Significance
Photosynthetic water oxidation by the multisubunit membrane protein complex PSII is an important process that sustains all aerobic life on Earth by producing molecular oxygen from sunlight and water. Understanding the mechanism of this process is crucial toward advancing fundamental knowledge as well as providing a blueprint for the development of solar fuel devices. Important pieces of information required for solving the mechanism of biological water oxidation are the oxidation states of the manganese ions forming the catalytic site of water oxidation in PSII. Here, we resolve a long-standing controversy between 2 competing schools of thought, by providing a clear-cut determination of overall manganese oxidation states using a simple counting experiment.
Keywords: photosynthesis, oxygen evolving cluster, photoassembly, manganese oxidation state, mechanism of water oxidation
Abstract
Knowledge of the manganese oxidation states of the oxygen-evolving Mn4CaO5 cluster in photosystem II (PSII) is crucial toward understanding the mechanism of biological water oxidation. There is a 4 decade long debate on this topic that historically originates from the observation of a multiline electron paramagnetic resonance (EPR) signal with effective total spin of S = 1/2 in the singly oxidized S2 state of this cluster. This signal implies an overall oxidation state of either Mn(III)3Mn(IV) or Mn(III)Mn(IV)3 for the S2 state. These 2 competing assignments are commonly known as “low oxidation (LO)” and “high oxidation (HO)” models of the Mn4CaO5 cluster. Recent advanced EPR and Mn K-edge X-ray spectroscopy studies converge upon the HO model. However, doubts about these assignments have been voiced, fueled especially by studies counting the number of flash-driven electron removals required for the assembly of an active Mn4CaO5 cluster starting from Mn(II) and Mn-free PSII. This process, known as photoactivation, appeared to support the LO model since the first oxygen is reported to evolve already after 7 flashes. In this study, we improved the quantum yield and sensitivity of the photoactivation experiment by employing PSII microcrystals that retained all protein subunits after complete manganese removal and by oxygen detection via a custom built thin-layer cell connected to a membrane inlet mass spectrometer. We demonstrate that 9 flashes by a nanosecond laser are required for the production of the first oxygen, which proves that the HO model provides the correct description of the Mn4CaO5 cluster’s oxidation states.
Photosynthesis is essential for life as it not only stores solar energy within chemical bonds, but also provides the molecular oxygen required for unlocking the stored energy with high efficiency. The photosynthetic reaction sequence begins with the light-driven oxidation of water that is catalyzed in the biosphere exclusively by PSII. Paradoxically, PSII is also inactivated by light exposure, necessitating a repair process that includes the disassembly and reassembly of the catalytic site for water oxidation in a PSII protein every half hour under full sunlight. Revealing the water oxidation mechanism by PSII, as well as the reassembly process of PSII, is vital for enhancing our knowledge of this key process and may provide inspiration toward the development of artificial water oxidation catalysts needed for the sustainable production of solar fuels, such as hydrogen, ammonia, or methane.
The kinetic scheme describing water oxidation by PSII is known as the Kok cycle (1). It comprises 1 stable (S1), 3 semistable (S0, S2, and S3), and 1 transient (S4) intermediate(s) where higher numbers denote successive oxidation of the catalytic center that contains a Mn4CaO5 cluster. Recent serial femtosecond X-ray crystallography data provided detailed information about structural changes in the Mn4CaO5 cluster as it cycles through the S0, S1, S2, and S3 states (2, 3), yet the water oxidation mechanism remains controversial (4, 5). One of the long-standing discussion points is the overall oxidation state of the Mn4CaO5 cluster (6). Historically, the debate on the Mn oxidation states of the Mn4CaO5 cluster arose from the continuous wave X-band electron paramagnetic resonance (EPR) based discovery that the S2 state gives rise to a multiline signal with an effective total spin of S = 1/2 that has 18–20 hyperfine lines (7, 8). This is a signature of a Mn(III)Mn(IV) mixed valence state within a complex with more than 2 Mn ions, which lead to the overall oxidation state assignments of either Mn(III)3Mn(IV) or Mn(III)Mn(IV)3 for the Mn4CaO5 cluster (6). As recent results confirm that each S-state advancement corresponds to a manganese centered one-electron oxidation (9), the oxidation states for all S-state intermediates in the Kok cycle can be calculated if one of the states is known. Thus, starting from the 2 options for the S2 state, 2 competing sets of oxidation state assignment have been proposed and are commonly known as the LO or HO models. Subsequent 55Mn electron nuclear double resonance (ENDOR) studies support the assignment of Mn(III)3Mn(IV) for the S0 state (10) and of Mn(III)Mn(IV)3 for S2 (10, 11). The HO model is, furthermore, consistent with recent 55Mn electron–electron double resonance detected NMR (EDNMR) studies of the S3 state that support the Mn(IV)4 assignment for this state (12). Nonetheless, there are alternative interpretations of the S = 1/2 EPR multiline signal (13) and a 55Mn ENDOR study (14) that supports the LO model.
