Abstract
Background
Kidney transplantation is the therapy of choice for many patients with end‐stage kidney disease (ESKD) with an improvement in survival rates and satisfactory short term graft survival. However, there has been little improvement in long‐term survival. The place of target of rapamycin inhibitors (TOR‐I) (sirolimus, everolimus), which have different modes of action from other commonly used immunosuppressive agents, in kidney transplantation remains uncertain. This is an update of a review first published in 2006.
Objectives
To evaluate the short and long‐term benefits and harms of TOR‐I (sirolimus and everolimus) when used in primary immunosuppressive regimens for kidney transplant recipients.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 20 September 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register were identified through searches of CENTRAL, MEDLINE and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
All randomised controlled trials (RCTs) and quasi‐RCTs in which drug regimens, containing TOR‐I commenced within seven days of transplant, were compared to alternative drug regimens, were included without age restriction, dosage or language of report.
Data collection and analysis
Three authors independently assessed study eligibility, risk of bias, and extracted data. Results were reported as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes and mean difference (MD) with 95% CI for continuous outcomes. Statistical analyses were performed using the random‐effects model. The certainty of the evidence was assessed using GRADE
Main results
Seventy studies (17,462 randomised participants) were included; eight studies included two comparisons to provide 78 comparisons. Outcomes were reported at six months to three years post transplant.
Risk of bias was judged to be low for sequence generation in 25 studies, for allocation concealment in 23 studies, performance bias in four studies, detection bias in 65 studies, attrition bias in 45 studies, selective reporting bias in 48 studies, and for other potential bias in three studies. Risk of bias was judged to be at high risk of bias for sequence generation in two studies, allocation concealment in two studies, performance bias in 61 studies, detection bias in one study, attrition bias in four studies, for selective reporting bias in 11 studies and for other potential risk of bias in 46 studies.
Compared with CNI and antimetabolite, TOR‐I with antimetabolite probably makes little or no difference to death (RR 1.31, 95% CI 0.87 to 1.98; 19 studies) or malignancies (RR 0.86, 95% CI 0.50 to 1.48; 10 studies); probably increases graft loss censored for death (RR 1.32, 95% CI 0.96 to 1.81; 15 studies), biopsy‐proven acute rejection (RR 1.60, 95% CI 1.25 to 2.04; 15 studies), need to change treatment (RR 2.42, 95% CI 1.88 to 3.11; 14 studies) and wound complications (RR 2.56, 95% CI 1.94 to 3.36; 12 studies) (moderate certainty evidence); but reduces CMV infection (RR 0.43, 95% CI 0.29 to 0.63; 13 studies) (high certainty evidence).
Compared with antimetabolites and CNI, TOR‐I with CNI probably makes little or no difference to death (RR 1.06, 95% CI 0.84 to 1.33; 31 studies), graft loss censored for death (RR 1.09, 95% CI 0.82 to 1.45; 26 studies), biopsy‐proven acute rejection (RR 0.95, 95% CI 0.81 to 1.12; 24 studies); and malignancies (RR 0.83, 95% CI 0.64 to 1.07; 17 studies); probably increases the need to change treatment (RR 1.56, 95% CI 1.28 to 1.90; 25 studies), and wound complications (RR 1.56, 95% CI 1.28 to 1.91; 17 studies); but probably reduces CMV infection (RR 0.44, 95% CI 0.34 to 0.58; 25 studies) (moderate certainty evidence).
Lower dose TOR‐I and standard dose CNI compared with higher dose TOR‐I and reduced dose CNI probably makes little or no difference to death (RR 1.07, 95% CI 0.64 to 1.78; 9 studies), graft loss censored for death (RR 1.09, 95% CI 0.54 to 2.20; 8 studies), biopsy‐proven acute rejection (RR 0.87, 95% CI 0.67 to 1.13; 8 studies), and CMV infection (RR 1.42, 95% CI 0.78 to 2.60; 5 studies) (moderate certainty evidence); and may make little or no difference to wound complications (RR 0.95, 95% CI 0.53 to 1.71; 3 studies), malignancies (RR 1.04, 95% CI 0.36 to 3.04; 7 studies), and the need to change treatments (RR 1.18, 95% CI 0.58 to 2.42; 5 studies) (low certainty evidence).
Lower dose of TOR‐I compared with higher doses probably makes little or no difference to death (RR 0.84, 95% CI 0.67 to 1.06; 13 studies), graft loss censored for death (RR 0.92, 95% CI 0.71 to 1.19; 12 studies), biopsy‐proven acute rejection (RR 1.26, 95% CI 1.10 to 1.43; 11 studies), CMV infection (RR 0.87, 95% CI 0.63 to 1.21; 9 studies), wound complications (RR 0.92, 95% CI 0.66 to 1.29; 7 studies), and malignancy (RR 0.84, 95% CI 0.54 to 1.32; 10 studies) (moderate certainty evidence); and may make little or no difference to the need to change treatments (RR 0.91, 95% CI 0.78 to 1.05; 10 studies) (low certainty evidence).
It is uncertain whether sirolimus and everolimus differ in their effects on kidney function and lipid levels because the certainty of the evidence is very low based on a single small study with only three months of follow‐up.
Authors' conclusions
In studies with follow‐up to three years, TOR‐I with an antimetabolite increases the risk of graft loss and acute rejection compared with CNI and an antimetabolite. TOR‐I with CNI potentially offers an alternative to an antimetabolite with CNI as rates of graft loss and acute rejection are similar between interventions and TOR‐I regimens are associated with a reduced risk of CMV infections. Wound complications and the need to change immunosuppressive medications are higher with TOR‐I regimens. While further new studies are not required, longer‐term follow‐up data from participants in existing methodologically robust RCTs are needed to determine how useful immunosuppressive regimens, which include TOR‐I, are in maintaining kidney transplant function and survival beyond three years.
Plain language summary
Target of rapamycin inhibitors (TOR‐I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients
What is the issue?
Kidney transplantation is the treatment of choice for many patients with end‐stage kidney disease. However, some kidney transplants do not work for long periods so it is important to find ways to improve long‐term transplant function by choosing the best therapies to maintain kidney function and keep transplant recipients healthy with minimal side effects.
What did we do?
We reviewed 70 studies, with 17,462 randomised participants, which compared TOR‐1 (everolimus or sirolimus) with other agents for initial immunosuppressive therapy for kidney transplant recipients.
What did we find?
We found that everolimus or sirolimus combined with cyclosporin or tacrolimus prevented kidney transplant failure and rejection as effectively as mycophenolate (an antimetabolite) with cyclosporin or tacrolimus in studies with follow‐up from six months to three years. The risk for viral infections (CMV and BK) was lower with TOR‐I. However, wound complications were more common with TOR‐I and more people had to stop TOR‐I and change to other immunosuppressive medications.
Conclusions
Although the results indicate that TOR‐I were effective in preventing transplant failure and rejection in the short term, studies do not follow‐up participants beyond six months to three years. Therefore, we do not need further studies but we do need much longer periods of follow‐up of participants in existing studies to determine how useful these medications are for maintaining kidney transplant function in the longer term.
Summary of findings
Background
Description of the condition
Kidney transplantation is the treatment of choice for many patients with end‐stage kidney disease (ESKD) providing improved patient survival rates (95% one‐year survival) and satisfactory short‐term graft survival. To maintain long‐term graft survival our challenge is the need to suppress the host immune system. Immunosuppressive therapies used in kidney transplantation inhibit one or more steps in the allo‐immune response that would otherwise result in rejection. Long‐term graft survival beyond five years has shown little improvement since the 1970s. Transplant waiting lists continue to grow with demand exceeding organ availability. Strategies to increase donor organ availability and to prolong kidney allograft survival have become priorities in kidney transplantation (ANZDATA 2017; NHS Blood and Transplant 2019 "Taking Organ Transplantation to 2020 Strategy", USRDS 2018).
Description of the intervention
Standard immunosuppressive therapy consists of initial induction and maintenance regimens to prevent rejection. Most current immunosuppressive regimens in the immediate post‐operative period typically involve three drug groups each directed to a site in the T‐cell activation or proliferation cascade which are central to the rejection process: calcineurin inhibitors (CNI; e.g. cyclosporin, tacrolimus), antimetabolite agents (azathioprine (AZA), mycophenolate mofetil (MMF), mycophenolate sodium (MPS)) and corticosteroids (prednisolone) with 93% recipients in the USA, and 70% in Australia, being discharged from hospital after transplantation on these agents. Following the introduction of CNI (cyclosporin in the early 1980s and tacrolimus the 1990s), one‐year graft survival improved to the current level at of over 90% though long‐term graft survival ranges between 34% and 56% across different population groups in Europe and the USA (KDIGO 2009; Gondos 2013).
Target of rapamycin inhibitors (TOR‐I) (sirolimus, everolimus) are immunosuppressive agents with a mode of action different from other commonly used immunosuppressive agents. Sirolimus is a macrocyclic lactone antibiotic produced from Streptomyces hygroscopicus initially discovered as an antifungal agent. The immunosuppressive properties were deemed an undesirable effect and led to the development of a useful drug. Everolimus is a derivative of sirolimus. Both bind to the same intracellular immunophilin as tacrolimus (FKBP12), but instead of inhibiting calcineurin, the drug‐receptor complex then binds to proteins known as the "mammalian targets of rapamycin" (mTOR). This causes inhibition of a multifunctional serine‐threonine kinase, preventing both DNA and protein synthesis resulting in arrest of the cell cycle (Hernandez 2011, Dumont 2001; Saunders 2001).
Based upon laboratory data, the early expectation was that TOR‐I would provide synergistic immunosuppression when combined with CNI (Schuurman 1997; Stepkowski 1997). The absence of nephrotoxicity in animal models increased expectations of significant clinical benefit (Viklicky 2000). Clinical studies dispelled some of the early optimism as synergistic nephrotoxicity was demonstrated when either sirolimus or everolimus were combined with cyclosporin (Kahan‐301 2000; MacDonald‐302 2001; Vitko‐201 2001). Since then studies have been undertaken to explore strategies that avoid this interaction and clarify other potential benefits such as vascular protection (Ponticelli 2004) and a reduction in malignancy (Stallone 2005), and the impact of harms such as hyperlipidaemia and wound complications. Nevertheless the ANZDATA 2017 report indicates that fewer than 1% of transplant recipients receive everolimus or sirolimus in the initial post transplant regimen and fewer than 4% receive TOR‐I at one year post transplant.
How the intervention might work
The major cause of long‐term graft loss is chronic allograft nephropathy a complex, multifactorial process characterised clinically by a progressive decline in graft function, proteinuria and hypertension, and pathologically characterised by interstitial fibrosis/tubular atrophy. Chronic allograft nephropathy is a consequence of immunological and non‐immunological injury. Immunological factors include human leukocyte antigen (HLA) matching, episodes of acute rejection and suboptimal immunosuppression. Important non‐immunological factors implicated are donor organ characteristics, delayed graft function, recipient‐related factors, hypertension, hyperlipidaemia and viral infections. CNI are linked to nephrotoxicity contributing to long‐term graft failure, hypertension, hyperlipidaemia, and new‐onset diabetes mellitus. The TOR‐I have increased treatment options that produce adequate immunosuppression, allow reduced CNI dose with a reduction in CNI‐associated side effects and reduced incidence of viral infections (Hernandez 2011; Kumar 2017).
Why it is important to do this review
Despite being in use for many years, the place of these agents in kidney transplantation remains uncertain. The aim of this study was to identify and summarise the currently available evidence of the short and long‐term benefits and harms of sirolimus and everolimus when used in primary immunosuppressive regimens for kidney transplant recipients. Since the review, which included 33 studies, was first published in 2006, an additional 37 studies have been identified. Their inclusion in the review should provide a more comprehensive assessment of the place of TOR‐I in immunosuppressive regimens. In this update we have only added studies where participants were commenced on a TOR‐I less than seven days from date of transplant. Studies in which participants commenced TOR‐I after seven days will be considered in a subsequent systematic review.
Objectives
To evaluate the short and long‐term benefits and harms of TOR‐I (sirolimus and everolimus) when used in primary immunosuppressive regimens for kidney transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) where drug regimens containing sirolimus or everolimus were compared to an alternative drug regimen in the immediate post transplant period (less than seven days post transplant) were included.
Types of participants
Inclusion criteria
All patients of all ages with ESKD, who were the recipient of a first or subsequent deceased donor or living donor kidney transplant, were included. There was no restriction by age of recipients, or dosage of immunosuppressive drugs.
Exclusion criteria
Studies in which participants commenced TOR‐I agents seven days or more post transplant were excluded. Studies in which transplant recipients received another solid organ in addition to a kidney transplant (e.g. kidney and pancreas) were excluded.
Types of interventions
Sirolimus or everolimus, given in combination with any other immunosuppressive agents, at any stage in the intra‐operative or immediate post‐transplant period. All dosage regimens were included. Sirolimus and everolimus were considered together to estimate 'class effect'.
Types of outcome measures
The outcome measures relate to those used by transplant registries to assess patient and graft survival. Outcome events were reported at the end of follow up or at two to three years post transplant depending on data availability.
Primary outcomes
Death (all causes)
All‐cause graft loss (death with functioning allograft or dependence on dialysis)
Graft loss censored for death with functioning allograft
All acute rejection and biopsy‐proven acute rejection
Incidence of cytomegalovirus (CMV) infections (all definitions), with diagnosis by culture, serology, antigen or antibody testing, or as specified by authors.
All adverse wound outcomes and lymphocoele
All malignancies
Number needing to change treatment.
Secondary outcomes
New‐onset diabetes mellitus
Lymphoma/post transplant lymphoproliferative disorder (PTLD)
Number with BK virus infection (all definitions)
Graft function (measured as absolute value or change in serum creatinine (SCr), glomerular filtration rate (GFR), creatinine clearance (CrCl)
Incidence of treatment‐related adverse reactions related to TOR‐I (specifically anaemia, thrombocytopenia, leucopenia, hypercholesterolaemia, hypertriglyceridaemia) and/or to CNI.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 20 September 2019 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The original review was undertaken by four authors (ACW, VWSL, JRC, JCC). The 2019 update was undertaken by three authors (LH, DH, EH) with support from ACW and VWSL. Disagreement about inclusion of studies in the review was resolved by discussion between authors.
Data extraction and management
Data extraction was performed independently by three authors (LH, DH, EH) using a standardised form. Where possible, authors of published work were contacted for clarification of unclear data.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2011 (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Studies were grouped and analysed according to the following comparisons.
TOR‐I versus CNI
TOR‐I versus antimetabolite
Variable dosages of TOR‐I and/or CNI
Low versus higher doses of TOR‐I.
For dichotomous outcomes (e.g. death, graft loss, acute rejection) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement are used (e.g. SCr, GFR), the mean difference (MD) with 95% CI was used.
Where sufficient RCTs were identified, publication bias was investigated using funnel plots (Egger 1997).
Unit of analysis issues
No cross‐over studies were identified for this review. If we had identified any cross‐over studies, we would only have included data from the first period of treatment in cross‐over studies (Higgins 2011).
Dealing with missing data
Any further information or clarification required from the authors was requested by written or electronic correspondence and relevant information obtained in this manner was included in the review. We aimed to analyse available data in meta‐analyses using intention‐to‐treat (ITT) data. However, where only ITT data were available graphically or were not provided and additional information could not be obtained from the study authors, per‐protocol (PP) data was used in analyses. We imputed standard deviations if necessary based on those from other studies included in meta‐analyses.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
The search strategy applied aimed to reduce publication bias caused by lack of publication of studies with negative results. We investigated for publication bias using funnel plots if there were sufficient studies of each comparison (Higgins 2011).
Data synthesis
Data were summarised using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen. Where there were multiple publications of the same study, all reports were reviewed to ensure that all details of methods and results were included.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity by assessing the P‐value for subgroup differences provided in RevMan analyses. Subgroups, defined a priori, were publication type (abstract or full publication), study methodological quality (sequence generation and allocation concealment), CNI used (whether tacrolimus or cyclosporin), whether or no induction with antibody was included in the immunosuppressive co‐interventions, the TOR‐I used (whether sirolimus or everolimus) and the antimetabolite used (whether mycophenolate or azathioprine).
Sensitivity analysis
Sensitivity analyses tested decisions where inclusion of a study may have altered the results of the meta‐analysis or when it may have led to heterogeneity.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
Primary outcomes
Death
Graft loss (censored for death)
Biopsy‐proven acute rejection
CMV infection
All adverse wound outcomes
All malignancies
Number needing to change treatment (for adverse effects, unsatisfactory response, other medical event. Does not include poor compliance, withdrawal of consent, death, graft loss, protocol violation, loss to follow up, non‐medical events)
Secondary outcomes
New‐onset diabetes mellitus
Number with BK infection
Glomerular filtration rate
Number with hypercholesterolaemia
Number with hypertriglyceridaemia
Number with leucopenia
Number with thrombocytopenia
Results
Description of studies
Results of the search
The initial review published in 2006 included 33 studies (142 reports). Further searches up to 30 September 2019 identified 37 new included studies (294 reports), 27 excluded studies (61 reports), and five ongoing studies (EVER TWIST 2013; Ferreira 2019; NCT02077556; NCT03468478; Traitanon 2019). Prior to publication of this review, two of these ongoing studies (Ferreira 2019; Traitanon 2019) were published and shall be included in a future update of this review (Figure 1).
1.
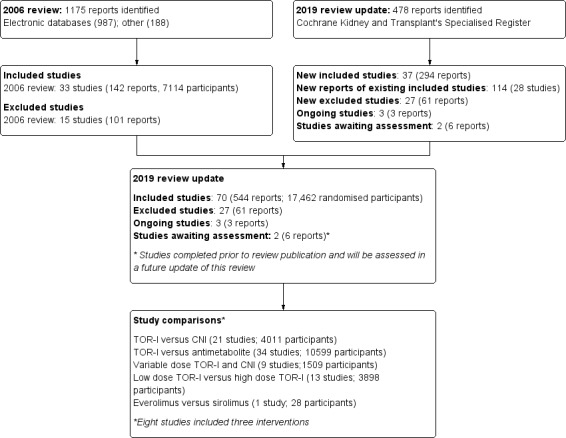
Flow diagram.
Included studies
See Characteristics of included studies.
The 70 completed studies included 17,462 randomised participants; eight studies (Gelens 2006; Kahan‐301 2000; Kandaswamy 2005; Kovarik‐251 2001; ORION 2011; Tedesco‐Silva 2010; Vitko‐201 2001; Vitko‐TERRA 2004) included three interventions so that 78 comparisons were included in the review. Twenty‐two studies compared TOR‐I (sirolimus or everolimus) with a CNI (tacrolimus or cyclosporin). Thirty‐three studies compared TOR‐I with an antimetabolite (MMF, MPS or AZA). Nine studies compared variable doses of TOR‐I with variable doses of a CNI. Thirteen studies compared low doses with higher doses of TOR‐I. One study compared everolimus with sirolimus (Rostaing 2001). Duration of follow‐up ranged from six months to three years.
TOR‐I versus calcineurin inhibitor
The 22 studies of TOR‐I compared with a CNI included 4011 participants (CALFREE 2006; Cattaneo 2005; Durlik 2008; Durrbach 2008; EVEROLD 2014; Fernandes‐Charpiot 2014; FIBRASIC 2009; Flechner 2013; Flechner‐318 2002; Gelens 2006; Glotz 2010; Groth‐207 1999; Kreis‐210 2000; Lebranchu‐132 2004; Martinez‐Mier 2006; Morelon 2010; ORION 2011; Pescovitz 2007; Riad 2007; Schaefer 2006; Stegall 2003; SYMPHONY 2007).
One study (EVEROLD 2014) did not report the participant numbers in each group so 1523 participants were included in the TOR‐I group and 2184 in the CNI group. All participants also received an antimetabolite.
TOR‐I versus antimetabolite
The 33 studies of TOR‐I compared with an antimetabolite included 10,599 participants (Anil Kumar 2005; Anil Kumar 2008; ATHENA 2016; AVESTA 2017; Bertoni 2011; Burke 2002; Ciancio 2016; Esmeraldo 2015; Favi 2009; Favi 2012; Gallon 2006; Gelens 2006; Gonwa‐PSG 2003; Kahan‐301 2000; Kandaswamy 2005; Kovarik‐251 2001; Machado 2001; ORION 2011; Paoletti 2012; Qazi 2017; RECORD 2017; Sampaio 2008; Shetty 2015; Souza 2017; Spagnoletti 2017; Stallone 2004; Takahashi 2013a; Tedesco‐Silva 2010; Tedesco‐Silva 2015; TRANSFORM 2018; van Gurp 2010; Vitko‐201 2001; Vitko‐TERRA 2004).
Two studies (AVESTA 2017; Spagnoletti 2017) did not report the participant numbers in each group so 6123 participants were included in the TOR‐I group and 4318 in the antimetabolite group. All study participants also received a CNI (tacrolimus or cyclosporin). Participants in the antimetabolite group received MMF or MPS except in two studies where azathioprine was administered (Kahan‐301 2000; Machado 2001).
Variable doses of TOR‐I and CNI
The nine studies comparing variable doses of TOR‐I and CNI included 1509 participants with 744 in the higher dose TOR‐I with reduced dose CNI group and 765 in the lower dose TOR‐I with standard dose CNI group (Bertoni 2011; Cohen 2002; EVEREST 2009; Grinyo 2004; Kahan‐203 1999; Kandaswamy 2005; MacDonald‐302 2001; Russ 2003; Velosa‐212 2001).
Lower versus higher doses of TOR‐I
The thirteen studies of lower versus higher doses of TOR‐I included 3898 participants with 1951 in the lower dose TOR‐I group and 1947 participants in the higher dose TOR‐I group (Hamdy 2005; Kahan‐157 2001; Kahan‐301 2000; Kovarik‐2306 2004; Kovarik‐251 2001; Kramer‐2307 2003; MacDonald‐302 2001; Pascual 2010; Tedesco‐Silva 2003; Tedesco‐Silva 2010; van Hooff 2003; Vitko‐201 2001; Vitko‐TERRA 2004).
Sirolimus versus everolimus
One study (28 participants) compared sirolimus (16 participants) with everolimus (12 participants) (Rostaing 2001).
Excluded studies
See Characteristics of excluded studies.
For the 2019 update, 27 studies (61 reports) were excluded. Seventeen studies were excluded because TOR‐I was commenced seven days or more post transplant. TOR‐I were commenced after day 14 in one study; the remaining 16 studies commenced TOR‐I four weeks or more after study commencement. Six studies were excluded because they: 1) compared early with delayed administration of TOR‐I (two studies); 2) studied steroid withdrawal (one study); 3) compared liquid with tablet formulation of sirolimus (one study); 4) studied the effect of increasing the dose of TOR‐I at one year (one study); or 5) compared increased dose of TOR‐I at three months as TAC ceased (one study). Three studies were excluded because it was unclear whether they were RCTS and one study was terminated because of inability to recruit participants.
Risk of bias in included studies
Risk of bias attributes are summarised for all studies in Figure 2 and Figure 3. Risk of bias attributes are reported for each of the five groups of comparisons below
2.
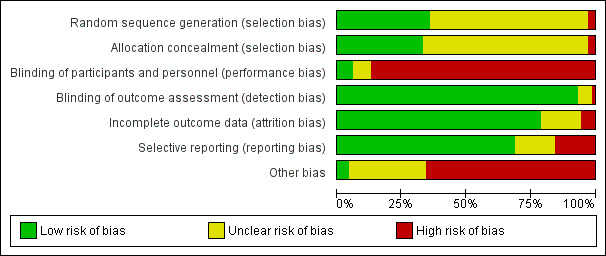
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
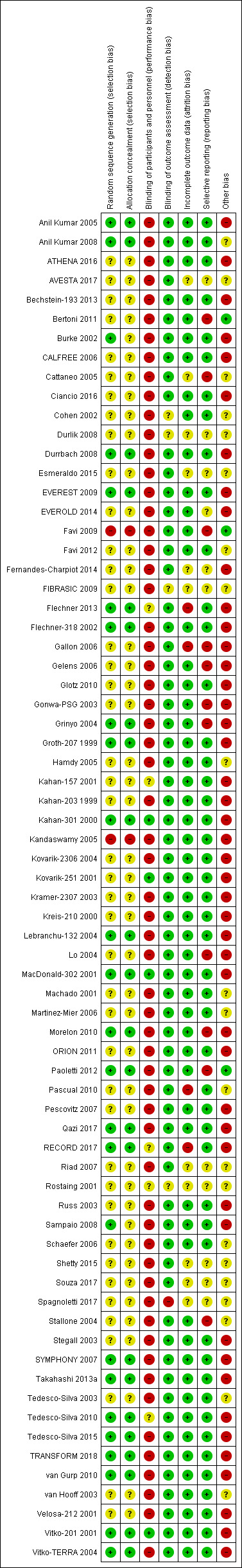
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
TOR‐1 versus calcineurin inhibitor
Of 22 studies, 14 were at low risk for sequence generation and allocation concealment. The remaining seven were at high risk of bias for both sequence generation and random allocation concealment.
TOR‐I versus antimetabolite
Of 33 studies, 14 were at low risk of bias for sequence generation and 18 were at unclear risk. Twelve comparisons were at low risk of bias for allocation concealment and 20 studies were at unclear risk. Two comparisons were at high risk of sequence generation and allocation concealment (Favi 2009; Kandaswamy 2005).
Variable dosage of TOR‐I and calcineurin inhibitor
Of nine studies, two comparisons were at low risk of bias for sequence generation and allocation concealment (EVEREST 2009; Grinyo 2004), one was at high risk of bias (Kandaswamy 2005) while six studies were at unclear risk.
Lower versus higher doses of TOR‐1
Of 13 studies, five were at low risk of bias and the remaining eight studies were assessed as unclear for sequence generation and allocation concealment.
Sirolimus versus everolimus
Rostaing 2001 was judged to be at unclear risk of bias for both sequence generation and allocation concealment.
Blinding
TOR‐1 versus calcineurin inhibitor
Twenty studies were at high risk of bias for performance bias and one study was assessed as unclear (Flechner 2013).
All studies were assessed as at low risk for detection bias as the primary outcomes (GFR and/or biopsy‐proven acute rejection) were laboratory based and unlikely to be influenced by detection bias.
TOR‐I versus antimetabolite
Three studies were at low risk of performance bias (Kahan‐301 2000; Kovarik‐251 2001; Vitko‐201 2001), 29 comparisons were at high risk and one comparison was at unclear risk (RECORD 2017).
In most comparisons, the primary outcomes were laboratory based so were considered unlikely to be influenced by detection bias. Thirty‐two studies were at low risk and one study was at unclear risk of detection bias (Durlik 2008).
Variable dosage of TOR‐I and calcineurin inhibitor
All nine studies were at high risk of performance bias.
In most comparisons, the primary outcomes were laboratory based so were considered unlikely to be influenced by detection bias. Eight studies were at low risk while one study (Cohen 2002) was at unclear risk of detection bias.
Lower versus higher doses of TOR‐I
Four comparisons were assessed at low risk of performance bias (Kahan‐301 2000; Kovarik‐251 2001; MacDonald‐302 2001; Vitko‐201 2001), nine studies were at high risk of bias and two was assessed as at unclear risk (Kahan‐157 2001; Tedesco‐Silva 2010).
All studies were assessed at low risk of detection bias as the primary outcomes were laboratory based and unlikely to be influenced by detection bias.
Sirolimus versus everolimus
Rostaing 2001 was judged to be at unclear risk of bias for both performance and detection bias.
Incomplete outcome data
TOR‐1 versus calcineurin inhibitor
Seventeen studies were considered at low risk of attrition bias, with four at unclear risk (Cattaneo 2005; Durlik 2008; FIBRASIC 2009; Riad 2007) and one at high risk of bias (Flechner 2013).
TOR‐I versus antimetabolite
Twenty‐six comparisons were considered to be at low risk of attrition bias while two were at high risk (Gallon 2006: RECORD 2017) and five were at unclear risk (AVESTA 2017; Esmeraldo 2015; Shetty 2015; Souza 2017; Spagnoletti 2017).
Variable dosage of TOR‐I and CNI
Eight studies were considered to be at low risk of attrition bias while one study (Russ 2003) was at high risk.
Lower versus higher doses of TOR‐I
Twelve studies were considered to be at low risk of attrition bias while one study was at high risk (Pascual 2010).
Sirolimus versus everolimus
Rostaing 2001 was judged to be at unclear risk of bias for attrition bias.
Selective reporting
TOR‐1 versus calcineurin inhibitor
Fourteen studies were considered at low risk of bias for selective reporting, with three assessed as at high risk of bias (Cattaneo 2005; Gelens 2006; Morelon 2010) and the remaining five assessed as at unclear risk (Durlik 2008; EVEROLD 2014; Fernandes‐Charpiot 2014; FIBRASIC 2009; Riad 2007).
TOR‐I versus antimetabolite
Twenty‐one studies were considered to be at low risk of attrition bias while seven were at high risk (Bertoni 2011; Favi 2009; Gallon 2006; Gelens 2006; Gonwa‐PSG 2003; Paoletti 2012; Stallone 2004) and five studies were at unclear risk (AVESTA 2017; Esmeraldo 2015; Shetty 2015; Souza 2017; Spagnoletti 2017).
Variable dosage of TOR‐I and calcineurin inhibitor
Nine studies were considered to be at low risk of reporting bias.
Lower versus higher doses of TOR‐I
All 13 studies were considered to be at low risk of reporting bias
Sirolimus versus everolimus
Rostaing 2001 was judged to be at unclear risk of bias for selection bias.
Other potential sources of bias
TOR‐1 versus CNI
Sixteen studies were industry funded studies and assessed as high risk of bias and the remaining six studies were assessed as unclear (Cattaneo 2005; Durlik 2008; FIBRASIC 2009; Martinez‐Mier 2006; Riad 2007; Schaefer 2006).
TOR‐I versus antimetabolite
Three studies were at low risk (Bertoni 2011; Favi 2009; Paoletti 2012) and 22 studies reporting on industry funded studies were considered to be at high risk of bias. Eight studies did not report funding sources and were considered to be at unclear risk of bias (Anil Kumar 2008; Esmeraldo 2015; Favi 2012; Machado 2001; Shetty 2015; Souza 2017; Spagnoletti 2017; Stallone 2004).
Variable dosage of TOR‐I and CNI
Eight studies reporting on industry funded studies were considered to be at high risk while one study (Cohen 2002) was at unclear risk as it did not report funding sources.
Lower versus higher doses of TOR‐I
Nine studies reported industry funding and were assessed at high risk of bias.
Sirolimus versus everolimus
Rostaing 2001 was judged to be at unclear risk of bias as it did not report funding sources.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Summary of findings for the main comparison. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): main outcomes for primary immunosuppression in kidney transplant recipients.
| TOR‐I versus CNI: outcomes up to 2 years (main outcomes) for primary immunosuppression in kidney transplant recipients | |||||
|
Patient or population: primary immunosuppression in kidney transplant recipients
Setting: kidney transplant services Intervention: TOR‐I Comparison: CNI | |||||
| Outcomes (up to 2 years for primary outcomes) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with CNI | Risk with TOR‐I | ||||
| Death (all causes) | 25 per 1,000 | 33 per 1,000 (22 to 50) | RR 1.31 (0.87 to 1.98) | 3618 (19) | ⊕⊕⊕⊝ MODERATE 1 |
| Graft loss censored for death | 51 per 1,000 | 68 per 1,000 (49 to 93) | RR 1.32 (0.96 to 1.81) | 3277 (14) | ⊕⊕⊕⊝ MODERATE 1 |
| Biopsy‐proven acute rejection | 196 per 1,000 | 333 per 1,000 (258 to 429) | RR 1.70 (1.32 to 2.19) | 3309 (15) | ⊕⊕⊕⊝ MODERATE1 |
| CMV infection | 157 per 1,000 | 68 per 1,000 (46 to 99) | RR 0.43 (0.29 to 0.63) | 2026 (13) | ⊕⊕⊕⊕ HIGH |
| Adverse wound outcomes: all complications | 77 per 1,000 | 198 per 1,000 (150 to 260) | RR 2.56 (1.94 to 3.36) | 1679 (12) | ⊕⊕⊕⊝ MODERATE 1 |
| All malignancies | 24 per 1,000 | 20 per 1,000 (12 to 35) | RR 0.86 (0.50 to 1.48) | 2584 (10) | ⊕⊕⊕⊝ MODERATE 1 |
| Number needing to change treatment | 132 per 1,000 | 320 per 1,000 (249 to 412) | RR 2.42 (1.88 to 3.11) | 3148 (14) | ⊕⊕⊕⊝ MODERATE 1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; CMV: cytomegalovirus | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 few events leading to wide confidence intervals
Summary of findings 2. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes for primary immunosuppression in kidney transplant recipients.
| TOR‐I versus CNI: outcomes up to two years (secondary outcomes) for primary immunosuppression in kidney transplant recipients | |||||
|
Patient or population: primary immunosuppression in kidney transplant recipients
Setting: kidney transplant services Intervention: TOR‐I Comparison: CNI: outcomes up to two years (secondary outcomes) | |||||
| Outcomes (up to 2 years for secondary outcomes) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with CNI | Risk with TOR‐I | ||||
| New‐onset diabetes mellitus | 60 per 1,000 | 56 per 1,000 (42 to 76) | RR 0.93 (0.69 to 1.26) | 2791 (13) | ⊕⊕⊕⊝ MODERATE 1 |
| Lymphoma/PTLD | 2 per 1,000 | 6 per 1,000 (2 to 19) | RR 2.47 (0.78 to 7.86) | 2537 (8) | ⊕⊕⊕⊝ MODERATE 1 |
| Tremor | 204 per 1,000 | 51 per 1,000 (31 to 83) | RR 0.25 (0.15 to 0.41) | 799 (6) | ⊕⊕⊕⊕ HIGH |
| GFR (mL/min) | The mean GFR was 2.2 mL/min higher with TOR‐I (1.29 lower to 5.68 higher) than CNI |
‐‐ | 2983 (15) | ⊕⊕⊝⊝ LOW 2 3 | |
| Cholesterol (mmol/L) | The mean cholesterol level was 0.77 mmol/L higher with TOR‐I (0.45 higher to 1.09 higher) than CNI |
‐‐ | 579 (7) | ⊕⊕⊝⊝ LOW 1 2 | |
| Triglycerides (mmol/L) | The mean triglyceride level 0.57 mmol/L higher with TOR‐I (0.28 higher to 0.86 higher) than CNI |
‐‐ | 843 (8) | ⊕⊕⊝⊝ LOW 1 2 | |
| Thrombocytopenia | 38 per 1,000 | 200 per 1,000 (109 to 367) | RR 5.26 (2.87 to 9.63) | 593 (4) | ⊕⊕⊝⊝ LOW 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; PTLD: post‐transplant lymphoproliferative disease; GRF: glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Small studies/ few events with wide confidence intervals
2 Unexplained heterogeneity
Summary of findings 3. Target of rapamycin inhibitors (TOR‐I) versus antimetabolites: primary outcomes for primary immunosuppression in kidney transplant recipients.
| TOR‐I versus antimetabolites: outcomes up to 2 years (primary outcomes) for primary immunosuppression in kidney transplant recipients | |||||
| Patient or population: primary immunosuppression in kidney transplant recipients Setting: kidney transplant services Intervention: TOR‐I Comparison: antimetabolites | |||||
| Outcomes (up to 2 years for primary outcomes) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with antimetabolites | Risk with TOR‐I | ||||
| Death (all causes) | 29 per 1,000 | 31 per 1,000 (24 to 38) | RR 1.06 (0.84 to 1.33) | 10,482 (31) | ⊕⊕⊕⊝ MODERATE 1 |
| Graft loss (censored) | 35 per 1,000 | 38 per 1,000 (29 to 51) | RR 1.09 (0.82 to 1.45) | 8966 (26) | ⊕⊕⊕⊝ MODERATE 1 |
| Biopsy‐proven acute rejection | 141 per 1,000 | 134 per 1,000 (113 to 158) | RR 0.95 (0.81 to 1.12) | 10,101 (24) | ⊕⊕⊕⊝ MODERATE 2 |
| CMV infection | 136 per 1,000 | 59 per 1,000 (46 to 78) | RR 0.44 (0.34 to 0.58) | 10,049 (26) | ⊕⊕⊕⊝ MODERATE 2 |
| Adverse wound outcomes: all complications | 155 per 1,000 | 241 per 1,000 (199 to 297) | RR 1.56 (1.28 to 1.90) | 6913 (17) | ⊕⊕⊕⊝ MODERATE 2 |
| All malignancies | 34 per 1,000 | 28 per 1,000 (22 to 36) | RR 0.83 (0.64 to 1.07) | 8799 (17) | ⊕⊕⊕⊝ MODERATE 1 |
| Number needing to change treatment | 174 per 1,000 | 248 per 1,000 (203 to 302) | (RR 1.56, 1.28 to 1.90) | 9747 (25) | ⊕⊕⊕⊝ MODERATE 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; CMV: cytomegalovirus | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Few events leading to wide confidence intervals
2 Significant heterogeneity present
Summary of findings 4. Target of rapamycin inhibitors (TOR‐I) versus antimetabolites: secondary outcomes for primary immunosuppression in kidney transplant recipients.
| TOR‐I compared to antimetabolites: outcomes to 2 years (secondary outcomes) for primary immunosuppression in kidney transplant recipients | |||||
| Patient or population: primary immunosuppression in kidney transplant recipients Setting: kidney transplant units Intervention: TOR‐I Comparison: antimetabolites | |||||
| Outcomes (up to 2 years for secondary outcomes) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with antimetabolites | Risk with TOR‐I | ||||
| New‐onset diabetes mellitus | 85 per 1,000 | 103 per 1,000 (86 to 124) | RR 1.28, (1.07 to 1.54) | 8728 (23) | ⊕⊕⊕⊝ MODERATE 1 |
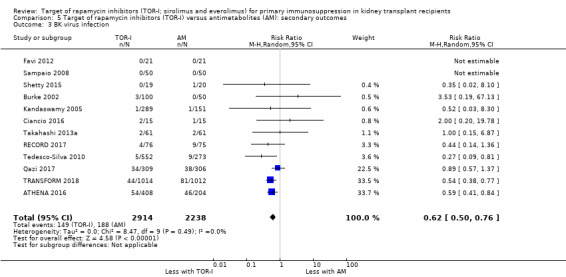
| BK virus infection | 84 per 1,000 | 52 per 1,000 (42 to 64) | RR 0.62 (0.50 to 0.76) | 5152 (12) | ⊕⊕⊕⊕ HIGH |
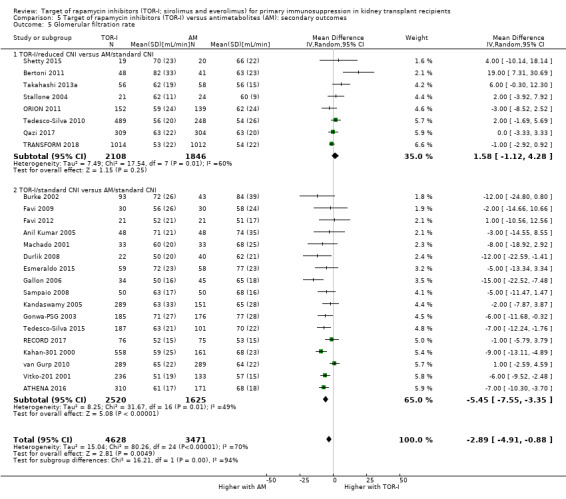
| GFR (mL/min) | The mean GFR was 2.89 mL/min lower with TOR‐I (4.91 lower to 0.88 lower) than with antimetabolites | ‐‐ | 7099 (25) | ⊕⊕⊕⊝ MODERATE 2 | |
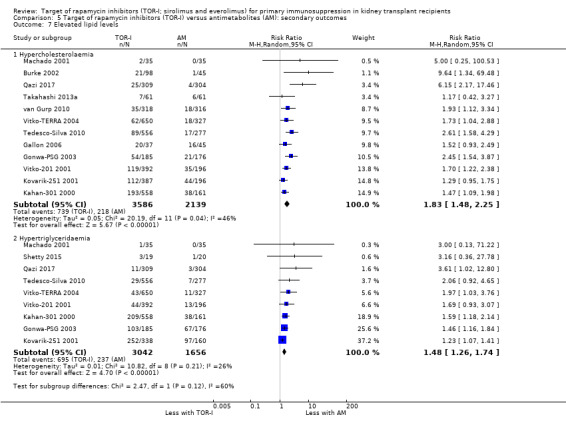
| Hypercholesterolaemia | 102 per 1,000 | 187 per 1,000 (151 to 229) | RR 1.83 (1.48 to 2.25) | 5725 (12) | ⊕⊕⊝⊝ LOW 1 2 |
| Hypertriglyceridaemia | 143 per 1,000 | 212 per 1,000 (180 to 249) | RR 1.48 (1.26 to 1.74) | 4698 (9) | ⊕⊕⊕⊝ MODERATE1 |
| Leucopenia | 123 per 1,000 | 50 per 1,000 (38 to 65) | RR 0.43 (0.33 to 0.56) | 8396 (15) | ⊕⊕⊝⊝ LOW 1 2 |
| Thrombocytopenia | 33 per 1,000 | 65 per 1,000 (46 to 92) | RR 1.96 (1.38 to 2.79) | 5028 (8) | ⊕⊕⊝⊝ LOW 1 3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; GFR: glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Funnel plot shows few studies reporting participants without events suggesting publication bias
2 Significant heterogeneity between studies
3 Few events with wide confidence intervals
Summary of findings 5. Variable target of rapamycin inhibitor (TOR‐I) and calcineurin inhibitor (CNI): primary outcomes for primary immunosuppression in kidney transplant recipients.
| Variable TOR‐I and CNI: primary outcomes for primary immunosuppression in kidney transplant recipients | |||||
| Patient or population: primary immunosuppression in kidney transplant recipients Setting: kidney transplant centres Intervention: lower dose TOR‐I and standard CNI Comparison: higher dose TOR‐I and reduced CNI | |||||
| Outcomes (up to 2 years for primary outcomes) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with higher dose TOR‐I | Risk with low dose TOR‐I | ||||
| Death (all causes) | 39 per 1,000 | 41 per 1,000 (25 to 69) | RR 1.07 (0.64 to 1.78) | 1501 (9) | ⊕⊕⊕⊝ MODERATE 1 |
| Graft loss (censored) | 36 per 1,000 | 39 per 1,000 (19 to 79) | RR 1.09 (0.54 to 2.20) | 1385 (8) | ⊕⊕⊕⊝ MODERATE 1 |
| Biopsy‐proven acute rejection | 155 per 1,000 | 135 per 1,000 (104 to 175) | RR 0.87 (0.67 to 1.13) | 1381 (8) | ⊕⊕⊕⊝ MODERATE 1 |
| CMV infection | 40 per 1,000 | 57 per 1,000 (32 to 105) | RR 1.42 (0.78 to 2.60) | 865 (5) | ⊕⊕⊕⊝ MODERATE 2 |
| Adverse wound outcomes: all complications | 135 per 1,000 | 128 per 1,000 (72 to 231) | RR 0.95 (0.53 to 1.71) | 291 (3) | ⊕⊕⊝⊝ LOW 3 |
| All malignancies | 15 per 1,000 | 16 per 1,000 (5 to 46) | RR 1.04 (0.36 to 3.04) | 1163 (7) | ⊕⊕⊝⊝ LOW 1 |
| Number needing to change treatment | 186 per 1,000 | 219 per 1,000 (108 to 450) | RR 1.18 (0.58 to 2.42) | 734 (5) | ⊕⊕⊝⊝ LOW 4 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; CMV: cytomegalovirus | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Few events leading to wide confidence intervals
2 Few events in only five studies; wide confidence intervals
3 Only reported in three studies; wide confidence intervals
4 Significant heterogeneity
Summary of findings 6. Variable target of rapamycin inhibitor (TOR‐I) and calcineurin inhibitor (CNI): secondary outcomes for primary immunosuppression in kidney transplant recipients.
| Variable TOR‐I and CNI: secondary outcomes for primary immunosuppression in kidney transplant recipients | |||||
| Patient or population: primary immunosuppression in kidney transplant recipients Setting: kidney transplant centres Intervention: lower dose TOR‐I and standard CNI Comparison: higher dose TOR‐I and reduced CNI | |||||
| Outcomes (up to 2 years for secondary outcomes) | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with higher dose TOR‐I | Risk with lower dose TOR‐I | ||||
| New‐onset diabetes mellitus: TAC | 57 per 1,000 | 102 per 1,000 (56 to 183) | RR 1.79 (0.99 to 3.23) | 580 (5) | ⊕⊕⊝⊝ LOW 1 2 |
| New‐onset diabetes mellitus: CSA | 63 per 1,000 | 36 per 1,000 (17 to 75) | RR 0.57 (0.27 to 1.20) | 606 (3) | ⊕⊕⊝⊝ LOW 1 2 |
| GFR (mL/min) | The mean GFR was 5.96 mL/min lower with low dose TOR‐I (9.54 lower to 2.38 lower) compared to higher dose TOR‐I | ‐‐ | 1305 (7) | ⊕⊕⊝⊝ LOW1 3 | |
| Hypercholesterolaemia | 251 per 1,000 | 241 per 1,000 (188 to 307) | RR 0.96 (0.75 to 1.22) | 734 (4) | ⊕⊕⊕⊝ MODERATE 2 |
| Hypertriglyceridaemia | 521 per 1,000 | 443 per 1,000 (380 to 526) | RR 0.85 (0.73 to 1.01) | 734 (4) | ⊕⊕⊕⊝ MODERATE 2 |
| Anaemia | 339 per 1,000 | 315 per 1,000 (271 to 366) | RR 0.93 (0.80 to 1.08) | 1074 (6) | ⊕⊕⊕⊝ MODERATE 2 |
| Leucopenia | 107 per 1,000 | 106 per 1,000 (75 to 150) | RR 0.99 (0.70 to 1.40) | 1012 (5) | ⊕⊕⊕⊝ MODERATE2 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; TAC: tacrolimus; CSA: cyclosporin; GFR: glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Few events leading to wide confidence intervals
2 Over 50% of included studies have unclear sequence generation and allocation concealment
3 Significant heterogeneity
Summary of findings 7. Low versus higher dose target of rapamycin inhibitor (TOR‐I): primary outcomes for primary immunosuppression in kidney transplant recipients.
| Low versus higher dose TOR‐I: primary outcomes for primary immunosuppression in kidney transplant recipients | |||||
| Patient or population: primary immunosuppression in kidney transplant recipients Setting: kidney transplant centres Intervention: lower dose TOR‐I Comparison: higher dose TOR‐I | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with higher dose TOR‐I | Risk with lower dose TOR‐I | ||||
| Death (all causes) | 35 per 1,000 | 31 per 1,000 (22 to 44) | RR 0.89 (0.63 to 1.25) | 3894 (13) | ⊕⊕⊕⊝ MODERATE 1 |
| Total graft loss (with death) | 85 per 1,000 | 72 per 1,000 (57 to 90) | RR 0.84 (0.67 to 1.06) | 3476 (11) | ⊕⊕⊕⊝ MODERATE 1 |
| Biopsy‐proven acute rejection | 179 per 1,000 | 226 per 1,000 (197 to 257) | RR 1.26 (1.10 to 1.43) | 3731 (11) | ⊕⊕⊕⊝ MODERATE 1 |
| CMV infection | 49 per 1,000 | 43 per 1,000 (31 to 60) | RR 0.87 (0.63 to 1.21) | 3099 (9) | ⊕⊕⊕⊝ MODERATE 2 |
| All malignancy | 29 per 1,000 | 24 per 1,000 (15 to 38) | RR 0.84 (0.54 to 1.32) | 3175 (10) | ⊕⊕⊕⊝ MODERATE 1 |
| Number needing to change treatment | 325 per 1,000 | 296 per 1,000 (254 to 341) | RR 0.91 (0.78 to 1.05) | 3652 (10) | ⊕⊕⊝⊝ LOW 1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; CMV: cytomegalovirus | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 few events leading to wide confidence intervals
2 Significant heterogeneity
Summary of findings 8. Low versus higher dose target of rapamycin inhibitor (TOR‐ I): secondary outcomes for primary immunosuppression in kidney transplant recipients.
| Low versus higher dose TOR‐ I: secondary outcomes for primary immunosuppression in kidney transplant recipients | |||||
| Patient or population: primary immunosuppression in kidney transplant recipients Setting: kidney transplant centres Intervention: low dose TOR‐I Comparison: higher dose TOR‐ I | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with higher dose TOR‐ I | Risk with low dose TOR‐I | ||||
| Diabetes | 119 per 1,000 | 82 per 1,000 (61 to 111) | RR 0.69 (0.51 to 0.93) | 2125 (6) | ⊕⊕⊕⊝ MODERATE 1 |
| Lymphoma/PTLD | 9 per 1,000 | 6 per 1,000 (2 to 15) | RR 0.66 (0.25 to 1.73) | 2792 (7) | ⊕⊕⊕⊝ MODERATE 1 |
| Acne/rash | 152 per 1,000 | 131 per 1,000 (95 to 185) | RR 0.86 (0.62 to 1.21) | 2958 (6) | ⊕⊕⊝⊝ LOW 1 2 |
| GRF (mL/min) | The mean GFR was 2.88 mL/min higher with low dose TOR‐I (0.71 lower to 6.48 higher) compared to higher dose TOR‐I | ‐‐ | 1863 (7) | ⊕⊕⊝⊝ LOW 1 3 | |
| Hypercholesterolaemia | 266 per 1,000 | 232 per 1,000 (208 to 261) | RR 0.87 (0.78 to 0.98) | 3250 (9) | ⊕⊕⊕⊝ MODERATE 1 |
| Anaemia | 294 per 1,000 | 238 per 1,000 (212 to 267) | RR 0.81 (0.72 to 0.91) | 3179 (10) | ⊕⊕⊝⊝ LOW 1 3 |
| Thrombocytopenia | 145 per 1,000 | 84 per 1,000 (64 to 109) | RR 0.58 (0.44 to 0.75) | 2242 (9) | ⊕⊕⊝⊝ LOW 1 3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; PTLD: post‐transplant lymphoproliferative disease; GFR: glomerular filtration rate | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 few events leading to wide confidence intervals
2 unexplained heterogeneity
3 over 50% of included studies have unclear sequence generation and allocation concealment
TOR‐1 versus CNI
Primary outcomes
Up to two years post kidney transplant, TOR‐I with an antimetabolite compared to a CNI with an antimetabolite:
Probably makes little or no difference to death (Analysis 1.1 (19 studies, 3618 participants): RR 1.31, 95% CI 0.87 to 1.98; I2 = 0%) (moderate certainty evidence).
Probably increases graft loss uncensored for death (Analysis 1.2 (20 studies, 3619 participants): RR 1.41, 95% CI 1.11 to 1.80; I2 = 0%) and censored for death (Analysis 1.3 (15 studies, 3277 participants): RR 1.32, 95% CI 0.96 to 1.81; I2 = 0%) (moderate certainty of evidence). When graft loss was reported for subgroups according to CNI administered, TOR‐I compared with tacrolimus probably slightly increases graft loss while TOR‐I compared with cyclosporin probably makes little or no difference to graft loss uncensored for death (Analysis 1.2.1; Analysis 1.2.2) or censored for death (Analysis 1.3.1; Analysis 1.3.2).
Probably increases all acute rejection (Analysis 1.4 (19 studies, 3019 participants): RR 1.58, 95% CI 1.30 to 1.91; I2 = 21%) and biopsy‐proven rejection (Analysis 1.5 (15 studies, 2708 participants): RR 1.60, 95% CI 1.25 to 2.04; I2 = 35%) (moderate certainty evidence).
Reduces the risk of CMV infection (Analysis 1.6 (13 studies, 2026 participants): RR 0.43, 95% CI 0.29 to 0.63; I2 = 27%) (high certainty evidence).
Probably increases the risk of all wound complications (Analysis 1.7.1 (12 studies, 1679 participants): RR 2.56, 95% CI 1.94 to 3.36; pI2 = 0%) and of lymphocoele (Analysis 1.7.2 (8 studies, 2538): RR 2.29, 95% CI 1.73 to 3.02; I2 = 0%) (moderate certainty evidence).
Probably increases the need to change immunosuppressive therapy‐related to adverse events (Analysis 1.9 (14 studies, 3148 participants): RR 2.42, 95% CI 1.88 to 3.11; I2 = 52%) (moderate certainty evidence).
