Abstract
Flat epithelial atypia (FEA) is an alteration of terminal duct-lobular units by a proliferation of ductal epithelium with low grade atypia. No consensus exists on whether the diagnosis of FEA in core needle biopsy (CNB) requires excision (EXC). We retrospectively identified all in-house CNBs obtained between 1/2012-7/2018 with FEA. We reviewed all CNB slides and assessed radiologic-pathologic concordance. An upgrade was defined as invasive carcinoma (IC) and/or ductal carcinoma in situ in the EXC. The EXC slides of all upgraded cases were re-reviewed. Out of ~15,700 consecutive CNBs in the study period, 106 CNBs from 106 patients yielded FEA alone or with classic lobular neoplasia (LN). We excluded 52 CNBs (40 patients with prior/concurrent carcinoma and 12 without EXC). After re-review, we reclassified 14 cases (2 marked nuclear atypia, 10 focal ADH, 2 benign). The final FEA study cohort consisted of 40 CNBs from 40 women. The CNB targeted mammographic calcifications in 36 (90%) cases, MRI non-mass enhancement in 3 (8%), and 1 (2%) sonographic mass. All CNBs were deemed radiologic-pathologic concordant. FEA was present alone in 34 CNBs and with LN in 6. EXC yielded 2 low grade IC, each spanning less than 2 mm, identified in tissue sections without biopsy site changes. The remaining 38 cases had no upgrade. Classic LN did not affect the upgrade. The upgrade rate of FEA was 5%; both minute, low-grade “incidental” IC. We conclude that non-surgical management may be considered in patients without prior/concurrent carcinoma and radiologic-pathologic concordant CNB diagnosis of FEA.
Keywords: invasive carcinoma, ductal carcinoma in situ, columnar cell change, lobular carcinoma in situ, atypical ductal hyperplasia, upgrade
Introduction
The widespread implementation of screening mammography has had many consequences, including an increase in the identification of flat epithelial atypia (FEA) in core needle biopsy (CNB) specimens from 1.3% of breast CNBs before 1981 to 3% thereafter.1 Nonetheless, the finding of FEA as the only atypical lesion in CNB remains rare overall, with the reported incidence ranging from 1% to 8% of all CNBs.2,3
FEA is defined by the World Health Organization (WHO)4 as a flat proliferation composed of one to several layers of cells which lack polarity and display low grade cytologic atypia. The cells range from cuboidal to columnar in shape, with round, monomorphic, hyperchromatic and uniform nuclei without prominent nucleoli, similar in appearance to the nuclei of low grade ductal carcinoma in situ (DCIS). The cells tend to have abundant pale and eosinophilic cytoplasm; apical cytoplasmic snouts or blebs may protrude into the lumen. The terminal duct lobular units (TDLUs) involved by FEA are enlarged, have dilated acini with smooth contours and often contain inspissated and calcified secretions (Figure 1). Along with low grade atypia, the diagnosis of FEA requires the absence of any architectural complexity, such as focal trabeculae, Roman arches or micropapillae. When these complex patterns are present the diagnosis of atypical ductal hyperplasia (ADH) should be used instead.4
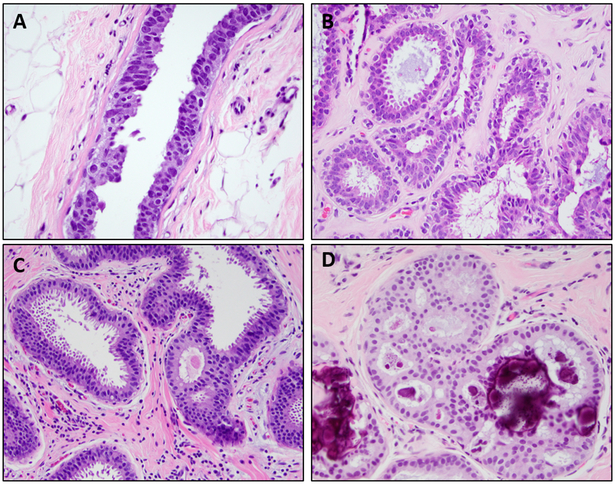
Figure 1: Examples of histologic features of flat epithelial atypia.
A: Dilated terminal duct lobular units with secretions and calcifications (H&E, 100X); B: Ductal cells with low grade atypia which lack polarity (H&E, 400X); C: Tall apical cytoplasmic snouts (H&E, 400X)
The rate of upgrade at excision of FEA varies widely in different series, ranging from 0% to 36% (Table 1).3,5 However, caution is warranted when interpreting the reported upgrade rates as their applicability is contingent on radiologic-pathologic concordance of the CNB findings and relies on the reproducibility of FEA as a diagnostic entity. Samples et al6 assessed interobserver agreement in the diagnosis of six reference cases of FEA, each reviewed independently by 29 to 30 pathologists. Agreement with the reference diagnosis of FEA ranged from 17% to 52%. The authors concluded that there is substantial and somewhat concerning variability among pathologists in the diagnosis of FEA. These results document challenges in the application of the diagnostic criteria of FEA into clinical practice and patient management.
