Abstract
Background
Early detection would improve upper gastrointestinal cancer prognosis. We aimed to identify serum protein biomarker for the detection of early-stage upper gastrointestinal cancer.
Methods
We performed a three-tiered study including 2028 participants from three medical centres. First, we applied two different antibody arrays to screen candidate serum proteins that increased in 20 patients with oesophageal squamous cell carcinoma (ESCC) compared with 20 normal controls. We then evaluated the selected protein by enzyme-linked immunosorbent assay in 1064 participants including 731 upper gastrointestinal cancer patients (287 ESCCs, 237 oesophagogastric junction adenocarcinomas (EJAs), and 207 stomach cancers) and 333 normal controls. The diagnostic value of the selected protein was finally validated in two independent cohorts of ESCC patients and controls (n=472 and 452, respectively). The receiver operating characteristic was used to calculate diagnostic accuracy.
Findings
Serum insulin-like growth factor binding protein-1 (IGFBP-1) identified in both antibody arrays showed significantly elevated levels in upper gastrointestinal cancers, compared with normal controls. Serum IGFBP-1 provided high diagnostic accuracy of early-stage ESCC, EJA, stomach and cancer (areas under the curve: 0·898, 0·936 and 0·864, respectively). This protein maintained diagnostic performance for early-stage ESCC in independent cohorts 1 and 2 (0·849 and 0·911, respectively). Additionally, serum levels of IGFBP-1 dropped significantly after surgical resection of primary tumours, compared with the corresponding pre-operative ESCC samples (p < 0·05).
Interpretation
Serum IGFBP-1 represents a promising diagnostic biomarker to detect early-stage upper gastrointestinal cancer.
Keywords: Upper gastrointestinal cancer, IGFBP-1, Early diagnosis, Sensitivity, Specificity
Research in context.
Evidence before this study
We systemically searched PubMed for reports with the terms: “serum OR plasma” AND “insulin-like growth factor binding protein-1 OR IGFBP-1” AND “oesophageal cancer OR oesophagogastric junction adenocarcinoma (EJA) OR stomach cancer” AND “diagnosis OR detection”, without date restriction or limitation to English language publications. We found that no studies began with high-throughput screening and verified with large-scale, multicentre validations to assessed diagnostic value of serum insulin-like growth factor binding protein-1 (IGFBP-1) in these upper gastrointestinal cancers. Whether serum IGFBP-1 could be used as a diagnostic biomarker for upper gastrointestinal cancer remained to be revealed.
Added value of this study
We used two high-throughput antibody arrays for screening, and designed a large study to assess the diagnostic value of serum IGFBP-1 in patients with upper gastrointestinal cancers from three medical centres and verify in two independent cohort validation of patients with oesophageal squamous cell carcinoma (ESCC) and normal controls. Our data showed that serum IGFBP-1 levels were increased in ESCC, EJA and stomach cancer. Serum IGFBP-1 provided a high diagnostic accuracy of ESCC, EJA, and stomach cancer, including early-stage disease. Importantly, compared with carcino-embryonic antigen, Cyfra21-1 and squamous cell carcinoma antigen tested alone or together, serum IGFBP-1 had significantly improved diagnostic accuracy for early stage ESCC. Moreover, this serum protein has the potential for post-operation monitoring of ESCC. These findings indicate that IGFBP-1 is a promising serum biomarker for the early detection of upper gastrointestinal cancer. To our knowledge, this is the first comprehensive study to evaluate the diagnostic performance of serum IGFBP-1 in upper gastrointestinal cancer.
Implications of all the available evidence
Our findings offer strong evidence that serum IGFBP-1 could detect early stage upper gastrointestinal cancer, which makes more tumours resectable and treatment more efficacious. The ability of this serum protein to detect upper gastrointestinal cancer should be verified in more medical institutions.
Alt-text: Unlabelled box
1. Introduction
Upper gastrointestinal cancers, mainly including oesophageal cancer, oesophagogastric junction cancer, and stomach cancer, are a huge threat to human health and are known as one of the most common cancer types with a high mortality worldwide [1]. According to GLOBOCAN 2018, it was estimated that 57 thousand cases and 1·03 million cases were diagnosed with oesophageal cancer and stomach cancer, respectively [2]. Despite huge investment in gastrointestinal cancer research, especially in drug development, advances in treatment and improvements in prognosis have been modest over the past few decades. A considerable part of localized upper gastrointestinal cancers can be treated by means of surgical operation alone, without any systemic treatment [3]. Once cancers have spread to distant organs, however, surgical resection is rarely effective. Most upper gastrointestinal cancer patients are diagnosed in metastatic stage with poor prognosis, but patients at early stage could be curable with the five-year survival rate of higher than 70% [[4], [5], [6]]. Thus, early detection is one of the most promising ways to reduce cancer-related deaths [[7], [8], [9]]. However, up to now, relatively few methods for early detection of upper gastrointestinal cancers have proven adequately valid and practical for wide use in clinical practice.
The approved approach for early detection of upper gastrointestinal cancer is endoscopy [10,11]. Upper gastrointestinal cancer lacks non-invasive test (e.g. serum biomarker) for early diagnosis. In the field of tumour diagnosis, the widely used blood-based biomarkers are CA125 and prostate-specific antigen for ovarian cancer and prostate cancer screening, respectively, but the proper use of these biomarkers is still being debated [12,13]. Although blood tests for early detection of cancer remain an elusive target, rapid advances in proteomics have changed the landscape of early cancer detection, promising to massively extend the pool of potentially useful biomarkers as blood tests for screening [[14], [15], [16]]. Among the serum-based proteomics methods, antibody microarrays occupy a pivotal space in the discovery of cancer biomarkers. Several groups have studied serum protein panels developed from antibody arrays, which have shown the ability to discriminate pancreatic cancer patients from healthy individuals [17,18]. To date, most studies on serum protein biomarkers, initially identified from antibody microarrays, involve a small number of patients with early-stage cancer and lack independent cohort validation.
We designed a multicentre study and identified insulin-like growth factor binding protein-1 (IGFBP-1) as a serum biomarker with high diagnostic accuracy for upper gastrointestinal cancers. Firstly, we identified serum IGFBP-1 by screening two kinds of antibody microarrays. We then evaluated IGFBP-1 as a biomarker by enzyme-linked immunosorbent assay (ELISA) in four types of gastrointestinal cancers, including oesophageal squamous cell carcinoma (ESCC), oesophagogastric junction adenocarcinoma (EJA), and stomach cancer. Finally, we used two independent validation cohorts comprised of ESCC patients and normal controls to verify the diagnostic value of IGFBP-1.
