Abstract
Suicide is the second leading cause of death worldwide for adolescents. Despite decades of research on correlates and risk factors for adolescent suicide, we know little about why suicidal ideation and behavior frequently emerge in adolescence and how to predict, and ultimately prevent, suicidal behavior among youths. In this review, we first discuss knowledge regarding correlates, risk factors, and theories of suicide. We then review why adolescence is a period of unique vulnerability, given changing biology and social network reorganization. Next, we present a conceptual model through which to interpret emerging findings in adolescent suicide research. We suggest that a promising area for future research is to examine adolescent suicide as a failure of biological responses to acute stress in the proximal moments of a suicidal crisis. After reviewing initial evidence for this conceptualization, we review future directions for studies on adolescent suicide.
Keywords: suicidal thoughts, stress response, suicidal behavior, acute suicidal crisis, proximal risk factors
INTRODUCTION
Suicide is the second leading cause of death worldwide among individuals between 10 and 24 years of age (Curtin et al. 2016, Mokdad et al. 2016), representing an alarming public health crisis. Suicidal ideation and behaviors are even more common than death by suicide. Prevalence rates range from 19.8% to 24% for suicidal ideation and from 3.1% to 8.8% for suicide attempts among youths (Nock et al. 2008). Suicidal ideation and behaviors are the most common mental health emergencies among adolescents (King et al. 2009). Despite these disturbing statistics, our ability to accurately predict suicidal ideation and behavior, as well as death by suicide, remains remarkably limited (Franklin et al. 2017). In this review, we offer a synthesis and framework for understanding the proximal processes involved in adolescent suicidal ideation and behavior. Specifically, we suggest that failures in biological responses to acute stress may underlie acute suicidal crises in youth. We first review prior research on adolescent suicide before offering an assessment of contemporary research and future directions.
Scope of Review
Self-injurious thoughts and behaviors include a wide range of actions used by individuals to deliberately inflict nonfatal or fatal bodily harm. Constructs include nonsuicidal self-injury (NSSI), defined as self-inflicted tissue damage without suicidal intent, as well as suicidal ideation, suicidal plans, suicidal gestures, and a range of suicidal behaviors (i.e., interrupted attempts; aborted attempts; suicidal attempts, ranging from minor to those that require medical attention; and death by suicide) (O’Carroll et al. 1996, Silverman et al. 2007). Notably, the distinction between these constructs is sometimes unclear, particularly among youths who may not be able to articulate their intent to die before considering or engaging in suicidal behavior (Hawton et al. 2012, Prinstein 2008). Nevertheless, prior research clearly has revealed NSSI as both a correlate and risk factor for suicidal ideation and behavior; yet the predictors and developmental course of NSSI differ from that of suicidal ideation and behaviors (see reviews by Fox et al. 2015 and Liu et al. 2017). Thus, we elected to focus this review specifically on suicidal self-injurious thoughts and behaviors and do not review literature on NSSI.
Moreover, it should be noted that far more research has examined suicidal ideation and suicidal attempts than other suicidal constructs (i.e., plans, gestures, interrupted or aborted attempts, and death by suicide), particularly among youths. Thus, readers are cautioned against generalizing findings across suicide constructs or assuming equivalence in the theories and predictors for various types of suicidal ideation and behaviors. For simplicity in prose, we use the terms suicidal ideation and suicidal behavior. We note where findings have been studied in relation to suicide death, where appropriate.
Notably, the prevalence of suicidal ideation, suicidal behavior, and death by suicide all increase dramatically during the adolescent transition, a unique period of developmental vulnerability for suicidal ideation and behaviors (Curtin et al. 2016, Glenn et al. 2017b, Kõlves & de Leo 2017). Attempts to explain and ultimately prevent suicide require a developmentally informed framework to identify the emergence of novel risk factors or developmental transactions between intra- and interpersonal risk factors unique to the adolescent period that elucidate why suicidal ideation and behaviors are more likely to appear within this period than any other period in the life span (Nock et al. 2008, 2013).
Organization of Review
This review is divided into three sections. Recently, several theoretical (Glenn et al. 2017a), systematic (Cha et al. 2018), and meta-analytic reviews (Franklin et al. 2017, Glenn et al. 2018, Ribeiro et al. 2016) have been published on the topic of suicide, with one review by Cha and colleagues (2018) focusing specifically on youths. In this article, we first summarize these reviews and comment briefly on their implications for understanding suicidal ideation and behaviors in adolescence. We also discuss the conceptual frameworks previously offered to understand suicide and the limitations of each theory for explaining the onset of suicidal ideation and behavior in adolescence.
Second, this review considers current trends in research on adolescent suicide, with a specific focus on emerging biological markers. In this section, we offer a heuristic framework for interpreting extant research and suggest targeted areas for future work. We suggest that adolescent suicidal crises may result from failures of biological responses to acute stress. Findings pertaining to each of several biological stress-response systems are reviewed to offer a foundation for understanding the processes that may occur immediately prior to adolescents’ suicidal behavior and to guide future research. Last, we highlight some important questions suggested from emerging research on biological markers that have relevance for future research and treatment.
Our focus on acute stress-response systems is designed to address a central limitation in prior work on suicide. For more than six decades, research on suicide predominantly has focused on distal risk factors that are associated with an increased likelihood of suicidal ideation, behavior, or death by suicide months, years, or even decades later (Franklin et al. 2017). This research tradition has offered valuable insight for understanding putative preconditions and identifying possible risk groups for later suicidal ideation and behaviors. Unfortunately, this focus on distal risk factors is limited in three critical ways that may ultimately impede progress toward preventing and identifying effective treatments to reduce suicide.
First, most risk factors previously identified are remarkably weak predictors of later suicidal ideation and behavior. Of all individuals who have experienced each risk factor (e.g., depression, substance use, childhood maltreatment), very few will ever report suicidal ideation or attempt suicide. Second, many prior conceptual frameworks and identified risk factors lack specificity; many elucidate risks relevant to psychopathology more generally, rather than to suicidal ideation and behaviors. Last, most suicidal behavior among youths occurs in response to a discrete stressful precipitant (King & Merchant 2008). Yet prior work has not elucidated the biological or psychological processes that immediately follow stressful experiences and occur within the hours, minutes, or seconds prior to suicidal behavior. This review focuses on processes that may represent proximal risk factors, differentiating risks for general distress from those that may be most relevant to adolescents’ suicidal ideation and behavior. Importantly, our goal is not to explain every occurrence of adolescent suicidal ideation or behavior, but rather to offer a framework for exploring the processes that may be most relevant for understanding why some distressed youths may elect to consider or attempt suicide and why this becomes especially more likely during the adolescent transition.
CORRELATES, RISK FACTORS, AND THEORIES OF SUICIDE
Published papers on the onset, course, correlates, and risk factors for suicidal ideation and behaviors have steadily increased since the early 1980s, with nearly half of empirical studies being published within the past 10 years (Franklin et al. 2017). Across time, far fewer empirical studies have been published on adolescent suicide compared with adult suicide (Franklin et al. 2017).
Current Knowledge About Correlates and Risk Factors
Many recent reviews and meta-analyses have offered summaries of current knowledge regarding the predictors of suicidal ideation and behaviors (Cha et al. 2018; Franklin et al. 2017; Glenn et al. 2017a, 2018; Ribeiro et al. 2016). Remarkably, all of these reviews demonstrate that we know surprisingly little about the etiology of adolescent suicidal ideation and behaviors and that predictors of suicidal ideation, suicidal behavior, and death by suicide are surprisingly weak.
For instance, Cha and colleagues (2018) offer a helpful catalog of correlates and risk factors for adolescent suicide across environmental, psychological, and biological domains, differentiating those factors that appear to prospectively predict suicidal ideation and behaviors beyond known predictors (e.g., depression and prior suicidal ideation or behaviors) from those that have been associated only concurrently with suicide. Results from this review suggest that beyond demographic characteristics, only childhood maltreatment, peer victimization, feelings of worthlessness, and low self-esteem increase the risk for suicidal ideation or behaviors over time, after accounting for depression and prior suicidal ideation or behaviors as predictors (Cha et al. 2018). While these risk factors provide information about global risk for any suicidal ideation or behaviors in the future, they are nonspecific. In other words, they do not provide information about which youths are more vulnerable to these risk factors or when a given youth will experience suicidal ideation or engage in suicidal behavior. Further, it is not clear how these factors confer risk in the proximal moments of a suicidal crisis.
Meta-analytic reviews of the broader suicide literature across the life span yield remarkably low odds ratios in the prospective prediction of suicidal ideation or behaviors. For example, they note that while internalizing risk factors accounted for nearly 66% of all predictors examined in the literature, they were not strongly related to future risk for suicidal ideation or behaviors when omnibus effect sizes are considered in meta-analyses (odds ratios between 1.17 and 1.71; Franklin et al. 2017). Similarly, Glenn and colleagues (2018) revealed significant, but low, effect sizes for markers of the National Institute of Mental Health’s Research Domain Criteria Negative Valence Systems domain (i.e., depressed mood, rumination, and hopelessness) in predicting suicidal ideation or behaviors. Even the effects of prior suicidal ideation and behaviors are relatively weak in predicting future suicidal behavior (Ribeiro et al. 2016), reflecting that in most studies, only a minority of those who attempt suicide are likely do so again.
Together, these reviews highlight a fundamental limitation in the suicide literature: By focusing on distal risk factors, it has been remarkably difficult to identify robust predictors of suicidal ideation, behaviors, or death by suicide. Consequently, little is known regarding the processes that may immediately precede suicide, and few effective strategies designed to intervene in the hours or minutes before individuals engage in suicidal behavior are available (Asarnow et al. 2017, Cha et al. 2018, Miller et al. 2017a), perhaps particularly among youths. Only three randomized controlled trials have demonstrated efficacy in temporarily reducing the risk for suicidal behavior among adolescents with a history of suicide attempts or self-harm (Asarnow et al. 2017, Esposito-Smythers et al. 2011, McCauley et al. 2018).
Theories of Suicide
Given that suicidal behavior runs counter to the basic biological instinct to survive that is present even in single-celled organisms, there has been no shortage of theories offered to explain the conditions under which this perplexing behavior occurs, and these date as far back as the ancient Greeks in writings by Plato and Aristotle. Rarely do these theories offer insight regarding developmental factors that may make adolescence a period of unique vulnerability for suicidal ideation and behaviors.
