Abstract
Background
Multiple sclerosis (MS) is one of the most prevalent diseases of the central nervous system with recent prevalence estimates indicating that MS directly affects 2.3 million people worldwide. Fall rates of 56% have been reported among people with MS in a recent meta‐analysis. Clinical guidelines do not outline an evidence‐based approach to falls interventions in MS. There is a need for synthesised information regarding the effectiveness of falls prevention interventions in MS.
Objectives
The aim of this review was to evaluate the effectiveness of interventions designed to reduce falls in people with MS. Specific objectives included comparing: (1) falls prevention interventions to controls and; (2) different types of falls prevention interventions.
Search methods
We searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group, Cochrane Central Register of Controlled Trials (2018 Issue 9); MEDLINE (PubMed) (1966 to 12 September 2018); Embase (EMBASE.com) (1974 to 12 September 2018); Cumulative Index to Nursing and Allied Health Literature (EBSCOhost) (1981 to 12 September 2018); Latin American and Caribbean Health Science Information Database (Bireme) (1982 to 12 September 2018); ClinicalTrials.gov; and World Health Organization International Clinical Trials Registry Platform; PsycINFO (1806 to 12 September 2018; and Physiotherapy Evidence Database (1999 to 12 September 2018).
Selection criteria
We selected randomised controlled trials or quasi‐randomised trials of interventions to reduce falls in people with MS. We included trials that examined falls prevention interventions compared to controls or different types of falls prevention interventions. Primary outcomes included: falls rate, risk of falling, number of falls per person and adverse events.
Data collection and analysis
Two review authors screened studies for selection, assessed risk of bias and extracted data. We used a rate ratio (RaR) and 95% confidence interval to compare falls rate between groups. For risk of falling, we used a risk ratio (RR) and 95% CI based on the number of fallers in each group.
Main results
A total of 839 people with MS (12 to 177 individuals) were randomised in the 13 included trials. The mean age of the participants was 52 years (36 to 62 years). The percentage of women participants ranged from 59% to 85%. Studies included people with all types of MS. Most trials compared an exercise intervention with no intervention or different types of falls prevention interventions. We included two comparisons: (1) Falls prevention intervention versus control and (2) Falls prevention intervention versus another falls prevention intervention. The most common interventions tested were exercise as a single intervention, education as a single intervention, functional electrical stimulation and exercise plus education. The risk of bias of the included studies mixed, with nine studies demonstrating high risk of bias related to one or more aspects of their methodology.
The evidence was uncertain regarding the effects of exercise versus control on falls rate (RaR of 0.68; 95% CI 0.43 to 1.06; very low‐quality evidence), number of fallers (RR of 0.85; 95% CI 0.51 to 1.43; low‐quality evidence) and adverse events (RR of 1.25; 95% CI 0.26 to 6.03; low‐quality evidence).
Data were not available on quality of life outcomes comparing exercise to control. The majority of other comparisons between falls interventions and controls demonstrated no evidence of effect in favour of either group for all primary outcomes.
For the comparison of different falls prevention interventions, the heterogeneity of intervention types across studies prohibited the pooling of data.
In relation to secondary outcomes, there was evidence of an effect in favour of exercise interventions compared to controls for balance function with a SMD of 0.50 (95% CI 0.09 to 0.92), self‐reported mobility with a SMD of 16.30 (95% CI 9.34 to 23.26) and objective mobility with a SMD of 0.28 (95% CI 0.07 to 0.50). Secondary outcomes were not assessed under the GRADE criteria and results must be interpreted with caution.
Authors' conclusions
The evidence regarding the effects of interventions for preventing falls in MS is sparse and uncertain. The evidence base demonstrates mixed risk of bias, with very low to low certainty of the evidence. There is some evidence in favour of exercise interventions for the improvement of balance function and mobility. However, this must be interpreted with caution as these secondary outcomes were not assessed under the GRADE criteria and as the results represent data from a small number of studies. Robust RCTs examining the effectiveness of multifactorial falls interventions on falls outcomes are needed.
Plain language summary
Falls interventions in multiple sclerosis
Review question
Do people with multiple sclerosis (MS) who received interventions to reduce falls show better falls outcomes than those who received no treatment? In addition, do different types of falls interventions result in different outcomes for people with MS?
Background
Due to damage to the central nervous system among people with MS, difficulties in thinking, muscle strength, muscle tone, sensation, coordination and mobility can lead to an increased risk of falling, compared to people without MS. Interventions to prevent falls are offered to people with MS and often include: exercises, medication, surgery, management of urinary incontinence, fluid or nutrition therapy, psychological intervention, environment/assistive technology, environment (social environment), knowledge interventions and other interventions. The risk of falling in people with MS is three times higher than that in older people, yet it is unclear whether falls interventions are effective in reducing falls in MS. Currently there are a few good‐quality studies that have investigated the effectiveness of falls interventions in people with MS.
Study characteristics
This review included 13 studies with 839 participants involving various types of falls interventions, most comparing an exercise intervention with no intervention or two or more falls prevention interventions.
Key results and quality of the evidence
There is uncertainty on the effect of exercises on prevention of falls due to the low to very low quality of the evidence for some of the primary outcomes. Our confidence in these results is low for the prevention of falls because this has been evaluated in only a few small trials that we judged as having some risk of bias and methodological shortcomings. There are still relatively few large, good‐quality studies to base our findings on, so more are needed.
Summary of findings
Summary of findings for the main comparison. Exercise compared to control (post‐intervention) for preventing falls in people with multiple sclerosis.
| Exercise compared to control (post‐intervention) for preventing falls in people with multiple sclerosis | ||||||
| Patient or population: people with multiple sclerosis (including people with relapsing‐remitting, secondary progressive or primary progressive types of MS), mean age: 53 years Setting: community or home Intervention: exercise (community‐based or home‐based exercise interventions), ranging from 6 to 24 weeks in duration, ranging from once to 5 times weekly frequency Comparison: usual care treatment or wait‐list control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control (post‐intervention) | Risk with Exercise | |||||
| Falls rate Falls were measured using prospective daily diaries, prospective monthly calendars or retrospectively. Falls rate calculation= number of falls/number in group * (number of days/365) The timing of measurement was post‐intervention for all studies |
Not applicable | Not applicable | Rate ratio 0.68 (0.43 to 1.06) | 399 (5 RCTs) | ⊕⊕⊕⊝ VERY LOW |
|
| Number of fallers Falls were measured using prospective daily diaries, prospective monthly calendars or retrospectively The timing of measurement was post‐intervention for all studies |
305 per 1,000 | 259 per 1,000 (156 to 436) | RR 0.85 (0.51 to 1.43) | 355 (5 RCTs) | ⊕⊕⊕⊝ LOW |
|
| Adverse events The timing of measurement was post‐intervention for all studies |
44 per 1,000 | 56 per 1,000 (12 to 268) | RR 1.25 (0.26 to 6.03) | 97 (3 RCTs) | ⊕⊕⊕⊝ LOW |
|
| Quality of life | see comments | see comments | not estimable | Studies included in this analysis did not report data on quality of life | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Falls rate: GRADE assessment‐ Downgraded one level due to risk of bias and one level due to imprecision and one level due to inconsistency. Four studies at high risk of bias in allocation concealment domain; five studies at unclear risk of bias in blinding of participants domain; two studies at high risk of bias and one study at unclear risk of bias in selective reporting domain; wide CI; I2= 59%
Number of fallers: GRADE assessment‐ Downgraded one level due to risk of bias and one level due to imprecision, One study at high risk of bias in attrition domain; two study at unclear risk of bias in allocation concealment domain; five studies at unclear risk of bias in blinding of participants domain; and four studies at high risk of bias in selective reporting domain; wide CI
Adverse events: GRADE assessment‐ Downgraded one level due to risk of bias and one level due to imprecision, One study at unclear risk of bias in allocation concealment domain; three studies at unclear risk of bias in blinding of participants domain; and three studies at high risk of bias in selective reporting domain; wide CI
Background
Multiple sclerosis (MS) is one of the most prevalent diseases of the central nervous system (CNS) with recent prevalence estimates indicating that MS directly affects 2.3 million people worldwide (Browne 2014). Global annual incidence estimates range from 0.07 to 13.75 per 100,000 people (Browne 2014). Wide variations occur in relation to the prevalence and incidence of MS, according to geographic location, with parts of Northern Europe and Canada being the most commonly affected (Browne 2014). It is the most common disabling neurological disorder among young people.
Traditionally MS has been categorised according to clinical phenotype as primary‐progressive, relapsing‐remitting, secondary‐progressive and progressive‐relapsing (Lublin 1996). However, it has been suggested that a classification based on clinical and radiological activity be instigated (Lublin 2014). MS is an immune‐mediated disease characterised by inflammatory demyelination and neurodegeneration within the CNS. This damage to the CNS structures in turn leads to impairments in cognition, muscle strength, muscle tone, sensation, coordination and gait, all of which are associated with an increased risk for falls. Despite the recent increased availability of disease‐modifying medical treatments and their potential to delay the clinical progression of MS, falls continue to present as a common and serious health concern in people with this disease.
Description of the condition
Fall rates of 56% have been reported among people with MS (measured using prospective measures) in a recent meta‐analysis of 537 individuals, with 37% of the study population falling recurrently (Nilsagard 2015). This study demonstrated that most falls occurred indoors (65%) between 6 a.m. and 6 p.m. (75%). In addition, primary progressive MS and Expanded Disability Severity Scale (EDSS) (Kurtzke 1983) levels of 4.0 and 6.0 were associated with significantly increased odds of falls (P < 0.05). The falls rate was also lower in women than men (relative risk (RR) 0.80; 95% confidence interval (CI) 0.67 to 0.94) and decreased with increasing age (RR 0.97 for each year, CI 0.95 to 0.98). In a study by Matsuda 2011, 28% of people with MS who had reported to have fallen (265 of a total of 455 respondents) suffered a fracture. A population‐based European study reported that the incidence rate of fracture was significantly higher among people with MS than age‐ and gender‐matched peers without MS (Bazelier 2011). People with MS with a history of falls report significantly poorer physical and psychological health status compared with non‐fallers with MS (Coote 2013b). Falls can further have an adverse impact on fear of falling and falls self‐efficacy, and can contribute to activity curtailment, physiological deconditioning, loss of independence, and institutionalisation (Finlayson 2010; Matsuda 2012). A systematic review with meta‐analysis identified four factors significantly associated with falls in people with MS: balance dysfunction, the use of a mobility aid, cognitive dysfunction, and progressive MS subtype (Gunn 2013). Given the high prevalence of falls among people with MS and the associated serious and wide‐ranging consequences, an increased number of randomised controlled trials have evaluated the effect of falls prevention interventions among people with MS.
Description of the intervention
To our knowledge there currently is no classification of falls prevention interventions in the MS literature. The effectiveness of several categories of falls prevention interventions has been reviewed systematically among older adults (Gillespie 2003; Gillespie 2012) and people post‐stroke (Verheyden 2013) by Cochrane. These categories are also used by the few researchers that have examined fall prevention or management in MS. However the Prevention of Falls Network Europe (ProFaNE) (Lamb 2005; Lamb 2011) proposes the following categories for older adults: exercises, medication, surgery, management of urinary incontinence, fluid or nutrition therapy, psychological intervention, environment/assistive technology, environment (social environment), knowledge interventions and other interventions. In the ProFaNE taxonomy, interventions are also classified as single interventions, multiple interventions or multifactorial interventions. A single intervention consists of only one intervention component which is delivered to all participants in the intervention group, (e.g. exercise). Multiple interventions consist of a combination of two or more intervention components, delivered to all of the participants in the intervention group, (e.g. exercise plus psychological interventions). Multifactorial interventions consist of more than one intervention component, but participants receive different combinations of interventions based on an individual assessment to identify potential risk factors for falls.
How the intervention might work
Falls prevention interventions are designed to minimise known modifiable personal, task and environmental risk factors for falling, and thereby reduce risk in order to prevent falls and associated injuries. Interventions are designed to reduce the falls rate by targeting improvement in personal risk factors, e.g. reduced balance function, and incorporate exercises to improve joint flexibility, muscle strength, reaction times and coordination. Other interventions are aimed at improving non‐physical personal risk factors and include strategies to promote risk awareness, planning and attention. Interventions are also designed to reduce falls by promoting improved task performance, e.g. safe mobility aid use, and include participant education regarding task analysis and planning. Interventions are additionally designed to ameliorate the falls rate by addressing environmental risk factors, e.g. home environmental modifications, and include the provision of aids for personal care.
Single component interventions are designed to address and ameliorate specific risk factors for falling. For example, in Cochrane Reviews focusing on falls prevention interventions among older adults, vitamin D prescription interventions have been shown to be effective in reducing falls rates among older adults in care facilities (Cameron 2012) and exercise interventions have been shown to be effective in reducing falls rates among older adults living in the community (Gillespie 2012). There is potential for this improvement to be mediated indirectly through the effect of exercise on balance function and mobility functions. To date in the MS literature, of the few falls interventions that have been evaluated, most have predominantly used combinations of education and exercise, targeting mobility, balance, and falls self‐efficacy outcomes. The association between balance, mobility impairments, and falls in MS is complex. Programmes focused on balance and stability in older adult populations have been shown to decrease falls in other populations (Gillespie 2012) whereas those that target mobility alone have tended to be either ineffective or to increase falls in older adult populations (Gillespie 2012).
Multiple component interventions aim to reduce several components of falls risk rather than dealing with single risk factors. Commonly, multiple component interventions focus on two or more common risk factors and provide these to all participants, regardless of their exact risk status. However, there is no assessment and individual tailoring of the intervention to risk factors. There is some evidence that multiple component interventions may reduce the rate of falls and the risk of falling in older people living in the community (Gillespie 2012).
The rationale underlying multifactorial interventions is that participants undergo an assessment for risk of falling, and a tailored intervention is provided based on their modifiable risk factors. Gillespie 2012 found some evidence that multifactorial interventions may reduce the rate of falls (i.e. the total number of falls per unit of person time that falls were monitored), but not the risk of falling (i.e. the number of people who fell once or more among older people living in the community).
Why it is important to do this review
The incidence of falls in people with MS is three times higher than that in older people, yet recently published clinical guidelines (NGC 2014) do not outline an evidence‐based approach to falls interventions among people with MS. This topic has been examined and reviewed systematically among older adults (Gillespie 2003; Cameron 2012; Gillespie 2012) and people post‐stroke (Verheyden 2013) by Cochrane. Therefore there is a clear clinical need for synthesised information regarding the effectiveness of falls prevention interventions among people with MS. This clinical need is relevant across multiple disciplines and multiple settings (home, community, clinical setting). A Cochrane systematic review of this topic has the potential to guide clinical decisions regarding care pathways for people with MS who are at risk of falling, and ultimately to improve quality of life of people with MS.
Objectives
The aim of this review was to evaluate the effectiveness of interventions designed to reduce falls in people with multiple sclerosis (MS). Specific objectives included comparing: (1) falls prevention interventions to controls and; (2) different types of falls prevention interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised trials, including randomised and quasi‐randomised cluster and cross‐over trials. We included all trials regardless of methodological quality.
Types of participants
We included adults 18 years of age or older, male and female, with clinically definite MS. People with the clinical diagnosis of MS according to the ICD‐8 (code 340) (ICD‐8 1965), and the McDonald criteria (Schumacher 1965; Poser 1983; McDonald 2001; Polman 2005; Polman 2011) were included. All subgroups of MS such as relapsing remitting, primary progressive and secondary progressive MS, and people at any time since diagnosis were included. People with neurological and non‐neurological co‐morbidities that may affect falls, e.g. dementia, Parkinson's disease, and recent orthopaedic surgery, were excluded, wherein separate MS data could not be extracted from the trial results.
Types of interventions
Falls prevention interventions were considered to be any programme in which the primary or secondary aim is to reduce falls‐ whether stated explicitly by the authors or not. Most fall prevention interventions can be classified according to the taxonomy developed by the Prevention of Falls Network Europe (ProFANE) (Lamb 2007; Lamb 2011). We decided to include studies wherein the authors tested interventions which may have an effect on falls (as defined by ProFANE), e.g. exercise programmes, even if the authors did not explicitly state that the intervention being tested was a falls prevention intervention. This inclusion of such interventions relates to our aim to capture all relevant evidence in this area. Some anticipated falls prevention interventions included: exercise (e.g. aerobic, strengthening, balance), medical intervention (e.g. supplementation with vitamin D), psychological (e.g. cognitive behavioural interventions), environment modifications (e.g. the provision of hip protectors, adaptations to homes), assistive technology interventions (e.g. provision of aids for personal care and protection and personal mobility, eyeglasses, hearing aids, personal alarm systems), surgical interventions (e.g. surgery to address a comorbidity such as hip or knee replacement for osteoarthritis) or other interventions (e.g. educational interventions designed to increase knowledge relating to falls prevention). This review included all interventions tested in trials that measured one or more of the primary falls outcomes (rate of falls, risk of falling).
Acceptable control interventions included: wait‐list control, usual care control, another type of intervention.
Types of outcome measures
Outcome measures were examined at the end of the intervention period in all included studies (post‐intervention) at the end of follow‐up (e.g. 3‐, 6‐ or 12‐month follow‐up periods).
Primary outcomes
The rate of falls (the number of falls per person year), baseline measure using retrospective (e.g. retrospective falls diary) and prospective measures, recommended by the International MS Falls Prevention Research Network (IMSFPRN) was the primary outcome for falls prevention trials (Sosnoff 2014b).
The risk of falling, i.e. the number of fallers: number of participants who fell at least once during the study.
The number of adverse events resulting from the intervention.
Secondary outcomes
Physiological falls risk, measured using measures including, but not restricted to, the Physiological Profile Assessment (PPA). The PPA measures five aspects of physiological components including contrast sensitivity, position sense, muscles strength, reaction time and postural sway (Lord 2003).
Quality of life, measured using measures including, but not restricted to, the Multiple Sclerosis Impact Scale‐29 (Hobart 2001).
Balance function, measured using measures including, but not restricted to, the Berg Balance Scale (Berg 1989), Mini‐BEST test (Franchignoni 2010).
Cognition, measured using measures including, but not restricted to, the Symbols Digit Modalities Test (SDMT) (Smith 1982).
Measures of MS disease progression, including but not restricted to the Expanded Disease Severity Scale (EDSS) (Kurtzke 1983), and Patient Determined Disease Steps (PDDS) (Hohol 1995).
Measures of mobility including, but not restricted to the Six Minute Walk Test (Fry 2006), and MS Walking Scale‐12 (Hobart 2003).
Measures of functional outcome, including but not restricted to the Functional Independence Measure (FIM) (Keith 1987).
Self‐reported fatigue, measured using measures including, but not restricted to, the Modified Fatigue Impact Scale (MFIS) (Fischer 1999).
Measures of participation, including but not restricted to the Community Integration Measure (CIM) (McColl 2001).
Outcomes that reflect cost, service utilisation and care burden.
Search methods for identification of studies
A systematic search without language or date restrictions was conducted using the optimally‐sensitive strategy developed for Cochrane to identify all relevant published and unpublished RCTs (Lefebvre 2011). We employed the services of a professional translator for the translation of one full text, for study screening.
Electronic searches
The Information Specialist searched the Trials Register of the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group, which, among other sources, contains trials from:
Cochrane Central Register of Controlled Trials (CENTRAL) (2018 Issue 12);
MEDLINE (PubMed) (1966 to 12 December 2018);
Embase (EMBASE.com) (1974 to 12 December 2018);
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (1981 to 12 December 2018);
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 to 12 December 2018);
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch);
PsycINFO (1806 to 12 December 2018); and
Physiotherapy Evidence Database (PEDro) (1999 to 12 December 2018).
Information on the Trials Register of the Review Group and details of the search strategies used to identify trials can be found in the 'Specialised Register' section within the Cochrane Multiple Sclerosis and Rare Diseases of the CNS Group (https://msrdcns.cochrane.org/).
The keywords that were used to search for trials for this review are listed in Appendix 1.
In addition, we performed separate searches to ensure we retrieved the most up‐to‐date results. The search strategies run are in Appendix 2
Searching other resources
We also:
handsearched the reference lists of all retrieved articles, texts and other reviews on the topic;
contacted researchers active in this field for additional data, for example we sent the list of included studies to the researchers within the International Falls Research Network (IFRN) in order to acquire further potentially suitable studies that our search did not highlight;
contacted principal authors of unpublished manuscripts to ask if they are willing to disclose their unpublished data, for example Prof Sheila Lennon (Lennon 2013a) for the data from an unpublished trial (Lennon 2013).
Data collection and analysis
Selection of studies
Titles and abstracts of the citations retrieved by the literature search were screened independently by two review authors (SH, SC) for inclusion or exclusion, based on predetermined inclusion criteria. The full text of potentially relevant studies were selected for further assessment and two authors (SH, SC) ascertained and agreed on eligibility based on the full article. The eligibility (on the basis of the information available in the published data) of these studies was evaluated independently. Papers assessed in full text that did not meet the inclusion criteria are listed in the 'Characteristics of excluded studies' table with the reasons for exclusion. Any disagreement regarding inclusion was resolved by discussion.