2. Materials and methods
2.1. Study design and participants
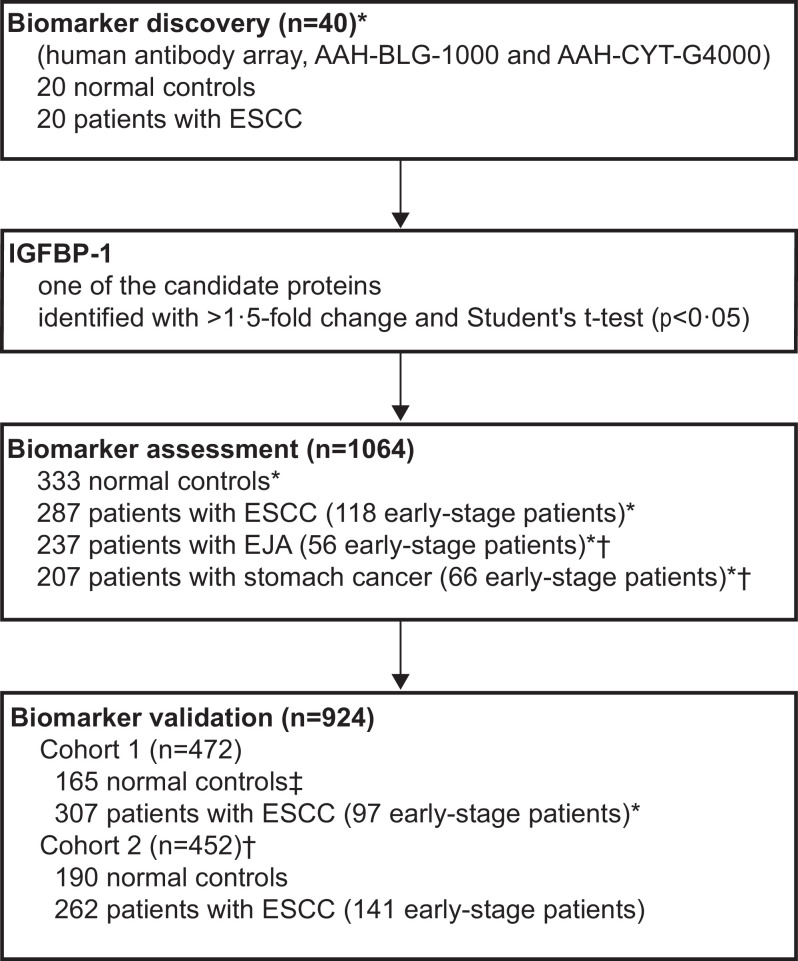
Participants were consecutively enrolled from the Cancer Hospital of Shantou University Medical College (SUMC), Cancer Centre of Sun Yat-sen University (SYSU), and Shantou Nan'ao People's Hospital (Fig. 1). In the biomarker discovery stage, we selected the blood samples from ESCC patients and age- and gender-matched normal controls, between Dec 10, 2012 and Feb 7, 2013, at the Cancer Hospital of SUMC (Supplementary Table S1). In the biomarker assessment stage, the sera of patients diagnosed with ESCC, EJA, or stomach cancer, and normal controls were collected at the Cancer Hospital of SUMC or the Cancer Centre of SYSU between Mar 4, 2013, and Apr 22, 2018. These three gastrointestinal cancer types were selected because they are common in East Asians or in Western populations, and because no clear evidence has been provided to clarify the early diagnostic value of serum IGFBP-1 among them, and because there are no blood-based biomarkers in common clinical use for their earlier detection. The validation stage had two cohorts comprised of controls and patients with ESCC. Cohort 1 was composed of ESCC patients at Cancer Hospital of SUMC enrolled from Dec 23, 2014, to Jun 21, 2017, and normal controls at Shantou Nan'ao People's Hospital who had been from part of a population-based cohort study of oesophageal cancer and precancerous lesions from the high-risk area (Nan'ao Island) [19]. Cohort 2 enrolled participants of ESCC patients and normal controls between Aug 1, 2016, and Apr 5, 2018 at the Cancer Centre of SYSU.
Fig. 1.
Study design.
Serum samples from 20 ESCC patients and 20 normal controls were used to screen protein candidates by using two different antibody arrays in the biomarker discovery stage. The diagnostic values of the candidate IGFBP-1 using ELISA were further evaluated in 1064 serum samples in the biomarker assessment stage, and were finally validated in 924 serum samples from two independent cohorts. *Participants at the Cancer Hospital of Shantou University Medical College. †Participants at The Sun Yat-sen University Cancer Centre. ‡Participants from part of a population-based cohort study of oesophageal cancer and precancerous lesions from a high-risk area (Nan'ao Island). ESCC, oesophageal squamous cell carcinoma.
This study was performed after approval from the institutional ethics review committee of each medical centre, and informed consent was obtained from all participants. This work complied with the principles laid down in the Declaration of Helsinki. Samples from patients with various types of cancers collected in this study met the following eligibility criteria: they did not have a history of cancer, type 1 and type 2 diabetes or cardiovascular diseases regarding acute coronary syndrome, acute myocardial infarction, abdominal aortic aneurysm, ischaemic heart disease and hypertrophic cardiomyopathy, and were confirmed histopathologically, and were obtained prior to any anti-cancer treatment. General demographics and American Joint Committee on Cancer (AJCC) stage (8th edition) were documented. We classified tumours with AJCC stage 0+I+II as early-stage cancer. Normal control samples, which were obtained from individuals with no history of cancer and without diabetes or cardiovascular disease and were matched as far as possible to the patient group with respect to age and gender, were eligible for inclusion in the study.
2.2. Collection of blood samples
Fasting blood samples from the cancer patients and normal controls were processed in an identical manner, which were collected into anticoagulant-free tubes and centrifuged at 1250g for 5 min. Then the serum was stored at –80°C in the biobank.
2.3. Antibody arrays detection
We sent sera of 20 ESCC patients and 20 normal controls to RayBiotech Company for antibody microarrays detection (AAH-BLG-1000 and AAH-CYT-G4000) to identify candidate biomarkers. Briefly, the Human Antibody Array AAH-BLG-1000 and the Human Cytokine Array AAH-CYT-G4000 contain 1000 human target proteins and 274 cytokines, respectively. The primary amine of the proteins was biotinylated in serum samples that were dialyzed. The glass slide arrays were then blocked, and the biotin-labelled samples were added onto the glass slide, which was pre-printed with capture antibodies, and incubated to allow for interaction of target proteins. Streptavidin-conjugated fluorescent dye (Cy3 equivalent) was then applied to the array. Finally, the glass slide was dried, and laser fluorescence scanning (GenePix 4000B scanner) was used to visualize the signals. Signal intensity data after background subtraction and normalization were exported directly into the RayBio® Analysis Tool software.
