Abstract
Aim
Plasmodium falciparum malaria predominantly affects children residing in endemic areas. However, recently a significant number of Malawian adults, who otherwise should have attained some malaria-specific immunity, have been observed to succumb to either uncomplicated malaria (UM) or severe malaria (SM). In addition, it is still unknown whether HIV is a contributing factor to SM in Malawian non-pregnant adults. We conducted this study to determine clinical and demographic features that characterize Malawian adults presenting with UM or SM.
Methods
Study participants were recruited when they presented at Queen Elizabeth Central Hospital (QECH), Blantyre, Malawi with UM or SM and had their demographic details recorded after consenting to participate in the study. Malaria infection was confirmed by Malaria Rapid Diagnostic Test (MRDT) and malaria slide microscopy. A venous blood sample was collected before treatment for various analyses.
Results
Both HIV-infected and HIV-uninfected participants were at risk of presenting with either UM or SM although there were more (37%) SM cases among those who were HIV-infected compared to those who were HIV-uninfected (28%) but the difference was not significant but similar numbers of UM cases (61% for HIV-uninfected and 60% for HIV-infected). Previous visit to areas of high malaria transmission was not associated with presenting with either UM or SM. HIV/malaria co-infected participants were more likely to be found with a positive blood culture result (Diphtheriods, Stenotophomonas maltophilia, Salmonella typhimurium and Staphylococcus aureus) and were at a higher risk of dying compared to HIV-uninfected malaria patients.
Conclusion
Although HIV-infection was associated with high mortality in those co-infected with HIV and malaria, recurrent malaria episodes and having other diseases co-existing with malaria infection were the main factors associated with the development of SM in this study cohort. Further studies need to be done to determine how the host immunity is affected in those co-infected with HIV and malaria.
Keywords: Plasmodium falciparum malaria, HIV-infection, Malawian adults
Introduction
Malaria continues to cause serious health concerns in low- and middle-income countries (LMICs)1 since in 2016 over a billion people were at risk from contracting malaria worldwide with and as many as 212 million clinical episodes of malaria were reported leading to as many as 445,000 deaths, the majority of whom were African children presenting with P. falciparum malaria.2 Clinical P. falciparum malaria presents either as uncomplicated malaria (UM) or as one of the following severe forms of the disease: cerebral malaria (CM), severe malarial anemia (SMA), metabolic acidosis (MA) or respiratory distress (RD) and other complications including some overlap syndromes.2,3 Immunity to malaria disease, but not to infection, is both humoral and cell-mediated with various mechanisms involved.4 Antibodies that develop through exposure to P. falciparum play a role,4 and the involvement of different cell subsets has been implicated in both protection against and pathogenesis of malaria.5,6 Malaria-specific protective immunity develops with age and exposure.7 Thus, adults under continuous exposure to P. falciparum antigens should normally have an effective immunity against malaria disease.8 This being the case, much attention is given to investigating interventions aimed at preventing and treating malaria in children.9,10
Lately, some countries in which malaria is endemic including Malawi, have observed that a substantial number of adults are getting infected with both UM and SM.9–11 Considering that HIV has been shown to increase the risk of malaria infection in adults and malaria-related in-hospital mortality,12,13 some had suspected that most of those adults presenting with different forms of malaria could be HIV-infected.
Alternatively, it is possible that a change in transmission patterns may mean that adults in some areas are not as exposed to P. falciparum parasite as before resulting in the loss of immunity against malaria. It is also possible that various transmission reduction initiatives currently being implemented in Malawi might be reducing exposure of individuals of various age-groups to the malaria parasite earlier on in life thereby reducing and delaying the development of the malaria-specific immunity. In addition, traveling to malaria high-risk areas could also be putting otherwise semi-immune adults at an additional risk of acquiring malaria.
This study was therefore conducted with the aim of investigating factors that are contributing toward an observed increase in the burden of UM and SM in adults in Blantyre, Malawi and to clinically characterize the adult malaria patients treated at the hospital, screening for other possible co-morbidities.
