Abstract
In contrast to the decreasing incidence of colorectal cancer (CRC) in older populations, the incidence has nearly doubled in younger adults since the early 1990s. Approximately 1 in 10 new diagnoses of CRC are now made in individuals 50 years or younger. Patients’ risk of CRC has been calculated largely by age and family history, yet 3 of 4 patients with early-onset CRC have no family history of the disease. Rapidly increasing incidence rates in younger persons could result from generational differences in diet, environmental exposures, and lifestyle factors. We review epidemiologic trends in CRC, data on genetic and non-genetic risk factors, and new approaches for determining CRC risk. These may identify individuals likely to benefit from early screening and specialized surveillance.
Keywords: Colorectal cancer, early-age onset, epidemiology, risk factors
Colorectal cancer (CRC) incidence and mortality rates have changed substantially in the United States (US) over the past 4 decades. Incidence and mortality have each decreased among adults older than 50 years since the early 1990s.1 These improvements are likely due to a combination of screening, shifts in distribution of risk factors (less smoking, more aspirin use), and improvements in treatment. However, data from epidemiology studies demonstrate an alarming and continued increase in CRC incidence among adults younger than 50 years.2,3 This early-onset CRC now accounts for 10–12% of all new CRC diagnoses. Little is known about the mechanisms of and factors that contribute to development of CRC in younger adults. Important questions to answer are: does early-onset CRC have distinct features from CRC that develops in older adults, and what environmental and lifestyle-related factors have contributed to increases in CRC incidence in younger, but not older, adults? Are there different risk factors for CRC in young vs old adults? We review the epidemiology and pathogenesis of CRC in young adults, including trends in incidence; genetic clinical, histopathologic, and molecular features; risk factors; and implications for screening.
Epidemiology
Trends in Incidence
After the adoption of population-based screening for CRC in the 1990s, CRC incidence has decreased in the total population by more than 35%.4 Yet, in contrast to the great decreases among older adults, the incidence of CRC among younger adults has nearly doubled in the same time period.2 Incidence rates have risen rapidly among persons ages 20–49 years in the US, from 8.6 per 100,000 in 1992 to 13.1 per 100,000 in 2016, with the largest increases among adults 40–49 years old.4 Although CRC mortality has also decreased in older adults, largely due to screening and advances in treatment,5, 6 mortality among younger adults has remained stable, at 2.8 per 100,000.7
Similar increases in early-onset CRC have been reported across the West, including Canada, Australia, and the United Kingdom (UK)., and in Asia. Despite overall population trends in aging, by 2030, approximately 11% of colon cancers and 23% of rectal cancers will occur in adults younger than age 50 years.3
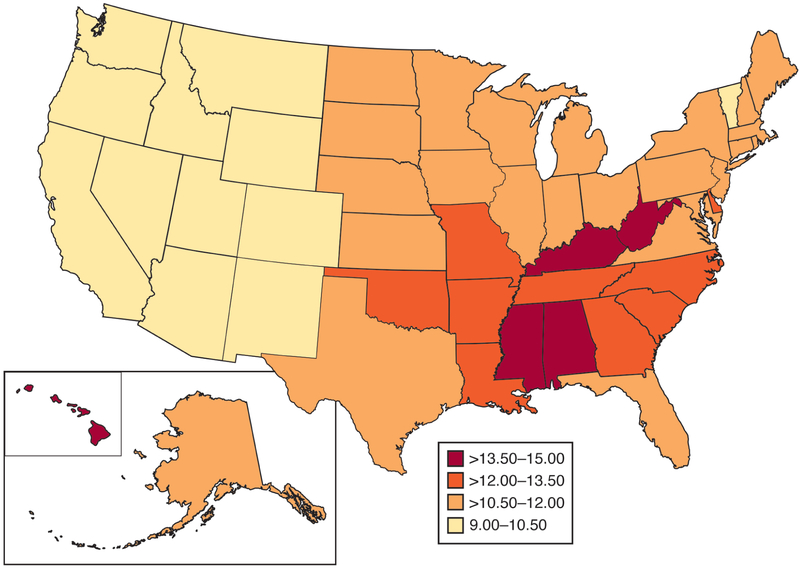
In the US, rates of early-onset CRC vary widely by state (Figure 1). Rates are lowest (about 9.5 per 100,000) in western states and higher in the Mississippi Delta Region and Appalachia (about 14.0 per 100,000). Specifically, Mississippi and Kentucky have the highest incidence rates at 15.1 per 100,000 and 14.2 per 100,000, respectively. The Mississippi Delta and Appalachia are geographically diverse regions, characterized by poverty, unemployment, and poor access to healthcare. Incidence of all gastrointestinal cancers is higher in these regions compared to other parts of the US,8 and CRC mortality rates (across age groups) are also strikingly high.9 These geographic differences in incidence rates suggest environmental exposures (such as agricultural runoff, industrial pollution),10 lifestyle-related factors (such as diet, obesity),11 and/or occupational exposures (such as mineral dust, trace elements)12 prevalent in these regions might contribute to pathogenesis.
Figure 1.
Incidence rates of early-onset CRC (ages 20–49 years) by US state, National Program of Cancer Registries, 2001 – 2015
Early-onset CRC has increased across successive birth cohorts (Figure 2),2, 13—persons born in and after the 1960s are at higher risk of CRC when compared to older generations. For example, in the US, CRC incidence is higher among 40-year old persons born in 1970 (24.4 per 100,000) than 40-year old persons born in 1950 (18.3 per 100,000).4 Interestingly, increases in early-onset CRC by birth cohort have also occurred worldwide, although risk varies by birth cohort within each country. In the US, incidence rates began to increase among Baby Boomers2 and are highest among Generation X.13 In Canada14 and Australia,15 the increased incidence of colon (vs. rectal) cancer was first noted among persons born in the 1970s. In Asian populations from Japan, Hong Kong, and Shanghai, increasing incidence rates appeared in later birth cohorts.16 Birth cohort effects point to exposures occurring in early life, or exposures frequently experienced by younger generations, that may increase risk of early-onset CRC.17
Figure 2.
Incidence rate ratios for CRC by birth cohort
Clinical features
Early-onset CRC is a challenge to study because most young adults diagnosed with CRC have no obvious risk factors (such as family history). In fact, most patients with early-onset CRC would be classified as average risk by current CRC screening and management algorithms. Age and family history of cancer remain the cornerstone of CRC risk stratification algorithms. However, only a minority of patients with early-onset CRC report having a first-degree relative with CRC, and even fewer have a predisposing condition (such as inflammatory bowel disease [IBD]).18,19, 20 Failure to consider the possibility of CRC, by patients and their providers, contributes to delays in diagnosis in younger adults – even in the presence of red flag symptoms (such as hematochezia or iron deficiency anemia). A substantial proportion of younger patients have metastatic disease at the time of diagnosis.
