Abstract
Stress responses differ by sex, and females are more susceptible to developing mental illnesses because of past stress, including alcohol use disorder. Investigation of neuroadaptations governing the interaction between past stress and future alcohol intake remains understudied in females. A history of footshock stress previously was shown to increase alcohol self-administration under relapse-like conditions in male rats, associated with elevated phosphodiesterase 10A (PDE10A) mRNA expression in the dorsomedial prefrontal cortex and basolateral amygdala. To identify sex differences in long-term stress effects, male and female Wistar rats were exposed to light-cued footshock stress prior to alcohol self-administration training. While past stress did not alter acquisition or extinction, reacquisition self-administration was oppositely impacted by past stress. Stress history slightly increased reacquisition self-administration in males, but reduced alcohol self-administration in females, relative to same-sex controls. Control females self-administered less alcohol following glucocorticoid receptor inhibition by mifepristone, which did not significantly alter alcohol consumption in the other groups. PDE10A expression in synaptically enriched fractions also differed by sex and stress history in a brain region-specific manner. Females expressed more synaptic PDE10A than males in basolateral amygdala and dorsolateral striatum, regardless of stress history, whereas dorsomedial prefrontal cortex PDE10A protein levels matched group differences in reacquisition drinking, but also were expressed at much lower levels than all other regions examined. Together, these data show stress history differentially impacts alcohol self-administration and PDE10A expression by sex, with control females consuming alcohol in a glucocorticoid receptor-sensitive fashion that may relate to sex differences in PDE10A expression.
Keywords: ethanol, sex differences, stress history, relapse, footshock, mifepristone
1. INTRODUCTION1
Alcohol use disorder (AUD) is a highly prevalent chronic disease afflicting 14% of the American population over the age of 15 (World Health Organization, 2018) and is characterized by high rates of recidivism following periods of abstinence (Dawson et al., 2007; Moos and Moos, 2006). Stress is a risk factor for AUD, contributing to both development of and relapse to excessive alcohol consumption (Armstrong et al., 2018; Sinha, 2012). The impact of biological sex on stress remodeling of the brain and how this alters alcohol use remain incompletely understood. Studies suggest greater susceptibility of females to stress potentiation of alcohol consumption (Armstrong et al., 2018; Peltier et al., 2019), with traumatic experiences more often preceding AUD development (Sonne et al., 2003) and associated with self-reported craving and relapse in females vs. males (Heffner et al., 2011; Lehavot et al., 2014). Understanding how past stress differentially alters alcohol self-administration and identifying underlying differences in neurochemistry are highly important for improving treatments for both sexes.
While exploration of sex differences in stress-alcohol interactions has received insufficient attention, existing studies show varied results (Logrip et al., 2018). Depending on stress modality and timing relative to alcohol access, drinking has been increased (Cozzoli et al., 2014; Finn et al., 2018), decreased (Chester et al., 2006; Quadir et al., 2019; Wille-Bille et al., 2017), or unchanged (Finn et al., 2018; Quadir et al., 2019) by stress in females, relative to controls, at times in opposition to the same stressors’ effects on male drinking. This divergence in stress effects across both sexes highlights the importance of elucidating sex differences, or lack thereof, in stress-alcohol interactions across a variety of stress parameters. Given the epidemiological support for stronger ties between past stress and relapse in females, it is critical to determine the long-term impact of stress on relapse-like alcohol intake in rodent models.
Unlike more limited data for sex differences in the longitudinal effects of stress on alcohol consumption, several stressors have been investigated for effects on male drinking over protracted time frames. A history of repeated footshock stress increased reacquisition of operant alcohol self-administration in a subset of male rats, relative to controls, without affecting acquisition, maintenance, or extinction (Logrip and Zorrilla, 2012). Footshock stress also increased alcohol intake in males when the stress exposure preceded the onset of alcohol access, but did not alter drinking patterns after escalation had occurred (Meyer et al., 2013). These models of footshock stress as a pre-existing factor contrast with predator odor, which increased alcohol intake in a subset of male rats (Edwards et al., 2013), but only in animals with significant prior drinking experience (Finn et al., 2018; Zoladz et al., 2018). These findings highlight the importance of modality and timing in the stress-alcohol relationship and support the use of repeated footshock stress to investigate sex differences in longitudinal effects of distal past stress on relapse-like reacquisition of alcohol self-administration and its molecular underpinnings.
Whereas sex differences in rodent alcohol intake and stress response have been demonstrated, the neuroadaptations which underlie these behavioral differences remain incompletely understood. One system demonstrating differential response magnitude in males vs. females is the hypothalamic-pituitary-adrenal axis response to stress or alcohol, resulting in release of corticosterone (Rivier, 1993, 1999). The contribution of corticosterone to relapse-like drinking modulated by a distal stress exposure, and whether this differs by sex, remains unknown. Previous studies showed differential effects of pharmacologically inhibiting corticosterone’s actions at its two receptor subtypes. Inhibition of the mineralocorticoid receptor reduced operant alcohol self-administration in both male and female rats (Makhijani et al., 2018), whereas blockade of the glucocorticoid receptor (GR) specifically decreased alcohol self-administration in alcohol-dependent, but not nondependent, male rats (Somkuwar et al., 2017; Vendruscolo et al., 2012; Vendruscolo et al., 2015). The GR antagonist mifepristone also reduced stress-induced reinstatement of alcohol-seeking, but not stress-potentiated alcohol self-administration, in male rats (Simms et al., 2012), but these GR effects have not been examined in females. Sex differences have been demonstrated for corticosterone modulation of glutamatergic activity, with the magnitude of excitatory postsynaptic potentials reduced by corticosterone application in female but not male lateral central amygdala neurons (Logrip et al., 2017), an effect that occluded further responsiveness to alcohol. Stress alters glucocorticoid responsiveness for a longer duration in females vs. males, with GR levels elevated for at least 1 week after predator stress (Finn et al., 2018). These studies raise the possibility that females and rats with a stress history may display sensitivity to GR regulation of reacquisition alcohol self-administration in the absence of alcohol dependence.
While corticosterone presents a general mechanism that may regulate relapse-like alcohol self-administration, sex differences in neuroadaptations following stress that persist to modulate alcohol consumption weeks or months later must be elucidated to establish the molecular bases for sex differences in stress-alcohol interactions. One candidate for regulation of both stress history and dependence effects on alcohol self-administration is phosphodiesterase 10A (PDE10A), a dual-specificity phosphodiesterase capable of deactivating both cyclic adenosine (cAMP) and cyclic guanosine monophosphate (cGMP) (Fujishige et al., 1999; Loughney et al., 1999; Soderling et al., 1999). PDE10A is most abundant in the striatum, where it localizes to the postsynaptic membrane (Kotera et al., 2004; Xie et al., 2006) adjacent to the activation sites of various signaling cascades it can regulate. PDE10A is expressed at lower levels in multiple brain regions (Seeger et al., 2003), including the amygdala and prefrontal cortex (Logrip and Zorrilla, 2014), components of the circuitry thought to underlie stress potentiation of alcohol drinking (Gilpin and Weiner, 2017). Pde10a mRNA expression was elevated in the basolateral amygdala (BLA) of male Wistar rats during acute withdrawal from 5 weeks’ alcohol vapor-induced dependence, an effect that persisted at least 6 weeks into abstinence, relative to alcohol-naïve males (Logrip and Zorrilla, 2014). Pde10a mRNA also was higher in the BLA of males with a history of stress, relative to stress-naïve males, as well as in the dorsomedial prefrontal cortex (dmPFC) of stress history male rats whose self-administration increased following renewed access in reacquisition, but not in controls or rats whose intake was unchanged after extinction (Logrip and Zorrilla, 2012). Inhibition of PDE10A reduced alcohol self-administration in male rats, including those with a history of stress, alcohol dependence, or a genetic predisposition to consume large amounts of alcohol (Logrip et al., 2014). Together, these data support a role for PDE10A as a stress-induced protein which may potentiate alcohol intake in males, yet no data exist regarding sex differences in PDE10A expression or mediation of stress-alcohol interactions.
Given the paucity of information regarding sex differences in the impact of footshock stress as a pre-existing factor on alcohol self-administration, as well as the lack of data regarding PDE10A expression or function in females, particularly with respect to stress history and alcohol intake, we proposed three hypotheses to address these gaps in the literature. First, we hypothesized that females with a history of stress would show greater increases in relapse-like reacquisition of alcohol self-administration than males. Second, we hypothesized that alcohol self-administration during reacquisition would be sensitive to GR inhibition in rats with a history of stress, and that these effects would be more marked in females. Finally, we hypothesized that increased alcohol self-administration would be accompanied by higher levels of PDE10A at the synapse in the BLA and dmPFC, and that this effect would differ by sex and stress history. These studies aim not only to elucidate sex differences in the impact of past stress on future alcohol intake and PDE10A expression, but also to determine whether the distal effects of past stress on relapse-like alcohol intake are due to corticosterone’s action on the GR.
