Abstract.
Hypophosphatasia (HPP) is a rare bone disease caused by inactivating mutations in the ALPL gene, which encodes tissue-nonspecific alkaline phosphatase (TNSALP). Patients with HPP have varied clinical manifestations and are classified based on the age of onset and severity. Recently, enzyme replacement therapy using bone-targeted recombinant alkaline phosphatase (ALP) has been developed, leading to improvement in the prognosis of patients with life-threatening HPP. Considering these recent advances, clinical practice guidelines have been generated to provide physicians with guides for standard medical care for HPP and to support their clinical decisions. A task force was convened for this purpose, and twenty-one clinical questions (CQs) were formulated, addressing the issues of clinical manifestations and diagnosis (7 CQs) and those of management and treatment (14 CQs). A systematic literature search was conducted using PubMed/MEDLINE, and evidence-based recommendations were developed. The guidelines have been modified according to the evaluations and suggestions from the Clinical Guideline Committee of The Japanese Society for Pediatric Endocrinology (JSPE) and public comments obtained from the members of the JSPE and a Japanese HPP patient group, and then approved by the Board of Councils of the JSPE. We anticipate that the guidelines will be revised regularly and updated.
Keywords: hypophosphatasia, systematic review, enzyme replacement therapy, guideline
*The Japanese version of these guidelines were published on the website of the JSPE (http://jspe.umin.jp/medical/files/guide20190111.pdf) on January 11, 2019.
List of Recommendations
[Issues and Clinical Questions (CQs) Related to Clinical Manifestations and Diagnosis]
CQ1: What differences are there in the symptoms and prognoses among the clinical forms of hypophosphatasia (HPP)?
HPP varies in age at onset and severity and is usually classified into 6 forms. Symptoms differ according to age, and all patients with the perinatal severe (lethal) form and approximately half of the patients with the infantile form have poor life prognoses without treatment, but the life prognoses are favorable for the other forms of the disease. (Recommendation grade 1, evidence level B)
CQ2: What are the diagnostic methods recommended for HPP?
HPP is diagnosed according to clinical symptoms, radiological findings, and biochemical test results. Although low serum alkaline phosphatase (ALP) activity is an important finding, comparison with reference values according to age and sex is necessary. For the definitive diagnosis, ALPL gene testing is recommended. (Recommendation grade 1, evidence level B)
CQ3: What are examples of complications of HPP?
In patients with HPP, numerous complications, including fracture, bone deformities, respiratory insufficiency, convulsion, premature craniosynostosis, hypercalcemia/hypercalciuria, ectopic calcification, premature loss of deciduous teeth, muscle weakness, and delayed motor development, are observed. (Recommendation grade 1, evidence level B)
CQ4: What are the plain bone X-ray findings in HPP?
In patients with HPP, plain bone X-ray examination findings vary depending on age and severity, and include bone mineralization disorder, rickets-like change, metaphyseal “tongues” of radiolucency, bone deformity, fracture, and pseudofracture. (Recommendation grade 1, evidence level B)
CQ5: What are the characteristics of blood tests and urinalysis findings in HPP?
In patients with HPP, the serum ALP activity is lower than the age- and sex-specific reference values. The urinary phosphoethanolamine (PEA) excretion and the blood pyridoxal 5’-phosphate (PLP) levels are increased. Furthermore, hypercalcemia and hypercalciuria may be observed. (Recommendation grade 1, evidence level B)
CQ6: Is fetal ultrasound recommended for the early diagnosis or prognostic improvement of HPP?
Early diagnosis of HPP by fetal ultrasound is recommended because it is considered to lead to early treatment and an improved outcome. (Recommendation grade 1, evidence level C)
CQ7: Are genetic tests recommended for the definitive diagnosis and severity assessment of HPP?
Genetic tests are recommended for the definitive diagnosis of HPP and genetic counseling. The accuracy of the severity evaluation by genetic testing is limited. (Recommendation grade 1, evidence level B)
[Issues related to treatment and management]
CQ8: What are the indications for ALP enzyme replacement therapy?
ALP enzyme replacement therapy is indicated if the patient is definitively diagnosed to have HPP and is expected to have a poor prognosis. Forms of the disease with a relatively favorable life prognosis are also relative indications for enzyme replacement therapy if symptoms based on HPP, such as bone symptoms and muscle weakness, are present because they are expected to be improved by the therapy. (Recommendation grade 1, evidence level C)
CQ9: What methods are recommended for the efficacy assessment of ALP enzyme replacement therapy?
The efficacy of ALP enzyme replacement therapy for HPP is assessed according to improvements in clinical symptoms and bone X-ray findings. (Recommendation grade 1, evidence level C)
CQ10: Is ALP enzyme replacement therapy recommended for improving the survival time in HPP?
The perinatal severe (lethal) and infantile forms, in which the outcomes are expected to be poor, ALP enzyme replacement therapy is recommended because it is expected to sufficiently improve life prognosis. (Recommendation grade 1, evidence level B)
CQ11: Is ALP enzyme replacement therapy also recommended for perinatal benign HPP?
At present, there is no evidence regarding the efficacy of ALP enzyme replacement therapy for perinatal benign HPP. Further accumulation and analysis of cases will be necessary in the future. (No recommendation grade, evidence level C)
CQ12: Is ALP enzyme replacement therapy recommended for improving premature craniosynostosis in HPP?
Presently, the effects of ALP enzyme replacement therapy on premature craniosynostosis in HPP are unclear. (No recommendation grade, evidence level D)
CQ13: Is ALP enzyme replacement therapy recommended for improving motor function in HPP?
ALP enzyme replacement therapy is recommended to improve the motor function of HPP patients. (Recommendation grade 1, evidence level C)
CQ14: Is ALP enzyme replacement therapy recommended to be initiated as early as possible?
In perinatal severe (lethal) and infantile HPP, the earliest possible initiation of enzyme replacement therapy is recommended to improve the life prognosis. As time is needed to improve respiratory function, and as intensive care may be necessary during this period, it is recommended to initiate enzyme replacement therapy as early as possible. (Recommendation grade 1, evidence level B)
CQ15: Can the reduction or withdrawal of ALP enzyme replacement therapy affect the therapeutic effect?
There have been no reports that evaluate the dose and therapeutic effects of asfotase alfa in ALP enzyme replacement therapy, and the evidence is lacking. (Recommendation grade 2, evidence level C)
CQ16: What are examples of adverse effects and adverse reactions of ALP enzyme replacement therapy? What are the recommendations for their management?
As the administration of asfotase alfa may cause injection site reactions, it is recommended that repeated injections at the same site be avoided and to change the injection site each time. In addition, due to the possibility of fluctuations in the serum calcium and phosphate levels, their monitoring is recommended. (Recommendation grade 1, recommendation level C)
CQ17: What are the recommendations for monitoring during ALP enzyme replacement therapy?
During ALP enzyme replacement therapy for HPP, it is necessary to monitor the efficacy and safety. It has been proposed to periodically perform biochemical tests, bone X-ray scans, respiratory function tests, growth assessment, pain and motor function assessments, quality of life (QOL) assessment, dental examinations, and evaluation of the presence of ectopic calcification according to age. (Recommendation grade 2, evidence level C)
CQ18: Are bisphosphonates contraindicated in HPP?
Although there is little evidence that bisphosphonates increase the atypical femoral fracture rate in HPP, it is recommended that their administration be avoided because they are not expected to improve bone symptoms. (Recommendation grade 1, evidence level C)
CQ19: What treatments are recommended for convulsions in HPP?
Convulsions in HPP are usually vitamin B6-dependent seizures and are treated by pyridoxine administration; however, some patients are not responsive to this therapy. (Recommendation grade 1, evidence level C)
CQ20: What are the recommendations for the management and treatment of hypercalcemia in HPP?
Enzyme replacement therapy can be a radical treatment for hypercalcemia in HPP. Restriction of calcium intake using low-calcium milk, fluid infusion, loop diuretics administration, and calcitonin administration are performed as temporary symptomatic treatments. However, as these treatments may exacerbate bone symptoms, concurrent enzyme replacement therapy is recommended. (Recommendation grade 1, evidence level C)
CQ21: Is dental follow-up and treatment recommended in HPP?