A higher proportion of younger patients have primary tumors in the distal colon or rectum compared with older patients, among whom proximal colon tumors predominate. Increasing incidence rates of early-onset CRC have been driven by increases in rectal (vs colon) cancer, particularly among whites.21 Across all racial/ethnic groups, cases of rectal cancer increased by more than 90% from the early 1990s through 2016 (2.6 to 5.1 per 100,000),4 compared to an increase of about 40% for cases of colon cancer. The largest relative increases in rectal cancer incidence have occurred in women (2.7 to 4.6 per 100,000). Differences in incidence by anatomic subsite indicate the importance of differentiating risk factors for colon vs rectal cancer.
Differences among races and ethnicities
Early-onset CRC disproportionately affects racial and ethnic minorities compared with non-Hispanic whites. Although 10%–12% of all patients diagnosed with CRC are younger than 50 years, the proportion is nearly doubled among non-Hispanic blacks (16%) compared with non-Hispanic whites (9%).22 Incidence rates of early-onset CRC have been persistently higher among blacks,4 although the gap with whites has recently narrowed.21 Incidence rates have also increased rapidly among young Hispanics.23, 24
Differences in survival by age of onset
Although most studies report no difference in CRC survival between younger and older patients with CRC, younger patients are more likely to be treated aggressively with surgery, multimodality chemotherapy, and/or radiation therapy.25–29 Even among patients with stage 2 colon cancer, for whom guidelines recommend against use of adjuvant chemotherapy,30 a large proportion of younger patients receive therapy.31–34 Studies have found racial differences in survival times of younger adults diagnosed with CRC.21, 35 Although these analyses are often limited by lack of detailed treatment information, blacks diagnosed with stage 2 CRC were 60% more likely to die of their disease compared with young non-Hispanic whites.35 If younger patients with CRC have no survival advantage, despite more aggressive treatment, this could mean that young patients with lower risk-disease (limited nodal involvement, stage 2) are over-treated, or that young patients have tumors that are more aggressive and/or respond differently to treatment regimens developed for older patients with CRC.
Pathogenesis
Genetic factors
Among all age groups, germline variants in a number of genes are associated with increased risk for CRC (Table 1). As many as 10% of unselected patients with CRC carry germline variants in genes associated with high and moderate cancer susceptibility.36 However, some of these variants have not been previously associated with CRC (such as BRCA1, BRCA2, PALB2, ATM, NBN, CHEK2, BARD1, BRIP1), and their role in pathogenesis and associated risk are unclear. Genetic predisposition for early-onset CRC appears to differ from that of older-onset disease.
Table 1.
Genes containing variants associated with CRC risk
| Genes | Hereditary Syndrome | Penetrance1 | Population Prevalence | Proportion of CRC (all ages) | Lifetime risk of CRC |
|---|---|---|---|---|---|
| DNA mismatch repair (MLH1, MSH2, MSH6, PMS2, EPCAM) | Lynch syndrome | High | 1 in 279 | 3% | 25–75% |
| APC | Familial adenomatous polyposis | High | 3 in 10,000 | 1% | 90–100% |
| MUTYH | MUTYH-associated polyposis (biallelic autosomal recessive) | High | 1 in 100 | 1% | 40–90% |
| Monoallelic MUYH | Low | 1 in 100 | 3% | 5–10% | |
| SMAD4, BMPR1A | Juvenile polyposis | High | 1 in 100,000 | <0.1% | 36–68% |
| PTEN | Cowden/ multiple hamartoma syndrome | High | 1 in 200,000 | <0.1% | 9–16% |
| STK11 | Peutz Jeghers syndrome | High | 1 in 50,000 – 200,000 | <0.1% | 39% |
| POLE, POLD1 | Polymerase proofreading associated polyposis | High | Unknown | <0.1% | Unknown |
| NTHL1, MSH3 | Autosomal recessive polyposis | Moderate/high | Unknown | <0.1% | Unknown |
| TP53 | Li Fraumeni syndrome | High | 1 in 5,000 – 20,000 | <1% | 10–20% |
| CHEK2 | Moderate/low | 2 in 100 | <1% | 5–10% |
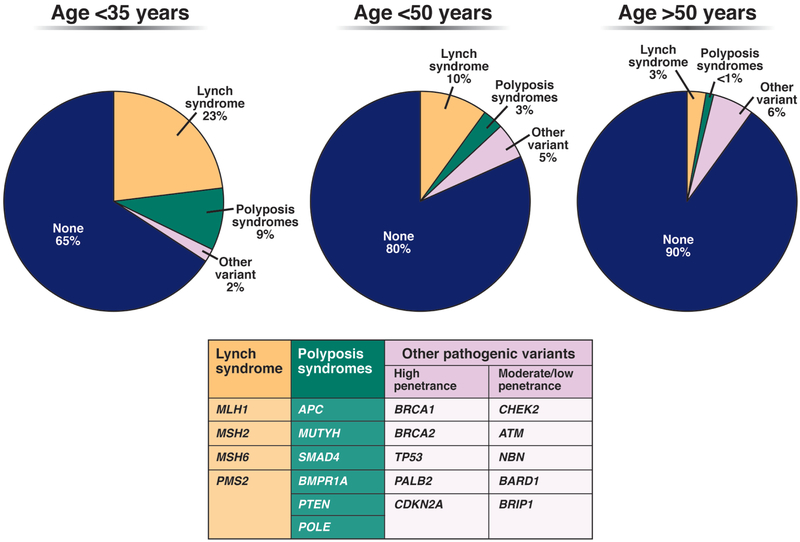
Specifically, younger patients have nearly double the prevalence (17%–35%) of pathogenic germline variants, and approximately half of these mutations are in DNA mismatch repair (MMR) genes associated with Lynch syndrome (Figure 3).19, 37, 38 Population-based studies conducted in the US and Iceland estimate the population prevalence of Lynch Syndrome at approximately 1 in 279–300, and most individuals remain undiagnosed and unaware of their increased risk for cancer.39, 40 Although universal testing of CRC tumors for MMR deficiency (MMRd) has been instrumental in identifying persons with Lynch Syndrome, tumor phenotypes vary—especially in patients with germline mutations in MSH6 and PMS2. Constitutional MMR deficiency (CMMRd), resulting from biallelic germline mutations in MMR genes, has been implicated in development of CRC in very young patients (children and adolescents).41
Figure 3:
Prevalence of pathogenic variants by age at CRC diagnosis. Based on findings reported in19, 36, 37, 131
Genes that contain pathogenic variants by age at CRC diagnosis
Familial adenomatous polyposis, associated with germline mutations in APC, is perhaps the most easily recognizable of the hereditary syndromes. However, it is important to note that as many as half of patients with CRC found to have a pathogenic germline variant do not meet clinical diagnostic criteria for the corresponding hereditary syndrome.19, 38 Although clinical practice guidelines deferred genetic testing for young patients without a family history or a polyposis phenotype, guidelines now recommend multigene panel testing of all young patients with CRC.42
Non-syndromic genetic factors
Multigene panel tests did not identify a germline mutation in approximately 80% of individuals with early-onset CRC. Whole-exome and whole-genome sequencing of large cohorts of patients with early-onset CRC have not identified common or rare genetic events associated with CRC with high penetrance. Genome-wide association studies are underway to investigate the potential impact of common variants associated with small (yet statistically significant) increases in CRC.43–47 Modeling studies found that adding these common variants to CRC risk prediction models improved model performance compared to using family history alone.48
Molecular and histopathologic features
Large-scale tumor profiling initiatives have demonstrated that CRC is a heterogeneous disease. Although early-onset CRC is often underrepresented in large cohorts, including the Cancer Genome Atlas,49 studies have found these cancers to have features that are distinct from those of older-onset, sporadic CRCs. Young adults diagnosed with CRC are more likely to have poorly differentiated tumors with features such as signet ring cell histology, lymphovascular invasion, and perineural invasion than older adults with CRC. Many of these features are associated with more aggressive tumors and worse prognosis.20, 50 Although MMRd tumors are slightly more prevalent in patients with early-onset CRC, most tumors are microsatellite stable and have chromosome stability.