2. MATERIALS AND METHODS
2.1. Animals
Adult male and female Wistar rats were obtained from Charles River Laboratories (CRL; Sections 3.1, 3.3: Kingston, NY, USA and Saint Constant, Canada) or ENVIGO (Section 3.2: Indianapolis, IN, USA) at 8–9 weeks old. The CRL Wistar line has been maintained according to International Genetic Standardization protocols to reduce genetic drift in the outbred lines between facilities since 2012, so behavioral results are anticipated to be comparable among colonies, while similar behavioral effects in CRL and ENVIGO Wistars suggest that the current findings do not result from genetic drift between CRL and ENVIGO Wistar lines. Upon arrival in the facility, rats were maintained on a 12h:12h reverse light-dark cycle, with food and water available ad libitum, and housed in a temperature- and humidity-controlled room. Rats were pair-housed until the beginning of experimental manipulation, at which point they were individually housed for the remainder of the experiment. All experimental procedures were according to the Guide for the Care and Use of Laboratory Animals and were approved by the IUPUI Institutional Animal Care and Use Committee.
2.2. Behavioral chambers
Self-administration sessions were performed in standard operant chambers equipped with two retractable levers flanking a central recessed reward port, with liquid reinforcer delivery controlled by a motorized syringe pump (Med Associates, Inc., Fairfax, VT). Behavioral sessions were controlled and recorded by Med-PC IV software (Med Associates), with operant sessions commencing upon lever insertion into the chamber and concluding with retraction of the levers into the wall. For footshock sessions, the reward port was replaced with a flat wall panel and levers maintained the retracted position throughout the session. As for operant sessions, cue and footshock delivery were regulated by Med PC-IV.
2.3. Behavioral procedures
Procedures were performed, with minor modifications, as in Logrip & Zorrilla (2012), with pre-stress components (see Section 2.3.1) designed to prevent confounds from stress-induced neophobia (Job and Barnes, 1995). No operant self-administration of alcohol occurred until after the conclusion of footshock stress exposure, and thus stress effects are designated throughout as “Stress History”. Rats were run in 4 cohorts, with 2 cohorts per behavioral experiment.
2.3.1. Pre-exposure:
Rats were given 48-h continuous access to two bottles in their home cages, one containing water and the other 10% (v/v) alcohol (2-bottle choice), followed by 4 sessions of 1-h 2-bottle choice access to alcohol vs. water provided once daily and time-matched to future operant self-administration sessions. Across the 1-h access days, rats acquired operant lever pressing behavior in a single, 12-h, water-reinforced session, in which responses on the right (active) lever delivered 0.1 mL water on a fixed-ratio 1 schedule (FR1; 1 press = 1 reinforcer delivery), and presses on the left (inactive) lever produced no consequences. Rats had ad libitum access to standard chow in the chamber during the session. As this training spanned an entire dark cycle, the house light was not illuminated. Sessions terminated upon completing 300 responses on the active lever or after 12 h, whichever occurred first. Lever retraction into the walls signaled the end of the session. This training paradigm resulted in robust acquisition of lever-pressing behavior in most rats.
2.3.2. Stress exposure:
Rats were separated into stress or control groups, balanced for home-cage alcohol consumption and performance on both levers in the water training session. On the day after the final 1-h 2-bottle choice exposure, rats in the stress group experienced footshock sessions once per day for three consecutive days. For each session, rats were placed in chambers with physically distinct locations, relative to the chamber used for each rat’s operant training sessions, and shocks were administered through electrification of the rod floor on a variable interval schedule (11–50 sec ITI). Each shock was preceded by 5 sec of house light illumination, co-terminating with a 1-sec, 0.4-mA footshock (total light illumination time = 6 sec per presentation). Stress sessions terminated after sixty light-shock presentations. Control animals were exposed to the same pattern of house light illumination in the absence of footshock.
2.3.3. Operant alcohol self-administration:
At least 36 h after the final footshock session, rats began operant self-administration training for 0.1-ml alcohol reinforcers (10%, v/v). A timeout interval of 0.84 sec was incorporated for the time required to run the syringe pump; presses on the active lever during the timeout intervals are included in the active lever data presented but did not count toward subsequent reinforcement. Operant training sessions lasted 1 h and were administered every 2–3 days. The first 5 sessions were reinforced on a FR1 schedule, with the effort requirement increased to FR3 for 10 sessions to produce stable baseline active lever responding. Rats then commenced extinction training, during which lever presses on the active lever were not reinforced. This resulted in low but nonzero responding on the active lever by the end of 15 extinction training sessions. The final training phase, reacquisition, examined changes in willingness to work for alcohol reinforcement after a period of forced deprivation (extinction), modeling some features of relapse-like behavior. During reacquisition, the active lever was alcohol-reinforced at FR3, and rats robustly reacquired their lever-pressing behavior over the course of 9 sessions. Electrical problems with 5 female boxes over 1 or more sessions of FR3 acquisition resulted in exclusion of a subset of rats in cohort 1 due to premature extinction training. Additionally, 3 female rats failed to acquire sufficient active lever pressing behavior for water or alcohol reinforcement and were excluded from the study (active lever presses: 12-h water session, 9, 22 and 39; across alcohol-reinforced or extinction sessions: single-day maxima, 3, 9 and 16; averages 0.5 – 5.6 active lever presses per session).
2.3.4. Mifepristone effects on reacquisition of alcohol self-administration:
Male and female Control and Stress History rats were exposed and trained as above, and systemic injections of the glucocorticoid receptor antagonist mifepristone (0 vs. 30 mg/kg) delivered subcutaneously 90 min prior to an alcohol-reinforced operant session during the reacquisition phase of training (dosing at minimum effective dose of Vendruscolo et al., 2015). Mifepristone (Millipore Sigma, St. Louis, MO, USA) was prepared in a vehicle of 5% dimethyl sulfoxide (Millipore Sigma), 5% Kolliphor EL (Millipore Sigma), and 90% saline (preservative-free 0.9% sodium chloride, Hospira, Lake Forest, IL, USA). Doses were delivered using a within-subjects design, with vehicle vs. mifepristone treatment day randomly assigned, counterbalanced by sex and stress history across the treatment days. A no-treatment operant session intervened between the two treatment days.
2.4. Euthanasia, sample collection & protein analysis
Rats were euthanized 48 h after the final self-administration session and tissue punches were collected for later protein analysis. Rats were lightly anesthetized with isoflurane, rapidly decapitated, brains removed, and prefrontal, striatal and amygdalar tissue punches collected from 2-mm coronal sections prepared on an ice-cold metal block, frozen on dry ice and stored at −80°C until fractionation. Trunk blood was collecte d for measurement of circulating corticosteroids. To avoid potential confounds from hormonal regulation of protein expression on female measurements, female rats’ estrous cycles were synchronized via injection of a synthetic gonadotropin releasing hormone (GnRH) analog ([Des-Gly10,D-Trp6,Pro-NHEt9]-LHRH, Bachem Americas, Inc., Torrance, CA, USA) as in Logrip et al. (2017), with brains collected during diestrus. Briefly, female rats received two, 2-μg doses of the GnRH analog, prepared in a vehicle of sterile phosphate buffered saline containing 2 mM acetic acid. Doses were administered 3 and 8 h into the light cycle to mimic hormone surges that trigger the onset of proestrus (Ogilvie and Rivier, 1997). Due to the reverse light cycle, these during the overnight hours before the final alcohol self-administration session, 2.5 days prior to euthanasia.
2.4.1. Synaptic fractionation:
To isolate postsynaptic density-enriched proteins, tissue punches were processed according to Hallett et al. (2008), with volume modifications for small tissue size (Logrip and Zorrilla, 2014), and fractions named below per conventions set out by Hallett and colleagues. Briefly, samples were homogenized via rotor-stator homogenizer (TissueTearor, Cole-Parmer Instrument Co., Vernon Hills, IL, USA) in TEVP homogenization buffer, containing, in mM: 10 Tris base, 1 EDTA, 1 EGTA, 5 NaF, 1 Na3VO4, 320 sucrose and Roche cOmplete and PhosSTOP protease and phosphatase inhibitors (Millipore Sigma). Some lots of TEVP did not contain sodium phosphate or sodium orthovanadate in the stock buffer since these are somewhat redundant with cOmplete and PhosSTOP inhibitors. Lysates (LYS) were cleared in an initial 800 × g spin, with supernatant (S1) fractionated into membrane-associated (pellet P2) and cytosolic (supernatant S2) proteins by a subsequent 9,600 × g spin. The P2 pellet was rinsed and hypo-osmotically lysed in low-sucrose TEVP buffer, identical to TEVP homogenization buffer but containing 35.6 mM, rather than 320 mM, sucrose. After 30-min incubation on ice to complete hypo-osmotic lysis, samples were centrifuged at 25,000 × g for 20 min to pellet postsynaptic density-enriched membranes (LP1), which were resuspended in TEVP and quantified using a detergent-compatible Lowry assay (RC-DC, Bio-Rad Laboratories, Hercules, CA).
2.4.2. Polyacrylamide gel electrophoresis and western blotting:
LP1 [5 μg except BLA (4.5 μg) and nucleus accumbens (NAc, 7.5 μg)] or LYS (16 μl) samples were resolved on Novex Tris-Glycine 8–16% gradient gels (ThermoFisher Scientific, Pittsburgh, PA, USA) and transferred to low-fluorescent PVDF membranes (Immobilon-FL, Millipore Sigma, or Immun-Blot Low-Fluorescence PVDF, Bio-rad Laboratories). Membranes were blocked in tris-buffered saline containing 0.1% tween-20 (Millipore Sigma) and either 3% (LYS) or 5% (LP1) milk (Carnation Nonfat Dry Milk, Nestle USA, Rosslyn, VA, USA). PDE10A primary antibodies (SAB2700582, Millipore Sigma) were prepared in milk blocking buffer at concentrations based on quantity of protein loaded and brain region abundance: 1:500, LYS; 1:1000, amygdala and prefrontal cortex LP1; 1:2500, dorsolateral striatum (DLS) and dorsomedial striatum (DMS) LP1; 1:5000, NAc LP1. Membranes were incubated overnight in primary antibodies at 4°C and for 1 h at room temperature in anti-rabbit AffiniPure Alexa Fluor 790-conjugated secondary antibodies (1:20,000, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Membranes were visualized on a LI-COR CFx scanner (Lincoln, NE, USA), and protein loading was normalized via 0.4% Coomassie staining (Varodayan et al., 2017) at the end of the experiment. Low protein yield from the first behavior cohort limited Western blot results to samples from second cohort rats, and minimal volume of LYS kept out of the fractionation (to maximize LP1 yield) precluded quantification of protein concentration before blotting.