In contemporary work, however, most theories reflect at least one of three themes that many consider central to the experience of suicide, particularly within the moments that immediately precede suicidal behavior. First, past theoretical work highlights the relevance of interpersonal distress as perhaps uniquely relevant to an individual’s decision to engage in suicidal behavior. For instance, Durkheim’s [1951 (1897)] well-cited theory from the late nineteenth century first highlighted the important role of a lack of social integration as a key factor associated with the desire to engage in self-inflicted injury. Baumeister’s (1990) escape theory similarly suggested that following realization about the self’s social inadequacies and interpersonal failures, individuals turn to suicide to escape resulting emotional distress. Shneidman (1993) asserted that suicide emerges from psychache, or mental pain, partly caused by social isolation. More recent theories also have emphasized negative interpersonal states that give rise to suicidal thinking (Joiner 2005, Klonsky & May 2015, O’Connor 2011, Van Orden et al. 2010), including thwarted belongingness or perceived burdensomeness.
Second, most theories suggest that suicidal ideation and behaviors are an expression of affective distress, perhaps resulting in cognitive inflexibility. Menninger (1933) suggested that suicide was a coping strategy to reduce extreme pain and self-blame. Neuringer (1964) noted that suicidal individuals were characterized by rigid thinking. Linehan (1993) highlighted the role of emotional dysregulation preceding suicide, and both Baumeister (1990) and Beck (1987) posited that increasingly distorted thinking leads an individual to view destructive behaviors as a reasonable option for dealing with intolerable distress. Recent work has suggested that dysfunctional cognitions related either to stressor appraisals or more generally to self-worth may be relevant predictors for later suicidal behavior (Klonsky & May 2015, Van Orden et al. 2010).
Last, some theories of suicide have attempted to link the exposure to stressors and the affective or cognitive experience of distress with engagement in specific behaviors that may increase the risk for suicidal behavior. For example, cognitive and dialectical behavioral theories of suicide suggest that distorted thinking often leads to narrowed problem-solving abilities and risky or self-regulatory behavior, such as alcohol use or self-harm, that may make future suicidal behavior more likely (Linehan 1993, Rudd 2010, Spirito et al. 2012).
Naturally, the exposure to stress and the experience of cognitive–affective distress have biological concomitants as well, although few theories acknowledge biological underpinnings in the etiology for suicide. One notable exception is Linehan’s (1993) theory of suicidal behavior, suggesting that abnormal biological signaling subsequent to stress, such as increased heart rate, rapid breathing, and general agitation, may increase perceptions of distress, leading individuals to engage in self-harm behaviors as a strategy for self-regulation. In addition, Joiner’s (2005) interpersonal–psychological theory posits that a person’s ability to enact lethal self-harm is acquired throughout the lifetime, with potentially dangerous or near-fatal prior experiences changing the body’s biological response to self-harm, resulting in a greater likelihood of eventual death by suicide (Van Orden et al. 2010).
These biological processes may be especially pertinent in adolescence, a developmental period characterized not only by increased exposure to interpersonally themed stressors but also by a recalibration of acute stress-response systems. We argue that in order to understand the course of suicidal ideation and behavior across the lifetime, we first must understand why adolescence represents a critical time of vulnerability.
ADOLESCENCE: A CRITICAL TIME OF VULNERABILITY TO SUICIDE
As reviewed above, few developmentally based theories have been offered to help explain why suicidal ideation and behaviors increase in prevalence at the adolescent transition (Nock et al. 2013). In this section, we offer an overview of the unique social and biological processes that occur in adolescence that may help explain the period of unique vulnerability for suicide. First, we briefly review biological and social changes that occur during adolescence. Next, we discuss four biological systems related to typical stress responses [i.e., the autonomic nervous system (ANS), the hypothalamic–pituitary–adrenal (HPA) axis, peripheral stress responses, and neural responses within the brain] and review theory and data regarding how these systems change during adolescence. We then review research on markers of acute stress-response systems that have been associated with suicidal ideation, behavior, and death by suicide. Finally, we discuss our conceptualization of a developmentally informed framework that highlights two potential moderators of the relationship between dysfunctional acute stress-response systems and suicide among youths.
The Adolescent Transition
Adolescence is marked by dramatic changes in biological and social systems that simultaneously increase acute stress-response system reactivity and adolescents’ orientation toward social stimuli, thus creating a uniquely vulnerable time frame for the emergence of suicidal ideation and behavior. The onset of puberty, characterized by rapid external physical and internal biological changes, includes extensive neural network changes in the brain and alterations in hormonal systems as adolescents move toward sexual maturity (Gunnar et al. 2009, Rudolph 2014, Susman & Dorn 2009). However, neural development is localized within specific regions of the brain throughout development. Early in this process, beginning before physical manifestations of puberty are evident, changes within the limbic system include synaptic pruning, increased myelinization, and a proliferation of dopamine and oxytocin receptors that facilitate more rapid and dramatic responses to social rewards and avoidance of social punishment (Casey et al. 2008, Chein et al. 2011). Throughout puberty, changes in social and mentalizing neural networks allow for more sophisticated social comparisons and reflected appraisals, allowing adolescents to form a more stable sense of self-concept that is heavily dependent on perceived peer appraisals (Casey et al. 2008, Harter et al. 1996, Somerville 2013). Comparable changes in development of the prefrontal cortex (PFC), allowing for emotional regulation skills and goal-directed behavior, do not occur until years later. In sum, findings from developmental affective neuroscience suggest that adolescents experience several years during the pubertal transition with newly developed and robust responses to their social environment and a more sophisticated capacity to allow these social experiences to shape their self-concept, but without mature abilities to regulate their emotions or inhibit impulsive responses (Casey et al. 2008). Research confirms that adolescents experience increased physiological responses to stressors (Stroud et al. 2009), increased prefrontal brain activation during social evaluation (Somerville 2013), and increased difficulty implementing cognitive control skills in the context of emotionally evocative events (Cohen et al. 2016, Somerville et al. 2011). Interestingly, epidemiological data suggest that the most dramatic increase in the incidence of suicidal ideation and behavior occurs between 12 and 17 years of age. (Nock et al. 2013).
These biological changes occur in parallel to notable alterations in adolescents’ peer milieu and in the frequency and quality of adolescents’ social experiences. Note that in many cultures, the pubertal transition coincides with a school transition and the introduction of a larger peer group, requiring adolescents to navigate more complex social roles and novel peer relationships. In recent years, adolescence has also been associated with an increase in online interactions with peers, offering remarkably frequent opportunities for new forms of interpersonal relationships and social expectations (Nesi et al. 2018). Indeed, research suggests that the pubertal transition is associated with marked increases in adolescents’ interactions with peers, concomitant decreases in parental supervision, and more severe parent–child conflict (Prinstein & Giletta 2016). At the peer group level, adolescence is marked by the introduction of a new form of peer status, reflecting adolescents’ dominance, power, influence, and visibility (Cillessen & Rose 2005, Parkhurst & Hopmeyer 1998). Adolescents’ bids to enhance or maintain this form of peer status frequently involve the use of overt or relational aggression strategies that often are cited as powerful predictors of adolescent suicide (Juvonen & Graham 2014, Massing-Schaffer et al. 2018). Adolescence also is marked by an increase in the frequency and emotional intimacy of dyadic peer experiences, with both best friendships and emerging romantic relationships occupying more of adolescents’ attention and interest (Rose & Rudolph 2006, Steinberg & Morris 2001). These dyadic relationships can be a significant source of stress. As compared with children and adults, adolescents report higher levels of peer-related interpersonal stressors and greater emotional reactivity to these types of stressors (Rudolph 2014). Note also that these peer-related stressors (e.g., friendship alienation, romantic breakups, bullying) are among the most frequently reported precipitants to adolescents’ suicidal behavior (Juvonen & Graham 2014, King & Merchant 2008, Massing-Schaffer et al. 2018).
In sum, the adolescent transition offers a potentially risky person–environment transaction that may be especially relevant for understanding suicide. Biologically equipped with an increased investment in social interactions and enhanced social sensitivity, adolescents enter a world involving novel, complex, and unfamiliar social interactions with increased stakes for success, but with immature biological resources to adequately regulate social stress. Indeed, difficulties navigating this unique combination of their changing environmental context, developing self- and social competencies, and biological vulnerabilities has been associated with a range of psychological symptoms and adjustment difficulties (see Prinstein et al. 2018 for a review). Suicidal ideation and behavior may occur following particularly severe social stressors, especially among those with atypical biological responses to acute stress. Below, we discuss biological responses to acute stress and how these responses change in adolescence, and we also briefly note why these responses may develop atypically among some youths.
Typical Biological Responses to Acute Stress
Adolescence is associated with a recalibration of biological responses to acute stress that may further create a period of developmental vulnerability for suicide. This notion has been recognized only recently, however, through emerging work on the nature of biological responses to acute stress and the way that each of the systems that support these responses may evolve across the pubertal transition. In this section, we briefly review acute stress responses and physiological markers in the ANS, HPA axis, peripheral stress-response systems, and neural networks. Following this brief overview, we discuss knowledge regarding the specific changes that occur within each of these in adolescence.
The ANS and HPA axis represent two hormone systems that act to mobilize the body to act flexibly and adaptively in response to a stressor. The fast-acting sympathetic branch (sympathetic nervous system; SNS) of the ANS releases epinephrine and norepinephrine into the bloodstream, activating the flight-or-fight response to a stressor. This system is modulated by the parasympathetic branch (parasympathetic nervous system; PNS) of the ANS, which has been specifically implicated in self-regulatory processes (Beauchaine 2015). The PNS exerts control of heart rate via the vagus nerve (Porges 2007), and its influence is measurable via respiratory sinus arrhythmia (RSA). RSA reflects the ebbing and flowing of the heart rate across the respiratory cycle (Beauchaine 2015, Porges 2007), with increased inhibitory PNS signaling during exhalation and decreased inhibitory PNS signaling during inhalation. In this way, the PNS acts as a brake on the SNS by reducing the heart rate and facilitating alert engagement in the environment (Porges 2003, 2007). When an individual is faced with threatening or emotional stressors, PNS withdrawal (indexed via RSA decreases or RSA suppression) occurs almost instantaneously, thereby facilitating the fight-or-flight response of the SNS. Dynamic and flexible responses of RSA across environmental contexts are essential for adaptive physiological self-regulation (Hastings et al. 2014).
