Abstract
Chemokines are a superfamily of ~50 small secreted proteins (8–12 kDa) involved in a host of physiological processes and disease states, with several previously shown to have direct antimicrobial activity comparable to defensins in efficacy. XCL1 is a unique metamorphic protein that interconverts between the canonical chemokine fold and a novel all β-sheet dimer. Phylogenetic analysis suggests that, within the chemokine family, XCL1 is most closely related to CCL20, which exhibits antibacterial activity. The in vitro antimicrobial activity of WT-XCL1 and structural variants was quantified using a radial diffusion assay (RDA) and in solution bactericidal assays against Gram-positive and Gram-negative species of bacteria. Comparisons of WT-XCL1 with variants that limit metamorphic interconversion showed a loss of antimicrobial activity when restricted to the conserved chemokine fold. These results suggest that metamorphic folding of XCL1 is required for potent antimicrobial activity.
In the past decade there has been a dramatic rise in the clinical incidence of drug-resistant disease arising from previously treatable infections, highlighting the need for new antibiotic drugs not easily evaded by adaptation under selective pressure1–5. Antimicrobial peptides (AMPs) are molecules of the innate immune system that possess broad-spectrum activity against invading pathogens with potential utility for development as new antibiotic therapies5–8. For example, defensins are a class of AMPs found on the mucosal surfaces in the gut, respiratory, and urogenital systems of mammals that contribute to innate defense against pathogens and shape the commensal microbiota9–13. Mammalian defensins range from 2–6 kDa in size, are cysteine-rich, positively charged, and certain peptides are chemotactic for several types of leukocytes including dendritic cells expressing the G protein-coupled receptor CCR614–16.
Chemokines, or chemotactic cytokines, share several structural and functional characteristics with the defensins including a high pI, cysteine-rich amino acid sequences, and robust chemotactic activity17, 18. Chemokines are divided into four subfamilies based upon the position of conserved cysteine residues (C, CC, CXC, and CX3C) and are involved in a host of physiological processes and disease states including cellular migration, tumor progression, and metastasis19, 20. Along with well-established roles in leukocyte trafficking some chemokines have broad-spectrum antimicrobial activities similar to those of defensins15, 20–22. In particular, CCL20 (Macrophage Inflammatory Protein-3, MIP-3α) and CCL28 (Mucosae-associated Epithelial Chemokine, MEC) are potent, well-studied, antimicrobial chemokines with activities against both Gram-positive and Gram-negative bacterial species.
XCL1 (lymphotactin) is a unique metamorphic chemokine that undergoes an unfolding transition during conversion between two unrelated folded conformations. One is a highly conserved monomeric structure (XCL1mon/Ltn10) consisting of an unstructured N-terminus followed by a three-stranded anti-parallel β-sheet and a C-terminal α-helix (the canonical chemokine fold). The alternative structure is an all-β-sheet dimer (XCL1dim/Ltn40). The dual native-state XCL1 structures are encoded by the same mRNA transcript and polypeptide and exist in equilibrium. The conserved chemokine fold (XCL1mon) and XCL1dim readily interconvert (kex ~ 1 s–1) and are equally abundant in physiological solution conditions23, 24. XCL1 binds and activates its G protein-coupled receptor XCR1 only in the monomeric form, while the alternative dimeric form binds glycosaminoglycans (GAGs); both functional interactions are vital to chemotactic activity in vivo25. As a first step toward discerning the evolutionary origins of metamorphic XCL1 folding we examined the patterns of conservation across the entire chemokine superfamily. This analysis confirmed a previously suggested evolutionary relationship with CCL20, a known antimicrobial agent26, 27.
Previous surveys of chemokine antibacterial activity reported that XCL1 had either modest or minimal potency as an antimicrobial agent against E. coli15, 28. XCL1 was recently shown to have direct HIV inhibitory activity29, providing additional evidence that chemokines can contribute to innate immune function as antimicrobial molecules. These observations prompted us to characterize the antibacterial properties of XCL1 in the context of its metamorphic structure. We found that human XCL1 has bactericidal activity against several species that is comparable to that of Defa4 (cryptdin-4), a mouse Paneth cell α-defensin, and that this antimicrobial activity relies on the native-state unfolding of XCL1 to access non-chemokine conformations. These results suggest that metamorphic XCL1 folding may be a result of selective pressure from microbial pathogens.
EXPERIMENTAL PROCEDURES
Chemokine Sequence Alignment and Phylogenetic Analysis:
457 chemokine sequences from 30 vertebrate species were used to generate a protein sequence alignment using the Promals3D alignment algorithm, which uses a combination of sequence identity, predicted secondary structural boundaries, and known protein structures to produce highly accurate sequence alignments30, 31. The alignment incorporated 14 chemokine structures (PDB codes: 1EL0, 3IFD, 1ESR, 2Q8R, 1M8A, 2EOT, 2KUM, 1LV9, 1RJT, 2HDL, 2KEC, 1J8I, and 1B2T) comprising both monomeric and dimeric states. After removal of initial alignment insertion-deletions (indels) from the N and C termini of the proteins, the resulting sequence alignment consists of the canonical chemokine fold (i.e. disulfide bonds, β-strands, and helical region) consisting of approximately 70 amino acid residues. Phylogenetic models were generated from the indel edited sequence alignment using the PhyML v3.0 software package32. The final phylogenetic model used to infer relationships between chemokine subfamily members employed the JTT amino-acid replacement matrix, with branch support values calculated as the approximate likelihood ratio statistic33.
Purification of Recombinant Chemokines:
Human XCL1, CCL28, CCL20, CXCL11, and CXCL12 were each expressed and purified as an N-terminal His6SUMO fusion protein in E. coli as previously described23, 25, 34–37. Briefly, cells were grown to OD600 = 1.0 at 37°C in Terrific Broth induced with 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG) before being harvested and stored at −80°C until further purification. Cell pellets were resuspended, lysed using a French press, and lysates were clarified by centrifugation (12000 × g for 20 min). The supernatant and resolubilized inclusion body pellets were loaded onto Ni-NTA resin. After 1 h, columns were washed and proteins were eluted with 6 M guanidinium chloride, 50 mM Na2PO4 (pH 7.4), 300 mM NaCl, 500 mM imidazole, 0.2% sodium azide, and 0.1% β-mercaptoethanol. The peptides were refolded via dialysis (XCL1, and all variants) or dilution (CCL28, CCL20, and CXCL11) overnight before cleavage of the His6SUMO fusion tags by Ulp1 protease for 4 hours. The His6SUMO fusion tags and chemokines were separated by cation-exchange chromatography on SP Sepharose Fast Flow resin (GE Healthcare UK Ltd.), and chemokines were subjected to reverse-phase high-performance liquid chromatography as a final purification using a C18 column with a 30 min gradient from 30% to 60% acetonitrile in aqueous 0.1% trifluoroacetic acid. Proteins were frozen, lyophilized and stored at −20°C. Purification and structural homogeneity of recombinant proteins was verified by SDS-PAGE, MALDI-TOF spectroscopy, and NMR spectroscopy.
