Abstract
The negative immune regulator Tollip inhibits the proinflammatory response to rhinovirus (RV) infection, a contributor to airway neutrophilic inflammation and asthma exacerbations, but the underlying molecular mechanisms are poorly understood. Tollip may inhibit IRAK1, a signaling molecule downstream of ST2, the receptor of IL-33. This study was carried out to determine whether Tollip downregulates ST2 signaling via inhibition of IRAK1, but promotes soluble ST2 (sST2) production, thereby limiting excessive IL-8 production in human airway epithelial cells during RV infection in a type 2 cytokine milieu (e.g., IL-13 and IL-33 stimulation). Tollip- and IRAK1-deficient primary human tracheobronchial epithelial (HTBE) cells and Tollip knockout (KO) HTBE cells were generated using the shRNA knockdown and CRISPR/Cas9 approaches, respectively. Cells were stimulated with IL-13, IL-33, and/or RV16. sST2, activated IRAK1, and IL-8 were measured. A Tollip KO mouse model was utilized to test if Tollip regulates the airway inflammatory response to RV infection in vivo under IL-13 and IL-33 treatment. Following IL-13, IL-33, and RV treatment, Tollip-deficient (vs. -sufficient) HTBE cells produced excessive IL-8, accompanied by decreased sST2 production but increased IRAK1 activation. IL-8 production following IL-13/IL-33/RV exposure was markedly attenuated in IRAK1-deficient HTBE cells, as well as in Tollip KO HTBE cells treated with an IRAK1 inhibitor or a recombinant sST2 protein. Tollip KO (vs. wild-type) mice developed exaggerated airway neutrophilic responses to RV in the context of IL-13 and IL-33 treatment. Collectively, these data demonstrate that Tollip restricts excessive IL-8 production in type 2 cytokine-exposed human airways during RV infection by promoting sST2 production and inhibiting IRAK1 activation. sST2 and IRAK1 may be therapeutic targets for attenuating excessive neutrophilic airway inflammation in asthma, especially during RV infection.
Keywords: Tollip, ST2, Airway epithelial cells, Rhinovirus, Type 2 cytokines, Interleukin-8
Introduction
Rhinovirus (RV) infection increases neutrophilic airway inflammation and triggers asthma exacerbations in both children and adults [1, 2, 3], but the underlying mechanisms are not completely understood. Toll-interacting protein (Tollip), an adaptor protein, serves as a negative regulator of Toll-like receptor (2, 4) and IL-1 receptor signaling [4, 5]. Tollip inhibits IL-1 and LPS-induced NF-κB activation and proinflammatory cytokine responses [6]. In humans, the Tollip rs5743899 single nucleotide polymorphism has been associated with increased susceptibility to tuberculosis infection [7] and cutaneous leishmaniasis [8]. Our research group has shown that Tollip rs5743899 is associated with reduced Tollip expression in human airway epithelial cells and worse lung function in asthmatic subjects [9]. However, the mechanisms whereby Tollip regulates airway inflammation during RV infection in asthma or allergic airway diseases remain to be fully established.
Asthma is mainly driven by type 2 immunity, as both IL-13 and IL-33 play key roles in the disease [10, 11]. While Tollip inhibits IL-1R-mediated signaling, IL-33 enhances this signaling pathway and drives type 2 as well as non-type 2 (e.g., neutrophilic) inflammation [12]. The receptor of IL-33 consists of ST2 (IL-1RL1) and IL-1 receptor accessory protein (IL-1RAP). ST2 exists in two major isoforms: membrane ST2 (ST2, ST2L) and soluble ST2 (sST2), both arising from a single gene (Il1rl1) by alternative splicing [13]. Polymorphism in the Il1rl1 gene is associated with an increased risk of asthma [14], and epithelial ST2 expression is increased in severe asthma in association with type 2 inflammation [15]. Interestingly, Tollip-deficient mice under intranasal IL-13 challenge develop excessive eosinophilic airway inflammation through a mechanism that involves ST2 signaling in lung macrophages [16]. Furthermore, IL-13 has been reported to increase ST2 expression in human airway epithelial cells [17], and we have previously shown that Tollip deficiency in airway epithelial cells promotes IL-8 production in response to IL-13 especially during RV infection [9]. Nonetheless, the functional role of Tollip in airway inflammation (e.g., regulation of epithelial sST2) during viral infection remains unclear.
Because Tollip interacts directly with IRAK1 and inhibits its activation [18], deficient Tollip expression in epithelial cells could lead to increased IRAK1 signaling under type 2 cytokine stimulation and RV infection, which would increase IL-8 (a neutrophil chemokine) production and promote excessive airway inflammation. Therefore, we hypothesized that Tollip downregulates RV-mediated IL-8 production in a type 2 cytokine milieu by modulating the IL-33/ST2 signaling axis (e.g., sST2 expression and IRAK1 activation) in human airways. Human primary airway epithelial cell cultures and mouse models were used to test our hypothesis in order to provide a novel mechanism detailing how Tollip inhibits IL-8 production to limit excessive inflammation during RV infection in a type 2 cytokine milieu that is reflective of a subset of asthma.
Materials and Methods
Reagents
Recombinant human IL-13, IL-33, and human ST2-Fc chimeras were purchased from R&D Systems (Minneapolis, MN, USA). Recombinant mouse IL-13 and IL-33 proteins were from PeproTech (Rocky Hill, NJ, USA). Goat and mouse anti-human Tollip, mouse anti-human IRAK1, and mouse anti-β-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). A rabbit anti-human IRAK1 (Phospho-Thr209) antibody was obtained from Aviva Systems Biology (San Diego, CA, USA). The goat anti-human ST2 antibody was obtained from R&D Systems. The IRAK1/4 inhibitor N-(2-morpholinylethyl)-2-(3-nitrobenzoylamido)-benzimidazole [19] was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Isolation of Human Tracheobronchial Epithelial Cells
Human tracheobronchial epithelial (HTBE) cells were isolated, as previously described [20], from deidentified donor lungs under a protocol approved by the Institutional Review Board at National Jewish Health. The selected donor lungs, obtained from the International Institute for the Advancement of Medicine (Edison, NJ, USA), were from nonsmokers with no prior history of lung disease and who died from accident-related causes including trauma or cerebrovascular injury. The HTBE cells were isolated by enzymatic digestion from the distal region of the trachea and proximal parts of the main bronchi.