Table 1:
Upgrade rate of FEA at surgical excision in selected series with pathology review
| Study | CNBs with FEA and follow-up EXC |
Rad-Path correlation |
Total Upgrades (%) |
Upgrades to Invasive Carcinoma |
Upgrades to DCIS |
Recommendations |
|---|---|---|---|---|---|---|
| Martel (2007)3 | 19 | No | 7/19 (36%) | 7 | 0 | EXC is not mandatory; close imaging follow up is advised |
| Kunju and Kleer (2007)32 | 12 | No | 3/12 (25%) | 2 | 1 | EXC is warranted |
| Piubello (2009)12 | 20 | Yes | 0/20 (0%) | 0 | 0 | EXC is not mandatory; close imaging follow up is advised |
| Lavoué (2011)33 | 60 | Yes | 8/60 (13%) | 2 | 6 | EXC is warranted |
| Uzoaru (2012)17 | 95 | No | 3/95 (3%) | 2 | 1 | EXC is not mandatory; close imaging follow up is advised |
| Dialani (2015)34 | 29 | Yes | 2/29 (6.9%) | 0 | 2† | EXC is warranted ONLY if the target lesion is not entirely removed on CNB |
| Calhoun (2015)35 | 94 | Yes | 5/94 (7%) | 2§ | 3 | EXC is not mandatory if target lesion entirely removed by CNB |
| McCroskey (2018)26 | 43 | Yes | 1/43 (2%) | 1§ | 0 | EXC is not mandatory |
| Ouldamer (2018)25 | 20 | Yes | 3/20 (15%) | 1 | 2 | EXC is warranted ONLY if target lesion is Ca2+ spanning >10 mm, with > 4 foci of FEA on CNB in patients > age 57 years |
| Hugar (2019)19 | 111* | Yes | 1/111 (1%) | 1§ | 0 | EXC is not mandatory; close imaging follow up is advised |
| Current study | 40‡ | Yes | 2/40 (5%) | 2§ | 0 | EXC is not mandatory if no personal history of breast carcinoma; close imaging follow up is advised |
| Totals | 543 | as above | 35/543 (6%) | 20/543 (3%) | 15/543 (3%) | as above |
Upgrades consisted of one ductal carcinoma in situ (DCIS) and one pleomorphic lobular carcinoma in situ (LCIS)
Excluding CNBs done for indications other than calcifications
Excluding patients with prior and/or concurrent invasive carcinoma and/or DCIS
Invasive carcinoma deemed incidental finding
CNB – core needle biopsy, EXC – excision, NA – not available, Ca2+ - calcifications
The 2012 WHO consensus group acknowledged the wide variation in the reported upgrade rate at excision of FEA and the limitations in the available data, and emphasized the importance of radiologic-pathologic correlation for determining further patient management, but left the decision for follow up surgical excision somewhat open-ended.4 In practice, given the uncertainties regarding upgrade rates and the observed difficulty in reproducibility of the diagnosis, most patients with diagnosis of FEA at CNB currently undergo follow-up surgical excision, regardless of radiologic-pathologic concordance. Our study aimed to evaluate the upgrade rate at excision of FEA in radiologic-pathologic concordant breast CNBs and assess the severity of the upgrades to determine whether in the context of radiologic-pathologic concordance, surgical excision might be safely spared in a subset of patients.
Materials and Methods
After obtaining institutional review board approval, we identified all consecutive in-house breast CNBs with the diagnosis of FEA obtained between January 2012 and July 2018 through a retrospective search of our pathology department database (Figure 2). FEA was defined according to the WHO 2012 criteria, as a flat proliferation of cells with low grade cytologic atypia involving variably dilated TDLUs without any evidence of architectural atypia.4 However, while the WHO 2012 criteria require “one or more layers” of atypical cells, we further restricted the diagnosis of FEA to cases with at least two layers of atypical ductal cells. We introduced these more stringent diagnostic criteria in our practice a few years ago with the aim to increase interobserver reproducibility.
Figure 2: Summary of study design and results.
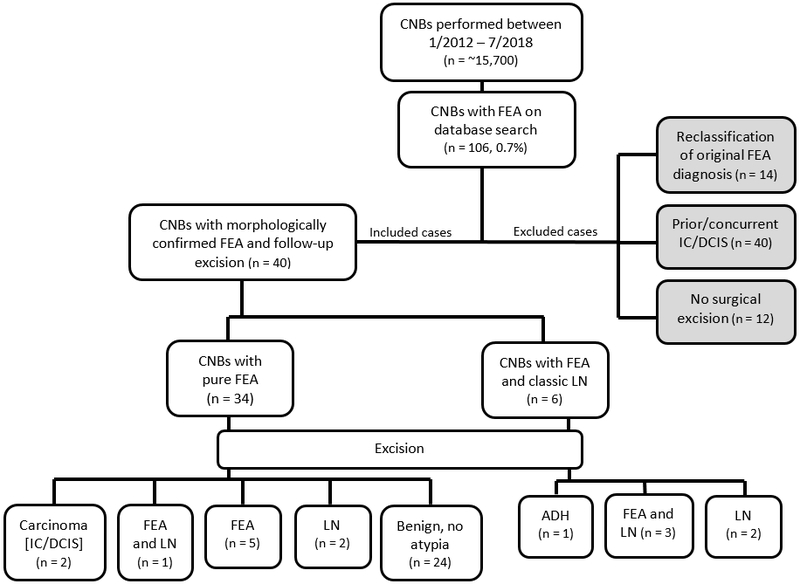
CNB – core needle biopsy, FEA – flat epithelial atypia, IC – invasive carcinoma, DCIS – ductal carcinoma in situ, LN – lobular neoplasia, ADH – atypical ductal hyperplasia
Clinicopathologic information was obtained from pathology reports. Only patients with no prior or concurrent ipsilateral and/or contralateral invasive carcinoma or DCIS were included in the study. CNBs with any concurrent higher risk lesion (i.e. ADH) which by itself would mandate excision were excluded. The presence of classic LN, namely ALH and classic LCIS in the CNB was not an exclusion criteria, as the finding of classic LN in a radiologic-pathologic concordant CNB does not mandate excision at our Center, based on the results of a previous study.7
We included in our series only cases with available follow up excision in women without a personal history of breast carcinoma. Fifty-two CNBs with FEA were excluded from the study: 40 CNBs were from patients with prior and/or concurrent invasive carcinoma and/or DCIS and 12 CNBs had no available follow up surgical excision for various reasons (5 patients opted for conservative management, 2 patients received treatment for a non-mammary malignancy, 3 patients relocated and/or transferred their medical care elsewhere and 2 patients were lost to follow up).