Determination of Antimicrobial Activity by Radial Diffusion Assay:
Chemokine antibacterial activity was initially measured in radial diffusion assays38. Briefly, trypticase soy broth (TSB) inoculated with a single colony of E. coli BL21 cells was incubated overnight at 37°C. Overnight cultures were transferred to 50 mL of fresh TSB and grown to OD600 = 0.6 (mid-log phase) with shaking at 37°C. Bacterial cells were deposited by centrifugation at 880 × g for 10 min, washed twice with 10 mL of 10 mM sodium phosphate buffer, pH = 7.4, and resuspended in 4 mL of buffer and colony-forming units (CFU) were calculated (CFU/mL = (2.5 × 108) × OD600). Twelve mL of molten sterile underlay, 1% w/v agarose prepared with low electroendosmosis (EEO)-type agarose (Sigma, St. Louis MO) at pH = 7.4, was inoculated with 4 × 106 CFU of E. coli and poured into gridded square plates (thirty-six 13 × 13mm squares, Fisher Scientific) and allowed to cool and solidify. When solidified, 9 × 3mm wells were created in the agarose, and filled with 5 μL of peptide solutions at concentrations ranging from 10 mg/mL to 70 μg/mL. Plates were incubated agar side up 3 h at 37°C to allow protein diffusion into the underlay agarose; then 12 mL of a 2× TSB molten 1% w/v agarose overlay was applied to each plate. After overlay agarose solidified, plates were incubated overnight at 37°C, disinfected with 12 mL of 5% acetic acid, 25% methanol for 20 minutes, and zones of inhibition of bacterial growth were measured at 6× magnification (0.53 mm/division) on a WILD Heerbrug microscope. All measurements were taken using the same microscope and at the same level of magnification. Measurements for the zones of clearance were corrected to exclude the diameter of the initial 3mm well. Due to differences in the concentration and diffusion coefficients of the molecules tested the diameters of the observed zones of clearance cannot be directly compared. Raw data were transformed and subjected to semi-linear fitting (plot corrected diameter vs. log [concentration]) and the x-intercept calculated to determine a minimal effective concentration (MEC). Analyses were carried out in GraphPad Prism 6.
Bactericidal Peptide Assays:
Chemokines were tested against E. coli ML35, Listeria monocytogenes 10403s, S. aureus 710a, and Salmonella enterica serovar Typhimurium 14028s for in vitro bactericidal activity as previously described39. Bacteria were grown to mid-exponential phase in TSB, deposited by centrifugation, and washed with 10 mM piperazine-1, 4-bis(2-ethanesulfonic acid) (PIPES), pH 7.4, supplemented with 1% (vol/vol) of TSB (10 mM PIPES-TSB, pH 7.4). In triplicate, 1–5 × 106 bacterial colony forming units (CFU)/mL were exposed to proteins in 50μL 10 mM PIPES-TSB in 96-well polystyrene plates. Samples were incubated at 37°C with shaking for 1 h, diluted 1:50 in 10 mM PIPES (pH 7.4), and plated using an Autoplate 4000 (Spiral Biotech Inc., Bethesda, MD). Bacterial cell survival was determined as a function of protein exposure by counting CFU after overnight growth at 37°C. After 1 h of peptide exposure, replicate peptide-bacterial mixtures were plated with a plating stylus enabling bacteria not killed by peptide to be easily enumerated according to the manufacturer’s protocol. Due to the dilution factors involved, plates on which no CFU were detected after overnight incubation theoretically may have between 1 to 999 viable CFU in the peptide-bacterial mixture. Therefore, the limit of detection for the assay is ≤ 103 CFU/mL and bacterial survival curves are labeled accordingly.
Peptide-induced Permeabilization of E. coli ML35.
Log-phase E. coli ML35 cells were washed and resuspended in 10 mM PIPES-TSB. In triplicate, bacteria (5 × 106 cells/ml) were exposed to peptides in the presence of 2.5 mM o-nitrophenyl-β-D-galactopyranoside (ONPG) for 2 h at 37 °C40–42. The kinetics of ONPG hydrolysis was measured by absorbance at 405 nm using a Spectra-Max plate spectrophotometer (Molecular Devices, Sunnyvale, CA), and the data were analyzed using GraphPad Prism.
RESULTS
Phylogenetic analysis of the chemokine family.
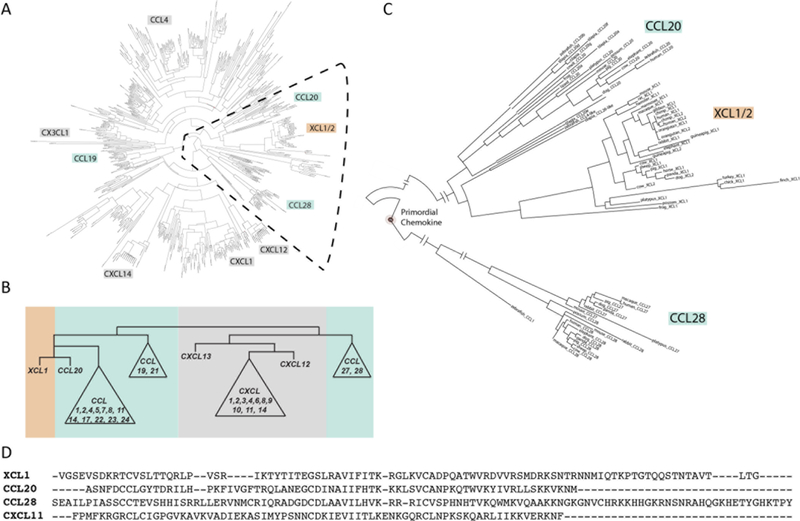
Previous genetic analysis has shown that XCL1 likely arose from a gene duplication involving a CC-type chemokine based on intron / exon boundaries27. To further explore the evolutionary relationships of XCL1 with the other chemokines and the possible origins of its metamorphic folding, we performed a comprehensive phylogenetic analysis of the available chemokine sequences. The phylogeny of the chemokine protein superfamily was inferred using maximum likelihood phylogenetics from 457 vertebrate chemokine sequences representing all four subfamily members30–33 (Figure 1A). The tree was rooted using a Cypriniformes CXCL12 sequence as the outgroup, thought to be the most primordial chemokine family member43. Inspection of the phylogenetic topology revealed that the majority of chemokine family members clustered together by subfamily with branch support values > 0.6 indicating a reliable topology. Aside from CCL27 and the closely related antimicrobial chemokine CCL28 that are evolutionarily quite distant from the rest of the subfamily, most CC chemokines form a distinct group separate from the CXC chemokines (Figure 1B). Inspection of the XCL subfamily members showed a tight grouping of sequences that diverged from a CC-type chemokine indicating common ancestry between the two subfamilies consistent with previous investigations26, 27. However, our current phylogenetic analysis suggests that XCL1 and CCL20, another well-studied antimicrobial chemokine, may have evolved from a common ancestor (Figure 1C). Based on this apparent phylogenetic relationship to CCL20, we investigated the ability of XCL1 to retard bacterial growth in comparison to other chemokines both evolutionarily close (CCL20) and more distant (CCL28 and CXCL11) (Figure 1D).
FIGURE 1:
XCL1 is most closely related to the antimicrobial chemokine CCL20. A: Phylogeny of the chemokine protein superfamily. B: View of branches demonstrating relationship of XCL1 with the antimicrobial CC chemokine, CCL20 compared to a more distant evolutionary relationship with CCL28. C: Simplified diagram of chemokine superfamily phylogeny highlighting the position of XCL1. Orange: XCL1. Green: CC chemokines. Gray: CXC chemokines. D: Sequence alignment of XCL1 with close and distant evolutionarily related chemokines tested for antimicrobial activity.
