Abstract
Neuroinflammation underlies the etiology of multiple neurodegenerative diseases and stroke. Our understanding of neuroinflammation has evolved in the last few years and major players have been identified. Microglia, the brain resident macrophages, are considered sentinels at the forefront of the neuroinflammatory response to different brain insults. Interestingly, microglia perform other physiological functions in addition to their role in neuroinflammation. Therefore, an updated approach in which modulation, rather than complete elimination of microglia is necessary. In this review, the emerging roles of microglia and their interaction with different components of the neurovascular unit are discussed. In addition, recent data on sex differences in microglial physiology and in the context of stroke will be presented. Finally, the multiplicity of roles assumed by microglia in the pathophysiology of ischemic stroke, and in the presence of co-morbidities such as hypertension and diabetes are summarized.
Graphical Abstract
Introduction
Ischemic stroke is a leading cause of death and disability worldwide. Fortunately, recent advancements in acute stroke care including the use of tissue plasminogen activator and thrombectomy over a wider therapeutic window led to a decline in stroke mortality. Unfortunately, stroke has become a disease of survivors that suffer from functional and cognitive deficits and there is no approved treatment for stroke recovery. Thus, better understanding of the pathophysiology that restricts the endogenous reparative ability of the brain, offers potential to identify novel therapeutic targets. In this regard, emerging evidence has put neuroinflammation in the spotlight because, depending on the severity and the spatial and temporal regulation of pro and anti-inflammatory signals, neuroinflammation may play both protective and detrimental role(s) in stroke injury and recovery. There are also dynamic interactions between the innate and adaptive immune responses in both the central nervous system and the periphery, and this has been elegantly detailed in several recent review articles (1–3).
In preclinical models, post-stroke inflammation begins shortly after occlusion of a vessel and peaks in the first few days after stroke onset (4). Following an ischemic stroke, innate and adaptive cellular immune responses occur and influence stroke pathology (5). Major players of the innate immune responses after stroke are microglia, circulating monocytes, tissue macrophages, and neutrophils, while major players of the adaptive immune responses include B cells and T-cells such as CD4+ T-lymphocytes, CD8+ T-lymphocytes, γδ T cells and T regulatory cells (Tregs). In addition, dendritic cells serve as the bridge between innate and adaptive immune responses (6). Ischemic inflammation is characterized by the breakdown of the blood brain barrier (BBB), infiltration of peripheral leukocytes, activation of glial cells, and the release of damage-associated molecular patterns (DAMPs) from injured cells to trigger the activation of immune cells (7). The activated immune cells, in turn, release inflammatory cytokines and cytotoxic mediators leading to the exacerbation of the ischemic injury (7). Weeks to months after stroke onset, tissue remodeling and repair take place leading to the formation of a cavity which prevents full functional recovery (8, 9). As the first immune responders to ischemic injury, in this review we will focus mainly on the brain-resident microglial cells and refer the readers to review articles introduced above for more general neuroinflammation in stroke. We will first briefly introduce microglia morphology and physiology and then review the impact of hypertension and diabetes, major comorbidities of stroke, on microglial responses before and after a stroke in experimental models. Final discussion will focus on the relevance of the preclinical data to the failed anti-inflammatory strategies in stroke treatment and identify potential opportunities.
Microglia Biology
At embryonic day 9, yolk sac progenitors start to enter the central nervous system (CNS) before the closure of the blood brain barrier at embryonic day 13 in mice. Once these progenitors reside in the CNS, they are called microglia and self-renew for the entire lifespan of the animal (10, 11). Microglia constitute 5–10% of adult brain cells and they represent the largest population of immune cells in the brain (12). Under physiological conditions, microglia are located within brain parenchyma in contact with other brain cells such as neurons, astrocytes and oligodendrocytes (12). The microglial population is distributed in different regions in the brain ranging from 5% in the cerebral cortex and corpus callosum to 12% in the substantia nigra (13). This heterogeneity across different brain regions could stem in part from local cues produced by neural cells (14–17). In addition, microglia show heterogeneity according to age, sex, and species, and in neurological disorders such as stroke.
There is accumulating evidence that suggests that microglia could be involved in more than the response to brain injury (18). It is clear that microglia play a role in regulation of BBB (19), synaptic reorganization, postsynaptic spine formation, regulation of neurogenesis, and early wiring of neuronal circuits (12, 18, 20–23). In addition, there is evidence for a mutual interaction between microglia and astrocytes. It has been shown that microglia produce signaling molecules that regulate astrogenesis (24, 25) and maturation of astrocytes (25). In support of these data, a lag time has been described between the appearance of microglia and astrogenesis in human and mice (26–28). Astrocytes, in turn, secrete soluble factors that increase proliferation of microglia (29, 30). A comprehensive summary of the roles of glia during brain development is reviewed by Reemst et al (25).
The BBB is a dynamic and metabolic interface that separates the CNS from the periphery and regulates the trafficking of solutes, fluid and cells, playing a crucial role in the maintenance of CNS homeostasis. The BBB is formed and stabilized by components of the neurovascular unit (NVU), which is composed of endothelial cells, astrocytes, pericytes, neurons and extracellular matrix (ECM) (19). While microglia were not traditionally considered part of the NVU, evolving NVU concept now incorporates microglia and peripheral macrophage as they certainly participate in important processes regulated by the NVU (31). Microglia regulate the BBB during development and after injury (19). Also, microglia play an important role in the stabilization and fusion of brain endothelial cells and in the sprouting, migration, and anastomosis of the cerebral and retinal vasculature during development (32, 33). There is evidence from ex vivo studies that microglia secrete soluble factors that can stimulate vessel sprouting without direct contact with endothelial cells (34). The loss of structural integrity and normal function of the BBB takes place in neurological disorders such as stroke (35). While it has been suggested that the opening of the BBB can be exploited therapeutically to deliver drugs that otherwise do not cross the BBB (36, 37), some studies have shown that normal diffusion of drugs into the CNS can be prevented by the accumulation of blood-derived debris and cells into enlarged perivascular spaces after BBB breakdown (38).
