Abstract
Background
The optimal treatment of superficial thrombophlebitis (ST) of the legs remains poorly defined. While improving or relieving the local painful symptoms, treatment should aim at preventing venous thromboembolism (VTE), which might complicate the natural history of ST. This is the third update of a review first published in 2007.
Objectives
To assess the efficacy and safety of topical, medical, and surgical treatments for ST of the leg in improving local symptoms and decreasing thromboembolic complications.
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register (March 2017), CENTRAL (2017, Issue 2), and trials registries (March 2017). We handsearched the reference lists of relevant papers and conference proceedings.
Selection criteria
Randomised controlled trials (RCTs) evaluating topical, medical, and surgical treatments for ST of the legs that included people with a clinical diagnosis of ST of the legs or objective diagnosis of a thrombus in a superficial vein.
Data collection and analysis
Two authors assessed the trials for inclusion in the review, extracted the data, and assessed the quality of the studies. Data were independently extracted from the included studies and any disagreements resolved by consensus. We assessed the quality of the evidence using the GRADE approach.
Main results
We identified three additional trials (613 participants), therefore this update considered 33 studies involving 7296 people with ST of the legs. Treatment included fondaparinux; rivaroxaban; low molecular weight heparin (LMWH); unfractionated heparin (UFH); non‐steroidal anti‐inflammatory drugs (NSAIDs); compression stockings; and topical, intramuscular, or intravenous treatment to surgical interventions such as thrombectomy or ligation. Only a minority of trials compared treatment with placebo rather than an alternative treatment and many studies were small and of poor quality. Pooling of the data was possible for few outcomes, and none were part of a placebo‐controlled trial. In one large, placebo‐controlled RCT of 3002 participants, subcutaneous fondaparinux was associated with a significant reduction in symptomatic VTE (risk ratio (RR) 0.15, 95% confidence interval (CI) 0.04 to 0.50; moderate‐quality evidence), ST extension (RR 0.08, 95% CI 0.03 to 0.22; moderate‐quality evidence), and ST recurrence (RR 0.21, 95% CI 0.08 to 0.54; moderate‐quality evidence) relative to placebo. Major bleeding was infrequent in both groups with very wide CIs around risk estimate (RR 0.99, 95% CI 0.06 to 15.86; moderate‐quality evidence). In one RCT on 472 high‐risk participants with ST, fondaparinux was associated with a non‐significant reduction of symptomatic VTE compared to rivaroxaban 10 mg (RR 0.33, 95% CI 0.03 to 3.18; low‐quality evidence). There were no major bleeding events in either group (low‐quality evidence). In another placebo‐controlled trial, both prophylactic and therapeutic doses of LMWH (prophylactic: RR 0.44, 95% CI 0.26 to 0.74; therapeutic: RR 0.46, 95% CI 0.27 to 0.77) and NSAIDs (RR 0.46, 95% CI 0.27 to 0.78) reduced the extension (low‐quality evidence) and recurrence of ST (low‐quality evidence) in comparison to placebo, with no significant effects on symptomatic VTE (low‐quality evidence) or major bleeding (low‐quality evidence). Overall, topical treatments improved local symptoms compared with placebo, but no data were provided on the effects on VTE and ST extension. Surgical treatment combined with elastic stockings was associated with a lower VTE rate and ST progression compared with elastic stockings alone. However, the majority of studies that compared different oral treatments, topical treatments, or surgery did not report VTE, ST progression, adverse events, or treatment adverse effects.
Authors' conclusions
Prophylactic dose fondaparinux given for 45 days appears to be a valid therapeutic option for ST of the legs for most people. The evidence on topical treatment or surgery is too limited and does not inform clinical practice about the effects of these treatments in terms of VTE. Further research is needed to assess the role of rivaroxaban and other direct oral factor‐X or thrombin inhibitors, LMWH, and NSAIDs; the optimal doses and duration of treatment in people at various risk of recurrence; and whether a combination therapy may be more effective than single treatment. Adequately designed and conducted studies are required to clarify the role of topical and surgical treatments.
Plain language summary
Treatment for superficial thrombophlebitis of the leg
Background
Superficial thrombophlebitis (ST) is a relatively common inflammatory process associated with a blood clot (thrombus) that affects the superficial veins (veins that are close to the surface of the body). Symptoms and signs include local pain, itching, tenderness, reddening of the skin, and hardening of the surrounding tissue. There is some evidence to suggest a link between ST and venous thromboembolism (VTE; a condition where blood clots form (most often) in the deep veins of the leg and can travel in the circulation and lodge in the lungs). Treatment aims to relieve the local symptoms and to prevent the extension of the clot into a deep vein, ST recurrence, or the development of more serious events caused by VTE. This is the third update of a review first published in 2007. The evidence is current to March 2017.
Study characteristics and key results
This update included 33 randomised controlled trials (clinical trials where people are randomly put into one of two or more treatment groups) involving 7296 participants. Treatments included rivaroxaban (a medicine called a direct oral inhibitor of activated factor X), injections of medicines under the skin to prevent blood clotting (e.g. fondaparinux, low molecular weight heparin, or unfractionated heparin), elastic compression stockings, oral non‐steroidal anti‐inflammatory drugs (NSAIDs; a pain killer medicine), topical treatment (medicine applied to the skin), and surgery.
One large study, accounting for half of the participants included in the review, showed that treatment with fondaparinux for 45 days was associated with a significant reduction in symptomatic VTE (where symptoms indicate there is a VTE), ST extension (where the clot moves further up the leg), and recurrence of ST (where clots return) compared to placebo. Major bleeding was infrequent in both groups. In one study in people with ST at high risk of recurrent thromboembolic events, fondaparinux was associated with a non‐significant reduction of symptomatic VTE compared to rivaroxaban. There were no major bleeding events in either group. Both low molecular weight heparin and NSAIDs reduced the occurrence of extension or recurrence of ST with no effect on symptomatic VTE or major bleeding. Topical treatments relieved local symptoms but the trials did not report on progression to VTE. Surgical treatment and wearing elastic stockings were associated with a lower rate of VTE and progression of the ST compared with elastic stockings alone.
Quality of the evidence
Overall, the quality of evidence was very low for most treatments due to poor study design, imprecision of results, lack of a placebo (non‐treated) group and only one study in some comparison. The quality of evidence was low to moderate for comparisons in two placebo‐controlled trials.
In conclusion, fondaparinux appears to be an adequate treatment for most people with ST. The optimal dose and duration of treatment need to be established in people at high risk as well as people at low risk for recurrent thrombotic events. Further research is needed to assess the role of rivaroxaban and other such medicines, or thrombin, low molecular weight heparin or NSAIDs and to demonstrate the effectiveness, if any, of topical treatment, or surgery in terms of VTE.
Summary of findings
Background
Description of the condition
The term superficial thrombophlebitis (ST), also known as superficial venous thrombosis, refers to a pathological state characterised by an inflammatory‐thrombotic process in a superficial vein. Distinctive clinical findings include pain and a reddened, warm, tender cord extending along the vein. The surrounding area may show signs of erythema (reddening of the skin) and oedema (swelling of the tissue). ST is a relatively common disease and, although its incidence has never been properly determined, it is estimated to be higher than that of deep vein thrombosis (DVT), which is about 1 per 1000 cases (De Weese 1991; Nordstrom 1992). In one community‐based study conducted in a population of 265,687 adults in France, the annual diagnosis rate of symptomatic, confirmed ST was 0.64% (95% confidence interval (CI) 0.55% to 0.74%) (Frappé 2014).
While the majority of ST occurs in varicose veins, additional predisposing risk factors similar to those for venous thromboembolism (VTE) include immobilisation, trauma, postoperative states, pregnancy, puerperium (the period immediately following childbirth), active malignancy, autoimmune diseases, use of oral contraceptive pills or hormonal replacement therapy, advanced age, obesity, and a history of previous VTE (Barrelier 1993; Bergqvist 1986; Chengelis 1996; de Moerloose 1998; Lutter 1991; Samlaska 1990a). Furthermore, the presence of inherited thrombophilia (a disorder where there is a tendency for thrombosis to occur, for example factor V Leiden, the prothrombin 20210A mutation, and deficiencies of the natural anticoagulant proteins C and S) in ST suggests a similar pathophysiology as VTE (de Moerloose 1998; Hanson 1998; Martinelli 1999; Samlaska 1990a; Samlaska 1990b). Traditionally, ST has been considered a relatively benign disease, but several studies have described an association between ST and VTE (Bergqvist 1986; Blumenberg 1998; Bounameaux 1997; Chengelis 1996; Jorgensen 1993; Krunes 1999; Lutter 1991; Quenet 2003; Unno 2002; Verlato 1999). In people with a diagnosis of ST, 6% to 44% have an associated (or develop) DVT, 20% to 33% have asymptomatic pulmonary embolism (PE), and 2% to 13% have symptomatic PE (Bergqvist 1986; Blumenberg 1998; Bounameaux 1997; Chengelis 1996; Frappé 2014; Jorgensen 1993; Krunes 1999; Lutter 1991; Plate 1985; Quenet 2003; Skillman 1990; Unno 2002; Verlato 1999). ST located in the saphenous main trunk seems to have the strongest association with VTE (Bergqvist 1986; Blumenberg 1998; Chengelis 1996; Jorgensen 1993; Lutter 1991; Quenet 2003; Unno 2002; Verlato 1999). The variations in estimates reported in the literature are probably due to the retrospective design of most studies, the small number of participants included, and the fact that ST was often diagnosed in vascular laboratories where people were referred for suspected DVT. In one cross‐sectional and prospective epidemiological cohort study, ST at diagnosis was associated with VTE in 25% of the cases (Decousus 2010a). During a three‐month follow‐up, 10% of people with ST developed thromboembolic complications despite 90% having received anticoagulant drugs, and about 98% had used elastic compression stockings. In one nationwide population‐based cohort study of 10,973 people with a first diagnosis of ST, the incidence of VTE in the first three months after ST diagnosis was 3.4%, which was estimated to be over 70 times higher compared to the general population without ST (Cannegieter 2015).
Description of the intervention
There is no consensus on the optimal treatment of ST in clinical practice as suggested by a survey in 2011 among practitioners mostly from North America showing a large variability in the management of ST (Dua 2014). Several therapies have been proposed in the literature, including surgery (ligation or stripping of the affected veins), elastic stockings, non‐steroidal anti‐inflammatory drugs (NSAIDs) that aim to reduce pain and inflammation, and several anticoagulant agents. It is unclear whether different locations of ST may influence the choice of treatment. The thrombus location in trunks of either the great saphenous vein (saphena magna) or small saphenous vein (saphena parva) may have the highest risk of extension into the deep vein system and thus could require an aggressive form of treatment, whereas other locations may be associated with a lower risk of extension and thus may warrant a less aggressive approach. Validated risk stratification tools based on ST location and patient characteristics are currently unavailable.
Why it is important to do this review
While the estimates of VTE prevalence in people with ST vary, management of ST should consider the prevention of this scaring complication beyond the mere resolution of local symptoms (Cannegieter 2015; Decousus 2010a; Wichers 2005). Conservative management, mainly focusing on the painful symptoms of disease, might therefore be insufficient. While provision of adequate treatment for ST may help prevent (fatal) VTE, the efficacy of the intervention needs to be balanced against the potential associated risks, such as (major) bleeding events with anticoagulants.
Objectives
To assess the efficacy and safety of topical, medical, and surgical treatments for ST of the leg in improving local symptoms and decreasing thromboembolic complications.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) evaluating topical, medical, and surgical treatments for ST of the legs.
Types of participants
Hospitalised and non‐hospitalised people with a diagnosis of ST of the lower extremities based on signs and symptoms of ST (e.g. pain, tenderness, induration (hardening of the tissue), or erythema (redness of the skin)) in a superficial vein, and objective diagnosis of the thrombus in the superficial vein by means of compression ultrasonography that excludes any concomitant DVT.
Types of interventions
Interventions included any treatment to relieve the symptoms and signs or to prevent complications of ST, such as topical treatments, compression stockings, compression bandages, leg elevation, medical treatments (e.g. NSAIDs, anticoagulants such as fondaparinux, low molecular weight heparin (LMWH) or the oral direct inhibitors of factor Xa or thrombin), and surgical intervention (e.g. ligation, vein stripping, crossectomy). Each treatment could be compared with another form of treatment, placebo, or no intervention. Combinations of therapies could be used.
Types of outcome measures
We included RCTs assessing any of the following outcome measures for any of the reviewed interventions.
Primary outcomes
Primary efficacy outcome:
symptomatic VTE (i.e. the combined of symptomatic PE and symptomatic DVT).
Primary safety outcome:
major bleeding.
The presence of PE or DVT had to be confirmed by an objective test, namely pulmonary angiography, ventilation/perfusion lung scan, or spiral computed tomography for PE; and ultrasonography, venography, or plethysmography for DVT.
Secondary outcomes
The secondary outcomes considered for the review were:
symptomatic PE;
symptomatic DVT or the progression of ST into DVT;
extension (symptomatic and asymptomatic) of ST;
recurrence (symptomatic and asymptomatic) of ST;
symptoms (e.g. pain);
signs (e.g. induration and erythema);
quality of life (assessed by means of disease‐specific and non‐specific questionnaires);
mortality;
adverse effects of treatment (e.g. minor bleeding, thrombocytopenia (reduced platelet count), allergic reactions, or surgery complications);
arterial thromboembolic events.
Search methods for identification of studies
We searched for RCTs comparing any treatment versus placebo or another treatment in people with ST of the legs. There was no restriction on language.
Electronic searches
For this update, the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
Cochrane Vascular Specialised Register (March 2017);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 2) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, and AMED, and through handsearching of relevant journals. The full list of the databases, journals, and conference proceedings that have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trials registries for details of ongoing and unpublished studies in March 2017:
ClinicalTrials.gov (www.clinicaltrials.gov);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch);
ISRCTN Register (www.isrctn.com/).
See Appendix 2.
Searching other resources
We searched reference lists of relevant papers and conference proceedings of the International Society for Thrombosis and Hemostasis (2003 to 2016) and American Society of Hematology (2004 to 2014), and we attempted to contact known experts in the field.
Data collection and analysis
Selection of studies
Two authors (MDN and IMW) independently reviewed titles and abstracts identified from the database searches to determine whether the inclusion criteria were satisfied. Two authors (MDN and IMW) independently assessed trials for inclusion in the review, and resolved any disagreements through discussion or involvement of a third author (SM). We independently reviewed the full text of identified articles, including those where there was disagreement in the initial title or abstract scanning stage, to ensure that the inclusion criteria were met. We obtained hard copies of the full text of studies that fulfilled the selection criteria. We were not blinded to the journal, institution, or results of the study. Titles and abstracts of non‐English articles were translated into English and assessed for inclusion. We documented reasons for excluding studies and resolved disagreements by consensus. One author (MDN) scanned conference proceedings, identified articles from other sources (experts or reference lists), and contacted trialists for further information if required.
Data extraction and management
Two authors (MDN and IMW) independently extracted the data from the included studies using an agreed format. We resolved any disagreements by consensus and, if necessary, by the involvement of the third author (SM). For any study published twice, we extracted the data from the more complete study. Collected information included methodological quality, characteristics of participants, type of intervention and control, and outcomes.
Assessment of risk of bias in included studies
Two authors independently assessed randomisation, blinding, and adequacy of analyses (Juni 2001). We resolved disagreements by consensus.
Two components of randomisation were assessed: generation of allocation sequences and concealment of allocation. Generation of allocation sequences was considered adequate if it resulted in an unpredictable allocation schedule. Mechanisms considered adequate included random‐number tables, computer‐generated random numbers, minimisation, coin tossing, shuffling cards, and drawing lots. Trials using an unpredictable allocation sequence were considered randomised. Trials using potentially predictable allocation mechanisms, such as alternation or the allocation of participants according to date of birth, were considered quasi‐randomised.
Concealment of allocation was considered adequate if participants and investigators responsible for participants selection were unable to predict, before allocation, which treatment was next. Methods considered adequate included central randomisation; pharmacy‐controlled randomisation using identical prenumbered containers; and sequentially numbered, sealed, opaque envelopes.
Blinding of participants and therapists was considered adequate if experimental and control preparations were explicitly described as indistinguishable or if a double‐dummy technique was used. Assessors were considered blinded if this was explicitly mentioned by the investigators.
Analyses were considered adequate if all randomised participants were included in the analysis according to the intention‐to‐treat (ITT) principle. The item 'free of selective reporting' was classified as at 'low risk of bias' if we had both the protocol and the full report of a given study, where the full report presented results for all outcomes listed in the protocol. We classified a study as at 'high risk of bias' if a report did not present data on all outcomes reported in either the protocol or the methods section. The risk of bias item 'free of other bias' was not considered in this review. We assessed the reporting of primary outcomes and sample size calculations.
Data synthesis
Prior to obtaining the global effect estimators (a balanced mean of the effect in different trials), we planned to evaluate the heterogeneity of treatment effects between trials using the I2 statistic (Higgins 2003), which describes the percentage of total variation across trials that is attributable to heterogeneity rather than chance. I2 values of 25%, 50%, and 75% may be interpreted as low, moderate, and high between‐trial heterogeneity, although the interpretation of the I2 statistic depends on the size and number of trials included (Rücker 2008). In the presence of no or low heterogeneity, we planned to use the fixed‐effect model (Mantel‐Haenszel method) and the random‐effects model to pool and analyse summary effect sizes. Where possible, we presented results as summary risk ratios (RR) or hazard ratios (HRs) for dichotomous variables and mean differences (MD) for all continuous variables. We determined the 95% CI for each estimate. The unit of analysis was the number of participants with the outcome of interest. Where possible, we analysed the results by ITT, including every individual in the randomly assigned treatment group regardless of whether they completed the treatment or withdrew from the trial.
We planned to evaluate publication bias and other biases related to small study size using funnel plots, plotting effect sizes on the vertical axis against their standard errors on the horizontal axis. We planned to assess asymmetry using the asymmetry coefficient: the difference in effect size per unit increase in standard error (Sterne 2001), which is mainly a surrogate for sample size. Symmetry would be expected in the absence of any bias related to small study size.
We used Review Manager 5 for data analysis (RevMan 2014).
'Summary of findings' tables
We presented the main findings of the review concerning the quality of evidence and the magnitude of treatment effects in the 'Summary of findings' tables, according to the GRADE principles described by Higgins 2011 and Guyatt 2008. We used GRADEproGDT software (GRADEproGDT 2015) to create the tables. We included the primary efficacy and safety outcomes of the review as well as the major secondary outcomes (i.e. extension and recurrence of ST, minor bleeding, adverse effects of treatment, and mortality). We focused on the active treatments fondaparinux, LMWH, and NSAIDs with placebo as comparator and at least one primary outcome comparison.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
See Figure 1.
1.
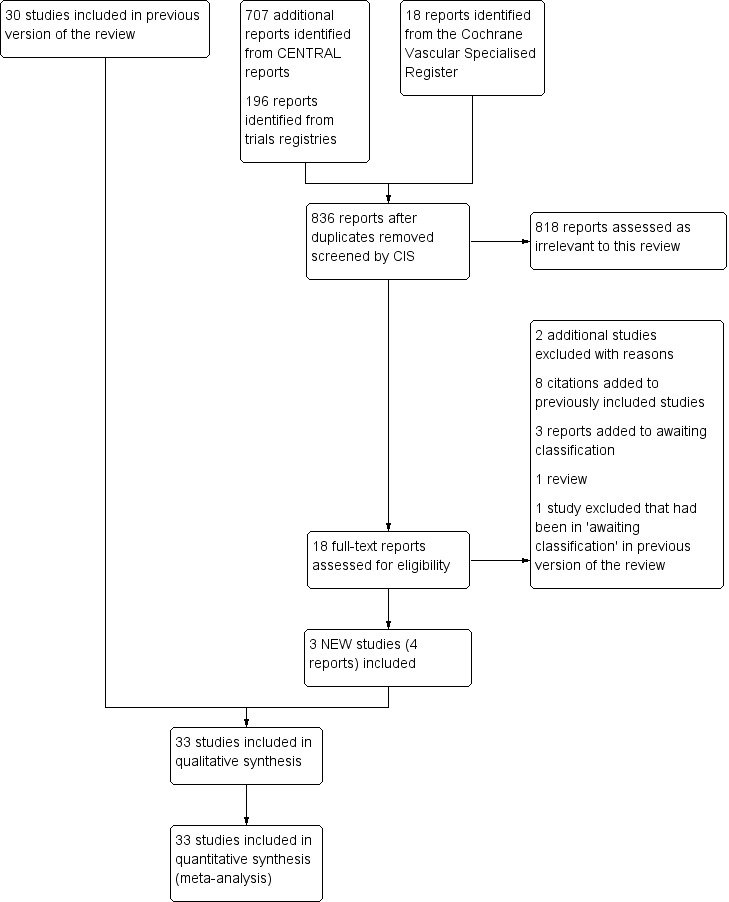
Study flow diagram.
There were three new included studies (Beyer‐Westendorf 2017; Boehler 2014; Spirkoska 2015).
Included studies
Three additional studies were included in this third update (Beyer‐Westendorf 2017; Boehler 2014; Spirkoska 2015), giving a total of 33 studies involving 7296 participants (Andreozzi 1996; Anonymous 1970; Archer 1977; Belcaro 1989; Belcaro 1990; Belcaro 1999; Belcaro 2011; Beyer‐Westendorf 2017; Boehler 2014; Cosmi 2012; Decousus 2010b; De Sanctis 2001; Ferrari 1992; Gorski 2005; Holzgreve 1989; Incandela 2001; Katzenschlager 2003; Koshkin 2001; Kuhlwein 1985; Lozano 2003; Marchiori 2002; Marshall 2001; Messa 1997; Nocker 1991; Nusser 1991; Pinto 1992; Rathbun 2012; Spirkoska 2015; Stenox Group 2003; Titon 1994; Uncu 2009; Vesalio Group 2005; Winter 1986). Nine studies reported data for 50 participants or fewer, 13 trials for 50 to 100 participants, and 11 studies for 100 participants or more.
Interventions and comparisons varied greatly among the studies. Nine trials included a topical treatment group (Belcaro 2011; De Sanctis 2001; Gorski 2005; Holzgreve 1989; Incandela 2001; Katzenschlager 2003; Nocker 1991; Pinto 1992; Winter 1986); three used a surgical treatment group (Belcaro 1989; Belcaro 1999; Lozano 2003); 14 used LMWH (Belcaro 1989; Belcaro 1990; Belcaro 1999; Cosmi 2012; Gorski 2005; Katzenschlager 2003; Lozano 2003; Marchiori 2002; Rathbun 2012; Spirkoska 2015; Stenox Group 2003; Titon 1994; Uncu 2009; Vesalio Group 2005); six used NSAIDs (Anonymous 1970; Ferrari 1992; Nusser 1991; Rathbun 2012; Stenox Group 2003; Titon 1994); two used fondaparinux (Beyer‐Westendorf 2017; Decousus 2010b); one used rivaroxaban (Beyer‐Westendorf 2017), and nine studies used another oral (Archer 1977; Belcaro 1989; Belcaro 1999; Koshkin 2001; Kuhlwein 1985; Messa 1997), intramuscular (Andreozzi 1996), intravenous (Marshall 2001), or non‐pharmacological (Boehler 2014) treatment.
Excluded studies
Two additional studies were excluded in this update (Ng 2010; Supe 2013) and one study which had previously been in the Studies awaiting classification section (Bijuan 2003) was also excluded making a total of 37 excluded studies. The reasons for exclusion are listed in the Characteristics of excluded studies table. Twenty‐one studies included a mixed population and it was not possible to extract data separately for ST (Allegra 1981; Annoni 1991; Argenteri 1983; Bagliani 1983; Becherucci 2000; Bergqvist 1990; Bracale 1996; Bruni 1979; Della Marchina 1989; Luttichau 1989; Mari 1982; Marsala 1985; Mauro 1992; Paciaroni 1982; Porters 1981; Pozza 1980; Seccia 1989; Seghezzi 1972; Seligman 1969; Stolle 1986; Tomamichel 1983). In one study it was not possible to extract outcome data separately for the two study treatment groups (Agus 1993). Four studies included people without a diagnosis of ST of the legs (Bernicot 1980; Gandhi 1984; Resta 1967; van Cauwenberge 1972), and two studies included people with DVT (Di Perri 1986; Rea 1981). In one study, it was unclear whether the study was randomised or not (Giorgetti 1990). Six studies included people with ST of the arm (Gouping 2003; Mehta 1975; Ng 2010; Rozsos 1994; Supe 2013; van der Knaap 1988), and in one study, the evaluated outcomes were not among those considered in the present review (Ibanez‐Bermudez 1996). For one study, we were unable to retrieve sufficient information to judge eligibility fully (Bijuan 2003).
