Abstract
Nicotinamide riboside kinase-2 (NRK-2), a muscle-specific β1 integrin binding protein, predominantly expresses in skeletal muscle with a trace amount expressed in healthy cardiac tissue. NRK-2 expression dramatically increases in mouse and human ischemic heart however, the specific role of NRK-2 in the pathophysiology of ischemic cardiac diseases is unknown.
We employed NRK2 knockout (KO) mice to identify the role of NRK-2 in ischemia-induced cardiac remodeling and dysfunction. Following myocardial infarction (MI), or sham surgeries, serial echocardiography was performed in the KO and littermate control mice. Cardiac contractile function rapidly declined and left ventricular interior dimension (LVID) was significantly increased in the ischemic KO vs. control mice at 2 weeks post-MI. An increase in mortality was observed in the KO vs. control group. The KO hearts displayed increased cardiac hypertrophy and heart failure reflected by morphometric analysis. Consistently, histological assessment revealed an extensive and thin scar and dilated LV chamber accompanied with elevated fibrosis in the KOs post-MI. Mechanistically, we observed that loss of NRK-2 enhanced p38α activation following ischemic injury. Consistently, ex vivo studies demonstrated that the gain of NRK-2 function suppresses the p38α as well as fibroblast activation (α-SMA expression) upon TGF-β stimulation, and limits cardiomyocytes death upon hypoxia/ re-oxygenation.
Collectively our findings show, for the first time, that NRK-2 plays a critical role in heart failure progression following ischemic injury. NRK-2 deficiency promotes post-MI scar expansion, rapid LV chamber dilatation, cardiac dysfunction and fibrosis possibly due to increased p38α activation.
Keywords: NMRK2, β1-integrin binding protein, MIBP, Dilated Cardiac Remodeling, Fibrosis, P38 MAP Kinase Signaling
1. Introduction:
Despite continuing therapeutic advancements, prognosis of dilated cardiomyopathy and heart failure is poor and remains one of the leading cause of deaths worldwide[1]. Myocardial ischemia (MI) promotes cardiac injury and scar formation which generates volume overload, to which the heart responds via myocyte lengthening, resulting in eccentric hypertrophy [2]. Post-MI cardiac remodeling is often associated with cardiomyopathy and extracellular matrix (ECM) deposition [3]. However, hemodynamic overload exerts biomechanical stress on left ventricle wall, resulting in the heart working harder to adapt to this condition, also leading to an increase in LV hypertrophy. Dilated cardiomyopathy (DCM) is the most common cause of heart failure, and with a limited ability of current therapies to prevent remodeling indefinitely, heart transplantation remains the last line of therapy [4].
We previously observed nicotinamide riboside kinase-2 (NRK-2) as a top upregulated protein in a model of DCM [5]. However, the impact of NRK-2 on cardiac structure and function during heart failure is unknown. NRK-2 is a muscle specific β1-integrin binding protein-3 (MIBP) that is expressed abundantly in skeletal muscle and at lower level in healthy cardiac muscle [6]. A recent study observed an aberrant NAD+ homeostasis concurrent with the induction of NRK-2 expression in a DCM model of heart failure [7]. Studies have shown that NRK-2 plays a role in the NAD+ salvage pathway however, recent reports have identified that nicotinamide phosphoribosyltransferase (NAMPT) plays a dominant role in the NAD+ synthesis in both cardiac and skeletal muscle, and the role of NRK-2 in NAD+ synthesis is dispensable[7, 8]. These studies suggest that NRK-2 may regulate cardiac pathophysiology of heart failure through novel mechanisms.
In our current study, we identify that NRK-2 expression increases several fold in both mouse and human ischemic hearts. Increased expression levels were associated with the progression of cardiac remodeling post-MI. Employing NRK2 KO mice for the first time, we show that NRK-2 deficiency induces p38α activation and promotes fibrotic scar formation, LV wall thinning and rapid LV dysfunction post-MI. Thus, NRK-2 plays a protective role against cardiac remodeling and dysfunction during the heart failure development post-ischemia.
2. Method:
2.1. NRK2 knockout mice generation and maintenances:
NRK2 knockout mouse was generated by The Jackson Laboratory (stock# 018683) using embryonic stem cells. Briefly, the ZEN-UB1 Velocigene cassette containing polyadenylation signal; promoter from the human ubiquitin C gene; loxP site; beta-galactosidase coding sequence from E. coli lacZ gene; neomycin phosphotransferase; polyadenylation signal; loxP site) was inserted to disrupt all coding exons and intervening sequences. The construct was introduced into VGB6 embryonic stem (ES) cells derived from C57BL/6N, and postive ES cells were injected into B6(Cg)-Tyrc−2J/J blastocysts. The C57BL/6NJ females mice were crossed with chimeric male mice followed by cross with B6N.Cg-Tg(Sox2-cre)1Amc/J to remove neo cassette. The resulting mice were crossed with C57BL/6NJ to remove the cre-expressing transgene.
The experimental NRK2 knockout (KO) mice were generated by crossing heterozygous male with heterozygous female mice. The mouse strain was maintained on the C57BL/6 background. The Institutional Animal Care and Use Committee of Vanderbilt University Medical Center approved all animal procedures and treatments.
2.2. Human Heart tissue sample:
The flash frozen human heart tissue samples were obtained from Vanderbilt Heart and Vascular Institute (VHVI) Registry/Repository. The remote LV myocardial tissue was obtained from ischemic end-stage cardiomyopathic HF male patients and LV sample from donor’s normal hearts not used for transplantation were used as control. The tissue lysates preparation for immunoblotting was done at 4°C. Sample procurement, and preparation were performed according to a human research subject protocol approved by Vanderbilt University Institutional Review Board (IRB).
2.3. Myocardial infarction:
Following baseline echocardiography, KO and littermate control mice were subjected to permanent ligation of proximal left anterior descending (LAD) coronary artery (myocardial infarction), or sham surgery as described previously[9]. Post 4-week echo examination, mice were euthanized and tissue was harvested for different studies.
2.4. Echocardiography
Echocardiography was performed as described previously[10, 11]. Briefly, transthoracic two-dimensional motion mode-echocardiography was performed 0, 2 and 4 weeks post-MI using 12-mHz probe (visualSonic, Vevo2100). During echocardiography mice anesthesia was maintained by inhalation of 1.5% isoflurane. LV end-diastolic interior dimension (LVID;d) and end-systolic interior dimension (LVID;s) was measured and ejection fraction (EF) and fractional shortening (FS) values were calculated using the Vevo2100 program.
2.5. Histochemistry:
Cardiac tissue was harvested from anesthetized mice and histochemistry was performed following a protocol described previously[5, 10]. Briefly, 4 week post-MI or sham surgeries, whole heart was excised and fixed them using 4% paraformaldehyde. Tissues were dehydrated through serial concentrations of ethanol, and then embedded in paraffin. Heart sections (5μm) were stained using Masson’s trichrome kit (Sigma-Aldrich# HT15–1KT) as per kit recommendation. A 0.8X objective of a Nikon Eclipse 80i microscope was used to capture the whole heart image and, a 20X objective was applied to take five images from the LV region of heart sections (remote area of ischemic LV). The fibrotic area from images was quantified using NIS Elements software in a blinded manner and percentage of fibrosis was calculated.
2.6. Measurement of cardiomyocyte circumference:
The 5μm thick sections from 4 week post-MI heart were subjected to Masson’s trichrome as well as Wheat germ agglutinin (WGA) staining as described previously [12]. A total of 5 images/ section were captured from the LV region using a 20X objective and circumference was measured in 250– 300 cells in each heart. The measurement of cardiomyocyte cross-sectional area was done using NIS Element software. The myocytes that were round (i.e., were cut in cross section) were only included in the analysis. Cardiomyocyte circumference measurement and analysis were performed in a blinded manner.