Data extraction and management
For each included study, two of the following four review authors (SH, SC, LH or RG) independently extracted data from the selected trials using standardised forms and SH entered the data into the RevMan software (Review Manager 2014). We extracted data on the following:
study design;
characteristics of participants (number, age, type of MS, EDSS score);
inclusion and exclusion criteria;
brief description of experimental intervention;
brief description of control intervention;
methodological quality of studies;
description of setting;
description of outcomes.
Disagreements were discussed and resolved by consensus among the review authors.
Assessment of risk of bias in included studies
The risk of bias for all included studies was independently assessed by two review authors (SH, SC) using the 'Risk of bias' tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) . The domains are: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome and other biases. Disagreements among the review authors on the methodological quality of the identified studies was resolved by discussion, or by referral to a third assessor (RG) if necessary.
We used the summary quality assessment at the analysis stage as a means of interpreting the results. For each dimension and for the summary assessment we assigned the 'Risk of bias' categories (Higgins 2011) as:
low risk of bias, plausible bias unlikely to seriously alter the results;
unclear risk of bias, plausible bias that raises some doubt about the results; and
high risk of bias, plausible bias that seriously weakens confidence in the results.
We also rated the overall risk of bias (low, unclear, high) specific for each outcome included in the Summary of Findings table.
Assessing the quality of the body of evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons:
rate of falls;
number of fallers: number of participants who fell at least once during the study;
adverse events;
quality of life.
Summary of findings table
We used the GRADEpro to import data from Review Manager 5.3 (Review Manager 2014) in order to create a 'Summary of findings' (SOF) table. As per the Cochrane Handbook for Systematic Reviews of Interventions guidelines, the SOF tables include the following information (Higgins 2011): a list of all important outcomes; absolute and relative magnitude of intervention effect; numbers of participants and studies addressing these outcomes; a rating of the overall quality of evidence for each outcome and a space for comments.
Due to the heterogeneity of the included trials, in terms of intervention types, this review comprised 21 comparisons, within the main comparison headings of: 1) Falls interventions versus control and 2) Falls intervention versus another falls intervention. As exercise is a very commonly‐prescribed falls prevention intervention in clinical practice, wherein the alternative is often usual care treatment, we present the data from the exercise versus control comparison in the SoF table only (Table 1). The findings from the other comparisons are presented within the text of this review.
We created the 'Summary of findings' table for the following outcomes:
rate of falls
number of fallers
adverse events
quality of life
A summary of the intervention effect and a measure of quality for each outcomes was produced using the GRADE approach. The GRADE approach uses five domains (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. We graded the quality of evidence as high, moderate, low, or very low upon considering within‐study risk of bias, directness of evidence, heterogeneity, precision of effect estimates, and risk of publication bias. Assumed baseline risks used in calculating absolute risks were based on the range of outcomes measured in comparison groups in the included studies.
Measures of treatment effect
According to the study characteristics, we determined the treatment effect of:
falls prevention interventions versus no treatment control, e.g. exercise versus control;
falls prevention intervention versus another falls prevention intervention, e.g. exercise versus functional electrical stimulation plus exercise.
According to the type of outcomes reported we used the following effect measures:
dichotomous data: risk ratio (RR). For the number of fallers and recurrent fallers (risk of falling) and number of adverse events we used a risk ratio (RR) and 95% CI based on the number of people falling and the number of people reporting adverse events in each group.
continuous data: mean difference (MD) or standardised mean difference (SMD) in the studies that assessed the same outcome but measured it in a variety of ways (for example, 10m walk speed and 25ft walk speed). For the pooling of continuous data, wherein studies used different measures of the same outcomes, e.g. the Berg Balance Scale and Four Step Square Test to measure balance, to ensure the accurate pooling of data and representation of the different outcome measures on the same standardised scale, we applied the rule that lower scores indicated poorer performance and higher scores indicated better performance. For the individual scales wherein the opposite was the case, we multiplied the mean estimate by minus 1;
A rate ratio (RaR) and 95% confidence interval (CI) was used to compare the rate of falls between intervention and control groups. Falls rate was reported by one study only (Taylor 2014). Therefore, where possible, by receiving additional data from study authors, we calculated the falls rate for individual studies. If a rate ratio was not reported, but appropriate raw data (number of falls in each group, number of participants in each group, length of assessment period) were available, the author SH used excel to calculate the falls rate (total number of falls, number of participants in each group and duration of falls data collection period (number of falls per person per year) for both groups in the included studies. The Generic Inverse Variance option in Revman requires entering the natural log of the rate ratio and its standard error for each study. The author CW calculated these in Stata.
Unit of analysis issues
Data analysis took into account the level at which randomisation occurred (e.g. cross‐over trials). Two cross‐over randomised trials were included in the review (Prosperini 2013; Taylor 2014). We consulted a statistician (CW), in addition to the guidance from the Cochrane Handbook Chapter 16.4.3 (Higgins 2011) to determine the most appropriate methods to meta‐analyse these data. Both trials included an exercise intervention component and given the nature of exercise, and potential for more sustained exercise behaviour change after crossing over to another intervention component, we decided to extract and analyse data from both groups, only for the first period of the two crossover studies. Therefore these data have been analysed in the same manner as a parallel group standard trial design (Cochrane Handbook Chapter 16.4.3) (Higgins 2011). With regard to dealing with studies with multiple arms: Cattaneo 2007 included three arms and the two other studies included four arms (Coote 2013; Sosnoff 2015); we did not include multiple arms in any of the included meta‐analyses in our review. Therefore, we did not need to take measures to account for double counting of participants.
Dealing with missing data
If trial data were insufficient or missing, we attempted to obtain additional information from the authors of included studies by personal communication. We analysed only the available data (ignoring the missing data).
Assessment of heterogeneity
Prior to the assessment of statistical heterogeneity, as an interdisciplinary reviewer team, we determined the clinical heterogeneity of included studies. Specifically, we considered the types of participants, interventions and outcomes before making a decision to pool data in meta‐analyses. After the pooling of appropriate data we calculated the I2 statistic for each pooled estimate to assess the impact on statistical heterogeneity. When the I2 was > 30% we used random‐effects models to take account of the between‐study variation in our findings (Higgins 2011). Where there was substantial clinical heterogeneity (e.g. in the nature of interventions) then these were analysed in homogenous subgroups.
Assessment of reporting biases
Due to the lack of unpublished suitable studies identified for inclusion in this review, it was not possible to examine the influence of unpublished papers on the overall effects.
Data synthesis
We performed separate analyses for trials comparing: (1) falls prevention interventions with control interventions and; (2) trials comparing two different types of falls prevention interventions. This outlines the two main comparisons we aimed to make across primary and secondary outcomes in this review and meta‐analysis. Data extraction from the included studies however, demonstrated that there were a wide variety of falls interventions, with less potential for pooling of data than anticipated. For example, due to the clinical heterogeneity evident in the included studies wherein the effectiveness of different types of falls prevention interventions were compared, we were not able to pool data from more than one study.
We analysed the data using Review Manager 5.3. We decided whether or not to perform meta‐analyses based on the similarity of the included trials. Where we could not carry out meta‐analysis because of substantial differences between studies and when only one study was identified, we presented results in a forest plot (with the pooled summary of outcomes suppressed) and provided a narrative review. The data of individual trials was pooled for each outcome using a fixed‐effect model (if heterogeneity was not present (I2 < 30) and using a random‐effects model if heterogeneity was present (I2≥ 30).
Subgroup analysis and investigation of heterogeneity
We had planned on undertaking subgroup analyses to establish if the following subgroups affected the overall effects:
participant‐related characteristics (e.g. type of impairment at baseline: participants with muscle weakness, participants with ataxia, etc.; age; time since diagnosis of MS; type of MS, level of impairment at baseline; adherence to intervention);
intervention‐related characteristics (e.g. type of falls prevention intervention, duration of intervention; frequency of intervention; intensity of intervention);
study design characteristics (e.g. type of comparison, type of falls outcome measurement, retrospective falls rate versus prospective falls rate). Retrospective data may have been reported by trials wherein retrospective falls diaries were used as an outcome measure.
However, due to insufficient number of studies, we were not able to perform these analyses. Given the clinical heterogeneity of many of the included studies within the comparison "Falls prevention intervention versus another falls prevention intervention", we conducted separate analyses across various intervention‐related characteristics.
Sensitivity analysis
We considered risk of bias of included studies when interpreting evidence using the GRADE approach. Sensistivity analysis was not performed.
Results
Description of studies
Results of the search
We screened a total of 728 citations; this includes duplicates. The results of our searching activities are summarised in Figure 1 Thirteen studies were identified for inclusion in this review based on previously‐outlined search strategy.
1.
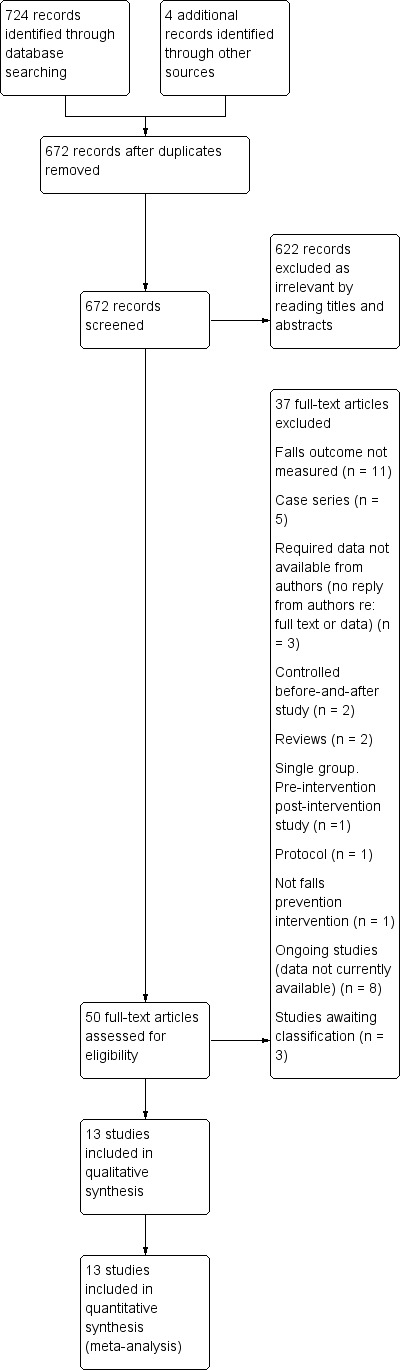
Study flow diagram.
We included 13 completed trials
We excluded 26 studies
We identified eight ongoing trials
We identified three trials that are awaiting classification.
Included studies
Thirteen studies met the inclusion criteria for this review (Stephens 2001; Cattaneo 2007; Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Taylor 2014; Gandolfi 2015; Sosnoff 2015; Cattaneo 2016; Hoang 2016; Carling 2017). We contacted authors on 11 of the included studies for additional participant data (Stephens 2001a; Esnouf 2010a; Coote 2013a; Lennon 2013a; Prosperini 2013a; Sosnoff 2014a; Taylor 2014a; Sosnoff 2015a; Cattaneo 2016a; Hoang 2016a; Carling 2017a). We received additional requested data relating to falls outcomes from the authors of the following studies (Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Taylor 2014; Carling 2017).
Participants
A total of 839 people with MS (range 12 to 177 individuals) were randomised to falls interventions or control interventions in the 13 included trials. The mean age of the participants was 52 years, ranging from 36 years (Prosperini 2013) to 62 years (Sosnoff 2015). Participants were diagnosed with MS using the McDonald criteria in three studies (Prosperini 2013; Hoang 2016; Carling 2017), eight studies did not report the criteria used to diagnose MS, but reported that participants were diagnosed with clinically‐definite MS (Stephens 2001; Cattaneo 2007; Esnouf 2010; Coote 2013; Lennon 2013; Sosnoff 2014; Sosnoff 2015; Gandolfi 2015) and two studies did not outline the criteria used to confirm MS diagnosis (Taylor 2014; Cattaneo 2016). Nine studies included participants with mixed types of MS (Stephens 2001; Cattaneo 2007; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Sosnoff 2015; Hoang 2016; Carling 2017), two studies included participants with secondary progressive MS only (Esnouf 2010; Taylor 2014), Gandolfi 2015 included people with relapsing‐remitting MS only and one study did not outline the type of MS included (Cattaneo 2016). The percentage of women participants ranged from 59% (Sosnoff 2015) to 85% (Sosnoff 2014). All trials delivered interventions in the community setting.
Interventions
Ten studies used two‐group comparisons including those that compared a falls intervention and control (Lennon 2013; Prosperini 2013; Sosnoff 2014; Hoang 2016; Carling 2017) or studies that compared two active falls interventions (Stephens 2001; Esnouf 2010; Taylor 2014; Gandolfi 2015; Cattaneo 2016). One study employed three‐group comparisons (Cattaneo 2007) and two studies used four‐group comparisons (Coote 2013; Sosnoff 2015). Cattaneo 2007 examined the effectiveness of motor and sensory, motor and conventional rehabilitation interventions; Coote 2013 compared the effectiveness of group exercise, individual physiotherapy exercise, yoga and control interventions and; Sosnoff 2015 examined the effect of home‐based exercise, exercise plus education, education and control interventions. Carling 2017 compared an exercise intervention with a wait list control group, wherein participants in the control group were delivered the exercise intervention at week seven of a 12‐week intervention. Therefore to make a comparison between an exercise falls intervention and control, we extracted data from Carling 2017 at seven weeks. Taylor 2014 and Prosperini 2013 used cross‐over trial designs. However, we included the pre‐cross‐over phase of these trials only. We did not combine the first and second phases of these trials because of uncertainty about the carryover effects in such trials, given that they are exercise and education interventions, wherein the wash‐out period is difficult to determine
Interventions to reduce falls varied across studies. Exercise interventions included interventions to promote improvements in: strength and balance function (Coote 2013; Sosnoff 2014; Sosnoff 2015; Carling 2017); balance function (Cattaneo 2007; Prosperini 2013; Gandolfi 2015; Cattaneo 2016; Hoang 2016); mobility and balance function (Stephens 2001; Lennon 2013); strength (Esnouf 2010; Taylor 2014). The majority of exercise interventions lasted from 6 to 12 weeks in duration (Stephens 2001; Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Taylor 2014; Sosnoff 2015; Cattaneo 2016; Hoang 2016; Carling 2017), while Gandolfi 2015 and Cattaneo 2007 delivered 5‐ and 3‐week exercise interventions, respectively. Frequency of exercise interventions ranged from once weekly (Coote 2013) to five times weekly (Prosperini 2013). Stephens 2001; Esnouf 2010; Coote 2013; Lennon 2013; Gandolfi 2015; Cattaneo 2016 and Carling 2017 evaluated group‐based exercise interventions, while home‐based exercise interventions were used in many of the included studies (Prosperini 2013; Taylor 2014; Sosnoff 2014; Sosnoff 2015 and Hoang 2016). Cattaneo 2007 and Coote 2013 also tested the effectiveness of individual exercise programmes delivered by Physiotherapists. Two studies compared the effectiveness of exercise interventions using home‐based step training systems with exercise games to group and home‐based exercise (Prosperini 2013 and Hoang 2016, respectively). Two studies compared the effectiveness of functional electrical stimulation (common peroneal nerve stimulation) to exercise interventions ( Esnouf 2010 and Taylor 2014).
Comparisons
The comparisons included wait list controls (Lennon 2013; Prosperini 2013; Sosnoff 2014; Sosnoff 2015; Carling 2017), treatment as usual controls (Coote 2013, Hoang 2016), other interventions that may reduce falls (Stephens 2001; Cattaneo 2007; Esnouf 2010; Coote 2013; Taylor 2014; Sosnoff 2015; Gandolfi 2015, Cattaneo 2016).
Outcomes
The 13 studies included a range of primary and secondary outcome measures. Outcomes were measured at the end of intervention for all included studies and at the end of 1‐month (Gandolfi 2015), 2‐month (Cattaneo 2016), 3‐month (Lennon 2013) and 6‐month (Lennon 2013) follow‐up periods. With regard to the primary outcomes; falls rate was reported by one study only (Taylor 2014). We therefore calculated the falls rate for each individual trial (number of falls per person per year) for the other included trials, wherein the required data were available. While it was not outlined in the protocol we have included the number of fallers (one or more fall) as an outcome in the review and was reported by six studies (Cattaneo 2007; Lennon 2013; Sosnoff 2014; Sosnoff 2015; Cattaneo 2016; Carling 2017). There were a range of definitions used for a fall and a variety of ways of collecting and reporting falls data in the included studies. Eight studies used prospective falls diaries to collect falls data (Stephens 2001; Esnouf 2010; Prosperini 2013; Taylor 2014; Gandolfi 2015; Sosnoff 2015; Hoang 2016; Carling 2017), three studies used retrospective methods of collecting self‐report falls data (Coote 2013; Sosnoff 2014; Sosnoff 2015 and two studies did not outline how falls data were collected (Cattaneo 2007; Cattaneo 2016). A variety of secondary outcome measures were used in the included studies; but only some trials shared the same outcomes and measures suitable for pooling.
Excluded studies
The most common reasons for exclusion were: a controlled trial in which the intervention did not meet the criteria for falls intervention or did not include a suitable comparison, or no falls outcomes were included. See Characteristics of excluded studies wherein we have outlined reasons for exclusion of each study.
Risk of bias in included studies
The risk of bias in the 13 included studies was generally mixed (Figure 2, Figure 3), with a high risk of selection bias associated with allocation concealment in one study (Lennon 2013), detection bias associated with lack of blinding of outcome assessment in two studies (Cattaneo 2007, Cattaneo 2016), attrition bias due to incomplete outcome data in two studies (Lennon 2013; Taylor 2014) and reporting bias due to selective reporting in seven studies (Coote 2013; Lennon 2013; Sosnoff 2014; Gandolfi 2015; Sosnoff 2015; Hoang 2016; Cattaneo 2016). We judged the risk of bias to be unclear in some instances mainly due to insufficient reporting of the methods used for random sequence generation (Stephens 2001; Coote 2013; Cattaneo 2016), allocation concealment (Stephens 2001; Cattaneo 2007; Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Taylor 2014; Hoang 2016; Cattaneo 2016), blinding of participants and personnel (Stephens 2001; Esnouf 2010; Coote 2013; Sosnoff 2014; Taylor 2014; Gandolfi 2015; Sosnoff 2015; Cattaneo 2016; Hoang 2016; Carling 2017), blinding of outcome assessment (Stephens 2001; Coote 2013; Taylor 2014), handling of incomplete outcome data (Stephens 2001; Coote 2013), and selective reporting (Stephens 2001; Cattaneo 2007; Esnouf 2010; Prosperini 2013; Taylor 2014; Gandolfi 2015; Cattaneo 2016). We judged that nine studies to have an unclear risk of other bias related to the fact that inferential statistics were computed without completing a formal sample size calculation, potentially exposing the study to a Type II statistical error (Stephens 2001; Cattaneo 2007; Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Taylor 2014; Sosnoff 2015).
2.
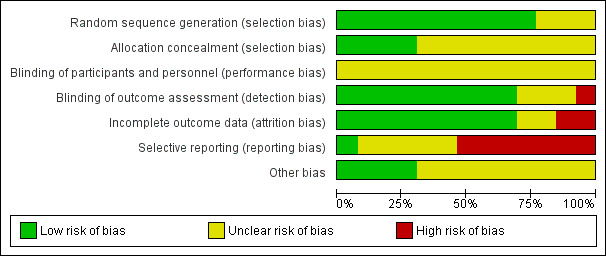
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
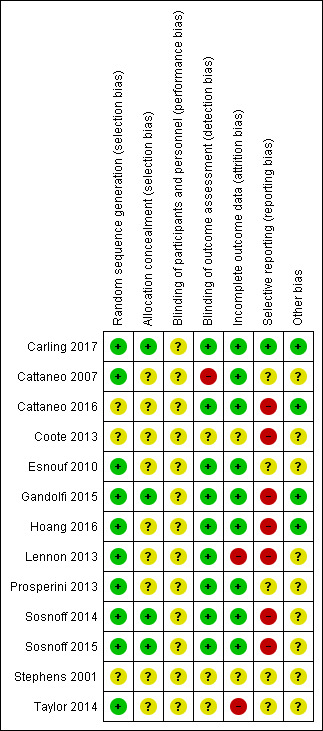
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten studies were judged to have a low risk of selection bias due to having adequate random sequence generation, having used a computerised random number generator by an independent unit (Cattaneo 2007; Esnouf 2010; Lennon 2013; Prosperini 2013; Sosnoff 2014; Taylor 2014; Gandolfi 2015; Sosnoff 2015; Hoang 2016; Carling 2017). The method used for random sequence generation and the risk of bias in three other studies was unclear (Stephens 2001; Coote 2013; Cattaneo 2016).