Antibacterial activity of human XCL1 relative to other cytokines.
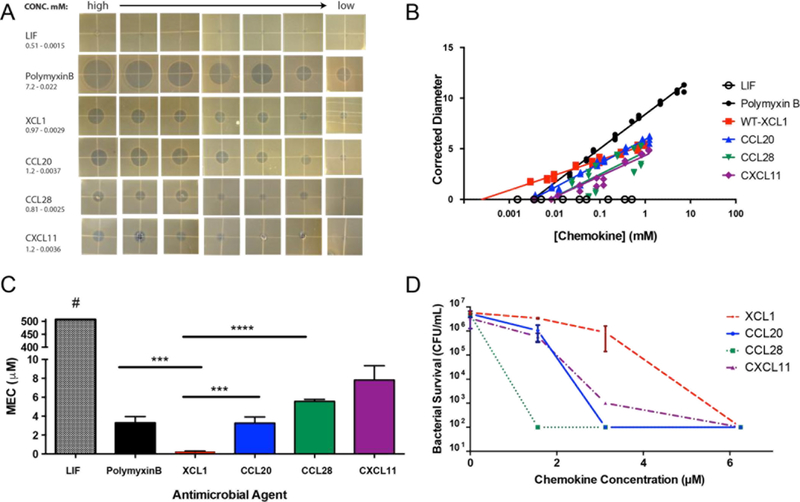
We initially used a Radial Diffusion Assay (RDA) to assess the ability of human chemokines to inhibit the growth of E. coli. Previous studies reported conflicting results on the antimicrobial activity of human XCL115, 28. The determination of antimicrobial activity by RDA is accomplished through the measurement of the diameters of zones of clearance after an overnight incubation following protein exposure (Figure 2A). We compared the antibacterial activity of human chemokines XCL1, CCL20, CCL28 and CXCL11 (I-TAC) with polymyxin B and the cytokine leukemia inhibitory factor (LIF) at a standard range of concentrations from 0.1–10 mg/mL. For each chemokine, the minimal effective concentration (MEC) for antimicrobial activity was estimated in molar units by extrapolating the diameters of zone clearance from the x-intercept as determined by semi-linear fitting factoring in peptide molecular weight (Figure 2B)38. The CXC-chemokine CXCL11 was the least potent antimicrobial agent with a MEC of 7.8 ± 1.5 μM. Human XCL1 had a significantly lower MEC (0.32 ± 0.14 μM) than polymyxin B (MEC = 3.3 ± 0.64 μM) or the antimicrobial chemokines CCL20 (MEC = 3.1 ± 0.87 μM) and CCL28 (MEC = 5.7 ± 0.18 μM), evidence of substantial antimicrobial activity against E. coli (Figure 2C).
FIGURE 2:
XCL1 exhibits potent antimicrobial activity against E. coli. A: Comparison of zones of clearance used to determine Minimal Effective Concentration (MEC) against E. coli BL21 for each protein analyzed. All photos were sized identically and have a resolution of 150 ppi. B: Representative example of semi-linear fitting of corrected diameters plotted as a function of concentration used to extrapolate the x-intercept and determine MEC. C: MEC of XCL1 in μM as determined by Radial Diffusion Assay (RDA) (n = 3 biological replicates). LIF exhibited no antimicrobial activity at the highest tested concentration of 507 μM. D: Bactericidal activity of XCL1 as determined by colony forming units (CFU) versus concentration of antimicrobial agent against E. coli ML35. Data were analyzed using a one-way ANOVA; comparisons were corrected with Sidak’s multiple comparison test (**** = p < 0.0001). Error is reported as ± standard deviation.
In addition to the influence of diffusion coefficients, the zones of clearance observed in the RDAs could result from bacteriostatic or bactericidal activities, or both. Therefore, we also employed more stringent in vitro solution assays to measure the direct bactericidal activities of individual chemokines (Figure 2D). All chemokines tested were bactericidal at 6 μM peptide levels. XCL1 was less effective than CCL20, CCL28 and CXCL11 at lower concentrations in contrast to the RDA results, suggesting that XCL1 may exert bacteriostatic activity at lower concentrations than those required for bactericidal effects.
Broad-spectrum bactericidal activity of XCL1.
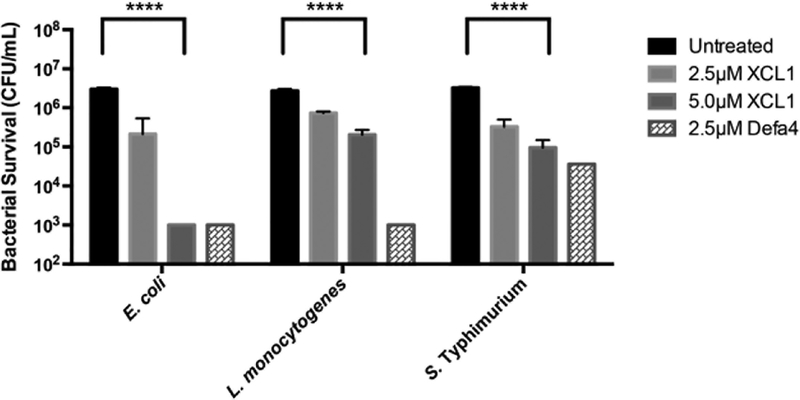
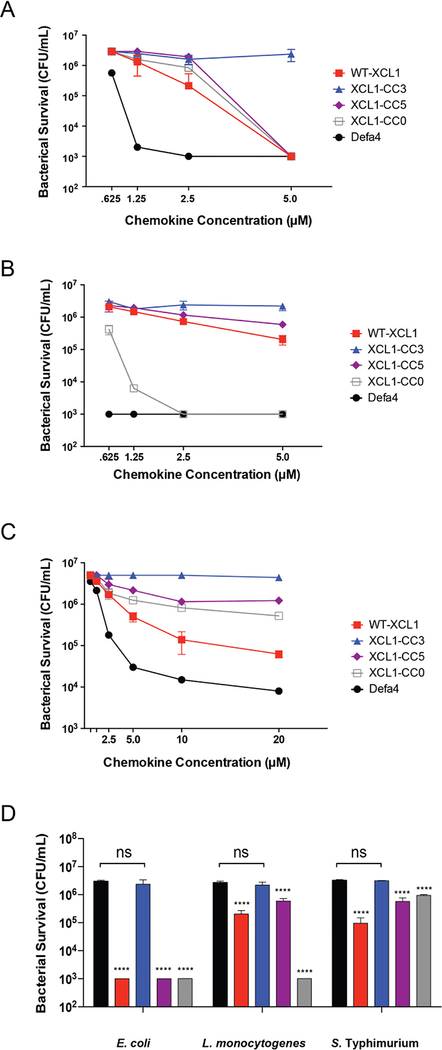
To determine whether the activity of XCL1 against E. coli was representative of a broader spectrum of antimicrobial function, we measured its bactericidal activity against Gram-positive Staphylococcus aureus, Listeria monocytogenes, and Gram-negative Salmonella enterica serovar Typhimurium and E. coli, and the activities were compared to the mouse Paneth cell α-defensin Defa439 (Figure 3). Native XCL1 had significant bactericidal activity at 5 μM and 2.5 μM protein concentrations against E. coli, L. monocytogenes, and S. Typhimurium. At the peptide concentrations tested, XCL1 and Defa4 lacked activity against S. aureus (data not shown). These results demonstrate that human XCL1 is bactericidal against both Gram-negative and Gram-positive bacteria with activity comparable to an α-defensin.
