Abstract
Spinal trauma can cause simultaneous injury of intervertebral discs (IVD) and anterior longitudinal ligaments (ALL). Injury of IVD is an important factor causing intervertebral disc degeneration (IDD). However, the relationship between ALL injury and IDD has rarely been discussed. Therefore, the purpose of this study was to investigate the effects of ALL injury on degeneration of injured IVD. Thirty-two rabbits were randomly and evenly divided into four groups including sham group, Group A (simple IVD punctured), Group B (IVD punctured with half transverse injury of ALL), and Group C (IVD punctured with entirely transverse injury of ALL). Then, computed tomography, HE staining, intraoperative exploration, immunohistochemistry, and TUNEL staining were used in detecting the degenerative changes in corresponding IVD. At 2 weeks postoperatively, in response to the extent of ALL injury, the middle height of the punctured intervertebral space was reduced. The IVD structure was disorganized and the number of IVD cells was decreasing. The percentage of IL-1β- and TNF-α-immunopositive cells was increased and the percentage of TUNEL-positive IVD cells was also increased. There was a significant difference between Group C and the other groups in the results of immunohistochemistry and TUNEL staining (P<0.05). At 8 weeks postoperatively, the middle height of intervertebral space was significantly lower in Group C than in other groups (P<0.05). Intraoperative exploration found that there was obvious instability of intervertebral space in Group C. Compared with 2 weeks postoperation, the pathological changes were severe. The percentage of IL-1β- and TNF-α-immunopositive cells was decreased and the percentage of TUNEL-positive cells was increased in the corresponding groups. There was a significant difference between Group C and the other groups in the results of immunohistochemistry and TUNEL staining (P<0.05). These findings indicate that IVD injury companied with completed ALL injury might cause obvious spinal instability, which might correspond to severe IDD.
Keywords: Intervertebral disc degeneration, anterior longitudinal ligament injury, intervertebral disc injury, inflammatory cytokines, apoptosis
Introduction
Spinal trauma is one of the main causes of intervertebral disc (IVD) and spinal ligaments injury [1-5]. Many studies have reported the effects of IVD injury caused by vertebral fractures on intervertebral disc degeneration (IDD) [6-8]. However, the effects of spinal ligaments injury on IDD have rarely been discussed.
As magnetic resonance imaging (MRI) has been widely used recently in checking patients suffering from spinal trauma, more and more spinal ligaments injuries have been found such as anterior longitudinal ligament (ALL), posterior longitudinal ligament (PLL), and posterior ligamentous complex (PLC) [3-5]. All of these ligaments are important structures in maintaining spinal stability. Spinal instability is a crucial factor resulting in IDD [9-11].
ALL injury is one of the most common types of spinal ligaments injury after cervical trauma. Because ALL and IVD are closely interlinked in anatomic structure, injury of IVD often accompanies with ALL injury, especially in cervical hyperextension injury [3-5,12]. Many studies have reported that ALL injury might decrease spinal stability [13-15]. However, to the best of our knowledge, there have not been any studies focusing on the effects of ALL injury on IDD. Thus, the aim of this study was to build punctured IVD with varying degrees of ALL injury models of rabbit. This model permited further investigation of the effects of ALL injury on degeneration of injured IVD by analyzing the changes in the imaging examination, histology examination, the expression of pro-inflammatory cytokines including IL-1β and TNF-α, and apoptosis of IVD cells.
Materials and methods
Animals and groups
All experimental approaches were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The study’s approach was approved by the Institutional Animal Care Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Thirty-two healthy adult male New Zealand rabbits (10-12 weeks, weight 2.5-3.5 kg) obtained from the Animal Laboratory of Shanghai Song-Lian Co (Shanghai, China) were randomly and evenly divided into four groups. (1) sham group (n=8): exposing ALL and IVD without injury; (2) Group A (n=8): simple IVD punctured without ALL injured; (3) Group B (n=8): IVD punctured with half transverse injury of ALL; (4) Group C (n=8): IVD punctured with entirely transverse injury of ALL. The rabbits were housed individually and fed on a twelve-hour light-dark cycle with food and water available ad libitum.