2.4. ELISA for IGFBP-1
ELISA (CUSABIO, Wuhan, China) for serum IGFBP-1 was performed by four researchers (Yi-Wei Xu, Yu-Hui Peng, Hao Chen and Ling-Yu Chu) at the Shantou University Medical College or Cancer Centre of SYSU, who were blinded to the status of the samples. Samples of patients and normal controls were assayed together in the same batch. Quality control (QC) samples were pooled serum samples randomly selected from 100 cancer patients, and were placed in each batch of study samples to ensure quality control monitoring of the assay runs by using Levey–Jennings plots, as previous described [20]. Briefly, 100μl of serum sample and QC at 20 fold-dilution and standard were added into each 96-microwell plate, and incubated for 2 h at 37°C, followed by the addition of 100μl biotin-antibody (1X) for 1 h. After removing the liquid and washing, 100μl horseradish peroxidase (HRP)-avidin (1X) for each well was incubated for 1 h at 37°C. Colour development was achieved with 90μl per well TMB Substrate, and stop solution was added to stop the reaction. The optical density (OD) value of each well was measured at 450 nm and referenced to 570 nm on a microplate reader (Thermo Fisher Scientific, Boston, MA). The serum IGFBP-1 concentrations were obtained by plotting a standard curve with a four-parameter logistic curve manner, and multiplied by the dilution factor. All measurements were done in duplicate.
2.5. Measurement of tumour markers in clinical use
The concentrations of Carcino-embryonic antigen (CEA) and Cyfra21-1 in serum were measured by an automatic electrochemical luminescence analyzer (Cobas e602, Roche, Germany). The serum level of squamous cell carcinoma antigen (SCCA) was detected by an Architect i2000 chemiluminescence analyzer (Abbott, USA). All tests for the three tumour markers were performed at the Department of Clinical Laboratory Medicine, Cancer Centre of SYSU and conducted according to instrument operating manuals. The recommended clinical cutoff values of CEA, Cyfra21-1 and SCCA were 5·0 ng/mL, 3·3 ng/mL and 1·5 ng/mL, respectively, in this study.
2.6. Statistical analysis
Statistical analyses were done with SigmaPlot (version 10·0), GraphPad Prism (version 5) and SPSS for Windows (version 19·0). For the antibody microarray analysis, significance analysis of microarrays [21] and Student's t test (p<0·05) were used to identify the candidate proteins with differential levels between normal controls and individuals with ESCC. We used the Mann–Whitney U test to compare the differences of IGFBP-1 levels in two groups. The correlation between positive rates of serum IGFBP-1 and clinicopathological features was analyzed with Pearson's χ² test. We assessed sensitivity, specificity, and areas under the curves (AUCs) with 95% confidence interval (CI) by plotting the receiver operating characteristic (ROC) curves. The optimum cutoff value for IGFBP-1 diagnosis was obtained by achieving the maximum sensitivity when the specificity was > 90% which could make a test beneficial to early cancer detection [22], and by minimising the distance of the cut-off value to the top-left corner of the ROC curve. To investigate the combined use of CEA, Cyfra21-1, and SCCA measurement, a new variable predicted probability (p) of the combined marker obtained by binary logistic regression was subjected to ROC analysis, as reported previously [23]. We used the Wilcoxon signed-ranks test for comparison of serum IGFBP-1 levels before and after tumour resection in ESCC patients. Overall survival (OS) time was assessed by the Kaplan–Meier method and compared by the log-rank test. Overall survival was defined as the interval between the date of surgical resection of tumour and death. The data were censored for patients who were alive at the last follow-up. In all statistical tests, we considered p values (two sided) of lower than 0·05 to be statistically significant.
2.7. Data sharing
Data about antibody arrays generated in this research were shared in Mendeley Data (DOI: 10.17632/mb38dzj6c2.2).
3. Results
3.1. Patient characteristics
We recruited 2028 participants in total, 40 in the biomarker discovery stage, 1064 in the biomarker assessment stage, and 924 in the biomarker validation stage (Fig. 1). The characteristics of the study participants in each group are summarised in the Supplementary Tables S1–S3.
3.2. Biomarker discovery by serum-based antibody arrays
To identify novel blood-based protein biomarkers that can detect the presence of cancer at an early stage, we performed a high-throughput proteomics analysis of ESCC, using two different antibody arrays. There were 86 and 21 serum proteins identified in Human Antibody Array AAH-BLG-1000 and Human Cytokine Array AAH-CYT-G4000, respectively, with significantly higher levels in ESCC patients than those in normal patients (Supplementary Tables S4 and S5). Among these proteins, we focused on IGFBP-1, which was one of only two candidate biomarkers (i.e., IGFBP-1 and uPAR) showing elevated levels in both antibody microarrays in the ESCC samples (Fig. 2, Supplementary Tables S4 and S5).
Fig. 2.
Results of human antibody arrays to measure serum proteins in ESCC patients.
Volcano plots of candidate protein expression in ESCC and normal control from the Human Antibody Array AAH-BLG-1000 (a) and the Human Cytokine Array AAH-CYT-G4000 (b). X-axis: log2 ratio of protein expression levels (fold change) between normal control and ESCC. Y-axis: the p value (−log10 transformed) of proteins. The vertical dotted lines correspond to 1·5-fold up and down, respectively, and the horizontal dotted lines represent a p value of 0·05. Hierarchical clustering results of serum proteins displaying higher levels in patients with ESCC than normal controls (fold change > 1·5) were measured by the Human Antibody Array AAH-BLG-1000 (c) and the Human Cytokine Array AAH-CYT-G4000 (d). Each row represents an individual protein, while each column indicates an individual serum sample. The colour scale bar is on the right of the heat map, with the highest and lowest values in red and blue, respectively. Representative results of IGFBP1 from AAH-CYT-G4000 were also shown (e). N1 to N4 are normal controls, and C1 to C4 are ESCC patients. ESCC, oesophageal squamous cell carcinoma.
3.3. Biomarker diagnosis assessment in upper gastrointestinal cancers
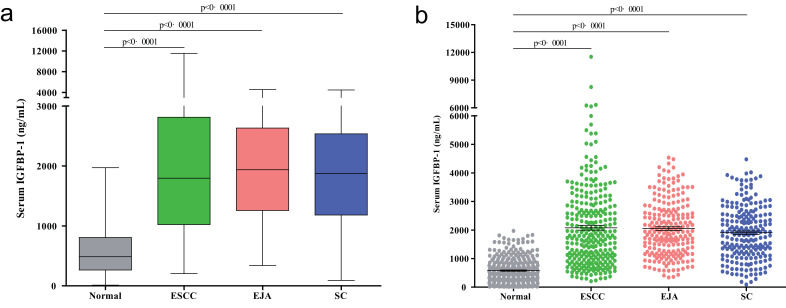
To evaluate whether IGFBP-1 could be served as a serum protein biomarker for the early detection of ESCC or even other gastrointestinal cancers, we used it to study 333 normal controls and 731 patients who had been diagnosed with ESCC, EJA, or stomach cancer. Compared with the normal controls, IGFBP-1 was elevated in the sera of patients with ESCC, EJA, or stomach cancer (Fig. 3, Mann–Whitney U test, all p<0·0001).
Fig. 3.
Levels of serum IGFBP-1 in gastrointestinal cancers.
(a) Box plot illustrates median levels and interquartile ranges and the whiskers show minimum and maximum value of serum IGFBP-1 in normal controls and patients with different tumour types. (b) Scatter plots of serum IGFBP-1 from patients with different tumour types and normal controls. Black horizontal lines are means, and error bars are SEs. Mann–Whitney U test was conducted to assess the differences.