FIGURE 3:
XCL1 has broad-spectrum antimicrobial activity comparable to the α-defensin Defa4. Against the three species of bacteria tested (E. coli ML35, L. monocytogenes 1043s, and S. Typhimurium 14028s), XCL1 exhibited significant antimicrobial activity at both 2.5 μM and 5.0 μM concentrations as compared to the untreated cultures. n = 3 (technical replicates) for all concentrations in all species except Defa4 where n=1. Data were analyzed using a two-way ANOVA; comparisons were corrected with Sidak’s multiple comparison test (**** = p < 0.0001). Error is reported as ± standard deviation.
Mechanism of XCL1 bactericidal action.
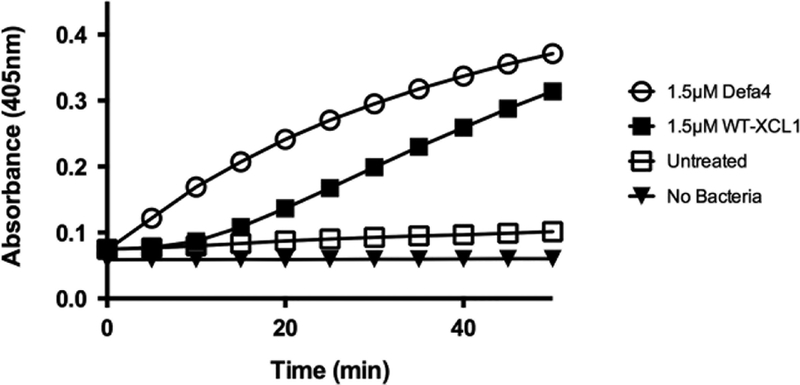
The bactericidal activity of Defa4 is mediated by selective disruption of bacterial cell membranes39, 44. To determine if human XCL1 was killing bacteria by a similar mechanism, E. coli ML35 cells were exposed to 1.5 μM concentrations of either human XCL1 or Defa4 in the presence of the lactose analog o-nitrophenyl-β-D-galactopyranoside (ONPG). Permease-negative E. coli strain ML35 (lacZc, lacY-) does not take up ONPG unless permeabilized by membrane disruptive agents, including defensins45. Upon membrane disruption, ONPG diffuses into the bacterial cell, and is converted by cytosolic β-galactosidase to ONP, which is measured by absorbance at 405 nm45. An increase in absorbance is indicative of ONPG hydrolysis and subsequent to membrane disruption and permeabilization, and untreated cells do not convert ONPG to ONP significantly (Figure 4). However, treatment of live E. coli ML35 cells with 1.5 μM human XCL1 results in ONPG conversion, indicating that XCL1 exhibits membrane disruptive activity under these in vitro conditions.
FIGURE 4:
XCL1 induces permeabilization of E. coli ML35 cells with kinetics comparable to the α-defensin Defa4. E. coli ML35 cells were exposed to 1.5 μM of either Defa4 or XCL1 and changes in absorbance at 405 nm were monitored every minute for 1 h. An increase in absorbance is indicative of ONPG hydrolysis upon contact with β-galactosidase within permeabilized cells.
Restriction of XCL1 to the monomeric state causes a loss of antimicrobial activity.
To elucidate the effect of the unique metamorphic nature of XCL1 on its antimicrobial activity, a panel of well-characterized structural variants was employed to determine which form is bactericidal. The XCL1 V21C V59C (CC3) variant possesses a second engineered disulfide bond that locks the protein into the canonical monomeric chemokine fold25. In contrast, XCL1 W55D (W55D) is a point mutant that favors dimer formation while still allowing access to the unfolded state, and XCL1 A36C A49C (CC5) has a second engineered intramolecular disulfide bond that locks XCL1 into the all-β-sheet conformation23, 46. The fourth variant we investigated, XCL1 C11A C48A (CC0), eliminates the native disulfide bond resulting in an unfolded protein similar to the unfolded transition state. The amino acid substitutions and structural consequences for each variant analyzed are summarized in Figure 5A. Using the RDA, which reports on both bactericidal and bacteriostatic function, MECs were calculated using semi-linear fitting for XCL1 variants, polymyxin B, and LIF, as positive and negative controls, respectively (Figure 5B). In these assays, monomeric XCL1 CC3 had the least activity (MEC = 250 ± 23 μM), reduced by two or more orders of magnitude relative to wild type XCL1 (MEC = 0.86 ± 0.29 μM), dimeric W55D (MEC = 2.0 ± 0.60 μM) and CC5 (MEC = 1.7 ± 0.45 μM), and the unfolded CC0 variant (2.4 μM). No significant differences were observed in the activities of dimeric or unfolded variants (Figure 5C).
FIGURE 5:
Alternative XCL1 conformations encode antimicrobial activity. A: Summary of sequence changes and structural consequences for each variant tested. B: Representative example of semi-linear fitting of corrected diameters to determine MEC for 1 biological replicate. C: MEC of WT-XCL1 or conformational variants in μM as determined by Radial Diffusion Assay (RDA) against E. coli BL21, n = 3 biological replicates each with 3 technical replicates, # indicates that LIF (leukocyte inhibitory factor) was found to exhibit no antimicrobial activity at the highest tested concentration of 507 μM, XCL1-CC3 was found to have significantly less antimicrobial activity than the other variants. Data were analyzed using a one-way ANOVA; comparisons were corrected with Sidak’s multiple comparison test (**** = p < 0.0001). Error is reported as ± standard deviation. Colors and symbols for each peptide are consistent throughout the figure.
Antibacterial activities of the CC3 variant and the other XCL1 variants were also measured in bactericidal assays against E. coli, L. monocytogenes, and S. Typhimurium. Against these three species, CC3 lacked significant bactericidal activity and was less active than variants with access to the dimeric or unfolded states. The variants of XCL1 with access to the dimeric or unfolded states retained bactericidal activity against E. coli (Figure 6A). Interestingly, L. monocytogenes was most sensitive to the completely unfolded CC0 variant (Figure 6B). Because S. Typhimurium has high inherent resistance to antimicrobial peptides, α-defensins in particular (see Defa4 in Figure 3) the maximum chemokine concentration in the assays was increased to 20 μM so that differences between XCL1 variants might be more evident (Figure 6C). As observed at lower concentrations against other bacterial species, XCL1 CC3 lacked significant bactericidal activity while variants with access to the dimeric or unfolded state maintain this function (Figure 6D). XCL1 CC5, the locked dimeric variant, also showed attenuated bactericidal activity against S. Typhimurium, showing that certain species may be resistant to killing by the dimeric form of XCL1 or that the dimeric form is primarily bacteriostatic rather than bactericidal.
FIGURE 6:
Alternative XCL1 conformations exhibit broad-spectrum antimicrobial activity similar to wild type. A: Bactericidal activity of WT-XCL1 or conformational variants against E. coli ML35. B: Bactericidal activity of WT-XCL1 or conformational variants against L. monocytogenes 10403s. C: Bactericidal activity of WT-XCL1 or conformational variants against S. Typhimurium 14028s, an organism with inherent resistance to defensins, to a maximum [peptide] of 20 μM. D: Bactericidal activity of XCL1 and conformational variants in E. coli, L. monocytogenes, and S. Typhimurium at 5 μM. Black: untreated, red: WT-XCL1, blue: XCL1-CC3, purple: XCL1-CC5, and gray: XCL1-CC0. Data were analyzed using a one-way ANOVA; comparisons were corrected with Sidak’s multiple comparison test (**** = p < 0.0001). Error is reported as ± standard deviation.
