relief, scale 1 to 5 Adverse effects | |
| Notes | Trial in Portuguese, translated by Fabio Guidugli | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | Not stated |
| Potential bias related to study funding | Unclear risk | Not stated |
Osathanondh 1985.
| Methods | Randomisation/allocation method unclear Parallel, double‐blind trial 96 women randomised, 85 analysed Withdrawals: fenoprofen 200 mg group ‐ 1 amenorrhoeic, 5 decided not to participate prior to treatment; fenoprofen 400 mg group ‐ 2 amenorrhoeic, 2 decided not to participate, 1 had treatment stolen Method of assessing adverse effects: nurse phoned daily during menses to ask for report on adverse effects | |
| Participants | Inclusion: women with primary dysmenorrhoea who usually require analgesics, medical evaluation Exclusion: use of other anti‐inflammatory, analgesic, antispasmodic or tranquillising drugs on a daily basis, OCP use, allergies to any drugs Age: 21 to 30 Location: USA | |
| Interventions | Fenoprofen calcium 200 mg or fenoprofen calcium 400 mg up to 4 times daily during menses) Aspirin (as control, 650 mg, taken as above) Placebo Duration: 4 cycles Codeine sulphate or pethidine hydrochloride was provided as a rescue analgesic | |
| Outcomes | Pain scale 0 to 4 (5 points) Adverse effects | |
| Notes | The 200 mg dose of fenoprofen has been used for the purpose of comparison for pain relief in this review. Results for both doses of fenoprofen (200 mg and 400 mg) have been pooled for adverse effects data Where necessary, the placebo group of this study were halved, to avoid double‐counting in pooled analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Low risk | Adverse effect data collected systematically |
| Complete follow‐up? | High risk | 96 women randomised, 85 analysed (88%) |
| Potential bias related to study funding | High risk | Lilly sponsored |
Osinusi 1986.
| Methods | Randomisation/allocation method unclear Double‐blind Cross‐over design 50 women randomised, 47 completed all 4 cycles/analysed, 1 left group A after cycle 1 and 2 left group B after cycles 2 and 3; 48 or 49 analysed in each group Method of assessing adverse effects: not stated | |
| Participants | Inclusion: primary dysmenorrhoea; no organic cause on examination; older than 12 years; no history of peptic ulcer, hepatic, renal or haematological disease Age: mean 18.6, range 16 to 24 Source: medical, midwifery and nursing students Location: Nigeria | |
| Interventions | Piroxicam 20 mg Placebo 2 capsules on day 1 and day 2, then 1 capsule daily until end of menses Duration: 4 cycles; ABBA, or BAAB treatment design Paracetamol was allowed as additional medication, all use was recorded | |
| Outcomes | Abdominal cramps Pain‐related symptoms Minor symptoms Overall pain Paracetamol consumption | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all adverse effects data reported |
| Complete follow‐up? | Low risk | 50 women randomised, 47 completed all 4 cycles, 1 left group A after cycle 1 and 2 left group B after cycles 2 and 3, 48 or 49 analysed in each group (98%) |
| Potential bias related to study funding | Unclear risk | Pfizer provided drugs and placebo |
Pasquale 1988.
| Methods | Randomised by computer‐generated schedule Allocation method: not stated Double‐blind, parallel trial 74 women randomised, 68 analysed (4 violated protocol, 2 for personal reasons) Method of assessing adverse effects: by prospective self report on report card | |
| Participants | Inclusion: primary dysmenorrhoea; at least a 6‐month history of moderate to severe pain; physical and pelvic exam; effective method of birth control Exclusion: secondary dysmenorrhoea; nursing mothers; OCP use for less than 6 months; IUD use; systemic disease; women planning to donate blood during the study period; addiction to alcohol or drugs; treated with coagulants etc; use of long‐acting NSAIDs Age: 16 to 40 Location: USA | |
| Interventions | Piroxicam (3 groups; 20 mg daily; 40 mg loading dose then 20 mg for subsequent days; 40 mg for day 1 and 2, 20 mg for days 3, 4 and 5) Ibuprofen 400 mg 4 x daily Duration: 1 cycle | |
| Outcomes | Pain relief Supplemental medication Adverse reactions | |
| Notes | This review has pooled the results of all 3 doses of piroxicam, for the purpose of comparison. No numerical data reported on adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse effects prospectively solicited |
| Complete follow‐up? | Unclear risk | 74 women randomised, 68 analysed (4 violated protocol, 2 for personal reasons) 68/74 analysed (92%) |
| Potential bias related to study funding | Unclear risk | Pfizer provided assistance with the statistics |
Pauls 1978.
| Methods | Randomisation/allocation method unclear. Double‐blind, parallel trial Random, method unclear 17 women (experimental n = 9, placebo n = 8), 17/17 analysed Method of assessing adverse effects: not stated | |
| Participants | Exclusion: hormonal or intrauterine contraception Age: experimental mean 23.5, control mean 20.1 Parity: all nulligravidae Source: private practice Location: Canada | |
| Interventions | Naproxen sodium (550 mg initially then 275 mg every 6 hours as needed) Placebo Duration: 3 cycles Supplemental analgesics allowed | |
| Outcomes | Pain relief: 6‐point scale Reported as mean relief scores for each group Adverse effects | |
| Notes | Authors state that no adverse effects were observed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data on adverse effects prospectively solicited |
| Complete follow‐up? | Low risk | 17/17 analysed |
| Potential bias related to study funding | Unclear risk | Not stated |
Pedron 1995.
| Methods | Randomisation not stated Parallel, double‐blind trial 60 women randomised, number analysed unclear Method of assessing adverse effects: unclear | |
| Participants | Inclusion: severe dysmenorrhoea which interfered with daily activities, nulliparous, no IUD or OCP, healthy Age: 18 to 25 Location: Mexico | |
| Interventions | Ibuprofen (200 mg, every 8 hours while pain persisted for max. 5 days, start at pain onset) Mefenamic acid (500 mg, as above) Duration: 2 cycles | |
| Outcomes | Pain intensity (10‐point visual scale) | |
| Notes | Spanish, translation by Anne Lethaby | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | High risk | Adverse effects not reported |
| Complete follow‐up? | Unclear risk | Not stated |
| Potential bias related to study funding | Unclear risk | Not stated |
Powell 1981.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over design 77 women randomised 69 women analysed after 1 cycle, 65 after completing 6 cycles 8 women withdrew (moved, became pregnant, started oral contraceptive pill) Method of assessing adverse effects: reported retrospectively at follow‐up | |
| Participants | Inclusion: women with primary dysmenorrhoea, in good general health, "emotionally stable", regular menstrual cycles Exclusion: organic cause for dysmenorrhoea, oral or intrauterine contraception, actively seeking pregnancy, endocrine disorders affecting genitalia or menstruation Age: not stated Location: USA | |
| Interventions | Mefenamic acid 250 mg Placebo 1 capsule 4 times daily for a maximum of 3 days | |
| Outcomes | Pain 1 to 4 (4 points) Supplemental medication Adverse effects | |
| Notes | Codeine prescribed if required for extra analgesia, otherwise no extra analgesia permitted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical appearing" placebo |
| Selective reporting (reporting bias) | High risk | Only included adverse effects data that were "considered by the investigator as attributable to study medications" |
| Complete follow‐up? | Unclear risk | 77 women randomised 69 women analysed after 1 cycle (90%), 65 after completing 6 cycles |
| Potential bias related to study funding | Unclear risk | Not stated |
Pulkkinen 1987.
| Methods | Randomisation using sealed, opaque, sequentially numbered envelopes Double‐blind, cross‐over study 14 women randomised and analysed (55 cycles) Power calculation was performed by the pharmaceutical company Helsinn SA Method of assessing adverse effects: reported retrospectively at follow‐up | |
| Participants | Inclusion: history of dysmenorrhoea for several cycles, regular cycles, general good health; physical and pelvic exams Exclusion: OCP, IUD use, contraindications or hypersensitivity to NSAIDs, organic causes of dysmenorrhoea, irregular cycles Age: 17 to 28, median 22 Location: Finland | |
| Interventions | Nimesulide (100 mg bid at onset of pain, as needed) Placebo Duration: 4 cycles, 2 cycles nimesulide, 2 cycles placebo or vice versa | |
| Outcomes | Pain Adverse effects | |
| Notes | Groups comparable at baseline Extra information supplied by authors. No numerical data reported for adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using sealed, opaque, sequentially numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects data not systematically reported |
| Complete follow‐up? | Low risk | 14 women randomised and analysed (55 cycles) |
| Potential bias related to study funding | Unclear risk | Power calculation was performed by the pharmaceutical company Helsinn SA |
Riihiluoma 1981.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 35 women randomised 29 analysed Withdrawals: 2 pregnancies; 1 moved from district; 2 insufficient compliance; 1 excluded due to OCP use Method of assessing adverse effects: self reported prospectively on cards | |
| Participants | Inclusion: primary dysmenorrhoea; gynaecological exam Exclusion: secondary dysmenorrhoea; wish for pregnancy; contraindications to NSAIDs Age: mean 21.7 (SD 3.2), range 17 to 28 Location: Finland | |
| Interventions | Diclofenac sodium (Voltaren) 25 mg Placebo Taken 3 times a day for 2 to 7 days following first symptoms Duration: 4 cycles, 1 treatment per alternate cycle | |
| Outcomes | Pain relief: 6‐point scale Reported as sums of pain relief scores in graph form Additional analgesics required Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | High risk | Adverse effects data not collected |
| Complete follow‐up? | High risk | 35 women randomised 29 analysed (83%) |
| Potential bias related to study funding | Unclear risk | Ciba‐Geigy provided the drug |
Rondel 1984.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over study 12 women randomised and analysed Method of assessing adverse effects: recorded retrospectively at end of each menstrual cycle | |
| Participants | Inclusion: primary dysmenorrhoea, medical and gynaecological exam to rule out pathology Exclusion: clinical pathology, use of IUD, trying to conceive, lactating, allergic to other NSAIDs, history of chronic or severe dyspepsia Age: 18 to 42, mean 29 Location: Germany | |
| Interventions | Nimesulide (200 mg per day, from 3 days prior to menstruation to 5th day of menstruation) Placebo Duration: 4 cycles (2 each treatment) Women were instructed to try not to take other analgesic compounds, but if they had to they needed to record their use | |
| Outcomes | Pain relief ‐ 5‐point scale Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, drugs not described |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects data not systematically reported |
| Complete follow‐up? | Low risk | No losses |
| Potential bias related to study funding | Unclear risk | Birex Solaris |
Salmalian 2014.
| Methods | Triple‐blind, parallel‐group RCT 84 women randomised and analysed of whom 56 received ibuprofen or placebo (third group received thymus vulgaris) |
|
| Participants | Iranian medical students aged 18 to 24 Inclusion: women with primary dysmenorrhoea, grade 1 or 2 in current cycle and at least in past 2 cycles having used no analgesia in 48 hours prior to entering study Exclusion: women with history of abdominal or pelvic surgery, liver or kidney disease, severe stress, non‐compliance |
|
| Interventions | 1. 200 mg ibuprofen + 25 ml placebo essential oil 2. Placebo capsule + 25 ml placebo essential oil [3. Thymus vulgaris + placebo capsule] |
|
| Outcomes | 1. Rate of satisfaction from pain relief 2. Menstrual pain intensity on 0‐10 VAS (data not entered as dichotomous data available) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | All packages of medication were coded by the pharmacists and given to the participants in 3 groups; A, B, C |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blinded ‐ outcomes self assessed |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects data not systematically reported: unclear whether data on "clinical symptoms" refers to pre‐existing dysmenorrhoea symptoms or new symptoms |
| Complete follow‐up? | Low risk | All 56 women included in analysis |
| Potential bias related to study funding | Unclear risk | Supported by Babol University grant, but thymus vulgaris supplied by commercial firm |
Saltveit 1985.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 92 women randomised, 90 analysed Withdrawals: 1 pregnancy, 1 excluded as wrong participant number was used in records Method of assessing adverse effects: self reported prospectively on diary card | |
| Participants | Inclusion: primary dysmenorrhoea for at least 6 months of a severity which limits normal activities Exclusion: attempts to conceive; breastfeeding Age: 15 to 45 Location: Norway | |
| Interventions | Piroxicam 20 mg Placebo Taken as 2 capsules as a single dose on day 1 and 2, then 1 capsule on day 3 if necessary Duration: 4 cycles | |
| Outcomes | Abdominal cramps Pain‐related symptoms Overall pain Paracetamol consumption | |
| Notes | Paracetamol used as a rescue medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Low risk | Adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | 92 women randomised, 90 analysed (98%) |
| Potential bias related to study funding | Unclear risk | Pfizer supplied the drug |
Saltveit 1989.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 198 women randomised, 174 analysed Withdrawals: 2 during piroxicam due to nausea; 2 during naproxen due to stomach pain, dizziness and headaches; 20 withdrew for a variety of reasons such as moving house, vacation etc Method of assessing adverse effects: self reported prospectively on diary card | |
| Participants | Inclusion: primary dysmenorrhoea for at least 6 months to such a degree that daily activities reduced during menstruation Exclusion: secondary dysmenorrhoea; attempting to get pregnant; breastfeeding; blood, liver or kidney disease; asthma, ulcers or serious dyspepsia during the last 12 months; sensitivity towards aspirin or NSAIDs Age: range 15 to 47 Location: Norway | |
| Interventions | Piroxicam 20 mg (2 capsules as 1 dose on day 1 and day 2, and 1 capsule on day 3 if needed) Naproxen 250 mg (500 mg as a loading dose then 250 mg as second and third doses) Duration: 4 cycles | |
| Outcomes | Pain intensity Additional treatment Ability to work Overall effect Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, matching placebo |
| Selective reporting (reporting bias) | Low risk | "Side effect recorded every night" by the woman |
| Complete follow‐up? | High risk | 198 women randomised, 174 analysed |
| Potential bias related to study funding | Unclear risk | Unclear |
Sande 1978.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel study 37 randomised, data available for all Method of assessing adverse effects: not stated | |
| Participants | Inclusion: primary dysmenorrhoea, medical, gynaecological and physical examination to confirm lack of pathology Exclusion: organic pathology causing dysmenorrhoea Location: Norway | |
| Interventions | Naproxen sodium (550 mg initially then 275 mg every 6 hours as required) Placebo Duration: 3 cycles Additional analgesics allowed, if pain relief was scored as 1 (a lower score than worse pain) | |
| Outcomes | Pain relief: 6‐point scale Reported as sums of pain relief scores in graph form Additional analgesics required Adverse effects | |
| Notes | Authors state "Few reported side effects" ‐ no numerical data given | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded, "identical" placebo |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | Low risk | 37/37 analysed |
| Potential bias related to study funding | Unclear risk | Not stated |
Soares 1993.
