Summary
Regulatory T (Treg) cell‐specific deletion of a gene of interest is a procedure widely used to study mechanisms controlling Treg development, homeostasis and function. Accordingly, several transgenic mouse lines have been generated that bear the Cre recombinase under control of the Foxp3 promoter either as a random transgene insertion or knocked into the endogenous Foxp3 locus, with the Foxp3YFP‐Cre strain of mice being one of the most widely used. In an attempt to generate Treg cells that lacked expression of the insulin receptor (Insr), we crossed Foxp3YFP‐Cre mice with Insrfl/fl mice. Using a conventional two‐band PCR genotyping method we found that offspring genotypes did not correspond to the expected Mendelian ratios. We therefore developed a quantitative PCR‐based genotyping method to investigate possible ectopic recombination outside the Treg lineage. With this method we found that ~50% of the F1‐generation mice showed evidence of ectopic recombination and that ~10% of the F2‐generation mice had germline Cre recombination activity leading to a high frequency of offspring with global Insr deletion. Use of the quantitative PCR genotyping method enabled accurate selection of mice without ectopic recombination and only the desired Treg cell‐specific Insr deletion. Our data highlight the need to use genotyping methods that allow for assessment of possible ectopic recombination driven by the Foxp3YFP‐Cre allele, particularly when studying genes that are systemically expressed.
Keywords: Cre recombination, Foxp3, mouse, regulatory T cells
Foxp3‐YFP‐cre mice are commonly used to generate regulatory T (Treg) cell‐specific knockout mice but when crossed with insulin receptor floxed mice, we found that some offspring had recombination outside the Treg lineage. Ectopic recombination could not be identified using a conventional two‐band‐based PCR genotyping method so we developed a quantitative PCR method to discriminate between mice that had Treg cell‐specific versus ectopic recombination. Our data highlight the need to use genotyping methods that allow for assessment of possible ectopic recombination driven by the Foxp3‐YFP‐Cre allele.

Abbreviations
- Insr
insulin receptor
- Tconv
conventional T cells
- Treg
regulatory T cells
Introduction
Generation of conditional gene knockout animals using Cre‐lox technology is a powerful tool for studying gene function in specific tissues and cell types. This technology exploits the ability of the Cre recombinase, initially discovered in bacteriophage P1, to recombine segments of DNA flanked by a pair of palindromic 34‐base‐pair DNA sequences called loxP sites.1 If Cre expression is under the control of tissue‐ and/or cell‐lineage‐specific promoters and enhancers, then recombination‐mediated deletion of the loxP‐flanked sequence can be limited to specific tissues or cell types.2 A multitude of different strains of mice encoding tissue/cell‐type‐specific Cre transgenes or loxP‐flanked (floxed) alleles have been generated, many of which are commercially available. For most researchers today, generating conditional gene knockout mice simply requires purchasing and breeding the desired Cre and floxed mice.
Although Cre‐lox technology is a powerful way to investigate gene function, precautions must be taken during breeding and genotyping to detect and avoid common pitfalls, such as Cre toxicity and ectopic Cre expression.3, 4 For example, Cre expression can cause DNA damage even in the absence of loxP sites;5 this may be because mammalian genomes contain pseudo‐loxP sites at the frequency of 1.2 sites per 1 megabase.6, 7 Apart from this inherent Cre recombinase toxicity, introduction of Cre as a transgene may change the expression of proximal genes;3 this problem can be avoided if Cre is directly knocked into a specific locus in a way that minimizes disruption of gene expression. Additionally, ectopic Cre expression and recombination are frequently described, and in extreme cases can entail germline deletion.8, 9, 10, 11, 12 Therefore, it is important to employ thorough genotyping methods during breeding and include the relevant details in publications.8
FOXP3+ regulatory T (Treg) cells are essential for immune tolerance and homeostasis, leading to interest in studying the mechanisms controlling their development and function. Mice expressing Cre under control of the Foxp3 promoter are widely used to generate Treg‐specific gene knockouts. Currently, three strains of C57BL/6 mice expressing Cre under control of a Foxp3 promoter are commercially available: (i) Foxp3‐EGFP‐hCre mice with an EGFP‐hCre fusion protein driven by a transgenic Foxp3 promoter;13 (ii) Foxp3YFP‐Cre mice, which encode a YFP‐Cre fusion protein knocked‐in at the endogenous Foxp3 locus;14 and (iii) Foxp3EGFP‐Cre‐ERT2 mice encoding a form of Cre that is only active in the presence of tamoxifen.15 Of all of these strains, the Foxp3YFP‐Cre mice are the most widely used with hundreds of publications on PubMed.
Here we present a cautionary case report showing that Foxp3YFP‐Cre mice have the potential to exhibit spontaneous ectopic recombination in both somatic and germ tissues. These findings underscore the importance of testing for ectopic recombination events when using Foxp3YFP‐Cre mice and highlight the need for more thorough reporting of data validating the fidelity of Treg‐specific recombination when using this strategy.
Materials and methods
Animals
All animal protocols were approved by the University of British Columbia Animal Care Committee. Foxp3YFP‐Cre mice (Jackson Laboratory, Bar Harbor, ME; B6.129(Cg)‐Foxp3tm4(YFP/Cre)Ayr/J; 016959) and Insrfl/fl mice (Jackson Laboratory, 006955) were purchased and crossed with the intention of generating progeny with a Treg‐specific deletion of Insr.
Tissue harvest and DNA extraction
At the time of weaning, at 3 weeks of age, ear tissue was notched and processed with the KAPA Express Extract Kit (Sigma‐Aldrich, St Louis, MO; KK7101) according to the manufacturer's instructions. Briefly, it was enzymatically digested at 75° for 10 min and denatured at 95° for 5 min; the resultant tissue lysate was diluted 10‐fold with 10 mm Tris–HCl (pH 8.0–8.5) and stored at −20° before genotyping. To assess the relative degree of ectopic recombination in metabolic tissues, mice were killed and immediately perfused with phosphate‐buffered saline. Ear, brain, heart, visceral adipose tissue, liver and quadricep muscles were collected and homogenized in gentleMACS M tubes using the gentleMACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany), then processed with the KAPA Express Extract Kit.
