Abstract
Co-occurrence of aberrant hepatocyte growth factor (HGF)/MET proto-oncogene receptor tyrosine kinase (MET) and Wnt/β-catenin signaling pathways has been observed in advanced and metastatic prostate cancers. This co-occurrence positively correlates with prostate cancer progression and castration-resistant prostate cancer development. However, the biological consequences of these abnormalities in these disease processes remain largely unknown. Here, we investigated the aberrant activation of HGF/MET and Wnt/β-catenin cascades in prostate tumorigenesis by using a newly generated mouse model in which both murine Met transgene and stabilized β-catenin are conditionally co-expressed in prostatic epithelial cells. These compound mice displayed accelerated prostate tumor formation and invasion compared with their littermates that expressed only stabilized β-catenin. RNA-Seq and quantitative RT-PCR analyses revealed increased expression of genes associated with tumor cell proliferation, progression, and metastasis. Moreover, Wnt signaling pathways were robustly enriched in prostate tumor samples from the compound mice. ChIP-qPCR experiments revealed increased β-catenin recruitment within the regulatory regions of the Myc gene in tumor cells of the compound mice. Interestingly, the occupancy of MET on the Myc promoter also appeared in the compound mouse tumor samples, implicating a novel role of MET in β-catenin–mediated transcription. Results from implanting prostate graft tissues derived from the compound mice and controls into HGF-transgenic mice further uncovered that HGF induces prostatic oncogenic transformation and cell growth. These results indicate a role of HGF/MET in β-catenin–mediated prostate cancer cell growth and progression and implicate a molecular mechanism whereby nuclear MET promotes aberrant Wnt/β-catenin signaling–mediated prostate tumorigenesis.
Keywords: prostate cancer, hepatocyte growth factor/scatter factor (HGF/SF), Wnt signaling, beta-catenin (B-catenin), mouse, cell signaling, MET proto-oncogene receptor tyrosine kinase (MET), metastasis, transgenic mice, tumorigenesis
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer mortality in men in the United States (1). Approximately 90% of patients with metastatic castrate-resistant prostate cancer (CRPC)3 develop distal secondary bone metastasis, and nearly every patient with bone metastasis eventually succumbs to the disease, resulting in 250,000 deaths worldwide each year (2). Emerging evidence has shown the critical role of the interaction between tumor cells and their surrounding microenvironment in prostatic tumorigenesis. Hepatocyte growth factor (HGF) plays a critical role in the regulation of cell growth, cell motility, morphogenesis, and angiogenesis (3). It has been shown that HGF derived from prostate stroma significantly increases proliferation, motility, and invasion of malignant cells through its receptor, Met (4, 5). The Met receptor tyrosine kinase (RTK) is encoded by Met, a proto-oncogene, and has been shown to play a promotional role in the proliferation and progression of a wide variety of human malignancies, including prostate cancer (4, 6). The aberrant expression of HGF and Met often correlates with poor prognosis in cancer patients (7). HGF is abundantly expressed in the tumor microenvironment, leading to Met activation and downstream signaling that promotes several properties of tumor progression and metastasis. Up-regulation of Met expression was observed in a majority of metastatic prostate cancer lesions (6, 8–11). A nuclear form of MET, nMET, has been identified in human CRPC samples (12). Androgen deprivation can induce nMET expression and promotes cell proliferation and stemlike cell self-renewal in androgen-independent prostate cancer cells (12), implicating a novel role of Met in prostate cancer progression and CRPC development.
Whereas increased expression of Met is frequently observed in advanced and metastatic prostate cancer (13, 14), the effect of up-regulation of Met expression in the pathogenesis of prostate cancer is largely unknown. Previously, we developed a conditional Met transgenic mouse strain, H11Met/+:PB-Cre4 (15), in which the murine Met transgene was targeted into the H11b locus (16), and its expression is activated by a modified probasin promoter-driven Cre expression (17). H11Met/+:PB-Cre4 mice only developed low-grade prostatic intraepithelial neoplasia (PIN) with exogenous HGF administration but failed to develop prostate carcinomas (15), suggesting that other additional factors may be required in initiating prostate tumor development.
Wnt signaling pathways play a significant role in prostate tumorigenesis (18, 19). Aberrant activation of Wnt signaling pathways was revealed as one of the most frequent abnormalities in advanced human prostate cancer (20). Abnormal expression of Wnt ligands, receptors, and effectors has been identified in prostate tumors and surrounding cells, suggesting paracrine regulatory mechanisms in prostate tumorigenesis (21, 22). Castration can elevate Wnt signaling and promote cell survival in the mouse prostate (23). An increase in nuclear β-catenin expression has been shown to promote prostate cancer cell proliferation (24). Conditional expression of stabilized β-catenin in prostate epithelium induces the development of squamous metaplasia and PIN (25, 26). An interaction between the androgen receptor (AR) and β-catenin proteins has been shown in prostate cancer cells (27–29). Interestingly, it has been shown that nMET regulates SRY (sex-determining region Y)-box9, β-catenin, and Nanog homeobox proteins predominately in CRPC cells (12).
Aberrant activation of HGF/Met and Wnt/β-catenin signaling pathways has been observed in advanced and metastatic prostate cancers and positively correlates with prostate cancer progression and CRPC development (20, 30). However, the biological roles of these abnormalities in prostate cancer progression and CRPC development are still largely unknown. In this study, we developed a compound mouse line through intercrossing our previously developed H11Met/+ mouse strain with Ctnnb1(Ex3)L/+:PB-Cre4 mice (15, 31), in which conditional expression of both murine Met transgene and stabilized β-catenin simultaneously co-occur in prostatic epithelial cells. The H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice mimic the condition of human prostate cancer cells with increased Met and β-catenin expression. Using this biologically relevant mouse model, we directly assessed the aberrant activation of HGF/Met and Wnt/β-catenin cascades in prostate tumorigenesis. The compound mice showed accelerated prostate cancer development, progression, and aggressive tumor invasion. RNA-Seq and RT-qPCR analyses showed a robust induction of β-catenin downstream target gene expression, including Myc, Lef1, Onecut2, Sox2, Sp6, and Ccnd1 in samples isolated from the H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice compared with those from Ctnnb1(Ex3)L/+:PB-Cre4 littermate controls. Using ChIP-PCR approaches, we demonstrated increased recruitment of β-catenin within the regulatory region of the Myc gene in tumor cells of the compound mice. Interestingly, the occupancy of Met on the above regulator region of the Myc gene was also observed in the above compound mouse tumor samples, implicating the involvement of Met in β-catenin–containing transcriptional complexes. These results demonstrate a promotional role of Met in β-catenin signaling–mediated tumorigenesis and provide fresh mechanistic insight into aberrant activation HGF/Met in regulating Wnt/β-catenin activation.
Results
Development of a mouse model with conditional expression of Met and stabilized β-catenin in the mouse prostate epithelium
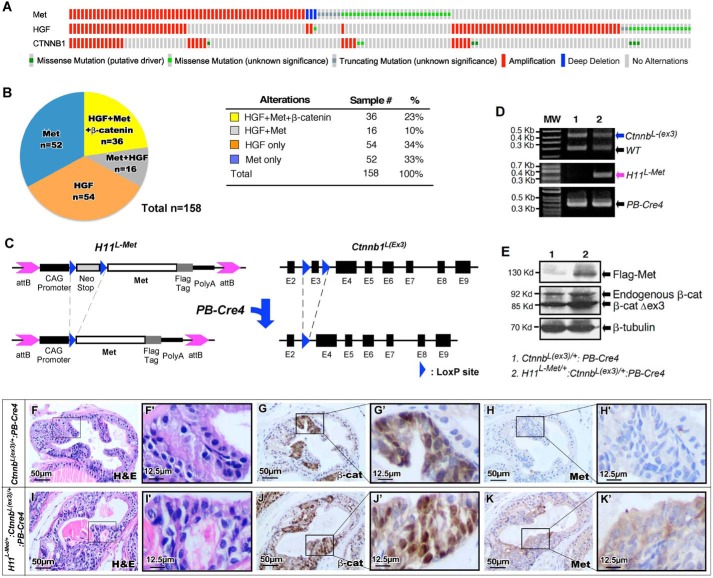
Both aberrant activation of HGF/MET axis and alterations in Wnt/β-catenin signaling have been observed in advanced and metastatic prostate cancers (4, 8). Recent studies further suggest that these two alterations co-exist in human prostate cancer samples (32). Aberrant activation of HGF/Met-mediated signaling pathways was detected in 158 of 2687 patients (∼6%) analyzed in seven different studies (33, 34) (Fig. 1A and Table S1). Among those patients, ∼36 patients (∼23%) also bore aberrant alteration of Wnt/β-catenin signaling pathways (Fig. 1B). Specifically, a statistically significant correlation was revealed in the patient cohorts bearing aberrant activation of Wnt/β-catenin with abnormal HGF/MET activation (Fig. S1). Given the significance and prevalence of the HGF/Met and Wnt/β-catenin abnormalities in human prostate cancers, we generated a new mouse model, H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4, and directly assessed the effect of increased expression of Met and stabilized β-catenin in the mouse prostate. As detailed in Fig. 1C, we intercrossed Ctnnb1(Ex3)L/+:PB-Cre4 mice (31, 35, 36) with our recently developed Met transgenic mice, H11Met/+ (15) to generate the H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice. In this mouse model, both conditional expression of murine Met transgene and stabilized β-catenin co-occur simultaneously in mouse prostate epithelia (Fig. 1C). H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice were born at the expected Mendelian ratios and appeared normal with no obvious differences from their WT littermates at birth. Using genomic PCR approaches, we assessed the presence of PB-Cre4 as well as both the Met transgene and Ctnnb1 exon 3–floxed alleles in H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 samples. A 450-bp (Fig. 1D, blue arrow) PCR fragment, corresponding to the exon 3–targeting allele of the Ctnnb gene, was detected in tissues of both H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice. A 340-bp fragment, corresponding to the Met transgene allele, was only detected in tissues of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice (Fig. 1D, purple arrow). Immunoblotting analyses further demonstrated the expression of transgenic Met protein with the FLAG antibody in prostate tissues of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 (Fig. 1E, top). Both endogenous and stabilized β-catenin protein were detected in prostate tissues of H11L-Met/+:Ctnnb1(Ex3)L/+PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 1E, middle). The expression of both transgenic Met and stabilized β-catenin was further assessed using immunohistochemistry analyses in adjacent prostate tissue sections from age- and sex-matched H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice. Cytoplasmic and nuclear β-catenin expression was detected within PIN areas in samples isolated from either H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 (Fig. 1, panels G and G′ and panels J and J′). Positive staining for Met protein was only revealed in H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice (Fig. 1, K and K′). The above results demonstrated the co-expression of transgenic Met and stabilized β-catenin in the H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mouse prostatic tissues.