Microglia Phenotypes and Neuroinflammation after Stroke
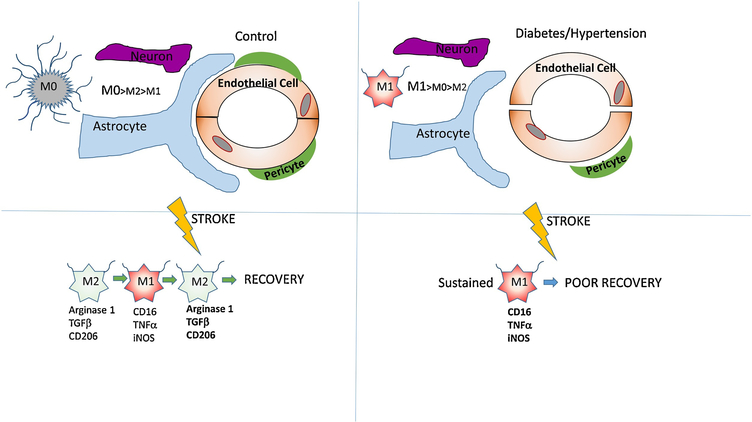
Microglia develop a spectrum of functional phenotypes with different effects on neuroinflammation (Fig. 1). These phenotypes are traditionally classified as surveillance (M0), pro-inflammatory (M1) and anti-inflammatory (M2) modes and dynamically change from one mode to another (31, 39, 40).
Figure 1.
A schematic model of the interaction between microglia and different components of the neurovascular unit in the absence and presence of comorbidities. In the left panel, microglia are in a resting state (M0) characterized by a higher number of process endpoints per cell that constantly probe the environment. After stroke, microglia transiently shift to the (M2) phenotype before shifting to (M1) pro-inflammatory phenotype. In the right panel, microglia are already primed for a pro-inflammatory phenotype in the presence of comorbidities and exhibit lower number of process endpoints per cell. After stroke, microglia shift directly to the (M1) phenotype and exhibit poorer recovery compared to microglia from animals with no comorbidities.
At homeostasis, microglia are considered to be in a surveillance mode and classified as quiescent microglia (M0). These quiescent microglia are characterized by a ramified morphological phenotype with low phagocytic properties (41). However, as highlighted in a recent review, even surveillance microglia are in a very dynamic state, constantly changing their ramified processes to continuously monitor the brain parenchyma (31, 42). The mediators and markers of M1 and M2 microglia phenotypes in the context of ischemic stroke are discussed below.
The role of microglia in the acute phase of neuroinflammation after stroke
At the early stages after stroke, resident microglia and recruited macrophages assume an M2 phenotype. Although counterintuitive, this M2 phenotype is needed to remove cell debris and curb brain damage (43, 44). Following that, a proinflammatory phenotype starts to dominate in the peri-infarct area (43). This proinflammatory phenotype, known as M1 or classically activated phenotype with an ameboid morphology, is induced by stimulation with IFN-γ and LPS in addition to other factors (40, 45). The M1 phenotype is identified by a number of markers (CD16, inducible nitric oxide synthase-iNOS and tumor necrosis factor α), and secretes a number of pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α, NO, and the proteolytic enzymes matrix metalloproteinases 3 and 9 (41, 46–48). This leads to the disruption of BBB and degradation of ECM (49) resulting in the infiltration of peripheral leukocytes and plasma-derived factors such as plasma-fibronectin and fibrinogen into the brain tissue (50). All these events lead to the exacerbation of the ischemic injury (7).
The activated form of microglia can also be beneficial, however, through the phagocytosis of cellular debris and secretion of beneficial neutrotrophic factors (40). In a neonatal stroke model, depletion of microglia exacerbated vascular permeability and increased intracerebral hemorrhage (51). This indicates that modulation of microglial function rather than inhibiting microglia altogether could be a viable therapeutic approach.
In addition to resident microglia, other myeloid cells, for example, bone marrow-derived monocytes, are recruited to the CNS from the periphery after stroke. These recruited peripheral cells have similar morphology and express similar surface markers as resident microglia (6, 52). Yet, infiltrating macrophages and resident microglia show distinct gene signatures and different temporal contributions to brain injury (53). It has been shown that infiltrating monocytes can either potentiate stroke damage or differentiate into microglia-like cells to participate in post-stroke repair processes (53–55). Taken together, the role of infiltrating macrophages during ischemic stroke is controversial. One reason for the difficulty in delineating the contribution of resident microglia compared to infiltrating macrophages is the difficulty in differentiating the two cell populations (6). The recent discovery of the resident microglia pool-specific marker, transmembrane protein 119 (TMEM119), has helped in differentiating resident microglia from other myeloid cells (52). For example, after stroke in humans and using TMEM119 as a biomarker, a substantial proportion of macrophage-like cells in the lesion has been shown to be derived from the original microglia pool (56).
The role of microglia in the delayed phase of neuroinflammation after stroke
Processes such as BBB repair, neurogenesis and angiogenesis are important for functional recovery after stroke (4). Microglia can either switch to an anti-inflammatory and neuroprotective M2-like phenotype to promote a tissue repair mechanism (57) or stay in sustained M1-like phenotype aggravating injury and impeding repair.
The alternatively activated phenotype, known as the M2 anti-inflammatory phenotype, is further divided into three subtypes, M2a, M2b and M2c, according to function and stimulating factors (40, 43, 58–60). The M2a subtype is induced by stimulation with IL-4 and IL-13 and express Arginase-1, Ym1, CD206, Fizz1 and IGF-1 (59, 61). The M2b phenotype is categorized as immunomodulatory microglia. While counterintuitive, it can induced by LPS and IL-1R agonists and express CD86 and SOCS3 (40, 58, 61). The M2c subtype, referred as deactivated microglia, is induced by TGFβ, IL-10 and glucocorticoids. Phenotypic markers include SOCS3, chemokine CXCL 13 and surface receptor (SR-A1) (40). It is involved in tissue regeneration (58, 61,62). In contrast to M1 microglia, M2 microglia enhance neuronal survival in vivo and in vitro (63).
Microglia influence neurogenesis differently under different physiologic/pathologic conditions and according to different observation time points (64). In neonatal mice, minocycline inhibits microglial function and at the same time reduces neurogenesis in the subventricular zone (SVZ) (65, 66). In aged mice, the number of microglia increases, and the removal of microglia reduces the activity of neural progenitor cells (67). After middle cerebral artery occlusion (MCAO), treatment with minocycline for 4 weeks enhances neurogenesis in the dentate gyrus (68). In another study, however, minocycline administered after MCAO reduced the number of neuroblasts and neurogenesis in the SVZ at 4 and 7 days respectively (69). These results indicate a biphasic role for microglia on neurogenesis in vivo (64, 70).
