Abstract
Fibroblast growth factor 2 (FGF2) and fibroblast growth factor receptors (FGFRs) are key regulatory factors in osteoarthritis (OA). HMWTg mice overexpress the high molecular weight FGF2 isoforms (HMWFGF2) in osteoblast lineage and phenocopy both Hyp mice (which overexpress the HMWFGF2 isoforms in osteoblasts and osteocytes) and humans with X-linked hypophosphatemia (XLH). We previously reported that, similar to Hyp mice and XLH subjects who develop OA, HMWTg mice also develop an OA phenotype associated with increased degradative enzymes and increased FGFR1 compared with VectorTg mice. Therefore, in this study, we examined whether in vivo treatment with the FGFR tyrosine kinase inhibitor NVP-BGJ398 (BGJ) would modulate development of the OA phenotype in knee joints of HMWTg mice. VectorTg and HMWTg mice (21 days of age) were treated with vehicle or BGJ for 13 weeks. Micro–computed tomography images revealed irregular shape and thinning of the subchondral bone with decreased trabecular number and thickness within the epiphyses of vehicle-treated HMWTg knees, which was partially rescued following BGJ treatment. Articular cartilage thickness was decreased in vehicle-treated HMWTg mice, and was restored to the cartilage thickness of VectorTg mice in the BGJ-treated HMWTg group. Increased OA degradative enzymes present in HMWTg vehicle-treated joints decreased after BGJ treatment. OA in HMWTg mice was associated with increased Wnt signaling that was rescued by BGJ treatment. This study demonstrates that overexpression of the HMWFGF2 isoforms in preosteoblasts results in osteoarthropathy that can be partially rescued by FGFR inhibitor via reduction in activated Wnt signaling.
Keywords: FGF receptor inhibitor, osteoarthritis, cartilage, FGF2 HMWTg mice
Osteoarthritis (OA) is the most common form of arthritis and has a major negative impact on human health and cost to the health care system. Degenerative osteoarthropathy, a form of OA, is prevalent in young individuals with X-linked hypophosphatemia (XLH) (1) and common to all older XLH patients; it is associated with significant morbidity (2, 3), and there is no effective therapy. The Hyp mouse homolog of XLH also develops severe OA (4, 5). We previously reported that transgenic mice that overexpress the high molecular weight (HMW) fibroblast growth factor 2 (FGF2) isoforms (HMWFGF2) in osteoblast lineage cells (HMWTg mice) phenocopy the Hyp mouse (which overexpress HMWFGF2 in osteoblasts and osteocytes) (6); the HMWTg mice spontaneously develop OA, as do Hyp mice and XLH patients (7). The OA phenotype in HMWTg is associated with increased FGF23 and increased fibroblast growth factor receptor 1 (FGFR1) expression in their joints (7).
Classical findings in the early stages of OA include degeneration of the articular cartilage (8), osteophyte formation (9), and thinning of the subchondral bone due to increases in bone resorption, followed by sclerosis of bone in later stages (10). In the articular cartilage, increased catabolic activity results in breakdown of the matrix due to upregulation of metalloprotease enzymes, including matrix metalloproteinase 13 (MMP-13) and a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5) (11–13).
Several reports have indicated a role for members of the fibroblast growth factor (FGF) family, especially FGF2, in modulating cartilage and joint homeostasis (14, 15). There is conflicting evidence which demonstrates both catabolic and anabolic potential of FGF2 in the joint. Specifically, exogenous FGF2 simulates MMP-13 and ADAMTS5 production in articular cartilage explants in vitro (14, 16) and the level of FGF2 in OA synovial fluid is markedly increased compared with that of healthy knees (15), consistent with a catabolic function. However as previously reported, Fgf2 knockout (Fgf2ALLKO) mice develop spontaneous and surgically-induced OA more frequently and severely compared with their wild-type (WT) littermates (16, 17), providing support for an anabolic role for FGF2 in the joint.
Of significance, the FGF2 gene encodes multiple protein isoforms, including several HMW isoforms as well as a low molecular weight (LMW) isoform. The LMW isoform (18 kDA) is translated from the methionine AUG start codon, whereas the HMW isoforms are initiated from CUG codons upstream of the LMW start site (18). While there are 3 HMWFGF2 isoforms (22, 22.5, 24 kDa) in humans, there are only 2 HMWFGF2 isoforms (21, 22 kDa) in rodents. All HMWFGF2 isoforms contain a nuclear localization sequence where they function as intracrine factors (19). In humans and rodents there is 1 LMWFGF2 isoform, which does not have a signal peptide but is transported out of the cell to act as an autocrine/paracrine growth factor (19). Of relevance, we previously reported that mice that overexpress the HMWFGF2 isoforms in osteoprogenitor cells develop degenerative joint disease (7), associated with altered FGFR signaling.
The scientific literature reveals that the role of FGFRs in OA is also controversial. FGFR1 is known to have an important role in OA development in humans and mice (20, 21). Binding of FGF2 to FGFR1 leads to a cascade of events that are typically catabolic in articular chondrocytes, including activation of mitogen-activated protein kinase (MAPK) pathways (22). FGF2 also binds to FGFR3 with high affinity, and activation of FGFR3 has been shown to have anabolic effects in articular cartilage (23, 24). Previous studies have shown that the development of OA could be due to the activation of hypertrophic differentiation of articular chondrocytes (25) and increased/activation of multiple signaling pathways (26).
There are many receptors and ligands that contribute to the complexity of OA, and little knowledge of their downstream signaling pathways contributing to the osteoarthropathy observed in Hyp mice or humans with XLH. As noted, FGFRs activate multiple downstream signaling pathways including Wnt/β-catenin signaling in chondrocytes (27). Modulation of Wnt signaling can have differing effects on cartilage homeostasis (28–30). We previously reported (31) that there was a reduction in the Wnt inhibitors Sost and Dkk1in the joints of HMWTg mice, associated with increased canonical Wnt pathway activation. These findings were consistent with reports by other investigators that increased Sost and Dkk1 levels are protective against cartilage degradation in OA in mice (28, 29). We also reported that the OA phenotype in HMWTg joints was associated with an increase in the Wnt receptor low-density lipoprotein receptor-related protein 5 (LRP5) and decreased LRP6 (31), supporting the studies of other investigators that LRP5 is necessary for Wnt-induced cartilage degradation via upregulation of MMPs (30).
The aim of this study was to examine whether treatment with an FGFR tyrosine kinase inhibitor would modulate the development of OA phenotype in HMWTg mice as well as the WNT downstream signaling pathways that induce activation of transcription factors and genes that regulate expression of hypertrophic genes/protein. The results of this novel study are of particular importance given the fact that there are no reports of the efficacy of FGFR inhibitors in modulating OA phenotype in XLH or Hyp mice, and this study for the first time shows the potential efficacy of FGFR blockade in OA treatment.
Materials and Methods
Experimental animals
The UConn Health Institute of Animal Care and Use Committee approved all animal protocols. We previously reported on the generation and characterization of the long bone phenotype of the HMWTg mice, which overexpress HMW isoforms of hFGF2 in a bone-specific manner (6). Col3.6-HMWFgf2 isoform-IRES-GFPsaph was constructed by replacing a CAT fragment in previously made Col3.6-CAT-IRES-GFPsaph with a human HMWFgf2 cDNA between AfeI and ScaI sites. This expression construct is capable of concurrently overexpressing the human HMWFGF2 isoforms (32) and GFPsaph from a single bicistronic mRNA. Generation of the Fgf2 cDNAs was previously described (32). As a control, a Col3.6-IRES/GFPsaph (VectorTg) construct was created and purified according to standard protocols. Microinjections into the pronucleus of fertilized oocytes were performed at the Gene Targeting & Transgenic Facility at UConn Health. Founder mice of the F2 (FVBN) strain were mated with wild-type mice to establish individual transgenic lines. Homozygote mice were generated by mating heterozygote male with heterozygote female. Both male and female mice were used in this study.
FGFR inhibitor treatment
NVP-BGJ398 (BGJ) is a novel selective pan-specific FGFR inhibitor. BGJ (2 mg/kg body weight; Chemie Tek, Indianapolis, IN) or vehicle only (hydrochloric acid 3.5 mM, dimethyl sulfoxide 5%) was administered to VectorTg and HMWTg mice by daily subcutaneous injection for 13 weeks. Injection was initiated at 21 days of age. The mice were sacrificed at 4 months of age for sample collection. Most data reported in this study are from both male and female mice, unless otherwise specified. Mice were euthanized by carbon dioxide for sample collection.