Fluorescence‐activated cell sorting
To purify immune cells, spleens were minced through a 100‐µm cell strainer, then incubated with 1 ml red blood cell lysis buffer (0.8% NH4Cl, StemCell Technologies, Vancouver, BC, Canada). Cells were washed and stained using the antibodies listed in the Supplementary material (Appendix S1). To quantify ectopic recombination in major immune cell types, B cells (CD19+ CD3−), myeloid cells (CD19− CD3− NK1.1−), NK cells (CD19− CD3− NK1.1+), CD8+ T cells (CD3+ CD8+), conventional T (Tconv) cells (CD3+ CD4+ Foxp3‐YFP−), and Tregs (CD3+ CD4+ Foxp3‐YFP+) were sorted, then lyzed with the KAPA Express Extract Kit and diluted 10‐fold for genotyping.
PCR‐based genotyping
Polymerase chain reaction (PCR) was performed on tissue and cell lysates containing genomic DNA (gDNA) using the KAPA2G Fast HotStart Genotyping Mix (Sigma‐Aldrich, KK5621) according to the manufacturer's cycling protocol. Primers are listed in the Supplementary material (Appendix S1). PCR products were resolved on a 2% agarose gel containing SYBR Safe (ThermoFisher Scientific, Waltham, MA) and imaged using a UV gel documentation system.
Quantitative PCR
To assess the relative quantity of each allele in a gDNA sample, three sets of primers, each amplifying only one of the possible alleles, were used (see Supplementary material, Appendix S1). Quantitative PCR (qPCR) was performed with PerfeCTa SYBR Green FastMix (QuantaBio, Beverley, MA; 95074) on a ViiA 7 Real‐Time PCR System (ThermoFisher Scientific). Ccr5 and Il10rb were used as gDNA housekeeping genes. Data were normalized to ear tissue lysates from Foxp3YFP‐Cre mice, Insrfl/fl mice, and Foxp3YFP‐CreInsrwt/rcb mice (where wt denotes wild‐type and rcb denotes the allele created by Cre‐mediated recombination). To quantify Insr mRNA expression, total RNA was isolated from flow‐sorted immune cells using the OMEGA E.Z.N.A Total RNA Kit (Omega Bio‐Tek, Norcross, GA; R6834), reverse‐transcribed with qScript cDNA SuperMix (QuantaBio, 95048), and qPCR was performed as described above.
Results
Unexpected offspring genotype ratios during the breeding of Foxp3YFP‐Cre to Insrfl/fl mice
We previously found evidence suggesting that the Insr may have a Treg‐intrinsic function,16 and aimed to test the hypothesis that Treg cell function would be altered in the absence of Insr signaling. In a pilot cohort, we intended to compare Treg cells from Foxp3YFP‐CreInsrfl/fl mice (experimental) and Foxp3YFP‐CreInsrfl/wt littermates (control). To generate these mice, we purchased female Foxp3YFP‐Cre mice14 and male Insrfl/fl mice17 from the Jackson Laboratory and bred them according to the scheme shown in Fig. 1(a). As Foxp3 is located on the X chromosome, female mice require homozygous Cre expression to achieve recombination in 100% of their Treg cells. Therefore, the ultimate desired experimental genotypes were hemizygous Foxp3YFP‐Cre/y males and homozygous Foxp3YFP‐Cre/YFP‐Cre females. According to our breeding scheme, all mice of generation F2 and beyond were predicted to carry the Foxp3YFP‐Cre allele on all their X chromosomes (Fig. 1a).
Figure 1.
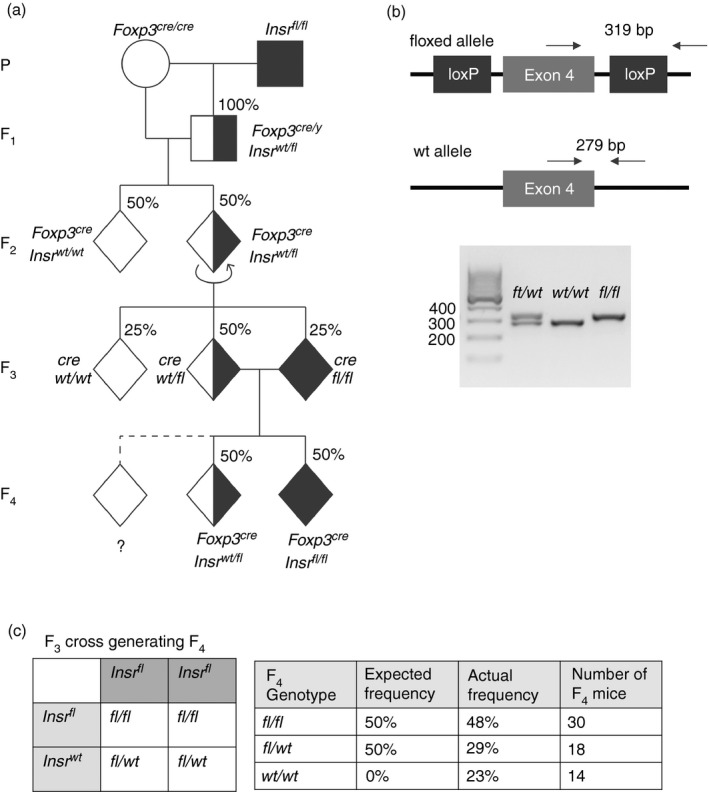
Unexpected offspring genotype ratio during the breeding of Foxp3YFP‐Cre to Insrfl/fl mice. (a) Breeding scheme to generate Foxp3YFP‐CreInsrfl/fl mice and Foxp3YFP‐CreInsrfl/wt littermate controls, with expected genotype ratios. Circles represent females, squares represent males, and diamonds represent mice of either sex; shading indicates the expected number of Insrfl alleles. The dashed line indicates progeny in the F4‐generation with an unexpected genotype. (b) Depiction of the conventional two‐band genotyping PCR strategy, in which primers flanking the loxP site that is 3′ of Insr exon 4 amplify different PCR products depending on the presence of a floxed or a wild‐type allele (top). Representative PCR products are resolved on a 2% agarose gel, with each lane representing one mouse (bottom). (c) Punnett square showing the expected F4‐generation genotype ratio from Foxp3YFP‐CreInsrfl/fl mice and Foxp3YFP‐CreInsrfl/wt parents (left). Table compares expected and actual F3 offspring ratios (right). n = 62 in the F4 generation.