Freshly isolated HTBE cells were cultured 4–5 days in collagen I-coated 60-mm tissue culture dishes containing 4 mL of BronchiaLifeTM culture medium (Lifeline Cell Technology, Frederick, MD, USA) containing the following supplements: L-glutamine (6 mM), human serum albumin (500 µg/mL), linoleic acid (0.6 µM), lecithin (0.6 µg/mL), extract PTM LifeFactor (0.4%), epinephrine (1 µM), transferrin (5 µg/mL), triiodothyronine (10 nM), hydrocortisone (0.1 µg/mL), recombinant human epidermal growth factor (5 ng/mL), recombinant human insulin (5 µg/mL), gentamycin (30 mg/mL), and amphotericin B (15 µL) (Lifeline Cell Technology). Stocks of HTBE cells were harvested and frozen in liquid nitrogen, at a density of 5 × 105 cells/mL in complete BronchiaLifeTM medium supplemented with 20% FBS (fetal bovine serum) and 10% dimethyl sulfoxide.
Preparation of RV
Human RV type 16 (RV16) and RV type 1B (RV1B) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The viruses were propagated in H1-HeLa cells, purified, and titrated as previously described [21].
Preparation of shRNA-Encoding Lentiviruses
Lentiviral expression vectors encoding human Tollip or IRAK1-targeting shRNA, or control (scrambled) shRNA, were obtained from GeneCopoeia (Rockville, MD, USA) and were packaged into lentivirus using the Lenti-PacTM HIV Expression Packaging Kit (GeneCopoeia). Briefly, 293FT cells were seeded at 0.8 × 106 cells/60-mm culture dish in 4 mL of Dulbecco's modified Eagle's medium containing 10% FBS but no antibiotics. At 70–80% confluence, the cells were cotransfected with the lentiviral constructs and packaging plasmids following the manufacturer's instructions. Culture supernatants containing freshly packaged lentivirus were serially harvested 40 and 60 h after transfection and were centrifuged at 1,000 g for 10 min at 4°C to remove cell debris. The clarified lentivirus supernatants were supplemented with 8 µg/mL polybrene and used immediately for transduction of shRNA in HTBE cells.
Generation of Tollip-Deficient and Tollip-Sufficient Primary HTBE Cells
HTBE cells from 4 individual donors were seeded at passage 1 into collagen-coated 6-well plates at a density of 105 cells/well in 3 mL of BronchiaLifeTM medium containing all supplements but no gentamycin or amphotericin B. After 48 h, the cultures reached 60% confluence. At this time, the cells were transduced twice, 24 h apart, with freshly harvested lentivirus encoding Tollip-specific shRNA or control (scrambled) shRNA (GeneCopoeia) to generate Tollip-deficient and Tollip-sufficient HTBE cells, respectively. Specifically, at each time point, the culture medium was removed from each well and was replaced with 1.5 mL of freshly harvested lentivirus supernatant supplemented with polybrene (8 µg/mL). The plates were then centrifuged at 2,000 rpm for 30 min at 4°C, and incubated at 37°C for 1 h, followed by removal of the lentivirus suspension and addition of 3 mL of BronchiaLifeTM medium to each well. The transduced cells were allowed to grow for 48 h, reaching about 90% confluence, before harvest and seeding into 12-well plates for the experiments.
Generation of IRAK1-Deficient Primary HTBE Cells
To establish a stable IRAK1-deficient primary HTBE cell line, we transduced primary HTBE cells with shRNA targeting human IRAK1 (GeneCopoeia), using lentivirus as described above for Tollip knockdown. Control HTBE cells were transduced with lentivirus encoding scrambled (control) shRNA. After transduction, the cells were harvested and seeded onto irradiated puromycin-resistant 3T3 fibroblasts in culture medium supplemented with 5 µM of the Rho-associated protein kinase inhibitor Y-27632 (APExBIO, Houston, TX, USA). Puromycin (1 µg/mL) was added to the culture medium for selection, and the medium with supplements was changed every other day. After 6 days of expansion, the selected HTBE cells were harvested and established as the IRAK1-deficient cell line after confirming IRAK1 knockdown by Western blotting.
Generation of Tollip CRISPR Knockout Primary HTBE Cells
Tollip knockout (KO) HTBE cells were generated using the CRISPR/Cas9 method [22], and a single guide (sg) RNA (sgRNA sequence: 5′-TCACCGCGTAGGACATGACG-3′) was designed to target human Tollip. A scrambled sgRNA guide was used for control CRISPR HTBE cells [23]. The designed sgRNAs were cloned into “all-in-one” pLenti-CRISPR vector coexpressing the scaffold RNA and Cas9 nuclease along with a puromycin resistance gene. The construct was sequenced to confirm the correct sgRNA sequence and packaged into lentivirus by cotransfection with VSV-G and psPAX2 plasmids in 293FT cells. The packaged lentivirus, freshly harvested in the culture supernatant, was transduced into 60% confluent HTBE cells, as described above (under Generation of Tollip-Deficient and Tollip-Sufficient Primary HTBE Cells). After 48 h of recovery, the transduced cells were harvested and seeded onto irradiated, puromycin-resistant 3T3 fibroblasts for expansion and selection with puromycin (1 µg/mL). Tollip KO was confirmed by Western blotting.
Treatment of HTBE Cells
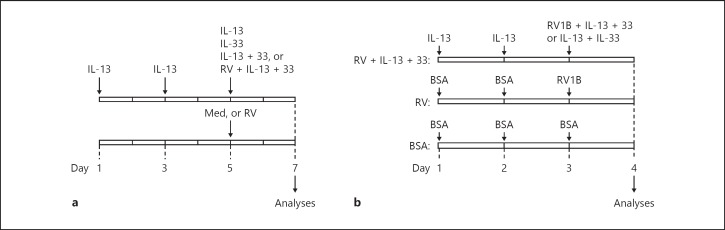
For submerged cultures, HTBE cells (at passage 2) were seeded into collagen-coated 12-well plates at a density of 1 × 105 cells/well in 1 mL of complete BronchiaLifeTM medium and grown for 1 day before treatment. For differentiated cell cultures, HTBE cells (at passage 2) were seeded into collagen-coated Transwell inserts in PneumaCult-ALI Medium (STEMCELL Technologies, Vancouver, BC, Canada) and grown at the air-liquid interface for 14 days to induce mucociliary function. Thereafter, the cells were pretreated with medium alone (control) or with IL-13 (10 ng/mL, on days 1 and 3), to mimic an asthma-like environment with upregulated ST2 on airway epithelial cells, and were subsequently treated (on day 5) with IL-13 (10 ng/mL), IL-33 (10 ng/mL), or a combination of IL-13 and IL-33 (10 ng/mL each) in the absence (IL-13 + IL-33) or in the presence of RV16 infection at a suboptimal dose of 0.2 MOI (RV + IL-13 + IL-33). Control cultures remained untreated or were infected only with RV16 (Fig. 1a). The doses of cytokine and RV were selected from preliminary dose-response experiments (online suppl. Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000497072).
Fig. 1.
