Abstract
Background
Neuromuscular diseases (NMDs) are a heterogeneous group of diseases affecting the anterior horn cell of spinal cord, neuromuscular junction, peripheral nerves and muscles. NMDs cause physical disability usually due to progressive loss of strength in limb muscles, and some NMDs also cause respiratory muscle weakness. Respiratory muscle training (RMT) might be expected to improve respiratory muscle weakness; however, the effects of RMT are still uncertain. This systematic review will synthesize the available trial evidence on the effectiveness and safety of RMT in people with NMD, to inform clinical practice.
Objectives
To assess the effects of respiratory muscle training (RMT) for neuromuscular disease (NMD) in adults and children, in comparison to sham training, no training, standard treatment, breathing exercises, or other intensities or types of RMT.
Search methods
On 19 November 2018, we searched the Cochrane Neuromuscular Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and Embase. On 23 December 2018, we searched the US National Institutes for Health Clinical Trials Registry (ClinicalTrials.gov), the World Health Organization International Clinical Trials Registry Platform, and reference lists of the included studies.
Selection criteria
We included randomized controlled trials (RCTs) and quasi‐RCTs, including cross‐over trials, of RMT in adults and children with a diagnosis of NMD of any degree of severity, who were living in the community, and who did not need mechanical ventilation. We compared trials of RMT (inspiratory muscle training (IMT) or expiratory muscle training (EMT), or both), with sham training, no training, standard treatment, different intensities of RMT, different types of RMT, or breathing exercises.
Data collection and analysis
We followed standard Cochrane methodological procedures.
Main results
We included 11 studies involving 250 randomized participants with NMDs: three trials (N = 88) in people with amyotrophic lateral sclerosis (ALS; motor neuron disease), six trials (N = 112) in Duchenne muscular dystrophy (DMD), one trial (N = 23) in people with Becker muscular dystrophy (BMD) or limb‐girdle muscular dystrophy, and one trial (N = 27) in people with myasthenia gravis.
Nine of the trials were at high risk of bias in at least one domain and many reported insufficient information for accurate assessment of the risk of bias. Populations, interventions, control interventions, and outcome measures were often different, which largely ruled out meta‐analysis. All included studies assessed lung capacity, our primary outcome, but four did not provide data for analysis (1 in people with ALS and three cross‐over studies in DMD). None provided long‐term data (over a year) and only one trial, in ALS, provided information on adverse events. Unscheduled hospitalisations for chest infection or acute exacerbation of chronic respiratory failure were not reported and physical function and quality of life were reported in one (ALS) trial.
Amyotrophic lateral sclerosis (ALS)
Three trials compared RMT versus sham training in ALS. Short‐term (8 weeks) effects of RMT on lung capacity in ALS showed no clear difference in the change of the per cent predicted forced vital capacity (FVC%) between EMT and sham EMT groups (mean difference (MD) 0.70, 95% confidence interval (CI) ‐8.48 to 9.88; N = 46; low‐certainty evidence). The mean difference (MD) in FVC% after four months' treatment was 10.86% in favour of IMT (95% CI ‐4.25 to 25.97; 1 trial, N = 24; low‐certainty evidence), which is larger than the minimal clinically important difference (MCID, as estimated in people with idiopathic pulmonary fibrosis). There was no clear difference between IMT and sham IMT groups, measured on the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALFRS; range of possible scores 0 = best to 40 = worst) (MD 0.85, 95% CI ‐2.16 to 3.85; 1 trial, N = 24; low‐certainty evidence) or quality of life, measured on the EuroQol‐5D (0 = worst to 100 = best) (MD 0.77, 95% CI ‐17.09 to 18.62; 1 trial, N = 24; low‐certainty evidence) over the medium term (4 months). One trial report stated that the IMT protocol had no adverse effect (very low‐certainty evidence).
Duchenne muscular dystrophy (DMD)
Two DMD trials compared RMT versus sham training in young males with DMD. In one study, the mean post‐intervention (6‐week) total lung capacity (TLC) favoured RMT (MD 0.45 L, 95% CI ‐0.24 to 1.14; 1 trial, N = 16; low‐certainty evidence). In the other trial there was no clear difference in post‐intervention (18 days) FVC between RMT and sham RMT (MD 0.16 L, 95% CI ‐0.31 to 0.63; 1 trial, N = 20; low‐certainty evidence). One RCT and three cross‐over trials compared a form of RMT with no training in males with DMD; the cross‐over trials did not provide suitable data. Post‐intervention (6‐month) values showed no clear difference between the RMT and no training groups in per cent predicted vital capacity (VC%) (MD 3.50, 95% CI ‐14.35 to 21.35; 1 trial, N = 30; low‐certainty evidence).
Becker or limb‐girdle muscular dystrophy
One RCT (N = 21) compared 12 weeks of IMT with breathing exercises in people with Becker or limb‐girdle muscular dystrophy. The evidence was of very low certainty and conclusions could not be drawn.
Myasthenia gravis
In myasthenia gravis, there may be no clear difference between RMT and breathing exercises on measures of lung capacity, in the short term (TLC MD ‐0.20 L, 95% CI ‐1.07 to 0.67; 1 trial, N = 27; low‐certainty evidence). Effects of RMT on quality of life are uncertain (1 trial; N = 27).
Some trials reported effects of RMT on inspiratory and/or expiratory muscle strength; this evidence was also of low or very low certainty.
Authors' conclusions
RMT may improve lung capacity and respiratory muscle strength in some NMDs. In ALS there may not be any clinically meaningful effect of RMT on physical functioning or quality of life and it is uncertain whether it causes adverse effects. Due to clinical heterogeneity between the trials and the small number of participants included in the analysis, together with the risk of bias, these results must be interpreted very cautiously.
Plain language summary
Respiratory muscle training in children and adults with neuromuscular disease
Review question
Does respiratory muscle training have beneficial effects for children and adults with neuromuscular disease?
Background
Neuromuscular disease is a very broad term that covers many diseases that either directly or indirectly affect muscles or nerves. Children and adults with neuromuscular diseases can present with muscle weakness, loss of movement control, and muscle wasting. Some neuromuscular diseases cause weakness of respiratory muscles (diaphragm and accessory muscles of respiration). The decline of respiratory muscle function in these diseases affects activities of daily living and quality of life. Respiratory muscle training could potentially be considered as an extra therapy for people with suspected or confirmed respiratory muscle weakness.
Study characteristics
This review included 11 studies with a total of 250 randomized participants with neuromuscular disease. Six studies included 112 young males (including children) with Duchenne muscular dystrophy, which is an inherited muscle disease. One trial involved 23 adults with other muscle diseases (Becker muscular dystrophy and limb‐girdle muscular dystrophy). Three trials involved 88 people with amyotrophic lateral sclerosis, a progressive condition that affects the nerves controlling movement. One trial involved 27 people with myasthenia gravis, a condition that affects the signals between nerves and muscles.
Key results
The studies showed that respiratory muscle training may result in some improvements in lung function for people with amyotrophic lateral sclerosis and Duchenne muscular dystrophy. However, this finding was not consistent between studies. Physical function and quality of life were only assessed in one amyotrophic lateral sclerosis trial, which indicated that RMT may have no clear effect. One trial reported on adverse events, but the certainty of evidence was too low for conclusions to be drawn. The studies did not report the number of unscheduled hospitalisations for sudden infection or worsening of chronic respiratory failure.
Certainty of the evidence
The certainty of the evidence examined as part of this review was low or very low. Low‐certainty evidence means that our confidence in the effect of respiratory muscle training is limited, and the true effect may be substantially different. When the evidence is of very low‐certainty, the true effect is likely to be substantially different. Given the low or very low‐certainty of the evidence presented in the studies, we believe that there is a need for more well‐conducted studies in order to assess the efficacy of respiratory muscle training in people with NMD.
The evidence is current to November 2018.
Summary of findings
Summary of findings for the main comparison. Respiratory muscle training versus sham training in ALS.
| Respiratory muscle training compared to sham training in ALS | ||||||
| Patient or population: people with ALS Intervention: respiratory muscle training Comparison: sham training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk or value with sham training | Risk or value with respiratory muscle training | |||||
|
Measures of lung capacity over the short term (less than 3 months) (change from baseline in % predicted FVC) Follow‐up: 8 weeks |
The mean change in % predicted FVC in the control group was ‐8.3% | The mean change in % predicted FVC in the training group was 0.70% less of a decline than in the sham training group (8.48 more of a decline to 9.88 less of a decline) | ‐ | 46 (1 RCT) |
⊕⊕⊝⊝ Lowa | |
| Measures of lung capacity over the medium term (greater than 3 months but less than 1 year) (change from baseline in % predicted FVC) Follow‐up: 4 months | The mean change in % predicted FVC in the control group was ‐5.20%b | The mean change in % predicted FVC in the training group was 10.86% less of a decline than in the sham training group (4.25 more of a decline to 25.97 less of a decline) | ‐ | 24 (1 RCT) | ⊕⊕⊝⊝ Lowa | RMT may improve lung capacity in comparison to sham training in the medium term The MCID for FVC, based on estimations in idiopathic pulmonary fibrosis is 2% to 6% (du Bois 2011). |
| Measures of lung capacity over the long term (greater than 1 year) | ‐ | Not measured | ||||
| Physical function in carrying out activities of daily living over the medium term (3 to 12 months; change from baseline) Assessed with: ALSFRS (maximum possible total score 40) Follow‐up: 4 months | The estimated mean change in the control group was ‐3.80b | The mean change in physical function assessed with ALSFRS was 0.85 points less of a decline than in the sham training group (2.16 more of a decline to 3.85 less of a decline) |
‐ | 24 (1 RCT) | ⊕⊕⊝⊝ Lowa | There may be no clinically meaningful difference in ALSFRS score between training and sham training groups in the medium term. Higher scores on the ALSFRS indicate better physical functioning. There is no established MCID for ALSFRS, but as the scale ranges from 0 to 40, we judged less than 1 point to be too small to make a difference. |
| Quality of life (change from baseline) in the medium term Assessed with: EuroQol‐5D, a 0 to 100 point visual analogue scale. The bottom rate (0) corresponds to " the worst health you can imagine", and the highest rate (100) corresponds to "the best health you can imagine" Follow‐up: 4 months | The mean change in the control group was not available | The mean change in quality of life score in the training group was MD 0.77 less of a decline (17.09 more of a decline to 18.62 less of a decline) | ‐ | 24 (1 RCT) | ⊕⊕⊝⊝ Lowa | There may be no clinically important difference in EuroQol score between the training and sham training groups in the medium term. |
| Number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure within 1 year of randomization | ‐ | Not measured | ||||
| All adverse events | One 4‐month trial of IMT in 24 people with ALS reported that no adverse event occurred. A second ALS trial, in 14 participants with ALS, did not provide information on adverse events. |
‐ | 38 (2 RCTs) | ⊕⊝⊝⊝ Very lowc | The certainty of the available evidence on RMT in ALS was too low for conclusions to be drawn about adverse events. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ALS: amyotrophic lateral sclerosis; ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale; CI: confidence interval; FEV₁: forced expiratory volume in one second; FVC: forced vital capacity; IMT: inspiratory muscle training; MCID: minimum clinically important difference; MD: mean difference; RCT: randomized controlled trial; RMT: respiratory muscle training | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe control group data were obtained from graphical representation of some outcomes, which included all participants who completed the study period (N = 9). Another study (N = 14) measured FVC and FEV₁ in the short term but was only published as an abstract and provided no data. bWe downgraded the evidence twice for serious imprecision due to small sample size and because the CIs included both an important effect and no effect. cWe downgraded the evidence three times: once because this outcome was at high risk of bias due to reporting bias, and twice for serious imprecision due to small sample and low event rate (no events).
Summary of findings 2. Respiratory muscle training versus sham training in DMD.
| Respiratory muscle training compared to sham training in DMD | ||||||
| Patient or population: children and young males with DMD Intervention: respiratory muscle training Comparison: sham training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk or value with sham traininga | Risk or value with respiratory muscle training | |||||
|
Measures of lung capacity over the short term (less than 3 months) Measured by: post‐intervention TLC Follow‐up: 6 weeks |
The mean post‐intervention TLC in the sham training group was 2.79 L | The mean post‐intervention TLC was 0.45 L higher than in the sham training group (0.24 lower to 1.14 higher) | ‐ | 16 (1 RCT) | ⊕⊕⊝⊝ Lowb | RMT may slightly increase TLC in comparison to sham training. For this study, a difference of 0.45 L represents approximately a 16% difference from the control group. A second trial did not report TLC but found no clear difference in FVC between RMT and sham training groups after 18 days (MD 0.16 L, 95% CI ‐0.31 to 0.63; N = 20). |
| Measures of lung capacity over the medium term (greater than 3 months but less than 1 year) | ‐ | Not measured | ||||
| Measures of lung capacity over the long term (greater than 1 year) | ‐ | Not measured | ||||
| Physical function in carrying out activities of daily living | ‐ | Not measured | ||||
| Quality of life | ‐ | Not measured | ||||
| Number of unscheduled hospitalizations for episodes of chest infection or acute exacerbation of chronic respiratory failure within 1 year of randomization | ‐ | Not measured | ||||
| All adverse events | ‐ | Two trials with 16 and 20 participants with DMD did not provide information on adverse events | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMD: Duchenne muscular dystrophy; MD: mean difference; RCT: randomized controlled trial; TLC: total lung capacity | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe control group data were obtained from graphical representation of some outcomes, which included all participants who completed the study period (N = 9). bWe downgraded the evidence twice for serious imprecision due to a small sample size and because CIs included both an important effect and no effect.
Summary of findings 3. Respiratory muscle training versus no training in DMD.
| Respiratory muscle training compared to no training in DMD | ||||||
| Patient or population: children and young males with DMD Intervention: respiratory muscle training Comparison: no training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk or value with no training | Risk or value with respiratory muscle training | |||||
| Measures of lung capacity over the short term (less than 3 months) | ‐ | Three cross‐over trials measured short‐term outcomes but did not provide data for each study period. | ||||
|
Measures of lung capacity over the medium term (greater than 3 months but less than 1 year) Measured by: post‐intervention % predicted VC Follow‐up: 6 months |
The mean post‐intervention % predicted VC was 44.4% | The mean post‐intervention % predicted VC was 3.50% higher than in the no training group (14.35% lower to 21.35% higher) | ‐ | 30 (1 RCT) | ⊕⊕⊝⊝ Lowa | There may be no clear difference in % predicted VC with RMT in comparison to no training. For more lung capacity outcomes, see text. |
| Measures of lung capacity over the long term (greater than 1 year) | ‐ | Not measured | ||||
| Physical function in carrying out activities of daily living | ‐ | Not measured | ||||
| Quality of life | ‐ | Not measured | ||||
| Number of unscheduled hospitalizations for episodes of chest infection or acute exacerbation of chronic respiratory failure within 1 year of randomization | ‐ | Not measured | ||||
| All adverse events | ‐ | Not measured | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMD: Duchenne muscular dystrophy; MD: mean difference; RCT: randomized controlled trial; VC: vital capacity | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the evidence twice for serious imprecision due to small sample size and the CIs included both an important effect and no effect.
Summary of findings 4. Respiratory muscle training versus breathing exercises in limb‐girdle muscular dystrophy or Becker muscular dystrophy.
| Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle) | ||||||
|
Patient or population: participants with limb‐girdle muscular dystrophy or Becker muscular dystrophy Intervention: respiratory muscle training Comparison: breathing exercises | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk or value with breathing exercises | RMT | |||||
|
Measures of lung capacity over the short term (less than 3 months) Measured as change from baseline in FVC (L) follow‐up: 12 weeks |
The mean change in FVC in the breathing exercises group was 0 | FVC decreased on average 0.01 L more (0.11 less to 0.13 more) in the RMT group than the breathing exercises group. (The mean change in FVC in the RMT group was a decrease of 0.01 L) | ‐ | 21 (1 RCT) | ⊕⊝⊝⊝ Very lowa | The effect of RMT on FVC, when compared to breathing exercises, is uncertain |
| Measures of lung capacity over the medium term (greater than 3 months but less than 1 year) | ‐ | Not measured | ||||
| Measures of lung capacity over the long term (greater than 1 year) | ‐ | Not measured | ||||
| Physical function in carrying out activities of daily living | ‐ | Not measured | ||||
| Quality of life | ‐ | Not measured | ||||
| Number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure within 1 year of randomization | ‐ | Not measured | ||||
| All adverse events | ‐ | Not measured | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FVC: forced vital capacity; RCT: randomized controlled trial; RMT: respiratory muscle training | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the certainty of evidence for this outcome to very low, downgrading two levels for very serious imprecision as the sample size was very small and because the CI included both an important effect and no effect, and once for study limitations as the trial was quasi‐randomized (alternate allocation).
Summary of findings 5. Respiratory muscle training versus breathing exercises in myasthenia gravis.
| Respiratory muscle training compared to breathing exercises in myasthenia gravis | ||||||
| Patient or population: people with myasthenia gravis Intervention: respiratory muscle training Comparison: breathing exercises | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk or value with breathing exercises | Risk or value with respiratory muscle training | |||||
|
Measures of lung capacity over the short term (less than 3 months) Measured by: post‐intervention TLC Follow‐up: 8 weeks |
The mean post‐intervention TLC was 4.7 L | The mean post‐intervention TLC was 0.20 lower than in the breathing exercises group (‐1.07 lower to 0.67 higher) | ‐ | 27 (1 RCT) | ⊕⊕⊝⊝ Lowa | RMT, compared to breathing exercises, may have no clear effect on lung capacity. For other lung capacity outcomes, see text. |
| Measures of lung capacity over the medium term (greater than 3 months but less than 1 year) | ‐ | Not measured | ||||
| Measures of lung capacity over the long term (greater than 1 year) | ‐ | Not measured | ||||
| Physical function in carrying out activities of daily living | ‐ | Not measured | ||||
| Quality of life | The trial authors reported narratively that a change in one of the nine SF‐36 domains (physical role functioning) showed a improvement in the training group compared to the breathing exercises group | ‐ | 27 (1 RCT) |
⊕⊝⊝⊝ Very lowb | It is uncertain whether RMT has an effect on quality of life in comparison to breathing exercises | |
| Number of unscheduled hospitalizations for episodes of chest infection or acute exacerbation of chronic respiratory failure within 1 year of randomization | ‐ | Not measured | ||||
| All adverse events | ‐ | Not measured | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RMT: respiratory muscle training; SF‐36: 36‐Item Short Form Health Survey; TLC: total lung capacity | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded the evidence three times: once because the trials providing data for this outcome were at high risk of bias, and twice for serious imprecision due to small sample size and the CIs included both an important effect and no effect. bWe downgraded the evidence three times: twice because this outcome was at high risk of bias due to reporting bias, and once for serious imprecision due to the small sample.
Background
Description of the condition
Neuromuscular diseases (NMDs) are a heterogeneous group of conditions that impair muscle function through pathologies of the anterior horn cell of spinal cord, neuromuscular junction, peripheral nerves and muscles (Anziska 2013). The clinical characteristics of NMDs are based on where the lesion occurs and these lesions can be found anywhere between the anterior horn cells of the spinal cord and the skeletal muscle (Rezania 2012). People with NMDs may present with muscle weakness, loss of spontaneous movement, involuntary muscle activity, and muscle atrophy (Wijdicks 2009).
Generally, children are affected by hereditary NMDs (Estournet‐Mathiaud 2003; MacDonald 2002; Reed 2002), while acquired NMDs are more common in adults (Reed 2002). A conservative estimate of overall prevalence among both sexes for the most common forms of muscular dystrophy, myotonic dystrophy and congenital myotonias, proximal spinal muscular atrophies, and hereditary motor and sensory neuropathies is 1 in 3500 of the general population (Emery 1991). If numbers include severe disorders that manifest only in infancy and early childhood, and the rare forms of dystrophy and myopathy, the overall prevalence could well exceed 1 in 3000 (Emery 1991).
NMDs cause physical disability, usually through progressive skeletal muscle weakness, and in some conditions this includes respiratory muscle (diaphragm and accessory muscles of respiration) weakness (Finder 2004; McDonald 2012; Pustavoitau 2008). Dysfunction at any level of the respiratory pathway, from the central nervous system, peripheral nerves, or neuromuscular junction, to the muscles themselves can cause respiratory failure, a condition in which the respiratory system fails in one or both of its gas exchange functions: oxygenation and carbon dioxide elimination (McCool 1995). NMDs that cause respiratory muscle weakness include muscular dystrophies, such as Becker muscular dystrophy (BMD), Duchenne muscular dystrophy (DMD), limb‐girdle, Emery‐Dreifuss and facioscapulohumeral muscular dystrophy, myotonic dystrophy, metabolic and congenital myopathies, inflammatory myopathies, myasthenia gravis, neuropathies (hereditary and acquired), amyotrophic lateral sclerosis (ALS), poliomyelitis, and spinal muscular atrophy (Paschoal 2007).
NMDs have variable effects on respiratory muscles with regard to the site of the pathology and the severity, yet the major complication is respiratory failure (Wirth 1999). Respiratory impairment includes ventilatory difficulty, decreased vital capacity and reduced chest wall expansion due to inspiratory muscle weakness. Signs of respiratory failure may include dyspnoea (shortness of breath) from slight effort, dyspnoea and tachypnoea (abnormally fast breathing) at rest, use of respiratory accessory muscles (indicating effortful breathing), paradoxical respiration (abnormal movement of the diaphragm), orthopnoea (shortness of breath lying down), poor sleep, morning headache, daytime fatigue or daytime sleepiness, and an ineffective cough (Pinto 2014).
Difficulty coughing due to weakness of expiratory, inspiratory and upper airway muscles can cause atelectasis (closure or collapse of lung tissue) and infections. Upper airway weakness can raise the risk of fluid aspiration (Benditt 2006; McCool 1995). Both inspiratory and expiratory muscles are needed to produce a cough strong enough to maintain upper airway patency (Park 2010). In people with NMD, inspiratory and expiratory muscle weakness is thus related to inadequate alveolar ventilation and poor airway clearance, which increase the risk of atelectasis, pneumonia, and chronic respiratory insufficiency (Ambrosino 2009; D'Angelo 2011; Misuri 2000).
The deterioration of respiratory muscle function in these diseases, in addition to effects on lung function, reduces functional capacity, limits activities of daily living, and limits quality of life (Yeldan 2008). Furthermore, it precipitates the onset of respiratory failure (Fitting 2006; Ramirez‐Sarmiento 2008), and contributes significantly to morbidity and mortality (Cup 2007; Hapke 1972; Pontes 2012).
Respiratory muscle training (RMT) could be considered a possible adjunctive therapy for people with suspected or confirmed respiratory muscle weakness (Nici 2006).
Description of the intervention
RMT is a technique that aims to increase the strength or endurance of respiratory muscles (Enright 2011;Moodie 2011). RMT can be classified into inspiratory muscle training (IMT) and expiratory muscle training (EMT).
Two different forms of RMT have predominantly been employed: respiratory muscle endurance training (RMET) and respiratory muscle strength training (RMST). RMET involves low pressure and high flow loads of both inspiratory and expiratory muscles (Hill 2004). This training is undertaken by means of normocapnic hyperpnoea, which requires maintenance of high levels of ventilation for an extended period of time (Pine 2005). In contrast, RMST involves high pressure and low flow loading of specific inspiratory or expiratory muscles (Hill 2004). According to Illi 2012, "RMST is performed by breathing against an external inspiratory or expiratory load. This load consists either of a flow‐dependent resistance or a pressure threshold that needs to be overcome and sustained to generate flow".
The type of RMT used has been targeted to the type of muscle weakness present, thus, IMT is used for inspiratory muscle weakness and EMT for expiratory weakness (Aslan 2014). RMT can be performed from the early stages of the disease (Pinto 2012), and can be undertaken with children (Topin 2002). Training sessions can be held in rehabilitation centers or at home (Aslan 2014; Cheah 2009; Fregonezi 2005; Pinto 2012).
The results of RMT have been mixed, with some studies showing improvement in respiratory muscle performance, while others report minimal or insignificant changes (Aboussouan 2009; Finder 2004; Fregonezi 2005). In children with DMD, the protective mechanism of nitric oxide during exercise is defective. The data indicate that sympathetic vasoconstriction and defective modulation in the exercising muscle can produce functional muscle ischemia (Sander 2000). Thus, for children with DMD, the implementation of respiratory training protocols could possibly result in an increase in muscle damage (Finder 2004), because progressive muscle fibrosis may be accelerated when muscles deficient in dystrophin and neuronal nitric oxide synthase undergo repeated bouts of ischemic exercise (Sander 2000).
How the intervention might work
The respiratory muscles are morphologically and functionally skeletal muscles, and respond to training in the same way as any muscle of the locomotor system (Romer 2003). Thus, RMT follows the same principles as those employed in training skeletal muscles: specificity, intensity, frequency, duration, and reversibility (Leith 1976). Specificity refers to adapting the training to be specific to the system or to the muscles being trained (Hoffman 2002). Training conducted at high load and with a low speed of muscle contraction promotes an increase in inspiratory muscle strength, while training employing high speed and low load has been shown to increase endurance (Romer 2003; Tzelepis 1994; Tzelepis 1999). The principle of intensity indicates that the exercise load must be greater than the muscular capacity to overcome it and it therefore must be adjusted during the training protocol (Pinto 2014). Training loads above 22% of maximal inspiratory pressure (MIP) are able to improve the endurance of inspiratory muscles, while loads of at least 30% of MIP are necessary to increase the strength of these muscles (Hill 2004). The duration and frequency of training sessions determines the magnitude of muscle response and the time needed for benefits to accrue (Pinto 2014). Reversibility means that fitness levels will eventually return to baseline when a training stimulus is removed (Hoffman 2002).
The strength that skeletal muscle can generate depends on the effective cross‐sectional area and the geometry of the way in which the tension force is applied (Sartori 2008). The imposition of loads by RMT promotes greater muscle strength through neural adaptations (recruitment of additional motor units and an increase in frequency of muscle fibre contraction), adaptations of the muscle itself (hypertrophy), or both (Epstein 1994; Huang 2011). The response of muscle to training is specific: strength training will enhance the number and volume of muscle fibres (hypertrophy), while endurance training will increase the number of oxidative fibres and capillary density (Pinto 2014).
In people with neurological and neurodegenerative diseases (e.g. multiple sclerosis, Parkinson's disease, spinal cord injury, and stroke), meta‐analysis shows that RMT increases inspiratory and expiratory muscle strength (Berlowitz 2013; Pollock 2013; Reyes 2013; Rietberg 2017; Van Houtte 2006; Xiao 2012), as well as improving vital capacity and residual volume (Berlowitz 2013; Van Houtte 2006). RMT has also been shown to promote greater exercise tolerance in healthy people and athletes (HajGhanbari 2013; Illi 2012; McConnell 2009).
Why it is important to do this review
The effects of RMT in people with NMD are uncertain. Some studies claim that after RMT, people with NMD have increased respiratory muscle strength, improved lung function, and reduced muscle fatigue (Fregonezi 2005; Yeldan 2008), and that RMT promotes a transient improvement in maximal voluntary ventilation, peak expiratory flow, and sniff inspiratory pressure (Pinto 2012). Some have claimed that participation in RMT is a significant independent predictor of survival in people in the early stages of ALS (Pinto 2012). Other studies, however, have discouraged the use of RMT because of the possibility of exceeding the force threshold and thereby damaging muscle fibres (Aboussouan 2009; de Godoy 2012; Eagle 2002).
To our knowledge, the published systematic reviews of RMT in NMDs included a mix of types of studies (i.e. randomized and non‐randomized studies; Eidenberger 2014); a mix of neurodegenerative diseases (for example, multiple sclerosis and ALS; Ferreira 2016), or did not include adults with NMDs (Human 2017). Thus, a review is necessary to synthesize the best available evidence on the effectiveness and safety of RMT in people with NMD, to inform clinical practice.
Objectives
