Abstract
Background
Strength training or aerobic exercise programmes, or both, might optimise muscle and cardiorespiratory function and prevent additional disuse atrophy and deconditioning in people with a muscle disease. This is an update of a review first published in 2004 and last updated in 2013. We undertook an update to incorporate new evidence in this active area of research.
Objectives
To assess the effects (benefits and harms) of strength training and aerobic exercise training in people with a muscle disease.
Search methods
We searched Cochrane Neuromuscular's Specialised Register, CENTRAL, MEDLINE, Embase, and CINAHL in November 2018 and clinical trials registries in December 2018.
Selection criteria
Randomised controlled trials (RCTs), quasi‐RCTs or cross‐over RCTs comparing strength or aerobic exercise training, or both lasting at least six weeks, to no training in people with a well‐described muscle disease diagnosis.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 14 trials of aerobic exercise, strength training, or both, with an exercise duration of eight to 52 weeks, which included 428 participants with facioscapulohumeral muscular dystrophy (FSHD), dermatomyositis, polymyositis, mitochondrial myopathy, Duchenne muscular dystrophy (DMD), or myotonic dystrophy. Risk of bias was variable, as blinding of participants was not possible, some trials did not blind outcome assessors, and some did not use an intention‐to‐treat analysis.
Strength training compared to no training (3 trials)
For participants with FSHD (35 participants), there was low‐certainty evidence of little or no effect on dynamic strength of elbow flexors (MD 1.2 kgF, 95% CI −0.2 to 2.6), on isometric strength of elbow flexors (MD 0.5 kgF, 95% CI −0.7 to 1.8), and ankle dorsiflexors (MD 0.4 kgF, 95% CI −2.4 to 3.2), and on dynamic strength of ankle dorsiflexors (MD −0.4 kgF, 95% CI −2.3 to 1.4).
For participants with myotonic dystrophy type 1 (35 participants), there was very low‐certainty evidence of a slight improvement in isometric wrist extensor strength (MD 8.0 N, 95% CI 0.7 to 15.3) and of little or no effect on hand grip force (MD 6.0 N, 95% CI −6.7 to 18.7), pinch grip force (MD 1.0 N, 95% CI −3.3 to 5.3) and isometric wrist flexor force (MD 7.0 N, 95% CI −3.4 to 17.4).
Aerobic exercise training compared to no training (5 trials)
For participants with DMD there was very low‐certainty evidence regarding the number of leg revolutions (MD 14.0, 95% CI −89.0 to 117.0; 23 participants) or arm revolutions (MD 34.8, 95% CI −68.2 to 137.8; 23 participants), during an assisted six‐minute cycle test, and very low‐certainty evidence regarding muscle strength (MD 1.7, 95% CI −1.9 to 5.3; 15 participants).
For participants with FSHD, there was low‐certainty evidence of improvement in aerobic capacity (MD 1.1 L/min, 95% CI 0.4 to 1.8, 38 participants) and of little or no effect on knee extension strength (MD 0.1 kg, 95% CI −0.7 to 0.9, 52 participants).
For participants with dermatomyositis and polymyositis (14 participants), there was very low‐certainty evidence regarding aerobic capacity (MD 14.6, 95% CI −1.0 to 30.2).
Combined aerobic exercise and strength training compared to no training (6 trials)
For participants with juvenile dermatomyositis (26 participants) there was low‐certainty evidence of an improvement in knee extensor strength on the right (MD 36.0 N, 95% CI 25.0 to 47.1) and left (MD 17 N 95% CI 0.5 to 33.5), but low‐certainty evidence of little or no effect on maximum force of hip flexors on the right (MD −9.0 N, 95% CI −22.4 to 4.4) or left (MD 6.0 N, 95% CI −6.6 to 18.6). This trial also provided low‐certainty evidence of a slight decrease of aerobic capacity (MD −1.2 min, 95% CI −1.6 to 0.9).
For participants with dermatomyositis and polymyositis (21 participants), we found very low‐certainty evidence for slight increases in muscle strength as measured by dynamic strength of knee extensors on the right (MD 2.5 kg, 95% CI 1.8 to 3.3) and on the left (MD 2.7 kg, 95% CI 2.0 to 3.4) and no clear effect in isometric muscle strength of eight different muscles (MD 1.0, 95% CI −1.1 to 3.1). There was very low‐certainty evidence that there may be an increase in aerobic capacity, as measured with time to exhaustion in an incremental cycle test (17.5 min, 95% CI 8.0 to 27.0) and power performed at VO2 max (maximal oxygen uptake) (18 W, 95% CI 15.0 to 21.0).
For participants with mitochondrial myopathy (18 participants), we found very low‐certainty evidence regarding shoulder muscle (MD −5.0 kg, 95% CI −14.7 to 4.7), pectoralis major muscle (MD 6.4 kg, 95% CI −2.9 to 15.7), and anterior arm muscle strength (MD 7.3 kg, 95% CI −2.9 to 17.5). We found very low‐certainty evidence regarding aerobic capacity, as measured with mean time cycled (MD 23.7 min, 95% CI 2.6 to 44.8) and mean distance cycled until exhaustion (MD 9.7 km, 95% CI 1.5 to 17.9).
One trial in myotonic dystrophy type 1 (35 participants) did not provide data on muscle strength or aerobic capacity following combined training. In this trial, muscle strength deteriorated in one person and one person had worse daytime sleepiness (very low‐certainty evidence).
For participants with FSHD (16 participants), we found very low‐certainty evidence regarding muscle strength, aerobic capacity and VO2 peak; the results were very imprecise.
Most trials reported no adverse events other than muscle soreness or joint complaints (low‐ to very low‐certainty evidence).
Authors' conclusions
The evidence regarding strength training and aerobic exercise interventions remains uncertain. Evidence suggests that strength training alone may have little or no effect, and that aerobic exercise training alone may lead to a possible improvement in aerobic capacity, but only for participants with FSHD. For combined aerobic exercise and strength training, there may be slight increases in muscle strength and aerobic capacity for people with dermatomyositis and polymyositis, and a slight decrease in aerobic capacity and increase in muscle strength for people with juvenile dermatomyositis. More research with robust methodology and greater numbers of participants is still required.
Plain language summary
Strength training or aerobic exercise training for muscle disease
Review question
What are the effects (benefits and harms) of strength training and aerobic exercise training in people with muscle disease?
Background
Strength training, which is performed to improve muscle strength and muscle endurance, or aerobic exercise programmes, which are designed to improve aerobic (cardiovascular) fitness, might optimise physical fitness and muscle strength in people with muscle disease. The number of training studies in people with muscle diseases is increasing steadily. This is an updated review that includes nine newly added studies.
Study characteristics
The review includes three trials of strength training in people with facioscapulohumeral muscular dystrophy (FSHD) and myotonic dystrophy (136 participants), five trials of aerobic exercise (cardiovascular training) in people with dermatomyositis and polymyositis (14 participants), Duchenne muscular dystrophy (DMD; 30 participants) and FSHD (111 participants), and six trials of strength training combined with aerobic exercise in people with mitochondrial myopathy (18 participants), myotonic dystrophy type I (35 participants), dermatomyositis and polymyositis (68 participants), and FSHD (16 participants).
Key results and certainty of the evidence
The findings of this review should be interpreted with caution due to the variable quality of the included studies, variation in exercise interventions, and outcomes measured. It was not possible for participants to be blinded (unaware of whether or not they were in the exercise group). We have, at best, low confidence in the results because of the small numbers of people included in the studies, a variability in results across studies, differences in populations and interventions across studies, and some issues regarding the conduct and design of the studies, in addition to the lack of blinding.
We have little confidence in findings that strength training has little or no effect on dynamic strength (during movement) of the elbow flexors and ankle dorsiflexors or on isometric (static contraction) strength of elbow flexors and ankle dorsiflexors in people with FSHD; and that the combination of strength training and aerobic exercise may have a positive effect on right and left knee extensor strength but no effect on right and left hip flexor strength in people with juvenile dermatomyositis. (Flexors are muscles that tend to bend the joint and extensors straighten or extend the joint).
We have very little confidence in findings that in people with myotonic dystrophy type 1 there may be a slight improvement in isometric wrist extensor strength and little or no effect on hand grip force, pinch grip force or isometric wrist extensor strength after strength training; that participants with dermatomyositis, polymyositis and juvenile dermatomyositis may experience a positive effect of the combination of strength training and aerobic exercise on dynamic strength of right and left knee extensors; that people with dermatomyositis and polymyositis may have a positive effect of aerobic exercise training on aerobic capacity; and that there may be a slight decrease in aerobic capacity after aerobic exercise training in people with juvenile dermatomyositis.
We found evidence that was too uncertain for conclusions to be drawn regarding the effect of strength training on shoulder muscle strength, pectoralis major muscle strength and anterior arm muscle strength in mitochondrial myopathy, the effect of aerobic exercise training in people with mitochondrial myopathy, in the effect of aerobic exercise training on maximal workload in people with FSHD, and on the number of arm and leg revolutions in a six‐minute cycle test in boys with DMD.
We have limited or very little confidence in findings of the absence of adverse events (side effects) in most studies. Additional high‐quality studies with a high number of participants is needed.
Date up to date
The most recent search for evidence was in November 2018.
Summary of findings
Background
Description of the condition
The term 'muscle disease' comprises a large group of conditions. Skeletal muscles are primarily affected, but in some disorders other organ systems may also be involved. Most conditions are progressive, causing the muscles to gradually weaken over time. When a person is diagnosed as having a muscle disease, questions arise about the prognosis, possible interventions, and genetics. However, people with muscle disease are usually also concerned about everyday issues, such as participation in sports, work and hobbies. To answer these concerns, there is a need for controlled trials of aerobic exercise and strength training in people with a muscle disease.
Description of the intervention
Training, or physical fitness training is defined as a planned, structured regimen of regular physical exercise deliberately performed to improve one or more of the following components of physical fitness: cardiorespiratory fitness, body composition, muscle strength and endurance, and flexibility (Garber 2011).
Strength training is defined as a systematic programme of exercises designed to increase an individual's ability to exert or resist force using, for example, weights, weight machines or elastic cords (Garber 2011).
Aerobic exercise training, or cardiorespiratory fitness training, is defined as training that is designed to improve the capacity and efficiency of aerobic energy‐producing systems and is effective for improving cardiorespiratory endurance. It consists of an activity or combination of activities that uses large muscle groups, can be maintained continuously, and is rhythmical and aerobic in nature, for example walking, running, cycling, rowing, aerobic dance exercise, or swimming (Garber 2011).
How the intervention might work
A progressive loss of muscle strength and muscle endurance is common in people with muscle disease and often leads to loss of functional abilities and mobility. Pain and fatigue may also be common symptoms, all of which contribute to a decreased quality of life. Low physical activity levels may lead to even more deconditioning, greater weakness and atrophy of skeletal muscles, which cause a vicious circle of disuse and increased fatigue (McDonald 2002). In healthy people, the best intervention to improve strength and cardiorespiratory function is physical training. Strength training or aerobic exercise programmes in people with muscle disease might maximise muscle and cardiorespiratory function and prevent additional disuse atrophy (Vignos 1983). The question of whether muscle exercise is beneficial or harmful for people with muscle disease has been debated for many years. In the past, reports of progression of weakness after exercise in people with myopathies have encouraged a cautious approach to training (Johnson 1971; Fowler 1984; Brouwer 1992). Traditionally, many people with a muscle disease were advised to avoid physical exertion (Fowler 1982). However, the previous update of this review showed that moderate‐intensity strength training in myotonic dystrophy and facioscapulohumeral dystrophy (FSHD), and aerobic exercise training in dermatomyositis and polymyositis and myotonic dystrophy appeared to do no harm, but there was insufficient evidence to conclude that they offered benefit. Moreover, it showed that in mitochondrial myopathy, aerobic exercise combined with strength training may be effective in increasing submaximal endurance capacity. Limitations in the design of studies in other muscle diseases prevented more general conclusions in these disorders. Although the number of exercise studies in people with a muscle disease is now gradually increasing, the overall number of studies is still scarce.
Why it is important to do this review
In this review, we systematically analysed evidence from randomised controlled trials (RCTs) and quasi‐RCTs on the effectiveness and safety of strength training and aerobic exercise training in people with specified muscle diseases. The review was first published in 2005 (Van der Kooi 2005), and previously updated in 2010 and 2013 (Voet 2010a; Voet 2013). We undertook this update to incorporate evidence from recent trials in this active area of research.
Objectives
To assess the effects (benefits and harms) of strength training and aerobic exercise training in people with a muscle disease.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs, quasi‐RCTs, or cross‐over RCTs that made any of the following comparisons:
strength training versus no training;
aerobic exercise training versus no training;
combined strength training and aerobic exercise versus no training.
Quasi‐RCTs are trials that allocate participants to experimental or control groups based on a method that is not truly random. The method of allocation is known, but is not considered strictly random, for example, it may be based on a hospital record number or date of birth. (There is a greater risk of selection bias in quasi‐randomised trials where allocation is not adequately concealed compared with randomised controlled trials with adequate allocation concealment.) We included eligible studies regardless of publication status or language of publication.
Types of participants
We selected all trials that included participants with a well‐described diagnosis of a muscle disease, such as inflammatory myopathies, metabolic myopathies, muscular dystrophies, muscle diseases with myotonia. We decided not to include studies looking at strength training or aerobic exercise training for people in whom muscle weakness was not the primary feature, but might have been secondary to chronic renal insufficiency, chronic heart failure, renal or heart transplantation, or corticosteroid use. We did not review the effects of respiratory muscle training. We did not include studies regarding aerobic exercise training for McArdle disease because there is a separate Cochrane Review available for this metabolic myopathy (Quinlivan 2011). We excluded studies in which participants had a variety of muscle diseases if we could not obtain results for each condition separately. We assessed the diagnostic criteria of each study; diagnosis had to be confirmed by muscle biopsy or genetic testing.
Types of interventions
To date, there is no evidence or recommendation for a minimum duration of training in muscle disease. However, in the first six weeks, the change in muscle strength or aerobic capacity is generally caused by neural adaptation. Therefore, we included all forms of strength training and aerobic exercise training lasting at least six weeks.
We excluded studies using a within‐participant design, with the non‐exercised limb as a control. If exercises are performed to increase muscle strength on one side of the body, voluntary strength can increase on the contralateral side. This concept is called cross‐education, and has been described with different forms of exercises. A meta‐analysis of 16 randomised studies concluded that, on average, the magnitude of cross‐education is eight per cent of the initial strength of the untrained limb (Munn 2004). Neural adaptations to training and learning effects due to testing are postulated as explanations (Sale 1988; Shima 2002; Munn 2005; Lee 2007 ). Moreover, the results may well be confounded by the presence of asymmetric weakness of both limbs, as the absolute gain in muscle strength resulting from strength training is related to pre‐exercise muscle weakness (Kilmer 2002). For this reason, a non‐exercised limb is not an appropriate control, even if training is randomly assigned. For this reason, we excluded studies using such a within‐participant design.
Types of outcome measures
Primary outcomes
Primary outcome measure specific to strength training:
muscle strength, expressed as change in measures of static (i.e. isometric) or dynamic strength between baseline and post‐training/control period.
Primary outcome measure specific to aerobic exercise training:
aerobic capacity, expressed as change in measures of (physical) work capacity between baseline and post‐training/control period.
Secondary outcomes
Secondary outcome measure specific to strength training:
muscle endurance or muscle fatigue, expressed as change between baseline and post‐training/control period.
Secondary outcome measure specific to aerobic exercise training:
aerobic capacity, expressed in measures of oxygen consumption, parameters of cardiac function or parameters of respiratory function, expressed as change between baseline and post‐training/control period.
Secondary outcome measures applicable to both strength training and aerobic exercise training, expressed as change between baseline and post‐training/control period:
timed‐scored functional assessments of muscle performance, such as a six‐minute walk test (Florence 2008);
quality‐of‐life measures, such as the Short Form 36 (SF‐36) Health Survey (Ware 2000);
pain assessed by an analogue pain scale (Kahl 2005);
experienced fatigue, assessed by questionnaires, e.g. Checklist Individual Strength (CIS‐fatigue; Vercoulen 1999);
Secondary outcome measures specific to assess the safety of the interventions:
parameters of muscle membrane permeability (serum creatine kinase (CK) level, myoglobin level);
adverse effects requiring withdrawal of the participant from the study, for example, acute rhabdomyolysis, increasing muscle pain, injury, etc.
We compared data on outcome measures at baseline with those obtained after at least six weeks of training. When there were assessments at more than one time (e.g. during the intervention, after cessation of the intervention), our preference was for data on outcome measures obtained at the end of the intervention. When a trial measured an outcome in multiple ways, we reported them all.
We did not use the reporting of specific outcomes as a study selection criterion.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web; 16 November 2018; Appendix 1);
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web; 16 November 2018; Appendix 1);
MEDLINE (1946 to 15 November 2018; Appendix 3);
Embase (1974 to 15 November 2018; Appendix 4);
CINAHL (1937 to November 2018; Appendix 5);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 22 December 2018; Appendix 6);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 22 December 2018; Appendix 7).
Searching other resources
We reviewed the bibliographies of the trials identified and other reviews of the subject, and contacted some of the authors in the field to clarify trial eligibility or to identify additional published and unpublished data.
Data collection and analysis
Selection of studies
Two review authors (Voet, Van der Kooi) independently checked the references identified by the search strategy. We obtained the full text of all potentially relevant studies for independent assessment by both review authors. We decided which trials fitted the inclusion criteria. We resolved disagreements through discussion, and a third review author (Geurts) acted as arbitrator where necessary.
Data extraction and management
We collected study data in sufficient detail to complete Characteristics of included studies tables: details of participants, interventions and comparators, outcomes and study design. We also collected details of funding source for each study and the declarations of interest for the primary investigators.
Two review authors (Voet, Van der Kooi) independently extracted the data from the included trials onto a specially designed data extraction form, and graded the risk of bias and certain other aspects of the design of the included trials. We resolved disagreements through discussion, and a third review author (Geurts) acted as arbitrator where necessary. Authors of primary studies did not extract data from their own studies. Voet entered data into Review Manager 5 (RevMan 5) and Van der Kooi checked the data entry (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (Voet, Van der Kooi) independently assessed the risk of bias in included studies according to guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the included studies for randomisation sequence generation, allocation concealment, blinding (participants and outcome assessors), incomplete outcome data, selective outcome reporting and other sources of bias. When there was uncertainty, we contacted study authors for clarification. We resolved disagreements about fulfilment of 'Risk of bias' criteria by discussion between the two review authors. We made a judgement on each of the 'Risk of bias' criteria, of 'high risk of bias', 'low risk of bias' or 'unclear risk of bias'. Whenever characteristics of study design or dropout rates were likely to cause a high risk of bias, we planned to make a note of this and investigate the possibility of differences in treatment effects varying with the degree of this problem.
Measures of treatment effect
When possible we expressed the results as mean differences (MD) with 95% confidence intervals (CI) for continuous outcomes, and risk ratios (RR) with 95% CI for dichotomous outcome measures.
When studies used a variety of instruments (for example rating scales) to measure the same continuous outcome, we calculated standardised mean differences (SMDs) with corresponding 95% CIs instead of MDs. We took data from the post‐training and post‐control period measurements.
For the interpretation of the Cohen’s SMD, we used a rule of thumb to interpret the magnitude of effect for the SMD using the following criteria (Schünemann 2011a):
less than 0.40 represents a small effect;
0.40 to 0.70 represents a moderate effect;
greater than 0.70 represents a large effect.
Unit of analysis issues
Cross‐over trials
The effects of an intervention given in one period persist into a subsequent period. A major concern of cross‐over trials is the potential for carry‐over effect. This occurs when an effect (e.g. pharmacological, physiological, psychological) of treatment in the first phase is carried over to the second phase. As a consequence of entry to the second phase, participants can differ systematically from their initial state despite a wash‐out phase (Higgins 2011b). As the effects of training given in one period can persist into a subsequent period, we only used data from eligible randomised cross‐over studies up to the point of first cross‐over. We did not consider data from the subsequent (second) period of cross‐over trials for analysis.
Studies with multiple treatment groups
Where multiple trial arms are reported in a single trial, we will include only the data for interventions eligible for inclusion in this review. If more than one comparison (e.g. treatment A versus placebo and treatment B versus the same placebo group) are combined in the same meta‐analysis, we will follow guidance in the Cochrane Handbook for Systematic Reviews of interventions, and, for example, combine groups to create a single pair‐wise comparison where clinically appropriate, or split the shared group into two or more groups (Higgins 2011b).
Dealing with missing data
We sought relevant missing data by contacting the primary study author or the corresponding study author. To optimise the strategy for dealing with missing data, we used an intention‐to‐treat (ITT) analysis when possible. ITT analysis includes all participants, including those who did not receive the assigned intervention according to the protocol as well as the participants who were lost to follow‐up. We investigated attrition rates, for example dropouts and withdrawals, to optimise data analyses.
Assessment of heterogeneity
We assessed heterogeneity both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is, the Chi² test and the I² statistic (Higgins 2003). As recommended in the Cochrane Handbook for Systematic Reviews of Interventions, we interpreted an I² value from 0% to 40% as might “not be important”; from 30% to 60% as may represent “moderate” heterogeneity; from 50% to 90% as may represent “substantial” heterogeneity; and from 75% to 100% as representing “considerable” heterogeneity (Deeks 2011). We considered P values less than 0.10 to be statistically significant heterogeneity. When we found heterogeneity, we assessed potential reasons for the differences by examining the study characteristics.
Data synthesis
We performed analyses according to Cochrane recommendations (Deeks 2011). We combined trial results for appropriate pairings of treatments using the Cochrane statistical package RevMan 5 (Review Manager 2014). Even though the outcome measures used, as well as the type and duration of intervention, might differ, we adapted, if applicable, the pooled SMD as an overall measure of the effect. We excluded studies at high risk of bias from the meta‐analysis (other than a high risk of performance bias, which is a feature of most or all exercise studies). In the presence of small sample bias, the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate (Deeks 2011). We reported data narratively in the absence of meta‐analysis.
'Summary of findings' tables
We described the main outcome differences between study groups in the 'Summary of findings' tables for all studies except for studies with a high risk of bias. We included the following outcomes:
muscle strength, expressed as change in measures of static (i.e. isometric) or dynamic strength;
aerobic capacity, expressed as change in measures of (physical) work capacity;
We included the following outcomes (expressed as change between baseline and post‐training control period)
timed‐scored functional assessments of muscle performance, such as a six‐minute walk test;
quality‐of‐life measures, such as the Short Form 36 (SF‐36) Health Survey;
pain, assessed by an analogue pain scale;
experienced fatigue, assessed by questionnaires, e.g. Checklist Individual Strength (CIS‐fatigue);
adverse effects requiring withdrawal of the participant from the study.
When there were assessments at more than one time (e.g. during the intervention, after cessation of the intervention), we took data from the post‐training and post‐control period measurements.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of a body of evidence (studies that contribute data for the prespecified outcomes). We used methods and recommendations described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011a; Schünemann 2011b), using GRADEpro software (GRADEpro GDT). We considered RCTs as high‐certainty evidence if the five factors above were not present to any serious degree, but downgraded the certainty to moderate, low or very low. We downgraded evidence once if a given GRADE consideration was serious and twice if very serious. We justified all decisions to downgrade or upgrade the certainty of evidence using footnotes and we made comments to aid readers' understanding of the review where necessary. We downgraded underpowered studies, or studies without power analysis, once for 'serious' imprecision,
No exercise guidelines exist for people with a muscle disease. We therefore assessed the training programmes according to the American College of Sports Medicine (ACSM) guidelines. We provided a description of the training programmes in the Characteristics of included studies.
Subgroup analysis and investigation of heterogeneity
We presented data for individual muscle diseases separately. As the pathophysiology of each muscle disease differs, we considered that their reaction to training might be different.
We decided at protocol stage not to make subgroups based on sex or age, for we expected that the large differences in disease severity in a specific muscle disease would be of much more influence on outcome than sex or age. Moreover, the ACSM state in their Stand Position (ACSM 1998), that relative improvements resulting from aerobic and resistance training are similar for young and old, male and female.
Where P value was less than 0.10 or I² statistic value greater than 50% (or both), we compared the fixed‐effect estimate against the random‐effects model to assess the possible presence of small sample bias (i.e. by which the intervention effect is more beneficial in smaller studies) in the published literature. In addition, in the case of statistical heterogeneity, we scrutinised the studies for sources of clinical heterogeneity and methodological differences.
Sensitivity analysis
We plan to carry out the following sensitivity analyses if meta‐analysis is possible in future.
Repeat the analysis excluding unpublished studies (if there were any)
Repeat the analysis excluding studies at high risk of bias (in any domain)
Repeat the analysis excluding other types of studies (e.g. to determine the effects of borderline decisions on inclusion)
Results
Description of studies
Results of the search
The searches for previous publications of this review found approximately 7400 references in total. The previous version of the review included five randomised trials. The database searches for this update found 628 new references, reduced to 492 after deduplication. We found 19 records in other sources (see Figure 1 for a chart illustrating the study selection process (Moher 2009)). After assessing the titles and abstracts, we identified 78 articles for potential inclusion: 32 articles describing completed trials that studied strength training as an intervention, 27 that studied aerobic exercise training, and 19 that studied combined strength training and aerobic exercise, sometimes incorporated in more comprehensive rehabilitation programmes. From these we selected 10 new articles reporting nine new studies for inclusion at this update. Most strength training studies included people with the following muscle diseases: slowly progressive dystrophies (mostly myotonic dystrophy and facioscapulohumeral muscular dystrophy (FSHD)) and in the older studies, non‐specified progressive muscular dystrophies and inflammatory myopathies. Studies on the effects of aerobic exercise training mainly included people with slowly progressive dystrophies and inflammatory and metabolic myopathies (mostly unspecified mitochondrial myopathies).
1.
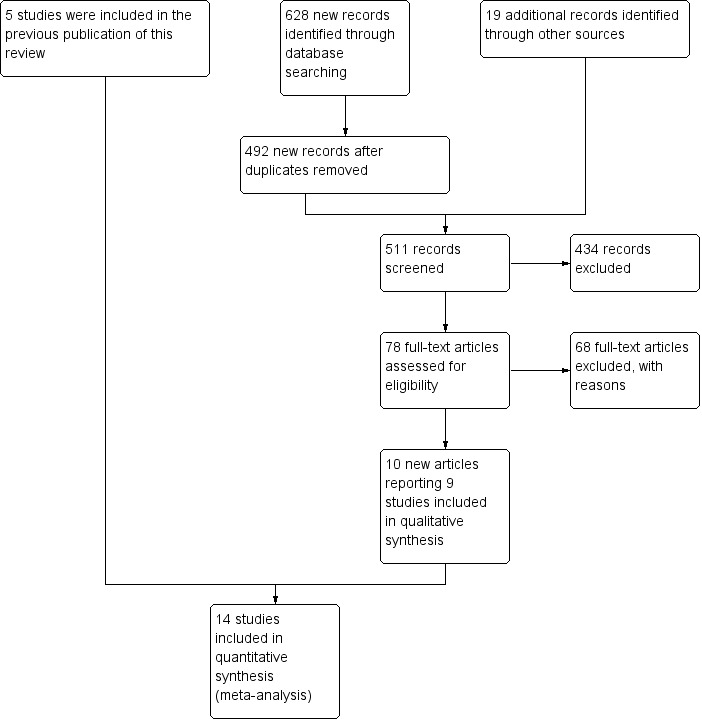
Study selection flow diagram
Only 16 studies were RCTs making a comparison between training and non‐training participants (Lindeman 1995; Wiesinger 1998a; Wiesinger 1998b; Van der Kooi 2004; Cejudo 2005; Dawes 2006; Kierkegaard 2011; Aldehag 2013; Jansen 2013; Munters 2013; Voet 2014; Alexanderson 2014; Andersen 2015; Bankolé 2016; Habers 2016; Andersen 2017). Regrettably, the extension of the initially randomised, controlled, six‐week aerobic exercise study in people with dermatomyositis and polymyositis by Wiesinger and colleagues lost its randomised controlled design due to a decision of the ethics committee, so we had to exclude it (Wiesinger 1998b). We also excluded a randomised controlled strength training combined with aerobic exercise study that compared eight weeks of walking and strengthening exercises to no training in 20 participants with different muscle diseases, as both study groups consisted of participants with various muscle diseases and the study authors did not present outcome measures for each condition separately (Dawes 2006). As the pathophysiology of each muscle disease differs, their reaction to training might be different. It is not known if the effect of strength training and aerobic exercise training is the same for every muscle disease. Therefore, data should be presented and analysed for each disease individually, and the power should be sufficient for each individual disorder. For this reason, we cannot draw any conclusions with regard to the effect of exercise training for each specific muscle disease in the study. Finally, the report provided no specific details about the exercise programme and the risk of bias of the study was high.
In conclusion, we included a total of 14 studies: three strength training studies (Lindeman 1995; Van der Kooi 2004, Aldehag 2013); five aerobic exercise studies (Wiesinger 1998a; Jansen 2013; Voet 2014; Andersen 2015; Andersen 2017); and six strength training combined with aerobic exercise studies (Cejudo 2005; Kierkegaard 2011; Munters 2013; Alexanderson 2014; Bankolé 2016; Habers 2016) (see Characteristics of included studies).
Included studies
See Characteristics of included studies.
Strength training studies
Lindeman 1995 compared the effect of 24 weeks of training versus no training in 36 adults with myotonic dystrophy and 30 adults with hereditary motor and sensory neuropathy types I or II. As this review is concerned with muscle disease, we have not discussed the results of the hereditary motor and sensory neuropathy participant group. Participants were recruited via neurologists, physiatrists, and the Dutch association for neuromuscular diseases (Spierziekten Nederland), and were selected on clinical grounds, without genetic verification.
Van der Kooi 2004 compared 52 weeks of strength training versus no training in a factorial study that also compared albuterol with placebo after the first 26 weeks of training in 65 adults with FSHD. Participants or a first‐degree relative had to have the associated deletion at chromosome 4 (described by Deidda 1996), and clinical symptoms of FSHD. We only discuss the results for the comparison of strength training versus no training (35 adults with FSHD).
Aldehag 2013 was a cross‐over trial that compared 12 weeks of hand training versus no training in 35 adults with myotonic dystrophy type 1. We have only included data from the first period in the analysis. The diagnosis was genetically confirmed in all participants.
None of the training schemes used in the strength training studies were adequate with respect to the number of muscle groups trained, as the ACSM recommends eight to 10 exercises of all the major muscle groups. Only four muscle groups were trained in the Lindeman 1995 myotonic dystrophy study and two muscle groups were trained in the Van der Kooi 2004 FSHD strength training study. The Aldehag 2013 myotonic dystrophy study consisted of strength training only of hand muscles. A physiotherapist supervised the training in Lindeman 1995 and Van der Kooi 2004, and in Aldehag 2013, an occupational therapist supervised training.
Aerobic exercise training studies
Wiesinger 1998a was an aerobic exercise study that compared six weeks of cycle and step aerobic exercise with no training in nine adults with dermatomyositis and five adults with polymyositis. All the participants had an established diagnosis of primary inflammatory muscle disease, as defined by the established criteria of Bohan and Peter (Bohan 1975a; Bohan 1975b), with a disease duration of at least six months. Muscle biopsies, electromyograms and laboratory studies had been performed in all participants to establish the diagnosis. We therefore considered the quality of the diagnostic criteria to be adequate.
Jansen 2013 compared 24 weeks of assisted bicycle training of the arms and legs with no training in 30 boys with Duchenne muscular dystrophy (DMD), all of whom had a DNA‐established diagnosis of DMD.
Voet 2014 compared 16 weeks of cycling exercises with no training in a factorial trial, which also compared 16 weeks of cognitive behaviour therapy in 57 adults with FSHD. We only discussed the results for cycling exercises versus no training comparison in this review. Participants or a first‐degree relative had to have the associated deletion at chromosome 4 (Deidda 1996), and clinical symptoms of FSHD.
Andersen 2015 compared 12 weeks of aerobic training with a placebo supplement versus no training in a factorial trial in 41 adults with FSHD that also included a group of participants with training and a protein supplement. This review only discusses the results for the comparison of aerobic training with a placebo supplement versus no training. Participants had clinical symptoms of FSHD, and they or a first‐degree relative had to have the associated deletion at chromosome 4 (Deidda 1996).
Andersen 2017 compared eight weeks of high‐intensity cycling exercises versus no training in 13 adults with FSHD, followed by eight weeks of unsupervised training. We have only discussed the results of the first eight weeks' training in this review. Participants had clinical symptoms of FSHD, and they or a first‐degree relative had to have the associated deletion at chromosome 4 (Deidda 1996).
The training programmes of all FSHD studies (Voet 2014; Andersen 2015;Andersen 2017), and the Wiesinger 1998a dermatomyositis and polymyositis study fulfilled most of the minimum requirements for aerobic exercise in healthy people, as defined by the ACSM Position Stand (Garber 2011). In Wiesinger 1998a the training frequency in the first two weeks was only twice a week, and in Andersen 2015, once to twice a week, but frequency increased to three times a week in the remaining four weeks. According to the ACSM, (healthy) children and adolescents should participate in activities that promote muscle strength on two or three days per week (ACSM 2015). In the DMD study, however, the duration of each training session was only 15 minutes, with a frequency of five days a week (Jansen 2013).
In the Andersen 2015 aerobic exercise in FSHD study, participants were supervised via phone calls. In the Jansen 2013 DMD study, parents or teachers or both were instructed to assist the boys. Training intensity and posture were monitored and adjusted by the primary investigator, if necessary. In the Andersen 2017 FSHD study, participants received live instructions from an unknown professional. In Wiesinger 1998a and Voet 2014 a physiotherapist supervised the training.
Combined aerobic exercise and strength training studies
Cejudo 2005 was a combined aerobic exercise and strength training study that compared 12 weeks of cycle exercises and dynamic and isokinetic strength training to no training in 18 adults with mitochondrial myopathy. Diagnosis was based on clinical and muscle biopsy data. Biopsy findings were determined by biochemical and histological techniques, without genetic verification. One participant in each group had only a probable diagnosis of mitochondrial myopathy.
Kierkegaard 2011 compared 14 weeks of balance exercises, aerobic activities, flexibility exercises, strength exercises, and brisk walks versus no training in 35 people with genetically‐confirmed myotonic dystrophy type 1.
Munters 2013 compared 12 weeks of cycle exercises and endurance exercises of the knee extensors versus no training in 12 adults with dermatomyositis and 11 adults with polymyositis. Two publications described the same RCT but included different numbers of participants. According to the study author, one publication focused on microdialysis data. Because microdialysis membranes broke inside the muscles, data from some participants needed to be excluded, which explains the apparent discrepancy. Participants had a diagnosis of definite or probable dermatomyositis or polymyositis (Bohan 1975a; Bohan 1975b).
Alexanderson 2014 compared a 24‐week resistive home exercise programme and brisk walking with range of motion exercises in nine adults with dermatomyositis and 10 adults with polymyositis. Participants had a diagnosis of definite or probable dermatomyositis or polymyositis according to established criteria (Bohan 1975a; Bohan 1975b).
Bankolé 2016 compared 24 weeks of strength training, high‐intensity interval, and low‐intensity aerobic training to no training in 16 adults with FSHD. Participants had clinical symptoms of FSHD and they or a first‐degree relative had to have the associated deletion at chromosome 4 (Deidda 1996).
Habers 2016 compared 12 weeks of treadmill interval training and strength exercises with usual care in 26 children and adolescents with juvenile dermatomyositis (see Characteristics of included studies). Participants were diagnosed with juvenile dermatomyositis by a paediatric rheumatologist/immunologist, according to the Bohan and Peter criteria (Bohan 1975a; Bohan 1975b).
The aerobic exercise part of the combined aerobic exercise and strength training studies (Cejudo 2005; Kierkegaard 2011; Munters 2013; Alexanderson 2014; Bankolé 2016;Habers 2016), were all congruent with the ACSM guidelines (Garber 2011). In the myotonic dystrophy study, the intervention consisted of a comprehensive group exercise training programme supported by music (Kierkegaard 2011).
In the Alexanderson 2014 dermatomyositis and polymyositis study, participants were supervised by phone calls. In the mitochondrial myopathy study (Cejudo 2005), there was no published information regarding supervision. In all other studies, a physiotherapist supervised the training (Kierkegaard 2011; Munters 2013; Bankolé 2016).
The strength training part of the Kierkegaard 2011 combined aerobic exercise and strength training study in myotonic dystrophy met the requirements of the ACSM guidelines (Garber 2011). The study author could not give the exact training load of each strength training exercise as a percentage of repetition maximum (RM), as it was not tested that way. However, all major muscle groups were trained: arm, back, leg and abdominal muscles (Kierkegaard 2011). Only three muscle groups were trained in the mitochondrial myopathy study (Cejudo 2005). Alexanderson 2014, the dermatomyositis and polymyositis study, defined the exercise intensity level only for the aerobic walks, not for the resistive home exercise programme.
All studies except the Kierkegaard 2011 myotonic dystrophy type I study and the Alexanderson 2014 dermatomyositis and polymyositis study focused on a limited number of muscle groups.
Although there is no single optimal combination of sets and repetitions for strength training in children and adolescents, one to three sets of six to 15 repetitions performed two to three times per week on nonconsecutive days is reasonable, according to the ACSM (Faigenbaum 2017). The Habers 2016 juvenile dermatomyositis study fulfilled these requirements and focused on the proximal muscle groups, since these tend to be most affected in juvenile dermatomyositis. The training was supervised by a physiotherapist or researcher.
Excluded studies
We excluded 62 studies because there was no randomised controlled comparison between training and non‐training participants, and six RCTs that made a comparison between two different training regimes (see Characteristics of excluded studies).
Ongoing studies
The database searches for this update found six ongoing studies: one strength training study in people with sporadic inclusion body myositis (Jorgensen 2016), one strength training study in DMD (NCT02421523), one aerobic exercise study in oculopharyngeal muscular dystrophy (NCT02158156), one aerobic exercise study in myotonic dystrophy type 1 (Van Engelen 2015), and two aerobic exercise studies in participants with (mixed) neuromuscular diseases (Veenhuizen 2015; Wallace 2016).
Risk of bias in included studies
The 'Risk of bias' assessments across included studies are displayed graphically in Figure 2 and Figure 3.
2.
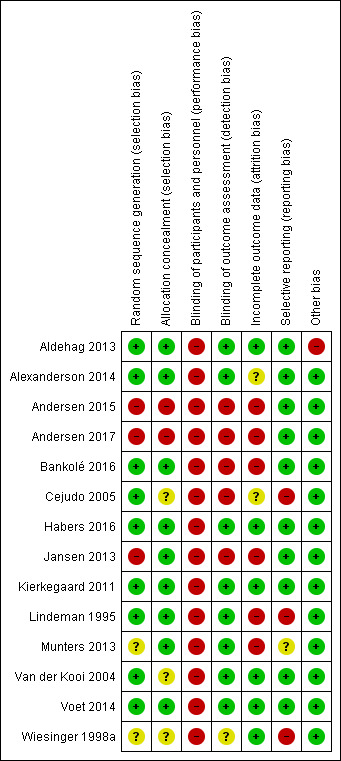
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.
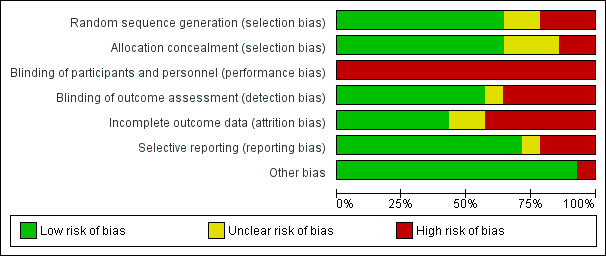
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
Allocation
Seven studies described the methods used for generation of the randomisation sequence as well as for allocation concealment and we judged them to have a low risk of bias (Lindeman 1995; Kierkegaard 2011; Aldehag 2013; Alexanderson 2014; Voet 2014; Bankolé 2016; Habers 2016). We judged two studies to be at unclear risk of selection bias because they failed to adequately report the methods used to generate a random sequence (Wiesinger 1998a; Munters 2013). We judged three studies to be at unclear risk of selection bias because they failed to adequately report the methods of allocation concealment (Wiesinger 1998a; Van der Kooi 2004; Cejudo 2005). We rated three studies at high risk of selection bias as they used a quasi‐randomisation method (Jansen 2013; Andersen 2015; Andersen 2017). In two studies, the investigators who enrolled and assigned participants were not blinded to the allocation and these were at high risk of bias for allocation concealment (Andersen 2015; Andersen 2017). The authors of the Lindeman 1995 myotonic dystrophy study considered the baseline comparability of the groups as suboptimal because the training group had longer time scores for stair climbing (a measure of functional ability) and had higher knee torques (a measure of muscle strength). However, we considered the way the authors presented and discussed the baseline differences as adequate.