Figure 1.
Generation of mice with conditional expression of Met and stabilized β-catenin in the mouse prostate. A, Oncoprint (http://www.cbioportal.org) (please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site) generated from human prostate cancer samples showing significant co-occurrence of aberrations in HGF/MET signaling pathways and aberrant alterations and mutations in Ctnnb1. B, numbers and percentages of prostate cancer samples in the patient cohort showing alterations of HGF and/or Met and β-catenin mutations. C, schematic of the conditional mouse Met transgene and Ctnnb1 exon 3–targeting constructs. Blue triangles, LoxP sequences. D, genomic PCR analysis of expression of Met transgene and Ctnnb1 exon 3–targeting allele. E, immunoblotting of whole prostate extracts from 6-month-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mice with the indicated antibody. F–J, histological and immunohistochemical analysis of 8-week-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mice. F and I, low and high magnification of H&E staining. Shown is immunohistochemical analysis of β-catenin (G and J) and Met (H and K) expression on sequential sections. Scale bar, 50 μm (12.5 μm for high-magnification images).
Synergistic activity of MET and β-catenin accelerates mouse PIN development in the prostate
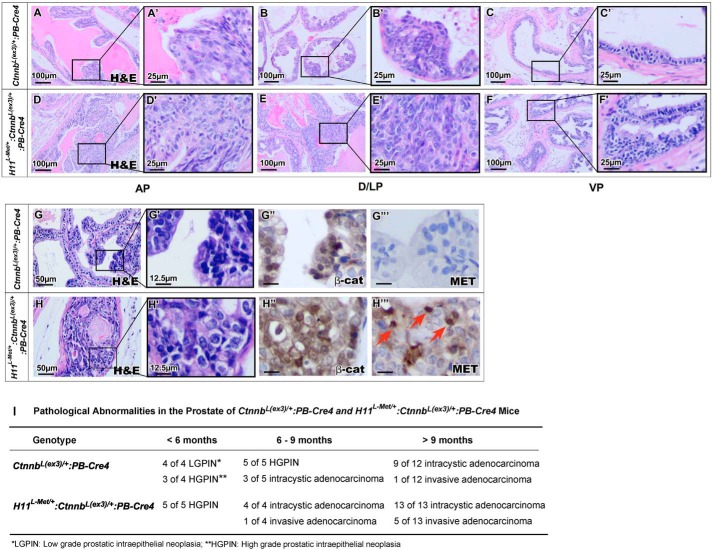
To evaluate the collaborative role of Met and stabilized β-catenin expression in the mouse prostate, we examined both H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice, adhering to recommendations of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee (37). Although both H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice developed PIN lesions starting at ages of 4–6 weeks, H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice developed much more severe PIN lesions and revealed faster disease progression (Fig. 2, D–F′). Lesions in the anterior (AP), dorsal (DP), and lateral (LP) prostate lobes appear more severe than in the ventral prostate (VP) (Fig. 2, panels D and D′ and panels E and E′ versus panels F and F′). Typical cribriform and papilliferous structures completely filled the lumen of prostatic glands of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice (Fig. 2, panels D and D′ and panels E and E′). In contrast, relatively mild pathological changes were observed in prostatic tissues of age-matched Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 2, panels A and A′, panels B and B′, and panels C and C′). Positive staining for stabilized β-catenin was observed in atypical cells within PIN lesions in samples of both H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 2, G″ and H″). In addition, positive staining for Met also appeared within PIN lesions in prostate tissues of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 2H‴) but not in those of Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 2G‴). These data provide a direct link between the expression of stabilized β-catenin and murine Met transgene with PIN development in the above mice. Interestingly, some atypical cells in H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice showed positive nuclear staining for Met (Fig. 2H‴, arrows). As age progresses, H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice showed faster disease progression compared with Ctnnb1(Ex3)L/+:PB-Cre4 mice and developed adenocarcinomas and invasive adenocarcinomas (Fig. 2I). The above data demonstrate a collaborative role of aberrant activation of Met and β-catenin in enhancing prostate cancer progression.
Figure 2.
Synergistic activity of Met and β-catenin accelerates PIN formation in the mouse prostate. A–F, histology of different lobes of 10-week-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mouse prostates. G and H, immunohistochemical analysis of 10-week-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mouse prostates with different antibodies as labeled. I, table of pathological abnormalities in the prostate of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mice. AP, anterior prostate; D/LP, dorsolateral prostate; VP, ventral prostate.
Development of invasive adenocarcinoma is promoted by Met and β-catenin expression in the mouse prostate
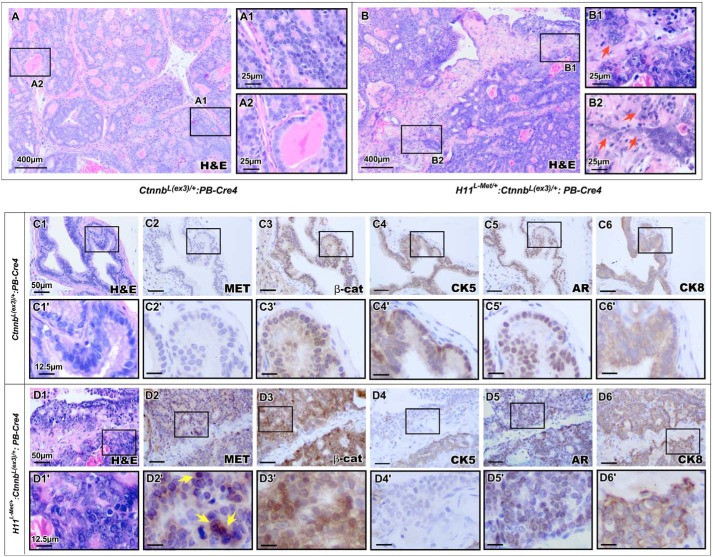
Our scrutiny of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice revealed an invasive tumor phenotype with aggressive adenocarcinoma that occurred with much higher frequency than seen in prostatic lesions of Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 2I). We then continued our analysis of aged H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice up to 16 months of age. We observed that H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice developed prostatic invasive adenocarcinomas as early as 7 months of age. However, Ctnnb1(Ex3)L/+:PB-Cre4 mice developed less frequent invasive adenocarcinomas than the H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice in this cohort (Fig. 2I). Representative images from 12-month-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice display large, poorly differentiated carcinomas with areas of invasion (Fig. 3B), where the epithelial tumor cells have crossed the basement membrane into the surrounding stroma (Fig. 3 (B1 and B2), arrows). In contrast, prostatic tumor tissues isolated from Ctnnb1(Ex3)L/+:PB-Cre4 mice showed pathological changes consistent with prostatic intracystic adenocarcinomas (37) (Fig. 3, A and A2). Immunohistochemistry analyses of the above tumor regions in both genotype mice showed positive staining for stabilized β-catenin (Fig. 3, panels C3 and C3′ and panels D3 and D3′). However, positive staining for Met was only observed in prostatic tumor tissues of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice (Fig. 3, D2 and D2′). Interestingly, as observed previously, positive nuclear staining for Met was revealed in some tumor cells (Fig. 3D2′, arrows). Similar immunoreactivities with AR, CK8, and CK5 were observed in prostate tissues of both genotype mice (Fig. 3, C4–C6′ and D4–D6′). These data further demonstrate a promotional role of transgenic Met protein in enhancing β-catenin–induced prostate tumor growth and progression.
Figure 3.
