Abstract
Background
Frey's syndrome is characterised by transient flushing and sometimes facial sweating in the area of the auriculotemporal nerve. It most commonly occurs after parotidectomy, but other causes may include submandibular gland surgery, mandibular condylar fracture, obstetric (forceps) trauma, sympathectomy and metabolic disease. Although the pathophysiology of Frey's syndrome remains controversial, the generally accepted hypothesis is that it occurs as the result of injury to the auriculotemporal nerve.
There is currently no clear evidence to establish the efficacy and safety of the different methods used for the treatment of Frey's syndrome, therefore the prevention of this symptom during surgery is important. The main method used for prevention is the interposition of a graft between the skin flap and the parotid bed during surgery. Biomaterials, allograft or autograft can be used for this purpose.
Objectives
To evaluate the effects and safety of biomaterial, allograft or autograft interposition for the prevention of Frey's syndrome in patients undergoing parotidectomy, and to identify its effect on prevention and delayed occurrence.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Cochrane Register of Controlled Trials (CENTRAL; 2019, Issue 2); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 5 February 2019.
Selection criteria
We included randomised controlled trials (RCTs) in patients with parotid disease (including tumours, inflammation, trauma etc.) undergoing parotidectomy with a minimal follow‐up period of six months. We planned to include trials with interventions including biomaterial, allograft or autograft interposition alone or in combination with other surgical techniques. We included trials that compared any graft interposition and no graft interposition, or different graft interpositions.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcome measures were incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) and other complications (postoperative infection, subjective painful or restricted cervical movement, scar spread, rejection of the graft, complications related to the donor site such as accessory nerve injury and haematoma). Our secondary outcome measures were incidence rate of Frey's syndrome assessed by participants (by questionnaire) and sweating area assessed by Minor's starch‐iodine test. We used GRADE to assess the certainty of the evidence for each outcome.
Main results
We included three RCTs (124 participants), two of which we assessed as at high risk of bias and one at unclear risk of bias. All studies were hospital‐based and recruited participants undergoing superficial parotidectomy. Most participants were diagnosed with benign lesions of the parotid gland. Participants were followed up for more than six months. The studies evaluated the two comparisons shown below:
Sternocleidomastoid muscle flap versus no flap
Two studies assessed this comparison. Both assessed the effects of the sternocleidomastoid muscle flap procedure on the incidence rate of Frey's syndrome assessed clinically but neither showed a significant difference between groups (risk ratio (RR) 0.08, 95% confidence interval (CI) 0.00 to 1.23; 24 participants and RR 1.23, 95% CI 0.88 to 1.73; 36 participants; very low‐certainty evidence). We did not pool the data due to the high heterogeneity (I² = 87%).
One study found that the sternocleidomastoid muscle flap may result in little or no difference in other complications including haematoma (RR 2.18, 95% CI 0.09 to 50.16; 36 participants; low‐certainty evidence), subjective painful or restricted cervical movement (RR 0.54, 95% CI 0.14 to 2.05; 36 participants; low‐certainty evidence) and scar spread in the cervical region (RR 0.71, 95% CI 0.05 to 10.54; 36 participants; low‐certainty evidence). Both studies reported the incidence rate of Frey's syndrome assessed by participants, with one reporting no events in either group and the other finding no evidence of a difference (RR 0.63, 95% CI 0.32 to 1.26; 36 participants; low‐certainty evidence).
Acellular dermal matrix versus no graft
Only one study assessed this comparison. Use of an acellular dermal matrix graft may result in little or no difference to the incidence rate of Frey's syndrome (assessed clinically) in comparison with the no graft group, but the evidence is very uncertain (RR 0.08, 95% CI 0.00 to 1.25; 30 participants; very low‐certainty evidence).
Acellular dermal matrix may slightly increase the wound infection rate compared with control (RR 17.00, 95% CI 1.02 to 282.67; 64 participants; low‐certainty evidence). Acellular dermal matrix may result in little or no difference to the incidence of seromas or sialoceles (RR 2.33, 95% CI 0.66 to 8.23; 64 participants; low‐certainty evidence). Acellular dermal matrix may result in little or no difference to the incidence rate of Frey's syndrome (assessed by participants) in comparison with the no graft group (RR 0.33, 95% CI 0.04 to 3.04; 64 participants; low‐certainty evidence).
Authors' conclusions
The evidence for the effectiveness of graft interposition in preventing Frey's syndrome is of low or very low certainty. The use of acellular dermal matrix may be associated with an increase in the wound infection rate, and little or no difference in the incidence of seromas or sialoceles. Further studies are needed to draw reliable conclusions.
Plain language summary
Grafts for preventing Frey's syndrome after surgery to the parotid (salivary) glands
Review question
Does the use of a graft during surgery to the parotid glands prevent Frey's syndrome?
Background
The clinical symptoms of Frey's syndrome include sweating and flushing of the cheek when eating and chewing. It results from abnormal regrowth of damaged autonomic nerve fibres of the parotid glands, for example during surgery for parotid gland tumours. It is unclear whether placing a graft between the skin flap and the parotid bed during surgery can prevent this syndrome. Various types of grafts can be used, including biomaterial or skin, muscle or other tissue from the patient. These grafts may possibly hinder the abnormal connections of the nerves controlling the sweat glands and parotid glands when the cut nerves are re‐linking after surgery.
Study characteristics
We included three studies with 124 participants in this review, but the quality of these studies was not ideal. All of the participants in the studies had tumours of the parotid glands and were undergoing surgery to part of the glands. The studies assessed two types of grafts, tissue obtained from the sternocleidomastoid muscle and a biomaterial (a collagen framework without cells).
Key results
Two studies compared a tissue graft obtained from the sternocleidomastoid muscle to no graft. It is not known whether this type of tissue graft can prevent Frey's syndrome because the available evidence is very uncertain.
One study compared a biomaterial graft to no graft. This type of graft may result in little or no difference to the incidence rate of Frey's syndrome, but the evidence is very uncertain. It may make the patient's wound slightly more likely to become infected.
Certainty of the evidence
The evidence in this review is mostly of low or very low certainty, because of the small number of studies on this question and the risk of bias in these studies. The findings must therefore be treated with caution and further studies are needed to draw reliable conclusions.
The evidence in this review is up to date to 5 February 2019.
Summary of findings
Summary of findings for the main comparison. Sternocleidomastoid muscle flap versus no flap.
| Sternocleidomastoid muscle flap for patients undergoing parotidectomy | |||||||
|
Patient or population: patients undergoing parotidectomy
Settings: hospitals
Intervention: sternocleidomastoid muscle flap Comparison: no flap | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Control | Sternocleidomastoid muscle flap | ||||||
| Incidence rate of Frey's syndrome assessed clinically Follow‐up: 9 to 72 months | Asal 2005 reported that sternocleidomastoid muscle flap could reduce the incidence rate of Frey's syndrome assessed clinically (RR 0.08, 95% CI 0.00 to 1.23; 24 participants), while Kerawala 2002 showed no significant difference between groups (RR 1.23, 95% CI 0.88 to 1.73; 36 participants). | 60 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | We did not pool the data due to the high heterogeneity (I2 = 87%). | |||
|
Other complications As measured by: clinical methods and subjective perception Follow‐up: 12 to 72 months |
Haematoma | 0 out of 15 | 1 out of 21 | RR 2.18 (0.09 to 50.16) | 36 (1 study) | ⊕⊕⊝⊝ low3 | There were no events in the control group. |
| Subjective painful or restricted cervical movement | 267 per 1000 | 144 per 1000 (37 to 547) | RR 0.54 (0.14 to 2.05) | 36 (1 study) | ⊕⊕⊝⊝ low3 | — | |
| Scar spread in cervical region | 67 per 1000 | 47 per 1000 (3 to 703) | RR 0.71 (0.05 to 10.54) | 36 (1 study) | ⊕⊕⊝⊝ low3 | — | |
| Incidence rate of Frey's syndrome assessed by participants Follow‐up: 12 to 72 months | 600 per 1000 | 378 per 1000 (192 to 756) | RR 0.63 (0.32 to 1.26) | 36 (1 study) | ⊕⊕⊝⊝ low3 | Asal 2005 reported that no events occurred in either group. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||||
1Downgraded two levels due to serious risk of bias (performance bias). 2Downgraded two levels due to serious inconsistency. 3Downgraded two levels due to serious imprecision.
Summary of findings 2. Acellular dermal matrix versus control.
| Acellular dermal matrix for patients undergoing parotidectomy | |||||||
|
Patient or population: patients undergoing parotidectomy
Settings: hospitals
Intervention: acellular dermal matrix Comparison: no graft | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Control | Acellular dermal matrix | ||||||
| Incidence rate of Frey's syndrome assessed clinically Follow‐up: > 6 months | 6 out of 15 | 0 out of 15 | RR 0.08 (0.00 to 1.25) | 30 (1 studies) | ⊕⊝⊝⊝ very low1 | There were no events in the acellular dermal matrix group. | |
|
Other complications As measured by clinical methods Follow‐up: > 6 months |
Wound infection | 0 out of 32 | 8 out of 32 | RR 17.00 (1.02 to 282.67) | 64 (1 study) | ⊕⊕⊝⊝ low1 | There were no events in the control group. |
| Seroma/sialoceles | 94 per 1000 | 218 per 1000 (62 to 772) | RR 2.33 (0.66 to 8.23) | 64 (1 study) | ⊕⊕⊝⊝ low1 | — | |
|
Incidence rate of Frey's syndrome assessed by participants Follow‐up: > 6 months |
94 per 1000 |
31 per 1000 (0 to 288) |
RR 0.33 (0.04 to 3.04) | 64 (1 study) | ⊕⊕⊝⊝ low1 | — | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | |||||||
1Downgraded two levels due to serious imprecision (single study of 64 participants). 2Downgraded two levels due to serious risk of bias.
Background
Description of the condition
Frey's syndrome, or gustatory sweating, is named after Łucja Frey, who first described it as 'auriculotemporal syndrome' in 1923 (Frey 1923). It is characterised by transient flushing and sometimes facial sweating in the area of the auriculotemporal nerve (Blanc 2016). Frey's syndrome most commonly occurs after parotidectomy, but other causes may include submandibular gland surgery, mandibular condylar fracture, obstetric (forceps) trauma, sympathectomy and metabolic disease (de Bree 2007).
Although the physiopathology of Frey's syndrome remains controversial, the generally accepted hypothesis is that it occurs as the result of injury to the auriculotemporal nerve. This is a branch of the mandibular nerve of the trigeminal nerve complex. Aberrant neuronal regeneration of the auriculotemporal nerve results in parasympathetic cholinergic innervation of cutaneous sympathetic receptors (Prattico 2006). Due to this abnormal communication, the skin glands and vessels are stimulated when eating and masticating (Singh 2011). In response to such nerve impulses, acetylcholine is released from the presynaptic nerve endings to postsynaptic cholinergic receptors, which results in sweating and flushing. As sweating is controlled by sympathetic cholinergic pathways, treatments have traditionally involved anticholinergics (Watkins 1973). However, recent anatomical research has indicated that the great auricular nerve may be the principal nerve underlying Frey's syndrome (Toure 2015). This hypothesis could explain why manifestations of the syndrome can be located outside the area of the auriculotemporal nerve. A case series of temporoparietal Frey's syndrome has also recently been reported, which proposes novel challenges to the traditional hypothesis of its aetiology (Wood 2019).