| Methods | Randomisation/allocation method unclear Double‐blind, parallel trial 40 women (also states 37) Method of assessing adverse effects: reported to doctor retrospectively at follow‐up | |
| Participants | Inclusion: primary dysmenorrhoea; clinical and gynaecological exam prior to study Exclusion: secondary dysmenorrhoea; IUD use; allergy to medications; peptic ulcer or hepatic or renal disease Age: 18 to 40 Mean age: 28 Location: Brazil | |
| Interventions | Nimesulide 100 mg Placebo Taken every 12 hours for 3 days, beginning at start of menses No additional medication was allowed during the trial Duration: 1 cycle | |
| Outcomes | Global evaluation | |
| Notes | Portuguese ‐ partially translated using altavista Babelfish website. No numerical data on adverse effects reported for placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | Denominators inconsistent in study |
| Potential bias related to study funding | Unclear risk | Not stated |
Villasenor 1984.
| Methods | Randomised, using a random numbers table Allocation method: not stated Double‐blind, parallel trial 40 women randomised ‐ text implies that all were analysed but not completely clear Method of assessing adverse effects: self reported prospectively | |
| Participants | Inclusion: primary dysmenorrhoea Exclusion: gastroduodenal ulcer, hepatic and severe renal insufficiency; known allergy to NSAIDs or prostaglandin inhibitors; IUD use Age: 17 to 30, mean diclofenac group 21 years, mean placebo group 19.6 years Location: Mexico | |
| Interventions | Diclofenac (loading dose of 100 mg, then next 2 doses 50 mg, then all subsequent doses 50 mg) Placebo Taken 3 times a day for 3 days Duration: 3 cycles | |
| Outcomes | Pain (100 mm VAS) Adverse effects | |
| Notes | Spanish ‐ translated by Fabio Guidugli, Brazilian Cochrane Centre | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects data prospectively solicited |
| Complete follow‐up? | Unclear risk | Unclear |
| Potential bias related to study funding | Unclear risk | Unclear |
Wilhelmsson 1985a.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 83 women randomised, 69 analysed Method of assessing adverse effects: self reported prospectively on questionnaire | |
| Participants | Inclusion: clinical diagnosis of primary dysmenorrhoea for at least 6 months; gynaecological exam to exclude clinical pathology; over the age of 15 Exclusion: potential pregnancy; IUD or OCP use; contraindications to NSAIDs Source: outpatients Location: Sweden | |
| Interventions | Naproxen sodium 1000 mg per day Piroxicam 40 mg per day for day 1 and 2, then 20 mg for days 3 and 4 | |
| Outcomes | Pain intensity Additional treatment Ability to work Overall effect Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, drugs not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects prospectively solicited |
| Complete follow‐up? | High risk | 69/83 analysed (83%) |
| Potential bias related to study funding | Unclear risk | Pfizer |
Wilhelmsson 1985b.
| Methods | Randomisation/allocation method unclear Double‐blind, cross‐over trial 23 women randomised, 21 analysed Method of assessing adverse effects: self reported prospectively on questionnaire | |
| Participants | Inclusion: clinical diagnosis of primary dysmenorrhoea for at least 6 months; gynaecological exam to exclude clinical pathology; over the age of 15 Exclusion: potential pregnancy; IUD or OCP use; contraindications to NSAIDs Source: outpatients Location: Sweden | |
| Interventions | Piroxicam 40 mg per day for day 1 and 2, then 20 mg for days 3 and 4 Placebo Duration: 2 cycles, 1 of each treatment | |
| Outcomes | Pain intensity Additional treatment Ability to work Overall effect Adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blinded, placebo not described |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether adverse effects prospectively solicited |
| Complete follow‐up? | Unclear risk | 21/23 analysed (91%) |
| Potential bias related to study funding | Unclear risk | Pfizer |
Yu 2014.
| Methods | Multicentre, randomised, double‐blinded, cross‐over study 139 women randomised, 133 completed |
|
| Participants | Chinese women aged at least 18 years, with moderate or severe primary dysmenorrhoea during a minimum of 4 of the previous 6 menstrual cycles. Moderate defined as "Over‐the‐counter analgesics provide significant relief in most menstrual cycles; discomfort interferes with usual activity". Severe defined as "Over‐the‐counter analgesics not consistently effective, or prescription analgesics required in at least some menstrual cycles; discomfort is incapacitating causing an inability to work or do usual activity" | |
| Interventions | Etoricoxib 120 mg + placebo 1 dose Ibuprofen 600 mg _ placebo up to four times a day Acetaminophen, isopropylantipyrine and anhydrous caffeine (Saridon) as rescue medication Randomised to one of 2 possible sequences of treatment regimens, over 2 menstrual cycles |
|
| Outcomes | Primary outcome TOTPAR6 Secondary outcomes: include SPID6 Global evaluation of pain at 6 hours Use of rescue medication Number evaluating good very good or excellent at 24 hours No mention of side effects |
|
| Notes | Conducted in China by Merck Sharp and Dohme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators blinded, placebo‐controlled |
| Selective reporting (reporting bias) | Unclear risk | Reports of adverse advents were solicited retrospectively |
| Complete follow‐up? | Unclear risk | 139 randomised, 130 analysed (94%). 3 withdrew, 2 not eligible, 1 withdrawn by physician |
| Potential bias related to study funding | Unclear risk | Merck Sharp and Dohme |
AE = adverse effect
bid = twice daily
GI = gastrointestinal
IUD = intrauterine device
LD = loading dose, a larger dose of medication the first time it is taken in a cycle
NSAID = nonsteroidal anti‐inflammatory drug
OCP = oral contraceptive pill
OTC = over the counter
PID = pelvic inflammatory disease
prn = as needed
RCT = randomised controlled trial
SD = standard deviation
SPID = sum of pain intensity difference over time
TOPAR (or TOTPAR) = total pain relief score
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Waili 2001 | NSAID (tenoxicam) given intramuscularly |
| Anderson 1978 | Mefenamic acid, dextropropoxyphene and paracetamol, flufenamic acid, 56 women, cross‐over trial 50% of participants not analysed |
| Baldi 1983 | Pyrasanone, placebo, 20 women, cross‐over trial No mention of randomisation |
| Baracat 1991 | Nimesulide, piroxicam, 26 women, parallel trial There is no mention of randomisation and the study is also described as open (no blinding) |
| Barbosa 2007 | Compares valdecoxib and piroxicam. Valdecoxib now withdrawn. Comparison with placebo not reported |
| Bonnar 1996 | Ethamsylate, mefenamic acid, tranexamic acid, 76 women, parallel trial Although one of the outcome measures in this trial was dysmenorrhoea, the main purpose of the study was to investigate treatments for menorrhagia and/or dysfunctional uterine bleeding. Therefore the included participants were selected along those criteria, they were not necessarily all dysmenorrhoeic and pain was a secondary problem to dysmenorrhoea |
| Bowen 1996 | Bromfenac sodium, placebo, 143 women, cross‐over trial Bromfenac sodium withdrawn by manufacturer for safety reasons |
| Budoff 1982 | Zomepirac sodium, placebo, 47 women, cross‐over trial Zomepirac sodium withdrawn by manufacturer for safety reasons |
| Buttram 1979 | Naproxen versus placebo, 35 women, parallel design Participants had dysmenorrhoea secondary to IUD insertion |
| Campana 1986 | Naproxen lysinate, naproxen, 32 women, parallel trial Study compared 2 forms of naproxen, therefore does not fit into the included interventions |
| Catalan 1991 | Diclofenac, placebo No mention of randomisation and no blinding used |
| Chan 1979 | Ibuprofen 200 mg, placebo, cross‐over trial, 7 women 28% not analysed |
| Chan 1980 | Ibuprofen, placebo, 6 women, cross‐over design No mention of randomisation |
| Cornely 1978 | Indomethacin, placebo, 54 participants, parallel design, German Allocation not stated as random, no blinding used |
| Corson 1978 | Ibuprofen, aspirin, 40 participants, cross‐over design Included women using IUD. No separate analysis |
| Csapo 1977 | No mention of randomisation Focus on uterine activity rather than pain |
| Daniels 2005 | Less than 80% of randomised participants followed up |
| Dawood 1988 | Suprofen versus placebo. This NSAID was withdrawn in 1987 for the treatment of dysmenorrhoea as it was found to cause transient renal failure and flank pain. Therefore it has been excluded from this review as it is now only prescribed for ophthalmic uses |
| Dawood 2007a | Suprofen withdrawn |
| De Almeida Prado 2004 | Compares meloxicam with rofecoxib. Rofecoxib now withdrawn |
| De la Boullaye 1971 | Alclofenac was taken off the market due to a negative risk/benefit ratio |
| DeLia 1982 | Flurbiprofen, aspirin, placebo, 87 women, cross‐over trial 32% not analysed |
| Di Girolamo 1996 | Lysine clonixinate versus placebo, 24 women, cross‐over design Includes IUD users, no separate analysis |
| Donadio 1987 | No mention of randomisation |
| Doubova 2007 | Less than 80% of ibuprofen/placebo groups followed up |
| Dreher 1980 | Mefenamic acid versus placebo, 18 women, cross‐over design Includes women using IUDs, no separate analysis |
| Du Rant 1985 | Trial compared 5 different doses of naproxen, therefore does not fit into the included interventions |
| DuRant 1988 | Naproxen, placebo, 54 women, parallel The trial included women with pain, not just dysmenorrhoea; cannot separate out dysmenorrhoeic women |
| Eccles 2010 | Uses co‐intervention: ibuprofen combined with paracetamol, versus placebo |
| Ertungealp 1985 | Naproxen, placebo, 81 women, parallel No mention of random allocation (Translated by Metin Gulmetzoglu, Cochrane Pregnancy and Childbirth Group) |
| EUCTR2004‐003809‐25‐HU | Compares NSAID (ibuprofen) with antispasmodic (drotaverine) in women with primary or secondary dysmenorrhoea |
| EUCTR2008‐006762‐29‐GB | Co‐intervention: NSAID (ibuprofen) is combined with paracetamol |
| Frank 1983 | Flurbiprofen versus paracetamol, 30 participants, cross‐over design No mention of randomisation |
| Fraser 1987 | Ibuprofen versus placebo, 47 women, cross‐over design Includes women using IUDs, no separate analysis |
| Fuchs 1979 | Ibuprofen, placebo, control, 5 women, cross‐over design No mention of randomisation, and the main outcome was prostaglandin levels rather than pain relief |
| Gookin 1983 | Ibuprofen, indomethacin, placebo, 42 women, cross‐over design 26% women not included in analysis |
| Grossi 1986 | Diclofenac, 878 women, parallel Trial compared 4 different doses of diclofenac, therefore does not fit into the included interventions |
| Halbert 1978 | Indomethacin, ibuprofen, 40 participants No mention of randomisation or blinding |
| Hamann 1977 | Indomethacin, placebo, 60 women, cross‐over trial 31% not analysed |
| Hanson 1982 | Ibuprofen, naproxen, 76 women, parallel study Included women using IUD, no separate analysis |