Blinding
Performance bias
None of the studies was obviously blinded for group assignment, as it is impossible to blind exercise training compared to no exercise training, so we judged all studies at high risk of bias. In Jansen 2013, two boys were originally allocated to the intervention group, but moved to the control group after trying the intervention.
Detection bias
Five studies were at high risk of detection bias as neither participants nor outcome assessors were blinded to the exercise intervention (Cejudo 2005; Jansen 2013; Andersen 2015; Bankolé 2016; Andersen 2017). In one study, there was only published information about blinding of the assessor of one measurement and we judged it at unclear risk of detection bias (Wiesinger 1998a). Eight studies were at low risk of detection bias (Lindeman 1995; Van der Kooi 2004; Kierkegaard 2011; Aldehag 2013; Munters 2013; Alexanderson 2014; Voet 2014; Habers 2016).
Incomplete outcome data
We considered six studies to be at low risk of attrition bias, as they had no missing data, low rates of missing data (10% or less) that were evenly distributed across groups, or the study authors performed an ITT analysis (Van der Kooi 2004; Kierkegaard 2011; Aldehag 2013; Voet 2014; Habers 2016; Wiesinger 1998a).
Poor adherence, compliance, or both, is another potential source of bias in exercise studies. In six studies, the dropout rate was high: up to 39% (Lindeman 1995; Cejudo 2005; Aldehag 2013; Voet 2014; Andersen 2015; Bankolé 2016). In the Alexanderson 2014 dermatomyositis and polymyositis study, there was no objective assessment of physical activity or exercise level to ensure compliance. In seven studies, analysis was not done by ITT (Lindeman 1995; Cejudo 2005; Jansen 2013; Munters 2013; Andersen 2015; Bankolé 2016; Andersen 2017). We judged the risk of bias as unclear in Cejudo 2005 and Alexanderson 2014, and as high in the other six studies (Lindeman 1995; Jansen 2013; Munters 2013; Andersen 2015; Bankolé 2016; Andersen 2017).
Selective reporting
Five studies referenced published protocols, and when we checked these against the published results, we found that reporting was adequate, and so our judgement was low risk of bias (Jansen 2013; Voet 2014; Andersen 2015; Habers 2016; Andersen 2017). In five studies, although RCT protocols were not available, it was clear that published reports included all expected outcomes and these studies were also at low risk of bias (Van der Kooi 2004; Kierkegaard 2011; Aldehag 2013; Alexanderson 2014; Bankolé 2016).
Munters 2013 changed the primary outcome on the basis of a prespecified interim analysis, and we considered the risk of bias unclear. Three studies were at high risk of bias. In two of them, the study authors defined no primary or secondary outcomes (Wiesinger 1998a; Cejudo 2005), and in the third, the authors stated that they selected a time point for reporting on the basis of "the most relevant differences between groups" (Lindeman 1995). .
Other potential sources of bias
All but one study was at low risk from other potential sources of bias. Aldehag 2013 was a study of strength training in myotonic dystrophy study, which had a cross‐over design. A cross‐over trial has various weaknesses: participants dropping out after the first period complicating the ITT analysis, and carry‐over effects of treatment across study periods. Therefore, we only included data from the first period in this review and considered the study at high risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Summary of findings for the main comparison. Strength training compared to no training for myotonic dystrophy.
| Strength training compared to no training for myotonic dystrophy | ||||||
| Patient or population: people with myotonic dystrophy Setting: hospital Intervention: strength training Comparison: without strength training | ||||||
| Outcomes | Mean (SD) without strength training | Mean (SD) with strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength assessed with electronic dynamometer Follow‐up: mean 12 weeks 35 participants (1 RCT)a |
Hand grip force | The mean change in hand grip force without strength training was an increase of 3.0 N (17.0) | The mean change in hand grip force with strength training was an increase of 9.0 N (17.0) | MD 6.0 N higher (6.7 lower to 18.7 higher) | ⊕⊝⊝⊝ Very lowb | May have little or no effect on hand grip force |
| Pinch grip force | The mean change in pinch grip force without strength training was an increase of 3.0 N (7.0) | The mean change in pinch grip force with strength training was an increase of 4.0 N (6.0) | MD 1.0 N higher (3.3 lower to 5.3 higher) | ⊕⊝⊝⊝ Very lowb | May have little or no effect on pinch grip force | |
| Isometric wrist flexor force | The mean change in isometric wrist flexor force without strength training 0.0 N (17.0) | The mean change in isometric wrist flexor force with strength training was an increase of 7.0 N (14.0) | MD 7.0 N higher (3.3 lower to 17.4 higher) | ⊕⊝⊝⊝ Very lowb | May have little or no effect on isometric wrist flexor force | |
| Isometric wrist extensor force | The mean change in isometric wrist extensor force without strength training was 0.0 N (10.0) | The mean change in isometric wrist extensor force with strength training was an increase of 8.0 N (12.0) | MD 8.0 N higher (0.7 higher to 15.3 higher) | ⊕⊝⊝⊝ Very lowb | May slightly improve isometric wrist extensor force | |
| Aerobic capacity | No data were provided for this outcome | |||||
|
Time‐scored functional assessments of muscle performance Follow‐up: 24 weeks 36 participants (1 RCT)a |
A study with a matched‐pair design presented data from multiple functional tests (see text). There were no statistically significant differences between the training and control groups. | ‐ | ⊕⊝⊝⊝ Very lowb | The effect on time‐scored functional assessments of muscle performance are uncertain | ||
| Quality of life | No data were provided for this outcome | |||||
| Pain | No data were provided for this outcome | |||||
| Experienced fatigue | No data were provided for this outcome | |||||
|
Adverse effects requiring withdrawal Follow‐up: 24 weeks 71 participants (2 RCTs) |
1 participant in the training group withdrawn by GP from an exercise session because of back problems and did not complete final test session because of knee pain (relatedness to the exercise intervention unclear). In the other trial (36 participants), a few participants complained of muscle soreness and transient strength reduction after 8 weeks, but no signs of muscle damage were found after 24 weeks. |
⊕⊕⊝⊝ Very lowc | May have few or no adverse effects requiring withdrawal | |||
| CI: confidence interval; GP: General Practitioner; MD: mean difference RCT: randomised controlled trial; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aData from a second myotonic dystrophy trial with 36 randomised participants was not suitable for inclusion in this analysis. bDowngraded three levels: two levels for imprecision and for study limitations. Quote: "The study sample was small and heterogeneous which lead to an underpowered study". Sample size of 35. Blinding of participants and personnel was not possible and the trial was also at a high risk of selective reporting bias. cDowngraded three levels: one level for imprecision and two levels for study limitations. The trial was unblinded and at a high risk of selective reporting and attrition bias.
Summary of findings 2. Strength training compared to no training for facioscapulohumeral muscular dystrophy.
| Strength training compared to no training for facioscapulohumeral muscular dystrophy | ||||||
| Patient or population: people with facioscapulohumeral muscular dystrophy Setting: at home Intervention: strength training Comparison: without strength training | ||||||
| Outcomes | Mean (SD) without strength training | Mean (SD) with strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength: maximum voluntary isometric contraction Assessed with Quantitative Muscle Assessment fixed myometry testing system Follow‐up: mean 52 weeks 35 participants (1 RCT) |
Elbow flexors ‐ maximum voluntary isometric contraction | The mean change in maximum voluntary isometric contraction of the elbow flexors without strength training was a decrease of 0.6 (1.9) kg | The mean change in maximum voluntary isometric contraction of the elbow flexors with strength training was a decrease of 0.1 (1.9) kg | MD 0.5 kg higher (0.7 lower to 1.8 higher) | ⊕⊕⊝⊝ Lowa,b | May be little or no effect on isometric muscle strength of elbow flexors |
| Elbow flexors ‐ dynamic strength | The mean change in dynamic strength of the elbow flexors without strength training was an increase of 1.4 (2.0) kg | The mean change in dynamic strength of the elbow flexors with strength training was an increase of 2.5 (2.1) kg | MD 1.2 kg higher (0.2 lower to 2.6 higher) | ⊕⊕⊝⊝ Lowa,b | May be little or no effect on dynamic muscle strength of elbow flexors | |
| Ankle dorsiflexors ‐ maximum isometric voluntary contraction | The mean change in maximum isometric voluntary contraction of the ankle dorsiflexors without strength training was a decrease of 1.6 (4.2) kg | The mean change in maximum isometric voluntary contraction of the ankle dorsiflexors with strength training was a decrease of 1.1 (4.3) kg | MD 0.4 kg higher (2.4 lower to 3.2 higher) | ⊕⊕⊝⊝ Lowa,b | May be little or no effect on isometric muscle strength of ankle dorsiflexors | |
| Ankle dorsiflexors ‐ dynamic strength | The mean change in dynamic strength of the ankle dorsiflexors without strength training was a decrease of 11 (2.8) kg | The mean change in dynamic strength of the ankle dorsiflexors with strength training was a decrease of 1.5 (2.7) kg | MD 0.4 kg lower (2.3 lower to 1.4 higher) | ⊕⊕⊝⊝ Lowa,b | May be little or no effect on dynamic muscle strength of ankle dorsiflexors | |
| Elbow flexors ‐ muscle endurance | The mean change in muscle endurance of the elbow flexors without strength training was a decrease of 3.0 (35.5) kgF/s | The mean change in muscle endurance of the elbow flexors with strength training was a decrease of 11.0 (65.0) kgF/s | MD 8.0 kgF/s lower (42.0 lower to 26.0 higher) | ⊕⊕⊝⊝ Lowa,b | May be little or no effect on muscle endurance of elbow flexors | |
| Ankle dorsiflexors ‐ muscle endurance | The mean change in muscle endurance of the ankle dorsiflexors without strength training was a decrease of 29.0 (28.0) kgF/s | The mean change in muscle endurance of the ankle dorsiflexors with strength training was a decrease of 46.0 (25.3) kgF/s | MD 17.0 kgF/s lower (34.8 lower to 0.8 higher) | ⊕⊕⊝⊝ Lowa,b | May be little or no effect on muscle endurance of ankle dorsiflexors | |
| Aerobic capacity | No data were provided for this outcome | |||||
| Time‐scored functional assessments of muscle performance | No data were provided for this outcome | |||||
| Quality of life | No data were provided for this outcome | |||||
|
Pain Follow‐up: mean 52 weeks 34 participants (1 RCT) |
11 out of 34 participants in the training group reported pain in neck and shoulder region to the physical therapist during his home visits. 5 mentioned a period with elbow complaints. The number of neck‐shoulder and elbow complaints did not differ between groups at baseline and at the final visit | ⊕⊕⊝⊝ Lowa,b | May be no effect on pain experience | |||
| Experienced fatigue | No data were provided for this outcome | |||||
|
Adverse effects requiring withdrawal Follow‐up: mean 52 weeks 35 participants: 35 (1 RCT) |
1 participant stopped training because of recurring, training‐related muscle soreness and fatigue. She had a second diagnostic workup, revealing a mitochondrial myopathy as well as FSHD. The training programme was well tolerated. Participants experienced no notable general fatigue or muscle soreness. The training‐induced muscle fatigue lasted less than an hour, so daily activities could be carried out normally afterwards. | ⊕⊕⊝⊝ Lowa,b | May be few or no adverse effects requiring withdrawal | |||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations: participants and personnel were not blinded, as blinding of participants and personnel was not possible. bDowngraded one level for imprecision: sample size of 35.
Summary of findings 3. Aerobic exercise compared to no training for dermatomyositis and polymyositis.
| Aerobic exercise compared to no training for dermatomyositis and polymyositis | |||||
| Patient or population: people with dermatomyositis and polymyositis Setting: hospital Intervention: aerobic exercise Comparison: without aerobic exercise training | |||||
| Outcomes | Mean (SD) without aerobic exercise | Mean (SD) with aerobic exercise | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens |
| Muscle strength | No data were provided for this outcome | ||||
|
Aerobic capacity: VO2 max (defined as the highest O2 consumption) Assessed with an incremental cycle test on a cycle ergometer Follow‐up: mean 6 weeks 14 participants (1 RCT) |
The mean change in VO2 max without aerobic exercise was a decrease of 2.6 (16.9) mL/min/kg | The mean change in VO2 max with aerobic exercise was an increase of 12.0 (12.4) mL/min/kg | MD 14.6 mL/min/kg higher (1.0 lower to 30.2 higher) | ⊕⊝⊝⊝ Very lowa,b,c | The effect on aerobic capacity (VO2 max) is uncertain |
|
Time‐scored functional assessments of muscle performance: disability Assessed with the modified Functional Assessment Screening Questionnaire Follow‐up: mean 6 weeks 14 participants (1 RCT) |
The mean change in disability without aerobic exercise was an increase of 2.9 (29.3) | The mean change in disability with aerobic exercise was an increase of 20.5 (10.9) | MD 17.6 higher (5.6 lower to 40.8 higher) | ⊕⊝⊝⊝ Very lowa,b,c | The effect on disability is uncertain |
| Quality of life | No data were provided for this outcome | ||||
| Pain | No data were provided for this outcome | ||||
| Experienced fatigue | No data were provided for this outcome | ||||
|
Adverse effects requiring withdrawal Follow‐up: mean 6 weeks 14 participants (1 RCT) |
No adverse effects were described | ⊕⊝⊝⊝ Very lowa,b,c | The presence or absence of adverse effects requiring withdrawal is uncertain | ||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation; VO2 max: maximal oxygen uptake | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level for study limitations: there was no information about the generation of the list. It is not clear what is meant by "distinct randomisation lists", there was no published information on the method of allocation concealment, there was no published information about blinding of the assessor of the other measurements and no primary or secondary outcomes were defined. Blinding of participants and personnel was not possible. bDowngraded one level for indirectness: there was no objective assessment of physical activity or exercise level to ensure compliance. cDowngraded one level for imprecision, due to sample size.
Summary of findings 4. Aerobic exercise compared to no training for Duchenne muscular dystrophy (DMD).
| Aerobic exercise compared to no training for Duchenne muscular dystrophy (DMD) | ||||||
| Patient or population: boys with DMD Setting: at home or at school, depending on the preferences of the participants Intervention: aerobic exercise Comparison: without aerobic exercise training | ||||||
| Outcomes | Mean (SD) without aerobic exercise | Mean (SD) with aerobic exercise | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength: hip extensors, knee extensors, ankle dorsiflexors, shoulder abductors and elbow extensors Assessed with MRC (sum scores) Follow‐up: mean 14 weeks 15 participants (1 RCT) |
The mean change in muscle strength of hip extensors, knee extensors, ankle dorsiflexors, shoulder abductors and elbow extensors in MRC sum score without aerobic exercise was a decrease of 0.7 (5.7) | The mean change in muscle strength of hip extensors, knee extensors, ankle dorsiflexors, shoulder abductors and elbow extensors in MRC sum score with aerobic exercise was an increase of 1.0 (1.4) | MD 1.7 higher (1.9 lower to 5.3 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on muscle strength of hip extensors, knee extensors, ankle dorsiflexors, shoulder abductors and elbow extensors is uncertain | |
|
Aerobic capacity Assessed with Assisted 6‐minute cycle test Follow‐up: mean 14 weeks 23 participants (1 RCT) |
Number of leg revolutions | The mean change in number of leg revolutions without aerobic exercise was an increase of 30.9 (131.9) | The mean change in number of leg revolutions with aerobic exercise was an increase of 44.9 (107.6) | MD 14 revolutions higher (89.0 lower to 117.0 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on aerobic capacity (number of leg revolutions) is uncertain |
| Number of arm revolutions | The mean change in number of arm revolutions without aerobic exercise was an increase of 30.9 (131.9) | The mean change in number of arm revolutions with aerobic exercise was an increase of 65.7 (107.6) | MD 34.8 revolutions higher (68.2 lower to 137.8 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on aerobic capacity (number of arm revolutions) is uncertain | |
|
Time‐scored functional assessments of muscle performance: functional abilities in 3 different dimensions, standing positions and transfers, axial and proximal motor functions and distal motor function Assessed with Motor Function Measure total Scale from 0% to 100% Follow‐up: mean 14 weeks 29 participants (1 RCT) |
The mean change in MFM total score without aerobic exercise was a decrease of 6.4 (13.0) % | The mean change in MFM total score with aerobic exercise was a decrease of 0.8 (16.9) % | MD 7.2 % higher (3.7 lower to 18.1 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on overall functional abilities is uncertain See Table 13 for MFM in standing positions and transfers; axial and proximal motor functions and distal motor functions |
|
| Quality of life | No data were provided for this outcome | |||||
| Pain | No data were provided for this outcome | |||||
| Experienced fatigue | No data were provided for this outcome | |||||
|
Adverse effects requiring withdrawal Follow‐up: mean 14 weeks 29 participants (1 RCT) |
No serious adverse events were observed or reported. During the training phase, postural adjustments were made in 3/24 participants who reported pain at the lateral side of the knee or foot due to an external rotation of the hip during training. 2 boys had injuries unrelated to training. |
‐ | ⊕⊝⊝⊝ Very lowa,b | The presence or absence of adverse effects requiring withdrawal is uncertain | ||
| CI: confidence interval; MD: mean difference; MFM: Motor Function Measure; MRC: Medical Research Council; RCT: randomised controlled trial; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for imprecision as it is not known if the sample size of 29 is sufficient. Quote: "No historical data were available at the start of this study in 2008. The sample size was therefore not based on statistical analysis. We arbitrarily chose to include 20 to 30 participants". bDowngraded one level for study limitations: participants and outcome assessor had no information about previous test results at each assessment but were not blinded to treatment allocation. Moreover, boys were originally allocated to the intervention group, but replaced to the control group within two weeks after trying the intervention. One boy discontinued the training and assessment after 12 weeks and was excluded from the analysis, so the analysis was not intention‐to‐treat. Blinding of participants and personnel was not possible.
Summary of findings 5. Aerobic exercise compared to no training for facioscapulohumeral muscular dystrophy.
| Aerobic exercise compared to no training for facioscapulohumeral muscular dystrophy | |||||
| Patient or population: people with facioscapulohumeral muscular dystrophy Setting: at home and in rehabilitation centres Intervention: aerobic exercise Comparison: without aerobic exercise training | |||||
| Outcomes | Mean (SD) without aerobic exercise | Mean (SD) with aerobic exercise | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens |
|
Muscle strength: maximum voluntary isometric knee extension strength Assessed with Quantitative Muscle Assessment fixed myometry testing system Follow‐up: mean 16 weeks 52 participants (1 RCT)a |
The mean change in maximum voluntary isometric knee extension strength without aerobic exercise was a decrease of 1.8 (1.4) kg | The mean change in maximum voluntary isometric knee extension strength with aerobic exercise was a decrease of 1.7 (1.4) kg | MD 0.1 kg higher (0.7 lower to 0.9 higher) | ⊕⊕⊝⊝ Lowb,c | May have little or no effect on quadriceps strength |
|
Aerobic capacity: VO2 peak Assessed with submaximal cycling test Follow‐up: mean 16 weeks 38 participants (1 RCT)a |
The mean change in VO2 peak without aerobic exercise was a decrease of 0.4 (0.8) L/min | The mean change in VO2 peak with aerobic exercise was an increase of 0.7 (1.3) L/min | MD 1.1 L/min higher (0.4 higher to 1.8 higher) | ⊕⊕⊝⊝ Lowb,c | May increase aerobic capacity (VO2 peak) slightly |
|
Time‐scored functional assessments of muscle performance: distance walked Assessed with 6‐minute walk test Follow‐up: mean 16 weeks 52 participants (1 RCT)a |
The mean change in distance walked in the 6‐min walk test without aerobic exercise was an increase of 0.0 (15.0) m | The mean change in distance walked in the 6‐min walk test with aerobic exercise was an increase of 31.0 (27.0) m | MD 31.0 higher (19.3 higher to 42.7 higher) | ⊕⊕⊝⊝ Lowb,c | May improve distance walked in a 6‐min walk test |
|
Quality of life Assessed with Sickness Impact Profile Scale from 0 to 572 Follow‐up: mean 16 weeks 52 participants (1 RCT)a |
The mean change in quality‐of‐life score without aerobic exercise was an increase of 8.0 (19.0) | The mean change in quality‐of‐life score with aerobic exercise was a decrease of 2.0 (16.0) | MD 10.0 lower (19.6 lower to 0.4 lower) | ⊕⊕⊝⊝ Lowb,c | May improve quality of life slightly |
|
Pain Asssesed with a Visual Analogue Scale Scale from 0 to 100 Follow‐up: mean 16 weeks 52 participants (1 RCT)a |
The mean change in pain score without aerobic exercise was an increase of 1.0 (2.8) | The mean change in pain score with aerobic exercise was an increase of 0.0 (4.5) | MD 1.0 lower (3.0 lower to 1.0 higher) | ⊕⊕⊝⊝ Lowb,c | May have little or no effect on pain |
|
Experienced fatigue Assesed with Checklist Individual Strength Scale from 7 to 56 Follow‐up: mean 16 weeks 52 participants (1 RCT)a |
The mean change in fatigue score without aerobic exercise was a decrease of 1.2 (1.0) | The mean change in fatigue score with aerobic exercise was a decrease of 8.5 (2.0) | MD 7.3 lower (8.1 lower to 6.5 lower) | ⊕⊕⊝⊝ Lowb,c | May improve experienced fatigue |
|
Adverse effects requiring withdrawal Follow‐up: mean 16 weeks 52 participants (1 RCT)a |
There were no adverse events leading to withdrawal 15 participants who had received aerobic exercise training reported 1 to 5 adverse events: 4 participants experienced knee pain, 9 saddle soreness, 7 neck and shoulder pain, and 6 back pain. All these complaints resolved spontaneously during the study period |
⊕⊕⊝⊝ Lowb,c | May be no adverse effects requiring withdrawal | ||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation; VO2 peak: peak oxygen uptake | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe did not include data from two other small studies in this summary. These trials had 13 and 19 participants, respectively. Both were at high risk of bias due to their methods of randomisation, lack of allocation concealment, lack of blinding, and attrition. bDowngraded one level for imprecision: the sample size was 52 participants. cDowngraded one level for study limitations: blinding of participants and personnel was not possible.
Summary of findings 6. Aerobic exercise and strength training compared to no training for mitochondrial myopathy.
| Aerobic exercise and strength training compared to no training for mitochondrial myopathy | ||||||
| Patient or population: people with mitochondrial myopathy Setting: rehabilitation unit Intervention: aerobic exercise and strength training Comparison: without aerobic exercise and strength training | ||||||
| Outcomes | Mean (SD) without aerobic exercise and strength training | Mean (SD) with aerobic exercise and strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength: the heaviest weight that could be lifted throughout the complete range of movement (1RM test) Follow‐up: mean 12 weeks 18 participants (1 RCT) |
Assessed with shoulder press exercise | The mean change in shoulder muscle strength, without aerobic exercise and strength training was an increase of 10.7 (10.0) kg | The mean change in pectoralis major muscle strength, with aerobic exercise and strength training was an increase of 5.7 (11.0) kg | MD 5.0 kg lower (14.7 lower to 4.7 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on shoulder press muscle strength is uncertain |
| Assessed with butterfly exercise | The mean change in pectoralis major muscle strength without aerobic exercise and strength training was an increase of 0.6 (11.0) kg | The mean change in pectoralis major muscle strength with aerobic exercise and strength training was an increase of 7.0 (9.0) kg | MD 6.4 kg higher (2.89 lower to 15.7 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on strength of pectoralis major muscle is uncertain | |
| Assessed with biceps curls exercise | The mean change in anterior arm muscle strength, without aerobic exercise and strength training was an increase of 0.7 (12.0) kg | The mean change in anterior arm muscle strength, with aerobic exercise and strength training was an increase of 8.0 (10.0) kg | MD 7.3 kg higher (2.9 lower to 17.5 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on anterior arm muscle strength is uncertain | |
|
Aerobic capacity: measures of (physical) work capacity Assessed with incremental cycle test Follow‐up: mean 12 weeks 18 participants (1 RCT) |
Mean time cycled till exhaustion | The mean change in mean time cycled till exhaustion without aerobic exercise and strength training was a decrease of 2.7 (16.0) min | The mean change in mean time cycled till exhaustion with aerobic exercise and strength training was an increase of 21.0 (28.0) min | MD 23.7 min higher (2.6 higher to 44.8 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on mean cycle time until exhaustion is uncertain |
| Mean distance cycled until exhaustion | The mean change in distance cycled until exhaustion without aerobic exercise and strength training was a decrease of 0.9 (6.0) km | The mean change in distance cycled until exhaustion with aerobic exercise and strength training was an increase of 8.8 (11.0) km | MD 9.7 km higher (1.5 higher to 17.9 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on mean distance until exhaustion is uncertain | |
|
Aerobic capacity: distance walked until exhaustion Follow‐up: mean 12 weeks 18 participants (1 RCT) |
Assessed with shuttle walking test | The mean change in distance walked until exhaustion without aerobic exercise and strength training was an increase of 17.0 m | The mean change in distance walked until exhaustion with aerobic exercise and strength training was an increase of 95.0 m | MD 78.0 m higher (144.9 lower to 300.9 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on distance walked until exhaustion is uncertain |
| Time‐scored functional assessments of muscle performance | No data were provided for this outcome | |||||
|
Quality of life Assessed with Nottingham Health Profile Scale from 0 to 100 Follow‐up: mean 12 weeks 18 participants (1 RCT) |
The mean change in quality‐of‐life score without aerobic exercise and strength training was an increase of 1.5 (17.7) | The mean change in quality‐of‐life score with aerobic exercise and strength training was a decrease of 8.3 (16.8) | MD 9.8 lower (25.7 lower to 6.1 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on quality of life is uncertain | |
| Pain | No data were provided for this outcome | |||||
| Experienced fatigue | No data were provided for this outcome | |||||
|
Adverse effects requiring withdrawal Follow‐up: mean 12 weeks 18 participants (1 RCT) |
There were no adverse events leading to withdrawal. Every participant was able to tolerate the exercise training regimen without complications. Most cancellations happened because of muscle soreness associated with the unaccustomed exercise activity. |
⊕⊝⊝⊝ Very lowa,b | The presence or absence of adverse effects requiring withdrawal is uncertain | |||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation; 1RM: one repetition maximum | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for study limitations: no published information on the blinding of outcome assessors. The study author (Cejudo) told us that the evaluators knew to which group each participant was assigned, one participant (of 10) was missing from the intervention group and one participant (of 10) was missing from the control group. The analysis was not intention‐to‐treat and the article defined no primary and secondary outcomes. Blinding of participants and personnel was not possible. bDowngraded two levels for imprecision: no power analysis performed before start of the study. Moreover, the sample size was 18 participants.
Summary of findings 7. Aerobic exercise and strength training compared to no training for myotonic dystrophy type 1.
| Aerobic exercise and strength training compared to no training for myotonic dystrophy type 1 | |||||
| Patient or population: people with myotonic dystrophy type 1 Setting: department of physical therapy of a hospital Intervention: aerobic exercise and strength training Comparison: without aerobic exercise and strength training | |||||
| Outcomes | Mean (SD) without aerobic exercise and strength training | Mean (SD) with aerobic exercise and strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens |
| Muscle strength | No data were provided for this outcome | ||||
| Aerobic capacity | No data were provided for this outcome | ||||
|
Time‐scored functional assessments of muscle performance: distance walked Assessed with: 6‐minute walk test (m) Follow‐up: mean 14 weeks 35 participants (1 RCT) |
The mean change in distance walked without aerobic exercise and strength training was a decrease of 2.0 (119.0) m | The mean change in distance walked with aerobic exercise and strength training was an increase of 9.0 (116.0) m | MD 11 m higher (66.9 lower to 88.9 higher) | ⊕⊝⊝⊝ Very lowa | May have little or no effect on distance walked in a 6‐minute walk test |
| Quality of life | No data were provided for this outcome | ||||
| Pain | No data were provided for this outcome | ||||
| Experienced fatigue | No data were provided for this outcome | ||||
|
Adverse effects requiring withdrawal Follow‐up: mean 14 weeks 35 participants (1 RCT) |
No adverse effects required withdrawal The study‐specific questionnaire on perceived effects showed that one person reported deterioration in muscle strength and another a worsening in daytime sleepiness. |
⊕⊝⊝⊝ Very lowa | May have few or no adverse effects requiring withdrawal | ||
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels for imprecision: no power analysis performed before start of the study. Moreover, the sample size was 35 participants; and one level for study limitations (blinding of participants and personnel was not possible).
Summary of findings 8. Aerobic exercise and strength training compared to no training for dermatomyositis and polymyositis.
| Aerobic exercise and strength training compared to no training for dermatomyositis and polymyositis | ||||||
| Patient or population: people with dermatomyositis and polymyositis Setting: at home and at the department of physical therapy of 3 participating hospitals Intervention: aerobic exercise and strength training Comparison: without aerobic exercise and strength training | ||||||
| Outcomes | Mean (SD) without aerobic exercise and strength training | Mean (SD) with aerobic exercise and strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength 21 participants (1 RCT) |
Muscle strength: MMT‐8 (maximal isometric strength of neck flexors, middle deltoid, gluteus maximus, gluteus medius, biceps brachii, wrist extensors, wrist flexors, ankle dorsiflexors) Scale from 0 (no movement) to 80 (normal) Follow‐up: mean 12 weeks |
The mean change in MMT‐8 without aerobic exercise and strength training was an increase of 4.0 (1.5) | The mean change in MMT‐8 with aerobic exercise and strength training was an increase of 3.0 (3.0) | MD 1.0 higher (1.1 lower to 3.1 higher) | ⊕⊝⊝⊝ Very lowa,b | May have no clear effect on maximal isometric strength of neck flexors, middle deltoid, gluteus maximus, gluteus medius, biceps brachii, wrist extensors, wrist flexors, ankle dorsiflexors |
| Muscle strength: maximum load in 5RM, for right knee extensors Assessed with hand‐held dynamometer Follow‐up: mean 12 weeks | The mean change in 5RM for right knee extensors without aerobic exercise and strength training was an increase of 1.3 (0.9) kg | The mean change in 5RM for right knee extensors with aerobic exercise and strength training was an increase of 3.8 (0.9) kg | MD 2.5 kg more (1.75 more to 3.25 more) | ⊕⊝⊝⊝ Very lowa,b | May slightly increase muscle strength of right knee extensors | |
|
Muscle strength: maximum load in 5RM, for left knee extensors
Assessed with hand‐held dynamometer Follow‐up: mean 12 weeks |
The mean change in 5RM for left knee extensors without aerobic exercise and strength training was an increase of 1.1 (0.7) kg | The mean change in 5RM for left knee extensors with aerobic exercise and strength training was an increase of 3.8 (1.1) kg | MD 2.7 kg more (2.0 more to 3.4 more) | ⊕⊝⊝⊝ Very lowa,b | May slightly increase muscle strength of left knee extensors | |
| Aerobic capacity, expressed in measures of (physical) work capacity |
Aerobic capacity: time cycled till exhaustion Assessed with change from baseline in incremental cycle test on a cycle ergometer Follow‐up: mean 12 weeks 15 participants (1 RCT) |
The mean change in time cycled till exhaustion without aerobic exercise and strength training was an increase of 0.8 (4.8) min | The mean change in time cycled till exhaustion with aerobic exercise and strength training was an increase of 18.3 (13.3) min | MD 17.5 min higher (8.0 higher to 27.0 higher) | ⊕⊝⊝⊝ Very lowa,b | May slightly extend time to exhaustion |
|
Aerobic capacity: power performed at VO2 max Assessed with exhaustion incremental cycle test on a cycle ergometer Follow‐up: mean 12 weeks 21 participants (1 RCT) |
The mean change in power performed at VO2 max without aerobic exercise and strength training was a decrease of 4.0 (3.5) W | The mean change in power performed at VO2 max with aerobic exercise and strength training was an increase of 14.0 (3.5) W | MD 18.0 W higher (15.0 higher to 21.0 higher) | ⊕⊝⊝⊝ Very lowa,b | May slightly increase aerobic capacity (power performed at VO2 max) | |
|
Aerobic capacity: VO2 max Assessed with exhaustion incremental cycle test on a cycle ergometer Follow‐up: mean 12 weeks 40 participants: 40 (2 RCTs) |
Aerobic capacity improved on average 0.27 SDs (0.35 less to 0.90 more) in the aerobic exercise and strength training group than in the group without training | SMD 0.27 higher (0.35 lower to 0.90 higher) | ⊕⊝⊝⊝ Very lowb,c | The effect on aerobic capacity (VO2 max) is uncertain. As a rule of thumb, a SMD of 0.2 represents a small effect |
||
|
Time‐scored functional assessments of muscle performance: muscle performance Assessed with disease‐specific Functional Index Scale from 0 to 64 Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in Functional Index without aerobic exercise and strength training was an increase of 11.6 (8.5) | The mean change in Functional Index with aerobic exercise and strength training was an increase of 17.1 (10.2) | MD 5.5 higher (2.9 lower to 13.9 higher) | ⊕⊝⊝⊝ Very lowb,c | The effect on change in muscle performance is uncertain | |
|
Quality of life Assessed with SF‐36 General Health scale from 0 to 100 (where 100 is optimal) Follow‐up: mean 12 weeks 21 participants (1 RCT) |
The mean change in SF‐36 General Health score without aerobic exercise and strength training was an increase of 6.0 (4.9) | The mean change in SF‐36 General Health score with aerobic exercise and strength training was an increase of 15.5 (4.4) | MD 9.5 higher (5.5 higher to 13.5 higher) | ⊕⊝⊝⊝ Very lowa,b | May improve quality of life‐general health See Table 14 for GRADE assessment of other quality‐of‐life measures |
|
| Pain | No data were provided for this outcome | |||||
| Experienced fatigue | No data were provided for this outcome | |||||
|
Adverse effects requiring withdrawal Follow‐up: 12 or 24 weeks 40 participants (2 RCTs) |
No withdrawals due to adverse events In a 12‐week study, no adverse events were noted. In a 24‐week study, 19 participants reported no adverse effects other than short‐term muscle soreness. None developed inflammatory infiltrates. |
⊕⊕⊝⊝ Very lowd | May have few or no adverse effects requiring withdrawal | |||
| 5RM: 5 voluntary repetitions; CI: confidence interval; MD: mean difference; MMT‐8: manual muscle testing of eight muscle groups; RCT: randomised controlled trial; SD: standard deviation; SMD: standardised mean difference; SF‐36: Short Form 36; VO2 max: maximal oxygen uptake | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for study limitations. Quote: "One patient in the exercise group was not able to perform the exercise programme and was excluded from the analysis". Follow‐up was therefore incomplete and analysis was not by intention‐to‐treat. Quote: "We aimed for nine patients in the exercise group, but some analyses were performed with N = 7 (VO2 max measurements) or N = 3 (mitochondrial enzyme activities)." (...) "All measurements were not successfully performed both before and after training in each subject". Blinding of participants and personnel was not possible. bDowngraded one level for imprecision: small samples. cDowngraded two levels for serious imprecision. Quote: "An important limitation is the lack of power analysis and the low number of patients, conditions that may explain lack of significant between‐group differences, with frequent dropouts further hampering the analyses and conclusion.(...) The exercise intensity level was defined only for the aerobic walks, not for the resistive home exercise programme". dDowngraded two levels for serious imprecision and study limitations. The combined sample size was too small to rule out less common adverse effects requiring withdrawal. Blinding of participants and personnel was not possible.
Summary of findings 9. Aerobic exercise and strength training compared to no training for facioscapulohumeral muscular dystrophy.
| Aerobic exercise and strength training compared to no training for facioscapulohumeral muscular dystrophy | ||||||
| Patient or population: people with facioscapulohumeral muscular dystrophy Setting: at home Intervention: aerobic exercise and strength training Comparison: without aerobic exercise and strength training | ||||||
| Outcomes | Mean (SD) without aerobic exercise and strength training | Mean (SD) with aerobic exercise and strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength: MVC quadriceps at rest
Assessed with femoral nerve magnetic stimuli delivered during isometric maximum voluntary contractions and at rest Follow‐up: mean 24 weeks 16 participants (1 RCT) |
The mean change in MVC quadriceps at rest without aerobic exercise and strength training was a decrease of 1.0 (40.0) Nm | The mean change in MVC quadriceps at rest with aerobic exercise and strength training was an increase of 14.0 (47.0) Nm | MD 15 Nm higher (27.77 lower to 57.77 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on MVC is uncertain | |
|
Aerobic capacity Assessed with incremental cycling test Follow‐up: mean 24 weeks 16 participants (1 RCT) |
Maximal aerobic power | The mean change in maximal aerobic power without aerobic exercise and strength training was an increase of 0.0 (37.0) W | The mean change in maximal aerobic power with aerobic exercise and strength training was an increase of 45.0 (87.0) W | MD 45 W higher (20.51 lower to 110.51 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on maximal aerobic power is uncertain |
| VO2 peak | The mean change in VO2 peak without aerobic exercise and strength training was a decrease of 0.1 (7.9) mL/min/kg | The mean change in VO2 peak with aerobic exercise and strength training was an increase of 12.3 (12.4) mL/min/kg | MD 12.4 mL/min/kg higher (2.21 higher to 22.59 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on oxygen uptake is uncertain | |
|
Time‐scored functional assessments of muscle performance: distance walked Assessed with 6‐minute walk test Follow‐up: mean 24 weeks 16 participants (1 RCT) |
The mean change in distance walked without aerobic exercise and strength training was a decrease of 2.0 (103.0) m | The mean change in distance walked with aerobic exercise and strength training was an increase of 62.0 (130.0) m | MD 64.0 m higher (50.9 lower to 178.9 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on distance walked in a 6‐min walk test is uncertain | |
|
Quality of life Assessed with SF‐36 Health Survey Scale from 0 to 100 Follow‐up: mean 24 weeks 16 participants (1 RCT) |
The mean change in SF‐36 score without aerobic exercise and strength training was a decrease of 5.0 (17.0) | The mean change in SF‐36 score with aerobic exercise and strength training was an increase of 9 (20) | MD 14.0 higher (4.2 lower to 32.2 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on quality of life is uncertain | |
| Pain | No data were provided for this outcome | |||||
|
Experienced fatigue Assessed with Fatigue Severity Scale (FSS) Scale from 9 to 63 Follow‐up: mean 24 weeks 16 participants (1 RCT) |
The mean change in FSS score without aerobic exercise and strength training was an increase of 5.0 (11.0) | The mean change in FSS score with aerobic exercise and strength training was a decrease of 10.0 (15.0) | MD 15.0 lower (27.9 lower to 2.1 lower) | ⊕⊝⊝⊝ Very lowa,b | The effect on experienced fatigue is uncertain | |
|
Adverse effects requiring withdrawal Follow‐up: mean 24 weeks 16 participants (1 RCT) |
No training complications were reported. | ⊕⊝⊝⊝ Very lowa,b | The presence of adverse effects requiring withdrawal is uncertain | |||
| CI: confidence interval; FSS: Fatigue Severity Score; MD: mean difference; MVC: maximal voluntary contraction; RCT: randomised controlled trial; SD: standard deviation; SF‐36: Short‐Form‐36; VO2 peak: peak oxygen uptake | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for study limitations: investigators involved in training and testing participants were not blinded, "Two patients dropped out in the TG [training] group before starting the training programme, (...). Another patient, included in CG [the control group], withdrew for unknown reasons before starting the training program. In total, 16 patients completed the study...and analysis was not according intention‐to‐treat." Blinding of participants and personnel was not possible. bDowngraded two levels for imprecision: the number of participants in the study was smaller (16 participants) than the calculated sample size.