Ongoing studies
One study is still ongoing (Rabe 2009). See Characteristics of ongoing studies table.
Risk of bias in included studies
Details of the methodological quality for each trial are reported in the Characteristics of included studies table. A risk of bias summary is presented in Figure 2.
2.
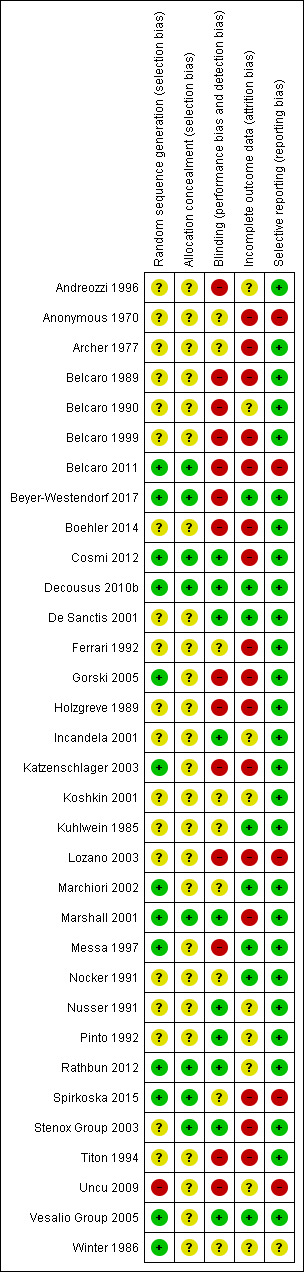
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen studies adequately generated the randomisation sequence (Belcaro 2011; Beyer‐Westendorf 2017; Cosmi 2012; Decousus 2010b; Gorski 2005; Katzenschlager 2003; Marchiori 2002; Marshall 2001; Messa 1997; Rathbun 2012; Spirkoska 2015; Vesalio Group 2005; Winter 1986), one was not adequate (Uncu 2009), and the remaining 18 studies were unclear. Eighteen studies adequately concealed allocation (Belcaro 2011; Beyer‐Westendorf 2017; Cosmi 2012; Decousus 2010b; Marshall 2001; Rathbun 2012; Spirkoska 2015; Stenox Group 2003), and the remaining 25 studies were unclear.
Blinding
Ten studies had a double‐blinded design (Cosmi 2012; Decousus 2010b; De Sanctis 2001; Incandela 2001; Marshall 2001; Nusser 1991; Pinto 1992; Rathbun 2012; Stenox Group 2003; Vesalio Group 2005), and in nine studies it was unclear whether blinding was attempted (Anonymous 1970; Archer 1977; Ferrari 1992; Koshkin 2001; Kuhlwein 1985; Marchiori 2002; Nocker 1991; Spirkoska 2015; Winter 1986). The remaining 14 studies did not attempt to blind the assessment of the outcomes or did not report whether blinding was used.
Incomplete outcome data
Seven studies performed the analysis according to the ITT principle (Beyer‐Westendorf 2017; Decousus 2010b; De Sanctis 2001; Kuhlwein 1985; Marchiori 2002; Messa 1997; Nocker 1991; Vesalio Group 2005); in nine this was unclear, while in the remaining studies the percentage of participants randomised and subsequently excluded from the analysis ranged from 2% to 33% (Anonymous 1970; Archer 1977; Belcaro 1989; Belcaro 1999; Belcaro 2011; Boehler 2014; Cosmi 2012; Ferrari 1992; Gorski 2005; Holzgreve 1989; Katzenschlager 2003; Lozano 2003; Marshall 2001; Spirkoska 2015; Stenox Group 2003; Titon 1994). In Beyer‐Westendorf 2017 the primary analysis was originally planned as ITT analysis, but later modified into a per‐protocol analysis. Although 7.6% (36/471) participants randomised were excluded from the primary analysis authors report results also according to the ITT principle.
Selective reporting
Most studies were free of selective reporting except Anonymous 1970; Belcaro 2011; Lozano 2003; Spirkoska 2015; and Uncu 2009 (all high risk), which did not provide data on some of the specified outcomes and Winter 1986 (unclear risk), which was reported as an abstract only and did not prespecify outcomes.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Fondaparinux compared to placebo for superficial thrombophlebitis of the leg.
| Fondaparinux compared to placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with ST of the leg Settings: hospitalised or non‐hospitalised Intervention: fondaparinux Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Fondaparinux | |||||
| Symptomatic VTE Ventilation‐perfusion scanning, helical computed tomography, pulmonary angiography, autopsy, ultrasonography, or venography Follow‐up: 77 days | 13 per 1000 | 2 per 1000 (1 to 7) | RR 0.15 (0.04 to 0.5) | 3002 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Major bleeding Follow‐up: 77 days | 1 per 1000 | 1 per 1000 (0 to 11) | RR 0.99 (0.06 to 15.86) | 2987 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Extension of ST Ultrasonography Follow‐up: 77 days | 34 per 1000 | 3 per 1000 (1 to 7) | RR 0.08 (0.03 to 0.22) | 3002 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Recurrence of ST Ultrasonography Follow‐up: 77 days | 16 per 1000 | 3 per 1000 (1 to 9) | RR 0.21 (0.08 to 0.54) | 3002 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Mortality Follow‐up: 77 days | 1 per 1000 | 1 per 1000 (0 to 15) | RR 2 (0.18 to 22) | 3002 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Minor bleeding Follow‐up: 77 days | 4 per 1000 | 6 per 1000 (2 to 17) | RR 1.49 (0.53 to 4.17) | 2987 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Adverse effects of treatment Follow‐up: 77 days | 33 per 1000 | 37 per 1000 (26 to 54) | RR 1.13 (0.78 to 1.65) | 2987 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; ST: superficial thrombophlebitis; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Evidence downgraded one level for imprecision due to a low number of events.
Summary of findings 2. Prophylactic LMWH versus placebo for superficial thrombophlebitis of the leg.
| Prophylactic LMWH versus placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with ST of the leg Settings: hospitalised and non‐hospitalised Intervention: prophylactic LMWH versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Prophylactic LMWH | |||||
| Symptomatic VTE Follow‐up: 97 days | 45 per 1000 | 54 per 1000 (17 to 174) | RR 1.22 (0.38 to 3.89) | 222 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Major bleeding Follow‐up: 97 days | See comment | See comment | Not estimable | 222 (1 study) | ⊕⊕⊝⊝ Low1,2 | 0 episodes of major bleeding |
| Extension or recurrence (or both) of ST Follow‐up: 97 days | 330 per 1000 | 145 per 1000 (86 to 244) | RR 0.44 (0.26 to 0.74) | 222 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Mortality | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Minor bleeding | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Adverse effects of treatment | See comment | See comment | See comment | ‐ | See comment | Data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low molecular weight heparin; RR: risk ratio; ST: superficial thrombophlebitis; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Evidence downgraded one level due to unclear random sequence generation and incomplete outcome data. 2Evidence downgraded one level for imprecision due to a low number of events.
Summary of findings 3. Therapeutic LMWH versus placebo for superficial thrombophlebitis of the leg.
| Therapeutic LMWH versus placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with ST of the leg Settings: hospitalised and non‐hospitalised Intervention: therapeutic LMWH versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Therapeutic LMWH | |||||
| Symptomatic VTE Follow‐up: 97 days | 45 per 1000 |
38 per 1000 (10 to 137) |
RR 0.85 (0.23 to 3.06) | 218 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Major bleeding Follow‐up: 97 days | See comment | See comment | Not estimable | 218 (1 study) | ⊕⊕⊝⊝ Low1,2 | No episodes of major bleeding |
| Extension or recurrence (or both) of ST Follow‐up: 97 days | 330 per 1000 |
152 per 1000 (89 to 254) |
RR 0.46 (0.27 to 0.77) | 218 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Mortality | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Minor bleeding | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Adverse effects of treatment | See comment | See comment | See comment | ‐ | See comment | Data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low molecular weight heparin; RR: risk ratio; ST: superficial thrombophlebitis; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Evidence downgraded one level due to an unclear random sequence generation and incomplete outcome data. 2Evidence downgraded one level for imprecision due to a low number of events.
Summary of findings 4. NSAIDs versus placebo for superficial thrombophlebitis of the leg.
| NSAIDs versus placebo for superficial thrombophlebitis of the leg | ||||||
| Patient or population: people with superficial thrombophlebitis of the leg Settings: hospitalised and non‐hospitalised Intervention: NSAIDs versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | NSAIDs | |||||
| Symptomatic VTE Follow‐up: 97 days | 45 per 1000 | 41 per 1000 (11 to 146) | RR 0.91 (0.25 to 3.28) | 211 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Major bleeding Follow‐up: 97 days | See comment | See comment | Not estimable | 211 (1 study) | ⊕⊕⊝⊝ Low1,2 | 0 episodes of major bleeding |
| Extension or recurrence (or both) of ST Follow‐up: 97 days | 330 per 1000 | 152 per 1000 (89 to 258) | RR 0.46 (0.27 to 0.78) | 211 (1 study) | ⊕⊕⊝⊝ Low1,2 | ‐ |
| Mortality | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Minor bleeding | See comment | See comment | See comment | ‐ | See comment | Data not available |
| Adverse effects of treatment | See comment | See comment | See comment | ‐ | See comment | Data not available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NSAIDs: non‐steroidal anti‐inflammatory drugs; RR: risk ratio; ST: superficial thrombophlebitis; VTE: venous thromboembolism. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Evidence downgraded one level due to an unclear random sequence generation and incomplete outcome data. 2Evidence downgraded one level for imprecision due to a low number of events.
None of the studies evaluated similar treatments on the same study outcomes. Treatment included fondaparinux, rivaroxaban, LMWH, unfractionated heparin (UFH), NSAIDs, topical treatment, oral treatment, intramuscular treatment, and intravenous treatment to surgery.
Fondaparinux
The CALISTO study, a large double‐blinded, placebo‐controlled RCT, evaluated a prophylactic dose (2.5 mg subcutaneously (sc) once daily) of fondaparinux given for 45 days (Decousus 2010b). The primary efficacy outcome of this RCT (i.e. composite of death from any cause, symptomatic PE, symptomatic DVT, or symptomatic extension to the saphenofemoral junction or symptomatic recurrence of ST up to day 47) was reduced by 85% by fondaparinux (RR 0.15, 95% CI 0.08 to 0.26) with a number needed to treat for an additional beneficial outcome (NNTB) of 20. The incidence of each component of the primary efficacy outcome was significantly reduced in the fondaparinux group compared with the placebo group except for the incidence of PE and of death, which did not differ significantly between the two groups (Analysis 1.1; Analysis 1.6). The risk of the composite of symptomatic DVT or PE was reduced by 85% with fondaparinux compared with placebo (RR 0.15, 95% CI 0.04 to 0.50) with a NNTB of 88. Fondaparinux was associated with lower rates of extension (RR 0.08, 95% CI 0.03 to 0.22) and recurrence of ST (RR 0.21, 95% CI 0.08 to 0.54). By day 47, major bleeding had occurred in one participant (0.1%) in each group (RR 0.99, 95% CI 0.06 to 15.86; P = 1.00). The rate of clinically relevant non‐major, minor, and total bleeding; arterial thromboembolic complications; and adverse effects of treatment did not differ significantly between the two groups.
1.1. Analysis.
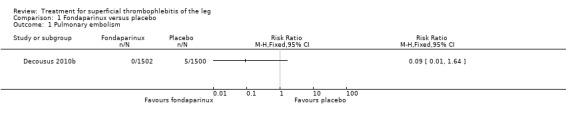
Comparison 1 Fondaparinux versus placebo, Outcome 1 Pulmonary embolism.
1.6. Analysis.
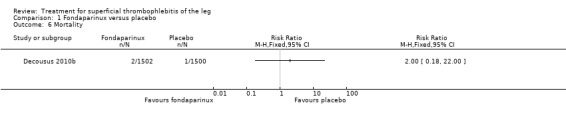
Comparison 1 Fondaparinux versus placebo, Outcome 6 Mortality.
In the SURPRISE study, 472 people with ST and one or more risk factors for thromboembolic complications (older than 65 years, male sex, previous ST or DVT or PE, active cancer or history of cancer, autoimmune disease, or involvement of non‐varicose veins) were randomised to fondaparinux (2.5 mg sc once daily) or the oral direct factor Xa inhibitor rivaroxaban (10 mg once daily) (Beyer‐Westendorf 2017). In the per‐protocol analysis, the incidence of the primary efficacy outcome (i.e. composite of symptomatic DVT or PE, progression or recurrence of ST, and all‐cause mortality at 45 days) was comparable in the rivaroxaban and fondaparinux groups at day 45 (3% with rivaroxaban versus 2% with fondaparinux; HR 1.9, 95% CI 0.6 to 6.4) and at 90 days, (7% with rivaroxaban versus 7% with fondaparinux; HR 1.1, 95% CI 0.5 to 2.2).
There were similar results when the analysis was performed according to the ITT principle. Fondaparinux was associated with a non‐statistically significant reduction of symptomatic VTE, DVT, recurrence of ST, mortality, clinically relevant non‐major bleeding, serious adverse events, or adverse effects of treatment compared with rivaroxaban (Analysis 2.2; Analysis 2.3; Analysis 2.5; Analysis 2.6; Analysis 2.8; Analysis 2.10; Analysis 2.11). There were no cases of PE, extension of ST or major bleeding in either treatment arm.
2.2. Analysis.
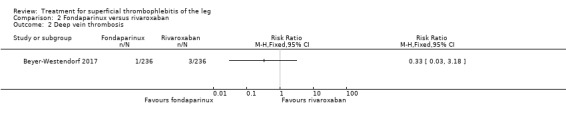
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 2 Deep vein thrombosis.
2.3. Analysis.
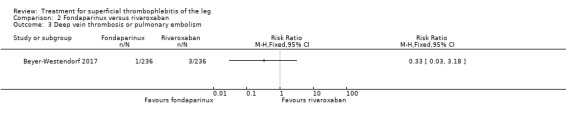
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 3 Deep vein thrombosis or pulmonary embolism.
2.5. Analysis.
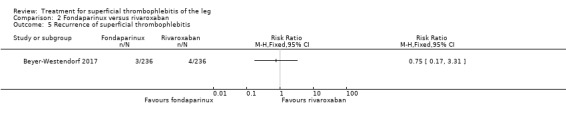
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 5 Recurrence of superficial thrombophlebitis.
2.6. Analysis.
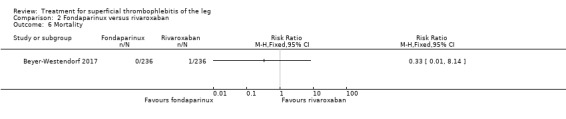
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 6 Mortality.
2.8. Analysis.
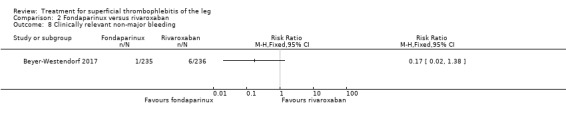
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 8 Clinically relevant non‐major bleeding.
2.10. Analysis.
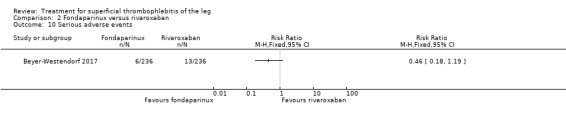
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 10 Serious adverse events.
2.11. Analysis.
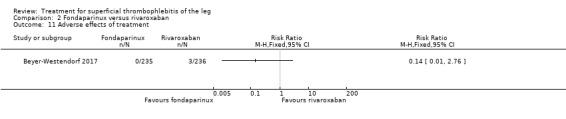
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 11 Adverse effects of treatment.
Low molecular weight heparin and unfractionated heparin
Fourteen studies included a LMWH group (Belcaro 1989; Belcaro 1990; Belcaro 1999; Cosmi 2012; Gorski 2005; Katzenschlager 2003; Lozano 2003; Marchiori 2002; Rathbun 2012; Spirkoska 2015; Stenox Group 2003; Titon 1994; Uncu 2009; Vesalio Group 2005).
Although not statistically significant, the incidence of VTE tended to be lower with both prophylactic and therapeutic LMWH compared with placebo shortly after treatment (prophylactic: RR 0.25, 95% CI 0.03 to 2.24; therapeutic: RR 0.26, 95% CI 0.03 to 2.33). However, at the end of the three‐month follow‐up, this difference was even less evident suggesting a catch‐up phenomenon (Analysis 3.2; Analysis 4.2) (Stenox Group 2003). Prophylactic and therapeutic LMWH given for eight to 12 days significantly reduced ST extension or recurrence, or both, compared with placebo (prophylactic: RR 0.44, 95% CI 0.26 to 0.74; therapeutic: RR 0.46, 95% CI 0.27 to 0.77). There were no episodes of major bleeding or heparin‐induced thrombocytopenia (HIT) in any treatment group (Stenox Group 2003).
3.2. Analysis.
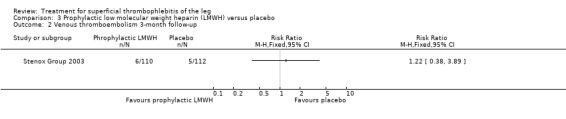
Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 2 Venous thromboembolism 3‐month follow‐up.
4.2. Analysis.
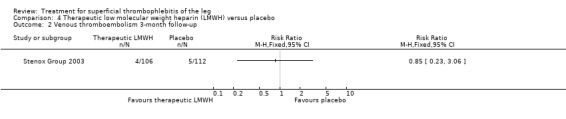
Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 2 Venous thromboembolism 3‐month follow‐up.
Combined therapy with LMWH plus elastic compression stockings seemed to reduce the incidence of VTE and ST extension or recurrence, or both, compared with elastic stockings alone (VTE: RR 0.08, 95% CI 0.00 to 1.38; ST extension or recurrence, or both: RR 0.08, 95% CI 0.01 to 0.59), although the former difference was not statistically significant (Belcaro 1999). This study did not provide data on safety outcomes.
Two studies randomised participants to topical treatment with heparin spray gel or LMWH (Gorski 2005; Katzenschlager 2003). There was a non‐significant decrease in DVT with LMWH (RR 0.30, 95% CI 0.03 to 2.70) and relief of local symptoms of ST was similar between both treatments at 21 days (Gorski 2005).
One study evaluated LMWH versus surgical treatment (saphenofemoral disconnection) (Lozano 2003). There was a comparable reduction of VTE events and a similar safety profile in the two study groups. There were numerically more cases of ST extension or recurrence with LMWH, although numbers were low and differences were not statistically significant (RR 3.00, 95% CI 0.33 to 27.23).
Three studies evaluated LMWH versus NSAIDs (Rathbun 2012; Stenox Group 2003; Titon 1994). Compared to NSAIDs, both fixed‐dose LMWH and weight‐adjusted LMWH seemed to have a similar effect on VTE (RR 0.93, 95% CI 0.24 to 3.63) and ST recurrence (RR 1.01, 95% CI 0.58 to 1.78). In the study by Rathbun 2012, there was one case of ST progression into the posterior tibial veins and one symptomatic PE, both in the LMWH group. Rathbun 2012 was not pooled with the other two studies for the outcome VTE since the administration of therapeutic LMWH in any person with thrombus progression during follow‐up could have introduced significant confounding. In Stenox Group 2003, which used placebo as a control group, an indirect comparison between prophylactic LMWH and NSAIDs suggested a non‐statistically significant reduction in VTE at the end of treatment (RR 0.45, 95% CI 0.04 to 4.89). There were no major bleeding events or HIT in either group. Rathbun 2012 reported two episodes of cutaneous rash with LMWH. In addition, there was a significant reduction in pain with both LMWH and NSAIDs with no differences between the groups.
One study compared LMWH alone versus LMWH combined with the anti‐inflammatory agent acemetacin (Uncu 2009). There were no cases of VTE, extension of ST, or major bleeding in either group. The effects on signs and symptoms of ST were not statistically different (Analysis 14.4; Analysis 14.5; Analysis 14.6; Analysis 14.7).
14.4. Analysis.
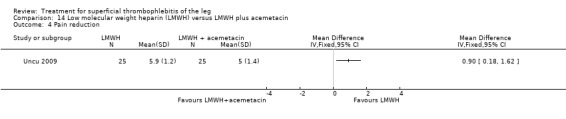
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 4 Pain reduction.
14.5. Analysis.
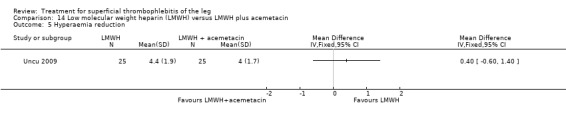
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 5 Hyperaemia reduction.
14.6. Analysis.
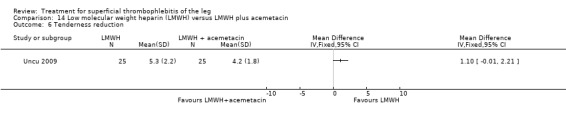
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 6 Tenderness reduction.
14.7. Analysis.
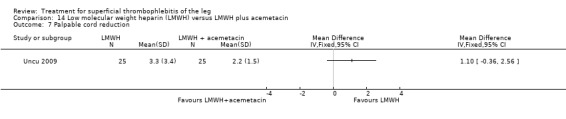
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 7 Palpable cord reduction.
Three studies compared different regimens of LMWH head‐to‐head but without using a placebo or inactive control group (Cosmi 2012; Spirkoska 2015; Vesalio Group 2005). In Cosmi 2012 (the STEFLUX study), the incidence of symptomatic VTE at the end of treatment and at the three‐month follow‐up was not different in the 30‐day intermediate‐dose LMWH and 30‐day prophylactic dose LMWH groups (Analysis 5.1; Analysis 5.2), and it was lower in the 30‐day intermediate‐dose LMWH compared with the 10‐day intermediate‐dose LMWH (VTE end‐of‐treatment: 0.46% with 30‐day intermediate‐dose LMWH versus 4.72% with 10‐day intermediate‐dose LMWH, RR 0.10, 95% CI 0.01 to 0.75; VTE 3‐month follow‐up: 1.82% with 30‐day intermediate‐dose LMWH versus 5.19% with 10‐day intermediate‐dose LMWH, RR 0.35, 95% CI 0.11 to 1.09; Analysis 7.1; Analysis 7.2). At the three‐month follow‐up, symptomatic PE had occurred in none of the participants of the 30‐day intermediate‐dose group and in one participant of both the 10‐day intermediate‐dose and 30‐day prophylactic‐dose LMWH groups. The incidence of symptomatic DVT did not differ between the three groups (Analysis 5.4; Analysis 6.4; Analysis 7.4). ST extension at three months was significantly reduced by the 30‐day intermediate‐dose LMWH in comparison to both the 30‐day prophylactic (2.28% with 30‐day intermediate‐dose versus 8.29% with 30‐day prophylactic dose LMWH; RR 0.28, 95% CI 0.10 to 0.73) and 10‐day intermediate dose LMWH (10.38%, RR 0.22, 95% CI 0.08 to 0.57), while recurrence of ST was similar in the 30‐day intermediate‐dose LMWH and the other two groups (Analysis 5.6; Analysis 7.6). There were no cases of major bleeding or HIT. The intensity of local symptoms evaluated by visual analogue scales (VAS) was comparable in the 30‐day intermediate‐dose, 10‐day intermediate‐dose, and the 30‐day prophylactic‐dose LMWH at the start of treatment (5.0 with 30‐day intermediate‐dose, 5.1 with 10‐day intermediate‐dose, 5.1 with 30‐day prophylactic‐dose; P = 0.97) as well as at the end of treatment (0.7 with 30‐day intermediate‐dose, 0.6 with 10‐day intermediate‐dose, 0.8 with 30‐day prophylactic‐dose; P = 0.10) and at three months (0.2 with 30‐day intermediate‐dose, 0.3 with 10‐day intermediate‐dose, 0.4 with 30‐day prophylactic‐dose; P = 0.47). Allergic reactions occurred in 0.4% with 30‐day intermediate‐dose, 1.4% with 10‐day intermediate‐dose, and 0% with 30‐day prophylactic‐dose.
5.1. Analysis.
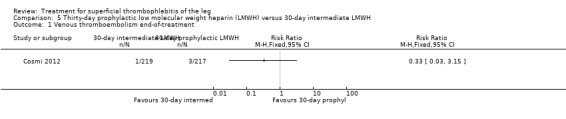
Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 1 Venous thromboembolism end‐of‐treatment.