| Hebert 1986 | Ketoprofen, mefenamic acid, 43 women, cross‐over design Includes women using IUDs, no separate analysis |
| Henzl 1977a | Naproxen versus placebo, 20 women, parallel design Includes women using IUDs, no separate analysis |
| Henzl 1979 | Naproxen, placebo No mention of randomisation Paper describes a number of separate trials: unclear if they cross over with Henzl 1980 |
| Henzl 1979b | Naproxen, placebo, 24 women, parallel trial Main focus is intrauterine pressure. Uses single megadose (1100 mg) of naproxen, above recommended therapeutic dose for dysmenorrhoea |
| Henzl 1980 | Naproxen, placebo No mention of randomisation Paper describes a number of separate trials: unclear if they cross over with Henzl 1979 |
| Ingemanson 1981 | Diclofenac, placebo, 30 women, parallel design Included women using IUD, no separate analysis |
| IRCT201304096790N4 | Not a RCT |
| Islas Perez 1981 | Mefenamic acid, placebo, 30 women No mention of randomisation Spanish trial |
| ISRCTN32847177 | No comparison of interest: study compares vaginal and oral doses of mefenamic acid |
| Iyagba 1987 | No mention of randomisation Trial only single‐blind |
| Jakubowicz 1984 | Mefenamic acid versus placebo. 80 women analysed but only 19 women of these were randomised. There are no separate outcome data for the randomised group of women: all outcome data are combined |
| Janbu 1978 | Aspirin, paracetamol, placebo, 35 women, cross‐over design Includes IUD users, no separate analysis |
| Jansen 1984 | No mention of randomisation |
| Jay 1986 | Naproxen, placebo, 50 women, parallel design Compares 2 types of primary dysmenorrhoea |
| Joelsson 1979 | Hysterometry was used in addition to naproxen, naproxen sodium or placebo |
| Kajanoja 1979 | Naproxen, indomethacin, 30 women, cross‐over trial 20% women not included in analysis |
| Kapadia 1987 | Naproxen, ibuprofen, 56 women Trial only single‐blind |
| Kauppila 1977 | Indomethacin, tolfenamic acid, placebo, 27 women, cross‐over trial 25% women not included in analysis |
| Kauppila 1979 | Ketoprofen, indomethacin, 30 women, cross‐over trial 23% women not included in analysis |
| Kauppila 1979b | Acetylsalicylic acid, indomethacin, tolfenamic acid, placebo, 18 women, parallel Participants also had endometriosis so they do not fit the criteria of primary dysmenorrhoea |
| Kauppila 1985 | Naproxen sodium, placebo, 24 women, cross‐over design Inclusion criteria for this study was women with secondary dysmenorrhoea (endometriosis) |
| Kauppila 1986 | Tiaprofenic acid, naproxen, placebo, 42 women, cross‐over trial 26% women not included in analysis |
| Kemp 1972 | Aspirin, Buscopam, placebo, 20 women, cross‐over trial 60% women not included in analysis |
| Killick 1990 | Azapropazone, placebo, 46 women, cross‐over trial 28% women not included in analysis |
| Kintis 1980 | Not randomised |
| Klein 1981 | Aspirin, placebo, 47 women, cross‐over trial 38% women not included in analysis |
| Kollenz 2009 | Compares 2 forms of ibuprofen |
| Krishna 1980 | Flurbiprofen, aspirin, placebo, 39 women, cross‐over trial No mention of randomisation |
| Kunz 1981 | No mention of randomisation German trial |
| Lalos 1983 | Naproxen versus placebo, 21 women, cross‐over design All participants had dysmenorrhoea associated with IUD use |
| Langrick 1982 | Naproxen sodium, dextropropoxyphene/paracetamol, 39 women Trial only single‐blind |
| Langrick 1983 | Naproxen sodium, mefenamic acid, 50 women Trial only single‐blind |
| Langrick 1989 | Mebeverine, mefenamic acid, placebo, 64 women, cross‐over trial 24% women not included in analysis |
| Larkin 1979 | Ibuprofen, propoxyphene, placebo, 22 women, cross‐over Trial not randomised |
| Lundstrom 1978 | Naproxen, placebo, 28 women, cross‐over trial 22% women not included in analysis |
| Lundstrom 1979 | Naproxen, placebo Trial excluded as it is not a RCT and focuses on uterine contractility rather than pain relief |
| Maclean 1983 | Flurbiprofen, paracetamol, 23 participants, cross‐over design No mention of randomisation |
| Makarainen 1983 | Proquazone, indomethacin, 47 women No mention of blinding |
| Mannix 2009 | Naproxen‐sumatriptan, placebo. 2 studies (n = 311, n = 310) Co‐intervention: NSAID was combined with sumatriptan |
| Marchini 1987 | Pirprofen, placebo, 82 women, parallel trial Pirprofen withdrawn from market |
| Mehlisch 1988 | Ketoprofen, ibuprofen, placebo, 43 women, cross‐over trial 40% to 72% women not included in analysis |
| Milsom 1984 | Ibuprofen, paracetamol, 12 women, parallel design No mention of randomisation |
| Milsom 1988 | Flurbiprofen, naproxen, 8 women, parallel design No mention of randomisation |
| Milsom 2002a | Naproxen (2 doses), placebo, 81 women, cross‐over design Some women used IUD, no separate analysis |
| Milsom 2002b | Naproxen (2 doses), placebo, 82 women, cross‐over design Some women used IUD, no separate analysis |
| Milsom 2002c | Naproxen (2 doses), placebo, 76 women, cross‐over design Some women used IUD, no separate analysis |
| Montrull 1987 | Ketoprofen, placebo, 20 women Translated from Spanish by Anne Lethaby. No mention of randomisation |
| NCT00380627 2006 | Not RCT |
| Nor Azlin 2008 | Single‐blinded |
| Ogden 1970 | Randomised, double‐blind, cross‐over trial, analgesic agents containing small amounts of acetaminophen and acetylsalicylate ‐ not included compounds 2 trials: 1) 202 women 2) 217 women |
| Ozbay 2006 | Does not mention randomisation ‐ unable to contact author |
| Ozgoli 2009 | Not RCT |
| Palmisano 1988 | Ketoprofen, ibuprofen, placebo, 36 women, cross‐over trial 33% women not included in analysis |
| Peixoto 1984 | Ibuprofen, placebo, 30 women, cross‐over trial 26% women not included in analysis |
| Pendergrass 1984 | Aspirin, paracetamol, placebo, 75 women, parallel design Trial population was not dysmenorrhoeic women but any women with regular periods |
| Pendergrass 1985 | Aspirin, paracetamol, placebo, 90 women, parallel trial Trial population was not dysmenorrhoeic women but any women with regular periods |
| Petti 1985 | Glucamethacin appears to be no longer available |
| Pirhonen 1986 | Design unclear ‐ unable to contact author |
| Plantema 1986 | Piroxicam, naproxen 85 women Not randomised Also mentions another study Wilhelmsson, which is included and republished elsewhere |
| Pogmore 1980 | Flurbiprofen, aspirin, placebo, 80 women, cross‐over study 51% women not included in analysis |
| Prasad 1980 | Benorylate versus placebo, 91 women, cross‐over study Benorylate (aspirin/paracetamol) not a NSAID |
| Pulkkinen 1978 | Naproxen sodium, placebo, 6 women Half the women in the trial single‐blinded only; focus was on prostaglandin concentrations rather than pain |
| Pulkkinen 1978b | Ibuprofen, placebo, 12 women, parallel trial Not randomised |
| Pulkkinen 1979 | Ibuprofen, placebo, 15 women Only single‐blinded and focuses on prostaglandins levels |
| Rawal 1987 | Naproxen, placebo 46% women not included in analysis |
| Rosenwaks 1981 | Naproxen sodium, aspirin, placebo, 32 women, cross‐over design. Described as controlled comparative trial No mention of randomisation |
| Roy 1981 | Ibuprofen, placebo, 20 women, cross‐over design Not a population of dysmenorrhoeic women |
| Roy 1983 | Ibuprofen, mefenamic acid, placebo, 48 women, cross‐over design No mention of randomisation |
| Sahin 2003 | Not described as double‐blinded ‐ attempts to contact author unsuccessful |
| Sauer 1994 | Diclofenac, naproxen, placebo, 102 women, cross‐over trial 23% women not included in analysis |
| Schulman 1985 | Piroxicam, placebo, 7 women, cross‐over design Pain relief is not an outcome measure, the trial focuses on uterine contractibility. Randomisation not mentioned |
| Schwartz 1974 | Flufenamic acid, placebo, 16 women No randomisation |
| Sedgwick 1985 | Meptazinol, d‐propoxyphene/paracetamol versus placebo Meptazinol not a NSAID |
| Serfaty 1986 | Piroxicam, diclofenac, mefenamic acid, 91 women No blinding used |
| Shapiro 1981 | Ibuprofen, aspirin, placebo, 72 women, cross‐over trial 22% women not analysed |
| Shapiro 1986 | Flurbiprofen, aspirin, placebo, 58 women, cross‐over design 25% women not included in analysis |
| Shishegar 1997 | Piroxicam, mefenamic acid, placebo Abstract only, no mention of blinding |
| Smith 1980 | Mefenamic acid versus placebo 81 women Only outcome was intrauterine pressure |
| Smith 1987 | Meclofenamate versus placebo 18 women Only outcome was uterine pressure |
| Szigeti 1981 | Indomethacin, placebo, 13 women No mention of randomisation or blinding |
| Tampakoudis 1997 | Tolfenamic acid, placebo, 50 women No mention of blinding |
| Tilyard 1992 | Tiaprofenic acid, mefenamic acid, placebo, 50 women, cross‐over trial 20% women not included in analysis |
| Villasenor 1985 | Pirprofen, diflunisal, zomepirac, 90 women, parallel design Pirprofen and zomepirac withdrawn |
| Von Graffenried 1981 | Fluproquazone, placebo, 42 women, cross‐over trial Fluproquazone no longer available |
| Williams 1982 | Naproxen, dextropropoxyphene hydrochloride/paracetamol, 59 women No mention of blinding |
| Ylikorkala 1980 | Naproxen tablets and naproxen suppositories, 32 women, cross‐over trial Compares 2 forms of the same NSAID, 20% withdrawals |
| Ylikorkala 1981 | Fluproquazone, indomethacin, 42 women, cross‐over trial Fluproquazone no longer available |
IUD = intrauterine device NSAID = nonsteroidal anti‐inflammatory drug RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
CTRI2188.
| Methods | Randomised, cross‐over trial, computer‐generated randomisation, double‐blinded |
| Participants | Women with primary dysmenorrhoea |
| Interventions | Lornoxicam 8 mg 500 tablets, ibuprofen 400 mg, placebo 400 mg twice a day for 3 days of each cycle for 2 consecutive cycles |
| Outcomes | Total pain relief score, safety, tolerability |
| Notes | Have emailed author in India asking whether results available ‐ Dr Patel replied 20 March 2014 to say that data are still being analysed, may take a month, he will contact us when data available. No data sent (July 2015) |
Contributions of authors
Jane Marjoribanks: Took the lead in writing and updating the review in 2009 and 2015, performed independent data extraction and 'Risk of bias' assessment of the included trials, was responsible for statistical analysis and interpretation of the data.
Reuben Ayeleke: Checked study eligibility and data extraction of newly included studies in the 2015 update. Cindy Farquhar: Initiated and conceptualised the review, commented on drafts of the protocol and review, checked trial quality for the update.
Michelle Proctor: Took the lead in writing the protocol, performed independent data extraction and quality assessment of the included trials for the original review, commented on the draft of subsequent versions.
Sources of support
Internal sources
University of Auckland, School of Medicine, Auckland, New Zealand.
External sources
Princess of Wales Memorial Trust Fund administered by the Mercia Barnes Fund, New Zealand.