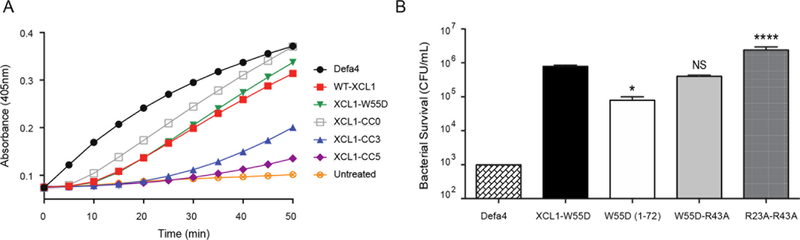
The ability of the XCL1 structural variants to induce membrane permeabilization similar to WT-XCL1 was monitored once again through the conversion of ONPG to ONP in E. coli ML35 cells (Figure 7A). Of the variants tested, the monomerically restricted CC3 proved to be one of the least potent at inducing membrane disruption as monitored by ONPG hydrolysis over the course of 1 hour. The CC5 variant, which is restricted to the all-β -sheet conformation, was the least effective variant at inducing the conversion of ONPG to ONP indicative of membrane permeabilization. These data support a primarily bacteriostatic, rather than bactericidal role for the all-β -sheet conformation of XCL1. In contrast those variants with access to the unfolded state (W55D, and CC0) were able to significantly accelerate ONPG hydrolysis and thus disrupt membranes in a manner similar to that of WT-XCL1.
FIGURE 7:
Alternative XCL1 conformations induce differential levels of antimicrobial activity. A: E. coli ML35 were exposed to 1.5 μM of WT-XCL1 or XCL1 variants and monitored for changes in absorbance at 405 nm every minute for 1 h. B: S. Typhimurium 14028s was exposed to 20 μM of WT-XCL1 or XCL1-W55D variants previously shown to alter anti-HIV activity Data were analyzed using a one-way ANOVA; comparisons were corrected with Sidak’s multiple comparison test (NS = not significant, * = p < 0.05, **** = p ≤ 0.0001). Error is reported as ± standard deviation. Defa4 was used as a positive control.
Using S. Typhimurium, we assessed the impact of C-terminal truncation (XCL1-W55D (1–72)), single (XCL1-W55D-R43A), and double (XCL1-W55D-R23AR43A) charge removal on the bactericidal activity of XCL1-W55D (Figure 7B). The dimeric-favoring W55D background was used to eliminate the monomeric conformation while maintaining access to the unfolded state. At a concentration of 20 μM, the C-terminally truncated XCL1-W55D (1–72) had significantly higher bactericidal activity than XCL1-W55D. There was no significant difference observed in the bactericidal activity of the single charge neutralized variant XCL1-W55D-R43A at 20 μM. However, removal of two positive charges in the XCL1-W55D-R23AR43A mutant resulted in a significant loss of bactericidal activity compared to XCL1-W55D.
DISCUSSION
In our efforts to elucidate the possible evolutionary origins of the metamorphic nature of XCL1 we demonstrated that the closest phylogenetic relative of XCL1 is CCL20, a known antimicrobial agent, validating previous investigations into the phylogeny of the chemokine superfamily15, 26, 27. Many chemokines are reported to exhibit antimicrobial activities, the most potent and well-studied being CXCL14, CXCL17, CCL28, and CCL2015, 20–22, 28, 39, 47. We examined the effect of XCL1 on bacterial cell growth and survival and established that this metamorphic chemokine exhibits significant antimicrobial activity much like its closest phylogenetic relative, CCL20.
This novel function of XCL1 as an antimicrobial agent was observed against both Gram-positive (L. monocytogenes) and Gram-negative (E. coli, and S. Typhimurium) bacteria, similar to other chemokines with established antimicrobial properties. We demonstrated that XCL1 was capable of inducing membrane disruption in E. coli and that this function was dependent on access to the unfolded metamorphic state. In turn, we found that the antimicrobial activity of XCL1 is lost when restricted to the canonical chemokine fold in all analyses performed. The all-β-sheet dimeric conformation was unable to induce membrane permeabilization despite near WT activity in the RDA. However, it is important to note that the potency of XCL1 as an antimicrobial agent may vary depending on the assay.
The increased antimicrobial activity of XCL1 observed by RDA relative to other assays may result from peptide exposure times that are longer in the RDA than in the in vitro bactericidal and ONPG hydrolysis assays. The RDA also measures combined bacteriostatic and bactericidal peptide activities, which are not recapitulated in assays of the effect of XCL1 on bacterial cell survival. The results suggest that the metamorphic structure of XCL1 may harbor significant bacteriostatic character in addition to bactericidal activity that may contribute to its antimicrobial function in vivo. Taken together, our results suggest that the metamorphic folding of XCL1 may have arisen as a result of selective pressure from microbial pathogens.
The structural determinants for the antimicrobial activities of individual chemokines are poorly understood, except for a positively charged patch on the surfaces of the peptides, or, in the case of CCL28, a unique highly cationic C-terminal extension that is essential for its antimicrobial effects15, 48. Here, CCL28 was compared with XCL1 because of its well-documented antimicrobial function, but, like CXCL11, it is evolutionarily quite distant from XCL1. Like CCL20 and CCL28, XCL1 has a significant number of positively charged residues on its surface resulting in a protein with a net positive charge, however, the metamorphic nature of native XCL1 is unique among chemokines23. Combining this unique structural property of XCL1 with its characterization as an antimicrobial peptide provided an opportunity to probe the relationship between chemokine structure and bactericidal activity. Membrane disruption is a common mechanism by which many AMPs, including defensins such as Defa4, exert their bactericidal effects7, 8, 12, 44. Similarly, XCL1 induced the permeabilization of live E. coli ML35 cell membranes, evidence that the mechanism of its bactericidal action is similar to that of many α-defensins44. Since the structural determinants of CCL20 bactericidal activity, the closest phylogenetic relative of XCL1, remain obscure, it is unclear whether CCL20 and XCL1 function as antimicrobial peptides via a common mechanism of action. However, because both chemokines are structurally unique and are expressed at different sites in different tissues and interact with different microbiomes, it is plausible that their mechanisms of action differ22, 49.
