Abstract
Background:
A community-wide outbreak of Legionnaires’ disease (LD) occurred in Genesee County, Michigan, in 2014 and 2015. Previous reports about the outbreak are conflicting and have associated the outbreak with a change of water source in the city of Flint and, alternatively, to a Flint hospital.
Objective:
The objective of this investigation was to independently identify relevant sources of Legionella pneumophila that likely resulted in the outbreak.
Methods:
An independent, retrospective investigation of the outbreak was conducted, making use of public health, health care, and environmental data and whole-genome multilocus sequence typing (wgMLST) of clinical and environmental isolates.
Results:
Strong evidence was found for a hospital-associated outbreak in both 2014 and 2015: a) 49% of cases had prior exposure to Flint hospital A, significantly higher than expected from Medicare admissions; b) hospital plumbing contained high levels of L. pneumophila; c) Legionella control measures in hospital plumbing aligned with subsidence of hospital A-associated cases; and d) wgMLST showed Legionella isolates from cases exposed to hospital A and from hospital plumbing to be highly similar. Multivariate analysis showed an increased risk of LD in 2014 for people residing in a home that received Flint water or was located in proximity to several Flint cooling towers.
Discussion:
This is the first LD outbreak in the United States with evidence for three sources (in 2014): a) exposure to hospital A, b) receiving Flint water at home, and c) residential proximity to cooling towers; however, for 2015, evidence points to hospital A only. Each source could be associated with only a proportion of cases. A focus on a single source may have delayed recognition and remediation of other significant sources of L. pneumophila. https://doi.org/10.1289/EHP5663
Introduction
Legionnaires’ disease (LD) is a severe pneumonia caused by Legionella pneumophila, a bacterium that grows in warm water systems and is transmitted via aerosols. Over the past 15 y, the incidence in the United States increased 4.5-fold to 1.89 per 100,000 people in 2015 (Shah et al. 2018). The associated burden of disease is substantial: of the cases are hospitalized, and the case fatality rate is . Although most LD cases are sporadic (CDC 2011), our understanding of the sources of LD comes almost entirely from outbreaks. L. pneumophila is now responsible for the majority () of waterborne disease outbreaks in the United States and for all of the outbreak-associated deaths (Benedict et al. 2017; McClung et al. 2017). Most outbreaks of LD have been associated with exposure to aerosols from warm water systems in large buildings that house vulnerable populations, such as hospitals and long-term care facilities (Garrison et al. 2016). Cooling towers and other aerosol sources also have frequently been associated with large LD outbreaks (van Heijnsbergen et al. 2015; Petzold et al. 2017; Caicedo et al. 2019).
In 2014 and 2015, a marked increase of reported LD cases was observed in Genesee County, Michigan, United States. Contemporaneously, a crisis with the potable water supply of the city of Flint, Michigan, occurred (Ruckart et al. 2019). In April 2014, the source of drinking water for Flint was switched from water provided by the Detroit Water and Sewerage Department to Flint River water, which was treated and distributed without corrosion inhibitor (Masten et al. 2016; Pieper et al. 2017). Shortly after the switch, residents noted changes in the color, odor, and taste of their tap water, as well as skin rashes. Following reports of high lead levels in Flint water (Edwards 2015) and in the blood of young children residing in Flint (Hanna-Attisha et al. 2016), a public health emergency was declared. Public health agencies investigated lead exposure as well as the LD cases. The Michigan Department of Health and Human Services (MDHHS) reported an outbreak of LD in Genesee County in 2014 and 2015 and associated the majority of cases with one hospital in Genesee County (MDHHS 2018). Tap water surveys conducted by academic researchers showed L. pneumophila DNA markers were detectable in Flint hospital plumbing in October 2015 but not in Flint homes and small buildings in August 2015 (Schwake et al. 2016; Rhoads et al. 2017). However, a modeling study (Zahran et al. 2018) suggested the outbreak was caused by system-wide proliferation of L. pneumophila in the potable water supply of the City of Flint, enabled by low chlorine levels in the distribution network after the switch to Flint River water as the source. Given the lack of clarity about the cause(s) of the 2014–2015 LD outbreak, this study was initiated to investigate the LD outbreak in Genesee County in 2014 and 2015. The objective of this investigation was to independently identify relevant sources of L. pneumophila that likely resulted in the outbreak. Based on the descriptive epidemiology and published literature, we focused on three potential sources: a) a hospital, b) residences receiving City of Flint water, and c) cooling towers or other outdoor aerosol sources.
Methods
Epidemiological Investigation
This retrospective study followed the outbreak investigation elements described by the U.S. Centers for Disease Control and Prevention (CDC) (CDC 2012) and used the original medical records to independently confirm the existence of the outbreak, verify the diagnosis, develop a case definition, and establish disease onset dates. Data for the evaluation of the three hypothesized sources were collected and analyzed independently. The study design, with access to medical records, was approved by the Institutional Review Board for the Protection of Human Subjects of the MDHHS. Requirement of informed consent was waived because the described use of existing documents involved no more than minimal risk, the research could not be practicably carried out without the waiver, and the waiver did not adversely impact the rights and welfare of the human subjects.
Case data.
A case was defined as a person who met the CDC’s definition of a confirmed LD case (CDC 2005; fever, myalgia, cough, and clinical or radiographic pneumonia and confirmed by detection of L. pneumophila serogroup 1 antigen in urine using validated reagents) with residence in Genesee County and onset of symptoms of LD in 2014 or 2015. The surveillance data for LD in the Michigan Disease Surveillance System (MDSS) provided general characteristics of the cases (age, sex, county of residence). Medical records, a report by the Genesee County Health Department (GCHD), and a supplementary questionnaire were thoroughly reviewed to confirm diagnosis, onset date, exposure window of 14 d prior to onset (CDC 2018), residential address, health care, and Flint water exposure. Two team members, of whom at least one was a physician, independently reviewed the case files to confirm the diagnosis via the presence of clinical findings and diagnostic tests (usually only the Urinary Antigen Test). They assigned a disease onset date based on the information in the medical records and the supplemental questionnaire. The supplementary LD questionnaire went into use in April 2015, many months after the onset of a large proportion of cases. Therefore, except place of residence, only data elements collected within 1 month of symptom onset were used. Adjudicating discrepancies of more than 1 d in onset date between team members was done by additional review and consensus.
Information abstracted about health care exposure included occurrence and type of health care facility exposure (inpatient, outpatient, visitor); duration of inpatient exposure; and dates, number of exposures, and location(s). The study methodology required that both reviewers agreed that a health care exposure occurred within 14 d of LD symptom onset for the exposure to be confirmed. The recorded information was compared with that obtained from the hospital line lists and the MDHHS line list. We had access to two line lists from Flint hospital A that contained information about previous visits to hospital A from cases admitted for LD in hospital A from June 2014 to February 2015. These hospital line lists were constructed after a request of the GCHD to investigate potential nosocomial LD in hospital A in July 2014.
Case addresses were available in the MDSS, medical records, the supplementary questionnaire, and the MDHHS line list. A residential address (case residence) during the exposure period could be extracted for 84 of the 86 cases (98%). Two addresses were classified as unclear because of inconsistent information in the supplementary questionnaire and indications that the case may be without permanent residence, or inconsistency in the medical records. We assigned geocodes to each of the addresses using a geocoding tool from Map Developers (https://www.mapdevelopers.com/batch_geocode_tool.php) and confirmed each of the geocodes by satellite imagery from Genesee County from 2014 and 2015 in Google Earth Pro.