Declarations of interest
None known for any author
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akerlund 1989 {published data only}
- Akerlund M, Stromberg P. Comparison of ketoprofen and naproxen in the treatment of dysmenorrhoea, with special regard to the time of onset of pain relief. Current Medical Research & Opinion 1989;11:485‐90. [DOI] [PubMed] [Google Scholar]
- Stromberg P, Akerlund M. Pain relief in dysmenorrhea can be achieved more rapidly with ketoprofen than with naproxen [Snabbare smartlindring vid dysmenorre med ketoprofen an med naproxen]. Lakartidningen 1990;87(4):191‐3. [PubMed] [Google Scholar]
Akinluyi 1987 {published data only}
- Akinluyi EM. Piroxicam in primary dysmenorrhea. Current Therapeutic Research 1987;41(6):609‐13. [Google Scholar]
al‐Waili 1990 {published data only}
- Al‐Waili NS, Khalaf ZD. Efficacy of indomethacin suppository in primary dysmenorrhoea. Indian Journal of Medical Research 1990;92:298‐301. [PubMed] [Google Scholar]
Andersch 1989 {published data only}
- Andersch B, Milsom I. A double‐blind cross‐over study comparing flurbiprofen with naproxen‐sodium for the treatment of primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica 1988;67:645‐8. [PubMed] [Google Scholar]
Arnold 1983 {published data only}
- Arnold JD, Iber FL, Burt RA, Gruber CM. Comparison of fenoprofen calcium, ibuprofen and placebo in primary dysmenorrhea. Journal of Medicine 1983;14:337‐50. [PubMed] [Google Scholar]
Balsamo 1986 {published data only}
- Balsamo SB. Double‐blind clinical trial with potassium diclofenac versus placebo in primary dysmenorrhoea [Estudo clinico comparativo, duplo‐cego com diclofenaco potassico versus placebo na dismenorreia primaria]. Jornal Brasileiro de Ginecologia 1986;96(8):427‐30. [Google Scholar]
Benassi 1993 {published data only}
- Benassi L, Bertani D, Avanzini A. An attempt at real prophylaxis of primary dysmenorrhea: comparison between meclofenamate sodium and naproxen sodium. Clinical & Experimental Obstetrics & Gynaecology 1993;20:102‐7. [PubMed] [Google Scholar]
Bitner 2004 {published data only (unpublished sought but not used)}
- Bitner M, Katternhorn J, Gao J, Kellstein D. Efficacy and tolerability of lumiracoxib in the treatment of primary dysmenorrhoea. International Journal of Clinical Practice 2004;58(4):340‐5. [DOI] [PubMed] [Google Scholar]
- Katternhorn J, Hatfield C, Gao J, Kellstein D. Lumiracoxib, a novel cyclooxygenase‐2 selective inhibitor, is an effective and well‐tolerated treatment for nausea. International Journal of Obstetrics and Gynaecology 2003;83(S3)(Book 2):102. [Google Scholar]
Budoff 1979 {published data only}
- Budoff PW. Mefenamic acid therapy in dysmenorrhea. Advances in Prostaglandin & Thomboxane Research 1980;8:1449‐53. [PubMed] [Google Scholar]
- Budoff PW. Use of mefenamic acid in the treatment of dysmenorrhea. JAMA 1979;241:2713‐6. [PubMed] [Google Scholar]
Cash 1982 {published data only}
- Cash HC, Humpstom D, Kasap HS. Feldene in the symptomatic treatment of primary dysmenorrhoea. Practitioner 1982;22:1338‐41. [PubMed] [Google Scholar]
Chan 1983 {published data only}
- Chan WY, Fuchs F, Powell AM. Effects of naproxen sodium on menstrual prostaglandins and primary dysmenorrhea. Obstetrics & Gynecology 1983;61:285‐91. [PubMed] [Google Scholar]
Chantler 2008 {published data only (unpublished sought but not used)}
- Chantler I, Mitchell D, Fuller A. The effect of three cyclo‐oxygenase inhibitors on intensity of primary dysmenorrhoeic pain. Clinical Journal of Pain 2008;24(1):39‐44. [DOI] [PubMed] [Google Scholar]
Chantler 2009 {published data only}
- Chantler I, Mitchell D, Fuller A. Diclofenac potassium attenuates dysmenorrhea and restores exercise performance in women with primary dysmenorrhea. Journal of Pain 2009;10(2):191‐200. [DOI] [PubMed] [Google Scholar]
Costa 1987a {published data only}
- Costa S, Mioli M, Ravaioli R, Bufalino L, Gardini F. Prostaglandin synthetase inhibitor piroxicam beta‐cyclodextrin in the treatment of primary dysmenorrhea. Current Therapeutic Research 1987;42(1):156‐64. [Google Scholar]
Costa 1987b {published data only}
- Costa S, Mioli M, Ravaioli R, Bufalino L, Gardini F. Prostaglandin synthetase inhibitor piroxicam beta‐cyclodextrin in the treatment of primary dysmenorrhea. Current Therapeutic Research 1987;42(1):156‐64. [Google Scholar]
Dandenell 1979 {published data only}
- Dandenell LO, Lalos O, Lisciak J, Sandstrom B, Barany S, Nilsson B. Clinical experience of naproxen in the treatment of primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:95‐100. [DOI] [PubMed] [Google Scholar]
Daniels 2002 {published data only}
- Daniels SE, Talwalker S, Torri S, Snabes MC, Recker DP, Verburg KM. Valdecoxib, a cyclo‐oxygenase‐2‐specific inhibitor, is effective in treating primary dysmenorrhea. Obstetrics and Gynecology 2002;100:350‐8. [DOI] [PubMed] [Google Scholar]
Daniels 2008 {published data only (unpublished sought but not used)}
- Daniels S, Gitton X, Zhou W, Stricker K, Barton S. Efficacy and tolerability of lumiracoxib 200 mg once daily for treatment of primary dysmenorrhea: results from two randomized controlled trials. Journal of Women's Health 2008;17(3):423‐37. [DOI] [PubMed] [Google Scholar]
Daniels 2009a {published data only}
- Daniels S, Robbins J, West CR, Nemeth MA. Celecoxib in the treatment of primary dysmenorrhea: results from two randomized, double‐blind, active‐ and placebo‐controlled crossover studies. Clinical Therapeutics 2009;31(6):1192‐8. [DOI] [PubMed] [Google Scholar]
Daniels 2009b {published data only}
- Daniels S, Robbins J, West CR, Nemeth MA. Celecoxib in the treatment of primary dysmenorrhea: results from two randomized, double‐blind, active‐ and placebo‐controlled crossover studies. Clinical Therapeutics 2009;31(6):1192‐8. [DOI] [PubMed] [Google Scholar]
Dawood 1999a {published data only}
- Dawood MY. Efficacy and safety of piroxicam‐B‐cyclodextrin (PBCD, Brexidol). Comparison studies with ibuprofen, naproxen sodium and placebo in the relief of moderate to severe abdominal pain associated with primary dysmenorrhoea. The Brexidol Study Group. Today's Therapeutic Trends 1999;17(4):273‐88. [Google Scholar]
Dawood 1999b {published data only}
- Dawood MY. Efficacy and safety of piroxicam‐B‐cyclodextrin (PBCD, Brexidol). Comparison studies with ibuprofen, naproxen sodium and placebo in the relief of moderate to severe abdominal pain associated with primary dysmenorrhoea. The Brexidol Study Group. Today's Therapeutic Trends 1999;17(4):273‐88. [Google Scholar]
Dawood 2007 {published data only (unpublished sought but not used)}
- Dawood MY, Khan‐Dawood FS. Clinical efficacy and differential inhibition of menstrual fluid prostaglandin F2a in a randomized, double blind crossover treatment with placebo, acetaminophen and ibuprofen in primary dysmenorrhea. American Journal of Obstetrics and Gynecology 2007;196(35):e1‐35e5. [DOI] [PubMed] [Google Scholar]
Delgado 1994 {published data only}
- Delgado J, Simonin G, Servier C, Garcia R, Yoma J. Tolfenamic acid and mefenamic acid in the treatment of primary dysmenorrhoea. Pharmacology & Toxicology 1994;75 Suppl(2):89‐91. [DOI] [PubMed] [Google Scholar]
de Mello 2004 {published data only}
- Martinez Alcala FO, Velasco JA, Cortes RJR, Mello NR, Baracat EC, Tomaz G, et al. Efficacy and safety of Cox‐2 selective inhibitor versus non‐selective NSAID in the symptomatic treatment of primary dysmenorrhea [Efficacia e seguranca do uso do inibidor seletivo da Cox‐2 versus antiinflamatorio nao esteroide classico no tratamento sintomatico da dismenorreia primaria]. Revista Brasileira de Medicina 2003;60(11):882‐7. [Google Scholar]
- Mello NR, Baracat EC, Tomaz G, Bedone AJ, Camargos, Barbosa IC, et al. Double‐blind study to evaluate efficacy and safety of meloxicam 7.5 mg and 15 mg versus mefenamic acid 1500 mg in the treatment of primary dysmenorrhea. Acta Obstetrica et Gynecologica Scandinavica 2004;83:667‐73. [DOI] [PubMed] [Google Scholar]
De Souza 1991 {published data only}
- Souza AZ, Tacla M, Ribeiro RM, Hegg R, Aguiar LF. Etodolac in primary dysmenorrhea [Etodolac em dismenorreia primaria]. Jornal Brasileiro de Ginecologia 1991;101(10):451‐9. [Google Scholar]
Di Girolamo 1999 {published data only}
- Girolamo G, Gimeno MAF, Faletti A, Santos AR, Marti ML, Zmijanovich R. Menstrual prostaglandins and dysmenorrhea: modulation by non‐steroidal antiinflammatory drugs [Prostaglandinas menstruales y dismenorrea su modulacion por antiinflamatorios no esteroideos]. Medicina 1999;59:259‐64. [PubMed] [Google Scholar]
Elder 1979 {published data only}
- Elder MG, Kapadia L. Indomethacin in the treatment of primary dysmenorrhoea. British Journal of Obstetrics & Gynaecology 1979;86:645‐7. [DOI] [PubMed] [Google Scholar]
Ezcurdia 1998 {published data only}
- Ezcurdia M, Javier Cortejoso F, Lanzon R, Javier Ugalde F, Herruzo A, Artigas R, et al. Comparison of the efficacy and tolerability of dexketoprofen and ketoprofen in the treatment of primary dysmenorrhoea. Journal of Clinical Pharmacology 1998;38:65S‐73S. [PubMed] [Google Scholar]
Facchinetti 2001 {published data only}
- Facchinetti F, Piccinini F, Sgarbi L, Renzetti D, Volpe A. Nimesulide in the treatment of primary dysmenorrhoea: a double blind study versus diclofenac. Drugs of Today 2001;37 Suppl(B):39‐45. [Google Scholar]
Fedele 1989 {published data only}
- Fedele L, Marchini M, Acaia B, Garagiola U, Tiengo M. Dynamics and significance of placebo response in primary dysmenorrhea. Pain 1989;36:43‐7. [DOI] [PubMed] [Google Scholar]
Gleeson 1983 {published data only}
- Gleeson S, Sorbie J. Efficacy of ketoprofen in treating primary dysmenorrhea. Canadian Medical Association Journal 1983;129:842‐4. [PMC free article] [PubMed] [Google Scholar]
Hamann 1980 {published data only}
- Hamann GO. Severe primary dysmenorrhea treated with naproxen: a prospective, double‐blind crossover investigation. Prostaglandins 1980;19:651‐7. [DOI] [PubMed] [Google Scholar]
Hanson 1978 {published data only}
- Hanson FW, Izu A, Henzl MR. Naproxen sodium, ibuprofen and a placebo in dysmenorrhea. Its influence in allowing continuation of work/school activities. Obstetrics & Gynecology 1978;52:583‐7. [PubMed] [Google Scholar]
Heidarifar 2014 {unpublished data only}
- Heidarifar R, Mehran N, Heidari A, Ahmari Tehran H, Koohbor M, Mansourabad MK. Effect of Dill (Anethum graveolens) on the severity of primary dysmenorrhea in compared with mefenamic acid: a randomized, double‐blind trial. Journal of Research in Medical Sciences 2014;19:326‐30. [PMC free article] [PubMed] [Google Scholar]
- IRCT201110205543N2. Comparing the influence of mefenamic acid and dill on primary dysmenorrhea [2011]. http://apps.who.int/trialsearch/Trial.aspx?TrialID=IRCT201110205543N2.
Henzl 1977b {published data only}
- Henzl MR, Buttram V, Segre EJ, Bessler S. The treatment of dysmenorrhea with naproxen sodium: a report on two independent double‐blind trials. American Journal of Obstetrics & Gynecology 1977;127:818‐23. [DOI] [PubMed] [Google Scholar]
Iacovides 2014 {published data only}
- Iacovides S, Avidon I, Bentley A, Baker FC. The 24‐h progression of menstrual pain in women with primary dysmenorrhea when given diclofenac potassium: a randomized,double‐blinded, placebo‐controlled crossover study. Archives of Gynecology and Obstetrics 2014;289(5):993‐1002. [DOI] [PubMed] [Google Scholar]
Ingemanson 1984 {published data only}
- Ingemanson CA, Sikstrom B. Comparison between diclofenac and naproxen in the treatment of primary dysmenorrhoea. Current Therapeutic Research 1984;36(6):1203‐9. [Google Scholar]
Jacobson 1979 {published data only}
- Jacobson J, Cavalli‐Bjokman K, Lundstrom V, Nilsson B, Norbeck M. Prostaglandin synthetase inhibitors and dysmenorrhea. A survey and personal clinical experience. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:73‐9. [DOI] [PubMed] [Google Scholar]
Jacobson 1983 {published data only}
- Jacobson J, Lunstrom V, Nilsson B. Naproxen in the treatment of OC‐resistant primary dysmenorrhea: a double‐blind cross‐over study. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1983;113:87‐9. [DOI] [PubMed] [Google Scholar]
Kajanoja 1978 {published data only}
- Kajanoja P. Indomethacin in the treatment of primary dysmenorrhea. Archiv fur Gynakologie 1978;225:1‐5. [DOI] [PubMed] [Google Scholar]
Kajanoja 1984 {published data only}
- Kajanoja P. Difusinal compared with naproxen in the treatment of dysmenorrhea. Prostaglandins, Leukotrienes and Medicine 1984;15:153‐8. [DOI] [PubMed] [Google Scholar]
Kapadia 1978 {published data only}
- Kapadia L, Elder MG. Flufenamic acid in treatment of primary spasmodic dysmenorrhoea: a double‐blind crossover study. Lancet 1978;1:348‐50. [DOI] [PubMed] [Google Scholar]
Kintigh 1995 {published data only}
- Kintigh JW. A multicenter, randomized, double‐blind, placebo controlled study of diclofenac potassium versus naproxen sodium in the treatment of primary dysmenorrhea. Today's Therapeutic Trends 1995;12 Suppl(1):47‐61. [Google Scholar]
Layes Molla 1974 {published data only}
- Layes Molla A, Donald J. A comparative study of ibuprofen and paracetamol in primary dysmenorrhoea. Journal of International Medical Research 1974;2:395‐9. [Google Scholar]
Legris 1997 {published data only}
- Legris M, Duhon R, Larue F, Lacoste C. Double‐blind placebo‐controlled study of the efficacy of niflumic acid (750 mg/day in three divided doses) in dysmenorrhea [Essai controle en double aveugle de l'efficacite de l'acide niflumique (750 mg en 3 prises par jour) versus placebo dans les dysmenorrhees]. Revue Francaise de Gynecologie et d'Obstetrique 1997;92(4):288‐96. [Google Scholar]
Letzel 2006 {published data only}
- Letzel H, Megard Y, Lamarca R, Raber A, Fortea J. The efficacy and safety of aceclofenac versus placebo and naproxen in women with primary dysmenorrhoea. European Journal of Obstetrics and Gynaecology and Reproductive Biology 2006;129:162‐8. [DOI] [PubMed] [Google Scholar]
Lopez Rosales 1989 {published data only}
- Lopez Rosales C, Cisneros Lugo JH, Romo Enciso LJ, Garcia Sandoval MG. Nimesulide in the treatment of primary dysmenorrhea: comparative clinical evaluation with mefenamic acid and fentiazac [Nimesulide en el tratamiento de la dismenorrea primaria: Evaluacion clinica comparativa con acido mefanamico y fentiazac]. Ginecologia y Obstetricia de Mexico 1989;57:196‐201. [PubMed] [Google Scholar]
Malmstrom 2003 {published data only (unpublished sought but not used)}
- Malmstrom K, Kotey P, Cichanowitz N, Daniels S, Desjardins PJ. Analgesic efficacy of etoricoxib in primary dysmenorrhea: results of a randomized, controlled trial. Gynecologica and Obstetric Investigation 2003;56(2):65‐9. [DOI] [PubMed] [Google Scholar]
- NCT0092729. An investigational drug study in the treatment of primary dysmenorrhea. http://clinicaltrials.gov/ct2/show/NCT00092729 2004.