Sex Differences in Microglia Biology
Under physiological conditions, there is difference between male- and female-derived microglia at the transcriptomic and proteomic levels. This gene signature is maintained in culture and after transplant into mice of opposite sex and is not dependent on circulating hormones, because ovariectomy did not affect these observed gene signatures (71). Also, these differences have functional consequences. Microglia density and soma size are higher in the hippocampus, cortex and amygdala in 13-week-old male mice compared to age matched female mice. In contrast, the number of microglia in female Sprague-Dawley rats are higher than in males (72). This indicates that microglial density varies according to species and sex. Naive male microglia show higher antigen presenting capacity as evidenced by higher major histocompatibility complex (MHC) class I and II in the cortex. In addition, the proteomic analysis of male-derived microglia indicates higher responsiveness to immunological stimuli and higher motility capacity (73).
Sex differences in microglial functions after stroke
In the context of ischemic stroke, female microglia transplanted in male mice reduced ischemic damage indicating that there is an intrinsic sex-specific phenotype that is maintained in the absence of female hormones (71).
The ability of microglia to phagocytose endogenous structures and debris after ischemia, is mediated in part by the integrin receptor CD11b (41, 74). Females express constitutively higher levels of CD11b that remains unchanged after stroke. Whereas males express lower levels of CD11 b at baseline that significantly increases after stroke. It is suggested that the higher baseline expression of CD11b in females enhances the microglial capacity to remove the necrotic debris and ameliorate the neuronal injury after stroke (75).
Interestingly, microglia in the normal neonatal brain are active with an amoeboid morphology. In the preoptic area, there are twice as many active microglia in males as compared to females, while in the neonatal hippocampus, there are no sex differences in the number of amoeboid microglia (18, 76–78). However, in females, the hippocampus has more phagocytic microglia than that observed in male (79).
One protein that plays an important role in the regulation of inflammatory processes and immune response is S100a8, which is a TLR4-binding protein that regulates the expression of pro-inflammatory cytokines (64). Sex differences in S100a8 has been described in earlier studies. In male-derived microglia, S100a8 has been shown to be upregulated in whole brain homogenate at homeostasis. Surprisingly, in another study, S100a8 has been shown to be upregulated at the mRNA level in female mice (80). It was suggested that differences in sample processing and microbiome could have contributed to these differences between the two studies (73).
Results from sex difference studies, however, should always be carefully interpreted. Different stages of the estrus cycle in females can affect the gene expression patterns (81). Differences in species, age, methods of isolation of different cell types and microbiome should be always be considered. In addition, it has been shown in some studies that microglia can rapidly lose epigenetic and transcriptional identity upon separation from their tissue environment (70, 82)
The impact of co-morbidities on microglia
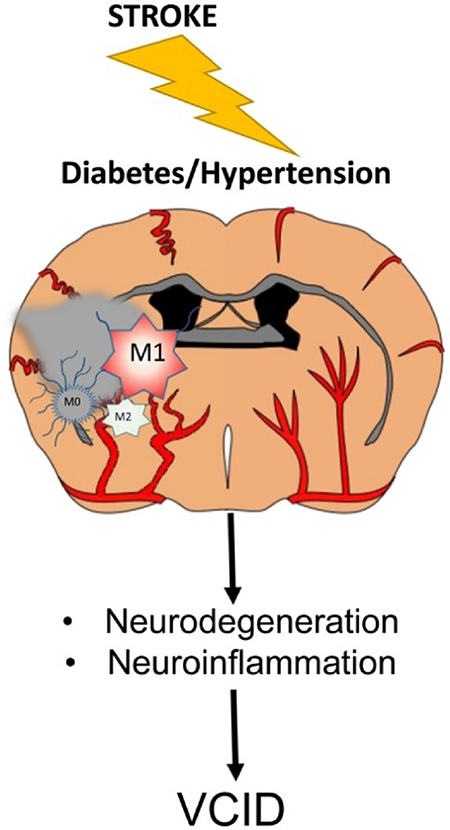
It is recognized that one potential factor that may have contributed to the failure of successful translation of potential treatments identified in preclinical studies into clinical use is the limited use of comorbid disease models in stroke research. In this regard, hypertension and diabetes are leading contributors to the risk, severity and poor recovery of ischemic stroke. Both diseases are considered as states of chronic systemic and vascular inflammation. While the brain has been long considered an immune “privileged” organ, emerging evidence suggests that even in the absence of a brain injury, microglia sense and respond to this systemic inflammation. An intriguing example of this is the robust hippocampal microglial activation that is associated with cognitive dysfunction after a peripheral surgical intervention (83). This neuroinflammatory response is further aggravated in diabetic animals and depletion of microglia prevents the development of cognitive impairment. Thus, it is highly likely that hypertension and diabetes can directly prime the microglia through the systemic inflammatory state.
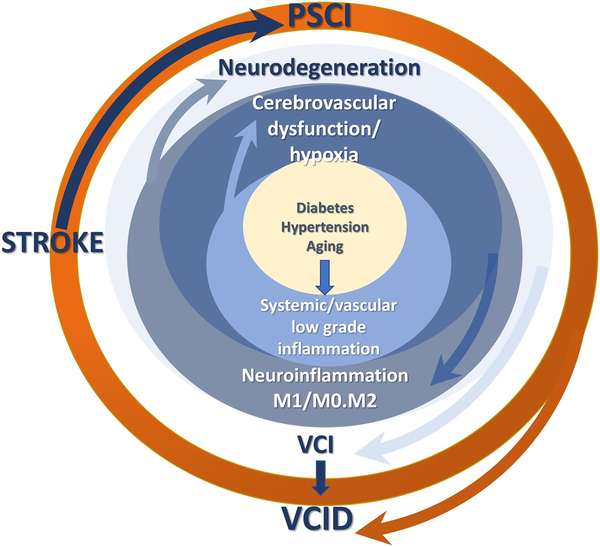
Both diseases can also indirectly influence neuroinflammation via regulation of cerebral blood flow (CBF). Diabetes and hypertension have profound effects on cerebrovascular function and structure resulting in dysregulation of autoregulatory and neurovascular coupling mechanisms (84–86) that are essential for continuous and “on demand” delivery of blood to the brain. It has been postulated that decreased CBF and a hypoxic milieu precedes development of overt symptoms of cognitive deficits in neurodegenerative diseases. As depicted in Fig 2, comorbid factors can initiate low grade systemic and vascular inflammation that causes early cerebrovascular dysfunction. Resultant decrease in CBF leads to microglial activation and neuroinflammation mediating neurodegeneration and ensuing cognitive deficits. An ischemic injury added to this pathology amplifies this vicious neuroinflammatory loop with microglia being a key player.