The oxidation states of the Mn4CaO5 cluster have been studied over the past 3 decades by a large number of additional techniques. Mn K-edge X-ray absorption near edge structure (15–17) and Mn Kβ X-ray emission spectroscopy (XES) (9, 15, 18) studies by various groups unanimously support the HO model. Yet these studies are challenged by alternative interpretations (19, 20) that suggest that the available data are better suited toward the LO model.
Beyond spectroscopic methods, the oxidation state of Mn4CaO5 can be inferred by an inventory of the redox equivalence between the Mn2+ and the functional Mn4CaO5 cluster. Titration of reductant to functional Mn4CaO5 while monitoring the amount of Mn2+ formation (21) or the progressive shift of the first maximum in flash-induced oxygen oscillation patterns allow assessments of the overall oxidation states (22, 23). Most of these data have been interpreted to support the HO model, but the study by Kretschmann et al. (23) supports the LO model.
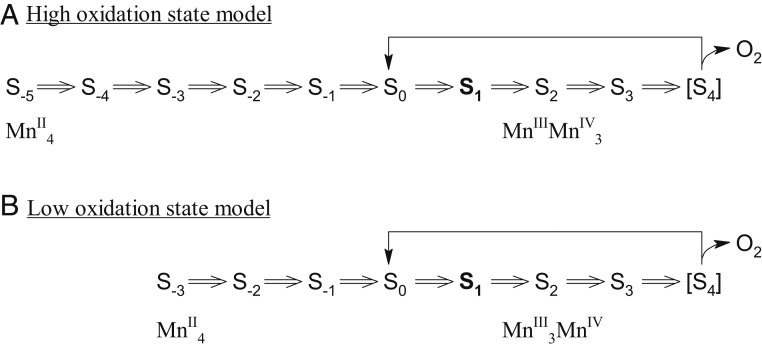
The converse approach is the photooxidation of exogenously supplied Mn2+ by PSII depleted of the Mn4CaO5 cluster (apo-PSII) to restore O2-evolving functionality in a process known as photoactivation (refs. 24–33; for reviews, see refs. 34 and 35). When using single turnover flashes, the photooxidation of Mn is a one-electron oxidation event as seen, for example, in intact dark-adapted (S1 state) PSII samples where such an illumination leads to a period 4 oscillation with the first O2 evolution after the third flash (1, 36, 37). Thus, by counting the number of single turnover flashes required to evolve the first O2 during photoactivation of apo-PSII, the overall oxidation state of the Mn4CaO5 cluster can be established. Starting from exogenously supplied Mn2+, O2 evolution after 7 flashes corresponds to the LO model, while O2 evolution after 9 flashes corresponds to the HO model (see Fig. 1). In contrast to the majority of the results presented above, previous reports of the oxidation state determination via photoactivation support the LO model (33, 35, 38). However, the light flashes used in these studies were not strictly single turnover but rather consisted of trains of short LED pulses. While these flashes, which were employed for increasing the yield of photoactivation to a level that O2 evolution could be detected, were designed by taking independently determined kinetics of the photoactivation process into account (33), this opens the possibility of multiple oxidation’s per flash. One indication for this may be the unexpected observation of O2 evolution below the minimum number of 7 light flashes (33).
Fig. 1.
Schematic of photoactivation of PSII based on the (A) high oxidation model and (B) low oxidation model. S0–S4 states are intermediate states of the Kok cycle; S−5 to S−1 states are photoactivation intermediate states. The dark stable S1 state is labeled in bold. Each double arrow corresponds to a flash-induced one-electron oxidation.