Marchini 1995 {published data only}
- Marchini M, Rozzi L, Bakshi R, Pistai R, Fedele L. Comparative study of diclofenac dispersible 50 mg and ibuprofen 400 mg in patients with primary dysmenorrhea. A randomized, double‐blind, within‐patient, placebo‐controlled study. International Journal of Clinical Pharmacology & Therapeutics 1995;33:491‐7. [PubMed] [Google Scholar]
Mehlisch 1990 {published data only}
- Mehlisch DR. Double‐blind crossover comparison of ketoprofen, naproxen and placebo in patients with primary dysmenorrhea. Clinical Therapeutics 1990;12:398‐409. [PubMed] [Google Scholar]
Mehlisch 1997 {published data only}
- Mehlisch DR, Fulmer RI. A crossover comparison of bromfenac sodium, and placebo for relief of pain from primary dysmenorrhea. Journal of Women's Health 1997;6:83‐92. [DOI] [PubMed] [Google Scholar]
Mehlisch 2003 {published data only}
- Mehlisch DRD, Ardia A, Pallotta T. Analgesia with ibuprofen arginate versus conventional ibuprofen for patients with dysmenorrhea: a crossover trial. Current Therapeutic Research 2003;64(6):327‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milsom 1985 {published data only}
- Milsom I, Andersch B. Ibuprofen and naproxen‐sodium in the treatment of primary dysmenorrhea: a double‐blind cross‐over study. International Journal of Gynaecology & Obstetrics 1985;23:305‐10. [DOI] [PubMed] [Google Scholar]
Milsom 2002d {published and unpublished data}
- Milsom I, Minic M, Dawood MY, Akin MD, Spann J, Niland NF, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clinical Therapeutics 2002;24(9):1384‐400. [DOI] [PubMed] [Google Scholar]
- Minic M. Personal correspondence April 2003.
Milsom 2002e {published and unpublished data}
- Milsom I, Minic M, Dawood MY, Akin MD, Spann J, Niland NF, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clinical Therapeutics 2002;24(9):1384‐400. [DOI] [PubMed] [Google Scholar]
- Minic M. Personal correspondence April 2003.
Moggian 1986 {published data only}
- Moggian G, Pellegri E, Tamburini E, Pini P, Tunidei U. A new pharmacologic treatment of primary dysmenorrhea [Un nuova trattamento farmacologico nella dismenorrea enenziale]. Clinica Terapeutica 1986;117:481‐92. [PubMed] [Google Scholar]
Morrison 1979 {published data only}
- Morrison JC, Jenning JC. Primary dysmenorrhea treated with indomethacin. Southern Medical Journal 1979;72:425‐8. [DOI] [PubMed] [Google Scholar]
Morrison 1980 {published data only}
- Morrison JC, Ling FW, Forman EK, Bates GW, Blake PG, Vecchio TJ, et al. Analgesic efficacy of ibuprofen for treatment of primary dysmenorrhea. Southern Medical Journal 1980;73:999‐1002. [DOI] [PubMed] [Google Scholar]
Morrison 1999 {published data only}
- Morrison BW, Daniels SE, Kotey P, Cantu N, Seidenberg B. Rofecoxib, a specific cyclooxygenase‐2 inhibitor, in primary dysmenorrhea: a randomized controlled trial. Obstetrics and Gynecology 1999;94:504‐8. [DOI] [PubMed] [Google Scholar]
Nahid 2009 {published data only}
- Nahid K, Fariborz M, Ataolah G, Solokian S. The effect on an Iranian herbal drug on primary dysmenorrhea: a clinical controlled trial. Journal of Midwifery and Women's Health 2009;54:401‐4. [DOI] [PubMed] [Google Scholar]
Onatra 1994 {published data only}
- Onatra W, Bautista AA, Calis O. Double blind cross‐evaluation of effects and tolerance of etodolac versus piroxicam in the treatment of primary dysmenorrhea [Valoracion doble‐ciega cruzada de la eficacia y tolerancia de etodolac vs prioxicam en el tratamiento de la dismenorrea primaria]. Arquivos Brasileiros de Medicina 1994;68:199‐202. [Google Scholar]
Osathanondh 1985 {published data only}
- Osathanondh R, Caldwell BV, Kaul AF, Sokoloff BJ, White RM, Scavone JM, et al. Efficacy of fenoprofen in the treatment of primary dysmenorrhea. Journal of Reproductive Medicine 1985;30:915‐9. [PubMed] [Google Scholar]
Osinusi 1986 {published data only}
- Osinusi BO, Bamgboye EA. Piroxicam in the symptomatic treatment of primary dysmenorrhea. Current Therapeutic Research 1986;39(5):715‐8. [Google Scholar]
Pasquale 1988 {published data only}
- Pasquale SA, Rathauser R, Dolese HM. A double‐blind, placebo‐controlled study comparing three single‐dose regimens of piroxicam with ibuprofen in patients with primary dysmenorrhea. American Journal of Medicine 1988;84:30‐4. [DOI] [PubMed] [Google Scholar]
Pauls 1978 {published data only}
- Pauls F. Naproxen in dysmenorrhea. Lancet 1978;2:159‐60. [DOI] [PubMed] [Google Scholar]
Pedron 1995 {published data only}
- Pedron Nuevo DN. Treatment of primary dysmenorrhea. Comparative study of ibuprofen and mefenamic acid [Tratamiento de la dismenorrea primeria. Estudio comparativo de ibuprofen y acido mefenamico]. Ginecologica y Obstetricia de Mexico 1995;63:4‐9. [PubMed] [Google Scholar]
Powell 1981 {published data only}
- Powell JR, Smith RP. Mefenamic acid (Ponstel) for treating primary spasmodic dysmenorrhea. Current Therapeutic Research 1981;29(3):544‐50. [Google Scholar]
Pulkkinen 1987 {published and unpublished data}
- Pulkkinen MO. Alterations in intrauterine pressure, menstrual fluid prostaglandin F levels, and pain in dysmenorrheic women treated with nimesulide. Journal of Clinical Pharmacology 1987;27:65‐9. [DOI] [PubMed] [Google Scholar]
Riihiluoma 1981 {published data only}
- Riihiluoma P, Wuolijoki E, Pulkkinen MO. Treatment of primary dysmenorrhea with diclofenac sodium. European Journal of Obstetrics, Gynecology & Reproductive Biology 1981;12:189‐94. [DOI] [PubMed] [Google Scholar]
Rondel 1984 {published data only}
- Rondel RK, Eberhardt R, Koch J, Schurmann W. Treatment of primary essential dysmenorrhoea with nimesulide; a double‐blind cross over study. Current Therapeutic Research 1984;35(1):123‐9. [Google Scholar]
Salmalian 2014 {published data only}
- Salmalian H, Saghebi R, Moghadamnia AA, Bijani A, Faramarzi M, Nasiri Amiri F, et al. Comparative effect of Thymus vulgaris and ibuprofen on primary dysmenorrhea: a triple‐blind clinical study. Caspian Journal of Internal Medicine 2014;5(2):82‐8. [PMC free article] [PubMed] [Google Scholar]
Saltveit 1985 {published data only}
- Saltveit T. Piroxicam in primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica 1985;68:635‐7. [DOI] [PubMed] [Google Scholar]
- Saltveit T. Piroxicam in primary dysmenorrhoea (Abstract). Archives of Gynecology 1985;237:206. [Google Scholar]
Saltveit 1989 {published data only}
- Saltveit T. Piroxicam versus naproxen for primary dysmenorrhoea [Piroxicam versus naproxen ved primore dysmenore]. Tidskrift Norske Loegeforening 1989;109(5):576‐8. [PubMed] [Google Scholar]
Sande 1978 {published data only}
- Sande HA, Salvesen T, Izu A. Treating dysmenorrhea with anti‐inflammatory agents: a double‐blind trial with naproxen sodium. International Journal of Gynaecology & Obstetrics 1978;16:240‐1. [DOI] [PubMed] [Google Scholar]
Soares 1993 {published data only}
- Soares A, Cabrera T. Clinical efficacy of nimesulide in the treatment of primary dysmenorrhoea [Tratamento clinico da dismenorreia primaria com nimesulide (um antiinflamatorio nao hormonal)]. Jornal Brasileiro de Ginecologia 1993;103(8):311‐3. [Google Scholar]
Villasenor 1984 {published data only}
- Villasenor FJM. Potassium diclofenac in the treatment of primary dysmenorrhea [Traramiento de la dismenorrea primaria con diclofenac potaico]. Investigacion Medica Internacional 1984;11(1):49‐52. [Google Scholar]
Wilhelmsson 1985a {published data only}
- Wilhelmsson L, Jonsson K, Halling L, Hermann M, Jaderling J, Lindell C, et al. Piroxicam in the treatment of primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica 1985;64:317‐21. [DOI] [PubMed] [Google Scholar]
Wilhelmsson 1985b {published data only}
- Wilhelmsson L, Jonsson K, Halling L, Hermann M, Jaderling J, Lindell C, et al. Piroxicam in the treatment of primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica 1985;64:317‐21. [DOI] [PubMed] [Google Scholar]
Yu 2014 {unpublished data only}
- Merck Sharp, Dohme Corp. Study to assess the safety and efficacy of etoricoxib versus ibuprofen in the treatment of dysmenorrhea (MK‐0663‐145 AM1). http://clinicaltrials.gov/ct2/show/record/NCT01462370?sect=X543012 2013.
- Yu Q, Zhu X, Zhang X, Zhang Y, Li X, Hua Q, et al. Etoricoxib in the treatment of primary dysmenorrhea in Chinese patients: a randomized controlled trial. Current Medical Research & Opinion 2014;30(9):1863‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Waili 2001 {published data only}
- Al‐Waili NSD. Intramuscular tenoxicam to treat primary dysmenorrhea: double‐blind study. Current Opinion in Clinical Experimental Research 2001;3(2):108‐22. [Google Scholar]
Anderson 1978 {published data only}
- Anderson AB, Haynes PJ, Fraser IS, Turnbull AC. Trial of prostaglandin‐synthetase inhibitors in primary dysmenorrhoea. Lancet 1978;1(8060):345‐8. [DOI] [PubMed] [Google Scholar]
Baldi 1983 {published data only}
- Baldi C, D'Ajello M, Balbi GC. Treatment of dysmenorrhea with pyrasanone [Trattamento della dismenorrea con pyrasanone]. Minerva Ginecologica 1983;35:247‐50. [PubMed] [Google Scholar]
Baracat 1991 {published data only}
- Baracat EC, Motta ELA, Lima GR. Clinical evaluation of the efficacy and tolerability of nimesulide versus piroxicam in the therapeutic of primary dysmenorrhea [Avaliacao clinica da eficacia e tolerabilidade do nimesulide versus piroxicam na terapeutica da dismenorreia primaria]. Jornal Brasileiro de Ginecologia 1991;101(10):467‐70. [Google Scholar]
Barbosa 2007 {published data only}
- Barbosa IC, Fernandes CE, Filho CI, Tadini V, Camargos AF, Finotti MCCF, et al. Comparative study of the efficacy and safety of valdecoxib and piroxicam in the treatment of patients with primary dysmenorrhea [Comparacao de eficacia e seguranca de valdecoxibe e piroxicam no tratamento da dismenorreia primaria]. Revista Brasileira de Medicina 2007;64(7):318‐22. [Google Scholar]
- NCT00649415. A double blind, double dummy, randomized, comparative study of the efficacy and safety of valdecoxib 40 mg twice daily, as needed in the first menstrual cycle day and then once a day, and piroxicam 40 mg once a day in the treatment of patients with primary dysmenorrhea. http://clinicaltrials.gov/ct2/show/NCT00649415 2008.