5.2. Analysis.
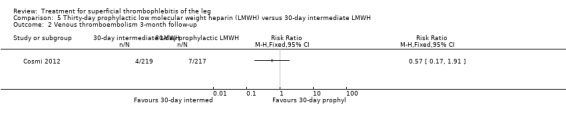
Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 2 Venous thromboembolism 3‐month follow‐up.
7.1. Analysis.
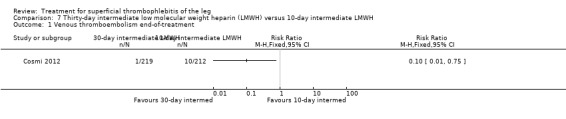
Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 1 Venous thromboembolism end‐of‐treatment.
7.2. Analysis.
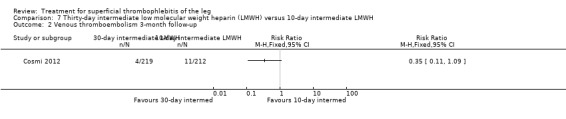
Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 2 Venous thromboembolism 3‐month follow‐up.
5.4. Analysis.

Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 4 Symptomatic deep vein thrombosis 3‐month follow‐up.
6.4. Analysis.
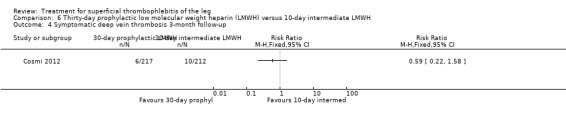
Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 4 Symptomatic deep vein thrombosis 3‐month follow‐up.
7.4. Analysis.
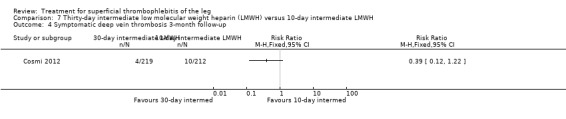
Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 4 Symptomatic deep vein thrombosis 3‐month follow‐up.
5.6. Analysis.
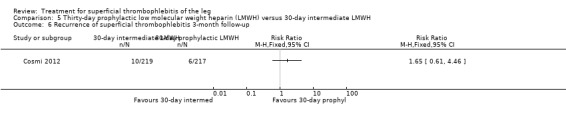
Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up.
7.6. Analysis.
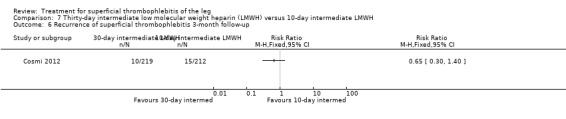
Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up.
In Spirkoska 2015, one participant in the intermediate‐dose LMWH group developed a symptomatic PE versus none in the prophylactic‐dose LMWH group. Asymptomatic ST progression into DVT occurred in one participant in each group. There were no major bleeding events. The authors reported a regression of the thrombus at the end of the study period in 66% of participants receiving the prophylactic‐dose LMWH compared to 80% in the intermediate‐dose LMWH and a complete thrombus resolution with recanalisation in three participants (9.7%) in the prophylactic‐dose LMWH and six (19.4%) participants in the intermediate‐dose LMWH. These differences were not statistically significant.
In the Vesalio Group 2005, one month of weight‐adjusted full therapeutic dose of LMWH or fixed prophylactic‐dose LMWH led to a similar reduction in ST extension or recurrence, or VTE (RR 1.20, 95% CI 0.42 to 3.40) over a three‐month follow‐up. In the prophylactic‐dose LMWH group most of the VTE events (77%) occurred while participants were still on treatment, whereas only 33% of participants on therapeutic‐dose LMWH developed VTE during LMWH administration. This advantage was lost after drug discontinuation with no difference at the end of the study period. There was no major bleeding or HIT during the study. Local symptoms and signs regressed faster with therapeutic dose LMWH although the difference was not statistically significant.
Two studies used sc UFH at prophylactic doses as the comparator treatment (Belcaro 1999; Marchiori 2002). Relative to elastic stockings alone, prophylactic sc UFH plus elastic stockings was associated with a statistically non‐significant lower VTE rate (RR 0.08, 95% CI 0.00 to 1.47) and a 83% reduction in ST extension or recurrence (RR 0.17, 95% CI 0.04 to 0.72) (Belcaro 1999). One study compared high‐ versus low‐dose sc UFH. There was a non‐significant 83% reduction in VTE (RR 0.17, 95% CI 0.02 to 1.30) and a 27% (RR 0.73, 95% CI 0.34 to 1.55) reduced rate of ST extension or recurrence among participants treated with high‐dose UFH (Marchiori 2002). There were no episodes of major bleeding or HIT in either study group.
Two studies evaluated sc heparin calcium (Belcaro 1989; Belcaro 1990). The combination of elastic stockings plus heparin calcium did not significantly improve local symptoms and signs compared with elastic stockings alone. Treatment with heparin calcium was correlated with a faster reduction of the analogue score and the area at maximum temperature than with defibrotide, although the difference was not significant. There were no adverse effects.
Non‐steroidal anti‐inflammatory drugs
Six studies included an NSAID group (Anonymous 1970; Ferrari 1992; Nusser 1991; Rathbun 2012; Stenox Group 2003; Titon 1994). Of these, two compared NSAIDs with placebo (Anonymous 1970; Stenox Group 2003), three NSAID with LMWH (Rathbun 2012; Stenox Group 2003; Titon 1994), and two randomised participants to two different NSAIDs (Ferrari 1992; Nusser 1991). The trials comparing NSAIDs versus LMWH have been discussed previously (Rathbun 2012; Stenox Group 2003; Titon 1994).
NSAIDs significantly reduced the risk of ST extension or recurrence, or both, by 54% compared with placebo (RR 0.46, 95% CI 0.27 to 0.78) (Stenox Group 2003). However, there were no differences in the incidence of VTE or in the resolution of local symptoms and signs. While there were no major bleeding episodes recorded in any of the NSAID or placebo groups, indomethacin tended to increase the rate of adverse effects compared with placebo (RR 2.60, 95% CI 0.95 to 7.08) (Anonymous 1970).
In one study, oral acemetacin led to a better resolution of the local clinical picture than diclofenac (Nusser 1991). Another trial compared nimesulide with diclofenac sodium (Ferrari 1992). Local symptoms were similarly improved by both treatments. In the group of participants randomised to nimesulide, there was a lower incidence of gastric pain episodes (RR 0.25, 95% CI 0.03 to 2.08) although this difference was not statistically significant (Ferrari 1992).
Topical treatment
Nine studies included a topical treatment group (Belcaro 2011; De Sanctis 2001; Gorski 2005; Holzgreve 1989; Incandela 2001; Katzenschlager 2003; Nocker 1991; Pinto 1992; Winter 1986). The comparison of heparin spray gel versus LMWH has been discussed earlier (see Low molecular weight heparin and unfractionated heparin section; Gorski 2005; Katzenschlager 2003).
Belcaro 2011 randomised participants to three doses of heparin spray gel versus placebo for seven to 14 days. After one week, there was a significant reduction in pain assessed by the VAS with heparin spray gel (‐93.13% reduction with heparin spray gel versus ‐61.35% reduction with placebo; P < 0.0001), a lower erythema extension (‐92% with heparin spray gel versus ‐26% with placebo; P < 0.012), and thrombus length (‐40.81 with heparin spray gel versus ‐4.22 with placebo; P < 0.0001). There were no adverse events or drug‐related reactions reported.
One study randomised participants to receive topical methylthioadenosine or placebo (Pinto 1992). Methylthioadenosine was associated with a non‐significant reduction in local signs and symptoms relative to placebo.
A significant improvement in the local symptomatology was observed with diclofenac gel (Nocker 1991) and essaven gel (De Sanctis 2001; Incandela 2001) compared with placebo.
Holzgreve 1989 and Winter 1986 compared two different types of gel. Holzgreve 1989 evaluated diclofenac gel versus etofenak gel and showed a comparable efficacy profile of the two topical medications. Winter 1986 compared diclofenac gel and heparin gel and found a better efficacy with diclofenac gel.
None of the studies evaluating a topical treatment reported data on VTE or ST recurrence.
Surgery
Three studies included a surgical treatment (Belcaro 1989; Belcaro 1999; Lozano 2003). One study compared surgery (saphenofemoral disconnection) with LMWH (see Low molecular weight heparin and unfractionated heparin section; Lozano 2003). The remaining two studies compared surgery combined with elastic stockings with elastic stockings alone (Belcaro 1989; Belcaro 1999).
One trial found that thrombectomy plus elastic stockings with or without venoruton led to an improvement of the local clinical signs and a greater reduction in the number of veins with ST compared with elastic compression bandages alone (Belcaro 1989). There were no cases of DVT in either group. One trial found that ligation of the vein plus elastic stockings was associated with a non‐significant reduction in VTE events (RR 0.33, 95% CI 0.07 to 1.60) and ST recurrence and extension (RR 0.46, 95% CI 0.18 to 1.15) relative to the control treatment (Belcaro 1999).
Compared with elastic stockings alone, venous stripping plus elastic stockings decreased the risk of ST extension and recurrence (RR 0.09, 95% CI 0.01 to 0.64) and seemed to be associated with a lower, non‐significant, incidence of VTE (RR 0.37, 95% CI 0.08 to 1.78) (Belcaro 1999).
Other
Nine studies evaluated an oral (Archer 1977; Belcaro 1989; Belcaro 1999; Koshkin 2001; Kuhlwein 1985; Messa 1997), intramuscular (Andreozzi 1996), intravenous (Marshall 2001), or non‐pharmacological (Boehler 2014) treatment. Beyer‐Westendorf 2017 compared oral rivaroxaban with fondaparinux and has been presented above (see Fondaparinux section).
Compared with placebo, oral vasotonin was associated with a higher proportion of participants who were cured or improved (Kuhlwein 1985). The criteria to determine the response to study treatment were not described. Vasotonin seemed to be well tolerated, with one case of poor tolerability among participants treated with vasotonin (3%) versus five cases (13%) in the placebo arm (RR 0.20, 95% CI 0.02 to 1.63).
The combination of venoruton, thrombectomy, and elastic stockings versus elastic stockings alone has been discussed above (see Low molecular weight heparin and unfractionated heparin section; Belcaro 1989). In the same trial, venoruton combined with elastic stockings led to an improvement of local symptoms compared with elastic stockings alone.
One study evaluating oral heparansulphate versus oral sulodexide suggested a greater decrease in local pain, itching, and redness in participants receiving oral heparansulphate than in the group receiving sulodexide (Messa 1997).
Compared with placebo, oxyphenbutazone reduced local tenderness four‐fold and halved the intensity of pain and erythema (Archer 1977).
One study evaluated oral vitamin K antagonists in combination with elastic stockings, which suggested a non‐significant reduction in VTE events (RR 0.08, 95% CI 0.00 to 1.47) and ST extension or recurrence (RR 0.42, 95% CI 0.16 to 1.13) with vitamin K antagonists plus elastic stockings compared with elastic stockings alone (Belcaro 1999).
Two studies addressed the use of enzyme therapy versus placebo (Koshkin 2001; Marshall 2001). Enzyme treatment seemed to improve local symptoms although the criteria to evaluate the response to study treatment were not reported.
One trial assessed the efficacy of three doses of desmin (Andreozzi 1996). There was a better control of local symptoms with higher doses of desmin without any increase in the risk of adverse events.
One study evaluated LMWH plus compression stockings versus LMWH alone (Boehler 2014). At the three‐week follow‐up, there was no difference between the two groups with regard to pain intensity (mean difference ‐0.15 cm, 95% CI ‐0.95 to 0.65), skin erythema (‐6.54 cm2, 95% CI ‐17.94 to 4.86), or quality of life evaluated through the 36‐item Short Form (SF‐36) Physical score (mean difference 2.92, 95% CI ‐1.04 to 6.88) and SF‐36 Mental score (‐2.98, 95% CI ‐7.30 to 1.34). There was no DVT or HIT.
Discussion
Uncertainty still exists around the optimal treatment of ST of the legs. The therapeutic approach for ST should aim at the resolution or improvement of the local symptoms but also, and even more importantly, at preventing the possible extension of the superficial vein thrombosis into the deep venous system (Wichers 2005).
This review summarised data from 7296 people with ST of the legs. About half of the participants were included in the CALISTO study, which compared 45 days of fondaparinux versus placebo using a double‐blind method (Decousus 2010b). Fondaparinux reduced the incidence of symptomatic VTE by 85%, ST extension by 92%, and the recurrence of ST by 79%. A total of 88 participants would need to be treated with fondaparinux to prevent one PE or DVT. These benefits were achieved without apparently increasing the risk of bleeding and they were maintained at one‐month follow‐up after discontinuation of treatment. However, CIs around bleeding estimate were broad and did not exclude a significantly higher risk with the drug. In the SURPRISE study, people with ST and one or more risk factors for thromboembolic complications were randomised to 45 days of fondaparinux or rivaroxaban 10 mg (Beyer‐Westendorf 2017). The results suggested that rivaroxaban was as effective as fondaparinux; however, the study was not powered to prove non‐inferiority. In addition, the authors observed a non‐statistically significant increase of the primary composite outcome as well as of clinically relevant non‐major bleedings in the rivaroxaban group which require further evaluation in appropriately sized studies. In contrast to the CALISTO study, the risk of thromboembolic events seemed to increase after treatment withdrawal at 45 days suggesting that a longer treatment may be required for people at high risk.
Compared with placebo or topical treatments, both NSAIDs and LMWH could help preventing ST extension while effectively controlling local symptoms (Stenox Group 2003; Titon 1994). When compared with each other, LMWH and NSAIDs seemed to be associated with a similar reduction in the incidence of VTE and worsening of ST. However, these conclusions need to be taken cautiously due to the methodological drawbacks, the low incidence of VTE, and the sample size of the available studies, which did not have enough power for a direct comparison between LMWH and NSAIDs. Thus, these data remain preliminary and further research is required to determine which treatment works better in terms of VTE prevention, and whether a combination may be more effective. Moreover, the benefits of LMWH and NSAIDs should be balanced against the associated adverse effects such as bleeding and gastric complications. None of the studies reported major bleeding episodes in participants randomised to LMWH. NSAIDs increased the risk of gastric pain three‐fold compared with placebo. To date, no study has evaluated NSAIDs versus surgery whereas one trial directly compared LMWH with surgical treatment, showing a comparable efficacy and safety (Lozano 2003). Despite the methodological limitations of this study, the results would suggest that a medical approach with LMWH would be as effective and safe as an invasive surgical treatment.
Cosmi 2012 and Vesalio Group 2005 compared different regimens of LMWH head‐to‐head. While symptomatic VTE occurred at a similar rate with prophylactic and higher (intermediate or therapeutic) dose LMWH, the higher‐dose LMWH seemed to be associated with a significant 70% reduction in ST progression (Cosmi 2012). Furthermore, the findings of Cosmi 2012 suggested that treatment with LMWH should be prolonged for at least 30 days to reduce the incidence of symptomatic VTE, compared to shorter usage of LMWH. However, it should be noted that neither Cosmi 2012 nor Vesalio Group 2005 had a placebo or inactive control group and Cosmi 2012 was prematurely interrupted, which may have led to an overestimation of the differences between the groups.
In the study of Boehler 2014, the addition of compression stockings to prophylactic dose LMWH seemed to carry no additional benefit in terms of clinical improvement.
Preliminary data suggested that high‐dose UFH can be effective in the treatment of ST although this needs to be confirmed in larger studies (Marchiori 2002). While not directly evaluated against UFH, fondaparinux and LMWH may still be preferable due to the easier mode of administration and the more predictable response not requiring laboratory monitoring as for UFH.
Most of the studies comparing oral treatment, topical treatment, or surgery did not report VTE, ST progression, adverse events, or treatment adverse effects. In addition, the methodological quality of these studies was often poor, with major study design flaws such as an unclear method of allocation or randomisation, the lack of a placebo as control group, or an unacceptably high dropout rate. All these limitations weaken the clinical applicability of the results and cast doubt about the actual efficacy and safety of these treatments.
Summary of main results
Fondaparinux was associated with a significant lower incidence of VTE, ST extension, or ST recurrence relative to placebo with similar risk of bleeding. Rivaroxaban 10 mg requires further evaluation. As compared to placebo, LMWH and NSAIDs appeared to reduce the extension or recurrence of ST, or both, whereas the available data did not show any significant effect on VTE. The evidence on oral treatments, topical treatment, or surgery was too limited and did not inform clinical practice about the effects of these treatments in terms of VTE.
Quality of the evidence
Our systematic approach to searching, study selection, and data extraction followed that of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The methodological quality of the included studies varied from low to high (see Figure 2). Poor reporting did not allow proper scoring of relevant study design features, such as sequence generation and allocation concealment, in the majority of included studies. Overall, the quality of evidence was very low for most intervention outcomes due to limitations in study design, imprecision of results and single study comparisons. The quality of evidence was low to moderate for outcomes in the two placebo‐controlled studies included in this review (Decousus 2010b; Stenox Group 2003). See Table 1; Table 2; Table 3; and Table 4.
Potential biases in the review process
One limitation of this review is that, despite the relatively large number of comparisons found, only a few studies compared the same treatment on the same study outcomes. The 'no difference' findings on a specific outcome may thus be the result of insufficient power of the analysis to show a difference between treatment groups as well as the absence of a true effect. For similar reasons, it was not possible to conduct subgroup analyses for the primary efficacy outcomes to evaluate the interaction of trial characteristics with treatment effects.
Agreements and disagreements with other studies or reviews
Since our previous systematic review on the prevention of VTE in people with ST of the leg (Wichers 2005), the results of two large RCTs on the efficacy of fondaparinux for the treatment of ST have become available (Beyer‐Westendorf 2017; Decousus 2010b). The high methodological quality and the size of the CALISTO study (Decousus 2010b), which alone accounted for half of the overall review population, made it a landmark investigation in the field. The SURPRISE study was the first to compare one of the direct oral factor Xa inhibitors, rivaroxaban, with fondaparinux and to select people with ST based on their underlying risk of thromboembolic events.
Authors' conclusions
Implications for practice.
Given the available evidence, prophylactic‐dose fondaparinux appears to be a valid treatment option in most people with ST. Fondaparinux should be given at a dose of 2.5 mg subcutaneously once daily for 45 days. Final recommendations cannot be drawn for rivaroxaban, low molecular weight heparin (LMWH), unfractionated heparin, or non‐steroidal anti‐inflammatory drugs (NSAID). Data are still too preliminary to draw firm conclusions on the role of surgery and the topical, oral, and parenteral treatments evaluated this far.
Implications for research.
Several questions about the treatment of ST remain unsolved. The role of rivaroxaban and other oral direct factor Xa inhibitors for the management of ST requires further evaluation. As suggested by the results of the SURPRISE study, the efficacy of anticoagulant treatment may vary according to the presence of underlying risk factors for recurrent thromboembolic events (Beyer‐Westendorf 2017). Individuals at increased risk could benefit of higher intensity or longer anticoagulant treatment. The usefulness, potential healthcare benefits and cost‐savings of risk stratification tools needs to be evaluated. Additional studies should assess the costs, effects on quality of life, and the cost‐effectiveness of fondaparinux (Goldman 2010). Large and adequately designed randomised controlled trials would be required to assess the actual role of NSAIDs and LMWH, and how these drugs compare with fondaparinux. Whether topical treatment might add some benefit if given in combination with fondaparinux remains unclear.
Feedback
Comment on data analysis, 12 February 2009
Summary
There are some analyses that are difficult to interpret and generate more statistics than data. This type of analysis is recommended in the Cochrane manual, but I am sure there must be a better way. See "Comparison 23. Exhirud ointment versus placebo". This has one study. One outcome "efficacy" was split into 4 categories, and there were thus 4 analyses for 'excellent/good/some/no efficacy'. (Analysis 23.2)
Reply
We fully agree with these comments, however, we felt that reporting these analysis in the dedicated section seemed the only way to inform the reader about these outcomes while avoiding to increase the confusion of the Results section caused by the already long list of comparisons as well as the endless list of studies cited after any statement. We welcome any advice.
Contributors
Feedback: Michael Power, Guideline author
Reply: Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp
Comment on Belcaro papers, 13 February 2009
Summary
This review includes a number of papers by Gianni Belcaro who was erased from the UK medical register in June 2007. This was for "misconduct", which seems to have been that he included as co‐authors on his papers people who were not involved in the research. The GMC report does not suggest that data was falsified. http://webcache.gmc‐uk.org/minutesfiles/3313.HTML Should you mention in the systematic reviews that the data may be suspect in Belcaro's papers?
Reply
We understand and share the suspicion on the reliability of the data. However, it does not seem that the reason for misconduct would influence the quality of the data which, in any case, the GMC report suggested not to be falsified. Therefore we do not think that this misconduct should be explicitly mentioned in the text. Moreover, the data from the studies of Belcaro do not affect the main conclusions of the review. Finally the significant methodological limitations of these studies are underlined in the text.
Contributors
Feedback: Michael Power, Guideline author
Reply: Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp
Best therapeutic options for ST of the legs, 27 October 2010
Summary
The authors concluded that 'low molecular weight heparin and NSAIDs appear as the current best therapeutic options for ST of the legs.' This statement does not fully capture the data presented in the review. In their discussion section the authors note several serious limitations in the studies presented in the review. These include unclear methods of allocation or randomisation, lack of a placebo group as control, high drop out rates, and poor reporting of serious adverse events. In addition, study data could not be pooled due to a high level of heterogeneity and thus data remains underpowered to show any difference in VTE between treatment groups. In the implications for practice section the authors concede that "the data are still too preliminary to make any recommendation". Yet the authors proceed to state that one month of therapy with LMWH may be appropriate to prevent VTE events as well the extension and/or recurrence of ST. Given these drawbacks coupled with the fact that individual trials fail to show significant differences between treatment groups, a final conclusion should not be drawn regarding therapeutic options. Perhaps the question that should be asked is not what the best treatment for ST is, but rather whether or not ST requires treatment at all. The authors note that ST is estimated to be more common than DVT and go on to say that ST is associated with DVT in 6 to 44% of patients, but this does nothing to answer the question of how prevalent ST is in the general population. Given that limited data is available on the prevalence of ST and its clinically relevant outcomes, it is not clear to us whether or not treatment of ST is required to improve patient outcomes.
Reply
We agree with these comments and have modified the text accordingly. Since our previous review, the CALISTO study has been published (Decousus 2010b). The results of this large and methodologically robust RCT provide good answers to some of the reviewer's concerns.
Contributors
Feedback: Michelle Co, BScPharm; Hayley Coe, BScPharm; Sarah West, BSc, BScPharm; Aaron Tejani BScPharm, PharmD
Reply: Marcello Di Nisio, Iris M Wichers, Saskia Middeldorp
What's new
| Date | Event | Description |
|---|---|---|
| 9 March 2017 | New search has been performed | Searches re‐run. Three new trials included and three trials excluded. Ten additional publications to previously included studies. |
| 9 March 2017 | New citation required but conclusions have not changed | Searches re‐run. Three new trials included and three trials excluded. Ten additional publications to previously included studies. Review updated including the addition of Summary of Findings tables. Conclusions not changed. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 15 October 2013 | Amended | Amendments made to the 'Risk of bias' tables and minor data errors corrected. Outcomes reordered to reflect clinical importance. |
| 23 November 2012 | New search has been performed | Searches re‐run. Four new trials included and one new trial excluded. Conclusions unchanged. |
| 23 November 2012 | New citation required but conclusions have not changed | Review updated. Four new trials included and one new trial excluded. Conclusions unchanged. |
| 30 November 2011 | Feedback has been incorporated | Feedback addressed. |
| 30 November 2011 | New search has been performed | Review updated, searches rerun. Two new trials included, one being a large RCT with fondaparinux. |
| 30 November 2011 | New citation required and conclusions have changed | Review updated. Conclusions changed. |
| 27 October 2010 | Feedback has been incorporated | Feedback added |
| 26 April 2010 | Amended | Contact details updated |
| 1 September 2008 | Amended | Converted to new review format. |
| 19 February 2007 | New citation required and minor changes | Updated to correct error in citation. Searches re‐run and no new trials found. |
Acknowledgements
We would like to thank the external peer referee Dr Benilde Cosmi for her comments and Mrs Carole Gibson for acting as the consumer on this review. We would also like to thank the Cochrane Consumer Network for their contribution to the 'Plain language summary.' We would like to thank the personnel from Cochrane Vascular, especially Marlene Stewart and Karen Welch for their invaluable assistance and advice.