Methodology
Study Setting and Sample Size
This study was conducted in the Accidents, Emergency and Trauma (AET) Centre and general medical male and female wards of Queen Elizabeth Central Hospital (QECH), Blantyre, Malawi from July 2016 to March 2017. QECH is the main referral hospital in the city of Blantyre, the southern part of Malawi. Blantyre is located on the Shire Highlands and as such malaria is seasonal coinciding with the rains and transmission is lower compared to some of the surrounding districts such as Chikwawa, Nsanje (in the lower Shire), Thyolo and Mulanje (on the Eastern side) and Mwanza (on the western side) where malaria is highly endemic throughout the year.14 The intended sample size was 160, 80 presenting with UM and 80 presenting with SM but we only managed to screen 116 potential participants. Of the 116 malaria-infected adults who were screened, 107 (57 females and 50 males). Of the 107 participants recruited, 30 (28%) were HIV-infected and on ARTs, 76 (71%) were HIV un-infected and the HIV status of one participant was unknown with discordant results from the follow-up two HIV tests and was therefore excluded from the final data analysis.
Study Design
This was a prospective cross-sectional study, in which adults with a diagnosis of UM or SM were recruited after obtaining consent from the patient or a legally able guardian. Case report forms (CRFs) were used to collect demographic information, history of malaria infection, HIV status from the patients’ health passports and history of travel. A 10 mL venous blood sample was collected from each participant for confirmation of malaria parasitaemia using Malaria Rapid Diagnostic Test (MRDT), malaria microscopy of thick and thin films, for HIV test, for analysis of full blood count (proportions and absolute cell counts for neutrophils, monocytes, platelets, basophils, eosinophils and lymphocytes), liver and renal function tests (creatinine, urea, bilirubin levels) and electrolytes (calcium, sodium, potassium, chloride), to assess for end-organ function and for standard blood cultures that would indicate any blood bacterial infections. HIV testing was performed using two rapid tests; Determine HIV1/HIV2 (Abbott Laboratories, Japan) and Unigold (Trinity Brotch, Dublin), according to the manufacturer’s instructions. Data on liver function, electrolyte tests and hematological analyses on this study cohort have already been published.15
Inclusion and Exclusion Criteria
Malaria was defined as a clinical syndrome without an apparent alternative cause, in the presence of a positive MRDT and thick blood film positive for P. falciparum asexual parasites on microscopy. Based on WHO guidelines, study participants with cerebral malaria, one form of SM, had a Glasgow Coma Score (GCS) of <11, with unarousable coma not attributable to any other cause, unable to localize, incomprehensible while those presenting with UM or other forms of SM had a score of greater than 11 at both times. Study participants presenting with severe malarial anemia (SMA) had a blood hemoglobin concentration of 7 g/dL or less or a hematocrit concentration of 20% or less together with a parasite count of more than 10,000/μL, while the rest had a hemoglobin concentration above this level. Study participants with liver or renal dysfunction were also included in the SM group. Study participants were checked on during the time they were still in the admission wards until the day of discharge or death. All outcomes for the respective admission were documented.
The inclusion criteria were adult (≥18 years) out- and in-patients at QECH that presented with a temperature >37.5°C, a positive MRDT with or without parasites on microscopy and signs consistent with malaria disease according to the WHO guidelines.16 Pregnant women and those who failed to give consent were not recruited.
Data Storage and Statistical Analysis
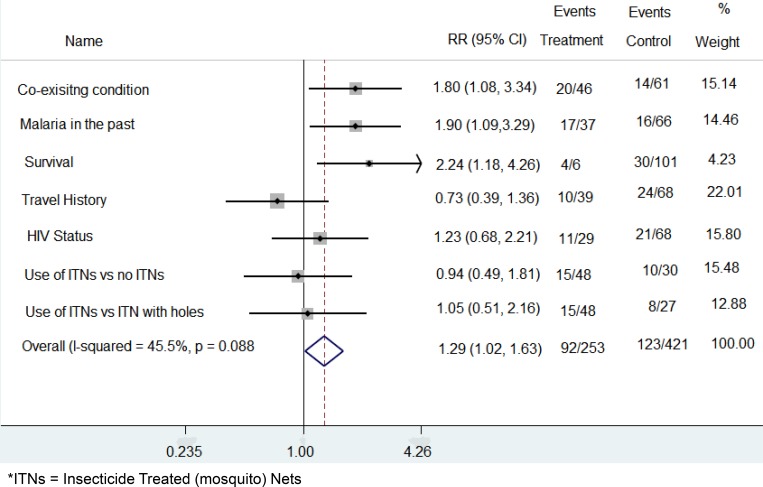
CRFs and consent forms were kept separately in folders which were stored in lockable drawers. Data were anonymized using study numbers and entered into an excel spreadsheet and statistical analysis was done using STATA software version 12. Data were stratified based on HIV status. Risk ratio for all the exposures in the study was calculated and graphically displayed using Forest Scatter Plots (Figure 1).