Radiological analyses and micro–computed tomography (microCT)
As previously described (31), digital x-ray images of murine knee joints taken in the sagittal plane were obtained with a UltraFocus from Faxitron Bioptics LLC (Tucson, AZ) and taken under constant conditions. Imaging of knee architecture was performed using ex vivo microCT (μCT40, ScanCo Medical, Bruttisellen, Switzerland). The regions of subchondral trabecular bone of the epiphysis in the femur and tibia were analyzed by 3-dimensional microCT for the following morphometric parameters: bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular spacing (Tb.Sp).
Histologic assessment
Whole knee joints were removed and fixed in 4% paraformaldehyde for 48 hours, then decalcified with 14% EDTA solution at 4°C for 7 days. Samples were processed for paraffin embedding in a frontal orientation and 6-μm sections were obtained. Following de-paraffinization and rehydration of the sections, Safranin-O staining of glycosaminoglycans was performed using 0.1% aqueous Safranin-O and counterstained with Weigert’s iron hematoxylin and 0.02% aqueous Fast Green. Alkaline phosphatase staining was performed by incubating tissue with 100 mM Tris-HCl buffer (pH, 8.2) for 10 minutes, followed by incubation with Vector Blue Alkaline Phosphatase Substrate (Vector Laboratories Inc, Burlingame, CA) for 30 minutes. All histological images were captured using a Nikon TS100 microscope interfaced with SPOT software. Articular cartilage thickness was determined by measuring the mean distance from the articular cartilage surface to the subchondral bone interface in Safranin-O stained sections using the OsteoMeasure image analysis system (R & M Biometrics, Nashville, TN) equipped with a Nikon E400 microscope (Nikon Inc., Melville, NY). The severity of cartilage destruction was assessed histologically using Safranin-O stained sections using the Osteoarthritis Research Society International (OARSI) histologic scoring system (33) on all articular surfaces within each representative section from each knee.
RNA isolation and real time PCR
After removal of skin and muscle from the mice, total RNA was isolated from whole knee joints, including all structures of the joint above the growth plate as previously described (7). Samples were snap-frozen by liquid nitrogen and TRIzol reagent (Invitrogen, Carlsbad, CA) was used to extract RNA from the tissue. For real‐time quantitative RT-PCR (qRT-PCR) analysis, RNA was reverse‐transcribed using the RNA to cDNA EcoDry™ Premix (Oligo dT) (Takara Bio USA Inc., Mountain View, CA). qPCR was carried out using the iTaq™ Universal SYBR® Green Supermix on a MyiQ™ instrument (BIO‐RAD Laboratories Inc., Hercules, CA). β-Actin was used as an internal reference for each sample. mRNA was normalized to the β‐Actin mRNA level and expressed as the fold‐change relative to the first sample for each experimental group. The primers for the genes of interest are listed in Table 1.
Table 1.
Primer Used in qRT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| βActin | 5’-atggctggggtgttgaaggt-3’ | 5’-atctggcaccacaccttctacaa-3’ |
| Fgfr1c | 5’-gactgctggagttaatacca-3’ | 5’-ctggtctctcttccagggct-3’ |
| Fgfr3 | 5’-gttctctctttgtagactgc-3’ | 5’-agtacctggcagcacca-3’ |
| Fgf18 | 5’-aaggagtgcgtgttcattgag-3’ | 5’-agcccacataccaaccagagt-3’ |
| Col10a1 | 5’-gggaccccaaggacctaaag-3’ | 5’-gcccaactagacctatctcacct-3’ |
| Mmp13 | 5’-ctttggcttagaggtgactgg-3’ | 5’-aggcactccacatcttggttt-3’ |
Immunohistochemistry
Immunohistochemical staining was performed as previously described (31) on 6-μm paraffin embedded sections using the ImmPRESSTM HRP REAGENT KITs (VectorTg Laboratories, Burlingame, CA). After sections were de-paraffinized and rehydrated, slides underwent antigen retrieval by incubation at 60°C with 10 mM sodium citrate buffer for overnight. Endogenous peroxidase activity was then blocked by incubating sections with 3% hydrogen peroxide in water for 15 minutes. Following blocking sections with 10% serum for 30 minutes at room temperature, the slides were incubated with primary antibodies in blocking buffer overnight at 4°C. A negative control slide was used each time, which was instead incubated with blocking serum overnight. Details of the primary antibodies used, which include antibodies against FGF2 (34), FGF23 (35), pFGFR1 (36), pFGFR3 (37), SOX9 (38), MMP13 (39), ADAMTS5 (40), WNT7b (41), pLRP5 (42), pLPR6 (43), DKK1 (44), SOST (45), AXIN2 (46), pGSK3β (47), and active β-actin (48), are in Table 2. Slides were then washed with phosphate-buffered saline and incubated with the appropriate biotinylated secondary antibody at room temperature for 30 minutes. Finally, slides were washed and developed with DAB Peroxidase Substrate kit (VectorTg Laboratories, Burlingame, CA), and counterstained with Harris hematoxylin.The percentage of positive staining area was quantified using ImageJ64.
Table 2.
Antibody Table
| Protein Target | RRID | Antibody Name | Manufacture/Catalog Number | Species Raised (Monoclonal/Polyclonal) | Dilution |
|---|---|---|---|---|---|
| FGF-2 | AB_631497 | FGF-2 (147) antibody | Santa Cruz, sc-79 | Rabbit, polyclonal | 1/50 |
| FGF-23 | AB_2104623 | Anti-Mouse Fgf-23 antibody | R&D Systems, MAB26291 | Rat, monoclonal | 1/50 |
| pFGFR1 | AB_963497 | Anti-Human FGFR1, phospho (Tyr654) antibody | Abnova, PAB0471 | Rabbit, polyclonal | 1/50 |
| pFGFR-3 | AB_2103530 | p-FGFR-3 (Tyr 724) antibody | Santa Cruz, sc-33 041 | Rabbit, polyclonal | 1/100 |
| SOX9 | AB_2665492 | Sox9 (D8G8H) antibody | Cell Signaling Technology, #82630 | Rabbit, monoclonal | 1/600 |
| MMP13 | AB_776416 | Anti-Human MMP13 antibody | Abcam, ab39012 | Rabbit, polyclonal | 1/100 |
| ADAMTS5 | AB_2222327 | ADAMTS5 antibody | Abcam, ab41037 | Rabbit, polyclonal | 1/100 |
| WNT7b | AB_11172941 | Wnt7b antibody | GeneTex, GTX114881 | Rabbit, polyclonal | 1/100 |
| pLRP5 | AB_2721918 | LRP5 (phospho T1492) antibody | Abcam, ab203306 | Rabbit, polyclonal | 1/100 |
| pLRP6 | AB_10858068 | Phospho-LRP6 (Ser1490) Antibody | Bioss, bs-3253R | Rabbit, polyclonal | 1/100 |
| DKK1 | AB_2091333 | DKK1 antibody | Abcam, ab61034 | Rabbit, polyclonal | 1/100 |
| SOST | AB_2195345 | Goat Anti-Mouse Sost antibody | R&D Systems, AF1589 | Goat, polyclonal | 1/100 |
| AXIN2 | AB_10900607 | AXIN2 antibody | Sigma-Aldrich, SAB3500619 | Rabbit, polyclonal | 1/100 |
| pGSK3β | AB_1310290 | GSK3 beta (phospho Y216) antibody | Abcam, ab75745 | Rabbit, polyclonal | 1/50 |
| Active βActin | AB_2798251 | Non-phospho (Active) β-Catenin (Ser33/37/Thr41) (D13A1) antibody | Cell Signaling Technology, #8814 | Rabbit, monoclonal | 1/100 |
Serum intact FGF23 and estradiol measurement
Serum was prepared from blood that was collected from euthanized animals by cardiac puncture. Mice were euthanized at 24 hours after the last injection. Serum intact FGF23 level was measured suing a Mouse/Rat FGF-23 (Intact) ELISA Kit (Immutopics Inc, San Clemente, CA, USA) (49). Serum estradiol was measured using a Estradiol Parameter Assay Kit (R&D STSTEMS, Minneapolis, MN, USA) following the manufacturer’s instruction.
Statistical analysis
Experimental values are reported as mean ± standard error (SE). ANOVA followed by least significant difference (LSD) for post hoc multiple comparisons was used. SPSS software was used for statistical analysis (IBM Corp., Armonk, NY), and the results were considered significantly different at P < 0.05.
