Abstract
Acute myeloid leukemia (AML) is an aggressive hematologic neoplasm, and patients with an internal tandem duplication (ITD) mutation of the FMS-like tyrosine kinase-3 (FLT3) receptor gene have a poor prognosis. FLT3-ITD interacts with DOCK2, a G effector protein that activates Rac1/2. Previously, we showed that knockdown of DOCK2 leads to decreased survival of FLT3-ITD leukemic cells. We further investigated the mechanisms by which Rac1/DOCK2 activity affects cell survival and chemotherapeutic response in FLT3-ITD leukemic cells. Exogenous expression of FLT3-ITD led to increased Rac1 activity, reactive oxygen species, phosphorylated STAT5, DNA damage response factors and cytarabine resistance. Conversely, DOCK2 knockdown resulted in a decrease in these factors. Consistent with the reduction in DNA damage response factors, FLT3-ITD cells with DOCK2 knockdown exhibited significantly increased sensitivity to DNA damage response inhibitors. Moreover, in a mouse model of FLT3-ITD AML, animals treated with the CHK1 inhibitor MK8776 + cytarabine survived longer than those treated with cytarabine alone. These findings suggest that FLT3-ITD and Rac1 activity cooperatively modulate DNA repair activity, the addition of DNA damage response inhibitors to conventional chemotherapy may be useful in the treatment of FLT3-ITD AML, and inhibition of the Rac signaling pathways via DOCK2 may provide a novel and promising therapeutic target for FLT3-ITD AML.
Introduction
Acute myeloid leukemia (AML) is an aggressive hematologic neoplasm characterized by clonal expansion of myeloid blasts. Over 30% of AML patients harbor activating mutations in the FMS-like tyrosine kinase-3 (FLT3) gene, and those who carry an internal tandem duplication (ITD) mutation in the juxtamembrane domain have a particularly poor prognosis.1,2 FLT3 is a receptor tyrosine kinase that plays important roles in the survival, proliferation and differentiation of hematopoietic stem/progenitor cells.3–5 The FLT3-ITD mutation confers constitutive autophosphorylation and activation of downstream signaling pathways, including PI-3-kinase/AKT, RAS/ERK and STAT5.2,6
FLT3 interacts with Dedicator of Cytokinesis 2 (DOCK2), which is a guanine nucleotide exchange factor for Rac1 and Rac2.7–10 Rac1 is widely expressed and plays key regulatory roles in various cellular functions, including actin cytoskeleton reorganization, cell proliferation, DNA damage response (DDR), angiogenesis and glucose uptake.11–16 Unlike Rac1, DOCK2 is expressed predominantly in hematopoietic tissues.10 DOCK2 is known to regulate several crucial processes, including lymphocyte migration, activation and differentiation of T cells, cell-cell adhesion, and bone marrow homing of various immune cells.17–28 Patients with DOCK2 deficiency exhibit pleiotropic immune defects, often characterized by early-onset invasive bacterial and viral infections with T- and/or B-cell lymphopenia, as well as defective T-cell, B-cell, and natural killer-cell responses.29,30
We previously demonstrated that suppression of DOCK2 expression in FLT3-ITD-positive leukemic cells led to a concomitant decrease of STAT5 and Rac1 activity, and that DOCK2 knockdown (KD) in a FLT3-ITD leukemia cell line prolonged disease progression in a mouse xenograft model.7 Additionally, we found that DOCK2 KD leads to increased sensitivity to the chemotherapeutic agent cytarabine (ara-C), which is the backbone of AML therapy.7
In the current study we further investigated the mechanisms by which Rac1/DOCK2 activity affects cell survival and response to ara-C in FLT3-ITD leukemia cells. We found that DOCK2 KD in FLT3-ITD cells resulted in decreased expression and activity of FLT3-ITD itself, as well as decreased expression of both mismatch repair (MMR) and DDR factors. Additionally, exogenous expression of FLT3-ITD resulted in elevated expression of DDR factors, increased Rac1 activity, and increased resistance to ara-C in TF-1 cells. Furthermore, DOCK2 KD significantly enhanced the sensitivity of FLT3-ITD leukemic cells to combined treatment with ara-C and DDR inhibitors, both in vitro and in a mouse xenograft model. These findings suggest that FLT3-ITD and Rac1/DOCK2 are key modulators of a coordinated regulatory network that controls DDR activity in FLT3-ITD leukemic cells, and also indicate that modification of DDR pathways may be of value in the treatment of FLT3-ITD AML.
Methods
Additional methods are detailed in the Online Supplement.
Cell culture assays
All assays were performed according to the manufacturers’ instructions. To measure cell proliferation after drug treatments, 0.5 × 106 cells/mL were placed in 24-well plates in triplicate, and cell densities were measured. Apoptosis assays were performed using annexin V-APC and 7-amino-actinomycin D (7-AAD; BD Biosciences, San Jose, CA, USA). Late apoptosis was defined as cells positive for both 7-AAD and annexin V, and apoptotic cells were cells positive for annexin V. The half maximal inhibitory concentration (IC50) values of the drugs for each cell line were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche Diagnostics, Indianapolis, IN, USA) (Online Supplementary Figure S1). Rac-1 activation (Rac1-GTP) was assessed using the G-LISA activation assay (Cytoskeleton, Inc., Denver, CO, USA). The levels of reactive oxygen species in cells were measured using CM-H2DCFDA (ThermoFisher Scientific, Waltham, MA, USA). The cell cycle was analyzed using a BD Pharmingen™ BrdU Flow Kit (BD Biosciences), and flow cytometric analysis of cellular gH2AX level was performed using Alexa Fluor 647-anti phospho-Histone H2AX (S139) antibodies (613408; BD Biosciences) in combination with the BD Pharmingen™ BrdU Flow Kit.
Mouse transplantation experiments
NSG (NOD/Shi-scid/IL-2Rgnull) mice were provided by the Johns Hopkins Research Animal Resources. Each mouse (female, 6-8 weeks) was injected with 0.6 × 106 cells via the lateral tail vein. Engraftment was assessed by flow cytometric measurement of human and mouse CD45 expression on the cell surface (APC mouse anti-human CD45 and FITC rat anti-mouse CD45, BD Biosciences). Treatments of mice transplanted with control MV4;11 (MV4;11-C) cells started on day 12 after transplantation, while treatments of mice transplanted with DOCK2 KD MV4;11 (MV4;11-KD) cells started on day 49 after transplantation. The starting times for treatments were determined based on pilot experiments that revealed the difference in disease progression in these two groups of mice. Engraftment in peripheral blood was assessed immediately prior to the start of treatment to ensure that the two groups of mice had similar peripheral blood blast levels (Online Supplementary Figure S6). Each mouse was given daily intraperitoneal injections of vehicle, ara-C (50 mg/kg), MK8776 (10 mg/kg), MK1775 (15 mg/kg), ara-C+MK8776 or ara-C+MK1775 for 3 consecutive days. When administered in combination with ara-C, MK8776 and MK1775 were injected 30 min after the ara-C injection. Each treatment group contained at least ten mice, three to five of which were sacrificed for bone marrow engraftment analysis 7 days after the start of treatment, and the rest were monitored for survival. All animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institute of Health, Bethesda, MD, USA) and were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
Statistics
Statistical analyses were performed with the Student t test (two-tailed), repeated measure analysis of variance, and log-rank tests using GraphPad (GraphPad Software, Inc., La Jolla, CA, USA). Each data point represents the average of at least three biological replicates. All data are presented as the mean ± standard error of the mean. P values <0.05 were considered to be statistically significant.