Figure 1.
Forest scatter plot showing the relationship between the different exposure and severe malaria. Event is the number of severe malaria patients in that group. The treatment group had exposure to the variable of interest, while the participants in the control group were not exposed. The weights depict how much each variable contributes to the overall exposures.
Exposures which were studied included HIV status, history of travel to areas of higher malaria transmission outside Blantyre city, use of insecticide-treated bed nets (ITNs) and the state in which the ITNs were in and presence of other disease conditions, and the outcomes of interest were UM, SM and death.
Participants were liable to recall bias and to address this potential bias, collateral sources of information were used to verify the past history, and these included the study participant and the guardian plus the information captured in the study participant’s health passport.
Ethical Approval
The study was approved by the College of Medicine Research and Ethics Committee (COMREC), University of Malawi. Each participant or an appropriate guardian provided informed written consent. This study was conducted in accordance with the Declaration of Helsinki.
Results
The data from these study participants have previously been published.15 In total, 107 participants were recruited who presented with UM and SM, of whom 57 (53%) were female. Of the participants, 30 (28%) were HIV-infected, 76 (71%) were HIV un-infected and the HIV status of one participant was unknown and was therefore excluded from the final data analysis. The HIV-uninfected population was much younger (mean age of 25 years) compared to the HIV-infected population (mean age of 35.5 years). All 30 HIV-infected participants already knew their HIV status at the time of recruitment and had been on antiretroviral treatment for variable durations although we did not collect data on the types regiments the different participants were on.
We analyzed the effect of various factors on severe malaria by Logical Regression Analysis and presented the results as raw figures (Supplementary Table 1) and in a forest scatter plot (Figure 1) respectively. Having one or more malaria episodes in the previous 12 months and having co-existing conditions were the two factors that positively and significantly contributed toward one presenting with SM (confidence interval of 95%). HIV infection and the other exposures which were measured, which did not show a significant association with SM.
There was a high rate of co-existing conditions such as influenza, diarrhea, coughing and non-malarial febrile illnesses in HIV-infected group, up to 56.7% in comparison to 36.8% in the HIV-uninfected group (Table 1). All deaths occurred only in the HIV-infected group with a mortality rate of 23.3% among HIV-infected participants. There were four blood-positive blood cultures in the HIV-infected group (one with Diphtheriods, one with Stenotophomonas maltophilia, one with Salmonella typhimurium and one with Staphylococcus aureus), while the HIV-uninfected group had only one recorded positive growth (Coagulase negative Staphylococcus).
Table 1.
Exposure and Outcome of the Study Participants Who Presented with Malaria in Relation to HIV Infection
| Total Participants = 107 | HIV Uninfected (n=76) | HIV Infected (n=30) | |
|---|---|---|---|
| Gender | Female (n=39) | Female (n=18) | |
| Median age (years) (range) | 25 (18–66) | 35.5 (20–67) | |
| Uncomplicated malaria | 47 | 18 | |
| Severe malaria | 21 | 11 | |
| Culture results | Coagulase negative Staphylococcus (n=1) |
Diphtheriods (n=1) Stenotophomonas maltophilia (n=1) Salmonella typhimurium (n=1) Staphylococcus aureus (n=1) |
|
| Survival | Dead | 0 | 7 |
| Alive | 76 | 23 | |
| Malaria past year | No malaria | 58 | 25 |
| At least one | 18 | 5 | |
| Traveled outside Blantyre | 29 | 11 | |
| Number with Other conditions* | 28/76 | 17/30 | |
Note: *Other conditions included influenza, diarrhea, dry cough and non-malaria febrile condition.
Discussion
Although more children than adults residing in endemic areas are expected to present with symptomatic malaria,4 recent retrospective data from archives and observed admission numbers in the hospital wards suggests that there is an increase in the proportion of adults presenting with malaria in Blantyre, Malawi. Unlike in children, it is always difficult to single out malaria as the main cause of morbidity which results in a particular individual being admitted. In this study, therefore, we carefully screened all the recruited participants for any other possible causes of illness and divided the participants into those who were HIV-infected and those who were HIV-uninfected.