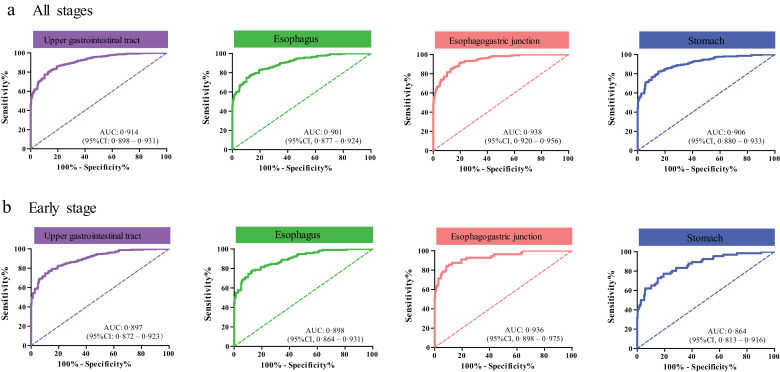
With an optimum diagnostic cutoff of 1228 ng/ml, ROC analysis showed that serum IGFBP-1 had an AUC 0·914 (95% CI 0·898–0·931) to distinguish all upper gastrointestinal cancer patients from controls with 73·46% sensitivity and 91·29% specificity (Fig. 4 and Table 1). Similar data were obtained when we compared the control group with patients with early-stage or advanced-stage upper gastrointestinal cancers (Fig. 4 and Table 1). We further assessed the diagnostic performance of serum IGFBP-1 among the three cancer types and found that the sensitivity of serum IGFBP-1 ranged from 70·38% in ESCC to 77·22% in EJA (Fig. 4 and Table 1). At this sensitivity, the specificity was 91·29%, with the AUC ranging from 0·901 in ESCC to 0·938 in EJA (Fig. 4 and Table 1). Clearly, serum IGFBP-1 had good diagnostic efficiency for ESCC, EJA, and stomach cancer (Table 1). A similar trend of early diagnosis performance was observed for serum IGFBP-1 in the identification of these three cancer types (Fig. 4). For the relationship between IGFBP-1 and the clinicopathological variables of tumours, IGFBP-1 was significantly associated with age, gender or T stage in some tumour types (Supplementary Table S6, Chi-squared test, p<0·05).
Fig. 4.
Performance of serum IGFBP-1 in gastrointestinal cancers.
(a) ROC curve for IGFBP-1 in all stages of different cancer types vs. normal control. (b) ROC curve for IGFBP-1 in early stage of different cancer types vs. normal control.
Table 1.
Diagnostic performance of serum IGFBP-1 by tumour type in the biomarker assessment stage.
| All stages | AUC (95%CI) | Sensitivity (95%CI) | Specificity (95%CI) | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|
| Upper Gastrointestinal Cancer | 0·914 (0·898–0·931) | 73·46% (70·10–76·63%) | 91·29% (87·73–94·09%) | 94·87% | 61·05% | 8·434 | 0·291 |
| ESCC | 0·901 (0·877–0·924) | 70·38% (64·74–75·61%) | 91·29% (87·73–94·09%) | 87·45% | 78·14% | 8·080 | 0·324 |
| EJA | 0·938 (0·920–0·956) | 77·22% (71·34–82·40%) | 91·29% (87·73–94·09%) | 86·18% | 85·07% | 8·866 | 0·250 |
| Stomach cancer | 0·906 (0·880–0·933) | 73·43% (66·86–79·31%) | 91·29% (87·73––94·09%) | 83·96% | 84·70% | 8·431 | 0·291 |
| Early stage | |||||||
| Upper Gastrointestinal Cancer | 0·897 (0·872–0·923) | 71·67% (65·51–77·28%) | 91·29% (87·73–94·09%) | 85·58% | 81·72% | 8·228 | 0·310 |
| ESCC | 0·898 (0·864–0·931) | 71·19% (62·13–79·15%) | 91·29% (87·73–94·09%) | 74·37% | 89·93% | 8·173 | 0·316 |
| EJA | 0·936 (0·898–0·975) | 83·93% (71·67–92·38%) | 91·29% (87·73–94·09%) | 61·85% | 97·12% | 9·636 | 0·176 |
| Stomach cancer | 0·864 (0·813–0·916) | 62·12% (49·34–73·78%) | 91·29% (87·73–94·09%) | 58·49% | 92·42% | 7·132 | 0·415 |
| Advanced stage | |||||||
| Upper Gastrointestinal cancer | 0·923 (0·906–0·940) | 74·34% (70·23–78·15%) | 91·29% (87·73–94·09%) | 92·64% | 70·69% | 8·535 | 0·281 |
| ESCC | 0·903 (0·874–0·932) | 69·86% (62·30–76·63%) | 91·29% (87·73–94·09%) | 80·30% | 85·63% | 8·021 | 0·330 |
| EJA | 0·939 (0·919–0·958) | 75·14% (68·18–81·25%) | 91·29% (87·73–94·09%) | 82·41% | 87·11% | 8·627 | 0·272 |
| Stomach cancer | 0·926 (0·899–0·953) | 78·72% (71·04–85·16%) | 91·29% (87·73–94·09%) | 79·25% | 91·03% | 9·038 | 0·233 |
Upper gastrointestinal cancers include oesophageal squamous cell carcinoma, oesophagogastric junction adenocarcinoma, and stomach cancer. CI, exact confidence interval; NC, normal controls; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value. ESCC, oesophageal squamous cell carcinoma. EJA, oesophagogastric junction adenocarcinoma.
We next evaluated the effect of the serum IGFBP-1 on survival of ESCC, EJA and stomach cancer. The median follow-up time for ESCC, EJA and stomach cancer was 48·3, 25·7 and 16·6 months, respectively. With the use of the same cutoff value of 1228 ng/ml in the diagnosis evaluation, Kaplan–Meier analysis and log-rank test revealed that there were no statistically significant differences of 5-year overall survival rates between patients with positive IGFBP-1 expression and negative IGFBP-1 (58·9% vs. 63·2% in ESCC, 49·9% vs. 50·4% in EJA, and 41·4% vs. 59·3% in ESCC, respectively, all p > 0·05, Supplementary Fig. S1). In addition, serum IGFBP-1 did not show prognostic value in selective patient subgroups stratified according to the depth of tumour invasion, lymph node status, or TNM stage (data not shown). These results indicated that serum IGFBP-1 may not be a prognostic predictor for upper gastrointestinal cancer.