Bonnar 1996 {published data only}
- Bonnar J, Sheppard B. Treatment of menorrhagia during menstruation: randomised controlled trial of ethamsylate, mefanamic acid and tranexamic acid. BMJ 1996;313:579‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 1996 {published data only}
- Bowen AJ, Fillingim JM, Macy VL, McDonald TA, McNeil QA. Comparison of bromfenac sodium and placebo in women with primary dysmenorrhea. Advances in Therapy 1996;13(3):167‐77. [Google Scholar]
Budoff 1982 {published data only}
- Budoff PW. Zomepirac sodium in the treatment of primary dysmenorrhea syndrome. New England Journal of Medicine 1982;307:714‐9. [DOI] [PubMed] [Google Scholar]
Buttram 1979 {published data only}
- Buttram V, Izu A, Henzl MR. Naproxen sodium in uterine pain following intrauterine contraceptive device insertion. American Journal of Obstetrics & Gynaecology 1979;134:575‐8. [DOI] [PubMed] [Google Scholar]
Campana 1986 {published data only}
- Campana A, Ruspa M. Therapeutic efficacy of naproxen‐lysine in dysmenorrhoea. Clinical Trials Journal 1986;23(4):249‐56. [Google Scholar]
Catalan 1991 {published data only}
- Catalan M, Azanza JR, Suarez JR, Honorato J. Piroxicam in the treatment of primary dysmenorrhea. Cross‐over comparative study with ibuprofen [Piroxicam en el tratamiento de la dismenorrea primaria. Estudio comparativo cruzado frente a ibuprofeno]. Clinica e Investigacion en Ginecologia y Obstetricia 1991;18(7):270‐5. [Google Scholar]
Chan 1979 {published data only}
- Chan W, Dawood MY, Fuchs F. Prostaglandins in primary dysmenorrhea. American Journal of Medicine 1981;70:535‐41. [DOI] [PubMed] [Google Scholar]
- Chan WY, Dawood MY, Fuchs F. Relief of dysmenorrhea with the prostaglandin synthetase inhibitor ibuprofen: effect on prostaglandin levels in menstrual fluid. American Journal of Obstetrics & Gynaecology 1979;135:102‐8. [PubMed] [Google Scholar]
Chan 1980 {published data only}
- Chan WY, Dawood MY. Prostaglandin levels in menstrual fluid of nondysmenorrheic and of dysmenorrheic subjects with and without oral contraceptive or ibuprofen therapy. Advances in Prostaglandin & Thromboxane Research 1980;8:1443‐7. [PubMed] [Google Scholar]
Cornely 1978 {published data only}
- Cornely M, Beutnagel H, Schonhofer PS. Symptomatic therapy of primary dysmenorrhoea by inhibition of prostaglandin synthesis with indomethacin [Symptomatische therapie der primaren dysmenorrhoe durch prostaglandinsynethesehemmung mit indomethacin]. Geburtshilfe und Fraeenheikunder 1978;38:18‐24. [PubMed] [Google Scholar]
Corson 1978 {published data only}
- Corson SL, Bolognese RJ. Ibuprofen therapy for dysmenorrhea. Journal of Reproductive Medicine 1978;20:246‐52. [PubMed] [Google Scholar]
Csapo 1977 {published data only}
- Csapo AI, Pulkkinen MO, Henzl MR. The effect of naproxen‐sodium on the intrauterine pressure and menstrual pain of dysmenorrheic patients. Prostaglandins 1977;13(1):193‐9. [DOI] [PubMed] [Google Scholar]
Daniels 2005 {published data only (unpublished sought but not used)}
- Daniels SE, Torri S, Desjardins PJ. Valdecoxib for treatment of primary dysmenorrhoea. Journal of General Internal Medicine 2005;20(1):62‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawood 1988 {published data only}
- Dawood MY. Efficacy and safety of suprofen in the treatment of primary dysmenorrhea: a multicentre, randomized, double‐blind study. Current Therapeutic Research 1988;44:257‐66. [Google Scholar]
Dawood 2007a {published data only}
- Dawood MY, Khan‐Dawood FS. Differential suppression of menstrual fluid prostaglandin F2a, prostaglandin E2, 6‐keto prostaglandin F1a and thromboxan B2 by suprofen in women with primary dysmenorrhea. Prostaglandins and other Lipid Mediators 2007;83:146‐53. [DOI] [PubMed] [Google Scholar]
De Almeida Prado 2004 {published data only}
- Almeida Prado RA, Silva EN, Guterman T, Leite TG. Meloxicam clinical trial, in carriers of primary dysmenorrhea, compared to rofecoxib [Ensaio clinico de meloxicam em muleres portadoras de dismenorreia primaria, comparado ao rofecoxib]. Revista Brasileira de Medicina 2004.;61(10):672‐5. [Google Scholar]
De la Boullaye 1971 {published data only}
- Boullaye H, Liesse AE, Cavrot E, Lamiroy H, Broeckhoven J. [Etude comparative en double insu de l'alclofenac et d'un placebo dans le traitement symtomatique re la dysmenorrhee]. Revue Francaise de Gynecologie et d'Obstetrique 1971;66(8‐9):525‐9. [PubMed] [Google Scholar]
DeLia 1982 {published data only}
- DeLia JE, Emery MG, Taylor RH, Scott JR. Flurbiprofen in dysmenorrhea. Clinical Pharmacology & Therapeutics 1982;32:76‐80. [DOI] [PubMed] [Google Scholar]
Di Girolamo 1996 {published data only}
- Girolamo G, Zmijanovich R, Santos AR, Marti ML, Terrangno A. Lysine clonixinate in the treatment of primary dysmenorrhea. Acta Physiologica Pharmacologica et Therapeutica Latinamericana 1996;46(4):223‐32. [PubMed] [Google Scholar]
Donadio 1987 {published data only}
- Donadio N. A double‐blind study with floctafenin, mefenamic acid, diclofenac and piroxicam in patients with primary dysmenorrhea [Estudo duplo‐cego entre a floctafenina, ac. mefenamico, diclofenac e piroxicam em pacientes com dismenorreia primaria]. Jornal Brasileiro de Ginecologia 1987;97(8):435‐9. [Google Scholar]
Doubova 2007 {published data only}
- Doubova SV, Morales HR, Hernandez SF, Martinez‐Garcia MC, Cossio Ortiz MG, Chavez Soto MA, et al. Effect of a Psidii guajavae folium extract in the treatment of primary dysmenorrhea: a randomized clinical trial. Journal of Ethnopharmacology 2007;110:305‐10. [DOI] [PubMed] [Google Scholar]
Dreher 1980 {published data only}
- Dreher E, Fischer B. Treatment of primary dysmenorrhea and dysmenorrhea because of IUP with PG‐synthetase inhibitors. Advances in Prostaglandin, Thromboxane and Leukotriene Research 1980;8:1487‐93. [PubMed] [Google Scholar]
Du Rant 1985 {published data only}
- Du Rant RH, Jay MS, Shoffitt T, Linder CW, Taylor W. Factors influencing adolescents' responses to regimens of naproxen for dysmenorrhea. American Journal of Diseases of Children 1985;139(5):489‐93. [DOI] [PubMed] [Google Scholar]
DuRant 1988 {published data only}
- DuRant RH, Jay S, Jerath R, Fink S. The influence of anxiety and locus of control on adolescents' response to naproxen sodium for mild to moderate pain. Journal of Adolescent Health Care 1988;9:424‐30. [DOI] [PubMed] [Google Scholar]
Eccles 2010 {published data only}
- Eccles R, Holbrook A, Jawad M. A double‐blind, randomised, crossover study of two doses of a single‐tablet combination of ibuprofen/paracetamol and placebo for primary dysmenorrhoea. Current Medical Research and Opinion 2010;26(11):2689‐99. [DOI] [PubMed] [Google Scholar]
Ertungealp 1985 {published data only}
- Ertungealp E, Colgar U, Arvas M, Topcuoglu D. The treatment of primary dysmenorrhoea with naproxen [Primer dismenore tedavisinde Naproksen]. Cerrahpaşa Tip Fakültesi Dergisi 1985;16:141‐8. [Google Scholar]
EUCTR2004‐003809‐25‐HU {unpublished data only}
- Sanofi‐Synthelabo Co. Ltd. Comparison of the efficacy and tolerability of drotaverine 80 mg or ibuprofen 400 mg administered alone with their combination for the treatment of primary and secondary dysmenorrhea. http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2004‐003809‐25‐HU 2004.
EUCTR2008‐006762‐29‐GB {unpublished data only}
- Reckitt Benckiser Healthcare (UK) Ltd. A double‐blind, randomised, crossover, single dose, single centre, study examining the analgesic efficacy and tolerability of fixed‐dose combinations of ibuprofen 200mg and acetaminophen 500mg, ibuprofen 400mg and acetaminophen 1,000mg and placebo in primary dysmenorrhoea. http://apps.who.int/trialsearch/Trial.aspx?TrialID=EUCTR2008‐006762‐29‐GB 2008.
Frank 1983 {published data only}
- Frank GJ, Keford RH. Report of a double‐blind crossover study to compare flurbiprofen with paracetamol in the treatment of primary dysmenorrhoea. Journal of International Medicine 1983;11 Suppl(2):6‐10. [PubMed] [Google Scholar]
Fraser 1987 {published and unpublished data}
- Fraser IS, McCarron G. Ibuprofen is a useful treatment for primary dysmenorrhoea. Australian & New Zealand Journal of Obstetrics & Gynaecology 1987;27(27):244‐7. [DOI] [PubMed] [Google Scholar]
Fuchs 1979 {published data only}
- Fuchs F, Chan WY, Dawood MY. Suppression of menstrual prostaglandins and relief of dysmenorrhea with ibuprofen. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:91‐2. [DOI] [PubMed] [Google Scholar]
Gookin 1983 {published data only}
- Gookin KS, Forman ES, Vecchio TJ, Wiser WL, Morrison JC. Comparative efficacy of ibuprofen, indomethacin and placebo in the treatment of primary dysmenorrhoea. Southern Medical Journal 1983;76(11):1361. [DOI] [PubMed] [Google Scholar]
Grossi 1986 {published data only}
- Grossi E, Borghi C. Randomized multicentre study on the use of diclofenac in the treatment of primary dysmenorrhea. An analysis of the data relative to the 878 patients [Studio multicentrico randomizzato sull'impiego del diclofenac nel trattamento della dismenorrea primaria. Un'analisi dei dati relativi z 878 pazienti]. Minerva Ginecologica 1986;38:637‐45. [PubMed] [Google Scholar]
Halbert 1978 {published data only}
- Halbert DR, Demers LM. A clinical trial of indomethacin and ibuprofen in dysmenorrhea. Journal of Reproductive Medicine 1978;21:219‐22. [PubMed] [Google Scholar]
Hamann 1977 {published data only}
- Hamann GO, Laursen B. Primary dysmenorrhea treated with indomethacin [Primaer dysmenore behandlet med indometacin]. Ugeskrift for Laeger 1977;139:1899‐901. [PubMed] [Google Scholar]
Hanson 1982 {published data only}
- Hanson FW. Naproxen sodium, ibuprofen and a placebo in dysmenorrhoea. Journal of Reproductive Medicine 1982;27(7):423‐7. [PubMed] [Google Scholar]
Hebert 1986 {published data only}
- Hebert JG, Morvan P, Bourgouin J. Double‐blind comparison of ketoprofen and mefenamic acid in the treatment of primary dysmenorrhea. Clinical Therapeutics 1986;8:329‐35. [PubMed] [Google Scholar]
Henzl 1977a {published data only}
- Henzl MR, Buttram V, Segre EJ, Bessler S. The treatment of dysmenorrhea with naproxen sodium: a report on two independent double‐blind trials. American Journal of Obstetrics & Gynecology 1977;127:818‐23. [DOI] [PubMed] [Google Scholar]
Henzl 1979 {published data only}
- Henzl MR, Izu A. Naproxen and naproxen sodium in dysmenorrhea: development from in vitro inhibition of prostaglandin synthesis to suppression of uterine contractions in women and demonstration of clinical efficacy. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:105‐17. [DOI] [PubMed] [Google Scholar]
Henzl 1979b {published data only}
- Henzl MR, Ortega‐Herrera E, Rodriguez C, Izu A. Anaprox in dysmenorrhea: reduction of pain and intrauterine pressure. American Journal of Obstetrics & Gynecology 1979;135:445‐60. [DOI] [PubMed] [Google Scholar]
Henzl 1980 {published data only}
- Henzl MR, Massey S, Hanson FW, Buttram VC, Rosenwaks Z, Pauls FD. Primary dysmenorrhea: the therapeutic challenge. Journal of Reproductive Medicine 1980;25:226‐35. [PubMed] [Google Scholar]
Ingemanson 1981 {published data only}
- Ingemanson CA, Carringtom B, Sikstrom B, Bjorkman R. Diclofenac in the treatment of primary dysmenorrhoea. Current Therapeutic Research 1981;30(5):632‐9. [Google Scholar]
IRCT201304096790N4 {unpublished data only}
- IRCT201304096790N4. The effect of celecoxib and ibuprofen on the treatment of primary dysmenorrhea on students of lam University of Medical Sciences. http://apps.who.int/trialsearch/Trial.aspx?TrialID=IRCT201304096790N4 2013.
Islas Perez 1981 {published data only}
- Islas Perez M, Rodriguez S. Mefenamic acid in primary dysmenorrhea [Acido mefenamico en dismenorrea primaria]. Ginecologia y Obstetricia de Mexico 1981;48(291):33‐6. [PubMed] [Google Scholar]
ISRCTN32847177 {unpublished data only}
- Bell D, Plethora Solutions Ltd (UK). A multi‐centre, double‐blind, placebo‐controlled, multiple‐dose crossover proof of concept study to compare the efficacy of mefenamic acid administered vaginally and orally in healthy menstruating women with primary dysmenorrhoea requiring analgesia. http://apps.who.int/trialsearch/Trial.aspx?TrialID=ISRCTN32847177 2007.