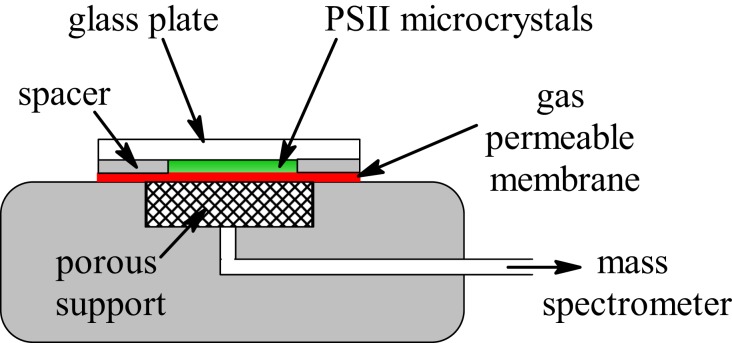
Recently, a crystal structure of a Mn-depleted PSII core complex from Thermosynechococcus elongatus was reported at 2.55 Å resolution that is based on a complete depletion of the Mn4CaO5 cluster in PSII crystals via NH2OH and EDTA treatments (39). Importantly, the resulting apo-PSII crystals retained all 20 protein subunits, including the 3 extrinsic proteins that stabilize the Mn4CaO5 cluster. This preparation of highly purified and well-defined apo-PSII crystals present a new opportunity to assess the overall oxidation state of Mn4CaO5 by photoactivation. In this paper, we use this preparation in combination with saturating nanosecond (ns) laser pulses that fully exclude multiple oxidation’s of the reaction center’s during one flash. Highly sensitive detection of the evolved O2 was achieved by a thin-layer membrane inlet cell (Fig. 2) that was connected to an isotope ratio mass spectrometer (IRMS). This unique experimental design enabled us to detect O2 release after the first S3 → [S4] → S0 transition reached during photoactivation. Consequently, the oxidation state determination did not require any complicated analysis beyond the straightforward count of the number of laser flashes required to observe O2 evolution.
Fig. 2.
Schematic of the thin-layer membrane inlet mass spectrometer cuvette. The sample is confined within a 6-mm-diameter chamber that has a height of 100 µm.
Results and Discussion
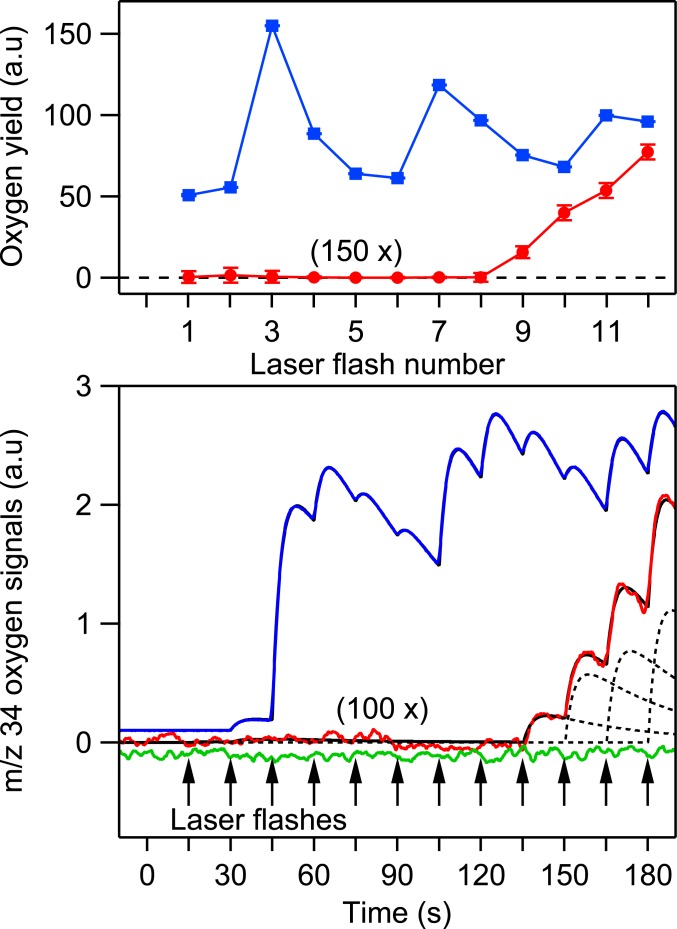
Flash-induced oxygen release patterns (FIOPs) of PSII microcrystals of T. elongatus core preparations were recorded at 20 °C in the presence of the artificial electron acceptor phenyl-p-benzoquinone (PPBQ) employing a thin-layer membrane inlet mass spectrometry (MIMS) cuvette (Fig. 2) and nanoseconds duration green (532 nm) laser flashes. The FIOPs of native PSII microcrystals (blue trace in Fig. 3) exhibited the typical period 4 oscillation of dark-adapted PSII samples with maxima at the third, seventh, and 11th flashes. The damping of this oscillation can be described by a miss parameter of 10%, which demonstrates that the laser light was saturating and that the exogenous electron acceptor allowed sustaining oxygen evolution well beyond the number of flashes needed to determine the overall Mn oxidation state of the oxygen-evolving complex. The excellent oscillation is remarkable given the fact that, due to the slow mass transport of O2 from the MIMS cuvette into the ion source of the IRMS, the laser flashes were spaced 15 s apart to resolve individual flash-induced oxygen peaks. In this context, we note that the small oxygen yield after the second flash is not due to double oxidation of a minor fraction of PSII centers (double hits) but to the extremely long S2 and S3 state lifetimes in the presence of PPBQ that led to an incomplete S1 state synchronization within the 60 min dark-adaptation period after the preflash employed to oxidize tyrosine D (40–44).