Results
Decreased DOCK2 expression in MV4;11 cells leads to differential responses to ara-C and 5-fluorouracil treatment
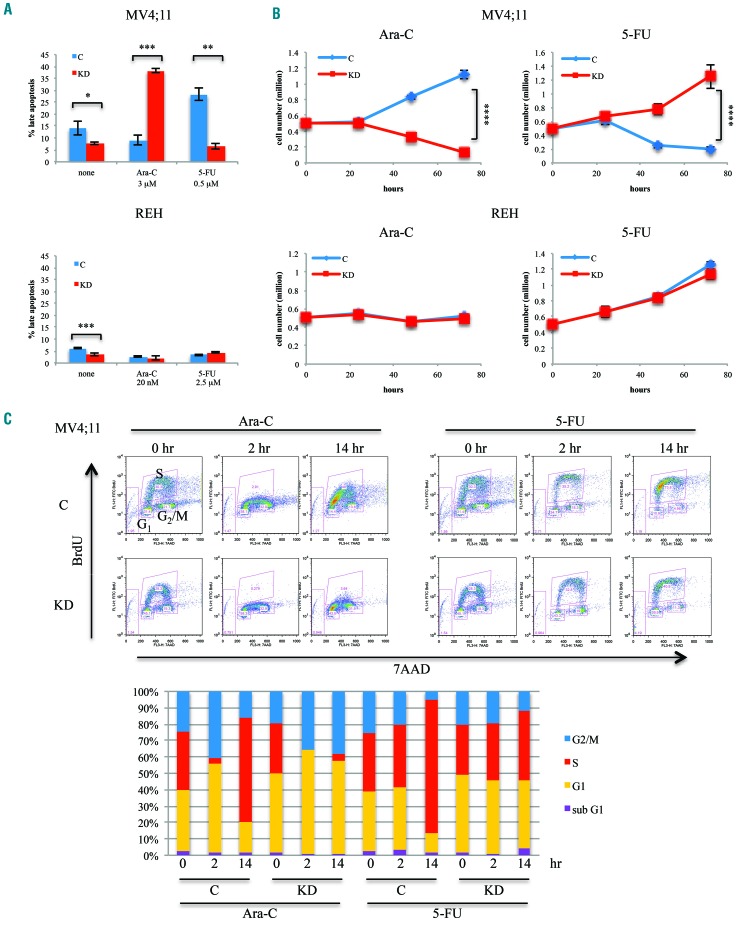
The antimetabolite ara-C interferes with the synthesis of DNA, and is the backbone of both induction and consolidation regimens in the treatment of AML. KD of DOCK2 expression via stable expression of a short hairpin (sh)RNA in the FLT3-ITD MV4;11 leukemic cell line resulted in increased sensitivity to ara-C (3 μM), as indicated by increased apoptosis (Figure 1A) and reduced cell proliferation (Figure 1B). However, when the same cell lines were treated with the thymidylate synthase inhibitor 5-fluorouracil (5-FU; 0.5 μM) they exhibited a markedly different response to treatment, with DOCK2 KD MV4;11 cells showing decreased apoptosis and increased cell proliferation. These differential effects were not seen in REH cells, a leukemia cell line that expresses wildtype (WT) FLT3 (Figure 1A,B), or K562 cells, a leukemia cell line that does not express FLT3 (Online Supplementary Figure S2), suggesting that the FLT3-ITD mutation is responsible for the effect.
Figure 1.
Suppression of DOCK2 expression in MV4;11 cells resulted in differential response to ara-C and 5-fluorouracil treatment. (A) Stable knockdown (KD) of DOCK2 expression increased the fraction of cells in apoptosis upon treatment with ara-C and decreased the fraction of cells in apoptosis upon treatment with 5-flu-orouracil (5-FU) in MV4;11 cells, but not REH cells. Cells were treated for 72 h. The concentration of ara-C and 5-FU used for each cell line was the IC50 as determined by MTT assay. (B) DOCK2 KD resulted in increased cell death upon treatment with ara-C and decreased cell death upon treatment with 5-FU in MV4;11 cells, but not REH cells. (C) DOCK2 KD MV4;11 cells exhibited greater impairment in cell cycling after ara-C treatment, and less disruption of cell cycling after 5-FU treatment, compared to control MV4;11 cells. A bromodeoxyuridine (BrdU) incorporation assay revealed the cell cycle status of MV4;11 cells at 0, 2 and 14 h after treatment with ara-C (3 μM) or 5-FU (0.5 μM). At each time point, cells were pulse-labeled with 10 μM BrdU for 30 min before harvesting. The percentage of cells in each phase of the cell cycle is indicated in the bottom panel. **P<0.01; ***P<0.001; ****P<0.0001. C: cells expressing control short hairpin (sh)RNA; KD: cells expressing shRNA against DOCK2.
We further investigated the differential effects of ara-C and 5-FU treatment in the proliferation and cell cycling of FLT3-ITD-positive cells using a bromodeoxyuridine (BrdU) incorporation assay. Both control and DOCK2 KD MV4;11 cells showed arrested DNA synthesis in response to ara-C (Figure 1C). While control cells continued to synthesize DNA, albeit following a brief partial arrest and at a reduced rate, DNA synthesis was completely abrogated in DOCK2 KD MV4;11 cells within 2 h of ara-C treatment. DNA replication recovered faster in the control MV4;11 cells, while an overall reduction in replication persisted in the DOCK2 KD cells throughout the 26 h observation period (Online Supplementary Figure S3A). In contrast, 5-FU treatment of control cells resulted in progressive accumulation of cells in the early S phase of the cell cycle throughout the 26 h observation period. The DOCK2 KD MV4;11 cells showed only a slight increase in the percentage of cells in S phase at later time points when treated with 5-FU (Figure 1C, Online Supplementary Figure S3B). These findings indicate that when leukemic cells are stressed via treatment with cytotoxic agents, DOCK2 KD affects cell proliferation and cell cycle differently in FLT3-ITD versus WT FLT3 cells.
DOCK2 and FLT3-ITD cooperate to regulate the DNA damage response in FLT3-ITD leukemic cells
5-FU is a thymidylate synthase inhibitor that blocks the synthesis of thymidine, and is utilized in the treatment of solid tumors including colorectal adenocarcinoma. MMR-deficient colorectal adenocarcinoma cells are reported to exhibit markedly decreased sensitivity to 5-FU treatment with a concurrent increase in sensitivity to ara-C, which is a profile similar to that seen in FLT3-ITD leukemic cells with DOCK2 KD.31,32 This suggests that DOCK2 may exert its effects on FLT3-ITD leukemic cell growth via DDR pathways. To verify this, we evaluated the effects of DOCK2 KD on components of MMR and DDR in FLT3-ITD cells.