Analyses of colorectal tumors from multiple cohorts have identified striking differences in mutation and molecular profiles among patients with different ages of CRC diagnosis. Compared with tumors from older patients, few colorectal tumors from young patients have the somatic BRAF encoding V600E, whereas the prevalence of mutations in other genes involved in the MAPK pathway appears to increase with patient age.18 Wnt pathway dysregulation is a common feature of non-hypermutated colorectal tumors, yet somatic mutations in APC are detected less frequently in early onset tumors compared to those from older patients.51 Tumors from very young patients (younger than 30 years) have an increased prevalence of somatic mutations in CTNNB1.18
The prevalence of different tumor subtypes, based on molecular features, also appears to vary with age. The highest proportion of consensus molecular subtype-1 (microsatellite instability/immune) tumors are found in adults younger than age 40 years.18 Although this molecular subtype encompasses hypermutated and MMRd tumors, it also includes neoplasms with markers of inflammation and/or the immune response. This observation supports the concept that longstanding colon inflammation contributes to their pathogenesis.
Epigenetic alterations, such as changes in DNA methylation patterns, have also been associated with early-onset CRC. Hypomethylation at long interspersed nuclear elements has been detected in a significantly higher proportion of tumors from young patients with non-hereditary CRC than from older patients. Hypomethylation at these elements has been associated with shorter survival times of patients with CRC and other cancers.52, 53 The distinct molecular features of early-onset colorectal tumors indicates that they have unique mechanisms of pathogenesis. These features might be used in determining patient prognoses and selecting treatment.
Mechanisms of pathogenesis
The reasons for the increasing incidence of early-onset CRC are poorly understood. Differences in clinical presentation and tumor phenotypes raise the question of whether early-onset CRC is a different disease, with a different mechanisms of pathogenesis from CRC in older adults. Although family history and/or hereditary cancer syndromes account for some cases of early-onset CRC, lifestyle and environmental factors are also likely to contribute. Increases in incidence have occurred more rapidly than can be accounted for by changes in population genetics.13
Some risk factors have been associated with CRC at all ages (Table 2). These include obesity, smoking, alcohol, red or processed meat, non-steroidal inflammatory drugs (including aspirin), diet, micronutrients (such as calcium or vitamin D), physical activity, and chronic conditions (such as diabetes). Only a few studies have examined their effects on risk for early-onset CRC.54–59 No study has examined the effect of diet, micronutrients, or non-steroidal inflammatory drugs, arguably some of the more important risk factors to study in early-onset CRC because their distribution in the population has shifted as incidence rates have increased. It is therefore important to conduct population-based studies to identify lifestyle-related and environmental risk factors associated with early-onset CRC, informed by trends in incidence.
Table 2.
Lifestyle-related and environmental risk factors associated with CRC and relationship with early-onset disease
| Risk factor (direction of risk) | Hypothesized mechanism | Supporting evidence | Studied in earlyonset CRC? |
|---|---|---|---|
| Established risk factors | |||
| Obesity (+) | Metabolic syndrome; insulin resistance; chronic inflammation; altered levels of adipocytokines134 | Meta-analyses show association between adiposity (including measures of body mass index, waist circumference, and waist-to-hip ratio) and CRC60, 135, 136 | See ref.54, 59 |
| Smoking (+) | Direct ingestion of or indirect exposure (via circulatory system) to known carcinogens contained in tobacco products137 | Meta-analyses demonstrate association between cigarette smoking and CRC incidence and mortality,138, 139 as well as risk of adenomas140 | See ref.59 |
| Alcohol (+) | Consumption adversely affects folate metabolism; genotoxic effects of acetaldehyde141 | Meta-analyses of cohort and case-control studies report moderately increased risk of CRC associated with alcohol consumption142, 143 | See ref.57 |
| Red or processed meat (+) | Consumption induces N-nitroso-compound formation; contains heterocyclic aromatic amines and polycyclic aromatic hydrocarbons, known carcinogenic chemicals144 | Prospective cohort and case-control studies demonstrate CRC associated with high vs. low consumption of red and processed meat145, 146 | See ref.57 |
| Aspirin/ NSAID (−) | Inhibits cyclooxygenase and phospholipase activity, enzymes involved in tumor growth and intracellular signaling147 | Decreases incidence of CRC148 and adenomas149 in randomized trials conducted in U.S. and Europe | -- |
| Dietary patterns (+) | Synergistic effect of highly correlated nutrients and other compounds in food, including vitamins, carotenoids, calcium, and folate; chronic inflammation150 | Some studies show “western” diets (more processed foods, sugar, fat, and refined grains) increase risk,151–153 although findings are inconsistent across all studies | See ref.154 |
| Micronutrients (−) | Calcium inhibits carcinogenic effects of bile acids in colorectum; Vitamin D lowers risk via antiproliferative, proapoptotic, and anti-angiogenic properties155 | Calcium156 decreases incidence of colorectal adenomas in randomized trials; inconsistent evidence supporting effect of vitamin D or folate | -- |
| Physical activity (−) | Less weight gain and body fatness; lower insulin levels and inflammation; stimulate digestion and reduce transit time85 | Prospective cohort and case-control studies show reduced risk of colon cancer among physically active men and women;82, 83 fewer data support association with rectal cancer103 | See ref.89, 157 |
| Diabetes (+) | Insulin resistance; chronic insulin therapy; shared risk factors (e.g., obesity); elevated concentration of fecal bile acids71 | Meta-analyses of case-control and cohort studies demonstrates 30% increase in CRC risk among those with type I and II diabetes71, 158 | See ref.59 |
| Inflammatory bowel disease (+) | Chronic inflammation and altered immune response; oxidative stress; microbiota-induced carcinogenesis159 | Large cohort studies report increased risk of CRC among persons with IBD; risk increases with duration and severity of symptoms160 | -- |
| Other possible risk factors | |||
| Antibiotics (+) | Alters patterns of microbiota assembly;161–164 may give rise to biological pathways initiating or promoting CRC97, 165, 166 | Antibiotic use in adults associated with increased risk of CRC (ages ≥50 years) in U.S.90 and European91, 92 studies | -- |
| Birth weight (+) | Pregnancy hormones (e.g., growth and steroid hormones, insulin) associated with birth size;68 large birth size associated with increased risk of obesity in adulthood167, 168 | Prospective cohort studies in Nordic countries report association between birth size and CRC68, 169, 170 | -- |
| Childhood obesity (+) | Increased body fatness in childhood and adolescence associated with higher insulin levels;171 obesity in childhood correlated with body fatness in adulthood172 | Early life body fatness increases risk of CRC in large, prospective cohort studies63,173 | See ref.54 |
| Cesarean delivery (?) | Thwarts vertical transmission of vaginal and gut microbiota from mother to infant111, 174; delivery by C-section increases risk of metabolic diseases175 and obesity176 | -- | -- |
| Breastfeeding (?) | Initial breastfeeding reduces risk of obesity later in life;177 influences composition of infant microbiome178 | -- | -- |
