Abstract
Purpose:
There is an urgent need for the development of novel positron emission tomography (PET) tracers for glioma imaging. In this study, we developed a novel PET probe ([18F]VUIIS1018A) by targeting translocator protein (TSPO), an imaging biomarker for glioma. The purpose of this preclinical study was to evaluate this novel TSPO probe for glioma imaging.
Procedures:
In this study, we synthesized [19F]VUIIS1018A and the precursor for radiosynthesis of [18F]VUIIS1018A. TSPO binding affinity was confirmed using a radioligand competitive binding assay in C6 glioma cell lysate. Further, dynamic imaging studies were performed in rats using a microPET system. These studies include displacement and blocking studies for ligand reversibility and specificity evaluation, and compartment modeling of PET data for pharmacokinetic parameter measurement using metabolite-corrected arterial input functions and PMOD.
Results:
Compared to previously reported TSPO tracers including [18F]VUIIS1008 and [18 F]DPA-714, the novel tracer [18F]VUIIS1018A demonstrated higher binding affinity and BPND. Pretreatment with the cold analog [19F]VUIIS1018A could partially block tumor accumulation of this novel tracer. Further, compartment modeling of this novel tracer also exhibited a greater tumor-to-background ratio, a higher tumor binding potential and a lower brain binding potential when compared with other TSPO probes, such as [18F]DPA-714 and [18F]VUIIS1008.
Conclusions:
These studies illustrate that [18F]VUIIS1018A can serve as a promising TSPO PET tracer for glioma imaging and potentially imaging of other solid tumors.
Keywords: TSPO, PET, Molecular imaging, Glioma, VUIIS1018A, DPA-714, Cancer imaging
Introduction
Malignant gliomas are the most common primary brain tumors and are characterized by invasive growth and recalcitrance to therapy. The use of pathology for the diagnosis, grading, and classification of gliomas is confounded by tumor heterogeneity and sampling errors associated with obtaining tissue specimens through biopsy or surgical resection. Hence, imaging is frequently used to support clinical decisions by providing a non-invasive method to characterize the whole tumor and metastases [1]. Though ubiquitously employed clinically, X-ray computed tomography (CT) and magnetic resonance imaging (MRI) provide little molecular information attributable to the pathology of disease and tend to poorly discriminate margins, particularly where tumors are highly infiltrative. As another non-invasive imaging modality, positron emission tomography (PET) imaging overcomes many of the limitations associated with conventional brain tumor imaging modalities and offers unique advantages including improved sensitivity and the ability to differentiate tissues based upon molecular characteristics. Currently, 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) is the most widely used PET tracer for brain tumor imaging in the clinic [2]. However, due to tracer accumulation in normal brain tissues, PET imaging using [18F]FDG can only generate modest to poor tumor-to-background ratios. Thus, there remains a strong unmet clinical need to develop and validate novel molecular imaging ligands for the detection and characterization of gliomas.
Translocator protein (TSPO) is an 18 kDa membrane protein typically localized to the outer mitochondrial membrane. Formerly known as the peripheral benzodiazepine receptor (PBR), TSPO participates in a variety of cellular functions, including steroid biosynthesis, cholesterol metabolism, cell proliferation, and apoptosis. [3]. Accordingly, TSPO expression is elevated in numerous clinically important diseased states, including neuroinflammation [4–8] and cancer [9–12]. As a hallmark of aggressive tumors, TSPO expression has been linked with disease progression and diminished survival in oncology.
A variety of TSPO ligands have been reported for imaging a number of cancers, including colon cancer [13], breast cancer [14–16], and glioma [17–21]. Since TSPO tends to have elevated expression in brain tumor tissues while maintaining low expression in normal brain, it is ideal to use TSPO PET tracers to visualize and quantify glioma [22]. In our previous studies, N-[18F]fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline ([18F]PBR06) [18 ] and N, N-diethyl-2-(2-(4-(2-[18F]fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide ([18F]DPA-714) [17] have been used for glioma imaging. Further, we reported the synthesis and structure activity relationship (SAR) analysis of a library of novel pyrazolopyrimidines [23], which led to the discovery of a novel ligand 2-(5,7-diethyl-2-(4-(2-fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide (VUIIS1008) that exhibited a 36-fold enhancement in binding affinity compared to the previously reported TSPO ligand, DPA-714 [17, 23]. Herein, we further modified the five and seven positions of the pyrazolopyrimidinal scaffold and discovered a novel TSPO ligand VUIIS1018A (Fig. 1), which features a 700-fold and a 20-fold enhancement in binding affinity when compared with DPA-714 and VUIIS1008, respectively [17, 23].
Fig. 1.