To assess the effects of respiratory muscle training (RMT) for neuromuscular disease (NMD) in adults and children, in comparison to sham training, no training, standard treatment, breathing exercises, or other intensities or types of RMT.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and quasi‐RCTs (including cross‐over trials) and included studies reported as full text, those published as abstract only, and unpublished data. No restrictions were applied on language. Quasi‐RCTs are studies in which participants are allocated to groups by a method that is not completely random, for example, by odd or even medical record number, or by alternation.
Types of participants
The participants in the studies included in this review were adults (age ≥18 years) and children (age < 18 years) of both sexes with a diagnosis of neuromuscular disease (NMD) of any degree of severity, confirmed by an appropriate consensus definition or using diagnostic criteria defined by the trial authors. Participants were living in the community without the need for mechanical ventilation (invasive or non‐invasive), since positive pressure ventilation would be a confounding factor for some outcomes (i.e. lung capacity, physical functioning and quality of life) (Hannan 2014; Radunovic 2017). Trials including participants with and without ventilatory support were excluded if we were not able to obtain data separately. We considered for inclusion participants with myopathies, disorders of the neuromuscular junction and neuropathies and excluded people with acute respiratory failure and cognitive impairment. We also excluded studies that assessed more than one type of NMD (for example, myopathies and neuropathies) if we were not able to obtain results for each condition separately, because the effects of respiratory muscle training (RMT) could be different for each type of disease.
Types of interventions
We considered trials for inclusion in which the intervention was RMT (inspiratory muscle training (IMT) or expiratory muscle training (EMT), or both) involving normocapnic hyperpnoea, resistive training, and pressure threshold loading, and where there was comparison with a control group using a sham, no training, standard treatment, different intensities of RMT (e.g. low versus high intensity), or different types of RMT (e.g. IMT versus IMT plus EMT), or breathing exercises (singing, deep breathing, diaphragmatic breathing, etc.).
We considered all intervention protocols, regardless of the duration of training.
Types of outcome measures
The outcomes listed below are not selection criteria for this review, but they are outcomes of interest within the included studies.
Primary outcomes
Measures of lung capacity (e.g. total lung capacity (TLC), forced vital capacity (FVC)) over the short term (less than 3 months), medium term (greater than 3 months but less than 1 year), and long term (greater than 1 year).
Secondary outcomes
Inspiratory muscle strength over the short term (less than 3 months), medium term (greater than 3 months but less than 1 year), and long term (greater than 1 year), measured by maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP).
Expiratory muscle strength over the short term (less than 3 months), medium term (greater than 3 months but less than 1 year), and long term (greater than 1 year), measured by maximal expiratory pressure (MEP).
Physical function in carrying out activities of daily living over the short term (less than 3 months), medium term (greater than 3 months but less than 1 year), and long term (greater than 1 year), measured by a validated instrument (e.g. Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised (ALSFRS‐R; Cedarbaum 1999) and ACTIVLIM questionnaire; Vandervelde 2009).
Quality of life over the short term (less than 3 months), medium term (greater than 3 months but less than 1 year), and long term (greater than 1 year), as measured by a validated questionnaire (e.g. 36‐Item Short Form Health Survey (SF‐36); Ware 1992).
Number of unscheduled hospitalisations for episodes of chest infections or acute exacerbation of chronic respiratory failure within the first year post‐randomization.
Adverse events: including all adverse events (e.g. respiratory muscle fatigue during or after the training), measured by clinical criteria (e.g. increased respiratory rate, use of accessory respiratory muscles, and decrease in oxygen saturation); adverse events that require discontinuation of treatment; and serious adverse events, namely those that are life threatening, require or prolong a hospital stay, or are fatal.
We specified that we would report the continuous outcomes as the change from baseline, and did so when these data were available. We otherwise reported final measurements.
Search methods for identification of studies
Electronic searches
We searched the following databases on 19 November 2018.
The Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; Appendix 1).
The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web; Appendix 2).
MEDLINE (1946 to 18 November 2018; Appendix 3).
Embase (1974 to 18 November 2018; Appendix 4).
On 13 December 2018, we also searched the following clinical trials registries.
US National Institutes for Health Clinical Trials Registry, ClinicalTrials.gov (www.clinicaltrials.gov/; Appendix 5).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/; Appendix 5).
We searched all databases from their inception to the present, and we imposed no restriction on language of publication or publication status.
Searching other resources
We searched reference lists of all relevant studies and review articles for additional references. We searched relevant device manufacturers' websites for trial information.
Data collection and analysis
Selection of studies
Two review authors (RP, IGA) independently screened titles and abstracts of all the potential studies identified for inclusion in the review. We coded studies as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text reports and two review authors (RP, IGA) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author (GMHF). We identified and excluded duplicate papers. We also clustered multiple reports relating to the same study and considered them as only one included study. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and provide a 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data extraction form for study characteristics and outcome data that we piloted on at least one study in the review. Two review authors (ISS and IGA) extracted the following study characteristics from the included studies.
Methods: study design, duration of study, details of any 'run‐in' period, number of study centers and locations, study settings, withdrawals, and date of study.
Participants: number (total and in each intervention group), mean age, age range, gender, severity of condition, diagnostic criteria, baseline characteristics, inclusion criteria, and exclusion criteria.
Interventions: intervention and comparison.
Outcomes: primary and secondary outcomes specified and collected, and time points reported. When the change from baseline was not reported, we extracted the final values.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (ISS, IGA) independently extracted outcome data from the included studies. We noted if outcome data were not reported in a usable way in the 'Characteristics of included studies' table. We resolved disagreements by consensus or by involving a third review author (GMHF). One review author (ISS) transferred data into Review Manager 5 (Review Manager 2014). A second review author checked the outcome data entries. Another review author (GMHF) spot‐checked study characteristics for accuracy against the trial report.
We would have used scaling to combine results from studies using different periods. In the analysis, this would have required values from studies using periods not equal to one month to be divided by the period expressed in months. For example, for studies using a three‐week interval between measurement points, we would have divided the totals by 0.75; as no meta‐analysis was possible, this was not done.
If reports had required translation, the translator would have extracted data directly using a data extraction form, or authors would have extracted data from the translation provided. When possible, a review author would have checked numerical data in the translation against the original study report.
Assessment of risk of bias in included studies
Two review authors (ISS, RP) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (GMHF). We assessed and classified the risk of bias according to each of the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and have provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We have summarized the 'Risk of bias' judgements across different studies for each of the domains listed. When information on risk of bias related to unpublished data or correspondence with a trialist, we also noted in the 'Risk of bias' table. 'Other bias' was a category of exclusion, for bias that did not fall into other domains. Where none was apparent we assessed the risk low unless information was very limited (e.g. an abstract), when we preferred unclear.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome. In addition, we planned to perform a sensitivity analysis in order to exclude studies at high risk of bias for allocation concealment.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Pedrosa 2015), and reported any deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We analysed continuous data as mean difference (MD), and would have reported a standardized mean difference (SMD) for results across studies with outcomes that were conceptually the same but measured in different ways, and dichotomous data as risk ratios (RRs).
When means and SD for the analysis of changes from baseline were not available or calculable, we reported MDs between groups at the given time points.
If the trials had not reported the mean and standard deviation (SD) for each group, we would have used generic inverse variance (GIV) to enter data in the analysis. We provided corresponding 95% confidence intervals (CIs) for measures of effect. We entered the data presented as a scale with a consistent direction of effect.
We undertook a meta‐analysis only when this was meaningful (i.e. if the treatments, participants, and the underlying clinical questions were similar enough for pooling to be logical).
Unit of analysis issues
We included cross‐over trials and reported data from the first treatment arm only. When the trials did not provide first period data, we contacted authors to request them.
If a single trial had reported multiple trial arms, we planned to include only relevant arms, that is, those in which participants had received our prespecified interventions and comparators. If two comparisons (e.g. IMT versus placebo and EMT versus placebo) were combined in the same meta‐analysis, we would have halved the control group to avoid double‐counting.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data (e.g. when a study was available as an abstract only). If we had assessed missing data as introducing serious bias, we would have explored the impact of including such studies in the overall assessment of results by employing a sensitivity analysis; however this would not have been possible as no more than two studies were included in any meta‐analysis.
Assessment of heterogeneity
We would have used the I² statistic to measure heterogeneity among the trials. If we had identified substantial unexplained heterogeneity (I² > 50%), we would have reported it and explored possible causes of clinical or methodological heterogeneity by undertaking prespecified subgroup analyses (Deeks 2011).
Assessment of reporting biases
If we had been able to pool more than 10 trials, we would have created and examined a funnel plot in order to explore possible small‐study biases.
Data synthesis
We would have used a fixed‐effect model to determine the effects of an intervention and performed a sensitivity analysis using a random‐effects model if there had been unexplained heterogeneity (Higgins 2011).
As the review included more than one comparison that could not be considered in the same analysis, we reported the results for each comparison separately. Moreover, we decided against combining various types of NMDs. Thus, we entered data from studies with different types of NMDs into a forest plot for visual interpretation of the results but did not pool the data (i.e. the meta‐analysis diamond was turned off).
Where meta‐analysis was not possible we reported results narratively.
'Summary of findings' table
We created a 'Summary of findings' table for each main comparison using the following outcomes.
Measures of lung capacity over the short term (less than 3 months), medium term (3 to 12 months), and long term (greater than 1 year). The order of choice for the presentation of the measures was as follows: total lung capacity (TLC), forced vital capacity (FVC), functional residual capacity (FRC), residual volume (RV), vital capacity (VC), and forced expiratory volume in one second (FEV₁).
Physical function in carrying out the activities of daily living in the medium term (3 to 12 months).
Quality of life in the medium term (3 to 12 months).
Number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure within the first year post‐randomization.
All adverse events.
We specified, when formulating outcomes, that we would report continuous outcomes as the change from baseline. When insufficient data were available to present the change from baseline, we reported the final values.
We used five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence (studies that contribute data for prespecified outcomes). We employed methods and followed recommendations described in Chapter 11 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the certainty of evidence using footnotes and we made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Duration of intervention (less than 6 weeks and 6 weeks or more).
Participant age (under 18 years of age and 18 years or above).
We were intending to use the following outcomes in subgroup analyses.
Frequency of unscheduled hospitalisation for episodes of acute exacerbation of chronic respiratory failure.
Physical function in carrying out activities of daily living.
We planned to use the formal test for subgroup interactions in Review Manager 5 (Review Manager 2014).
As the review included only one trial that assessed physical function, it was not possible to perform subgroup analyses.
Sensitivity analysis
We planned to perform the following sensitivity analyses.
Repeat the analysis, excluding unpublished studies (if there were any).
Repeat the analysis, excluding those studies at high risk of bias for allocation concealment.
If there were one or more very large studies (100 or more participants per group), repeat the analysis, excluding these particular studies to determine their effect on the overall results.
Repeat the analysis, excluding quasi‐RCTs.
Most of the analyses were based on data from a single study, therefore we did not perform sensitivity analyses.
Reaching conclusions
We based our conclusions only on the findings from the synthesis of the quantitative and narrative data from the studies included in this review.
Results
Description of studies
Results of the search
We identified 504 references for possible inclusion in the review by the searches outlined in the appendices, of which 375 remained after deduplication. We identified 10 additional references by searching other resources (i.e. bibliographies of all relevant studies and international trials registers). After deduplication, there were 385 references. From these 385 references, two review authors selected 38 abstracts as potentially appropriate for inclusion in the review. After reading the full texts of these articles, we excluded 19 as not being relevant. Thus, 11 studies (reported in 19 references) fulfilled the inclusion criteria and are included in this review.
One trial was ongoing (NCT02710110).
We present a PRISMA diagram in Figure 1.
1.
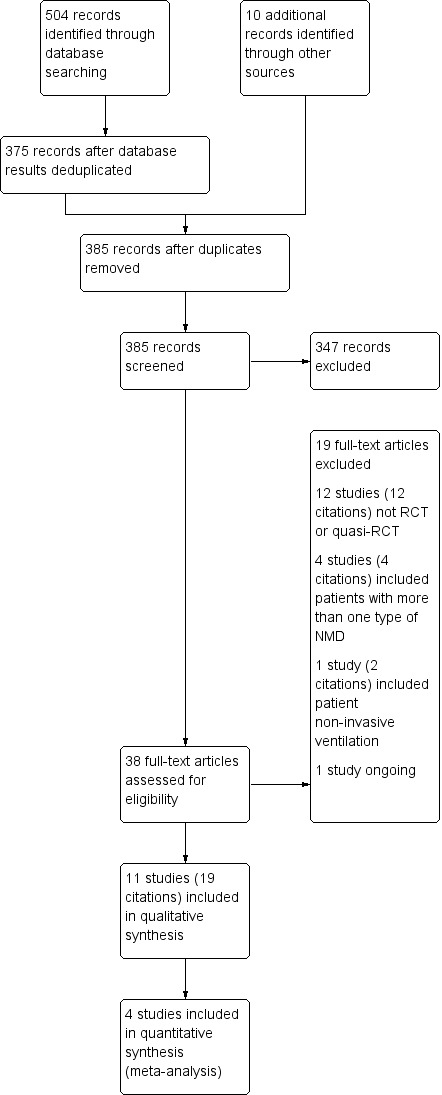
Study flow diagram.
Included studies
Of the 11 included studies, seven were randomized controlled trials (RCTs) and four were cross‐over trials (Martin 1986; Rodillo 1989; Smith 1988; Stern 1989). They were published between 1986 and 2019. The nine fully published studies were conducted in Spain (Fregonezi 2005), France (Topin 2002), Austria (Wanke 1994), Portugal (Pinto 2012), UK (Rodillo 1989), Australia (Martin 1986; Stern 1989), USA (Plowman 2019), and Turkey (Yeldan 2008). One paper was published only as abstracts and was conducted in the UK (Suleman 2003). The Smith 1988 trial was conducted in the UK and published as a letter to the editor.
All papers had been published in English language journals. We wrote to all trial authors for further information. We have provided complete details of the 11 included studies in the Characteristics of included studies table. For the cross‐over trials, we reported data from the first treatment arm only.
Participants
Eleven studies involving 250 randomized people with neuromuscular disease (NMD) met the inclusion criteria. The trialists excluded 13 participants from data analysis, so 237 participants provided data. The sample size of the included studies varied from 8 to 48 participants.
Three trials involved people with amyotrophic lateral sclerosis/motor neuron disease (ALS/MND; Pinto 2012; Plowman 2019; Suleman 2003). In Pinto 2012, the mean age in the training group was 57.14 ± 9.3 years and ranged from 41.5 to 72.5 years; in the control group the mean was 56.8 ± 8.7 years (38.3 to 73.4). The training group in Plowman 2019 had a mean age of 63.1 ± 10.0 years, and the control group had a mean age of 60.1 ± 10.3 years. Suleman 2003 did not provide information about the age of participants.
Seven trials included young males (including children) with myopathies: Duchenne muscular dystrophy (DMD), limb‐girdle muscular dystrophy, and Becker muscular dystrophy (BMD) (Martin 1986; Rodillo 1989; Smith 1988; Stern 1989; Topin 2002; Wanke 1994; Yeldan 2008). In Wanke 1994, all participants had the onset of DMD between three and five years of age and were free from respiratory tract infections. None of them had: symptoms or signs of inspiratory muscle fatigue (i.e. exertional dyspnoea, orthopnoea, or paradoxic breathing), sleep disturbance, daytime hypersomnolence, morning headache, or episodes of acute respiratory failure requiring endotracheal ventilation. The age in the training group ranged from 10 to 24 years (mean 13.6 ± 4.5 years), and in the control group from 9 to 20 years (mean 14.5 ± 3.8 years). In a second DMD trial, all participants were clinically stable at the time of evaluation, free of any medication, free from respiratory tract infection, and had no history of acute respiratory failure requiring endotracheal ventilation, neither symptoms or signs of inspiratory muscle fatigue (Topin 2002). The mean age was 14.7 ± 4.5 years in the training group and 12.63 ± 1.8 years in the control group. In Martin 1986, all participants attended a center for physically handicapped children and the mean age was 14.2 years (range 7 to 20). The age of participants in Rodillo 1989 was between 9 and 14 years (mean 11.6 yrs) and they were recruited from two special schools. Smith 1988 included eight participants with mean age 12.3 years (range 8 to 16). In Stern 1989, ages ranged from 10.4 to 23.4 years (mean 15 years).
Yeldan 2008 included outpatient participants with limb‐girdle muscular dystrophy and BMD that had no visible spinal deformities; had no symptoms or signs of cardiomyopathy, heart failure symptoms or physical findings; had no symptoms or signs of inspiratory muscle fatigue, shortness of breath, orthopnoea or dyspnoea during bathing or swimming, short sentences during speech, tachypnoea, paradoxical movement of abdominal or thoracic wall, problems with cough; and free from respiratory tract infections. The mean age of participants was 22.50 ± 7.50 years and 24.27 ± 9.40 years in the training and control groups, respectively.
One trial involved participants with a disorder of the neuromuscular junction (myasthenia gravis), the age of participants ranged 33 to 75 years (mean age 64 ± 10 years) (Fregonezi 2005).
Diagnostic criteria and disease classification
Six of the included studies reported the diagnostic criteria used. Seven trials did not mention the disease classification (Martin 1986; Rodillo 1989; Smith 1988; Stern 1989; Suleman 2003; Topin 2002; Yeldan 2008).
Pinto 2012 included participants with definite or probable ALS, using the revised El Escorial criteria (Brooks 2000). Plowman 2019 included participants with possible, probable or definite ALS, according to the revised El Escorial criteria. Pinto 2012 included participants with Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) scores greater than 24/40 (Cedarbaum 1997). In Plowman 2019, participants had mild to moderately severe symptoms of ALS.
DMD diagnosis had been confirmed from clinical, enzymatic and muscle biopsy criteria in two DMD studies (Topin 2002; Wanke 1994). Martin 1986 confirmed the diagnosis of DMD from the typical clinical presentation and features, raised creatine phosphokinase, electromyograms and muscle biopsy. In Stern 1989, the diagnosis of DMD was based on clinical findings and muscle biopsy. In Topin 2002, all participants were wheelchair dependent. In Martin 1986, 17 boys were in wheelchairs and one was still ambulant, and in Stern 1989, 16 were in wheelchairs and two were ambulant. In Yeldan 2008, the neurologist who referred the patients made the diagnosis of muscular dystrophy (limb‐girdle or BMD) using diagnostic criteria defined by Emery 1994. In Wanke 1994, 11 of the 30 participants were wheelchair dependent, corresponding to a stage 9 functional capacity, using the criteria of Inkley 1974.
Fregonezi 2005 categorized participants as subclass IIa and IIb according to the myasthenia gravis classification of Osserman and Genkins (Osserman 1971).
Interventions and comparisons
Eight studies assessed inspiratory muscle training (IMT); the control groups were sham IMT (Pinto 2012; Rodillo 1989; Topin 2002), no training (Smith 1988; Stern 1989; Wanke 1994), or breathing exercises (Fregonezi 2005; Yeldan 2008). In four studies that performed threshold IMT, the training load ranged from 15% to 60% of maximal inspiratory pressure (MIP) (Fregonezi 2005; Pinto 2012; Topin 2002; Wanke 1994). The training in one of these trials consisted of 10 minutes of diaphragmatic breathing, 10 minutes of interval‐based IMT and 10 minutes of pursed lip breathing (Fregonezi 2005). Pinto 2012 applied a delayed start design. The IMT group received an active IMT protocol for eight months and the control group received sham IMT for the first four months, followed by an identical active IMT training protocol for the last four months. Thus, we considered data from the first four months for analysis. The frequency of threshold IMT, i.e. the number of days per week dedicated to the RMT program, ranged from three times a week to twice daily for 10 to 15 minutes. The duration of the interventions was between six weeks and four months. Another four trials performed resistive IMT. In Rodillo 1989, the participants from this trial used an inspirometer device that entailed forced inspiration against a resistance, which increased as inspiratory flow increased to a total of 20 inspirations/day. In one study, the inspiratory resistance was varied to give a subjectively heavy but tolerable load for 10 to 15 minutes (Smith 1988). The participants in Wanke 1994 had to perform both resistive breathing manoeuvres and maximal static inspiratory efforts against the almost occluded resistance. The inspiratory resistive breathing training consisted of 10 loaded breathing cycles of one minute duration each, twice daily. Fifteen minutes after the resistive breathing training, the participants had to perform 10 maximal static inspiratory efforts and reach a certain minimal pressure value. In Stern 1989, to exercise the respiratory muscles, the participants were required to inhale through a mask while playing a video game. The training consisted of 20‐minute sessions, five days a week, with the participants choosing the computer game they wanted to play. Inspiratory effort was increased by their having to breathe through a mask to both start and continue the games. The resistive IMT duration ranged from 18 days to six months.
Two trials studied expiratory muscle training (EMT). One trial compared EMT with sham IMT (Suleman 2003). Participants trained with 90% of maximal expiratory pressure (MEP), twice a day, for two months. In Plowman 2019, participants completed eight weeks of training at home, five days a week, with weekly home therapy visits. The trial compared EMT (50% of MEP) to training using a sham device (internal spring removed).
Martin 1986 performed a combined RMT (strength training plus endurance training) over two months. For strength training, maximum static inspiratory and expiratory manoeuvres at approximately 20% intervals over the vital capacity (VC) range were performed. The boys trained for about 30 minutes per day. For endurance training, the participants ventilated to exhaustion three times with recovery intervals. The initial resistances selected were those that led to exhaustion within three minutes. When each subject was able to ventilate without exhaustion through a resistance for three minutes or longer, the resistance was increased. In the control group, the participants were not trained.
Outcomes
All the included studies assessed our primary outcome, lung capacity, however four studies did not provide data for analysis (Martin 1986; Smith 1988; Stern 1989; Suleman 2003). Inspiratory muscle strength was measured in 10 trials, but only five studies reported sufficient numerical data and were entered in our analysis (Pinto 2012; Rodillo 1989; Topin 2002; Wanke 1994; Yeldan 2008). Seven trials assessed expiratory muscle strength, and we included five in quantitative analysis (Fregonezi 2005; Pinto 2012; Plowman 2019; Suleman 2003; Yeldan 2008). Physical function in carrying out activities of daily living was reported in two studies. Pinto 2012 assessed this using the ALS Functional Rating Scale (ALSFRS; Cedarbaum 1997), and Plowman 2019 used the revised ALSFRS (ALSFRS‐R; Cedarbaum 1999). Two trials measured quality of life. Pinto 2012 assessed this using EuroQol‐5D (Rabin 2001), and the trial report provided sufficient numerical data. Fregonezi 2005 evaluated quality of life using the Short Form‐36 Health Survey questionnaire (SF‐36; Alonso 1995), but the data (mean and SD) were reported for three domains (physical role functioning, physical functioning, and emotional role functioning) in the training group and for one domain (bodily pain) in the control groups. Thus, we did not present the data. Pinto 2012 stated that exercise protocol employed in their study had no adverse effects. Other trials did not provide data on adverse events. None of the included studies evaluated the number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure.
Excluded studies
We excluded 18 studies (reported in 19 articles), which are listed in the Characteristics of excluded studies table. We excluded 12 studies that were not RCTs or quasi‐RCTs; five studies because they included participants with more than one type of NMD (myopathies and neuropathies) or more than one type of neurological disorder, or because a participant used non‐invasive ventilation.
Ongoing studies
We found one ongoing trial, of respiratory training versus sham training in people with ALS (NCT02710110). See the Characteristics of ongoing studies table for details.
Risk of bias in included studies
See Figure 2 for an illustration of the review authors' 'Risk of bias' judgements across all studies and the 'Risk of bias' tables (in the Characteristics of included studies table) for further information.
2.
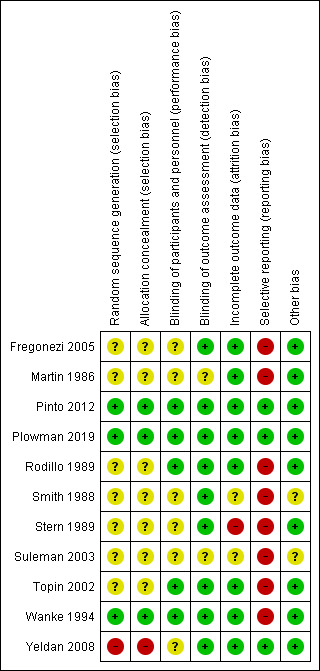
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study. Red (‐) = high risk of bias; yellow (?) = unclear risk of bias; green (+) = low risk of bias.
Allocation
Yeldan 2008 allocated participants to either the training or control group alternately, according to the order of their arrival in hospital. We therefore judged this trial to be at a high risk of bias. Three trials were at low risk of bias. Wanke 1994 used a computer program to generate the randomization sequence and numbered containers to conceal allocation (this information is from correspondence). Pinto 2012 randomized the participants in blocks of six, and then used numbered containers to implement the random allocation sequence (we ascertained this information from correspondence). Plowman 2019 employed a permuted block randomization schedule and concealed the sequence until the intervention was assigned (we ascertained this information from correspondence). Thus, we judged them to be at low risk of bias. The remaining seven trials were at unclear risk of bias, as they did not report the randomization method used.
Blinding
Five studies were described as double‐blind (assessors and participants) and we judged them to be at low risk of performance bias and detection bias (Pinto 2012; Plowman 2019; Rodillo 1989; Topin 2002; Wanke 1994). Four trials reported insufficient information about blinding of participants and personnel and we judged the risk of performance bias to be unclear (Fregonezi 2005; Smith 1988; Stern 1989; Yeldan 2008). However, the outcome assessors were blind to the intervention and we classified these trials as being at low risk of detection bias. Suleman 2003 and Martin 1986 did not mention blinding, so we judged them to have an unclear risk of performance and detection bias.
Incomplete outcome data
In two studies, all participants who started the training finished it and had their data included in the analysis (Fregonezi 2005; Topin 2002). Plowman 2019 and Wanke 1994 imputed missing data using an appropriate method (intention‐to‐treat analysis). A small number of participants dropped out of four studies, but the reasons for the missing outcome data were unrelated to the intervention (Martin 1986; Pinto 2012; Rodillo 1989; Yeldan 2008). In one trial (Stern 1989), six of 18 (33%) participants were excluded from analysis, either due to imbalance in numbers or reasons of missing data across intervention groups. We judged Suleman 2003 and Smith 1988 to have an unclear risk of attrition bias, as the reports provided no information about exclusions from the analysis.
Selective reporting
Two studies reported data for all outcomes (Pinto 2012; Yeldan 2008). We also considered Plowman 2019 at low risk of selective reporting bias. The remaining eight studies did not report one or more outcomes appropriately, and we were unable to extract or calculate the mean difference (MD) and standard deviation (SD) for each group separately. Therefore, we judged them to have a high risk of reporting bias.
Other potential sources of bias
We considered 'other bias' a category of exclusion, for bias that did not fall into other categories. Where no bias was apparent we considered the risk low. We did not identify other sources of bias in nine studies and judged them as being at low risk of bias for this domain. One was published only as abstract (Suleman 2003), and another as a letter to the editor (Smith 1988), therefore we judged these to have an unclear risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS)
Three trials compared a form of respiratory muscle training (RMT) with sham training in people with amyotrophic lateral sclerosis/motor neuron disease (ALS/MND; Pinto 2012; Plowman 2019; Suleman 2003). Pinto 2012, Suleman 2003 and Plowman 2019 involved 24, 14 and 48 participants with ALS/MND, respectively. See Table 1.
None of the trials of RMT versus sham training in ALS provided long‐term data (at time points greater than 1 year). Plowman 2019 and Suleman 2003 measured short‐term outcomes (at less than 3 months) and Pinto 2012 provided medium‐term data (between 3 months and 1 year).
Primary outcome: measures of lung capacity (e.g. total lung capacity (TLC), forced vital capacity (FVC))
Short term (less than 3 months)
Suleman 2003 (N = 14) was published only as an abstract and reported neither numerical nor narrative data for FVC and forced expiratory volume in 1 second (FEV₁).
Plowman 2019 showed no clear difference between the RMT and sham groups with respect to change in the per cent predicted FVC (from baseline to 8 weeks) (mean difference (MD) 0.70, 95% confidence interval (CI) ‐8.48 to 9.88; 1 trial, N = 46; low‐certainty evidence; Analysis 1.1). We graded the certainty of evidence as low, downgrading twice for very serious imprecision as the study was small and the CI was very wide and included the possibility of no effect.
1.1. Analysis.

Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 1 Change in FVC (% of predicted): short term.
Medium term (greater than 3 months but less than 1 year)
In Pinto 2012, the mean change in sitting FVC (from baseline to 4 months) favoured inspiratory muscle training (IMT) over sham IMT, but the CI included the possibility of no effect (MD 10.86% of predicted, 95% CI ‐4.25 to 25.97; 1 trial, N = 24; low‐certainty evidence; Analysis 1.2). The minimum clinically important difference (MCID) for FVC in NMDs has not yet been established. However, du Bois 2011 estimated the MCID for FVC% in people with idiopathic pulmonary fibrosis, another restrictive respiratory disorder, as 2% to 6%, based on a trial involving 1156 participants. Thus, the effect size in Pinto 2012 was potentially clinically important, but imprecision limited our confidence in the result. We graded the certainty of evidence as low, downgrading twice for very serious imprecision as the study was small and the CI was very wide and included the possibility of no effect.
1.2. Analysis.

Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 2 Change in FVC (% of predicted): medium term.
Inspiratory muscle strength, measured by maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP)
Short term (less than 3 months)
Suleman 2003 reported neither numerical nor narrative data for this outcome. Plowman 2019 did not assess this outcome.
Medium term (greater than 3 months but less than 1 year)
Pinto 2012 showed no clear difference in the change in per cent predicted sitting MIP (from baseline to 4 months) between the IMT and sham IMT group (MD ‐8.15%, 95% CI ‐29.85 to 13.54; 1 trial, N = 24; Analysis 1.3), nor was there a clear difference in change in per cent predicted sitting SNIP over the same period (MD ‐10.38%, 95% CI ‐30.44 to 9.67; 1 trial, N = 24; Analysis 1.4). There is no established MCID for evaluating the clinical significance of changes in MIP or SNIP. The sample size was small and the wide CI included no effect; these results were therefore very imprecise.
1.3. Analysis.

Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 3 Change in MIP (% of predicted): medium term.
1.4. Analysis.

Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 4 Change in SNIP (% of predicted): medium term.
Expiratory muscle strength, measured by maximal expiratory pressure (MEP)
Short term (less than 3 months)
Two ALS studies provided short‐term data on MEP (Plowman 2019; Suleman 2003). Analysis of pooled data found that MEP was higher with expiratory muscle training (EMT) than sham EMT (MD 20.24, 95% CI 6.58 to 33.90; I² = 0%; 2 trials, N = 60; Analysis 1.5). The sample size was small and was less than the targeted sample size generated by the power calculation.
1.5. Analysis.
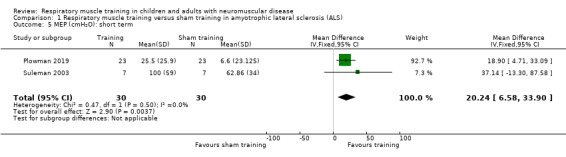
Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 5 MEP (cmH₂O): short term.
Medium term (greater than 3 months but less than 1 year)
Pinto 2012 found little or no difference in change in MEP between IMT and sham IMT groups at four months (MD ‐7.62% of predicted, 95% CI ‐32.06 to 16.83; 1 trial, N = 24; Analysis 1.6). The sample size was small and the wide CI included no effect; the results were therefore very imprecise.
1.6. Analysis.
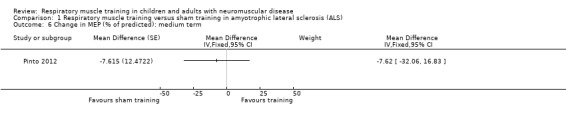
Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 6 Change in MEP (% of predicted): medium term.
Physical function in carrying out activities of daily living, measured by a validated instrument
No data were available on physical function in the long term from any trial. Plowman 2019 and Pinto 2012 provided short‐ and medium‐term data, respectively.
Short term (less than 3 months)
Plowman 2019 showed no clear difference in the change in Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised (ALSFRS‐R) score between EMT and sham EMT groups at eight weeks (MD 0.80, 95% CI ‐1.41 to 3.01; 1 trial, N = 46; low‐certainty evidence; Analysis 1.7). The ALSFRS‐R includes 12 questions and each task is rated on a five‐point scale from 0 (cannot do) to 4 (normal ability). Individual item scores are summed to produce a reported score of between 0 (worst) and 48 (best).
1.7. Analysis.
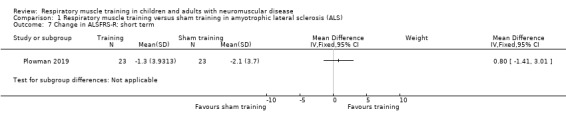
Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 7 Change in ALSFRS‐R: short term.
This result was very imprecise as the study was small and the CI was very wide, and included the possibility of no effect. Additionally, an intervention period of two months may not be adequate to document disease‐related progression or the potential impact of EMT on this outcome, and the trial included participants with ALSFRS‐R score > 30.
Medium term (greater than 3 months but less than 1 year)
Pinto 2012 assessed physical functioning in people with ALS on the ALSFRS (a scale with a range from 0 to 40), and reported the change in score between baseline and four months. There was no clear difference between IMT and sham IMT groups in the mean change in ALSFRS score (MD 0.85, 95% CI ‐2.16 to 3.85; 1 trial, N = 24; low‐certainty evidence; Analysis 1.8). We downgraded the certainty of the evidence to low because of very serious imprecision. The study was small and the CI included potentially clinically relevant effects in either direction. There is no established MCID for the ALSFRS, but as the scale ranges from 0 to 40, we judged less than 1 point to be too small a change to make a difference clinically.
1.8. Analysis.
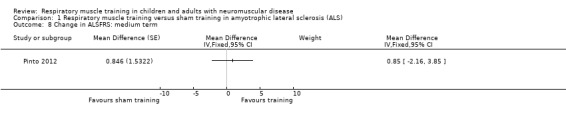
Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 8 Change in ALSFRS: medium term.
Quality of life, measured by a validated questionnaire
Data were not available for this outcome at either short‐ or long‐term time points from the studies of RMT versus sham training.
Medium term (greater than 3 months but less than 1 year)
Pinto 2012 measured quality of life using EuroQol‐5D in 24 people with ALS (Rabin 2001). In this study, the participants evaluated their overall health status using a VAS (visual analog scale; a vertical scale with end points of 0 and 100). The MD for the change in EuroQol‐5D score between IMT and sham IMT groups after a four‐month intervention was 0.77 (95% CI ‐17.09 to 18.62; 1 trial;, N = 24; low‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1 Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS), Outcome 9 Change in EuroQol‐5D: medium term.
We downgraded the evidence two levels for serious imprecision because the sample size was small and the CI included clinically important effects in either direction.
Adverse events
Pinto 2012 (N = 24) stated that their exercise protocol had no adverse effect (very low‐certainty evidence). The other ALS trial did not provide information on adverse events. We downgraded the evidence three times for study limitations (reporting bias) and imprecision (small sample size and no events).
Other secondary outcomes
No study of RMT versus sham training in people with ALS evaluated the number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure.
Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD)
Two trials in people with DMD compared training versus sham training: Rodillo 1989 (N = 20) and Topin 2002 (N = 16). Both only reported short‐term outcomes (at less than 3 months). See Table 2.
Primary outcome: measures of lung capacity (e.g. total lung capacity (TLC), forced vital capacity (FVC))
Short term (less than 3 months)
Rodillo 1989 found no clear difference between RMT and sham training groups in FVC measured 18 days after the intervention (MD 0.16 L, 95% CI ‐0.31 to 0.63; 1 trial, N = 20; Analysis 2.2) or FEV₁ (MD 0.18 L, 95% CI ‐0.28 to 0.64; 1 trial, N = 20; low‐certainty evidence; Analysis 2.5).
2.2. Analysis.