Surgical approach
Before the experiments, the rabbits were routinely housed for at least one week. Rabbits were deeply anaesthetized with 3% sodium pentobarbital (1 ml/kg, ear vein injection) and placed in the supine position. The anterolateral retroperitoneal approach was used to expose the disc of the lumbar spine. After sterilization using povidone iodine, an 8 cm skin incision was cut between the iliac crest and twelfth rib, about halfway between the ventral and dorsal midline. Fascia and muscles were carefully separated. The white intervertebral disc of the L2-L3 segment was exposed. The corresponding ALL of the L2-L3 segment was carefully separated by vessel clamp, ensuring no additional injury to the anterior annulus fibrosus (AF). In the sham group, ALL and IVD were fully exposed without injury; in Group A, the ventrolateral aspect of the intervertebral disk was punctured to a depth of 3 mm by a 16G needle; in Group B, the ALL was half transversely cut with eye scissors after puncturing the IVD using the above method; in Group C, the ALL was completely transversely cut with eye scissors after puncturing the IVD using the above method (Figure 1). The abdominal wall was then closed.
Figure 1.
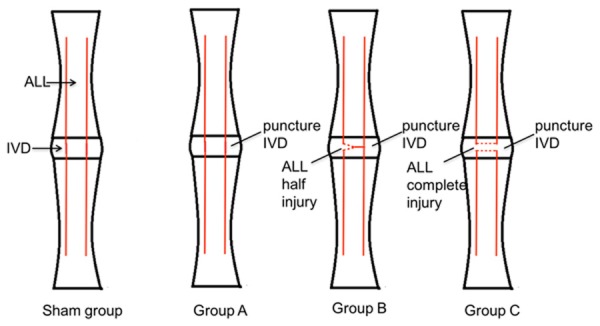
The illustrations of IVD puncture and ALL injury. Sham group: Exposing ALL and IVD without injury; Group A: Simple IVD punctured without ALL injured; Group B: IVD punctured with half transverse injury of ALL; Group C: IVD punctured with entirely transverse injury of ALL.
Examination time
Inflammation of the punctured IVD usually occurs early (within 2 weeks). In addition, according to Martin JT, IDD was apparent at 8 weeks after the puncture injury [16]. Therefore, we chose 2 weeks and 8 weeks postoperatively to conduct the following examinations.
Computed tomography (CT) scan
Computed tomography (Philips Briliance iCT, Amsterdam, Netherlands) scans of the lumbar spine were performed at 2 weeks and 8 weeks postoperatively for each rabbit and the middle height of the punctured intervertebral space was calculated on CT sagittal reconstruction images. The following technical specifications were used: 5.2-sec anatomical scan time with 0.75-sec rotation time, a 500-mm maximum diameter field of view (FOV) with 120 kV voltage and 30 mA current.
Intraoperative exploration
After intravenous anesthesia, four randomly selected rabbits in each group were routinely sacrificed by injecting air into the ear vein at 2 weeks and at 8 weeks postoperatively. Operators tugged the vertebral bodies by using forceps to confirm the motion range of punctured intervertebral space. If there was obvious increasing mobilization range of the vertebral bodies we believed that there was instability of punctured intervertebral space.
H&E staining
After intraoperative exploration, the corresponding IVD was completely removed and kept in 4% PFA for fixation overnight. After dehydration, the IVDs were embedded in paraffin. Serial coronal sections were then collected at 5 µm thickness and were further subjected to H&E staining using well established methods. Two independent observers blinded to the experiment collected images using an inverted fluorescence microscope (NIKON ECLIPSE T3-S, Tokyo, Japan).
Immunohistochemistry and imaging analysis
The paraffin-embedded sections of the IVD were subjected to immunohistochemical staining. Briefly, tissue sections of 5 µm were deparaffinized, rehydrated in a series of descending concentrations of ethanol, and were placed in an antigen retrieval solution (10 min microwave irradiation in 0.05 M tris buffer, pH 9.0). Endogenous peroxidase was blocked using 3% H2O2. After three rinses in phosphate-buffered saline for 5 minutes each, non-specific binding was blocked by incubation in 10% normal rabbit serum for 30 minutes at room temperature. Sections were incubated with primary polyclonal anti-rabbit IL-1β antibody (Abcam, Shanghai, China; 1:200 dilution) and primary polyclonal anti-rabbit TNF-α antibody (Boster, Wuhan, China; 1:200 dilution), followed by secondary goat anti-rabbit antibody (Boster, Wuhan, China; 1:1 dilution) labelled with horseradish peroxidase. Negative controls in which IgGs replaced the primary antibody at an equal protein concentration were used. Immunoreactivity was visualized using the DAB chromogenic reagent (DAKO). Sections were counterstained with haematoxylin. Two independent observers blinded to the experiment collected images using an inverted fluorescence microscope (NIKON ECLIPSE T3-S, Tokyo, Japan). At least 100 IVD cells were counted for each IVD specimen. IL-1β- and TNF-α-immunopositive cells were counted with respect to the total number of IVD cells. A positive immunohistochemical examination was reported as the percentage of immunopositive cells out of the total number of IVD cells. The mean score was obtained by two observers.