To confirm the water source of the residence of the cases, we used a list of addresses connected to the City of Flint water supply (2013–2016), City of Flint utility billing records (https://bsaonline.com/?uid=1158), a database of addresses outside municipal boundary that received Flint water during that time [Water_Customers_Outside_of_City (Feature Server) https://services5.arcgis.com/lqqWNtSxx8Akj04A/arcgis/rest/services/Water_Customers_Outside_of_City/FeatureServer], and the supplementary questionnaire. All sources were combined to determine whether the case’s residence received Flint water.
We relied on MDHHS line-list data (MDHHS 2018) for information regarding comorbidities and smoking and for sporadic exposures, such as travel.
Confirmation of the outbreak.
Crude LD incidence in Genesee County and in Michigan (without Genesee County) was calculated as LD cases per population count, using U.S. Census Bureau population data (U.S. Census Bureau 2015). To confirm the existence of the LD outbreak in Genesee County in 2014 and 2015, a Bayesian Poisson regression framework was applied to all LD cases in Michigan for the years 2009–2017 and U.S. Census Bureau population data for each county by year. Random effects modeling was used to allow for county-specific averages and trends and to account for potential overdispersion. Trends were estimated using data from 2009–2013 and 2016–2017, and the discrepancy between observed and predicted incidence in 2014 and 2015 was evaluated by adding indicator variables for these years (and for each county) to the model.
Analysis of spatial clustering.
To analyze the distribution of the LD incidence over Genesee County, LD incidence was calculated per census block group using U.S. Census Bureau population data (U.S. Census Bureau 2015). To address the imprecision of incidence data based on small numbers of cases at that small geographic scale, we applied empirical Bayesian smoothing using two approaches: a) Global Empirical Bayes, using the overall crude incidence of Genesee county as prior; and b) Local Empirical Bayes, using the crude incidence of neighboring areas as prior. The average of the block incidence and the prior is calculated, weighted by the population size per block group. Spatial autocorrelation was evaluated with Moran’s I.
Hospital exposure.
Hospital exposure in the exposure window was classified as inpatient, outpatient, or visitor. Observed inpatient exposure was compared with expected inpatient exposures, given the proportion of total Medicare inpatient admissions (Centers for Medicare and Medicaid Services 2015) at each of the three Genesee County hospitals for 2014 and 2015, using a test. The Medicare population was used as reference because 85% of that population is over 65 y old, it has a high proportion of chronic conditions, and inpatient admission data were available for the hospitals. Hospital A records provided data about Legionella occurrence in their plumbing system and their remedial actions from September 2014 onward. The occurrence of hospital-exposed cases was compared with the timing of these remedial actions.
Residential Flint water exposure.
Incidence Rate Ratios (IRRs) were calculated by dividing the incidence rates in Census block groups in which residences are connected to the City of Flint water distribution network by the incidence rates in Census block groups in which residences are not connected to the City of Flint water distribution network, using Genesee County population data (U.S. Census Bureau 2015). A multilevel Poisson model was nested in block groups and adjusted for age group, sex, and an interaction factor for residential Flint water exposure and year. Year and residence in a Census block within 1 or 2 miles from a cooling tower AS5 (see below) were included as interaction factors. The model was also run adjusted for poverty level (U.S. Census Bureau 2015) and with hospital A exposed cases excluded. Free chlorine testing data from the City of Flint from eight monitoring sites in the Flint water distribution network were obtained through City of Flint (2015, 2016).
Cooling tower exposure.
Case addresses and LD onset dates were combined with weather data to create a heat map with areas where, if present, aerosol sources could affect multiple cases. Wind speed and direction were extracted from the U.S. National Oceanographic and Atmospheric Administration (NOAA) map tool (https://www.ncdc.noaa.gov/cdo-web/datatools/lcd) data recorded at Bishop International Airport, Michigan. Hourly average wind direction and speed in the exposure window of each case were calculated from these data. Missing wind direction (6.5% of the data) were imputed with the daily average wind direction. The hourly wind direction was used to create upwind vectors in ArcMap 10.5 (Esri). In the NOAA data, wind directions are rounded at 10-degree intervals. To represent the variation in wind direction, a 10-degree wedge was created around the hourly average vector. Vector length was limited to 1 mile; a 2-mile limit was used to check the sensitivity of the outcome to the distance. Although some studies reported LD cases were exposed miles downwind of aerosol sources (Nygård et al. 2008), most outbreaks reported that exposure of LD cases occurred within 1–2 miles from the cooling tower (García-Fulgueiras et al. 2003; Ulleryd et al. 2012; Weiss, et al. 2017) or wastewater treatment plant (Loenenbach et al. 2018; Caicedo et al. 2019). The density of wind vectors was calculated in ArcMap, in units of length per unit of area. This calculation resulted in heat maps with “hot” areas in which an aerosol source would have a higher probability to affect one or more cases. The inpatient stays in any of the hospitals were excluded from the analysis, both in terms of time (because the cases were not at their residences at that time) and in terms of location (because this factor would bias toward aerosol sources in the vicinity of the hospitals). The period from 1 December to 1 March was excluded because cooling towers in Genesee County are generally not operational during those months.
The hot areas were systematically inspected for the presence of aerosol sources (wet cooling towers and wastewater treatment) in satellite imagery from Genesee County using Google Maps, (maps.google.com) and Google Earth Pro, and geocodes and general characteristics of aerosol sources were documented. Expert judgment was used on Google Streetview images to determine whether cooling towers were using evaporative cooling (wet cooling towers). For each of these aerosol sources, the concentration of aerosols downwind was calculated for the exposure windows of each of the LD cases, using a Gaussian plume model (Korsakissok and Mallet 2009):
| (1) |
where C is the concentration at steady state (g/m2), Q is the source emission rate (g/s), u is the wind speed (m/s), y is the crosswind distance from the point source (m), z is the height of the receptor (), H is the release height of the source (m, estimated using Google Maps street view), is the horizontal stability parameter, and is the vertical stability parameter. For the atmospheric stability, the Pasquill–Guifford classes were used. The class ranges from A (extremely unstable) to F (extremely stable). The values of stability parameter increase with increasing downwind distance, and the magnitude of the increases depends on the stability class. The parameter values are formulated following the EPA’s ICS model (EPA 1995) as:
where x is the downwind distance from the point source (km); a, b, c, and d are the stability-class–specific parameter values (see Tables S1 and S2). The aerosol dispersion distance was limited to 2 mi. As the source emission rates were not known, we assumed a constant emission rate of (based on Nygård et al. 2008) for each source. Therefore, we interpreted the modeled aerosol concentrations at case (receptor) residences as relative rather than absolute values.
Exposure–response trends with proximity to the aerosol source were evaluated using a multilevel Poisson model nested in Census block groups adjusting for age group, sex, interaction factor for proximity and year and an interaction factor for City of Flint water at the residence and year. LD cases were assigned to the Census block group of their residential address and LD cases in the period 1 December to 1 March were excluded because cooling towers in Genesee County are not generally operational during these months. Because five cooling towers were included, a Bonferroni adjustment was done to determine if trends were significant ().
Comparative Genomic Analysis
Clinical L. pneumophila isolates were available from eight cases from 2015, as well as from four cases from 2016. Environmental isolates were available from one cooling tower from 2015 and from hospital A’s plumbing system from 2015 to 2017. Genomic DNA was extracted and sequenced by the MDHHS laboratory as described in Mercante et al. (2018). Genome sequences were deposited in the National Center for Biotechnology Information database (see Tables S3 and S4). Sequence types (ST) were derived by BLAST in silico extraction of the sequence of the seven alleles used in the international sequence-based typing scheme (Gaia et al. 2005). Whole-genome multilocus sequence typing (wgMLST) analysis was performed by finding alleles present in the genome sequences of current strains using a database of 5,777 loci from 32 reference genomes of various serogroups of L. pneumophila that were available in Bionumerics 7.6.3 (Applied Maths NV), including seven EWGLI sequence-based typing loci (Gaia et al. 2005), 1,521 core genome loci (Moran-Gilad et al. 2015), and 4,249 accessory loci (Applied Maths NV). Sequence types of clinical and environmental L. pneumophila isolates were compared using UPGMA. Genome sequences of L. pneumophila ST1 isolates were compared with sequences of USA ST1 isolates from the CDC archive (NCBI Bioproject PRJNA423272).
