Significance
Nectar feeding by mosquitoes is important for survival and reproduction, and hence disease transmission. However, we know little about the sensory mechanisms that mediate mosquito attraction to sources of nectar, like those of flowers, or how this information is processed in the mosquito brain. Using a unique mutualism between Aedes mosquitoes and Platanthera obtusata orchids, we reveal that the orchid’s scent mediates this mutualism. Furthermore, lateral inhibition in the mosquito’s antennal (olfactory) lobe—via the neurotransmitter GABA—is critical for the representation of the scent. These results have implications for understanding the olfactory basis of mosquito nectar-seeking behaviors.
Keywords: Platanthera, mosquitoes, Aedes aegypti, olfaction, nectar
Abstract
Mosquitoes are important vectors of disease and require sources of carbohydrates for reproduction and survival. Unlike host-related behaviors of mosquitoes, comparatively less is understood about the mechanisms involved in nectar-feeding decisions, or how this sensory information is processed in the mosquito brain. Here we show that Aedes spp. mosquitoes, including Aedes aegypti, are effective pollinators of the Platanthera obtusata orchid, and demonstrate this mutualism is mediated by the orchid’s scent and the balance of excitation and inhibition in the mosquito’s antennal lobe (AL). The P. obtusata orchid emits an attractive, nonanal-rich scent, whereas related Platanthera species—not visited by mosquitoes—emit scents dominated by lilac aldehyde. Calcium imaging experiments in the mosquito AL revealed that nonanal and lilac aldehyde each respectively activate the LC2 and AM2 glomerulus, and remarkably, the AM2 glomerulus is also sensitive to N,N-diethyl-meta-toluamide (DEET), a mosquito repellent. Lateral inhibition between these 2 glomeruli reflects the level of attraction to the orchid scents. Whereas the enriched nonanal scent of P. obtusata activates the LC2 and suppresses AM2, the high level of lilac aldehyde in the other orchid scents inverts this pattern of glomerular activity, and behavioral attraction is lost. These results demonstrate the ecological importance of mosquitoes beyond operating as disease vectors and open the door toward understanding the neural basis of mosquito nectar-seeking behaviors.
Mosquitoes are important vectors of disease, such as dengue, malaria, or Zika, and are considered one of the deadliest animal on earth (1); for this reason, research has largely focused on mosquito–host interactions, and in particular, the mosquito’s sensory responses to those hosts (2–5). Nectar feeding is one such aspect of mosquito sensory biology that has received comparatively less attention, despite being an excellent system in which to probe the neural bases of behavior (6). For instance, nectar and sugar feeding is critically important for both male and female mosquitoes, serving to increase their lifespan, survival rate, and reproduction, and for males, it is required for survival (6, 7).
Mosquitoes are attracted to, and feed on, a variety of plant nectar sources, including those from flowers (8–12). Although most examples of mosquito–plant interactions have shown that mosquitoes contribute little in reproductive services to the plant (13), there are examples of mosquitoes being potential pollinators (9, 10, 14–17). However, few studies have identified the floral cues that serve to attract and mediate these decisions by the mosquitoes and how these behaviors influence pollination.
The association between the Platanthera obtusata orchid and Aedes mosquitoes is one of the few examples that shows mosquitoes as effective pollinators (14–17) and thus provides investigators a unique opportunity to identify the sensory mechanisms that help mosquitoes locate sources of nectar. The genus Platanthera has many different orchid species having diverse morphologies and specialized associations with certain pollinators (SI Appendix, Table S1), with P. obtusata being an exemplar with its association with mosquitoes (14–17). Although mosquito visitation has been described in this species (15), the cues that attract mosquitoes to the flowers, and the importance of mosquito visitation for orchid pollination, are unknown.
In this article, we examine the neural and behavioral processes mediating mosquito floral preference. We present findings from 1) pollination studies in P. obtusata by Aedes mosquitoes, 2) analyses of floral scent compounds that attract diverse mosquito species, and 3) antennal and antennal lobe (AL) recordings showing how these floral scents and compounds are represented in the mosquito brain (SI Appendix, Fig. S1). Using this integrative approach, we demonstrate that Aedes discrimination of Platanthera orchids is mediated by the balance of excitation and inhibition in the mosquito antennal lobe.
Results
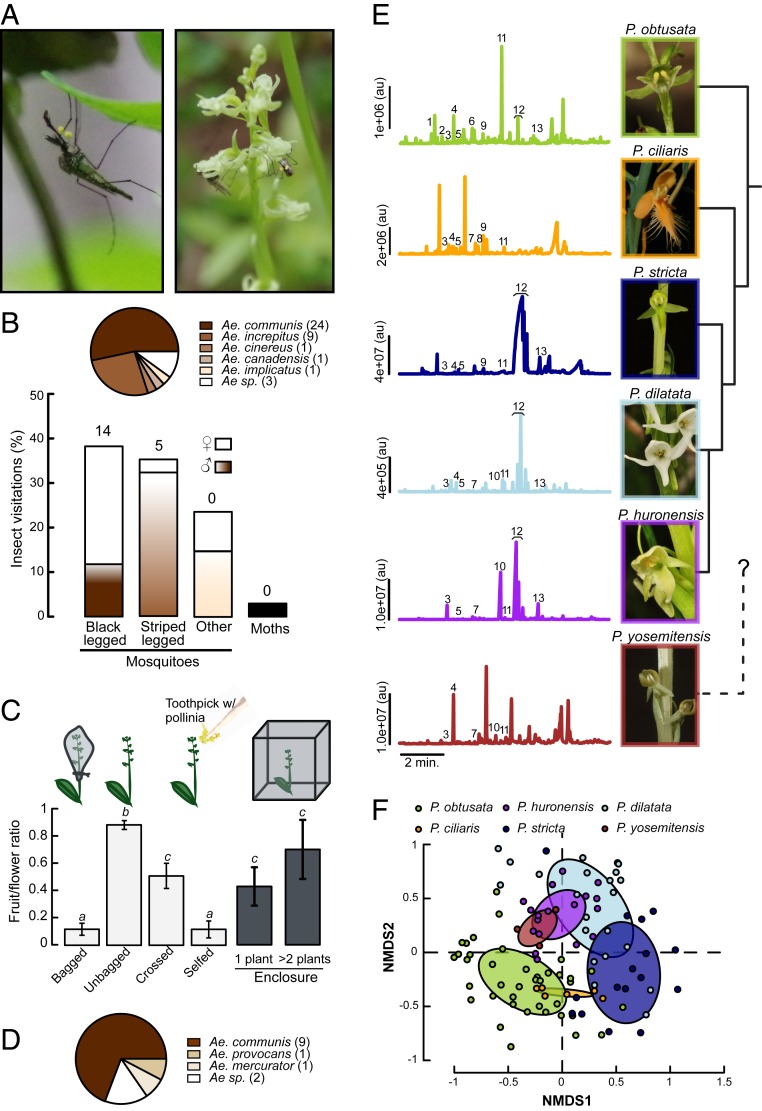
To understand the importance of various pollinators, including mosquitoes, on P. obtusata, we first conducted pollinator observation and exclusion experiments in northern Washington State where Platanthera orchids and mosquitoes are abundant. Using a combination of video recordings and focal observations by trained participants, more than 581 P. obtusata flowers were observed for a total of 47 h, with 57 floral feeding events by mosquitoes. During our observations, flowers were almost solely visited by various mosquito species (both sexes) that mainly belonged to the Aedes group (Fig. 1 A and B and SI Appendix, Table S2), with the only other visitor being a single geometrid moth. Mosquitoes quickly located these rather inconspicuous flowers, even on plants that were bagged and thus lacked a visual display. After landing on the flower, the mosquito’s probing of the nectar spur resulted in pollinia attachment to its eyes (Fig. 1A and Movies S1 and S2). Most of the pollinia-bearing mosquitoes had 1 or 2 pollinia, but we found up to 4 pollinia on a single female. To assess the impact of the mosquitoes’ visits on the orchid fruit set, we conducted a series of pollination experiments, such as bagging (thus preventing mosquito visitations) and cross- and self-pollinating the plants. We found significantly higher fruit-to-flower ratios and seed sets in unbagged plants compared with those in bagged or self-pollinated plants (Fig. 1C and SI Appendix, Fig. S2; Mann–Whitney U test, P < 0.001), and elevated fruit ratios in our cross-pollinated plants compared with bagged or self-pollinated plants (Fig. 1C). In the field, we released field-caught mosquitoes into cages containing either a single plant or 2 to 3 plants (Fig. 1 C and D). Once released into the cages, the mosquitoes fed from the P. obtusata flowers, and ∼10% of the mosquitoes showed pollinia attachment (Fig. 1D). There was a strong trend for cages with 2 or more plants to have higher fruit-to-flower ratios than those with 1 plant (Mann–Whitney U test, P = 0.07), although our low sample size for locations with 2 to 3 plants (rare at these sites; n = 8) may explain the lack of significance at α = 0.05. Nonetheless, cages containing 2 or more plants had significantly higher fruit-to-flower ratios than bagged plants (Mann–Whitney U test, P < 0.001), but were not statistically different from the unbagged plants (Mann–Whitney U test, P = 0.84), further suggesting that cross-pollination is important in this orchid species.
