Abstract
Study Objectives
To examine economic aspects of insomnia and insomnia medication treatment among a nationally representative sample of older adult Medicare beneficiaries.
Methods
Using a random 5% sample of Medicare administrative data (2006–2013), insomnia was defined using International Classification of Disease, Version 9, Clinical Modification diagnostic codes. Treatment was operationalized as one or more prescription fills for an US Food and Drug Administration (FDA)-approved insomnia medication following diagnosis, in previously untreated individuals. To evaluate the economic impact of insomnia treatment on healthcare utilization (HCU) and costs in the year following insomnia diagnosis, a difference-in-differences approach was implemented using generalized linear models.
Results
A total of 23 079 beneficiaries with insomnia (M age = 71.7 years) were included. Of these, 5154 (22%) received one or more fills for an FDA-approved insomnia medication following insomnia diagnosis. For both treated and untreated individuals, HCU and costs increased during the 12 months prior to diagnosis. Insomnia treatment was associated with significantly increased emergency department visits and prescription fills in the year following insomnia diagnosis. After accounting for pre-diagnosis differences between groups, no significant differences in pre- to post-diagnosis costs were observed between treated and untreated individuals.
Conclusions
These results advance previous research into economics of insomnia disorder by evaluating the impact of medication treatment and highlighting important differences between treated and untreated individuals. Future studies should seek to understand why some individuals diagnosed with insomnia receive treatment but others do not, to identify clinically meaningful clusters of older adults with insomnia, and to explore the economic impact of insomnia and insomnia treatment among subgroups of individuals with insomnia, such as those with cardiovascular diseases, mood disorders, and neurodegenerative disease.
Keywords: sleep, insomnia, treatment, healthcare utilization, health economics, costs, Medicare, older adults
Statement of Significance.
To our knowledge, this is the first empirical study to evaluate the economic impact of insomnia medication treatment among Medicare beneficiaries. This study employed a randomly selected, nationally representative 5% sample of Medicare administrative claims data for years 2006–2013. A minority of beneficiaries diagnosed with insomnia were treated with an FDA-approved medication. Relative to untreated individuals and controlling for pre-diagnosis differences, beneficiaries who received insomnia-related medications subsequently demonstrated higher prescription and emergency department utilization. After controlling for pre-diagnosis differences, no differences in post-diagnosis costs were observed between treated and untreated individuals. This study adds to the growing body of literature regarding economic aspects of insomnia and insomnia treatments among older adults.
Introduction
Sleep complaints increase with age, with half of older adults reporting poor quality sleep. Among older adults, the prevalence of insomnia disorder, defined as difficulty initiating or maintaining sleep with daytime impairment, ranges from 25% to 40% [1–4], a rate more than double that of the general population [5]. Further, the prevalence of insomnia among older adults appears to be increasing [3, 6–8]. In this population, insomnia is associated with worsened health outcomes, including increased risk for depression [9–11], pain [9, 12], cognitive decline [13, 14], fall risk [15, 16], pulmonary and cardiovascular disease [17–20], and worsened health-related quality of life [12, 21].
In addition to these adverse health consequences, insomnia is also associated with increased healthcare utilization (HCU) and costs among older adults [2, 22–25]. For example, relative to matched controls without insomnia, Ozminkowski et al. found older adults with insomnia (n = 75 558) to have $1143 (in 2007 USD) greater direct medical expenditures over 6 months prior to insomnia diagnosis or first medication fill [23]. More recently, Wickwire and colleagues found insomnia to be associated with increased all-cause HCU and costs among a national sample of Medicare beneficiaries [25]. Others have found insomnia to be associated with increased economic burden among vulnerable geriatric subpopulations, including nursing home residents [2] and individuals with comorbid depression [22]. Although longitudinal data are scant, evidence suggests insomnia-related costs are increasing. For example, Gamaldo and colleagues found total costs associated with insomnia-related hospital admissions increased from $22 500 in 2002 to $31 527 in 2012 [26].
Given the elevated prevalence of insomnia among older adults and the substantial associated economic burden, it is surprising how little is known about the economic impact of insomnia treatments among this population. In light of the rapidly aging population in the United States, such insight could provide needed evidence-based guidance to payers, policy-makers, and health systems leaders seeking to allocate limited resources to manage population health [22, 27, 28]. To date, only one study has evaluated the economic impact of insomnia treatments among older adults. Tannenbaum and colleagues [29] conducted a cost-effectiveness analysis to compare the cost-effectiveness of cognitive-behavioral treatment for insomnia (CBTI) and insomnia pharmacotherapies. These authors estimated that due to increased costs associated with medication-induced falls and fractures, CBTI was more cost-effective than insomnia medication among older adults. However, this was a modeling study that relied on published data from multiple sources; the literature will benefit from an empirical analysis of actual claims data typically available to the health payer.
Our prior work has examined all-cause HCU and costs in the 12 months prior to insomnia diagnosis among a nationally representative sample of older adult Medicare beneficiaries [25]. However, whereas we previously considered the additive costs of insomnia during the 12 months prior to insomnia diagnosis [25], the purpose of the present study was to evaluate the impact of insomnia treatment on HCU and costs during the 12 months after insomnia diagnosis, in previously untreated individuals. Our hypothesis was that relative to untreated individuals and the pre-diagnosis period, beneficiaries who receive insomnia-related medications would demonstrate decreased HCU and costs during the year following diagnosis.