In HPP, dental follow-up and treatment are recommended. (Recommendation grade 1, evidence level C)
Introduction
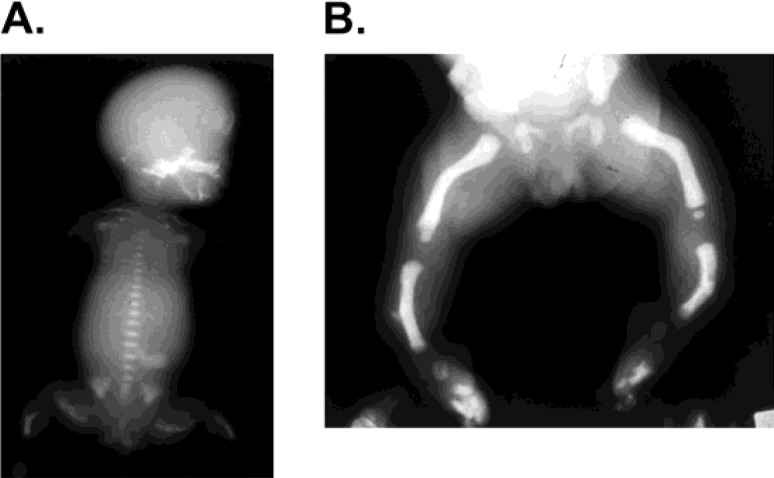
Hypophosphatasia (HPP) is an inherited skeletal disease caused by defects in tissue-nonspecific alkaline phosphatase (TNSALP). Reduced serum alkaline phosphatase (ALP) activity despite demonstration of impaired mineralization of bones and rickets-like changes on bone X-ray scans is a characteristic of this disease (1, 2). Due to the loss of TNSALP activity, its substrates, such as phosphoethanolamine (PEA), inorganic pyrophosphate, and pyridoxal 5’-phosphate (PLP), are not degraded and, therefore, accumulate in the body. The disease is transmitted as an autosomal recessive trait in many families and as an autosomal dominant trait in some families. The incidence of severe HPP in Japan is estimated to be 1 out of 150,000 people (3, 4). The frequencies of other forms of HPP are unclear, and there may be undiagnosed cases. Clinical symptoms vary with age and severity, and the disease is usually classified into 6 clinical disease forms (perinatal severe [lethal], perinatal benign, infantile, childhood, adult, and odontohypophosphatasia) (5, 6), but other classification methods are also used. Findings on plain bone X-ray scans include generalized hypomineralization of bones, deformity of long bones, and metaphyseal irregularity (Fig. 1). Symptoms including convulsions, hypercalcemia, poor weight gain, and premature loss of deciduous teeth are also observed.
Fig. 1.
X-ray images of pediatric patients with hypophosphatasia (HPP). (A) Bone X-ray image of perinatal lethal HPP. Marked hypomineralization of all bones in the body, including the cranium, vertebrae, and long bones, and deformation of long bones are observed. Rickets-like metaphyseal irregularity is noted. The thorax is small, and respiratory disorder is inevitable. (B) Bone X-ray image of perinatal benign HPP. Hypomineralization is very minimal, and no metaphyseal irregularity is noted. Long bones are deformed. Bone spurs are noted sporadically.
HPP can be diagnosed by clinical symptoms and bone X-ray findings that are accompanied by a serum ALP activity lower than the age-specific normal value (2, 7). For the definitive diagnosis, analysis of the ALPL gene that encodes TNSALP is useful. The diagnostic criteria have been prepared by the “Basic and Clinical Studies for Individual Optimization of Treatment for Hypophosphatasia” group as part of a research project funded by a Health and Labour Sciences Research Grant on Intractable Diseases from the Ministry of Health, Labour and Welfare, Japan, and are presented on the website of the Japan Intractable Diseases Information Center (http://www.nanbyou.or.jp/entry/4565). In addition, information provided on the website of the Japanese edition of GeneReviews (GeneReviews Japan; http://grj.umin.jp) is also useful as a reference.
HPP is caused by loss-of-function mutations of the ALPL gene. The ALPL gene is located in the short arm of chromosome 1 and consists of 12 exons. In HPP, ALPL mutations are observed in all exons. Many of them are missense mutations, but some are frameshift mutations due to deletions or insertions of bases, in-frame mutations, and intron mutations.
In Japanese patients with HPP, the deletion of T at nucleotide 1559 (c.1559delT) and p.F327L mutation due to the substitution of the phenylalanine at codon 327 (numbered from the translation initiation site) by leucine are commonly found (5). In particular, carriers with c.1559delT in one allele are reported to be present at a frequency of 1/480 in the general Japanese population (3).
In families with autosomal-recessive HPP, patients with compound heterozygous mutations having a different mutation in each allele are relatively common. The patients’ parents are carriers having a mutation in one allele alone and have a low serum ALP level. Carriers may exhibit mild symptoms but are usually asymptomatic. In families with autosomal-dominant HPP, which is often observed among families with relatively mild HPP, symptoms are caused by a mutation of one allele alone (1, 2). Some of the mutations identified in autosomal-dominant families have been confirmed to have a dominant-negative effect (1, 8).
Although the core manifestation of HPP is impaired bone mineralization, the mechanism by which low TNSALP activity leads to hypomineralization is not fully understood (9). Bone gains strength by mineralization, the process in which bone minerals (mainly calcium and phosphate) deposit on the bone matrix consisting of type I collagen, osteocalcin, etc. TNSALP produces inorganic phosphate by degrading inorganic pyrophosphate, a mineralization inhibitor. The produced inorganic phosphate is taken into matrix vesicles released from the osteoblasts. Phosphate and calcium concentrated in the matrix vesicles are crystallized and form hydroxyapatite, and mineralization progresses as hydroxyapatite deposits on collagen fibers. In HPP, hypomineralization is believed to be caused by the accumulation of inorganic pyrophosphate and a decrease in the local phosphate concentration associated with reduced TNSALP activity. Inhibition of the accumulation of calcium in bones by impaired bone mineralization may cause hypercalcemia and hypercalciuria.
Furthermore, in HPP, dephosphorylation of PLP, a metabolite of vitamin B6, into pyridoxal (PL) is inhibited due to the low TNSALP activity. As PL can, but PLP cannot, pass through the cell membrane, the central nervous system is considered to become deficient in vitamin B6, leading to convulsions (1).
Until recently, management of HPP was limited to the treatment of symptoms, including respiratory insufficiency and convulsions in patients with severe HPP and hypercalcemia, and dental care. Recently, however, treatment for HPP has changed greatly with the development of ALP enzyme replacement therapy (asfotase alfa). In 2012, favorable results were reported by a clinical trial of this drug in patients with severe HPP (10), and marked improvements in rickets-like changes on bone X-ray scans were observed. In Japan, manufacturing and sale of this drug received approval in 2015. According to the final report of an international collaborative clinical trial released in 2016, the overall survival of patients administered asfotase alfa was 84% at the age of 5 yr. On the contrary, the survival rate of untreated patients followed up in a study of the natural history of HPP at the age of 5 yr was 27%, suggesting that asfotase alfa improves the survival rate of patients with HPP (11).
In the international collaborative clinical trial, disorders, including pneumonitis, respiratory disorders, and convulsions, were observed as adverse events associated with the administration of asfotase alfa, but their relationships with the treatment were judged to be weak. Although adverse drug reactions were observed in 60 of a total of 71 patients administered the drug in clinical studies in Japan and abroad, many were injection site reactions. An important point of concern is the possibility of hypocalcemia due to the acceleration of calcium deposition on bone by asfotase alfa (12). It is recommended to periodically monitor the serum calcium level, and to replenish calcium and vitamin D, if necessary. Presently, there is no evidence regarding the effects of asfotase alfa on extraskeletal symptoms, such as convulsions and dental symptoms, or optimization of its dose, and further study is necessary.
The objective of these guidelines is to provide clinicians with guides for standard medical care for HPP and to support their clinical decisions. The present practice guidelines aim not to restrict the clinical strategies of physicians but to help with their practice. Judgments about actual treatments should be made by the physician in charge according to the condition of each patient.
Intended users of these guidelines may include physicians who treat neonates/children, physicians specializing in endocrine diseases/metabolic abnormalities, orthopedists and obstetricians, medical workers in general, dentists, and patients and their families.
Methods
Task force for hypophosphatasia guidelines
The task force was organized by the study group for the research project “Creation of a network for skeletal dysplasia research and care to develop clinical guidelines (Principal Investigator: Keiichi Ozono),” which was funded by the Japan Agency for Medical Research and Development (AMED).