Probably makes little or no difference to all malignancies (Analysis 1.8 (10 studies, 2584 participants): RR 0.86, 95% CI 0.50 to 1.48; I2 = 0%) (moderate certainty evidence).
A small substudy of SYMPHONY 2007 involving 156 participants found no difference in health‐related quality of life between participants receiving TOR‐I and those receiving CNI.
1.1. Analysis.
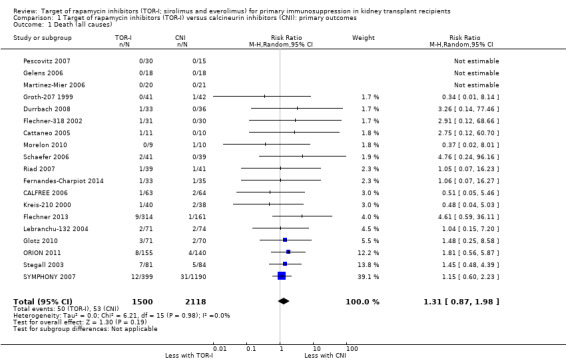
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 1 Death (all causes).
1.2. Analysis.
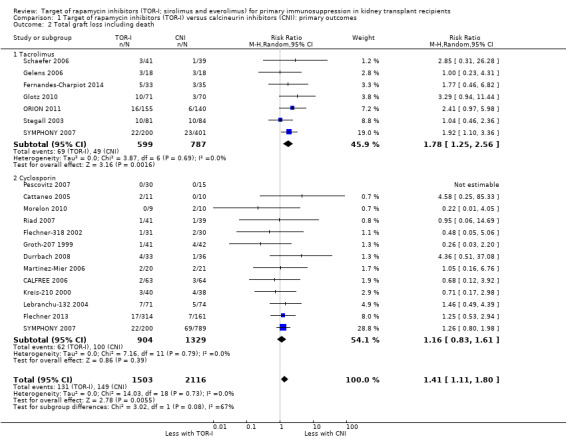
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 2 Total graft loss including death.
1.3. Analysis.
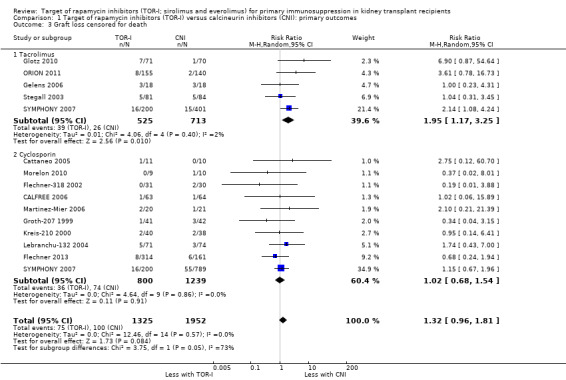
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 3 Graft loss censored for death.
1.4. Analysis.
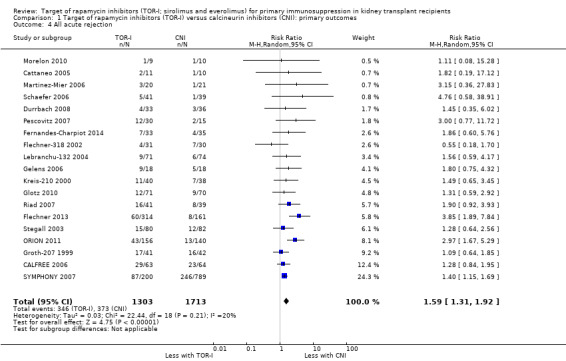
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 4 All acute rejection.
1.5. Analysis.
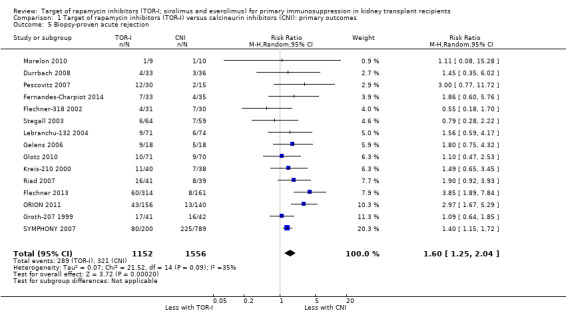
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 5 Biopsy‐proven acute rejection.
1.6. Analysis.
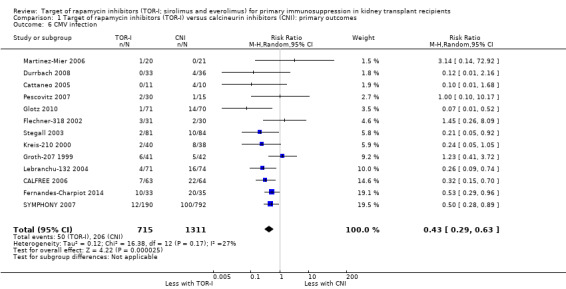
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 6 CMV infection.
1.7. Analysis.
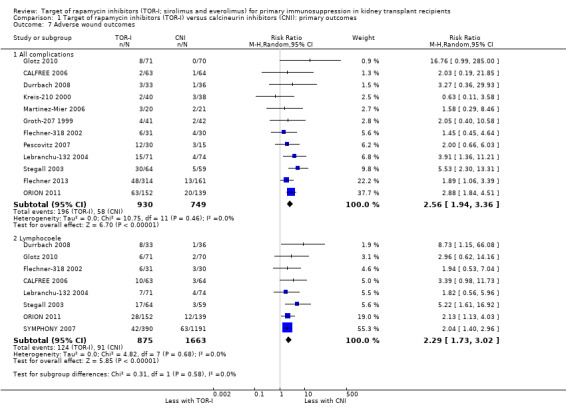
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 7 Adverse wound outcomes.
1.9. Analysis.
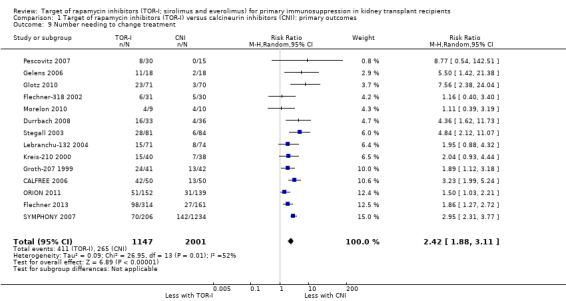
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 9 Number needing to change treatment.
1.8. Analysis.
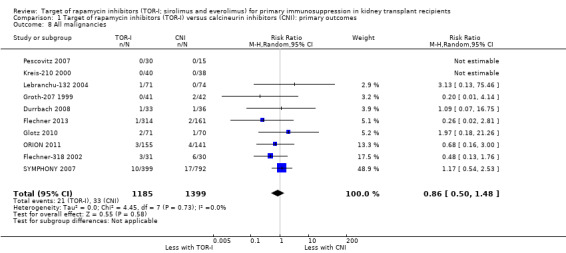
Comparison 1 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes, Outcome 8 All malignancies.
Outcomes were downgraded for imprecision (Table 1).
Secondary outcomes
All outcomes were assessed by GRADE as shown in the results below but only the seven most important outcomes (bold) are included in Table 2,
TOR‐I with an antimetabolite compared with CNI with an antimetabolite:
Probably makes little or no difference in the risk of new‐onset diabetes mellitus (Analysis 2.1 (15 studies, 2791 participants): RR 0.93, 95% CI 0.69 to 1.26; I2 = 0%) regardless of CNI used (Analysis 2.1.1; Analysis 2.1.2) (moderate certainty of evidence).
Probably makes little or no difference to the risk for lymphoma/PTLD (Analysis 2.2 (8 studies, 2537 participants): RR 2.47, 95% CI 0.78 to 7.86; I2 = 0%) (moderate certainty of evidence).
May make little or no difference to the risk for BK virus infection (Analysis 2.3 (3 studies, 386 participants): RR 0.46, 95% CI 0.16 to 1.29; I2 = 0%) (low certainty evidence).
Reduces the risk of adverse cosmetic outcomes including tremor (Analysis 2.4.1 (6 studies, 799 participants): RR 0.25, 95% CI 0.15 to 0.41; I2 = 0%) (high certainty evidence) and may make little or no difference to hirsutism (Analysis 2.4 (1 study, 78 participants): RR 0.24, 95% CI 0.03 to 2.03; I2 = 0%) (low certainty evidence).
Probably slightly reduces serum creatinine (Analysis 2.6 (10 studies, 672 participants): MD ‐10.64 µmol/L, 95% CI ‐19.19 to ‐2.10; I2 = 34%) and may increase GFR (Analysis 2.5 (15 studies, 2983 participants: MD 2.20 mL/min, 95% CI ‐1.29 to 5.68; I2 = 74%) (low certainty evidence).
It is uncertain whether TOR‐I increases the number of participants with elevated cholesterol levels (Analysis 2.7.1 (4 studies, 1877 participants): RR 1.74, 95% CI 1.17 to 2.59; I2 = 51%) because the evidence is very uncertain but may increase the number of participants with elevated triglyceride levels (Analysis 2.7.2 (5 studies, 1922 participants): RR 1.72, 95% CI 1.20 to 2.46; I2 = 39%) (low certainty evidence).
May increase the mean levels of cholesterol (Analysis 2.8.1 (7 studies, 579 participants): MD 0.77 mmol/L, 95% CI 0.45 to 1.09; I2 = 56%) (low certainty evidence) and may increase the mean levels of triglycerides (Analysis 2.8.2 (8 studies, 853 participants): MD 0.57 mmol/L, 95% CI 0.28 to 0.86; I2 = 63%).
May increase the number of participants with anaemia (Analysis 2.9.1 (6 studies, 2216 participants): RR 1.47, 95% CI 1.28 to 1.70; I2 = 0%) (low certainty evidence), leucopenia (Analysis 2.9.2 (5 studies, 1922 participants): RR 1.52, 95% CI 0.95 to 2.44; I2 = 50%) or thrombocytopenia (Analysis 2.9.3 (4 studies, 592 participants): RR 5.26, 95% CI 2.87 to 9.63; I2 = 0%) (low certainty evidence).
2.1. Analysis.
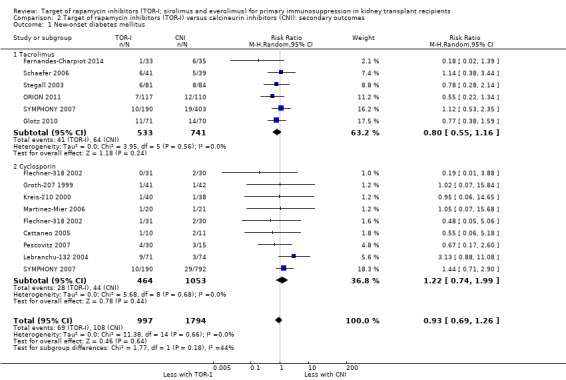
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 1 New‐onset diabetes mellitus.
2.2. Analysis.
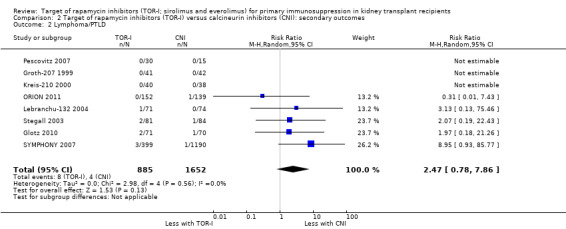
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 2 Lymphoma/PTLD.
2.3. Analysis.
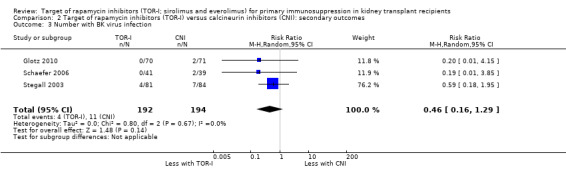
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 3 Number with BK virus infection.
2.4. Analysis.
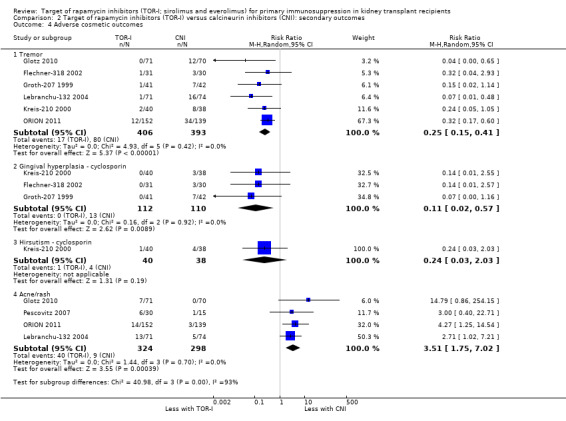
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 4 Adverse cosmetic outcomes.
2.6. Analysis.
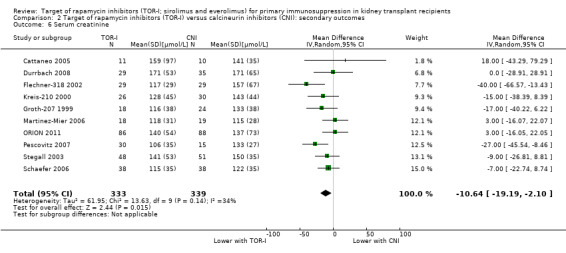
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 6 Serum creatinine.
2.5. Analysis.
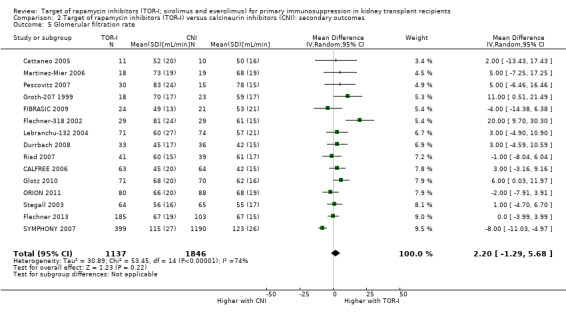
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 5 Glomerular filtration rate.
2.7. Analysis.
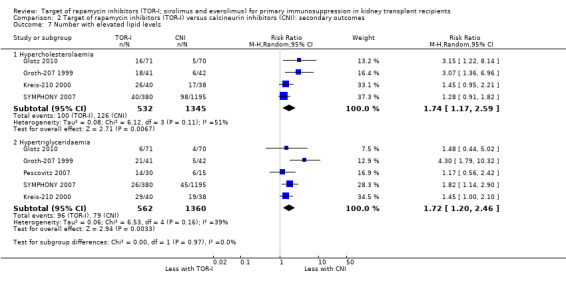
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 7 Number with elevated lipid levels.
2.8. Analysis.
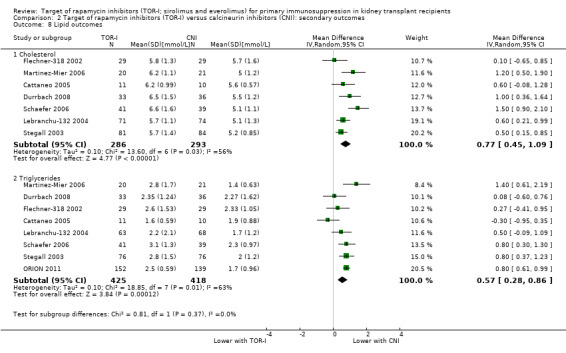
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 8 Lipid outcomes.
2.9. Analysis.
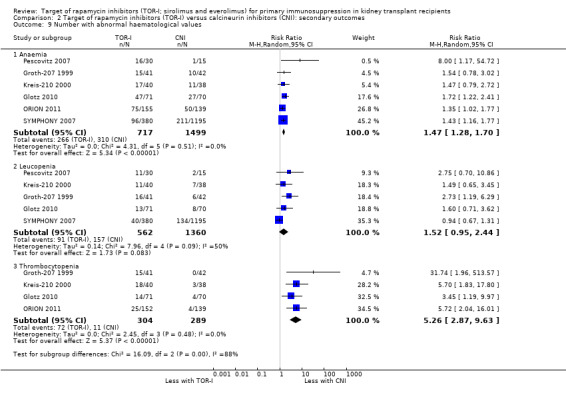
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 9 Number with abnormal haematological values.
Outcomes were downgraded for heterogeneity and imprecision (Table 2).
Longer term follow‐up
Two studies (Flechner‐318 2002; Lebranchu‐132 2004) reported outcomes at five and three years respectively. TOR‐I compared with CNI may make little or no difference to the number dying (Analysis 6.1), the number with graft loss (overall (Analysis 3.2) and censored for death with a functioning graft (Analysis 3.3)) and malignancies (Analysis 3.4). It is uncertain whether TOR‐I compared with CNI increases GFR because the certainty of the evidence is very low (Analysis 3.5 (2 studies, 163 participants): MD 13.51 mL/min, 95% CI 6.94 to 20.08; I2 = 65%)
6.1. Analysis.
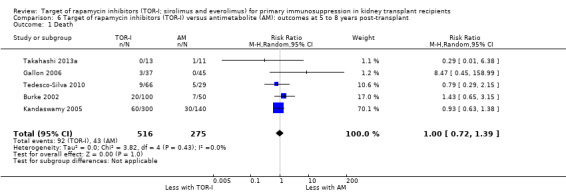
Comparison 6 Target of rapamycin inhibitors (TOR‐I) versus antimetabolite (AM): outcomes at 5 to 8 years post‐transplant, Outcome 1 Death.
3.2. Analysis.
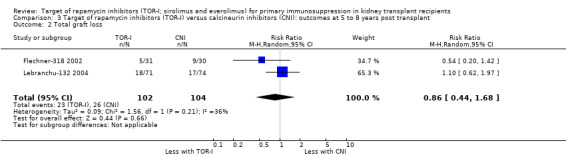
Comparison 3 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): outcomes at 5 to 8 years post transplant, Outcome 2 Total graft loss.
3.3. Analysis.
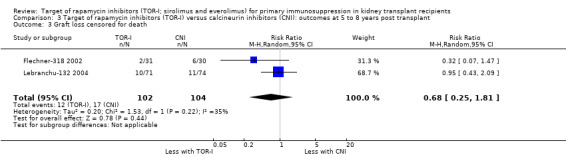
Comparison 3 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): outcomes at 5 to 8 years post transplant, Outcome 3 Graft loss censored for death.
3.4. Analysis.
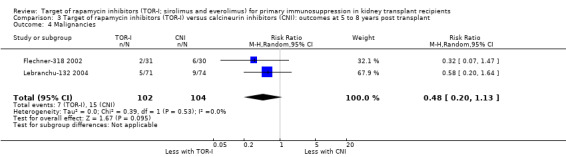
Comparison 3 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): outcomes at 5 to 8 years post transplant, Outcome 4 Malignancies.
3.5. Analysis.
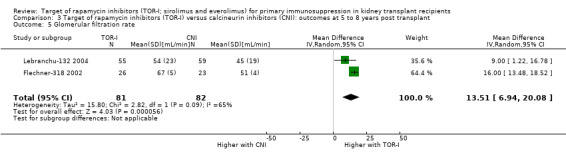
Comparison 3 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): outcomes at 5 to 8 years post transplant, Outcome 5 Glomerular filtration rate.
TOR‐I versus antimetabolite
Primary outcomes
Up to two years post kidney transplant, TOR‐I with CNI compared with an antimetabolite with CNI (Table 3):
Probably makes little or no difference to death (Analysis 4.1 (31 studies, 10,482 participants): RR 1.06, 95% CI 0.84 to 1.33; I2 = 0%) (moderate certainty evidence).
Probably makes little or no difference to graft loss (uncensored) (Analysis 4.2 (27 studies, 7626 participants): RR 1.14, 95% CI 0.93 to 1.40; I2 = 8%) or graft loss (censored for death) (Analysis 4.3 (26 studies, 8966 participants): RR 1.09, 95% CI 0.82 to 1.45; I2 = 25%) (moderate certainty evidence).
Probably makes little or no difference to all acute rejection (Analysis 4.4 (31 studies, 10,075 participants): RR 0.90, 95% CI 0.79 to 1.02; I2 = 35%) or to biopsy‐proven acute rejection (Analysis 4.5 (24 studies, 10,101 participants): RR 0.95, 95% CI 0.81 to 1.12; I2 = 51%) (moderate certainty evidence). In sensitivity analyses for both outcomes, heterogeneity was reduced below 30% by exclusion of ATHENA 2016 and Qazi 2017. These studies showed reduced biopsy‐proven acute rejection with TOR‐I in contrast to other studies, which showed no differences. Subgroup analysis demonstrated that TOR‐I with reduced dose CNI, compared with antimetabolite and CNI, probably makes little or no difference to the number with biopsy‐proven acute rejection (Analysis 4.5)
Probably reduces the risk of CMV infection (Analysis 4.6 (26 studies, 10,049 participants): RR 0.44, 95% CI 0.34 to 0.58; I2 = 68%) (moderate certainty evidence). Heterogeneity of the results may have been due to different reporting of CMV infection and/or disease in different studies.
Probably increases the risk of all wound complications (Analysis 4.7.1 (17 studies, 6913 participants): RR 1.56, 95% CI 1.28 to 1.91; I2 = 59%) and the risk of lymphocoele (Analysis 4.7.2 (16 studies, 8415 participants): RR 1.55, 95% CI 1.32 to 1.81; I2 = 0%) (moderate certainty evidence). Heterogeneity in the risk of all wound complications was reduced by exclusion of ATHENA 2016.
Probably makes little or no difference to the risk of malignancies (Analysis 4.8 (17 studies, 8799 participants): RR 0.83, 95% CI 0.64 to 1.07; I2 = 7%) (moderate certainty evidence).
Probably increases the need to change immunosuppressive treatment because of adverse effects (Analysis 4.9 (25 studies, 9747 participants): RR 1.56, 95% CI 1.28 to 1.90; I2 = 71%) (moderate certainty evidence). Heterogeneity between studies was reduced by exclusion of Anil Kumar 2008, Kahan‐301 2000 and Tedesco‐Silva 2015,which found that TOR‐I were not associated with an increase in the need to change immunosuppressive therapy.
4.1. Analysis.
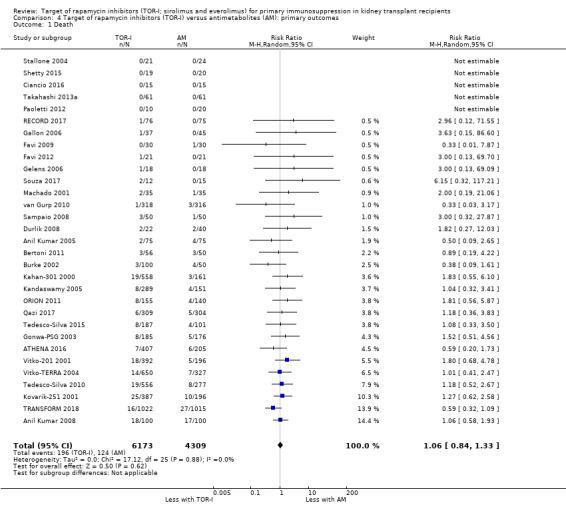
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 1 Death.
4.2. Analysis.
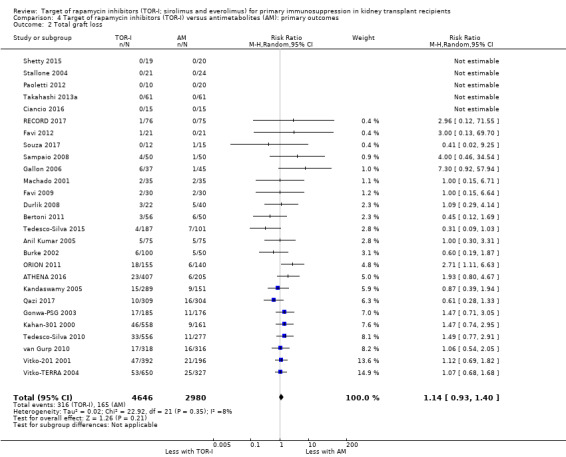
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 2 Total graft loss.
4.3. Analysis.
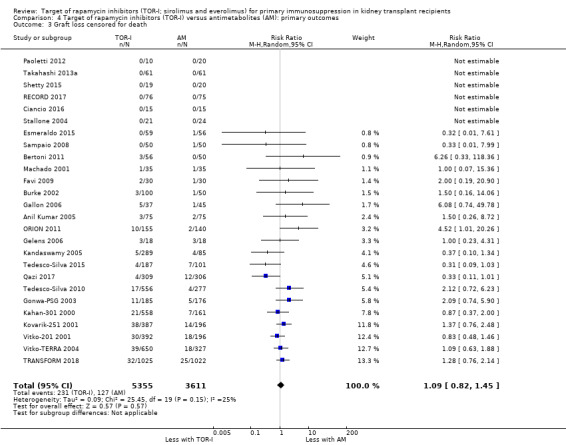
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 3 Graft loss censored for death.
4.4. Analysis.
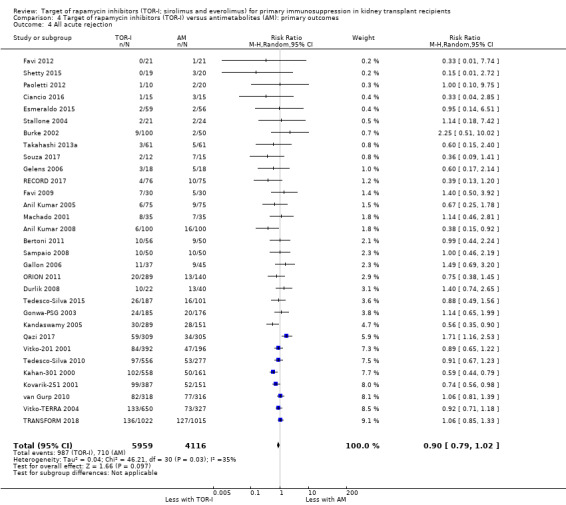
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 4 All acute rejection.
4.5. Analysis.
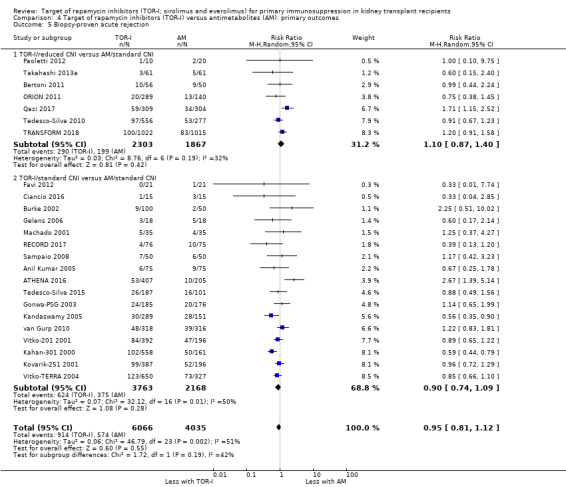
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 5 Biopsy‐proven acute rejection.
4.6. Analysis.

Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 6 CMV infection.
4.7. Analysis.
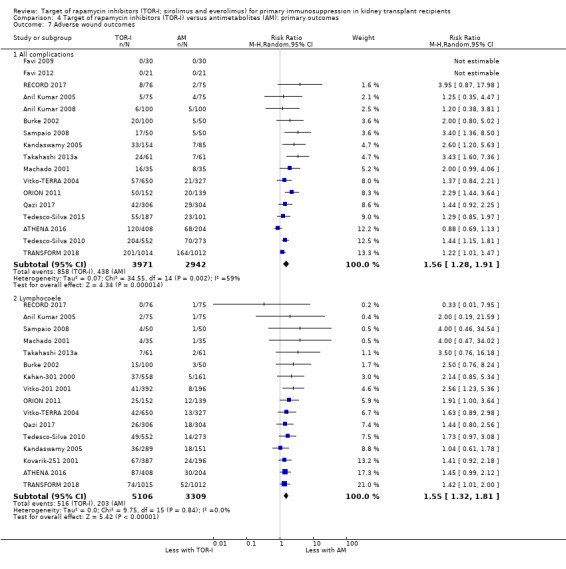
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 7 Adverse wound outcomes.
4.8. Analysis.
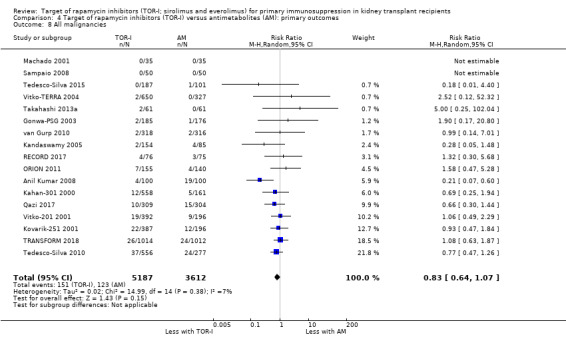
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 8 All malignancies.
4.9. Analysis.
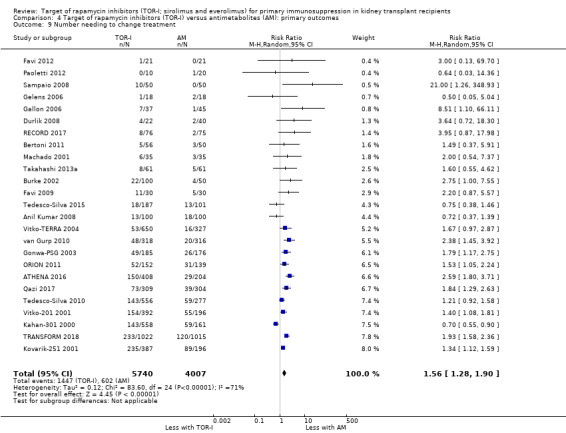
Comparison 4 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes, Outcome 9 Number needing to change treatment.
Outcomes were downgraded for imprecision or heterogeneity (Table 3).
Secondary outcomes
All outcomes were assessed by GRADE as shown in the results below but only the seven most important outcomes (bold) are included in Table 4,
TOR‐I with CNI compared with an antimetabolite with CNI:
Probably increases the risk of new‐onset diabetes mellitus (Analysis 5.1 (23 studies, 8728 participants): RR 1.28, 95% CI 1.07 to 1.54; I2 = 22%) (moderate certainty evidence).
Probably makes little or no difference to the risk of PTLD (Analysis 5.2 (14 studies, 5415 participants): RR 1.52, 95% CI 0.62 to 3.72; I2 = 0%) (moderate certainty evidence).
Reduces the risk of BK virus infection (Analysis 5.3 (12 studies, 5152 participants): RR 0.62, 95% CI 0.50 to 0.76; I2 = 0%) (high certainty evidence).
May make little or no difference to GFR overall (Analysis 5.5 (25 studies, 8099 participants): MD ‐2.89 mL/min, 95% CI ‐4.91 to ‐0.88; I2 = 70%) (low certainty evidence). Subgroup analysis demonstrated that TOR‐I with reduced dose CNI, compared with antimetabolite and CNI, may make little or no difference to GFR (Analysis 5.5.1 (8 studies, 3954 participants): MD 1.58 mL/min (95% CI ‐1.12 to 4.28; I2 = 60%). However TOR‐I with standard dose CNI, compared with antimetabolite and CNI, may lead to a reduction in GFR (Analysis 5.5.2 (17 studies, 4145 participants): MD ‐5.45 mL/min, 95% CI ‐7.55 to ‐3.35; I2 = 49%).
May increase the number of participants with elevated cholesterol levels (Analysis 5.7.1 (12 studies, 5725 participants): RR 1.83, 95% CI 1.48 to 2.25; I2 = 46%) (low certainty evidence) and may increase the number with elevated triglyceride levels (Analysis 5.7.2 (9 studies, 4698 participants): RR 1.48, 95% CI 1.26 to 1.74; I2 = 26%) (low certainty evidence).
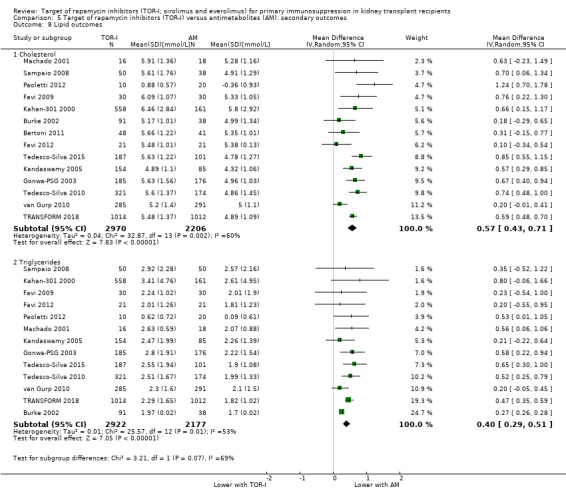
May increase mean levels of cholesterol Analysis 5.8.1 (14 studies, 5176 participants): MD 0.57 mmol/L, 95% CI 0.43 to 0.71; I2 = 60%) and triglycerides (Analysis 5.8.2 (13 studies, 5099 participants): MD 0.40 mmol/L, 95% CI 0.29 to 0.51; I2 = 53%) (low certainty evidence).
May make little or no difference to the number of participants with anaemia (Analysis 5.9.1 (15 studies, 8595 participants): RR 1.06, 95% CI 0.92 to 1.23; I2 = 67%) or to haemoglobin levels (Analysis 5.10.1 (6 studies, 1035 participants): MD ‐0.38 g/dL, 95% CI ‐0.63 to ‐0.12; I2 = 15%) (low certainty evidence).
May reduce the number of participants with leucopenia (Analysis 5.9.2) or may increase the number of participants with thrombocytopenia (Analysis 5.9.3) (low certainty evidence). It is uncertain whether TOR‐I compared with antimetabolite makes any difference to white blood or platelet counts (Analysis 5.10.2; Analysis 5.10.3) because the certainty of the evidence is very low.
May reduce the number of participants with tremor (Analysis 5.4.1 (5 studies, 3803 participants): RR 0.87, 95% CI 0.66 to 1.15; I2 = 62%) and the number with gingival hyperplasia (Analysis 5.4.2 (2 studies, 903 participants): RR 0.30, 95% CI 0.15 to 0.60; I2 = 0%) but increase the number with acne/rash (Analysis 5.4.4 (5 studies, 2022 participants): RR 1.74, 95% CI 1.08 to 2.81; I2 = 67%) (low certainty evidence).
It is uncertain whether TOR‐I compared with antimetabolite makes any difference to the number of participants with hirsutism (Analysis 5.4,3) because the certainty of the evidence is very low.
5.1. Analysis.
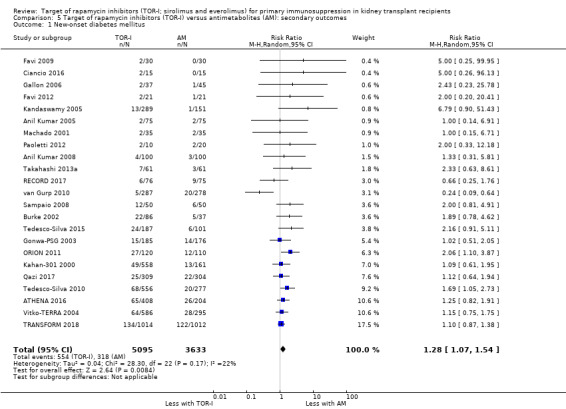
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 1 New‐onset diabetes mellitus.
5.2. Analysis.
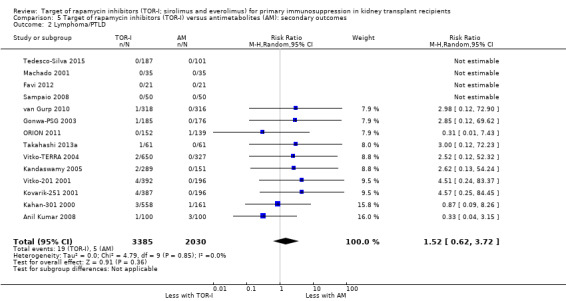
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 2 Lymphoma/PTLD.
5.3. Analysis.
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 3 BK virus infection.
5.5. Analysis.
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 5 Glomerular filtration rate.
5.7. Analysis.
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 7 Elevated lipid levels.
5.8. Analysis.
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 8 Lipid outcomes.
5.9. Analysis.
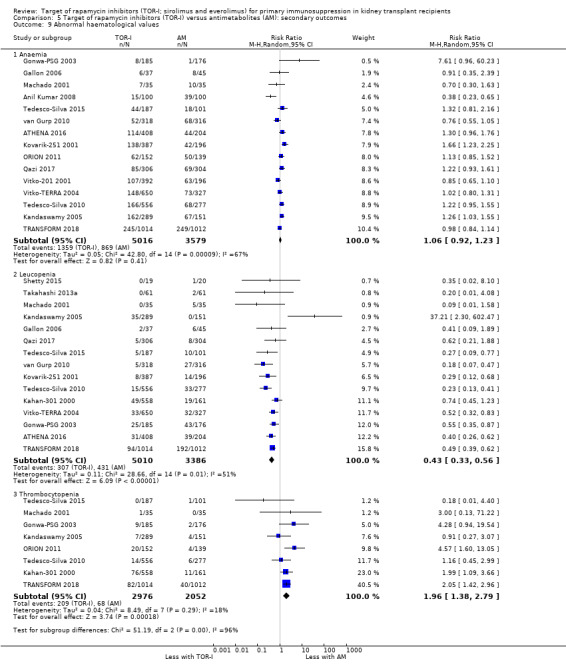
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 9 Abnormal haematological values.
5.10. Analysis.
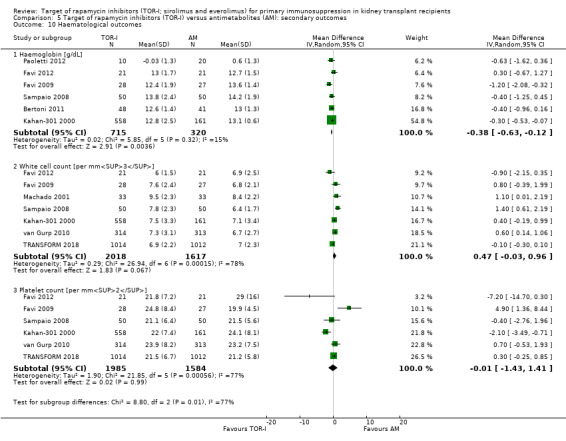
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 10 Haematological outcomes.
5.4. Analysis.
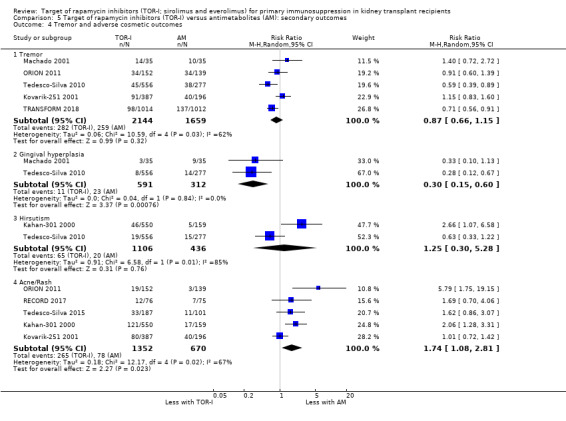
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 4 Tremor and adverse cosmetic outcomes.
Outcomes were downgraded for heterogeneity, imprecision and publication bias (Figure 4) (Table 4).
4.
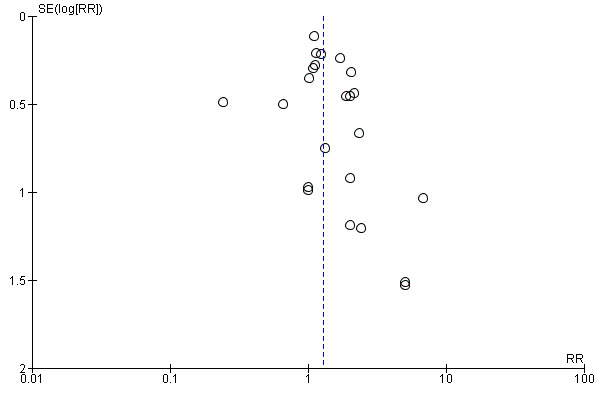
Funnel plot of comparison: 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, outcome: 5.1 New‐onset diabetes mellitus.
Longer term follow‐up
Five studies (Burke 2002; Gallon 2006; Kandaswamy 2005; Takahashi 2013a; Tedesco‐Silva 2010) reported outcomes at five to eight years post‐transplant. Limited data from single centres were available for these meta‐analyses for the multicentre studies of Takahashi 2013a and Tedesco‐Silva 2010. TOR‐I compared with antimetabolite may make little or no difference to the number dying (Analysis 6.1), the number with graft loss overall (Analysis 6.2) and censored for death with a functioning graft (Analysis 6.3) and with malignancies (Analysis 6.4). It is uncertain whether TOR‐I compared with antimetabolites influences GFR (Analysis 6.5). There was significant heterogeneity in the analyses for all outcomes except death. In sensitivity analyses removal of Gallon 2006 abolished the heterogeneity.
6.2. Analysis.
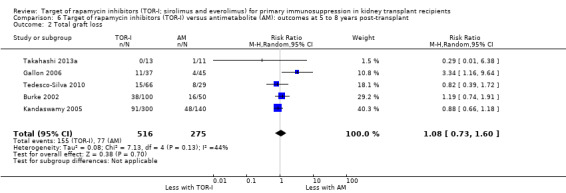
Comparison 6 Target of rapamycin inhibitors (TOR‐I) versus antimetabolite (AM): outcomes at 5 to 8 years post‐transplant, Outcome 2 Total graft loss.
6.3. Analysis.
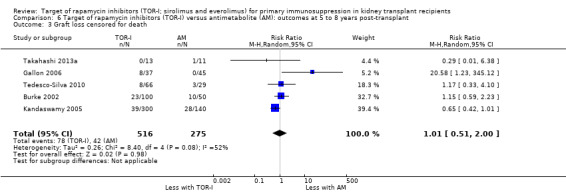
Comparison 6 Target of rapamycin inhibitors (TOR‐I) versus antimetabolite (AM): outcomes at 5 to 8 years post‐transplant, Outcome 3 Graft loss censored for death.
6.4. Analysis.
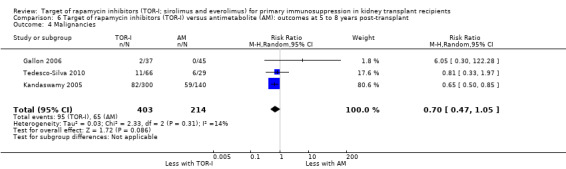
Comparison 6 Target of rapamycin inhibitors (TOR‐I) versus antimetabolite (AM): outcomes at 5 to 8 years post‐transplant, Outcome 4 Malignancies.
6.5. Analysis.
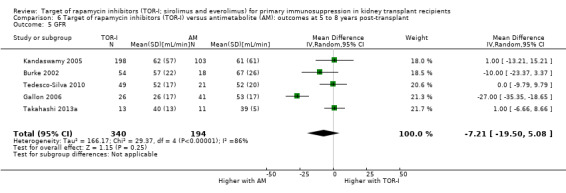
Comparison 6 Target of rapamycin inhibitors (TOR‐I) versus antimetabolite (AM): outcomes at 5 to 8 years post‐transplant, Outcome 5 GFR.
Variable doses of TOR‐I and CNI
Primary outcomes
Lower dose TOR‐I and standard dose CNI compared with higher dose TOR‐I and reduced dose CNI:
Probably makes little or no difference to death (all causes) (Analysis 7.1 (9 studies, 1501 participants): RR 1.07, 95% CI 0.64 to 1.78; I2 = 0%) (moderate certainty evidence).
Probably makes little of no difference to all graft loss (Analysis 7.2 (8 studies, 1385 participants): RR 1.09, 95% CI 0.68 to 1.75; I2 = 21%) and graft loss censored for death (Analysis 7.3 (8 studies, 1385 participants): RR 1.09, 95% CI 0.54 to 2.20; I2 = 25%) (moderate certainty evidence).
Probably makes little or no difference to all acute rejection (Analysis 7.4 (9 studies, 1509 participants): RR 0.84, 95% CI 0.67 to 1.07; I2 = 0%) and biopsy‐proven acute rejection (Analysis 7.5 (8 studies, 1381 participants): RR 0.87, 95% CI 0.67 to 1.13; I2 = 0%) (moderate certainty evidence).
May make little or no difference to CMV infection (Analysis 7.6 (5 studies, 865 participants): RR 1.42, 95% CI 0.78 to 2.60; I2 = 0%) (low certainty evidence).
May make little or no difference to all wound complications (Analysis 7.7.1 (3 studies, 291 participants): RR 0.95, 95% CI 0.53 to 1.71; I2 = 0%) or lymphocoele Analysis 7.7.2 (3 studies, 702 participants): RR 0.86, 95% CI 0.45 to 1.63; I2 = 46%) (low certainty evidence).
May make little or no difference to malignancies (Analysis 7.8 (7 studies, 1163 participants): RR 1.04, 95% CI 0.36 to 3.04; I2 = 0%) (low certainty evidence).
May make little of no difference to the number of participants needing to change treatment (Analysis 7.9 (5 studies, 734 participants): RR 1.18, 95% CI 0.58 to 2.42; I2 = 76%) (low certainty evidence).
7.1. Analysis.
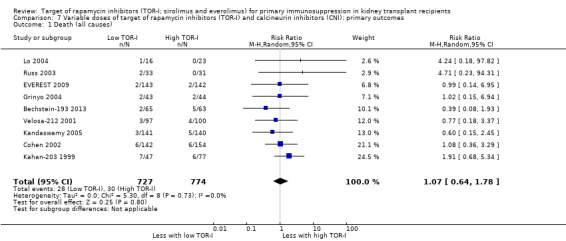
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 1 Death (all causes).
7.2. Analysis.
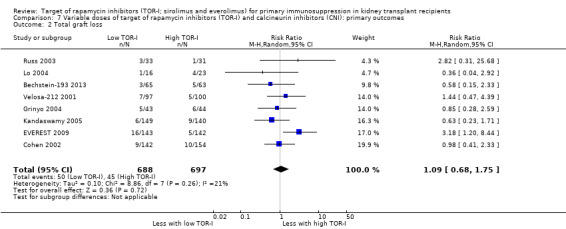
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 2 Total graft loss.
7.3. Analysis.
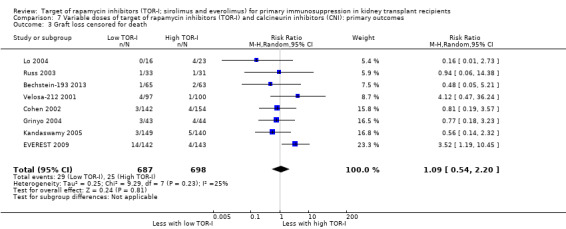
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 3 Graft loss censored for death.
7.4. Analysis.
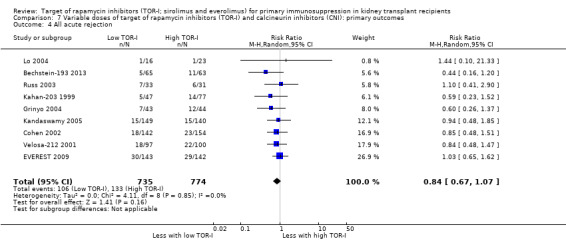
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 4 All acute rejection.
7.5. Analysis.
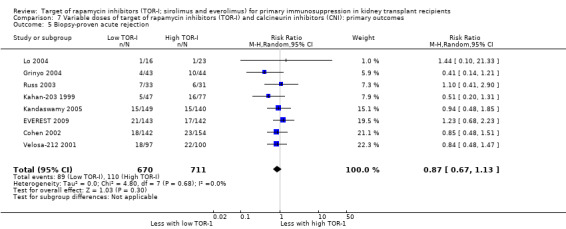
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 5 Biopsy‐proven acute rejection.
7.6. Analysis.
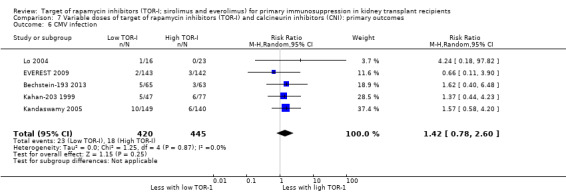
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 6 CMV infection.
7.7. Analysis.
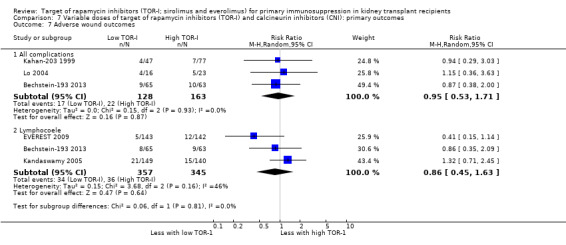
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 7 Adverse wound outcomes.
7.8. Analysis.
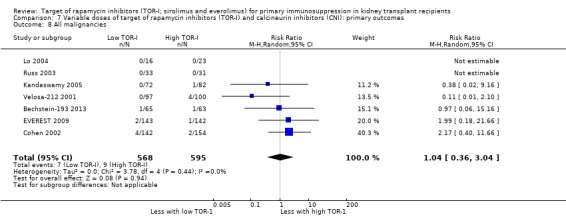
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 8 All malignancies.
7.9. Analysis.
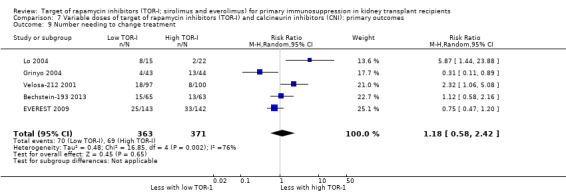
Comparison 7 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes, Outcome 9 Number needing to change treatment.
Outcomes were downgraded for risk of bias issues, imprecision or heterogeneity (Table 5).
Secondary outcomes
All outcomes were assessed by GRADE as shown in the results below but only the seven most important outcomes (bold) are included in Table 6
Lower dose TOR‐I and standard dose CNI compared with higher dose TOR‐I and reduced dose CNI"
May make little or no difference to the risk of new‐onset diabetes mellitus whether participants also received tacrolimus (Analysis 8.1.1 (5 studies, 580 participants): RR 1.79, 95% CI 0.99 to 3.23; I2 = 0%) or cyclosporin (Analysis 8.1.2 (3 studies, 606 participants): RR 0.57, 95% CI 0.27 to 1.20; I2 = 0%) (low certainty evidence).
May make little or no difference to the risk of lymphoma/PTLD (Analysis 8.2 (7 studies, 1298 participants): RR 0.68, 95% CI 0.15 to 3.07; I2 = 0%).
May slightly reduce GFR (Analysis 8.4 (7 studies, 1305 participants): MD ‐5.96 mL/min, 95% CI ‐9.54 to ‐2.38; I2 = 48%) (low certainty evidence) or serum creatinine (Analysis 8.5 (9 studies, 1368 participants). MD 1.53 µmol/L, 95% CI ‐8.82 to 11.89; I2 = 69%) (low certainty evidence).
Probably makes little or no difference to the number of participants with increased cholesterol (Analysis 8.6.1 (4 studies, 734 participants). RR 0.96, 95% CI 0.75 to 1.22; I2 = 0%) or triglyceride levels (Analysis 8.6.2 (4 studies, 734 participants): RR 0.85, 95% CI 0.73 to 1.01; I2 = 16%) (moderate certainty of evidence).
Probably makes little or no difference in the number with anaemia (Analysis 8.8.1 (6 studies, 1074 participants): RR 0.93, 95% CI 0.80 to 1.08; I2 = 0%) (moderate certainty evidence).
Probably makes little or no difference to the number of participants with leucopenia (Analysis 8.8.2 (5 studies, 1012 participants): RR 0.99, 95% CI 0.70 to 1.40; I2 = 0%) (moderate certainty evidence) or thrombocytopenia (Analysis 8.8.3 (5 studies, 888 participants): RR 0.67, 95% CI 0.43 to 1.07; I2 = 0%) (moderate certainty evidence).
It is uncertain whether variable levels of TOR‐I and CNI makes any difference to the number of participants with hirsutism (Analysis 8.3.1); gum hypertrophy (Analysis 8.3.2); mean levels of creatinine (Analysis 8.5), cholesterol (Analysis 8.7.1) and triglycerides (Analysis 8.7.2); or to mean levels of haemoglobin (Analysis 8.9.1), white blood count (Analysis 8.9.2), or platelet count (Analysis 8.9.3) because the certainty of the evidence is very low.