Methods
Data source and study design
Data for this study were derived from a 5% sample of Medicare administrative claims for years 2006–2013 obtained from the Centers for Medicare & Medicaid Services (CMS) Chronic Condition Data Warehouse (CCW). A nested cohort design using a difference-in-difference (DID; see Analytic plan, below) estimation approach was employed to evaluate the impact of insomnia treatment on HCU and costs among individuals diagnosed with insomnia.
Study population
Beneficiaries were included in this study if they (1) received one or more physician-assigned insomnia diagnosis; (2) possessed continuous Medicare Parts A, B, and D, with no Medicare Part C (Medicare Advantage) coverage for at least a 25-month period comprising the 12 months pre- and post-insomnia diagnosis, as well as the month of diagnosis; and (3) for treated individuals, if insomnia diagnosis preceded insomnia treatment, and they had no previous insomnia treatment. Beneficiaries were excluded from this study if they were diagnosed with other non-insomnia sleep disorders such as obstructive sleep apnea, restless syndrome, or narcolepsy. Similarly, because we were interested in maximizing specificity of insomnia treatment, individuals were also excluded if they received one or more prescription fills for non–US Food and Drug Administration (FDA)-approved medications (i.e. medications commonly used off-label to treat insomnia; see Table 1).
Table 1.
List of included and excluded medications
| Included: FDA-approved sleep medications | Excluded: off-label sleep medications |
|---|---|
| Butabarbital | Amobarbital |
| Secobarbital | Pentobarbital |
| Estazolam | Phenobarbital |
| Flurazepam | Mephobarbital |
| Quazepam | Lorazepam |
| Temazepam | Oxazepam |
| Triazolam | Chloral hydrate |
| Eszopiclone | Hydroxyzine |
| Zaleplon | Amitriptyline |
| Ramelteon | Nortriptyline |
| Zolpidem | Clomipramine |
| Doxepin | Trazodone |
| Nefazodone | |
| Mirtazapine |
Insomnia diagnoses
Insomnia diagnoses were identified by searching inpatient and outpatient claims for the presence of at least one International Classification of Disease, Version 9, Clinical Modification (ICD-9-CM) code for insomnia (307.41, 307.42, 307.49, 327.00, 327.01, 327.09, 780.52, and V69.4). As in our prior work, the date of first insomnia diagnosis was considered the index date [25].
Insomnia treatment
The Part D prescription drug event files were searched for FDA-approved medications for the treatment of insomnia (butabarbital, doxepin, estazolam, eszopiclone, flurazepam, quazepam, ramelteon, secobarbital, temazepam, triazolam, zaleplon, zolpidem) during the study period. Insomnia treatment was defined as at least one fill of such an FDA-approved insomnia medication during the 12 months following insomnia diagnosis. In addition, monthly indictors were created to capture subsequent fills.
HCU and costs
HCU was operationalized as counts of claims over the year prior to and following the month of the index date. To avoid including claims associated with the insomnia diagnosis itself, the month of the index date was excluded. As in our prior work, HCU was categorized by point of service (inpatient, emergency department [ED], and outpatient care, as well as prescription fills) using information available on the claims [25]. Total all-cause costs (i.e. all costs paid out by Medicare) were computed by summing costs occurring during the year prior to and following the insomnia diagnosis, excluding the month of insomnia diagnosis. Costs were also categorized by point of service. To account for inflation, all costs were converted to 2013 dollars using the consumer price index produced by the US Bureau of Labor Statistics.
Covariates
Information on beneficiary demographic characteristics was obtained from the claims files. The CCW contains information on 27 comorbid conditions, with an annual flag for each condition as well as the date of first diagnosis [30]. We used the date of first diagnosis to determine if a condition was present at the date of insomnia diagnosis (i.e. index date). Other comorbidities of interest were identified by searching all claim types for relevant ICD-9-CM codes during the study period. Any diagnoses received during the year prior to insomnia diagnosis were assumed to be present at the index date. A comorbidity index based on the Deyo adaptation of the Charlson comorbidity index was calculated and included in subsequent analyses [31].
Analytic plan
Following examination of the distributions and frequencies of all variables, differences between the treated and untreated cohorts were evaluated using chi-square Goodness of Fit and Student’s t-tests. Within each cohort and for all point-of-service categories, unadjusted annual mean HCU and costs and their standard deviations were calculated by year before and year after insomnia diagnosis. Monthly costs were plotted against time for the treated and untreated groups.
To accommodate overdispersion of HCU counts, generalized linear models with a negative binomial distribution and a log link were employed to model HCU by point of service. DID facilitates estimation of causal effects by using a quasi-experimental design, in which changes within a treated group are compared to changes within a control group over time [32, 33]. In this case, beneficiaries with insomnia who received insomnia treatment were compared with beneficiaries with insomnia who did not receive insomnia treatment (i.e. [Treatedpost − Treatedpre] − [Untreatedpost − Untreatedpre]). The DID estimator is obtained by creating an interaction term between post-insomnia diagnosis status and treatment group [32, 33]. Thus, the unadjusted models contained indicator variables for post-insomnia diagnosis status, treatment, the interaction between post-insomnia diagnosis and treatment, and index year. The fully adjusted model was generated using stepwise selection with a p-value for entry/exit set at 0.001. Final models were generated separately for each point of service.