Results
Epidemiological Investigation
Confirmation of the outbreak.
The LD surveillance data in MDSS, combined with population data, confirmed the existence of an outbreak of LD in Genesee County in 2014 and 2015. Accounting for time trends, the observed incidence in Genesee County was 3.49 times higher than expected (95% credibility interval (CI): 1.98, 6.25) in 2014 and 3.67 times higher than expected (95% CI: 2.10, 6.48) in 2015. After exclusion of cases exposed to hospital A, the observed incidence in Genesee county was still 2.04 times higher than expected (95% CI: 1.08, 3.84) in 2014 and 1.78 times higher than expected (95% CI: 0.93, 3.33) in 2015 (see Figure S1). A total of 86 patients met the case definition. General case characteristics were similar for both years (Table 1).
Table 1.
Characteristics of Legionnaires’ disease (LD) cases in Genesee County, MI, in 2014 and 2015.
| Case characteristic | No. of cases (%) | ||
|---|---|---|---|
| 2014 + 2015 () | 2014 () | 2015 () | |
| Sex | |||
| Female | 43 (50) | 22 (56) | 21 (45) |
| Male | 43 (50) | 17 (44) | 26 (55) |
| Age (y) | |||
| Mean | 65 | 63 | 67 |
| Median | 65 | 64 | 66 |
| Range | 26–94 | 26–94 | 35–89 |
| 25–50 | 15 (17) | 7 (18) | 8 (17) |
| 51–64 | 27 (31) | 13 (33) | 14 (30) |
| 65–74 | 20 (23) | 11 (28) | 9 (19) |
| 24 (28) | 8 (21) | 16 (34) | |
| Symptomsa | |||
| Fever | 49/61 (80) | 15/18 (83) | 34/43 (79) |
| Chills | 46/60 (77) | 14/18 (78) | 32/42 (76) |
| Cough | 47/62 (76) | 14/20 (70) | 33/42 (79) |
| Shortness of breath | 48/65 (74) | 15/20 (75) | 33/45 (73) |
| Aches/muscle pains | 35/58 (60) | 12/18 (67) | 23/40 (58) |
| Diarrhea | 32/59 (54) | 8/17 (47) | 24/42 (57) |
| Nausea | 30/63 (48) | 9/20 (45) | 21/43 (49) |
| Vomiting | 35/61 (60) | 6/19 (32) | 16/42 (38) |
| Headaches | 28/60 (47) | 7/20 (35) | 21/40 (53) |
| Comorbiditiesa | |||
| Any chronic health condition | 59/71 (83) | 20/24 (83) | 39/47 (83) |
| Heart disease | 39/69 (57) | 10/24 (42) | 29/45 (64) |
| Diabetes | 28/68 (41) | 11/24 (46) | 17/44 (39) |
| Chronic kidney disease | 22/68 (32) | 11/24 (46) | 11/44 (25) |
| Chronic lung disease | 20/67 (30) | 8/24 (33) | 12/43 (28) |
| Immunocompromised | 19/63 (30) | 8/22 (36) | 11/41 (27) |
| Asthma/chronic bronchitis | 12/67 (18) | 5/24 (21) | 7/43 (18) |
| Smokera | |||
| Current + former | 53/70 (76) | 19/24 (79) | 34/46 (74) |
| Current | 22/70 (31) | 8/24 (33) | 14/46 (30) |
| Not current or former | 17/70 (24) | 5/24 (21) | 12/46 (26) |
| Number of days hospitalized for LD | |||
| Mean | 11 | 14 | 9.5 |
| Median | 10 | 11 | 9 |
| Range | 1–46 | 2–46 | 1–24 |
| Deaths | 10 | 4 | 6 |
Data on symptoms, comorbidities, and smoking were not available for all of the cases; the denominator shows the number of cases for which data were available.
Analysis of spatial clustering.
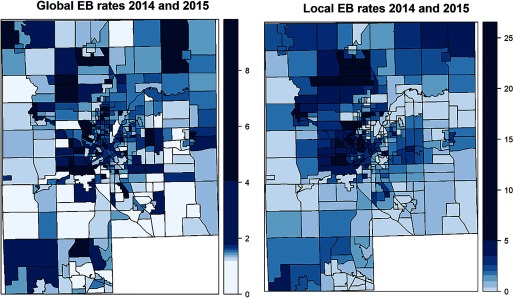
Cases resided throughout Genesee County. No evident geographical clustering in specific areas of Genesee County was observed from Empirical Bayes smoothed incidence rates per Census block group (Figure 1) and Moran’s I test (, ). The most frequently reported exposure was to hospital A (49%), of which 88% was inpatient (Table 2). Although only 34% of the cases received Flint water at their residence, this percentage was higher in 2014 (51%) than in 2015 (19%).
Figure 1.
EB smoothed incidence (Legionnaires’ disease cases per 10,000) in 2014 and 2015 per Genesee County block group. Note: EB incidence rates smooth the intrinsic high variability of rates within block groups that are the result of low numbers of cases per block group. These are weighted averages of individual Census block group and county rates (Global EB, left), or of census block group and neighboring census block group rates (Local EB, right). Weights are proportional to the underlying population in the block groups (U.S. Census Bureau 2015). The incidence color scale is depicted on the right, units are Legionnaires’ disease cases per 10,000 people. EB, empirical Bayes.
Table 2.
Exposure characteristics of Legionnaires’ disease cases in Genesee County in 2014 and 2015.
| Exposure type | No. of cases (%) | ||
|---|---|---|---|
| 2014 + 2015 () | 2014 () | 2015 () | |
| Residence served by Flint water | 29 (34) | 20 (51) | 9 (19) |
| Hospital Exposurea | 46 (53) | 19 (49) | 27 (57) |
| Hospital Aa | 42 (49) | 17 (44) | 25 (53) |
| Inpatientb | 37 (88) | 14 (82) | 23 (92) |
| Outpatientb | 3 (7) | 1 (6) | 2 (8) |
| Visitorb | 5 (12) | 2 (12) | 3 (12) |
| Hospital Ba | 5 (6) | 4 (10) | 1 (2) |
| Inpatient | 3 (60) | 3 (75) | 0 (0) |
| Outpatient | 0 (0) | 0 (0) | 0 (0) |
| Visitor | 2 (40) | 1 (25) | 1 (100) |
| Hospital C | 0 (0) | 0 (0) | 0 (0) |
Exposure of cases to multiple hospitals in the exposure window resulted in sums .
Multiple exposure types per case resulted in sums .
Hospital exposure.
