Abstract
Background
Renal vasculitis presents as rapidly progressive glomerulonephritis and comprises of a group of conditions characterised by acute kidney injury (AKI), haematuria and proteinuria. Treatment of these conditions involve the use of steroid and non‐steroid agents in combination with plasma exchange. Although immunosuppression overall has been very successful in treatment of these conditions, many questions remain unanswered in terms of dose and duration of therapy, the use of plasma exchange and the role of new therapies. This 2019 publication is an update of a review first published in 2008 and updated in 2015.
Objectives
To evaluate the benefits and harms of any intervention used for the treatment of renal vasculitis in adults.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 21 November 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
Randomised controlled trials investigating any intervention for the treatment of renal vasculitis in adults.
Data collection and analysis
Two authors independently assessed study quality and extracted data. Statistical analyses were performed using a random effects model and results expressed as risk ratio (RR) with 95% confidence intervals (CI) for dichotomous outcomes or mean difference (MD) for continuous outcomes.
Main results
Forty studies (3764 patients) were included. Studies conducted earlier tended to have a higher risk of bias due to poor (or poorly reported) study design, broad inclusion criteria, less well developed disease definitions and low patient numbers. Later studies tend to have improved in all areas of quality, aided by the development of large international study groups.
Induction therapy: Plasma exchange as adjunctive therapy may reduce the need for dialysis at three (2 studies: RR 0.43, 95% CI 0.23 to 0.78; I2 = 0%) and 12 months (6 studies: RR 0.45, 95% CI 0.29 to 0.72; I2 = 0%) (low certainty evidence). Plasma exchange may make little or no difference to death, serum creatinine (SCr), sustained remission or to serious or the total number of adverse events. Plasma exchange may increase the number of serious infections (5 studies: RR 1.26, 95% CI 1.03 to 1.54; I2 = 0%; low certainty evidence). Remission rates for pulse versus continuous cyclophosphamide (CPA) were equivalent but pulse treatment may increase the risk of relapse (4 studies: RR 1.79, 95% CI 1.11 to 2.87; I2 = 0%) (low certainty evidence) compared with continuous cyclophosphamide. Pulse CPA may make little or no difference to death at final follow‐up, or SCr at any time point. More patients required dialysis in the pulse CPA group. Leukopenia was less common with pulse treatment; however, nausea was more common. Rituximab compared to CPA probably makes little or no difference to death, remission, relapse, severe adverse events, serious infections, or severe adverse events. Kidney function and dialysis were not reported. A single study reported no difference in the number of deaths, need for dialysis, or adverse events between mycophenolate mofetil (MMF) and CPA. Remission was reported to improve with MMF however more patients relapsed. A lower dose of steroids was probably as effective as high dose and may be safer, causing fewer infections; kidney function and relapse were not reported. There was little of no difference in death or remission between six and 12 pulses of CPA. There is low certainty evidence that there were less relapses with 12 pulses (2 studies: RR 1.57, 95% CI 0.96 to 2.56; I2 = 0%), but more infections (2 studies: RR 0.79, 95% CI 0.36 to 1.72; I2 = 45%). One study reported severe adverse events were less in patients receiving six compared to 12 pulses of CPA. Kidney function and dialysis were not reported. There is limited evidence from single studies about the effectiveness of intravenous immunoglobulin, avacopan, methotrexate, immunoadsorption, lymphocytapheresis, or etanercept.
Maintenance therapy: Azathioprine (AZA) has equivalent efficacy as a maintenance agent to CPA with fewer episodes of leucopenia. MMF resulted in a higher relapse rate when tested against azathioprine in remission maintenance. Rituximab is an effective remission induction and maintenance agent. Oral co‐trimoxazole did not reduce relapses in granulomatosis with polyangiitis. There were fewer relapses but more serious adverse events with leflunomide compared to methotrexate. There is limited evidence from single studies about the effectiveness of methotrexate versus CPA or AZA, cyclosporin versus CPA, extended versus standard AZA, and belimumab.
Authors' conclusions
Plasma exchange was effective in patients with severe AKI secondary to vasculitis. Pulse cyclophosphamide may result in an increased risk of relapse when compared to continuous oral use but a reduced total dose. Whilst CPA is standard induction treatment, rituximab and MMF were also effective. AZA, methotrexate and leflunomide were effective as maintenance therapy. Further studies are required to more clearly delineate the appropriate place of newer agents within an evidence‐based therapeutic strategy.
Plain language summary
Interventions for renal vasculitis in adults
What is the issue? Renal vasculitis is a rapidly progressing form of kidney disease that causes damage to the small structures (glomeruli) inside the kidneys that help filter waste and fluids from blood to form urine. The disease means a rapid loss of kidney function. Steroids and cyclophosphamide are recommended to help suppress the immune system.
What did we do? We searched the Cochrane Kidney and Transplant Register of Studies up to 21 November 2019 for randomised controlled trials investigating any intervention for the treatment of renal vasculitis in adults.
What did we find? Forty studies (3764 patients) were identified. Plasma exchange reduces the risk of end‐stage kidney disease in patients presenting with severe acute kidney failure (AKI). The use of pulse cyclophosphamide results in good remission rates but there was an increased risk of relapse. Other appropriate induction agents include rituximab and mycophenolate. Azathioprine is effective as maintenance therapy once remission has been achieved. A lower dose of steroids is just as effective as high dose and may be safer, causing fewer infections. One study shows that a new complement inhibitor can be used to replace steroids in the initial treatment of vasculitis. These are early data. The drug is likely to be very expensive so its place in treatment is not yet clearly defined. Mycophenolate mofetil has also been tested in maintenance treatment and was found to result in a higher rate of disease relapse, when compared to Azathioprine. Methotrexate and leflunomide are useful in maintenance therapy but their relative effectiveness are not clearly defined. Patients on immunosuppression for up to four years after diagnosis have a lowered relapse rate to those in whom treatment is ceased by three years.
Conclusions Plasma exchange was effective in patients with severe AKI. Pulse cyclophosphamide may result in an increased risk of relapse when compared to continuous oral use but a reduced total dose. Whilst cyclophosphamide is used as standard induction treatment, rituximab and mycophenolate mofetil were also effective. Lower dose steroids can now be safely used in initial treatment protocols. Azathioprine, rituximab, mycophenolate, methotrexate and leflunomide are effective maintenance therapy. More trials are required to understand these drugs and new therapies for quickly treating renal vasculitis.
Summary of findings
Background
Description of the condition
Renal vasculitis presents as rapidly progressive glomerulonephritis (RPGN) which comprises of a group of conditions characterised by acute kidney injury (AKI), haematuria and proteinuria. Histological examination of the kidney reveals severe inflammation in the form of crescent formation, glomerular necrosis and vasculitis of small and medium sized vessels within the kidney. These conditions include the anti‐neutrophil cytoplasmic antibody (ANCA) associated vasculitides, anti‐glomerular basement membrane (anti‐GBM) disease and idiopathic RPGN (Savage 1997). ANCA‐associated vasculitides are generally small vessel vasculitides and include granulomatosis with polyangiitis (GPA; previously called Wegener's granulomatosis (WG)), microscopic polyangiitis (MPA) and renal‐limited vasculitis (Seo 2004). GPA is characterised by granulomatous inflammation usually involving the sinuses, lungs and kidneys. It is usually associated with the detection of cytoplasmic‐ANCA (c‐ANCA) specific for proteinase‐3 (PR3) in the serum of the patient (Jennette 2003). MPA is a small to medium vessel vasculitis in the presence of perinuclear‐ANCA (p‐ANCA) specific for myeloperoxidase (MPO). Studies often include GPA, MPA and renal‐limited vasculitis together as ANCA‐associated vasculitides though there is some evidence that they have distinct genetic backgrounds and therefore pathogenesis (Lyons 2012). Eosinophilic GPA is also classified as an ANCA‐associated vasculitides (Jennette 2013), but is not specifically included in this review. In the majority of studies, it is excluded. It is a less well defined condition with overlap with other eosinophilic diseases. Evidence increasingly points to the pathogenicity of ANCA (Jennette 2008). Other conditions also cause vasculitis in the kidney such as Henoch Schonlein Purpura and cryoglobulinaemia resulting in immune deposits visible on electron microscopic examination of renal tissue. The treatment of Goodpasture's disease and other forms of RPGN with granular immune deposits (which have an entirely separate pathogenesis to the pauci‐immune (no immune deposits) forms of the disease) has not been addressed in this review.
Description of the intervention
Treatments for vasculitis involve suppression of the immune system and have been highly successful. Death of untreated vasculitis was 80% at one year (Phillip 2008). Recent figures suggest 80% five‐year survival with modern immunosuppression (Harper 2011). Induction protocols have historically been based around the use of cyclophosphamide (CPA), either daily oral dosing or monthly intravenous (IV) pulses (Bolton 1989; Savage 1997). More recently anti‐CD20 monoclonal antibody treatment has gained some popularity as a primary treatment, though supported by a considerably smaller body of evidence. In the presence of kidney failure, plasma exchange is often used as an adjunct to pharmacological treatment (Lockwood 1976; Pusey 1991; Rondeau 1989). Once remission of the disease is achieved, treatment is scaled back with lower doses of steroids and the induction agent is replaced by a less potent immunosuppressive, such as azathioprine (AZA). Co‐trimoxazole has been used in GPA mainly to prevent the occurrence of pneumocystis infection, upper respiratory tract infection and subsequent relapse of disease. Various guidelines are available which summarise available treatment options and some of the evidence for their use (Lapraik 2007; Menahem 2008; Mukhtyar 2009).
How the intervention might work
There are multiple interventions deployed in this condition. The majority of these interventions work by suppression of the immune system in various ways. Some of these are well defined whereas others are not. For instance, rituximab works by specifically binding to CD20 a molecule expressed on B cell subsets. It works to inhibit the actions of these cells and reduce levels of antibodies that are thought to be pathogenic in this disease. CPA also is directed against B cells. AZA is an anti‐metabolite which inhibits cell proliferation and tends to inhibit lymphocytes, since they have a high rate of cell division. Steroids, also known as glucocorticoids, have a broad immunosuppressive effect via multiple cellular pathways.
Why it is important to do this review
These treatments are well established but many questions remain unanswered. Though recent guidelines are comprehensive (KDIGO 2012), optimal agent, dose, duration, route and frequency of treatment are uncertain. CPA can be given as a daily oral dose or in intermittent oral or IV doses (Adu 1997). IV regimens tend to give a lower total dose and have fewer side effects, but give a higher rate of relapse (de Groot 2001; Harper 2011). Treatment may also include IV methylprednisolone or plasma exchange but their place in therapy is debated (Kerr 2001; Levey 1994). Other therapies including mycophenolate mofetil (MMF), anti‐TNF alpha therapy, leflunomide, methotrexate (MTX), anti‐adhesion molecule (CD52) therapy and IV immunoglobulin (IVIg) have been suggested (Jayne 2000a; Nowack 1997; Tervaert 2001) but the randomised controlled trial (RCT) data are limited.
Death from this condition remains significant with more than 10% of patients with severe ANCA‐associated vasculitides dying in the first 12 months after diagnosis (Little 2010). Fifty percent of these are caused by treatment side effects.
Objectives
To evaluate the benefits and harms of any intervention used for the treatment of renal vasculitis in adults.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at any intervention used for the treatment of renal vasculitis in adults.
Types of participants
Inclusion criteria
All adult patients suffering from vasculitis with renal involvement. Renal involvement includes an episode of AKI, proteinuria and haematuria, or both, with a kidney biopsy showing severe acute glomerulonephritis with crescents, glomerular necrosis or other histological evidence of vasculitis or a positive test for ANCA antibodies. AKI was defined by the included studies.
Exclusion criteria
RPGN with granular immune deposits such as systemic lupus erythematosus, cryoglobulinaemia, Henoch‐Schonlein Purpura
RPGN secondary to infections
Polyarteritis nodosa (PAN)
Eosinophilic GPA
Goodpasture's disease (or anti‐GBM antibody disease).
Types of interventions
Any pharmacological intervention covering:
Corticosteroids versus placebo
Non‐corticosteroid agents, including CPA, AZA, plasma exchange and immunoadsorption, with or without concurrent use of other immunosuppressive agents
Different doses and duration of corticosteroid treatment
Different doses, duration and route of administration of non‐corticosteroid treatment
Any other agents evaluated in an RCT.
Types of outcome measures
Primary outcomes
Death at 1, 2 and 5 years
Kidney function: serum creatinine (SCr), glomerular filtration rate (GFR) at 1, 2, 3, 6 and 12 months then annually
Need for kidney replacement therapy (KRT) at 1, 2, 3, 6 and 12 months then annually.
Secondary outcomes
Number of patients achieving remission
Number of patients relapsing (as defined by the study)
Adverse effects of each drug (e.g. nausea, leukopenia, infections)
Cumulative doses of steroid and other agents.
Relapse of disease was defined by the included studies, but typically included an increase in Birmingham Vasculitis Activity Score (BVAS) score or a recurrence of symptoms of vasculitis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 21 November 2019 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete trials to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that did not meet inclusion criteria although studies and reviews that might include relevant data or information on studies were retained initially. Two authors independently assessed abstracts and, if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out by the same authors independently using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study exists, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions those data were used.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
Dichotomous outcomes were expressed as a risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used.
The summary measure data were translated into number needed to treat (NNT) and number needed to harm (NNH) for the observed overall baseline risks. Adverse effects were tabulated and assessed with descriptive techniques. The risk differences with 95% CI were to be calculated for each adverse effect, either compared to no treatment or compared to another agent, unfortunately there were insufficient studies to do this.
Dealing with missing data
Any further information required from the original author was requested by written correspondence and any relevant data obtained in this manner were included in the review.
Assessment of heterogeneity
For this update we first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was as follows:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a CI for I2) (Higgins 2011).
Assessment of reporting biases
Although we planned to construct funnel plots to assess for the potential existence of small study bias, we did not identify sufficient studies to enable analysis (Higgins 2011).
Data synthesis
Data were pooled using the random effects model but the fixed effects model were also analysed to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Because there were insufficient studies comparing the same pair of interventions we were unable to explore whether there were differences in the following study level characteristics; participants (age, gender and kidney function at presentation), treatments and study quality variability The review reports the therapeutic agent used, its dose and duration of therapy.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The certainty of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). Two review authors independently rated the certainty of the evidence for each outcome. We used the GRADE system to rank the certainty of the evidence using the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). See Appendix 3 for steps for assessing GRADE and reasons for upgrading or downgrading the certainty of the evidence. We presented the following outcomes in the 'Summary of findings' tables:
Death (one year; end of study; end of follow‐up)
Kidney function (one year; end of study; end of follow‐up)
Dialysis (one year; end of study)
Remission (induction only; six months; one year)
Relapse (any time point)
Serious adverse events (e.g. causing death or study drug discontinuation)
Infections (serious; any).
Results
Description of studies
Results of the search
A PRISMA flow chart combining all searches and screening results is shown in Figure 1.
1.
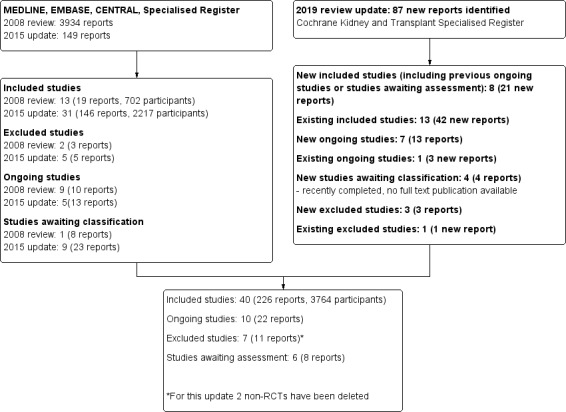
Study flow diagram
For this 2019 update, we searched the Cochrane Kidney and Transplant Specialised Register up to November 2019. Since 2008, inclusive of all updates of this review, we have identified a total of 4170 reports and examined 63 full‐text studies (267 reports). We included 40 studies (226 reports) (see Included studies). We have excluded seven studies (11 reports) (Basu 2017; CHUSPAN 2 2017; De Vita 2012; Harper 2018; Imai 2006; Ribi 2010; Rifle 1990), six studies (eight reports) are awaiting classification (Chen 2011c; CLASSIC 2016; Henderson 2009; MAINTANCAVAS 2017; Pagnoux 2003; RATTRAP 2015), and ten studies (22 reports) are ongoing (ADVOCATE 2019; ALEVIATE 2018; CANVAS 2016; COMBIVAS 2019; MAINRITSAN 3 2015; MUPIBAC 2004; NCT03323476; RITAZAREM 2013; Tuin 2019).
Included studies
Forty studies (3764 participants) were included in this review. See Characteristics of included studies. Nine new studies have been included since the 2015 update.
Types of treatments for remission induction
Plasma exchange adjunctive therapy (Cole 1992; Glockner 1988; Mauri 1985; MEPEX 2007; PEXIVAS 2013; Pusey 1991; Rifle 1980; Szpirt 2011; Zauner 2002)
Pulse versus continuous CPA treatment (Adu 1997; CYCLOPS 2004; Guillevin 1997; Haubitz 1998)
Ten studies considered other potential treatments including: rituximab (RAVE 2010; RITUXVAS 2010), mycophenolate mofetil (Han 2011b; Hu 2008b; MYCYC 2012), methotrexate (NORAM 2005), avacopan (CLEAR 2013), intravenous immunoglobulin for refractory disease (Jayne 2000), immunoadsorption (Stegmayr 1999), lymphocytapheresis (Furuta 1998)
Six to 12 pulses of CPA for vasculitis with poor prognostic factors (Guillevin 2003; CORTAGE 2015)
Reduced dose to standard dose of steroids (PEXIVAS 2013)
Etanercept and placebo (WGET 2002).
As the inclusion and exclusion criteria and treatment regimens varied so widely they have been listed in separate tables (see Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9).
Types of maintenance therapies
Maintenance treatment was considered by sixteen studies including comparisons of:
Azathioprine after three months of remission induction with continued cyclophosphamide (CYCAZAREM 2003);
Azathioprine and mycophenolate mofetil (IMPROVE 2003)
Azathioprine and methotrexate (WEGENT 2008)
Azathioprine and rituximab (MAINRITSAN 2014)
Co‐trimoxazole and placebo (Stegeman 1996; Zycinska 2009)
Cyclosporin and cyclophosphamide (Szpirt 2011)
Extended and standard azathioprine (AZA‐ANCA 2016; REMAIN 2003)
Methotrexate and leflunomide (Metzler 2007)
Methotrexate and cyclophosphamide (Maritati 2017)
Tailored and fixed rituximab (MAINRITSAN 2 2018)
Pre‐emptive therapy for relapse (Boomsma 2003; Tervaert 1990)
Belimumab with placebo (BREVAS 2019).
As the inclusion and exclusion criteria and treatment regimens varied so widely they have been listed in separate tables (see Appendix 10; Appendix 11).
No quasi‐RCTs were identified.
Diagnoses
The vast majority of the studies included patients now recognised as having ANCA‐associated vasculitis in the forms of GPA, MPA and renal‐limited vasculitis.
Other included diagnoses were mainly in earlier studies and included extracapillary and endo‐extracapillary proliferative GN (Rifle 1980), Goodpasture's disease (Stegmayr 1999; only 6/52 patients had this diagnosis), lymphomatoid granulomatosis (Mauri 1985), necrotizing angiitis (Mauri 1985), post‐infectious disease RPGN (Cole 1992), PAN (Adu 1997; Glockner 1988; Mauri 1985), scleroderma (Glockner 1988), and systemic lupus erythematous (Glockner 1988).
Excluded studies
Seven studies have been excluded, five due to the wrong participant population to fit our criteria (CHUSPAN 2 2017; De Vita 2012; Imai 2006; Ribi 2010; Rifle 1990), and two studies were not induction or maintenance studies (Basu 2017; Harper 2018). See Characteristics of excluded studies.
Non‐RCTs have been removed from this review update (Figure 1).
Risk of bias in included studies
For a summary of the risk of bias assessments see Figure 2.
2.
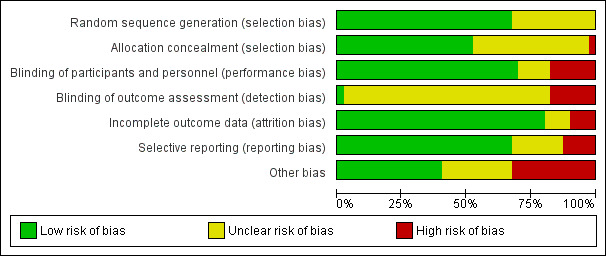
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Studies conducted earlier tended to have a higher risk of bias due to poor (or poorly reported) study design, broad inclusion criteria, less well‐developed disease definitions and low patient numbers. Later studies tend to have improved in all areas of quality, aided by the development of large transnational study groups.
Allocation
Random sequence generation
Randomisation methods were not clearly reported in 13 studies (Boomsma 2003; Furuta 1998; Guillevin 1997; Haubitz 1998; Hu 2008b; Mauri 1985; Pusey 1991; Rifle 1980; Stegmayr 1999; Tervaert 1990; WGET 2002; Zauner 2002; Zycinska 2009).
Randomisation methods were adequately reported in the remaining 27 studies. Such methods included:
Computer‐generated random numbers and stratified for kidney function, urine volume, and by country and disease;
Stratified for oliguria or dialysis;
Stratified by disease severity and by recruiting centre;
Telephone with a statistician;
Stratified by hospital;
Stratified for diagnosis; and centrally performed.
Allocation concealment
Allocation concealment was not performed in Maritati 2017, (high risk). Allocation concealment was unclear in 18 studies (Boomsma 2003; Cole 1992; CORTAGE 2015; Furuta 1998; Guillevin 1997; Guillevin 2003; Han 2011b; Haubitz 1998; Hu 2008b; Mauri 1985; Metzler 2007; Pusey 1991; REMAIN 2003; Rifle 1980; Stegmayr 1999; Tervaert 1990; Zauner 2002; Zycinska 2009), and at low risk of bias for the remaining 21 studies (Adu 1997; AZA‐ANCA 2016; BREVAS 2019; CLEAR 2013; CYCAZAREM 2003; CYCLOPS 2004; Glockner 1988; IMPROVE 2003; Jayne 2000; MAINRITSAN 2014; MAINRITSAN 2 2018; MEPEX 2007; MYCYC 2012; NORAM 2005; PEXIVAS 2013; RAVE 2010; RITUXVAS 2010; Stegeman 1996; Szpirt 2011; WEGENT 2008; WGET 2002).
Blinding
Performance bias
Five studies provided adequate descriptions of blinding both the participants and study personnel (BREVAS 2019; CLEAR 2013; Jayne 2000; Stegeman 1996; WGET 2002) (low risk of performance bias). For 22 studies, blinding of participants or study personnel was not possible however the risk of bias was judged to be low as this was unlikely to affect the outcomes of the studies (Adu 1997; Boomsma 2003; CYCLOPS 2004; Glockner 1988; Guillevin 1997; Guillevin 2003; Han 2011b; Haubitz 1998; Hu 2008b; IMPROVE 2003; MEPEX 2007; Metzler 2007; MYCYC 2012; NORAM 2005; Pusey 1991; Rifle 1980; RITUXVAS 2010; Stegmayr 1999; Szpirt 2011; Tervaert 1990; WEGENT 2008; Zauner 2002). Cole 1992 blinded the participants and review of the initial biopsies (low risk).
Blinding or participants or investigators could not be determined in five studies and judged to be an unclear risk (CYCAZAREM 2003; Furuta 1998; Mauri 1985; RAVE 2010; Zycinska 2009).
Six studies were open‐label and the methods used were judged to be high risk (AZA‐ANCA 2016; CORTAGE 2015; MAINRITSAN 2014; MAINRITSAN 2 2018; Maritati 2017; PEXIVAS 2013; REMAIN 2003).
Detection bias
BREVAS 2019 was judged to be low risk for adequately blinding all outcome assessors.
In 32 studies there was a lack of information regarding blinding of the outcome assessors and judged to be unclear risk. Cole 1992 blinded the review of the final biopsies and Stegeman 1996 blinded the participant's physician. In CYCLOPS 2004 outcomes were classified by non‐blinded investigators and validated by an independent observer. Two studies had centralised computer entry from data books (CYCAZAREM 2003; CYCLOPS 2004). For the rest of the studies no further information was reported.
Seven studies reported a clear indication that the outcome assessors were not blinded and judged to be high risk (AZA‐ANCA 2016; CORTAGE 2015; MAINRITSAN 2014; MAINRITSAN 2 2018; Maritati 2017; MYCYC 2012; PEXIVAS 2013).
Incomplete outcome data
The completeness of follow‐up ranged from 82% to 100%. In most studies follow‐up was generally good with few patients being lost to follow‐up or being withdrawn from the studies.
Thirty‐two studies were judged to be at low risk of attrition bias. Four studies were judged unclear risk due to insufficient details to judge (BREVAS 2019; MYCYC 2012; REMAIN 2003; Rifle 1980), and four studies were judged to be at high risk due to either high attrition rates, or too many missing participants who were not accounted for at the end of the trials (Haubitz 1998; Hu 2008b; Mauri 1985; Metzler 2007).
Selective reporting
Selective reporting bias was generally not detected. These were mostly small studies with very limited reporting measures. The larger studies had very clearly defined outcomes which were clearly reported.
Twenty‐seven studies were judged to be low risk of reporting bias. Eight studies were judged to be unclear risk (Cole 1992; CORTAGE 2015; Glockner 1988; Guillevin 2003; Maritati 2017; Pusey 1991; REMAIN 2003; Tervaert 1990), and five studies were judged to be at high risk of reporting bias (Boomsma 2003; Furuta 1998; Mauri 1985; Zauner 2002; Zycinska 2009).
Other potential sources of bias
Sixteen studies were judged to be at low risk of other sources of bias.
Potential biases were unclear in 11 studies (Boomsma 2003; CORTAGE 2015; Furuta 1998; Hu 2008b; Mauri 1985; PEXIVAS 2013; REMAIN 2003; Rifle 1980; Stegmayr 1999; Szpirt 2011; Zauner 2002).
Thirteen studies were judged to be at high risk of other sources of bias:
Groups appeared to be unbalanced (age, kidney function, BVAS score) (Adu 1997; BREVAS 2019; Guillevin 2003; Zycinska 2009).
Studies were terminated early (interim analyses showed increased side effects; higher rate of relapses; significant differences between the groups) (AZA‐ANCA 2016; BREVAS 2019; Guillevin 1997; Haubitz 1998; Metzler 2007).
Funded by pharmaceutical industry (BREVAS 2019; CLEAR 2013; Jayne 2000; MAINRITSAN 2 2018).
Patients crossed from one treatment arm to the other after four weeks of treatment (Glockner 1988)
Time taken to complete the study (10 years) subject to biases involved in changing physician perceptions of the efficacy of treatment (Pusey 1991).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9
Summary of findings for the main comparison. Plasma exchange as adjunctive therapy for renal vasculitis.
| Plasma exchange as adjunctive therapy for renal vasculitis | |||||
|
Patient or population: adults with renal vasculitis
Settings: inpatients then outpatients
Intervention: plasma exchange as adjunctive therapy Comparison: standard therapy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Plasma exchange | ||||
| Death at one year | 189 per 1000 | 197 per 1000 (108 to 364) | RR 1.04 (0.57 to 1.92) | 267 (5) | ⊕⊕⊝⊝ low1,2 |
| Serum creatinine at 1 year | Mean serum creatinine in the plasma exchange group was 23.52 µmol/L higher (17.19 lower to 64.22 higher) than the control group | ‐‐ | 156 (4) | ⊕⊕⊝⊝ low1,3 | |
| Dialysis at one year | 376 per 1000 | 169 per 1000 (109 to 271) | RR 0.45 (0.29 to 0.72) | 235 (6) | ⊕⊕⊝⊝ low1,2 |
| Sustained remission | 560 per 1000 |
571 per 1000 (498 to 649) |
RR 1.02 0.89 to 1.16) |
704 (1) | ⊕⊕⊝⊝ low1,2 |
| Relapse | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Total number of adverse events | 577 per 1000 |
583 per 1000 (525 to 646) |
RR 1.01 (0.91 to 1.12) |
956 (5) | ⊕⊕⊝⊝ low1,2 |
| Serious infections | 253 per 1000 | 318 per 1000 (260 to 389) | RR 1.26 (1.03 to 1.54) | 956 (5) | ⊕⊕⊝⊝ low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Though some of the studies are of high quality, others have very significant problems (e.g. Mauri 1985; Pusey 1991) 2 Event rate and sample size are small
3 High heterogeneity across groups
Summary of findings 2. Pulse cyclophosphamide versus continuous cyclophosphamide for remission induction.
| Pulse cyclophosphamide (CPA) versus continuous CPA for remission induction | |||||
| Patient or population: adults with renal vasculitis Settings: inpatients then outpatients Intervention: pulse CPA Comparison: continuous CPA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Continuous CPA | Pulse CPA | ||||
| Death at final follow‐up | 206 per 1000 | 158 per 1000 (90 to 271) | RR 0.77 (0.44 to 1.32) | 278 (4) | ⊕⊕⊝⊝ low1,2 |
| Serum creatinine at 12 months | Mean serum creatinine in the pulse CPA group was 9.78 µmol/L lower (53.16 lower to 33.61 higher) than the continuous CPA group | ‐‐ | 52 (2) | ⊕⊕⊝⊝ low2,3 | |
| Dialysis at end of study | 74 per 1000 | 140 per 1000 (68 to 288) | RR 1.90 (0.92 to 3.91) | 245 (4) | ⊕⊕⊝⊝ low1,2 |
| Remission at 6 months | 880 to 1000 |
906 per 1000 (808 to 994) |
RR 1.03 (0.93 to 1.13) |
176 (2) | ⊕⊕⊝⊝ low1,2 |
| Relapse at the end of follow‐up | 181 per 1000 | 324 per 1000 (201 to 519) | RR 1.79 (1.11 to 2.87) | 235 (4) | ⊕⊕⊝⊝ low1,2 |
| Adverse events ‐ treatment failure | 140 per 1000 |
190 per 1000 (21 to 1000) |
RR 1.36 (0.115 to 12.56) |
82 (2) | ⊕⊕⊝⊝ low1,2 |
| Serious infections | 348 per 1000 | 247 per 1000 (132 to 462) | RR 0.71 (0.38 to 1.33) | 278 (4) | ⊕⊕⊝⊝ low1,4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Two of these studies had a high risk of bias. 2 Sample size and/or event rate were low. 3 Wide 95% CI 4 Very different event rates across studies
Summary of findings 3. Rituximab versus cyclophosphamide for renal vasculitis for remission induction.
| Rituximab compared to cyclophosphamide (CPA) for remission induction | |||||
| Patient or population: adults with renal vasculitis Settings: inpatients then outpatients Intervention: rituximab Comparison: CPA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| CPA | Rituximab | ||||
| Death at 6 months | 28 per 1000 |
28 per 1000 (6 to 129) |
RR 1.00 (0.21 to 4.70) |
241 (2) | ⊕⊕⊕⊝ moderate1 |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Remission at 6 months | 661 per 1000 | 674 per 1000 (522 to 872) | RR 1.02 (0.79 to 1.32) | 236 (2) | ⊕⊕⊕⊝ moderate1 |
| Relapse at 12 months | 100 per 1000 |
143 per 1000 (18 to 1000) |
RR 1.43 (0.18 to 11.31) |
38 (1) | ⊕⊕⊝⊝ low1,2 |
| Serious adverse events | 826 per 1000 |
971 per 1000 (594 to 1000) |
RR 1.11 (0.72 to 1.71) |
241 (2) | ⊕⊕⊕⊝ moderate1 |
| Serious Infections | 92 per 1000 | 82 per 1000 (39 to 176) | RR 0.89 (0.62 to 1.92) | 241 (2) | ⊕⊕⊕⊝ moderate3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Number of events overall is low
2 One small study
3 Different event rates in the 2 studies
Summary of findings 4. Mycophenolate mofetil versus cyclophosphamide for remission induction.