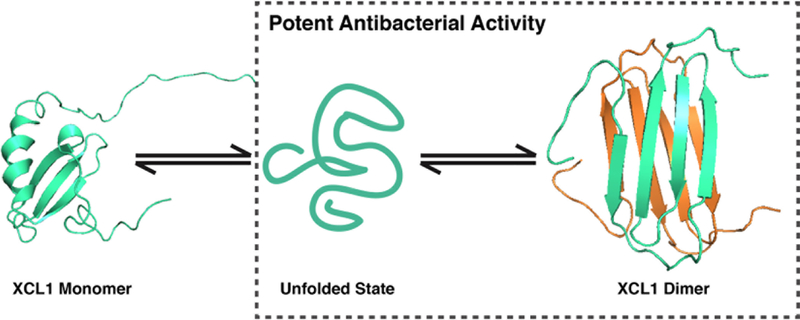
Dissection of the metamorphic XCL1 native state equilibrium through the use of conformationally restricted variants revealed that the alternative dimer and the unfolded protein harbor bacteriostatic and bactericidal activity, respectively, while the monomeric, conserved, chemokine fold does not contribute to antimicrobial activity (Figure 8). Chemokines use this conserved tertiary structural motif to bind the extracellular N-terminus of their GPCR targets, an interaction that contributes to ligand-receptor affinity and specificity ultimately stimulating downstream signaling and cellular responses. GPCR agonist activity is preserved in the CC3 variant, which restricts XCL1 to the canonical chemokine fold adopted by all members of the family but is lost in the W55D variant indicating that XCL1 binds and activates XCR1, its cognate G protein-coupled receptor while in the canonical chemokine conformation25. The loss of antimicrobial activity upon being restricted to the active XCR1 signaling conformation demonstrates that GPCR agonist activity and antimicrobial activity are encoded by distinct XCL1 conformations, perhaps including the unfolded state.
FIGURE 8:
The metamorphic nature of WT-XCL1 is necessary for potent antimicrobial activity. Restricting XCL1 to its monomeric form (CC3) abolishes antimicrobial activity, while variants with access to the unfolded or alternative dimeric conformations retain activity. XCL1 monomer and dimer structures are represented from PDB ID 1J9O and 2JP1, respectively.
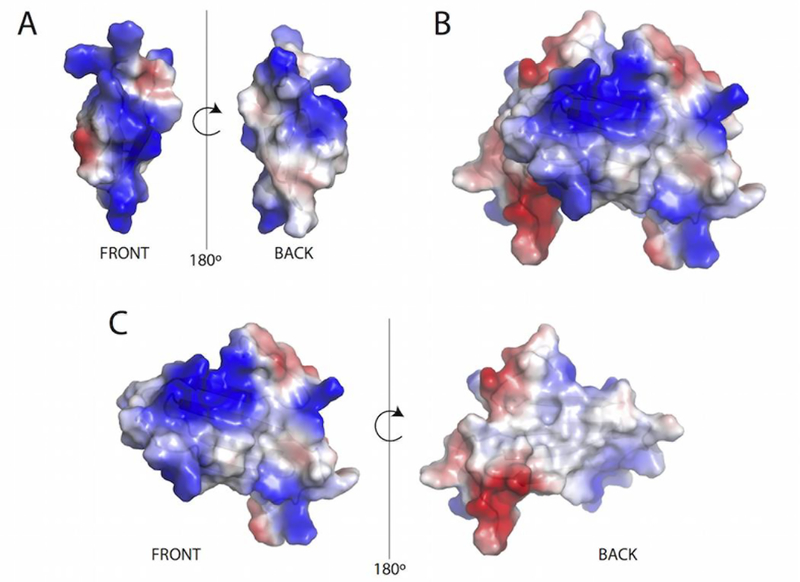
Defensins, like the majority of AMPs, are amphipathic, and most exert their antimicrobial effect through the disruption of membranes8, 11, 12, 50. In the case of Defa4, one face of the β-sheet is hydrophobic while the other is cationic allowing it to insert into the membrane and form transient defects (Figure 9A). Against all organisms tested, the dimeric forms of XCL1 were as potent antimicrobial agents as wild type XCL1 in both the RDA and in vitro bactericidal assays. The all-β-sheet dimeric conformation of XCL1 was recently demonstrated to possess a large, positively charged patch vital to its anti-HIV activity46, 51. In the intact dimer these positively charged patches are solvent exposed resulting in a highly charged molecule lacking the amphipathic character necessary to insert into a membrane and induce pore formation (Figure 9B). Dissociation of the XCL1 dimer into individual monomers would expose a hydrophobic surface that constitutes much of the dimer interface (Figure 9C).
FIGURE 9:
The all-β-sheet conformation of XCL1 exhibits significant amphipathic character similar to the α-defensin Defa4. A: Structure of mouse Defa4 (PDB ID 1TV0) with electrostatic surfaces mapped to highlight amphipathic character. B: Human XCL1 dimeric structure (PDB ID 2JP1) showing electrostatic potential across the surface. C: Individual subunit of human XCL1 mapping electrostatic potential demonstrating that one half of the subunit has a large patch of positive charge while the other half, usually obscured by the second subunit, has a large hydrophobic patch resulting in an amphipathic molecule
Based on the significant amphipathic character of the individual subunits of the all-β-sheet XCL1 conformation, we speculated that the dimeric all-β -sheet conformation of XCL1 would be capable of insertion and disruption of the bacterial membrane. However, similar to CC3, CC5 induced minimal membrane permeabilization in the ONPG hydrolysis assay, suggesting that restriction to the dimeric conformation limits the membrane permeabilization activity of XCL1. Therefore, it appears that the dimeric form facilitates bacterial killing primarily by impairing further cell growth rather than through membrane disruption. In contrast, the W55D variant favors the dimeric conformation but also accesses the unfolded state, and this is reflected in its ability to induce ONPG hydrolysis in a manner comparable to wild type XCL1. Together these data suggest a mechanism by which XCL1 interacts with the surface of the bacterial membrane, disrupting membrane curvature and leading to cell death.
It is possible that the primary stimulus for XCL1 production varies depending on the context or compartment of expression, with its antimicrobial function playing a larger role in epithelial cells rich in γδ-intraepithelial T cells, including the epidermis, during challenges like infection11. The specific release of XCL1, capable of simultaneous chemotactic and antimicrobial activities, may in part explain the previously described regulatory role for γδ T cells upon the infection of mice with L. monocytogenes12. In the same vein, our previous studies showed that treatment of CD4+ and CD8+ T cells with bacterial super antigens, specifically, Staphylococcus toxic shock syndrome toxin-1 (TSST- 1) and Staphylococcus enterotoxin B (SEB) but not LPS or inflammatory cytokines, stimulated high levels of XCL1 production by CD8+ T cells. In contrast, in the same study a broad range of inflammatory stimuli induced CCL3 and CCL5 expression13, 14. Therefore unlike other chemokines, XCL1 is only produced when T cells encounter bacterial pathogens. Thus, in addition to its chemoattractant function, XCL1 may be an important molecular effector of T cell-mediated host defense.
The anti-HIV activity of XCL1 is dependent on the dimeric form of the molecule with the unfolded CC0 protein unable to inhibit HIV infection of peripheral blood mononuclear cells (PBMCs)46. In contrast, CC0 was as potent an antimicrobial agent as the wild type protein in all species tested, and, unlike CC5, induced membrane permeabilization in a manner similar to WT-XCL1. Similarly, structure-function analysis of the antimicrobial activity of Defa4 revealed that the disulfide linkages were not necessary for bactericidal activity, and in fact their loss enhanced activity52. Unlike XCL1, Defa4 is not metamorphic and therefore may not have access to this unfolded state with enhanced antimicrobial activity in vivo. On the other hand, XCL1 with its inherent metamorphic nature interconverts between the canonical chemokine fold, the unique all-β-sheet conformation, and the unfolded state at physiological conditions. This permits frequent switching between GPCR signaling, GAG binding and anti-HIV activity, and bactericidal activity.