8.1. Analysis.
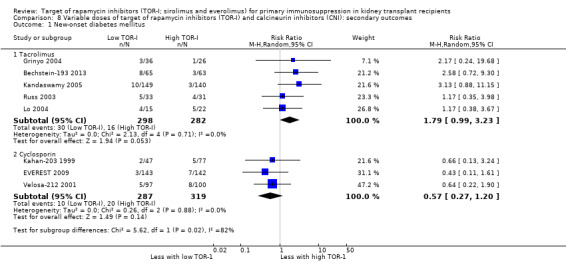
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 1 New‐onset diabetes mellitus.
8.2. Analysis.
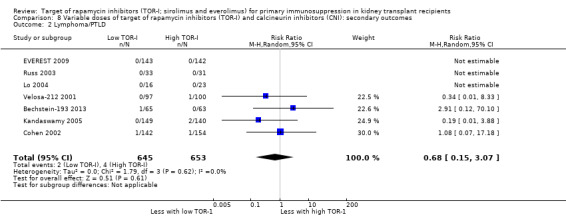
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 2 Lymphoma/PTLD.
8.4. Analysis.
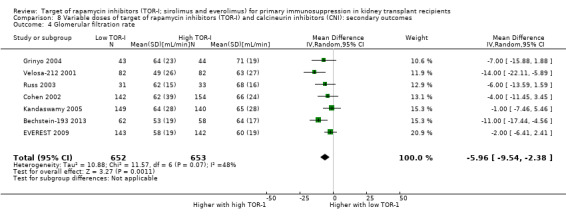
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 4 Glomerular filtration rate.
8.5. Analysis.
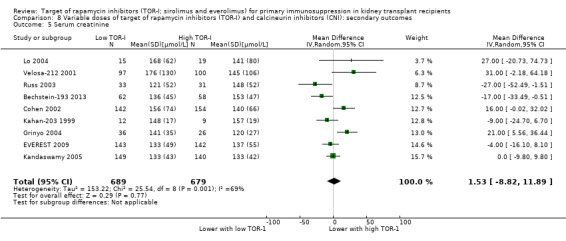
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 5 Serum creatinine.
8.6. Analysis.
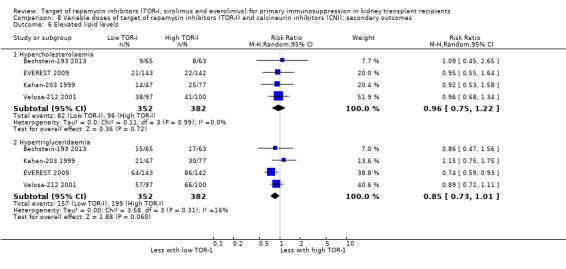
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 6 Elevated lipid levels.
8.8. Analysis.
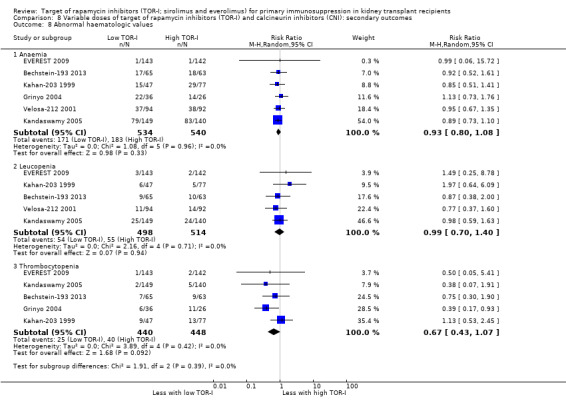
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 8 Abnormal haematologic values.
8.3. Analysis.
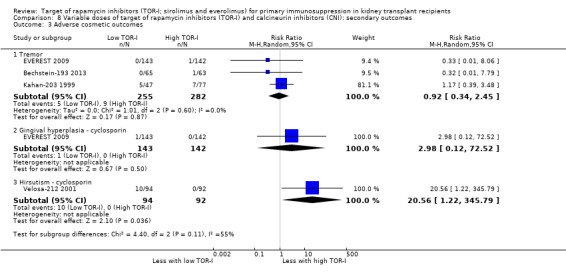
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 3 Adverse cosmetic outcomes.
8.7. Analysis.
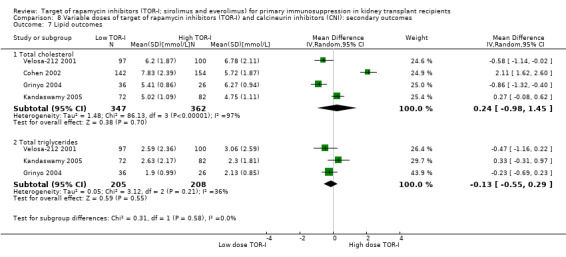
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 7 Lipid outcomes.
8.9. Analysis.
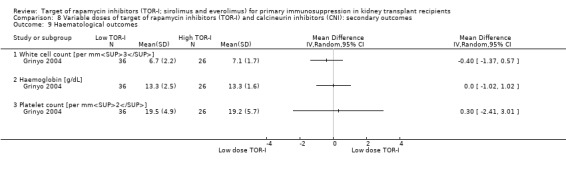
Comparison 8 Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes, Outcome 9 Haematological outcomes.
Outcomes were downgraded for risk of bias issues, heterogeneity and imprecision (Table 6).
Lower versus higher dose of TOR‐I
Primary outcomes
Up to two years post kidney transplant, lower dose TOR‐I with CNI versus higher dose TOR‐I with CNI:
Probably makes little or no difference to death (Analysis 9.1 (13 studies, 3894 participants): RR 0.89, 95% CI 0.63 to 1.25; I2 = 0%) (moderate certainty evidence).
Probably makes little or no difference to overall graft loss (Analysis 9.2 (11 studies, 3476 participants): RR 0.84, 95% CI 0.67 to 1.06; I2 = 0%) and graft loss censored for death (Analysis 9.3 (12 studies, 3863 participants): RR 0.92, 95% CI 0.71 to 1.19; I2 = 0%) (moderate certainty evidence).
Probably slightly increases the risk of acute rejection (Analysis 9.4 (13 studies, 3898 participants): RR 1.25, 95% CI 1.10 to 1.42; I2 = 0%) and biopsy‐proven acute rejection (Analysis 9.5 (11 studies, 3731 participants): RR 1.26, 95% CI 1.10 to 1.43; I2 = 0%) (moderate certainly evidence).
Probably makes little or no difference to the risk of CMV infection (Analysis 9.6 (9 studies, 2099 participants): RR 0.87, 95% CI 0.63 to 1.21; I2 = 0%) (moderate certainty evidence).
Probably makes little or no difference to the risk of malignancy (Analysis 9.7 (10 studies, 3175 participants): RR 0.84, 95% CI 0.54 to 1.32; I2 = 0%) (moderate certainty evidence).
Probably makes little or no difference to the risk of all wound complications (Analysis 9.8.1 (7 studies, 2792 participants): RR 0.92, 95% CI 0.66 to 1.29; I2 = 61%) or lymphocoele (Analysis 9.8.2 (10 studies, 3302 participants): RR 0.81, 95% CI 0.63 to 1.04; I2 = 29%) (moderate certainty evidence).
May make little or no difference to the need for treatment change (Analysis 9.9 (10 studies, 3652 participants): RR 0.91, 95% CI 0.78 to 1.05; I2 = 52%) (low certainty evidence).
9.1. Analysis.
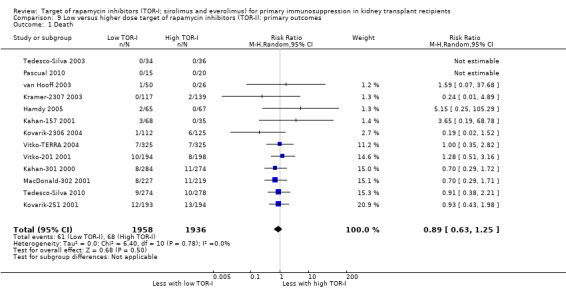
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 1 Death.
9.2. Analysis.
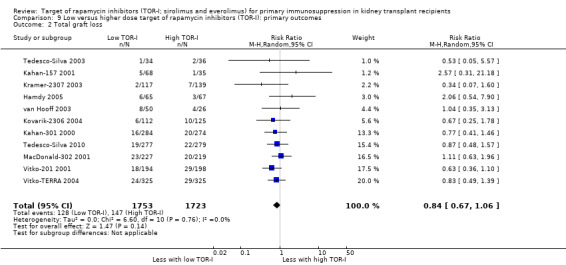
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 2 Total graft loss.
9.3. Analysis.
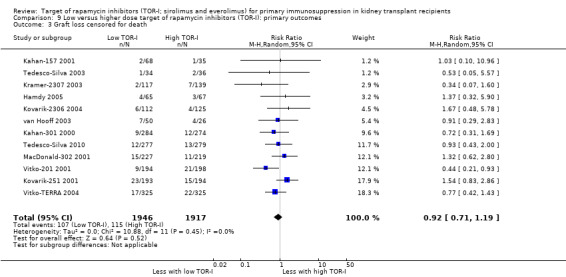
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 3 Graft loss censored for death.
9.4. Analysis.
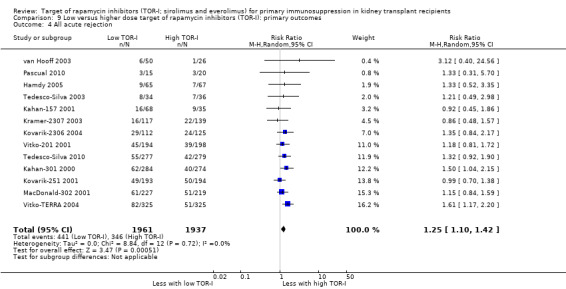
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 4 All acute rejection.
9.5. Analysis.
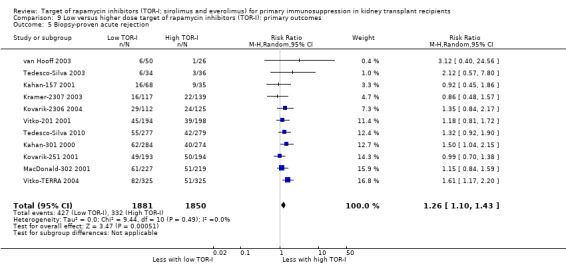
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 5 Biopsy‐proven acute rejection.
9.6. Analysis.

Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 6 CMV infection.
9.7. Analysis.
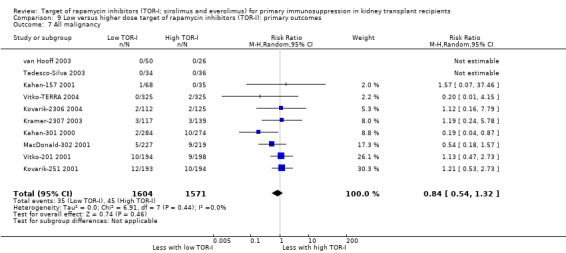
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 7 All malignancy.
9.8. Analysis.
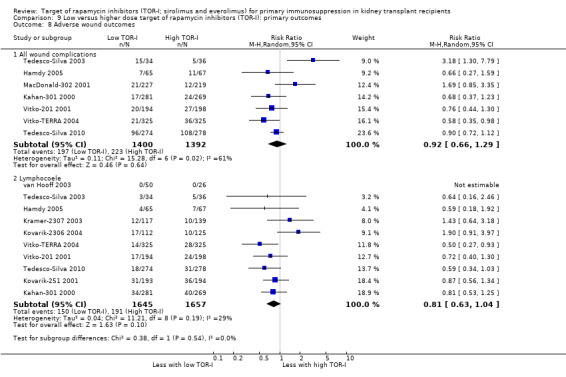
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 8 Adverse wound outcomes.
9.9. Analysis.
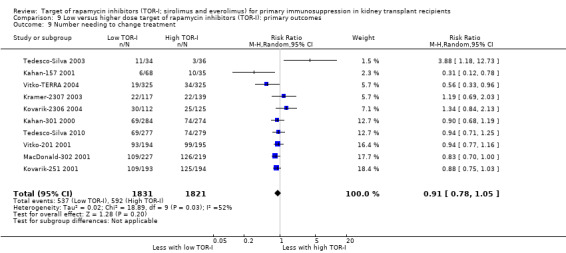
Comparison 9 Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes, Outcome 9 Number needing to change treatment.
Outcomes were downgraded for heterogeneity or imprecision (Table 7).
Secondary outcomes
All outcomes were assessed by GRADE as shown in the results below but only the seven most important outcomes (bold) are included in Table 8.
Lower dose TOR‐I and standard dose CNI compared with higher dose TOR‐I and reduced dose CNI:
Probably reduces the risk of new‐onset diabetes mellitus (Analysis 10.1 (6 studies, 2125 participants): RR 0.69, 95% CI 0.51 to 0.93; I2 = 16%) (moderate certainty evidence).
Probably makes little or no difference to the risk of lymphoma (Analysis 10.2 (7 studies, 2792 participants): RR 0.66, 95% CI 0.25 to 1.73; I2 = 0%) (moderate certainty evidence).
May make little or no difference to the risk of adverse outcomes including tremor (Analysis 10.3.1 (1 study, 387 participants): RR 0.90, 95% CI 0.63 to 1.29), gum hyperplasia (Analysis 10.3.2 (2 studies, 622 participants): RR 1.45, 95% CI 0.48 to 4.42; I2 = 0%) or acne/rash (Analysis 10.3.4 (6 studies, 2408 participants): RR 0.86, 95% CI 0.62 to 1.21; I2 = 71%) (low certainty evidence) though it may reduce the risk of hirsutism (Analysis 10.3.3 (2 studies, 1102 participants): RR 0.50, 95% CI 0.30 to 0.85; I2 = 5%) (low certainty evidence).
May make little or no difference to GFR (Analysis 10.4 (7 studies, 1863 participants): MD 2.88 mL/min, 95% CI ‐0.71 to 6.48; I2 = 70%) (low certainty evidence) or to serum creatinine (Analysis 10.5 (5 studies, 1951 participants): MD ‐2.21 µmol/L, 95% CI ‐13.68 to 9.26; I2 = 65% (low certainty evidence).
Probably makes little or no difference to the number of participants with hypercholesterolaemia (Analysis 10.6.1 (9 studies, 3250 participants): RR 0.87, 95% CI 0.78 to 0.98; I2 = 0%) (moderate certainty evidence), hypertriglyceridaemia (Analysis 10.6.2 (5 studies, 1064 participants): RR 0.71, 95% CI 0.47 to 1.07; I2 = 0%) (moderate certainty evidence), mean cholesterol (Analysis 10.7.1 (5 studies, 1041 participants): MD ‐0.13 mmol/L, 95% CI ‐0.35 to 0.08; I2 = 0%) or mean triglycerides (Analysis 10.7.2 (4 studies, 1041 participants): MD ‐0.37 mmol/L, 95% CI ‐0.72 to ‐0.03; I2 = 22%) (moderate certainty evidence).
May make little or no difference to the number of participants with anaemia (Analysis 10.8.1) (low certainty evidence), leucopenia (Analysis 10.8.2) (low certainty evidence), or thrombocytopenia (Analysis 10.8.3) (low certainty evidence).
10.1. Analysis.
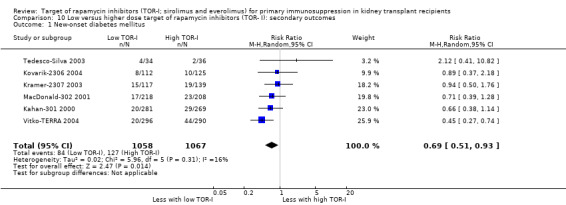
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 1 New‐onset diabetes mellitus.
10.2. Analysis.
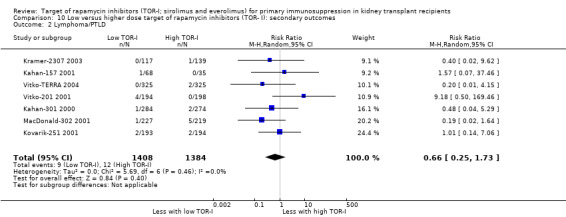
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 2 Lymphoma/PTLD.
10.3. Analysis.
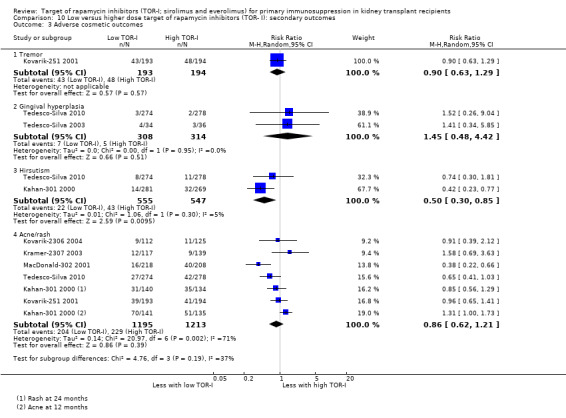
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 3 Adverse cosmetic outcomes.
10.4. Analysis.
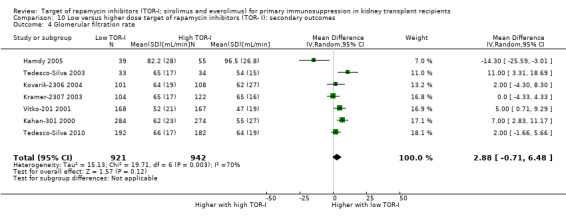
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 4 Glomerular filtration rate.
10.5. Analysis.
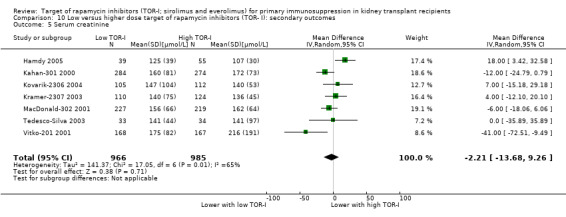
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 5 Serum creatinine.
10.6. Analysis.
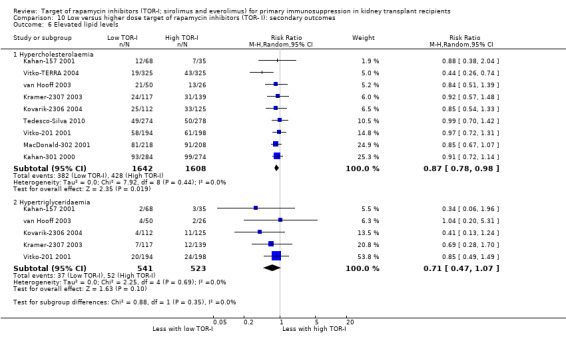
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 6 Elevated lipid levels.
10.7. Analysis.
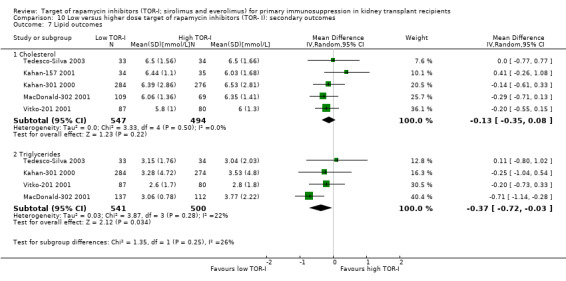
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 7 Lipid outcomes.
10.8. Analysis.
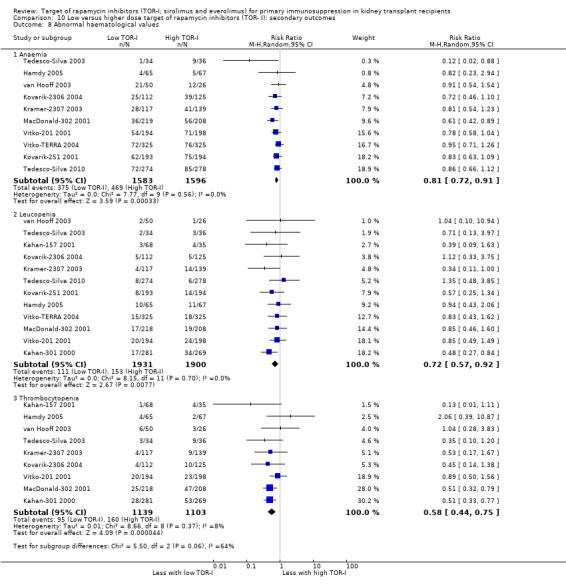
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 8 Abnormal haematological values.
Outcomes were downgraded for heterogeneity, imprecision and risk of bias related to sequence generation or allocation.(Table 8).
Comparative efficacy of sirolimus versus everolimus
Only one small study (28 recipients), reported as an abstract, compared sirolimus (mean dose 1.94 mg/d) to everolimus (mean dose 2.37 mg/d), with cyclosporin (mean dose 203 mg/d and 223 mg/d respectively) and prednisolone co‐interventions (Rostaing 2001). Preliminary results for limited outcomes at three months showed higher GFR (Analysis 11.2: MD ‐17.00 mL/min, 95% CI ‐28.98 to ‐5.02) and lower mean SCr (Analysis 11.1: MD 33.00 µmol/L, 95% CI 2.00 to 64.00) for everolimus‐treated patients, but lower total cholesterol (Analysis 11.3.1: MD ‐1.00 mmol/L, 95% CI ‐1.18 to ‐0.82) and triglycerides (Analysis 11.3.2: MD ‐0.30 mmol/L, 95% CI ‐0.44 to ‐0.16) for sirolimus‐treated patients. In view of the small patient numbers, limited outcomes and short follow‐up, it is uncertain whether sirolimus and everolimus differ in their effects on these outcomes.
11.2. Analysis.
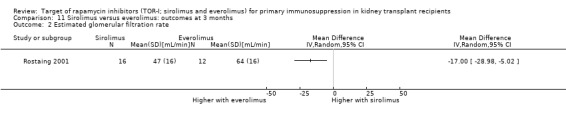
Comparison 11 Sirolimus versus everolimus: outcomes at 3 months, Outcome 2 Estimated glomerular filtration rate.
11.1. Analysis.
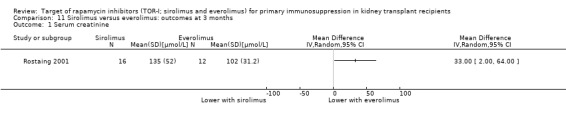
Comparison 11 Sirolimus versus everolimus: outcomes at 3 months, Outcome 1 Serum creatinine.
11.3. Analysis.
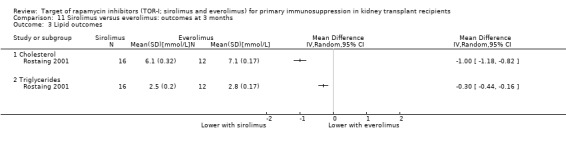
Comparison 11 Sirolimus versus everolimus: outcomes at 3 months, Outcome 3 Lipid outcomes.
Subgroup analyses
Stratified analysis was performed for the most commonly reported outcome, all acute rejection, to examine whether key study design features modified the overall results.
For studies of TOR‐I versus CNI, P‐values were greater than 0.05 for all analyses indicating no differences in the risk of acute rejection for the subgroups analysed (Analysis 12.1, Analysis 12.2, Analysis 12.3, Analysis 12.4) (Table 24). Only one study used everolimus and one study used azathioprine so different TOR‐I and antimetabolites could not be assessed.
12.1. Analysis.
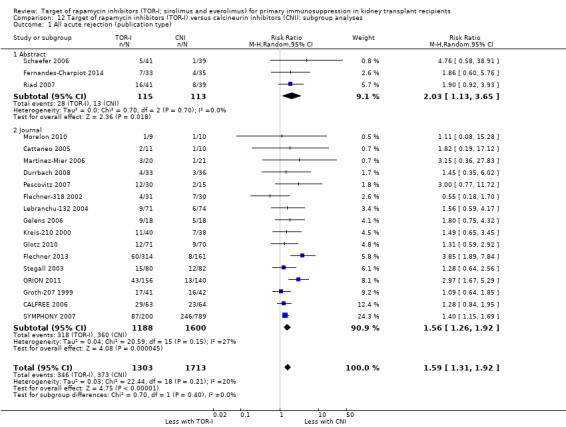
Comparison 12 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses, Outcome 1 All acute rejection (publication type).
12.2. Analysis.
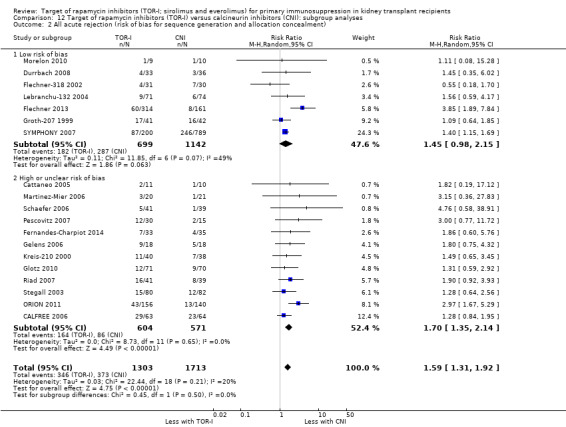
Comparison 12 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses, Outcome 2 All acute rejection (risk of bias for sequence generation and allocation concealment).
12.3. Analysis.
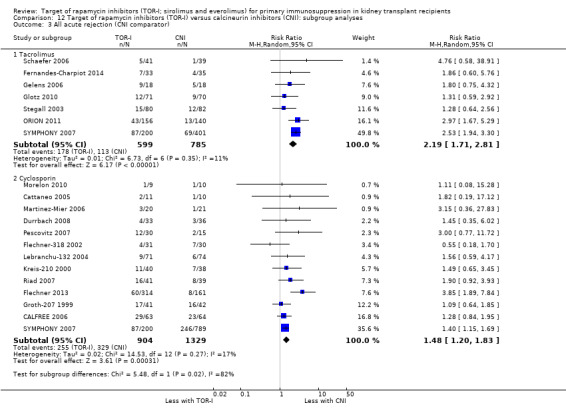
Comparison 12 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses, Outcome 3 All acute rejection (CNI comparator).
12.4. Analysis.
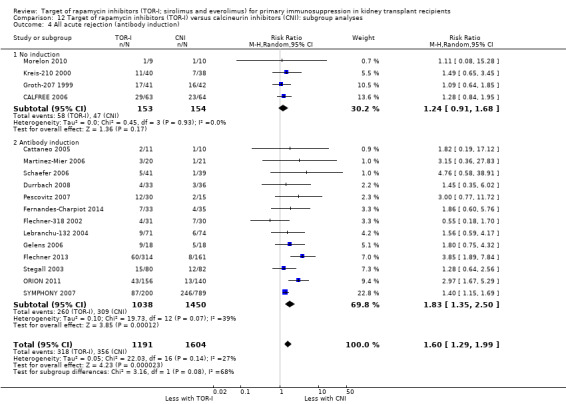
Comparison 12 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses, Outcome 4 All acute rejection (antibody induction).
1. Target of rapamycin inhibitor (TOR‐I) versus calcineurin inhibitor (CNI) or antimetabolite: subgroup analyses of study methodology and design features for all acute rejection.
| Variable | TOR‐I versus CNI^ | TOR‐I versus antimetabolite* | ||||
| Studies | RR (95% CI) | P‐value for subgroup differences | Studies | RR (95% CI) | P‐value for subgroup differences | |
| Publication type | ||||||
| Abstract | 3 | RR 2.03 (95% CI 1.13 to 3.65) | 0.35 | 5 | RR 0.68 (95% CI 0.29 to 1.61) | 0.49 |
| Journal | 16 | RR 1.53 (95% CI 1.23 to 1.90) | 27 | RR 0.93 (95% CI 0.80 to 1.08) | ||
| Risk of bias | ||||||
| Low risk | 7 | RR 1.64 (95% CI 1.31 to 2.06) | 0.65 | 12 | RR 0.85 (95% CI 0.50 ‐ 1.46) | 0.21 |
| High or unclear risk | 12 | RR 1.61 (95% CI 1.28 to 2.03) | 21 | RR 1.04 (95% CI 0.87 to 1.26) | ||
| CNI co‐intervention | ||||||
| Tacrolimus | 7^^ | RR 2.09 (95% CI 1.56 to 2.78) | 0.06 | 18** | RR 0.93 (95% CI 0.76 to 1.14) | 0.25 |
| Cyclosporin | 13 | RR 1.48 (95% CI 1.20 to 1.83) | 9 | RR 0.85 (95% CI 0.64 ‐ 1.14) | ||
| TOR‐I | ||||||
| Everolimus | 1 | Not analysed^^^ | ‐‐ | 16 | RR 1.04 (95% CI 0.85 to 1.28) | 0.17 |
| Sirolimus | 18 | Not analysed^^^ | 16 | RR 0.85 (95% CI 0.70 ‐ 1.04) | ||
| Antimetabolite comparator | ||||||
| Azathioprine | 1 | Not analysed^^^ | ‐‐ | 2 | RR 0.71 (95% CI 0.39 ‐ 1.28) | 0.30 |
| Mycophenolate | 18 | Not analysed^^^ | 30 | RR 0.98 (95% CI 0.86 to 1.12) | ||
| Antibody induction | ||||||
| No induction | 4 | RR 1.24 (95% CI 0.91 to 1.68) | 0.13 | 10*** | RR 0.93 (95% CI 0.77 ‐ 1.12) | 0.88 |
| Antibody induction | 13^^^^ | RR 1.81 (95% CI 1.29 to 2.53) | 21 | RR 0.90 (95% CI 0.70 to 1.16) | ||
* Includes 32 studies, which compared TOR‐I with an antimetabolite and reported the outcome of all acute rejection
**CNI co‐intervention: 5 studies excluded as they used both tacrolimus and cyclosporin
*** One study excluded as it did not report whether antibody induction was administered
^ Includes 19 studies, which compared TOR‐I with a CNI and reported the outcome of all acute rejection
^^ Includes 20 studies as one study (SYMPHONY 2007) had separate groups receiving cyclosporin and tacrolimus
^^^ Analyses not carried out as only one study used the TOR‐I, everolimus, and only one study used the antimetabolite, azathioprine
^^^^ Two studies only used induction in TOR‐I arm
For studies comparing TOR‐I with antimetabolite, P‐values were greater than 0.05 for all analyses indicating no differences in the risk of acute rejection for the subgroups analysed (Analysis 13.1, Analysis 13.2, Analysis 13.3, Analysis 13.4; Analysis 13.5; Analysis 13.6) (Table 24).
13.1. Analysis.
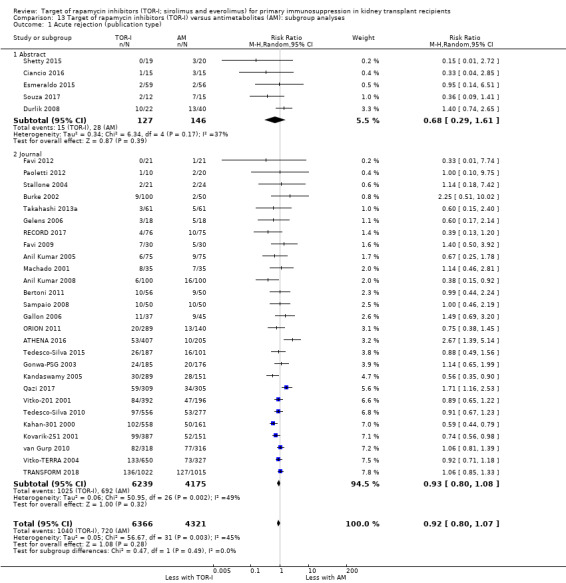
Comparison 13 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses, Outcome 1 Acute rejection (publication type).
13.2. Analysis.
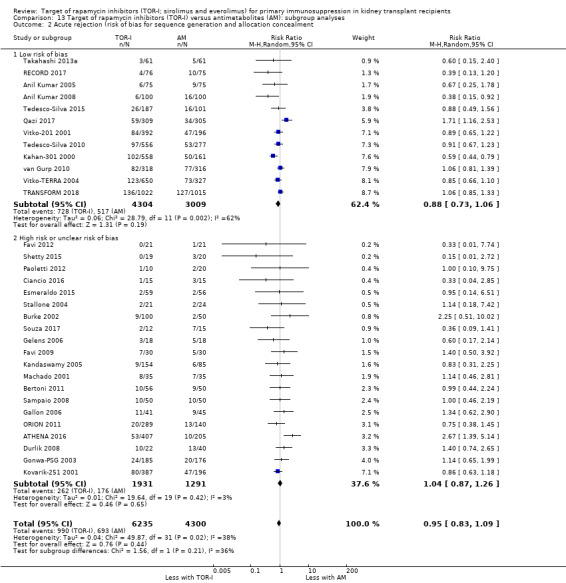
Comparison 13 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses, Outcome 2 Acute rejection (risk of bias for sequence generation and allocation concealment.
13.3. Analysis.
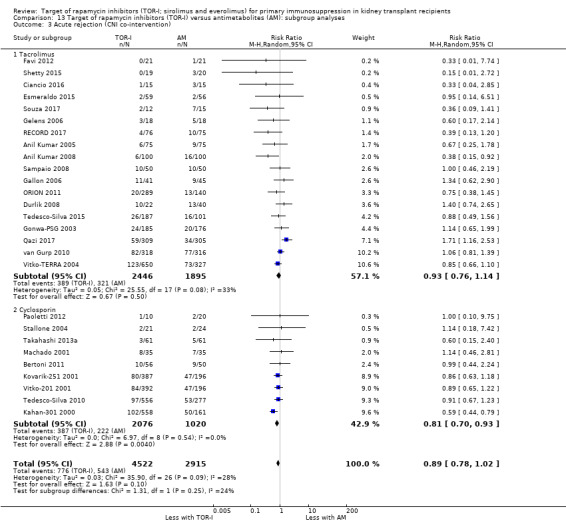
Comparison 13 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses, Outcome 3 Acute rejection (CNI co‐intervention).
13.4. Analysis.
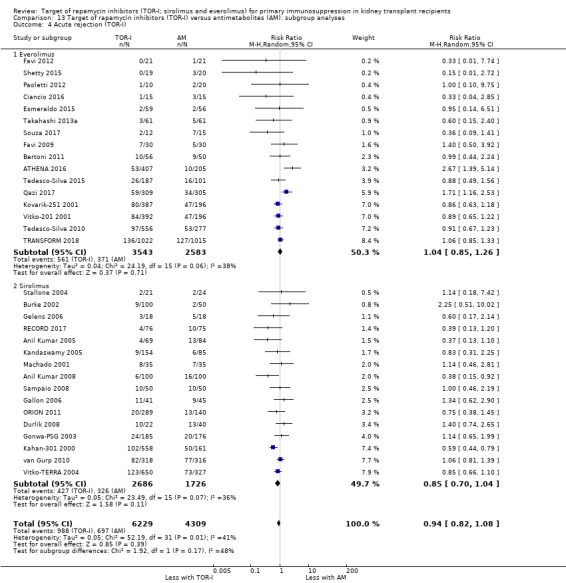
Comparison 13 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses, Outcome 4 Acute rejection (TOR‐I).
13.5. Analysis.
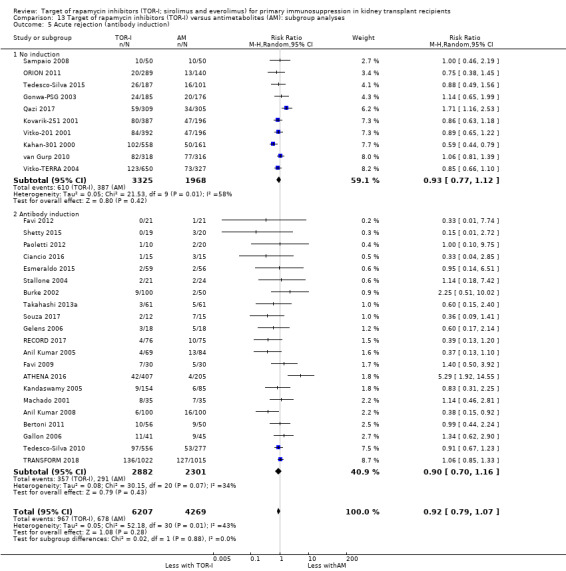
Comparison 13 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses, Outcome 5 Acute rejection (antibody induction).
13.6. Analysis.
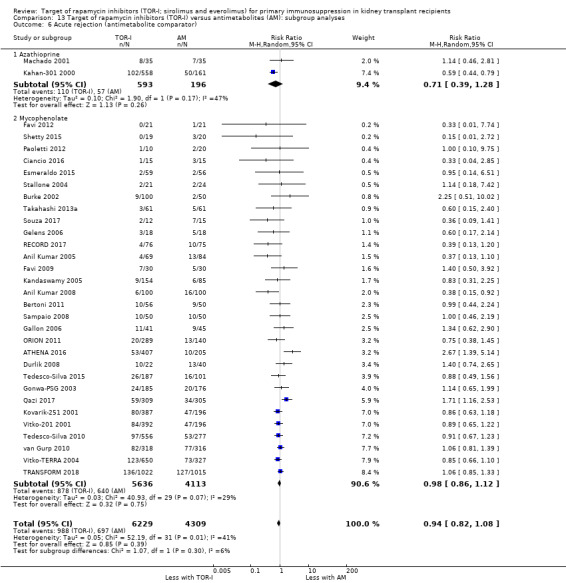
Comparison 13 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses, Outcome 6 Acute rejection (antimetabolite comparator).
For studies evaluating variable doses of TOR‐I in combination with variable doses of CNI, P‐values were greater than 0.05 for all analyses indicating no difference in the risk of acute rejection for the subgroups analysed (Analysis 14.1; Analysis 14.2; Analysis 14.3; Analysis 14.4; Analysis 14.5). (Table 25). All studies used mycophenolate mofetil or mycophenolate sodium so different antimetabolites could not be assessed.
14.1. Analysis.
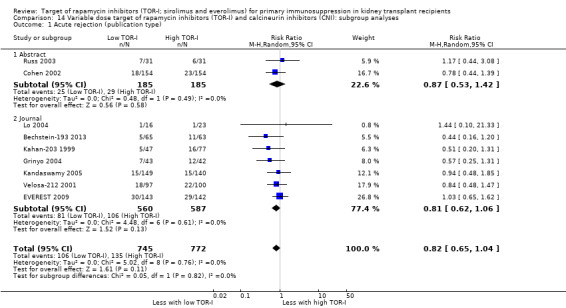
Comparison 14 Variable dose target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): subgroup analyses, Outcome 1 Acute rejection (publication type).
14.2. Analysis.
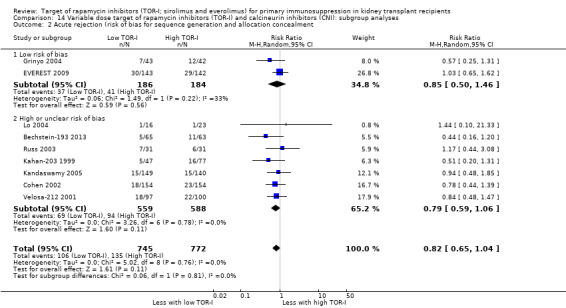
Comparison 14 Variable dose target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): subgroup analyses, Outcome 2 Acute rejection (risk of bias for sequence generation and allocation concealment.
14.3. Analysis.
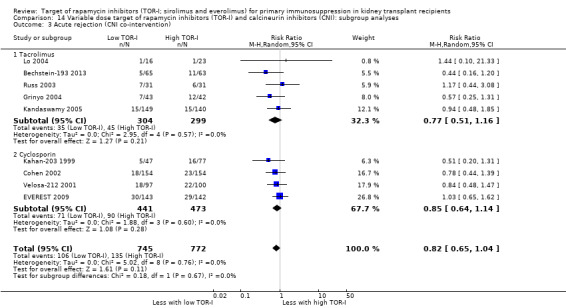
Comparison 14 Variable dose target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): subgroup analyses, Outcome 3 Acute rejection (CNI co‐intervention).
14.4. Analysis.
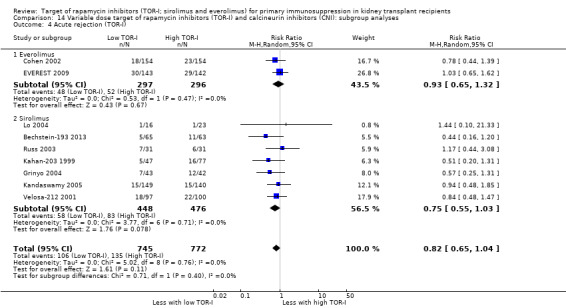
Comparison 14 Variable dose target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): subgroup analyses, Outcome 4 Acute rejection (TOR‐I).
14.5. Analysis.
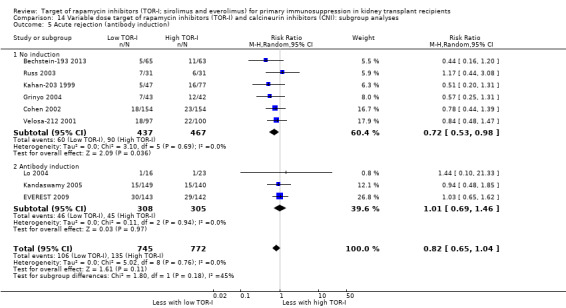
Comparison 14 Variable dose target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): subgroup analyses, Outcome 5 Acute rejection (antibody induction).
2. Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI) and lower versus higher doses of TOR‐I: subgroup analysis of study methodology and design features for all acute rejection.
| Variable | Variable doses of TOR‐I and CNI* | Lower versus higher doses of TOR‐I** | ||||
| Studies | RR (95% CI) | P‐value for subgroup differences | Studies | RR (95% CI) | P‐value for subgroup differences | |
| Publication type | ||||||
| Abstract | 2 | RR 0.85 (95% CI 0.50 ‐ 1.46) | 0.7 | 0 | No studies | Not applicable |
| Journal | 7 | RR 0.83 (95% CI 0.63 ‐ 1.08) | 13 | (RR 1.24, 95% CI 1.10 to 1.41) | ||
| Risk of bias | ||||||
| Low risk | 2 | RR 0.85 (95% CI 0.50 ‐ 1.46) | 0.81 | 5 | (RR 1.33, 95% CI 1.15 to 1.55) | 0.10 |
| High or unclear risk | 7 | RR 0.79 (95% CI 0.59 ‐ 1.06) | 8 | (RR 1.06, 95% CI 0.84 to 1.33) | ||
| CNI co‐intervention | ||||||
| Tacrolimus | 5 | RR 0.77 (95% CI 0.51 ‐ 1.16) | 0.67 | 3 | (RR 1.62, 95% CI 1.19 to 2.19) | 0.06 |
| Cyclosporin | 4 | RR 0.85 (95% CI 0.64 ‐ 1.14) | 9 | (RR 1.17, 95% CI 1.02 to 1.35) | ||
| Antibody induction | ||||||
| No induction | 6 | RR 0.72 (95% CI 0.53 ‐ 0.98) | 0.97 | 10 | (RR 1.25, 95% CI 1.09 to 1.45) | 0.81 |
| Antibody induction | 3 | RR 1.01 (95% CI 0.69 ‐ 1.46) | 3 | (RR 1.20, 95% CI 0.90 to 1.62) | ||
| TOR‐I | ||||||
| Everolimus | 2 | (RR 0.93, 95% CI 0.65 to 1.32) | 0.4 | 7 | (RR 1.11, 95% CI 0.93 to 1.33) | 0.09 |
| Sirolimus | 7 | (RR 0.75, 95% CI 0.55 to 1.03) | 6 | (RR 1.39, 95% CI 1.16 to 1.66) | ||
* Includes 9 studies, which reported the outcome of all acute rejection
** Includes 13 studies, which reported the outcome of all acute rejection
For studies comparing low with higher doses of TOR‐I, P‐values were greater than 0.05 for all analyses indicating no difference in the risk of acute rejection for the subgroups analysed (Analysis 15.1; Analysis 15.2; Analysis 15.3; Analysis 15.4; Analysis 15.5). (Table 25). All studies used mycophenolate mofetil or mycophenolate sodium so different antimetabolites could not be assessed.
15.1. Analysis.
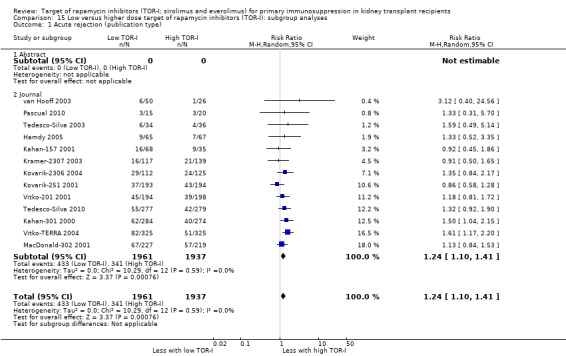
Comparison 15 Low versus higher dose target of rapamycin inhibitors (TOR‐I): subgroup analyses, Outcome 1 Acute rejection (publication type).
15.2. Analysis.
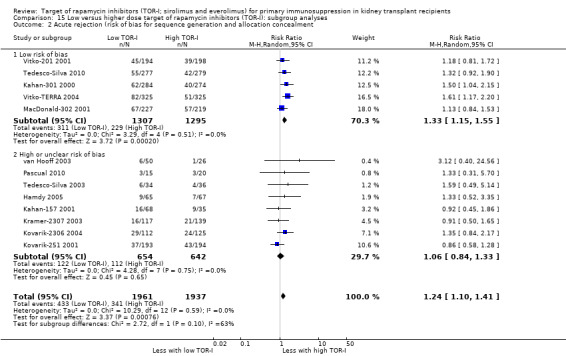
Comparison 15 Low versus higher dose target of rapamycin inhibitors (TOR‐I): subgroup analyses, Outcome 2 Acute rejection (risk of bias for sequence generation and allocation concealment.
15.3. Analysis.
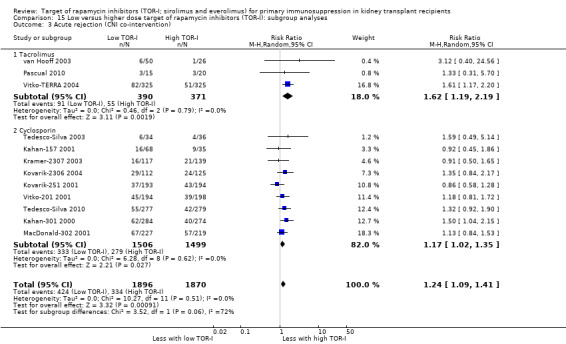
Comparison 15 Low versus higher dose target of rapamycin inhibitors (TOR‐I): subgroup analyses, Outcome 3 Acute rejection (CNI co‐intervention).
15.4. Analysis.
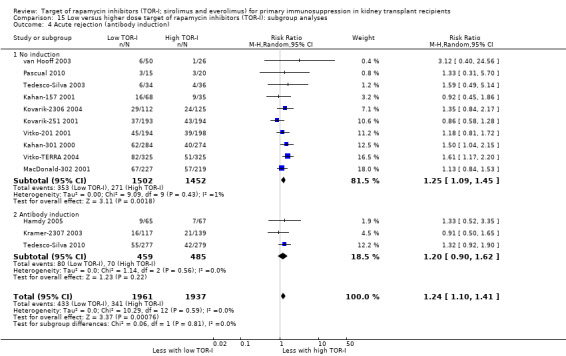
Comparison 15 Low versus higher dose target of rapamycin inhibitors (TOR‐I): subgroup analyses, Outcome 4 Acute rejection (antibody induction).
15.5. Analysis.
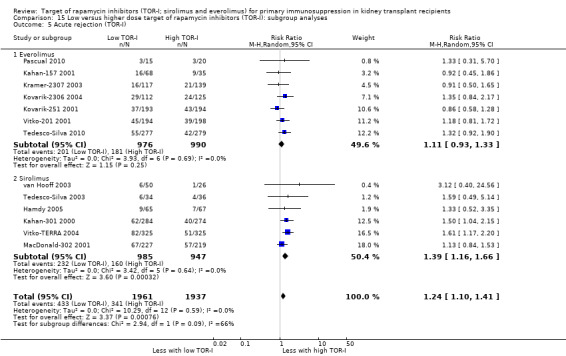
Comparison 15 Low versus higher dose target of rapamycin inhibitors (TOR‐I): subgroup analyses, Outcome 5 Acute rejection (TOR‐I).
Discussion
Summary of main results
Seventy studies (544 reports) with 17,462 participants were included in this review; 33 studies were included in the original review published in 2006 and 37 were added for the 2019 update. The studies were divided into four groups of comparisons. Data on outcomes in deceased donor and living donor transplant recipients was not reported separately in the included studies.
TOR‐I compared with CNI
Twenty‐one studies compared TOR‐I with CNI with both groups receiving antimetabolites. For outcomes for up to three years, TOR‐I compared with CNI probably makes little or no difference to death, graft loss and the number with malignancies but it probably increases the risk of biopsy‐proven acute rejection compared with CNI (all moderate certainty evidence). TOR‐I reduces the risk of CMV infection (high certainty evidence) but it probably increases the risk of wound complications and the number of participants who need to change immunosuppressive medications (moderate certainty evidence). Subgroup analyses of study methodology and design features for the outcome of all acute rejection identified no differences between groups (Table 24).
The outcomes in the 2019 review update are compared with those in the 2006 review in Table 26. In this update, the risk for all acute rejection and BPAR were increased with TOR‐I and the risk for CMV disease was reduced while these risks did not differ in the 2006 review.
3. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): comparison in outcomes between 2006 review and 2019 update.
| Outcomes | 2006 review (8 studies) | 2019 update (22 studies) |
| Death | No difference | No difference |
| All graft loss | No difference | No difference |
| Graft loss censored for death | No difference | No difference |
| All acute rejection | No difference | Increased with TOR‐I |
| Biopsy‐proven acute rejection | No difference | Increased with TOR‐I |
| CMV infection | No difference | Reduced with TOR‐I |
| Wound complications | Increased with TOR‐I | Increased with TOR‐I |
| Malignancies | No difference | No difference |
| Need to change treatment | Increased with TOR‐I | Increased with TOR‐I |
| New‐onset diabetes mellitus | No difference | No difference |
| Lymphoma/PTLD | No difference | No difference |
| BK virus infection | No difference (1 study) | No difference |
| Tremor | Reduced with TOR‐I | Reduced with TOR‐I |
| Acne/rash | Increased with TOR‐I | Increased with TOR‐I |
| GFR | Increased with TOR‐I | No difference |
| SCr | Reduced with TOR‐I | Reduced with TOR‐I |
| Hypercholesterolaemia | No difference | Increased with TOR‐I |
| Hypertriglyceridaemia | No difference | Increased with TOR‐I |
| Bone marrow suppression | Increased with TOR‐I | Increased with TOR‐I |
Change in results have been highlighted
CMV ‐ cytomegalovirus; GFR ‐ glomerular filtration rate; PTLD ‐ post‐transplant lymphoproliferative disease; SCr ‐ serum creatinine
TOR‐I compared with antimetabolite
Thirty‐four studies compared TOR‐I with antimetabolite with both groups receiving CNI. For outcomes for up to three years, TOR‐I compared with antimetabolite probably makes little or no difference to death, graft loss, biopsy‐proven acute rejection, and the risk for malignancies (all moderate certainty evidence). TOR‐I probably reduces the risk of CMV infection (moderate certainty evidence) and the risk for BK virus infection (high certainty evidence). It probably increases the risk of wound complication and the number of participants who need to change immunosuppressive medications (moderate certainty evidence). Subgroup analyses of study methodology and design features for the outcome of all acute rejection identified no differences (Table 24).
The outcomes in the 2019 review update are compared with those in the 2006 review in Table 27. In this update, no differences were identified in the risk for all acute rejection and BPAR while the risks were lower with TOR‐I compared with antimetabolite in the 2006 review.