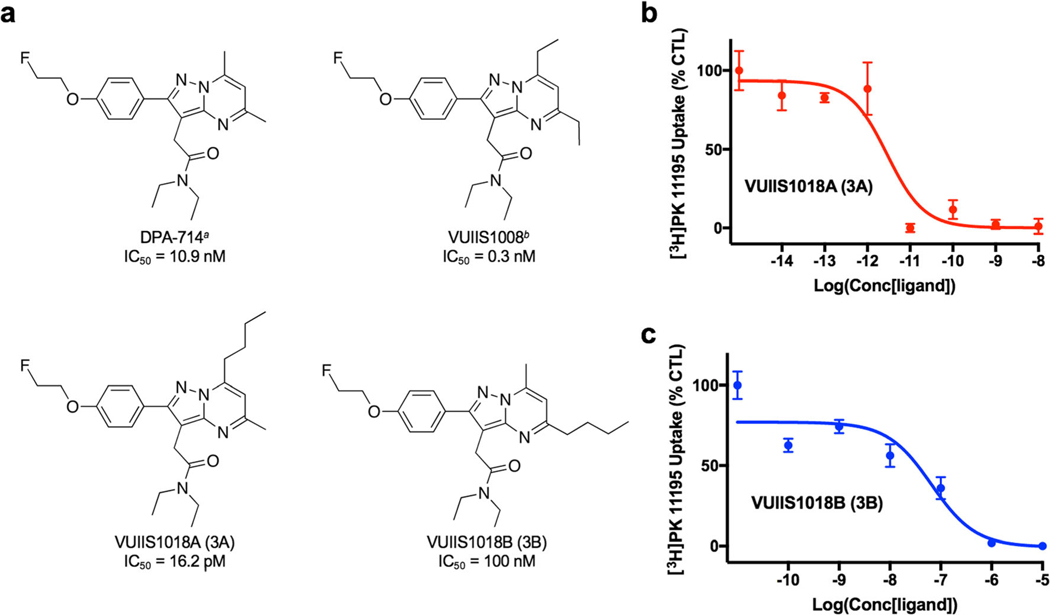
a Chemical structure and IC50 values of DPA-714 (asee ref. [17]), VUIIS1008 (bsee ref. [23]), VUIIS1018A, and VUIIS1018B. Equilibrium competitive binding of [3H]PK 11195 using b VUIIS1018A and c VUIIS1018B in C6 glioma cell lysate.
To evaluate its in vivo performance in C6-glioma imaging, we prepared the radiolabeled ligand [18F]VUIIS1018A and performed a series of analyses including in vivo PET imaging, radiometabolite analysis, and pharmacokinetic modeling.
Materials and Methods
Chemicals
[3H]PK 11195 was purchased from PerkinElmer. Phosphate-buffered saline (PBS) and CytoScint ES Liquid Scintillation Cocktail were purchased from MP biomedicals. 2,4-Octanedione was purchased from Alfa Aesar. All other synthesis reagents were purchased from Sigma-Aldrich Chemical Company and used as supplied.
Ligand and Radioligand Precursor Preparation
As shown in Scheme 1 (see Electronic Supplementary Material: ESM), 2A and 2B can be prepared via a two-step synthetic route starting from previously reported compound 1 [23, 24]. Compound 1 is combined with octane-2,4-dione to afford the desired pyrimidine ring heterocycle, followed by de-protection of the methyl ether to reveal the phenol moiety via reaction with hydrogen bromide, HBr. Further reaction of 2A and 2B with the appropriate tosylates produced 3A (VUIIS1018A), as the cold analog of the F-18 labeled probe, 3B (VUIIS1018B) and 4A, as a precursor for the radiosynthesis of the PET tracer [18F]VUIIS1018A. Structure characterization was performed using liquid chromatography-mass spectrometry (LCMS), H-1 and C-13 NMR (see ESM).
In Vitro TSPO-Binding Assay
C6 cells were collected and washed with PBS buffer three times, then frozen and thawed three times in lysis buffer (5.0 mM HEPES, 0.21 M D-mannitol, 0.07 M sucrose, 2.0 mM benzamidine, 2.0 mM toluenesulfonyl fluoride, 4.0 mM MgCl2, pH 7.4) to produce C6 cell lysate (0.5 mg/ ml). The obtained C6 cell lysate (0.3 ml) was then incubated with [3H]PK 11195 (final concentration = 0.6 nM) and TSPO ligands (10−5 to 10−14 M) in a total volume of 1.0 ml for 2 h at 25 °C. The reaction was terminated by rapid filtration through a Brandel harvester (Gaithersburg, MD, USA) and collection onto a filter presoaked with 0.3 % polyethylenimine. Filters were then punched out into scintillation vials and bound radioactivity measured on a Beckman LS 6000 Scintillation Counter (Brea, California, USA). Binding affinity (IC50) was calculated using GraphPad Prism (La Jolla, CA, USA) in triplicates.
Radiosynthesis
Radiosynthesis was carried out by the Center for Molecular Probes Radiochemistry Core (VUMC). [18F]VUIIS1018A was synthesized from the tosylate ester precursor (4A in Suppl. Scheme 1, ESM). In brief, using a commercial apparatus (TRACERlab FXF-N; GE Healthcare, Little Chalfont, UK), aqueous [18F]fluoride (~ 3.0 Ci; 111 GBq) was dried by iterative cycles of addition/evaporation of acetonitrile in the presence of potassium carbonate and Kryptofix 2.2.2 to form the complexation mixture of [18F]K+-K2.2.2/K2CO3. This complex was then reacted with 2-(4-(7-butyl-3-(2-(diethylamino)-2-oxoethyl)-5-methylpyrazolo[1,5-a]pyrimidin-2-yl)phenoxy)ethyl 4-methylbenzenesulfonate (4.0 mg) (4A) at 99 °C for 20 min in anhydrous dimethyl sulfoxide (0.6 ml). [18F]VUIIS1018A was purified using RP-HPLC (C18, Dynamax 250 × 21.4 mm; Varian), eluting with 10 mM NaH2PO4 buffer (pH 6.7) and ethanol (47.5/52.5, v/v) at 6.0 ml/min. The product was collected directly into 140 ml of water (deionized), passed through a C-18 Sep-Pak Plus (Waters, Milford, MA, USA), and eluted with 200 proof ethanol (1.0 ml) then saline (9.0 ml) into a sterile vial. Radiochemical purities were greater than 90 % in all cases and the typical specific activities were 4203 Ci/mmol (156 TBq/mmol) or greater.
Animals
All studies involving animals were conducted in compliance with federal and institutional guidelines. For glioma studies, healthy male Wistar rats were stereotactically inculcated in the right hemisphere with 1.0 × 105 C6 glioma cells (American Type Culture Collection, Manassas, VA, USA) 2 weeks prior to imaging. Rats were affixed with venous and arterial catheters prior to the MRI and PET/CT study.