Frey's syndrome may occur three to six months, or even as long as 14 years, after surgery to the parotid gland (Bakke 2006; Wenzel 2004). It has been reported to develop after an average of 12 months following parotidectomy (Rustemeyer 2008). The incidence of Frey's syndrome varies among studies (O'Neill 2008). A survey has reported patients' self‐reported incidence of Frey's syndrome to be 23%, while a positive Minor's starch‐iodine test was observed in 62% of cases following parotidectomy (Neumann 2011). A recent study identified tumour size as a predictor of the incidence of Frey's syndrome, whereas disease pathology, type of resection and previous treatments such as radiotherapy or parotidectomy did not seem to be associated with its development (Lee 2017).
In a questionnaire evaluation of patients who had undergone any type of parotidectomy for benign salivary diseases, Frey's syndrome was identified as the most serious self‐perceived sequela and was of the greatest concern, resulting in discomfort that worsened with time ‐ even more than five years postoperatively (Baek 2009).
Description of the intervention
There is currently no clear evidence to establish the efficacy and safety of the methods used for the treatment of Frey's syndrome (Li 2015). Botulinum toxin may have a potential role in its treatment (Xie 2015b), but no high‐certainty evidence has yet supported its effects. The prevention of this symptom during surgery is therefore important. The main method for prevention is the interposition of a graft between the skin flap and the parotid bed during surgery. Biomaterials (such as acellular dermal matrix from a different species), allograft (transplantation of tissues or related biomaterials from a genetically non‐identical donor of the same species, including acellular dermal matrix from the same species) or autograft (transplantation of tissue from the same patient) can be used. These procedures have become quite common in clinical practice.
The main allograft or biomaterial used is acellular dermal matrix, which is derived from skin or other tissues from human beings or other animals. The cells and antibodies are removed using special techniques, leaving a collagen framework. It is reported that acellular dermal matrix could reduce the risk of Frey's syndrome by 85% (by objective assessment) and 68% (by subjective assessment) (Zeng 2012). Animal pericardium membrane can also be used (Gennaro 2013). Some studies have shown that acellular dermal matrix might be associated with a higher incidence of local wound complications such as salivary fistula compared with no graft (Wang 2013; Zeng 2012). However, the limited number of studies means that this is inconclusive.
Frequently used autografts include the sternocleidomastoid muscle flap procedure (Sanabria 2012), the temporal fascial flap (Sharma 2014), free fat grafting (Chan 2014), the superficial musculoaponeurotic system flap (Barberá 2014; Dulguerov 2016), and the platysma muscle flap (Wang 2013). These autografts may have some effect in the prevention of Frey's syndrome (Li 2013; Sanabria 2012). Serious adverse events from these autografts have been rarely reported.
Specific surgical procedures may also be used in combination with graft interposition to prevent Frey's syndrome. Preservation of the parotid masseteric fascia during flap elevation may reduce the risk (Yang 2013). It has been suggested that extracapsular dissection reduces the risk of Frey's syndrome when compared with superficial parotidectomy or partial superficial parotidectomy (Foresta 2014; Lin 2019; Xie 2015a). Recently, a novel technique, extracapsular dissection via a sternocleidomastoid muscle‐parotid space approach, has demonstrated a lower incidence of subjective Frey's syndrome (Yang 2019). As different surgical approaches significantly impact the incidence of Frey's syndrome, particular attention needs to be paid to them when evaluating the effects of graft interposition.
How the intervention might work
As discussed above, a potential strategy for the prevention of Frey's syndrome is the placement of a physical barrier between the cheek skin flap and the parotid bed during surgery. In a healthy individual, the auriculotemporal branch of the trigeminal nerve passes through the parotid gland and carries sympathetic fibres to the sweat glands of the scalp and parasympathetic fibres to the parotid gland. According to the currently accepted hypothesis (Prattico 2006), during parotidectomy postganglionic parasympathetic fibres are exposed. The parotidomasseteric fascia, which could prevent them from aberrantly innervating the sweat glands, is always destroyed during parotidectomy. Therefore these postganglionic parasympathetic fibres can switch courses and aberrantly innervate the cutaneous sweat glands instead of the parotid salivary gland. Thus, when eating, with the parasympathetic fibres activated, the switched fibres accelerate the secretion of the sweat glands instead of the parotid gland and cause vasodilatation, resulting in Frey's syndrome (Frey 1923; Glaister 1958; Laage‐Hellman 1958). Frey's syndrome can therefore potentially be prevented by inserting a barrier between the parotid bed and the skin flap (Bonanno 1992), because the barrier may prevent the auriculotemporal nerve from aberrantly innervating the sweat glands of the skin (de Bree 2007). The preventive effect of graft interposition may also be affected by whether the surgical area can be totally covered and the thickness of the graft (Durgut 2013); the effect of different grafts therefore varies. Another important issue focused on by investigators is the possibility that graft interposition may only delay the aberrant innervation of the parasympathetic fibres instead of preventing it (Zhao 2005). As none of the current methods of graft interposition have avoided the incidence of Frey's syndrome in all patients undergoing parotidectomy, the credibility of the mechanism hypothesis cannot currently be confirmed.
Why it is important to do this review
Graft interposition is now widely used in clinical settings, but some problems remain. There is a lack of high‐certainty evidence to support the use of graft interposition; in addition, its safety has not been fully confirmed. It is also unclear whether graft interposition can prevent Frey's syndrome or just delay its occurrence. We therefore conducted a Cochrane systematic review to attempt to clarify these issues.
Objectives
To evaluate the effects and safety of biomaterial, allograft or autograft interposition for the prevention of Frey's syndrome in patients undergoing parotidectomy, and to identify its effect on prevention and delayed occurrence.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a minimal follow‐up period of six months. We excluded quasi‐RCTs as well as cluster‐RCTs, cross‐over studies and split‐mouth studies (within‐patient controlled).
As Wu 2009 has shown, studies carried out in China often use the terminology of randomisation more broadly than in other countries. We therefore contacted trial authors to request a description of the randomisation method used if it was unclearly described and we only included those trials that had clearly been properly randomised.
Types of participants
Patients with parotid disease (including tumours, inflammation, trauma etc.) undergoing parotidectomy. We set no limitation on the types of parotidectomy or the age of patients.
Types of interventions
Participants in the intervention groups received either biomaterial, allograft or autograft interposition alone or in combination with other surgical techniques that might help to prevent Frey's syndrome (such as preservation of the parotidomasseteric fascia during flap elevation).
Participants in the control groups received either a different type of allograft or autograft interposition, or no graft interposition, or no graft interposition plus the same combination of other surgical techniques that might help to prevent Frey's syndrome.
The main possible comparison pairs were:
graft interposition versus no graft interposition;
autograft versus allograft.
Other possible comparisons were:
between different autografts with different thickness or materials;
between different autografts.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies. The expected time points of outcome assessment were three months, six months, one year, three years and over five years.
Primary outcomes
Incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test, the iodine‐sublimated paper histogram method or blotting paper technique). Incidence rate was defined as the proportion of patients with symptoms/signs as measured by the various tests (attention was also paid to the follow‐up data to check whether graft interposition prevents the symptom or delays its occurrence).
Other complications (wound infection, salivary fistula, seromas, sialoceles, facial nerve palsy, complications related to the donor site such as accessory nerve injury and haematoma).
Secondary outcomes
Incidence rate of Frey's syndrome assessed by participants (by questionnaire).
Sweating area assessed by Minor's starch‐iodine test.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 5 February 2019.
Electronic searches
The Information Specialist searched the following databases with the search strategy presented in Appendix 1:
the Cochrane ENT Trials Register (searched via the Cochrane Register of Studies 5 February 2019);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2) (searched via CRS Web 5 February 2019);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 5 February 2019);
Ovid Embase (1974 to 5 February 2019);
Ovid CAB Abstracts (1910 to 5 February 2019);
EBSCO CINAHL (1982 to 5 February 2019);
LILACS, lilacs.bvsalud.org (searched 5 February 2019);
KoreaMed (searched via Google Scholar 5 February 2019);
Web of Knowledge, Web of Science (1945 to 5 February 2019);
ClinicalTrials.gov (searched via the Cochrane Register of Studies and https://clinicaltrials.gov/ 5 February 2019);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) www.who.int/ictrp (searched 5 February 2019).
In searches prior to December 2017, we also searched PubMed (1946 to 22 August 2016) as a top‐up to Ovid MEDLINE, and PakMediNet and ISRCTN (searched 22 August 2016).
The author team searched:
CNKI (in Chinese) (searched 12 February 2019) (Appendix 2);
CBM (in Chinese) (searched 12 February 2019) (Appendix 3);
VIP (in Chinese) (searched 12 February 2019) (Appendix 4).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
For selection of studies, data extraction and 'Risk of bias' assessment, two review authors were involved and worked in duplicate. We resolved any disagreements by discussion.
Selection of studies
Initially, two review author independently screened the titles and abstracts of the search records in duplicate. They recorded any potentially eligible studies. The same two review authors retrieved the full texts of these studies and carefully assessed them independently according to the inclusion criteria. Any disagreements were resolved by discussion. We illustrated the whole process of study selection in a PRISMA flow diagram (Figure 1). For studies with insufficient data, if we could not make definite decisions on inclusion or exclusion we sent emails or letters to the original authors for further information; meanwhile we recorded them as studies awaiting classification.
1.
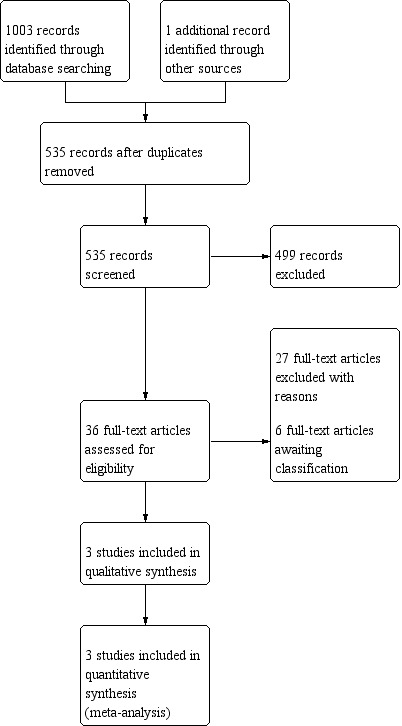
Process for sifting search results and selecting studies for inclusion.
Data extraction and management
We used the standard data extraction form recommended by Cochrane ENT. Two authors independently collected the following data in duplicate during the data extraction process, with any differences resolved by discussion:
Source: study ID, citation and contact details.
Eligibility: reasons for inclusion or exclusion.
Methods of the study: centres and their location, study duration, ethics, study registration, inclusion and exclusion criteria for participants, study design, sequence generation, allocation concealment, blinding and statistical methods.
Participants: setting and number, age and sex, type of parotid disease.
Interventions: number of patients in intervention groups, intervention details, control treatment and other active treatment including details of parotidectomy, and dose and usage of drugs if administered.
Outcomes: definition of outcomes and units of measurement, time points of measurement, sample size calculation, number of participants allocated to each group, number lost follow‐up and reasons, detailed summary data for each group.
Miscellaneous: funding, key conclusions of each report, correspondence required and miscellaneous comments from the review authors.
We requested any missing information from the original authors if possible through emails and letters.
Assessment of risk of bias in included studies
We undertook assessment of the risk of bias of the included trials independently, with the following taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding of the participants and personnel;
blinding of the outcome assessors;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane 'Risk of bias' tool in RevMan 5.3 (RevMan 2014), which involved describing each of these domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias. We resolved any disagreements by discussion.
Measures of treatment effect
The measures of treatment effect differed according to the data type and outcome variables. We treated incidence rate and adverse events as dichotomous data. We planned to express all dichotomous data as a risk ratio (RR) with 95% confidence interval (CI). We planned to treat sweating area as continuous data using the mean difference (MD) with 95% CI.