TUNEL staining
TUNEL staining examination was performed using an In Situ Apoptosis Detection Kit (Roche, Switzerland). The paraffin-embedded sections of IVD were subjected to antigen retrieval proteinase K (Roche, Switzerland) for 30 minutes, then washed 3 times with PBS. The preparation of the TUNEL solution and the staining were performed according to the assay kit’s instruction manual. Two independent observers blinded to the experiment collected images using an inverted fluorescence microscope (NIKON ECLIPSE T3-S, Tokyo, Japan). At least 100 IVD cells were counted for each IVD specimen and TUNEL-positive cells were counted with respect to the total number of IVD cells. A positive examination was counted as the percentage of TUNEL-positive cells out of the total number of IVD cells. The mean score was obtained by two observers.
Statistical analysis
Data analyses were performed using the SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and results were represented as the mean ± standard deviation (SD). A one-way analysis of variance with Bonferroni correction was performed to compare the middle height, the percentage of immunopositive cells, and the percentage of TUNEL-positive cells in corresponding IVD between all groups. Significance was accepted at P<0.05.
Results
CT examination
All experimental animals survived until they were sacrificed. At 2 weeks postoperatively, a sagittal reconstruction CT examination revealed that the middle height of the punctured intervertebral space was 1.45 ± 0.06 mm in the sham group, 1.38 ± 0.10 mm in Group A, 1.30 ± 0.08 mm in Group B, and 1.15 ± 0.13 mm in Group C. The height in Group C was significantly lower than that in sham group and Group A (P<0.05). At 8 weeks postoperatively, the middle height of the punctured intervertebral space was 1.40 ± 0.08 mm in sham group, 1.30 ± 0.12 mm in Group A, 1.10 ± 0.14 mm in Group B, and 0.83 ± 0.10 mm in Group C. The height in sham group was significantly higher than that in Group B and Group C (P<0.05) and the height in Group C was significantly lower than that in Group A and Group B (P<0.05). It is worth noting that there was calcification in Group C (Figure 2).
Figure 2.
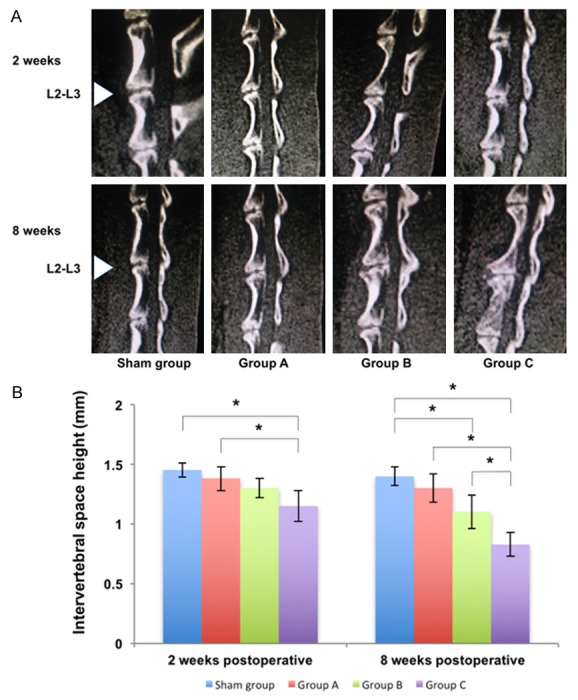
CT sagittal reconstruction images and the middle height of punctured intervertebral space in different groups at 2 weeks and 8 weeks postoperatively. A. CT sagittal reconstruction images of the rabbit lumbar spine in different groups. The intervertebral space gradually narrowed in the L2-L3 IVD from sham group to Group C. There was calcification in Group C at 8 weeks postoperatively. B. The middle height of punctured intervertebral space in different groups. There was a significant difference between Group C and other groups at 8 weeks postoperatively (*P<0.05, unit: mm).
Intraoperative exploration finding
At 2 weeks postoperatively, there was not obvious instability of punctured intervertebral space in all groups confirmed by operators. At 8 weeks postoperatively, there was not obvious instability of punctured intervertebral space in sham group, Group A, and Group B. In contrast, there was obvious instability of punctured intervertebral space in Group C confirmed by operators.