Experimental design. a Cell culture system. Cells were pretreated with IL-13 on days 1 and 3, and were subsequently exposed (on day 5) to IL-13, IL-33, IL-13 + IL-33, or rhinovirus (RV) + IL-13 + IL-33. Controls were pretreated with medium (Med) alone, and subsequently exposed to Med or RV. Analyses were carried out 48 h later. b Mouse model. Tollip knockout and wild-type mice were pretreated with IL-13 (on days 1 and 2), and were subsequently (on day 3) administered IL-13 + IL-33 with RV1B (RV + IL-13 + IL-33 group) or without (IL-13 + IL-33 group). Control groups consisted of mice similarly pretreated with bovine serum albumin (BSA) and subsequently administered BSA (BSA group) or inoculated with RV1B (RV group). Airway inflammation was assessed 24 h later (on day 4).
For infection, the cells were washed with Dulbecco's phosphate-buffered saline (D-PBS) and incubated with the virus, suspended in 200 µL D-PBS supplemented with 0.25% bovine serum albumin (BSA), for 30 min at room temperature, followed by 90-min incubation at 34.5°C to allow for virus adsorption and entry into the cells. Uninfected cultures were similarly exposed to 200 µL D-PBS containing 0.25% BSA. After this, the inoculum was removed and the cells were washed twice with D-PBS before addition of fresh culture medium (1 mL/well) without or with cytokines. Analyses were carried out 48 h after RV infection.
ST2 Promoter Activity
HTBE cells were transfected with luciferase plasmid constructs expressing the distal or proximal promoter of human ST2 (provided by Dr. Tago, Jichi Medical University, Shimotsuke, Japan) [24], or promoter-less luciferase plasmid, using Effectene Transfection Reagent (Qiagen, Germantown, MD, USA), and pRL-TK plasmid containing Renilla luciferase (Promega, Madison, WI, USA) to standardize for transfection efficiency. Luminescence was measured as relative luminescence units in a Promega Glomax Multi Detection Plate Reader (Promega). Luciferase activity was measured in cell lysates, harvested 48 h after treatment, using the Dual-Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity for each sample, and normalized values were expressed as fold luminescence intensity relative to promoter-less luciferase intensity.
Western Blotting
Cells were lysed with cold radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Thermo Fisher). The protein lysates were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and probed with the following specific primary antibodies: goat or mouse anti-human Tollip (Santa Cruz Biotechnology, Dallas, TX, USA), goat anti-human ST2 (R&D Systems), rabbit anti-human p-IRAK1 (Aviva Systems Biology), mouse anti-human IRAK1 (Santa Cruz), and mouse anti-β-actin (Santa Cruz). The primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies against goat, rabbit, and mouse antibodies (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Protein expression was quantified by densitometry.
Enzyme-Linked Immunosorbent Assay
IL-8 was measured in cell culture supernatants using a commercial kit, per the manufacturer's instructions (R&D Systems).
Mouse Model of IL-13/IL-33 Treatment and RV1B Infection
Tollip KO mice on a C57/BL6 background were obtained from Dr. Liwu Li at Virginia Polytechnic Institute and State University, and bred at the National Jewish Health Biological Research Center. Wild-type (WT) C57/BL6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were maintained and bred in the animal facility at the Biological Resources Center of National Jewish Health. All animals and procedures were used under an approved Institutional Animal Care and Use protocol.
Tollip KO and WT C57BL/6 mice (n = 5–8 per group) were pretreated with recombinant mouse IL-13 (250 ng) or BSA on days 1 and 2 (Fig. 1b) to mimic the human epithelial cell culture model. On day 3, the mice were either administered 500 ng BSA (BSA group), inoculated with RV1B (106 PFU) (RV group), inoculated with RV1B and 250 ng each of IL-13 and IL-33 (RV + IL-13 + IL-33 group), or treated with IL-13 + IL-33 (250 ng each) (IL-13 + IL-33 group). All treatments were administered intranasally in 50 µL PBS. Airway inflammation was assessed on day 4, by total and differential counting of inflammatory cells recovered in the bronchoalveolar lavage (BAL) fluids. All animals and procedures were used under an approved Institutional Animal Care and Use protocol.
Statistical Analysis
Normally distributed data were analyzed using two-way ANOVA with Tukey's test for multiple comparisons of the groups. Data that were not normally distributed were analyzed using a nonparametric Kruskal-Wallis test. A p value < 0.05 was considered statistically significant.
Results
Tollip Restricts Excessive IL-8 Production during RV Infection in Type 2 Cytokine-Treated HTBE Cells
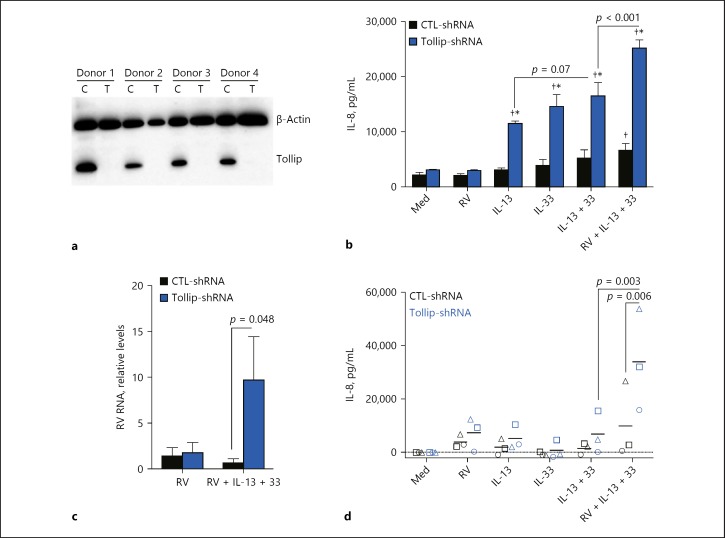
To define the role of Tollip in epithelium-driven airway inflammation, we generated Tollip-deficient and Tollip-sufficient primary HTBE cells using the shRNA knockdown approach and examined IL-8 production in response to type 2 cytokines and RV infection. Western blot analysis confirmed that Tollip was effectively abolished in HTBE cells, from all 4 donors, following lentiviral transduction with Tollip-specific shRNA as compared with control shRNA (Fig. 2a).
Fig. 2.
IL-8 production by Tollip-deficient (Tollip-shRNA) and Tollip-sufficient (control [CTL]-shRNA) primary human tracheobronchial epithelial (HTBE) cells. a Tollip protein expression in HTBE cells following lentiviral transduction with control (scrambled) shRNA or Tollip-specific shRNA. C, control-shRNA; T, Tollip-shRNA. b Effect of Tollip knockdown on IL-8 production in submerged culture, at baseline (cell culture medium [Med]); after rhinovirus (RV) 16 infection (RV); after treatment with IL-13, IL-33, and IL-13 + IL-33 (IL13 + 33); and after RV infection in the presence of IL-13 + IL-33 (RV + IL-13 + 33). Data are means ± SEM of n = 4 donors. * p < 0.0001, compared to CTL-shRNA for the same treatment; † p < 0.05, compared to Med. c Viral load in supernatant from submerged cultures. Data are means ± SEM of n = 4 donors. d Effect of Tollip knockdown on IL-8 production by HTBE cells grown at the air-liquid interface for 14 days to induce mucociliary differentiation. Individual data from the same donor are labeled with the same symbol. Data are from n = 3 donors. Mean values are represented by short lines.