Finally, we performed a preliminary analysis of the structure-activity profile of XCL1-W55D against S. Typhimurium using C-terminal truncation and reduced charge mutants previously shown to alter anti-HIV activity of XCL1-W55D. Removal of the XCL1-W55D C-terminal extension significantly increased its bactericidal activity, which is in contrast to the C-terminal truncation of CCL28 that results in the loss of antimicrobial activity48. This result demonstrates the importance of additional structural characteristics in the differential antimicrobial activity of chemokines and suggests that multiple mechanisms may be responsible for the widespread appearance of antimicrobial activity in the chemokine family. The removal of two positively charged residues from XCL1-W55D had the opposite effect, resulting in a loss of antimicrobial activity. This suggests that in addition to the importance of the metamorphic unfolded state to the antimicrobial activity of XCL1, positively charged regions of the chemokine also contribute to this novel function. This preliminary assessment of the structure-activity relationships between XCL1-W55D and its bactericidal activity resulted in a profile that is more responsive to alteration than that of many other AMPs, including Defa4, which will be further investigated in later studies41.
In conclusion, data presented here establish the metamorphic protein XCL1 as a potent antimicrobial agent similar to CCL20 and Defa4 against both Gram-positive and Gram-negative bacteria. Exposing E. coli ML35 cells to XCL1 induces cell permeabilization and membrane disruption. Furthermore, we have shown that stabilization of the chemokine fold of XCL1 results in the loss of its novel function as an antimicrobial agent. Upon closer inspection of the all-β-sheet dimeric fold of XCL1, we observed that dissociation of the dimer into individual subunits revealed a highly amphipathic molecule reminiscent of the Defa4 structure. The all-β-sheet dimeric fold was unable to disrupt cell membranes, but variants with access to the unfolded state did permeabilize cell membranes, similar to the wild type protein. This observation suggests that the antimicrobial activity of XCL1 is enhanced by its unusual structural interconversion and access to the unfolded state at physiologic conditions. Using the dimer-favoring XCL1-W55D we performed a limited mutational analysis establishing an initial SAR profile for the bactericidal activity of the protein that will inform future studies. We speculate that selective pressure by microbial pathogens may have contributed to the development of the unique metamorphic nature of XCL1. By increasing our understanding of the structural origins of the antimicrobial activity of chemokines and the evolution of the metamorphic nature of XCL1 across species, these results will guide future development of antimicrobial peptides as therapeutic agents.
ACKNOWLEDGEMENTS
AMN conceived the idea for the project, purified proteins, conducted the radial diffusion assays, analyzed the results, and wrote the paper. AS, JLT, DPD conducted the in vitro bactericidal and cell permeabilization experiments. RCT performed phylogenetic analysis. DRJ aided in set up and optimization of the radial diffusion assays. AJO and BFV supervised the project, analyzed results, and edited the manuscript. We thank Dr. Jamie Fox for providing plasmids for protein production in addition to protein for analysis and Anthony Getschman for providing recombinant CCL20 for analysis. This work was supported by NIH grants AI013325 and AI058072 to B.F.V. and AI105057 to A.J.O.
Funding Information: This work was supported by NIH grants AI013325 and AI058072 to B.F.V. and AI105057 to A.J.O. B.F.V. is a co-founder and has a significant financial interest in Protein Foundry, LLC.
Abbreviations and Textual Footnotes:
- RDA
Radial Diffusion Assay
- AMP
Antimicrobial Peptide
- MIP-3α
macrophage inflammatory protein 3α
- MEC
mucosae-associated epithelial chemokine
- GPCR
G-Protein Coupled Receptor
- GAG
glycosaminoglycan
- Defa4
Defensin alpha 4
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- TSB
Trypticase Soy Broth
- CFU
Colony Forming Units
- MEC
Minimal Effective Concentration
- PIPES
piperazine-1, 4-bis(2-ethanesulfonic acid)
- ONPG
o-nitrophenyl-β-D-galactopyranoside
- I-TAC
Interferon-inducible T-cell alpha chemoattractant
- LIF
Leukemia Inhibitory Factor
- S. Typhimurium
Salmonella enterica serovar Typhimurium
- PBMC
peripheral blood mononuclear cells
REFERENCES
- 1.Butler MS, Blaskovich MA, and Cooper MA (2013) Antibiotics in the clinical pipeline in 2013, The Journal of antibiotics 66, 571–591. [DOI] [PubMed] [Google Scholar]
- 2.Lee CR, Cho IH, Jeong BC, and Lee SH (2013) Strategies to minimize antibiotic resistance, International journal of environmental research and public health 10, 4274–4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenna M (2013) Antibiotic resistance: the last resort, Nature 499, 394–396. [DOI] [PubMed] [Google Scholar]
- 4.Scanlon TC, Dostal SM, and Griswold KE (2013) A high-throughput screen for antibiotic drug discovery, Biotechnology and bioengineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wimley WC, and Hristova K (2011) Antimicrobial peptides: successes, challenges and unanswered questions, The Journal of membrane biology 239, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haney EF, and Hancock RB (2013) Peptide design for antimicrobial and immunomodulatory applications, Biopolymers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilhelmelli F, Vilela N, Albuquerque P, Derengowski Lda S, Silva-Pereira I, and Kyaw CM (2013) Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance, Frontiers in microbiology 4, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez Cascales JJ, Garro A, Porasso RD, and Enriz RD (2014) The dynamic action mechanism of small cationic antimicrobial peptides, Physical chemistry chemical physics : PCCP 16, 21694–21705. [DOI] [PubMed] [Google Scholar]
- 9.Kudryashova E, Quintyn R, Seveau S, Lu W, Wysocki VH, and Kudryashov DS (2014) Human Defensins Facilitate Local Unfolding of Thermodynamically Unstable Regions of Bacterial Protein Toxins, Immunity 41, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaal JB, Tran D, Tran P, Osapay G, Trinh K, Roberts KD, Brasky KM, Tongaonkar P, Ouellette AJ, and Selsted ME (2012) Rhesus macaque theta defensins suppress inflammatory cytokines and enhance survival in mouse models of bacteremic sepsis, PLoS One 7, e51337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai KP, Le VV, Selsted ME, and Ouellette AJ (2014) Hydrophobic determinants of alpha-defensin bactericidal activity, Infect Immun 82, 2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wimley WC, Selsted ME, and White SH (1994) Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores, Protein Sci 3, 1362–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanniarachchi YA, Kaczmarek P, Wan A, and Nolan EM (2011) Human defensin 5 disulfide array mutants: disulfide bond deletion attenuates antibacterial activity against Staphylococcus aureus, Biochemistry 50, 8005–8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Territo MC, Ganz T, Selsted ME, and Lehrer R (1989) Monocyte-chemotactic activity of defensins from human neutrophils, J Clin Invest 84, 2017–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, and Oppenheim JJ (2003) Many chemokines including CCL20/MIP-3alpha display antimicrobial activity, J Leukoc Biol 74, 448–455. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, and Oppenheim JJ (1999) Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6, Science 286, 525–528. [DOI] [PubMed] [Google Scholar]
- 17.Raman D, Sobolik-Delmaire T, and Richmond A (2011) Chemokines in health and disease, Exp Cell Res 317, 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Biragyn A, Kwak LW, and Oppenheim JJ (2002) Mammalian defensins in immunity: more than just microbicidal, Trends in immunology 23, 291–296. [DOI] [PubMed] [Google Scholar]