Healthcare costs are also skewed with multiple zero values. Although a gamma distribution typically provides a good fit to cost data, due to the interaction terms, these models do not permit accurate estimation of the value of the DID and its confidence interval. Therefore, a generalized linear model with a normal distribution and identity link was initially employed to model mean total and point-of-service charges, which were used to produce the DID estimates. Next, to test the p-value of the DID term using a better fitting model, a generalized linear model with a gamma distribution and log link was employed. The p-value from the gamma model is reported along with the DID estimates. As with HCU, final models were generated separately for each point of service and total costs by starting off with all covariates in the model and removing those with p-value ≥0.001 whose removal did not change the effect estimate of case by >10%.
To assess whether increasing number of insomnia medication fills was associated with decreased HCU, additional analyses were performed. To implement the DID estimator, a binary exposure variable was required. Therefore, beneficiaries without a medication fill during the study period were excluded from the model. Next, the exposure was dichotomized (i.e. one fill or more than one fill), and the generalized linear models for HCU were rerun.
Analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC) and Stata 14 (StatCorp LP, College Station, TX). This study was approved by the Institutional Review Board of the University of Maryland, Baltimore.
Results
Participants
We identified 50 848 beneficiaries with a diagnosis of insomnia and meeting continuous coverage criteria during the study period. We excluded individuals who received a non-FDA approved sleep-related medication (n = 10 567), individuals who received treatment prior to diagnosis of insomnia (n = 12 408), and those who had any other sleep-related diagnosis such as obstructive sleep apnea, restless legs syndrome, or narcolepsy (n = 4794). A total of 23 079 (M age = 71.7 years) Medicare beneficiaries diagnosed with insomnia and meeting inclusion criteria were included in the final sample. As presented in Table 2, 5154 (22%) of these individuals were treated with at least one FDA-approved insomnia medication over the 12 months following insomnia diagnosis, and 17 925 (78%) did not receive any medication for insomnia. Among those who received treatment, the median time between insomnia diagnosis and first insomnia medication fill was 6 days (inter-quartile range 0, 399). The cohort was predominantly female (70.7%) and white (81.8%). Relative to beneficiaries not treated for insomnia, treated individuals were younger (71.6 [SD 14.6] vs. 72.3 [SD 12.1] years, p < 0.001) and more likely to be male (32.4% vs. 28.5%, p < 0.001). At the time of insomnia diagnosis and relative to beneficiaries not treated for insomnia, individuals who ultimately received treatment demonstrated lower prevalence of multiple psychiatric comorbidities including Alzheimer’s disease and related dementias (10.0% vs. 13.9%), anxiety (15.2% vs. 24.8%), and depression (16.4% vs. 24.4%; all ps < 0.001).
Table 2.
Baseline characteristics of Medicare beneficiaries with an insomnia diagnosis, 2007–2011, by receipt of at least one FDA-approved medication fill, N = 23 079
| Total, N =23 079 | ≥1 FDA medication for insomnia, n = 5154 | No medication for insomnia, n = 17 925 | p-valuea | |
|---|---|---|---|---|
| Age, mean (SD) | 71.7 (14.1) | 71.6 (14.6) | 72.3 (12.1) | <0.001 |
| Sex | <0.001 | |||
| Female | 16 306 (70.7) | 3486 (67.6) | 12 820 (71.5) | |
| Male | 6773 (29.3) | 1668 (32.4) | 5105 (28.5) | |
| Race | <0.001 | |||
| White | 18 884 (81.8) | 4138 (80.3) | 14 746 (82.3) | |
| Black | 2086 (9.0) | 450 (8.7) | 1636 (9.1) | |
| Hispanic | 795 (3.5) | 198 (3.8) | 597 (3.3) | |
| Other | 1314 (5.7) | 368 (7.1) | 946 (5.3) | |
| Comorbid conditions | ||||
| ADRD | 3007 (13.0) | 516 (10.0) | 2491 (13.9) | <0.001 |
| Anemia | 11 300 (49.0) | 2527 (49.0) | 8773 (48.9) | 0.91 |
| Anxiety | 5234 (22.7) | 783 (15.2) | 4451 (24.8) | <0.001 |
| Asthma | 2706 (11.7) | 584 (11.3) | 2122 (11.8) | 0.32 |
| Atrial fibrillation | 2608 (11.3) | 603 (11.7) | 2005 (11.2) | 0.30 |
| Cancer | 2928 (12.7) | 734 (14.2) | 2194 (12.2) | <0.001 |
| Cataracts | 14 504 (62.9) | 3245 (63.0) | 11 259 (62.8) | 0.85 |
| Chronic kidney disease | 3486 (15.1) | 815 (15.8) | 2671 (14.9) | 0.11 |
| COPD | 5787 (25.1) | 1321 (25.6) | 4466 (24.9) | 0.30 |
| Depression | 5213 (22.6) | 846 (16.4) | 4367 (24.4) | <0.001 |
| Diabetes | 6878 (29.8) | 1579 (30.6) | 5299 (29.6) | 0.14 |
| Glaucoma | 5002 (21.7) | 1172 (22.7) | 3830 (21.4) | 0.04 |
| Heart failure | 5440 (23.6) | 1217 (23.6) | 4223 (23.6) | 0.94 |
| Hip fracture | 898 (3.9) | 169 (3.3) | 729 (4.1) | 0.01 |
| Hyperlipidemia | 16 174 (70.1) | 3742 (72.6) | 12 432 (69.4) | <0.001 |
| Hypertension | 17 600 (76.3) | 4003 (77.7) | 13 597 (75.9) | 0.007 |
| Ischemic heart disease | 10 376 (45.0) | 2395 (46.5) | 7981 (44.5) | 0.01 |
| Osteoporosis | 8867 (38.4) | 1938 (37.6) | 6929 (38.7) | 0.17 |
| Rheumatoid/osteoarthritis | 12 643 (54.8) | 2864 (55.6) | 9779 (54.6) | 0.20 |
| Stroke | 3230 (14.0) | 690 (13.4) | 2540 (14.2) | 0.15 |
| Deyo CCI | 0.50 | |||
| 0 | 4845 (21.0) | 1049 (20.4) | 3796 (21.2) | |
| 1 | 7092 (30.7) | 1619 (31.4) | 5473 (30.5) | |
| 2 | 5440 (23.6) | 1218 (23.6) | 4222 (23.6) | |
| ≥3 | 5702 (24.7) | 1268 (24.6) | 4434 (24.7) |
a p-value from chi-square Goodness of Fit or Student’s t-test.
