Abstract
Proper function of the female pelvic floor requires intact pelvic floor muscles (PFMs). The prevalence of pelvic floor disorders (PFDs) increases substantially with age, in part due to clinically identified deterioration of PFM function with age. However, the etiology of this decline remains largely unknown. We previously demonstrated that PFMs undergo age-related fibrotic changes. This study sought to determine whether aging also impacts PFMs’ passive mechanical properties that are largely determined by the intramuscular extracellular matrix. Biopsies from younger (≤52y) and older (>52y) female cadaveric donors were procured from PFMs, specifically coccygeus (C) and two portions of the levator ani - iliococcygeus (IC) and pubovisceralis (PV), and the appendicular muscles - obturator internus (OI) and vastus lateralis (VL). Muscle bundles were subjected to a passive loading protocol, and stress-sarcomere length (Ls) relationships calculated. Muscle stiffness was compared between groups using 2-way ANOVA and Sidak pairwise comparisons, α < 0.05. The mean age was 43.4 ± 11.6y and 74.9 ± 11.9y in younger (N = 5) and older (N = 10) donors, respectively. In all PFMs, the quadratic coefficient of parabolic regression of the stress-Ls curve, a measure of stiffness, was lower in the younger versus older group: C: 33.7 ± 13.9 vs 87.2 ± 10.7, P = 0.02; IC: 38.3 ± 12.7 vs 84.5 ± 13.9, P = 0.04; PV: 24.7 ± 8.8 vs 74.6 ± 9.6, P = 0.04. In contrast, non-PFM stiffness was not affected by aging: OI: 14.5 ± 4.7 vs 32.9 ± 6.2, P = 0.8 and VL: 13.6 ± 5.7 vs 30.1 ± 5.3, P = 0.9. Age-associated increase in PFM stiffness is predicted to negatively impact PFM function by diminishing muscle load-bearing, excursional, contractile, and regenerative capacity, thus predisposing older women to PFDs.
Keywords: Passive mechanics, Aging, Pelvic floor muscles, Pelvic floor disorders, Stiffness
1. Introduction
Pelvic floor disorders (PFDs) include pelvic organ prolapse and urinary and fecal incontinence. Due to high prevalence and negative impact on quality of life, PFDs pose a large social and economic burden (DeLancey, 1993; Lawrence et al., 2008; Subak et al., 2001; Wilson et al., 2001). The specific etiology of these disorders is incompletely understood; risk factors that have been identified include vaginal parity, aging, and obesity. Interestingly, after menopause, age becomes the strongest risk factor for PFDs (Gregory and Nygaard, 2004; Nygaard et al., 2008; Olsen et al., 1997).
The female pelvic floor skeletal muscles (PFMs) span the pelvic outlet, supporting pelvic and abdominal viscera and contributing to urinary and fecal continence. The human PFMs are comprised of posteriorly located coccygeus (C) and a more anterior levator ani. Levator ani, in turn, is composed of iliococcygeus (IC) and pubovisceralis (PV), with the latter muscle subdivided into a more lateral pubococcygeus and a more medial puborectalis (Fig. 1). Due to their anatomic location deep in the pelvis, these muscles are inherently difficult to study directly in living women. Consequently, investigations of the human PFMs have predominantly relied on indirect assessments via clinical examination, imaging modalities, or computational modeling (Brink et al., 1989; Chiarelli, 1989; DeLancey et al., 2007; DeLancey et al., 2012; Sampselle et al., 1989; Theofrastous and Swift, 1998). Although these studies have been and continue to be critical for furthering our knowledge regarding the role of PFMs in the normal function of the female pelvic floor, many unanswered questions remain, emphasizing the need for direct assessment of PFMs. Fresh cadaveric tissue is a great source of human PFM tissue as PFMs are not readily accessible during pelvic surgeries and surgery is performed in the clinical setting of pathology or dysfunction.
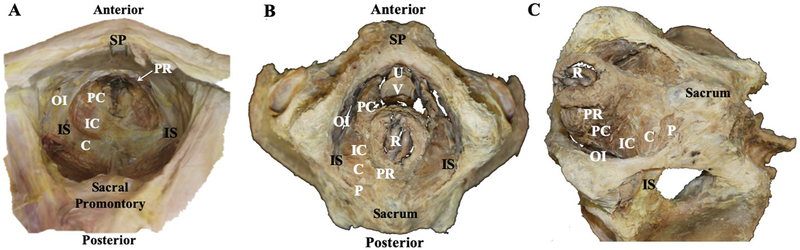
Fig. 1.