The second, slower-acting stress response by the HPA axis begins in a group of neurons, the paraventricular nucleus (PVN), within the hypothalamus. The PVN stimulates the release of corticotropin-releasing hormone, which signals the pituitary gland to release adrenocorticotropic hormone (ACTH). ACTH acts on the adrenal glands to synthesize and release glucocorticoids. Glucocorticoids (cortisol in humans) are the end point of the HPA axis system and are responsible for many of the adaptive acute stress responses, such as enhanced memory (Barsegyan et al. 2010) and regulation of inflammatory immune signaling (Slavich & Irwin 2014). Operating in a negative feedback loop, glucocorticoids signal the HPA axis to shut off following the end of a stressor via targeted brain structures, including the PVN, hippocampus, amygdala, and PFC regions (Herman et al. 2003, Tasker & Herman 2011).
In addition to these two central acute stress-response systems, peripheral stress-response systems, including the immune system, polyamine system, and cellular-level processes (including gene expression), are activated following exposure to an acute stressor to prepare the body for adaptation and recovery after a stressor has passed. The body’s adaptive immune response to a stressor triggers proinflammatory activity that promotes signaling of intracellular transcription. This transcription ultimately drives the activity of intracellular, proinflammatory immune response genes [e.g., tumor necrosis factor alpha (TNF-alpha) and interleukin 1 beta (IL1B)] that release protein molecules called cytokines, which are the primary mediators of systemic inflammation (Raison et al. 2006, Slavich et al. 2014). Further, the end products of the SNS (norepinephrine) and HPA axis (cortisol) interact with immune cells in the body, leading to increased inflammatory activity (Raison et al. 2006, Slavich & Irwin 2014).
Polyamines are aliphatic molecules that are stress responsive (Gilad & Gilad 2003), have a major role in homeostatic regulation, and function to influence neurotransmitter systems, including catecholamines, γ-aminobutyric acid, glutamate, and nitric oxide (for an excellent review, see Gross & Turecki 2013). Polyamine levels increase following exposure to stress, and the magnitude of this response is associated with the intensity of the stressor and the behavioral response to the stressor (Gilad & Gilad 2003, Turecki 2014). Although still an emerging area of research, cellular-level responses to acute stress, such as free circulating mitochondrial DNA (mtDNA), result in downstream systemic inflammation (Picard et al. 2014).
Of course, stress responses are triggered, regulated, and terminated in neural networks within the brain. The vagus nerve modulates the faster-acting SNS and transmits afferent signals to subcortical structures of the brain, including the amygdala, via the nucleus tractus solitarius (Berthoud & Neuhuber 2000). Glucocorticoids, the end products of the slower-acting stress-response system, signal the HPA axis to shut off via targeted brain structures, including the PVN, hippocampus, amygdala, and PFC regions (Herman et al. 2003, Tasker & Herman 2011). Peripheral inflammation is similarly controlled via subcortical neural structures, including the hypothalamus (Sternberg 2006).
Although data from studies in developmental affective neuroscience are still emerging, accumulating evidence suggests that pubertal adaptations in acute stress-response systems likely contribute to a protracted period of increased stress reactivity and prolonged stress responses (i.e., a slower return to baseline) in adolescence, which is during the same developmental period associated with greater exposure to interpersonal stress (Dahl & Gunnar 2009). For example, across rodent and human studies, adolescents demonstrate changes in ANS responses, including increased SNS reactivity and PNS withdrawal to acute stressors (Allen & Matthews 1997, Choi & Kellogg 1996, Kurtz & Campbell 1994, Stroud et al. 2009), suggesting that adolescents are more physiologically reactive to stressors and less able to flexibly regulate these increased signals. Similarly, changes in the HPA axis during adolescence result in heightened cortisol reactivity to acute stressors and a longer recovery relative to childhood and adult years (Gunnar et al. 2009, Romeo 2017). Less is known regarding adolescent-specific changes in peripheral stress-response systems, although research has begun examining typical adolescent levels of immune system markers, such as cytokines (Riis et al. 2014). Evidence from neuroimaging research demonstrates increased neural reactivity to negative stimuli during adolescence relative to reactivity in children and adults (Silvers et al. 2012) and increased social sensitivity among adolescents, as reflected by activity in the anterior cingulate cortex, compared with sensitivity in children and adults (Somerville 2013, Somerville et al. 2013). Thus, normative adolescent development is defined by increased sensitivity and responsiveness across these acute stress-response systems, and recent data suggest that atypical presentations of these same systems have implications for understanding emotion dysregulation (Miller et al. 2017b, Tottenham et al. 2010) and a range of both internalizing and externalizing symptoms (Burghy et al. 2012, Chen et al. 2015, Nederhof et al. 2015, Slavich & Irwin 2014).
Although a full discussion of the factors that lead to the development of atypical biological responses to acute stress is beyond the scope of this review, we note that many of the same factors that have been identified as distal predictors for suicide (e.g., depression, childhood maltreatment) also have been associated with dysfunctional acute stress responses in adolescence. For instance, a rich literature has demonstrated that depressive symptoms are associated with the development of hyporeactive responses to stress in the HPA axis (see Guerry & Hastings 2011 for a review). Research also indicates that exposure to chronic stress during early development has lasting effects on stress responses (see McEwen & Gianaros 2010 for a review). Findings from rodent studies and human neuroimaging research have revealed that early chronic stress is linked with increased amygdala reactivity and with a lack of prefrontal regulation to acute stressors (Tottenham & Galván 2016). Rodent models also have demonstrated that chronic exposure to stress, particularly during early childhood and adolescent development, is associated with structural and functional changes in both the amygdala and hippocampus, thus increasing the risk for altered acute stress responses (Radley et al. 2015).
Associations Among Biological Markers of Stress Response and Suicide
Although data on adolescents is sorely lacking, research suggests that each of these biological stress-response systems may be linked with suicidal ideation and behaviors. We believe this is a critical direction for future research: to identify the proximal processes that occur between individuals’ experience of stress and the decision to engage in suicidal behavior. Ideally, to capture actual acute stress responses, this research would use experience sampling procedures, short-term longitudinal designs, and in vivo assessments. However, unique methodological and ethical limitations make the study of suicide more challenging than, perhaps, the examination of almost every other clinical phenomenon. Thus, findings remain sparse, particularly among adolescents. Below, we review preliminary associations between suicide and markers of the two central acute stress-response systems, the ANS and the HPA axis. Then, we move on to genomic markers of peripheral stress-response systems. Finally, we discuss neural markers, emphasizing that stress responses are initiated and terminate in the brain.
Markers of Central Stress-Response Systems
Markers of central stress-response systems may be promising predictors of proximal suicide risk in adolescents. To date, most research has been cross-sectional and predominantly conducted with adults. Below, we review findings related to markers of the fast-acting ANS and then the slower-acting HPA axis.
Autonomic nervous system.
Although still an emerging area of research in the literature on adolescent suicide, a handful of studies have examined RSA as an ANS correlate of suicide risk, with findings suggesting that a lower resting RSA or excessive RSA withdrawal may be linked with emotion dysregulation and suicidal ideation and behavior. However, findings have been mixed, suggesting that more research is needed, particularly among youths.
In general, findings from concurrent studies that examined individuals with and without lifetime histories of self-injury have been promising. For instance, in an early concurrent study, 23 adolescent females aged 14 to 18 years with a history of suicidal behavior or NSSI, or both, evinced lower resting RSA and excessive RSA withdrawal (i.e., greater release of SNS suppression) following exposure to evocative stimuli compared with controls (N = 23, ages 14–18 years) (Crowell et al. 2005). Another study demonstrated that children ages 7–11 years with a history of suicidal ideation and who had parents rated high on expressed negative emotion did not show flexible RSA responses to a stressful discussion, suggesting a poor adaptive stress response (James et al. 2017). Evidence from studies of adults also suggests that a lower resting RSA is associated with suicidal ideation (Rottenberg et al. 2002) and distinguishes individuals with and without a history of suicide attempt (Tsypes et al. 2018).
However, studies examining RSA as a prospective predictor of later suicidal ideation and behavior have yielded more equivocal findings. In a community-based sample of early adolescents (mean age = 12.82 years), Wielgus and colleagues (2016) revealed that resting RSA and greater RSA withdrawal to a noninterpersonal frustration task did not predict engagement in self-injurious thoughts and behavior (defined as a composite of NSSI, suicidal ideation, and suicidal behavior) during the following 6 months. However, slower recovery of RSA (indexed by lower resting RSA during recovery from the task) was associated with a prospective risk for this same self-injury composite over time (Wielgus et al. 2016). In contrast, Giletta and colleagues (2017) revealed that greater RSA withdrawal following a social stressor task was associated with a prospective risk for suicidal ideation during the following 9 months in a sample of adolescents with a history of mental health concerns, even after controlling for lifetime history of suicidal ideation and current depressive symptoms. More research examining RSA indices in response to interpersonal and noninterpersonal stress across different developmental periods may be especially important for understanding whether suicide may be predicted by dysregulated arousal or recovery following stress. However, these findings offer preliminary promise, suggesting that ANS responses to acute stressors may provide insight into adolescents’ risk for suicide.
Hypothalamic–pituitary–adrenal axis.
Researchers also have recently indexed markers of the HPA axis as potential predictors of suicidal ideation and behavior. Notably, this has involved examining baseline or resting salivary cortisol levels; chronic cortisol output as measured by hair cortisol concentrations; and reactive cortisol through repeated salivary sampling before, during, and after an experimentally induced stressor. Unfortunately, little work has been done with adolescents.
Regarding baseline cortisol, both higher and lower levels of cortisol have been associated with suicide. For instance, in some studies in adults, elevated baseline levels of cortisol have been associated with greater frequencies of suicidal behavior (Mann & Currier 2007). Yet in more recent studies with combined samples of adults and youths, individuals with a lifetime history of suicidal behavior have had lower baseline levels or hair concentrations of cortisol compared with individuals with only suicidal ideation or with healthy controls (Keilp et al. 2016, Melhem et al. 2017).
Findings regarding cortisol levels following a stressor similarly reveal that both hypo- and hyperreactivity may be relevant for suicide. In a cross-sectional study of depressed and/or self-harming adolescent girls, lower levels of cortisol after a dexamethasone suppression test (suggesting cortisol hyporeactivity) were associated with greater current suicidal ideation (Beauchaine et al. 2015). Some studies have revealed that adults with a history of suicide attempts have more blunted cortisol reactivity to a Trier Social Stress Test (TSST) than those with only suicidal ideation history or than healthy controls (Melhem et al. 2016, O’Connor et al. 2017).