2.5. Corticosterone Measurement
Blood samples collected at euthanasia were centrifuged to isolate plasma (10 min, 1,000 × g, 4°C), and corticosterone levels were assessed using the DetectX® Corticosterone Enzyme Immunoassay (Arbor Assays, Ann Arbor, MI, USA).
2.6. Data analysis
2.6.1. Behavioral analyses:
Operant data were analyzed for changes in three parameters: (1) active lever pressing, defined as all presses on the reinforced lever, regardless of reinforcement; (2) inactive lever pressing, defined as all presses on the unreinforced lever; (3) weight-normalized alcohol intake during FR3-reinforced sessions, calculated as g alcohol reinforcers earned divided by the rat’s body weight in kg (g/kg alcohol intake). Data were analyzed by repeated measures analyses of variance (RM-ANOVA), with the between-subjects factors of Sex (Male vs. Female) and Group (Control vs. Stress History) and the within-subjects factors Session or Dose, as appropriate. Because dosing occurred only on two days, it was necessary to examine whether the order of dosing impacted our findings. It was determined that Dose Order for mifepristone treatments did not significantly affect our results (active lever presses: F’s < 2.26, p’s > 0.14; alcohol intake: F’s < 3.04, p’s > 0.09), and thus was excluded from the analyses presented.
To assess changes in alcohol consumption during reacquisition, data were expressed as fold change in alcohol intake during each reacquisition session, relative to baseline, calculated as an average of the final 5 FR3-reinforced sessions prior to extinction training, and analyzed by RM-ANOVA as above. Significant interactions in which sex was an interacting factor were followed by separate ANOVAs by sex. Post hoc comparisons were performed via Tukey’s test. Data are presented as mean ± standard error of the mean (S.E.M.). For ease of visualization, data are graphically represented on separate panels by sex, with matched scales.
To determine whether the efficacy of mifepristone treatment was related to rats’ pre-treatment level of alcohol consumption, Pearson correlations were used to compare weight-normalized alcohol intake (g/kg) on vehicle treatment day with the percent change in active lever presses after mifepristone treatment, relative to vehicle treatment. The percent change in active lever responding was calculated as [(active lever presses, mifepristone) – (active lever presses, vehicle)] / (active lever presses, vehicle).
2.6.2. Molecular analyses:
Western blot data were quantified using Image Studio Lite (LI-COR) for fluorescent PDE10A signals and ImageJ (National Institutes of Health, Bethesda, MD, USA) for Coomassie total-protein staining. Proteins were normalized within-sample to total protein loading and across blots to a 10-μg standard rat brain lysate sample run on every gel for cross-blot normalization. Grubbs test was used to identify Coomassie outliers, which eliminated one DMS sample. Data violating ANOVA assumptions were transformed and re-analyzed, where indicated, as follows: for normality violations, data were log10 transformed; for heteroscedasticity violations, data were square root transformed. Statistics are provided for both the non-transformed and transformed analyses. Corticosterone ELISA standards were used to generate an optimal fit 4-parameter standard curve from which sample values were extrapolated (Sigma Plot 14.0, Systat Inc., Chicago, IL, USA). Both Western blot and corticosterone data were analyzed via 2-way ANOVA, with Sex and Group as the between-subjects variables. Significant interactions were explored with post hoc Tukey’s tests. Data are presented as mean ± S.E.M., with individual data points overlaid on the mean histograms to demonstrate the spread of the data.
2.6.3. Comparison of behavioral and molecular data:
Average alcohol intake across the reacquisition phase was compared to corticosterone levels at euthanasia via Pearson correlation. Protein expression was compared to reacquisition self-administration and corticosterone levels where ANOVA findings supported an a priori expectation of relationship between the two data modalities, also via Pearson correlation.
2.6.4. Statistical Software:
Statistical analyses and graphical representation of data were generated using SigmaPlot 14.0, Systat 13.1 (Systat Software, Inc., San Jose, CA, USA) and GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA).
3. RESULTS
3.1. Stress history oppositely modulates alcohol self-administration by sex
Male and female Control and Stress History rats self-administered 10% (v/v) alcohol in 1-h operant sessions, with reinforcement earned after 1 (FR1) or 3 (FR3) presses, or unreinforced (EXT), as indicated in Figures 1 and 2. Stress History differentially affected male and female operant behavior and alcohol intake, particularly during the reacquisition phase.
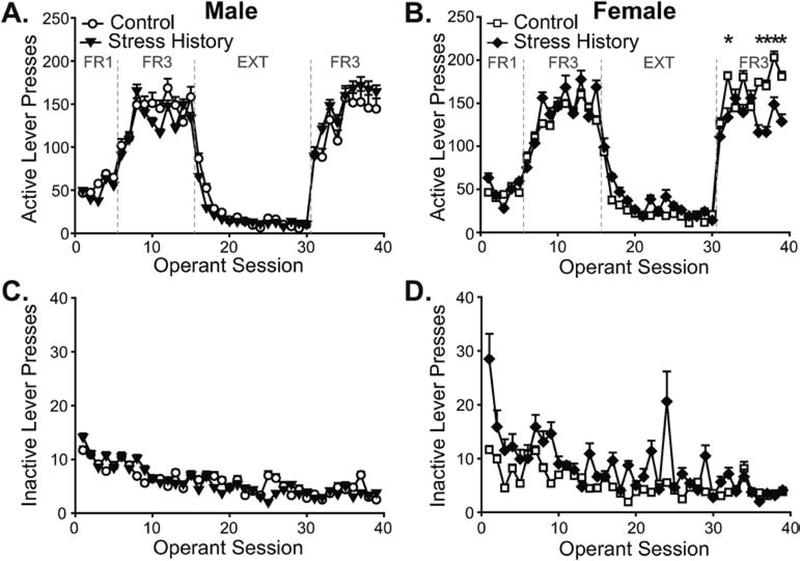
Figure 1. Stress history differentially alters alcohol-reinforced operant behavior by sex.
Male (A,C) and female (B,D) rats without (Control, open shapes) and with (Stress History, filled shapes) previous exposure to light-cued footshocks performed operant responses over 1-h sessions to earn alcohol reinforcers. Presses on the active lever (A,B) were reinforced as labeled in panels A-B, with alcohol delivery after 1 (fixed ratio 1, FR1) or 3 (FR3) lever presses, or with no alcohol delivery during extinction (EXT). Data are presented separately by sex for ease of visualization. Presses on the inactive lever (C,D) were never reinforced. Data are displayed as mean ± S.E.M. and shown across all 39 operant sessions. * p < 0.05, Control vs. Stress History. n = 10, Control Males; n = 12, Stress History Males; n = 11, Control Females; n = 8, Stress History Females.
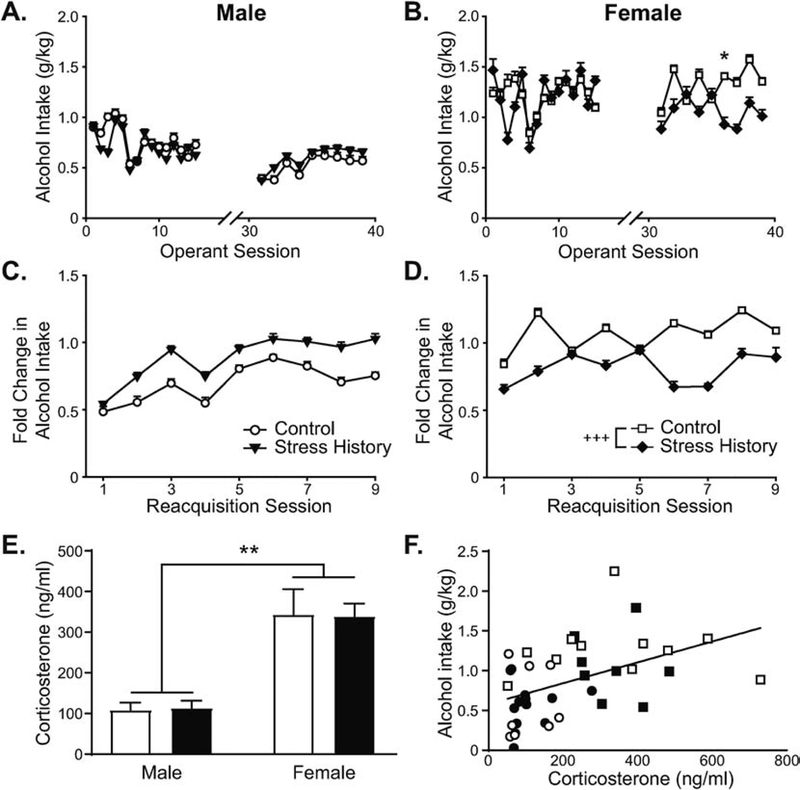
Figure 2. Alcohol intake during FR3-reinforced operant sessions differs by sex, and stress history bi-directionally changes reacquisition alcohol intake in male vs. female rats.