2.7. LV lysate preparation and immunoblotting:
LV lysate preparation and immunoblotting was done as describe previously [13]. Briefly, heart tissue was harvested from anesthetized mice one week post-MI. Both atrium and right ventricle were removed and LV tissue was homogenized in 1X lysis buffer (Cell Signaling #9803) with protease and phosphatase inhibitor cocktail. After tissue homogenization, samples were centrifuged at 15,000g for 15 minutes at 4°C. Supernatant was transferred to a fresh tubes and protein concentration was quantified with the bicinchoninic acid (BCA) protein assay (Pierce# 23225). An equal amount of proteins were loaded to SDS-PAGE and subsequently were transferred to nitrocellulose membranes. A primary antibody incubations was performed at 1:1,000 dilution for anti-GSK-3α/β (Cell Signaling# 5676), phospho-GSK-3α/β (Cell Signaling #9327), phospho-GSK-3β (S9) (Cell Signaling #5558), anti-p38α (Cell Signaling# 9218), phospho-p38 (Cell Signaling #9211), Bax (Cell Signaling #2772), Akt (Cell Signaling #9272), phospho-Akt (Cell Signaling #4060), phospho-ERK1/2 (Cell Signaling #9101), ERK1/2 (Cell Signaling #4695) anti-HA High Affinity, (Roche #11867423001), 1:2000 for α-SMA (Sigma Aldrich #A2547), 1:100 for NRK-2 (Santa Cruz #sc-240681), and 1:10,000 for GAPDH (Fitzgerald #10R-G109a). All incubations were done at 4°C, overnight. The secondary antibody used was Alexa Fluor 680 (Molecular Probes), at 1:3,000 dilution for 1 hour at room temperature. Membranes were scanned with the Odyssey Infrared Imaging System (LI-COR).
2.8. Primary cell isolation and culture:
Adult mouse cardiac fibroblasts were isolated, as described earlier [14]. In brief, 10–12 weeks old mice were euthanized, hearts were excised and rinsed with cold Hank’s Balanced Salt Solutions (HBSS, Corning, Cat#MT21023CV). Ventricles were cut in small pieces (1mm) and digested in HBSS containing 150 unit/mL of Collagenase Type 2 (Worthington, Cat# LS004176) and 0.6 mg/mL Trypsin (USB Corp., Cat#22705) at 37°C for 15 minutes. The digested supernatant was collected and spun down at 400xg for 5 minutes. Cell pellets were resuspended in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% Non-Essential Amino Acids. The cell suspension was passed through a 70-micron cell strainer, and flow-through cell suspension is cultured in a cell culture dish at 37°C and 5% CO2. After 2h, media was removed and replaced with fresh media. The neonatal rat ventricular myocytes (NRVMs) were isolated and cultured as described earlier [15].
2.9. Adenoviral transduction and cell lysate preparation:
Adult mouse cardiac fibroblasts (AMCFs) and NRVMs were cultured as culture conditions mentioned above. Cells were infected with adenovirus encoding control β-galactosidase (Ad-βgal) or NRK2-HA (Applied BioMaterial #205052A). Post 24h of viral infections, cells were treated with 10 ng/ml recombinant mouse transforming growth factor-β (TGFβ, R&D Systems, Cat #7666-MB-005) for 24h in reduced serum (1%) conditions. Cells were collected, washed in 1X PBS, and lysed in 1X RIPA lysis buffer (Millipore, Cat #20–188) supplemented with SIGMAFAST(TM) protease inhibitor cocktail (Sigma-Aldrich, Cat #S8830–20TAB) and PhosSTOP™ phosphatase inhibitor cocktail (Roche, Cat #04906837001). Cell lysates were centrifuged at 13,000g for 10 min at 4°C. The supernatant was collected, and protein concentrations were determined by Pierce 660nm protein assay (Thermo Fisher Scientific, Cat #22660) according to manufacturer’s protocol. An equal amount of proteins was used for immunoblotting.
2.10. RNA isolation, reverse transcription, and quantitative Real-Time polymerase chain reaction (qRT-PCR):
RNA isolation and qRT-PCR was performed as described previously [11]. Briefly, total RNA was isolated from LV tissue using RNeasy kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. The quality of total RNA was assessed by the ratios OD260/OD280 and OD260/OD230. Complimentary DNA (cDNA) was synthesized using iScript™ cDNA Synthesis kit (BioRad #170–8891). Quantitative real-time polymerase chain reaction (PCR) for NRK-1, NRK-2 and Col1α1 was carried out in thermocycler with the use of Taqman Universal PCR Master Mix. The FAM-labeled TaqMan gene expression assays (ABI, Carlsbad, CA) was used for mouse NRK-1 (ID: Mm00521051_m1), NRK-2 (ID: Mm01172899_g1), atrial natriuretic peptide (ID: Mm01255748_g1) and Col1α1 (ID: Mm00801666_g1) respectively. The NRK-2 expression in human heart was evaluated using another FAM-labeled TaqMan gene expression assay (ID: Hs01043681_m1). The VIC-labeled 18S rRNA assay probes (ABI #4319413E) was included in each reaction as an endogenous control and used for normalization. Relative gene expression was determined by using the δ-δ Ct method.
2.11. Hypoxia/re-oxygenation and Annexin V/ Propidium iodide Staining:
NRVMs were plated on Glass Bottom Microwell Dishes (MatTek Corporation, Cat# P35G-1.5–10-C) and infected with indicated protein-expressing adenovirus. Post 24 h of infection, cells were placed in hypoxia chamber for 8 h and then 24 h reoxygenation. Cells were stained with 1X Annexin V Alexa Fluor™ 488 conjugate (Thermo Fisher Scientific, Cat# V13241), Propidium iodide (1μg/ml), and Hoechst 33342 (1μM) solution for 30 min. Fluorescent images were obtained at 405, 488 and 561 nm excitation using a 40X oil objective (Nikon Eclipse TiE microscope). Images were analyzed and quantified using Image J.
2.12. Statistics:
Differences between data groups were evaluated for significance using unpaired t-test or ANOVA (Graph Pad Prism Software Inc., San Diego, CA). Data are expressed as mean±SEM. For all tests, a P value <0.05 was considered for statistical significance
3. Results:
3.1. Heart failure coincides with upregulated expression of cardiac NRK-2 in mice and humans:
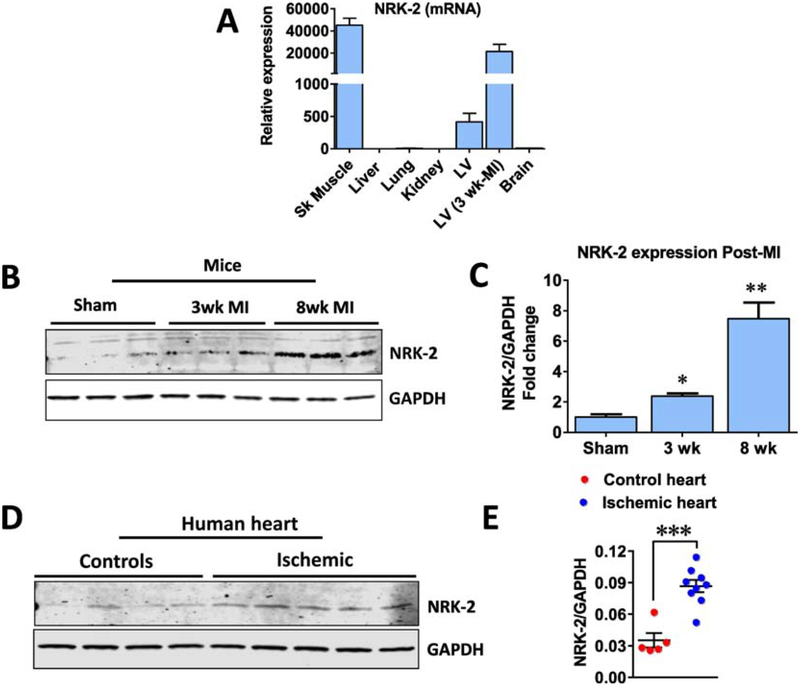
Recently, we and others have identified NRK-2 as a top upregulated protein in heart failure models [5, 7]. To take this further, first we assessed the expression level of NRK-2 in different major murine organs including lung, liver, brain, skeletal muscle and cardiac tissues, and compared with the expression levels in the ischemic heart. The NRK-2 level was undetectable in lung, liver, brain and kidney (Fig. 1A). NRK-2 protein expression was abundant in skeletal muscle and a lower level was detected in healthy cardiac tissue, however, a higher level was identified in post-myocardial infarction (MI) hearts. Notably, NRK-2 expression was upregulated by ~75 fold in the ischemic heart vs. healthy cardiac muscle (Fig. 1A).