| Infectious agents (?) | Colon is frequently exposed to both pathogenic and nonpathogenic viruses, including H. pylori, HPV, and JC virus | Inconsistent evidence support the effect of infectious agents on risk of CRC (reviewed in110) | -- |
Birth cohort effects (see Figure 2) reveal the importance of reconsidering the timing and duration of well-established risk factors, such as obesity. Rather than assessing risk factors occurring in the few years before diagnosis, we might need to study these risk factors across a lifetime. Researchers have proposed that obesity might account for the increasing incidence of early-onset CRC.60 However, only 10% of CRCs in all age groups can be attributed to obesity in adulthood.61 Examining obesity during vulnerable windows of growth and development (such as birth weight62 or childhood obesity63, 64) might provide more information on risk factors for early-onset CRC and identify periods of exposure that have the greatest effects on risk. Childhood obesity has increased by more than 200% since the early 1960s.65–67 Although evidence is limited, studies have found a high body mass index (BMI) during childhood and/or puberty to increase risk of CRC.64 Others have demonstrated a J-shape relationship between birth weight and subsequent CRC, whereby the lowest (<2000 g) and highest (>4000 g) weights are associated with CRC.68
Adult-onset obesity, which has a shorter latent period, might also contribute to risk. For example, weight gain during young adulthood (vs maintaining stable weight) is associated with a higher risk of CRC, and weight loss during this period is associated with lower risk.69 Accumulating abdominal fat in adulthood appears to have a similar effect on risk.70 A recent analysis of data from the Nurses’ Health Study 2 reported BMI at age 18 years, as well as weight gain since adolescence, were associated with higher risk of early-onset CRC compared to normal body weight.54
The prevalence of chronic conditions associated with CRC, including type 2 diabetes71 and IBD,72 has increased in the U.S.,73, 74 and there is some concern that these conditions now occur at a greater frequency in youth and young adults.75, 76 For example, mean age at diabetes diagnosis decreased from 52 years in the late 1980s to 46 years in the early 2000s.75 Incidence of pediatric-onset IBD has nearly doubled since the late 1980s.76, 77 Eosinophilic gastrointestinal disorders have also increased during this period,78 particularly among children and young adults. Increasing prevalence may be an artifact of changes in diagnostic criteria, but younger age at diagnosis may also reflect a true population trend in conditions marked by inflammation and injury. This is important because, at least in patients with IBD, the risk of CRC increases with duration, extent, and degree of colon inflammation,79, 80 indicating an effect of altered immune regulation or surveillance. For persons with diabetes, prolonged exposure to hyperinsulinemia can shift the age-related acceleration in CRC incidence to an earlier age.81 Only 1 study59 has examined the association of early-onset CRC and diabetes; in a large population of Koreans undergoing colonoscopy examinations, those with younger than age 50 years and with type 2 diabetes had a modest increase in risk of CRC and advanced adenomas.
Among all age groups, studies82–84 have found a reduced risk of CRC among physically active men and women, although the association appears stronger for colon cancer compared to rectal cancer. Risk reduction may occur through several mechanisms: less weight gain and body fatness, lower insulin levels and inflammation, and reduced transit time.85 Prevalence of physical activity has remained consistent in the US over time (at about 25%),86, 87 but sedentary behavior has increased, largely driven by deskbound working hours.88 Evidence from the Nurses’ Health Study 2 indicates that sedentary television viewing time (>14 hours per week) increases risk of early-onset CRC,89 even after adjusting for confounding effects of physical activity, BMI, and smoking.
Possible risk factors
Although not specific to early-onset CRC, several prospective cohort and case-control studies have associated antibiotic use with advanced adenomas90 and CRC (in all age groups91, 92), mediated by their effect on gut microbiota. The human intestine contains a diverse community of microbes that affect health.93 Diet and environmental exposures determine their diversity, and alterations in microbiota induce changes in gene expression, metabolism, and local and systemic immune responses.94 In the US, use of broad-spectrum antibiotics nearly tripled through the 1980s,95 due to inappropriate prescriptions for ear and upper respiratory infections in children.96 Antibiotics given at weaning to mice increase adiposity and alter the composition of intestinal microbiota.97 Other studies show even small doses of antimicrobial agents affect the microbiota. For example, brief exposure to triclosan, an antimicrobial agent added to hundreds of consumer products in the 1970s,98 causes inflammation, increases colitis, and exacerbates colitis-associated cancer in mice.99
Beyond antibiotics, other dysbiosis-related factors, such as periodontal disease, modestly increase risk of CRC, so pathogens might be involved in carcinogensis.100 Mucosal colonization with specific pathogens (including fusobacterium nucleatum) has been associated with CRC risk, and other organisms might modify risk, either individually or in aggregate.101 We have only recently begun to understand the complexity of intestinal microbiota, but studies of microbiota-related risk factors might increase our understanding of early-onset CRC.
Risk factors more strongly associated with rectal cancer and increasing in prevalence are likely to contribute to development of early-onset CRC. Studies that examined the risk of colon and rectal cancer separately found that family history, obesity, physical activity, and smoking had different effects on risk.102, 103 The reasons for these differences are poorly understood. Concentrations of bile salts and metabolites, level of oxygenation, and microbial environments vary along the colorectum.104 These might affect susceptibility to risk factors and create better or worse conditions for carcinogenesis.105 There are different embryonic origins of the proximal (midgut) colon and distal (hindgut) colorectum,106 which might also affect carcinogenesis. For example, the right colon is exposed to bile acids and other metabolites that have escaped the small intestine, and in a form that the left colon is not, after bacterial action on metabolites. Unrecognized toxins, either dietary or environmental, might also have systemic and/or local effects on rectal mucosa.
Studies of racial and ethnic differences in the prevalence of risk factors might provide additional insights into development of early-onset CRC. For example, blacks have more constant exposure to type 2 diabetes75 and childhood obesity67 compared with the increases in exposure that have only recently occurred among whites. Young Hispanics have a greater burden of other gastrointestinal cancers (such as liver or gastric cancer) compared with non-Hispanic whites,107 and shared risk factors among these cancers, such as metabolic syndrome,108 could account for the rapidly increasing incidence rates. Immune responses and inflammation differ among races and ethnicities (stronger lymphocytic reactions in tumors of African Americans109). Monitoring changes in the relative presence or absence of risk factors and differences in tumor molecular phenotypes by race/ethnicity will increase our understanding early-onset CRC, particularly as the demographic landscape of the US continues to evolve.