Comparison 2 Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD), Outcome 2 Post‐intervention FVC (L): short term.
2.5. Analysis.

Comparison 2 Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD), Outcome 5 Post‐intervention FEV₁ (L): short term.
In Topin 2002, the mean post‐intervention (6‐week) total lung capacity (TLC) between IMT and sham training groups favoured IMT (MD 0.45 L, 95% CI ‐0.24 to 1.14; 1 trial, N = 16; Analysis 2.1). The mean control TLC was 2.79 L; the MD of 0.45 L represents approximately a 16% difference between the groups. The mean forced residual capacity (FRC) at six weeks also favoured IMT (MD 0.40 L, 95% CI ‐0.12 to 0.92; 1 trial, N = 16; Analysis 2.3). The mean control FRC was 1.47 L; the MD of 0.4 L therefore corresponds to an improvement of 27%. The post‐intervention vital capacity (VC) suggested no clear difference between IMT and sham training groups in VC (MD 0.02 L, 95% CI ‐0.57 to 0.61; 1 trial, N = 16; Analysis 2.4).
2.1. Analysis.

Comparison 2 Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD), Outcome 1 Post‐intervention TLC (L): short term.
2.3. Analysis.

Comparison 2 Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD), Outcome 3 Post‐intervention FRC (L): short term.
2.4. Analysis.

Comparison 2 Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD), Outcome 4 Post‐intervention VC (L): short term.
We downgraded the certainty of evidence for all these outcomes two levels to low certainty for very serious imprecision, as the sample size was small and the CI was wide, including both an important effect and no effect.
Medium term (greater than 3 months but less than 1 year)
Neither study measured lung capacity in the medium term.
Inspiratory muscle strength, measured by maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP)
Short term (less than 3 months)
Topin 2002 assessed MIP at six weeks and Rodillo 1989 at 18 days. The MD for MIP indicated no clear difference between IMT and sham IMT groups (MD 2.84 cmH₂O, 95% CI ‐1.47 to 7.15; I² = 0%; 2 trials, N = 36; Analysis 2.6). However, in Topin 2002, the training protocol used a low‐intensity IMT specifically designed to improve inspiratory muscle endurance. Since the adaptations to training are specific, inspiratory endurance training would not be sufficient to improve MIP. Indeed, in this study there was no change in MIP, but respiratory muscle endurance showed a considerable increase. Rodillo 1989 performed only 18 days of RMT and this period of training could be too short to improve the MIP.
2.6. Analysis.
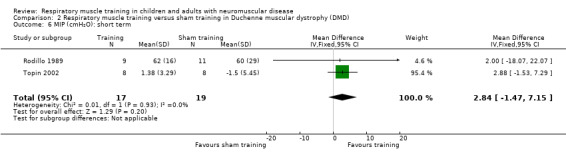
Comparison 2 Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD), Outcome 6 MIP (cmH₂O): short term.
There was very serious imprecision, as the sample size was small, and the CI included no effect.
Medium term (greater than 3 months but less than 1 year)
Neither study measured inspiratory muscle strength in the medium term.
Other secondary outcomes
Neither trial reported change in inspiratory or expiratory muscle strength, physical function, quality of life, unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure, or adverse events.
Respiratory muscle training versus no training in DMD
Wanke 1994 (N = 30), Martin 1986 (N = 17), Smith 1988 (N = 8) and Stern 1989 (N = 12) compared a form of RMT with no training in participants with DMD. Martin 1986 and Smith 1988 measured short‐term outcomes (at less than 3 months). Two studies measured medium‐term data (between 3 months and 1 year) (Stern 1989; Wanke 1994). None of these trials provided long‐term data (at time points greater than 1 year). Table 3.
Primary outcome: measures of lung capacity (e.g. total lung capacity (TLC), forced vital capacity (FVC)) over the short term
Short term (less than 3 months)
Martin 1986 and Smith 1988, which were cross‐over studies, did not provide data for each study period, therefore, the outcomes could not be meta‐analysed.
Medium term (greater than 3 months but less than 1 year)
Wanke 1994 assessed VC and FEV₁ in young males with DMD who underwent training or no training. Post‐intervention (6‐month) VC values showed no clear difference between groups, whether expressed as absolute values (MD 0.14 L, 95% CI ‐0.44 to 0.72; 1 trial, N = 30; Analysis 3.1) or as per cent predicted (MD 3.50%, 95% CI ‐14.35 to 21.35; 1 trial, N = 30; Analysis 3.2). For FEV₁, the final value (after a 6‐month intervention) increased slightly in the training group compared to the no training group (MD 0.18 L, 95% CI ‐0.29 to 0.65; 1 trial, N = 30; low‐certainty evidence; Analysis 3.3). There is no established MCID for FEV₁ in NMDs. Changes greater than 12% and 0.2 L in the FEV₁ may be clinically important (Pellegrino 2005). In people with chronic obstructive pulmonary disease (COPD), a 100 mL improvement in FEV₁ was associated with reduction of 5.9 units in St. George's Respiratory Questionnaire (SGRQ; Jones 1991; Jones 1992; Jones 2005), a disease‐specific instrument designed to measure impact on overall health, daily life, and perceived well‐being in patients with obstructive airways disease (de la Loge 2016). A mean change of 4 units on the SGRQ is associated with slightly efficacious treatment (Jones 2002). However, the use of MCIDs from studies of chronic respiratory diseases in NMDs has significant limitations.
3.1. Analysis.

Comparison 3 Respiratory muscle training versus no training in Duchenne muscular dystrophy (DMD), Outcome 1 Post‐intervention VC (L): medium term.
3.2. Analysis.

Comparison 3 Respiratory muscle training versus no training in Duchenne muscular dystrophy (DMD), Outcome 2 Post‐intervention VC (% of predicted): medium term.
3.3. Analysis.

Comparison 3 Respiratory muscle training versus no training in Duchenne muscular dystrophy (DMD), Outcome 3 Post‐intervention FEV₁ (L): medium term.
We downgraded the certainty of evidence two levels to low for very serious imprecision as the sample size was small and CIs were wide).
Stern 1989 was a cross‐over study that did not provide individual data for the six‐month time point.
Inspiratory muscle strength, measured by maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP)
Short term (less than 3 months)
Martin 1986 and Smith 1988 did not provide data for each study period, therefore, we could not assess the results.
Medium term (greater than 3 months but less than 1 year)
In Wanke 1994, the maximal sniff assessed esophageal pressure (Pesmax) and maximal transdiaphragmatic pressure (Pdimax) values served as outcome measures for global inspiratory muscle strength and diaphragmatic strength, respectively. These measures were obtained in 30 participants with DMD; however, eight people (5 in the training group and 3 in the control group) had VC values less than 25% of those predicted and/or a partial pressure of carbon dioxide in the arterial blood (PaCO₂) of more than 45 mmHg, indicating severe pulmonary function impairment. We did not consider data from these eight participants for analysis. The final values (at 6 months) demonstrated an improvement in Pesmax (MD 22.53 cmH₂O, 95% CI 13.33 to 31.73; 1 trial, N = 22; Analysis 3.4) and Pdimax (MD 24.39 cmH₂O, 95% CI 14.65 to 34.13; 1 trial, N = 22; Analysis 3.5) in the IMT group in comparison to the no training group.
3.4. Analysis.

Comparison 3 Respiratory muscle training versus no training in Duchenne muscular dystrophy (DMD), Outcome 4 Post‐intervention Pesmax (cmH₂O): medium term.
3.5. Analysis.

Comparison 3 Respiratory muscle training versus no training in Duchenne muscular dystrophy (DMD), Outcome 5 Post‐intervention Pdimax (cmH₂O): medium term.
These outcomes were subject to serious imprecision, as the sample size was small and CI wide.
Stern 1989 did not provide individual data for the six‐month time point.
Expiratory muscle strength, measured by maximal expiratory pressure (MEP)
Short term (less than 3 months)
Martin 1986 and Stern 1989 reported neither numerical nor narrative data from the first treatment arm.
Other secondary outcomes
No study of respiratory training versus no training evaluated the following secondary outcomes: change in physical function in carrying out activities of daily living, quality of life, number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure, and adverse events.
Respiratory muscle training versus breathing exercises in limb‐girdle muscular dystrophy or Becker muscular dystrophy (BMD)
Yeldan 2008 involved 21 participants with limb‐girdle muscular dystrophy and BMD, and reported results after 12 weeks of IMT or breathing exercises. The trial did not report medium‐term or long‐term outcomes.
Primary outcome: measures of lung capacity (e.g. total lung capacity (TLC), forced vital capacity (FVC))
Short term (less than 3 months)
Yeldan 2008 provided numerical data for the change from baseline in each intervention group. The MD showed no clear difference between the IMT and breathing exercises group for FVC (MD 0.01 L, 95% CI ‐0.11 to 0.13; N = 21; Analysis 4.1), VC (MD ‐0.02 L, 95% CI ‐0.15 to 0.11; N = 21; Analysis 4.2) or FEV₁ (MD 0.03 L, 95% CI ‐0.09 to 0.15; N = 21; Analysis 4.3). There was very serious imprecision in the analysis as the sample size was small and CIs were wide. Additionally, the trial was at high risk of selection bias.
4.1. Analysis.

Comparison 4 Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle), Outcome 1 FVC (L): short term.
4.2. Analysis.

Comparison 4 Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle), Outcome 2 Change in VC (L): short term.
4.3. Analysis.
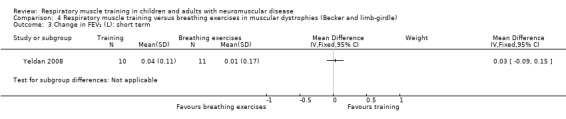
Comparison 4 Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle), Outcome 3 Change in FEV₁ (L): short term.
Inspiratory muscle strength, measured by maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP)
Short term (less than 3 months)
In Yeldan 2008, 12 weeks of IMT improved MIP in comparison to breathing exercises (MD 18.50 cmH₂O, 95% CI 1.29 to 35.71; N = 21; Analysis 4.4). The sample size was small and the result very imprecise. The trial was at high risk of selection bias.
4.4. Analysis.

Comparison 4 Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle), Outcome 4 Change in MIP (cmH₂O): short term.
Expiratory muscle strength, measured by maximal expiratory pressure (MEP)
Short term (less than 3 months)
In Yeldan 2008, 12 weeks of RMT in people with muscular dystrophies produced no clear improvement in MEP in comparison to breathing exercises (MD ‐2.47 cmH₂O, 95% CI ‐13.82 to 8.88; N = 21; Analysis 4.5). The sample size was small and the result very imprecise. The trial was at high risk of selection bias.
4.5. Analysis.
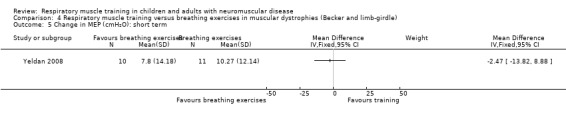
Comparison 4 Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle), Outcome 5 Change in MEP (cmH₂O): short term.
Other secondary outcomes
The following secondary outcomes were not evaluated: quality of life, physical function in carrying out activities of daily living, number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure, adverse events.
Respiratory muscle training (RMT) versus breathing exercises in myasthenia gravis
Fregonezi 2005 (N = 27) compared a form of RMT with breathing exercises in people with myasthenia gravis and reported only short‐term outcomes (after 8 weeks of RMT). See Table 5.
Primary outcome: measures of lung capacity (e.g. total lung capacity (TLC), forced vital capacity (FVC))
Short term (less than 3 months)
Fregonezi 2005 reported numerical data as mean and standard deviation (SD) for each evaluation period. The final values from Fregonezi 2005 showed no clear difference between the RMT group and the breathing exercises group in TLC (MD ‐0.20 L, 95% CI ‐1.07 to 0.67; N = 27; Analysis 5.1), FVC (‐0.20 L, 95% CI ‐0.80 to 0.40; N = 27; Analysis 5.2), residual volume (RV) (MD 0.00 L, 95% CI ‐0.30 to 0.30; N = 27; Analysis 5.3), inspiratory capacity (IC) (MD ‐0.10 L, 95% CI ‐0.63 to 0.43; N = 27; Analysis 5.4), or FEV₁ (MD ‐0.30 L, 95% CI ‐0.90 to 0.30; N = 27; Analysis 5.5). We downgraded the evidence for all of these outcomes two levels to low certainty for very serious imprecision as the sample size was small and the CIs wide.
5.1. Analysis.
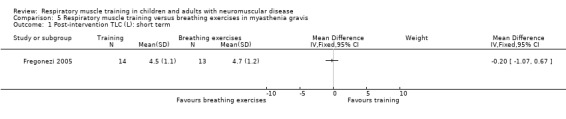
Comparison 5 Respiratory muscle training versus breathing exercises in myasthenia gravis, Outcome 1 Post‐intervention TLC (L): short term.
5.2. Analysis.

Comparison 5 Respiratory muscle training versus breathing exercises in myasthenia gravis, Outcome 2 FVC (L): short term.
5.3. Analysis.

Comparison 5 Respiratory muscle training versus breathing exercises in myasthenia gravis, Outcome 3 Post‐intervention RV (L): short term.
5.4. Analysis.
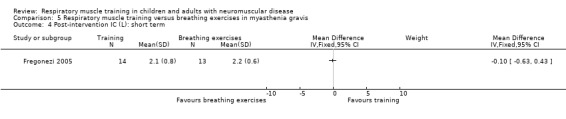
Comparison 5 Respiratory muscle training versus breathing exercises in myasthenia gravis, Outcome 4 Post‐intervention IC (L): short term.
5.5. Analysis.

Comparison 5 Respiratory muscle training versus breathing exercises in myasthenia gravis, Outcome 5 FEV₁ (L): short term.
Inspiratory muscle strength, measured by maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP)
Short term (less than 3 months)
Fregonezi 2005 did not provide sufficient numerical data on MIP (only final values were reported in graphs) and we were unable to calculate MD and SD. The trial authors reported narratively that eight weeks of RMT in people with myasthenia gravis increased MIP in comparison to the control group.
Expiratory muscle strength, measured by maximal expiratory pressure (MEP)
Short term (less than 3 months)
Fregonezi 2005 provided mean MEP and SD for each intervention group. We calculated the change from baseline and obtained the SD from P values for differences in means. The results showed that eight weeks' RMT improved MEP in comparison to breathing exercises in people with myasthenia gravis (MD 15.00 cmH₂O, 95% CI 4.45 to 25.55; N = 27; Analysis 5.6). The data were imprecise, the sample size small, and the risk of bias from randomization, allocation concealment and blinding of participants was unclear. The trial protocol was not directly targeted to expiratory muscles; however, the training group performed diaphragmatic breathing, followed interval‐based IMT and performed pursed lip breathing. The authors of this trial suggest that pursed lip breathing could have influenced the functional improvement of expiratory muscles in the control group.
5.6. Analysis.