Other than research from our own group, we are unaware of any other studies examining cortisol reactivity to social stressors as a prospective predictor of suicidal ideation and behavior among adolescents. Our research examined cortisol reactivity to a TSST in a sample of adolescent girls (ages 13–17 years) at elevated risk for future suicide due to a history of mental health concerns. Following the lab-based stressor, participants were followed quarterly for 18 months to examine cortisol reactivity as a prospective predictor of adolescent suicidal ideation and suicide attempts. Initial findings revealed a significant effect for cortisol hyperreactivity (and a marginal trend for hyporeactivity) to the TSST on the risk for suicidal ideation 3 months later, even after controlling for the lifetime history of suicidal ideation and other distal risk factors (e.g., impulsivity) (Giletta et al. 2015). Subsequent analyses using a multilevel model to capture within-person deviations in chronic peer stress revealed that higher-than-usual peer stress predicted suicidal ideation over 18 months, regardless of HPA axis function, but it predicted suicidal behavior only among girls with blunted cortisol responses to the TSST at baseline (Eisenlohr-Moul et al. 2018a).
In sum, findings suggest that dysregulated cortisol levels, as markers of HPA axis responses to stress, may be relevant markers of suicide risk. Consistent with allostatic load theories (McEwen 2007) regarding moderate levels of cortisol facilitating flexible, adaptive responses to environmental stressors, findings suggest that both hyper- and hypocortisol responses reflect disruptions in the HPA axis system that may increase the vulnerability to suicide in the context of acute stress.
Peripheral Stress-Response Systems and Functional Genomics
Markers of several peripheral response systems also may be relevant correlates of adolescent suicide. Recent research efforts have focused on epigenetic mechanisms, with far more examining cytokines than other peripheral response markers. Below, we review some of the promising findings related to cytokines, followed by a review of preliminary work linking suicide with polyamines, mtDNA, and other epigenetic markers.
Cytokines.
Recall that the immune system responds to acute stressors, resulting in increased inflammation aimed at promoting healing and recovery. Following acute stress, proinflammatory cytokines signal the brain to engage in sickness behaviors closely mimicking depression and, in some cases, leading to clinical depression among physically ill people (Dantzer et al. 2008). Because work has demonstrated that adverse social experiences characterized by loss or threat activate proinflammatory cytokine activity (Giletta et al. 2018, Slavich & Cole 2013) and trigger alterations in the genes regulating proinflammatory cytokine production (Slavich et al. 2010), increased inflammatory brain signaling in the context of exposure to social stressors may distinguish adolescents at risk for suicidal ideation and behavior.
This hypothesis has some support from emerging studies demonstrating that compared with controls, individuals with suicidal ideation and behavior have increased proinflammatory cytokine activity (Black & Miller 2015). Findings indicate that adults with a history of suicidal behavior show increased levels of interleukin6 (IL-6) (Janelidze et al. 2011, Lindqvist et al. 2009, Serafini et al. 2013), increased tumor necrosis factor-α (TNF-α) (Janelidze et al. 2011), decreased levels of IL-2 (Janelidze et al. 2011, Serafini et al. 2013), and increased levels of cytokine-stimulated plasma kynurenine (Sublette et al. 2011) and quinolinic acid (Erhardt et al. 2013) in cerebrospinal fluid.
Results linking cytokines to suicidal ideation or behavior among adolescents are rare, but findings are promising. For instance, one study revealed that adolescents with major depressive disorder and suicidal ideation (N = 12) showed higher levels of interferon-γ compared with controls (N = 15) (Gabbay et al. 2009). In a sample of adolescents and emerging adults (age range 15–30 years), individuals with a history of suicide attempt had higher levels of TNF-α and C-reactive protein compared with those with a history of suicidal ideation and with healthy controls (Melhem et al. 2017). In another study, youths who died by suicide (N = 24) compared with matched healthy controls (N = 24) had higher levels of IL-6, IL-1β, and TNF-α in the PFC (Pandey et al. 2012), suggesting that proinflammatory cytokines may be a useful avenue for further exploration of adolescent suicide risk.
Other epigenetic markers.
Preliminary associations have also been revealed between suicide and polyamines, mtDNA, and DNA methylation. The polyamine system has been implicated in the risk for suicide (see Lutz et al. 2017 for a detailed discussion), with preliminary empirical findings coming from rodent studies and postmortem studies of human adults. For example, one study revealed that administration of the polyamine agmatine ameliorated experimentally (TNF-α)-induced depression-like behaviors in mice (Neis et al. 2014). Altered polyamine-related genes, suggesting system dysregulation, also were revealed in a postmortem study of adults who died by suicide (Gross & Turecki 2013). Both studies suggest that the polyamine system may be relevant for regulating depressed affect and perhaps also suicidal ideation or behavior. Results on mtDNA similarly suggest that dysregulation may be linked with suicide. In a recent study of adults, free circulating mtDNA measured via blood plasma was significantly higher in individuals with a history of suicide attempt compared with healthy controls (Lindqvist et al. 2016). Researchers have found that DNA methylation systematically alters gene expression, resulting in an increased risk for psychopathology and, specifically, suicide (Haghighi et al. 2014). There is also some preliminary evidence for autonomic dysregulation in insulin and glucagon secretion among individuals with a history of suicide attempt (Bendix et al. 2017). Further examination of markers of the peripheral stress system in the context of suicide will be an important direction for future work.
Neural Markers
Emerging research in neuroscience supports a link between suicide risk and altered neural structure and function. Subcortical brain structures, which mediate acute stress responses, are modulated and regulated via prefrontal brain regions. Altered frontolimbic connectivity could help explain why some youths who experience an acute stressor are unable to leverage higher-order skills, such as cognitive reappraisal (Buhle et al. 2014), to modulate subcortical activation and, subsequently, begin experiencing suicidal ideation. Examinations of both subcortical and cortical brain structures, neural activation, and network connectivity preliminarily have revealed associations with suicide risk.
Subcortical brain regions, including the amygdala and hippocampus, are primitive brain structures that monitor the environment for salient cues for reward or threat. In particular, these structures have an important role in the perception of stressors, and they signal the body to enact and modulate stress responses. Thus, altered structure and function in these areas may engender risk for altered acute stress responses. Two recent studies suggest that youths at risk for suicide demonstrate significant differences in subcortical brain regions. Johnston and colleagues (2017) revealed that compared with nonsuicidal controls, youths with a history of suicidal behavior had decreased gray matter volumes in the hippocampus and cerebellum and decreased functional connectivity between the amygdala and the left ventral and right rostral PFC when viewing emotional faces (Johnston et al. 2017), suggesting altered signaling between subcortical and PFC control regions. Another study, by Quevedo and colleagues (2016), found that youths with a history of suicidal ideation and behavior demonstrated blunted responses to happy faces in the hippocampus and amygdala, suggesting altered processing of potential stressors in the environment. Thus, early evidence suggests there is altered signaling in subcortical regions among youths at risk for suicide.
The PFC serves a critical role in leveraging higher-order cognitive skills in the service of modulating subcortical activation. In other words, the PFC can assist in managing subcortical activation resulting from exposure to a stressor to achieve an efficient and effective acute stress response. An emerging line of research points to altered neural activation (Just et al. 2017, Miller et al. 2018, Pan et al. 2013, Quevedo et al. 2016) and neural network connectivity (Chase et al. 2017; Ordaz et al. 2018; Zhang et al. 2014, 2016) in PFC regions among individuals with a history of suicidal ideation or behavior. For instance, work from our group demonstrated that compared with controls, youths with a history of suicidal ideation show decreased dorsolateral PFC activation when passively viewing negative stimuli (Miller et al. 2018), suggesting that youths with a history of suicidal ideation may not automatically engage skills to regulate negative emotions. With regard to functional connectivity, individuals with a history of suicidal ideation appear to show abnormal resting state connectivity between PFC control regions and subcortical brain regions (Chase et al. 2017, Ordaz et al. 2018), potentially signaling a vulnerability to increased reactivity and decreased ability to modulate this reactivity when faced with a stressor. In adult samples, similar findings have emerged, suggesting decreased frontolimbic functional connectivity in individuals with suicidal ideation (Du et al. 2017, Kim et al. 2017, Minzenberg et al. 2015). Together, these findings suggest that activation of the PFC and subcortical regions during emotionally evocative tasks may be associated with suicidal ideation and behavior among adolescents.
Although more research is needed in the area of the neural underpinning of suicidal ideation and behavior among youths, emerging evidence points to altered activation and connectivity patterns that may have specific effects on subcortical activation, which are instrumental in mediating and modulating acute stress-response systems. Notably, the PFC is a relatively large region, and findings have not yet converged on one specific structure within the PFC. Further, the data are not consistent enough to distinguish neural markers of risk for suicidal ideation from risk for suicidal behavior. This is an area in critical need of future research as the neural processes underlying suicidal ideation are likely related to but different from those underlying suicidal behavior. Further, none of these studies examines the prospective risk for suicidal ideation and behavior.
SYNTHESIS AND FUTURE DIRECTIONS
In this review, we have suggested that our understanding and, ultimately, the successful treatment of suicidal thoughts and behaviors and the prevention of suicide would be aided by addressing one of the central facts about this perplexing and troubling clinical phenomenon: namely, the worldwide trend for suicidal ideation and behavior to increase dramatically in prevalence at the adolescent transition (Nock et al. 2013), a fact that, unfortunately, has been unaddressed in almost all prior theories regarding suicide. We have reviewed a constellation of biological and social adaptations to adolescence that occur at precisely the same period when the onset of suicidal ideation and behavior is most likely. Also, consistent with dynamic systems theories, we elucidated how reciprocal intra- and interpersonal transactions between social–environmental, cognitive, and biological systems may confer a unique risk for the types of interpersonally themed stress that most adolescents report as precipitants to suicidal behavior and the impulsive suicidal urges that are more common among adolescents than among suicidal adults (Orbach 1997).
With this review, we also have encouraged a greater focus on biological responses to acute stress that may help explain the proximal processes occurring in the moments between adolescents’ exposure to stress and their experience of suicidal ideation and behavior (see also Cha et al. 2018; Chang et al. 2016; Lutz et al. 2017; Pan et al. 2011, 2016; Turecki & Brent 2016). Four biological systems that are implicated in acute stress responses have been discussed, with a summary of evidence suggesting that each (a) may undergo important recalibration in adolescence, increasing vulnerability during this developmental period; (b) may be especially dysregulated among adolescents who possess known distal risk factors for suicide; and (c) is associated with relevant suicidal outcomes, at least preliminarily, based on limited empirical studies.
