Abstract
In contrast to amidines bearing ionizable α-CH bonds, which react with nitric oxide (NO) to add diazeniumdiolate groups at their α-carbons, benzamidine forms an N-bound diazeniumdiolate that can be further derivatized at the other amidine nitrogen and/or the terminal oxygen to form caged NO compounds as potential NO prodrugs.
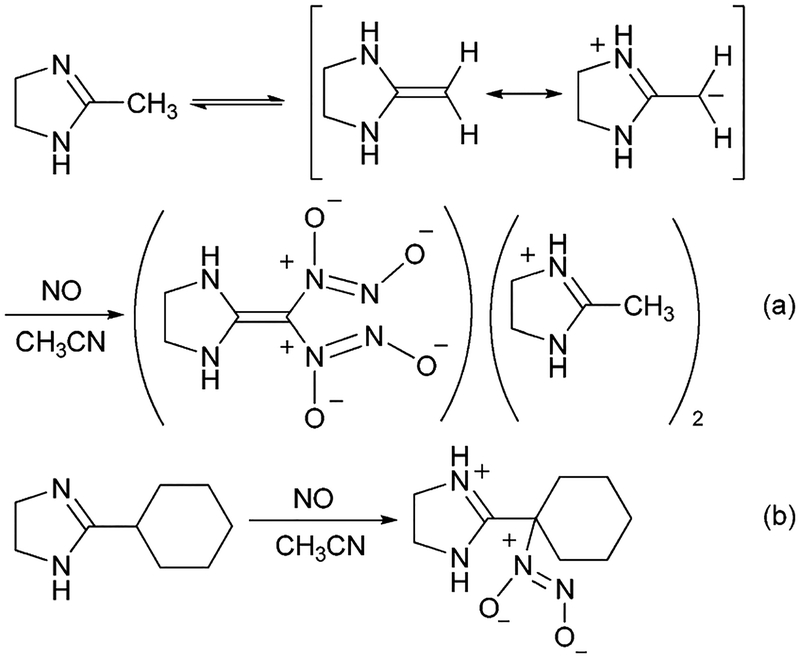
Preparation of carbon-bound diazeniumdiolates1 by the treatment of aliphatic amidines with nitric oxide (NO) has been reported.2 Aliphatic amidine free bases containing α-hydrogen atom(s) react with NO to give C-diazeniumdiolates. The α-hydrogen atom(s) of these amidines are far more acidic than the amine protons, resulting in the reaction of an enediamine tautomer in solution as illustrated in Scheme 1a using lysidine as the example. Thus, the reaction of these amidines with NO has been found to be analogous to the reaction of enamines reported earlier.3 Amidines, such as lysidine, containing more than one replaceable α-hydrogen atom were found to produce polydiazeniumdiolated intramolecular salts, such as the imidazoline bis(diazeniumdiolate) bis(imidazolinium) salt shown in Scheme 1a. In contrast, reaction of 2-cyclohexyl-2-imidazoline containing only one replaceable α-hydrogen atom with NO results in the corresponding C–diazeniumdiolated product as a zwitterionic salt (Scheme 1b).2 As a part of our ongoing research into the synthesis and properties of diazeniumdiolated compounds, we desired to study the reactivity of the N-atom(s) of the amidines as a means for investigating the preparation of new N-diazeniumdiolated products.
Scheme 1.
Preparation of (a) C-diazeniumdiolated lysidine, (b) C-diazeniumdiolated 2-cyclohexyl-2-imidazoline.