To determine the genotype at the Insr locus, PCR was performed with ear‐notch tissue lysate using a recommended set of genotyping primers.17 As shown in Fig. 1(b), these primers flank the loxP site located 3′ to Insr exon 4, such that a floxed Insr allele yields a larger PCR product than the wild‐type allele (full annotated sequence shown in the Supplementary material, Appendix S1). Mice with both PCR products were considered heterozygous Insrfl/wt, whereas mice with only a smaller or a larger PCR product were considered homozygous Insrwt/wt or Insrfl/fl, respectively (Fig. 1b, bottom).
While tracking genotypes through the generations of breeding we observed unexpected genotype ratios in our F4‐generation mice, which are the offspring of F3‐generation Foxp3YFP‐CreInsrfl/wt and Foxp3YFP‐CreInsrfl/fl mice. We anticipated 50% homozygous Insrfl/fl mice and 50% heterozygous Insrfl/wt mice in F4 (Fig. 1c, left); instead, 48% were Insrfl/fl, 29% were Insrfl/wt, and 23% appeared to be Insrwt/wt (Fig. 1c, right; n = 62), which would have been an impossibility had the genotyping of the of the F3 parents been correct (Fig. 1a, dashed line). These results were verified by genotyping of repeat samples of ear‐notch tissue lysates from F3 parents and F4 offspring (data not shown), and Sanger sequencing was used to confirm that the unexpected PCR product was indeed the 279‐bp sequence of the Insrwt allele.
The conventional two‐band PCR strategy does not identify mice with germline‐recombined alleles
The conventional two‐band PCR strategy failed to detect one possible genotype, i.e. the recombined allele, which would yield no PCR product with these primers because the forward primer targeted sequence between the two loxP sites, which would be excised after Cre‐lox recombination (Fig. 2a). Therefore, using the conventional genotyping protocol and ear‐notch lysates, mice with a global Insrfl/rcb genotype (where rcb denotes the allele created by Cre‐mediated recombination) would appear the same as Insrfl/fl mice, with a single 319‐bp PCR product from only the floxed allele. Similarly, globally heterozygous Insrwt/rcb mice would appear the same as homozygous Insrwt/wt mice.
Figure 2.
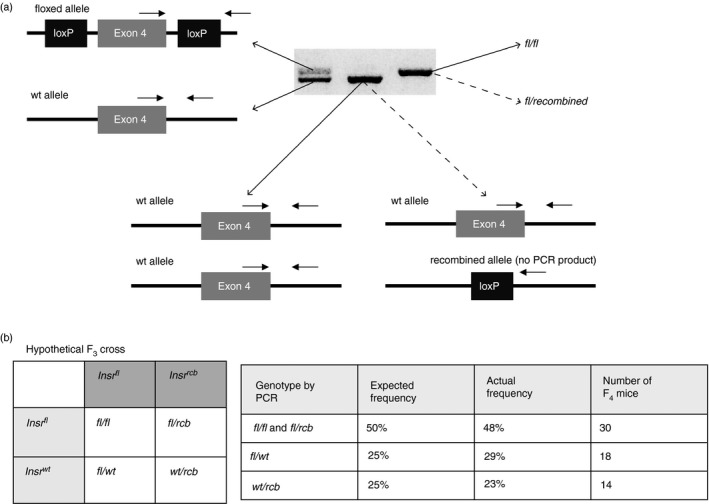
The conventional two‐band genotyping PCR strategy does not distinguish mice with germline‐recombined alleles. (a) Schematic diagram of the conventional two‐band genotyping PCR primers illustrating that mice with a germline‐recombined (rcb) Insr allele would yield no PCR product and that Insrwt/wt and Insrwt/rcb mice would appear identical as would Insrfl/fl and Insrfl/rcb mice. (b) Punnett square with a hypothetical F3 cross, in which Foxp3YFP‐CreInsrfl/rcb mice (mistaken as Foxp3YFP‐CreInsrfl/fl) are crossed with Foxp3YFP‐CreInsrfl/wt mice (left). Table compares hypothetical and actual F4 genotype ratios (right). n = 62 in the F4 generation.
Taking this possible misleading genotyping result into consideration, it was possible that Foxp3YFP‐CreInsrfl/rcb mice instead of Foxp3YFP‐CreInsrfl/fl mice were mistakenly used as parents in the F3 cross. Indeed, an F3 cross of Foxp3YFP‐CreInsrfl/wt with Foxp3YFP‐CreInsrfl/rcb would produce F4‐generation PCR product ratios consistent with the observed ratios with an equal distribution of Insrfl/fl, Insrfl/rcb, Insrfl/wt and Insrwt/rcb genotypes (all with Foxp3YFP‐Cre) (Fig. 2b, left). Using the conventional two‐band PCR ear‐notch lysate strategy, the Insrfl/fl and Insrfl/rcb alleles would appear the same and account for up to 50% of offspring; and 25% of mice would be Insrwt/rcb and appear the same as Insrwt/wt mice; these ratios are in line with our actual F4 genotype ratios (Fig. 2b, right). Taken together, this hypothetical F3 cross could have explained the unexpected F4 genotype ratios as well as the appearance of mice with Insrwt alleles, and suggested that both Insrfl/rcb and Insrwt/rcb genotypes may be present in our breeding cohort.
Ectopic germline recombination confirmed with a three‐band PCR strategy
To more accurately genotype our mice, we designed new PCR primers so that all three Insr alleles (wild‐type, floxed and recombined) could be distinguished. These primers flanked both loxP sites surrounding exon 4 such that each allele yielded a PCR product of a different size: wild‐type (1983 bp), floxed (2150 bp), and recombined (209 bp; Fig. 3a; and see Supplementary material, Appendix S1). To validate the primer design, PCR was performed with the cell lysates of Foxp3‐Cre‐YFP‐ Tconv cells and Foxp3‐Cre‐YFP+ Treg cells, which were sorted from spleens of what we had previously presumed to be Foxp3YFP‐creInsrwt/wt, Foxp3YFP‐creInsrfl/wt or Foxp3YFP‐creInsrfl/fl mice (Fig. 3b). In Treg cells, expected PCR products were observed in all three genotypes, validating the three‐band PCR strategy. However, in Tconv cells from Foxp3YFP‐CreInsrfl/fl mice, there was a 209‐bp PCR product from the recombined allele in addition to the 2150‐bp product from the floxed allele. The presence of the recombined allele in Tconv cells could have been due to: (i) contamination of the Tconv population with Treg cells; (ii) ‘ex‐Treg’ cells, which are Foxp3− cells that previously expressed Foxp3;18 and/or (iii) ectopic recombination activity of the YFP‐Cre.
Figure 3.