Fig. 1.
Association between the P. obtusata orchid and mosquito pollinators. (A) Picture (Left image) of a black legged male mosquito bearing 2 pollinia on its head, and (Right image) a male mosquito feeding on P. obtusata and a female with 2 pollinia attached to its head after having visited a flower. (B) Insect visitations (barplot; % insect visitation, calculated by the total number of insect visits to P. obtusata) and distribution of the mosquito species found in the field with pollinia (pie chart; numbers in legend denote the number of mosquitoes with pollinia). Both males (dark brown bars) and females (white bars) of different mosquito species visited the plants. Black-legged mosquitoes were predominantly Ae. communis, and striped legged were Ae. increpitus. Numbers above the bars indicate the number of individuals observed with pollinia. (C) Fruit-to-flower ratio for bagged (using organza bags around P. obtusata plants to prevent pollinator visitation), unbagged, self-crossed, out-crossed plants, and plants in the enclosure. Bagged and self-pollinated plants produced similar fruit-to-flower ratios (0.11 ± 0.04, 0.12 ± 0.06, respectively; Mann–Whitney U test, P = 0.99), but were significantly lower than the unbagged plants (0.89 ± 0.03; Mann–Whitney U test, P < 0.001). Although fruit weight did not differ between treatments (Student’s t test, P = 0.082), bagged plants produced significantly fewer viable seeds per fruit per flower than unbagged plants (SI Appendix, Fig. S1; Wilcoxon rank sum test, P < 0.05). Letters above bars show statistical differences between experimental conditions (Mann–Whitney U test, P < 0.05). Bars are the mean ± SEM (n = 8 to 20 plants/treatment). (D) Pie chart of the species of mosquitoes which removed pollinia from the plants in the enclosures (numbers in legend denote the number of mosquitoes with pollinia). (E) GCMS analyses of the floral volatiles emitted by P. obtusata, P. ciliaris, P. stricta, P. dilatata, P. huronensis, and P. yosemitensis. Pictures of the floral species, and their phylogenetic relationship, are shown on the Right. P. obtusata flowers emitted a low emission rate scent that is dominated by aliphatic compounds (including octanal [No. 7], 1-octanol [No. 9], and nonanal [No. 11]; 54% of the total emission), whereas the moth-visited species P. dilatata, P. huronensis, and P. stricta emit strong scents dominated by terpenoid compounds (75%, 76%, and 97% of the total emission for the 3 species, respectively), and the butterfly-visited P. ciliaris orchid is dominated by nonanal and limonene (24% and 12% of the total emission, respectively) (SI Appendix, Table S3). Numbers in the chromatograms correspond to: 1, α-pinene; 2, camphene; 3, benzaldehyde; 4, β-pinene; 5, β-myrcene; 6, octanal; 7, d-limonene; 8, eucalyptol; 9, 1-octanol; 10, (±)linalool; 11, nonanal; 12, lilac aldehydes (D and C isomers); and 13, lilac alcohol. Orchid images courtesy of G. Van Velsir, R. Coleman, and T. Nelson (photographers). (F) Nonmetric multidimensional scaling (NMDS) plot (stress = 0.265) of the chemical composition of the scent of all of the orchid species presented in B. Each dot represents a sample from a single individual plant collected in the field. The ellipses represent the SD around the centroid of their respective cluster. Differences in scent composition and emission rate are significantly different between species (composition: ANOSIM, R = 0.25, P = 0.001; emission rate: Student’s t tests, P < 0.05). au, arbitrary units.
Platanthera Orchids Differ in Their Floral Scents.
P. obtusata has a short (∼12 cm) inflorescence (Fig. 1A), and flowers emit a faint grassy- and musky-type of scent. The height and green coloration of the flowers make this plant difficult to pick out from neighboring vegetation, but throughout our observations, we noticed that mosquitoes readily oriented and flew to the flowers, exhibiting a zig-zagging flight typical of odor-conditioned optomotor anemotaxis (5). Moreover, even when the plants were bagged (thereby preventing the visual display of the flowers) mosquitoes would still land and attempt to probe the plants through the bag.
In the Platanthera genus, species differ in their floral advertisements, including their scent, and this is reflected in the different pollinators visiting each orchid species (SI Appendix, Table S1). Often these species can co-occur in the same sedge, such as P. obtusata, Platanthera stricta, Platanthera dilatata, and Platanthera huronensis, although hybridization can be rare (18, 19). Mosquitoes have sensitive olfactory systems that are used to locate important nutrient sources, including nectar (2–4, 11). Our observations on the strength of the association between P. obtusata and the mosquitoes, and how mosquitoes were able to locate the P. obtusata orchids, motivated us to examine the scent of closely related Platanthera species and identify the putative volatiles that mosquitoes might be using to detect and discriminate between the different orchid species.
The floral scents of the 6 orchid species were collected and subsequently characterized using gas chromatography with mass spectrometry (GCMS) (Fig. 1E). These analyses showed that species differed in both scent emissions and compositions (Fig. 1 E and F and SI Appendix, Table S3; composition: analysis of similarities [ANOSIM], R = 0.25, P = 0.001; emission rate: Student’s t tests, P < 0.05). Mosquito-pollinated P. obtusata flowers predominantly emitted nonanal and octanal, and low levels of terpene compounds (linalool, lilac aldehyde), whereas the other orchid species, which are pollinated by other insect taxa (SI Appendix, Table S1), emitted scents that were enriched in terpene compounds, such as lilac aldehyde (e.g., P. dilatata, P. huronensis, and P. stricta), or aromatic compounds, such as phenylacetaldehyde (e.g., Platanthera yosemitensis).
Divergent Mosquitoes Show Similar Antennal and Behavioral Responses to the P. obtusata Orchid Scent.