4. Target of rapamycin inhibitors (TOR‐I) versus antimetabolite: comparison in outcomes between 2006 review and 2019 update.
| Outcomes | 2006 review (11 studies) | 2019 update (33 studies) |
| Death | No difference | No difference |
| All graft loss | No difference | No difference |
| Graft loss censored for death | No difference | No difference |
| All acute rejection | Reduced with TOR‐I | No difference |
| Biopsy‐proven acute rejection | Reduced with TOR‐I | No difference |
| CMV infection | Reduced with TOR‐I | Reduced with TOR‐I |
| Wound complications | Increased with TOR‐I | Increased with TOR‐I |
| Malignancies | No difference | No difference |
| Need to change treatment | No difference | Increased with TOR‐I |
| New‐onset diabetes mellitus | No difference | Increased with TOR‐I |
| Lymphoma/PTLD | No difference | No difference |
| BK virus infection | Not reported | Lower with TOR‐I |
| Tremor | No difference (1 study) | No difference |
| Acne/rash | Increased with TOR‐I (1 study) | Increased with TOR‐I |
| GFR | Reduced with TOR‐I | Reduced with TOR‐I |
| SCr | Increased with TOR‐I | Increased with TOR‐I |
| Hypercholesterolaemia | Increased with TOR‐I | Increased with TOR‐I |
| Hypertriglyceridaemia | Increased with TOR‐I | Increased with TOR‐I |
| Leucopenia | Reduced with TOR‐I | Reduced with TOR‐I |
| Thrombocytopenia | Increased with TOR‐I | Increased with TOR‐I |
Change in results have been highlighted
CMV ‐ cytomegalovirus; GFR ‐ glomerular filtration rate; PTLD ‐ post‐transplant lymphoproliferative disease; SCr ‐ serum creatinine
Variable TOR‐I and CNI
Nine studies compared a lower TOR‐I with standard CNI regimen with a higher TOR‐I with reduced CNI regimen. For outcomes to two years the lower TOR‐I regimen probably made little or no difference to death, graft loss, biopsy‐proven acute rejection, and CMV infection (moderate certainty evidence). The lower TOR‐I regimen may make little or no difference to the number of wound complications, the number with malignancies and the number needing to change immunosuppressive regimens (low certainty evidence). Subgroup analyses of study methodology and design features for the outcome of all acute rejection identified no differences (Table 25).
The outcomes in the 2019 review update are compared with those in the 2006 review in Table 28. In this update, no differences were identified in the risk for all acute rejection and BPAR while the risks were lower with TOR‐I compared with antimetabolite in the 2006 review.
5. Variable target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): comparison in outcomes between 2006 review and 2019 update.
| Outcome | 2006 review (8 studies) | 2019 update (9 studies) |
| Death | No difference | No difference |
| All graft loss | No difference | No difference |
| Graft loss censored for death | No difference | No difference |
| All acute rejection | Reduced in low TOR‐I | No difference |
| Biopsy‐proven acute rejection | Reduced in low TOR‐I | No difference |
| CMV infection | No difference | No difference |
| Wound complications | No difference | No difference |
| Malignancies | No difference | No difference |
| Need to change treatment | No difference | No difference |
| New‐onset diabetes mellitus | No difference | No difference |
| Lymphoma/PTLD | No difference | No difference |
| BK virus infection | Not reported | No difference |
| Tremor | No difference (1 study) | No difference |
| Acne/rash | Not reported | No difference |
| GFR | Increased in low TOR‐I | Increased in low TOR‐I |
| SCr | No difference | No difference |
| Hypercholesterolaemia | No difference | No difference |
| Hypertriglyceridaemia | No difference | No difference |
| Leucopenia | No difference | No difference |
| Thrombocytopenia | No difference | No difference |
Change in results have been highlighted
CMV ‐ cytomegalovirus; GFR ‐ glomerular filtration rate; PTLD ‐ post‐transplant lymphoproliferative disease; SCr ‐ serum creatinine
Low compared with higher doses of TOR‐I
Thirteen studies compared a lower TOR‐I with a higher TOR‐I dose regimen. For outcomes to two years, the lower TOR‐I dose probably makes little or no difference to death, graft loss, biopsy‐proven acute rejection, CMV infection or the number with malignancies (moderate certainty evidence). Lower TOR‐I dose compared with a higher dose probably reduces the number of participants with wound complications. It may make little or no difference to the number of participants needing to change immunosuppressive regimens (low certainty evidence). Subgroup analyses of study methodology and design features for the outcome of all acute rejection did not identify any differences (Table 25).
The outcomes in the 2019 review update are compared with those in the 2006 review in Table 29. In this update, the risk for hypercholesterolaemia was increased with higher doses of TOR‐I while no difference was identified in the 2006 review.
6. Low versus high target of rapamycin inhibitors (TOR‐I): comparison in outcomes between 2006 review and 2019 update.
| Outcome | 2006 review (8 studies) | 2019 update (13 studies) |
| Death | No difference | No difference |
| All graft loss | No difference | No difference |
| Graft loss censored for death | No difference | No difference |
| All acute rejection | Reduced in high TOR‐I | Reduced in high TOR‐I |
| Biopsy‐proven acute rejection | Reduced in high TOR‐I | Reduced in high TOR‐I |
| CMV infection | No difference | No difference |
| Wound complications | No difference | No difference |
| Malignancies | No difference | No difference |
| Need to change treatment | No difference | No difference |
| New‐onset diabetes mellitus | Increased in high TOR‐I | Increased in high TOR‐I |
| Lymphoma/PTLD | No difference | No difference |
| BK virus infection | Not reported | Not reported |
| Tremor | Not reported | No difference |
| Acne/rash | No difference | No difference |
| GFR | Reduced in high TOR‐I | Reduced in high TOR‐I |
| SCr | No difference | No difference |
| Hypercholesterolaemia | No difference | Increased in high TOR‐I |
| Hypertriglyceridaemia | No difference | No difference |
| Leucopenia | Increased in high TOR‐I | Increased in high TOR‐I |
| Thrombocytopenia | Increased in high TOR‐I | Increased in high TOR‐I |
Change in results have been highlighted
CMV ‐ cytomegalovirus; GFR ‐ glomerular filtration rate; PTLD ‐ post‐transplant lymphoproliferative disease; SCr ‐ serum creatinine
Overall completeness and applicability of evidence
Most studies did not report on outcomes beyond three years. To determine efficacy outcomes in these short‐term studies, the primary outcome was frequently a composite of outcomes and the studies were designed as non‐inferiority studies. For example, Qazi 2017 used a composite efficacy endpoint of biopsy‐proven acute rejection, graft loss, death and loss to follow up rather than individual components. In other studies, eGFR was the primary outcome of the study with or without biopsy‐proven acute rejection. In the large study TRANSFORM 2018, the triallists used a composite primary outcome of the number of participants with eGFR < 50 mL/min calculated from the MDRD formula or with treated biopsy‐proven acute rejection at 12 months. Because of the short duration of studies, outcomes of death or graft loss are unlikely to differ between treatment groups. Any identified differences between treatments are likely to be adverse effects of treatment. Therefore, the more important outcomes in short‐term studies are adverse effects such as wound complications, CMV, lipid abnormalities and the number needing to change immunosuppressive medication. In the comparisons, these outcomes were reported less commonly than the outcomes of death, graft loss or biopsy‐proven acute rejection. For example, in the comparison of TOR‐I compared with CNI, CMV infection was reported in 13/21 studies while in the comparison of TOR‐I compared with antimetabolite, CMV infection was reported in 24/34 studies.
Although this review included 70 studies, many studies did not report on outcomes important to participants including cosmetic complications and tremor. Health‐related quality of life was only reported in a substudy of 156 participants of the SYMPHONY 2007. Because few studies reported separately on cardiovascular death or reported the number of cardiovascular events, we were not able to include an assessment of these outcomes in this review.
Quality of the evidence
Most studies did not report on how the sequence generation was derived or whether there was adequate allocation concealment. However, where these items were reported, they were generally at low risk of bias. Most studies were open label with only four studies being at low risk of performance bias. Almost all studies were considered at low risk of detection bias because the primary outcome was laboratory based and unlikely to be influenced by lack of blinding. Most studies were at low risk of incomplete outcome reporting or selective reporting though these quality outcomes were unclear in some studies available only in abstract form. Many studies were industry funded and considered at high risk for other bias.
GRADE assessment was used for 14 outcomes reported in summary of findings tables. In the comparisons of TOR‐I versus CNI and TOR‐I versus antimetabolite, GRADE assessment concluded that there was moderate certainty evidence for all primary outcomes except for CMV infection (high certainty evidence) in the TOR‐I versus CNI comparison. In the comparisons of variable TOR‐I and CNI and low versus higher TOR‐I, GRADE assessment concluded that there was also moderate certainty evidence for most primary outcomes. Outcomes were downgraded for imprecision and heterogeneity. GRADE assessment for secondary outcomes was more likely to be considered low or very low particularly for laboratory outcomes. Outcomes were downgraded for imprecision, heterogeneity, publication bias and risk of bias for sequence generation and allocation concealment.
Potential biases in the review process
For this update a comprehensive search of the Cochrane Kidney and Transplant’s Specialised Register was performed, which reduced the likelihood that eligible published studies were omitted from the review. Eligible studies published after the last search date of 20 September 2019 or published in congress proceedings not routinely searched could have been missed. Twelve studies were available in abstract form and provided limited information on study methods and results. Inclusion of these studies could be a source of bias.
The review was completed independently by at least two authors, who participated in all steps of the update. This limited the risk of errors in determining study eligibility, data extraction, risk of bias assessment and data synthesis. Some outcomes were reported in only a few studies which increased the risk of bias. In particular, adverse effects important to participants such as cosmetic effects and tremor were reported in few studies. The authors determined the outcomes that they considered were the most important for a review of TOR‐I medications in kidney transplant recipients and did not report every outcome reported in each study. Therefore, some outcomes considered of importance by others could have been excluded from the review.
Agreements and disagreements with other studies or reviews
Recent systematic reviews of RCTs have evaluated TOR‐I in kidney transplant recipients. Reviews included studies in which participants were converted to TOR‐I weeks to months after kidney transplant as well as those commencing TOR‐I at transplant while our review only included studies in which TOR‐I was commenced at transplant or within six days of transplant. Kumar 2017 examined the role of TOR‐I as an alternative to CNI and included 20 RCTs. Ten of these were also included in this review while the other 10 studies concerned later conversion to TOR‐I regimens. As in this review they identified an increased risk of acute rejection among participants receiving de novo TOR‐I compared with those receiving CNI but no difference in deaths or graft loss. Similarly, another review (Mallet 2017) including 24 RCTs (11 included in our review) found an increased risk of acute rejection in participants receiving de novo TOR‐I compared with those receiving CNI but no difference in participants, who received TOR‐I and reduced dose CNI compared with mycophenolic acid (MPA) and standard dose CNI. Wound complications were higher in all groups receiving TOR‐I as in our review but graft loss did not differ between groups.
Mallet 2017 also examined the risk of CMV and BK virus infections in kidney transplant recipients receiving TOR‐I. Among studies comparing TOR‐I with CNI and studies comparing TOR‐I and a reduced dose of CNI with MPA and standard dose CNI, CMV infection was reduced by 46% and 57% respectively. In equivalent analyses in this review, CMV infection was reduced by 57% and 58% respectively. Mallet 2017 found no difference in the number of patients with BK virus in studies comparing TOR‐I with CNI (12 studies) or those comparing TOR‐I with reduced dose CNI with standard dose CNI and MPA (two studies). In our review with additional studies, the risk for BK virus infection was reduced in participants receiving TOR‐I compared with participants receiving MPA (high certainty evidence). However, in our review, it was unclear whether TOR‐I compared with CNI reduced the number with BK virus infection because few studies addressed this outcome (very low certainty evidence).
Authors' conclusions
Implications for practice.
Data included in this review show that TOR‐I combined with an antimetabolite increases the risk for acute rejection compared with CNI combined with an antimetabolite suggesting that as initial immunosuppression for kidney transplant recipients, TOR‐I should be given with a CNI rather than with an antimetabolite alone. More recent data confirm that TOR‐I with CNI may offer a satisfactory alternative to an antimetabolite with CNI as rates of acute rejection are similar between interventions and TOR‐I regimens are associated with a reduced risk of CMV and BK infections though wound complications and the need to change immunosuppressive medications are higher with TOR‐I regimens. In addition, TOR‐I regimens using a reduced dose of CNI compared with antimetabolite regimens result in similar GFR outcomes. However, most studies do not provide follow up beyond six months to three years. In the absence of long‐term data particularly on graft survival, we are limited to reporting on the short‐term outcomes including acute rejection, GFR and CMV disease and the adverse effects of each regimen and are unable to report on long term patient survival (particularly associated with cardiovascular disease and malignancy) and graft survival.
Implications for research.
SYMPHONY 2007, which randomised 1645 participants, confirmed that TOR‐I with an antimetabolite was inferior in terms of acute rejection rates to CNI with an antimetabolite so that few further studies evaluated this comparison. Similarly following the publication of TRANSFORM 2018, which enrolled 2037 participants and confirmed the relative efficacies of TOR‐I with reduced dose CNI and mycophenolate sodium with standard dose CNI, there appears to be no requirement for further short‐term studies comparing de novo use of TOR‐I. There should be longer term follow‐up of participants in the TRANSFORM 2018 and other large studies to provide longer term information about graft loss and graft function and help to assess the value of the outcomes of acute rejection and GFR reported for short follow up periods. Linkage to registry data can be used to provide longer follow up data on participants in RCTs. For example in a recent publication, Ying 2018 linked data from the Australian trial participants in four RCTs evaluating everolimus with registry data to determine the outcomes of incident cancers and cancer‐related deaths.
To date studies have generally excluded sensitised recipients with PRA levels > 20%. Further studies are required in this group of transplant recipients.
This systematic review did not include studies where a TOR‐I was added to the immunosuppressive regimen a week or more post‐transplant. Further reviews are required to investigate the relative efficacies and adverse effects of TOR‐I when introduced later after transplant.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2019 | New citation required and conclusions have changed | New studies added ‐ some changes to direction of results |
| 11 November 2019 | New search has been performed | 37 new studies added |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 15 October 2008 | Amended | Converted to new review format. |
Acknowledgements
ACW would like to acknowledge the help and support of all members of Cochrane Kidney and Transplant, and particularly to Jane Powell, a visiting elective student, who contributed early administrative support to the 2006 review. ACW also wishes to thank all report authors who responded to our enquiries about their work and those who provided further information about their studies, particularly Drs S Vitko, L Gallon and Profs A Kumar and Y Lebranchu.
The 2006 review was co‐published with Transplantation May, 2006, (Webster 2006b).
The authors of the 2019 update are grateful to the following peer reviewers for their time and comments: Rowan Walker (Renal Medicine Alfred Health/Department of Medicine Monash University), John Asher (Glasgow Renal and Transplant Unit, Queen Elizabeth University Hospital, Glasgow, Scotland), Daniel Abramowicz, MD, PhD (University of Antwerp, Belgium), Dr Ashley Irish (Nephrologist, Fiona Stanley Hospital, Murdoch, Western Australia and Faculty of Medicine, University of Western Australia). We would also like to thank the Cochrane Kidney and Transplant editorial staff and the review Editor Colin Wilson.
Appendices
Appendix 1. Electronic search strategies
| Electronic databases | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all causes) | 19 | 3618 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.87, 1.98] |
| 2 Total graft loss including death | 19 | 3619 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.11, 1.80] |
| 2.1 Tacrolimus | 7 | 1386 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.25, 2.56] |
| 2.2 Cyclosporin | 13 | 2233 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.83, 1.61] |
| 3 Graft loss censored for death | 14 | 3277 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.96, 1.81] |
| 3.1 Tacrolimus | 5 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.17, 3.25] |
| 3.2 Cyclosporin | 10 | 2039 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.54] |
| 4 All acute rejection | 19 | 3016 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.31, 1.92] |
| 5 Biopsy‐proven acute rejection | 15 | 2708 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.25, 2.04] |
| 6 CMV infection | 13 | 2026 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.29, 0.63] |
| 7 Adverse wound outcomes | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 All complications | 12 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [1.94, 3.36] |
| 7.2 Lymphocoele | 8 | 2538 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [1.73, 3.02] |
| 8 All malignancies | 10 | 2584 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.50, 1.48] |
| 9 Number needing to change treatment | 14 | 3148 | Risk Ratio (M‐H, Random, 95% CI) | 2.42 [1.88, 3.11] |
Comparison 2. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New‐onset diabetes mellitus | 13 | 2791 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.26] |
| 1.1 Tacrolimus | 6 | 1274 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.16] |
| 1.2 Cyclosporin | 8 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.74, 1.99] |
| 2 Lymphoma/PTLD | 8 | 2537 | Risk Ratio (M‐H, Random, 95% CI) | 2.47 [0.78, 7.86] |
| 3 Number with BK virus infection | 3 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.16, 1.29] |
| 4 Adverse cosmetic outcomes | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Tremor | 6 | 799 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.41] |
| 4.2 Gingival hyperplasia ‐ cyclosporin | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.57] |
| 4.3 Hirsutism ‐ cyclosporin | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 2.03] |
| 4.4 Acne/rash | 4 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [1.75, 7.02] |
| 5 Glomerular filtration rate | 15 | 2983 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.29, 5.68] |
| 6 Serum creatinine | 10 | 672 | Mean Difference (IV, Random, 95% CI) | ‐10.64 [‐19.19, ‐2.10] |
| 7 Number with elevated lipid levels | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypercholesterolaemia | 4 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.17, 2.59] |
| 7.2 Hypertriglyceridaemia | 5 | 1922 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.20, 2.46] |
| 8 Lipid outcomes | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Cholesterol | 7 | 579 | Mean Difference (IV, Random, 95% CI) | 0.77 [0.45, 1.09] |
| 8.2 Triglycerides | 8 | 843 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.28, 0.86] |
| 9 Number with abnormal haematological values | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Anaemia | 6 | 2216 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.28, 1.70] |
| 9.2 Leucopenia | 5 | 1922 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.95, 2.44] |
| 9.3 Thrombocytopenia | 4 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 5.26 [2.87, 9.63] |
| 10 Haematological outcomes | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Haemoglobin [g/dL] | 5 | 481 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.17, ‐0.11] |
| 10.2 White cell count [per mm3] | 5 | 433 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.21, ‐0.41] |
| 10.3 Platelet count [per mm2] | 3 | 247 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.79, 1.85] |
2.10. Analysis.
Comparison 2 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): secondary outcomes, Outcome 10 Haematological outcomes.
Comparison 3. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): outcomes at 5 to 8 years post transplant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.60, 3.08] |
| 2 Total graft loss | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.44, 1.68] |
| 3 Graft loss censored for death | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.81] |
| 4 Malignancies | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.20, 1.13] |
| 5 Glomerular filtration rate | 2 | 163 | Mean Difference (IV, Random, 95% CI) | 13.51 [6.94, 20.08] |
3.1. Analysis.
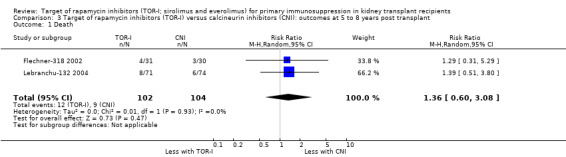
Comparison 3 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): outcomes at 5 to 8 years post transplant, Outcome 1 Death.
Comparison 4. Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 31 | 10482 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.33] |
| 2 Total graft loss | 27 | 7626 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.93, 1.40] |
| 3 Graft loss censored for death | 26 | 8966 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.82, 1.45] |
| 4 All acute rejection | 31 | 10075 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 5 Biopsy‐proven acute rejection | 24 | 10101 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.81, 1.12] |
| 5.1 TOR‐I/reduced CNI versus AM/standard CNI | 7 | 4170 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.87, 1.40] |
| 5.2 TOR‐I/standard CNI versus AM/standard CNI | 17 | 5931 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.74, 1.09] |
| 6 CMV infection | 25 | 10049 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.34, 0.58] |
| 7 Adverse wound outcomes | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 All complications | 17 | 6913 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.28, 1.91] |
| 7.2 Lymphocoele | 16 | 8415 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.32, 1.81] |
| 8 All malignancies | 17 | 8799 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.64, 1.07] |
| 9 Number needing to change treatment | 25 | 9747 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.28, 1.90] |
Comparison 5. Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New‐onset diabetes mellitus | 23 | 8728 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.07, 1.54] |
| 2 Lymphoma/PTLD | 14 | 5415 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.62, 3.72] |
| 3 BK virus infection | 12 | 5152 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.50, 0.76] |
| 4 Tremor and adverse cosmetic outcomes | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Tremor | 5 | 3803 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.15] |
| 4.2 Gingival hyperplasia | 2 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.60] |
| 4.3 Hirsutism | 2 | 1542 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.30, 5.28] |
| 4.4 Acne/Rash | 5 | 2022 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.08, 2.81] |
| 5 Glomerular filtration rate | 25 | 8099 | Mean Difference (IV, Random, 95% CI) | ‐2.89 [‐4.91, ‐0.88] |
| 5.1 TOR‐I/reduced CNI versus AM/standard CNI | 8 | 3954 | Mean Difference (IV, Random, 95% CI) | 1.58 [‐1.12, 4.28] |
| 5.2 TOR‐I/standard CNI versus AM/standard CNI | 17 | 4145 | Mean Difference (IV, Random, 95% CI) | ‐5.45 [‐7.55, ‐3.35] |
| 6 Serum creatinine | 16 | 4453 | Mean Difference (IV, Random, 95% CI) | 10.22 [1.72, 18.72] |
| 7 Elevated lipid levels | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Hypercholesterolaemia | 12 | 5725 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.48, 2.25] |
| 7.2 Hypertriglyceridaemia | 9 | 4698 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.26, 1.74] |
| 8 Lipid outcomes | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Cholesterol | 14 | 5176 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.43, 0.71] |
| 8.2 Triglycerides | 13 | 5099 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.29, 0.51] |
| 9 Abnormal haematological values | 18 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Anaemia | 15 | 8595 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.23] |
| 9.2 Leucopenia | 15 | 8396 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.33, 0.56] |
| 9.3 Thrombocytopenia | 8 | 5028 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.38, 2.79] |
| 10 Haematological outcomes | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Haemoglobin [g/dL] | 6 | 1035 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.63, ‐0.12] |
| 10.2 White cell count [per mm3] | 7 | 3635 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.03, 0.96] |
| 10.3 Platelet count [per mm2] | 6 | 3569 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.43, 1.41] |
5.6. Analysis.
Comparison 5 Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): secondary outcomes, Outcome 6 Serum creatinine.
Comparison 6. Target of rapamycin inhibitors (TOR‐I) versus antimetabolite (AM): outcomes at 5 to 8 years post‐transplant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 5 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.72, 1.39] |
| 2 Total graft loss | 5 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.73, 1.60] |
| 3 Graft loss censored for death | 5 | 791 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.51, 2.00] |
| 4 Malignancies | 3 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.05] |
| 5 GFR | 5 | 534 | Mean Difference (IV, Random, 95% CI) | ‐7.21 [‐19.50, 5.08] |
Comparison 7. Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (all causes) | 9 | 1501 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.64, 1.78] |
| 2 Total graft loss | 8 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.68, 1.75] |
| 3 Graft loss censored for death | 8 | 1385 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.54, 2.20] |
| 4 All acute rejection | 9 | 1509 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.07] |
| 5 Biopsy‐proven acute rejection | 8 | 1381 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 6 CMV infection | 5 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.78, 2.60] |
| 7 Adverse wound outcomes | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 All complications | 3 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.53, 1.71] |
| 7.2 Lymphocoele | 3 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.45, 1.63] |
| 8 All malignancies | 7 | 1163 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.36, 3.04] |
| 9 Number needing to change treatment | 5 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.58, 2.42] |
Comparison 8. Variable doses of target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New‐onset diabetes mellitus | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Tacrolimus | 5 | 580 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.99, 3.23] |
| 1.2 Cyclosporin | 3 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.27, 1.20] |
| 2 Lymphoma/PTLD | 7 | 1298 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.15, 3.07] |
| 3 Adverse cosmetic outcomes | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tremor | 3 | 537 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.34, 2.45] |
| 3.2 Gingival hyperplasia ‐ cyclosporin | 1 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [0.12, 72.52] |
| 3.3 Hirsutism ‐ cyclosporin | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 20.56 [1.22, 345.79] |
| 4 Glomerular filtration rate | 7 | 1305 | Mean Difference (IV, Random, 95% CI) | ‐5.96 [‐9.54, ‐2.38] |
| 5 Serum creatinine | 9 | 1368 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐8.82, 11.89] |
| 6 Elevated lipid levels | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Hypercholesterolaemia | 4 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.75, 1.22] |
| 6.2 Hypertriglyceridaemia | 4 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 1.01] |
| 7 Lipid outcomes | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Total cholesterol | 4 | 709 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.98, 1.45] |
| 7.2 Total triglycerides | 3 | 413 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.55, 0.29] |
| 8 Abnormal haematologic values | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Anaemia | 6 | 1074 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.08] |
| 8.2 Leucopenia | 5 | 1012 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.70, 1.40] |
| 8.3 Thrombocytopenia | 5 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.07] |
| 9 Haematological outcomes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 White cell count [per mm3] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Haemoglobin [g/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Platelet count [per mm2] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Low versus higher dose target of rapamycin inhibitors (TOR‐I): primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 13 | 3894 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.25] |
| 2 Total graft loss | 11 | 3476 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.67, 1.06] |
| 3 Graft loss censored for death | 12 | 3863 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.19] |
| 4 All acute rejection | 13 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.10, 1.42] |
| 5 Biopsy‐proven acute rejection | 11 | 3731 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.10, 1.43] |
| 6 CMV infection | 9 | 3099 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.21] |
| 7 All malignancy | 10 | 3175 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.32] |
| 8 Adverse wound outcomes | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 All wound complications | 7 | 2792 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.29] |
| 8.2 Lymphocoele | 10 | 3302 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 9 Number needing to change treatment | 10 | 3652 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.78, 1.05] |
Comparison 10. Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 New‐onset diabetes mellitus | 6 | 2125 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.51, 0.93] |
| 2 Lymphoma/PTLD | 7 | 2792 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.25, 1.73] |
| 3 Adverse cosmetic outcomes | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tremor | 1 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.63, 1.29] |
| 3.2 Gingival hyperplasia | 2 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.48, 4.42] |
| 3.3 Hirsutism | 2 | 1102 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.30, 0.85] |
| 3.4 Acne/rash | 6 | 2408 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.62, 1.21] |
| 4 Glomerular filtration rate | 7 | 1863 | Mean Difference (IV, Random, 95% CI) | 2.88 [‐0.71, 6.48] |
| 5 Serum creatinine | 7 | 1951 | Mean Difference (IV, Random, 95% CI) | ‐2.21 [‐13.68, 9.26] |
| 6 Elevated lipid levels | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Hypercholesterolaemia | 9 | 3250 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.78, 0.98] |
| 6.2 Hypertriglyceridaemia | 5 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.47, 1.07] |
| 7 Lipid outcomes | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Cholesterol | 5 | 1041 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.35, 0.08] |
| 7.2 Triglycerides | 4 | 1041 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.72, ‐0.03] |
| 8 Abnormal haematological values | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Anaemia | 10 | 3179 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.91] |
| 8.2 Leucopenia | 12 | 3831 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.57, 0.92] |
| 8.3 Thrombocytopenia | 9 | 2242 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.44, 0.75] |
| 9 Haematological outcomes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Haemoglobin [g/dL] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 White cell count [per mm3] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Platelet count [per mm2] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
10.9. Analysis.
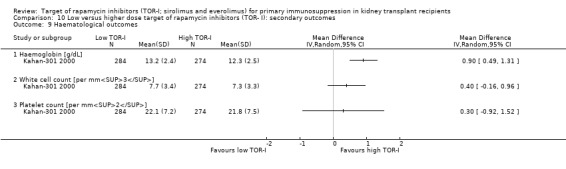
Comparison 10 Low versus higher dose target of rapamycin inhibitors (TOR‐ I): secondary outcomes, Outcome 9 Haematological outcomes.
Comparison 11. Sirolimus versus everolimus: outcomes at 3 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Estimated glomerular filtration rate | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Lipid outcomes | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Cholesterol | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Triglycerides | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All acute rejection (publication type) | 19 | 3016 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.31, 1.92] |
| 1.1 Abstract | 3 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [1.13, 3.65] |
| 1.2 Journal | 16 | 2788 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.92] |
| 2 All acute rejection (risk of bias for sequence generation and allocation concealment) | 19 | 3016 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.31, 1.92] |
| 2.1 Low risk of bias | 7 | 1841 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.98, 2.15] |
| 2.2 High or unclear risk of bias | 12 | 1175 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.35, 2.14] |
| 3 All acute rejection (CNI comparator) | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tacrolimus | 7 | 1384 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.71, 2.81] |
| 3.2 Cyclosporin | 13 | 2233 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.20, 1.83] |
| 4 All acute rejection (antibody induction) | 17 | 2795 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.29, 1.99] |
| 4.1 No induction | 4 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.68] |
| 4.2 Antibody induction | 13 | 2488 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.35, 2.50] |
| 5 Graft loss censored for death (CNI comparator) | 14 | 3277 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.96, 1.81] |
| 5.1 Tacrolimus | 5 | 1238 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.17, 3.25] |
| 5.2 Cyclosporin | 10 | 2039 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.54] |
| 6 Acute rejection (antimetabolite co‐intervention) | 6 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.48] |
| 6.1 Azathioprine | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.85] |
| 6.2 Mycophenolate | 5 | 587 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.75, 1.59] |
12.5. Analysis.
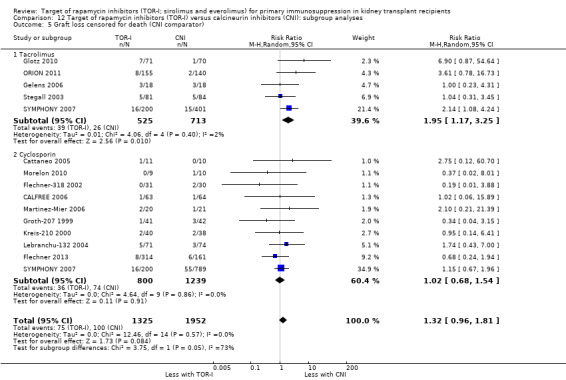
Comparison 12 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses, Outcome 5 Graft loss censored for death (CNI comparator).
12.6. Analysis.
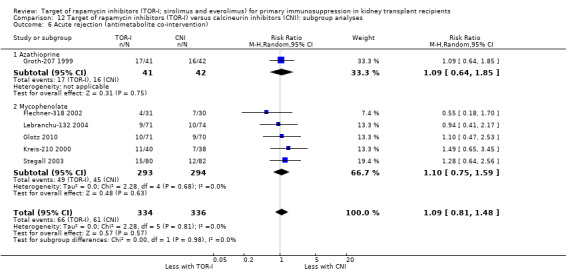
Comparison 12 Target of rapamycin inhibitors (TOR‐I) versus calcineurin inhibitors (CNI): subgroup analyses, Outcome 6 Acute rejection (antimetabolite co‐intervention).
Comparison 13. Target of rapamycin inhibitors (TOR‐I) versus antimetabolites (AM): subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection (publication type) | 32 | 10687 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.07] |
| 1.1 Abstract | 5 | 273 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.29, 1.61] |
| 1.2 Journal | 27 | 10414 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.80, 1.08] |
| 2 Acute rejection (risk of bias for sequence generation and allocation concealment | 32 | 10535 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.09] |
| 2.1 Low risk of bias | 12 | 7313 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 2.2 High risk or unclear risk of bias | 20 | 3222 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.26] |
| 3 Acute rejection (CNI co‐intervention) | 27 | 7437 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.02] |
| 3.1 Tacrolimus | 18 | 4341 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.14] |
| 3.2 Cyclosporin | 9 | 3096 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.70, 0.93] |
| 4 Acute rejection (TOR‐I) | 32 | 10538 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 4.1 Everolimus | 16 | 6126 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.85, 1.26] |
| 4.2 Sirolimus | 16 | 4412 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.70, 1.04] |
| 5 Acute rejection (antibody induction) | 31 | 10476 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.07] |
| 5.1 No induction | 10 | 5293 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.77, 1.12] |
| 5.2 Antibody induction | 21 | 5183 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.70, 1.16] |
| 6 Acute rejection (antimetabolite comparator) | 32 | 10538 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 6.1 Azathioprine | 2 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.39, 1.28] |
| 6.2 Mycophenolate | 30 | 9749 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.86, 1.12] |
Comparison 14. Variable dose target of rapamycin inhibitors (TOR‐I) and calcineurin inhibitors (CNI): subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection (publication type) | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 1.1 Abstract | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.53, 1.42] |
| 1.2 Journal | 7 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 2 Acute rejection (risk of bias for sequence generation and allocation concealment | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 2.1 Low risk of bias | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.50, 1.46] |
| 2.2 High or unclear risk of bias | 7 | 1147 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.59, 1.06] |
| 3 Acute rejection (CNI co‐intervention) | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 3.1 Tacrolimus | 5 | 603 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.16] |
| 3.2 Cyclosporin | 4 | 914 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 4 Acute rejection (TOR‐I) | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 4.1 Everolimus | 2 | 593 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.32] |
| 4.2 Sirolimus | 7 | 924 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 5 Acute rejection (antibody induction) | 9 | 1517 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
| 5.1 No induction | 6 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.53, 0.98] |
| 5.2 Antibody induction | 3 | 613 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.69, 1.46] |
Comparison 15. Low versus higher dose target of rapamycin inhibitors (TOR‐I): subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Acute rejection (publication type) | 13 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.10, 1.41] |
| 1.1 Abstract | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Journal | 13 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.10, 1.41] |
| 2 Acute rejection (risk of bias for sequence generation and allocation concealment | 13 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.10, 1.41] |
| 2.1 Low risk of bias | 5 | 2602 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.15, 1.55] |
| 2.2 High or unclear risk of bias | 8 | 1296 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.33] |
| 3 Acute rejection (CNI co‐intervention) | 12 | 3766 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.09, 1.41] |
| 3.1 Tacrolimus | 3 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.19, 2.19] |
| 3.2 Cyclosporin | 9 | 3005 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.02, 1.35] |
| 4 Acute rejection (antibody induction) | 13 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.10, 1.41] |
| 4.1 No induction | 10 | 2954 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.45] |
| 4.2 Antibody induction | 3 | 944 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.90, 1.62] |
| 5 Acute rejection (TOR‐I) | 13 | 3898 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.10, 1.41] |
| 5.1 Everolimus | 7 | 1966 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.93, 1.33] |
| 5.2 Sirolimus | 6 | 1932 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.16, 1.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anil Kumar 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Co‐interventions
Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was determined using the First Generator Plan (www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was determined using the First Generator Plan (www.randomization.com) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/personnel reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary endpoint was acute rejection diagnosed on biopsy by pathologist without knowledge of the patient’s clinical diagnosis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. Complete follow‐up to 6 months |
| Selective reporting (reporting bias) | Low risk | Expected primary outcomes reported |
| Other bias | High risk | This clinical study was supported by an unrestricted financial grant from Fujisawa Healthcare and clinical revenue of Division of Transplantation, Drexel University College of Medicine |
Anil Kumar 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (MMF/CSA)
Treatment group 2 (SRL/CSA)
Treatment group 3 (MMF/TAC)
Treatment group 4 (SRL/TAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was determined using the First Generator Plan (http://www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | Patients allocated using the First Generator Plan (http://www.randomization.com) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/personnel reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes of death, graft loss, biopsy‐confirmed acute rejection were unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All surviving patients completed 4 years of follow up; analysed in groups to which randomised |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported for all four treatment groups |
| Other bias | Unclear risk | Funding source not reported |
ATHENA 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/TAC)
Treatment group 2 (EVL/CSA)
Treatment group 3 (MPA/TAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "validated system to ensure an unbiased treatment assignment in a 1:1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "validated system to ensure an unbiased treatment assignment in a 1:1:1 ratio" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome is GFR and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 655 randomised; 43 did not receive medication. ITT population 612/safety population 612 |
| Selective reporting (reporting bias) | Low risk | Expected outcomes were reported |
| Other bias | High risk | The study was funded by Novartis Pharma GmbH, Nürnberg, Germany |
AVESTA 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/rCSA)
Treatment group 2 (MPA/sCNI)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible patients randomised 1:1 prior to transplantation |
| Allocation concealment (selection bias) | Unclear risk | Eligible patients randomised 1:1 prior to transplantation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided to suggest study was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was GFR so unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to permit judgement ‐ abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to permit judgement ‐ abstract only |
| Other bias | Unclear risk | Insufficient data to permit judgement ‐ abstract only |
Bechstein‐193 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (rTAC; high dose SRL)
Treatment group 2 (sTAC; low dose SRL)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment 1:1; no further information |
| Allocation concealment (selection bias) | Unclear risk | Random assignment 1:1; no further information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome is laboratory based (CrCl) and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Expected primary outcomes reported |
| Other bias | High risk | At the time of this study, Anthony J. Zygmunt was an employee of Wyeth Research |
Bertoni 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (sCSA/MMF)
Treatment group 2 (rCSA/EVL)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes (graft loss, BPAR) unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | No report on wound complications |
| Other bias | Low risk | Quote: "no financial support" |
Burke 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (SRL/TAC)
Treatment group 2 (MMF/TAC)
Treatment group 3 (SRL/CS)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation scheme; equally divided into three groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | National Institutes of Health (grant R01DK25243‐25), Miami Veterans Affairs Medical Center research support, Astellas Pharma US, Roche Laboratories, and Wyeth |
CALFREE 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "127 patients were randomly assigned before transplant..(in a masked fashion)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "127 patients were randomly assigned before transplant..(in a masked fashion)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome (kidney function) was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/127 (3%) did not complete follow up |
| Selective reporting (reporting bias) | Low risk | Expected outcomes (graft function, rejection, death, adverse effects) reported |
| Other bias | High risk | Sponsored by Wyeth |
Cattaneo 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were allocated to one of the following two study groups according to a randomization design" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "They were allocated to one of the following two study groups according to a randomization design" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was MPA levels & these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether all patients randomised were included in analyses |
| Selective reporting (reporting bias) | High risk | Only GFR and SCr reported |
| Other bias | Unclear risk | No report on funding |
Ciancio 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/sTAC)
Treatment group 2 (MPS/sTAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be open‐label RCT but no other data provided |
| Allocation concealment (selection bias) | Unclear risk | Said to be open‐label RCT but no other data provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was Biopsy proven rejection & unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Novartis educational grant CRAD001AUS103T |
Cohen 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (rSRL/sCSA)
Treatment group 2 (sSRL/rCSA)
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcome unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% participants did not complete 1 year follow‐up |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Durlik 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (TAC, MMF)
Treatment group 2 (TAC, SRL)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Durrbach 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised |
| Allocation concealment (selection bias) | Low risk | Centrally randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were patient/graft survival and these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified primary outcomes reported |
| Other bias | High risk | Funded by Wyeth. EUDRACT trial number: 0468E1‐100969 |
Esmeraldo 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/sTAC)
Treatment group 2 (MPS/sTAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised 1:1 within 24 hours post transplant. No further information |
| Allocation concealment (selection bias) | Unclear risk | Randomised 1:1 within 24 hours post transplant. No further information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was CMV infection diagnosed by lab tests routinely done to 6 months and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Said to be ITT population |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Other bias | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
EVEREST 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (rEVL/sCSA)
Treatment group 2 (sEVL/rCSA)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization codes were generated at Novartis Farma SpA (Origgio, Varese, Italy), using a validated computer method" |
| Allocation concealment (selection bias) | Low risk | Quote: "Each center was assigned an adequate number of sealed envelops, each of them labeled with a unique patient number, that were opened after transplantation immediately before the administration of the first EVL dose" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were CrCl estimated from Cockcroft and Gault equation and the proportion of patients with BPAR. Unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted. All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Outcomes mentioned in methods are reported |
| Other bias | High risk | Sponsored by Novartis. Several authors also had affiliations or were authors of Novartis |
EVEROLD 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised but no other information provided. |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised but no other information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported on 94% of participants. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Other bias | High risk | Novartis, Roche, Genzyme listed on clinical trials as sponsors |
Favi 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/sCSA)
Treatment group 2 (MMF/sTAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "consecutively assigned 1:1 to one of the two immunosuppressive regimens" |
| Allocation concealment (selection bias) | High risk | Quote: "consecutively assigned 1:1 to one of the two immunosuppressive regimens" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed follow‐up |
| Selective reporting (reporting bias) | High risk | Adverse effects incompletely reported |
| Other bias | Low risk | This study was partially supported by UCSC grant MIUR 2007 |
Favi 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding likely to influence performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft loss, BPAR and lack of blinding likely to influence outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients (5%) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Fernandes‐Charpiot 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/MPS)
Treatment group 2 (TAC/MPS)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised but no details provided |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised but no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Other bias | High risk | Novartis Research Support listed in disclosures |
FIBRASIC 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (SRL/MMF)
Treatment group 2 (CSA/MMF)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be a RCT but no details provided. |
| Allocation concealment (selection bias) | Unclear risk | Said to be a RCT but no details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
| Other bias | Unclear risk | Insufficient information to permit judgement ‐ abstract only |
Flechner 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned (2:1) by a computerized randomisation/enrolment system |
| Allocation concealment (selection bias) | Low risk | Patients were randomly assigned (2:1) by a computerized randomisation/enrolment system |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were BPAR and GFR; unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated prematurely because of increased risk of rejection in SRL group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Wyeth. Authors were employees/received funding from drug companies |
Flechner‐318 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | Comparison: TOR‐I versus CNI. 3 switched from Cyclosporin ‐ 1 severe hirsutism and gum hypertrophy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised via computer‐generated cards" |
| Allocation concealment (selection bias) | Low risk | Quote "randomised via computer‐generated cards" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes of kidney function and number of acute rejection episodes between group were laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported (death, graft loss, rejection) |
| Other bias | High risk | Sponsored by Wyeth |
Gallon 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised but no information provided |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised but no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was graft loss by 3 years & unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 82/94 (87%) randomised were analysed ‐ per protocol analysis only |
| Selective reporting (reporting bias) | High risk | Limited information on adverse effects |
| Other bias | High risk | Partly funded by Astellas‐USA |
Gelens 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 12‐month, open label, prospective, parallel group, randomised (1‐1‐1), single‐centre study; no other information provided |
| Allocation concealment (selection bias) | Unclear risk | 12‐month, open label, prospective, parallel group, randomised (1‐1‐1), single‐centre study; no other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death/graft loss and these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients included in analyses |
| Selective reporting (reporting bias) | High risk | Reported on death, graft loss, rejection‐free survival. Inadequate reporting of adverse effects |
| Other bias | High risk | Sponsored by Roche, Astellas (Fujisawa Beneleux) |
Glotz 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Open‐label randomised study. No further information provided |
| Allocation concealment (selection bias) | Unclear risk | Open‐label randomised study. No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft loss & these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 141/149 included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes |
| Other bias | High risk | Wyeth Research, Paris, France |
Gonwa‐PSG 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised 1:1 to receive corticosteroids and either TAC plus SRL or TAC plus MMF. No other information |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised 1:1 to receive corticosteroids and either TAC plus SRL or TAC plus MMF. No other information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft survival and these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | Limited information on adverse effects. No information on wound complications |
| Other bias | High risk | Sponsored by Fujisawa |
Grinyo 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated in a 1:1 proportion to one of two groups using computer‐generated randomisation envelopes prepared by Wyeth without stratification |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated in a 1:1 proportion to one of two groups using computer‐generated randomisation envelopes prepared by Wyeth without stratification |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was kidney function: Laboratory outcome unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | No information on wound complications & limited information on other adverse effects |
| Other bias | High risk | Assistance provided by Wyeth |
Groth‐207 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer based randomisation |
| Allocation concealment (selection bias) | Low risk | Central computer based randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft loss and biopsy confirmed acute rejection. These unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data addressed (ITT analysis) |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Supported by Wyeth‐Ayerst |
Hamdy 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomised controlled trial where they were divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A prospective, randomised controlled trial where they were divided into two groups" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death and graft survival and these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | No report of funding |
Kahan‐157 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Said to be double‐blinded but no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft survival and BPAR and these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes |
| Other bias | High risk | Sponsored by Novartis. Study authors employed by Novartis |
Kahan‐203 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Treatment group 5
Treatment group 6
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised but no further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Single blind study. Patients not investigators blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft survival and BPAR and these unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients appear to be accounted for; 149/151 received study drug and reported |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Wyeth‐Ayerst |
Kahan‐301 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomisation schedule" |
| Allocation concealment (selection bias) | Low risk | Study drugs assigned after transplant by computer generated randomisation schedule |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Drug Study code could only be broken in event of emergency" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Drug Study code could only be broken in event of emergency" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in ITT analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Funded by Wyeth‐Ayerst |
Kandaswamy 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Baseline immunosuppression
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised by non‐blinded card pull |
| Allocation concealment (selection bias) | High risk | Randomised by non‐blinded card pull |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were death, graft loss and biopsy confirmed acute rejection. These unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Each arm analysed according to ITT |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Funded by Fujisawa and Genzyme |
Kovarik‐2306 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective, multicenter, randomised study. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Prospective, multicenter, randomised study. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary endpoint was GFR and creatinine measured in central laboratory. Unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Novartis |
Kovarik‐251 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was 1:1:1. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was 1:1:1. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind double‐dummy for 1 year; then open label, when amendment protocol introduced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind double‐dummy for 1 year; then open label, when amendment protocol introduced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Novartis, some authors employed by Novartis |
Kramer‐2307 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective, multicenter, randomised study. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Prospective, multicenter, randomised study. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary endpoint was GFR and creatinine measured in central laboratory. Unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Supported by Novartis Pharma AG |
Kreis‐210 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Open‐label, parallel group, multicenter RCT. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Open‐label, parallel group, multicenter RCT. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of participants or personnel but primary outcome was laboratory based and is unlikely to influence blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All primary outcomes mentioned |
| Other bias | High risk | Funded by Wyeth‐Ayerst Research, Paris, France |
Lebranchu‐132 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned prior to transplantation by computer‐generated selection |
| Allocation concealment (selection bias) | Low risk | Patients were randomly assigned prior to transplantation by computer‐generated selection |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of participants or personnel reported but primary outcome laboratory based and unlikely to influence judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Wyeth |
Lo 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective, randomised, comparative pilot study. No details provided |
| Allocation concealment (selection bias) | Unclear risk | Prospective, randomised, comparative pilot study. No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary endpoint of the study was a composite of patient death, graft lost, or biopsy‐confirmed AR at 6 months post‐transplantation; unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed 6 months of study |
| Selective reporting (reporting bias) | High risk | Limited information on adverse effects |
| Other bias | High risk | Sponsored by Wyeth and SangStat |
MacDonald‐302 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomly assigned to one of three treatment groups in a 2:2:1 ratio. A computerized system was used to randomise and stratify patients by centre and donor source (living or cadaver) |
| Allocation concealment (selection bias) | Low risk | A computerized system was used to randomise and stratify patients by centre and donor source (living or cadaver) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and investigators blinded to treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients and investigators blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Wyeth‐Ayerst |
Machado 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants/personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes of graft loss, BPAR and death unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Martinez‐Mier 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes mentioned |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Morelon 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2 (CSA)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Randomised using sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes of death/graft loss/AR unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | not all prespecified outcomes mentioned, limited information on adverse events |
| Other bias | High risk | Funded by Genzyme |
ORION 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned" insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for. Efficacy/safety analyses based on ITT comprising all patients, who were transplanted and received ≥ 1 dose of treatment medications. 1.6% excluded |
| Selective reporting (reporting bias) | Low risk | Primary endpoints mentioned |
| Other bias | High risk | Study sponsored by Wyeth and several investigators were employees of Wyeth at the time of the study |
Paoletti 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated block; 1:2 randomisation was adopted |
| Allocation concealment (selection bias) | Low risk | Allocation was implemented using sequentially numbered, opaque sealed envelopes that were kept by an employee of the Regione Liguria Transplant Coordination Office who was not involved in the clinical study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Kidney outcomes unlikely to be influenced by lack of blinding. Primary outcome (cardiac) was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no drop‐outs in of the groups, and all patients completed the 1‐year observation period |
| Selective reporting (reporting bias) | High risk | Reporting of adverse events incomplete |
| Other bias | Low risk | The Italian National Health Service (Servizio Sanitario Nazionale) (Italy) and San Martino University Hospital (Azienda Ospedaliera Universitaria San Martino), Genoa (Italy) |
Pascual 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised; insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome lab based and unlikely to influence blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/35 withdrawn (22%) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes mentioned |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Pescovitz 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Enrolled 2:1 before transplant. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes BPAR, death, graft loss unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Roche |
Qazi 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Novartis Drug supply Management generated a randomization list, using a validated system with a fixed‐block design that automated treatment‐arm randomization in the specified ratio' |
| Allocation concealment (selection bias) | Low risk | Investigators received treatment allocation cards with sequential randomisation numbers and treatment group information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures were unlikely to be influenced by lack of blinding (death, graft loss, BPAR) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients only excluded from everolimus group |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Funded by Novartis |
RECORD 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was centrally released by an electronic case report form before transplantation. Randomization code was generated and performed using a block designed stratified by each site" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was centrally released by an electronic case report form before transplantation. Randomization code was generated and performed using a block designed stratified by each site" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All blinded before randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome BPAR, graft loss and patient death and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patients accounted for but greater than 10% not analysed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes mentioned |
| Other bias | High risk | Pharma funded study. Funded by Astellas |
Riad 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised; insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome lab based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Rostaing 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Russ 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | Funded by Wyeth |
Sampaio 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2 (SRL/sTAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised 1:1 using a computer generated sequence number" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was computer generated but unclear whether sequence was known to investigators |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All studies pre‐specified outcomes mentioned |
| Other bias | High risk | Grant sponsored by Jansen‐Cilag |
Schaefer 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes mentioned |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Shetty 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomised; insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomised; insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes lab based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement; abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement, abstract only |
| Other bias | Unclear risk | Insufficient information to permit judgement; abstract only |
Souza 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (dosage not reported)
Treatment group 2 (dosage not reported)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Single centre, prospective, randomised, controlled pilot study |
| Allocation concealment (selection bias) | Unclear risk | Single centre, prospective, randomised, controlled pilot study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were CMV diagnosis and BPAR; unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Spagnoletti 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (dosage/levels not reported)
Treatment group 2 (dosage/levels not reported)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary outcome composite outcome ‐ included clinical rejection, need to change medication |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgment; abstract only |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgment; abstract only |
| Other bias | Unclear risk | insufficient information to permit judgment; abstract only |
Stallone 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All recipients accounted for |
| Selective reporting (reporting bias) | High risk | No report of adverse effects |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Stegall 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A prospective, randomised trial". No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A prospective, randomised trial". No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three (1.8%) excluded from final analysis as did not receive transplants |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | This study was supported in part by research contracts from Wyeth Research, Philadelphia, PA, Genzyme Corporation, Cambridge, MA, and Roche Laboratories Inc., Nutley, NJ |
SYMPHONY 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients underwent randomisation... with the use of a centralized interactive voice response system (ClinIT)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients underwent randomisation... with the use of a centralized interactive voice response system (ClinIT)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was eGFR; laboratory outcome so unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% randomised patients not included in ITT analyses |
| Selective reporting (reporting bias) | Low risk | Expected outcomes for this review (death, graft loss and acute rejection) have been reported. No protocol but outcomes specified in methods reported in results |
| Other bias | High risk | Funding for the study was provided by Hoffmann‐La Roche |
Takahashi 2013a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified by donor type deceased/living; randomised 1:1. Independent clinical research company using a validated system that automated the random assignment of treatment arms to randomisation numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation list was produced by an independent clinical research organization using a validated system that automated the random assignment of treatment arms to randomisation numbers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome is GFR. Lab measure unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT and less than 10% lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | Author list includes Novartis employees |
Tedesco‐Silva 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective, 12‐month, randomised, two‐arm, concentration‐controlled study. Randomised 7 days after transplant |
| Allocation concealment (selection bias) | Unclear risk | Prospective, 12‐month, randomised, two‐arm, concentration‐controlled study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tedesco‐Silva 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned a randomisation number, which was linked to one of the three treatment groups, using an interactive voice‐response system. The randomisation scheme was reviewed and approved by the Biostatistics Quality Assurance Group |
| Allocation concealment (selection bias) | Low risk | Patients were assigned a randomisation number, which was linked to one of the three treatment groups, using an interactive voice‐response system. The randomisation scheme was reviewed and approved by the Biostatistics Quality Assurance Group |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Funded by Novartis. Authors received money from drug companies |
Tedesco‐Silva 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation sequence, stratified living/deceased donor, randomised 1:1 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelops. Transplant surgeons were blinded to treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory based & unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | Investigator‐initiated study that was partially supported by Novartis |
TRANSFORM 2018.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (EVL/rCNI)
Treatment group 2 (MPA/sCNI)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A randomization sequence, stratified within treatment groups by donor type (living, deceased standard criteria, or deceased expanded criteria) and by the type of CNI (CsA or tacrolimus), was generated by a computer program and implemented by telephone‐based interactive response technology.” |
| Allocation concealment (selection bias) | Low risk | Quote: “A randomization sequence, stratified within treatment groups by donor type (living, deceased standard criteria, or deceased expanded criteria) and by the type of CNI (CsA or tacrolimus), was generated by a computer program and implemented by telephone‐based interactive response technology.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes (GFR, BPAR) were laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Pharma funded by Novartis |
van Gurp 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2 (MMF/sTAC)
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation 1:1, stratified by centre before first dose of medication. Sealed randomisation envelopes were supplied by study sponsor |
| Allocation concealment (selection bias) | Low risk | Sealed randomisation envelopes were supplied by study sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded and primary outcome (GFR) was laboratory based and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes mentioned or reported |
| Other bias | High risk | Funded by Astelllas Pharma Europe, involved in study design, analysis of data and preparation of manuscript |
van Hooff 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment/control group 1
Treatment group 2
Treatment group 3
Treatment group 4
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective RCT. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Prospective RCT. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/106 (1.9%) not transplanted and excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Velosa‐212 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised assigned to two groups. No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients were randomised assigned to two groups. No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Funded by Wyeth |
Vitko‐201 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised according to a computer‐generated schedule that ensured equal distribution among the three treatment groups within each centre |
| Allocation concealment (selection bias) | Low risk | Patients were randomised according to a computer‐generated schedule that ensured equal distribution among the three treatment groups within each centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double dummy for 12 months |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, double dummy for 12 months |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Supported by Novartis |
Vitko‐TERRA 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was 1:1:1 and was performed locally at each centre using sealed randomisation envelopes supplied by the study sponsor |
| Allocation concealment (selection bias) | Low risk | Randomisation was 1:1:1 and was performed locally at each centre using sealed randomisation envelopes supplied by the study sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Fujiawa |
ARB ‐ angiotensin receptor blocker; ATN ‐ acute tubular necrosis; ACEi ‐ angiotensin‐converting enzyme inhibitor; ALG ‐ antilymphocyte globulin; ATG ‐ antithymocyte globulin; AZA ‐ azathioprine; BMI ‐ body mass index; BP ‐ blood pressure; BPAR ‐ biopsy‐proven acute rejection; CAN ‐ chronic allograft nephropathy; CCB ‐ calcium channel blockers; CMV ‐ cytomegalovirus; CNI ‐ calcineurin inhibitor; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin; DD ‐ deceased donor; ER ‐ extended release; ESKD ‐ end‐stage kidney disease; EVL ‐ everolimus; FSGS ‐ focal segmental glomerulosclerosis; (e)GFR ‐ (estimated) glomerular filtration rate; GI ‐ gastrointestinal; Hb ‐ haemoglobin; HbSAg ‐ hepatitis B surface antigen; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; HLA ‐ human leukocyte antigen; IL2 ‐ interleukin 2; ITT ‐ intention‐to‐treat; IV ‐ intravenous(ly); LD ‐ living donor; LRD ‐ living‐related donor; M/F‐ male/female; MI ‐ myocardial infarction; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; MPA ‐ mycophenolic acid; MPS ‐ mycophenolate sodium; NODM ‐ new‐onset diabetes mellitus; PRA ‐ panel reactive antibodies; r ‐ reduced dose (rCSA; rTAC); RCT ‐ randomised controlled trial; s ‐ standard dose (sCSA; sTAC); SCr ‐ serum creatinine; SD ‐ standard deviation; SRL ‐ sirolimus; TAC ‐ tacrolimus; TOR‐I ‐ target of rapamycin inhibitor; WBC ‐ white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ADHERE 2017 | SRL not started till day 28 post transplant |
| Barsoum 2007 | Cannot compare SRL with MMF till 3 months or more post transplant |
| CALLISTO 2009 | Compared SRL commenced at transplant with SRL commenced at week 5 |
| Carmellini 2010 | TOR‐I use in stable transplant patients (> 6 months); not primary immunosuppression |
| CENTRAL 2012 | Patient randomised at week 7 |
| CERTITEM 2015 | Randomised at 3 months based on protocol biopsy at 3 months. Therefore not primary Immunosuppression |
| Citterio 2004 | Unclear if this is a RCT |
| Cruzado 2016 | Switch to EVL as secondary immunosuppression more than 1 year post transplant |
| EVIDENCE 2014 | Study of non‐inferiority of steroid withdrawal |
| Fior 2015 | Unclear if this is a RCT |
| Libetta 2007 | Patients selected into study at 3 months; unclear whether RCT |
| Libetta 2015 | Late conversion to SRL |
| Mathew 2006 | Compares same dose of SRL using oral solution or tablets |
| Nafar 2012 | Quasi‐RCT comparing TOR‐I with CNI but TOR‐I not commenced till 3 months post transplant |
| NCT00005113 | Paediatric study terminated due to inability to recruit sufficient patients |
| NCT00965094 | Patients not randomised until month 3 |
| nEVEROLD 2017 | Conversion to TOR‐I at 1 month |
| NEVERWOUND 2014 | Compares immediate with delayed administration of EVL |
| Novoa 2011 | Patients not randomised till 3 months |
| Oh 2012 | EVL commenced at 1 month post transplant |
| Pretagostini 2016 | EVL commenced at 1 month post transplant |
| Rivelli 2014 | Both groups received SRL; dose increased in one group at 3 months when TAC ceased |
| SOCRATES 2014 | EVL not commenced till 14 days post transplant |
| Tamashiro 2017 | Late conversion at 3 months to TOR‐I; not clear whether this is an RCT |
| van Gelder 2003 | Conversion to TOR‐I at 12 months |
| Wojciechowski 2017 | Late conversion to TOR‐I after diagnosis of BK viruria |
| Wyrley‐Birch 2009 | Randomisation between pairs of recipients of kidneys from same donor not groups of recipients |
CNI ‐ calcineurin inhibitor; EVL ‐ everolimus; MMF ‐ mycophenolate mofetil; RCT ‐ randomised controlled trial; SRL ‐ sirolimus; TAC ‐ tacrolimus; TOR‐I ‐ target of rapamycin inhibitor
Characteristics of studies awaiting assessment [ordered by study ID]
Ferreira 2019.