Unadjusted HCU and costs
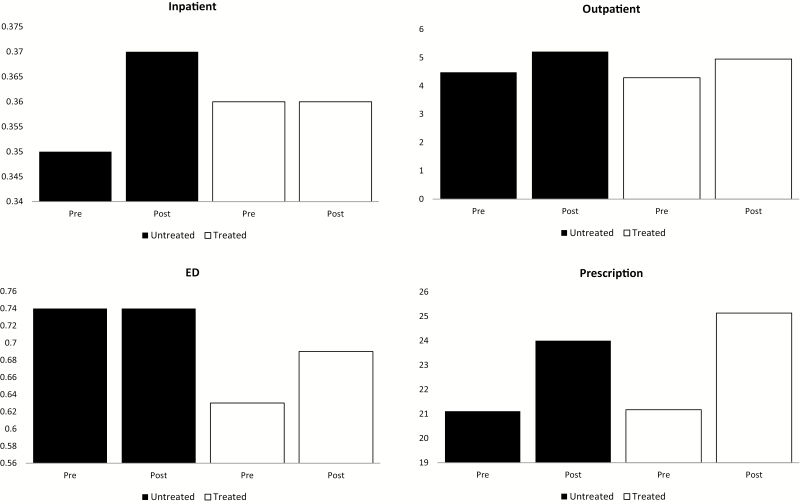
Table 3 presents unadjusted mean annual counts of HCU as well as mean costs by point of service. Mean counts of HCU by point of service were similar across treatment and year groups for inpatient stays, which increased in the year following insomnia diagnosis among both treated and untreated groups. Compared to the year pre-diagnosis, costs post-diagnosis were elevated. Mean total costs during the year post-insomnia diagnosis were $113 739 (SD $405 285) in the treated group and $108 599 (SD $337 277) in the untreated group. Inpatient costs were the primary drivers of total costs, followed by outpatient costs.
Table 3.
Mean annual HCU and costs among Medicare beneficiaries over 1 year pre-insomnia diagnosis and 1 year post-insomnia diagnosis, 2007–2011, by receipt of at least one FDA-approved medication, N = 23 079
| ≥1 FDA medication for insomnia, n = 5154 | No medication for insomnia, n = 17 925 | ||||
|---|---|---|---|---|---|
| 1 year pre-insomnia diagnosis | 1 year post-insomnia diagnosis | 1 year pre-insomnia diagnosis | 1 year post-insomnia diagnosis | ||
| HCU count, mean (SD) | |||||
| Inpatient | 0.37 (0.91) | 0.36 (0.90) | 0.37 (0.86) | 0.35 (0.86) | |
| ED | 0.63 (1.42) | 0.69 (1.65) | 0.74 (1.76) | 0.74 (1.61) | |
| Outpatient | 4.29 (5.89) | 4.95 (6.65) | 4.58 (6.20) | 5.21 (7.11) | |
| Prescriptions | 21.17 (15.37) | 25.13 (17.07) | 21.11 (15.79) | 23.99 (17.38) | |
| Costs in 2013 dollars, mean (SD) | |||||
| Inpatient | 78 626 (288 811) | 70 374 (329 535) | 68 525 (268 139) | 58 454 (222 101) | |
| ED | 2198 (8128) | 2159 (8089) | 2120 (7528) | 2075 (6797) | |
| Outpatient | 25 167 (130 222) | 38 193 (178 869) | 22 717 (121 273) | 33 731 (164 214) | |
| Prescriptions | 2611 (3913) | 3014 (4286) | 2632 (4046) | 2961 (5059) | |
| Total | 108 599 (337 277) | 113 739 (405 285) | 95 992 (314 459) | 97 219 (298 654) | |
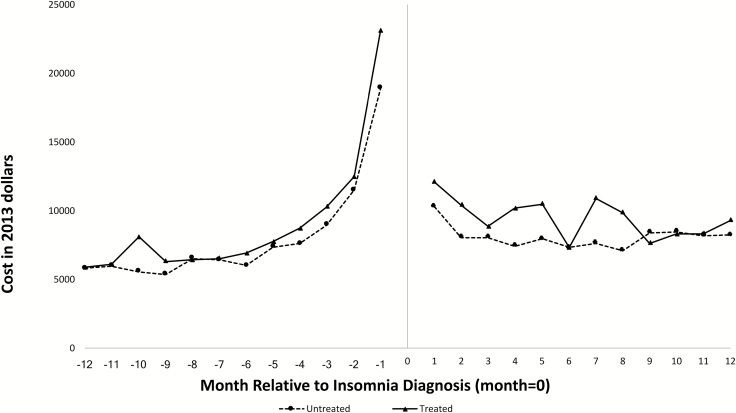
Figure 1 presents differences in HCU by point of service, before and after insomnia diagnosis, among both treated and untreated individuals. Figure 2 illustrates mean total all-cause monthly healthcare costs over the 12 months before and 12 months after insomnia diagnosis. Among both treated and untreated individuals, pre-diagnosis costs increased over time, with an exponential increase beginning approximately 4 months prior to insomnia diagnosis. Costs stabilized during the post-diagnosis period.