MRI
PET was co-registered with MRI for anatomical comparison. In this study, rats were secured prone in a radiofrequency coil (38-mm inner diameter) and placed in a 4.7T MRI imaging system (Varian Inc., Palo Alto, CA, USA). Body temperature was maintained at 37 °C using heated airflow. In order to properly position the rats for subsequent scans, a multi-slice gradient-echo sequence (repetition time, 150 ms; echo time, 3.5 ms; matrix, 128 × 128; field of view, 40 × 40 mm2; slice thickness, 2 mm) was employed to acquire seven slices in each imaging plane (axial, coronal, and sagittal direction). Following this, MRI imaging was performed using a T2-weighted fast spin-echo scan with 8 echoes and 8-ms echo spacing (effective echo time, 32 ms). The 8 coronal slices of 2 mm thickness (16 acquisitions) were collected using a repetition time of 2000 ms; field of view 32 × 32 mm2 and a matrix of 128 × 128.
PET and CT Acquisition
In rats with confirmed tumors, PET and CT were performed within 24 h of MRI. Rats were administered [18F]VUIIS1018A via jugular catheter while in a microPET Focus 220 scanner (Siemens, Knoxville, TN, USA). Data were collected in listmode format for 60 or 90 min, followed by CT (microCAT II; Siemens) for attenuation correction. The dynamic PET acquisition was divided into 32 frames (12 frames (10 s per frame) for the first 2 min, 3 frames (1 min per frame) for the following 3 min, and 17 frames (5 min per frame) for the remainder of the scan). Within each frame, the raw data were binned into 3D sinograms with a span of three and ring difference of 47. The scatter and attenuation corrections were applied. The tomographic images (128 × 128 × 95) with voxel sizes of 0.095 × 0.095 × 0.08 cm3 were obtained by reconstructing the sinograms using a two-dimensional ordered-subsets expectation-maximization algorithm with 16 subsets and 4 iterations. Attenuation correction was accomplished by generating an attenuation map from the CT images. The CT images were first co-registered with PET, then segmented and projected into sinogram space with a span of 47 and ring difference of 23. Three-dimensional volumes of interest were drawn over tumor and contralateral brain using ASIPro (Siemens) in order to generate time-activity curves (TACs).
In vivo Uptake, Blocking, and Displacement of [18F]VUIIS1018A in C6 Glioma-Bearing Rats
In vivo uptake (n = 7), blocking (n = 3), and displacement studies (n = 3) were carried out in glioma-bearing rats. Rats were administered 1.1 ± 0.3 mCi (40.9 ± 10.1 MBq, mean ± SD) of [18F]VUIIS1018A for PET imaging. For blocking studies, [19F]VUIIS1018A (10 mg/kg) was injected 5 min [18F]VUIIS1018A administration prior to [18F]VUIIS1018A administration and initiation of a 60-min dynamic PET scan. For displacement studies, [19F]VUIIS1018A (10 mg/kg) was injected 30 min following [18F]VUIIS1018A administration during a 60-min dynamic PET scan. Regions of interest (ROIs) were selected for tumor and brain using ASIPro (Siemens).
TLC Radiometabolite Analysis
Plasma metabolites of [18F]VUIIS1018A were evaluated by thin layer chromatography (TLC). Arterial blood (200 μl) was collected at 2, 12, 30, 60, and 90 min following administration of [18F]VUIIS1018A in rats. Following centrifugation, plasma was extracted with a mixture of acetonitrile/water (340 μl, 7/1 v/v). The mixture was again centrifuged and the supernatant spotted on silica/glass TLC plates (Waterman, GE healthcare, USA). TLC plates were scanned using an AR-2000 radio-TLC imaging scanner (Bioscan, France) according to published methods [25].
Image Analysis and Modeling
For PET imaging analysis, TACs were generated by manually drawing three-dimensional volumes of interest over contralateral brain and tumor using ASIPro (Siemens). The arterial input function (AIF) was generated from plasma sampling (15 μl) during the dynamic imaging and corrected for metabolism of the parent ligand. For the following analysis, a two-tissue, four-rate-constant kinetic model was explored using PMOD (PMOD technologies LLC, Zurich) (Fig. 4). In this model, Cp represents the concentration of [18F]VUIIS1018A in the plasma. Ct represents non-specific and free tracer in the tissue. Cm represents the specific binding of the tracer to TSPO in the outer-mitochondria membrane. The four parameters of K1, k2, k3, and k4 reflect the transport or binding rates of the tracer. In this specific study, K1 indicates the perfusion rate of the probe from plasma to tissue (ml/min/g), while k2 reflects the clearance rate for the tracer from tissue to plasma (1/min). k3 and k4 represent the specific binding rates and dissociation rates of the tracer (1/min), respectively.
Fig. 4.
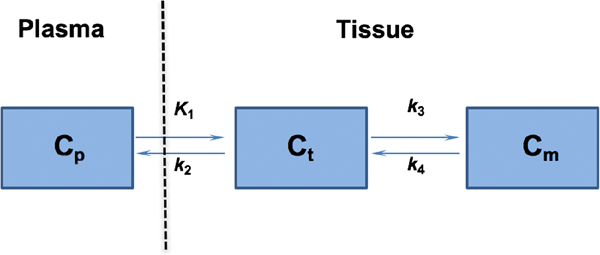
The 2-tissue, 4-kinetic-parameter model used for describing [18F]VUIIS1018A kinetics in reference tissues, including tumor and brain. In this model, Cp represents the concentration of [18F]VUIIS1018A in the plasma. Ct represents non-specific and free tracer in the tissue. Cm represents the specific binding of the tracer to TSPO in the outer-mitochondria membrane. The four parameters of K1, k2, k3, and k4 reflect the transport or binding rates of the tracer. In this specific study, K1 indicates the perfusion rate of the probe from plasma to tissue, while k2 reflects the clearance rate for the tracer from tissue to plasma. k3 and k4 represent the specific binding rates and dissociation rates of the tracer, respectively.