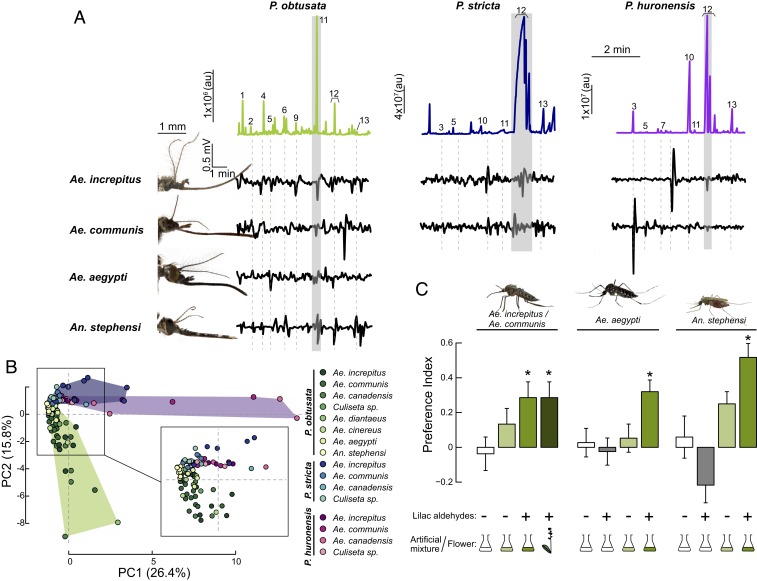
To identify volatile compounds that mosquitoes might use to detect the plants, we performed gas chromatography coupled with electroantennographic detection (GC-EAD) using various species of mosquitoes that visit P. obtusata flowers in the field (SI Appendix, Table S2). Several chemicals evoked antennal responses in the Aedes mosquitoes, including aliphatic (nonanal and octanal) and terpenoid compounds (e.g., lilac aldehydes, camphene, and α- and β-pinene) (Fig. 2A and SI Appendix, Fig. S3). For example, across the Aedes–Ochlerotatus group, nonanal elicited consistent responses and one of the strongest relative responses within a given mosquito species (Fig. 2A and SI Appendix, Fig. S3). Interestingly, Culiseta mosquitoes, which also visited P. obtusata but did not have pollinia attachment, showed very little response to nonanal. Although mosquito species showed differences in their response magnitude to the chemicals (Fig. 2A and SI Appendix, Fig. S3), the responses were relatively consistent, which was reflected in their overlapping distribution in multivariate (principal component analysis [PCA]) space (ANOSIM, R = 0.076, P = 0.166) (Fig. 2B). This similarity in evoked responses by Aedes mosquitoes led us to examine whether these chemicals also evoked similar responses in other mosquitoes. We thus used 2 species of mosquitoes that are not native to the area, but are closely (Aedes aegypti) or distantly (Anopheles stephensi) related to the other Aedes species. The nonnative mosquitoes (Ae. aegypti and An. stephensi) also responded to these volatiles and were not significantly different in their responses to the other Aedes species (ANOSIM, R = 0.087, P = 0.09) (Fig. 2B).
Fig. 2.
Identification of behaviorally effective orchid volatiles in mosquitoes. (A) Gas chromatogram traces for the P. obtusata (Left), P. stricta (Middle), and P. huronensis (Right) headspaces, with electroantennogram responses to the GC peaks for 4 mosquito species (Ae. communis, Ae. increpitus, Ae. aegypti, and An. stephensi) immediately below. See chromatogram number correspondence in Fig. 1 legend. Mosquito images courtesy of A. Jewiss-Gaines (photographer) and F. Hunter (Brock University, St. Catharines, Canada). (B) PCA plot based on the antennal responses of individual mosquitoes from the different Aedes species to the peaks from the P. obtusata, P. stricta, and P. huronensis scents. Each dot corresponds to the responses of an individual mosquito; shaded areas and dots are color coded according to mosquito species and flower scent (green, P. obtusata; blue, P. stricta; and purple, P. huronensis). Antennal responses to the 3 tested orchid scents were significantly different from one another (ANOSIM, R = 0.137, P < 0.01) (n = 3 to 16 mosquitoes per species per floral extract). (C) Behavioral preferences by snow mosquitoes (Ae. communis and Ae. increpitus), Ae. aegypti, and An. stephensi mosquitoes to the P. obtusata scent and scent mixture, with and without the lilac aldehyde (at the concentration found in the P. obtusata headspace). A y-maze olfactometer was used for the behavioral experiments in which mosquitoes are released and have to fly upwind and choose between 2 arms carrying the tested compound/mixture or no odorant (control). A preference index (PI) was calculated based on these responses (see SI Appendix, Supplementary Methods for details). The colored flask denotes the use of an artificial mixture (dark green is with lilac aldehyde; light green is without); empty flask denotes the negative (solvent) control. The plant motif is the positive control (orchid flowers), and the + and − symbols represent the presence or absence of the lilac aldehyde in the stimulus, respectively. Bars are the mean ± SEM (n = 17 to 53 mosquitoes/treatment); asterisks denote a significant difference between treatments and the mineral oil (no odor) control (binomial test: P < 0.05). au, arbitrary units.
P. obtusata occurs in sympatry with P. huronensis, P. dilatata, and P. stricta, but we did not observe Aedes mosquitoes visiting these orchids. To examine whether these differences in orchid visitation arise from differences in antennal responses, we performed GC-EADs using the scents of P. stricta and P. huronensis, which are predominantly pollinated by bees, moths, and butterflies (SI Appendix, Table S1). Results showed that the mosquitoes (Aedes increpitus, Aedes communis, Aedes canadensis, and Culiseta sp.), which coexist with these orchids in the same habitat, all responded to several compounds, including linalool, nonanal, benzaldehyde, β-myrcene, and lilac aldehydes (Fig. 2). In particular, the high concentration of lilac aldehydes in the scent of P. stricta, and to a lesser extent in P. huronensis, elicited relatively strong responses in the antennae of Ae. increpitus and Ae. communis. Despite occurring in sympatry and overlapping in their scent composition, mosquito antennal responses to the 3 different orchid scents were significantly different from one another (Fig. 2B; ANOSIM, R = 0.137, P < 0.01), suggesting that the orchid species pollinated by other insects were activating distinct olfactory channels in the mosquitoes.
To evaluate if the P. obtusata orchid scent attracts mosquitoes, we tested the behavior of Ae. increpitus and Ae. communis mosquitoes (both important pollinators of P. obtusata) in response to the scent emitted by live P. obtusata flowers, as well as by an artificial mixture composed of the floral volatiles that elicited strong antennal responses in mosquitoes. Both the artificial mixture and the scent from the flowers significantly attracted these mosquitoes (Fig. 2C; binomial tests: P < 0.05). However, upon removal of lilac aldehyde (∼5.4 ng) from the mixture emissions, the attraction was reduced (binomial test: P = 0.292).
The similarity between mosquito species in their antennal responses to volatiles in the P. obtusata scent (Fig. 2) raised the question of whether closely related (Ae. aegypti) and more distantly related (An. stephensi) mosquitoes might also be attracted to the orchid scent. When tested in the olfactometer, both Ae. aegypti and An. stephensi mosquitoes exhibited significant attraction to the orchid scent with the lilac aldehydes (binomial tests: P < 0.05). By contrast, and similar to responses by Aedes–Ochlerotatus mosquitoes, once the lilac aldehydes were removed from the mixture, this attraction was reduced to levels approaching the mineral oil (no odor) control (Fig. 2C). Nonetheless, the attraction by these other mosquito species may not indicate that pollinia also attaches to their eyes, or that they may serve as pollinators. To address this question, we released both male and female Ae. aegypti mosquitoes into cages with flowering P. obtusata plants. Once entering the cage, the mosquitoes immediately fed from the flowers, and pollinia attached to their eyes similar to the other Aedes species (SI Appendix, Fig. S4).
The P. obtusata Orchid Scent Evokes Strong Responses in the Mosquito Antennal Lobe.