Histological examination
Sham animals did not show abnormalities of morphology. At 2 weeks postoperatively, H&E staining showed minor inflammatory cell infiltration and vasculogenesis in the AF. Mild disorganization was found in the peripheral layer structure of the AF and the number of nucleus pulposus (NP) cells was slightly decreased. The above pathological changes were obvious in Group C. At 8 weeks postoperatively, H&E staining showed new blood vessels in the AF. There was obvious disorganization in the peripheral layer structure of the AF and the number of NP cells was decreased. The boundary between the AF and NP could be distinguished with difficulty. The above pathological changes were also observed in Group C (Figure 3).
Figure 3.
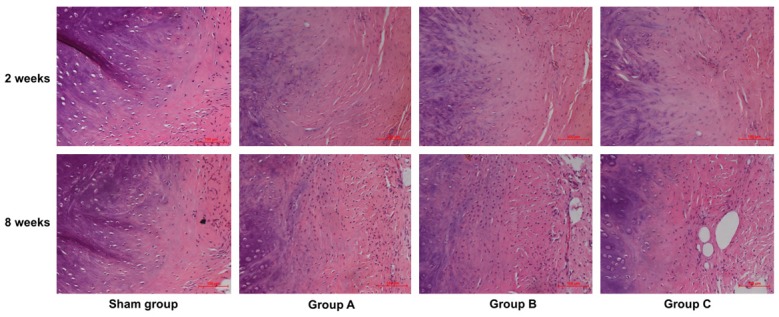
H&E staining of the boundary between the AF and NP of punctured IVDs in different groups at 2 weeks and 8 weeks postoperatively ×200. At 2 weeks postoperatively, minor inflammatory cell infiltration and vasculogenesis was observed in the AF. At 8 weeks postoperatively, the disorganization of the peripheral layer structure of the AF was gradually increased, and the number of NP cells gradually decreased from sham group to Group C. New blood vessels were found in the AF. The above pathological changes were obvious in Group C.
Immunohistochemical examination and calculation of immunopositive cells
The immunopositive cells were stained yellow and observed in the IVD (Figures 4 and 5). At 2 weeks postoperatively, the percentage of IL-1β-immunopositive cells in the punctured IVD was 3.87 ± 0.82% in sham group, 27.35 ± 2.35% in Group A, 31.62 ± 2.30% in Group B, and 43.73 ± 2.26% in Group C. The percentage of TNF-α-immunopositive cells in the punctured IVD was 4.75 ± 1.44% in sham group, 32.71 ± 1.61% in Group A, 35.54 ± 2.41% in Group B, and 46.84 ± 1.52% in Group C. There was a significant difference between the sham group and other groups (P<0.05) and there was also a significant difference between Group C and other groups (P<0.05). At 8 weeks postoperatively, the percentage of immunopositive cells in the corresponding group was lower than that at 2 weeks postoperatively. The percentage of IL-1β-immunopositive cells in the punctured IVD was 3.66 ± 0.74% in sham group, 14.46 ± 2.41% in Group A, 18.02 ± 1.48% in Group B, and 26.65 ± 3.00% in Group C. The percentage of TNF-α-immunopositive cells in the punctured IVD was 4.70 ± 1.03% in sham group, 17.48 ± 1.84% in Group A, 19.79 ± 2.49% in Group B, and 30.04 ± 2.64% in Group C. There was a significant difference between the sham group and other groups (P<0.05) and there was also a significant difference between Group C and other groups (P<0.05).
Figure 4.