| Mycophenolate mofetil (MMF) versus cyclophosphamide (CPA) for remission induction | |||||
| Patient or population: adults with renal vasculitis Settings: inpatients then outpatients Intervention: MMF Comparison: CPA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| CPA | MMF | ||||
| Death at 6 months | 57 per 1000 |
71 per 1000 (20 to 255) |
RR 1.25 (0.35 to 4.46) |
140 (1) | ⊕⊕⊝⊝ low1,2 |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis | 29 per 1000 |
29 per 1000 (4 to 197) |
RR 1.00 (0.14 to 6.90) |
140 (1) | ⊕⊕⊝⊝ low1,2 |
| Relapse at any time point | 203 per 1000 |
366 per 1000 (203 to 654) |
RR 1.80 (1.00 to 3.22) |
127 (1) | ⊕⊕⊝⊝ low1,2 |
| Remission at 6 months | 716 per 1000 | 837 per 1000 (723 to 966) | RR 1.17 (1.01 to 1.35) | 216 (3) | ⊕⊕⊕⊝ moderate3 |
| Serious adverse events | 400 per 1000 |
500 per 1000 (344 to 724) |
RR 1.25 (0.86 to 1.81 |
140 (1) | ⊕⊕⊝⊝ low1,2 |
| Infection | 183 per 1000 |
233 per 1000 (138 to 396) |
RR 1.27 (0.75 to 2.16) |
216 (3) | ⊕⊕⊕⊝ moderate3 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Single study results 2 Wide CI 3 Some inconsistency in results between studies
Summary of findings 5. Intravenous immunoglobulin versus placebo for renal vasculitis in adults.
| Intravenous immunoglobulin (IVIg) compared to placebo for renal vasculitis in adults | |||||
| Patient or population: adults with renal vasculitis Settings: inpatients then outpatients Intervention: IVIg Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | IVIg | ||||
| Death | 118 per 1000 |
24 per 1000 (1 to 456) |
RR 0.20 (0.01 to 3.88) |
34 (1) | ⊕⊕⊝⊝ low1,2 |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Response at 3 months | 353 per 1000 | 822 per 1000 (416 to 1000) | RR 2.33 (1.18 to 4.61) | 34 (1) | ⊕⊕⊝⊝ low1,2 |
| Relapse at 3 months | 267 per 1000 |
312 per 1000 (104 to 949) |
RR 1.17 (0.39 to 3.56) |
34 (1) | ⊕⊕⊝⊝ low1,2 |
| Adverse events | 235 per 1000 |
706 per 1000 (285 to 1000) |
RR 3.00 1.21 to 7.45) |
34 (1) | ⊕⊕⊝⊝ low1,2 |
| Serious infection | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Small sample size; single study results 2 Wide CI
Summary of findings 6. Azathioprine versus cyclophosphamide for maintenance therapy.
| Azathioprine (AZA) versus cyclophosphamide (CPA) for maintenance therapy | |||||
| Patient or population: adults with renal vasculitis for maintenance therapy Settings: inpatients then outpatients Intervention: AZA Comparison: CPA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| CPA | AZA | ||||
| Death (median follow‐up time 8.5 years) | 164 per 1000 |
127 per 1000 (58 to 283) |
RR 0.77 (0.35 to 1.72) |
144 (1) | ⊕⊕⊕⊝ moderate1 |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis (median follow‐up time 8.5 years) | 110 per 1000 | 181 per 1000 |
RR 1.65 (0.57 to 4.79) |
144 (1) | ⊕⊕⊕⊝ moderate1 |
| Relapse at 18 months | 137 per 1000 | 155 per 1000 (70 to 342) | RR 1.13 (0.51 to 2.50) | 144 (1) | ⊕⊕⊕⊝ moderate1 |
| Relapse (median follow‐up time 8.5 years) | 356 per 1000 | 520 per 1000 (356 to 762) | RR 1.46 (1.00 to 2.14) | 144 (1) | ⊕⊕⊕⊝ moderate1 |
| Serious adverse events | 96 per 1000 |
113 per 1000 (43 to 294) |
RR 1.18 (0.45 to 3.07) |
144 (1) | ⊕⊕⊕⊝ moderate1 |
| Infections | 178 per 1000 | 183 per 1000 (91 to 367) | RR 1.03 (0.51 to 2.06) | 144 (1) | ⊕⊕⊕⊝ moderate1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Single study results 2 Wide CI
Summary of findings 7. Azathioprine versus methotrexate for maintenance therapy.
| Azathioprine (AZA) versus methotrexate (MTX) for renal vasculitis for maintenance therapy | |||||
| Patient or population: adults with renal vasculitis for maintenance therapy Settings: inpatients then outpatients Intervention: AZA Comparison: MTX | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| MTX | AZA | ||||
| Death | 16 per 1000 |
5 per 1000 (0 to 127) |
RR 0.33 (0.01 to 8.03 |
126 (1) | ⊕⊕⊝⊝ low1,2 |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Relapse | 333 per 1000 | 367 per 1000 (227 to 590) | RR 1.10 (0.68 to 1.77) | 126 (1) | ⊕⊕⊝⊝ low1,2 |
| Adverse events causing death or study drug discontinuation | 190 per 1000 | 110 per 1000 (48 to 263) | RR 0.58 (0.25 to 1.38) | 126 (1) | ⊕⊕⊝⊝ low1,2 |
| Severe adverse events | 175 per 1000 |
79 per 1000 (30 to 215) |
RR 0.58 0.25 to 1.38) |
126 (1) | ⊕⊕⊝⊝ low1,2 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Single study results 2 Wide CI
Summary of findings 8. Antibiotics versus placebo for maintenance therapy.
| Antibiotics versus placebo for maintenance therapy | |||||
|
Patient or population: adults with renal vasculitis for maintenance therapy
Settings: inpatients then outpatients
Intervention: antibiotics Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Antibiotics | ||||
| Death at 6 months | 25 per 1000 |
8 per 1000 (0 to 194) |
RR 0.33 (0.01 to 7.76) |
81 (1) | ⊕⊕⊝⊝ low1 |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Remission at one year | 796 per 1000 | 908 per 1000 (780 to 1000) | RR 1.14 (0.98 to 1.33) | 111 (2) | ⊕⊕⊝⊝ low2,3 |
| Relapse | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Adverse events causing study drug discontinuation | 50 per 1000 |
195 per 1000 (44 to 863) |
RR 3.90 (0.88 to 17.26) |
81 (1) | ⊕⊕⊝⊝ low1 |
| Infection (urinary tract infection) | 25 per 1000 |
8 per 1000 (0 to 194) |
RR 0.33 (0.01 to 7.76 |
81 (1) | ⊕⊕⊝⊝ low1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgrade 2 levels for study size and limitations with risk of bias assessment 2 One study had multiple limitations in the reporting of the study design 3 Two small studies
Summary of findings 9. Leflunomide versus methotrexate for maintenance therapy.
| Leflunomide compared to methotrexate (MTX) for maintenance therapy | |||||
| Patient or population: adults with renal vasculitis for maintenance therapy Settings: inpatients then outpatients Intervention: leflunomide Comparison: MTX | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| MTX | Leflunomide | ||||
| Death | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Kidney function | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Dialysis | not reported | not reported | ‐‐ | ‐‐ | ‐‐ |
| Relapse | 464 per 1000 | 232 per 1000 (102 to 515) | RR 0.50 (0.22 to 1.11) | 54 (1) | ⊕⊕⊝⊝ low1 |
| Serious adverse events* | no events | 5/26* |
RR 11.81 (0.69 to 203.68) |
54 (1) | ⊕⊕⊝⊝ low1 |
| Infection | 429 per 1000 |
501 per 1000 (283 to 887) |
RR 1.17 (0.27 to 106.88) |
54 (1) | ⊕⊕⊝⊝ low1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgrade 2 levels for study size and limitations with risk of bias assessment
* Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group
Remission induction studies
1: Plasma exchange as adjunctive therapy
Ten studies investigated plasma exchange as adjunctive therapy (Cole 1992; Glockner 1988; Mauri 1985; MEPEX 2007; PEXIVAS 2013; Pusey 1991; Rifle 1980; Stegmayr 1999; Szpirt 2011; Zauner 2002). Zauner 2002 contained no extractable data and could not be included in the meta‐analyses.
Plasma exchange may reduce the need for KRT at three months (Analysis 1.3.2 (2 studies, 147 participants): RR 0.43, 95% CI 0.23 to 0.78; I2 = 0%; NNT = 5; low certainty evidence) and 12 months (Analysis 1.3.4 (6 studies, 235 participants): RR 0.45, 95% CI 0.29 to 0.72; I2 = 0%; NNT = 5; low certainty evidence) post‐treatment. The MEPEX 2007 included patients with SCr > 500 µM and reported a reduction in the need for dialysis at three and 12 months. A subgroup analysis included in Pusey 1991 showed that plasma exchange is effective in patients with severe AKI requiring dialysis. PEXIVAS 2013 reported no difference in the need for dialysis at any time point (Analysis 1.3.6). Currently there are no data available at specific time points for PEXIVAS 2013.
1.3. Analysis.
Comparison 1 Plasma exchange as adjunctive therapy, Outcome 3 Dialysis.
Plasma exchange may increase the number of serious infections compared to control (Analysis 1.5.1 (5 studies, 956 participants): RR 1.26, 95% CI 1.03 to 1.54; I2 = 0%; low certainty evidence).
1.5. Analysis.
Comparison 1 Plasma exchange as adjunctive therapy, Outcome 5 Adverse events.
Plasma exchange may make little or no difference to death (Analysis 1.1), SCr (Analysis 1.2), sustained remission (Analysis 1.4), serious or total number of adverse events (Analysis 1.5.7; Analysis 1.5.8) (low certainty evidence).
1.1. Analysis.
Comparison 1 Plasma exchange as adjunctive therapy, Outcome 1 Death.
1.2. Analysis.
Comparison 1 Plasma exchange as adjunctive therapy, Outcome 2 Kidney function: serum creatinine.
1.4. Analysis.
Comparison 1 Plasma exchange as adjunctive therapy, Outcome 4 Sustained remission.
2: Pulse versus continuous cyclophosphamide
Four studies (Adu 1997; Guillevin 1997; Haubitz 1998; CYCLOPS 2004) investigated the use of pulse and continuous administration of CPA for remission induction. Patients with systemic, rather than specifically renal, vasculitis were included in these studies. Raw data has been obtained from Adu 1997 and those patients with PAN have been excluded from these analyses.
Compared to continuous CPA, pulse CPA may make little or no difference to death at final follow‐up (Analysis 2.1.5 (4 studies, 278 participants): RR 0.77, 95% CI 0.44 to 1.32; I2 = 15%; low certainty evidence) or SCr at any time point (Analysis 2.2).
2.1. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 1 Death.
2.2. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 2 Kidney function: serum creatinine.
There were more patients requiring KRT at the end of the study period in the pulse CPA group than the continuous CPA group however further studies are required (Analysis 2.3.4 (4 studies, 245 participants): RR 1.90, 95% CI 0.92 to 3.91; I2 = 0%; low certainty evidence).
2.3. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 3 Dialysis.
Pulse CPA may make little or no difference to remission compared to continuous CPA (Analysis 2.4).
2.4. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 4 Remission.
Pulse CPA may increase the risk of relapse compared to continuous CPA at the end of follow‐up (Analysis 2.5.3 (4 studies, 235 participants): RR 1.79, 95% CI 1.11 to 2.87; I2 = 0%; NNH = 5; low certainty evidence).
2.5. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 5 Relapse.
Leukopenia was less common with pulse treatment (Analysis 2.7.2 (4 studies, 278 participant): RR 0.53, 95% CI 0.36 to 0.77; I2 = 0%; NNH = 5), however nausea was more common (Analysis 2.7.3 (2 studies, 97 participants): RR 2.51, 95% CI 1.07 to 5.89; I2 = 0%; NNH = 7).
2.7. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 7 Adverse events.
Pulse CPA compared to continuous CPA may make little or no difference to either treatment failure (Analysis 2.6) or serious infections (Analysis 2.7.1).
2.6. Analysis.
Comparison 2 Pulse versus continuous cyclophosphamide, Outcome 6 Treatment failure.
3: Rituximab versus cyclophosphamide
Two studies compared rituximab versus CPA for remission induction (RAVE 2010; RITUXVAS 2010).
Rituximab compared to CPA probably makes little or no difference to death (Analysis 3.1), remission (Analysis 3.2), relapse (Analysis 3.3), severe adverse events (Analysis 3.4.1), serious infections (Analysis 3.4.2), or severe adverse events (episodes/patient‐months) (Analysis 3.5).
3.1. Analysis.
Comparison 3 Rituximab versus cyclophosphamide, Outcome 1 Death.
3.2. Analysis.
Comparison 3 Rituximab versus cyclophosphamide, Outcome 2 Remission.
3.3. Analysis.
Comparison 3 Rituximab versus cyclophosphamide, Outcome 3 Relapse.
3.4. Analysis.
Comparison 3 Rituximab versus cyclophosphamide, Outcome 4 Adverse events.
3.5. Analysis.
Comparison 3 Rituximab versus cyclophosphamide, Outcome 5 Adverse events (episodes/patient‐months).
Kidney function and dialysis were not reported.
4: Mycophenolate mofetil versus cyclophosphamide
Three studies compared MMF and CPA for remission induction (Han 2011b; Hu 2008b; MYCYC 2012).
MYCYC 2012 reported 5/70 deaths in the MMF group and 4/70 in the CPA group (Analysis 4.1). Two patients in each group required dialysis at six months (Analysis 4.2).
4.1. Analysis.
Comparison 4 Mycophenolate mofetil versus cyclophosphamide, Outcome 1 Death.
4.2. Analysis.
Comparison 4 Mycophenolate mofetil versus cyclophosphamide, Outcome 2 Dialysis.
MMF improved remission at six months compared to CPA (Analysis 4.3.1 (3 studies, 217 participants): RR 1.17, 95% CI 1.01 to 1.35; I2 = 4%; high certainty evidence).
4.3. Analysis.
Comparison 4 Mycophenolate mofetil versus cyclophosphamide, Outcome 3 Remission.
MYCYC 2012 reported more patients relapsed with MMF (21/63) than CPA (12/56) at 18 months (Analysis 4.4).
4.4. Analysis.
Comparison 4 Mycophenolate mofetil versus cyclophosphamide, Outcome 4 Relapse.
There were no differences in adverse events (GI symptoms, infections, leukopenia, serious adverse events) between the two groups (Analysis 4.5).
4.5. Analysis.
Comparison 4 Mycophenolate mofetil versus cyclophosphamide, Outcome 5 Adverse events.
Kidney function was not reported.
5: Methotrexate versus cyclophosphamide
NORAM 2005 compared MTX with CPA.
There were no deaths in the MTX group at 6 months and 1/49 deaths in the CPA group. There were two deaths in each group at 18 months (Analysis 5.1)
5.1. Analysis.
Comparison 5 Methotrexate versus cyclophosphamide, Outcome 1 Death.
Remission at 6 months was similar (MTX: 44/49; CPA: 43/46) (Analysis 5.2.1). The authors reported longer time to remission for MTX in patients with a higher disease activity index. Relapse post‐remission rates were higher for the MTX group (32/46) than the CPA group (20/43) (Analysis 5.3). Relapse figures quoted here are end of study numbers, not a specific time point.
5.2. Analysis.
Comparison 5 Methotrexate versus cyclophosphamide, Outcome 2 Remission.
5.3. Analysis.
Comparison 5 Methotrexate versus cyclophosphamide, Outcome 3 Relapse.
Adverse event rates were reported to be similar with leukopenia more frequent with CPA treatment (6 events in the CPA group and 0 events in the MTX group) and more liver dysfunction in MTX (7 events in the MTX group and 1 in the CPA group).
Kidney function and dialysis were not reported.
6: Avacopan versus prednisolone
CLEAR 2013 reported the mean eGFR at three months for patients receiving avacopan was 56.1 ± 5.2 mL/min/1.73 m2 and 52.8 ± 3.6 mL/min/1.73 m2 for those receiving prednisolone (Analysis 6.2). There were no deaths reported in either group (Analysis 6.1)
6.2. Analysis.
Comparison 6 Avacopan versus prednisolone, Outcome 2 Kidney function: eGFR [mL/min/1.73 m2].
6.1. Analysis.
Comparison 6 Avacopan versus prednisolone, Outcome 1 Death.
Remission (avacopan: 7/21; prednisolone: 8/20) (Analysis 6.3) and relapse (avacopan: 3/22; prednisolone: 2/23) (Analysis 6.4) were similar.
6.3. Analysis.
Comparison 6 Avacopan versus prednisolone, Outcome 3 Remission.
6.4. Analysis.
Comparison 6 Avacopan versus prednisolone, Outcome 4 Relapse.
There were more serious adverse events in the avacopan group (avacopan: 8/22; prednisolone: 4/23) (Analysis 6.5).
6.5. Analysis.
Comparison 6 Avacopan versus prednisolone, Outcome 5 Adverse events.
Dialysis was not reported.
7: Intravenous immunoglobulin use in persistent disease
Jayne 2000 reported the use of IVIg demonstrated a therapeutic response in more patients at three months when compared with placebo. Response was defined as a reduction in BVAS of > 50% (IVIg: 14/17; control: 6/17) Analysis 7.2. Benefit was not demonstrated beyond three months. There were no deaths in the IVIg group and 2/17 deaths in the control group (Analysis 7.1). There were 5/16 relapses in the IVIg group and 4/15 in the control group (Analysis 7.3). There were more adverse events in the IVIg group (IVIg: 12/17; control: 4/17) (Analysis 7.4).
7.2. Analysis.
Comparison 7 Intravenous immunoglobulin versus placebo, Outcome 2 Response.
7.1. Analysis.
Comparison 7 Intravenous immunoglobulin versus placebo, Outcome 1 Death.
7.3. Analysis.
Comparison 7 Intravenous immunoglobulin versus placebo, Outcome 3 Relapse.
7.4. Analysis.
Comparison 7 Intravenous immunoglobulin versus placebo, Outcome 4 Adverse events.
Kidney function and dialysis were not reported.
8: Immunoadsorption versus plasma exchange
Stegmayr 1999 reported 3/21 deaths in the immunoadsorption group and 2/23 deaths in the plasma exchange group (Analysis 8.1).
8.1. Analysis.
Comparison 8 Plasma exchange versus immunoadsorption, Outcome 1 Death.
At six months SCr was 164.5 ± 94.1 µmol/L in the immunoadsorption group and 187.8 ± 61.2 µmol/L in the plasma exchange group (Analysis 8.2). Two of 18 needed dialysis in the immunoadsorption group and 3/21 in the plasma exchange group (Analysis 8.3).
8.2. Analysis.
Comparison 8 Plasma exchange versus immunoadsorption, Outcome 2 Kidney function: serum creatinine.
8.3. Analysis.
Comparison 8 Plasma exchange versus immunoadsorption, Outcome 3 Dialysis.
Remission, relapse, and adverse events were not reported.
9: Lymphocytapheresis
Furuta 1998 reported a reduction in SCr with lymphocytapheresis (2.1 ± 0.3 mg/dL) compared to control (4.2 ± 0.9 mg/dL) at four weeks (Analysis 9.2). There were 2/12 deaths in the lymphocytapheresis group and 5/12 in the control group (Analysis 9.1). One of 12 patients required dialysis in the lymphocytapheresis group and 3/12 in the control group (Analysis 9.3).
9.2. Analysis.
Comparison 9 Lymphocytapheresis versus control, Outcome 2 Kidney function: serum creatinine.
9.1. Analysis.
Comparison 9 Lymphocytapheresis versus control, Outcome 1 Death.
9.3. Analysis.
Comparison 9 Lymphocytapheresis versus control, Outcome 3 Dialysis.
Remission, relapse, and adverse events were not reported.
10: Duration (6 versus 12 pulses) of cyclophosphamide induction
Two studies compared six versus 12 pulses of cyclophosphamide for remission induction (CORTAGE 2015; Guillevin 2003).
Guillevin 2003 reported 6/19 deaths in the 6‐pulse group compared to 6/28 in the 12‐pulse group at the end of the study. CORTAGE 2015 reported 9/53 deaths in the 6‐pulse group and 12/53 in the 12‐pulse group at 3 years (Analysis 10.1).
10.1. Analysis.
Comparison 10 Six versus 12 cyclophosphamide pulses, Outcome 1 Death.
There was little or no difference in remission between 6 and 12 pulses of CPA (Analysis 10.2 (2 studies, 151 participants): RR 0.99, 95% CI 0.85 to 1.15; I2 = 11%; low certainty evidence). There is low certainty evidence that there were less relapses with 12 pulses (Analysis 10.3 (2 studies, 133 participants): RR 1.57, 95% CI 0.96 to 2.56; I2 = 0%), but more infections (Analysis 10.4.1 (2 studies, 169 participants): RR 0.79, 95% CI 0.36 to 1.72; I2 = 45%).
10.2. Analysis.
Comparison 10 Six versus 12 cyclophosphamide pulses, Outcome 2 Remission.
10.3. Analysis.
Comparison 10 Six versus 12 cyclophosphamide pulses, Outcome 3 Relapse.
10.4. Analysis.
Comparison 10 Six versus 12 cyclophosphamide pulses, Outcome 4 Adverse events.
CORTAGE 2015 reported severe adverse events were less in patients receiving 6 (32/53) compared to 12 pulses (40/51) of CPA (Analysis 10.4.2).
Kidney function and dialysis were not reported.
11: Reduced versus standard dose steroids
PEXIVAS 2013 reported 46/353 death in the reduced dose group and 53/351 in the standard dose group (Analysis 11.1). There were 70/353 requiring dialysis in the reduced dose group and 68/351 in the standard dose group (Analysis 11.2).
11.1. Analysis.
Comparison 11 Reduced dose versus standard dose steroids, Outcome 1 Death.
11.2. Analysis.
Comparison 11 Reduced dose versus standard dose steroids, Outcome 2 Dialysis.
In the reduce dose group 204/353 had sustained remission and there were 193/353 in the standard dose group (Analysis 11.3).
11.3. Analysis.
Comparison 11 Reduced dose versus standard dose steroids, Outcome 3 Sustained remission.
There were 231/353 severe adverse events and 96/353 serious infections in the reduced dose group and 218/351 and 116/351 in the standard dose group (Analysis 11.4.1) (Analysis 11.4.2).
11.4. Analysis.
Comparison 11 Reduced dose versus standard dose steroids, Outcome 4 Adverse events.
Kidney function and relapse were not reported.
12: Etanercept versus placebo
WGET 2002 reported 4/89 deaths in the etanercept group and 2/85 in the placebo group (Analysis 12.1). There were 62/89 sustained remissions in the etanercept group and 64/85 in the placebo group (Analysis 12.2); 19/62 relapses in the etanercept group and 21/64 in the placebo group (Analysis 12.3). There were 44/89 infections and 6/89 cancers in the etanercept group and 42/85 and 0/85 in the placebo group (Analysis 12.4).
12.1. Analysis.
Comparison 12 Etanercept versus placebo, Outcome 1 Death.
12.2. Analysis.
Comparison 12 Etanercept versus placebo, Outcome 2 Sustained remission.
12.3. Analysis.
Comparison 12 Etanercept versus placebo, Outcome 3 Relapse.
12.4. Analysis.
Comparison 12 Etanercept versus placebo, Outcome 4 Adverse events.
Kidney function and relapse were not reported.
Maintenance therapy studies
13: Azathioprine versus cyclophosphamide
CYCAZAREM 2003 reported there were 37/71 relapses after the introduction of AZA compared to 26/73 for the group who remained on CPA (Analysis 13.3). There were 35 episodes/1095 patient‐months of leukopenia reported in patients treated with CPA and 22 episodes/1065 patient‐months in the AZA group (Analysis 13.5.1).
13.3. Analysis.
Comparison 13 Maintenance therapy: azathioprine versus cyclophosphamide, Outcome 3 Relapse.
13.5. Analysis.
Comparison 13 Maintenance therapy: azathioprine versus cyclophosphamide, Outcome 5 Adverse events (episodes/patient‐months).
Leukopenia was more frequent in the CPA group (35/73) compared to the AZA group (22/71) (Analysis 13.5) but no difference in infection (AZA: 13/71; CPA: 13/73) (Analysis 13.5.2) or serious adverse events (AZA: 8/71; CPA: 7/73) (Analysis 13.4.3).
13.4. Analysis.
Comparison 13 Maintenance therapy: azathioprine versus cyclophosphamide, Outcome 4 Adverse events.
Long‐term follow‐up (median time 8.5 years) showed no difference in death (AZA: 9/71; CPA: 12/73) (Analysis 13.1.1) or need for dialysis (AZA: 8/71; CPA: 5/73) Analysis 13.2).
13.1. Analysis.
Comparison 13 Maintenance therapy: azathioprine versus cyclophosphamide, Outcome 1 Death.
13.2. Analysis.
Comparison 13 Maintenance therapy: azathioprine versus cyclophosphamide, Outcome 2 Dialysis.
Kidney function was not reported.
14: Mycophenolate mofetil versus azathioprine
IMPROVE 2003 reported no difference in death (Analysis 14.1) between MMF (1/76) and AZA (1/80). More patients were reported to relapse in the MMF group (42/76) compared to the AZA group (30/80) (Analysis 14.2.1). There were no differences between major (Analysis 14.2.2) and minor relapses (Analysis 14.2.3). In the MMF group there were 3/76 serious infections and 8/80 in the AZA group (Analysis 14.3.2). In the MMF group there 4/76 reports of leukopenia and 7/80 reports in the AZA group (Analysis 14.3.3).
14.1. Analysis.
Comparison 14 Maintenance therapy: mycophenolate mofetil versus azathioprine, Outcome 1 Death.
14.2. Analysis.
Comparison 14 Maintenance therapy: mycophenolate mofetil versus azathioprine, Outcome 2 Relapse.
14.3. Analysis.
Comparison 14 Maintenance therapy: mycophenolate mofetil versus azathioprine, Outcome 3 Adverse events.
Kidney function and dialysis were not reported.
15: Azathioprine versus methotrexate
WEGENT 2008 reported no differences between the treatments for death (AZA: 0/63; MTX: 1/63) (Analysis 15.1), relapse (AZA: 23/63; MTX: 21/63) (Analysis 15.2), and event‐free survival (AZA: 17/24; MTX: 15/25) (Analysis 15.4).
15.1. Analysis.
Comparison 15 Maintenance therapy: azathioprine versus methotrexate, Outcome 1 Death.
15.2. Analysis.
Comparison 15 Maintenance therapy: azathioprine versus methotrexate, Outcome 2 Relapse.
15.4. Analysis.
Comparison 15 Maintenance therapy: azathioprine versus methotrexate, Outcome 4 Event‐free survival at 24 months.
There were more patients with relapse‐free survival at 18 (AZA: 30/43; MTX 40/43) and 24 months (AZA: 13/25; MTX: 22/30) in the MTX group (Analysis 15.3) but not at 36 months. There were more adverse events (AZA: 26/63; MTC: 35/63) (Analysis 15.5.1), severe adverse events (AZA: 5/63; MTX: 11/63) (Analysis 15.5.2), and adverse events causing death or study drug withdrawal (AZA: 7/63; MTTX: 12/63) (Analysis 15.5.2) in the MTX group.
15.3. Analysis.
Comparison 15 Maintenance therapy: azathioprine versus methotrexate, Outcome 3 Relapse‐free survival.
15.5. Analysis.
Comparison 15 Maintenance therapy: azathioprine versus methotrexate, Outcome 5 Adverse events.
Kidney function and dialysis were not reported.
16: Rituximab versus azathioprine
MAINRITSAN 2014 reported less major relapses in rituximab compared to azathioprine at one year (RTX: 1/57; AZA: 8/58) (Analysis 16.2.1), two years (RTX: 1/59; AZA: 10/58) (Analysis 16.2.2), and 28 months (RTX: 3/57; AZA: 17/58) (Analysis 16.2.3).
16.2. Analysis.
Comparison 16 Maintenance therapy: rituximab versus azathioprine, Outcome 2 Major relapse.
No differences were found between the two treatments for death (Analysis 16.1), minor relapse at 12, 24 and 28 months (Analysis 16.3), or serious infection (Analysis 16.4).
16.1. Analysis.
Comparison 16 Maintenance therapy: rituximab versus azathioprine, Outcome 1 Death.
16.3. Analysis.
Comparison 16 Maintenance therapy: rituximab versus azathioprine, Outcome 3 Minor relapse.
16.4. Analysis.
Comparison 16 Maintenance therapy: rituximab versus azathioprine, Outcome 4 Adverse events.
Kidney function and dialysis were not reported.
17: Co‐trimoxazole (antibiotics) versus placebo for relapse prevention
Two studies investigated co‐trimoxazole for relapse prevention (Stegeman 1996; Zycinska 2009). Stegeman 1996 reported death at six months and remission at 12 and 24 months; Zycinska 2009 reported remission at 12 and 18 months.
Stegeman 1996 reported no difference in death at six months (Analysis 17.1).
17.1. Analysis.
Comparison 17 Maintenance therapy: co‐trimoxazole (antibiotics) versus placebo, Outcome 1 Death.
At 12 months antibiotics may make little or no difference to remission (Analysis 17.2.1 (2 studies, 111 participants): RR 1.14, 95% CI 0.98 to 1.33; I2 = 0%; low certainty evidence).
17.2. Analysis.
Comparison 17 Maintenance therapy: co‐trimoxazole (antibiotics) versus placebo, Outcome 2 Remission.
Zycinska 2009 reported no improvement in remission at 18 months (antibiotics: 12/16; placebo: 8/15) (Analysis 17.2.2) and Stegeman 1996 reported no improvement at 24 months (antibiotics: 31/41; placebo: 23/39) (Analysis 17.2.3).
Stegeman 1996 reported more adverse events causing study drug discontinuation (antibiotics: 8/41; placebo: 2/40) (Analysis 17.3.6).
17.3. Analysis.
Comparison 17 Maintenance therapy: co‐trimoxazole (antibiotics) versus placebo, Outcome 3 Adverse events.
There were some significant difficulties with the reporting of Zycinska 2009 along with unbalanced groups at baseline which would bias in favour of the treatment being effective.
Kidney function and dialysis were not reported.
18: Cyclosporin versus cyclophosphamide
Szpirt 2011 reported no difference in the number of relapses with cyclosporin (10/16) compared to CPA (8/16) (Analysis 18.1).
18.1. Analysis.
Comparison 18 Maintenance therapy: cyclosporin versus cyclophosphamide, Outcome 1 Relapse.
Death, kidney function, dialysis, remission, adverse events, and infection were not reported.
19: Extended versus standard azathioprine
Two studies compared an extended azathioprine with a standard AZA treatment (AZA‐ANCA 2016; REMAIN 2003). There were more relapses in the standard AZA group (Analysis 19.3 (2 studies, 162 participants): RR 0.41, CI 0.26 to 0.64).
19.3. Analysis.
Comparison 19 Maintenance therapy: extended versus standard azathioprine, Outcome 3 Relapse.
No differences were found for death between the two groups (Analysis 19.1). REMAIN 2003 reported 0/61 in the extended AZA group and 4/56 in the standard AZA group needed dialysis (Analysis 19.2).
19.1. Analysis.
Comparison 19 Maintenance therapy: extended versus standard azathioprine, Outcome 1 Death.
19.2. Analysis.
Comparison 19 Maintenance therapy: extended versus standard azathioprine, Outcome 2 Dialysis.
AZA‐ANCA 2016 reported no differences in serious infections (Analysis 19.4.1) and leukopenia (Analysis 19.4.2) between the two treatments.
19.4. Analysis.
Comparison 19 Maintenance therapy: extended versus standard azathioprine, Outcome 4 Adverse events.
Kidney function was not reported.
20: Leflunomide versus methotrexate
Metzler 2007 reported more relapses in the MTX group (13/28) compared to the leflunomide group (6/26) (Analysis 20.1.1). More major relapses were also reported in the MTX group (7/28) compared to the leflunomide group (1/26) (Analysis 20.1.2). There were more severe adverse events in the leflunomide group (5/26) than the MTX group (0/28) (Analysis 20.2.1). There were no differences between the groups for infection (Analysis 20.2.2) or leukopenia (Analysis 20.2.3).
20.1. Analysis.
Comparison 20 Maintenance therapy: leflunomide versus methotrexate, Outcome 1 Relapse.
20.2. Analysis.
Comparison 20 Maintenance therapy: leflunomide versus methotrexate, Outcome 2 Adverse events.
There were multiple methodological difficulties with this study addressed in the discussion section.
Death, kidney function, and dialysis were not reported.
21: Methotrexate versus cyclophosphamide
Maritati 2017 reported no differences in death (Analysis 21.1), relapse (Analysis 21.2.1), major relapse (Analysis 21.2.2), minor relapse (Analysis 21.2.3), or serious infection (Analysis 21.3.1) at 12 months. Leukopenia was more frequent in the CPA group (7/33) compared to the MTX group (3/38) (Analysis 21.3.2)
21.1. Analysis.
Comparison 21 Maintenance therapy: methotrexate versus cyclophosphamide, Outcome 1 Death.