Male (A,C,E,F) and female (B,D,E,F) rats without (Control, open shapes) and with (Stress History, filled shapes) previous exposure to light-cued footshock self-administered 0.1-mL 10% (v/v) alcohol reinforcers in 1-h operant sessions. (A,B) Alcohol intake, expressed as g alcohol consumed normalized to body weight in kg, is shown for all FR3 sessions across the experiment. (C,D) Change in alcohol intake after extinction training, displayed as g/kg alcohol intake normalized to each rat’s pre-extinction average g/kg intake, is shown for each reacquisition session. (E) Corticosterone levels were measured from plasma collected at euthanasia, expressed as ng corticosterone per ml plasma. (F) Average alcohol intake across the reacquisition period was positively related to corticosterone levels at euthanasia. Data are presented as mean ± S.E.M. (A-E) or individual values with sex indicated by shape (circles – males; squares – females) and group indicated by fill (open – Control; filled – Stress History) (F). Multi-session data are displayed on separate panels by sex for ease of visualization (A-D). * p < 0.05, Control vs. Stress History; ** p < 0.01, Male vs. Female; +++ p < 0.001, Control vs. Stress History (main effect of group across the reacquisition period). n = 8–12 per group.
3.1.1. Lever pressing:
Analysis of active lever pressing across all 39 sessions by 3-way ANOVA yielded a main effect of session (F38,1406 = 65.69, p < 0.001), as well as a 3-way interaction between Session, Sex and Group (F38,1406 = 1.58, p = 0.014). Subsequent within-sex analyses by 2-way RM-ANOVA produced differential findings by sex. For males, only the main effect of Session was significant (F38,760 = 31.60, p < 0.001; Figure 1A), without effect of Group or interaction between the factors (F’s < 0.54, p’s > 0.96). Females showed a significant interaction between Session and Group (F38,646 = 1.71, p = 0.006; Figure 1B) due to greater active lever pressing in Control vs. Stress History females over multiple sessions in the reacquisition phase [Group effects by Tukey test for sessions 32 (p = 0.032), 36 (p = 0.010), 37 (p = 0.017), 38 (p = 0.015), 39 (p = 0.020)]. Analysis of data by training phase demonstrated that whereas early FR1-reinforced learning differed by stress history, independent of sex (Group by Session interaction, F4,148 = 2.55, p = 0.041), sex impacted how active lever pressing changed across FR3 acquisition (Session by Sex interaction, F9,333 = 2.12, p = 0.027) and reacquisition (Session by Sex interaction, F8,296 = 2.91, p = 0.004). A main effect of Sex also was observed for extinction responding (F1,37 = 6.35, p = 0.016), independent of Group (F’s < 1.91, p’s > 0.17), and without significant interaction with Session (F’s < 1.69, p’s > 0.055). Females responded more across all extinction sessions, decreasing from 95.6 ± 13.7 active lever presses on extinction day 1 to 17.9 ± 3.5 presses on day 15, compared to 75.5 ± 9.5 presses on day 1 and 10.6 ± 1.7 presses on day 15 for males.
Unlike active lever responding, inactive lever presses (males, Figure 1C; females, Figure 1D) only significantly differed by session when analyzed by 3-way RM-ANOVA (main effect of Session, F38,1406 = 6.91, p < 0.001) without significant effects of Sex, Group, or interactions between the factors (F’s < 1.40, p’s > 0.054). Focusing on patterns within individual training sectors yielded no significant effects or interactions by Group (F’s < 2.36, p’s > .055), although sex affected how inactive lever pressing changed across sessions, with Session by Sex interactions observed during FR1 acquisition (F4,148 = 2.81, p = 0.028), extinction (F14,518 = 1.82, p = 0.034), and reacquisition (F8,296 = 2.10, p = 0.036).
3.1.2. Alcohol intake:
Similar to active lever pressing, weight-normalized alcohol intake differed by Sex and Group, with 3-way RM-ANOVA yielding main effects of Sex (F1,37 = 28.63, p < 0.001) and Session (F18,666 = 6.41, p < 0.001), as well as interactions between Session and Sex (F18,666 = 2.81, p < 0.001) and between Session, Sex, and Group (F18,666 = 2.63, p < 0.001). Breaking down this 3-way interaction by sex, 2-way RM-ANOVAs demonstrated main effects of Session for males (F18,360 = 6.72, p < 0.001; Figure 2A) and females (F18,306 = 3.60, p < 0.001; Figure 2B) and a significant interaction between Session and Group only for females (F18,306 = 1.93, p = 0.014). Post hoc analyses identified higher intake in Control vs. Stress History females only for session 36 (p = 0.045), although group differences were just above the cutoff for significance in sessions 37 (p = 0.055) and 38 (p = 0.071). Separate analyses of alcohol intake by training sector yielded significant main effects of Sex (F’s > 24.93, p’s <0.001) and Sex by Session interactions (F’s > 2.07, p’s < 0.04) during both FR3-reinforced acquisition and reacquisition. A trend towards a 3-way interaction between Sex, Group, and Session also was observed for reacquisition (F8,296 = 1.84, p = 0.069).
3.1.3. Alcohol intake changes after extinction training:
Previous experiments in male rats (Logrip et al., 2014; Logrip and Zorrilla, 2012) demonstrated a critical function of stress history to change alcohol self-administration after extinction training, relative to baseline, in a subset of rats, such that looking only at weight-normalized mean alcohol intake levels might obscure visualization of stress history effects on drinking. This a priori expectation supported further examination of the change in alcohol self-administration during reacquisition, relative to pre-extinction intake. Analysis of the change in alcohol consumed during operant sessions, expressed as fold change from baseline, by 3-way ANOVA produced a main effect of Sex (F1,37 = 4.91, p = 0.033) and a Sex by Group interaction (F1,37 = 11.18, p = 0.002). While Sex interacted with Session (F8,296 = 2.77, p = 0.006), the Sex by Group interaction did not change across sessions (F8,296 = 0.99, p = 0.44). Analysis of Group effects within sex by 2-way ANOVA produced a significant effect of Session (F8,160 = 6.72, p < 0.001) but only a weak trend for stress history to increase reacquisition alcohol intake in males (F1,20 = 3.02, p = 0.098, Figure 2C). Conversely, females with a stress history displayed significantly less reacquisition alcohol self-administration, relative to baseline, than same-sex controls (main effect of Group, F1,17 = 14.89, p = 0.001), without effects of Session or interaction (F’s < 1.86, p’s > 0.07). Together these results support differential effects of stress history on post-extinction alcohol self-administration, with reacquisition of alcohol self-administration slightly increased in males but substantially blunted in females, relative to same-sex controls, suggesting divergent, long-lasting molecular adaptations to stress may differentially regulate male and female operant alcohol intake.
3.1.4. Corticosterone levels relate to past alcohol intake:
The impact of stress history on corticosterone levels and their relationship to reacquisition self-administration were assessed using serum samples collected at euthanasia, which was timed to approximate the expected onset of the next self-administration session (Figure 2E). Analysis of corticosterone levels by 2-way ANOVA revealed a main effect of Sex (F1,36 = 33.81, p < 0.0001), without effect of Group or interaction between the factors (F’s < 0.015, p’s > 0.90). Plasma corticosterone levels positively correlated with average alcohol intake during reacquisition (Figure 2F; R = 0.459, p = 0.003), suggesting the possibility that corticosterone may be a driver of reacquisition alcohol self-administration.