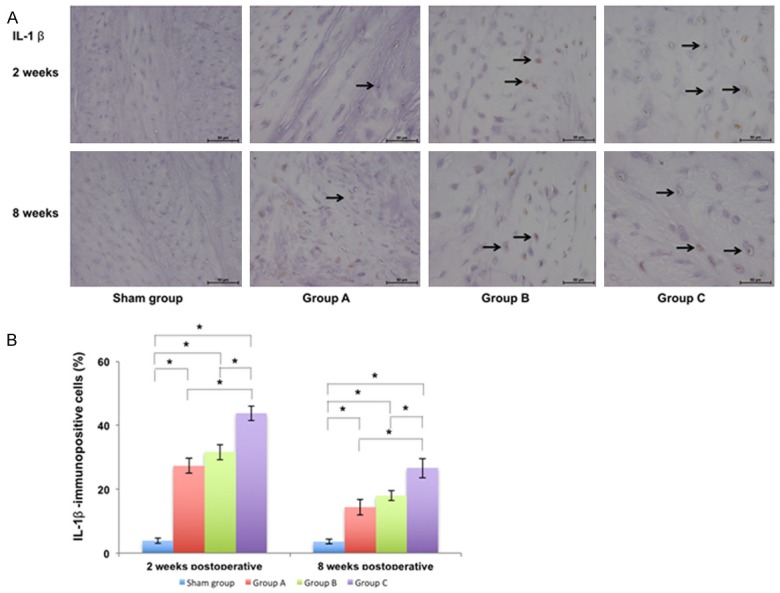
Immunohistochemical examination of punctured IVDs and calculation of immunopositive cells with respect to IL-1β in different groups at 2 weeks and 8 weeks postoperatively ×400. A. The number of IVD cells gradually decreased and the percentage of immunopositive cells (marked by the arrow) gradually increased from sham group to Group C. B. The percentage of IL-1β-immunopositive cells in punctured IVDs in different groups and there was also a significant difference between Group C and other groups (P<0.05) (*P<0.05).
Figure 5.
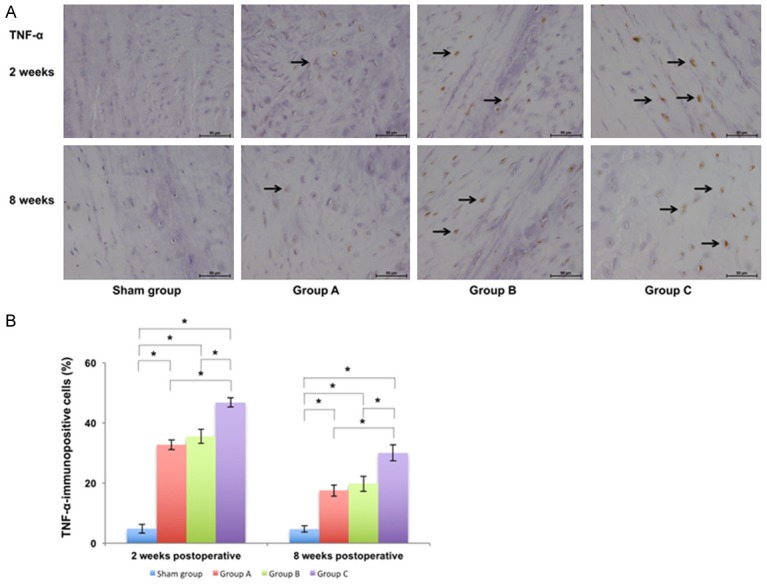
Immunohistochemical examination of punctured IVDs and calculation of immunopositive cells with respect to TNF-α in different groups at 2 weeks and 8 weeks postoperatively ×400. A. The number of IVD cells gradually decreased and the percentage of immunopositive cells (marked by the arrow) gradually increased from sham group to Group C. B. The percentage of TNF-α-immunopositive cells in punctured IVDs in different groups and there was a significant difference between Group C and other groups (P<0.05) (*P<0.05).
Apoptosis examination
TUNEL-positive cells were stained yellow and observed in the IVD (Figure 6). At 2 weeks postoperatively, the percentage of TUNEL-positive cells was 3.23 ± 0.91% in sham group, 13.67 ± 1.81% in Group A, 16.68 ± 2.32% in Group B, and 25.50 ± 2.05% in Group C. There was a significant difference between sham group and other groups (P<0.05) and there was also a significant difference between Group C and other groups (P<0.05). At 8 weeks postoperatively, the percentage of TUNEL-positive cells in the corresponding group was higher than that at 2 weeks postoperatively. The percentage of positive cells was 3.86 ± 1.15% in sham group, 18.35 ± 2.94% in Group A, 21.81 ± 1.73% in Group B, and 32.50 ± 2.17% in Group C. There was a significant difference between sham group and other groups (P<0.05) and there was also a significant difference between Group C and other groups (P<0.05).
Figure 6.