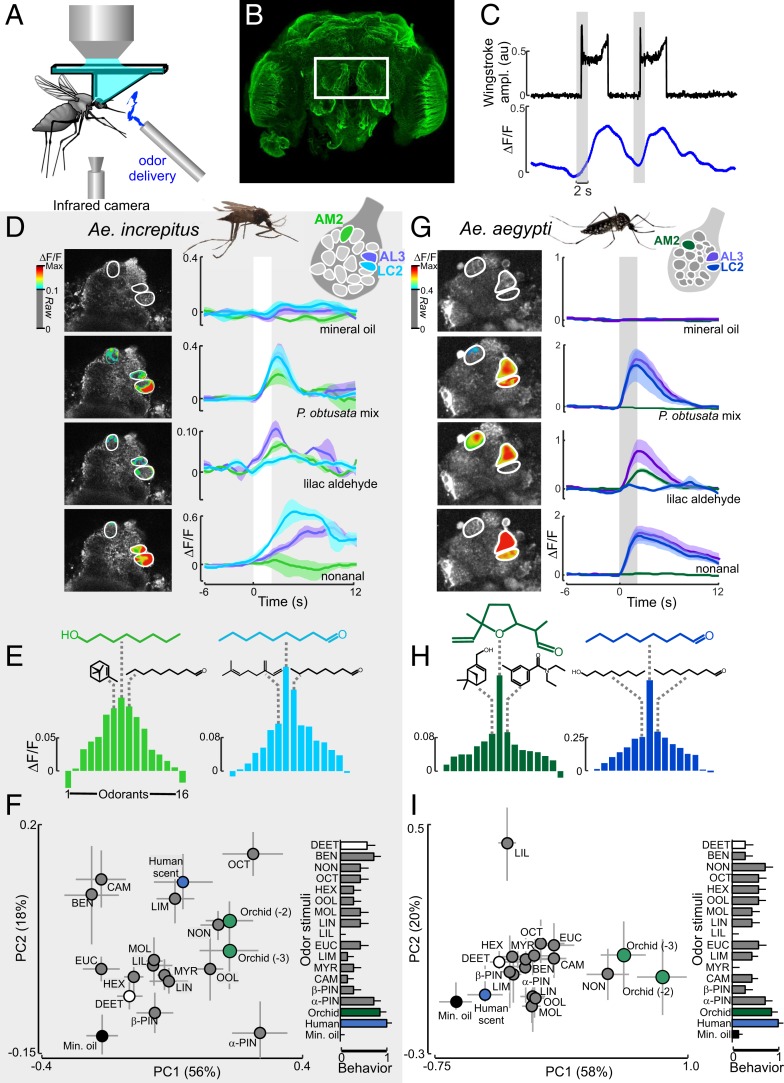
The differences in floral scents between the orchid species, and the behavioral responses by different mosquito species to the P. obtusata scent, raised the question of how this chemical information was represented in the mosquito’s primary olfactory center, the AL. Therefore, we used bath application of a calcium indicator (Fluo4) in Ae. increpitus and our PUb-GCaMP6s line of Ae. aegypti mosquitoes (20, 21). Although both calcium indicators do not allow explicit recording of specific cell types in the AL, they do provide an ability to record and characterize the responses of individual glomeruli to odor stimuli. Mosquitoes were glued to holders that permitted 2-photon imaging of calcium responses in the AL during tethered flight (21, 22) and tentative registration and naming of glomeruli (Fig. 3 A and B). For both mosquito species, odor stimulation evoked distinct calcium dynamics in the glomerular regions of the AL that were time locked to stimulus onset (Fig. 3 C, D, and G). The orchid mixture evoked flight responses and strong (>20% ΔF/F) multiglomerular patterns of activity in both mosquito species, particularly in the anterior-medial glomeruli (the putative AM2, AM3, and V1 glomeruli) and the anterior-lateral glomeruli (AL3 and LC2) (Fig. 3 D and G and SI Appendix, Figs. S5 and S6). In addition, certain odorants elicited overlapping patterns of glomerular activity similar to those elicited by the orchid scent (Fig. 3 F and I), such as nonanal in the AL3 and LC2 glomeruli (Fig. 3 D and G), with the LC2 glomerulus showing the strongest response to nonanal, octanal, and 1-octanol (Fig. 3 E and H). Although the anterior-medial glomeruli showed broader tuning in Ae. increpitus than in Ae. aegypti, these glomeruli were sensitive to terpene compounds in both species and the AM2 glomerulus often exhibited inhibition when stimulated with nonanal (Fig. 3 D, E, G, and H and SI Appendix, Figs. S5 and S6). Interestingly, for Ae. aegypti, the AM2 glomerulus showed the strongest response to lilac aldehyde, followed by DEET, a strong mosquito repellent (23–26) (SI Appendix, Fig. S7), although these responses were suppressed when stimulated with the orchid mixture (Fig. 3 G and H and SI Appendix, Fig. S6). However, other odor stimuli, including human scent, evoked a dissimilar pattern of glomerular activity compared with the orchid mixture (Fig. 3 F and I).
Fig. 3.
Mosquito antennal lobe responses to the P. obtusata scent. (A) Schematic of the 2-photon setup used to record calcium dynamics in the mosquito AL. (B) Ae. aegypti brain (α-tubulin stain). The white rectangle surrounds the 2 ALs that are accessible for calcium imaging. Optical sectioning using the 2-photon microscope and subsequent immunohistochemical characterization allowed us to register glomeruli to an AL atlas as well as repeatably image from the same glomeruli. Although the AL between species differed in volume (0.0029 ± 0.0001 and 0.0062 ± 0.0004 mm3 for Ae. aegypti and Ae. increpitus, respectively), they consisted of similar numbers of glomeruli (18 to 22 glomeruli) in the ventral region of the AL, ∼40 µm from the surface. (C) Representative time traces of behavioral (wing-stroke amplitude) (Top, black) and AL LC2 glomerulus response (Bottom, blue) to 2 P. obtusata odor stimulations (gray bars). (D) For Ae. increpitus mosquitoes with bath application of Fluo4, schematic of AL glomeruli imaged at the 40-µm depth (Top) and pseudocolor plot overlying the raw grayscale image (Left) and mean ∆F/F time traces (Right) for Ae. increpitus AL glomerular (AM2 [green], LC2 [blue], and AL3 [purple]) responses to mineral oil (no odor) control (Top); P. obtusata mix (Middle, Top); lilac aldehyde (Middle, Bottom); and nonanal (Bottom). White bars are the odor stimulations. Traces are the mean from 3 to 9 mosquitoes; shaded areas denote the SEM. Pseudocolor images were generated by subtracting the frame before stimulus onset from the frames during the stimulus window; only those glomerular regions of interest that were >0.1 ∆F/F are shown. (E) Response curves for the Ae. increpitus AM2 (green) and LC2 (blue) glomeruli based on a panel of 16 odorants. AM2 is most responsive to octanol (green chemical structure), followed by α-pinene and nonanal (black chemical structures). LC2 is most responsive to nonanal (blue), followed by octanal and β-myrcene (black chemical structures). Bars are the mean (n = 3 to 9). (F, Left) PC plot from responses of 20 glomeruli to the odorants. PC1 and PC2 explain 56% and 18% of the variance, respectively. The orchid mixture at 2 concentrations (1:100 and 1:1,000 dilution) and nonanal evoked stronger responses than the mineral oil (no odor) control (Kruskal–Wallis test: P < 0.05) and were significantly different in the multivariate analysis (ANOSIM: P < 0.05). Error bars represent SEM. (F, Right) Behavioral responses of the tethered mosquitoes to the odor stimuli. Responses were significantly different between the mineral oil control and the human and orchid scents (Kruskal–Wallis test: P < 0.05), although they were not significantly correlated with the glomerular representations (Spearman rank correlation: ρ = 0.35; P = 0.16). (G) As in D, but for PUb-GCaMP6s Ae. aegypti mosquitoes and the AM2 (green), LC2 (blue), and AL3 (purple) AL glomeruli. Traces are the mean (n = 7 to 14 mosquitoes); shaded area is the SEM. (H) As in E, but for the Ae. aegypti AM2 and LC2 glomeruli. AM2 is the most responsive to lilac aldehyde (green), followed by DEET and myrtenol (black chemical structures). LC2 is the most responsive to nonanal (blue), followed by octanal and octanol (black chemical structures). Bars are the mean (n = 7 to 14 mosquitoes). (I, Left) As in F, but for the Ae. aegypti mosquito and the 18 imaged glomerular responses to the panel of odorants. PC1 and PC2 explain 58% and 20% of the variance, respectively. (I, Right) Behavioral responses for the orchid and human scents were significantly different from control (P < 0.05), although the correlation with the glomerular responses was not significant (Spearman rank correlation: ρ = 0.46; P = 0.07). AM, anterior-medial; LC, lateral-central; AD, anterior-lateral.