A. A superior view of eviscerated pelvis with pelvic floor muscles in situ. Bony landmarks include the symphysis pubis (SP), sacral promonatory and ischial spines (IS). Pelvic floor muscles include pubovisceralis, composed of puborectalis (PR) and pubococcygeus (PC); iliococcygeus (IC); and coccygeus (C). Obturator internus (OI) is labeled for reference. B. An inferior view of a pelvis with viscera and pelvic floor muscles, demonstrating the individual components of the pelvic floor muscles described in A. Bony landmarks include the symphysis pubis (SP), sacrum and ischial spine (IS). Openings for the urethra (U), vagina (V) and rectum/anus (R) are shown. Obturator internus (OI) and piriformis (P) are labeled for reference. C. An inferiolateral view of the pelvic floor muscles. Sacrum (S), ischial spines (IS), and Obturator internus (OI) and piriformis (P) muscles are labeled for reference.
Aging is well-known to negatively impact the functional capacity of the limb and trunk muscles. This is attributed to alterations in the contractile myofibers, responsible for muscle’s active mechanical properties, and non-contractile intramuscular extracellular matrix (ECM), which drives the passive mechanical properties of skeletal muscles. Accumulation of collagen and advanced glycation end products in ECM of the limb muscles has been implicated in the increased stiffness associated with decline in muscle force transmission that occurs with aging (Alnaqeeb et al., 1984; Prado et al., 2005; Rowe, 1981; Wood et al., 2014). We have previously demonstrated that aging, uncoupled from parity, is associated with a significant decrease in PFM physiological cross-sectional area that is predictive of muscle’s force generating capacity, similar to appendicular muscles. Furthermore, we have observed a concomitant dramatic increase in intramuscular collagen content (Alperin et al., 2016a; Cook et al., 2017). The goal of the current study was to determine whether aging impacts the passive mechanical properties of PFMs, which can have a marked effect on muscle force, power, and excursion (Roberts, 2016). We hypothesized that PFM stiffness increases with age.
2. Methods
2.1. Cadaveric donor selection
PFM biopsies were obtained from human cadaveric donors with documented medical, surgical, and obstetrical history, including number and mode of deliveries, through collaboration with the Bequest Body Donation Program at the University of Minnesota. The study was exempt from the Institutional Review Board approval, as it excluded living subjects. Donors were categorized as younger (≤52y) or older (>52y) based on the average age of menopause in the United States. Menopause is considered a marker of biological senescence in women and existing epidemiologic studies indicate that after menopause, aging is the leading risk factor for PFDs (Gregory and Nygaard, 2004; Nygaard et al., 2008; Olsen et al., 1997). Donors were also characterized in terms of vaginal parity and body mass index (BMI), other well-known risk factors for PFDs. Donors with history of gynecologic or colorectal malignancy, pelvic metastasis, pelvic radiation, connective tissue disorder, myopathy, surgery for pelvic organ or rectal prolapse, colectomy, or proctatectomy were excluded.
2.2. Tissue procurement
As feasibility of obtaining PFM tissue from living women is substantially constrained, we had previously tested the post-mortem stability of muscle’s passive mechanical properties up to 7 days following death and determined that these properties are unaltered by the post-mortem state within this time period (Tuttle et al., 2014). Therefore, biopsies were obtained from the mid-belly of each component of the PFM complex within 7 days post-mortem. Biopsies were also obtained from the pelvic portion of the obturator internus (OI) and from vastus lateralis (VL). Biopsies, labeled with study identification numbers assigned to each donor, were submerged into a glycerinated storage solution and stored at −20 °C until further testing as previously described (Lieber et al., 2003; Tuttle et al., 2014).
2.3. Determination of passive mechanical properties
To explore functional changes with aging, passive mechanical properties of younger and older PFMs and non-PFMs were tested by the investigators blinded to the group assignment of the individual specimens, labeled with study IDs. Determination of muscle stiffness was performed at the bundle level, previously demonstrated to reflect mechanical behavior driven by ECM (Meyer and Lieber, 2011). Small muscle bundles were micro-dissected from the biopsies and secured in a testing chamber between a force transducer (405 A; Aurora Scientific Aurora, ON, Canada) and a fixed pin connected to a rotational bearing (MT-RS; Newport Irvine, CA). Testing was performed in a relaxing solution in the presence of the protease inhibitor leupeptin, as has been previously described (Shah et al., 2004; Tuttle et al., 2014). Laser diffraction, coupled to the mechanical testing apparatus, was used for sarcomere length (Ls) measurements to provide objective quantification of muscle deformation. Baseline Ls and bundle diameter were measured at the minimum bundle length that produced measurable forces. The range of baseline stress values was 0.1–0.9 kPa. Fiber cross-sectional area was calculated based on bundle diameter, assuming cylindrical shape of fiber bundles. Force-strain data were generated for 3 bundles/muscle. Tissue was subjected to an established stress-relaxation protocol consisting of successive bouts of bundle elongation in ~10% strain increments at rate of 100 fiber lengths/second followed by a 3-min relaxation period either until failure or until Ls reached 4.0 μm (Brown et al., 2012; Shah et al., 2004; Thacker et al., 2012; Ward et al., 2009). Stress was calculated by dividing force by baseline cross-sectional area (i.e., engineering/nominal stress). Ls was measured at each strain. Stress was plotted as a function of Ls and the curve was fit using a quadratic regression with r2 value for each curve > 0.96. Bundle stiffness was indicated by the quadratic coefficient of parabolic regression for each curve that captures the non-linear nature of muscle stiffness over an expanded range of sarcomere lengths (Shah et al., 2004). This metric provides a mathematical description of the curve shape, enabling an objective and direct comparison of a variety of trajectories.