The affiliation and specialty of the task force members and conflict of interest (COI) disclosures are shown in the Appendix.
Funding source for guideline preparation
The funds necessary for the preparation of these guidelines were provided by the AMED Practical Research Project for Rare/Intractable Disease Project of Japan “Creation of a network for skeletal dysplasia research and care to develop clinical guidelines (Principal Investigator: Keiichi Ozono).”
Process of the guideline preparation
In preparing these guidelines, using the “Minds Handbook for Clinical Practice Guideline Development 2014” as a reference, clinical questions (CQs) concerning the clinical manifestation, diagnosis, treatment, and management of HPP were formulated, recommendations were prepared, and the strength and interpretation of recommendations were drafted for each CQ based on the total evidence obtained by a systematic review. The final strength of each recommendation was determined by discussion among all members and cooperators of the Task Force.
1. Formulation of CQs
The components of CQs were based on “PICO” (P: patients, problem, population, I: interventions, C: comparisons, controls, comparators, O: outcomes). CQs were prepared concerning the patient outcome, and the relative importance of each of the selected outcomes was evaluated using a scale of 1–9 points, with a higher score reflecting a more important outcome for the patient. The outcomes scored 1–3 were classified as “not important,” 4–6 as “important,” and 7–9 as “critical,” and an actual systematic review was performed for outcomes judged to be “critical” which were scored as 6 and judged to be more important than the others.
2. Literature retrieval
For each CQ, the Task Force members selected search keywords, prepared search formulas, and carried out searches using PubMed/MEDLINE. In the primary screening, titles and abstracts that were not consistent with the CQ were excluded. In the secondary screening, each Task Force member selected papers that fulfilled the selection criteria by reading the full text of each paper. Thereafter, the literature list was supplemented with papers judged to be useful.
3. Evaluation of the evidence level of the literature and all evidence
HPP is a rare disease, and the life prognosis of patients with severe forms is poor without enzyme replacement therapy. Therefore, there have been no reports of randomized controlled trials, and the literature comprised primarily cross-sectional studies and case reports. These guidelines defined the strength of evidence as stated in Table 1. The strength of recommendations was determined by discussing the balance between benefits and harms of the recommendations prepared by each committee member among the members, and by all members of the Task Force and cooperators proofreading the text (Table 2).
Table 1. Strength and definition of evidence.
Table 2. Strength of recommendation.
4. External evaluation
(1) Hearing opinions from members of the Japanese Society for Pediatric Endocrinology (JSPE) (August 16–September 30, 2018)
(2) Evaluation and proposal by the guideline committee of the JSPE (September 25, 2018)
(3) Review by the Board of Councils of the JSPE (December 7–21, 2018)
(4) Approved by the Board of Councils of the JSPE (December 21, 2018)
5. Hearing opinions from a patient group
Opinions were collected from a patient group (HypoPhosPhatasia Support Association). (August 29, 2018)
Time of revision
These guidelines are expected to be revised within 5 years after their release. The task force responsible for the revision will be organized by the Board of Councils of the JSPE. If a new situation that is considered to markedly affect the content of these guidelines develops, and if the Board of Councils of the JSPE judges it to be urgent, a revision may be made as a “proposal.”
Clinical Manifestations and Diagnosis of HPP (CQ1–CQ7)
CQ1: What differences are there in the symptoms and prognoses among the clinical forms of HPP?
[Recommendation]
HPP varies in age at onset and severity and is usually classified into 6 forms. Symptoms differ according to age, and all patients with the perinatal severe (lethal) form and approximately half of the patients with the infantile form have poor life prognoses without treatment, but the life prognoses are favorable for the other forms of the disease. (Recommendation grade 1, evidence level B)
[Interpretation]
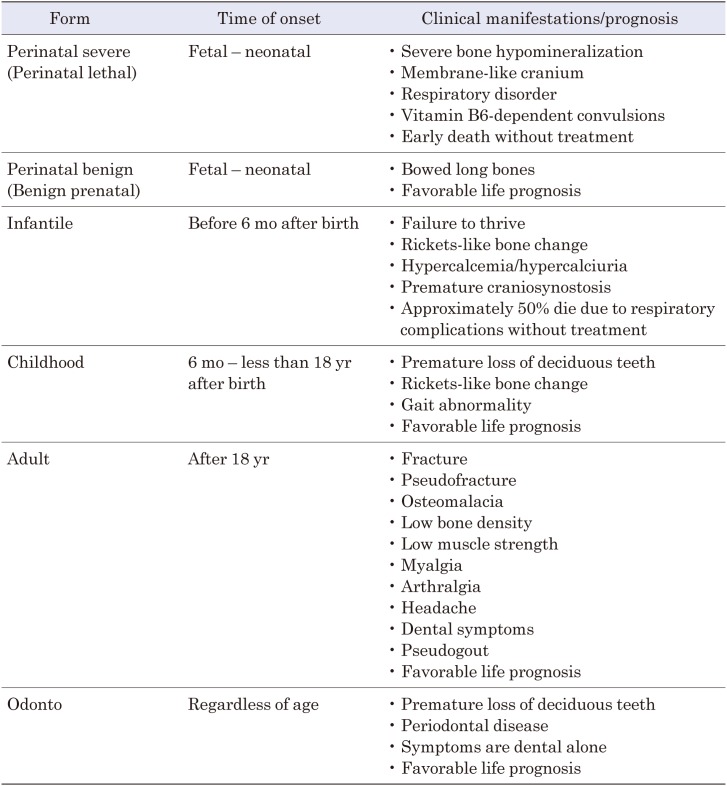
HPP varies in age at onset and severity and is usually classified into 6 forms: perinatal severe (lethal) form, perinatal benign form (also called the prenatal benign form or benign prenatal form), infantile form, childhood form, adult form, and odontohypophosphatasia (13, 14). The time of onset, major manifestations, and prognosis of each disease form are shown in Table 3 (11, 13,14,15). Formerly, HPP was classified into 5 forms without subdivision of the perinatal type, but recently, as the presence of the perinatal benign form, in which the life prognosis is favorable despite the perinatal onset, has been demonstrated (16), the classification into 6 forms with the distinction between the perinatal severe (lethal) and perinatal benign forms has begun to be used. However, in the Nosology and Classification of Genetic Skeletal Disorders (2015 revision), there is no reference to the perinatal benign form or odontohypophosphatasia, and only the perinatal lethal form, infantile form, juvenile form, and adult form are mentioned (17). The perinatal benign form is often referred to as the benign “prenatal” form because bone deformity is detected prenatally by fetal ultrasound examination (16). In HPP, symptoms are considered to vary according to age, and nearly all patients with the perinatal lethal form and approximately half of the patients with the infantile form will die without treatment, though the recent introduction of ALP enzyme replacement therapy can improve the prognoses. According to the report by Whyte et al. in 2016, the 1-yr survival rate was 42% and the 5-yr survival rate was 27% for 48 patients with perinatal severe (lethal) or infantile HPP without treatment (11). Although the life prognoses are favorable in the other disease forms, complications that may affect physical functions and quality of life (QOL) may develop in all forms of the disease. In a 2017 retrospective observational study of 38 patients with the adult form of HPP reported that 39% had a history of fracture, 55% had recurrent headache, 61% had recurrent myalgia, 11% had severe muscle weakness, and 47% had dental symptoms (18). The clinical forms of the disease are part of a continuous spectrum, and laboratory data overlap among the forms. The forms of disease may change during the course of a patient’s life as, for example, patients classified as having odontohypophosphatasia may develop bone symptoms during their lifetime (13).
Table 3. Forms of hypophosphatasia, time of onset, clinical manifestations, and prognosis.
CQ2: What are the diagnostic methods recommended for HPP?