Compared to Tollip-sufficient cells, Tollip-deficient cells produced excessive amounts of IL-8 following stimulation with type 2 cytokines (Fig. 2b). Interestingly, while IL-33 increased IL-8 production in Tollip-deficient (vs. -sufficient) cells pretreated with IL-13, this IL-8 response was further enhanced with RV infection (p < 0.001). Of note, RV infection alone at the low dose we used did not significantly increase IL-8 in untreated cells, or after pretreatment with IL-13 (data not shown). In parallel, the amounts of virus recovered in the culture supernatants were significantly higher for Tollip-deficient cells than for Tollip-sufficient cells, following RV infection under IL-13 + IL-33 stimulation (Fig. 2c). In fully differentiated HTBE cultures, the effect of Tollip deficiency on an exaggerated IL-8 response was significant only in IL-13/IL-33/RV-treated cells (Fig. 2d), but did not reach statistical significance with the other treatments, a limitation that may be explained by the small number of donors (n = 3) and the low dose of RV (0.2 MOI) used. Overall, these data indicate that Tollip restricts excessive human airway IL-8 production in response to type 2 cytokines, especially during RV infection.
IRAK1 Activation Is Increased in Tollip-Deficient HTBE Cells following RV Infection in the Presence of Type 2 Cytokines
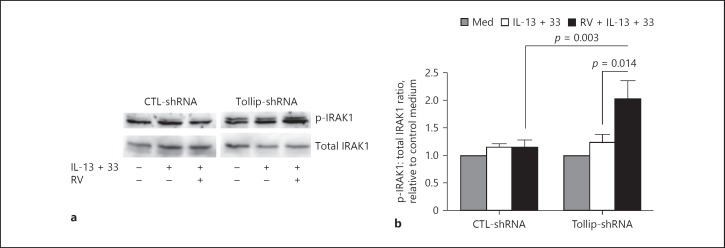
Tollip is known to interact directly with IRAK1 and inhibits its activity [17]. We next examined whether IRAK1 activation was increased in parallel with IL-8 overproduction in Tollip-deficient cells under type 2 cytokines, particularly during RV infection. As shown in Figure 3, IRAK1 activity, as detected by the p-IRAK1/IRAK1 ratio, was significantly increased in Tollip-deficient cells compared to Tollip-sufficient cells during RV infection in the presence of IL-13 and IL-33. These data revealed that IL-8 overproduction in Tollip-deficient cells was associated with increased IRAK1 activation.
Fig. 3.
IRAK1 activation in Tollip-deficient (Tollip-shRNA) and Tollip-sufficient (CTL-shRNA) primary human tracheobronchial epithelial cells. a Representative Western blot showing p-IRAK1 and total IRAK1 expression levels. RV, rhinovirus. b Densitometric analysis of p-IRAK1 expression that was normalized to total IRAK1 expression. Results are expressed as fold change relative to unstimulated control (Med). Data are means ± SEM of n = 4 donors.
IL-8 Production Is Dependent on IRAK1 Activation in HTBE Cells
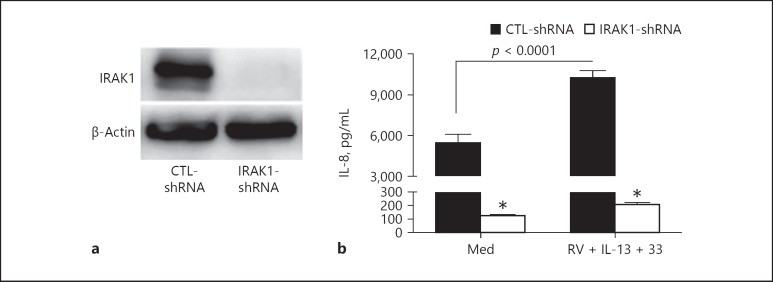
To determine if IL-8 production is dependent on IRAK1 levels in HTBE cells, we generated a stable IRAK1-deficient primary HTBE cell line and examined IL-8 production following RV infection in the presence of IL-13 and IL-33. As confirmed by Western blotting, IRAK1 expression was completely knocked down in HTBE cells following lentiviral transduction of IRAK1-specif ic shRNA (Fig. 4a). Baseline IL-8 production was significantly reduced in IRAK1-deficient cells compared to IRAK1-sufficient cells (Fig. 4b). However, unlike in IRAK1-sufficient cells, RV infection and IL-13 + IL-33 stimulation failed to increase IL-8 production in IRAK1-deficient cells, further demonstrating the requirement of IRAK1 for IL-8 production by these cells.
Fig. 4.
IRAK1 requirement for IL-8 production by primary human tracheobronchial epithelial (HTBE) cells. a Western blot confirming IRAK1-shRNA knockdown in primary HTBE cells. b IL-8 levels measured in IRAK1-deficient (IRAK1-shRNA) and IRAK1-sufficient (CTL-shRNA) HTBE cell culture supernatants at baseline (Med) and after infection with rhinovirus (RV) 16 in the presence of IL-13 + IL-33 (RV + IL-13 + 33). Data are means ± SEM of n = 4 replicates for each condition. * p < 0.001, compared to CTL-shRNA for similar treatment.
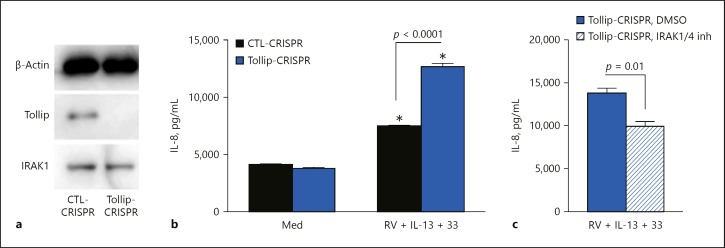
To further establish a role for IRAK1 activation in Tollip-regulated IL-8 production, we generated a Tollip KO HTBE cell line using the CRISPR/Cas9 method and examined the effects of an IRAK1/4 inhibitor on IL-8 production following infection with RV in the presence of IL-13 + IL-33. Tollip KO HTBE cells expressed normal levels of IRAK1 but lacked Tollip protein expression, confirming the Tollip KO phenotype (Fig. 5a). When infected with RV in the presence of IL-13 + IL-33, Tollip KO HTBE cells produced significantly higher amounts of IL-8 than control CRISPR cells (Fig. 5b), similar to those observed with Tollip shRNA knockdown. Importantly, IL-8 production was significantly reduced by treating Tollip KO HTBE cells with an IRAK1/4 inhibitor (Fig. 5c), further implicating IRAK1 in the exaggerated IL-8 response by Tollip-deficient cells.