We judged four studies to have a low risk of selection bias due to effectively concealing allocation into groups using a concealed envelope system (Sosnoff 2014; Sosnoff 2015; Carling 2017) or having a separate staff member who was not otherwise involved in the study complete allocation (Gandolfi 2015). The remaining nine studies provided insufficient information in the paper to permit a judgement of high or low risk of bias and were deemed to have an unclear risk of bias relating to allocation concealment.
Blinding
Twelve studies were judged as having an unclear risk risk of performance bias due to lack of information provided on blinding procedures among participants or personnel blinding (Stephens 2001; Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Taylor 2014; Gandolfi 2015; Sosnoff 2015; Cattaneo 2016; Hoang 2016; Carling 2017).
Incomplete outcome data
We judged two studies to have a high risk of attrition bias due to incomplete outcome data (Lennon 2013; Taylor 2014). Nine studies were deemed to have a low risk of attrition bias as reasons for attrition were adequately explained in the paper (Cattaneo 2007; Esnouf 2010; Prosperini 2013; Sosnoff 2014; Sosnoff 2015; Gandolfi 2015; Cattaneo 2016; Hoang 2016; Carling 2017). Two studies did not provide sufficient information to permit a judgement of low or high risk of bias relating to incomplete outcome data ( Stephens 2001; Coote 2013).
Selective reporting
Eight studies provided a trial registration number that enabled the examination of the domain of selective reporting of outcomes. In five studies where no reference to a trial registration number or published protocol were provided, these studies were deemed to have an unclear risk of bias under this domain (Stephens 2001; Cattaneo 2007; Esnouf 2010; Prosperini 2013; Taylor 2014).
Of the eight studies that provided a trial registration number, one study was deemed to have a low risk of bias (Carling 2017) as all outcomes reported in the trial mapped to those presented in the protocol. The remaining seven studies were deemed to have a high risk of selective reporting of outcomes (Stephens 2001; Cattaneo 2007; Esnouf 2010; Prosperini 2013; Taylor 2014; Gandolfi 2015; Cattaneo 2016). In three studies, additional outcomes are presented in the paper that are not documented in the protocol (Lennon 2013; Cattaneo 2016; Hoang 2016) and in four studies, not all outcomes presented in the protocol are reported in the paper (Coote 2013; Sosnoff 2014; Gandolfi 2015; Sosnoff 2015).
Other potential sources of bias
In terms of other potential sources of bias, we focused on whether studies had reported the completion of a formal sample size calculation a priori. Nine studies had an unclear risk of bias related to the fact that inferential statistics were computed without completing a formal sample size calculation, potentially exposing the study to a Type II statistical error (Stephens 2001; Cattaneo 2007; Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Taylor 2014; Sosnoff 2015). The remaining studies were deemed to have a low risk of bias as a formal sample size was calculated a priori and the required number of participants were recruited.
Effects of interventions
See: Table 1
Effect of falls interventions on primary outcome measures
We present the results below according to the comparison being tested. Not all studies included all outcomes and therefore we report the results for primary and secondary outcomes, where available from the included studies. Therefore we have presented outcomes under two main comparison types:
Falls prevention intervention versus control
Falls prevention intervention versus another falls prevention intervention
Effect of interventions on primary outcomes
Comparison 1: Falls prevention interventions versus control
• Exercise versus control
See Table 1 for comparison of main outcomes.
The rate of falls
Of the seven trials that compared the effect of exercise interventions and controls, none included a measure of falls rate post‐intervention. The post‐intervention findings we reported relate to the assessment time points immediately after the interventions were delivered in the individual included studies. We used data (where available in trial publications and through contacting trial authors) from five studies (Coote 2013; Lennon 2013; Prosperini 2013; Hoang 2016; Carling 2017) in order to calculate falls rate. There was no significant effect of exercise compared to control on falls rate (Rate Ratio [RaR] 0.68, 95% CI 0.43 to 1.06, I2= 59%, n = 399, very low GRADE evidence) (Analysis 1.1).
1.1. Analysis.
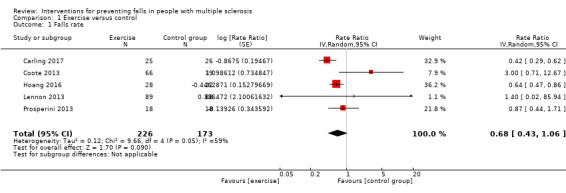
Comparison 1 Exercise versus control, Outcome 1 Falls rate.
The number of fallers
Five studies reported the number of fallers per group post‐intervention (Coote 2013; Lennon 2013; Sosnoff 2014; Sosnoff 2015; Carling 2017). There was no significant effect of treatment on the number of fallers post‐intervention (Risk Ratio [RR] 0.85, 95% CI 0.51 to 1.43, I2= 45%, n = 355, low GRADE evidence) (Analysis 1.2). Only one study (Lennon 2013) examined this outcome at 3‐month and 6‐month follow‐up, respectively. Results demonstrated no evidence of an effect in favour of exercise (RR 1.16, 95% CI 0.78 to 1.73 and RR 1.02, 95% CI 0.69 to 1.52) (Analysis 17.1 and Analysis 18.1 respectively).
1.2. Analysis.
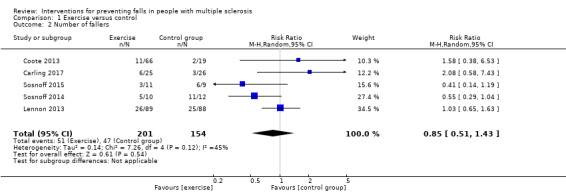
Comparison 1 Exercise versus control, Outcome 2 Number of fallers.
17.1. Analysis.
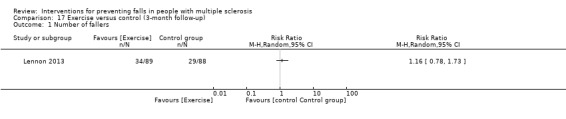
Comparison 17 Exercise versus control (3‐month follow‐up), Outcome 1 Number of fallers.
18.1. Analysis.
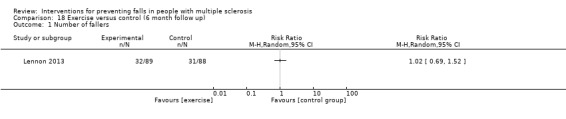
Comparison 18 Exercise versus control (6 month follow up), Outcome 1 Number of fallers.
Adverse events
Three studies reported the number of participants with adverse events per group (Sosnoff 2014; Sosnoff 2015; Hoang 2016). There was no evidence of an effect of the intervention (RR 1.25, 95% CI 0.26 to 6.03, I2=0%, n = 97, low GRADE evidence) (Analysis 1.3).
1.3. Analysis.
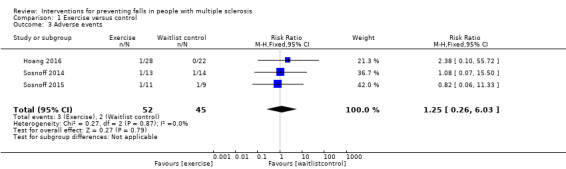
Comparison 1 Exercise versus control, Outcome 3 Adverse events.
• Education versus control
The number of fallers
One study examined the effect of an education intervention versus a wait list control on number of fallers (Sosnoff 2015). There was no evidence of an effect in favour of either group (RR 0.83, 95% CI 0.40 to 1.76) (Analysis 2.1).
2.1. Analysis.
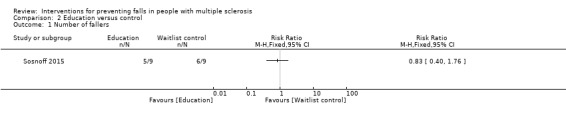
Comparison 2 Education versus control, Outcome 1 Number of fallers.
Adverse events
One study reported the number of participants with adverse events per group (Sosnoff 2015). There was no significant effect of education on the number of people reporting adverse events during the intervention (RR 2.00, 95% CI 0.22 to 18.33) (Analysis 2.2).
2.2. Analysis.
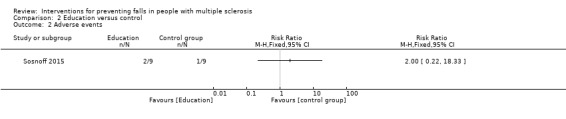
Comparison 2 Education versus control, Outcome 2 Adverse events.
• Exercise plus education versus control
The number of fallers
One study examined the effect of an exercise plus education intervention versus a wait list control on the number of fallers (Sosnoff 2015). There was no evidence of an effect in favour of either group (RR 0.30, 95% CI 0.04 to 2.20) (Analysis 3.1).
3.1. Analysis.
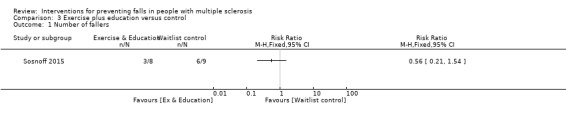
Comparison 3 Exercise plus education versus control, Outcome 1 Number of fallers.
Adverse events
One study reported the number of participants with adverse events per group (Sosnoff 2015). There was no significant effect of exercise plus education on the number of people reporting adverse events during the intervention (RR 3.38, 95% CI 0.43 to 26.30) (Analysis 3.2).
3.2. Analysis.
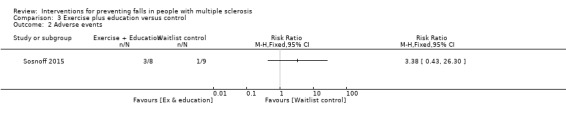
Comparison 3 Exercise plus education versus control, Outcome 2 Adverse events.
• Individual exercise versus control
Falls rate
One study examined the effect of individual exercise versus control on the rate of falls (Coote 2013). There was evidence of an effect in favour of control group (RaR 4.50, 95% CI 1.04 to 19.48) (Analysis 4.1).
4.1. Analysis.
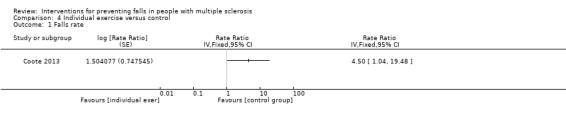
Comparison 4 Individual exercise versus control, Outcome 1 Falls rate.
The number of fallers
One study examined the effect of individual exercise versus control on the number of fallers (Coote 2013). There was no evidence of an effect in favour of either group (RR 2.11, 95% CI 0.51 to 8.74) (Analysis 4.2).
4.2. Analysis.
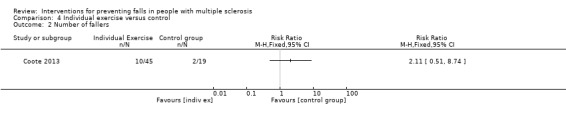
Comparison 4 Individual exercise versus control, Outcome 2 Number of fallers.
• Yoga versus control
Falls rate
One study examined the effect of yoga versus control on the rate of falls (Coote 2013). There was no evidence of an effect in favour of either group (RaR 4.67, 95% CI 0.99 to 21.99) (Analysis 5.1).
5.1. Analysis.
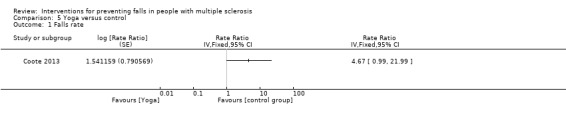
Comparison 5 Yoga versus control, Outcome 1 Falls rate.
Comparison 2: Falls intervention versus another falls intervention
• Functional Electrical Stimulation (FES) versus exercise
Falls rate
Two studies examined the effect of FES versus exercise on the rate of falls post‐intervention (Esnouf 2010; Taylor 2014). There was no evidence of an effect in favour of either group (RaR 0.91, 95% CI 0.78 to 1.06, I2 54%) (Analysis 6.1).
6.1. Analysis.
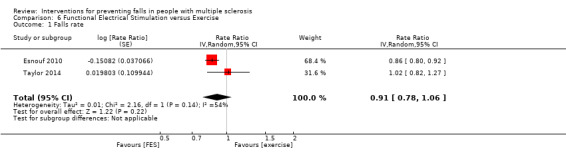
Comparison 6 Functional Electrical Stimulation versus Exercise, Outcome 1 Falls rate.
Adverse events
One study reported the number of participants with adverse events per group (Esnouf 2010). There was no significant effect of FES or exercise on the number of people reporting adverse events during the intervention (RR 2.00, 95% CI 0.39 to 10.16) (Analysis 6.2).
6.2. Analysis.
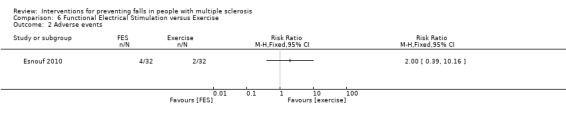
Comparison 6 Functional Electrical Stimulation versus Exercise, Outcome 2 Adverse events.
• Exercise versus education
Falls rate
One study examined the effect of exercise versus education on the rate of falls (Stephens 2001). There was no evidence of an effect in favour of either group (RaR 0.71, 95% CI 0.39 to 1.28) (Analysis 7.1).
7.1. Analysis.
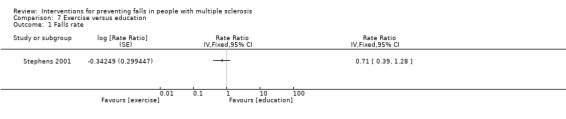
Comparison 7 Exercise versus education, Outcome 1 Falls rate.
The number of fallers
One study examined the effect of an exercise versus education on the number of fallers per group (Sosnoff 2015). There was no evidence of an effect in favour of the intervention (RR 0.49, 95% CI 0.16 to 1.52) (Analysis 7.2).
7.2. Analysis.
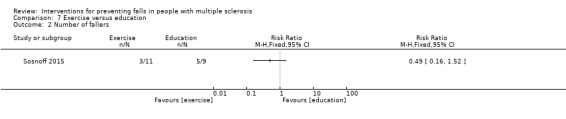
Comparison 7 Exercise versus education, Outcome 2 Number of fallers.
Adverse events
One study reported the number of participants with adverse events per group (Sosnoff 2015). There was no significant effect of either intervention on the number of people reporting adverse events during the intervention (RR 0.41, 95% CI 0.04 to 3.82) (Analysis 7.3).
7.3. Analysis.
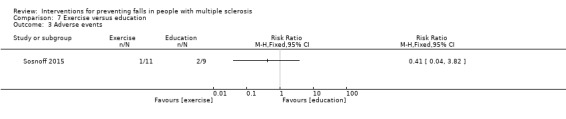
Comparison 7 Exercise versus education, Outcome 3 Adverse events.
Cost effectiveness
None of the studies reported data on cost‐effectiveness.
• Exercise versus exercise plus education
The number of fallers
One study examined the effect of an exercise plus education intervention versus exercise on the number of fallers (Sosnoff 2015). There was no evidence of an effect in favour of the intervention (RR 0.73, 95% CI 0.20 to 2.71) (Analysis 8.1).
8.1. Analysis.
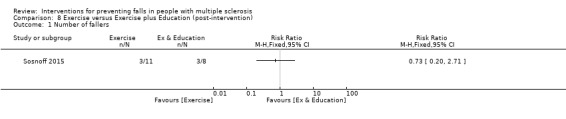
Comparison 8 Exercise versus Exercise plus Education (post‐intervention), Outcome 1 Number of fallers.
Adverse events
One study reported the number of participants with adverse events per group (Sosnoff 2015). There was no significant effect of either intervention on the number of people reporting adverse events during the intervention (RR 0.24, 95% CI 0.03 to 1.92) (Analysis 8.2).
8.2. Analysis.
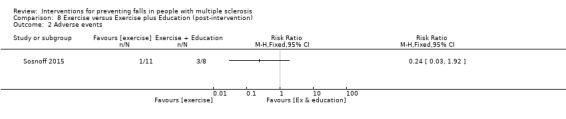
Comparison 8 Exercise versus Exercise plus Education (post‐intervention), Outcome 2 Adverse events.
• Education versus exercise plus education
The number of fallers
One study examined the effect of an exercise plus education intervention versus education on the number of fallers per group (Sosnoff 2015). There was no evidence of an effect in favour of the intervention (RR 2.08, 95% CI 0.30 to 14.55) (Analysis 9.1).
9.1. Analysis.
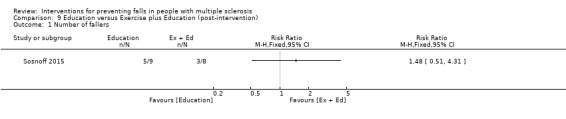
Comparison 9 Education versus Exercise plus Education (post‐intervention), Outcome 1 Number of fallers.
Adverse events
One study reported the number of participants with adverse events per group (Sosnoff 2015). There was no significant effect of either intervention on the number of people reporting adverse events during the intervention (RR 0.67, 95% CI 0.15 to 2.98) (Analysis 9.2).
9.2. Analysis.
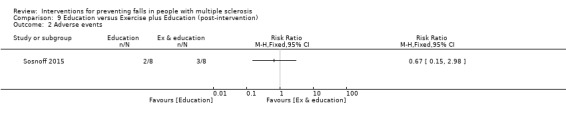
Comparison 9 Education versus Exercise plus Education (post‐intervention), Outcome 2 Adverse events.
• Sensory integration balance training versus conventional rehabilitation
Falls rate
One study examined the effect of sensory integration balance training (SIBT) versus conventional rehabilitation on the rate of falls (Gandolfi 2015). There was evidence of an effect in favour of the SIMT group (RaR 0.10, 95% CI 0.01 to 0.67) (Analysis 10.1).
10.1. Analysis.
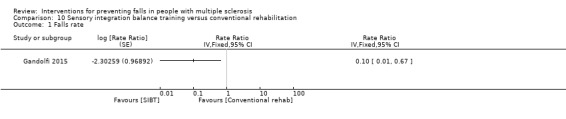
Comparison 10 Sensory integration balance training versus conventional rehabilitation, Outcome 1 Falls rate.
• Motor and sensory balance rehabilitation versus motor balance rehabilitation
Falls rate
One study examined the effect of motor and sensory balance rehabilitation versus motor balance rehabilitation on the rate of falls (Cattaneo 2007). There was no evidence of an effect in favour of either group (RaR 6.00, 95% CI 0.38 to 95.93) (Analysis 11.1).
11.1. Analysis.
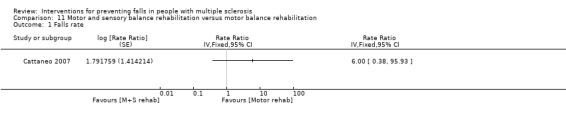
Comparison 11 Motor and sensory balance rehabilitation versus motor balance rehabilitation, Outcome 1 Falls rate.
The number of fallers
One study examined the effect of motor and sensory balance rehabilitation versus motor balance rehabilitation on the number of fallers (Cattaneo 2007). There was no evidence of an effect in favour of either group (RR 0.48, 95% CI 0.03 to 6.96) (Analysis 11.2).
11.2. Analysis.
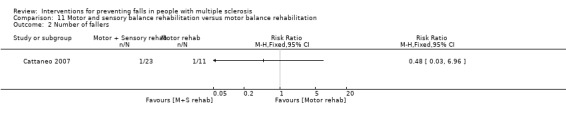
Comparison 11 Motor and sensory balance rehabilitation versus motor balance rehabilitation, Outcome 2 Number of fallers.
• Motor and sensory balance rehabilitation versus conventional rehabilitation
Falls rate
One study examined the effect of motor and sensory balance rehabilitation versus conventional rehabilitation on the rate of falls (Cattaneo 2007). There was no evidence of an effect in favour of either group (RaR 3.00, 95% CI 0.31 to 28.84) (Analysis 12.1).
12.1. Analysis.
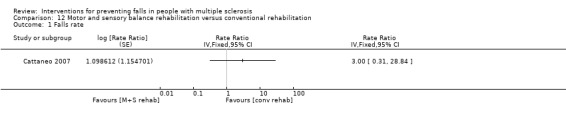
Comparison 12 Motor and sensory balance rehabilitation versus conventional rehabilitation, Outcome 1 Falls rate.
The number of fallers
One study examined the effect of a motor and sensory balance rehabilitation intervention versus conventional rehabilitation on the number of fallers (Cattaneo 2016). There was no evidence of an effect in favour of the intervention (RR 1.05, 95% CI 0.49 to 2.25 post‐intervention) (Analysis 12.2). However, there was evidence of an effect in favour of the conventional rehabilitation at 2‐month follow‐up with a RR of 9.46, 95% CI 1.31 to 68.38 (Analysis 19.1).
12.2. Analysis.
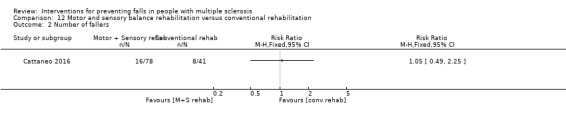
Comparison 12 Motor and sensory balance rehabilitation versus conventional rehabilitation, Outcome 2 Number of fallers.
19.1. Analysis.
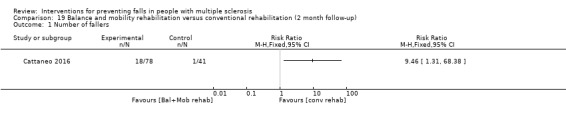
Comparison 19 Balance and mobility rehabilitation versus conventional rehabilitation (2 month follow‐up), Outcome 1 Number of fallers.