[Recommendation]
HPP is diagnosed according to clinical symptoms, radiological findings, and biochemical test results. Although low serum ALP activity is an important finding, comparison with reference values according to age and sex is necessary. For the definitive diagnosis, ALPL gene testing is recommended. (Recommendation grade 1, evidence level B)
[Interpretation]
HPP is diagnosed according to clinical symptoms, radiological findings, and biochemical test results, and must be differentiated from other bone diseases, including rickets, osteomalacia, and osteogenesis imperfecta. Although low serum ALP activity is an important finding, comparison with reference values according to age and sex is necessary (13). It is also necessary to confirm consistently low serum ALP activity by multiple measurements. As a low ALP level is associated with numerous diseases, such as a nutritional disorder, zinc deficiency, Cushing’s syndrome, and cleidocranial dysplasia, in addition to HPP, their exclusion is also important (13). Moreover, as the substrates of TNSALP accumulate in HPP, urinary PEA and blood PLP are measured (13, 15). PEA is included as an item of an amino acid analysis. PLP, a metabolite of vitamin B6, is commonly examined abroad and is considered to have high sensitivity for the diagnosis of HPP. However, Japanese commercial laboratories cannot differentiate PLP and pyridoxal (PL) because they measure the vitamin B6 group after dephosphorylation. Differential measurements of PLP and PL are performed at the research level. Inorganic pyrophosphate is also a substrate of ALP, but it is not widely used for the diagnosis because its measurement is performed at limited facilities and is not covered by health insurance in Japan. For the definitive diagnosis of HPP, ALPL gene testing, which is covered by health insurance in Japan, is recommended (13).
The diagnostic criteria (see below) prepared by the “Basic and Clinical Studies for Individual Optimization of Treatment for Hypophosphatasia” Group (Principal Investigator: Keiichi Ozono), a research project funded by a Health and Labour Sciences Research Grant, are presented on the website of the Japan Intractable Diseases Information Center (http://www.nanbyou.or.jp/entry/4565).
<<Diagnostic criteria for hypophosphatasia>>
Primary symptoms
1. Bone mineralization disorder
Hypomineralization of bones, deformity of long bones, and rickets-like epiphyseal irregularity as plain X-ray findings
2. Premature loss of deciduous teeth (loss before the age of 4 yr)
Primary laboratory findings
1. A low serum alkaline phosphatase (ALP) level (Pay attention to age-specific normal values: Judgments are made according to age-specific normal values at each institution, but this disease must be suspected if the serum ALP level is < 300 IU/L in a growing child.)
Reference symptoms
1. Vitamin B6-dependent convulsions
2. Short, deformed limbs
Reference laboratory findings
1. High urinary phosphoethanolamine level (PEA; included as an item of a urinary amino acid analysis)
2. High serum inorganic pyrophosphate level
3. Hypercalcemia in infants
Genetic testing
1. For the definitive diagnosis and diagnosis of the disease form, tissue-nonspecific ALP (TNSALP) gene testing is recommended.
Reference findings
1. Familial history
2. Low serum ALP level in parents.*
*The ALP level increases in pregnant women due to ALP from the placenta.
Diagnostic category
If one or more primary symptoms and a low serum ALP level are observed, HPP should be suspected, and gene testing should be performed for the definitive diagnosis (Definite). A definite diagnosis is a condition for certification as an intractable disease patient.*
*The Japanese government has a program to promote research related to intractable diseases and to financially support the patients affected with these diseases. The criteria for the designated intractable disease in Japan include (1) a rarity (affecting less than 0.1% of the population), (2) an unknown etiology, (3) a lack of effective treatment, (4) a necessity of long-term treatment, and (5) an existence of objective diagnostic criteria.
CQ3: What are examples of complications of HPP?
[Recommendation]
In patients with HPP, numerous complications, including fracture, bone deformities, respiratory insufficiency, convulsion, premature craniosynostosis, hypercalcemia/hypercalciuria, ectopic calcification, premature loss of deciduous teeth, muscle weakness, and delayed motor development, are observed. (Recommendation grade 1, evidence level B)
[Interpretation]
In HPP, many complications, such as those listed in Table 4, are observed depending on age (15, 19,20,21,22,23,24). A wide variety of symptoms develop not only in bones but also in extraskeletal systems, such as muscles, joints, respiratory organs, the nervous system, and the renourinary system, and can affect the patient’s physical functions and QOL.
Table 4. Complications in hypophosphatasia.
CQ4: What are the plain bone X-ray findings in HPP?
[Recommendation]
In patients with HPP, plain bone X-ray examination findings vary depending on age and severity, and include bone mineralization disorder, rickets-like change, metaphyseal “tongues” of radiolucency, bone deformity, fracture, and pseudofracture. (Recommendation grade 1, evidence level B)
[Interpretation]
In patients with HPP, in the fetal period to childhood, plain bone X-ray examination findings vary depending on the severity, including impaired bone mineralization, rickets-like change, bone deformity, bowed long bones, and epiphyseal “tongues” of radiolucency (13, 25). In patients with a perinatal onset, osteophytes called spurs are often observed in the ulna and fibula (25). X-ray images differ among patients with the same disease form (25). In addition, plain X-ray examination of the head may reveal copper beaten skull associated with premature craniosynostosis. In adult patients with HPP, plain bone X-ray can demonstrate findings such as fracture, poor healing of fatigue fracture of metatarsal bones, pseudofracture (Looser’s zone), and atypical femoral fracture (13, 26).
CQ5: What are the characteristics of blood tests and urinalysis findings in HPP?
[Recommendation]
In patients with HPP, the serum ALP activity is lower than the age- and sex-specific reference values. The urinary PEA excretion and the blood PLP levels are increased. Furthermore, hypercalcemia and hypercalciuria may be observed. (Recommendation grade 1, evidence level B)
[Interpretation]
In patients with HPP, the serum ALP level is reduced (13, 27, 28). The ALP level measured on standard blood tests is the ALP activity. It must be noted that the reference serum ALP level varies depending on age and sex. In addition, the urinary PEA and blood PLP level increase as PEA, PLP, and inorganic pyrophosphate, which are the substrates of TNSALP, accumulate in the body without being degraded (13, 27, 28). PEA is included as an item of amino acid analysis, and its value is usually corrected for the creatinine concentration for the assessment of its urinary excretion. PLP is a metabolite of vitamin B6 and is measured at the research level in Japan. The measurement of inorganic pyrophosphate is performed at limited institutions and is not widely practiced. In addition, patients with HPP may exhibit hypercalcemia and hypercalciuria because of impaired calcium deposition in bones (13).
CQ6: Is fetal ultrasound recommended for the early diagnosis or prognostic improvement of HPP?
[Recommendation]
Early diagnosis of HPP by fetal ultrasound is recommended because it is considered to lead to early treatment and an improved outcome. (Recommendation grade 1, evidence level C)
[Interpretation]
In perinatal severe (lethal) and perinatal benign HPP, impaired bone mineralization and shortened or deformed long bones can be detected by fetal ultrasonography, and this modality is useful for the early diagnosis (16, 29). In particular, for patients with perinatal severe (lethal) HPP, early diagnosis is considered to lead to early therapeutic intervention and an improved prognosis. In addition to fetal ultrasound, a positive familial history and low serum ALP level in parents are important information. In women in the late pregnancy period, it must be noted that the serum ALP level is higher than in the non-pregnant period because of the presence of placental ALP (28).
CQ7: Are genetic tests recommended for the definitive diagnosis and severity assessment of HPP?
[Recommendation]
Genetic tests are recommended for the definitive diagnosis of HPP and genetic counseling. The accuracy of the severity evaluation by genetic testing is limited. (Recommendation grade 1, evidence level B)
[Interpretation]
HPP, if typical, can be diagnosed based on the clinical manifestations, X-ray findings, and biochemical test results. However, as a reduced serum ALP level is also observed in diseases other than HPP, ALPL gene testing is recommended for the definitive diagnosis, evaluation of the risk of recurrence, and genetic counseling (13, 30, 31).
Severe HPP, such as the perinatal severe (lethal) and infantile forms, is usually inherited in an autosomal recessive pattern with mutations in both alleles. In mild HPP, such as the childhood form, adult form, and odontohypophosphatasia, there are families with autosomal dominant inheritance and those with autosomal recessive inheritance. Therefore, if a mutation is detected in one allele alone by genetic testing, the disease is usually expected to be mild. Thus, although the course of the disease can be predicted to an extent from the results of genetic testing, the severity of symptoms may vary among patients with the same genotype (32), and the accuracy of the severity assessment by genetic testing is limited.
Treatment and Management of HPP(CQ8–CQ21)
CQ8: What are the indications for ALP enzyme replacement therapy?
