Abstract
Motivation
Transcription factors (TFs) are key regulators of gene expression, and can activate or repress multiple target genes, forming regulatory units, or regulons. Understanding downstream effects of these regulators includes evaluating how TFs cooperate or compete within regulatory networks. Here we present RTNduals, an R/Bioconductor package that implements a general method for analyzing pairs of regulons.
Results
RTNduals identifies a dual regulon when the number of targets shared between a pair of regulators is statistically significant. The package extends the RTN (Reconstruction of Transcriptional Networks) package, and uses RTN transcriptional networks to identify significant co-regulatory associations between regulons. The Supplementary Information reports two case studies for TFs using the METABRIC and TCGA breast cancer cohorts.
Availability and implementation
RTNduals is written in the R language, and is available from the Bioconductor project at http://bioconductor.org/packages/RTNduals/.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Gene regulation in eukaryotes integrates a large number of interconnected regulatory influences. Some of the major contributors in gene regulation are transcription factors (TFs): proteins that can act as activators or repressors of gene expression, typically by binding to regulatory DNA regions and recruiting the transcriptional apparatus (Yamaguchi et al., 2017). TFs are widely used in methods that reconstruct transcriptional networks, and algorithms that reconstruct such networks consider both positive and negative target associations, consistent with mechanistic studies that have demonstrated the dual-function roles of TFs (Lubelsky and Shaul, 2019). In such a network, each regulator and its target genes form a regulatory unit, or regulon (Margolin et al., 2006). Target genes can belong to multiple regulons, and regulators may co-operate and compete in influencing target gene expression.
In previous studies, we have used regulon activities to identify TFs associated with variant risk loci in breast cancer (Castro et al., 2016), and to characterize differences between molecular subtypes in muscle-invasive bladder cancer (Robertson et al., 2017). Because regulators can co-operate and compete, we anticipated that identifying pairs of regulons that share targets could be informative. Here, we report RTNduals, an R/Bioconductor package that automates the search for co-regulation between regulons, assessing all targets shared by pairs of regulators; when it identifies that a pair has more shared targets than expected by chance, which we assess by overlap and permutation analyses, it defines this pair as a dual regulon.
2 A method for identifying dual regulons
Figure 1a gives an overview of how RTNduals infers dual regulons. The package can take two types of data as input. The first type consists of a gene expression matrix (e.g. a cancer cohort’s transcriptome) and, from prior biological information, a list that indicates which genes should be regarded as regulators. The second consists of a transcriptional regulatory network pre-computed by the RTN package (Castro et al., 2016). The package architecture allows the input of different classes of regulators (e.g. TFs, miRNAs).
Fig. 1.
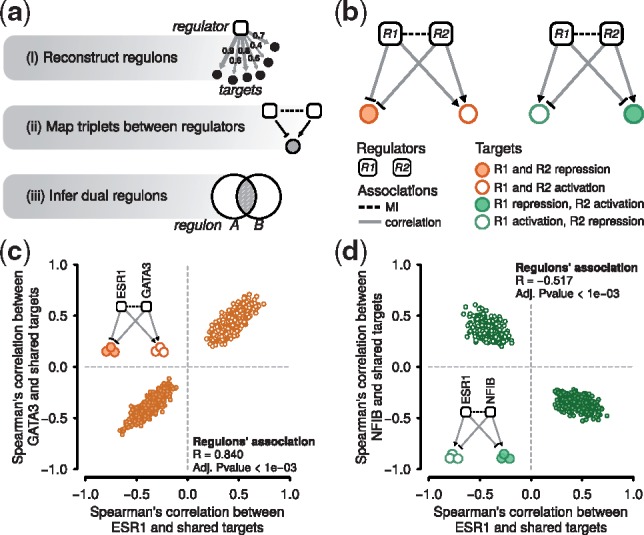
Inference of dual regulons. (a) RTNduals computes dual regulons using: (i) MI between a regulator and targets; (ii) triplets consisting of pairs of regulators and a shared target; (iii) whether the number of shared targets is statistically significant. (b) Examples showing two associated regulators and two regulator-target triples. Left: an example in which the regulators co-operate by influencing shared target genes in the same direction (i.e. either co-activating or co-repressing the shared targets). Right: regulators compete by influencing shared target genes in opposite directions. (c, d) Distribution of correlation coefficients between regulators and shared targets in two example dual regulons, computed from the expression profiles of METABRIC breast cancer data, n = 997 for cohort 1 (Curtis et al., 2012).
RTNduals uses three complementary statistics to identify dual regulons (Fig. 1a). (i) Targets are assigned to regulators based on mutual information (MI), forming regulons. The statistical significance of the MI values is assessed by permutation and bootstrap analysis. Because regulators can target each other, associations between pairs of regulators are also identified. (ii) Triplets formed by two regulators and a shared target gene are identified, and the direction of regulation is determined by correlation analysis (e.g. Pearson or Spearman). (iii) A Fisher’s exact test assesses the number of triplets shared between two regulators, and permutation analysis tests the statistical significance of the correlation between shared targets. The schematics in Figure 1b show the two cases that RTNduals identifies: regulator pairs (left) that co-operate, influencing shared target genes in the same direction (co-activation or co-repression), and (right) that compete, influencing targets in opposite directions. Figure 1c shows the distribution of Spearman correlations of targets shared between ESR1 and GATA3 regulons, indicating that these TFs either co-activate or co-repress their shared targets, while Figure 1d shows a contrasting case with ESR1 and NFIB regulons.
3 Case studies
RTNduals allows high-throughput screening for co-regulators and their shared targets. The Supplementary Information provides two detailed case studies that demonstrate the package’s workflow, using tumor samples from breast cancer cohorts. The first study analyses a regulatory network generated by RTN from METABRIC microarray data (Curtis et al., 2012), while the second case study shows how to prepare harmonized RNA-seq data from the National Cancer Institute’s Genomic Data Commons (GDC) for analysis (TCGA, 2012).
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq) [407090/2016-9]; and the Cancer Research UK (CRUK), the Breast Cancer Research Foundation (BCRF) [BCRF-17-127]. V.S.C., C.S.G., K.G.O. and S.T. were funded by the Coordination for the Improvement of Higher Education Personnel (CAPES). S.J.M.J. and A.G.R. are funded by the National Cancer Institute of the National Institutes of Health [U24CA210952]. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest: none declared.
Supplementary Material
References
- Castro M.A.A. et al. (2016) Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat. Genet., 48, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. et al. (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature, 486, 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubelsky Y., Shaul Y. (2019) Recruitment of the protein phosphatase-1 catalytic subunit to promoters by the dual-function transcription factor RFX1. Biochem. Biophys. Res. Commun., 509, 1015–1020. [DOI] [PubMed] [Google Scholar]
- Margolin A.A. et al. (2006) ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics, 7, 1471–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.G. et al. (2017) Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell, 171, 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network. (2012) Comprehensive molecular portraits of human breast tumours. Nature, 490, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N. et al. (2017) Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through induction of interleukin 6. J. Biol. Chem., 292, 8136–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