Figure 1.
Mean annual counts of HCU for inpatient encounters, outpatient encounters, ED visits, and prescription medication use over 12 months prior to insomnia diagnosis and the 12 months following insomnia diagnosis, stratified by receipt of an FDA-approved insomnia medication after diagnosis, 2007–2013.
Figure 2.
Mean total healthcare costs over 12 months prior to insomnia diagnosis and the 12 months post-insomnia diagnosis by month, stratified by receipt of an FDA-approved insomnia medication after diagnosis, 2007–2013.
DID results
Results from the DID models are presented in Table 4. In fully adjusted negative binomial models (Table 4), the DID for ED visits was significantly elevated (rate ratio [RR] 1.09; 95% CI = 1.01% to 1.16%), meaning that even accounting for baseline differences between treatment groups, time trends, and potential cofounding variables, insomnia treatment was associated with increased ED utilization. Similarly, accounting for baseline differences between treatment groups, time trends, and potential cofounding variables, insomnia treatment was associated with increased prescription drug utilization (DID estimate RR 1.05; 95% CI = 1.03% to 1.07%) post-insomnia diagnosis. There were no differences in inpatient or outpatient HCU between the groups.
Table 4.
DID estimators (95% confidence intervals) of the effect of insomnia treatment among Medicare beneficiaries diagnosed with insomnia on HCU, 2006–2013, N = 23 079
| Model 1a | Model 2 | |
|---|---|---|
| Inpatient | ||
| DID | 1.05 (0.96–1.14) | 1.03 (0.95–1.13)b |
| Treated vs. untreated group prior to insomnia diagnosis | 1.02 (0.95–1.10)b | |
| Pre- vs. post-insomnia diagnosis in untreated group | 1.02 (0.98–1.07)b | |
| ED | ||
| DID | 1.09 (1.02–1.17) | 1.09 (1.01–1.16)c |
| Treated vs. untreated group prior to insomnia diagnosis | 0.92 (0.87–0.98)c | |
| Pre- vs. post-insomnia diagnosis in untreated group | 1.07 (1.03–1.10)c | |
| Outpatient | ||
| DID | 1.01 (0.98–1.05) | 1.02 (0.99–1.06)d |
| Treated vs. untreated group prior to insomnia diagnosis | 0.95 (0.91–0.98)d | |
| Pre- vs. post-insomnia diagnosis in untreated group | 1.16 (1.14–1.18)d | |
| Prescription | ||
| DID | 1.04 (1.03–1.06) | 1.05 (1.03–1.07)e |
| Treated vs. untreated group prior to insomnia diagnosis | 1.02 (1.00–1.04)e | |
| Pre- vs. post-insomnia diagnosis in untreated group | 1.15 (1.14–1.16)e |
aAdjusted for index year of diagnosis.
bAdjusted for index year of diagnosis, age, sex, race, Alzheimer’s disease and related dementias, anemia, anxiety, asthma, atrial fibrillation, chronic kidney disease, COPD, depression, glaucoma, heart failure, hip fracture, hyperlipidemia, hypertension, ischemic heart disease, rheumatoid/osteoarthritis, stroke, and Deyo CCI.
cAdjusted for index year of diagnosis, age, sex, race, Alzheimer’s disease and related dementias, anemia, anxiety, asthma, atrial fibrillation, cancer, chronic kidney disease, COPD, depression, diabetes, heart failure, hip fracture, hyperlipidemia, ischemic heart disease, stroke, and Deyo CCI.
dAdjusted for index year of diagnosis, age, sex, race, Alzheimer’s disease and related dementias, anemia, anxiety, asthma, atrial fibrillation, cancer, cataracts, chronic kidney disease, depression, diabetes, hip fracture, hyperlipidemia, hypertension, ischemic heart disease, stroke, and Deyo CCI.
eAdjusted for index year of diagnosis, age, sex, race, Alzheimer’s disease and related dementias, anemia, anxiety, asthma, atrial fibrillation, cataracts, chronic kidney disease, COPD, depression, diabetes, glaucoma, heart failure, hip fracture, hyperlipidemia, hypertension, ischemic heart disease, osteoporosis, rheumatoid/osteoarthritis, stroke, and Deyo CCI.
DID estimates for healthcare costs overall and by point of service obtained from the linear models are presented in Table 5. Wald chi-square p-values obtained from the gamma models are also presented. After accounting for differences in pre-diagnosis costs between treatment groups, time trends, and potential confounders, no significant differences in post-diagnosis costs were observed among individuals who received treatment for insomnia.
Table 5.