21.2. Analysis.
Comparison 21 Maintenance therapy: methotrexate versus cyclophosphamide, Outcome 2 Relapse.
21.3. Analysis.
Comparison 21 Maintenance therapy: methotrexate versus cyclophosphamide, Outcome 3 Adverse events.
Kidney function and dialysis were not reported.
22: Tailored versus fixed rituximab
MAINRITSAN 2 2018 compared a tailored schedule of 500 mg rituximab infusion with a fixed schedule of 500 mg rituximab infusion and reported no differences for death (Analysis 22.1) and serious infection (Analysis 22.3.2) at 18 months. There were 6/81 major relapses in the tailored group and 3/81 in the fixed group (Analysis 22.2) and 26/81 serious adverse events in the tailored group and 31/81 in the fixed group (Analysis 22.3.1).
22.1. Analysis.
Comparison 22 Maintenance therapy: tailored versus fixed rituximab, Outcome 1 Death.
22.3. Analysis.
Comparison 22 Maintenance therapy: tailored versus fixed rituximab, Outcome 3 Adverse events.
22.2. Analysis.
Comparison 22 Maintenance therapy: tailored versus fixed rituximab, Outcome 2 Relapse.
Kidney function and dialysis were not reported.
23: Pre‐emptive therapy for relapse
Two studies investigated pre‐emptive therapy for relapse (Boomsma 2003; Tervaert 1990).
For patients with a rising ANCA, fewer relapses occur for those randomised to increased immunosuppression in both studies (Analysis 23.1 (2 studies, 60 participants): RR 0.23, 95% CI 0.03 to 1.59; I2 = 53%).
23.1. Analysis.
Comparison 23 Maintenance therapy: pre‐emptive therapy for relapse, Outcome 1 Relapse.
Death, kidney function, dialysis, adverse events, and infection were not reported.
24: Belimumab versus placebo
BREVAS 2019 compared belimumab to placebo and reported no difference to relapse (Analysis 24.1), any adverse event (Analysis 24.2.1) or infection (Analysis 24.2.2).
24.1. Analysis.
Comparison 24 Maintenance therapy: belimumab versus placebo, Outcome 1 Relapse.
24.2. Analysis.
Comparison 24 Maintenance therapy: belimumab versus placebo, Outcome 2 Adverse events.
Death, kidney function, and dialysis were not reported.
Discussion
Summary of main results
In this 2019 review update, an additional nine studies have been included since the 2015 update (a total of 40 studies, 3764 participants).
Remission induction
Plasma exchange as adjunctive therapy
This meta‐analysis shows that plasma exchange confers a significant benefit to many patients with RPGN by reducing the risk of ESKD at both three and 12 months from diagnosis. Szpirt 2011 supports this effect and also suggests that the benefit may be present at five years follow‐up. The 12‐month RR of 0.45 suggests that the number of patients requiring dialysis may be halved by this intervention. Previous studies have shown an effect in the most severely ill patients. A subgroup analysis in Pusey 1991 showed a benefit for patients requiring dialysis at presentation. More recently, MEPEX 2007 has shown a benefit for patients with SCr > 500 µM with ANCA‐associated vasculitis. The majority of patients included in these studies would meet the criteria for having severe AKI (SCr > 500 µM or dialysis required at presentation). It is therefore not clear whether plasma exchange has any impact in patients whose kidney failure is not severe. There was little statistical heterogeneity in all outcomes of these studies with the single exception of SCr at 12 months. Whilst the PEXIVAS results have been included in the review, they do not directly impact the outcomes at particular time points. At time of writing the final manuscript is not released and no data are available to the authors to enter data appropriately. The survival curves published suggest that there is an early effect of plasma exchange on the combined end point of death and dialysis but this has yet to be clarified by the research team. Currently the detailed evidence continues to suggest an effect of plasma exchange on reducing the requirement for dialysis treatment.
Pulse versus continuous cyclophosphamide
Pulse treatment with CPA was equivalent to continuous treatment for remission induction, but may increase the risk of relapse at the end of follow‐up. None of the studies were powered to answer the question of relapse rate since this would require either much larger studies or significantly longer follow‐up. We are therefore reliant on the results of meta‐analysis to attempt to provide an answer. This answer is less than perfect since it is a meta‐analysis of results at different times post treatment across studies with significantly different protocols. In spite of this, there is no evidence of heterogeneity in the outcome, suggesting that the final result is likely to be valid. This analysis is supported by the recent follow‐up data from CYCLOPS 2004 showing that patients treated with pulse CPA suffered a 39.5% relapse rate as opposed to 19.8% for continuous daily treatment. Though the rates of relapse with pulse CPA treatment are perhaps discouraging, this does not invalidate this mode of treatment. Pulse therapy still delivers a significantly lower total dose of CPA. For those patients who remain in remission, they have likely benefited in terms of risk of long term side effects. The MAINRITSAN study has unexpectedly provided an interesting insight into the increased relapse rate with pulse therapy. This has been the only study to date that has utilised only pulse cyclophosphamide for induction treatment. Patients were then randomised to rituximab or azathioprine for maintenance, with the study showing an improved relapse rate on rituximab. However, the overall rate of relapse in the rituximab limb was similar to the rate in the azathioprine limb of the IMPROVE study. The IMPROVE study patients were treated with oral cyclophosphamide for induction. This shows that relapse rate is highly dependent on the induction treatment, as well as the maintenance therapy used.
There is a trend towards more patients requiring dialysis with the use of pulse CPA therapy. This is currently not statistically significant, but the fact remains that there were twice as many patients requiring dialysis after pulse therapy and that this effect is present in all studies. This effect was not confirmed in the long term follow‐up of CYCLOPS 2004. CPA treatment was given for three months, approximately six months, one and two years in the four relevant studies. This difference may account for the significant level of statistical heterogeneity detected in death and the incidence of serious infections. Pulse therapy also caused significantly more nausea but less leukopenia and serious infections. In the light of data from CYCAZAREM 2003, it would seem reasonable to suggest that continuous oral CPA should be limited to three months treatment if the patient has achieved a sustained remission with a change to AZA for maintenance therapy. The optimal regimen for CPA administration for remission induction in ANCA‐associated vasculitis remains unclear.
Rituximab versus cyclophosphamide alone for remission induction
RITUXVAS 2010 and RAVE 2010 are two well‐designed studies showing that Rituximab is equivalent to CPA therapy for remission induction whilst side effects occur at a similar frequency, albeit possibly in a smaller number of patients with rituximab. The difference in remission rates of over 90% in RITUXVAS 2010 and 605 to 70% in RAVE 2010 at six months is of interest. The studies differed in their patient population (new versus new and relapsed), treatment protocols (rituximab with pulse CPA versus IV pulse CPA (RITUXVAS 2010) and rituximab alone against oral CPA (RAVE 2010)), and remission definitions. The patient populations differed in that all patients in RITUXVAS 2010 had kidney involvement as opposed to 52% in RAVE 2010. In a subgroup analysis of these patients in RAVE 2010, 61% of the rituximab group and 63% of the CPA group reached the primary endpoint. This does not account for the difference in remission rates between the studies. In RITUXVAS 2010, rituximab was given in conjunction with IV pulses of CPA whereas RAVE 2010 gave rituximab without concomitant CPA. CPA was given orally at 2 mg/kg/day in RAVE 2010 as opposed to the IV pulses in RITUXVAS 2010. There are no data to support a higher remission rate from pulse therapy per se. RITUXVAS 2010 defined remission as a BVAS of 0 for at least two months whereas RAVE 2010 defined remission as a BVAS of 0 and either no steroid treatment or less than 10 mg/day prednisolone. The latter figures from RAVE 2010 are included in this review. The inclusion of steroid doses in the definition of remission may be one of the main influences reducing the apparent remission rate in RAVE 2010.
Mycophenolate mofetil for remission induction
The data currently available on this question have improved markedly with the publication of the initial results of MYCYC 2012. The data now suggests that MMF is an equivalent induction agent to CPA. The next question is the subsequent relapse rate and this has not so far been addressed. If the relapse rate is particularly high, MMF may simply turn out to be an expensive prelude to subsequent CPA. Data from MYCYC 2012 will be available later on the relapse rate in their population.
The populations of patients studied in Han 2011b and Hu 2008b are significantly different from those in other studies, most obviously in the proportion of patients with MPO‐ANCA and MPA at 87%. This is significantly different from that reported from Europe where the majority of patients are PR3‐ANCA positive. The remission rate is also lower than that achieved in similar studies from Europe with only 44% of patients achieving remission (CYCAZAREM 2003) as opposed to over 90% in MYCYC 2012. The external validity of these studies and wider applicability of their results have improved with MYCYC 2012 data showing very similar findings in terms of the comparison with CPA. The predominantly European cohort has again shown a higher level of remission induction on both agents.
Han 2011b is very similar to Hu 2008b. A very similar population of patients diagnosed with MPA were randomised who were almost exclusively MPO‐ANCA positive. Hu 2008b excluded patients with severe and dialysis‐dependent kidney failure whereas Han 2011b did not, though there were only nine patients in this subgroup. Both studies treated with CPA for six months regardless of time to remission and outcomes quoted are at six months only. MYCYC 2012 treated to remission with a minimum of three months CPA and a maximum of six.
Methotrexate for remission induction
The single study (NORAM 2005) comparing the use of oral MTX with oral CPA showed that in patients with early disease and SCr < 150 µM, MTX is an effective induction agent. Time to remission may be a little longer with MTX though this was not conclusively shown. Side effects were similar on the two agents. The relapse rate in this study was high and the MTX group had a significantly higher rate than the CPA group. These data have been used to argue that 12 months of treatment is probably not adequate for patients with these diseases, especially for those with GPA who have a greater tendency to relapse. Considering the data on the utility of MTX as a maintenance agent, this study shows that MTX is a useful induction agent for patients with early systemic vasculitis. Longer term follow‐up of these patients showed that those treated with MTX had longer periods of treatment with other agents than those initially treated with CPA.
Avacopan versus prednisolone for remission induction
A single study (CLEAR 2013) comparing avacopan with prednisolone reported a higher mean eGFR (mL/min/1.73 m2) at three months with avacopan, but no differences between the two treatments for remission. It is currently not clear how a complement inhibitor might fit into a treatment strategy for vasculitis or what the costs and benefits of such a treatment might be.
Intravenous immunoglobulin use for refractory vasculitis
The single study in this area suggests a short‐term benefit lasting no more than three months (Jayne 2000). The treatment can be viewed as a therapy available to help induce remission but has little bearing on the longer term problem of remission maintenance.
Lymphocytapheresis and immunoadsorption
Lymphocytapheresis, described by Furuta 1998, gives some benefit when compared with three weeks of IV pulse methylprednisolone with a significantly lower SCr in treated patients. There was however no change in either the need for dialysis treatment or death at six months. Considering the lack of a comparison with plasma exchange and the recent data suggesting the use of plasma exchange is superior to pulse methylprednisolone, there is currently no compelling reason to consider using this therapy in these conditions. Immunoadsorption, similarly, appears to have no benefit over the use of plasma exchange.
Duration (6 versus 12) of cyclophosphamide induction for remission induction
Two studies compared six pulses with 12 pulses of CPA and no significant differences were found between the two treatments in measuring death, remission, relapse, or infections (CORTAGE 2015; Guillevin 2003). CORTAGE 2015 reported less severe adverse events in patients receiving six pulses.
The numbers used in this review include only MPA patients whereas the original paper also included PAN. The relapse rate was high in the PAN patients treated with six pulses and this gave a significant result on survival analysis. Including all patients in our analysis still did not quite reach statistical significance. This study does not reflect current practice and has in some ways been superseded by CYCAZAREM 2003 which compared a short to a long course of CPA but also included maintenance therapy in the form of AZA. With the inclusion of patients with PAN, the absence of maintenance therapy and its inadequate size, this study is rather difficult to interpret.
Reduced dose versus standard dose steroids for remission induction
PEXIVAS 2013 compared two different doses of steroid and found no differences between the two treatments in terms of remission induction, but did show a reduction in infections with the lower dose steroids. The lower dose of steroids therefore appears to be effective and safer.
Etanercept for remission induction
The stated aim of the single study into the use of etanercept in systemic vasculitis was to demonstrate that the relapse rate would be reduced (WGET 2002). The study failed to show this and also suggested an increase in the incidence of malignancy in treated patients. There are currently no RCT data on the use of infliximab or other anti‐TNF agents. There is some possibility that alternative agents may produce significantly different outcomes since their mechanism of action is distinct from that of etanercept. At this point in time there is no RCT data supporting their use.
Maintenance therapy
Azathioprine versus cyclophosphamide for maintenance therapy
The use of AZA as maintenance therapy after an initial three month treatment with CPA is strongly supported by the data from CYCAZAREM 2003. The number of relapses on AZA is similar to CPA with fewer episodes of leukopenia and similar numbers of infections. As well as the data on reduced leukopenia, the reduction in total dose of CPA is presumed to reduce longer term side effects from CPA such as infertility and neoplasia.
Azathioprine versus mycophenolate mofetil for maintenance therapy
IMPROVE 2003 was designed to test the hypothesis that MMF would be superior to AZA in remission maintenance but showed the opposite with an increased risk of relapse with MMF. Interestingly the separation of the groups started within the first 12 months of maintenance therapy when patients were treated at full dose of MMF. Major relapses appeared after the first year and could perhaps be due to a reduction in therapy. The study is clear, however, in rejecting MMF as a superior alternative to AZA for maintenance therapy.
Azathioprine versus methotrexate for maintenance therapy
WEGENT 2008 showed that the safety and efficacy profiles of MTX and AZA are comparable. The dosing regimen for MTX in this study was superior to that in the leflunomide/MTX study since the rate of rise in dose was faster and the final dose higher (Metzler 2007).
Rituximab versus azathioprine for maintenance therapy
MAINRITSAN 2014 found less major relapses when comparing rituximab to azathioprine, at one and two years and 28 months. There are, however some concerns with the data from this study. As mentioned above, this is one of the first studies where on pulse CPA was used for induction treatment, rather than a mix of oral and pulse CPA and rituximab. The relapse rate was high for the study with the relapse rate achieved with rituximab equivalent to that achieved with AZA in the IMPROVE study. The second problem is that the majority of relapses in this study were major relapses. This has not been reported previously. Whilst rituximab is clearly a superior agent compared to AZA, the final relapse rate achieved is a function of both the induction and maintenance regimens. The relapse rate achieved in the MAINRITSAN study is no better than that achieved previously with different induction regimens.
Co‐trimoxazole (antibiotics) versus placebo for maintenance of remission
The use of co‐trimoxazole to maintain remission was examined by Stegeman 1996. This study showed a benefit in reducing the risk of relapse but not on other outcomes. Analysis in the paper by life table analysis showed this result to be statistically significant. Our analysis found no difference (P = 0.12). Relapses detected in the study were mainly respiratory in nature but 11/23 patients with a relapse also had progressive glomerulonephritis. Zycinska 2009 adds some data to this but still does not clearly answer the question. There were some major limitations in the reporting of this study. Patients were said to be in remission at randomisation but the mean BVAS of the placebo group was 11 (remission is 0). There was no reporting of relapse, only numbers of patients in remission. A firm conclusion is not possible on the available data.
Cyclosporin versus cyclophosphamide for maintenance therapy
The limited data available suggest that there may be a higher relapse rate with the use of cyclosporin. The single trial was small. It is not possible to be conclusive.
Extended versus standard length Azathioprine maintenance therapy
The data strongly support continuation of immunosuppressive treatment with Azathioprine out to approximately 4 years from diagnosis. The REMAIN study was conducted in patients at fairly low risk of relapse. All patients had gone into remission with induction treatment and had no relapses by 18 months post diagnosis. When treatment was tapered, the relapse rate was high over the subsequent two years. This would suggest that patients are safer to continue immunosuppression for at least 4 years after diagnosis and possibly longer for patients at higher risk of relapse.
Leflunomide versus methotrexate for maintenance therapy
The single study of leflunomide suggests that this may be an appropriate treatment for patients who are intolerant of AZA (Metzler 2007). There are problems with interpretation and the external validity of this study. The dose of MTX was increased very slowly. Many commentators felt this to be an inadequate dose, potentially causing the higher relapse rate and inadequately reflecting the potential of MTX in this area. There were also a high number of adverse events in the leflunomide arm. The study does however give some data on the use of leflunomide and grounds for its clinical use. Final conclusions are difficult to draw. Further study of leflunomide is warranted as induction therapy and in comparison to AZA as maintenance therapy
Methotrexate versus cyclophosphamide for maintenance therapy
In a single study, no differences were found when comparing methotrexate with cyclophosphamide for the outcomes of death or relapse.
Tailored versus fixed rituximab for maintenance therapy
A single study compared a tailored schedule of 500mg rituximab infusion with a fixed schedule of 500mg rituximab infusion and reported no differences between treatment groups for death, major relapse, or serious infection at 18 months, but did find severe adverse events to be higher in the fixed schedule group. It is not easy to make clear conclusions from this study. The event rate was low, so the study was under‐powered. There were numerically more relapses (both total and major) in the tailored therapy arm. Also 25% more of the patients were left ANCA positive at the end of treatment compared to the fixed schedule infusion group
Pre‐emptive therapy for relapse
Two studies showed that patients will relapse less often on low level immunosuppression with either AZA or CPA plus prednisolone rather than no change in their treatment (Boomsma 2003; Tervaert 1990). It is difficult to interpret anything else from these studies. At the time they were undertaken, there was some suggestion that an asymptomatic rise in ANCA titre was likely to be a good predictor of imminent relapse. Current literature does not support that hypothesis. Boomsma 2003 was published as an abstract only. It would be interesting to know how many of the patients without an asymptomatic rise had a subsequent relapse. The abstract notes that after immunosuppression, patients went on to have relapses. Those treated did not appear to benefit from a long term protection from relapse.
Belimumab versus placebo for maintenance therapy
BREVAS 2019 compared belimumab to placebo and reported no difference to relapse, any adverse event, or infection. There is currently no evidence for the use of belimumab in the treatment of vasculitis
Overall completeness and applicability of evidence
The data on the treatment of vasculitis remain incomplete. However, this review summarises a significant body of research that represents some high quality data which clearly goes some way to giving guidance on treatment. The areas of the review with data from multiple studies are probably the most helpful and applicable. In these areas, the earlier studies carried multiple problems in disease ascertainment and methodology but their overall conclusions have generally been borne out by the later larger studies. This is reassuring on applicability. In some areas there are many further questions over how to deploy expensive and potentially harmful treatments. One example here is the data on the use of plasma exchange. This review suggests that it is a highly effective therapy when deployed in a particular group of patients, however this result rests on a relatively small number of outcomes. Questions remain as to whether it is a true effect and which patient groups will benefit. The PEXIVAS study should go some way to clarifying this. Data released so far are analyses over the whole seven‐year course of the study and suggest that plasma exchange is not effective in the long term for reducing either death or dialysis. However, the published survival analyses suggest there may well be some effect within the first year on both death and the need for dialysis treatment. The majority of the data in this review is in patients with very poor kidney function. It may also work for patients with good kidney function. This hypothesis is still to be tested. For each of the areas of this review there are multiple such questions still to be answered.
Quality of the evidence
The strength of this review rests on the breadth of the literature search which included non‐English language studies. Unpublished individual patient data were obtained from Adu 1997. This is the first systematic review to cover all areas of renal vasculitis. The review is limited by the small number of available studies answering particular questions and some design features of the included studies. Several older studies included diagnoses other than renal vasculitis. Some date prior to the development of the ANCA assay. This will limit the validity of the data and diagnoses included in those studies. Other differences include those between interventions, notably the regimens of immunosuppressive drugs and the number and volume of plasma exchanges utilised. As noted above, some maintenance treatment studies appear to be influenced by the induction regimen dictated by the protocol, making relapse rates difficult to compare across studies. Some of these issues may have had a very significant impact on the outcomes of studies and may explain the level of heterogeneity in some of the results. Studies of renal vasculitis are notoriously difficult to carry out due to the low incidence of the disease and consequent need for broad collaboration to attain patient numbers for adequately powered studies.
The earlier studies included in this review suffered from some significant methodological problems and inclusion of a wide range of diagnoses which may not have been well validated. The more recent data are of much higher quality. These have generated a significant body of high quality data.
Potential biases in the review process
We have attempted to avoid bias in our review process, including all studies that are available in the area. They have been examined with standard processes. For each update, we have followed our methods from the original protocol, or used revised methods as recommended by Cochrane (e.g. Risk of Bias and GRADE assessment).
Agreements and disagreements with other studies or reviews
Three previous reviews have covered some of the subjects addressed in this review.
Bosch 2007 provides a broad review of the treatment of ANCA‐associated vasculitis. This includes patients with localized disease and those without renal vasculitis. They include a large number of uncontrolled studies and there was no attempt at meta‐analysis. In the area of severe vasculitis with kidney involvement, there is a brief summary of the RCT data as included in this review. Their conclusions are similar to ours.
de Groot 2001 is a review of the data relating to the use of pulse or continuous CPA for induction of remission of ANCA‐associated vasculitis and includes a meta‐analysis of the RCT data. As such, it performs a similar meta‐analysis to ours in this area. There are, however, a number of differences. de Groot 2001 has utilised all the data from Adu 1997. We have extracted the data only for patients without PAN and with some evidence of glomerular involvement. This accounts for some of the differences but not all. de Groot 2001 reports that treatment failure is more likely with continuous treatment with CPA. This is based on 8/25 patients, in Adu 1997, failing treatment. Adu 1997 reports 4/30 patients failed remission induction. Of those, 1/20 suffered treatment failure in the data we have extracted. de Groot 2001 quotes 4/25 patients failing treatment on continuous treatment in Haubitz 1998. Our understanding of this paper suggests that of those four patients, three had in fact died, mostly of sepsis. This is not entirely clear from the paper and we have been so far unable to substantiate this further. Each of these changes contributes to the overall effect in de Groot 2001 showing an increased risk of treatment failure on continuous treatment. Currently we do not believe that a close inspection of the data bears this out. The difference in figures here is also reflected in the results for relapse rate. Our results show that continuous treatment is significantly better at preventing relapse. We have studied relapse as related to the initial number of patients whereas de Groot 2001 have recorded relapses as related to achieved remissions. With the higher treatment failure figures in the continuous arm, de Groot 2001 does not show a significant difference in the overall relapse rate.
Walsh 2011 performed a similar systematic review to ours in the area of plasma exchange and its effect on ESKD, death and a combined endpoint of both. They demonstrated similar results to ours with a profound reduction in the development of ESKD and no effect on death. They then combined those two endpoints to argue that there was little conclusive evidence for the overall effect of plasma exchange on the "hard" endpoint of ESKD and death combined. It is currently our view that combining two outcomes with markedly different results does not serve to appropriately highlight the efficacy of plasma exchange. The current data suggests a striking reduction in the numbers of patients requiring KRT with no change in the risk of death.
Authors' conclusions
Implications for practice.
Plasma exchange is effective in patients with severe AKI secondary to vasculitis. It is not yet clear whether the results of the PEXIVAS study alter this conclusion. On current data, the use of pulse CPA results in an increased risk of relapse when compared to continuous use but a reduced total CPA dose. Rituximab and MMF are comparable to CPA as induction agents. IVIg is useful but only as a short‐term measure. The PEXIVAS protocol for lower dose glucocorticoid induction treatment appears to be safer than the higher dose with equivalent efficacy for remission induction.
AZA and MTX are effective as maintenance therapy once remission has been achieved. The use of MMF in remission maintenance should be third line after failure, or contraindication, of other agents such as AZA and MTX. Rituximab is a superior agent to AZA for maintenance treatment. The use of fixed interval dosing of rituximab may be more efficacious than tailored interval dosing but requires further study. Patients are likely to benefit from at least four years immunosuppression post diagnosis of renal vasculitis.
The use of co‐trimoxazole is not supported for prevention of relapse of vasculitis. Etanercept and belimumab are not recommended for use in vasculitis. Leflunomide may be useful as maintenance therapy but requires further evaluation.
Implications for research.
The exact place of plasma exchange in the treatment of vasculitis requires further analysis of the huge dataset behind the PEXIVAS study. Clarity is required over the effect of treatments within the first 12 months of treatment. Whilst the "hard endpoints" at the end of the study are of great clinical and administrative concern, patients will find an extra 6 months alive and off dialysis of significant benefit. Also the use of plasma exchange in pulmonary haemorrhage has not been finally clarified.
The optimal dose of steroids to be used in induction treatment requires further study. Can the PEXIVAS study lower dose be halved without loss of efficacy? Should steroid treatment be more "front‐loaded" with a higher percentage of the dose being given in the first 2 to 4 weeks of treatment with a more rapid dose reduction? Previously the currently side‐lined MEPEX study clearly showed improved outcomes with plasma exchange rather than methylprednisolone. Should the first two weeks of prednisolone be replaced with alternate day plasma exchange with or without a complement inhibitor?
The data from the MAINRITSAN study seem to confirm previous findings that the use of pulse CPA induction therapy increase the risk of subsequent relapse. Whilst this was an appropriate strategy to increase the event rate in this study, it also gave a relapse rate that was no improvement upon the rate achieved in the IMPROVE study with induction using oral CPA and maintenance with AZA. The IMPROVE study regimen would then be the cheaper option for countries unable to access rituximab treatment. Studies are required that examine the long term effects of the main induction regimens (now oral and pulse CPA, rituximab and MMF) on both remission and relapse rate. One hypothesis would be that there are optimal induction/maintenance combinations that are likely to give equivalent outcomes over the longer term.
The use of AVACOPAN in treatment protocols needs further research. An initial study using the drug as steroid replacement has been successful, but this currently seems an unlikely clinical strategy in the short term due to clinicians' familiarity with steroids and their very low price. Equivalence with glucocorticoid seems unlikely to be an adequate reason for complement inhibitors to feature significantly in treatment protocols in vasculitis.
The AVACOPAN study does illustrate that the strategy of comparing gold standard treatment with a new treatment is potentially more likely to reveal new treatment modalities rather than the adjunctive treatment approach where gold standard therapy is given to both treatment arms and the new therapy is added. The etanercept and belimumab studies are "adjunctive therapy" approaches that have failed. The Avacopan study approach requires more courage, more complex ethical consideration and, potentially, a graded experimental design but may be more likely to be successful.
The studies in this review reflect the solid success of the collaborative efforts of the vasculitis networks which have culminated in the recent PEXIVAS study. Building on this success will hopefully continue to provide answers to the many questions remaining.
What's new
| Date | Event | Description |
|---|---|---|
| 19 December 2019 | New citation required and conclusions have changed | New interventions added |
| 19 December 2019 | New search has been performed | New studies added; SoF tables added |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 25 August 2015 | New citation required and conclusions have changed | New interventions included |
| 25 August 2015 | New search has been performed | Multiple new studies included; methodology updated |
| 20 March 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to thank,
Dr Peter Kerr for his contribution to the protocol of this review.
The referees for their editorial advice during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Appendix 3. GRADE approach: rating the certainty of the evidence
The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome (GRADE 2008; GRADE 2011; Higgins 2011). The GRADE system uses the following criteria for assigning the grade of evidence.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Reasons for decreasing grade are due to:
Serious (–1) or very serious (–2) limitation to study quality;
Important inconsistency (–1);
Some (–1) or major (–2) uncertainty about directness;
Imprecise or sparse data (–1);
High probability of reporting bias (–1).