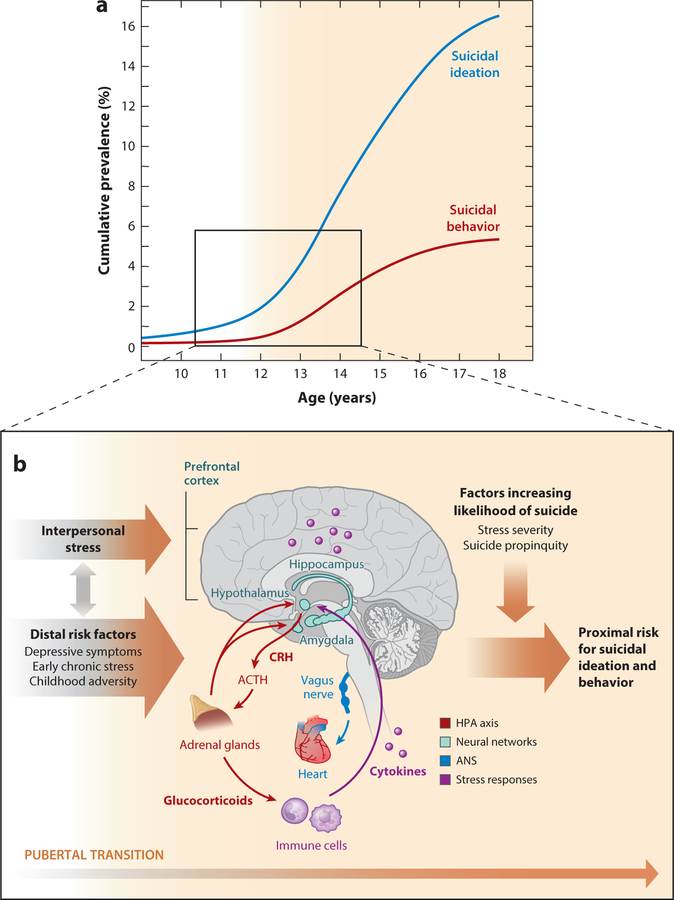
In sum, we have suggested a heuristic model to guide further research (Figure 1). This model suggests that the vulnerability conferred by the pubertal transition and from preexisting distal risk factors combine to affect biological stress-response systems in adolescence, leaving some youths especially ill-prepared to cope with the increase in interpersonal stressors that characterize this developmental period. Note, however, that it remains unclear why these distressed adolescents exhibit suicidal ideation and behavior.
Figure 1.
Heuristic model of adolescent suicidal ideation and behavior as a failure of biological responses to acute stress. (a) Developmental onset and prevalence of suicidal ideation and behavior (data from Nock et al. 2013). (b) Risks for suicidal ideation and behavior begin to increase exponentially at the pubertal transition. This increase occurs during a period of significant changes in biological systems that respond to acute stress. Our model suggests that interpersonal stressors and distal risk factors directly influence biological stress-response systems. In turn, failures of biological responses to acute stress confer risks for suicidal ideation and behavior, particularly among those facing stress exceeding their own personal threshold and those with prior exposure to suicidal ideation and behavior. Key stress-response systems that have been linked with suicidal ideation and behavior are depicted. Abbreviations: ACTH, adrenocorticotropic hormone; ANS, autonomic nervous system; CRH, corticotropin-releasing hormone; HPA, hypothalamic–pituitary–adrenal.
Indeed, the problem with this model, like so many others proposed to explain suicide, is the multifinality of the risk pathways we have articulated thus far. In short, many other domains of psychopathology also may be predicted by these same distal factors, the onset of social–biological adaptations to puberty, and atypical stress-response systems. In short, why suicide? We propose that compared with a host of other possible dysfunctional outcomes, suicide is most likely to occur when at least one of two conditions is met.
Stress Threshold
First, we posit that suicide may become more likely when individuals experience distress that is unusual, severe, and exceeds their actual or perceived capacity to activate cognitive and social coping resources. In other words, suicide may follow from the same stress-response processes that confer risk for other forms of psychopathology when stressors exceed an individual’s own stress threshold.
For healthy individuals, stress thresholds may be quite high. Yet even healthy individuals with intact acute stress-response systems might conceive of suicidal behavior if faced with a stressor perceived to be unusually intense, life-altering, or overwhelming (see Orbach 1997 for a detailed discussion of people who attempted suicide and exhibited high levels of perfectionism but no other apparent psychopathology). However, for those with depressive symptoms, chronic or early childhood stress, or other distal risks for suicide, thresholds may be lower, making suicide a more likely option.
Although a rich literature exists examining the association between stress and suicide, almost all research studies have employed analytical approaches that prohibit testing of a stress-threshold hypothesis. Note that traditional tests of associations among suicide risk factors and self-injurious constructs traditionally utilize a between-subjects design, with resulting statistical indices revealing whether those high in stress compared with an entire study sample may also exhibit higher levels of suicidal ideation or behavior than others in the sample. Moreover, the vast majority of research on suicide is concurrent in nature, precluding the ability to understand how an individual’s experience of stress or suicidal ideation or behavior at one time point might compare with an individual’s enduring level of stress.
A series of multiwave prospective studies conducted within our research group using a within-person analytical approach has offered some evidence to support a stress-threshold hypothesis. In a large sample of adolescent girls, we found modest between-person associations between interpersonally themed stress and suicidal ideation or suicide attempts during the course of an 18-month follow-up period. However, our ability to predict when adolescents may attempt suicide was notably enhanced by computing individual stress and suicide trajectories, allowing us to examine how girls’ stress within each 3-month follow-up period compared with their average (person-centered) level of stress throughout their participation in our study. Findings suggested that among adolescent girls who have been exposed to child abuse, for instance, periods of higher-than-usual stress are also times of enhanced risk for suicidal behavior (Miller et al. 2017a). Moreover, results revealed a social–biological transactional effect, suggesting that suicidal attempts were most likely among girls who demonstrated hyporeactive cortisol responses to a social speech task and only during the period when they experienced higher-than-usual peer stress (Eisenlohr-Moul et al. 2018a). We have replicated a similar pattern of results in a separate sample of adolescents and in a sample of emerging adults (Miller et al. 2018).
Suicide Propinquity
A second factor that may increase the likelihood that vulnerable (i.e., stress-exposed) adolescents might exhibit suicidal ideation or suicidal behavior reflects the extent to which they have been exposed to suicide as an option for ameliorating distress. While for many distressed individuals, suicide may seem like a remote consideration—an option that may not even enter consciousness—there are several factors that may increase the likelihood that adolescents will readily consider suicide, including (a) their own prior experiences with self-injury and suicide, such as prior NSSI, ideation, or attempts; (b) their knowledge of others’ (a relative’s or celebrity’s) suicidal behavior; and (c) peer socialization toward self-injurious thoughts and behaviors. The notion of suicide propinquity is not novel, but rather represents an integration of substantial theory and empirical data. Adolescents who engage in NSSI or make suicide attempts are significantly more likely to engage in future suicidal behavior (Ribeiro et al. 2016), especially within the first few months following an attempt (Goldston et al. 2001). The effects of exposure to well-known individuals’ suicidal behavior on adolescents’ own suicidal behavior also have been well documented through research on suicide clusters (see Brent et al. 1989, Gould 1990, Gould et al. 1990, Joiner 1999, Phillips & Carstensen 1986), yielding best practice recommendations for the media and school personnel when covering the death by suicide of a well-known person (Pirkis et al. 2006).
A growing body of data also suggests that adolescents may be particularly susceptible toward the socialization of self-injury when a close friend endorses depressive symptoms, NSSI, or suicidal ideation and behavior (Bearman & Moody 2004, Kandel et al. 1991, Rew et al. 2001, Yoder 1999). Although most studies have examined concurrent associations, making it difficult to determine whether adolescents select friends who are similarly suicidal or are socialized toward greater suicidal ideation and behaviors through affiliations with other distressed teens, some emerging longitudinal evidence suggests that a friend’s suicide is a meaningful predictor of an adolescent’s own risk for future NSSI (Giletta et al. 2013, Prinstein et al. 2010, You et al. 2013) and suicidal behavior (e.g., Liu 2006).
Thus, suicide propinquity may moderate the association between adolescents’ acute stress response and suicidal ideation or behavior. Although many adolescents with developmental risk factors may experience psychological symptoms following stress, those who become suicidal might be predicted by understanding whether adolescents have recently been exposed to and, perhaps, consequently possess less-aversive attitudes toward suicide (Nock et al. 2010).
CONCLUSIONS
In conclusion, this integrative review has offered several new directions for research on adolescent suicide aimed at better understanding a phenomenon that has remained poorly understood for too long. High-priority areas for future research are to focus on adolescence, proximal risk factors, biological responses to acute stress, longitudinal designs, within-person analytical approaches, moderators of the short-term association between stress and suicidal ideation and behaviors, and the identification of factors that promote suicide but not other psychological symptoms, as unique outcomes among youths with multiple risk factors.
Yet it deserves mention that these suggested research directions should be considered in the context of many other highly salient factors that are beyond the scope of this review. For example, the acute stress-response risk model suggested herein should be considered in the context of biological sex, particularly given adolescent girls’ increased experience of social stress, greater biological and emotional reactivity to interpersonal stress (Rose & Rudolph 2006, Stroud et al. 2017), and emerging data suggesting unique biological factors among females (i.e., circulating reproductive hormones) that may modulate stress-response systems (see Eisenlohr-Moul et al. 2018b). Adolescent suicide also must be considered in the context of cultural factors, such as race, ethnicity, and religiosity, that are remarkably relevant but often neglected determinants of suicidal outcomes (Goldston et al. 2008). Our review also did not address a myriad of protective factors that may buffer the effects of risk on adolescents’ suicidal behavior; this remains a critically understudied yet important direction for research (see Gallagher & Miller 2018). Last, adolescent suicide may be best understood in the context of the macrosystem (Bronfenbrenner 1979) to better address the cultural and political factors that may prove especially relevant to adolescent groups traditionally at risk for suicide (e.g., lesbian, gay, bisexual, transgender, questioning, and/or queer youths) and may at least partially explain generational differences in the rates of adolescent suicide.