Results
Radiological and morphometric changes in knee joints of VectorTg and HMWTg mice treated with vehicle or BGJ
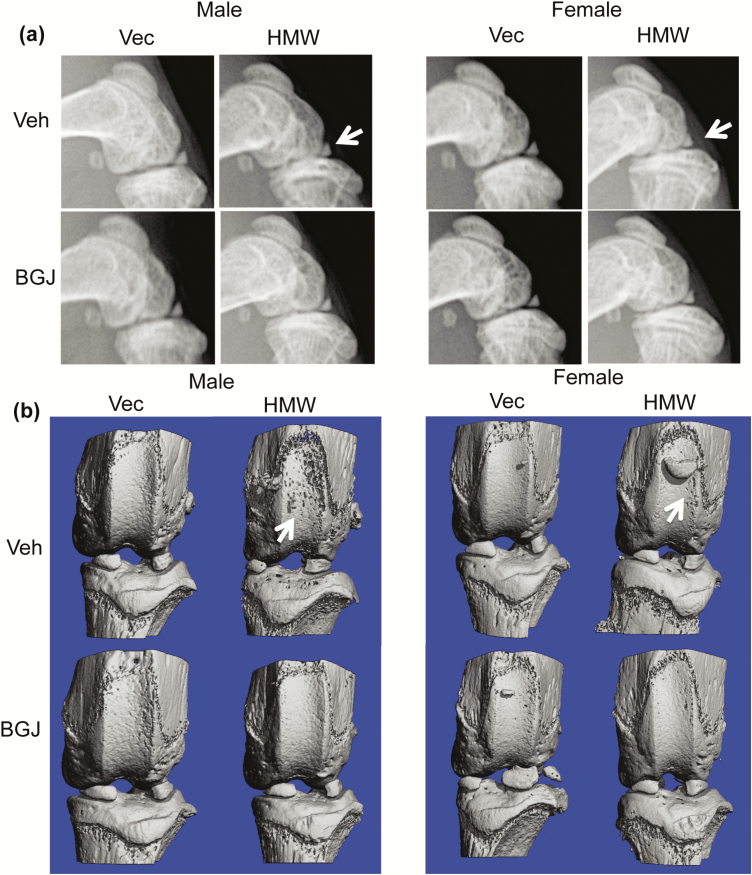
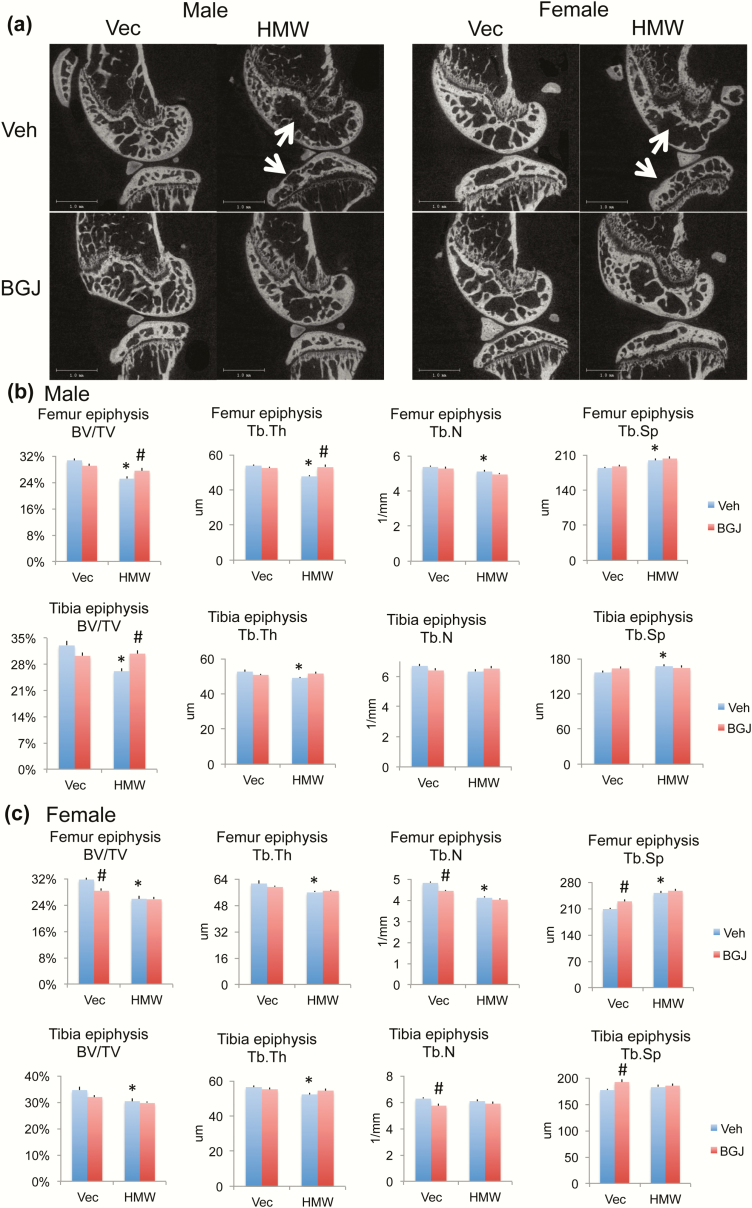
We previously reported that HMWTg knees displayed an OA phenotype and increased FGFR1 expression, and since FGFRs play a role in the pathogenesis of OA, we assessed whether blocking FGFRs could rescue the OA phenotype in HMWTg mice. As shown by digital radiography, the flattened tibia plateau, narrowing of the joint space, osteophytes, and uneven joint surfaces in the HMWTg vehicle-treated group was rescued following BGJ treatment in both male and female mice (Fig. 1a, arrows). Three-dimensional microCT images showed erosion of articular cartilage in the vehicle-treated HMWTg group, whereas BGJ treatment resulted in a phenotype resembling that of the VectorTg groups in both sexes (Fig. 1b, arrows). Two-dimensional sagittal microCT images reveal irregular shape and thinning of the subchondral bone in HMWTg vehicle-treated mice, which was rescued following BGJ treatment in both sexes (Fig. 2a, arrows). Three-dimensional morphometric parameters calculated from microCT of femoral and tibiae epiphyses showed the altered trabecular architecture indicative of early OA in both sexes. Specifically, there was significantly decreased BV/TV, Tb.Th, and Tb.N, as well as increased Tb.Sp in the femoral epiphysis of HMWTg vehicle-treated knees compared with VectorTg vehicle-treated epiphyses in both sexes. BGJ treatment significantly increased abnormal BV/TV and Tb.Th in the femoral epiphyses of HMWTg male mice. (Fig. 2b and 2c). There was significantly decreased BV/TV and Tb.Th in the tibial epiphyses of HMWTg vehicle-treated knees compared with VectorTg vehicle-treated epiphyses of both sexes. Trabecular spacing was significantly increased in the tibial epiphysis of HMWTg vehicle-treated knees compared with VectorTg vehicle-treated epiphyses in male mice. BGJ treatment significantly increased BV/TV of tibial epiphyses of HMWTg male mice (Fig. 2b and c). BGJ reduced BV/TV, Tb.N, and increased Tb.Sp in the femoral epiphysis of female VectorTg mice. BGJ also reduced Tb.N, and increased Tb.Sp in tibial epiphysis of female VectorTg mice.
Figure 1.
Radiographic and microCT images of knee joint from VectorTg and HMWTg mice treated with vehicle or BGJ. (a) Sagittal digital x-ray images of HMWTg knees show the flattened tibia plateau, narrowing of the joint space, osteophytes, and uneven joint surfaces in the HMWTg vehicle-treated group in both male and female mice, which was rescued following BGJ treatment in both sexes. (b) Representative 3-dimensional microCT images show erosion of articular cartilage in the HMWTg vehicle-treated group in both male and female mice, whereas BGJ treatment resulted in a phenotype resembling that of the VectorTg groups in both sexes. Representative images from n = 7–9 males/group, n = 7–8 females/group.
Figure 2.
MicroCT analysis of subchondral bone of knee joint from VectorTg and HMWTg mice treated with vehicle or BGJ. (a) Representative 2-dimensional sagittal microCT images reveal irregular shape and thinning of the subchondral bone in HMWTg vehicle-treated group in both male and female mice, which was rescued following BGJ treatment in both sexes. Morphometric parameters calculated from 3-dimensional microCT of femoral and tibiae epiphyses of (b) male and (c) female mice. There is significantly decreased bone volume per total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and increased trabecular spacing (Tb.Sp) in femoral epiphysis of HMWTg vehicle-treated knees compared with VectorTg vehicle-treated epiphyses in both sexes. BGJ treatment significantly increased abnormal BV/TV and Tb.Th in male mice. There were significantly decreased BV/TV and Tb.Th in tibia epiphysis of HMWTg vehicle-treated knees compared with VectorTg vehicle-treated epiphyses in both genders. BGJ treatment significantly increased abnormal BV/TV in male mice. n = 7–9 males/group, n = 7–8 females/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA.