Comparison 5 Respiratory muscle training versus breathing exercises in myasthenia gravis, Outcome 6 Change in MEP (cmH₂O): short term.
Quality of life, measured by a validated questionnaire
Short term (less than 3 months)
Fregonezi 2005 assessed quality of life using the SF‐36 Health Survey questionnaire (Alonso 1995), but the data (mean and SD) were reported for three domains (physical role functioning, physical functioning and emotional role functioning) in the training group and for one domain (bodily pain) in the control groups. Thus, we did not present the data.
The trial authors reported narratively that, in people with myasthenia gravis, a change in one of the nine SF‐36 domains (physical role functioning) showed an improvement in the RMT group compared to the breathing exercises group (N = 27; very low‐certainty evidence). We downgraded the evidence twice for serious imprecision and once for study limitations.
Other secondary outcomes
The following secondary outcomes were not evaluated: physical function in carrying out activities of daily living, number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure and adverse events.
Discussion
Summary of main results
We assessed the effects of respiratory muscle training (RMT) (inspiratory muscle training (IMT) or expiratory muscle training (EMT), or both) for neuromuscular disease (NMD) in children and adults. Eleven studies satisfied the inclusion criteria. These studies included 250 randomized participants (237 evaluable) with myopathies (Duchenne muscular dystrophy (DMD; 6 studies), limb‐girdle muscular dystrophy or Becker muscular dystrophy (BMD; 1 study), amyotrophic lateral sclerosis (ALS; 3 studies), and myasthenia gravis (1 study). Eight trials investigated the effects of IMT, two studied EMT, and a single trial investigated IMT plus EMT. RMT was compared with sham training, no training, or breathing exercises. Heterogeneity in interventions, populations, comparators and outcome measures meant that no meta‐analysis was possible for most comparisons.
Measures of lung capacity
All included studies assessed lung capacity, our primary outcome. In people with ALS, IMT may lead to some benefit over sham IMT, based on the change in the per cent predicted forced vital capacity (FVC) from baseline to four months in one study. Another trial in ALS showed no clear difference in the change in per cent predicted FVC (from baseline to 8 weeks) between participants treated with EMT compared to those treated with sham EMT.
Four studies performed in people with myopathies reported data on lung capacity. In the short term, RMT may produce a small improvement in TLC (6 weeks) in young males with DMD, but may lead to no effect on FVC (18 days) in boys with DMD when compared to sham RMT. Moreover, RMT may lead to no clear difference in the per cent predicted vital capacity (VC%) in comparison to no training (at 6 months follow‐up), or FVC in comparison to breathing exercises (at 12 weeks follow‐up), respectively.
In people with myasthenia gravis, based on a single trial, RMT may lead to little difference in TLC (at 8 weeks follow‐up) in comparison to respiratory exercises.
The remaining four trials (1 in ALS and 3 in myopathies) did not provide data on lung capacity.
Inspiratory muscle strength
In people with ALS, the effect of IMT on the change in inspiratory muscle strength between baseline and four months may result in little difference from sham IMT.
In people with DMD, RMT may improve inspiratory muscle strength in comparison to no training in the medium term (6 months); but when compared to sham training, in the short term, may lead to no clear difference between groups, based on two studies with six weeks and 18 days of training. In comparison to breathing exercises, it is uncertain whether RMT improves inspiratory muscle strength in people with myopathies, because the certainty of the evidence is very low.
Expiratory muscle strength
In ALS, there was a higher expiratory muscle strength with EMT when compared to sham EMT in the short term. However, IMT may lead to no effect on expiratory muscle strength in the medium term (4 months) in people with ALS/MND.
The effects of RMT in comparison to breathing exercises in the short term (12 weeks) in people with limb‐girdle muscular dystrophy and BMD are unclear because the certainty of evidence is very low.
In myasthenia gravis, IMT may improve expiratory muscle strength post‐8 weeks in comparison to breathing exercises.
Physical functioning
Two trials assessed physical functioning in people with ALS; one in the short term (8 weeks) and another in the medium term (4 months). Both trials showed that there may be no clinically meaningful difference in ALSFRS between IMT and sham IMT groups.
Quality of life
In relation to quality of life, four months of IMT may lead to no clear difference in EuroQol‐5D in comparison to sham IMT in participants with ALS.
A trial that assessed quality of life in people with myasthenia gravis using the SF‐36 reported an improvement in one of the nine SF‐36 domains (physical role functioning) in the IMT group compared to the breathing exercises group after eight weeks.
Adverse events
One trial involving people with ALS compared IMT to sham IMT and stated that their exercise protocol had no adverse effect. Due to very low‐certainty evidence it is uncertain whether IMT may have adverse effects in this group. Other trials did not provide data on adverse events.
No included study provided long‐term data (at time points greater than 1 year) or evaluated the number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure.
Overall completeness and applicability of evidence
The effect of RMT in people with NMDs has long been controversial.
The trials included participants with DMD, limb‐girdle muscular dystrophy, BMD, ALS/MND, and myasthenia gravis. The results may not be generalized to other NMDs. The small number of trials did not allow for meaningful subgroup analyses. Thus we did not investigate the effects of RMT by age of the participants (children versus adults). Children are not little adults and these groups are pathophysiologically distinct (Landrigan 2005). Thus, children may have different responses to therapies in comparison to adults. Moreover, the power of a study to detect a real difference between interventions depends on sample size. Very small samples undermine the internal and external validity of a trial, while very large samples tend to transform small differences into statistically significant differences, even when they are clinically insignificant (Faber 2014). The CONSORT (Consolidated Standards of Reporting Trials) statement recommends that the clinical trials should indicate how the sample size was determined (CONSORT 2010). However, no study included in this review reported how sample size was determined.
For RMT benefits to be achieved, the principles of exercise prescription must be considered to ensure an adequate RMT 'dose'. The prescription of the RMT program should ideally be reported according to the so‐called FITT components (Frequency, Intensity, Time and Type of exercise) (Riebe 2014). However, the ideal FITT components for RMT for people with NMD are uncertain. In the majority of the included studies in this systematic review, interventions were performed once or twice per day, for five to seven days per week. The RMT programs involved low‐to‐high intensity exercise and the duration of interventions ranged from six weeks to seven months. Our findings are based mainly on pressure threshold IMT and cannot be extrapolated to any other type of training.
In addition to dose‐dependent effects of IMT, impaired ventilatory function may alter the effects of RMT on inspiratory muscle strength. In participants whose respiratory system involvement is slowly progressive, i.e. in whom VC declined by less than 10% in the year before the start of training, there was a significant positive correlation between the number of successfully completed IMT programs and improvement in maximal inspiratory pressure (MIP). In people whose VC decline is more rapidly progressive (more than 10%), no significant correlation between the improvement of MIP and the intensity of training was found (Winkler 2000). Moreover, Wanke 1994 showed that in people with VC < 25% of predicted and/or PaCO2 > 45 mmHg, inspiratory muscle function had not improved with IMT.
Most included studies in our review compared a form of RMT with a control group using sham training. No trial included in this review compared different intensities of RMT (e.g. low versus high intensity) or different types of RMT (e.g. IMT versus IMT plus EMT).
Five (71%), two (29%) and four (57%) of our seven prespecified outcomes were addressed in the analysis comparing RMT with sham training, no training and breathing exercises, respectively. Moreover, the data were not always presented in a suitable format. We had planned to develop the analysis using the change from baseline measures, but some trials reported only final values. Thus when the change from baseline was not reported, we extracted final values and both final values and change from baseline were reported and would have been used in any meta‐analysis. The number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure was not measured or reported in any of the included studies. Only one study reported whether participants experienced adverse events (Pinto 2012).
Nitric oxide is a potent regulator of skeletal muscle metabolism, mass, function and regeneration. In DMD, there is loss of neuronal nitric oxide synthase and the capacity of the muscles for endogenous nitric oxide synthesis (Timpani 2017). During exercise, people with DMD do not increase blood flow in the working muscles by attenuating sympathetic (i.e. α‐adrenergic) vasoconstriction. This impairment of the protective mechanism results in functional muscle ischemia due to unopposed sympathetic vasoconstriction (Thomas 2013). Therefore, we have concerns about ischemic muscle damage in this subgroup of patients with NMD during RMT due to the absence of high‐certainty evidence on RMT in people with DMD.
Certainty of the evidence
Eleven studies met the inclusion criteria. These trials involved 250 randomized people with NMD, and data from 237 participants was included in the quantitative analysis. Therefore, there was a sample size loss of only 5.2%.
All but two of the included studies were at high risk of bias in at least one domain, and many reported insufficient information for accurate assessment of the risk of bias. The major methodological limitations were lack of information about random sequence generation and allocation concealment, lack of blinding (particularly with subjective outcomes highly susceptible to biased assessment), and selective outcome reporting. Trials rarely described the methods used to assign participants to groups or for concealment of allocation (Savović 2012). Three included trials had a proper sequence generation and allocation concealment. In studies in which sequence generation and allocation concealment are inadequate or unclear (versus adequate), intervention effect estimates have been found to be exaggerated by approximately 7% and 10%, respectively (Page 2016). Moreover, our analyses relied on subjective outcomes, which appear to be at greater risk of bias than objective outcomes (Page 2016). Eight included trials were judged to be at high risk of reporting bias. We experienced some difficulties in entering the data of studies into our analysis because some numerical data were not reported and other results were not always presented in a suitable format (e.g. values reported only in graphs). The CONSORT (Consolidated Standards of Reporting Trials) statement recommends that, for each outcome, trial data should be reported as a summary of the outcome in each group together with the effect size, which for continuous data is usually the difference in means and standard deviation for the difference (CONSORT 2010).
The certainty of evidence across the different outcomes was low to very low. For all outcomes, imprecision of results contributed to a downgrading when we applied GRADE criteria (Schünemann 2013). Most findings in our review came from single studies with small numbers of participants, i.e. the sample size of trials included in the analysis varied from 14 to 48 participants. Moreover there is a wide CI around the estimate of the effect, including both an important effect and no effect. Although there were methodological limitations, we judged all but two of the included studies to have no serious limitations and we did not downgrade them.
Participants, interventions, and outcomes were not substantially different from those considered in the question in this systematic review. In addition, we have no reason to suspect publication bias, since the search strategy found studies with a small sample and 'negative studies', i.e. trials reporting statistically insignificant findings.
Taking into account the small number of participants included, differences in the training protocols and the imprecision in most of the analyses limit our confidence in the results. Moreover, data from the trials were not always presented in a suitable format for meta‐analysis. Thus, any conclusions must be drawn very cautiously. New, high quality evidence is likely to have had an important impact on our confidence in the estimates of effect for the outcomes investigated and potentially could affect our assessment of the effects of interventions of this type.
Potential biases in the review process
Dr Fregonezi was not involved in assessment of his own study for inclusion or bias, nor did he assist in the extraction and analysis of the data (Fregonezi 2005).
NMDs are generally fairly rare in single centers, thus some studies have included a heterogeneous sample with different NMD in order to gain sufficient power. However, diseases affecting the anterior horn cells, peripheral nerves, and/or muscles would not respond in the same way to exercise training (Abresch 2012). As the pathophysiology of each NMD is different, we considered that the effects of RMT might differ between different types of NMD. Therefore, we excluded trials in which participants had a variety of NMDs since we could not obtain results for each condition separately. Because of this, when studies with different types of NMDs were included in the same comparison, we entered study data into a forest plot for visual interpretation of the results but did not pool them. These approaches may introduce bias in this review.
We had difficulty performing and interpreting the comparisons due to substantial differences between the studies, including the populations, FITT components and data presentation (for example, absolute and predicted values). In order to minimise heterogeneity between studies, we performed three comparisons according to the control group (sham training, no training, and breathing exercises). Accordingly there were a few studies for each comparison in the meta‐analysis. Therefore, we were unable to undertake the proposed subgroup and sensitivity analyses. If we had been able to include more studies in our meta‐analysis, we might have demonstrated possible differences between different types of NMD (myopathies, disorders of the neuromuscular junction, and neuropathies) and differences related to the duration of the interventions. Moreover, sensitivity analysis could have identified the influence of some factors (such as random sequence generation and allocation concealment) on the results, thus revealing a more accurate estimate of the effect of the interventions.
Some studies did not report methodology in sufficient detail. We tried to minimise possible biases by contacting the authors to verify study characteristics and to request data, but some authors had no data available, while others did not respond to our emails. Furthermore, despite our attempts to apply a systematic process in assessment of the risk of bias, the final decisions are necessarily subject to a level of interpretation. Methods have not been substantially modified from the protocol (Pedrosa 2015). We have reported any deviations in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
We identified three systematic reviews that evaluated the effects of RMT in people with neuromuscular and neurological conditions generally.
Eidenberger 2014 reviewed the efficacy of IMT in ALS, and found four studies: two RCTs, one pre‐experimental study and one with a historical control group. The authors concluded that there was limited evidence that IMT induces strengthening of inspiratory muscles in participants with ALS. Moreover, in the Eidenberger 2014 review no statistical analysis was performed.
Ferreira 2016 reviewed nine RCTs and observed that RMT significantly increased respiratory muscle strength and FEV₁ in people with multiple sclerosis and ALS.
Recently, Human 2017 systematically reviewed the effects of RMT with an external device compared to control group. The population was children aged between 5 and 18 years with NMDs. The authors identified seven studies, all but one of which we also included in our review. We excluded the seventh trial included in Human 2017 because the trial report did not provide separate data for different types of NMD (spinal muscular atrophy and DMD) (Gozal 1999). Six of these studies showed no significant improvement in pulmonary function tests after IMT. Moreover, two trials reported significant increases in inspiratory muscle endurance and four studies found significantly greater improvement in inspiratory muscle strength in the training groups. The review concluded that in the population under review, although RMT might benefit respiratory muscle strength and endurance, evidence was lacking for other outcomes and adverse events, and there was no clear evidence for or against its use.
Our analysis (7 RCTs and 4 cross‐over trials providing data) showed that RMT may improve lung capacity and respiratory muscle strength in some NMDs. To our knowledge, this is the first systematic review that has included participants with disorders of the neuromuscular junction (myasthenia gravis).
Authors' conclusions
Implications for practice.
We found low‐certainty evidence that respiratory muscle training (RMT) may improve lung capacity and respiratory muscle strength in some neuromuscular diseases (NMDs). Moreover there may be no meaningful difference in physical functioning or in quality of life in people with amyotrophic lateral sclerosis (ALS). It is uncertain whether RMT causes adverse effects, as the quality of evidence is very low. Thus, there is no definitive evidence in this review about the effect of RMT for NMDs.
Due to clinical heterogeneity between the trials and small number of participants included in the analysis, together with a high risk of bias, the results must be interpreted very cautiously. In the future, the inclusion of more randomized controlled trials (RCTs) with a low risk of bias would be likely to have an important impact on our confidence in the estimate of effect for the outcomes investigated. Thus, the assessment of the effects of RMT in people with NMDs could change.
Implications for research.
The quality of current evidence on this topic is low, thus we need well‐conducted RCTs to evaluate the clinical benefit of RMT in the management of people with NMDs. More attention needs to be paid to high‐quality study design and reporting, including items such as determination of the trial sample size before the beginning of the study, adequate random sequence generation and allocation concealment, blinding of the outcome assessor and, when possible, of participants. If there is attrition of participants, an intention‐to‐treat analysis must be performed. Moreover, results should be reported following CONSORT guidelines (CONSORT 2010). Studies that assess more than one type of NMD should present results for each condition separately, because the effects of RMT may be different for each type of disease.
In order to draw firm conclusions, future trials should enrol people with NMDs that cause impairment of respiratory muscles, since we identified only seven eligible studies, with small sample size for this review. NMDs that cause impairment of respiratory muscles include muscular dystrophies such as Becker muscular dystrophy (BMD), Duchenne muscular dystrophy (DMD), limb‐girdle, Emery‐Dreifuss, and facioscapulohumeral muscular dystrophy, myotonic dystrophy, metabolic and congenital myopathies, inflammatory myopathies, myasthenia gravis, neuropathies (hereditary and acquired), ALS, poliomyelitis, and spinal muscular atrophy. The diagnostic criteria, disease stage, sex, and age of participants, respiratory muscle weakness (inspiratory, expiratory or both) and inclusion and exclusion criteria must also be specified.
Few trials have performed expiratory muscle training (EMT) or inspiratory muscle training (IMT) plus EMT, and the main comparisons of interest in this review were sham training and no training. More adequate RCTs comparing RMT with sham training and no training are necessary, because there was not sufficient evidence about efficacy of the intervention. Particular attention should be given to FITT components (Frequency, Intensity, Time and Type of exercise).
The trials should investigate important outcomes, including physical functioning in activities of daily living, quality of life, and the number of unscheduled hospitalisations for episodes of chest infection or acute exacerbation of chronic respiratory failure. Furthermore, attention must be paid to adverse effects that could arise during training, i.e. dyspnoea, tachypnoea, desaturation, haemodynamic instability, and respiratory fatigue.
Acknowledgements
We would like to thank members of Cochrane Neuromuscular. The 'Methods' section of this protocol is based on a standard template produced by Cochrane Airways and adapted by Cochrane Neuromuscular.
We gratefully thank Angela Gunn for running the searches and her help in devising the search strategies.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease and the Motor Neurone Disease Association.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web) search strategy
Search date = 19 November 2018
#1 MeSH DESCRIPTOR Glycogen Storage Disease Explode All AND INREGISTER #2 "metabolic disease*" AND INREGISTER #3 "muscular disease*" AND INREGISTER #4 (#1 or #2) and #3 AND INREGISTER #5 MeSH DESCRIPTOR Muscular Dystrophies Explode All AND INREGISTER #6 (metabolic or congenital) near2 myopath* AND INREGISTER #7 inflammatory near2 myopath*:ti AND INREGISTER #8 MeSH DESCRIPTOR Muscular Diseases WITH CI CN GE AND INREGISTER #9 MeSH DESCRIPTOR Muscular Atrophy, Spinal Explode All AND INREGISTER #10 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND INREGISTER #11 "motor neuron disease*" or "motor neurone disease*" AND INREGISTER #12 "motoneuron disease*" or "motoneurone disease*" AND INREGISTER #13 "motorneuron disease*" or "motorneurone disease*" AND INREGISTER #14 "amyotrophic lateral sclerosis" AND INREGISTER #15 als:ti or als:ab or nmd:ti or mnd:ab AND INREGISTER #16 poliomyelitis or "muscular dystroph*" or "myotonic dystroph*" or myasthen* or myelopath* AND INREGISTER #17 dystrophy near3 (becker or Duchenne or "limb girdle" or "emery dreifuss" or facioscapulohumeral) AND INREGISTER #18 "peripheral nervous system disease*" AND INREGISTER #19 neuropathy or neuropathies or polyneuropathy or polyneuropathies AND INREGISTER #20 "neuromuscular disease*" or "neuromuscular weakness" or "respiratory insufficiency" AND INREGISTER #21 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 AND INREGISTER #22 MeSH DESCRIPTOR Breathing Exercises Explode All AND INREGISTER #23 (respir* or inspirat*) near3 (training or exercise*) AND INREGISTER #24 "chest physiotherapy" AND INREGISTER #25 "physical therapy techni*" AND INREGISTER #26 "physical therapy modalities" AND INREGISTER #27 (#25 or #26) and (breath* or respir* or inspir* or chest) AND INREGISTER #28 (respir* or inspirat* or expiratory or ventilatory or chest) near4 (training or exercise* or endurance) AND INREGISTER #29 threshold near3 (load or device*) AND INREGISTER #30 "resistive breathing" AND INREGISTER #31 #22 or #23 or #24 or #27 or #28 or #29 or #30 AND INREGISTER #32 #21 and #31 AND INREGISTER
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) search strategy
Search date = 19 November 2018