Fig. 5.
Effect of Tollip gene knockout and IRAK1 inhibitor on IL-8 production by primary human tracheobronchial epithelial (HTBE) cells. a Tollip and IRAK1 protein expression in Tollip knockout (KO) (Tollip-CRISPR) and control (CTL-CRISPR) HTBE cells. b IL-8 production at baseline (Med) and after rhinovirus (RV) infection in the presence of IL-13 + IL-33 (RV + IL-13 + 33). c Effect of an IRAK1 inhibitor on IL-8 production by Tollip KO HTBE cells after infection with RV16 in the presence of IL-13 + IL-33 (RV + IL-13 + 33). IRAK1 inhibitor (5 µM) was added to the culture 2 h before RV infection. IL-8 was measured 24 h after the infection. Data are means ± SEM of n = 4 replicates for each condition. * p < 0.01, compared to the respective baseline control (Med).
Tollip Promotes sST2 Production in HTBE Cells
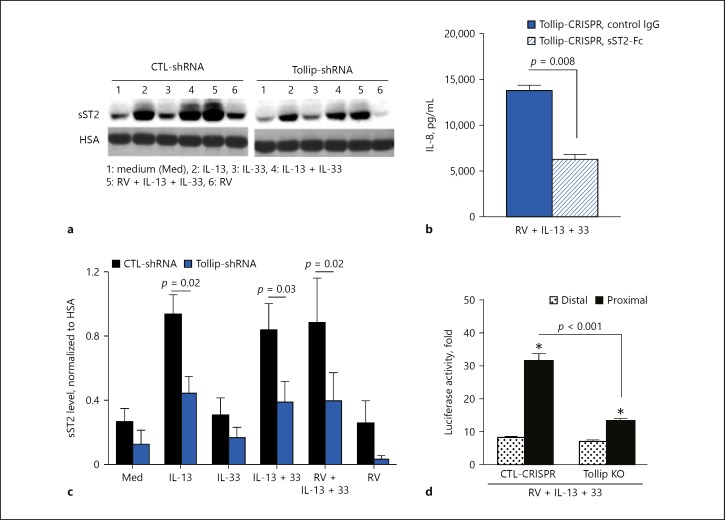
To determine whether Tollip may regulate IL-8 production in the context of type 2 cytokines by modulating ST2 expression in HTBE cells, we examined ST2L and sST2 expression and compared the results between Tollip-deficient and Tollip-sufficient cells. We found low levels of ST2L protein expression that were comparable between the two cell types (data not shown). However, Tollip-deficient cells produced significantly less sST2 protein compared to Tollip-sufficient cells under type 2 cytokine stimulation and RV infection (Fig. 6a, b). This finding suggests that Tollip's suppressive effect on IL-8 production could be linked to its promoting effect on sST2 production in HTBE cells.
Fig. 6.
a Representative Western blot showing soluble ST2 (sST2) expression levels in culture supernatants of Tollip-deficient (Tollip-shRNA) and Tollip-sufficient (CTL-shRNA) primary human tracheobronchial epithelial (HTBE) cells. HSA, human serum albumin. b Effect of exogenous sST2 (sST2-Fc) on IL-8 production by Tollip knockout (KO) HTBE cells. sST2-Fc (1 µg/mL) was added to the culture 2 h before infection. IL-8 was measured 24 h after the infection. c Densitometric analysis of sST2 levels in culture supernatants of Tollip-deficient (Tollip-shRNA) and Tollip-sufficient (CTL-shRNA) HTBE cells (means ± SEM of n = 4 donors). d Differential usage of the ST2 promoter in control and Tollip KO HTBE cells after rhinovirus (RV) infection in the presence of IL-13 and IL-33. Data are means ± SEM of n = 4 replicates for each condition. * p < 0.05, compared to CTL-shRNA.
Exogenous sST2 Attenuates IL-8 Overproduction in Tollip-Deficient Cells
Since Tollip-deficient cells secreted low amounts of sST2 but produced high levels of IL-8, we postulated that Tollip-regulated sST2 production might play a protective role by antagonizing IL-33, thereby reducing IL-8 production in HTBE cells. To test this concept, we pretreated Tollip KO HTBE cells with human sST2-Fc fusion protein or control IgG and examined the effects on IL-8 production after infection of these cells with RV in the presence of IL-13 and IL-33. Treatment of Tollip KO HTBE cells with exogenous sST2-Fc strongly inhibited IL-8 production (Fig. 6c), implying a significant role for sST2 in the Tollip-regulated suppression of excessive IL-8 production by airway epithelial cells.
Tollip Promotes Proximal ST2 Promoter Activity in HTBE Cells
The transcription of ST2 can be initiated from two distinct promoters on the Il1rl1 gene: the distal promoter and the proximal promoter. Since Tollip promoted sST2 production (Fig 6a, b), we sought to determine which promoter of the Il1rl1 gene is preferentially activated in Tollip-deficient HTBE cells under RV infection in the presence of type 2 cytokines. As shown in Figure 6d, the proximal promoter of ST2 was preferentially activated under these conditions, establishing a predominant role for this promoter in driving sST2 production in HTBE cells. However, in Tollip KO HTBE cells, as compared to control HTBE cells, this proximal ST2 promoter activity was significantly reduced under IL-13 + IL-33 with RV infection. These findings are in line with the reduced sST2 production by Tollip-deficient cells and further demonstrate that Tollip promotes the activity of the proximal ST2 promoter, which was shown to contribute to sST2 production in fibroblasts [23].
Tollip Inhibits the Airway Neutrophilic Response to RV Infection in Mice Treated with IL-13 and IL-33
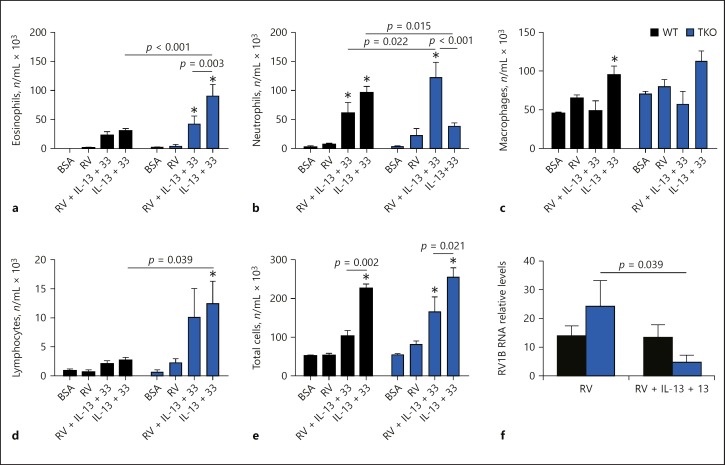
To test if Tollip limits the development of an exaggerated airway neutrophilic response to type 2 cytokines during RV infection in vivo, we examined the cellular inflammatory response that developed in the airways of Tollip KO and WT mice following intranasal administration of IL-13 and IL-33 and RV1B infection. No statistically significant difference was detected between Tollip KO and WT mice in total, or differential cell counts following intranasal administration of BSA or RV1B infection (Fig. 7).