Histological changes in articular cartilage of VectorTg and HMWTg mice treated with vehicle or BGJ
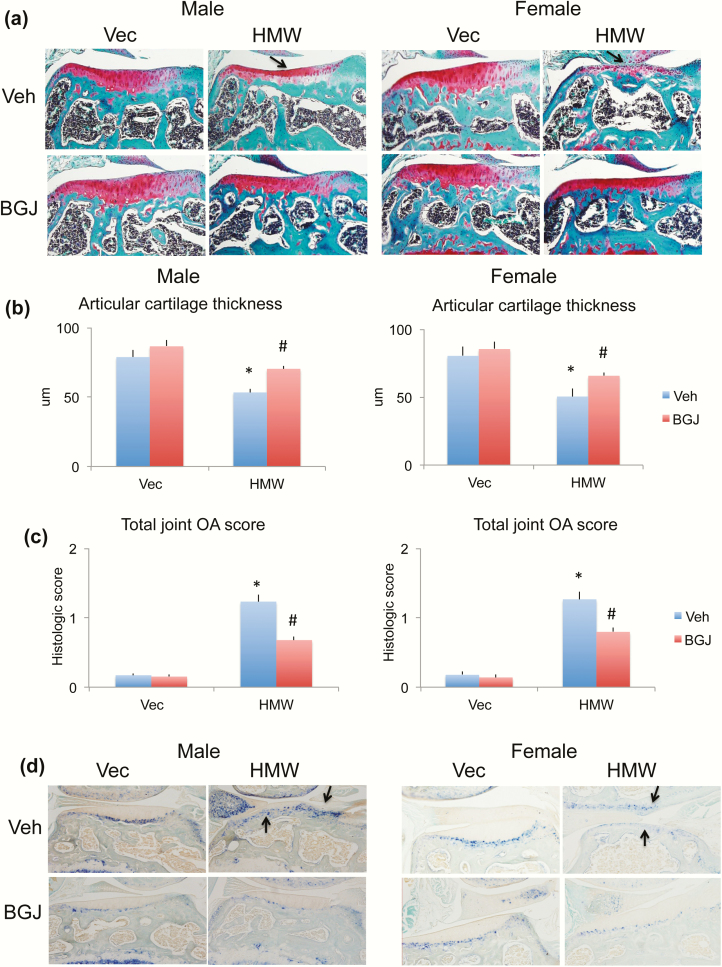
Articular cartilage integrity was determined histologically using Safranin-O staining to examine proteoglycan content in VectorTg and HMWTg mice with and without BGJ treatment. A dramatic decrease in Safranin-O staining (proteoglycan content) was found in HMWTg knees, particularly on the lateral aspect (Fig. 3a, arrows), which was partially rescued with BGJ treatment in both sexes. OsteoMeasure analysis showed that the average articular cartilage thickness was significantly decreased in HMWTg vehicle-treated knees compared with VectorTg vehicle-treated knees, which was partially rescued with BGJ treatment in both sexes (Fig. 3b). Mean total joint OA score was significantly increased in the HWTg-vehicle group compared with the VectorTg-vehicle group in both male and female mice, which was partially reversed with BGJ treatment in both sexes (Fig. 3c). Alkaline phosphatase staining was used to visualize the expression and localization of calcified cartilage along with hypertrophic chondrocytes, which was markedly increased in HMWTg vehicle-treated joints, invading the noncalcified area of the articular cartilage (indicating that the tidemark or mineralization front is moving towards the superficial layer of the cartilage, a sign of OA development), compared with the VectorTg vehicle-treated group in both sexes. These were partially normalized with BGJ treatment (Fig. 3d, arrows).
Figure 3.
Comparison of proteoglycan content and alkaline phosphatase of knee joint from VectorTg and HMWTg mice treated with vehicle or BGJ. (a) Safranin-O stained representative images showed a dramatic decrease in proteoglycan content in HMWTg knees (arrows) in both male and female mice, which was partially rescued with BGJ treatment in both sexes. (b) Mean lateral tibial articular cartilage thickness analyzed by OsteoMeasure showed a decrease in cartilage thickness in both male and female HMWTg mice, which was partially rescued with BGJ treatment in both sexes. n = 7–9 males/group, n = 7–8 females/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA. (c) Mean total joint OA score was significantly increased HWTg-vehicle group compared with VectorTg-vehicle group in both male and female mice, which was partially rescued with BGJ treatment in both sexes. n = 7–9 males/group, n = 7–8 females/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA. (d) Alkaline phosphatase staining showed more alkaline phosphatase-positive articular chondrocytes in HMWTg vehicle-treated joints of both male and female mice, these were partially normalized with BGJ treatment in both sexes. Representative images from n = 7–9 males/group, n = 7–8 females/group.
Expression of OA markers in knees of VectorTg and HMWTg mice treated with vehicle or BGJ
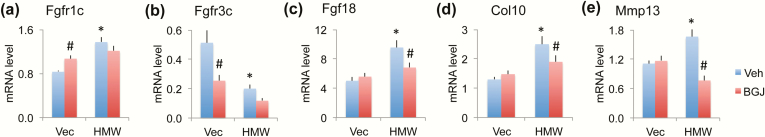
Since FGF2 signal through the receptors FGFR1 and FGFR3, which have been implicated in OA disease, we examined their mRNA and protein expression in knees. FGFR1c mRNA expression was significantly increased in whole joints of HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice; BGJ treatment significantly increased FGFR1c mRNA expression in VectorTg mice, but not in HMWTg mice (Fig. 4a). FGFR3c mRNA was significantly decreased in HMWTg mice treated with vehicle, and BGJ treatment significantly decreased FGFR3c mRNA in VectorTg mice but not in HMWTg mice (Fig. 4b). FGF18 has been reported to protect against OA, and thus increased levels could reflect an early repair process. We found that Fgf18 mRNA was significantly increased by 47% in HMWTg vehicle-treated joints compared with VectorTg vehicle-treated joints; this effect was rescued (counteracted) by BGJ treatment (Fig. 4c).
Figure 4.
Effect of BGJ on OA regulatory genes and OA marker genes in knees of VectorTg and HMWTg mice. RNA extracted from whole knee joint was used for gene expression. qPCR analysis of (a) Fgfr1c, (b) Fgfr3c, (c) Fgf18, (d) Col10, and (e) Mmp13. n = 7–9 males/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA.
We also examined the OA marker genes that are typically upregulated in OA articular cartilage, including type X collagen (Col10, hypertrophic chondrocyte marker) and MMP13 (the major aggrecan degrading enzyme in murine cartilage). Both Col10 and Mmp13 mRNA expression were significantly increased in HMWTg vehicle-treated joints compared with VectorTg vehicle-treated joints, which was rescued with BGJ treatment (Fig. 4d and 4e).