[Recommendation]
ALP enzyme replacement therapy is indicated if the patient is definitively diagnosed to have HPP and is expected to have a poor prognosis. Forms of the disease with a relatively favorable life prognosis are also relative indications for enzyme replacement therapy if symptoms based on HPP, such as bone symptoms and muscle weakness, are present because they are expected to be improved by the therapy. (Recommendation grade 1, evidence level C)
[Interpretation]
The efficacy of asfotase alfa, a recombinant ALP enzyme replacement drug with an increased affinity for bone, in patients with HPP was first reported in 2012 (10). Subsequently, through international collaborative clinical trials and investigator-initiated trials, the manufacture and sale of this drug in Japan received approval in 2015, and its distribution was started under the trade name of Strensiq®.
An accurate diagnosis of HPP is essential for implementing ALP enzyme replacement therapy (33). As a low serum ALP level is also observed in numerous other disorders, they must be excluded. ALPL gene tests are useful for the definitive diagnosis of HPP (34). However, it must be noted that a person with a mutation in the ALPL gene does not necessarily have HPP. In families with autosomal-recessive HPP, the parents of the patient have a mutation in one allele of the ALPL gene, but they are usually asymptomatic carriers and not affected. However, some carriers exhibit biochemical abnormalities or mild symptoms. Individuals with mutations in the ALPL gene and clinical manifestations consistent with HPP are diagnosed as patients (33).
If the patient is confirmed to have HPP and to have a disease form with a poor life prognosis, such as the perinatal severe (lethal) and infantile forms, the patient has an absolute indication for ALP enzyme replacement therapy (11). Other disease forms are also relative indications if motor function and QOL are impaired due to bone symptoms and muscle weakness because they are expected to be alleviated by the therapy (7), but there are presently no clear criteria for recommendation of the therapy, and it should be evaluated in consideration of the expected therapeutic effects and risks in individual patients.
CQ9: What methods are recommended for the efficacy assessment of ALP enzyme replacement therapy?
[Recommendation]
The efficacy of ALP enzyme replacement therapy for HPP is assessed according to improvements in clinical symptoms and bone X-ray findings. (Recommendation grade 1, evidence level C)
[Interpretation]
The efficacy of ALP enzyme replacement therapy for HPP is assessed according to improvements in clinical symptoms and bone X-ray findings (7, 11, 35). Presently, no biomarkers useful for efficacy assessment have been established.
In the perinatal and infantile periods, the efficacy is evaluated according to prolongation of the survival time, improvements in respiratory function, improvements in bone symptoms, improvements in growth and development, improvements in mental/motor development, improved control of convulsions, etc. During childhood, the efficacy is evaluated according to improvements of motor function, improvements of bone symptoms, improvements in growth, improvements in mental/motor development, prevention of kidney calcification, etc. In adulthood, the evaluation is according to improvement in fracture healing, decrease in the number of fractures, decrease in pseudofractures, improvement in endurance capacity and gait, etc. At all ages, evaluation of pain and QOL are recommended (7, 11, 35).
Improvements in morbid changes on bone X-ray scans are evaluated using the Rickets Severity Score and the Radiographic Global Impression of Changes. Examinations, such as the 6-minute walking test, are useful to assess motor function (11, 12, 35).
CQ10: Is ALP enzyme replacement therapy recommended for improving the survival time in HPP?
[Recommendation]
The perinatal severe (lethal) and infantile forms, in which the outcomes are expected to be poor, ALP enzyme replacement therapy is recommended because it is expected to sufficiently improve the life prognosis. (Recommendation grade 1, evidence level B)
[Interpretation]
In the past, although nearly all patients with perinatal severe (lethal) HPP and approximately half of the patients with infantile HPP died due to respiratory insufficiency in early childhood, the life prognoses of these severe forms of HPP have improved after the introduction of ALP enzyme replacement therapy using asfotase alfa.
In a paper published in 2016 that summarized the results of international collaborative clinical trials, the survival time of 37 patients with perinatal severe (lethal) or infantile HPP treated by enzyme replacement therapy using asfotase alfa (median duration of treatment: 2.7 yr) was compared with that of 48 untreated historical controls. The survival rate at the age of 5 yr was 27% in the historical controls but was improved to 84% in those treated by enzyme replacement therapy (11). Moreover, although only 5% of those who underwent artificial respiratory management during the clinical course survived in the historical control group, 76% survived in the enzyme replacement therapy group, and 75% of them were able to be weaned from the respirator (11). In forms of the disease with a poor prognosis, shortening of the period in which intensive care such as respiratory management is necessary, improvement in the life prognosis can also be expected by the prompt diagnosis and early initiation of enzyme replacement therapy (11, 36). In an investigator-initiated clinical trial in Japan, 5 patients needed artificial respiratory management and 3 needed oxygen administration at the initiation of treatment, but the respiratory function was improved in all patients by asfotase alfa, and respiratory support became unnecessary in 3 patients (12).
CQ11: Is ALP enzyme replacement therapy also recommended for perinatal benign HPP?
[Recommendation]
At present, there is no evidence regarding the efficacy of ALP enzyme replacement therapy for perinatal benign HPP. Further accumulation and analysis of cases will be necessary in the future. (No recommendation grade, evidence level C)
[Interpretation]
In the perinatal benign form, bone deformities are present at birth, but bone mineralization is only slightly impaired, and the life prognosis is favorable. This form is observed relatively frequently among Japanese patients with HPP, and the first case was reported in 1996 (37), but it was internationally recognized after a literature review was published in 2011 (16). As bone deformities can be detected before birth by methods such as fetal ultrasound, this form of the disease is often referred to as the “prenatal” benign form (16). The efficacy of ALP enzyme replacement therapy for the perinatal benign form has not been evaluated sufficiently at present. In this disease form, improvements in bone symptoms are often observed after birth even without treatment, and its clinical features vary from those in cases close to the infantile form or to odontohypophosphatasia; therefore, it is necessary to accumulate patients treated and not treated by enzyme replacement therapy for further analyses.
CQ12: Is ALP enzyme replacement therapy recommended for improving premature craniosynostosis in HPP?
[Recommendation]
Presently, the effects of ALP enzyme replacement therapy on premature craniosynostosis in HPP are unclear (No recommendation grade, evidence level D)
[Interpretation]
In the first report that described the effects of asfotase alfa on severe HPP, premature craniosynostosis was observed in 2 of the 11 patients treated for a maximum duration of 6 mo (10). In the 10 patients treated for 7–12 mo, 4 exhibited premature craniosynostosis and 2 required surgery (10). This suggests that enzyme replacement therapy using asfotase alfa is unlikely to improve premature craniosynostosis in HPP. In a multicenter physician-initiated clinical trial carried out in Japan, 2 of the 13 patients aged 0–34 yr exhibited premature craniosynostosis during a treatment period of 868 days at the longest, and a relationship with treatment was suggested in 1 patient (12). To reach a conclusion on the effects of asfotase alfa on premature craniosynostosis, further follow-up and accumulation of patients will be necessary.
CQ13: Is ALP enzyme replacement therapy recommended for improving motor function in HPP?
[Recommendation]
ALP enzyme replacement therapy is recommended to improve the motor function of HPP patients. (Recommendation grade 1, evidence level C)
[Interpretation]
In a report summarizing the effects of enzyme replacement therapy by the 5-yr administration of asfotase alfa in children with HPP (aged 6–12 yr), improvements in the walked distance on the 6-min walking test, the score of the Bruininks-Oseretsky Test of Motor Proficiency™–Second Edition, which is a test of motor function, and the score of the Childhood Health Assessment Questionnaire, which is an index of QOL in children that primarily reflects physical functions, were reported (35). Therefore, ALP enzyme replacement therapy is expected to improve reduced motor function in patients with HPP.
CQ14: Is ALP enzyme replacement therapy recommended to be initiated as early as possible?
[Recommendation]
In perinatal severe (lethal) and infantile HPP, the earliest possible initiation of enzyme replacement therapy is recommended to improve the life prognosis. As time is needed to improve respiratory function, and as intensive care may be necessary during this period, it is recommended to initiate enzyme replacement therapy as early as possible. (Recommendation grade 1, evidence level B)
[Interpretation]
As mentioned in CQ10, according to an international collaborative clinical trial, the survival rate of children with the perinatal severe (lethal) or infantile HPP at the age of 5 yr was markedly higher, 84%, for those treated by asfotase alfa enzyme replacement therapy than the 27% for historical controls (11). Furthermore, the respiratory function improved with improvement in bone mineralization, and 76% of those who underwent artificial respiratory management in the enzyme replacement therapy group survived, with 75% of them having been weaned from artificial ventilation (11). However, as time is necessary to improve bone mineralization, some patients who did not require respiratory management at the initiation of treatment exhibited deteriorated respiratory function early after the initiation of treatment and temporarily required artificial ventilation. Therefore, the earliest possible initiation of enzyme replacement therapy is recommended for patients expected to have a poor life prognosis. Shortening of the period requiring intensive care and an improved life prognosis are expected from the prompt diagnosis and early initiation of enzyme replacement therapy (11, 36).