Fig. 3.
(Top) Representative FIOPs of native PSII microcrystals (blue trace) and during the photoactivation of apo-PSII microcrystals using equal flash spacings of 15 s (red trace). The red trace magnified by 150 times; the blue trace offset from zero for clarity. Error bars are 95% confidence interval derived from the fitting procedure and smaller than the marker symbols. (Bottom) Original oxygen signals from the MIMS system during laser flashing of apo-PSII microcrystals incubated in an assembly buffer (red trace); apo-PSII microcrystals without Mn2+ and Ca2+ (green trace) and a native PSII microcrystal (blue trace). The dashed line: fitted oxygen peaks after each laser flash; the black line: sum of all fitted oxygen peaks. The vertical arrows indicate laser flashes. The red and green traces are magnified by 100 times. The green and blue traces were offset from zero for clarity.
The green trace obtained with Mn-free (Fig. 3) apo-PSII microcrystals in the absence of added Mn2+ and Ca2+ but with the exogenous electron acceptor PPBQ shows that, in contrast to initial trials with Clark and Joliot type electrodes, the laser pulses did not cause any flash-induced artifacts in the MIMS system. Importantly, no O2 was produced by apo-PSII in the absence of added Mn and Ca cofactors.
On the basis of these controls, photoactivation experiments were performed by adding 10 mM MnCl2, 400 mM CaCl2, 5 mM NaHCO3, and the same acceptor as above (assembly buffer) to dark-adapted apo-PSII microcrystals (no preflash treatment), which were then flashed in the same way as above. No molecular oxygen was observed in the first 8 flashes, but the ninth flash induced a clearly resolved O2 signal indicating the successful assembly of the Mn4CaO5 cluster in a fraction of the centers (red trace in Fig. 3). The subsequent rise of the flash-induced O2 signal shows the photoactivation of further PSII centers. It does not display any oscillation, indicating a significant miss factor causing mixing of S states during photoactivation.
The O2 yield after the ninth flash was ∼0.1% of that obtained from native PSII microcrystals after the third flash, indicating that the S1 state population was 0.1% after 6 photoactivation flashes. This implies that the average quantum yield of photoactivation for the first 6 flashes is close to 32% (SI Appendix), i.e., higher than the typically reported quantum yield of 1% based on noncrystalline Mn4CaO5 depleted PSII preparations (24, 25, 27, 28, 34). This comparison also allows the estimation that the absolute O2 yield induced by the ninth flash was about 15 nM, i.e., well above the estimated oxygen detection limit of 3 nM in the current experimental setup (SI Appendix, Figs. S1 and S2). We note that the observation of O2 evolution after 9 flashes can be made consistent with the LO model if the O2 patterns of photoactivation are shifted by 2 electrons due to the presence of auxiliary electron donors that donate electrons more efficiently to or P680+ than Mn. The most likely auxiliary electron donors are tyrosine D, YD, and cytochrome b 559, cyt b559. However, this scenario is considered highly unlikely due to the presence of a high affinity Mn2+ binding site at or near the assembled Mn4CaO5 cluster involving the D1-A170 and D1-E333 residues (29, 45–47). Transient variable fluorescence (48, 49) and EPR measurements (49, 50) during single flash experiments on Mn depleted PSII samples have demonstrated that electron donation from this specifically bound Mn2+ to is very efficient. This is expected given the much longer distances (>30 Å) of YD or cyt b559 as compared to Mn2+ at the high affinity binding site (7 Å). Moreover, the rate of YZ oxidation by P680+ at pH < 7 is, at least, 2 orders of magnitude faster compared to the oxidation of YD or cyt b559 based on optical and EPR measurements in Mn depleted PSII samples (51, 52).