• Motor balance rehabilitation vs conventional non balance rehabilitation
Falls rate
One study examined the effect of motor balance rehabilitation versus conventional non‐balance rehabilitation on the rate of falls (Cattaneo 2007). There was no evidence of an effect in favour of either group (RaR 0.50, 95% CI 0.05 to 4.81) (Analysis 13.1).
13.1. Analysis.
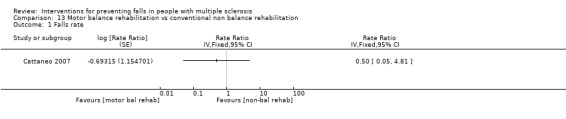
Comparison 13 Motor balance rehabilitation vs conventional non balance rehabilitation, Outcome 1 Falls rate.
The number of fallers
One study examined the effect of a motor balance intervention vs conventional non balance rehabilitation on the number of fallers (Cattaneo 2007). There was no evidence of an effect in favour of the intervention (RR 1.20, 95% CI 0.07 to 21.72 post‐intervention) (Analysis 13.2).
13.2. Analysis.
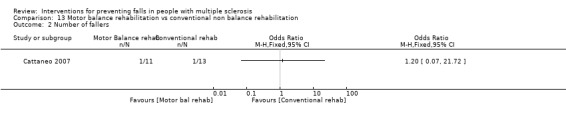
Comparison 13 Motor balance rehabilitation vs conventional non balance rehabilitation, Outcome 2 Number of fallers.
• Group exercise versus Yoga
Falls rate
One study examined the effect of group exercise versus yoga on the rate of falls (Coote 2013). There was no evidence of an effect in favour of either group (RaR 0.75, 95% CI 0.34 to 1.66) (Analysis 14.1).
14.1. Analysis.
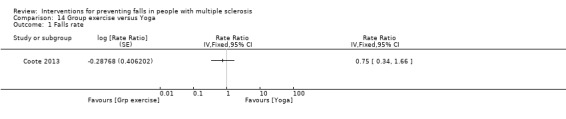
Comparison 14 Group exercise versus Yoga, Outcome 1 Falls rate.
The number of fallers
One study examined the effect of group exercise versus yoga on the number of fallers (Coote 2013). There was no evidence of an effect in favour of either group (RR 0.87, 95% CI 0.21 to 3.56) (Analysis 14.2).
14.2. Analysis.
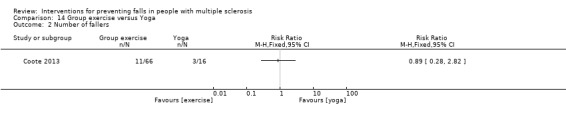
Comparison 14 Group exercise versus Yoga, Outcome 2 Number of fallers.
• Group exercise versus individual exercise
Falls rate
One study examined the effect of group exercise versus individual exercise on the rate of falls (Coote 2013). There was no evidence of an effect in favour of either group (RaR 1.00, 95% CI 0.54 to 1.85) (Analysis 15.1).
15.1. Analysis.
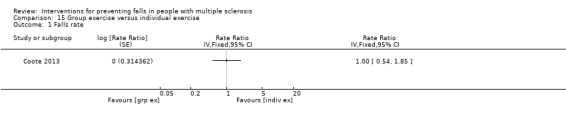
Comparison 15 Group exercise versus individual exercise, Outcome 1 Falls rate.
The number of fallers
One examined the effect of group exercise versus individual exercise on the number of fallers (Coote 2013). There was no evidence of an effect in favour of either group (RR 0.70, 95% CI 0.27 to 1.82) (Analysis 15.2).
15.2. Analysis.
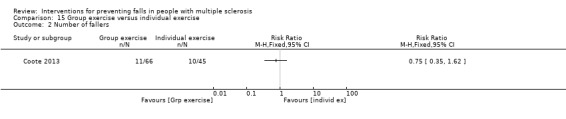
Comparison 15 Group exercise versus individual exercise, Outcome 2 Number of fallers.
• Individual exercise versus yoga
The rate of falls
One study examined the effect of individual exercise versus yoga on the rate of falls (Coote 2013). There was no evidence of an effect in favour of either group (RaR 0.75, 95% CI 0.32 to 1.74) (Analysis 16.1).
16.1. Analysis.
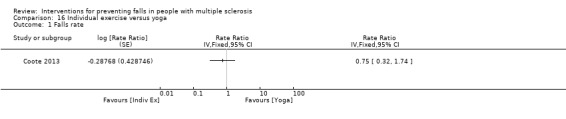
Comparison 16 Individual exercise versus yoga, Outcome 1 Falls rate.
The number of fallers
One examined the effect of individual exercise versus yoga on the number of fallers (Coote 2013). There was no evidence of an effect in favour of either group (RR 1.19, 95% CI 0.37 to 3.77) (Analysis 16.2).
16.2. Analysis.
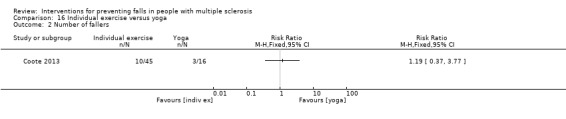
Comparison 16 Individual exercise versus yoga, Outcome 2 Number of fallers.
Effect of interventions on secondary outcome measures
Comparison 1: Falls intervention versus control
• Exercise versus control
Physiological falls risk
Two studies (Sosnoff 2014; Sosnoff 2015),examined the effect of exercise versus control on physiological falls risk post‐intervention measured using the Physiological Profile Assessment (PFA). There was no evidence of an effect in favour of either group with a mean difference of 0.68 (95% CI ‐0.27 to 1.63, I2= 42%).
Balance function
One study examined the effect of exercise versus control on postural sway in standing post‐intervention using a swaymeter (eyes open) (Carling 2017). There was no evidence of an effect in favour of the intervention with a mean difference of ‐ 512.12 (95% CI ‐ 6357.16 to 5332.92). One study (Sosnoff 2014) examined the effect of exercise versus control on balance confidence post‐intervention, using the Activities‐Specific Balance Confidence (ABC) scale. There was no evidence of an effect in favour of the intervention with a standardised mean difference of 5.70 (95% CI ‐11.07 to 22.47). Five studies (Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Carling 2017) examined the effect of exercise versus control on balance function post‐intervention, measured using the Berg Balance Scale (BBS) (Coote 2013; Lennon 2013; Sosnoff 2014; Carling 2017) and the Four Step Square Test (FSST) (Prosperini 2013). There was evidence of an effect in favour of exercise with a standardised mean difference of 0.50 (95% CI 0.09 to 0.92, I2=66%). One study examined the effect of exercise versus control on postural sway in standing post‐intervention using a swaymeter (eyes closed) (Carling 2017). There was no evidence of an effect in favour of the intervention with a mean difference of ‐ 615.43 (95% CI ‐7458.57 to 6227.71). Two studies examined the effect of exercise versus control on dynamic balance post‐intervention (Four Step Square Test [4SST] and choice step reaction test) (Prosperini 2013; Hoang 2016). There was no evidence of an effect in favour of the intervention with a mean difference of 0.65 (95% CI ‐0.04 to 1.34, I2=59%).
Psychological measures
Two studies (Coote 2013; Lennon 2013) examined the effect of exercise versus control on the psychological impact of MS post‐intervention, using the Multiple Sclerosis Impact Scale (psychological sub‐component). There was no evidence of an effect in favour of either group with a mean difference of 5.52 (95% CI ‐3.90 to 14.95, I2=83%). One study (Lennon 2013) examined the effect of exercise versus control on MS self‐efficacy, post‐intervention using the Multiple Sclerosis Self‐Efficacy (MSSE) scale . There was evidence of an effect in favour of the control group with a mean difference of ‐7.58 (95% CI –12.57 to ‐2.59). One study (Carling 2017) examined the effect of exercise versus control on falls self‐efficacy post‐intervention, using the Falls Efficacy Scale‐International (FES‐I). There was no evidence of an effect in favour of the intervention with a mean difference of ‐0.57 (95% CI ‐26.35 to 25.21). One study (Prosperini 2013) examined the effect of exercise versus control on the physical and psychological impact of MS post‐intervention, using the Multiple Sclerosis Impact Scale (physical and psychological sub‐components). There was no evidence of an effect in favour of the intervention with a mean difference of 9.00 (95% CI ‐5.73 to 23.73).
Cognition
One study (Hoang 2016) examined the effect of exercise versus control on cognition post‐intervention using the Timed Up and Go‐Cognitive (TUG‐Cog) measure . There was no evidence of an effect in favour of the exercise with a mean difference of 0.70 (95% CI ‐2.21 to 3.61). One study (Hoang 2016) examined the effect of exercise versus control on cognition post‐intervention using the Symbols Digit Modality Test (SDMT) measure. There was no evidence of an effect in favour of the exercise with a mean difference of ‐1.00 (95% CI –6.96 to 4.96). One study (Hoang 2016) examined the effect of exercise versus control on cognition post‐intervention using the Trail Making Test (TMT) measure. There was no evidence of an effect in favour of the exercise with a mean difference of ‐7.10 (95% CI –25.72 to 11.52). One study (Hoang 2016) examined the effect of exercise versus control on cognition post‐intervention using the Stroop stepping test measure . There was evidence of an effect in favour of exercise with a mean difference of 16.40 (95% CI 5.34 to 27.46).
Mobility
Self‐reported mobility
Three studies (Lennon 2013; Sosnoff 2014; Carling 2017) examined the effect of exercise versus control on self‐reported mobility post‐intervention using the Multiple Sclerosis Walking Scale‐12 (MSWS‐12). There was evidence of an effect in favouring exercise with a mean difference of 16.30 (95% CI 9.34 to 23.26, I2=0%). At 3‐month and 6‐month follow‐up points only one study (Lennon 2013) examined this outcome demonstrating no evidence of an effect in favour of either group with a mean difference of 2.89 (95% CI ‐5.09 to 10.87) at 3‐month follow‐up and a mean difference of 0.70 (95% CI ‐ 7.71 to 9.11).
Long walking measures of mobility
Four studies examined the effect of exercise versus control on long walking tests of mobility post‐intervention using the Six Minute Walk Test (6MWT) (Coote 2013; Sosnoff 2014; Hoang 2016) and the Two Minute Walk Test (2MWT) Carling 2017). There was no evidence of an effect in favour of either group with a standardised mean difference of 0.18 (95% CI ‐ 0.24 to 0.60, I2=49%).
Short walking measures of mobility
Five studies (Lennon 2013; Prosperini 2013; Sosnoff 2014; Hoang 2016; Carling 2017) examined the effect of exercise versus control on short walking tests of mobility post‐intervention (25Ft walk and 10m walk) There was evidence of an effect in favour of exercise with a standardised mean difference of 0.28 (95% CI 0.07 to 0.50, I2=0%). At 3‐month and 6‐month follow‐up points only one study (Lennon 2013) examined this outcome demonstrating no evidence of an effect in favour of either group with a mean difference of ‐ 0.10 (95% CI ‐ 0.22 to 0.02, 95% CI ‐ 0.01 to 0.21). Three studies (Sosnoff 2014; Hoang 2016; Carling 2017) examined the effect of exercise versus control on short walking tests of mobility using the TUG measure. There was no evidence of an effect in favour of either group with a mean difference of 2.26 (95% CI ‐ 3.24 to 7.75, I2= 81%).
Functional outcome
One study (Lennon 2013) examined the effect of exercise versus control on basic activities of daily living post‐intervention using the Barthel Activities of Daily Living scale. There was no effect in favour of exercise with a mean difference of 0.63 (95% CI 0.07 to 1.19).
Fatigue
Two studies examined the effect of exercise versus control on fatigue post‐intervention using the Modified Fatigue Impact Scale (MFIS) (Coote 2013) and the Fatigue scale for Motor and Cognitive functions (Carling 2017). There was no effect in favour of the intervention (standardised mean difference 0.24, 95% CI ‐ 0.14 to 0.61, I2 = 16%).
Cost effectiveness
None of the studies reported data on cost‐effectiveness.
• Education versus control
Physiological falls risk
One study examined the effect of education versus control on physiological falls risk post‐intervention using the PPA (Sosnoff 2015). There was no effect in favour of the intervention with a mean difference of 0.40 (95% CI ‐ 1.37 to 0.57).
• Exercise and education versus control
Physiological falls risk
One study examined the effect of exercise plus education versus control on physiological falls risk post‐intervention using the PPA (Sosnoff 2015). There was no effect in favour of the intervention with a mean difference of 0.50 (95% CI ‐ 0.79 to 1.79).
• Yoga versus control
Balance function
One study examined the effect of yoga exercise versus control on balance function post‐intervention using the BBS (Coote 2013). There was no e effect in favour of the intervention with a MD of 6.10, 95% CI ‐1.67 to 13.87, p=0.12.
Psychological measures
One study examined the effect of yoga exercise versus control on the psychological impact of MS post‐intervention using the MSIS‐psychological sub‐component (Coote 2013). There was no effect in favour of the intervention with a mean difference of 2.05 (95% CI ‐ 1.89 to 5.99).
Fatigue
One study examined the effect of yoga exercise versus control on fatigue post‐intervention using the MFIS (Coote 2013). There was no effect in favour of the intervention with a mean difference of 10.10 (95% CI ‐ 2.16 to 22.36).
• Individual exercise versus control
Balance function
One study examined the effect of individual exercise versus control on balance function post‐intervention using the BBS (Coote 2013). There was an effect in favour of the individual exercise group with a mean difference of 12.40 (95% CI 6.33 to 18.47).
Psychological measures
One study examined the effect of individual exercise versus control on the psychological impact of MS post‐intervention using the MSIS‐psychological sub‐component (Coote 2013). There was no effect in favour of the intervention with a mean difference of 0.44 (95% CI ‐ 3.06 to 3.94).
Fatigue
One study examined the effect of individual exercise versus control on fatigue post‐intervention using the MFIS (Coote 2013). There was no effect in favour of the intervention with a mean difference of 3.10 (95% CI ‐ 5.57 to 11.77).
Comparison 2: Falls intervention versus another falls intervention
• Functional electrical stimulation versus exercise
Psychological measures
One study examined the effect of FES versus exercise on the psychological impact of MS post‐intervention using the MSIS‐29 (Taylor 2014). There was no effect in favour of either group with a mean difference of ‐13.90 (95% CI ‐ 30.29 to 2.49).
Mobility
One study examined the effect of FES versus exercise on mobility post‐intervention using 10m walking speed (Taylor 2014). There was no effect in favour of either group with a mean difference of 0.22 (95% CI ‐ 0.57 to 1.02).
• Exercise versus education
Physiological falls risk
One study examined the effect of exercise versus education on the physiological falls risk post‐intervention using the PFA (Sosnoff 2015). There was no effect in favour of the intervention with a mean difference of 0.60 (95% CI ‐ 0.41 to 1.61).
Balance function
One study examined the effect of exercise versus education on computerised balance assessment post‐intervention (Stephens 2001). There was no effect in favour of the intervention with a mean difference of 0.37 (95% CI ‐ 0.19 to 0.92). One study examined the effect of exercise versus education on balance confidence post‐intervention using the ABC scale (Stephens 2001).There was no effect in favour of the intervention with a mean difference of ‐ 5.73 (95% CI ‐ 25.37 to 13.91).
Psychological measures
One study examined the effect of exercise versus education on self‐efficacy post‐intervention using the MSSE (Stephens 2001). There was no effect in favour of the intervention with a mean difference of 12.80 (95% CI ‐ 23.70 to 49.30).
• Exercise plus education versus exercise
Physiological falls risk
One study examined the effect of exercise plus education versus exercise on the physiological falls risk post‐intervention using the PFA (Sosnoff 2015). There was non effect in favour of either group with a mean difference of ‐ 0.90 (95% CI ‐ 2.22 to 0.42).
•Exercise plus education versus education
Physiological falls risk
One study examined the effect of exercise plus education versus exercise on the physiological falls risk post‐intervention using the PFA (Sosnoff 2015). There was no effect in favour of either group with a mean difference of ‐ 0.90 (95% CI ‐ 2.22 to 0.42).
• Sensory integration balance training versus conventional rehabilitation
Quality of life
One study examined the effect of Sensory Integration Balance Training (SIBT) versus conventional rehabilitation on quality of life post‐intervention using the MS Quality of Life‐54 scale (mental component) (Gandolfi 2015). There was no effect in favour of either group with a mean difference of 2.23 (95% CI ‐ 4.62 to 9.08). No evidence of an effect was evident at one‐month follow‐up with a mean difference of ‐ 0.06 (95% CI ‐ 6.99 to 6.87). One study examined the effect of SIBT versus conventional rehabilitation on quality of life post‐intervention using the MS Quality of Life‐54 scale (physical component) (Gandolfi 2015). There was no effect in favour of either group with a mean difference of 5.92 (95% CI 1.51 to 10.33). The effect was in favour of the SIBT group at one‐month follow‐up (mean difference 5.02, 95% CI 0.2 to 9.82).
Balance function
One study examined the effect SIBT versus conventional rehabilitation on balance function using the BBS (Gandolfi 2015). There was an effect in favour of SIBT with a mean difference of 4.98 (95% CI 2.88 to 7.08). This effect was also evident at one‐month follow‐up with a mean difference of 4.59 (95% CI 2.56 to 6.62). One study examined the effect of sensory integration balance training versus conventional rehabilitation on balance confidence post‐intervention using the ABC scale (Gandolfi 2015). There was an effect in favour of SIBT with a mean difference of 8.97 (95% CI 0.94 to 17.00). This effect was maintained at one‐month follow‐up (mean difference 8.43, 95% CI 0.92 to 15.94).
Fatigue
One study examined the effect of sensory integration balance training versus conventional rehabilitation on fatigue post‐intervention using the Fatigue Severity Scale (FSS) (Gandolfi 2015). There was no effect in favour of either group with a mean difference of 0.73 (95% CI ‐ 0.01 to 1.45). There was no evidence of an effect in favour of the SIBT at one‐month follow‐up with a mean difference of 1.25 (95% CI 0.55 to 1.95).
• Motor and sensory balance rehabilitation versus motor balance rehabilitation
Balance function
One study examined the effect of motor and sensory balance rehabilitation versus motor balance rehabilitation on balance function (BBS) (Cattaneo 2007). There was no effect in favour of either group with a mean difference of 1.65 (95% CI ‐ 2.06 to 5.36). One study examined the effect of motor and sensory balance rehabilitation versus motor balance rehabilitation on balance confidence post‐intervention using the ABC scale (Cattaneo 2007).There was no effect in favour either group with a mean difference of ‐ 10.78 (95% CI ‐ 23.27 to 1.71).
• Motor and sensory balance rehabilitation versus conventional rehabilitation
Balance function
Two studies examined the effect of motor and sensory balance rehabilitation versus conventional rehabilitation on balance function post‐intervention using the BBS (Cattaneo 2007; Cattaneo 2016). There was no effect in favour of either group with a mean difference of 4.01 (95% CI ‐ 3.90 to 11.92, I2= 89%). Two studies examined the effect of motor and sensory balance rehabilitation versus conventional rehabilitation on balance confidence post‐intervention using the ABC scale (Cattaneo 2007; Cattaneo 2016). There was no effect in favour of either group with a mean difference of ‐ 3.82 (95% CI ‐ 8.63 to 0.98, I2= 0%).
Mobility
Two studies examined the effect of of motor and sensory balance rehabilitation versus conventional rehabilitation on mobility (DGI) (Cattaneo 2007, Cattaneo 2016). There was no effect in favour of either group with a mean difference of 2.01 (95% CI ‐ 3.48 to 7.49, I2=86%). One study examined the effect of motor and sensory balance rehabilitation versus conventional rehabilitation on mobility (TUG) (Cattaneo 2016). There was no effect in favour of either group with a mean difference of 0.10 (95% CI ‐ 1.70 to 1.90).
• Motor balance rehabilitation vs conventional non balance rehabilitation
Balance function
One study examined the effect of motor balance rehabilitation versus conventional non balance rehabilitation on balance function (BBS) post‐intervention (Cattaneo 2007). There was an effect in favour of the motor balance rehabilitation group with a mean difference of 6.75 (95% CI 1.09 to 12.41). One study examined the effect of motor balance rehabilitation versus conventional non balance rehabilitation on balance confidence (ABC) (Cattaneo 2007). There was no effect in favour of either group with a mean difference of 6.81 (95% CI ‐ 6.54 to 20.16).
Mobility
One study examined the effect of motor and sensory balance rehabilitation versus conventional non balance rehabilitation on mobility (DGI) (Cattaneo 2007). There was no effect in favour of either group with a mean difference of 1.83 (95% CI ‐ 2.83 to 6.49).
• Group exercise versus Yoga
Balance function
One study examined the effect of group exercise versus yoga on balance function (BBS) (Coote 2013). There was an effect in favour of the exercise group with a mean difference of 6.60 (95% CI 0.49 to 12.71).
Psychological measures
One study examined the effect of group exercise versus yoga on the psychological impact of MS post‐intervention using the MSIS‐29 scale (Coote 2013). There was no effect in favour of either group with a mean difference of ‐ 0.84 (95% CI ‐ 3.62 to 1.94).