#1 MeSH DESCRIPTOR Glycogen Storage Disease Explode All AND CENTRAL:TARGET #2 "metabolic disease*" AND CENTRAL:TARGET #3 "muscular disease*" AND CENTRAL:TARGET #4 (#1 or #2) and #3 AND CENTRAL:TARGET #5 MeSH DESCRIPTOR Muscular Dystrophies Explode All AND CENTRAL:TARGET #6 (metabolic or congenital) near2 myopath* AND CENTRAL:TARGET #7 inflammatory near2 myopath*:ti AND CENTRAL:TARGET #8 MeSH DESCRIPTOR Muscular Diseases WITH CI CN GE AND CENTRAL:TARGET #9 MeSH DESCRIPTOR Muscular Atrophy, Spinal Explode All AND CENTRAL:TARGET #10 MeSH DESCRIPTOR Motor Neuron Disease Explode All AND CENTRAL:TARGET #11 "motor neuron disease*" or "motor neurone disease*" AND CENTRAL:TARGET #12 "motoneuron disease*" or "motoneurone disease*" AND CENTRAL:TARGET #13 "motorneuron disease*" or "motorneurone disease*" AND CENTRAL:TARGET #14 "amyotrophic lateral sclerosis" AND CENTRAL:TARGET #15 als:ti or als:ab or nmd:ti or mnd:ab AND CENTRAL:TARGET #16 poliomyelitis or "muscular dystroph*" or "myotonic dystroph*" or myasthen* or myelopath* AND CENTRAL:TARGET #17 dystrophy near3 (becker or Duchenne or "limb girdle" or "emery dreifuss" or facioscapulohumeral) AND CENTRAL:TARGET #18 "peripheral nervous system disease*" AND CENTRAL:TARGET #19 neuropathy or neuropathies or polyneuropathy or polyneuropathies AND CENTRAL:TARGET #20 "neuromuscular disease*" or "neuromuscular weakness" or "respiratory insufficiency" AND CENTRAL:TARGET #21 #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 AND CENTRAL:TARGET #22 MeSH DESCRIPTOR Breathing Exercises Explode All AND CENTRAL:TARGET #23 (respir* or inspirat*) near3 (training or exercise*) AND CENTRAL:TARGET #24 "chest physiotherapy" AND CENTRAL:TARGET #25 "physical therapy techni*" AND CENTRAL:TARGET #26 "physical therapy modalities" AND CENTRAL:TARGET #27 (#25 or #26) and (breath* or respir* or inspir* or chest) AND CENTRAL:TARGET #28 (respir* or inspirat* or expiratory or ventilatory or chest) near4 (training or exercise* or endurance) AND CENTRAL:TARGET #29 threshold near3 (load or device*) AND CENTRAL:TARGET #30 "resistive breathing" AND CENTRAL:TARGET #31 #22 or #23 or #24 or #27 or #28 or #29 or #30 AND CENTRAL:TARGET #32 #21 and #31 AND CENTRAL:TARGET
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to November 16, 2018> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (471524) 2 controlled clinical trial.pt. (92755) 3 randomized.ab. (426757) 4 placebo.ab. (193277) 5 drug therapy.fs. (2062820) 6 randomly.ab. (300483) 7 trial.ab. (444977) 8 groups.ab. (1852440) 9 or/1‐8 (4320215) 10 exp animals/ not humans.sh. (4515931) 11 9 not 10 (3734956) 12 exp Glycogen Storage Disease/ (5824) 13 metabolic diseases/ (12474) 14 Muscular Diseases/ (23945) 15 (12 or 13) and 14 (598) 16 exp Muscular Dystrophies/ (24950) 17 ((metabolic or congenital) adj2 myopath$).mp. (2135) 18 inflammatory myopath$.mp. (2700) 19 Muscular Diseases/ci, cn, ge [Chemically Induced, Congenital, Genetics] (5621) 20 exp Muscular Atrophy, Spinal/ (4461) 21 exp motor neuron disease/ (25062) 22 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (8349) 23 amyotrophic lateral sclerosis.tw. (20242) 24 (poliomyelitis or muscular dystroph$ or myotonic dystroph$ or myasthen$ or myelopath$).mp. (86809) 25 (dystrophy adj3 (becker or duchenne or limb girdle or emery dreifuss or facioscapulohumeral)).tw. (12698) 26 Peripheral Nervous System Diseases/ (21886) 27 (neuropathy or neuropathies or polyneuropathy or polyneuropathies).tw. (76820) 28 (neuromuscular disease$1 or neuromuscular weakness or respiratory insufficiency).mp. (47134) 29 or/15‐28 (251575) 30 exp Breathing Exercises/ (3282) 31 ((respir$ or inspirat$) adj3 (training or exercise$1)).mp. (3163) 32 chest physiotherapy.mp. (788) 33 physical therapy technique$1.mp. (98) 34 Physical Therapy Modalities/ (34303) 35 (33 or 34) and (breath$3 or respir$5 or inspir$5 or chest).mp. (2070) 36 ((respir$ or inspirat$ or expiratory or ventilatory or chest) adj4 (training or exercise$1 or endurance)).mp. (6997) 37 (threshold adj3 (load or device$)).mp. (779) 38 resistive breathing.mp. (121) 39 or/30‐32,35‐38 (12486) 40 11 and 29 and 39 (167) 41 remove duplicates from 40 (166)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1974 to 2018 November 16> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (57280) 2 double‐blind procedure.sh. (155259) 3 single‐blind procedure.sh. (33057) 4 randomized controlled trial.sh. (523119) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1570865) 6 trial.ti. (256140) 7 controlled clinical trial/ (458477) 8 or/1‐7 (1879186) 9 exp animal/ or exp invertebrate/ or animal.hw. or non human/ or nonhuman/ (25109133) 10 human/ or human cell/ or human tissue/ or normal human/ (19104097) 11 9 not 10 (6058846) 12 8 not 11 (1670365) 13 limit 12 to (conference abstracts or embase) (1417329) 14 exp glycogen storage disease/ (8775) 15 metabolic disorder/ (58220) 16 (14 or 15) and muscle disease/ (642) 17 exp muscular dystrophy/ (39870) 18 ((metabolic or congenital) adj2 myopath$).mp. (2778) 19 (inflammatory myopath* or myositis).mp. (21670) 20 muscle disease/cn, et [Congenital Disorder, Etiology] (1921) 21 exp spinal muscular atrophy/ (47616) 22 motor neuron disease/ or amyotrophic lateral sclerosis/ (39752) 23 (moto$1 neuron$1 disease$1 or moto?neuron$1 disease$1).mp. (13004) 24 amyotrophic lateral sclerosis.tw. (27080) 25 (poliomyelitis or muscular dystroph$ or myotonic dystroph$ or myasthen$ or myelopath$).mp. (110713) 26 (dystrophy adj3 (becker or Duchenne or limb girdle or emery dreifuss or facioscapulohumeral)).tw. (17382) 27 peripheral neuropathy/ (41267) 28 (neuropathy or neuropathies or polyneuropathy or polyneuropathies).tw. (110326) 29 (neuromuscular disease$1 or neuromuscular weakness or respiratory insufficiency).mp. (28912) 30 or/16‐29 (325726) 31 breathing exercise$1.mp. (6810) 32 ((respir$ or inspirat$) adj3 (training or exercise$1)).mp. (4480) 33 chest physiotherapy.mp. (1359) 34 exp physiotherapy/ (76380) 35 physical therapy.mp. (25520) 36 (34 or 35) and (breath$3 or respir$5 or inspir$5 or chest).mp. (7152) 37 ((respir$ or inspirat$ or expiratory or ventilatory or chest) adj4 (training or exercise$1 or endurance)).mp. (9732) 38 (threshold adj3 (load or device$)).mp. (1009) 39 resistive breathing.mp. (174) 40 or/31‐33,36‐39 (22587) 41 13 and 30 and 40 (118) 42 remove duplicates from 41 (117)
Appendix 5. Clinical trials registries search strategies
US National Institutes for Health Clinical Trials Registry, ClinicalTrials.gov (www.clinicaltrials.gov/), and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
| Database or resource | Strategy |
| Clinicaltrials.gov |
[Advanced Search] CONDITION: Neuromuscular Diseases STUDY TYPE: Interventional/Clinical Trial INTERVENTION: Exercise OR Training |
| ICTRP |
[Basic Search] Neuromuscular Diseases AND Exercise OR Neuromuscular Diseases AND Training |
Data and analyses
Comparison 1. Respiratory muscle training versus sham training in amyotrophic lateral sclerosis (ALS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FVC (% of predicted): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Change in FVC (% of predicted): medium term | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3 Change in MIP (% of predicted): medium term | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4 Change in SNIP (% of predicted): medium term | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5 MEP (cmH₂O): short term | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 20.24 [6.58, 33.90] |
| 6 Change in MEP (% of predicted): medium term | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7 Change in ALSFRS‐R: short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Change in ALSFRS: medium term | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9 Change in EuroQol‐5D: medium term | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only |
Comparison 2. Respiratory muscle training versus sham training in Duchenne muscular dystrophy (DMD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐intervention TLC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Post‐intervention FVC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Post‐intervention FRC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Post‐intervention VC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Post‐intervention FEV₁ (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 MIP (cmH₂O): short term | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 2.84 [‐1.47, 7.15] |
Comparison 3. Respiratory muscle training versus no training in Duchenne muscular dystrophy (DMD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐intervention VC (L): medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Post‐intervention VC (% of predicted): medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Post‐intervention FEV₁ (L): medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Post‐intervention Pesmax (cmH₂O): medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Post‐intervention Pdimax (cmH₂O): medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 4. Respiratory muscle training versus breathing exercises in muscular dystrophies (Becker and limb‐girdle).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FVC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Change in VC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Change in FEV₁ (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Change in MIP (cmH₂O): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Change in MEP (cmH₂O): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 5. Respiratory muscle training versus breathing exercises in myasthenia gravis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐intervention TLC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 FVC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Post‐intervention RV (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Post‐intervention IC (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 FEV₁ (L): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Change in MEP (cmH₂O): short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fregonezi 2005.
| Methods | A single‐blind (assessors) RCT, which took place in Hospital de la Santa Creu i Sant Pau, Spain | |
| Participants | Participants had stable generalized myasthenia gravis (subclass IIa and IIb) according to the Classification of Osserman and Genkins (Osserman 1971). 29 people were recruited. 2 withdrew during the preprogram training period: 1 due to a myasthenic crisis and 1 due to associated disease (lung tumor). The remaining 27 participants were randomized into two groups Inclusion criteria Age ≤ 75 years; 60% of MIP, not surpassing the maximum value of valve resistance (41 cmH₂O); stable respiratory and neurologic clinical condition without myasthenic crises in the last 2 months; and no other significant diseases that could inhibit completion of training Control group N = 13 M/F = 6/7 Age (mean ± SD) = 61 ± 12 yrs Severity of myasthenia gravis, 6 subclass IIa, 8 subclass IIb Training group N = 14 M/F = 5/9 Age (mean ± SD) = 67 ± 10 yrs Severity of myasthenia gravis, 7 subclass IIa, 6 subclass IIb There were no statistical differences in baseline values between groups |
|
| Interventions | Participants completed a preprogram training period of 3 days to introduce interval‐based training prior to the 8‐week intervention Training group 45‐min session 3 times per week, consisting of 10 min of diaphragmatic breathing, followed by 10 min interval‐based IMT and 10 min of pursed lip breathing. All participants began with an initial load of 20% of MIP values, increased to 30% in week 3, 45% in week 5, and 60% in week 7. In the 1st, 2nd, and 3rd weeks, training consisted of 5 series of 2 min. In the 4th, 5th, and 6th weeks, training consisted of 4 series of 2, 3, 3, and 2 min. In the 7th and 8th weeks, training consisted of 3 series of 3, 4, and 3 min. The series were separated by 2 min recovery intervals Control group 1 breathing retraining (diaphragmatic breathing and pursed lip breathing) session and education about energy conservation at the 1st visit. Participants were encouraged to use these techniques and contact the team when necessary. |
|
| Outcomes | Pulmonary function (included FVC and FEV₁ indexes), respiratory pattern, MIP and MEP, thoracic mobility and SF‐36 questionnaire The outcomes were reported at the beginning and after 8 weeks of the intervention |
|
| Funding | Dr. Fregonezi was a doctoral fellow supported by CNPq‐ Brazil (process:200005/01– 4) | |
| Conflicts of interest | Information not available | |
| Notes | Author written to for further details. Reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "In this prospective randomized trial, participants were allocated to either a control group or a training group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "The technicians who collected data for outcome measures were blinded to a patient's allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "Two subjects withdrew during the preprogram training period, one due to a myasthenia crisis and the other due to associated disease (lung tumor)" |
| Selective reporting (reporting bias) | High risk | Report did not provide numerical outcome data for MIP and SF‐36 and we were unable to extract SD for lung function, therefore, these outcomes could not be meta‐analysed |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Martin 1986.
| Methods | A cross‐over trial which took place in Australia | |
| Participants | 18 boys with DMD were selected. The diagnoses were made from the typical clinical presentations and features, raised creatinine phosphokinase, electromyograms and muscle biopsy. 17 boys were in wheelchairs and 1 was still ambulant. The mean age was 14.2 yrs (range 7 to 20) and mean VC was 47.7% predicted (range 9 to 82). All attended a center for physically handicapped children and were under close supervision 5 days a week. 1 participant had very poor function at the initial period of the study and he died during his training period from respiratory failure Group A Participants performed respiratory muscle strength and endurance training exercises for two months, rested for two months and were observed for a further two‐month period without specific training N = 8 Group B Boys were not trained for the first two months, rested for the second two months and performed respiratory muscle strength and endurance training exercises during the last two months N = 9 |
|
| Interventions |
Training group Respiratory muscle strength For 5 days each week during the training period, maximum static inspiratory and expiratory manoeuvres at approximately 20% intervals over the VC range were performed. The boys trained for about 30 minutes per day Endurance training For 5 days each week during the training periods, the boys ventilated to exhaustion 3 times with recovery intervals. A simple acrylic mouthpiece was used, into which 4 different resistances could be fitted. Each resistance consisted of a hollow acrylic tube 6‐7 cm in length with central bore diameters of 1/4 inch, 3/16 inch, 1/8 inch and 3/32 inch. The initial resistances selected were those that led to exhaustion within 3 minutes. When each subject was able to ventilate without exhaustion through a resistance for 3 minutes or longer, the resistance was increased Control group No training |
|
| Outcomes | VC, MIP, MEP and maximal time that each MIP and MEP could be held for | |
| Funding | Information not available | |
| Conflicts of interest | Information not available | |
| Notes | Trial author contacted for further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "The boys were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "Patient 5 had very poor function at the initial period of the study and he died during his training period from respiratory failure" |
| Selective reporting (reporting bias) | High risk | Data not provided for each study period, therefore, the outcomes could not be meta‐analysed |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Pinto 2012.
| Methods | A double‐blind (assessors and participants) RCT which took place in Portugal | |
| Participants | 26 participants with definite (4 participants) or probable (22 participants) ALS, as defined by the revised El Escorial criteria, recruited immediately after diagnosis Disease onset: bulbar 4 participants, spinal in 22 participants (10 in upper limbs and 12 in lower limbs) Inclusion criteria Age at disease onset between 18 and 75 years; disease duration < 24 months at entry; ALSFRS > 24/40 at entry; and informed consent Exclusion criteria Familial ALS, pregnant women and people with additional problems (e.g. diabetes, lung disorders, frontal dementia, perioral weakness preventing adequate lip sealing, and upper limb weakness sufficient to prevent respiratory training), people with FVC < 70% or MIP and MEP < 50% of the predicted value, and ulnar lesion or absent ulnar nerve motor response 1 person in each group dropped out less than 2 months after study entry: 1 with rapidly progressive spasticity (active treatment group); the other (sham IMT group) was lost to follow‐up. 24 participants finished the first 4 months of the study and were included in the statistical analysis Group 1 (active IMT) N = 13 M/F = 7/6 Age (mean ± SD) = 57.14 ± 9.3 yrs ALSFRS (mean ± SD) = 34.39 ± 3.64 Group 2 (sham IMT) N = 13 M/F = 11/2 Age (mean ± SD) = 56.8 ± 8.7 yrs ALSFRS (mean ± SD) = 33.5 ± 3.8 There was no significant difference between the two groups at entry |
|
| Interventions | Active or sham IMT twice daily for a 10‐min period Training group (group 1) Active IMT protocol for 8 months Control group (group 2) Sham training for the first 4 months, followed by an identical active IMT protocol for 4 months In the active exercise period, both in group 1 and group 2, the threshold load was set to 30% to 40% of the MIP. The sham‐period exercise (first 4 months for participants in group 2) consisted of breathing through the respiratory device with the lowest possible load, i.e. 9 cmH₂O |
|
| Outcomes | Participants were evaluated 3 times: at entry (T0), end of the 1st 4‐month period (T1), and at the end (T2) of the study (8 months) Primary outcome: ALSFRS Secondary outcomes: respiratory function tests (sitting and supine FVC, MIP and MEP, PEF, MVV, SNIP), P0.1, nocturnal pulse oximetry, diaphragmatic motor responses by percutaneous bipolar phrenic stimulation, Neurophysiological Index, dyspnoea by a visual analogue scale, Fatigue Severity Scale, Borg scale, Hamilton Rating Scale for Depression, Epworth Daytime Sleepiness Scale, Functional Independent Measure, quality of life (EuroQol‐5D) and participants were assessed clinically and asked to say whether they felt better, worse or unchanged |
|
| Funding | The 1st author (SP) had a grant from the “Fundação para a Ciência e a Tecnologia”, SFRH/BD/30714/2006 This work was supported by “Fundação para a Ciência e Tecnologia” ‐PIC/IC/82765/2007 | |
| Conflicts of interest | The authors had no conflicts of interest | |
| Notes | Trial author contacted for further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): "all subjects were independently randomized, in blocks of six, into two treatment groups" |
| Allocation concealment (selection bias) | Low risk | The randomization was done by an external independent randomizer. At the time of each inclusion, the envelope with the number of inclusion was opened and the allocated group study was known by one of the authors, SP, who trained the participants (details from correspondence with authors) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from report): "They were all informed that the trial was devised to determine the best respiratory training protocol. Those included in group 2 were not aware of the placebo training during the first half of this study, as
the device was set for them at the minimum load value" Quote (from correspondence): "the patients were scientifically blinded to which group was 'the best'" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): the person "who performed the functional evaluation and the phrenic nerve studies, was blinded to the allocated study group" Quote (from trial report): "The ALSFRS was applied by a blinded evaluator (MdeC) to derive the total score" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "One patient in each group dropped out less than two months after study entry: one (group 1) had rapidly progressive spasticity; the other (group 2) was lost to follow‐up" |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Plowman 2019.
| Methods | Double‐blind (participants and assessors) randomized sham‐controlled trial | |
| Participants | 52 individuals with a diagnosis of probable or definite ALS based on Revised El‐Escorial criteria were screened for potential enrollment in this study. The diagnosis was confirmed in all participants by academic neuromuscular neurologists specializing in ALS. 48 individuals met the inclusion criteria and were enrolled in this study. Over the 2‐month study period, 2 participants dropped out (1 from each group). One was due to a diagnosis of cancer and the need to undergo radiation therapy and chemotherapy, and another participant no longer wished to participate Inclusion criteria Confirmed ALS diagnosis; FVC > 65% predicted; ALSFRS‐R score > 30; adequate cognition to follow simple commands as evidenced by a score of > 24 points on the Mini‐Mental Status Examination; no allergies to barium; no tracheostomy or mechanical ventilation; and no diaphragmatic pacer Sham group N = 24 M/F = 12/12 Age (mean ± SD) = 60.1 ± 10.3 yrs Training group N = 24 M/F = 17/7 Age (mean ± SD) = 63.1 ± 10.0 yrs Participants' characteristics were well balanced across groups with no statistically significant baseline differences across treatment groups for any of the demographic variables |
|
| Interventions | Participants completed 8 weeks of daily sessions at home on 5 days of the participants choosing per week. A single daily training session consisted of 25 targeted forced exhalations through the trainer, performed in 5 sets of 5 repetitions Training group Training was performed with the trainer set at 50% of the participant's individual MEP Sham group Internal spring was removed from the device. Therefore, these patients performed exercises against no physiologic load |
|
| Outcomes |
Primary outcome MEP (cmH₂O) Secondary outcomes Include: kinematic and temporal swallowing indices; cough spirometry measures; and the Penetration Aspiration Scale score (an index of airway safety during swallowing); patient‐reported measures using validated scales for: swallow‐related quality of life (SWAL‐QOL); dysphagia severity (Eating Assessment Tool; EAT‐10); and functional oral intake (Functional Oral Intake Scale; FOIS); FVC; impact of expiratory muscle strength training on disease progression over time via the ALSFRS‐R |
|
| Funding | This study was funded by a grant from the National Institutes of Child Health and Development (1R21 HDO75327) | |
| Conflicts of interest | The authors had no conflicts of interest | |
| Notes | Author written to for further details. Reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): "Participants were assigned to the EMST or control (sham EMST) group using a permuted block randomization schedule" |
| Allocation concealment (selection bias) | Low risk | Concealed until intervention assigned (from correspondence) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from report): "Participants and the clinical researchers performing and interpreting evaluations were blinded to group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "Participants and the clinical researchers performing and interpreting evaluations were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no exclusion after randomization, since the authors described their analysis as an intention‐to‐treat analysis (from correspondence) |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes that are of interest in the review |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Rodillo 1989.
| Methods | A double‐blind (participants and assessors) cross‐over trial which took place in the UK | |
| Participants | Twenty‐two boys with DMD aged 9‐14 yrs (mean 11.6 yrs) were recruited from two special schools. Of the 22 children recruited to the study two were withdrawn because of illness during the study period Group 1 Children started with 'placebo' training and crossed over to the training during the second period N = 11 Group 2 Children performed the training in the reverse order N = 9 |
|
| Interventions |
Training period Participants used an inspirometer device that entailed forced inspiration against a resistance that increased as inspiratory flow increased to a total of 20 inspirations/day 'Placebo' period The 'placebo' training was performed by a mini peak flow meter to a total of 10 expirations/day. Both training and 'placebo' were administered by the school physiotherapists After 18 days using either inspirometer or placebo, each child 'crossed over' to the other form of treatment for a further 18 days |
|
| Outcomes | FVC, FEV1 and PEFR in sitting, supine, and sitting position, and MIP | |
| Funding | Study was supported by a center grant from the Muscular Dystrophy Group of Great Britain | |
| Conflicts of interest | Information not available | |
| Notes | Trial author contacted for further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "Twenty‐two boys with Duchenne muscular dystrophy were entered into a randomised double‐blind crossover trial" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from report): "Twenty‐two boys with Duchenne muscular dystrophy were entered into a randomised double‐blind crossover trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "Lung function was assessed initially and at the end of each training period by two of the authors (ER, FM) who were unaware of the nature of the randomisation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "Of the 22 children recruited to the study two were withdrawn because of illness during the study period" |