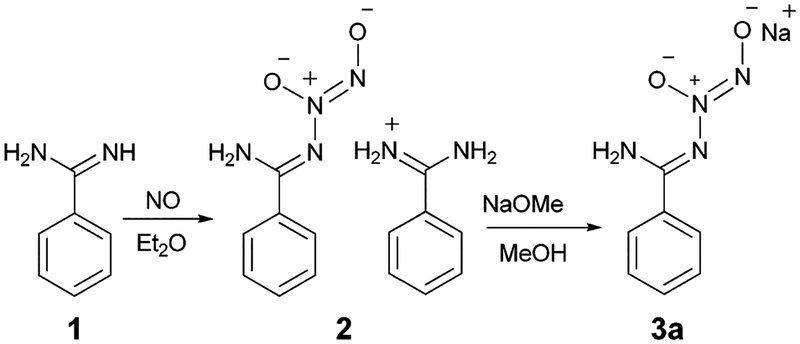
It was envisaged that amidines containing no α-hydrogen atom might possess different reactivity towards NO as compared to those bearing one or more α-protons because reaction of these compounds could not proceed via enediamine tautomers. It seemed that diazeniumdiolation would most likely occur at the amidine N-atom(s). In fact, reaction of benzamidine (1) with NO produced the corresponding N-diazeniumdiolated benzamidine accompanied by benzamidinium counter ion (2) in 46% yield as a stable white powder (Scheme 2). Treatment of a solution of 2 in basified methanol with a stoichiometric quantity of sodium methoxide afforded the sodium salt of N-diazeniumdiolated benzamidine (3a) in 84% yield.
Scheme 2.
Preparation of N-diazeniumdiolated benzamidine.
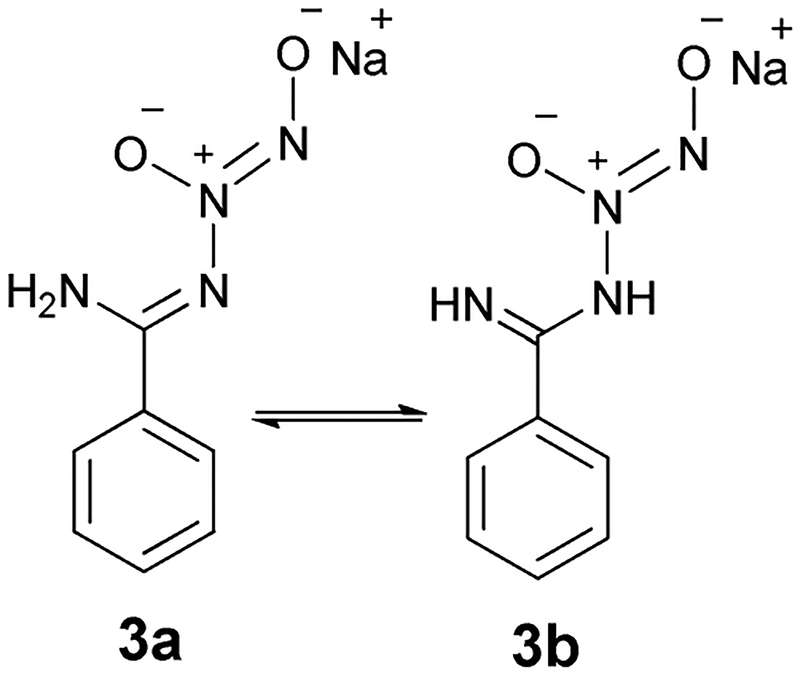
The amidine moiety in benzamidine consists of a primary amine N-atom and also an azomethine N-atom, and accordingly N-diazeniumdiolated benzamidine can exist in either of the two tautomeric forms (3a and 3b, depicted in Fig. 1) depending on their relative stability. We presume the imine-diazeniumdiolate tautomers would predominate due to their higher resonance stabilization as compared to their amine-diazeniumdiolate tautomers (for example 3b). However, due to the possibility of tautomerization occurring after the reaction, it is not possible to determine which N-atom of benzamidine actually reacts with NO.
Fig. 1.
Tautomeric forms of N-diazeniumdiolated benzamidine.