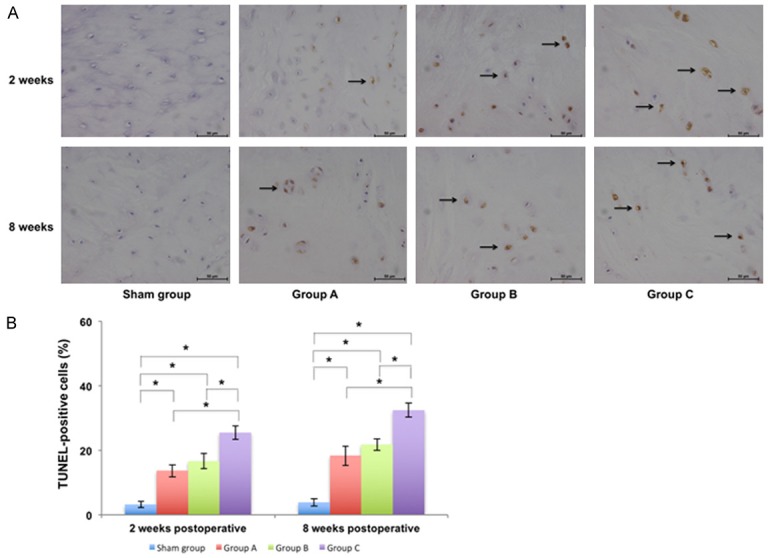
Apoptosis examination of punctured IVDs and calculation of TUNEL-positive cells in different groups at 2 weeks and 8 weeks postoperatively ×400. A. The number of IVD cells gradually decreased and the percentage of TUNEL-positive cells (marked by the arrow) gradually increased from Group A to Group C. B. The percentage of TUNEL-positive cells of punctured IVDs indifferent groups and there was a significant difference between Group C and other groups (P<0.05) (*P<0.05).
Discussion
In our present study, we found that there was obvious spinal instability in Group C at 8 weeks postoperatively and there was more serious degeneration of IVD in Group C than the other three groups including significant middle height loss, histological deterioration, upregulated pro-inflammatory cytokines including IL-1β and TNF-α, and increased apoptosis of IVD cells in corresponding IVDs. Therefore, we considered that IVD injury companied with complete ALL injury could cause obvious spinal instability, which might lead to corresponding severe IDD.
In this needle-puncturing IVD model of the rabbit model, we investigated IVD injury [17]. After puncture injury of the IVD, hydrostatic pressure was reduced. This caused changes in proteoglycan and collagen content, further accelerating IDD. In Group A, after simple puncture of the rabbits’ IVD, CT examination showed that the middle height of the punctured intervertebral space was reduced. H&E staining showed that the IVD structure was disorganized and the number of IVD cells was decreased. These imaging and pathological changes coincided with the known process of IVD injury [16,18,19]. Therefore, we suggest that the IVD injury model of rabbit is successfully built in our study.
Increasing expression of pro-inflammatory cytokines including IL-1β and TNF-α and increasing apoptosis of IVD cells may play roles in IDD [20-25]. Previous experimental studies have demonstrated that the disruption of extracellular matrix is one meaningful characteristic of IDD [26-28]. IL-1β and TNF-α can promote the expression of catabolic enzymes such as ADAMTSs and MMPs, which simultaneously decrease the expression of anabolic extracellular matrix proteins aggrecan and collagen II, finally accelerating IDD [29,30]. In addition, the increased apoptosis of IVD cells can reduce the production of type II collagen, proteoglycans, nitric oxide, prostaglandins, and metalloproteinases which can reduce the osmotic forces in the NP. This results in the loss of water content and the disruption of complete structure, finally accelerating IDD [21,25]. Therefore, we believe that expression of pro-inflammatory cytokines including IL-1β and TNF-α and the number of apoptosis of IVD cells are effective indications that reflect the degree of IDD and the increasing expression of above cytokines and the increasing apoptosis of IVD cells coincide with the changes of reduction of intervertebral space and deteriorated pathology in our study.
Spinal stability is maintained by the bones, joints, IVDs, and spinal ligaments including ALL, PLL, and PLC. Many studies have suggested that instability of intervertebral space is an important factor causing IDD [9-11]. In our present study, at 8 weeks postoperatively, the results of Group C show that the injury of IVD companied with completed ALL injury might result in obvious instability of injured intervertebral space, further leading to deteriorate IDD. The results of Group A show that there is limited effect on stability of intervertebral space with the simple injury of IVD. In addition, the results of Group B show the injury of IVD companied with partial ALL injury might not result in obvious instability of corresponding intervertebral space, for the remains of ALL might be helpful in maintaining the stability of injured intervertebral space and injured ALL might be repaired by fibrous tissue or scar soon.