Figure 2.
A schematic model of the role of microglia phenotypes in the vicious neuroinflammatory loop initiated by comorbid conditions. Hypoxic conditions resulting from cerebrovascular dysfunction and altered CBF causes an imbalance of pro- (M1) and anti-inflammatory (M2) microglia leading to neurodegeneration and vascular cognitive impairment (VCI). An ischemic stroke overlaid on this pathology and amplifies neurodegeneration via sustained M2 activation and causes poststroke cognitive impairment (PSCI), collectively contributing vascular contributions to cognitive impairment and dementia (VCID).
Diabetes and Microglia
Diabetes-mediated activation of microglia in the absence of an ischemic event has been reported in a number of studies. Drake et al. investigated microglial activation and neurovascular inflammation in the corpulent JCR:LA-cp (cp/cp) model of diabetes using an interesting positron emission tomography (PET) approach, as well as traditional immunohistological methods (87). This model of diabetes presents with insulin resistance and obesity. As animals aged (12–15 months), there was a significant activation of microglia in the diabetic animals, as evidenced by increased uptake of the translocator protein TSPO radiotracer [18F]DPA-714. Augmented IBA-1 staining in the diabetic animals supported this finding. Vascular inflammation was evident by increased ICAM-1 and VCAM-1 staining in the cerebrovascular cross sections. The same study also reported increased microglial activation in diabetic individuals that did not have any intracranial pathology detected by MRI, collectively suggesting that diabetes can cause neuroinflammation in the absence of an ischemic event. While this particular study did not provide information with respect to different microglia phenotypes, a recent study showed that high glucose alone can increase M1/M2 ratio shifting isolated microglia to a proinflammatory phenotype. Furthermore, sham-operated diabetic animals exhibited lower M2-like microglia in the brain homogenates analyzed by flow cytometry (88).
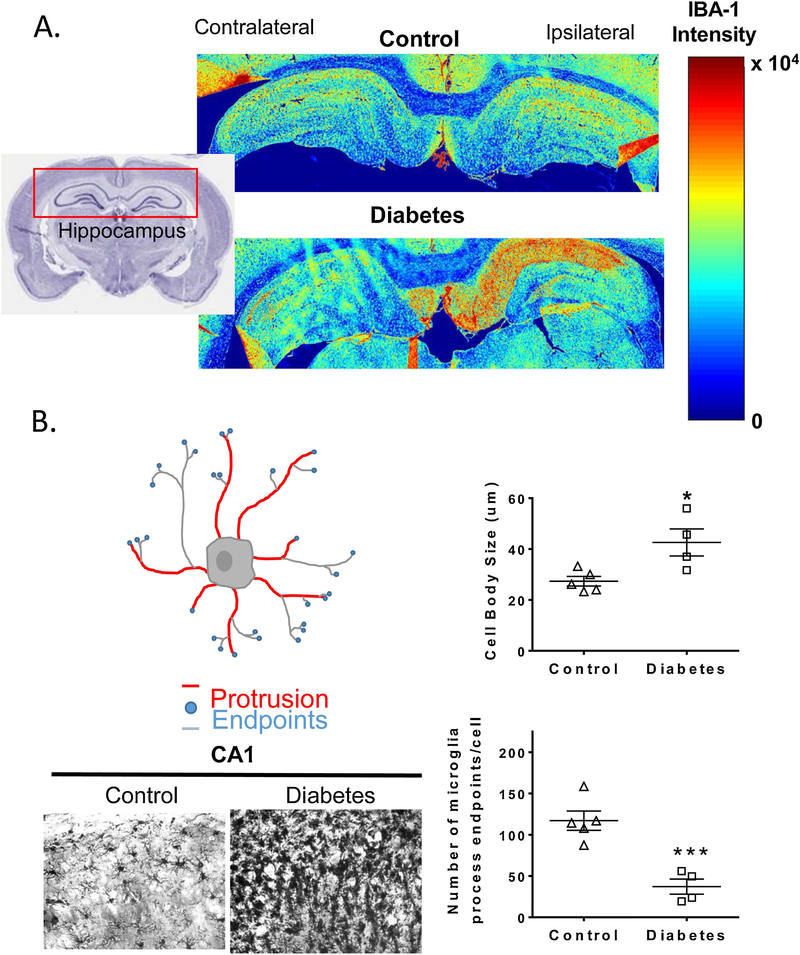
As one would expect, an ischemic injury in the setting of diabetes further exacerbates deleterious microglial activation. In an earlier study, Hu and colleagues reported that in control animals an early beneficial M2 activation after stroke dynamically shifts to an M1 phenotype (43). A follow-up study by the same group recently expanded these findings and showed that in the db/db mouse model of type 2 diabetes, M2 microglia density is much lower and the increase in M1 microglia is much more robust. This increased activation of the deleterious microglia phenotype is associated with poor white matter integrity and worse functional outcomes in diabetic animals. Moreover, this increase in M1/M2 ratio under diabetic conditions suppresses oligodendrocyte precursor cell differentiation explaining the mechanistic basis of poor myelination while highlighting the importance of microglia phenotype in recovery. We have also shown that stroke caused by either suture or embolic occlusion of middle cerebral artery (MCAO) activates microglia to a greater extent in diabetic rats in a diet-induced model of diabetes and this effect is more pronounced in embolic stroke which causes greater hemorrhagic transformation (Fig 3) (89). While we did not use specific phenotypic markers, morphological studies showed increased cell body swelling, reduced number of microglial protrusions and summed process length in the hippocampus in both 60 min and embolic MCAO compared to control animals. Sensorimotor and cognitive deficits follow a similar pattern. In a follow-up study we reported that iron chelation by deferoxamine in the recovery period after ischemic stroke attenuates microglial activation and this is accompanied by improved functional outcomes (90). However, in these studies, microglial activation was based on morphology and microglia phenotype was not assessed by classical flow cytometry analyses.
Figure 3.