3.2. Glucocorticoid receptor inhibition reduces self-administration in control females
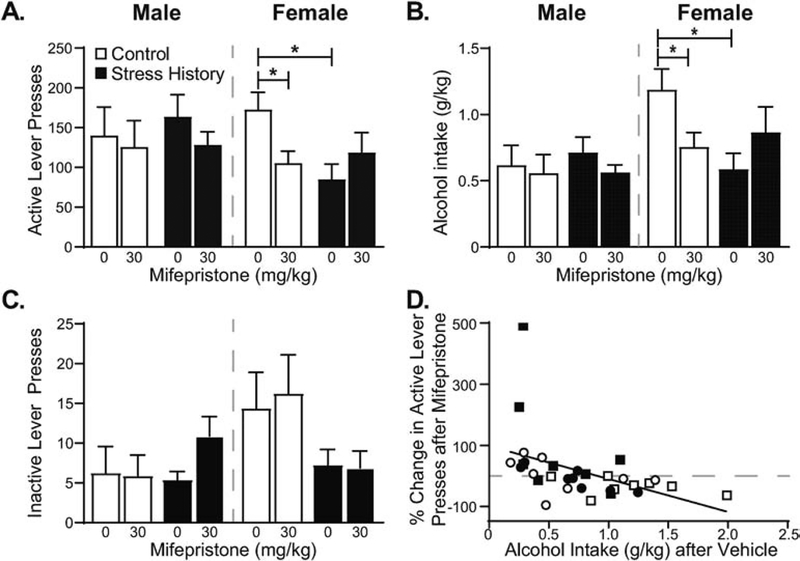
To investigate the functional contribution of corticosterone to reacquisition alcohol self-administration, rats were treated subcutaneously with the glucocorticoid receptor inhibitor mifepristone (0, 30 mg/kg) 90 min prior to reacquisition self-administration sessions, as in (Vendruscolo et al., 2015). Analysis of lever pressing data by 3-way RM-ANOVA yielded an interaction between Sex, Group, and Dose (F1,28 = 6.73, p = 0.015; Figure 3A), borne out in 2-way RM-ANOVAs by sex as a lack of treatment effect in males (F’s < 2.46, p’s > 0.13) but a significant Dose by Group interaction in females (F1,14 = 8.53, p = 0.011) due to decreased active lever presses in Control, but not Stress History, females after mifepristone treatment. Weight-normalized alcohol intake similarly was reduced only in control females after mifepristone treatment (Figure 3B), yielding a Dose by Sex by Group interaction in the 3-way ANOVA (F1,28 = 8.24, p = 0.008) and a Dose by Group interaction in the female-only 2-way ANOVA (F1,14 = 8.48, p = 0.011). Post hoc testing confirmed significant effects of mifepristone treatment in Control females (active lever presses, p = 0.016; alcohol intake, p = 0.026), as well as significant differences between Control and Stress History females following vehicle treatment (active lever presses, p = 0.006; alcohol intake, p = 0.009). Interestingly, inactive lever presses (Figure 3C) also changed after mifepristone treatment in a dose- and sex-dependent fashion (F1,28 = 4.56, p = 0.042). However, follow-up within-sex analyses yielded no significant effects, although males showed a trend toward a Dose by Group interaction (F1,14 = 3.76, p = 0.073) not observed in females (F’s < 2.75, p’s > 0.11), likely due to increased inactive lever pressing after mifepristone treatment in Stress History males. Examination of the relationship between alcohol intake on vehicle treatment day and the change in active lever pressing caused by mifepristone treatment, expressed as percent change normalized to vehicle baseline, yielded a significant negative correlation (Figure 3D; R = −0.446, p = 0.0088). Together these data show GR regulation of alcohol self-administration in nondependent females, suggesting that sex differences in molecular adaptations to alcohol intake and past stress may underlie these differential drinking patterns.
Figure 3. Alcohol intake is selectively reduced by mifepristone in the highest-drinking, footshock stress-naïve females.
Male and female rats were treated with mifepristone (0, 30 mg/kg, subcutaneous) 90 min prior to reacquisition self-administration sessions using a within subjects design, counterbalanced by group across treatment days. Mifepristone treatment significantly reduced active lever presses (A) and weight-normalized alcohol intake (B) in Control females, but minimally change inactive lever pressing (C). Data are expressed as mean ± S.E.M., with sex, group and dose as indicated. (D) Rats consuming more alcohol (g/kg) on vehicle treatment day showed greater reductions in active lever pressing after mifepristone treatment, expressed as percent change in active lever presses, [(active lever presses, mifepristone) – (active lever presses, vehicle)] / (active lever presses, vehicle). Individual data points by rat are displayed with sex indicated by shape (circles – males; squares – females) and group indicated by fill (open – Control; filled – Stress History). * p < 0.05, Vehicle vs. Mifepristone or Control, Vehicle vs. Stress History, Vehicle, as indicated. n = 8 per group.
3.3. Region-specific effects of sex and stress history on synaptic phosphodiesterase 10A
PDE10A mRNA expression previously showed similar patterns in alcohol-dependent and stress history males following the cessation of alcohol access (Logrip et al., 2014; Logrip and Zorrilla, 2012). To determine whether stress history similarly impacts PDE10A protein levels in a synaptically enriched sample, as well as to examine sex differences in PDE10A expression profiles, synaptic fractionation and Western blotting techniques were employed, as described in Methods, on tissue punches obtained 48 h after the final reacquisition self-administration session in rats from the second behavioral cohort.
3.3.1. Striatum:
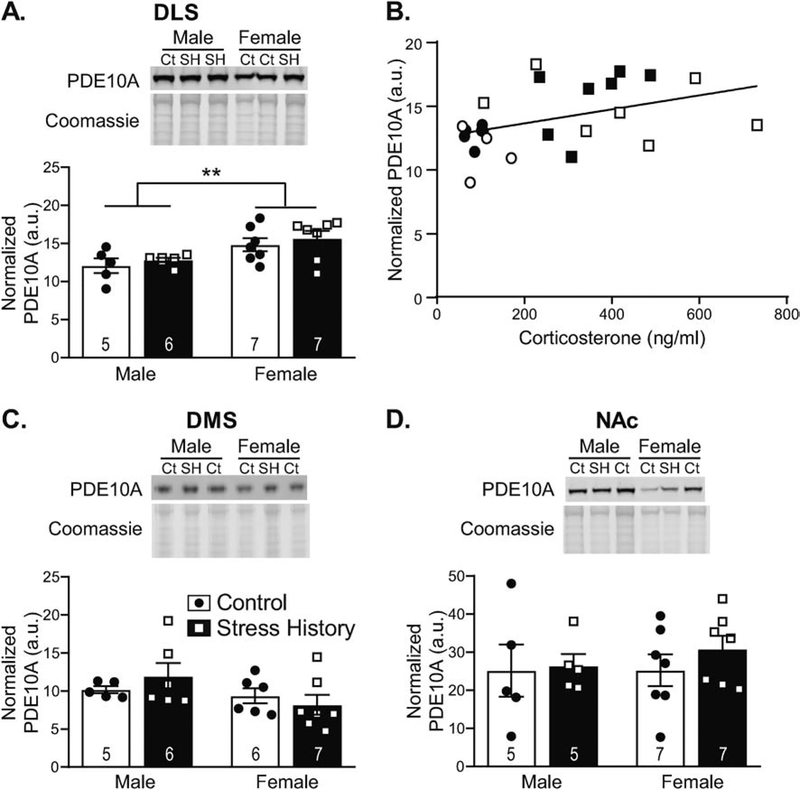
As the site of greatest PDE10A expression in the brain (Seeger et al., 2003) and given indications of possible sex differences in striatal PDE10A function (Hsu et al., 2011), PDE10A levels were assessed in 3 subdivisions of the striatum: DLS, DMS and NAc. PDE10A levels in synaptically enriched protein lysates from these striatal subdivisions supported sex-, but not stress history-, specific differences in PDE10A protein levels that diverged among the subnuclei. As shown in Figure 4, DLS and DMS, but not NAc, samples demonstrated sex differences in PDE10A expression. In the DLS (Figure 4A), analysis by 2-way ANOVA showed a main effect of Sex (F1,21 = 10.64, p = 0.0037), without effect of Group or interaction between the factors (F’s < 0.81, p’s > 0.37), due to greater expression of PDE10A in the synaptically enriched DLS fractions from female vs. male rats. DLS PDE10A levels significantly correlated with plasma corticosterone (R = 0.417, p = 0.042; Figure 4B), a feature not observed for PDE10A expression in other brain regions. Conversely, in DMS, PDE10A levels were slightly, though not significantly, lower in females vs. males (Figure 4C). Analysis by 2-way ANOVA yielded a trend for Sex (F1,20 = 3.19, p = 0.089), without Group or interaction effects (F’s < 1.34, p’s > 0.26), but data were not normally distributed. Log transformation normalized the data, but Sex effects remained at trend level (F1,20 = 4.21, p = 0.054). No sex-specific expression pattern for PDE10A was observed in NAc (Figure 4D), with 2-way ANOVA generating no significant effects (F’s < 0.56, p’s > 0.46).
Figure 4. Striatal PDE10A near the synapse differs by sex in a subregion-specific pattern, with DLS PDE10A correlated with corticosterone and expressed at higher levels in females.
Tissue punches from dorsolateral striatum (DLS), dorsomedial striatum (DMS), and nucleus accumbens (NAc) were collected 48 h after the final alcohol self-administration session and were enriched for synaptic content via centrifugal fractionation. Western blot membranes were probed for PDE10A, then Coomassie stained for total protein normalization. Data are calculated as a ratio of PDE10A to total protein load as measured by Coomassie staining, normalized across blots via a 10 μg rat brain lysate standard, and expressed in histograms as mean ± S.E.M. (in arbitrary units, a.u.), with individual data points overlaid. Representative images are included in the top panels, displaying PDE10A and Coomassie staining, as labeled. Samples are designated as control (Ct) or stress history (SH), as indicated above the blot images. (A) DLS PDE10A expression was higher in females compared to males, regardless of Stress History. (B) DLS PDE10A levels significantly correlated with serum corticosterone levels at the time of brain collection, with data presented as individual data points by rat, with sex indicated by shape (circles – males; squares – females) and group indicated by fill (open – Control; filled – Stress History). (C) PDE10A expression was slightly, but not significantly, lower in DMS female vs. male samples. (D) No differences were observed in NAc samples. **p < 0.005 Male vs. Female, all n’s as indicated on histograms. Full size blot images can be found in Supplemental Figure 1.