Currently, it is easy to diagnose injury and degeneration of IVD by MRI in clinical practice but the method of detecting the injured degree of ALL is still difficult. The main changes in MRI imaging reflect the injured degree of ALL including the increasing gap size of anterior intervertebral space, the hemorrhage degree of prevertebral fasciae, and the discontinuity degree of ALL. According to the results of our study, we suggest that surgeons should pay more attention to patients who have suffered from injury of IVD companied with suspiciously serious ALL injury, for those patients have high risk of secondary spinal instability and deteriorating IDD. Patients with suspected injury of spinal ligaments confirmed by MRI should get early strict cervical external fixator and surgeons should inform them that there is an increasing risk of secondary spinal instability and deteriorating IDD.
There are several limitations in this study. First, MRI is one of the most important means for confirming the degree of IDD and ALL injury. However, because of limitations in experimental equipment, we used CT in performing imaging examinations of IVD. Second, based on ethical demands, we only selected earlier and later time points. Subsequent research could be conducted to add time intervals of observation after establishing the above models, further improving the accuracy.
In summary, our experiment confirms that IVD injury companied with complete ALL injury might cause obvious spinal instability, which might lead to corresponding severe IDD, including significant middle height loss, histological deterioration, upregulation of pro-inflammatory cytokines including IL-1β and TNF-α, and increased apoptosis of IVD cells in corresponding IVDs. Our suggestion for clinical work is that surgeons should pay more attention to patients suffering from basic injury of IVD companied with suspiciously serious ALL injury and for those patients at high risk of secondary spinal instability and deteriorating IDD.
Acknowledgements
This research was supported by the Natural Science Research Fund Project of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine of China, No. syz2015-024.
Disclosure of conflict of interest
None.
References
- 1.Maeda T, Ueta T, Mori E, Yugue I, Kawano O, Takao T, Sakai H, Okada S, Shiba K. Soft-tissue damage and segmental instability in adult patients with cervical spinal cord injury without major bone injury. Spine (Phila Pa 1976) 2012;37:E1560–1566. doi: 10.1097/BRS.0b013e318272f345. [DOI] [PubMed] [Google Scholar]
- 2.Mesfar W, Moglo K. Effect of the transverse ligament rupture on the biomechanics of the cervical spine under a compressive loading. Clin Biomech (Bristol, Avon) 2013;28:846–852. doi: 10.1016/j.clinbiomech.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Zhuge W, Ben-Galim P, Hipp JA, Reitman CA. Efficacy of MRI for assessment of spinal trauma: correlation with intraoperative findings. J Spinal Disord Tech. 2015;28:147–151. doi: 10.1097/BSD.0b013e31827734bc. [DOI] [PubMed] [Google Scholar]
- 4.Malham GM, Ackland HM, Varma DK, Williamson OD. Traumatic cervical discoligamentous injuries: correlation of magnetic resonance imaging and operative findings. Spine (Phila Pa 1976) 2009;34:2754–2759. doi: 10.1097/BRS.0b013e3181b6170b. [DOI] [PubMed] [Google Scholar]
- 5.Goradia D, Linnau KF, Cohen WA, Mirza S, Hallam DK, Blackmore CC. Correlation of MR imaging findings with intraoperative findings after cervical spine trauma. AJNR Am J Neuroradiol. 2007;28:209–215. [PMC free article] [PubMed] [Google Scholar]
- 6.Sitte I, Klosterhuber M, Lindtner RA, Freund MC, Neururer SB, Pfaller K, Kathrein A. Morphological changes in the human cervical intervertebral disc post trauma: response to fracture-type and degeneration grade over time. Eur Spine J. 2016;25:80–95. doi: 10.1007/s00586-015-4089-5. [DOI] [PubMed] [Google Scholar]
- 7.Kerttula LI, Serlo WS, Tervonen OA, Paakko EL, Vanharanta HV. Post-traumatic findings of the spine after earlier vertebral fracture in young patients: clinical and MRI study. Spine (Phila Pa 1976) 2000;25:1104–1108. doi: 10.1097/00007632-200005010-00011. [DOI] [PubMed] [Google Scholar]