The impact of stroke and diabetes on microglial morphology and density. A. Heatmaps generated by IBA-1 staining after embolic MCAO show that microglial density is significantly higher in stroked animals in the ipsilateral side in the presence of diabetes. B. Cell swelling, number of protrusions from microglia cell body (red lines), number of endpoints at the tips of microglia processes (blue circles) and process length (grey lines) calculated from 40x images show that microglia of stroked diabetic animals exhibit larger cell body size and lower number of process endpoints per cell compared to control animals. (Adapted with permission from Ward, B. et al AJP: Heart and Circ Physiol.2018 (89)).
Several other studies offer hope that detrimental microglial responses can be therapeutically modulated to improve outcomes. Exendin 4, a glucagon-like receptor 1 agonist that is clinically used for blood glucose control in diabetic patients, provides neuroprotection and improves outcomes by promoting M2 polarization even when treatment is started 4.5 h after stroke (91). We have evidence that stimulation of the Angiotensin II type 2 receptor (ATR2) by C21 improves M1/M2 ratio in favor of M2 microglia in diabetic animals, while increasing surveillance and M2 like microglia in control animals (92). Interestingly, an earlier study revealed that diabetic animals fail to launch an inflammatory response as evidenced by reduced inflammatory cytokine expression and microglial activation resulting in impaired repair (93). The PPARγ agonist darglitazone improved outcomes by restoring the inflammatory response (94). However, it has to be acknowledged that this study employed a hypoxia-ischemia model in diabetic mice, and not a stroke model.
Hypertension and Microglia
Microglia may play a dual role in hypertension. Emerging evidence suggests that microglial activation contributes to the central regulation of blood pressure and development of hypertension as recently reviewed in two excellent review articles (31, 95). In this self-perpetuating cycle, the delicate balance of microglia phenotype can be tilted towards deleterious chronically activated M1 microglia.
Evidence for microglial activation in hypertension has been shown in different models of hypertension (96, 97). A recent study reported that chronic Angiotensin (Ang) II infusion in mice causes astrogliosis and hippocampal microglial activation and these are associated with parallel increases in TRPV4-mediated currents and Ca2+ events in astrocytes emphasizing the close communication between these cells (96). Central administration of a tetracycline derivative with potent anti-inflammatory activity (CMT-3) has been demonstrated to reduce microglial activation and attenuate increases in blood pressure in both spontaneously hypertensive rats (SHR) as well as in Ang II-induced hypertensive rats (97). We have shown that Ang II type 1 receptor (AT1R) blockade with candesartan or AT2R stimulation with Compound 21 prevents microglial activation and accompanying cognitive decline in aged SHRs (98). Moreover, Bhat and colleagues demonstrated that candesartan-mediated attenuation of microglial activation in SHRs is independent of its blood pressure lowering effects (99).
While limited, there is evidence for exacerbated microglial activation after stroke in the hypertensive setting. A 2001 study reported more quiescent and activated microglia in the stroke-prone spontaneously hypertensive rats (SHRSP) as compared to normotensive controls. Ischemic injury further increased activated microglia (100). Pires and colleagues showed that ischemic stroke in SHRSPs chronically treated with TNFα inhibitor etanercept caused greater infarct sizes that is characterized by reduced number of microglia and upregulation of the expression of M1 markers in the infarct core (101). Interestingly, another study reported reduced microglial activation after endothelin-1-induced stroke in SHRs (102). While microglial phenotype was not identified, microglia were reported to be dominating cell type in the ischemic hemisphere at Day 4 after permanent distal MCAO (103). A more recent report showed that estradiol-mediated neuroprotection observed in SHRs was independent of changes in microglial activation (104). Minocycline administration at reperfusion was reported to promote a protective microglial phenotype and improve recovery in the SHR model (105). While minocycline is a known microglia inhibitor, it also inhibits matrix metalloproteases (MMPs). This may be an indirect effect and may stem from inhibition of by MMPs. Our own studies also showed a robust microglial activation exacerbated by stroke in this model of hypertension. AT2R activation by C21 or AT1R blockade by candesartan, even when started at day 7 after stroke, reduced neuroinflammation and improved cognitive outcomes suggesting that modulation of the Ang II system may be a therapeutic target with high translational potential (106). Notably, all these studies utilized the SHR or SHRSP models of hypertension. There is a need to investigate post-stroke recovery and microglial activation in other preclinical models of hypertension.
Microglia and Post-stroke Cognitive Impairment
Cognitive impairment is a common cause of disability after stroke, occurring in up to 60% of individuals and progressing to dementia in up to a third of its victims (107). Recent evidence from a large, population-based, epidemiological study in the United States revealed the chronically progressive nature of cognitive impairment, even in the absence of additional events (108). Post stroke cognitive impairment and vascular cognitive impairment, the leading diseases in the vascular contributions to cognitive impairment and dementia (VCID) spectrum of disorders, are the leading causes of Alzheimer’s Disease Related Dementias (100).
In animal models of stroke, although recovery from the immediately obvious sensorimotor deficits occurs in the first week, cognitive impairment can appear later and may be progressive (106). This latter phenomenon has only recently been appreciated and can only be detected when animals are allowed to survive for weeks to months after stroke. The recognition of chronically progressive secondary neurodegeneration after stroke is not new, however, it was originally described in both animals and humans in areas remote from the infarct, including the thalamus (109, 110). More recently, this secondary neurodegeneration has been linked to neuroinflammation (111–113), in general, and microglial activation, in particular (114). This secondary neurodegeneration has been associated with development of post-stroke cognitive impairment (115) but whether interference with microglial activation is a viable therapeutic target in humans remains unknown (116).
Conclusions and Therapeutic Implications
Chronic microglial activation after stroke is a promising area of therapeutics research. In addition to the studies discussed above under diabetes and hypertension models, in preclinical models of secondary neurodegeneration, microglial modulation in favor of the M2 phenotype, with either osteopontin (117) or the CSF1R inhibitor, PLX3397 (118), has been shown to decrease tissue damage and improve functional outcomes, including cognition. In stroke patients, perispinal administration of the TNF inhibitor, etanercept, has been shown in case series to reduce functional impairment even years after stroke, by a mechanism involving reduction in microglial activation (119). Interfering with microglial function is to be undertaken carefully, however, due to an incomplete understanding of the biphasic nature of microglial response (70), the multiple involved phenotypes (39), and sex differences in microglia physiology/pathophysiology. Strategies to enhance the surveilling M0 properties as well as anti-inflammatory M2 phenotype need to be better developed. An interesting recent study by Xing and colleagues reported that while conditioned medium from endothelial cells exposed to oxygen glucose deprivation promoted an M1 like phenotype, conditioned medium from astrocytes mediated a protective/restorative M2 like microglial activation suggesting a gliovascular switch (120). Thus, given these complex interactions, we support the evaluation of microglia modulation in the context of the NVU.