Fig. 7.
Profile of inflammatory cells recovered in the bronchoalveolar lavage fluids from Tollip knockout (TKO) and wild-type (WT) mice. Mice were intranasally administered bovine serum albumin (BSA) (protein control), rhinovirus (RV) 1B in the absence (RV alone) or presence of IL-13 + IL-33 (RV + IL-13 + 33), or IL-13 + IL-33 (IL-13 + 33). Data are presented as means ± SEM of n = 5–8 mice/group. * p < 0.05, compared to BSA control. a Eosinophils. b Neutrophils. c Macrophages. d Lymphocytes. e Total cells. f RV1B RNA.
In the absence of infection, IL-13 + IL-33 treatment elicited a predominant eosinophilic response in the airways of Tollip KO mice (Fig. 7a), whereas neutrophils predominated in the airways of WT mice similarly treated with IL-13 + IL-33 (Fig. 7b). However, after RV1B infection, IL-13 + IL-33-treated Tollip KO mice developed an exaggerated airway neutrophilic (not eosinophilic) response that was significantly greater than in WT mice treated with IL-13 + IL-33 (Fig. 7b). The RV1B load in BAL fluid was not significantly different in the absence or presence of IL13 + IL-33 treatment (Fig. 7f).
Discussion
The current study demonstrated that in primary human airway epithelial cells, Tollip deficiency led to excessive production of IL-8 in the presence of IL-13 and IL-33 that was further enhanced with RV infection. In vivo, Tollip KO mice developed an exaggerated airway neutrophilic inflammation following concomitant RV infection and treatment with IL-13 and IL-33, establishing an important role for Tollip in limiting excessive neutrophilic airway inflammation during RV infection, especially in a type 2 cytokine environment. Using several genetic and molecular approaches, we further revealed potential mechanisms in airway epithelial cells. We found that the enhanced IL-8 response in Tollip-deficient cells was associated with less sST2 and increased IRAK1 activation. Importantly, administration of sST2 and inhibition of IRAK1 reversed the promoting effect of Tollip deficiency on IL-8 production. These results identified the upregulation of sST2 and downregulation of IRAK-1 by Tollip as new mechanisms for Tollip-regulated suppression of IL-8 production in human airway epithelial cells.
IRAK1 is typically associated with Toll-like receptor and IL-1 receptor signaling [25] and plays an important role in the innate immune response to pathogens. IRAK1 is also involved in IL-33-mediated signaling via its receptor consisting of ST2 (IL-1RL1) and IL-1 receptor accessory protein (IL-1RAP) [26]. In the present study, we found low levels of ST2L that were similar for both Tollip-sufficient and Tollip-deficient HTBE cells (data not shown). However, the levels of secreted sST2 were significantly reduced in Tollip-deficient cells, which produced excessive amounts of IL-8 compared to Tollip-sufficient cells. This finding led us to propose that sST2 might play a negative feedback role, thus contributing to Tollip-regulated control of IL-8 production. Indeed, provision of exogenous human sST2 (sST2-Fc protein) to Tollip-deficient HTBE cells attenuated IL-8 overproduction during RV infection in the presence of IL-13 and IL-33. These data identified a novel mechanism whereby Tollip may restrict IL-8 production in airway epithelial cells, which suggests the need of a combinative approach (blocking IRAK1 and adding sST2) to prevent excessive neutrophilic inflammation during RV infection in airways exposed to type 2 cytokines, such as in asthma.
sST2 acts mainly as a decoy receptor to IL-33 [27]; hence, it can protect ST2-expressing immune cells − namely, type 2 innate lymphoid cells (ILC2), mast cells, and a subset of Th2 cells − from producing excessive amounts of type 2 cytokines in the presence of IL-33. Conceivably, while sST2 from epithelial cells may act in an autocrine fashion to attenuate IL-33 signaling within the epithelium, it may also act in a paracrine fashion to inhibit IL-33-mediated type 2 cytokine production by effector immune cells in vivo. In this regard, given its extensive coverage of the airways, the epithelium is expected to play a major role in this regulation by secreting large amounts of sST2 that would dampen IL-33-driven airway inflammation. In our study, the strong inhibition of IL-8 production with exogenous sST2 is likely mediated through direct antagonism of exogenous IL-33 added to the culture.
How sST2 production by human airway epithelial cells is regulated has not yet been established. The transcription of ST2 can be initiated from two regions of the Il1rl1 gene promoter: the distal promoter and the proximal promoter. In the human fibroblast cell line TM1, ST2 transcription appears to be initiated at least partly by STAT3 binding to the proximal promoter, leading to sST2 production [28]. Our finding that HTBE cells produced mainly sST2 suggested a preferential activation of the proximal ST2 promoter in these cells. This was further confirmed by the demonstration of selective activation of the proximal ST2 promoter in HTBE cells under RV infection with type 2 cytokines. We also found that IL-13 can directly activate STAT3 (online suppl. Fig. S2), a previously known effect on monocytes [29], which provides a link to IL-13-mediated activation of the proximal ST2 promoter in HTBE cells. Since Tollip appears to promote sST2 production in these cells, one might speculate that Tollip may also facilitate STAT3 activation, perhaps through interaction with − and targeted proteasomal degradation of − SOCS3, a known inhibitor of STAT3 activation [30].
The functional role of sST2 in asthma, whether protective or deleterious, has not yet been addressed. Some studies reported increased levels of sST2 in serum and induced sputum of young asthmatic subjects in correlation with disease severity [31], while others found that serum sST2 levels were predictive of subsequent asthma development in wheezing preschool-age children [32]. A recent study found reduced levels of sST2 in serum of asthmatic children with documented RV infection, and these levels were further decreased in association with lower vitamin D3 levels [33], suggesting either that RV downregulates sST2 production or that low sST2 levels may predispose to RV infection/colonization of asthmatic airways. In animal models of allergic airway disease, sST2 showed beneficial anti-inflammatory effects [34, 35]. Our in vitro data, in which exogenous human sST2 inhibited IL-8 production, are in support of such a protective anti-inflammatory role. Our finding that IL-13 drives an anti-inflammatory response through sST2 production may seem paradoxical given its well-known proinflammatory roles in asthma. However, this may not be totally surprising, since other cytokines (e.g., IL-1 and TNF) can trigger soluble decoy receptors that counterregulate their proinflammatory activities [36].