| Methods | Single centre RCT enrolling adult recipients of ECD donors |
| Participants | 171 enrolled |
| Interventions | All receive single dose of 1 gm methylprednisolone & then oral prednisone & induction with 4 doses of ATG Group 1
Group 2
|
| Outcomes |
|
| Notes | The institution “Hospital do Rim” have received research grants from Novartis and Sanofi to conduct this study |
Traitanon 2019.
| Methods | RCT enrolling adults 18 to 70 years; recipients of living donor transplants; PRA < 20%; patients with ESKD from primary FSGS excluded |
| Participants | 88 enrolled by June 2014 |
| Interventions | Group
Group 2
|
| Outcomes | Change in transplant function Change in T cell and B cell immune response |
| Notes | Study supported by Novartis |
ATG ‐ antithymocyte globulin; BPAR ‐ biopsy‐proven acute rejection; CMV ‐ cytomegalovirus; ECD ‐ extended criteria donors; ESKD ‐ end‐stage kidney disease; EVL ‐ everolimus; FSGS ‐ focal segmental glomerulosclerosis; MMF ‐ mycophenolate mofetil; MPA ‐ mycophenolic acid; PRA ‐ panel reactive antibodies; RCT ‐ randomised controlled trial; TAC ‐ tacrolimus
Characteristics of ongoing studies [ordered by study ID]
EVER TWIST 2013.
| Trial name or title | EVER TWIST study |
| Methods | Open‐label RCT enrolling de novo kidney transplant recipients |
| Participants | 31 enrolled by 2013 |
| Interventions | Group A
Group B
|
| Outcomes | Immunological data |
| Starting date | Not reported |
| Contact information | Dr Carmelo Libetta |
| Notes |
NCT02077556.
| Trial name or title | The effect of everolimus on the pharmacokinetics of tacrolimus in renal transplant patients, and the effect of ABCB1, CYP3A4, CYP3A5, POR genetic polymorphism on the two drugs |
| Methods | RCT; parallel assignment; open‐label |
| Participants | 70 adult recipients (20 to 65 years) of de novo kidney transplants; aspartate aminotransferase/alanine aminotransferase within 2 times the upper limit of normal range |
| Interventions | Group 1
Group 2
|
| Outcomes | Pharmacokinetic profiles (8 to 10 days post transplant); acute rejection (within 2 weeks) |
| Starting date | April 2014; Estimated study completion date January 2018 |
| Contact information | Fe‐Lin Lin Wu; flwu@ntu.edu.tw. Taiwan |
| Notes | Duration of follow‐up not reported but only clinical outcome to be reported is acute rejection within 2 weeks of transplant. Primary outcomes are pharmacological as aim of study is to investigate for drug interactions |
NCT03468478.
| Trial name or title | Comparison of the efficacy and safety of sirolimus versus everolimus versus mycophenolate in kidney transplantation (SEM) |
| Methods | RCT; parallel assignment; open label |
| Participants | 400 adult recipients (18 years and older) of their first living or deceased donor kidney transplant. Patients with FSGS/history of nephrotic syndrome excluded |
| Interventions | Group 1 (SRL + rTAC)
Group 2 (EVL + rTAC)
Group 3 (MMF + sTAC)
|
| Outcomes | Incidence of CMV disease or infection by 12 months; no other outcomes provided |
| Starting date | June 16, 2017; expected completion date June 18, 2021 |
| Contact information | Helio Tedesco Silva Jr, Hospital do Rim e Hipertensão, Brazil |
| Notes |
ATG ‐ antithymocyte globulin; CMV ‐ cytomegalovirus; ESKD ‐ end‐stage kidney disease; EVL ‐ everolimus; FSGS ‐ focal segmental glomerulosclerosis; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; MPS ‐ mycophenolate sodium; PRA ‐ panel reactive antibodies; RCT ‐ randomised controlled trial; reduced dose ‐ r (rCSA, rTAC); SRL ‐ sirolimus; standard dose ‐ s (sCSA, sTAC); TAC ‐ tacrolimus
Differences between protocol and review
For this update, risk of bias assessment and GRADE have been used
Contributions of authors
Writing of protocol and review ‐ AW, VSWL, JRC, JCC Screening of titles and abstracts ‐ AW, VSWL, DH, EH, LH Assessment for inclusion ‐ AW, VSWL, DH, EH, LH Quality assessment ‐ AW, VSWL, DH, EH, LH Data extraction ‐ AW, VSWL,DH, EH, LH Data entry into RevMan ‐ AW, VSWL, DH, EH, LH Data analysis ‐ AW, VSWL, DH, EH Disagreement resolution ‐ AW, JRC, JCC, DH, EH Writing of the update review ‐ LH, DH, EH, AW, VSWL Review procedures for update ‐ LH, DH, EH, AW
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anil Kumar 2005 {published and unpublished data}
- Anil Kumar MS, Fyfe B, Sierka D, Heifets M, Saeed MI, Parikh MH. Comparison of efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) as adjunct to calcineurin inhibitor (CNI) based steroid free immunosuppression in kidney transplantation [abstract no: 1530]. American Journal of Transplantation 2004;4(Suppl 8):578. [CENTRAL: CN‐00509056] [Google Scholar]
- Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, et al. Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 2005;80(6):807‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Anil Kumar MS, Heifets M, Fyfe B, Sierka D, Saeed MI, Parekh M, et al. A prospective randomized study to compare the efficacy and safety of sirolimus (SLR) and mycophenolate mofetil (MMF) monitored by protocol biopsies in tacrolimus (TAC) based steroid free immunosuppression [abstract no: 212]. American Journal of Transplantation 2004;4(Suppl 8):216. [CENTRAL: CN‐00509296] [Google Scholar]
- Kumar A, Lee D, Xiao SG, Moritz MJ, Fyfe B, Heifets M, et al. Comparison of tacrolimus (FK506) and sirolimus (SRL) combination with FK506 and mycophenolate mofetil (MMF) in kidney transplant recipients with steroid avoidance [abstract no: 771]. American Journal of Transplantation 2003;3(Suppl 5):350. [CENTRAL: CN‐00446223] [Google Scholar]
Anil Kumar 2008 {published data only}
- Anil Kumar MS, Heifets M, Fyfe B, Moritz MJ, Saeed MI, Parikh MH, et al. Comparison of four different steroid avoidance protocols in kidney transplantation: tacrolimus (TAC)/mycophenolate mofetil (MMF) versus Tac/sirolimus (SLR) versus cyclosporine (CSA)/MMF versus CSA/SLR [abstract no: 512]. American Journal of Transplantation 2005;5(Suppl 11):286‐7. [CENTRAL: CN‐00679049] [Google Scholar]
- Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento‐Reodica N, Kumar AM, et al. Comparison of four different immunosuppression protocols without long‐term steroid therapy in kidney recipients monitored by surveillance biopsy: five‐year outcomes. Transplant Immunology 2008;20(1‐2):32‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
ATHENA 2016 {published data only}
- Dragun D, Sommerer C, Hauser IA, Suwelack BM, Schenker P, Witzke O, et al. Everolimus [EVR]‐based versus tacrolimus [TAC]‐MPA regimen in de novo kidney transplant recipients: 12 months safety and efficacy data from the ATHENA study [abstract no: SA‐PO510]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):810. [CENTRAL: CN‐01657414] [Google Scholar]
- Dragun D, Sommerer C, Suwelack B, Schenker P, Hauser I, Witzke O, et al. ATHENA study outcomes on allograft function after 12 months with everolimus‐CNI vs tacrolimus‐MPA regimen in de novo renal transplant recipients [abstract no: PLB099]. Transplant International 2017;30(Suppl 2):562. [EMBASE: 618769585] [Google Scholar]
- Dragun D, Suwelack B, Sommerer C, Hauser IA, Witzke O, Hugo C, et al. Significant anti‐CMV/BKV effect of a modern everolimus‐based regimen comparted to standard tacrolimus‐MPA regimen in De novo kidney transplant recipients: ATHENA 12 months data on infections [abstract no: 401.2]. Transplantation 2018;102(7 Suppl 1):S128. [EMBASE: 623701445] [Google Scholar]
- Hauser A, Sommerer C, Suwelack B, Dragun D, Witzke O, Hugo C, et al. Significant anti‐CMV/BKV effect of a modern everolimus‐based regimen comparted to a standard tacrolimus‐MPA regimen in de novo kidney transplant recipients: ATHENA 12 months data on infections [abstract no: 183]. American Journal of Transplantation 2018;18(Suppl 4):318. [EMBASE: 622280450] [Google Scholar]
- Hauser IA, Marx S, Sommerer C, Suwelack B, Dragun D, Witzke O, et al. Differences in CMV‐specific CD4 T cell population in de novo kidney transplant recipients treated with everolimus‐based regimen compared to a standard tacrolimus‐MPA regimen: Results from ATHENA [abstract]. American Journal of Transplantation 2019;19(Suppl 3):520. [EMBASE: 628454558] [Google Scholar]
- Hauser IA, Sommerer C, Suwelack BM, Dragun D, Schenker P, Witzke O, et al. Significantly less CMV‐and BKV‐events with everolimus‐based versus tacrolimus‐MPA regimen in de novo renal transplant recipients: 12 months data on infections from the ATHENA study [abstract no: SA‐PO484]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):803. [CENTRAL: CN‐01657413] [Google Scholar]
- Kamar N, Merville P, Sommerer C, Suwelack B, Dragun D, Hauser IA, et al. 12 months data on infections from ATHENA study show significantly less CMV‐and BKV‐events with everolimus‐based versus tacrolimus‐MPA regimen in de novo renal transplant recipients [abstract no: O41]. Transplant International 2018;31(Suppl 1):14. [EMBASE: 619997616] [Google Scholar]
- Kamar N, Merville P, Suwelack B, Sommerer C, Dragun D, Hauser IA, et al. 12 months safety and efficacy data from ATHENA study on everolimus‐based vs tacrolimus‐MPA regimen in de novo kidney transplant recipients [abstract no: O85]. Transplant International 2018;31(Suppl 1):25. [EMBASE: 619997548] [Google Scholar]
- Merville P, Kamar N, Dragun D, Sommerer C, Suwelack B, Schenker P, et al. 12 months outcomes on allograft function with everolimus‐CNI vs tacrolimus‐MPA regimen in de novo renal transplant recipients: the ATHENA study [abstract no: O43]. Transplant International 2018;31(Suppl 1):15. [EMBASE: 619997638] [Google Scholar]
- Nashan B, Sommerer C, Suwelack B, Dragun D, Hauser I, Schenker P, et al. Significantly less CMV‐and BKV‐events with everolimus‐based vs. tacrolimus‐MPA regimen in de novo renal transplant recipients: 12 months data on infections from ATHENA study [abstract no: LOS009]. Transplant International 2017;30(Suppl 2):162. [EMBASE: 618769221] [Google Scholar]
- Nashan B, Sommerer C, Suwelack B, Dragun D, Hauser IA, Witzke O, et al. The ATHENA study: 12 months safety and efficacy data for everolimus combined with tacrolimus or cyclosporine compared to a standard tacrolimus‐mycophenolate regimen in de novo kidney transplant patients [abstract no: 517.1]. Transplantation 2018;102(7 Suppl 1):S279. [EMBASE: 623702422] [Google Scholar]
- Nashan B, Sommerer C, Suwelack B, Dragun D, Witzke O, Hugo C, et al. Allograft function after 12 months with everolimus plus either tacrolimus or cyclosporine compared to a standard regimen of tacrolimus with MPA in de novo renal transplant recipients: the ATHENA study [abstract no: 38]. American Journal of Transplantation 2018;18(Suppl 4):260. [EMBASE: 622280707] [Google Scholar]
- Schenker P, Sommerer C, Suwelack B, Dragun D, Hauser IA, Witzke O, et al. Outcomes on allograft function after 12 months with everolimus plus either tacrolimus or cyclosporine compared to a standard tacrolimus‐MPA regimen in de novo renal transplant recipients: the ATHENA study [abstract no: 517.3]. Transplantation 2018;102(7 Suppl 1):S280. [EMBASE: 623702498] [Google Scholar]
- Sommerer C, Suwelack B, Dragun D, Hauser I, Schenker P, Baumer D, et al. 12‐month ATHENA study: everolimus versus standard regimen in de novo renal transplant recipients [abstract no: P251]. Transplant International 2015;28(Suppl 4):450. [EMBASE: 72112244] [Google Scholar]
- Sommerer C, Suwelack B, Dragun D, Hauser I, Schenker P, Witzke O, et al. Everolimus‐based vs. tacrolimus‐MPA regimen in de novo kidney transplant recipients: 12 months safety and efficacy data from ATHENA study [abstract no: LOS003]. Transplant International 2017;30(Suppl 2):160. [EMBASE: 618768794] [Google Scholar]
- Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Nashan B, et al. Design and rationale of the ATHENA study ‐ a 12‐month, multicentre, prospective study evaluating the outcomes of a de novo everolimus‐based regimen in combination with reduced cyclosporine or tacrolimus versus a standard regimen in kidney transplant patients: study protocol for a randomised controlled trial. Trials [Electronic Resource] 2016;17:92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Witzke O, et al. ATHENA study: outcomes on allograft function after 12 months of modern everolimus‐based vs. conservative tacrolimus‐MPA regimen in de novo renal transplant recipients [abstract no: PV18]. Transplant International 2017;30(Suppl 4):20. [EMBASE: 619082507] [Google Scholar]
- Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, Witzke O, et al. An open‐label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney International 2019;96(1):231‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suwelack B, Dragun D, Sommerer C, Schenker P, Hauser IA, Witzke O, et al. Reduced incidence of CMV‐events with modern everolimus‐based vs conservative tacrolimus‐MPA‐based regimen in denovo renal transplant recipients: 12 months data on infections from ATHENA study [abstract no: V128]. Transplant International 2017;30(Suppl 4):15. [EMBASE: 619082395] [Google Scholar]
- Suwelack B, Sommerer C, Dragun D, Witzke O, Hugo C, Kamar N, et al. The ATHENA study: 12 months safety and efficacy data from everolimus combined with tacrolimus or cyclosporine compared to a standard tacrolimus‐mycophenolate regimen in de novo kidney transplant patients [abstract no: 37]. American Journal of Transplantation 2018;18(Suppl 4):259‐60. [EMBASE: 622280671] [Google Scholar]
- Thaiss F, Sommerer C, Suwelack B, Dragun D, Hauser IA, Witzke O, et al. European kidney transplant trial ATHENA‐patient and allograft outcome by country [abstract no: PV01]. Transplant International 2018;31(Suppl 5):18. [EMBASE: 625099157] [Google Scholar]
- Thaiss F, Sommerer C, Suwelack B, Dragun D, Schenker P, Hauser IA, et al. 12 months data from ATHENA study show comparable safety and efficacy of modern everolimus‐based regimen vs. conservative tacrolimus‐MPA‐based regimen in de novo renal transplant recipients [abstract no: PV18]. Transplant International 2017;30(Suppl 4):20. [EMBASE: 619082512] [Google Scholar]
- Thaiss F, Sommerer C, Suwelack BM, Dragun D, Hauser IA, Schenker P, et al. Design and rationale of ATHENA study: a 12‐month study evaluating everolimus versus standard regimen in de novo renal transplant recipients‐baseline data [abstract no: FR‐PO1067]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):614A. [Google Scholar]
- Thaiss F, Suwelack BM, Sommerer C, Dragun D, Hauser IA, Schenker P, et al. ATHENA study outcomes on allograft function after 12 months with everolimus‐based versus tacrolimus‐MPA regimen in de novo renal transplant recipients. [abstract no: FR‐OR078]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):57. [Google Scholar]
AVESTA 2017 {published data only}
- Gholi FP, Dalili N, Alinezhad M, Moosavi M. AVESTA study‐an Iranian verification of everolimus success in transplantation aspects [abstract no: SP779]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii406‐7. [EMBASE: 617290880] [Google Scholar]
Bechstein‐193 2013 {published data only}
- Bechstein W, Paczek L, European Rapamune‐FK Study Group. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract no: 1321]. American Journal of Transplantation 2002;2(Suppl 3):471. [CENTRAL: CN‐00415252] [Google Scholar]
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, Zygmunt AJ, European Rapamune Tacrolimus Study Group. A comparative, randomized trial of concentration‐controlled sirolimus combined with reduced‐dose tacrolimus or standard‐dose tacrolimus in renal allograft recipients. Transplantation Proceedings 2013;45(6):2133‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Bechstein WO, Paczek L, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus + steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract no: W739]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):785. [CENTRAL: CN‐00444369] [Google Scholar]
- Paczek L, Bechstein W, Wramner L, Squifflet JP. A phase II, open‐label, concentration‐controlled, randomized 6‐month study of standard‐dose tacrolimus + sirolimus + corticosteroids compared to reduced‐dose tacrolimus + sirolimus + corticosteroids in renal allograft recipients [abstract no: 170]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami, USA. 2002. [CENTRAL: CN‐01912222]
- Paczek L, Bechstein WO, Wramner L, Squifflet JP, European Sirolimus‐Tacrolimus Study Group. An open‐label, concentration‐controlled, randomised 6‐month study of standard‐dose tacrolimus + sirolimus +steroids compared to reduced‐dose tacrolimus + sirolimus + steroids in renal allograft recipients [abstract no: 1219]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐01912354] [Google Scholar]
- Whelchel J, Paczek L, Bechstein WO, Russ G. A regimen of sirolimus and reduced‐dose tacrolimus results in improved renal allograft function: combined analysis of the North American target, European and Australian sirolimus‐tacrolimus trials [abstract no: 1218]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐00448351] [Google Scholar]
Bertoni 2011 {published data only}
- Bertoni E, Becherelli P, Salvadori M. Triple therapy with neoral, steroids and enteric‐coated mycophenolate acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. a monocentric experience [abstract no: F‐PO614]. Journal of the American Society of Nephrology 2007;18(Abstracts):234A. [CENTRAL: CN‐00716078] [Google Scholar]
- Bertoni E, Larti A, Farsetti S, Rosso G, Maria L, Zanazzi M. Cyclosporine (CyA) very low dose with everolimus (E) high dose is associated with better outcomes in renal transplant patients with respect to standard treatment with EC‐MPS (M) [abstract no: LB‐1]. Transplant International 2009;22(Suppl 2):91. [CENTRAL: CN‐01657256] [Google Scholar]
- Bertoni E, Larti A, Rosso G, Zanazzi M, Maria L, Salvadori M. Good outcomes with cyclosporine very low exposure with everolimus high exposure in renal transplant patients. Journal of Nephrology 2011;24(5):613‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosati A, Bertoni E, Rosso G, Larti A, Mehmetaj A, Salvadori M. Triple therapy with neoral, steroids and enteric‐coated mycophenolic acid vs everolimus: efficacy, side effects and pharmaco‐economic aspects. a monocentric experience [abstract no: SaP477]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi396. [CENTRAL: CN‐00725011] [Google Scholar]
Burke 2002 {published data only}
- Burke GW, Ciancio C, Blomberg BB, Rosen A, Suzart K, Roth D, et al. Randomized trial of three different immunosuppressive regimens to prevent chronic renal allograft rejection. Transplantation Proceedings 2002;34(5):1610‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long‐term trial of tacrolimus and sirolimus versus tacrolimus and mycophenolate mofetil versus cyclosporine (NEORAL) and sirolimus in renal transplantation. I. Drug interactions and rejection at one year. Transplantation 2004;77(2):244‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Mattiazzi A, Roth D, Kupin W, et al. A randomized long‐term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate mofetil versus cyclosporine (NEORAL)/sirolimus in renal transplantation. II. Survival, function, and protocol compliance at 1 year. Transplantation 2004;77(2):252‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Gaynor JJ, Ruiz P, Roth D, Kupin W, et al. A randomized long‐term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine/sirolimus in renal transplantation: three‐year analysis. Transplantation 2006;81(6):845‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Burke GW, Mattiazzi AD, Gaynor JJ, Roohipour R, Dowdy L, et al. Prophylaxis for cytomegalovirus disease in a randomized trial of three different immunosuppressive regimens in renal transplantation [abstract no: 1228]. American Journal of Transplantation 2004;4(Suppl 8):494. [CENTRAL: CN‐00509136] [Google Scholar]
- Ciancio G, Gaynor JJ, Sageshima J, Chen L, Roth D, Kupin W. Machine perfusion in kidney transplantation: better outcomes in the presence of longer pump time [abstract no: 1011]. American Journal of Transplantation 2010;10(Suppl 4):333. [CENTRAL: CN‐01912201] [Google Scholar]
- Ciancio G, Gaynor JJ, Sageshima J, Roth D, Kupin W, Guerra G, et al. Machine perfusion following static cold storage preservation in kidney transplantation: donor‐matched pair analysis of the prognostic impact of longer pump time. Transplant International 2012;25(1):34‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ciancio G, Sageshima J, Burke GW, Gaynor JJ, Herrada E, Roth D, et al. A randomized long‐term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine (Neoral©)/sirolimus in renal transplantation: three year analysis [abstract no: 385]. American Journal of Transplantation 2006;6(Suppl 2):196. [CENTRAL: CN‐00676056] [Google Scholar]
- Guerra G, Ciancio G, Gaynor JJ, Zarak A, Brown R, Hanson L, et al. Randomized trial of immunosuppressive regimens in renal transplantation. Journal of the American Society of Nephrology 2011;22(9):1758‐68. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra G, Gaynor JJ, Ciancio G, Zarak A, Sageshima J, Roth D, et al. Randomized trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine (Neoral®/sirolimus in renal transplantation: seven year results [abstract no: 461]. American Journal of Transplantation 2009;9(Suppl 2):325. [Google Scholar]
- Mattiazzi A, Burke GW, Miller J, Ciancio G. Surgical complications after kidney transplantation: comparing three different immunosuppresive regimens [abstract]. Journal of the American Society of Nephrology 2003;3(Suppl 5):484. [CENTRAL: CN‐00446660] [Google Scholar]
- Miller J, Burke GW, Ciancio G, Blomberg BB, Rosen A, Gaynor J, et al. Randomized trial of three different immunosuppressive regimens to prevent chronic renal allograft rejection [abstract no: 1222]. American Journal of Transplantation 2003;3(Suppl 5):465. [CENTRAL: CN‐00446775] [DOI] [PubMed] [Google Scholar]
- Miller J, Burke GW, Ciancio G, Blomberg BB, Rosen A, Roth D, et al. Randomized trial of three different immunosuppressive regimens to prevent chronic renal allograft rejection [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [DOI] [PubMed]
CALFREE 2006 {published data only}
- Franz S, Regeniter A, Hopfer H, Mihatsch M, Dickenmann M. Tubular toxicity in sirolimus and cyclosporine based transplant immunosuppression strategies: an ancillary study from a randomized controlled trial. American Journal of Kidney Diseases 2010;55(2):335‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Franz SE, Bengue O, Dickenmann MJ, Brunner F, Steiger J. Tubulotoxicity of sirolimus [abstract no: O60]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):19. [CENTRAL: CN‐00509200] [Google Scholar]
- Franz SE, Regeniter A, Mihatsch M, Steiger JU. Tubulotoxic properties of sirolimus [abstract no: F‐FC0057]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):12A. [CENTRAL: CN‐00445379] [Google Scholar]
- Giannini O, Dickenmann M, Kim MJ, Franz S, Mayr M, Mihatsch MJ, et al. The CALFREE Study ‐ an open, prospective, randomized single center study to investigate calcineurin free immunosuppression in 100 de novo, normal risk renal transplant recipients: preliminary results [abstract no: P04.02]. Kidney & Blood Pressure Research 2004;27(5‐6):329. [CENTRAL: CN‐00615861] [Google Scholar]
- Kim MJ, Dickenmann MJ, Moll S, Mihatsch MJ, Brunner F, Steiger J. Protocol biopsies: do they improve patient management? [abstract no: T466]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):317. [CENTRAL: CN‐00509278] [Google Scholar]
- Kim MJ, Mayr M, Pechula M, Steiger J, Dickenmann M. Marked erythrocyte microcytosis under primary immunosuppression with sirolimus. Transplant International 2006;19(1):12‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cattaneo 2005 {published data only}
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Gotti E, Remuzzi G, et al. Influence of co‐medication with sirolimus or cyclosporine on mycophenolic acid pharmacokinetics in kidney transplantation. American Journal of Transplantation 2005;5(12):2937‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cattaneo D, Merlini S, Zenoni S, Baldelli S, Perico N, Gotti E, et al. Effects of sirolimus on the pharmacokinetics of mycophenolic acid in kidney transplantation [abstract no: 650]. American Journal of Transplantation 2005;5(Suppl 11):322. [CENTRAL: CN‐00724878] [DOI] [PubMed] [Google Scholar]
- Noris M, Casiraghi F, Todeschini M, Cravedi P, Cugini D, Monteferrante G, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. Journal of the American Society of Nephrology 2007;18(3):1007‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perico N, Gotti E, Cravedi P, D'Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation 2007;84(8):956‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low‐dose rabbit anti‐thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor‐specific anti‐HLA antibody development. Journal of Immunology 2013;191(5):2818‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ciancio 2016 {published data only}
- Ciancio G, Tryphonopoulos P, Gaynor JJ, Guerra G, Sageshima J, Roth D, et al. Pilot randomized trial of tacrolimus/everolimus vs tacrolimus/enteric‐coated mycophenolate sodium in adult, primary kidney transplant recipients at a single center. Transplantation Proceedings 2016;48(6):2006‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cohen 2002 {published data only}
- Cohen DJ, Vincenti F. A comparative, open‐label study to evaluate graft function in de novo renal allograft recipients treated with reduced‐dose or standard‐dose cyclosporine in combination with sirolimus and corticosteroids. [abstract no: 394]. American Journal of Transplantation 2002;2(Suppl 3):237. [CENTRAL: CN‐01912453] [Google Scholar]
- Cohen DJ, Vincenti F, US Rapamune‐CSA Study Group. A comparative, open‐label study to evalute graft function in de novo renal allograft recipients treated with reduced‐dose or standard‐dose cyclosporine in combination with sirolimus and corticosteroids [abstract no: 1223]. American Journal of Transplantation 2003;3(Suppl 5):465. [CENTRAL: CN‐00444868] [Google Scholar]
- Vincenti F, Cohen DJ. A comparative, open‐label study to evaluate graft function in de novo renal allograft recipients treated with reduced‐dose or standard‐dose cyclosporine in combination with sirolimus and corticosteroids [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00420865]
Durlik 2008 {published data only}
- Durlik M, Tronina O, Baczkowska T, Klinger M, Rutkowski B, Wiecek A, et al. Sirolimus is associated with worsen outcome compared to mycophenolate mofetil in high immunologic risk renal transplant recipients receiving tacrolimus combined with ATG induction [abstract no: 533]. Transplantation 2008;86(Suppl 2):186. [CENTRAL: CN‐00740488] [Google Scholar]
Durrbach 2008 {published data only}
- Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil‐Noble C, Kessler M, et al. Use of sirolimus as initial therapy after renal transplantation: preliminary results of a randomized pilot study in patient receiving marginal kidneys [abstract no: P102]. Transplantation 2004;78(2 Suppl):228. [Google Scholar]
- Durrbach A, Rostaing L, Tricot L, Ouali N, Wolf P, Pouteil‐Noble C, et al. Prospective comparison of the use of sirolimus and cyclosporine in recipients of a kidney from an expanded criteria donor. Transplantation 2008;85(3):486‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Thervet E, Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil‐Noble C, et al. Use of sirolimus as initial therapy after renal transplantation: preliminary results of a randomized pilot study in patient receiving marginal kidneys [abstract no: 686]. American Journal of Transplantation 2004;4(Suppl 8):345. [CENTRAL: CN‐00509509] [Google Scholar]
- Thervet E, Durrbach A, Rostaing L, Ouali N, Wolf P, Pouteil‐Noble C, et al. Use of sirolimus as initial therapy in renal transplant recipients of marginal kidneys [abstract no: F‐PO1051]. Journal of the American Society of Nephrology 2004;15(Oct):295A. [CENTRAL: CN‐00583227] [Google Scholar]
Esmeraldo 2015 {published data only}
- Esmeraldo R, Oliveira M, Pinheiro P, Girao C, Freitas T. Prospective randomized open trial designed to reduce the incidence of cytomegalovirus (CMV) infection in de novo kidney transplant recipients two‐year results [abstract no: 39]. American Journal of Transplantation 2016;16(Suppl 3):218. [EMBASE: 611700413] [Google Scholar]
- Esmeraldo R, Oliveira MLM, Pinheiro PM, Girao CM. One‐year results of a prospective randomized open trial designed to reduce the incidence of cytomegalovirus (CMV) infection in de novo kidney transplant recipients [abstract no: O05]. Transplant International 2015;28(Suppl 4):2. [EMBASE: 72111250] [Google Scholar]
- Esmeraldo RM, Sandes‐Freitas TM, Girao CM, Oliveira‐Sales ML, Pinheiro PM. Everolimus enables tacrolimus and steroid minimization with reduced incidence of CMV infection and preserved graft function in de novo renal transplant recipients at 12 and 24 months post‐transplant [abstract no: OS164]. Transplant International 2017;30(Suppl 2):64. [EMBASE: 618769705] [Google Scholar]
EVEREST 2009 {published data only}
- Citterio F, Scolari MP, Salvadori M, Castagneto M, Rigotti P, Albertazzi A, et al. A randomized trial comparing standard everolimus plus cyclosporine with higher blood everolimus levels plus very low cyclosporine levels in renal transplant recipients: preliminary results of the Everest Study [abstract no: P118]. Transplant International 2007;20(Suppl 2):124. [CENTRAL: CN‐00724884] [Google Scholar]
- Corbetta G, Salvadori M, Scolari MP, Citterio F, Rigotti P, Cossu M, et al. Exposure to everolimus, and not to cyclosporine, is associated with freedom from acute rejection in de novo renal recipients [abstract no: 447]. Transplantation 2008;86(Suppl 2):157. [CENTRAL: CN‐00740485] [Google Scholar]
- Ponticelli C, Salvadori M, Scolari MP, Citterio F, Rigotti P, Veneziano A, et al. Everolimus and minimization of cyclosporine in renal transplantation: 24‐month follow‐up of the EVEREST study. Transplantation 2011;91(10):e72‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salvadori M, Scolari M, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Targeting upper everolimus blood levels with very low‐dose cyclosporine is effective and safe in de novo renal transplantation [abstract no: 448]. Transplantation 2008;86(Suppl 2):158. [CENTRAL: CN‐00740453] [Google Scholar]
- Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M. Upper everolimus blood levels with very low‐dose cyclosporin: 12 months follow up of the Everest Study [abstract no: 1085]. American Journal of Transplantation 2009;9(Suppl 2):497. [CENTRAL: CN‐00776867] [Google Scholar]
- Salvadori M, Scolari MP, Bertoni E, Citterio F, Rigotti P, Cossu M, et al. Everolimus with very low‐exposure cyclosporine A in de novo kidney transplantation: a multicenter, randomized, controlled trial. Transplantation 2009;88(10):1194‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
EVEROLD 2014 {published data only}
- Meur Y, Buchler M, Mousson C, Moal M, Albano L, Merville P, et al. EVEROLD: a multicenter randomized study for the use of everolimus in "old‐for‐old" renal transplantation [abstract no: 2155]. Transplantation 2014;98(Suppl 1):114. [EMBASE: 71543890] [Google Scholar]
Favi 2009 {published data only}
- Favi E, Citterio F, Spagnoletti G, Gargiulo A, Delreno F, Romagnoli J, et al. Prospective clinical trial comparing two immunosuppressive regimens, tacrolimus and mycophenolate mofetil versus everolimus and low‐dose cyclosporine, in de novo renal transplant recipients: results at 6 months follow‐up. Transplantation Proceedings 2009;41(4):1152‐5. [EMBASE: 2009227250] [DOI] [PubMed] [Google Scholar]
- Favi E, Citterio F, Spagnoletti G, Romagnoli J, Gargiulo A, Castagneto M. Tacrolimus and MMF vs everolimus and low dose cyclosporine: 2 year results of a prospective clinical trial [abstract no: Su665]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00784295]
- Favi E, Citterio F, Spagnoletti G, Tondolo V, Romagnoli J, Castagneto M. A prospective trial comparing tacrolimus with everolimus and low dose cyclosporine [abstract no: 1155]. American Journal of Transplantation 2007;7(Suppl 2):443. [CENTRAL: CN‐00653736] [Google Scholar]
- Favi E, Spagnoletti G, Gargiulo A, Romagnoli J, Castagneto M, Citterio F. The combination of everolimus and low dose cyclosporine allows similar results as the standard tacrolimus and MMF regimen: 3 year results of a prospective clinical trial in renal transplant recipients [abstract no: 1645]. American Journal of Transplantation 2010;10(Suppl 4):506. [CENTRAL: CN‐00776468] [Google Scholar]
- Favi E, Spagnoletti G, Salerno MP, Pedroso JA, Romagnoli J, Citterio F. Tacrolimus plus mycophenolate mofetil vs. cyclosporine plus everolimus in deceased donor kidney transplant recipients: three‐yr results of a single‐center prospective clinical trial. Clinical Transplantation 2013;27(4):E359‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salerno M, Bianchi V, Favi E, Spagnoletti G, Pedroso J, Toscano A, et al. 3 years outcomes in a randomized trial evaluating everolimus plus CNI reduction in kidney transplant recipients [abstract no: B987]. Transplantation 2014;98(Suppl 1):546. [EMBASE: 71545369] [Google Scholar]
Favi 2012 {published data only}
- Favi E, Silvestrini N, Pedroso J, Salerno M, Spagnoletti G, Bianchi V, et al. Extended‐release tacrolimus plus everolimus vs extended‐release tacrolimus plus micophenolate mofetil in primary deceased donor kidney transplant recipients: 1‐year results of an open label, randomized phase 2 clinical trial [abstract no: B950]. American Journal of Transplantation 2013;13(Suppl 5):316. [EMBASE: 71057526] [Google Scholar]
- Favi E, Silvestrini N, Pedroso JA, Salerno MP, Spagnoletti G, Romagnoli J, et al. ER‐tacrolimus plus everolimus vs ER‐tacrolimus plus MMF in primary deceased donor kidney transplantation: 1‐year results of single center, open label, prospective, randomized clinical trial [abstract no: P288]. Transplant International 2013;26(Suppl 2):241. [EMBASE: 71359898] [Google Scholar]
- Favi E, Silvestrini N, Salerno MP, Romagnoli J, Citterio F. Extended‐release tacrolimus plus everolimus or micophenolate mofetil in deceased donor kidney transplant recipients: 6‐month results of a prospective randomized clinical trial [abstract no: 55]. American Journal of Transplantation 2012;12(Suppl 3):42‐3. [EMBASE: 70746002] [Google Scholar]
Fernandes‐Charpiot 2014 {published data only}
- Abbud‐Filho M, Mazeti C, Caldas H, Fernandes I, Razera J, Baptista MA. Identifying the cytokine molecular profile in renal transplant recipients of extended criteria donor kidneys [abstract no: P461]. Transplant International 2015;28(Suppl 4):590. [EMBASE: 72112425] [Google Scholar]
- Fernandes‐Charpiot I, Caldas H, Mazeti C, Baptista M, Razera J, Abbud‐Filho M. Efficacy and safety of early use of everolimus in standard and extended criteria deceased donor kidney transplant recipients: 12‐month preliminary results of a randomized single center trial [abstract no: C1855]. Transplantation 2014;98(Suppl 1):597‐8. [EMBASE: 71545554] [Google Scholar]
FIBRASIC 2009 {published data only}
- Woestenburg AT, Peeters P, Sennesael J, Abramowicz D, Wissing KM, Geers C, et al. Interstitial fibrosis and fibrous intimal thickening in de novo renal allografts under sirolimus or cyclosporine: results of a randomised, controlled trial (FIBRASIC) [abstract no: O‐300]. Transplant International 2009;22(Suppl 2):79. [CENTRAL: CN‐01658203] [Google Scholar]
Flechner 2013 {published data only}
- Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al. A randomized, open‐label study of sirolimus versus cyclosporine in primary de novo renal allograft recipients. Transplantation 2013;95(10):1233‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Gurkan A, See Tai S, Schulman SL. Incidence of delayed graft function (DGF) in sirolimus (SRL)‐based versus cyclosporine (CsA)‐based regimen in de novo renal allograft recipients [abstract no: 300]. American Journal of Transplantation 2009;9(Suppl 2):278. [CENTRAL: CN‐00775180] [Google Scholar]
Flechner‐318 2002 {published data only}
- Campistol JM, Glyda M, Gurkan A, Flechner SM, Schulman S, See Tai S. Incidence of delayed graft function (DGF) in sirolimus (SRL)‐based regimens compared with calcineurin inhibitors (CNIs) and mycophenolate mofetil (MMF) in de novo renal allograft recipients [abstract no: P‐105]. Transplant International 2009;22(Suppl 2):121. [CENTRAL: CN‐01657955] [Google Scholar]
- Flechner SM, Burke JT, Cook DJ, Mastroianni B, Savas K, Goldfarb D, et al. A randomized prospective trial of sirolimus vs cyclosporine in kidney transplantation: renal function and histology at two years [abstract no: 1164]. American Journal of Transplantation 2003;3(Suppl 5):450. [CENTRAL: CN‐00445351] [Google Scholar]
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches [abstract no: 0378]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415657]
- Flechner SM, Cook DJ, Goldfarb D, Modlin C, Mastroianni B, Savas K, et al. A randomized trial of sirolimus vs cyclosporine in kidney transplantation: impact on blood cells, lymphocyte subsets, and flow crossmatches. [abstract no: 1317]. American Journal of Transplantation 2002;2(Suppl 3):470. [CENTRAL: CN‐01912448] [Google Scholar]
- Flechner SM, Goldfarb D, Modlin C, Feng J, Krishnamurthi V, Mastroianni B, et al. Kidney transplantation without calcineurin inhibitor drugs: a prospective, randomized trial of sirolimus versus cyclosporine. Transplantation 2002;74(8):1070‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil‐based immunosuppression: 5‐year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007;83(7):883‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Kurian S, Solez K, Cook DJ, Burke JT, Rollin H, et al. Kidney transplantation with sirolimus and mycophenolate mofetil based immunosuppression preserves renal structure and function at two years compared to calcineurin inhibitor drugs [abstract no: O361]. Transplantation 2004;78(2 Suppl):141. [CENTRAL: CN‐00509193] [Google Scholar]
- Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. American Journal of Transplantation 2004;4(11):1776‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Solez K, Cook DJ, Burke JT, Rollin H, Mastoianni B, et al. Kidney transplantation with sirolimus and mycophenolate mofetil based immunosuppression preserves renal structure and function compared to calcineurin inhibitor (CNI) drugs [abstract no: 502]. American Journal of Transplantation 2004;4(Suppl 8):296. [CENTRAL: CN‐00509194] [Google Scholar]
Gallon 2006 {published and unpublished data}
- Chhabra D, Skaro AI, Leventhal JR, Dalal P, Shah G, Wang E, et al. Long‐term kidney allograft function and survival in prednisone‐free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Clinical Journal of the American Society of Nephrology: CJASN 2012;7(3):504‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal P, Xu L, Joseph L, Shah G, Chhabra D, Wang E, et al. Prospective randomized study to evaluate the long term impact on graft survival and function of two pred‐free, CNI based maintenance immunosuppression: FK/MMF vs. FK/SRL [abstract no: 1670]. American Journal of Transplantation 2010;10(Suppl 4):512. [CENTRAL: CN‐00775927] [Google Scholar]
- Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, et al. Long‐term renal allograft function on a tacrolimus‐based, pred‐free maintenance immunosuppression comparing sirolimus vs MMF. American Journal of Transplantation 2006;6(7):1617‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gallon L, Perico N, Winoto J, Dimitrov B, Leventhal J, Gaspari F, et al. Prospective randomized single center study comparing the impact on long term renal transplant function of two prednisone free maintenance immunosuppressive combinations with tacrolimus (TAC), mycophenolate mofetil (MMF) versus tacrolimus/sirolimus (SLR) [abstract no: 517]. American Journal of Transplantation 2005;5(Suppl 11):288. [CENTRAL: CN‐00583215] [Google Scholar]
- Gallon LG, Kaufman DB, Leventhal JR, Abecassis M, Fryer J, Koffron A, et al. A prospective randomized single center study of prednisone free immunosuppression that compares two maintenance combinations: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. [abstract no: SU‐PO598]. Journal of the American Society of Nephrology 2003;14(Nov):664A. [CENTRAL: CN‐00583857] [Google Scholar]
- Gallon LG, Leventhal JR, Kaufman DB, Koffron AJ, Fryer JP, Abescassis MM, et al. A prospective randomized single center study of rapid corticosteroid elimination comparing two maintenance immunosuppressive protocols: tacrolimus (FK506)/mycophenolate mofetil (MMF) versus tacrolimus/sirolimus [abstract no: 1217]. American Journal of Transplantation 2003;3(Suppl 5):463. [CENTRAL: CN‐00583421] [Google Scholar]
Gelens 2006 {published data only}
- Gelens M, Christiaans M, Hooff J. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: P‐3]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00583729]
- Gelens M, Christiaans M, Hooff J. Incidence of post transplant diabetes mellitus during calcineurin‐free and calcineurin‐based immunosuppression with limited steroid exposure [abstract no: 1210]. American Journal of Transplantation 2005;5(Suppl 11):465. [CENTRAL: CN‐01657297] [Google Scholar]
- Gelens M, Christianns M, Heurn E, Hooff J. Calcineurin‐free immunosuppression and limited steroid exposure in renal transplantation [abstract no: 865]. American Journal of Transplantation 2005;5(Suppl 11):376. [CENTRAL: CN‐01657298] [Google Scholar]
- Gelens MA, Christiaans MH, Heurn EL, Berg‐Loonen EP, Peutz‐Kootstra CJ, Hooff JP. High rejection rate during calcineurin inhibitor‐free and early steroid withdrawal immunosuppression in renal transplantation. Transplantation 2006;82(9):1221‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glotz 2010 {published data only}
- Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. 6 months preliminary results of a randomized trial comparing sirolimus (SRL) versus tacrolimus (FK) in 141 transplant patients receiving a cadaveric renal graft [abstract no: 1191]. American Journal of Transplantation 2005;5(Suppl 11):460. [CENTRAL: CN‐00583237] [Google Scholar]
- Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation 2010;89(12):1511‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gonwa‐PSG 2003 {published data only}
- Fitzsimmons WE, First MR, Gao J. Correlation of tacrolimus trough blood levels to rejection and adverse events after kidney transplantation [abstract no: 649]. American Journal of Transplantation 2005;5(Suppl 11):322. [CENTRAL: CN‐01657974] [Google Scholar]
- Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S. One year results for the first prospective multi‐center kidney transplant study comparing tacrolimus+sirolimus vs. tacrolimus+MMF combination therapy [abstract no: P202]. Transplantation 2004;78(2 Suppl):263‐4. [CENTRAL: CN‐00509213] [Google Scholar]
- Gonwa T, Mendez R, Yang HC, Weinstein S, Jensik S, Steinberg S, et al. Randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 6 months. Transplantation 2003;75(8):1213‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gonwa T, Rice K, Langnas A, Tomlandovich S, Danovitch G, Inokuchi S, et al. Sirolimus vs MMF‐first report of a US multicenter kidney transplant study with tacrolimus combination therapy [abstract no: 1320]. American Journal of Transplantation 2002;2(Suppl 3):471. [CENTRAL: CN‐00415747] [Google Scholar]
- Gonwa TA, Fujisawa Study Group. Final results for the first prospective, multi‐center kidney transplant study comparing tacrolimus +sirolimus vs tacrolimus+MMF combination therapy [abstract no: F‐FC040]. Journal of the American Society of Nephrology 2003;14(Program & Abstracts):9‐10A. [CENTRAL: CN‐00550649] [Google Scholar]
- Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S, et al. A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: results at 1 year. Transplantation 2005;80(3):303‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mendez R, Fujisawa Study Group. Six‐month results of the first prospective, randomized, multi‐center kidney transplant study comparing tacrolimus+rapamune vs tacrolimus+MMF combination therapy [abstract no: 1552]. American Journal of Transplantation 2003;3(Suppl 5):550. [CENTRAL: CN‐00446736] [Google Scholar]
- Steinberg SM, Mendez R, Yang HC, Weinstein S, Jensik S, Gonwa T, et al. One year results for the first prospective multi‐center kidney transplant study comparing tacrolimus+sirolimus vs tacrolimus+MMFcombination therapy [abstract no: 24]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004.