A collaborative role of Met and β-catenin in enhancing prostatic tumor formation and invasion. Representative histology of a 12-month-old Ctnnb1(Ex3)L/+:PB-Cre4 (A) or H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 (B) mouse prostate. High-magnification images show invasive prostatic adenocarcinoma (B1 and B2). C and D, histological and immunohistochemical analyses of Ctnnb1(Ex3)L/+:PB-Cre4 (C) or H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 (D) mouse prostate using the indicated antibodies: Met (C2 and D2), β-catenin (β-cat) (C3 and D3), CK5 (C4 and D4), AR (C5 and D5), and CK8 (C6 and D6). Scale bars, 400 μm (A and B), 25 μm (panels A1 and A2 and panels B1 and B2), 50 μm (C1–D6), or 12.5 μm (C1′–D6′).
Met enhances β-catenin–mediated tumor progression in a xenograft model
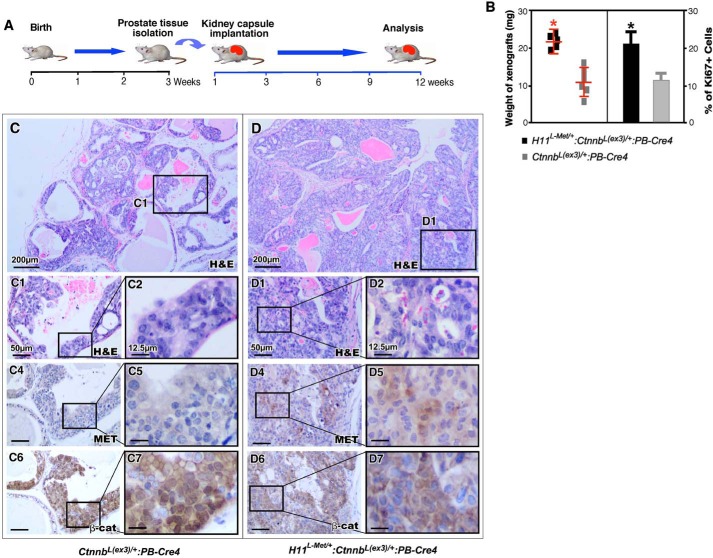
The expression of stabilized β-catenin was initiated by ARR2PB, a modified probasin promoter–driven Cre in the above compound mice (36). It has been shown that β-catenin is an AR activator and enhances AR-mediated transcription (18, 29). To reduce additional factors and specifically assess the effect of Met and stabilized β-catenin in prostate tissues, we implanted prostate tissues that were isolated from 3-week-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice under the kidney capsule of naive SCID male mice (Fig. 4A). The tissue grafts were harvested and analyzed after 12 weeks (Fig. 4A). The weight of tissue grafts derived from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 was significantly higher than those from Ctnnb1(Ex3)L/+PB-Cre littermates (Fig. 4B). Histological analyses of prostatic graft tissues of the compound mice showed severe pathologic changes resembling prostatic adenocarcinoma (Fig. 4D). Tumor cells showed cellular abnormalities, including loss of normal polarity and an increase in nuclear to cytoplasmic ratio as well as nuclear pleiomorphism (Fig. 4, D1 and D2). An increase in Ki67-positive cells was also observed in prostatic graft samples of the compound mice compared with those of Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 4B). The graft tissues isolated from Ctnnb1(Ex3)L/+:PB-Cre4 mice showed less severe pathologic changes than those of the compound mice (Fig. 4, C–C2), resembling PIN lesions. Graft tissues from both genotype mice showed positive staining for β-catenin (Fig. 4, panels C6 and C7 and panels D6 and D7). However, only graft tissues from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice showed positive staining for Met (Fig. 4, D4 and D5). These results provided an additional line of evidence demonstrating the promotional role of MET expression in enhancing stabilized β-catenin–initiated prostate tumor development and progression.
Figure 4.
Met enhances β-catenin–mediated tumor progression in xenograft model for prostate cancer. A, schematic representation of experimental design. B, graphical representation of weights of xenografts derived from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mouse prostate tissues (left) or Ki67 expression (right) in prostate tissues of the indicated genotypes. Shown are representative images of H&E-stained prostate tumors from Ctnnb1(Ex3)L/+:PB-Cre4 (C) or H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 (D) mice. C1–D2, high-magnification images from the indicated mice. Immunohistochemical analysis of Met (panels C4 and C5 and panels D4 and D5) and β-catenin (panels C6 and C7 and panels D6 and D7) in prostate tissues from the indicated mice. Scale bar, 50 μm (12.5 μm for high-magnification images).
Conditional expression of Met enhances Wnt signaling to promote cellular survival, proliferation, and migration
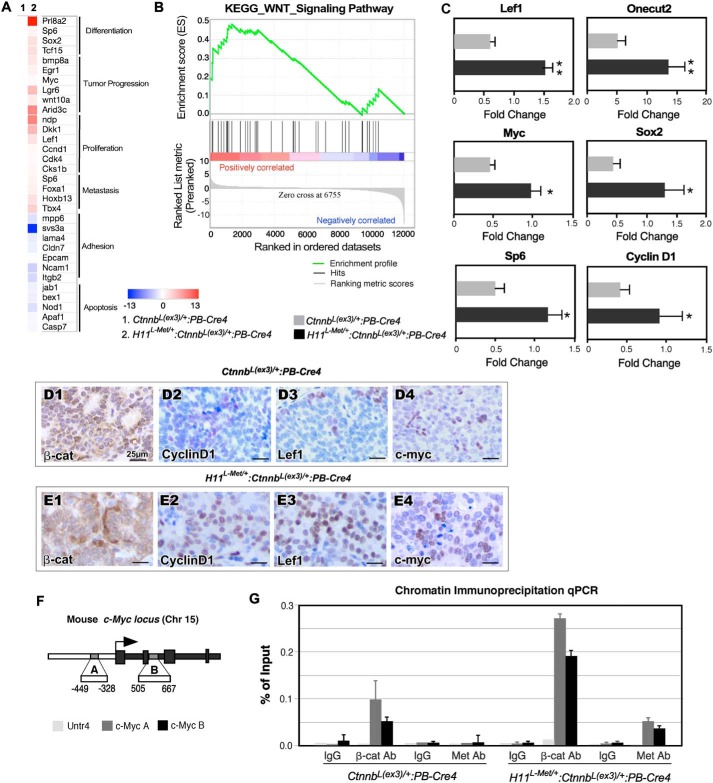
In search of the molecular basis for the collaborative role of transgenic Met and stabilized β-catenin expression in prostate tumorigenesis, we performed RNA-Seq to examine the global transcriptome profiles in the tumor tissue of different genotype mice. We microscopically confirmed that the tumor tissues used to prepare RNA samples were composed of more than 80% tumor cells. Analyses of the gene expression profiles of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compared with Ctnnb1(Ex3)L/+:PB-Cre4 mice yielded 3240 differentially expressed genes (DEGs), of which 894 genes were up-regulated (>1 log2 -fold change) and 2346 genes were down-regulated (<−1 log2 -fold change) (Table S2). A heat map (Fig. 5A) depicts potential target genes that are associated with prostate differentiation and growth, tumor progression, proliferation, metastasis, and apoptosis within the context of prostate cancer (39–60) In support of our previous observations, GSEA analyses with hallmark gene sets revealed significant enrichment of the Wnt signaling pathway based on the DEGs of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 versus Ctnnb1(Ex3)L/+:PB-Cre4 prostate tumor tissues (Fig. 5B and Table S3), suggesting a promotional role of Met in β-catenin–mediated signaling pathways. Using RT-qPCR, we further investigated the expression of β-catenin downstream target genes using RNA samples isolated from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 versus Ctnnb1(Ex3)L/+:PB-Cre4. As shown in Fig. 5C, an increase in the expression of β-catenin downstream target genes involved in cell proliferation and tumor progression, including Lef1, Myc, Sp6, Onecut2, Sox2, and Ccnd1, was observed in RNA samples isolated from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice compared with those of Ctnnb1(Ex3)L/+:PB-Cre4 littermates. Using immunohistochemistry, we further demonstrated increased expression of β-catenin, cyclin D1, Lef1, and Myc proteins in prostatic tumor samples of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice compared with the samples of Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 5, D1–D4 and E1–E4). To directly determine the role of Met in β-catenin–mediated transcription, we performed ChIP-qPCR analyses using the immunoprecipitated genomic DNA samples isolated from prostatic tumor cells of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice to examine the occupancy of stabilized β-catenin and Met on the mouse Myc locus (Fig. 5F), a bona fide β-catenin downstream target gene (61). We observed a significant increase in recruitment of β-catenin within both binding sites in the regulatory region of the Myc in tumor samples isolated from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice compared with the ones from Ctnnb1(Ex3)L/+:PB-Cre4 only mice (Fig. 5G). However, there is no significant recruitment with IgG or on the locus of Untr4, used as a negative control (62). A previous report has shown a nuclear localization of Met in prostate tumor cells (12). In this study, we also observed nuclear staining of Met in prostate tumor cells of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice (Figs. 2H‴ and 3D2′). To further explore the potential role of Met in the nuclei of prostatic tumor cells in the above mouse models, we further examined the involvement of Met in β-catenin–mediated transcription using the above immunoprecipitated genomic DNA samples. Interestingly, we observed the occupancy of Met on both binding sites within the regulatory region of the Myc in tumor samples of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice but not in those of Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 5G). The recruitment of Met in β-catenin–mediated transcriptional complexes on the promoters of β-catenin–regulated downstream target genes, Lef1 and Ccnd1, was also identified in the tumor samples of the above compound mice using ChIP-qPCR approaches (Fig. S2). These data suggest a potential role of Met in β-catenin–involved transcription complexes, providing new mechanistic insight into the effect of Met in promoting β-catenin–mediated tumor growth and progression.