Acknowledgments
This work was supported in part by a Veterans Affairs (VA) Merit Award (BX000347), VA Senior Research Career Scientist Award (IK6 BX004471) and National Institutes of Health (NIH) award (R01NS083559) to Adviye Ergul, and R01NS104573 multi-PI grant to Susan C. Fagan and Adviye Ergul.
Disclosures: Adviye Ergul is a Senior Research Career Scientist at the Ralph H. Johnson Veterans Affairs Medical Center in Charleston, SC. The contents do not represent the views of the Department of Veterans Affairs or the US Government.
Footnotes
Declaration of Interests: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2:136–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambertsen KL, Finsen B, Clausen BH. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcet P, Santos N, Borlongan CV. When friend turns foe: central and peripheral neuroinflammation in central nervous system injury. Neuroimmunol Neuroinflamm. 2017;4:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajkovic O, Potjewyd G, Pinteaux E. Regenerative Medicine Therapies for Targeting Neuroinflammation After Stroke. Front Neurol. 2018;9:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. [DOI] [PubMed] [Google Scholar]
- 6.Rayasam A, Hsu M, Kijak JA, Kissel L, Hernandez G, Sandor M, Fabry Z. Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology. 2018;154(3):363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anrather J, ladecola C. Inflammation and Stroke: An Overview. Neurotherapeutics. 2016;13(4):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Adv Funct Mater. 2014;24(44):7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewell SS, Churilov L, Sidon TK, Aleksoska E, Cox SF, Macleod MR, Howells DW. Evolution of ischemic damage and behavioural deficit over 6 months after MCAo in the rat: Selecting the optimal outcomes and statistical power for multi-centre preclinical trials. PLoS One. 2017;12(2):e0171688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeffel G, Ginhoux F. Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol. 2018;330:5–15. [DOI] [PubMed] [Google Scholar]
- 11.Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, Ishikawa-Ankerhold HC, Margraf A, Hutter S, Vagnozzi R, Klapproth S, Frampton J, Yona S, Scheiermann C, Molkentin JD, Jeschke U, Moser M, Sperandio M, Massberg S, Geissmann F, Schulz C. Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat Commun. 2018;9(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thion MS, Ginhoux F, Garel S. Microglia and early brain development: An intimate journey. Science. 2018;362(6411):185–189. [DOI] [PubMed] [Google Scholar]
- 13.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. [DOI] [PubMed] [Google Scholar]
- 15.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018–1027. [DOI] [PubMed] [Google Scholar]
- 16.Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, de Boer-Bergsma JJ, Martin NA, Karram K, Kramer I, Boddeke EW, Waisman A, Eggen BJ, Owens T. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 2017;36(22):3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, Prinz M. Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol. 2017;134(3):441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33(7):2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer DP, Stevens B. Microglia Function in Central Nervous System Development and Plasticity. Cold Spring Harb Perspect Biol. 2015;7(10):a020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33(10):4216–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9(1):1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375(6526):68–73. [DOI] [PubMed] [Google Scholar]
- 25.Reemst K, Noctor SC, Lucassen PJ, Hol EM. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front Hum Neurosci. 2016;10:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23(5):1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95(1):134–143. [DOI] [PubMed] [Google Scholar]
- 28.Bechade C, Pascual O, Triller A, Bessis A. Nitric oxide regulates astrocyte maturation in the hippocampus: involvement of NOS2. Mol Cell Neurosci. 2011;46(4):762–769. [DOI] [PubMed] [Google Scholar]
- 29.Frei K, Bodmer S, Schwerdel C, Fontana A. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol. 1986;137(11):3521–3527. [PubMed] [Google Scholar]
- 30.Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12(4):309–318. [DOI] [PubMed] [Google Scholar]
- 31.Morrison HW, Filosa JA. Stroke and the neurovascular unit: glial cells, sex differences, and hypertension. Am J Physiol Cell Physiol. 2019;316(3):C325–C339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc Cell. 2013;5(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS One. 2011;6(1):e15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. [DOI] [PubMed] [Google Scholar]
- 36.Borlongan CV, Emerich DF. Facilitation of drug entry into the CNS via transient permeation of blood brain barrier: laboratory and preliminary clinical evidence from bradykinin receptor agonist, Cereport. Brain Res Bull. 2003;60(3):297–306. [DOI] [PubMed] [Google Scholar]
- 37.Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20(2):217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. [DOI] [PubMed] [Google Scholar]
- 40.Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. [DOI] [PubMed] [Google Scholar]
- 41.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. [DOI] [PubMed] [Google Scholar]
- 42.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15(5):300–312. [DOI] [PubMed] [Google Scholar]
- 43.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–3070. [DOI] [PubMed] [Google Scholar]
- 44.Perego C, Fumagalli S, Zanier ER, Carlino E, Panini N, Erba E, De Simoni MG. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol Dis. 2016;96:284–293. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa Y, Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel). 2014;7(12):1028–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. [DOI] [PubMed] [Google Scholar]
- 47.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–787. [DOI] [PubMed] [Google Scholar]
- 48.Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp (Warsz). 2012;60(4):251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haley MJ, Lawrence CB. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab. 2017;37(2):456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dejonckheere E, Vandenbroucke RE, Libert C. Matrix metalloproteinases as drug targets in ischemia/reperfusion injury. Drug Discov Today. 2011;16(17–18):762–778. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Lopez D, Faustino J, Klibanov AL, Derugin N, Blanchard E, Simon F, Leib SL, Vexler ZS. Microglial Cells Prevent Hemorrhage in Neonatal Focal Arterial Stroke. J Neurosci. 2016;36(10):2881–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA. 2016;113(12):E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Bonilla L, Faraco G, Moore J, Murphy M, Racchumi G, Srinivasan J, Brea D, Iadecola C, Anrather J. Spatio-temporal profile, phenotypic diversity, and fate of recruited monocytes into the post-ischemic brain. J Neuroinflammation. 2016;13(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117(3):531–539. [DOI] [PubMed] [Google Scholar]