All CNB hematoxylin and eosin stained (H&E) slides were reviewed by two breast pathologists (AG and EB). Upon re-review, we further excluded all lesions that did not fulfill the strict diagnostic criteria of FEA described above. Atypical lesions that were flat throughout but showed even a single intraluminal structure such as trabecular bar, Roman bridge or an atypical micropapillary projection were reclassified as focal ADH and excluded from the study. We assessed the number and size of the foci of FEA in each CNB specimen. As defined by Ely et al8 the number of foci corresponded to the number of TDLUs with FEA. The microscopic span of the largest focus of FEA in each case was measured and recorded. An upgrade was defined as invasive carcinoma and/or DCIS in the surgical excision specimen. All H&E slides from the excision specimens of cases with an upgrade were reviewed. In cases without upgrade, we recorded the histologic findings in the excision specimen, including the presence of ADH. The presence of the biopsy site and its relation to the lesion with upgrade were assessed in all cases.
A dedicated breast radiologist (SB) determined adequacy of tissue sampling by review of the pertinent imaging findings obtained before, at the time of, and after the CNB yielding FEA. Information regarding imaging modality, type and diameter of the imaging target, gauge of biopsy needle, number of tissue cores obtained, and whether the target lesion was entirely removed by the CNB was assessed and recorded. For CNB targeting calcifications, the distribution and type of calcifications were noted. Radiologic-pathologic concordance was assessed for all cases.
Results
Among approximately 15,700 consecutive in-house CNBs performed during the study period, 106 (0.7%) had the diagnosis FEA in the absence of a higher risk lesion mandating surgical excision. Fifty-four CNBs met the study inclusion criteria and the slides were re-reviewed by the two study pathologists. The diagnosis of FEA was confirmed in 40 (74%) CNBs. In 14 (26%) CNBs the highest risk lesion present did not fulfill the diagnostic criteria of FEA and the lesions were reclassified.
FEA study cohort: 40 core needle biopsy specimens with confirmed diagnosis of FEA
The final FEA study cohort consisted of 40 CNBs from 40 women with a median age of 52 years (range 35-73) and no personal history of breast carcinoma. FEA was present alone in 34 (85%) CNBs and with classic LN in 6 (15%) cases (5 ALH and 1 LCIS). The median number of FEA foci per case was 2 (range 1-4). The median size of the largest focus of FEA was 1.58 mm (range 0.5-4.5).
There were two upgrades at surgical excision; both were invasive carcinoma (5% upgrade rate) (Table 2). The CNB of one of the two cases with upgrade targeted a 15 mm area of coarse heterogenous calcifications in the breast of a 73-year-old woman. The CNB material contained two foci of FEA, with the largest focus spanning 3 mm. Follow-up surgical excision yielded a 2.2 mm focus of invasive ductal carcinoma (IDC) grade II/III with lobular growth pattern. A minute focus of low-grade DCIS, solid type, was also present with the IDC (Figure 3). Residual foci of FEA with associated calcifications were present in the tissue section with biopsy site changes, but the invasive carcinoma did not harbor calcifications and was present in a tissue section that did not contain biopsy site changes or FEA.
Table 2:
Clinicopathologic features of upgraded cases
| Case | Age | Mammographic calcifications |
CNB Findings | EXC Findings |
|---|---|---|---|---|
| 1 | 73 | 15 mm coarse heterogenous | 2 FEA foci largest 3 mm | IDC, grade II/III 2.2 mm |
| 2 | 46 | 22 mm amorphous | 1 FEA focus 3.7 mm | Tubular carcinoma 2.0 mm and 1.0 mm |
CNB – core needle biopsy, EXC – excision, IDC – invasive ductal carcinoma
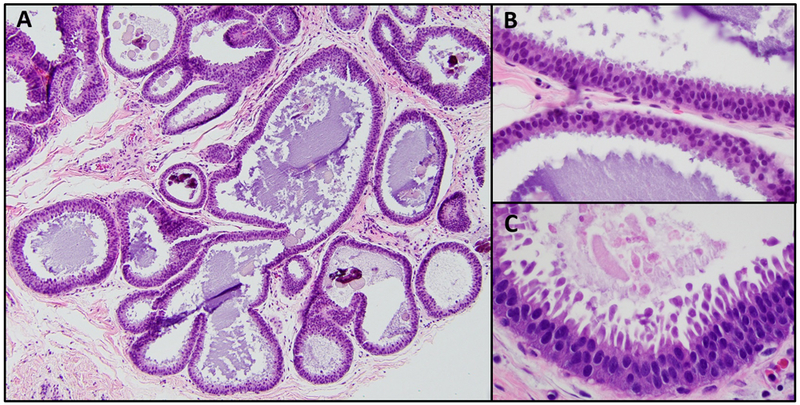
Figure 3: Example of case with upgrade at excision.