Inhibition in the Mosquito AL Plays An Important Role in the Processing of the Orchid Scents.
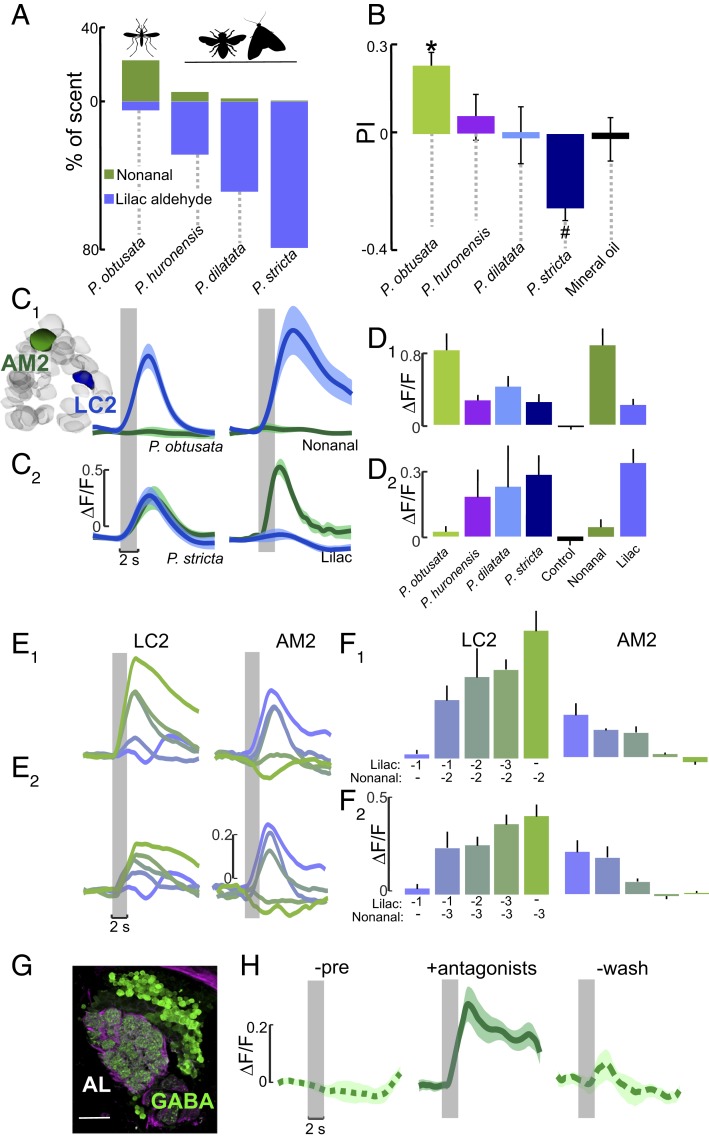
Results from our calcium imaging and behavioral experiments suggested that certain volatile compounds, such as nonanal and lilac aldehyde, are particularly important for mosquito responses to P. obtusata. However, the other Platanthera species that are primarily pollinated by different insects (but avoided by Aedes mosquitoes), also emit these volatile compounds, but at different ratios (Fig. 4A). To examine how mosquitoes respond to the scents of the other Platanthera species and to determine the importance of odorant ratios for the behavioral preferences, we increased the ratio of lilac aldehyde in the artificial P. obtusata mixture to the levels found in the different Platanthera species. However, in these mixtures we kept the other odor constituents (including nonanal) at the same levels as in P. obtusata, thus allowing us to examine how changing the concentration of 1 component (lilac aldehyde) altered the behavior (Fig. 4A and SI Appendix, Fig. S8A). Data showed that the increase in lilac aldehyde elicited behavioral responses that were not significantly different from the solvent control (binomial tests: P > 0.05) or elicited an aversive response when compared with the P. obtusata mixture (Fig. 4B; binomial tests: P < 0.05). Similarly, when we decreased the nonanal ratio in the mixture to the levels of the other Platanthera species, the behavioral efficacy of these mixtures decreased to levels that were not significantly different from the solvent control (SI Appendix, Fig. S8 C and D; binomial tests: P > 0.05). To examine the relationship between mosquito behavior and AL response, we compared glomerular responses to the odors of the different orchid species. Stimulation with the P. obtusata mixture evoked strong glomerular responses in the AL, particularly in the AL3 and LC2 glomeruli, whereas stimulation with the other Platanthera scents (containing much higher lilac aldehyde: nonanal ratios) showed decreased responses in the LC2 glomerulus; however, the AM2 glomerulus (responsive to lilac aldehyde and DEET) showed much stronger responses (Fig. 4 C and D; Kruskal–Wallis test with multiple comparisons: P < 0.05).
Fig. 4.
Glomeruli encoding the orchid scents are sensitive to odorant ratios. (A) Percentage of nonanal and lilac aldehyde concentrations in the different Platanthera orchid scents, which have 6- to 40-fold higher lilac aldehyde concentrations than P. obtusata. (B) Behavioral preferences by Ae. aegypti mosquitoes to scent mixtures containing lilac aldehydes at the concentrations quantified in the different Plathanthera species. Similar to Fig. 2C, mosquitoes were released in a y-olfactometer and had to choose between 2 arms carrying the scent mixture or no odorant (control). Asterisk denotes a significant difference from the mineral oil control (binomial test: P < 0.05); number symbol denotes a significant difference from the P. obtusata scent (binomial test: P < 0.05). (C and C1) Mean ∆F/F time traces for LC2 (blue) and AM2 (green) glomeruli to P. obtusata (Left) and nonanal (Right). (C2) Same as in C1, except to the P. stricta scent (Left) and lilac aldehyde (Right). The P. obtusata and P. stricta mixtures contain the same concentration of nonanal and other constituents but differ in their lilac aldehyde concentrations (see A). Traces are the mean (n = 6 to 10 mosquitoes); shaded areas denote ± SEM. (D and D1) Responses of the LC2 glomerulus to the different Platanthera orchid mixtures, and the single odorants nonanal and lilac aldehyde. The increasing concentration of lilac aldehyde in the other orchid mixtures caused a significant suppression of LC2 response to the nonanal in the scents (Kruskal–Wallis test: P < 0.05), even though nonanal was at the same concentration as in the P. obtusata mixture. (D2) Responses of the AM2 glomerulus to the different Platanthera orchid scents and nonanal and lilac aldehyde constituents. The increasing concentration of lilac aldehyde in the other orchid scents caused a significant increase in AM2 responses compared with responses to P. obtusata (Kruskal–Wallis test: P < 0.05). Bars are the mean ± SEM. (E) ∆F/F time traces for the LC2 (Left) and AM2 (Right) glomeruli. The preparation was simultaneously stimulated using separate vials of lilac aldehyde and nonanal at different concentrations to create 10 different mixture ratios. (E1) Each trace is a different ratio of lilac aldehyde to nonanal, ranging from green (10−2 nonanal: 0 lilac aldehyde) to purple (0 nonanal: 10−1 lilac aldehyde); 10−3 to 10−1 lilac aldehyde, and 10−2 nonanal concentrations were tested. (E2) As in E1, except tested concentrations were 10−3 to 10−1 for lilac aldehyde, and 10−3 for nonanal. (F and F1) Mean ∆F/F during 2 s of odor presentation for the LC2 glomerulus (Left) and the AM2 glomerulus (Right). Bars are color coded according to the ratio of lilac aldehyde to nonanal traces in E1. (F2) As in F1, except the concentrations of lilac aldehyde and nonanal in the ratio mixtures correspond to those in E2. Bars are the mean (n = 6) ± SEM. (G) Antibody labeling against GABA (green) in the right Ae. aegypti AL; background label (α-tubulin) is purple. (Scale bar, 20 µm.) (H) Mean ∆F/F time traces for the AM2 glomerulus. GABA receptor antagonists block the suppressive effect of nonanal to AM2’s response to the lilac aldehyde in the P. obtusata mixture, causing a significantly higher response than the preapplication and wash periods (Kruskal–Wallis test: P < 0.05). Traces are the mean (n = 4 mosquitoes) ± SEM.