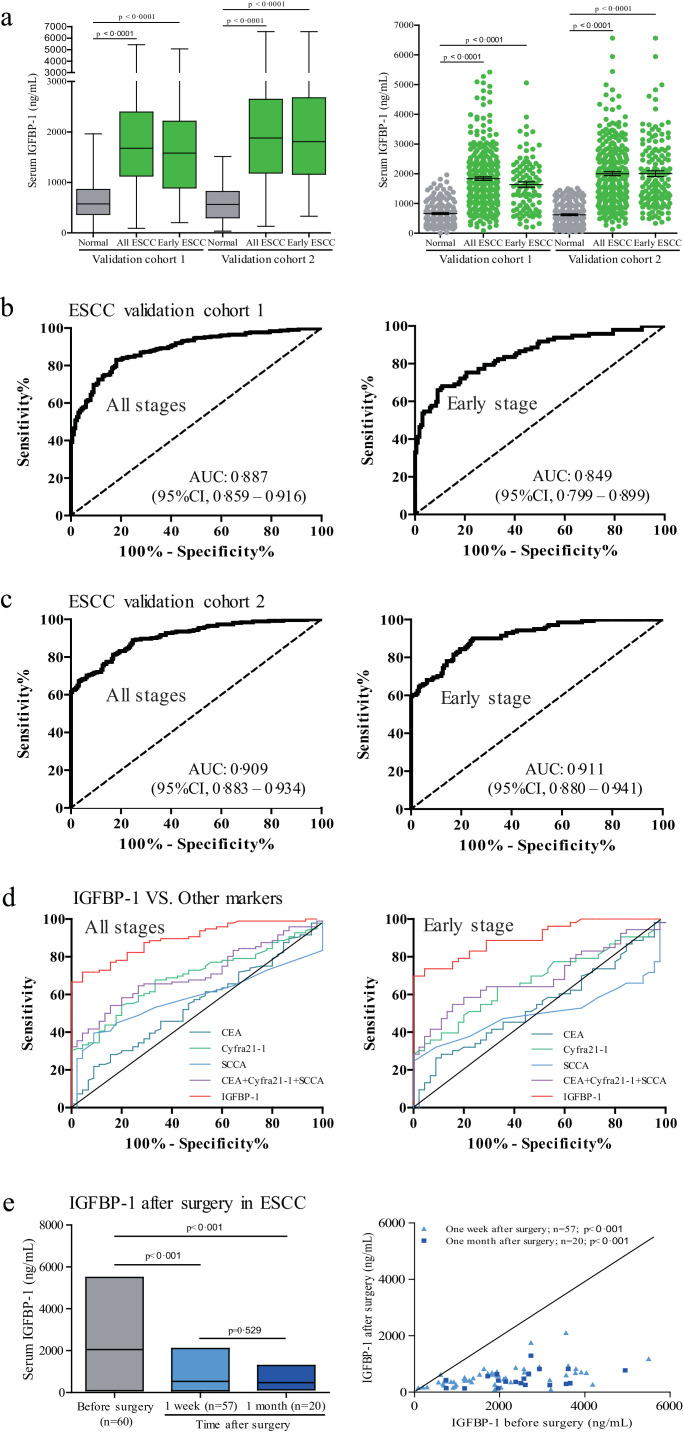
3.4. Biomarker diagnosis validation in ESCC
To confirm the diagnostic value of serum IGFBP-1, we carried out a validation study using ESCC, as representative of gastrointestinal tumours, in two independent cohorts (cohorts 1 and 2). We observed similar results in both cohorts 1 and 2. Serum IGFBP-1 levels were significantly higher in both ESCC groups and early-stage ESCC group than in the control group (Fig. 5, Mann–Whitney U test, p<0·0001). Using the same threshold (1228 ng/ml), serum IGFBP-1 showed good diagnostic accuracy for all ESCC patients, including early-stage and advanced-stage ESCC (Fig. 5, Supplementary Table S7). The correlation of serum IGFBP-1 with clinical variables in ESCC patients in both cohorts is shown in Supplementary Table S8.
Fig. 5.
Performance of serum IGFBP-1 as diagnostic biomarker in ESCC.
(a) Box plot illustrates median levels and interquartile ranges and the whiskers show minimum and maximum value of serum IGFBP-1 in validation cohorts. Scatter plots show serum IGFBP-1 from ESCC patients and normal controls. Black horizontal lines are means, and error bars are SEs. Mann–Whitney U test was conducted to assess differences of serum IGFBP-1 between patients and normal controls. (b) ROC curve for serum IGFBP-1 in validation cohort 1. (c) ROC curve for serum IGFBP-1 in validation cohort 2. (d) ROC curves for different biomarkers in discriminating ESCC patients and early-stage ESCC patients from normal controls. (e) Serum IGFBP-1 Level after surgical resection of ESCC. Floating bar of serum IGFBP-1 levels in the ESCC patients before and after surgery is shown on the left (black horizontal lines are means), and scatter plot of serum IGFBP-1 in paired serum samples from before and one week and one month after surgery from the same patients with ESCC is shown on the right. Wilcoxon signed-ranks test was used for comparison.
We next compared the performance of serum IGFBP-1 with the performance of CEA, Cyfra21-1, SCCA and the three-biomarker combined panel (i.e., CEA+ Cyfra21-1+ SCCA) in the ability to diagnose cancer in a group of ESCC patients (n=96) versus normal controls (n=45), which were selected from cohort 2 between Aug 2016, and Mar 2017. The three-biomarker panel was established using a logistical regression model with the predicted probability (p) for ESCC calculated by the equation: ln(p/(1–p)) = 0·048 × (CEA)+0·551 × (Cyfra21-1)+0·432 × (SCCA) -1·486. The AUC for serum IGFBP-1 in this setting was significantly greater than those for CEA, Cyfra21-1, SCCA or the three-biomarker panel (Fig. 5, Supplementary Table S9).
To monitor serum IGFBP-1 in response to therapy, 77 serum samples were obtained from 60 ESCC patients, of which 57 samples were taken at 1 week and 20 samples taken at 1 month after tumour resection. As shown in Fig. 5, the mean concentration of serum IGFBP-1 in preoperative ESCC samples was 2049·257±1224·524 ng/ml, and levels dropped after surgical resection of primary tumours to 529·923±364·292 ng/ml at one week (Wilcoxon signed-ranks test, p<0·001) and to 472·855±291·449 ng/ml at one month (Wilcoxon signed-ranks test, p<0·001) after surgery.
4. Discussion
In this study, we identify a blood-based biomarker test in a large number of participants (n=2028), including three types of upper gastrointestinal cancers and normal controls. Patients with upper gastrointestinal tumours showed significantly higher levels of serum IGFBP-1, compared to normal controls. Serum IGFBP-1 could identify early-stage upper gastrointestinal tumours with high diagnostic accuracy (AUC 0·897, sensitivity 71·67% and specificity 91·29%). Our findings highlight serum IGFBP-1 as a potential biomarker for non-invasive detection of early-stage upper gastrointestinal tumours.