Summary of findings 10. Aerobic exercise and strength training compared to no training for juvenile dermatomyositis.
| Aerobic exercise and strength training compared to no training for juvenile dermatomyositis | ||||||
| Patient or population: children and adolescents with juvenile dermatomyositis Setting: at home Intervention: aerobic exercise and strength training Comparison: without aerobic exercise and strength training | ||||||
| Outcomes | Mean (SD) without aerobic exercise and strength training | Mean (SD) with aerobic exercise and strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens | |
|
Muscle strength Assessed with hand‐held dynamometer Follow‐up: mean 12 weeks 26 participants (1 RCT) |
Maximum force of right knee extensors | The mean change in maximum force of right knee extensors without aerobic exercise and strength training was a decrease of 5.0 (15.0) N | The mean change in maximum force of right knee extensors with aerobic exercise and strength training was an increase of 31.0 (13.5) N | MD 36.0 N higher (25.0 higher to 47.1 higher) | ⊕⊕⊝⊝ Lowa,b | May increase dynamic muscle strength of right knee extensors |
| Maximum force of left knee extensors | The mean change in maximum force of left knee extensors without aerobic exercise and strength training was a decrease of 4.0 (27.0 ) N | The mean change in maximum force of left knee extensors with aerobic exercise and strength training was an increase of 13.0 (12.0) N | MD 17.0 N higher (0.5 higher to 33.5 higher) | ⊕⊕⊝⊝ Lowa,b | May increase dynamic muscle strength of left knee extensors slightly | |
| Maximum force of right hip flexors | The mean change in maximum force of right hip flexors without aerobic exercise and strength training was an increase of 5.0 (18.0) N | The mean change in maximum force of right hip flexors with aerobic exercise and strength training was a decrease of 4.0 (16.5) N | MD 9.0 N lower (22.4 lower to 4.4 higher) | ⊕⊕⊝⊝ Lowa,b | May have little or no effect on dynamic muscle strength of the right hip flexors | |
| Maximum force of left hip flexors | The mean change in maximum force of left hip flexors without aerobic exercise and strength training was an increase of 3.0 (17.0) N | The mean change in maximum force of left hip flexors with aerobic exercise and strength training was an increase of 9.0 (15.5) N | MD 6.0 N higher (6.6 lower to 18.6 higher) | ⊕⊕⊝⊝ Lowa,b | May have little or no effect on change in dynamic muscle strength of the left hip flexors | |
|
Aerobic capacity Assessed with treadmill‐based incremental maximal exercise test Follow‐up: mean 12 weeks 26 participants (1 RCT) |
Endurance time | The mean change in endurance time without aerobic exercise and strength training was an increase of 1.1 (0.5) min | The mean change in endurance time with aerobic exercise and strength training was a decrease of 0.1 (0.4) min | MD 1.2 min lower (1.55 lower to 0.85 lower) | ⊕⊕⊝⊝ Lowa,b | May slightly decrease endurance time |
| VO2 peak | The mean change in aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 peak) without aerobic exercise and strength training was an increase of 2.1 (1.6) mL/kg/min | The mean change in aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 peak) with aerobic exercise and strength training was an increase of 0.0 (1.4) mL/kg/min | MD 2.1 mL/kg/min lower (3.3 lower to 0.9 lower) | ⊕⊕⊝⊝ Lowa,b | May decrease aerobic capacity slightly | |
|
Time‐scored functional assessments of muscle performance: distance walked in meters Assessed with 6‐min walk test Follow‐up: mean 12 weeks 26 participants (1 RCT) |
The mean change in distance walked in a 6‐min walk test without aerobic exercise and strength training was an increase of 9.0 m (20.0) | The mean change in distance walked in a 6‐min walk test with aerobic exercise and strength training was an increase of 2.0 m (17.5) | MD 7.0 m lower (21.6 lower to 7.6 higher) | ⊕⊕⊝⊝ Lowa,b | May have little or no effect on distance walked in a 6‐min walk test | |
|
Quality of life Assessed with PedsQL Generic Core Scale Scale from 0 to 100 Follow‐up: mean 12 weeks 26 participants (1 RCT) |
The mean change in PedsQL Generic Core Scale score without aerobic exercise and strength training was an increase of 5.0 (2.5) | The mean change in PedsQL Generic Core Scale score with aerobic exercise and strength training was a decrease of 3.0 (2.0) | MD 8.0 lower (9.8 lower to 6.2 lower) | ⊕⊕⊝⊝ Lowa,b | May improve quality of life slightly | |
|
Pain Assessed with 10‐cm VAS Scale from: 0 to 100 Follow‐up: mean 12 weeks 26 participants (1 RCT) |
The mean change in score on VAS without aerobic exercise and strength training was an increase of 4.0 (3.5) | The mean change in score on VAS with aerobic exercise and strength training was a decrease of 3.0 (3.5) | MD 7.0 lower (9.7 lower to 4.3 lower) | ⊕⊕⊝⊝ Lowa,b | May decrease pain level slightly | |
|
Experienced fatigue Assessed with PedsQL Multidimensional Fatigue Scale Scale from 0 to 100 (higher scores indicate less fatigue) Follow‐up: mean 12 weeks 26 participants (1 RCT) |
The mean change in PedsQL Multidimensional Fatigue Scale score without aerobic exercise and strength training was an increase (improvement) of 4.0 (2.0) | The mean change in PedsQL Multidimensional Fatigue Scale score with aerobic exercise and strength training was a decrease of 1.0 (2.0) | MD 5.0 lower (6.5 lower to 3.5 lower) | ⊕⊕⊝⊝ Lowa,b | May increase experienced fatigue slightly | |
|
Adverse effects requiring withdrawal Follow‐up: mean 12 weeks 26 participants (1 RCT) |
There were no adverse events leading to withdrawal. No hospitalisation occurred in the participants who participated in the intervention. In all participants who started the intervention, immune suppressive therapy remained stable or decreased during the study period. |
⊕⊕⊝⊝ Lowa,b | May have no adverse effects requiring withdrawal | |||
| CI: confidence interval; MD: mean difference RCT: Randomised controlled trial; SD: standard deviation; VAS: visual analogue scale VO2 peak: peak oxygen uptake | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aStudy limitations: blinding of participants and personnel was not possible. bDowngraded one level for imprecision: 26 participants, according to the authors of the juvenile dermatomyositis study, there was a high variation in aerobic fitness and disease characteristics between the participants at baseline.
Judging the clinical meaningfulness and effect size of the mean differences (MDs) was not straightforward, as there are no internationally agreed standards on which level of change is clinically meaningful or even which can be considered ’small’ or 'large' in size.
Strength training versus no training in myotonic dystrophy
This comparison includes two studies with 36 and 35 participants, respectively (Lindeman 1995; Aldehag 2013). See Table 1. We intended to combine study results for appropriate pairings of treatments by calculating a mean of the difference between their effects using the Cochrane statistical package RevMan 5 (Review Manager 2014). We were unable to produce MDs and 95% CIs for Lindeman 1995, the myotonic dystrophy study, because of the matched‐pair design. We therefore reported the findings of the study as given in the paper.
Primary outcome: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Two studies reported this outcome (Lindeman 1995; Aldehag 2013).
Lindeman and colleagues measured differences in muscle strength isokinetically, on a dynamometer as maximum concentric knee torques at three velocities, and isometrically, as maximum voluntary contraction (MVIC; Lindeman 1995). There were no statistically significant differences in knee torques between the training and control groups, as found with a paired t‐test. After 24 weeks, the mean change in isokinetic knee torque extension was 5.3 (SD 12.9) Newton meters (Nm) in the training group and 1.4 Nm (SD 8.2) in the control group, P = 0.34, 28 participants; low‐certainty evidence. One Newton is defined as the force that will accelerate a mass of 1 kg at the rate of one meter per second per second. Mean (SD) change in isokinetic knee torque flexion was 7.4 Nm (11.4) in the training group and 3.7 Nm (8.6) in the control group, P = 0.34, 28 participants. Mean change in MVIC was 8.7 Nm (14.7) in the training group and 6.6 Nm (11.0) in the control group (P = 0.67; 28 participants; low‐certainty evidence, downgraded for imprecision and study limitations (not shown in Table 1)).
In Aldehag 2013, the primary outcome measures were hand‐grip and pinch‐grip force measured with an electronic dynamometer, which gave the average force in N over a period of 10 seconds. Analysis showed no clear effect of strength training on hand grip force after 12 weeks (MD 6.0 N, 95% CI −6.7 to 18.7; 35 participants, very low‐certainty evidence; Analysis 1.1) or on pinch grip force (MD 1.0 N, 95% CI −3.3 to 5.3; 35 participants, very low‐certainty evidence; Analysis 1.2). Secondary outcome measures were isometric force in wrist flexors and wrist extensors as measured with a hand‐held myometer. Twelve weeks of strength training had no clear effect on isometric wrist flexor force compared to no training (MD 7.0 N, 95% CI −3.4 to 17.4; 35 participants, very low‐certainty evidence; Analysis 1.3). Twelve weeks of strength training improved isometric wrist extension force slightly compared to no training (MD 8.0 N. 95% CI 0.7 to 15.3; 35 participants, very low‐certainty evidence; Analysis 1.4). The overall certainty of evidence from Aldehag 2013 was very low, downgraded two levels for imprecision and once for study limitations: the study was underpowered (sample size 35) and blinding was not possible.
1.1. Analysis.
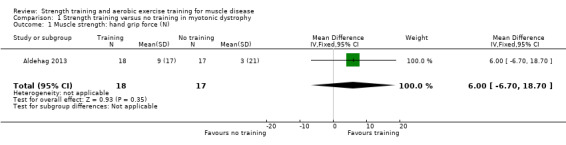
Comparison 1 Strength training versus no training in myotonic dystrophy, Outcome 1 Muscle strength: hand grip force (N).
1.2. Analysis.
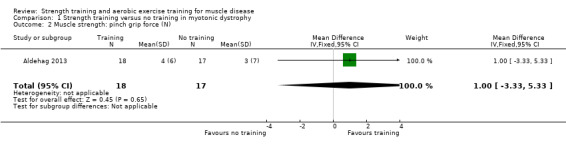
Comparison 1 Strength training versus no training in myotonic dystrophy, Outcome 2 Muscle strength: pinch grip force (N).
1.3. Analysis.
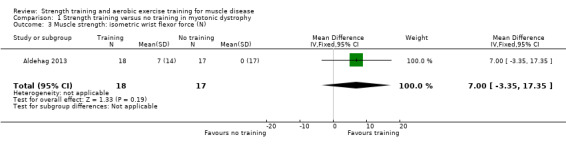
Comparison 1 Strength training versus no training in myotonic dystrophy, Outcome 3 Muscle strength: isometric wrist flexor force (N).
1.4. Analysis.
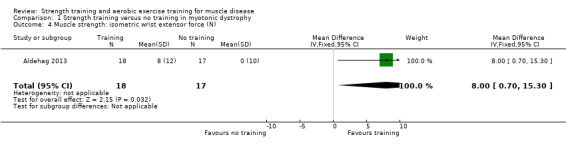
Comparison 1 Strength training versus no training in myotonic dystrophy, Outcome 4 Muscle strength: isometric wrist extensor force (N).
Secondary outcome measures
Muscle strength, expressed in measures of endurance or muscle fatigue
Lindeman 1995 measured endurance as maximum duration of knee flexion and extension contraction at 80% of MVIC on an isokinetic dynamometer. After 24 weeks, the change in MVIC for the control group was −7.4 s (SD 12.0) and for the training group 5.7 s (SD 17.0; P = 0.09, 36 participants; very low‐certainty evidence, downgraded for imprecision and very serious study limitations). This difference between groups was mainly due to a decrease in endurance in the non‐training group.
Aldehag 2013 did not provide data for this outcome.
Time‐scored functional assessments of muscle performance
Functional assessments in Lindeman 1995 comprised the following time‐scored activities: ascending and descending stairs, rising from a chair, rising from supine, walking 50 m as fast as possible, and walking 6 m at natural speed. They reported no clear differences in change in the functional outcome measures after 24 weeks (very low‐certainty evidence; downgraded for imprecision and very serious study limitations; Table 11).
1. Lindeman 1995: functional tests.
| Functional test | No training n = 14 | Training n = 14 | |||
| Mean change (0 to week 24)a | SD | Mean change (0 to week 24)a | SD | P valueb | |
| Descending stairs (s) | 0.5 | 3.6 | 2.5 | 7.2 | 0.43 |
| Climbing stairs (s) | 0.3 | 1.8 | 1.1 | 5.8 | 0.66 |
| Standing up from a chair (s) | 0.2 | 0.8 | 1.2 | 4.0 | 0.4 |
| Standing up from lying supine (s) | 0.5 | 2.2 | ‐0.4 | 1.4 | 0.11 |
| Walking 6 m (comfortably) (s) | 0.5 | 0.8 | 0.3 | 0.8 | 0.52 |
| Walking 50 m (fast) (s) | 3.5 | 5.8 | 2.7 | 6.3 | 0.75 |
| SD: standard deviation | |||||
aPositive values indicate improvement. bP values from paired t‐tests (matched pair design).
Aldehag 2013 did not provide data for this outcome.
Quality‐of‐life measures
No data were provided for this outcome (Lindeman 1995; Aldehag 2013).
Pain assessed by an analogue pain scale
Lindeman 1995 and Aldehag 2013 did not provide data for this outcome.
Experienced fatigue
Lindeman 1995 and Aldehag 2013 did not provide data for this outcome.
Parameters of muscle membrane permeability
Lindeman 1995 assessed serum myoglobin levels just before and one hour after the measurement session at the baseline visit and at the final visit in 36 participants. Changes in serum myoglobin activity one hour after a standardised test should reflect changes in muscle fibre permeability due to muscle damage. The mean rise in serum myoglobin levels did not differ between the training and the non‐training group (31 participants; Table 12).
2. Lindeman 1995: muscle permeability (change in serum myoglobin ng/L).
| Initial value at baseline | Increase due to test session at baseline | Initial value at week 24 | Increase due to test session at week 24 | |
| Control | 263 (149) | 41(42) | 248 (120) | 28 (28) |
| Training | 203 (84) | 20 (34) | 175 (74) | 26 (35) |
Data show the increase in serum myoglobin levels one hour after test sessions at baseline and week 24 in the training and control groups. Changes between baseline and 24 weeks were not significant. Myoglobin levels were determined with a radioimmunoassay. 31 participants, numbers in each group not given.
Aldehag 2013 did not provide data for this outcome.
Adverse effects requiring withdrawal of the participant from the study
In Lindeman 1995 (36 participants), study authors report "No serious side effects of the training occurred." “One MyD [myotonic dystrophy] patient stopped training during the second training period on the advice of his general practitioner because of back complaints. This patient also failed to attend the last test session because of knee problems." These appear not to have been considered adverse events leading to withdrawal.
A few participants in Lindeman 1995 complained of muscle soreness and transient strength reduction after eight weeks. However, no signs of muscle damage were found at the final visit after 24 weeks.
Aldehag 2013 (35 participants) did not find other signs of overuse, such as a decline in strength measures.
Strength training versus no training in facioscapulohumeral muscular dystrophy (FSHD)
This comparison includes one study with 35 participants (Van der Kooi 2004). See: Table 2.
The certainty of evidence for all outcomes from the Van der Kooi 2004 study was low; we downgraded one level for imprecision and one level for study limitations, as blinding was not possible.
Primary outcome: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
The primary outcome measure in the Van der Kooi 2004 FSHD strength training study was the change in maximum voluntary isometric strength of the elbow flexors and ankle dorsiflexors, measured on a Quantitative Muscle Assessment fixed myometry testing system.
After 52 weeks, strength training had no clear effect on isometric strength of the elbow flexors (MVIC) compared to no training (MD for the right side 0.5 kgF, 95% CI −0.8 to 1.8; 35 participants, low‐certainty evidence; Analysis 2.1). One kgF is 9.8 N.
2.1. Analysis.
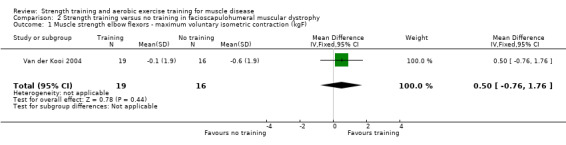
Comparison 2 Strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 1 Muscle strength elbow flexors ‐ maximum voluntary isometric contraction (kgF).
Van der Kooi 2004 evaluated dynamic strength using the one‐repetition maximum (1RM), which is the weight a person can lift once, but not twice, at a steady controlled pace through the full range of joint motion. After 52 weeks, there was no clear difference in dynamic strength of the elbow flexors in the training group compared to the non‐training group (right side MD 1.2 kgF (95% CI −0.2 to 2.6; 35 participants, low‐certainty evidence; Analysis 2.2).
2.2. Analysis.
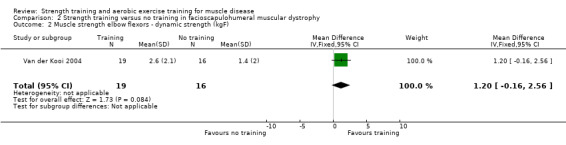
Comparison 2 Strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 2 Muscle strength elbow flexors ‐ dynamic strength (kgF).
Both strength measures of the ankle dorsiflexors decreased significantly and markedly in all treatment groups after 52 weeks. Training had no clear effect on isometric strength (MVIC) of the ankle dorsiflexors (right side MD 0.4 kgF, 95% CI −2.4 to 3.2; 35 participants, low‐certainty evidence; Analysis 2.3) or on dynamic strength of the ankle dorsiflexors in 1RM (right side MD −0.4 kg, 95% CI −2.3 to 1.4;35 participants, low‐certainty evidence; Analysis 2.4).
2.3. Analysis.
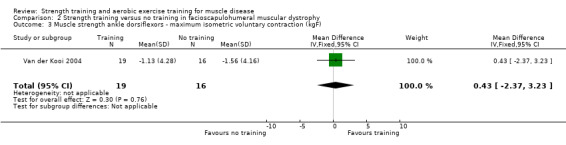
Comparison 2 Strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 3 Muscle strength ankle dorsiflexors ‐ maximum isometric voluntary contraction (kgF).
2.4. Analysis.
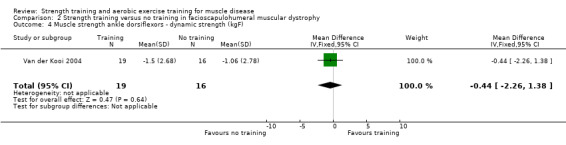
Comparison 2 Strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 4 Muscle strength ankle dorsiflexors ‐ dynamic strength (kgF).
Differences between groups for the trained muscles on the left side did not significantly differ from those on the right side after 52 weeks.
Secondary outcomes
Muscle strength, expressed in measures of endurance or muscle fatigue
Muscle endurance was expressed as a Force‐Time Integral (FTI30) of a sustained 30 second maximal isometric contraction measured on a Quantitative Muscle Assessment fixed myometry testing system (Van der Kooi 2004). After 52 weeks of strength training, there was no clear difference between the training group and the non‐training group in FTI30 of the elbow flexors and ankle dorsiflexors (low‐certainty evidence; Analysis 2.5; Analysis 2.6).
2.5. Analysis.
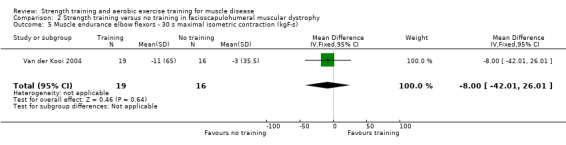
Comparison 2 Strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 5 Muscle endurance elbow flexors ‐ 30 s maximal isometric contraction (kgF‐s).
2.6. Analysis.
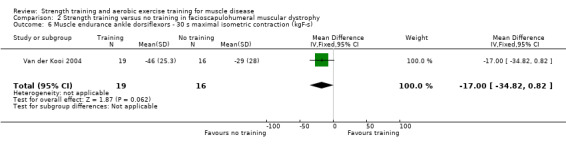
Comparison 2 Strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 6 Muscle endurance ankle dorsiflexors ‐ 30 s maximal isometric contraction (kgF‐s).
Time‐scored functional assessments of muscle performance
The functional tests consisted of the assessment of a functional upper extremity grade and functional lower extremity grade (Personius 1994), and the following time‐scored tasks: standing from lying supine, standing from sitting, walking 30 feet (9.14 m), and climbing three standard stairs (Personius 1994; Van der Kooi 2004). After 52 weeks, Van der Kooi 2004 reported no differences between groups in functional assessments; 35 participants, low‐certainty evidence.
Quality‐of‐life measures
Van der Kooi 2004 assessed quality of life using the Sickness Impact Profile (SIP). The mean total of the SIP and its subscales did not demonstrate relevant or significant changes for either the training or non‐training groups. In addition, for both groups the mean SIP total did not change between the baseline and final visit after 52 weeks (35 participants, low‐certainty evidence).
Pain assessed by an analogue pain scale
Eleven out of 34 participants in the training group reported pain in the neck and shoulder region to the physical therapist during home visits (Van der Kooi 2004). Five people mentioned a period with elbow complaints. However, the number of people with neck‐shoulder and elbow complaints did not differ between treatment groups at baseline nor at the final visit. Moreover, the number of participants with neck‐shoulder and elbow complaints slightly decreased in both groups. There was no clear difference between the exercise and no‐training group in the number of participants with neck‐shoulder complaints at the final visit (RR 1.0, 95% CI 0.7 to 1.6; 35 participants, low‐certainty evidence) but more people had elbow complaints in the exercise group than in the no‐training group (RR 1.8, 95% CI 0.2 to 19.1; 35 participants, low‐certainty evidence). Although not formally quantified, the study authors mentioned that participants experienced no notable muscle soreness after training. At the final visit, scores on the visual analogue scale (VAS) for pain and the mean daily rated pain scores did not demonstrate significant changes in either group.
Experienced fatigue
Van der Kooi 2004 measured experienced fatigue by the subscale 'fatigue severity' of the Checklist Individual Strength (CIS‐fatigue). Scores range from 7 (no fatigue) to 56 (worst fatigue). The mean score on the CIS‐fatigue did not change between the baseline and final visit (52 weeks) for either group (35 participants, low‐certainty evidence).
Parameters of muscle membrane permeability
One participant stopped training because of recurring, training‐related muscle soreness and fatigue (Van der Kooi 2004). A diagnostic work‐up revealed a mitochondrial myopathy in addition to FSHD.
Adverse effects requiring withdrawal of the participant from the study
There were no adverse events leading to withdrawal. Van der Kooi 2004 found neither signs of overuse, such as a decline in strength measures, nor training‐related increases in pain or fatigue (Van der Kooi 2004; Voet 2014).
Aerobic exercise training versus no training in dermatomyositis and polymyositis
We included one study with 14 participants in total in this comparison (Wiesinger 1998a). See: Table 3.
The overall certainty of evidence from the Wiesinger 1998a study was very low, downgraded by two levels for study limitations (see Characteristics of included studies), one level for indirectness, as there was no objective assessment of physical activity or exercise level to ensure compliance, and one level for imprecision, as the study had 14 participants. Therefore, the effect of aerobic training in dermatomyositis and polymyositis for all the outcomes below, based on this study, is uncertain.
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
Wiesinger 1998a did not provide data for this outcome.
Secondary outcome measures
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Wiesinger 1998a measured aerobic capacity during an incremental cycle test on a cycle ergometer, defining maximal oxygen uptake (VO2 max) as the highest oxygen consumption obtained during the symptom‐limited exercise test. After six weeks, the effect of aerobic exercise on the change in mean VO2 max (mL/min/kg) was uncertain (MD 14.6, 95% CI −1.0 to 30.2; 14 participants, very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.
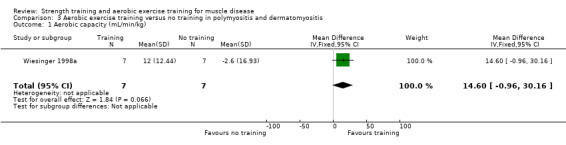
Comparison 3 Aerobic exercise training versus no training in polymyositis and dermatomyositis, Outcome 1 Aerobic capacity (mL/min/kg).
Time‐scored functional assessments of muscle performance
Wiesinger 1998a used the modified Functional Assessment Screening Questionnaire (Millard 1989), for evaluating disability. After six weeks, the effect of aerobic exercise training on disability was uncertain (MD 17.6, 95% CI −5.6 to 40.8; 14 participants, very low‐certainty evidence; Analysis 3.2).
3.2. Analysis.
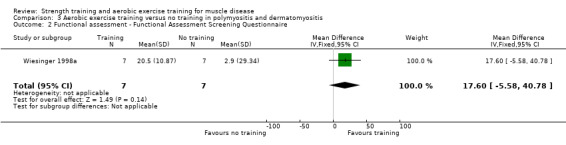
Comparison 3 Aerobic exercise training versus no training in polymyositis and dermatomyositis, Outcome 2 Functional assessment ‐ Functional Assessment Screening Questionnaire.
Quality‐of‐life measures
Wiesinger 1998a did not provide data for this outcome.
Pain assessed by an analogue pain scale
Wiesinger 1998a did not provide data for this outcome.
Experienced fatigue
Wiesinger 1998a did not provide data for this outcome.
Parameters of muscle membrane permeability
Wiesinger 1998a took weekly measurements of serum levels of creatine kinase (CK) and aldolase on Mondays after a weekend recovery phase without exercise. After six weeks, the effect of aerobic exercise training on change in serum CK level and serum aldolase level (%) during the observation period was uncertain (MD 7.9, 95% CI −24.2 to 40.0;14 participants, very low‐certainty evidence; Analysis 3.3).
3.3. Analysis.
Comparison 3 Aerobic exercise training versus no training in polymyositis and dermatomyositis, Outcome 3 Creatine kinase and aldolase serum level.
Adverse effects requiring withdrawal of the participant from the study
Wiesinger 1998a did not describe adverse effects.
Aerobic exercise training versus no training in Duchenne muscular dystrophy
We included one study with 30 participants in this comparison (Jansen 2013). See Table 4 and Table 13.
3. Aerobic exercise compared to control for Duchenne muscular dystrophy (DMD): GRADE assessments for other functional outcome measures (supplementary to 'Summary of findings' table 4).
| Aerobic exercise compared to control for Duchenne muscular dystrophy (DMD) | |||||
| Patient or population: boys with DMD Setting: at home or at school, depending on the preferences of the participants Intervention: aerobic exercise Comparison: control without aerobic exercise training | |||||
| Time‐scored functional assessments of muscle performance | Mean (SD) without aerobic exercise | Mean (SD) with aerobic exercise | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens |
|
Functional ability in standing positions and transfers Assessed with Motor Function Measure D1 Scale from 0% to 100% Follow‐up: mean 14 weeks 29 participants (1 RCT) |
The mean difference in motor Function Measure D1 without aerobic exercise was a decrease of 9.1% (22.1) | The mean difference in motor Function Measure D1 with aerobic exercise was an increase of 0.8% (29.2) | MD 9.9% higher (8.8 lower to 28.6 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on functional ability in standing positions and transfers is uncertain |
|
Functional ability in axial and proximal motor functions Assessed with Motor Function Measure D2 Scale from: 0% to 100% Follow‐up: mean 14 weeks 29 participants (1 RCT) |
The mean difference in Motor Function Measure D2 without aerobic exercise was a decrease of 4.1% (13.8) | The mean difference in Motor Function Measure D2 with aerobic exercise was an increase of 0.3% (15.3) | MD 4.4% higher (6.2 lower to 15.0 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on functional ability in axial and proximal motor functions is uncertain |
|
Functional ability in distal motor function Assessed with Motor Function Measure D3 Scale from: 0% to 100% Follow‐up: mean 14 weeks 29 participants (1 RCT) |
The mean difference in Motor Function Measure D3 without aerobic exercise was a decrease of 5.2% (8.0) | The mean difference in Motor Function Measure D3 with aerobic exercise was an increase of 1.5% (7.6) | MD 6.7% higher (1.0 higher to 12.4 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on functional ability in distal motor function is uncertain |
| CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; SD: standard deviation | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels for imprecision as it is not known if the sample size is sufficient. Quote: "No historical data were available at the start of this study in 2008. The sample size was therefore not based on statistical analysis. We arbitrarily chose to include 20 to 30 participants". CI consistent with both effects in favour of training and little or no effect. bDowngraded one level for study limitations: participants and outcome assessor had no information about previous test results at each assessment but were not blinded to treatment allocation. Moreover, boys were originally allocated to the intervention group, but replaced to the control group within two weeks after trying the intervention. One boy discontinued the training and assessment after 12 weeks and was excluded from the analysis, so the analysis was not intention‐to‐treat. Blinding of participants and personnel was not possible.
The overall certainty of evidence of the Jansen 2013 study was very low, downgraded by two levels for serious study limitations (see Characteristics of included studies) and by two levels for imprecision, as we do not know if the sample size was sufficient. Participants and outcome assessor had no information about previous test results at each assessment, but were not blinded to treatment allocation. Moreover, boys were originally allocated to the intervention group, but replaced to the control group within two weeks after trying the intervention. One boy discontinued the training and assessment after 12 weeks and was excluded from the analysis, so the analysis was not ITT. Therefore, the effect of aerobic exercise training in Duchenne muscular dystrophy for all the outcomes below, based on this study, is uncertain.
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
One of the primary outcomes was the assisted six‐minute cycling test (A6MCT). Jansen 2013 used the A6MCT to assess endurance using both legs and arms. Participants were instructed to perform as many revolutions as possible in six minutes. After a baseline period of eight weeks, and 14 weeks of training, the effect of aerobic exercise on the change in number of leg revolutions (MD 14.0, 95% CI −89.0 to 117.0; 23 participants, very low‐certainty evidence; Analysis 4.4) and arm revolutions (MD 34.8, 95% CI −68.12 to 137.8; 23 participants, very low‐certainty evidence; Analysis 4.5) was uncertain. There is very serious imprecision.
4.4. Analysis.
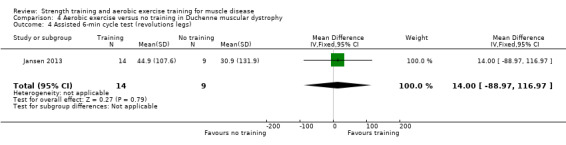
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 4 Assisted 6‐min cycle test (revolutions legs).
4.5. Analysis.
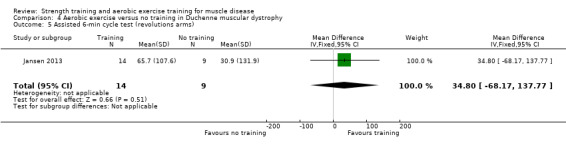
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 5 Assisted 6‐min cycle test (revolutions arms).
Secondary outcome measures
Muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Jansen 2013 scored muscle strength of the hip extensors, knee extensors, ankle dorsiflexors, shoulder abductors and elbow extensors bilaterally on the Medical Research Council (MRC) scale. The baseline period was eight weeks, followed by 14 weeks of training. There were small changes in muscle strength scores in favour of the training group, but the evidence was uncertain: the MRC sum score (MD 1.7, 95% CI −1.9 to 5.3; 15 participants, very low‐certainty evidence; Analysis 4.1); lower limb score (MD 1.3, 95% CI −1.5 to 4.1; 26 participants, very low‐certainty evidence; Analysis 4.2); and upper limb score (MD 0.40, 95% CI −1.42 to 2.22; 27 participants, very low‐certainty evidence; Analysis 4.3).
4.1. Analysis.
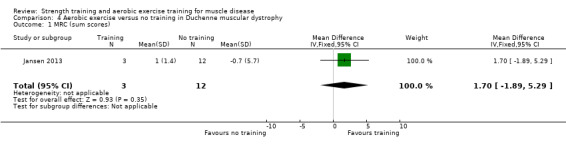
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 1 MRC (sum scores).
4.2. Analysis.
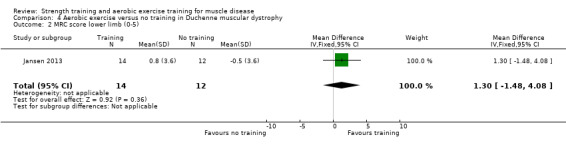
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 2 MRC score lower limb (0‐5).
4.3. Analysis.
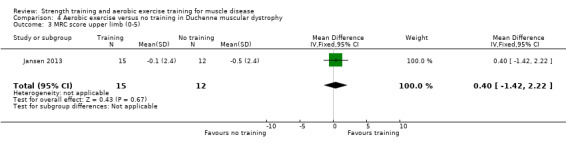
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 3 MRC score upper limb (0‐5).
Time‐scored functional assessments of muscle performance
One of the primary outcome measures in Jansen 2013 was the Motor Function Measure (MFM). The MFM assesses functional abilities (range 0% to 100%) in three different dimensions; the effect of aerobic exercise on all dimensions was uncertain. D1, standing positions and transfers (MD 9.90, 95% CI −8.78 to 28.58; 29 participants, very low‐certainty evidence; Analysis 4.7); D2, axial and proximal motor functions (MD 4.40, 95% CI −6.21 to 15.01; 29 participants, very low‐certainty evidence; Analysis 4.8); and D3, distal motor function (MD 6.7%, 95% CI 1.0 to 12.4; 29 participants, very low‐certainty evidence; Analysis 4.9). The statistically significant difference in D3 was partly due to a decrease in distal motor function in the control group.
4.7. Analysis.
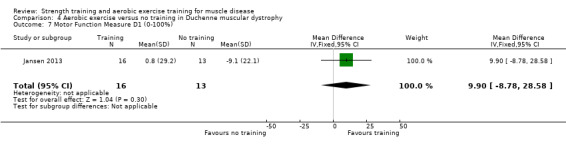
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 7 Motor Function Measure D1 (0‐100%).
4.8. Analysis.
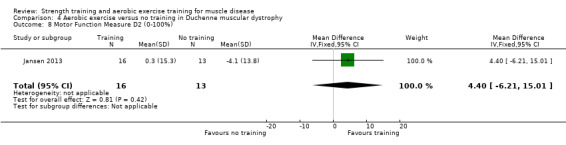
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 8 Motor Function Measure D2 (0‐100%).
4.9. Analysis.
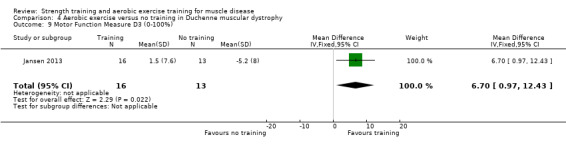
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 9 Motor Function Measure D3 (0‐100%).
Secondary outcome measures for this study were timed tests: rise from floor, 10‐m run and 9‐hole peg test. The effect of aerobic exercise training for these secondary outcome measures was uncertain (Analysis 4.10; Analysis 4.11; Analysis 4.12; respectively 12, 14 and 29 participants; very low‐certainty evidence).
4.10. Analysis.
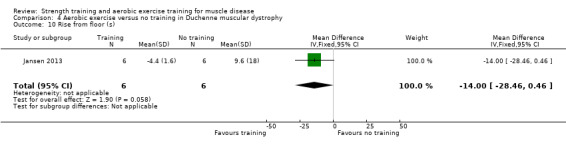
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 10 Rise from floor (s).
4.11. Analysis.
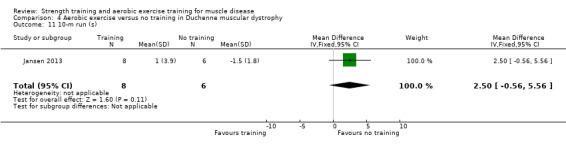
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 11 10‐m run (s).
4.12. Analysis.
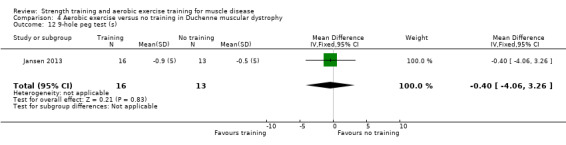
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 12 9‐hole peg test (s).
Quality‐of‐life measures
Jansen 2013 did not provide data for this outcome.
Pain assessed by an analogue pain scale
Jansen 2013 did not provide data for this outcome.
Experienced fatigue
Jansen 2013 did not provide data for this outcome.
Parameters of muscle membrane permeability
Jansen 2013 did not provide data for this outcome.
Adverse effects requiring withdrawal of the participant from the study
Jansen 2013 did not observe or report any serious adverse events.
During the training phase, postural adjustments were made in three out of 24 participants who reported pain at the lateral side of the knee or foot due to an external rotation of the hip during training. In addition, one ambulant boy in the intervention group had an inversion trauma of his ankle after 12 weeks of training and subsequently stopped walking, but he continued cycling. Another boy, who was wheelchair‐dependent, fractured his femur after 12 weeks of training. This boy continued cycling with his arms and he resumed cycling with his legs after three weeks of immobilisation. Injuries were unrelated to the training activities (Jansen 2013).
Aerobic exercise training versus no training in facioscapulohumeral muscular dystrophy (FSHD)
We included three studies with 57, 23 and 13 participants, respectively, in this comparison (Voet 2014; Andersen 2015; Andersen 2017). See: Table 5.
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
The Andersen 2015 and Andersen 2017 aerobic exercise studies in FSHD defined the maximal workload as the highest workload completed for at least 30 seconds in an incremental cycling test. After 12 weeks of aerobic exercise training, the change in maximal workload was greater in the training than the no‐training group (MD 21.5 W, 95% CI 2.2 to 40.8; 19 participants, very low‐certainty evidence; Analysis 5.8; Andersen 2015). After eight weeks of high‐intensity‐training, the change in mean maximal workload was also greater in the training group (MD 18.8 W, 95% CI 13.7 to 23.9; 12 participants, very low‐certainty evidence; Analysis 5.9; Andersen 2017).
5.8. Analysis.
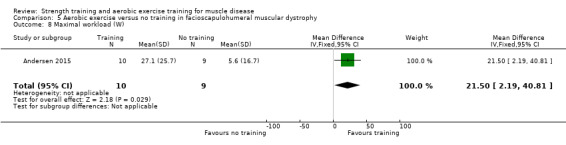
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 8 Maximal workload (W).
5.9. Analysis.
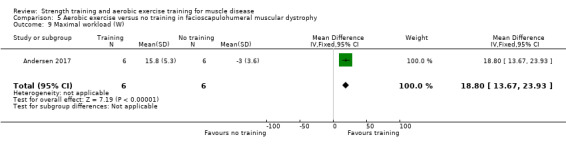
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 9 Maximal workload (W).
The Voet 2014 aerobic exercise in FSHD study did not provide data for this outcome.
Secondary outcome measures
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Voet 2014 estimated VO2 peak noninvasively with the Åstrand test, a submaximal cycling test. After 16 weeks, there was a slight increase in aerobic capacity (change in VO2 max) with training compared to no training (MD 1.1 L/min, 95% CI 0.5 to 1.7; 38 participants, low‐certainty evidence; Analysis 5.12). Andersen 2015 and Andersen 2017 measured VO2 max during an exhaustion incremental test on a cycle ergometer, measuring gas exchanges and ventilation continuously and presenting VO2 max as 30‐second average at peak exercise. After 12 weeks of aerobic exercise training and eight weeks of high‐intensity training, respectively, the change in VO2 peak was greater with training in both studies (MD 3.6 mL/min/kg, 95% CI 0.6 to 6.6; 19 participants, very low‐certainty evidence; Analysis 5.10; and MD 3.3 mL/min/kg, 95% CI 2.5 to 4.1; 12 participants, very low‐certainty evidence; Analysis 5.11). Because of the overall high risk of bias of both Andersen 2015 and Andersen 2017, we did not perform a meta‐analysis.
5.12. Analysis.
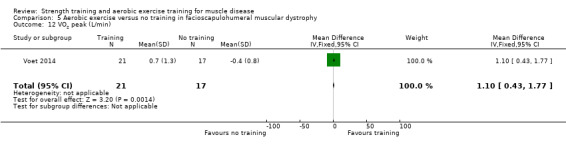
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 12 VO2 peak (L/min).
5.10. Analysis.
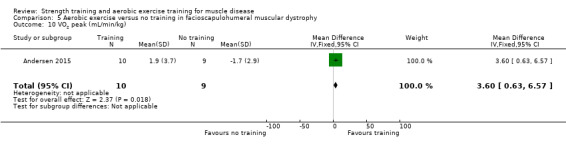
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 10 VO2 peak (mL/min/kg).
5.11. Analysis.
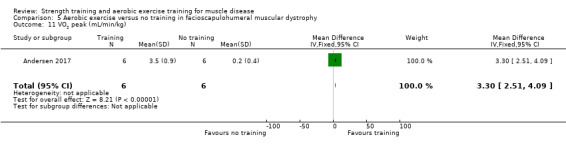
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 11 VO2 peak (mL/min/kg).