The field of suicide research has grown extensively during the past two decades, marked by an increasing number of publications (Franklin et al. 2017), an increased commitment to federal funding (Gordon 2016), and even public awareness of suicidal ideation and behaviors as an urgent public health crisis. A developmentally informed understanding of suicide may reveal mechanisms and processes that are relevant for reducing the frequency of suicidal deaths across the life span.
SUMMARY POINTS.
Suicide remains a leading cause of death among adolescents despite decades of research on correlates and risk factors.
The developmental period of adolescence creates a unique vulnerability for the emergence of suicidal ideation and behavior as adolescents undergo biological changes and experience substantial social reorganization. Suicidal crises are often triggered by interpersonal stressors, which increase in frequency and intensity during adolescence.
Emerging evidence points to a failure in biological responses to acute stress as a potential mechanism of adolescent suicide.
Data suggest that biological markers of acute stress responses in the autonomic nervous system, hypothalamic–pituitary–adrenal axis, peripheral stress systems (e.g., cytokines), and neural systems may differ among adolescents at risk for suicide.
While abnormal acute stress responses may confer a risk for psychopathology more generally, we hypothesize that youths who exceed their own typical levels of stress are so remarkably overwhelmed in a suicidal crisis that they turn to suicide as a means of ending distress. Further, we hypothesize that failures in acute stress-response systems are particularly likely to precipitate risk for youths exposed to previous suicidal ideation and behaviors, either through exposure to other’s suicidal behavior or through their own past suicidal ideation or behavior.
Drawing from a developmental psychopathology perspective, we suggest that failures of acute stress responses and suicidal crisis are transactionally linked. Adolescents’ perceptions of a stressor and their ability to cope are likely altered by failures of acute stress responses, which, in turn, lower their threshold for stress tolerance in the future.
It is unlikely that failures of acute stress responses explain every type of risk for adolescent suicide. However, we believe that across yet-to-be-elucidated phenotypes of suicidal behavior, stress-response systems are likely altered.
FUTURE ISSUES.
Adolescent suicide research must move toward focusing on proximal processes (i.e., in the hours, minutes, seconds) that characterize suicidal crises.
Overall, we believe that any future research must consider the unique biological and social changes that characterize adolescence.
Leveraging methodologies across multiple units of analysis, such as those suggested by the Research Domain Criteria matrix, is likely to sharpen our understanding of the complex behavior of adolescent suicide.
We believe that the most promising research designs involve combining careful biological assays with rigorous behavioral assessments. An example could include repeated assessments of acute stress responses via laboratory-based visits together with ecological momentary assessments of behaviors between sessions.
A crucial step facing future research on adolescents is to engage in more careful phenotyping of suicidal behavior. We are not the first to call for examining subtypes of behavior, but we believe that it is imperative to move toward a more individualized model of risk rather than treating all suicidal ideation and behavior as the same outcome.
ACKNOWLEDGMENTS
Preparation of this manuscript was supported by grants from the National Institute of Mental Health (R01MH107479 to M.J.P., F32MH108238 to A.B.M., and K01MH116325 to A.B.M.).
Glossary
- Suicidal ideation
includes consideration of, or desire for, death that can range from passive (e.g., “I would be better off dead”) to active (e.g., “I plan to kill myself”)
- Suicide attempt
refers to a deliberate action taken to end one’s life
- Suicidal behavior
sometimes used to encompass both actual and interrupted suicide attempts
- Suicide death
results from fatal, deliberate self-harm; frequently determined by a medical examiner or coroner
- ANS
autonomic nervous system
- HPA
hypothalamic–pituitary–adrenal
- PFC
prefrontal cortex
- SNS
sympathetic nervous system
- PNS
parasympathetic nervous system
- RSA
respiratory sinus arrhythmia
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Allen MT, Matthews KA. 1997. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology 34(3):329–39 [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Hughes JL, Babeva KN, Sugar CA. 2017. Cognitive-behavioral family treatment for suicide attempt prevention: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 56(6):506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. 2010. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. PNAS 107(38):16655–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF. 1990. Suicide as escape from self. Psychol. Rev 97(1):90–113 [DOI] [PubMed] [Google Scholar]
- Bearman PS, Moody J. 2004. Suicide and friendships among American adolescents. Am. J. Public Health 94(1):89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP. 2015. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Curr. Opin. Psychol 3:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Crowell SE, Hsiao RC. 2015. Post-dexamethasone cortisol, self-inflicted injury, and suicidal ideation among depressed adolescent girls. J. Abnorm. Child Psychol. 43(4):619–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. 1987. Cognitive models of depression. J. Cogn. Psychother 1(1):5–37 [Google Scholar]
- Bendix M, Uvnäs-Moberg K, Petersson M, Kaldo V, Åsberg M, Jokinen J. 2017. Insulin and glucagon in plasma and cerebrospinal fluid in suicide attempters and healthy controls. Psychoneuroendocrinology 81:1–7 [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Neuhuber WL. 2000. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci 85(1–3):1–17 [DOI] [PubMed] [Google Scholar]
- Black C, Miller BJ. 2015. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol. Psychiatry 78(1):28–37 [DOI] [PubMed] [Google Scholar]
- Brent DA, Kerr MM, Goldstein C, Bozigar J, Wartella M, Allan MJ. 1989. An outbreak of suicide and suicidal behavior in a high school. J. Am. Acad. Child Adolesc. Psychiatry 28(6):918–24 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U 1979. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard Univ. Press [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, et al. 2014. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex 24(11):2981–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, et al. 2012. Developmental pathways to amygdala–prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci 15(12):1736–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. 2008. The adolescent brain. Ann. N. Y. Acad. Sci 1124:111–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha CB, Franz PJ, M Guzmán E, Glenn CR, Kleiman EM, Nock MK. 2018. Suicide among youth—epidemiology, (potential) etiology, and treatment. J. Child Psychol. Psychiatry 59(4):460–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BP, Franklin JC, Ribeiro JD, Fox KR, Bentley KH, et al. 2016. Biological risk factors for suicidal behaviors: a meta-analysis. Transl. Psychiatry 6(9):e887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Segreti AM, Keller TA, Cherkassky VL, Just MA, et al. 2017. Alterations of functional connectivity and intrinsic activity within the cingulate cortex of suicidal ideators. J. Affect. Disord 212:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. 2011. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci 14(2):F1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FR, Raine A, Soyfer L, Granger DA. 2015. Interaction of adrenocortical activity and autonomic arousal on children’s externalizing and internalizing behavior problems. J. Abnorm. Child Psychol. 43(1):189–202 [DOI] [PubMed] [Google Scholar]
- Choi S, Kellogg CK. 1996. Adolescent development influences functional responsiveness of noradrenergic projections to the hypothalamus in male rats. Dev. Brain Res. 94(2):144–51 [DOI] [PubMed] [Google Scholar]
- Cillessen AHN, Rose AJ. 2005. Understanding Popularity in the Peer System. Curr. Dir. Psychol. Sci 14(2):102–5 [Google Scholar]
- Cohen AO, Dellarco DV, Breiner K, Helion C, Heller AS, et al. 2016. The impact of emotional states on cognitive control circuitry and function. J. Cogn. Neurosci 28(3):446–59 [DOI] [PubMed] [Google Scholar]
- Crowell SE, Beauchaine TP, McCauley E, Smith CJ, Stevens AL, Sylvers P. 2005. Psychological, autonomic, and serotonergic correlates of parasuicide among adolescent girls. Dev. Psychopathol 17(4):1105–27 [DOI] [PubMed] [Google Scholar]
- Curtin S, Warner M, Hedegaard H. 2016. Increase in suicide in the United States, 1999–2014. NCHS Data Brief 241:1–8 [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. 2009. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev. Psychopathol 21(1):1–6 [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9(1):46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Zeng J, Liu H, Tang D, Meng H, et al. 2017. Fronto-limbic disconnection in depressed patients with suicidal ideation: A resting-state functional connectivity study. J. Affect. Disord 215:213–17 [DOI] [PubMed] [Google Scholar]
- Durkheim E 1951. (1897). Suicide: A Study in Sociology. London: Routledge. 2nd ed. [Google Scholar]
- Eisenlohr-Moul TA, Miller AB, Giletta M, Hastings PD, Rudolph KD, et al. 2018a. HPA axis response and psychosocial stress as interactive predictors of suicidal ideation and behavior in adolescent females: a multilevel diathesis–stress framework. Neuropsychopharmacology 43(13):2564–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Prinstein M, Rubinow D, Young S, Walsh E, et al. 2018b. Ovarian steroid withdrawal underlies perimenstrual worsening of suicidality: evidence from a crossover steroid stabilization trial. Biol. Psychiatry 83(9 Suppl):S387 [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, et al. 2013. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 38(5):743–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito-Smythers C, Spirito A, Kahler CW, Hunt J, Monti P. 2011. Treatment of co-occurring substance abuse and suicidality among adolescents: a randomized trial. J. Consult. Clin. Psychol 79(6):728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KR, Franklin JC, Ribeiro JD, Kleiman EM, Bentley KH, Nock MK. 2015. Meta-analysis of risk factors for nonsuicidal self-injury. Clin. Psychol. Rev 42:156–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Ribeiro JD, Fox KR, Bentley KH, Kleiman EM, et al. 2017. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol. Bull 143(2):187–232 [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, et al. 2009. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J. Child Adolesc. Psychopharmacol 19(4):423–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher ML, Miller AB. 2018. Suicidal thoughts and behavior in children and adolescents: an ecological model of resilience. Adolesc. Res. Rev 3(2):123–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. 