Unit of analysis issues
We considered the patient to be the unit of analysis. We did not include any cluster‐RCTs, cross‐over studies or split‐mouth (within‐patient controlled) studies.
Studies with multiple treatment groups
As each meta‐analysis would address only a single pair‐wise comparison, for any trials with multiple treatment groups we planned to combine some groups to create a single pair‐wise comparison if possible or, if not, we would have selected the most related pair of interventions.
Dealing with missing data
We tried to obtain any missing information from the original authors through emails, letters or both. If there was no reply, we planned to adopt the methods in the Cochrane Handbook for Systematic Reviews of Interventions for dealing with important missing data (Handbook 2011). If these methods failed, we would have described the outcomes narratively.
Assessment of heterogeneity
Clinical heterogeneity might be due to different participant types (participants with different kinds of parotid disease, etc.), or different interventions (different surgical procedures and types of graft) and comparisons. Detailed methods for the assessment of clinical heterogeneity are presented in Subgroup analysis and investigation of heterogeneity.
Any statistical heterogeneity would appear during the meta‐analysis. To assess statistical heterogeneity we planned to use the I² statistic to determine the range as follows:
0% to 40% slight heterogeneity;
30% to 60% moderate heterogeneity;
50% to 90% substantial heterogeneity;
75% to 100% considerable heterogeneity.
If there was considerable heterogeneity for an outcome, we did not carry out a meta‐analysis.
Assessment of reporting biases
If there had been more than 10 studies included in one single meta‐analysis, we would have assessed reporting bias for each outcome by drawing funnel plots. Asymmetric funnel plots could indicate reporting bias. We would then have conducted statistical analysis. We planned to test the asymmetry of the funnel plot using the methods introduced by Begg 1994 (using STATA 11.0) at the level of α = 0.10 via STATA 11.0.
Data synthesis
We considered two types of analysis model: random‐effects and fixed‐effect. We adopted a random‐effects model if the I² statistic was > 50% and the P value was ≤ 0.10. If not, we chose a fixed‐effect model. The statistical methods used for meta‐analysis were the Mantel‐Haenszel (M‐H) method for dichotomous data and the inverse variance (IV) method for continuous data. Statistical significance for the hypothesis test was set at P value < 0.05 (two‐tailed z tests).
If it had been necessary we would have carried out network meta‐analysis via R software to compare the effect of different grafts indirectly.
Subgroup analysis and investigation of heterogeneity
We considered the following items as contributing to slight clinical heterogeneity and we would have used meta‐regression to detect their influence on the outcome via STATA 11.0 if the included studies in one meta‐analysis had exceeded 10:
methods of parotidectomy (partial parotidectomy, superficial parotidectomy and total parotidectomy etc.);
conservation of parotidomasseteric fascia;
thickness of graft used;
radiotherapy.
We planned to conduct subgroup analysis based on any significant clinical heterogeneity detected. We also planned to carry out subgroup analysis according to the different grafts used. Such methods were mainly planned to reduce the clinical heterogeneity in each outcome.
Sensitivity analysis
We planned to carry out sensitivity analysis in order to test the stability of each outcome. We planned two sensitivity analyses:
including high‐quality studies only; and
intention‐to‐treat (ITT) analysis ('worst‐case scenario' analysis versus 'best‐case scenario' analysis).
We would have reported the results of the sensitivity analyses and analysed the stability of the outcome.
GRADE and Summary of findings' table
Two independent authors used the GRADE approach to rate the overall certainty of evidence (Atkins 2004; Guyatt 2008; Handbook 2011). The certainty of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: high, moderate, low and very low. A rating of high certainty of evidence implies that we were confident in our estimate of effect and that further research was very unlikely to change our confidence in the estimate of effect. A rating of very low certainty implies that any estimate of effect obtained was very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision; and
publication bias.
We included a 'Summary of findings' table for each comparison, constructed according to the recommendations in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). We included the following outcomes in the 'Summary of findings' tables: incidence rate of Frey's syndrome assessed clinically, other complications and incidence rate of Frey's syndrome assessed by participants.
Results
Description of studies
Results of the search
Through our electronic searches and handsearches, we identified 1004 references. There were 535 records after we removed duplicates. After scanning the titles and abstracts, we considered 36 reports to be potentially eligible and obtained the full texts for further review. We included three studies (reported in three articles) in this systematic review. We excluded 27 studies (see Excluded studies). The remaining six studies are awaiting classification (see below). A study selection flow diagram is shown in Figure 1.
Included studies
This review includes three randomised controlled trials (RCTs), which were published between 2001 and 2005 (Asal 2005; Govindaraj 2001; Kerawala 2002). The details of the included studies are shown in the Characteristics of included studies table.
Design
All included studies used a two‐arm, parallel‐group design.
The duration of follow‐up of participants varied among studies: this was 9 to 48 months in Asal 2005, 12 to 72 months in Kerawala 2002, and at least 6 months in Govindaraj 2001.
It is unclear whether any of the three studies received industrial or commercial funding, or involved any conflict of interest.
Sample sizes
This review included a total of 124 randomised participants. Sample sizes ranged from 24 to 64. No participants were lost during follow‐up but only 30 participants (46.9%) were assessed for the primary outcomes in Govindaraj 2001.
Setting
The setting for all included studies was a hospital. Two studies were conducted in a single centre (Asal 2005; Kerawala 2002), while one study was performed in two centres (Govindaraj 2001). The studies were conducted in Turkey (Asal 2005), the United States (Govindaraj 2001) and the United Kingdom (Kerawala 2002).
Participants
The mean age of participants was 51 years. The proportion of males was 41.1%.
The inclusion criteria for participants in Asal 2005 specified that participants should have benign lesions of the parotid gland, while Govindaraj 2001 and Kerawala 2002 did not specify the type of parotid lesions. All of the included studies specified that the type of surgery was superficial parotidectomy.
Interventions
We classified the identified interventions into two groups:
sternocleidomastoid muscle flap; and
acellular dermal matrix.
Comparisons
Kerawala 2002 performed a standard cervicofacial incision with the skin flap raised by sham dissection in a plane immediately above the parotid fascia for the participants in the control group. In the other studies, the control group participants received parotidectomy without any graft interposition or incision.
Outcomes
Primary outcomes
Incidence rate of Frey's syndrome assessed clinically
The incidence rate of Frey's syndrome assessed clinically was reported in all of the included studies (Asal 2005; Govindaraj 2001; Kerawala 2002).
Other complications
Other complications were reported in two studies (Govindaraj 2001; Kerawala 2002). Sialoceles and wound infection were reported in Govindaraj 2001. Haematoma, subjective painful or restricted cervical movement and scar spread in the cervical region were reported in Kerawala 2002.
Secondary outcomes
Incidence rate of Frey's syndrome assessed by participants
Two studies reported the incidence rate of Frey's syndrome assessed by participants (Asal 2005; Kerawala 2002).
Sweating area assessed by Minor's starch‐iodine test
None of the studies reported this outcome.
Excluded studies
We excluded 27 studies for the reasons listed below. The reasons for exclusion are also shown in the Characteristics of excluded studies table.
Not a randomised controlled trial: Chan 2014; Chen 2004; Chen 2007; Chen 2008; Ding 2010; Dong 2008; Gennaro 2013; Gou 2018; Grosheva 2016; Jiang 2010; Jin 2008; Jin 2013; Li 2006; Liao 2008; Liao 2012; Luo 2012; Mao 2018; Ren 2010; Sinha 2003; Wang 2016; Wille‐Bischofberger 2007; Xie 2011; Zeng 2010 (Mao 2018 was a quasi‐randomised study).
No graft interposition applied: Durgut 2013; Jiang 2018.
Less than six months follow‐up: Elgammal 2017; Yu 2007.
Awaiting assessment studies
There are six studies awaiting further assessment (Ding 2018; Hao 2008; Sun 2008; Xue 2010; Yang 2018; Yu 2011). As mentioned above, studies carried out in China often use the terminology of randomisation more broadly than is usual in other countries. All the studies awaiting assessment were conducted in China. As we were not able to make contact with the authors, we could not judge whether they are true RCTs. The details of these studies are therefore presented in the Characteristics of studies awaiting classification table.
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph (our judgements as percentages across studies) and Figure 3 for a 'Risk of bias' summary (each individual judgement).
2.
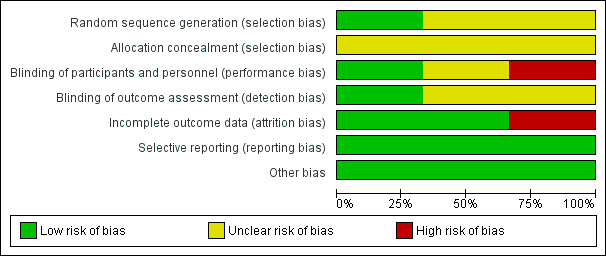
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
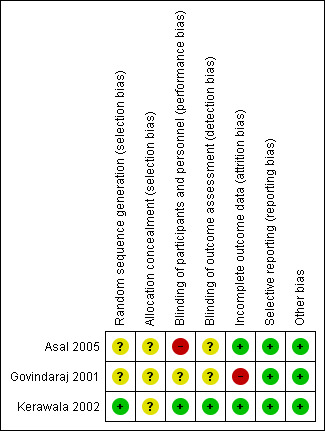
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Kerawala 2002 used a random number table to achieve random sequence generation, which we considered to have a low risk of bias. Asal 2005 and Govindaraj 2001 stated that allocation was random but provided no further details and we therefore assessed them at unclear risk of bias for this domain.
Allocation concealment
In all of the included studies allocation concealment was not described in sufficient detail to determine the risk of bias and we rated them all at unclear risk of bias.
Blinding
In all of the included studies, blinding of personnel was not possible but it could not have influenced the outcome.
Blinding of participants was described in Kerawala 2002, which we assessed at low risk of performance bias. Blinding of participants was impossible in Asal 2005, which we evaluated at high risk of performance bias. There was insufficient information provided in Govindaraj 2001 and we judged the risk of performance bias to be unclear.
Blinding of outcome assessment was possible in all of the included studies and was described in Kerawala 2002, which we assessed at low risk of detection bias. There was insufficient information provided in Asal 2005 and Govindaraj 2001, thus we judged the risk of detection bias to be unclear.
Incomplete outcome data
In Govindaraj 2001, only 30 participants (46.9%) were assessed by the modified Minor's starch‐iodine test, thus we judged the risk of attrition bias to be high. In Asal 2005 and Kerawala 2002 no participants were lost to follow‐up, thus there was a low risk of attrition bias.
Selective reporting
All of the included studies reported the outcomes specified in their methods section in full and we assessed them at low risk of reporting bias. We did not find any study protocols in clinical trials registers.
Other potential sources of bias
We assessed all the included studies at low risk of other possible sources of bias.
Effects of interventions
Sternocleidomastoid muscle flap versus no flap
Two included studies evaluated the sternocleidomastoid muscle flap procedure versus no flap (Asal 2005; Kerawala 2002).
Incidence rate of Frey's syndrome assessed clinically
Both studies reported the incidence rate of Frey's syndrome assessed clinically (Asal 2005; Kerawala 2002). We did not pool the data due to the high heterogeneity between the two studies (I² = 87%). Neither study showed a significant difference between groups (risk ratio (RR) 0.08, 95% confidence interval (CI) 0.00 to 1.23; 24 participants (Asal 2005) and RR 1.23, 95% confidence interval (CI) 0.88 to 1.73; 36 participants) (Kerawala 2002) (Analysis 1.1) (very low‐certainty evidence).