Muscle strength, expressed in measures of endurance or fatigue
Voet 2014 measured MVIC for the quadriceps using Quantitative Muscle Assessment, calculating the average value of the left and right quadriceps strength. After 16 weeks of training, there was no clear difference in mean changes in quadriceps strength between the training and no‐training groups (MD 0.10, 95% CI −0.66 to 0.86; 52 participants; low‐certainty evidence; Analysis 5.2).
5.2. Analysis.
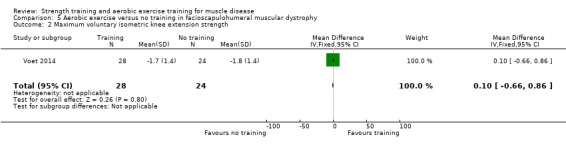
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 2 Maximum voluntary isometric knee extension strength.
Andersen 2015 measured static muscle strength of knee and elbow flexion and extension with a custom‐made dynamometer testing box. After 12 weeks of aerobic exercise training, the effect on muscle strength was uncertain and there was very serious imprecision (Analysis 5.1; Analysis 5.3; Analysis 5.4; Analysis 5.5; 19 participants, very low‐certainty evidence).
5.1. Analysis.
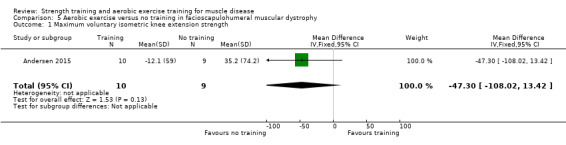
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 1 Maximum voluntary isometric knee extension strength.
5.3. Analysis.

Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 3 Maximum voluntary isometric knee flexion strength (N).
5.4. Analysis.
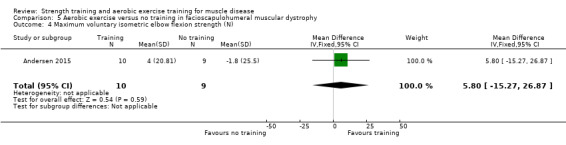
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 4 Maximum voluntary isometric elbow flexion strength (N).
5.5. Analysis.
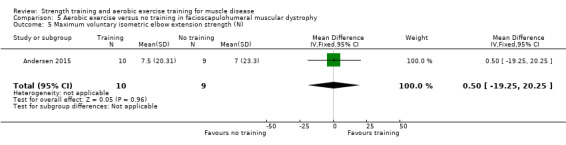
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 5 Maximum voluntary isometric elbow extension strength (N).
Andersen 2017 assessed static muscle strength of hip flexion, knee extension and knee flexion and elbow flexion by hand‐held dynamometer. After eight weeks of high‐intensity‐training, changes in muscle strength did not differ between groups (Analysis 5.6; Analysis 5.7; 12 participants, very low‐certainty evidence).
5.6. Analysis.
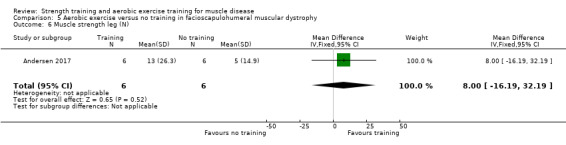
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 6 Muscle strength leg (N).
5.7. Analysis.
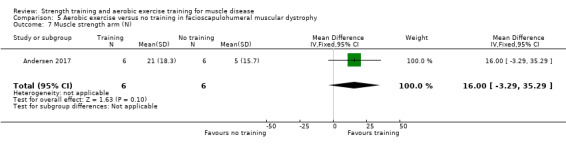
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 7 Muscle strength arm (N).
Because of the overall high risk of bias of Andersen 2015 and Andersen 2017, we did not perform meta‐analysis.
Time‐scored functional assessments of muscle performance
All three aerobic exercise in FSHD studies measured the distance walked in a six‐minute walk test (Voet 2014; Andersen 2015; Andersen 2017).
In Voet 2014 and Andersen 2015, the distance walked in metres in six minutes was greater in the training groups than the control groups: MD 28.9 m (95% CI 4.2 to 53.6; 19 participants, very low‐certainty evidence; Analysis 5.13) after 12 weeks of aerobic exercise in Andersen 2015; and MD 31.0 (95% CI 19.3 to 42.7; 52 participants, low‐certainty evidence; Analysis 5.14) after 16 weeks of aerobic exercise in Voet 2014. In Andersen 2017, after eight weeks of high‐intensity‐training, there was no clear difference in distance walked between groups (MD 7.90, 95% CI −18.37 to 34.17; 12 participants, very low‐certainty evidence; Analysis 5.15). Because of the overall high risk of bias of Andersen 2015 and Andersen 2017, we performed no meta‐analysis. Andersen 2017 performed the 5‐time sit‐to‐stand‐test after eight weeks of high‐intensity‐training, but the time taken for this test did not differ between groups (MD −0.03, 95% CI −7.61 to 7.55; 12 participants, very low‐certainty evidence; Analysis 5.16).
5.13. Analysis.
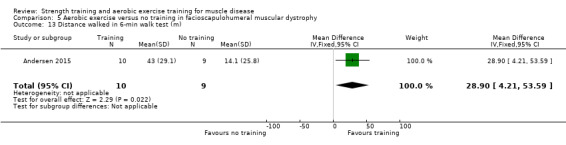
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 13 Distance walked in 6‐min walk test (m).
5.14. Analysis.
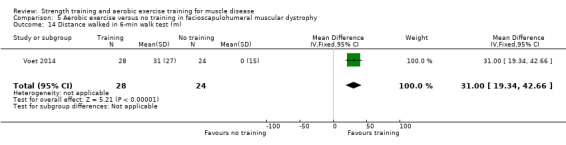
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 14 Distance walked in 6‐min walk test (m).
5.15. Analysis.
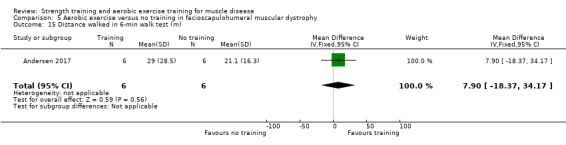
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 15 Distance walked in 6‐min walk test (m).
5.16. Analysis.
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 16 5‐times sit to stand (s).
Quality‐of‐life measures
Andersen 2015 measured quality of life by the SF‐36 Health Survey, in which scales range from 0 to 100 (optimal health). The scores on all subscales of the SF‐36 did not demonstrate relevant changes in either the training or non‐training group after 12 weeks.
Voet 2014 assessed this outcome using the Sickness Impact Profile (SIP), which is a weighted score ranging from 0 to 572; a higher score indicates a poorer outcome. The MD in the score on subscale social behaviour of the SIP after 16 weeks was slightly better in the training group than the no‐training group (MD −10.0, 95% CI −19.6 to −0.4; 52 participants, low‐certainty evidence; Analysis 5.17).
5.17. Analysis.
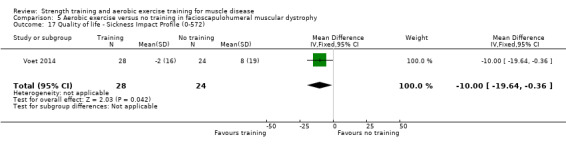
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 17 Quality of life ‐ Sickness Impact Profile (0‐572).
Andersen 2017 did not provide data for this outcome.
Pain assessed by an analogue pain scale
In Voet 2014, scores on the VAS pain did not demonstrate relevant changes for either the training or non‐training group after 16 weeks and data comparing the training and no‐training groups were very imprecise (MD −1.00, 95% CI −3.00 to 1.00; 52 participants, low‐certainty evidence; Analysis 5.18).
5.18. Analysis.
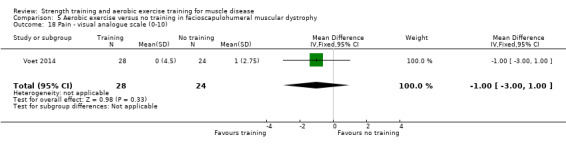
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 18 Pain ‐ visual analogue scale (0‐10).
Andersen 2015 and Andersen 2017 did not provide data for this outcome.
Experienced fatigue
The primary outcome in Voet 2014 was experienced fatigue, which they measured by the "fatigue severity" subscale of the Checklist Individual Strength (CIS‐fatigue). Scores ranged from 7 (no fatigue) to 56 (worst fatigue). After 16 weeks, aerobic exercise slightly decreased the level of fatigue: (MD −7.3, 95% CI −8.1 to −6.5; 52 participants, low‐certainty evidence; Analysis 5.20) in favour of the training group. Andersen 2015 measured fatigue by grading fatigue three days before and after the intervention on a scale from 0 (none) to 10 (worst). After 12 weeks, MD for fatigue was slightly lower in the training group (MD −1.2, 95% CI −3.0 to −0.6 ; 19 participants, very low‐certainty evidence; Analysis 5.19). Because of the overall high risk of bias of Andersen 2015, we did not perform a meta‐analysis.
5.20. Analysis.
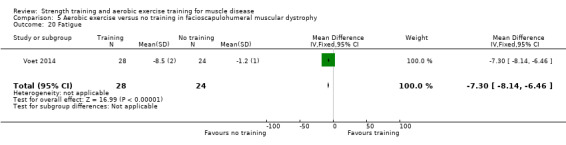
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 20 Fatigue.
5.19. Analysis.
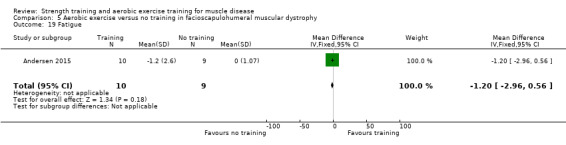
Comparison 5 Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy, Outcome 19 Fatigue.
Andersen 2017 did not provide data for this outcome.
Parameters of muscle membrane permeability
None of the included studies provided data for this outcome (Voet 2014; Andersen 2015; Andersen 2017).
Adverse effects requiring withdrawal of the participant from the study
There were no other signs of overuse, such as a decline in strength measures (Andersen 2015; Andersen 2017), or training‐related increases in pain or fatigue (Voet 2014). There were no adverse events leading to withdrawal.
No episodes of muscle damage occurred according to participant reports and plasma CK levels. Self‐rated levels of muscle pain before training did not change during high‐intensity training. Maximum scores of activity and fatigue increased, but the mean scores were unchanged (Andersen 2017).
Combined aerobic exercise and strength training versus no training in mitochondrial myopathy
We included one study with 20 participants in this comparison (Cejudo 2005). See: Table 6.
The certainty of evidence from Cejudo 2005 was very low for all outcomes: we downgraded by two levels for very serious study limitations (see Characteristics of included studies) and by two levels for very serious imprecision. The study authors did not perform a power analysis before the start of the study, therefore, the effects of aerobic exercise and strength training in mitochondrial myopathy for all the outcomes below, based on this study, are uncertain.
Primary outcome measure: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Cejudo 2005 measured weight‐lifting capacity as the heaviest weight that could be lifted throughout the complete range of movement (1RM test). After the study period, participants in both groups showed increases in all 1RM tests. After 12 weeks, the MD for weight‐lifting capacity between the training and non‐training group were too imprecise for conclusions to be drawn: for the shoulder press exercise (MD −5.0 kg, 95% CI −14.7 to 4.7; 18 participants; very low‐certainty evidence; Analysis 6.1); for the butterfly exercise (MD 6.4 kg, 95% CI −2.9 to 15.7; 18 participants; very low‐certainty evidence; Analysis 6.2) and for the biceps curls exercise (MD 7.3 kg, 95% CI −2.9 to 17.5; 18 participants; very low‐certainty evidence; Analysis 6.3).
6.1. Analysis.
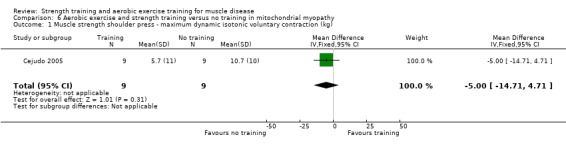
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 1 Muscle strength shoulder press ‐ maximum dynamic isotonic voluntary contraction (kg).
6.2. Analysis.
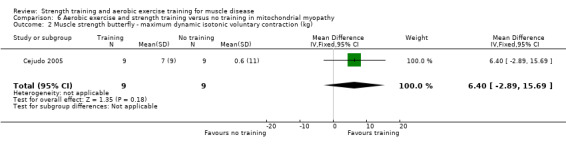
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 2 Muscle strength butterfly ‐ maximum dynamic isotonic voluntary contraction (kg).
6.3. Analysis.
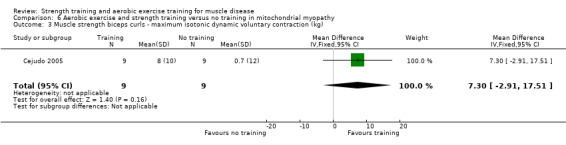
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 3 Muscle strength biceps curls ‐ maximum isotonic dynamic voluntary contraction (kg).
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
Cejudo 2005 measured work capacity in a cycle test and in the shuttle walking test. They measured endurance time in a submaximal cycling test at a constant workload of 70% of the maximum power output achieved during the baseline incremental cycle test. After 12 weeks, the differences in mean time and distance cycled till exhaustion and leg fatigue or breathlessness exhaustion differed between groups, but effects were uncertain. Mean time and distance cycled till exhaustion were slightly better in the training group than the no‐training group (time: MD 23.7 min, 95% CI 2.6 to 44.8; 18 participants; very low‐certainty evidence; Analysis 6.4; distance: MD 9.7 km, 95% CI 1.5 to 17.9; 18 participants; very low‐certainty evidence; Analysis 6.5). The mean distance walked until exhaustion measured in the shuttle walking test was 78.0 m further in the training group than the no‐training group (95% CI −144.9 to 301.0; 18 participants; very low‐certainty evidence; Analysis 6.6).
6.4. Analysis.
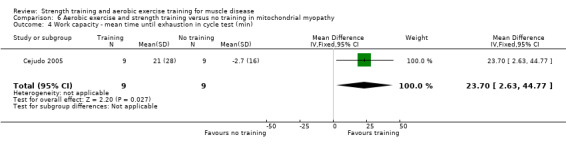
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 4 Work capacity ‐ mean time until exhaustion in cycle test (min).
6.5. Analysis.
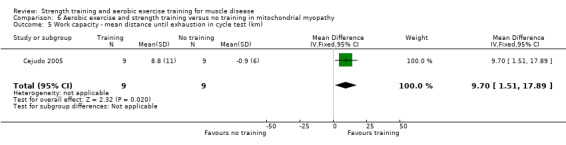
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 5 Work capacity ‐ mean distance until exhaustion in cycle test (km).
6.6. Analysis.
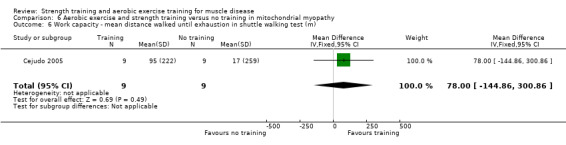
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 6 Work capacity ‐ mean distance walked until exhaustion in shuttle walking test (m).
Secondary outcome measures
Muscle strength, expressed in measures of endurance or fatigue
Cejudo 2005 did not provide data for this outcome.
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Cejudo 2005 noninvasively determined VO2 max in a maximal incremental cycle exercise test. After 12 weeks, the difference between the training and the no‐training group in mean VO2 max was very uncertain (MD 400 mL/min, 95% CI 62.0 to 862.0; 18 participants; very low‐certainty evidence; Analysis 6.7).
6.7. Analysis.
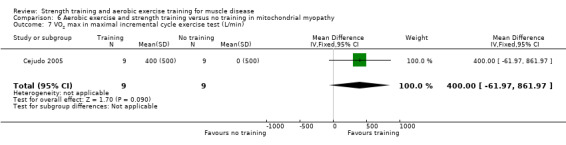
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 7 VO2 max in maximal incremental cycle exercise test (L/min).
Time‐scored functional assessments of muscle performance
Cejudo 2005 did not provide data for this outcome.
Quality‐of‐life measures
Cejudo 2005 used the Nottingham Health Profile (NHP) questionnaire to measure quality of life. Scores range from 0 (no problem) to 100 (maximum problem). The evidence was very uncertain (MD −9.8 points, 95% CI −25.7 to 6.1; 18 participants; very low‐certainty evidence; Analysis 6.8).
6.8. Analysis.
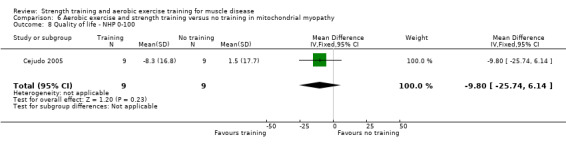
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 8 Quality of life ‐ NHP 0‐100.
Pain assessed by an analogue pain scale
Cejudo 2005 recorded participants' arm and leg myalgia by a simple questionnaire and scored it as mild, moderate or severe. Two of nine people in the exercise group and three of nine people in the control group reported severe myalgia in arms and legs. Seven of nine people in the exercise group and five of nine people in the control group reported moderate myalgia in arms and legs. After the 12‐week training programme, no participants of nine in the exercise group and five of nine participants in the control group still reported symptoms of myalgia (very low‐certainty evidence).
Experienced fatigue
Cejudo 2005 recorded participants' usual fatigability in a simple questionnaire and scored as mild, moderate or severe. Three of nine participants in the exercise group and five of nine participants in the control group reported severe fatigue in arms and legs. At the end of the study period, none of the nine participants in the exercise group and five of the nine participants in the control group reported severe fatigue in arms or legs. Six of nine participants in the exercise group and two of nine participants in the control group reported moderate fatigue. After the intervention period, five of the nine participants in the exercise group and two of the nine participants in the control group still reported moderate fatigue (very low‐certainty evidence).
Parameters of muscle membrane permeability
The study authors stated that the participants' serum creatine kinase (CK) levels remained unaltered after the intervention period (Cejudo 2005). However, they did not publish data for the serum CK level. Participants cancelled exercise sessions because of muscle soreness associated with the exercise activity. However, every participant was able to tolerate the exercise training regimen without complications.
Adverse effects requiring withdrawal of the participant from the study
In Cejudo 2005, participants related cramps to exercise, accompanying the feeling of fatigue. After the 12‐week training programme, symptoms were generally less severe or had disappeared.
There were no adverse events leading to withdrawal.
Combined aerobic exercise and strength training versus no training in myotonic dystrophy type 1
We included one study with 35 participants in this comparison (Kierkegaard 2011). See: Table 7.
The certainty of evidence for outcomes from Kierkegaard 2011 was very low, downgraded by two levels for imprecision: no power analysis was performed before the start of the study, and one level for study limitations, as blinding was not possible.
Primary outcome measure: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Kierkegaard 2011 did not provide data for this outcome.
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
Kierkegaard 2011 did not provide data for this outcome.
Secondary outcome measures
Muscle strength, expressed in measures of endurance or fatigue
Kierkegaard 2011 did not provide data for this outcome.
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Kierkegaard 2011 did not provide data for this outcome.
Time‐scored functional assessments of muscle performance
The primary outcome in Kierkegaard 2011 was the distance walked in the six‐minute walk test. A difference above or equal to 6% in distance walked between the baseline measurement and the measurement after the intervention period of 14 weeks was considered as a minimally clinically important change. After 14 weeks, the distance walked on the six‐minute walk test after aerobic exercise and strength training was slightly longer than in the no‐training group (MD 11.0 m, 95% CI −66.9 to 88.9; 35 participants; very low‐certainty evidence for study limitations and very serious imprecision; Analysis 7.1).
7.1. Analysis.
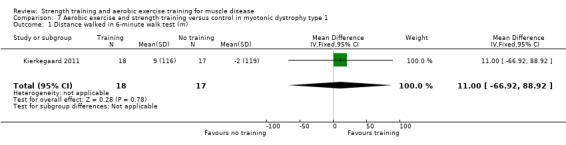
Comparison 7 Aerobic exercise and strength training versus control in myotonic dystrophy type 1, Outcome 1 Distance walked in 6‐minute walk test (m).
Aerobic exercise and strength training had no clear effect on the timed‐stands test (MD −1.00, 95% CI −6.76 to 4.76; 35 participants; very low‐certainty evidence; Analysis 7.2), and the timed‐up‐and‐go test (MD −0.50, 95% CI −1.86 to 0.86; 35 participants; very low‐certainty evidence; Analysis 7.3) reported after 14 weeks, compared to no training (Kierkegaard 2011).
7.2. Analysis.
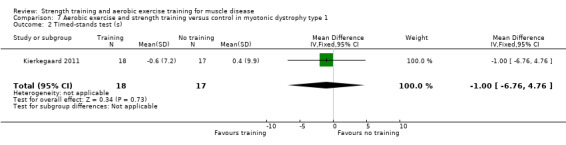
Comparison 7 Aerobic exercise and strength training versus control in myotonic dystrophy type 1, Outcome 2 Timed‐stands test (s).
7.3. Analysis.
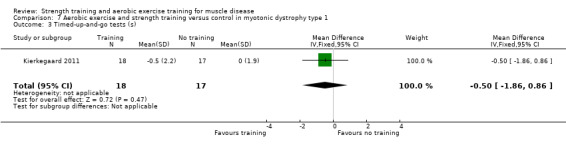
Comparison 7 Aerobic exercise and strength training versus control in myotonic dystrophy type 1, Outcome 3 Timed‐up‐and‐go tests (s).
Quality‐of‐life measures
Kierkegaard 2011 measured quality of life by the SF‐36 Health Survey. All SF‐36 scales range from 0 to 100 (optimal health). After 14 weeks, the scores on all subscales of the SF‐36 did not demonstrate relevant changes from baseline in either the training or non‐training group (35 participants; very low‐certainty evidence).
Pain assessed by an analogue pain scale
Kierkegaard 2011 did not provide data for this outcome.
Experienced fatigue
Kierkegaard 2011 did not provide data for this outcome.
Parameters of muscle membrane permeability
Kierkegaard 2011 did not provide data for this outcome.
Adverse effects requiring withdrawal of the participant from the study
In Kierkegaard 2011, one person had periods of atrial arrhythmia; however, this was not in connection with the training and the participant was allowed by a cardiologist to complete the study. No other adverse effects were reported and none leading to withdrawal.
Combined aerobic exercise and strength training versus no training in dermatomyositis and polymyositis
We included two studies with 23 and 19 participants respectively in this comparison (Munters 2013; Alexanderson 2014). See: Table 8 and Table 14.
4. Aerobic exercise and strength training compared to control for dermatomyositis and polymyositis: GRADE assessments for other quality of life measures (supplementary to 'Summary of findings' table 8).
| Aerobic exercise and strength training compared to control for dermatomyositis and polymyositis | |||||
| Patient or population: people with dermatomyositis and polymyositis Setting: at home and at the department of physical therapy of 3 participating hospitals Intervention: aerobic exercise and strength training Comparison: control without aerobic exercise and strength training | |||||
| Outcomes | Mean (SD) without aerobic exercise and strength training | Mean (SD) with aerobic exercise and strength training | Difference (95% CI) | Certainty of the evidence (GRADE) | What happens |
|
Quality of life Assessed with: SF‐36 Physical Function Scale from 0 to 100 (where 100 is optimal) and NHP ‐ Physical Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up (mean): 18 weeks 40 participants (2 RCTs) |
Quality of life (SF‐36 Physical Function) improved on average 1.5 SDs (0.8 higher to 2.2 higher) in the aerobic exercise and strength training group than in the group without training | SMD 1.5 higher (0.8 higher to 2.2 higher) | ⊕⊝⊝⊝ Very lowa,b | The effect on quality of life‐physical function is uncertain | |
|
Quality of life Assessed with SF‐36 Vitality Scale from 0 to 100 (where 100 is optimal) Follow‐up: mean 24 weeks 21 participants (1 RCT) |
The mean change in SF‐36 Vitality score without aerobic exercise and strength training was a decrease of 0.5 (5.1) | The mean change in SF‐36 vitality score with aerobic exercise and strength training was an increase of 11.8 (4.6) | MD 12.3 higher (8.2 higher to 16.5 higher) | ⊕⊝⊝⊝ Very lowa,c | May improve quality of life‐vitality |
|
Quality of life Assessed with SF‐36 Physical Function Scale from 0 to 100 (where 100 is optimal) Follow‐up: mean 12 weeks 21 participants (1 RCT) |
The mean change in SF‐36 Physical Function score without aerobic exercise and strength training was −0.2 (6.6) | The mean change in SF‐36 Physical Function score with aerobic exercise and strength training was 8.9 (3.2) | MD 1.5 higher (0.8 higher to 2.2 higher) | ⊕⊝⊝⊝ Very lowa,c | May have little or no effect on quality of life‐physical function |
|
Quality of life Assessed with SF‐36 Mental Health Scale from 0 to 100 (where 100 is optimal) Follow‐up: mean 24 weeks 21 participants (1 RCT) |
The mean change in SF‐36 Mental Health score without aerobic exercise and strength training was an increase of 3.1 (4.1) | The mean change in SF‐36 Mental Health score with aerobic exercise and strength training was an increase of 8.1 (3.7) | MD 5.0 higher (1.7 higher to 8.4 higher) | ⊕⊝⊝⊝ Very lowa,c | May improve quality of life‐mental health slightly |
|
Quality of life Assessed with NHP‐Energy Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in NHP‐Energy score without aerobic exercise and strength training was −3.1 (23.9) | The mean change in NHP‐Energy score with aerobic exercise and strength training was 21.1 (37.3) | MD 18.0 lower (45.9 lower to 9.9 higher) | ⊕⊝⊝⊝ Very lowa,c | The effect on quality of life‐energy is uncertain |
|
Quality of life Assessed with NHP‐Pain Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in NHP‐Pain score without aerobic exercise and strength training was −4.3 (0.1) | The mean change in NHP‐Pain score with aerobic exercise and strength training was a decrease of 7.4 (14.7) | MD 3.1 lower (12.22 lower to 6.0 higher) | ⊕⊝⊝⊝ Very lowa,c | The effect on quality of life‐pain is uncertain |
|
Quality of life Assessed with NHP‐Sleep Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in NHP‐Sleep score without aerobic exercise and strength training was −13.7 (6.1) | The mean change in NHP‐Sleep score with aerobic exercise and strength training was a decrease of 6.4 (27.1) | MD 7.3 higher (10.0 lower to 24.6 higher) | ⊕⊝⊝⊝ Very lowb,c | The effect on quality of life‐sleep is uncertain |
|
Quality of life Assessed with NHP‐Social Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in NHP‐Social score without aerobic exercise and strength training was −5.4 (0.1) | The mean change in NHP‐Social score with aerobic exercise and strength training was a decrease of 4.3 (25.0) | MD 1.1 higher (14.4 lower to 16.6 higher) | ⊕⊝⊝⊝ Very lowa,c | The effect on quality of life‐social is uncertain |
|
Quality of life Assessed with NHP‐Emotional Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in NHP‐Emotional score without aerobic exercise and strength training was −7.1 (9.7) | The mean change in NHP‐Emotional score with aerobic exercise and strength training was an increase of 29.1 (29.4) | MD 22.3 lower (41.4 lower to 3.2 lower) | ⊕⊝⊝⊝ Very lowa,c | The effect on quality of life‐emotional is uncertain |
|
Quality of life Assessed with NHP‐Physical Scale from 0 to 100 (where 0 is no perceived problems and 100 is maximum problems) Follow‐up: mean 24 weeks 19 participants (1 RCT) |
The mean change in NHP‐Physical score without aerobic exercise and strength training was a decrease of 8.5 (1.7) | The mean change in NHP‐ Physical score with aerobic exercise and strength training was a decrease of 10.3 (0.9) | MD 1.8 lower (3.0 lower to 0.6 lower) | ⊕⊝⊝⊝ Very lowa,c | The effect on quality of life‐physical is uncertain |
| 5RM: 5 voluntary repetitions; CI: confidence interval; MD: mean difference; MMT‐8: manual muscle testing of eight muscle groups; NHP: Nottingham Health Profile; RCT: randomised controlled trial SD: standard deviation; SMD: standardised mean difference; SF‐36: Short Form 36 Health Survey | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded two levels for study limitations. In one trial, quote: "One patient in the exercise group was not able to perform the exercise programme and was excluded from the analysis". Follow‐up was therefore incomplete and analysis was not by intention‐to‐treat. Quote: "We aimed for nine patients in the exercise group, but some analyses were performed with N = 7 (VO2 max measurements) or N = 3 (mitochondrial enzyme activities). (...). "All measurements were not successfully performed both before and after training in each subject". Blinding of participants and personnel was not possible in either trial. bDowngraded one level for imprecision: small samples. cDowngraded two levels for serious imprecision: Quote: "An important limitation is the lack of power analysis and the low number of patients, conditions that may explain lack of significant between‐group differences, with frequent dropouts further hampering the analyses and conclusion.(...). Possible indirectness: the exercise intensity level was defined only for the aerobic walks, not for the resistive home exercise programme".
The overall certainty of evidence for all outcomes in Munters 2013 is low: we downgraded the certainty by two levels for study limitations (see Characteristics of included studies) and by one level for imprecision.
The overall certainty of evidence for outcomes reported from Alexanderson 2014 is very low for serious imprecision: there was no power analysis, with a sample size of 19.
Primary outcome measure: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Munters 2013 performed manual muscle testing of eight muscle groups: the MMT‐8 (maximal isometric strength of neck flexors, middle deltoid, gluteus maximus, gluteus medius, biceps brachii, wrist extensors, wrist flexors, ankle dorsiflexors, on a scale from 0 (no movement) to 80 (normal)). After 12 weeks, the aerobic exercise and strength training had no clear effect on the MMT‐8 score (MD 1.0, 95% CI −1.1 to 3.0; 21 participants; very low‐certainty evidence for very serious imprecision; Analysis 8.1). In addition, they assessed the maximum load a participant can lift in a full range of motion in five repetitions (5RM) for the left and right knee extensor. After 12 weeks, aerobic exercise and strength training slightly increased muscle strength (kg) of the right knee extensors (MD 2.5, 95% CI 1.8 to 3.3; 21 participants; very low‐certainty evidence; Analysis 8.2) and left knee extensors (MD 2.7, 95% CI 2.0 to 3.4; 21 participants; very low‐certainty evidence; Analysis 8.3).
8.1. Analysis.
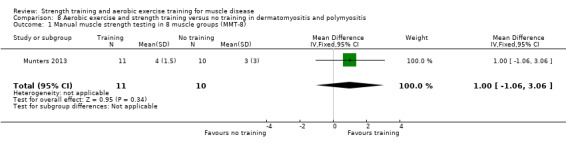
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 1 Manual muscle strength testing in 8 muscle groups (MMT‐8).
8.2. Analysis.
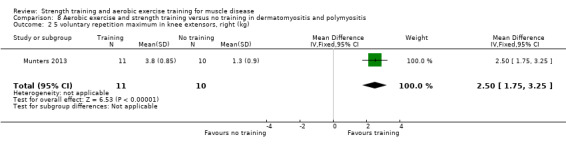
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 2 5 voluntary repetition maximum in knee extensors, right (kg).
8.3. Analysis.
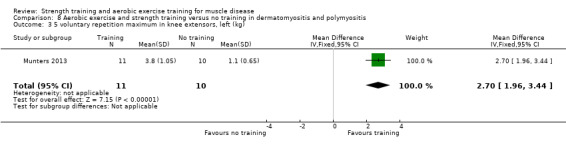
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 3 5 voluntary repetition maximum in knee extensors, left (kg).
Alexanderson 2014 did not provide data for this outcome.
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
Munters 2013 defined aerobic capacity as the power performed in Watts (W) at the time VO2 max was recorded in an ergometer cycle incremental maximal exercise test. In addition, during a cycling endurance test, performed at a power requiring 65% of the VO2 max obtained in the maximal exercise test, the time from the start to exhaustion was determined. After 12 weeks of training, aerobic exercise and strength training may slightly increase aerobic capacity (MD 18.0 W, 95% CI 15.0 to 21.0; 21 participants; very low‐certainty evidence; Analysis 8.4) and time to exhaustion in the endurance cycling test (MD 17.5 min, 95% CI 8.0 to 27.0; 15 participants; very low‐certainty evidence; Analysis 8.5).
8.4. Analysis.
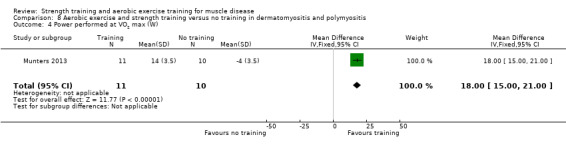
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 4 Power performed at VO2 max (W).
8.5. Analysis.
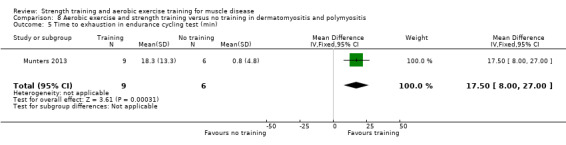
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 5 Time to exhaustion in endurance cycling test (min).
Alexanderson 2014 did not provide data for this outcome.
Secondary outcome measures
Muscle strength, expressed in measures of endurance or fatigue
Munters 2013 and Alexanderson 2014 did not provide data for this outcome.
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Munters 2013 measured VO2 max during an exhaustion incremental test on a cycle ergometer, defined as the highest O2 uptake rate measured during the test. After 12 weeks, aerobic exercise and strength training had no clear effect on VO2 max compared to no training (21 participants; low‐certainty evidence; Analysis 8.6). Alexanderson 2014 noninvasively estimated aerobic capacity using an eight‐minute submaximal treadmill test. After 24 weeks, there was no clear difference in change in VO2 max between the training and no‐training groups (19 participants; low‐certainty evidence; Analysis 8.6). The pooled data indicated that training slightly improved aerobic capacity, but the result was very imprecise (SMD 0.27, 95% CI −0.35 to 0.90; I2 = 0%, P = 0.50; 2 RCTs; 40 participants; very low‐certainty evidence; Analysis 8.6). Based on rule‐of‐thumb interpretation, an SMD of 0.2 represents a small effect.
8.6. Analysis.
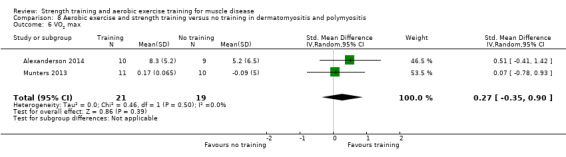
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 6 VO2 max.
Time‐scored functional assessments of muscle performance
Alexanderson 2014 used the disease‐specific Functional Index (FI) as a primary outcome to assess muscle performance. The FI includes testing of repetitions in 11 muscle groups: elbow flexion, shoulder flexion and abduction, hip flexion and abduction, step test, heel and toe lifts, neck flexion and trunk flexion, with additional tests of ability to transfer from side to side lying down, transfer up to sitting, and peak expiratory flow. After 24 weeks, the effect of aerobic exercise and strength training on muscle performance was uncertain (MD 5.5, 95% CI −2.91 to 13.91; 19 participants; very low‐certainty evidence; Analysis 8.7).
8.7. Analysis.
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 7 Disease‐specific functional index 0‐64.
Munters 2013 did not provide data for this outcome.
Quality‐of‐life measures
Alexanderson 2014 measured quality of life with the NHP questionnaire (range of possible scores from 0 (no perceived problems) to 100 (maximum problems)). After 24 weeks, the NHP Emotional domain score favoured training over no training (MD −22.3, 95% CI −41.4 to −3.2; 21 participants; low‐certainty evidence; Analysis 8.16). The NHP Physical domain score slightly favoured training over no training (MD −1.8, 95% CI −3.0 to −0.6; 19 participants; very low‐certainty evidence; Analysis 8.17). Results for other subdomains of the NHP were very imprecise: Energy (MD −18.0, 95% CI −45.9 to 9.9; Analysis 8.12); Pain (MD −3.10, 95% CI −12.2 to 6.0; Analysis 8.13); Sleep (MD 7.3, 95% CI −10.0 to 24.6; Analysis 8.14); and Social (MD 1.1, 95% CI −14.4 to 16.6; Analysis 8.15). All data were from 19 participants and the evidence was very low certainty.
8.16. Analysis.
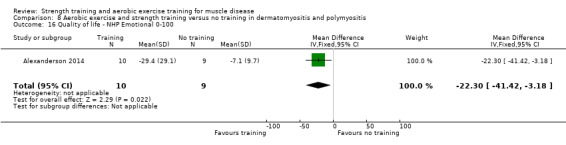
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 16 Quality of life ‐ NHP Emotional 0‐100.
8.17. Analysis.
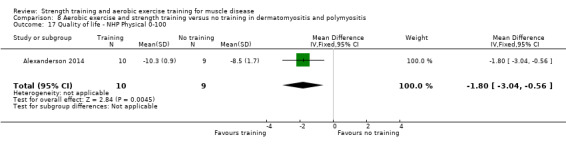
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 17 Quality of life ‐ NHP Physical 0‐100.
8.12. Analysis.
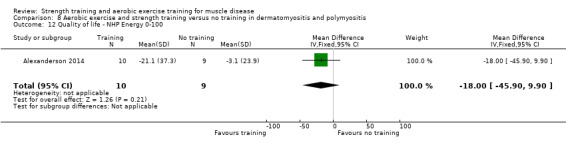
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 12 Quality of life ‐ NHP Energy 0‐100.
8.13. Analysis.
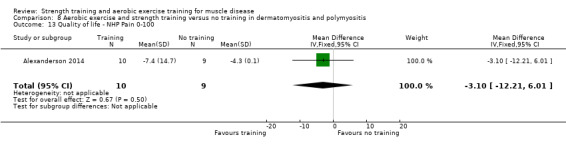
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 13 Quality of life ‐ NHP Pain 0‐100.
8.14. Analysis.
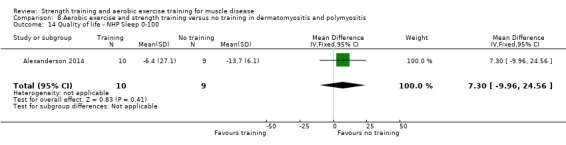
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 14 Quality of life ‐ NHP Sleep 0‐100.
8.15. Analysis.
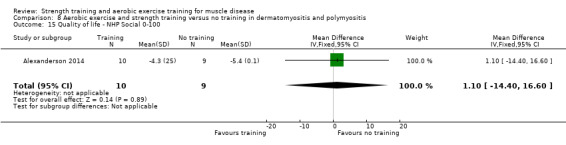
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 15 Quality of life ‐ NHP Social 0‐100.
Munters 2013 measured quality of life by the SF‐36 Health Survey. All SF‐36 scales range from 0 to 100 (optimal health). Twelve weeks of combined strength and aerobic exercise training improved quality of life, assessed by the SF‐36 General Health scale (MD 9.5 points, 95% CI 5.5 to 13.5; 19 participants; low‐certainty evidence; Analysis 8.9) and the subscales Mental Health (MD 5.0 points, 95% CI 1.7 to 8.4; 21 participants; low‐certainty evidence; Analysis 8.11) and Vitality (MD 12.3 points, 95% CI 8.2 to 16.5; 21 participants; low‐certainty evidence; Analysis 8.10) compared to the no‐training group.
8.9. Analysis.
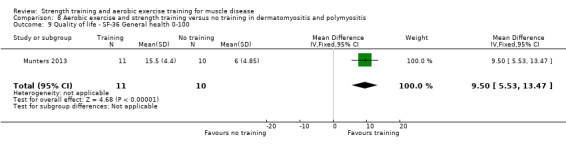
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 9 Quality of life ‐ SF‐36 General health 0‐100.
8.11. Analysis.
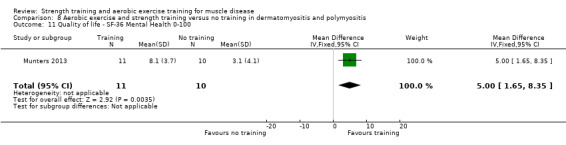
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 11 Quality of life ‐ SF‐36 Mental Health 0‐100.
8.10. Analysis.
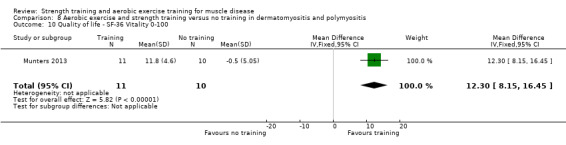
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 10 Quality of life ‐ SF‐36 Vitality 0‐100.