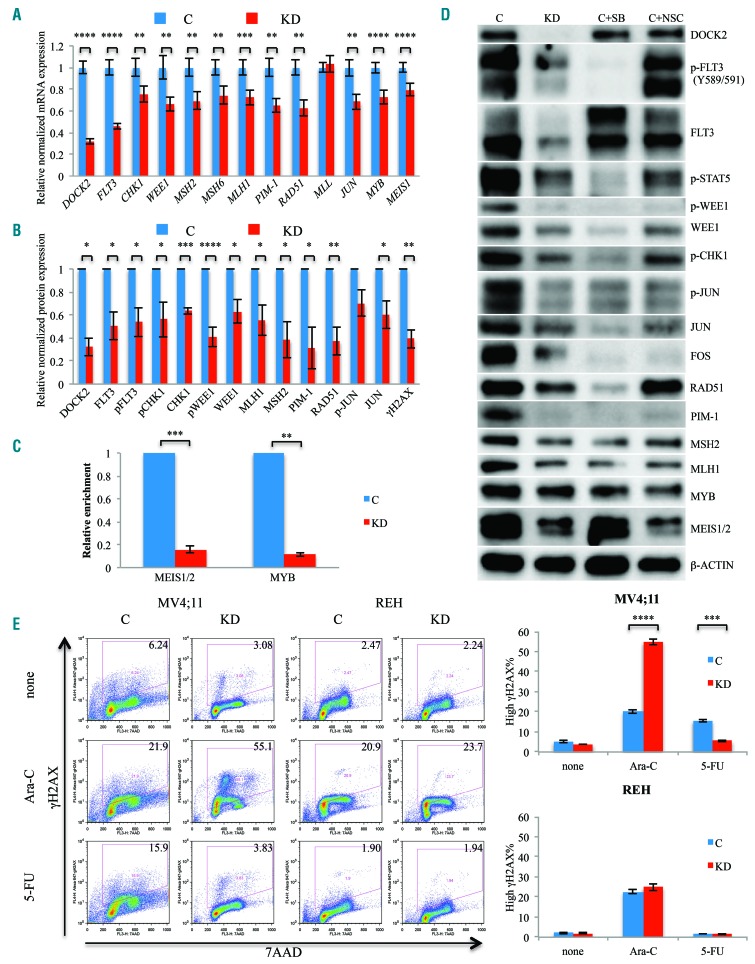
We first investigated the effects of DOCK2 KD on mRNA levels of key MMR and DDR factors. Decreased DOCK2 expression in MV4;11 cells resulted in significantly reduced mRNA levels of key MMR factors MLH1, MSH2 and MSH6, as well as DDR factors including CHK1, WEE1, RAD51 and PIM-1, although MLL (KMT2A) was not affected (Figure 2A). Accordingly, western blot analysis demonstrated that protein levels of MLH1, MSH2, RAD51, PIM-1, CHK1, WEE1 and JUN were also markedly decreased in DOCK2 KD MV4;11 cells, as was the expression of activated (phosphorylated) CHK1, WEE1 and JUN (Figure 2B, D). Of note, JUN is part of the AP1 complex that regulates the transcription of MMR factors.33
Figure 2.
DOCK2 knockdown in MV4;11 cells resulted in decreased expression and activity of FLT3 and DNA damage response factors. (A) Quantitative reverse transcriptase polymerase chain reaction assays revealed decreased mRNA levels of FLT3, CHK1, WEE1, MSH2, MSH6, MLH1, PIM-1, RAD51, JUN, MYB and MEIS1 in DOCK2 knockdown (KD) MV4;11 cells. The levels of the transcripts were normalized based on that of GAPDH, and the relative expression of each transcript in KD cells compared to control cells is shown. (B) Western blot analysis revealed significantly decreased levels of total and phosphorylated FLT3, CHK1, WEE1, JUN, total MSH2, MLH1, RAD51, PIM-1, and phosphorylated histone H2AX (gH2AX). The level of expression of each protein was normalized to the expression level of β-actin, and the relative expression of each protein in KD cells compared to control cells is shown. (C) DOCK2 KD resulted in decreased binding of MEIS1/2 and MYB to the regulatory element located -15 kb from the FLT3 initiation codon. Relative enrichments were normalized against those in control cells. (D) The reduction in DNA damage response (DDR) activity in DOCK2 KD MV4;11 cells was due to the decrease in Rac1 and FLT3 activity. MV4;11 cells treated with NSC23766 (NSC; 40 μM) or sorafenib (SB; 25 nM) for 20 h exhibited decreased levels of MEIS1, MYB, MSH2, MLH1, RAD51, PIM-1, and phosphorylation of STAT5, CHK1, WEE1, JUN and FOS. (D) Compared with control cells, the percentage of cells harboring elevated gH2AX levels in DOCK2 KD MV4;11 cells was increased upon treatment with ara-C (3 μM) and decreased upon treatment with 5-fluorouracil (5-FU; 0.5 μM). Cells were treated for 18 h. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. C: cells expressing control short hairpin (sh)RNA; KD: cells expressing shRNA against DOCK2.
DOCK2 KD MV4;11 cells also exhibited significantly reduced expression of MEIS1 and MYB, which are known regulators of FLT3 expression (Figure 2A, B, D).34,35 Accordingly, the binding of MEIS1/2 and MYB to the regulatory element located -15 kb from the FLT3 initiating codon was significantly reduced, as indicated by chromatin immunoprecipitation assays (Figure 2C), and the expression level and activity of FLT3 were markedly decreased in DOCK2 KD cells (Figure 2A, B, D). Similar changes in expression levels of FLT3 and DDR factors were also observed in the FLT3-ITD-positive Molm14 leukemia cell line (Online Supplementary Figure S4A). However, the expression of most of the DDR factors examined was not significantly altered in REH cells, which express WT FLT3 (Online Supplementary Figure S4B).
Since DOCK2 KD leads to decreased Rac1 activity and FLT3 expression in MV4;11 cells, we investigated whether a pharmacological reduction in Rac1 and FLT3 activity would also lead to downregulation of DDR factors. After treatment with the Rac1 inhibitor NSC23766 (40 μM) or the FLT3 inhibitor sorafenib (25 nM), MV4;11 cells exhibited a similar profile of protein expression changes as those seen in DOCK2 KD cells, including decreased phospho-STAT5 as well as AP1 and DDR factors (Figure 2D). These findings suggest that the downregulation of DDR activity observed in DOCK2 KD MV4;11 cells is likely due to reduced Rac1 and/or FLT3 activity in these cells. Furthermore, this observation is consistent with our previous finding that FLT3 inhibitors markedly sensitized DOCK2 KD MV4;11 cells to ara-C treatment, while control cells were not significantly affected.7
We further investigated the downstream effects of the reduction of MMR and DDR factors seen in association with DOCK2 KD in FLT3-ITD leukemic cells by assessing the phosphorylation of histone H2AX (gH2AX), which is triggered by DNA damage. Western blot analysis revealed a significantly reduced level of gH2AX in DOCK2 KD MV4;11 cells compared with the level in control cells (Figure 2B), suggesting a lower level of DNA damage and/or decreased baseline DNA repair activity in DOCK2 KD cells. This finding was confirmed by flow cytometric analysis of cellular gH2AX levels, indicating a greater percentage of cells with a high gH2AX signal (above the baseline level observed during normal DNA replication) in control MV4;11 cells versus DOCK2 KD cells (Figure 2E). Control MV4;11 cells showed increased DNA damage upon treatment with either ara-C (3 μM; 18 h) or 5-FU (0.5 μM; 18 h). In contrast, DOCK2 KD MV4;11 cells exhibited a significant increase in the gH2AX-high proportion only in response to ara-C but not 5-FU treatment. The 5-FU-treated DOCK2 KD MV4;11 cells demonstrated a gH2AX profile similar to that of untreated cells (Figure 2E). Meanwhile, both control and DOCK2 KD REH cells exhibited similar levels of gH2AX after treatment with either ara-C or 5-FU (Figure 2E). These data indicate that DOCK2 KD impedes the cells’ ability to repair damaged DNA upon ara-C but not 5-FU treatment.