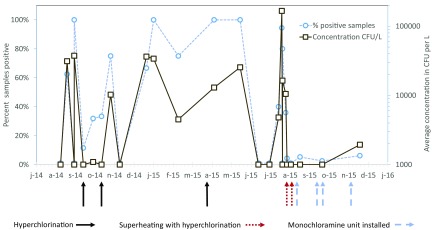
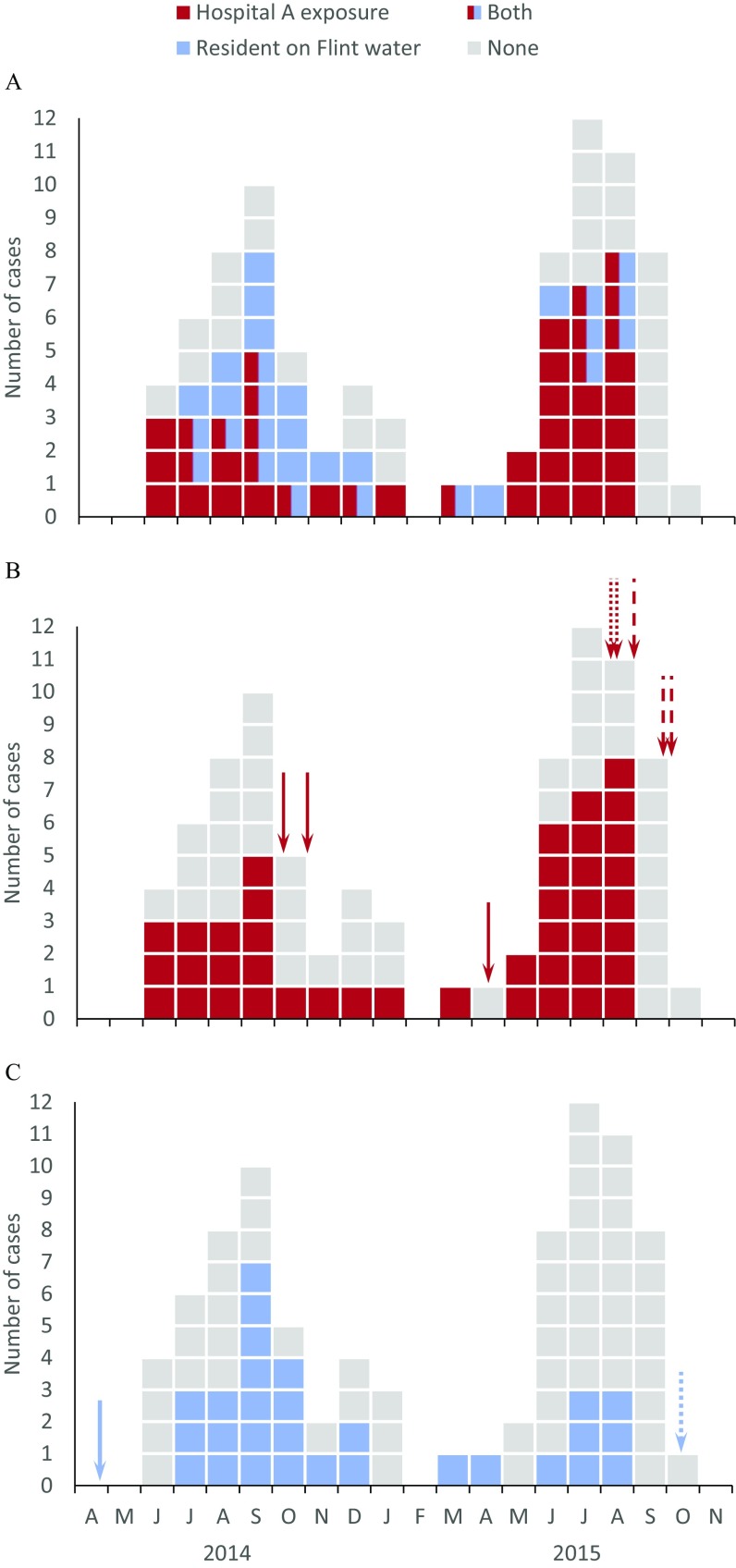
Admission to hospital A within the exposure window was higher than expected, based on the distribution of Medicare admissions among the three Genesee County hospitals (40 vs. 19; , ; Table 3). High concentrations ( CFU/L) of L. pneumophila SG1 were detected in hospital A’s plumbing system on several sampling dates in 2014 and 2015 at several locations throughout the hospital. Several rounds of hyperchlorination (4 October and 1 November 2014; 19 April 2015) did not sustainably reduce L. pneumophila SG1 in the plumbing system (Figure 2), and hospital A–exposed cases continued to occur (Figure 3). Superheating with hyperchlorination in August 2015 and subsequent installation of monochloramination units consistently reduced the presence of L. pneumophila, and no further hospital A–exposed cases appeared in Sep.–Dec. 2015 (Figure 3).
Table 3.
Expected versus observed inpatient visits of Legionnaires’ disease cases to the three main hospitals in Genesee County in 2014 and 2015.
| Hospital | Percentage of Medicare admissions in 2014 + 2015 | Expected # cases | Observed # cases | Chi-square | p-Value |
|---|---|---|---|---|---|
| A | 43.8 | 19 | 40 | 41.2 | |
| B | 18.7 | 8 | 4 | 2.8 | 0.093 |
| C | 37.5 | 17 | 0 | 23.8a |
Assuming one case was inpatient in hospital C. Expected inpatient exposures were calculated from the proportion of total Medicare inpatient admissions (Centers for Medicare and Medicaid Services 2015) at each of the three Genesee County hospitals in 2014 and 2015, and compared with the observed number of inpatient exposures among the Legionnaires’ disease cases in Genesee County in 2014 and 2015 using a test.
Figure 2.
Legionella pneumophila serogroup 1 in the plumbing system of Genesee County hospital A in 2014 and 2015 and alignment with treatment interventions. Note: Different locations throughout the hospital buildings (patient room hot and cold water taps at handwashing sinks and showers; staff room hot and cold water taps at handwashing sinks; technical room sampling taps; hot water returns) and different numbers of samples (2–55, average 11) were collected on the sampling days. Depicted are the percentage of samples from which L. pneumophila serogroup 1 was detected by culture (dashed line, left axis) and the concentration of L. pneumophila serogroup 1 by culture (colony forming units per liter, solid line, right axis). Arrows represent the time of Legionella control interventions in the plumbing system of hospital A: hyperchlorination events (solid), superheating and hyperchlorination (dotted), and the start of monochloramination (dashed).
Figure 3.
Epidemic curves of Legionnaires’ disease cases in Genesee County in 2014 and 2015 by month. (A) Cases with exposure to hospital A, residential exposure to Flint water, both exposure to hospital A and residential exposure to Flint water and no exposure to hospital A or residential exposure to Flint water. (B) Cases with and without exposure to hospital A. Arrows represent the date of Legionella control interventions in the plumbing system of hospital A: hyperchlorination events (solid), superheating and hyperchlorination (dotted), and the start of monochloramination (dashed). (C) Cases with and without residence on Flint water. Arrows indicate the date the water source was switched from Detroit water to Flint River (solid) and back (dotted).
Hospital A exposure was reported for 42 cases (49%). Even after exclusion of these cases, the incidence was still higher in Genesee County than it was in other Michigan counties, both in 2014 and in 2015 (see Figure S1), suggesting additional sources.
Residential Flint water exposure.
In 2014, residents receiving Flint water were significantly more at risk of acquiring LD than were other Genesee County residents [IRR 3.9 (95% CI: 2.0, 7.7), ]. No excess risk was observed in 2015 [IRR 0.9 (95% CI: 0.4, 1.9), ]. The interaction term for Flint water and year was statistically significant (). The IRR was attenuated when adjusting for proximity to the cooling tower AS5, which had the highest probability of affecting cases [IRR 2.25 (95% CI: 1.03, 4.94), ] or for the percentage of the population who lived below the poverty line [IRR 3.0 (95% CI: 1.3, 6.6)], but remained clearly elevated. When hospital A–exposed cases were excluded from the analysis, the increased risk for residents on Flint water persisted for 2014 [IRR 3.2 (95%CI: 1.4, 7.5)], , whereas data from 2015 showed no excess risk. Significantly more samples with low () free chlorine concentration were reported in the Flint water network (see Figure S2) in 2014 than in 2015 (; ). The first LD case in 2014 was observed 2 months after the 25 April drinking-water source change. The last case in 2015 with residence connected to Flint water was in August, 2 months before the switch back to Detroit water.