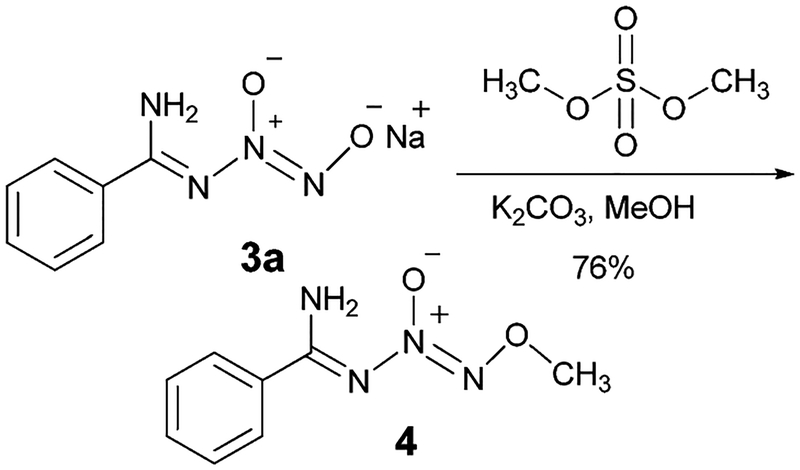
In order to elucidate the structural attributes of N-diazeniumdiolated benzamidine, preparation of an O2-alkylated derivative of this compound was attempted. Accordingly, O2-methyl N-diazeniumdiolated benzamidine (4) was prepared in 76% yield from 3a (Scheme 3). The product was carefully re-crystallized using a solvent combination of ethyl acetate – hexanes and single-crystal X-ray diffraction data indeed revealed that the N-diazeniumdiolated benzamidine exists as the imine tautomer 4 in the solid state.
Scheme 3.
Preparation of O2-methylated N-diazeniumdiolated benzamidine.
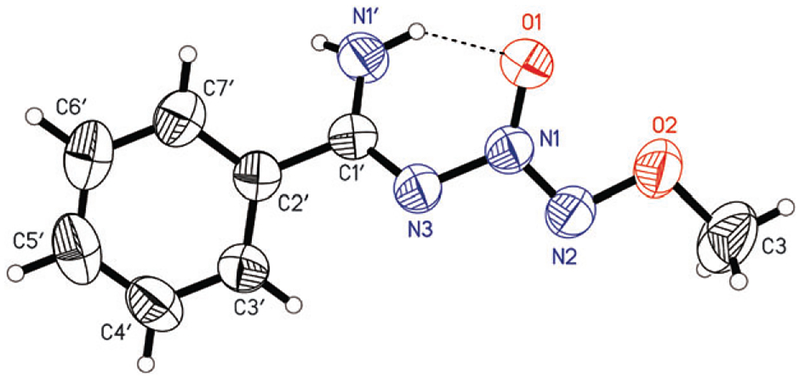
There have been reports indicating preferential E stereo-chemistry for imines of monosubstituted amidines.4 However, the crystal structure of 4 (Fig. 2) reveals that the presence of a hydrogen bond between the amino proton and the O1-atom results in an unusual Z-conformation of the amidine double bond (C1′ = N3 in Fig. 2). While we have postulated the existence of this type of hydrogen bond,5 this is the first confirmed observation. In this amidine, the diazeniumdiolate moiety exists in syn-periplanar conformation which is consistent with the previously reported6 alkylated N-based diazeniumdiolated compounds. Examination of the crystal structure also reveals comparable C–N bond lengths suggesting the relatively low double-bond character of the imine group.
Fig. 2.
Z-conformation molecular structure and numbering scheme for 4.
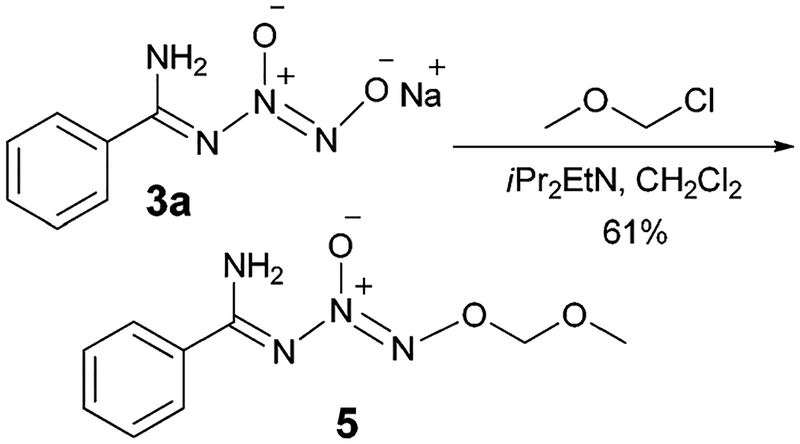
Next, we investigated the preparation of several other O2-protected N-diazeniumdiolated benzamidine prodrugs for potential therapeutic applications as NO-donating agents under suitable conditions. It has been reported that O2-alkoxyalkyl-protected diazeniumdiolates are acid labile7 and thus it was envisaged that the use of this derivative would provide the parent N-diazeniumdiolated amidine as a potential NO source under acidic hydrolytic conditions. Accordingly, O2-methoxymethyl N-diazeniumdiolated benzamidine (5) was prepared in 61% yield (Scheme 4).