Fatigue
One study examined the effect of group exercise versus yoga on fatigue post‐intervention using the MFIS (Coote 2013). There was no effect in favour of either group with a mean difference of ‐ 3.10 (95% CI ‐13.37 to 7.17).
• Group exercise versus individual exercise
Balance function
One study examined the effect of group exercise versus individual exercise on balance function (BBS) post‐intervention (Coote 2013). There was no effect in favour of either group with a mean difference of 0.30 (95% CI ‐ 3.41 to 4.01).
Psychological measures
One study examined the effect of group exercise versus individual exercise on the psychological impact of MS post‐intervention using the MSIS‐29 scale (Coote 2013). There was no e effect in favour of either group with a mean difference of 0.77 (95% CI ‐ 1.34 to 2.88).
Fatigue
One study examined the effect of group exercise versus individual exercise on fatigue post‐intervention using the MFIS (Coote 2013). There was no effect in favour of either group with a mean difference of 3.90 (95% CI ‐ 1.59 to 9.39).
• Individual exercise versus yoga
Balance function
One study examined the effect of individual exercise versus yoga on balance function (BBS) (Coote 2013). There was no e effect in favour of either group with a mean difference of 6.30 (95% CI ‐ 0.02 to 12.62).
Psychological measures
One study examined the effect of individual exercise versus yoga on the psychological impact of MS post‐intervention using the MSIS‐29 scale (Coote 2013). There was no effect in favour of either group with a mean difference of ‐ 1.61 (95% CI ‐ 4.63 to 1.41).
Discussion
Summary of main results
Despite the fact that the development and evaluation of interventions to reduce falls for people with MS has received increased scientific interest in the last decade, the evidence base presented in this review demonstrates many important unanswered questions and methodological short‐comings.
We included 13 RCTs or cross‐over RCTs in this review. These studies included either exercise interventions (of various delivery mechanism), functional electrical stimulation interventions, education interventions or interventions comprising multiple intervention components, e.g. exercise plus education. The included trials were of mixed quality, with many of them not adhering to the CONSORT guidelines (Moher 2012) and demonstrating mixed risk of bias throughout (see "Assessment of risk of bias in included studies").
Comparison 1: Falls prevention interventions versus control
Previous Cochrane reviews of falls interventions for older adults grouped trials by exercise modality into six categories using the ProFaNE taxonomy (Gillespie 2012). However for the first comparison, comparing falls prevention interventions to controls, in the current review we treated exercise as a single intervention and did not report results based on different sub‐groupings of exercise modality. The rationale for pooling different types of exercise interventions and comparing to controls, relates to the scarcity of studies within this comparison.
Pooled comparisons post‐intervention (immediately post‐intervention in the included studies) between exercise and controls for any of the primary falls outcomes, demonstrated no evidence of an effect in favour of exercise compared to controls (Table 1). Relating to the pooled rate of falls outcome, which is recommended as the gold standard measurement of falls in MS trials (Coote 2014), there was a non‐significant effect in favour of exercise compared to controls. However, due to the "very low" and "low" GRADE grading for the primary outcomes, there is uncertainty on the effect of exercises on prevention of falls compared to control.
For all of the other comparisons between falls interventions and controls, pooling of falls data across studies was not possible due to the heterogeneity of the interventions being tested. Results demonstrated that there was no evidence of an effect in favour of education, exercise plus education or yoga over control interventions for any of the falls outcomes. To our knowledge, this is the first review to aim to include falls prevention interventions, other than exercise‐only interventions.
Mobility dysfunction has been demonstrated to be an independent risk factor for falls in MS (Gunn 2013). Our results demonstrated that there was pooled evidence from five studies demonstrating a positive effect of exercise compared with controls for self‐reported mobility (Lennon 2013; Prosperini 2013; Sosnoff 2014; Hoang 2016; Carling 2017) and three studies for short objective walking tests (Lennon 2013; Sosnoff 2014; Carling 2017). Again, caution is needed in the interpretation of these results, given the wide nature of the confidence intervals presented.
There was no evidence from the included studies to demonstrate support of exercise interventions for the improvement of other secondary outcomes such as physiological falls risk, fatigue, long walking tests of mobility/walking endurance, and cognition.
Comparison 2: Falls prevention interventions versus other intervention
Due to the scarcity of data for pooling across all comparisons, there were data available from only two studies (Esnouf 2010; Taylor 2014), for two of our main outcomes only (number of falls per person and rate of falls), for the comparison between FES and exercise interventions. Summary results demonstrate no evidence in favour of FES or exercise for falls prevention among people with MS.
The substantial heterogeneity evident in the other types of interventions included in the studies in this comparison precluded pooling data for all other outcomes, and therefore we have presented results of individual studies per outcome (see the Effects of interventions section).
Overall completeness and applicability of evidence
Participants
As the majority of trials specifically excluded people who presented with severe disability due to MS, the results of this review may not be applicable to this group of people at risk. Participant characteristics did not vary greatly due to the recruitment methods used, and the inclusion and exclusion criteria applied. Participants in the majority of studies included people with mild to moderate severity of MS. Some trials recruited people being treated in hospital clinics, while the majority included people living in the community. None of the trials exclude people based on their falls history.
Interventions
Interventions to reduce falls are complex in nature and therefore more detail is required in published trials regarding what participants are experiencing in both the intervention and comparison groups. The description of the intervention and control groups in many of the studies could have been improved upon. Guidelines such as the Template for Intervention Description and Replication (TIDieR) (Hoffmann 2014) are essential for future trials including falls interventions. At protocol stage we planned to examine the effectiveness of single, multiple‐ and multifactorial interventions to reduce falls rate. However, given the low number of studies available for inclusion, the scarcity of multiple‐component interventions (only one study included a combined exercise and education intervention (Sosnoff 2015), and the complete lack of multi‐factorial interventions, this was not possible in the current review. Given the established effectiveness of multi‐factorial interventions to target known risk factors for falls in older adult populations (Gillespie 2012), there is strong rationale for the evaluation of such multi‐factorial interventions in MS populations. The effectiveness of interventions targeting most risk factors for falls in MS has not been well researched or established. While the identification of risk factors for falling in MS has received increased scientific interest in the last decade, there is still much clarification needed regarding the most suitable factors to target in interventions. Gunn 2013 conducted a systematic review and meta‐analysis of falls risk factors in MS and identified 20 risk factors. Due to the heterogeneity of included studies, pooled meta‐analysis was feasible for only four individual risk factors: impairments of balance (Odds ratio: 1.07, 95% CI 1.04 to 1.10); use of a mobility aid (OR: 2.5, 95% CI 2.21 to 2.83); cognitive impairments (OR:1.28, 95% CI 1.2 to 1.36) and; progressive versus relapse‐remitting MS (OR:1.98, 95% CI 1.39 to 2.80). This is in notable contrast to the older adult literature, wherein over 400 risk factors for falls have been identified Oliver 2004). Gaps include the examination of interventions addressing the management of urinary incontinence, medical interventions, and environmental modifications among people with MS.
Outcomes
We sought data for rate of falls and number of people falling. Few studies provided falls rate data. As the analyses demonstrate, some studies provided data for both falls and fallers, as recommended by the ProFaNE network (Lamb 2005; Lamb 2011) and the International MS FAlls Prevention Research Network (IMSFPRN) (Coote 2014; Sosnoff 2014b). Other studies provided data only for one or other fall outcome.
The selection of outcomes in the included trials also highlights some limitations. The fact that the outcome of interest, falling, was not always defined, is notable. Comparability of future research findings would be facilitated by adoption of the consensus definition of a fall as "an unexpected event in which the participants come to rest on the ground, floor, or lower level" developed for trials in clinical populations by the ProFaNE network (Lamb 2005; Lamb 2011) and recommended for use in MS trials by the the IMSFPRN (Coote 2014; Sosnoff 2014b). The included studies also illustrated the wider problems of variation in the methods of ascertaining, recording, analysing, and reporting falls. Studies should use consensus recommendations (IMSFPRN) for conducting fall prevention trials which include the daily recording of falls, with bi‐weekly phone reminders, monthly returns and follow‐up by the researchers blind to group allocation, with a falls data collection period of at least three months in duration (Coote 2014). In the current review, only one study included a measure of falls rate (Taylor 2014) and only eight studies used a prospective method to collect falls data (Esnouf 2010; Lennon 2013; Prosperini 2013; Taylor 2014; Gandolfi 2015; Sosnoff 2015; Hoang 2016; Carling 2017), and seven studies collected data for three months in duration (Esnouf 2010; Coote 2013; Lennon 2013; Prosperini 2013; Sosnoff 2014; Sosnoff 2015; Hoang 2016).
The current review aimed to examine the effectiveness of falls interventions on a large breadth of secondary outcomes, including balance function, function, mobility, fatigue, cognition, physiological falls risk. The results demonstrate that while balance and mobility outcomes were relatively well‐addressed in the included studies, there are substantial gaps in terms of potentially important patient‐oriented and cost‐related outcomes which need to be considered in subsequent trials of falls interventions in MS. Of the 13 included studies only one study included measures of cognition (Hoang 2016) and only three studies included falls self‐efficacy as an outcome (Carling 2017). Given that cognitive impairment is an established risk factor for falling in MS (Gunn 2013) and reduced self‐efficacy related to falls has been identified as an independent risk factor for falling in MS (Matsuda 2012), future falls prevention trials need to consider these important outcomes. Of note, the cost‐effectiveness of included interventions was not examined by any of the included studies, demonstrating an important outcome for inclusion in future trials of falls prevention interventions in MS.
Quality of the evidence
This review containing 13 trials (839 participants) provides uncertain evidence regarding the effect in favour of exercise interventions compared to treatment as usual control for reducing falls rate, number of fallers and adverse events in people with MS. Results have highlighted some evidence in favour of exercise interventions compared with controls for balance function and mobility. However, there are some methodological flaws in the included studies to be considered when interpreting the results. The quality of the evidence base investigating the effectiveness of interventions to reduce falls for people with MS is mixed, with nine studies demonstrating high risk of bias for various methodological short‐comings.
We identified a high risk of selection bias associated with allocation concealment in one study (Lennon 2013), detection bias associated with lack of blinding of outcome assessment in one study (Cattaneo 2007), attrition bias due to incomplete outcome data in two studies (Lennon 2013; Taylor 2014) and reporting bias due to selective reporting in seven studies ( Coote 2013; Lennon 2013; Sosnoff 2014; Gandolfi 2015; Sosnoff 2015; Cattaneo 2016; Hoang 2016). Many of the included studies were also judged to demonstrate an unclear RoB, mostly owing to inadequate reporting of methods used. This demonstrates the need for increased focus on issues related to bias when conducting future trials of falls preventions interventions in MS.
Using the GRADE criteria, we examined the overall quality of the evidence for this summary comparison. It is worth noting that the certainty of evidence across all of the main outcomes in this comparison is very low to low, indicating limited confidence in the estimate of the effects. In addition to the scarcity of available studies to meta‐analyse, therefore the results need to be interpreted with caution. Using GRADE criteria, we considered the evidence to be of very low to low certainty for all of the main falls outcomes included in this review. Given the relatively small evidence base presented for many of the outcomes, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate of these outcomes.
Potential biases in the review process
One of the review authors was a lead investigator in one of the included studies (Coote 2013). However, two authors independently extracted data and determined risk of bias in included studies, that of which was cross‐checked by a third reviewer, limiting any potential bias.One our the review author, SC, is the author of one of the included studies and the RoB assessment for this study was completed independently by two other authors (SH and RG).
We attempted to minimise publication bias in the review by searching multiple databases and contacting authors of studies identified in trials registers that were ongoing or completed, but for which full reports had not been identified. We included one study not published as full reports (Lennon 2013) and obtained supplementary information from the authors of many of the included studies, in particular around the acquisition of additional falls outcomes. We also availed of translation services to aid screening of studies published in languages other than English.
Agreements and disagreements with other studies or reviews
This is the first systematic review and meta‐analysis of interventions to examine the effectiveness of any intervention to reduce falls in people with MS. Gunn 2015 conducted a robust systematic review and meta‐analysis of exercise interventions and therefore the current review differs by extending the search beyond exercise or physical therapy only interventions. Unlike the Gunn 2015 review, we have excluded studies that did not include a falls outcome. The current review used data available in published papers, in addition to accessing unpublished data (Lennon 2013) and thus provides a more exhaustive picture of the falls prevention intervention evidence base. Gunn 2015 did not report falls rate using rate ratios, which is the recommended primary outcome for falls in MS trials (Coote 2014). Our findings compare with the meta‐analysis conducted by Gunn 2015, wherein the number of fallers data from two studies (Cattaneo 2007; Coote 2013) were pooled to demonstrate no evidence of effect in favour of exercise. We have accessed additional data from an unpublished study (Lennon 2013) and three more recent trials (Sosnoff 2014; Sosnoff 2015; Carling 2017) to add to this evidence base. Similarly, our meta‐analysis demonstrates no evidence of an effect in favour of exercise over controls for number of fallers.
In relation to secondary outcomes, the current results demonstrated that there was evidence of a positive effect of exercise interventions compared to controls for the improvement of balance function. This finding is in line with the meta‐analysis conducted by Gunn 2015. While we have added new data to the meta‐analysis completed by Gunn et al (2015), the demonstrated effect size is moderate in nature and the statistical heterogeneity noted (I2=66%) is likely due to the differences in exercise types across studies. Of further note, the CI's presented in the current review are wide in nature, and therefore the pooled, significant SMD needs to be interpreted with caution. A systematic review and meta‐analysis of cross‐sectional, cohort and experimental trial studies (Gunn 2013) demonstrates that the impairment of balance function is an independent risk factor for falls among people with MS and therefore provides rationale for the targeting of improved balance function in falls prevention interventions.
The conclusions based on the data presented in the current review do not allow for recommendations in favour of falls interventions to reduce falls outcomes in people with MS. This is in agreement with the conclusion of Gunn 2015, wherein the authors reported a non‐significant modest reduction in risk of falling (RR= 0.75, 95% CI 0.12 to 4.80)‐ based on data from two exercise studies (Coote 2013; Cattaneo 2007). Similar to the pooled risk ratio reported by Gunn 2015, the current review reported a non‐significant reduction in risk of falling among people with MS, based on five studies (Coote 2013; Lennon 2013; Sosnoff 2014; Sosnoff 2015; Carling 2017) of exercise interventions.
Authors' conclusions
Implications for practice.
People with MS fall frequently and are often offered clinical interventions to reduce falls rate. However, their effectiveness has not been established. We used a comprehensive, transparent, and pragmatic system for rating the quality of the evidence (i.e. the GRADE approach) for falls rate, risk of falls and adverse events outcomes; and according to this approach, any estimate of effect based on very low to low quality evidence is uncertain, and further research may change the estimate. The current systematic review and meta‐analysis has found that evidence in support of interventions for preventing falls in MS is sparse and does not currently demonstrate significantly positive results for falls outcomes. The evidence base demonstrates mixed risk of bias, with very low to low certainty of the evidence for the primary outcomes. There is some evidence to suggest that exercise‐based falls interventions are effective in improving balance function and mobility outcomes among people with MS. The results did not demonstrate that the included interventions caused harm among people with MS, however, this is to be interpreted with caution as the majority of the studies did not report adverse events.
Implications for research.
Of note, a significant gap in the current evidence base relates to the fact that none of the included studies included an economic evaluation, which has particular implications for future trials of falls interventions in MS, in terms of establishing the cost‐effectiveness of interventions. This involves measuring health‐ related quality of life as an outcome, defining the perspective and timeframe for costs, collecting data on healthcare use, costing healthcare resources, calculating cost‐effectiveness ratios (if the intervention is effective in reducing falls), and evaluating uncertainty.
Given the high risk of bias and methodological limitations demonstrated in nine of the included studies and the very low to low quality GRADE findings for selected clinically‐important, falls‐related outcomes, there are some methodological considerations for future trials in this area:
· Studies evaluating fall prevention should be adequately powered and use a contemporary standard definition of a fall (Lamb 2005).
· Falls should be measured prospectively, recorded daily and monitored monthly.
· Fall events should be reported by group as total number of falls, fallers, and people sustaining a fall‐related injury; rate of falls (falls per person year); and number in each analysis.
· Design and reporting of trials should meet the contemporary standards of the CONSORT statement (Moher 2012) and the Tidier statement (Hoffmann 2014), respectively.
A substantial gap identified by the current systematic review is the lack of multifactorial falls prevention interventions for people with MS. Given the effectiveness of multifactorial interventions for reducing falls rate in older adult populations (Gillespie 2012), there is strong theoretical rationale for the design, delivery and evaluation of such interventions that specifically target physiological falls risk factors among people with MS. Further robust RCTs of high methodological quality examining the effectiveness of multifactorial falls prevention interventions on falls outcomes are needed.
Another gap identified by this systematic review relates to the sparsity of evidence on falls prevention interventions among older people with MS. Falls and fall‐related injuries are common and a serious problem in older people. People over 65 years of age have the highest risk of falling. The rate of fall‐related injuries also increases with age (Peel 2002). However, none of the included studies targeted an older MS population. There is a breadth of research examining the effectiveness of falls prevention interventions, classified according to the ProFANE taxonomy (Gillespie 2012), and future trials in MS need to be cognisant of the increased risk of falls with age.
Acknowledgements
Ms. Isabella Delunois‐ Medical librarian at University Hospital Limerick, Ireland who contributed to the search strategy.
Mr. Lonan Hughes, School of Allied Health, University of Limerick who contributed to data extraction from included studies.