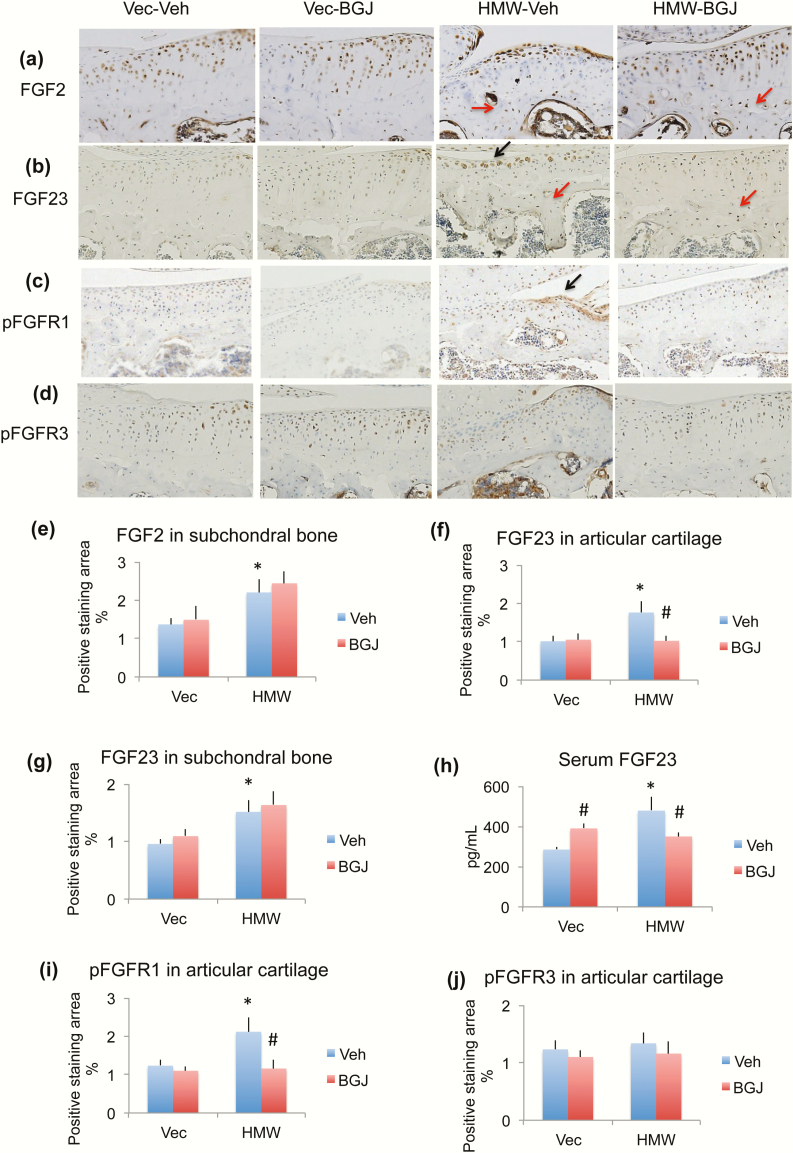
Since the Col1 promoter drives FGF2 overexpression in osteoblast lineage in HMWTg mice, we examined FGF2 protein in the knees by immunohistochemistry staining. As shown in Fig. 5a and 5e, FGF2 expression in chondrocytes of articular cartilage was similar among 4 groups. However, FGF2 expression in the osteocytes of subchondral bone was markedly increased in HMWTg vehicle-treated mice. BGJ treatment did not alter FGF2 expression in either VectorTg mice or HMWTg mice (red arrows).
Figure 5.
Effect of BGJ on OA regulatory proteins in knees of VectorTg and HMWTg mice. Representative immunohistochemical staining images for (a) FGF2, (b) FGF23, (c) pFGFR1, and (d) pFGFR3. Quantitative analysis of percentage of positive staining area for (e) FGF2 in subchondral bone, (f) FGF23 in articular cartilage, (g) FGF23 in subchondral bone, (i) pFGFR1 in articular cartilage, and (j) pFGFR3 in articular cartilage. (h) Serum FGF23 level. (a and e) FGF2 immunohistochemical staining showed that FGF2 expression in cartilage was similar among 4 groups. However, FGF2 expression in the osteocytes of subchondral bone was markedly increased in the HMWTg vehicle-treated group; BGJ treatment did not alter FGF2 expression in either VectorTg mice or HMWTg mice, n = 4–5 males/group. (b, f, and g) FGF23 protein expression was significantly increased in chondrocyte of articular cartilage and osteocytes of subchondral bone in knees from HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice. BGJ treatment significantly rescued abnormal FGF23 expression in articular cartilage, but not in subchondral bone, n = 7–9 males/group. (h) Serum FGF23 level was significantly increased in HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice. BGJ treatment significantly increased FGF23 serum level in VectorTg mice, but significantly decreased serum FGF23 level in HMWTg mice, n = 7–9 males/group. (c and i) phosphorylated FGFR1 (pFGFR1) was significantly increased in HMWTg-vehicle group compared with VectorTg-vehicle group, which was rescued with BGJ treatment, n = 7–9 males/group. (d and j) pFGFR3 protein expression was similar among all 4 groups, n = 4–5 males/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA.
Since we previously reported that HMWTg mice overexpress the HMWFGF2 isoforms, which results in increased FGF23 expression in serum and bone, we examined FGF23 expression in knees and serum. FGF23 protein expression was significantly increased in the chondrocytes of articular cartilage and osteocytes of subchondral bone in knees from HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice. BGJ treatment significantly rescued abnormal FGF23 expression in articular cartilage (black arrow), but not in subchondral bone (red arrows) (Fig. 5b, f, and g). We also found significantly increased serum FGF23 levels in HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice. BGJ treatment significantly increased FGF23 serum level in VectorTg mice, but significantly decreased serum FGF23 level in HMWTg mice (Fig. 5h).
OA associated with XLH is seen in both sexes; however, to ensure that the FGFR inhibition did not modulate estrogen expression, which is important in bone homeostasis, we measured serum estrogen levels and found no significant differences either by genotype or treatment. In vehicle-treated and BGJ-treated VectorTg mice, estradiol levels were 61 pg/mL and 52 pg/mL, respectively. In vehicle-treated HMWTg and BGJ-treated HMWTg mice, serum estradiol levels were 73 pg/mL and 63 pg/mL respectively.
Since FGFR1c mRNA was increased, we performed immunohistochemistry for the phosphorylated active form of FGFR1. Immunohistochemistry staining revealed that phosphorylated FGFR1 protein was localized in the articular cartilage and meniscus of the joints, and there is significantly increased phosphorylated FGFR1 (pFGFR1) expression in HMWTg-vehicle group compared with VectorTg-vehicle group, which was rescued with BGJ treatment (Fig. 5c and 5i, arrow). We also assessed phosphorylated FGFR3 protein expression and show that this was similar among all four groups (Fig. 5d and 5j.
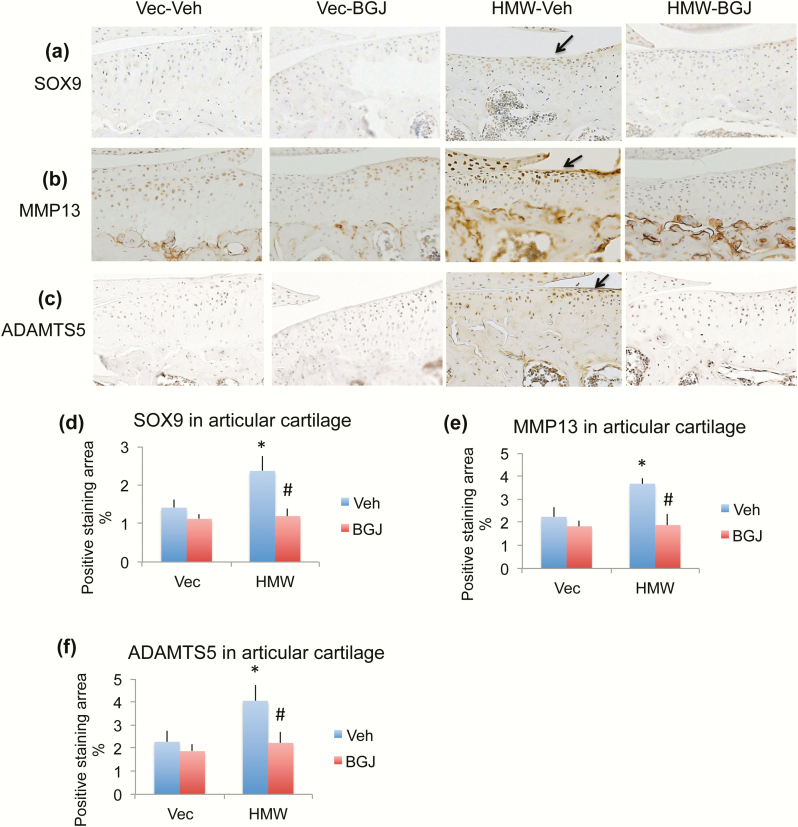
As noted, studies have shown there is an increase in SOX9 expression in human chondrocytes of early-stage OA, as well as in murine osteoarthritic cartilage, particularly in areas of developing osteophytes, therefore we examined the SOX9 protein by immunohistochemistry. As shown in Fig. 6a and 6d, SOX9 protein expression was significantly increased in chondrocytes of articular cartilage of knees from the HMWTg-vehicle group compared with the VectorTg-vehicle group, which was rescued with BGJ treatment (arrow). In addition, immunohistochemistry was utilized to determine the localization and expression of MMP13 and ADAMTS5. In HMWTg vehicle-treated knee joints, expression of MMP13 and ADAMTS5 was markedly increased particularly in the articular cartilage, compared with VectorTg vehicle-treated mice; these were rescued with BGJ treatment (Fig. 6b, 6c, 6e, 6f, arrows).
Figure 6.
Effect of BGJ on OA marker proteins in knees of VectorTg and HMWTg mice. (a and d) Representative immunohistochemical staining images for (a) SOX9, (b) MMP13, and (c) ADAMTS5. Quantitative analysis of percentage of positive staining area for (d) SOX9, (e) MMP13, and (f) ADAMTS5 in articular cartilage. (a and d) SOX9 protein expression was significantly increased in chondrocytes of articular cartilage of knees from HMWTg-vehicle group compared with VectorTg-vehicle group, which was rescued with BGJ treatment, n = 7–9 males/group. (b and e) MMP13 and (c and f) ADAMTS5 expression were increased in knees of HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice; these were rescued with BGJ treatment, n = 7–9 males/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA.