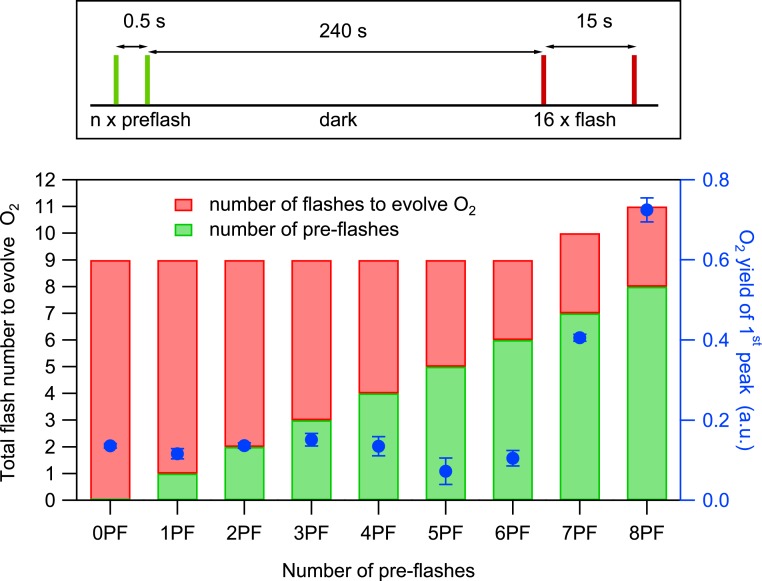
According to the two-quantum model of photoactivation (25, 34), a second photon is required to stabilize the intermediate formed after the first flash; the optimal flash spacing between the first and the second flash is reported to be between 0.5 and 1 s. Thus, the long dark time (15 s) between flashes used above may allow significant back reaction of the first photooxidized intermediate, potentially reducing the O2 yield that may originate from the seventh flash to below our detection limit. In the results presented in Fig. 4, we, therefore, applied a modified flash sequence in which a preflash train was used with 0.5 s spacing (green), followed by a dark time of 240 s before a second flash train (15 s spacing, red) was applied. This modified flash sequence should allow optimal formation of the 2-photon stabilized intermediate by minimizing the amount of back reaction thereby allowing the detection of O2 evolution from the seventh flash (if any). For apo-PSII microcrystal suspensions that were subjected to 2 preflashes (2PF), the total number of flashes (sum of preflashes and flashes) required to evolve O2 was, however, still 9, further supporting the HO model (Fig. 4). This test additionally excludes the scenario where residual NH2OH that remained in the samples after 10 washing steps, calculated to be below 0.1 μM (SI Appendix), leads to back reactions of photoactivation intermediates between the dark times of the flashes. We further confirmed this observation by examining the sum of flashes required to evolve O2 from apo-PSII that were subjected to 0PF to 8PF. Between 0PF and 6PF, in total, 9 laser flashes were required in each case to evolved O2 and the O2 yields induced by the ninth flash were similar in each case (blue symbols in Fig. 4). At 6PF, 7PF, and 8PF, the number of additional laser flashes (red) required to evolve O2 remained constant at 3 flashes, while the O2 yield increased with each additional preflash. These observations can be rationalized by the formation of the S1 state after 6PF and of the S2 and S3 states after 7PF and 8PF, which decay back to the dark stable S1 during the 240 s dark time via electron donation by, for example, tyrosine YD (see below). Therefore, 3 additional flashes were required in each case to complete the first catalytic cycle to evolve O2, and the larger number of total flashes led to the expected increased photoactivation yields (compare to Fig. 3). EPR experiments showed that, in the dark-adapted apo-PSII samples, indeed, 94% of YD was reduced due to the NH2OH treatment (SI Appendix, Fig. S4). Thus, the data in Fig. 3 provide additional strong evidence that the S2 state was formed for the first time after 7 flashes and that the first O2 was produced after 9 flashes during the first S3 → [S4] → S0 transition.
Fig. 4.
(Top) Laser flash spacing’s used for photoactivation of apo-PSII microcrystals suspension by various combinations of tightly spaced preflashes (green line) and monitoring flashes (red line) with 15-s spacing. The dark time of 240 s was employed to allow the back reaction of the S2 and S3 states to S1. (Bottom) Total number of flashes required to observe the first O2 evolution (left axis) during the photoactivation of apo-PSII crystals with various combinations of preflashes and monitoring flashes (see Top). The yields of the first O2 peaks, plotted in blue, are the averages of 2 repeat measurements, and the error bars are SDs.
To summarize, in contrast to previous photoactivation studies that employed complicated LED pulse trains as flashes, our data obtained with single turnover ns-laser pulses provides strong support for the HO state model of the Mn4CaO5 cluster. Together with the spectroscopic evidence for the HO model, this provides the required firm basis for deriving the mechanism of water oxidation. Furthermore, this study demonstrates the feasibility of the stepwise photoactivation of the Mn4CaO5 cluster depleted PSII microcrystals, which will allow detailed time resolved structural characterization of photoactivation intermediates using serial femtosecond X-ray crystallography.
Materials and Methods
T. elongatus cells were cultivated, and PSII core complexes purified as described previously (39). Intact PSII microcrystals with a size between 20 and 80 µm were generated according to literature methods (53). Apo-PSII microcrystals were obtained as in ref. 39 by incubating intact PSII microcrystals for 30 min with 50 mM NH2OH and 50 mM EDTA, but the crystals were, subsequently, washed 8 times and dialyzed 2 times to achieve rigorous removal of NH2OH and EDTA. The estimated residual NH2OH and EDTA concentrations are no more than 0.1 µM (SI Appendix). EPR spectra of the apo-PSII microcrystal suspension in the g = 2.0 region were completely devoid of features associated with Mn2+ before and after heat treatment at 363 K to release any residual Mn, confirming the complete absence of Mn in apo-PSII preparations (SI Appendix, Fig. S3).