3.3.2. Amygdala:
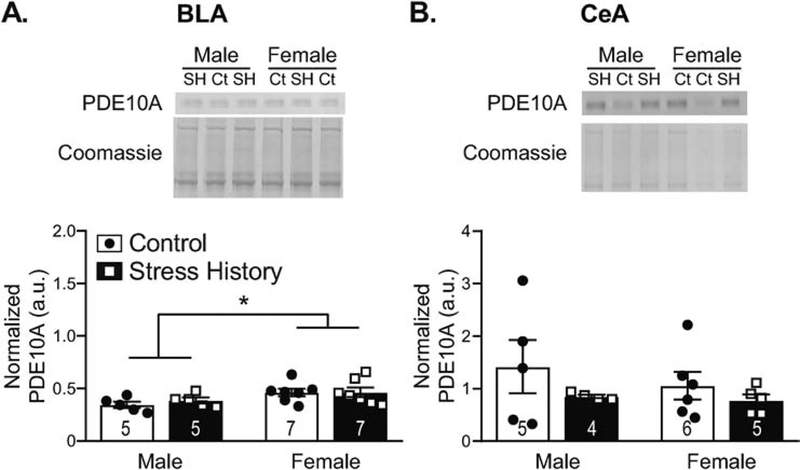
PDE10A levels in synaptically enriched fractions differed in a subdivision-specific way in the amygdala (Figure 5), based on analyses by 2-way ANOVA. In the BLA, greater PDE10A was seen in female vs. male rats (main effect of Sex, F1,20 = 6.53, p = 0.019; Figure 5A), without effects of Group or interaction between the factors (F’s < 0.30, p’s > 0.59). Unlike DLS, where PDE10A also showed sex-specific elevation, the relationship between BLA PDE10A and corticosterone levels did not achieve significance (R = 0.355, p = 0.097). In the adjacent central amygdala (CeA), no significant differences in PDE10A levels were observed (F’s < 1.86, p’s > 0.19; Figure 5B).
Figure 5. Basolateral (BLA), but not central (CeA), amygdala PDE10A is elevated adjacent to the synapse in females, relative to males, independent of stress history.
Synaptically enriched protein samples processed from tissue punches collected 48 h after the final alcohol self-administration session were resolved by Western blotting. Membranes were probed for PDE10A, then Coomassie stained for total protein normalization. Data are calculated as a ratio of PDE10A to total protein load as measured by Coomassie staining, normalized across blots via a 10 μg rat brain lysate standard, and expressed in histograms as mean ± S.E.M. (in arbitrary units, a.u.), with individual data points overlaid. Representative images (top panels) are included for each region to show PDE10A protein bands and Coomassie staining, as labeled. Samples are designated as control (Ct) or stress history (SH), as indicated above the blot images. Quantification (bottom panels) shows higher PDE10A expression in females compared to males for BLA (A), a pattern not seen in CeA (B), independent of Stress History. * p < 0.05, Male vs. Female. All n’s as indicated on histograms. Full size blot images can be found in Supplemental Figure 2.
3.3.3. Medial prefrontal cortex:
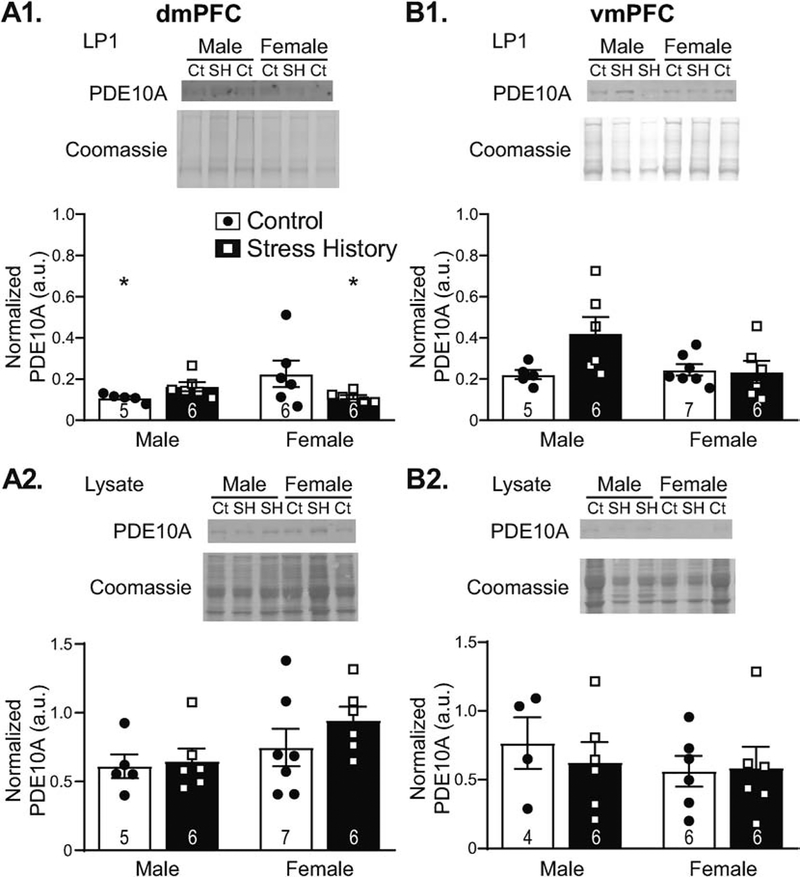
Samples from the dmPFC and ventromedial prefrontal cortex (vmPFC) displayed sex-, stress history-, and region-specific expression patterns, in line with changes in alcohol self-administration observed during reacquisition. Across regions of investigation, PDE10A levels at the synapse were visibly lowest in dmPFC (Figure 6A1), despite previous indications that mRNA expression in the region is comparable with other non-striatal brain regions (Logrip and Zorrilla, 2014). This may be due to minimal membrane localization of the protein, as PDE10A was more visible in pre-fractionation lysates (Figure 6A2). Nonetheless, analysis of dmPFC LP1 PDE10A expression levels revealed a significant interaction between Sex and Group (F1,19 = 5.22, p = 0.034), but data failed normality testing. Subsequent 2-way ANOVA analysis of log10-normalized dmPFC data, which passed normality testing, similarly displayed a significant interaction between Sex and Group (F1,19 = 6.53, p = 0.019). Post hoc Tukey tests indicated significant Sex differences in the Control group (p = 0.046), due to higher PDE10A expression in females vs. males, as well as significant Group effects in females (p = 0.047), with Control females expressing PDE10A at higher levels than Stress History females. Similar effects were not observed for dmPFC LYS samples, which displayed a slight elevation in expression in females vs. males (trend for Sex, F1,20 = 3.76, p = 0.067) but no significant Group or interaction effects (F’s < 1.11, p’s > 0.30). In the neighboring vmPFC (Figure 6B), no significant Sex or Group differences were observed in LP1 PDE10A levels (Figure 6B1), although analysis by two-way ANOVA showed trends for both the interaction between Sex and Group (F1,20 = 4.03, p = 0.059) and a main effect of Group (F1,20 = 3.32, p = 0.083). Because vmPFC data were not heteroscedastic, subsequent 2-way ANOVA of square root-transformed data confirmed the trending, but not significant, interaction between Sex and Group (F1,20 = 4.29, p = 0.052). No effects were observed for vmPFC LYS PDE10A (F’s < 0.69, p’s > 0.41; Figure 6B2).
Figure 6. Prefrontal cortex PDE10A expression differs by sex and stress exposure in the dorsomedial (dmPFC) but not the ventromedial (vmPFC) subdivision.
Synaptically-enriched protein samples (A1,B1) or whole lysate (A2,B2) processed from tissue punches collected 48 h after the final alcohol self-administration session were resolved by Western blotting. Membranes were probed for PDE10A, then Coomassie stained for total protein normalization. Data are calculated as a ratio of PDE10A to total protein load, as measured by Coomassie staining, normalized to a 10 μg rat brain lysate standard, and expressed in histograms as mean ± S.E.M. (in arbitrary units, a.u.), with individual data points overlaid. Representative images (top panel) show PDE10A and Coomassie staining, as labeled. Samples are designated as control (Ct) or stress history (SH), as indicated above the blot images. Quantification of PDE10A (bottom panels) in synaptically enriched dmPFC fractions (A1) shows very low levels overall, with higher expression in females vs. males in the Control group, as well as reduced PDE10A levels in Stress History females relative to same-sex Controls. This difference was not observed in dmPFC lysates from the same rats (A2), where PDE10A levels were somewhat higher overall and showed a trend towards higher expression in females. In the neighboring vmPFC, PDE10A did not significantly differ by Sex or Stress History in either the synaptically enriched fraction (B1) or the total lysate (B2), and PDE10A levels were more prominent after synaptic enrichment than in lysates. *p < 0.05, vs. Control Female. All n’s as indicated on histograms. Full size blot images can be found in Supplemental Figure 3.
4. DISCUSSION
These studies demonstrate the importance of investigating sex differences at the intersection of past stress and alcohol use, highlighting the potential centrality of sex for neuroadaptations and treatment efficacy. Here we demonstrate that stress history differentially modulated relapse-like alcohol self-administration by sex, slightly increasing alcohol intake in males but significantly decreasing alcohol-directed behavior and consumption in females, relative to same-sex controls. Circulating corticosterone levels at euthanasia, timed to approximate the anticipated onset of the next operant session, correlated with average alcohol intake across the preceding weeks of reacquisition. Corticosterone levels also correlated with PDE10A expression in synaptically enriched DLS samples, with higher overall expression in females vs. males, irrespective of stress history. BLA displayed a similar pattern but lacked significance in relation to corticosterone, whereas the very low expression at dmPFC synapses nonetheless displayed PDE10A expression aligned with group differences in alcohol consumption. Importantly, to our knowledge this is the first study showing mifepristone reduction of alcohol self-administration in female rats without a history of alcohol dependence, a feature required to generate GR-sensitive drinking in males (Somkuwar et al., 2017; Vendruscolo et al., 2012; Vendruscolo et al., 2015). Together, these data enumerate distinct, sex-specific effects of past stress on alcohol self-administration, differential patterns of PDE10A expression, and GR regulation of alcohol-related behaviors in the absence of physical dependence, demonstrating a novel and possibly important role for corticosterone in driving high levels of alcohol intake in females in the absence of overt physiological dependence.