1.1. Analysis.
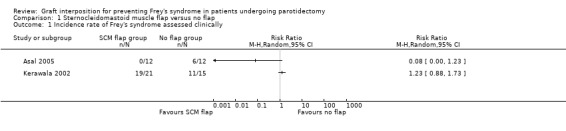
Comparison 1 Sternocleidomastoid muscle flap versus no flap, Outcome 1 Incidence rate of Frey's syndrome assessed clinically.
Other complications
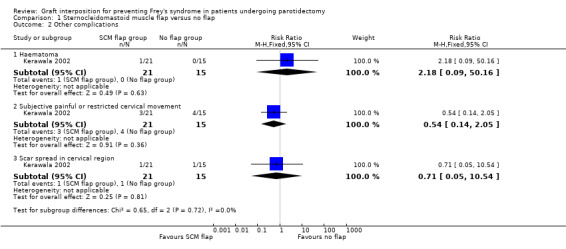
Only one small study reported other complications (Kerawala 2002). This found that the sternocleidomastoid muscle flap may result in little or no difference in other complications including haematoma (RR 2.18, 95% CI 0.09 to 50.16; 36 participants; low‐certainty evidence), subjective painful or restricted cervical movement (RR 0.54, 95% CI 0.14 to 2.05; 36 participants; low‐certainty evidence) and scar spread in the cervical region (RR 0.71, 95% CI 0.05 to 10.54; 36 participants; low‐certainty evidence) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Sternocleidomastoid muscle flap versus no flap, Outcome 2 Other complications.
Incidence rate of Frey's syndrome assessed by participants
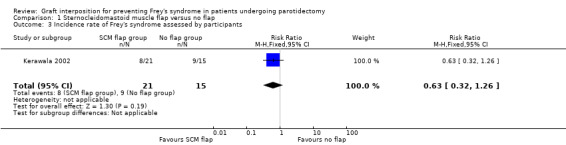
Two studies reported the incidence rate of Frey's syndrome assessed by participants (Asal 2005; Kerawala 2002). Asal 2005 reported no events in either group, which could not be pooled. No evidence of a difference was found between groups who received sternocleidomastoid muscle flap or no flap (RR 0.63, 95% CI 0.32 to 1.26; 36 participants) (low‐certainty evidence) (Analysis 1.3).
1.3. Analysis.
Comparison 1 Sternocleidomastoid muscle flap versus no flap, Outcome 3 Incidence rate of Frey's syndrome assessed by participants.
Sweating area assessed by Minor's starch‐iodine test
Neither of the included studies for this comparison assessed this outcome.
Acellular dermal matrix versus no graft
Only one included study evaluated an acellular dermal matrix versus control (Govindaraj 2001).
Incidence rate of Frey's syndrome assessed clinically
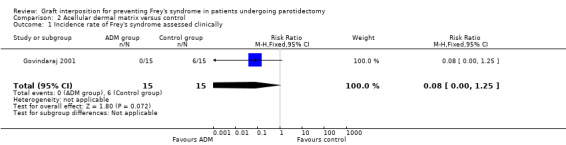
Acellular dermal matrix may result in little or no difference in the incidence rate of Frey's syndrome (assessed clinically) in comparison with the no graft group, but the evidence is very uncertain (RR 0.08, 95% CI 0.00 to 1.25; 30 participants) (very low‐certainty evidence) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Acellular dermal matrix versus control, Outcome 1 Incidence rate of Frey's syndrome assessed clinically.
Other complications
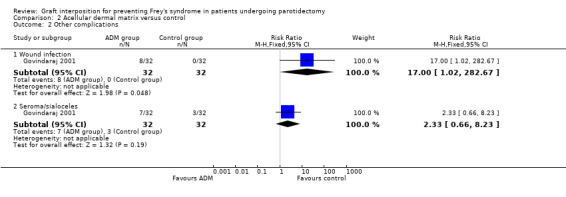
Low‐certainty evidence indicates that acellular dermal matrix may slightly increase the incidence rate of wound infection compared with control (RR 17.00, 95% CI 1.02 to 282.67; 64 participants) (Analysis 2.2). Acellular dermal matrix may result in little or no difference to the incidence of seromas or sialoceles (RR 2.33, 95% CI 0.66 to 8.23; 64 participants) (Analysis 2.2) (low‐certainty evidence).
2.2. Analysis.
Comparison 2 Acellular dermal matrix versus control, Outcome 2 Other complications.
Incidence rate of Frey's syndrome assessed by participants
Acellular dermal matrix may result in little or no difference in the incidence rate of Frey's syndrome (assessed clinically) in comparison with the no graft group (RR 0.33, 95% CI 0.04 to 3.04; 64 participants) (low‐certainty evidence) (Analysis 2.3).
2.3. Analysis.
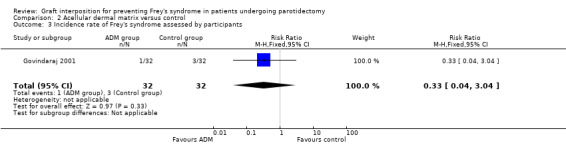
Comparison 2 Acellular dermal matrix versus control, Outcome 3 Incidence rate of Frey's syndrome assessed by participants.
Sweating area assessed by Minor's starch‐iodine test
The included study for this comparison did not assess this outcome.
Discussion
Summary of main results
The aim of this review was to evaluate the effects and safety of biomaterial, allograft or autograft interposition for the prevention of Frey's syndrome in patients undergoing parotidectomy, and to identify its effect on prevention and delayed occurrence. We found three eligible randomised controlled trials (RCTs) for the review with a total of 124 participants. The studies focused on the comparison between graft interposition and no graft interposition. The graft interposition included the sternocleidomastoid muscle flap procedure and the use of an acellular dermal matrix graft.
We could not draw any conclusions about the effects of the sternocleidomastoid muscle flap on the incidence rate of Frey's syndrome assessed clinically compared with no flap (very low‐certainty evidence).
It was not possible to establish the effects of sternocleidomastoid muscle flap on other complications compared with no flap (low‐certainty evidence).
It remains impossible to establish the effects of acellular dermal matrix on the incidence rate of Frey's syndrome assessed clinically in comparison with no graft (very low‐certainty evidence).
Acellular dermal matrix may increase the wound infection rate compared with control (low‐certainty evidence).
Acellular dermal matrix may result in little or no difference to the incidence of seromas or sialoceles (low‐certainty evidence).
Due to the limited number of included studies and the low or very low certainty of the evidence, we should treat these results with caution.
Overall completeness and applicability of evidence
Studies included in the review recruited participants who needed parotidectomy. Asal 2005 specified that participants had benign lesions of the parotid gland, while the other two studies did not. Neoplasms of the parotid gland are commonly benign lesions, thus most of the recruited participants were diagnosed with benign lesions of the parotid gland. Hence, we should apply these results to malignant lesions of the parotid gland with caution, particularly if adjuvant radiotherapy is applied.
All of the included studies adopted superficial parotidectomy, thus it remains unclear whether these results are applicable to other types of parotidectomy. For modified superficial parotidectomy (Chang 2017; Li 2014) or segmental parotidectomy (Eski 2018), which have emerged recently, the results of this review might be not applicable.
The follow‐up duration varied from individual to individual in all of the included studies. In the past it was thought that Frey's syndrome mostly occurred within six months postoperatively. However, Rustemeyer et al reported that the Frey's syndrome developed after an average of 12 months following parotidectomy in their institution (Rustemeyer 2008). Moreover, none of the included studies reported time‐to‐event data to show when Frey's syndrome occurred in an individual. Therefore, it remains unclear whether these interventions might prevent or only delay the incidence of Frey's syndrome.
Despite the issues mentioned above, our review did not set any limitation on the demographic characteristics of participants and the included studies were conducted in diverse locations. We consider that the results have acceptable external validity.
Overall, the evidence provided by this review might be applicable to most patients undergoing parotidectomy; however, we should be cautious when extending the conclusions to situations not described in our review. We should adequately consider the limitations and biases of the studies included in the review when interpreting the results and applying the evidence.
Quality of the evidence
We assessed one included study as at high risk of bias due to the lack of blinding of participants (Asal 2005), and one at high risk of bias because not all participants were clinically assessed for incidence rate of Frey's syndrome (Govindaraj 2001). Due to the high risk of bias in the studies, we downgraded the certainty of evidence by two levels for incidence rate of Frey's syndrome assessed clinically in both of the comparisons. In Govindaraj 2001, all participants were evaluated for all outcomes except for incidence rate of Frey's syndrome assessed clinically; we therefore did not downgrade the certainty of evidence for attrition bias for those outcomes.
In most analyses, there was only one study that measured the outcome or the subgroup. For sternocleidomastoid muscle flap versus no flap, the analysis of incidence rate of Frey's syndrome assessed clinically consisted of two studies with significant heterogeneity (Analysis 1.1; I² = 87%); thus, we downgraded the evidence by two levels due to serious inconsistency.
The number of events was mostly insufficient, reflected in the wide confidence intervals. Therefore, we downgraded the evidence for imprecision for all of the outcomes.
We did not downgrade any of the evidence for indirectness.
Due to the limited number of included studies we were not able to generate a funnel plot to examine publication bias across studies, thus we did not downgrade any of the evidence for this reason.
We did not upgrade any of the evidence due to a large effect, plausible confounding or dose response gradient.
In summary, for sternocleidomastoid muscle flap versus no flap, we downgraded the certainty of the evidence to low for incidence rate of haematoma, subjective painful or restricted cervical movement, scar spread in the cervical region and incidence rate of Frey's syndrome assessed by participants, and to very low for incidence rate of Frey's syndrome assessed clinically (Table 1). For acellular dermal matrix versus control, we downgraded the certainty of the evidence to low for incidence rate of wound infection and seromas/sialoceles and Frey's syndrome assessed by participants, and to very low for incidence rate of Frey's syndrome assessed clinically (Table 2).
Potential biases in the review process
In order to reduce the risk of publication bias in our review, we conducted an exhaustive electronic search and a manual search of the reference lists of the included studies. In addition, we did not include all of the identified studies that had been conducted in China, because these studies often use the terminology of randomisation more broadly than is usual in other countries (Wu 2009). Instead, as planned in our protocol (Li 2016), we contacted trial authors to request a description of the randomisation method if it was unclearly described and we only included those trials that had clearly been properly randomised. According to the information acquired from our contact with study authors, we found only one trial that was a true RCT in China; most were excluded due to severe flaws in their randomisation methods. However, we failed to acquire information from the contact authors of six possible RCTs in China and these studies are currently categorised as awaiting assessment (Characteristics of studies awaiting classification).
We set restrictions on our inclusion criteria that were as limited as possible, which resulted in the potential clinical heterogeneity among studies. We included all types of biomaterial, allograft or autograft interposition. After screening the search results, we found that diverse methods and materials had been investigated for their effect on the prevention of Frey's syndrome. Although only three studies were ultimately included in this review, we had to divide them into two comparisons due to the significant clinical heterogeneity. The statistical efficacy was thus limited in each analysis, which was associated with imprecision. In addition, we restricted neither the diagnosis or reason for parotidectomy, nor the type of parotidectomy. The clinical heterogeneity decreases the credibility of data pooling.
In the data synthesis, we did not assess the effect of diverse durations of follow‐up. Although all of the included studies followed the participants for an adequate duration, this duration varied from half a year to six years. It remains unclear to us whether Frey's syndrome would alleviate or disappear over time. However, due to the limited information reported in the included studies, we could not analyse further the possible negative impact of diverse follow‐up on our results. Further studies with a fixed follow‐up duration may be needed. In addition, it has been reported that Frey's syndrome develops after an average of 12 months following parotidectomy (Rustemeyer 2008), thus the duration of follow‐up in the included studies might be inadequate. We could not confirm whether Frey's syndrome was prevented or just delayed because of the short‐term nature of the evidence.