- 19.Allen SJ, Crown SE, and Handel TM (2007) Chemokine: receptor structure, interactions, and antagonism, Annual review of immunology 25, 787–820. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen LT, and Vogel HJ (2012) Structural perspectives on antimicrobial chemokines, Front Immunol 3, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf M, and Moser B (2012) Antimicrobial activities of chemokines: not just a side-effect?, Front Immunol 3, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yung SC, and Murphy PM (2012) Antimicrobial chemokines, Front Immunol 3, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuinstra RL, Peterson FC, Kutlesa S, Elgin ES, Kron MA, and Volkman BF (2008) Interconversion between two unrelated protein folds in the lymphotactin native state, Proc Natl Acad Sci U S A 105, 5057–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuloglu ES, McCaslin DR, Markley JL, and Volkman BF (2002) Structural rearrangement of human lymphotactin, a C chemokine, under physiological solution conditions, J Biol Chem 277, 17863–17870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuinstra RL, Peterson FC, Elgin ES, Pelzek AJ, and Volkman BF (2007) An engineered second disulfide bond restricts lymphotactin/XCL1 to a chemokine-like conformation with XCR1 agonist activity, Biochemistry 46, 2564–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomiyama H, Osada N, and Yoshie O (2013) Systematic classification of vertebrate chemokines based on conserved synteny and evolutionary history, Genes to cells : devoted to molecular & cellular mechanisms 18, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida T, Imai T, Takagi S, Nishimura M, Ishikawa I, Yaoi T, and Yoshie O (1996) Structure and expression of two highly related genes encoding SCM-1/human lymphotactin, FEBS letters 395, 82–88. [DOI] [PubMed] [Google Scholar]
- 28.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, and Strieter RM (2001) Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity, J Immunol 167, 623–627. [DOI] [PubMed] [Google Scholar]
- 29.Guzzo C, Fox J, Lin Y, Miao H, Cimbro R, Volkman BF, Fauci AS, and Lusso P (2013) The CD8-derived chemokine XCL1/lymphotactin is a conformation-dependent, broad-spectrum inhibitor of HIV-1, PLoS pathogens 9, e1003852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei J, Kim BH, and Grishin NV (2008) PROMALS3D: a tool for multiple protein sequence and structure alignments, Nucleic acids research 36, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei J, Tang M, and Grishin NV (2008) PROMALS3D web server for accurate multiple protein sequence and structure alignments, Nucleic acids research 36, W30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guindon S, and Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood, Systematic biology 52, 696–704. [DOI] [PubMed] [Google Scholar]
- 33.Guindon S, Delsuc F, Dufayard JF, and Gascuel O (2009) Estimating maximum likelihood phylogenies with PhyML, Methods in molecular biology 537, 113–137. [DOI] [PubMed] [Google Scholar]
- 34.Takekoshi T, Ziarek JJ, Volkman BF, and Hwang ST (2012) A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability, Mol Cancer Ther 11, 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veldkamp CT, Ziarek JJ, Peterson FC, Chen Y, and Volkman BF (2010) Targeting SDF-1/CXCL12 with a ligand that prevents activation of CXCR4 through structure-based drug design, J Am Chem Soc 132, 7242–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veldkamp CT, Ziarek JJ, Su J, Basnet H, Lennertz R, Weiner JJ, Peterson FC, Baker JE, and Volkman BF (2009) Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12, Protein Sci 18, 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach H. H. t., Wong YM, Tripathi A, Nevins AM, Gamelli RL, Volkman BF, Byron KL, and Majetschak M (2014) Chemokine (C-X-C motif) receptor 4 and atypical chemokine receptor 3 regulate vascular alpha(1)-adrenergic receptor function, Mol Med 20, 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg DA, and Lehrer RI (1997) Designer assays for antimicrobial peptides. Disputing the “one-size-fits-all” theory, Methods in molecular biology 78, 169–186. [DOI] [PubMed] [Google Scholar]
- 39.Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuniga J, Selman M, Ouellette AJ, and Zlotnik A (2012) CXCL17 is a mucosal chemokine elevated in idiopathic pulmonary fibrosis that exhibits broad antimicrobial activity, J Immunol 188, 6399–6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weeks CS, Tanabe H, Cummings JE, Crampton SP, Sheynis T, Jelinek R, Vanderlick TK, Cocco MJ, and Ouellette AJ (2006) Matrix metalloproteinase-7 activation of mouse paneth cell pro-alpha-defensins: SER43 down arrow ILE44 proteolysis enables membrane-disruptive activity, The Journal of biological chemistry 281, 28932–28942. [DOI] [PubMed] [Google Scholar]
- 41.Hadjicharalambous C, Sheynis T, Jelinek R, Shanahan MT, Ouellette AJ, and Gizeli E (2008) Mechanisms of alpha-defensin bactericidal action: comparative membrane disruption by Cryptdin-4 and its disulfide-null analogue, Biochemistry 47, 12626–12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueredo SM, Weeks CS, Young SK, and Ouellette AJ (2009) Anionic amino acids near the pro-alpha-defensin N terminus mediate inhibition of bactericidal activity in mouse pro-cryptdin-4, The Journal of biological chemistry 284, 6826–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zlotnik A, Yoshie O, and Nomiyama H (2006) The chemokine and chemokine receptor superfamilies and their molecular evolution, Genome biology 7, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt NW, Mishra A, Lai GH, Davis M, Sanders LK, Tran D, Garcia A, Tai KP, McCray PB, Ouellette AJ, Selsted ME, and Wong GC (2011) Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization, J Am Chem Soc 133, 6720–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehrer RI, Barton A, and Ganz T (1988) Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry, J Immunol Methods 108, 153–158. [DOI] [PubMed] [Google Scholar]
- 46.Fox JC, Tyler RC, Guzzo C, Tuinstra RL, Peterson FC, Lusso P, and Volkman BF (2015) Engineering the metamorphic chemokine Lymphotactin/XCL1 into the GAG-binding, HIV-inhibitory dimer conformation, ACS chemical biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai C, Basilico P, Cremona TP, Collins P, Moser B, Benarafa C, and Wolf M (2015) CXCL14 Displays Antimicrobial Activity against Respiratory Tract Bacteria and Contributes to Clearance of Streptococcus pneumoniae Pulmonary Infection, J Immunol. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, and Wilson E (2010) The antimicrobial activity of CCL28 is dependent on C-terminal positively-charged amino acids, Eur J Immunol 40, 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tikhonov I, Kitabwalla M, Wallace M, Malkovsky M, Volkman B, and Pauza CD (2001) Staphylococcal superantigens induce lymphotactin production by human CD4+ and CD8+ T cells, Cytokine 16, 73–78. [DOI] [PubMed] [Google Scholar]
- 50.White SH, Wimley WC, and Selsted ME (1995) Structure, function, and membrane integration of defensins, Current opinion in structural biology 5, 521–527. [DOI] [PubMed] [Google Scholar]
- 51.Guzzo C, Fox JC, Miao H, Volkman BF, and Lusso P (2015) Structural Determinants for the Selective Anti-HIV-1 Activity of the All-beta Alternative Conformer of XCL1, Journal of virology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maccari G, Di Luca M, Nifosi R, Cardarelli F, Signore G, Boccardi C, and Bifone A (2013) Antimicrobial peptides design by evolutionary multiobjective optimization, PLoS computational biology 9, e1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]