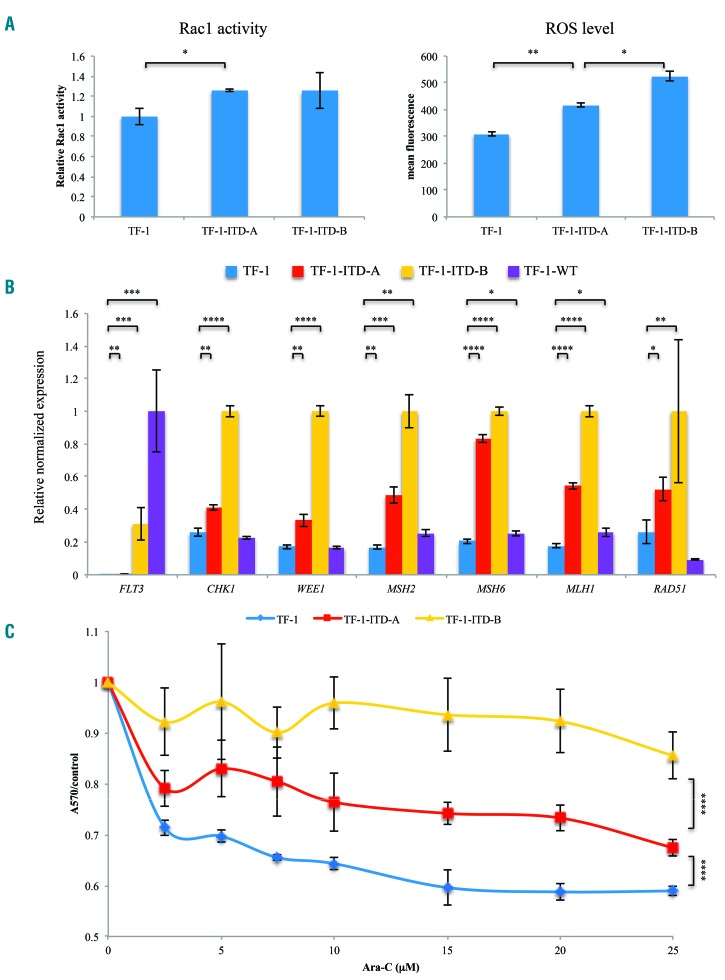
In order to confirm that FLT3-ITD affects expression of DDR factors, we utilized a TF-1 leukemia cell line that does not normally express FLT3. Consistent with previous reports, TF-1 cells exogenously expressing a moderate (TF-1-ITD-A) or relatively high level of FLT3-ITD (TF-1-ITD-B) both exhibited elevated Rac1 activity and an increase in the level of reactive oxygen species (Figure 3A). Downstream targets of FLT3-ITD signaling include STAT5 and ERK1/2. The STAT5 pathway is known to regulate the expression of DDR factors (CHK1, WEE1, RAD51), and the ERK1/2 pathway is known to affect the generation of MMR and DDR factors. Thus, we would expect both DDR and MMR factors to be enhanced by exogenous expression of FLT3-ITD in TF-1 cells.33,36–41 Quantitative reverse transcriptase polymerase chain reaction studies of TF-1 cell lines confirmed that expression of FLT3-ITD resulted in a significant increase in CHK1, WEE1, MSH2, MSH6, MLH1 and RAD51 expression, which positively correlated with the level of FLT3-ITD expression in these cells (Figure 3B). In contrast, relatively high expression of WT FLT3 in TF-1 cells resulted in only minor increases in the expression of MMR factors (MSH2, MSH6, MLH1), with no change in CHK1, WEE1 or RAD51 expression (Figure 3B). Consistent with increased DNA repair activity in FLT3-ITD-expressing TF-1 cells, these cells exhibited markedly increased resistance to ara-C treatment, which also correlated positively with the level of expression of FLT3-ITD (Figure 3C).
Figure 3.
Exogenous expression of FLT3-ITD led to increased DNA repair activity in TF-1 cells. (A) TF-1 cells expressing FLT3-ITD exhibited increased Rac1 activity and reactive oxygen species (ROS) levels compared to parental TF-1 cells. (B) Quantitative reverse transcriptase polymerase chain reaction assays revealed increased expression of CHK1, WEE1, MSH2, MSH6, MLH1, and RAD51 in TF-1 cells expressing FLT3-ITD, but not in cells expressing wildtype (WT) FLT3. The levels of the transcripts were normalized based on that of GAPDH. To better visualize the differences in expression, the relative levels of FLT3 compared to that of TF-1-WT cells are shown, while for other genes, the relative levels of transcripts compared to those of TF-1-ITD-B cells are exhibited. (C) MTT assays revealed increased survival of FLT3-ITD-expressing TF-1 cells in the presence of ara-C (48 h). *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. C: cells expressing control short hairpin (sh)RNA; KD: knockdown cells expressing shRNA against DOCK2.
Taken together, these results indicate that DOCK2 expression affects the level of FLT3-ITD expression, with associated changes in the expression of DDR factors and DNA damage.