Overall, although we have done our best to conduct an exhaustive search and used unbiased selection, correct analyses and suitable interpretation we cannot ignore the impact of limited study quantity and quality. We must therefore treat the results of this review with caution.
Agreements and disagreements with other studies or reviews
Other reviews of the effects of graft interposition on preventing Frey's syndrome in patients undergoing parotidectomy have been published (Li 2013; Liu 2013; Sanabria 2012; Zeng 2012). These reviews differ from ours in the following ways:
Types of studies: Sanabria 2012 included both RCTs and non‐RCTs while the others only included RCTs. However, these three other reviews did not contact the original authors of RCTs conducted in China, thus they included many badly performed RCTs, which we have confirmed by author contact.
Types of interventions: Liu 2013 and Sanabria 2012 focused on the sternocleidomastoid muscle flap, Zeng 2012 focused on acellular dermal matrix and Li 2013 included all kinds of graft.
Types of outcomes: Li 2013, Liu 2013 and Sanabria 2012 did not address any other complications except Frey's syndrome. All of the reviews studied both objective and subjective assessment of Frey's syndrome.
Results and quality of the evidence: Sanabria 2012 concluded that the currently reported evidence is inconclusive for the use of the sternocleidomastoid muscle flap, which is consistent with the results of our review. Liu 2013 and Zeng 2012 advocated the use of the sternocleidomastoid muscle flap and acellular dermal matrix respectively, without any evaluation of evidence quality. Li 2013 reported a high quality of evidence to support the effects of the sternocleidomastoid muscle flap and acellular dermal matrix. In our review, however, although the results favoured the use of acellular dermal matrix, it was impossible to form a definite conclusion due to the low certainty of the evidence.
Overall, there are many discrepancies between the previously published reviews and our review. Only Sanabria 2012 had similar findings. The inclusion of low‐quality RCTs from China may be the main reason why there are differences between the other three reviews and our review.
Authors' conclusions
Implications for practice.
The evidence for the use of the sternocleidomastoid muscle flap procedure is inconclusive. Very low‐certainty evidence suggests that the use of acellular dermal matrix may result in little or no difference to the incidence of Frey's syndrome after parotidectomy but this is very uncertain. Low‐certainty evidence shows that it may slightly increase the wound infection rate. We found no high‐certainty evidence to determine which graft interposition may be the most effective for preventing Frey's syndrome. Further randomised controlled trials (RCTs) are needed to draw reliable conclusions.
Implications for research.
Considering the limited number of true RCTs in this field, more RCTs are needed that focus on the effects of graft interposition for preventing Frey's syndrome. Future studies could address the issues described below:
Types of studies: RCTs that are well designed and performed. Especially for RCTs conducted in China, authors should report the methodological design of their studies transparently. If randomisation is not strictly performed in the participant allocation, a non‐RCT design should be clearly noted in the final publication. As it is comparatively difficult to conduct an RCT in surgery, careful design and conduct are essential.
Types of participants: Almost all of the current studies have looked at benign lesions of the superficial lobes of the parotid glands. More patients with deep parotid glands could be included in future studies, to confirm whether the conclusions can be applied to parotidectomy to any lobes. In addition, the size and position of tumours should be recorded and balanced between groups.
Types of interventions and comparisons: Sternocleidomastoid muscle flap and acellular dermal matrix grafts were included in our review, however there are many other types of graft including the temporalis myofascial flap (Jin 2008) and parotidomasseteric fascia (Ding 2018). Future studies could either further confirm the effects of traditional grafts or explore the function of novel grafts. In addition, all of the current RCTs have compared parotidectomy with a graft and without a graft. None have compared parotidectomy with different grafts. Future studies could be designed as two‐arm or multi‐arm parallel RCTs comparing different types of graft. In addition, future studies could record the participants' history of chronic disorders, the size of tumours and grafts, tumour pathology and previous ENT surgery history, all of which might be associated with the development of Frey's syndrome.
Types of outcomes: The time to the incidence of Frey's syndrome could be recorded, so that an analysis of time‐to‐event data might give us more information about the preventive or delaying effects of grafts. In addition, complications should be recorded and reported more comprehensively.
Risk of bias: Investigators in future studies should find ways to reduce the risk of bias. Although blinding of personnel is impossible, blinding of participants and outcome assessment should be achievable. When the graft is obtained from the patient, sham dissection could help to blind the participants (Kerawala 2002).
Acknowledgements
We are grateful to Professor Dr Pavel Dulguerov for peer reviewing the draft review and to Joan Blakley for her consumer review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL (Cochrane Register of Studies) | MEDLINE (Ovid) | Embase (Ovid) | Web of Science (Web of Knowledge) |
| 1 MESH DESCRIPTOR Parotid Gland EXPLODE ALL AND CENTRAL:TARGET 2 MESH DESCRIPTOR Parotid Diseases EXPLODE ALL AND CENTRAL:TARGET 3 (parotid*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 4 #1 OR #2 OR #3 5 MESH DESCRIPTOR Facial Nerve Injuries EXPLODE ALL AND CENTRAL:TARGET 6 MESH DESCRIPTOR Taste EXPLODE ALL AND CENTRAL:TARGET 7 MESH DESCRIPTOR Taste Disorders EXPLODE ALL AND CENTRAL:TARGET 8 (gustatory):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 9 #5 OR #6 OR #7 OR #8 10 MESH DESCRIPTOR Sweat EXPLODE ALL AND CENTRAL:TARGET 11 MESH DESCRIPTOR Sweat Glands EXPLODE ALL AND CENTRAL:TARGET 12 MESH DESCRIPTOR Sweating EXPLODE ALL AND CENTRAL:TARGET 13 MESH DESCRIPTOR Hyperhidrosis EXPLODE ALL AND CENTRAL:TARGET 14 MESH DESCRIPTOR Erythema EXPLODE ALL AND CENTRAL:TARGET 15 MESH DESCRIPTOR Paresthesia EXPLODE ALL AND CENTRAL:TARGET 16 (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 17 #16 OR #14 OR #13 OR #12 OR #11 OR #10 OR #15 18 #9 AND #17 19 (((eat* OR masticat* OR meal*) NEAR (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*))):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 20 #18 OR #19 21 #20 AND #4 22 MESH DESCRIPTOR Sweating, Gustatory EXPLODE ALL AND CENTRAL:TARGET 23 ((frey OR frey's OR baillarger* OR dupuy OR dupuy's OR auriculotemporal) NEAR syndrome):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 24 (gustatory next sweat*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET 25 #21 OR #22 OR #23 OR #24 |
1 exp Parotid Gland/ or exp Parotid Diseases/ 2 "parotid*".ab,ti. 3 1 or 2 4 exp Facial Nerve Injuries/ 5 exp Taste Disorders/ or exp Taste/ 6 gustatory.ab,ti. 7 4 or 5 or 6 8 exp Sweat Glands/ or exp Sweat/ 9 exp Hyperhidrosis/ 10 exp Paresthesia/ 11 exp Erythema/ 12 exp Sweating/ 13 (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*).ab,ti. 14 8 or 9 or 10 or 11 or 12 or 13 15 7 and 14 16 ((eat* or masticat* or meal*) adj5 (sweat* or flush* or redness or erythema or salivat*)).ab,ti. 17 15 or 16 18 3 and 17 19 exp Sweating, Gustatory/ 20 ((frey* or baillarger* or dupuy or dupuy's or auriculotemporal) adj3 syndrome).ab,ti. 21 (gustatory adj3 sweat*).ab,ti. 22 18 or 19 or 20 or 21 |
1 exp parotid gland/ or exp parotid gland disease/ 2 "parotid*".ti,ab. 3 exp parotidectomy/ 4 1 or 2 or 3 5 exp facial nerve injury/ 6 exp taste/ or exp taste disorder/ 7 gustatory.ti,ab. 8 5 or 6 or 7 9 exp sweat/ or exp sweat gland/ 10 exp hyperhidrosis/ 11 exp paresthesia/ 12 exp sweating/ 13 exp erythema/ 14 (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*).ab,ti. 15 9 or 10 or 11 or 12 or 13 or 14 16 8 and 15 17 ((eat* or masticat* or meal*) adj5 (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*)).ab,ti. 18 16 or 17 19 4 and 18 20 exp sweat gland disease/ and 7 21 ((frey* or baillarger* or dupuy or dupuy's or auriculotemporal) adj3 syndrome).ab,ti. 22 (gustatory adj3 sweat*).ab,ti. 23 19 or 20 or 21 or 22 |
#1 TOPIC: (parotid*) #2 TOPIC: (gustatory) #3 TOPIC: (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*) #4 TOPIC: ((eat* OR masticat* OR meal*) NEAR/5 (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*)) #5 #3 AND #2 #6 #5 OR #4 #7 #6 AND #1 #8 TOPIC: ((frey OR frey's OR baillarger* OR dupuy OR dupuy's OR auriculotemporal) NEAR/3 syndrome) #9 TOPIC: (gustatory NEAR/3 sweat*) #10 #7 OR #8 OR #9 |
| CINAHL (EBSCO) | ICTRP | ClinicalTrials.gov | LILACS |
| S22 S18 OR S19 OR S20 OR S21 S21 TX gustatory N3 sweat* S20 TX (frey OR frey's OR baillarger* OR dupuy OR dupuy's OR auriculotemporal) N3 syndrome S19 (MH "Frey's Syndrome") S18 S4 AND S17 S17 S15 OR S16 S16 TX (eat* OR masticat* OR meal*) N5 (sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia*) S15 S8 AND S14 S14 S9 OR S10 OR S11 OR S12 OR S13 S13 TX sweat* or hyperhidrosis or erythema* or redness or flush* or Paresthesia* or Formication or Dysesthesia* S12 (MH "Paresthesia") S11 (MH "Erythema+") S10 (MH "Hyperhidrosis") S9 (MH "Sweat") OR (MH "Sweat Glands") OR (MH "Sweating") S8 S5 OR S6 OR S7 S7 TX gustatory S6 (MH "Taste") OR (MH "Taste Disorders") S5 (MH "Facial Nerve Diseases") S4 S1 OR S2 OR S3 S3 TX parotid* S2 (MH "Parotid Diseases+") S1 (MH "Parotid Gland") |
gustatory AND sweat* OR frey* AND syndrome |
Via ClinicalTrials.gov (gustatory AND sweating) OR (frey's AND syndrome) Via Cochrane Register of Studies 1 (gustatory AND sweating) OR (frey's AND syndrome) AND INSEGMENT 2 nct* AND INSEGMENT 3 #1 AND #2 4 (gustatory AND sweating) OR (frey's AND syndrome) AND STUDY:CRSTYPE AND INSEGMENT |
((TW:frey* OR TW:auriculotemporal OR TW: baillarger) AND TW:syndrome) OR (TW:gustatory AND (TW: sweat* OR TW:hyperhidrosis)) OR ((TW:Sudoración OR TW:Sudorese) AND TW:gustativa) |
Appendix 2. CNKI search strategy
1. 摘要= Frey or 摘要=味觉出汗
2. 摘要=腮腺
3. 摘要=随机
4. 1 and 2 and 3
Appendix 3. CBM search strategy
#1. 主题词:出汗, 味觉性/全部树/全部副主题词 ‐限定:‐
#2. 缺省[智能]:Frey ‐限定:‐
#3. 缺省[智能]:味觉出汗 ‐限定:‐
#4. #3 or #2 or #1 ‐限定:‐
#5. 主题词:腮腺/全部树/全部副主题词 ‐限定:‐
#6. 主题词:腮腺疾病/全部树/全部副主题词 ‐限定:‐
#7. #5 or #6 ‐限定:‐
#8. 中文摘要:随机 ‐限定:‐
#9. #8 and #7 and #4 ‐限定:‐
Appendix 4. VIP search strategy
1. 题名或关键词=frey 或者 题名或关键词=味觉出汗
2. 题名=腮腺
3. 任意字段=随机
4. 1 and 2 and 3
Data and analyses
Comparison 1. Sternocleidomastoid muscle flap versus no flap.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence rate of Frey's syndrome assessed clinically | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Other complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Haematoma | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.09, 50.16] |
| 2.2 Subjective painful or restricted cervical movement | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.14, 2.05] |
| 2.3 Scar spread in cervical region | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.05, 10.54] |
| 3 Incidence rate of Frey's syndrome assessed by participants | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.32, 1.26] |
Comparison 2. Acellular dermal matrix versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence rate of Frey's syndrome assessed clinically | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.25] |
| 2 Other complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Wound infection | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [1.02, 282.67] |
| 2.2 Seroma/sialoceles | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.66, 8.23] |
| 3 Incidence rate of Frey's syndrome assessed by participants | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asal 2005.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with at least 9 to 48 months of follow‐up | |
| Participants |
Location: Turkey Setting of recruitment and treatment: Gazi University Hospital Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland Exclusion criteria: not stated |
|
| Interventions |
Intervention group (flap group): superficial parotidectomy + sternocleidomastoid muscle flap Comparator group (no flap group): superficial parotidectomy Use of additional interventions: not stated |
|
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at least 9 months after surgery); complications Secondary outcomes: incidence rate of Frey's syndrome assessed by participants (assessed at least 9 months after surgery) | |
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | Participants lost to follow‐up: 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twelve patients were randomly selected to undergo superficial parotidectomy with sternocleidomastoid muscle flap reconstruction and 12 without reconstruction." Comment: the authors did not provide detailed information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors did not provide detailed information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not possible to blind the participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the authors did not provide detailed information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the authors reported all the variables |
| Other bias | Low risk | Comment: no other bias detected |
Govindaraj 2001.