Figure 5.
Conditional expression of mouse Met transgene enhances β-catenin–mediated transcription in prostatic tumor cells. A, heat map of representative gene sets that are altered in Ctnnb1(Ex3)L/+:PB-Cre4 and H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 tumors. B, enrichment plot for expression of genes involved in the Wnt signaling pathway. C, RT-qPCR validation of the gene expression in Ctnnb1(Ex3)L/+:PB-Cre4 and H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 tumors. The data are presented as the mean ± S.D. (error bars) for three independent samples: *, p < 0.05; **, p < 0.01. D and E, immunohistochemical analysis of β-catenin (D1 and E1), CCND1 (D2 and E2), Lef1 (D3 and E3), and Myc (D4 and E4) expression in prostate tumors of Ctnnb1(Ex3)L/+:PB-Cre4 and H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice, respectively. F, schematic of the two β-catenin–binding sites in the mouse Myc locus. G, chromatin immunoprecipitation qPCR results from ChIP with different antibodies and IgG using the indicated conditions. Scale bar, 25 μm.
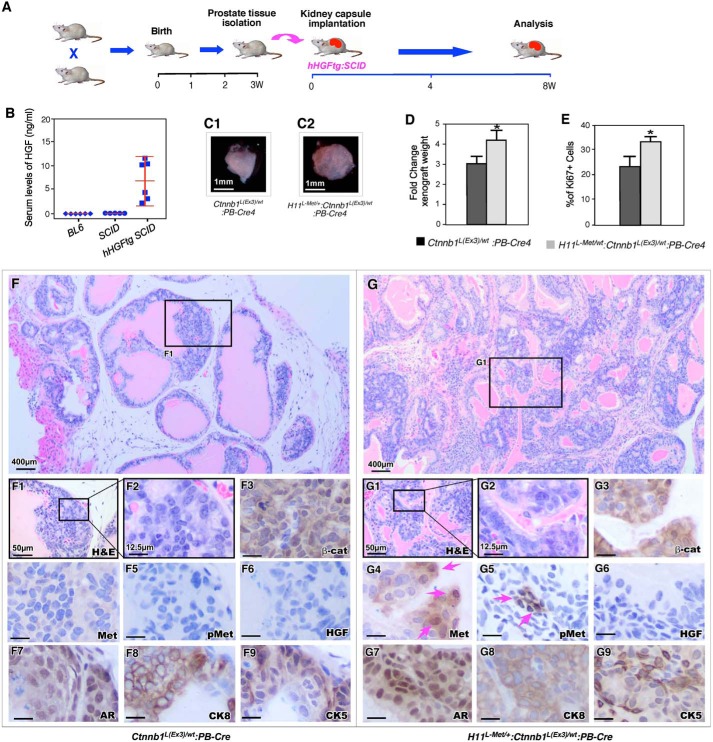
HGF facilitates β-catenin–mediated tumor progression in a xenograft model
Met is the RTK for HGF/SF and is activated by HGF/SF (3). Conditional expression of the murine Met gene did not shown notable abnormalities in the prostate of H11Met/+:PB-Cre4 mice (15). In this study, we assessed the direct role of activation of Met through HGF in promoting β-catenin–mediated prostate tumor growth in H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice. We implanted prostate tissues isolated from 3-week-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice and their Ctnnb1(Ex3)L/+:PB-Cre4 littermates under the kidney capsule of hHGF transgenic SCID male mice (63) (Fig. 6A). Examination of 6–9-week-old hHGF transgenic SCID mice showed higher levels of HGF in sera of these mice than those of age- and sex-matched BL6/C57 and regular SCID mice (Fig. 6B), which is consistent with the previous report (63). Gross examination showed that the grafts derived from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice were larger and heavier than those derived from Ctnnb1(Ex3)L/+:PB-Cre4 (Fig. 6, C and D). Increased Ki67-positive cells were revealed in grafts from the above compound mice than those from the controls (Fig. 6E). Histologically, graft tissues derived from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice showed pathological lesions resembling HGPIN lesions (Fig. 6, G–G2). In contrast, only LGPIN lesions were revealed in graft tissues of Ctnnb1(Ex3)L/+:PB-Cre4 mice (Fig. 6, F–F2). Whereas graft tissues from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice showed strong cytoplasmic and nuclear staining for β-catenin (Fig. 6, F3 and G3), positive staining for Met and pMet only appeared in focal cells in grafts of the compound mice. Specifically, some cells showed clear nuclear staining of Met (Fig. 6, G4 and G5, arrows). There was no visible staining for HGF in both graft tissues (Fig. 6, F6 and G6). Positive staining for other prostate cellular markers, such as AR, CK8, and CK5, appeared in both graft tissues (Fig. 6, F7–F9 and G7–G9). Taken together, these results provide direct evidence demonstrating that the activation of Met by HGF promotes β-catenin–mediated prostatic tumor growth.
Figure 6.
Expression of HGF promotes prostatic tumor formation in H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice. A, schematic representation of experimental design. B, ELISA results for serum levels of HGF in mice of the indicated genotypes. C, representative gross images for xenografts derived from prostate tissues of the indicated genotypes. D, graphical representation of the average weight of xenografts from the indicated genotypes. E, graphical representation of Ki67 expression in xenografts derived from Ctnnb1(Ex3)L/+:PB-Cre4 and H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice. F and G, representative images of H&E-stained prostate tumors from Ctnnb1(Ex3)L/+:PB-Cre4 (F-F2) or H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 (G-G2) mice. Shown is immunohistochemical analysis of β-catenin (F3 and G3), MET (F4 and G4), phosphorylated Met (F5 and G5), HGF (F6 and G6), AR (F7 and G7), CK8 (F8 and G8), or CK5 (F9 and G9) expression in prostate tumors of Ctnnb1(Ex3)L/+:PB-Cre4 and H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice, respectively. Scale bar, 1 mm (C), 400 μm (F and G), 50 μm (F1 and G1), and 12.5 μm (F2–F9 and G2–G9). The data are presented as the mean S.D. + (error bars) for three independent samples. *, p < 0.05.
Discussion
The HGF/Met signaling pathway plays a critical role in prostate tumorigenesis. Up-regulation of Met expression appeared in a majority of advanced and metastatic prostate cancer lesions (6, 8–11). In addition, aberrant activation of Wnt signaling pathways has also been shown to be one of the most frequent abnormalities in advanced human prostate cancer (20). Recent studies from human prostate cancer samples further suggest that these two alterations co-exist in human prostate cancer, particularly in the late stages of disease (32). Particularly, aberrant co-amplification and activation of Wnt/β-catenin with abnormal MET or HGF activation were also seen in the above-referenced prostate cancer samples, and an inverse correlation exists between these abnormalities and survival rates (Fig. S1). Given this biological significance and clinical relevance, we directly assessed the collaborative role of aberrant activation Met and β-catenin in prostate tumorigenesis using a newly generated mouse model, H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4. As described above, conditional expression of the murine Met gene and stabilized β-catenin co-occurred in prostatic epithelial cells of the above mice. This clinically relevant mouse model enables us to recapitulate the aberrant activation of Met and β-catenin during prostate cancer development and progression. The H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice showed accelerated prostate tumor development and progression compared with their Ctnnb1(Ex3)L/+:PB-Cre4 littermates, demonstrating a promotional role of the HGF/Met signaling axis in Wnt/β-catenin–mediated prostate tumorigenesis. The H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mouse model will be a biologically relevant and useful tool for further characterizing the molecular mechanisms underlying HGF/Met signaling in prostate cancer development, progression, and metastasis.
Emerging evidence has shown the significance of the HGF/Met signaling pathway in prostate cancer progression and CRPC development (64). HGF plays a critical role in the regulation of cell growth, cell motility, morphogenesis, and angiogenesis (3). The HGF-Met signaling axis is known to be important to bone remodeling. An increase in expression of both the Met receptor and the HGF ligand has been observed at sites of prostate cancer bone metastasis, suggesting that this pathway may be active during bone metastasis (8). Up-regulation of Met expression has been observed in most metastatic prostate cancer lesions (6, 8–11). A significant challenge within the field of prostate cancer has been the lack of clinically relevant models for examining the biological role of Met in prostate tumor progression and metastasis. Therefore, we recently developed H11Met/+:PB-Cre4 mice, where expression of the mouse Met gene is specifically activated in prostatic epithelial cells through Cre-LoxP–mediated recombination (15). Although activation of murine Met gene expression with the addition of HGF administration induced prostatic intraepithelial neoplasia development, no prostate tumor formation was revealed in H11Met/+:PB-Cre4 mice. These data suggest that other oncogenic hits may be required in HGF/Met signaling axis–induced prostate tumor formation and progression. Therefore, in this study, we used stabilized β-catenin mice (31), a well-established Wnt signaling tumor model, to directly assess the promotional role of Met in prostate tumor formation and progression. We observed the development of more aggressive and invasive prostate tumors in H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice compared with their Ctnnb1(Ex3)L/+:PB-Cre4 littermates. However, we did not observe tumor metastasis in the above compound mice. Despite the evidence indicating that HGF/Met activation is closely associated with bone metastases in human prostate cancer, the failure of metastatic prostate tumor development in the above mouse models indicates that many biological differences may exist between human and murine prostate tissues. The data collected in this study have led us to pursue several more in-depth characterizations of the HGF/Met signaling axis.