Cooling tower exposure.
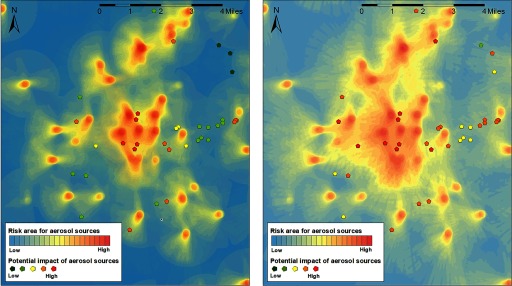
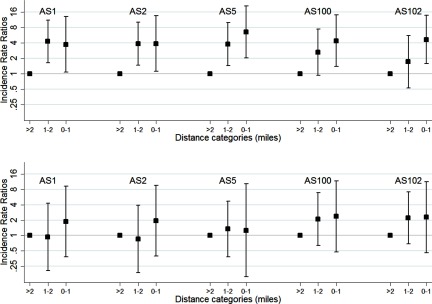
The heat map of the upwind areas for the LD cases was combined with the geocodes of the aerosol sources (Figure 4). With a 2-mile upwind search radius, nine aerosol sources were present in the zones with the highest impact probability. When the upwind radius was reduced to 1 mile, five of these nine aerosol sources (all cooling towers) were present in the zones with the highest impact probability. These five cooling towers are located within a mile from each other (see Figure S3); thus, it was not possible to distinguish among exposure to aerosols generated by these cooling towers. The Gaussian plume models (using neutral stability class D) showed that very few case residences were located in the zones with the highest concentration of aerosols from individual aerosol sources, up to 9 case residences were in the zone with the intermediate concentration, and up to 13 case residences in the zone with the lower concentration (Table 4). Modeling with other stability classes had little impact on the outcome. The IRR in block groups increased in the proximity of each of these five cooling towers in 2014, but not in 2015 (Table S5). When adjusting for Flint water at the residence, the IRRs for each of the cooling towers were attenuated, but the linear effects across distance remained significant (all p-values for linear effects across distance in 2014 ; Figure 5). When hospital A–exposed cases are excluded, the proximity effect is still visible, but the number of cases in each distance category became too low to analyze this effect with sufficient statistical power.
Figure 4.
Heat map with upwind area of Legionnaires’ disease case residences (excluding hospital inpatient stays) during the exposure window and aerosol sources, using a 1-mile (left) or 2-mile (right) upwind search radius. Heat maps were generated with ArcGIS (Esri). The heat map depicts the potential impact of a (virtual) aerosol source could have had on a case residence in the exposure window of each case (the closer to the residence, the higher the potential impact). The pentagonal shapes depict the actual aerosol sources, with the number of cases they could potentially have reached in their individual exposure windows from low () to high ().
Table 4.
Number of Legionnaires’ disease cases in Genesee County in 2014 and 2015 that was potentially affected by each of the five highest impact aerosol sources using three different aerosol concentration levels (, and ).
| Aerosol source ID | Number of cases potentially affected at aerosol concentration | ||
|---|---|---|---|
| AS01 | 0 | 9 | 13 |
| AS02 | 1 | 8 | 11 |
| AS05 | 1 | 8 | 13 |
| AS100 | 0 | 7 | 13 |
| AS102 | 2 | 6 | 12 |
: The concentration of aerosols downwind of each aerosol source was calculated for the exposure windows of each of the Legionnaires’ disease cases, using a Gaussian plume model (Korsakissok and Mallet 2009) and the wind speed and direction. We maximized transport of Legionella in aerosols to 2 miles. Because no data were available on emission rates, we assumed a constant emission rate of (based on Nygård et al. 2008) for each source. The modeled aerosol concentrations at case residences is therefore relative rather than absolute. Aerosol concentrations are relative, assuming each source emitted aerosols at the same rate. Hospital inpatient stays and visits were excluded.
Figure 5.
Incidence Rate Ratio (IRR) of Legionnaires’ disease in Census block groups with a centroid miles, 1–2 miles, and mile from aerosol sources (AS) in (A) 2014 and (B) 2015. Cases with onset between 1 December 2014 and 1 March 2015 were excluded as, generally, cooling towers are not operational in winter. A multilevel Poisson model was nested in Census block groups and adjusted for age group, sex, and interaction factors for age group and sex, and included an interaction factor for cooling tower and year and for residential Flint water exposure and year. Data are available in Table S5A.
Comparative Genomic Analysis
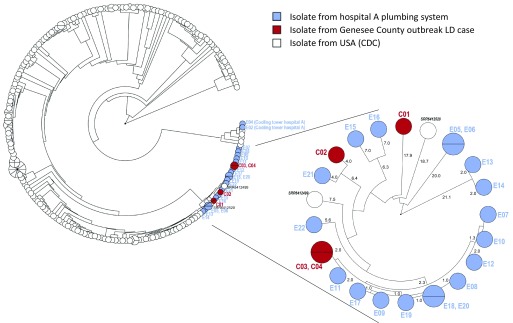
The eight clinical isolates that were cultured from LD cases from 2015 were of five different sequence types: ST1 (2), ST44 (1) ST222 (1), ST213 (3), and ST1941 (1). We also had access to clinical isolates of four Genesee County resident cases that were diagnosed with LD in 2016: these were ST1 (1 case, 2 isolates), ST222 (2), and ST1941 (1). Both ST1 cases from 2015 and the ST1 case from 2016 had been exposed to hospital A. All isolates from hospital A’s plumbing system in 2016 (2) and 2017 (10) that had been submitted for sequence typing were also ST1. In addition, wgMLST showed that these clinical ST1 and plumbing system ST1 isolates compose a cluster of highly similar ST1 strains, distinct from the other 254 USA ST1 isolates (Figure 6), except two isolates that were not associated with this outbreak [one isolated from a case in Washington in 1989 and one from a case in Arizona in 2009 (Mercante et al. 2018)]. No shared exposures were recorded for the three 2015 cases with ST213. ST222 (2) and ST1941 (1) recurred in 2016, but no epidemiological connection was found in the data of these cases from 2015 and 2016. Two ST1 isolates were recovered from hospital A’s cooling towers in October 2015, but these isolates did not cluster closely with the clinical ST1 isolates.
Figure 6.
Circular representation of dendrograms after UPGMA clustering of whole-genome multilocus sequence typing (wgMLST) profiles of Legionella pneumophila Sequence Type 1 (ST1) isolates, with the allele calls considered as categorical data. Clinical, hospital A plumbing, and hospital A cooling tower isolates from Genesee County, compared with USA ST1 isolates from the CDC archive (Mercante et al. 2018). The ST1 isolates from the hospital A–exposed cases and the isolates from hospital A’s plumbing system form a unique cluster of highly similar wgMLST within the USA ST1 isolates. Inlay shows the Genesee County L. pneumophila ST1 isolates from outbreak cases in 2015 (C01, C02), a case in 2016 (C03, C04), and from hospital A plumbing in 2016 and 2017, as well as the two unrelated clinical isolates from Arizona in 2009 (SRR6412520) and Washington in 1989 (SRR6412499) (Mercante et al. 2018). The numbers show the average number of the 5,777 loci that are different between isolates.