Despite evidence supporting the association of lifestyle-related risk factors with early-onset CRC, case studies have shown that many young adults with CRC led previously active and healthy lives. Studying risk factors not traditionally considered to be associated CRC, or with limited evidence (Table 2), could provide additional clues of pathogenesis. For example, given the number of microbes found in the colorectum, infectious agents (such as human papillomavirus or Helicobacter pylori) associated with other types of cancer might also contribute to early-onset CRC.110 Other microbiota-related events, such as cesarean delivery, pre-and perinatal antibiotics, or breastfeeding could create an imbalance of infants’ microbiome111 that affect susceptibility to CRC in young adulthood. These factors112 have also increased among generations at higher risk of early-onset CRC.113 Finally, some have suggested environmental toxins may be related to early-onset disease, although evidence is still very limited.114
Treatment and Prevention
Reducing incidence
Because CRC screening has demonstrated benefits for older adults (age >50 years),115 the American Cancer Society recently recommended lowering the age to initiate screening to 45 years.116 In addition to simulation modelling, the American Cancer Society based its recommendation on the observation that CRC risk among persons 45–49 year old is now similar to that of persons 50–54 years old in the early 1990s. The implications of widening the age-eligible screening pool has generated controversy for several reasons. It is important to recognize that the large relative increase in early-onset CRC corresponds to a smaller absolute increase in incidence.117 Incidence increased by 30% among 40-year old persons from 1992 to 2015, and the absolute difference in incidence rates over the same period is only 8.2 cases per 100,000. It has been estimated that lowering the screening age will result in an additional 22 million persons eligible, overwhelming endoscopic capacity, incurring substantial costs, and perhaps diverting resources away from older adults more likely to benefit.118–122 We lack empirical data supporting the efficacy of screening younger adults; most of the landmark screening trials are limited to adults older than 50 years. This makes it difficult to determine expected benefits and harms, yield, and test performance. Earlier screening may exacerbate disparities because younger adults most likely to be screened (health-seeking and early adopters) are also unlikely to belong to racial/ethnic and socioeconomic subgroups at higher risk.121 Finally, half of persons with early-onset CRC are younger than age 45 years, so lowering the screening age will provide little or no benefit to these patients.
Improving risk assessment
Because we lack evidence on the benefits and harms of initiating screening at age 45 years (vs 50 years), we should instead focus on improving how we define persons at higher risk. Precision cancer screening uses a combination of genetic factors, environmental and lifestyle exposures, and prior screening to determine the expected benefit of screening for any individual person.123 Better risk assessment – and personalized screening regimens based on risk – may improve the ratio of benefit to harm over conventional age-based screening strategies. For example, risk prediction models43, 124 that include information on genetics and environmental risk factors determine the starting age for screening with greater accuracy than models including family history or age alone. The starting age in these models ranges from 38 years for men with a family history of CRC and higher risk score to 71 years for women with no family history and lower risk score.124 Risk prediction models may also inform choice of screening test (such as colonoscopy vs FIT).125 For example, because younger adults have proportionately more left-sided cancers, stool-based tests or sigmoidoscopy may be an appropriate, less invasive option. As genetic data become increasingly available, and we learn more about environmental risk factors of early-onset CRC, we will have opportunities to develop and validate risk prediction models that expand precision screening efforts. In the meantime, improving recognition of family history (of CRC and advanced adenomas),126–128 IBD, and red flag symptoms may facilitate earlier screening for individuals at higher risk of early-onset CRC.
Future Directions
Incidence rates of early-onset CRC have increased in the US and across much of the West since the 1990s, for unknown reasons. Although genetic factors can affect risk of early-onset CRC, most young adults diagnosed with CRC have no hereditary syndrome or germline mutation associated with CRC,19 and traditional clinical criteria (family history, tumor phenotype) fail to identify those at higher risk.129, 130 Many patients lack the classic family history or disease phenotype and/or have genetic mutations that have not been associated with CRC.129
Genetic testing of all patients diagnosed with early-onset CRC could guide treatment and facilitate testing and earlier cancer screening for at-risk relatives. Beyond genetic factors, increases in incidence by birth cohort indicate that environmental and lifestyle-related risk factors, particularly exposures during early life, contribute to early-onset CRC. Further studies of genetic and molecular features of colorectal tumors, as well as the effects of exposures on colorectal mucosa, will provide additional information about carcinogenesis and new treatment approaches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no conflicts of interest or financial disclosures.
References
- 1.Murphy CC, Sandler RS, Sanoff HK, et al. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clin Gastroenterol Hepatol 2017;15:903–909.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg 2015;150:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stat S.
- 5.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Ann Epidemiol 2015;25:208–213.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Colorectal Cancer Mortality Rates in Adults Aged 20 to 54 Years in the United States, 1970–2014. Jama 2017;318:572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whatley Z, Daram SR, Yousuf S, et al. Gastrointestinal cancers in Mississippi. South Med J 2014;107:229–34. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Sahar L, Robbins A, et al. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev 2015;24:1151–6. [DOI] [PubMed] [Google Scholar]
- 10.James W, Jia C, Kedia S. Uneven magnitude of disparities in cancer risks from air toxics. Int J Environ Res Public Health 2012;9:4365–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Zhang L, Penman A, et al. Using small-area estimation method to calculate county-level prevalence of obesity in Mississippi, 2007–2009. Prev Chronic Dis 2011;8:A85. [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson N, Shelton BJ, Hopenhayn C, et al. Concentrations of arsenic, chromium, and nickel in toenail samples from Appalachian Kentucky residents. J Environ Pathol Toxicol Oncol 2011;30:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy CC, Singal AG, Baron JA, et al. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner DR, Ruan Y, Shaw E, et al. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med 2017;105:345–349. [DOI] [PubMed] [Google Scholar]
- 15.Feletto E, Yu XQ, Lew JB, et al. Trends in Colon and Rectal Cancer Incidence in Australia from 1982 to 2014: Analysis of Data on Over 375,000 Cases. Cancer Epidemiol Biomarkers Prev 2019;28:83–90. [DOI] [PubMed] [Google Scholar]
- 16.Chung RY, Tsoi KKF, Kyaw MH, et al. A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer Epidemiol 2019;59:29–36. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Land KC. Age-period-cohort analysis: new models, methods, and empirical applications: CRC Press, 2013. [Google Scholar]
- 18.Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoffel EM, Koeppe E, Everett J, et al. Germline Genetic Features of Young Individuals With Colorectal Cancer. Gastroenterology 2018;154:897–905.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012;25:1128–39. [DOI] [PubMed] [Google Scholar]