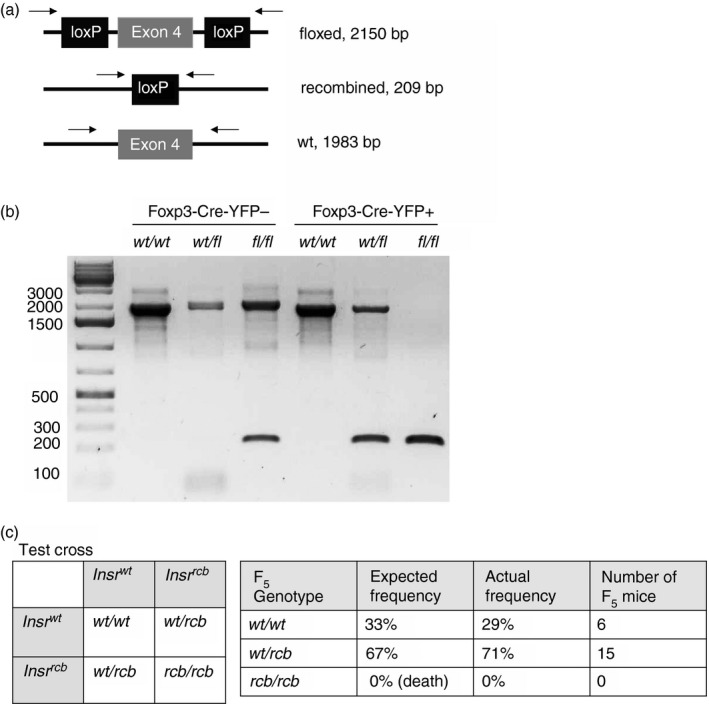
A three‐band PCR strategy confirms the presence of mice with ectopic germline recombination. (a) Schematic diagram depicting the expected PCR products from primers flanking both loxP sites. (b) Foxp3‐YFP‐Cre– conventional T (Tconv) cells and Foxp3‐YFP‐Cre+ regulatory T (Treg) cells were sorted from the spleens of Foxp3YFP‐Cre mice, Foxp3YFP‐CreInsrfl/wt mice, and Foxp3YFP‐CreInsrfl/fl mice. PCR was performed with cell lysates, and PCR products were resolved on a 1% agarose gel. Each lane represents one mouse. (c) Punnett square showing the expected offspring genotype ratio of a test cross of Foxp3YFP‐CreInsrwt/rcb parents (left). Table compares expected and actual test‐cross offspring ratios (right). (b) Data from two independent experiments. (c) n = 21 in the F5 generation.
PCR of ear‐notch tissue lysates of F4‐generation mice yielded the 209‐bp recombined product in nearly all samples assayed, confirmed by Sanger sequencing (data not shown). This suggests that even Foxp3YFP‐creInsrfl/fl or Foxp3YFP‐creInsrfl/wt mice without a predicted germline‐recombined Insrrcb allele exhibit frequent ectopic recombination in their ear tissues.
Having validated that the three‐band PCR genotyping approach could distinguish Insrwt/rcb and Insrwt/wt genotypes, we re‐genotyped the F4‐generation mice, identified putative Foxp3YFP‐CreInsrwt/rcb mice, and performed a test cross among themselves to ask if there was vertical transmission of the Insrrcb allele (Fig. 3c, left). As complete knockout of the Insr leads to postnatal diabetes and death,19 no homozygous Foxp3YFP‐CreInsrrcb/rcb mice would survive. Consistent with the predicted frequencies, the test cross produced 29% of Foxp3YFP‐CreInsrwt/wt and 71% of Foxp3YFP‐CreInsrwt/rcb mice (Fig. 3c, right; n = 21). This result confirms the presence of Foxp3YFP‐CreInsrwt/rcb mice in the F4 generation, supports the hypothetical F3 cross in Fig. 2(b), and demonstrates that ectopic germline recombination occurred before the F4 generation.
A quantitative PCR strategy quantifies the extent of ectopic recombination in genomic DNA
In order to determine the extent of ectopic recombination within each animal, we turned to a qPCR‐based method. We designed three pairs of primers, each amplifying only a single allele: wild‐type, floxed or recombined (Fig. 4a). Primers A and B amplify only the wild‐type allele, because the primer A binding site is disrupted by the 5′ loxP site; primers C and D amplify only the floxed allele; and primers E and F amplify only the recombined allele, as the potential PCR product from a floxed allele would be too large to form a product within the timeframe of qPCR (Fig. 4a). Note that all primers targeted introns, so only genomic DNA and not mRNA was amplified. For reference genes, we chose wild‐type Ccr5 and Il10rb, which should be homozygous in all mice.
Figure 4.
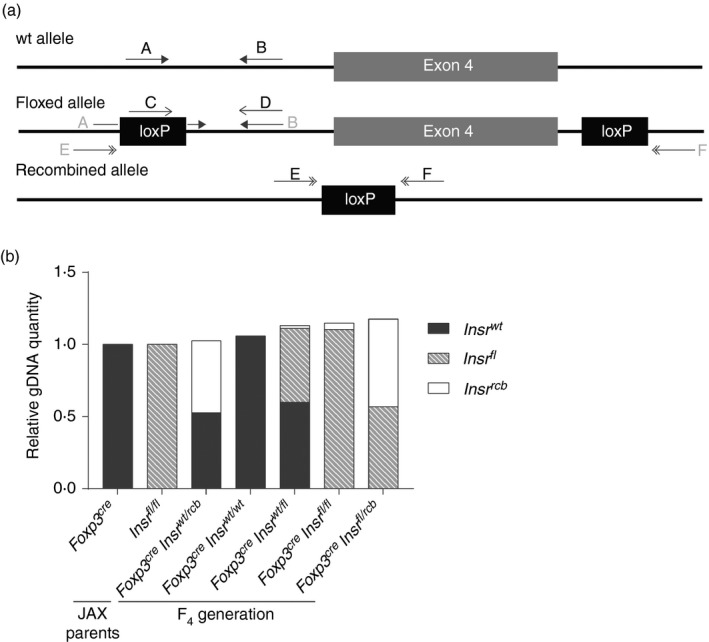
A quantitative PCR strategy quantifies the extent of unwanted ectopic recombination in gDNA. (a) Schematic diagram of the location of three sets of qPCR primers specifically designed to amplify one Insr allele: wild‐type, floxed or recombined. (b) Quantitative PCR was performed with ear‐notch tissue lysates with these three primer pairs. The relative amounts of each allele were quantified and normalized to those of a Foxp3YFP‐Cre mouse, an Insrfl/fl mouse or a Foxp3YFP‐CreInsrwt/rcb mouse. Each column represents one mouse; data are representative of two independent experiments.