Iyagba 1987 {published data only}
- Iyagba N, Agboola A. Controlled, single blind crossover study of piroxicam and placebo in primary dysmenorrhoea. Current Therapeutic Research 1987;42(1):48‐54. [Google Scholar]
Jakubowicz 1984 {published data only}
- Jakubowicz DL, Godard E, Dewhurst J. The treatment of premenstrual tension with mefanamic acid: analysis of prostaglandin concentrations. British Journal of Obstetrics & Gynaecology 1984;91:78‐84. [DOI] [PubMed] [Google Scholar]
Janbu 1978 {published data only}
- Janbu T, Lokken P, Nesheim BI. Effect of acetylsalicylic acid, paracetamol, and placebo on pain and blood loss in dysmenorrhoeic women. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:81‐5. [DOI] [PubMed] [Google Scholar]
- Janbu T, Lokken P, Nesheim BI. Effect of acetylsalicylic acid, paracetamol, and placebo on pain and blood loss in dysmenorrhoeic women. European Journal of Clinical Pharmacology 1978;14(6):413‐6. [DOI] [PubMed] [Google Scholar]
Jansen 1984 {published data only}
- Jansen CH, Spinelli CAP, Santos AG, Martins ACP, Mendez‐Villasenor J. Efficacy and tolerability evaluation for diclofenac potassium in patients with primary dysmenorrhoea [Avaliacao da eficacia e da tolerabilidade do diclofenaco potassico em pacientes com dismenorreia primaria]. Arquivos Brasileiros de Medicina 1984;58(4):267‐71. [Google Scholar]
Jay 1986 {published data only}
- Jay MS, Durant RH, Shoffitt T, Linder CW. Differential response by adolescents to naproxen sodium therapy for spasmodic and congestive dysmenorrhea. Journal of Adolescent Health Care 1986;7:395‐400. [DOI] [PubMed] [Google Scholar]
Joelsson 1979 {published data only}
- Joelsson I, Lalos O. The effect of inhibitors of prostaglandin synthesis in primary dysmenorrhea studied with hysterometry. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:45‐9. [DOI] [PubMed] [Google Scholar]
Kajanoja 1979 {published data only}
- Kajanoja P, Vestano T. Naproxen and indomethacin in the treatment of primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:87‐9. [DOI] [PubMed] [Google Scholar]
Kapadia 1987 {published data only}
- Kapadia L. A study of naproxen sodium and ibuprofen in primary dysmenorrhoea. Journal of the Society of Occupational Medicine 1987;37:777‐80. [DOI] [PubMed] [Google Scholar]
Kauppila 1977 {published data only}
- Kauppila A, Ylikorkala O. Indomethacin and tolfenamic acid in primary dysmenorrhea. European Journal of Obstetrics Gynecology & Reproductive Biology 1977;7(2):59‐64. [Google Scholar]
Kauppila 1979 {published data only}
- Kauppila A, Puolakka J, Ylikorkala O. The relief of primary dysmenorrhea by ketoprofen and indomethacin. Prostaglandins 1979;18:647‐53. [DOI] [PubMed] [Google Scholar]
Kauppila 1979b {published data only}
- Kauppila A, Puolakka J, Ylikorkala P. Prostaglandin biosynthesis inhibitors and endometriosis. Prostaglandins 1979;18:655‐61. [DOI] [PubMed] [Google Scholar]
Kauppila 1985 {published data only}
- Kauppila A, Ronnberg L. Naproxen sodium in dysmenorrhea secondary to endometriosis. Obstetrics & Gynaecology 1985;65(3):379‐83. [PubMed] [Google Scholar]
Kauppila 1986 {published data only}
- Kauppila A, Makila UM, Makarainen L, Puolakka J, Seppala A. Tiaprofenic acid in the treatment of primary dysmenorrhoea. European Journal of Obstetrics, Gynaecology & Reproductive Biology 1986;22:359‐63. [DOI] [PubMed] [Google Scholar]
Kemp 1972 {published data only}
- Kemp JH. "Buscopan" in spasmodic dysmenorrhoea. Current Medical Research & Opinion 1972;1:19‐25. [DOI] [PubMed] [Google Scholar]
Killick 1990 {published data only}
- Killick S, Faragher B, Elstein M. Azapropazone: an alternative agent for the treatment of primary dysmenorrhoea. Journal of Obstetrics & Gynaecology 1990;10:406‐10. [Google Scholar]
Kintis 1980 {published data only}
- Kintis GA, Coutifaris B. Treatment of primary dysmenorrhea with mefenamic acid. International Journal of Gynaecology & Obstetrics 1980;18:172‐5. [DOI] [PubMed] [Google Scholar]
Klein 1981 {published data only}
- Klein JR, Litt IF, Rosenberg A, Udall L. The effect of aspirin on dysmenorrhea in adolescents. Journal of Pediatrics 1981;98:987‐90. [DOI] [PubMed] [Google Scholar]
Kollenz 2009 {published data only}
- Kollenz C, Phleps W, Kaehler ST. ADIDAC trial: analgesia with dexibuprofen versus ibuprofen in patients suffering from primary dysmenorrhea: a crossover trial. Gynecologic and Obstetric Investigation 2009;67:25‐31. [DOI] [PubMed] [Google Scholar]
Krishna 1980 {published data only}
- Krishna UR, Naik S, Mandlkar A, Gupta KC, Kulkarni VN, Sheth UK. Flurbiprofen in the treatment of primary dysmenorrhoea. British Journal of Clinical Pharmacology 1980;9:605‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunz 1981 {published data only}
- Kunz J, Schneider W. Therapy of dysmenorrhea with prostaglandin synthetase inhibitors: experiences with fluproquazone. Schweizerische Medizinische Wochenschrift. Journal Suisse de Medecine 1981;111(39):1451‐5. [PubMed] [Google Scholar]
Lalos 1983 {published data only}
- Lalos O, Nilsson B. Dysmenorrhea in women with intrauterine contraceptive device: treatment with a prostaglandin synthetase inhibitor, naproxen. International Journal of Gynaecology & Obstetrics 1983;21:33‐7. [DOI] [PubMed] [Google Scholar]
Langrick 1982 {published data only}
- Langrick AF, Gunn AD. A comparison of naproxen sodium and a dextropropoxyphene/paracetamol combination in the treatment of primary dysmenorrhoea in University Health Centres. British Journal of Clinical Practice 1992;36:181‐4. [PubMed] [Google Scholar]
Langrick 1983 {published data only}
- Langrick AF. A study of naproxen sodium and mefenamic acid in primary dysmenorrhoea. British Journal of Clinical Practice 1985;35(10):342‐7. [PubMed] [Google Scholar]
Langrick 1989 {published data only}
- Langrick AF, Gunn AD, Livesy H, Whitehead AM. A double‐blind placebo‐controlled crossover study of mebeverine and mefenamic acid in the treatment of primary dysmenorrhoea. British Journal of Clinical Practice 1989;3:317‐21. [PubMed] [Google Scholar]
Larkin 1979 {published data only}
- Larkin RM, Orden DE, Poulson AM, Scott JR. Dysmenorrhea: treatment with an antiprostaglandin. Obstetrics & Gynecology 1979;54:456‐60. [PubMed] [Google Scholar]
Lundstrom 1978 {published data only}
- Lundstrom V. Treatment of primary dysmenorrhea with prostaglandin synthetase inhibitors ‐ a promising therapeutic alternative. Acta Obstetricia et Gynecologica Scandinavica 1978;57:421‐8. [DOI] [PubMed] [Google Scholar]
Lundstrom 1979 {published data only}
- Lundstrom V, Green K, Svanborg K. Endogenous prostaglandins in dysmenorrhea and the effect of prostaglandin synthetase inhibitors (PGSI) on uterine contractility. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:51‐6. [DOI] [PubMed] [Google Scholar]
Maclean 1983 {published data only}
- Maclean D. A comparison of flurbiprofen and paracetamol in the treatment of primary dysmenorrhoea. Journal of International Medical Research 1983;11 Suppl(2):1‐5. [PubMed] [Google Scholar]
Makarainen 1983 {published data only}
- Makarainen L, Ylikorkala O. Menstrual blood loss in dysmenorrhoea: effects of proquazone and indomethacin. British Journal of Obstetrics & Gynaecology 1983;90:570‐2. [DOI] [PubMed] [Google Scholar]
Mannix 2009 {published data only}
- Mannix LK, Martin VT, Cady RK, Diamond ML, Lener SE, White JD, et al. Combination treatment for menstrual migraine and dysmenorrhea using sumatriptan–naproxen. Obstetrics and Gynecology 2009;114:106‐13. [DOI] [PubMed] [Google Scholar]
Marchini 1987 {published data only}
- Marchini M, Fedele L, Garagiola U, Maresca V. Pirprofen, naproxen and placebo in the treatment of primary dysmenorrhoea. Drugs under Experimental & Clinical Research 1987;13:699‐705. [PubMed] [Google Scholar]
Mehlisch 1988 {published data only}
- Mehlisch DR. Ketoprofen, ibuprofen, and placebo in the treatment of primary dysmenorrhea: a double‐blind crossover comparison. Journal of Clinical Pharmacology 1988;28:S29‐33. [DOI] [PubMed] [Google Scholar]
Milsom 1984 {published data only}
- Milsom I, Andersch B. Effect of ibuprofen, naproxen sodium and paracetamol on intrauterine pressure and menstrual pain in dysmenorrhoea. British Journal of Obstetrics & Gynaecology 1984;91:1129‐35. [DOI] [PubMed] [Google Scholar]
Milsom 1988 {published data only}
- Milsom I, Andersch B, Sundell G. The effect of flurbiprofen and naproxen sodium on intra‐uterine pressure and menstrual pain in patients with primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavica 1988;67:711‐6. [DOI] [PubMed] [Google Scholar]
Milsom 2002a {published and unpublished data}
- Milsom I, Minic M, Dawood MY, Akin MD, Spann J, Niland NF, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clinical Therapeutics 2002;24(9):1384‐400. [DOI] [PubMed] [Google Scholar]
- Minic M. Personal correspondence April 2003.
Milsom 2002b {published and unpublished data}
- Milsom I, Minic M, Dawood MY, Akin MD, Spann J, Niland NF, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clinical Therapeutics 2002;24(9):1384‐400. [DOI] [PubMed] [Google Scholar]
- Minic M. Personal correspondence April 2003.
Milsom 2002c {published and unpublished data}
- Milsom I, Minic M, Dawood MY, Akin MD, Spann J, Niland NF, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clinical Therapeutics 2002;24(9):1384‐400. [DOI] [PubMed] [Google Scholar]
- Minic M. Personal correspondence April 2003.
Montrull 1987 {published data only}
- Montrull HL, Meirovich C, Rubio A, Brizuela NY. Prostaglandins in primary dysmenorrhea. Effect of ketoprofen [Prostaglandinas en dismenorrea primaria. Accion del ketoprofen]. Revista de la Facultad de Ciencias Medicas 1987;45:21‐4. [PubMed] [Google Scholar]
NCT00380627 2006 {unpublished data only}
- Merck Sharp, Dohme Corp. Quality of life with arcoxia in women with dysmenorrhea. http://clinicaltrials.gov/ct2/show/NCT00380627 2006.