To better understand how the ratio of lilac aldehyde and nonanal altered the activation of the LC2 and AM2 glomeruli, we tested mixtures of lilac aldehyde and nonanal at different concentration ratios and found that lilac aldehyde suppressed the response of LC2 to nonanal, suggesting lateral inhibition between these 2 glomeruli. Higher lilac aldehyde concentrations increased LC2 suppression, but reciprocally increased AM2 activation (Fig. 4 E and F). By contrast, nonanal caused suppression of AM2 responses to lilac aldehyde, with higher nonanal concentrations causing increased AM2 suppression, while increasing the activation of LC2 (Fig. 4 E and F). To determine whether this suppression of glomerular activity is mediated by γ-aminobutyric acid (GABA), an important inhibitory neurotransmitter in insect olfactory systems (27–29), we used antisera against GABA in the Ae. aegypti brain and found widespread labeling in AL glomeruli, including AM2 and LC2 (Fig. 4G). Next, we pharmacologically manipulated the inhibition by focally applying GABA-receptor antagonists (1 µM CGP54626; 10 µM picrotoxin) onto the AL during our experiments. During application of the vehicle (saline) control, LC2 and AM2 responses to the P. obtusata scent were similar to those described above (Fig. 4 E, F, and H and SI Appendix, Fig. S9), whereas during antagonist application, the effect of nonanal was blocked and the small amount of lilac aldehyde in the scent was sufficient to evoke a strong response in AM2 (Fig. 4H). The antagonists blocked the symmetrical inhibition by nonanal and lilac aldehyde in the P. stricta scent, causing increased response in both glomeruli, with the LC2 response levels similar to those evoked by P. obtusata (SI Appendix, Fig. S9). Together, these results support the hypothesis that the ratios of volatile compounds in the orchid scents, and the resulting balance of excitation and inhibition in the mosquito AL, play an important role in mediating mosquito attraction to P. obtusata and, possibly, reproductive isolation between orchid species.
Discussion
In this study, we use a unique mutualism between P. obtusata orchids and Aedes mosquitoes to show the importance of mosquito pollination for this orchid and the role of scent in mediating this association. Olfactory cues play important roles in a variety of biological processes for mosquitoes, including locating suitable hosts (4), oviposition sites (30), and nectar sources (31). For Aedes mosquitoes to efficiently locate sources of nutrients, they must distinguish between complex floral scents in a dynamic chemical environment (6). In the case of sympatric Platanthera orchids—which share the same scent constituents but differ in their ratios of nonanal and lilac aldehydes—their scents evoke distinct patterns of activation in AL glomeruli. How is this occurring? Our results suggest that GABA-mediated lateral inhibition from the LC2 glomerulus that encodes nonanal (found in higher abundance in P. obtusata) suppresses responses of glomeruli encoding lilac aldehydes (abundant in the scent of the other Platanthera species) which allows mosquitoes to distinguish between orchids.
There are only a handful of mosquito-pollinated flowers, but some of these species have been shown to emit similar volatile profiles as P. obtusata (7, 8, 31–33). Our results showed that certain terpene volatiles, like lilac aldehyde, were important in the discrimination of the P. obtusata scent, and at low concentrations, this volatile was important for attracting diverse mosquito species. In other mosquitoes, oxygenated terpene compounds that are derivatives of linalool, like lilac aldehyde and linalool oxide, were shown to elicit attraction to nectar sources (12, 34, 35). The qualitative similarities in the scent profiles of attractive nectar sources, and the attractiveness of the P. obtusata scent across mosquito species, raises the question of whether flower scents may be activating conserved olfactory channels, such as homologous odorant receptors (34). Our results will hopefully motivate research to identify the odorant receptors that are responsive to floral compounds, and their projections to the AL, such as the LC2 and AM2 glomeruli (34).
Our results also demonstrate the importance of mixtures and the processing of odorant ratios in Aedes. Interestingly, some of the volatile compounds emitted from blood hosts also occur in the P. obtusata scent, including nonanal (36, 37). However, in both Ae. increpitus and Ae. aegypti mosquitoes, the AL representations of host and orchid scents were different, suggesting that these odors may be processed via distinct olfactory channels. Despite the different glomerular ensemble responses, the complex nectar and host odors may share some of the same coding processes by AL circuits, including lateral inhibition of glomeruli. Similar to floral scents, human odors are complex mixtures that can differ between individuals in their constituent ratios, which may explain why mosquitoes often show behavioral preferences for certain individuals over others (4, 38). These dissimilarities have important epidemiological implications for disease transmission (4, 39, 40), and could be related to the subtle differences in the ratios of key compounds in an individual’s scent (38). Future work may explore if mosquito AL circuits process other complex odors, like those of human scent or other nectar sources, in a manner similar to that of the orchid scents, and whether the identified odorants and corresponding glomerular channels and modulatory systems can be leveraged in control interventions.
Materials and Methods
Procedures for floral volatile organic compound (VOC) collection and analysis, mosquito rearing, the preparation used for GC-EAD experiments, behavior experiments and associated stimuli, olfactory stimuli and pharmacological reagents used in calcium imaging experiments, and immunohistochemistry are described in SI Appendix, Supplementary Methods.
Orchid-Pollinator Observations and Pollination Experiments.
Flower observations.
Pollinator activity was monitored in the Okanogan-Wenatchee National Forest (47.847° N, 120.707° W; WA) from late June to early July in 2016 and 2017 when the flowers of P. obtusata were in full bloom. Multiple direct and video observations of varying lengths from 30 min to 2.5 h were made for a total of 46.7 h (15 h of direct and 31.7 h of video recordings). The observations were conducted from 10 AM to 8 PM when mosquitoes were found to visit the flowers. Observations were recorded by visually inspecting each plant, with the trained observer ∼1 m away from the plant—this distance did not influence the feeding and mosquito–flower visitation since no mosquito took off from the plant in the field and instead remained busy feeding from flower after flower. To further prevent the potential for observer interference, video observations were made using GoPro Hero4 Silver (San Mateo, CA) fitted with a 128 GB Lexar High-Performance 633× microSD card. Videos were set at 720-pixel resolution, 30 frames per second, and “narrow” field of view. These settings were optimized for the memory capacity, battery life, and best resolution by the camera. Both observation methods, direct and video, provided similar visitation rates. The visitation time, insect identity, leg color, and sex (for mosquitoes), were recorded from both direct and video observations. The number of feedings (defined by the probing into the flower using the proboscis) and visits (nonfeeding or resting) were quantified per hour per flower for each pollinator type. Over the course of the experiments and observations, temperatures ranged from 9.6 °C to 32.3 °C, with a relative humidity range of 13.4 to 100% (iButtons; Maxim Integrated, San Jose, CA, DS1923). These experiments, therefore, captured both sunny and rainy weather conditions that were common in this area at this time of the year.
Pollinator addition experiments.
To evaluate the contribution of mosquitoes to the pollination of P. obtusata orchids, we performed pollinator addition experiments during June through July in 2016. Mosquitoes were collected from the Okanogan-Wenatchee National Forest using Centers for Disease Control Wilton traps baited with carbon dioxide (John W. Hock Company, Gainesville, FL). Carbon dioxide traps provide a standardized method to sample the mosquito assemblages near and among wetland habitats (41, 42). Traps were placed within the sedge habitat, but more than 60 m from the nearest focal flower patch to prevent any disturbance.
P. obtusata from the same site was enclosed in BugDorm cages (30 cm × 30 cm × 30 cm; BioQuip Products, Rancho Dominguez, CA, 1452) for which the bottom panel was removed to cover the orchid. Thirty mosquitoes were introduced into each cage through a sleeve located on the front panel and left without human interference for a duration of 48 h, after which the mosquitoes were collected from the enclosures and identified. The number and species of mosquitoes with pollinium attached were recorded, and the plant was bagged for determination of the fruit-to-flower ratio at the end of the field season. A total of 19 enclosures were used, 11 enclosures with a single plant and 8 enclosures with 2 to 3 plants.