A: Core needle biopsy with flat epithelial atypia (FEA) and associated calcifications (H&E, 40X); inset: higher power showing duct involved by FEA (H&E, 200X); B: Tissue slice from excision specimen with biopsy site changes (upper right) and residual FEA (lower center) (H&E, 20X); C: Tissue slice from excision specimen with no biopsy site changes and focus of invasive carcinoma, 2.2 mm (H&E, 20X); D: Higher power of focus of invasive ductal carcinoma with lobular growth pattern and focal DCIS (H&E, 100X)
The other CNB with upgrade at excision targeted a 22 mm area of amorphous calcifications in the breast of a 46-year-old woman. The CNB material contained one focus of FEA, spanning 3.7 mm. Surgical excision yielded two foci of tubular carcinoma measuring 2.0 mm and 1.0 mm microscopically. The tubular carcinomas did not harbor calcifications and were identified in two separate tissue sections, neither of which contained biopsy site changes. A focus of FEA was present near the 2 mm focus of tubular carcinoma.
Surgical excision of the remaining 38 cases of FEA yielded no upgrade to carcinoma. One case yielded a single focus of ADH. The corresponding CNB targeted a 10 mm area of punctate calcifications and sampled two foci of FEA, with the largest focus spanning 2.5 mm in greatest dimension, and ALH. No residual FEA or ALH was identified in the excision specimen. The remaining cases without upgrade yielded FEA in 9 cases, and no residual atypia in 24. Four excisions contained classic LN without FEA; the corresponding CNBs contained FEA and classic LN in 2 of these cases and FEA only in 2 (Table 3).
Table 3:
Radiographic features of breast core needle biopsies
| Case | Age (years) |
Type of target lesion | Target lesion diameter (mm) |
Target removed by CNB |
Needle gauge |
Number of specimens |
Excision Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 73 | Coarse heterogenous Ca2+ | 15 | N | 11 | 8 | IDC, DCIS, ALH, FEA |
| 2 | 46 | Amorphous Ca2+ | 22 | N | 9 | 4 | Tubular carcinoma, FEA |
| 3 | 50 | Pleomorphic Ca2+ | 4 | N | 9 | 8 | Benign |
| 4 | 49 | Amorphous Ca2+ | 5 | N | 9 | 9 | Benign |
| 5 | 47 | Pleomorphic Ca2+ | 5 | N | 9 | 10 | Benign |
| 6 | 57 | Amorphous Ca2+ | 5 | N | 9 | 6 | Benign |
| 7 | 45 | Coarse heterogenous Ca2+ | 7 | N | 9 | 6 | FEA |
| 8 | 51 | Pleomorphic Ca2+ | 7 | N | 9 | 8 | Benign |
| 9 | 62 | Amorphous Ca2+ | 7 | N | 9 | 9 | FEA, ALH† |
| 10 | 52 | Pleomorphic Ca2+ | 8 | N | 8 | 8 | Benign |
| 11 | 50 | Amorphous Ca2+ | 8 | N | 9 | 9 | Benign |
| 12 | 46 | Amorphous Ca2+ | 8 | N | 9 | 10 | ADH† |
| 13 | 52 | Amorphous Ca2+ | 9 | N | 9 | 7 | FEA |
| 14 | 42 | Coarse heterogenous Ca2+ | 10 | N | 9 | 10 | ALH† |
| 15 | 42 | Pleomorphic Ca2+ | 10 | N | 9 | 7 | Benign |
| 16 | 51 | Amorphous Ca2+ | 12 | N | 9 | 8 | Benign |
| 17 | 71 | Pleomorphic Ca2+ | 12 | N | 9 | 7 | Benign |
| 18 | 47 | Coarse heterogenous Ca2+ | 13 | N | 9 | 6 | FEA, ALH‡ |
| 19 | 64 | Punctate Ca2+ | 2 | Y | 9 | 8 | Benign |
| 20 | 44 | Punctate Ca2+ | 2 | Y | 9 | 8 | LCIS‡ |
| 21 | 45 | Coarse heterogenous Ca2+ | 3 | Y | 9 | 6 | FEA, LCIS§ |
| 22 | 56 | Coarse heterogenous Ca2+ | 3 | Y | 9 | 7 | Benign |
| 23 | 54 | Amorphous Ca2+ | 3 | Y | 9 | 5 | FEA, ALH† |
| 24 | 44 | Pleomorphic Ca2+ | 3 | Y | 9 | 10 | FEA |
| 25 | 44 | Pleomorphic Ca2+ | 3 | Y | 9 | 7 | Benign |
| 26 | 49 | Pleomorphic Ca2+ | 3 | Y | 9 | 7 | FEA |
| 27 | 73 | Punctate Ca2+ | 3 | Y | 9 | 6 | ALH† |
| 28 | 41 | Amorphous Ca2+ | 3 | Y | 9 | 12 | Benign |
| 29 | 47 | Amorphous Ca2+ | 4 | Y | 9 | 9 | Benign |
| 30 | 59 | Amorphous Ca2+ | 4 | Y | 9 | 4 | ALH‡ |
| 31 | 70 | Punctate Ca2+ | 5 | Y | 9 | 9 | Benign |
| 32 | 65 | Coarse heterogenous Ca2+ | 5 | Y | 9 | 14 | Benign |
| 33 | 49 | Pleomorphic Ca2+ | 7 | Y | 9 | 8 | Benign |
| 34 | 52 | Pleomorphic Ca2+ | 8 | Y | 9 | 8 | Benign |
| 35 | 46 | Pleomorphic Ca2+ | 9 | Y | 9 | 7 | FEA |
| 36 | 50 | Punctate Ca2+ | 10 | Y | 9 | 8 | Benign |
| 37 | 35 | Non-mass enhancement | 6 | N | 9 | 10 | Benign |
| 38 | 53 | Non-mass enhancement | 15 | N | 9 | 7 | Benign |
| 39 | 49 | Non-mass enhancement | 31 | N | 9 | 8 | Benign |
| 40 | 67 | Sonographic mass | 11 | N | 12 | 9 | Benign |
corresponding CNB: FEA and ALH
corresponding CNB: FEA only
corresponding CNB: FEA and LCIS
CNB – core needle biopsy, Y – yes, N – no, IDC – invasive ductal carcinoma, DCIS – ductal carcinoma in situ, ALH – atypical lobular hyperplasia, FEA – flat epithelial atypia; Ca2+ - calcifications
There were no appreciable differences in the histologic characteristics of the CNBs with or without upgrade. The median number of FEA foci in CNBs with upgrade was 2 (range 1-2), comparable to the number of FEA foci in CNBs without upgrade (median number 2; range 1-4). The median size of the largest focus of FEA in CNBs with upgrade was 1.5 mm (3-3.7) and in CNBs without upgrade was 1.5 mm (0.5-4.5).