Nor Azlin 2008 {published data only}
- Nor Azlin MI, Maryasalwati I, Norzilawati MN, Mahdy ZA, Jamil MA, Zainul Rashid MR. The efficacy of etoricoxib vs mefenamic acid in the treatment of primary dysmenorrhea: a randomised comparative trial. Journal of Obstetrics and Gynaecology 2008;28(4):424‐6. [DOI] [PubMed] [Google Scholar]
Ogden 1970 {published data only}
- Ogden JA, Wade ME, Anderson G, Davis CD. Treatment of dysmenorrhea. A comparative double blind study. American Journal of Obstetrics & Gynecology 1970;106:838‐42. [DOI] [PubMed] [Google Scholar]
Ozbay 2006 {published data only}
- Ozbay K, Inanmis RA, Deveci S, Yardim T. [Primer dismenore tedavisinde plasebo, lornoxicam, ve alverine citrate + simethicone'un etkinliklerinin karsilastirilmasi]. Jinekoloji ve Obstetrik Dergisi 2006;20(3):158‐61. [Google Scholar]
Ozgoli 2009 {published data only}
- Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid and ibuprofen on pain in women with primary dysmenorrhea. Journal of Alternative and Complementary Medicine 2009;15(2):129‐32. [DOI] [PubMed] [Google Scholar]
Palmisano 1988 {published data only}
- Palmisano GP, Lamb EJ. Double‐blind crossover comparison of ketoprofen, ibuprofen and placebo in the treatment of patient with primary dysmenorrhea. Advances in Therapy 1988;5(4):128‐37. [Google Scholar]
Peixoto 1984 {published data only}
- Peixoto S, Pontes AC, Fittipaldi JAS. Treatment of dysmenorrhea with antiprostaglandin agent: comparative study with ibuprofen and placebo [Tratamento da dismenorreia com agente antiprostaglandina: estudo comparativo com ibuprofen e placebo]. Jornal Brasileiro de Ginecologia 1984;94(11‐12):521‐3. [Google Scholar]
Pendergrass 1984 {published data only}
- Pendergrass PB, Ream LJ, Scott JN, Agna MA. Do aspirin and acetaminophen affect total menstrual loss?. Gynecologic & Obstetric Investigation 1984;18:129‐33. [DOI] [PubMed] [Google Scholar]
Pendergrass 1985 {published data only}
- Pendergrass PB, Scott JN, Ream LJ, Agna MA. Effect of small doses of aspirin and acetaminophen on total menstrual loss and pain of cramps and headache. Gynecologic & Obstetric Investigation 1985;19:32‐7. [DOI] [PubMed] [Google Scholar]
Petti 1985 {published data only}
- Petti DA, Naiberg E. Glucametacin vs acetylsalicylic acid in the treatment of primary dysmenorrhea: a double‐blind cross‐over study [Glucametacina vs acido acetilsalicilico no tratamento da dismenorreia primaria: estudo duplo‐cego cruzado]. Jornal Brasileiro de Ginecologia 1985;95(5):199‐201. [Google Scholar]
Pirhonen 1986 {published data only}
- Pirhonen J, Pulkkinen M. The effect of nimesulide and naproxen on the uterine and ovarian arterial blood flow velocity. A Doppler study. Acta Obstetricia et Gynecologica Scandinavica 1995;74:549‐53. [DOI] [PubMed] [Google Scholar]
Plantema 1986 {published data only}
- Plantema F. Worldwide studies comparing piroxicam and naproxen. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1986;138:15‐7. [DOI] [PubMed] [Google Scholar]
Pogmore 1980 {published data only}
- Pogmore JR, Filshie GM. Flurbiprofen in the management of dysmenorrhea. British Journal of Obstetrics & Gynaecology 1980;87:326‐9. [DOI] [PubMed] [Google Scholar]
Prasad 1980 {published data only}
- Prasad R. Treatment of primary dysmenorrhoea with benorylate. Practitioner 1980;224:325‐7. [PubMed] [Google Scholar]
Pulkkinen 1978 {published data only}
- Pulkkinen MO, Henzl MR, Csapo AI. The effect of naproxen‐sodium on the prostaglandin concentrations of the menstrual blood and uterine jet‐washings in dysmenorrheic women. Prostaglandins 1978;15(3):543‐50. [DOI] [PubMed] [Google Scholar]
Pulkkinen 1978b {published data only}
- Pulkkinen MO, Csapo AI. The effect of ibuprofen on the intrauterine pressure and menstrual pain of dysmenorrheic patients. Prostaglandins 1978;15:1055‐62. [DOI] [PubMed] [Google Scholar]
Pulkkinen 1979 {published data only}
- Pulkkinen MO, Csapo AI. Effect of ibuprofen on menstrual blood prostaglandin levels in dysmenorrheic women. Prostaglandins 1979;18:137‐42. [DOI] [PubMed] [Google Scholar]
Rawal 1987 {published data only}
- Rawal MY, Dave D, Shah SK, Daftary SN, Lal HM, Phaterpekar SJ, et al. Double‐blind comparison of the efficacy and safety of naproxen and placebo in the treatment of dysmenorrhea. Current Therapeutic Research 1987;42(6):1073‐80. [Google Scholar]
Rosenwaks 1981 {published data only}
- Rosenwaks Z, Jones GS, Henzl MR, Dubin NH, Ghodgaonkar RB, Hoffman S. Naproxen sodium, aspirin, and placebo in primary dysmenorrhea. Reduction of pain and blood levels of prostaglandin F2‐alpha metabolite. American Journal of Obstetrics & Gynecology 1981;140:592‐8. [DOI] [PubMed] [Google Scholar]
Roy 1981 {published data only}
- Roy S, Shaw ST Jr. Role of prostaglandins in IUD‐associated uterine bleeding ‐ effect of a prostaglandin synethetase inhibitor (ibuprofen). Obstetrics & Gynecology 1981;58:101‐6. [PubMed] [Google Scholar]
Roy 1983 {published data only}
- Roy S. A double‐blind comparison of a propionic acid derivative (ibuprofen) and a fenamate (mefenamic acid) in the treatment of dysmenorrhea. Obstetrics & Gynecology 1983;61:628‐32. [PubMed] [Google Scholar]
Sahin 2003 {published data only}
- Sahin I, Saracoglu F, Kurban Y, Turkkani B. Dysmenorrhea treatment with a single daily dose of rofecoxib. International Journal of Gynecology and Obstetrics 2003;83:285‐91. [DOI] [PubMed] [Google Scholar]
Sauer 1994 {published data only}
- Sauer MV, Bernstein RI, Gaudiani LM, Tredway DR. A double‐blind, placebo‐controlled, crossover study of diclofenac potassium versus naproxen sodium in the treatment of primary dysmenorrhea. Today's Therapeutic Trends 1994;12:63‐79. [Google Scholar]
Schulman 1985 {published data only}
- Schulman H, Duvivier R, Blattner P. The uterine contractility index: a research and diagnostic tool in dysmenorrhea. American Journal of Obstetrics & Gynecology 1983;145(8):1049. [DOI] [PubMed] [Google Scholar]
Schwartz 1974 {published data only}
- Schwartz A, Zor U, Lindner HR, Naor S. Primary dysmenorrhea; alleviation by an inhibitor of prostaglandin synthesis and action. Obstetrics & Gynecology 1974;44(5):709‐12. [PubMed] [Google Scholar]
Sedgwick 1985 {published data only}
- Sedgwick JP, Daily HR, Langrick AF, Hill RC. Double‐blind study of meptazinol, d‐propoxyphene/paracetamol and placebo in patients with primary dysmenorrhoea. Current Therapeutic Research, Clinical & Experimental 1985;38:528‐35. [Google Scholar]
Serfaty 1986 {published data only}
- Serfaty D. A comparative crossover study of piroxicam vs mefenamic acid and diclofenac in France. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1986;138:19‐20. [DOI] [PubMed] [Google Scholar]
Shapiro 1981 {published data only}
- Shapiro SS, Diem K. The effect of ibuprofen in the treatment of dysmenorrhea. Current Therapeutic Research, Clinical & Experimental 1981;30:327‐34. [Google Scholar]
Shapiro 1986 {published data only}
- Shapiro SS. Flurbiprofen for the treatment of primary dysmenorrhea. American Journal of Medicine 1986;80 Suppl(3A):71‐5. [DOI] [PubMed] [Google Scholar]
Shishegar 1997 {published data only}
- Shishegar F, Modares M. Comparison of piroxicam and mefenamic acid in treatment of primary dysmenorrhea. Acta Obstetricia et Gynecologica Scandinavia Supplementum 1997;76(167(2)):61. [Google Scholar]
Smith 1980 {published data only}
- Smith RP, Powell JR. The objective evaluation of dysmenorrhea therapy. American Journal of Obstetrics & Gynecology 1980;137:314‐9. [DOI] [PubMed] [Google Scholar]
Smith 1987 {published data only}
- Smith RP. Objective changes in intrauterine pressure during placebo treatment of dysmenorrhea. Pain 1987;29:59‐66. [DOI] [PubMed] [Google Scholar]
- Smith RP. The dynamics of nonsteroidal anti‐inflammatory therapy for primary dysmenorrhea. Obstetrics and Gynaecology 1987;70:785‐8. [PubMed] [Google Scholar]
- Smith RP, Powell JR. Simultaneous objective and subjective evaluation of meclofenamate sodium in the treatment of primary dysmenorrhea. American Journal of Obstetrics & Gynecology 1987;157:611‐8. [DOI] [PubMed] [Google Scholar]
Szigeti 1981 {published data only}
- Szigeti L, Bolgar J. Effectiveness of indomethacin in the management of childhood and juvenile primary dysmenorrhea (letter) [Az indomethacinum hatekonysaga a gyermet ‐ ill. ifjukori primer dysmenorrhoeak kezelesben]. Orvosi Hetilap 1981;122(30):1871. [PubMed] [Google Scholar]
Tampakoudis 1997 {published data only}
- Tampakoudis P, Tantanassis T, Kellatizis D, Moussas D, Venetis C, Lazaridis P, et al. Tolfenamic acid in primary dysmenorrhoea. Long treatment protocol and 1‐year follow up. Acta Obstetricia et Gynecologica Scandinavia Supplementum 1997;76:59. [Google Scholar]
Tilyard 1992 {published data only}
- Tilyard MW, Dovey SM. A comparison of tiaprofenic acid, mefenamic acid and placebo in the treatment of dysmenorrhoea in general practice. Australian & New Zealand Journal of Obstetrics & Gynaecology 1992;32:165‐8. [DOI] [PubMed] [Google Scholar]
Villasenor 1985 {published data only}
- Villasenor FJM. Oral pirprofen in primary dysmenorrhea [Pirprofen oral en dismenorrea primaria]. Compendium de Investigaciones Clinicas Latioamericanas 1985;5(1):8‐11. [Google Scholar]
Von Graffenried 1981 {published data only}
- Graffenried B, Nuesch E. Fluproquazone in the treatment of primary dysmenorrhoea. A double‐blind, cross‐over study. Arzneimittel‐Forschung 1981;31(5a):932‐3 (Abstract only). [Google Scholar]
Williams 1982 {published data only}
- Williams AA, Backhouse CI. A general practice study of naproxen sodium and a dextropropoxyphene‐paracetamol combination in primary dysmenorrhoea. British Journal of Clinical Practice 1982;36:383‐5. [PubMed] [Google Scholar]
Ylikorkala 1980 {published data only}
- Ylikorkala O, Puolakka J, Kauppila A. Comparison between naproxen tablets and suppositories in primary dysmenorrhea. Prostaglandins 1980;20(3):463‐8. [DOI] [PubMed] [Google Scholar]
Ylikorkala 1981 {published data only}
- Ylikorkala O, Puolakka J, Kauppila A. Comparison between fluproquazone and indomethacin in treatment of primary dysmenorrhoea. Prostaglandins & Medicine 1981;6:217‐21. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
CTRI2188 {unpublished data only}
- Patel JC. Comparative efficacy, safety and cost effectiveness of lornoxicam with ibuprofen in patient with primary dysmenorrhoea: a randomized, double‐blind, active‐controlled study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3443 Registered 2/12/2011, retrospectively.
Additional references
Banikarim 2000
- Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Archives of Pediatrics and Adolescent Medicine 2000;154(12):1226‐9. [DOI] [PubMed] [Google Scholar]
Brooks 1999
- Brooks PM, Day RO. Non‐steroidal anti‐inflammatory drugs ‐ differences and similarities. New England Journal of Medicine 1999;324:1716‐25. [DOI] [PubMed] [Google Scholar]
Brown 2010
- Brown J, Brown S. Exercise for dysmenorrhoea. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD004142.pub2] [DOI] [PubMed] [Google Scholar]
Chan 1978
- Chan WY, Dawood MY, Fuchs F. Relief of dysmenorrhea with the prostaglandin synthetase inhibitor ibuprofen: effect on prostaglandin levels in menstrual fluid. American Journal of Obstetrics and Gynecology 1979;135:102‐8. [PubMed] [Google Scholar]
Coco 1999
- Coco AS. Primary dysmenorrhea. American Family Physician 1999;60:489‐96. [PubMed] [Google Scholar]
CONSORT 2001
- Consort statement. http://www.consort‐statement.org/consort‐statement/ 2001.
Dawood 1984
- Dawood MY. Ibuprofen and dysmenorrhea. American Journal of Medicine 1984;77(1A):87‐94. [DOI] [PubMed] [Google Scholar]
Dawood 2006
- Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstetrics and Gynecology 2006;108:428‐41. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
EMEA 2002
- The European Agency for the Evaluation of Medicinal Products. Press release EMEA website 26 April 2002. http://www.emea.eu.int/pdfs/human/press/pr/174702en.pdf (accessed 7 April 2003; link no longer operating July 2015).
FDA 2015
- US Food, Drug Administration. COX‐2 selective (includes Bextra, Celebrex, and Vioxx) and non‐selective non‐steroidal anti‐inflammatory drugs (NSAIDs). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm429364.htm 2015.
Fedorowicz 2012
- Fedorowicz Z, Nasser M, Jagannath VA, Beaman JH, Ejaz K, Zuuren EJ. Beta2‐adrenoceptor agonists for dysmenorrhoea. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD008585.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 1984
- Hart FD, Huskisson EC. Non‐steroidal anti‐inflammatory drugs. Current status and rational therapeutic use. Drugs 1984;27(2):232‐55. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillen 1999
- Hillen TI, Grbavac SL, Johnston PJ, Straton JA, Keogh JM. Primary dysmenorrhea in young Western Australian women: prevalence, impact and knowledge of treatment. Journal of Adolescent Health 1999;25(1):40‐5. [DOI] [PubMed] [Google Scholar]
Laszlo 2008
- László KD, Gyrffy Z, Ádám S, Csoboth C, Kopp MS. Work‐related stress factors and menstrual pain: a nation‐wide representative survey. Journal of Psychosomatic Obstetrics and Gynecology 2008;29(2):133‐8. [DOI] [PubMed] [Google Scholar]
Latthe 2006
- Latthe P, Latthe M, Say L, Gülmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006;6(177):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichten 1987
- Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. Journal of Reproductive Medicine 1987;32:37‐41. [PubMed] [Google Scholar]
Pickles 1979
- Pickles VR. Prostaglandins and dysmenorrhea: historical survey. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1979;87:7‐12. [DOI] [PubMed] [Google Scholar]
Pitts 2008
- Pitts MK, Ferris JA, Smith AMA, Shelley JM, Richters J. Prevalence and correlates of three types of pelvic pain in a nationally representative sample of Australian women. Medical Journal of Australia 2008;189(3):138‐43. [DOI] [PubMed] [Google Scholar]
Proctor 2001
- Proctor M, Murphy PA. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002124] [DOI] [PubMed] [Google Scholar]
Proctor 2002
- Proctor M, Farquhar C, Stones W, He L, Zhu X, Brown J. Transcutaneous electrical nerve stimulation for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2005
- Proctor M, Latthe P, Farquhar C, Khan K, Johnson N. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhoea. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2006
- Proctor M, Hing W, Johnson TC, Murphy PA, Brown J. Spinal manipulation for dysmenorrhoea. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002119.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2007
- Proctor M, Murphy PA, Pattison HM, Suckling JA, Farquhar C. Behavioural interventions for dysmenorrhoea. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002248.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shi 2008
- Shi S, Klotz U. Clinical use and pharmacological properties of selective COX‐2 inhibitors. European Journal of Clinical Pharmacology 2008;64:233‐52. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith CA, Zhu X, He L, Song J. Acupuncture for dysmenorrhoea. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD007854.pub2] [DOI] [PubMed] [Google Scholar]
Wong 2009
- Wong CL, Farquhar C, Roberts H, Proctor M. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002120.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zahradnik 2010
- Zahradnik HP, Hanjalic‐Beck A, Groth K. Nonsteroidal anti‐inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception 2010;81:185‐96. [DOI] [PubMed] [Google Scholar]
Zhang 1998
- Zhang WY, Li Wan Po A. Efficacy of minor analgesics in primary dysmenorrhoea: a systematic review. British Journal of Obstetrics and Gynaecology 1998;105:780‐9. [DOI] [PubMed] [Google Scholar]
Zhu 2008
- Zhu X, Proctor M, Bensoussan A, Wu E, Smith CA. Chinese herbal medicine for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005288] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wilson 1999
- Wilson ML, Sinclair OJ, Farquhar C, Ivanova V, Stones W. Nonsteroidal anti‐inflammatory drugs for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001751] [DOI] [PubMed] [Google Scholar]