Having taken other possible causes of febrile conditions into account, we observed that presenting with several episodes of malaria in a year was a risk factor for developing SM. This observation is counterintuitive since one would have expected that the malaria-specific immunity would have developed more as those individuals were infected more frequently with malaria. However, one possible explanation for frequent exposure being a risk factor for developing severe malaria could be that the affected individual is being infected with different strains of P. falciparum during each infection thereby rendering the previously attained immunity against other strains non-effective. Additionally, participants with SM were more likely to have other co-existing conditions compared to those presenting with UM and these co-existing factors could be contributing factors in the transition from UM to SMA.17
In a study conducted during seven separate weeks from February 2011 through January 2012, of which four 1-week surveys were conducted during the peak malaria transmission period (wet season) in Blantyre Malawi, there were 61 adult malaria-related admissions, of which 28 (46%) fulfilled the criteria for SM. Although the HIV serostatus of the majority of these patients was unknown, the prevalence of SM was unexpectedly higher among adults and could also have been attributed to frequent exposure to the P. falciparum parasite.11 Retrospective data from QECH’s archives showed that between November 2014 and October 2015, 169 adult patients (2.6% of total admissions) were admitted with malaria, of which 32 cases (19%) fulfilled the criteria for SM. Twelve in-hospital deaths were recorded, of which four were HIV-positive and presenting with SM, three with SM but unknown HIV status and five with unknown severity of malaria and unknown HIV status.
The observation that all fatalities were from the group co-infected with HIV and malaria builds on the results of one of our previous studies which showed that co-infection of HIV and SM presenting as cerebral malaria (CM) exacerbated pan-lymphopenia in Malawian children18 which had previously been reported to characterize CM.19,20 The observation that all deaths occurred in the HIV-infected group suggests that although it may not directly affect the malaria disease severity, HIV-infection does influence morbidity and mortality in malaria-infected individuals. This could be as the result of the HIV’s direct effect on both the cell-mediated and antibody-mediated immunity since previous studies have shown that HIV infection induces depletion and early abnormalities of CD4+ T cells, causes deterioration of antigen-specific humoral responses and leads to alteration of innate immunity through impairment of cytolytic activity and cytokine production by NK cells.21,22
In contrast to our findings, a study from Maputo, Mozambique showed that HIV and malaria co-infection was associated with disease severity and in-hospital mortality in adults.13 In addition, a number of studies summarized in a Systematic Review by Flateau et al23 also support the possible link between HIV-infection and severe malaria. The reason for the disparity in the results of our study and those of previous studies could be because, according to current guidelines, all HIV-infected people in Malawi are given prophylactic Co-trimoxazole after diagnosis, regardless of HIV stage.24 The protective effect of interventions for malaria prophylaxis in adults with HIV was shown in one study, in which a combination of Co-trimoxazole, antiretroviral therapy and insecticide-treated bednets (ITNs) was offered to the affected individuals and this substantially reduced the frequency of malaria in adults with HIV by 95%.25 Therefore, most HIV-infected adults could be protected from frequent and SM episodes through such interventions. Follow-up studies need to be conducted to thoroughly investigate the effect of Co-trimoxazole prophylaxis on the development of severe malaria in HIV-infected patients. As far as we know our study is the first of its kind that has shown the numbers of UM and SM in Malawian adults stratified by their HIV status.
The study had several limitations the most important of which was the small sample sizes and the huge difference in the sample size of those co-infected with malaria and HIV (n=30) and those only infected with malaria (n=76). If we had larger sample sizes, the HIV-infected group could have been further stratified by duration on HAART, CD4 count and viral load. Follow-up studies will need to capture data on the duration of symptoms before hospital presentation and evaluate the immune status of the HIV-infected participants.
Conclusions
Although HIV-infection was associated with high mortality in those co-infected with HIV and malaria, recurrent malaria episodes and having other diseases co-existing with malaria infection were the main factors associated with the development of SM in this study cohort. Further studies need to be done to determine how the host immunity is affected in those co-infected with HIV and malaria.