- 55.Schilling M, Besselmann M, Leonhard C, Mueller M, Ringelstein EB, Kiefer R. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol. 2003;183(1):25–33. [DOI] [PubMed] [Google Scholar]
- 56.Zrzavy T, Machado-Santos J, Christine S, Baumgartner C, Weiner HL, Butovsky O, Lassmann H. Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol. 2018;28(6):791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Machado-Pereira M, Santos T, Ferreira L, Bernardino L, Ferreira R. Anti-Inflammatory Strategy for M2 Microglial Polarization Using Retinoic Acid-Loaded Nanoparticles. Mediators Inflamm. 2017;2017:6742427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chhor V, Le Charpentier T, Lebon S, Ore MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Savman K, Mallard C, Gressens P, Fleiss B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Latta CH, Sudduth TL, Weekman EM, Brothers HM, Abner EL, Popa GJ, Mendenhall MD, Gonzalez-Oregon F, Braun K, Wilcock DM. Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-beta using BV2 microglial cells and APP/PS1 transgenic mice. J Neuroinflammation. 2015;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol Aging. 2013;34(4):1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mecha M, Feliu A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutierrez S, de Sola RG, Guaza C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233–245. [DOI] [PubMed] [Google Scholar]
- 62.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. [DOI] [PubMed] [Google Scholar]
- 63.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma L, Sun P, Zhang JC, Zhang Q, Yao SL. Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int J Mol Med. 2017;40(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34(6):2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vukovic J, Colditz MJ, Blackmore DG, Ruitenberg MJ, Bartlett PF. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J Neurosci. 2012;32(19):6435–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adachi M, Abe M, Sasaki T, Kato H, Kasahara J, Araki T. Role of inducible or neuronal nitric oxide synthase in neurogenesis of the dentate gyrus in aged mice. Metab Brain Dis. 2010;25(4):419–424. [DOI] [PubMed] [Google Scholar]
- 68.Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38(1):146–152. [DOI] [PubMed] [Google Scholar]
- 69.Kim BJ, Kim MJ, Park JM, Lee SH, Kim YJ, Ryu S, Kim YH, Yoon BW. Reduced neurogenesis after suppressed inflammation by minocycline in transient cerebral ischemia in rat. J Neurol Sci. 2009;279(1–2):70–75. [DOI] [PubMed] [Google Scholar]
- 70.Holtman IR, Skola D, Glass CK. Transcriptional control of microglia phenotypes in health and disease. J Clin Invest. 2017;127(9):3220–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, Lolli F, Marcello E, Sironi L, Vegeto E, Maggi A. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018;23(12):3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA. Sex differences in glia reactivity after cortical brain injury. Glia. 2015;63(11):1966–1981. [DOI] [PubMed] [Google Scholar]
- 73.Guneykaya D, Ivanov A, Hernandez DP, Haage V, Wojtas B, Meyer N, Maricos M, Jordan P, Buonfiglioli A, Gielniewski B, Ochocka N, Comert C, Friedrich C, Artiles LS, Kaminska B, Mertins P, Beule D, Kettenmann H, Wolf SA. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep. 2018;24(10):2773–2783 e2776. [DOI] [PubMed] [Google Scholar]
- 74.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morrison HW, Filosa JA. Sex differences in astrocyte and microglia responses immediately following middle cerebral artery occlusion in adult mice. Neuroscience. 2016;339:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120(6):948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munn NA. Microglia dysfunction in schizophrenia: an integrative theory. Med Hypotheses. 2000;54(2):198–202. [DOI] [PubMed] [Google Scholar]
- 79.Nelson LH, Warden S, Lenz KM. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav Immun. 2017;64:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, Lledo PM, Greter M, Chan JKY, Amit I, Beyer M, Schultze JL, Schlitzer A, Pettersson S, Ginhoux F, Garel S. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell. 2018;172(3):500–516 e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yagi S, Drewczynski D, Wainwright SR, Barha CK, Hershorn O, Galea LAM. Sex and estrous cycle differences in immediate early gene activation in the hippocampus and the dorsal striatum after the cue competition task. Horm Behav. 2017;87:69–79. [DOI] [PubMed] [Google Scholar]
- 82.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17(1):18–25. [DOI] [PubMed] [Google Scholar]
- 83.Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2(7):e91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coucha M, Abdelsaid M, Ward R, Abdul Y, Ergul A. Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr Physiol. 2018;8(2):773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hardigan T, Ward R, Ergul A. Cerebrovascular complications of diabetes: focus on cognitive dysfunction. Clin Sci (Lond). 2016;130(20):1807–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cipolla MJ, Liebeskind DS, Chan SL. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. 2018;38(12):2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011;25(6):1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma S, Wang J, Wang Y, Dai X, Xu F, Gao X, Johnson J, Xu N, Leak RK, Hu X, Luo Y, Chen J. Diabetes Mellitus Impairs White Matter Repair and Long-Term Functional Deficits After Cerebral Ischemia. Stroke. 2018;49(10):2453–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, Ergul A. Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. Am J Physiol Heart Circ Physiol. 2018;315(5):H1402–H1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abdul Y, Jamil S, Ward R, Dong G, Li W, Fagan S, Ergul A. Abstract WP152: Iron Chelation Improves Stroke Outcomes in Diabetes: Impact on Neurovascular Remodeling, Microglial Activation and Ferroptotic Endothelial Cell Death. Stroke. 2019;50(Suppl_1):AWP152–AWP152. [Google Scholar]