It is well known that endoscopic examination could help identify early-stage upper gastrointestinal cancer, [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]] but the invasive nature of endoscopy limits its widespread use as a screening tool in the asymptomatic population. Moreover, by using endoscopy, it is difficult to identify precancerous lesions of upper gastrointestinal cancer, such as areas of mucosal thickening and early cancer. In recent decades, tremendous efforts have been made to identify protein biomarkers of clinical utility for early cancer detection [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]]. In addition, serum protein biomarkers are thought to be the most promisingly applicable test for population studies and routine clinical work [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]]. Recently, several serum-based protein biomarker tests (e.g. OVA2 composed of CA125, transferrin, APOA1, follicle-stimulating hormone and human epididymis protein 4) have been approved by the FDA and exhibited improved performance clinically in early detection of certain types of tumours [28,29]. In this study, the sensitivity of serum IGFBP-1, among gastrointestinal cancers evaluated, ranged from 77·22% in EJA to 70·38% in ESCC (Fig. 4 and Table 1). At this sensitivity, the specificity was 91·29%, which is sufficient for use as a screening test for upper gastrointestinal cancers. For screening purposes, one of the most important attributes of a biomarker should be the ability to identify cancers at relatively early stages. The sensitivity of serum IGFBP-1 to detect early-stage upper gastrointestinal cancers was similar to its ability to detect upper gastrointestinal cancers at all stages, indicating the performance of serum IGFBP-1 is independent of tumour stage (Table 1). Importantly, compared with CEA, Cyfra21-1, SCCA or the combined panel, serum IGFBP-1 shows higher sensitivity and AUC value to diagnose ESCC from early to late stages. Thus, we believe that such a serum protein test should be helpful for detecting upper gastrointestinal cancer cases in early stage, thereby resulting in earlier treatment and more effective therapy. On the other hand, thinking about the process of diagnosis, serum biomarker tests are a kind of screening, which requires high sensitivity to reduce false negative. The sensitivity of the IGFBP-1 test might limit the clinical application in screening purposes in general or high-risk individuals. In the future studies, we will seek biomarkers of high sensitivity and specificity for further optimisation with the test of serum IGFBP-1. Obviously, serum uPAR may be another promising candidate biomarker identified in both antibody arrays. Since a study reported by Usnarska–Zubkiewicz et al showed the serum level of uPAR was elevated in patients with gastrointestinal cancer [30], in the present study, we only selected serum IGFPB-1 for evaluation and validation. Further studies could be carried out to establish an optimised protein panel for early diagnosis by combining serum IGFBP-1 and other potential candidates (e.g., uPAR) identified in the antibody arrays.
IGFBP-1 is one of the key IGFBPs and belongs to the insulin-like growth factor (IGF) signalling pathway that plays a critical role in cell growth, differentiation, and apoptosis [31]. IGFBP-1 is closely related to gynecological physiology. On the other hand, by interacting with cell surface molecules, IGFBP-1 has effects on proliferation, migration and apoptosis of different cells in an IGF-independent manner [32]. Increasing results indicate the expression pattern of IGFBP-1 in cancer tissues remains equivocal and even controversial [[33], [34], [35]], but there is little evidence concerning IGFBP-1 expression in upper gastrointestinal cancers. Interestingly, a recent study on tumour-stroma interaction in glioblastoma indicated that the increase in IGFBP-1 secretion by microglial cells in response to glioma-secreted MCSF is an important mediator to promote tumour angiogenesis [36]. Whether this mechanism exists in upper gastrointestinal cancer needs to be further explored. But based on this evidence, it is reasonable to accept that the secreted IGFBP-1 is likely to play an important role in the tumourigenesis and progression, and that the elevated serum levels of IGFBP-1 could be detected in some types of tumours. As a secreted protein, the serum levels of IGFBP-1 in cancers have also been explored. Most studies have focused on the association between prediagnostic serum levels of IGFBP-1 and cancer risk, and the results in colorectal cancer seem to be contradictory [[37], [38], [39], [40], [41]]. Several reports demonstrated that serum IGFBP-1 levels were increased in some cancers, such as ovarian cancer, hepatocellular carcinoma, and NPC [[42], [43], [44]]. However, these studies were limited by the small sample size and lacked early diagnostic assessment. A biomarker study needs high throughput screening followed by large sample size evaluation, and independent cohort validation. So far, none of published studies on serum IGFBP-1 in cancer diagnosis followed this procedure. The present study was designed according to the above criteria, which began with high-throughput screening, recruited more than 2000 participants, and had independent cohort validation.
There are other strengths in the present study. First, we began screening candidate biomarkers by using two different sets of high-throughput antibody arrays, of which the methodology could increase opportunities to identify biomarkers appropriate for early diagnosis. Both sets of antibody arrays revealed elevated levels of serum IGFBP-1in ESCC patients, thus making the data more reliable and stable. Second, a biomarker with different cutoff values to distinguish different types of cancers from normal controls would raise concerns about its robustness and practicality for early diagnosis. In this study, serum IGFBP-1 with the same cutoff had the ability to differentiate patients with upper gastrointestinal cancers from normal individuals, and could have diagnostic value for robust detection of early-stage upper gastrointestinal cancers. These results indicate the availability of serum IGFBP-1 for potential clinical utility. Third, the biomarker study of early cancer diagnosis should be directed specifically at the group of patients with early-stage cancer. Our study possesses a large sample size of patients with early-stage gastrointestinal cancers (a total of 478 cases) for stable estimates of biomarker sensitivity and specificity. Furthermore, studies have showed serum levels of IGFBP-1 might be associated with cardiovascular disease or diabetes [[45], [46], [47], [48], [49], [50], [51]]. We excluded participants diagnosed with cardiovascular disease or diabetes in this study thus to maximally reduce the bias from other factors, which may influence the interpretation of our results.
Notably, the serum levels of IGFBP-1 are significantly decreased after tumour resection in ESCC patients, suggesting that this serum protein might have the potential for the post-operative surveillance of ESCC patients. To further explore whether serum IGFBP-1 could be a useful biomarker for therapy monitoring of upper gastrointestinal cancers, we need to enlarge the sample size and perform long-term follow-up of the upper gastrointestinal cancer patients with surgical treatment.
Few limitations of this study should be also acknowledged: all patients in our study were recruited from individuals with known cancers, and most were diagnosed on the basis of disease symptoms. It is conceivable that most persons in a true screening setting would be individuals with asymptomatic disease or precancerous lesions, and the detection sensitivity is likely to be less than reported here and needs to be validated further. Furthermore, the independent validation was limited to ESCC patients. Since the diagnostic value of serum IGFBP-1 was well validated in two large cohorts of participants from two different clinical centres, we believe that the diagnostic performance will be equally applicable to cases of EJA, or stomach cancer.
Our study assessing large sample sizes finds that IGFBP-1 as a serum protein biomarker has potential clinical value for the early diagnosis of upper gastrointestinal cancer. Such a noninvasive biomarker test is not meant to replace other non-blood-based screening tests like endoscopy, but to help diagnose patients who might harbour upper gastrointestinal cancer at an earlier stage. Further validation studies in cohorts of larger samples of early-stage patients from different institutions are needed to assess the diagnostic power of serum IGFBP-1 in upper gastrointestinal cancer.
Declaration of Competing Interest
We declare that we have no conflicts of interest.