Expression of Wnt signaling components in VectorTg and HMWTg knee joints treated with vehicle or BGJ
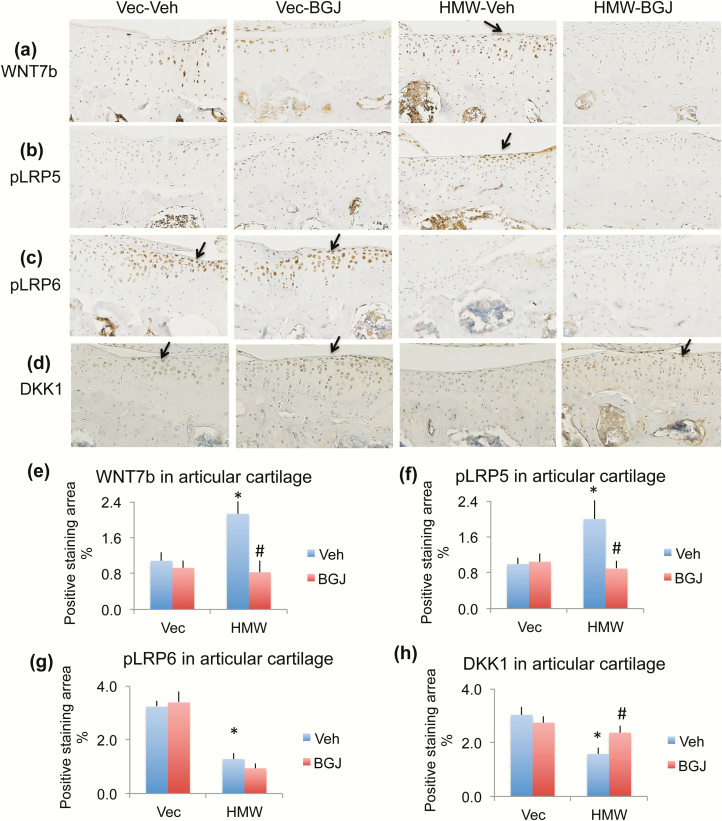
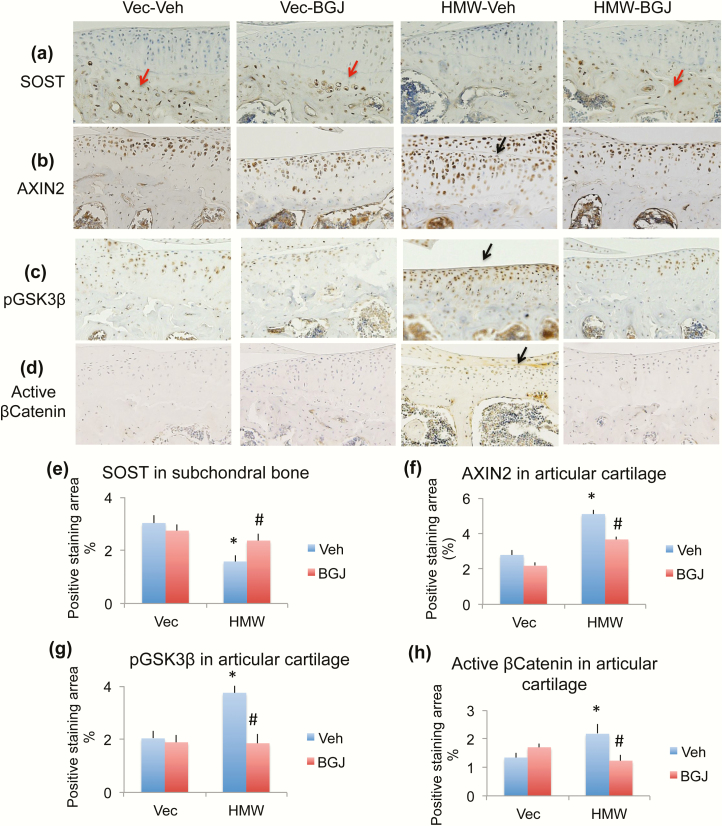
Previously we reported that canonical Wnt signaling contributes to the OA phenotype observed in HMWTg mice (31). To determine if the rescue effect of BGJ on OA in HMWTg is through regulating Wnt signaling, we examined protein expression of components of the Wnt signaling pathway. Ligand WNT7B was greatly increased in HMWTg vehicle-treated cartilage compared with VectorTg vehicle-treated group, which was rescued with BGJ treatment (Fig. 7a and 7e, arrow). Phosphorylated receptor LRP5 was strongly expressed in HMWTg vehicle-treated cartilage compared with VectorTg vehicle-treated group, which was rescued with BGJ treatment (Fig. 7b and 7f, arrow). Interestingly, phosphorylated receptor LRP6 protein was decreased in HMWTg vehicle-treated and HMWTg BGJ-treated articular cartilage, compared with VectorTg cartilage (Fig. 7c and 7g, arrows). DKK1, an inhibitor of Wnt signaling, protein expression was significantly decreased in chondrocytes of articular cartilage of HMWTg-vehicle knees compared with VectorTg vehicle-treated mice, which was rescued with BGJ treatment (Fig. 7d and 7h, arrows). SOST, a Wnt inhibitor, was only expressed in osteocytes in the subchondral bone, but not expressed in articular cartilage. SOST expression in the subchondral bone of HMWTg vehicle-treated mice was much lower than VectorTg vehicle-treated mice, which was rescued with BGJ treatment (Fig. 8a and 8e, red arrows). AXIN2 (a member of the ß-catenin destruction complex, ultimately leading to the degradation of ß-catenin) was increased in the cartilage of HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice, indicating Wnt signaling activation. BGJ treatment markedly inhibited the increased AXIN2 in HMWTg mice (Fig. 8b and 8f, arrow). Phosphorylated inactive GSK3β, a downstream component of canonical Wnt signaling, was strongly expressed in HMWTg vehicle-treated cartilage compared with VectorTg vehicle-treated and VectorTg BGJ-treated groups, and was rescued with BGJ treatment (Fig. 8c and 8g, arrow). To determine the effect of blocking FGFR signaling on the specifically canonical Wnt signaling, in which β-catenin does not become degraded, we examined nonphosphorylated ß-catenin (active form) by immunohistochemistry. As shown in Fig. 8d and 8h (arrow), active β-catenin protein expression was significantly increased in articular cartilage of knees from HMWTg-vehicle group compared with VectorTg-vehicle group, which was rescued with BGJ treatment.
Figure 7.
Effect of BGJ on Wnt signaling component proteins in knees of VectorTg and HMWTg mice. Representative immunohistochemical staining images for (a) WNT7b, (b) phosphorylated LRP5 (pLRP5), (c) pLRP6, and (d) DKK1. Quantitative analysis of percentage of positive staining area for (e) WNT7b, (f) pLRP5, (g) pLRP6, and (h) DKK1 in articular cartilage. (a and e) WNT7b was greatly increased in HMWTg vehicle-treated cartilage compared with VectorTg vehicle-treated group, which was rescued with BGJ treatment, n = 7–9 males/group. (b and f) pLRP5 was strongly expressed in HMWTg vehicle-treated cartilage compared with VectorTg vehicle-treated group, which was rescued with BGJ treatment, n = 7–9 males/group. (c and g) pLRP6 protein was decreased in HMWTg vehicle-treated and HMWTg BGJ-treated articular cartilage, compared with VectorTg cartilage, n = 7–9 males/group. (d and h) DKK1 was significantly decreased in chondrocytes of articular cartilage of HMWTg-vehicle knees compared with VectorTg vehicle-treated mice, which was rescued with BGJ treatment, n = 4–5 males/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA.
Figure 8.