When data from Alexanderson 2014 and Munters 2013 were pooled, they indicated that the effect of combined aerobic and strength training on the physical domain of quality‐of‐life measures in dermatomyositis and polymyositis was uncertain (SMD 1.50 95% CI 0.78 to 2.22; I2 = 0%, P = 0.55; 2 studies, 40 participants, very low‐certainty evidence; Analysis 8.8). We downgraded the certainty of evidence three times: once for study limitations (see Characteristics of included studies) and twice for imprecision.
8.8. Analysis.
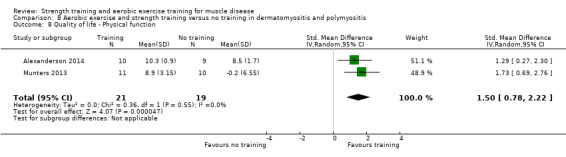
Comparison 8 Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis, Outcome 8 Quality of life ‐ Physical function.
Pain assessed by an analogue pain scale
Munters 2013 and Alexanderson 2014 did not provide data for this outcome.
Experienced fatigue
Munters 2013 and Alexanderson 2014 did not provide data for this outcome.
Parameters of muscle membrane permeability
Alexanderson 2014 measured disease by analysis of CK levels. After 24 weeks, both groups had similar CK levels.
Munters 2013 did not provide data for this outcome.
Adverse effects requiring withdrawal of the participant from the study
Munters 2013 found no decline in strength measures. There were no adverse events leading to withdrawal.
In Alexanderson 2014, participants did not report side effects of exercise, other than short‐term muscle soreness, especially in the beginning, and shortly after increasing the exercise loads. After 24 weeks of aerobic exercise and strength training, no participants had inflammatory infiltrates.
We considered the adverse effects evidence to be low certainty. We downgraded the evidence twice, for serious imprecision, as the combined data were from 40 participants, and for study limitations, because the studies were at high risk of bias.
Combined aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy (FSHD)
We included one study with 16 participants in this comparison (Bankolé 2016). See: Table 9.
The overall certainty of evidence for outcomes reported in Bankolé 2016 is very low; we downgraded the evidence twice for study limitations (see Characteristics of included studies), and twice for imprecision as the number of participants in the study was smaller than the calculated sample size. Therefore, the effects of aerobic exercise and strength training in FSHD for all the outcomes below, based on this study, are uncertain.
Primary outcome measure: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Bankolé 2016 measured isometric maximal quadriceps strength with femoral nerve magnetic stimuli delivered during isometric maximum voluntary contractions (MVCs) and at rest. After 24 weeks, the effect of aerobic exercise and strength training on change in strength was uncertain (MD 15.0 N, 95% CI −27.8 to 57.8; 16 participants; very low‐certainty evidence; Analysis 9.1).
9.1. Analysis.
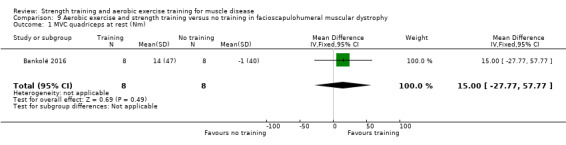
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 1 MVC quadriceps at rest (Nm).
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
Bankolé 2016 measured maximal aerobic power (MAP) in an incremental cycling test. After 24 weeks, effect of aerobic exercise and strength training on change in MAP between groups was uncertain (MD 45.0 W, 95% CI −20.5 to 110.5; 16 participants; very low‐certainty evidence; Analysis 9.3).
9.3. Analysis.
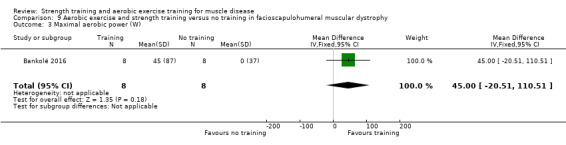
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 3 Maximal aerobic power (W).
Secondary outcome measures
Muscle strength, expressed in measures of endurance or fatigue
Bankolé 2016 assessed quadriceps neuromuscular function (strength, fatigability, endurance) by the quadriceps intermittent fatigue test, which was composed of isometric MVCs with femoral nerve magnetic stimuli delivered during MVCs and at rest. After six months of training, voluntary activation at rest did not clearly differ between the training and non‐training group (MD −0.5, 95% CI −2.62 to 1.62; 16 participants; very low‐certainty evidence; Analysis 9.2). The number of repetitions (muscle endurance) during the test increased statistically significantly in the training group, compared to the control group (MD 12.0, 95% CI 0.8 to 23.2; 16 participants; very low‐certainty evidence; Analysis 9.4).
9.2. Analysis.
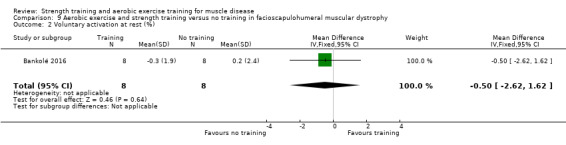
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 2 Voluntary activation at rest (%).
9.4. Analysis.
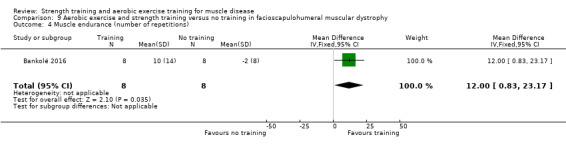
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 4 Muscle endurance (number of repetitions).
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Bankolé 2016 assessed VO2 peak using an incremental cycling test. After the training period of 24 weeks, the change in VO2 peak was higher in the exercise than the no‐training group, but this effect was uncertain (MD 12.4 mL/min/kg, 95% CI 2.2 to 22.6; 16 participants; very low‐certainty evidence; Analysis 9.5).
9.5. Analysis.
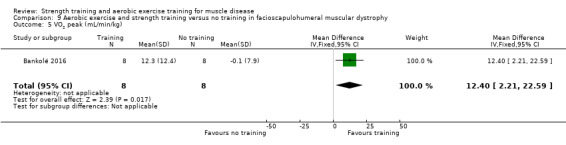
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 5 VO2 peak (mL/min/kg).
Time‐scored functional assessments of muscle performance
Bankolé 2016 determined the distance walked in a six‐minute walk test. The distance walked (m) was greater in the training than the no‐training group but the data were highly imprecise and the evidence therefore uncertain (MD 64.0, 95% CI −50.9 to 178.9; 16 participants; very low‐certainty evidence; Analysis 9.6).
9.6. Analysis.
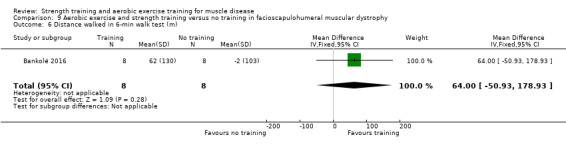
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 6 Distance walked in 6‐min walk test (m).
Quality‐of‐life measures
Bankolé 2016 measured quality of life by the SF‐36 General Health Survey, in which scales range from 0 to 100 (optimal health). The scores on all subscales of the SF‐36 did not demonstrate any changes for either the training or non‐training group (16 participants; very low‐certainty evidence; Analysis 9.7).
9.7. Analysis.
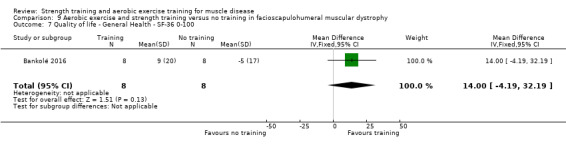
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 7 Quality of life ‐ General Health ‐ SF‐36 0‐100.
Pain assessed by an analogue pain scale
Bankolé 2016 did not provide data for this outcome.
Experienced fatigue
Bankolé 2016 measured fatigue by the Fatigue Severity Scale (FSS). Scores ranged from 9 (no fatigue) to 63 (worst fatigue). After 24 weeks, there was a decrease in fatigue score, in favour of the training group (MD −15.0 (95% CI −27.9 to −2.1; 16 participants; very low‐certainty evidence; Analysis 9.8).
9.8. Analysis.
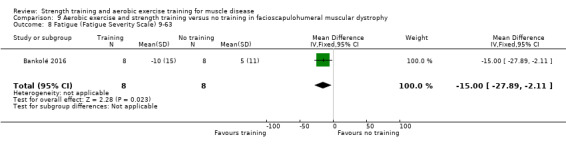
Comparison 9 Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy, Outcome 8 Fatigue (Fatigue Severity Scale) 9‐63.
Parameters of muscle membrane permeability
Serum CK concentrations 24 hours after maximal cycling and quadriceps intermittent fatigue tests were less than 1000 IU‐L‐1 for all groups (Bankolé 2016).
Adverse effects requiring withdrawal of the participant from the study
There were no adverse events leading to withdrawal in Bankolé 2016.
Combined aerobic exercise and strength training versus no training in juvenile dermatomyositis
We included one study with 26 participants in this comparison (Habers 2016). See: Table 10.
The overall certainty of evidence for outcomes from Habers 2016 is low, downgraded once for imprecision and once for study limitations (as blinding was not possible).
Primary outcome measure: muscle strength, expressed in measures of static (i.e. isometric) or dynamic strength
Habers 2016 measured maximal isometric muscle strength (MVIC) of the proximal muscle groups in the lower extremities on both the right and the left sides with a hand‐held dynamometer. After 12 weeks, the change in strength of the right and left knee extensors was better in the training group than the no‐training group: right knee extensors MD 36.0 N (95% CI 24.95 to 47.05; Analysis 10.1); left knee extensors MD 17.0 N (95% CI 0.5 to 33.5; Analysis 10.2). Both analyses had 26 participants and the evidence was low certainty. The mean changes from baseline in maximal force of right and left hip flexors showed no clinically important differences between the training group and the no‐training group: right hip flexors MD −9.0 N (95% CI −22.4 to 4.4; Analysis 10.3); and left hip flexors MD 6.0 N (95% CI −6.6 to 18.6; Analysis 10.4). Both analyses had 26 participants and the evidence was low certainty.
10.1. Analysis.
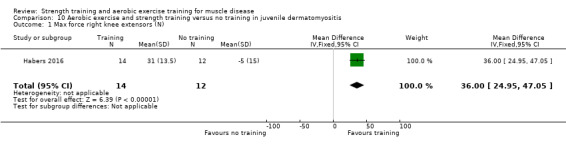
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 1 Max force right knee extensors (N).
10.2. Analysis.
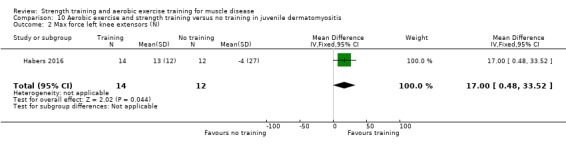
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 2 Max force left knee extensors (N).
10.3. Analysis.
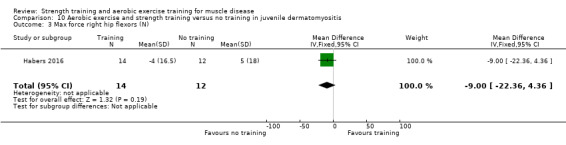
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 3 Max force right hip flexors (N).
10.4. Analysis.
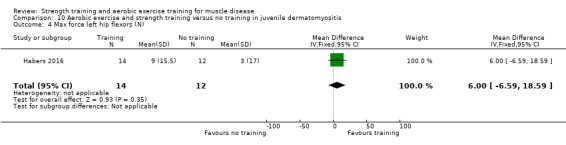
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 4 Max force left hip flexors (N).
Primary outcome measure: aerobic capacity, expressed in measures of (physical) work capacity
Habers 2016 defined aerobic capacity as endurance time: the time from the start to the end of a treadmill‐based incremental maximal exercise test. After 12 weeks of training, the change in mean endurance time between groups slightly favoured the control group (MD −1.2 min, 95% CI − 1.6 to −0.9; 26 participants, low‐certainty evidence; Analysis 10.5). According to the study authors, they found higher average baseline values in the training group than the control group for several outcome measures (e.g. aerobic fitness, perception of fatigue, quality of life, and functional ability). This reduced the possibility for improvement in the training group compared with the control group, thus decreasing the opportunity to measure significant benefits from training.
10.5. Analysis.
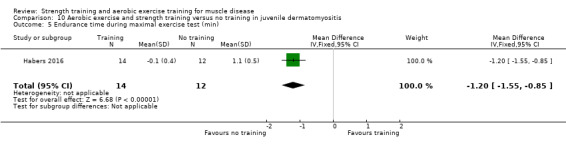
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 5 Endurance time during maximal exercise test (min).
Secondary outcome measures
Muscle strength, expressed in measures of endurance or fatigue
Habers 2016 did not provide data for this outcome.
Aerobic capacity, expressed in measures of oxygen uptake (i.e. VO2 max)
Habers 2016 measured VO2 peak with a treadmill‐based incremental maximal exercise test. After 12 weeks of training, the change in mean VO2 peak was slightly lower in the exercise group (MD −2.1 mL/kg/min, 95% CI −3.3 to −0.9; 26 participants, low‐certainty evidence; Analysis 10.6).
10.6. Analysis.
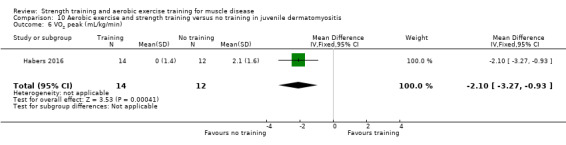
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 6 VO2 peak (mL/kg/min).
Time‐scored functional assessments of muscle performance
Habers 2016 assessed muscle function via Subscale 8 (Strength) of the Bruininks‐Osteretsky Test of Motor Proficiency, Second Edition, which includes five items that are related to the strength training programme of the participant. After 12 weeks of training, the MD in time wall sit in the training group was slightly lower (worse) than in the no‐training group (MD −3.00 s. 95% CI −5.5 to 0.5; 26 participants, low‐certainty evidence; (Analysis 10.10) and time V‐up was also lower (worse) in the training group than in the no‐training group (MD −10.0 s, 95% CI −12.7 to −7.3; 26 participants, low‐certainty evidence; Analysis 10.11). However, the results of other functional assessments favoured the training group over the no‐training group: distance of standing long jump (MD 18.0 cm, 95% CI 15.3 to 20.7; 26 participants, low‐certainty evidence; Analysis 10.7), number of push‐ups in 30 seconds (MD 5.0, 95% CI 3.6 to 6.4; 26 participants, low‐certainty evidence; Analysis 10.8) and number of sit‐ups in 30 seconds (MD 3.0, 95% CI 2.0 to 4.0; 26 participants, low‐certainty evidence; Analysis 10.9). The distance walked in a six‐minute walk test showed no clear difference between groups after 12 weeks (MD −7.0 m, 95% CI −21.6 to 7.6; 26 participants, low‐certainty evidence; Analysis 10.12).
10.10. Analysis.
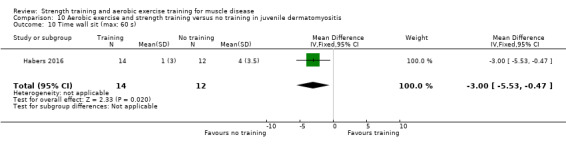
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 10 Time wall sit (max: 60 s).
10.11. Analysis.
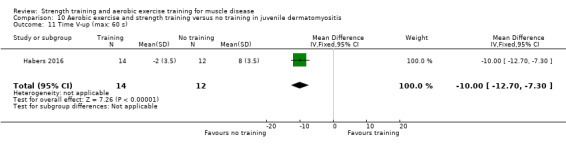
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 11 Time V‐up (max: 60 s).
10.7. Analysis.
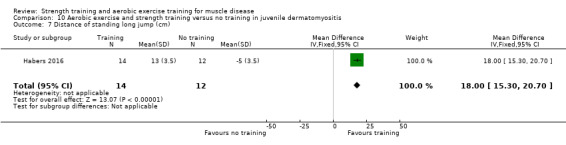
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 7 Distance of standing long jump (cm).
10.8. Analysis.
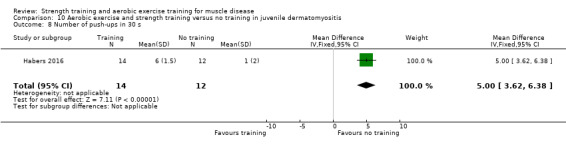
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 8 Number of push‐ups in 30 s.
10.9. Analysis.
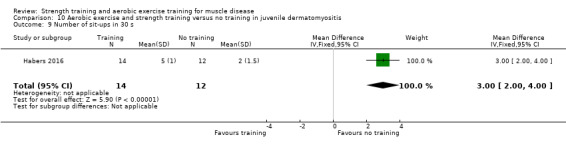
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 9 Number of sit‐ups in 30 s.
10.12. Analysis.
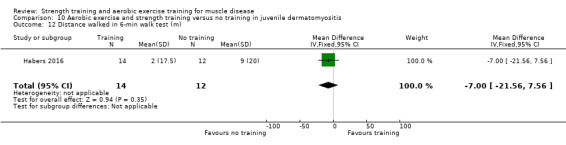
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 12 Distance walked in 6‐min walk test (m).
Quality‐of‐life measures
Habers 2016 measured quality of life with the patient form of the PedsQL Generic Core Scale. Scores ranged from 0 (no problem) to 100 (maximum problem). After 12 weeks, mean difference in total score was higher (better) in the training group than the no‐training group (MD 8.00, 95% CI 6.24 to 9.76; 26 participants, low‐certainty evidence; Analysis 10.13).
10.13. Analysis.
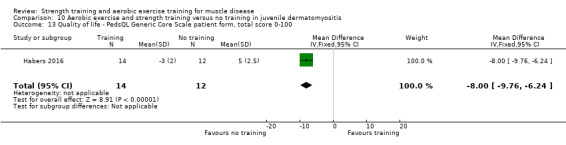
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 13 Quality of life ‐ PedsQL Generic Core Scale patient form, total score 0‐100.
Pain assessed by an analogue pain scale
Habers 2016 measured muscle pain on a 10‐cm VAS. After 12 weeks of training, the VAS score increased (mean 4.0 (SD 3.5) in the no‐training group and decreased (mean −3 (SD 3.5)) in the training group (MD −7.0 cm, 95% CI−9.7 to −4.3; 26 participants, low‐certainty evidence; Analysis 10.14).
10.14. Analysis.
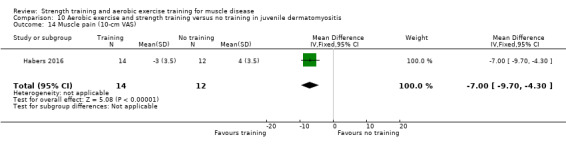
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 14 Muscle pain (10‐cm VAS).
Experienced fatigue
Habers 2016 measured perception of fatigue using the PedsQL Multidimensional Fatigue Scale (a scale from 0 to 100 on which higher scores indicate less fatigue). After 12 weeks of training, MD in PedsQL score were slightly lower (worse) in the training group (MD −5.0, 95% CI −6.5 to −3.5; 26 participants, low‐certainty evidence; Analysis 10.15).
10.15. Analysis.
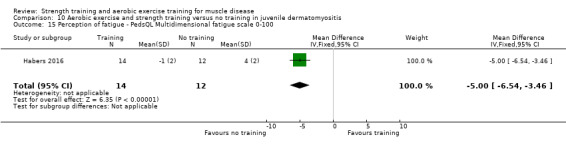
Comparison 10 Aerobic exercise and strength training versus no training in juvenile dermatomyositis, Outcome 15 Perception of fatigue ‐ PedsQL Multidimensional fatigue scale 0‐100.
Parameters of muscle membrane permeability
Habers 2016 did not provide data for this outcome.
Adverse effects requiring withdrawal of the participant from the study
Two participants experienced increasing complaints at their heel or knee, both after 16 training sessions (Habers 2016).
There were no adverse events leading to withdrawal.
Discussion
Summary of main results
In total, this review includes 14 studies regarding strength training aerobic exercise training, or both, in 428 people with muscle disease (Lindeman 1995; Wiesinger 1998a; Van der Kooi 2004; Cejudo 2005; Kierkegaard 2011; Aldehag 2013; Jansen 2013; Munters 2013; Alexanderson 2014; Voet 2014; Andersen 2015; Bankolé 2016; Habers 2016; Andersen 2017). Exercise duration ranged from eight to 52 weeks. One study reported heel and knee complaints in two participants with juvenile dermatomyositis but no other study found signs of overuse. In general, the studies found little or no evidence of efficacy for strength training but some studies reported benefit from strength training in combination with aerobic exercise training or aerobic exercise training alone. Although the number of exercise studies in muscle disease is slightly increasing, still, many studies are uncontrolled, of relatively short duration or with a low number of participants. Studies remain insufficient for subgroup analyses or evaluation of dose‐response relationships. Judging the clinical meaningfulness and effect size of the mean differences (MDs) was not straightforward, as there are no internationally agreed standards on which level of change is clinically meaningful or even which can be considered ’small’ or 'large' in size.
Strength training versus no training
In the last decade, only one RCT on strength training has been carried out (Aldehag 2013). There is very low‐certainty evidence of little or no effect on hand grip force, pinch grip force, isometric wrist flexor force and of a positive effect on isometric wrist extensor force in people with myotonic dystrophy type 1, compared with no training (Aldehag 2013). Compared with no training, there is low‐certainty evidence for little or no effect on dynamic and isometric strength of the elbow flexors and ankle dorsiflexors in people with FSHD (Van der Kooi 2004).
An absence of, or limited positive effects of strength training on muscle strength could reflect the inability of the diseased muscular system to respond with normal neural and trophic adaptations to the applied training stimuli. However, part of this lack of response could be due to the specificity or the intensity of the training (Lindeman 1995; Cup 2007). All adaptations to training are specific to the stimuli applied. Specific strength training essentially involves exercising the muscles in the same manner as the expected use (Kraemer 2002). This means that a training programme with dynamic exercises increases dynamic strength more than isometric strength, and vice versa. This phenomenon of specificity of training has implications for the sensitivity of the outcome measures; for example, the positive effect of a dynamic strength training programme may be captured by using a dynamic evaluation technique, but might be missed using an isometric strength measure. The size of the carry‐over effect from, for example, dynamic strength to isometric strength cannot be predicted and it may be that there is a diminished ability of the diseased muscular system to transfer effects of a specific training programme from one strength modality to another (Van der Kooi 2004).
In people with a muscle disease, it is assumed that absolute gain in muscle strength resulting from strength training is probably related to pre‐exercise muscle strength, and that severely weakened muscles (< 10% of normal strength) may not be able to improve. However, this widely reported assumption is based on one published observation only (Milner‐Brown 1988a). In the FSHD strength training study (Van der Kooi 2004), training did not influence strength of the ankle dorsiflexors, in contrast to the elbow flexors. The study authors thought that a difference in grade of muscle weakness at baseline between elbow and ankle dorsiflexors might provide the explanation for the difference in their response to training. In this study, elbow flexors were eligible for testing and training when strength according to the MRC scale grade was 3 or more, whereas ankle dorsiflexors were eligible when the muscles moved the ankle joint in a position between dorsiflexion and plantarflexion, which potentially includes MRC grades less than 3 (Medical Research Council 1981). Therefore, pre‐exercise weakness might have been more severe in ankle dorsiflexors compared with elbow flexors.
An exercise intervention should be of sufficient intensity and duration to provide a training stimulus. The strength training studies had an exercise duration of, in general, 30 minutes. Overall, in the aerobic exercise or the combined aerobic exercise and strength training studies, the exercise duration was longer (60 minutes). This could explain why some studies of strength training combined with aerobic exercise training measured an increase in muscle strength (Munters 2013; Habers 2016).
Aerobic exercise versus no training
Compared with no training, there is low‐certainty evidence that there may be a positive effect of aerobic exercise on aerobic capacity in FSHD (Voet 2014), little or no effect on quadriceps muscle strength (Voet 2014), a positive effect on distance walked in a six‐minute walk test (Voet 2014), a slightly positive effect on quality of life (Voet 2014), little or no effect on pain (Voet 2014), and a positive effect on fatigue (Voet 2014). The certainty of evidence for outcomes in all the other aerobic exercise studies is too low for results to be meaningful (Wiesinger 1998a; Jansen 2013; Andersen 2015; Andersen 2017).
Combined aerobic exercise and strength training versus no training
Compared with no training, there is very low‐certainty evidence that combined aerobic and strength training has little or no effect on maximal isometric strength of neck flexors, middle deltoid, gluteus maximus, gluteus medius, biceps brachii, wrist extensors, wrist flexors and ankle dorsiflexors, aerobic capacity, time to exhaustion and quality of life in people with dermatomyositis and polymyositis. It may increase muscle strength of right knee and left extensors slightly in people with dermatomyositis and polymyositis (Munters 2013). In juvenile dermatomyositis, combined aerobic exercise and strength training may increase dynamic muscle strength of right knee extensors and left knee extensors slightly, have little or no effect on change in dynamic muscle strength of the left hip flexors, decrease endurance time and aerobic capacity slightly, increase muscle performance, have little or no effect on distance walked in a six‐minute walk test, increase quality of life slightly, decrease pain level slightly, and may decrease experienced fatigue slightly (Habers 2016). The certainty of evidence for all the other aerobic exercise and strength training studies is too low for results to be meaningful (Cejudo 2005; Bankolé 2016; Kierkegaard 2011; Alexanderson 2014).
Adverse effects
In some studies, a few participants complained of muscle soreness and transient strength reduction (Lindeman 1995; Cejudo 2005; Habers 2016). All complaints resolved spontaneously. In Habers 2016, aerobic capacity, endurance time on the maximal exercise test, time wall‐sit and time V‐up were higher in the control group than in the training group. However, according to the study authors, higher average baseline values were found in the training group compared with the control group for several outcome measures (e.g. aerobic fitness, perception of fatigue, quality of life, and functional ability). This reduced the possibility for improvement in the training group as compared with the control group, thus decreasing the opportunity to detect significant benefits from training. In all other studies, scores in the control group were no better than in the training group, and we found no evidence for any decline in primary or secondary outcome measures. Most studies did not report adverse events leading to withdrawal. The included studies were small and the evidence was largely low or low certainty; therefore, we can make no definitive statements regarding safety.
Overall completeness and applicability of evidence
The number of recent studies lacking a randomised controlled design is striking. At least for the relatively common muscle diseases, one should aim for randomised controlled training studies. Preferably, homogeneous groups of people with the same muscle disease should be included. To facilitate meaningful comparisons among studies and statistical power by effective pooling of study results, more uniformity is needed in type of interventions, intensity of exercise therapy, and type of outcome measures. Because we cannot perform sensitivity analyses in this review, it is not possible to define the optimal exercise duration for people with a specific muscle disease.
We found no RCTs regarding strength training or aerobic exercise training in Becker muscular dystrophy or inclusion body myositis. Included studies were performed in Austria, Denmark, France, the Netherlands, Spain, and Sweden, which represent only Western European countries. In five studies, participants exercised at home, in five studies in a hospital or rehabilitation centre, and in four studies with an alternating frequency at home or in a rehabilitation centre or hospital, which suggests easy generalisability. In all studies, exercise was supervised. Clinical experience shows that a high compliance with exercise training is usually achieved as long as the participant’s exercise is supervised, but when the individual is to continue to exercise on his or her own, compliance decreases. Only two studies had a long‐term follow‐up without supervised exercise (Alexanderson 2014; Voet 2014). In the aerobic exercise in FSHD study (Voet 2014), more than 70% of participants continued exercising. In the combined aerobic exercise and strength training study in dermatomyositis and polymyositis (Alexanderson 2014), five of eight participants in the exercise group kept exercising one to three times per week throughout the rest of the study. All participants kept their frequent walking habits. Future studies should focus on the long‐term benefits of regular exercise training, on developing beneficial exercise and behavioural modification interventions with high compliance (also following the intervention), and include a long‐term follow‐up after the intervention. Strength training and aerobic exercise training are already available worldwide for people with a muscle disease, and many existing treatment programmes and guidelines for people with a muscle disease already include exercise training. However, evidence regarding the optimal exercise programme is still lacking. The most effective dose of exercise for people with muscle diseases is currently unknown, making it difficult to prescribe exercise in this population. This is reflected in the large variation in the frequency, duration and intensity of exercise prescribed.
As most aerobic exercise studies define aerobic capacity instead of work capacity as the primary outcome measure, we intend to change the primary outcome measure for aerobic exercise training and the combination of aerobic exercise and strength training from work capacity into aerobic capacity in the next update.
Certainty of the evidence
The risk of bias differed widely between the included studies. Blinding of participants and personnel was not possible in any study, because of the nature of the intervention, which placed all studies at high risk of performance bias. The risk of bias for the Andersen 2015 and Andersen 2017 aerobic exercise training studies in FSHD was high for most items. Seven other studies were at high risk of bias in at least one additional domain, and the risk of bias in the other included training studies was unclear or low.
The most prominent issue with regards to validity of evidence from these exercise and physical activity intervention studies is the sample sizes used. Three studies were underpowered (Aldehag 2013; Bankolé 2016; Andersen 2017), and three studies did not use a power analysis (Cejudo 2005; Kierkegaard 2011; Jansen 2013). Overall, the number of participants in the studies was low: between nine and 65 participants. Recruiting large samples in rarer diseases, such as muscle diseases, is challenging. Small studies are known to overestimate the treatment effect by up to 32% in comparison with larger studies (Deschartes 2013). We note the occurrence of a large number of results that lack precision and demonstrate neither an effect or lack of effect with any certainty. The most reasonable explanation for this finding is the low number of included participants. However, because of the low prevalence of all muscle diseases, studies with a sample size of at least 100 participants are not expected, unless multinational studies are undertaken.
Exercise adherence is an important contributor to the efficacy of exercise. Non‐adherence to an exercise plan is an ever‐present threat to the validity and outcome of any intervention study. Exercise adherence and compliance should be documented to further understand the dose‐response relationship between exercise and outcome measures. Some studies reported a high dropout rate (Aldehag 2013; Alexanderson 2014; Andersen 2015; Habers 2016), and a low adherence (Aldehag 2013; Voet 2014; Andersen 2015).
Lack of blinding of participants and outcome assessors was a prevalent risk of bias. Blinding of participants in exercise studies is not possible, however blinding of outcome assessors is strongly recommended. Moreover, participants in an active training group may experience additional non‐specific benefits (that is, Hawthorne effects), for instance from regular interaction with a skilled therapist, in contrast to those in a non‐treatment or usual care group. As it is well known that such Hawthorne effects may affect outcome (Parsons 1974), future studies should preferably have an appropriate control intervention rather than 'no training' in order to assess the specific benefits of aerobic exercise and strength training. For example, the control group might receive weekly counselling sessions with general information about exercise.
Minimum clinically important differences (MCIDs) for outcomes have only been reported in the literature for people with DMD, not for any other muscle disease (Henricson 2013). However, as all muscle diseases are slowly progressive, any decrease in progression can potentially be beneficial.
We used the GRADE methodology to assess the certainty of evidence for key outcomes for all comparisons. By grading the evidence according to the GRADE criteria (Guyatt 2008), the overall certainty of findings for individual outcomes varied between low and very low. We downgraded the evidence for study limitations (risk of bias), indirectness, imprecision, or a combination of these. The impossibility of participant blinding meant that studies could at best provide moderate‐certainty evidence. Other study limitations and the lack of a power analysis or an underpowered study were the main reasons for further downgrading.
The recommendations from the ACSM Position Stand on 'The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults' can be used as requirements for an effective, safe and individualised exercise prescription, taking into account the pre‐training level of fitness (Garber 2011). The ACSM recommendations were almost all adhered to by most of the included and excluded studies in this review. The only criterion that was rarely met was that eight to 10 major muscle groups should be exercised in strength training programmes. This is probably partly due to limitations in time available to evaluate the effects of training by multiple assessments covering the different outcome measures. In addition, expenses for (adjusted) training equipment can be high. Thirdly, investigators were perhaps too cautious in order not to strain participants too much. Moreover, strength training for fewer than eight muscle groups could be adequate in people with a muscle disease, who are generally untrained.
Potential biases in the review process
As there are few experts in this field and as we supplemented our search strategy with checking references, searching study registers and contacting experts, we are likely to have identified all relevant studies in this review. Given that there was nearly complete consensus between the two review authors responsible for study selection, the risk of selection bias in this part of the review process is probably low. All but one study author responded to our requests for data and further information, and the study authors who responded provided most of the requested information. Two out of four authors of this Cochrane Review conducted two of the 14 included studies. To minimise this conflict of interest, other review authors examined these studies to determine the suitability for inclusion in this review and analysed the data.
Agreements and disagreements with other studies or reviews
No previous reviews have examined the effect of strength training and aerobic exercise training in people with a muscle disease.
Authors' conclusions
Implications for practice.
The evidence regarding strength training and aerobic exercise interventions remains uncertain. Low‐certainty evidence suggests that strength training alone may have little or no effect, and that aerobic exercise training alone may lead to a possible improvement in aerobic capacity, but only for participants with FSHD. For combined aerobic exercise and strength training, very‐low certainty evidence shows that there may be slight increases in muscle strength and aerobic capacity for people with dermatomyositis and polymyositis, and there is low‐certainty evidence for a slight decrease in aerobic capacity and increase in muscle strength for people with juvenile dermatomyositis. The included studies in this review reported no negative side effects of either strength or exercise training in people with a muscle disease. The optimal exercise modality and intensity of exercise for people with a muscle disease is still unclear.
Implications for research.
There is a need for more research to establish whether strength training is beneficial in all forms of muscle disease, and whether exercise training is beneficial in Becker muscular dystrophy and inclusion body myositis, and to define the optimal aerobic exercise programmes for people with a muscle disease.
Specific diagnostic criteria should be used and reported for all muscle diseases included. Information on the severity of the muscle disease in participants should also be presented so as to allow readers to assess the generalisability of the results to other people with a similar type and severity of muscle disease. In studies with a small sample size, participants should be stratified for disease severity. Another related characteristic that may influence outcome is the level of activity (sedentary versus active) at baseline, because in the healthy population untrained persons respond with higher percentages and rates of gain in strength, compared with trained individuals (Garber 2011). Activity level and change in activity level for each participant should be monitored objectively during the study period, for example with an accelerometer.
When people with different neuromuscular disorders but with similar distribution and severity of muscle weakness participate in the same study, the data should also be presented for each major type of muscle disease separately, to detect possible disease‐specific trends.
In strength training and aerobic exercise intervention studies, the training programme should be described in detail, just as with the prescription of drugs. Study authors should provide information about the type(s) of exercise, the intensity (including progression rate), frequency, duration per exercise session, the duration of the entire programme, as well as the trained muscle groups, and the supervision of training. Studies including well‐described interventions, and more specifically harmonisation of outcome measures across studies, may improve the quality and comparability of the evidence. These studies would ultimately facilitate the development of uniform exercise guidelines for people with muscle diseases. Although some studies measured the same domains, they used different test protocols for strength, aerobic capacity, muscle endurance, fatigue, quality of life and pain, which impeded pooling of data. The large variety in outcome measures that the studies used underscores the need for a general agreement about most important measures to assess effects of exercise intervention. A core set of outcome measures to determine the effect of exercise therapy would enable comparison of the magnitude of effect of different exercise regimens.
In summary, the review authors' recommendations for future studies are as follows.
Participants with different muscle disorders can participate in one study, but data should be presented for each major type of muscle disease separately if possible.
Randomised controlled comparisons should be made with participants having the same types of muscle disease. The effect of training in people with a muscle disease should be compared to a non‐exercising control group of people with the same muscle disease and not to healthy individuals or to contralateral non‐exercised limbs.
An appropriate placebo intervention is recommended in order to measure exercise‐specific benefits.
Stratified randomisation is strongly advised with regard to disease severity, particularly in studies with a small sample size. It should also be considered for pre‐training level of activity (sedentary versus active), particularly in aerobic intervention studies.
The following aspects of the training intervention should be specified: type(s) of exercise training, intensity and progression rate, frequency, duration per exercise session and of the entire programme, trained muscle groups, supervision of training, and adherence. Duration of the training intervention should be at least six weeks.
Outcomes should at least include measures of muscle function (for example, strength, endurance measured by the maximum duration of contraction) and aerobic capacity (for example, work capacity measured by an incremental cycle test), and functional assessments such as a six‐minute walk test. Researchers should be aware of the specificity of training effects in their choice of outcome measures. The following evaluations are strongly advised: measures of quality of life, pain and experienced fatigue.
Outcome measures should be standardised in order to compare study results.
Outcomes assessors should be blinded to interventions, to avoid measurement bias.
Activity levels of participants in the control group should be monitored objectively in order to assess the specific benefits of aerobic exercise and strength training exercise.
A long‐term follow‐up without supervised exercise is recommended in order to measure the proportion of the population that maintains the programme of exercise employed in the intervention, or some other form of exercise.
What's new
| Date | Event | Description |
|---|---|---|
| 16 November 2018 | New search has been performed | The searches were updated to 16 November 2018. The search strategies were revised to increase specificity. |
| 16 November 2018 | New citation required and conclusions have changed | This update includes nine new trials. The previous version of the review concluded that "moderate‐intensity strength training in myotonic dystrophy and facioscapulohumeral dystrophy, aerobic exercise training in dermatomyositis and polymyositis, and myotonic dystrophy type I appear to do no harm, but there is insufficient evidence to conclude that they offer benefit. In mitochondrial myopathy, aerobic exercise combined with strength training appears to be safe and may be effective in increasing submaximal endurance capacity." In this update we concluded that although more trials have been published, the evidence for these interventions is still limited, insufficient, or both. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 26 August 2012 | New citation required and conclusions have changed | Review updated to include a study of people with dermatomyositis and polymyositis and a study with people with myotonic dystrophy type I. The results and conclusions of the review amended accordingly. |
| 2 July 2012 | New search has been performed | Searches updated to July 2012. One new trial identified from searches. In this update we have included studies with a exercise programme duration of at least six, instead of 10, weeks. Therefore, one trial which was previously excluded in the former update is now also included. |
| 15 June 2011 | Amended | Additional acknowledgement added. |
| 20 July 2009 | New citation required and conclusions have changed | Search updated to July 2009. Review updated to include a new study of people with mitochondrial myopathy (Cejudo 2005). The results and conclusions of the review have been amended accordingly. |
| 2 July 2008 | Amended | Converted to new review format. |
| 23 September 2004 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
The Netherlands Organisation for Scientific Research (NWO), the Netherlands Organisation for Health Research and Development (ZonMw) and the Princess Beatrix Fund (the Dutch Public Fund for Neuromuscular Disorders) are acknowledged for supporting three of the authors (Voet, Van der Kooi, Lindeman) in related neuromuscular research projects.
We thank M Janssen for her help with examining two studies written by the review authors
A Gunn from Cochrane Neuromuscular searched all databases.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease.