Discussion
We found evidence for three sources of a large, community-wide LD outbreak among Genesee County residents in 2014 and 2015. There was strong evidence (CDC class I; CDC 2019) for a hospital-associated outbreak in 2014 and 2015, as well as indications (CDC class II; CDC 2019) that in 2014 a proportion of cases was associated with residences served by City of Flint water and select cooling towers in Flint. The data did not allow us to determine the attributable risk of the three sources, but multivariate analysis showed an increased risk with residential exposure to Flint water and select cooling towers in 2014 only. Both sources remained associated with LD occurrence after mutual adjustment in the Poisson model. For hospital A exposure, we could not compute incidence rates of the exposed and nonexposed population in the absence of an appropriate population denominator, but the association was supported by: a) the large proportion of cases with prior exposure to hospital A, of which the inpatient exposure was significantly higher than expected on the basis of Medicare admissions among local hospitals; b) the presence of high levels of L. pneumophila SG1 in the hospital plumbing system; c) the alignment of the implementation of effective Legionella control measures with the subsidence of hospital A–associated cases; and d) the clustering of the two ST1 clinical isolates from cases exposed to hospital A with ST1 isolates from the hospital water system in 2016 and 2017, as well as from a LD case exposed to the same hospital in 2016. The appearance of two epidemiologically unrelated ST1 isolates from other U.S. regions in this cluster is not uncommon (Mercante et al. 2018; David et al. 2017). Although the isolates examined in this study span several years, it has been noted in previous hospital-associated outbreaks of ST1 that hospitals were colonized by a limited number of distinct ST1 populations and that highly genetically similar strains could be isolated from the same hospital water systems over multiple years (Oberdorfer et al. 2008; David et al. 2017). For the other sources, no environmental Legionella data and isolates were available, except four isolates from one of the cooling towers, which were genotypically distinct from the clinical isolates. The limitation of having only a few isolates and predominantly from one of the sources underscores the importance of timely sampling of all potential L. pneumophila sources to support identification of source(s) that gave rise to the outbreak.
The aerosol model we used was simple and did not take plume rise, terrain roughness, or buildings into account. The emission rate of each aerosol source was assumed equal and constant, which was very likely not the actual situation. Hence, the aerosol model outcome reflects potential rather than actual concentrations and impact on case residences. The significant increase in IRR when a case residence was closer to the cooling towers was reflecting the actual situation in 2014.
Our investigation sheds new light on the outbreak of LD in Genesee County. The two earlier studies of this same outbreak (MDHHS 2018; Zahran et al. 2018) did not report multiple sources. A descriptive epidemiological evaluation (MDHHS 2018) identified hospital exposure but not the associations with residential exposure to Flint water and cooling towers, underlining the need to include analytical epidemiological approaches in outbreak investigations. Zahran et al. (2018) recognized 25 (rather than 42; Table 2) cases exposed at hospital A, conducted a modeling study focusing on Flint water, and reported an association between LD risk and the low free chlorine concentrations after the switch to Flint River as source, and a temporal association between LD risk and the switch and switch-back and the boil water advisories. However, several assumptions in their models do not seem to be supported by available data and literature. They did not use the actual LD onset date from the medical records to determine the time window for Legionella exposure of the cases but an approximation that differed from the onset date we established from the medical records by four or more days for 31% of the cases. They assumed residents living in Flint Census tracts received City of Flint water, whereas our data sources indicate that this is not always the case. They used the boil-water advisories to assume change in (shower) behavior, which is not supported by data. They used commuter data to associate the LD cases who lived outside Flint with exposure to City of Flint water, whereas the LD case data show that, of the 57 cases that were living outside the City of Flint water-supply zone, the vast majority (72%, 41 cases) did not work (they were disabled or retired); 21% (12 cases) did work, of whom only 2 worked in Flint (no data about 4 cases). Zahran et al. also translated data from the free chlorine monitoring sites to the (Census tract of the) case residences, whereas literature suggests that this cannot be done reliably in a highly looped network, particularly under the dynamic water-treatment operation and distribution water quality in the period of the water switch and without accurate data about residence time and chlorine decay (Blokker et al. 2014; Masten et al. 2016). The last LD cases who received City of Flint water at their residence did so in August 2015, 2 months before the switch-back (see figure 3). These findings emphasizes the need for accurate case history evaluation to reconstruct and assign exposure status for each case as adequately as possible prior to statistical modeling of outbreak data to avoid bias in associations between case status and potential determinants.
Previous LD outbreaks have been associated with a single source by a single ST (Phin et al. 2014; George et al. 2016; David et al. 2017; Weiss et al. 2017). Spatial and geographical clusters of otherwise unrelated LD outbreaks have been reported in New York City, USA, and Sydney, Australia (MacIntyre et al. 2018). An outbreak of ST345 in Germany was associated with aerosols from wastewater treatment plants and cooling towers (Petzold et al. 2017). One previous outbreak in a hotel in Spain was associated with two different STs in two different sources: ST23 in a spa pool and air-conditioning system and ST578 in the hotel plumbing system (Sánchez-Busó et al. 2016). This study is the first report of a multiple ST LD outbreak in the United States with epidemiological evidence to link the outbreak to three sources. The retrospective nature of the investigation precluded the collection of additional evidence regarding possible relationships among sources. Hospital A, the residences on City of Flint water, and possibly the cooling towers were connected to the City of Flint water network, and the high iron and low chlorine content of the water may have created more favorable conditions in water systems that were vulnerable to proliferation of L. pneumophila. Even so, other cooling towers and another large hospital B in Flint were not associated with the LD cases. High L. pneumophila concentrations were detected also in hospital B’s plumbing system in October 2015 (Schwake et al. 2016). No L. pneumophila was detected in small buildings, including single-family residences, in Flint in August 2015. Therefore, if the Flint water switch created more favorable conditions for L. pneumophila to grow in connected water systems (Schwake et al. 2016; Rhoads et al. 2017), the local conditions of the building water system and water use probably determined whether it could lead to an outbreak. Hence, key to LD outbreak prevention is proper design, operation, and maintenance of specific water systems in buildings. Guidelines for Legionella control in building water systems have been made available (Parr et al. 2015; ASHRAE 2018), and health care facilities are now called on to implement them (Centers for Medicare and Medicaid Services 2017). Implementing hyperchlorination alone was not effective in controlling the nosocomial outbreak in hospital A. This lack of effectiveness had been reported before (Lin et al. 2011) and emphasizes the importance of applying evidence-based methods (Lin et al. 2011; EPA 2016) to ensure sustainable Legionella control. Country-wide implementation of guidelines to control Legionella in public buildings and in cooling towers has been successful in France in stopping further increase in LD incidence and (large) outbreaks and was associated with a decrease in nosocomial LD (Hartemann and Hautemaniere 2011; Campèse et al. 2015; French Public Health Agency 2019). Nevertheless, as in the United States, community-acquired LD remains substantial in France (French Public Health Agency 2019), and guidance to homeowners to control Legionella in their home water systems may help reduce future occurrence (Government of Western Australia 2019). LD is largely preventable; the increasing incidence over past years and the high associated burden of disease and fatality rate warrant implementation of prevention and control measures, as scientists have suggested (U.S. National Academies of Sciences, Engineering, and Medicine Committee 2019). Cases of LD need rapid follow-up, with L. pneumophila testing of LD cases and suspected environmental sources, to identify the source(s) and implement evidence-based control measures to prevent further spread of LD.