Adjusted DID estimators (95% confidence interval) of effect of treatment following insomnia diagnosis on healthcare costs among Medicare beneficiaries in 2013 dollars, N = 23 079
| p-valuea | ||
|---|---|---|
| Inpatientb | $1481 (−$9753, $12 715) | 0.52 |
| EDc | $11 (−$301, $324) | 0.48 |
| Outpatientd | $2025 (−$4334, $8385) | 0.39 |
| Prescriptione | $76 (−$112, $263) | 0.43 |
| Totalf | $3593 (−$10 128, $17 314) | 0.87 |
ap-value of the DID term is derived from a generalized linear model with a gamma distribution and log link.
bAdjusted for index year of diagnosis, age, sex, race, depression, acute myocardial infarction, asthma, atrial fibrillation, congestive heart failure, COPD, depression, diabetes, hip fracture, hyperlipidemia, hypertension, ischemic heart disease, and stroke.
cAdjusted for index year of diagnosis, age, sex, race, depression, acute myocardial infarction, Alzheimer’s disease and related dementias, anemia, asthma, atrial fibrillation, congestive heart failure, COPD, diabetes, hip fracture, hyperlipidemia, hypertension, ischemic heart disease, rheumatoid arthritis, and stroke.
dAdjusted for index year of diagnosis, age, sex, race, substance abuse, anemia, asthma, congestive heart failure, depression, diabetes, glaucoma, hip fracture, hypertension, ischemic heart disease, rheumatoid arthritis, and stroke.
eAdjusted for index year of diagnosis, age, sex, race, depression, Alzheimer’s disease and related dementias, anemia, asthma, cataracts, congestive heart failure, COPD, diabetes, glaucoma, hyperlipidemia, hypertension, hypothyroidism, ischemic heart disease, osteoporosis, rheumatoid arthritis, and stroke.
fAdjusted for index year of diagnosis, age, sex, race, depression, acute myocardial infarction, Alzheimer’s disease and related dementias, anemia, atrial fibrillation, congestive heart failure, COPD, diabetes, glaucoma, hip fracture, hyperlipidemia, hypertension, ischemic heart disease, and stroke.
Additional analyses conducted only among beneficiaries who received at least one fill of an insomnia medication revealed no significant between-group differences in HCU.
Discussion
In this large national analysis of Medicare beneficiaries with physician-diagnosed insomnia, HCU and costs increased during the 12 months prior to insomnia diagnosis. Further, compared to individuals who did not receive treatment, beneficiaries who received FDA-approved insomnia medication fills demonstrated higher total costs before insomnia diagnosis. Contrary to our hypothesis, after controlling for pre-diagnosis differences in costs and any time trends, no between-group differences were observed during the 12 months following insomnia diagnosis. Thus, insomnia treatment neither decreased nor increased most HCU and costs. To our knowledge, these are the first empirical data to highlight the economic trajectories of older adults diagnosed with insomnia both before and after diagnosis and to evaluate the economic impact of insomnia treatments among older adults. In addition to highlighting the increased costs associated with insomnia 12 months prior to diagnosis, our results and suggest important differences between individuals diagnosed with insomnia who receive and who do not receive insomnia treatment.
The most important finding of our study is the increasing cost trajectory of older adults diagnosed with insomnia during the 12 months before insomnia diagnosis. Although prior work from our group [25] and others [2, 22–24] has demonstrated increased costs among individuals with insomnia relative to matched non-insomnia controls, these studies have frequently employed “pre-index” methodology [2, 22–25]. To our knowledge, the present results are the first empirical data to highlight the differences in costs and HCU between treated and untreated individuals, both before and after insomnia diagnosis. As in our past research [25], inpatient costs accounted for the bulk of observed expenditures. Because insomnia is unlikely to be the sole cause of an inpatient hospital stay, these results raise important questions about the clinical and economic trajectories of individuals diagnosed with insomnia. For example, why do individuals with insomnia demonstrate such elevated costs prior to insomnia diagnosis? Is insomnia a marker of worsening severity of comorbid disease? We speculate that increasing costs in the months prior to diagnosis reflect increasing health events/contact with the healthcare system, but this suggestion requires empirical evaluation in future studies.
In addition to increasing costs associated with insomnia, our results also provide novel insight into the economic impact of insomnia treatment. In the present study, insomnia treatment was not associated with decreased HCU or costs and in fact increased HCU in two points-of-service (i.e. ED visits and prescription fills). Notably, our study design did not enable us to determine the cause of the observed increase in ED visits. However, consistent with past findings demonstrating increased risk of falls from physician-prescribed sleep medications older adults [34], our past work among Medicare beneficiaries has demonstrated an increased risk from non-benzodiazepine sedative hypnotics for fall-related hospitalizations (i.e. for hip fracture and traumatic brain injury) [35]. Similarly, it is likely that the observed increase in prescription costs was due to costs of insomnia medications themselves, although we were unable to test this explicitly. Notably, these results are inconsistent with a recent comprehensive review, which found both pharmacologic and non-pharmacologic insomnia treatments to be associated with favorable economic outcomes [28]. However, studies included in that review employed different methodologies (e.g. study designs, samples, insomnia treatments, and economic outcomes). To our knowledge, the current project is the first national-scale, empirical study to analyze the population-level impact of insomnia treatment on HCU and costs within actual administrative claims data.
Results from this study suggest two major directions for future research. First, it is necessary to understand the mechanisms by which insomnia so dramatically increases costs during the months preceding insomnia diagnosis. Whereas our past research indicated that the bulk of insomnia-related medical expenditures are associated with inpatient costs [25], present results suggest the need for greater understanding of the insomnia disease process. For example, does insomnia result from or exacerbate the comorbid medical conditions or accidents that are leading to inpatient hospital stays? Or, does insomnia develop during hospitalization and then progress into a chronic condition [36]? To address these and similar questions, more granular analyses of insomnia trajectories and economic outcomes will be required. Given the heterogeneous nature of insomnia itself, we propose that identifying clusters and subpopulations of insomnia patients based on demographic, comorbid disease, and medication usage profiles (including number of fills) is likely to help to phenotype insomnia patients, advance understanding of insomnia disease trajectories, and identify patients particularly at risk for poor outcomes, including economic outcomes.