- 91.Darsalia V, Hua S, Larsson M, Mallard C, Nathanson D, Nystrom T, Sjoholm A, Johansson ME, Patrone C. Exendin-4 reduces ischemic brain injury in normal and aged type 2 diabetic mice and promotes microglial M2 polarization. PLoS One. 2014;9(8):e103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson L, Dong G, Fagan SC, Ergul A. Abstract TP565: Delayed Administration of Angiotensin Receptor (AT2R) Agonist C21 Downregulates Diabetes Induced Pro-Inflammatory Microglia Activation to Improve Cognitive and Functional Recovery Post-Stroke. Stroke. 2019;50(Suppl_1):ATP565–ATP565. [Google Scholar]
- 93.Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab. 2007;27(4):710–718. [DOI] [PubMed] [Google Scholar]
- 94.Kumari R, Willing LB, Patel SD, Krady JK, Zavadoski WJ, Gibbs EM, Vannucci SJ, Simpson IA. The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic ob/ob mouse. J Cereb Blood Flow Metab. 2010;30(2):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biancardi VC, Bomfim GF, Reis WL, Al-Gassimi S, Nunes KP. The interplay between Angiotensin II, TLR4 and hypertension. Pharmacol Res. 2017;120:88–96. [DOI] [PubMed] [Google Scholar]
- 96.Diaz JR, Kim KJ, Brands MW, Filosa JA. Augmented astrocyte microdomain Ca(2+) dynamics and parenchymal arteriole tone in angiotensin II-infused hypertensive mice. Glia. 2019;67(3):551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S, Richards EM, Pepine CJ, Sumners C, Raizada MK. Microglial Cells Impact Gut Microbiota and Gut Pathology in Angiotensin II-Induced Hypertension. Circ Res. 2019;124(5):727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmed HA, Ishrat T, Pillai B, Bunting KM, Vazdarjanova A, Waller JL, Ergul A, Fagan SC. Angiotensin receptor (AT2R) agonist C21 prevents cognitive decline after permanent stroke in aged animals-A randomized double-blind pre-clinical study. Behav Brain Res. 2019;359:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhat SA, Goel R, Shukla S, Shukla R, Hanif K. Angiotensin Receptor Blockade by Inhibiting Glial Activation Promotes Hippocampal Neurogenesis Via Activation of Wnt/beta-Catenin Signaling in Hypertension. Mol Neurobiol. 2018;55(6):5282–5298. [DOI] [PubMed] [Google Scholar]
- 100.Marks L, Carswell HV, Peters EE, Graham DI, Patterson J, Dominiczak AF, Macrae IM. Characterization of the microglial response to cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 2001;38(1):116–122. [DOI] [PubMed] [Google Scholar]
- 101.Pires PW, Girgla SS, Moreno G, McClain JL, Dorrance AM. Tumor necrosis factor-alpha inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats. Am J Physiol Heart Circ Physiol. 2014;307(5):H658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Geyter D, Stoop W, Zgavc T, Sarre S, Michotte Y, De Keyser J, Kooijman R. Spontaneously hypertensive rats display reduced microglial activation in response to ischemic stroke and lipopolysaccharide. J Neuroinflammation. 2012;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moller K, Boltze J, Posel C, Seeger J, Stahl T, Wagner DC. Sterile inflammation after permanent distal MCA occlusion in hypertensive rats. J Cereb Blood Flow Metab. 2014;34(2):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stoop W, De Geyter D, Verachtert S, Brouwers S, Verdood P, De Keyser J, Kooijman R. Post-stroke treatment with 17beta-estradiol exerts neuroprotective effects in both normotensive and hypertensive rats. Neuroscience. 2017;348:335–345. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahmed HA, Ishrat T, Pillai B, Fouda AY, Sayed MA, Eldahshan W, Waller JL, Ergul A, Fagan SC. RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial. J Neuroinflammation. 2018;15(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mijajlovic MD, Pavlovic A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, Kliper E, Shin YI, Kim YH, Choi S, Jung S, Lee YB, Sinanovic O, Levine DA, Schlesinger I, Mead G, Milosevic V, Leys D, Hagberg G, Ursin MH, Teuschl Y, Prokopenko S, Mozheyko E, Bezdenezhnykh A, Matz K, Aleksic V, Muresanu D, Korczyn AD, Bornstein NM. Post-stroke dementia - a comprehensive review. BMC Med. 2017; 15(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, Wadley VG. Trajectory of Cognitive Decline After Incident Stroke. JAMA. 2015;314(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujie W, Kirino T, Tomukai N, Iwasawa T, Tamura A. Progressive shrinkage of the thalamus following middle cerebral artery occlusion in rats. Stroke. 1990;21(10):1485–1488. [DOI] [PubMed] [Google Scholar]
- 110.lizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke. 1990;21(5):790–794. [DOI] [PubMed] [Google Scholar]
- 111.Block F, Dihne M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75(5):342–365. [DOI] [PubMed] [Google Scholar]
- 112.Weishaupt N, Zhang A, Deziel RA, Tasker RA, Whitehead SN. Prefrontal Ischemia in the Rat Leads to Secondary Damage and Inflammation in Remote Gray and White Matter Regions. Front Neurosci. 2016;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jones KA, Maltby S, Plank MW, Kluge M, Nilsson M, Foster PS, Walker FR. Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav Immun. 2018;67:299–307. [DOI] [PubMed] [Google Scholar]
- 114.Kluge MG, Abdolhoseini M, Zalewska K, Ong LK, Johnson SJ, Nilsson M, Walker FR. Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke. J Cereb Blood Flow Metab. 2018:271678X18797346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schaapsmeerders P, van Uden IW, Tuladhar AM, Maaijwee NA, van Dijk EJ, Rutten-Jacobs LC, Arntz RM, Schoonderwaldt HC, Dorresteijn LD, de Leeuw FE, Kessels RP. Ipsilateral hippocampal atrophy is associated with long-term memory dysfunction after ischemic stroke in young adults. Hum Brain Mapp. 2015;36(7):2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Zhang Y, Xing S, Liang Z, Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke. 2012;43(6):1700–1705. [DOI] [PubMed] [Google Scholar]
- 117.Ladwig A, Rogall R, Hucklenbroich J, Willuweit A, Schoeneck M, Langen KJ, Fink GR, Adele Rueger M, Schroeter M. Osteopontin Attenuates Secondary Neurodegeneration in the Thalamus after Experimental Stroke. J Neuroimmune Pharmacol. 2018. [DOI] [PubMed] [Google Scholar]
- 118.Rice RA, Spangenberg EE, Yamate-Morgan H, Lee RJ, Arora RP, Hernandez MX, Tenner AJ, West BL, Green KN. Elimination of Microglia Improves Functional Outcomes Following Extensive Neuronal Loss in the Hippocampus. J Neurosci. 2015;35(27):9977–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ignatowski TA, Spengler RN, Dhandapani KM, Folkersma H, Butterworth RF, Tobinick E. Perispinal etanercept for post-stroke neurological and cognitive dysfunction: scientific rationale and current evidence. CNS Drugs. 2014;28(8):679–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xing C, Li W, Deng W, Ning M, Lo EH. A potential gliovascular mechanism for microglial activation: differential phenotypic switching of microglia by endothelium versus astrocytes. J Neuroinflammation. 2018;15(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]