The Met is an RTK for HGF (3, 4, 6). It has been shown that Met activation through binding HGF regulates prostate cell proliferation, motility, and invasion (4, 5). Low and inconsistent levels of HGF have been reported in mice (65). To address this caveat, we implanted prostatic tissues isolated from either 3-week-old H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+:PB-Cre4 mice under the kidney capsules of HGF transgenic SCID male mice (63). We analyzed graft tissues after 8 weeks of implantation, rather than the 12-week period that we routinely use. We observed robust atypical cell growth and pathologic changes resembling HGPIN lesions in grafts derived from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice. In contrast, only a few local LGPIN lesions were revealed in graft tissues of Ctnnb1(Ex3)L/+:PB-Cre4 mice. The above pathological differences clearly reflect a promotional role of transgenic HGF expression in activating Met in inducing oncogenic transformation in the mouse prostate and promoting tumor cell growth. Establishing a new mouse strain with both transgenic HGF and Met expression may more closely mimic the pathologic conditions of human prostate cancer, and this should be developed and further investigated. Particularly, using this double transgenic mouse strain in the presence of other oncogenic hits may produce more aggressive and metastatic prostate tumor phenotypes in future mouse models.
To further understand the molecular mechanism underlying Met-mediated tumor progression and metastasis, we examined the transcriptional profile in prostate tumor tissues isolated from both H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 or Ctnnb1(Ex3)L/+: PB-Cre4 mice using RNA-Seq approaches. Increased expression of genes related to tumor development and progression was observed in the samples from H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice. An enrichment in Wnt signaling was also identified in DEGs between H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 and Ctnnb1(Ex3)L/+:PB-Cre4 mice, suggesting a promotional role of transgenic Met expression in enhancing β-catenin mediated transcription. An increase in β-catenin downstream target genes was further shown in tumor samples from the compound mice. Interestingly, a significant increase of Onecut2, a newly defined master regulator in prostate tumor progression (66), was revealed in tumor samples of the compound mice. A previous study has shown a nuclear form of Met in castrated Pten/Trp53 null prostate tumor cells, which can activate Sox9, β-catenin, and Nanog transcription factors (12). Using ChIP-qPCR approaches, we observed the recruitment of Met in β-catenin–involved transcription complexes on the promoters and other regulatory regions of β-catenin–regulated downstream target genes, including Myc, cyclin D1, and Lef1, in prostatic tumor cells of the H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 compound mice. Our data support the previous observation of nuclear Met and provide additional scientific evidence demonstrating a novel role of Met in facilitating β-catenin–mediated transcription in prostate cancer cells. Identification of the nuclear role of transgenic Met protein beyond its canonical role on the membrane in tumor cells of H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 is novel and interesting. Further characterization of nuclear Met using H11L-Met/+:Ctnnb1(Ex3)L/+:PB-Cre4 mice will provide fresh insight into the role and the regulatory mechanism for HGF/Met-mediated oncogenic signaling in promoting prostate tumor development and progression.
Experimental procedures
Mouse breeding and genotyping
The founder mice were bred with WT C57Bl6/J, and progenies were genotyped to confirm the presence of the transgene. To generate conditional Met transgenic mice, the LSL-Met transgenic mice (H11Met/+) were intercrossed with the PB-Cre4 strain (15), carrying the Cre transgene under the control of a modified probasin promoter (ARR2PB) (36, 67). Mice homozygous for floxed β-catenin exon 3, Ctnnb1Ex3(L)/Ex3(L), were obtained from the Jackson Laboratory (Bar Harbor, ME) (strain 004597). All animals used in this study were on a C57BL/6 background, and all experiments were performed in accordance with animal care guidelines approved by the Institutional Animal Care and Use Committee at Beckman Research Institute and City of Hope.
For genotyping, mouse tail tips were incubated in lysis buffer (catalog no. 102-T, VIAGEN Biotech, Los Angeles, CA) for genomic DNA. The conditional expression of H11Met/+ was detected with the forward 5′-AGCGCATCGCCTTCTATCGCCTTC-3′ and reverse primers 5′-AAACAATCTGGGTGTTCC-3′. PCR was performed as follows: 5 min at 94 °C and then 34 cycles of 94 °C for 30 s, 62 °C for 30 s, and 72 °C for 70 s, followed by a final step at 72 °C for 5 min. For Ctnnb1(ex3) allele, the forward (5′-AACTGGCTTTTGGTGTCGGG-3′) and reverse (5′-TCGGTGGCTTGCTGATTATTTC-3′) primers were used to distinguish the WT and floxed alleles (68). For PB-Cre4, the forward (5′-GCAGGAAGCTACTCTGCACCTTG-3′) and reverse (5′-GATCCTGGCAATTTCGGCTAT-3′) primers were used (69).
In vivo prostate regeneration assay
Prostatic tissues were collected from 3-week-old mice and implanted under the renal capsule of SCID mice or hHGFtg SCID mice (35, 70). The SCID or hHGFtg SCID mice were sacrificed after 12 or 8 weeks, respectively, and the grafted tissues were collected and used for histological analyses.
Western blotting
Prostates from H11Met/+/Ctnnb1(Ex3)L/+PB-Cre4 and Ctnnb1(Ex3)L/+PB-Cre4 mice were cut into small pieces, homogenized, and isolated with radioimmune precipitation assay buffer (0.5% Nonidet P-40, 0.3% Triton X-100, 15 mm MgCl2, 5 mm EDTA, 150 mm NaCl, 50 mm Tris-HCl, pH 7.8) as described previously (69, 71). Whole-cell lysates were denatured by boiling in SDS-sample buffer and then resolved by 8% SDS-PAGE. The proteins were transferred onto a nitrocellulose membrane and probed with anti-FLAG antibody (F3165, Sigma-Aldrich), anti-β-catenin (BD 610154, BD Transduction Laboratories, Sparks, MD), or anti-tubulin antibody (MS-581, Thermo Scientific). Detection was performed with ECL reagents (Amersham Biosciences).
Histological analyses and immunostaining
Mouse tissues were fixed and processed as described in our previous study (72). For histological analysis, 5-μm serial sections were processed from Clearify to water through a decreasing ethanol gradient, stained with 5% (w/v) Harris hematoxylin and eosin, and processed back to Clearify through an increasing ethanol gradient.
For immunohistochemical assays, 5-μm sections were boiled in 0.01 m citrate buffer (pH 6.0) for 20 min after rehydration from Clearify to water, placed in 0.3% H2O2/methanol for 15 min, and blocked by 5% goat serum or 5% donkey serum. Tissue slides were then exposed to first antibodies in PBS with 1% goat (or donkey serum) at 4 °C overnight. The following dilutions were used: 1:150 dilution of anti-MET (AF527, R&D Systems, Minneapolis, MN), 1:100 anti-pMET (3077, Cell Signaling, Danvers, MA), 1:1000 anti-mouse/human AR (PA5-16750, Invitrogen, Carlsbad, CA), 1:200 anti-E-cadherin (c20820, BD Transduction Laboratories), 1:2000 anti-CK8 (MMS-162P, Covance, Brea, CA), 1:2600 anti-CK5 (PRB-160P, Covance), 1:400 anti-Ki67 (D3B5, Cell Signaling, Danvers, MA), 1:150 anti-HGF (52445, Cell Signaling), 1:200 anti-Lef1 (sc374522, Santa Cruz Biotechnology, Dallas, TX). Tissues were then incubated with biotinylated goat anti-mouse, goat anti-rabbit (Vector Laboratories, BA-1000 or BA-9200), or donkey anti-goat (ab6987, Abcam, Cambridge, MA) at 1:750 dilution for 1 h at room temperature followed by a 45-min incubation with horseradish peroxidase–conjugated streptavidin (Vector Laboratories, SA-5004). Immunostainings were visualized using a DAB kit (Vector Laboratories, SK-4100). Slides were counterstained with hematoxylin, and coverslips were mounted with Permount Mounting Medium (SP15-500, Fisher).
RNA isolation, RNA-Seq, and RT-qPCR
RNA samples were isolated from age-matched mice of different genotypes. The prostate tissues were homogenized in RNA-Bee (TEL-TEST, Inc., Friendswood, TX), and total RNA was isolated as recommended by the manufacturer. The purified RNA libraries were then sequenced using the Illumina HiSeq 2000 at the City of Hope Integrative Genomics Core. Pathway analysis of hallmark gene sets was performed using preranked gene set enrichment analysis (GSEA 4.0.1).