Appendix 4. Study criteria ‐ plasma exchange
| Study ID | Inclusion criteria | Exclusion criteria |
| Cole 1992 | RPGN of undefined aetiology (idiopathic or post‐infectious disease) with specific pathologic criteria; adults (16 to 75 years); normal sized kidneys SCr > 170 μmol/L, increasing by 44 μmol/week or both; no evidence of systemic disease or anti‐GBM antibody‐induced disease; renal biopsy within 5 days of study entry | Cellular crescents in < 50% non‐obsolescent glomeruli; evidence of serious infection or active ulcer disease |
| Glockner 1988 | RPGN with >70% crescents on kidney biopsy; CrCl < 50 mL/min; urine output >200 mL/24 h | Anti‐GBM disease; life threatening conditions; contraindications to immunosuppression; previous treatment with AZA or CPA for > 14 days |
| Mauri 1985 | Histologically proven crescentic GN and rapidly progressive kidney impairment | < 60% glomerular involvement, primary glomerulopathies, transplanted kidneys, SLE, HSP |
| MEPEX 2007 | Biopsy‐proven ANCA‐associated necrotizing GN with AKI (SCr > 500 μmol/L) | Aged < 18 years or > 80 years; inadequate contraception; pregnancy; previous malignancy; hepatitis B antigenaemia or hepatitis C antibody or HIV infection; other multisystem autoimmune disease; circulating anti‐GBM antibody or linear staining of GBM on histology; life‐threatening non‐renal manifestations of vasculitis; dialysis for > 2 weeks before entry; Cr > 200 µM more than 1 year before entry; > 2 weeks treatment with CPA or AZA; > 500 mg of IV MP; PE within the preceding year; > 3 months treatment with oral prednisolone; allergy to study medications |
| PEXIVAS 2013 | New or previous clinical diagnosis of granulomatosis with polyangiitis or microscopic polyangiitis consistent with the Chapel‐Hill consensus definitions AND positive test for proteinase 3‐ANCA or myeloperoxidase‐ANCA AND severe vasculitis defined by at least one of the following: Renal involvement with both: renal biopsy demonstrating focal necrotizing glomerulonephritis or active urine sediment characterized by glomerular haematuria or red cell casts and proteinuria AND eGFR <50 mL/min/1.73 m2. Pulmonary haemorrhage due to active vasculitis defined by a compatible chest X‐ray or CT scan (diffuse pulmonary infiltrates) AND the absence of an alternative explanation for all pulmonary infiltrates (e.g. volume overload or pulmonary infection) AND At least one of the following: evidence of alveolar haemorrhage on bronchoscopic examination or increasingly bloody returns with bronchoalveolar lavage; observed haemoptysis. Unexplained anaemia (< 10 g/dL) or documented drop in Hb >1 g/dL); increased diffusing capacity of carbon dioxide |
Diagnosis of vasculitis other than granulomatosis with polyangiitis or microscopic polyangiitis; positive anti‐glomerular basement membrane antibody test or renal biopsy demonstrating linear glomerular immunoglobulin deposition; receipt of dialysis for > 21 days immediately prior to randomisation or prior renal transplant; aged < 15 years; pregnancy; inability or unwillingness to comply with birth control/abstinence; treatment with > 1 IV dose of CPA and/or > 14 days of oral CPA and/or > 14 days of prednisone/prednisolone (> 30 mg/day) and/or >1 dose of RTX within the 28 days immediately prior to randomisation; a comorbidity that, in the opinion of the investigator, precludes the use of CPA, glucocorticoids, or PE or absolutely mandates the use of PE |
| Pusey 1991 | Focal necrotizing GN with crescents (WG, systemic vasculitis, polyarteritis, idiopathic RPGN) | Anti‐GBM disease; SLE; HSP; chronic GN; previously treated with IV MP, oral CPA or PE |
| Rifle 1980 | New onset RPGN with > 50% glomerular crescents | Goodpasture's syndrome; IgA nephropathies; SLE; systemic disease |
| Szpirt 2011 | All patients with a new diagnosis of WG who were c‐ANCA or PR3‐ANCA positive; clinical manifestations as defined by Fauci 1973 from at least 2 organ systems, histology proven WG and positive ANCE by IIF and ELISA; all patients fulfilled the ACR 1990 classification for WG | Not reported |
| Zauner 2002 | Patients with a clinical picture of RPGN and Couser Type II or III (immune deposits or pauci‐immune respectively) | Couser Type I GN (linear GBM Ab staining on biopsy), previous immunosuppression or PE |
Footnotes: ACR ‐ albumin creatinine ration; AKI ‐ acute kidney injury; ANCA ‐ anti‐neutrophil cytoplasmic antibody; anti‐GBM ‐ anti‐glomerular basement membrane; AZA ‐ azathioprine; CPA ‐ cyclophosphamide; Cr ‐ creatinine; CrCl ‐ creatinine clearance; GN ‐ glomerulonephritis; HSP ‐ Henoch‐Schonlein Purpura; IV ‐ intravenous; MP ‐ methylprednisolone; PE ‐ plasma exchange; PR3 ‐ proteinase‐3; RPGN ‐ rapidly progressive glomerulonephritis; SCr ‐ serum creatinine; SLE ‐ systemic lupus erythematosus; WG ‐ Wegener's granulomatosis
Appendix 5. Treatment regimens and study outcomes ‐ plasma exchange
| Study ID | Treatment | Control | Study outcomes |
| Cole 1992 | Immunosuppression as for control group PE: at least 10 PE treatments within 16 days of study entry; 1 plasma volume with complete replacement using 5% albumin + crystalloid |
Immunosuppression IV MP: 10 mg/kg/day for 3 days followed by prednisone 1.4 mg/kg/day for next 4 days and then tapered to 1 mg/kg/day over 2 weeks; 0.35 mg/kg/day at 1 month and 025 mg/kg/day at 2 months AZA: 1.5 to 3.0 mg/kg/day with dose adjustment as necessary to ensure neutrophil count of ≥ 2.0 x 109/L |
Kidney pathology Patients on dialysis at randomisation: dialysis at 1, 3, 6, 12 months Kidney function in patients not on dialysis: 1, 3, 6, 12 months Change in SCr Adverse events (serious infections, gastrointestinal bleeding) Death |
| Glockner 1988 | PE: 9 x 50 mL/kg over 4 weeks replaced with 3% to 5% albumin solution Immunosuppression as for control group |
Immunosuppression
|
Death at 6 months Dialysis at 6 months SCr at 4 weeks, 8 weeks and 6 months Adverse events including serious infections, GI haemorrhage and anaphylaxis |
| Mauri 1985 | CPA and prednisolone as for control group PE alternate days for 6 treatments
|
CPA: 2 mg/kg/day
Prednisolone: 1 mg/kg/day
|
Death Dialysis post treatment, at 3 months and 12 months after treatment SCr after treatment and 6 months later |
| MEPEX 2007 | Immunosuppression as for the control group PE: 7 x 60 mL/kg in first 2 weeks after diagnosis |
Immunosuppression
|
Death at 3 and 12 months Dialysis at 3 and 12 months Side effects SCr at 12 months |
| PEXIVAS 2013 | Treatment group 1 (a, b)
Adjunctive PE: 7 exchanges over 14 days 60 mL/kg Treatment group 2 (a, b) No plasma exchange |
Treatment group 1a, 2a
Reduced dose prednisolone Treatment group 1b, 2b Full dose prednisolone |
Time to the composite of death from any cause and ESKD Death Dialysis Quality of life Serious infections Serious adverse events Sustained remission |
| Pusey 1991 | Induction/maintenance therapy as for control group PE: 5 x 4 L exchanges of 5% albumin (plasma protein fraction) within first week. Two units of fresh frozen plasma were given at end of exchange. Total number of exchanges determined by clinical response |
Induction therapy, 8 weeks of:
Maintenance therapy
|
Improvement (fall in SCr > 25% or rise in CrCl > 25%; recovery of kidney function in those initially on dialysis) SCr Dialysis Death Adverse events |
| Rifle 1980 | Immunosuppression as per control group PE: 5 sessions during 5 successive days, then 3 sessions/week until 15 days after SCr reached a plateau
|
Immunosuppression
CPA: 2 to 3 mg/kg/day for 2 months Calcium heparinate 9 days after kidney biopsy for the duration of the study |
Dialysis: 2, 6 12, 24 months CrCl: 2, 6 and 12 months. Recovery (off dialysis) according to initial SCr level Recovery (off dialysis) according to initial % of crescents Death Circulating immune complexes Pathology changes Adverse events (septicaemia) |
| Szpirt 2011 | Immunosuppression as for control group PE: 6 sessions of 4L PE with 3% albumin in Ringer's lactate solution replacement on alternate days. Performed using Gambro F‐1000 filters. If c‐ANCA titres > 320 or PR3‐ANCA > 25 U/mL on ELISA after 6 sessions the additional 3 to 6 sessions performed |
Immunosuppression
|
Kidney outcomes: progression, remission and dialysis at 1, 3 and 12 months
Relapse: clinical symptoms of active disease and at least 2 of: a 2‐fold increase in ANCA titre, 20% increase in Cr, increase in proteinuria and increase in ESR or CRP Kidney and patient survival to 5 years |
| Zauner 2002 | Immunosuppression as for control group PE: 40 mL/kg with FFP replacement daily for 3 exchanges, continued if no response to a maximum of 12 exchanges; mean number of PE = 6 |
Immunosuppression
|
Death ESKD SCr Adverse events |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; c‐ANCA ‐ cytoplasmic‐ANCA; AZA ‐ azathioprine; CPA ‐ cyclophosphamide; Cr ‐ creatinine; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; ESKD ‐ end‐stage kidney disease; ESR ‐ erythrocyte sedimentation rate; IV ‐ intravenous; MP ‐ methylprednisolone; PE ‐ plasma exchange; PR3 ‐ proteinase‐3; SCr ‐ serum creatinine; WG ‐ Wegener's granulomatosis
Appendix 6. Study criteria ‐ pulse versus continuous cyclophosphamide
| Study ID | Inclusion criteria | Exclusion criteria |
| Adu 1997 | Patients 15 to 70 years with new‐onset systemic necrotizing vasculitis. WG, classical PAN and MPA diagnosed by histological or radiological evidence | Not reported |
| CYCLOPS 2004 | Newly diagnosed WG, MPA, or renal‐limited MPA, kidney involvement: at least one of: SCr > 150 µmol/L and < 500 µmol/L, biopsy evidence of necrotizing GN, erythrocyte casts, or haematuria and proteinuria, confirmatory histology or ANCA positivity | Other multisystem autoimmune disease; hepatitis B or C virus or HIV infection/ SCr > 500 µmol/L; previous cancer; pregnancy; < 18 years or > 80 years |
| Guillevin 1997 | Aged > 15 years; new diagnosis of systemic WG diagnosed clinically based on the presence of multiorgan involvement; monovisceral involvement representing a potential risk or severe morbidity of fatality; histopathologic characterization of necrotizing granulomatosis vasculitis or evidence of either granulomatous inflammation and vasculitis or segmental necrotizing GN | Not reported |
| Haubitz 1998 | New diagnoses of WG and MPA and renal involvement; biopsy performed | < 18 years; pregnancy; HIV; malignancy; SCr > 200 μmol/L more than 1 year before presentation; cytotoxic drug therapy for > 1 week before start of study; HD for > 10 days before start of study |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; GN ‐ glomerulonephritis; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; MPA ‐ microscopic polyangiitis; PAN ‐ polyarteritis nodosa; SCr ‐ serum creatinine; WG ‐ Wegener's granulomatosis
Appendix 7. Treatment regimens and study outcomes ‐ pulse versus continuous cyclophosphamide
| Study ID | Treatment | Control | Study outcomes |
| Adu 1997 | PCYP
|
CCAZP
|
Complete and partial remission Relapse Adverse events Treatment failure Chronic dialysis |
| CYCLOPS 2004 | Immunosuppression as for control group Pulse CPA
|
Immunosuppression
Continuous CPA
|
Time to remission Proportion of patients who achieved remission at 6 and 9 months Proportion with major and minor relapses Death Change in kidney function Adverse events Cumulative dose of CPA and prednisolone. calculated at 3, 6, 9, 12, 15 and 18 months |
| Guillevin 1997 | Initial regimen as for control group IV pulse CPA
|
Initial regimen
Oral CPA
|
Treatment failure Complete remission Partial remission Relapse Death Side effects |
| Haubitz 1998 | Steroid regime as for control group IV pulse CPA
Antiemetic drugs were given immediately before and 8 h after treatment. At least 3 L of fluid was administered on day of CPA treatment |
Steroid regime
Oral daily CPA
|
Complete remission Partial remission Relapse Serious infection ESR, CRP, ANCA, Hb, WBC platelet count, SCr, urea, CrCl, quantitative proteinuria, urinary microscopy, alanine aminotransferase, aspartate aminotransferase, gonadal toxicity (FSH) |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; AZA ‐ azathioprine; CCAZP ‐ continuous CPA, then AZA and prednisolone; CPA ‐ cyclophosphamide; Hb ‐ haemoglobin; IV ‐ intravenous; MP ‐ methylprednisolone; PCYP ‐ pulse cyclophosphamide and prednisolone; SCr ‐ serum creatinine
Appendix 8. Study criteria ‐ other remission induction studies
| Study ID | Inclusion criteria | Exclusion criteria |
| BREVAS 2019 | Eligible patients were ages ≥ 18 years, had a clinical diagnosis of GPA or MPA according to the 2012 Chapel Hill Consensus Conference definitions, and tested positive (current or historical) for either PR3‐ANCAs or MPO‐ANCAs. Patients must have experienced either new‐onset or relapsing GPA or MPA in the 26 weeks prior to day 0, that required treatment under one of the following remission induction regimens: a single course of rituximab (375 mg/m2 /week for 4 weeks) plus high‐dose glucocorticoids; 2 doses of IV rituximab (1 gm), separated by a 2‐week interval, plus high‐dose glucocorticoids; oral cyclophosphamide (2 mg/kg/day); or pulses of IV cyclophosphamide (15 mg/kg), administered 2 weeks apart for 3 doses followed by further pulses every 3 weeks, plus high‐dose glucocorticoids. Additionally, patients had to be in remission on day 0 (with remission defined as a BVAS score; of 0) and receiving glucocorticoids (presented as prednisone‐equivalent doses) at ≤ 10 mg/day (on 2 consecutive measurements ≥ 14 days apart, and 6–26 weeks after the first dose of induction therapy) | Coexistence of another autoimmune disease; any known intolerance or contraindications to azathioprine and methotrexate; receipt of any B cell–targeted therapy (excluding rituximab) at any time, or any other investigational agent within 60 days of day 0 or 5 half‐lives of the agent (whichever was longest); any acute or chronic infections requiring hospitalisation (within 60 days of day 0) and/or receipt of parenteral antibacterial drugs, antiviral drugs, antifungal drugs, or antiparasitic drugs (within 60 days of day 0); and serologic evidence of infection with human immunodeficiency virus, hepatitis B virus, or hepatitis C virus |
| CLEAR 2013 | ≥ 18 years with newly diagnosed or relapsing granulomatosis with polyangiitis (Wegener's) or microscopic polyangiitis according to the Chapel Hill Consensus Conference definitions 1) required CPA treatment (steps 1 and 2), CPA or rituximab (step 3), were PR3 or MPO‐ANCA positive or ANCA positive by indirect immunofluorescence, had an eGFR ≥ 20 mL/min/1.73 m2, biopsy‐proven renal vasculitis or haematuria (> 30 RBC/HPF or greater than 2+ by urine dipstick) plus albuminuria (at least 0.5 g/g creatinine) for steps 1 and 2, or had at least one major or three non‐major items, or at least two renal items on the BVAS version 3 2 for step 3. | Severe disease (including RPGN, alveolar haemorrhage leading to grade 3 hypoxia, rapid‐onset mononeuritis multiplex, or central nervous system involvement); any other autoimmune disease, coagulopathy or bleeding disorder; received CPA within 12 weeks, rituximab within 12 months prior to screening (or 6 months with B‐cell reconstitution, CD19 count > 0.01 x 109/L); cumulative dose of IV glucocorticoids > 3 g within 12 weeks, or oral glucocorticoids > 10 mg/day prednisone equivalent for more than 6 weeks prior to screening. |
| CORTAGE 2015 | Patients with newly diagnosed PAN not related to hepatitis B virus infection, EGPA, GPA, or MPA; 2) to satisfy the 1990 American College of Rheumatology criteria and/or 1994 Chapel Hill nomenclature definitions (10–13); 3) to be in or after the year of their 65th birthday at the time of SNV diagnosis; and 4) to provide written informed consent. Patients could have started corticosteroids, but for no more than 1 month prior to enrolment, and could not have started CYC and/or received any other immunosuppressant before inclusion | Not reported |
| Furuta 1998 | Biopsy‐proven RPGN | Not reported |
| Guillevin 2003 | New diagnosis of PAN or MPA with at least one of five factors: Cr > 1.58 mg/dL (140 µM), proteinuria > 1 g/day, severe GI involvement, cardiomyopathy, CNS involvement | Not reported |
| Han 2011b | Patients with MPA with moderate to severe renal involvement (MPA by Chapel Hill Nomenclature) | Severe lung haemorrhage (haemoptysis > 300 mL/24 h or hypoxaemia); CNS involvement; other life‐threatening situations; cytotoxic drug in the previous 6 months; severe infection in the last month; active hepatitis or abnormal liver function; pregnancy; malignancies; > 70 years |
| Hu 2008b | Newly diagnosed active ANCA‐associated vasculitis; > 18 years of age with kidney involvement with SCr < 500 µM; ANCA positive or ANCA negative with confirmatory kidney biopsy | Cytotoxic drug treatment in 6 months prior; HBV, HCV, HIV or active CMV viral infection; acquired immune deficiency; severe kidney failure with Cr > 500 µM or on KRT; life‐threatening organ manifestations (lung haemorrhage or CNS involvement); active TB; liver dysfunction; pregnancy or inadequate contraception if female; < 18 years or > 65 years |
| Jayne 2000 | Prior diagnosis of WG or MPA; ANCA positivity at diagnosis; active vasculitis with a requirement for further therapy; at least 2 months treatment with prednisolone and CPA or AZA; ≥ 18 years | IVIg in previous 3 months; history of anaphylaxis to matched blood products; selective IgA deficiency; RPGN (20% rise in SCr in 2 weeks) or pulmonary haemorrhage |
| MYCYC 2012 | New diagnosis of ANCA‐associated systemic vasculitis (WG or MPA) (within the previous six months); active disease (defined by at least one major or three minor BVAS 2003 items); ANCA positivity (c‐ANCA and PR3‐ANCA or p‐ANCA and MPO‐ANCA) or histology confirming active vasculitis from any organ. | Previous treatment with MMF (more than two weeks ever), CPA (more than two weeks daily oral or more than 1 pulse of IV CPA 15 mg/kg), rituximab or high dose IVIg within the last 12 months; active infection (including HBV, HCV, HIV and TB); known hypersensitivity to MMF, AZA or CPA; cancer or an individual history of cancer (other than resected basal cell skin carcinoma); pregnant, breast feeding, or at risk of pregnancy and not using a medically acceptable form of contraception; any condition judged by the investigator that would cause the study to be detrimental to the patient; any other multi‐system autoimmune disease including Churg Strauss angiitis, SLE, anti‐GBM disease and cryoglobulinaemia; active serious digestive system disease (e.g. inflammatory bowel disease); imminently life threatening vasculitis (diffuse alveolar haemorrhage, intestinal perforation or major haemorrhage, cerebral vasculitis and cardiac vasculitis); RPGN and declining kidney function; eGFR fall > 20% in previous 2 weeks; GFR < 15 mL/min at entry or on dialysis. |
| NORAM 2005 | New diagnosis of WG or MPA in 1 or more organ systems; elevated ESR or CRP or both or ANCA positivity, or a non‐renal biopsy demonstrating small vessel vasculitis | Organ or life‐threatening vasculitis (severe haemoptysis with bilateral pulmonary infiltrates, cerebral infarction due to vasculitis, rapidly progressive neuropathy, orbital pseudotumour, massive GI bleeding, heart failure due to pericarditis or myocarditis; Cr > 150 µM, urinary red cell casts or proteinuria >1 g/day; skin vasculitis only; another multisystem autoimmune disease; malignancy; hepatitis B or HIV infection; < 18 years or >75 years |
| RAVE 2010 | Weight ≥ 88 lbs (40 kg); diagnosis of WG or MPA; newly diagnosed patient of WG or MPA OR must be experiencing a disease flare characterized by: (a) active disease with a BVAS for WG of ≥ 3 that would normally require treatment with CPA; OR (b) disease severe enough to require treatment with CPA; OR (c) must be positive for either PR3‐ANCA or MPO‐ANCA at the screening; willing to use acceptable forms of contraception for the duration of the study and for up to 1 year after stopping study medications; willing to report pregnancies (female participants or male participants' partners) occurring at any time during the study and for up to 1 year after stopping study medications; parent or guardian willing to provide informed consent, if applicable | Diagnosis of Churg‐Strauss Syndrome; limited disease that would not normally be treated with CPA; requires mechanical ventilation because of alveolar haemorrhage; history of severe allergic reactions to human or chimeric monoclonal antibodies; active systemic infection; deep‐space infection, such as osteomyelitis, septic arthritis, or pneumonia complicated by pleural cavity or lung abscess, within 6 months prior to study entry; history of or current HBV or HCV; HIV; acute or chronic liver disease that, in the opinion of the investigator, may interfere with the study; history of or active cancer diagnosed within the last 5 years; history of anti‐GBM disease; other uncontrolled disease, including drug and alcohol abuse; pregnancy or breastfeeding |
| RITUXVAS 2010 | New diagnosis of ANCA‐associated vasculitis, ANCA positivity, and kidney involvement, as evidenced by necrotizing GN on biopsy or red‐cell casts or haematuria (30 red cells per high‐power field) on urinalysis | Previous CPA (> 2 weeks of an oral or IV pulse CPA regimen); co‐existence of another multisystem autoimmune disease, e.g. SLE, Churg Strauss syndrome, HSP, rheumatoid vasculitis, essential mixed cryoglobulinaemia, anti‐GBM antibody positivity; Hepatitis B antigen positive or hepatitis C antibody positive; known HIV positive; previous malignancy (usually exclude unless agreed with trial co‐ordinator; pregnancy, breast feeding or inadequate contraception; allergy to a study medication; live vaccine within last four weeks |
| Stegmayr 1999 | Goodpasture's disease; ANCA positive vasculitis; idiopathic RPGN | HIV; hepatitis A, HBV, HCV, severe cardiac failure; malignancy; septicaemia |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; anti‐GBM ‐ anti‐glomerular basement membrane; AZA ‐ azathioprine; BVAS ‐ Birmingham Vasculitis Activity Score; CMV ‐ cytomegalovirus; CNS ‐ central nervous system; CPA ‐ cyclophosphamide; Cr ‐ creatinine; eGFR ‐ estimated glomerular filtration rate; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HIV ‐ human immunodeficiency virus; HSP ‐ Henoch‐Schonlein Purpura; IV ‐ intravenous; IVIg ‐ IV immunoglobulin; KRT ‐ kidney replacement therapy; MMF ‐ mycophenolate mofetil; MPA ‐ microscopic polyangiitis; MPO ‐ myeloperoxidase; p‐ANCA ‐ perinuclear‐ANCA; PR3 ‐ proteinase‐3; RPGN ‐ rapidly progressive glomerulonephritis; SCr ‐ serum creatinine; SLE ‐ systemic lupus erythematosus; TB ‐ tuberculosis; WG ‐ Wegener's granulomatosis
Appendix 9. Treatment regimens and study outcomes ‐ other remission induction studies
| Study ID | Treatment | Control | Study Outcomes |
| BREVAS 2019 | Belimumab (IV): 10 mg/kg Co‐interventions: azathioprine (2 mg/kg/day) and low‐dose oral glucocorticoids (≤ 10 mg/day) |
Placebo (IV) Co‐interventions: azathioprine (2 mg/kg/day) and low‐dose oral glucocorticoids (≤ 10 mg/day) |
Relapse (BVAR score) Infection Adverse events |
| CLEAR 2013 | Treatment group 1
Avacopan: 40 mg/day Prednisone: 20 mg/day Treatment group 2 Avacopan: 30 mg/day Prednisone placebo |
Prednisone: 60 mg/day Avacopan placebo |
Proportion of patients with a treatment response at week 12 defined as a BVAS decrease from baseline of at least 50% plus no worsening in any body system
Proportion of patients with a renal response, defined as an improvement in eGFR calculated using the MDRD equation, haematuria, and albuminuria at week 12 Proportion of patients with disease remission (BVAS 0); and change from baseline in BVAS, eGFR, UACR, urinary RCC, urinary MCP‐1‐to‐creatinine ratio, vasculitis damage index, SF‐36 version 2, EQ‐5D‐5L, and rescue glucocorticoid use |
| CORTAGE 2015 | Corticosteroids: started at 1 mg/kg then progressively tapered after 3 weeks and stopped at 9 months CPA pulses: 500 mg every 2 weeks for first 3 pulses then every 3 weeks until remission Patients switched to MTX or AZA (or MMF for those with intolerance to MTX or AZA) |
Corticosteroids: started at 1 mg/kg the progressive tapered after 3 weeks until stopped at 26 months CPA pulses: 500 mg/m2 for six doses with a further 3 doses for consolidation prior to maintenance therapy |
Occurrence of 1 or more serious adverse events defined as potentially life‐threatening adverse events, requiring hospitalisation or its prolongation, causing significant disability, or resulting in death. Death Remission Relapse Serious adverse event‐free survival Progression free survival |
| Furuta 1998 | Lymphocytapheresis: 3 x 1 hour sessions on alternate days in each of 3 consecutive weeks Prednisolone: 20 mg/day CPA: 50 mg/day |
IV MP: 1 g for 3 consecutive days in each of 3 consecutive weeks Prednisolone: 20 mg/day CPA: 50 mg/day |
SCr 4 weeks post treatment Death |
| Guillevin 2003 | Initial regimen
Tapering dose of steroids: 3 daily IV pulses of MP 15 mg/kg. Prednisolone (1 mg/kg/day) for 3 weeks, gradually tapered to stop CPA pulses given at 0, 2 and 4 weeks, then monthly Treatment group 1 CPA dose: 6 pulses |
Treatment group 2 CPA dose: 12 pulses | Complete remission Death Relapse |
| Han 2011b | MMF (oral): 1.0 g/day (1.5 g/day if weight > 70 kg) MP (IV): 360 to 500 mg/day for 3 days, then oral prednisone 0.6 to 0.8 mg/kg/day, gradually tapered |
CPA (IV): 1.0 g/pulse (0.8G if body weight < 50 kg) monthly MP (IV): 360 to 500 mg/day for 3 days, then oral prednisone 0.6 to 0.8 mg/kg/day, gradually tapered |
Remission at 6 months Kidney function at 6 months Adverse events |
| Hu 2008b | IV MP 0.5 g daily for 3 days followed by oral prednisolone at 0.6 to 0.8 mg/kg/day for 4 weeks tapered by 5 mg/week to 10 mg/day MMF: 2 g/day (1.5 g if weight < 50 kg) for 6 months |
IV MP 0.5 g daily for 3 days followed by oral prednisolone at 0.6 to 0.8 mg/kg/day for 4 weeks tapered by 5 mg/week to 10 mg/day IV CPA: 0.75 to 1.0 g/m2 for 6 months, modified depending on WCC nadir |
Remission rate at 6 months Changes in kidney function Side effects |
| Jayne 2000 | CPA and prednisolone for remission induction then AZA for maintenance
2 week observation period IVIg: 0.4 g/kg/day for 5 days |
CPA and prednisolone for remission induction then AZA for maintenance
2 week observation period Placebo (identical injections) for 5 days |
Treatment response Fall in BVAS, CRP and ANCA Relapse frequency between 3 and 12 months Reduction in immunosuppressive drug doses Adverse effects |
| MYCYC 2012 | MMF: 2 g/day or maximum tolerated dose between 1 and 2 g/day, for 3 to 6 months until remission (BVAS = 0 for 2 consecutive study assessments), then switch to AZA maintenance regimen
|
CPA: 15 mg/kg at weeks 0, 2 and 4 then pulses every 3 weeks for 3 to 6 months (6 to 10 doses) until remission (BVAS = 0 for 2 consecutive study assessments), then switch to AZA maintenance regimen | Remission at 6 months Time to remission (months) Adverse events: mild/moderate/severe and infections Relapse (relapse rates at 18 months and relapse free survival) Cumulative dose of corticosteroids Improvement in calculated GFR at 18months Cumulative VDI scores Change in SF‐36 at 12 and 18 months BVAS (AUC) between entry and 18 months ANCA status at 6 months |
| NORAM 2005 | MTX: 15 mg/week oral MTX increasing to 2 5mg/week at week 12, continued to month 10 then tapered to stop at month 12 | Oral CPA: group: 2 mg/kg/day (max 150 mg/day) until remission, minimum of 3 months, maximum of 6 months. At remission, dose reduced to 1.5 mg/kg/day continued to month 10 then tapered to stop at month 12. Dose adjusted for age and low white cell count Co‐interventions (both groups) Oral prednisolone: 1 mg/kg/day tapered to 15 mg/day at 12 weeks and 7.5 mg/day by 6 months, stopped at 12 months | Remission at 6 months
Disease relapse Adverse effects |
| RAVE 2010 | Rituximab: 375 mg/m2 infusions once weekly for 4 weeks CPA placebo daily for 3 to 6 months During the remission maintenance phase participants will discontinue CPA placebo and start oral AZA placebo daily until month 18 |
Rituximab placebo: infusions once weekly for 4 weeks CPA daily for 3 to 6 months During the remission maintenance phase, participants will discontinue CPA and start AZA daily until month 18 |
Complete remission during the first 6 months after randomisation Rate of selected adverse events experienced by participants receiving rituximab versus those receiving conventional therapy |
| RITUXVAS 2010 | Immunosuppression as for control group IV Rituximab: 375 mg/m2 IV once a week for 4 weeks CPA: 15 mg/kg 2 weeks apart given with the 1st and 3rd rituximab dose |
Immunosuppression
Remission induction
Remission maintenance
|
Sustained remission Severe adverse events Response rate at 6 weeks Remission at 6 months Time to remission Relapses (all relapses and major/minor) BVAS AUC Change in GFR Change in SF‐36 Change in VDI Severe adverse events at 6 weeks and 6 months All adverse events Death Prednisolone cumulative dose CPA cumulative dose Human anti‐chimeric antibody testing Correlation of B cells with disease activity Change in ANCA and disease activity Histopathology predictors of outcome |
| Stegmayr 1999 | Immunosuppression as for control group Immunoadsorption of at least 2 plasma volumes. Median of six sessions |
Immunosuppression
PE: 3 in first 5 days of at least 1 plasma volume, 4% albumin as replacement. Median of six sessions |
Death at 6 months SCr at 3 and 6 months |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; AUC ‐ area under the curve; AZA ‐ azathioprine; BVAS ‐ Birmingham Vasculitis Activity Score; CPA ‐ cyclophosphamide; CRP ‐ C‐reactive protein; GFR ‐ glomerular filtration rate; IV ‐ intravenous; IVIg ‐ IV immunoglobulin; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; PE ‐ plasma exchange; SCr ‐ serum creatinine; VDI ‐ vasculitis damage index; WCC ‐ white cell count
Appendix 10. Study criteria ‐ maintenance treatment
| Study ID | Inclusion criteria | Exclusion criteria |
| AZA‐ANCA 2016 | Patients > 18 years with newly diagnosed PR3 ANCA vasculitis recruited between diagnosis and remission. Those found to be cANCA positive at stable remission after 3 months were randomised. The other patients were treated with standard AZA treatment | Intolerance for AZA or inability to give informed consent |
| Boomsma 2003 | Patients with PR3 ANCA‐associated vasculitis in remission on ≤ 50 mg daily CPA and ≤ 15 mg daily prednisolone; rise in ANCA titre of more than 75% from previous sample. 100 patients followed; 40 patients developed ANCA rise and were randomised | Not reported |
| CYCAZAREM 2003 | Diagnosis of WG, MPA or RLV. Kidney involvement, other threatened loss of function of vital organ, or both. ANCA positivity. ANCA negative patients enrolled with biopsy evidence of vasculitis | Cytotoxic drug in previous year; other multisystem autoimmune disease; hepatitis B e antigenaemia; hepatitis C; HIV infection; SCr > 500 μmol/L; cancer; pregnancy; aged < 18 years or > 75 years |
| IMPROVE 2003 | Newly diagnosed patients with WG, MPA or RLV; ANCA positivity. ANCA positivity requires PR3‐ANCA or a typical c‐ANCA pattern by indirect immunofluorescence (IIF), preferably confirmed by anti‐PR3 ELISA; MPO‐ANCA determined by ELISA requires demonstration of p‐ANCA, and p‐ANCA by IIF requires confirmation by anti‐MPO ELISA; optionally, central review of ANCA serology can be performed; 18 to 75 years | Any cytotoxic drug within previous year, unless started within one months of entry and according to the protocol design; co‐existence of another systemic autoimmune disease (e.g. SLE, HBV, HCV, HIV positivity); failure to achieve remission after 6 months of CPA therapy; failure to control progressive disease with induction protocol; malignancy (usually exclude unless agreed with trial co‐ordinator); pregnancy or inadequate contraception; < 18y and > 75 years; ESKD unless active extrarenal disease requires treatment (temporal dependency of HD is not an exclusion criterion); inability for informed consent |
| MAINRITSAN 2 2018 | > 18 years old; had newly diagnosed or relapsing GPA or MPA (defined by the Chapel Hill Consensus nomenclature); in complete remission after induction therapy, combining glucocorticoids and cyclophosphamide, rituximab or methotrexate (as decided by each investigator), in accordance with French and international recommendations; Birmingham Vasculitis Activity Score V.3 (BVAS) of 0 (score range: 0–63, with higher scores indicating more active disease) defined complete remission. | Another systemic vasculitis; induction with an agent not recommended; active disease; incapacity for informed consent; non‐compliance; allergy to the study medication; pregnancy; breastfeeding; HIV, hepatitis B or C; severe infection declared during the 3 months before randomisation; cancer or malignant blood disease diagnosed during the 5 years preceding vasculitis diagnosis; participation in another clinical research protocol during the 4 weeks before inclusion; any clinical or psychiatric disorder that could expose the patient to a greater risk of an adverse event (AE) or could prevent treatment administration and patient follow‐up according to the protocol; severe immunosuppression; administration of live vaccine during the 4 weeks before inclusion; severe chronic obstructive pulmonary diseases (maximum expiratory volume <50% or dyspnoea grade III); chronic heart failure (dyspnoea NYHA III or IV); history of recent acute coronary syndrome unrelated to vasculitis; patients not enrolled in the French national health insurance. |
| MAINRITSAN 2014 | Patients aged 18 to 75 years of age with newly diagnosed or relapsing granulomatosis with polyangiitis, microscopic polyangiitis, or renal‐limited ANCA‐associated vasculitis in complete remission after combined treatment with glucocorticoids and pulse CPA; patients had to be ANCA‐positive at diagnosis or during the course of their disease; have histologically confirmed necrotizing small‐vessel vasculitis with a clinical phenotype of granulomatosis with polyangiitis, microscopic polyangiitis, or renal‐limited ANCA‐associated vasculitis; or both | Other systemic vasculitis; secondary vasculitis (following neoplastic disease or an infection in particular); induction treatment with a regimen not corresponding to that recommended in France; patient who has not achieved remission; already received a treatment by biological agents (monoclonal antibody); incapacity or refusal to understand or sign the informed consent form; incapacity or refusal to adhere to treatment or perform the follow‐up examinations required by the study; non‐compliance; allergy, documented hypersensitivity or contraindication to the study medication (CPA, corticosteroids, AZA, RTX); history of severe allergic or anaphylactic reactions to humanized or murine monoclonal antibodies; patients receiving allopurinol cannot be included if the allopurinol must absolutely be maintained; pregnancy, breastfeeding; women of childbearing age must use a reliable method of contraception throughout the duration of immunosuppressive treatment up to 1 year after the last infusion of RTX; infection by HIV, HCV or HBV; progressive, uncontrolled infection requiring a prolonged treatment; severe infection declared during the 3 months before randomisation (CMV, HBV, HHV8, HCV, HIV, TB); progressive cancer or malignant blood disease diagnosed during the 5 years before the diagnosis of vasculitis; patients presenting a systemic disease receiving protocolized treatments (AZA, RTX) which could have unexpected and inappropriate side effects; participation in another clinical research protocol during the 4 weeks before inclusion; any medical or psychiatric disorder which, in the investigator's opinion, may prevent the administration of treatment and patient follow‐up according to the protocol, and/or which may expose the patient to a too greater risk of an adverse effect; no social security; Churg and Strauss syndrome; viral, bacterial or fungic or mycobacterial infection uncontrolled in the 4 weeks before the inclusion; history of deep tissue infection (fasciitis, osteomyelitis, septic arthritis) in the first year before the inclusion; history of chronic and severe or recurrent infection or history of pre‐existing disease predisposing to severe infection; severe immunodepression Administration of live vaccine in the four weeks before inclusion; severe chronic obstructive pulmonary diseases (VEMS < 50 % or dyspnoea grade III); chronic heart failure stage III and IV (NYHA); history of recent acute coronary syndrome |
| Maritati 2017 | Diagnosis of clinically active SNV; aged 18 to 80 years; life‐expectancy > 1 year; written informed consent; randomisation performed only if GFR > 30 | CrCl < 10 mL/min/1.73 m2 |
| Metzler 2007 | Patients aged 18 and 75 years with a diagnosis of generalized WG after successful induction therapy with prednisolone and CPA | Bone marrow insufficiency (leukopenia <4000/µL, Hb < 10 g/dL, thrombocytopenia > 100,000/µL); SCr > 1.3 mg/dL (115 µM); malignancies; HBV, HCV or HIV positivity; pregnancy or breast feeding; inadequate contraception; chronic liver disease or alcohol abuse; active gastric ulcer; lack of compliance; further coexisting autoimmune diseases or treatments interfering with the study medication |
| REMAIN 2003 | Males or females > 18 years, and (1, 2 and 3 are required); (1) a diagnosis of MPA, GPA or renal‐limited vasculitis; (2) renal involvement and/or other threatened loss of function of avital organ (lung, brain, eye, motor nerve or gut) and ANCA positivity, and ANCA‐negative patients were eligible for enrolment in the study only when there was histological confirmation of pauci‐immune vasculitis; (3) remission‐induction therapy with CPA and prednisolone for at least 3 months, with or without PE; and (4) stable remission on AZA/prednisolone. | < 18 years; pregnancy; previous malignancy; known HIV infection; previous life‐threatening relapse; ESKD at inclusion; allergy to study medications |
| Stegeman 1996 | Three groups of patients
|
Allergy or adverse reactions to co‐trimoxazole or one of its components; long term (> 6 weeks) antibiotic treatment; impaired kidney function (CrCl < 30 mL/min/24 h) |
| Tervaert 1990 | Patients with ANCA‐associated vasculitis in remission with a significant rise in ANCA titre | Not reported |
| WEGENT 2008 | Aged > 18 years; newly diagnosed WG and MPA | Use of steroids for more than 1 months prior to CPA therapy; co‐existence of another systemic disease; cancer (unless in remission for more than 3 years); HIV, HBV or HCV infection; contraindication to study drugs; pregnancy, absence of contraception in premenopausal women; mental or physical disabilities abrogating ability to consent; patients not entering remission were not randomised |
| WGET 2002 | At least 2 of 5 modified criteria of the American College of Rheumatology for classification of WG; either new or established disease; patients with BVAS/WG score of 3 or more; stratified to severe (life‐threatening manifestations including RPGN, alveolar haemorrhage or neuropathy) or limited (skin, joints, sinus or mild renal abnormalities) | Not reported |
| Zycinska 2009 | Patients with WG in remission after treatment with CPA and steroids | Not reported |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; BVAS ‐ Birmingham Vasculitis Activity Score; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; ESKD ‐ end‐stage kidney disease; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; MPA ‐ microscopic polyangiitis; MPO ‐ myeloperoxidase; p‐ANCA ‐ perinuclear‐ANCA; PR3 ‐ proteinase‐3; RLV ‐ renal‐limited vasculitis; RPGN ‐ rapidly progressive glomerulonephritis; SCr ‐ serum creatinine; SLE ‐ systemic lupus erythematosus; WG ‐ Wegener's granulomatosis
Appendix 11. Treatment regimens and study outcomes ‐ maintenance treatment
| Study ID | Treatment | Control | Study outcomes |
| AZA‐ANCA 2016 | Extended AZA therapy: 1.5 to 2.0 mg/kg/day until 4 years after diagnosis, then tapered by 25 mg every 3 months. | Standard AZA therapy: 1.5 to 2.0 mg/kg/day until 12 months after diagnosis, then tapered by 25 mg every 3 months Other treatment All patients received TMP/SMX 400/80 mg prophylaxis | Relapse‐free survival at 4 years Cumulative dosages of CPA, prednisolone and AZA Cumulative organ damage Side effects due to study medication and severity of relapses. |
| Boomsma 2003 | Pre‐emptive therapy AZA: 75 mg/day for 9 months Prednisolone: 30 mg/day tapered over 4.5 months |