Appendices
Appendix 1. CENTRAL search strategy
| Search run on Thur 9 Mar 2017 | Hits | |
| #1 | MESH DESCRIPTOR Thrombophlebitis EXPLODE ALL TREES | 1046 |
| #2 | MESH DESCRIPTOR Phlebitis | 145 |
| #3 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES WITH QUALIFIERS CO | 197 |
| #4 | (superficial near thrombo*):TI,AB,KY | 152 |
| #5 | (superficial near phleb*):TI,AB,KY | 33 |
| #6 | SVT:TI,AB,KY | 175 |
| #7 | *phleb*:TI,AB,KY | 3102 |
| #8 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 | 3452 |
| #9 | * NOT SR‐PVD:CC AND 12/12/2012 TO 28/02/2017:DL | 349958 |
| #10 | #8 AND #9 | 707 |
Appendix 2. Trials registries searches
ClinicalTrials.gov
30 studies found for: thrombophlebitis
World Health Organization International Clinical Trials Registry Platform
136 records for 69 trials found for: thrombophlebitis AND leg
ISRCTN Register
30 results thrombophlebitis
Data and analyses
Comparison 1. Fondaparinux versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis or pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Extension of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Recurrence of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Clinically relevant non‐major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Minor bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Arterial thromboembolic complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse effects of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Non‐fatal serious adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.2. Analysis.
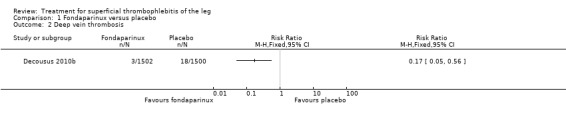
Comparison 1 Fondaparinux versus placebo, Outcome 2 Deep vein thrombosis.
1.3. Analysis.
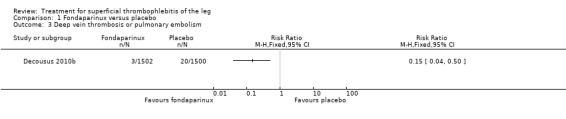
Comparison 1 Fondaparinux versus placebo, Outcome 3 Deep vein thrombosis or pulmonary embolism.
1.4. Analysis.

Comparison 1 Fondaparinux versus placebo, Outcome 4 Extension of superficial thrombophlebitis.
1.5. Analysis.
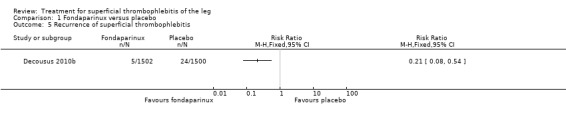
Comparison 1 Fondaparinux versus placebo, Outcome 5 Recurrence of superficial thrombophlebitis.
1.7. Analysis.
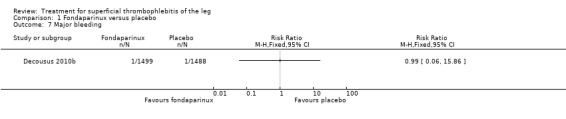
Comparison 1 Fondaparinux versus placebo, Outcome 7 Major bleeding.
1.8. Analysis.
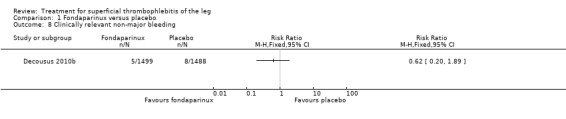
Comparison 1 Fondaparinux versus placebo, Outcome 8 Clinically relevant non‐major bleeding.
1.9. Analysis.
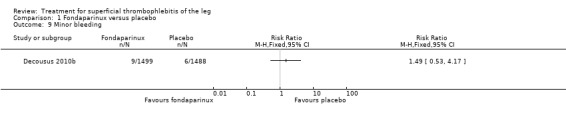
Comparison 1 Fondaparinux versus placebo, Outcome 9 Minor bleeding.
1.10. Analysis.
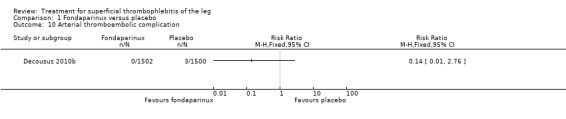
Comparison 1 Fondaparinux versus placebo, Outcome 10 Arterial thromboembolic complication.
1.11. Analysis.
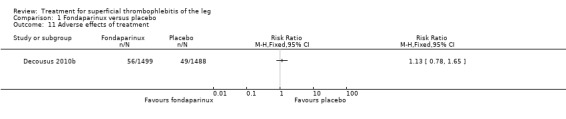
Comparison 1 Fondaparinux versus placebo, Outcome 11 Adverse effects of treatment.
1.12. Analysis.
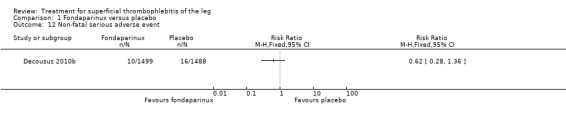
Comparison 1 Fondaparinux versus placebo, Outcome 12 Non‐fatal serious adverse event.
Comparison 2. Fondaparinux versus rivaroxaban.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Deep vein thrombosis or pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Extension of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Recurrence of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Clinically relevant non‐major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Minor bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse effects of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 1 Pulmonary embolism.
2.4. Analysis.
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 4 Extension of superficial thrombophlebitis.
2.7. Analysis.
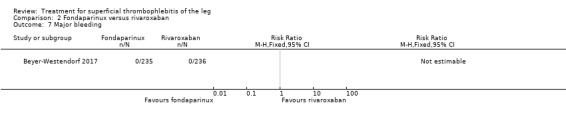
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 7 Major bleeding.
2.9. Analysis.
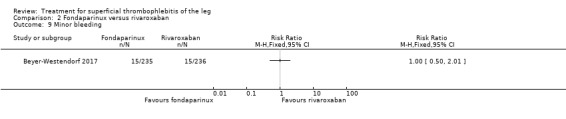
Comparison 2 Fondaparinux versus rivaroxaban, Outcome 9 Minor bleeding.
Comparison 3. Prophylactic low molecular weight heparin (LMWH) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heparin‐induced thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
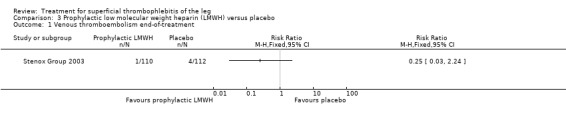
Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 1 Venous thromboembolism end‐of‐treatment.
3.3. Analysis.
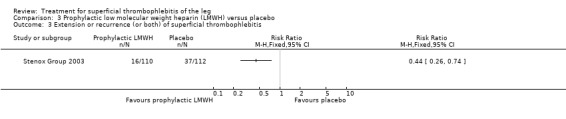
Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 3 Extension or recurrence (or both) of superficial thrombophlebitis.
3.4. Analysis.
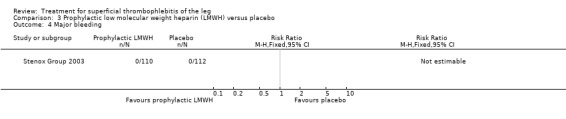
Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 4 Major bleeding.
3.5. Analysis.
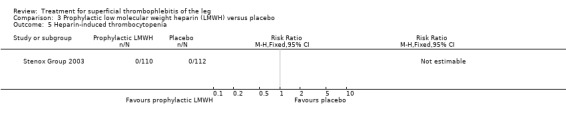
Comparison 3 Prophylactic low molecular weight heparin (LMWH) versus placebo, Outcome 5 Heparin‐induced thrombocytopenia.
Comparison 4. Therapeutic low molecular weight heparin (LMWH) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heparin‐induced thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
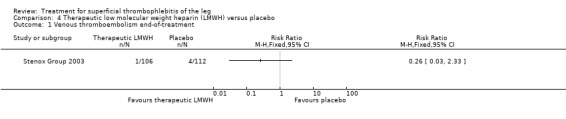
Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 1 Venous thromboembolism end‐of‐treatment.
4.3. Analysis.

Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 3 Extension or recurrence (or both) of superficial thrombophlebitis.
4.4. Analysis.
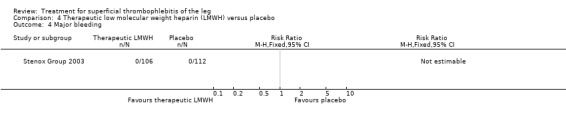
Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 4 Major bleeding.
4.5. Analysis.
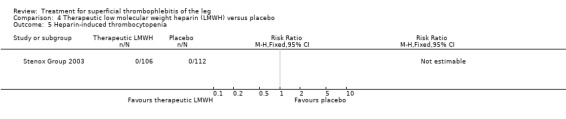
Comparison 4 Therapeutic low molecular weight heparin (LMWH) versus placebo, Outcome 5 Heparin‐induced thrombocytopenia.
Comparison 5. Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic deep vein thrombosis end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptomatic deep vein thrombosis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extension of superficial thrombophlebitis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
5.3. Analysis.
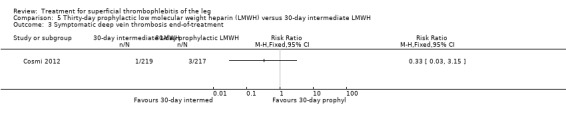
Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 3 Symptomatic deep vein thrombosis end‐of‐treatment.
5.5. Analysis.
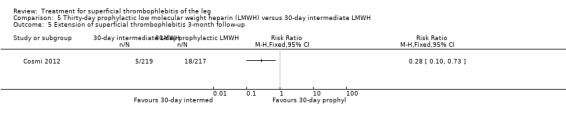
Comparison 5 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 30‐day intermediate LMWH, Outcome 5 Extension of superficial thrombophlebitis 3‐month follow‐up.
Comparison 6. Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic deep vein thrombosis end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptomatic deep vein thrombosis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extension of superficial thrombophlebitis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.
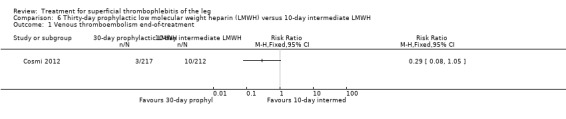
Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 1 Venous thromboembolism end‐of‐treatment.
6.2. Analysis.
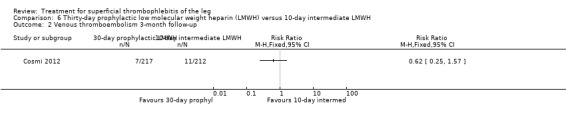
Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 2 Venous thromboembolism 3‐month follow‐up.
6.3. Analysis.
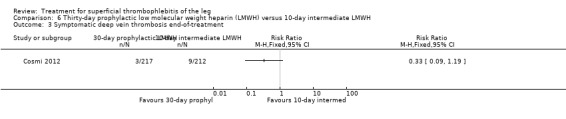
Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 3 Symptomatic deep vein thrombosis end‐of‐treatment.
6.5. Analysis.
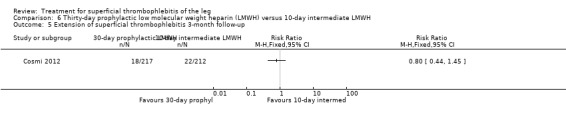
Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 5 Extension of superficial thrombophlebitis 3‐month follow‐up.
6.6. Analysis.
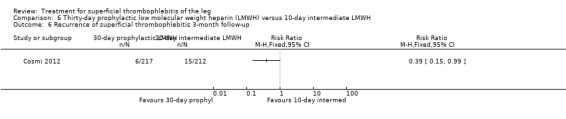
Comparison 6 Thirty‐day prophylactic low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up.
Comparison 7. Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptomatic deep vein thrombosis end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptomatic deep vein thrombosis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Extension of superficial thrombophlebitis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Recurrence of superficial thrombophlebitis 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
7.3. Analysis.
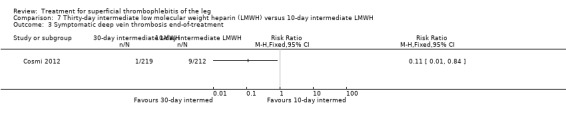
Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 3 Symptomatic deep vein thrombosis end‐of‐treatment.
7.5. Analysis.
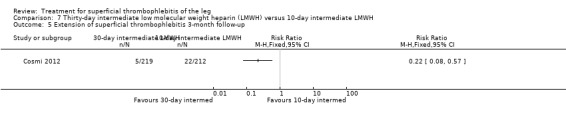
Comparison 7 Thirty‐day intermediate low molecular weight heparin (LMWH) versus 10‐day intermediate LMWH, Outcome 5 Extension of superficial thrombophlebitis 3‐month follow‐up.
Comparison 8. Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptomatic pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Superficial thrombophlebitis progression into deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
8.1. Analysis.
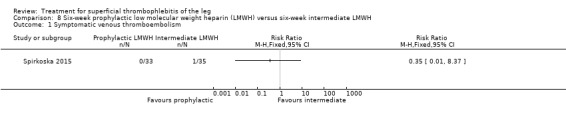
Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 1 Symptomatic venous thromboembolism.
8.2. Analysis.
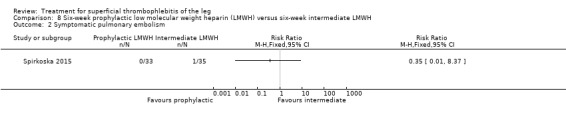
Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 2 Symptomatic pulmonary embolism.
8.3. Analysis.
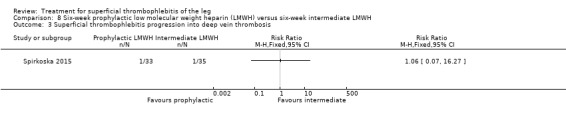
Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 3 Superficial thrombophlebitis progression into deep vein thrombosis.
8.4. Analysis.
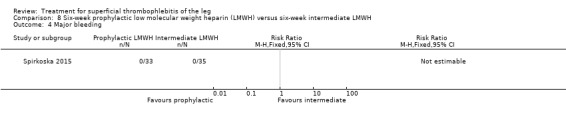
Comparison 8 Six‐week prophylactic low molecular weight heparin (LMWH) versus six‐week intermediate LMWH, Outcome 4 Major bleeding.
Comparison 9. Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Superficial thrombophlebitis or venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.72] |
| 3 Superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Swelling disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Tenderness disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Pain disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Pitting oedema disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Collateral veins disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Redness disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Palpable cord disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Major bleeding | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Heparin‐induced thrombocytopenia | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.07, 16.11] |
9.1. Analysis.
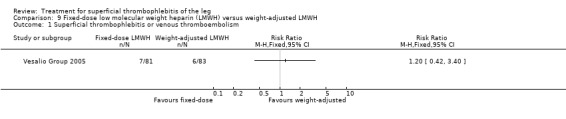
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 1 Superficial thrombophlebitis or venous thromboembolism.
9.2. Analysis.
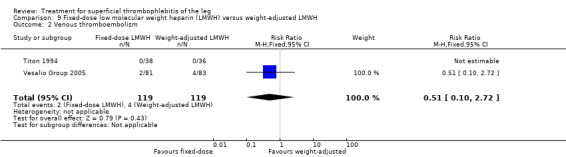
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 2 Venous thromboembolism.
9.3. Analysis.
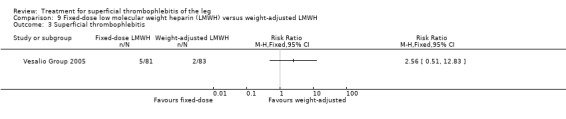
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 3 Superficial thrombophlebitis.
9.4. Analysis.
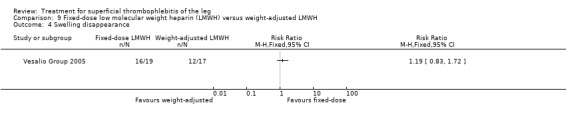
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 4 Swelling disappearance.
9.5. Analysis.
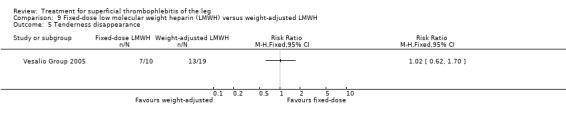
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 5 Tenderness disappearance.
9.6. Analysis.
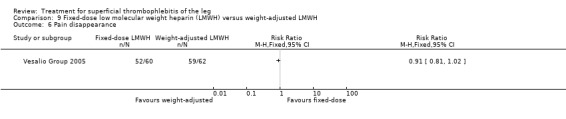
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 6 Pain disappearance.
9.7. Analysis.
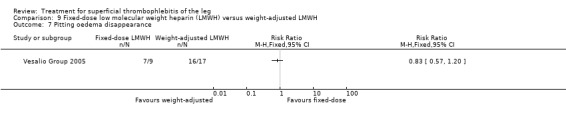
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 7 Pitting oedema disappearance.
9.8. Analysis.
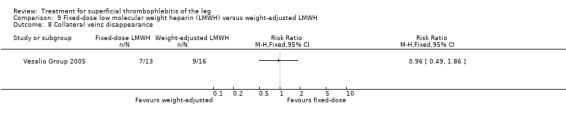
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 8 Collateral veins disappearance.
9.9. Analysis.
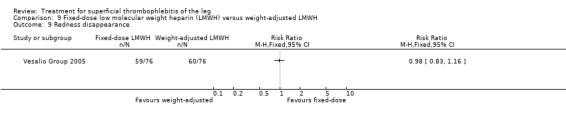
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 9 Redness disappearance.
9.10. Analysis.
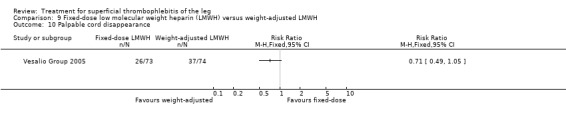
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 10 Palpable cord disappearance.
9.11. Analysis.
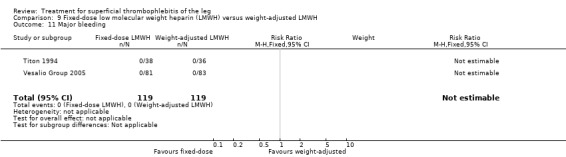
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 11 Major bleeding.
9.12. Analysis.
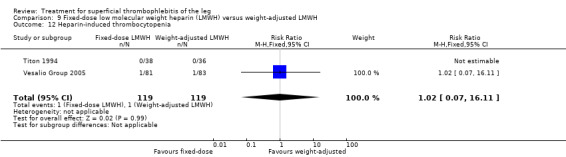
Comparison 9 Fixed‐dose low molecular weight heparin (LMWH) versus weight‐adjusted LMWH, Outcome 12 Heparin‐induced thrombocytopenia.
Comparison 10. Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.
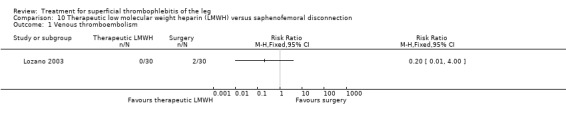
Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 1 Venous thromboembolism.
10.2. Analysis.
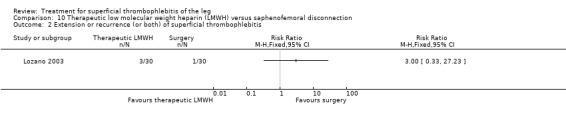
Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
10.3. Analysis.
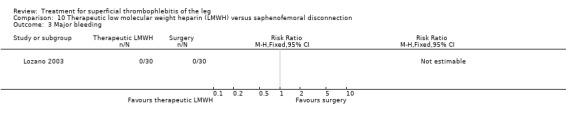
Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 3 Major bleeding.
10.4. Analysis.
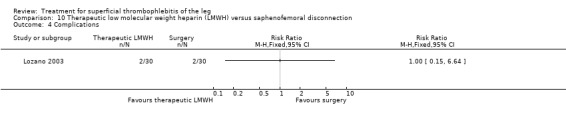
Comparison 10 Therapeutic low molecular weight heparin (LMWH) versus saphenofemoral disconnection, Outcome 4 Complications.
Comparison 11. Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.24, 3.63] |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 3 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.58, 1.78] |
| 3 Major bleeding | 3 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Heparin‐induced thrombocytopenia | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.
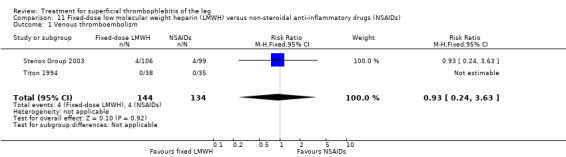
Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 1 Venous thromboembolism.
11.2. Analysis.
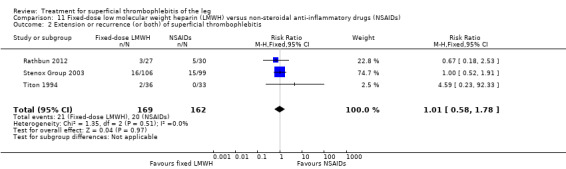
Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
11.3. Analysis.
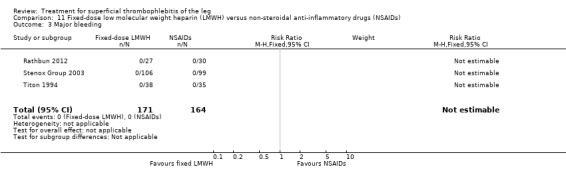
Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 3 Major bleeding.
11.4. Analysis.
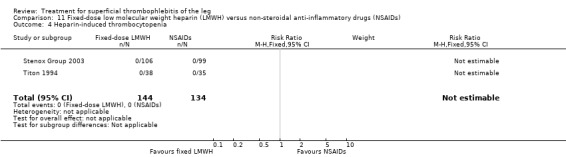
Comparison 11 Fixed‐dose low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 4 Heparin‐induced thrombocytopenia.
Comparison 12. Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Heparin‐induced thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
12.1. Analysis.
Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 1 Venous thromboembolism.
12.2. Analysis.
Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
12.3. Analysis.
Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 3 Major bleeding.
12.4. Analysis.
Comparison 12 Weight‐adjusted low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 4 Heparin‐induced thrombocytopenia.
Comparison 13. Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism end‐of‐treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Venous thromboembolism 3‐month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heparin‐induced thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
13.1. Analysis.
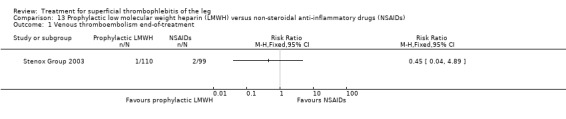
Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 1 Venous thromboembolism end‐of‐treatment.
13.2. Analysis.
Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 2 Venous thromboembolism 3‐month follow‐up.
13.3. Analysis.
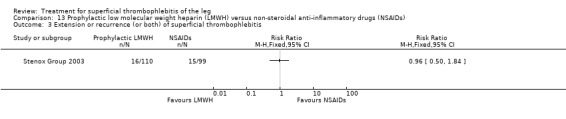
Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 3 Extension or recurrence (or both) of superficial thrombophlebitis.
13.4. Analysis.
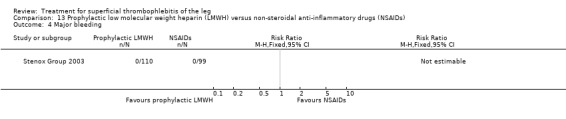
Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 4 Major bleeding.
13.5. Analysis.
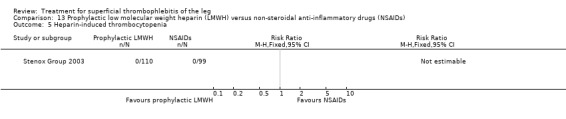
Comparison 13 Prophylactic low molecular weight heparin (LMWH) versus non‐steroidal anti‐inflammatory drugs (NSAIDs), Outcome 5 Heparin‐induced thrombocytopenia.
Comparison 14. Low molecular weight heparin (LMWH) versus LMWH plus acemetacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary embolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Extension of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pain reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Hyperaemia reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Tenderness reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Palpable cord reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Minor bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
14.1. Analysis.
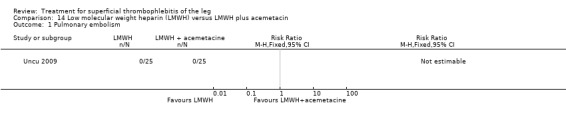
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 1 Pulmonary embolism.
14.2. Analysis.
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 2 Deep vein thrombosis.
14.3. Analysis.
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 3 Extension of superficial thrombophlebitis.
14.8. Analysis.
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 8 Mortality.
14.9. Analysis.
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 9 Major bleeding.
14.10. Analysis.
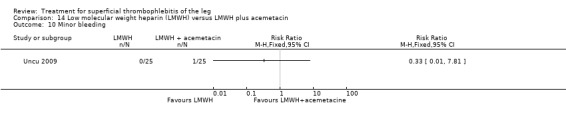
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 10 Minor bleeding.