Pollen limitation studies.
To determine the importance of pollination and out-crossing on P. obtusata fruit set, plants were subjected to 4 different experimental treatments during the June through July summer months. For 2 wk, plants were either unbagged (n = 20 plants) or bagged to prevent pollinator visitation (n = 19 plants). Organza bags (model B07735-1; Housweety, Causeway Bay, Hong Kong) were used to prevent pollinators from visiting the flowers. In addition, we determined the importance of cross- and self-pollination for P. obtusata. For cross-pollination, 6 pollinia were removed from 2 plants using a toothpick and gently brushed against the stigma of a neighboring plant (n = 11 plants). To examine the effects of self-pollination, 6 pollinia were removed from 3 flowers and gently brushed the flowers on the same plant (n = 9 plants). At the end of the field season, the number of flowers and the number of fruits produced per individual plants were recorded and the fruit-to-flower ratios were calculated. For comparing the fruit weights and the seed set for each treatment, up to 4 fruits from each individual of P. obtusata were collected. The weights were measured with a digital scale (Mettler Toledo, Columbus, OH), and the number of viable seeds per fruit were counted using an epifluorescent microscope (60× magnification; Nikon Ti4000). Fruit weights and seed sets were compared using a Student’s t test; fruit-to-flower ratios were compared using a Mann–Whitney U test.
GC-EAD.
Electroantennogram signals were filtered and amplified (100×; 0.1 to 500 Hz) using an A-M 1800 amplifier (Sequim, WA) connected to a personal computer via a BNC-2090A analog-to-digital board (National Instruments, Austin, TX) and digitized at 20 Hz using WinEDR software (Strathclyde Electrophysiology Software, Glasgow, UK). A Hum Bug noise eliminator (Quest Scientific, Vancouver, Canada) was used to decrease electrical noise. The antennal responses to peaks eluting from the GC were measured for each mosquito preparation and each peak and mosquito species. Bioactive peaks were those that elicited strong EAD responses, corresponding to deflections beyond the average noise floor of the baseline EAD signal. Responses by each individual preparation were used for principal component analysis (Ade4 package, R). The responses of 8 different mosquito species were tested to the scent extracts of 3 orchid species (n = 8 mosquito species for P. obtusata and n = 4 mosquito species each for P. stricta and P. huronensis, with 3 to 17 replicates per mosquito species per orchid, for a total of 109 GC-EAD experiments).
Two-Photon Excitation Microscopy.
Calcium imaging in the Ae. increpitus mosquito AL.
Odor-evoked responses in the Ae. increpitus mosquito AL were imaged with 9 female mosquitoes at the beginning of the season when mosquitoes were relatively young (as defined by wing and scale appearance). Calcium imaging experiments were conducted using application of the calcium indicator Fluo4 to the mosquito brain and using a stage that allows simultaneous calcium imaging and tethered flight (22). The mosquito was cooled on ice and transferred to a Peltier-cooled holder that enables the mosquito head to be fixed to the stage using UV glue. The custom stage permits the superfusion of saline to the head capsule and space for movement by the wings and proboscis (22) (Fig. 3). Once the mosquito was fixed to the stage, a window in its head was cut to expose the brain, and the brain was continuously superfused with physiological saline (21, 22). Next, the perineural sheath was gently removed from the AL using fine forceps, and 75 µL of the Fluo4 solution—made by 50 mg of Fluo4 in 30 µL Pluronic F-127 and then subsequently diluted in 950 µL of mosquito physiological saline—was pipetted to the holder allowing the brain to be completely immersed in the dye. Mosquitoes were kept in the dark at 15 °C for 1.5 h (the appropriate time for adequate penetration of the dye into the tissue), after which the brain was washed 3 times with physiological saline. After the rinse, mosquitoes were kept in the dark at room temperature for ∼10 to 20 min before imaging.
Wing stroke amplitudes were acquired and analyzed using a custom camera-based computer vision system at frame rates of 100 Hz (22, 43), where the mosquito was illuminated with infrared LEDs (880 nm), and images were collected with an infrared-sensitive camera synched to the 2-photon system. Stimulus-evoked initiation of flight and changes in the amplitude of the wing-stroke envelope were characterized for each odor stimulus (sensu ref. 22). Calcium-evoked responses in the AL were imaged using the Prairie Ultima IV 2-photon excitation microscope (Prairie Technologies) and Ti-Sapphire laser (Chameleon Ultra; Coherent). Experiments were performed at a depth of 40 µm from the ventral surface of the AL, allowing the calcium dynamics from ∼18 to 22 glomeruli to be repeatedly imaged across preparations. Images were collected at 2 Hz, and for each odor stimulus images were acquired for 35 s, starting 10 s before the stimulus onset. Imaging data were extracted in Fiji/ImageJ and imported into Matlab (v2017; Mathworks, Natick, MA) for Gaussian filtering (2 × 2 pixel; σ = 1.5 to 3) and alignment using a single frame as the reference at a given imaging depth and subsequently registered to every frame to within 1/4 pixel. Trigger-averaged ΔF/F was used for comparing glomerular responses between odor stimuli. After an experiment, the AL was sequentially scanned at 1-µm depths from the ventral to dorsal surface. Ventral glomeruli to the 40-µm depth were 3-dimensionally (3D) reconstructed using Reconstruct software or Amira v5 (Indeed-Visual Concepts, Houston TX) to provide glomerular assignment and registration between preparations. Glomeruli in the ventral region of the AL, based on their positions, were tentatively assigned names similar to those in Ae. aegypti (22, 44).
Calcium imaging in the Ae. aegypti mosquito AL.
Odor-evoked responses in the Ae. aegypti AL were imaged taking advantage of our genetically encoded PUb-GCaMPs mosquito line (20). A total of 20 preparations were used: 10 for single odorant and orchid mixture experiments, 6 for ratio experiments, and 4 for experiments using GABA-receptor antagonists. Glomeruli were imaged at 40 µm from the ventral surface, as glomeruli at this depth show strong responses to odorants in the orchid headspace, including nonanal, octanal, and lilac aldehyde, and at this depth, ∼14 to 18 glomeruli can be neuroanatomically identified and registered between preparations. The expression of GCaMP occurred in glia, local interneurons, and projection neurons. Nevertheless, double-labeling for GFP (GCaMPs) and glutamine synthase (GS; glial marker) revealed broad GFP labeling that did not always overlap with the glial stain, with GS staining often occurring on astroglial-like processes on the rind around glomeruli, and strong GFP occurring within the glomeruli (SI Appendix, Fig. S10). Thus, in our calcium imaging experiments we took care to image from the central regions of the glomeruli and avoid the sheaths and external glomerular loci. Moreover, strong GFP staining occurred in soma membranes located in the medial and lateral cell clusters, which contain the projection neurons and GABAergic local interneurons, respectively; the vast majority of these cell bodies did not stain for GS (SI Appendix, Fig. S10). Relatedly, GCaMP6s expression is very high in AL local interneurons and projection neurons (PNs), such that during odor stimulation the PNs and axonal processes can often be imaged, and 3D reconstructions can take place through simultaneous optical sections with odor stimulation. Nonetheless, we assume the glomerular responses are a function of multiple cell types. In other insects, GABAergic modulation has been shown to operate on olfactory receptor neurons, local interneurons, and PNs (27–29).
Similar to experiments with Ae. increpitus, the majority the mosquitoes were UV glued to the stage to allow free movement of their wings and proboscis; however, for experiments using GABA-receptor antagonists the proboscis was glued to the stage for additional stability. Once the mosquito was fixed to the stage, a window in its head was cut to expose the brain, and the brain was continuously superfused with physiological saline (21).
Data and Resource Availability.