2.4. Statistical analysis
Statistical comparisons between muscles and conditions were performed by repeated measures 1 or 2-way analysis of variance (ANOVA), followed by pairwise comparisons with Sidak’s multiple comparisons test, as appropriate. Demographic variables were compared by either 1-way ANOVA, followed by pairwise comparisons with Sidak’s multiple comparisons test, or Student t-test, depending on the number of groups considered in the specific analysis. Power calculation (G*Power) (Buchner et al., 1997) yielded N1 = 5/younger group and N2 = 9/older group (α of 0.05; β of 0.8, N2/N1 = 2), based on Cohen’s d effect size of 1.8 calculated for pubovisceralis muscle, presumed to be the most important component of the PFM complex for the proper female pelvic floor function. Results are presented as mean ± standard error of the mean (SEM), unless indicated otherwise. Planned analyses of muscle stiffness included 1) comparisons between muscles harvested within age groups with subdivision based on parity and 2) between age groups. All statistical analyses were performed with GraphPad Prism version 8.00 (GraphPad Software, San Diego, CA).
3. Results
3.1. Demographics and anthropometries
The mean age of younger donors (N = 5, 43.4 ± 11.6 (SD) years) differed significantly from the mean age of older donors (N = 10, 74.9 ± 11.9 (SD) years), P < 0.001, allowing a meaningful evaluation of the age impact. Donors were predominantly Caucasian (17/18) with one African American donor. All younger donors were vaginally nulliparous. Two out of five younger donors had a history of Cesarean deliveries, with the overall median parity of 0 (range 0–2) in this group. Cesarean delivery, in contrast to vaginal delivery, is not associated with PFM muscle injury (Albrich et al., 2012; Shek and Dietz, 2010) or decrease in PFM strength later in life (Hilde et al., 2013). As such, all young donors were grouped together as none had vaginal deliveries. Among older donors, 3 were nulliparous and 7 had a history of vaginal deliveries with an overall median parity of 2 (range 0–7). Parity did not differ between younger donors and all older donors combined (P = 0.2) but differed significantly between younger and older parous donors (P = 0.04) (Table 1). The mean age of older nulliparous (n = 3, 79.3 ± 8.1 years) and older parous (n = 7, 73.0 ± 13.3 years) donors did not differ (P = 0.7) from each other (Table 1). The groups were similar with respect to body mass index (BMI). Mean BMI of younger donors (20.6 ± 1.7 (SD) kg/m2) was not significantly different from that of older nulliparous (27.2 ± 3.9 kg/m2, P = 0.1) or older parous (21.3 ± 4.5 kg/m2, P = 0.9) donors. BMI of older nulliparous and older parous donors was also not significantly different (P = 0.1), (Table 1).
Table 1.
Demographic variables of cadaveric donors.
| Group | Age (years) mean ± SD |
BMI (kg/m2) mean ± SD |
Parity median (range) |
|---|---|---|---|
| Younger (n = 5) | 43.4 ± 11.6 | 20.6 ± 1.7 | 0 (0–2) |
| Older (n = 10) | 74.9 ± 11.9 | 23.1 ± 5.0 | 2 (0–7) |
| Older Nulliparous (n = 3) | 79.3 ± 8.1 | 27.2 ± 3.9 | NA |
| Older Parous(n = 7) | 73.0 ± 13.3 | 21.3 ± 4.5 | 3 (1–7) |
| P value* | |||
| Younger vs Older | <0.001 | 0.3 | 0.2 |
| Younger vs Older Nulliparous | 0.004 | 0.1 | 0.8 |
| Younger vs Older Parous | 0.003 | 0.9 | 0.04 |
| Older Parous vs Older Nulliparous | 0.7 | 0.1 | NA |
P values were derived from one-way analysis of variance followed by pairwise comparisons with Sidak’s multiple comparisons test or Student t-test, with the significance level set to 5%. Abbreviations: Body mass index (BMI), Standard Deviation (SD).