- Yang H, Gonwa T, Rice K, Mendez R, Filo R, Holman M, et al. Sirolimus vs mycophenolate mofetil ‐ results of the first U.S. multicenter kidney transplant study with tacrolimus combination therapy [abstract no: O557]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416968]
Grinyo 2004 {published data only}
- Campistol JM, Grinyo JM, Paul J, Garcia J, Arias M, Morales JM, et al. Sirolimus, TAC and corticosteroids in the postoperative period. Preliminary results [abstract no: 2108]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00415380]
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open, multicenter, trial comparing tacrolimus (TAC) withdrawal with TAC dose reduction in de novo renal transplants, receiving sirolimus (SIR), TAC and steroids in the postoperative time. Initial results [abstract no: SA‐PO0491]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):363A. [CENTRAL: CN‐00445561] [Google Scholar]
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open, multicenter, trial comparing tacrolimus (TAC) withdrawal with TAC dose reduction in de novo renal transplants, receiving sirolimus (SIR), TAC and steroids in the postoperative time. Initial results [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):101. [Google Scholar]
- Grinyo JM, Campistol JM, Paul J, Garcia J, Arias M, Morales JM, et al. A randomised, open‐label, pilot study to compare the safety and efficacy of tacrolimus (TAC) elimination with tac dose reduction in de novo renal allograft recipients, receiving sirolimus, tac and corticosteroids in the postoperative period. Preliminary results [abstract no: 1018]. American Journal of Transplantation 2002;2(Suppl 3):394. [CENTRAL: CN‐00415775] [Google Scholar]
- Grinyo JM, Campistol JM, Paul J, Garcia‐Martinez J, Morales JM, Prats D, et al. Pilot randomized study of early tacrolimus withdrawal from a regimen with sirolimus plus tacrolimus in kidney transplantation. American Journal of Transplantation 2004;4(8):1308‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Grinyo JM, Campistol JM, Garcia J, Arias M, Paul J, et al. FK‐506 withdrawal from a regimen of sirolimus plus FK‐506 results at 2 years in an improvement in renal function, blood pressure and proteinuria in ongoing kidney recipients [abstract no: 1192]. American Journal of Transplantation 2005;5(Suppl 11):460. [CENTRAL: CN‐00724880] [Google Scholar]
- Morales JM, Grinyo JM, Campistol JM, Garcia‐Martinez J, Arias M, Paul J, et al. Improved renal function, with similar proteinuria, after two years of early tacrolimus withdrawal from a regimen of sirolimus plus tacrolimus. Transplantation 2008;86(4):620‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paul J, Grinyo JM, Campistol JM, Garcia J, Morales JM, Arias M, et al. A randomised, open‐label, pilot study to compare the safety and efficacy of tacrolimus (tac) elimination with tac dose reduction in de novo renal allograft recipients, receiving sirolimus, TAC and corticosteroids in the postoperative period: Preliminary results [abstract no: O58]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):18‐9. [CENTRAL: CN‐00550490] [Google Scholar]
Groth‐207 1999 {published data only}
- Campistol JM, Backman L, Brattstrom C, Kreis H, Morales JM, Sirolimus European Renal Transplant Study Group. Influence of immunosupressive treatment on markers of bone remodeling in renal transplantation: comparison between cyclosporine and sirolimus [abstract no: A3814]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):754A. [CENTRAL: CN‐00550450] [Google Scholar]
- Campistol JM, Holt DW, Epstein S, Gioud‐Paquet M, Rutault K, Burke JT, et al. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transplant International 2005;18(9):1028‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charpentier B, Groth CG, Backman L, Morales JM, Calne R, Kreis H, et al. Bicentre hospital experience with sirolimus‐based therapy in human renal transplantation: the Sirolimus European Renal Transplant Study. Transplantation Proceedings 2003;35(3 Suppl):58S‐61S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)‐based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation 1999;67(7):1036‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groth CG, Brattstrom C, Claesson K, Backman L. New trails in transplantation: how to exploit the potential of sirolimus in clinical transplantation. Transplantation Proceedings 1998;30(8):4064‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre C, Campistol JM, Squifflet JP, Burke JT, Sirolimus European Renal Transplant Study Group. Cardiovascular risk factors of sirolimus compared with cyclosporine: early experience from two randomized trials in renal transplantation. Transplantation Proceedings 2003;35(3 Suppl):151‐3S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre C, Squifflet JP, Campistol JM, Sirolimus European Renal Transplant Study Group. Comparison of long term CSA ‐ and sirolimus‐based therapies on lipid parameters [abstract no: 976]. Transplantation 2000;69(8 Suppl):S365. [CENTRAL: CN‐00446314] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Malignancy in renal transplant recipients receiving sirolimus (rapamune) maintenance therapy: 2‐year follow up from 5 multicenter studies [abstract no: 58]. American Journal of Transplantation 2002;2(Suppl 3):152. [CENTRAL: CN‐00416243] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Two‐year incidence of malignancy in sirolimus (rapamune)‐treated renal transplant recipients: follow‐up results from 5 multicenter studies [abstract no: 0374]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416244]
- Mathew T, Kreis H, Friend P. Two‐year incidence of malignancy in sirolimus‐treated renal transplant recipients: results from five multicenter studies. Clinical Transplantation 2004;18(4):446‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Wramner L, Kreis H, Durand D, Campistol JM, Andres A, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. American Journal of Transplantation 2002;2(5):436‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hamdy 2005 {published data only}
- Hamdy A, El‐Baz M, Bakr M, Ghoneim M. The incidence of chronic allograft nephropathy among live‐donor renal transplant recipients primarily treated with sirolimus‐based regimens [abstract no: Sa734]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐00776624]
- Hamdy AF, Bakr MA, Ghoneim MA. Long‐term efficacy and safety of a calcineurin inhibitor‐free regimen in live‐donor renal transplant recipients. Journal of the American Society of Nephrology 2008;19(6):1225‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy AF, Bakr MA, Ghoneim MA. Proteinuria among primarily sirolimus treated live‐donor renal transplant recipients' long‐term experience. Experimental & Clinical Transplantation 2010;8(4):283‐91. [MEDLINE: ] [PubMed] [Google Scholar]
- Hamdy AF, El‐Agroudy AE, Bakr MA, Mostafa A, El‐Baz M, El‐Shahawy El‐M, et al. Comparison of sirolimus with low‐dose tacrolimus versus sirolimus‐based calcineurin inhibitor‐free regimen in live donor renal transplantation. American Journal of Transplantation 2005;5(10):2531‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hamdy AF, Elhadedy MA, Donia AF, Taha NM, Bakr MA. Outcome of sirolimus‐based immunosuppression, fifteen years post‐live‐donor kidney transplantation: single‐center experience. Clinical Transplantation 2019;33(2):e13463. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kahan‐157 2001 {published data only}
- Kahan BD, Kaplan B, Lorber M, Winkler M, Cambon N, Maca J, et al. Multicenter, randomized, double‐blind, dose‐finding study evaluating the efficacy and safety of RAD001 (RAD) in de novo renal transplant recipients [abstract no: 954]. Transplantation 2000;69(8 Suppl):S359. [CENTRAL: CN‐00445976] [Google Scholar]
- Kahan BD, Kaplan B, Lorber M, Winkler M, Campbell M, Maca J, et al. Safety and preliminary efficacy of RAD in de novo renal transplant recipients evaluated in a multicenter, randomized, double‐blind, dose‐finding study [abstract no: 0662]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00445977]
- Kahan BD, Kaplan B, Lorber MI, Winkler M, Cambon N, Boger RS. RAD in de novo renal transplantation: comparison of three doses on the incidence and severity of acute rejection. Transplantation 2001;71(10):1400‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Kaplan B, Vitko S, McMahon L, Attinger M, Boger R, et al. Longitudinal influence of everolimus on cyclosporine assessed over 6 months in two blinded de novo kidney transplant trials [abstract no: 1341]. American Journal of Transplantation 2001;1(Suppl 1):475. [CENTRAL: CN‐00583198] [Google Scholar]
- Kovarik JM, Rordorf C, Kahan BD, Kaplan B, Lorber M, Winkler M, et al. Longitudinal assessment of the steady‐state pharmacokinetics of RAD in de novo renal transplant patients [abstract no: 160]. Transplantation 2000;69(8 Suppl):S154. [CENTRAL: CN‐00446175] [Google Scholar]
- Rordorf C, Kovarik JM, Kahan BD, Kaplan B, Lorber M, Winkler M, et al. Double‐blind, longitudinal assessment of the influence of RAD on cyclosporine pharmacokinetics in 87 de novo renal transplant patients [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00447458]
- Rordorf C, Kovarik JM, Kahan BD, Kaplan B, Lorber M, Winkler M, et al. Steady‐state disposition of RAD in 91 de novo renal transplant patients assessed longitudinally over a 6‐month period [abstract]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00447459]
Kahan‐203 1999 {published data only}
- Groth CG, Brattstrom C, Claesson K, Backman L. New trails in transplantation: how to exploit the potential of sirolimus in clinical transplantation. Transplantation Proceedings 1998;30(8):4064‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in Caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation 1999;68(10):1526‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kahan BD, Kramer WG. Median effect analysis of efficacy versus adverse effects of immunosuppressants. Clinical Pharmacology & Therapeutics 2001;70(1):74‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kahan‐301 2000 {published data only}
- Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. American Journal of Transplantation 2002;2(6):551‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Blum CB, Rapamune US Study Group. Cholesterol and triglyceride levels in sirolimus‐treated renal transplant recipients [abstract no: A3577]. Journal of the American Society of Nephrology 2000;11(Sept):680A. [CENTRAL: CN‐00550429] [Google Scholar]
- Campistol JM, Cole E, Gioud‐Paquet M, US European and Global Renal Transplant Study Groups. Sirolimus (rapamune): effects on bone in renal transplant patients [abstract no: 681]. Transplantation 2000;69(8 Suppl):S289. [CENTRAL: CN‐00444659] [Google Scholar]
- Campistol JM, Legendre C, Castegneto M, Hartmann A, European US Global and Tri‐Continental Study Groups. The effect of sirolimus on lipids on renal transplant recipients: results from clinical trials [abstract no: A4606]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):880A. [CENTRAL: CN‐00550752] [Google Scholar]
- Campistol JM, Legendre C, Hartmann A, Castagneto M, European US Global and Tri‐Continental Study Group. Lipid effects in renal allograft recipients receiving sirolimus‐based therapies [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00444660] [Google Scholar]
- Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet 2000;356(9225):194‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kahan BD. Two‐year results of multicenter phase III trials on the effect of the addition of sirolimus to cyclosporine‐based immunosuppressive regimens in renal transplantation. Transplantation Proceedings 2003;35(3 Suppl):37‐51S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kahan BD, Kramer WG. Median effect analysis of efficacy versus adverse effects of immunosuppressants. Clinical Pharmacology & Therapeutics 2001;70(1):74‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kahan BD, US and the Global Multicenter Sirolimus Trial Groups. Two‐year follow‐up of the pivotal multicenter trials of sirolimus [abstract no: 960]. Transplantation 2000;69(8 Suppl):S361. [Google Scholar]
- Kahan BD, USA Rapamune Study Group. Pivotal phase III multicenter, randomized, blinded trial of sirolimus versus azathioprine in combination with cyclosporine and prednisone in primary renal transplants [abstract]. Transplantation 1999;67(9):S559. [Google Scholar]
- Manninen D, Dong F, Wang F, Rapamune US and Global Study Groups. Pharmacoeconomic evaluation of sirolimus treatment 12 months following kidney transplantation [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):173A. [CENTRAL: CN‐00550742] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Malignancy in renal transplant recipients receiving sirolimus (rapamune) maintenance therapy: 2‐year follow up from 5 multicenter studies [abstract no: 58]. American Journal of Transplantation 2002;2(Suppl 3):152. [CENTRAL: CN‐00416243] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Two‐year incidence of malignancy in sirolimus (rapamune)‐treated renal transplant recipients: follow‐up results from 5 multicenter studies [abstract no: 0374]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416244]
- Mathew T, Kreis H, Friend P. Two‐year incidence of malignancy in sirolimus‐treated renal transplant recipients: results from five multicenter studies. Clinical Transplantation 2004;18(4):446‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathew TH, Rapamune Global Study Group. The safety and efficacy of sirolimus/cyclosporine for the prevention of acute rejection in primary renal allograft recipients [abstract no: 957]. Transplantation 2000;69(8 Suppl):S360. [CENTRAL: CN‐00446653] [Google Scholar]
- Neylan JF, Jain S, Rapamune US Study Group. Racial effects on the safety and efficacy of sirolimus in renal transplantation [abstract no: 962]. Transplantation 2000;69(8 Suppl):S361. [CENTRAL: CN‐00446941] [Google Scholar]
- Neylan JF, Pescovitz MD, Julian BA, Chan G, Kahan BD, Sirolimus Multi‐Center Study Group. Multi‐center phase II trial of sirolimus in renal transplantation: safety and efficacy for black patients [abstract no: P1003]. Nephrology 1997;3(Suppl 1):S324. [CENTRAL: CN‐00583430] [Google Scholar]
- Neylan JF, Wickersham PS, Jaffe J. Effect of race on efficacy & safety of sirolimus vs AZA + standard immunotherapy in renal transplantation [abstract no: 924]. Transplantation 1999;67(7):S237. [CENTRAL: CN‐00446942] [Google Scholar]
- Neylan JF, Rapamune US Study Group. African‐Americans may benefit from increased sirolimus dosing in renal transplantation [abstract no: PO549]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00446939]
- Neylan JF, Rapamune US Study Group. Safety and efficacy of sirolimus after renal transplantation: one‐year follow‐up [abstract no: 0659]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00446940]
- Pescovitz M, Rapamune US Study Group. One year safety and efficacy of sirolimus after renal transplant [abstract no: 7]. Transplantation 2000;69(8 Suppl):S112. [CENTRAL: CN‐00447185] [Google Scholar]
- Sindhi R, US Rapamune Study Group. Phase III multicenter, randomized, blinded trial of sirolimus versus azathioprine in combination with cyclosporine and prednisone in primary renal transplants [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):747A. [CENTRAL: CN‐00550433] [Google Scholar]
- Wang F, Revicki DA, Sillup G, Ganoczy D, Rapamune US Study Group. Health‐related quality‐of‐life outcomes in kidney transplant patients treated with sirolimus [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):184A. [CENTRAL: CN‐00550707] [Google Scholar]
- Wasielewski S. Sirolimus reduces acute rejection after kidney transplantation [Sirolimus verringert akute Abstossungs‐reaktionen nach Nierentransplantation]. Deutsche Apotheker Zeitung 2000;140(38):42‐4. [EMBASE: 2000353677] [Google Scholar]
Kandaswamy 2005 {published data only}
- Kandaswamy R, Humar A, Dunn T, Gross E, Hughes M, Hill M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: 5‐year results [abstract no: 286]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00677752] [Google Scholar]
- Kandaswamy R, Humar A, Khwaja K, Asolati M, Harmon J, Gillingham K, et al. A prospective randomized study of cyclosporine (CsA)/Cellcept (MMF) vs. tacrolimus (TAC)/sirolimus (SIR) with rapid discontinuation of prednisone (P) [abstract no: 182]. American Journal of Transplantation 2003;3(Suppl 5):198. [CENTRAL: CN‐00445992] [Google Scholar]
- Kandaswamy R, Humar A, Sturdevant M, Garcia‐Roca R, Casingal V, Tan M, et al. Prospective randomized trial of maintenance immunosuppression (IS) in prednisone (P)‐free recipients: mixed results [abstract no: 329]. American Journal of Transplantation 2006;6(Suppl 2):178. [CENTRAL: CN‐00766092] [Google Scholar]
- Kandaswamy R, Humar A, Sutherland DE, Gillingham K, Matas A. A prospective, randomized study of cyclosporine (CsA)/mycophenolate mofetil (MMF) versus tacrolimus (TAC)/sirolimus(SIR) with rapid discontinuation of prednisone (P) [abstract no: 084]. Transplantation 2004;78(2 Suppl):32. [CENTRAL: CN‐00509261] [Google Scholar]
- Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid‐free maintenance regimens in kidney transplant recipients‐‐an interim analysis. American Journal of Transplantation 2005;5(6):1529‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suszynski TM, Gillingham KJ, Matas AJ, Kandaswamy R. Prospective randomized trial of maintenance immunosuppression (IS) with rapid discontinuation of prednisone (RDP) in adult kidney transplantation (KTx): 10‐year results [abstract no: 1587]. American Journal of Transplantation 2012;12(Suppl 3):493. [EMBASE: 70747569] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszynski TM, Gillingham KJ, Rizzari MD, Dunn TB, Payne WD, Chinnakotla S, et al. Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. American Journal of Transplantation 2013;13(4):961‐70. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovarik‐2306 2004 {published data only}
- Kovarik JM, Tedesco H, Pascual J, Civati G, Bizot MN, Geissler J, et al. Everolimus therapeutic concentration range defined from a prospective trial with reduced‐exposure cyclosporine in de novo kidney transplantation. Therapeutic Drug Monitoring 2004;26(5):499‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Tedesco H, Pascual J, Civati G, Schmidli H, Geissler J. Everolimus therapeutic concentration range derived from prospective concentration‐controlled trial in de novo kidney transplantation [abstract no: 510]. American Journal of Transplantation 2004;4(Suppl 8):298. [CENTRAL: CN‐00509289] [DOI] [PubMed] [Google Scholar]
- Magee J, Tedesco H, Pascual J, Civati G, Filho G, Garcia V, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose neoral® in de novo kidney transplant recipients: 12 months analysis [abstract no: 504]. American Journal of Transplantation 2004;4(Suppl 8):296‐7. [CENTRAL: CN‐00509335] [Google Scholar]
- Pascual J. Concentration‐controlled everolimus (Certican): combination with reduced dose calcineurin inhibitors. Transplantation 2005;79(9 Suppl):S76‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pascual J, Cambi V, Dissegna D, Esmeraldo R, Lao M, Durlik M, et al. Efficacy and safety of 2 doses of everolimus combined with reduced dose Neoral® in de novo kidney transplant recipients: 24 months analysis [abstract no: 1010]. American Journal of Transplantation 2005;5(Suppl 11):414. [CENTRAL: CN‐00725012] [Google Scholar]
- Pascual J, Tedesco H, Civati G, Magee J, Haas T, Bernhardt P. Excellent renal function with concentration‐controlled everolimus, reduced exposure to neoral, and steroids in de novo kidney transplant recipients: results of a 6 months efficacy and safety analysis [abstract no: W740]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):786. [CENTRAL: CN‐00447112] [Google Scholar]
- Pascual J, Tedesco H, Magee J, Civati G, Mazzali M, Garcia V, et al. 12 month clinical trial results of safety and efficacy of trough‐controlled Certican® dosing with reduced neoral® exposure in de novo kidney transplant recipients [abstract no: P218]. Transplantation 2004;78(2 Suppl):269. [CENTRAL: CN‐00509404] [Google Scholar]
- Russ G, Eris J, Walker R, Campbell S, Stambe C, RAD2306/7 Study Group. Therapeutic drug monitoring (TDM) for everolimus: modeling of efficacy based on two kidney transplantation trials with implemented TDM [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:97. [CENTRAL: CN‐00509450]
- Tedesco‐Silva H Jr, Vitko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12‐month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transplant International 2007;20(1):27‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6‐month safety and efficacy results of two randomized studies. American Journal of Transplantation 2004;4(4):626‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kovarik‐251 2001 {published data only}
- Kaplan B, Tedesco‐Silva H, Mendez R, Kahan B, Buren D, Boger R, et al. North/South American, double‐blind, parallel group study of the safety and efficacy of Certican (RAD) versus mycophenolate mofetil (MMF) in combination with neoral and corticosteroids [abstract no: 1339]. American Journal of Transplantation 2001;1(Suppl 1):475. [CENTRAL: CN‐00446004] [Google Scholar]
- Kaplan B, Tedesco‐Silva H, Mendez R, Kahan BD, Buren D, Boger R, et al. Everolimus (RAD) ‐ 12 month pivotal study results; the efficacy and safety in conjunction with neoral® and steroids [abstract no: A4700]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):899A. [CENTRAL: CN‐00583195] [Google Scholar]
- Kovarik JM, Hartmann S, Berthier S, Hubert M, Rordorf C, Somberg K. Statin therapy in renal transplant patients receiving everolimus: screening for clinical and pharmacokinetic drug interactions [abstract no: 187]. American Journal of Transplantation 2002;2(Suppl 3):185. [CENTRAL: CN‐00416055] [Google Scholar]
- Kovarik JM, Hsu CH, McMahon L, Berthier S, Rordorf C. Population pharmacokinetics of everolimus in de novo renal transplant patients: impact of ethnicity and comedications. Clinical Pharmacology & Therapeutics 2001;70(3):247‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Kaplan B, Silva HT, Kahan BD, Dantal J, McMahon L, et al. Pharmacokinetics of an everolimus‐cyclosporine immunosuppressive regimen over the first 6 months after kidney transplantation. American Journal of Transplantation 2003;3(5):606‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Kaplan B, Tedesco Silva H, Kahan BD, Dantal J, Vitko S, et al. Exposure‐response relationships for everolimus in de novo kidney transplantation: defining a therapeutic range. Transplantation 2002;73(6):920‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Kaplan B, Vitko S, McMahon L, Attinger M, Boger R, et al. Longitudinal influence of everolimus on cyclosporine assessed over 6 months in two blinded de novo kidney transplant trials [abstract no: 1341]. American Journal of Transplantation 2001;1(Suppl 1):475. [CENTRAL: CN‐00583198] [Google Scholar]
- Kovarik JM, Rordorf C, McMahon L, Berthier SB. Exposure‐response relationships for everolimus in de novo kidney transplantation: defining a therapeutic range [abstract no: 1336]. American Journal of Transplantation 2001;1(Suppl 1):474. [DOI] [PubMed] [Google Scholar]
- Lorber MI, Mulgaonkar S, Butt KM, Elkhammas E, Mendez R, Rajagopalan PR, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3‐year randomized, multicenter, phase III study. Transplantation 2005;80(2):244‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lorber MI, Ponticelli C, Whelchel J, Mayer HW, Kovarik J, Li Y, et al. Therapeutic drug monitoring for everolimus in kidney transplantation using 12‐month exposure, efficacy, and safety data. Clinical Transplantation 2005;19(2):145‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mendez R, Kovarik JM, Kaplan B, Tedesco‐Silva H, Kahan BD, McMahon L, et al. Everolimus pharmacokinetics in de novo kidney allograft recipients: dose‐proportional, stable exposure over 6 months [abstract no: 149]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487730]
- Tedesco‐Silva H, Boger R, Buren D, Kahan B, Kaplan B, Mendez R, et al. Certican (RAD; Everolimus) is complementary with neoral in preventing organ rejection as part of triple therapy in de novo renal transplant recipients [abstract no: 1625]. 2001 A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00487768]
- Tedesco‐Silva H, Kaplan B, Mendez R, Kahan BD, Buren D, Boger R, et al. Certican (RAD; everolimus) is complementary with neoral in preventing organ rejection as part of triple therapy in denovo renal transplant recipients [abstract no: 405]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487769]
Kramer‐2307 2003 {published data only}
- Campbell S, Eris J, Brown F, Russ G, Caicedo L, Walker R, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced Neoral® exposure: 24 month result [abstract no: FC‐50002]. Nephrology 2005;10(Suppl 1):A1. [CENTRAL: CN‐01912374] [Google Scholar]
- Campbell S, Eris J, Walker R, Russ G, Kanellis J, RAD2307 International Study Group. 36 month result showing excellent graft function in de novo kidney transplant recipients treated with everolimus, basiliximab and reduced cyclosoporin exposure [abstract]. Immunology & Cell Biology 2007;85(4):A35. [Google Scholar]
- Campbell S, Eris J, Walker R, Russ G, Kanellis J, RAD2307 International Study Group. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CsA and basiliximab at 24 months [abstract no: 36]. Transplantation Society of Australia & New Zealand (TSANZ). 24th Annual Scientific Meeting; 2006 Mar 29‐31; Canberra, Australia. 2006:53. [CENTRAL: CN‐00583469]
- Eris J, Campbell S, Burbigott B, Leone J, Kraemer B, Rigotti P, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced neoral® exposure: 12‐month results [abstract no: O82]. Transplantation 2004;78(2 Suppl):31. [CENTRAL: CN‐01912456] [Google Scholar]
- Eris J, Campbell S, Walker R, Russ G, Stambe C, RAD2307 International Study Group. Excellent graft function in de novo transplant recipients treated with everolimus, reduced dose Neoral and Simulect: 6 months analysis [abstract no: 5]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:33. [CENTRAL: CN‐00509178]
- Kraemer BK, Bourbigot B, Vitko S, Rigotti P, Caicedo L, Boccardo G. Excellent graft function in kidney transplant recipients treated with everolimus, low‐CSA and basiliximab at 24 months [abstract no: PO‐437]. 12th Congress of the European Society for Organ Transplantation (ESOT); 2005 Oct 15‐19; Geneva, Switzerland. 2005. [CENTRAL: CN‐00653704]
- Kramer BK, Neumayer HH, Stahl R, Pietrzyk M, Kruger B, Pfalzer B, et al. Graft function, cardiovascular risk factors, and sex hormones in renal transplant recipients on an immunosuppressive regimen of everolimus, reduced dose of cyclosporine, and basiliximab. Transplantation Proceedings 2005;37(3):1601‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kramer BK, Whelchel J, Eris J, Campbell S, Vitko S, Haas T, et al. Excellent graft function in de novo kidney transplant recipients treated with concentration controlled everolimus, reduced Neoral exposure and basiliximab: 6 months analysis [abstract no: W741]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):786. [CENTRAL: CN‐00446190] [Google Scholar]
- Leone J, Vitko S, Whelchel J, Eris J, Campbell S, Boubigott B, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced Neoral® exposure: 24 month result [abstract no: 1011]. American Journal of Transplantation 2005;5(Suppl 11):414. [CENTRAL: CN‐00602128] [Google Scholar]
- Pascual J. Concentration‐controlled everolimus (Certican): combination with reduced dose calcineurin inhibitors. Transplantation 2005;79(9 Suppl):S76‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rigotti P, Balden N, Vitko S, Cretin N. Excellent graft function in de novo kidney transplant recipients treated with concentration controlled everolimus, reduced neoral exposure and simulect: 6 months analysis [abstract no: 294]. European Society of Transplantation; 2003 Sept; Venice, Italy. 2003. [CENTRAL: CN‐00527155]
- Russ G, Eris J, Walker R, Campbell S, Stambe C, RAD2306/7 Study Group. Therapeutic drug monitoring (TDM) for everolimus: modeling of efficacy based on two kidney transplantation trials with implemented TDM [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 22nd Annual Scientific Meeting; 2004 Mar 31‐Apr 2; Canberra, Australia. 2004:97. [CENTRAL: CN‐00509450]
- Tedesco H, Pascual J, Civati G, Filho G, Garcia V, Haas T. Efficacy and safety of 2 doses of everolimus combined with reduced dose neoral in de novo kidney transplant recipients: 6 months analysis [abstract no: 1210]. American Journal of Transplantation 2003;3(Suppl 5):462. [CENTRAL: CN‐00447959] [Google Scholar]
- Tedesco‐Silva H Jr, Vitko S, Pascual J, Eris J, Magee JC, Whelchel J, et al. 12‐month safety and efficacy of everolimus with reduced exposure cyclosporine in de novo renal transplant recipients. Transplant International 2007;20(1):27‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6‐month safety and efficacy results of two randomized studies. American Journal of Transplantation 2004;4(4):626‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Whelchel J, Eris J, Campbell S, Bourbigot B, Haas T, et al. Excellent graft function in de novo kidney transplant recipients treated with everolimus, reduced dose neoral and simulect: 6 months analysis [abstract no: 625]. American Journal of Transplantation 2003;3(Suppl 5):311. [CENTRAL: CN‐00448221] [Google Scholar]
- Whelchel J, Vitko S, Eris J, Campbell S, Burbigott B, Leone J, et al. Excellent graft function in de novo kidney transplant recipients treated with Certican®, Simulect® and reduced Neoral® exposure: 12‐month results [abstract no: 507]. American Journal of Transplantation 2004;4(Suppl 8):297. [CENTRAL: CN‐00509179] [Google Scholar]
Kreis‐210 2000 {published data only}
- Abramowicz D, Kreis H, Campistol JM, Morales JM, Mourad G, Holt DW, et al. Prednisolone pharmacokinetics in sirolimus ‐ and cyclosporine ‐ treated renal transplant recipients [abstract no: PO551]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00444096]
- Campistol JM, Holt DW, Epstein S, Gioud‐Paquet M, Rutault K, Burke JT, et al. Bone metabolism in renal transplant patients treated with cyclosporine or sirolimus. Transplant International 2005;18(9):1028‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 2000;69(7):1252‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kreis H, Cisterne JM, Land W, Wramner L, Sirolimus European Renal Transplant Study Group. Sirolimus versus cyclosporine in association with mycophenolate mofetil [abstract no: 959]. Transplantation 2000;69(8 Suppl):360. [CENTRAL: CN‐00446198] [Google Scholar]
- Legendre C, Campistol JM, Squifflet JP, Burke JT, Sirolimus European Renal Transplant Study Group. Cardiovascular risk factors of sirolimus compared with cyclosporine: Early experience from two randomized trials in renal transplantation. Transplantation Proceedings 2003;35(3 Suppl):151‐3S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Legendre C, Squifflet JP, Campistol JM, Sirolimus European Renal Transplant Study Group. Comparison of long term CSA ‐ and sirolimus‐based therapies on lipid parameters [abstract no: 976]. Transplantation 2000;69(8 Suppl):S365. [CENTRAL: CN‐00446314] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Malignancy in renal transplant recipients receiving sirolimus (rapamune) maintenance therapy: 2‐year follow up from 5 multicenter studies [abstract no: 58]. American Journal of Transplantation 2002;2(Suppl 3):152. [CENTRAL: CN‐00416243] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Two‐year incidence of malignancy in sirolimus (rapamune)‐treated renal transplant recipients: follow‐up results from 5 multicenter studies [abstract no: 0374]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416244]
- Mathew T, Kreis H, Friend P. Two‐year incidence of malignancy in sirolimus‐treated renal transplant recipients: results from five multicenter studies. Clinical Transplantation 2004;18(4):446‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales JM, Wramner L, Kreis H, Durand D, Campistol JM, Andres A, et al. Sirolimus does not exhibit nephrotoxicity compared to cyclosporine in renal transplant recipients. American Journal of Transplantation 2002;2(5):436‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ostraat O, Grinyo JM, Cisterne JM, Land W, Squifflet JP, Johnston A, et al. Mycophenolic acid plasma levels are higher with sirolimus than cyclosporine when given with MMF in renal transplant recipients [abstract no: 0496]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00447058]
Lebranchu‐132 2004 {published and unpublished data}
- Al Najjar A, Etienne I, Toupance O, Westeel PF, Hurault de Ligny B, Rerolle JP, et al. Long term follow‐up of a multicenter randomized trial comparing a CNI‐free regimen with sirolimus (SRL) to a cyclosporine based regimen: the Spiesser study [abstract no: 1642]. American Journal of Transplantation 2010;10(Suppl 4):505. [EMBASE: 70465017] [Google Scholar]
- Buchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, et al. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6‐month course of steroids. American Journal of Transplantation 2007;7(11):2522‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Buchler M, Lebranchu Y, Beneton M, Meur Y, Heng AE, Westeel PF, et al. Higher exposure to mycophenolic acid with sirolimus than with cyclosporine cotreatment. Clinical Pharmacology & Therapeutics 2005;78(1):34‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gatault P, Bertrand D, Buchler M, Colosio C, Hurault de Ligny B, Weestel PF, et al. Eight‐year results of the Spiesser study, a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation. Transplant International 2016;29(1):41‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Joannides R, Etienne I, Iacob M, Hurault de Ligny B, Barbier S, Bellien J, et al. Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transplant International 2010;23(11):1135‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Joannides R, Etienne I, Iacob M, Hurault de Ligny B, Barbier S, Lebranchu Y, et al. Comparative effects of sirolimus and cyclosporine on conduit arteries endothelial function in renal transplant recipients [abstract no: 1488]. American Journal of Transplantation 2005;5(Suppl 11):535. [CENTRAL: CN‐00724874] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y. Can we eliminate both calcineurin inhibitors and steroids?. Transplantation Proceedings 2010;42(9 Suppl):S25‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lebranchu Y, Buchler M, Etienne I, Toupance O, Westell P, Legendre C, et al. 12 month results of a randomized trial comparing sirolimus (SRL) versus cyclosporine (CsA) in 150 transplant patients receiving a cadaveric renal graft [abstract no: 1509]. American Journal of Transplantation 2005;5(Suppl 11):540. [Google Scholar]
- Lebranchu Y, Buchler M, Etienne I, Westeel P, Legendre C, Hurault de Ligny B, et al. 12 month results of a randomized trial comparing sirolimus (SRL) versus cyclosporine (CsA) in 150 transplant patients receiving a cadaveric renal graft [abstract no: TH‐FC161]. Journal of the American Society of Nephrology 2005;16(Abstracts):34A. [CENTRAL: CN‐00583240] [Google Scholar]
- Lebranchu Y, Etienne I, Toupance O, Westeel P, Legendre C, Hurault de Ligny B, et al. Preliminary results of a randomized trial comparing sirolimus (SRL) versus cyclosporine (CSA) in 150 transplant patients receiving a cadaveric renal graft [abstract no: 463]. American Journal of Transplantation 2004;4(Suppl 8):285. [CENTRAL: CN‐00509310] [Google Scholar]
- Lebranchu Y, Etienne I, Toupance O, Westeel PF, Hurault de Ligny B, Rerolle JP, et al. CNI avoidance and steroid withdrawal in renal transplantation. Results at three years of a prospective multicenter randomized trial comparing sirolimus (SRL) and cyclosporine (CsA): the SPIESSER study [abstract no: P‐599]. Transplant International 2009;22(Suppl 2):244. [Google Scholar]
- Lebranchu Y, Etienne I, Toupance O, Westeel PF, Legendre C, Hurault de Ligny B, et al. Preliminary results of a randomized trial comparing sirolimus (SRL) versus cyclosporine (CsA) in 150 transplant patients receiving a cadaveric renal graft [abstract no: P746]. Transplantation 2004;78(2 Suppl):463. [Google Scholar]
- Lebranchu Y, Snanoudj R, Toupance O, Weestel PF, Hurault de Ligny B, Buchler M, et al. Five‐year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: the SPIESSER study. American Journal of Transplantation 2012;12(7):1801‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lo 2004 {published data only}
- Gaber AO, Egidi MF, Lo A, Gaber LW, Shokouh‐Amiri MH, Grewal HP, et al. Defining the optimal doses of sirolimus and tacrolimus in high‐risk cadaveric renal transplant recipients [abstract no: 541]. American Journal of Transplantation 2002;2(Suppl 3):274. [CENTRAL: CN‐00415690] [Google Scholar]
- Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus‐based calcineurin inhibitor‐sparing and calcineurin inhibitor‐free regimens in cadaveric renal transplantation. Transplantation 2004;77(8):1228‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lo A, Egidi MF, Gaber LW, Gaber AO. Observations on the use of sirolimus and tacrolimus in high‐risk renal transplant recipients. Transplantation Proceedings 2003;35(3 Suppl):105‐8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lo A, Egidi MF, Gaber LW, Shokouh‐Amiri MH, Nazakatgoo N, Fisher JS, et al. Observations regarding the use of sirolimus and tacrolimus in high‐risk cadaveric renal transplantation. Clinical Transplantation 2004;18(1):53‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lo A, Egidi MF, Gross B, Amiri HS, Vera S, Nezakatgoo N, et al. The use of sirolimus and mycophenolate mofetil is associated with improved renal function, but similar metabolic profiles to sirolimus and tacrolimus [abstract no: 1408]. American Journal of Transplantation 2004;4(Suppl 8):544. [CENTRAL: CN‐00509328] [Google Scholar]
MacDonald‐302 2001 {published data only}
- Blum CB. Effects of sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. American Journal of Transplantation 2002;2(6):551‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Campistol JM, Cole E, Gioud‐Paquet M, US European and Global Renal Transplant Study Group. Sirolimus (rapamune): effects on bone in renal transplant patients [abstract no: 681]. Transplantation 2000;69(8 Suppl):S289. [CENTRAL: CN‐00444659] [Google Scholar]
- Campistol JM, Legendre C, Castegneto M, Hartmann A, European US Global and Tri‐Continental Study Group. The effect of sirolimus on lipids on renal transplant recipients: results from clinical trials [abstract no: A4606]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):880A. [CENTRAL: CN‐00550752] [Google Scholar]
- Campistol JM, Legendre C, Hartmann A, Castagneto M, European US Global and Tri‐Continental Study Group. Lipid effects in renal allograft recipients receiving sirolimus‐based therapies [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00444660] [Google Scholar]
- Kahan BD, Jaffe JS. Triglyceride (TG) elevations in renal transplant recipients treated with sirolimus (rapamycin, RAPA) added to a cyclosporine (CSA)/prednisone (PRED) regimen [abstract no: 172]. Transplantation 1999;67(9):S585. [CENTRAL: CN‐00445975] [Google Scholar]
- Kahan BD, Kramer WG. Median effect analysis of efficacy versus adverse effects of immunosuppressants. Clinical Pharmacology & Therapeutics 2001;70(1):74‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- MacDonald A, Rapamune Global Study Group Transplant Service. A randomized trial of sirolimus, cyclosporine and prednisone vs cyclosporine ‐ prednisone alone in recipients of mismatched first kidney grafts: results at one year [abstract no: 2]. Transplantation 1999;67(9):S543. [CENTRAL: CN‐00446517] [Google Scholar]
- MacDonald AS, RAPAMUNE Global Study Group. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation 2001;71(2):271‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Malignancy in renal transplant recipients receiving sirolimus (rapamune) maintenance therapy: 2‐year follow up from 5 multicenter studies [abstract no: 58]. American Journal of Transplantation 2002;2(Suppl 3):152. [CENTRAL: CN‐00416243] [Google Scholar]
- Mathew T, Kreis H, Doyen K. Two‐year incidence of malignancy in sirolimus (rapamune)‐treated renal transplant recipients: follow‐up results from 5 multicenter studies [abstract no: 0374]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416244]
- Mathew T, Kreis H, Friend P. Two‐year incidence of malignancy in sirolimus‐treated renal transplant recipients: results from five multicenter studies. Clinical Transplantation 2004;18(4):446‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mathew TH, Rapamune Global Study Group. The safety and efficacy of sirolimus/cyclosporine for the prevention of acute rejection in primary renal allograft recipients [abstract no: 957]. Transplantation 2000;69(8 Suppl):S360. [CENTRAL: CN‐00446653] [Google Scholar]
- McEvoy JR, Sindhi R, Bunke M, Uber L, Baillie M, Baliga P, et al. Hypercholesterolemia in sirolimus treated renal transplant patients (single center results of phase III protocol 0468‐302US) [abstract no: A3505]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):687A. [CENTRAL: CN‐00446684] [Google Scholar]
- Ponticelli C. Sirolimus is effective for prevention of acute rejection in primary mismatched renal allograft recipients. Transplantation Proceedings 2001;33(1‐2):1031‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ponticelli C. Sirolimus is safe and effective at one year for prevention of acute rejection in primary mismatched renal allograft recipients [abstract no: 0426]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00447260]
- Ponticelli C, MacDonald AS, Rajagopalan P, Sindhi R, Mathew T. Phase III trial of rapamune versus placebo in primary renal allograft recipients. Transplantation Proceedings 2001;33(3):2271‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rajagopalan PR, Rapamune Global Study Group. A randomized, placebo‐controlled trial of rapamune in primary renal allograft recipients [abstract no: A3759]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):743A. [CENTRAL: CN‐00724881] [Google Scholar]
- Salm P, Taylor PJ, Pillans PI. The quantification of sirolimus by high‐performance liquid chromatography‐tandem mass spectrometry and microparticle enzyme immunoassay in renal transplant recipients. Clinical Therapeutics 2000;22 Suppl B:B71‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sindhi R, LaVia MF, Paulin E, Shaw S, Sindhi LA, Livingston R, et al. Stimulated response of peripheral lymphocytes may distinguish cyclosporine effect in renal transplant recipients on a cyclosporine+rapamycin regimen (phase III protocol 0468602US) [abstract no: S861]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):698‐9A. [CENTRAL: CN‐00447755] [Google Scholar]
- Sindhi R, LaVia MF, Paulling E, McMichael J, Burckart G, Shaw S, et al. Stimulated response of peripheral lymphocytes may distinguish cyclosporine effect in renal transplant recipients receiving a cyclosporine+rapamycin regimen. Transplantation 2000;69(3):432‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sindhi R, McEvoy JR, Bunke M, Baliga P, Rajagopalan PR. A single center 6 month follow up of a phase III trial of sirolimus in a predominantly African‐American renal transplant population [abstract no: T882]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):699A. [CENTRAL: CN‐00447756] [Google Scholar]
- Sindhi R, Wickersham PS, Jaffee JS, Scarola J. Outcomes of African Americans (AA) in a phase III randomized placebo‐controlled trial of sirolimus (SRL) in renal transplantation [abstract no: 925]. Transplantation 1999;67(7):S238. [CENTRAL: CN‐00447757] [Google Scholar]
Machado 2001 {published data only}
- Machado PG, Felipe CR, Hanzawa NM, Park SI, Garcia R, Alfieri F, et al. An open‐label randomized trial of the safety and efficacy of sirolimus vs. azathioprine in living related renal allograft recipients receiving cyclosporine and prednisone combination. Clinical Transplantation 2004;18(1):28‐38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Machado PG, Felipe CR, Park SI, Garcia R, Moreira S, Casarini D, et al. Preservation of graft function in low‐risk living kidney transplant recipients treated with a combination of sirolimus and cyclosporine. Brazilian Journal of Medical & Biological Research 2004;37(9):1303‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Machado PG, Garcia C, Felipe CR, Garcia R, Franco M, Delcelo R, et al. A single‐center open label randomized trial of the safety and efficacy of the use of sirolimus versus azathioprine in one‐haplotype living related kidney transplant recipients‐preliminary results. Transplantation Proceedings 2001;33(1‐2):1074‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Machado PP, Junior HT, Felipe CR, Garcia C. A single‐center open label randomized trial of the safety and efficacy of the use of rapamune versus azathioprine one haplotype living related kidney recipients ‐ preliminary results [abstract no: PO545]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00446521]
- Medina‐Pestana J, Felipe C, Franco M, Garcia R, Machado P, Tedesco H. A single‐center open label randomized trial of the safety and efficacy of the use of sirolimus versus azathioprine in one haplotype living related kidney recipients: 6 months analysis [abstract no: 1666]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00716080]
- Medina‐Pestana JO, Felipe CR, Garcia R, Alfieri F, Tedesco‐Silva H, Franco M, et al. An‐open label randomized trial of the safety and efficacy of rapamune vs azathioprine in renal allograft recipients [abstract no: 0439]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416261]
- Tedesco H, Felipe CR, Garcia C, Machado PP, Medina JO. A single‐center open label randomized trial of the safety and efficacy of the use of rapamune versus azathioprine in one haplotype living related kidney recipients ‐ preliminary results [abstract no: 163]. Transplantation 2000;69(8 Suppl):S155. [CENTRAL: CN‐00447958] [DOI] [PubMed] [Google Scholar]
- Tedesco‐Silva H Jr, Felipe CR, Machado PG, Garcia R, Motegi S, Hosaka BH, et al. Safety and efficacy of sirolimus in kidney transplant patients and in patients with coronary artery disease undergoing angioplasty. Transplantation Proceedings 2003;35(3 Suppl):177‐80S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martinez‐Mier 2006 {published data only}
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Avila‐Pardo SF, Zamudio‐Morales C. Living related kidney transplantation without calcineurin inhibitors: 3‐year results of a randomized prospective trial in a Mexican center [abstract no: 1098]. American Journal of Transplantation 2009;9(Suppl 2):500. [CENTRAL: CN‐01657972] [Google Scholar]
- Martinez‐Mier G, Mendez‐Lopez MT, Budar‐Fernandez LF, Estrada‐Oros J, Franco‐Abaroa R, George‐Micelli E, et al. Living related kidney transplantation without calcineurin inhibitors: initial experience in a Mexican center. Transplantation 2006;82(11):1533‐6. [EMBASE: 2006628001] [DOI] [PubMed] [Google Scholar]
Morelon 2010 {published data only}
- Morelon E, Lefrancois N, Besson C, Prevautel J, Brunet M, Touraine JL, et al. Preferential increase in memory and regulatory subsets during CD4+ T‐cell immune reconstitution after thymoglobulin induction therapy in renal transplant patients receiving sirolimus vs cyclosporine [abstract no: O‐259]. Transplant International 2009;22(Suppl 2):68‐9. [DOI] [PubMed] [Google Scholar]
- Morelon E, Lefrancois N, Besson C, Prevautel J, Brunet M, Touraine JL, et al. Preferential increase in memory and regulatory subsets during T‐lymphocyte immune reconstitution after Thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transplant Immunology 2010;23(1‐2):53‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morelon E, Lefrancois N, Besson C, Prevautel J, Kollop‐Sarda MN, Brunet M, et al. Preferential increase in memory and regulatory subsets during CD4+ T‐cell immune reconstitution after thymoglobulin induction therapy in renal transplant patients receiving sirolimus vs cyclosporine [abstract no: 622]. American Journal of Transplantation 2009;9(Suppl 2):371. [EMBASE: 70010495] [Google Scholar]
- Morelon E, Lefrancois N, Besson C, Prevautel J, Kollop‐Sarda MN, Brunet M, et al. Preferential increase in memory and regulatory subsets during CD4+ T‐cell immune reconstitution after thymoglobulin induction therapy in renal transplant patients receiving sirolimus vs cyclosporine [abstract no: P‐58]. Transplant International 2009;22(Suppl 2):109. [Google Scholar]
- Morelon E, Lefrancois N, Besson C, Prevautel J, Kolopp‐Sarda MN, Brunet M, et al. Immune reconstitution in human renal allograft recipients treated by sirolimus or cyclosporin after thymoglobulin induction therapy [abstract no: 797]. Transplantation 2008;86(Suppl 2):278. [CENTRAL: CN‐00740582] [Google Scholar]
ORION 2011 {published data only}
- Campistol JM, Glyda M, Gurkan A, Flechner SM, Schulman S. Incidence of delayed graft function (DGF) in sirolimus (SRL)‐based regimens compared with calcineurin inhibitors (CNIs) and mycophenolate mofetil (MMF) in de novo renal allograft recipients [abstract no: P‐105]. Transplant International 2009;22(Suppl 2):121. [CENTRAL: CN‐01657955] [Google Scholar]
- Flechner S, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with tacrolimus (TAC)+ mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year safety results from the Orion Trial [abstract no: 444]. Transplantation 2008;86(Suppl 2):156. [CENTRAL: CN‐00740477] [Google Scholar]
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil (MMF) regimen in de novo renal allograft recipients: renal function results from the ORION study [abstract no: 1141]. American Journal of Transplantation 2007;7(Suppl 2):440. [Google Scholar]
- Flechner S, Glyda M, Steinberg S, Harler MB. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (Tac) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from the ORION study [abstract no: 52]. American Journal of Transplantation 2007;7(Suppl 2):160. [Google Scholar]
- Flechner S, Glyda M, Steinberg S, Harler MB, ORION Trial Investigators. A randomized, open‐label study to compare the safety and efficacy of two different sirolimus (SRL) regimens with a tacrolimus (TAC) and mycophenolate mofetil regimen (MMF) in de novo renal allograft recipients: acute rejection and graft survival results from The ORION Study [abstract no: P477]. Transplant International 2007;20(Suppl 2):209. [CENTRAL: CN‐01657180] [Google Scholar]
- Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. A randomized, open‐label study to compare the efficacy and safety of two different sirolimus (SRL) regimens with tacrolimus (TAC) + mycophenolate mofetil (MMF) in de novo renal allograft recipients: preliminary 2‐year efficacy results from the ORION trial [abstract no: 287]. American Journal of Transplantation 2008;8(Suppl 2):254. [CENTRAL: CN‐00653710] [Google Scholar]
- Flechner SM, Cockfield S, Grinyo J, Russ G, Wissing KM, Legendre C, et al. Preliminary two‐year efficacy results from a randomized, open‐label, comparative study of two different sirolimus (SRL) regimens versus a tacrolimus (TAC) + mycophenolate regimen (MMF) in de novo renal allograft recipients [abstract no: 531]. Transplantation 2008;86(Suppl 2):186. [CENTRAL: CN‐00740478] [Google Scholar]
- Flechner SM, Glyda M, Cockfield S, Grinyo J, Legendre C, Russ G, et al. The ORION Study: comparison of two sirolimus‐based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients.[Erratum in: Am J Transplant. 2012 Jan;12(1):268]. American Journal of Transplantation 2011;11(8):1633‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Flechner SM, Glyda M, See Tai S, ORION Trial Investigators. Delayed graft function (DGF) in two sirolimus (SRL)‐based regimens compared with tacrolimus (TAC) and mycophenolate mofetil (MMF) in de novo renal allograft recipients [abstract no: 299]. American Journal of Transplantation 2009;9(Suppl 2):277. [CENTRAL: CN‐00774294] [Google Scholar]
- Flechner SM, Glyda M, Steinberg S, Copley JB, ORION Trial Investigators. The efficacy of sirolimus (SRL) and tacrolimus (TAC) withdrawal versus SRL and mycophenolate mofetil (MMF) compared with TAC and MMF in de novo renal allograft recipients: interim results from the Orion Study [abstract no: SU‐FC124]. Journal of the American Society of Nephrology 2007;18(Abstracts):95A. [CENTRAL: CN‐00716077] [Google Scholar]
- Russ G, Flechner SM, Cockfield S, Grinyo J, Wissing KM, Legendre C, et al. Cardiovascular risk profile of renal transplant recipients enrolled in an open‐label, randomized study comparing 2 sirolimus (SRL) regimens with a tacrolimus (TAC)+ mycophenolate regimen (MMF): preliminary results from the Orion Trial [abstract no: 445]. Transplantation 2008;86(Suppl 2):156. [CENTRAL: CN‐00740479] [Google Scholar]
Paoletti 2012 {published data only}
- Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation 2012;93(5):503‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paoletti E, Marsano L, Bellino D, Cassottana P, Rolla D, Maio G. Everolimus for regression of left ventricular hypertrophy of renal transplant recipients: a randomized controlled trial [abstract no: 16]. American Journal of Transplantation 2012;12(Suppl 3):31. [EMBASE: 70745963] [Google Scholar]
Pascual 2010 {published data only}
- Castillo D, Pascual J, Cabello M, Pallardo L, Grinyo JM, Fernandez AM, et al. A phase II randomized pharmacokinetic (PK) study of tacrolimus (TAC)‐everolimus (EVL) combination for kidney transplantation (KT) [abstract no: 449]. Transplantation 2008;86(Suppl 2):158. [CENTRAL: CN‐00740487] [Google Scholar]
- Pascual J, Castillo D, Cabello M, Pallardo L, Grinyo JM, Fernandez A, et al. A phase II dose‐comparison randomized pharmacokinetic (PK) study on tacrolimus (TAC)‐everolimus (EVL) combination for kidney transplantation (KT) [abstract no: SP502]. NDT Plus 2008;1(Suppl 1):ii201. [Google Scholar]
- Pascual J, Castillo D, Cabello M, Pallardo L, Grinyo JM, Fernandez AM, et al. Interaction between everolimus and tacrolimus in renal transplant recipients: a pharmacokinetic controlled trial. Transplantation 2010;89(8):994‐1000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pescovitz 2007 {published data only}
- Pescovitz M, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety and efficacy of mycophenolate mofetil in combination with sirolimus vs cyclosporine in renal transplant patients [abstract no: 341]. American Journal of Transplantation 2004;4(Suppl 8):251. [CENTRAL: CN‐00509411] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Vincenti F, Hart M, Melton L, Whelchel J, Mulgaonkar S, et al. Pharmacokinetics, safety, and efficacy of mycophenolate mofetil in combination with sirolimus or ciclosporin in renal transplant patients. British Journal of Clinical Pharmacology 2007;64(6):758‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Qazi 2017 {published data only}
- Peddi V, Qazi Y, Shaffer D, Luan F, Shihab F, Tomlanovich S, et al. Effect of everolimus with low dose tacrolimus vs mycophenolate with standard tacrolimus regimen in African‐American de novo renal transplant recipients [abstract no: B955]. Transplantation 2014;98(Suppl 1):536. [EMBASE: 71545337] [Google Scholar]
- Qazi Y, Shaffer D, Kaplan B, Kim D, Luan F, Peddi V, et al. Efficacy and safety of everolimus with low‐dose tacrolimus in de novo renal transplant recipients: 12‐month randomized study [abstract no: 713]. Transplantation 2014;98(Suppl 1):80. [EMBASE: 71543789] [DOI] [PubMed] [Google Scholar]
- Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR, et al. Efficacy and safety of everolimus plus low‐dose tacrolimus versus mycophenolate mofetil plus standard‐dose tacrolimus in de novo renal transplant recipients: 12‐month data. American Journal of Transplantation 2017;17(5):1358‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shaffer D, Qazi Y, Kim D, Mulgaonkar S, Shihab F, Tomlanovich S, et al. Management of the wound complications in de novo renal transplant recipients: US92 12‐month randomized study [abstract no: B975]. Transplantation 2014;98(Suppl 1):542‐3. [EMBASE: 71545357] [Google Scholar]
- Shihab F, Qazi Y, Kaplan B, Kim D, Mulgaonkar S, Peddi V, et al. Everolimus‐facilitated tacrolimus minimization preserves renal function in de novo renal transplant recipients [abstract no: B962]. Transplantation 2014;98(Suppl 1):538‐9. [EMBASE: 71545344] [Google Scholar]
- Shihab F, Qazi Y, Mulgaonkar S, McCague K, Patel D, Peddi V, et al. Everolimus does not increase risk of delayed graft function or prolong duration of dialysis in renal transplantation [abstract no: D64]. American Journal of Transplantation 2017;17(Suppl 3):732. [EMBASE: 615704509] [Google Scholar]
- Shihab F, Qazi Y, Mulgaonkar S, McCague K, Patel D, Peddi VR, et al. Association of clinical events with everolimus exposure in kidney transplant patients receiving low doses of tacrolimus. American Journal of Transplantation 2017;17(9):2363‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihab F, Qazi Y, Peddi V, Shaffer D, McCague K, Patel D, et al. Post‐hoc analysis of everolimus trough levels around the onset of related adverse events and treatment discontinuation in de novo kidney transplant recipients from the US92 study [abstract no: D64]. American Journal of Transplantation 2018;18(Suppl 4):628. [EMBASE: 622279504] [Google Scholar]
- Shihab F, Shaffer D, Qazi Y, Kaplan B, Vincenti F, McCague K, et al. Reduced incidence of CMV and BK virus infection with everolimus vs mycophenolate based regimen in de novo renal transplant recipients [abstract no: B959]. Transplantation 2014;98(Suppl 1):537. [EMBASE: 71545341] [Google Scholar]
- Vincenti F, Qazi Y, Kaplan B, Kim D, Shihab F, McCague K, et al. Influence of induction therapy on the efficacy of everolimus vs mycophenolate based regimen in de novo renal transplant recipients [abstract no: B966]. Transplantation 2014;98(Suppl 1):539‐40. [EMBASE: 71545348] [Google Scholar]
- Yilmaz S, Shaffer D, Qazi Y, McCague K, Patel D, Peddi V, et al. The effect of everolimus and tacrolimus exposure levels on renal histology parameters 6 months post‐transplantation [abstract no: A245]. American Journal of Transplantation 2016;16(Suppl 3):484. [EMBASE: 611699510] [Google Scholar]
- Yilmaz S, Shaffer D, Shihab F, McCague K, Patel D, Qazi Y, et al. Everolimus with low‐dose tacrolimus vs a standard immunosuppressive regimen: renal histology at 6 months in de novo renal transplant patients [abstract no: 34]. American Journal of Transplantation 2016;16(Suppl 3):216. [EMBASE: 611700205] [Google Scholar]
RECORD 2017 {published data only}
- Huh KH, Lee JG, Ha J, Oh CK, Ju MK, Kim CD, et al. De novo low‐dose sirolimus versus mycophenolate mofetil in combination with extended‐release tacrolimus in kidney transplant recipients: a multicentre, open‐label, randomized, controlled, non‐inferiority trial. Nephrology Dialysis Transplantation 2017;32(8):1415‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee J, Huh K, Ha J, Oh CK, Ju M, Kim CD, et al. De novo low‐dose sirolimus versus mycophenolate mofetil in combination with extended‐release tacrolimus in kidney transplant recipients [abstract no: 72]. American Journal of Transplantation 2017;17(Suppl 3):231‐2. [EMBASE: 615704942] [DOI] [PubMed] [Google Scholar]
Riad 2007 {published data only}
- Riad H, Griffin C, Parrott N, Augustine T, Campbell B, Pararajasingam R, et al. Randomised prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard ciclosporin based triple therapy for rejection prophylaxis in renal transplantation: results at year 4 [abstract no: O39]. British Transplantation Society (BTS).13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
- Riad H, Tavakoli A, Hamer C, Augustine T, Parrott N, Nimako L, et al. A randomized prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard cyclosporin based triple therapy for rejection prophylaxis in renal transplantation [abstract no: P086]. Transplant International 2007;20(Suppl 2):116. [CENTRAL: CN‐00740555] [Google Scholar]
- Tavakoli A, Riad H, Hamer C, Augustine T, Parrott N, Nimako L, et al. A randomised prospective trial of daclizumab induction followed by sirolimus in association with mycophenolate mofetil and steroids versus standard cyclosporin based triple therapy for rejection prophylaxis in renal transplantation [abstract no: O17]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
Rostaing 2001 {published data only}
- Rostaing L, Tran‐Van T, Cointault O, Lavayssiere L, Durand D, Ader JL. Assessment of renal function in de novo renal transplant patients receiving either sirolimus or everolimus in addition to ciclosporine A [abstract no: A4785]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):916A. [CENTRAL: CN‐00550538] [Google Scholar]
Russ 2003 {published data only}
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Comparison of reduced‐and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results [abstract]. Transplantation Society of Australia & New Zealand (TSANZ). 21st Annual Scientific Meeting; 2003 Apr 9‐11; Canberra, Australia. 2003:64. [CENTRAL: CN‐00447521]
- Russ G, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. The safety and efficacy of reduced‐ and standard‐target concentration tacrolimus plus sirolimus in renal allograft recipients: preliminary 6‐month results from Australia [abstract no: SA‐P0496]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):364A. [CENTRAL: CN‐00447522] [Google Scholar]
- Russ GR, Campbell S, Chadban S, Eris J, O'Connell P, Pussell B, et al. Reduced and standard target concentration tacrolimus with sirolimus in renal allograft recipients. Transplantation Proceedings 2003;35(3 Suppl):115‐7S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Whelchel J, Paczek L, Bechstein WO, Russ G. A regimen of sirolimus and reduced‐dose tacrolimus results in improved renal allograft function: combined analysis of the North American target, European and Australian sirolimus‐tacrolimus trials [abstract no: 1218]. American Journal of Transplantation 2003;3(Suppl 5):464. [CENTRAL: CN‐00448351] [Google Scholar]
Sampaio 2008 {published data only}
- Park SI, Felipe CR, Pinheiro‐Machado PG, Garcia R, Fernandes FB, Casarini DE, et al. Tacrolimus pharmacokinetic drug interactions: effect of prednisone, mycophenolic acid or sirolimus. Fundamental & Clinical Pharmacology 2009;23(1):137‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Sampaio EL, Pinheiro‐Machado PG, Garcia R, Felipe CR, Park SI, Casarini DE, et al. Mycophenolate mofetil vs. sirolimus in kidney transplant recipients receiving tacrolimus‐based immunosuppressive regimen. Clinical Transplantation 2008;22(2):141‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaefer 2006 {published data only}
- Schaefer HM, Kizilisik AT, Feurer I, Nylander WA, Langone AJ, Helderman JH, et al. Short‐term results under three different immunosuppressive regimens at one center. Transplantation Proceedings 2006;38(10):3466‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shetty 2015 {published data only}
- Gallon L, Leventhal J, Maluf D, Sai B, Shetty A, Traitanon O, et al. Molecular profiles of renal allograft biopsies at 12 months post renal transplant in patients exposed to low dose CNI and everolimus vs. patients exposed to full dose CNI and MMF [abstract no: 69]. American Journal of Transplantation 2017;17(Suppl 3):230. [EMBASE: 615704855] [Google Scholar]
- Shetty A, Leventhal J, Traitanon O, Alvarado A, Mas V, Mathew J, et al. Prospective study of a steroid free, low dose tacrolimus and everolimus combination regimen in kidney transplant [abstract no: D133]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71954083] [Google Scholar]
- Shetty A, Opas T, Mathew J, Mas V, Leventhal J, Sustento‐Reodica N, et al. Impact of low dose tacrolimus with everolimus regimen on renal pathology and t‐regulatory cells in kidney transplant [abstract no: 38]. American Journal of Transplantation 2016;16(Suppl 3):218. [EMBASE: 611700367] [Google Scholar]
- Shetty A, Traitanon O, Ansari M, Mathew J, Leventhal J, Mas V, et al. Prospective randomized study of a steroid free, low dose tacrolimus with everolimus regimen in kidney transplant [abstract no: B110]. American Journal of Transplantation 2016;16(Suppl 3):535‐6. [EMBASE: 611700126] [Google Scholar]
Souza 2017 {published data only}
- Souza P, Ventura C, Agena F, Barreto N, Reusing JO, Onusic V, et al. Impact of everolimus added to tacrolimus/ mycophenolate/prednisone on CMV disease and acute rejection in sensitized kidney transplant recipients. A single center, randomized and controlled, pilot study [abstract no: P341]. Transplant International 2017;30(Suppl 2):482. [EMBASE: 618770379] [Google Scholar]
Spagnoletti 2017 {published data only}
- Spagnoletti G, Gesualdo L, Pisani F, Gruttadauria S, Caputo F, Frasca G, et al. Multicenter, randomized, open label, prospective clinical study to compare the efficacy and safety of steroid withdrawal on day 5 in a combination of tacrolimus plus everolimus vs tacrolimus plus MMF [abstract no: OS163]. Transplant International 2017;30(Suppl 2):64. [EMBASE: 618769692] [Google Scholar]
Stallone 2004 {published data only}
- Stallone G, Paolo S, Schena A, Infante B, Battaglia M, Ditonno P, et al. Addition of sirolimus to cyclosporine delays the recovery from delayed graft function but does not affect 1‐year graft function. Journal of the American Society of Nephrology 2004;15(1):228‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Schena A, Paolo S, Gesualdo L, Ditonno P, et al. Sirolimus prolongs delayed graft function in recipients of sub‐optimal cadaveric kidney donors [abstract no: 1601]. American Journal of Transplantation 2003;3(Suppl 5):563. [CENTRAL: CN‐00447829] [Google Scholar]
Stegall 2003 {published data only}
- Dean PG, Grande JP, Sethi S, Park WD, Griffin MD, Cosio FG, et al. Kidney transplant histology after one year of continuous therapy with sirolimus compared with tacrolimus. Transplantation 2008;85(8):1212‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effect of immunosuppression on renal function and graft histology: a comparison of tacrolimus and sirolimus [abstract no: 379]. American Journal of Transplantation 2005;5(Suppl 11):252. [CENTRAL: CN‐00725015] [Google Scholar]
- Dean PG, Larson TS, Rea DJ, Griffin MD, Textor SC, Schwab TR, et al. The effects of calcineurin inhibitor avoidance on renal function and graft histology after kidney transplantation: a prospective, randomized comparison of tacrolimus and sirolimus [abstract no: O228]. Transplantation 2004;78(2 Suppl):89. [CENTRAL: CN‐00509154] [Google Scholar]
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus [abstract no: 259]. American Journal of Transplantation 2004;4(Suppl 8):229. [CENTRAL: CN‐01912356] [DOI] [PubMed] [Google Scholar]
- Dean PG, Lund WJ, Larson TS, Prieto M, Nyberg SL, Ishitani MB, et al. Wound‐healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77(10):1555‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hamad A, Buehrig CK, Kreps MA, Lager DJ, Fidler ME, Gloor JM, et al. Frequency of polyomavirus‐associated nephropathy in a randomized controlled trial of tacrolimus versus sirolimus‐based immunosuppression [abstract no: 147]. American Journal of Transplantation 2003;3(Suppl 5):189. [CENTRAL: CN‐00445626] [Google Scholar]
- Kudva YC, Basu A, Larson TS, Stegall MD. Metabolic complications after renal transplantation: a comparison of sirolimus vs tacrolimus‐based immunosuppression [abstract no: 256]. American Journal of Transplantation 2003;3(Suppl 5):217. [CENTRAL: CN‐00446214] [Google Scholar]
- Larson TS, Dean PG, Griffin MD, Prieto M, Schwab TR, Cosio FG, et al. Preserving renal function by avoiding calcineurin‐inhibitors in renal transplantation: a randomized trial of sirolimus vs. tacrolimus [abstract no: 461]. American Journal of Transplantation 2004;4(Suppl 8):285. [CENTRAL: CN‐00509307] [Google Scholar]
- Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. American Journal of Transplantation 2006;6(3):514‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Larson TS, Griffin MD, Prieto M, Schwab TR, Lund WJ, Nyberg SL, et al. Avoiding calcineurin‐inhibitors in renal transplantation: a randomized trial of sirolimus vs tacrolimus [abstract no: 1261]. Journal of the American Society of Nephrology 2003;3(Suppl 5):475. [CENTRAL: CN‐00446279] [Google Scholar]
- Larson TS, Velosa JA, Prieto M, Lund WJ, Griffin MD, Gloor JM. Kidney transplantation without calcineurin‐inhibitors: a randomized trial of sirolimus vs tacrolimus. [abstract no: 396]. American Journal of Transplantation 2002;2(Suppl 3):237. [CENTRAL: CN‐01912451] [Google Scholar]
- Larson TS, Velosa JA, Prieto M, Lund WJ, Griffin MD, Gloor JM, et al. Kidney transplantation without calcineurin‐inhibitors: a randomized trial of sirolimus vs tacrolimus [abstract no: 0552]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416102]
- Lund WJ, Larson TS, Stegall MD, Prieto M, Kremers WK. Obesity increases wound complications in sirolimus‐treated renal allograft recipients [abstract no: 246]. American Journal of Transplantation 2003;3(Suppl 5):214. [CENTRAL: CN‐00446497] [Google Scholar]
- Qian Q, Du H, King BF, Kumar S, Cosio FG, Torres VE. Sirolimus reduces polycystic liver volume in ADPKD patients after renal transplantation [abstract no: SA‐PO094]. Journal of the American Society of Nephrology 2007;18(Abstracts):365A. [CENTRAL: CN‐00716079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q, Du H, King BF, Kumar S, Dean PG, Cosio FG, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. Journal of the American Society of Nephrology 2008;19(3):631‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegall MD, Larson TS, Prieto M, Gloor J, Textor S, Nyberg S, et al. Kidney transplantation without calcineurin inhibitors using sirolimus. Transplantation Proceedings 2003;35(3 Suppl):125‐7S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SYMPHONY 2007 {published data only}
- Bagul A, Nicholson M, Chavez R, Grinyo J, Frei U, Vanrentergehm Y, et al. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY study [abstract no: P23]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007. [CENTRAL: CN‐01657142]
- Chavez R, Nicholson M, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, et al. SYMPHONY ‐ Comparing efficacy of standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation. Sub analysis of GFR in cases that completed one year within an intended regime [abstract no: O06]. British Transplantation Society (BTS).10th Annual Congress; 2007 Mar 28‐30; Manchester, UK. 2007.