By using Foxp3YFP‐Cre, Insrfl/fl and Foxp3YFP‐CreInsrwt/rcb mice as the normalizing controls for Insrwt, Insrfl and Insrrcb alleles, respectively, we were able to estimate the relative abundance of the three Insr alleles within each animal (Fig. 4b). With this qPCR method, we genotyped all F4‐generation mice, and were not only able to distinguish Insrfl/fl from Insrfl/rcb mice, but were also able to quantify the relative amount of ectopic recombination (2%–40% of total gDNA) in the ear‐tissue gDNA of Foxp3YFP‐CreInsrfl/fl mice (data not shown), enabling us to select mice with minimal ectopic recombination for further breeding and experimentation.
Quantification of ectopic recombination across metabolic tissues and immune cell types
We found that there were varying degrees of ectopic recombination in the ear‐tissue gDNA of Foxp3YFP‐CreInsrfl/fl mice. As we intended to use these mice to study the role of Treg cells in the context of metabolism, we also asked whether ectopic recombination was present in immune cell types and/or key metabolic tissues, namely the brain, fat, liver and muscle. We selected four F4‐generation mice with the least (< 5%) ectopic recombination in their ear tissue and subjected them to whole‐animal perfusion with phosphate‐buffered saline to eliminate possible contamination with circulating Treg cells, then extracted DNA from brain, visceral adipose tissue, liver and skeletal muscle. Using the qPCR method, we observed a small amount of ectopic recombination (2%–5% of total gDNA) in all tissues examined in Foxp3YFP‐CreInsrfl/fl mice (Fig. 5a), which might be at least partially attributable to tissue‐resident Treg cells that were not removed by perfusion.
Figure 5.
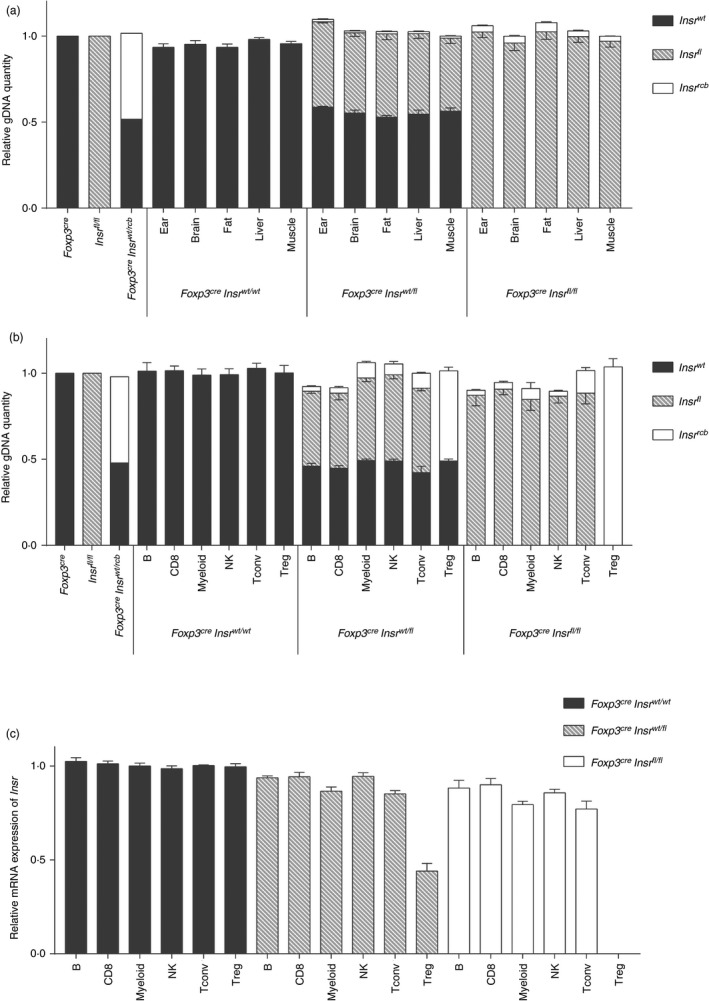
Quantification of ectopic recombination across metabolic tissues and immune cell types. (a,b) Quantitative PCR was performed using three pairs of allele‐specific primers on the gDNA obtained from (a) ear, brain, fat, liver and muscle tissue lysates and (b) Sorted B cells, CD8+ T cells, myeloid cells, natural killer (NK) cells, conventional T (Tconv) cells and regulatory T (Treg) cells. Data were normalized to the gDNA from ear‐tissue lysates of a Foxp3YFP‐Cre mouse, an Insrfl/fl mouse, or a Foxp3YFP‐CreInsrwt/rcb mouse. Error bars represent standard errors (n = 4 per genotype). (c) Insr mRNA expression by qPCR with mRNA from sorted immune cells. Data were normalized within each immune cell group to those obtained from Foxp3YFP‐CreInsrwt/wt mice. Error bars represent standard errors (n = 4 per genotype).
We also examined the extent of ectopic recombination in several immune cell types sorted from the spleens of Foxp3YFP‐CreInsrfl/fl mice. All examined immune cell types exhibited a low amount of Insr recombination, with the greatest in myeloid cells and Tconv cells (10%–15% of their total gDNA) (Fig. 5b). Analysis of Insr mRNA showed a similar trend (Fig. 5c). Collectively, these data show that mice with < 5% ectopic recombination in their ear tissue also exhibited limited recombination across multiple metabolic tissues and immune cell types. Therefore, the use of more precise PCR strategies is an effective way to screen mice to identify those with the desired tissue‐restricted gene deletions.