Imaging studies review
The CNB imaging target consisted of calcifications in 36 (90%) cases, MRI non-mass enhancement in 3 (8%), and a sonographic mass in 1 (2%). The average number of cores removed for each CNB was 8 (range 4-14). A 9-gauge needle was used in 34 (94%) stereotactic CNBs and 8-gauge and 11-gauge needles in two stereotactic CNBs; the MRI-guided biopsies used 9-gauge needles and the US-guided CNB used a 12-gauge needle. The radiographic features of the study cases are summarized in Table 3. The pathologic assessments of all CNBs were deemed to be concordant with the imaging findings in all cases.
In cases targeting calcifications the average lesion diameter was 9.0 mm (range 2-22). Calcifications were amorphous in 12 cases, pleomorphic in 12, coarse and heterogenous in 7 and punctate in 5. The CNB removed the target calcifications in 18 (50%) cases; the target lesion diameter ranged from 2-10 mm. None of these 18 cases yielded an upgrade. Residual calcifications were identified on post-biopsy images in 18 (50%) cases, including the two cases with upgrade. The diameter of the target calcifications ranged from 4-22 mm. Both CNBs with upgrade at excision targeted ≥15 mm of calcifications. In our series, none of the CNB targeting <15 mm of calcifications yielded an upgrade at excision.
In the three cases of non-mass enhancement (NME) the average lesion diameter was 8.7 mm (range 6-31). CNB of the 31 mm NME yielded FEA and pseudoangiomatous stromal hyperplasia (PASH). The CNB targeting a 15 mm NME revealed FEA near changes of a remote procedure. This patient had undergone an ipsilateral CNB one year earlier, which yielded benign findings and was deemed radiologic-pathologic concordant. The sonographic mass measured 11 mm. Dense stromal fibrosis was present in the CNB and excision specimens in this case. Residual target lesion was present in post-biopsy images for all cases targeting NME and the sonographic mass. No upgrades were associated with any of these CNBs.
CNBs reclassified on re-review
In 26% (14/54) of CNBs the lesion originally diagnosed as FEA was reclassified upon re-review (Figure 4). Two CNBs had flat proliferations with marked nuclear atypia, prominent nucleoli, irregular nuclear outline and increased nuclear to cytoplasmic ratio. Excision of both lesions yielded focal DCIS, spanning 3 mm in one case, and present as a single focus arising in the background of ADH in the other.
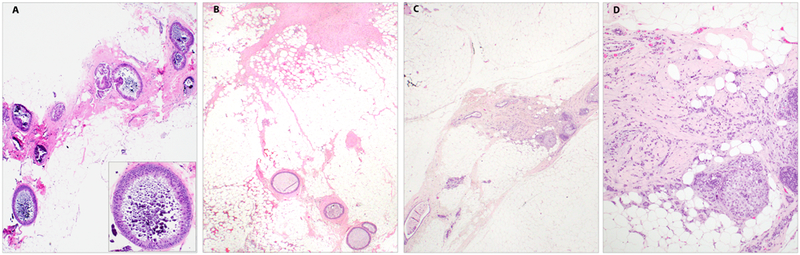
Figure 4: Examples of cases reclassified following morphologic review.
A: Flat proliferation with marked nuclear atypia – flat lesion shows prominent nucleoli, irregular nuclear outlines and increased nuclear to cytoplasmic ratio (H&E, 400X); B: Benign without atypia – columnar cell changes with prominent myoepithelial cell layer (H&E, 200X); C, D: Focal atypical ductal hyperplasia (ADH) – demonstrating a spectrum of architectural complexity ranging from minimal (C, H&E, 200X) to more developed ADH (D, H&E, 400X)
Low-grade epithelial proliferations with focal minimal architectural complexity spanning less than 2 mm microscopically were reclassified as “focal” ADH in 10 CNBs. The subsequent surgical excisions yielded: one IDC, grade II/III (12 mm); one tubular carcinoma (6 mm); one DCIS, low nuclear grade (2 mm); ADH in 6 excisions; and benign breast parenchyma without residual atypia in one excision.
Two CNBs were reclassified as benign breast tissue without atypia. At surgical excision, one case yielded focal ADH and the other benign breast tissue without atypia.