3.2. Passive mechanical properties
To compare muscle bundle stiffness, stress-Ls curves were fit using parabolic regression and the quadratic coefficients were compared. Baseline Ls was not significantly different (P > 0.05) between younger and older specimens in any of the muscles examined (Table 2).
Table 2.
Starting sarcomere lengths (μm) of the individual components of the pelvic floor muscle complex and the limb muscles, obturator internus and vastus lateralis, determined by laser diffraction.
| Groups | Coccygeus | Iliococcygeus | Pubovisceralis | Obturator Internus | Vastus Lateralis |
|---|---|---|---|---|---|
| Younger (n = 5) | 2.81 ± 0.18 | 2.79 ± 0.08 | 2.74 ± 0.13 | 2.70 ± 0.11 | 2.71 ± 0.1 |
| Older (n = 10) | 2.57 ± 0.05 | 2.49 ± 0.06 | 2.52 ± 0.05 | 2.58 ± 0.04 | 2.52 ± 0.05 |
| P value* | 0.18 | 0.06 | 0.27 | 0.87 | 0.57 |
P values were derived from two-way analysis of variance, followed by pairwise comparisons with Sidak’s multiple comparisons test, with the significance level set to 5%. All data presented in mean ± standard error of the mean.
Since the pubovisceralis (PV) muscle is comprised of two distinct portions, pubococcygeus and puborectalis, we initially examined stiffness parameters between the two, but there were no statistically significant differences within either the younger or the older group, P > 0.2 (Table 3). Thus, subsequent analyses were performed with PV muscle as a whole. We also examined whether muscle stiffness differed between older nulliparous and older parous donors. We did not identify difference in muscle stiffness between these groups for any of the muscles examined, therefore, the data were combined for subsequent analyses (Table 3).
Table 3.
Stiffness of pelvic floor muscles, obturator internus, and vastus lateralis in younger and older groups.
| Muscle | Younger | Olrder | Older Nulliparous | Older Parous | |
|---|---|---|---|---|---|
| Pelvic Floor Muscles | |||||
| Coccygeus | 33.7 ± 13.9 | 87.2 ±10.7 | 94.1 ± 34.2 | 83.8 ± 6.1 | |
| Iliococcygeus | 38.3 ± 12.7 | 84.5 ± 13.9 | 76.4 ± 23.5 | 88.1 ± 18.1 | |
| Pubovisceralis | 24.7 ± 8.8 | 74.6 ± 9.6 | 79.5 ± 11.1 | 72.53 ± 13.39 | |
| Pubococcygeus | 31.2 ± 17.5 | 62.4 ± 8.6 | 64.8 ± 4.5 | 60.0 ± 12.7 | |
| Puborectalis | 22.4 ± 9.5 | 90.9 ± 22.2 | 94.2 ± 26.6 | 87.5 ± 17.9 | |
| Non-Pelvic Muscles | |||||
| Obturator Internus | 14.5 ± 4.7 | 32.9 ± 6.2 | 35.4 ± 0.6 | 31.7 ± 9.7 | |
| Vastus Lateralis | 13.6 ± 5.7 | 30.1 ± 5.3 | 44.4 ± 8.8 | 22.9 ± 2.5 | |
| P value* | |||||
| Coccygeus | Iliococcygeus | Pubovisceralis | Obturator Internus | Vastus Lateralis | |
| Younger vs Older | 0.02 | 0.04 | 0.04 | 0.82 | 0.91 |
| Older Nulliparous vs Older Parous | 0.99 | 0.99 | 0.99 | 0.99 | 0.96 |
P values were derived from two-way analysis of variance followed by Sidak’s multiple comparisons test, with the significance levels set to 5%.
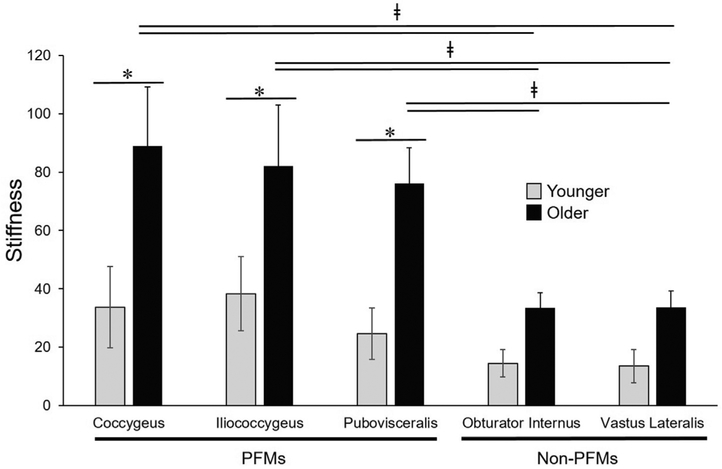
First, we focused on within muscle group comparisons for each age group. In the younger group, muscle stiffness did not differ either between the individual components of the PFM complex, P > 0.5, or between OI and VL, P > 0.9 . PFM stiffness was also similar to non-pelvic OI and VL, P > 0.2. Similarly, neither the stiffness of the individual PFMs, nor the stiffness of OI vs VL differed from each other in the older group, (P > 0.5). However, in contrast to the younger specimens, aged PFMs were significantly stiffer than both non-pelvic muscles procured from the same donors, P < 0.05 (Table 3).