- 21.Murphy CC, Wallace K, Sandler RS, et al. Racial Disparities in Incidence of Young-Onset Colorectal Cancer and Patient Survival. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy CC, Sanoff HK, Stitzenberg KB, et al. Patterns of Sociodemographic and Clinicopathologic Characteristics of Stages II and III Colorectal Cancer Patients by Age: Examining Potential Mechanisms of Young-Onset Disease. J Cancer Epidemiol 2017;2017:4024580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia S, Pruitt SL, Singal AG, et al. Colorectal cancer incidence among Hispanics and non-Hispanic Whites in the United States. Cancer Causes Control 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang DY, Thrift AP, Zarrin-Khameh N, et al. Rising Incidence of Colorectal Cancer Among Young Hispanics in Texas. J Clin Gastroenterol 2017;51:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schellerer VS, Merkel S, Schumann SC, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer : CRC in patients under 50 years of age. Int J Colorectal Dis 2012;27:71–9. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CC, Harlan LC, Lund J, et al. Patterns of colorectal cancer care in the United States: 1990–2010. J Natl Cancer Inst 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kneuertz PJ, Chang GJ, Hu CY, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg 2015;150:402–9. [DOI] [PubMed] [Google Scholar]
- 28.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer 2016;122:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manjelievskaia J, Brown D, McGlynn KA, et al. Chemotherapy Use and Survival Among Young and Middle-Aged Patients With Colon Cancer. JAMA Surg 2017;152:452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson AB 3rd, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw 2014;12:1028–59. [DOI] [PubMed] [Google Scholar]
- 31.Quah HM, Joseph R, Schrag D, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol 2007;14:2759–65. [DOI] [PubMed] [Google Scholar]
- 32.Schrag D, Cramer LD, Bach PB, et al. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst 2001;93:850–7. [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick HM, Aitelli CL, Qin H, et al. Referral patterns and adjuvant chemotherapy use in patients with stage II colon cancer. Clin Colorectal Cancer 2010;9:150–6. [DOI] [PubMed] [Google Scholar]
- 34.Wirtzfeld DA, Mikula L, Gryfe R, et al. Concordance with clinical practice guidelines for adjuvant chemotherapy in patients with stage I-III colon cancer: experience in 2 Canadian provinces. Can J Surg 2009;52:92–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol 2016;34:2148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer Susceptibility Gene Mutations in Individuals With Colorectal Cancer. J Clin Oncol 2017;35:1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol 2017;3:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mork ME, You YN, Ying J, et al. High Prevalence of Hereditary Cancer Syndromes in Adolescents and Young Adults With Colorectal Cancer. J Clin Oncol 2015;33:3544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851–60. [DOI] [PubMed] [Google Scholar]
- 40.Haraldsdottir S, Rafnar T, Frankel WL, et al. Comprehensive population-wide analysis of Lynch syndrome in Iceland reveals founder mutations in MSH6 and PMS2. Nat Commun 2017;8:14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Durno CA, Holter S, Sherman PM, et al. The gastrointestinal phenotype of germline biallelic mismatch repair gene mutations. Am J Gastroenterol 2010;105:2449–56. [DOI] [PubMed] [Google Scholar]
- 42.NCCN. Genetic/Familial High Risk Assessment: Breast and/or Ovarian and Colorectal. NCCN Clinical Practice Guidelines in Oncology 2018; [Google Scholar]
- 43.Hsu L, Jeon J, Brenner H, et al. A model to determine colorectal cancer risk using common genetic susceptibility loci. Gastroenterology 2015;148:1330–9.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huyghe JR, Bien SA, Harrison TA, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet 2019;51:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemire M, Qu C, Loo LWM, et al. A genome-wide association study for colorectal cancer identifies a risk locus in 14q23.1. Hum Genet 2015;134:1249–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmit SL, Edlund CK, Schumacher FR, et al. Novel Common Genetic Susceptibility Loci for Colorectal Cancer. J Natl Cancer Inst 2019;111:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hang D, Joshi AD, He X, et al. Colorectal cancer susceptibility variants and risk of conventional adenomas and serrated polyps: results from three cohort studies. Int J Epidemiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon J, Du M, Schoen RE, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology 2018;154:2152–2164 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol 2009;33:572–82. [DOI] [PubMed] [Google Scholar]
- 51.Xicola RM, Manojlovic Z, Augustus GJ, et al. Lack of APC somatic mutation is associated with early-onset colorectal cancer in African Americans. Carcinogenesis 2018;39:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antelo M, Balaguer F, Shia J, et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS One 2012;7:e45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinberg AP. The Key Role of Epigenetics in Human Disease Prevention and Mitigation. N Engl J Med 2018;378:1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol 2019;5:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen LH, Liu PH, Zheng X, et al. Sedentary Behaviors, TV Viewing Time, and Risk of Young-Onset Colorectal Cancer. JNCI Cancer Spectr 2018;2:pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imperiale TF, Kahi CJ, Stuart JS, et al. Risk factors for advanced sporadic colorectal neoplasia in persons younger than age 50. Cancer Detect Prev 2008;32:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosato V, Bosetti C, Levi F, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control 2013;24:335–41. [DOI] [PubMed] [Google Scholar]
- 58.Jung YS, Ryu S, Chang Y, et al. Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years. Gastrointest Endosc 2015;81:637–645.e7. [DOI] [PubMed] [Google Scholar]
- 59.Kim JY, Jung YS, Park JH, et al. Different risk factors for advanced colorectal neoplasm in young adults. World J Gastroenterol 2016;22:3611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533–47. [DOI] [PubMed] [Google Scholar]
- 61.Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin 2019;69:88–112. [DOI] [PubMed] [Google Scholar]
- 62.Smith NR, Jensen BW, Zimmermann E, et al. Associations between birth weight and colon and rectal cancer risk in adulthood. Cancer Epidemiol 2016;42:181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang X, Wu K, Giovannucci EL, et al. Early life body fatness and risk of colorectal cancer in u.s. Women and men-results from two large cohort studies. Cancer Epidemiol Biomarkers Prev 2015;24:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Celind J, Ohlsson C, Bygdell M, et al. Childhood Body Mass Index is associated with risk of adult colon cancer in men-an association modulated by pubertal change in Body Mass Index. Cancer Epidemiology and Prevention Biomarkers 2019:cebp. 1077.2018. [DOI] [PubMed] [Google Scholar]
- 65.Troiano RP, Flegal KM, Kuczmarski RJ, et al. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med 1995;149:1085–91. [DOI] [PubMed] [Google Scholar]
- 66.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogden CL, Carroll MD, Lawman HG, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988–1994 Through 2013–2014. Jama 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandhu MS, Luben R, Day NE, et al. Self-reported birth weight and subsequent risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2002;11:935–8. [PubMed] [Google Scholar]
- 69.Song M, Hu FB, Spiegelman D, et al. Adulthood Weight Change and Risk of Colorectal Cancer in the Nurses’ Health Study and Health Professionals Follow-up Study. Cancer Prev Res (Phila) 2015;8:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song M, Hu FB, Spiegelman D, et al. Long-term status and change of body fat distribution, and risk of colorectal cancer: a prospective cohort study. Int J Epidemiol 2016;45:871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. Journal of the National Cancer Institute 2005;97:1679–1687. [DOI] [PubMed] [Google Scholar]