Scheme 4.
Preparation of O2-methoxymethyl N-diazeniumdiolated benzamidine.
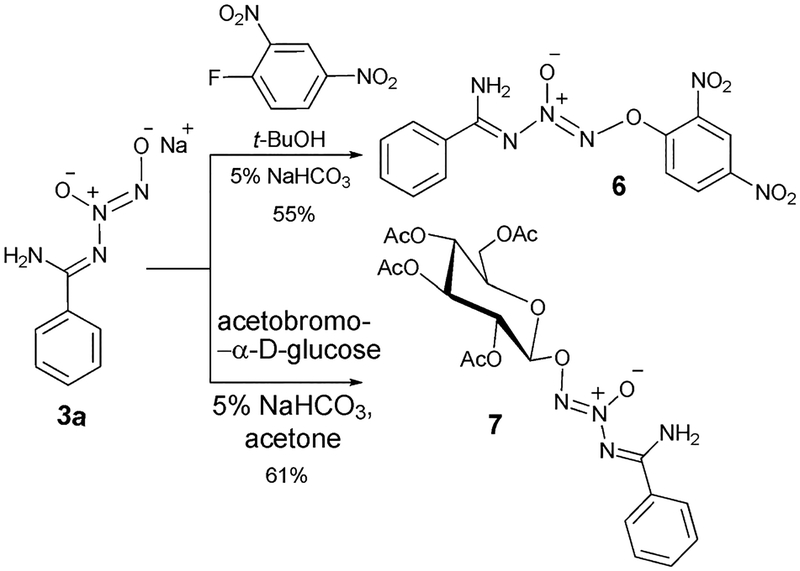
In the past few years, preparation of a diverse class of O2-2,4-dinitrophenyl-protected diazeniumdiolates as glutathione-activated NO-releasing prodrugs8 has been reported and a few have proved to be substantial anticancer lead compounds.9 Thus, we prepared O2-2,4-dinitrophenyl N-diazeniumdiolated benzamidine (6) from 3a as a new addition to this class of compounds (Scheme 5). O2-Glycosylated diazeniumdiolates10 have also emerged as a significant class of glycosidase-activated NO-releasing prodrug whose decomposition products are normal mammalian metabolites, several compounds of this kind having been reported.11 Reaction of acetobromo-α-d-glucose with 3a produced O2-glucosylated N-diazeniumdiolated benzamidine peracetate (7) in 61% yield (Scheme 5).
Scheme 5.
Preparation of O2-2,4-dinitrophenyl N-diazeniumdiolated benzamidine and O2-glucosylated N-diazeniumdiolated benzamidine peracetate.
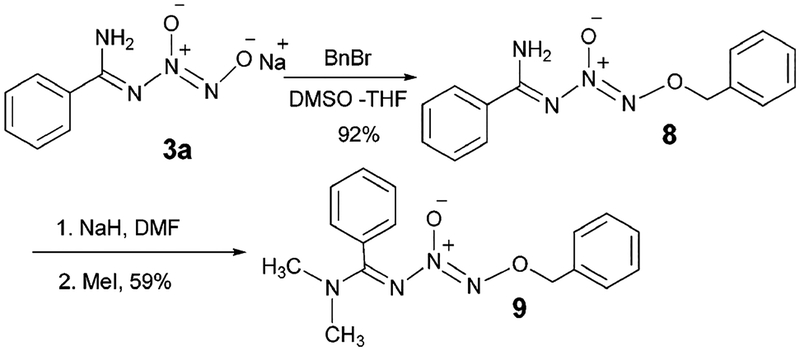
In an effort to prepare more structurally diverse N-diazeniumdiolated amidines that can act as precursors for the preparation of other bio-active molecules, we explored the possibility of multiple functionalization of an analog of 3a. O2-Benzylated N-diazeniumdiolated benzamidine (8) was prepared by the treatment of 3a with benzyl bromide as an acid- and base-stable analog and was subsequently used for further functionalization. Treatment of 8 with sodium hydride and an excess of methyl iodide produced a crystalline white solid in 59% yield which could potentially be either O2-benzyl N′,N′-dimethyl N-diazeniumdiolated benzamidine (9) or the product with a methyl group on each of the amidine N-atoms (Scheme 6). Recrystallization of the crude product using a solvent mixture of ethyl acetate and hexanes and single-crystal X-ray diffraction analysis revealed that the product solely exists in the N′,N′-dimethyl form (9) in the solid state (see Supporting Information). Unlike the O2-methylated analog (4), lack of hydrogen bonding in 9 due to the presence of a secondary amine moiety and the steric bulk of the the dimethyl group results in the more common E-conformation for the imine substituents.