Appendices
Appendix 1. Keywords for searching the MS Group Register
(((((("falls"[Title/Abstract]) OR "recurrent falls"[Title/Abstract]) OR "reduced falls"[Title/Abstract]) OR "falls prevention"[Title/Abstract])) AND ((("Tertiary Prevention"[Mesh]) OR "intervention"[Title/Abstract]) OR "prevention"[Title/Abstract]))
Appendix 2. Appendix 2
MEDLINE (PubMed) (1966 to 23 October 2017)
((((((((((("Multiple Sclerosis"[Mesh:noexp] OR "Multiple Sclerosis, Chronic Progressive"[Mesh]) OR "Multiple Sclerosis, Relapsing‐Remitting"[Mesh]) OR "Demyelinating Diseases"[Mesh:noexp]) OR "Optic Neuritis"[Mesh]) OR "Demyelinating Autoimmune Diseases, CNS"[Mesh:noexp]) OR "Encephalomyelitis, Acute Disseminated"[Mesh]) OR "Myelitis, Transverse"[Mesh]) OR ((((((((((((((((("multiple sclerosis"[Title/Abstract]) OR "chronic progressive multiple sclerosis"[Title/Abstract]) OR "progressive relapsing multiple sclerosis"[Title/Abstract]) OR "secondary progressive multiple sclerosis"[Title/Abstract]) OR "primary progressive multiple sclerosis"[Title/Abstract]) OR "relapsing remitting multiple sclerosis"[Title/Abstract]) OR "remitting‐relapsing multiple sclerosis"[Title/Abstract]) OR "acute relapsing multiple sclerosis"[Title/Abstract]) OR "neuromyelitis optica"[Title/Abstract]) OR "optic neuritis"[Title/Abstract]) OR "devic disease"[Title/Abstract]) OR "demyelinating disease"[Title/Abstract]) OR adem[Title/Abstract]) OR "demyelinating disorder"[Title/Abstract]) OR "clinically isolated syndrome"[Title/Abstract]) OR "transverse myelitis"[Title/Abstract]) OR "acute disseminated encephalomyelitis"[Title/Abstract] OR ("encephalomyelitis"[Title/Abstract])))))) AND (((((((("Accidental Falls"[Mesh]) OR "falls"[Title/Abstract]) OR "recurrent falls"[Title/Abstract]) OR "reduced falls"[Title/Abstract]) OR "falls prevention"[Title/Abstract]) OR "faller"[Title/Abstract])) AND (("Tertiary Prevention"[Mesh]) OR (Intervention[Title/Abstract] OR prevention[Title/Abstract])))
Embase (EMBASE.com) (1974 to 23 October 2017)
#1 'multiple sclerosis'/exp
#2 'demyelinating disease'/exp OR 'demyelinating disease'
#3 'optic neuritis'/exp OR 'optic neuritis'
#4 'acute disseminated encephalomyelitis'/exp
#5 'myelooptic neuropathy'/exp
#6 'myelitis'/exp
#7 'multiple sclerosis' OR 'chronic progressive multiple sclerosis' OR 'progressive relapsing multiple sclerosis' OR 'secondary progressive multiple sclerosis' OR 'primary progressive multiple sclerosis' OR 'relapsing remitting multiple sclerosis':ti,ab
#8 'remitting‐relapsing multiple sclerosis' OR 'acute relapsing multiple sclerosis' OR 'optic neurities' OR 'neuromyelitis optica' OR encephalomyelitis OR 'clinically isolated syndrome':ti,ab
#9 'transverse myelitis' OR 'devic disease' OR 'demyelinating disease' OR 'demyelinating disorder' OR 'acute disseminated encephalomyelitis' OR adem:ti,ab
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#11 'falls'/exp OR 'falls'
#12 'accidental falls' OR 'falls' OR 'recurrent falls' OR 'reduced falls' OR faller:ti,ab
#13 falls NEAR/4 (prevention OR intervention)
#14 #11 OR #12 OR #13
#15 #10 AND #14
#16 #10 AND #14 AND [humans]/lim AND [embase]/lim
#17 #16 AND ('clinical article'/de OR 'clinical trial'/de OR 'controlled study'/de OR 'major clinical study'/de OR 'randomized controlled trial'/de)
Cochrane Central Register of Controlled Trials (CENTRAL) (2017 Issue 10)
#1 MeSH descriptor Multiple Sclerosis, this term only #2 MeSH descriptor Multiple Sclerosis, Chronic Progressive, this term only #3 MeSH descriptor Multiple Sclerosis, Relapsing‐Remitting, this term only #4 MeSH descriptor Myelitis, Transverse explode trees 3, 5 and 7 #5 MeSH descriptor Optic Neuritis explode all trees #6 MeSH descriptor Encephalomyelitis, Acute Disseminated, this term only #7 MeSH descriptor Demyelinating Autoimmune Diseases, CNS, this term only #8 "multiple sclerosis":ti,ab,kw or "chronic progressive multiple sclerosis":ti,ab,kw or "progressive relapsing multiple sclerosis":ti,ab,kw or "secondary progressive multiple sclerosis":ti,ab,kw or "primary progressive multiple sclerosis":ti,ab,kw #9 "relapsing remitting multiple sclerosis":ti,ab,kw or "remitting‐relapsing multiple sclerosis":ti,ab,kw or "acute relapsing multiple sclerosis":ti,ab,kw or "neuromyelitis optica":ti,ab,kw or "optic neuritis":ti,ab,kw #10 "devic disease":ti,ab,kw or "demyelinating disease":ti,ab,kw or (adem):ti,ab,kw or "demyelinating disorder":ti,ab,kw or "clinically isolated syndrome":ti,ab,kw #11 "transverse myelitis":ti,ab,kw or "acute disseminated encephalomyelitis":ti,ab,kw or (encephalomyelitis):ti,ab,kw #12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 MeSH descriptor: [Accidental Falls] explode all trees
#14 "falls" or "recurrent falls" or "reduced falls" or "falls prevention" or "faller":ti,ab,kw
#15 #13 OR #14
#16 MeSH descriptor: [Tertiary Prevention] explode all trees
#17 "Tertiary Prevention" or Intervention or prevention:ti,ab,kw
#18 #16 OR #17
#19 #12 AND #15 AND #18
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (1981 23 October 2017)
"Multiple sclerosis" AND ("accidental falls" OR falls OR faller OR "falls prevention") AND ("tertiary prevention" OR prevention OR intervention)
Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (1982 23 October 2017)
"Multiple sclerosis" AND "accidental falls"
Data and analyses
Comparison 1. Exercise versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 5 | 399 | Rate Ratio (Random, 95% CI) | 0.68 [0.43, 1.06] |
| 2 Number of fallers | 5 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.43] |
| 3 Adverse events | 3 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.26, 6.03] |
Comparison 2. Education versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Exercise plus education versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Individual exercise versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Yoga versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 6. Functional Electrical Stimulation versus Exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 2 | Rate Ratio (Random, 95% CI) | 0.91 [0.78, 1.06] | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Exercise versus education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Exercise versus Exercise plus Education (post‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Education versus Exercise plus Education (post‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Sensory integration balance training versus conventional rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected |
Comparison 11. Motor and sensory balance rehabilitation versus motor balance rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Motor and sensory balance rehabilitation versus conventional rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 13. Motor balance rehabilitation vs conventional non balance rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Group exercise versus Yoga.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 15. Group exercise versus individual exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 16. Individual exercise versus yoga.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Falls rate | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 17. Exercise versus control (3‐month follow‐up).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 18. Exercise versus control (6 month follow up).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 19. Balance and mobility rehabilitation versus conventional rehabilitation (2 month follow‐up).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of fallers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carling 2017.
| Methods | Multi‐centre single‐blinded randomised controlled pilot study, computerised random allocation with varied block sizes | |
| Participants | n=51, Randomised: (E=25, C=26), Anaylsed (E=23, C=25) Groups differed at baseline for age, gender and MS subtype ‐ E group older, higher proportion of females and no participants with relapsing remitting MS |
|
| Interventions | Core stability exercise, dual tasking and sensory strategies individualised and progressed by physiotherapists in groups of 2‐5. 14 sessions over 7 weeks, 60 minute per session. 2‐5 individualised and progressive home exercises, twice a week |
|
| Outcomes | Number of falls per group, number of fallers, number of multiple fallers, Fatigue, trunk impairment, timed sit‐to‐stand, postural sway, balance function, falls efficacy, walking mobility, timed mobility, walking velocity | |
| Notes | E: Exercise, C: Control, BBS: Berg Balance Scale, ES: Effect Size, MSWS: Multiple Sclerosis Walking Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An external statistician conducted a computerized random allocation sequence with varied block sizes |
| Allocation concealment (selection bias) | Low risk | Concealed allocation was achieved using sealed envelopes, which were opened right after baseline measure by the physiotherapist in charge at each site |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not outlined in paper that participants or healthcare providers were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blinding was accomplished with the raters travelling to different centres, unaware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three were lost to follow‐up, (early intervention group= 2 due to fall‐related fracture; 1 in late intervention group due to lost to follow‐up) |
| Selective reporting (reporting bias) | Low risk | Compared with planned outcome reporting outlined in the registered trial (NCT 02209467) all outcomes are reported in the trial |
| Other bias | Low risk | Sample size calculated a priori and required number of participants recruited. |
Cattaneo 2007.
| Methods | Randomised controlled trial, participants allocated to groups by matching order of admission to hospital with pre‐study randomised list | |
| Participants | n=50, randomised & analysed groups: M&S=23, M=12, C=15 No statistically significant difference between groups for characteristics or baseline measures. For total group, mean age 46 years +/‐ 10.2, MS onset mean 13.8 years +/‐ 8.1 & n=15 used a walking aid. |
|
| Interventions | M&S: Strategies that challenged the motor and sensory system to maintain equilibrium. Biofeedback incorporated. Progressive difficulty. 1 to 1 with experienced therapist. 10‐12 sessions fo 45 minutes over 3 weeks. M: As above with motor strategies only. C: Conventional therapy not directly targeted at balance improvements. Dosage as above. |
|
| Outcomes | Number of fallers, balance function, mobility, balance confidence, | |
| Notes | M&S: Motor & Sensory Rehabilitation, M: Motor Rehabilitation, C: Control, BBS: Berg Balance Scale, DGI: Dynamic Gait Index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following initial assessment, the subjects were randomly assigned to three subgroups using computer‐ generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment mechanism not outlined in the paper. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not enough information is presented in the study to determine if participants or personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Whenever possible an independent rater not directly involved in the treatment rated both the initial and the final assessment. The rater was not masked with respect to the subject’s group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced across both groups |
| Selective reporting (reporting bias) | Unclear risk | Results reported are in line with planned analysis, however, published protocol or trial registration is not available |
| Other bias | Unclear risk | No sample size calculated and inferential statistics computed. Study may be at risk of a type II error |
Cattaneo 2016.
| Methods | Multi‐centre, single‐blinded randomised controlled trial, independent clinician within clinical centre allocated participants with pre‐study randomised list | |
| Participants | n=119 Randomised: I=78, C=41 Analysed Post: I=69, C=36 Analysed Follow‐Up: I=58, C=26 Baseline characteristics were similar between groups and centres. Less participants in I group had more than 2 falls in two months prior to study. |
|
| Interventions | I: Balance treatment to improve control of posture, moving centre of mass and body segments during static, dynamic and transitional tasks. 1 to 1 with experienced physical therapist for 45 minutes. 20 sessions over 7‐10 weeks. C: Treatments that reduce limitations of body function and activity levels, with max of 10 minutes for balance. Dosage as above. |
|
| Outcomes | Number of fallers, number of multiple fallers, balance function, dynamic balance, mobility, balance confidence | |
| Notes | I: Intervention, C: Control, BBS: Berg Balance Scale, DGI: Dynamic Gait Index, TUG: Timed Up and Go, ABC: Activities‐specific Balance Confidence scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomisation list made before the beginning of the study‐ randomisation procedure is not identified |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described in enough detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not aware of group assignment.Not enough information is presented in the study to determine if personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessor took assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing data in groups provided |
| Selective reporting (reporting bias) | High risk | Trial registration number NCT02390830. Additional outcomes reported in the paper that weren't included in the protocol including the TUG, DGI, ABC and BBS |
| Other bias | Low risk | Sample size calculated and required number of participants recruited |
Coote 2013.
| Methods | Multi‐centre single‐blinded block randomised controlled trial. Allocation of blocks by sealed envelope with a piece of paper for each group, emitted once selected. | |
| Participants | n=111, randomised & analysed groups: 1. Group physiotherapy (GP) = 48; 2. 1‐to‐1 physiotherapy = 35; Yoga = 13, C = 15 Baseline characteristics of intervention groups not reported. Differences between fallers & non‐fallers were: significantly greater physical and psychological impact of MS and impact of fatigue for fallers |
|
| Interventions | All interventions were for 1 hour per week for 10 weeks GP: physiotherapist supervised circuit class of 6 strength & balance exercises with progressions 1‐to‐1 physiotherapy: at discretion of physiotherapist (focus was exercise to improve balance and strength) Yoga instructor classes, focus on yoga postures, stretching, breathing, meditation and relaxation exercises |
|
| Outcomes |
Participants were asked retrospectively about the number of falls in the 3 months before the baseline assessment. They were reassessed at week 12, during which they were asked about the number of falls in the 3 months before that assessment.
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information presented in the paper to permit a judgement of low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information presented in the paper to permit a judgement of low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information presented in the paper to permit a judgement of low or high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information presented in the paper to permit a judgement of low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information presented in the paper to permit a judgement of low or high risk of bias |
| Selective reporting (reporting bias) | High risk | Some outcome data not reported, e.g. PCI and HHD |
| Other bias | Unclear risk | No sample size calculation reported in the paper. Study may be exposed to a type II error |
Esnouf 2010.
| Methods | Randomized controlled study, allocation by computer‐generated randomisation | |
| Participants | n=64 Randomised: (ODFS=32, E=32) Anaylsed (ODFS=26, E=27) No statistical difference between groups for performance or satisfaction on COPM. ODFS = 53 years (mean), 62% female E: 57 years (mean), 66% female Similar proportion of participants in each group on Kurtze scale |
|
| Interventions | ODFS: Wear ODFS daily for mobility for 18 weeks E: Physiotherapist prescribed exercises, individualised, to improve gait and strength (core, hip). 1‐2 per day at home for 30 minutes, for 18 weeks |
|
| Outcomes | Number of falls per person, adverse events, occupational performance | |
| Notes | ODFS: Oddstock drop foot stimulator, E: Exercise, COPM: Canadian Occupational Performance Measure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Following recruitment, the research volunteers were randomly assigned by computer‐generated randomisation to ODFS and exercise groups |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The occupational therapist was not involved in any aspect of the study intervention and was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | No published trial protocol or registered trial is cited in the paper, therefore unclear |
| Other bias | Unclear risk | No sample size calculated and inferential statistics computed. The study may be exposed to a type II error |
Gandolfi 2015.
| Methods | Single‐blinded block randomised controlled trial, allocation by an 8 block list using computer‐generated random numbers tables | |
| Participants | n= 80, randomised & analysed: E = 39, C = 41 No statistically significant differences between groups at baseline. C was slightly older, longer disease duration, higher EDSS & BBS scores. |
|
| Interventions | 50 minutes a day, 3 days/week, for 5 weeks E: Physiotherapist supervised, sensory integration balance training, individualised & progressive C: Physiotherapist supervised, mobilization, stretching and strengthening in accordance with MS rehabilitation guidelines |
|
| Outcomes | Number of falls per person, balance function, balance confidence, quality of life, fatigue, sensory organisation balance test | |
| Notes | E: Exercise, C: Control, EDSS: Expanded Disability Status Scale, BBS: Berg Balance Scale, FSS: Fatigue Severity Scale, SOT: Sensory Organisation Balance Test, ABC: Activities Balance Confidence Scale, MSQOL‐54 PHC: Multiple Sclerosis Quality of Life‐54 Physical Health Component | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number tables were used |
| Allocation concealment (selection bias) | Low risk | "According to the assignment in the block by a second blinded physician" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The examiner was blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | High risk | Trial registration number NCT01040117. No outcomes from GAITRite, platform stabilometry or postural evaluation reported |
| Other bias | Low risk | Sample size calculated and recruitment of required sample |
Hoang 2016.
| Methods | Single‐blinded block randomised controlled trial. Allocation by computer‐generated random number schedule | |
| Participants | n=50 Randomised: E = 28, C = 22 Analysed: E = 23, C = 21 No differences at baseline between groups on primary (CSRT & SST) & secondary (CSRT decision & movement time, postural sway, TUG, 10MWT, 6MWT, TMT, SDMT, 9‐HPT, MSFC & number of falls) measures |
|
| Interventions | E: 30 minutes, twice per week for 12 weeks at home, of 2 interactive exergames requiring stepping in multiple directions to cues C: no intervention, continued their normal physical activity |
|
| Outcomes | Number of falls per group, mobility, dual task mobility, cognition, MS function, stepping reaction time | |
| Notes | E: Exercise, C: Control, CSRT: Choice Stepping Reaction Time, SST: Stroop Stepping Test, TUG: Timed Up and Go, 10MWT: 10 meter walk test, 6MWT: 6 minute walk test, TMT: trail making test, SDMT” symbol digit modalities test, 9‐HPT: 9 hole peg test, MSFC: multiple sclerosis functional composite | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer generated random number schedule” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Reassessments were conducted by a physiotherapist who was blind to group allocation”… |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced across groups and reasons for missing data unlikely to be related to outcome |
| Selective reporting (reporting bias) | High risk | Trial registration number ACTRN12612001139864.Additional outcomes presented in the paper to those presented in the protocol including MSFC, TUG, TMT and SDMT |
| Other bias | Low risk | Sample size calculated and required number of participants recruited |
Lennon 2013.
| Methods | Single‐blinded stratified randomised controlled trial. Allocation by computer generated randomisation code & stratification using RMI score | |
| Participants | n=177, randomised & analysed: E = 89, C: 88 No significant differences between groups at baseline for characteristics or outcome measures. |
|
| Interventions | E: Physiotherapy supervised group exercise (warm‐up/stretch, functional mobility & cool‐down), individualised & progressed. 2 hours twice a week for 6 weeks C: Weekly 10 minute call with structured questions about balance and mobility, for 6 weeks |
|
| Outcomes | Number of falls per group, number of fallers, number of multiple fallers, mobility, activities of daily living, balance function, MS impact (physical and psychological), MS self‐efficacy, walking velocity | |
| Notes | RMI: Rivermead Index, E: Exercise, C: Control, BBS: Berg Balance Scale, MSIS‐Phy: Multiple Sclerosis Impact Scale Physical, MSIS‐Psy: Multiple Sclerosis Impact Scale Psychological, MSWS: Multiple Sclerosis Walking Scale, MSSES: Multiple Sclerosis Self‐Efficacy Scale, 10MWT: 10 meter walk test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer generated randomisation code stratified according to .their scores on the primary outcome” |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Member of the research team who was blinded to the intervention” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “A total of 177 participants were randomised into the trial. 89 participants were randomised into the exercise group and 88 to the control group. Within the exercise group 5 failed to complete post intervention outcomes measurements and 8 were lost to 3 a 6 month follow‐up. Seven participants were lost to all outcome assessment within the control group." No data on reasons for missing data given |
| Selective reporting (reporting bias) | High risk | Trial registration ISRCTN78227711‐ all primary and secondary outcomes reported but additional outcomes reported in the study include the 10m walking speed and number of fallers |
| Other bias | Unclear risk | No sample size calculated and inferential statistics calculated. Study may be exposed to a type II error |
Prosperini 2013.
| Methods | Single‐blinded randomised cross‐over trial. Allocation by computer‐generated random numbers in a 1:1 ratio. | |
| Participants | n = 36 Randomised: GA = 18, GB = 18 Analysed: GA = 17, GB = 17 No significant difference between groups at baseline for demographic & clinical characteristics |
|
| Interventions | 30 minutes/day, 5 days/week for 12 weeks use of Wii Fit Plus balance games of progressive difficulty, at home | |
| Outcomes | Number of falls per group, static balance function, dynamic balance function, mobility, MS impact (physical and psychological) | |
| Notes | GA: Group A, GB: Group B, COP Path Centre of Pressure Path, FSST: Four Step Square Test, 25‐FWT: 25 Foot Walk Test, MSIS‐29: Multiple Sclerosis Impact Scale‐29, WBBS: Wii Balance Board System | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients who met all eligibility criteria underwent study assessments and were randomly assigned in a 1:1 ratio to 2 counterbalanced arms by computer‐generated random numbers. Randomization procedure was performed by an operator (LL) not involved in study measurements” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The following outcome measures were collected at each scheduled visit (T0, T1, and T2) by 2 neurologists (LP and CG) unaware of the training order allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | Registered trial protocol is not cited in paper so it is not possible to check against planned a‐priori analyses |
| Other bias | Unclear risk | No sample size calculated and inferential statistics calculated. Study may be exposed to a type II error |
Sosnoff 2014.
| Methods | Single‐blinded randomised controlled pilot trial. Allocation by computer‐generated random numbers, 1:1 ratio | |
| Participants | n= 27 Randomised: E = 13, C = 14 Analysed: E = 10, C = 12 No differences in characteristics between groups at baseline |
|
| Interventions | E: Progressive home exercise programme to improve balance, walking, lower limb and trunk strength and spasticity. 45‐60 minutes, 3 times/week for 3 months C: No intervention or contact, on wait‐list |
|
| Outcomes | Number of fallers, adverse events, physiological falls risk, mobility, walking endurance, balance function, balance confidence | |
| Notes | E: Exercise, C: Control, PPA: Physiological Profile Assessment, T25FW: Timed 25 Foot Walk, ABC: Activities‐Specific Balance Confidence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “After baseline assessment, participants were randomised into two groups (exercise and control) using a simple randomisation method with a 1:1 allocation ratio (independent of baseline assessment) by computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | “Group allocation for each participant was concealed in opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The outcome assessors were blinded to group allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from five participants who were randomised were not analysed die to withdrawal for various reasons. Acceptable reasons for incomplete date are outlined and balanced across groups and do not suggest bias |
| Selective reporting (reporting bias) | High risk | The self‐reported falls at baseline and incidence of falls throughout the intervention were not reported‐ this was outlined as planned outcomes in the methods section of the paper. All other planned outcomes (outlined in methods section of paper) were reported. However, the registered trial (NCT01837017) outlined a planned outcome reporting of spasticity at 3‐months using the Modified Ashworth Scale‐ this outcome is not reported in the trial paper |
| Other bias | Unclear risk | No sample size calculated and inferential statistics calculated. Study may be exposed to a type II error |
Sosnoff 2015.
| Methods | Single‐blinded randomised controlled trial. Allocation by computer generated numbers with a 1:1:1:1 ratio. | |
| Participants | n = 37 Randomized: Ex + Ed = 8, Ex = 11, Ed = 9, C = 9 Analysed: Ex + Ed = 8, Ex = 10, Ed = 8, C = 8 No statistically significant difference between groups at baseline MS duration (in years) was greater in the C (19 +/‐ 9.3) & Ex + Ed (20 +/‐ 7.4) groups, compared to the Ex (15 +/‐ 5.6) & Ed (14.6 +/‐ 10.9) groups |
|
| Interventions | Ex: Standard set of progressive exercises for balance, lower limb & trunk strength & stretching, performed at home, 3 times/week for 12 weeks, with 4 clinic visits for guidance Ed: Group education led by trained interventionist involving self‐management & self‐efficacy enhancement techniques. 1 hour, 4 times over 12 weeks Ex + Ed: Combination of Ex & Ed groups C: No intervention, wait‐list control |
|
| Outcomes | Number of fallers, adverse events, fall prevention strategy, physiological profile assessment | |
| Notes | Ex: Exercise, Ed: Education, Ex + Ed: Exercise and Education, C: Control, PPA: Physiological Profile Assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “After baseline assessment, participants were randomised into groups (exercise, education, exercise plus education, and control) using a simple randomisation method with a 1:1:1:1 allocation ratio (independent of baseline assessment) by computer generated random numbers" |
| Allocation concealment (selection bias) | Low risk | “Group allocation for each participant was concealed in opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The outcome assessors were blinded to group allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Three participants withdrew and were not included in analysis. The reasons are reported clearly, are acceptable and do not suggest bias" |
| Selective reporting (reporting bias) | High risk | Stusy is lacking detail on the falls incidence data in the trial groups, with only the p‐value reported. In addition, some planned outcomes, outlined in the trial registration (NCT01956227), are not reported in the trial paper, including timed 25ft walk at 3 months, 6MWT at 3 months, TUG at 3 months, BBS at 3 months, ABC at 3 months |
| Other bias | Unclear risk | No sample size calculated and inferential statistics calculated. Study may be exposed to a type II error |
Stephens 2001.