- 72.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854–862. [DOI] [PubMed] [Google Scholar]
- 73.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. Jama 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 74.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet 2017;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 75.Koopman RJ, Mainous AG, Diaz VA, et al. Changes in Age at Diagnosis of Type 2 Diabetes Mellitus in the United States, 1988 to 2000. Ann Fam Med 2005;3:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghione S, Sarter H, Fumery M, et al. Dramatic Increase in Incidence of Ulcerative Colitis and Crohn’s Disease (1988–2011): A Population-Based Study of French Adolescents. Am J Gastroenterol 2018;113:265–272. [DOI] [PubMed] [Google Scholar]
- 77.Benchimol EI, Manuel DG, Guttmann A, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: a population-based cohort study of epidemiology trends. Inflamm Bowel Dis 2014;20:1761–9. [DOI] [PubMed] [Google Scholar]
- 78.Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 2009;7:1055–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol 2008;14:3937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y-X, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clinical Gastroenterology and Hepatology 2005;3:587–594. [DOI] [PubMed] [Google Scholar]
- 82.Samad A, Taylor R, Marshall T, et al. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal disease 2005;7:204–213. [DOI] [PubMed] [Google Scholar]
- 83.Wolin K, Yan Y, Colditz G, et al. Physical activity and colon cancer prevention: a meta-analysis. British journal of cancer 2009;100:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolin KY, Lee IM, Colditz GA, et al. Leisure-time physical activity patterns and risk of colon cancer in women. International Journal of Cancer 2007;121:2776–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Slattery ML. Physical activity and colorectal cancer. Sports Medicine 2004;34:239–252. [DOI] [PubMed] [Google Scholar]
- 86.Ussery EN, Fulton JE, Galuska DA, et al. Joint Prevalence of Sitting Time and Leisure-Time Physical Activity Among US Adults, 2015–2016. Jama 2018;320:2036–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prevalence of physical activity, including lifestyle activities among adults--United States, 2000–2001. MMWR Morb Mortal Wkly Rep 2003;52:764–9. [PubMed] [Google Scholar]
- 88.Clark B, Sugiyama T. Prevalence, trends, and correlates of sedentary behavior. Physical Activity, Exercise, Sedentary Behavior and Health: Springer, 2015:79–90. [Google Scholar]
- 89.Nguyen LH, Liu P-H, Zheng X, et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectrum 2019;2:pky073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 2018;67:672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dik VK, van Oijen MG, Smeets HM, et al. Frequent Use of Antibiotics Is Associated with Colorectal Cancer Risk: Results of a Nested Case-Control Study. Dig Dis Sci 2016;61:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boursi B, Haynes K, Mamtani R, et al. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf 2015;24:534–42. [DOI] [PubMed] [Google Scholar]
- 93.Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell 2012;148:1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Song M, Chan AT. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. Jama 1995;273:214–9. [PubMed] [Google Scholar]
- 96.Soyka LF, Robinson DS, Lachant N, et al. The misuse of antibiotics for treatment of upper respiratory tract infections in children. Pediatrics 1975;55:552–6. [PubMed] [Google Scholar]
- 97.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weatherly LM, Gosse JA. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev 2017;20:447–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang H, Wang W, Romano KA, et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Science translational medicine 2018;10:eaan4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Momen-Heravi F, Babic A, Tworoger SS, et al. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. International journal of cancer 2017;140:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehta RS, Nishihara R, Cao Y, et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol 2017;3:921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei EK, Colditz GA, Giovannucci EL, et al. A Comprehensive Model of Colorectal Cancer by Risk Factor Status and Subsite Using Data From the Nurses’ Health Study. Am J Epidemiol 2017;185:224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murphy N, Ward HA, Jenab M, et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carethers JM. Risk factors for colon location of cancer. Transl Gastroenterol Hepatol 2018;3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004;108:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carethers JM. One colon lumen but two organs. Gastroenterology 2011;141:411–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haile RW, John EM, Levine AJ, et al. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila) 2012;5:150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aguilar M, Bhuket T, Torres S, et al. Prevalence of the metabolic syndrome in the United States, 2003–2012. Jama 2015;313:1973–4. [DOI] [PubMed] [Google Scholar]
- 109.Wallace K, Lewin DN, Sun S, et al. Tumor Infiltrating Lymphocytes and Colorectal Cancer Survival in African American and Caucasian Patients. Cancer Epidemiol Biomarkers Prev 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev 2008;17:2970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010;107:11971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Friede A, Reid JA, Ory HW. CDC WONDER: a comprehensive on-line public health information system of the Centers for Disease Control and Prevention. American Journal of Public Health 1993;83:1289–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petitti D, Olson RO, Williams RL. Cesarean section in California--1960 through 1975. Am J Obstet Gynecol 1979;133:391–7. [DOI] [PubMed] [Google Scholar]
- 114., In Fifth Annual Early Age Onset Colorectal Cancer Summit, New York, NY, 2019. [Google Scholar]
- 115.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. Jama 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
- 117.Murphy CC, Sanoff HK, Stitzenberg KB, et al. RE: Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Imperiale TF, Kahi CJ, Rex DK. Lowering the Starting Age for Colorectal Cancer Screening to 45 Years: Who Will Come…and Should They? Clin Gastroenterol Hepatol 2018;16:1541–1544. [DOI] [PubMed] [Google Scholar]
- 119.Anderson JC, Samadder JN. To Screen or Not to Screen Adults 45–49 Years of Age: That is the Question. Am J Gastroenterol 2018;113:1750–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bretthauer M, Kalager M, Weinberg DS. From Colorectal Cancer Screening Guidelines to Headlines: Beware! Ann Intern Med 2018;169:405–406. [DOI] [PubMed] [Google Scholar]
- 121.Liang PS, Allison J, Ladabaum U, et al. Potential Intended and Unintended Consequences of Recommending Initiation of Colorectal Cancer Screening at Age 45 Years. Gastroenterology 2018;155:950–954. [DOI] [PubMed] [Google Scholar]
- 122.Ladabaum U, Mannalithara A, Meester RGS, et al. Cost-Effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-Risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marcus PM, Pashayan N, Church TR, et al. Population-Based Precision Cancer Screening: A Symposium on Evidence, Epidemiology, and Next Steps. Cancer Epidemiol Biomarkers Prev 2016;25:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeon J, Du M, Schoen RE, et al. Determining Risk of Colorectal Cancer and Starting Age of Screening Based on Lifestyle, Environmental, and Genetic Factors. Gastroenterology 2018;154:2152–2164.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robertson DJ, Ladabaum U. Opportunities and Challenges in Moving From Current Guidelines to Personalized Colorectal Cancer Screening. Gastroenterology 2019;156:904–917. [DOI] [PubMed] [Google Scholar]