Follow‐up only | Relapse of vasculitis |
| CYCAZAREM 2003 | Remission induction as for control group After remission induction
Switched to AZA (2 mg/kg/day) and prednisolone (7.5 mg/day) 12 months after study entry |
Remission induction
After remission induction
From 12 months received AZA (1.5 mg/kg/day) and prednisolone (7.5 mg/day) |
Relapse by 18 months Side effects including leukopenia and infections |
| IMPROVE 2003 | Initial treatment as for control group MMF: 2 g/day; reduced to 1500 mg/day after 12 months, 1000 mg/day after 18 months, and withdrawn after 42 months |
Initial treatment
AZA: 2 mg/kg/day (max 200 mg), rounded down to the nearest 25 mg increment. The dose was reduced to 1.5 mg/kg/day after 12 months, 1 mg/kg/day after 18 months, and withdrawn after 42 months |
Time to first relapse
Relapse rate Rate of side‐effects and intolerance Cumulative doses (AZA, steroids, MMF) AUC for BVAS SF‐36 or VDI Evolution of titres of ANCA and CRP |
| MAINRITSAN 2 2018 | Rituximab regimens: given according to ANCA status and/or circulating CD19 B‐cell reconstitution vs systematically infused (controls). 'Tailored schedule' patients received fixed 500 mg RTX infusions on day 0 post‐randomisation, then every 3 months until month 18, when CD19 lymphocytes exceeded 0/mm3 or ANCA status (reappearance)/titre (higher) differed from the previous determination |
'Fixed schedule' controls received 500 mg of RTX on days 0 and 14 post‐randomisation, then 6, 12 and 18 months after the first infusion. | Major relapse Death Severe adverse events Serious infections |
| MAINRITSAN 2014 | Initial regimen (both groups)
Standard induction with prednisolone and IV CPA pulses. Prednisolone started at 1 mg/kg/day preceded by IV MP pulses of 500 to 1000 mg for 1 to 3 days. CPA pulses 0.6 g/m2 day 0, 14 and 28 followed by 0.7 g/m2 every 3 weeks for 3 to 6 pulses until remission. Patients were randomised after remission Treatment group RTX: 500 mg day 0, 14 then at month 6, 12 and 18 |
Control group AZA: 2 mg/kg/day orally for 12 months, then 1.5 mg/kg/day for 6 months, then 1 mg/kg/day for 4 months before ceasing | Major relapse Minor relapse Death Serious adverse event |
| Maritati 2017 | MTX: 15 mg/week increased to 0.3 mg/kg/week Patients with eGFR of 30 to 50 mL/min/1.73 m2 received 75% of the full CPA dose and half of the full MTX dose |
CPA: 1.5 mg/kg/day orally; treatment continued for 12 months | Relapse at 12 months, 18 and 24 months Major and minor relapses Change in eGFR Death Adverse events |
| Metzler 2007 | Immunosuppression as for control group PE: 7 x 60 mL/kg in first 2 weeks after diagnosis |
Immunosuppression
|
Death at 3 and 12 months Dialysis at 3 months and 12 months Total number of side effect Serious infections SCr at 12 months |
| REMAIN 2003 | Continued limb: continues treatment at least until 30 months after start of REMAIN trial regimen European Vasculitis Study Group (EUVAS) AVERT project | Withdrawal arm: discontinues all treatment 4 months after start of REMAIN trial regimen
Both groups
AZA and prednisolone from cessation of CPA Relapse treated according to guidelines for treatment of relapse |
Relapse Major relapse Minor relapse Death Dialysis Severe adverse events Vasculitis Damage Index eGFR ANCA status |
| Stegeman 1996 | TMP/SMX: 160/800 mg twice/day for 24 months | Placebo tablets for 24 months | Death Remission at 24 months Number of infections/patient/years |
| Tervaert 1990 | CPA: 1 mg/kg/day tapered over 9 months Prednisolone: 30 mg/day tapered over 3 months |
No change to current treatment | Relapse Cumulative dose Side effects: infection Death |
| WEGENT 2008 | Remission induction therapy as for control group AZA: 2 mg/kg/day Maintenance therapy as for control group |
Remission induction therapy
MTX: 0.3 mg/kg/week, increasing every week by 2.5 mg to 25 mg/week Folinic acid 25 mg or folic acid 5 mg given 48 hours after MTX Maintenance therapy
|
Adverse reaction causing death or leading to discontinuation of the study drug
Any adverse event Severe adverse event Relapse Relapse‐free survival Event‐free survival Quality of life |
| WGET 2002 | SC etanercept: 25 mg twice weekly Co‐interventions as for control group |
Twice weekly placebo injection Co‐interventions
|
Sustained remission Number and rate of flares during treatment Percentage of patients with sustained low level of disease activity Percentage of patients with a remission Cumulative AUC for the BVAS/WG Adverse events Quality of life |
| Zycinska 2009 | TMP/SMX: 160/800 mg 3 times/week for 18 months | Placebo tablets for 18 months | Remission Relapse Infection Side effects |
Footnotes: ANCA ‐ anti‐neutrophil cytoplasmic antibody; AUC ‐ area under the curve; AZA ‐ azathioprine; BVAS ‐ Birmingham Vasculitis Activity Score; CPA ‐ cyclophosphamide; Cr ‐ creatinine; CRP ‐ C‐reactive protein; IV ‐ intravenous; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; MTX ‐ methotrexate; PE ‐ plasma exchange; SC ‐ subcutaneous; SCr ‐ serum creatinine; TMP/SMX ‐ trimethoprim‐sulphamethoxazole; VDI ‐ vasculitis disease index; WG ‐ Wegener's granulomatosis
Appendix 12. Patients randomised per study
| Study ID | Treatment | Control | Total |
| Adu 1997 | 24 | 30 | 54 |
| AZA‐ANCA 2016 | 21 | 24 | 45 |
| Boomsma 2003 | 20 | 20 | 40 |
| BREVAS 2019 | 81 | 81 | 81 |
| CLEAR 2013 | Group 1: 22 Group 2: 22 |
23 | 67 |
| Cole 1992 | 16 | 16 | 32 |
| CORTAGE 2015 | 53 | 51 | 104 |
| CYCAZAREM 2003 | 79 | 76 | 155 |
| CYCLOPS 2004 | 76 | 73 | 149 |
| Furuta 1998 | 12 | 12 | 24 |
| Glockner 1988 | 16 | 15 | 31 |
| Guillevin 1997 | 27 | 23 | 50 |
| Guillevin 2003 | 31 | 34 | 65 |
| Han 2011b | 19 | 22 | 41 |
| Haubitz 1998 | 22 | 25 | 47 |
| Hu 2008b | 18 | 17 | 35 |
| IMPROVE 2003 | 76 | 80 | 156 |
| Jayne 2000 | 17 | 17 | 34 |
| MAINRITSAN 2 2018 | 81 | 81 | 162 |
| MAINRITSAN 2014 | 57 | 58 | 115 |
| Maritati 2017 | 38 | 33 | 71 |
| Mauri 1985 | 12 | 10 | 22 |
| MEPEX 2007 | 70 | 67 | 137 |
| Metzler 2007 | 26 | 28 | 54 |
| MYCYC 2012 | 70 | 70 | 140 |
| NORAM 2005 | 49 | 46 | 95 |
| PEXIVAS 2013 | 352 | 352 | 704 |
| Pusey 1991 | 25 | 23 | 48 |
| RAVE 2010 | 99 | 98 | 197 |
| REMAIN 2003 | 61 | 56 | 117 |
| Rifle 1980 | 6 | 8 | 14 |
| RITUXVAS 2010 | 33 | 11 | 44 |
| Stegeman 1996 | 41 | 40 | 81 |
| Stegmayr 1999 | 21 | 23 | 44 |
| Szpirt 2011 | 16 | 16 | 32 |
| Tervaert 1990 | 9 | 11 | 20 |
| WEGENT 2008 | 63 | 63 | 126 |
| WGET 2002 | 89 | 91 | 180 |
| Zauner 2002 | 21 | 18 | 39 |
| Zycinska 2009 | 16 | 15 | 31 |
| TOTAL | 1907 | 1857 | 3764 |
Footnotes
Data and analyses
Comparison 1. Plasma exchange as adjunctive therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Three months | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.49, 2.15] |
| 1.2 Six months | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.13] |
| 1.3 One year | 5 | 267 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.92] |
| 1.4 Two years | 4 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.49, 3.38] |
| 1.5 Five years | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.71, 2.04] |
| 1.6 Death at any time point | 6 | 957 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.72, 1.29] |
| 2 Kidney function: serum creatinine | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 One month | 3 | 73 | Mean Difference (IV, Random, 95% CI) | ‐111.37 [‐318.19, 95.45] |
| 2.2 Two months | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐79.70 [‐198.76, 39.36] |
| 2.3 Three months | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 36.62 [‐23.32, 96.57] |
| 2.4 Six months | 2 | 49 | Mean Difference (IV, Random, 95% CI) | 9.82 [‐180.10, 199.74] |
| 2.5 Twelve months | 4 | 156 | Mean Difference (IV, Random, 95% CI) | 23.52 [‐17.19, 64.22] |
| 3 Dialysis | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 One month | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.52] |
| 3.2 Three months | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.78] |
| 3.3 Six months | 4 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.13, 1.10] |
| 3.4 Twelve months | 6 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.29, 0.72] |
| 3.5 Five years | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.64] |
| 3.6 At any time point | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.70, 1.27] |
| 4 Sustained remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Serious infections | 5 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.03, 1.54] |
| 5.2 Myocardial infarction | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.31, 24.99] |
| 5.3 Lung haemorrhage | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.11, 3.39] |
| 5.4 Subarachnoid haemorrhage | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.26] |
| 5.5 Gastrointestinal haemorrhage | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.69] |
| 5.6 Anaphylaxis | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.12, 64.39] |
| 5.7 Serious adverse events | 1 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.89, 1.11] |
| 5.8 Total number of adverse events | 5 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.12] |
Comparison 2. Pulse versus continuous cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Three months | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.10, 4.73] |
| 1.2 Six months | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.22, 1.69] |
| 1.3 One year | 3 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.34, 1.31] |
| 1.4 Two years | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.21, 2.61] |
| 1.5 Death at final follow‐up | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.44, 1.32] |
| 2 Kidney function: serum creatinine | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 One month | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Two months | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Three months | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐4.58 [‐97.77, 88.61] |
| 2.4 Six months | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 51.69 [‐81.03, 184.41] |
| 2.5 Twelve months | 2 | 52 | Mean Difference (IV, Random, 95% CI) | ‐9.78 [‐53.16, 33.61] |
| 2.6 Two years | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 4.46 [‐67.90, 76.82] |
| 3 Dialysis | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Three months | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.11, 65.43] |
| 3.2 Six months | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 7.02 [0.90, 54.80] |
| 3.3 Twelve months | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [0.41, 30.80] |
| 3.4 Dialysis end of study | 4 | 245 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.92, 3.91] |
| 4 Remission | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Three months | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.30] |
| 4.2 Six months | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.93, 1.13] |
| 4.3 Nine months | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.92, 1.02] |
| 4.4 Twelve months | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.03] |
| 4.5 Eighteen months | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.94, 1.03] |
| 4.6 Untimed | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.98, 1.42] |
| 5 Relapse | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 One year | 2 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.57, 3.69] |
| 5.2 Two years | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.51, 7.03] |
| 5.3 Untimed | 4 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.11, 2.87] |
| 6 Treatment failure | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.15, 12.56] |
| 7 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Serious infections | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.33] |
| 7.2 Leukopenia | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.36, 0.77] |
| 7.3 Nausea | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.07, 5.89] |
Comparison 3. Rituximab versus cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Six months | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.21, 4.70] |
| 1.2 Two years | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.24, 4.25] |
| 2 Remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Sustained remission at 12 months (censored for death) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Any remission at 12 months (uncensored) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Twelve months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Serious adverse events | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.72, 1.71] |
| 4.2 Serious infections | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.42, 1.92] |
| 5 Adverse events (episodes/patient‐months) | 2 | 1710 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.62, 1.32] |
Comparison 4. Mycophenolate mofetil versus cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Dialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Remission | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Six months | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.35] |
| 3.2 Any time point | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.09] |
| 4 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 18 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At any time point | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Minor relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Major relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Infections | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.75, 2.16] |
| 5.2 GI symptoms | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.17, 1.59] |
| 5.3 Leukopenia | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 5.15] |
| 5.4 Serious adverse events | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.86, 1.81] |
Comparison 5. Methotrexate versus cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Eighteen months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Untimed | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Avacopan versus prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Kidney function: eGFR [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Any adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Serious infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Intravenous immunoglobulin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Response | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Plasma exchange versus immunoadsorption.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Kidney function: serum creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Three months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Six months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Lymphocytapheresis versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Kidney function: serum creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 One month | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Dialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Six versus 12 cyclophosphamide pulses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 One year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Three years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Untimed | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Untimed | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.85, 1.15] |
| 3 Relapse | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Untimed | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.96, 2.56] |
| 4 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Infection | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.36, 1.72] |
| 4.2 Severe adverse events | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.59, 1.00] |
| 4.3 Cytopenia | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.28] |
Comparison 11. Reduced dose versus standard dose steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Dialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Sustained remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Serious infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Etanercept versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Sustained remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Cancer | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Maintenance therapy: azathioprine versus cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Long‐term follow‐up (median time 8.5 years) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Dialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Long‐term follow‐up (median time 8.5 years) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Eighteen months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Long‐term follow‐up (median time 8.5 years) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Leukopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events (episodes/patient‐months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Leukopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Maintenance therapy: mycophenolate mofetil versus azathioprine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Untimed | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Any relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Major relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Minor relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Any adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Serious infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Leukopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Maintenance therapy: azathioprine versus methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Death due to study drug | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Relapse‐free survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 18 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 36 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Event‐free survival at 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Any adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Severe adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Adverse event causing death or study drug discontinuation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Maintenance therapy: rituximab versus azathioprine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Major relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 One year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Two years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 End of study (28 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Minor relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 One year | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Two years | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 End of study (28 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Serious infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Maintenance therapy: co‐trimoxazole (antibiotics) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Remission | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 One year | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.98, 1.33] |
| 2.2 18 months | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.81, 2.44] |
| 2.3 Two years | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.94, 1.76] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Anorexia and nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Rash | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Interstitial nephritis | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Asymptomatic hepatotoxic effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Recurrent urinary tract infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Adverse events causing study drug discontinuation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Maintenance therapy: cyclosporin versus cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 19. Maintenance therapy: extended versus standard azathioprine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 162 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [‐0.01, 0.13] |
| 2 Dialysis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Relapse | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.26, 0.64] |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Serious infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Leukopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Total side effects (episodes/patient‐months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
19.5. Analysis.
Comparison 19 Maintenance therapy: extended versus standard azathioprine, Outcome 5 Total side effects (episodes/patient‐months).
Comparison 20. Maintenance therapy: leflunomide versus methotrexate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 All relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Major relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Severe adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Leukopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 21. Maintenance therapy: methotrexate versus cyclophosphamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 All relapses | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Major relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Minor relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Serious infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Leukopenia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 22. Maintenance therapy: tailored versus fixed rituximab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Major relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Severe adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Serious infections | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 23. Maintenance therapy: pre‐emptive therapy for relapse.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.03, 1.59] |
Comparison 24. Maintenance therapy: belimumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Any adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adu 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other treatment
Duration of treatment: 72 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers, stratified for kidney function (< 250, 251 to 500, > 500 mmol/L) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study could not be blinded to investigators or participants; unlikely to affect results |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes clearly stated |
| Other bias | High risk | Patients in PCYP had worse kidney function than the CCAZP group (median SCr 234, range 60 to1082 mmol/L vs 139, range 72 to 1255 mmol/L (P = 0.3)) |
AZA‐ANCA 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other treatment
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of four. Patients were stratified according to hospital, i.e. patients from the UMCG versus patients from other hospitals." |
| Allocation concealment (selection bias) | Low risk | Quote: "Closed envelopes with the randomised treatment duration were produced before inclusion of the first patient" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessed by unblinded observers by BVAS which is a subjective assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes appear to be comprehensively reported |
| Selective reporting (reporting bias) | Low risk | Organ damage was quoted in the secondary outcomes but no damage index was reported in the results. This is not a high risk for the overall study result |
| Other bias | High risk | Early termination of the study due to poor recruitment. Negative outcomes |
Boomsma 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding not possible (pre‐emptive versus follow‐up treatment); unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 40 patients had outcomes reported |
| Selective reporting (reporting bias) | High risk | Abstract only with no protocol to detect reporting bias; no full‐text publication identified 12 years after conference abstracts presented |
| Other bias | Unclear risk | Insufficient information to permit judgement |
BREVAS 2019.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization schedule was produced by Human Genome Sciences (HGS)" |
| Allocation concealment (selection bias) | Low risk | Quote: " both the sites and study sponsor remained blinded with regard to treatment allocation at all times" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "both the sites and study sponsor remained blinded with regard to treatment allocation at all times" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes measured "Quote: "... at double‐blind week 48 of year 1 and double‐blind week 24 of year 2, as well as by visit" Comment: outcome assessors appear to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All patients had outcomes reported |
| Selective reporting (reporting bias) | Low risk | All outcomes clearly stated |
| Other bias | High risk | Funded by GlaxoSmithKline; study terminated early; imbalance across the age categories: Quote: "the proportion of elderly patients (age ≥65 years) was higher in the belimumab group (18 [34.0%] of 53 patients) than in the placebo group (8 [15.4%] of 52 patients)." |
CLEAR 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, stratified by new or relapsing disease, PR3/MPO, induction therapy with cyclophosphamide or RTX. Stratification and randomisation were performed centrally via an interactive voice response system using a minimization algorithm to maintain balance among the treatment groups with respect to strata and study centre |
| Allocation concealment (selection bias) | Low risk | Randomisation performed centrally via interactive voice system. allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and all study personnel were masked to treatment allocation. All study drugs had matching active and placebo capsules, and identical bottles and boxes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | BVAS is a subjective assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | High risk | Funded by ChemoCentryx, Inc., Mountain View, California; 4 authors are employees of the funder |
Cole 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers, stratified at entry with respect to urine volume, need for dialysis and > 50% glomeruli sclerosed |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient randomised did not receive treatment due to GI bleed at time of randomisation |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not reported in the methods |
| Other bias | Low risk | "Supported by grants from Health & Welfare Canada and the Kidney Foundation of Canada, and the Metropolitan Toronto Community Foundation." |
CORTAGE 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally. The randomisation list was computer generated, using random blocks of 6, with a between‐arm randomisation ratio of 1:1 |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported |
| Other bias | Unclear risk | Study appears free of other biases |
CYCAZAREM 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally with the use of permuted blocks of four within each country, with stratification according to diagnosis." |
| Allocation concealment (selection bias) | Low risk | Not reported, however assumed to be performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Supported by contracts (BMH1‐CT93‐1078, CIPD‐CT94‐0307, BMH4‐CT97‐2328, and IC20‐CT97‐0019) with the European Union |
CYCLOPS 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignments were computer‐generated and performed centrally by permuted blocks of 4, stratified by country and disease." randomised 1:1 to treatments. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were enrolled by their treating physician and registered with the central trial coordinating office by fax submission of a form that contained information on centre, date of birth, sex, disease, and creatinine level." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study, unable to blind interventions; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eleven patients were withdrawn before random assignment: 1 declined further participation, 8 were withdrawn by their physician, and 2 did not meet the entry criteria |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Quote: "This trial was funded by the European Union (European Community Systemic Vasculitis Trial project, contract BMH1‐CT93‐1078 and CIPD‐CT94‐0307, and Associated Vasculitis European Randomised Trial project, contract BMH4‐CT97‐2328 and IC20‐CT97‐0019)." |
Furuta 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | High risk | Planned outcomes were not reported; our outcomes of need for KRT, relapse, adverse effects and cumulative dose were not reported |
| Other bias | Unclear risk | Funding not reported |
Glockner 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Telephone consultation with a statistician |
| Allocation concealment (selection bias) | Low risk | Telephone consultation with a statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients in Group A and 2 in Group B did not complete study (GI bleed, death, sepsis or anaphylactic reaction to PE) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not clearly identified in the methods |
| Other bias | High risk | The study allowed cross‐over from one treatment arm to another after four weeks of therapy |
Guillevin 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Initial regimen
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients were excluded post randomisation from oral CPA due to wrong initial diagnosis (MPA) |
| Selective reporting (reporting bias) | Low risk | All study and review outcomes were reported |
| Other bias | High risk | Recruitment was terminated early at 30 months due to an interim analysis suggesting higher incidence of side effects and relapse rate between the groups. |
Guillevin 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Initial regimen
Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was centralized at the coordinating centre and made by phone, fax, or E‐mail." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No major missing data |
| Selective reporting (reporting bias) | Unclear risk | The outcomes were not clearly defined in the methods. No kidney outcomes reported |
| Other bias | High risk | The groups did not appear well balanced at the start of the study, very different levels of renal involvement and Cr level though this was not assessed as statistically significant |
Han 2011b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions (both groups)
|
|
| Outcomes | Primary outcome
Secondary outcomes
Maintenance dialysis defined as HD or PD for at least 6 weeks without subsequent kidney recovery signs |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients entered into the study were divided into two groups randomly according to the randomised number table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 patients lost to follow‐up and presumed failed treatment, 3 in CPA and 2 in MMF groups |
| Selective reporting (reporting bias) | Low risk | All study and review outcomes were reported |
| Other bias | Low risk | Study was supported by grants from the National Natural Science Foundation of PR China (30801148) to Fei Han and the Key Projects in the National Science & Technology Pillar Program in the Eleventh Five‐Year Plan Period (2008BAI60B04) to Jianghua Chen |
Haubitz 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation was stratified by the diagnosis, however the method was not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This is an "as treated" as opposed to an intention to treat analysis. 4 patients were excluded due to protocol violations with the interventions |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | High risk | The study was terminated when the analysis showed a difference. Quote: "The prospective, multicenter study was terminated in June 1997, since significant differences between the 2 treatment groups were found." p1836 |
Hu 2008b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions (both groups)
|
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/17 patients on CPA were lost to follow‐up at 3 months. The intention to treat analysis assumes that they did badly |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
IMPROVE 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Initial treatment (both groups)
|
|
| Outcomes | Primary outcome measure
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "1:1 ratio with the use of a minimized central computerized randomisation procedure. Randomization was stratified for age, diagnosis (Wegener granulomatosis vs MPA), and route of cyclophosphamide administration (daily oral vs intravenous pulse)." |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | Funding source stated and had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript |
Jayne 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Initial treatment (both groups)
Treatment group
Control group
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation and distribution of trial medication was centrally controlled by Novartis UK" |
| Allocation concealment (selection bias) | Low risk | Medication distributed by Novartis UK |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "patients and physicians were blinded to the treatment limb" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | High risk | Funding source: Novartis |
MAINRITSAN 2 2018.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes | Quote: "2 new relapses occurred after the followup (month 28) and were censured from the main analysis". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised at a 1:1 ratio". "An independent statistician provided the computer‐generated randomisation sequence, stratified by newly diagnosed or relapsing AAV" |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent statistician provided the computer‐generated randomisation sequence, stratified by newly diagnosed or relapsing AAV. Randomisation was centralised through electronic case‐report forms (eCRF) to assure allocation concealment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study, no blinding of participants or clinicians |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors not blinded, open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for and all outcome data complete |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | High risk | Pharmaceutical funding of study drug |
MAINRITSAN 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Initial regimen (both groups)
Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation stratified according to disease flare category |
| Allocation concealment (selection bias) | Low risk | Centrally allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Maritati 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer algorithm |
| Allocation concealment (selection bias) | High risk | Not performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported |
| Other bias | Low risk | Study appears free of other biases |
Mauri 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/22 patients unaccounted for |
| Selective reporting (reporting bias) | High risk | Unlikely since outcomes are not clearly defined in the study |
| Other bias | Unclear risk | Funding not reported |
MEPEX 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation in permuted blocks of four stratified by country and oliguria or likely to require dialysis |
| Allocation concealment (selection bias) | Low risk | Performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Low risk | Quote: "This trial was designed and launched as part of the European Community Systemic Vasculitis Trial project (contract nos. BMH1‐CT93‐1078 and CIPD‐CT94‐0307) and finished as part of the Associated Vasculitis European randomised Trial project (contract nos. BMH4‐CT97‐2328 and IC20‐CT97‐0019) funded by the European Union." |
Metzler 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes | Primary outcome
Secondary outcome measures
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed centrally (outside of all participating centres) with the use of permuted blocks of four." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study terminated early |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | High risk | Study was terminated early due to a high rate of relapses in the MTX group |
MYCYC 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation algorithm utilised |
| Allocation concealment (selection bias) | Low risk | Faxed form returned in 24 hours |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Remission assessment performed by unblinded clinicians |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study yet to be published in full |
| Selective reporting (reporting bias) | Low risk | All outcomes have been pre‐stated |
| Other bias | Low risk | Funding source stated; "Data will be collected, analysed and published independently from the source of funding." |
NORAM 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes | Primary endpoint
Secondary endpoints
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally in blocks of 4 by country and stratified by diagnosis |
| Allocation concealment (selection bias) | Low risk | Centrally performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | States unblinded; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up losses all stated and counted as treatment failure |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Low risk | Funding source stated |
PEXIVAS 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1 (a, b)
Treatment group 2 (a, b)
Treatment group 1a, 2a
Treatment group 1b, 2b
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central web‐based sequence generation with minimisation stratified by severity of renal disease, age, ANCA subtype, severity of pulmonary haemorrhage and induction therapy |
| Allocation concealment (selection bias) | Low risk | Central web‐based system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Study appears free of other biases |
Pusey 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group Induction therapy, 8 weeks of:
Maintenance therapy
Study duration
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation procedure ensured that patients in different arms of the trial were equally distributed throughout its course" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | This trial took 10 years to complete and is therefore subject to biases involved in changing physician perceptions of the efficacy of PE |
RAVE 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
During the remission maintenance phase
|
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Highly likely. stratified by clinical site and ANCA type. |
| Allocation concealment (selection bias) | Low risk | Centrally performed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Low risk | Funding source and study conduct disclosed |
REMAIN 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Both groups
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation performed centrally with block randomisation per country (blocks of four) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Rifle 1980.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were divided into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to determine numbers randomised and if all were analysed |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
RITUXVAS 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Other interventions
Progressive disease treatment
Relapse treatment
|
|
| Outcomes | Primary outcomes
Secondary outcomes
Tertiary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer minimization algorithm stratified by age diagnosis and renal function; 3:1 randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "maintained concealment of study group assignments for the investigators" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Low risk | All study and review outcomes were reported |
| Other bias | Low risk | Funding source and study conduct stated |
Stegeman 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified according to disease group. sequence generation not reported but all parties blinded |
| Allocation concealment (selection bias) | Low risk | Treatment assignment not known to investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment assignment not known to investigators, patients or physicians |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Low risk | Funding source stated |
Stegmayr 1999.