| Methods | Two‐arm, multicentre, parallel‐group RCT, with at least 6 months of follow‐up | |
| Participants |
Location: USA Setting of recruitment and treatment: Departments of Otolaryngology, Mount Sinai Medical Center; and the Center for Cranial Base Surgery, Department of Otolaryngology, St. Luke's‐Roosevelt Hospital Center Sample size:
Participant (baseline) characteristics:
Inclusion criteria: patients needed superficial parotidectomy, 18 years of age or older, non‐pregnant or breast‐feeding, and not on immunosuppressive therapy Exclusion criteria: required postoperative radiation therapy |
|
| Interventions |
Intervention group (acellular dermal matrix group): superficial lobe parotidectomy + acellular dermal matrix Comparator group (control group): superficial lobe parotidectomy Use of additional interventions: not stated |
|
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Modified minor's starch‐iodine test) (assessed at least 6 months after surgery); complications Secondary outcomes: incidence rate of Frey's syndrome assessed by participants (assessed at least 6 months after surgery) | |
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | Participants lost to follow‐up: 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Sixty‐four patients were randomly assigned to two groups. Group I consisted of 32 patients who underwent a superficial lobe parotidectomy. Group II consisted of 32 patients who underwent a superficial lobe parotidectomy and underwent intraoperative placement of acellular dermis within the parotid bed, between the skin flap and the remaining parotid tissue." Comment: the authors did not provide detailed information on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: the authors did not provide detailed information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: the authors did not provide detailed information on blinding of participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: the authors did not provide detailed information on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Group I consisted of 32 patients who underwent a superficial lobe parotidectomy without place‐ ment of a barrier between the exposed parotid bed and overlying soft tissue, whereas group II consisted of 32 patients who under‐ went a superficial lobe parotidectomy and intraoperative place‐ ment of acellular dermis within the parotid bed, between the skin flap and the remaining parotid tissue. Objective testing with the Modified Minor's Starch‐Iodine Test of 30 patients (15 from each respective group) revealed the presence of gustatory sweating in 6." Comment: not all participants were assessed with the modified Minor's starch‐iodine test |
| Selective reporting (reporting bias) | Low risk | Comment: the authors reported all the variables |
| Other bias | Low risk | Comment: no other bias detected |
Kerawala 2002.
| Methods | Two‐arms, double‐blind, single‐centre, parallel‐group RCT, with 12 to 72 months of follow‐up | |
| Participants |
Location: UK Setting of recruitment and treatment: Sunderland Royal Hospital Sample size:
Participant (baseline) characteristics:
Inclusion criteria: patients needed superficial parotidectomy Exclusion criteria: not stated |
|
| Interventions |
Intervention group (flap group): delivery of the superficial portion of parotid gland + anteriorly based sternocleidomastoid flaps were raised and sutured to the resected margin of the superficial parotid fascia Comparator group (no flap group): delivery of the superficial portion of parotid gland + a standard cervicofacial incision with the skin flap raised by sham dissection in a plane immediately above the parotid fascia Use of additional interventions: not stated |
|
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at least 1 year after surgery); complications Secondary outcomes: incidence rate of Frey's syndrome assessed by participants (assessed at least 1 year after surgery) | |
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | Participants lost to follow‐up: 0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation was by a list of random numbers, with even numbers having flaps and odd numbers no flap." Comment: the randomisation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: authors did not provide any information on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Postoperatively patients and clinicians were unaware of the allocation." Comment: patients were unaware of the allocation, but for the personnel this was unclear. The blinding of personnel could not influence the outcome. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Postoperatively patients and clinicians were unaware of the allocation." Comment: the blinding of outcome assessors was adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: all related variables were reported |
| Other bias | Low risk | Comment: no other bias detected |
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chan 2014 | Not randomised |
| Chen 2004 | Not randomised. Information retrieved from author contact. |
| Chen 2007 | Not randomised. Information retrieved from author contact. |
| Chen 2008 | Not randomised |
| Ding 2010 | Not randomised. Information retrieved from author contact. |
| Dong 2008 | Not randomised |
| Durgut 2013 | Randomised, but no graft interposition applied |
| Elgammal 2017 | The follow‐up duration was unclear |
| Gennaro 2013 | Not randomised |
| Gou 2018 | Not randomised. Information retrieved from author contact. |
| Grosheva 2016 | Not randomised |
| Jiang 2010 | Not randomised. Information retrieved from author contact. |
| Jiang 2018 | Randomised, but no graft interposition applied |
| Jin 2008 | Not randomised. Information retrieved from author contact. |
| Jin 2013 | Not randomised |
| Li 2006 | Not randomised |
| Liao 2008 | Not randomised. Information retrieved from author contact. |
| Liao 2012 | Not randomised. Information retrieved from author contact. |
| Luo 2012 | Not randomised. Information retrieved from author contact. |
| Mao 2018 | Quasi‐randomised. Information retrieved from author contact. |
| Ren 2010 | Not randomised |
| Sinha 2003 | Not randomised. |
| Wang 2016 | Not randomised. Information retrieved from author contact. |
| Wille‐Bischofberger 2007 | Not randomised |
| Xie 2011 | Not randomised. Information retrieved from author contact. |
| Yu 2007 | The follow‐up duration was less than 6 months. |
| Zeng 2010 | Not randomised. Information retrieved from author contact. |
Characteristics of studies awaiting assessment [ordered by study ID]
Ding 2018.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with 6 to 12 months of follow‐up |
| Participants |
Location: China Setting of recruitment and treatment: Affiliated Hospital of Jiangsu University Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland Exclusion criteria: too large tumours or malignant tumours |
| Interventions |
Intervention group (flap group): superficial parotidectomy + parotid masseter fascia reconstruction Comparator group (no flap group): superficial parotidectomy Use of additional interventions: not stated |
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at least 6 months after surgery); complications Secondary outcomes: none |
| Notes |
|
Hao 2008.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with at least 3 months of follow‐up |
| Participants |
Location: China Setting of recruitment and treatment: Baoding Third People Hospital Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland Exclusion criteria: not stated |
| Interventions |
Intervention group (flap group): superficial parotidectomy + acellular dermal matrix Comparator group (no flap group): superficial parotidectomy Use of additional interventions: not stated |
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at least 3 months after surgery); complications Secondary outcomes: none |
| Notes | We could not make contact with the authors to confirm whether randomisation was strictly performed |
Sun 2008.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with 12 months of follow‐up |
| Participants |
Location: China Setting of recruitment and treatment: Shenzhen People's Hospital Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland; aged over 18 years Exclusion criteria: not stated |
| Interventions |
Intervention group (flap group): superficial parotidectomy + acellular dermal matrix Comparator group (no flap group): superficial parotidectomy Use of additional interventions: not stated |
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at 1 year after surgery); complications Secondary outcomes: incidence rate of Frey's syndrome assessed by participants (assessed at 1 year after surgery) |
| Notes | We could not make contact with the authors to confirm whether randomisation was strictly performed |
Xue 2010.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with 12 to 18 months of follow‐up |
| Participants |
Location: China Setting of recruitment and treatment: the Affiliated Hospital of Medical College Qingdao University Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland; aged over 18 years Exclusion criteria: not stated |
| Interventions |
Intervention group (flap group): superficial parotidectomy + acellular dermal matrix Comparator group (no flap group): superficial parotidectomy Use of additional interventions: not stated |
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at 1 year after surgery) Secondary outcomes: incidence rate of Frey's syndrome assessed by participants (assessed at 1 year after surgery) |
| Notes | We could not make contact with the authors to confirm whether randomisation was strictly performed |
Yang 2018.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with 3 to 15 months of follow‐up |
| Participants |
Location: China Setting of recruitment and treatment: Xiangtan Center Hospital Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland; aged over 18 years Exclusion criteria: not stated |
| Interventions |
Intervention group (flap group): superficial parotidectomy + sternocleidomastoid muscle flap Comparator group (no flap group): superficial parotidectomy Use of additional interventions: not stated |
| Outcomes | Primary outcome: complications Secondary outcomes: incidence rate of Frey's syndrome assessed by participants (assessed at least 3 months after surgery) |
| Notes | We could not make contact with the authors to confirm whether randomisation was strictly performed |
Yu 2011.
| Methods | Two‐arm, single‐centre, parallel‐group RCT, with 5 to 14 months of follow‐up |
| Participants |
Location: China Setting of recruitment and treatment: General Hospital of Tianjin Medical University Sample size:
Participant (baseline) characteristics:
Inclusion criteria: benign lesions of the parotid gland; aged over 18 years Exclusion criteria: not stated |
| Interventions |
Intervention group (flap group): parotidectomy + acellular dermal matrix Comparator group (no flap group): parotidectomy Use of additional interventions: not stated |
| Outcomes | Primary outcome: incidence rate of Frey's syndrome assessed clinically (Minor's starch‐iodine test) (assessed at least 5 months after surgery) Secondary outcomes: none |
| Notes | We could not make contact with the authors to confirm whether randomisation was strictly performed |
RCT: randomised controlled trial
Differences between protocol and review
In Assessment of risk of bias in included studies, the blinding domain was further divided into blinding of the participants and personnel, and blinding of the outcome assessors.
In Types of outcome measures, we changed "adverse events" into "other complications". Complications may be a more suitable expression to describe the undesired postoperative events that can occur in both the intervention and control groups.