- Claes K, Meier‐Kriesche HU, Schold JD, Vanrenterghem Y, Halloran PF, Ekberg H. Effect of different immunosuppressive regimens on the evolution of distinct metabolic parameters: evidence from the Symphony study. Nephrology Dialysis Transplantation 2012;27(2):850‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Colom H, Fernandez De Troconiz I, Caldes A, Oppenheimer F, Sanchez Plumed J, Gentil MA, et al. Population pharmacokinetics of mycophenolic acid in combination with free or reduced doses of calcineurin inhibitors during the first week in renal transplant: the Symphony Study [abstract no: 105]. Transplantation 2008;86(2 Suppl):37. [CENTRAL: CN‐00678981] [Google Scholar]
- Daloze P, Ekberg H, Vincenti F, Tedesco‐Silva H, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the SYMPHONY Study [abstract no: F‐PO1078]. Journal of the American Society of Nephrology 2006;17(Abstracts):563A. [CENTRAL: CN‐01912358] [Google Scholar]
- Demirbas A, Hugo C, Grinyo J, Frei U, Gurkan A, Marcen R, et al. Low toxicity regimens in renal transplantation: a country subset analysis of the Symphony study. Transplant International 2009;22(12):1172‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Demirbas A, Hugo C, Grinyo J, Frei U, Gurkan A, Marcen R, et al. Results of the Symphony study are applicable across transplant populations; experience from Spain, Turkey and Germany [abstract no: P581]. Transplant International 2007;20(Suppl 2):234. [CENTRAL: CN‐01657143] [Google Scholar]
- Ekberg H, Bernasconi C, Halloran P. CNI minimisation with 2 G mycophenolate mofetil ‐ what have we learned from the Symphony Study [abstract no: 964]. Transplantation 2008;86(2 Suppl):334. [CENTRAL: CN‐00671785] [Google Scholar]
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrology Dialysis Transplantation 2010;25(6):2004‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Bernasconi C, Noldeke J, Yussim A, Mjornstedt L, Erken U, et al. Cyclosporine, tacrolimus and sirolimus retained their distinct toxicity profiles despite low doses in the Symphony Study [abstract no: 55]. American Journal of Transplantation 2007;7(Suppl 2):160. [CENTRAL: CN‐00653721] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Halloran P, Vanrenterghem Y, Schold J, Meier‐Kriesche H. Renal function progression following DGF and early AR is not impaired if function is restored: evidence from the Symphony Study [abstract no: P583]. Transplant International 2007;20(Suppl 2):235. [Google Scholar]
- Ekberg H, Mamelok R, Bernasconi C, Vincenti F, Tedesco‐Silva H, Daloze P, et al. The challenge of meeting target drug concentrations: experience from the Symphony study [abstract no: 58]. American Journal of Transplantation 2007;7(Suppl 2):161. [CENTRAL: CN‐00615834] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Bitko S, Klempnauer J, Gurkan A, et al. Improved outcomes after de novo renal transplantation: 2‐year results from the Symphony Study [abstract no: 531]. American Journal of Transplantation 2008;8(Suppl 2):320. [CENTRAL: CN‐00653754] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Klempnauer J, Gurkan A, et al. CNI sparing in de novo renal transplantation: 3‐year results from the Symphony Study [abstract no: LB04]. American Journal of Transplantation 2008;8(Suppl 2):336. [CENTRAL: CN‐00653755] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Klempnauer J, Gurkan A, et al. CNI sparing with mycophenolate mofetil in de novo renal transplantation: 3‐year results from the Symphony Study [abstract no: 623]. Transplantation 2008;86(2 Suppl):218. [CENTRAL: CN‐00676055] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. New England Journal of Medicine 2007;357(25):2562‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, Gurkan A, et al. Symphony ‐ comparing standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation [abstract no: 49]. American Journal of Transplantation 2006;6(Suppl 2):83. [CENTRAL: CN‐01912360] [Google Scholar]
- Ekberg H, Vincenti F, Tedesco Silva H, Daloze P, Pearson T. Low‐dose sirolimus in the first 8 weeks following renal transplantation accompanied by daclizumab induction, MMF and steroids: the experience of the Symphony Study [abstract no: 691]. American Journal of Transplantation 2006;6(Suppl 2):300. [CENTRAL: CN‐00602015] [Google Scholar]
- Frei U, Daloze P, Vitko S, Klempnauer J, Reyes‐Acevedo R, Titiz I, et al. Acute rejection in low‐toxicity regimens: clinical impact and risk factors in the Symphony study. Clinical Transplantation 2010;24(4):500‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frei U, Daloze P, Vitko S, Klempnauer J, Reyes‐Acevedo R, Titiz I, et al. Characterization of acute rejections and associated relative risk factors in the Symphony Study [abstract no: 245]. American Journal of Transplantation 2007;7(Suppl 2):210. [CENTRAL: CN‐00653713] [Google Scholar]
- Frei U, Ekberg H, Tedesco‐Silva H, Demirbas A, Vitko S, Nashan B, et al. SYMPHONY ‐ comparing standard immunosuppression to low‐dose cyclosporine, tacrolimus or sirolimus in combination with MMF, daclizumab and corticosteroids in renal transplantation [abstract no: F‐FC152]. Journal of the American Society of Nephrology 2006;17(Abstracts):69A. [CENTRAL: CN‐00602018] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Gentil MA, Hernandez D, Plumed JS, et al. Pharmacokinetics of total and free mycophenolic acid in renal transplantation receiving standard‐dose cyclosporine, low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony PK sub‐study [abstract no: P342]. Transplant International 2007;20(Suppl 2):177. [CENTRAL: CN‐00653737] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid (MPA) when mycophenolate mofetil (MMF) is administered with low‐dose tacrolimus, low‐dose cyclosporine, low‐dose sirolimus or standard‐dose cyclosporine in renal transplantation. Results of the Symphony PK substudy [abstract no: 824]. American Journal of Transplantation 2006;6(Suppl 2):345. [CENTRAL: CN‐00602016] [Google Scholar]
- Grinyo J, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid in renal transplantation receiving standard‐dose cyclosporine, low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony PK sub‐study [abstract no: 1151]. American Journal of Transplantation 2007;7(Suppl 2):443. [CENTRAL: CN‐01912452] [Google Scholar]
- Grinyo JM, Ekberg H, Mamelok RD, Oppenheimer F, Sanchez‐Plumed J, Gentil MA, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard‐dose or low‐dose cyclosporine, low‐dose tacrolimus or low‐dose sirolimus: the Symphony pharmacokinetic substudy. Nephrology Dialysis Transplantation 2009;24(7):2269‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Halloran P, Meier‐Kriesche HU, Schold J, Vanrenterghem Y. Blood pressure in the first year following renal transplantation is associated with immunosuppressive regimens: evidence from the Symphony study [abstract no: 1137]. American Journal of Transplantation 2007;7(Suppl 2):439. [CENTRAL: CN‐00615835] [Google Scholar]
- Hugo C, Frei U, Margreiter R, Peeters P, Ok E, Viebahn R, et al. Elderly kidney transplant recipients are a high‐risk group for death, infections and PTx diabetes: evidence from the Symphony study [abstract no: 154]. American Journal of Transplantation 2007;7(Suppl 2):186. [CENTRAL: CN‐00724927] [Google Scholar]
- Hugo C, Frei U, Margreiter R, Peeters P, Toz H, Viebahn R, et al. Marginal donor quality transmits a high risk profile in elderly but not young renal transplant patients ‐ a subgroup analysis of the Symphony Study [abstract no: F‐PO1471]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):436A. [Google Scholar]
- Kuypers D, Ekberg H, Oppenheimer F, Plumed J, Rodriguez G, Hernandez D, et al. Pharmacokinetics of total and free mycophenolic acid (MPA) when mycophenolate mofetil (MMF) is administered with low‐dose tacrolimus, low‐dose cyclosporine, low‐dose sirolimus or standard‐dose cyclosporine in renal transplantation. Results of the SYMPHONY PK substudy [abstract no: TH‐PO564]. Journal of the American Society of Nephrology 2006;17(Abstracts):227A. [CENTRAL: CN‐01912359] [Google Scholar]
- Llaudo I, Colom H, Gimenez‐Bonafe P, Torras J, Caldes A, Sarrias M, et al. Do drug transporter (ABCB1) SNPs and P‐glycoprotein function influence cyclosporine and macrolides exposure in renal transplant patients? Results of the pharmacogenomic substudy within the SYMPHONY study. Transplant International 2013;26(2):177‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Brunet M, Torras J, Cruzado JM, Oppenheimer F, Sanchez‐Plumed J, et al. Influence of MRP2 and UGT1A9 polimorphisms in the MPA pharmacokinetics in renal transplant recipients. Results of the pharmacogenonic substudy within the Symphony Study [abstract no: TH‐PO508]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):220A. [CENTRAL: CN‐00724892] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Brunet M, Torras J, Oppenheimer F, Sanchez‐Plumed J, Gentil MA, et al. Influence of MRP2 and UGT1A9 polimorphisms in the MPA pharmacokinetics in renal transplant substudy within the Symphony Study [abstract no: 2243]. Transplantation 2008;86(2 Suppl):733. [CENTRAL: CN‐00671787] [DOI] [PubMed] [Google Scholar]
- Lloberas N, Llaudo I, Torras J, Caldes A, Cruzado JM. Polymorphisms of the MDR1 gene in renal transplant recipients affect the PGP activity. Results of the pharmacogenetic substudy within the Symphony Study [abstract no: 2244]. Transplantation 2008;86(2 Suppl):734. [CENTRAL: CN‐00671786] [Google Scholar]
- Lloberas N, Llaudo I, Torras J, Cruzado JM, Caldes A, Oppenheimer F, et al. Polymorphisms of the MDR1 gene in renal transplant recipients affect the PGP activity. Results of the pharmacogenomic substudy (Symphony Study) [abstract no: TH‐PO507]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):220A. [CENTRAL: CN‐00724893] [Google Scholar]
- Lloberas N, Torras J, Cruzado JM, Andreu F, Oppenheimer F, Sanchez‐Plumed J, et al. Influence of MRP2 on MPA pharmacokinetics in renal transplant recipients‐results of the Pharmacogenomic Substudy within the Symphony Study. Nephrology Dialysis Transplantation 2011;26(11):3784‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Meier‐Kriesche H, Halloran P, Vanrenterghem Y, Schold J. Early immunosuppression exposure and incidence of acute rejection and renal function progression: evidence from the Symphony Study [abstract no: 244]. American Journal of Transplantation 2007;7(Suppl 2):210. [CENTRAL: CN‐00724894] [Google Scholar]
- Meier‐Kriesche H, Schold J, Vanrenterghem Y, Halloran P, Ekberg H. Factors associated with BMI increases early after renal transplantation: evidence from the Symphony Study [abstract no: P582]. Transplant International 2007;20(Suppl 2):234. [CENTRAL: CN‐01912362] [Google Scholar]
- O'Connell PJ, Walker RG, Ierino F, Eris J, Russ G, Grinyo J, et al. Results of the SYMPHONY trial in renal transplantation ‐ comparing standard immunosuppression to low dose cyclosporine (low‐CSA), tacrolimus (low‐TAC) or sirolimus (low‐SRL with MMF, daclizumab and corticosteroids [abstract]. Immunology & Cell Biology 2007;85(4):A33. [CENTRAL: CN‐01657144] [Google Scholar]
- Oppenheimer F, Rebello P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Health‐related quality of life of patients receiving low toxicity immunosuppressive regimens [abstract no: TH‐PO566]. Journal of the American Society of Nephrology 2006;17(Abstracts):228A. [CENTRAL: CN‐00747305] [DOI] [PubMed] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez J, Gonzalez M, et al. Health care resource consumption (HCRC) of patients recieving low toxicity immunosuppressive regimens. One year follow up results of the quality of life substudy within the Symphony study [abstract no: P108]. Transplant International 2007;20(Suppl 2):121. [CENTRAL: CN‐00724895] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Cost‐effectiveness analysis of adverse events in low toxicity immunosuppressive regimens, preliminary results of the quality of life substudy within the Symphony Study [abstract no: 107]. Transplantation 2008;86(2 Suppl):38. [CENTRAL: CN‐00677744] [Google Scholar]
- Oppenheimer F, Rebollo P, Grinyo JM, Ortega F, Sanchez‐Plumed J, Gonzalez‐Molina M, et al. Health‐related quality of life of patients receiving low‐toxicity immunosuppressive regimens: a substudy of the Symphony study. Transplantation 2009;87(8):1210‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vanrenterghem Y, Meier‐Kriesche H, Schold J, Halloran P, Ekberg H. Levels and progression of parameters associated with metabolic syndrome by immunosuppressive regimen: evidence from the Symphony Study [abstract no: P580]. Transplant International 2007;20(Suppl 2):234. [CENTRAL: CN‐01912361] [Google Scholar]
Takahashi 2013a {published data only}
- Goto N, Watarai Y, Narumi S, Yamamoto T, Tsujita M, Hiramitsu T, et al. Lower incidence of cytomegalovirus infection with everolimus based immunosuppression versus mycophenolate in de novo renal transplants with 5 years follow‐up [abstract no: D2449]. Transplantation 2014;98(Suppl 1):621. [EMBASE: 71545639] [Google Scholar]
- Hiramitsu T, Okada M, Futamura K, Yamamoto T, Tsujita M, Goto N, et al. 5‐year follow‐up of a randomized clinical study comparing everolimus plus reduced‐dose cyclosporine with mycophenolate mofetil plus standard‐dose cyclosporine in de novo kidney transplantation: Retrospective single center assessment. International Immunopharmacology 2016;39:192‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Kenmochi T, Watarai Y, Kamisawa O. Efficacy and safety of everolimus plus reduced‐dose cyclosporine in recipients of living‐related and unrelated kidney donors: A 24‐month sub‐analysis from the A1202 study [abstract no: P314]. Transplant International 2017;30(Suppl 2):474. [EMBASE: 618769145] [Google Scholar]
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Okada M, Tsujita M, et al. Long‐term efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 9 years follow‐up [abstract no: C86]. American Journal of Transplantation 2018;18(Suppl 4):797. [EMBASE: 622280454] [Google Scholar]
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Tsujita M, Okada M, et al. Everolimus‐based Immunosuppression Possibly Suppresses Mean Fluorescence Intensity Values of De Novo Donor‐specific Antibodies After Primary Kidney Transplantation. Transplantation Proceedings 2019;51(5):1378‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Tsujita M, Yamamoto T, et al. Efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 5 years follow‐up especially in protocol biopsy findings and donor specific antibody production [abstract no: B958]. Transplantation 2014;98(Suppl 1):537. [EMBASE: 71545340] [Google Scholar]
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Tsujita M, Yamamoto T, et al. Impacts of mycophenolate mofetile addition to very low exposure everolimus and calcineurine inhibitor based immunosuppression in de novo kidney transplantation [abstract no: BO414]. Transplant International 2015;28(Suppl 4):268‐9. [EMBASE: 72112005] [Google Scholar]
- Narumi S, Watarai Y, Goto N, Hiramitsu T, Yamamoto T, Tsujita M, et al. Long‐term efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 7 years follow‐up [abstract no: MP703]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i573. [EMBASE: 72327554] [Google Scholar]
- Saito K, Uchida K, Takahara S, Yoshimura N, Teraoka S, Cornu‐Artis C, et al. Efficacy of everolimus with reduced cyclosporine in Japanese de novo renal transplant recipients: 24‐month, randomized, multicenter study [abstract no: B944]. American Journal of Transplantation 2013;13(Suppl S5):314. [EMBASE: 71057520] [Google Scholar]
- Takahara S, Uchida K, Yoshimura N, Teraoka S, Kobayashi E, Teshima R, et al. Efficacy and safety of concentration controlled everolimus with reduced dose cyclosporine in Japanese adult de‐novo renal transplant patients: 12 month results [abstract no: 935]. American Journal of Transplantation 2012;12(Suppl 3):300. [EMBASE: 70746888] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al. Efficacy and safety of concentration‐controlled everolimus with reduced‐dose cyclosporine in Japanese de novo renal transplant patients: 12‐month results. Transplantation Research 2013;2(1):14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, Watarai Y, Narumi S, Yamamoto T, Tsujita M, Hiramitsu T, et al. Proteinuria and histopathological change of everolimus based immunosuppression on de novo kidney transplantation with 5 years follow‐up in protocol biopsies [abstract no: A194]. Transplantation 2014;98(Suppl 1):454. [EMBASE: 71545049] [Google Scholar]
- Uchida K, Hoshinaga K, Watarai Y, Goto N, Kusaka M, Sasaki H, et al. Pharmacokinetics of everolimus when combined with cyclosporine in Japanese de novo renal transplant recipients. Transplantation Proceedings 2014;46(5):1314‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Watarai Y, Akutsu N, Saito K, Nakagawa Y, Kamisawa O, Kenmochi T. Everolimus plus reduced‐exposure calcineurin inhibitor versus mycophenolate mofetil plus standard‐exposure calcineurin inhibitor: 2‐year results in living donor kidney transplant recipients [abstract no: D127]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953410] [Google Scholar]
- Watarai Y, Narumi S, Goto N, Hiramitsu T, Tsujita M, Yamamoto T, et al. Efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 5 years follow‐up especially in protocol biopsy findings and donor specific antibody production [abstract no: MP686]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii560. [EMBASE: 71493087] [Google Scholar]
- Watarai Y, Okada M, Futamura K, Ito K, Yamamoto T, Tsujita M, et al. Impacts of mycophenolate mofetile addition to very low exposure everolimus and calcineurine inhibitor based immunosuppression in de novo kidney transplantation [abstract no: B71]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953820] [Google Scholar]
- Watarai Y, Okada M, Futamura K, Nagai T, Yamamoto T, Tsujita M, et al. Long‐term efficacy and safety of everolimus based immunosuppression on de novo kidney transplantation with 7 years follow‐up [abstract no: B115]. American Journal of Transplantation 2016;16(Suppl 3):537. [EMBASE: 611700338] [Google Scholar]
- Yoshida K, Watarai Y, Nakagawa K, Kamisawa O. Effect of everolimus plus reduced‐dose cyclosporine on graft outcomes in kidney transplant recipients with >=3 HLA mismatches: 24‐month results from the A1202 study [abstract no: P322]. Transplant International 2017;30(Suppl 2):476‐7. [EMBASE: 618769624] [Google Scholar]
Tedesco‐Silva 2003 {published data only}
- Ferreira A, Medina‐Pestana J, Pinheiro‐Machado P, Felipe C, Motegi S, Hosaka B, et al. Concentration‐controlled use of sirolimus (SRL) associated with reduced exposure of cyclosporine (CSA) in black patients: 12‐month results [abstract no: P321]. Transplantation 2004;78(2 Suppl):307. [CENTRAL: CN‐00509189] [Google Scholar]
- Ferreira AN, Machado PG, Felipe CR, Motegi SA, Hosaka BH, Tanaka MK, et al. Concentration‐controlled use of sirolimus (SRL) associated with reduced exposure of cyclosporine (CSA) in black patients: 12‐month results [abstract no: 458]. American Journal of Transplantation 2004;4(Suppl 8):284. [CENTRAL: CN‐01912454] [Google Scholar]
- Ferreira AN, Machado PG, Felipe CR, Motegi SA, Hosaka BH, Tanaka MK, et al. Concentration‐controlled use of sirolimus associated with reduced exposure of cyclosporine in black recipients of primarily living renal allograft donors: 12‐month results. Clinical Transplantation 2005;19(5):607‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Machado PG, Felipe CR, Park SI, Garcia R, Franco M, Alfieri F, et al. Concentration‐controlled use of sirolimus (SRL) associated with reduced exposure of cyclosporine (CSA) in black patients [abstract no: 777]. American Journal of Transplantation 2003;3(Suppl 5):351. [CENTRAL: CN‐00446520] [Google Scholar]
- Machado PG, Hosaka BH, Felipe CR, Garcia R, Franco M, Alfieri F, et al. A concentration‐controlled study of the use of sirolimus (SRL)‐cyclosporine (CSA) combination in black patients [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00416199]
- Machado PG, Hosaka BH, Felipe CR, Garcia R, Franco M, Alfieri F, et al. A concentration‐controlled study of the use of sirolimus (SRL)‐cyclosporine(CSA) combination in black patients [abstract no: 205]. American Journal of Transplantation 2002;2(Suppl 3):190. [CENTRAL: CN‐01912455] [Google Scholar]
- Tedesco Silva H Jr, Felipe CR, Machado PG, Garcia R, Motegi S, Hosaka BH, et al. Safety and efficacy of sirolimus in kidney transplant patients and in patients with coronary artery disease undergoing angioplasty. Transplantation Proceedings 2003;35(3 Suppl):177‐80S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tedesco‐Silva 2010 {published data only}
- Campbell S, Walker R, Pilmore H, Kanellis J, Russ G, Hutchison B, et al. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation [abstract no: 43]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 June 29‐Jul 1; Canberra (ACT). 2011:64. [CENTRAL: CN‐01657183]
- Carmellini M, Garcia V, Wang Z, Vergara M, Escrig C, Russ G. Everolimus de novo with reduced‐exposure cyclosporine in renal transplant recipients at high risk of efficacy failure: results of a post‐HOC analysis [abstract no: BO234]. Transplant International 2015;28(Suppl 4):209. [EMBASE: 72111829] [Google Scholar]
- Carmellini M, Garcia V, Wang Z, Vergara M, Russ G. Efficacy of everolimus with reduced‐exposure cyclosporine in de novo kidney transplant patients at increased risk for efficacy events: analysis of a randomized trial. Journal of Nephrology 2015;28(5):633‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Carmellini M, Garcia V, Wong Z, Vergara M, Escrig C, Russ G. Treatment with everolimus and reduced‐exposure cyclosporine is efficacious in de novo renal transplant recipients at increased risk for efficacy failure: post‐hoc analysis from the A2309 study [abstract no: D125]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71954404] [Google Scholar]
- Chadban S, Pilmore H, Russ G, John K, Campbell S, O'Connell P, et al. Everolimus plus reduced‐exposure cyclosporin versus mycophenolic acid plus cyclosporin: Long‐term follow‐up of Australia and New Zealand kidney transplant recipients in the A2309 randomised controlled trial [abstract no: 450.9]. Transplantation 2016;100(7 Suppl 1):S250. [EMBASE: 613005419] [Google Scholar]
- Chadban S, Pilmore H, Russ G, Kanellis J, Campbell S, O'Connell P, et al. Everolimus plus reduced‐exposure cyclosporin versus mycophenolic acid plus cyclosporin: Seven‐year follow‐up of Australia and New Zealand kidney transplant recipients in the A2309 study [abstract no: 287]. American Journal of Transplantation 2016;16(Suppl 3):305. [EMBASE: 611700282] [Google Scholar]
- Chadban S, Pilmore H, Russ G, Kanellis J, Campbell S, O'Connell P, et al. Everolimus plus reduced‐exposure cyclosporin versus mycophenolic acid plus cyclosporin: seven year follow‐up of ANZ patients from a randomised controlled trial [abstract no: 079]. Nephrology 2015;30(Suppl 3):39. [EMBASE: 71995868] [Google Scholar]
- Cibrik D, Johnston T, Kim YS, Walker R, Zibari G, Mange K, et al. Everolimus allows for around 60% reduction in CsA exposure over 12 months: results from a multicenter, prospective study in renal transplantation [abstract no: 1667]. American Journal of Transplantation 2010;10(Suppl 4):511. [EMBASE: 70465042] [Google Scholar]
- Cibrik D, Johnston T, Kim YS, Walker RG, Zibari G, Cornu‐Artis C, et al. Everolimus exposure and relationship to efficacy and safety: results from a multicenter study in renal transplantation using reduced CsA exposure [abstract no: LB25]. American Journal of Transplantation 2010;10(Suppl 4):567‐8. [EMBASE: 70465240] [Google Scholar]
- Cibrik D, Kim YS, Johnston T, Walker R, Zibari G, Mange K, et al. Benefits of everolimus with reduced CsA exposure on renal function: a multicenter, prospective study in renal transplantation [abstract no: 378]. American Journal of Transplantation 2010;10(Suppl 4):151‐2. [EMBASE: 70463739] [Google Scholar]
- Cibrik D, Kim YS, Johnston T, Walker R, Zibari GB, Cornu‐Artis C, et al. Renal function stability in renal transplant recipients receiving concentration‐controlled everolimus with reduced cyclosporine exposure: 24 month results from the A2309 study [abstract no: 1293]. American Journal of Transplantation 2011;11(Suppl 2):406‐7. [EMBASE: 70406341] [Google Scholar]
- Cibrik D, Tedesco Silva H Jr, Vathsala A, Lackova E, Cornu‐Artis C, Walker RG, et al. Randomized trial of everolimus‐facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 2013;95(7):933‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Colussi G, Vergara M, Wang Z, Roland R. Risk factors associated with efficacy outcomes in kidney transplantation: analysis of a contemporary cohort of patients from the A2309 trial. Clinical Nephrology 2015;83(6):338‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dong G, Akacha M, Ng J, Escrig C, Bernhardt P, Witte S. Different methods for imputing missing renal function data: evidence from a large clinical trial in renal transplant recipients [abstract no: B957]. Transplantation 2014;98(Suppl 1):537. [EMBASE: 71545339] [Google Scholar]
- Escrig C, Bernhardt P, Dong G, Junge G. Changes in the lipid profile in the first year after renal transplantation: Risk factors and influence of immunosuppressive regimens [abstract no: B917]. Transplantation 2014;98(Suppl 1):524‐5. [EMBASE: 71545299] [Google Scholar]
- Escrig C, Colussi G, Roland R, Vergara M, Wang Z. Factors affecting efficacy outcomes at 12 and 24‐months after kidney transplantation in patients from the A2309 study [abstract no: D126]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953480] [Google Scholar]
- Escrig C, Colussi G, Roland R, Vergara M, Wang Z, Bernhardt P. Donor and recipient factors that influence efficacy outcomes: 12 and 24‐month multivariate analyses from kidney transplant recipients receiving everolimus with reduced calcineurin inhibitor [abstract no: O272]. Transplant International 2015;28(Suppl 4):101. [EMBASE: 72111514] [Google Scholar]
- Kanellis J, Walker R, Pilmore H, Russ G, Hutchison B, Chadban S, et al. Benefits of everolimus with reduced CSA exposure on renal function: a multicenter, prospective study in renal transplantation [abstract no: 6]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:40. [CENTRAL: CN‐01657145]
- Kim YS, Tedesco‐Silva H, Johnston T, Lee P, Zibari G, Walker R, et al. Lower incidence of cytomegalovirus and BK virus with everolimus versus mycophenolate in de novo renal transplant patients: results from a multicenter, prospective study [abstract no: 1615]. Transplantation 2010;90(Suppl):256. [EMBASE: 71531587] [Google Scholar]
- Lim WH, Russ GR, Wong G, Pilmore H, Kanellis J, Chadban SJ. The risk of cancer in kidney transplant recipients may be reduced in those maintained on everolimus and reduced cyclosporine. Kidney International 2017;91(4):954‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mange KC, Tedesco H, Johnston T, Zibari G, Li Y, Wang Z. Lack of variation in serum creatinine values between central study laboratory and Cleveland Clinic in kidney transplant recipients [abstract no: 1565]. Transplantation 2008;86(2S):521. [CENTRAL: CN‐01657939] [Google Scholar]
- Pattison J, Riad H, Yaqoob M, Chan L. Correlation of everolimus exposure with reduced CsA exposure to efficacy and safety outcomes: results from a multicentre study in renal transplantation [abstract no: 19]. British Transplantation Society (BTS).14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Pattison J, Riad H, Yaqoob M, Tedesco‐Silva H. Preserved efficacy and renal function from 12 to 24 months with everolimus facilitated CsA reduction [abstract no: 47]. British Transplantation Society (BTS).14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Pattison J, Riad H, Yaqoob M, Tedesco‐Silva H. Renal function in de novo kidney transplant patients receiving everolimus with reduced dose ciclosporin or enteric‐coated mycophenolic acid with standard‐dose ciclosporin: results from a large‐scale, randomised, international trial [abstract no: O36]. British Transplantation Society (BTS).13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
- Riad H, Pattison J, Yaqoob M, Chan L. 12 month analysis of effects of graft type, donor criteria and gender on improved renal allograft function with everolimus facilitated CNI reduction [abstract no: 21]. British Transplantation Society (BTS).14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Riad H, Pattison J, Yaqoob M, Kim YS. Lower incidence of CMV and BK virus with everolimus vs. mycophenolate in de novo renal transplant patients at 12 months [abstract no: 20]. British Transplantation Society (BTS).14th Annual Congress; 2011 Mar 9‐11; Bournemouth, UK. 2011.