| Methods |
|
|
| Participants |
Only 6 patients had Goodpasture's disease; therefore we included this study |
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Unclear risk | Economical support stated |
Szpirt 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
After 3 months of induction treatment, all patients underwent a second randomisation to either continue CPA or to change to CSA for 9 months. Dose initiated 5 mg/kg/day with trough levels 150 to 200 µmol/L |
|
| Outcomes | Kidney outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by minimisation method stratified by sex, age, biopsy results, lung Infiltrates, c‐ANCA level and kidney function Second randomisation at 3 months to assign continued CPA or CSA |
| Allocation concealment (selection bias) | Low risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants or personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Low risk | Study and review outcomes reported |
| Other bias | Unclear risk | Funding source not reported |
Tervaert 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...block randomisation (block length four) was made after stratification for present treatment at the time of ANCA rise." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind participants and personnel; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients randomised were analysed |
| Selective reporting (reporting bias) | Unclear risk | Study and review outcomes reported |
| Other bias | Low risk | Funding source stated |
WEGENT 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Initial treatment
Treatment group 1
Treatment group 2
Maintenance therapy (both groups)
|
|
| Outcomes | Primary end point
Secondary end points
Remission defined as BVAS of 0, relapse required increased BVAS attributable to vasculitis Follow‐up to discontinuation of maintenance therapy on final patient |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks of six |
| Allocation concealment (selection bias) | Low risk | Central site |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Funding source stated |
WGET 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment assignments are generated in permuted blocks of varying lengths. Randomization is stratified by clinic and by disease severity (either severe or limited). The randomisation schedule is designed to yield an assignment ratio of 1:1" |
| Allocation concealment (selection bias) | Low risk | Performed centrally and stratified according to severity of disease and the centre |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment assignment not known to investigators, patients or their physicians |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for; 7 excluded from control group (6 lost to follow‐up, 1 wrong diagnosis) |
| Selective reporting (reporting bias) | Low risk | Protocol available; all outcomes reported |
| Other bias | Low risk | Funding source stated |
Zauner 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Subsequent maintenance therapy is not mentioned |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open‐label study, unable to blind interventions; unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | Data could not be extracted for analysis |
| Other bias | Unclear risk | Funding source not reported |
Zycinska 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo controlled, but blinding not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for |
| Selective reporting (reporting bias) | High risk | Results presented in a form that could not be meta‐analysed |
| Other bias | High risk | The groups were not balanced. Patients in the placebo group were older, had worse kidney function and a higher mean BVAS score at baseline |
ACR ‐ albumin‐creatinine ratio; AKI ‐ acute kidney injury; anti‐GBM ‐ antiglomerular basement membrane; ANCA ‐ anti‐neutrophil cytoplasmic antibody; AUC ‐ area under the curve; AZA ‐ azathioprine; BVAS ‐ Birmingham Vasculitis Activity Score; c‐ANCA ‐ cytoplasmic‐ANCA; CMV ‐ cytomegalovirus; CPA ‐ cyclophosphamide; Cr ‐ creatinine; CrCl ‐ creatinine clearance; CRP ‐ C‐reactive protein; CSA ‐ cyclosporin A; DEI ‐ Disease Extent Index; ELISA ‐ Enzyme‐Linked Immunosorbent Assay; ESKD ‐ end‐stage kidney disease; ESR ‐ erythrocyte sedimentation rate; FFP ‐ Fresh Frozen Plasma; GBM ‐ glomerular basement membrane; GFR ‐ glomerular filtration rate; GI ‐ gastrointestinal; GN ‐ glomerulonephritis; Hb ‐ haemoglobin; HBV ‐ hepatitis B virus; HCV ‐ hepatitis C virus; HD ‐ haemodialysis; HIV ‐ human immunodeficiency virus; HSP ‐ Henoch Schonlein Purpura; IIF ‐ indirect immunofluorescence; IV ‐ intravenous; IVIg ‐ IV immunoglobulin; KRT ‐ kidney replacement therapy; M/F ‐ male/female; MMF ‐ mycophenolate mofetil; MP ‐ methylprednisolone; MPA ‐ microscopic polyangiitis; MPO‐ myeloperoxidase; MTX ‐ methotrexate; NYHA ‐ New York Heart Association; PAN ‐ polyarteritis nodosa; PD ‐ peritoneal dialysis; PE‐ plasma exchange; p‐ANCA ‐ perinuclear‐ANCA; PR3 ‐ proteinase‐3; RCT ‐ randomised controlled trial; RLV ‐ renal‐limited vasculitis; RPGN ‐ rapidly progressive glomerulonephritis; RTX ‐ rituximab; SC ‐ subcutaneous injection; SCr ‐ serum creatinine; SLE ‐ systemic lupus erythematosus; TB‐ tuberculosis; TMP/SMX ‐ trimethoprim‐sulphamethoxazole; VDI ‐ Vasculitis Damage Index; WCC ‐ white cell count; WG ‐ Wegener's granulomatosis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Basu 2017 | Not induction or maintenance therapy, treating fatigue |
| CHUSPAN 2 2017 | Mixed population: EGPA, MPA or PAN |
| De Vita 2012 | Wrong population: patients with cryoglobulinaemic vasculitis |
| Harper 2018 | Not induction or maintenance therapy, treating fatigue |
| Imai 2006 | Wrong patient population, mostly IgA; there were 5 patients with likely ANCA vasculitis in one group |
| Ribi 2010 | Excluded patients with kidney disease (raised Cr and proteinuria) and they were only randomised after a period of steroid treatment. It is a study of treatment failure and relapse treatment rather than induction or maintenance therapy |
| Rifle 1990 | Wrong diagnosis for most patients. Immunoglobulin deposits demonstrated in renal biopsies |
ANCA ‐ anti‐neutrophil cytoplasmic antibody; RCT ‐ randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Chen 2011c.
| Methods | Parallel RCT |
| Participants | 19 patients with newly diagnosed ANCA‐associated small vessel vasculitis |
| Interventions | MMF versus CPA for 6 months |
| Outcomes | BVAS, renal insufficiency, anaemia, pulmonary infection, severe infection |
| Notes | Abstract‐only publication; data cannot be extracted/meta‐analysed |
CLASSIC 2016.
| Methods | Parallel RCT |
| Participants | Patients with relapsing AAV and PR3 or MPO ANCA |
| Interventions | Standard care + placebo (n = 13) Standard care + 10 mg CCX168 twice daily (n = 13) Standard care + 30 mg CCX168 twice daily (n = 16) All patients received RTX or CPA |
| Outcomes | BVAS at 12 weeks Serious adverse events: infection‐related |
| Notes | Abstract‐only publication; data cannot be extracted/meta‐analysed Completed but no results posted as of November 2019 |
Henderson 2009.
| Methods | Pilot parallel RCT |
| Participants | Patients with AAC |
| Interventions |
Additional therapies: all patients subsequently receiving a standard reducing regime of MTX and prednisolone |
| Outcomes |
|
| Notes | Abstract‐only publication; data cannot be extracted/meta‐analysed |
MAINTANCAVAS 2017.
| Methods | Parallel, open‐label RCT |
| Participants | ANCA vasculitis as defined by a positive MPO‐ and/or PR3‐ANCA test together with clinical features characteristic of ANCA‐positive diseases as detailed in the 2012 Chapel Hill Consensus Conference Definitions eGFR > 30 mL/min/1.73 m2; 18 to 82 years; treated with RTX‐induced continuous B cell depletion and in remission (defined by a modified BVAS‐WG=0 AND a prednisone dose ≤ 7.5 mg) for at least 24 months. CD20 (B cells) undetectable at time of enrolment/randomisation; urine HCG negative for women of child bearing potential and not planning to become pregnant for at least 12 months from enrolment and at least 12 months after any study related RTX dose; judged to be otherwise healthy by the Investigator, based on medical history and physical examination (no known active disease process for which life expectancy is less than 36 months) Exclusion criteria: secondary disease: disease suspected to be induced by levamisole‐adulterated cocaine; all transplanted patients Treatment: additional immunosuppressive agents other than RTX and/or total daily prednisone dose > 7.5 mg; hypogammaglobulinaemia: IgG level < 250 mg/dL; terminal cancer or other primary illness with life expectancy < 36 months; active anti‐GBM disease and other known autoimmune disease for which the need for additional immunosuppression is likely; pregnancy or breastfeeding |
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Notes |
|
Pagnoux 2003.
| Methods | Parallel, open‐label RCT |
| Participants | Patients with newly diagnosed Churg Strauss Syndrome |
| Interventions | 6 versus 12 pulse CPA |
| Outcomes | Remission Relapse Death Severe adverse effects |
| Notes | Abstract‐only publication; data cannot be extracted/meta‐analysed |
RATTRAP 2015.
| Methods | Parallel RCT |
| Participants | 18 years and older |
| Interventions | Infliximab versus RTX |
| Outcomes | Primary outcome measures
Partial or complete remission of the vasculitides Secondary outcome measures To study the safety and adverse effects of both regimens MPA, WG, Churg‐Strauss syndrome |
| Notes | Study completed, last updated 19 November 2017; no data available |
AAV ‐ ANCA Associated Vasculitis; ANCA ‐ anti‐neutrophil cytoplasmic antibody; AZA ‐ azathioprine; BVAS ‐ Birmingham Vasculitis Activity Score; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; FFP ‐ Fresh Frozen Plasma; GBM ‐ glomerular basement membrane; GN ‐ glomerulonephritis; HD ‐ haemodialysis; HSP ‐ Henoch Schonlein Purpura; IV ‐ intravenous; IVIg ‐ IV immunoglobulin; MP ‐ methylprednisolone; MPA ‐ microscopic polyangiitis; RCT ‐ randomised controlled trial; RPGN ‐ rapidly progressive glomerulonephritis; SLE ‐ systemic lupus erythematosus; WG ‐ Wegener's Granulomatosis.
Characteristics of ongoing studies [ordered by study ID]
ADVOCATE 2019.
| Trial name or title | A randomised phase 3 trial evaluating the safety and efficacy of avacopan in patients with new or relapsing ANCA‐associated vasculitis |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary
|
| Starting date | December 2016 |
| Contact information | Study Director: Cass Kelleher, MD. ChemoCentryx, Inc. |
| Notes | Abstract only publication. Recruitment status: Active, not recruiting. |
ALEVIATE 2018.
| Trial name or title | Alemtuzumab for ANCA Associated Refractory Vasculitis (ALEVIATE) |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group A
Treatment group B
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | February 2011 |
| Contact information | Dr David Jayne, Cambridge University Hospitals NHS Foundation Trust |
| Notes | Abstract only publication. Recruitment status: unknown last updated July 2011. |
CANVAS 2016.
| Trial name or title | CMV Modulation of the Immune System in ANCA‐associated Vasculitis (CANVAS) |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | 4 July 2012 |
| Contact information | Professor Lorraine Harper, University of Birmingham |
| Notes |
|
COMBIVAS 2019.
| Trial name or title | Rituximab and Belimumab Combination Therapy in PR3 COMBIVAS |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | February 2019 |
| Contact information | Rachel Jones, Cambridge University Hospitals NHS Foundation Trust |
| Notes | Recruiting. Estimated completion February 2022 |
MAINRITSAN 3 2015.
| Trial name or title | Comparison Between a Long Term and a Conventional Maintenance Treatment With Rituximab (MAINRITSAN3) |
| Methods |
|
| Participants | Inclusion criteria
MAINRITSAN 2 inclusion criteria
Patients must also meet all of the following criteria:
Exclusion criteria:
|
| Interventions | Treatment group
Control group
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | March 31, 2015 |
| Contact information | Study Chair: Loic GUILLEVIN, MD‐PhD. Assistance Publique ‐ Hôpitaux de Paris. |
| Notes | Ongoing. Recruitment finalised. Estimated completion September 2018. No outcome data published. |
MUPIBAC 2004.
| Trial name or title | The prevention of relapses of Wegeners granulomatosis by the elimination of nasal S aureus carriage: a multicentre randomised study |
| Methods | RCT |
| Participants | Early systemic or generalized WG with GFR > 50 mL/min (in remission after 18 months) |
| Interventions | Mupirocin ointment vs placebo |
| Outcomes | Relapse and infection rates between 18‐42 months |
| Starting date | |
| Contact information | www.bantao.org/2_2/2_2_1.pdf |
| Notes | Recruitment suspended due to problems with the supply of the trial ointment. The protocol is presently being redesigned |
NCT03323476.
| Trial name or title | Maintaining or stopping immunosuppressive therapy in patients with ANCA vasculitis and end‐stage renal disease: a prospective, multicenter, randomised, open‐label, clinical trial |
| Methods | Parallel, open‐label RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes | The primary end point will be the time between inclusion and the first severe prejudicial event (measured in days) during 24 months of follow‐up (24 months) Severe prejudicial event is defined by the occurrence of: severe infection, major AAV relapse, death |
| Starting date | 2 February 2018 |
| Contact information | Chloé MOREAU chloe.moreau@chd‐vendee.fr |
| Notes |
RITAZAREM 2013.
| Trial name or title | An international, open label, randomised controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA‐associated vasculitis |
| Methods | Parallel, open‐label RCT |
| Participants | Patients with established ANCA vasculitis whose disease has come back 'relapsing vasculitis' |
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | April 2013 |
| Contact information | David Jayne: Cambridge University Hospitals NHS Foundation Trust Peter Merkel: University of Pennsylvania |
| Notes | Active, not recruiting |
Tuin 2019.
| Trial name or title | Comparative study of the efficacy of induction therapy with cyclophosphamide or mycophenolate mofetil for non‐life‐threatening relapses of PR3‐ or MPO‐ANCA associated vasculitis |
| Methods | Open RCT, safety and efficacy study |
| Participants | Males and females ≥ 18 years |
| Interventions | When relapses occur, patients will be randomised for either the standard therapy with CPA or for MMF |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | December 2004 |
| Contact information | Patricia M. Stassen, M.D. +31503611295 p.m.stassen@int.umcg.nl |
| Notes |
ANCA ‐ anti‐neutrophil cytoplasmic antibody; AZA ‐ azathioprine; BVAS ‐ Birmingham Vasculitis Activity Score; CPA ‐ cyclophosphamide; eGFR ‐ estimated glomerular filtration rate; IVIg ‐ intravenous immunoglobulin; MMF ‐ mycophenolate mofetil; MPO ‐ myeloperoxidase; MTX ‐ methotrexate; PR3 ‐ proteinase‐3; RCT ‐ randomised controlled trial; rituximab ‐ RTX; WG ‐ Wegener's granulomatosis
Differences between protocol and review
Risk of bias assessment tool has replaced quality assessment checklist (Walters 2001).
Contributions of authors
GW: study selection, quality assessment, data extraction, review writing
NW: study selection, quality assessment, data extraction, review writing
TC: quality assessment, data extraction, review writing
JC: review writing, resolution of disagreements
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This review is funded by the National Institute for Health Research (NIHR) Cochrane Incentive Scheme 2018 (NIHR128416). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declarations of interest
None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Adu 1997 {published data only}
- Adu D, Pall A, Luqmani RA, Richards NT, Howie AJ, Emery P, et al. Controlled trial of pulse versus continuous prednisolone and cyclophosphamide in the treatment of systemic vasculitis. Qjm 1997;90(6):401‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pall A, Lugmani RA, Adu D, Richards N, Howie AJ, Emery P, et al. Controlled trial of pulse cyclophosphamide (PCY) and prednisolone (PP) versus continuous cyclophosphamide (CCY) and prednisolone (CP) in the treatment of systemic vasculitis [abstract]. Journal of the American Society of Nephrology 1992;3(3):317. [CENTRAL: CN‐00461459] [DOI] [PubMed] [Google Scholar]
AZA‐ANCA 2016 {published data only}
- Sanders JF, Joode AA, DeSevaux RG, Broekroelofs J, Voskuyl AE, Paassen P, et al. Extended versus standard azathioprine maintenance therapy in newly diagnosed proteinase‐3 anti‐neutrophil cytoplasmic antibody‐associated vasculitis patients who remain cytoplasmic anti‐neutrophil cytoplasmic antibody‐positive after induction of remission: a randomized clinical trial. Nephrology Dialysis Transplantation 2016;31(9):1453‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boomsma 2003 {published data only}
- Boomsma MM, Stegeman CA, Hermans J, Kallenberg CG, Hene RJ, Limburg PC, et al. Prevention of relapses in PR3 anti‐neutrophil cytoplasmic antibody (ANCA) associated vasculitis by treatment with azathioprine and corticosteroids: a multi‐centre, randomized study [abstract no: 020]. 11th International Vasculitis & ANCA Workshop; 2003 Oct 2‐5; Prague, Czech Republic. 2003:29‐30. [CENTRAL: CN‐00460433]
- Boomsma MM, Stegeman CA, Hermans J, Kallenberg CGM, Hene RJ, Limburg PC, et al. Prevention of relapses in PR3 anti‐neutrophil cytoplasmic antibody associated vasculitis by treatment with azathioprine and corticosteroids: a multi‐centre, randomized study [abstract no: T208]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):347‐8. [CENTRAL: CN‐00444485] [Google Scholar]
BREVAS 2019 {published data only}
- Jayne D, Blockmans D, Luqmani R, Ji B, Green Y, Hall L, et al. Belimumab in combination with azathioprine for remission maintenance in granulomatosis with polyangiitis and microscopic polyangiitis: effect on biomarkers [abstract]. Annals of the Rheumatic Diseases 2018;77(Suppl 2):1488. [EMBASE: 623994409] [Google Scholar]
- Jayne D, Blockmans D, Luqmani R, Ji B, Green Y, Hall L, et al. Efficacy and safety of belimumab in combination with azathioprine for remission maintenance in granulomatosis with polyangiitis and microscopic polyangiitis: a multicenter randomized, placebo‐controlled study [abstract no: 893]. Arthritis and Rheumatology 2017;69(Suppl 10):NA. [EMBASE: 618912517] [Google Scholar]
- Jayne D, Blockmans D, Luqmani R, Moiseev S, Ji B, Green Y, et al. Efficacy and safety of belimumab and azathioprine for maintenance of remission in antineutrophil cytoplasmic antibody‐associated vasculitis: a randomized controlled study. Arthritis & Rheumatology 2019;71(6):952‐63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CLEAR 2013 {published data only}
- Bekker P, Jayne D, Bruchfeld A, Schaier M, Ciechanowski K, Harper L, et al. CCX168, an orally administered C5aR inhibitor for treatment of patients with antineutrophil cytoplasmic antibody‐associated vasculitis [abstract no: 1863]. Arthritis & Rheumatology 2014;66(Suppl 10):S820. [EMBASE: 71737855] [Google Scholar]
- Bekker P, Potarca A, Dairaghi D, Miao S, Powers JP, Jaen JC, et al. Oral C5A receptor antagonist CCX168 in a phase 2 clinical trial in ANCA‐associated renal vasculitis [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii419‐20. [EMBASE: 70766535] [Google Scholar]
- Bruchfeld A, Schaier M, Harper L, Jadoul MY, Segelmark M, Szombati I, et al. Phase 2 clinical trial of CCX168, an orally administered C5aR antagonist, in patients with ANCA‐associated renal vasculitis (CLEAR) [abstract no: SA‐PO695]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):788A. [Google Scholar]
- Bruchfeld A, Schaier M, Jadoul M, Selga D, Szombati I, Venning M, et al. C5aR inhibitor on leukocytes exploratory ANCA associated renal vasculitis (CLEAR) clinical trial with orally administered CCX168 [abstract no: P206]. Presse Medicale 2013;42(4):768. [EMBASE: 71944014] [Google Scholar]
- Bunch DO, McInnis EA, Bekker P, Hillson JL, Schall TJ, Jennette CJ, et al. Effect of a selective C5AR antagonist, avacopan (CCX168), on plasma complement levels in ANCA associated vasculitis (AAV) [abstract]. Annals of the Rheumatic Diseases 2018;77(Suppl 2):74‐5. [EMBASE: 623992027] [Google Scholar]
- Jayne D, Bruchfeld A, Harper L, Schaier M, Venning M, Hamilton P, et al. Successful steroid replacement in ANCA‐associated vasculitis with c5a receptor inhibitor CCX168 in phase 2 randomised trial (CLEAR) [abstract no: MO039]. Nephrology Dialysis Transplantation 2016;31(Suppl 1):i45. [EMBASE: 72326021] [Google Scholar]
- Jayne D, Potarca A, Schall T, Hilson J, Becker P. Adverse events with glucocorticoid standard of care versus avacopan in ANCA‐associated vasculitis: observations from the CLEAR trial [abstract]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):i85‐6. [EMBASE: 622606065] [Google Scholar]
- Jayne DR, Bruchfeld A, Schaier M, Ciechanowski K, Harper L, Jadoul M, et al. Phase 2 randomised trial of oral C5A receptor antagonist CCX168 in ANCA‐associated renal vasculitis [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii27. [EMBASE: 71491530] [Google Scholar]
- Jayne DR, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA‐associated vasculitis. Journal of the American Society of Nephrology 2017;28(9):2756‐67. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayne DRW, Bruchfeld A, Schaier M, Ciechanowski K, Harper L, Jadoul M, et al. Phase 2 randomised trial of oral C5A receptor antagonist CCX168 in ANCA‐associated renal vasculitis [abstract no: MO012]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii27. [EMBASE: 71491530] [Google Scholar]
- Schall T, Potarca A, Hillson J, Bekker P. C5ar inhibitor avacopan in anti‐neutrophil cytoplasmic autoantibody (ANCA) vasculitis phase II outcomes of safety, renal parameters, and quality of life [abstract]. American Journal of Kidney Diseases 2018;71(4):582. [EMBASE: 621595967] [Google Scholar]
- Tesar V, Jayne D, Bruchfeld A, Harper L, Schaier M, Hamilton P, et al. Rapid onset of orally administered C5aR inhibitor CCX168 in randomized clinical trial in ANCA‐associated vasculitis (CLEAR) [abstract no: TH‐OR037]. Journal of the American Society of Nephrology 2016;27(Abstracts):9A. [CENTRAL: CN‐01658058] [Google Scholar]
Cole 1992 {published data only}
- Cole E, Cattran D, Magil A, Greenwood C, Churchill D, Sutton D, et al. A prospective randomized trial of plasma exchange as additive therapy in idiopathic crescentic glomerulonephritis. The Canadian Apheresis Study Group. American Journal of Kidney Diseases 1992;20(3):261‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CORTAGE 2015 {published data only}
- Pagnoux C, Quemeneur T, Ninet J, Diot E, Kyndt X, Wazieres B, et al. Treatment of systemic necrotizing vasculitides in patients aged sixty‐five years or older: results of a multicenter, open‐label, randomized controlled trial of corticosteroid and cyclophosphamide‐based induction therapy. Arthritis & Rheumatology 2015;67(4):1117‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pagnoux C, Rollot F, Vanhille P, Ninet J, Diot E, Kyndt X, et al. Baseline data and interim induction analysis based on the 101 patients aged ≥ 65 years with SNV enrolled in the CORTAGE multi‐centre trial [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):63. [EMBASE: 70622655] [Google Scholar]
CYCAZAREM 2003 {published data only}
- Flossmann O, Jayne DR. Demographic data in ANCA associated vasculitis [abstract no: 90]. Kidney & Blood Pressure Research 2005;28(3):189. [CENTRAL: CN‐00583623] [Google Scholar]
- Flossmann O, Jayne DR. Demographic data in ANCA associated vasculitis [abstract no: F‐PO998]. Journal of the American Society of Nephrology 2005;16:554A. [CENTRAL: CN‐01912399] [Google Scholar]
- Goceroglu A, Berden AE, Fiocco M, Flosmann O, Westman KW, Ferrario F, et al. ANCA‐associated glomerulonephritis: risk factors for renal relapse. PLoS ONE [Electronic Resource] 2016;11(12):e0165402. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goek ON, Stone JH. Randomized controlled trials in vasculitis associated with anti‐neutrophil cytoplasmic antibodies. Current Opinion in Rheumatology 2005;17(3):257‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Harper L, Verburgh K, Savage CO, Jayne D. Adverse events in patients with primary systemic vasculitis [abstract no: SA‐PO271]. Journal of the American Society of Nephrology 2004;15(Oct):360A. [CENTRAL: CN‐00583612] [Google Scholar]
- Hauer HA, Bajema IM, Houwelingen HC, Ferrario F, Noel LH, Waldherr R, et al. Determinants of outcome in ANCA‐associated glomerulonephritis: a prospective clinico‐histopathological analysis of 96 patients. Kidney International 2002;62(5):1732‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jayne D, Gaskin G, European Vasculitis Study Group (EUVAS). Randomised trial of cyclophosphamide versus azathioprine during remission in ANCA‐associated systemic vasculitis (CYCAZAREM) [abstract no: A0540]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):105A. [CENTRAL: CN‐00583620] [Google Scholar]
- Jayne D, Rasmussen N, Andrassy K, Bacon P, Tervaert JW, Dadoniene J, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. New England Journal of Medicine 2003;349(1):36‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jayne DR, Pusey CD, European Vasculitis Study Group (EUVAS). European randomised trials in ANCA‐associated systemic vasculitis: a progressive report [abstract]. 35th Congress of the European Renal Association European Dialysis & Transplant Association; 1998 Jun 6‐9; Rimini (Italy). 1998:106. [CENTRAL: CN‐00550613]
- Rahmattulla C, Lind van Wijngaarden RA, Berden AE, Hauer HA, Flosmann O, Jayne DR, et al. Renal function and ear, nose, throat involvement in anti‐neutrophil cytoplasmic antibody‐associated vasculitis: prospective data from the European Vasculitis Society clinical trials. Rheumatology 2015;54(5):899‐907. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salmela A, Rasmussen N, Tervaert JW, Jayne DR, Ekstrand A, European Vasculitis Study Group. Chronic nasal Staphylococcus aureus carriage identifies a subset of newly diagnosed granulomatosis with polyangiitis patients with high relapse rate. Rheumatology 2017;56(6):965‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suppiah R, Hadden RD, Batra R, Arden NK, Collins MP, Guillevin L, et al. Peripheral neuropathy in ANCA‐associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology 2011;50(12):2214‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vankova Z, Rihova Z, Jancova E, Rysava R, Merta M, Tesar V. Optimizing the therapeutic strategies in ANCA‐associated vasculitis‐‐single centre experience with international randomized trials. Prague Medical Report 2006;107(2):199‐212. [MEDLINE: ] [PubMed] [Google Scholar]
- Walsh M, Faurschou M, Berden A, Flossmann O, Bajema I, Hoglund P, et al. Long‐term follow‐up of cyclophosphamide compared with azathioprine for initial maintenance therapy in ANCA‐associated vasculitis. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(9):1571‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CYCLOPS 2004 {published data only}
- Harper L, Morgan M, Flossman O, Westman K, Groot K, Flores Suarez LF, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA‐associated vasculitis [abstract no: 079]. Renal Association & British Renal Society Joint Meeting; 2011 Jun 6‐9; Birmingham, UK. 2011:908.
- Harper L, Morgan MD, Flossman O, Westman K, Groot K, Flores Suarez LF, et al. Pulse versus daily oral CyP for induction of remission in ANCA‐associated vasculitis. A European, multi‐centre randomized controlled trial: long‐term follow up [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):56. [EMBASE: 70622641] [Google Scholar]
- Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA‐associated vasculitis: long‐term follow‐up. Annals of the Rheumatic Diseases 2012;71(6):955‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Morgan MD, Szeto M, Marsh J, Walsh M, Jayne D, Westman K, et al. Remaining anti‐neutrophil cytoplasm antibody positive at switch to maintenance therapy is associated with an increased risk of relapse: observation from the long term follow up of the CYCLOPS and IMPROVE trials [abstract no: P20]. Nephron 2015;129(Suppl 2):115. [EMBASE: 623780483] [Google Scholar]
- Morgan MD, Szeto M, Walsh M, Jayne D, Westman K, Rasmussen N, et al. Negative anti‐neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Research & Therapy 2017;19(1):129. [EMBASE: 616622020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmattulla C, Lind van Wijngaarden RA, Berden AE, Hauer HA, Flosmann O, Jayne DR, et al. Renal function and ear, nose, throat involvement in anti‐neutrophil cytoplasmic antibody‐associated vasculitis: prospective data from the European Vasculitis Society clinical trials. Rheumatology 2015;54(5):899‐907. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rihova Z, Jancova E, Merta M, Zabka J, Rysava R, Bartunkova J, et al. Daily oral versus pulse intravenous cyclophosphamide in the therapy of ANCA‐associated vasculitis‐‐preliminary single center experience. Prague Medical Report 2004;105(1):64‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Suppiah R, Hadden RD, Batra R, Arden NK, Collins MP, Guillevin L, et al. Peripheral neuropathy in ANCA‐associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology 2011;50(12):2214‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody‐associated vasculitis: a randomized trial. Annals of Internal Medicine 2009;150(10):670‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groot K, Jayne DR, Tesar V, Savage CO, European Vascular Study Group. European, multicenter randomised controlled trial of daily oral versus pulse cyclophosphamide for induction of remission in ANCA‐associated systemic vasculitis [abstract no: TH‐FC033]. Journal of the American Society of Nephrology 2005;16:7A. [Google Scholar]
- Groot K, Jayne DR, Tesar V, Savage CO, EUVAS Group. Randomised controlled trial of daily oral versus pulse cyclophosphamide for induction of remission in ANCA‐associated systemic vasculitis [abstract no: 103]. Kidney & Blood Pressure Research 2005;28(3):195. [CENTRAL: CN‐00644300] [Google Scholar]
Furuta 1998 {published data only}
- Furuta T, Hotta O, Chiba S, Horigome I, Taguma Y. Lymphocytapheresis (LCP) in rapidly progressive glomerulonephritis (RPGN) a comparison with steroid pulse therapy [abstract no: P208]. Nephrology 1997;3(Suppl 1):S127. [CENTRAL: CN‐00460781] [Google Scholar]
- Furuta T, Hotta O, Yusa N, Horigome I, Chiba S, Taguma Y. Lymphocytapheresis to treat rapidly progressive glomerulonephritis: a randomised comparison with steroid‐pulse treatment. Lancet 1998;352(9123):203‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glockner 1988 {published data only}
- Glockner WM, Sieberth HG, Wichmann HE, Backes E, Bambauer R, Boesken WH, et al. Plasma exchange and immunosuppression in rapidly progressive glomerulonephritis: a controlled, multi‐center study. Clinical Nephrology 1988;29(1):1‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Guillevin 1997 {published data only}
- Girard T, Mahr A, Noel LH, Cordier JF, Lesavre P, Andre MH, et al. Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener's granulomatosis? A prospective study. Rheumatology 2001;40(2):147‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, Mahr A, Pagnoux C, Cohen P, Mouthon L, Guillevin L, et al. Long‐term outcome of 37 patients with Wegener's granulomatosis with renal involvement. Presse Medicale 2007;36(5 Pt 1):771‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guillevin L, Cordier JF, Lhote F, Cohen P, Jarrousse B, Royer I, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis. Arthritis & Rheumatism 1997;40(12):2187‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guillevin 2003 {published data only}
- Guillevin L, Cohen P, Mahr A, Arene JP, Mouthon L, Puechal X, et al. Treatment of polyarteritis nodosa (PAN) and microscopic polyangiitis (MPA) with poor prognosis factors: a prospective trial comparing steroids (CS) and 6 or 12 cyclophosphamide (CYC) pulses in 65 patients [abstract no: 112‐113]. Cleveland Clinic Journal of Medicine 2002;69(Suppl II):SII‐189. [CENTRAL: CN‐01912272] [Google Scholar]
- Guillevin L, Cohen P, Mahr A, Arene JP, Mouthon L, Puechal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis with poor prognosis factors: a prospective trial comparing glucocorticoids and six or twelve cyclophosphamide pulses in sixty‐five patients. Arthritis & Rheumatism 2003;49(1):93‐100. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Han 2011b {published data only}
- Han F, Liu G, Zhang X, Li Xi, He Q, He X, et al. Effects of mycophenolate mofetil combined with corticosteroids for induction therapy of microscopic polyangiitis. American Journal of Nephrology 2011;33(2):185‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haubitz 1998 {published data only}
- Haubitz M, Brunkhorst R, Schellong S, Gobel U, Schurek HJ, Koch KM. ANCA‐associated vasculitis and renal involvement: preliminary results of a prospective randomised study comparing daily oral versus monthly I.V. cyclophosphamide application [abstract]. Journal of the American Society of Nephrology 1995;6(3):922. [CENTRAL: CN‐00484270] [Google Scholar]
- Haubitz M, Schellong S, Gobel U, Schurek HJ, Schaumann D, Koch KM, et al. Intravenous pulse administration of cyclophosphamide versus daily oral treatment in patients with antineutrophil cytoplasmic antibody‐associated vasculitis and renal involvement: a prospective, randomized study. Arthritis & Rheumatism 1998;41(10):1835‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hu 2008b {published data only}
- Hu W, Liu C, Xie H, Chen H, Liu Z, Li L. Mycophenolate mofetil versus cyclophosphamide for inducing remission of ANCA vasculitis with moderate renal involvement. Nephrology Dialysis Transplantation 2008;23(4):1307‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
IMPROVE 2003 {published data only}
- Hiemstra T, Walsh M, Andrews P, Short A, Savage C, Harper L, et al. Randomized controlled trial of mycophenolate mofetil versus azathioprine for maintenance therapy in ANCA‐associated vasculitis (IMPROVE) [abstract no: O23]. British Renal Society Conference; 2010 May 17‐May 20; Manchester, UK. 2010. [CENTRAL: CN‐01912269]
- Hiemstra TF, Walsh M, Mahr A, Savage CO, Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody‐associated vasculitis: a randomized controlled trial. JAMA 2010;304(21):2381‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hiemstra TF, Walsh MW, Schmitt W, Jayne DR. Randomised controlled trial of mycophenolate mofetil versus azathioprine for maintenance therapy in ANCA associated vasculitis (IMPROVE) [abstract no: SA‐FC331]. Journal of the American Society of Nephrology 2009;20(Abstracts):77A. [CENTRAL: CN‐01912270] [Google Scholar]
- IMPROVE Co‐ordinators. International mycophenolate mofetil protocol to reduce outbreaks of vasculitides (IMPROVE). www.vasculitis.org/images/documents/improve.pdf (accessed 16 December 2019).