DOCK2 knockdown renders MV4;11 cells more sensitive to treatment with DNA damage response inhibitors
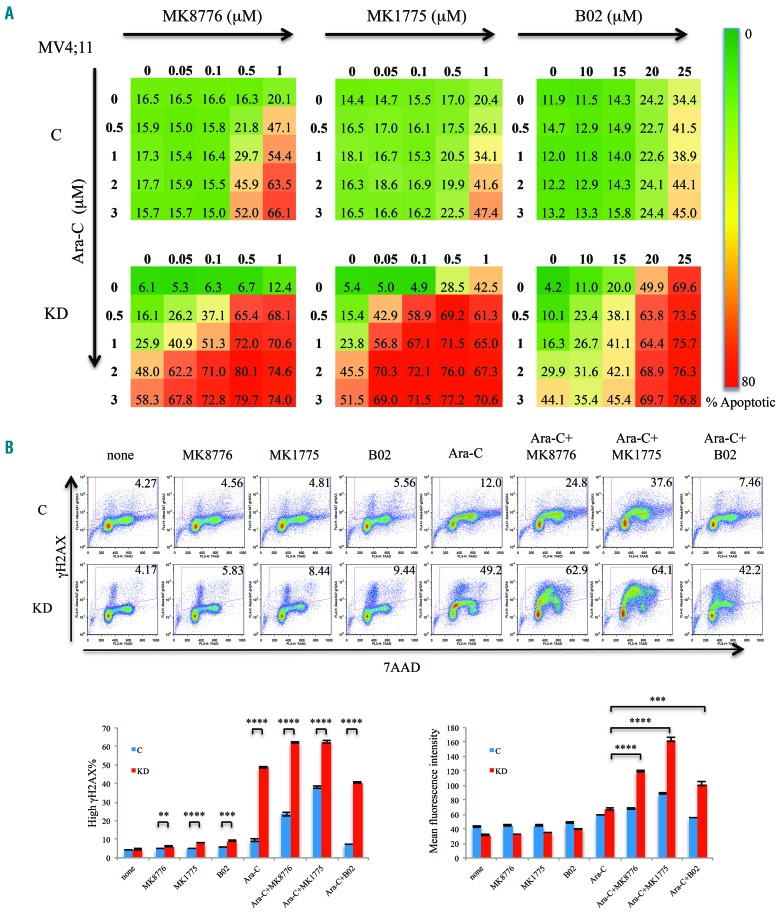
Since DOCK2 KD MV4;11 cells exhibit downregulation of CHK1, WEE1 and RAD51, we further investigated whether these cells are more sensitive to treatment with DDR inhibitors. CHK1 and WEE1 are activated in response to DNA damage and replication stress, and arrest cells in the S and G2 phases of the cell cycle. Both CHK1 and WEE1 are overexpressed in more than 50% of myeloid leukemias and are important determinants of ara-C sensitivity in AML cells.42 RAD51 is one of the key factors in the homology-directed DNA repair pathway and has been shown to play important roles in DNA repair in FLT3-ITD leukemic cells.43,44 MV4;11 cells with DOCK2 KD showed an increase in the percentage of apoptotic cells after treatment with the CHK1 inhibitor MK8776, WEE1 inhibitor MK1775 and RAD51 inhibitor B02 (Figure 4A). Furthermore, synergistic effects between these DDR inhibitors and ara-C were observed at markedly lower concentrations in DOCK2 KD MV4;11 cells (Online Supplementary Figure S4C).
Figure 4.
Suppression of DOCK2 expression rendered MV4;11 cells more sensitive to MK8776, MK1775 and B02. (A) Compared to control cells, DOCK2 knokcdown (KD) MV4;11 cells exhibited increased percentages of apoptotic cells upon treatment with MK8776, MK1775 and B02, both alone and in the presence of ara-C. Cells were treated for 48 h. The assays were performed in triplicate. (B) Compared to control cells, a higher percentage of DOCK2 KD MV4;11 cells harbored elevated DNA damage (as indicated by an elevated gH2AX signal) upon treatment with ara-C (2 μM), as well as with MK8776 (0.1 μM), MK1775 (0.1 μM), and B02 (20 μM), both alone and in combination with ara-C. Treatment with MK8776, MK1775 and B02 in combination with ara-C resulted in an increased mean gH2AX signal in DOCK2 KD MV4;11 cells compared to cells treated with ara-C alone. Cells were treated for 16 h. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001. C: cells expressing control short hairpin (sh)RNA; KD: cells expressing an shRNA against DOCK2.
Flow cytometric analysis of cellular gH2AX levels revealed that increased DNA damage (% high γH2AX) was significantly more frequent in DOCK2 KD MV4;11 cells than in control MV4;11 cells after treatment with ara-C (2 μM), alone or in combination with MK8776 (0.1 μM), MK1775 (0.1 μM) or B02 (20 μM) (Figure 4B).
This assay indicates not only the overall percentage of cells that harbored elevated DNA damage, but also the extent of the damage as measured by the mean fluorescence intensity of gH2AX. The differences in these measurements were particularly notable when cells were treated with ara-C with or without DDR inhibitors. In the presence of ara-C, the percentage of cells with elevated gH2AX level was much higher among DOCK2 KD cells than among the control cells. In contrast, the mean fluorescence intensity of gH2AX was not markedly increased in the DOCK2 KD cells as compared to the control cells. This indicates that the DDR was activated in both control and DOCK2 KD cells after a low level of DNA damage was induced by ara-C, resulting in the arrest of DNA replication and cell cycle to prevent further damage. As shown in Online Supplementary Figure S3, after ara-C treatment, control cells were able to overcome the cell cycle arrest due to higher DNA repair activity. In contrast, DOCK2 KD cells were unable to repair the damage and remained arrested. Therefore, at the point of measurement (16 h after ara-C treatment), when the majority of control cells had repaired the damage and resumed DNA replication and cell cycling, a much higher percentage of DOCK2 KD cells still harbored DNA damage. As expected, when a DDR inhibitor (MK8776 or MK1775) was added, the number of cells (gH2AX %) that exhibited DNA damage increased significantly over that following treatment with ara-C alone. Notably, the extent of DNA damage (gH2AX mean fluorescence intensity) in DOCK2 KD cells showed a marked increase over that of cells treated with ara-C alone, while a modest increase was observed in control cells. These findings are consistent with an increase in DNA damage level and a loss of DNA damage checkpoint response in cells treated with both ara-C and a DDR inhibitor, which are enhanced by suppression of DOCK2 (Figure 4B, Online Supplementary Table S2).
We further investigated whether suppression of Rac1 activity affects sensitivity to ara-C in primary mouse leukemic samples. Whole bone marrow cells from moribund Flt3+/ITD; NHD1345 and Flt3+/+; NHD13 mice46 that had developed acute leukemia were treated in vitro with ara-C and the Rac1 inhibitor NSC23766. As shown in Online Supplementary Figure S5, NSC23766 and ara-C acted synergistically to promote apoptosis in Flt3+/ITD; NHD13 leukemic bone marrow cells, but not in Flt3+/+; NHD13 bone marrow cells.