In Types of outcome measures, we deleted "etc." and described the other complications as wound infection, salivary fistula, seromas, sialocele, facial nerve palsy, complications related to the donor site such as accessory nerve injury and haematoma.
Contributions of authors
Li Ye, Yubin Cao and Wenbin Yang contributed equally to this work.
Study selection: Li Ye, Yubin Cao, Wenbin Yang and Chunjie Li.
Data extraction: Li Ye, Yubin Cao, Wenbin Yang and Chunjie Li.
Risk of bias assessment: Li Ye, Yubin Cao, Wenbin Yang and Chunjie Li.
Data analysis: Yubin Cao and Chunjie Li.
Data interpretation: Li Ye, Yubin Cao, Longjiang Li and Chunjie Li.
Manuscript preparation: Li Ye, Yubin Cao and Chunjie Li.
Revision of the manuscript: Li Ye, Yubin Cao, Wenbin Yang, Fanglong Wu, Jie Lin, Longjiang Li and Chunjie Li.
Sources of support
Internal sources
West China Hospital of Stomatology, Sichuan University, China.
Chinese Cochrane Center, China.
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Li Ye: none known.
Yubin Cao: none known.
Wenbin Yang: none known.
Fanglong Wu: none known.
Jie Lin: none known.
Longjiang Li: none known.
Chunjie Li: none known.
Li Ye, Yubin Cao and Wenbin Yang contributed equally to this work.
New
References
References to studies included in this review
Asal 2005 {published data only}
- Asal K, Köybaşioğlu A, Inal E, Ural A, Uslu SS, Ceylan A, et al. Sternocleidomastoid muscle flap reconstruction during parotidectomy to prevent Frey's syndrome and facial contour deformity. Ear, Nose, & Throat Journal 2005;84(3):173‐6. [PubMed] [Google Scholar]
Govindaraj 2001 {published data only}
- Govindaraj S, Cohen M, Genden EM, Costantino PD, Urken ML. The use of acellular dermis in the prevention of Frey's syndrome. Laryngoscope 2001;111(11 Pt 1):1993‐8. [DOI] [PubMed] [Google Scholar]
Kerawala 2002 {published data only}
- Kerawala CJ, McAloney N, Stassen LF. Prospective randomised trial of the benefits of a sternocleidomastoid flap after superficial parotidectomy. British Journal of Oral & Maxillofacial Surgery 2002;40(6):468‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chan 2014 {published data only}
- Chan LS, Barakate MS, Havas TE. Free fat grafting in superficial parotid surgery to prevent Frey's syndrome and improve aesthetic outcome. Journal of Laryngology and Otology 2014;128(Suppl 1):S44‐9. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen R, Yang K. Clinical observation of efficacy of sternocleidomastoid muscle flap reconstruction after parotid surgery on prevention of Frey's syndrome. Chongqing Medical Journal 2004;33(1):94‐5. [Google Scholar]
Chen 2007 {published and unpublished data}
- Chen WL, Yang ZH, Huang ZQ, Wang YJ, Li JS, Zhang B, et al. SMAS folded flap and allograft dermal matrix repairing parotid bed following parotidectomy to prevent facial deformity and Frey's syndrome. China Journal of Oral and Maxillofacial Surgery 2007;5(4):265‐9. [Google Scholar]
- You YH, Chen WL, Huang ZQ, Chai Q. SMAS fold flap and allograft dermal matrix repairing parotid bed following parotidectomy to prevent facial deformity and Frey's syndrome. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology 2012;24(1):18‐22. [Google Scholar]
Chen 2008 {published data only}
- Chen W, Li J, Yang Z, Yongjie W, Zhiquan W, Wang Y. SMAS fold flap and ADM repair of the parotid bed following removal of parotid haemangiomas via pre‐ and retroauricular incisions to improve cosmetic outcome and prevent Frey's syndrome. Journal of Plastic, Reconstructive & Aesthetic Surgery 2008;61(8):894‐9. [DOI] [PubMed] [Google Scholar]
Ding 2010 {published data only}
- Ding YF, Zhou PG, Gu YC, Chen Y. Application of sternocleidomastoid muscle flap in benign parotidectomies. Journal of Dental Prevention and Treatment 2010;18(2):88‐90. [Google Scholar]
Dong 2008 {published data only}
- Dong ZQ, Zhang F, Wei FC, Peng HH, Tan WY. Acellular dermal matrix in the prevention of Frey syndrome after parotidectomy. Journal of Shandong University (Health Sciences) 2008;46(8):801‐3. [Google Scholar]
Durgut 2013 {published data only}
- Durgut O, Basut O, Demir UL, Ozmen OA, Kasapoglu F, Coskun H. Association between skin flap thickness and Frey's syndrome in parotid surgery. Head & Neck 2013;35(12):1781‐6. [DOI] [PubMed] [Google Scholar]
Elgammal 2017 {published data only}
- Elgammal AS, Sisi A, Rageh T, Gaber A. The role of covering the facial nerve and parotid surface in prevention of the postparotidectomy complications. Egyptian Journal of Surgery 2017;36:446‐50. [DOI: 10.4103/ejs.ejs_77_17] [DOI] [Google Scholar]
Gennaro 2013 {published data only}
- Gennaro P, Curzio P, Mitro V, Facchini A, Saponaro G, Cascino F, et al. Use of irradiate animal pericardium membrane for prevention of Frey's syndrome after parotidectomy. European Review for Medical and Pharmacological Sciences 2013;17(4):548‐51. [PubMed] [Google Scholar]
Gou 2018 {published data only}
- Guo SY. Analysis of the effect of oral repair membrane on the postoperative taste sweating syndrome of parotid gland tumors. China & Foreign Medical Treatment 2018;1:90‐2. [DOI: 10.16662/j.cnki.1674-0742.2018.01.090] [DOI] [Google Scholar]
Grosheva 2016 {published data only}
- Grosheva M, Horstmann L, Volk GF, Holler C, Ludwig L, Weiss V, et al. Frey's syndrome after superficial parotidectomy: role of the sternocleidomastoid muscle flap: a prospective nonrandomized controlled trial. American Journal of Surgery 2016;212(4):740‐7. [DOI] [PubMed] [Google Scholar]
Jiang 2010 {published data only}
- Jiang YJ, Yang K. Clinical research of prosthodontics membrane in prevention of Frey's syndrome after parotidectomy. Guide of China Medicine 2010;8(21):9‐10. [Google Scholar]
Jiang 2018 {published data only}
- Jiang QK, Cao ZY, Wei YC, Zhang Q, Yan JF, Zhang J. The application of modified cosmetic incision and skin adhesive in parotid benign tumor functional surgery. Journal of Clinical Otorhinolaryngology Head and Neck Surgery 2018;32(16):1255‐9. [DOI] [PubMed] [Google Scholar]
Jin 2008 {published data only}
- Jin S. Application of temporalis flap in treatment of benign parotid tumours. Shangdong Medical Journal 2008;48(36):96. [Google Scholar]
Jin 2013 {published data only}
- Jin X, Xu WH, Chen X. To observe the efficacy of haiao bio‐membrane in preventing gustatory sweating syndrome. Anhui Medical and Pharmaceutical Journal 2013;17(7):1128‐9. [Google Scholar]
Li 2006 {published data only}
- Li DZ, Wu YH, Wang XL, Liu SY, Li ZJ. Prospective cohort study on prevention of Frey syndrome in parotid surgery. Zhonghua Wai Ke Za Zhi 2006;44(15):1033‐5. [PubMed] [Google Scholar]
Liao 2008 {published data only}
- Liao XL, Wu B, Zhang Y, Yin P, Ding N. The use of acellular dermal matrix in the prevention of post‐parotidectomy Frey syndrome. Journal‐Hebei Medical University 2008;29(2):223‐5. [Google Scholar]
Liao 2012 {published data only}
- Liao XM, Yang K, Liu XH, Zhang FJ. Clinical research of bioabsorbable membrane in prevention of Frey syndrome after parotidectomy. Chinese Journal of Primary Medicine and Pharmacy 2012;19(2):171‐2. [Google Scholar]
Luo 2012 {published and unpublished data}
- Luo W, Zheng X, Chen L, Jing W, Tang W, Long J, et al. The use of human acellular dermal matrix in the prevention of infra‐auricular depressed deformities and Frey's syndrome following total parotidectomy. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology 2012;114(2):e9‐13. [DOI] [PubMed] [Google Scholar]
Mao 2018 {published data only}
- Mao Y, Chang YJ, Zhang XL, Xu FF, Yu SQ, Ji ZW. Functional parotid surgery for removal of benign parotid tumors. Journal of Clinical Otorhinolaryngology Head and Neck Surgery 2018;32(23):1810‐2. [DOI: 10.13201/j.issn.1001-1781.2018.23.010] [DOI] [PubMed] [Google Scholar]
Ren 2010 {published data only}
- Ren WH, Zhi KQ, Gao L, Xu Y, Li XQ, Shi MJ. Clinical utilization of veiled incision and sternocleidomastoid flap in parotidectomy of parotid benign tumors. Shanghai Kou Qiang Yi Xue 2010;19(3):232‐5. [PubMed] [Google Scholar]
Sinha 2003 {published data only}
- Sinha UK, Saadat D, Doherty CM, Rice DH. Use of AlloDerm implant to prevent Frey syndrome after parotidectomy. Archives of Facial Plastic Surgery 2003;5(1):109‐12. [DOI] [PubMed] [Google Scholar]
Wang 2016 {published and unpublished data}
- Wang S, Li L, Chen J, Li X, Yin J, Liu K, et al. Effects of free fat grafting on the prevention of Frey's syndrome and facial depression after parotidectomy: a prospective randomized trial. Laryngoscope 2016;126(4):815‐9. [DOI] [PubMed] [Google Scholar]
Wille‐Bischofberger 2007 {published data only}
- Wille‐Bischofberger A, Rajan GP, Linder TE, Schmid S. Impact of the SMAS on Frey's syndrome after parotid surgery: a prospective, long‐term study. Plastic & Reconstructive Surgery 2007;120(6):1519‐23. [DOI] [PubMed] [Google Scholar]
Xie 2011 {published data only}
- Xie LC, Xu XM, Zeng XT, Ni XB, Zhang WF. Application of xenogenic acellular dermal matrix in preventing Frey's syndrome and facial concave deformity. Journal of Hubei University of Medicine 2011;30(2):141‐4. [Google Scholar]
Yu 2007 {published and unpublished data}
- Yu K, Yang J, Li M J, Ma H B. Clinical application of acellular dermal matrix to prevent gustatory sweating syndrome. Zhonghua Kou Qiang Ke Za Zhi 2007;42(9):570‐1. [PubMed] [Google Scholar]
Zeng 2010 {published data only}
- Zeng ZX. The clinical value of parotid masseter fasscia and acellular dermal matrix in prevention of Frey's syndrome after parotidectomy. Journal of Clinical Stomatology 2010;26(1):40‐2. [Google Scholar]
References to studies awaiting assessment
Ding 2018 {published data only}
- Ding F, Wu X, Li J, Yang XH, Ding XJ, Zhou FJ. Prevention of postoperative complications of parotid gland surgery by reconstruction of parotid masseter fascia. Journal of Practical Stomatology 2018;34(5):632‐5. [DOI: 10.3969/j.issn.1001-3733.2018.05.012] [DOI] [Google Scholar]
Hao 2008 {published data only}
- Hao Z, Bai H, Cui JY. The effect of acellular dermal matrix in prevention of Frey syndrome in parotid surgery. Chinese Clinical Oncology 2008;13(2):150‐2. [Google Scholar]
Sun 2008 {published data only}
- Sun HP, Feng L, Weng RL. Clinical study of acellular dermal matrix to prevent Frey's syndrome and facial deformity after parotidectomy. Journal of Dental Prevention and Treatment 2008;16(4):161‐3. [Google Scholar]