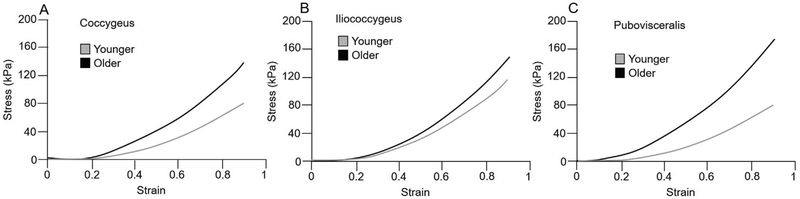
Comparisons between younger and older groups revealed a dramatic increase in muscle stiffness of all aged PFMs relative to younger specimens: C: 33.7 ± 13.9 vs 87.2 ± 10.7, P = 0.02; IC: 38.3 ± 12.7 vs 84.5 ± 13.9, P = 0.04; PV: 24.7 ± 8.8 vs 74.6 ± 9.6, P = 0.04 (Table 3, Figs. 2, 3). We then compared stiffness of non-PFMs of younger vs older donors. The stiffness of non-PFMs increased with age, however, the magnitude of this increase was substantially lower than that observed in PFMs and did not reach statistical significance: OI: 14.5 ± 4.7 vs 32.9 ± 6.2, P = 0.8 and VL: 13.6 ± 5.7 vs 30.1 ± 5.3, P = 0.9 (Table 3, Fig. 2, Fig. 3).
Fig. 2.
Stress (KPa) strain curves in younger (gray curves) and older (black curves) specimens. All pelvic floor muscles, including coccygeus (A), iliococcygeus (B) and pubovisceralis (C), were significantly stiffer in older compared to younger donors.
Fig. 3.
Graphical comparison of the pelvic floor and non-pelvic muscles’ stiffness. The quadratic coefficient of regression derived from stress-sarcomere length curves of younger (gray bars) and older (black bars) specimens. Pelvic floor muscles (PFMs) were significantly stiffer in older compared to younger donors (*) and stiffer than appendicular muscles within older group (ǂ). In contrast, stiffness of non-pelvic appendicular muscles was not significantly impacted by aging. Footnote Fig. 3: * ǂP values (*between groups and ǂwithin group) were derived from two-way and one-way analysis of variance, respectively, followed by Sidak’s multiple comparisons test, with the significance levels set to 5%.
4. Discussion
Based on the studies conducted in the limb muscles, aging is inextricably linked to the decline in skeletal muscle function, with many factors thought to contribute to this process (Boirie, 2009). In the limb, fibrotic changes that occur with aging are associated with increased muscle, tendon, and aponeurosis stiffness using both experimental and computational approaches (Danos et al., 2016; Holt et al., 2016; Jarvinen et al., 2002; Lieber et al., 2003; Lieber and Ward, 2013). Although PFMs are skeletal muscles similar in many ways to those of the limb, a growing body of work suggests that PFMs are uniquely adapted to respond to hormonal and physiological cues (Alperin et al., 2016b; Alperin et al., 2015; Burnett et al., 2019; Catanzarite et al., 2018). The above suggests that these muscles may be preferentially at risk for functional alterations associated with hormonal deprivation and aging.
Existing clinical studies highlight the correlation of aging with PFM weakness and diminished response to rehabilitation (Lewicky-Gaupp et al., 2010; Sherburn et al., 2011), however the mechanisms responsible for these alterations are largely unknown. In our previous investigations conducted in muscles fixed in situ, we elucidated age-mediated changes, uncoupled from parity, in the PFM architecture and collagen content (Alperin et al., 2016a). Physiologic cross-sectional area, a predictor of muscle’s active force generating capacity, decreased drastically in aged PFMs compared to the younger specimens. With respect to ECM, collagen content significantly increased (from 44% in C to 72% in PV) in aged, relative to younger, PFMs (Alperin et al., 2016a).