- 8.Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A. Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol. 2006;6:5. doi: 10.1186/1472-6890-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herkowitz HN, Rothman RH. Subacute instability of the cervical spine. Spine (Phila Pa 1976) 1984;9:348–357. doi: 10.1097/00007632-198405000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara A, Tamai K, An HS, Kurihashi T, Lim TH, Yoshida H, Saotome K. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J Spinal Disord. 2000;13:444–450. doi: 10.1097/00002517-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Aikawa T, Shibata M, Sadahiro S. Hemilaminectomy and vertebral stabilization for thoracolumbar intervertebral disc associated dynamic compression in 11 dogs. Vet Comp Orthop Traumatol. 2013;26:498–504. doi: 10.3415/VCOT-12-12-0150. [DOI] [PubMed] [Google Scholar]
- 12.Davis SJ, Teresi LM, Bradley WG Jr, Ziemba MA, Bloze AE. Cervical spine hyperextension injuries: MR findings. Radiology. 1991;180:245–251. doi: 10.1148/radiology.180.1.2052703. [DOI] [PubMed] [Google Scholar]
- 13.Oxland TR, Panjabi MM, Southern EP, Duranceau JS. An anatomic basis for spinal instability: a porcine trauma model. J Orthop Res. 1991;9:452–462. doi: 10.1002/jor.1100090318. [DOI] [PubMed] [Google Scholar]
- 14.Richter M, Wilke HJ, Kluger P, Claes L, Puhl W. Load-displacement properties of the normal and injured lower cervical spine in vitro. Eur Spine J. 2000;9:104–108. doi: 10.1007/s005860050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leahy PD, Puttlitz CM. The effects of ligamentous injury in the human lower cervical spine. J Biomech. 2012;45:2668–2672. doi: 10.1016/j.jbiomech.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JT, Gorth DJ, Beattie EE, Harfe BD, Smith LJ, Elliott DM. Needle puncture injury causes acute and long-term mechanical deficiency in a mouse model of intervertebral disc degeneration. J Orthop Res. 2013;31:1276–1282. doi: 10.1002/jor.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, An HS. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976) 2005;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 18.Issy AC, Castania V, Castania M, Salmon CE, Nogueira-Barbosa MH, Bel ED, Defino HL. Experimental model of intervertebral disc degeneration by needle puncture in Wistar rats. Braz J Med Biol Res. 2013;46:235–244. doi: 10.1590/1414-431X20122429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issy AC, Castania V, Silveira JW, Nogueira-Barbosa MH, Salmon CE, Del-Bel E, Defino HL. Does a small size needle puncture cause intervertebral disc changes? Acta Cir Bras. 2015;30:574–579. doi: 10.1590/S0102-865020150080000009. [DOI] [PubMed] [Google Scholar]
- 20.Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20141191. doi: 10.1098/rsif.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding F, Shao ZW, Xiong LM. Cell death in intervertebral disc degeneration. Apoptosis. 2013;18:777–785. doi: 10.1007/s10495-013-0839-1. [DOI] [PubMed] [Google Scholar]
- 22.Ma X, Lin Y, Yang K, Yue B, Xiang H, Chen B. Effect of lentivirus-mediated survivin transfection on the morphology and apoptosis of nucleus pulposus cells derived from degenerative human disc in vitro. Int J Mol Med. 2015;36:186–194. doi: 10.3892/ijmm.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudli S, Haschtmann D, Ferguson SJ. Fracture of the vertebral endplates, but not equienergetic impact load, promotes disc degeneration in vitro. J Orthop Res. 2012;30:809–816. doi: 10.1002/jor.21573. [DOI] [PubMed] [Google Scholar]
- 24.Alkhatib B, Rosenzweig DH, Krock E, Roughley PJ, Beckman L, Steffen T, Weber MH, Ouellet JA, Haglund L. Acute mechanical injury of the human intervertebral disc: link to degeneration and pain. Eur Cell Mater. 2014;28:98–110. doi: 10.22203/ecm.v028a08. discussion 110-111. [DOI] [PubMed] [Google Scholar]
- 25.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNF-alpha and IL-1beta in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–116. doi: 10.22203/ecm.v030a08. discussion 116-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang MH, Kim KS, Yoo CM, Shin JH, Nam HG, Jeong JS, Kim JH, Lee KH, Choi H. Photobiomodulation on human annulus fibrosus cells during the intervertebral disk degeneration: extracellular matrix-modifying enzymes. Lasers Med Sci. 2016;31:767–777. doi: 10.1007/s10103-016-1923-x. [DOI] [PubMed] [Google Scholar]
- 28.Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Wang H, Yang H, Li J, Cai Q, Shapiro IM, Risbud MV. Tumor necrosis factor-alpha- and interleukin-1beta-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-kappaB axis: implications in inflammatory disc disease. Am J Pathol. 2014;184:2560–2572. doi: 10.1016/j.ajpath.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]