RT was carried out as described in our previous report (73). For quantitative PCR, cDNA samples were mixed with Power SYBR Green qPCR Mix (4367569, Applied Biosystems) and specific primers, and quantitative PCR was performed according to the manufacturer's protocol. Relative mRNA levels were calculated by the ΔΔC(T) method (74). Reactions were done in triplicate, and the values were normalized by PPIA (peptidylprolyl isomerase A) expression levels. Primers for Ccnd1 (5′-GTGACACTTATGAGCGCCCTA-3′; 5′-CCACTTGTCGCCAATCTTGTA-3′), Lef1 (5′-CCAGCAGATTTCAAGGTGGAC-3′; 5′-TTACAGCTACCTGCCACTTTTC-3′), Myc (5′-CCACGCAGTGAGCATCGAA-3′; 5′-CAGGTGGCAGGTCATTTTCTT-3′), Sox2 (5′-AGACGGACACACATGGAGGT-3′; 5′-AAAGACTCAATGCATGCCAC-3′), Onecut2 (5′-AGACGGACACACATGGAGGT-3′; 5′-AAAGACTCAATGCATGCCAC-3′), Sp6 (5′-AGACGGACACACATGGAGGT-3′; 5′-AAAGACTCAATGCATGCCAC-3′), and Ppia (5′-TGTGCCAGGGTGGTGACTTT-3′; 5′-CGTTTGTGTTTGGTCCAGCAT-3′) were synthesized and used in the qPCRs, respectively.
ChIP assays
ChIP assays were performed as described previously (75). Briefly, mouse tissues were minced and incubated with 1% formaldehyde for 15 min and quenched with 0.150 m glycine for 10 min. Samples were washed sequentially with cold PBS and resuspended in cell lysis buffer (50 mm Tris-HCl (pH 8.0), 140 mm NaCl, 1 mm EDTA, 10% glycerol, 0.5% Nonidet P-40, and 0.25% Triton X-100) and then homogenized. The chromatin was sheared in nuclear lysis buffer (10 mm Tris-HCl, pH 8.0, 1 mm EDTA, 0.5 mm EGTA, and 0.2% SDS) to an average size of 200–500 bp by sonication and then diluted 3-fold in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl, pH 8.1, and 167 mm NaCl) and was subjected to immunoprecipitation by magnetic protein G beads (Invitrogen) conjugated with β-catenin (BD 610154) or Met antibody (3D4, R&D Systems, Minneapolis, MN). Cross-links were reversed, and chromatin DNA fragments were analyzed by real-time qPCR with the indicated primers: Myc-A (5′-ACTCATTCGTTCGTCCTTC-3′; 5′-CCTCGCTCCACACAATAC-3′); Myc-B (5′-CTCACTGGAACTTACAATCTG-3′; 5′-CAACGCCCAAAGGAAATC-3′); Ccnd1 (5′-CCGGCTTTGATCTCTGCTTA-3′; 5′-CGCGGAGTCTGTAGCTCTCT-3′); Lef1 (5′-CTGCGGGCTGGAACATTT-3′; 5′-CGGAGGAGGAGGGGAGAA-3′).
ELISA
Mouse sera were collected from different genotype mice and separated by centrifugation from total blood following cardiac puncture as described previously (38). The serum concentration of HGF was measured by the Quantikine ELISA human HGF immunoassay (DHG00B, R&D Systems). Measurements were performed in in accordance with the manufacturer's instructions.
Statistical analyses
Data are shown as the mean ± S.D. Differences between groups were examined by two-tailed Student's t test or two-way analysis of variance for comparisons among multiple groups. For all analyses, p < 0.05 was considered statistically significant.
Author contributions
J. A., J. M., A. P., W. K. K., A. O., E. H., Y. H., E.-J. Y., V. L., J. G., and Z. S. validation; J. A., J. M., W. K. K., and Z. S. investigation; J. A., J. M., A. P., W. K. K., A. O., Y. H., E.-J. Y., V. L., D.-H. L., J. G., and Z. S. methodology; J. A. and Z. S. writing-original draft; J. A., A. O., and Z. S. writing-review and editing; J. M. and Z. S. conceptualization; J. M., E. H., Y. H., V. L., D.-H. L., and Z. S. resources; J. M. and Z. S. data curation; J. M., W. K. K., A. O., E. H., E.-J. Y., V. L., and Z. S. formal analysis; J. M. and Z. S. supervision; J. M., A. P., W. K. K., A. O., Y. H., V. L., D.-H. L., J. G., and Z. S. visualization; Z. S. funding acquisition; Z. S. project administration.
Supplementary Material
This work was supported in part by National Institutes of Health Grants R01CA070297, R01CA166894, and R01DK104941. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Tables S1–S3 and Figs. S1 and S2.
- CRPC
- castration-resistant prostate cancer
- HGF
- hepatocyte growth factor
- hHGF
- human HGF
- nMET
- nuclear MET
- PIN
- prostate intraepithelial neoplasia
- HGPIN
- high-grade PIN
- LGPIN
- low-grade PIN
- AR
- androgen receptor
- PB
- probasin
- CK
- cytokeratin
- DEGs
- differentially expressed genes
- RTK
- receptor tyrosine kinase
- SF
- scatter factor
- qPCR
- quantitative PCR
- H&E
- hematoxylin and eosin.
References
- 1. DeSantis C. E., Miller K. D., Goding Sauer A., Jemal A., and Siegel R. L. (2019) Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 69, 211–233 10.3322/caac.21555 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A., Bray F., Center M. M., Ferlay J., Ward E., and Forman D. (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto K., and Nakamura T. (1996) Emerging multipotent aspects of hepatocyte growth factor. J. Biochem. 119, 591–600 10.1093/oxfordjournals.jbchem.a021283 [DOI] [PubMed] [Google Scholar]
- 4. Humphrey P. A., Zhu X., Zarnegar R., Swanson P. E., Ratliff T. L., Vollmer R. T., and Day M. L. (1995) Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am. J. Pathol. 147, 386–396 [PMC free article] [PubMed] [Google Scholar]
- 5. Kasai S., Sugimura K., Matsumoto K., Nishi N., Kishimoto T., and Nakamura T. (1996) Hepatocyte growth factor is a paracrine regulator of rat prostate epithelial growth. Biochem. Biophys. Res. Commun. 228, 646–652 10.1006/bbrc.1996.1710 [DOI] [PubMed] [Google Scholar]
- 6. Pisters L. L., Troncoso P., Zhau H. E., Li W., von Eschenbach A. C., and Chung L. W. (1995) c-met proto-oncogene expression in benign and malignant human prostate tissues. J. Urol. 154, 293–298 10.1016/S0022-5347(01)67297-5 [DOI] [PubMed] [Google Scholar]
- 7. Birchmeier C., Birchmeier W., Gherardi E., and Vande Woude G. F. (2003) Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4, 915–925 10.1038/nrm1261 [DOI] [PubMed] [Google Scholar]
- 8. Knudsen B. S., Gmyrek G. A., Inra J., Scherr D. S., Vaughan E. D., Nanus D. M., Kattan M. W., Gerald W. L., and Vande Woude G. F. (2002) High expression of the Met receptor in prostate cancer metastasis to bone. Urology 60, 1113–1117 10.1016/S0090-4295(02)01954-4 [DOI] [PubMed] [Google Scholar]
- 9. Watanabe M., Fukutome K., Kato H., Murata M., Kawamura J., Shiraishi T., and Yatani R. (1999) Progression-linked overexpression of c-Met in prostatic intraepithelial neoplasia and latent as well as clinical prostate cancers. Cancer Lett. 141, 173–178 10.1016/S0304-3835(99)00102-0 [DOI] [PubMed] [Google Scholar]
- 10. van Leenders G. J., Sookhlall R., Teubel W. J., de Ridder C. M., Reneman S., Sacchetti A., Vissers K. J., van Weerden W., and Jenster G. (2011) Activation of c-MET induces a stem-like phenotype in human prostate cancer. PLoS One 6, e26753 10.1371/journal.pone.0026753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russo A. L., Jedlicka K., Wernick M., McNally D., Kirk M., Sproull M., Smith S., Shankavaram U., Kaushal A., Figg W. D., Dahut W., Citrin D., Bottaro D. P., Albert P. S., Tofilon P. J., and Camphausen K. (2009) Urine analysis and protein networking identify met as a marker of metastatic prostate cancer. Clin. Cancer Res. 15, 4292–4298 10.1158/1078-0432.CCR-09-0599 [DOI] [PubMed] [Google Scholar]
- 12. Xie Y., Lu W., Liu S., Yang Q., Carver B. S., Li E., Wang Y., Fazli L., Gleave M., and Chen Z. (2014) Crosstalk between nuclear MET and SOX9/β-catenin correlates with castration-resistant prostate cancer. Mol. Endocrinol. 28, 1629–1639 10.1210/me.2014-1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta S., Li J., Kemeny G., Bitting R. L., Beaver J., Somarelli J. A., Ware K. E., Gregory S., and Armstrong A. J. (2017) Whole genomic copy number alterations in circulating tumor cells from men with abiraterone or enzalutamide-resistant metastatic castration-resistant prostate cancer. Clin. Cancer Res. 23, 1346–1357 10.1158/1078-0432.CCR-16-1211 [DOI] [PubMed] [Google Scholar]
- 14. Cannistraci A., Federici G., Addario A., Di Pace A. L., Grassi L., Muto G., Collura D., Signore M., De Salvo L., Sentinelli S., Simone G., Costantini M., Nanni S., Farsetti A., Coppola V., et al. (2017) C-Met/miR-130b axis as novel mechanism and biomarker for castration resistance state acquisition. Oncogene 36, 3718–3728 10.1038/onc.2016.505 [DOI] [PubMed] [Google Scholar]