Supplementary Material
Acknowledgments
This study was financed by the Department of Development, Technology and Budget of the State of Michigan. The authors thank the Michigan Department of Health and Human Services (MDHHS) for agreeing to use the data they had available and MDHHS and the Genesee County Health Department for sharing information about the data collection process at the time of the outbreak. In addition, the authors thank M.S. Dworkin (University of Illinois at Chicago), L. Fitzpatrick (George Washington University), P. Hartemann (University of Lorraine, France), P. Hunter (University of East Anglia, England), and T. Wade (U.S. Environmental Protection Agency) for the helpful discussions and recommendations related to study design, outcomes, and the manuscript. The authors also thank L. Portengen of IRAS for statistical support and L. Rosenthal and N. Slaats of KWR for management of the project.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5663).
Independent Epidemiology Consultant.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers). 2018. Legionellosis: risk management for building water systems. ANSI/ASHRAE Standard 188. Atlanta, GA https://www.ashrae.org/technical-resources/bookstore/ansi-ashrae-standard-188-2018-legionellosis-risk-management-for-building-water-systems [accessed 14 September 2019].
- Benedict KM, Reses H, Vigar M, Roth DM, Roberts VA, Mattioli M, et al. 2017. Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2013–2014. MMWR Morb Mortal Wkly Rep 66(44):1216–1221, PMID: 29121003, 10.15585/mmwr.mm6644a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokker M, Vreeburg J, Speight V. 2014. Residual chlorine in the extremities of the drinking water distribution system: the influence of stochastic water demands. Procedia Engineering 70:172–180, 10.1016/j.proeng.2014.02.020. [DOI] [Google Scholar]
- Caicedo C, Rosenwinkel KH, Exner M, Verstraete W, Suchenwirth R, Hartemann P, et al. 2019. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: review. Water Res 149:21–34, PMID: 30445393, 10.1016/j.watres.2018.10.080. [DOI] [PubMed] [Google Scholar]
- Campèse C, Descours G, Lepoutre A, Beraud L, Maine C, Che D, et al. 2015. Legionnaires’ disease in France. Med Mal Infect 45(3):65–71, PMID: 25722040, 10.1016/j.medmal.2015.01.015. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2005. Legionellosis/Legionnaires’ Disease or Pontiac Fever 2005 case definition. Atlanta, GA, USA: https://wwwn.cdc.gov/nndss/conditions/legionellosis/case-definition/2005/ [accessed 14 September 2019]. [Google Scholar]
- CDC. 2011. Legionellosis—United States, 2000–2009. MMWR Morb Mortal Wkly Rep 60(32):1083–1086, PMID: 21849965. [PubMed] [Google Scholar]
- CDC. 2012. Principles of Epidemiology in Public Health Practice. https://www.cdc.gov/csels/dsepd/ss1978/ [accessed 14 September 2019].
- CDC. 2018. Legionellosis Case Report Form Instructions. https://www.cdc.gov/legionella/health-depts/surv-reporting/form-instructions.html [accessed 14 September 2019].
- CDC. 2019. Strength-of-Evidence Classification for Waterborne Disease & Outbreaks https://www.cdc.gov/healthywater/surveillance/outbreak-classifications.html [accessed 14 September 2019].
- Centers for Medicare and Medicaid Services. 2015. Inpatient Prospective Payment System (IPPS) Provider Summary for All Diagnosis-Related Groups (DRG)–FY2014 and FY2015. https://data.cms.gov/inpatient-provider-lookup/view-data [accessed 14 September 2019].
- Centers for Medicare and Medicaid Services. 2017. Requirement to reduce Legionella risk in healthcare facility water systems to prevent cases and outbreaks of Legionnaires’ disease. Baltimore, MD: Centers for Medicare & Medicaid Services, Department of Health and Human Services; https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Policy-and-Memos-to-States-and-Regions-Items/Survey-And-Cert-Letter-17-30-.html [accessed 11 November 2019]. [Google Scholar]
- City of Flint, 2015. City of Flint Water Treatment Plant monthly report January–December 2014. https://www.michigan.gov/documents/flintwater/2014_Flint_MORs_-_Identifying_Data_Removed_per_Order_512526_7.pdf [accessed 11 November 2019].
- City of Flint, 2016. City of Flint Water Treatment Plant monthly report January–December 2015. https://www.michigan.gov/documents/flintwater/2015_Flint_MORs_-_Identifying_Data_Removed_per_Order_512527_7.pdf [accessed 11 November 2019].
- David S, Afshar B, Mentasti M, Ginevra C, Podglajen I, Harris SR, et al. 2017. Seeding and establishment of Legionella pneumophila in hospitals: implications for genomic investigations of nosocomial Legionnaires’ Disease. Clin Infect Dis 64(9):1251–1259, PMID: 28203790, 10.1093/cid/cix153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. 2015. Our sampling of 252 homes demonstrates a high lead in water risk: Flint should be failing to meet the EPA Lead and Copper Rule. http://flintwaterstudy.org/2015/09/our-sampling-of-252-homes-demonstrates-a-high-lead-in-water-risk-flint-should-be-failing-to-meet-the-epa-lead-and-copper-rule/ [accessed 14 September 2019].
- EPA (U.S. Environmental Protection Agency). 1995. User’s guide for the industrial source complex (ISC3) dispersion models. Volume II—Description of model algorithms. EPA EPA-454/B-95-003b. https://www3.epa.gov/scram001/userg/regmod/isc3v2.pdf [accessed 14 September 2019].
- EPA. 2016. Technologies for Legionella control in premise plumbing systems: scientific literature review. Washington, DC: Office of Water, U.S. Environmental Protection Agency; https://www.epa.gov/sites/production/files/2016-09/documents/legionella_document_master_september_2016_final.pdf [accessed 14 September 2019]. [Google Scholar]
- French Public Health Agency. 2019. Données de surveillance de la légionellose de 1988 á 2018. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/legionellose/articles/donnees-de-surveillance-de-la-legionellose-de-1988-a-2018 [accessed 5 September 2019].
- Gaia V, Fry NK, Afshar B, Lück PC, Meugnier H, Etienne J, et al. 2005. Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J Clin Microbiol 43(5):2047–2052, PMID: 15872220, 10.1128/JCM.43.5.2047-2052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Fulgueiras A, Navarro C, Fenoll D, García J, González-Diego P, Jiménez-Buñuales T, et al. 2003. Legionnaires’ disease outbreak in Murcia, Spain. Emerg Infect Dis 9(8):915–921, PMID: 12967487, 10.3201/eid0908.030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison LE, Kunz JM, Cooley LA, Moore MR, Lucas C, Schrag S, et al. 2016. Vital signs: deficiencies in environmental control identified in outbreaks of Legionnaires’ disease—North America, 2000–2014. MMWR Morb Mortal Wkly Rep 65(22):576–584, PMID: 27281485, 10.15585/mmwr.mm6522e1. [DOI] [PubMed] [Google Scholar]
- George F, Shivaji T, Pinto CS, Serra L, Valente J, Albuquerque M, et al. 2016. A large outbreak of Legionnaires’ disease in an industrial town in Portugal. Revista Portuguesa de Saúde Pública 34(3):199–208, 10.1016/j.rpsp.2016.10.001. [DOI] [Google Scholar]
- Government of Western Australia. 2019. Minimising the risk of a Legionella infection at home. https://www.healthywa.wa.gov.au/Articles/J_M/Minimising-the-risk-of-a-Legionella-infection-at-home [accessed 14 September 2019].
- Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. 2016. elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health 106(2):283–290, PMID: 26691115, 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartemann P, Hautemaniere A. 2011. Legionellosis prevention in France. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 54(6):724–727, PMID: 21626377, 10.1007/s00103-011-1290-5. [DOI] [PubMed] [Google Scholar]
- Korsakissok I, Mallet V. 2009. Comparative study of Gaussian dispersion formulas within the Polyphemus platform: evaluation with prairie grass and Kincaid experiments. J Appl Meteor Climatol 48(12):2459–2473, 10.1175/2009JAMC2160.1. [DOI] [Google Scholar]
- Lin YE, Stout JE, Yu VL. 2011. Controlling Legionella in hospital drinking water: an evidence-based review of disinfection methods. Infect Control Hosp Epidemiol 32(2):166–173, PMID: 21460472, 10.1086/657934. [DOI] [PubMed] [Google Scholar]
- Loenenbach AD, Beulens C, Euser SM, van Leuken JPG, Bom B, van der Hoek W, et al. 2018. Two community clusters of Legionnaires’ Disease directly linked to a biologic wastewater treatment plant, the Netherlands. Emerg Infect Dis 24(10):1914–1918, PMID: 30226165, 10.3201/eid2410.180906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre CR, Dyda A, Bui CM, Chughtai AA. 2018. Rolling epidemic of Legionnaires’ disease outbreaks in small geographic areas. Emerg Microbes Infect 7(1):36, PMID: 29559643, 10.1038/s41426-018-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten SJ, Davies SH, McElmurry SP. 2016. Flint water crisis: what happened and why? J Am Water Works Assoc 108(12):22–34, PMID: 28316336, 10.5942/jawwa.2016.108.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung RP, Roth DM, Vigar M, Roberts VA, Kahler AM, Cooley LA, et al. 2017. Waterborne disease outbreaks associated with environmental and undetermined exposures to water—United States, 2013–2014. MMWR Morb Mortal Wkly Rep 66(44):1222–1225, PMID: 29120997, 10.15585/mmwr.mm6644a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDHHS (Michigan Department of Health and Human Services). 2018. Epidemiology of Legionnaires’ Disease in Genesee County, Michigan, 2014–2017, Chart book. Lansing, MI: Michigan Department of Health and Human Services; https://www.michigan.gov/documents/flintwater/GC_Legionella_ChartBook_FINAL_624171_7.pdf [accessed 14 September 2019]. [Google Scholar]
- Mercante JW, Caravas JA, Ishaq MK, Kozak-Muiznieks NA, Raphael BH, Winchell JM. 2018. Genomic heterogeneity differentiates clinical and environmental subgroups of Legionella pneumophila sequence type 1. PLoS One 13(10):e0206110, PMID: 30335848, 10.1371/journal.pone.0206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Gilad J, Prior K, Yakunin E, Harrison TG, Underwood A, Lazarovitch T, et al. 2015. Design and application of a core genome multilocus sequence typing scheme for investigation of Legionnaires’ disease incidents. Euro Surveill 20(28), PMID: 26212142, 10.2807/1560-7917.ES2015.20.28.21186. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2019. Management of Legionella in Water Systems. Washington, DC: The National Academies Press; 10.17226/25474 [accessed 11 November 2019]. [DOI] [PubMed] [Google Scholar]
- Nygård K, Werner-Johansen Ø, Rønsen S, Caugant DA, Simonsen Ø, Kanestrøm A, et al. 2008. An outbreak of Legionnaires disease caused by long-distance spread from an industrial air scrubber in Sarpsborg, Norway. Clin Infect Dis 46(1):61–69, PMID: 18171215, 10.1086/524016. [DOI] [PubMed] [Google Scholar]
- Oberdorfer K, Müssigbrodt G, Wendt C. 2008. Genetic diversity of Legionella pneumophila in hospital water systems. Int J Hyg Environ Health 211(1–2):172–178, PMID: 17652025, 10.1016/j.ijheh.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Parr A, Whitney EA, Berkelman RL. 2015. Legionellosis on the rise: a review of guidelines for prevention in the United States. J Public Health Manag Pract 21(5):E17–26, PMID: 25203696, 10.1097/PHH.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold M, Prior K, Moran-Gilad J, Harmsen D, Lück C. 2017. Epidemiological information is key when interpreting whole genome sequence data—lessons learned from a large Legionella pneumophila outbreak in Warstein, Germany, 2013. Euro Surveill 22(45), PMID: 29162202, 10.2807/1560-7917.ES.2017.22.45.17-00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phin N, Parry-Ford F, Harrison T, Stagg HR, Zhang N, Kumar K, et al. 2014. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis 14(10):1011–1021, PMID: 24970283, 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- Pieper KJ, Tang M, Edwards MA. 2017. Flint water crisis caused by interrupted corrosion control: investigating “Ground Zero” home. Environ Sci Technol 51(4):2007–2014, PMID: 28145123, 10.1021/acs.est.6b04034. [DOI] [PubMed] [Google Scholar]
- Rhoads WJ, Garner E, Ji P, Zhu N, Parks J, Schwake DO, et al. 2017. Distribution system operational deficiencies coincide with reported Legionnaires’ disease clusters in Flint, Michigan. Environ Sci Technol 51(20):11986–11995. Epub 2017 Sep 26, PMID: 28849909, 10.1021/acs.est.7b01589. [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Ettinger AS, Hanna-Attisha M, Jones N, Davis SI, Breysse PN. 2019. The Flint water crisis: a coordinated public health emergency response and recovery initiative. J Public Health Manag Pract 25 (suppl 1, Lead Poisoning Prevention):S84–S90, PMID: 30507775, 10.1097/PHH.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Busó L, Guiral S, Crespi S, Moya V, Camaró ML, Olmos MP, et al. 2016. Genomic investigation of a Legionellosis outbreak in a persistently colonized hotel. Front Microbiol 6(1556), PMID: 26834713, 10.3389/fmicb.2015.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake DO, Garner E, Strom OR, Pruden A, Edwards MA. 2016. Legionella DNA markers in tap water coincident with a spike in Legionnaires’ disease in Flint, MI. Environ Sci Technol Lett 3(9):311–315, 10.1021/acs.estlett.6b00192. [DOI] [Google Scholar]
- Shah P, Barskey A, Binder A, Edens C, Lee S, Smith J, Schrag S, Whitney C, Cooley L. 2018. Legionnaires’ disease surveillance summary report, United States 2014–2015. https://www.cdc.gov/legionella/health-depts/surv-reporting/2014-15-surv-report-508.pdf [accessed 14 September 2019].
- Ulleryd P, Hugosson A, Allestam G, Bernander S, Claesson BE, Eilertz I, et al. 2012. Legionnaires’ disease from a cooling tower in a community outbreak in Lidköping, Sweden—epidemiological, environmental and microbiological investigation supported by meteorological modelling. BMC Infect Dis 12:313, PMID: 23171054, 10.1186/1471-2334-12-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2015. TIGER/Line Geodatabases. https://www.census.gov/geographies/mapping-files/time-series/geo/tiger-geodatabase-file.2015.html [accessed 14 September 2019].
- van Heijnsbergen E, Schalk JA, Euser SM, Brandsema PS, den Boer JW, de Roda Husman AM. 2015. Confirmed and Potential Sources of Legionella Reviewed. Environ Sci Technol 49(8):4797–4815, PMID: 25774976, 10.1021/acs.est.5b00142. [DOI] [PubMed] [Google Scholar]
- Weiss D, Boyd C, Rakeman JL, Greene SK, Fitzhenry R, McProud T, Musser K, South Bronx Legionnaires’ Disease Investigation Team, et al. 2017. A large community outbreak of Legionnaires’ Disease associated with a cooling tower in New York City, 2015. Public Health Rep 132(2):241–250, PMID: 28141970, 10.1177/0033354916689620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran S, McElmurry SP, Kilgore PE, Mushinski D, Press J, Love NG, et al. 2018. Assessment of the Legionnaires’ disease outbreak in Flint, Michigan. Proc Natl Acad Sci USA 115(8):E1730–E1739, PMID: 29432149, 10.1073/pnas.1718679115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.