Discussion
The management of FEA as the only atypical lesion found in a radiology pathology concordant needle core biopsy is debated. Most series analyzing the upgrade rate at excision of FEA are relatively small, with only a few studies having over 100 cases.2,9-11 Furthermore, some published series9 did not specify whether radiologic-pathologic concordance of the CNB cases was assessed or did not include dedicated re-review of histologic findings.2,10 A recent systematic review of “16 higher-quality studies” found an estimated risk of upgrade to carcinoma of 7.5% and an estimated risk of upgrade to ADH of 18.6%. Based on these findings the authors concluded that approximately 25% of patients with FEA on CNB would have pathologic findings in the surgical excision specimen which would impact patient management, leading to a recommendation for routine excision.12 Despite the strength of the analysis, these results should be interpreted with caution, as most of the series included in the systematic review were retrospective case series that involved re-review of the pathologic findings but did not include re-review of the imaging studies, and/or did not take into account radiologic-pathologic concordance. In this context, our series provides a more comprehensive and informative assessment, as all CNB slides and all pertinent imaging studies for the cases included in our study were re-reviewed by dedicated pathologists and a radiologist and radiologic-pathologic concordance was assessed for each case.
Using this methodology, we found that only 5% (2/40) of radiologic-pathologic concordant CNBs were upgraded to carcinoma. Both upgrades consisted of minute foci of low-grade invasive carcinoma not associated with the biopsy site and not harboring calcifications, which we deemed to be ‘incidental’ findings. Additionally, one excision yielded a single focus of ADH and three CNBs containing only FEA yielded classic LN at excision, including two cases of ALH and one of classic LCIS.
Lamb et al10 accrued 208 CNBs with pure FEA over a nine-year period and observed an upgrade rate to carcinoma of 2.4% but they reported finding a higher risk lesion in 29.8% of cases (ADH 18.3%, LCIS 4.8%, ALH 6.7%). While Hugar et al11 identified only one upgrade to carcinoma out of 111 CNBs with FEA they observed a higher risk lesion in 36% of cases (ADH 18%, ALH/LCIS 18%). Both studies concluded that the rate of upgrade to carcinoma is low, but surveillance is warranted along with consideration of chemoprevention for risk reduction. Boulos et al13 also reported an increased prevalence of atypical hyperplasia in the presence of FEA, notably this association was also seen with columnar cell lesions without atypia. In a follow up study14, the authors again observed that columnar cell lesions, both with and without atypia, commonly co-occur with atypical hyperplasia but found no association between FEA and subsequent carcinomas, arguing against the role of FEA as a precursor to more significant risk lesions. Overall, they found that the relative risk (RR) of subsequent breast cancer in women with only FEA was approximately 1.5 times that of women without FEA, similar to the RR of usual ductal hyperplasia and lower than the RR of ADH and ALH.4,13,15,16 Likewise, a long-term follow-up study at the Mayo Clinic, reported that women with a diagnosis of pure FEA developed breast carcinoma with a similar incidence to that of women with proliferative changes without atypia and, additionally, when present with established forms of epithelial atypia, FEA did not significantly impact the incidence of carcinoma. Based on these observations the authors concluded that FEA appears to have no impact on breast cancer risk, whether present alone or with atypical hyperplasia.1 In this setting, it seems the implementation of anti-estrogen preventative therapy should be discussed in the context of a woman’s cumulative risk of breast cancer rather than due to a diagnosis of FEA alone.
In their analysis, Lamb et al further identified that having a genetic mutation associated with breast cancer was significantly associated with an upgrade to carcinoma while a personal history of breast cancer was the single significant risk factor associated with an upgrade to a higher risk lesion. Similarly, Berry et al17 observed that women with a personal history of breast carcinoma were significantly more likely to show an upgrade at excision compared to those without such a history, with upgrade rates of 50% and 0%, respectively. We excluded from our series CNBs from patients with prior and/or concurrent ipsilateral and/or contralateral carcinoma, under the assumption that any lesion yielding atypia at CNB in patients with past or current carcinoma is likely to undergo excision. Our strict selection criteria may in part account for the low rate of upgrade to carcinoma and ADH in our series.
No radiologic features are diagnostic of FEA, but usually FEA presents as an area of mammographic calcifications. In most of our cases the calcifications were clustered and amorphous, but all suspicious and/or indeterminate morphologies were observed. The sonographic features of FEA are even more ambiguous, and may resemble those associated with DCIS or ADH, including irregular masses with microlobulated borders.18
Schiaffino et al19 examined the upgrade rate following conservative (non-surgical) management of patients who had a biopsy with FEA which targeted a single group of calcifications, completely removed on biopsy. Only cases with pure FEA without any other atypia and in patients with no prior or concurrent history of carcinoma were included. One of 48 (2%) patients developed new calcifications adjacent to the prior biopsy site 26 months later with the pathology yielding ADH. The authors concluded that surgical excision may be unnecessary when there are no residual calcifications following CNB and close mammographic follow-up is possible. In contrast, Bianchi et al20 report an upgrade rate of 4% in cases where the targeted calcifications were entirely removed at CNB suggesting the absence of residual lesion on post-biopsy images does not necessarily exclude an upgrade to carcinoma in the excision specimen.