The apo-PSII microcrystals were stored in 0.1 M MES pH 6.5, 0.1 M NH4Cl, and 20% PEG 5000 (MIMS buffer). All manipulations of apo-PSII microcrystals were performed in complete darkness with the aid of an IR camera and 940 nm illumination. Apo-PSII microcrystal suspensions (0.5 ± 0.15 mM) in an assembly buffer (MIMS buffer with 10 mM MnCl2, 400 mM CaCl2, 5 mM NaHCO3, and 0.3 mM PPBQ, 6% H218O) were confined within a 6 mm diameter chamber that is 100 µm thick (thin-layer MIMS cuvette; Fig. 2) and allowed to degas for 4 min. Saturating single turnover flashes were provided using a 10 ns duration laser (532 nm and 60 mJ/cm2 per pulse). The thin-layer MIMS cuvette was connected to the source vacuum of a Thermo Delta XP IRMS via a dry ice/ethanol cooling trap (54). Oxygen evolution was monitored via the m/z 34 signal from the IRMS. The oxygen yield for each flash-induced peak was calculated using a multipeak fitting procedure implemented in the software Igor Pro-6.3 (WaveMetrics) (for details see the SI Appendix).
Data Availability Statement.
All data discussed in the paper are available at http://uu.diva-portal.org under accession number diva2:1368663.
Supplementary Material
Acknowledgments
We thank Casper de Lichtenberg and Gesine Bartels for technical assistance. This project was supported by the Swedish Research Council (Grant 2016-05183). A.Z. and M.Z. acknowledge financial support funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy- EXC 2008/1 - 390540038.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data discussed in the paper are available at http://uu.diva-portal.org under accession no. diva2:1368663.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1915879117/-/DCSupplemental.
References
- 1.Kok B., Forbush B., McGloin M., Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 11, 457–475 (1970). [DOI] [PubMed] [Google Scholar]
- 2.Suga M., et al. , Light-induced structural changes and the site of O=O bond formation in PSII caught by XFEL. Nature 543, 131–135 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Kern J., et al. , Structures of the intermediates of Kok’s photosynthetic water oxidation clock. Nature 563, 421–425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantazis D. A., Missing pieces in the puzzle of biological water oxidation. ACS Catal. 8, 9477–9507 (2018). [Google Scholar]
- 5.Lubitz W., Chrysina M., Cox N., Water oxidation in photosystem II. Photosynth. Res. 142, 105–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krewald V., et al. , Metal oxidation states in biological water splitting. Chem. Sci. 6, 1676–1695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dismukes G. C., Siderer Y., Intermediates of a polynuclear manganese center involved in photosynthetic oxidation of water. Proc. Natl. Acad. Sci. U.S.A. 78, 274–278 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson Ö., Andréasson L.-E., EPR-detectable magnetically interacting manganese ions in the photosynthetic oxygen-evolving system after continuous illumination. Biochim. Biophys. Acta Bioenerg. 679, 261–268 (1982). [Google Scholar]
- 9.Zaharieva I., et al. , Room-temperature energy-sampling Kβ X-ray emission spectroscopy of the Mn4Ca complex of photosynthesis reveals three manganese-centered oxidation steps and suggests a coordination change prior to O2 formation. Biochemistry 55, 4197–4211 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Kulik L. V., Epel B., Lubitz W., Messinger J., Electronic structure of the Mn4OxCa cluster in the S0 and S2 states of the oxygen-evolving complex of photosystem II based on pulse 55Mn-ENDOR and EPR spectroscopy. J. Am. Chem. Soc. 129, 13421–13435 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Peloquin J. M., et al. , 55Mn ENDOR of the S2-state multiline EPR signal of photosystem II: Implications on the structure of the tetranuclear Mn cluster. J. Am. Chem. Soc. 122, 10926–10942 (2000). [Google Scholar]
- 12.Cox N., et al. , Photosynthesis. Electronic structure of the oxygen-evolving complex in photosystem II prior to O-O bond formation. Science 345, 804–808 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Zheng M., Dismukes G. C., Orbital configuration of the valence electrons, ligand field symmetry, and manganese oxidation states of the photosynthetic water oxidizing complex: Analysis of the S(2) state multiline EPR signals. Inorg. Chem. 35, 3307–3319 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Jin L., et al. , Electronic structure of the oxygen evolving complex in photosystem II, as revealed by 55Mn Davies ENDOR studies at 2.5 K. Phys. Chem. Chem. Phys. 16, 7799–7812 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Messinger J., et al. , Absence of Mn-centered oxidation in the S(2) –> S(3) transition: Implications