Spontaneous ectopic Cre recombination occurs as early as the first generation of breeding
Finally, to exclude the possibility that ectopic Cre recombination was the result of a problem with the original breeders, we purchased new Foxp3YFP‐Cre mice and Insrfl/fl mice from the Jackson Laboratory. In a second experimental cohort, we intended to use Foxp3YFP‐CreInsrfl/fl (experimental) as before, and Foxp3YFP‐CreInsrwt/wt mice as controls. We established a revised breeding scheme (Fig. 6) and incorporated the qPCR‐based method to select mice with low ectopic recombination as breeders for the next generation. All genotype ratios and the incidence of ectopic recombination were documented throughout all four generations (Table 1). Other than the Foxp3YFP‐CreInsrwt/wt offspring, all mice generated displayed varying degrees of ectopic recombination (2%–65% of total gDNA). We considered mice with > 40% ectopic recombination to harbor a germline Insrrcb allele, mice with < 5% ectopic recombination to be Insrfl, and mice with 5%–40% ectopic recombination to have a mixed population of cells with varying proportions of cells with Insrrcb and/or Insrfl alleles, denoted as Insrfl‐r. Ectopic Cre‐mediated recombination was observed as early as the F1 generation, with up to half of male heterozygous Foxp3YFP‐CreInsrfl/wt mice exhibiting some degree of ectopic recombination in ear tissues. Spontaneous germline recombination first appeared in the F2 generation with the emergence of Foxp3YFP‐CreInsrwt/rcb mice, despite the stringent selection of breeders (Table 1). Moreover, the F3 generation yielded a low proportion of mice with the desired genotypes: only 5% of mice were truly Foxp3YFP‐CreInsrfl/fl, and 14% were truly Foxp3YFP‐CreInsrwt/wt, instead of the predicted 25% each (Table 1).
Figure 6.
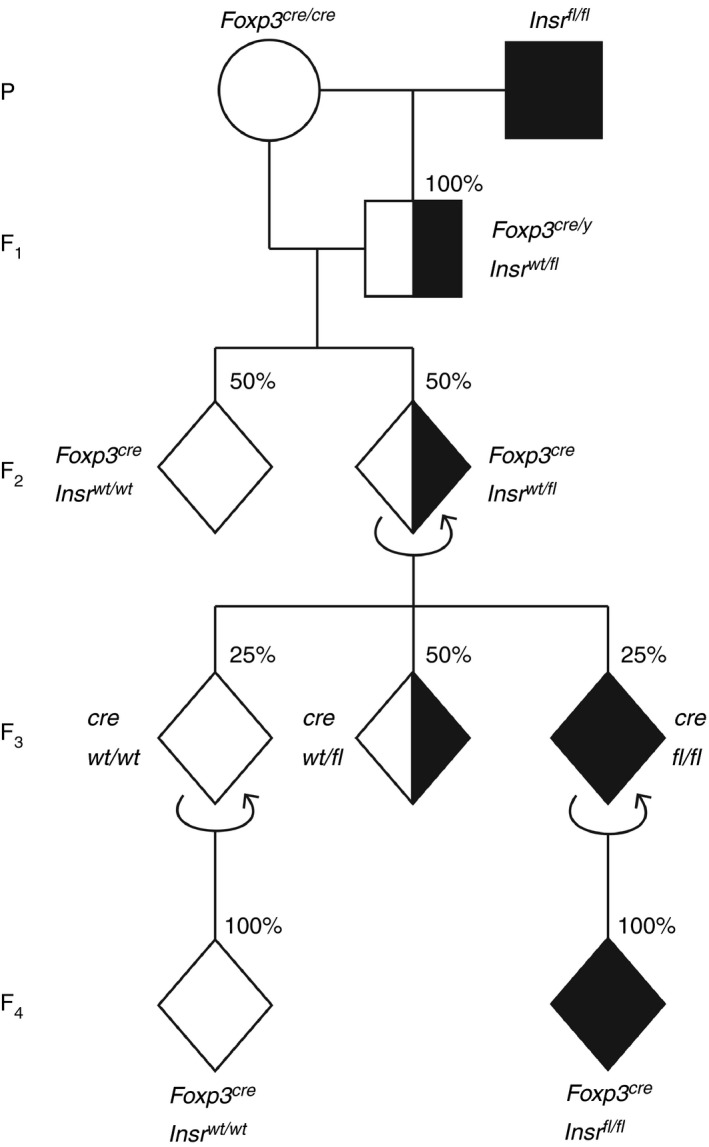
A second cohort reveals spontaneous ectopic Cre‐mediated recombination as early as the first generation of breeding. Breeding scheme to generate a second cohort of Foxp3YFP‐CreInsrfl/fl mice (experimental) and Foxp3YFP‐CreInsrwt/wt mice (control). Circles represent females, squares represent males, and diamonds represent mice of either sex; shading indicates the number of Insrfl alleles. The expected genotype ratios, assuming no ectopic recombination, are shown. Experimental outcomes are shown in Table 1.
Table 1.
Genotype ratios of all mice in the second breeding cohort from Figure 6
| F1 | Male (n = 18) | Female | Total (n = 18) |
|---|---|---|---|
| Foxp3YFP‐Cre/yInsrwt/fl | 9 (50%) | No data | 9 (50%) |
| Foxp3YFP‐Cre/yInsrwt/fl‐r | 9 (50%) | No data | 9 (50%) |
| F2 | Male (n = 66) | Female (n = 53) | Total (n = 119) |
|---|---|---|---|
| Foxp3YFP‐CreInsrwt/fl | 8 (12%) | 8 (15%) | 16 (13%) |
| Foxp3YFP‐CreInsrwt/fl‐r | 23 (35%) | 18 (34%) | 41 (34%) |
| Foxp3YFP‐CreInsrwt/wt | 29 (44%) | 21 (40%) | 50 (42%) |
| Foxp3YFP‐CreInsrwt/rcb | 6 (9%) | 6 (11%) | 12 (10%) |
| F3 | Male (n = 81) | Female (n = 86) | Total (n = 167) |
|---|---|---|---|
| Foxp3YFP‐CreInsrfl/fl | 4 (5%) | 4 (5%) | 8 (5%) |
| Foxp3YFP‐CreInsrfl/fl‐r | 5 (6%) | 12 (14%) | 17 (10%) |
| Foxp3YFP‐CreInsrfl/rcb | 11 (14%) | 4 (5%) | 15 (8%) |
| Foxp3YFP‐CreInsrwt/fl | 4 (5%) | 7 (8%) | 11 (7%) |
| Foxp3YFP‐CreInsrwt/fl‐r | 34 (42%) | 39 (45%) | 73 (43%) |
| Foxp3YFP‐CreInsrwt/wt | 11 (14%) | 11 (13%) | 22 (13%) |
| Foxp3YFP‐CreInsrwt/rcb | 12 (15%) | 9 (10%) | 21 (12%) |
| F4 (knockout) | Male (n = 88) | Female (n = 75) | Total (n = 163) |
|---|---|---|---|
| Foxp3YFP‐CreInsrfl/fl | 43 (49%) | 36 (48%) | 79 (48%) |
| Foxp3YFP‐CreInsrfl/fl‐r | 45 (51%) | 39 (52%) | 84 (52%) |
Using a qPCR genotyping strategy, mice with > 40% ear tissue gDNA containing ectopic recombination were considered to have a Insrrcb allele; ectopic recombination within 5–40% of ear tissue gDNA was denoted as Insrfl‐r; and < 5% ectopic recombination was considered normal (Insrfl)
Discussion
Here we have shown that breeding of Foxp3YFP‐Cre mice and Insrfl/fl mice led to ectopic recombination of the floxed Insr allele. Half of the immediate offspring of the cross had severe ectopic recombination (up to 40% of non‐Treg gDNA) as identified by analysis of their ear‐notch tissues. Germline recombination occurred as early as the F2 generation, despite efforts to stringently select animals for breeding in each generation. Collectively, our experience with the Foxp3YFP‐Cre mice highlights the importance of careful breeding and genotyping at every generation to identify and eliminate mice with unwanted germline and/or somatic cell recombination.