There are some limitations to our current study. First, because a selective IRAK1 inhibitor is currently unavailable, we used an IRAK1/4 inhibitor, which may also inhibit IRAK4 activity. The partial inhibition of IL-8 production by this inhibitor in our study is likely due to its relatively low activity on IRAK1 phosphorylation [37]. However, our IRAK1 shRNA knockdown approach demonstrated that IL-8 production was highly dependent on IRAK1 in our cell culture system. Moreover, IRAK4 activity is not required for IRAK1 activation [38]. Second, in the mouse model, although IL-13 + IL-33 treatment and exposure to RV translated the in vitro data in terms of airway inflammation (e.g., exaggerated neutrophilic response in Tollip KO mice), we have not been able to find a significant difference in sST2 levels between BAL fluid of Tollip KO mice and that of WT mice, because sST2 levels in murine BAL fluid are very low. In addition, we have not examined the in vivo effect of exogenous sST2 on the inflammatory response in the present study. Future work is needed to further establish the role of sST2 in this model.
Conclusions
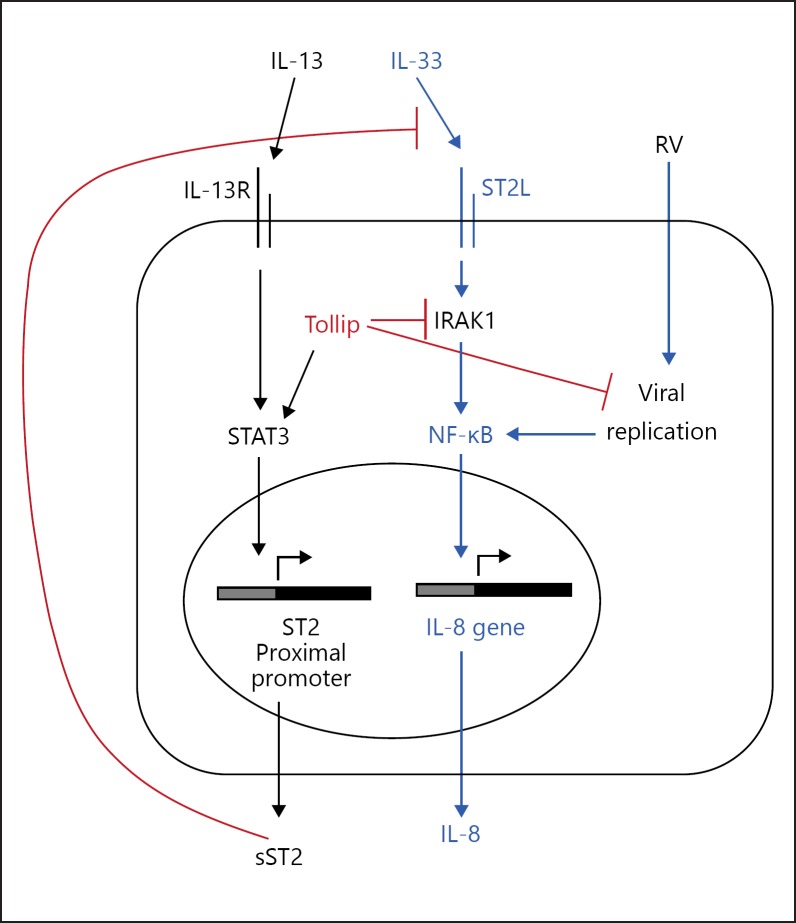
The results of this study, together with our previous data [9], emphasize the importance of Tollip in limiting the extent of neutrophilic inflammation in response to RV infection, especially in a type 2 cytokine milieu. We have previously shown that Tollip restricts RV replication through enhancement of autophagy [9], which is in line with our observation of an increased viral load in RV-infected Tollip-deficient cells treated with IL-13 and IL-33. Both RV infection and IRAK1 are known to activate NF-κB, which in turn activates IL-8 transcription. Our current study identified further mechanisms whereby Tollip limits excessive IL-8 production in airway epithelial cells. One such mechanism is through the enhancement of sST2 production. A second mechanism involves the inhibition of IRAK1, a signaling molecule shown in this study to be essential for IL-8 production by human airway epithelial cells (Fig. 8). These new findings may provide support for using sST2 and targeting IRAK1 as potential avenues for the therapeutic control of excessive inflammation in airway diseases such as asthma.
Fig. 8.
Proposed mechanism of Tollip-regulated IL-8 production in human airway epithelial cells. During rhinovirus (RV) infection in the presence of the pro-asthma cytokines IL-13 and IL-33, Tollip promotes soluble ST2 (sST2) production but limits IRAK1 activation, thereby restricting excessive IL-8 production and the development of detrimental inflammation. Targeting ST2 signaling with exogenous sST2 and inhibiting IRAK1 may be potential avenues for the therapeutic control of excessive airway inflammation during RV-mediated asthma exacerbations.
Statement of Ethics
The HTBE cells were used under a protocol that has been approved by the Institutional Review Board on Human Research at National Jewish Health.
All animal experiments described in this study conform with internationally accepted standards and have been approved by the Institutional Animal Care and Use Committee at National Jewish Health.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the following NIH grants: 1U19AI125357, R01HL122321, R01AI106287, and R01HL125128. These funding sources were not involved in the preparation of data or the manuscript.
Author Contributions
A.D. and H.W.C. designed the study, analyzed the data, interpreted the results, and drafted the manuscript; M.S., D.V., J.G.L., M.K., and L.L. contributed to data analysis and data interpretation; R.A.M. and N.P. participated in data collection.
Supplementary Material
Supplementary data
Acknowledgments
The authors thank Niccolette Schaefer, Amelia Sanchez, and Reena Berman for technical assistance.