DOCK2 knockdown enhances the efficacy of ara-C treatment in a mouse xenograft model of FLT3-ITD acute myeloid leukemia, both alone and in combination with MK8776
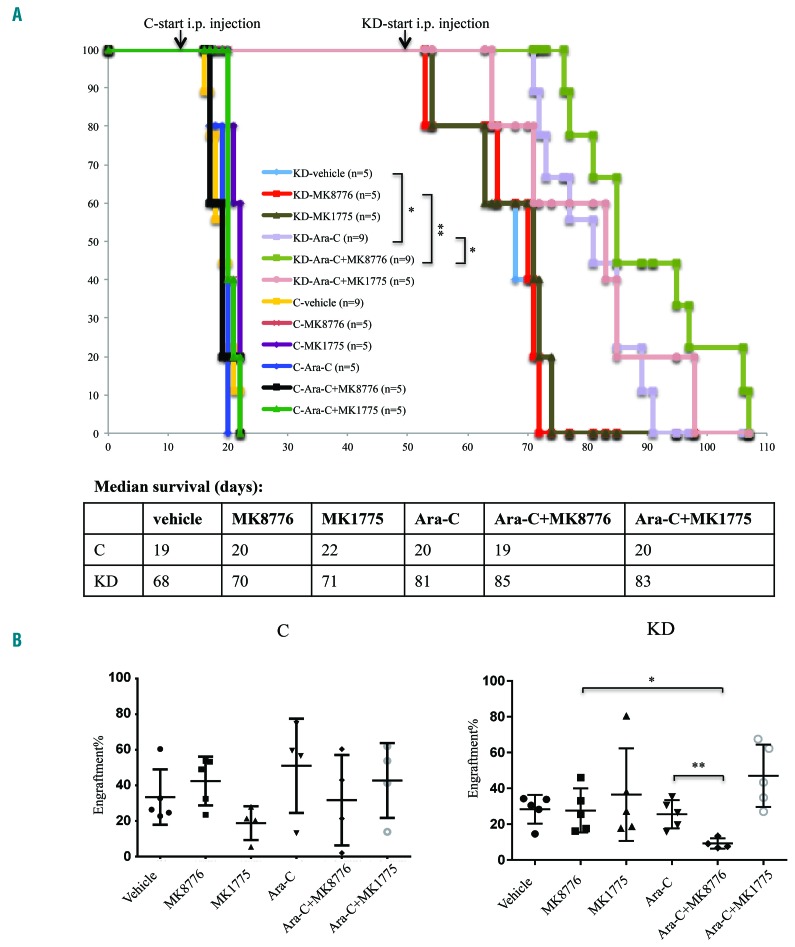
As previously reported, NSG mice transplanted with MV4;11 cells displayed markedly extended survival when expression of DOCK2 was suppressed.7 Since DOCK2 KD MV4;11 cells exhibit significantly increased sensitivity to treatments with ara-C and DDR inhibitors in vitro, we further investigated the effects of DOCK2 KD on the sensitivity of FLT3-ITD leukemic cells to these treatments in a mouse xenograft model. Mice were injected with 0.6 × 106 MV4;11 cells with or without DOCK2 KD cells via a lateral tail vein, and engraftment of the cells was monitored over time. Treatment with ara-C and/or DDR inhibitors was initiated when mice transplanted with control and DOCK2 KD cells reached similar levels of engraftment (day 12 after transplantation for control mice and day 49 after transplantation for DOCK2 KD mice) (Online Supplementary Figure S6). Each mouse received daily intraperitoneal injections of vehicle, ara-C (50 mg/kg), MK8776 (10 mg/kg), MK1775 (15 mg/kg), ara-C+MK8776, or ara-C+MK1775 for 3 consecutive days. DOCK2 KD mice treated with ara-C showed extended survival that was statistically significant as compared with vehicle-treated mice (Figure 5). Furthermore, DOCK2 KD mice treated with ara-C+MK8776 showed slightly prolonged survival that was statistically significant as compared with mice treated with either single agent alone (Figure 5A). Examination of the bone marrow 7 days after the start of treatment revealed a significantly reduced blast percentage in DOCK2 KD mice treated with the combination of ara-C and MK8776, as compared with mice in other treatment groups (Figure 5B). In contrast, no significant difference in survival (Figure 5A) or bone marrow blast percentage (Figure 5B) was observed among mice transplanted with control MV4;11 cells and treated with any of the individual drugs or combinations.
Figure 5.
DOCK2 knockdown in transplanted MV4;11 cells enhanced the treatment benefit of ara-C in NSG mice, both alone and in combination with MK8776. (A) Survival of immunodeficient NSG mice transplanted with MV4;11 cells (0.6 × 106 cells) after daily intraperitoneal (i.p.) injections of vehicle, ara-C (50 mg/kg), MK8776 (10 mg/kg), MK1775 (15 mg/kg), ara-C+MK8776, or ara-C+MK1775 for 3 consecutive days. When combined with ara-C, MK8776 and MK1775 were injected 30 min after the ara-C injection. (B) Bone marrow blast percentage was measured 7 days after the start of treatment. The combined treatment with ara-C and MK8776 resulted in significantly reduced bone marrow blast percentage in NSG mice transplanted with DOCK2 KD MV4;11 cells, compared with mice treated with either single agent. *P<0.05; **P<0.01. C: mice transplanted with MV4;11 cells expressing control short hairpin (sh)RNA; KD: mice transplanted with MV4;11 cells expressing shRNA against DOCK2.
Discussion
The treatment of AML with FLT3-ITD mutations represents a significant clinical challenge. Although remission in patients harboring FLT3-ITD mutations can be achieved with cytarabine-based conventional induction chemotherapy with a frequency similar to other AML patients, the remission is often shorter and the relapse rates are higher.
One well-established mechanism of chemoresistance is the enhancement of DNA damage repair activity by oncogenic kinases, which promotes cancer cell survival in the presence of genotoxic stress. The elevated FLT3 kinase activity in FLT3-ITD leukemic cells leads to increased STAT5 activity, which regulates the activity of several key DDR regulators, including PIM-1, CHK1, WEE1, and RAD51. Furthermore, ERK, another downstream target of FLT3-ITD signaling, regulates expression of MMR factors via AP-1. Accordingly, we found that exogenous expression of FLT3-ITD in TF-1 cells led to elevated activity of Rac1, increased expression of CHK1, WEE1, RAD51 and MMR factors, as well as significantly increased resistance to ara-C treatment. The increased expression of these MMR and DDR pathway components in FLT3-ITD cells is likely crucial for the cells’ survival, since FLT3-ITD drives an increase in reactive oxygen species resulting in increased DNA damage.
Our previous study revealed that decreased DOCK2 expression in FLT3-ITD leukemic cells leads to increased sensitivity to ara-C treatment. FLT3-ITD is known to activate Rac1, which controls a variety of cellular functions.47 Of particular interest, Rac1 has been implicated in chemoresistance in cancer cells due to its regulatory roles in DDR pathways.48 Since DOCK2 functions as a guanine nucleotide exchange factor for Rac1, DOCK2 KD results in decreased Rac1 activity, thereby decreasing STAT5 and ERK phosphorylation, as well as markedly reducing the expression of downstream DDR factors. Interestingly, KD of DOCK2 also resulted in reduced expression and activity of FLT3-ITD. The mechanism by which FLT3-ITD is regulated by DOCK2 is not completely clear. However, the expression of Meis1 and Myb, two known transcription regulators of FLT3, was also significantly downregulated when DOCK2 was knocked-down in FLT3-ITD leukemic cells. Thus, DOCK2/Rac1 and FLT3-ITD appear to form a positive feedback loop, and cooperate to modulate cellular DDR activities (Figure 6).