Here we expand on the existing studies to demonstrate the functional impact of aging on PFM passive mechanical properties in fresh post-mortem specimens. The key findings of this study are: (1) PFM stiffness increases dramatically with age and (2) significant age-related increase in stiffness is not observed in the appendicular muscles of the same donors. Studies of appendicular muscle in males demonstrate increased muscle stiffness and functional impairment associated with aging (Palmer and Thompson, 2017; Rosant et al., 2007). Limited studies exist examining the effect of aging on muscle stiffness in females. In contrast to the published literature on aging effects in male appendicular muscles, a clinical study using myotonometry did not find an increase in muscle stiffness with aging in women (Ikezoe et al., 2012). Consistent with these clinical findings, we find no difference in the age-related limb muscle stiffness in our female donors. Divergent from our results in the obturator internus and vastus lateralis, we find increased stiffness with aging selectively in PFMs. These findings highlight potentially sex-mediated differences in the effect of aging on skeletal muscles stiffness. Additional studies are needed to understand the impact of sex on changes in passive biomechanical properties of various muscle groups with increasing age.
In addition to identifying dramatically higher collagen content of aged PFMs (Alperin et al., 2016a), we have previously found a significant increase in collagen content of aged obturator internus muscles compared to younger specimens (Cook et al., 2017). However, the absolute collagen content of OI is several fold lower than that of PFMs. Taken together, our previous and current findings suggest that despite significant age-related increase in collagen content of the limb muscles, in contrast to PFMs, their overall low ECM content contributes minimally to the passive mechanical properties of these muscles. Previous studies have demonstrated age-related increase in muscle stiffness associated with variables other than collagen content, including alterations in the arrangement and size of collagen fibrils and altered muscle stem cell fate favoring fibrogenesis (Gao et al., 2008; Stearns-Reider et al., 2017). Many drivers of intramuscular ECM alterations in limb have been identified including collagen synthesis, regulation of matrix metalloproteinase enzymes and mechanical loading (Kjaer, 2004). Future studies are needed to determine whether mechanisms underlying intramuscular ECM remodeling and muscle stiffness differ by sex or muscle group.
In the limb muscles, increased stiffness associated with aging has been correlated with decrease in muscle cross-sectional area, peak torque and rate of torque development. (Palmer and Thompson, 2017) The increased PFM stiffness is similarly predicted to negatively impact muscle function by diminishing load-bearing capacity, excursion, and active contractile properties, in turn, potentially predisposing older women to PFDs. These findings parallel the existing epidemiologic literature that identifies aging as the strongest risk factor for PFD development after menopause independent of parity (Kepenekci et al., 2011; Quiroz et al., 2013; Quiroz et al., 2012). The current study serves as a first step in characterizing passive muscle mechanics in our system, however, only equilibrium passive properties have been considered. In future studies, it will be important to evaluate viscoelastic properties such as hysteresis, stress relaxation, and creep, as these properties dictate how much active work would be needed to return a muscle to a resting state, as well as to understand the effects of strain rate on loads borne by passively loaded structures. The effect of aging on these parameters is not well characterized in skeletal muscles of both the limb and pelvic floor.
Our study was constrained by donor availability and as such, no vaginally parous younger donors were included in this analysis. However, vaginally nulliparous younger donors, who are the least likely to have PFM compromise based on clinical epidemiologic studies, were included and compared to older specimens. In the older group, no differences were observed between nulliparous vs parous donors. Despite the limitation of the small sample size in the older nulliparous group, these results are consistent with our previous study, where we had demonstrated that age-related changes in PFM architecture and collagen content are independent of parity, indicating that aging is an independent risk factor for the pathological changes in PFMs (Alperin et al., 2016a). Our study also excluded donors with history of PFDs to avoid the potential confounding effect of PFDs, especially pelvic organ prolapse, on muscle properties; however, these findings may be different in women with symptomatic PFDs dependent on type and severity of PFD.
In conclusion, our work provides the foundational knowledge that has a high potential to change the practice of women’s health care, as it serves as an important impetus for the development of preventative strategies to mitigate the untoward age-related changes in these integral components of the female pelvic floor, potentially reducing the incidence of PFDs. The mechanism underlying the relatively increased susceptibility of PFMs to age-related increase in muscle stiffness is currently unknown. Further studies are needed to examine the potential role of hormonal signaling and regenerative potential in this unique susceptibility of PFMs to aging effects.
Acknowledgements
The authors thank the individuals who donated their bodies to the University of Minnesota’s Anatomy Bequest Program for the advancement of education and research.
Funding
This research was supported by the National Institute of Health/National Institute of Aging grant (R03AG050951) and National Institute of Health/National Institute of Child Health and Human Development (K12HD001259) awarded to Marianna Alperin, MD MS. Sponsors were not involved in the study design, collection, analysis, interpretation of the data, in writing the manuscript, or the decision to submit the manuscript for publication.
Footnotes
All authors have read and concur with the content of the manuscript.