- Riad H, Pattison J, Yaqoob M, Tedesco‐Silva H. Everolimus with reduced‐dose ciclosporin as a strategy to optimise long‐term renal function: results from a randomised study in 833 de novo kidney transplant recipients [abstract no: P43]. British Transplantation Society (BTS).13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
- Russ G, Chadban S, Campbell S, Hutchison B, Kanellis J, O'Connell P, et al. Everolimus plus reduced‐dose cyclosporine: results from a randomized, phase III study in 833 de‐novo renal transplant recipients [abstract no: 70]. Immunology & Cell Biology 2010;88(6):A22. [EMBASE: 70313681] [Google Scholar]
- Russ G, Walker R, Pilmore H, Kanellis J, Hutchison B, Chadban S, et al. Everolimus plus reduced CSA exposure: efficacy results from a multicenter, randomized prospective study in renal transplantation [abstract no: 1]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:36. [CENTRAL: CN‐01657148]
- Russ G, Walker R, Pilmore H, Kanellis J, Hutchison B, Chadban S, et al. Lower incidence of cytomegalovirus and BK virus with everolimus versus mycophenolate in de novo renal transplant patients: results from a multicenter, prospective study [abstract no: 70]. Transplantation Society of Australia & New Zealand (TSANZ). 29th Annual Scientific Meeting; 2011 Jun 29‐Jul 1; Canberra (ACT). 2011:82. [CENTRAL: CN‐01657149]
- Shihab F, Cibrik D, Chan L, Kim YS, Carmellini M, Walker R, et al. Exposure‐response analysis of everolimus with reduced cyclosporine in renal transplant recipients at 24 months in a randomized trial [abstract no: 929]. American Journal of Transplantation 2012;12(Suppl 3):298‐9. [EMBASE: 70746882] [Google Scholar]
- Shihab F, Cibrik D, Kim YS, Johnston T, Walker R, Roland R, et al. De novo everolimus combined with reduced cyclosporine exposure ‐ analysis of renal function at 12 months in renal transplant recipients [abstract no: 2023]. Transplantation 2010;90(Suppl 1):110. [EMBASE: 71531314] [Google Scholar]
- Shihab F, Tedesco‐Silva H, Johnston T, Kim YS, Zibari GB, Walker R, et al. Lower incidence of cytomegalovirus and BK virus adverse events with everolimus versus mycophenolate was maintained over 24 months in de novo renal transplant recipients [abstract]. American Journal of Transplantation 2011;11(Suppl 2):45. [EMBASE: 70405089] [Google Scholar]
- Shihab FS, Cibrik D, Chan L, Kim YS, Carmellini M, Walker R, et al. Association of clinical events with everolimus exposure in kidney transplant patients receiving reduced cyclosporine. Clinical Transplantation 2013;27(2):217‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tedesco‐Silva H Jr, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al. Everolimus plus reduced‐exposure CsA versus mycophenolic acid plus standard‐exposure CsA in renal‐transplant recipients. American Journal of Transplantation 2010;10(6):1401‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tedesco‐Silva H, Johnston T, Kim YS, Zibari G, Walker R, Mange K, et al. Everolimus‐treated renal transplant patients have a lower incidence of CMV and BKV: results from a multicenter, prospective study [abstract: 1659]. American Journal of Transplantation 2010;10(Suppl 4):509. [EMBASE: 70465034] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Johnston T, Walker R, Tufvetson G, Zibari G, et al. Everolimus with reduced exposure of cyclosporine: efficacy results from a randomized prospective study in 833 de novo renal transplant recipients [abstract no: 427]. Transplantation 2010;90(Suppl 1):110. [EMBASE: 71531315] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Johnston T, Walker R, Zibari G, Mange K, et al. Everolimus plus reduced CsA exposure: efficacy results from a multicenter, randomized prospective study in renal transplantation [abstract no: 374]. American Journal of Transplantation 2010;10(Suppl 4):150. [EMBASE: 70463735] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Johnston T, Walker R, Zibari GB, Cornu‐Artis C, et al. Concentration‐controlled everolimus with reduced cyclosporine concentration in de novo renal transplant recipients: efficacy results at 24 months [abstract no: 57]. American Journal of Transplantation 2011;11(Suppl 2):46. [EMBASE: 70405092] [Google Scholar]
- Tedesco‐Silva H, Kim YS, Lackova E, Johnston T, Zibari G, Panis C, et al. Everolimus with reduced‐dose cyclosporine as a strategy for optimizing long‐term renal function: results from a randomized study in 833 de‐novo renal‐transplant recipients [abstract no: P‐371]. Transplant International 2009;22(Suppl 2):186‐7. [CENTRAL: CN‐01657151] [Google Scholar]
- Vathsala A, Zibari G, Kim YS, Cibrik D, Johnston T, Walker R, et al. Dose related incidences of wound healing events in renal transplant recipients treated with everolimus and cyclosporine [abstract no: 2060]. Transplantation 2012;90(Suppl 1):615. [EMBASE: 71532274] [Google Scholar]
- Walker R, Cibrik D, Tedesco‐Silva H, Johnston T, Kim YS, Zibari G, et al. Everolimus allows for a 60% reduction in cyclosporine exposure in subject cohorts defined by geography [abstract no: 1616]. Transplantation 2010;90(Suppl 1):610. [EMBASE: 71532265] [Google Scholar]
- Walker R, Vathsala A, Zibari GB, Kim YS, Cibrik D, Johnston T, et al. Class related adverse events in renal transplant recipients treated with everolimus: 24 month results from the A2309 study [abstract no: 1295]. American Journal of Transplantation 2011;11(Suppl 2):407. [EMBASE: 70406343] [Google Scholar]
- Wiseman AC, McCague K, Kim Y, Geissler F, Cooper M. The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcomes. American Journal of Transplantation 2013;13(2):442‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yaqoob M, Pattison J, Riad H, Cornu‐Artis C, Wang Z, Shihab F. Cytomegalovirus and BK virus infections are less frequent with everolimus versus mycophenolate immunosuppression: 24‐month update from the 2309 study in de novo renal transplant recipients [abstract]. Transplant International 2011;24(Suppl 2):40‐1. [EMBASE: 70527224] [Google Scholar]
- Zibari G, Kim YS, Cibrik D, Johnston T, Walker R, Mange K, et al. Wound healing events are dose related: a multicenter, prospective study on everolimus in renal transplantation [abstract no: 1661]. American Journal of Transplantation 2010;10(Suppl 4):510. [EMBASE: 70465036] [Google Scholar]
Tedesco‐Silva 2015 {published data only}
- Basso G, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes‐Freitas T, et al. Kinetics of cytomegalovirus load in kidney transplant recipients receiving everolimus or mycophenolate sodium and no pharmacological prophylaxis [abstract no: C274]. American Journal of Transplantation 2016;16(Suppl 3):688. [EMBASE: 611699076] [Google Scholar]
- Basso G, Felipe CR, Cristelli MP, Mansur Silviano J, Viana L, Ferreira Brigido AN, et al. The effect of anti‐thymocyte globulin and everolimus on the kinetics of cytomegalovirus viral load in seropositive kidney transplant recipients without prophylaxis. Transplant Infectious Disease 2018;20(4):e12919. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brigido A, Tedesco H, Felipe C, Cristelli M, Franco M, Medina‐Pestana J. Efficacy and renal function in kidney transplant recipients receiving tacrolimus (TAC)‐based immunosuppressive regimens in combination with everolimus (EVR) versus mycophenolate (MPA) [abstract no: 64]. American Journal of Transplantation 2016;16(Suppl 3):227. [EMBASE: 611699395] [Google Scholar]
- Cristelli M, Felipe C, Oliveira N, Gusukuma L, Ferreira A, Sandes‐Freitas T, et al. De novo everolimus (EVR) versus mycophenolate (MPA) in kidney transplant recipients receiving tacrolimus (TAC) [abstract no: 2908]. Transplantation 2014;98(Suppl 1):141. [EMBASE: 71543976] [Google Scholar]
- Cristelli M, Ueno P, Felipe C, Hannun P, Tedesco H, Medina‐Pestana J. Wound healing complications in kidney transplant recipients receiving everolimus (EVR) [abstract no: D2530]. Transplantation 2014;98(Suppl 1):644. [EMBASE: 71545720] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felipe C, Oliveira N, Bessa A, Gusukuma L, Ferreira A, Cristelli M, et al. Association between immunosuppressive drug concentration and the incidence of CMV infection or biopsy proven acute rejection (BPAR) in kidney transplant recipients [abstract no: D2410]. Transplantation 2014;98(Suppl 1):775‐6. [EMBASE: 71546189] [Google Scholar]
- Ferreira A, Felipe C, Cristelli M, Viana L, Basso G, Stopa S, et al. Donor‐specific anti‐human leukocyte antigens antibodies, acute rejection, renal function, and histology in kidney transplant recipients receiving tacrolimus and everolimus. American Journal of Nephrology 2017;45(6):497‐508. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferreira A, Felipe C, Ueno P, Hannun P, Gusukuma L, Cristelli M, et al. Effects of three immunosuppressive regimens on renal function in kidney transplant recipients [abstract no: B967]. Transplantation 2014;98(Suppl 1):540. [EMBASE: 71545349] [Google Scholar]
- Ferreira A, Felipe C, Ueno P, Hannun P, Ruppel P, Sandes‐Freitas T, et al. Comparison of efficacy and renal function in kidney transplant recipients receiving tacrolimus (TAC)‐based immunosuppressive regimens in combination with everolimus (EVR) or mycophenolate (MPA) [abstract no: D121]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953176] [Google Scholar]
- Grenzi PC, Campos EF, Tedesco‐Silva H, Felipe CR, Franco MF, Medina‐Pestana JO, et al. Influence of immunosuppression on SCD30 levels and association of SCD30 levels with CMV infection/disease in kidney transplant recipients under different immunosuppressive regimens [abstract no: MO6]. HLA 2017;89(6):375‐6. [EMBASE: 616279203] [Google Scholar]
- Gusukuma L, Felipe C, Sandes‐Freitas T, Ferreira A, Cristelli M, Oliveira N, et al. Kinetics of cytomegalovirus (CMV) viral replication in tacrolimus (TAC) treated kidney transplant recipients receiving everolimus (EVR) or mycophenolate (MPA) [abstract no: D2417]. Transplantation 2014;98(Suppl 1):777‐8. [EMBASE: 71546196] [Google Scholar]
- Tedesco H, Felipe C, Sandes‐Freitas T, Oliveira N, Gusukuma L, Ferreira A, et al. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant recipients [abstract no: D2371]. Transplantation 2014;98(Suppl 1):765. [EMBASE: 71546150] [Google Scholar]
- Tedesco‐Silva H, Felipe C, Ferreira A, Cristelli M, Oliveira N, Sandes‐Freitas T, et al. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. American Journal of Transplantation 2015;15(10):2655‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tedesco‐Silva H, Felipe C, Ferreira A, Cristelli M, Sandes‐Freitas T, Oliveira N, et al. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus [abstract no: 212]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71954236] [DOI] [PubMed] [Google Scholar]
- Tedesco‐Silva H, Felipe CR, Ferreira AN, Cristelli M, Oliveira NI, Mansur JB, et al. Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus [abstract no: BO347]. Transplant International 2015;28(Suppl 4):247. [EMBASE: 72111939] [Google Scholar]
- Tedesco‐Silva H, Sandes‐Freitas T, Felipe C, Cristelli M, Rodrigues C, Gusukuma L, et al. A prospective randomized trial aimed to reduce the incidence of cytomegalovirus (CMV) infection in kidney transplant (KT) recipients [abstract no: BA296]. Transplant International 2013;26(Suppl 2):154‐5. [EMBASE: 71359536] [Google Scholar]
- Ueno P, Felipe C, Ferreira A, Cristelli M, Viana L, Mansur J, et al. Wound healing complications in kidney transplant recipients receiving everolimus. Transplantation 2017;101(4):844‐50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno P, Tedesco H, Brigido A, Felipe C, Hannun P, Cristelli M, et al. Wound healing complications in kidney transplant recipients receiving everolimus [abstract no: C242]. American Journal of Transplantation 2016;16(Suppl 3):679. [EMBASE: 611699551] [DOI] [PMC free article] [PubMed] [Google Scholar]
TRANSFORM 2018 {published data only}
- Berger S, Sommerer C, Bakr M, Soo KM, Danguilan R, Fijter J, et al. TRANSFORM study: Impact of donor type on 12‐month outcomes of everolimus and reduced calcineurin inhibitor versus mycophenolate and standard calcineurin inhibitor in de novo kidney transplant patients [abstract no: LOS011]. Transplant International 2017;30(Suppl 2):162‐3. [EMBASE: 618769281] [Google Scholar]
- Berger SP, Sommerer C, Witzke O, Tedesco H, Chadban S, Mulgaonkar S, et al. Two‐year outcomes in de novo renal transplant recipients receiving everolimus‐facilitated calcineurin inhibitor reduction regimen from TRANSFORM study. American Journal of Transplantation 2019 Jun 1 [epub ahead of print]. [PUBMED: 31152476] [DOI] [PubMed] [Google Scholar]
- Buchler M, Sommerer C, Mulgaonkar S, Garcia V, Massari P, Kuypers D, et al. The TRANSFORM study: lower viral infections with everolimus and reduced calcineurin inhibitor versus mycophenolate and standard calcineurin inhibitor in de novo kidney transplant patients at month 12 [abstract no: O40]. Transplant International 2018;31(Suppl 1):14. [EMBASE: 619997597] [Google Scholar]
- Chadban S, Hughes P, Campbell S, Irish A, Lim W, O'Connell P, et al. TRANSFORM trial design: a randomized, multicentre, open‐label study of everolimus with reduced calcineurin inhibitors in over 20000 de novo renal transplant recipients [abstract no: 48]. Transplantation Society of Australia & New Zealand (TSANZ). 32nd Annual Scientific Meeting; 2014 Jun 11‐13; Canberra (ACT). 2014:61. [CENTRAL: CN‐01657286]
- Chadban S, Mulgaonkar S, Pascual J, Tedesco H, Berger S, Qazi Y, et al. Everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplant recipients: efficacy and safety outcomes from the TRANSFORM study [abstract no: 597]. American Journal of Transplantation 2018;18(Suppl 4):475. [EMBASE: 622280975] [Google Scholar]
- Chadban S, Oppenheimer F, Citterio F, Witzke O, Garcia VD, Gutierrez‐Dalmau A, et al. Effcacy and safety outcomes with de novo use of everolimus‐based regimen in renal transplant recipients with delayed graft function from the TRANSFORM study [abstract]. American Journal of Transplantation 2019;19(Suppl 3):357‐8. [EMBASE: 628453504] [Google Scholar]
- Citterio F, Watarai Y, Mulgaonkar S, Huynh‐Do U, Bernhardt P, Henry M. Effects of the de novo use of everolimus with reduced calcineurin inhibitor on wound healing events in the TRANSFORM study [abstract no: C391.6]. Transplantation 2018;102(7 Suppl 1):S94. [EMBASE: 623701238] [Google Scholar]
- Cruzado J, Mulgaonkar S, Garcia V, Massari P, Kuypers D, Buchler M, et al. The TRANSFORM study: Lower viral infections with everolimus and reduced calcineurin inhibitor versus mycophenolate and standard calcineurin inhibitor in de novo kidney transplant patients at month 12 [abstract no: LOS002]. Transplant International 2017;30(Suppl 2):159‐60. [EMBASE: 618770367] [Google Scholar]
- Henry M. TRANSFORM: A modern approach to evaluate long‐term outcomes of everolimus with reduced‐calcineurin inhibitors in de novo kidney transplant recipients‐baseline characteristics [abstract no: B129]. American Journal of Transplantation 2016;16(Suppl 3):542. [EMBASE: 611699077] [Google Scholar]
- Henry M, Citterio F, Kim M, Kim D, Bernhardt P, Gupta A, et al. Effect of de novo use of everolimus with reduced calcineurin inhibitor versus standard mycophenolic acid‐based regimen on wound healing events in kidney transplant recipients from TRANSFORM study [abstract no: C67]. American Journal of Transplantation 2018;18(Suppl 4):790. [EMBASE: 622279816] [Google Scholar]
- Lee PC, Chadban SJ. Baseline characteristics of Asian and non‐Asian renal transplant recipients: Findings from the TRANSFORM trial [abstract no: P.1530]. Transplantation 2016;100(7 Suppl 1):S725. [EMBASE: 613005011] [Google Scholar]
- Legendre C, Berger S, Oppenheimer F, Wiseman A, Steinberg S, Viklicky O, et al. Assessment of proteinuria reported as adverse events in de novo renal transplant recipients receiving an everolimns‐based regimen: 24‐month results from TRANSFORM [abstract]. American Journal of Transplantation 2019;19(Suppl 3):693‐4. [EMBASE: 628454964] [Google Scholar]
- Legendre C, Chadban S, Tedesco H, Berger S, Qazi Y, Cruzado JM, et al. Efficacy and safety of everolimus [EVR] with reduced‐dose calcineurin inhibitor [rCNI] in de novo kidney transplant recipients [KTxRs]: M24 results from the TRANSFORM study [abstract no: O57]. Transplant International 2019;32(Suppl 1):18‐9. [EMBASE: 625831383] [Google Scholar]
- Legendre C, Sommerer C, Bakr MA, Soo KM, Danguilan R, Fijter J, et al. TRANSFORM study: Impact of donor type on 12‐month outcomes of everolimus and reduced calcineurin inhibitor versus mycophenolate and standard calcineurin inhibitor in De novo kidney transplant patients [abstract no: O42]. Transplant International 2018;31(Suppl 1):14‐5. [EMBASE: 619997625] [Google Scholar]
- Legendre C, Srinivas T, Pascual J, Chadban S, Citterio F, Henry M, et al. The TRANSFORM trial design: A large randomized, multicenter, open‐label study of everolimus with reduced calcineurin inhibitors in de novo renal transplantation [abstract no: P16]. Transplant International 2013;26(Suppl 3):23‐4. [EMBASE: 71356170] [Google Scholar]
- Mulgaonkar S, Qazi Y, Kim D, Peddi V, McCague K, Patel D, et al. Effect of everolimus with reduced calcineurin inhibitor on efficacy and safety in de novo kidney transplant recipients: 12‐month results from the us cohort of TRANSFORM study [abstract no: 35]. American Journal of Transplantation 2018;18(Suppl 4):259. [EMBASE: 622280595] [Google Scholar]
- Oberbauer R, Vincenti F, Viklicky O, Huynh‐Do U, Kim D, Cruzado JM, et al. Effect of induction therapy on outcomes of de novo renal transplant recipients receiving everolimus with reduced‐dose calcineurin inhibitor: 24‐month results from the TRANSFORM study [abstract]. American Journal of Transplantation 2019;19(Suppl 3):558. [EMBASE: 628454072] [Google Scholar]
- Oppenheimer F, Legendre C, Cruzado J, Russ G, Viklicky O, Oberbauer R, et al. Everolimus with reduced‐dose calcineurin inhibitor versus mycophenolate with standard‐dose calcineurin inhibitor in de novo kidney transplant recipients: Renal function results from the TRANSFORM study [abstract no: 33]. American Journal of Transplantation 2018;18(Suppl 4):258. [EMBASE: 622280526] [Google Scholar]
- Oppenheimer F, Russ G, Viklicky O, Oberbauer R, Garcia V, Witzke O, et al. Renal function outcomes in de novo kidney transplant recipients receiving everolimus with reduced‐exposure calcineurin inhibitor versus mycophenolate with standard‐exposure calcineurin inhibitor: results from the TRANSFORM study [abstract no: 591.2]. Transplantation 2018;102(7 Suppl 1):S363. [EMBASE: 623701955] [Google Scholar]
- Oppenheimer F, Witzke O, Viklicky O, Cassuto E, Fijter J, Huynh‐Do U, et al. The TRANSFORM study: Effect of everolimus with reduced‐exposure calcineurin inhibitor on 12‐month renal function outcomes in de novo kidney transplant recipients [abstract no: LOS010]. Transplant International 2017;30(Suppl 2):162. [EMBASE: 618769255] [Google Scholar]
- Pascual J. TRANSFORM: a novel study to evaluate the effect of everolimus with reduced calcineurin inhibitors in de novo kidney transplant recipients: baseline data [abstract no: P242]. Transplant International 2015;28(Suppl 4):442. [EMBASE: 72112235] [Google Scholar]
- Pascual J, Berger S, Witzke O, Chadban S, Oppenheimer F, Oberbauer R, et al. The TRANSFORM study: 12‐month efficacy and safety of everolimus with reduced calcineurin inhibitor in de novo kidney transplant recipients [abstract no: LOS001]. Transplant International 2017;30(Suppl 2):159. [EMBASE: 618770261] [Google Scholar]
- Pascual J, Berger SP, Witzke O, Tedesco H, Mulgaonkar S, Qazi Y, et al. Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. Journal of the American Society of Nephrology 2018;29(7):1979‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J, Chadban S, Citterio F, Henry M, Legendre C, Oppenheimer F, et al. TRANSFORM trial design: Effect of everolimus on long‐term outcomes after kidney transplantation [abstract no: P308]. Transplant International 2013;26(Suppl 2):247. [EMBASE: 71359928] [Google Scholar]
- Pascual J, Chadban S, Tedesco H, Berger S, Qazi Y, Cruzado JM, et al. Efficacy and safety of everolimus with reduced‐dose calcineurin inhibitor in de novo kidney transplant recipients: results from the TRANSFORM study [abstract no: 591.9]. Transplantation 2018;102(7 Suppl 1):S367‐8. [EMBASE: 623702178] [Google Scholar]
- Pascual J, Srinivas TR, Chadban S, Citterio F, Henry M, Legendre C, et al. Balancing efficacy and renal function preservation after kidney transplantation with everolimus and reduced calcineurin inhibitors for better graft outcomes: design of the TRANSFORM study [abstract no: MP612]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii535. [EMBASE: 71493013] [Google Scholar]
- Pascual J, Srinivas TR, Chadban S, Citterio F, Oppenheimer F, Tedesco H, et al. TRANSFORM: A novel study design to evaluate the effect of everolimus on long‐term outcomes after kidney transplantation. Open Access Journal of Clinical Trials 2013;6:45‐53. [EMBASE: 373306041] [Google Scholar]
- Pernin V, Buchler M, Cruzado J, Garcia V, Kuypers D, Citterio F, et al. The TRANSFORM study: Infection outcomes with everolimus plus reduced calcineurin inhibitor and mycophenolate plus standard calcineurin inhibitor regimens in de novo kidney transplant recipients [abstract no: O73]. Transplant International 2019;32(Suppl 1):23. [EMBASE: 625831569] [Google Scholar]
- Qazi YA, Kim DY, Wiseman AC, Peddi VR, Steinberg SM, Shihab FS, et al. Effect of everolimus with reduced‐exposure calcineurin inhibitor in de novo kidney transplant recipients: 24‐month results from the US cohort of the TRANSFORM study [abstract no: TH‐PO1155]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):B6. [Google Scholar]
- Shihab F, Qazi Y, Peddi V, Shaffer D, Kim D, McCague K, et al. Effect of early attainment of everolimus target trough levels on efficacy‐safety outcomes in de novo kidney transplant recipients: 12‐month results from US92 and TRANSFORM studies [abstract no: 36]. American Journal of Transplantation 2018;18(Suppl 4):259. [EMBASE: 622280636] [Google Scholar]
- Srinivas T, Legendre C, Oppenheimer F, Tedesco‐Silva H, Luo WL, Bernhardt P, et al. A novel endpoint for evaluating the effect of everolimus with reduced calcineurin inhibitor on long‐term kidney graft outcome: The TRANSFORM study protocol [abstract no: D95]. American Journal of Transplantation 2017;17(Suppl 3):742‐3. [EMBASE: 615705499] [Google Scholar]
- Srinivas T, Pascual J, Chadban SJ, Citterio F, Henry ML, Legendre CM, et al. The TRANSFORM trial design: a large randomized, multicenter, open‐label study of everolimus with reduced calcineurin inhibitors in de novo renal transplantation [abstract no: PUB462]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):976A. [Google Scholar]
- Tedesco SH, Citterio F, Henry M, Srinivas TR, Watarai Y, Steinberg S, et al. Antirejection efficacy in de novo renal transplant recipients receiving everolimus with a reduced‐exposure calcineurin inhibitor‐based regimen: 24‐month results from the TRANSFORM study [abstract]. American Journal of Transplantation 2019;19(Suppl 3):358‐9. [EMBASE: 628453592] [Google Scholar]
- Tedesco‐Silva H, Pascual J, Viklicky O, Basic‐Jukic N, Cassuto E, Kim DY, et al. Safety of everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplants: an analysis from the randomized TRANSFORM study. Transplantation 2019;103(9):1953‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vincenti F, Cruzado J, Mulgaonkar S, Garcia V, Kuypers D, Buchler M, et al. The TRANSFORM study: Infection outcomes with everolimus plus reduced calcineurin inhibitor and mycophenolate plus standard calcineurin inhibitor regimens in de novo kidney transplant recipients [abstract no: 34]. American Journal of Transplantation 2018;18(Suppl 4):258. [EMBASE: 622280564] [Google Scholar]
- Watarai Y, Berger SP. TRANSFORM: everolimus with reduced calcineurin inhibitors vs mycophenolate with standard calcineurin inhibitors in de novo kidney transplant recipients‐baseline data according to donor type [abstract no: P.1416]. Transplantation 2016;100(7 Suppl 1):S673‐4. [EMBASE: 613005289] [Google Scholar]
- Witzke O, Arns W, Weithofer P, Lehner F, Banas B, Giet M, et al. Everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplant recipients: Infection and wound healing outcomes from the TRANSFORM study [abstract no: PV03]. Transplant International 2018;31(Suppl 5):18. [EMBASE: 625099371] [Google Scholar]
van Gurp 2010 {published data only}
- Gurp E, Bustamante J, Franco A, Rostaing L, Becker T, Rondeau E, et al. Comparable renal function at 6 months with tacrolimus combined with fixed‐dose sirolimus or MMF: results of a randomized multicenter trial in renal transplantation. Journal of Transplantation 2010;2010. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hooff 2003 {published data only}
- Wlodarczyk Z, Squifflet JP, Hooff JP, Vanrentergheim Y, Paczek L. Tacrolimus in combination with rapamycin: results of a multicentre, randomised, dose‐finding study [abstract no: 1314]. American Journal of Transplantation 2002;2(Suppl 3):469. [CENTRAL: CN‐00416943] [Google Scholar]
- Wlodarczyk Z, Hooff JP, Vanrenterghem Y, Squifflet JP, Paczek L. Tacrolimus in combination with various dosages of rapamycin in renal recipients: safety and efficacy of the first 6‐month multicenter randomised trial [abstract]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002. [CENTRAL: CN‐00725007]
- Hooff JP, Squifflet JP, Wlodarczyk Z, Vanrenterghem Y, Paczek L. A prospective randomized multicenter study of tacrolimus in combination with sirolimus in renal‐transplant recipients. Transplantation 2003;75(12):1934‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Velosa‐212 2001 {published data only}
- Campistol JM, Alveranga D, Hricik DE, Velosa J, Grinyo JM, Mourad G, et al. Sirolimus‐treated renal transplant recipients experience improved renal function with cyclosporine elimination: one‐year results from a phase II trial [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A208. [CENTRAL: CN‐00487674] [Google Scholar]
- Gonwa T, Alveranga D, Ancona G, Brinker K, Cambi V, Campistol J, et al. Sirolimus (rapamune) permits early elimination of cyclosporine in recipients of cadaveric renal allografts [abstract no: 958]. Transplantation 2000;69(8 Suppl):S360. [CENTRAL: CN‐00445514] [Google Scholar]
- Gonwa TA. Sirolimus (SRL) treated patients demonstrate improved renal function after withdrawal of cyclosporine (CSA) [abstract no: 4658]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):891A. [CENTRAL: CN‐00550647] [Google Scholar]
- Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP, Sirolimus Renal Function Study Group. Improved renal function in sirolimus‐treated renal transplant patients after early cyclosporine elimination. Transplantation 2002;74(11):1560‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hricik DE, Rapamune Renal Function Study Group. Sirolimus improves renal function without increasing acute rejection after cyclosporine elimination in renal transplant patients [abstract no: 0430]. XVIII International Congress of the Transplantation Society; 2000 Aug 27 ‐ Sep 1; Rome, Italy. 2000. [CENTRAL: CN‐00583304]
- Velosa JA, Larson TS, Gloor JM, Stegall MD. Cyclosporine elimination in the presence of TOR inhibitors: effects on renal function, acute rejection, and safety. American Journal of Kidney Diseases 2001;38(4 Suppl 2):S3‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vitko‐201 2001 {published and unpublished data}
- Boger R, Dantal J, Margreiter R, Viljoen H, Vitko S, Weimar W, et al. CERTICAN (TM) (RAD; Everolimus), the proliferation signal inhibitor, is complementary with Neoral in preventing acute allograft rejection [abstract no: 1626]. 2001 A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00487752]
- Dantal J, Boger R, RAD 201 Study Group, Margreiter R, Viljoen H, Vitko S, et al. Incidence of cytomegalovirus and other viral infections is significantly less in patients receiving Certican(® (RAD; Everolimus) versus MMF while maintaining good efficacy [abstract no: 1624]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00583727]
- Dantal J, Vitko S, Margreiter R, Weimar W, Viljoen H, Boger R, et al. Incidence of cytomegalovirus and other viral infections is significantly less in patients receiving certican (RAD; everolimus) versus MMF while maintaining good efficacy [abstract no: 404]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00527106]
- Dantal J, Vitko S, Margreiter R, Weimar W, Viljoen H, Somberg K. Everolimus (certican (TM), RAD) is associated with a reduced incidence of CMV infection following renal transplantation [abstract no: 963]. American Journal of Transplantation 2002;2(Suppl 3):380. [CENTRAL: CN‐00415492] [Google Scholar]
- Holmes M, Chilcott J, Walters S, Whitby S, Akehurst R. Economic evaluation of everolimus versus mycophenolate mofetil in combination with cyclosporine and prednisolone in de novo renal transplant recipients. Transplant International 2004;17(4):182‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Holmes MW, Chilcott JB, Walters SJ, Whitfield MD, Whitby S, Akehurst RL. Economic evaluation of everolimus (certican) versus mycophenolate mofetil as part of triple immunosuppressive therapy in de novo renal transplant recipients [abstract]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487705]
- Holmes MW, Chilcott JB, Walters SJ, Whitfield S, Whitby RL. Economic evaluation of everolimus (Certican) versus mycophenolate mofetil as part of triple immunosuppressive therapy in de novo renal transplant recipients [abstract no: 2148]. XIXth International Congress of the Transplantation Society; 2002 Aug 25‐30; Miami (FL). 2002.
- Kovarik JM, Kahan BD, Kaplan B, Lorber M, Winkler M, Rouilly M, et al. Longitudinal assessment of everolimus in de novo renal transplant recipients over the first post‐transplant year: pharmacokinetics, exposure‐response relationships, and influence on cyclosporine. Clinical Pharmacology & Therapeutics 2001;69(1):48‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Kaplan B, Silva HT, Kahan BD, Dantal J, McMahon L, et al. Pharmacokinetics of an everolimus‐cyclosporine immunosuppressive regimen over the first 6 months after kidney transplantation. American Journal of Transplantation 2003;3(5):606‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kovarik JM, Rordorf C, McMahon L, Berthier S, Boger R. Toward defining a therapeutic range for everolimus in de novo kidney transplantation based on exposure‐response relationships [abstract no: 151]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487720]
- Lorber MI, Ponticelli C, Whelchel J, Mayer HW, Kovarik J, Li Y, et al. Therapeutic drug monitoring for everolimus in kidney transplantation using 12‐month exposure, efficacy, and safety data. Clinical Transplantation 2005;19(2):145‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Margreiter R, Vitko S, Weimar W, Dantal J, Viljoen H, Boger R, et al. Certican (RAD; everolimus), the proliferation signal inhibitor, is complementary with neoral in preventing acute allograft rejection [abstract no: 725]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487726]
- Neumayer HH, Oppenheimer F, Viljoen OO, Vitko SV, Budde K, Cretin N. 36‐month results of an international study with everolimus for the prevention of allograft rejection in de novo kidney transplant recipients [abstract no: T681]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):499. [Google Scholar]
- Noronha IL, Eisen HJ, Pedersen TR, Rivero E. Long‐term predicted probability of coronary heart disease in renal transplant recipients treated with everolimus or MMF: an estimation based on the first year of treatment by applying a Framingham prediction model [abstract no: 2411]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487745]
- Oppenheimer F, Oyen O, Viljoen H, Vitko S, Falcone A, Cremer M. 36‐month results of an international study with everolimus for the prevention of allograft rejection in de novo kidney transplant recipients [abstract no: 1201]. American Journal of Transplantation 2003;3(Suppl 5):459. [CENTRAL: CN‐00446927] [Google Scholar]
- Viljoen H, Boger R, Dantal J, Margreiter R, Vitko S, RAD 201 Study Group. Hyperlipidemia associated with a certican (TM) (RAD; everlimus) containing immunosuppressive regimen is reversible and responsive to lipid lowering therapies [abstract no: 1628]. A Transplant Odyssey; 2001 Aug 20‐23; Istanbul, Turkey. 2001. [CENTRAL: CN‐00583311]
- Vitko S, Margreiter R, Weimar W, Dantal J, Kuypers D, Winkler M, et al. Three‐year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. American Journal of Transplantation 2005;5(10):2521‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Margreiter R, Weimar W, Dantal J, Viljoen H, Cambon N, et al. International, double‐blind, parallel group study of the safety and efficacy of certican (RAD) versus mycophenolate mofetil (MMF) in combination with neoral and steroids [abstract no: 1337]. American Journal of Transplantation 2001;1(Suppl 1):474. [CENTRAL: CN‐00448220] [Google Scholar]
- Vitko S, Margreiter R, Weimar W, Dantal J, Viljoen HG, Li Y, et al. Everolimus (Certican) 12‐month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation 2004;78(10):1532‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Tedesco H, Eris J, Pascual J, Whelchel J, Magee JC, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6‐month safety and efficacy results of two randomized studies. American Journal of Transplantation 2004;4(4):626‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weimar W, Vitko S, Margreiter R, Dantal J, Viljoen H, Boger R, et al. Hyperlipidemia associated with a certican (RAD; everolimus) containing immunosuppressive regimen is reversible and responsive to lipid lowering therapies [abstract no: 555]. 10th ESOT & 12th ETCO Congress. Bridging the Future; 2001 Oct 6‐11; Lisboa, Portugal. 2001. [CENTRAL: CN‐00487777]
Vitko‐TERRA 2004 {published and unpublished data}
- Margreiter R, Czajkowski Z, Vitko S, Wlodarczyk W, Salmela K, TERRA Study Group. Renal graft function and proteinuria in patients receiving tacrolimus/sirolimus combination therapy [abstract no: P‐62]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550627]
- Salmela K, Vitko S, Wlodarczyk Z, Czajkowski Z, Margreiter R, TERRA Study Group. Tacrolimus with MMF or two different doses of sirolimus in kidney transplantation: a large randomised multicentre study [abstract no: 1630]. American Journal of Transplantation 2005;5(Suppl 11):571. [CENTRAL: CN‐00583243] [Google Scholar]
- Vitko S, Wlodarczyk Z, Kyllonen L, Czajkowski Z, Margreiter R, Backman L, et al. Tacrolimus combined with two different dosages of sirolimus in kidney transplantation: results of a multicenter study. American Journal of Transplantation 2006;6(3):531‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vitko S, Wlodarczyk Z, Salmela K, Czajkowski Z, Margreiter R. Tacrolimus in combination with two different sirolimus doses versus a tacrolimus/MMF‐based regimen: a large, randomised clinical study in renal transplantation [abstract no: 0286]. Transplantation 2004;78(2 Suppl):112‐3. [CENTRAL: CN‐00509547] [Google Scholar]
- Wlodarczyk Z, Vitko S, Salmela K, Czajkowski Z, Margreiter R, TERRA Study Group. Lipid metabolism in renal transplant patients receiving tacrolimus/sirolimus combination therapy. Transplantation Proceedings 2005;37(4):1871‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wlodarczyk Z, Vitko S, Salmela K, Czajkowski Z, Margreiter R, TERRA Study Group. A randomised multicentre study of tacrolimus in combination with two different doses of sirolimus compared with tacrolimus plus MMF [abstract no: 23]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550680]
References to studies excluded from this review
ADHERE 2017 {published data only}
- Rummo OO, Carmellini M, Rostaing L, Oberbauer R, Christiaans MH, Mousson C, et al. ADHERE: randomized controlled trial comparing renal function in de novo kidney transplant recipients receiving prolonged‐release tacrolimus plus mycophenolate mofetil or sirolimus. Transplant International 2017;30(1):83‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barsoum 2007 {published data only}
- Barsoum RS, Morsey AA, Iskander IR, Morgan MM, Fayad TM, Atalla NT, et al. The Cairo Kidney Center protocol for rapamycin‐based sequential immunosuppression in kidney transplant recipients: 2‐year outcomes. Experimental & Clinical Transplantation 2007;5(2):649‐57. [MEDLINE: ] [PubMed] [Google Scholar]
CALLISTO 2009 {published data only}
- Albano L, Berthoux F, Moal MC, Rostaing L, Legendre C, Blanc AS, et al. Immediate versus delayed everolimus treatment in de novo renal transplant recipients at risk of delayed graft function: 1‐year results of the CALLISTO Study [abstract no: P‐99]. Transplant International 2009;22(Suppl 2):119. [Google Scholar]
- Albano L, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, et al. Incidence of delayed graft function and wound healing complications after deceased‐donor kidney transplantation is not affected by de novo everolimus. Transplantation 2009;88(1):69‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cassuto E, Kamar N, Berthoux F, Moal M, Legendre C, Blanc A, et al. Renal function and wound healing in kidney transplant recipients at risk of delayed graft function receiving everolimus [abstract no: 1089]. American Journal of Transplantation 2009;9(Suppl 2):498. [CENTRAL: CN‐00776097] [Google Scholar]
- Dantal J, Berthoux F, Moal M, Rostaing L, Legendre C, Blanc A, et al. Immediate vs delayed everolimus treatment in de novo renal transplant patients at risk of delayed graft function: results of a 12‐month randomized, multicentre trial [abstract no: 239]. American Journal of Transplantation 2009;9(Suppl 2):259. [CENTRAL: CN‐00775108] [Google Scholar]
- Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Blanc AS, et al. Immediate versus delayed everolimus: comparable renal function and wound healing complications in kidney transplant recipients at risk of delayed graft function [abstract no: O‐301]. Transplant International 2009;22(Suppl 2):79. [Google Scholar]
- Dantal J, Berthoux F, Moal MC, Rostaing L, Legendre C, Genin R, et al. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased‐donor kidney transplant recipients at specified risk of delayed graft function: 12‐month results of a randomized, multicenter trial.[Erratum in: Transpl Int. 2012 Jan;25(1):138]. Transplant International 2010;23(11):1084‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carmellini 2010 {published data only}
- Carmellini M, Bernini M, Ruggieri G, Collini A. Conversion of stable kidney transplant recipients from a twice‐daily everolimus regimen to a once‐daily everolimus regimen [abstract no: 1109]. American Journal of Transplantation 2009;9(Suppl 2):503. [CENTRAL: CN‐00774238] [DOI] [PubMed] [Google Scholar]
- Carmellini M, Collini A, Ruggieri G, Bernini M. Conversion of stable kidney transplant recipients from a twice‐daily to once‐daily everolimus regimen. Transplantation Proceedings 2010;42(4):1312‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CENTRAL 2012 {published data only}
- Mjornstedt L, Schwartz Sorensen S, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Renal function three years after early conversion from a calcineurin inhibitor to everolimus: results from a randomized trial in kidney transplantation. Transplant International 2015;28(1):42‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mjornstedt L, Sorensen SS, Zur Muhlen B, Jespersen B, Hansen JM, Bistrup C, et al. Improved renal function after early conversion from a calcineurin inhibitor to everolimus: a randomized trial in kidney transplantation. American Journal of Transplantation 2012;12(10):2744‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murbraech K, Holdaas H, Massey R, Undset LH, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients: an echocardiographic substudy of the randomized controlled CENTRAL trial. Transplantation 2014;97(2):184‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Murbraech K, Massey R, Undset LH, Midtvedt K, Holdaas H, Aakhus S. Cardiac response to early conversion from calcineurin inhibitor to everolimus in renal transplant recipients ‐ a three‐yr serial echocardiographic substudy of the randomized controlled CENTRAL trial. Clinical Transplantation 2015;29(8):678‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CERTITEM 2015 {published data only}
- Hertig A, Kamar N, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: 717]. Transplantation 2014;98(Suppl 1):81. [EMBASE: 71543793] [Google Scholar]
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: The CERTITEM trial [abstract no: O5]. Transplant International 2013;26(Suppl 3):2. [EMBASE: 71356080] [Google Scholar]
- Hertig A, Kamar N, Anglicheau D, Moulin B, Hazzan M, Hurault De Ligny B, et al. Epithelial to mesenchymal transition markers in kidney transplant recipients: the CERTITEM trial [abstract no: P081]. Transplant International 2013;26(Suppl 2):201. [EMBASE: 71359712] [Google Scholar]
- Rostaing L, Hertig A, Albano L, Anglicheau D, Durrbach A, Vuiblet V, et al. Fibrosis progression according to epithelial‐mesenchymal transition profile: a randomized trial of everolimus versus CsA. American Journal of Transplantation 2015;15(5):1303‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Snanoudj R, Suberbielle C, Kamar N, Cassuto E, Caillard S, Taupin JL, et al. Epitope load is predictive of de novo donor specific antibodies occurrence in renal transplant recipients after conversion from cyclosporine to everolimus [abstract no: 279]. American Journal of Transplantation 2016;16(Suppl 3):301‐2. [EMBASE: 611699953] [Google Scholar]
Citterio 2004 {published data only}
- Citterio F, Rossi E, Tondolo V, Romagnoli J, Castagneto M. Better cardiovascular risk profile with tacrolimus based immunosuppression [abstract no: P‐60]. 3rd International Congress on Immunosuppression; 2004 Dec 8‐11; San Diego (CA). 2004. [CENTRAL: CN‐00550362]
Cruzado 2016 {published data only}
- Cruzado JM, Pascual J, Sanchez‐Fructuoso A, Seron D, Diaz JM, Rengel M, et al. Controlled randomized study comparing the cardiovascular profile of everolimus with tacrolimus in renal transplantation. Transplant International 2016;29(12):1317‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morales J, Pascual J, Sanchez‐Fructuoso A, Seron D, Diaz J, Oppenheimer F, et al. Effect of everolimus versus tacrolimus on left ventricular hypertrophy in renal transplant patients on maintenance [abstract no: A176]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953375] [Google Scholar]
EVIDENCE 2014 {published data only}
- Carmellini M, Todeschini P, Manzia TM, Valerio F, Messina M, Sghirlanzoni MC, et al. Twelve‐month outcomes from EVIDENCE trial (everolimus once‐a‐day regimen with cyclosporine versus corticosteroid elimination) in adult kidney transplant recipients [abstract no: O193]. Transplant International 2013;26(Suppl 2):100. [EMBASE: 71359334] [Google Scholar]
- Ponticelli C, Carmellini M, Tisone G, Sandrini S, Segoloni G, Rigotti P, et al. A randomized trial of everolimus and low‐dose cyclosporine in renal transplantation: with or without steroids?. Transplantation Proceedings 2014;46(10):3375‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fior 2015 {published data only}
- Fior F, Momo R, Nacchia F, Sagliocca A, Pessolano G, Boschiero L. Everolimus based/calcineurin inhibitors free protocol in marginal donor kidney transplantation: Results at five years of a cohort study [abstract no: P336]. Transplant International 2015;28(Suppl 4):504. [EMBASE: 72112316] [Google Scholar]
Libetta 2007 {published data only}
- Libetta C, Portalupia V, Sepe V, Cosmai L, Bellotti N, Rossi N, et al. Effects of sirolimus or cyclosporine‐based immunosuppression on in vitro allostimulation of CD4+ [abstract no: FP542]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi203. [CENTRAL: CN‐00724875] [Google Scholar]
- Libetta C, Sepe V, Zucchi M, Portalupi V, Meloni F, Rampino T, et al. The effect of sirolimus‐ or cyclosporine‐based immunosuppression effects on T‐cell subsets in vivo. Kidney International 2007;72(1):114‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Libetta 2015 {published data only}
- Libetta C, Esposito P, Gregorini M, Margiotta E, Martinelli C, Borettaz I, et al. Sirolimus vs cyclosporine after induction with basiliximab does not promote regulatory T cell expansion in de novo kidney transplantation: results from a single‐center randomized trial. Transplant Immunology 2015;33(2):117‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathew 2006 {published data only}
- Hariharan S, Zimmerman JJ, Rapamune Study Group. A comparative study of the pharmacokinetic profiles of sirolimus oral solution and tablets in renal allograft patients [abstract no:157]. Transplantation 2000;69(8 Suppl):S154. [Google Scholar]
- Hariharan S, Rapamune US Study Group. Equivalent safety and efficacy of sirolimus oral solution and tablet formulations in primary renal allograft recipients [abstract no: A3627]. Journal of the American Society of Nephrology 2000;11(Sept):690A. [CENTRAL: CN‐00550609] [Google Scholar]
- Mathew TH, Buren C, Kahan BD, Butt K, Hariharan S, Zimmerman JJ, et al. A comparative study of sirolimus tablet versus oral solution for prophylaxis of acute renal allograft rejection. Journal of Clinical Pharmacology 2006;46(1):76‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Buren CT, Rapamune US Study Group. Sirolimus oral solution and tablet formulations demonstrate equivalent safety and efficacy in renal allograft recipients [abstract no:P0533]. XVIII International Congress of the Transplantation Society; 2000 Aug 27‐Sept 1; Rome (Italy). 2000.
- Buren CT, Rapamune Study Group. Sirolimus oral solution and tablets demonstrate equivalent safety and efficacy in renal allograft [abstract no: 157]. Transplantation 2000;69(8 Suppl):S153‐4. [CENTRAL: CN‐00448115] [Google Scholar]
Nafar 2012 {published data only}
- Nafar M, Alipour B, Ahmadpoor P, Pour‐Reza‐Gholi F, Samadian F, Samavat S, et al. Sirolimus versus calcineurin inhibitor‐based immunosuppressive therapy in kidney transplantation: a 4‐year follow‐up. Iranian Journal of Kidney Diseases 2012;6(4):300‐6. [MEDLINE: ] [PubMed] [Google Scholar]
NCT00005113 {published data only}
- NCT00005113. A study to compare treatment with sirolimus versus standard treatment in patients who have received a kidney transplant. www.clinicaltrials.gov/ct2/show/NCT00005113 (first received 14 April 2000).
NCT00965094 {published data only}
- NCT00965094. Efficacy and safety of everolimus+EC‐MPS after early CNI elimination vs EC‐MPS +tacrolimus in renal transplant recipients. www.clinicaltrials.gov/ct2/show/NCT00965094 (first received 24 August 2009).
nEVEROLD 2017 {published data only}
- David‐Neto E, Agena F, Ramos F, Triboni A, Altona M, Coelho V, et al. Everolimus/low tacrolimus (TAC) compared to MPA/regular TAC for renal transplantation in the elderly recipient‐preliminary analysis of the NEVEROLD trial [abstract no: 288]. American Journal of Transplantation 2016;16(Suppl 3):305. [EMBASE: 611700340] [Google Scholar]
- David‐Neto E, Agena F, Ramos F, Triboni AH, Altona M, Coelho V, et al. Everolimus/low tacrolimus (TAC) compared to MPA/regular TAC for renal transplantation in the elderly recipient‐preliminary analysis of the nEverOld trial [abstract no: P1481]. Transplantation 2016;100(7 Suppl 1):S694. [EMBASE: 613005024] [Google Scholar]
- David‐Neto E, Agena F, Ramos F, Triboni AH, Romano P, Almeida Rezende Ebner P, et al. Longitudinal pharmacokinetics of everolimus when combined with low‐level of tacrolimus in elderly renal transplant recipients. Transplantation 2017;101(9):2133‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- David‐Neto E, Romano P, Kamada Triboni AH, Ramos F, Agena F, Almeida Rezende Ebner P, et al. Longitudinal pharmacokinetics of tacrolimus in elderly compared with younger recipients in the first 6 months after renal transplantation. Transplantation 2017;101(6):1365‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Romano P, Agena F, Almeida Rezende EP, Massakazu SN, Kamada Triboni AH, Ramos F, et al. Longitudinal pharmacokinetics of mycophenolic acid in elderly renal transplant recipients compared to a younger control group: data from the nEverOld trial. European Journal of Drug Metabolism & Pharmacokinetics 2019;44(2):189‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NEVERWOUND 2014 {published data only}
- Carmellini M, Boschiero L, Rigotti P, Sandrini S, Frasca GM, Minetti E, et al. Immediate introduction of everolimus does not affect wound healing and delayed graft function in kidney transplant recipients: 12‐months results from NEVERWOUND study [abstract no: BOS299]. Transplant International 2017;30(Suppl 2):252. [EMBASE: 618770335] [Google Scholar]
- Carmellini M, Ruggieri G, Sforza D, Veroux M, Secchi A, Todeschini P, et al. Immediate introduction of everolimus does not affect wound healing and delayed graft function in renal transplant patients: Preliminary results from NEVERWOUND study [abstract no: D2430]. Transplantation 2014;98(Suppl 1):615. [EMBASE: 71545620] [Google Scholar]
- Carmellini M, Todeschini P, Secchi A, Sandrini S, Minetti E, Furian L, et al. Immediate introduction of everolimus does not affect wound healing and delayed graft function in kidney transplant recipients: 3‐months results from NEVERWOUND study [abstract no: 67]. American Journal of Transplantation 2016;16(Suppl 3):228. [EMBASE: 611699536] [Google Scholar]
Novoa 2011 {published data only}
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus CSA elimination compared with CSA reduction [abstract no: P‐359]. Transplant International 2009;22(Suppl 2):183. [CENTRAL: CN‐01657137] [Google Scholar]
- Grinyo JM, Paul J, Novoa P, Errasti P, Franco A, Aldana G, et al. Better renal function in renal‐transplant recipients treated with everolimus plus cyclosporine elimination compared with cyclosporine minimisation [abstract no: 1636]. American Journal of Transplantation 2010;10(Suppl 4):503. [EMBASE: 70465011] [Google Scholar]
- Novoa PA, Grinyo JM, Ramos FJP, Errasti P, Franco A, Aldana G, et al. De novo use of everolimus with elimination or minimization of cyclosporine in renal transplant recipients. Transplantation Proceedings 2011;43(9):3331‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oh 2012 {published data only}
- Oh CK, Ha JW, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus (Certican) with low dose of cyclosporine in de novo kidney recipients after 1 month of transplantation (preliminary results). Journal of the Korean Society for Transplantation 2012;26(2):83‐91. [CENTRAL: CN‐01046131] [Google Scholar]
- Oh CK, Huh KH, Ha J, Kim YH, Kim YL, Kim YS. Safety and efficacy of the early introduction of everolimus with reduced‐exposure cyclosporine a in de novo kidney recipients. Transplantation 2015;99(1):180‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pretagostini 2016 {published data only}
- Pretagostini R, Poli L, Pettorini L, Lai Q, Garofalo M, Melandro F, et al. Delayed introduction of everolimus in de novo renal transplanted patients: a single‐center experience. Transplantation Proceedings 2016;48(2):326‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rivelli 2014 {published data only}
- Rivelli RF, Goncalves RT, Leite M Jr, Santos MA, Delgado AG, Cardoso LR, et al. Early withdrawal of calcineurin inhibitor from a sirolimus‐based immunosuppression stabilises fibrosis and the TGF‐beta signalling pathway in kidney transplant. Nephrology 2014;20(3):168‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SOCRATES 2014 {published data only}
- Chadban SJ, Eris JM, Kanellis J, Pilmore H, Lee PC, Lim SK, et al. A randomized, controlled trial of everolimus‐based dual immunosuppression versus standard of care in de novo kidney transplant recipients. Transplant International 2014;27(3):302‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ G, Eris J, Kanellis J, Hutchison B, Hibberd A, Pilmore H, et al. Multicentre RCT of early switch to everolimus plus steroids or everolimus plus CSA versus CSA, MPA and steroids in de novo kidney transplant recipients: 12 month analysis [abstract no: 93]. Transplantation Society of Australia & New Zealand (TSANZ). 30th Annual Scientific Meeting; 2012 Jun 27‐29; Canberra (ACT). 2012:103. [CENTRAL: CN‐01912372]
Tamashiro 2017 {published data only}
- Tamashiro EY, Felipe CR, Genvigir FD, Rodrigues AC, Campos AB, Hirata RD, et al. Influence of CYP3A4 and CYP3A5 polymorphisms on tacrolimus and sirolimus exposure in stable kidney transplant recipients. Drug Metabolism & Personalized Therapy 2017;32(2):89‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Gelder 2003 {published data only}
- Gelder T, ter Meulen CG, Hene R, Weimar W, Hoitsma A. Oral ulcers in kidney transplant recipients treated with sirolimus and mycophenolate mofetil. Transplantation 2003;75(6):788‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wojciechowski 2017 {published data only}
- Webber A, Wojciechowski D, Leung C, Chandran S, Hirose R, Vincenti F. Pharmacodynamic monitoring of NFAT‐regulated gene expression and P70S6 kinase activity in kidney transplant patients with BKV infection [abstract]. Transplantation 2014;98(Suppl 1):880. [EMBASE: 71546539] [Google Scholar]
- Wojciechowski D, Chandran S, Webber A, Hirose R, Vincenti F. Mycophenolate mofetil withdrawal with conversion to everolimus to treat BK virus infection in kidney transplant recipients. Transplantation Proceedings 2017;49(8):1773‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wojciechowski D, Webber A, Chandran S, Hirose R, Vincenti F. Everolimus conversion to treat BK virus infection in renal transplant recipients: Interim analysis of a pilot study [abstract]. Transplantation 2014;98(Suppl 1):558‐9. [EMBASE: 71545412] [Google Scholar]
- Wojciechowski D, Webber A, Chandran S, Vincenti F. Everolimus conversion to treat BK virus infection in kidney transplant recipients [abstract]. American Journal of Transplantation 2015;15(Suppl 3). [EMBASE: 71953446] [DOI] [PubMed] [Google Scholar]
Wyrley‐Birch 2009 {published data only}
- Wyrley‐Birch H, Kanwar A, Dakshinamoorty V, Navarro A, Reddy M, Asher J, et al. A prospective randomised paired trial of sirolimus versus tacrolimus as primary immunosuppression following non heart beating donor kidney transplantation [abstract no: O41]. British Transplantation Society (BTS). 13th Annual Congress; 2010 Mar 17‐19; Kensington, UK. 2010.
- Wyrley‐Birch H, Kanwar A, Vijayanand D, Ray C, Moir J, Navarro A, et al. Early results from a trial comparing sirolimus with tacrolimus as primary immunosuppression for recipients of non heart beating donor (NHBD) kidneys after anti‐IL2 monoclonal antibody [abstract no: P‐580]. Transplant International 2009;22(Suppl 2):239. [Google Scholar]
References to studies awaiting assessment
Ferreira 2019 {published data only}
- Ferreira AN, Felipe CR, Cristelli M, Viana L, Mansur J, Paula M, et al. Prospective randomized study comparing everolimus and mycophenolate sodium in de novo kidney transplant recipients from expanded criteria deceased donor. Transplant International 2019;32(11):1127‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tedesco H, Nicolau FA, Cristelli M, Oliveira NI, Rosso FC, Pestana JO. Everolimus versus mycophenolate for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin and tacrolimus [abstract no: BO5]. Transplant International 2015;28(Suppl 4):135. [EMBASE: 72111605] [Google Scholar]
- Tedesco‐Silva H, Felipe C, Brigido A, Bessa A, Paula M, Ruppel P, et al. Everolimus (EVR) versus mycophenolate sodium (MPS) for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin (r‐ATG) and tacrolimus (TAC) [abstract no: 222]. American Journal of Transplantation 2016;16(Suppl 3):281. [EMBASE: 611699738] [Google Scholar]
- Tedesco‐Silva H, Felipe C, Ferreira A, Cristelli M, Bessa A, Ueno P, et al. Everolimus versus mycophenolate for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin and tacrolimus [abstract no: 294]. American Journal of Transplantation 2015;15(Suppl 3):N/A. [EMBASE: 71953354] [Google Scholar]
- Tedesco‐Silva H, Felipe CR, Cristelli MP, Viana LA, Ferreira AN, Bessa AB, et al. Everolimus (EVR) versus mycophenolate sodium (MPS) for recipients of kidney transplants from expanded criteria donors (ECD) receiving anti‐thymocyte globulin (r‐ATG) and tacrolimus (TAC) [abstract no: 620.5]. Transplantation 2016;100(7 Suppl 1):S398. [EMBASE: 613004941] [Google Scholar]
Traitanon 2019 {published data only}
- Traitanon O, Mathew JM, Shetty A, Bontha SV, Maluf DG, Kassis Y, et al. Mechanistic analyses in kidney transplant recipients prospectively randomized to two steroid free regimen‐Low dose Tacrolimus with Everolimus versus standard dose Tacrolimus with Mycophenolate Mofetil. PLoS ONE 2019;14(5):e0216300. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
EVER TWIST 2013 {published data only}
- Libetta C, Margiotta E, Borettaz I, Canevari M, Martinelli C, Lainu E, et al. Everolimus and low dose of tacrolimus combined with thymoglobulin induction induces regulatory T cells expansion in de novo kidney transplant recipients: Preliminary data of controlled randomized study (EVER TWIST) [abstract no: SP646]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i277. [EMBASE: 71075839] [Google Scholar]
NCT02077556 {published data only}
- Tsai MK. Effect of everolimus on the pharmacokinetics of tacrolimus in renal transplant patients. www.clinicaltrials.gov/ct2/show/NCT02077556 (first received 2 March 2014).
NCT03468478 {published data only}
- Tedesco Silva H Jr. Comparison of the efficacy and safety of sirolimus versus everolimus versus mycophenolate in kidney transplantation (SEM). www.clinicaltrials.gov/ct2/show/NCT03468478 (first received 9 March 2018).
Additional references
ANZDATA 2017
- ANZDATA Registry. 40th Report, Chapter 7: Transplantation. Australia and New Zealand Dialysis and Transplant Registry, Adelaide, Australia. 2018. www.anzdata.org.au/report/anzdata‐40th‐annual‐report‐2017/ (accessed 24 July 2019).
Dumont 2001
- Dumont FJ. Everolimus Novartis. Current Opinion in Investigational Drugs 2001;2(9):1220‐34. [MEDLINE: ] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gondos 2013
- Gondos A, Dohler B, Brenner H, Opelz G. Kidney graft survival in Europe and the United States: strikingly different long‐term outcomes. Transplantation 2013;95(2):267‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hernandez 2011
- Hernandez D, Martinez D, Gutierrez E, Lopes V, Gutierrez C, Garcia P, et al. Clinical evidence on the use of anti‐mTOR drugs in renal transplantation. Nefrologia 2011;31(1):27‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
KDIGO 2009
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guidelines for the care of kidney transplant recipients. American Journal of Transplantation 2009;9(Suppl 3):S1‐157. [DOI: 10.1111/j.1600-6143.2009.02834.x] [DOI] [PubMed] [Google Scholar]
Kumar 2017
- Kumar J, Bridson JM, Sharma A, Halawa A. Systematic review on role of mammalian target of rapamycin inhibitors as an alternative to calcineurin inhibitors in renal transplant: Challenges and Window to Excel. Experimental & Clinical Transplantation 2017;15(3):241‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mallet 2017
- Mallat SG, Tanios BY, Itani HS, Lotfi T, McMullan C, Gabardi S, et al. CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor‐based regimen versus a CNI‐based regimen: a systematic review and meta‐analysis of randomized, controlled trials. Clinical Journal of The American Society of Nephrology: CJASN 2017;12(8):1321–36. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS Blood and Transplant 2019
- NHS Blood and Transplant. Organ utilisation evidence. www.odt.nhs.uk/transplantation/organ‐utilisation‐evidence/ (accessed 24 July 2019).
Ponticelli 2004
- Ponticelli C. The pleiotropic effects of mTor inhibitors. Journal of Nephrology 2004;17(6):762‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Saunders 2001
- Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney International 2001;59(1):3‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schuurman 1997
- Schuurman HJ, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, et al. SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation 1997;64(1):32‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stallone 2005
- Stallone G, Schena A, Infante B, Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi's sarcoma in renal‐transplant recipients. New England Journal of Medicine 2005;352(13):1317‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stepkowski 1997
- Stepkowski SM, Tian L, Napoli KL, Ghobrial R, Wang ME, Chou TC, et al. Synergistic mechanisms by which sirolimus and cyclosporin inhibit rat heart and kidney allograft rejection. Clinical & Experimental Immunology 1997;108(1):63‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
USRDS 2018
- United Stated Renal Data System. 2018 Annual Data Report. www.usrds.org/adr.aspx (accessed 24 July 2019).
Viklicky 2000
- Viklicky O, Zou H, Muller V, Lacha J, Szabo A, Heemann U. SDZ‐RAD prevents manifestation of chronic rejection in rat renal allografts. Transplantation 2000;69(4):497‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ying 2018
- Ying T, Wong G, Lim W, Kanellis J, Pilmore H, Campbell S, et al. De novo or early conversion to everolimus and long‐term cancer outcomes in kidney transplant recipients: a trial‐based linkage study. American Journal of Transplantation 2018;18(12):2977–86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Webster 2003
- Webster AC, Higgins G, Chapman JR, Craig JC. Sirolimus and everolimus for kidney transplant recipients. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004290] [DOI] [PubMed] [Google Scholar]
Webster 2006a
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (TOR‐I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004290.pub2] [DOI] [PubMed] [Google Scholar]
Webster 2006b
- Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta‐analysis of randomized trials. Transplantation 2006;81(9):1234‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