Calculation of the total distribution volume (VT) was performed based on the estimated kinetic parameters of K1, k2, k3, and k4 for different tissues. Normally, VT is regarded as the sum of specific (VS) and non-specific distribution (VND) (Eqs. 1, 2, and 3).
| (1) |
| (2) |
| (3) |
The binding potential (BPND) can be characterized as:
| (4) |
For receptor binding:
| (5) |
where Bmax is the concentration of the receptor in the tissue, while Kd is the equilibrium dissociation constant, which represents the concentration of radioligand occupying half of the maximum receptor population.
Calculation of the BPND parameter map was done using the Simplified Reference Tissue Model 2 (SRTM2) method [26] in the PMOD software package for the normal 60-min dynamic PET scan with [18F]VUIIS1018A and for the 60-min dynamic PET scan in the blocking study with [19F]VUIIS1018A administered prior to [18F]VUIIS1018A. The normal cerebrum is selected as the TSPO-devoid tissue, and the tumor was selected as the TSPO-rich tissue for this analysis.
Results
SARs and Binding Affinity
The TSPO affinities of a series of pyrazolopyrimidine probes, including DPA-714, VUIIS1008, VUIIS1018A, and VUIIS1018B were analyzed by performing competitive binding assays against the isoquinoline carboxamide [3H]PK 11195, a well-known TSPO ligand, in C6 glioma cell lysates (Fig. 1). Strikingly, the new probe VUIIS1018A exhibits an exceptional TSPO binding affinity (IC50 = 16.2 pM), which is almost 700-fold higher than DPA-714 (IC50 = 10.9 nM) and 20-fold higher than VUIIS1008 (IC50 = 0.3 nM). More interestingly, VUIIS1018B, the regioisomer of VUIIS1018A, featured an IC50 value of 0.1 μM, which is 6000-fold lower than VUIIS1018A. The significant difference in binding affinity indicated that modification of the 5-position of the pyrazolopyrimidine ring with a bulky n-butyl group can decrease the binding affinity dramatically, while the same modification on the 7-position leads to elevated binding affinity as compared to the parent compounds DPA-714 and VUIIS1008.
[18F]VUIIS1018A Accumulation in Glioma-Bearing Rats
Accumulation of [18F]VUIIS1018A was evaluated in C6 glioma-bearing rats. Uptake (n = 7) of [18F]VUIIS1018A was evaluated using 60-min dynamic PET scans (Fig. 2). Similar to what was observed in earlier studies [23], the C6 glioma can be detected with [18F]VUIIS1018A PET imaging (Fig. 2b, c) and the anatomic location of the PET signal corresponded to the tumor location as seen with T2-weighted MRI (Fig. 2a). [18F]VUIIS1018A exhibited robust accumulation in C6 gliomas with negligible accumulation observed in normal brain (Fig. 2c, d). This result is consistent with the elevated levels of TSPO in this model compared with surrounding, non-tumor brain as demonstrated through TSPO immunohistochemistry (IHC) [17–19, 23, 25, 27] and autoradiography [28, 29] in previous studies. As shown in the time-activity curves (TACs), rapid delivery of the tracer to both tumor and normal brain can be observed right after the tracer injection (Fig. 2e). [18F]VUIIS1018A cleared very rapidly and did not accumulate to an appreciable extent in normal brain. In contrast, [18F]VUIIS1018A rapidly accumulated in tumor tissue and increased modestly over time. Ultimately, a tumor-to-background ratio of 3:1 was achieved at the end of the 60-min scan (Fig. 2e).
Fig. 2.
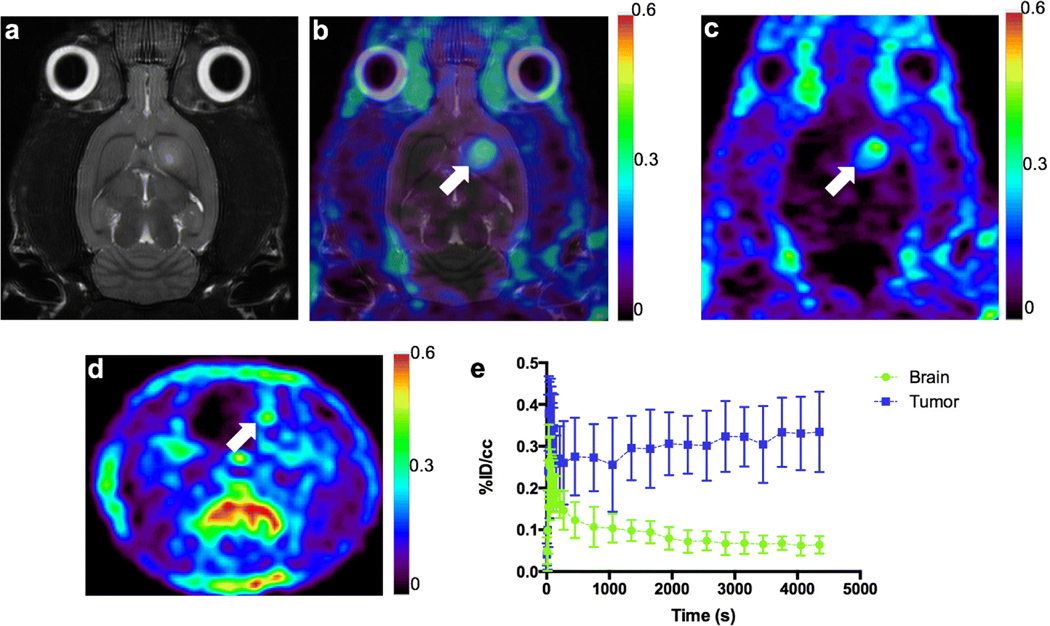
a T2-weighted MR image (coronal) of a C6 glioma-bearing rat. b Co-registration of [18F]VUIIS1018A PET image with the T2-weighted MR image for the same C6 glioma-bearing rat. c Coronal PET image of the same C6 glioma-bearing rat obtained from dynamic scan with [18F]VUIIS1018A. d Transverse PET image of the same C6 glioma-bearing rat obtained from dynamic scan with [18F]VUIIS1018A. e Time activity curves (TACs) for the tumor (blue) and contralateral brain (green) in the 60-min dynamic scan (n = 7). %ID/cm3 = percentage injected dose per cubic centimeter. In TACs, data = mean ± SD. Tumor is marked by the white arrow on the image.