Declaration of Competing Interest
All the authors declare no conflict of interest related to the manuscript.
References
- Albrich SB, Laterza RM, Skala C, Salvatore S, Koelbl H, Naumann G, 2012. Impact of mode of delivery on levator morphology: a prospective observational study with three-dimensional ultrasound early in the postpartum period. BJOG 119, 51–60. [DOI] [PubMed] [Google Scholar]
- Alnaqeeb MA, Al Zaid NS, Goldspink G, 1984. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J. Anat 139 (Pt 4), 677–689. [PMC free article] [PubMed] [Google Scholar]
- Alperin M, Cook M, Tuttle LJ, Esparza MC, Lieber RL, 2016a. Impact of vaginal parity and aging on the architectural design of pelvic floor muscles. Am. J. Obstet. Gynecol 215 (312), e311–e319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperin M, Kaddis T, Pichika R, Esparza MC, Lieber RL, 2016b. Pregnancy-induced adaptations in intramuscular extracellular matrix of rat pelvic floor muscles. Am. J. Obstet. Gynecol 215 (210), e211–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperin M, Lawley DM, Esparza MC, Lieber RL, 2015. Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. Am. J. Obstet. Gynecol 213 (191), e191–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie Y, 2009. Physiopathological mechanism of sarcopenia. J. Nutr. Health Aging 13, 717–723. [DOI] [PubMed] [Google Scholar]
- Brink CA, Sampselle CM, Wells TJ, Diokno AC, Gillis GL, 1989. A digital test for pelvic muscle strength in older women with urinary incontinence. Nurs. Res 38, 196–199. [PubMed] [Google Scholar]
- Brown SH, Carr JA, Ward SR, Lieber RL, 2012. Passive mechanical properties of rat abdominal wall muscles suggest an important role of the extracellular connective tissue matrix. J. Orthop. Res 30, 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner A, Erdfleder E, Faul F, 1997. How to Use G*Power. [Google Scholar]
- Burnett LA, Boscolo FS, Laurent LC, Wong M, Alperin M, 2019. Uncovering changes in proteomic signature of rat pelvic floor muscles in pregnancy. Am. J. Obstet. Gynecol 221, 130. e131–130 e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzarite T, Bremner S, Barlow CL, Bou-Malham L, O’Connor S, Alperin M, 2018. Pelvic muscles’ mechanical response to strains in the absence and presence of pregnancy-induced adaptations in a rat model. Am. J. Obstet. Gynecol 218, 512. e511–512 e519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli PE, 1989. Incontinence. The pelvic floor function. Aust. Fam. Phys 18, 949 953–944 956–947. [PubMed] [Google Scholar]
- Cook MS, Bou-Malham L, Esparza MC, Alperin M, 2017. Age-related alterations in female obturator internus muscle. Int. Urogynecol. J 28, 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos N, Holt NC, Sawicki GS, Azizi E, 2016. Modeling age-related changes in muscle-tendon dynamics during cyclical contractions in the rat gastrocnemius. J. Appl. Physiol 1985 (121), 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLancey JO, 1993. Anatomy and biomechanics of genital prolapse. Clin. Obstet. Gynecol 36, 897–909. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA, 2007. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet. Gynecol 109, 295–302. [DOI] [PubMed] [Google Scholar]
- DeLancey JO, Sorensen HC, Lewicky-Gaupp C, Smith TM, 2012. Comparison of the puborectal muscle on MRI in women with POP and levator ani defects with those with normal support and no defect. Int. Urogynecol. J 23, 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Kostrominova TY, Faulkner JA, Wineman AS, 2008. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J. Biomech 41, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory WT, Nygaard I, 2004. Childbirth and pelvic floor disorders. Clin. Obstet. Gynecol 47, 394–403. [DOI] [PubMed] [Google Scholar]
- Hilde G, Staer-Jensen J, Siafarikas F, Engh ME, Braekken IH, Bo K, 2013. Impact of childbirth and mode of delivery on vaginal resting pressure and on pelvic floor muscle strength and endurance. Am. J. Obstet. Gynecol 208 (50), e51–e57. [DOI] [PubMed] [Google Scholar]
- Holt NC, Danos N, Roberts TJ, Azizi E, 2016. Stuck in gear: age-related loss of variable gearing in skeletal muscle. J. Exp. Biol 219, 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezoe T, Asakawa Y, Fukumoto Y, Tsukagoshi R, Ichihashi N, 2012. Associations of muscle stiffness and thickness with muscle strength and muscle power in elderly women. Geriatr. Gerontol. Int 12, 86–92. [DOI] [PubMed] [Google Scholar]
- Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Jarvinen M, 2002. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J. Muscle Res. Cell Motil 23, 245–254. [DOI] [PubMed] [Google Scholar]
- Kepenekci I, Keskinkilic B, Akinsu F, Cakir P, Elhan AH, Erkek AB, Kuzu MA, 2011. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis. Colon Rectum 54, 85–94. [DOI] [PubMed] [Google Scholar]