- 15. Mi J., Hooker E., Balog S., Zeng H., Johnson D. T., He Y., Yu E. J., Wu H., Le V., Lee D. H., Aldahl J., Gonzalgo M. L., and Sun Z. (2018) Activation of hepatocyte growth factor/MET signaling initiates oncogenic transformation and enhances tumor aggressiveness in the murine prostate. J. Biol. Chem. 293, 20123–20136 10.1074/jbc.RA118.005395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tasic B., Hippenmeyer S., Wang C., Gamboa M., Zong H., Chen-Tsai Y., and Luo L. (2011) Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl. Acad. Sci. U.S.A. 108, 7902–7907 10.1073/pnas.1019507108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu X., Noda A., Noda H., Eto S., Muro H., Ono Y., and Inokuchi K. (2001) Enantioselective metabolism of propanolol in isolated hepatocytes prepared from untreated, PB- or 3-MC-pretreated rats. J. UOEH 23, 23–34 10.7888/juoeh.23.23 [DOI] [PubMed] [Google Scholar]
- 18. Verras M., and Sun Z. (2006) Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 237, 22–32 10.1016/j.canlet.2005.06.004 [DOI] [PubMed] [Google Scholar]
- 19. Murillo-Garzón V., and Kypta R. (2017) WNT signalling in prostate cancer. Nat. Rev. Urol. 14, 683–696 10.1038/nrurol.2017.144 [DOI] [PubMed] [Google Scholar]
- 20. Robinson D., Van Allen E. M., Wu Y.-M., Schultz N., Lonigro R. J., Mosquera J.-M., Montgomery B., Taplin M.-E., Pritchard C. C., Attard G., Beltran H., Abida W., Bradley R. K., Vinson J., Cao X., et al. (2015) Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 10.1016/j.cell.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun Y., Campisi J., Higano C., Beer T. M., Porter P., Coleman I., True L., and Nelson P. S. (2012) Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 18, 1359–1368 10.1038/nm.2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wissmann C., Wild P. J., Kaiser S., Roepcke S., Stoehr R., Woenckhaus M., Kristiansen G., Hsieh J. C., Hofstaedter F., Hartmann A., Knuechel R., Rosenthal A., and Pilarsky C. (2003) WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J. Pathol. 201, 204–212 10.1002/path.1449 [DOI] [PubMed] [Google Scholar]
- 23. Placencio V. R., Sharif-Afshar A. R., Li X., Huang H., Uwamariya C., Neilson E. G., Shen M. M., Matusik R. J., Hayward S. W., and Bhowmick N. A. (2008) Stromal transforming growth factor-β signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res. 68, 4709–4718 10.1158/0008-5472.CAN-07-6289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chesire D. R., and Isaacs W. B. (2002) Ligand-dependent inhibition of β-catenin/TCF signaling by androgen receptor. Oncogene 21, 8453–8469 10.1038/sj.onc.1206049 [DOI] [PubMed] [Google Scholar]
- 25. Bierie B., Nozawa M., Renou J. P., Shillingford J. M., Morgan F., Oka T., Taketo M. M., Cardiff R. D., Miyoshi K., Wagner K. U., Robinson G. W., and Hennighausen L. (2003) Activation of β-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene 22, 3875–3887 10.1038/sj.onc.1206426 [DOI] [PubMed] [Google Scholar]
- 26. Gounari F., Signoretti S., Bronson R., Klein L., Sellers W. R., Kum J., Siermann A., Taketo M. M., von Boehmer H., and Khazaie K. (2002) Stabilization of β-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelia. Oncogene 21, 4099–4107 10.1038/sj.onc.1205562 [DOI] [PubMed] [Google Scholar]
- 27. Mulholland D. J., Cheng H., Reid K., Rennie P. S., and Nelson C. C. (2002) The androgen receptor can promote β-catenin nuclear translocation independently of adenomatous polyposis coli. J. Biol. Chem. 277, 17933–17943 10.1074/jbc.M200135200 [DOI] [PubMed] [Google Scholar]
- 28. Truica C. I., Byers S., and Gelmann E. P. (2000) β-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 60, 4709–4713 [PubMed] [Google Scholar]
- 29. Yang F., Li X., Sharma M., Sasaki C. Y., Longo D. L., Lim B., and Sun Z. (2002) Linking β-catenin to androgen-signaling pathway. J. Biol. Chem. 277, 11336–11344 10.1074/jbc.M111962200 [DOI] [PubMed] [Google Scholar]
- 30. Gundem G., Van Loo P., Kremeyer B., Alexandrov L. B., Tubio J. M., Papaemmanuil E., Brewer D. S., Kallio H. M., Högnäs G., and Annala M., Kivinummi K., Goody V., Latimer C., O'Meara S., Dawson K. J., et al. (2015) The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 10.1038/nature14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., and Taketo M. M. (1999) Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 18, 5931–5942 10.1093/emboj/18.21.5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armenia J., Wankowicz S. A. M., Liu D., Gao J., Kundra R., Reznik E., Chatila W. K., Chakravarty D., Han G. C., Coleman I., Montgomery B., Pritchard C., Morrissey C., Barbieri C. E., Beltran H., et al. (2018) The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 50, 645–651 10.1038/s41588-018-0078-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., Aksoy B. A., Jacobsen A., Byrne C. J., Heuer M. L., Larsson E., Antipin Y., Reva B., Goldberg A. P., Sander C., and Schultz N. (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., and Schultz N. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee S. H., Luong R., Johnson D. T., Cunha G. R., Rivina L., Gonzalgo M. L., and Sun Z. (2016) Androgen signaling is a confounding factor for β-catenin-mediated prostate tumorigenesis. Oncogene 35, 702–714 10.1038/onc.2015.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu X., Wu J., Huang J., Powell W. C., Zhang J., Matusik R. J., Sangiorgi F. O., Maxson R. E., Sucov H. M., and Roy-Burman P. (2001) Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 101, 61–69 10.1016/S0925-4773(00)00551-7 [DOI] [PubMed] [Google Scholar]
- 37. Ittmann M., Huang J., Radaelli E., Martin P., Signoretti S., Sullivan R., Simons B. W., Ward J. M., Robinson B. D., Chu G. C., Loda M., Thomas G., Borowsky A., and Cardiff R. D. (2013) Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 73, 2718–2736 10.1158/0008-5472.CAN-12-4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parasuraman S., Raveendran R., and Kesavan R. (2010) Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 1, 87–93 10.4103/0976-500X.72350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barrett R. T., Erikstad K. E., Sandvik H., Myksvoll M., Jenni-Eiermann S., Kristensen D. L., Moum T., Reiertsen T. K., and Vikebø F. (2015) The stress hormone corticosterone in a marine top predator reflects short-term changes in food availability. Ecol. Evol. 5, 1306–1317 10.1002/ece3.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhardwaj A., Rao M. K., Kaur R., Buttigieg M. R., and Wilkinson M. F. (2008) GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol. Cell Biol. 28, 2138–2153 10.1128/MCB.01170-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang W. C., Chou C. K., Tsou C. C., Li S. H., Chen C. H., Zhuo Y. X., Hsu W. L., and Chen C. H. (2010) Comparative proteomic analysis of proteins involved in the tumorigenic process of seminal vesicle carcinoma in transgenic mice. Int. J. Proteomics 2010, 726968 10.1155/2010/726968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen C., Zhang Q., Liu S., Parajuli K. R., Qu Y., Mei J., Chen Z., Zhang H., Khismatullin D. B., and You Z. (2015) IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate 75, 883–895 10.1002/pros.22971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen K., Luo Z., Li Z., Liu Y., and Zhao Q. (2011) PERP gene therapy attenuates lung cancer xenograft via inducing apoptosis and suppressing VEGF. Cancer Biol. Ther. 12, 1114–1119 10.4161/cbt.12.12.18435 [DOI] [PubMed] [Google Scholar]
- 44. Chimento A., Sirianni R., Casaburi I., Ruggiero C., Maggiolini M., Andò S., and Pezzi V. (2012) 17β-Estradiol activates GPER- and ESR1-dependent pathways inducing apoptosis in GC-2 cells, a mouse spermatocyte-derived cell line. Mol. Cell Endocrinol. 355, 49–59 10.1016/j.mce.2012.01.017 [DOI] [PubMed] [Google Scholar]
- 45. De Marzo A. M., Platz E. A., Sutcliffe S., Xu J., Grönberg H., Drake C. G., Nakai Y., Isaacs W. B., and Nelson W. G. (2007) Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7, 256–269 10.1038/nrc2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ding Z., Wu C. J., Chu G. C., Xiao Y., Ho D., Zhang J., Perry S. R., Labrot E. S., Wu X., Lis R., Hoshida Y., Hiller D., Hu B., Jiang S., Zheng H., et al. (2011) SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 470, 269–273 10.1038/nature09677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hahn-Strömberg V., Askari S., Befekadu R., Matthiessen P., Karlsson S., and Nilsson T. K. (2014) Polymorphisms in the CLDN1 and CLDN7 genes are related to differentiation and tumor stage in colon carcinoma. APMIS 122, 636–642 10.1111/apm.12211 [DOI] [PubMed] [Google Scholar]