Appendices
Appendix 1. Cochrane Neuromuscular Specialised Register via the Cochrane Register of Studies (CRS‐Web) search strategy
Search date = 16 November 2018
#1 (muscular or myotonic) NEAR4 dystroph* AND INREGISTER #2 myositis or dermatomyositis or polymyositis AND INREGISTER #3 (muscular or myotonic) NEAR4 dystroph* AND INREGISTER #4 myopathy or myopathies AND INREGISTER #5 MESH DESCRIPTOR neuromuscular diseases WITH QUALIFIER RH TH AND INREGISTER #6 #1 OR #2 OR #3 OR #4 OR #5 AND INREGISTER #7 "exercise therap*" or "exercise program*" or "exercise training" or "strength training" or "aerobic training" or "aerobic exercis*" or "training program*" or "resistive exercis*" or "resistive training" or "endurance exercis*" or "endurance training" or "muscle exercis*" AND INREGISTER #8 MESH DESCRIPTOR exercise therapy EXPLODE ALL AND INREGISTER #9 MESH DESCRIPTOR Physical Education and Training EXPLODE ALL AND INREGISTER #10 #7 OR #8 OR #9 AND INREGISTER #11 #6 AND #10 AND INREGISTER
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) search strategy
Search date = 16 November 2018
#1 (muscular or myotonic) NEAR4 dystroph* AND CENTRAL:TARGET #2 myositis or dermatomyositis or polymyositis AND CENTRAL:TARGET #3 (muscular or myotonic) NEAR4 dystroph* AND CENTRAL:TARGET #4 myopathy or myopathies AND CENTRAL:TARGET #5 MESH DESCRIPTOR neuromuscular diseases WITH QUALIFIER RH TH AND CENTRAL:TARGET #6 #1 OR #2 OR #3 OR #4 OR #5 AND CENTRAL:TARGET #7 "exercise therap*" or "exercise program*" or "exercise training" or "strength training" or "aerobic training" or "aerobic exercis*" or "training program*" or "resistive exercis*" or "resistive training" or "endurance exercis*" or "endurance training" or "muscle exercis*" AND CENTRAL:TARGET #8 MESH DESCRIPTOR exercise therapy EXPLODE ALL AND CENTRAL:TARGET #9 MESH DESCRIPTOR Physical Education and Training EXPLODE AND CENTRAL:TARGET #10 #7 OR #8 OR #9 AND CENTRAL:TARGET #11 #6 AND #10 AND CENTRAL:TARGET
Appendix 3. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to November 15, 2018> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 ((muscular or myotonic) adj4 dystroph*).mp. (33875) 2 (myositis or dermatomyositis or polymyositis).mp. (23499) 3 ((muscular or myotonic) adj4 dystroph*).mp. (33875) 4 (myopathy or myopathies).mp. (24002) 5 neuromuscular diseases/rh, th (1446) 6 or/1‐5 (74821) 7 (exercise therap* or exercise program* or exercise training or strength training or aerobic training or aerobic exercis* or training program* or resistive exercis* or resistive training or endurance exercis* or endurance training or muscle exercis*).mp. or exp Exercise therapy/ or exp "Physical education and training"/ (117908) 8 (Trial* or random*).mp. or Clinical trial.pt. or Controlled clinical trial.pt. or Randomised controlled trial.pt. or Meta‐analysis.pt. or Multicenter study.pt. or exp Clinical trials as topic/ (2186732) 9 6 and 7 and 8 (175) 10 exp animals/ not humans.sh. (4515620) 11 9 not 10 (163) 12 remove duplicates from 11 (161)
Appendix 4. Embase (OvidSP) search strategy
Database: Embase <1974 to 2018 November 15> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 ((muscular or myotonic) adj4 dystroph*).mp. (44207) 2 (myositis or dermatomyositis or polymyositis).mp. (34980) 3 ((muscular or myotonic) adj4 dystroph*).mp. (44207) 4 (myopathy or myopathies).mp. (41908) 5 neuromuscular disease/rh, th (385) 6 or/1‐5 (107828) 7 (exercise therap* or exercise program* or exercise training or strength training or aerobic training or aerobic exercis* or training program* or resistive exercis* or resistive training or endurance exercis* or endurance training or muscle exercis*).mp. or exp exercise/ or exp muscle exercise/ or exp excessive training/ or exp kinesiotherapy/ (386382) 8 trial*.ti. or random*.mp. or exp clinical trial/ or controlled study/ (7670206) 9 6 and 7 and 8 (1272) 10 remove duplicates from 9 (1257) 11 exp animal/ not human/ (4478813) 12 10 not 11 (995) 13 limit 12 to (conference abstracts or embase) (961)
Appendix 5. Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) search strategy
Friday, November 16, 2018 5:08:40 PM
S10 S9 18 S9 S6 AND S7 AND S8 72 S8 TX Trial* OR TX random* OR PT "Systematic review" OR PT "Clinical trial" OR MH "Clinical trials+" 589,189 S7 TX "exercise therapy" OR TX "exercise program" OR TX "exercise training" OR TX "strength training" OR TX "aerobic training" OR TX "aerobic exercise" OR TX "training program" OR TX "resistive exercise" OR TX "resistive training" OR TX "endurance exercise" OR TX "endurance training" OR TX "muscle exercise" OR TX "exercise therapies" OR TX "exercise programs" OR TX "aerobic exercises" OR TX "training programs" OR TX "resistive exercises" OR TX "endurance exercises" OR TX "muscle exercises" OR MH "Therapeutic exercise+"TX "exercise therapy" OR TX "exercise program" OR TX "exercise training" OR TX "strength training" OR TX "aerobic training" OR TX "aerobic exercise" OR TX "training program" OR TX "resistive exercise" OR TX "resistive training" OR TX "endurance exercise" OR TX "endurance training" OR TX "muscle exercise" OR TX "exercise therapies" OR TX "exercise programs" OR TX "aerobic exercises" OR TX "training programs" OR TX "resistive exercises" OR TX "endurance exercises" OR TX "muscle exercises" OR MH "Therapeutic exercise+" 70,261 S6 S1 OR S2 OR S3 OR S4 OR S5 9,631 S5 (MH "Neuromuscular Diseases/RH/TH") 463 S4 myopathy or myopathies 2,994 S3 (muscular or myotonic) N4 dystroph* 3,873 S2 myositis or dermatomyositis or polymyositis 3,365 S1 (muscular or myotonic) N4 dystroph* 3,873
Appendix 6. International Clinical Trials Registry Platform
Saturday, December 22, 2018 12:44:20 AM
Database: International Clinical Trials Registry Platform Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 muscular dystrophy AND exercise 2 muscular dystrophy AND training 3 myositis AND exercise 4 myositis AND training 5 myopathy AND exercise 6 myopathy AND training
7 neuromuscular disease AND exercise 8 neuromuscular disease AND training
Appendix 7. ClinicalTrials.gov
Saturday, December 22, 2018 13:53:30 AM
Database: ClinicalTrials.gov Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 muscular dystrophy AND exercise 2 muscular dystrophy AND training 3 myositis AND exercise 4 myositis AND training 5 myopathy AND exercise 6 myopathy AND training
7 neuromuscular disease AND exercise 8 neuromuscular disease AND training
Data and analyses
Comparison 1. Strength training versus no training in myotonic dystrophy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Muscle strength: hand grip force (N) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐6.70, 18.70] |
| 2 Muscle strength: pinch grip force (N) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐3.33, 5.33] |
| 3 Muscle strength: isometric wrist flexor force (N) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [‐3.35, 17.35] |
| 4 Muscle strength: isometric wrist extensor force (N) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [0.70, 15.30] |
Comparison 2. Strength training versus no training in facioscapulohumeral muscular dystrophy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Muscle strength elbow flexors ‐ maximum voluntary isometric contraction (kgF) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.76, 1.76] |
| 2 Muscle strength elbow flexors ‐ dynamic strength (kgF) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.16, 2.56] |
| 3 Muscle strength ankle dorsiflexors ‐ maximum isometric voluntary contraction (kgF) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐2.37, 3.23] |
| 4 Muscle strength ankle dorsiflexors ‐ dynamic strength (kgF) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐2.26, 1.38] |
| 5 Muscle endurance elbow flexors ‐ 30 s maximal isometric contraction (kgF‐s) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐42.01, 26.01] |
| 6 Muscle endurance ankle dorsiflexors ‐ 30 s maximal isometric contraction (kgF‐s) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐17.0 [‐34.82, 0.82] |
Comparison 3. Aerobic exercise training versus no training in polymyositis and dermatomyositis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Aerobic capacity (mL/min/kg) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 14.6 [‐0.96, 30.16] |
| 2 Functional assessment ‐ Functional Assessment Screening Questionnaire | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 17.6 [‐5.58, 40.78] |
| 3 Creatine kinase and aldolase serum level | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 7.9 [‐24.20, 40.00] |
Comparison 4. Aerobic exercise versus no training in Duchenne muscular dystrophy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MRC (sum scores) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 1.7 [‐1.89, 5.29] |
| 2 MRC score lower limb (0‐5) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 1.3 [‐1.48, 4.08] |
| 3 MRC score upper limb (0‐5) | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐1.42, 2.22] |
| 4 Assisted 6‐min cycle test (revolutions legs) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 14.00 [‐88.97, 116.97] |
| 5 Assisted 6‐min cycle test (revolutions arms) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 34.80 [‐68.17, 137.77] |
| 6 Motor Function Measure total (0‐100%) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 7.2 [‐3.69, 18.09] |
| 7 Motor Function Measure D1 (0‐100%) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 9.9 [‐8.78, 28.58] |
| 8 Motor Function Measure D2 (0‐100%) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 4.40 [‐6.21, 15.01] |
| 9 Motor Function Measure D3 (0‐100%) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [0.97, 12.43] |
| 10 Rise from floor (s) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐28.46, 0.46] |
| 11 10‐m run (s) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [‐0.56, 5.56] |
| 12 9‐hole peg test (s) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐4.06, 3.26] |
4.6. Analysis.
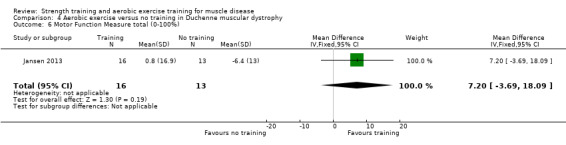
Comparison 4 Aerobic exercise versus no training in Duchenne muscular dystrophy, Outcome 6 Motor Function Measure total (0‐100%).
Comparison 5. Aerobic exercise versus no training in facioscapulohumeral muscular dystrophy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum voluntary isometric knee extension strength | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐47.30 [‐108.02, 13.42] |
| 2 Maximum voluntary isometric knee extension strength | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.66, 0.86] |
| 3 Maximum voluntary isometric knee flexion strength (N) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐7.5 [‐55.63, 40.63] |
| 4 Maximum voluntary isometric elbow flexion strength (N) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 5.8 [‐15.27, 26.87] |
| 5 Maximum voluntary isometric elbow extension strength (N) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐19.25, 20.25] |
| 6 Muscle strength leg (N) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 8.0 [‐16.19, 32.19] |
| 7 Muscle strength arm (N) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [‐3.29, 35.29] |
| 8 Maximal workload (W) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 21.5 [2.19, 40.81] |
| 9 Maximal workload (W) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 18.8 [13.67, 23.93] |
| 10 VO2 peak (mL/min/kg) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [0.63, 6.57] |
| 11 VO2 peak (mL/min/kg) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [2.51, 4.09] |
| 12 VO2 peak (L/min) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.43, 1.77] |
| 13 Distance walked in 6‐min walk test (m) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 28.9 [4.21, 53.59] |
| 14 Distance walked in 6‐min walk test (m) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 31.00 [19.34, 42.66] |
| 15 Distance walked in 6‐min walk test (m) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐18.37, 34.17] |
| 16 5‐times sit to stand (s) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐7.61, 7.55] |
| 17 Quality of life ‐ Sickness Impact Profile (0‐572) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐19.64, ‐0.36] |
| 18 Pain ‐ visual analogue scale (0‐10) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.00, 1.00] |
| 19 Fatigue | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐2.96, 0.56] |
| 20 Fatigue | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐7.3 [‐8.14, ‐6.46] |
Comparison 6. Aerobic exercise and strength training versus no training in mitochondrial myopathy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Muscle strength shoulder press ‐ maximum dynamic isotonic voluntary contraction (kg) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐14.71, 4.71] |
| 2 Muscle strength butterfly ‐ maximum dynamic isotonic voluntary contraction (kg) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 6.4 [‐2.89, 15.69] |
| 3 Muscle strength biceps curls ‐ maximum isotonic dynamic voluntary contraction (kg) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 7.3 [‐2.91, 17.51] |
| 4 Work capacity ‐ mean time until exhaustion in cycle test (min) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 23.7 [2.63, 44.77] |
| 5 Work capacity ‐ mean distance until exhaustion in cycle test (km) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 9.70 [1.51, 17.89] |
| 6 Work capacity ‐ mean distance walked until exhaustion in shuttle walking test (m) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 78.0 [‐144.86, 300.86] |
| 7 VO2 max in maximal incremental cycle exercise test (L/min) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 400.0 [‐61.97, 861.97] |
| 8 Quality of life ‐ NHP 0‐100 | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐9.8 [‐25.74, 6.14] |
| 9 Myoglobin | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐21.0 [‐48.35, 6.35] |
6.9. Analysis.
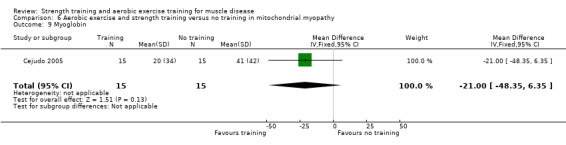
Comparison 6 Aerobic exercise and strength training versus no training in mitochondrial myopathy, Outcome 9 Myoglobin.
Comparison 7. Aerobic exercise and strength training versus control in myotonic dystrophy type 1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Distance walked in 6‐minute walk test (m) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐66.92, 88.92] |
| 2 Timed‐stands test (s) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.76, 4.76] |
| 3 Timed‐up‐and‐go tests (s) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.86, 0.86] |
Comparison 8. Aerobic exercise and strength training versus no training in dermatomyositis and polymyositis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Manual muscle strength testing in 8 muscle groups (MMT‐8) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.06, 3.06] |
| 2 5 voluntary repetition maximum in knee extensors, right (kg) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.75, 3.25] |
| 3 5 voluntary repetition maximum in knee extensors, left (kg) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [1.96, 3.44] |
| 4 Power performed at VO2 max (W) | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [15.00, 21.00] |
| 5 Time to exhaustion in endurance cycling test (min) | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 17.5 [8.00, 27.00] |
| 6 VO2 max | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.35, 0.90] |
| 7 Disease‐specific functional index 0‐64 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 5.50 [‐2.91, 13.91] |
| 8 Quality of life ‐ Physical function | 2 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 1.50 [0.78, 2.22] |
| 9 Quality of life ‐ SF‐36 General health 0‐100 | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 9.5 [5.53, 13.47] |
| 10 Quality of life ‐ SF‐36 Vitality 0‐100 | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 12.30 [8.15, 16.45] |
| 11 Quality of life ‐ SF‐36 Mental Health 0‐100 | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [1.65, 8.35] |
| 12 Quality of life ‐ NHP Energy 0‐100 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐45.90, 9.90] |
| 13 Quality of life ‐ NHP Pain 0‐100 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐12.21, 6.01] |
| 14 Quality of life ‐ NHP Sleep 0‐100 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 7.30 [‐9.96, 24.56] |
| 15 Quality of life ‐ NHP Social 0‐100 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐14.40, 16.60] |
| 16 Quality of life ‐ NHP Emotional 0‐100 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐22.30 [‐41.42, ‐3.18] |
| 17 Quality of life ‐ NHP Physical 0‐100 | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.04, ‐0.56] |
Comparison 9. Aerobic exercise and strength training versus no training in facioscapulohumeral muscular dystrophy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MVC quadriceps at rest (Nm) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 15.00 [‐27.77, 57.77] |
| 2 Voluntary activation at rest (%) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.62, 1.62] |
| 3 Maximal aerobic power (W) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 45.0 [‐20.51, 110.51] |
| 4 Muscle endurance (number of repetitions) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 12.0 [0.83, 23.17] |
| 5 VO2 peak (mL/min/kg) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 12.4 [2.21, 22.59] |
| 6 Distance walked in 6‐min walk test (m) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 64.0 [‐50.93, 178.93] |
| 7 Quality of life ‐ General Health ‐ SF‐36 0‐100 | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [‐4.19, 32.19] |
| 8 Fatigue (Fatigue Severity Scale) 9‐63 | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐27.89, ‐2.11] |
Comparison 10. Aerobic exercise and strength training versus no training in juvenile dermatomyositis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Max force right knee extensors (N) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 36.0 [24.95, 47.05] |
| 2 Max force left knee extensors (N) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [0.48, 33.52] |
| 3 Max force right hip flexors (N) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐22.36, 4.36] |
| 4 Max force left hip flexors (N) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐6.59, 18.59] |
| 5 Endurance time during maximal exercise test (min) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐1.55, ‐0.85] |
| 6 VO2 peak (mL/kg/min) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐3.27, ‐0.93] |
| 7 Distance of standing long jump (cm) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [15.30, 20.70] |
| 8 Number of push‐ups in 30 s | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [3.62, 6.38] |
| 9 Number of sit‐ups in 30 s | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [2.00, 4.00] |
| 10 Time wall sit (max: 60 s) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐5.53, ‐0.47] |
| 11 Time V‐up (max: 60 s) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐12.70, ‐7.30] |
| 12 Distance walked in 6‐min walk test (m) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐7.00 [‐21.56, 7.56] |
| 13 Quality of life ‐ PedsQL Generic Core Scale patient form, total score 0‐100 | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐9.76, ‐6.24] |
| 14 Muscle pain (10‐cm VAS) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐9.70, ‐4.30] |
| 15 Perception of fatigue ‐ PedsQL Multidimensional fatigue scale 0‐100 | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐6.54, ‐3.46] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Lindeman 1995.
| Methods | Evaluator‐blind, matched‐control RCT | |
| Participants |
Sample size Intervention group (number randomised): 21 adults with myotonic dystrophy type 1, control group 15 adults with myotonic dystrophy type 1 Inclusion criteria Participants diagnosed with myotonic dystrophy on the basis of their clinical picture, electromyography and nerve conduction studies Exclusion criteria Participants were excluded if there were contraindications for muscle strengthening exercises or if they had other disabling disorders that might influence the scoring in the functional tests. Baseline demographics Of the 33 myotonic dystrophy participants who ultimately started the trial, 2 had the congenital form; the others had the classical, adult type. All participants were ambulatory. |
|
| Interventions | Strength training vs no training Type of training and exercise Dynamic strength training with weights Intensity Individualised progressive overload, 3 sets from 25 repetitions at 60% of 1RM, via 15 repetitions at 70%, to 10 repetitions at 80% Frequency 3 times/week Setting At home (the Netherlands) Duration Session: within 30 min. Programme: 24 weeks Muscle groups Knee extensors and flexors, hip extensors and abductors Supervision Supervised home training programme |
|
| Outcomes |
Primary Muscle strength by isokinetically measured knee torques and isometrically as MVIC. Secondary Endurance by maximum duration of contraction at 80% of MVIC, functional performance by timed motor performance tests and by questionnaires. Serum myoglobin levels to detect changes in muscle fibre membrane permeability Time‐points measured Outcome measurements were performed at the start of the study period (t0) and after 8 (t8), 16 (t16), and 24 (t24) weeks of follow‐up. |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | Study authors have chosen not to select a disclosure statement | |
| Notes | Participants were matched based on muscle strength (knee extension torque/body weight) and on performance in a stair‐climbing test. Only complete pairs were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: there was no published information on the sequence generation but the study author (Lindeman) informed us that 2 independent persons drew a sealed lot per matched pair and allocated each pair to the training or non‐training group by tossing a coin. There was some baseline imbalance but we considered it consistent with chance. |
| Allocation concealment (selection bias) | Low risk | Comment: there was no published information on the method of allocation concealment but the study author (Lindeman) informed us that 2 independent persons allocated the training, after tossing the coin, to the training or non‐training group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded as it is impossible to blind exercise training compared to no exercise training |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "observers of the outcome measurements were blinded for treatment allocation" Comment: approximately 20% of the myotonic dystrophy participants revealed information to the clinical evaluators that resulted in unblinding during the course of the trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 3 of the initially 36 randomised participants withdrew before disclosure of treatment allocation. The 33 participants starting the trial made 15 matched pairs. During the trial 1 person dropped out. Because of the matched pair design only complete pairs were analysed, therefore eventually 28 of the initial 36 randomised participants were analysed. Follow‐up was therefore incomplete and analysis was not by ITT. However, the flow path of participants was well documented |
| Selective reporting (reporting bias) | High risk | Quote: "Differences were compared using paired T tests and two sample T tests (to look for differences because of the small number of complete pairs). Only results of the paired T tests at t0 and t24 will be presented because they showed the most relevant differences between the groups." Comment: appears to be a high risk of selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Wiesinger 1998a.
| Methods | Parallel‐group RCT | |
| Participants |
Sample size Intervention group: 2 adults with PM, 5 adults with DM, control group: 2 adults with PM, 5 adults with DM Inclusion criteria Established DM or PM with a disease duration of > 6 months, clinical activity defined as the presence of proximal muscle weakness, and/or the elevation of CK levels above the upper limit of normal on ≥ 3 consecutive observations during the preceding 3‐month period, the drug therapy had to be stable for at least 3 months before the start of the training programme Exclusion criteria Clinically manifest pulmonary or cardiac disorders, inclusion body myositis, fever, neoplasms, physical inability to exercise, or increase in muscle destruction during the past 3 months before the start of the training programme, as indicated by at least a 50% increase in CK levels over the baseline value Baseline demographics Mean age participants control group: 40 years, mean age participants training group: 56 years. Female/male ratio control group: 5/2, female/male ratio training group: 4/3. |
|
| Interventions | Aerobic exercise training vs no training Type of training and exercise Endurance bicycle training, endurance step aerobics Intensity Bicycle training: 30 min, slowly increased on an individual basis. Resistance was increased until a heart rate of 60% of maximum. Step aerobics: 30 min Frequency During the first 2 weeks, twice weekly, during the remaining 4 weeks, 3 times weekly Setting University Hospital of Vienna, Austria Duration Session: 60 min. Programme: 6 weeks Muscle groups Not applicable Supervision Supervised by a physiotherapist |
|
| Outcomes | No primary outcome or secondary outcomes defined. Study outcomes: ADL score, peak isometric torque of knee extensors and hip flexors, peak oxygen consumption and CK and aldolase levels Time‐points measured Before and after 6 weeks of control or training period |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | None reported | |
| Notes | Outcomes were not presented separately for DM and PM | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Distinct randomisation lists were used". Comment: there was no information about the generation of the list. It is not clear what is meant by "distinct randomisation lists" |
| Allocation concealment (selection bias) | Unclear risk | Comment: there was no published information on the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded for group assignment, as it is impossible to blind exercise training compared to no exercise training. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Muscle strength assessments were carried out by the same person who was unaware of the group to which the individual patients belonged". Comment: there was no published information about blinding of the assessor of the other measurements |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: complete follow‐up of all participants |
| Selective reporting (reporting bias) | High risk | Comment: no primary or secondary outcomes were defined. Outcomes were also not clearly specified and reported |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Van der Kooi 2004.
| Methods | Evaluator‐blind, parallel‐group RCT | |
| Participants |
Sample size Intervention group: 19 adults with FSHD type 1, control group 16 adults with FSHD type 1 Inclusion criteria Eligible participants were aged 18‐65 years, were willing to train if allocated to the training group, agreed to refrain from training if allocated to the non‐training group, and agreed to use the study medication as prescribed. Participants had to have at least 2 trainable muscle groups (fully supinated elbow flexion Medical Research Council (MRC) 3, ankle dorsiflexor 0° dorsiflexion from neutral ankle position). Exclusion criteria Inability to walk independently (ankle‐foot orthoses and canes were accepted); history of (treated) hypertension, heart failure, or ischaemic heart disease, arrhythmias, diabetes mellitus, or thyrotoxicosis; use of sympathicomimetics, beta‐blockers, or systemic corticosteroids during the last 3 months; (planned) pregnancy or breastfeeding; and articular diseases of the elbow or ankle joints. Participants with abnormalities on their electrocardiogram, but without a cardiovascular history or hypertension, were seen by the cardiologist to decide on their inclusion. Baseline demographics The training group comprised 8 women and 11 men, mean age 36 years (SD 9). The control group comprised 7 women and 9 men, mean age 39 years (SD 9). |
|
| Interventions | Strength training vs no training (and as add‐on in a double‐blind, randomised controlled design albuterol or placebo) Type of training and exercise Dynamic and isometric strength training with weights Intensity Individualised progressive overload, 2 sets dynamic from 10 repetitions at 10RM, via 8 repetitions at 8RM, to 5 repetitions at 5RM, and 30 s isometric with same weight Frequency 3 times/week Setting At home (the Netherlands) Duration Session: within 30 min. Programme: 52 weeks Muscle groups Elbow flexors, ankle dorsiflexors Supervision Supervised at home by a physical therapist |
|
| Outcomes |
Primary Difference in muscle strength of elbow flexors and ankle dorsiflexors after 52 weeks using the MVIC Secondary Muscle endurance (MVIC Force‐Time Integral) and dynamic muscle strength (1RM). Other measures included functional tests and timed motor performance tasks Time‐points measured Before and after 52 weeks of control or training period |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | "Supported by a government grant of the Health Research and Development Council of the Netherlands (ZON‐MW), the Prinses Beatrix Fonds, the Dutch Public Fund for Neuromuscular Disorders (VSN), and the Dutch FSHD Foundation" | |
| Notes | Outcomes are presented for the 4 treatment groups (i.e. the 4 combinations of training vs non‐training, and albuterol vs placebo). Effect sizes are presented by intervention as well | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " (...) participants were randomly assigned to one of the four treatment groups according to a computer generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "information on the assignment to training or non‐training was disclosed to the participants by the physical therapist" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded as it is impossible to blind exercise training compared to no exercise training. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The RM measurements were performed by the physical therapist, who was not blinded for the allocation to training or non‐training, as this specific measurement carried too great a risk of unblinding the clinical evaluator" Comment: adequate, although one of the main secondary outcome measures, the 1RM measurement for assessing dynamic strength, was performed by the physical therapist, who supervised the training, and was therefore not blinded to the allocation to training or non‐training. Unblinding during the trial was adequately registered. Allocation to training or non‐training was unmasked in 3 cases, due to unintentional remarks |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient stopped training because of recurring, training‐related muscle soreness and fatigue. Four participants stopped using their study medication because of side effects. Data for the participants who discontinued an intervention were analysed in the assigned treatment group" Although there was not total adherence, analysis was by ITT. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Cejudo 2005.
| Methods | Parallel‐group RCT | |
| Participants |
Inclusion criteria
Baseline demographics Mean age participants control group: 55 years, mean age participants training group: 45 years. Female/male ratio control group: 4/5, female/male ratio training group: 4/5 Sample size Intervention group: 9 adults with MM, control group 9 adults with MM |
|
| Interventions | Strength training and aerobic exercise training vs no training Type of training and exercise Endurance bicycle training, dynamic isotonic with weights Intensity Aerobic training: individualised work rate, 30‐min leg exercise on an ergo cycle, 70% of the peak work rate; strength training: 1 set dynamic and isotonic of 10‐15 repetitions at 50% 1RM load, to 2 or 3 sets. Adjustments on workload changed every 2 weeks Frequency 3 times/week Setting In a rehabilitation unit in Spain Duration Session: approximately 60 min. Programme: 12 weeks Muscle groups Shoulder, upper back, arm, pectoralis major, biceps brachii and brachialis muscles Supervision Supervised training programme by specialised nurses and a physiatrist specialist |
|
| Outcomes |
Primary Exercise capacity ‐ expressed in measures of oxygen uptake (i.e. VO2 max), endurance time and distance walked in the shuttle walking test Secondary Peripheral muscle strength (1RM test), quality of life, symptoms of myalgia, cramps and fatigability and functional exercise capacity Time‐points measured Before and after 12 weeks of control or training period |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | "This work was supported by grants from the Ministerio de Sanidad (FIS 97/0371), Consejería de Salud, Junta de Andalucía (96/28), Mitochondrial Disorders Network G 03/011, and Red Respira RTIC 03/011, Spain" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to a training group or control group". Comment: no published information on the sequence generation. Study author (Cejudo) informed us that participants were randomly assigned according to a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to a training group or control group". Comment: no published information on the allocation concealment. Study author (Cejudo) informed us that participants were randomly assigned according to a computer‐generated randomisation list |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participant blinding not possible and no published information on the blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no published information on the blinding of the outcome assessors. Study author (Cejudo) told us that the evaluators knew to which group each participant was assigned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "one patient in each group failed to finish the study for personal reasons". Comment: baseline outcome data assessed, but not available for these participants. So 1/10 missing from intervention group and 1/10 missing from control group and analysis was not ITT |
| Selective reporting (reporting bias) | High risk | Comment: no primary and secondary outcome(s) defined in the article and outcomes not clearly specified and reported |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Kierkegaard 2011.
| Methods | Evaluator‐blind, parallel‐group RCT | |
| Participants |
Sample size Intervention group: 18 adults with myotonic dystrophy type 1, control group: 17 adults with myotonic dystrophy type 1 Inclusion criteria Diagnosed DM1; living in the Stockholm County Council area; aged ≥ 18 years; ability to walk 50 m without assistance; permission from a cardiologist to take part in an exercise programme; and classified as grade 2–5 on the MIRS Exclusion criteria Inability to communicate in Swedish; clinically obvious severe cognitive impairment; and other diagnoses that could interfere with participation. Of 114 eligible persons, 35 did not fulfil the inclusion criteria (24 were unable to walk 50 m, 4 were classified as MIRS 1, 4 had severe cardiac arrhythmia, 2 had other concurrent diagnoses and 1 did not speak Swedish). Baseline demographics The training group comprised 10 women and 8 men, mean age 44 years, standard deviation (SD) 11, range 20–60 years. The control group comprised 10 women and 7 men, mean age 41 years, SD 15, ranging from 20‐65 |
|
| Interventions | Strength training and aerobic exercise training vs no training Type of training and exercise Strength training, aerobic exercise, balance exercises, supported by music Intensity Strength exercises for arm, leg, back and abdominal muscles 16‐20 repetitions, for 6‐7 min, balance exercises for 3‐4 min, aerobic activities for 11‐12 min at 60%‐80% of maximum heart rate. Once a week a 30‐min brisk walk Frequency 2 times/week and once a week a brisk walk Setting The department of physical therapy, Karolinska University Hospital (Sweden) Duration Session: 60 min and a 30‐min walk. Programme: 14 weeks Muscle groups Arm, leg, back and abdominal muscles Supervision All sessions were supervised by a specialised physiotherapist |
|
| Outcomes |
Primary Distance walked in the 6‐min walk test Secondary Timed‐stands test, timed up‐and‐go test Time‐points measured Before and after a control or training period of 14 weeks |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | "This research was supported by grants from the Einar Belvén Foundation, the Norrbacka‐Eugenia Foundation, Stockholm, the Swedish Association of Registered Physiotherapists and the Swedish Association for Persons with Neurological Disabilities (NHR). Financial support was also provided through the strategic research programme in Care Sciences (SFP‐V), Karolinska Institutet, and the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet." | |
| Notes | Participants were stratified before randomisation by their results in the 6‐min walk test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The lots were drawn by a person who was not involved in any other part of the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were recruited before randomisation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible: intervention was training vs no training |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data was collected before and after the intervention by two independent experienced physiotherapists, blinded to group allocation and each assessing the same participants on both occasions" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "one person in the control group did not attend the data collection after the intervention" |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Jansen 2013.
| Methods | Parallel‐group RCT | |
| Participants |
Sample size Intervention group: 17 boys with DMD, control group: 13 boys with DMD Inclusion criteria Boys were included if they needed more than 5 s to rise from the floor, were not able to rise from the floor, were not able to cycle without electric assistance, or needed a wheelchair to move over a long (> 500 m) distance. Wheelchair‐dependent boys were eligible if they were able to touch the top of their head with both hands or were able to use a hand‐operated wheelchair. Exclusion criteria Exclusion criteria were as follows: age < 6 years, a clinical cardiomyopathy, and other disabling diseases influencing mobility Baseline demographics At baseline, 8 boys were ambulant and 9 were wheelchair‐dependent in the intervention group, compared with 10 and 3 in the control group, respectively (P = .098). In the intervention group, 14 boys used corticosteroids compared with 9 in the control group. |
|
| Interventions | Aerobic exercise vs no training Type of training and exercise Assisted bicycle home training programme Intensity 15 min cycling with arms and legs using a mobility trainer with electrical motor support Frequency 5 days/week Setting At home or at school, depending on the preferences of the participants (the Netherlands) Duration Session: 15 min. Programme: 24 weeks Supervision Parents and/or teachers were instructed to assist the boys. Training intensity and posture were monitored and if necessary adjusted by the primary investigator |
|
| Outcomes |
Primary MFM and the 6‐min Cycling Test Secondary PEDI, timed tests (rise from floor, 10‐m run, 9‐hole peg test), muscle strength, passive joint ROM, and quantitative muscle ultrasound. Bilaterally muscle strength of the hip extensors, knee extensors, ankle dorsiflexors, shoulder abductors, and elbow extensors (MRC score), signs of overexertion by means of a questionnaire Time‐points measured Outcomes were assessed at baseline, after 4 weeks, 8 weeks, 32 weeks and 56 weeks. |
|
| Dates | Boys were recruited January 2009‐January 2010 | |
| Funding/ declarations of interest |
Funding Study author(s) "disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This study was financially supported by a grant from the patient organisation Duchenne Parent Project." Declarations of interest Study author(s) "declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article." |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Stratified randomisation (ambulant vs wheelchair‐dependent) was used to allocate participants to either the intervention or the control group in a 2:1 ratio. Two boys were not randomised, but directly allocated to the intervention group, because the study duration was ending. Comment: no published information on the sequence generation. Study author (Jansen) informed us that the randomisation list was provided by the statistician. |
| Allocation concealment (selection bias) | Low risk | Comment: no published information on the allocation concealment. Study author (Jansen) informed us that an independent secretary allocated the boys to one of both groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and outcome assessor were not blinded to treatment allocation but had no information about previous test results at each assessment. 2 boys were originally allocated to the intervention group, but replaced to the control group within 2 weeks after trying the intervention". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: participants and outcome assessor were not blinded to treatment allocation but had no information about previous test results at each assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: one boy discontinued the training and assessment after 12 weeks and was excluded from the analysis, so the analysis was not ITT. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Aldehag 2013.
| Methods | Randomised controlled cross‐over pilot study | |
| Participants |
Sample size: 35 Inclusion criteria Adults with genetically verified diagnosed DM1, with strength in wrist and hand muscles graded at least 3 on the 0–5‐scale of manual muscle testing Exclusion criteria Clinically obvious severe cognitive impairment, other diagnoses that could interfere with participation, or inability to communicate in Swedish Baseline demographics Group A (to start with a training period): n = 18, mean age 44 years (SD = 11) Group B (to start with a period of no training): n = 17, mean age 47 years (SD = 12) Participants’ hand function ranged from mildly to severely impaired. All but 2 participants were right‐handed and all but one were ambulatory. |
|
| Interventions |
Type of training and exercise Hand training programme: dynamic strength‐endurance exercises, i.e. mass wrist‐ and finger movements, and isolated finger movements, and stretching exercises for wrist and finger muscles vs no training Intensity The resistance of the putty was dependent on the baseline hand‐grip force of the participant and differed from supersoft to medium. Frequency Three times/week. The number of sets for each movement was progressively increased during the 12‐week training period, one set every 4th week. Thus, each mass movement consisted of 1 set of 10 repetitions during weeks 1‐4, 2 sets of repetitions during weeks 5‐8, and 3 sets of 10 repetitions during weeks 9‐12. Setting Every week, 1 session was performed at the Department of Occupational Therapy, Karolinska University Hospital, (Sweden). 2 sessions were performed at home. Duration Session: approximately 1 h. Programme: 12 weeks Supervision 1 session/week was a group‐training session, supervised by an occupational therapist. |
|
| Outcomes |
Primary Hand‐grip and pinch‐grip force measured with the Grippit instrument, an electronic dynamometer giving the average force in Newton (N) over a period of 10 seconds(s). Secondary Isometric force in wrist flexors and wrist extensors, manual dexterity was measured by counting the number of pegs placed on the Purdue Pegboard during 30 s. Change in self‐perception of occupational performance and satisfaction with performance (COPM); evaluation of instrumental ADL (AMPS) Time‐points measured Before and after the intervention or control period (12 weeks) |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | "Financial support was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Centre for Health Care Science, Department of Clinical Neuroscience and the strategic research program in Care Sciences (SFP‐V), Karolinska Institutet, the Swedish Association for Persons with Neurological Disabilities (NHR), Röda Korshemmets (Red Cross Home’s) Lori Lindahl Stipendium, Idrottens Forskningsråd (Swedish Sports Research Council) and the Department of Occupational Therapy, Karolinska University Hospital." | |
| Notes | Participants were stratified into 2 strata for level of functioning, based on hand‐grip force. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were divided into two strata from which they were assigned by lot to either intervention A or B. The group to start with a training period was then decided by lot." |
| Allocation concealment (selection bias) | Low risk | Quote: "The lots were drawn by the person who was also responsible for the training. Since participants were recruited before randomisation, concealed allocation procedures were applied". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded for group assignment, as it is impossible to blind exercise training compared to no exercise training |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Data collections were performed before and after training and control periods (...) by an experienced occupational therapist blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The dropout rate was high: 29%, however, an ITT analysis was applied. Quote: "Spontaneous given reasons for withdrawal were family and/or work matters, fatigue, lack of motivation and concerns about having to travel back‐and‐forth to the hospital for the group‐training sessions. A few also reported emotional difficulties with meeting people at the group training with worse symptoms than they had themselves. (...) An intention‐to‐treat approach was applied and missing data was replaced using the last‐observation‐carried‐forward (LOCF) method. (...) Altogether, 10 persons dropped out of the study. (...) Nine persons in group A and four persons in group B had acceptable adherence". |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | High risk | Quote: "A wash‐out period was incorporated between the periods of intervention in order to control for a possible carry‐over effect from the training period into the control period". Comment: a cross‐over trial has various weaknesses: participants dropping out after the first period complicating the ITT analysis, and carry‐over effects of treatment across study periods. |
Munters 2013.
| Methods | Evaluator‐blind, parallel‐group RCT | |
| Participants |
Sample size Intervention group: 5 adults with PM, 6 adults with DM, control group: 4 adults with PM, 6 adults with DM Inclusion criteria
Exclusion criteria
Baseline demographics 23 people with PM or DM (12 in the exercise group and 11 in the control group) were included in the 12‐week endurance exercise programme. All participants were in a stable disease phase with no to high damage and with unchanged medication for at least 1 month before inclusion in the study, as well as during the 12‐week intervention. |
|
| Interventions | Aerobic exercise and strength training vs no training Type of training and exercise Cycling exercises and muscular endurance exercises Intensity Aerobic exercise: during the first weeks, the exercise intensity was gradually increased from 50% up to 70% of the participants’ individual VO2 max. Strength training: 30%‐40% of 1RM Frequency 3 times/week, twice a week at a physical therapy department, once a week at home Setting Twice a week at the department of physical therapy at each of the 3 participating centres (Karolinska University Hospital, Sahlgrenska University Hospital, Uppsala University Hospital, Sweden) Duration Session: 1 h. Programme: 12 weeks Muscle groups Knee extensors Supervision Physical therapist at each participating centre |
|
| Outcomes |
Primary: primary outcome changed during the study from the FI2 to VO2 max Secondary FI2, SF‐36,The Myositis Activities Profile for limitations in ADL, 1RM measurements, disease activity was performed using the core set measures developed by the International Myositis Assessment and Clinical Studies, the maximum load a patient can lift in a full ROM in 5 repetitions (5 VRM) of knee extensors left and right. Secondary Power performed (in W) at the time of VO2 max was recorded, time cycling to exhaustion performed with a constant power at 65% of baseline VO2 max, manual muscle testing of 8 muscle groups was performed (maximal isometric strength of neck flexors, middle deltoid, gluteus maximus, gluteus medius, biceps brachii, wrist extensors, wrist flexors, ankle dorsiflexors) Time‐points measured Before and after 12 weeks of control or training period |
|
| Dates | Participants were recruited from 2007‐2011 | |
| Funding/ declarations of interest | Supported by grants from the Myositis Association, the Swedish Research Council, the Swedish Rheumatism Association, funds at the Karolinska Institutet, Promobilia, and through “The regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet.” | |
| Notes | Outcomes were not presented for DM and PM separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a randomisation list, patients were randomised by an independent nurse". |
| Allocation concealment (selection bias) | Low risk | Quote: "Using a randomisation list, patients were randomised by an independent nurse". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded as it is impossible to blind exercise training compared to no exercise training. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "assessments (...) were performed by a physical therapist at each respective centre, blinded to the type of intervention" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One patient in the exercise group was not able to perform the exercise programme and was excluded from the analysis". Follow‐up was therefore incomplete and analysis was not by ITT. Quote: "We aimed for nine patients in the exercise group, but some analyses were performed with N = 7 (VO2 max measurements) or N = 3 (mitochondrial enzyme activities). (...) All measurements were not successfully performed both before and after training in each subject". |
| Selective reporting (reporting bias) | Unclear risk | Quote: "Because PM [polymyositis] and DM [dermatomyositis] are rare conditions, the research group decided at the study onset to perform an interim analysis in case patient recruitment did not proceed as quickly as projected. VO2 max was determined to be more sensitive to change than the FI‐2 according to the type of exercise performed, and thereby was selected instead as the primary outcome and used for the interim analysis". Comment: primary outcome changed at the study onset from the FI2 to VO2 max |
| Other bias | Low risk | Comment:no risk of bias from other sources detected |
Alexanderson 2014.