14.11. Analysis.
Comparison 14 Low molecular weight heparin (LMWH) versus LMWH plus acemetacin, Outcome 11 Adverse events.
Comparison 15. Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus ECS alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
15.1. Analysis.
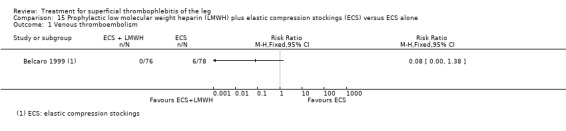
Comparison 15 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.
15.2. Analysis.
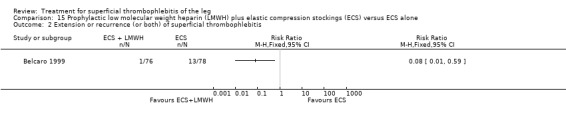
Comparison 15 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
Comparison 16. Low molecular weight heparin (LMWH) versus heparin spray gel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deep vein thrombosis | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.03, 2.70] |
| 2 Participants with thrombus at 21 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Allergic reaction or elevated sedimentation rate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
16.1. Analysis.
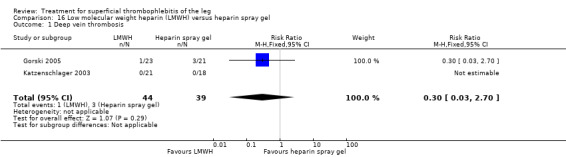
Comparison 16 Low molecular weight heparin (LMWH) versus heparin spray gel, Outcome 1 Deep vein thrombosis.
16.2. Analysis.
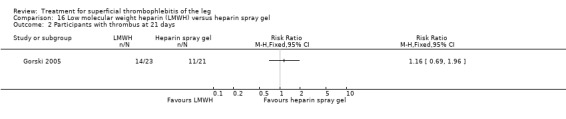
Comparison 16 Low molecular weight heparin (LMWH) versus heparin spray gel, Outcome 2 Participants with thrombus at 21 days.
16.3. Analysis.
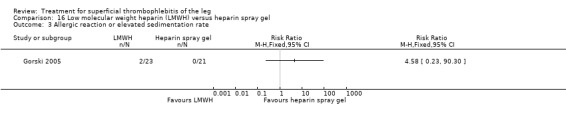
Comparison 16 Low molecular weight heparin (LMWH) versus heparin spray gel, Outcome 3 Allergic reaction or elevated sedimentation rate.
Comparison 17. High‐dose unfractionated heparin (UFH) versus low‐dose UFH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Heparin‐induced thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
17.1. Analysis.
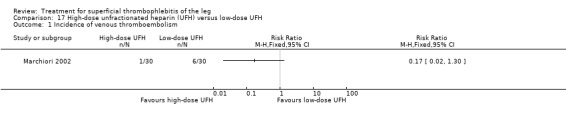
Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 1 Incidence of venous thromboembolism.
17.2. Analysis.
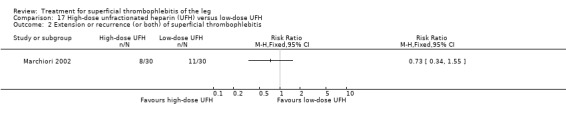
Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
17.3. Analysis.
Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 3 Major bleeding.
17.4. Analysis.
Comparison 17 High‐dose unfractionated heparin (UFH) versus low‐dose UFH, Outcome 4 Heparin‐induced thrombocytopenia.
Comparison 18. Heparin calcium plus elastic compression bandage (ECB) versus ECB alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
18.1. Analysis.
Comparison 18 Heparin calcium plus elastic compression bandage (ECB) versus ECB alone, Outcome 1 Deep vein thrombosis.
Comparison 19. Heparin subcutaneous (sc) versus defibrotide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decrease in the analogue score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse effects of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
19.1. Analysis.
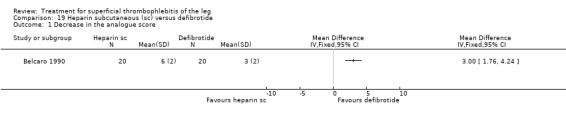
Comparison 19 Heparin subcutaneous (sc) versus defibrotide, Outcome 1 Decrease in the analogue score.
19.2. Analysis.
Comparison 19 Heparin subcutaneous (sc) versus defibrotide, Outcome 2 Adverse effects of treatment.
Comparison 20. Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Major bleeding | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Heparin‐induced thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
20.1. Analysis.
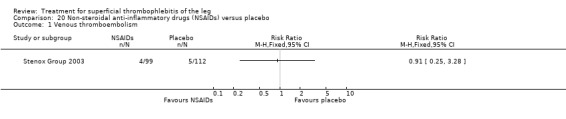
Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 1 Venous thromboembolism.
20.2. Analysis.
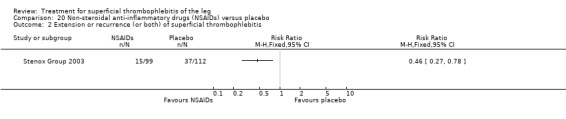
Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
20.3. Analysis.
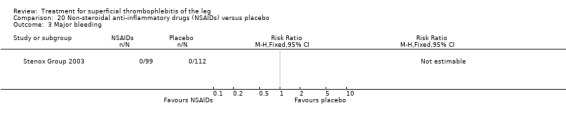
Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 3 Major bleeding.
20.4. Analysis.
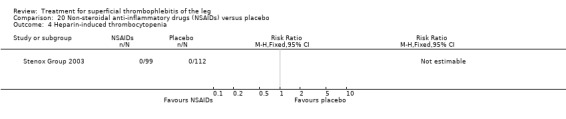
Comparison 20 Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo, Outcome 4 Heparin‐induced thrombocytopenia.
Comparison 21. Indomethacin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
21.1. Analysis.
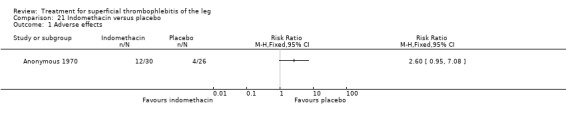
Comparison 21 Indomethacin versus placebo, Outcome 1 Adverse effects.
Comparison 22. Nimesulide versus diclofenac sodium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gastric pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
22.1. Analysis.
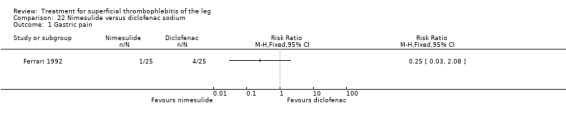
Comparison 22 Nimesulide versus diclofenac sodium, Outcome 1 Gastric pain.
Comparison 23. Essaven gel versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intolerance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
23.1. Analysis.
Comparison 23 Essaven gel versus placebo, Outcome 1 Intolerance.
Comparison 24. Thrombectomy plus venoruton plus elastic compression bandage (ECB) versus ECB alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
24.1. Analysis.
Comparison 24 Thrombectomy plus venoruton plus elastic compression bandage (ECB) versus ECB alone, Outcome 1 Deep vein thrombosis.
Comparison 25. Thrombectomy plus elastic compression bandage (ECB) versus ECB alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
25.1. Analysis.
Comparison 25 Thrombectomy plus elastic compression bandage (ECB) versus ECB alone, Outcome 1 Deep vein thrombosis.
Comparison 26. Ligation plus elastic compression stockings (ECS) versus ECS alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
26.1. Analysis.
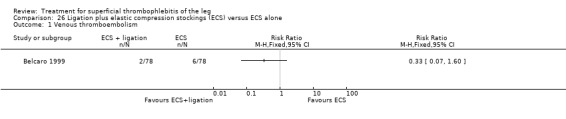
Comparison 26 Ligation plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.
26.2. Analysis.
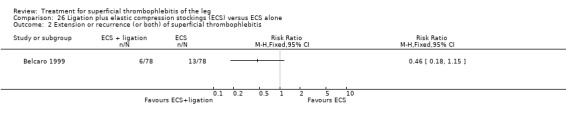
Comparison 26 Ligation plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
Comparison 27. Prophylactic unfractionated heparin (UFH) plus elastic compression stockings (ECS) versus ECS alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
27.1. Analysis.
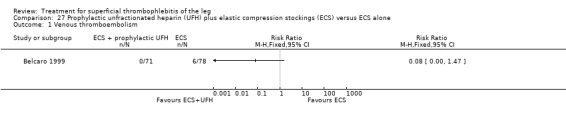
Comparison 27 Prophylactic unfractionated heparin (UFH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.
27.2. Analysis.
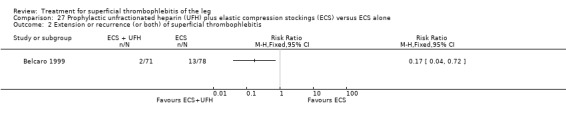
Comparison 27 Prophylactic unfractionated heparin (UFH) plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
Comparison 28. Oral vasotonin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cured or substantially better | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Poor tolerability | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
28.1. Analysis.
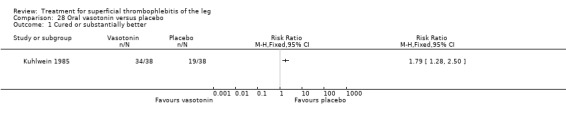
Comparison 28 Oral vasotonin versus placebo, Outcome 1 Cured or substantially better.
28.2. Analysis.
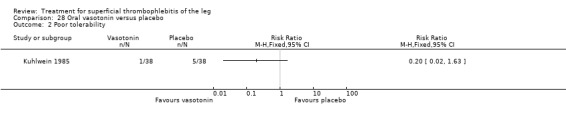
Comparison 28 Oral vasotonin versus placebo, Outcome 2 Poor tolerability.
Comparison 29. Elastic compression bandage (ECB) plus venoruton versus ECB alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deep vein thrombosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
29.1. Analysis.
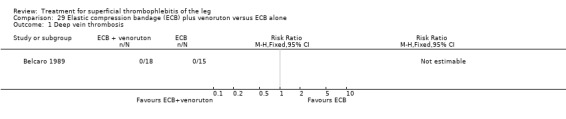
Comparison 29 Elastic compression bandage (ECB) plus venoruton versus ECB alone, Outcome 1 Deep vein thrombosis.
Comparison 30. Oral heparansulphate versus oral sulodexide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Redness disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pain disappearance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Disappearance of itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Oedema improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Trophism improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
30.1. Analysis.
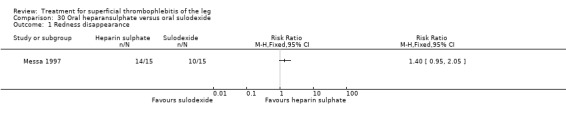
Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 1 Redness disappearance.
30.2. Analysis.
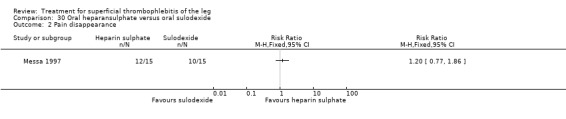
Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 2 Pain disappearance.
30.3. Analysis.

Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 3 Disappearance of itching.
30.4. Analysis.
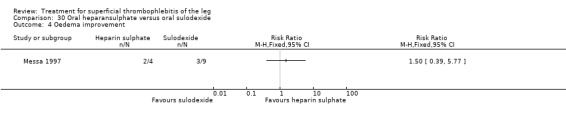
Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 4 Oedema improvement.
30.5. Analysis.
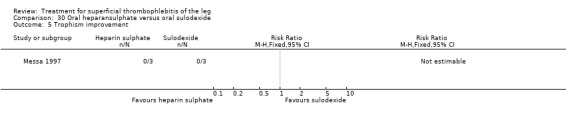
Comparison 30 Oral heparansulphate versus oral sulodexide, Outcome 5 Trophism improvement.
Comparison 31. Oxyphenbutazone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tenderness improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
31.1. Analysis.
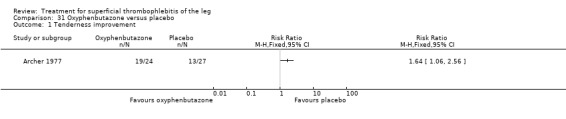
Comparison 31 Oxyphenbutazone versus placebo, Outcome 1 Tenderness improvement.
Comparison 32. Vitamin K antagonist (VKA) plus elastic compression stockings (ECS) versus ECS alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
32.1. Analysis.
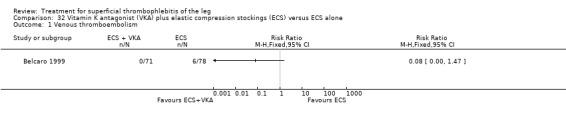
Comparison 32 Vitamin K antagonist (VKA) plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.
32.2. Analysis.
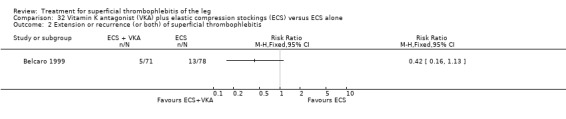
Comparison 32 Vitamin K antagonist (VKA) plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
Comparison 33. Stripping plus elastic compression stockings (ECS) versus ECS alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venous thromboembolism | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Extension or recurrence (or both) of superficial thrombophlebitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
33.1. Analysis.
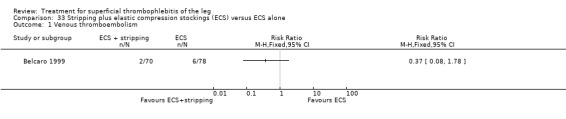
Comparison 33 Stripping plus elastic compression stockings (ECS) versus ECS alone, Outcome 1 Venous thromboembolism.
33.2. Analysis.
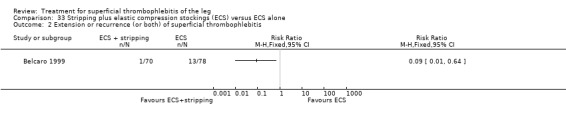
Comparison 33 Stripping plus elastic compression stockings (ECS) versus ECS alone, Outcome 2 Extension or recurrence (or both) of superficial thrombophlebitis.
Comparison 34. Enzyme therapy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain reduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Responders | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
34.1. Analysis.
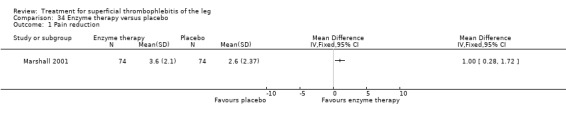
Comparison 34 Enzyme therapy versus placebo, Outcome 1 Pain reduction.
34.2. Analysis.
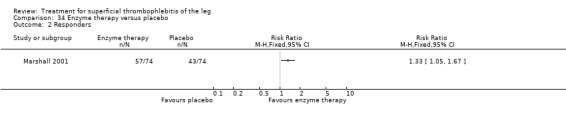
Comparison 34 Enzyme therapy versus placebo, Outcome 2 Responders.
Comparison 35. Desmin intramuscular (im) 200 versus desmin 100.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse drug reactions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
35.1. Analysis.
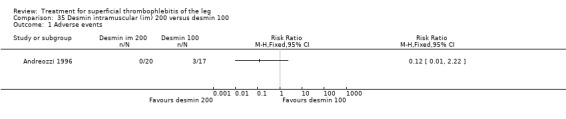
Comparison 35 Desmin intramuscular (im) 200 versus desmin 100, Outcome 1 Adverse events.
35.2. Analysis.
Comparison 35 Desmin intramuscular (im) 200 versus desmin 100, Outcome 2 Adverse drug reactions.
Comparison 36. Desmin subcutaneous (sc) 2 × 100 versus desmin 100.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse drug reactions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
36.1. Analysis.
Comparison 36 Desmin subcutaneous (sc) 2 × 100 versus desmin 100, Outcome 1 Adverse events.
36.2. Analysis.
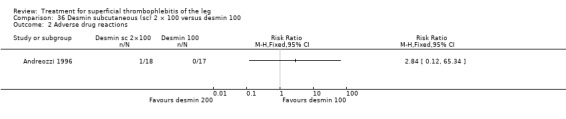
Comparison 36 Desmin subcutaneous (sc) 2 × 100 versus desmin 100, Outcome 2 Adverse drug reactions.
Comparison 37. Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS, cm) at 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Skin erythema (cm2) at 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 SF‐36 physical score at 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 SF‐36 mental score at 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
37.1. Analysis.
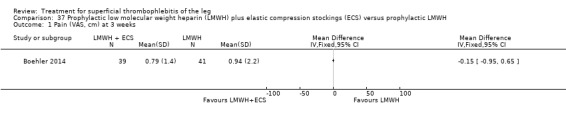
Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 1 Pain (VAS, cm) at 3 weeks.
37.2. Analysis.
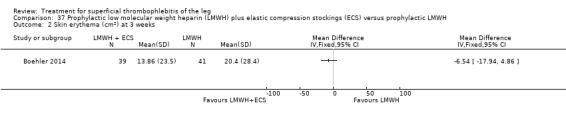
Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 2 Skin erythema (cm2) at 3 weeks.
37.3. Analysis.
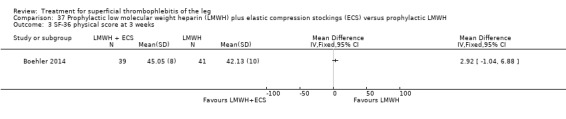
Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 3 SF‐36 physical score at 3 weeks.
37.4. Analysis.
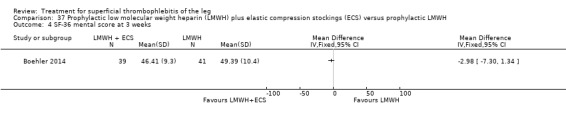
Comparison 37 Prophylactic low molecular weight heparin (LMWH) plus elastic compression stockings (ECS) versus prophylactic LMWH, Outcome 4 SF‐36 mental score at 3 weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andreozzi 1996.
| Methods | Multicentre, open RCT. | |
| Participants | 56 participants with ST or varicophlebitis of lower limbs; 19 males, 36 females; mean age range 48‐52 years. Diagnosis of ST objectively confirmed by Doppler compression ultrasonography. Not reported if the included participants were hospitalised or non‐hospitalised. | |
| Interventions | Desmin (Dermatan sulphate) (100 mg bid sc). Desmin (200 mg od im). Desmin (100 mg od sc). Study treatment given for 30 days. |
|
| Outcomes | Pain and functional inability, local oedema, palpable thrombophlebitic cord, fever, hyperaemia and cutaneous hyperthermia, adverse events or adverse drug reactions. | |
| Notes | Funding: Alfa Wassermann S.p.A (Bologna, Italy) supplied desmin. Disclosure of potential COI: 2 authors from Alfa Wassermann S.p.A., Bologna, Italy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study "open and multicenter." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants analysed unclear. 1 participant discontinued treatment and was excluded from baseline descriptive table. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the methods section were addressed in the results or discussion section. |
Anonymous 1970.
| Methods | Placebo‐controlled, double‐blind RCT. | |
| Participants | 56 participants with ST of the calf (68%), thigh (16%), both (16%). Not reported if included participants were hospitalised or non‐hospitalised. Not reported if diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Indomethacin (50 mg tid). Placebo. Study treatment given for 1 week. |
|
| Outcomes | Erythema, spontaneous pain, tenderness, oedema. | |
| Notes | Every participant received tetracyclines (250 mg 4 times daily), paracetamol when required, warm socks (5 participants in indomethacin group, 3 in placebo group). Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. "..comparison between indomethacin and inactive placebo..the choice being determined by random selection." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind study. "double‐blind comparison;" however, not reported who was blinded and how blinding was attempted. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not clear how many participants of those initially included had full assessment of the study endpoints. From Table 1 in Anonymous 1970, it was clear that ≥ 1 of the outcomes not all participants were evaluated at longest follow‐up. |
| Selective reporting (reporting bias) | High risk | No data provided for some of the outcomes. Adverse effects reported in the results section but not mentioned in the methods section. |
Archer 1977.
| Methods | Multicentre, double‐blind RCT. | |
| Participants | 54 non‐hospitalised participants with ST; 18 males, 36 females; mean age 55 years. Not reported if the diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Oxyphenbutazone (Tandacote) (100 mg 4 times daily). Placebo (4 times daily). Study treatment given for 7 days. |
|
| Outcomes | Pain, erythema, tenderness, length of indurated vein. | |
| Notes | Paracetamol and firm bandaging allowed. Outcome tenderness assessed in 24/26 participants in the oxyphenbutazone group. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. "Oxyphenbutazone..and matching placebo were allocated at random." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No clear information provided about blinding. "...none was a satisfactory double‐blind controlled study...". "...it was felt that such a study should be undertaken.." It could be a double blinded study, however, blinding is not clearly mentioned in the methods, results or discussion sections. Not reported who was blinded and how blinding was attempted. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant (from the placebo group) excluded postrandomisation (2%). Unclear if all the remaining participants were analysed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported. |
Belcaro 1989.
| Methods | Open RCT. | |
| Participants | 83 participants with ST without DVT on duplex ultrasonography; 36 males, 45 females; mean age 38.9 years (range 27‐46 years). Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | ST + ECB. Heparin calcium (0.5 mg bid sc) + ECB. Venoruton (1000 mg tid) + ECB. Venoruton (1000 mg tid) after ST + ECB. ECB alone. Non‐surgical treatment given for 8 weeks. |
|
| Outcomes | Area of maximum temperature, pain and tenderness (analogical score), DVT. Not specified if DVTs were symptomatic or asymptomatic. | |
| Notes | 6/9 participants excluded postrandomisation underwent surgery of the superficial venous system. DVT verified at 6 weeks by strain‐gauge plethysmography. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. Gianni Belcaro erased from the UK medical register in June 2007 for "misconduct," which seems to have been that he included as coauthors on his papers people who were not involved in the research. The General Medical Council report did not suggest that data were falsified webcache.gmc‐uk.org/minutesfiles/3313.HTML. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. "patients were randomized in 5 groups of treatment." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information provided about blinding, but it was likely an open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 participants excluded after inclusion in the study (10%): "6 patients underwent surgery of the superficial venous system and 3 failed to follow the prescribed treatments." |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Belcaro 1990.
| Methods | Single‐centre RCT. | |
| Participants | 40 participants with ST confirmed by colour duplex ultrasonography; 13 males, 27 females; aged 46‐48 years. Not reported if participants were hospitalised or non‐hospitalised. Angiodynography used to exclude the presence of obstruction and DVT. | |
| Interventions | Defibrotide (first week 400 mg bid; second and third weeks 400 mg od). Low‐dose heparin (5000 IU bid sc) for 3 weeks. |
|
| Outcomes | Area of maximum temperature, analogue score (redness, local tenderness, inflammation). | |
| Notes | All participants received compression bandages. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. Gianni Belcaro erased from the UK medical register in June 2007 for "misconduct," which seems to have been that he included as coauthors on his papers people who were not involved in the research. The General Medical Council report did not suggest that data were falsified webcache.gmc‐uk.org/minutesfiles/3313.HTML. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. "Patients were randomized in two treatment groups." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information provided about blinding, but it was likely an open study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all included participants were evaluated for study outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Belcaro 1999.
| Methods | Multicentre, open RCT; 6‐month follow‐up. | |
| Participants | 562 non‐hospitalised participants with ST, large varicose veins, and venous incompetence; 181 males, 263 females; mean age range across study groups 53‐56 years. Diagnosis of ST objectively confirmed by colour Doppler compression ultrasonography. | |
| Interventions | Surgery (ligation). Surgery (complete stripping). Low‐dose sc heparin. LMWH. Coumadin. |
|
| Outcomes | DVT and extension of ST after treatment and after 3 and 6 months. Not specified if DVT and extension of ST were symptomatic or asymptomatic. | |
| Notes | All participants received compression bandages. Duration of non‐surgical treatments unclear and dose of heparin or coumadin not specified. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. Gianni Belcaro erased from the UK medical register in June 2007 for "misconduct," which seems to have been that he included as coauthors on his papers people who were not involved in the research. The General Medical Council report did not suggest that data were falsified webcache.gmc‐uk.org/minutesfiles/3313.HTML. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information provided about blinding, but it was likely an open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 118 (21%) participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Belcaro 2011.