| Selective reporting (reporting bias) | High risk | We were unable to extract SD for all outcomes, therefore, we included post‐intervention values in the meta‐analysis |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Smith 1988.
| Methods | A single blind cross‐over trial which took place in the UK | |
| Participants | 8 participants with DMD, mean age 12.3 yrs (range 8 to 16) and mean VC 1.8 L (range 1.4 to 3.2) were studied. After 3 baseline assessments of ventilatory capacity, the subjects were reassessed after 5 weeks training Group 1 The participants trained for the first 5‐week period N = 4 Group 2 The participants trained for the second 5‐week period N = 4 |
|
| Interventions | The training was performed twice daily. The inspiratory resistance was varied to give a subjectively heavy but tolerable load for 10 to 15 minutes | |
| Outcomes | Total expiratory volumes, VC and MIP | |
| Funding | Information not available | |
| Conflicts of interest | Information not available | |
| Notes | Trial author contacted for further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "the subjects were randomized to two groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "Blind assessment of ventilatory capacity was made after each period" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | High risk | Report did not provide numerical data, therefore, the outcomes could not be meta‐analysed |
| Other bias | Unclear risk | Insufficient information to assess whether a significant risk of other bias |
Stern 1989.
| Methods | A single‐blind (assessors), cross‐over trial which took place in Australia | |
| Participants | 18 participants were enrolled in the trial following informed consent. The diagnosis of DMD had been confirmed clinically and by muscle biopsy. Their ages ranged from 10.4 to 23.4 yrs (mean 15 yrs). 16 were in wheelchairs and 2 were ambulant. 4 died during the trial due to respiratory failure with superimposed acute infections. Two boys moved to another state and did not complete the trial, and a third was moderately retarded and could not perform the endurance test
Group A
Participants performed IMT for the 1st six months of the trial N = 7 Group B Participants performed IMT for the 2nd six months of the trial N = 5 |
|
| Interventions | To exercise the respiratory muscles, the participants were required to inhale through a mask while playing a video game, which was connected to a microcomputer system. The mask was connected to valves to separate inhaled and exhaled air. A range of restriction holes was fitted to the intake tube (6.35, 4.76, 3.18 and 2.38 mm). To maintain control of the game, the boys were required to inhale so that the work done by their respiratory muscles exceeded a preset threshold value. The training consisted of 20‐min sessions, 5 days a week, with the participants choosing the computer game they wanted to play. Inspiratory effort was increased by their having to breathe through a mask to both start and continue the games. It was proportional to the transpulmonary pressure, and for a given restriction size was proportional to airflow through the restriction; hence measurement of airflow was related to the power developed by the inspiratory muscles. The aim was an increase of 10 beats per minute, equivalent to 11 W to 12 W of work. If little or no change in pulse rate occurred over a few sessions, the resistance was increased. | |
| Outcomes | FVC, MIP, MEP, ventilatory muscle endurance. Trialists measured outcomes at baseline, after each 6‐month intervention period and at 18 months. Results for training and no training from each group were pooled for analysis, i.e. not presented separately. | |
| Funding | Information not available | |
| Conflicts of interest | Information not available | |
| Notes | Trial author contacted for further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "The boys were randomly divided into two groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "The technician who performed the tests did not know to which group the boys belonged" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (from report): "Four participants died during the trial because of respiratory failure with superimposed acute infections. Two boys moved to another state and did not complete the trial, and a third was moderately retarded and could not perform the endurance test. These participants were excluded from analysis" |
| Selective reporting (reporting bias) | High risk | Data not provided for each study period, therefore, the outcomes could not be meta‐analysed |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Suleman 2003.
| Methods | RCT which took place in the UK | |
| Participants | 14 people with motor neuron disease were randomized into 2 groups: Active training group N = 7 Placebo (sham) group N = 7 |
|
| Interventions | 2‐month intervention. Both groups trained at home for 15 min twice a day. Active training group Trained by exhaling against resistance of approximately 90% of their MEP Placebo (sham) group Trained against negligible inspiratory resistance |
|
| Outcomes | Pulmonary function (including FVC and FEV₁), MIP, MEP, peak cough expiratory flow rate The outcomes were measured at baseline and after 2 months of training |
|
| Funding | Information not available | |
| Conflicts of interest | Information not available | |
| Notes | Published as an abstract Author contacted for further details. Reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "Seven patients were randomized to expiratory muscle training and seven patients to sham training” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | High risk | Data not available for lung function and MIP, therefore cannot be meta‐analysed |
| Other bias | Unclear risk | Insufficient information to assess whether a significant risk of bias exists |
Topin 2002.
| Methods | Double‐blind (assessors and participants) RCT which took place in France | |
| Participants | 16 male participants with DMD, confirmed from clinical, enzymatic and muscle biopsy criteria All participants were wheelchair dependent, clinically stable at the time of evaluation, free of any medication, free from respiratory tract infection and had no history of acute respiratory failure requiring endotracheal ventilation. None had symptoms or signs of inspiratory muscle fatigue Training group N = 8 Age (mean ± SD) = 14.7 ± 4.5 yrs No statistically significant differences were found in the two groups at baseline on mean age, anthropometric characteristics, or spirometric values Control group N = 8 Age (mean ± SD) = 12.6 ± 1.8 yrs |
|
| Interventions | 6‐week intervention. Participants were studied for 2 years Both groups had to breathe through a valve for 10 min twice daily. Training group In the training group, the threshold pressure load was equal to 30% of their individual MIP. Control (sham) group In the control group, the threshold pressure was set at 5% |
|
| Outcomes | Pulmonary function, breathing pattern, MIP and inspiratory muscle endurance Baseline measurements performed in the week prior to starting the training program and at the end of the training period |
|
| Funding | This investigation was supported by the 'Association Française contre les Myopathies' (AFM), (grant no. 5395) | |
| Conflicts of interest | Information not available | |
| Notes | Author contacted for further details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (from report): "double‐blind, placebo‐controlled study" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from report): "This study was double‐blinded in that neither the physician who performed the tests nor the children were aware of the nature of the randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "This study was double‐blinded in that neither the physician who performed the tests nor the children were aware of the nature of the randomization" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "All children who were included in the study finished it" |
| Selective reporting (reporting bias) | High risk | Mean difference data for pulmonary function were not presented and we were unable to extract SD, therefore, we included post‐intervention values in the meta‐analysis |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Wanke 1994.
| Methods | Single‐blind (assessor‐blinded) RCT which took place in Austria | |
| Participants | 30 participants with DMD Inclusion criterion Diagnosis of DMD on clinical, enzymatic, electromyographic, and muscle biopsy specimen criteria Exclusion criteria Respiratory tract infections, signs of inspiratory muscle fatigue and sleep disturbance In the training group, 12 participants were wheelchair dependent, and in the control group, 11 participants were wheelchair dependent, corresponding to functional capacity stage 9, according to the criteria of Inkley 1974. No participants had symptoms or signs of inspiratory muscle fatigue Control group N = 15 Age (mean ± SD) = 14.5 ± 3.8 yrs Training group N = 15 Age (mean ± SD) = 13.6 ± 4.5 yrs At the beginning of the study there were no significant differences between the 2 groups |
|
| Interventions | The trial had a 3‐month introductory period, a 6‐month intervention and a 6‐month post‐intervention phase Training group Participants had to perform both resistive breathing manoeuvres and maximal static inspiratory efforts against the almost occluded resistance. The inspiratory resistive breathing training consisted of 10 correctly completed breathing cycles of 1 min duration each, twice daily. Fifteen minutes after the resistive breathing training, the participants had to perform 10 maximal static inspiratory efforts and reach a certain minimal pressure value. The mean of the 5 highest pressure values during 10 maximal static inspiratory efforts against the almost occluded airway was used as the minimal pressure value which had to be reached during such efforts at home. The level of resistance was readjusted each month, if the participants could achieve higher Pdimax values or sustain more than 15 resistive breathing cycles Control group No training |
|
| Outcomes | Pulmonary function (included VC and FEV₁), blood gas analysis, maximal sniff assessed esophageal (Pesmax), transdiaphragmatic pressure (Pdimax) and inspiratory muscle endurance The outcomes were assessed 3 months before training, at the beginning of training, in the first and third month of training, at the end, and 6 months after its cessation |
|
| Funding | The trial was not funded | |
| Conflicts of interest | The authors had no conflicts of interest | |
| Notes | Author contacted for further details. Reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from report): “Before entering the study, they were randomly assigned to receive or not receive inspiratory muscle training” Computer generated (from correspondence) |
| Allocation concealment (selection bias) | Low risk | Numbered containers (from correspondence) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The personnel was not aware of the allocated study group. The participants did not know the aim of the study (from correspondence) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blind to the allocated intervention (from correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "Five of the 15 training patients and 3 of the 15 patients in control group showed VC values less than 25 percent of predicted and/or a PaCO2 of more than 45 mmHg. Inspiratory muscle function parameters of these 8 were therefore analyzed separately" There was no exclusion. Dr Wanke described their analysis as an intention‐to‐treat analysis (from correspondence) |
| Selective reporting (reporting bias) | High risk | Quote (from report): "Pulmonary and inspiratory muscle function parameters were assessed 3 months before and at the beginning of training, in the first and third month of training, at the end, and 6 months after its cessation" Comment: numerical data for all outcomes 1st and 3rd month of training were not presented, therefore, cannot be meta‐analysed |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
Yeldan 2008.
| Methods | A single‐blind (assessor‐blinded) quasi‐RCT which took place in Turkey | |
| Participants | 23 participants with muscular dystrophy (17 people with limb‐girdle muscular dystrophy and 6 with Becker muscular dystrophy) were included. The diagnosis was made by the neurologist who referred the patients, using diagnostic criteria defined by Emery 1994 2 participants (one from each group) dropped out because of difficulty in transportation and loss of ambulation. Thus, 21 participants completed the study Inclusion criteria
Threshold IMT group N = 11 M/F = 7/3 Age (mean ± SD) = 22.50 ± 7.50 years Breathing exercise group N = 12 M/F = 6/5 Age (mean ± SD) = 24.27 ± 9.40 Participant characteristics of the 2 groups were similar and there was no significant difference between the baseline values of mouth pressure and spirometry |
|
| Interventions |
Threshold IMT In the IMT group, the initial training load was 15% of the participant's baseline MIP at first week. In the following weeks, the threshold pressure load was equalized to 30% of weekly MIP measurements. Breathing exercise In the breathing exercise group, a physical therapist instructed participants to perform diaphragmatic and segmental exercises. The participants were encouraged to do deep inspiration and full expiration during all breathing exercises. Participants performed daily IMT or breathing exercise sessions for 15 min, twice a day for 5 days per week at home |
|
| Outcomes | Pulmonary function (included VC, FVC and FEV₁), MIP and MEP The outcomes were evaluated at baseline and after 12 weeks |
|
| Funding | This study was partially supported by Istanbul University Research Foundation (Project T‐888/17072000) | |
| Conflicts of interest | Information not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote (from report): "The first patient is allocated to the threshold inspiratory muscle training group and the next one is allocated to the breathing exercise group. With the others, this pattern is preserved" |
| Allocation concealment (selection bias) | High risk | Quote (from report): "patients were allocated to either threshold inspiratory muscle training or breathing exercise group alternately according to their arrival order in the hospital" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote (from report): "Both exercise programmes were supervised by the same trainer who was blind to initial and final assessments" Comments: there is insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "groups were evaluated (...) by the same examiner who was blind to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (from report): "Two subjects (one from each group) dropped the study because of difficulty in transportation and loss of ambulation" |
| Selective reporting (reporting bias) | Low risk | Data reported for all outcomes |
| Other bias | Low risk | The trial appears to be free of other sources of bias |
ALS: amyotrophic lateral sclerosis ALSFRS: Amyotrophic Lateral Sclerosis Functional Rating Scale ALSFRS‐R: Amyotrophic Lateral Sclerosis Functional Rating Scale‐Revised DMD: Duchenne muscular dystrophy FEV₁: forced expiratory volume in one second FVC: forced vital capacity IMT: inspiratory muscle training MEP: maximal expiratory pressure MIP: maximal inspiratory pressure MVV: maximal voluntary ventilation P0.1: inspiratory pressure 100 ms into an occluded inspiratory effort PEF: peak expiratory flow PEFR: peak expiratory flow rate RCT: randomized controlled trial SD: standard deviation SF‐36: 36‐Item Short Form Health Survey SNIP: sniff nasal inspiratory pressure VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 1998 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT |
| Aslan 2014 | Study evaluated more than one type of NMD (7 myotonic dystrophy, 6 myopathy, 4 oculopharyngodistal myopathy, 1 desminopathy, 1 multiminicore disease, 1 facioscapulohumeral dystrophy, 1 congenital myopathy, 2 limb‐girdle muscular dystrophy, 1 SMA type III) Three participants were receiving non‐invasive mechanical ventilation. We were not able to obtain results for each condition separately. |
| Cheah 2009 | One participant in the training group needed non‐invasive mechanical ventilation |
| DiMarco 1985 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT |
| Estrup 1986 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT Study evaluated more than one type of NMD (progressive muscular dystrophia and SMA) |
| Fregonezi 2010 | Study following an A‐B‐A design, phase A control time, phase B training time and phase A control time. There was no parallel group allocating each participant to a single intervention for comparison with an alternative intervention |
| Gozal 1999 | Study evaluated participants with more than one type of NMD (21 children with DMD or SMA). We are not able to obtain results for each condition separately |
| Gross 1993 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT. The participants were divided into three major groups according to their type of NMD |
| Koessler 2001 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT. Study evaluated participants with more than one type of NMD (18 with DMD and 9 with SMA) Participants were divided into 3 groups according to their vital capacity values |
| Litchke 2008 | Study evaluated participants with more than one type of neurological disorder (8 with spinal cord injury, 1 with a neurological disorder, and 1 with postpolio syndrome) |
| Núñez 2014 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT Study evaluated participants with more than one type of NMD (7 with DMD, 3 with SMA, 2 with myelomeningocele, 1 with facioscapulohumeral, 1 with BMD, 1 with Bethlem myopathy, 1 with congenital myopathy, 1 with Charcot‐Marie‐Tooth disease, and 1 with Guillain‐Barré syndrome |
| Plowman 2016 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT |
| Rassler 2007 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT |
| Rassler 2011 | Pre‐and post‐intervention study, not a RCT or quasi‐RCT |
| Raßler 2014 | Not a RCT or quasi‐RCT. The participants chose the group to which they would be assigned (information obtained by email) |
| Weiner 1998 | Not a RCT or quasi‐RCT. The participants with myasthenia gravis were divided into 2 groups: 10 participants that were mildly and moderately affected and received both IMT and EMT, and 8 patients with severe disease received IMT only |
| Zupan 2002 | Study evaluated more than one type of NMD (12 DMD, 11 SMA type II, 6 SMA type III) We were not able to obtain results for each condition separately |
BMD: Becker muscular dystrophy DMD: Duchenne muscular dystrophy EMT: expiratory muscle training IMT: inspiratory muscle training NMD: neuromuscular disease RCT: randomized controlled trial SMA: spinal muscular atrophy
Characteristics of ongoing studies [ordered by study ID]
NCT02710110.
| Trial name or title | Respiratory strength training in persons with amyotrophic lateral sclerosis (ALS) |
| Methods | Randomized, parallel‐assignment, single‐blind intervention trial |
| Participants | Estimated enrollment: 50 Adults 21 to 85 years old, either sex Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Respiratory training Active respiratory training (PowerLung trainer): single daily training session of 3 sets of 10 repetitions for a total of 30 inspiratory repetitions and 30 expiratory repetitions (i.e. 60 repetitions) for 5 days a week, for 3 months Sham training Same device without resistance, to the same schedule |
| Outcomes |
Primary outcome measures (change from baseline to month 3): Maximum expiratory pressure (highest of 3 trials) Maximum inspiratory pressure (highest of 3 trials) Pulmonary function: forced vital capacity (FVC) expressed as a percentage of predicted values Sniff nasal inspiratory pressure Pulmonary function: peak expiratory flow (PEF) expressed as a percentage of predicted values. Pulmonary function: forced expiratory volume (FEV₁) expressed as a percentage of predicted values Secondary outcome measures (change from baseline to month 3): Swallowing function: the penetration‐aspiration scale Lingual strength: peak performance using the Iowa Oral Pressure Instrument device (performed 3 times) and maximal lingual endurance time Lingual endurance: peak performance using the Iowa Oral Pressure Instrument device (placement of the bulb of the tongue on the roof of the mouth, maintained as long as the participant can at a threshold of 20% of the maximum anterior isometric pressure. Voluntary and reflexive cough function assessed using an oral pneumotachograph; reflexive cough response to 0 μM, 50 μM, 100 μM, 200 μM, and 500 μM capsaicin. |
| Starting date | April 2016, estimated completion date March 2020 |
| Contact information | University of Florida: ltabor@ufl.edu raerobison1@ufl.edu |
| Notes | Other study ID: IRB201501172 |
Differences between protocol and review
In 'Type of participants' section, we replaced the sentence 'participants with respiratory insufficiency' with 'participants with acute respiratory failure'.
We added a definition of children (< 18 years old).
We reordered outcomes from the protocol (Pedrosa 2015), to make lung capacity the primary outcome.
We noted that chest infections would be included in the definition of acute exacerbation in secondary outcome 5.
We reported results for each condition separately, therefore there was no need for subgroup analyses by condition.
We noted in the Methods that when the trials did not report the change from baseline, we extracted final values for analysis; where meta‐analysis was not possible we reported available results narratively. Moreover, when an included trial did not report mean and standard deviation (SD) for each group, we would have used generic inverse variance to enter data in the analysis. In the 'Summary of findings table' section, we added the order of choice for the presentation of the lung capacity measures.
We explained our approach to 'other bias'.
Ricardo Guerra withdrew from authorship at the review stage.
Contributions of authors
| Task | Contributing authors |
| Draft the protocol | RP, ISS, GAFF, GMHF, IGA, Ricardo Guerra, METDJ, SRHL, AMF |
| Develop criteria for a search strategy (in conjunction with the Trials Search Co‐ordinator) | RP, ISS, GAFF, GMHF, IGA, METDJ, SRHL |
| Search identified titles and abstracts for trials (usually 2 review authors) | RP, IGA |
| Obtain copies of trials | RP, IGA, ISS |
| Select which trials to include (2 review authors + 1 arbiter) | RP, IGA, GMHF |
| Extract data from trials (2 review authors) | ISS, IGA |
| Enter data into RevMan (1 review author + 1 review author to check) | ISS, GMHF |
| Carry out the analysis | ISS, RP, GMHF, IGA, METDJ, SRHL |
| Create 'Summary of findings' table(s) | ISS, RP |
| Interpret the analysis | ISS, GMHF, RP, GAFF, IGA METDJ, SRHL |
| Draft the final review | ISS, RP, GAFF, GMHF, IGA, METDJ, SRHL, AMF |
| Update the review | ISS, RP, GAFF, GMHF, IGA, METDJ, SRHL, AMF |
| Provide a consumer perspective | AMF |
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
ISS: none known. RP: none known. IGA: none known. AMF: none known. GAFF: none known. He is the author of a trial included in this Cochrane Review (Fregonezi 2005). METDJ: none known. He has received lecture fees from Sociedade Brasileira de Neurofisiologia Clínica. SRHL: none known. GMHF: none known.
New
References
References to studies included in this review
Fregonezi 2005 {published data only}
- Fregonezi GA, Guell R, Pradas J, Casan P. Effects of inspiratory muscle training program in generalized myasthenia gravis. European Respiratory Journal 2003;22(Suppl 45):2729. [Google Scholar]
- Fregonezi GA, Resqueti VR, Güell R, Pradas J, Casan P. Effects of 8‐week, interval‐based inspiratory muscle training and breathing retraining in patients with generalized myasthenia gravis. Chest 2005;128(3):1524‐30. [PUBMED: 16162753] [DOI] [PubMed] [Google Scholar]
Martin 1986 {published data only}
- Martin AJ, Stern L, Yeates J, Lepp D, Little J. Respiratory muscle training in Duchenne muscular dystrophy. Developmental Medicine & Child Neurology 1986;28(3):314‐8. [DOI] [PubMed] [Google Scholar]
Pinto 2012 {published data only}
- Pinto S, Carvalho M. Respiratory exercises in amyotrophic lateral sclerosis (reals). Amyotrophic Lateral Sclerosis 2009;10:59. [EMBASE: 70078384] [Google Scholar]
- Pinto S, Swash M, Carvalho M. Respiratory exercise in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2012;13(1):33‐43. [PUBMED: 22214352] [DOI] [PubMed] [Google Scholar]
- Pinto S, Carvalho M. Can inspiratory muscle training increase survival in early‐affected amyotrophic lateral sclerosis patients?. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2013;14(2):124‐6. [PUBMED: 23035780] [DOI] [PubMed] [Google Scholar]
Plowman 2019 {published data only}
- Plowman E, Tabor L, Rosado MK, Robison R, Gaziano J, Richter J, et al. A randomized sham control trial of EMST on bulbar function. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2015;16(Suppl 1):44. [DOI: 10.3109/21678421.2015.1089039/0065] [DOI] [Google Scholar]
- Plowman EK, Rosado M, Tabor L, Turner K, Gaziano J, Richter J, et al. Impact of expiratory muscle strength training on bulbar function in amyotrophic lateral sclerosis: updates from a randomized sham‐controlled clinical trial. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 2014;15:206. [Google Scholar]