Figure 1; NRK-2 expression profile in major organs and, ischemic heart.
(A) NRK-2 expression pattern at mRNA levels in major organs and ischemic heart from mouse. (B) Representative western blot and (C) quantification show NRK-2 protein expression levels at acute and chronic stages of MI. n=3 each (D) Blot image from human ischemic and healthy heart lysates and (E) quantification show an increased NRK-2 protein expression in human ischemic vs. healthy hearts. n=4–5, *p<0.05; **p<0.005; *** p=0.0001.
To further investigate if NRK-2 expression associates with the progression of heart failure, a group of C57BL/6 mice were subjected to MI or Sham surgeries. Cardiac tissue was harvested from sham mice, as well as 3 or 8 weeks post-MI mice and western blotting was performed using remote LV lysates. Notably, the protein level of NRK-2 increased as heart failure progressed. The expression was significantly higher in the early 3 wk post-MI time point in comparison to sham-operated hearts at both the mRNA and protein levels, while expression further increased by 8 weeks post-MI (Fig. 1B–C). We also evaluated NRK-2 expression in human ischemic and control (non-ischemic) LV tissues. Consistent with mice ischemic LV data, the NRK-2 protein expression was significantly elevated in human ischemic vs. control hearts (Fig. 1D–E). These observations indicate the clinical significance of NRK-2 in ischemic cardiac diseases.
3.2. Characterization of NRK2 knockout mice:
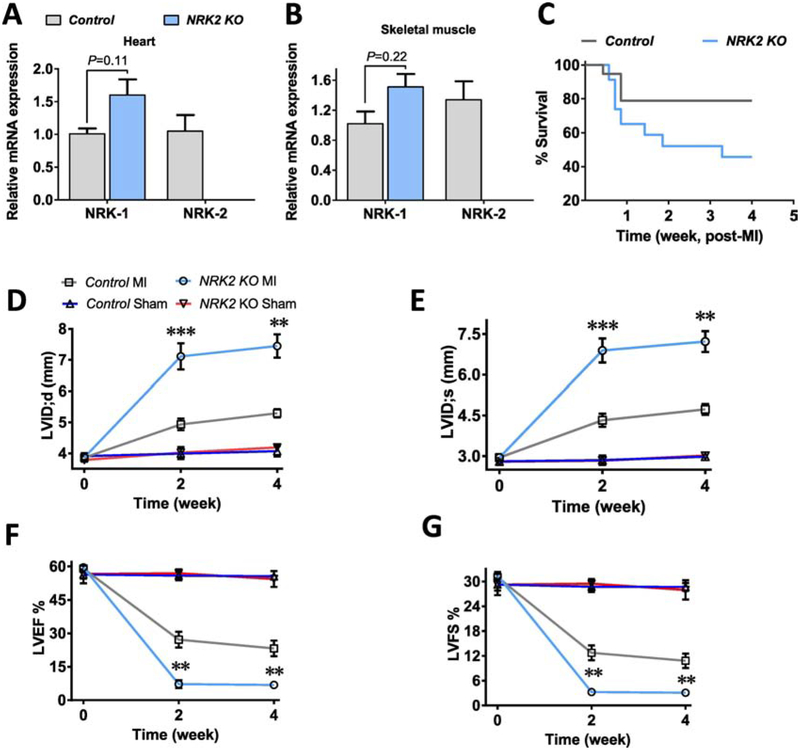
Considering the elevated NRK-2 expression level and potential NRK-2-mediated beneficial processes during heart failure [7], we employed NRK2 knockout (KO) mice to determine novel roles of NRK-2 in heart failure. Expression levels of NRK-1 and NRK-2 were confirmed in the NRK2 KO mice. Since NRK-2 found to express primarily in skeletal and cardiac muscle, we tested the expression levels of NRK-2 along with NRK-1 in these organs. Real time-quantitative PCR (RT-qPCR) shows undetectable NRK-2 in the KO cardiac and skeletal muscles (Fig. 2A–B). Notably, a trend of increased NRK-1 expression was observed in the KO cardiac as well as skeletal muscles which might be due to a compensatory effect. The body weight of 8–10 week old KO male mice was comparable to littermate control male mice (Suppl. Fig. 1A). Since, NRK-2 is a muscle-specific protein, we hypothesized that deletion of NRK-2 could lead to an adverse effect on skeletal muscle, however the percentage of total muscle mass in the KO mice was found to be comparable to the muscle mass in littermate controls (Suppl. Fig. 1B).
Figure 2; Genetic ablation of NRK2 exaggerates post-MI adverse cardiac remodeling and dysfunction:
The NRK-2 was undetectable in (A) cardiac as well as (B) skeletal muscle of KO mice and NRK-1 level was insignificantly elevated in the KO mice. (C) Survival curve shows a trend of increased post-myocardial infarction (MI) mortality in the KO vs. control groups post-MI, n=19–23, P value analyzed using Gehan-Breslow-Wilcoxan test. Cardiac function was assessed post-MI or Sham surgeries by transthoracic two-dimensional motion-mode echocardiography at 0, 2 and 4 week time points. (D) A significant increased left ventricle end-diastolic dimension (LVID;d) and (E) end-systolic dimension (LVID;s) at 2 and 4 weeks show rapid LV chamber dilatation post-MI. (F) LV ejection fraction (LVEF) and (G) fractional shortening (LVFS) were significantly declined in the KO vs. control animals at 2 and 4 weeks post-MI, n=5–13 for MI and n=3 each for Sham groups. Data presented as mean ±SEM and P values shown are for the comparison between Control vs. KO mice subjected to MI. *p<0.05; **p<0.005; ***p=0.0005.
Further, we assessed the baseline cardiac function in the KO mice using M-mode echocardiography. The LV chamber dimension at systole and diastole (LVID;s and LVID;d) and contractile function (LV ejection fraction and fractional shortening) were comparable in the KOs vs. controls at baseline (Suppl. Fig. 1C–D). Collectively, these data suggest that loss of NRK-2 does not induce an overt physiological effect, with comparable body weight and size, and cardiac contractile functions.
3.3. NRK-2 deficiency exaggerates adverse cardiac remodeling and promotes contractile dysfunction post-MI:
To identify the role of NRK-2 in the pathophysiology of ischemic heart disease, a batch of NRK2 KO and control male mice were subjected to sham or MI surgery. The post-MI mortality was observed and heart function was assessed using M-mode echocardiography at 0, 2 and 4 weeks post-MI. Overall a trend toward higher mortality was observed post-MI in the KO compared to the control group (Fig. 2C). An increased mortality (~40%) in the KO was observed in the early cardiac rupture phase of MI. The postmortem examination confirmed the cardiac rupture as the cause of the deaths as the chest cavity was found full of the blood. The LV chamber was quickly dilated in the KOs as LVID;s and LVID;d were significantly greater at 2 weeks post-MI (Fig. 2D–E). Consistent with increased pathological LV remodeling, cardiac contractile function was significantly reduced as reflected by decreased LVEF and LVFS in the KO mice (Fig. 2F–G). The dilated LV internal dimension and the cardiac dysfunction in the KO mice were consistent and remained significantly worse until the end of the study (4 weeks post-MI). Taken together, these data suggest that NRK-2 protects against MI-induced cardiac remodeling and preserves cardiac function, while deletion of NRK-2 exaggerates pathological cardiac remodeling and dysfunction post-MI.