4.1. Stress differentially alters reacquisition of alcohol intake by sex
The current findings extend previous understanding of stress history effects on alcohol self-administration in male rats into the important area of sex differences in stress-alcohol interactions. First, the data reaffirm the ability of stress history to elevate alcohol self-administration in males (Logrip et al., 2014; Logrip and Zorrilla, 2012), albeit at a trend level. The small effect size is unsurprising, as previous studies subdivided male rats by susceptibility to stress history-enhanced reacquisition self-administration, similar to susceptibility vs. resilience to predator odor (Edwards et al., 2013), whereas the current analysis explored effects across the entire population. More importantly, we demonstrate, contrary to hypothesis, that stress history suppressed alcohol intake in females during the relapse-like reacquisition period. These findings oppose reported stress effects on AUD development and relapse in women (Armstrong et al., 2018; Heffner et al., 2011), but are in line with effects of some stressors in female rodents.
Preclinical investigation of sex differences in the impact of stress on alcohol consumption has lagged behind studies employing male-only populations. Nonetheless, existing literature (reviewed in Logrip et al., 2018) shows that stress modality and timing relative to alcohol intake both affect stressors’ modulation of alcohol consumption. While sex differences in the modulation of alcohol intake by past footshock were previously unknown, data exist for adult stress exposure via several alternate modalities, with mixed effects. Similar to the data presented here, repeated restraint stress increased male but decreased female limited access alcohol consumption in mice selectively bred for high alcohol preference (Chester et al., 2006). Restraint also suppressed drinking in female Wistar rats (Wille-Bille et al., 2017), the strain used here, but not in WSC-1 mice of either sex (Tambour et al., 2008). C57BL/6J mice showing suppressed alcohol intake immediately after acute restraint in both sexes subsequently displayed sex differences in response to a single predator odor exposure, which increased female but suppressed male drinking (Cozzoli et al., 2014). Repeated predator stress potentiated drinking only in a subset of female C57BL/6J mice, regardless of alcohol history (Finn et al., 2018), but in all males with a history of binge drinking. While predator stress effects appear to diverge from the current findings, chronic mild stress exposure concurrent with binge alcohol access decreased future alcohol intake in females without significant effect on males (Quadir et al., 2019). Together, these studies exemplify the varied impact of sex on stress modulation of alcohol consumption, a body of literature to which our study adds important detail regarding the longitudinal effects of past stress as factor predating acquisition of operant alcohol self-administration.
Unlike previous studies, our work demonstrates sex differences in the effects of repeated footshock stress as a pre-existing factor on the development, extinction and reacquisition of alcohol self-administration. Like males, females fail to display significant effects of a history of repeated footshock until reacquisition. This suggests that abstinence or extinction training may be integral to the long-term impact of stress history on alcohol self-administration in our model, raising the possibility that neuroadaptations produced during the alcohol-free period, rather than at the initial stress exposure, vary by sex and stress history and produce the differential alcohol intake in reacquisition, an important future direction for these studies. Additionally, it would be of interest to determine whether female drinking is differentially modulated, relative to male intake, by stress enhanced fear learning, particularly whether it similarly diverges from controls in the early sessions of alcohol access (Meyer et al., 2013). Finally, it will be imperative to determine whether the opposing effects of past stress on drinking produce behavioral disinhibition in males but behavioral inhibition in females, suggestive of greater cumulative stress load in females vs. males (McEwen et al., 2015), in line with higher corticosterone expression in females. Regardless, the current model provides a framework for investigating neuroadaptations driving differential patterns of alcohol self-administration, with the goal of identifying novel treatment targets that may address sex differences in drinking regulation.
4.2. Heightened female sensitivity to GR modulation
Sexual dimorphism in circulating corticosteroid levels in rodents, with higher corticosterone production in females both at baseline (Critchlow et al., 1963) and following stress or alcohol challenge (Rivier, 1993, 1999), have long been established. However, sex differences in corticosteroid function with respect to alcohol intake and neural function are less completely understood. Female neurons of the lateral central amygdala display significant blunting of glutamatergic excitatory postsynaptic potentials after corticosterone treatment, preventing any further effect of alcohol application, whereas males show the opposite pattern, with alcohol, but not corticosterone, reducing response magnitude (Logrip et al., 2017). Whether this enhanced sensitivity of amygdala neurons to corticosterone generalizes to other brain regions, and if heightened neural corticosteroid sensitivity, rather than higher levels of circulating corticosterone, underlies the female-specific behavioral impact of mifepristone on alcohol intake remains to be determined. Regardless, the data presented herein indicate that females may display enhanced treatment efficacy for drugs targeting GR, a therapeutic approach previously demonstrated to reduce craving and drinking in a mixed sex population with a history of AUD without sex-specific effects (Vendruscolo et al., 2015). The sexually divergent response to mifepristone here contrasts with the broader efficacy of the mineralocorticoid receptor antagonist spironolactone to reduce alcohol self-administration in both sexes (Makhijani et al., 2018), suggesting a GR-specific, rather than general corticosterone, mechanism supporting female alcohol self-administration. Interestingly, mifepristone effects were specific to control females, despite data from a mouse predator stress model indicating increased PFC GR expression persisting at least one week post-stress (Finn et al., 2018). It should be noted that mifepristone is not a GR-specific inhibitor, but also has significant activity at the progesterone receptor (Peeters et al., 2004), and thus we cannot rule out progesterone receptor contribution to mifepristone’s reduction of alcohol consumption. It remains to be determined whether GR expression differs by sex, stress history, or brain region in our model, but such findings would enhance understanding of the possible connection between corticosterone, mifepristone-sensitive drinking, and PDE10A expression.
4.3. Sex differences in PDE10A expression: Implications for neuronal function
PDE10A, a dual specificity phosphodiesterase with the potential to broadly decrease cyclic nucleotide-directed signaling via deactivation of either cAMP or cGMP, may serve as a regulator of neuronal activity, particularly via actions at the synapse. In the striatum, PDE10A is primarily localized near the membrane, particularly within dendritic spines (Xie et al., 2006), and acts to reduce neuronal responses to cortical input (Threlfell et al., 2009). Striatal PDE10A function was differentially sensitive to the PDE10A-selective inhibitor papaverine, which increased phosphorylation of extracellular signal-regulated kinase (ERK) in male but not female striatal slices (Hsu et al., 2011). Our data indicate that this differential pharmacological response might arise from sexual dimorphism in striatal PDE10A expression, as higher PDE10A levels in female DLS could produce both lower basal ERK phosphorylation and the blunted papaverine response seen in the female slices. Subsequent studies assessing sex differences in PDE10A expression or function have been lacking, as has attention to extrastriatal PDE10A.
PDE10A has been implicated as a neuroadaptation to and possible mediator of experiences producing heightened stress states (Hebb et al., 2008; Le-Niculescu et al., 2011; Logrip and Zorrilla, 2014; Siuciak et al., 2008). Pde10a was suggested as a regulator of comorbidity between anxiety and other mental illnesses (Le-Niculescu et al., 2011) and as a putative modulator of stress-motivated alcohol consumption, based on elevated BLA Pde10a mRNA levels in males with a stress history or past alcohol dependence (Logrip and Zorrilla, 2012, 2014). Surprisingly, PDE10A protein levels in synaptically enriched BLA fractions were not significantly higher in stress history males, but rather were elevated in females, independent of stress history. The source of this discrepancy between mRNA and synaptic protein expression levels in male BLA remains unknown. Conversely, the current study suggests DLS PDE10A as a possible stress-modulated protein, given its significant correlation to corticosterone levels. It should be noted that neither DLS nor BLA PDE10A was most highly expressed in the control females whose drinking was sensitive to mifepristone treatment, suggesting that the correlation between corticosterone and PDE10A expression at euthanasia may not be an indicator of PDE10A involvement in mifepristone-sensitive alcohol intake.
Unlike BLA and DLS, which showed higher PDE10A in females regardless of stress history, dmPFC expression patterns aligned with changes in Pde10a mRNA expression in male rats under similar experimental conditions (Logrip and Zorrilla, 2012) and with relapse-like changes in alcohol intake. It should be noted that, despite significant findings, PDE10A expression in synaptically enriched dmPFC fractions was very low, a finding we have replicated in the laboratory with dmPFC samples from subsequent experiments (data not shown). Thus, these findings must be regarded with caution. It remains to be seen whether such minimal synaptic PDE10A expression is functionally relevant, since greater PDE10A expression in dmPFC lysates suggests minimal PDE10A membrane translocation in this region. Determining how these sex differences in PDE10A expression in synaptically enriched samples, measured at a single time point following both stress history and several months of operant alcohol consumption, correspond to differential synaptic function or contribute to alcohol-related behaviors present important directions for future investigation.