CQ15: Can the reduction or withdrawal of ALP enzyme replacement therapy affect the therapeutic effect?
[Recommendation]
There have been no reports that evaluate the dose and therapeutic effects of asfotase alfa in ALP enzyme replacement therapy, and the evidence is lacking. (Recommendation grade 2, evidence level C)
[Interpretation]
In severe HPP, the reduction or withdrawal of asfotase alfa before sufficient improvement in bone mineralization is considered to exacerbate symptoms. After improvement of symptoms or in mild forms of HPP, asfotase alfa may be reduced or stopped; however, evaluation of the effects due to a reduction or suspension of the therapeutic outcome is insufficient, and there is little evidence to support this.
CQ16: What are examples of adverse effects and adverse reactions of ALP enzyme replacement therapy? What are the recommendations for their management?
[Recommendation]
As the administration of asfotase alfa may cause injection site reactions, it is recommended that repeated injections at the same site be avoided and to change the injection site each time. In addition, due to the possibility of fluctuations in the serum calcium and phosphate levels, their monitoring is recommended. (Recommendation grade 1, evidence level C)
[Interpretation]
As the administration of asfotase alfa has been reported to cause injection site reactions (erythema, rash, discoloration, itching, pain, papules, nodules, atrophy, thickening, etc.) (10, 35), it is recommended to change the injection site at each injection and avoid repeated injections at the same site. It is important to sufficiently train the patient in the injection method before the initiation of self-injection. The risk of local reactions, such as pain, can be reduced by removing the vial from the refrigerator more than 15 min before injection and returning it to room temperature. However, the vial must be used within 1 h once removed from the refrigerator. The administration of asfotase alfa may cause fever, chills, irritability, nausea, headache, and anaphylaxis; therefore, the patient’s condition must be observed carefully, and the administration must be immediately discontinued and appropriate treatment given if such hypersensitivity reactions develop (7). Furthermore, as there is the possibility of fluctuations in the serum calcium and phosphate levels after the initiation of treatment, they should be monitored and corrected, as necessary (12, 36). In addition, neutralizing antibodies against asfotase alfa may appear during enzyme replacement therapy (35).
CQ17: What are the recommendations for monitoring during ALP enzyme replacement therapy?
[Recommendation]
During ALP enzyme replacement therapy for HPP, it is necessary to monitor the efficacy and safety. It has been proposed to periodically perform biochemical tests, bone X-ray scans, respiratory function tests, growth assessment, pain and motor function assessments, QOL assessment, dental examinations, and evaluation of the presence of ectopic calcification according to age. (Recommendation grade 2, evidence level C)
[Interpretation]
Although the items to be monitored during enzyme replacement therapy for HPP or the frequency of their monitoring have not been sufficiently evaluated, a guide prepared by an international expert panel was issued in 2017 (7), and follow-up appropriate for the patient’s age by a multidisciplinary team is recommended. In addition to biochemical tests and bone X-ray scans, the guide recommends periodic monitoring of the respiratory function, growth, pain, motor function, gait, muscle strength, and QOL. As to specific biochemical tests, the guide mentions the blood ALP activity, PLP, inorganic pyrophosphate, urinary PEA, calcium, phosphoric acid, parathyroid hormone, items related to renal function, and asfotase alfa neutralizing antibody. Radiologically, bone density testing is performed for older children and adults in addition to plain bone X-ray. Dental assessment and examination of the presence of ectopic calcification in the eyes and kidneys are also necessary. For the assessment of motor function, tests, such as the 6-min walking test, are performed. Questionnaires are used to assess pain and QOL.
CQ18: Are bisphosphonates contraindicated in HPP?
[Recommendation]
Although there is little evidence that bisphosphonates increase the atypical femoral fracture rate in HPP, it is recommended that their administration be avoided because they are not expected to improve bone symptoms. (Recommendation grade 1, evidence level C)
[Interpretation]
There are multiple case reports of exacerbation of bone symptoms and atypical femoral fracture in patients with HPP after the administration of bisphosphonates (38, 39). However, the results of a case-control study on whether HPP is a risk factor for atypical femoral fracture were negative (40), and there is little evidence that bisphosphonates increase the rate of atypical femoral fracture in HPP. Even so, it is recommended to avoid the administration of bisphosphonates if the patient is confirmed to have HPP because bisphosphonates are not expected to be effective for improving bone symptoms of HPP.
CQ19: What treatments are recommended for convulsions in HPP?
[Recommendation]
Convulsions in HPP are usually vitamin B6-dependent seizures and are treated by pyridoxine administration; however, some patients are not responsive to this therapy. (Recommendation grade 1, evidence level C)
[Interpretation]
In patients with HPP, dephosphorylation of PLP, a metabolite of vitamin B6, to PL is inhibited due to the loss of TNSALP activity. As PL can, but PLP cannot, pass through the cell membrane, the central nervous system becomes deficient in vitamin B6, which induces convulsions (41). Thus, convulsions in HPP are usually vitamin B6-dependent seizures and are treated by pyridoxine (not pyridoxal) administration (42). However, some patients respond only temporarily or are nonresponsive (43), and the administration of other anticonvulsive drugs may be necessary. In this event, the possibility of encephalopathy must also be considered (23). There is presently no evidence as to whether vitamin B6 administration can be stopped after the initiation of enzyme replacement therapy.
CQ20: What are the recommendations for the management and treatment of hypercalcemia in HPP?
[Recommendation]
Enzyme replacement therapy can be a radical treatment for hypercalcemia in HPP. Restriction of calcium intake using low-calcium milk, fluid infusion, loop diuretics administration, and calcitonin administration are performed as temporary symptomatic treatments. However, as these treatments may exacerbate bone symptoms, concurrent enzyme replacement therapy is recommended. (Recommendation grade 1, evidence level C)
[Interpretation]
The cause of hypercalcemia/hypercalciuria in HPP is a decrease in the accumulation of calcium in bones associated with impaired mineralization (13). Therefore, ALP enzyme replacement therapy is the radical treatment. As temporary symptomatic treatments for marked hypercalcemia, restriction of calcium intake using low-calcium milk, fluid infusion, loop diuretics administration, and calcitonin administration, etc., are performed (44); however, as they may exacerbate bone symptoms, concurrent enzyme replacement therapy is recommended. Although there have also been reports of the administration of steroids and bisphosphonates (45), they are not frequently recommended. If there is kidney insufficiency, correction of the calcium level by dialysis should be considered (46).
CQ21: Is dental follow-up and treatment recommended in HPP?
[Recommendation]
In HPP, dental follow-up and treatment are recommended. (Recommendation grade 1, evidence level C)
[Interpretation]
In HPP, mobility and premature loss of deciduous teeth are caused by cemental hypoplasia (47). Deciduous teeth are characteristically lost with the roots not being resorbed, and the use of a pediatric denture is often necessary. In general, the incisors are lost, but other teeth may also be lost in severe cases. In severe forms of HPP, enamel hypoplasia or delayed eruption of teeth may be observed. X-ray examination may demonstrate a decrease in alveolar bone and an enlargement of the pulp cavity. Increased mobility and premature loss of permanent teeth have also been reported (48). The management involves primary control of the periodontal condition. The deciduous teeth, even if loose, should be preserved as long as possible until they are replaced by permanent teeth by oral health guidance and periodontal treatment. In cases with premature loss of deciduous teeth, pediatric dentures are used for aesthetic restoration, acquisition of articulation function, and reduction of occlusal pressure on the residual deciduous teeth. In Japan, the use of pediatric dentures in HPP is covered by health insurance.
Acknowledgements
This work was supported by a grant from the AMED Practical Research Project for Rare/Intractable Disease Project of Japan 17ek010935h0003 (Principal Investigator, Keiichi Ozono), and by Research on Rare and Intractable Diseases, Health, Labour and Welfare Sciences Research Grants 19FC1006 (Principal Investigator, Hideaki Sawai).