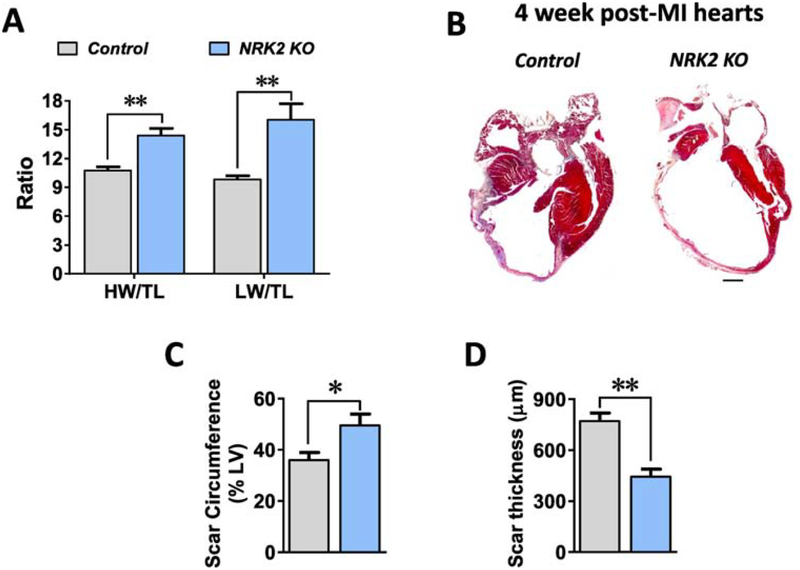
3.4. Loss of NRK-2 promotes post-MI scar expansion and thinning, LV dilatation, fibrosis and heart failure:
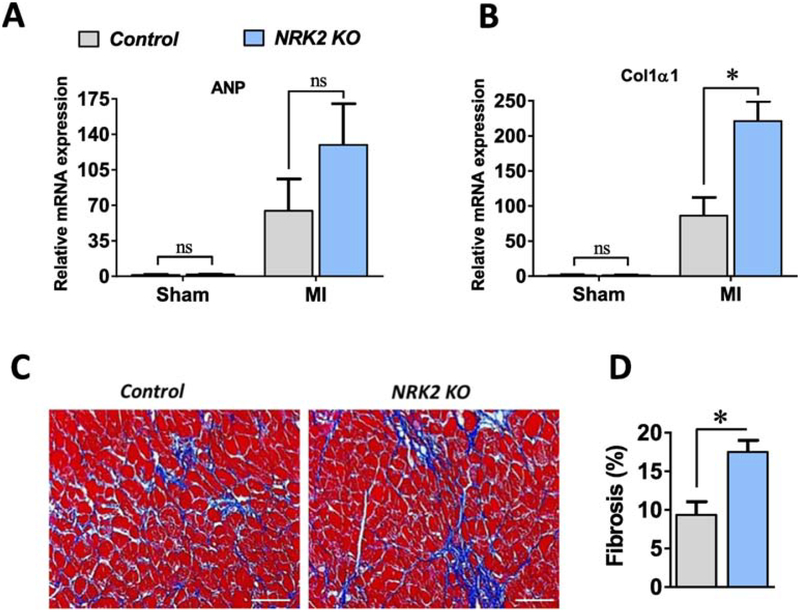
At 4 weeks post-MI, the remaining mice were sacrificed and heart and lung tissues were harvested from anesthetized mice. While heart weight and lung weight to tibia length ratios were greater in the KOs (Fig. 3A), WGA and trichrome staining have shown a comparable cardiomyocyte circumference in the KO vs. control hearts (Suppl. Fig. 2A–C) post-4 weeks of MI. To assess scar circumference, Masson’s trichrome staining was performed, revealing a noticeably thinner scar and significantly larger percent scar circumference of the left ventricle than in the controls (Fig. 3B–D). Next, we assessed the expression level of atrial natriuretic peptide (ANP), a molecular marker for heart failure, in the KO and littermate control hearts. Irrespective of genotype, ANP expression was significantly increased in the post-MI vs. Sham operated hearts, and, consistent with increased morphometric parameters in the KOs, ANP expression was higher in the KO vs. control hearts post-MI (Fig. 4A). Further, we evaluated the level of fibrosis via measurement of procollagen type-I alpha (Col1α1) expression level in the post-MI hearts. Similar to ANP levels, MI induced a significant increase in Col1α1 expression in both genotypes, however Col1α1 was found to be more highly expressed in the KO vs. control hearts post-MI (Fig. 4B). To further assess collagen deposition in the post-MI hearts, we performed Masson’s trichrome staining in the 4 week post-MI hearts sections. Consistent with the increased level of Col1α1 expression, a significantly increased fibrotic area was observed in the 4 week post-MI KO vs. control hearts (Fig. 4C–D). These observations strongly suggest that loss of NRK-2 promotes LV chamber dilatation, scar expansion and thinning, remote fibrosis and heart failure post-MI.
Figure 3; Loss of NRK2 promotes cardiac hypertrophy, scar expansion and heart failure post-MI.
(A) Morphometric parameters including heart weight and lung weight to tibia length ratios show an increased cardiac hypertrophy and heart failure in the KO vs control mice post-MI, n=5–6. (B) Representative images of Masson’s trichrome- stained control and KO heart sections show a dilated LV chamber and extensive scar in the KO post-MI. Bar diagram shows (C) an increased scar circumference and (D) decreased thickness in the post-MI KO hearts, n=5–6. ns, non-significant; *p<0.05; **p<0.005.
Figure 4; NRK2 deficiency promotes cardiac fibrosis post-MI.
(A) Fetal gene atrial natriuretic peptide (ANP) and (B) Collagen1α1 expression levels in the Sham and 1 week post-MI KO and control hearts, n=3–5 for Sham and n=4–6 for MI groups. (C) Representative images (20X objective) from Masson’s trichrome- stained KO and control hearts show (D) an increased fibrosis in remote heart post- 4 week of MI, n=5–6. ns, non-significant; *p<0.05.