| Methods | Randomised controlled trial | |
| Participants | n = 12, ATM = 6, EDU = 6 No statistically significant difference between groups for characteristics or outcomes at baseline |
|
| Interventions | ATM: Class led by Guild Certified Feldenkrais practitioners & based on the principles of this approach to developing functional movement awareness progressively through practice of tasks. 8 classes of 2‐4 hours totaling 20 hours over 10 weeks EDU: Group education on topics of acupuncture, exercise, social support and dealing with MS. 4 classes of 90 minutes over 10 weeks |
|
| Outcomes | NUmber of falls per person, sway velocity, balance confidence, MS self‐efficacy | |
| Notes | ATM: Awareness Through Movement, EDU: Education, ,CTSIB: modified Clinical Test of Sensory Interaction in Balance, ABC: Activities‐Specific Balance Confidence Scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Subjects were randomly assigned to the ATM group of the control group”. Therefore, there is insufficient information about the sequence generation process available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation concealment process available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | While some participants knew each other prior to the study, an attempt was made to prevent communication between groups by scheduling classes at different times”. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken. Therefore Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study did not address this outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome |
| Selective reporting (reporting bias) | Unclear risk | No registered trial cited in the paper‐ cannot compare with a‐priori plans for analyses |
| Other bias | Unclear risk | The output of the sample size is unclear in terms of how it relates to study participant numbers |
Taylor 2014.
| Methods | Single‐blinded randomised crossover trial. | |
| Participants | n = 25 Randomized: FES = 11, C = 14 Analysed: FES = 9, C = 11 No statistically significant difference between groups for baseline characteristics, although there was a higher proportion of walking aid users in C |
|
| Interventions | FES: The ODFS for perineal stimulation worn for 6 weeks, gluteal stimulation added from week 6. At week 12 core stability exercises weekly in clinic to week 18. Exercises continued at home till week 24 C: No intervention, wait list control |
|
| Outcomes | Gait analysis, 10m walk speed, MS impact (physical and psychological), falls rate | |
| Notes | FES: Functional Electrical Stimulation, C: Control, ODFS: Oddstock Drop Foot Stimulator, ROGA: Rivermead Observational Gait Analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using computer generated random numbers and group allocations were held by an independent medical statistician |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors is not outlined in the paper. To this end, Insufficient information available to permit a judgement of ‘low risk’ or ‘high risk’ except for the ROGA |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only volunteers who completed the protocol were included in the analysis.” There is discrepancy in the flow of participants throughout the trial‐ text on pg 77 outlines that 28 people were recruited, however, figure 3 demonstrates that 27 participants were enrolled. Of the 25 randomised participants, 5 participants withdrew for different reasons |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol was referenced in the paper so it is difficult to assess reporting bias |
| Other bias | Unclear risk | Potential risk of bias introduced as the intervention delivery mechanism (the Odstock Drop Foot Stimulator) is produced by a company owned by some of the authors‐ however, this potential conflict of interest is outlined by the authors. No sample size calculated and inferential statistics calculated. Study may be exposed to a type II error |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12612000218897 | Case series. Uncontrolled longitudinal study |
| Brichetto 2015 | Falls not measured |
| Cadorin 2015 | Case series. Uncontrolled longitudinal study |
| Cakt 2010 | Falls not measured |
| Eftekharsadat 2015 | Falls not measured |
| Forsberg 2016 | Falls not measured |
| Francavilla 2015 | Abstract only published. Authors contacted twice for unpublished data without reply |
| Goodman 2008 | Not falls prevention intervention |
| Kalgarfard 2013 | Falls not measured |
| Kalron 2016 | Falls not measured |
| Kramer 2014 | Controlled before‐and‐after study |
| Marzal Alfaro 2016 | Case series. Uncontrolled longitudinal study |
| McAuley 2007 | Falls not measured |
| Monjezi 2017 | Falls not measured |
| NCT01829776 | Case series. Uncontrolled longitudinal study |
| Nedeljkoviü 2014 | Review |
| Nilsagard 2014 | Single‐group. Pre‐intervention post‐intervention study |
| O'Hara 2002 | Falls not measured |
| Prosperini 2010 | Controlled before‐and‐after study |
| Prosperini 2014 | Case series. Uncontrolled longitudinal study |
| Sandroff 2014 | Falls not measured |
| Sebastiao 2017 | Study protocol |
| Segev‐Jacubovski 2011 | Review |
| Tarakci 2013 | Falls not measured |
| Ward 2004 | The study included 53 participants with Parkinson's disease and 45 participants with MS. Separate data for participants with MS were not reported in the published article and not available from authors |
| Zenginler 2016 | Abstract only published. Data unavailable from authors |
Characteristics of studies awaiting assessment [ordered by study ID]
Cattaneo 2018.
| Methods | A bi‐centre randomised rater‐blinded controlled trial |
| Participants | People with MS |
| Interventions | Participants in the intervention group received treatment aimed at improving balance and mobility. Participants in the control group received treatments to reduce limitations at activity and body function level |
| Outcomes | Primary measures were frequency of fallers (>1 fall in two months) and responders (>3 points improvement) at the Berg Balance Scale (BBS) |
| Notes | If suitable, we will include this study in the next iteration of this Cochrane review |
Kannan 2017.
| Methods | Randomised controlled trial |
| Participants | People with MS |
| Interventions | Participants in the intervention group will receive a "free from falls online" intervention, consisting of 8 weekly 30‐minute webinars with supplementary printable material and an hour long exercise video demonstrating exercises targeted for improving balance and posture |
| Outcomes | Outcomes are all assessed remotely and include fall frequency and self‐reported measures physical function, fatigue, self‐efficacy, psychosocial illness impact, social participation and satisfaction and perception of global health |
| Notes | This is published only in abstract format and we await the full text and suitable data for the next iteration of this Cochrane review |
Martini 2018.
| Methods | Randomised controlled trial |
| Participants | People with MS using a walking aid at baseline who had fallen in the previous year |
| Interventions | Participants in the intervention group were randomised to the Assistive Device Selection, Training and Education Program (ADSTEP) intervention (6 weekly, 40‐minute, 1‐on‐1 sessions with a physical therapist) and participants in the control group were randomised to usual medical care with the option of ADSTEP after the study |
| Outcomes | The following were assessed at baseline, intervention completion, and 3 months later: falls, timed up and go, timed 25‐foot walk, 2‐minute walk, four square step test, international physical activity questionnaire, Quebec user evaluation of satisfaction with assistive technologies, Multiple Sclerosis walking scale‐12, activities‐specific balance confidence scale, and Multiple Sclerosis impact scale‐29 |
| Notes | If suitable, we will include this study in the next iteration of this Cochrane review |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000415404.
| Trial name or title | Do interventions targeting proprioceptive feedback and exercise improve functional gait and reduce falls and falls risk in people with MS? |
| Methods | Randomised controlled trial |
| Participants | Participants with MS |
| Interventions | Experimental treatment: four exercises at home on a whole body vibration (WBV) board, at least 3 times a week for 10 weeks. Each exercise session should take ˜30 minutes Control treatment: the treatment will be the same as in the WBV group, except that participants will perform the same four exercises on a hard stable surface at least 3 times a week for 10 weeks |
| Outcomes | Primary: falls risk will be assessed using the Physiological Profile Assessment (PPA) fall risk index score. This is a series of tests composed of weighted values from five sensorimotor and balance domains: quadriceps muscle strength, hand reaction time, proprioception, postural sway, and visual contrast sensitivity. Falls risk will be measured at baseline and at 10 weeks after the commencement of the intervention. |
| Starting date | 18/04/2016 |
| Contact information | Dr David Kennedy. University of Sydney, East St Lidcombe. NSW. 2141 Australia phone: +61 2 9351 9589 email: david.kennedy@sydney.edu.au |
| Notes |
ACTRN12616001053415.
| Trial name or title | An interactive step training system to reduce falls in people with multiple sclerosis: a randomised controlled trial |
| Methods | Randomised controlled trial |
| Participants | Confirmed diagnosis of Multiple Sclerosis with Expanded Disability Status Scale score between 2 and 6 |
| Interventions | Balance and step training delivered through a novel home‐based step‐training system (smart +/‐ step). The system involves a computerised training mat measuring approximately one square metre with eight step panels and connected to a visual display (television or computer screen) that displays training instructions and game‐based stepping exercises. The control group: participants not engaging in the minimum weekly training dose for 2 consecutive weeks will be contacted by telephone to discuss any issues and to encourage adherence during the 6‐month intervention period |
| Outcomes | The proportion of fallers in each group: Falls will be monitored with monthly falls diaries for the 12 months after baseline assessment;The rate of fallers in each group: Falls will be monitored with monthly falls diaries for the 12 months after baseline assessment;Static and dynamic balance will be measured using the swaymeter device from the Physiological Profile Assessment;Clinical measures of lower leg strength will be measured using a dynamometer;Clinical measures of gait (velocity and distance) will be assessed with the 10‐metre and 6‐minute walk tests;Clinical measures of stepping will be measured using the Choice Stepping Reaction Time (CSRT) tests;MS disease severity will be measured using the Multiple Sclerosis Functional Composite (MSFC);Questionnaire measure of concern about falling using the Iconographical Falls Efficacy Scale;Questionnaire measure of physical activity levels using the Incidental Planned Exercise Questionnaire;Cognition measured using the Trails A and Trails B tests;Questionnaire measure of quality of life using the European Quality of Life ‐ 5 Dimensions and World Health Organisation Disability Assessment Schedule 2.0;Questionnaire measure of mood using the Patient Health Questionnaire‐9;Questionnaire measure of fatigue using the Modified Fatigue Impact Scale;Cost‐effectiveness of the intervention measured by data linkage to fall‐related medical health records;Movement detection threshold about the ankle joint measured using a motorised foot plate designed for this study;Maximal isometric voluntary force of the calf muscles measured with surface electrodes <br>;Measures of sleep disruption using a take‐home sleep testing device. Home testing will be performed using either a standard Type 2 (e.g. Nox A1 Polysomnography system) or Type 3 (e.g. ResMed ApneaLink plus) device.;Questionnaire measures of sleepiness and sleep quality using the Epworth Sleepiness Scale, Functional outcomes of sleep questionnaire, Karolinska sleepiness scale, and Pittsburgh sleepiness scale;Screening questionnaires for the presence of sleep apnoea measured using the STOP BANG, Berlin sleep questionnaire, and OSA50;Joint position sense about the ankle joint measured using a motorised foot plate designed for this study;Reaction time to small movements about the ankle joint using a motorised foot plate designed for this study;Voluntary activation of the calf muscles using twitch interpolation and measured with surface electrodes;Twitch force of the calf muscles elicited by electrical stimulation and measured with surface electrodes;Fatigue of the calf muscles with a sustained isometric contraction measured with electrical muscle stimulation and surface electrodes;Daily life walking patterns measured with a wearable physical activity monitor over a seven day period ;Sit to stand transitions measured with a wearable physical activity monitor over a seven day period;Number of near falls, slips or trips measured with a wearable physical activity monitor over a seven day period;Total energy expenditure from activities of daily living measured with a wearable physical activity monitor over a seven day period;Sedentary time measured with a wearable physical activity monitor over a seven day period;Daily life sleeping patterns measured with a wearable physical activity monitor over a seven day period;Dual task cost assessed with a dual task and 10‐metre walk |
| Starting date | 23/08/2016 |
| Contact information | s.lord@neura.edu.au |
| Notes | Ongoing study‐ recruiting |
Hatton 2016.
| Trial name or title | Do textured insoles alter gait, foot sensation & proprioception in people with Multiple Sclerosis? |
| Methods | Quasi‐randomised controlled trial |
| Participants | 176 community‐dwelling pwMS, across Brisbane, Australia. Adults with a diagnosis of MS (disease steps 1–4), who are ambulant over 100 meters, and meet specific inclusion criteria, will be recruited |
| Interventions | Participants will be randomised to a smooth control insole (N = 88) or textured insole (N = 88) group |
| Outcomes | The primary outcome measure will be mediolateral base of support when walking over even and uneven surfaces. Secondary outcome measures include: spatio temporal gait parameters, gait kinematics, foot sensation (light touch‐pressure, vibration, 2‐point discrimination) and proprioception (ankle joint position sense) |
| Starting date | |
| Contact information | a.hatton1@uq.edu.au |
| Notes | Study protocol published‐ Ongoing study‐ data collection stage |
ISRCTN13587999 (Gunn 2017).
| Trial name or title | Balance right in Multiple Sclerosis (BRiMS): a guided self‐management programme to reduce falls and improve quality of life, balance and mobility in people with secondary progressive multiple sclerosis: a protocol for a feasibility randomised controlled trial |
| Methods | Feasibility randomised controlled trial |
| Participants | Sixty ambulant people with secondary progressive MS who self‐report two or more falls in the previous 6‐months |
| Interventions | BRiMS programme plus usual care or to usual care alone |
| Outcomes | Feasibility outcomes, including trial recruitment, retention and completionAssessment of the proposed outcome measures for the anticipated definitive trial (including measures of walking, quality of life, falls, balance and activity level). Measures of adherence to the BRiMS programme and data to inform the economic evaluation in a future trial. Process evaluation (assessment of treatment fidelity) |
| Starting date | 09/01/2017 |
| Contact information | margie.berrow@plymouth.ac.uk |
| Notes | Ongoing study |
NCT02314585.
| Trial name or title | Fall risk reduction in multiple sclerosis: exercise intervention vs. attention control modification |
| Methods | Randomised controlled trial |
| Participants | People with an established definite diagnosis of MS. Ability to walk 25 feet with or without aid. Relapse free in the last 30 days. Being= 50 years of age. Having fallen at least once in the past year |
| Interventions | Exercise versus education |
| Outcomes | Primary: change in fall risk; secondary: identification of fall risk factors |
| Starting date | December 2014 |
| Contact information | jwajda@illinois.edu;jwajda@illinois.edu |
| Notes | Ongoing study, data collection |
NCT02583386.
| Trial name or title | Comprehensive fall prevention and detection in multiple sclerosis |
| Methods | Randomised controlled trial |
| Participants | The investigators will recruit 94 people with multiple sclerosis, who report having fallen at least twice in the previous 2 months. |
| Interventions | Participants will be randomised to be placed in either a group that receives classroom training during the study, or into a wait‐listed control group that will be offered the classroom training after their participation in the study is completed. In addition, 30 participants will be randomised to wear electronic fall detectors on their bodies for the duration of the study. These detectors will record when and where falls occur, and this data will be compared with the participants' self‐reported falls as recorded on the falls calendars |
| Outcomes | All participants will receive mobility and quality of life assessments at baseline, 9 weeks, 5 months, and 8 months. All participants will be asked to record any falls they have on falls calendars |
| Starting date | April 2016 |
| Contact information | cameromi@ohsu.edu |
| Notes | Ongoing study, data collection |
NCT02694666.
| Trial name or title | Vibration training for preventing falls in healthy population and multiple sclerosis |
| Methods | Randomised parallel controlled trial |
| Participants | Healthy population and people with multiple sclerosis |
| Interventions | Vibration training group: the vibration group will receive 8‐week controlled whole‐body vibration training as the intervention on the Galileo Med L device Placebo comparator: placebo training group The placebo group will receive 8‐week placebo training on the Galileo Med L device |
| Outcomes | Primary: real life falls. Time frame: up to 12 months |
| Starting date | April 2015 |
| Contact information | Georgia State University. PI: Feng Yang |
| Notes | Estimated study completion date: December 2020 |
NCT02885233.
| Trial name or title | Evaluation of a web‐based fall prevention program on people with multiple sclerosis |
| Methods | Randomised controlled trial |
| Participants | MS of any type with no relapse in the previous month. Self‐reported history of 2 or more falls in the previous 2 months. Ability to walk at least 100 meters with intermittent or unilateral constant assistance (Expanded Disability Severity Scale step <6.0) |
| Interventions | Experimental group (behavioral): free from falls online; control group: wait‐list |
| Outcomes | Primary outcome: difference in mean change in total falls between intervention and control arms; difference in mean change in total falls between intervention and control arms. Secondary outcomes: difference in patient reported outcomes including physical function, fatigue, self efficacy, psychosocial illness impact, social participation and satisfaction, and perception of global health between intervention and control arms |
| Starting date | August 2016 |
| Contact information | cameromi@ohsu.edu |
| Notes | Ongoing study‐ data collection |
Differences between protocol and review
We replaced the "number of recurrent or frequent fallers" outcome with the "number of fallers" outcome (number of participants who fell at least once during the study), which was omitted from the protocol‐ as the latter outcome also captures recurrent fallers. We removed the "number of falls per person" outcome‐ as this is also reflected in the rate of falls (the number of falls per person year) outcome.
As outlined in the review protocol (Hayes 2017), falls prevention interventions were considered to be any programme in which the primary or secondary aim was to reduce the rate of falls. The current review provides a more specific description of the types of interventions that were considered for inclusion and therefore a minor change from the protocol. This review included all interventions tested in trials that measured one or more of the primary falls outcomes (rate of falls, risk of falling). Trials that did not include a measure of falls (one or more of our primary falls outcomes), were excluded, as the review authors decided that falls prevention was not an aim of the intervention. Trials that focused on intermediate outcomes such as improved balance or strength, and did not report rate of falls or risk of falling as an outcome, were excluded.
Contributions of authors
SH and RG drafted the paper. All authors participated in reviewing and editing the manuscript as necessary . All authors read and approved the final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland.
Dr. Hayes has been awarded a two‐year part‐time Fellowship from the Irish Health Research Board to complete this Cochrane Review protocol and subsequent review.
Declarations of interest
SH ‐ none.
CK ‐ none.
RG ‐ none.
MF ‐ none.
CM ‐ none.
CDW ‐ none.
SC ‐ SC is an author of one of the included studies in this Cochrane Review (Coote 2013).
New
References
References to studies included in this review
Carling 2017 {published data only}
- Carling A, Forsberg A, Gunnarsson M, Nilsagård Y. CoDuSe group exercise programme improves balance and reduces falls in people with multiple sclerosis: a multi‐centre, randomised,controlled pilot study. Multiple Sclerosis Journal 2017;23:1394‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cattaneo 2007 {published data only}
- Cattaneo D, Jonsdottir J, Zocchi M, Regola A. Effects of balance exercises on people with multiples sclerosis: a pilot study. Clinical Rehabilitation 2007;21:771‐81. [DOI] [PubMed] [Google Scholar]
Cattaneo 2016 {published data only}
- Cattaneo D, Rasova K, Gervasoni E, Dobrovodská G, Montesano A, Jonsdottir J. Falls prevention and balance rehabilitation in multiple sclerosis: a bi‐centre randomised controlled trial, disability and rehabilitation. Disability and Rehabilitation 2016;40:522‐6. [DOI] [PubMed] [Google Scholar]
- NCT02390830. Falls Prevention and Balance Rehabilitation in Multiple Sclerosis. https://clinicaltrials.gov/ct2/show/NCT02390830 18/03/2015.
Coote 2013 {published data only}
- Coote S, Hogan N, Franklin S. Falls in people with multiple sclerosis who use a walking aid: prevalence, factors, and effect of strength and balance interventions. Archives of physical medicine and rehabilitation 2013;94:616‐21. [DOI] [PubMed] [Google Scholar]
Esnouf 2010 {published data only}
- Esnouf JE, Taylor PN, Mann GE, Barrett CL. Impact on activities of daily living using a functional electrical stimulation device to improve dropped foot in people with multiple sclerosis, measured by the Canadian Occupational Performance Measure. Multiple Sclerosis 2010;16:1141‐7. [DOI] [PubMed] [Google Scholar]
Gandolfi 2015 {published data only}
- Gandolfi M, Munari D, Geroin C, Gajofatto A, Donata Benedetti M, Midiri A, et al. Sensory integration balance training inpatients with multiple sclerosis: a randomised, controlled trial. Multiple Sclerosis Journal 2015;21:1453‐62. [DOI] [PubMed] [Google Scholar]
Hoang 2016 {published data only}
- ACTRN12612001139864. Balance exercise for multiple sclerosis [Exercise to improve balance and reduce fall risk in people with multiple sclerosis – a pilot randomized controlled trial]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=363148 26/10/2012.
- Hoang P, Schoene D, Gandevia S, Smith S, Lord SR. Effects of a home‐based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis – a randomised controlled trial. Multiple Sclerosis Journal 2016;22:94‐103. [DOI] [PubMed] [Google Scholar]
Lennon 2013 {published data only}
- Lennon S. Group exercise therapy for balance and mobility in people with multiple sclerosis: a randomised controlled trial. Personal communication with author.