Second and equally important, it is vital to understand why some Medicare beneficiaries with insomnia receive treatment but others do not. In the current study, a surprisingly low number of beneficiaries diagnosed with insomnia were treated with FDA-approved insomnia medications, despite our comprehensive search of Part D prescription drug files, raising important questions regarding insomnia treatment practices. It is certainly possible that individuals were treated or not treated based on symptom severity. However, this suggests a higher rate of provider diagnosis than has previously been suggested, as the literature has generally found insomnia to be much more frequently treated than diagnosed [28, 37]. Another possible explanation for why some individuals are treated, but others are not, is that individuals who were diagnosed but not treated for insomnia were more likely to possess psychiatric comorbidities. This possible disparity in access to care warrants research attention. Further, results from our study highlight clear differences in HCU and costs associated with treatment status, with treated individuals demonstrating lower ED and outpatient HCU and costs before insomnia diagnosis. That is, even within a cohort diagnosed with insomnia, there are differences in HCU and costs between individuals who eventually receive insomnia medication treatment and those who remain untreated. These results raise important questions about insomnia treatment patterns as well as economic impact of insomnia treatments. Future research should not only seek to characterize differences between treated and untreated individuals but also to elucidate the economic effects of insomnia treatments.
Several strengths warrant mention. First, this study presents the first empirical analysis of the economic impact of insomnia treatment among older adults to date. Second, we analyzed a large national sample of Medicare fee-for-service beneficiaries. Third, Medicare is the largest health payer for older adults in the United States and is considered a leader in federal health policy. Finally, as in our previous work, we captured a broad range of health expenditures from the payer perspective, including HCU and costs associated with outpatient, inpatient, ED, and medication prescriptions.
However, our administrative methodology has limitations. First, insomnia was operationalized based exclusively on physician-assigned diagnoses. To date, the literature lacks a validated algorithm or other evidence-based approach to identify individuals with insomnia within administrative claims data. Insomnia is underdiagnosed among older adults, and many Medicare beneficiaries with insomnia were undoubtedly undiagnosed in the claims and thus not included in our study. Second, ICD-9-CM codes do not provide insight into insomnia subtypes (e.g. acute or chronic insomnia), limiting our ability to understand the importance of duration of sleep complaints or how duration of sleep complaints might impact HCU. Third, our administrative methodology was unable to provide insight into other clinical variables of interest, such as insomnia symptoms or subjective or objective measures of sleep. Identifying and characterizing insomnia phenotypes based on sleep symptoms, daytime sequelae, comorbid disease characteristics, medication use patterns, and other factors is essential; understanding the trajectories of such insomnia phenotypes including their impact on HCU will facilitate population surveillance and enable more accurate identification of high-risk individuals. Fourth, our administrative design prohibited us from tracking actual medication usage, instead utilizing prescription fills as a proxy for medication use. Insomnia medications are typically prescribed on an as-needed basis (i.e. prn), and operational definitions of insomnia treatment warrant future research attention. Little is known about prescribing patterns in insomnia, such as single or multiple dosages, short-term and long-term usage, insomnia medications and medication classes, mediation switching, and so on—more nuanced definitions of insomnia treatment are clearly required. Fifth, several FDA-approved insomnia medications have multiple indications, and we were unable to determine clinical indications for the prescribed medications. Related to this, many insomnia medications are used off-label to treat insomnia but were not included in our study; to maximize the specificity of our definition of medication treatment, we only included FDA-approved medications here. Sixth, our data files did not enable us to assess multiple medications and polypharmacy, which have been associated with increased HCU [38]. Seventh, we were unable to account for other possible, concurrent insomnia treatment modalities, such as over the counter medications, herbal remedies, or CBTI. Finally, although administrative claims provide excellent insight into HCU and costs from the payer perspective, we were unable to assess the economic impact of insomnia treatment from other perspectives of interest, such as the patient perspective or employer perspective.
In summary, results from this study present the first empirical analysis of the economic impact of insomnia treatments among older adults. Contrary to our hypothesis, insomnia treatment was not associated with decreased HCU or costs and in fact was associated with increased ED visits and prescription fills within 12 months. However, significant differences in HCU and costs between treated and untreated individuals were evident before treatment, suggesting important differences in insomnia trajectories. To advance understanding, future research should seek to identify differences between these groups, including insomnia phenotypes based on demographic, disease, and medication usage characteristics.
Funding
This research was supported by an investigator-initiated grant awarded from Merck to The University of Maryland, Baltimore (PI: Wickwire). EMW, SMS, and JSA’s institution has received research funding from the AASM Foundation, Merck, and ResMed. JSA is supported by Agency for Healthcare Research and Quality grant K01HS024560. SET is supported by National Institute on Aging grant 1K01AG050723. AV is supported by National Institutes of Health grant T32AG000262.
Conflict of interest statement. EMW has served as a scientific consultant to DayZz, Merck, Purdue, and Eisai and is an equity shareholder in WellTap. No other conflicts of interests are declared.