5. CONCLUSION
These studies establish sex differences in the long-term impact of repeated footshock stress on alcohol self-administration, with relapse-like intake potentiated in males and reduced in females with a stress history, compared to same-sex controls. Additionally, PDE10A is identified as a protein displaying sexually divergent expression in the DLS, which aligns with corticosterone expression, as well as in brain regions central to stress adaptation and its promotion of alcohol use, such as the BLA and dmPFC. To our knowledge, this is the first study to demonstrate GR regulation of alcohol self-administration in females, but not males, in the absence of alcohol dependence, suggestive of a broader function of GR to modulate alcohol-related behaviors in females. Further exploration of sex differences in stress history and corticosteroid regulation of alcohol self-administration, as well as elucidation of their relationship to PDE10A function, has the potential to identify novel treatments for individuals with stress-potentiated relapse drinking that may show sex-specific therapeutic efficacy.
Supplementary Material
HIGHLIGHTS.
Past stress increases male but decreases female relapse-like alcohol intake
Mifepristone decreases alcohol intake in high-drinking, nondependent control females
Females have more synaptic PDE10A in basolateral amygdala and dorsolateral striatum
Stress history males have more and females have less PDE10A in dmPFC synapses
Dorsolateral striatum synaptic PDE10A correlates with corticosterone levels
ACKNOWLEDGMENTS
We thank Shannon Roy, Brittany Bogan, Kimberly Redd, and Jordon Reyes for technical assistance. This research was supported by internal funding from the Indiana University - Purdue University School of Science as well as by the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism [R00 AA021802 (MLL), P60 AA007611 (MLL), T32 AA007462 (SCG)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute of Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATIONS OF INTEREST
The authors have no conflicts to disclose.
Abbreviations: AUD, alcohol use disorder; BLA, basolateral amygdala; CeA, central amygdala; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; DLS, dorsolateral striatum; DMS, dorsomedial striatum; dmPFC, dorsomedial prefrontal cortex; ERK, extracellular signal-regulated kinase; FR, fixed ratio; GR, glucocorticoid receptor, NAc, nucleus accumbens; PDE10A, phosphodiesterase 10A; vmPFC, ventromedial prefrontal cortex
REFERENCES
- Armstrong JL, Ronzitti S, Hoff RA, Potenza MN, 2018. Gender moderates the relationship between stressful life events and psychopathology: Findings from a national study. J Psychiatr Res 107, 34–41. [DOI] [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, Finegan A, 2006. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol 41, 44–53. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Tanchuck-Nipper MA, Kaufman MN, Horowitz CB, Finn DA, 2014. Environmental stressors influence limited-access ethanol consumption by C57BL/6J mice in a sex-dependent manner. Alcohol 48, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS, 1963. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol 205, 807–815. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF, 2007. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res 31, 2036–2045. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW, 2013. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry 3, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Helms ML, Nipper MA, Cohen A, Jensen JP, Devaud LL, 2018. Sex differences in the synergistic effect of prior binge drinking and traumatic stress on subsequent ethanol intake and neurochemical responses in adult C57BL/6J mice. Alcohol 71, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K, 1999. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J Biol Chem 274, 18438–18445. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Weiner JL, 2017. Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes Brain Behav 16, 15–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ, Collins TL, Standaert DG, Dunah AW, 2008. Biochemical fractionation of brain tissue for studies of receptor distribution and trafficking. Curr Protoc Neurosci Chapter 1, Unit 1 16. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Robertson HA, Denovan-Wright EM, 2008. Phosphodiesterase 10A inhibition is associated with locomotor and cognitive deficits and increased anxiety in mice. Eur Neuropsychopharmacol 18, 339–363. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Blom TJ, Anthenelli RM, 2011. Gender differences in trauma history and symptoms as predictors of relapse to alcohol and drug use. Am J Addict 20, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Liao G, Bi X, Oka T, Tamura S, Baudry M, 2011. The PDE10A inhibitor, papaverine, differentially activates ERK in male and female rat striatal slices. Neuropharmacology 61, 1275–1281. [DOI] [PubMed] [Google Scholar]
- Job RF, Barnes BW, 1995. Stress and consumption: inescapable shock, neophobia, and quinine finickiness in rats. Behav Neurosci 109, 106–116. [DOI] [PubMed] [Google Scholar]
- Kotera J, Sasaki T, Kobayashi T, Fujishige K, Yamashita Y, Omori K, 2004. Subcellular localization of cyclic nucleotide phosphodiesterase type 10A variants, and alteration of the localization by cAMP-dependent protein kinase-dependent phosphorylation. J Biol Chem 279, 4366–4375. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Balaraman Y, Patel SD, Ayalew M, Gupta J, Kuczenski R, Shekhar A, Schork N, Geyer MA, Niculescu AB, 2011. Convergent functional genomics of anxiety disorders: translational identification of genes, biomarkers, pathways and mechanisms. Transl Psychiatry 1, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K, Stappenbeck CA, Luterek JA, Kaysen D, Simpson TL, 2014. Gender differences in relationships among PTSD severity, drinking motives, and alcohol use in a comorbid alcohol dependence and PTSD sample. Psychol Addict Behav 28, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Milivojevic V, Bertholomey ML, Torregrossa MM, 2018. Sexual dimorphism in the neural impact of stress and alcohol. Alcohol 72, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Oleata C, Roberto M, 2017. Sex differences in responses of the basolateral-central amygdala circuit to alcohol, corticosterone and their interaction. Neuropharmacology 114, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Vendruscolo LF, Schlosburg JE, Koob GF, Zorrilla EP, 2014. Phosphodiesterase 10A regulates alcohol and saccharin self-administration in rats. Neuropsychopharmacology 39, 1722–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, 2012. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addict Biol 17, 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, 2014. Differential changes in amygdala and frontal cortex Pde10a expression during acute and protracted withdrawal. Front Integr Neurosci 8, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K, Snyder PB, Uher L, Rosman GJ, Ferguson K, Florio VA, 1999. Isolation and characterization of PDE10A, a novel human 3’, 5’-cyclic nucleotide phosphodiesterase. Gene 234, 109–117. [DOI] [PubMed] [Google Scholar]
- Makhijani VH, Van Voorhies K, Besheer J, 2018. The mineralocorticoid receptor antagonist spironolactone reduces alcohol self-administration in female and male rats. Pharmacol Biochem Behav 175, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C, 2015. Mechanisms of stress in the brain. Nat Neurosci 18, 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I, 2013. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res 37, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS, 2006. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction 101, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie KM, Rivier C, 1997. Effect of alcohol on the proestrous surge of luteinizing hormone (LH) and the activation of LH-releasing hormone (LHRH) neurons in the female rat. J Neurosci 17, 2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters BW, Tonnaer JA, Groen MB, Broekkamp CL, van der Voort HA, Schoonen WG, Smets RJ, Vanderheyden PM, Gebhard R, Ruigt GS, 2004. Glucocorticoid receptor antagonists: new tools to investigate disorders characterized by cortisol hypersecretion. Stress 7, 233–241. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA, 2019. Sex differences in stress-related alcohol use. Neurobiol Stress 10, 100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadir SG, Guzelian E, Palmer MA, Martin DL, Kim J, Szumlinski KK, 2019. Complex interactions between the subject factors of biological sex and prior histories of binge-drinking and unpredictable stress influence behavioral sensitivity to alcohol and alcohol intake. Physiol Behav 203, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, 1993. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res 17, 854–859. [DOI] [PubMed] [Google Scholar]
- Rivier C, 1999. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav 64, 739–751. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA, Varghese AH, Williams RD, Wylie PG, Menniti FS, 2003. Immunohistochemical localization of PDE10A in the rat brain. Brain Res 985, 113–126. [DOI] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE, 2012. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology 37, 906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2012. How does stress lead to risk of alcohol relapse? Alcohol Res 34, 432–440. [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Martin AN, Harms JF, Schmidt CJ, 2008. Behavioral characterization of mice deficient in the phosphodiesterase-10A (PDE10A) enzyme on a C57/Bl6N congenic background. Neuropharmacology 54, 417–427. [DOI] [PubMed] [Google Scholar]
- Soderling SH, Bayuga SJ, Beavo JA, 1999. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci U S A 96, 7071–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Vendruscolo LF, Fannon MJ, Schmeichel BE, Nguyen TB, Guevara J, Sidhu H, Contet C, Zorrilla EP, Mandyam CD, 2017. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 84, 17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonne SC, Back SE, Diaz Zuniga C, Randall CL, Brady KT, 2003. Gender differences in individuals with comorbid alcohol dependence and post-traumatic stress disorder. Am J Addict 12, 412–423. [PubMed] [Google Scholar]
- Tambour S, Brown LL, Crabbe JC, 2008. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res 32, 2100–2106. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Sammut S, Menniti FS, Schmidt CJ, West AR, 2009. Inhibition of Phosphodiesterase 10A Increases the Responsiveness of Striatal Projection Neurons to Cortical Stimulation. J Pharmacol Exp Ther 328, 785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Logrip ML, Roberto M, 2017. P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology 125, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32, 7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ, 2015. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest 125, 3193–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Bille A, Ferreyra A, Sciangula M, Chiner F, Nizhnikov ME, Pautassi RM, 2017. Restraint stress enhances alcohol intake in adolescent female rats but reduces alcohol intake in adolescent male and adult female rats. Behav Brain Res 332, 269–279. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2018. Global status report on alcohol and health 2018. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Xie Z, Adamowicz WO, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, Strick CA, Williams RD, Menniti FS, 2006. Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 139, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Eisenmann ED, Rose RM, Kohls BA, Johnson BL, Robinson KL, Heikkila ME, Mucher KE, Huntley MR, 2018. Predator-based psychosocial stress model of PTSD differentially influences voluntary ethanol consumption depending on methodology. Alcohol 70, 33–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.