Data on the behavioral, chemical, ecological, and calcium imaging experiments can be found on Mendeley Data. Software is available at https://github.com/riffelllab. Mosquito lines are available upon request.
Supplementary Material
Acknowledgments
We are grateful for the advice and assistance provided by G. Thornton, J. Patt, B. Nguyen, E. Lutz, J. Lim, E. Mathis, M. Clifford, and K. Moosavi. Support for this project was provided by National Institutes of Health under grants RO1-DC013693 (J.A.R.) and R21-AI137947 (J.A.R.), Air Force Office of Scientific Research under grants FA9550-14-1-0398 (J.A.R.) and FA9550-16-1-0167 (J.A.R.), an Endowed Professorship for Excellence in Biology (J.A.R.), and the University of Washington Innovation Award (J.A.R.).
Footnotes
Competing interest statement: A provisional patent on the odor that mimics the orchid scent was recently filed (62/808,710).
This article is a PNAS Direct Submission. W.S.L. is a guest editor invited by the Editorial Board.
Data deposition: Data is accessible on Mendeley Data (https://data.mendeley.com/datasets/brrx42bjvg/1).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910589117/-/DCSupplemental.
References
- 1.World Health Organization , “A global brief on vector-borne diseases” (No. WHO/DCO/WHD/2014.1, WHO Press, Geneva, Switzerland, 2014).
- 2.Cardé R. T., Gibson G., Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues. Olfaction vector host Interact. 2010, 115–142 (2010). [Google Scholar]
- 3.McMeniman C. J., Corfas R. A., Matthews B. J., Ritchie S. A., Vosshall L. B., Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takken W., Verhulst N. O., Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 58, 433–453 (2013). [DOI] [PubMed] [Google Scholar]
- 5.van Breugel F., Riffell J., Fairhall A., Dickinson M. H., Mosquitoes use vision to associate odor plumes with thermal targets. Curr. Biol. 25, 2123–2129 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster W. A., Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40, 443–474 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Manda H., et al. , Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar. J. 6, 113 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhumur U. S., Dötterl S., Jürgens A., Floral odors of Silene otites: Their variability and attractiveness to mosquitoes. J. Chem. Ecol. 34, 14–25 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Peach D. A., Gries G., Nectar thieves or invited pollinators? A case study of tansy flowers and common house mosquitoes. Arthropod Plant Interact. 10, 497–506 (2016). [Google Scholar]
- 10.Brantjes N., Leemans J., Silene otites (Caryophyllaceae) pollinated by nocturnal Lepidoptera and mosquitoes. Acta Bot. Neerl. 25, 281–295 (1976). [Google Scholar]
- 11.Nikbakhtzadeh M. R., Terbot J. W. 2nd, Otienoburu P. E., Foster W. A., Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera: Culicidae) among common African plants. J. Vector Ecol. 39, 372–383 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Nyasembe V. O., Torto B., Volatile phytochemicals as mosquito semiochemicals. Phytochem. Lett. 8, 196–201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inouye D. W., Mosquitoes: More likely nectar thieves than pollinators. Nature 467, 27 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Dexter J. S., Mosquitoes pollinating orchids. Science 37, 867 (1913). [DOI] [PubMed] [Google Scholar]
- 15.Thien L. B., Mosquito pollination of Habenaria obtusata (Orchidaceae). Am. J. Bot. 56, 232–237 (1969). [Google Scholar]
- 16.Thien L. B., Utech F., The mode of pollination in Habenaria obtusata (Orchidaceae). Am. J. Bot. 57, 1031–1035 (1970). [Google Scholar]
- 17.Stoutamire W. P., Mosquito pollination of Habenaria obtusata (Orchidaceae). Mich. Bot. 7, 203–212 (1968). [Google Scholar]
- 18.Wallace L. E., Spatial genetic structure and frequency of interspecific hybridization in Platanthera aquilonis and P. dilatata (Orchidaceae) occurring in sympatry. Am. J. Bot. 93, 1001–1009 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Bateman R. M., et al. , Molecular phylogenetics and morphological reappraisal of the Platanthera clade (Orchidaceae: Orchidinae) prompts expansion of the generic limits of Galearis and Platanthera. Ann. Bot. 104, 431–445 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bui M., et al. , Live calcium imaging of Aedes aegypti neuronal tissues reveals differential importance of chemosensory systems for life-history-specific foraging strategies. BMC Neurosci. 20, 27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinauger C., et al. , Visual-olfactory integration in the human disease vector mosquito Aedes aegypti. Curr. Biol. 29, 2509–2516.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinauger C., et al. , Modulation of host learning in Aedes aegypti mosquitoes. Curr. Biol. 28, 333–344.e8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ditzen M., Pellegrino M., Vosshall L. B., Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319, 1838–1842 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Leal W. S., The enigmatic reception of DEET - the gold standard of insect repellents. Curr. Opin. Insect Sci. 6, 93–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowland M., et al. , DEET mosquito repellent provides personal protection against malaria: A household randomized trial in an Afghan refugee camp in Pakistan. Trop. Med. Int. Health 9, 335–342 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Syed Z., Leal W. S., Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. U.S.A. 105, 13598–13603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen S. R., Wilson R. I., Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silbering A. F., Galizia C. G., Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J. Neurosci. 27, 11966–11977 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldrop B., Christensen T. A., Hildebrand J. G., GABA-mediated synaptic inhibition of projection neurons in the antennal lobes of the sphinx moth, Manduca sexta. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 161, 23–32 (1987). [DOI] [PubMed] [Google Scholar]
- 30.Zhu F., Xu P., Barbosa R. M., Choo Y.-M., Leal W. S., RNAi-based demonstration of direct link between specific odorant receptors and mosquito oviposition behavior. Insect Biochem. Mol. Biol. 43, 916–923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster W. A., Hancock R. G., Nectar-related olfactory and visual attractants for mosquitoes. J. Am. Mosq. Control Assoc. 10, 288–296 (1994). [PubMed] [Google Scholar]
- 32.Jhumur U. S., Dötterl S., Jürgens A., Electrophysiological and behavioural responses of mosquitoes to volatiles of Silene otites (Caryophyllaceae). Arthropod Plant Interact. 1, 245–254 (2007). [Google Scholar]
- 33.Joseph S., Fruit feeding of mosquitoes in nature. Proceedings. N. J. Mosq. Exterm. Assoc. 57, 125–131 (1970). [Google Scholar]
- 34.Zeng F., Xu P., Leal W. S., Odorant receptors from Culex quinquefasciatus and Aedes aegypti sensitive to floral compounds. Insect Biochem. Mol. Biol. 113, 103213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyasembe V. O., Teal P. E., Mukabana W. R., Tumlinson J. H., Torto B., Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasit. Vectors 5, 234 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syed Z., Leal W. S., Acute olfactory response of Culex mosquitoes to a human-and bird-derived attractant. Proc. Natl. Acad. Sci. U.S.A. 106, 18803–18808 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernier U. R., Kline D. L., Barnard D. R., Schreck C. E., Yost R. A., Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem. 72, 747–756 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Leal H. M., Hwang J. K., Tan K., Leal W. S., Attraction of Culex mosquitoes to aldehydes from human emanations. Sci. Rep. 7, 17965 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly D. W., Why are some people bitten more than others? Trends Parasitol. 17, 578–581 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Simpson J. E., et al. , Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. Biol. Sci. 279, 925–933 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin D. C., Whelan P. I., Tropical mosquito assemblages demonstrate ‘textbook’ annual cycles. PLoS One 4, e8296 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silver J. B., Mosquito Ecology: Field Sampling Methods (Springer Science & Business Media, 2007). [Google Scholar]
- 43.Suver M. P., Huda A., Iwasaki N., Safarik S., Dickinson M. H., An array of descending visual interneurons encoding self-motion in Drosophila. J. Neurosci. 36, 11768–11780 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ignell R., Dekker T., Ghaninia M., Hansson B. S., Neuronal architecture of the mosquito deutocerebrum. J. Comp. Neurol. 493, 207–240 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.