- Morgan MD, Szeto M, Walsh M, Jayne D, Westman K, Rasmussen N, et al. Negative anti‐neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Research & Therapy 2017;19(1):129. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WH, Jayne D, Woude FJ, European Vasculitis Study Group (EUVAS). Mycophenolate mofetil (MMF) vs azathioprine (AZA) for the maintenance of remission in ANCA‐associated vasculitis: the IMPROVE protocol [abstract no: P90]. 11th International Vasculitis & ANCA Workshop; 2003 Oct 2‐5; Prague, Czech Republic. 2003:51. [CENTRAL: CN‐00461684]
Jayne 2000 {published data only}
- Jayne DR, Chapel H, Adu D, Misbah S, O'Donoghue D, Scott D, et al. Intravenous immunoglobulin for ANCA‐associated systemic vasculitis with persistent disease activity. Qjm 2000;93(7):433‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MAINRITSAN 2014 {published data only}
- Guillevin L, Karras A, Pagnoux C, Carron P, Quemeneur T, Gobert P, et al. Rituximab versus azathioprine for maintenance in ANCA‐associated vasculitis. A prospective study in 117 patients [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii28. [EMBASE: 71491532] [Google Scholar]
- Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA‐associated vasculitis. New England Journal of Medicine 2014;371(19):1771‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in antineutrophil cytoplasmic antibodies (ANCA)‐associated vasculitis [abstract]. Arthritis & Rheumatism 2012;64(10 Suppl):S706. [EMBASE: 70999648] [Google Scholar]
- Guillevin TL, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Relationship between infectious side effects and immunoglobulin levels in the maintenance rituximab vs azathioprine for ANCA‐associated vasculitides [abstract no: 744]. Arthritis & Rheumatism 2013;65(10 Suppl):S314. [EMBASE: 71318224] [Google Scholar]
- Montante A, Bras A, Terrier B, Cohen P, Puechal X, Karras A, et al. Economic evaluation of rituximab versus azathioprine for maintenance treatment of ANCA‐associated vasculitis. A prospective, multicenter study [abstract]. Arthritis & Rheumatology 2017;69(Suppl 10):na. [EMBASE: 618916781] [Google Scholar]
- Pugnet G, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for ANCA‐associated vasculitis maintenance therapy: impact in health‐related quality of life [abstract no: 1778]. Arthritis & Rheumatology 2014;66(10 Suppl):S780. [EMBASE: 71737772] [PubMed] [Google Scholar]
- Pugnet G, Pagnoux C, Terrier B, Perrodeau E, Puechal X, Karras A, et al. Rituximab versus azathioprine for ANCA‐associated vasculitis maintenance therapy: impact on global disability and health‐related quality of life. Clinical & Experimental Rheumatology 2016;34(3 Suppl 97):S54‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Terrier B, Pagnoux C, Geri G, Karras A, Khouatra C, Aumaitre O, et al. Factors predictive of ANCA‐associated vasculitis relapse in patients given rituximab‐maintenance therapy [abstract no: 1776]. Arthritis & Rheumatology 2014;66(10 Suppl):S779. [EMBASE: 71737770] [Google Scholar]
- Terrier B, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Factors predictive of ANCA‐associated vasculitis relapse in patients given rituximab‐maintenance therapy: data from the extended follow‐up of MAINRITSAN trial patients [abstract no: O19]. Nephron 2015;129(Suppl 2):55‐6. [CENTRAL: CN‐01646825] [Google Scholar]
- Terrier B, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in antineutrophil cytoplasmic antibodies (ANCA)‐associated vasculitis (MAINRITSAN): Follow‐up at 34 months [abstract no: P230]. Press Medicale 2013;42(4):778‐9. [EMBASE: 71944038] [Google Scholar]
- Terrier B, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in antineutrophil cytoplasmic antibodies (ANCA)‐associated vasculitis (MAINRITSAN): follow up at 34 months [abstract no: OP0213]. Annals of the Rheumatic Diseases 2013;72(Suppl 3):A124. [EMBASE: 71332503] [Google Scholar]
- Terrier B, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in antineutrophil cytoplasmic antibodies‐associated vasculitis: follow up at 39 months [abstract no: 2783]. Arthritis & Rheumatism 2013;65(10 Suppl):S1190‐1. [EMBASE: 71320239] [Google Scholar]
- Terrier B, Pagnoux C, Perrodeau E, Karras A, Khouatra C, Aumaitre O, et al. Long‐term efficacy of remission‐maintenance regimens for ANCA‐associated vasculitides. Annals of the Rheumatic Diseases 2018;77(8):1150‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Terrier B, Pagnoux C, Perrodeau E, Karras A, Khouatra C, Aumaitre O, et al. Rituximab versus azathioprine to maintain remission of ANCA‐associated vasculitides (MAINRITSAN): Follow‐up at 60 months [abstract]. Rheumatology 2017;56(Suppl 3):iii16. [EMBASE: 619334509] [Google Scholar]
MAINRITSAN 2 2018 {published data only}
- Charles P, Dechartres A, Terrier B, Guillevin L. Reducing the number of rituximab infusions at onset of maintenance therapy for ANCA‐associated vasculitides: results of a post hoc analysis from a randomized‐controlled trials [abstract]. Rheumatology 2019;58(Suppl 2):na. [EMBASE: 628291931] [Google Scholar]
- Charles P, Terrier B, Cohen P, Faguer S, Huart A, Hamidou M, et al. Comparison of systematic vs individually tailored rituximab regimen to maintain ANCA associated vasculitis remission [abstract]. Rheumatology 2017;56(Suppl 3):iii151. [EMBASE: 619334915] [Google Scholar]
- Charles P, Terrier B, Perrodeau E, Cohen P, Faguer S, Huart A, et al. Comparison of individually tailored versus fixed‐schedule rituximab regimen to maintain ANCA‐associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2).[Erratum appears in: Ann Rheum Dis. 2019 Sep;78(9):e101; PMID: 31405899]. Annals of the Rheumatic Diseases 2018;77(8):1143‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Charles P, Terrier B, Perrodeau E, Cohen P, Faguer S, Huart A, et al. Comparison of individually tailored vs systematic rituximab regimens to maintain ANCA‐associated vasculitis remissions: results of a prospective, randomized‐controlled, phase 3 trial [abstract]. Arthritis & Rheumatology 2017;69(Suppl 10). [EMBASE: 618916722] [Google Scholar]
Maritati 2017 {published data only}
- Maritati F, Alberici F, Oliva E, Urban ML, Palmisano A, Santarsia F, et al. Methotrexate versus cyclophosphamide for remission maintenance in ANCA‐associated vasculitis: a randomised trial. PLoS ONE [Electronic Resource] 2017;12(10):e0185880. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglio A, Casazza I, Grasselli C, Alberici F, Palmisano A, Andrulli S, et al. Methotrexate versus cyclophosphamide as maintenance therapy for ANCA‐associated vasculitides [abstract no: M318]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
Mauri 1985 {published data only}
- Mauri JM, Gonzalez MT, Poveda R. Therapeutic plasma exchange in the treatment of rapidly progressive glomerulonephritis. Plasma Therapy & Transfusion Technology 1985;6(3):587‐91. [EMBASE: 1986054364] [Google Scholar]
- Mauri JM, Gonzalez R, Poveda R, Seron D, Torras J, Andres E. Evaluation of plasma exchanges (PEX) in the treatment of rapidly progressive glomerulonephritis (RPGN) preliminary results of a randomized trial [abstract no: 231]. European Journal of Clinical Investigation 1985;15(2 (Pt II)):A39. [CENTRAL: CN‐00446666] [Google Scholar]
MEPEX 2007 {published data only}
- Casian A. Plasma exchange for severe renal vasculitis: long‐term follow‐up of the MEPEX trial [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):52. [EMBASE: 70622633] [Google Scholar]
- Casian AL, Walsh M, Jayne DR. A long‐term analysis of the MEPEX trial: plasma exchange for severe renal ANCA vasculitis [abstract no: FR‐OR290]. Journal of the American Society of Nephrology 2011;22(Abstract Suppl):70A. [Google Scholar]
- Flossmann O, Jayne DR. Demographic data in ANCA associated vasculitis [abstract no: 90]. Kidney & Blood Pressure Research 2005;28(3):189. [CENTRAL: CN‐00583623] [Google Scholar]
- Flossmann O, Jayne DR. Demographic data in ANCA associated vasculitis [abstract]. Journal of the American Society of Nephrology 2005;16(Abstracts):554A. [CENTRAL: CN‐01912399] [Google Scholar]
- Gaskin G, Jayne DR, European Vasculitis Study Group. Adjunctive plasma exchange is superior to methylprednisolone in acute renal failure due to ANCA‐associated glomerulonephritis [abstract no: F‐FC010]. Journal of the American Society of Nephrology 2002;13(Programs & Abstracts):2A. [CENTRAL: CN‐00445448] [Google Scholar]
- Goceroglu A, Berden AE, Fiocco M, Flosmann O, Westman KW, Ferrario F, et al. ANCA‐associated glomerulonephritis: risk factors for renal relapse. PLoS ONE [Electronic Resource] 2016;11(12):e0165402. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper L, Verburgh K, Savage CO, Jayne D. Adverse events in patients with primary systemic vasculitis [abstract no: SA‐PO271]. Journal of the American Society of Nephrology 2004;15(Oct):360A. [CENTRAL: CN‐00583612] [Google Scholar]
- Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, et al. Randomized trial of plasma exchange or high‐dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. Journal of the American Society of Nephrology 2007;18(7):2180‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jayne DR, Pusey CD, European Vasculitis Study Group (EUVAS). European randomised trials in ANCA‐associated systemic vasculitis: a progressive report [abstract]. 35th Congress of the European Renal Association European Dialysis & Transplant Association; 1998 Jun 6‐9; Rimini, Italy. 1998:106. [CENTRAL: CN‐00550613]
- Jayne DR, Rasmussen N. Treatment of antineutrophil cytoplasm autoantibody‐associated systemic vasculitis: initiatives of the European Community Systemic Vasculitis Clinical Trials Study Group. Mayo Clinic Proceedings 1997;72(8):737‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rahmattulla C, Lind van Wijngaarden RA, Berden AE, Hauer HA, Flosmann O, Jayne DR, et al. Renal function and ear, nose, throat involvement in anti‐neutrophil cytoplasmic antibody‐associated vasculitis: prospective data from the European Vasculitis Society clinical trials. Rheumatology 2015;54(5):899‐907. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suppiah R, Hadden RD, Batra R, Arden NK, Collins MP, Guillevin L, et al. Peripheral neuropathy in ANCA‐associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology 2011;50(12):2214‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vankova Z, Rihova Z, Jancova E, Rysava R, Merta M, Tesar V. Optimizing the therapeutic strategies in ANCA‐associated vasculitis‐‐single centre experience with international randomized trials. Prague Medical Report 2006;107(2):199‐212. [MEDLINE: ] [PubMed] [Google Scholar]
- Walsh M, Casian A, Flossmann O, Westman K, Hoglund P, Pusey C, et al. Long‐term follow‐up of patients with severe ANCA‐associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney International 2013;84(2):397‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lind van Wijngaarden RA, Hauer HA, Nagelkerke NJ, Jayne DR, Gaskin G, Rasmussen N, et al. Determinants of outcome in ANCA‐associated vasculitis: a prospective clinico‐histopathological analysis of 65 patients [abstract no: SA‐PO183]. Journal of the American Society of Nephrology 2004;15(Oct):340A. [CENTRAL: CN‐00583573] [Google Scholar]
- Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, et al. Chances of renal recovery for dialysis‐dependent ANCA‐associated glomerulonephritis. Journal of the American Society of Nephrology 2007;18(7):2189‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Gaskin G, Rasmussen N, et al. Clinical and histologic determinants of renal outcome in ANCA‐associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. Journal of the American Society of Nephrology 2006;17(8):2264‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Rasmussen N, Gaskin G, et al. Predictors of outcome in severe ANCA‐associated glomerulonephritis [abstract no: SA‐PO551]. Journal of the American Society of Nephrology 2005;16:677A. [CENTRAL: CN‐00583615] [Google Scholar]
- Lind van Wijngaarden RA, Hauer HA, Wolterbeek R, Jayne DR, Rasmussen N, Gaskin G, et al. Treatment decisions in severe ANCA‐associated glomerulonephritis [abstract no: F‐FC063]. Journal of the American Society of Nephrology 2005;16:52A. [CENTRAL: CN‐00583617] [Google Scholar]
Metzler 2007 {published data only}
- Metzler C, Miehle N, Manger K, Iking‐Konert C, Gross WL, Reinhold‐Keller E. Unexpected high relapse‐rate under oral methotrexate for maintenance of remission in Wegener's granulomatosis. The LEM‐trial comparing leflunomide versus methotrexat [abstract no: 106]. Kidney & Blood Pressure Research 2005;28:196. [CENTRAL: CN‐00653812] [Google Scholar]
- Metzler C, Miehle N, Manger K, Iking‐Konert C, Groot K, Hellmich B, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener's granulomatosis. Rheumatology 2007;46(7):1087‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MYCYC 2012 {published data only}
- Jayne D, Jones R, Harper L. MYCYC clinical trial protocol: A randomised clinical trial of mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA associated vasculitis [protocol Version 2A, 16th January 2007]. www.vasculitis.org/images/documents/mycyc.pdf (accessed 1 December 2019).
- Jones R, Harper L, Brogan P, Dahisveen K, Lanyon P, Jayne D. A randomised trial of mycophenolate mofetil versus cyclosphosphamide for remission induction of ANCA‐associated vasculitis: MYCYC [abstract no: O4]. British Transplantation Society & the Renal Association Joint Congress; 2013 Mar 13‐15; Bournemouth, UK. 2013.
- Jones R, Walsh M, European Vascular Study Group. A randomised trial of mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA‐associated vasculitis: MYCYC [abstract no: HI‐OR04]. Journal of the American Society of Nephrology 2012;23(Abstracts):3B. [CENTRAL: CN‐01912277] [Google Scholar]
- Jones RB, Harper L, Walsh M, Jayne DR. 18 month results from a randomised trial of mycophenolate mofetil versus cyclophosphamide for remission induction of severe ANCA‐associated vasculitis: MYCYC [abstract no: SA‐PO1077]. Journal of the American Society of Nephrology 2013;24(Abstract Suppl):2B. [Google Scholar]
- Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA‐associated vasculitis: a randomised, non‐inferiority trial. Annals of the Rheumatic Diseases 2019;78(3):399‐405. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NORAM 2005 {published data only}
- Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatism 2005;52(8):2461‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Faurschou M, Westman K, Rasmussen N, Groot K, Flossmann O, Hoglund P, et al. Brief Report: long‐term outcome of a randomized clinical trial comparing methotrexate to cyclophosphamide for remission induction in early systemic antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatism 2012;64(10):3472‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goek ON, Stone JH. Randomized controlled trials in vasculitis associated with anti‐neutrophil cytoplasmic antibodies. Current Opinion in Rheumatology 2005;17(3):257‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rasmussen N, Salmela A, Ekstrand A, Groot K, Gregorini G, Cohen Tervaert JW, et al. Changes in proteinase 3 anti‐neutrophil cytoplasm autoantibody levels in early systemic granulomatosis with polyangiitis (Wegener's) may reflect treatment rather than disease activity. Clinical & Experimental Rheumatology 2013;31(1 Suppl 75):S38‐44. [MEDLINE: ] [PubMed] [Google Scholar]
- Salmela A, Rasmussen N, Tervaert JW, Jayne DR, Ekstrand A, European Vasculitis Study Group. Chronic nasal Staphylococcus aureus carriage identifies a subset of newly diagnosed granulomatosis with polyangiitis patients with high relapse rate. Rheumatology 2017;56(6):965‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Suppiah R, Hadden RD, Batra R, Arden NK, Collins MP, Guillevin L, et al. Peripheral neuropathy in ANCA‐associated vasculitis: outcomes from the European Vasculitis Study Group trials. Rheumatology 2011;50(12):2214‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Groot K, Rasmussen N, Tervaert JW, Jayne D. Randomised trial of cyclophosphamide versus methotrexate for induction of remission in 'non‐renal' ANCA‐associated vasculitis [abstract no: F‐FC009]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):2A. [CENTRAL: CN‐01912278] [Google Scholar]
PEXIVAS 2013 {published data only}
- Casian A, Merkel P, Walsh M, Jayne D. PEXIVAS: design of a randomised controlled trial of plasma exchange and glucocorticoid dosing in severe ANCA associated vasculitis [abstract no: P259]. Renal Association & British Renal Society Joint Meeting; 2010 May 17‐20; Manchester, UK. 2010:363.
- European Vasculitis Study Group (EUVAS) and the Vasculitis Clinical Research Consortium (VCRC). Plasma exchange and glucocorticoid dosing in the treatment of anti‐neutrophil cytoplasm antibody associated vasculitis: an international randomized controlled trial (Protocol Version: 1.1). 2010. www.vasculitis.org/images/pexivas201.120protocol.pdf (accessed prior to 16 December 2019).
- Kono H, Uchida S, Muso E, Endo T, Kakita H, Hamano Y, et al. Demographic characteristics of Japanese participants in PEXIVAS as compared with REMIT‐JAV [abstract]. Rheumatology 2017;56(Suppl 3):iii150. [EMBASE: 619334902] [Google Scholar]
- Walsh M, Merkel P, Casian A, Jayne D. PEXIVAS: design of an RCT of PLEX and GC dosing in the treatment of severe ANCA‐AAV [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):67. [EMBASE: 70622662] [Google Scholar]
- Walsh M, Merkel P, Peh CA, Guillevin L, Fujimoto S, Ives N, et al. Plasma exchange and glucocorticoid dosing in the treatment of anti‐neutrophil cytoplasm antibody associated vasculitis: Baseline characteristics of a randomized controlled trial (PEXIVAS) [abstract]. Rheumatology 2017;56(Suppl 3):iii149. [EMBASE: 619334895] [Google Scholar]
- Walsh M, Merkel P, Peh CA, Szpirt W, Puechal X, Fujimoto S, et al. The effect of plasma exchange on end stage renal disease and death in patients with severe ANCA associated vasculitis [abstract no: LB01]. Nephrology Dialysis Transplantation 2018;33(Suppl 1):1636. [Google Scholar]
- Walsh M, Merkel PA, Jayne DR. The effect of plasma exchange and the effect of reduced‐dose oral glucocorticoids during remission induction in severe ANCA‐Associated vasculitis [abstract no: FR‐OR079]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):59. [Google Scholar]
- Walsh M, Merkel PA, Peh CA, Szpirt W, Guillevin L, Pusey CD, et al. Plasma exchange and glucocorticoid dosing in the treatment of anti‐neutrophil cytoplasm antibody associated vasculitis (PEXIVAS): protocol for a randomized controlled trial. Trials [Electronic Resource] 2013;14:73. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pusey 1991 {published data only}
- Pusey CD, Rees AJ, Evans DJ, Peters DK, Lockwood CM. Plasma exchange in focal necrotizing glomerulonephritis without anti‐GBM antibodies. Kidney International 1991;40(4):757‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RAVE 2010 {published data only}
- Abdulahad WH, Specks U, Bijma T, Phippard DJ, Karnell F, Lim N, et al. B‐cell depletion by rituximab affects the distribution of effector th‐cell subsets in patients with ANCA associated vasculitis [abstract]. Arthritis & Rheumatology 2017;69(Suppl 10):na. [EMBASE: 618916466] [Google Scholar]
- Berti A, Warner R, Johnson K, Cornec D, Schroder D, Kabat B, et al. Circulating cytokine profiles reflect ANCA specificity in patients with ANCA‐associated vasculitis [abstract]. Arthritis & Rheumatology 2017;69(Suppl 10):na. [EMBASE: 618916561] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti A, Warner R, Johnson K, Cornec D, Schroeder D, Kabat B, et al. Serum interleukin‐6 levels in antineutrophil cytoplasmic antibody‐associated vasculitis [abstract]. Annals of the Rheumatic Diseases 2018;77(Suppl 2):771. [EMBASE: 623991960] [Google Scholar]
- Cartin‐Ceba R, Indrakanti D, Fervenza F, Hoffman G, Kallenberg C, Langford C, et al. Association of Fc gamma receptor polymorphisms and outcomes in patients with ANCA‐associated vasculitis treated with rituximab [abstract no: O84]. Nephron 2015;129(Suppl 2):96. [CENTRAL: CN‐01912275] [Google Scholar]
- Clain J, Specks U, Stone J. The association of IgM ANCA and alveolar hemorrhage revisited [abstract no: P9]. Presse Medicale 2013;42(4 Pt 2):685. [EMBASE: 71943818] [Google Scholar]
- Cornec D, Kabat BF, Mills JR, Cheu M, Hummel AM, Schroeder DR, et al. Pharmacokinetics of rituximab and clinical outcomes in patients with anti‐neutrophil cytoplasmic antibody associated vasculitis. Rheumatology 2018;57(4):639‐50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draibe JB, Pepper RJ, Merkel PA, Salama AD. Serum calprotectin and disease relapse in ANCA‐associated vasculitis [abstract no: 1860]. Arthritis & Rheumatology 2014;66(10 Suppl):S818‐9. [EMBASE: 71737852] [Google Scholar]
- Fussner LA, Hummel AM, Schroeder D, Silva F, Cartin‐Ceba R, Snyder M, et al. Predictive value of a rise in PR3‐ANCA for relapse after complete remission in severe ANCA‐associated vasculitis [abstract no: P17]. Nephron 2015;129(Suppl 2):113‐4. [CENTRAL: CN‐01912274] [Google Scholar]
- Geetha D, Fervenza F. The efficacy of rituximab vs cyclophosphamide for the treatment of renal disease in ANCA‐associated vasculitis: the RAVE trial [abstract no: 1542]. Arthritis & Rheumatism 2012;64(Suppl 10):S660. [EMBASE: 70999538] [Google Scholar]
- Geetha D, Fervenza FC, RAVE‐ITN Research Group. The efficacy of rituximab versus cyclophosphamide for treatment of renal disease in ANCA‐associated vasculitis: the RAVE Trial [abstract no: SA‐OR099]. Journal of the American Society of Nephrology 2012;23(Abstracts):88A. [CENTRAL: CN‐01912263] [Google Scholar]
- Geetha D, Sethi S, Vriese AS, Specks U, Kallenberg CG, Lim N, et al. Interstitial immunostaining and renal outcomes in antineutrophil cytoplasmic antibody‐associated glomerulonephritis. American Journal of Nephrology 2017;46(3):231‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha D, Specks U, Stone JH, Merkel PA, Seo P, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis with renal involvement. Journal of the American Society of Nephrology 2015;26(4):976‐85. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland D, Naisbett‐Groet B, Chang S, Sungher D, Sawyer L, Diamantopoulos A. MabThera® (Rituximab) for the treatment of severe granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) ‐ a cost‐utility model for the United Kingdom [abstract no: P9]. Nephron 2015;129(Suppl 2):108‐9. [CENTRAL: CN‐01912264] [DOI] [PubMed] [Google Scholar]
- Hellmich B. Remission induction in ANCA‐associated vasculitis: Follow‐up data of the RAVE study [Remissionsinduktion bei ANCA‐assoziierten Vaskulitiden: Follow‐up‐Daten der RAVE‐Studie]. Zeitschrift fur Rheumatologie 2014;73(2):194‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Miloslavsky E, Specks U, Merkel P, Seo P, Spiera R, Langford C, et al. Outcomes of non‐severe relapses in ANCA‐associated vasculitis treated with glucocorticoids [abstract no: O61]. Nephron 2015;129(Suppl 2):81. [EMBASE: 623780476] [Google Scholar]
- Miloslavsky E, Specks U, Merkel P, Seo P, Spiera R, Langford C, et al. Retreatment with rituximab in the RAVE trial [abstract no: P229]. Presse Medicale 2013;42(4 Pt 2):778. [EMBASE: 71944037] [Google Scholar]
- Miloslavsky E, Specks U, Merkel P, Seo P, Spiera R, Langford C, et al. Safety of remission induction with rituximab versus cyclophosphamide in patients 65 and older with severe ANCA‐associated vasculitis [abstract no: P231]. Presse Medicale 2013;42(4 Pt 2):779. [EMBASE: 71944039] [Google Scholar]
- Miloslavsky E, Specks U, Merkel PA, Seo P, Spiera RF, Langford CA, et al. Efficacy of glucocorticoids to treat limited flares in ANCA‐associated vasculitis [abstract no: 747]. Arthritis & Rheumatism 2013;65(10 Suppl):S315‐6. [EMBASE: 71318227] [Google Scholar]
- Miloslavsky E, Specks U, Merkel PA, Seo P, Spiera RF, Langford CA, et al. Retreatment with rituximab in the Rituximab in ANCA‐Associated Vasculitis (RAVE) trial [abstract no: 2782]. Arthritis & Rheumatism 2013;65(10 Suppl):S1190. [EMBASE: 71320238] [Google Scholar]
- Miloslavsky E, Specks U, Merkel PA, Seo P, Spiera RF, Langford CA, et al. Safety of remission induction with rituximab versus cyclophosphamide in patients 65 and older with severe ANCA‐associated vasculitis [abstract no: 742]. Arthritis & Rheumatism 2013;65(10 Suppl):S313. [EMBASE: 71318222] [Google Scholar]
- Miloslavsky E, Specks U, Stone JH. Primary endpoint failure in the rituximab in ANCA‐associated vasculitis trial [abstract no: 1654]. Arthritis & Rheumatism 2012;64(10 Suppl):S707. [EMBASE: 70999650] [Google Scholar]
- Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatism 2013;65(9):2441‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Rituximab for the treatment of relapses in antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatology 2014;66(11):3151‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach P, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, et al. Serum proteins reflecting inflammation, injury, and repair as biomarkers of disease activity in ANCA‐associated vasculitis [abstract no: 792]. Arthritis & Rheumatism 2011;63(10 Suppl):S312. [EMBASE: 70786745] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach PA, Kumpers P, Lukasz A, Tomasson G, Specks U, Stone JH, et al. Circulating angiopoietin‐2 as a biomarker in ANCA‐associated vasculitis. PLoS One [Electronic Resource] 2012;7(1):e30197. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach PA, Tomasson G, Specks U, Stone JH, Cuthbertson D, Krischer J, et al. Circulating markers of vascular injury and angiogenesis in antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatism 2011;63(12):3988‐97. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monach PA, Warner RL, Tomasson G, Specks U, Stone JH, Ding L, et al. Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA‐associated vasculitis. Annals of the Rheumatic Diseases 2013;72(8):1342‐50. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah M, Pouliot Y, Hartmann B, Dunn P, Thomson E, Wiser J, et al. Reanalysis of the Rituximab in ANCA‐Associated Vasculitis trial identifies granulocyte subsets as a novel early marker of successful treatment. Arthritis Research & Therapy 2015;17:262. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owczarczyk K, Holweg C, Ortmann W, Behrens T, Brunetta P, Specks U, et al. The role of baseline FCRL5 mRNA expression in predicting response to rituximab (RTX) therapy in patients with granulomatous polyangiitis (GPA) or microscopic polyangiitis (MPA) [abstract no: O86]. Nephron 2015;129(Suppl 2):97. [EMBASE: 623780265] [Google Scholar]
- Owczarczyk K, Holweg C, Ortmann W, Behrens T, Brunetta P, Specks U, et al. The role of baseline FCRL5 mRNA expression in predicting response to rituximab (RTX) therapy in patients with granulomatous polyangiitis (GPA) or microscopic polyangiitis (MPA) [abstract no: OP0232]. Annals of the Rheumatic Diseases 2014;73(Suppl 2):150. [EMBASE: 71551187] [Google Scholar]
- Pepper RJ, Draibe JB, Caplin B, Fervenza FC, Hoffman GS, Kallenberg CG, et al. Association of serum calprotectin (S100A8/A9) Level with disease relapse in proteinase 3‐antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatology 2017;69(1):185‐93. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee RL, Davis JC, Ding L, Fervenza FC, Hoffman GS, Kallenberg CG, et al. The utility of urinalysis in determining the risk of renal relapse in ANCA‐associated vasculitis. Clinical Journal of the American Society of Nephrology: CJASN 2018;13(2):251‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specks U, Merkel PA, Hoffman GS, Langford CA, Spiera R, Seo P, et al. Design of the Rituximab in ANCA‐Associated Vasculitis (RAVE) Trial. Open Arthritis Journal 2011;4(1):1‐18. [EMBASE: 2011666161] [Google Scholar]
- Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission‐induction regimens for ANCA‐associated vasculitis. New England Journal of Medicine 2013;369(5):417‐27. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specks U, Merkel PA, Seo P, Spiera RF, Langford CA, Hoffman GS, et al. Immunoglobulin concentrations and infection risk among patients with ANCA‐associated vasculitis treated with rituximab or cyclophosphamide [abstract no: 789]. Arthritis & Rheumatism 2011;63(10 Suppl):S310. [EMBASE: 70785837] [Google Scholar]
- Specks U, Stone JH, RAVE‐ITN Research Group. Long‐term efficacy and safety results of the RAVE trial [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):65. [EMBASE: 70622659] [Google Scholar]
- Stone JH, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Extended follow‐up of treatment with rituximab versus cyclophosphamide for remission‐induction of ANCA‐associated vasculitis: Which subsets are at greatest risk for flare? [abstract no: 2432]. Arthritis & Rheumatism 2011;63(10 Suppl):S946. [EMBASE: 70785573] [Google Scholar]
- Stone JH, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for induction of remission of ANCA associated vasculitis: a randomized controlled trial (RAVE) [abstract no: 550]. Arthritis & Rheumatism 2009;60(Suppl 10):550. [EMBASE: 70374447] [Google Scholar]
- Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA‐associated vasculitis. New England Journal of Medicine 2010;363(3):221‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasson G, Monach PA, Tanriverdi K, Specks U, Stone JH, Ding L, et al. Predictive value of selected markers of inflammation and platelet activation for complete remission in ANCA‐associated vasculitis [abstract no: 2567]. Arthritis & Rheumatism 2012;64(10 Suppl):S1085‐6. [EMBASE: 71000563] [Google Scholar]
- Unizony S, Lim N, Carey V, Phippard DJ, Tchao N, Miloslavsky EM, et al. Peripheral CD5+ B‐cells in ANCA‐associated vasculitis [abstract no: 1754]. Arthritis & Rheumatology 2014;66(10 Suppl):S769‐70. [EMBASE: 71737748] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unizony S, Lim N, Phippard DJ, Carey VJ, Miloslavsky EM, Tchao NK, et al. Peripheral CD5+ B cells in antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatology 2015;67(2):535‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unizony S, Miloslavsky EE, Villarreal M, Merkel PA, Monach P, Clair EW, et al. Comparison of clinicopathologically‐ and serologically‐based classification systems for ANCA‐associated vasculitis [abstract no: O67]. Nephron 2015;129(Suppl 2):85. [EMBASE: 623780526] [Google Scholar]
- Unizony S, Miloslavsky EM, Villarreal M, Merkel PA, Monach P, Claire EW, et al. Comparison of clinicopathologically‐and serologically‐based classification systems for ANCA‐associated vasculitis [abstract no: 1766]. Arthritis & Rheumatology 2014;66(10 Suppl):S775‐6. [EMBASE: 71737760] [Google Scholar]
- Unizony S, Villarreal M, Miloslavsky EM, Lu N, Merkel PA, Spiera R, et al. Clinical outcomes of treatment of anti‐neutrophil cytoplasmic antibody (ANCA)‐associated vasculitis based on ANCA type. Annals of the Rheumatic Diseases 2016;75(6):1166‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ZS, Fu X, Liao K, Kallenberg CG, Langford CA, Merkel PA, et al. Disease activity, antineutrophil cytoplasmic antibody type, and lipid levels in antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis & Rheumatology 2019;71(11):1879‐87. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ZS, Miloslavsky EM, Cascino M, Unizony SH, Lu N, Hoffman GS, et al. Effect of disease activity, glucocorticoid exposure, and rituximab on body composition during induction treatment of antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis Care & Research 2017;69(7):1004‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
REMAIN 2003 {published data only}
- European Vasculitis Study Group (EUVAS). Clinical trial protocol ‐ REMAIN ‐ Randomised trial of prolonged remission‐maintenance therapy in systemic vasculitis [Version 4.2: January 2006]. www.vasculitis.nl/media/documents/remain.pdf (accessed 17 December 2019).