We chose to use Foxp3YFP‐Cre mice instead of transgenic Foxp3‐EGFP‐hCre mice to generate a Treg cell‐specific knockout to avoid unexpected phenotypes that could result from random transgene integration in the latter mouse. However, unlike the transgenic Foxp3‐EGFP‐hCre mice, which can be used as heterozygous animals, female Foxp3YFP‐Cre mice require homozygous Cre expression to achieve recombination in 100% of their Treg cells. Although there is evidence that maternal inheritance of the Cre allele can increase ectopic recombination frequency,11, 20, 21 a breeding scheme in which the YFP‐Cre allele is only transmitted paternally would not allow the generation of male offspring with the desired genotype. Hence, in our breeding strategy, maternal transmission of the Cre allele could have contributed to enhanced ectopic recombination.
Another consideration relevant to the use of Cre homozygous mice is that dose‐dependent Cre toxicity has been described in both mammalian cell lines22 and mouse models.23, 24, 25 However, in the case of X‐linked Cre expression, toxicity in females is decreased because of X‐inactivation.26 As previously reported,14 and consistent with our observation, the proportion of splenic Treg cells in female Foxp3YFP‐Cre/YFP‐Cre mice is similar to that in wild‐type mice, suggesting that the knock‐in of YFP‐Cre does not affect Treg cell development. It is possible that transient YFP‐Cre expression in homozygous female gametes and/or in early embryos before X‐inactivation, could favor ectopic Cre recombination. However, our data suggest otherwise because across all generations, genotype ratios with recombined Insr alleles were not different between males and females.
Quantitative PCR on ear‐tissue lysates revealed that, other than the offspring Foxp3YFP‐CreInsrwt/wt mice, all mice generated in the second experimental cohort displayed varying degrees of ectopic recombination, ranging from 2% to 65% of total gDNA. Therefore, a qPCR genotyping strategy is superior to the conventional PCR genotyping methods, allowing more accurate selection of mice with low ectopic recombination. We elected to only use mice with < 5% ectopic recombination for further experimentation (manuscript in preparation). Mice with > 40% ectopic recombination likely inherited the recombined allele (i.e. the recombination took place in the parents) whereas mice with 5%–40% ectopic recombination likely resulted from transient Cre expression during embryonic development. We did not find any mice with a Foxp3YFP‐CreInsrrcb/fl‐r genotype (i.e. > 65% ectopic recombination); such mice likely could not survive because of insufficient amounts of Insr.
A major question is how often ectopic Cre‐mediated recombination occurs when using Foxp3YFP‐Cre mice to generate Treg cell‐specific knockouts. In the original report of the Foxp3YFP‐Cre mice, crosses between Foxp3YFP‐Cre and Rosa26fl‐Stop‐fl‐YFP reporter mice showed varying degrees (2%–10%) of ectopic recombination in a number of immune cell types, including B cells, T cells, myeloid cells, and bone marrow precursor cells; although it was not clear whether the Cre was inherited paternally or maternally and data from non‐immune cells were not reported.14 More recently, a comprehensive study characterized offspring from Foxp3YFP‐Cre mice crossed to Rosa26fl‐Stop‐fl‐tdTomato reporter mice, found stochastic activity of the Foxp3 promoter and widespread expression of tdTomato in immune and non‐immune tissues.27 In line with our findings, this study found evidence of ectopic recombination during fetal development and vertical transmission of recombined alleles from Cre‐positive parents to Cre‐negative offspring.27 Moreover, in the progeny of Foxp3YFP‐Cre and Cd28fl/fl mice, ~30% of Tconv cells were found to be CD28‐deficient.28 In the same study, the authors noted unpublished data in which Foxp3YFP‐CreBcl6fl/fl mice also exhibited ectopic recombination.28 Hence, evidence for ectopic recombination with Foxp3YFP‐Cre mice is not limited to our observations with the Insr allele.
It is likely that the degree of Cre‐mediated ectopic recombination depends on the DNA accessibility of the floxed allele. Indeed it is known that a given Cre‐expressing mouse can have different recombination efficiencies for different floxed genes in different cell types. For example, transgenic CD19‐Cre mediates efficient recombination of Rosa26fl‐Stop‐fl‐YFP in mature B cells but very little recombination in B‐cell progenitors.29 However, the same CD19‐Cre achieves complete recombination of floxed Mcl1 or Myb genes in B‐cell progenitors.30, 31 The Insr is ubiquitously expressed at all stages of development, meaning that it is likely a highly accessible locus that is more susceptible to transient/low levels of Cre expression.
Overall, this case report of Cre expression outside the expected cell type highlights that a great degree of caution must be used when using Foxp3YFP‐Cre mice, or indeed any Cre‐based system, to generate tissue‐specific deletions. Had we failed to notice the non‐Mendelian genotype ratios in the F4 generation we would have proceeded to carry out experiments with incorrectly genotyped mice. As most studies using Foxp3YFP‐Cre mice do not provide detailed information on genotyping and breeding strategies, or consider the possibility of Cre activity outside the immune lineage, our findings raise the possibility that some published experiments using Foxp3YFP‐Cre mice may have produced misleading results.