| Methods | Single‐centre, randomised, placebo‐controlled study. | |
| Participants | 120 non‐hospitalised participants with ST of the legs within 72 hours prior to study inclusion. ST confirmed by colour‐Doppler ultrasonography. Mean age 46.3 years (11.51), 26 males, 94 females. | |
| Interventions | Heparin spray gel (Viatromb 2400 IU/g): 4122 IU sodium heparin applied as 3 times 3 spray puffs. Heparin spray gel (Viatromb 2400 IU/g): 5496 IU sodium heparin applied as 3 times 4 spray puffs. Heparin spray gel (Viatromb 2400 IU/g): 6879 IU sodium heparin applied as 3 times 5 spray puffs. Placebo applied as 3 times 5 puffs. Study medication applied for 7‐14 days. |
|
| Outcomes | Pain reduction (VAS scale and VRS), erythema extension, thrombus size, oedema reduction, adverse events, investigator's and participant's assessment of efficacy. | |
| Notes | Funding: study sponsored by CRO Opera S.r.L., Genoa, Italy. Disclosure of potential COI: authors reported to have no COI. Gianni Belcaro erased from the UK medical register in June 2007 for "misconduct," which seems to have been that he included as coauthors on his papers people who were not involved in the research. The General Medical Council report did not suggest that data were falsified webcache.gmc‐uk.org/minutesfiles/3313.HTML. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated: "The treatments were allocated according to a randomization list (randomization by blocks)." Block allocation sequences created at random by using numbers generated by a computer program. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation sequence. "Each subject enrolled into the study received the respective lowest randomization number available." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blind: "observer blinded design." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/150 (11.7%) dropped out, 68.3% completed treatment on day 7, 11.7% on day 14, and 8.3% had treatment failure. |
| Selective reporting (reporting bias) | High risk | Data not reported for all prespecified outcomes. DVT mentioned in methods but not reported in results or discussion. |
Beyer‐Westendorf 2017.
| Methods | Open‐label, masked endpoint, randomised, non‐inferiority phase 3b trial. | |
| Participants | 472 participants aged ≥ 18 years with symptomatic ST (≥ 5 cm in a supragenual superficial‐vein segment) and ≥ 1 additional risk factor for thromboembolic complications (aged ≥ 65 years, male sex, previous VTE, cancer, autoimmune disease, thrombosis of non‐varicose veins). | |
| Interventions | Rivaroxaban (10 mg oral od). Fondaparinux (2.5 mg sc od). Study treatment given for 45 days. |
|
| Outcomes | Primary efficacy outcome: composite of symptomatic DVT or PE, progression or recurrence of ST, and all‐cause mortality at 45 days. Secondary efficacy outcomes: composite primary efficacy outcome at 90 days, incidence of each component of the primary efficacy outcome at 45 and 90 days; major VTE (symptomatic PE, symptomatic proximal DVT, or VTE‐related death) at days 45 and 90; and surgery for ST within 45 and 90 days of initiation of study drug treatment. Primary safety outcome: major bleeding. Secondary safety outcomes: clinically relevant non‐major bleeding, and minor bleeding within 45 days of initiation of treatment with study drug, censored 2 days after the last dose of study drug. |
|
| Notes | Funding: GWT‐TUD and Bayer Vital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate method of randomisation. Quote: "Randomisation was done with a central block randomisation process, with a random block sequence of four numbers per block." |
| Allocation concealment (selection bias) | Low risk | Participants and investigators could not foresee treatment allocation. Quote: "The generation of the allocation sequence to assign all patients to the two treatment groups and the allocation were done at the sponsor’s site by the study manager, who had no role in the trial or in data collection or analysis." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study with blinded outcome assessment. Quote: "An independent committee, whose members were unaware of study group assignment, adjudicated the qualifying diagnosis, the anatomical extent of the initial superficial‐vein thrombosis, and all suspected outcomes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis originally planned as ITT, but modified into a per‐protocol analysis during study. 36/471 (7.6%) participants randomised were excluded from the primary analysis but the authors reported results also according to the ITT principle. Quote: "The original study protocol specified that the primary efficacy analysis would be done in the intention‐to‐treat population; however, the protocol was changed by the steering committee on June 15, 2016, to specify analysis in the per‐protocol population." |
| Selective reporting (reporting bias) | Low risk | All outcome described in the methods and protocol were presented in the results. |
Boehler 2014.
| Methods | Prospective, single‐centre, open‐label, randomised study. | |
| Participants | 73 outpatients with isolated superficial vein thrombosis of the legs. Thrombus length ≥ 5 cm and confirmed by compression ultrasonography. Mean age 56.3 years (SD 13.2) in compression stockings group and 60.3 years (SD 14.8) in control group. | |
| Interventions | Thigh‐length compression stockings, class II (23‐32 mmHg; Venotrain, Bauerfeind, Zeulenroda, Germany) for 3 weeks. No compression. |
|
| Outcomes | Primary outcome: reduction of spontaneous and induced pain as assessed by a VAS and Lowenberg test. Secondary outcomes: consumption of analgesics, thrombus length, skin erythema, D‐dimer, and quality of life through the SF‐36. Main safety outcomes: symptomatic or asymptomatic DVT and HIT. |
|
| Notes | All participants received LMWH at prophylactic dosage (enoxaparin 40 mg/day). NSAIDs (mefenamic acid, diclofenac, and dexibuprofen) were allowed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported. "The randomization procedure was performed in a 1:1 ratio in blocks of 20 with closed envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Unclear if envelopes were sealed and sequentially numbered. "The randomization procedure was performed in a 1:1 ratio in blocks of 20 with closed envelopes, with the investigators not being aware of the sequence within the envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/80 (8.7%) participants initially enrolled were excluded from the analysis due to missing data during follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section were reported in results section of the study publication. |
Cosmi 2012.
| Methods | STEFLUX: multicentre, randomised, double‐blind, placebo‐controlled study. | |
| Participants | 664 consecutive outpatients with ≥ 4 cm long ST of long or short saphenous veins or their collaterals confirmed by CUS. ST of the long saphenous or short saphenous vein within 3 cm to, respectively, the sapheno‐femoral or sapheno‐popliteal junction were excluded. Median age 69 years (range 20‐94 years); males 37% (246/664). | |
| Interventions | Intermediate‐dose LMWH (parnaparin 8500 IU od) for 10 days followed by placebo for 20 days. Intermediate‐dose LMWH (parnaparin 8500 IU od for 10 days followed by 6400 IU od for 20 days). Prophylactic‐dose LMWH (parnaparin 4250 IU od) for 30 days. |
|
| Outcomes | Primary efficacy outcomes: composite of symptomatic and asymptomatic DVT, symptomatic PE and relapse, symptomatic or asymptomatic SVT recurrence, or a combination in first 33 days. Secondary efficacy outcomes: reduction in local symptoms during treatment, combined efficacy endpoint during a follow‐up of 93 days after the start of treatment. Primary safety outcomes: major bleeding. Secondary safety outcomes: composite of minor bleeding, thrombocytopenia or any other adverse events (e.g. local allergic reactions). |
|
| Notes | Participants were instructed to wear graduated elastic stockings (knee high or thigh length) with a compression of 20‐40 mmHg at the ankle, unless contraindicated. Oral or topical NSAIDs were permitted for only 4 days after study inclusion. Only paracetamol, naproxen, or ibuprofen were allowed. Study prematurely interrupted according to predefined stopping rules. Funding: study supported by the non‐profit organisation Angioclot in the Department of Angiology and Blood Coagulation, S. Orsola Malpighi University Hospital through an unrestricted grant by Alfa Wassermann, Bologna, Italy which had no input into study design; analysis and interpretation of data; writing of the report; and decision to submit the article for publication. Alfa Wassermann provided the study drug and placebo. Disclosure of potential COI: 3 authors including the lead author reported to have received consulting fees from Alfa Wasserman. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer‐generated randomisation sequence: "The randomization sequence was computer generated centrally in blocks of six." |
| Allocation concealment (selection bias) | Low risk | Central computer‐generated randomisation sequence: "The randomization sequence was computer generated centrally in blocks of six and centers were provided with consecutively numbered boxes of identically appearing prefilled syringes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. "Consecutive outpatients were randomly assigned to receive in a double‐blind fashion one of the following subcutaneous treatments." "All primary and secondary outcomes were evaluated by a central adjudication committee, whose members were not involved in patient recruitment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16/664 (2.4%) participants excluded from the analysis, 8 lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
De Sanctis 2001.
| Methods | Placebo‐controlled RCT. | |
| Participants | 30 participants with ST confirmed by colour duplex ultrasonography and varicose veins; mean age 51 years; 17 males, 13 females. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | Essaven gel (5 cm of gel). Placebo (5 cm of gel). Study treatment given for 4 weeks. |
|
| Outcomes | Mean decrease in temperature, mean symptomatic score (local pain, disability, swelling). | |
| Notes | All participants received LMWH (clexane 0.1 mL/10 kg of bodyweight od) for 4 weeks and elastic compression stockings. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. "the randomization process was controlled by an external statistical controller according to GCP rules." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. "Placebo comparable to Essaven gel was used," "operators were unaware of the contents of the tube." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Decousus 2010b.
| Methods | CALISTO: multicentre, randomised, double‐blind, placebo‐controlled study, ITT analysis. | |
| Participants | 3002 hospitalised or non‐hospitalised participants, aged ≥ 18 years, with acute, symptomatic lower limb superficial‐vein thrombosis ≥ 5 cm long, as confirmed by standardised compression ultrasonography. Mean age 57.1 (SD 13.3) years fondaparinux and 56.9 (SD 13.6) years placebo. 1084 males, 1918 females. | |
| Interventions | Fondaparinux (2.5 mg sc od). Placebo. Study treatment given for 45 days. |
|
| Outcomes | Primary efficacy outcome: composite of death from any cause or symptomatic PE, symptomatic DVT, or symptomatic extension to the saphenofemoral junction or symptomatic recurrence of superficial‐vein thrombosis at day 47. Secondary efficacy outcomes: composite primary efficacy outcome up to day 77 and the following outcomes up to day 47 and 77: each component of the primary efficacy outcome, the composite of symptomatic PE or DVT, and surgery for ST. Primary safety outcome: major bleeding. Secondary safety outcomes: clinically relevant non‐major, minor, total (any) bleeding, arterial thromboembolic events, adverse events. |
|
| Notes | In the fondaparinux group, 83.0% of participants received graduated compression stockings, 41.5% topical NSAIDs, 3.9% topical anticoagulant drugs, 2.1% oral NSAIDs or COX‐2 inhibitors, 1.1% oral or parenteral anticoagulant drugs, 21.4% aspirin or other antiplatelet agents. The corresponding values in the placebo group were similar (83.1%, 41.8%, 3.3%, 3.7%, 6.4%, and 22.6%) except for anticoagulant drugs and oral NSAIDs, which were prescribed more frequently than in the fondaparinux group. Funding: study funded by GlaxoSmithKline, which collected and analysed the data. Disclosure of potential COI: all authors reported to have received grant support and supporting fees from GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer based. "computer‐generated randomisation list." |
| Allocation concealment (selection bias) | Low risk | Central allocation. "with the use of a central telephone system and a computer‐generated randomization list, consecutive participants were randomly assigned..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. "Fondaparinux and placebo were packaged in identical boxes containing visually identical, prefilled 0.5‐ml single‐dose syringes." "All symptomatic outcomes were reviewed by the central adjudication committee, whose members were unaware of the patients’ group assignments." "The database of adjudicated outcomes was managed by an independent central adjudication committee." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses performed on data from the ITT data. Safety analyses performed on data from the as‐treated population. Overall, 18 participants in fondaparinux group (1.2%) and 22 in placebo group (1.5%) did not have a primary efficacy assessment. Overall, 1.8% of randomised participants did not complete follow‐up. Safety analysis. "1499 patients in the fondaparinux group (99.8%) and 1488 in placebo group (99.2%) were included in the safety analyses." |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Ferrari 1992.
| Methods | Single centre, open RCT. | |
| Participants | 50 participants with acute ST; 22 males, 28 females; median age 52 years. Not reported if participants were hospitalised or non‐hospitalised or if the diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Nimesulide (100 mg bid). Diclofenac sodium (50 mg bid). Study treatment given for 10 days. |
|
| Outcomes | Spontaneous pain, hyperaemia, oedema, local hyperaemia, fever, tolerability. | |
| Notes | All participants received prophylaxis with heparin calcium. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. "randomly allocated to oral treatment." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote from abstract: "Double‐blind study." However, blinding not mentioned in methods, results or discussion sections. Not reported who was blinded and how blinding was attempted. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, 3 participants were not analysed for efficacy outcomes (6%): 2 participants in nimesulide group and 1 in diclofenac group. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Gorski 2005.
| Methods | Multicentre, open, RCT; 7‐ to 14‐day follow‐up. | |
| Participants | 46 non‐hospitalised participants with symptomatic ST confirmed by duplex ultrasonography with first symptoms not earlier than 72 hours before inclusion; 15 males, 31 females; mean age 52.5 years. | |
| Interventions | Topical liposomal heparin spray gel (4 puffs of 458 IU tid). LMWH (enoxaparin 40 mg sc od). Study treatment given for 7 or 14 days. |
|
| Outcomes | Primary outcomes: DVT, pain scoring, erythema, safety, tolerance. Not specified if DVTs were symptomatic or asymptomatic. Secondary outcomes: participant and investigator assessment of efficacy of treatment. |
|
| Notes | Paracetamol up to 1000 mg/day and compression therapy permitted and documented. Funding: study sponsored by CSC Pharmaceuticals Handelsges, Austria. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patient randomization was performed according to a prespecified randomization list." |
| Allocation concealment (selection bias) | Unclear risk | "Each patient...was allocated to a treatment group according to the next free number on the randomization list." Not reported if allocation done centrally. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study: "treatment were administered in a open way, there was no blinding." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants lost to follow‐up (10%). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Holzgreve 1989.
| Methods | RCT. | |
| Participants | 60 participants with ST of the legs; 17 males, 43 females; mean age 53.4 years (SD 12). Not reported if participants were hospitalised or non‐hospitalised or if diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Etofenak gel. Diclofenac gel. |
|
| Outcomes | Length of superficial venous thrombosis, pain, redness, palpable veins, oedema. | |
| Notes | All participants had compression therapy. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single‐blinded. Authors stated it was a single‐blind study, and the participants received medication that was labelled as "trial medication." Unclear from paper whether trial physician was blinded to treatment, and since the outcome was symptom improvement, there was a high risk of detection bias by imperfect blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20 (33%) participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Incandela 2001.
| Methods | Multicentre, placebo‐controlled RCT. | |
| Participants | 30 participants with ST confirmed by colour duplex ultrasonography and varices; 14 males, 16 females; mean age 54 years. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | Essaven gel (5 cm of gel). Placebo (5 cm of gel). Study treatment given for 8 weeks. |
|
| Outcomes | Analogue clinical/symptomatic score including pain, tenderness, disability, local swelling, erythema, presence of thrombosis. | |
| Notes | All participants received LMWH (enoxaparin 0.1 mL/10 kg of bodyweight od) for initial 4 weeks of study and elastic compression stockings for study period. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear method of random sequence generation: "randomisation process was controlled by an external statistical controller." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. "Placebo comparable to Essaven gel was used," "operators were unaware of the contents of the tube." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all participants included were analysed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported. |
Katzenschlager 2003.
| Methods | Multicentre, open RCT. | |
| Participants | 42 non‐hospitalised participants with ST diagnosed by duplex ultrasonography with signs and symptoms lasting < 72 hours; 11 males, 31 females; mean age 52 years. | |
| Interventions | Topical liposomal heparin spray gel (Lipohep 2400 IU/g, 4 spray puffs tid) plus compressive stockings. LMWH (enoxaparin 40 mg sc) plus compressive stockings. Study treatment given for 7‐14 days. |
|
| Outcomes | Median pain (VAS scale), median area of erythema, thrombus size. | |
| Notes | Participants received paracetamol (1000 mg/day) as pain rescue medication. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The assignment of patients to treatment was done accordingly to a randomisation list using a validated system." |
| Allocation concealment (selection bias) | Unclear risk | "Each valid subject...was assigned to the next number on the randomization list." Unclear if allocation was done centrally. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study: "treatments were administered in a open randomised way." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 (7%) participants, all in the heparin spray gel group, lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Koshkin 2001.
| Methods | Placebo‐controlled, double‐blinded RCT. | |
| Participants | 119 participants with acute ST confirmed by duplex ultrasonography; mean age 54.5 years. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | Systemic enzyme therapy (Wobenzym) (10 tablets tid). Placebo. Study treatment given for 16 days. |
|
| Outcomes | Positive changes for a combined outcome including pain, redness, oedema. | |
| Notes | Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind study; however, not reported who was blinded and how blinding was obtained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. Unclear if all included participants were evaluated for study outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. |
Kuhlwein 1985.
| Methods | Multicentre, placebo‐controlled RCT. | |
| Participants | 76 participants with ST, diagnosed on signs and symptoms only by general practitioners or internists. Not reported if some participants were hospitalised. No data on sex or age reported. | |
| Interventions | Vasotonin forte. Placebo. Study treatment given for 3 weeks. |
|
| Outcomes | Overall score including pain, redness, swelling, movement improvement. | |
| Notes | Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind study. Authors stated in abstract that study was double‐blind and placebo‐controlled. However, no details regarding study medication or placebo given in text. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appeared that after 3 weeks there were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Lozano 2003.
| Methods | Open RCT. | |
| Participants | 60 participants with saphenous proximal thrombophlebitis confirmed by Doppler ultrasonography; 22 males, 38 females; mean age 59 years. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | LMWH (enoxaparin 1 mg/kg bid for first week, then 1 mg/kg for 3 weeks) on an outpatient basis. Saphenofemoral disconnection with short hospital stay. |
|
| Outcomes | Resolution of symptoms and signs, ST recurrence, VTE, complications from treatment, socioeconomic assessment. Not specified if VTE and ST recurrence were symptomatic or asymptomatic. | |
| Notes | In the immediate postoperative period, compression bandages/elastic stockings used and early walking recommended. Pain was controlled with oral paracetamol 500 mg. Compressive bandage/elastic stockings continued at home by all participants. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 (5%) participants, all in saphenofemoral disconnection group, lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | No information provided on the resolution of signs and symptoms. |
Marchiori 2002.
| Methods | Single‐centre RCT. | |
| Participants | 60 consecutive participants with ST of great saphenous vein confirmed by ultrasonography; 26 males, 34 females; mean age 62 years. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | UFH (12,500 IU sc for 1 week then 10,000 IU). UFH (5000 IU). Study treatment given for 4 weeks. |
|
| Outcomes | Asymptomatic and symptomatic recurrence or extension of ST and VTE (or both recurrence and extension) after treatment and at 3 and 6 months. Safety outcomes: major bleeding, HIT, overall mortality. |
|
| Notes | Systemic or local anti‐inflammatory drugs (or both) allowed. Funding: not reported. Disclosure of potential COI: the authors report no COI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based: "List generated by a computer." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Ultrasound assessment performed by blinded investigators. However, not reported if participants and investigators evaluating all other events were blind to study treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Marshall 2001.
| Methods | Multicentre, placebo‐controlled RCT. | |
| Participants | 159 participants with acute ST of leg, diagnosed on symptoms and signs only; 40 males, 116 females; mean age 53.8 years. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | Wobenzym (4 tablets tid). Placebo. Study treatment given for 12‐16 days. |
|
| Outcomes | Reduction of pain to day 7 which had to amount to at least 4 points on the VRAS at baseline. | |
| Notes | LMWH was allowed and administered to 4 participants. Paracetamol (maximal 2 g/day) + compression stockings were given to all participants. 9 participants had already been treated with other medications (no further details), which were stopped before the administration of study treatment. 4 participants had received LMWH before inclusion and continued LMWH throughout study. Funding: not stated. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based. Participants assigned to treatment with a list generated by a computer. |
| Allocation concealment (selection bias) | Low risk | Participants assigned to treatment with a list generated by a computer. Concealment of allocation was present since randomisation took place with a computer program called "Random V.5" by Firma Wiedey GmbH Konstanz. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. Study medication described as "similarly looking study medication [placebo]." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant withdrew consent postrandomisation; 10 (6%) participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Messa 1997.
| Methods | Single‐centre, open RCT. | |
| Participants | 30 participants with ST; 7 males, 23 females; aged 32‐72 years. Not reported if participants were hospitalised or non‐hospitalised or if diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Heparansulphate (100 mg tid orally). Sulodexide (250 lipasemic units bid, orally). Study treatment given for 2 weeks. |
|
| Outcomes | Redness of the skin, pain, itching, oedema, trophism. | |
| Notes | Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned to treatment with a random list. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study. "The study was performed in a open‐label...design." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Nocker 1991.
| Methods | Placebo‐controlled RCT. | |
| Participants | 30 participants with unilateral ST of the legs diagnosed on symptoms and signs only; 8 males, 12 females; mean age 59.7 (SD 7.1) years in intervention group; 54.6 (SD 5.6) years in placebo group. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | Diclofenac gel (diclofenac 1 g/100 g gel) tid. Placebo tid. Study treatment given for 3 weeks. |
|
| Outcomes | Efficacy measured by volume change relative to baseline (difference between the affected and unaffected leg) and reduction in pain described by a VAS; tolerability. | |
| Notes | Heparin gel given to 10 participants, without randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind study. Authors stated that study was double‐blind and placebo‐controlled. However, no details regarding study medication or placebo given in main text. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Nusser 1991.
| Methods | RCT. | |
| Participants | 60 participants with ST diagnosed on symptoms and signs only; 25 males, 34 females; mean age 53 (SD 13) years in acemetacin group and 53 (SD 16) years in diclofenac group. Not reported if participants were hospitalised or non‐hospitalised. | |
| Interventions | Oral acemetacin (60 mg tid). Oral diclofenac (50 mg tid). Study treatment given until symptoms resolved for maximally 15 days. |
|
| Outcomes | Area of ST, pain, redness, palpable venous induration, oedema. | |
| Notes | All participants received compression stockings. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. "similarly looking study medication." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all included participants were evaluated for study outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Pinto 1992.
| Methods | Multicentre, placebo‐controlled RCT. | |
| Participants | 68 participants with ST; mean age 42 years. Not reported if participants were hospitalised or non‐hospitalised or if the diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Topical 5'‐methylthioadenosine 0.5% (0.1 mL/cm skin tid). Placebo. Study treatment given for 1 week. |
|
| Outcomes | Oedema, erythema, pain, functional impairment, sc induration, tolerability, VAS score. | |
| Notes | No compression therapy allowed during study. People with ST represent a subgroup of a larger RCT, which included also people with chronic venous insufficiency or second‐degree haemorrhoids. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. "indistinguishable placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all included participants were evaluated for study outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Rathbun 2012.
| Methods | Randomised, controlled, double‐blind, double‐dummy trial. | |
| Participants | 72 consecutive inpatient and outpatients with ST of the lower (57 participants) or upper extremities confirmed by ultrasonography in the absence of a current intravenous catheter. ST involved lower extremities in 27/37 participants in dalteparin group and in 30/35 in ibuprofen group. Mean age 51 years (range 28‐88) in dalteparin group and 52 years (range 19‐85) in ibuprofen group. 21 males, 51 females. | |
| Interventions | LMWH (dalteparin 200 IU/kg at presentation followed by a fixed dose of 10,000 units sc daily for additional 6‐13 days) + placebo given orally tid for 7 days. Ibuprofen 800 mg orally tid for up to 14 days + placebo injection od for 7 days. If symptoms of ST were not resolved at day 7‐9, in the absence of thrombus extension, participant received an additional 7 days of blind therapy. If symptoms of ST were resolved at day 7‐9 in the absence of thrombus extension, study medication was stopped. If at any time thrombus extended either superficially or into deep venous system, study treatment was discontinued and full therapeutic anticoagulation with iv heparin or LMWH started. |
|
| Outcomes | Primary efficacy outcomes: incidence of thrombus extension or new symptomatic VTE during 14‐day and 3‐month follow‐up period. Secondary efficacy outcomes: reduction in pain, evaluated through the 11‐point Box Pain scale, from baseline to day 7 and 14‐ to 16‐day follow‐up. Safety outcomes: incidence of major and minor bleeding. |
|
| Notes | Compression stockings not routinely prescribed. Funding: grant from the National Center for Research Resources, National Institute of Health and Pfizer Inc for provision of dalteparin, ibuprofen, and nurse personnel salary support. Disclosure of potential COI: the authors reported no COI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Balanced randomization blocks of four each consisting of equal numbers" for the 2 treatment groups. |
| Allocation concealment (selection bias) | Low risk | "Randomization...was performed by the investigational pharmacist within 24 h of presenting with a confirmed diagnosis..." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. "The patient, research assistant and principal investigator were blinded to the treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all included participants were evaluated for study outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Spirkoska 2015.