Acknowledgments
This work was supported by funding from the Natural Science Foundation of China (31600632 and 81972801), the Natural Science Foundation of Guangdong Province (2018A030307079 and 2019A1515011873), the Population-based prospective cohort study of oesophageal cancer and precancerous lesions in high risk areas (2016YFC0901404), the National Cohort of Oesophageal Cancer of China (2016YFC0901400), the National Key Research and Development Program of China (2018YFC1313101); the Guangdong Oesophageal Cancer Institute Science and Technology Program (M201713); the Innovative and Strong School Project of Guangdong (2018KTSCX068); and Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2019.11.027.
Contributor Information
Zhi-Yong Wu, Email: stwuzy@163.com.
Yu-Hui Peng, Email: pengyuhui666@163.com.
Li-Yan Xu, Email: lyxu@stu.edu.cn.
En-Min Li, Email: nmli@stu.edu.cn.
Appendix. Supplementary materials
References
- 1.Herszenyi L., Tulassay Z. Epidemiology of gastrointestinal and liver tumours. Eur Rev Med Pharmacol Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Wang G.Q., Jiao G.G., Chang F.B., Fang W.H., Song J.X., Lu N. Long-term results of operation for 420 patients with early squamous cell oesophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77:1740–1744. doi: 10.1016/j.athoracsur.2003.10.098. [DOI] [PubMed] [Google Scholar]
- 5.Karimi P., Islami F., Anandasabapathy S., Freedman N.D., Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maida M., Macaluso F.S., Ianiro G., Mangiola F., Sinagra E., Hold G. Screening of colorectal cancer: present and future. Expert Rev Anticancer Ther. 2017;17:1131–1146. doi: 10.1080/14737140.2017.1392243. [DOI] [PubMed] [Google Scholar]
- 7.Etzioni R., Urban N., Ramsey S., McIntosh M., Schwartz S., Reid B. The case for early detection. Nat Rev Cancer. 2003;3:243–252. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 8.Smith R.A., Andrews K.S., Brooks D., Fedewa S.A., Manassaram-Baptiste D., Saslow D. Cancer screening in the United States, 2018: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68:297–316. doi: 10.3322/caac.21446. [DOI] [PubMed] [Google Scholar]
- 9.Wender R.C., Brawley O.W., Fedewa S.A., Gansler T. A blueprint for cancer screening and early detection: advancing screening's contribution to cancer control. CA Cancer J Clin. 2019;69:50–79. doi: 10.3322/caac.21550. [DOI] [PubMed] [Google Scholar]
- 10.Dekker E., Rex D.K. Advances in CRC prevention: screening and surveillance. Gastroenterology. 2018;154:1970–1984. doi: 10.1053/j.gastro.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 11.Wei W.Q., Chen Z.F., He Y.T., Feng H., Hou J., Lin D.M. Long-term follow-up of a community assignment, one-time endoscopic screening study of oesophageal cancer in China. J Clin Oncol. 2015;33:1951–1957. doi: 10.1200/JCO.2014.58.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinsky P.F., Prorok P.C., Kramer B.S. Prostate cancer screening – a perspective on the current state of the evidence. N Engl J Med. 2017;376:1285–1289. doi: 10.1056/NEJMsb1616281. [DOI] [PubMed] [Google Scholar]
- 13.Matulonis U.A., Sood A.K., Fallowfield L., Howitt B.E., Sehouli J., Karlan B.Y. Ovarian cancer. Nat Rev Dis Primers. 2016;2:16061. doi: 10.1038/nrdp.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanash S.M., Pitteri S.J., Faca V.M. Mining the plasma proteome for cancer biomarkers. Nature. 2008;452:571–579. doi: 10.1038/nature06916. [DOI] [PubMed] [Google Scholar]
- 15.Borrebaeck C.A. Precision diagnostics: moving towards protein biomarker signatures of clinical utility in cancer. Nat Rev Cancer. 2017;17:199–204. doi: 10.1038/nrc.2016.153. [DOI] [PubMed] [Google Scholar]
- 16.Belczacka I., Latosinska A., Metzger J., Marx D., Vlahou A., Mischak H. Proteomics biomarkers for solid tumours: current status and future prospects. Mass Spectrom Rev. 2019;38:49–78. doi: 10.1002/mas.21572. [DOI] [PubMed] [Google Scholar]
- 17.Mirus J.E., Zhang Y., Li C.I., Lokshin A.E., Prentice R.L., Hingorani S.R. Cross-species antibody microarray interrogation identifies a 3-protein panel of plasma biomarkers for early diagnosis of pancreas cancer. Clin Cancer Res. 2015;21:1764–1771. doi: 10.1158/1078-0432.CCR-13-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingren C., Sandstrom A., Segersvard R., Carlsson A., Andersson R., Lohr M. Identification of serum biomarker signatures associated with pancreatic cancer. Cancer Res. 2012;72:2481–2490. doi: 10.1158/0008-5472.CAN-11-2883. [DOI] [PubMed] [Google Scholar]
- 19.Tan H.Z., Lin W.J., Huang J.Q., Dai M., Fu J.H., Huang Q.H. Updated incidence rates and risk factors of oesophageal cancer in Nan'ao Island, a coastal high-risk area in southern China. Dis Esophagus. 2017;30:1–7. doi: 10.1111/dote.12468. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y.W., Peng Y.H., Chen B., Wu Z.Y., Wu J.Y., Shen J.H. Autoantibodies as potential biomarkers for the early detection of oesophageal squamous cell carcinoma. Am J Gastroenterol. 2014;109:36–45. doi: 10.1038/ajg.2013.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tusher V.G., Tibshirani R., Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle P., Chapman C.J., Holdenrieder S., Murray A., Robertson C., Wood W.C. Clinical validation of an autoantibody test for lung cancer. Ann Oncol. 2011;22:383–389. doi: 10.1093/annonc/mdq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Y.H., Xu Y.W., Huang L.S., Zhai T.T., Dai L.H., Qiu S.Q. Autoantibody signatures combined with epstein-barr virus capsid antigen-IgA as a biomarker panel for the detection of nasopharyngeal carcinoma. Cancer Prev Res (Phila) 2015;8:729–736. doi: 10.1158/1940-6207.CAPR-14-0397. [DOI] [PubMed] [Google Scholar]
- 24.Ajani J.A., Barthel J.S., Bentrem D.J., D'Amico T.A., Das P., Denlinger C.S. Oesophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 25.Frantzi M., Bhat A., Latosinska A. Clinical proteomic biomarkers: relevant issues on study design & technical considerations in biomarker development. Clin Transl Med. 2014;3:7. doi: 10.1186/2001-1326-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan D.J., O'Connor D.P., Rexhepaj E., Ponten F., Gallagher W.M. Antibody-based proteomics: fast-tracking molecular diagnostics in oncology. Nat Rev Cancer. 2010;10:605–617. doi: 10.1038/nrc2902. [DOI] [PubMed] [Google Scholar]