2003. Overview of the brain polyamine-stress-response: regulation, development, and modulation by lithium and role in cell survival. Cell. Mol. Neurobiol 23(4–5):637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Burk WJ, Scholte RH, Engels RC, Prinstein MJ. 2013. Direct and indirect peer socialization of adolescent nonsuicidal self-injury. J. Res. Adolesc 23(3):450–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Calhoun CD, Hastings PD, Rudolph KD, Nock MK, Prinstein MJ. 2015. Multi-level risk factors for suicidal ideation among at-risk adolescent females: the role of hypothalamic–pituitary–adrenal axis responses to stress. J. Abnorm. Child Psychol. 43(5):807–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Hastings PD, Rudolph KD, Bauer DJ, Nock MK, Prinstein MJ. 2017. Suicide ideation among high-risk adolescent females: examining the interplay between parasympathetic regulation and friendship support. Dev. Psychopathol 29(4):1161–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Slavich GM, Rudolph KD, Hastings PD, Nock MK, Prinstein MJ. 2018. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J. Child Psychol. Psychiatry 59(2):129–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Cha CB, Kleiman EM, Nock MK. 2017a. Understanding suicide risk within the Research Domain Criteria (RDoC) framework: insights, challenges, and future research considerations. Clin. Psychol. Sci 5(3):568–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Kleiman EM, Cha CB, Deming CA, Franklin JC, Nock MK. 2018. Understanding suicide risk within the Research Domain Criteria (RDoC) framework: a meta-analytic review. Depress. Anxiety 35(1):65–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Lanzillo EC, Esposito EC, Santee AC, Nock MK, Auerbach RP. 2017b. Examining the course of suicidal and nonsuicidal self-injurious thoughts and behaviors in outpatient and inpatient adolescents. J. Abnorm. Child Psychol. 45(5):971–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldston DB, Daniel SS, Reboussin BA, Reboussin DM, Frazier PH, Harris AE. 2001. Cognitive risk factors and suicide attempts among formerly hospitalized adolescents: a prospective naturalistic study. J. Am. Acad. Child Adolesc. Psychiatry 40(1):91–99 [DOI] [PubMed] [Google Scholar]
- Goldston DB, Molock SD, Whitbeck LB, Murakami JL, Zayas LH, Hall GCN. 2008. Cultural considerations in adolescent suicide prevention and psychosocial treatment. Am. Psychol 63(1):14–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA. 2016. The push for suicide prevention. Natl. Inst. Mental Health https://www.nimh.nih.gov/about/director/messages/2016/the-push-for-suicide-prevention.shtml [Google Scholar]
- Gould MS. 1990. Suicide clusters and media exposure In Suicide Over the Life Cycle: Risk Factors, Assessment, and Treatment of Suicidal Patients, ed. Blumenthal SJ, Kupfer DJ, pp. 517–32. Arlington, VA: Am. Psychiatr. Assoc. [Google Scholar]
- Gould MS, Wallenstein S, Kleinman MH, O’Carroll P, Mercy J. 1990. Suicide clusters: an examination of age-specific effects. Am. J. Public Health 80(2):211–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JA, Turecki G. 2013. Suicide and the polyamine system. CNS Neurol. Disord. Drug Targets 12(7):980–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry JD, Hastings PD. 2011. In search of HPA axis dysregulation in child and adolescent depression. Clin. Child Fam. Psychol. Rev 14(2):135–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. 2009. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev. Psychopathol 21(1):69–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi F, Xin Y, Chanrion B, O’Donnell AH, Ge Y, et al. 2014. Increased DNA methylation in the suicide brain. Dialogues Clin. Neurosci 16(3):430–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S, Stocker C, Robinson NS. 1996. The perceived directionality of the link between approval and self-worth: the liabilities of a looking glass self-orientation among young adolescents. J. Res. Adolesc 6:285–308 [Google Scholar]
- Hastings PD, Kahle SS, Han GH-P. 2014. Developmental affective psychophysiology: using physiology to inform our understanding of emotional development. Child. Emotion 26:13–28 [Google Scholar]
- Hawton K, Saunders KE, O’Connor RC. 2012. Self-harm and suicide in adolescents. Lancet 379(9834):2373–82 [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, et al. 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol 24(3):151–80 [DOI] [PubMed] [Google Scholar]
- James KM, Woody ML, Feurer C, Kudinova AY, Gibb BE. 2017. Disrupted physiological reactivity among children with a history of suicidal ideation: moderation by parental expressed emotion-criticism. Biol. Psychol 130:22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin Å, Träskman-Bendz L, Brundin L. 2011. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain. Behav. Immun 25(2):335–39 [DOI] [PubMed] [Google Scholar]
- Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, et al. 2017. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am. J. Psychiatry 174(7):667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE Jr. 1999. The clustering and contagion of suicide. Curr. Dir. Psychol. Sci 8(3):89–92 [Google Scholar]
- Joiner TE Jr. 2005. Why People Die by Suicide. Cambridge, MA: Harvard Univ. Press [Google Scholar]
- Just MA, Pan L, Cherkassky VL, McMakin D, Cha C, et al. 2017. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat. Hum. Behav 1:911–19 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Juvonen J, Graham S. 2014. Bullying in schools: the power of bullies and the plight of victims. Annu. Rev. Psychol 65(1):159–85 [DOI] [PubMed] [Google Scholar]
- Kandel DB, Raveis VH, Davies M. 1991. Suicidal ideation in adolescence: depression, substance use, and other risk factors. J. Youth Adolesc. 20(2):289–309 [DOI] [PubMed] [Google Scholar]
- Keilp JG, Stanley BH, Beers SR, Melhem NM, Burke AK, et al. 2016. Further evidence of low baseline cortisol levels in suicide attempters. J. Affect. Disord 190:187–92 [DOI] [PubMed] [Google Scholar]
- Kim K, Kim S-W, Myung W, Han CE, Fava M, et al. 2017. Reduced orbitofrontal–thalamic functional connectivity related to suicidal ideation in patients with major depressive disorder. Sci. Rep 7(1):15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Merchant CR. 2008. Social and interpersonal factors relating to adolescent suicidality: a review of the literature. Arch. Suicide Res. 12(3):181–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, O’Mara RM, Hayward CN, Cunningham RM. 2009. Adolescent suicide risk screening in the emergency department. Acad. Emerg. Med 16(11):1234–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky ED, May AM. 2015. The three-step theory (3ST): a new theory of suicide rooted in the “ideationto-action” framework. Int. J. Cogn. Ther 8(2):114–29 [Google Scholar]
- Kõlves K, de Leo D. 2017. Suicide methods in children and adolescents. Eur. Child Adolesc. Psychiatry 26(2):155–64 [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Campbell BA. 1994. Paradoxical autonomic responses to aversive stimuli in the developing rat. Behav. Neurosci 108(5):962–71 [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Fernström J, Grudet C, Ljunggren L, Träskman-Bendz L, et al. 2016. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl. Psychiatry 6(12):e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, et al. 2009. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry 66(3):287–92 [DOI] [PubMed] [Google Scholar]
- Linehan MM. 1993. Cognitive–Behavioral Treatment of Borderline Personality Disorder. New York: Guilford Press [Google Scholar]
- Liu RT, Trout ZM, Hernandez EM, Cheek SM, Gerlus N. 2017. A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: meta-analysis and recommendations for future research. Neurosci. Biobehav. Rev 83:440–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RX. 2006. Vulnerability to friends’ suicide influence: the moderating effects of gender and adolescent depression. J. Youth Adolesc. 35(3):454 [Google Scholar]
- Lutz P-E, Mechawar N, Turecki G. 2017. Neuropathology of suicide: recent findings and future directions. Mol. Psychiatry 22(10):1395–1412 [DOI] [PubMed] [Google Scholar]
- Mann JJ, Currier D. 2007. A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Arch. Suicide Res. 11(1):3–16 [DOI] [PubMed] [Google Scholar]
- Massing-Schaffer M, Helms SW, Rudolph KD, Slavich GM, Hastings PD, et al. 2018. Preliminary associations among relational victimization, targeted rejection, and suicidality in adolescents: a prospective study. J. Clin. Child Adolesc. Psychol In press. 10.1080/15374416.2018.1469093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley E, Berk MS, Asarnow JR, Adrian M, Cohen J, et al. 2018. Efficacy of dialectical behavior therapy for adolescents at high risk for suicide: a randomized clinical trial. JAMA Psychiatry 75(8):777–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev 87(3):873–904 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. 2010. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci 1186(1):190–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem NM, Keilp JG, Porta G, Oquendo MA, Burke A, et al. 2016. Blunted HPA axis activity in suicide attempters compared to those at high risk for suicidal behavior. Neuropsychopharmacology 41(6):1447–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhem NM, Munroe S, Marsland A, Gray K, Brent D, et al. 2017. Blunted HPA axis activity prior to suicide attempt and increased inflammation in attempters. Psychoneuroendocrinology 77:284–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger KA. 1933. Psychoanalytic aspects of suicide. Int. J. Psychoanal 14:376–390 [Google Scholar]
- Miller AB, Eisenlohr-Moul T, Giletta M, Hastings PD, Rudolph KD, et al. 2017a. A within-person approach to risk for suicidal ideation and suicidal behavior: Examining the roles of depression, stress, and abuse exposure. J. Consult. Clin. Psychol 85(7):712–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA. 2018. Neural correlates of emotion regulation and adolescent suicidal ideation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3(2):125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Song J-H, Sturza J, Lumeng JC, Rosenblum K, et al. 2017b. Child cortisol moderates the association between family routines and emotion regulation in low-income children. Dev. Psychobiol 59(1):99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Lesh TA, Niendam TA, Yoon JH, Cheng Y, et al. 2015. Control-related frontal–striatal function is associated with past suicidal ideation and behavior in patients with recent-onset psychotic major mood disorders. J. Affect. Disord 188:202–9 [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Forouzanfar MH, Daoud F, Mokdad AA, Bcheraoui CE, et al. 2016. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 387(10036):2383–2401 [DOI] [PubMed] [Google Scholar]
- Nederhof E, Marceau K, Shirtcliff EA, Hastings PD, Oldehinkel AJ. 2015. Autonomic and adrenocortical interactions predict mental health in late adolescence: The TRAILS Study. J. Abnorm. Child Psychol. 43(5):847–61 [DOI] [PubMed] [Google Scholar]
- Neis VB, Manosso LM, Moretti M, Freitas AE, Daufenbach J, Rodrigues ALS. 2014. Depressive-like behavior induced by tumor necrosis factor-α is abolished by agmatine administration. Behav. Brain Res. 261:336–44 [DOI] [PubMed] [Google Scholar]
- Nesi J, Choukas-Bradley S, Prinstein MJ. 2018. Transformation of adolescent peer relations in the social media context: part 1—a theoretical framework and application to dyadic peer relationships. Clin. Child Fam. Psychol. Rev 21(3):267–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuringer C 1964. Rigid thinking in suicidal individuals. J. Consult. Psychol 28(1):54–58 [DOI] [PubMed] [Google Scholar]