3.5. Loss of NRK-2 specifically promotes p38α activation in ischemic heart:
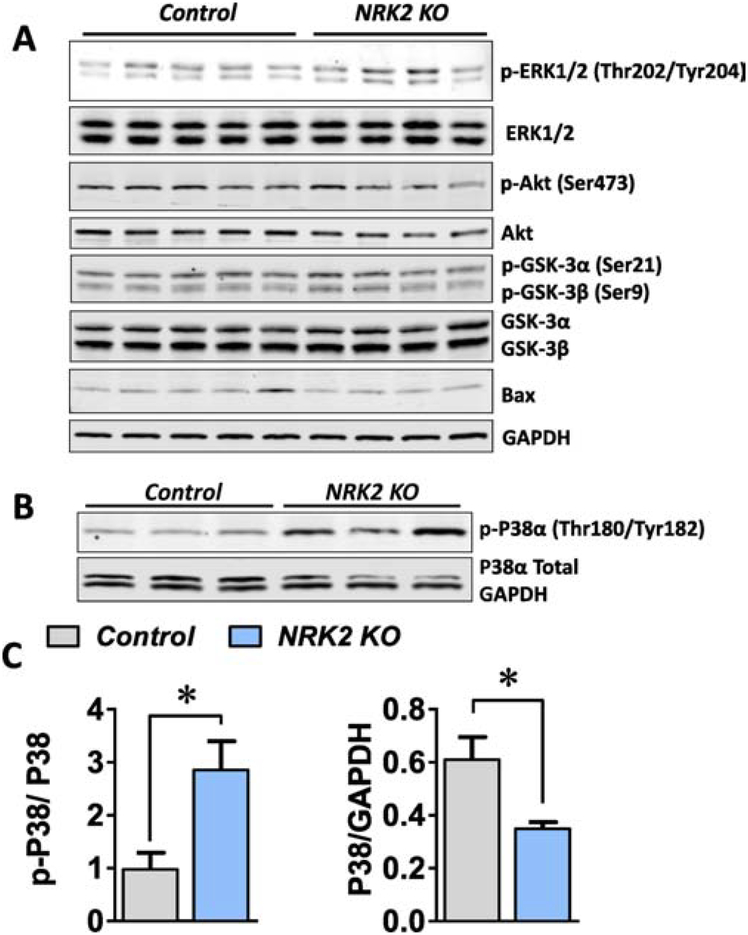
β1 integrin and its binding proteins including focal adhesion kinase (FAK), talin, integrin linked kinase (ILK) and melusin are well reported to propagate the mitogen activated protein (MAP) kinase and cell survival pathways in the heart [16–19]. Hence we hypothesized if NRK-2, an integrin binding protein, may regulate these cell signaling pathways under ischemic conditions. Since a rapid decline in cardiac function and increase in LV chamber dilatation was observed in the KO mice post-MI (Fig. 2D–G), the heart tissue was harvested at one week post-MI and protein lysate was prepared using the remote LV tissue. First we analyzed the Akt survival pathway in which the expression and phosphorylation level was assessed via western blot analysis. The phosphorylation level of Akt (Ser473) and its downstream targets Glycogen synthase kinase-3α (GSK-3α) (Ser21) and GSK-3β (Ser9) were comparable between KO and control hearts post-MI (Fig. 5A). Similarly, levels of the pro-apoptotic protein Bcl-2-associated X (Bax) were not different between KO and control hearts post-MI (Fig. 5A).
Figure 5; NRK-2 deficiency increases p38 MAP kinase activity in the ischemic hearts;
(A) Heart tissues were harvested from NRK-2 KO and control mice post one-week of MI and western blotting was performed using LV lysates. (A) Western blot images for phosphorylation and total Akt, GSK-3α, GSK-3β and ERK1/2, and Bax expression levels, n=4–5. (B) Representative blot images and (C) blot quantification show a significantly increased phosphorylation (activation) and decreased expression of p38α in the KO hearts post-MI, n=4–5.*P<0.05.
Next, we assessed MAP kinase pathways, specifically ERK1/2 and p38α kinase expression and activation. The phosphorylation level of ERK1/2 found to be comparable in the KO vs. control heart post-MI, however, ~3-fold increase in p38α (Thr180/Tyr182) phosphorylation (activation) was observed in the KO in comparison to control hearts at one week post-MI (Fig. 5B–C). Further, a significantly decreased level p38α protein expression was observed in the KO hearts (Fig. 5B–C), resulting in an exaggerated increase in phosphorylated-to-total p38α level. These data indicate that loss of NRK-2 specifically results in enhanced p38α activation following ischemic injury in the heart, which may contribute to an elevated level of fibrosis and worsened cardiac dysfunction and dilatative remodeling.
3.6. NRK-2 limits hypoxia/ re-oxygenation-induced cardiomyocytes death:
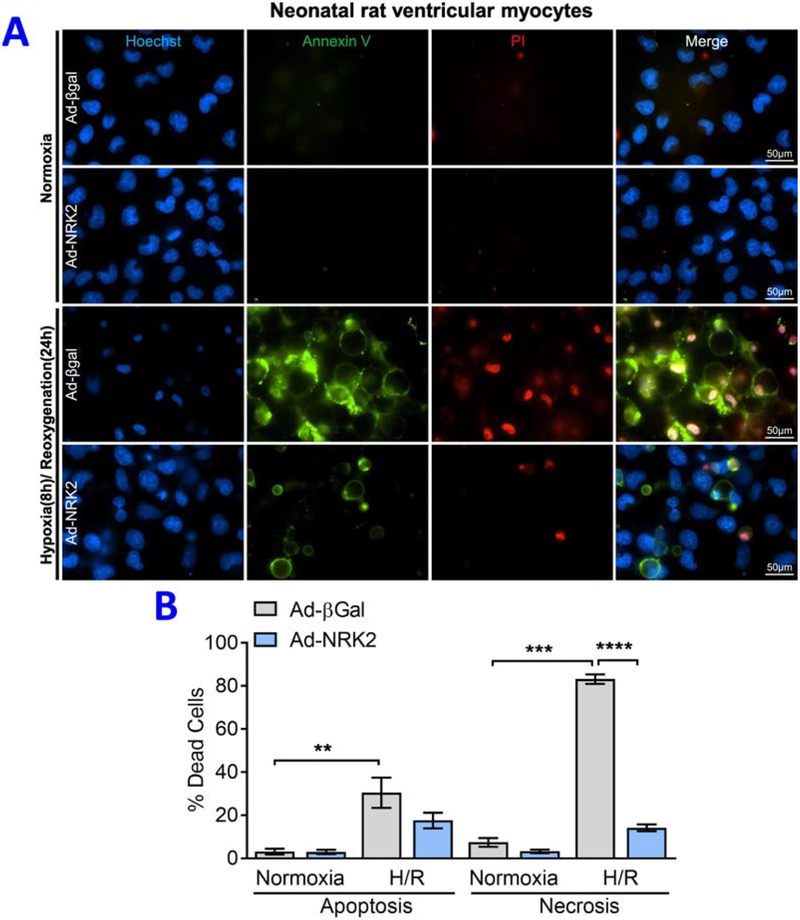
In vivo findings revealed that NRK-2 limits LV fibrosis and chamber dilatation post-MI which found to be largely regulated through increased p38α activation. However, it is unknown if NRK-2 modulates cardiomyocyte death in acute ischemic condition. To address the same, we performed ex vivo experiments employing gain of function model. NRVMs were isolated and transduced with either Ad-βgal or Ad-NRK2 adenovirus. Post-adenoviral transduction, cells were challenged with hypoxia/ re-oxygenation (H/R) and labeled with annexin V (AnnV) and propidium iodide (PI) to assess the level of cell death. Consistent with detrimental phenotype observed in the ischemic KO mice, a significantly less number of cardiomyocyte deaths were observed in the NRK-2 overexpression vs. Ad-βgal group post-H/R (Fig. 6A–B). An increased number of apoptotic (AnnV-positive) and necrotic (PI-positive) cardiomyocytes were seen in H/R. Unlike the level of apoptosis, the proportion of necrotic cardiomyocytes was found to be significantly higher in Ad-βgal in comparison to Ad-NRK2 group undergone H/R. These observations suggest that NRK-2 protects cardiomyocytes against acute H/R-induced necrotic death.
Figure 6; Gain of NRK-2 function limits hypoxia/re-oxygenation -induced cardiomyocyte death:
(A) Representative images of annexin V and propidium iodide stained neonatal rat ventricular myocytes (NRVMs) treated with control Ad-βgal or Ad-NRK2 adenovirus. (B) Quantification shows a decreased number of annexin V (apoptotic) and PI positive (necrotic) cardiomyocytes in Ad-NRK2 in comparison to Ad-βgal treated cells subjected to hypoxia/ reoxygenation, **p<0.005, ***p<0.0005, ****p<0.0001.