Binding Specificity and Reversibility in Tumor
To further evaluate the specificity of tracer accumulation, a blocking study (n = 3) was conducted by administering cold [19F]VUIIS1018A 5 min prior to the injection of [18F]VUIIS1018A and initiation of the dynamic PET study (Fig. 3a, b). A blocking dose of 10 mg/kg [19F]VUIIS1018A led to significantly elevated circulating plasma radioactivity compared with the unblocked scenario (Fig. 3c). Furthermore, uptake of [18F]VUIIS1018A in tumor tissue was significantly reduced in the blocking study. An overall reduction in tumor PET signal of 48.3 ± 2.8 % (mean ± SD, n = 3) was achieved with the injection of cold compound (Fig. 3c). However, the blocking dose had an insignificant effect on [18F]VUIIS1018A accumulation in the normal brain.
Fig. 3.
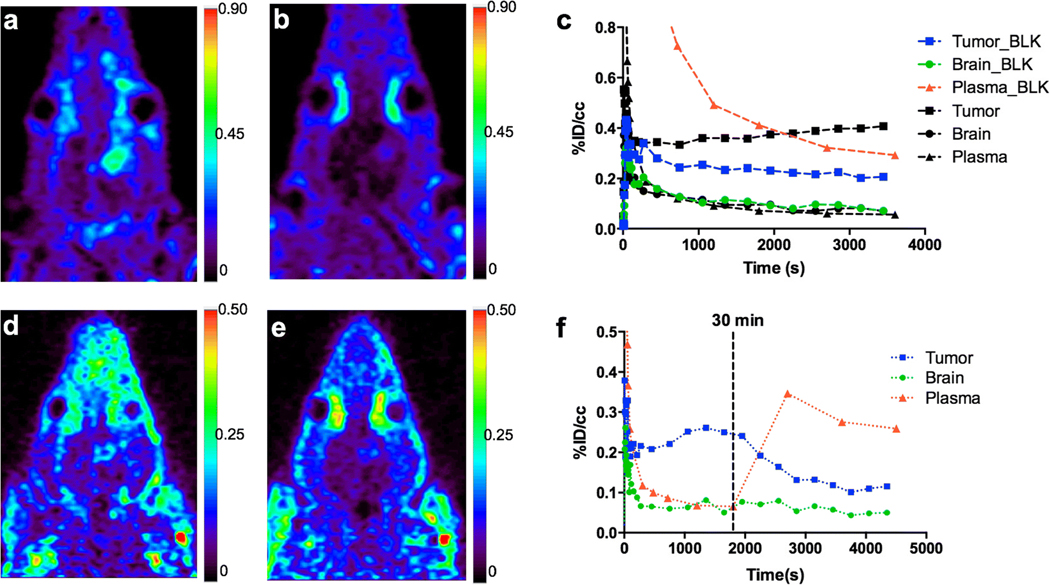
Blocking study with administration of [19F]VUIIS1018A (10 mg/kg) 5 min before administration of [18F]VUIIS1018A and a 60-min dynamic PET scan. a Coronal PET image of C6 glioma-bearing rat obtained from dynamic scan with [18F]VUIIS1018A (no blockade). b Uptake characteristics of [18F]VUIIS1018A following pre-blocking with [19F]VUIIS1018A (10 mg/kg) in the same rat (coronal view). c Representative TACs for tumor (black and blue squares), contralateral brain (black and green circles), and plasma (black and red triangles) in the normal (un-blocked) 60-min dynamic (black) and pre-blocked 60-min dynamic (blue, green, and red) PET scans in the same rat. Chase study with administration of [19F]VUIIS1018A (10 mg/kg) at 30 min during a 60-min dynamic PET scan. d Summation of the first 30 min of a 60-min dynamic PET acquisition following administration of [18F]VUIIS1018A, prior to injection of [19F]VUIIS1018A. e Summation of the last 30 min of the dynamic acquisition following administration of [18F]VUIIS1018A after injection of [19F]VUIIS1018A. f Representative TACs for tumor (blue), contralateral brain (green), and plasma (red) in the displacement assay. %ID/cm3 = percentage injected dose per cubic centimeter.
To evaluate the reversibility of [18F]VUIIS1018A accumulation in tumor and normal brain, a displacement study was conducted where cold [19F]VUIIS1018A was administered 30 min following [18F]VUIIS1018A administration during a dynamic PET study. Typical uptake characteristics of [18F]VUIIS1018A were observed before injection of [19F]VUIIS1018A in the first 30 min of the PET scan (0–30 min) (Fig. 3d). However, significant displacement of [18 F]VUIIS1018A in tumor tissue was observed in the final 30 min of the dynamic PET scan (30–60 min) (Fig. 3e). Accordingly, analysis of the time-activity curves (TACs) from this displacement study indicated that after the injection of [19F]VUIIS1018A, the radioactivity of the tumor was reduced approximately 62.0 ± 8.0 % (mean ± SD, n = 3), compared with the tumor uptake in the PET analysis without displacement (Fig. 3f).