Our data also show that PCR‐based methods can be easily adapted to avoid this problem by monitoring target gene recombination across multiple cell and tissue types. During breeding, one should employ PCR primers to detect the recombined allele, in addition to the traditional detection of the floxed and wild‐type alleles. Moreover, in at least a subset of experimental animals, (q)PCR should be performed with the gDNA from a series of cell types and tissues unrelated to the Cre‐driver, to rule out the possibility of non‐tissue/cell type‐specific deletion. With this more rigorous genotyping method, mice with the desired genotypes can be successfully identified and used to study the biology of Treg cells.
Disclosures
MKL received research funding from TxCell, Bristol‐Myers Squibb, Pfizer, Takeda and CRISPR Therapeutics for work unrelated to this report.
Author contributions
DW conceived the study, performed experiments, analyzed data, interpreted results and wrote the manuscript. QH performed experiments. PCO assisted in experimental design and interpretation and critically reviewed the manuscript. MKL conceived the study, assisted in experimental design and interpretation, revised the manuscript and secured funding.
Supporting information
Appendix S1. Annotated sequence of Insr gene exon 4, PCR primers, and antibodies used for florescence activated cell sorting.
Acknowledgements
This work was supported by the Canadian Diabetes Association (OG‐3‐14‐4460‐ML) and the Canadian Institutes of Health Research (CIHR) (FDN‐154304). DW is supported by a CIHR Graduate Award and MKL receives salary awards from BC Children's Hospital Research Institute.
References
- 1. Orban PC, Chui D, Marth JD. Tissue‐ and site‐specific DNA recombination in transgenic mice. Proc Natl Acad Sci USA 1992; 89:6861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis 2000; 26:99–109. [PubMed] [Google Scholar]
- 3. Schmidt‐Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol 2007; 8:665–8. [DOI] [PubMed] [Google Scholar]
- 4. Becher B, Waisman A, Lu LF. Conditional gene‐targeting in mice: problems and solutions. Immunity 2018; 48:835–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre‐dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci USA 2000; 97:13702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thyagarajan B, Guimaraes MJ, Groth AC, Calos MP. Mammalian genomes contain active recombinase recognition sites. Gene 2000; 244:47–54. [DOI] [PubMed] [Google Scholar]
- 7. Semprini S, Troup TJ, Kotelevtseva N, King K, Davis JR, Mullins LJ et al Cryptic loxP sites in mammalian genomes: genome‐wide distribution and relevance for the efficiency of BAC/PAC recombineering techniques. Nucleic Acids Res 2007; 35:1402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Song AJ, Palmiter RD. Detecting and avoiding problems when using the Cre‐lox system. Trends Genet 2018; 34:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liput DJ. Cre‐Recombinase dependent germline deletion of a conditional allele in the Rgs9cre mouse line. Front Neural Circuits 2018; 12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martens K, Bottelbergs A, Baes M. Ectopic recombination in the central and peripheral nervous system by aP2/FABP4‐Cre mice: implications for metabolism research. FEBS Lett 2010; 584:1054–8. [DOI] [PubMed] [Google Scholar]
- 11. Zhang J, Dublin P, Griemsmann S, Klein A, Brehm R, Bedner P et al Germ‐line recombination activity of the widely used hGFAP‐Cre and nestin‐Cre transgenes. PLoS ONE 2013; 8:e82818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He Y, Sun X, Wang L, Mishina Y, Guan JL, Liu F. Male germline recombination of a conditional allele by the widely used Dermo1‐cre (Twist2‐cre) transgene. Genesis 2017; 55: e23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT et al Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med 2008; 205:1983–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X et al Regulatory T cell‐derived interleukin‐10 limits inflammation at environmental interfaces. Immunity 2008; 28:546–58. [DOI] [PubMed] [Google Scholar]
- 15. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D et al Stability of the regulatory T cell lineage in vivo. Science 2010; 329:1667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Han JM, Patterson SJ, Speck M, Ehses JA, Levings MK. Insulin inhibits IL‐10‐mediated regulatory T cell function: implications for obesity. J Immunol 2014; 192:623–9. [DOI] [PubMed] [Google Scholar]
- 17. Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D et al A muscle‐specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 1998; 2:559–69. [DOI] [PubMed] [Google Scholar]
- 18. Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev 2014; 259:173–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu Rev Physiol 2003; 65:313–32. [DOI] [PubMed] [Google Scholar]
- 20. Heffner CS, Herbert Pratt C, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR et al Supporting conditional mouse mutagenesis with a comprehensive Cre characterization resource. Nat Commun 2012; 3:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cochrane RL, Clark SH, Harris A, Kream BE. Rearrangement of a conditional allele regardless of inheritance of a Cre recombinase transgene. Genesis 2007; 45:17–20. [DOI] [PubMed] [Google Scholar]
- 22. Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R et al Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci USA 2001; 98:9209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastru W, Sala V et al High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci 2006; 26:9593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T et al Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J Immunol 2009; 182:5633–40. [DOI] [PubMed] [Google Scholar]
- 25. Bersell K, Choudhury S, Mollova M, Polizzotti BD, Ganapathy B, Walsh S et al Moderate and high amounts of tamoxifen in αMHC‐MerCreMer mice induce a DNA damage response, leading to heart failure and death. Dis Model Mech 2013; 6:1459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Avner P, Heard E. X‐chromosome inactivation: counting, choice and initiation. Nat Rev Genet 2001; 2:59–67. [DOI] [PubMed] [Google Scholar]
- 27. Bittner‐Eddy PD, Fischer LA, Costalonga M. Cre‐loxP reporter mouse reveals stochastic activity of the Foxp3 promoter. Front Immunol 2019; 10:2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franckaert D, Dooley J, Roos E, Floess S, Huehn J, Luche H et al Promiscuous Foxp3‐cre activity reveals a differential requirement for CD28 in Foxp3+ and Foxp3– T cells. Immunol Cell Biol 2015; 93:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt‐Supprian M, Wunderlich FT, Rajewsky K. Excision of the Frt‐flanked neo (R) cassette from the CD19cre knock‐in transgene reduces Cre‐mediated recombination. Transgenic Res 2007; 16:657–60. [DOI] [PubMed] [Google Scholar]
- 30. Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL‐1. Nature 2003; 426:671–6. [DOI] [PubMed] [Google Scholar]
- 31. Thomas MD, Kremer CS, Ravichandran KS, Rajewsky K, Bender TP. c‐Myb is critical for B cell development and maintenance of follicular B cells. Immunity 2005; 23:275–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Annotated sequence of Insr gene exon 4, PCR primers, and antibodies used for florescence activated cell sorting.