- 48. Katoh M., and Katoh M. (2003) CLDN23 gene, frequently down-regulated in intestinal-type gastric cancer, is a novel member of CLAUDIN gene family. Int. J. Mol. Med. 11, 683–689 [PubMed] [Google Scholar]
- 49. Kim J. H., Kim T. W., and Kim S. J. (2011) Downregulation of ARFGEF1 and CAMK2B by promoter hypermethylation in breast cancer cells. BMB Rep. 44, 523–528 10.5483/BMBRep.2011.44.8.523 [DOI] [PubMed] [Google Scholar]
- 50. Kozakowski N., Hartmann C., Klingler H. C., Susani M., Mazal P. R., Scharrer A., and Haitel A. (2014) Immunohistochemical expression of PDGFR, VEGF-C, and proteins of the mToR pathway before and after androgen deprivation therapy in prostate carcinoma: significant decrease after treatment. Target Oncol. 9, 359–366 10.1007/s11523-013-0298-1 [DOI] [PubMed] [Google Scholar]
- 51. Kurashige J., Sawada G., Takahashi Y., Eguchi H., Sudo T., Ikegami T., Yoshizumi T., Soejima Y., Ikeda T., Kawanaka H., Uchiyama H., Yamashita Y., Morita M., Oki E., Saeki H., Sugimachi K., Watanabe M., Mori M., Baba H., and Mimori K. (2013) Suppression of MAL gene expression in gastric cancer correlates with metastasis and mortality. Fukuoka Igaku Zasshi 104, 344–349 [PubMed] [Google Scholar]
- 52. Lee J., Beliakoff J., and Sun Z. (2007) The novel PIAS-like protein hZimp10 is a transcriptional co-activator of the p53 tumor suppressor. Nucleic Acids Res. 35, 4523–4534 10.1093/nar/gkm476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mulholland D. J., Kobayashi N., Ruscetti M., Zhi A., Tran L. M., Huang J., Gleave M., and Wu H. (2012) Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 72, 1878–1889 10.1158/0008-5472.CAN-11-3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rao D. S., Hyun T. S., Kumar P. D., Mizukami I. F., Rubin M. A., Lucas P. C., Sanda M. G., and Ross T. S. (2002) Huntingtin-interacting protein 1 is overexpressed in prostate and colon cancer and is critical for cellular survival. J. Clin. Invest. 110, 351–360 10.1172/JCI0215529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saeki N., Usui T., Aoyagi K., Kim D. H., Sato M., Mabuchi T., Yanagihara K., Ogawa K., Sakamoto H., Yoshida T., and Sasaki H. (2009) Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer 48, 261–271 10.1002/gcc.20636 [DOI] [PubMed] [Google Scholar]
- 56. Sasnauskienė A., Jonušienė V., Krikštaponienė A., Butkytė S., Dabkevičienė D., Kanopienė D., Kazbarienė B., and Didz̆iapetrienė J. (2014) NOTCH1, NOTCH3, NOTCH4, and JAG2 protein levels in human endometrial cancer. Medicina 50, 14–18 10.1016/j.medici.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 57. Sathyanarayana U. G., Padar A., Suzuki M., Maruyama R., Shigematsu H., Hsieh J. T., Frenkel E. P., and Gazdar A. F. (2003) Aberrant promoter methylation of laminin-5-encoding genes in prostate cancers and its relationship to clinicopathological features. Clin. Cancer Res. 9, 6395–6400 [PubMed] [Google Scholar]
- 58. Singh V., Singh L. C., Vasudevan M., Chattopadhyay I., Borthakar B. B., Rai A. K., Phukan R. K., Sharma J., Mahanta J., Kataki A. C., Kapur S., and Saxena S. (2015) Esophageal cancer epigenomics and integrome analysis of genome-wide methylation and expression in high risk Northeast Indian population. OMICS 19, 688–699 10.1089/omi.2015.0121 [DOI] [PubMed] [Google Scholar]
- 59. Stucke V. M., Timmerman E., Vandekerckhove J., Gevaert K., and Hall A. (2007) The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol. Biol. Cell 18, 1744–1755 10.1091/mbc.e06-11-0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vadnais C., Shooshtarizadeh P., Rajadurai C. V., Lesurf R., Hulea L., Davoudi S., Cadieux C., Hallett M., Park M., and Nepveu A. (2014) Autocrine cctivation of the Wnt/β-catenin pathway by CUX1 and GLIS1 in breast cancers. Biol. Open 3, 937–946 10.1242/bio.20148193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mahmoudi T., Boj S. F., Hatzis P., Li V. S., Taouatas N., Vries R. G., Teunissen H., Begthel H., Korving J., Mohammed S., Heck A. J., and Clevers H. (2010) The leukemia-associated Mllt10/Af10-Dot1l are Tcf4/β-catenin coactivators essential for intestinal homeostasis. PLoS Biol. 8, e1000539 10.1371/journal.pbio.1000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wyce A., Bai Y., Nagpal S., and Thompson C. C. (2010) Research resource: the androgen receptor modulates expression of genes with critical roles in muscle development and function. Mol. Endocrinol. 24, 1665–1674 10.1210/me.2010-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang Y. W., Su Y., Lanning N., Gustafson M., Shinomiya N., Zhao P., Cao B., Tsarfaty G., Wang L. M., Hay R., and Vande Woude G. F. (2005) Enhanced growth of human met-expressing xenografts in a new strain of immunocompromised mice transgenic for human hepatocyte growth factor/scatter factor. Oncogene 24, 101–106 10.1038/sj.onc.1208181 [DOI] [PubMed] [Google Scholar]
- 64. Varkaris A., Corn P. G., Gaur S., Dayyani F., Logothetis C. J., and Gallick G. E. (2011) The role of HGF/c-Met signaling in prostate cancer progression and c-Met inhibitors in clinical trials. Expert Opin. Investig. Drugs 20, 1677–1684 10.1517/13543784.2011.631523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jeffers M. F., and Vande Woude G. F. (1999) Activating mutations in the Met receptor overcome the requirement for autophosphorylation of tyrosines crucial for wild type signaling. Oncogene 18, 5120–5125 10.1038/sj.onc.1202902 [DOI] [PubMed] [Google Scholar]
- 66. Rotinen M., You S., Yang J., Coetzee S. G., Reis-Sobreiro M., Huang W. C., Huang F., Pan X., Yáñez A., Hazelett D. J., Chu C. Y., Steadman K., Morrissey C. M., Nelson P. S., Corey E., et al. (2018) ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat. Med. 24, 1887–1898 10.1038/s41591-018-0241-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chlenski A., Nakashiro K., Ketels K. V., Korovaitseva G. I., and Oyasu R. (2001) Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate 47, 66–75 10.1002/pros.1048 [DOI] [PubMed] [Google Scholar]
- 68. Kwak M. K., Johnson D. T., Zhu C., Lee S. H., Ye D. W., Luong R., and Sun Z. (2013) Conditional deletion of the Pten gene in the mouse prostate induces prostatic intraepithelial neoplasms at early ages but a slow progression to prostate tumors. PLoS One 8, e53476 10.1371/journal.pone.0053476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu E. J., Hooker E., Johnson D. T., Kwak M. K., Zou K., Luong R., He Y., and Sun Z. (2017) LZTS2 and PTEN collaboratively regulate ss-catenin in prostatic tumorigenesis. PLoS One 12, e0174357 10.1371/journal.pone.0174357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee S. H., Johnson D. T., Luong R., Yu E. J., Cunha G. R., Nusse R., and Sun Z. (2015) Wnt/β-catenin-responsive cells in prostatic development and regeneration. Stem Cells 33, 3356–3367 10.1002/stem.2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sharma M., Chuang W. W., and Sun Z. (2002) Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3β inhibition and nuclear β-catenin accumulation. J. Biol. Chem. 277, 30935–30941 10.1074/jbc.M201919200 [DOI] [PubMed] [Google Scholar]
- 72. Zhu C., Luong R., Zhuo M., Johnson D. T., McKenney J. K., Cunha G. R., and Sun Z. (2011) Conditional expression of the androgen receptor induces oncogenic transformation of the mouse prostate. J. Biol. Chem. 286, 33478–33488 10.1074/jbc.M111.269894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hohaus S., Petrovick M. S., Voso M. T., Sun Z., Zhang D. E., and Tenen D. G. (1995) PU.1 (Spi-1) and C/EBPα regulate expression of the granulocyte- macrophage colony-stimulating factor receptor α gene. Mol. Cell Biol. 15, 5830–5845 10.1128/MCB.15.10.5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Livak K. J., and Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 75. Cotney J. L., and Noonan J. P. (2015) Chromatin immunoprecipitation with fixed animal tissues and preparation for high throughput sequencing. Cold Spring Harb Protoc 2, 191–199 10.1101/pdb.prot084848 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.