Prosperini 2013 {published data only}
- Prosperini L, Fortuna D, Giannì C, Leonardi L, Marchetti MR, Pozzilli C. Home‐based balance training using the wiibalance board: a randomized, crossover pilot study in multiple sclerosis. Neurorehabilitation and neural repair 2013;27:516‐25. [DOI] [PubMed] [Google Scholar]
Sosnoff 2014 {published data only}
- NCT01837017. Project FARMS: fall risk reduction in multiple sclerosis. https://clinicaltrials.gov/ct2/show/NCT01837017 22/04/2013.
- Sosnoff J, Finlayson M, McAuley E, Morrison S, Motl RW. Home‐based exercise program and fall‐risk reduction in older adults with multiple sclerosis: phase 1 randomised controlled trial. Clinical Rehabilitation 2014;28:254‐63. [DOI] [PubMed] [Google Scholar]
Sosnoff 2015 {published data only}
- NCT01956227. Fall risk reduction in multiple sclerosis: exercise versus behavior (FARMS‐2). https://clinicaltrials.gov/ct2/show/NCT01956227 08/10/2013.
- Sosnoff JJ, Moon Y, Wajda DA, Finlayson ML, McAuley E, Peterson EW, et al. Fall risk and incidence reduction in high risk individuals with multiple sclerosis: a pilot randomised control trial. Clinical Rehabilitation 2015;29:952‐60. [DOI] [PubMed] [Google Scholar]
Stephens 2001 {published data only}
- Stephens J, DuShuttle D, Hatcher C, Schumnes J, Slaninka C. Use of awareness through movement improves balance and balance confidence in people with multiple sclerosis: a randomised controlled study. Journal of neurological physical therapy 2001;25:39‐49. [Google Scholar]
Taylor 2014 {published data only}
- Taylor P, Barrett C, Mann G, Wareham W, Swain I. A feasibility study to investigate the effect of functional electrical stimulation and physiotherapy exercise on the quality of gait of people with multiple sclerosis. Neuromodulation 2014;17:75–84. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12612000218897 {published and unpublished data}
- ACTRN12612000218897. Investigating fatigue, balance, falls and mobility in people with multiple sclerosis. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000218897 21/02/2012.
Brichetto 2015 {published data only}
- Brichetto G, Piccardo E, Pedullà L, Battaglia MA, Tacchino A. Tailored balance exercises on people with multiple sclerosis: a pilot randomized, controlled study. Multiple sclerosis 2015;21(8):1055‐63. [DOI] [PubMed] [Google Scholar]
Cadorin 2015 {published and unpublished data}
- Cadorin C, Stabile MR, Rossi S, Boscolo E, Vendramin A, Campagnolo D, et al. A comparison between two technological tools for balance training in patients with progressive multiple sclerosis: a pilot study. Multiple sclerosis journal 2015;21:503. [Google Scholar]
Cakt 2010 {published data only}
- Cakt BD, Nacir B, Genç H, Saraçoğlu M, Karagöz A, Erdem HR, et al. Cycling progressive resistance training for people with multiple sclerosis: a randomized controlled study. American journal of physical medicine and rehabilitation 2010;89:446‐57. [DOI] [PubMed] [Google Scholar]
Eftekharsadat 2015 {published data only}
- Eftekharsadat B, Babaei‐Ghazani A, Mohammadzadeh M, Talebi M, Eslamian F, Azari E. Effect of virtual reality‐based balance training in multiple sclerosis. Neurological research 2015;37:539‐44. [DOI] [PubMed] [Google Scholar]
Forsberg 2016 {published and unpublished data}
- Forsberg A, Koch L, Nilsagård Y. Effects on balance and walking with the CoDuSe balance exercise program in people with multiple sclerosis: a multicenter randomized controlled trial. Multiple sclerosis international 2016;2016:7076265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francavilla 2015 {published and unpublished data}
- Francavilla G, Sgarito C, Brichetto G, Battaglia MA, Lopes de Carvalho MA. Randomized controlled trial about effectiveness of personalized treatment of balance disorders in multiple sclerosis: integration of visual, proprioceptive and vestibular components. American academy of physical medicine and rehabilitation 2015;7:s83. [Google Scholar]
Goodman 2008 {published data only (unpublished sought but not used)}
- Goodman AD, Brown TR, Cohen JA, Krupp LB, Schapiro R, Schwid SR, et al. Dose comparison trial of sustained‐release fampridine in multiple sclerosis. Neurology 2008;71:1134‐41. [DOI] [PubMed] [Google Scholar]
Kalgarfard 2013 {published data only}
- Kargarfard M, Mehrabi M, Hamidi‐Tehrani J, Rouzbahani R. Changes in speed, endurance and balance in women with multiple sclerosis after 4 and 8 weeks of aquatic exercise training. Journal of Isfahan medical school 2013;31:1628‐39. [Google Scholar]
Kalron 2016 {published data only}
- Kalron A, Fonkatz I, Frid L, Baransi H, Achiron A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: a pilot randomized controlled trial. Journal of neuroengineering and rehabilitation 2016;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2014 {published data only}
- Kramer A, Dettmers C, Gruber M. Exergaming with additional postural demands improves balance and gait in patients with multiple sclerosis as much as conventional balance training and leads to high adherence to home‐based balance training. Archives of physical medicine and rehabilitation 2104;95:1803‐9. [DOI] [PubMed] [Google Scholar]
Marzal Alfaro 2016 {published data only}
- Marzal Alfaro BM, Martin‐Barbero M, Ribed A, Revuelta JL, Tovar‐Pozo M, Lorenzo‐Pinto A, et al. Adherence, quality of life and patient satisfaction with dalfampridine in clinical practice. European journal of hospital pharmacy 2016;23:A141. [Google Scholar]
McAuley 2007 {published data only}
- McAuley E, Motl RW, Morris KS, Hu L, Doerksen SE, Elavsky S, et al. Enhancing physical activity adherence and well‐being in multiple sclerosis: a randomised controlled trial. Multiple sclerosis 2007;13:652‐9. [DOI] [PubMed] [Google Scholar]
Monjezi 2017 {published data only}
- Monjezi S, Negahban H, Tajali S, Yadollahpour N, Majdinasab N. Effects of dual‐task balance training on postural performance in patients with multiple sclerosis: a double‐blind, randomized controlled pilot trial. Clinical rehabilitation 2017;31:234‐41. [DOI] [PubMed] [Google Scholar]
NCT01829776 {published and unpublished data}
- NCT01829776. Pilot study of free from falls program in multiple sclerosis (FFF). https://clinicaltrials.gov/ct2/show/NCT01829776 11/04/2013.
Nedeljkoviü 2014 {published data only}
- Nedeljković U, Raspopović ED, Ilić N, Dačković J, Dujmović I. Endurance and resistance training in rehabilitation of patients with multiple sclerosis. Vojnosanit pregled 2014;71:963‐8. [PubMed] [Google Scholar]
Nilsagard 2014 {published and unpublished data}
O'Hara 2002 {published data only}
- O’Hara L, Cadbury H, Souza L, Ide L. Evaluation of the effectiveness of professionally guided self‐care for people with multiple sclerosis living in the community: a randomized controlled trial. Clinical rehabilitation 2002;16:119‐28. [DOI] [PubMed] [Google Scholar]
Prosperini 2010 {published data only}
- Prosperini L, Leonardi L, Carli P, ML Mannocchi, C Pozzilli. Visuo‐proprioceptive training reduces risk of falls in patients with multiple sclerosis. Multiple sclerosis 2010;16:491‐9. [DOI] [PubMed] [Google Scholar]
Prosperini 2014 {published data only}
- Prosperini L, Giannì C, Fortuna D, Marchetti MR, Pozzilli C. Oral Dalfampridine improves standing balance detected at static posturography in multiples sclerosis. Multiple sclerosis international 2014;2014:802307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandroff 2014 {published data only}
- Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RHB, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. Journal of neurology 2014;261:363‐72. [DOI] [PubMed] [Google Scholar]
Sebastiao 2017 {published data only}
- Sebastiãoa E, McAuley E, Shigematsu R, Motl RW. Feasibility study design and methods for a home‐based, square‐stepping exercise program among older adults with multiple sclerosis: The SSE‐MS project. Contemporary clinical trials communications 2017;7:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Segev‐Jacubovski 2011 {published data only}
- Segev‐Jacubovski O, Herman T, Yogev‐Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk?. Expert review of neurotherapeutics 2011;11:1057‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarakci 2013 {published data only}
- Tarakci E, Yeldan I, Huseyinsinoglu BE, Zenginler Y, Eraksoy M. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: a randomized controlled trial. Clinical rehabilitation 2013;27(9):813‐22. [DOI] [PubMed] [Google Scholar]
Ward 2004 {published data only}
- Ward CD, Turpin G, Dewey ME, Fleming S, Hurwitz B, Ratib S, et al. Education for people with progressive neurological conditions can have negative effects: evidence from a randomized controlled trial. Clinical rehabilitation 2004;18:717‐25. [DOI] [PubMed] [Google Scholar]
Zenginler 2016 {published data only}
- Zenginler Y, Tarakci E, Kurtuncu M, Razak Ozdincler A. The impact of Nintendo Wii fit games on the balance and functionality of multiple sclerosis patients: a randomized controlled study. ECTRIMS online library. Kürtüncü M 2016;145682:EP1587. [Google Scholar]
References to studies awaiting assessment
Cattaneo 2018 {published data only}
- Cattaneo D, Rasova K, Gervasoni E, Dobrovodska G, Montesano A, Jonsdottir J. Falls prevention and balance rehabilitation in multiple sclerosis: a bi‐centre randomised controlled trial. Disability and rehabilitation 2018;40:522‐6. [DOI] [PubMed] [Google Scholar]
Kannan 2017 {published data only}
- Kannan M, Hugos C, Hildebrand A, Wick K, Cameron M. Evaluation of a web‐based fall prevention program among people with multiple sclerosis. Multiple sclerosis journal 2017;23:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martini 2018 {published data only}
- Hildebrand A, Martini D, Fling B, Cameron M. Ambulation assistive device training prevents falls, increases device satisfaction and may decrease sitting and increase walking in MS: a randomized‐controlled pilot study. Neurology 2017;88(16 Supplement):S24.004. [Google Scholar]
- Martini DN, Zeeboer E, Hildebrand A, Fling BW, Hugos CL, Cameron MH. ADSTEP: preliminary investigation of a multicomponent walking aid program in people with multiple sclerosis. Archives of physical medicine and rehabilitation 2018;99:2050‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000415404 {published and unpublished data}
- ACTRN12616000415404. Do interventions targeting proprioceptive feedback and exercise improve functional gait and reduce falls and falls risk in people with MS?. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370297 31/03/2016.
ACTRN12616001053415 {unpublished data only}
- ACTRN12616001053415. An interactive step training system to reduce falls in people with multiple sclerosis: a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370671 05/08/2016.
Hatton 2016 {unpublished data only}
- Hatton AL, Dixon J, Rome K, Brauer SG, Williams K, Kerr G. The effects of prolonged wear of textured shoe insoles on gait, foot sensation and proprioception in people with multiple sclerosis: study protocol for a randomised controlled trial. Trials 2016;17:10.1186/s13063‐016‐1337‐x. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN13587999 (Gunn 2017) {published and unpublished data}
- ISRCTN13587999. Balance right in multiple sclerosis. https://doi.org/10.1186/ISRCTN13587999 29/09/2016.
NCT02314585 {published and unpublished data}
- NCT02314585. Fall risk reduction in multiple sclerosis (FIRMS). https://clinicaltrials.gov/ct2/show/NCT02314585 11/12/2014.
NCT02583386 {published and unpublished data}
- NCT02583386. Comprehensive fall prevention and detection in multiple sclerosis (FFF). https://clinicaltrials.gov/ct2/show/NCT02583386 22/10/2015.
NCT02694666 {published data only}
- NCT02694666. Vibration training for preventing falls in healthy population and multiple sclerosis. https://clinicaltrials.gov/ct2/show/NCT02694666 29/02/2016.
NCT02885233 {published and unpublished data}
- NCT02885233. Evaluation of a web‐based fall prevention program on people with multiple sclerosis. https://clinicaltrials.gov/ct2/show/NCT02885233 31/08/2016. [DOI] [PMC free article] [PubMed]
Additional references
Bazelier 2011
- Bazelier MT, Staa T, Uitdehaag BM, Cooper C, Leufkens HG, Vestergaard P, et al. The risk of fracture in patients with multiple sclerosis: the UK general practice research database. Journal of bone and mineral research 2011;26:2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 1989
- Berg K, Wood‐Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada 1989;41:304‐11. [Google Scholar]
Browne 2014
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014;83:1022‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2012
- Cameron ID, Gillespie LD, Robertson MC, Murray GR, Hill KD, Cumming RG, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD005465] [DOI] [PubMed] [Google Scholar]
Carling 2017a
- Carling A, Forsberg A, Gunnarsson M, Nilsagard Y. [Request for additional results [personal communication]]. Email to A Carling 20‐03‐2018.
Cattaneo 2016a
- Cattaneo D, Rasova K, Gervasoni E, Dobrovodska G, Montesano A, Jonsdottir J. [Request for additional results [personal communication]]. Email D Cattaneo 22‐06‐2018.
Coote 2013a
- Coote S, Hogan N, Franklin S. [Request for additional results [personal communication]]. Email to S Coote 06‐03‐2018.
Coote 2013b
- Coote SC, Hogan N, Franklin S. Falls in people with multiple sclerosis who use a walking aid: prevalence, factors and effect of balance and strength interventions. Archives of physical medicine and rehabilitation 2013;94:616‐21. [DOI] [PubMed] [Google Scholar]
Coote 2014
- Coote S, Sosnoff JJ, Gunn H. Fall incidence as the primary outcome in multiple sclerosis falls‐prevention trials: recommendation from the international MS falls prevention research network. International journal of multiple sclerosis care 2014;16:178‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Esnouf 2010a
- Esnouf JE, Taylor PN, Mann GE, Barrett CL. [Request for additional results [personal communication]]. Email to P Taylor 22‐06‐2018.
Finlayson 2010
- Finlayson M, Peterson E. Falls, aging and disability. Physical medicine and rehabilitation clinics of North America 2010;21:357‐73. [DOI] [PubMed] [Google Scholar]
Fischer 1999
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS). Multiple sclerosis 1999;5:251‐9. [DOI] [PubMed] [Google Scholar]
Franchignoni 2010
- Franchignoni FF, Horak M, Godi A, Nardone A, Giordano A. Using psychometric techniques to improve the balance evaluation systems test: the mini‐BESTest. Journal of rehabilitation medicine 2010;42:323‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fry 2006
- Fry DK, Pfalzer LA. Reliability of four functional tests and rating of perceived exertion in persons with multiple sclerosis. Physiotherapy Canada. Physiotherapie Canada 2006;58:212‐20. [Google Scholar]
Gillespie 2003
- Gillespie LD, Gillespie WJ, Robertson MC, Lamb SE, Cumming RG, Rowe BH. Interventions for preventing falls in elderly people. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000340] [DOI] [PubMed] [Google Scholar]
Gillespie 2012
- Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007146] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunn 2013
- Gunn HJ, Newell P, Haas B, Marsden JF, Freeman JA. Identification of risk factors for falls in multiple sclerosis: a systematic review and meta‐analysis. Physical therapy 2013;93:504‐13. [DOI] [PubMed] [Google Scholar]
Gunn 2015
- Gunn H, Markevics S, Haas B, Marsden J, Freeman J. Systematic review: the effectiveness of interventions to reduce falls and improve balance in adults with multiple sclerosis.. Archive of physical and medical rehabilitation 2015;96:1898‐912. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoang 2016a
- Hoang P, Schoene D, Gandevia S, Smith S, Lord SR. [Request for additional results [personal communication]]. Email to P Hoang 12‐07‐2018.
Hobart 2001
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS‐29): a new patient‐based outcome measure. Brain 2001;124:962‐73. [DOI] [PubMed] [Google Scholar]
Hobart 2003
- Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12‐Item MS Walking Scale (MSWS‐12). Neurology 2003;60:31‐6. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hohol 1995
- Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 1995;45:251‐5. [DOI] [PubMed] [Google Scholar]
ICD‐8 1965
- World Health Organization. Report of the international conference for the eighth revision of the international classification of diseases. Unpublished document WHO/ICD9/74.4; available on request from Strengthening of Epidemiological and Statistical Services, World Health Organization. Geneva: World Health Organization, 1965.
Keith 1987
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Advances in clinical rehabilitation 1987;1:6‐18. [PubMed] [Google Scholar]
Kurtzke 1983
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;11:1444‐52. [DOI] [PubMed] [Google Scholar]
Lamb 2005
- Lamb SE, Jørstad‐Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. Journal of the American geriatrics society 2005;53:618‐22. [DOI] [PubMed] [Google Scholar]
Lamb 2011
- Lamb SE, Becker C, Gillespie LD, Smith JL, Finnegan S, Potter R, et al. Reporting of complex interventions in clinical trials: development of a taxonomy to classify and describe fall‐prevention interventions. Trials 2011;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lennon 2013a
- Lennon S. [Request for additional results [personal communication]]. Email to S Lennon 03‐02‐2018.
Lord 2003
- Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Physical therapy 2003;83:237‐52. [PubMed] [Google Scholar]
Lublin 1996
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996;46:907‐11. [DOI] [PubMed] [Google Scholar]
Lublin 2014
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuda 2011
- Matsuda PN, Shumway‐Cook A, Bamer AM, Johnson SL, Amtmann D, Kraft GH. Falls in multiple sclerosis. PM & R: the journal of injury, function, and rehabilitation 2011;3(7):624‐32. [DOI] [PubMed] [Google Scholar]
Matsuda 2012
- Matsuda PN, Shumway‐Cook A, Ciol MA, Bombardier CH, Kartin DA. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Physical therapy 2012;92:407‐15. [DOI] [PubMed] [Google Scholar]
McColl 2001
- McColl MA, Davies D, Carlson P, Johnston J, Minnes P. The community integration measure: development and preliminary validation. Archives of physical medicine and rehabilitation 2001;82:429‐34. [DOI] [PubMed] [Google Scholar]
McDonald 2001
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Annals of neurology 2001;50:121‐7. [DOI] [PubMed] [Google Scholar]
Moher 2012
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International journal of surgery 2012;10:28‐55. [DOI] [PubMed] [Google Scholar]
NGC 2014
- National Guideline Clearinghouse (NGC). Guideline summary: management of multiple sclerosis in primary and secondary care. National Clinical Guideline Centre (UK).
Nilsagard 2015
- Nilsagård Y, Gunn H, Freeman J, Hoang P, Lord S, Mazumder R, et al. Falls in people with MS, an individual data meta‐analysis from studies from Australia, Sweden, United Kingdom and the United States. Multiple sclerosis (Houndmills, Basingstoke, England) 2015;21:92‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliver 2004
- Oliver D, Daly F, Martin FC, McMurdo ME. Risk factors and risk assessment tools for falls in hospital in‐patients: a systematic review. Age and ageing 2004;33:122‐30. [DOI] [PubMed] [Google Scholar]
Polman 2005
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Annals of neurology 2005;58:840‐6. [DOI] [PubMed] [Google Scholar]
Polman 2011
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology 2011;69:292‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poser 1983
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of neurology 1983;13:227‐31. [DOI] [PubMed] [Google Scholar]
Prosperini 2013a
- Prosperini L, Fortuna D, Gianni C, Leonardi L, Marchetti MR, Pozzilli C. [Request for additional results [personal communication]]. Email to L Prosperini 22‐06‐2018.
Review Manager 2014 [Computer program]
- Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schumacher 1965
- Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials in multiple sclerosis. Annals of the New York Academy of Sciences 1965;122:552–68. [DOI] [PubMed] [Google Scholar]
Smith 1982
- Smith A. Symbol Digits Modalities Test. Los Angeles: Western Psychological Services, 1982. [Google Scholar]
Sosnoff 2014a
- Sosnoff JJ, Finlayson M, McAuley E, Morrison S, Motl RW. [Request for additional results [personal communication]]. Email to J Sosnoff 22‐06‐2018.
Sosnoff 2014b
- Sosnoff JJ, Finlayson M. International MS falls prevention research network. International journal of MS care 2014;16:161‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sosnoff 2015a
- Sosnoff JJ, Moon Y, Wajda DA, Finlayson ML, McAuley E, Peterson EW, et al. [Request for additional results [personal communication]]. Email to JJ Sosnoff 22‐06‐2018.
Stephens 2001a
- Stephens J, DuShuttle D, Hatcher C, Shmunes J, Slaninka C. [Request for additional results [personal communication]]. Email to J Stephens 07‐03‐2018.
Taylor 2014a
- Taylor P, Barrett C, Mann G, Wareham W, Swain I. [Request for additional results [personal communication]]. Email to P Taylor 12‐07‐2018.
Verheyden 2013
- Verheyden GSAF, Weerdesteyn V, Pickering RM, Kunkel D, Lennon S, Geurts ACH, et al. Interventions for preventing falls in people after stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD008728] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hayes 2017
- Hayes S, Kennedy C, Galvin R, Finlayson M, McGuigan C, Walsh CD, et al. Interventions for preventing falls in people with multiple sclerosis. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012475] [DOI] [PMC free article] [PubMed] [Google Scholar]