| Methods | RCT | |
| Participants |
Sample size: 19 participants Intervention group: 5 adults with PM, 5 adults with DM Control group: 5 adults with PM, 4 adults with DM Inclusion criteria
Exclusion criteria Severe osteoporosis, concomitant malignancy, or cardiovascular disease contraindicating exercise Baseline demographics Median age 60.0 years, median diagnosis duration 3.0 months |
|
| Interventions |
Type of training and exercise Resistive home exercise programme Intensity Strength training: step up exercise for warm‐up, shoulder flexion and knee extension in a sitting position, hip flexion and abduction, pelvic lifts and sit‐ups lying down. Each exercise in 10 repetitions bilaterally, the programme ended with stretching. Exercise intensity was prescribed individually. Aerobic exercise: a 15‐min walk at an intensity level of 50%‐70% of participants' estimated maximal heart rate Frequency 5 times/week Setting The 1st 12 weeks at home, the second 12 weeks at home and/or at the gym, (Sweden) Duration Not described, but varied individually Supervision Weekly telephone support from the physical therapist |
|
| Outcomes |
Primary Muscle performance (the disease‐specific FI). The FI includes testing of correctly performed repetitions in 11 muscle groups: elbow flexion, shoulder flexion and abduction, hip flexion and abduction, step test, heel and toe lifts, neck flexion and trunk flexion, with additional tests of ability to transfer from side to side lying down, transfer up to sitting, and peak expiratory flow. Secondary Aerobic capacity using 8‐min submaximal treadmill test; quality of life (NHP); disease activity (CK and muscle biopsy) Time‐points measured At baseline, and after 24 weeks of training or control period |
|
| Dates | All patients with recent‐onset PM or DM from 1998‐2002 were consecutively invited to participate. | |
| Funding/ declarations of interest | "Supported by grants from the Swedish Research Council, the Swedish Rheumatism Association, King Gustaf V 80 Year Foundation, Funds at the Karolinska Institutet and through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet." | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were enrolled by any of two rheumatologists and randomised into an exercise group or a control group using a randomisation table". |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent nurse was responsible for the randomisation, which was concealed to the blinded assessors and the two rheumatologists responsible for patient enrolment throughout data collection. She informed the exercise supervisors about group allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded for group assignment, as it is impossible to blind exercise training compared to no exercise training. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Three well‐trained physical therapists blinded to group allocation, one at each participating centre, assessed patients recruited from their own centre and supervised the exercise for patients from any of the other centres (...) Study participants were not blinded to group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 2 participants were lost to follow‐up in the exercise group. However, analysis was by ITT. |
| Selective reporting (reporting bias) | Low risk | No evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Voet 2014.
| Methods | Evaluator‐blind, parallel‐group RCT | |
| Participants |
Sample size Intervention group: 20 adults with FSHD type 1, control group: 24 adults with FSHD type 1 Inclusion criteria Age ≥ 18 years, severe fatigue (CIS‐fatigue > 35), able to walk independently (ankle‐foot orthoses and canes are accepted), able to exercise on a bicycle ergometer, able to complete either type of intervention Exclusion criteria Cognitive impairment, insufficient mastery of the Dutch language, neurologic or orthopedic comorbidity interfering with the interventions or possibly influencing outcomes, pregnancy, use of psychotropic drugs (except simple sleeping medication), severe cardiopulmonary disease (chest pain, arrhythmia, pacemaker, cardiac surgery, severe exertional dyspnea, emphysema), epileptic seizures, poorly regulated diabetes mellitus or hypertension, clinical depression, as diagnosed with the Beck Depression Inventory for Primary Care. Baseline demographics Mean age participants control group: 52 years, mean age participants training group: 59 years. FSHD clinical score participants control group: 3.0, Ricci score training group: 3.0. Female/male ratio control group: 17/17, female/male ratio training group: 12/18. Duration of illness participants control group: 16.7 years, duration of illness participants training group: 13.0 years |
|
| Interventions | Aerobic exercise vs usual care (and a 3rd group with cognitive behaviour therapy) Type of training and exercise. Cycling exercises on an ergometer Intensity Resistance was increased until an increase of 50%‐65% in heart rate reserve was achieved. Frequency 3 times/week, twice a week at home, once a week at a rehabilitation centre Setting Once a week at the department of rehabilitation at one of 6 the participating centres (St Maartenskliniek Nijmegen, de Hoogstraat Utrecht, de Vogellanden Zwolle, het Roessingh Enschede, St Franciscus ziekenhuis Roosendaal, Reade Amsterdam, the Netherlands) Duration Session: 30 min, with additional warming‐up and cooling‐down periods of 5 and 3 min, respectively. Programme: 16 weeks Supervision Supervised by a physiotherapist |
|
| Outcomes |
Primary The subscale fatigue of the checklist individual strength Secondary Isometric strength for the quadriceps as MVIC, aerobic exercise tolerance (VO2 peak and distance walked in a 6‐min walk test), physical activity using actometers and a questionnaire, sleep quality, pain intensity, social participation restrictions Time‐points measured Before and after the intervention period of 16 weeks and after 12 weeks of follow‐up |
|
| Dates | Participants were enrolled from January 2009 through February 2012 | |
| Funding/ declarations of interest | N Voet received grants from Prinses Beatrix SpierFonds (PBF) (The Dutch Public Fund for Neuromuscular Disorders), the Netherlands Organisation for Health Research and Development (ID: ZonMW 89000003), and global FSH. G Bleijenberg, J Hendriks, I de Groot, and G Padberg report no disclosures relevant to the manuscript. B van Engelen was research director of the European Neuromuscular Centre (ENMC), received grants from global FSH, the Netherlands Organisation for Health Research and Development, Prinses Beatrix SpierFonds (PBF) (The Dutch Public Fund for Neuromuscular Disorders), and the Dutch FSHD Foundation. S. Geurts received grants from Prinses Beatrix SpierFonds (PBF) (The Dutch Public Fund for Neuromuscular Disorders), the Netherlands Organisation for Health Research and Development (ID: ZonMW 89000003), and global FSH. Go to Neurology.org for full disclosures. | |
| Notes | Outcomes were presented for the 3 treatment groups (i.e. aerobic exercise, cognitive behaviour therapy and usual care). Effect sizes were presented by group as well | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent research assistant allocated each participant to 16 weeks of AET [aerobic exercise training], CBT [cognitive behavioural therapy], or UC [usual care] using a computer‐generated randomisation block list". The block sizes varied randomly in order to prevent predictability of the allocation process. |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent research assistant allocated each participant to 16 weeks of AET [aerobic exercise training], CBT [cognitive behavioural therapy], or UC [usual care], using a computer‐generated randomisation block list". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded for group assignment, as it is impossible to blind exercise training compared to no exercise training. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All measurements were performed (...) by 2 blinded physical therapists" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 11 participants in the aerobic exercise group (39%) did not achieve the level of acceptable adherence. One participant in the aerobic exercise group withdrew due to time constraints. However, analysis was by ITT. Comment: in the usual care group, 4 participants withdrew just before the 2nd randomisation because they thought that the intervention would be too time‐consuming. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Andersen 2015.
| Methods | Evaluator blinded, parallel‐group RCT | |
| Participants |
Sample size: intervention group: 13 adults with FSHD type 1, control group n = 10 adults with FSHD type 1 Inclusion criteria Adults (18–65 years of age) with genetically verified FSHD type 1 (10 D4Z4 repeats in leukocytes DNA) Exclusion criteria Regular cardio exercise (> 2 h/week), pregnancy or breastfeeding, inability to cycle, or other disabilities than FSHD, which could confound the interpretation of the results Baseline demographics Mean age participants control group: 51.3 years, mean age participants training group: 45.7 years. FSHD clinical score participants control group: 5.9, FSHD clinical score participants training group: 6.2. Female/male ratio control group: 4/5, female/male ratio training group: 6/7 |
|
| Interventions | Aerobic exercise training vs no training (and as add‐on in a double blind randomised controlled design a protein supplement or placebo) Type of training and exercise Cycling exercises on an ergometer Intensity 70% of VO2 max Frequency 3 times/week Setting At the Copenhagen Neuromuscular Center (Denmark) Duration Session: 15 min in the first week, 20 min in the second week, 30 min thereafter. Programme: 12 weeks Supervision Participants reported in a diary and were supervised by phone. The number of phone contacts varied, depending on the individual need for supervision. |
|
| Outcomes |
Primary Fitness as VO2 max and maximal workload (Wmax) during an exhaustion test on a cycle ergometer, walking speed in a 6‐min walk test Secondary Mobility (5‐times sit‐to‐stand‐test, 14‐step‐stair‐test, standing balance test self‐assessed physical questionnaire, testing of knee and elbow flexion and extension strength; quality of life (SF‐36), fatigue and pain (VAS) Time‐points measured Before and after 12 weeks of control or training period |
|
| Dates | The study was performed from 21 March 2012, to 28 October 2013 | |
| Funding/ declarations of interest |
Funding The Aase and Einar Danielsens Foundation, Augustinus Foundation, AP Moeller Foundation, and The Danish Rheumatism Association Foundation. Declarations of interest "G Andersen, K Prahm, J Dahlqvist, and G Citirak report no disclosures relevant to the manuscript. J Vissing reports having received research support and honoraria from the Genzyme Corporation. He is a member of the Genzyme Pompe Disease Advisory Board. Go to Neurology.org for full disclosures." |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "We randomised patients to three groups. (..) The first participants were randomised with a dice to one of the three groups. To balance disease severity in groups, a stratification method for randomisation was then used called minimisation. Participants were randomised with discretion to essential factors that can influence outcomes: age (18‐40 to 41‐65 years), sex, and disease severity (FSHD clinical score; 0‐3 versus 4‐15). If an equal point in each group and number of participants was reached, participants were randomised with a dice to one of the three groups." Comment: taking into account the low number of participants in each group, we considered this method as inadequate. |
| Allocation concealment (selection bias) | High risk | Comment: study author (Andersen) informed us that 2 investigators enrolled and assigned participants. They were not blinded for the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: neither participants nor investigators were blinded to the exercise intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: neither participants nor investigators were blinded to the exercise intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: " In the analysis, we included data from seven patients who withdrew from the study at the 7‐week evaluation, but data from the six patients who were lost to follow‐up are only included in the intention‐to‐treat‐ analysis". Comment: in the control group, 1 participant was lost to follow‐up by an accidental fall. In the minimisation process, data from participants who were lost to follow‐up were excluded so analysis was not done by ITT. The total dropout rate is high: 39% |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Habers 2016.
| Methods | Parallel‐group RCT | |
| Participants |
Inclusion criteria People were eligible if they were diagnosed with juvenile DM by a paediatric rheumatologist/immunologist according to the Bohan and Peter criteria, and were aged 8‐18 years at time of enrolment in this study. Exclusion criteria People were excluded if: medical status contraindicated exercise testing; the patient (or the parents or caregivers) had an insufficient understanding of the Dutch language; a medical event that might interfere with the outcome of testing and/or the trial was present (such as a planned surgery); the rheumatologist advised against participation based on a recent relapse or the concurrent existence of other disease; and/or the person was already very active in sports without any restrictions and without a subjectively diminished exercise capacity. Baseline demographics The median (range) age at inclusion was 12.3 years and 62% were girls. The median (range) age at diagnosis was 7.1 years. The median (range) disease duration at inclusion was 4.4 years. Sample size Intervention group: 14 children and adolescents with juvenile DM, control group: 12 children and adolescents with juvenile DM |
|
| Interventions | Strength training and aerobic exercise training vs no training Type of training and exercise Interval treadmill training, strength training, at home Intensity Aerobic training: individualised work rate, 30 min leg exercise on an ergo cycle, 65%‐90% of the peak heart rate alternated with short periods of low‐intensity exercise (50%‐60% of peak heart rate) Strength training: the intensity of the strength training was set by time (20‐30 s for every single exercise), performing as many repetitions in 20‐30 s. Frequency 2‐3 times/week, total of 32 training sessions Setting At home (the Netherlands) Duration Session: 40 min‐60 min. Programme: 12 weeks Muscle groups Proximal muscle groups Supervision Parents were present for support and motivation. Every 2 weeks, a researcher or physiotherapist conducted a home visit for supervision, adjustments and motivation. |
|
| Outcomes |
Primary Aerobic exercise capacity Secondary Isometric muscle strength of knee and hip flexors and extensors, perception of fatigue, distance walked in a 6‐min walk test, quality of life, muscle function (distance standing long jump, amount of sit‐ups and push‐ups, time wall sit, time V‐up, muscle soreness, perception of fatigue (PedsQOL multidimensional fatigue scale), pain (VAS) Time‐points measured Before and after 12 weeks of control or training period and after 12 weeks of follow‐up |
|
| Dates | Participants were included from 2012‐2014 | |
| Funding/ declarations of interest |
Funding: "This work was supported by the Dutch Arthritis Foundation [11‐I‐202]." Disclosure statement: study authors have declared no conflicts of interest |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was a multicenter (four academic hospitals), stratified (age and gender), parallel‐group study (...) with a balanced randomisation performed by an independent and blinded person using computer generated lists of random numbers with randomly varying block sizes (2 or 4)". |
| Allocation concealment (selection bias) | Low risk | Quote: "This was a multicenter (four academic hospitals), stratified (age and gender), parallel‐group study (...) with a balanced randomisation performed by an independent and blinded person using computer generated lists of random numbers with randomly varying block sizes (2 or 4)". Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible: intervention was training vs no training |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researchers performing the assessments, as well as those charged with data analyses were blinded to treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all participants were included in the groups to which they were randomly assigned and the researchers made efforts too obtain outcome data for all participants, even if the intervention was not completed". Comment: 3 participants stopped the intervention prematurely. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Bankolé 2016.
| Methods | Parallel‐group RCT | |
| Participants |
Inclusion criteria Molecular FSHD Type 1 diagnosis, age ≥ 18 years, and the ability to perform the cycling programme in this study. Exclusion criteria A history of cardiovascular disease including cardiac arrhythmias and clinical cardiovascular anomaly, evidence of inflammatory syndrome or diabetes, abnormal coagulation, or BMI ≥ 35 kg m2 Baseline demographics Mean age participants control group: 41 years, mean age participants training group: 40 years. Female/male ratio control group: 1/7, female/male ratio training group: 3/5 Sample size Intervention group: 8 adults with FSHD type 1, control group: 8 adults with FSHD type 1 |
|
| Interventions | Strength training and aerobic exercise training vs no training Type of training and exercise Assisted bicycle home training programme Intensity 2 combined sessions consisted of aerobic exercise at a constant moderate intensity (60% of MAP) followed by steps of near‐maximal revolutions. Session 3 consisted of interval training with an intensity between 40% and 80% of MAP. The exercise intensity was individualised based on either observed heart rate reduction with training or the new MAP from incremental cycling sessions Frequency 3 times/week Session: 35 min. Programme: 24 weeks Setting At home (France) Muscle groups Muscles of the upper and lower leg Supervision The first 5‐10 training sessions were supervised by an experienced exercise physiologist. Every week, the exercise physiologist provided telephone support for 2 sessions and attended the 3rd session to supervise it and adjust the individualised exercise intensity |
|
| Outcomes |
Primary Peak oxygen uptake (the oxygen consumption during the last 30 s before task failure in an incremental cycling test) Secondary Maximal aerobic power in the last stage of an incremental cycling test; quadriceps neuromuscular function (isometric maximal voluntary contractions with femoral nerve magnetic stimuli delivered during MVCs and at rest); distance walked in a 6‐min walk test, quality of life (SF‐36); perceived fatigue (FSS) Time‐points measured Before and after 24 weeks of training or control period |
|
| Dates | Participants were enrolled October 2010‐August 2012 | |
| Funding/ declarations of interest |
Funding "This study was supported by the Association Française contre la Myopathie (AFM). The funding source had no role in study design, data collection, data analysis, data interpretation, or writing of the report. Landry‐Cyrille Bankolé was supported by a doctoral research grant from Örebro University and by the Association Française contre la Myopathie (AFM). John Temesi was supported by a doctoral research grant from the Rhône‐Alpes Region." The study authors have no conflicts of interest to disclose. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study administrative coordinator performed a computer‐generated pairwise randomisation by enrolment order, ensuring similar numbers of patients allocated to each group". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were informed of group allocation when a sealed envelope was opened in their presence". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"due to the nature of the training study, investigators involved in training and testing patients were unable to be blinded. All histological analyses were conducted by individuals blinded to training status". Comment: training interventions precluded participant blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "due to the nature of the training study, investigators involved in training and testing patients were unable to be blinded. All histological analyses were conducted by individuals blinded to training status". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Two patients dropped out in the training group before starting the programme, (...). Another patient, included in the control group, withdrew for unknown reasons (...). In total, 16 patients completed the study". Comment: analysis was not according ITT. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
Andersen 2017.
| Methods | Parallel‐group RCT | |
| Participants |
Sample size: 6 adults with FSHD1 randomised to intervention group, 7 adults with FSHD1 randomised to control group Inclusion criteria Age 18‐70 years and genetically verified FSHD1 Exclusion criteria Inability to cycle, regular cardio‐exercise (1 h/week), or factors that potentially could confound the results (pregnancy, breastfeeding, disabilities other than FSHD1, participation in other studies) Baseline demographics Mean age participants control group: 46 years, mean age participants training group: 53 years. FSHD clinical score participants control group: 7.5, FSHD clinical score participants control group: 6.3. Female/male ratio control group: 1/5, female/male ratio training group: 2/4 |
|
| Interventions | High‐intensity aerobic exercise training vs no training Type of training and exercise Cycling exercises on an ergometer Intensity Each min of HIT was performed at 3 different work intensities: 30 s of easy pedaling, 20 s of hard work and 10 s of all‐out, maximal intensity. Frequency 3 times/week Setting At the Copenhagen Neuromuscular Center (Denmark) and at home Duration Session: 21 min including an 8‐min standardised warm‐up and two sets of 5‐min HIT separated by a 3‐min break at very low intensity. Programme: 8 weeks Supervision 1 weekly session was performed in the clinic. All participants received live training instructions and a recorded training guide for home use. |
|
| Outcomes |
Primary Fitness as VO2 max and during an exhaustion test on a cycle ergometer Secondary Maximal workload (Wmax), meters walked in a 6‐min walk test, 5‐times sit‐to‐stand‐test, static muscle strength of hip and knee flexion and extension Time‐points measured Before and after 8 weeks of control or training period |
|
| Dates | Study dates not reported | |
| Funding/ declarations of interest | Study authors declare that they have no competing interests "We thank Aase and Einar Danielsens Foundation, Augustinus Foundation, and AP Moeller Foundation for financial support." |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "According to the zip‐code, patients living close to our clinic were randomised to supervised HIT." |
| Allocation concealment (selection bias) | High risk | Quote: "According to the zip‐code, patients living close to our clinic were randomised to supervised HIT." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: neither participants nor investigators were blinded to the exercise intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: neither participants nor investigators were blinded to the exercise intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "12 of the 13 patients completed the controlled part of the study." Comment: analysis was not done by ITT |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence found for selective reporting |
| Other bias | Low risk | Comment: no risk of bias from other sources detected |
1RM: one repetition maximum; ADL: activities of daily living; AMPS: Assessment of Motor and Process Skills; BMI: body‐mass index; CIS: Checklist Individual Strength; CK: creatine kinase; COPM: Canadian Occupational Performance Measure; DM: dermatomyositis; DM1: myotonic dystrophy type 1; DMD: Duchenne muscular dystrophy; FEV: forced expiratory volume; FI: functional index; FSHD: facioscapulohumeral muscular dystrophy; FSS: fatigue severity scale; FVC: forced vital capacity; HIT: high‐intensity training; ITT: intention‐to‐treat; MAP: maximal aerobic power; MFM: motor function measure; MIRS: muscular impairment rating scale; MM: mitochondrial myopathy; MRC: Medical Research Council; MVC: maximum voluntary contractions; MVIC: maximum voluntary isometric strength; N: Newton; NHP: Nottingham Health Profile; PM: polymyositis; PEDI: Pediatric Evaluation of Disability Inventory; RCT: randomised controlled trial; RM: repetition maximum; ROM: range of motion; SD: standard deviation; SF‐36: short form health survey; VAS: visual analogue scale; VO2 max: maximal oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramson 1952 | Not a RCT |
| Aitkens 1993 | Not a RCT. Exercised versus non‐exercised control limb (randomly assigned) and patients versus healthy volunteers |
| Aldehag 2005 | Not a RCT |
| Alemdaroglu 2015 | A RCT that makes a comparison between 2 different training regimes. No comparison of training versus non‐training participants |
| Alexanderson 1999 | Pilot study. Not a RCT |
| Alexanderson 2000 | Extension of a pilot study Alexanderson 1999. Not a RCT |
| Alexanderson 2007 | Not a RCT |
| Arnardottir 2003 | Not a RCT |
| Berthelsen 2014 | Not a RCT |
| Chung 2007 | No non‐exercising control group |
| Cudia 2016 | RCT that makes a comparison between 2 different training regimes. No comparison of training versus non‐training participants |
| Dastmalchi 2007 | Not a RCT |
| Dawes 2006 | Both study groups consisted of participants with various muscle diseases and the study authors did not present outcome measures for each muscle disease separately. |
| De Lateur 1979 | Not a RCT. Exercised versus non‐exercised control limb (randomly assigned) |
| Escalante 1993 | Not a RCT |
| Florence 1984a | Not a RCT |
| Florence 1984b | Not a RCT |
| Fowler 1965 | Not a RCT. Exercise combined with medication |
| Hedermann 2016 | Not a RCT |
| Heikkila 2001 | Not a RCT. Training programme duration of 3 weeks |
| Hicks 1989 | Not a RCT. Training programme duration of 1 month |
| Hoberman 1955 | Not a RCT. 3 drugs added to a comprehensive regimen of therapies, including breathing and resistive exercises |
| Jeppesen 2006 | Not a RCT |
| Jeppesen 2009 | Not a RCT |
| Johnson 2007 | Not a RCT |
| Johnson 2009 | Not a RCT |
| Kelm 2001 | Not a RCT |
| Kilinc 2015 | A RCT that makes a comparison between 2 different training regimes. No comparison of training versus non‐training participants |
| Kilmer 1994 | Not a RCT. Exercised versus non‐exercised control limb (randomly assigned) and patients versus healthy volunteers |
| Kilmer 2005 | Not a RCT |
| Lenman 1959 | Not a RCT. Training programme duration for participants with muscle disorders ranged from approximately 1 to 21 months |
| Mattar 2014 | Not a RCT |
| McCartney 1988 | Not a RCT. Exercised versus non‐exercised control limb (randomly assigned) |
| Mielke 1990 | Not a RCT |
| Milner‐Brown 1988a | Not a RCT. Training programme duration for participants with muscle disorders ranged from approximately 2‐48 months |
| Milner‐Brown 1988b | Not a RCT. Intervention is not training versus non‐training, but training added to electric stimulation or electric stimulation only in 1 limb versus a non‐stimulated, non‐exercised control limb |
| Milner‐Brown 1990 | Not a RCT. Intervention is not training versus no training, but amitriptyline added to strength training |
| Murphy 2008 | Not a RCT |
| Na 1996 | Not a RCT. Intervention is not training versus non‐training, but training and daily quinine sulfate |
| Nader 2010 | Not a RCT |
| Olsen 2005 | Not a RCT |
| Omori 2010 | Not a RCT |
| Omori 2012 | Not a RCT |
| Orngreen 2005 | Not a RCT |
| Regardt 2014 | Not a RCT |
| Riisager 2013 | Not a RCT |
| Scott 1981 | RCT that makes a comparison between 2 different training regimes. No comparison of training versus non‐training participants |
| Siciliano 2000 | Not a RCT |
| Siciliano 2012 | Not a RCT |
| Spector 1997 | Not a RCT |
| Sriram 2015 | A RCT that makes a comparison between 2 different training regimes. No comparison of training versus non‐training participants |
| Sunnerhagen 2004 | Not a RCT |
| Sveen 2007 | Not a RCT |
| Sveen 2008 | Not a RCT |
| Sveen 2013 | Not a RCT |
| Taivassalo 1998 | Not a RCT |
| Taivassalo 1999 | Not a RCT |
| Taivassalo 2001 | Not a RCT |
| Taivassalo 2006 | Not a RCT |
| Tiffreau 2017 | A RCT that makes a comparison between 2 different training regimes. No comparison of training versus non‐training participants |
| Tollbäck 1999 | Not a RCT. Exercised versus non‐exercised control limb (randomly assigned) |
| Trenell 2006 | Not a RCT |
| Van den Berg 2015 | Not a RCT |
| Varju 2003 | Not a RCT. Training programme duration of 3 weeks |
| Vignos 1966 | Not a RCT |
| Wiesinger 1998b | A non‐randomised extension of a RCT (Wiesinger 1998a) |
| Wright 1996 | Not a RCT |
| Yildirim 2007 | Not a RCT |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Jorgensen 2016.
| Trial name or title | Blood‐flow restricted exercise in inclusion body myositis |
| Methods | 22 participants diagnosed with sporadic IBM were tested for maximal unilateral isometric knee extensor muscle strength in both legs (mean of right and left leg is presented), using an isokinetic dynamometer (KinCom; Chattecx Corp., Chattanooga, TN, USA). Following baseline testing participants were randomised to a blood‐flow restricted (BFR)‐training group (BFR, n = 11) or to a non‐exercising control group (CON, n = 11). |
| Participants | 22 participants diagnosed with sporadic IBM (4 female, 18 male, 69.0+/‐5.6 years) |
| Interventions | The BFR group performed unilateral BFR training for both legs (leg press, knee extension, knee flexion, dorsal flexion and plantar flexion, 3‐4 sets per exercise) 2 times/week for 12 weeks. Exercise intensity (training loads) was ˜25 RM and blood‐flow restriction was achieved using an inflatable pneumatic cuff applied at the proximal part of the shank/thigh. Cuff pressure (110 mmHg) was maintained throughout all sets and pauses while released by the cessation of the final set of each exercise, before continuing with the next exercise. |
| Outcomes | Participant‐reported physical function on the (SF‐36) Health Survey subscale: Physical Function Myositis Disease Activity Assessment Tool Myositis Damage Index Physician/Patient Global activity (VAS) Physician/Patient Global Damage (VAS) 2‐min walk test Manual Muscle Testing Chair rise Timed up & go Health assessment questionnaire Inclusion Body Myositis Functional Rating Scale |
| Starting date | January 2015 |
| Contact information | Louise Pyndt Diederichsen, MD, PhD Louise.Diederichsen@syd.dk Per Aagaard, Prof, PhD, paagaard@health.sdu.dk |
| Notes | The results will be published soon |
NCT02158156.
| Trial name or title | Effect of aerobic training in patients with oculopharyngeal muscular dystrophy (OPMD) |
| Methods | No information available |
| Participants | Danish people with OPMD |
| Interventions | Aerobic exercise for 10 weeks |
| Outcomes |
Primary VO2 max Secondary 6‐min walk test, 14‐step stair test, 5‐time‐repetition‐sit‐to‐stand‐test, dynamometry, intensity in maximal load (Watt), level of plasma CK, level of plasma myoglobin, SF‐36 questionnaire |
| Starting date | February 2014 |
| Contact information | Not available |
| Notes |
NCT02421523.
| Trial name or title | Strength training in Duchenne muscular dystrophy (DMD) |
| Methods | Development of a strength training protocol in DMD |
| Participants | Children with DMD, aged 7‐10.5 years |
| Interventions | Procedure: Aim 1 exercise dosing Procedure: Aim 2 control group Procedure: Aim 2 exercise group |
| Outcomes |
Primary outcome Change from baseline in T2‐weighted MRI of skeletal muscle in leg for Aim 2 Change in baseline in T2 weighted MRI of skeletal muscle in leg for Aim 1 |
| Starting date | May 2015 |
| Contact information | djlottpt@phhp.ufl.edu |
| Notes |
Van Engelen 2015.
| Trial name or title | OPTIMISTIC |
| Methods | 2‐arm multi‐centre, randomised trial |
| Participants | 296 (male and female) people with myotonic dystrophy type 1 to be recruited |
| Interventions | At baseline participants will choose an activity programme with the counsellor, either a low‐intensity, graded physical activity programme and an exercise programme aimed at an increased physical fitness:
The intervention runs for 10 months but is front‐loaded, meaning the first 4 months can be considered the ‘active’ phase with the remaining 6 months in the ‘booster’ phase. In this period of 10 months a participant will receive 10‐14 sessions; at least 5 of them are face‐to‐face sessions. For the other sessions the therapist can decide, dependent on the travelling distance and the mobility of the participant, to use telephone contact or video conferencing as an alternative. In addition, all therapists will receive 1 support call every 2 weeks by telephone from an experienced cognitive behavioural therapist in the OPTIMISTIC team, with extra support available by email. |
| Outcomes |
Primary outcome The DM1‐Activ measured at the end of the 10‐month intervention period Secondary outcomes: 6‐min walk test with BORG Scale assessment Fatigue and Daytime Sleepiness Scale CIS subscale fatigue severity Individualised Neuromuscular Quality of Life Questionnaire |
| Starting date | Study start date: April 2014. Completion date: October 2016. Results expected to be published: from the end of 2017 |
| Contact information | CP Okkersen, MD. Kees.Okkersen@radboudumc.nl |
| Notes |
Veenhuizen 2015.
| Trial name or title | ENERGETIC |
| Methods | Multicentered, assessor‐blinded, 2‐armed RCT |
| Participants | A total of 50 adult participants with an established neuromuscular disease, preferably FSHD, IBM, or MM |
| Interventions | During 4 months (16 weeks) participants receive individually tailored aerobic exercise training from the physical therapist, amounting to sessions of 90 min with regular breaks as needed; during the first 9 weeks twice a week and during the last 7 weeks once weekly. Training intensity is aimed at 50%‐70% of the maximum heart rate, guided by a cardiac rhythm monitor mounted on the chest that is read out by a wrist watch. The training includes different exercises, such as walking on a treadmill, cycling on a home trainer, rowing, and using a cross‐trainer, depending on the preference and motor abilities of the individual. |
| Outcomes |
Primary The participant’s self‐rated performance in 3‐5 self‐identified problematic daily occupations assessed with the Canadian Occupational Performance Measure Secondary CIS ‐ subscale Fatigue 6‐min walk test Health‐related quality of life: SF‐36 |
| Starting date | Study start date: July 2014. Completion date: November 2016. Results expected to be published: from the end of 2017 |
| Contact information | Yvonne Veenhuizen MSc, yvonne.veenhuizen@radboudumc.nl |
| Notes |
Wallace 2016.
| Trial name or title | Evaluating the benefits of community based aerobic training on the physical health and well‐being of people with neuromuscular disease |
| Methods | A cross‐over RCT of aerobic exercise to ascertain the effect of training on fitness levels, muscle strength, walking abilities and general well‐being |
| Participants | 28 people with Charcot Marie Tooth 1A and 17 people with IBM |
| Interventions | Aerobic exercise |
| Outcomes | The primary outcome measure is oxygen uptake during a maximal exercise test and will be analysed using hierarchical ("random effect") models |
| Starting date | |
| Contact information | Gita Ramdharry, PhD g.ramdharry@ucl.ac.uk |
| Notes | Article with results submitted |
AET: aerobic exercise therapy; BFR: blood flow restricted; CBT: cognitive behavioural therapy; CIS: Checklist Individual Strength; CK: creatine kinase; DM1: myotonic dystrophy type 1; DMD: Duchenne muscular dystrophy; FSHD: facioscapulohumeral dystrophy; IBM: inclusion body myositis; MM: mitochondrial myopathy; MRI: magnetic resonance imaging; OPMD: oculopharyngeal muscular dystrophy; RCT: randomised controlled trial; RM: repetition maximum; SF‐36: Short‐Form 36; VAS: visual analogue scale
Differences between protocol and review
This review has a published protocol (Van der Kooi 2002). As stated in a previous update, we:
excluded all studies using a within‐subjects design with the non‐exercised limb as a control (De Lateur 1979; McCartney 1988; Aitkens 1993; Kilmer 1994; Tollbäck 1999);
included exercise programmes with a minimum duration of six, rather than 10 weeks as previously specified. Because of this change of protocol, we included one study which was excluded in the previous update (Wiesinger 1998a) and one new study (Kierkegaard 2011);
added a statement that we would exclude studies in which outcomes were not presented separately for each muscle disease. We excluded one randomised controlled strength training combined with aerobic exercise study for this reason. No specific details about the exercise programme were provided and we considered the risk of bias in the study high(Dawes 2006);
added a statement that we did not consider data from the subsequent (second) period of the cross‐over trial for analysis;
added a statement that we excluded studies with a high risk of bias from the meta‐analysis;
updated the definitions in Types of interventions;
updated and changed the diagnostic criteria to 'confirmed by muscle biopsy or genetic testing';
updated the exercise guidelines (Garber 2011);
searched the Cochrane Rehabilitation and Related Therapies Field Register in October 2002, August 2008, and July 2009. As, in the past, it yielded no results and is no longer available, it has been removed from the Methods section;
reported all forms of outcome measurement for a domain, when there were several forms of outcome measurement in one study.
For this update, for compliance with Cochrane Methodological Expectations for Cochrane Intervention Reviews standards (MECIR 2018), we:
separated performance and detection bias, as now recommended;
searched clinical trials registries;
included 'Summmary of findings' tables;
added a PRISMA flow chart.
Contributions of authors
NBM Voet and EL van der Kooi identified and assessed potentially relevant studies, and extracted the data from included studies. NBM Voet prepared the final draft. ACH Geurts, BGM van Engelen and EL van der Kooi edited each drafted and approved the final text of the review.
E Lindeman (deceased) and I Riphagen contributed to the original review and former updates.
Sources of support
Internal sources
-
Department of Rehabilitation, Radboud University Medical Centre, Nijmegen, Netherlands.
salary
-
Department of Neurology, Radboud University Medical Centre, Nijmegen, Netherlands.
Salary
-
Rehabilitation Centre Klimmendaal, Arnhem, Netherlands.
Salary
-
Department of Neurology, Medical Centre Leeuwarden, Leeuwarden, Netherlands.
Salary
External sources
No sources of support supplied
Declarations of interest
E van der Kooi carried out a randomised controlled trial (RCT) on the effect of strength training and albuterol in facioscapulohumeral muscular dystrophy (FSHD; Van der Kooi 2004).
NBM Voet received grant support from the Netherlands Organisation for Health Research and Development (ZonMw), Princess Beatrix Muscle Fund (PBS), and the Dutch FSHD Foundation.
NBM Voet, BGM van Engelen and ACH Geurts carried out a RCT on the effect of aerobic exercise in FSHD (Voet 2014).
BGM van Engelen was research director of the European Neuromuscular Centre and receives institutional support from the Radboud University Medical Centre and the ENMC, grant support from European Union’s Horizon 2020 research and innovation programme (Murab), European Union 7th Framework Programme (OPTIMISTIC), the Netherlands Organisation for Scientific Research (NWO), The Netherlands Organisation for Health Research and Development (ZonMw), Global FSH, Prinses Beatrix Fonds, Spieren voor Spieren, Association Francaise contre les Myopathies, and the Dutch FSHD Foundation. He is consultant and clinical advisor of Fulcrum.