Effect of BGJ on Wnt signaling component proteins in knees of VectorTg and HMWTg mice. Representative immunohistochemical staining images for (a) SOST, (b) AXIN2, (c) phosphorylated GSK3β (pGSK3β), and (d) active β-catenin. Quantitative analysis of percentage of positive staining area for (e) SOST in subchondral bone, (f) AXIN2 in articular cartilage, (g) pGSK3β in articular cartilage, and (h) active β-catenin in articular cartilage. (a and e) SOST was only expressed in osteocytes in the subchondral bone, but not expressed in articular cartilage. SOST expression in the subchondral bone of HMWTg vehicle-treated mice was much lower than VectorTg vehicle-treated mice, which was rescued with BGJ treatment, n = 4–5 males/group. (b and f) AXIN2 was increased in the cartilage of HMWTg vehicle-treated mice compared with VectorTg vehicle-treated mice. BGJ treatment markedly inhibited the increased AXIN2 in HMWTg mice, n = 4–5 males/group. (c and g) pGSK3β was strongly expressed in HMWTg vehicle-treated cartilage compared with VectorTg vehicle-treated and VectorTg BGJ-treated groups, and was rescued with BGJ treatment, n = 7–9 males/group. (d and h) Active β-Catenin protein expression was significantly increased in articular cartilage of knees from HMWTg-vehicle group compared with VectorTg-vehicle group, which was rescued with BGJ treatment, n = 7–9 males/group. *compared with VectorTg-vehicle group P < 0.05; #compared with corresponding vehicle group P < 0.05 by 2-way ANOVA.
Discussion
We previously reported that HMWTg mice demonstrated OA affecting the knees at 2 months of age and progressively worsening by 18 months of age to total destruction of the knee joints and complete loss of articular cartilage with osteophyte formation, as demonstrated by microCT and histomorphometry (7). We also reported a progressive decrease in proteoglycan content, thinning of the articular cartilage, and increased production of proteolytic degradative enzymes MMP13 and ADAMTS5 in the knee joints, as determined by gene and protein analysis (7). Since these earlier studies (7, 31) also demonstrated an increase in FGFR1 mRNA and protein, our goals were to (1) determine whether earlier in vivo treatment of weanling HMWTg mice with the FGFR inhibitor BGJ would modulate the development of the OA phenotype; and (2) whether FGFR inhibition would modulate aberrant Wnt signaling in the articular cartilage of HMWTg mice.
In the present studies we observed OA changes in all components of the knee joint, including the articular cartilage and subchondral bone of the HMWTg male mice, which were partially rescued by in vivo administration of the FGFR inhibitor BGJ. Consistent with this earlier report, we confirmed microCT abnormalities of the epiphysis in HMWTg male and now demonstrate similar abnormalities in the epiphysis of HMWTg female mice. BGJ rescued the epiphyseal bone abnormalities in male but not female HMWTg mice. Interestingly, we observed a negative effect of BGJ treatment in VectorTg female mice. Our observation that FGFR tyrosine kinase inhibitor negatively affected the bone parameters in VectorTg female mice is consistent with FGFR expression on the ovaries and this inhibition in normal bone homeostasis could alter FGF regulation of estrogen, which is important in maintaining bone mass (50). However, our result demonstrated no significant difference in serum estradiol level based on genotype or treatment, suggesting that there was no effect of BGJ on estradiol. As discussed earlier, previous studies have shown that the progression of OA may be caused by increased hypertrophic differentiation of articular chondrocytes and mineralization of the articular cartilage (25). Interestingly, increased alkaline phosphatase was observed in articular cartilage of vehicle-treated HMWTg knees, which was rescued by FGFR inhibition. These results suggest that FGFR inhibitor can block the increased chondrocytic differentiation and thus prevent the migration of the mineralization front/tidemark towards the nonmineralized articular cartilage in HMWTg knee joints.
XLH patients develop OA that can be severe enough to warrant joint replacement (51) and Hyp mice, a homolog of XLH that overexpresses HMWFGF2 isoforms in osteocytes, also develop OA (5). While FGFR1 is increased in human OA chondrocytes and mediates cartilage destruction (21), and genetic inhibition of FGFR1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice (22), there have been no reported studies of the efficacy of FGFR inhibition on the OA phenotype, either in XLH patients or in Hyp mice (which HMWTg mice phenocopy).
The interplay of FGFR1 and FGFR3 is important in cartilage homeostasis, with the preponderance of studies supporting FGFR1 being catabolic and FGFR3 being anabolic. Specifically, FGFR3 was found to be decreased in human OA chondrocytes (52), it increased anabolic activity in cartilage (53), and its ablation increased murine OA (54). Given that the FGFR inhibitor BGJ is not specific for FGFR1, we also examined the potential contribution of modulation of FGFR3 to the OA phenotype. We observed an increase in FGFR1 mRNA in the whole knee joint as well as phosphorylated FGFR1 protein in knee articular cartilage, which was reduced by BGJ. In contrast, FGFR3 mRNA was significantly reduced in vehicle-treated HMWTg knees and was not rescued by BGJ. This would suggest that in HMWTg mice, activation of FGFR1 was the major contributor to the observed OA phenotype.
Altered Wnts and Wnt-related proteins have been reported in OA and although quite complex, modulating the Wnt pathway is considered a promising target for OA therapy using small molecule inhibitors (55). However, small molecule FGFR inhibitors such as BGJ, have not typically been under consideration, since there are limited studies on FGFR modulation of Wnt signaling contributing to OA. Of relevance to this study, we previously reported by gene and protein analysis that there was increased Wnt signaling in HMWTg OA whole knee joints demonstrated by decreased Dkk1, Sost, and Lrp6 expression with increased Wnt5a, Wnt7b, Lrp5, Axin2, phospho-GSK3β, Lef1, and nuclear β-catenin accumulation (31) associated with increased FGFR1. In the same study, we also reported that there was increased FGFR1, increased canonical WNT7b, and increased GSK3β in articular chondrocytes harvested from HMWTg knee joints (31).
We also previously reported that FGF23 contributed to OA phenotype in HMWTg mice via aberrant Wnt signaling and that in vivo administration of FGF23 neutralizing antibody partially rescued the OA phenotype in HMWTg mice (31). Since FGF23 signals via FGFR, activation of FGF23/FGFR/Wnt could contribute to the OA phenotype in HMWTg mice. It has been reported that selective ablation of FGFR1 in osteocytes reduced FGF23 expression in bone (56), and we observed a significant decrease in serum FGF23 in the BGJ-treated HMWTg mice. Thus, it is possible that FGFR blockade resulted in reduced FGF23, leading to reduced Wnt activity with partial rescue of the OA phenotype observed in this study.
Although the FGF receptor inhibitor BGJ is currently in clinical use for treatment of certain diseases (57), and BGJ was shown by Wohrle et al (58) to ameliorate rickets and hypophosphatemia in the Hyp and Dmp1ko mouse models, there are no reports of its efficacy in osteoarthropathy. Since Hyp mice overexpress HMWFGF2 isoforms in osteocytes (6), it will be of interest to determine whether, consistent with the present study, FGFR inhibition also partially rescues the OA phenotype in Hyp mice.
Acknowledgments
Financial Support: This project is supported in part by National Institutes of Health grant NIH AR072985-05A1.