Appendix: Affiliation and Specialty of the Task Force Members and COI Disclosure
[Affiliation and specialty of Task Force Members and Cooperators]
Names, affiliations and the specialty of Task Force members and those of Task Force cooperators are listed in Appendix Tables 1
Appendix Table 1. Task Force members.
| Name | Affiliation | Specialty |
|---|---|---|
| Toshimi Michigami (Chairperson) | Department of Bone and Mineral Research,Osaka Women’s and Children’s Hospital | • Board certified pediatrician |
| Yasuhisa Ohata | Department of Pediatrics, Osaka University Graduate School of Medicine | • Board certified pediatrician |
| Makoto Fujiwara | Department of Pediatrics, Osaka University Graduate School of Medicine | • Board certified pediatrician • Japanese board of medical genetics and genomics |
| Hiroshi Mochizuki | Division of Endocrinology and Metabolism,Saitama Children’s Medical Center | • Board certified pediatrician • Board certified endocrinologist (pediatrics) • Board certified nephrologist • Fellow of the Japanese Society for Dialysis Therapy |
and 2
Appendix Table 2. Task Force cooperators.
| Name | Affiliation | Specialty |
|---|---|---|
| Masanori Adachi | Department of Endocrinology and Metabolism,Kanagawa Children’s Medical Center | • Board certified pediatrician • Board certified endocrinologist (pediatrics) |
| Keiichi Ozono | Department of Pediatrics, Osaka University Graduate School of Medicine | • Board certified pediatrician • Board certified endocrinologist (pediatrics) |
| Taichi Kitaoka | Department of Pediatrics, Osaka University Graduate School of Medicine | • Board certified pediatrician |
| Takuo Kubota | Department of Pediatrics, Osaka University Graduate School of Medicine | • Board certified pediatrician • Board certified endocrinologist (pediatrics) |
| Hideaki Sawai | Department of Obstetrics and Gynecology,Hyogo College of Medicine | • Medical specialist of the Japan Society of
Obstetrics and Gynecology • Japanese board of medical genetics and genomics • Board certified by Japan Society for Reproductive Medicine |
| Noriyuki Namba | Department of Pediatrics, Osaka Hospital,Japan Community Healthcare Organization (JCHO) | • Board certified pediatrician • Board certified endocrinologist (pediatrics) |
| Kosei Hasegawa | Department of Pediatrics, Okayama University Hospital | • Board certified pediatrician • Board certified endocrinologist (pediatrics) |
| Ikuma Fujiwara | Department of Pediatrics, Sendai City Hospital | • Board certified pediatrician • Board certified endocrinologist (pediatrics) |
, respectively.
[COI disclosure of the Task Force members and cooperators]
The following declarations were made by the members and cooperators according to the Japan Association of Medical Sciences COI Management Guidance on Eligibility Criteria for Clinical Practice Guideline Formulation (March 2017). The other members and cooperators had no COIs to disclose.
• Keiichi Ozono: Lecture fees, manuscript fees, research funds, scholarship funds (Alexion Pharmaceuticals, Inc)
• Takuo Kubota: Research funds, scholarship funds (Alexion Pharmaceuticals, Inc)
References
- 1.Mornet E. Hypophosphatasia. Best Pract Res Clin Rheumatol 2008;22: 113–27. doi: 10.1016/j.berh.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 2.Whyte MP. Hypophosphatasia: An overview For 2017. Bone 2017;102: 15–25. doi: 10.1016/j.bone.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 3.Watanabe A, Karasugi T, Sawai H, Naing BT, Ikegawa S, Orimo H, et al. Prevalence of c.1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasia in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet 2011;56: 166–8. doi: 10.1038/jhg.2010.161 [DOI] [PubMed] [Google Scholar]
- 4.Ozono K, Michigami T. Hypophosphatasia now draws more attention of both clinicians and researchers: a commentary on Prevalence of c. 1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasias in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet 2011;56: 174–6. doi: 10.1038/jhg.2011.6 [DOI] [PubMed] [Google Scholar]
- 5.Michigami T, Uchihashi T, Suzuki A, Tachikawa K, Nakajima S, Ozono K. Common mutations F310L and T1559del in the tissue-nonspecific alkaline phosphatase gene are related to distinct phenotypes in Japanese patients with hypophosphatasia. Eur J Pediatr 2005;164: 277–82. doi: 10.1007/s00431-004-1612-9 [DOI] [PubMed] [Google Scholar]
- 6.Whyte MP, Zhang F, Wenkert D, McAlister WH, Mack KE, Benigno MC, et al. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone 2015;75: 229–39. doi: 10.1016/j.bone.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 7.Kishnani PS, Rush ET, Arundel P, Bishop N, Dahir K, Fraser W, et al. Monitoring guidance for patients with hypophosphatasia treated with asfotase alfa. Mol Genet Metab 2017;122: 4–17. doi: 10.1016/j.ymgme.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 8.Müller HL, Yamazaki M, Michigami T, Kageyama T, Schönau E, Schneider P, et al. Asp361Val Mutant of alkaline phosphatase found in patients with dominantly inherited hypophosphatasia inhibits the activity of the wild-type enzyme. J Clin Endocrinol Metab 2000;85: 743–7. doi: 10.1210/jcem.85.2.6373 [DOI] [PubMed] [Google Scholar]
- 9.Millán JL, Whyte MP. Alkaline Phosphatase and Hypophosphatasia. Calcif Tissue Int 2016;98: 398–416. doi: 10.1007/s00223-015-0079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med 2012;366: 904–13. doi: 10.1056/NEJMoa1106173 [DOI] [PubMed] [Google Scholar]
- 11.Whyte MP, Rockman-Greenberg C, Ozono K, Riese R, Moseley S, Melian A, et al. Asfotase Alfa Treatment Improves Survival for Perinatal and Infantile Hypophosphatasia. J Clin Endocrinol Metab 2016;101: 334–42. doi: 10.1210/jc.2015-3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitaoka T, Tajima T, Nagasaki K, Kikuchi T, Yamamoto K, Michigami T, et al. Safety and efficacy of treatment with asfotase alfa in patients with hypophosphatasia: Results from a Japanese clinical trial. Clin Endocrinol (Oxf) 2017;87: 10–9. doi: 10.1111/cen.13343 [DOI] [PubMed] [Google Scholar]
- 13.Whyte MP. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 2016;12: 233–46. doi: 10.1038/nrendo.2016.14 [DOI] [PubMed] [Google Scholar]
- 14.Mornet E. Hypophosphatasia. Orphanet J Rare Dis 2007;2: 40. doi: 10.1186/1750-1172-2-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkseth KE, Tebben PJ, Drake MT, Hefferan TE, Jewison DE, Wermers RA. Clinical spectrum of hypophosphatasia diagnosed in adults. Bone 2013;54: 21–7. doi: 10.1016/j.bone.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 16.Wenkert D, McAlister WH, Coburn SP, Zerega JA, Ryan LM, Ericson KL, et al. Hypophosphatasia: nonlethal disease despite skeletal presentation in utero (17 new cases and literature review). J Bone Miner Res 2011;26: 2389–98. doi: 10.1002/jbmr.454 [DOI] [PubMed] [Google Scholar]
- 17.Bonafe L, Cormier-Daire V, Hall C, Lachman R, Mortier G, Mundlos S, et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am J Med Genet A 2015;167A: 2869–92. doi: 10.1002/ajmg.a.37365 [DOI] [PubMed] [Google Scholar]
- 18.Schmidt T, Mussawy H, Rolvien T, Hawellek T, Hubert J, Rüther W, et al. Clinical, radiographic and biochemical characteristics of adult hypophosphatasia. Osteoporos Int 2017;28: 2653–62. doi: 10.1007/s00198-017-4087-z [DOI] [PubMed] [Google Scholar]