- 126.Luba DG, DiSario JA, Rock C, et al. Community Practice Implementation of a Self-administered Version of PREMM1,2,6 to Assess Risk for Lynch Syndrome. Clin Gastroenterol Hepatol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guivatchian T, Koeppe ES, Baker JR, et al. Family history in colonoscopy patients: feasibility and performance of electronic and paper-based surveys for colorectal cancer risk assessment in the outpatient setting. Gastrointest Endosc 2017;86:684–691. [DOI] [PubMed] [Google Scholar]
- 128.Dinh TA, Rosner BI, Atwood JC, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila) 2011;4:9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vilar E, Stoffel EM. Universal Genetic Testing for Younger Patients With Colorectal Cancer. JAMA Oncol 2017;3:448–449. [DOI] [PubMed] [Google Scholar]
- 130.Stoffel EM. Colorectal Cancer in Young Individuals: Opportunities for Prevention. J Clin Oncol 2015;33:3525–7. [DOI] [PubMed] [Google Scholar]
- 131.Mork ME, You YN, Ying J, et al. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. Journal of Clinical Oncology 2015;33:3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223–62; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Easton DF, Pharoah PD, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 2015;372:2243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62:933–947. [DOI] [PubMed] [Google Scholar]
- 135.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556–65. [DOI] [PubMed] [Google Scholar]
- 136.Hong S, Cai Q, Chen D, et al. Abdominal obesity and the risk of colorectal adenoma: a meta-analysis of observational studies. Eur J Cancer Prev 2012;21:523–31. [DOI] [PubMed] [Google Scholar]
- 137.Giovannucci E An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiology and Prevention Biomarkers 2001;10:725–731. [PubMed] [Google Scholar]
- 138.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. Jama 2008;300:2765–2778. [DOI] [PubMed] [Google Scholar]
- 139.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406–15. [DOI] [PubMed] [Google Scholar]
- 140.Botteri E, Iodice S, Raimondi S, et al. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology 2008;134:388–395. e3. [DOI] [PubMed] [Google Scholar]
- 141.Boffetta P, Hashibe M. Alcohol and cancer. The lancet oncology 2006;7:149–156. [DOI] [PubMed] [Google Scholar]
- 142.Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol 2011;22:1958–72. [DOI] [PubMed] [Google Scholar]
- 143.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Annals of internal medicine 2004;140:603–613. [DOI] [PubMed] [Google Scholar]
- 144.Bouvard V, Loomis D, Guyton KZ, et al. Carcinogenicity of consumption of red and processed meat. The Lancet Oncology 2015;16:1599–1600. [DOI] [PubMed] [Google Scholar]
- 145.Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PloS one 2011;6:e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Norat T, Lukanova A, Ferrari P, et al. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. International journal of cancer 2002;98:241–256. [DOI] [PubMed] [Google Scholar]
- 147.Marnett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Research 1992;52:5575–5589. [PubMed] [Google Scholar]
- 148.Rothwell PM, Wilson M, Elwin C-E, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. The Lancet 2010;376:1741–1750. [DOI] [PubMed] [Google Scholar]
- 149.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 2009;101:256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miller PE, Lazarus P, Lesko SM, et al. Diet index-based and empirically derived dietary patterns are associated with colorectal cancer risk. J Nutr 2010;140:1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fung T, Hu FB, Fuchs C, et al. Major dietary patterns and the risk of colorectal cancer in women. Archives of internal medicine 2003;163:309–314. [DOI] [PubMed] [Google Scholar]
- 152.Williams CD, Satia JA, Adair LS, et al. Dietary patterns, food groups, and rectal cancer risk in Whites and African-Americans. Cancer Epidemiol Biomarkers Prev 2009;18:1552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shivappa N, Prizment AE, Blair CK, et al. Dietary inflammatory index and risk of colorectal cancer in the Iowa Women’s Health Study. Cancer Epidemiology and Prevention Biomarkers 2014;23:2383–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zheng X, Nguyen LH, Liu P-H, et al. 983–Comprehensive Assessment of Diet Quality and Risk of Early-Onset Colorectal Adenoma. Gastroenterology 2019;156:S-208. [Google Scholar]
- 155.McCullough ML, Zoltick ES, Weinstein SJ, et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts. J Natl Cancer Inst 2019;111:158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baron J, Beach Mf, Mandel J, et al. Calcium supplements for the prevention of colorectal adenomas. New England Journal of Medicine 1999;340:101–107. [DOI] [PubMed] [Google Scholar]
- 157.Peters RK, Garabrant DH, Yu MC, et al. A case-control study of occupational and dietary factors in colorectal cancer in young men by subsite. Cancer Res 1989;49:5459–68. [PubMed] [Google Scholar]
- 158.Deng L, Gui Z, Zhao L, et al. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Digestive diseases and sciences 2012;57:1576–1585. [DOI] [PubMed] [Google Scholar]
- 159.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol 2014;20:9872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Munkholm P The incidence and prevalence of colorectal cancer in inflammatory bowel disease. Alimentary pharmacology & therapeutics 2003;18:1–5. [DOI] [PubMed] [Google Scholar]
- 161.Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015;6:7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014;158:705–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006;118:511–521. [DOI] [PubMed] [Google Scholar]
- 164.Fouhy F, Guinane CM, Hussey S, et al. High-throughput sequencing reveals the incomplete, short-term, recovery of the infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamycin. Antimicrobial agents and chemotherapy 2012:AAC. 00789–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jernberg C, Lofmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010;156:3216–23. [DOI] [PubMed] [Google Scholar]
- 166.Lange K, Buerger M, Stallmach A, et al. Effects of Antibiotics on Gut Microbiota. Dig Dis 2016;34:260–8. [DOI] [PubMed] [Google Scholar]
- 167.Yu ZB, Han SP, Zhu GZ, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 2011;12:525–42. [DOI] [PubMed] [Google Scholar]
- 168.Schellong K, Schulz S, Harder T, et al. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One 2012;7:e47776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nilsen TI, Romundstad PR, Troisi R, et al. Birth size and colorectal cancer risk: a prospective population based study. Gut 2005;54:1728–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang P, He X, Wang B, et al. Birth weight and risk of colorectal cancer: a meta-analysis. International journal of colorectal disease 2014;29:1017. [DOI] [PubMed] [Google Scholar]
- 171.Cruz ML, Shaibi GQ, Weigensberg MJ, et al. Pediatric obesity and insulin resistance: chronic disease risk and implications for treatment and prevention beyond body weight modification. Annu Rev Nutr 2005;25:435–68. [DOI] [PubMed] [Google Scholar]
- 172.Cunningham SA, Kramer MR, Narayan KV. Incidence of childhood obesity in the United States. New England Journal of Medicine 2014;370:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Levi Z, Kark JD, Barchana M, et al. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1.1 million males. Cancer Epidemiol Biomarkers Prev 2011;20:2524–31. [DOI] [PubMed] [Google Scholar]
- 174.Blaser MJ, Dominguez-Bello MG. The Human Microbiome before Birth. Cell Host Microbe 2016;20:558–560. [DOI] [PubMed] [Google Scholar]
- 175.Cardwell CR, Stene LC, Joner G, et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studies. Diabetologia 2008;51:726–35. [DOI] [PubMed] [Google Scholar]
- 176.Huh SY, Rifas-Shiman SL, Zera CA, et al. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch Dis Child 2012;97:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Owen CG, Martin RM, Whincup PH, et al. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 2005;115:1367–1377. [DOI] [PubMed] [Google Scholar]
- 178.Azad MB, Bridgman SL, Becker AB, et al. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) 2014;38:1290–8. [DOI] [PubMed] [Google Scholar]