- 27.Locker G.Y., Hamilton S., Harris J., Jessup J.M., Kemeny N., Macdonald J.S. ASCO 2006 update of recommendations for the use of tumour markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 28.Coleman R.L., Herzog T.J., Chan D.W., Munroe D.G., Pappas T.C., Smith A. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215:82.e1–82.e11. doi: 10.1016/j.ajog.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Jett J.R., Peek L.J., Fredericks L., Jewell W., Pingleton W.W., Robertson J.F. Audit of the autoantibody test, EarlyCDT(R)-lung, in 1600 patients: an evaluation of its performance in routine clinical practice. Lung Cancer. 2014;83:51–55. doi: 10.1016/j.lungcan.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Usnarska-Zubkiewicz L., Strutyńska-Karpińska M., Zubkiewicz-Kucharska A., Zarębski P., Grabowski K. Soluble urokinase-type plasminogen activator receptor and ferritin concentration in patients with advanced alimentary tract carcinoma. Relationship to localization, surgical treatment and the stage of the disease–preliminary report. Adv Clin Exp Med. 2014;23:959–967. doi: 10.17219/acem/30817. [DOI] [PubMed] [Google Scholar]
- 31.Kashyap M.K. Role of insulin-like growth factor-binding proteins in the pathophysiology and tumourigenesis of gastrooesophageal cancers. Tumour Biol. 2015;36:8247–8257. doi: 10.1007/s13277-015-3972-3. [DOI] [PubMed] [Google Scholar]
- 32.Firth S.M., Baxter R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 33.Gong Y., Cui L., Minuk G.Y. The expression of insulin-like growth factor binding proteins in human hepatocellular carcinoma. Mol Cell Biochem. 2000;207:101–104. doi: 10.1023/a:1007010818094. [DOI] [PubMed] [Google Scholar]
- 34.Dai B., Ruan B., Wu J., Wang J., Shang R., Sun W. Insulin-like growth factor binding protein-1 inhibits cancer cell invasion and is associated with poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7:5645–5654. [PMC free article] [PubMed] [Google Scholar]
- 35.Pekonen F., Nyman T., Ilvesmaki V., Partanen S. Insulin-like growth factor binding proteins in human breast cancer tissue. Cancer Res. 1992;52:5204–5207. [PubMed] [Google Scholar]
- 36.Nijaguna M.B., Patil V., Urbach S., Shwetha S.D., Sravani K., Hegde A.S. Glioblastoma-derived macrophage colony-stimulating factor (MCSF) induces microglial release of insulin-like growth factor-binding protein 1 (IGFBP1) to promote angiogenesis. J Biol Chem. 2015;290:23401–23415. doi: 10.1074/jbc.M115.664037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei E.K., Ma J., Pollak M.N., Rifai N., Fuchs C.S., Hankinson S.E. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev. 2006;15:750–755. doi: 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 38.Le Marchand L., Wang H., Rinaldi S., Kaaks R., Vogt T.M., Yokochi L. Associations of plasma C-peptide and IGFBP-1 levels with risk of colorectal adenoma in a multiethnic population. Cancer Epidemiol Biomarkers Prev. 2010;19:1471–1477. doi: 10.1158/1055-9965.EPI-10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenab M., Riboli E., Cleveland R.J., Norat T., Rinaldi S., Nieters A. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European prospective investigation into cancer and nutrition. Int J Cancer. 2007;121:368–376. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 40.Lukanova A., Toniolo P., Akhmedkhanov A., Biessy C., Haley N.J., Shore R.E. A prospective study of insulin-like growth factor-I, IGF-binding proteins-1, -2 and -3 and lung cancer risk in women. Int J Cancer. 2001;92:888–892. doi: 10.1002/ijc.1265. [DOI] [PubMed] [Google Scholar]
- 41.Krajcik R.A., Borofsky N.D., Massardo S., Orentreich N. Insulin-like growth factor I (IGF-I), IGF-binding proteins, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1566–1573. [PubMed] [Google Scholar]
- 42.Gershtein E.S., Isaeva E.R., Kushlinsky D.N., Korotkova E.A., Ermilova V.D., Laktionov K.P. Insulin-like growth factors (IGF) and IGF-binding proteins (IGFBP) in the serum of patients with ovarian tumours. Bull Exp Biol Med. 2016;160:814–816. doi: 10.1007/s10517-016-3317-2. [DOI] [PubMed] [Google Scholar]
- 43.Hwang D.L., Huang S.P., Lan W.S., Lee P.D. Elevated insulin, proinsulin and insulin-like growth factor-binding protein-1 in liver disease. Growth Horm IGF Res. 2003;13:316–321. doi: 10.1016/s1096-6374(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 44.Feng X., Lin J., Xing S., Liu W., Zhang G. Higher IGFBP-1 to IGF-1 serum ratio predicts unfavourable survival in patients with nasopharyngeal carcinoma. BMC Cancer. 2017;17:90. doi: 10.1186/s12885-017-3068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gokulakrishnan K., Velmurugan K., Ganesan S., Mohan V. Circulating levels of insulin-like growth factor binding protein-1 in relation to insulin resistance, type 2 diabetes mellitus, and metabolic syndrome (Chennai Urban Rural Epidemiology Study 118) Metabolism. 2012;61:43–46. doi: 10.1016/j.metabol.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Hilding A., Brismar K., Degerblad M., Thorén M., Hall K. Altered relation between circulating levels of insulin-like growth factor-binding protein-1 and insulin in growth hormone-deficient patients and insulin-dependent diabetic patients compared to that in healthy subjects. J Clin Endocrinol Metab. 1995;80:2646–2652. doi: 10.1210/jcem.80.9.7545695. [DOI] [PubMed] [Google Scholar]
- 47.Arnetz L., Hage C., Ekberg N.R., Alvarsson M., Brismar K., Norhammar A. Improved glycemic control due to sitagliptin is not related to cortisol or the surrogate marker IGFBP-1 for hepatic insulin sensitivity. Growth Horm IGF Res. 2015;25:298–303. doi: 10.1016/j.ghir.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Hui L., Hui P., Feng-ying G. Advances in insulin like growth factor binding protein 1 in the prognosis of acute myocardial infarction. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011;33:22–24. doi: 10.3881/j.issn.1000-503X.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Yeap B.B., Chubb S.A., McCaul K.A., Flicker L., Ho K.K., Golledge J. Associations of IGF1 and its binding proteins with abdominal aortic aneurysm and aortic diameter in older men. Eur J Endocrinol. 2012;166:191–197. doi: 10.1530/EJE-11-0725. [DOI] [PubMed] [Google Scholar]
- 50.Borai A., Livingstone C., Ghayour-Mobarhan M., Abuosa A., Shafi S., Mehta S. Serum insulin-like growth factor binding protein-1 (IGFBP-1) phosphorylation status in subjects with and without ischaemic heart disease. Atherosclerosis. 2010;208:593–598. doi: 10.1016/j.atherosclerosis.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Saeki H., Hamada M., Hiwada K. Circulating levels of insulin-like growth factor-1 and its binding proteins in patients with hypertrophic cardiomyopathy. Circ J. 2002;66:639–644. doi: 10.1253/circj.66.639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.