References
- 1. Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10 (Suppl. 1):S7–11. [DOI] [PubMed] [Google Scholar]
- 2. Kaufmann CN, et al.. Trends in prescribing of sedative-hypnotic medications in the USA: 1993–2010. Pharmacoepidemiol Drug Saf. 2016;25(6):637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maust DT, et al.. National trends in antidepressant, benzodiazepine, and other sedative-hypnotic treatment of older adults in psychiatric and primary care. J Clin Psychiatry. 2017;78(4):e363–e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olfson M, et al.. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. [DOI] [PubMed] [Google Scholar]
- 5. Ford DE, et al.. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? J Am Med Assoc. 1989;262(11):1479–1484. [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, et al.. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care survey 1999–2010. Sleep. 2014;37(8):1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moloney ME, et al.. The medicalization of sleeplessness: a public health concern. Am J Public Health. 2011;101(8):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albrecht JS, et al.. Trends in insomnia diagnosis and treatment among Medicare beneficiaries, 2006–2013. Am J Geriatr Psychiatry. 2019;27(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dragioti E, et al.. Insomnia severity and its relationship with demographics, pain features, anxiety, and depression in older adults with and without pain: cross-sectional population-based results from the PainS65+ cohort. Ann Gen Psychiatry. 2017;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soehner AM, et al.. Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J Affect Disord. 2014;167:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sunderajan P, et al.. Insomnia in patients with depression: a STAR*D report. CNS Spectr. 2010;15(6):394–404. [DOI] [PubMed] [Google Scholar]
- 12. Dragioti E, et al.. Association of insomnia severity with well-being, quality of life and health care costs: a cross-sectional study in older adults with chronic pain (PainS65+). Eur J Pain. 2018;22(2):414–425. [DOI] [PubMed] [Google Scholar]
- 13. Altena E, et al.. Do sleep complaints contribute to age-related cognitive decline? Prog Brain Res. 2010;185:181–205. [DOI] [PubMed] [Google Scholar]
- 14. Dzierzewski JM, et al.. Sleep and cognition in older adults. Sleep Med Clin. 2018;13(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, et al.. Age- and gender-specific associations between insomnia and falls in Boston Puerto Rican adults. Qual Life Res. 2017;26(1):25–34. [DOI] [PubMed] [Google Scholar]
- 16. Helbig AK, et al.. Association between sleep disturbances and falls among the elderly: results from the German Cooperative Health Research in the region of Augsburg-Age study. Sleep Med. 2013;14(12):1356–1363. [DOI] [PubMed] [Google Scholar]
- 17. Helbig AK, et al.. Relationship between sleep disturbances and multimorbidity among community-dwelling men and women aged 65–93 years: results from the KORA Age Study. Sleep Med. 2017;33:151–159. [DOI] [PubMed] [Google Scholar]
- 18. Khan MS, et al.. The effects of insomnia and sleep loss on cardiovascular disease. Sleep Med Clin. 2017;12(2):167–177. [DOI] [PubMed] [Google Scholar]
- 19. Routledge FS, et al.. Insomnia symptoms are associated with abnormal endothelial function. J Cardiovasc Nurs. 2017;32(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sofi F, et al.. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64. [DOI] [PubMed] [Google Scholar]
- 21. Kyle SD, et al.. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69–82. [DOI] [PubMed] [Google Scholar]
- 22. Asche CV, et al.. The direct costs of untreated comorbid insomnia in a managed care population with major depressive disorder. Curr Med Res Opin. 2010;26(8):1843–1853. [DOI] [PubMed] [Google Scholar]
- 23. Ozminkowski RJ, et al.. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273. [DOI] [PubMed] [Google Scholar]
- 24. Pollack M, et al.. Insomnia-related comorbidities and economic costs among a commercially insured population in the United States. Curr Med Res Opin. 2009;25(8):1901–1911. [DOI] [PubMed] [Google Scholar]
- 25. Wickwire EM, et al. Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep. 2019;42(4). doi:10.1093/sleep/zsz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamaldo AA, et al.. Sleep disturbances among older adults in the United States, 2002–2012: nationwide inpatient rates, predictors, and outcomes. Front Aging Neurosci. 2016;8:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaudette É, et al.. Health and health care of Medicare beneficiaries in 2030. Forum Health Econ Policy. 2015;18(2):75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wickwire EM, et al.. Health economics of insomnia treatments: the return on investment for a good night’s sleep. Sleep Med Rev. 2016;30:72–82. [DOI] [PubMed] [Google Scholar]
- 29. Tannenbaum C, et al.. Sedative-hypnotic medicines and falls in community-dwelling older adults: a cost-effectiveness (decision-tree) analysis from a US Medicare perspective. Drugs Aging. 2015;32(4):305–314. [DOI] [PubMed] [Google Scholar]
- 30. Finan PH, et al.. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65(2):363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deyo RA, et al.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 32. GW I, et al. Recent developments in the econometrics of program evaluation. J. Econ. Lit. 2009;47(1):5–86. [Google Scholar]
- 33. Blundell R, et al. Alternative approaches to evaluation in empirical microeconomics. J Hum Resour. 2009;44(3):565–640. [Google Scholar]
- 34. Chen TY, et al. A greater extent of insomnia symptoms and physician-recommended sleep medication use predict fall risk in community-dwelling older adults. Sleep. 2017;40(11). doi:10.1093/sleep/zsx142 [DOI] [PubMed] [Google Scholar]
- 35. Tom SE, et al.. Non-benzodiazepine sedative hypnotics and risk of fall-related injury. Sleep. 2016;39(5):1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alessi CA, et al.. More daytime sleeping predicts less functional recovery among older people undergoing inpatient post-acute rehabilitation. Sleep. 2008;31(9):1291–1300. [PMC free article] [PubMed] [Google Scholar]
- 37. Albrecht JS, et al. Trends in insomnia diagnosis and treatment among Medicare beneficiaries, 2006–2013. Am J Geriatr Psychiatry. 2019;27(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fried TR, et al.. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]