Compartmental Modeling
Pharmacokinetic modeling was used to more closely examine the uptake and clearance characteristics of [18F]VUIIS1018A in tumor and normal brain. As with other TSPO PET ligands that we have evaluated in this setting [17, 18], the pharmacokinetics of [18F]VUIIS1018A more closely fit a 2-tissue, 4-rate constant kinetic model in both tumor and normal brain (Fig. 4). K1 and k2, terms reflecting delivery and efflux from a non-binding compartment, respectively, were similar for [18F]VUIIS1018A in normal brain and tumor tissue, both sharing a smaller K1 when compared with k2. Meanwhile, both tumor and brain have a higher specific binding rate (k3) when compared with the dissociation rate (k4) (Suppl. Table 1, ESM). The most notable difference in tracer pharmacokinetics between tumor and normal brain was observed in k3/k4, or the parameter describing binding potential BPND. The tumor features an 8-fold higher k3/k4 when compared with k3/k4 of healthy brain (Table 1). According to Eq. 5, these data are in agreement with the higher TSPO expression level in brain tumors relative to normal brain. Parametric images were constructed to compare the binding potential BPND of [18F]VUIIS1018A with and without blocking by [19F]VUIIS1018A (Fig. 5). As shown in Fig. 5b, d, blocking significantly lowers BPND in the glioma tumor.
Table 1.
Pharmacokinetic parameters obtained from analysis using the two-tissue, 4-rate constant kinetic model (Fig. 4) for [18F]DPA-714a,b (n = 11), [18F]VUIIS1008b (n = 5), and [18F]VUIIS1018A (n = 7). Results = mean ± SEM
| Probe | Region | K1/k2 | k3/k4 | VT |
|---|---|---|---|---|
| [18F]DPA-714a,b | Tumor | 6.867 ± 1.226 | 8.913 ± 1.155 | 70.033 ± 14.729 |
| Brain | 3.619 ± 0.551 | 4.024 ± 0.842 | 15.963 ± 3.566 | |
| [18F]VUIIS1008b | Tumor | 1.902 ± 0.816 | 12.634 ± 1.406 | 25.224 ± 10.035 |
| Brain | 0.855 ± 0.325 | 4.435 ± 1.387 | 4.210 ± 1.612 | |
| [18F]VUIIS1018A | Tumor | 0.532 ± 0.130 | 23.097 ± 4.757 | 11.036 ± 3.731 |
| Brain | 0.311 ± 0.036 | 2.913 ± 0.456 | 1.214 ± 0.190 |
Fig. 5.
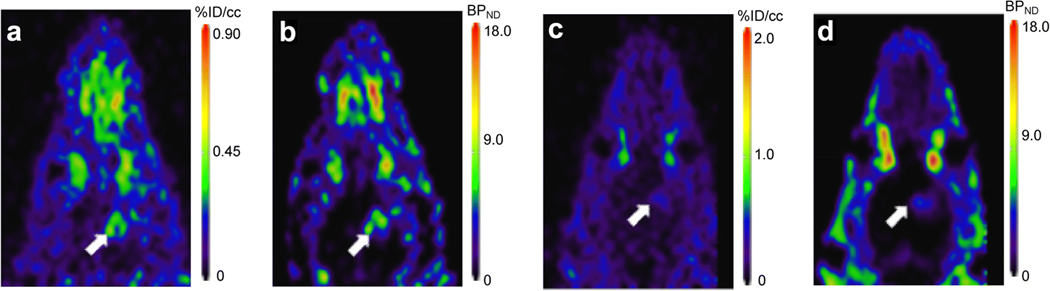
Sixty-minute dynamic PET scans of [18F]VUIIS1018A were obtained with and without blocking by [19F]VUIIS1018A. The blocking study was performed with administration of [19F]VUIIS1018A (10 mg/kg) 5 min before administration of [18F]VUIIS1018A and a 60-min dynamic PET scan. Binding potential BPND was calculated for both of these images. a Distribution of [18F]VUIIS1018A in the 60-min dynamic PET scan. b Calculated BPND parameter map for the 60-min dynamic PET scan. c Imaging for the 60-min dynamic PET scan in the blocking study. d Calculated BPND parameter map for the blocking study. Arrows indicate tumors.
Comparison of Pharmacokinetics of [18 F]VUIIS1018A, [18F]DPA-714, and [18F]VUIIS1008
The in vivo performance of [18F]VUIIS1018A compared favorably to two other TSPO PET agents we previously evaluated in this setting, [18F]DPA-714 and [18F]VUIIS1008. In both healthy brain and tumor tissue, [18F]VUIIS1018A exhibited a lower influx-to-efflux parameter (K1/k2) when compared with [18F]DPA-714 and [18F]VUIIS1008 (Table 1). In normal brain, [18F]VUIIS1018A features a lower k3/k4 when compared with [18F]DPA-714 (2.91 vs. 4.02) and [18 F]VUIIS1008 (2.91 vs. 4.43) (Table 1), suggesting a lower binding potential (BPND) and binding preference in healthy brain. However, the opposite trend was observed in tumor tissue, where the value for BPND (k3/k4) was higher for [18F]VUIIS1018A compared to [18F]DPA-714 (23.10 vs. 8.91) [17] and [18F]VUIIS1008 (23.10 vs. 12.63) [25] (Table 1), indicating a higher binding potential (BPND) and binding preference in tumor. Taken together, these results appear to be congruent with the relative affinities of these probes (Fig. 1) and suggest potentially higher tumor avidity and lower brain avidity of our new tracer.