- Kjaer M, 2004. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev 84, 649–698. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Lukacz ES, Nager CW, Hsu JW, Luber KM, 2008. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet. Gynecol 111, 678–685. [DOI] [PubMed] [Google Scholar]
- Lewicky-Gaupp C, Brincat C, Yousuf A, Patel DA, Delancey JO, Fenner DE, 2010. Fecal incontinence in older women: are levator ani defects a factor?. Am. J. Obstet. Gynecol 202 (491), e491–e496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Runesson E, Einarsson F, Friden J, 2003. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve 28, 464–471. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Ward SR, 2013. Cellular mechanisms of tissue fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. Am. J. Physiol. Cell Physiol 305, C241–C252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer GA, Lieber RL, 2011. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. J. Biomech 44, 771–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ, Pelvic Floor Disorders N, 2008. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300, 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL, 1997. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet. Gynecol 89, 501–506. [DOI] [PubMed] [Google Scholar]
- Palmer TB, Thompson BJ, 2017. Influence of age on passive stiffness and size, quality, and strength characteristics. Muscle Nerve 55, 305–315. [DOI] [PubMed] [Google Scholar]
- Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA, 2005. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J. Gen. Physiol 126, 461–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz LH, Shobeiri SA, White D, Wild RA, 2013. Does age affect visualization of the levator ani in nulliparous women?. Int. Urogynecol. J 24, 1507–1513. [DOI] [PubMed] [Google Scholar]
- Quiroz LH, White DE, Juarez D, Shobeiri SA, 2012. Age effects on pelvic floor symptoms in a cohort of nulliparous patients. Female Pelvic Med. Reconstr. Surg 18, 325–328. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, 2016. Contribution of elastic tissues to the mechanics and energetics of muscle function during movement. J. Exp. Biol 219, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosant C, Nagel MD, Perot C, 2007. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp. Gerontol 42, 301–308. [DOI] [PubMed] [Google Scholar]
- Rowe RW, 1981. Morphology of perimysial and endomysial connective tissue in skeletal muscle. Tissue Cell 13, 681–690. [DOI] [PubMed] [Google Scholar]
- Sampselle CM, Brink CA, Wells TJ, 1989. Digital measurement of pelvic muscle strength in childbearing women. Nurs. Res 38, 134–138. [PubMed] [Google Scholar]
- Shah SB, Davis J, Weisleder N, Kostavassili I, McCulloch AD, Ralston E, Capetanaki Y, Lieber RL, 2004. Structural and functional roles of desmin in mouse skeletal muscle during passive deformation. Biophys. J 86, 2993–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shek KL, Dietz HP, 2010. Intrapartum risk factors for levator trauma. BJOG 117, 1485–1492. [DOI] [PubMed] [Google Scholar]
- Sherburn M, Bird M, Carey M, Bo K, Galea MP, 2011. Incontinence improves in older women after intensive pelvic floor muscle training: an assessor-blinded randomized controlled trial. Neurourol. Urodyn 30, 317–324. [DOI] [PubMed] [Google Scholar]
- Stearns-Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C, Barchowsky A, Rando TA, Tuan RS, Ambrosio F, 2014. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 16, 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS, 2001. Cost of pelvic organ prolapse surgery in the United States. Obstet. Gynecol 98, 646–651. [DOI] [PubMed] [Google Scholar]
- Thacker BE, Tomiya A, Hulst JB, Suzuki KP, Bremner SN, Gastwirt RF, Greaser ML, Lieber RL, Ward SR, 2012. Passive mechanical properties and related proteins change with botulinum neurotoxin A injection of normal skeletal muscle. J. Orthop. Res 30, 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofrastous JP, Swift SE, 1998. The clinical evaluation of pelvic floor dysfunction. Obstet. Gynecol. Clin. North Am 25, 783–804. [DOI] [PubMed] [Google Scholar]
- Tuttle LJ, Alperin M, Lieber RL, 2014. Post-mortem timing of skeletal muscle biochemical and mechanical degradation. J. Biomech 47, 1506–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SR, Tomiya A, Regev GJ, Thacker BE, Benzl RC, Kim CW, Lieber RL, 2009. Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. J. Biomech 42, 1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Brown JS, Shin GP, Luc KO, Subak LL, 2001. Annual direct cost of urinary incontinence. Obstet. Gynecol 98, 398–406. [DOI] [PubMed] [Google Scholar]
- Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV, 2014. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol 1985 (117), 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]