Xue 2010 {published data only}
- Xue LF, Shang W, Feng YY, Jin XM, Liu FT, Jia MY, et al. Clinical study of heterogeneous acellular dermal matrix to prevent Frey's syndrome after parotidectomy. China Journal of Oral and Maxillofacial Surgery 2010;8(3):229‐31. [Google Scholar]
Yang 2018 {published data only}
- Yang HP, Zhang HW, Wang XB, Yang SX, Wang J, Chen HD. The application of sternocleidomastoid muscle flap and cosmetic incision for resection of parotid tumor. Stomatology 2018;38(8):704‐7. [DOI: 10.13591/j.cnki.kqyx.2018.08.007] [DOI] [Google Scholar]
Yu 2011 {published data only}
- Yu XL, Yang K, Zhang FJ, Lv XQ. Comparison of the effects of two types of SMAS reservations on tumor recurrence and Frey's syndrome prevention after parotidectomy. Journal of Chongqing Medical University 2011;36(10):1259‐61. [Google Scholar]
Additional references
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baek 2009
- Baek CH, Chung MK, Jeong HS, Son YI, Jung SC, Jeon HK, et al. Questionnaire evaluation of sequelae over 5 years after parotidectomy for benign diseases. Journal of Plastic, Reconstructive & Aesthetic Surgery 2009;62(5):633‐8. [DOI] [PubMed] [Google Scholar]
Bakke 2006
- Bakke M, Max Thorsen N, Bardow A, Dalager T, Eckhart Thomsen C, Regeur L. Treatment of gustatory sweating with low‐dose botulinum toxin A: a case report. Acta Odontologica Scandinavica 2006;64(3):129‐33. [DOI] [PubMed] [Google Scholar]
Barberá 2014
- Barberá R, Castillo F, D'Oleo C, Benítez S, Cobeta I. Superficial musculoaponeurotic system flap in partial parotidectomy and clinical and subclinical Frey's syndrome. Cosmesis and quality of life. Head and Neck 2014;36(1):130‐6. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Blanc 2016
- Blanc S, Bourrier T, Boralevi F, Sabouraud‐Leclerc D, Pham‐Thi N, Couderc L, et al. Frey Syndrome Collaborators. Frey syndrome. Journal of Pediatrics 2016;174:211‐7. [DOI: 10.1016/j.jpeds.2016.03.070] [DOI] [PubMed] [Google Scholar]
Bonanno 1992
- Bonanno PC, Casson PR. Frey's syndrome: a preventable phenomenon. Plastic and Reconstructive Surgery 1992;89(3):452‐6. [PubMed] [Google Scholar]
Chang 2017
- Chang JW, Leem SS, Choi HJ, Lee JH. Modified functional superficial parotidectomy with ligation of the major branch of the parotid duct extending to the superficial lobe. Annals of Plastic Surgery 2017;78(5):507‐10. [DOI: 10.1097/SAP.0000000000000885] [DOI] [PubMed] [Google Scholar]
de Bree 2007
- Bree R, Waal I, Leemans CR. Management of Frey syndrome. Head and Neck 2007;29(8):773‐8. [DOI] [PubMed] [Google Scholar]
Dulguerov 2016
- Dulguerov N, Makni A, Dulguerov P. The superficial musculoaponeurotic system flap in the prevention of Frey syndrome: a meta‐analysis. Laryngoscope 2016;126(7):1581‐4. [DOI: 10.1002/lary.25895] [DOI] [PubMed] [Google Scholar]
Eski 2018
- Eski E, Sökmen MF, Yilmaz I. Segmental superficial parotidectomy in the surgical treatment of benign parotid tumours. Journal of Laryngology and Otology 2018;132(4):356‐9. [DOI: 10.1017/S0022215118000245] [DOI] [PubMed] [Google Scholar]
Foresta 2014
- Foresta E, Torroni A, Nardo F, Waure C, Poscia A, Gasparini G, et al. Pleomorphic adenoma and benign parotid tumors: extracapsular dissection vs superficial parotidectomy‐‐review of literature and meta‐analysis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2014;117(6):663‐76. [DOI: 10.1097/00005537-200212000-00004] [DOI] [PubMed] [Google Scholar]
Frey 1923
- Frey L. Le syndrome du nerf auriculo‐temporal. Revue Neurologique 1923;2:97. [Google Scholar]
Glaister 1958
- Glaister DH, Hearnshaw JR, Heffron PF, Peck AW, Patey DH. The mechanism of post‐parotidectomy gustatory sweating (the auriculo‐temporal syndrome). British Medical Journal 1958;2(5102):942‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Laage‐Hellman 1958
- Laage‐Hellman JE. Gustatory sweating and flushing; aetiological implications of latent period and mode of development after parotidectomy. Acta Oto‐Laryngologica 1958;49(4):306‐14. [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee CC, Chan RC, Chan JY. Predictors for Frey syndrome development after parotidectomy. Annals of Plastic Surgery 2017;79(1):39‐41. [DOI: 10.1097/SAP.0000000000000993] [DOI] [PubMed] [Google Scholar]
Li 2013
- Li C, Yang X, Pan J, Shi Z, Li L. Graft for prevention of Frey syndrome after parotidectomy: a systematic review and meta‐analysis of randomized controlled trials. Journal of Oral and Maxillofacial Surgery 2013;71(2):419‐27. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li C, Xu Y, Zhang C, Sun C, Chen Y, Zhao H, et al. Modified partial superficial parotidectomy versus conventional superficial parotidectomy improves treatment of pleomorphic adenoma of the parotid gland. American Journal of Surgery 2014;208(1):112‐8. [DOI: 10.1016/j.amjsurg.2013.08.036] [DOI] [PubMed] [Google Scholar]
Li 2015
- Li C, Wu F, Zhang Q, Gao Q, Shi Z, Li L. Interventions for the treatment of Frey's syndrome. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009959.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2019
- Lin YQ, Wang Y, Ou YM, Dong SY, Wang YD. Extracapsular dissection versus partial superficial parotidectomy for the treatment of benign parotid tumours. International Journal of Oral and Maxillofacial Surgery 2019;48(7):895‐901. [DOI: 10.1016/j.ijom.2019.01.030] [DOI] [PubMed] [Google Scholar]
Liu 2013
- Liu DY, Tian XJ, Li C, Sun SS, Xiong YH, Zeng XT. The sternocleidomastoid muscle flap for the prevention of Frey syndrome and cosmetic deformity following parotidectomy: a systematic review and meta‐analysis. Oncology Letters 2013;5(4):1335‐42. [DOI: 10.3892/ol.2013.1179] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neumann 2011
- Neumann A, Rosenberger D, Vorsprach O, Dazert S. The incidence of Frey syndrome following parotidectomy: results of a survey and follow‐up. HNO 2011;59(2):173‐8. [DOI] [PubMed] [Google Scholar]
O'Neill 2008
- O'Neill JP, Condron C, Curran A, Walsh A. Lucja Frey‐‐historical relevance and syndrome review. Surgeon 2008;6(3):178‐81. [DOI] [PubMed] [Google Scholar]
Prattico 2006
- Prattico F, Perfetti P. Frey's syndrome. New England Journal of Medicine 2006;355(1):66. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rustemeyer 2008
- Rustemeyer J, Eufinger H, Bremerich A. The incidence of Frey's syndrome. Journal of Cranio‐maxillo‐facial Surgery 2008;36(1):34‐7. [DOI: 10.1016/j.jcms.2007.07.003] [DOI] [PubMed] [Google Scholar]
Sanabria 2012
- Sanabria A, Kowalski LP, Bradley PJ, Hartl DM, Bradford CR, Bree R, et al. Sternocleidomastoid muscle flap in preventing Frey's syndrome after parotidectomy: a systematic review. Head & Neck 2012;34(4):589‐98. [DOI] [PubMed] [Google Scholar]
Sharma 2014
- Sharma R. Prevention of Frey syndrome with superficial temporal fascia interpositioning: a retrospective study. International Journal of Oral and Maxillofacial Surgery 2014;43(4):413‐7. [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh N, Kohli M, Kohli H. Innovative technique to reduce incidence of Frey's syndrome after parotid surgery. American Surgeon 2011;77(3):351‐4. [PubMed] [Google Scholar]
Toure 2015
- Toure G. Intraparotid location of the great auricular nerve: a new anatomical basis for gustatory sweating syndrome. Plastic and Reconstructive Surgery 2015;136(5):1069‐81. [DOI: 10.1097/PRS.0000000000001726] [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang WH, Zhu J, Li M, Xia B, Xu B. Usefulness of platysma muscle flap following superficial parotidectomy. Journal of Cranio‐Maxillo‐Facial Surgery 2013;41(1):10‐4. [DOI] [PubMed] [Google Scholar]
Watkins 1973
- Watkins PJ. Facial sweating after food: a new sign of diabetic autonomic neuropathy. British Medical Journal 1973;1(5853):583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenzel 2004
- Wenzel GI, Draf W. Unusually long latency before the appearance of Frey's syndrome after parotidectomy. HNO 2004;52(6):554‐6. [DOI] [PubMed] [Google Scholar]
Wood 2019
- Wood CB, Netterville JL. Temporoparietal Frey syndrome: an uncommon variant of a common syndrome. Laryngoscope 2019;129(9):2071‐5. [DOI: 10.1002/lary.27632] [DOI] [PubMed] [Google Scholar]
Wu 2009
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2015a
- Xie S, Wang K, Xu H, Hua RX, Li TZ, Shan XF, et al. PRISMA‐extracapsular dissection versus superficial parotidectomy in treatment of benign parotid tumors: evidence from 3194 patients. Medicine (Baltimore) 2015;94(34):e1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2015b
- Xie S, Wang K, Xu T, Guo XS, Shan XF, Cai ZG. Efficacy and safety of botulinum toxin type A for treatment of Frey's syndrome: evidence from 22 published articles. Cancer Medicine 2015;4(11):1639‐50. [DOI: 10.1002/cam4.504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2013
- Yang W, Li C, Men Y, Han B, Li L, Pan J. A systematic review and meta‐analysis of effects of parotid masseteric fascia reservation on prevention of Frey's syndrome. International Journal of Stomatology 2013;40(6):730‐5. [Google Scholar]
Yang 2019
- Yang R, Guo Y, Mao C, Guo C, Wang D. Extracapsular dissection via sternocleidomastoid muscle‐parotid space approach‐a new operative technique for treating clinically benign tumor in the parotid tail. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2019;pii: S2212‐4403(19):30396‐7. [DOI: 10.1016/j.oooo.2019.03.006] [DOI] [PubMed] [Google Scholar]
Zeng 2012
- Zeng XT, Tang XJ, Wang XJ, Li MZ, Guo Y, Huang W, et al. AlloDerm implants for prevention of Frey syndrome after parotidectomy: a systematic review and meta‐analysis. Molecular Medicine Reports 2012;5(4):974‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2005
- Zhao HW, Li LJ, Han B, Liu H, Pan J. A retrospective study on the complications after modified parotidectomy in benign tumors of parotid gland. Hua Xi Kou Qiang Yi Xue Za Zhi 2005;23(1):53‐6. [PubMed] [Google Scholar]
References to other published versions of this review
Li 2016
- Li C, Yang W, Wu F, Men Y, Lin J, Li L. Graft interposition for preventing Frey's syndrome in patients undergoing parotidectomy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012323] [DOI] [PMC free article] [PubMed] [Google Scholar]