Furthermore, although calculated VT for tumor and normal brain were higher for [18F]DPA-714 and [18F]VUIIS1008, VT ratios between tumor and normal brain were higher for [18F]VUIIS1018A when compared with [18 F]DPA-714 (9.1 vs. 4.4) and [18F]VUIIS1008 (9.1 vs. 6.0), which led to the improved signal-to-noise ratio and imaging contrast with this tracer.
Discussion
In our previous studies, we reported the utilization of several probes for quantitative PET imaging of TSPO expression in preclinical glioma studies. These probes include [18F]PBR06 [18], [18F]DPA-714 [17] and [18F]VUIIS1008 [23, 25]. In these studies, the TSPO probes displayed significant capability for discriminating the tumors from the surrounding normal brain. More importantly, using compartmental analysis, the TSPO levels could be quantitatively assayed in tumors [17, 18, 25]. However, potential drawbacks associated with these prior radiotracers included (1) tracer accumulation in the normal brain which reached a sufficient level to prevent the detection of gliomas with modest TSPO expression, such as lower grade disease and (2) significant metabolism in vivo, which required further AIF correction for the following quantitative analysis. While demonstrating the potential of TSPO PET to detect gliomas, these studies motivated us to develop novel TSPO PET tracers with improved profiles for cancer imaging.
Herein, we quantitatively evaluated the in vivo performance of a novel TSPO probe [18F]VUIIS1018A in preclinical glioma imaging studies. [18F]VUIIS1018A was synthesized by modifying the 7-position of the pyrazolopyrimidine ring with an n-butyl group instead of the 7-methyl group found on [18F]DPA-714 (Suppl. Scheme 1 and Fig. 1). This resulted in a TSPO-probe with approximately 700-fold greater TSPO affinity when compared with [18F]DPA-714 [17, 23]. Furthermore, as illustrated in this study, this optimization also resulted in a variety of intriguing and improved properties for cancer imaging. For example, [18F]VUIIS1018A exhibits significantly lower accumulation in healthy brain, as illustrated by the TACs, VT and brain binding potential BPND, when compared to previously reported TSPO probes. This could prove advantageous when attempting to discriminate lower grade gliomas, which have low TSPO expression, from the healthy brain. More practically compared to the parent tra cer s [18F]DP A-71 4 a nd [18F]VUIIS100 8, [18F]VUIIS1018A exhibited an improved tumor-to-background ratio, a higher specific-to-non-specific binding ratio and a higher tumor binding potential, which made the new tracer a better candidate for detecting tumors with modest TSPO expression profiles.
Conclusion
In this study, we developed a novel TSPO PET tracer [18 F]VUIIS1018A by replacing the 7-methyl group on the pyrazolopyrimidine ring of DPA-714 with an n-butyl group. The new tracer exhibited an exceptional binding affinity to TSPO with an IC50 value of 16.2 pM, which is a 700-fold improvement compared with DPA-714. Taking advantage of this tracer’s significantly lower accumulation in healthy brain and higher tumor binding potential, the new tracer can serve as a better candidate to image low grade tumors or tumors with modest TSPO expression. We envision that [18F]VUIIS1018A can be a very promising PET ligand for glioma diagnostics, and could possibly be used in other TSPO related diseases.
Supplementary Material
Acknowledgements.
The authors thank George H. Wilson and Dan Colvin for assistance with microPET and MR imaging studies.
Funding The authors acknowledge funding from the National Institutes of Health (K25 CA127349, P50 CA128323, S10 RR17858, U24 CA126588, 1R01 CA163806) and grants from the National Natural Science Foundation of China (81601536), The Kleberg Foundation, and The Lustgarten Foundation.
Footnotes
Compliance with Ethical Standards
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11307-018-1198-7) contains supplementary material, which is available to authorized users.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- 1.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ (2010) Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol 9:906–920 [DOI] [PubMed] [Google Scholar]
- 2.Dimitrakopoulou-Strauss A, Seiz M, Tuettenberg J, Schmieder K, Eisenhut M, Haberkorn U, Strauss LG (2011) Pharmacokinetic studies of 68Ga-labeled Bombesin (68?Ga-BZH3) and F-18FDG PET in patients with recurrent gliomas and comparison to grading: preliminary results. Clin Nucl Med 36:101–108 [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M (2006) Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27:402–409 [DOI] [PubMed] [Google Scholar]
- 4.Chauveau F, Boutin H, Van Camp N et al. (2008) Nuclear imaging of neuroinflammation: a comprehensive review of [11?C]PK11195 challengers. Eur J Nucl Med Mol Imaging 35:2304–2319 [DOI] [PubMed] [Google Scholar]
- 5.Doorduin J, de Vries EF, Dierckx RA, Klein HC (2008) PET imaging of the peripheral benzodiazepine receptor: monitoring disease progression and therapy response in neurodegenerative disorders. Curr Pharm Des 14:3297–3315 [DOI] [PubMed] [Google Scholar]
- 6.Venneti S, Lopresti BJ, Wiley CA (2006) The peripheral benzodiazepine receptor (translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol 80:308–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeda J, Higuchi M, Inaji M, Ji B, Haneda E, Okauchi T, Zhang MR, Suzuki K, Suhara T (2007) Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res 1157:100–111 [DOI] [PubMed] [Google Scholar]