- Nock MK, Borges G, Bromet EJ, Cha CB, Kessler RC, Lee S. 2008. Suicide and suicidal behavior. Epidemiol. Rev 30(1):133–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, et al. 2013. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry 70(3):300–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR. 2010. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychol. Sci 21(4):511–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll PW, Berman AL, Maris RW, Moscicki EK, Tanney BL, Silverman MM. 1996. Beyond the Tower of Babel: a nomenclature for suicidology. Suicide Life Threat. Behav 26(3):237–252 [PubMed] [Google Scholar]
- O’Connor DB, Green JA, Ferguson E, O’Carroll RE, O’Connor RC. 2017. Cortisol reactivity and suicidal behavior: investigating the role of hypothalamic–pituitary–adrenal axis responses to stress in suicide attempters and ideators. Psychoneuroendocrinology 75:183–91 [DOI] [PubMed] [Google Scholar]
- O’Connor RC. 2011. Towards an integrated motivational–volitional model of suicidal behaviour International Handbook of Suicide Prevention: Research, Policy and Practice, ed. O’Connor RC, Platt S, Gordon J, pp. 181–98. Oxford: Wiley [Google Scholar]
- Orbach I 1997. A taxonomy of factors related to suicidal behavior. Clin. Psychol. Sci. Pract 4(3):208–24 [Google Scholar]
- Ordaz SJ, Goyer MS, Ho TC, Singh MK, Gotlib IH. 2018. Network basis of suicidal ideation in depressed adolescents. J. Affect. Disord 226:92–99 [DOI] [PubMed] [Google Scholar]
- Pan LA, Hassel S, Segreti AM, Nau SA, Brent DA, Phillips ML. 2013. Differential patterns of activity and functional connectivity in emotion processing neural circuitry to angry and happy faces in adolescents with and without suicide attempt. Psychol. Med 43(10):2129–42 [DOI] [PubMed] [Google Scholar]
- Pan LA, Martin P, Zimmer T, Segreti AM, Kassiff S, et al. 2016. Neurometabolic disorders: potentially treatable abnormalities in patients with treatment-refractory depression and suicidal behavior. Am. J. Psychiatry 174(1):42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan LA, McKain BW, Madan-Khetarpal S, Mcguire M, Diler RS, et al. 2011. GTP-cyclohydrolase deficiency responsive to sapropterin and 5-HTP supplementation: relief of treatment-refractory depression and suicidal behaviour. BMJ Case Rep. 10.1136/bcr.03.2011.3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, et al. 2012. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J. Psychiatr. Res 46(1):57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst JT, Hopmeyer A. 1998. Sociometric popularity and peer-perceived popularity: two distinct dimensions of peer status. J. Early Adolesc. 18(2):125–44 [Google Scholar]
- Phillips DP, Carstensen LL. 1986. Clustering of teenage suicides after television news stories about suicide. N. Engl. J. Med 315(11):685–89 [DOI] [PubMed] [Google Scholar]
- Picard M, Juster R-P, McEwen BS. 2014. Mitochondrial allostatic load puts the “gluc” back in glucocorticoids. Nat. Rev. Endocrinol 10(5):303–10 [DOI] [PubMed] [Google Scholar]
- Pirkis J, Blood RW, Beautrais A, Burgess P, Skehan J. 2006. Media guidelines on the reporting of suicide. Crisis 27(2):82–87 [DOI] [PubMed] [Google Scholar]
- Porges SW. 2003. Social engagement and attachment: a phylogenetic perspective. Ann. N. Y. Acad. Sci 1008:31–47 [DOI] [PubMed] [Google Scholar]
- Porges SW. 2007. The polyvagal perspective. Biol. Psychol 74(2):116–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ. 2008. Introduction to the special section on suicide and nonsuicidal self-injury: A review of unique challenges and important directions for self-injury science. J. Consult. Clin. Psychol 76(1):1–8 [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Giletta M. 2016. Peer relations and developmental psychopathology In Developmental Psychopathology, ed. Cicchetti D, pp. 527–79. Oxford: Wiley [Google Scholar]
- Prinstein MJ, Heilbron N, Guerry JD, Franklin JC, Rancourt D, et al. 2010. Peer influence and nonsuicidal self injury: longitudinal results in community and clinically-referred adolescent samples. J. Abnorm. Child Psychol. 38(5):669–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinstein MJ, Rancourt D, Aldeman CB, Ahlich E, Smith J, Guerry JD. 2018. Peer status and psychopathology In Handbook of Peer Interactions, Relationships, and Groups, ed. Bukowski W, Laursen B, Rubin KH, pp. 617–36. New York: Guilford Press; 2nd ed. [Google Scholar]
- Quevedo K, Ng R, Scott H, Martin J, Smyda G, et al. 2016. The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. J. Abnorm. Psychol 125(8):1185–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J, Morilak D, Viau V, Campeau S. 2015. Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci. Biobehav. Rev 58:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 27(1):24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rew L, Thomas N, Horner SD, Resnick MD, Beuhring T. 2001. Correlates of recent suicide attempts in a triethnic group of adolescents. J. Nurs. Scholarsh 33(4):361–67 [DOI] [PubMed] [Google Scholar]
- Ribeiro JD, Franklin JC, Fox KR, Bentley KH, Kleiman EM, et al. 2016. Self-injurious thoughts and behaviors as risk factors for future suicide ideation, attempts, and death: a meta-analysis of longitudinal studies. Psychol. Med 46(2):225–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Out D, Dorn LD, Beal SJ, Denson LA, et al. 2014. Salivary cytokines in healthy adolescent girls: intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Dev. Psychobiol 56(4):797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. 2017. The metamorphosis of adolescent hormonal stress reactivity: a focus on animal models. Front. Neuroendocrinol 49:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. 2006. A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychol. Bull 132(1):98–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Wilhelm FH, Gross JJ, Gotlib IH. 2002. Respiratory sinus arrhythmia as a predictor of outcome in major depressive disorder. J. Affect. Disord 71(1–3):265–72 [DOI] [PubMed] [Google Scholar]
- Rudd D 2010. The suicidal mode: a cognitive-behavioral model of suicidality. Suicide Life Threat. Behav 30(1):18–33 [PubMed] [Google Scholar]
- Rudolph KD. 2014. Puberty as a developmental context of risk for psychopathology In Handbook of Developmental Psychopathology, ed. Lewis M, Rudolph KD, pp. 331–54. Boston, MA: Springer [Google Scholar]
- Serafini G, Pompili M, Seretti ME, Stefani H, Palermo M, et al. 2013. The role of inflammatory cytokines in suicidal behavior: a systematic review. Eur. Neuropsychopharmacol 23(12):1672–86 [DOI] [PubMed] [Google Scholar]
- Shneidman ES. 1993. Suicide as Psychache: A Clinical Approach to Self-Destructive Behavior. Latham, MD: Jason Aronson [Google Scholar]
- Silverman MM, Berman AL, Sanddal ND, O’Carroll PW, Joiner TE Jr. 2007. Rebuilding the tower of Babel: a revised nomenclature for the study of suicide and suicidal behaviors. Part 1: Background, rationale, and methodology. Suicide Life Threat. Behav 37(3):248–263 [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. 2012. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion 12(6):1235–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. 2013. The emerging field of human social genomics. Clin. Psychol. Sci 1(3):331–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. 2014. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull 140(3):774–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, O’Donovan A, Epel ES, Kemeny ME. 2010. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci. Biobehav. Rev 35(1):39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Tartter MA, Brennan PA, Hammen C. 2014. Endogenous opioid system influences depressive reactions to socially painful targeted rejection life events. Psychoneuroendocrinology 49:141–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH. 2013. The teenage brain sensitivity to social evaluation. Curr. Dir. Psychol. Sci 22(2):121–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. 2011. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci 23(9):2123–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. 2013. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci 24(8):1554–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirito A, Esposito-Smythers C, Weismoore J, Miller A. 2012. Adolescent suicidal behavior In Child and Adolescent Therapy: Cognitive-Behavioral Procedures, ed. Kendall PC, pp. 234–56. New York: Guilford Press; 4th ed. [Google Scholar]
- Steinberg L, Morris AS. 2001. Adolescent development. Annu. Rev. Psychol 52:83–110 [DOI] [PubMed] [Google Scholar]
- Sternberg EM. 2006. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol 6(4):318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, et al. 2009. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev. Psychopathol 21(1):47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, D’Angelo CM, Brush B, Lloyd-Richardson EE. 2017. Sex differences in biological response to peer rejection and performance challenge across development: a pilot study. Physiol. Behav 169:224–33 [DOI] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, et al. 2011. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav. Immun 25(6):1272–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman E, Dorn L. 2009. Puberty: its role in development In Handbook of Adolescent Psychology, ed. Lerner RM, Steinberg L, pp. 116–51. Oxford: Wiley [Google Scholar]
- Tasker JG, Herman JP. 2011. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic–pituitary–adrenal axis. Stress 14(4):398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Galván A. 2016. Stress and the adolescent brain: amygdala–prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav. Rev 70:217–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, et al. 2010. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci 13(1):46–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsypes A, James KM, Woody ML, Feurer C, Kudinova AY, Gibb BE. 2018. Resting respiratory sinus arrhythmia in suicide attempters. Psychophysiology 55(2):e12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G 2014. The molecular bases of the suicidal brain. Nat. Rev. Neurosci 15(12):802–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, Brent DA. 2016. Suicide and suicidal behaviour. Lancet 387(10024):1227–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden KA, Witte TK, Cukrowicz KC, Braithwaite SR, Selby EA, Joiner TE Jr. 2010. The interpersonal theory of suicide. Psychol. Rev 117(2):575–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgus MD, Aldrich JT, Mezulis AH, Crowell SE. 2016. Respiratory sinus arrhythmia as a predictor of self-injurious thoughts and behaviors among adolescents. Int. J. Psychophysiol 106:127–34 [DOI] [PubMed] [Google Scholar]
- Yoder KA. 1999. Comparing suicide attempters, suicide ideators, and nonsuicidal homeless and runaway adolescents. Suicide Life Threat. Behav 29(1):25–36 [PubMed] [Google Scholar]
- You J, Lin MP, Fu K, Leung F. 2013. The best friend and friendship group influence on adolescent nonsuicidal self-injury. J. Abnorm. Child Psychol. 41(6):993–1004 [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen Z, Jia Z, Gong Q. 2014. Dysfunction of neural circuitry in depressive patients with suicidal behaviors: a review of structural and functional neuroimaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 53:61–66 [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen J-M, Kuang L, Cao J, Zhang H, et al. 2016. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry 16(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]