In most (86%, 31/36) CNBs in our study cohort targeting calcifications, the target lesion diameter was 10 mm or less and in less than half of those the CNB did not remove all calcifications (Table 3). In contrast, the two CNBs with carcinoma on excision targeted more than 15 mm of calcifications and residual calcifications were identified in post-biopsy x-rays. In both, FEA with associated calcifications was identified in the surgical excision specimens. Indeed, calcifications spanning more than 10 mm were found to be significantly associated with malignancy in one series.21 However, in our data set, the carcinomas themselves did not contain calcifications and were present on tissue sections not containing the CNB site. Our findings suggest that when the biopsy target is a lesion that is >10-15 mm and is not entirely removed at CNB, surgical excision may be considered.
When FEA is present in association with ADH, the latter mandates excision. Piubello et al5 observed no upgrades at excision of pure FEA but the upgrade rate was 30% if ADH was also present. Uzoaru et al9 found that carcinoma was six times more likely to be present in the surgical excision if the CNB contained ADH and FEA as opposed to FEA alone, with upgrade rates of 18.6% and 3%, respectively. However, McCroskey et al22 observed that when only limited ADH, defined as ADH involving less than 3 TDLUs, was associated with FEA the upgrade rate was not influenced. In our study, 10 CNBs originally diagnosed as FEA were reclassified as focal ADH on re-review due to the presence of focal architectural complexity (Figure 4). The upgrade rate of these 10 lesions was 30% (3/10). Although the number of cases is small, this observation highlights the importance of adhering to strict, defined criteria for the diagnosis of FEA: in particular, no architectural complexity should be allowed.
This also raises the question on whether ALH, which carries a similar risk as ADH for subsequent breast cancer, would have an impact on the upgrade rate when present with FEA on CNB. El Khoury et al23 identified an upgrade rate to carcinoma of 2% for pure FEA and 19% for FEA with LN. Additionally, the presence of LN with FEA in the CNB was significantly associated with the identification of ADH in the excision specimen when compared to CNB with FEA only. On the other end, McCroskey et al22 found no association between presence of ALH and upgrade rate to carcinoma in cases of FEA. In our series, the CNBs with upgrade to carcinoma contained FEA only. We observed no upgrades to carcinoma at excision of CNBs containing both FEA and LN.
Recommendation to forego surgical excision when FEA is found on CNB is highly dependent on the accurate interpretation and strict adherence to diagnostic criteria of FEA. Following morphologic review, we reclassified 26% of CNBs with index diagnosis of FEA which documents intrinsic difficulties in recognizing this lesion. Multiple studies have shown variable degrees of reproducibility. One study found that among trainees the agreements on the diagnosis of FEA were significantly improved following a tutorial on the diagnostic criteria24, while another examining practicing pathologists observed moderate to substantial agreement immediately following a tutorial however agreement fell from moderate to fair when participants reviewed the same images one week later, without the tutorial.25 In contrast, two studies found excellent overall agreement in the diagnosis among pathologists with expertise in breast pathology, however in both studies the assessments were done immediately following a tutorial.26,27 These observations suggest that appropriate training tutorials and follow up educational sessions should be conducted to maintain satisfactory consistency in the diagnosis. In our current practice, all CNB in which FEA appears to be the only pathologic finding, with or without associated classic LN, are reviewed at our intradepartmental consensus conference.
Our study has limitations. The sample size is small due to the rarity of pure FEA in CNB (only 0.7% of nearly 15,700 consecutive CNBs in the study period), particularly in women with no personal history of breast carcinoma. The relatively small number of cases precludes statistical analysis to identify factors that may be significantly associated with an upgrade on excision. Nonetheless, our series provides a comprehensive assessment of the imaging and histologic findings, possible pitfalls and risk of upgrade to carcinoma at excision of pure FEA in women without personal history of carcinoma that may be helpful for individual patient management.
In summary, current recommendations for the diagnosis of FEA in CNB are increasingly in favor of imaging surveillance over surgical excision.16 We demonstrate a low upgrade rate of FEA at excision of 5% which consist of minute, low grade invasive carcinomas not associated with the biopsy site. It is possible that these carcinomas would have been detected at follow-up. It is difficult to speculate on the implications of delayed diagnosis on patient management but given the small size and low-grade nature of the carcinomas we hypothesize minimal consequences. Additionally, we identified upgrades at excision only in those CNBs targeting more than a 15 mm span of calcifications suggesting that targets <15 mm which are adequately sampled at CNB can be managed conservatively. Our findings support that, in women without prior or concurrent breast carcinoma, and radiologic-pathologic concordant CNB yielding FEA diagnosed according to strict criteria, non-operative management of FEA may be safely considered.
Acknowledgements
The findings in this study were presented in part at United States and Canadian Academy of Pathology (USCAP) annual conference at National Harbor (MD) in March 2019.
Footnotes
Disclosure/Conflict of Interest
Monica Morrow has received honoraria from Roche and Genomic Health, not relevant to this study. All other authors declare no conflict of interest. All authors have read and approved the manuscript and have contributed sufficiently to the project to be included as authors.