- Plowman EK, Tabor‐Gray L, Rosado KM, Vasilopoulos T, Robison R, Chapin JL, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: Results of a randomized, sham‐controlled trial. Muscle & Nerve 2019;59(1):40‐6. [DOI: 10.1002/mus.26292] [DOI] [PubMed] [Google Scholar]
Rodillo 1989 {published data only}
- Rodillo E, Noble‐Jamieson CM, Aber V, Heckmatt JZ, Muntoni F, Dubowitz V. Respiratory muscle training in Duchenne muscular dystrophy. Archives of Disease in Childhood 1989;64(5):736‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1988 {published data only}
- Smith PE, Coakley JH, Edwards RH. Respiratory muscle training in Duchenne muscular dystrophy. Muscle & Nerve 1988;11(7):784‐7. [DOI] [PubMed] [Google Scholar]
Stern 1989 {published data only}
- Stern LM, Martin AJ, Jones N, Garrett R, Yeates J. Training inspiratory resistance in Duchenne dystrophy using adapted computer games. Developmental Medicine & Child Neurology 1993;31(4):494‐500. [DOI] [PubMed] [Google Scholar]
Suleman 2003 {published data only}
- Suleman M, Whitely A, Kinnear W. Expiratory muscle training in motor neurone disease [Abstract]. Thorax 2003;58(Suppl 3):iii77‐8. [Google Scholar]
Topin 2002 {published data only}
- Topin N, Matecki S, Bris S, Rivier F, Echenne B, Prefaut C, et al. Dose‐dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscular Disorders 2002;12(6):576‐83. [PUBMED: 12117483] [DOI] [PubMed] [Google Scholar]
Wanke 1994 {published data only}
- Merkle M, Wanke TH, Formanek D, Lahrmann H, Toifl K, Zwick H. Inspiratory muscle training in patients with neuromuscular disease [abstract]. European Respiratory Journal 1993;6(Suppl 17):310S. [Google Scholar]
- Ungar D, Gössler R, Toifl K, Wanke T. Innovative respiratory muscle training for patients with Duchenne muscular dystrophy‐a psychological evaluation. Wiener Medizinische Wochenschrift 1996;146(9‐10):213‐6. [PUBMED: 9012219] [PubMed] [Google Scholar]
- Wanke T, Toifl K, Merkle M, Formanek D, Lahrmann H, Zwick H. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest 1994;105(2):475‐82. [PUBMED: 8306750] [DOI] [PubMed] [Google Scholar]
- Wild M, Wanke T, Lahrmann H, Merkle M, Toifl K, Zwick H. Training of the respiratory musculature in muscle dystrophy Duchenne. Atemwegs ‐ und Lungenkrankheiten 1995;21:478. [Google Scholar]
Yeldan 2008 {published data only}
- Yeldan I, Gurses HN, Yuksel H. Comparison study of chest physiotherapy home training programmes on respiratory functions in patients with muscular dystrophy. Clinical Rehabilitation 2008;22(8):741‐8. [PUBMED: 18678574] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 1998 {published data only}
- Abe K, Matsuo Y, Kadekawa J, Inoue S, Yanagihara T. Respiratory training for patients with myotonic dystrophy. Neurology 1998;51(2):641‐2. [DOI] [PubMed] [Google Scholar]
Aslan 2014 {published data only}
- Aslan GK, Gurses HN, Issever H, Kiyan E. Effects of respiratory muscle training on pulmonary functions in patients with slowly progressive neuromuscular disease: a randomized controlled trial. Clinical Rehabilitation 2014;28(6):573‐81. [DOI] [PubMed] [Google Scholar]
Cheah 2009 {published data only}
- Cheah B, Boland R, Brodaty N, Zoing M, Jeffery S, McKenzie D, et al. "Inspirational" ‐ Inspiratory muscle training in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 20th International Symposium on ALS/MND; 2009 Dec 8‐10; Berlin, Germany. 2009. [DOI] [PubMed]
- Cheah BC, Boland RA, Brodaty NE, Zoing MC, Jeffery SE, McKenzie DK, et al. INSPIRATIonAL ‐ INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 2009;10(5‐6):384‐92. [DOI] [PubMed] [Google Scholar]
DiMarco 1985 {published data only}
- DiMarco AF, Kelling JS, DiMarco MS, Jacobs I, Shields R, Altose MD. The effects of inspiratory resistive training on respiratory muscle function in patients with muscular dystrophy. Muscle & Nerve 1985;8(4):284‐90. [DOI] [PubMed] [Google Scholar]
Estrup 1986 {published data only}
- Estrup C, Lyager S, Noeraa N, Olsen C. Effect of respiratory muscle training in patients with neuromuscular diseases and in normals. Respiration 1986;50(1):36‐43. [DOI] [PubMed] [Google Scholar]
Fregonezi 2010 {published data only}
- Fregonezi GA, Araujo TL, Azevedo IG, Severino FG, Dias FA, Bruno SS, et al. Effects of respiratory muscle training on strength and heart rate variability in myotonic dystrophy patients. Chest 2010;138(4):920A. [Google Scholar]
Gozal 1999 {published data only}
- Gozal D, Thiriet P. Respiratory muscle training in neuromuscular disease: long‐term effects on strength and load perception. Medicine & Science in Sports & Exercise 1999;31(11):1522‐7. [DOI] [PubMed] [Google Scholar]
Gross 1993 {published data only}
- Gross D, Meiner Z. The effect of ventilatory muscle training on respiratory function and capacity in ambulatory and bed‐ridden patients with neuromuscular disease. Monaldi Archives for Chest Disease 1993;48(4):322‐6. [PubMed] [Google Scholar]
Koessler 2001 {published data only}
- Koessler W, Wanke T, Winkler G, Nader A, Toifl K, Kurz H, et al. 2 Years' experience with inspiratory muscle training in patients with neuromuscular disorders. Chest 2001;120(3):765‐9. [DOI] [PubMed] [Google Scholar]
Litchke 2008 {published data only}
- Litchke LG, Russian CJ, Lloyd LK, Schmidt EA, Price L, Walker JL. Effects of respiratory resistance training with a concurrent flow device on wheelchair athletes. The Journal of Spinal Cord Medicine 2008;31(1):65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Núñez 2014 {published data only}
- Núñez IR, Araos DZ, Delgado CM. Effects of home‐based respiratory muscle training in children and adolescents with chronic lung disease. Jornal Brasileiro de Pneumologia 2014;40(6):626‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plowman 2016 {published data only}
- Plowman EK, Watts SA, Tabor L, Robison R, Gaziano J, Domer AS, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle & Nerve 2016;54(1):48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rassler 2007 {published data only}
- Rassler B, Hallebach G, Kalischewski P, Baumann I, Schauer J, Spengler CM. The effect of respiratory muscle endurance training in patients with myasthenia gravis. Neuromuscular Disorders 2007;17(5):385‐91. [DOI] [PubMed] [Google Scholar]
Rassler 2011 {published data only}
- Rassler B, Marx G, Hallebach S, Kalischewski P, Baumann I. Long‐term respiratory muscle endurance training in patients with myasthenia gravis: first results after four months of training. Autoimmune Diseases 2011;2011:808607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raßler 2014 {published data only}
- Raßler B, Hallebach S, Freitag S, Baumann I, Kalischewski P. Long‐term respiratory muscle endurance training in patients with myasthenia gravis. Acta Physiologica 2014;210(Suppl 695):138‐9. [EMBASE: 71389711] [Google Scholar]
Weiner 1998 {published data only}
- Weiner P, Gross D, Meiner Z, Ganem R, Weiner M, Zamir D, et al. Respiratory muscle training in patients with moderate to severe myasthenia gravis. Canadian Journal of Neurological Sciences 1998;25(3):236‐41. [DOI] [PubMed] [Google Scholar]
Zupan 2002 {published data only}
- Zupan A, Praznikar A, Sardoc M. Inspiratory versus expiratory muscle training in children with neuromuscular diseases. Journal of the Neurological Sciences 2002;199(Suppl 1):S32. [Google Scholar]
References to ongoing studies
NCT02710110 {published data only}
- NCT02710110. Respiratory strength training in persons with amyotrophic lateral sclerosis (ALS) [The impact of respiratory strength training in individuals with amyotrophic lateral sclerosis (ALS)]. clinicaltrials.gov/ct2/show/NCT02710110 (first posted 16 March 2016). [NCT02710110]
Additional references
Aboussouan 2009
- Aboussouan LS. Mechanisms of exercise limitation and pulmonary rehabilitation for patients with neuromuscular disease. Chronic Respiratory Disease 2009;6(4):231‐49. [DOI] [PubMed] [Google Scholar]
Abresch 2012
- Abresch RT, Carter GT, Han JJ, McDonald CM. Exercise in neuromuscular diseases. Physical Medicine Rehabilitation Clinics North America 2012;23(3):653‐73. [DOI] [PubMed] [Google Scholar]
Alonso 1995
- Alonso J, Prieto L, Antó JM. The Spanish version of the SF‐36 Health Survey (the SF‐36 health questionnaire): an instrument for measuring clinical results [La versión española del SF‐36 Health Survey (Cuestionario de Salud SF‐36): un instrumentopara la medida de los resultados clínicos]. Medicine Clinica 1995;104(20):771‐6. [PubMed] [Google Scholar]
Ambrosino 2009
- Ambrosino N, Carpene N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. European Respiratory Journal 2009;34(2):444‐51. [DOI] [PubMed] [Google Scholar]
Anziska 2013
- Anziska Y, Sternberg A. Exercise in neuromuscular disease. Muscle & Nerve 2013;48(1):3‐20. [DOI] [PubMed] [Google Scholar]
Benditt 2006
- Benditt JO. The neuromuscular respiratory system: physiology, pathophysiology, and a respiratory care approach to patients. Respiratory Care 2006;51(8):829‐39. [PubMed] [Google Scholar]
Berlowitz 2013
- Berlowitz DJ, Tamplin J. Respiratory muscle training for cervical spinal cord injury. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008507.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 2000
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Sclerosis and Other Motor Neuron Disorders 2000;1(5):293‐9. [DOI] [PubMed] [Google Scholar]
Cedarbaum 1997
- Cedarbaum JM, Stambler N. Performance of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) in multicenter clinical trials. Journal of Neurological Sciences 1997;152(Suppl 1):S1‐9. [DOI] [PubMed] [Google Scholar]
Cedarbaum 1999
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Thurmond B, Nakanishi A. The ALSFRS‐R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of the Neurological Sciences 1999;169(1‐2):13‐21. [DOI] [PubMed] [Google Scholar]
CONSORT 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. [DOI] [PubMed] [Google Scholar]
Cup 2007
- Cup EH, Pieterse AJ, Broek‐Pastoor JM, Munneke M, Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Archives of Physical Medicine and Rehabilitation 2007;88(11):1452‐64. [DOI] [PubMed] [Google Scholar]
D'Angelo 2011
- D'Angelo MG, Romei M, Lo Mauro A, Marchi E, Gandossini S, Bonato S. Respiratory pattern in an adult population of dystrophic patients. Journal of the Neurological Sciences 2011;306(1‐2):54‐61. [DOI] [PubMed] [Google Scholar]
de Godoy 2012
- Godoy VC, Lanzillotta P. Respiratory muscle training in Becker's muscular dystrophy ‐ critical literature review [Treinamento muscular respiratório na distrofia muscular de Becker ‐ revisão crítica de literatura]. Revista Neurociências 2012;20(1):138‐43. [Google Scholar]
de la Loge 2016
- Loge C, Tugaut B, Fofana F, Lambert J, Hennig M, Tschiesner U, et al. Relationship between FEV1 and patient‐reported outcomes changes: results of a meta‐analysis of randomized trials in stable COPD. Chronic Obstructive Pulmonary Disease 2016;3(2):519‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
du Bois 2011
- du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. American Journal of Respiratory Critical Care Medicine 2011;184(12):1382‐9. [DOI] [PubMed] [Google Scholar]
Eagle 2002
- Eagle M. Report on the Muscular Dystrophy Campaign workshop: exercise in neuromuscular diseases Newcastle, January 2002. Neuromuscular Disorders 2002;12(10):975‐83. [DOI] [PubMed] [Google Scholar]
Eidenberger 2014
- Eidenberger M, Nowotny S. Inspiratory muscle training in patients with amyotrophic lateral sclerosis: a systematic review. NeuroRehabilitation 2014;35(3):349‐61. [DOI] [PubMed] [Google Scholar]
Emery 1991
- Emery AE. Population frequencies of inherited neuromuscular diseases ‐ a world survey. Neuromuscular Disorders 1991;1(1):19‐29. [DOI] [PubMed] [Google Scholar]
Emery 1994
- Emery AE, editor. Diagnostic Criteria for Neuromuscular Disorders. Baarn, The Netherlands: European Neuromuscular Centre, 1994. [ISBN: ISBN 90 261 0719 6, 72 pp] [Google Scholar]
Enright 2011
- Enright SJ, Unnithan VB. Effect of inspiratory muscle training intensities on pulmonary function and work capacity in people who are healthy: a randomized controlled trial. Physical Therapy 2011;91(6):894‐905. [DOI] [PubMed] [Google Scholar]
Epstein 1994
- Epstein SK. An overview of respiratory muscle function. Clinics in Chest Medicine 1994;15(4):619‐39. [PubMed] [Google Scholar]
Estournet‐Mathiaud 2003
- Estournet‐Mathiaud B. Respiratory complications of neuromuscular diseases [Complications respiratoires des maladies neuromusculaires]. In: Dutau G, Labbé A editor(s). Pneumologie de l'Enfant. 2nd Edition. Paris: Arnette, Blackwell, 2003:273‐83. [Google Scholar]
Faber 2014
- Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press Journal of Orthodontics 2014;19(4):27‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2016
- Ferreira GD, Costa AC, Plentz RD, Coronel CC, Sbruzzi G. Respiratory training improved ventilatory function and respiratory muscle strength in patients with multiple sclerosis and lateral amyotrophic sclerosis: systematic review and meta‐analysis. Physiotherapy 2016;102(3):221‐8. [DOI] [PubMed] [Google Scholar]
Finder 2004
Fitting 2006
- Fitting FW. Sniff nasal inspiratory pressure: simple or too simple?. European Respiratory Journal 2006;27(5):881‐3. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
HajGhanbari 2013
- HajGhanbari B, Yamabayashi C, Buna TR, Coelho JD, Freedman KD, Morton TA, et al. Effects of respiratory muscle training on performance in athletes: a systematic review with meta‐analysis. Journal of Strength and Conditioning Research 2013;27(6):1643‐63. [DOI] [PubMed] [Google Scholar]
Hannan 2014
- Hannan LM, Dominelli GS, Chen YW, Darlene Reid W, Road J. Systematic review of non‐invasive positive pressure ventilation for chronic respiratory failure. Respiratory Medicine 2014;108(2):229‐43. [DOI] [PubMed] [Google Scholar]
Hapke 1972
- Hapke EJ, Meek JC, Jacobs J. Pulmonary function in progressive muscular dystrophy. Chest 1972;61(1):41‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2004
- Hill K, Jenkins SC, Hillman DR, Eastwood PR. Dyspnoea in COPD: can inspiratory muscle training help?. Australian Journal of Physiotherapy 2004;50(3):169‐80. [DOI] [PubMed] [Google Scholar]
Hoffman 2002
- Hoffman J. Physiological Aspects of Sport Training and Performance. Champaign, IL: Human Kinetics, 2002. [Google Scholar]
Huang 2011
- Huang CH, Yang GG, Wu YT, Lee CW. Comparison of inspiratory muscle strength training effects between older subjects with and without chronic obstructive pulmonary disease. Journal of the Formosan Medical Association 2011;110(8):518‐26. [DOI] [PubMed] [Google Scholar]
Human 2017
- Human A, Corten L, Jelsma J, Morrow B. Inspiratory muscle training for children and adolescents with neuromuscular diseases: A systematic review. Neuromuscular Disorders 2017;27(6):503‐17. [DOI] [PubMed] [Google Scholar]
Illi 2012
- Illi SK, Held U, Frank I, Spengler CM. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta‐analysis. Sports Medicine 2012;42(8):707‐24. [DOI] [PubMed] [Google Scholar]
Inkley 1974
- Inkley SR, Oldenburg FC, Vignos PJ Jr. Pulmonary function in Duchenne muscular dystrophy related to stage of disease. The American Journal of Medicine 1974;56(3):297‐306. [DOI] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St George's Respiratory Questionnaire. Respiratory Medicine 1991;85(suppl B):25‐31. [DOI: ] [DOI] [PubMed] [Google Scholar]
Jones 1992
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self‐complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145(6):1321‐7. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19(3):398‐404. [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2(1):75‐9. [DOI] [PubMed] [Google Scholar]
Landrigan 2005
- Landrigan PJ, Garg A. Children are not little adults. In: Pronczuk‐Garbino J editor(s). Children's Health and the Environment: a global perspective: a resource manual for the health sector. Geneva: World Health Organization, 2005:3‐16. [Google Scholar]
Leith 1976
- Leith DE, Bradley M. Ventilatory muscle strength and endurance training. Journal of Applied Physiology 1976;41(4):508‐16. [DOI] [PubMed] [Google Scholar]
MacDonald 2002
- MacDonald CM. Physical activity, health impairments, and disability in neuromuscular disease. Journal of American Journal of Physical Medicine and Rehabilitation 2002;81(11 Suppl):S108‐20. [DOI] [PubMed] [Google Scholar]
McConnell 2009
- McConnell AK. Respiratory muscle training as an ergogenic aid. Journal of Exercise Science & Fitness 2009;7(2):S18‐S27. [Google Scholar]
McCool 1995
- McCool FD, Tzelepis GE. Inspiratory muscle training in the patient with neuromuscular disease. Physical Therapy 1995;75(11):1006‐14. [DOI] [PubMed] [Google Scholar]
McDonald 2012
- McDonald CM. Clinical approach to the diagnostic evaluation of hereditary and acquired neuromuscular diseases. Physical Medicine & Rehabilitation Clinics of North America 2012;23(3):495‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Misuri 2000
- Misuri G, Lanini B, Gigliotti F, Iandelli I, Pizzi A, Bertolini MG. Mechanism of CO2 retention in patients with neuromuscular disease. Chest 2000;117(2):447‐53. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moodie 2011
- Moodie LH, Reeve JC, Vermeulen N, Elkins MR. Inspiratory muscle training to facilitate weaning from mechanical ventilation: protocol for a systematic review. BMC Research Notes 2011;4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nici 2006
- Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. ATS/ERS Pulmonary Rehabilitation Writing Committee. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2006;173(12):1390‐413. [DOI] [PubMed] [Google Scholar]
Osserman 1971
- Osserman KE, Genkins G. Studies in myasthenia gravis: review of a twenty‐year experience in over 1200 patients. The Mount Sinai Journal of Medicine 1971;38(6):497‐537. [PubMed] [Google Scholar]
Page 2016
- Page MJ, Higgins JP, Clayton G, Sterne JA, Hróbjartsson A, Savović J. Empirical evidence of study design biases in randomized trials: systematic review of meta‐epidemiological studies. PLoS One 2016;11(7):e0159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2010
- Park JH, Kang SW, Lee SC, Choi WA, Kim DH. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Medical Journal 2010;51(3):392‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paschoal 2007
- Paschoal IA, Villalba WO, Pereira MC. Chronic respiratory failure in patients with neuromuscular diseases: diagnosis and treatment [Insuficiência respiratória crônica nas doenças neuromusculares: diagnóstico e tratamento]. Jornal Brasileiro de Pneumologia 2007;33(1):81‐92. [DOI] [PubMed] [Google Scholar]
Pellegrino 2005
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. European Respiratory Journal 2005;26(5):948‐68. [DOI] [PubMed] [Google Scholar]
Pine 2005
- Pine M, Watsford M. Specific respiratory muscle training for athletic performance. Sports Coach 2005;27(4):1‐4. [Google Scholar]
Pinto 2014
- Pinto S, Carvalho M. Breathing new life into treatment advances for respiratory failure in amyotrophic lateral sclerosis patients. Neurodegenerative Disease Management 2014;4(1):83‐102. [DOI] [PubMed] [Google Scholar]
Pollock 2013
- Pollock RD, Rafferty GF, Moxham J, Kalra L. Respiratory muscle strength and training in stroke and neurology: a systematic review. International Journal of Stroke 2013;8(2):124‐30. [DOI] [PubMed] [Google Scholar]
Pontes 2012
- Pontes JF, Ferreira GM, Fregonezi GA, Sena‐Evangelista KC, Dourado ME Jr. Respiratory muscle strength, nutritional and postural profile in children with neuromuscular diseases [Força muscular respiratória e perfil postural e nutricional em crianças com doenças neuromusculares]. Fisioterapia em Movimento 2012;25(2):253‐61. [Google Scholar]
Pustavoitau 2008
- Pustavoitau A, Stevens RD. Mechanisms of neurologic failure in critical illness. Critical Care Clinics 2008;24(1):1‐24. [DOI] [PubMed] [Google Scholar]
Rabin 2001
- Rabin R, Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Annals of Medicine 2001;33(5):337‐43. [DOI] [PubMed] [Google Scholar]
Radunovic 2017
- Radunovic A, Annane D, Rafiq MK, Brassington R, Mustfa N. Mechanical ventilation for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD004427.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramirez‐Sarmiento 2008
- Ramirez‐Sarmiento A, Orozco‐Levi M. Pulmonary rehabilitation should be prescribed in the same way medications are prescribed. Archivos de Bronconeumología 2008;44(3):119‐21. [DOI] [PubMed] [Google Scholar]
Reed 2002
- Reed UC. Neuromuscular diseases [Doenças neuromusculares]. Jornal de Pediatria 2002;78(Suppl 1):S89‐103. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2013
- Reyes A, Ziman M, Nosaka K. Respiratory muscle training for respiratory deficits in neurodegenerative disorders: a systematic review. Chest 2013;143(5):1386‐94. [DOI] [PubMed] [Google Scholar]
Rezania 2012
- Rezania K, Goldenberg FD, White S. Neuromuscular disorders and acute respiratory failure: diagnosis and management. Neurologic Clinics 2012;30(1):161–85. [DOI] [PubMed] [Google Scholar]
Riebe 2014
- Riebe D. Exercise prescription. In: Pescatello LS editor(s). ACSM's Guidelines for Exercise Testing and Prescription. 9th Edition. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2014:161‐93. [Google Scholar]
Rietberg 2017
- Rietberg MB, Veerbeek JM, Gosselink R, Kwakkel G, Wegen EEH. Respiratory muscle training for multiple sclerosis. Cochrane Database of Systematic Reviews 2017, Issue 12. [DOI: 10.1002/14651858.CD009424.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romer 2003
- Romer LM, McConnell AK. Specificity and reversibility of inspiratory muscle training. Medicine and Science in Sports Exercise 2003;35(2):237‐44. [DOI] [PubMed] [Google Scholar]
Sander 2000
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, et al. Functional muscle ischemia in neuronal nitric oxide synthase‐deficient skeletal muscle of children with Duchenne muscular dystrophy. Proceedings of the National Academy of Sciences of the Unites States of America 2000;97(25):13818‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sartori 2008
- Sartori R, Barbi E, Poli F, Ronfani L, Marchetti F, Amaddeo A, et al. Respiratory training with a specific device in cystic fibrosis: a prospective study. Journal of Cystic Fibrosis 2008;7(4):313‐9. [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1–82. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Thomas 2013
- Thomas GD. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Frontiers in Physiology 2013;4:381. [DOI: 10.3389/fphys.2013.00381] [DOI] [PMC free article] [PubMed] [Google Scholar]
Timpani 2017
- Timpani CA, Hayes A, Rybalka E. Therapeutic strategies to address neuronal nitric oxide synthase deficiency and the loss of nitric oxide bioavailability in Duchenne Muscular Dystrophy. Orphanet Journal of Rare Diseases 2017;12:100. [DOI: 10.1186/s13023-017-0652-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzelepis 1994
- Tzelepis GE, Vega DL, Cohen ME, Fulambarker AM, Patel KK, McCool FD. Pressure‐flow specificity of inspiratory muscle training. Journal of Applied Physiology 1994;77(2):795‐801. [DOI] [PubMed] [Google Scholar]
Tzelepis 1999
- Tzelepis GE, Kasas V, McCool FD. Inspiratory muscle adaptations following pressure or flow training in humans. European Journal of Applied Physiology 1999;79(6):467‐71. [DOI] [PubMed] [Google Scholar]
Van Houtte 2006
- Houtte S, Vanlandewijck Y, Gosselink R. Respiratory muscle training in persons with spinal cord injury: a systematic review. Respiratory Medicine 2006;100(11):1886‐95. [DOI] [PubMed] [Google Scholar]
Vandervelde 2009
- Vandervelde L, Bergh PY, Goemans N, Thonnard JL. Activity limitations in patients with neuromuscular disorders: a responsiveness study of the ACTIVLIM questionnaire. Neuromuscular Disorders 2009;19:99‐103. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JEJ, Sherbourne CD. The MOS 36‐Item Short‐Form Health Survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wijdicks 2009
- Wijdicks EF. Generalized weakness in the intensive care unit. Neurological Complications of Critical Illness. Oxford: Oxford University Press, 2009:59‐74. [Google Scholar]
Winkler 2000
- Winkler G, Zifko U, Nader A, Frank W, Zwick H, Toifl K, et al. Dose‐dependent effects of inspiratory muscle training in neuromuscular disorders. Muscle & Nerve 2000;23(8):1257‐60. [DOI] [PubMed] [Google Scholar]
Wirth 1999
- Wirth B, Herz M, Wetter A, Moskau S, Hahnen E, Rudnik‐Schöneborn S, et al. Quantitative analysis of survival motor neuron copies: identification of subtle SMN1 mutations in patients with spinal muscular atrophy, genotype‐phenotype correlation, and implications for genetic counseling. American Journal of Human Genetics 1999;64(5):1340‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiao 2012
- Xiao Y, Luo M, Wang J, Luo H. Inspiratory muscle training for the recovery of function after stroke. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009360.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Pedrosa 2015
- Pedrosa R, Silva IS, Azevedo IG, Forbes A‐M, Fregonezi GA, Dourado Junior ME, et al. Respiratory muscle training in children and adults with neuromuscular disease. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011711] [DOI] [PMC free article] [PubMed] [Google Scholar]