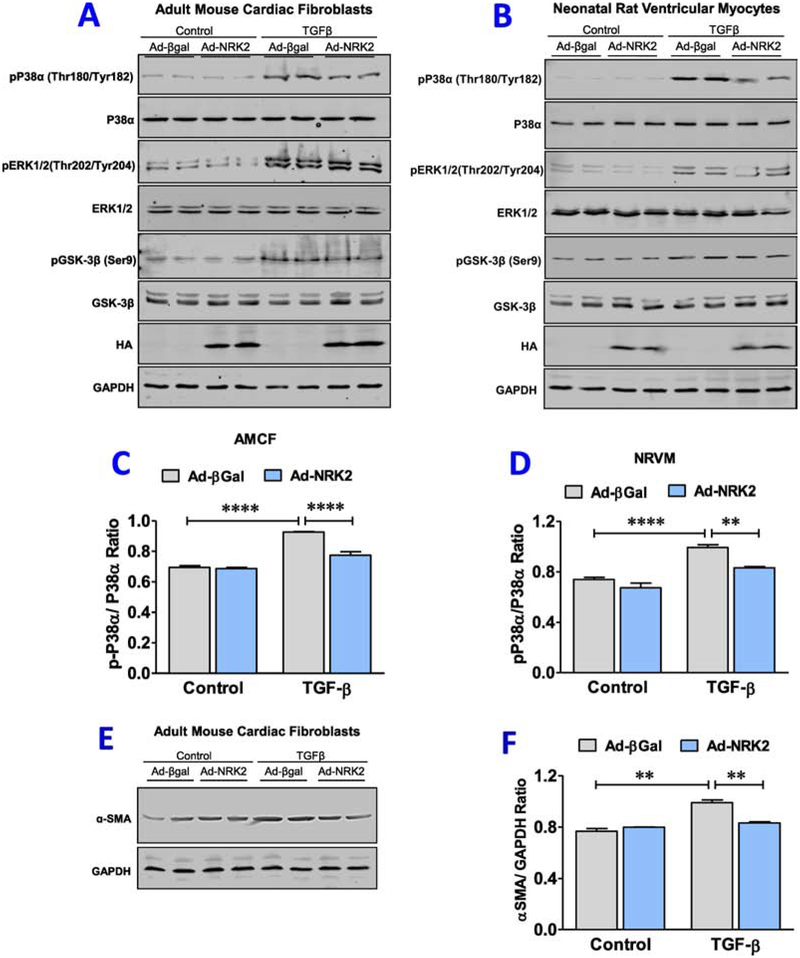
3.7. NRK-2 suppresses p38α activation in NRVMs and AMCFs post- TGF-β treatment:
To delineate the molecular mechanism and validate the results observed in the ischemic KO hearts, we employed gain of NRK-2 function model. In this series, NRVMs and AMCFs were isolated and transduced with Ad-βgal or Ad-NRK2 adenovirus to overexpress the NRK-2, and treated with TGF-β. Cells were harvested and activity of MAP Kinases and GSK-3β pathways was assessed. NRK-2 overexpression found to have no role in modulation of said signaling molecules at the baseline, however, irrespective of adenoviral transduction, TGF-β treatment induces dramatic increase in the phosphorylation levels of p38α, ERK1/2, GSK-3β both in NRVMs and AMCFs vs. untreated groups. The increased phosphorylation levels of ERK1/2, GSK-3β were comparable between Ad-βgal control and NRK-2 overexpressing NRVMs as well as AMCFs group treated with TGF-β. However, interestingly, a significantly less phosphorylation of p38α was identified in both types of cells overexpressing NRK-2 vs. Ad-βgal control groups treated with TGF-β (Fig. 7A–D). These ex vivo, and in vivo results from NRK-2 KOs strongly suggest that NRK-2 protects heart from adverse fibrotic and dilatative cardiac remodeling post-MI largely through suppressing p38α activity.
Figure 7; NRK-2 suppresses TGF-β -induced P38α activation in NRVMs and AMCFs cells:
Representative blot images for phosphorylation and total levels of P38α, ERK1/2 and GSK-3β in TGF-β treated (A) neonatal rat ventricular myocytes (NRVMs) and (B) adult mouse cardiac fibroblasts (AMCFs) cells. Blot quantification shows an increased phosphorylation level of P38α in (C) NRVMs as well as (D) AMCFs post-TGF-β treatment. (E) Representative blot images of α-SMA expression in AMCFs and (F) blot quantification shows a significantly less α-SMA expression (fibroblast activation) in NRK-2 overexpressing AMCFs treated with TGF-β, **p<0.005, ****p<0.0001.
3.8. NRK-2 alleviates myofibroblast transformation under cardiac pathological condition:
Since we identified an increased collagen deposition and collagen1α1 expression in the ischemic KO hearts, we hypothesized if NRK-2 overexpression modulates myofibroblast transformation. To address the same, ACMF were isolated and transduced with Ad-βgal or Ad-NRK2 adenoviruses. Post-adenoviral transduction, cells were treated with TGF-β and harvested to analyze the level of myofibroblast transformation. The evaluation of α-smooth muscle actin (α-SMA) shows a comparable expression between Ad-βgal and Ad-NRK2 groups at basal level, however, TGF-β treatment induces significantly increased myofibroblast transformation in Ad-βgal group however, it found to be blunted in Ad-NRK2 group(Fig. 7E–F). These observations suggest that NRK-2 mitigates TGF-β -induced myofibroblast transformation.
4. Discussion:
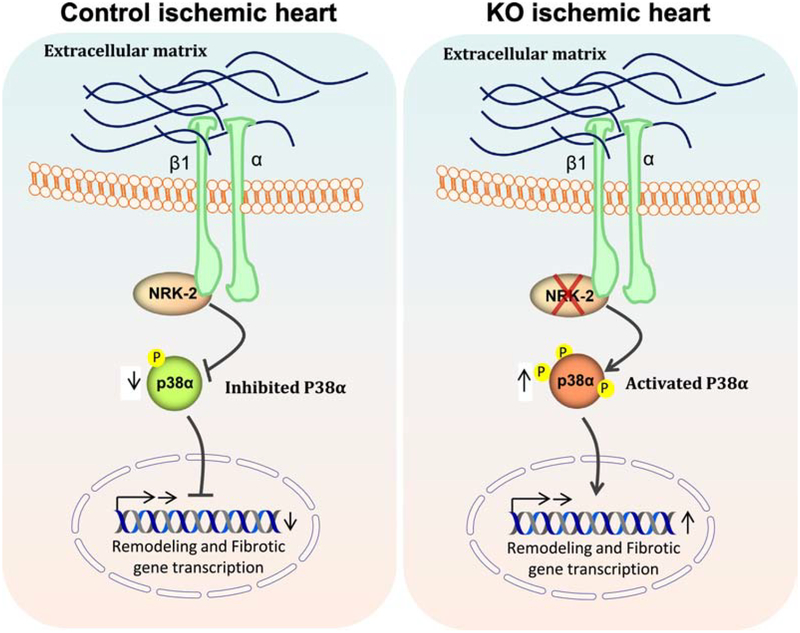
Here we observed a significant increase in NRK-2 expression in mouse as well as human ischemic hearts, consistent with a recent report showing enhanced NRK-2 expression in both mouse and human dilated cardiomyopathy hearts [7]. We have employed, for the first time, NRK2 KO mice to explore the impact of NRK-2 deletion on cardiac function and remodeling outcomes following ischemic injury-induced heart failure. We observed a rapid onset of cardiac dysfunction, more severe remodeling and increased mortality in the KO mice subjected to MI. These adverse phenotypes in the KO mice accompanied with extensive scar formation, dilatative cardiac remodeling and increased fibrosis post-MI. Mechanistically, an increased level of P38α activation was observed in the ischemic KO heart (Fig. 8). The observed detrimental phenotypes in the KO mice suggests that the identified elevated levels of NRK-2 in human and wildtype mouse ischemic hearts is compensatory which probably helps rescue heart from failure by inhibiting P38α MAPK activity.