ACH Geurts receives institutional support from the Radboud University Medical Centre, and grant support from the Netherlands Organisation for Scientific Research (NWO), The Netherlands Organisation for Health Research and Development (ZonMw), Princess Beatrix Muscle Fund (PBS), and the Dutch FSHD Foundation.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aldehag 2013 {published data only}
- Aldehag A, Jonsson H, Lindblad J, Kottorp A, Ansved T, Kierkegaard M. Effects of hand‐training in persons with myotonic dystrophy type 1 ‐ a randomised controlled cross‐over pilot study. Disability and Rehabilitation 2013;35(21):1798‐807. [PUBMED: 23480644] [DOI] [PubMed] [Google Scholar]
Alexanderson 2014 {published data only}
- Alexanderson H, Alemo Munters L, Dastmalchi M, Loell I, Heimbürger M, Opava CH, et al. Resistive home exercise in patients with recent‐onset polymyositis and dermatomyositis ‐ a randomized controlled single‐blinded study with a 2‐year followup. Journal of Rheumatology 2014;41(6):1124‐32. [PUBMED: 24786930] [DOI] [PubMed] [Google Scholar]
Andersen 2015 {published data only}
- Andersen G, Prahm KP, Dahlquist J, Citirak G, Vissing J. Does endurance training and protein supplementation improve fitness in patients with facioscapulohumeral muscle dystrophy (FSHD)?. Neuromuscular Disorders 2013;23(9‐10):824‐5. [EMBASE: 71938318] [Google Scholar]
- Andersen G, Prahm KP, Dahlqvist JR, Citirak G, Vissing J. Aerobic training and postexercise protein in facioscapulohumeral muscular dystrophy: RCT study. Neurology 2015;85(5):396‐403. [PUBMED: 26156512] [DOI] [PubMed] [Google Scholar]
Andersen 2017 {published data only}
- Andersen G, Heje K, Buch AE, Vissing J. High‐intensity interval training in facioscapulohumeral muscular dystrophy type 1: a randomized clinical trial. Journal of Neurology 2017;264(6):1099‐106. [PUBMED: 28470591] [DOI] [PubMed] [Google Scholar]
- Buch A, Andersen G, Borup Heje Pedersen K, Vissing J. High intensity training in patients with facioscapulohumeral muscular dystrophy. Neuromuscular Disorders 2015;25(Suppl 2):S215. [EMBASE: 72163848] [Google Scholar]
Bankolé 2016 {published data only}
- Bankole L, Millet GY, Temesi J, Wuyam B, Bachasson D, Kadi F, et al. 24‐weeks supervised and home‐based training program improves motor function in FSHD patients. Annals of Physical and Rehabilitation Medicine 2014;57:e96‐e97. [Google Scholar]
- Bankolé LC, Millet GY, Temesi J, Bachasson D, Ravelojaona M, Wuyam B, et al. Safety and efficacy of a 6‐month home‐based exercise program in patients with facioscapulohumeral muscular dystrophy. A randomized controlled trial. Medicine 2016;95(31):e4497. [PUBMED: 27495097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cejudo 2005 {published data only}
- Cejduo P, Bautista J, Montemayor T, Villagomez R, Jimenez L, Ortega F, et al. Exercise training in mitochondrial myopathy: a randomized clinical trial. Muscle & Nerve 2005;32(3):342‐50. [PUBMED: 15962332] [DOI] [PubMed] [Google Scholar]
Habers 2016 {published data only}
- Habers EA, Brussel M, Langbroek‐Amersfoort AC, Royen‐Kerkhof A, Takken T. Design of the Muscles in Motion study: a randomized controlled trial to evaluate the efficacy and feasibility of an individually tailored home‐based exercise training program for children and adolescents with juvenile dermatomyositis. BMC Musculoskeletal Disorders 2012;13:108. [PUBMED: 27018060] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habers GE, Bos GJ, Royen‐Kerkhof A, Lelieveld OT, Armbrust W, Takken T, et al. Muscles in Motion: a randomized controlled trial on the feasibility, safety and efficacy of an exercise training programme in children and adolescents with juvenile dermatomyositis. Rheumatology (Oxford, England) 2016;55(7):1251‐62. [PUBMED: 27018060] [DOI] [PubMed] [Google Scholar]
Jansen 2013 {published and unpublished data}
- Jansen M, Groot IJ, Alfen N, Geurts AC. Physical training in boys with Duchenne muscular dystrophy: the protocol of the No Use is Disuse study. BMC Pediatrics 2010;10:55. [PUBMED: 20691042] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, Alfen N, Geurts AC, Groot IJ. Assisted bicycle training delays functional deterioration in boys with Duchenne muscular dystrophy: the randomized controlled trial "no use is disuse". Neurorehabilitation and Neural Repair 2013;27(9):816‐27. [DOI: 10.1177/1545968313496326; PUBMED: 23884013] [DOI] [PubMed] [Google Scholar]
- Jansen M, Alfen N, Geurts AC, Groot IJ. Assisted bicycle training delays physical deterioration in boys with Duchenne muscular dystrophy: results of the randomized controlled trial "no use is disuse". Neuromuscular Disorders 2011;21:658. [EMBASE: 71943412] [Google Scholar]
Kierkegaard 2011 {published data only}
- Kierkaard M, Harms‐RIngdahl K, Edstrom L, Widen Holmqvist L, Tollback A. Feasibility and effects of a physical exercise programme in adults with myotonic dystrophy type 1: a randomized controlled pilot study. Journal of Rehabilitation Medicine 2011;43(8):695‐702. [PUBMED: 21670942] [DOI] [PubMed] [Google Scholar]
- Kierkegaard M, Harms‐Ringdahl K, Widen Holmqvist L, Edstrom L, Tollback A. Compliance and feasibility of a physical exercise programme in adults with myotonic dystrophy type 1. Physiotherapy (United Kingdom) 2011;97:eS608‐eS609. [EMBASE: 71883003] [DOI] [PubMed] [Google Scholar]
- Kierkegaard M, Widen Holmqvist L. Effects of a physical exercise programme in adults with myotonic dystrophy type 1 ‐ a one‐year follow‐up study. Neuromuscular Disorders 2012;22(9‐10):895‐6. [EMBASE: 71943283] [Google Scholar]
Lindeman 1995 {published and unpublished data}
- Lindeman E, Drukker J. Specificity of strength training in neuromuscular disorders. Journal of Rehabilitation Sciences 1994;7(3 Suppl):13‐5. [EMBASE: 24292073] [Google Scholar]
- Lindeman E, Leffers P, Spaans F, Drukker J, Reulen J, Kerckhoffs M, et al. Strength training in patients with myotonic dystrophy and hereditary motor and sensory neuropathy: a randomized clinical trial. Archives of Physical Medicine and Rehabilitation 1995;76(7):612‐20. [PUBMED: 7605179] [DOI] [PubMed] [Google Scholar]
- Lindeman E, Spaans F, Reulen J, Leffers P, Drukker J. Progressive resistance training in neuromuscular patients. Effects on force and surface EMG. Journal of Electromyography and Kinesiology 1999;9(6):379‐84. [PUBMED: 10597050] [DOI] [PubMed] [Google Scholar]
Munters 2013 {published data only}
- Alemo Munters L, Dastmalchi M, Andgren V, Emilson C, Bergegård J, Regardt M, et al. Improvement in health and possible reduction in disease activity using endurance exercise in patients with established polymyositis and dermatomyositis: a multicenter randomized controlled trial with a 1‐year open extension followup. Arthritis Care & Research 2013;65(12):1959‐68. [DOI: 10.1002/acr.22068; PUBMED: 23861241] [DOI] [PubMed] [Google Scholar]
- Alemo Munters L, Dastmalchi M, Katz A, Esbjörnsson M, Loell I, Hanna B, et al. Improved exercise performance and increased aerobic capacity after endurance training of patients with stable polymyositis and dermatomyositis. Arthritis Research & Therapy 2013;15(4):R83. [PUBMED: 23941324] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemo Munters L, Dastmalchi M, Lindroos E, Ottosson C, Alexanderson H, Crofford LJ, Lundberg IE, et al. Metabolic effects in skeletal muscle of endurance exercise in patients with polymyositis and dermatomyositis: a pilot study. Annals of the Rheumatic Diseases 2015;74(Suppl 2):828. [EMBASE: 72153241] [Google Scholar]
- Boehler J, Hogarth M, Barberio M, Ghimbovschi S, Brown K, Alemo Munters L, et al. A systems biology approach to investigating beneficial effects of endurance exercise in myositis patients. Arthritis & Rheumatology 2016;68(Suppl 10):76‐7. [EMBASE: 613886480] [Google Scholar]
- Boehler JF, Hogarth MW, Barberio MD, Novak JS, Ghimbovschi S, Brown KJ, et al. Effect of endurance exercise on microRNAs in myositis skeletal muscle‐a randomized controlled study. PLoS One 2017;12(8):e0183292. [PUBMED: 28829792] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munters L, Loell I, Ossipova E, Raouf J, Dastmalchi M, Lindroos E, et al. Endurance exercise improves molecular pathways of aerobic metabolism in patients with myositis. Arthritis & Rheumatology 2016;68(7):1738‐50. [PUBMED: 26867141] [DOI] [PubMed] [Google Scholar]
- Munters LA, Loell IM, Raouf J, Dastmalchi M, Lindroos E, Ottosso C, et al. A randomized controlled, clinical, histological and mRNA profiling pilot study of endurance exercise in myositis. Arthritis & Rheumatism 2013;65(Suppl 10):S757. [EMBASE: 71319242] [Google Scholar]
Van der Kooi 2004 {published and unpublished data}
- Kooi EL, Kalkman JS, Lindeman E, Hendriks JC, Engelen BG, Bleijenberg G, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. Journal of Neurology 2007;254(7):931‐40. [PUBMED: 17361345] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooi EL, Vogels OJ, Asseldonk RJ, Lindeman E, Hendriks JC, Padberg GW. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 2001;56(8 Suppl 3):A80. [DOI] [PubMed] [Google Scholar]
- Kooi EL, Vogels OJ, Asseldonk RJ, Lindeman E, Hendriks JC, Padberg GW. The efficacy of strength training in facioscapulohumeral muscular dystrophy. Neurology 2000;54(Suppl 3):A435. [DOI] [PubMed] [Google Scholar]
- Kooi EL, Vogels OJ, Asseldonk RJ, Lindeman E, Hendriks JC, Wohlgemuth M, et al. Strength training and albuterol in facioscapulohumeral muscular dystrophy. Neurology 2004;63(4):702‐8. [PUBMED: 15326246] [DOI] [PubMed] [Google Scholar]
Voet 2014 {published and unpublished data}
- Janssen B, Voet N, Geurts A, Engelen B, Heerschap A. Quantitative MRI reveals decelerated fatty infiltration in muscles of active FSHD patients. Neurology 2016;86(18):1700‐7. [PUBMED: 27037227] [DOI] [PubMed] [Google Scholar]
- Engelen BG, Janssen B, Voet N, Nabuurs C, Padberg GW, Geurts A, et al. Physical activity decreases fatty infiltration in FSHD, a quantitative muscle MR study. Neuromuscular Disorders 2012;22(9‐10):896. [EMBASE: 71943284] [Google Scholar]
- Engelen BG, Voet N, Janssen B, Bleijenberg G, Hendriks J, Groot I, et al. Aerobic exercise and cognitive behavior therapy reduce fatigue and slow progression of muscle MR fatty infiltration in FSHD. Neuromuscular Disorders 2014;24(9‐10):798‐9. [EMBASE: 71909243] [Google Scholar]
- Voet N, Bleijenberg G, Groot I, Padberg G, Engelen B, Geurts A. Both aerobic exercise training and cognitive behavior therapy reduce chronic fatigue in patients with facioscapulohumeral muscular dystrophy: a randomized controlled trial. Annals of Physical and Rehabilitation Medicine 2014;57:e96. [EMBASE: 71517165] [Google Scholar]
- Voet N, Bleijenberg G, Hendriks J, Groot I, Padberg G, Engelen B, et al. Both aerobic exercise and cognitive‐behavioral therapy reduce chronic fatigue in FSHD: an RCT. Neurology 2014;83(21):1914‐22. [PUBMED: 25339206] [DOI] [PubMed] [Google Scholar]
- Voet NB, Bleijenberg G, Hendriks JC, Groot IJ, Padberg GW, Engelen BG. Both aerobic exercise and cognitive‐behavioral therapy reduce fatigue in FSHD: an RCT [Minder vermoeid bij facioscapulohumerale dystrofie: RCT naar effect van aerobe training en cognitieve gedragstherapie]. Nederlands Tijdschrift Voor Geneeskunde 2015;159(12):A8806. [EMBASE: 604740746] [Google Scholar]
- Voet NB, Bleijenberg G, Padberg GW, Engelen BG, Geurts AC. Effect of aerobic exercise training and cognitive behavioural therapy on reduction of chronic fatigue in patients with facioscapulohumeral dystrophy: protocol of the FACTS‐2‐FSHD trial. BMC Neurology 2010;10:56. [PUBMED: 20591139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiesinger 1998a {published data only}
- Wiesinger GF, Quittan M, Aringer M, Seeber A, Volc‐Platzer B, Smolen J, et al. Improvement of physical fitness and muscle strength in polymyositis/dermatomyositis patients by a training programme. British Journal of Rheumatology 1998;37(2):196‐200. [PUBMED: 9569076] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abramson 1952 {published data only}
- Abramson AS, Rogoff J. An approach to the rehabilitation of children with muscular dystrophy. Physical treatment in muscular dystrophy. 1st and 2nd Medical Conferences, Muscular Dystrophy Association of America. New York, 1952:123‐5.
Aitkens 1993 {published data only}
- Aitkens SG, McCrory MA, Kilmer DD, Bernauer EM. Moderate resistance exercise program: its effect in slowly progressive neuromuscular disease. Archives of Physical Medicine and Rehabilitation 1993;74(7):711‐5. [DOI] [PubMed] [Google Scholar]
Aldehag 2005 {published data only}
- Aldehag AS, Jonsson H, Ansved T. Effects of a hand training programme in five patients with myotonic dystrophy type 1. Occupational Therapy International 2005;12(1):14‐27. [DOI] [PubMed] [Google Scholar]
Alemdaroglu 2015 {published data only}
- Alemdaroglu I, Karaduman A, Topaloglu H. Effects of upper extremity dynamic exercise on respiratory function and quality of life in Duchenne muscular dystrophy [Duchenne muskuler distrofi'de ust ekstremite dinamik egzersizinin solunum fonksiyonu ve yasam kalitesi uzerine etkisi]. Fizyoterapi Rehabilitasyon 2014;25(2):78‐85. [EMBASE: 600480405] [Google Scholar]
- Alemdaroglu I, Karaduman A, Yilmaz OT, Topaloglu H. Different types of upper extremity exercise training in Duchenne muscular dystrophy: effects on functional performance, strength, endurance, and ambulation. Muscle & Nerve 2015;51(5):697‐705. [PUBMED: 25196721] [DOI] [PubMed] [Google Scholar]
- Alemdaroglu I, Karaduman AA, Yulmaz O, Topaloglu H. Effects of upper extremity exercise training on respiratory function and quality of life in children with Duchenne muscular dystrophy. Neuromuscular Disorders 2013;23(9‐10):779. [EMBASE: 71938172] [Google Scholar]
Alexanderson 1999 {published data only}
- Alexanderson H, Stenström CH, Lundberg I. Safety of a home exercise programme in patients with polymyositis and dermatomyositis: a pilot study. Rheumatology 1999;38(7):608‐11. [DOI] [PubMed] [Google Scholar]
Alexanderson 2000 {published data only}
- Alexanderson H, Stenström CH, Jenner G, Lundberg I. The safety of a resistive home exercise program in patients with recent onset active polymyositis or dermatomyositis. Scandinavian Journal of Rheumatology 2000;29(5):295‐301. [DOI] [PubMed] [Google Scholar]
Alexanderson 2007 {published data only}
- Alexanderson H, Dastmalchi M, Esbjornsson‐Liljedahl M, Opava CH, Lundberg IE. Benefits of intensive resistance training in patients with chronic polymyositis or dermatomyositis. Arthritis & Rheumatism 2007;57(5):768‐77. [DOI] [PubMed] [Google Scholar]
Arnardottir 2003 {published data only}
- Arnardottir S, Alexanderson H, Lundberg IE, Borg K. Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. Journal of Rehabilitation Medicine 2003;35(1):31‐5. [DOI] [PubMed] [Google Scholar]
Berthelsen 2014 {published data only}
- Berthelsen MP, Husu E, Christensen SB, Prahm KP, Vissing J, Jensen BR. Anti‐gravity training improves walking capacity and postural balance in patients with muscular dystrophy. Neuromuscular Disorders 2014;24(6):492‐8. [PUBMED: 24684860] [DOI] [PubMed] [Google Scholar]
- Jensen BR, Berthelsen MP, Husu E, Christensen SB, Prahm KP, Vissing J. Body weight‐supported training in Becker and limb girdle 2I muscular dystrophy. Muscle & Nerve 2016;54(2):239‐43. [PUBMED: 26773840] [DOI] [PubMed] [Google Scholar]
Chung 2007 {published data only}
- Chung YL, Alexanderson H, Pipitone N, Morrison C, Dastmalchi M, Ståhl‐Hallengren C, et al. Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: six‐month, double‐blind, randomized, placebo‐controlled trial. Arthritis & Rheumatism 2007;57(4):694‐702. [PUBMED: 17471547] [DOI] [PubMed] [Google Scholar]
Cudia 2016 {published data only}
- Cudia P, Baba A, Pegoraro V, Angelini C. Clinical and molecular effects of functional electrical stimulation/lower extremity training in myotonic dystrophy. Neurology 2017;88(16 Suppl 1):P4.214. [EMBASE: 616551462] [Google Scholar]
- Cudia P, Weis L, Baba A, Kiper P, Marcante A, Rossi S, et al. Effects of functional electrical stimulation lower extremity training in myotonic dystrophy type I: a pilot controlled study. American Journal of Physical Medicine and Rehabilitation 2016;95(11):809‐17. [PUBMED: 27088471] [DOI] [PubMed] [Google Scholar]
Dastmalchi 2007 {published data only}
- Dastmalchi M, Alexanderson H, Loell I, Stahlberg M, Borg K, Lundberg IE, et al. Effect of physical training on the proportion of slow‐twitch type I muscle fibers, a novel nonimmune‐mediated mechanism for muscle impairment in polymyositis or dermatomyositis. Arthritis Care and Research 2007;57(7):1303‐10. [DOI] [PubMed] [Google Scholar]
Dawes 2006 {published data only}
- Dawes H, Korpershoek N, Freebody J, Elsworth C, Tintelen N, Wade DT, et al. A pilot randomised controlled trial of a home‐based exercise programme aimed at improving endurance and function in adults with neuromuscular disorders. Journal of Neurology, Neurosurgery and Psychiatry 2006;77(8):959‐62. [PUBMED: 16614008] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Lateur 1979 {published data only}
- Lateur BJ, Giaconi RM. Effect on maximal strength of submaximal exercise in Duchenne muscular dystrophy. American Journal of Physical Medicine 1979;58(1):26‐36. [PubMed] [Google Scholar]
Escalante 1993 {published data only}
- Escalante A, Miller L, Beardmore TD. An N‐of‐1 trial of resistive vs. non‐resistive exercise in inflammatory muscle disease (IMD). Arthritis & Rheumatism 1991;34:S173. [Google Scholar]
- Escalante A, Miller L, Beardmore TD. Resistive exercise in the rehabilitation of polymyositis/dermatomyositis. Journal of Rheumatology 1993;20(8):1340‐4. [PubMed] [Google Scholar]
Florence 1984a {published data only}
- Florence JM, Hagberg JM. Effect of training on the exercise responses of neuromuscular disease patients. Medicine and Science in Sports and Exercise 1984;16(5):460‐5. [DOI] [PubMed] [Google Scholar]
Florence 1984b {published data only}
- Florence JM, Brooke MH, Hagberg JM, Carroll JE. Endurance exercise in neuromuscular disease. In: Serratrice G editor(s). Neuromuscular Diseases. New York: Raven Press, 1984:577‐81. [Google Scholar]
Fowler 1965 {published data only}
- Fowler WM, Pearson CM, Egstrom GH, Gardner GW. Ineffective treatment of muscular dystrophy with an anabolic steroid and other measures. New England Journal of Medicine 1965;272(17):875‐82. [Google Scholar]
Hedermann 2016 {published data only}
- Hedermann G, Vissing CR, Heje K, Preisler N, Witting N, Vissing J. Aerobic training in patients with congenital myopathy. PLoS ONE 2016;11(1):e0146036. [PUBMED: 26751952] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heikkila 2001 {published data only}
- Heikkila S, Viitanen JV, Kautiainen H, Rajamaki T, Mantyvuo P, Harju T. Rehabilitation in myositis: preliminary study. Physiotherapy 2001;87(6):301‐9. [Google Scholar]
Hicks 1989 {published data only}
- Hicks J, Miller F, Plotz P, Zdrojewski M, Chen T, Gerber L. Exercise in patients with polymyositis. Arthritis & Rheumatism 1989;32:S149. [Google Scholar]
Hoberman 1955 {published data only}
- Hoberman M. Physical medicine and rehabilitation: its value and limitations in progressive muscular dystrophy. American Journal of Physical Medicine 1955;34:109‐15. [PubMed] [Google Scholar]
Jeppesen 2006 {published data only}
- Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Duno M, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 2006;129(12):3402‐12. [DOI] [PubMed] [Google Scholar]
Jeppesen 2009 {published data only}
- Jeppesen TD, Dunø M, Schwartz M, Krag T, Rafiq J, Wibrand F, et al. Short‐ and long‐term effects of endurance training in patients with mitochondrial myopathy. European Journal of Neurology 2009;16(12):1336‐9. [DOI] [PubMed] [Google Scholar]
Johnson 2007 {published data only}
- Johnson LG, Hons BS, Edwards DJ, Walters S, Thickbroom GW, Mastaglia FL. The effectiveness of an individualized, home‐based functional exercise program for patients with sporadic inclusion body myositis. Journal of Clinical Neuromuscular Disease 2007;8(4):187‐94. [Google Scholar]
Johnson 2009 {published data only}
- Johnson LG, Collier KE, Edwards DJ, Philippe DL, Eastwood PR, Walters SE, et al. Improvement in aerobic capacity after an exercise program in sporadic inclusion body myositis. Journal of Clinical Neuromuscular Disease 2009;10(4):178‐84. [DOI] [PubMed] [Google Scholar]
Kelm 2001 {published data only}
- Kelm J, Ahlhelm F, Regitz T, Pape D, Schmitt E. Controlled dynamic weight training in patients with neuromuscular disorders [Kontrolliertes dynamisches drafttraining bei patienten mit neuromuskulären erkrankungen]. Fortschritte der Neurologie‐Psychiatrie 2001;69(8):359‐66. [DOI] [PubMed] [Google Scholar]
Kilinc 2015 {published data only}
- Kilinç M, Yildirim SA, Tan E. The effects of electrical stimulation and exercise therapy in patients with limb girdle muscular dystrophy. A controlled clinical trial. Neurosciences (Riyadh, Saudi Arabia) 2015;20(3):259‐66. [PUBMED: 26166595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilmer 1994 {published data only}
- Kilmer DD, McCrory MA, Wright NC, Aitkens SG, Bernauer EM. The effect of a high resistance exercise program in slowly progressive neuromuscular disease. Archives of Physical Medicine and Rehabilitation 1994;75(5):560‐3. [PubMed] [Google Scholar]
Kilmer 2005 {published data only}
- Kilmer DD, Wright NC, Aitkens S. Impact of a home‐based activity and dietary intervention in people with slowly progressive neuromuscular diseases. Archives of Physical Medicine and Rehabilitation 2005;86(11):2150‐6. [DOI] [PubMed] [Google Scholar]
Lenman 1959 {published data only}
- Lenman JA. A clinical and experimental study of the effects of exercise on motor weakness in neurological disease. Journal of Neurology, Neurosurgery, and Psychiatry 1959;22:182‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattar 2014 {published data only}
- Mattar MA, Gualano B, Perandini LA, Shinjo SK, Lima FR, Sá‐Pinto AL, et al. Safety and possible effects of low‐intensity resistance training associated with partial blood flow restriction in polymyositis and dermatomyositis. Arthritis Research and Therapy 2014;16(5):473‐5. [PUBMED: 25344395] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCartney 1988 {published data only}
- McCartney N, Moroz D, Garner SH, McComas AJ. The effects of strength training in patients with selected neuromuscular disorders. Medicine and Science in Sports and Exercise 1988;20(4):362‐8. [DOI] [PubMed] [Google Scholar]
Mielke 1990 {published data only}
- Mielke U, Leipnitz, Holzer H, Schimrigk K. Dynamic muscular training in neuromuscular disease. Journal of the Neurological Sciences 1990;S98:388. [Google Scholar]
Milner‐Brown 1988a {published data only}
- Milner‐Brown HS, Miller RG. Muscle strengthening through high‐resistance weight training in patients with neuromuscular disorders. Archives of Physical Medicine and Rehabilitation 1988;69(1):14‐9. [PubMed] [Google Scholar]
Milner‐Brown 1988b {published data only}
- Milner‐Brown HS, Miller RG. Muscle strengthening through electric stimulation combined with low‐resistance weights in patients with neuromuscular disorders. Archives of Physical Medicine Rehabilitation 1988;69(1):20‐4. [PubMed] [Google Scholar]
Milner‐Brown 1990 {published data only}
- Milner‐Brown HS, Miller RG. Myotonic dystrophy: quantification of muscle weakness and myotonia and the effect of amitriptyline and exercise. Archives of Physical Medicine and Rehabilitation 1990;71(12):983‐7. [PubMed] [Google Scholar]
Murphy 2008 {published data only}
- Murphy JL, Blakely EL, Schaefer AM, He L, Wyrick P, Haller RG, et al. Resistance training in patients with single, large‐scale deletions of mitochondrial DNA. Brain 2008;131(Pt 11):2832‐40. [DOI] [PubMed] [Google Scholar]
Na 1996 {published data only}
- Na YM, Kang SW, Lee HS, Moon JH. The effect of home exercise program for patients with myotonic dystrophy. Journal of the Korean Academy of Rehabilitation Medicine 1996;20(1):33‐8. [Google Scholar]
Nader 2010 {published data only}
- Nader GA, Dastmalchi M, Alexanderson H, Grundtman C, Gernapudi R, Esbjörnsson M, et al. A longitudinal, integrated, clinical, histological and mRNA profiling study of resistance exercise in myositis. Molecular Medicine 2010;16(11‐12):455‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsen 2005 {published data only}
- Olsen DB, Orngreen MC, Vissing J. Aerobic training improves exercise performance in facioscapulohumeral muscular dystrophy. Neurology 2005;64(6):1064‐6. [DOI] [PubMed] [Google Scholar]
Omori 2010 {published data only}
- Omori C, Prado DM, Gualano B, Sallum AM, Sá‐Pinto AL, Roschel H, et al. Responsiveness to exercise training in juvenile dermatomyositis: a twin case study. BMC Musculoskeletal Disorders 2010;11:270. [DOI: 10.1186/1471-2474-11-270; PUBMED: 21106107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omori 2012 {published data only}
- Omori CH, Silva CA, Sallum AM, Rodrigues Pereira RM, Lúciade Sá Pinto A, Roschel H, et al. Exercise training in juvenile dermatomyositis. Arthritis Care & Research 2012;64(8):1186‐94. [PUBMED: 22505288] [DOI] [PubMed] [Google Scholar]
Orngreen 2005 {published data only}
- Orngreen MC, Olsen DB, Vissing J. Aerobic training in patients with myotonic dystrophy type 1. Annals of Neurology 2005;57(5):754‐7. [DOI] [PubMed] [Google Scholar]
Regardt 2014 {published data only}
- Regardt M, Schult ML, Axelsson Y, Aldehag A, Alexanderson H, Lundberg IE, et al. Hand exercise intervention in patients with polymyositis and dermatomyositis: a pilot study. Musculoskeletal Care 2014;12(3):106‐72. [PUBMED: 24623733] [DOI] [PubMed] [Google Scholar]
Riisager 2013 {published data only}
- Riisager M, Mathiesen PR, Vissing J, Preisler N, Ørngreen MC. Aerobic training in persons who have recovered from juvenile dermatomyositis. Neuromuscular Disorders 2013;23(12):962‐8. [PUBMED: 24120572] [DOI] [PubMed] [Google Scholar]
Scott 1981 {published data only}
- Dubowitz V, Hyde SA, Scott OM, Goddard C. Controlled trial of exercise in Duchenne muscular dystrophy. In: Serratrice G, Cross D, Desnuelle, C, Gastaut J‐L, Pellissier J‐F, Pouget J, et al. editor(s). Neuromuscular Diseases. New York: Raven Press, 1984:571‐5. [0‐89004‐070‐X] [Google Scholar]
- Scott OM, Hyde SA, Goddard C, Jones R, Dubowitz V. Effect of exercise in Duchenne muscular dystrophy. Physiotherapy 1981;67(6):174‐6. [PUBMED: 7029578] [PubMed] [Google Scholar]
Siciliano 2000 {published data only}
- Siciliano G, Manca ML, Renna M, Prontera C, Mercuri A, Murri L. Effects of aerobic training on lactate and catecholaminergic exercise responses in mitochondrial myopathies. Neuromuscular Disorders 2000;10(1):40‐5. [DOI] [PubMed] [Google Scholar]
Siciliano 2012 {published data only}
- Siciliano G, Simoncini C, Lo Gerfo A, Orsucci D, Ricci G, Mancuso M. Effects of aerobic training on exercise‐related oxidative stress in mitochondrial myopathies. Neuromuscular Disorders 2012;22 Suppl 3:S172‐7. [PUBMED: 23182634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spector 1997 {published data only}
- Spector SA, Lemmer JT, Koffman BM, Fleischer TA, Feuerstein IM, Hurley BF, et al. Safety and efficacy of strength training in patients with sporadic inclusion body myositis. Muscle & Nerve 1997;20(10):1242‐8. [DOI] [PubMed] [Google Scholar]
Sriram 2015 {published data only}
- Sriram S, Kavitha MM, Prembabu KR, Tamilselvam, TN, Rajeswari S. Endurance versus aerobic exercises in the rehabilitation of idiopathic inflammatory myositis. International Journal of Rheumatic Diseases 2015;18(Suppl 1):88. [EMBASE: 72004882] [Google Scholar]
Sunnerhagen 2004 {published data only}
- Sunnerhagen KS, Darin N, Tajsharghi H, Oldfors A. The effects of endurance training in persons with a hereditary myosin myopathy. Acta Neurologica Scandinavica 2004;110(2):80‐6. [DOI] [PubMed] [Google Scholar]
Sveen 2007 {published data only}
- Sveen ML, Jeppesen TD, Hauerslev S, Krag TO, Vissing J. Endurance training: an effective and safe treatment for patients with LGMD2I. Neurology 2007;68(1):59‐61. [DOI] [PubMed] [Google Scholar]
Sveen 2008 {published data only}
- Sveen ML, Jeppesen TD, Hauerslev S, Kober L, Krag TO, Vissing J. Endurance training improves fitness and strength in patients with Becker muscular dystrophy. Brain 2008;131(Pt 11):2824‐31. [DOI] [PubMed] [Google Scholar]
Sveen 2013 {published data only}
- Sveen ML, Andersen SP, Ingelsrud LH, Blichter S, Olsen NE, Jønck S, et al. Resistance training in patients with limb‐girdle and Becker muscular dystrophies. Muscle & Nerve 2013;47(2):163‐9. [PUBMED: 23169433] [DOI] [PubMed] [Google Scholar]
Taivassalo 1998 {published data only}
- Taivassalo T, Stefano N, Argov Z, Matthews PM, Chen J, Genge A, et al. Effects of aerobic training in patients with mitochondrial myopathies. Neurology 1998;50(4):1055‐60. [DOI] [PubMed] [Google Scholar]
Taivassalo 1999 {published data only}
- Taivassalo T, Stefano N, Chen J, Karpati G, Arnold DL, Argov Z. Short‐term aerobic training response in chronic myopathies. Muscle & Nerve 1999;22(9):1239‐43. [DOI] [PubMed] [Google Scholar]
Taivassalo 2001 {published data only}
- Taivassalo T, Shoubridge EA, Chen J, Eng M, Kennaway NG, DiMauro S, et al. Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Annals of Neurology 2001;50(2):133‐41. [DOI] [PubMed] [Google Scholar]
Taivassalo 2006 {published data only}
- Taivassalo T, Gardner JL, Taylor RW, Schaefer AM, Newman J, Barron MJ, et al. Endurance training and detraining in mitochondrial myopathies due to single large‐scale mtDNA deletions. Brain 2006;129(12):3391‐401. [DOI] [PubMed] [Google Scholar]
Tiffreau 2017 {published data only}
- Tiffreau V, Rannou F, Kopciuch F, Hachulla E, Mouthon L, Thoumie P, et al. Postrehabilitation functional improvements in patients with inflammatory myopathies: the results of a randomized, controlled trial. Archives of Physical Medicine and Rehabilitation 2017;98(2):227‐34. [PUBMED: 27789240] [DOI] [PubMed] [Google Scholar]
Tollbäck 1999 {published data only}
- Tollbäck A, Eriksson S, Wredenberg A, Jenner G, Vargas R, Borg K, et al. Effects of high resistance training in patients with myotonic dystrophy. Scandinavian Journal of Rehabilitation Medicine 1999;31(1):9‐16. [PubMed] [Google Scholar]
Trenell 2006 {published data only}
- Trenell MI, Sue CM, Kemp GJ, Sachinwalla T, Thompson CH. Aerobic exercise and muscle metabolism in patients with mitochondrial myopathy. Muscle & Nerve 2006;33(4):524‐31. [DOI] [PubMed] [Google Scholar]
Van den Berg 2015 {published data only}
- Berg LE, Favejee MM, Wens SC, Kruijshaar ME, Praet SF, Reuser AJ, et al. Safety and efficacy of exercise training in adults with Pompe disease: evaluation of endurance, muscle strength and core stability before and after a 12 week training program. Orphanet Journal of Rare Diseases 2015;10:87. [PUBMED: 26187632] [DOI] [PMC free article] [PubMed] [Google Scholar]
Varju 2003 {published data only}
- Varju C, Petho E, Kutas R, Czirjak L. The effect of physical exercise following acute disease exacerbation in patients with dermatomyositis/polymyositis. Clinical Rehabilitation 2003;17(1):83‐7. [DOI] [PubMed] [Google Scholar]
Vignos 1966 {published data only}
- Vignos PJ Jr, Watkins MP. The effect of exercise in muscular dystrophy. JAMA 1966;197(11):843‐8. [PubMed] [Google Scholar]
Wiesinger 1998b {published data only}
- Wiesinger GF, Quittan M, Graninger M, Seeber A, Ebenbichler G, Sturm B, et al. Benefit of 6 months long‐term physical training in polymyositis/dermatomyositis patients. British Journal of Rheumatology 1998;37(12):1338‐42. [DOI] [PubMed] [Google Scholar]
Wright 1996 {published data only}
- Wright NC, Kilmer DD, McCrory MA, Aitkens SG, Holcomb BJ, Bernauer EM. Aerobic walking in slowly progressive neuromuscular disease: effect of a 12‐week program. Archives of Physical Medicine and Rehabilitation 1996;77(1):64‐9. [DOI] [PubMed] [Google Scholar]
Yildirim 2007 {published data only}
- Yildirim S, Erden Z, Kilinc M. Comparison of the effects of proprioceptive neuromuscular facilitation techniques and weight training in patients with neuromuscular diseases. Fizyoterapi Rehabilitasyon 2007;18(2):65‐71. [Google Scholar]
References to ongoing studies
Jorgensen 2016 {published and unpublished data}
- Jørgensen AN, Aagaard P, Christiansen M, Frandsen U, Diederichsen LP. Effects of 12 weeks low‐intensity blood‐flow restricted resistance training on knee extensor strength in patients with sporadic inclusion body myositis. Arthritis & Rheumatology 2016;68(Suppl 10):3023‐4. [EMBASE: 613887953] [Google Scholar]
NCT02158156 {unpublished data only}
- NCT02158156. Effect of aerobic training in patients with oculopharyngeal muscular dystrophy. clinicaltrials.gov/show/NCT02158156 (first received 6 June 2014).
NCT02421523 {unpublished data only}
- NCT02421523. Strength training in Duchenne muscular dystrophy [Development of a strength training protocol in Duchenne muscular dystrophy]. clinicaltrials.gov/show/NCT02421523 (first received 20 April 2015).
Van Engelen 2015 {published data only}
- Jimenez‐Moreno AC, Wood L, Lochmuller H, Gorman, G. OPTIMISTIC: observational prolonged trial in myotonic dystrophy type 1 to improve quality of life‐standards, a target identification collaboration. Neuromuscular Disorders 2015;25:S40. [EMBASE: 71927154] [Google Scholar]
- Engelen B, OPTIMISTIC consortium. Cognitive behaviour therapy plus aerobic exercise training to increase activity in patients with myotonic dystrophy type 1 (DM1) compared to usual care (OPTIMISTIC): study protocol for randomised controlled trial. Trials 2015;16(1):224. [PUBMED: 26002596] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veenhuizen 2015 {published data only}
- Veenhuizen Y, Cup EH, Groothuis JT, Hendriks JC, Adang EM, Engelen BG, et al. Effectiveness and cost‐effectiveness of a self‐management group program to improve social participation in patients with neuromuscular disease and chronic fatigue: protocol of the Energetic study. BMC Neurology 2015;15:58. [PUBMED: 25913823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2016 {published data only}
- Wallace A, Pietrusz A, Dewar E, Dudziec M, Jones K, Hennis P, et al. Evaluating the benefits of community based aerobic training on the physical health and well‐being of people with neuromuscular disease. Muscle & Nerve 2016;54(Suppl 1):S5. [EMBASE: 613434266] [Google Scholar]
Additional references
ACSM 2015
- American College of Sports Medicine. Physical activity in children and adolescents. www.acsm.org/docs/default‐source/brochures/physical‐activity‐in‐children‐and‐adolescents.pdf (accessed 10 August 2017).
Bohan 1975a
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). New England Journal of Medicine 1975;292(7):344‐7. [PUBMED: 1090839] [DOI] [PubMed] [Google Scholar]
Bohan 1975b
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). New England Journal of Medicine 1975;292(8):403‐7. [PUBMED: 1089199] [DOI] [PubMed] [Google Scholar]
Brouwer 1992
- Brouwer OF, Padberg GW, Ploeg RJ, Ruys CJ, Brand R. The influence of handedness on the distribution of muscular weakness of the arm in facioscapulohumeral muscular dystrophy. Brain 1992;115(Pt 5):1587‐98. [PUBMED: 1422805] [DOI] [PubMed] [Google Scholar]
Cup 2007
- Cup EH, Pieterse AJ, Broek‐Pastoor JM, Munneke M, Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Archives of Physical Medicine and Rehabilitation 2007;88(11):1452‐64. [PUBMED: 47625324] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Deidda 1996
- Deidda G, Cacurri S, Piazzo N, Felicetti L. Direct detection of 4q35 rearrangements implicated in facioscapulohumeral muscular dystrophy (FSHD). Journal of Medical Genetics 1996;33(5):361‐5. [PUBMED: 26147865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deschartes 2013
- Deschartes A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faigenbaum 2017
- Faigenbaum A, Micheli L. Youth strength training. Indianapolis. American College of Sports Medicine 2017.
Florence 2008
- Florence JM, Ploeg A, Clemens PR, Escolar DM, Laforet P, Rosenbloom B, et al. Use of the 6min walk test as an endpoint in clinical trials for neuromuscular diseases. Neuromuscular Disorders 2008;18(9‐10):738‐9. [Google Scholar]
Fowler 1982
- Fowler WM Jr, Taylor M. Rehabilitation management of muscular dystrophy and related disorders: I. The role of exercise. Archives of Physical Medicine and Rehabilitation 1982;63(7):319‐21. [PubMed] [Google Scholar]
Fowler 1984
- Fowler WM Jr. Importance of overwork weakness. Muscle & Nerve 1984;7(6):496‐9. [PUBMED: 6543905] [PubMed] [Google Scholar]
Garber 2011
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Medicine & Science in Sports & Exercise 2011;43(7):1334‐59. [PUBMED: 21694556] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 10 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henricson 2013
- Henricson E, Abresch R, Han JJ, Nicorici A, Goude Keller E, Bie E, et al. The 6‐minute walk test and person‐reported outcomes in boys with Duchenne muscular dystrophy and typically developing controls: longitudinal comparisons and clinically‐meaningful changes over one year. PLoS Currents 2013;5:ecurrents.md.9e17658b007eb79fcd6f723089f79e06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johnson 1971
- Johnson EW, Braddom R. Over‐work weakness in facioscapulohumeral muscular dystrophy. Archives of Physical Medicine and Rehabilitation 1971;52(7):333‐6. [PUBMED: 5565885] [PubMed] [Google Scholar]
Kahl 2005
- Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the McGill pain questionnaire: an overview of psychometric properties. Physical Therapy Reviews 2005;10(2):123–8. [Google Scholar]
Kilmer 2002
- Kilmer DD. Response to aerobic exercise training in humans with neuromuscular disease. American Journal of Physical Medicine and Rehabilitation 2002;81 Suppl 11:148‐50. [PUBMED: 12409819] [DOI] [PubMed] [Google Scholar]
Kraemer 2002
- Kraemer WJ, Adams K, Cafarellil E, Dudley GA, Dooly C, Feigenbaum MS, et al. American College of Sports position stand. Progression models in resistance training for healthy adults. Medicine and Science in Sports and Exercise 2002;34(2):364‐80. [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee M, Carroll TJ. Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Medicine 2007;37(1):1‐14. [DOI] [PubMed] [Google Scholar]
McDonald 2002
- McDonald CM. Physical activity, health impairments, and disability in neuromuscular disease. American Journal of Physical Medicine and Rehabilitation 2002;81(Suppl 11):S108‐20. [DOI] [PubMed] [Google Scholar]
MECIR 2018
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version 1.06 2018.
Medical Research Council 1981
- Medical Research Council. Aids to investigation of peripheral nerve injuries. London: Her Majesty's Stationary Office, 1981. [Google Scholar]
Millard 1989
- Millard RW. The functional assessment screening questionnaire: application for evaluating pain‐related disability. Archives of Physical Medicine and Rehabilitation 1989;70(4):303‐7. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munn 2004
- Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta‐analysis. Journal of Applied Physiology 2004;96(5):1861‐6. [DOI] [PubMed] [Google Scholar]
Munn 2005
- Munn J, Herbert RD, Hancock MJ, Gandevia SC. Training with unilateral resistance exercise increases contralateral strength. Journal of Applied Physiology 2005;99(5):1880‐4. [DOI] [PubMed] [Google Scholar]
Parsons 1974
- Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science 1974;183(4128):922‐32. [DOI] [PubMed] [Google Scholar]
Personius 1994
- Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Physical Therapy 1994;74(3):253‐63. [DOI] [PubMed] [Google Scholar]
Quinlivan 2011
- Quinlivan R, Vissing J, Hilton‐Jones D, Buckley J. Physical training for McArdle disease. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD007931.pub2] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sale 1988
- Sale DG. Neural adaptation to resistance training. Medicine and Science in Sports and Exercise 1988;20 Suppl 5:135‐45. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Shima 2002
- Shima N, Ishida K, Katayama K, Morotome Y, Sato Y, Miyamura M. Cross education of muscular strength during unilateral resistance training and detraining. European Journal of Applied Physiology 2002;86(4):287‐94. [DOI] [PubMed] [Google Scholar]
Vercoulen 1999
- Vercoulen JH, Alberts M, Bleijenberg G. The checklist individual strength [De checklist individual strength (CIS)]. Gedragstherapie 1999;32(1):131–6. [Google Scholar]
Vignos 1983
- Vignos PJ Jr. Physical models of rehabilitation in neuromuscular disease. Muscle & Nerve 1983;6(5):323‐38. [PUBMED: 6888414] [DOI] [PubMed] [Google Scholar]
Ware 2000
- Ware JE Jr. SF‐36 Health Survey update. Spine 2000;25(24):3130‐9. Erratum in: Spine 2001; 26(18):2062. [PUBMED: 11124729] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van der Kooi 2002
- Kooi EL, Lindeman E, Riphagen I. Strength training and aerobic exercise training for muscle disease. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003907] [DOI] [PubMed] [Google Scholar]
Van der Kooi 2005
- Kooi EL, Lindeman E, Riphagen I. Strength training and aerobic exercise training for muscle disease. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD003907.pub2; PUBMED: 15674918] [DOI] [PubMed] [Google Scholar]
Voet 2010a
- Voet NB, Kooi EL, Riphagen IL, Lindeman E, Engelen BG, Geurts ACH. Strength training and aerobic exercise training for muscle disease. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003907.pub3; PUBMED: 20091552] [DOI] [PubMed] [Google Scholar]
Voet 2013
- Voet NB, Kooi EL, Riphagen II, Lindeman E, Engelen BG, Geurts AC. Strength training and aerobic exercise training for muscle disease. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003907.pub4; PUBMED: 23835682] [DOI] [PubMed] [Google Scholar]