References
- 1.Said SM, Visscher DW, Nassar A, et al. Flat epithelial atypia and risk of breast cancer: A Mayo cohort study. Cancer. 2015;121(10):1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoumais NA, Scaranelo AM, Moshonov H, et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann Surg Oncol. 2013;20(1):133–138. [DOI] [PubMed] [Google Scholar]
- 3.Martel M, Barron-Rodriguez P, Tolgay Ocal I, Dotto J, Tavassoli FA. Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992–1999). Virchows Arch. 2007;451(5):883–891. [DOI] [PubMed] [Google Scholar]
- 4.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ WHO Classification of Tumours of the Breast. IARC: Lyon; 2012. [Google Scholar]
- 5.Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, Iannucci A. Flat epithelial atypia on core needle biopsy: which is the right management? Am J Surg Pathol. 2009;33(7):1078–1084. [DOI] [PubMed] [Google Scholar]
- 6.Samples LS, Rendi MH, Frederick PD, et al. Surgical implications and variability in the use of the flat epithelial atypia diagnosis on breast biopsy specimens. Breast. 2017;34:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray MP, Luedtke C, Liberman L, Nehhozina T, Akram M, Brogi E. Classic lobular carcinoma in situ and atypical lobular hyperplasia at percutaneous breast core biopsy: outcomes of prospective excision. Cancer. 2013;119(5):1073–1079. [DOI] [PubMed] [Google Scholar]
- 8.Ely KA, Carter BA, Jensen RA, Simpson JF, Page DL. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. The American journal of surgical pathology. 2001;25(8):1017–1021. [DOI] [PubMed] [Google Scholar]
- 9.Uzoaru I, Morgan BR, Liu ZG, et al. Flat epithelial atypia with and without atypical ductal hyperplasia: to re-excise or not. Results of a 5-year prospective study. Virchows Arch. 2012;461(4):419–423. [DOI] [PubMed] [Google Scholar]
- 10.Lamb LR, Bahl M, Gadd MA, Lehman CD. Flat Epithelial Atypia: Upgrade Rates and Risk-Stratification Approach to Support Informed Decision Making. J Am Coll Surg. 2017;225(6):696–701. [DOI] [PubMed] [Google Scholar]
- 11.Hugar SB, Bhargava R, Dabbs DJ, Davis KM, Zuley M, Clark BZ. Isolated Flat Epithelial Atypia on Core Biopsy Specimens Is Associated With a Low Risk of Upgrade at Excision. Am J Clin Pathol. 2019;151(5):511–515. [DOI] [PubMed] [Google Scholar]
- 12.Rudin AV, Hoskin TL, Fahy A, et al. Flat Epithelial Atypia on Core Biopsy and Upgrade to Cancer: a Systematic Review and Meta-Analysis. Ann Surg Oncol. 2017;24(12):3549–3558. [DOI] [PubMed] [Google Scholar]
- 13.Boulos FI, Dupont WD, Simpson JF, et al. Histologic associations and long-term cancer risk in columnar cell lesions of the breast: a retrospective cohort and a nested case-control study. Cancer. 2008;113(9):2415–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulos FI, Dupont WD, Schuyler PA, et al. Clinicopathologic characteristics of carcinomas that develop after a biopsy containing columnar cell lesions: evidence against a precursor role. Cancer. 2012;118(9):2372–2377. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32(4):513–523. [DOI] [PubMed] [Google Scholar]
- 16.Rageth CJ, O’Flynn EAM, Pinker K, et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat. 2019;174(2):279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry JS, Trappey AF, Vreeland TJ, et al. Analysis of Clinical and Pathologic Factors of Pure, Flat Epithelial Atypia on Core Needle Biopsy to Aid in the Decision of Excision or Observation. J Cancer. 2016;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solorzano S, Mesurolle B, Omeroglu A, et al. Flat epithelial atypia of the breast: pathological-radiological correlation. AJR Am J Roentgenol. 2011;197(3):740–746. [DOI] [PubMed] [Google Scholar]
- 19.Schiaffino S, Gristina L, Villa A, et al. Flat epithelial atypia: conservative management of patients without residual microcalcifications post-vacuum-assisted breast biopsy. Br J Radiol. 2018;91(1081):20170484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi S, Bendinelli B, Castellano I, et al. Morphological parameters of flat epithelial atypia (FEA) in stereotactic vacuum-assisted needle core biopsies do not predict the presence of malignancy on subsequent surgical excision. Virchows Arch. 2012;461(4):405–417. [DOI] [PubMed] [Google Scholar]
- 21.Ouldamer L, Poisson E, Arbion F, et al. All pure flat atypical atypia lesions of the breast diagnosed using percutaneous vacuum-assisted breast biopsy do not need surgical excision. Breast. 2018;40:4–9. [DOI] [PubMed] [Google Scholar]
- 22.McCroskey Z, Sneige N, Herman CR, et al. Flat epithelial atypia in directional vacuum-assisted biopsy of breast microcalcifications: surgical excision may not be necessary. Mod Pathol. 2018;31(7):1097–1106. [DOI] [PubMed] [Google Scholar]
- 23.El Khoury M, Sanchez LM, Lalonde L, Trop I, David J, Mesurolle B. Is the outcome at surgery different when flat epithelial atypia and lobular neoplasia are found in association at biopsy? Br J Radiol. 2017;90(1072):20160750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haupt B, Schwartz MR, Xu QS, Ro JY. Columnar cell lesions: a consensus study among pathology trainees. Human Pathology. 2010;41(6):895–901. [DOI] [PubMed] [Google Scholar]
- 25.Tan PH, Ho BC, Selvarajan S, Yap WM, Hanby A. Pathological diagnosis of columnar cell lesions of the breast: are there issues of reproducibility? J Clin Pathol. 2005;58(7):705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darvishian F, Singh B, Simsir A, Ye W, Cangiarella JF. Atypia on breast core needle biopsies: reproducibility and significance. Ann Clin Lab Sci. 2009;39(3):270–276. [PubMed] [Google Scholar]
- 27.O’Malley FP, Mohsin SK, Badve S, et al. Interobserver reproducibility in the diagnosis of flat epithelial atypia of the breast. Mod Pathol. 2006;19(2):172–179. [DOI] [PubMed] [Google Scholar]