- Karras A, Pagnoux C, Haubitz M, Groot K, Puechal X, Tervaert JWC, et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA‐associated vasculitis. Annals of the Rheumatic Diseases 2017;76(10):1662‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Karras A, Segelmark M, Jayne DR. Randomized controlled trial of treatment withdrawal in the remission phase of ANCA vasculitis: the REMAIN study [abstract no: TH‐OR025]. Journal of the American Society of Nephrology 2015;26(Abstract Suppl):7a. [Google Scholar]
Rifle 1980 {published data only}
- Rifle G, Chalopin JM, Zech P, Deteix P, Ducret F, Vialtel P, et al. Treatment of idiopathic acute crescentic glomerulonephritis by immunodepression and plasma‐exchanges. A prospective randomised study. Proceedings of the European Dialysis & Transplant Association 1981;18:493‐502. [MEDLINE: ] [PubMed] [Google Scholar]
RITUXVAS 2010 {published data only}
- Berden AE, Jones RB, Erasmus DD, Walsh M, Noel LH, Ferrario F, et al. Tubular lesions predict renal outcome in antineutrophil cytoplasmic antibody‐associated glomerulonephritis after rituximab therapy. Journal of the American Society of Nephrology 2012;23(2):313‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jayne DR, Jones R. An international, randomised open label trial comparing a rituximab based regime with a standard cyclophosphamide/azathioprine regimen in the treatment of active 'generalised' ANCA‐associated vasculitis [protocol v1b Nov 2005]. www.vasculitis.nl/media/documents/rituxvas.pdf (accessed 1 December 2019).
- Jones R, Savage C, Peh CA, Hauser T, Cohen Tervaert JW, Tesar V, et al. Rituximab: a novel remission induction agent in ANCA associated vasculitis: the design of an international, randomised trials (RITUXVAS) [abstract no: PUB647]. Journal of the American Society of Nephrology 2007;18(Abstracts):972A. [CENTRAL: CN‐01912265] [Google Scholar]
- Jones R, Tervaert J, Hauser T, Luqmani R, Morgan M, Peh C, et al. Two year follow‐up results from a randomised trial of rituximab versus cyclophosphamide for ANCA‐associated renal vasculitis: RITUXVAS [abstract]. Renal Association & British Renal Society Joint Meeting; 2011 Jun 6‐9 ; Birmingham, UK. 2011:1157.
- Jones R, Tervaert JW, Hauser T, Luqmani R, Peh CA, Savage C, et al. A randomised trial of rituximab versus cyclophosphamide for ANCA‐associated renal vasculitis: RITUXVAS [abstract no: SA773]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐01912295]
- Jones R, Tervaert JW, Hauser T, Luqmani R, Peh CA, Savage C, et al. Randomised trial of rituximab versus cyclophosphamide for ANCA associated renal vasculitis: RITUXVAS [abstract no: O104]. British Transplantation Society (BTS).12th Annual Congress; 2009 Apr 21‐24; Liverpool, UK. 2009. [CENTRAL: CN‐01912296]
- Jones R, Walsh M, Jayne D, European Vascular Study. Randomised trial of rituximab versus cyclophosphamide for ANCA associated renal vasculitis: RITUXVAS [abstract no: F‐FC269]. Journal of the American Society of Nephrology 2008; Vol. 19, issue Abstracts Issue:61A. [CENTRAL: CN‐00757749]
- Jones RB, Furuta S, Tervaert JW, Hauser T, Luqmani R, Morgan MD, et al. Rituximab versus cyclophosphamide in ANCA‐associated renal vasculitis: 2‐year results of a randomised trial. Annals of the Rheumatic Diseases 2015;74(6):1178‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA‐associated renal vasculitis. New England Journal of Medicine 2010;363(3):211‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones RB, Walsh M, Jayne DR. Two‐year follow‐up results from a randomized trial of RTX versus CyP for ANCA‐ associated renal vasculitis: RITUXVAS [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):57‐8. [EMBASE: 70622643] [Google Scholar]
- Jones RB, Walsh MW, Jayne DR. Two‐year follow‐up results from a randomised trial of rituximab versus cyclophosphamide for ANCA‐associated renal vasculitis: RITUXVAS [abstract no: SA‐FC405]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):92A. [Google Scholar]
- Peh C, Jones R, Walsh M, Jayne D. Rituximab versus cyclophosphamide in ANCA associated renal vasculitis [abstract no: 087]. Nephrology 2010;15(Suppl 4):49. [CENTRAL: CN‐00831615] [Google Scholar]
Stegeman 1996 {published data only}
- Stegeman CA, Cohen Tervaert JW, Jong PE, Kallenberg CG. Prevention of relapses of Wegener's granulomatosis by treatment with co‐trimoxazole. A placebo controlled trial [abstract]. Journal of the American Society of Nephrology 1995;6(3):930. [CENTRAL: CN‐00486000] [Google Scholar]
- Stegeman CA, Tervaert JW, Jong PE, Kallenberg CG. Trimethoprim‐sulfamethoxazole (co‐trimoxazole) for the prevention of relapses of Wegener's granulomatosis. Dutch Co‐Trimoxazole Wegener Study Group. New England Journal of Medicine 1996;335(1):16‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stegmayr 1999 {published data only}
- Stegmayr BG, Almroth G, Berlin G, Fehrman I, Kurkus J, Norda R, et al. Plasma exchange or immunoadsorption in patients with rapidly progressive crescentic glomerulonephritis. A Swedish multi‐center study. International Journal of Artificial Organs 1999;22(2):81‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Stegmayr BG, Almroth G, Kurkus J, Olander R, Sterner G, Thysell H, et al. Plasma exchange versus immunoadsorption in the treatment of rapidly progressive glomerulonephritis (RPG). A multi‐center study [abstract]. XXXV Congress of the European Renal Association European Dialysis & Transplant Association; 1998 Jun 6‐9; Rimini (Italy). 1998:294. [CENTRAL: CN‐00486001]
Szpirt 2011 {published data only}
- Szpirt WM, Heaf JG, Petersen J. Plasma exchange for induction and cyclosporine A for maintenance of remission in Wegener's granulomatosis‐‐a clinical randomized controlled trial. Nephrology Dialysis Transplantation 2011;26(1):206‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Szpirt WM, Rasmussen N, Petersen J. Long term outcome and prognostic factors in randomized study of plasma exchange and cyclosporin‐A in Wegener's granulomatosis [abstract no: A0932]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):182A. [CENTRAL: CN‐01912294] [Google Scholar]
- Szpirt WM, Rasmussen N, Petersen J. Plasma exchange and cyclosporin A in Wegner`s granulomatosis a controlled study [abstract]. International Journal of Artificial Organs 1996;19(9):501. [CENTRAL: CN‐00322008] [Google Scholar]
- Szpirt WM, Rasmussen N, Petersen J. Plasma exchange for induction and cyclosporin A for maintenance of remission in Wegener's granulomatosis [abstract no: A2677]. Journal of the American Society of Nephrology 1996;7(9):1781. [CENTRAL: CN‐01912292] [Google Scholar]
Tervaert 1990 {published data only}
- Tervaert JW, Huitema MG, Hene RJ, Sluiter WJ, The TH, Hem GK, et al. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet 1990;336(8717):709‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WEGENT 2008 {published data only}
- Mahr A, Pagnoux C, Cohen C, Hamidou M, Ruivard M, Mouthon L, et al. Treatment of ANCA‐associated vasculitides: corticosteroids and pulse cyclophosphamide followed by maintenance therapy with methotrexate or azathioprine: a prospective multicenter randomized trial (WEGENT) [abstract no: 102]. Kidney & Blood Pressure Research 2005;28(3):194. [CENTRAL: CN‐01912293] [Google Scholar]
- Pagnoux C, Mahr A, Cohen P, Arene JP, Hamidou M, Guillevin L, et al. Treatment of ANCA‐associated systemic vasculitis (AASV) with corticosteroids (CS) and pulse cyclophosphamide (CYC), then azathioprine (AZA) or methotrexate (MTX) to maintain remission: interim analysis [abstract]. 11th International Vasculitis & ANCA Workshop; 2003 Oct 2‐5; Prague, Czech Republic. 2003:47. [CENTRAL: CN‐00461458]
- Pagnoux C, Mahr A, Hamidou MA, Boffa JJ, Ruivard M, Ducroix JP, et al. Azathioprine or methotrexate maintenance for ANCA‐associated vasculitis. New England Journal of Medicine 2008;359(26):2790‐803. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Puechal X, Pagnoux C, Perrodeau E, Hamidou M, Boffa JJ, Kyndt X, et al. Granulomatosis with polyangiitis or microscopic polyangiitis: long term outcomes of the prospective WEGENT trial comparing azathioprine vs methotrexate for remission maintenance in 126 patients [abstract no: O60]. Nephron 2015;129(Suppl 2):80. [EMBASE: 623780460] [Google Scholar]
- Puechal X, Pagnoux C, Perrodeau E, Hamidou M, Boffa JJ, Kyndt X, et al. Long‐term outcomes among participants in the WEGENT trial of remission‐maintenance therapy for granulomatosis with polyangiitis (Wegener's) or microscopic polyangiitis. Arthritis & Rheumatology 2016;68(3):690‐701. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Seror R, Pagnoux C, Ruivard M, Landru I, Wahl D, Riviere S, et al. Treatment strategies and outcome of induction‐refractory Wegener's granulomatosis or microscopic polyangiitis: analysis of 32 patients with first‐line induction‐refractory disease in the WEGENT trial. Annals of the Rheumatic Diseases 2010;69(12):2125‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WGET 2002 {published data only}
- Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, Clair EW, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Annals of Internal Medicine 2007;147(9):611‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, Clair EW, et al. Glycosylation of proteinase 3 (PR3) is not required for its reactivity with antineutrophil cytoplasmic antibodies (ANCA) in Wegener's granulomatosis. Clinical & Experimental Rheumatology 2009;27(1 Suppl 52):S45‐52. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Goek ON, Stone JH. Randomized controlled trials in vasculitis associated with anti‐neutrophil cytoplasmic antibodies. Current Opinion in Rheumatology 2005;17(3):257‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Merkel PA, Lo GH, Holbrook JT, Tibbs AK, Allen NB, Davis JC, et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener's Clinical Occurrence of Thrombosis (WeCLOT) Study. Annals of Internal Medicine 2005;142(8):620‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rhee RL, Davis JC, Ding L, Fervenza FC, Hoffman GS, Kallenberg CG, et al. The utility of urinalysis in determining the risk of renal relapse in ANCA‐associated vasculitis. Clinical Journal of the American Society of Nephrology: CJASN 2018;13(2):251‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener's granulomatosis and its treatment: prospective data from the Wegener's Granulomatosis Etanercept Trial (WGET). Arthritis & Rheumatism 2005;52(7):2168‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stone JH, Rajapakse VN, Hoffman GS, Specks U, Merkel PA, Spiera RF, et al. A serum proteomic approach to gauging the state of remission in Wegener's granulomatosis. Arthritis & Rheumatism 2005;52(3):902‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stone JH, Wegener's Granulomatosis Etanercept Trial Research Group. Limited versus severe Wegener's granulomatosis: baseline data on patients in the Wegener's granulomatosis etanercept trial. Arthritis & Rheumatism 2003;48(8):2299‐309. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tomasson G, Boers M, Walsh M, LaValley M, Cuthbertson D, Carette S, et al. Assessment of health‐related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener's). Arthritis Care & Research 2012;64(2):273‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- WGET Research Group. Design of the Wegener's Granulomatosis Etanercept Trial (WGET). Controlled Clinical Trials 2002;23(4):450‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wegener's Granulomatosis Etanercept Trial Research Group. Etanercept plus standard therapy for Wegener's granulomatosis. New England Journal of Medicine 2005;325(4):351‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Wung PK, Anderson T, Fontaine KR, Hoffman GS, Specks U, Merkel PA, et al. Effects of glucocorticoids on weight change during the treatment of Wegener's granulomatosis. Arthritis & Rheumatism 2008;59(5):746‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wung PK, Holbrook JT, Hoffman GS, Tibbs AK, Specks U, Min YI, et al. Herpes zoster in immunocompromised patients: incidence, timing, and risk factors. American Journal of Medicine 2005;118(12):1416‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zauner 2002 {published data only}
- Bohler J, Zauner I, Bach D, Braun N, Funfstuck R, Helmchen U, et al. Effect of initial histology and plasmapheresis treatment on prognosis of RPGN: results of the German prospective randomized multicenter study [abstract no: 947]. Journal of the American Society of Nephrology 1995;6(3):412. [CENTRAL: CN‐00483280] [Google Scholar]
- Zauner I, Bach D, Braun N, Kramer BK, Funfstuck R, Helmchen U, et al. Predictive value of initial histology and effect of plasmapheresis on long‐term prognosis of rapidly progressive glomerulonephritis. American Journal of Kidney Diseases 2002;39(1):28‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Zauner I, Bach D, Braun N, Kramer BK, Funfstuck R, Helmchen U, et al. Ten year follow up after treatment for rapidly progressive glomerulonephritis. Results from the German prospective randomized multicenter study [abstract no: A0918]. Journal of the American Society of Nephrology 2000;11(Sept):172A. [CENTRAL: CN‐00550632] [Google Scholar]
Zycinska 2009 {published data only}
- Zycinska K, Wardyn KA, Zielonka TM, Krupa R, Lukas W. Co‐trimoxazole and prevention of relapses of PR3‐ANCA positive vasculitis with pulmonary involvement. European Journal of Medical Research 2009;14 Suppl 4:265‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Basu 2017 {published data only}
- Basu G, Beasley M, Jones GT, Druce K, D'Allesandro M, Moffat H, et al. Psychological interventions delivered by allied health professionals (AHPS) can improve quality of life in patients with inactive ANCA associated vasculitis: results from a pilot randomised trial [abstract]. Rheumatology 2017;56(Suppl 3):iii164. [EMBASE: 619334569] [Google Scholar]
CHUSPAN 2 2017 {published data only}
- Puechal X, Pagnoux C, Baron G, Quemeneur T, Neel A, Agard C, et al. Adding azathioprine to remission‐induction glucocorticoids for eosinophilic granulomatosis with polyangiitis (Churg‐Strauss), microscopic polyangiitis, or polyarteritis nodosa without poor prognosis factors: a randomized, controlled trial. Arthritis & Rheumatology 2017;69(11):2175‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Vita 2012 {published data only}
- Vita S, Quartuccio L, Isola M, Masolini P, Sacco S, Marchi G, et al. A randomized, controlled, multicenter phase III study of the efficacy and safety of rituximab (RTX) monotherapy versus the best available treatment in patients with mixed cryoglobulinemia syndrome [abstract]. Arthritis & Rheumatism 2010;62(Suppl 10):2201. [EMBASE: 70380187] [Google Scholar]
- Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis & Rheumatism 2012;64(3):843‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vita S, Quartuccio L, Masolini P, Stefania S, Marchi G, Zabotti A, et al. A randomized, controlled, multicenter phase III study of the efficacy and safety of rituximab (RTX) monotherapy versus the best available treatment (BAT) in patients with mixed cryoglobulinemia syndrome [abstract no: OP0120]. Annals of Rheumatic Diseases 2010;69(Suppl 3):93. [Google Scholar]
- Quartuccio L, Zuliani F, Corazza L, Scaini P, Zani R, Lenzi M, et al. Retreatment regimen of rituximab monotherapy given at the relapse of severe HCV‐related cryoglobulinemic vasculitis: long‐term follow up data of a randomized controlled multicentre study. Journal of Autoimmunity 2015;63:88‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Quartuccio L, Zuliani F, Corazza L, Scaini P, Zani R, Lenzi M, et al. Rituximab monotherapy of severe HCV‐related cryoglobulinemic vasculitis for more than 2 years: follow‐up of a randomized controlled multicentre study [abstract no: OP0228]. Annals of the Rheumatic Diseases 2014;73(Suppl 2). [EMBASE: 71551183] [Google Scholar]
Harper 2018 {published data only}
- Harper L, Morgan MD, Chanouzas D, Caulfield HK, Coughlan L, Dean C, et al. Treatment of fatigue with physical activity and behavioural change support in vasculitis: study protocol for an open‐label randomised controlled feasibility study. BMJ Open 2018;8(10):e023769. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imai 2006 {published data only}
- Imai H, Hotta O, Yoshimura M, Konta T, Tsubakihara Y, Miyazaki M, et al. Deoxyspergualin, an immunosuppressant, in patients suffering from nephropathies with crescent formation: an open‐label trial to evaluate safety and efficacy. Clinical & Experimental Nephrology 2006;10(1):40‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ribi 2010 {published data only}
- Ribi C, Cohen P, Pagnoux C, Mahr A, Arene JP, Puechal X, et al. Treatment of polyarteritis nodosa and microscopic polyangiitis without poor‐prognosis factors: a prospective randomized study of one hundred twenty‐four patients. Arthritis & Rheumatism 2010;62(4):1186‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rifle 1990 {published data only}
- Rifle G, Dechelette E. Treatment of rapidly progressive glomerulonephritis by plasma exchange and methylprednisolone pulses. A prospective randomized trial of cyclophosphamide. Interim analysis. The French Cooperative Group. Progress in Clinical & Biological Research 1990;337:263‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies awaiting assessment
Chen 2011c {published data only}
- Chen N, Li X, Zhang W, Shen P, Yu H, Chen Y, et al. A randomized controlled trial of mycophenolate mofetil versus cyclophosphamide for induction therapy in ANCA associated systemic vasculitis with renal involvement [abstract]. Nephrology Dialysis Transplantation 2012;27:ii61‐2. [EMBASE: 70765469] [Google Scholar]
- Chen N, Yu H, Zhang W, Ren H, Shen P, Li X, et al. A randomized controlled trial of MMF versus CyP for induction therapy in ANCA‐ AASV with renal involvement [abstract]. Clinical & Experimental Immunology 2011;164(Suppl 1):52‐3. [EMBASE: 70622634] [Google Scholar]
CLASSIC 2016 {published data only}
- Merkel PA, Niles J, Jimenez R, Spiera RF, Rovin BH, Bomback A, et al. A randomized clinical trial of CCX168, an orally administered C5AR inhibitor for treatment of patients with ANCA‐associated vasculitis [abstract no: 978]. Arthritis & Rheumatology 2016;68(Suppl 10):1296‐7. [EMBASE: 613887152] [Google Scholar]
- Schall T, Potarca A, Hillson J, Bekker P. C5aR inhibitor avacopan in anti‐neutrophil cytoplasmic autoantibody (ANCA) vasculitis phase II outcomes of safety, renal parameters, and quality of life [abstract]. American Journal of Kidney Diseases 2018;71(4):582. [EMBASE: 621595967] [Google Scholar]
Henderson 2009 {published data only}
- Henderson S, Salama AD, EUVAS Study Group. CTLA4‐Ig in the treatment of ANCA‐associated vasculitis [abstract no: F‐PO1308]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):412A. [Google Scholar]
MAINTANCAVAS 2017 {published data only}
- Cortazar FB, Pendergraft WF, Dunbar CB, Laliberte KA, Niles J. Design of the maintenance of ANCA vasculitis remission by intermittent rituximab dosing based on B cell reconstitution versus a serologic ANCA flare (MAINTANCAVAS) trial [abstract no: SA‐PO257]. Journal of the American Society of Nephrology 2017;28(Abstract Suppl):744. [Google Scholar]
Pagnoux 2003 {published data only}
- Pagnoux C, Cohen P, Mahr A, Hauser T, Mouthon L, Arene JP, et al. Treatment of Churg Strauss syndrome (CSS) with poor prognosis factor(s): a prospective, randomized, multicenter trial comparing corticosteroids (CS) and 6 vs 12 cyclophosphamide [abstract]. 11th International Vasculitis & ANCA Workshop; 2003 Oct 2‐5; Prague, Czech Republic. 2003:43. [CENTRAL: CN‐00461457]
RATTRAP 2015 {published data only}
- Guillevin L. RATTRAP: Infliximab versus rituximab in systemic necrotizing vasculitides. www.clinicaltrials.gov/ct2/show/NCT00307593 (first received 28 March 2006).
References to ongoing studies
ADVOCATE 2019 {published data only}
- Merkel P, Jayne D, Harigai M, Schall T, Bekker P, Potarca A, et al. A randomized phase 3 trial evaluating the safety and efficacy of avacopan in patients with new or relapsing ANCA‐associated vasculitis [abstract]. Rheumatology 2019;58(Suppl 2):na. [EMBASE: 628291607] [Google Scholar]
ALEVIATE 2018 {published data only}
- Gopaluni S, Smith RM, Goymer D, Broadhurst E, Mcclure ME, Cahill HC, et al. Alemtuzumab for relapsing and refractory primary systemic vasculitis: a trial of efficacy and safety (ALEVIATE) [abstract no: TH‐PO1160]. Journal of the American Society of Nephrology 2018;29(Abstract Suppl):B8. [Google Scholar]
CANVAS 2016 {published data only}
- Chanouzas D, Dyall L, Dale J, Moss P, Morgan M, Harper L. CD4+CD28‐ T‐cell expansions in ANCA‐associated vasculitis and association with arterial stiffness: baseline data from a randomised controlled trial [abstract no: 58]. Lancet 2015;385 Suppl 1:S30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chanouzas D, Dyall L, Dale J, Moss P, Morgan MD, Harper L. CD4+CD28‐ T‐cell expansions in ANCA‐associated vasculitis and association with arterial stiffness [abstract no: P51]. Nephron 2015;129(Suppl 2):135. [DOI] [PubMed] [Google Scholar]
- Chanouzas D, Dyall L, Nightingale P, Ferro C, Moss P, Morgan MD, et al. Valaciclovir to prevent cytomegalovirus mediated adverse modulation of the immune system in ANCA‐associated vasculitis (CANVAS): study protocol for a randomised controlled trial. Trials [Electronic Resource] 2016;17(1):338. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanouzas D, Sagmeister M, Dyall L, Ferro C, Moss P, Morgan MD, et al. Valaciclovir to prevent Cytomegalovirus mediated adverse modulation of the immune system in ANca Associated VASculitis (CANVAS): results of a randomised controlled clinical trial [abstract no: TH‐PO816]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):282A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanouzas D, Sagmeister M, Dyall L, Nightingale P, Ferro C, Moss P, et al. Role of cytomegalovirus in the expansion of CD4+CD28‐T cells in patients with ANCA‐associated vasculitis: A proof‐of‐concept, randomised controlled trial [abstract]. Lancet 2017;389(Suppl 1):S17. [EMBASE: 618809848] [Google Scholar]
- Chanouzas D, Sagmeister M, Dyall L, Nightingale P, Ferro C, Moss P, et al. Subclinical cytomegalovirus reactivation drives the expansion of cytotoxic endothelial homing T‐cells in ANCA associated vasculitis that can be reversed by valaciclovir treatment [abstract]. Rheumatology 2017;56(Suppl 3):iii116. [EMBASE: 619334906] [Google Scholar]
COMBIVAS 2019 {published data only}
- McClure M, Gopaluni S, Wason J, Rosen AN, Henderson R, Salama A, et al. A randomised, double‐blind, controlled, mechanistic study of rituximab and belimumab combination therapy in pr3 ANCA‐associated vasculitis (COMBIVAS): study protocol [abstract]. Rheumatology 2019;58(Suppl 2):na. [EMBASE: 628291554] [Google Scholar]
MAINRITSAN 3 2015 {published data only}
- Guillevin L. Comparison between a long term and a conventional maintenance treatment with rituximab (MAINRITSAN3). www.clinicaltrials.gov/ct2/show/NCT02433522 (first received 5 May 2015).
MUPIBAC 2004 {published data only}
- Buhaescu I. Actualities in the treatment of systemic vasculitis. BANTAO Journal 2004;2(2):4‐9. [CENTRAL: CN‐01658416] [Google Scholar]
NCT03323476 {published data only}
- Couvrat‐Desvergnes G. Maintaining or stopping immunosuppressive therapy in patients with ANCA vasculitis and end‐stage renal disease (MASTER‐ANCA). www.clinicaltrials.gov/show/NCT03323476 (first received 27 October 2017).
RITAZAREM 2013 {published data only}
- Gopaluni S, Smith RM, Lewin M, McAlear CA, Mynard K, Jones RB, et al. Rituximab versus azathioprine as therapy for maintenance of remission for anti‐neutrophil cytoplasm antibody‐associated vasculitis (RITAZAREM): study protocol for a randomized controlled trial. Trials [Electronic Resource] 2017;18(1):112. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayne D, Smith R, Merkel P. An international, open‐label, randomised controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA‐associated vasculitis (RITAZAREM) [abstract no: P205]. Press Medicale 2013;42(4 Pt 2):768. [EMBASE: 71944013] [Google Scholar]
- Jayne DR, Smith RM, Merkel PA. RITAZAREM: an international, open label, randomized, controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA‐associated vasculitis [abstract no: SA‐PO307]. Journal of the American Society of Nephrology 2012;23(Abstracts):707A. [CENTRAL: CN‐01912286] [Google Scholar]
- Smith R, Jones R, Speckes U, McAlear CA, Mynard K, Bond S, et al. Rituximab as re‐induction therapy in relapsing ANCA‐associated vasculitis [abstract no: 18L]. Arthritis & Rheumatology 2017;69(Suppl 10):na. [Google Scholar]
- Smith R, Lewin M, Jones R, Merkel P, Jayne D. RITAZAREM: an international, open label, randomised controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA‐associated vasculitis [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i180‐1. [EMBASE: 71075527] [Google Scholar]
- Smith R, Merkel P, Jayne D. RITAZAREM: an international, open‐label, randomised controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA‐associated vasculitis [abstract no: 13]. Nephron 2015;129(Suppl 2):110‐1. [CENTRAL: 623780439] [Google Scholar]
- Smith RM, Arimura Y, Merkel PA, Jayne DR. RITAZAREM: An international, open‐label, randomised controlled trial comparing rituximab with azathioprine as maintenance therapy in relapsing ANCA‐associated vasculitis [abstract]. Rheumatology 2017;56(Suppl 3):iii150‐1. [EMBASE: 619334913] [Google Scholar]
Tuin 2019 {published data only}
- Tuin J, Stassen PM, Bogdan DI, Broekroelofs J, Paassen P, Cohen Tervaert JW, et al. Mycophenolate mofetil versus cyclophosphamide for the induction of remission in nonlife‐threatening relapses of antineutrophil cytoplasmic antibody‐associated vasculitis: randomized, controlled trial. Clinical Journal of the American Society of Nephrology: CJASN 2019;14(7):1021‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuin J, Stassen PM, Bogdan DI, Tervaert J, Sanders J, Stegeman CA. Mycophenolate mofetil versus cyclophosphamide for the induction of remission in non‐life‐threatening relapses of PR3 and MPOANCA associated vasculitis [abstract]. Rheumatology 2017;56(Suppl 3):iii16. [EMBASE: 619334519] [Google Scholar]
Additional references
Bolton 1989
- Bolton WK, Sturgill BC. Methylprednisolone therapy for acute crescentic rapidly progressive glomerulonephritis. American Journal of Nephrology 1989;9(5):368‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bosch 2007
- Bosch X, Guilabert A, Espinosa G, Miropeix E. Treatment of antineutrophil cytoplasmic antibody‐associated vasculitis. JAMA 2007;298(6):655‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Groot 2001
- Groot K, Adu D, Savage CO, EUVAS (European vasculitis study group). The value of pulse cyclophosphamide in ANCA‐associated vasculitis: meta‐analysis and critical review. Nephrology Dialysis Transplantation 2001;16(10):2018‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fauci 1973
- Fauci AS, Wolff SM. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. Medicine 1973;52(6):535‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harper 2011
- Harper L, Morgan MD, Walsh M, Hoglund P, Westman K, Flossmann O, et al. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA‐associated vasculitis: long‐term follow‐up. Annals of the Rheumatic Diseases 2011;71(6):955‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jayne 2000a
- Jayne DR, Chapel H, Adu D, Misbah S, O'Donoghue D, Scott D, et al. Intravenous immunoglobulin for ANCA‐associated systemic vasculitis with persistent disease activity. Qjm 2000;93(7):433‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jennette 2003
- Jennette JC, Falk RJ. Renal and systemic vasculitis. In: Johnson RJ, Feehally J editor(s). Comprehensive clinical nephrology. 2nd Edition. Edinburgh: Mosby, 2003:341‐57. [ISBN 0723432589] [Google Scholar]
Jennette 2008
- Jennette JC, Falk RJ. New insight into the pathogenesis of vasculitis associated with antineutrophil cytoplasmic autoantibodies. Current Opinion in Rheumatology 2008;20(1):55‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jennette 2013
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis & Rheumatism 2013;65(1):1‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
KDIGO 2012
- Chapter 13: Pauci‐immune focal and segmental necrotizing glomerulonephritis. Kidney International Supplements 2012;2(2):233‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 2001
- Kerr PG, Chadban SJ, Atkins RC. Is there a role for plasma exchange in rapidly progressive glomerulonephritis?. Nephrology 2001;6(3):141‐3. [EMBASE: 2001231297] [Google Scholar]
Lapraik 2007
- Lapraik C, Watts R, Bacon P, Carruthers D, Chakravarty K, D'Cruz D, et al. BSR and BHPR guidelines for the management of adults with ANCA associated vasculitis. Rheumatology 2007;46(10):1615‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levey 1994
- Levey JB, Winearls CG. Rapidly progressive glomerulonephritis: what should be first‐line therapy?. Nephron 1994;67(4):402‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Little 2010
- Little MA, Nightingale P, Verburgh CA, Hauser T, Groot K, Savage C, et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Annals of the Rheumatic Diseases 2010;69(6):1036‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lockwood 1976
- Lockwood CM, Rees AJ, Pearson TA, Evans DJ, Peters DK, Wilson CB. Immunosuppression and plasma exchange in the treatment of Goodpasture's syndrome. Lancet 1976;1(7962):711‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lyons 2012
- Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA‐associated vasculitis. New England Journal of Medicine 2012;367(3):214‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menahem 2008
- Menahem S, Hiremagalur B, Mudge D, Toussaint N, Walters G, Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Induction and maintenance therapy in ANCA‐associated systemic vasculitis. Nephrology 2008;13 Suppl 2:S24‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mukhtyar 2009
- Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, Groot K, Gross W, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Annals of the Rheumatic Diseases 2009;68(3):310‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nowack 1997
- Nowack, R, Birck R, Woude FJ. Mycophenolate mofetil for systemic vasculitis and IgA nephropathy. Lancet 1997;349(9054):774. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Phillip 2008
- Phillip R, Luqmani R. Mortality in systemic vasculitis: a systematic review. Clinical & Experimental Rheumatology 2008;26(5 Suppl 51):S94‐104. [MEDLINE: ] [PubMed] [Google Scholar]
Rondeau 1989
- Rondeau E, Levy M, Dosquet P, Ruedin P, Mougenot B, Kanfer A, et al. Plasma exchange and immunosuppression for rapidly progressive glomerulonephritis: prognosis and complications. Nephrology Dialysis Transplantation 1989;4(3):196‐200. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Savage 1997
- Savage CO, Harper L, Adu D. Primary systemic vasculitis. Lancet 1997;349(9051):553‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seo 2004
- Seo P, Stone JH. The antineutrophil cytoplasmic antibody‐associated vasculitides. American Journal of Medicine 2004;117(1):39‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tervaert 2001
- Tervaert JW, Stegeman CA, Kallenberg CG. Novel therapies for anti‐neutrophil cytoplasmic antibody‐associated vasculitis. Current Opinion in Nephrology & Hypertension 2001;10(2):211‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walsh 2011
- Walsh M, Catapano F, Szpirt W, Thorlund K, Bruchfeld A, Guillevin L, et al. Plasma exchange for renal vasculitis and idiopathic rapidly progressive glomerulonephritis: a meta‐analysis. American Journal of Kidney Diseases 2011;57(4):566‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Walters 2001
- Walters G, Willis N, Craig JC, Kerr P. Treatment for renal vasculitis and Goodpasture's disease in adults. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003232] [DOI] [Google Scholar]
Walters 2008
- Walters G, Willis NS, Craig JC. Interventions for renal vasculitis in adults. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003232.pub2] [DOI] [PubMed] [Google Scholar]
Walters 2010
- Interventions for renal vasculitis in adults. A systematic review. Walters GD, Willis NS, Craig JC. BMC Nephrology 2010;11:12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2015
- Walters G, Willis NS, Craig JC. Interventions for renal vasculitis in adults. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD003232.pub3] [DOI] [PubMed] [Google Scholar]