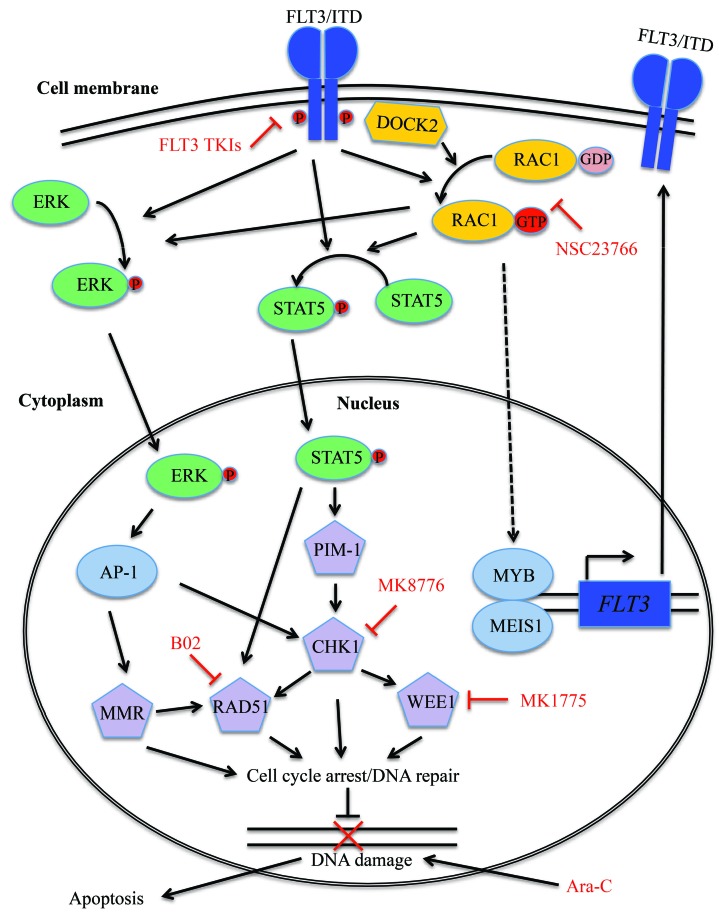
Figure 6.
Proposed mechanism through which Rac1/DOCK2 and FLT3-ITD cooperate to regulate the DNA damage response in FLT3-ITD leukemic cells. FLT3-ITD activates STAT5, directly or through activation of Rac1. Activated STAT5 leads to activation of CHK1, WEE1, PIM-1 and RAD51, which in turn increases DNA repair activity in the cell. FLT3-ITD also activates mismatch repair activity via activation of ERK1/2. DOCK2 activates Rac1 activity through its function as a guanine nucleotide exchange factor (GEF), and also modulates FLT3-ITD expression via regulation of Meis1 and Myb.
Rac1 itself is a challenging therapeutic target due to its widespread expression and diverse cellular functions.49 As a tissue-specific Rac1 effector, DOCK2 may prove to be a more feasible target in that its inhibition allows for hematopoietic-specific Rac1 inhibition. Although DOCK2 inhibitors are not currently widely available, small molecular inhibitors of DOCK2 have been reported.50 Moreover, screening of pre-existing drug libraries may be warranted to uncover potential novel DOCK2 inhibitors.
Various regulators of DDR have also been investigated as therapeutic targets to combat chemoresistance. Here we demonstrate that the suppression of DOCK2 significantly increases the sensitivity of FLT3-ITD cells to ara-C in combination with inhibitors of CHK1, WEE1 and RAD51 in vitro, and ara-C with a CHK1 inhibitor in vivo. While these results help to clarify the interplay between FLT3-ITD and DOCK2, they also suggest that DDR inhibitors may provide a useful addition to chemotherapeutic regimens in patients with FLT3-ITD AML, since control FLT3-ITD cells also showed modest increases in apoptosis and DNA damage when treated with DDR inhibitors in combination with ara-C.
The findings in this study suggest that DOCK2/Rac1 activity may play an important role in FLT3-ITD signaling, particularly with respect to DDR pathways. DOCK2 is a promising therapeutic target that allows for tissue-specific Rac1 inhibition, and perturbations in DDR pathways in FLT3-ITD AML could also be harnessed to provide novel strategies for the treatment of this aggressive neoplasm.
Acknowledgment
The authors would like to thank NIH/NCI for grants (R21 CA175667 to ASD, R01 CA090668 and P30 CA006973 to DS and T32 CA60441 to MW), Allegheny Health Network-Johns Hopkins Cancer Research Fund (to ASD), Johns Hopkins Catalyst Award (to ASD), Catherine and Constantinos J. Limas Research Award (to ASD) and the Giant Food Pediatric Cancer Research Fund (to DS) for research funding. DS is also supported by the Kyle Haydock Professorship.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/104/12/2418
References
- 1.Moreno I, Martin G, Bolufer P, et al. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88(1):19–24. [PubMed] [Google Scholar]
- 2.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752. [DOI] [PubMed] [Google Scholar]
- 3.Broxmeyer HE, Lu L, Cooper S, Ruggieri L, Li ZH, Lyman SD. Flt3 ligand stimulates/costimulates the growth of myeloid stem/progenitor cells. Exp Hematol. 1995;23(10):1121–1129. [PubMed] [Google Scholar]
- 4.Lyman SD, James L, Vanden Bos T, et al. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993;75(6):1157–1167. [DOI] [PubMed] [Google Scholar]
- 5.Small D, Levenstein M, Kim E, et al. STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34+ human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A. 1994;91(2):459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhary C, Muller-Tidow C, Berdel WE, Serve H. Signal transduction of oncogenic Flt3. Int J Hematol. 2005;82(2):93–99. [DOI] [PubMed] [Google Scholar]
- 7.Wu M, Hamaker M, Li L, Small D, Duffield AS. DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells. Leukemia. 2017;31(3):688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwofie MA, Skowronski J. Specific recognition of Rac2 and Cdc42 by DOCK2 and DOCK9 guanine nucleotide exchange factors. J Biol Chem. 2008;283(6):3088–3096. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara H, Maeda M, Oda A, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100(12):3968–3974. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi T, Kubonishi S, Shibakura M, et al. Dock2 participates in bone marrow lympho-hematopoiesis. Biochem Biophys Res Commun. 2008;367(1):90–96. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y, Oda H, Patrick MS, et al. Rac GTPases are involved in development, survival and homeostasis of T cells. Immunol Lett. 2009;124(1):27–34. [DOI] [PubMed] [Google Scholar]
- 12.Kalfa TA, Pushkaran S, Zhang X, et al. Rac1 and Rac2 GTPases are necessary for early erythropoietic expansion in the bone marrow but not in the spleen. Haematologica. 2010;95(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sylow L, Nielsen IL, Kleinert M, et al. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J Physiol. 2016;594(17):4997–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rassool FV, Gaymes TJ, Omidvar N, et al. Reactive oxygen species, DNA damage, and error-prone repair: a model for genomic instability with progression in myeloid leukemia? Cancer Res. 2007;67(18) 8762–8771. [DOI] [PubMed] [Google Scholar]
- 15.Fritz G, Henninger C. Rho GTPases: novel players in the regulation of the DNA damage response? Biomolecules. 2015;5(4): 2417–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancelas JA, Jansen M, Williams DA. The role of chemokine activation of Rac GTPases in hematopoietic stem cell marrow homing, retention, and peripheral mobilization. Exp Hematol. 2006;34(8):976–985. [DOI] [PubMed] [Google Scholar]
- 17.Reif K, Cyster J. The CDM protein DOCK2 in lymphocyte migration. Trends Cell Biol. 2002;12(8):368–373. [DOI] [PubMed] [Google Scholar]
- 18.Ackerknecht M, Gollmer K, Germann P, et al. Antigen availability and DOCK2-driven motility govern CD4(+) T cell interactions with dendritic cells in vivo. J Immunol. 2017;199(2):520–530. [DOI] [PubMed] [Google Scholar]