| Methods | Randomised, double‐blind, controlled, single‐centre trial. Recanalisation of veins in superficial vein thrombosis (REVETR) study. |
|
| Participants | 68 participants with ultrasonographically confirmed first symptomatic acute SVT of the lower extremities without concomitant DVT or PE. Mean age 60.2 (SD 11.2) years; males: 31 (46%). | |
| Interventions | LMWH (dalteparin 5000 IU) sc od. LMWH (dalteparin 10,000 IU) sc od. Both groups received treatment with study drug for 6 weeks. Quote: "All patients were advised to remain active and to use ECBs or elastic compression stockings up to the thigh (30‐40 mm Hg of compression) for the whole study period." |
|
| Outcomes | Primary: change in the diameter and length of thrombus. Secondary: recurrent ST, DVT, PE, major bleeding. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were individually randomized by a computer generated random allocation sequence." Comment: adequate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were individually randomized by a computer generated random allocation sequence." Comment: adequate method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "All participants and investigators were masked to treatment assignment and also blind to the size of each block." Comment: reported as a double‐blind study; however, not stated that the 2 dosages of study drug had identical appearance. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In all, 3 (5%) patients were excluded from further follow‐up after the diagnosis of active cancer." Comment: at least 5% of participants randomised were subsequently excluded from the analysis. |
| Selective reporting (reporting bias) | High risk | Comment: authors reported that ST recurrence was a secondary outcome; however, no information about occurrence of this outcome given in results section of study publication. |
Stenox Group 2003.
| Methods | STENOX: multicentre, placebo‐controlled RCT with 3‐month follow‐up, ITT analysis. | |
| Participants | 427 hospitalised or non‐hospitalised participants with ST of ≥ 5 cm on ultrasonography examination; 156 males, 271 females; mean age 62 years. | |
| Interventions | LMWH (enoxaparin 40 mg sc od). LMWH (enoxaparin 1.5 mg/kg sc od). Oral tenoxicam (20 mg od). Placebo. Study treatment given for 8‐12 days. |
|
| Outcomes | Primary efficacy outcome: symptomatic PE and symptomatic and asymptomatic DVT at 12 days. Secondary efficacy outcomes: symptomatic and asymptomatic recurrence or extension of ST (or both) at 12 days and 3 months; symptomatic PE and symptomatic and asymptomatic DVT at 12 days and 3 months (97 days). Safety outcomes: death, major and minor bleeding, thrombocytopenia, and any other adverse event. |
|
| Notes | All participants used elastic bandages or support stockings from day 1 of therapy and continued for at least 15 days. Participants requiring anticoagulant therapy, ligation of the saphenofemoral junction or thrombectomy, anticoagulants or NSAIDs for > 48 hours excluded from study. Study prematurely interrupted due to slow recruitment rate. Funding: Laboratoires Aventis, Paris and Association Française de Formation Continue en Angiologie, Paris. Disclosure of potential COI: authors reported no financial interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear method of sequence generation: "patients were centrally randomly assigned to receive..." |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study: "study medications were packaged in boxes of identical appearance." "all boxes had visually identical contents." "outcomes were reviewed blindly by an independent critical event committee." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/436 (2%) participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Titon 1994.
| Methods | Multicentre, open RCT. | |
| Participants | 117 non‐hospitalised participants with ST confirmed by ultrasonography; 25 males, 92 females; age range 54‐64 years. Mean ages varied between groups, particularly for the few men in the dose‐adjusted group of nadroparin who were much younger than the men in the other 2 groups. | |
| Interventions | Naproxen (oral 500 mg od). LMWH (nadroparin 0.6 mL/6150 anti‐Xa IU od). LMWH (nadroparin 61.5 anti‐Xa IU/kg sc od). Study treatment given for 6 days. |
|
| Outcomes | Primary efficacy: recurrence or extension (or both) of ST, VTE after treatment (day 7) and after 8 weeks. Secondary efficacy outcomes: symptoms (pain and functional disability) and signs (erythema, oedema). Safety outcomes: major and minor bleeding. |
|
| Notes | All participants received elastic stockings for the first 7 days. Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study. "open trial." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At day 7, 0 participants were lost to follow‐up. 4 (3.4%) participants were not evaluated by ultrasonography. At 8 weeks, 8 (6.8%) participants were lost to follow‐up and 25 (21%) participants were not evaluated by ultrasonography. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Uncu 2009.
| Methods | Open RCT | |
| Participants | 50 participants with ST of greater saphenous vein of ≥ 5 cm in length on duplex ultrasonography. Not reported if participants were hospitalised or non‐hospitalised. Mean age: 48.6 (range 25‐90) years in Ca‐nadroparin and 44.9 (28‐85) years in Ca‐nadroparin + acemetacin. 27 males, 23 females. | |
| Interventions | LMWH (Ca‐nadroparin 190 IU Axa/kg od). LMWH (Ca‐nadroparin 190 IU Axa/kg od) + acemetacin (60 mg oral bid). Study treatment given for 10 days. |
|
| Outcomes | Primary outcomes: spontaneous pain, erythema, local tenderness, palpable cord. | |
| Notes | Funding: not reported. Disclosure of potential COI: not reported, no COI forms available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation: "consecutive alternating method." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No information provided about blinding, but likely an open study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report if they performed an ITT analysis and unclear whether study outcomes were evaluated in all included participants. |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes reported. Symptomatic DVT, PE, ST extension, bleeding, death, and adverse events reported in results but not mentioned in methods section. |
Vesalio Group 2005.
| Methods | VESALIO: multicentre, double‐blind, double‐dummy RCT, ITT analysis. | |
| Participants | 164 hospitalised or non‐hospitalised participants with ST of great saphenous vein with the thrombosis extending up to 3 cm from the saphenous‐femoral junction; 60 males, 104 females; mean age 63 years. Diagnosis of ST objectively confirmed by compression ultrasonography. | |
| Interventions | Weight‐adjusted LMWH (nadroparin full dose for 10 days followed by half dose for 20 additional days). Fixed‐dose LMWH (nadroparin 2850 anti‐Xa IU). Study treatment given for 30 days. |
|
| Outcomes | Primary efficacy outcome: asymptomatic and symptomatic extension of ST or VTE (or both) in a 3‐month follow‐up period. Secondary efficacy outcomes: clinical signs and symptoms. Primary safety outcomes: major bleeding, HIT. |
|
| Notes | No aspirin or NSAIDs use throughout study. Study prematurely interrupted due to slow recruitment rate. Funding: grant from Sanofi‐Synthélabo, Sanofi Aventis Group, Milano and GlaxoSmithKline, Verona. Disclosure of potential COI: the authors declared no COI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer based: "each patient was assigned a unique sequential subject number, generated by a computer." |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes, not clear if opaque. "Each center received a initial fixed amount of randomization numbers and corresponding sealed envelopes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study. "Placebo identical in appearance to nadroparin." "all suspected outcome events were reviewed and classified by a Central Adjudication Committee whose members were unaware of treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
Winter 1986.
| Methods | Multicentre, randomised trial. | |
| Participants | 100 people with ST. Not reported if participants were hospitalised or non‐hospitalised or if the diagnosis of ST was objectively confirmed by ultrasonography. | |
| Interventions | Diclofenac emulgel. Heparin gel. Study treatment given 14 days. |
|
| Outcomes | Pain (spontaneous and after pressure), redness, palpable cord. | |
| Notes | Study only reported as an abstract for a scientific meeting. No full paper available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information on study medication or outcome assessment provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors did not report whether they performed an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not specified, only a general outcome of "efficacy" reported. |
bid: twice daily; COI: conflicts of interest; COX: cyclo‐oxygenase; CUS: compression ultrasound; DVT: deep vein thrombosis; ECB: elastic compression bandage; HIT: heparin‐induced thrombocytopenia; im: intramuscularly; ITT: intention to treat; IU: international units; LMWH: low molecular weight heparin; NSAID: non‐steroidal anti‐inflammatory drug; od: once daily; PE: pulmonary embolism; RCT: randomised controlled trial; sc: subcutaneously; SD: standard deviation; SF‐36: 36‐item Short Form; ST: superficial thrombophlebitis; tid: three times daily; UFH: unfractionated heparin; VAS: visual analogue scale;‐ VRAS: visual rating analogue scale; VRS: visual rating scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agus 1993 | Impossible to extract outcomes data separately for the 2 study treatment groups. |
| Allegra 1981 | Mixed population. Impossible to extract data separately for the ST. |
| Annoni 1991 | Mixed population. Impossible to extract data separately for ST. |
| Argenteri 1983 | Mixed population including people with DVT. Impossible to extract data separately for ST. |
| Bagliani 1983 | Mixed population including also people with DVT. Impossible to extract data separately for ST. |
| Becherucci 2000 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for ST of the lower limbs. |
| Bergqvist 1990 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for ST of the lower limbs. |
| Bernicot 1980 | People without a diagnosis of ST of the legs. |
| Bijuan 2003 | Likely people with acute ST of the upper limb. However, impossible to judge eligibility fully due to unavailability of the full text. |
| Bracale 1996 | Mixed population including also people with DVT. Impossible to extract data separately for the ST. |
| Bruni 1979 | Mixed population including also people with acute ST of the upper limb. Impossible to extract data separately for the ST of the lower limbs. |
| Della Marchina 1989 | Mixed population including also people with DVT and post‐phlebitic syndrome. Impossible to extract data separately for the ST. |
| Di Perri 1986 | People with DVT. |
| Gandhi 1984 | People without a diagnosis of ST of the legs. |
| Giorgetti 1990 | Single‐blind study of people with varicophlebitis who received either Seaprose S or placebo. Unclear whether study was randomised or not. |
| Gouping 2003 | People with postinfusion ST of the arm. |
| Ibanez‐Bermudez 1996 | Evaluated outcomes not among those evaluated in present review. |
| Luttichau 1989 | Mixed population. Impossible to extract data separately for ST. |
| Mari 1982 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for ST of the lower limbs. |
| Marsala 1985 | Mixed population. Impossible to extract data separately for ST. |
| Mauro 1992 | Mixed population including people with DVT. Impossible to extract data separately for ST. |
| Mehta 1975 | People with ST of the arm. |
| Ng 2010 | People with ST of the arm. |
| Paciaroni 1982 | Mixed population including people with chronic venous insufficiency. Impossible to extract data separately for ST. |
| Porters 1981 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for ST of the lower limbs. |
| Pozza 1980 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for ST of the lower limbs. |
| Rea 1981 | People with DVT. |
| Resta 1967 | People without a diagnosis of ST of the legs. |
| Rozsos 1994 | People with ST of the arm. |
| Seccia 1989 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for the ST of the lower limbs. |
| Seghezzi 1972 | Mixed population including people with DVT and recurrent postphlebitic syndromes. Impossible to extract data separately for ST of the lower limbs. |
| Seligman 1969 | Mixed population including people with DVT. Impossible to extract data separately for ST. |
| Stolle 1986 | Mixed population including people with acute ST of the upper limb. Impossible to extract data separately for the ST of the lower limbs. |
| Supe 2013 | People with ST of the arm. |
| Tomamichel 1983 | Mixed population including people with DVT. Impossible to extract data separately for ST. |
| van Cauwenberge 1972 | People without a diagnosis of ST of the legs. |
| van der Knaap 1988 | People with ST of the arm. |
DVT: deep vein thrombosis; ST: superficial thrombophlebitis.
Characteristics of studies awaiting assessment [ordered by study ID]
Cazaubon 2013.
| Methods | Not available. |
| Participants | Not available. |
| Interventions | Not available. |
| Outcomes | Not available. |
| Notes | Unable to retrieve the abstract or the full text. |
Wac 2001.
| Methods | Not available. |
| Participants | Not available. |
| Interventions | Not available. |
| Outcomes | Not available. |
| Notes | Unable to retrieve the abstract or the full text. |
Xu 2001.
| Methods | Not available. |
| Participants | Not available. |
| Interventions | Not available. |
| Outcomes | Not available. |
| Notes | Unable to retrieve the abstract or the full text. |
Characteristics of ongoing studies [ordered by study ID]
Rabe 2009.
| Trial name or title | DAPS‐Dalteparin in Patients with Superficial Leg Vein Phlebitis in Addition to Compression Treatment ‐ a Placebo‐Controlled Phase III Study. |
| Methods | Randomised, double‐blind, multicentre, phase III trial. |
| Participants | 276 participants with superficial leg vein phlebitis. |
| Interventions | Compression stockings (30 mmHg) for 3 months and either dalteparin 10,000 IU (group A) or placebo (group B) for 14 days. |
| Outcomes | Primary endpoint: progression of the thrombotic process during treatment period as confirmed by ultrasound. Sonographic assessment planned in all participants on days 1, 7, 14, and 90. Secondary endpoints: pain assessment by visual analogue scale and calculation of symptom scores (tension, heaviness, swelling). |
| Starting date | Not reported. |
| Contact information | Rabe E. |
| Notes |
Differences between protocol and review
We planned to evaluate publication bias using funnel plots and heterogeneity of treatment effects between trials using the Chi2 test and the I2 statistic. However, despite the relatively broad number of comparisons found, no funnel plots or tests of heterogeneity were performed since most studies did not evaluate the same treatment comparisons on the same study outcomes. For the same reason, subgroup analysis and sensitivity analysis to take into account possible sources of bias (e.g. open‐label design, incomplete follow‐up, high levels of exclusions unbalanced between the groups, or inadequate allocation concealment) were not possible. For most of the treatment comparisons, standardised mean differences (SMD) could not be calculated for continuous variables since the standard deviations of the means were not reported.
Contributions of authors
MDN: selected and assessed the quality of trials, extracted data, and wrote the review. IW: selected and assessed the quality of trials, extracted data, and commented on the review. SM: supervised the development of the review in all its phases.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
MDN: Dr Di Nisio reported participation to Advisory Boards for Daiichi‐Sankyo and Pfizer, and receiving consultancy fees from Daiichi‐Sankyo and Bayer Health Care. IW: none known. SM: Dr Middeldorp was a member of the Steering Committee of the CALISTO study, which was funded by GlaxoSmithKline (GSK) and which investigated the efficacy and safety of fondaparinux for superficial thrombophlebitis; funds were paid to Dr Middeldorp's institution. Dr Middeldorp's institution had also received funding from several pharmaceutical companies, including GSK, BMS, Bayer, Boehringer Ingelheim, Sanofi, and Pfizer to support some of her other educational and research activities. The first version of this review was written before the CALISTO study was designed.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andreozzi 1996 {published data only}
- Andreozzi GM, Signorelli S, Pino L, Martini R, Marchitelli E, Pinto A, et al. Tolerability and clinical efficacy of desmin in the treatment of superficial varicothrombophlebitis. Angiology 1996;47(9):887‐94. [DOI] [PubMed] [Google Scholar]
Anonymous 1970 {published data only}
- Anonymous. Indomethacin in superficial thrombophlebitis. Practitioner 1970;205(227):369‐72. [PubMed] [Google Scholar]
Archer 1977 {published data only}
- Archer DS, Fowler PD. Comparison of oxyphenbutazone and placebo in the treatment of superficial thrombophlebitis: an object lesson in clinical trial design. Practitioner 1977;218:712‐75. [PubMed] [Google Scholar]
Belcaro 1989 {published data only}
- Belcaro G, Errichi BM, Laurora G, Cesarone MR, Candiani C. Treatment of acute superficial thrombosis and follow‐up by computerized thermography. VASA. Zeitschrift fur Gefasskrankheiten. Journal for Vascular Diseases 1989;18(3):227‐34. [PubMed] [Google Scholar]
Belcaro 1990 {published data only}
- Belcaro G. Evolution of superficial vein thrombosis treated with defibrotide: comparison with low dose subcutaneous heparin. International Journal of Tissue Reactions 1990;12(5):319‐24. [PubMed] [Google Scholar]
Belcaro 1999 {published data only}
- Belcaro G, Nicolaides AN, Errichi BM, Cesarone MR, Sanctis MT, Incandela L, et al. Superficial thrombophlebitis of the legs: a randomized, controlled, follow‐up study. Angiology 1999;50(7):523‐9. [DOI] [PubMed] [Google Scholar]
Belcaro 2011 {published data only}
- Belcaro G, Cesarone MR, Dugall M, Feragalli B, Ippolito E, Corsi M, et al. Topical formulation of heparin is effective in reducing the symptoms of superficial venous thrombosis: a monocenter, observer‐blind, placebo‐controlled randomized study. Panminerva Medica 2011;53(3 Suppl 1):3‐11. [PubMed] [Google Scholar]
Beyer‐Westendorf 2017 {published data only}
- Beyer‐Westendorf J, Schellong SM, Gerlach H, Rabe E, Weitz JI, Jersemann K, et al. Prevention of thromboembolic complications in patients with superficial‐vein thrombosis given rivaroxaban or fondaparinux: the open‐label, randomised, non‐inferiority SURPRISE phase 3b trial. Lancet Haematology 2017;4(3):e105‐13. [DOI] [PubMed] [Google Scholar]
- Werth S, Bauersachs R, Gerlach H, Rabe E, Schellong S, Beyer‐Westendorf J. Superficial vein thrombosis treated for 45 days with rivaroxaban versus fondaparinux: rationale and design of the SURPRISE trial. Journal of Thrombosis and Thrombolysis 2016;42(2):197‐204. [DOI] [PubMed] [Google Scholar]
Boehler 2014 {published data only}
- Boehler K, Kittler H, Stolkovich S, Tzaneva S. Therapeutic effect of compression stockings versus no compression on isolated superficial vein thrombosis of the legs: a randomized clinical trial. European Journal of Vascular and Endovascular Surgery 2014;48(4):465‐71. [DOI] [PubMed] [Google Scholar]
Cosmi 2012 {published data only}
- Cosmi B. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial SteFlux (Superficial Thromboembolism Fluxum). Thrombosis Research 2014;133(2):196‐202. [DOI] [PubMed] [Google Scholar]
- Cosmi B, Filippini M, Tonti D, Avruscio G, Ghirarduzzi A, Bucherini E, et al. A randomized double‐blind study of low‐molecular‐weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). Journal of Thrombosis and Haemostasis 2012;10(6):1026‐35. [DOI] [PubMed] [Google Scholar]
- Cosmi B, Filippini M, Tonti D, Ghirarduzzi A, Avruscio G, Imberti D, et al. Risk factors for recurrent events in subjects with superficial vein thrombosis in the randomized clinical trial Steflux (Superficial Thromboembolism Fluxum). Hamostaseologie 2012;32:A29. [DOI] [PubMed] [Google Scholar]
- Cosmi B, Filippini M, Tonti D, Ghirarduzzi A, Avruscio G, Imberti D, et al. The Steflux (Superficial Thromboembolism and Fluxum) randomized double blind clinical study of different treatment doses and duration of low molecular weight heparin (parnaparin) in superficial vein thrombosis. Hamostaseologie 2012;32:A28. [DOI] [PubMed] [Google Scholar]
Decousus 2010b {published data only}
- Bauersachs RM. The CALISTO‐study. Phlebologie 2011;40(2):79‐83. [Google Scholar]
- Decousus H. Fondaparinux reduced a composite of VTE complications or death in superficial leg‐vein thrombosis. Annals of Internal Medicine 2011;154(4):JC2‐3. [DOI] [PubMed] [Google Scholar]
- Decousus H, Prandoni P, Mismetti P, Bauersachs RM, Boda Z, Brenner B, et al. CALISTO Study Group. Fondaparinux for the treatment of superficial‐vein thrombosis in the legs. New England Journal of Medicine 2010;363(13):1222‐32. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Becker F, Buchmuller A, Quere I, Prandoni P, Decousus H, et al. Clinical relevance of symptomatic superficial‐vein thrombosis extension: lessons from the CALISTO study. Blood 2013;122(10):1724‐9. [DOI] [PubMed] [Google Scholar]
- Leizorovicz A, Prandoni P, Decousus H. Fondaparinux reduces all types of symptomatic thromboembolic complications in patients with superficial‐vein thrombosis in the legs: data from the CALISTO study. ASH Annual Meeting Abstracts 2011;118:2310. [Google Scholar]
De Sanctis 2001 {published data only}
- Sanctis MT, Cesarone MR, Incandela L, Belcaro G, Griffin M. Treatment of superficial vein thrombosis with standardized application of Essaven gel ‐ a placebo‐controlled, randomized study. Angiology 2001;52 Suppl 3:S57‐62. [DOI] [PubMed] [Google Scholar]
Ferrari 1992 {published data only}
- Ferrari E, Pratesi C, Scaricabarozzi I, Trezzani R. A clinical study of efficacy and tolerability of nimesulide compared with diclofenac sodium in the treatment of acute superficial thrombophlebitis [Studio clinico sull'efficacia terapeutica e la tollerabilità della nimesulide in confronto a diclofenac sodio nel trattamento delle tromboflebiti acute superficiali]. Minerva Cardioangiologica 1992;40(11):455‐60. [PubMed] [Google Scholar]
Gorski 2005 {published data only}
- Gorski G, Szopinski P, Michalak J, Marianowska A, Borkowski M, Geremek M, et al. Liposomal heparin spray: a new formula in adjunctive treatment of superficial venous thrombosis. Angiology 2005;56(1):9‐17. [DOI] [PubMed] [Google Scholar]
Holzgreve 1989 {published data only}
- Holzgreve A, Kleine W, Stegmann W. Local treatment of superficial thrombophlebitis with nonsteroidal antiinflammatory agents. Zeitschrift fur Allgemeinmedizin 1989;65(27):663‐7. [Google Scholar]
Incandela 2001 {published data only}
- Incandela L, Sanctis MT, Cesarone MR, Ricci A, Errichi BM, Dugal M, et al. Treatment of superficial vein thrombosis: clinical evaluation of essaven gel ‐ a placebo‐controlled, 8‐week, randomized study. Angiology 2001;52 Suppl 3:69‐72. [DOI] [PubMed] [Google Scholar]
Katzenschlager 2003 {published data only}
- Katzenschlager R, Ugurluoglu A, Minar E, Hirschl M. Liposomal heparin‐spraygel in comparison with subcutaneous low molecular weight heparin in patients with superficial venous thrombosis. A randomized, controlled, open multicentre study. Journal fur Kardiologie 2003;10(9):375‐8. [Google Scholar]
Koshkin 2001 {published data only}
- Koshkin VM, Kirienko AI. Systemic enzyme therapy in the treatment of acute thrombosis of superficial veins in the lower extremities and postthrombophlebitic disease. International Journal of Immunotherapy 2001;17(2‐4):121‐4. [Google Scholar]
Kuhlwein 1985 {published data only}
- Kuhlwein A. Drug treatment of superficial thrombophlebitides [Medikamentose behandlung oberflachlicher thrombophlebitiden]. Therapiewoche 1985;35(36):4067‐70. [Google Scholar]