for the mechanism of photosynthetic water oxidation. J. Am. Chem. Soc. 123, 7804–7820 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dau H., Liebisch P., Haumann M., X-ray absorption spectroscopy to analyze nuclear geometry and electronic structure of biological metal centers–Potential and questions examined with special focus on the tetra-nuclear manganese complex of oxygenic photosynthesis. Anal. Bioanal. Chem. 376, 562–583 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Haumann M., et al. , Structural and oxidation state changes of the photosystem II manganese complex in four transitions of the water oxidation cycle (S0 –> S1, S1 –> S2, S2 –> S3, and S3,4 –> S0) characterized by X-ray absorption spectroscopy at 20 K and room temperature. Biochemistry 44, 1894–1908 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Davis K. M., et al. , X-ray emission spectroscopy of Mn coordination complexes toward interpreting the electronic structure of the oxygen-evolving complex of photosystem II. J. Phys. Chem. C 120, 3326–3333 (2016). [Google Scholar]
- 19.Pace R. J., Jin L., Stranger R., What spectroscopy reveals concerning the Mn oxidation levels in the oxygen evolving complex of photosystem II: X-ray to near infra-red. Dalton Trans. 41, 11145–11160 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Petrie S., Stranger R., Pace R. J., What Mn Kβ spectroscopy reveals concerning the oxidation states of the Mn cluster in photosystem II. Phys. Chem. Chem. Phys. 19, 27682–27693 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Kuntzleman T., Yocum C. F., Reduction-induced inhibition and Mn(II) release from the photosystem II oxygen-evolving complex by hydroquinone or NH2OH are consistent with a Mn(III)/Mn(III)/Mn(IV)/Mn(IV) oxidation state for the dark-adapted enzyme. Biochemistry 44, 2129–2142 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Messinger J., Seaton G., Wydrzynski T., Wacker U., Renger G., S(-3) state of the water oxidase in photosystem II. Biochemistry 36, 6862–6873 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Kretschmann H., Witt H. T., Chemical reduction of the water splitting enzyme system of photosynthesis and its light-induced reoxidation characterized by optical and mass spectrometric measurements: A basis for the estimation of the states of the redox active manganese and of water in the quaternary oxygen-evolving S-state cycle. Biochim. Biophys. Acta Bioenerg. 1144, 331–345 (1993). [Google Scholar]
- 24.Cheniae G. M., Martin I. F., Photoactivation of the manganese catalyst of O 2 evolution. I. Biochemical and kinetic aspects. Biochim. Biophys. Acta 253, 167–181 (1971). [DOI] [PubMed] [Google Scholar]
- 25.Radmer R., Cheniae G. M., Photoactivation of the manganese catalyst of O2 evolution. II. A two-quantum mechanism. Biochim. Biophys. Acta Bioenerg. 253, 182–186 (1971). [DOI] [PubMed] [Google Scholar]
- 26.Cheniae G. M., Martin I. F., Effects of hydroxylamine on photosystem II: II. Photoreversal of the NH(2)OH destruction of O(2) evolution. Plant Physiol. 50, 87–94 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura N., Cheniae G., Photoactivation of the water-oxidizing complex in photosystem II membranes depleted of Mn and extrinsic proteins. I. Biochemical and kinetic characterization. Biochim. Biophys. Acta Bioenerg. 890, 179–194 (1987). [Google Scholar]
- 28.Miyao-Tokutomi M., Inoue Y., Improvement by benzoquinones of the quantum yield of photoactivation of photosynthetic oxygen evolution: Direct evidence for the two-quantum mechanism. Biochemistry 31, 526–532 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Ananyev G. M., Dismukes G. C., High-resolution kinetic studies of the reassembly of the tetra-manganese cluster of photosynthetic water oxidation: Proton equilibrium, cations, and electrostatics. Biochemistry 35, 14608–14617 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Ananyev G. M., Dismukes G. C., Assembly of the tetra-Mn site of photosynthetic water oxidation by photoactivation: Mn stoichiometry and detection of a new intermediate. Biochemistry 35, 4102–4109 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Baranov S. V., et al. , Bicarbonate is a native cofactor for assembly of the manganese cluster of the photosynthetic water oxidizing complex. Kinetics of reconstitution of O2 evolution by photoactivation. Biochemistry 43, 2070–2079 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Hwang H. J., Burnap R. L., Multiflash experiments reveal a new kinetic phase of photosystem II manganese cluster assembly in Synechocystis sp. PCC6803 in vivo. Biochemistry 44, 9766–9774 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Kolling D. R. J., Cox N., Ananyev G. M., Pace R. J., Dismukes G. C., What are the oxidation states of manganese required to catalyze photosynthetic water oxidation? Biophys. J. 103, 313–322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao H., Burnap R. L., Photoactivation: The light-driven assembly of the water oxidation complex of photosystem II. Front. Plant Sci. 7, 578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasgupta J., Ananyev G. M., Dismukes G. C., Photoassembly of the water-oxidizing complex in photosystem II. Coord. Chem. Rev. 252, 347–360 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joliot P., Barbieri G., Chabaud R., Un nouveau modeles des centres photochimiques du systeme II. Photochem. Photobiol. 10, 309–329 (1969). [Google Scholar]