- 19.Collmann H, Mornet E, Gattenlöhner S, Beck C, Girschick H. Neurosurgical aspects of childhood hypophosphatasia. Childs Nerv Syst 2009;25: 217–23. doi: 10.1007/s00381-008-0708-3 [DOI] [PubMed] [Google Scholar]
- 20.Taketani T. Neurological Symptoms of Hypophosphatasia. Subcell Biochem 2015;76: 309–22. doi: 10.1007/978-94-017-7197-9_14 [DOI] [PubMed] [Google Scholar]
- 21.Whyte MP, Wenkert D, McAlister WH, Mughal MZ, Freemont AJ, Whitehouse R, et al. Chronic recurrent multifocal osteomyelitis mimicked in childhood hypophosphatasia. J Bone Miner Res 2009;24: 1493–505. doi: 10.1359/jbmr.090308 [DOI] [PubMed] [Google Scholar]
- 22.Chapple IL. Hypophosphatasia: dental aspects and mode of inheritance. J Clin Periodontol 1993;20: 615–22. doi: 10.1111/j.1600-051X.1993.tb00705.x [DOI] [PubMed] [Google Scholar]
- 23.Balasubramaniam S, Bowling F, Carpenter K, Earl J, Chaitow J, Pitt J, et al. Perinatal hypophosphatasia presenting as neonatal epileptic encephalopathy with abnormal neurotransmitter metabolism secondary to reduced co-factor pyridoxal-5′-phosphate availability. J Inherit Metab Dis 2010;33(Suppl 3): S25–33. doi: 10.1007/s10545-009-9012-y [DOI] [PubMed] [Google Scholar]
- 24.Seshia SS, Derbyshire G, Haworth JC, Hoogstraten J. Myopathy with hypophosphatasia. Arch Dis Child 1990;65: 130–1. doi: 10.1136/adc.65.1.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shohat M, Rimoin DL, Gruber HE, Lachman RS. Perinatal lethal hypophosphatasia; clinical, radiologic and morphologic findings. Pediatr Radiol 1991;21: 421–7. doi: 10.1007/BF02026677 [DOI] [PubMed] [Google Scholar]
- 26.Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009;24: 1132–4. doi: 10.1359/jbmr.081253 [DOI] [PubMed] [Google Scholar]
- 27.Riancho-Zarrabeitia L, García-Unzueta M, Tenorio JA, Gómez-Gerique JA, Ruiz Pérez VL, Heath KE, et al. Clinical, biochemical and genetic spectrum of low alkaline phosphatase levels in adults. Eur J Intern Med 2016;29: 40–5. doi: 10.1016/j.ejim.2015.12.019 [DOI] [PubMed] [Google Scholar]
- 28.Gehring B, Mornet E, Plath H, Hansmann M, Bartmann P, Brenner RE. Perinatal hypophosphatasia: diagnosis and detection of heterozygote carriers within the family. Clin Genet 1999;56: 313–7. doi: 10.1034/j.1399-0004.1999.560409.x [DOI] [PubMed] [Google Scholar]
- 29.Simon-Bouy B, Taillandier A, Fauvert D, Brun-Heath I, Serre JL, Armengod CG, et al. Hypophosphatasia: molecular testing of 19 prenatal cases and discussion about genetic counseling. Prenat Diagn 2008;28: 993–8. doi: 10.1002/pd.2088 [DOI] [PubMed] [Google Scholar]
- 30.Bianchi ML. Hypophosphatasia: an overview of the disease and its treatment. Osteoporos Int 2015;26: 2743–57. doi: 10.1007/s00198-015-3272-1 [DOI] [PubMed] [Google Scholar]
- 31.Reibel A, Manière MC, Clauss F, Droz D, Alembik Y, Mornet E, et al. Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J Rare Dis 2009;4: 6. doi: 10.1186/1750-1172-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann C, Girschick H, Mornet E, Schneider D, Jakob F, Mentrup B. Unexpected high intrafamilial phenotypic variability observed in hypophosphatasia. Eur J Hum Genet 2014;22: 1160–4. doi: 10.1038/ejhg.2014.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyte MP. Hypophosphatasia: Enzyme Replacement Therapy Brings New Opportunities and New Challenges. J Bone Miner Res 2017;32: 667–75. doi: 10.1002/jbmr.3075 [DOI] [PubMed] [Google Scholar]
- 34.Tenorio J, Álvarez I, Riancho-Zarrabeitia L, Martos-Moreno GA, Mandrile G, de la Flor Crespo M, et al. Molecular and clinical analysis of ALPL in a cohort of patients with suspicion of Hypophosphatasia. Am J Med Genet A 2017;173: 601–10. doi: 10.1002/ajmg.a.37991 [DOI] [PubMed] [Google Scholar]
- 35.Whyte MP, Madson KL, Phillips D, Reeves AL, McAlister WH, Yakimoski A, et al. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight 2016;1: e85971. doi: 10.1172/jci.insight.85971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okazaki Y, Kitajima H, Mochizuki N, Kitaoka T, Michigami T, Ozono K. Lethal hypophosphatasia successfully treated with enzyme replacement from day 1 after birth. Eur J Pediatr 2016;175: 433–7. doi: 10.1007/s00431-015-2641-2 [DOI] [PubMed] [Google Scholar]
- 37.Ozono K, Yamagata M, Michigami T, Nakajima S, Sakai N, Cai G, et al. Identification of novel missense mutations (Phe310Leu and Gly439Arg) in a neonatal case of hypophosphatasia. J Clin Endocrinol Metab 1996;81: 4458–61. [DOI] [PubMed] [Google Scholar]
- 38.Sutton RA, Mumm S, Coburn SP, Ericson KL, Whyte MP. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J Bone Miner Res 2012;27: 987–94. doi: 10.1002/jbmr.1565 [DOI] [PubMed] [Google Scholar]
- 39.Cundy T, Michigami T, Tachikawa K, Dray M, Collins JF, Paschalis EP, et al. Reversible deterioration in hypophosphatasia caused by renal failure with bisphosphonate treatment. J Bone Miner Res 2015;30: 1726–37. doi: 10.1002/jbmr.2495 [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharyya T, Jha S, Wang H, Kastner DL, Remmers EF. Hypophosphatasia and the risk of atypical femur fractures: a case-control study. BMC Musculoskelet Disord 2016;17: 332. doi: 10.1186/s12891-016-1191-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockler S, Plecko B, Gospe SM, Jr, Coulter-Mackie M, Connolly M, van Karnebeek C, et al. Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol Genet Metab 2011;104: 48–60. doi: 10.1016/j.ymgme.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 42.Baumgartner-Sigl S, Haberlandt E, Mumm S, Scholl-Bürgi S, Sergi C, Ryan L, et al. Pyridoxine-responsive seizures as the first symptom of infantile hypophosphatasia caused by two novel missense mutations (c.677T>C, p.M226T; c.1112C>T, p.T371I) of the tissue-nonspecific alkaline phosphatase gene. Bone 2007;40: 1655–61. doi: 10.1016/j.bone.2007.01.020 [DOI] [PubMed] [Google Scholar]
- 43.de Roo MGA, Abeling NGGM, Majoie CB, Bosch AM, Koelman JHTM, Cobben JM, et al. Infantile hypophosphatasia without bone deformities presenting with severe pyridoxine-resistant seizures. Mol Genet Metab 2014;111: 404–7. doi: 10.1016/j.ymgme.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 44.Barcia JP, Strife CF, Langman CB. Infantile hypophosphatasia: treatment options to control hypercalcemia, hypercalciuria, and chronic bone demineralization. J Pediatr 1997;130: 825–8. doi: 10.1016/S0022-3476(97)80029-7 [DOI] [PubMed] [Google Scholar]
- 45.Demirbilek H, Alanay Y, Alikaşifoğlu A, Topçu M, Mornet E, Gönç N, et al. Hypophosphatasia presenting with pyridoxine-responsive seizures, hypercalcemia, and pseudotumor cerebri: case report. J Clin Res Pediatr Endocrinol 2012;4: 34–8. doi: 10.4274/jcrpe.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whyte MP, Leelawattana R, Reinus WR, Yang C, Mumm S, Novack DV. Acute severe hypercalcemia after traumatic fractures and immobilization in hypophosphatasia complicated by chronic renal failure. J Clin Endocrinol Metab 2013;98: 4606–12. doi: 10.1210/jc.2013-1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollis A, Arundel P, High A, Balmer R. Current concepts in hypophosphatasia: case report and literature review. Int J Paediatr Dent 2013;23: 153–9. doi: 10.1111/j.1365-263X.2012.01239.x [DOI] [PubMed] [Google Scholar]
- 48.Atar M, Körperich EJ. Systemic disorders and their influence on the development of dental hard tissues: a literature review. J Dent 2010;38: 296–306. doi: 10.1016/j.jdent.2009.12.001 [DOI] [PubMed] [Google Scholar]