- 8.Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M (2010) Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov 9:971–988 [DOI] [PubMed] [Google Scholar]
- 9.Han Z, Slack RS, Li W, Papadopoulos V (2003) Expression of peripheral benzodiazepine receptor (PBR) in human tumors: relationship to breast, colorectal, and prostate tumor progression. J Recept Signal Transduct Res 23:225–238 [DOI] [PubMed] [Google Scholar]
- 10.Hardwick M, Fertikh D, Culty M, Li H, Vidic B, Papadopoulos V (1999) Peripheral-type benzodiazepine receptor (PBR) in human breast cancer: correlation of breast cancer cell aggressive phenotype with PBR expression, nuclear localization, and PBR-mediated cell proliferation and nuclear transport of cholesterol. Cancer Res 59:831–842 [PubMed] [Google Scholar]
- 11.Hardwick M, Rone J, Han Z, Haddad B, Papadopoulos V (2001) Peripheral-type benzodiazepine receptor levels correlate with the ability of human breast cancer MDA-MB-231 cell line to grow in SCID mice. Int J Cancer 94:322–327 [DOI] [PubMed] [Google Scholar]
- 12.Corsi L, Geminiani E, Baraldi M (2008) Peripheral benzodiazepine receptor (PBR) new insight in cell proliferation and cell differentiation review. Curr Clin Pharmacol 3:38–45 [DOI] [PubMed] [Google Scholar]
- 13.Deane NG, Manning HC, Foutch AC, Washington MK, Aronow BA, Bornhop DJ, Coffey RJ (2007) Targeted imaging of colonic tumors in smad3−/− mice discriminates cancer and inflammation. Mol Cancer Res 5:341–349 [DOI] [PubMed] [Google Scholar]
- 14.Wyatt SK, Manning HC, Bai M, Bailey SN, Gallant P, Ma G, McIntosh L, Bornhop DJ (2010) Molecular imaging of the translocator protein (TSPO) in a pre-clinical model of breast cancer. Mol Imaging Biol 12:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasdev N, Green DE, Vines DC, McLarty K, McCormick PN, Moran MD, Houle S, Wilson AA, Reilly RM (2013) Positron-emission tomography imaging of the TSPO with [18?F]FEPPA in a preclinical breast cancer model. Cancer Biother Radiopharm 28:254–259 [DOI] [PubMed] [Google Scholar]
- 16.Zheng J, Boisgard R, Siquier-Pernet K, Decaudin D, Dollé F, Tavitian B (2011) Differential expression of the 18 kDa translocator protein (TSPO) by neoplastic and inflammatory cells in mouse tumors of breast cancer. Mol Pharm 8:823–832 [DOI] [PubMed] [Google Scholar]
- 17.Tang D, Hight MR, McKinley ET et al. (2012) Quantitative preclinical imaging of TSPO expression in glioma using N,N-diethyl-2-(2-(4-(2-18?F-fluoroethoxy)phenyl)-5,7-dimethylpyrazolo[1,5-a]pyrimidin-3-yl)acetamide. J Nucl Med 53:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck JR, McKinley ET, Hight MR et al. (2011) Quantitative, preclinical PET of translocator protein expression in glioma using 18F-N-fluoroacetyl-N-(2,5-dimethoxybenzyl)-2-phenoxyaniline. J Nucl Med 52:107–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung YY, Nickels ML, Tang D, Buck JR, Manning HC (2014) Facile synthesis of SSR180575 and discovery of 7-chloro-N,N,5-trimethyl-4-oxo-3(6-[18?F]fluoropyridin-2-yl)-3,5-dihydro-4H-pyri dazino[4,5-b]in-dole-1-acetamide, a potent pyridazinoindole ligand for PET imaging of TSPO in cancer. Bioorg Med Chem Lett 24:4466–4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck JR, McKinley ET, Fu A et al. (2015) Preclinical TSPO ligand PET to visualize human glioma xenotransplants: a preliminary study. PLoS One 10:e0141659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkeler A, Boisgard R, Awde AR, Dubois A, Thézé B, Zheng J, Ciobanu L, Dollé F, Viel T, Jacobs AH, Tavitian B (2012) The translocator protein ligand [18?F]DPA-714 images glioma and activated microglia in vivo. Eur J Nucl Med Mol Imaging 39:811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venneti S, Lopresti BJ, Wiley CA (2013) Molecular imaging of microglia/macrophages in the brain. Glia 61:10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang D, McKinley ET, Hight MR et al. (2013) Synthesis and structure-activity relationships of 5,6,7-substituted pyrazolopyrimidines: discovery of a novel TSPO PET ligand for cancer imaging. J Med Chem 56:3429–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang D, Buck JR, Hight MR, Manning HC (2010) Microwave-assisted organic synthesis of a high-affinity pyrazolo-pyrimidinyl TSPO ligand. Tetrahedron Lett 51:4595–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang D, Nickels ML, Tantawy MN, Buck JR, Manning HC (2014) Preclinical imaging evaluation of novel TSPO-PET ligand 2-(5,7-Diethyl-2-(4-(2-[18?F]fluoroethoxy)phenyl)pyrazolo[1,5-a]pyrimidin-3-yl)-N,N-diethylacetamide ([18?F]VUIIS1008) in glioma. Mol Imaging Biol 16:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Carson RE (2002) Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22:1440–1452 [DOI] [PubMed] [Google Scholar]
- 27.Tang D, Li J, Buck JR, Tantawy MN, Xia Y, Harp JM, Nickels ML, Meiler J, Manning HC (2017) Evaluation of TSPO PET ligands [18?F]VUIIS1009A and [18?F]VUIIS1009B: tracers for cancer imaging. Mol Imaging Biol 19:578–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starosta-Rubinstein S, Ciliax BJ, Penney JB, McKeever P, Young AB (1987) Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc Natl Acad Sci U S A 84:891–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black KL, Ikezaki K, Toga AW (1989) Imaging of brain tumors using peripheral benzodiazepine receptor ligands. J Neurosurg 71:113–118 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