Glossary
Abbreviations:
- ADAMTS5
disintegrin and metalloproteinase with thrombospondin motifs 5
- BGJ
NVP-BGJ398
- BV/TV
bone volume/tissue volume
- Col10
type X collagen
- FGF2
fibroblast growth factor 2
- FGFR
fibroblast growth factor receptor
- HMW
high molecular weight
- HMWFGF2
high molecular weight FGF2 isoform
- LMW
low molecular weight
- LRP5
low-density lipoprotein receptor-related protein 5
- microCT
micro–computed tomography
- MMP-13
matrix metalloproteinase 13
- OA
osteoarthritis
- qRT-PCR
real‐time quantitative reverse-transcriptase polymerase chain reaction
- Tb.N
trabecular number
- Tb.Sp
trabecular spacing
- Tb.Th
trabecular thickness
- WT
wild-type
- XLH
X-linked hypophosphatemia
Author Contributions: MMH conceived and coordinated the study. MMH designed the study. LX performed and analyzed the experiments shown. MH and LX wrote the paper. DW provided technical assistance. All authors reviewed the results and approved the final version of the manuscript. MMH is the guarantor of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Information
Disclosure Summary: The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1. Econs MJ, Francis F. Positional cloning of the PEX gene: new insights into the pathophysiology of X-linked hypophosphatemic rickets. Am J Physiol. 1997;273(4):F489–F498. [DOI] [PubMed] [Google Scholar]
- 2. Hardy DC, Murphy WA, Siegel BA, Reid IR, Whyte MP. X-linked hypophosphatemia in adults: prevalence of skeletal radiographic and scintigraphic features. Radiology. 1989;171(2):403–414. [DOI] [PubMed] [Google Scholar]
- 3. Reid IR, Hardy DC, Murphy WA, Teitelbaum SL, Bergfeld MA, Whyte MP. X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine (Baltimore). 1989;68(6):336–352. [PubMed] [Google Scholar]
- 4. Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int. 2009;85(3):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang G, Vanhouten J, Macica CM. An atypical degenerative osteoarthropathy in Hyp mice is characterized by a loss in the mineralized zone of articular cartilage. Calcif Tissue Int. 2011;89(2):151–162. [DOI] [PubMed] [Google Scholar]
- 6. Xiao L, Naganawa T, Lorenzo J, Carpenter TO, Coffin JD, Hurley MM. Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J Biol Chem. 2010;285(4):2834–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meo Burt P, Xiao L, Dealy C, Fisher MC, Hurley MM. FGF2 high molecular weight isoforms contribute to osteoarthropathy in male mice. Endocrinology. 2016;159(6):2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karvonen RL, Negendank WG, Teitge RA, Reed AH, Miller PR, Fernandez-Madrid F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21(7):1310–1318. [PubMed] [Google Scholar]
- 9. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. [DOI] [PubMed] [Google Scholar]
- 10. Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38(2):234–243. [DOI] [PubMed] [Google Scholar]
- 11. Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. [DOI] [PubMed] [Google Scholar]
- 13. Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. [DOI] [PubMed] [Google Scholar]
- 14. Orito K, Koshino T, Saito T. Fibroblast growth factor 2 in synovial fluid from an osteoarthritic knee with cartilage regeneration. J Orthop Sci. 2003;8(3):294–300. [DOI] [PubMed] [Google Scholar]
- 15. Im HJ, Li X, Muddasani P, Kim GH, Davis F, Rangan J, et al. Basic fibroblast growth factor accelerates matrix degradation via a neuro‐endocrine pathway in human adult articular chondrocytes. J. Cell Physiol 2008;215(2):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis Rheum. 2009;60(7):2019–2027. [DOI] [PubMed] [Google Scholar]
- 17. Burt PM, Xiao L, Doetschman T, Hurley MM. Ablation of low-molecular-weight FGF2 isoform accelerates murine osteoarthritis while loss of high-molecular-weight FGF2 isoforms offers protection. J Cell Physiol. 2019;234(4):4418–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okada-Ban M, Thiery JP, Jouanneau J. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32(3):263–267. [DOI] [PubMed] [Google Scholar]
- 19. Azhar M, Yin M, Zhou M, Li H, Mustafa M, Nusayr E, Keenan JB, Chen H, Pawlosky S, Gard C, Grisham C, Sanford LP, Doetschman T. Gene targeted ablation of high molecular weight fibroblast growth factor-2. Dev Dyn. 2009;238(2):351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, Im HJ. Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem. 2013;114(4):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, Im HJ. Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther. 2011;13(4):R130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Weng T, Yi L, Huang J, Luo F, Wen X, Du X, Chen Q, Deng C, Chen D, Chen L. Genetic inhibition of fibroblast growth factor receptor 1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice. Arthritis Rheum. 2012;64(12):3982–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muddasani P, Norman JC, Ellman M, van Wijnen AJ, Im HJ. Basic fibroblast growth factor activates the MAPK and NFkappaB pathways that converge on Elk-1 to control production of matrix metalloproteinase-13 by human adult articular chondrocytes. J Biol Chem. 2007;282(43):31409–31421. [DOI] [PubMed] [Google Scholar]
- 24. Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, Buschmann MD, Henderson JE. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280(21):20509–20515. [DOI] [PubMed] [Google Scholar]
- 25. Li X, Ellman MB, Kroin JS, Chen D, Yan D, Mikecz K, Ranjan KC, Xiao G, Stein GS, Kim SG, Cole B, van Wijnen AJ, Im HJ. Species-specific biological effects of FGF-2 in articular cartilage: implication for distinct roles within the FGF receptor family. J Cell Biochem. 2012;113(7):2532–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong L, Huang X, Karperien M, Post JN. The regulatory role of signaling crosstalk in hypertrophy of MSCs and human articular chondrocytes. Int J Mol Sci. 2015;16(8):19225–19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchtova M, Oralova V, Aklian A, Masek J, Vesela I, Ouyang Z, Obadalova T, Konecna Z, Spoustova T, Pospisilova T, Matula P, Varecha M, Balek L, Gudernova I, Jelinkova I, Duran I, Cervenkova I, Murakami S, Kozubik A, Dvorak P, Bryja V, Krejci P. Fibroblast growth factor and canonical WNT/β-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage. Biochim Biophys Acta. 2015;1852(5):839–850. [DOI] [PubMed] [Google Scholar]
- 28. Chan BY, Fuller ES, Russell AK, Smith SM, Smith MM, Jackson MT, Cake MA, Read RA, Bateman JF, Sambrook PN, Little CB. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011;19(7):874–885. [DOI] [PubMed] [Google Scholar]
- 29. Oh H, Chun CH, Chun JS. Dkk-1 expression in chondrocytes inhibits experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2012;64(8):2568–2578. [DOI] [PubMed] [Google Scholar]
- 30. Shin Y, Huh YH, Kim K, Kim S, Park KH, Koh JT, Chun JS, Ryu JH. Low-density lipoprotein receptor-related protein 5 governs Wnt-mediated osteoarthritic cartilage destruction. Arthritis Res Ther. 2014;16(1):R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meo Burt P, Xiao L, Hurley MM. FGF23 regulates Wnt/β-catenin signaling-mediated osteoarthritis in mice overexpressing high-molecular-weight FGF2. Endocrinology. 2018;159(6):2386–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Florkiewicz RZ, Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989;86(11):3978–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. [DOI] [PubMed] [Google Scholar]
- 34.RRID: AB_631497, https://scicrunch.org/resolver/AB_631794.
- 35.RRIS: AB_2104623, https://scicrunch.org/resolver/AB_2104623.
- 36.RRID: AB_963497, https://scicrunch.org/resolver/AB_963497.
- 37.RRID: AB_2103530, https://scicrunch.org/resolver/AB_2103530.
- 38.RRID: AB_2665492, https://scicrunch.org/resolver/AB_2665492.
- 39.RRID: AB_776416, https://scicrunch.org/resolver/AB_776416.
- 40.RRID: AB_2222327, https://scicrunch.org/resolver/AB_2222327.
- 41.RRID: AB_11172941, https://scicrunch.org/resolver/AB_11172941.
- 42.RRID: AB_2721918, https://scicrunch.org/resolver/AB_2721918.
- 43.RRID: AB_10858068, https://scicrunch.org/resolver/AB_10858068.
- 44.RRID: AB_2091333, https://scicrunch.org/resolver/AB_2091333.
- 45.RRID: AB_2195345, https://scicrunch.org/resolver/AB_2195345.
- 46.RRID: AB_10900607, https://scicrunch.org/resolver/AB_10900607.
- 47.RRID: AB_1310290, https://scicrunch.org/resolver/AB_1310290.
- 48.RRID: AB_2798251, https://scicrunch.org/resolver/AB_2798251.
- 49.RRID: AB_2813726, https://scicrunch.org/resolver/AB_2813726.
- 50. Price CA. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J Endocrinol. 2016;228(2):R31–R43. [DOI] [PubMed] [Google Scholar]
- 51. Mills ES, Iorio L, Feinn RS, Duignan KM, Macica CM. Joint replacement in X-linked hypophosphatemia. J Orthop. 2019;16(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, Buschmann MD, Henderson JE. Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. J Biol Chem. 2005;280(21):20509–20515. [DOI] [PubMed] [Google Scholar]
- 53. Valverde-Franco G, Binette JS, Li W, Wang H, Chai S, Laflamme F, Tran-Khanh N, Quenneville E, Meijers T, Poole AR, Mort JS, Buschmann MD, Henderson JE. Defects in articular cartilage metabolism and early arthritis in fibroblast growth factor receptor 3 deficient mice. Hum Mol Genet. 2006;15(11):1783–1792. [DOI] [PubMed] [Google Scholar]
- 54. Tang J, Su N, Zhou S, Xie Y, Huang J, Wen X, Wang Z, Wang Q, Xu W, Du X, Chen H, Chen L. Fibroblast growth factor receptor 3 inhibits osteoarthritis progression in the knee joints of adult mice. Arthritis Rheumatol. 2016;68(10):2432–2443. [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. 2019;17(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiao Z, Huang J, Cao L, Liang Y, Han X, Quarles LD. Osteocyte-specific deletion of Fgfr1 suppresses FGF23. PLoS One. 2014;9(8):e104154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nogova L, Sequist LV, Perez Garcia JM, et al. Evaluation of BGJ398, a fibroblast growth factor receptor 1–3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. 2017;35(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wöhrle S, Henninger C, Bonny O, Thuery A, Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M, Graus Porta D. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2013;28(4):899–911. [DOI] [PubMed] [Google Scholar]