Figure 8; Loss of NRK-2 exaggerate P38 activation and cardiac remodeling in ischemic heart:
Schematic diagram shows that β1-integrin binding protein NRK-2 inhibits P38α activation however, loss of NRK-2 leads to exaggerated P38α activation in turn promotes dilatative cardiac remodeling and fibrosis post-MI.
Recent studies have demonstrated that the cellular level of NAD+ is regulated by the rate limiting enzymes NAMPT and NRK-2 in skeletal and cardiac muscle [7, 20]. Aberrant NAD+ homeostasis has been reported in heart failure models including MI, pressure-overload, angiotensin II infusion and ischemia/reperfusion[21–24]. Unlike NRK-2, NAMPT is reported to become down-regulated in heart failure [7, 22, 23]. Furthermore, a study demonstrated that the elevated level of NRK-2 in the failing heart is largely due to the inhibition of alternative NAD+ synthesis pathway i.e., NAMPT [7]. Recently, similar group has shown that the role of NRK-2 in maintaining the global NAD+ level in heart and skeletal muscle is dispensable [8]. Studies reveal that NRK-2 has limited role in NAD+ signaling compared to the NAMPT, both in cardiac and skeletal muscle, at least under maladaptive metabolic response to exercise [7, 8]. Thus, the impact of NRK-2 deletion on worsened cardiac outcomes following ischemic injury may be due to other functions, such as dysregulation of cell signaling events.
NRK-2 is a muscle-specific β1 integrin binding protein (ITGB1BP3) that interacts with the cytoplasmic domain of integrin [6, 25]. Integrin-mediated cell signaling is generally propagated through several cytoplasmic proteins including ILK, FAK, Talin, Vinculin and Melusin [26], and is known to regulate cardiac hypertrophy, cardiomyopathy and fibrosis, together contributing to adverse cardiac remodeling and heart failure [27, 28]. NRK2 deficient mice demonstrated eccentric cardiac hypertrophy and accelerated LV chamber dilatation post-MI, however, gain of NRK-2 function in NRVM protects cardiomyocytes against hypoxia/reoxygenation-induced death. Similarly, previous studies indicated that cardiomyocyte-specific deletion of β1 integrin leads to cardiac hypertrophy and fibrosis associated with DCM [29–31]. Importantly, muscle-specific β1-integrin-binding mechanosensitive protein melusin (ITGB1BP2) deficiency leads to DCM and fibrosis due to aberrant signaling through ERK1/2 and GSK-3β in pressure overload [16, 32, 33]. Moreover, a study on talin1 has shown that cardiomyocyte-specific loss of talin1 protects heart from pressure overload-induced hypertrophy and fibrosis by modulating p38α, ERK1/2, Akt and GSK-3β signaling [34]. In contrast, a recent study reveals that deficiency of both talin isoforms leads to myocyte instability and DCM [35].
In current study, we screened canonical signaling pathways involved in regulating cardiac survival and remodeling events in the NRK2 KO hearts which displayed comparable activation of Akt, GSK-3α/β and ERK1/2 compared to control hearts post-MI. However, significantly elevated p38α activation in the ischemic KO hearts suggests the specific repression of p38α activation by NRK-2 that is lost in the NRK2-deficient mice post-MI. Furthermore, ex vivo experiment employing NRK-2 over-expressing NRVMs and AMCFs challenged with TGF-β displayed similar cell signaling results with increased level of α-SMA expression in fibroblast. Therefore, unlike melusin, talin and FAK, NRK-2 does not regulate Akt or ERK signaling, but inhibits p38α activation to protect hearts from ischemic pathological insults.
p38α has been shown to regulate cardiac hypertrophy, cardiomyopathy and fibrosis upon cardiac pathologies [36, 37] and the role particularly in cardiac hypertrophy is controversial [38, 39]. Additionally, studies have shown that loss of p38α function protects the heart from apoptotic cell death in pathological conditions [40]. Similarly, studies from mice and humans have shown that increased activation of p38α leads to dilated cardiomyopathy [41] and increased fibrosis-related gene expression. Moreover, studies have shown that integrins in the cardiac fibroblast acts as a mechano-transducer and regulate the angiotensinogen gene through p38α MAPK signaling [27, 42]. Consistent with these observations, our ex vivo and in vivo studies implies that NRK-2 inhibits p38α activation and genetic ablation of NRK2 leads to scar expansion and thinning, dilatative cardiac remodeling, and excessive fibrosis post-MI.
In conclusion our studies, for the first time, demonstrate the important role of muscle-specific β1 integrin binding protein NRK-2 in the pathogenesis of ischemic cardiac disease. We show that NRK-2 deficiency leads to rapid heart failure post-ischemia which was accompanied by accelerated LV chamber dilatation, cardiac dysfunction and, extensive fibrotic scar formation. Mechanistically, we identified that NRK-2 specifically regulates p38α activation following ischemia, which likely contributes to dilatative fibrotic remodeling and cardiac dysfunction in ischemic heart. Hence, gain of NRK-2 function could be a novel therapeutic strategy to limit chronic MI-induced dilatative cardiac remodeling and heart failure.
Supplementary Material
Study highlights:
NRK-2 expression increases several fold in both mouse and human ischemic hearts
NRK-2 protects heart from dilatative cardiac remodeling in cardiac pathologies post-ischemia
Loss of NRK-2 promotes post-MI scar expansion and thinning, LV dilatation, fibrosis and heart failure
NRK-2 protects cardiomyocytes from hypoxia re-oxygenation
NRK-2 specifically inhibits p38α activation to protect heart from ischemic insult
5. Acknowledgment:
The work was supported by Targeted (VCRG/R.824/2018) and Seed (VCRG/R.449/2018) research grants from University of Sharjah to Firdos Ahmad. The study was also supported by grants from the National Heart, Lung, and Blood Institute to Thomas Force (HL061688 and HL114124), and from American Heart Association (17POST33660251, 19CDA34490009) to Dhanendra Tomar.