- 19.Gollmer K, Asperti-Boursin F, Tanaka Y, et al. CCL21 mediates CD4+ T-cell costimulation via a DOCK2/Rac-dependent pathway. Blood. 2009;114(3):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitrova D, Freeman AF. Current status of dedicator of cytokinesis-associated immunodeficiency: DOCK8 and DOCK2. Dermatol Clin. 2017;35(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, Chen SY. Dedicator of cytokinesis 2 in cell signaling regulation and disease development. J Cell Physiol. 2017;232(8): 1931–1940. [DOI] [PubMed] [Google Scholar]
- 22.Jiang H, Pan F, Erickson LM, et al. Deletion of DOCK2, a regulator of the actin cytoskeleton in lymphocytes, suppresses cardiac allograft rejection. J Exp Med. 2005;202(8):1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, Terasawa M, Miyano K, et al. DOCK2 and DOCK5 act additively in neutrophils to regulate chemotaxis, superoxide production, and extracellular trap formation. J Immunol. 2014;193(11):5660–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Meng F, Wang B, He L, Liu Y, Liu Z. Dock2 in the development of inflammation and cancer. Eur J Immunol. 2018;48(6):915–922. [DOI] [PubMed] [Google Scholar]
- 25.Kunisaki Y, Tanaka Y, Sanui T, et al. DOCK2 is required in T cell precursors for development of Valpha14 NK T cells. J Immunol. 2006;176(8):4640–4645. [DOI] [PubMed] [Google Scholar]
- 26.Nishihara H, Maeda M, Tsuda M, et al. DOCK2 mediates T cell receptor-induced activation of Rac2 and IL-2 transcription. Biochem Biophys Res Commun. 2002;296(3):716–720. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Nishihara H, Kimura T, et al. DOCK2 regulates cell proliferation through Rac and ERK activation in B cell lymphoma. Biochem Biophys Res Commun. 2010;395(1):111–115. [DOI] [PubMed] [Google Scholar]
- 28.Ushijima M, Uruno T, Nishikimi A, et al. The Rac activator DOCK2 mediates plasma cell differentiation and IgG antibody production. Front Immunol. 2018;9:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobbs K, Dominguez Conde C, Zhang SY, et al. Inherited DOCK2 deficiency in patients with early-onset invasive infections. N Engl J Med. 2015;372(25):2409–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alizadeh Z, Mazinani M, Shakerian L, Nabavi M, Fazlollahi MR. DOCK2 deficiency in a patient with hyper IgM phenotype. J Clin Immunol. 2018;38(1):10–12. [DOI] [PubMed] [Google Scholar]
- 31.Jardim MJ, Wang Q, Furumai R, Wakeman T, Goodman BK, Wang XF. Reduced ATR or Chk1 expression leads to chromosome instability and chemosensitization of mismatch repair-deficient colorectal cancer cells. Mol Biol Cell. 2009;20(17):3801–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hewish M, Martin SA, Elliott R, Cunningham D, Lord CJ, Ashworth A. Cytosine-based nucleoside analogs are selectively lethal to DNA mismatch repair-deficient tumour cells by enhancing levels of intracellular oxidative stress. Br J Cancer. 2013;108(4):983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humbert O, Achour I, Lautier D, Laurent G, Salles B. hP2 expression is driven by AP1-dependent regulation through phorbolester exposure. Nucleic Acids Res. 2003;31(19): 5627–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpe G, Walton DS, Del Pozzo W, et al. C/EBPα and MYB regulate FLT3 expression in AML. Leukemia. 2013;27(7):1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins CT, Hess JL. Deregulation of the HOXA9/MEIS1 axis in acute leukemia. Curr Opin Hematol. 2016;23(4):354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan LL, Green AS, Bertoli S, et al. Pim kinases phosphorylate Chk1 and regulate its functions in acute myeloid leukemia. Leukemia. 2014;28(2):293–301. [DOI] [PubMed] [Google Scholar]
- 37.Hasselbach L, Haase S, Fischer D, Kolberg HC, Sturzbecher HW. Characterisation of the promoter region of the human DNA-repair gene Rad51. Eur J Gynaecol Oncol. 2005;26(6):589–598. [PubMed] [Google Scholar]
- 38.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20(19): 2390–2400. [DOI] [PubMed] [Google Scholar]
- 39.Raleigh JM, O’Connell MJ. The G(2) DNA damage checkpoint targets both Wee1 and Cdc25. J Cell Sci. 2000;113(Pt 10):1727–1736. [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Cao Y, Pham V, et al. Ras-MEK signaling mediates a critical Chk1-dependent DNA damage response in cancer cells. Mol Cancer Ther. 2017;16(4):694–704. [DOI] [PubMed] [Google Scholar]
- 41.Schulze J, Lopez-Contreras AJ, Uluckan O, Grana-Castro O, Fernandez-Capetillo O, Wagner EF. Fos-dependent induction of Chk1 protects osteoblasts from replication stress. Cell Cycle. 2014;13(12):1980–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tibes R, Bogenberger JM, Chaudhuri L, et al. RNAi screening of the kinome with cytarabine in leukemias. Blood. 2012;119(12): 2863–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henning W, Sturzbecher HW. Homologous recombination and cell cycle checkpoints: Rad51 in tumour progression and therapy resistance. Toxicology. 2003;193(1-2):91–109. [DOI] [PubMed] [Google Scholar]
- 44.Seedhouse CH, Hunter HM, Lloyd-Lewis B, et al. DNA repair contributes to the drug-resistant phenotype of primary acute myeloid leukaemia cells with FLT3 internal tandem duplications and is reversed by the FLT3 inhibitor PKC412. Leukemia. 2006;20(12):2130–2136. [DOI] [PubMed] [Google Scholar]
- 45.Greenblatt S, Li L, Slape C, et al. Knock-in of a FLT3/ITD mutation cooperates with a NUP98-HOXD13 fusion to generate acute myeloid leukemia in a mouse model. Blood. 2012;119(12):2883–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2005;106(1):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 2008;270(1):1–9. [DOI] [PubMed] [Google Scholar]
- 48.Cardama GA, Alonso DF, Gonzalez N, et al. Relevance of small GTPase Rac1 pathway in drug and radio-resistance mechanisms: opportunities in cancer therapeutics. Crit Rev Oncol Hematol. 2018;124:29–36. [DOI] [PubMed] [Google Scholar]
- 49.Marei H, Malliri A. Rac1 in human diseases: The therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases. 2017;8(3):139–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishikimi A, Uruno T, Duan X, et al. Blockade of inflammatory responses by a small-molecule inhibitor of the Rac activator DOCK2. Chem Biol. 2012;19(4):488–497. [DOI] [PubMed] [Google Scholar]