Acknowledgments
We are grateful to the research nurse, Patricia Phula, for her assistance in the recruitment of the study participants. We also thank all the study participants without whom this study could not have been possible.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Malawi-Liverpool Wellcome Trust (MLW) Intern fellowship to Alinane Munyenyembe.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Murray CJL, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Malaria Report. Geneva: World Health Organisation 2017. Licence: CC BY-NC-SA 3.0 IGO Available from: http://www.who.int/malaria/publications/world-malaria-report-2017/en/. Accessed September10, 2018. [Google Scholar]
- 3.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102 [DOI] [PubMed] [Google Scholar]
- 4.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205 [DOI] [PubMed] [Google Scholar]
- 5.Riley EM. Is T-cell priming required for initiation of pathology in malaria infections? Immunol Today. 1999;20:228–233. doi: 10.1016/S0167-5699(99)01456-5 [DOI] [PubMed] [Google Scholar]
- 6.Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686 [DOI] [PubMed] [Google Scholar]
- 7.Carneiro I, Roca-Feltrer A, Griffin JT, et al. Age-patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One. 2010;5(2):e8988. doi: 10.1371/journal.pone.0008988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MJ. Observations on the natural history of malaria in the semi-resistant West African. Trans R Soc Trop Med Hyg. 1958;52:152–168. doi: 10.1016/0035-9203(58)90036-1 [DOI] [PubMed] [Google Scholar]
- 9.Jenkins R, Omollo R, Ongecha M, et al. Prevalence of malaria parasites in adults and its determinants in malaria endemic area of Kisumu County, Kenya. Malar J. 2015;14(263):. doi: 10.1186/s12936-015-0781-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayor A, Aponte JJ, Fogg C, et al. The epidemiology of malaria in adults in a rural area of southern Mozambique. Malar J. 2007;6(3):. doi: 10.1186/1475-2875-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segula D, Frosch AP, SanJoaquin M, et al. Prevalence and spectrum of illness among hospitalized adults with malaria in Blantyre, Malawi. Malar J. 2014;13:391. doi: 10.1186/1475-2875-13-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:18. doi: 10.1186/1756-3305-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg A, Patel S, Aukrust P, et al. Increased severity and mortality in adults co-infected with malaria and HIV in Maputo, Mozambique: a prospective cross-sectional study. PLoS One. 2014;9(2):e88257. doi: 10.1371/journal.pone.0088257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett A, Kazembe L, Mathanga DP, et al. Mapping malaria transmission intensity in Malawi, 2000–2010. Am J Trop Med Hyg. 2013;89:840–849. doi: 10.4269/ajtmh.13-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munyenyembe AU, Gausi K, Nyirenda TS, Hiestand J, Mallewa J, Mandala WL. HIV infection has a profound effect on hematological factors but not on electrolyte profile of Malawian adults presenting with uncomplicated malaria and severe malaria. J Blood Med. 2018;9:153–162. doi: 10.2147/JBM.S172869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyburn H. New WHO guidelines for the treatment of malaria. BMJ. 2010;340:c2637. doi: 10.1136/bmj.c2637 [DOI] [PubMed] [Google Scholar]
- 17.Greenwood B, Marsh K, Snow R. Why do some African children develop severe malaria? Parasitol Today. 1991;7(10):277–281. doi: 10.1016/0169-4758(91)90096-7 [DOI] [PubMed] [Google Scholar]
- 18.Mandala WL, Gondwe EN, Nyirenda TS, Drayson M, Molyneux ME, MacLennan CA. HIV infection compounds the lymphopenia associated with cerebral malaria in Malawian children. J Blood Med. 2018;10:9–18. doi: 10.2147/JBM.S187081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandala WL, Msefula C, Gondwe EN, et al. Lymphocyte perturbations in Malawian children with severe and uncomplicated malaria. Clin Vaccine Immunol. 2015;23(2):95–103. doi: 10.1128/CVI.00564-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hviid L, Kurtzhals JA, Goka BQ, Oliver-Commey JO, Nkrumah FK, Theander TG. Rapid reemergence of T cells into peripheral circulation following treatment of severe and uncomplicated plasmodium falciparum malaria. Infect Immun. 1997;65:4090–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finney CA, Ayi K, Wasmuth JD, et al. HIV infection deregulates innate immunity to malaria despite combination antiretroviral therapy. AIDS. 2013;27(3):325–335. doi: 10.1097/QAD.0b013e32835b3dfa [DOI] [PubMed] [Google Scholar]
- 22.Subramaniam KS, Skinner J, Ivan E, et al. HIV malaria co-infection is associated with atypical memory B cell expansion and a reduced antibody response to a broad array of Plasmodium falciparum antigens in rwandan adults. PLoS One. 2015;10(4):e0124412. doi: 10.1371/journal.pone.0124412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11(7):541–556. doi: 10.1016/S1473-3099(11)70031-7 [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health, Malawi Government. Clinical Management of HIV in Children and Adults. 2014. Available from: https://aidsfree.usaid.gov/sites/default/files/tx_malawi_2014.pdf. Accessed December 31, 2019. [Google Scholar]
- 25.Mermin J, Ekwaru JP, Liechty CA, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367(9518):1256–1261. doi: 10.1016/S0140-6736(06)68541-3 [DOI] [PubMed] [Google Scholar]