Footnotes
Declaration: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. Reference:
- [1].Savarese G, Lund LH, Global Public Health Burden of Heart Failure, Card Fail Rev, 3 (2017) 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].French BA, Kramer CM, Mechanisms of Post-Infarct Left Ventricular Remodeling, Drug Discov Today Dis Mech, 4 (2007) 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Konstam MA, Patterns of ventricular remodeling after myocardial infarction: clues toward linkage between mechanism and morbidity, JACC Cardiovasc Imaging, 1 (2008) 592–594. [DOI] [PubMed] [Google Scholar]
- [4].Begic E, Begic Z, Naser N, Clinical Course and Treatment of Dilated Cardiomyopathy During Twenty Years of Follow-up, Med Arch, 72 (2018) 68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, Song J, Yuan A, Shanmughapriya S, Guo Y, Gao E, Koch W, Woodgett JR, Madesh M, Kishore R, Lal H, Force T, Loss of Adult Cardiac Myocyte GSK-3 Leads to Mitotic Catastrophe Resulting in Fatal Dilated Cardiomyopathy, Circ Res, 118 (2016) 1208–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li J, Mayne R, Wu C, A novel muscle-specific beta 1 integrin binding protein (MIBP) that modulates myogenic differentiation, J Cell Biol, 147 (1999) 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J, Breton M, Decaux JF, Lavery GG, Baczko I, Zoll J, Garnier A, Li Z, Brenner C, Mericskay M, Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy, Circulation, 137 (2018) 2256–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deloux R, Tannous C, Ferry A, Li Z, Mericskay M, Aged Nicotinamide Riboside Kinase 2 Deficient Mice Present an Altered Response to Endurance Exercise Training, Front Physiol, 9 (2018) 1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ, A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse, Circ Res, 107 (2010) 1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ahmad F, Lal H, Zhou J, Vagnozzi RJ, Yu JE, Shang X, Woodgett JR, Gao E, Force T, Cardiomyocyte-specific deletion of gsk3alpha mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure, J Am Coll Cardiol, 64 (2014) 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lal H, Zhou J, Ahmad F, Zaka R, Vagnozzi RJ, Decaul M, Woodgett J, Gao E, Force T, Glycogen synthase kinase-3alpha limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death, Circulation, 125 (2012) 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bensley JG, De Matteo R, Harding R, Black MJ, Three-dimensional direct measurement of cardiomyocyte volume, nuclearity, and ploidy in thick histological sections, Sci Rep, 6 (2016) 23756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, Guo Y, Yu D, Tsai EJ, Woodgett J, Gao E, Force T, Cardiac fibroblast glycogen synthase kinase-3beta regulates ventricular remodeling and dysfunction in ischemic heart, Circulation, 130 (2014) 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lombardi AA, Gibb AA, Arif E, Kolmetzky DW, Tomar D, Luongo TS, Jadiya P, Murray EK, Lorkiewicz PK, Hajnoczky G, Murphy E, Arany ZP, Kelly DP, Margulies KB, Hill BG, Elrod JW, Mitochondrial calcium exchange links metabolism with the epigenome to control cellular differentiation, Nat Commun, 10 (2019) 4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ahmad F, Singh AP, Tomar D, Rahmani M, Zhang Q, Woodgett JR, Tilley DG, Lal H, Force T, Cardiomyocyte-GSK-3alpha promotes mPTP opening and heart failure in mice with chronic pressure overload, J Mol Cell Cardiol, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F, Silengo L, Tarone G, Lembo G, Melusin, a muscle-specific integrin b1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload, Nat. Med, 9 (2003) 68–75. [DOI] [PubMed] [Google Scholar]
- [17].Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G, Integrin signalling: the tug-of-war in heart hypertrophy, Cardiovasc Res, 70 (2006) 422–433. [DOI] [PubMed] [Google Scholar]
- [18].Li R, Wu Y, Manso AM, Gu Y, Liao P, Israeli S, Yajima T, Nguyen U, Huang MS, Dalton ND, Peterson KL, Ross RS, beta1 integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses, Am J Pathol, 180 (2012) 952–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Israeli-Rosenberg S, Manso AM, Okada H, Ross RS, Integrins and integrin-associated proteins in the cardiac myocyte, Circ Res, 114 (2014) 572–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, Garten A, Elhassan YS, Redpath P, Migaud ME, Philp A, Brenner C, Canto C, Lavery GG, Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells, Mol Metab, 6 (2017) 819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mericskay M, Nicotinamide adenine dinucleotide homeostasis and signalling in heart disease: Pathophysiological implications and therapeutic potential, Arch Cardiovasc Dis, 109 (2016) 207–215. [DOI] [PubMed] [Google Scholar]
- [22].Hsu CP, Yamamoto T, Oka S, Sadoshima J, The function of nicotinamide phosphoribosyltransferase in the heart, DNA Repair (Amst), 23 (2014) 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J, Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes, Circ Res, 105 (2009) 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP, Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway, J Biol Chem, 285 (2010) 3133–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li J, Rao H, Burkin D, Kaufman SJ, Wu C, The muscle integrin binding protein (MIBP) interacts with alpha7beta1 integrin and regulates cell adhesion and laminin matrix deposition, Dev Biol, 261 (2003) 209–219. [DOI] [PubMed] [Google Scholar]
- [26].Schwartz MA, Ginsberg MH, Networks and crosstalk: integrin signalling spreads, Nat Cell Biol, 4 (2002) E65–68. [DOI] [PubMed] [Google Scholar]
- [27].Chen C, Li R, Ross RS, Manso AM, Integrins and integrin-related proteins in cardiac fibrosis, J Mol Cell Cardiol, 93 (2016) 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schroer AK, Merryman WD, Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease, J Cell Sci, 128 (2015) 1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS, Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure, Circ Res, 90 (2002) 458–464. [DOI] [PubMed] [Google Scholar]
- [30].Keller RS, Shai S-Y, Babbitt CJ, Pham CG, Solaro RJ, Valencik ML, Loftus JC, Ross RS, Disruption of integrin function in the murine myocardium leads to perinatal lethality, fibrosis, and abnormal cardiac performance, Am. J. Pathol, 158 (2001) 1079–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ross RS, Pham CG, Shai S-Y, G. JI, Fenczik C, Glembotski CC, Ginsberg MH, Loftus JC, b1 integrins participate in the hypertrophic response of rat ventricular myocytes, Circ. Res, 82 (1998) 1160–1172. [DOI] [PubMed] [Google Scholar]
- [32].De Acetis M, Notte A, Accornero F, Selvetella G, Brancaccio M, Vecchione C, Sbroggio M, Collino F, Pacchioni B, Lanfranchi G, Aretini A, Ferretti R, Maffei A, Altruda F, Silengo L, Tarone G, Lembo G, Cardiac overexpression of melusin protects from dilated cardiomyopathy due to long-standing pressure overload, Circ Res, 96 (2005) 1087–1094. [DOI] [PubMed] [Google Scholar]
- [33].Penna C, Brancaccio M, Tullio F, Rubinetto C, Perrelli MG, Angotti C, Pagliaro P, Tarone G, Overexpression of the muscle-specific protein, melusin, protects from cardiac ischemia/reperfusion injury, Basic Res Cardiol, 109 (2014) 418. [DOI] [PubMed] [Google Scholar]
- [34].Manso AM, Li R, Monkley SJ, Cruz NM, Ong S, Lao DH, Koshman YE, Gu Y, Peterson KL, Chen J, Abel ED, Samarel AM, Critchley DR, Ross RS, Talin1 has unique expression versus talin 2 in the heart and modifies the hypertrophic response to pressure overload, J Biol Chem, 288 (2013) 4252–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Manso AM, Okada H, Sakamoto FM, Moreno E, Monkley SJ, Li R, Critchley DR, Ross RS, Loss of mouse cardiomyocyte talin-1 and talin-2 leads to beta-1 integrin reduction, costameric instability, and dilated cardiomyopathy, Proc Natl Acad Sci U S A, 114 (2017) E6250–E6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Marber MS, Rose B, Wang Y, The p38 mitogen-activated protein kinase pathway--a potential target for intervention in infarction, hypertrophy, and heart failure, J Mol Cell Cardiol, 51 (2011) 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yokota T, Wang Y, p38 MAP kinases in the heart, Gene, 575 (2016) 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, Takeda T, Osuka S, Morita T, Kondoh G, Uno Y, Kashiwase K, Taniike M, Nakai A, Matsumura Y, Miyazaki J, Sudo T, Hongo K, Kusakari Y, Kurihara S, Chien KR, Takeda J, HOri M, Otsu K, p38a mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload, Mol. Cell. Biol, 24 (2004) 10611–10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, Wang Y, Muslin AJ, The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis, J Clin Invest, 111 (2003) 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sharov VG, Todor A, Suzuki G, Morita H, Tanhehco EJ, Sabbah HN, Hypoxia, angiotensin-II, and norepinephrine mediated apoptosis is stimulus specific in canine failed cardiomyocytes: a role for p38 MAPK, Fas-L and cyclin D1, Eur J Heart Fail, 5 (2003) 121–129. [DOI] [PubMed] [Google Scholar]
- [41].Muchir A, Wu W, Choi JC, Iwata S, Morrow J, Homma S, Worman HJ, Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation, Hum Mol Genet, 21 (2012) 4325–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].MacKenna DA, Dolfi F, Vuori K, Ruoslahti E, Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts, J Clin Invest, 101 (1998) 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.