Lozano 2003 {published data only}
- Lozano FS, Almazan A. Low‐molecular‐weight heparin versus saphenofemoral disconnection for the treatment of above‐knee greater saphenous thrombophlebitis: a prospective study. Vascular and Endovascular Surgery 2003;37(6):415‐20. [DOI] [PubMed] [Google Scholar]
Marchiori 2002 {published data only}
- Marchiori A, Verlato F, Sabbion P, Camporese G, Rosso F, Mosena L, et al. High versus low doses of unfractionated heparin for the treatment of superficial thrombophlebitis of the leg. A prospective, controlled, randomized study. Haematologica 2002;87(5):523‐7. [PubMed] [Google Scholar]
Marshall 2001 {published data only}
- Marshall M, Kleine M‐W. Efficacy and tolerability of an oral enzyme therapy in the treatment of painful acute superficial thrombophlebitis [Wirksamkeit und vertraglichkeit einer oralen enzymtherapie bei der schmerzhaften akuten thrombophlebitis superficialis]. Phlebologie 2001;30(2):36‐43. [Google Scholar]
Messa 1997 {published data only}
- Messa G, Placa G, Puccetti L, Perri T. Heparansulphate. Effectiveness and tolerability of heparan sulphate in the treatment of superficial thrombophlebitis. Controlled clinical study vs sulodexide [Efficacia e tollerabilità dell'eparansolfato nel trattamento della tromboflebite superficiale. Studio clinico controllato vs sulodexide]. Minerva Cardioangiologica 1997;45(4):147‐53. [PubMed] [Google Scholar]
Nocker 1991 {published data only}
- Diebschlag W, Nocker W. Lokal treatment for superficial thrombophlebitis. Die Medizinische Welt 1990;41:651‐5. [Google Scholar]
- Nocker W, Diebschlag W, Lehmacher W. The efficacy of a diclofenac gel compared with placebo and heparin gel in the local treatment of superficial thrombophlebitis [Lokaltherapie bei oberflachlicher thrombophlebitis. Wirksamkeit eines diclofenac‐natrium‐gels im vergleich zu placebo‐ und heparin‐gel]. Zeitschrift fur Allgemeinmedizin 1991;67:2214‐22. [Google Scholar]
Nusser 1991 {published data only}
- Nusser C‐J, Schare W, Bernard I. The treatment of superficial thrombophlebitis with nonsteroidal antiphlogistic agents [Therapie superfizieller thrombophlebitiden mit nichtsteroidalen antiphlogistika]. Therapiewoche 1991;41(9):541‐4. [Google Scholar]
Pinto 1992 {published data only}
- Pinto G, Galati D, Bompiani GD, Corcione F, Califano G, Colucci S, et al. Topical 5'‐methylthioadenosine in the treatment of symptomatic chronic venous insufficiency, haemorrhoids and superficial phlebitis. A double‐blind placebo‐controlled trial. Drug Investigation 1992;4(3):205‐14. [Google Scholar]
Rathbun 2012 {published data only}
- Rathbun SW, Aston CE, Whitsett TL. A randomized trial of dalteparin compared with ibuprofen for the treatment of superficial thrombophlebitis. Journal of Thrombosis and Haemostasis 2012;10(5):833‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spirkoska 2015 {published data only}
- Spirkoska A, Jezovnik MK, Poredos P. Time course and the recanalization rate of superficial vein thrombosis treated with low‐molecular‐weight heparin. Angiology 2015;66:381‐6. [DOI] [PubMed] [Google Scholar]
Stenox Group 2003 {published data only}
- Superficial Thrombophlebitis Treated by Enoxaparin Study Group. A pilot randomized double‐blind comparison of a low‐molecular‐weight heparin, a nonsteroidal anti‐inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Archives of Internal Medicine 2003;163(14):1657‐63. [DOI] [PubMed] [Google Scholar]
Titon 1994 {published data only}
- Titon JP, Auger D, Grange P, Hecquet JP, Remond A, Ulliac P, et al. Therapeutic management of superficial venous thrombosis with calcium nadroparin. Dosage testing and comparison with an non‐steroidal anti‐inflammatory agent [Traitement curatif des thromboses veineuses superficielles par nadroparine calcique. Recherche posologique et comparaison à un anti‐inflammatoire non stéroidien]. Annales de Cardiologie et d'Angeiologie 1994;43(3):160‐6. [PubMed] [Google Scholar]
Uncu 2009 {published data only}
- Uncu H. A comparison of low‐molecular‐weight heparin and combined therapy of low‐molecular‐weight heparin with an anti‐inflammatory agent in the treatment of superficial vein thrombosis. Phlebology 2009;24(2):56‐60. [DOI] [PubMed] [Google Scholar]
Vesalio Group 2005 {published data only}
- Prandoni P. High versus low doses of low‐molecular‐weight heparin for the treatment of superficial vein thrombosis of the legs. A double‐blind, randomized trial. Journal of Thrombosis and Haemostasis 2005;3 (Suppl 1):Abstract number OR145. [http://onlinelibrary.wiley.com/doi/10.1111/j.1538‐7836.2005.0300b.x/full] [DOI] [PubMed] [Google Scholar]
- Prandoni P, Tormene D, Pesavento R, Vesalio Investigators Group. High vs. low doses of low‐molecular‐weight heparin for the treatment of superficial vein thrombosis of the legs: a double‐blind, randomized trial. Journal of Thrombosis and Haemostasis 2005;3(6):1152‐7. [DOI] [PubMed] [Google Scholar]
Winter 1986 {published data only}
- Winter WR, Rauhut K, Arnold S, Babiak D, Stoidner B. Local therapy of thrombophlebitis superficialis ‐ an inter‐individual comparison of Voltaren‐Emulgel versus a gel containing heparin [Lokale therapie der thrombophlebitis superficialis ‐ Ein individueller vergleich von Voltaren‐emulgel versus ein heparin‐haltiges gel]. Zeitschrift fur Rheumatologie 1986;45:180‐1. [Google Scholar]
References to studies excluded from this review
Agus 1993 {published data only}
- Agus GB, Angelis R, Mondani P, Moia R. Double‐blind comparison of nimesulide and diclofenac in the treatment of superficial thrombophlebitis with telethermographic assessment. Drugs 1993;46 Suppl 1:200‐3. [DOI] [PubMed] [Google Scholar]
Allegra 1981 {published data only}
- Allegra C, Pollari G, Criscuolo A, Bonifacio M, Tabassi D. Centella asiatica extract in venous disorders of the lower limbs. Comparative clinical‐instrumental trial against a placebo [L'estratto di centella asiatica nelle flebopatie degli arti inferiori. Ricerca clinico‐strumentale comparativa con un placebo]. Clinica Terapeutica 1981;99:507‐13. [PubMed] [Google Scholar]
Annoni 1991 {published data only}
- Annoni F, Stefano A, Pabisch S, Floresta M, Magnani P, Lietti F, et al. Efficacy and safety of topical treatment with heparan sulfate in superficial phlebitis. A double‐blind placebo‐controlled trial. Acta Therapeutica 1991;17:263‐72. [Google Scholar]
Argenteri 1983 {published data only}
- Argenteri A, Vittori F, Longoni A. Flurbiprofen and thrombophlebitis of lower limbs: a controlled clinical trial [Terapia antiinfiammatoria delle tromboflebiti degli arti inferiori con flurbiprofen: studio clinico controllato]. Giornale Italiano di Angiologia 1983;3:203‐8. [Google Scholar]
Bagliani 1983 {published data only}
- Bagliani A, Rosa A, Sarchi C. A new anti‐inflammatory drug, suprofen, in the treatment of thrombophlebitis [Un nuovo farmaco antiinfiammatorio, il suprofen, nel trattamento delle tromboflebiti]. Giornale Italiano di Angiologia 1983;3:57‐64. [Google Scholar]
Becherucci 2000 {published data only}
- Becherucci A, Bagilet D, Marenghini J, Diab M, Biancardi H. Effect of topical and oral diclofenac on superficial thrombophlebitis caused by intravenous infusion [Efecto del diclofenaco topico y oral sobre la tromboflebitis superficial inducida por infusion intravenosa]. Medicina Clinica 2000;114(10):371‐3. [DOI] [PubMed] [Google Scholar]
Bergqvist 1990 {published data only}
- Bergqvist D, Brunkwall J, Jensen N, Persson NH. Treatment of superficial thrombophlebitis. A comparative trial between placebo, hirudoid cream and piroxicam gel. Annales Chirurgiae et Gynaecologiae 1990;79(2):92‐6. [PubMed] [Google Scholar]
Bernicot 1980 {published data only}
- Bernicot J. The value of Eucatex in venous pathology in the young woman [Interet d'eucatex dans la pathologie veineuse de la jeune femme]. Quest Medical 1980;33(5):221‐2. [Google Scholar]
Bijuan 2003 {published data only}
- Bijuan L. Observation of aloe pigmentum in treatment of phlebitis. Nanfang Journal of Nursing 2003:3. [Google Scholar]
Bracale 1996 {published data only}
- Bracale G, Selvetella L. Controlled clinical trial comparing seaprose S to serratio‐peptidase in venous inflammatory disease. Efficacy and safety [Studio clinico sull'efficacia e la tollerabilità del seaprose S nelle flebopatie infiammatorie. Studio controllato verso serratio‐peptidasi]. Minerva Cardioangiologica 1996;44(10):515‐24. [PubMed] [Google Scholar]
Bruni 1979 {published data only}
- Bruni M, Quarti Trevano GM, Lochis D, Baresi A, Soletti L. Double‐blind assessment of the clinical and pharmacological results of administration of a preparation with trypsin/chymotrypsin and tetracycline hydrochloride base in cases of acute phlebitis [Analisi in doppio cieco dei risultati clinici e farmacologici dopom somministrazione di un preparato a base di tripsina/chimotripsina e tetraciclina cloridrato nelle flebiti acute]. Gazzetta Medica Italiana 1979;138(11):567‐70. [Google Scholar]
Della Marchina 1989 {published data only}
- Della Marchina M, Renzi G, Palazzini E. Treatment of phlebopathies with low molecular weight heparin as compared to heparin calcium. Riforma Medica 1989;104(4):99‐104. [Google Scholar]
Di Perri 1986 {published data only}
- Perri T, Vittoria A, Messa GL, Cappelli R. Defibrotide therapy for thrombophlebitis ‐ controlled clinical trial. Haemostasis 1986;16 Suppl 1:42‐7. [DOI] [PubMed] [Google Scholar]
Gandhi 1984 {published data only}
- Gandhi DB, Palmar JR, Lewis B, Schraibman IG. Clinical comparison of elastic supports for venous diseases of the lower limbs. Postgraduate Medical Journal 1984;60:349‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giorgetti 1990 {published data only}
- Giorgetti PL, Bortolani EM, Morbidelli A, Vandone PL, Ghilardi G, Mattioli A, et al. Use of a new anti‐inflammatory drug in the treatment of varicophlebitis of the lower limbs [L'uso di un nuovo farmaco antiinflammatorio nel trattamento delle varicoflebiti degli arti inferiori]. Minerva Chirurgica 1990;45(12):883‐6. [PubMed] [Google Scholar]
Gouping 2003 {published data only}
- Gouping Z, Wan‐Er T, Xue‐Ling W, Min‐Qian X, Kun F, Turale S, et al. Notoginseny cream in the treatment of phlebitis. Journal of Infusion Nursing 2003;26(1):49‐54. [DOI] [PubMed] [Google Scholar]
Ibanez‐Bermudez 1996 {published data only}
- Ibanez‐Bermudez S, Perez Martinez F, Llamas del Castillo MD, Sevilla Jiménez JC, Gonzalez Gonzalez EM. Randomized double‐blind clinical study on the efficacy of topical sodium heparin versus sodium polysulphate pentosan [Estudio clinico randomizado y doble ciego sobre la eficacia de heparina sodica topica frente a pentosan polisulfato sodico en patologias venosas superficiales]. Farmacia Clinica 1996;13(2):110‐5. [Google Scholar]
Luttichau 1989 {published data only}
- Luttichau U, Palazzini E. Antithrombotic therapy in phlebopathies of lower limbs: a controlled study of low molecular weight heparin versus heparin calcium. Rivista Europea Per Le Scienze Mediche e Farmacologiche 1989;11(4):351‐8. [PubMed] [Google Scholar]
Mari 1982 {published data only}
- Mari F, Cerreta G, Nardi V, Dialti L. Piroxicam as antiinflammatory therapy in thrombophlebitis: clinical experience [Il piroxicam nella terapia antiflogistica delle tromboflebiti. Nostra esperienza clinica]. Gazzetta Medica Italiana 1982;141(5):243‐5. [Google Scholar]
Marsala 1985 {published data only}
- Marsala F. A controlled double‐blind cross‐over study of a calcium heparin preparation [Studio controllato in doppio cieco cross‐over su un preparato a base di calcio‐eparina]. Clinica Terapeutica 1985;113(6):473‐7. [PubMed] [Google Scholar]
Mauro 1992 {published data only}
- Mauro M, Ferraro G, Palmieri G. Profibrinolytic and antithrombotic effects of sulodexide oral administration: a double‐blind, cross‐over, placebo‐controlled study. Current Therapeutic Research, Clinical and Experimental 1992;51(3):342‐50. [Google Scholar]
Mehta 1975 {published data only}
- Mehta PP, Sagar S, Kakkar VV. Treatment of superficial thrombophlebitis: a randomized double‐blind trial of heparinoid cream. BMJ 1975;3(5984):614‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ng 2010 {published data only}
- Ng IHL. Best treatment modality for superficial phlebitis. Annals of the Academy of Medicine Singapore 2010:S210. [Google Scholar]
Paciaroni 1982 {published data only}
- Paciaroni E, Marini M. Topical therapy for phlebophaties. Results of a controlled clinical study [Sulla terapia topica delle flebopatie. Risultati di uno studio clinico controllato]. Policlinico Sezione Medica 1982;89(3):255‐64. [Google Scholar]
Porters 1981 {published data only}
- Porters J, Roekaerts F, Reyntjens A. R 41 468, a specific serotonin antagonist, relieves symptoms of acute superficial thrombophlebitis. Current Therapeutic Research, Clinical and Experimental 1981;30(4):499‐506. [Google Scholar]
Pozza 1980 {published data only}
- Pozza E, Menghi R, Pansini GC, Duatti A, Carnovali M. Clinical trial with BPH 689 for the treatment of superficial phlebitis [Impiego clinico del BPH 689 nel trattamento delle flebiti superficiali]. Acta Chirurgica Italiana 1980;36(2):157‐66. [Google Scholar]
Rea 1981 {published data only}
- Rea WJ, Peters DW, Smiley RE, Edgar R, Greenberg M, Fenyves E. Recurrent environmentally triggered thrombophlebitis: a five‐year follow‐up. Annals of Allergy 1981;47(5):338‐44. [PubMed] [Google Scholar]
Resta 1967 {published data only}
- Resta V. Clinical comparative experiment on 2 ointments of different extractive heparinoid concentrations (double blind test). Arzneimittel‐Forschung 1967;17(8):1078‐82. [PubMed] [Google Scholar]
Rozsos 1994 {unpublished data only}
- Rozsos I, Kollar L, Scholz ME. The topical treatment of infusion thrombophlebitis with pentosan polysulfate sodium ointment. A randomised double‐blind study. Annals of Hematology 1994;68 Suppl 1:A92. [Google Scholar]
Seccia 1989 {published data only}
- Seccia M, Bortolotti P, Bellomini MG, Buccianti P, Chiarugi M, Cavina E. Use of defibrotide in the treatment of acute superficial thrombophlebitis of the legs [Impiego del defibrotide nel trattamento delle tromboflebiti acute superficiali degli arti]. Minerva Chirurgica 1989;44(9):1379‐84. [PubMed] [Google Scholar]
Seghezzi 1972 {published data only}
- Seghezzi R, Borri P, Chierichetti S, Ferrari P. Controlled clinical trial of 4‐prenyl‐1,2‐diphenyl‐3,5‐pyrazolidinedione (DA2370) and oxyphenbutazone in thrombophlebitis. Arzneimittel‐Forschung 1972;22(1):272‐4. [PubMed] [Google Scholar]
Seligman 1969 {published data only}
- Seligman B. Oral bromelains as adjuncts in the treatment of acute thrombophlebitis. Angiology 1969;20(1):22‐6. [DOI] [PubMed] [Google Scholar]
Stolle 1986 {published data only}
- Stolle A. Treatment of superficial thrombophlebitis with a new emulsion gel [Behandlung der thrombophlebitis superficialis mit einem neuartigen emulsiongel]. Die Medizinische Welt 1986;37:700‐2. [Google Scholar]
Supe 2013 {published data only}
- Supe A, Subnis BM, Rajeev RM, Panchal VH, Lakhani RJ, Mehtalia B, et al. Novel topical quick penetrating solution of heparin in management of superficial thrombophlebitis: results of randomized active controlled trial. International Journal of Pharmaceutical Sciences and Research 2013;4(11):4442‐7. [Google Scholar]
Tomamichel 1983 {published data only}
- Tomamichel M, Reiner M. Treatment of thrombophlebitis and superficial phlebitis. A comparison of nimesulide and oxyphenbutazone. Clinical Trials Journal 1983;20(3):148‐57. [Google Scholar]
van Cauwenberge 1972 {published data only}
- Cauwenberge H. Double‐blind study of the efficacy of a soluble rutoside derivative in the treatment of venous disease [Etude n double aveugle de l'efficacité de l'O‐(beta‐hydroxyéthyl)‐rutosides dans le traitement des affections veineuses]. Archives Internationales de Pharmacodynamie et de Therapie 1972;196:122‐8. [PubMed] [Google Scholar]
van der Knaap 1988 {published data only}
- Knaap JH, Ottolander GJH, Heerde LR. Research into efficacy of heparinoid cream [Onderzoek naar de effectiviteit van heparinodezalf]. Pharmaceutisch Weekblad 1988;123:973‐5. [Google Scholar]
References to studies awaiting assessment
Cazaubon 2013 {published data only}
- Cazaubon M. Effects of progressive versus degressive elastic compression stockings on superficial and deep veins of the calf. Angeiologie 2013;65:39‐47. [Google Scholar]
Wac 2001 {published data only}
- Wac ZX. Observation of the effect of Honghua injection in treating superficial thrombotic phlebitis. Chinese Journal of Information on Traditional Chinese Medicine 2001;8:60‐1. [Google Scholar]
Xu 2001 {published data only}
- Xu BH, Tang L, Tang WJ, Peng JH, Zheng WP, Peng JX. Clinical observation of Mai Luo Tong Granule for treating thrombophlebitis. Chinese Journal of Information on Traditional Chinese Medicine 2001;8:69. [Google Scholar]
References to ongoing studies
Rabe 2009 {published data only}
- Rabe E. DAPS‐dalteparin in patients with superficial leg vein phlebitis in addition to compression treatment ‐ a placebo‐controlled phase‐III study. XVIth World Meeting of the Union Internationale de Phlébologie (UIP); 2009 Aug 31‐Sept 4; Principality of Monaco.
Additional references
Barrelier 1993
- Barrelier MT. Superficial venous thrombosis of the legs. Phlebologie 1993;46(4):633‐9. [PubMed] [Google Scholar]
Bergqvist 1986
- Bergqvist D, Jaroszewski H. Deep venous thrombosis in patients with superficial thrombophlebitis of the leg. British Medical Journal 1986;292(6521):658‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenberg 1998
- Blumenberg RM, Barton E, Gelfand ML, Skudder P, Brennan J. Occult deep venous thrombosis complicating superficial thrombophlebitis. Journal of Vascular Surgery 1998;27(2):338‐43. [DOI] [PubMed] [Google Scholar]
Bounameaux 1997
- Bounameaux H, Reber‐Wasem MA. Superficial thrombophlebitis and deep venous thrombosis. A controversial association. Archives of Internal Medicine 1997;157(16):1822‐4. [PubMed] [Google Scholar]
Cannegieter 2015
- Cannegieter SC, Horvath‐Puhò E, Schmidt M, Dekkers OM, Pedersen L, Vandenbroucke JP, et al. Risk of venous and arterial thrombotic events in patients diagnosed with superficial vein thrombosis: a nationwide cohort study. Blood 2015;125(2):229‐35. [DOI] [PubMed] [Google Scholar]
Chengelis 1996
- Chengelis DL, Bendick PJ, Glover JL, Brown OW, Ranval TJ. Progression of superficial venous thrombosis to deep vein thrombosis. Journal of Vascular Surgery 1996;24(5):745‐9. [DOI] [PubMed] [Google Scholar]
de Moerloose 1998
- Moerloose P, Wutschert R, Heinzmann M, Perneger T, Reber G, Bounameaux H. Superficial vein thrombosis of lower limbs: influence of factor V Leiden, Factor II G20210A and overweight. Thrombosis and Haemostasis 1998;80(2):239‐41. [PubMed] [Google Scholar]
De Weese 1991
- Weese MS. Nonoperative treatment of acute superficial thrombophlebitis and deep femoral venous thrombosis. In: Ernst CB, Stanley JC editor(s). Current Therapy in Vascular Surgery. 2nd Edition. Philadelphia (PA): BC Decker, 1991. [Google Scholar]
Decousus 2010a
- Decousus H, Quéré I, Presles E, Becker F, Barrellier M, Chanut M, et al. POST (Prospective Observational Superficial Thrombophlebitis) Study Group. Superficial venous thrombosis and venous thromboembolism. Annals of Internal Medicine 2010;152(4):218‐24. [DOI] [PubMed] [Google Scholar]
Dua 2014
- Dua A, Heller JA, Patel B, Desai SS. Variability in the management of superficial venous thrombophlebitis across practitioners based in North America and the global community. Thrombosis 2014;2014:306018. [DOI: 10.1155/2014/306018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frappé 2014
- Frappé P, Buchmuller‐Cordier A, Bertoletti L, Bonithon‐Kopp C, Couzan S, Lafond P, et al. Annual diagnosis rate of superficial vein thrombosis of the lower limbs: the STEPH community‐based study. Journal of Thrombosis and Haemostasis 2014;12(6):831‐8. [DOI] [PubMed] [Google Scholar]
Goldman 2010
- Goldman L, Ginsberg J. Superficial phlebitis and phase 3.5 trials. New England Journal of Medicine 2010;363(13):1278‐80. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University. GRADEpro GDT. Version accessed May 2017. Hamilton (ON): McMaster University, 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanson 1998
- Hanson JN, Ascher E, Pippo P, Lorensen E, Scheinman M, Yorkovich W, et al. Saphenous vein thrombophlebitis (SVT): a deceptively benign disease. Journal of Vascular Surgery 1998;27(4):677‐80. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jorgensen 1993
- Jorgensen JO, Hanel KC, Morgan AM, Hunt JM. The incidence of deep venous thrombosis in patients with superficial thrombophlebitis of the lower limbs. Journal of Vascular Surgery 1993;18(1):70‐3. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krunes 1999
- Krunes U, Lindner F, Lindner R, Gnutzmann J. Is the physical diagnosis of below‐knee superficial thrombophlebitis reliable?. Phlebologie 1999;28(3):93‐6. [Google Scholar]
Lutter 1991
- Lutter KS, Kerr TM, Roedersheimer LR, Lohr JM, Sampson MG. Superficial thrombophlebitis diagnosed by duplex scanning. Surgery 1991;110(1):42‐6. [PubMed] [Google Scholar]
Martinelli 1999
- Martinelli I, Cattaneo M, Taioli E, Stefano V, Chiusolo P, Mannucci PM. Genetic risk factors for superficial vein thrombosis. Thrombosis and Haemostasis 1999;82(4):1215‐7. [PubMed] [Google Scholar]
Nordstrom 1992
- Nordstrom M, Lindblad B, Bergqvist D, Kjellstrom T. A prospective study of the incidence of deep‐vein thrombosis within a defined urban population. Journal of Internal Medicine 1992;232(2):155‐60. [DOI] [PubMed] [Google Scholar]
Plate 1985
- Plate G, Eklof B, Jensen R, Ohlin P. Deep venous thrombosis, pulmonary embolism and acute surgery in thrombophlebitis of the long saphenous vein. Acta Chirurgica Scandinavica 1985;151(3):241‐4. [PubMed] [Google Scholar]
Quenet 2003
- Quenet S, Laporte S, Decousus H, Leizorovicz A, Epinat M, Mismetti P, STENOX Group. Factors predictive of venous thrombotic complications in patients with isolated superficial vein thrombosis. Journal of Vascular Surgery 2003;38(5):944‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rücker 2008
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samlaska 1990a
- Samlaska CP, James WD. Superficial thrombophlebitis II. Secondary hypercoagulable states. Journal of the American Academy of Dermatology 1990;23(1):1‐18. [DOI] [PubMed] [Google Scholar]
Samlaska 1990b
- Samlaska CP, James WD. Superficial thrombophlebitis I. Primary hypercoagulable states. Journal of the American Academy of Dermatology 1990;22(6 Pt 1):975‐89. [DOI] [PubMed] [Google Scholar]
Skillman 1990
- Skillman JJ, Kent KC, Porter DH, Kim D. Simultaneous occurrence of superficial and deep thrombophlebitis in the lower extremity. Journal of Vascular Surgery 1990;11(6):818‐23. [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Unno 2002
- Unno N, Mitsuoka H, Uchiyama T, Yamamoto N, Saito T, Ishimaru K, et al. Superficial thrombophlebitis of the lower limbs in patients with varicose veins. Surgery Today 2002;32(5):397‐401. [DOI] [PubMed] [Google Scholar]
Verlato 1999
- Verlato F, Zucchetta P, Prandoni P, Camporese G, Marzola MC, Salmistraro G, et al. An expectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the thigh. Journal of Vascular Surgery 1999;30(6):1113‐5. [DOI] [PubMed] [Google Scholar]
Wichers 2005
- Wichers IM, Nisio M, Buller HR, Middeldorp S. Treatment of superficial vein thrombosis to prevent deep vein thrombosis and pulmonary embolism: a systematic review. Haematologica 2005;90(5):672‐7. [PubMed] [Google Scholar]
References to other published versions of this review
Di Nisio 2007a
- Di Nisio M, Middeldorp S, Wichers IM. Treatment for superficial thrombophlebitis of the leg. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004982.pub2] [DOI] [PubMed] [Google Scholar]
Di Nisio 2007b
- Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD004982.pub3] [DOI] [PubMed] [Google Scholar]
Di Nisio 2012
- Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD004982.pub4] [DOI] [PubMed] [Google Scholar]
Di Nisio 2013
- Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD004982.pub5] [DOI] [PubMed] [Google Scholar]