- 37.Joliot P., Kok B., “Oxygen evolution in photosynthesis” in Energetics of Photosynthesis, Govindjee , Ed. (Elsevier, 1975), pp. 387–412. [Google Scholar]
- 38.Zaltsman L., Ananyev G. M., Bruntrager E., Dismukes G. C., Quantitative kinetic model for photoassembly of the photosynthetic water oxidase from its inorganic constituents: Requirements for manganese and calcium in the kinetically resolved steps. Biochemistry 36, 8914–8922 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Zhang M., et al. , Structural insights into the light-driven auto-assembly process of the water-oxidizing Mn4CaO5-cluster in photosystem II. eLife 6, e26933 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diner B. A., Dependence of the deactivation reactions of Photosystem II on the redox state of plastoquinone pool a varied under anaerobic conditions. Equilibria on the acceptor side of Photosystem II. Biochim. Biophys. Acta Bioenerg. 460, 247–258 (1977). [DOI] [PubMed] [Google Scholar]
- 41.Nugent J. H. A., Demetriou C., Lockett C. J., Electron donation in photosystem II. Biochim. Biophys. Acta Bioenerg. 894, 534–542 (1987). [Google Scholar]
- 42.Vermass W. F. J., Rutherford A. W., Hansson O., Site-directed mutagenesis in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: Donor D is a tyrosine residue in the D2 protein. Proc. Natl. Acad. Sci. U.S.A. 85, 8477–8481 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pham L. V., Messinger J., Probing S-state advancements and recombination pathways in photosystem II with a global fit program for flash-induced oxygen evolution pattern. Biochim. Biophys. Acta 1857, 848–859 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Styring S., Rutherford A. W., Deactivation kinetics and temperature dependence of the S-state transitions in the oxygen-evolving system of Photosystem II measured by EPR spectroscopy. Biochim. Biophys. Acta Bioenerg. 933, 378–387 (1988). [Google Scholar]
- 45.Asada M., Mino H., Location of the high-affinity Mn(2+) site in photosystem II detected by PELDOR. J. Phys. Chem. B 119, 10139–10144 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Nixon P. J., Diner B. A., Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry 31, 942–948 (1992). [DOI] [PubMed] [Google Scholar]
- 47.Campbell K. A., et al. , Dual-mode EPR detects the initial intermediate in photoassembly of the photosystem II Mn cluster: The influence of amino acid residue 170 of the D1 polypeptide on Mn coordination. J. Am. Chem. Soc. 122, 3754–3761 (2000). [Google Scholar]
- 48.Ghirardi M. L., Lutton T. W., Seibert M., Interactions between diphenylcarbazide, zinc, cobalt, and manganese on the oxidizing side of photosystem II. Biochemistry 35, 1820–1828 (1996). [DOI] [PubMed] [Google Scholar]
- 49.Ono T. A., Mino H., Unique binding site for Mn2+ ion responsible for reducing an oxidized YZ tyrosine in manganese-depleted photosystem II membranes. Biochemistry 38, 8778–8785 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Hoganson C. W., Ghanotakis D. F., Babcock G. T., Yocum C. F., Mn(2+) reduces Yz+ in manganese-depleted photosystem II preparations. Photosynth. Res. 22, 285–293 (1989). [DOI] [PubMed] [Google Scholar]
- 51.Buser C. A., Thompson L. K., Diner B. A., Brudvig G. W., Electron-transfer reactions in manganese-depleted photosystem II. Biochemistry 29, 8977–8985 (1990). [DOI] [PubMed] [Google Scholar]
- 52.Faller P., et al. , Rapid formation of the stable tyrosyl radical in photosystem II. Proc. Natl. Acad. Sci. U.S.A. 98, 14368–14373 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibrahim M., et al. , Improvements in serial femtosecond crystallography of photosystem II by optimizing crystal uniformity using microseeding procedures. Struct. Dyn. 2, 041705 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beckmann K., Messinger J., Badger M. R., Wydrzynski T., Hillier W., On-line mass spectrometry: Membrane inlet sampling. Photosynth. Res. 102, 511–522 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in the paper are available at http://uu.diva-portal.org under accession number diva2:1368663.