References
- 1.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995 May;310((6989)):1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol. 2005 Aug;116((2)):267–73. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009 Feb;39((2)):193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001 Jul;167((2)):987–94. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002 Mar;277((9)):7059–65. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- 6.Didierlaurent A, Brissoni B, Velin D, Aebi N, Tardivel A, Käslin E, et al. Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol Cell Biol. 2006 Feb;26((3)):735–42. doi: 10.1128/MCB.26.3.735-742.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah JA, Vary JC, Chau TT, Bang ND, Yen NT, Farrar JJ, et al. Human TOLLIP regulates TLR2 and TLR4 signaling and its polymorphisms are associated with susceptibility to tuberculosis. J Immunol. 2012 Aug;189((4)):1737–46. doi: 10.4049/jimmunol.1103541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Araujo FJ, da Silva LD, Mesquita TG, Pinheiro SK, Vital WS, Chrusciak-Talhari A, et al. Polymorphisms in the TOLLIP Gene Influence Susceptibility to Cutaneous Leishmaniasis Caused by Leishmania guyanensis in the Amazonas State of Brazil. PLoS Negl Trop Dis. 2015 Jun;9((6)):e0003875. doi: 10.1371/journal.pntd.0003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Jiang D, Francisco D, Berman R, Wu Q, Ledford JG, et al. Tollip SNP rs5743899 modulates human airway epithelial responses to rhinovirus infection. Clin Exp Allergy. 2016 Dec;46((12)):1549–63. doi: 10.1111/cea.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015 Jan;15((1)):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake LY, Kita H. IL-33: biological properties, functions, and roles in airway disease. Immunol Rev. 2017 Jul;278((1)):173–84. doi: 10.1111/imr.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol C, Girard JP. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev. 2018 Jan;281((1)):154–68. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 13.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994 Mar;13((5)):1176–88. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. GABRIEL Consortium A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010 Sep;363((13)):1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traister RS, Uvalle CE, Hawkins GA, Meyers DA, Bleecker ER, Wenzel SE. Phenotypic and genotypic association of epithelial IL1RL1 to human TH2-like asthma. J Allergy Clin Immunol. 2015 Jan;135((1)):92–9. doi: 10.1016/j.jaci.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito Y, Schaefer N, Sanchez A, Francisco D, Alam R, Martin RJ, et al. Toll-Interacting Protein, Tollip, Inhibits IL-13-Mediated Pulmonary Eosinophilic Inflammation in Mice. J Innate Immun. 2018;10((2)):106–18. doi: 10.1159/000485850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe T, Shimokawaji T, Kanoh S, Rubin BK. IL-33 stimulates CXCL8/IL-8 secretion in goblet cells but not normally differentiated airway cells. Clin Exp Allergy. 2014 Apr;44((4)):540–52. doi: 10.1111/cea.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns K, Clatworthy J, Martin L, Martinon F, Plumpton C, Maschera B, et al. Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat Cell Biol. 2000 Jun;2((6)):346–51. doi: 10.1038/35014038. [DOI] [PubMed] [Google Scholar]
- 19.Wee ZN, Yatim SM, Kohlbauer VK, Feng M, Goh JY, Bao Y, et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat Commun. 2015 Oct;6((1)):8746. doi: 10.1038/ncomms9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Q, Jiang D, Smith S, Thaikoottathil J, Martin RJ, Bowler RP, Chu HW. IL-13 dampens human airway epithelial innate immunity through induction of IL-1 receptor-associated kinase M. J Allergy Clin Immunol. 2012;129:825–833. doi: 10.1016/j.jaci.2011.10.043. e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Q, van Dyk LF, Jiang D, Dakhama A, Li L, White SR, et al. Interleukin-1 receptor-associated kinase M (IRAK-M) promotes human rhinovirus infection in lung epithelial cells via the autophagic pathway. Virology. 2013 Nov;446((1-2)):199–206. doi: 10.1016/j.virol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dakhama A, Chu HW. The Use of CRISPR-Cas9 Technology to Reveal Important Aspects of Human Airway Biology. Methods Mol Biol. 2018;1799:371–80. doi: 10.1007/978-1-4939-7896-0_27. [DOI] [PubMed] [Google Scholar]
- 23.Chu HW, Rios C, Huang C, Wesolowska-Andersen A, Burchard EG, O'Connor BP, et al. CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 2015 Oct;22((10)):822–9. doi: 10.1038/gt.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tago K, Ohta S, Funakoshi-Tago M, Aoki-Ohmura C, Matsugi J, Tominaga SI, et al. STAT3 and ERK pathways are involved in cell growth stimulation of the ST2/IL1RL1 promoter. FEBS Open Bio. 2017 Jan;7((2)):293–302. doi: 10.1002/2211-5463.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottipati S, Rao NL, Fung-Leung WP. IRAK1: a critical signaling mediator of innate immunity. Cell Signal. 2008 Feb;20((2)):269–76. doi: 10.1016/j.cellsig.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Millar NL, O'Donnell C, McInnes IB, Brint E. Wounds that heal and wounds that don't - The role of the IL-33/ST2 pathway in tissue repair and tumorigenesis. Semin Cell Dev Biol. 2017 Jan;61:41–50. doi: 10.1016/j.semcdb.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007 Sep;282((36)):26369–80. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 28.Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999 Sep;264((2)):397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 29.Roy B, Bhattacharjee A, Xu B, Ford D, Maizel AL, Cathcart MK. IL-13 signal transduction in human monocytes: phosphorylation of receptor components, association with Jaks, and phosphorylation/activation of Stats. J Leukoc Biol. 2002 Sep;72((3)):580–9. [PubMed] [Google Scholar]
- 30.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014 Feb;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013 Oct;50((8)):803–9. doi: 10.3109/02770903.2013.816317. [DOI] [PubMed] [Google Scholar]
- 32.Ketelaar ME, van de Kant KD, Dijk FN, Klaassen EM, Grotenboer NS, Nawijn MC, et al. Predictive value of serum sST2 in preschool wheezers for development of asthma with high FeNO. Allergy. 2017 Nov;72((11)):1811–5. doi: 10.1111/all.13193. [DOI] [PubMed] [Google Scholar]
- 33.Haag P, Sharma H, Rauh M, Zimmermann T, Vuorinen T, Papadopoulos NG, et al. Soluble ST2 regulation by rhinovirus and 25(OH)-vitamin D3 in the blood of asthmatic children. Clin Exp Immunol. 2018 Aug;193((2)):207–20. doi: 10.1111/cei.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin H, Li XY, Liu T, Yuan BH, Zhang BB, Hu SL, et al. Adenovirus-mediated delivery of soluble ST2 attenuates ovalbumin-induced allergic asthma in mice. Clin Exp Immunol. 2012 Oct;170((1)):1–9. doi: 10.1111/j.1365-2249.2012.04629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee HY, Rhee CK, Kang JY, Byun JH, Choi JY, Kim SJ, et al. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res. 2014 Mar;40((2)):66–76. doi: 10.3109/01902148.2013.870261. [DOI] [PubMed] [Google Scholar]
- 36.Mantovani A, Locati M, Polentarutti N, Vecchi A, Garlanda C. Extracellular and intracellular decoys in the tuning of inflammatory cytokines and Toll-like receptors: the new entry TIR8/SIGIRR. J Leukoc Biol. 2004 May;75((5)):738–42. doi: 10.1189/jlb.1003473. [DOI] [PubMed] [Google Scholar]
- 37.Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell. 2013 Jul;24((1)):90–104. doi: 10.1016/j.ccr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vollmer S, Strickson S, Zhang T, Gray N, Lee KL, Rao VR, et al. The mechanism of activation of IRAK1 and IRAK4 by interleukin-1 and Toll-like receptor agonists. Biochem J. 2017 Jun;474((12)):2027–38. doi: 10.1042/BCJ20170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data