Scheme 6.
Preparation and methylation of O2-benzyl N-diazeniumdiolated benzamidine.
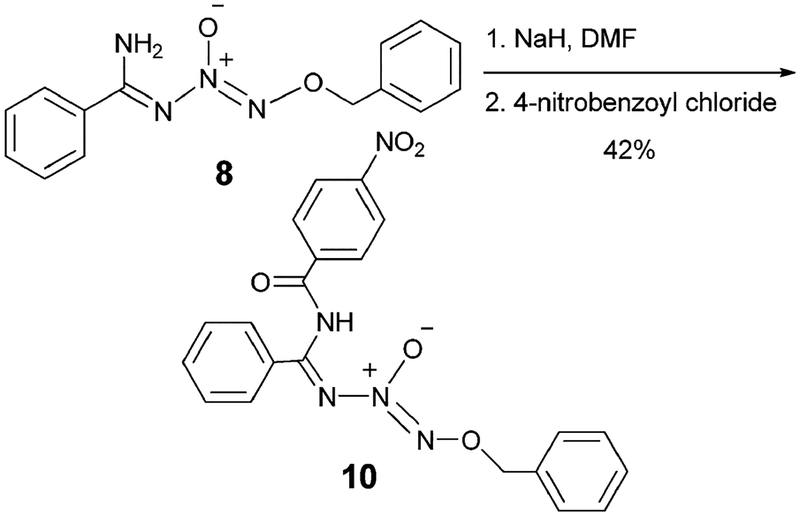
Diazeniumdiolated molecules containing free amine functionality can undergo amide coupling and this methodology has been utilized for the preparation of diazeniumdiolated proteins, such as bovine serum albumin and human serum albumin.7 A similar protocol was applied to our amidine chemistry and resulted in the preparation of a new α-amido N-diazeniumdiolated benzamidine. Reaction of 8 with sodium hydride, followed by the addition of 4-nitrobenzoyl chloride, afforded the desired α-amido product (10) in 43% yield (Scheme 7). Application of this protocol to the preparation of other α-amido and α-sulfonamido analogs is currently being pursued.
Scheme 7.
Preparation of an α-amido-N-diazeniumdiolated benzamidine.
Preliminary results show that certain of these N- diazeniumdiolated benzamidine derivatives regenerate NO in phosphate buffer under physiological pH at 37 °C. The NO-release profiles of these compounds are currently under investigation.
In conclusion, we have prepared N-diazeniumdiolated amidines as a new class of potential NO-donors. The crystal structures of two O2-derivatized analogs of this molecule have been determined and 1H and 13C NMR spectra of the bulk samples fully agree with the assigned structures. We synthesized several O2-protected derivatives of N-diazeniumdiolated benzamidine that can potentially act as useful NO-releasing prodrugs under appropriate conditions. N-Alkylation of O2-benzylated benzamidine diazeniumdiolate has been shown to produce a change in configuration about the imine bond.
Supplementary Material
Acknowledgments
This project has been funded with Federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E, and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Crystallographic studies were supported by the National Institute on Drug Abuse under contract Y1-DA6002.
Footnotes
Electronic supplementary information (ESI) available: Experimental procedures, 1H and 13C NMR spectra for all new compounds and X-ray crystallographic data for 4 and 9. CCDC 761561 and 761562. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc00849d6
Notes and references
- 1.Hrabie JA and Keefer LK, Chem. Rev, 2002, 102, 1135–1154. [DOI] [PubMed] [Google Scholar]
- 2.Hrabie JA, Citro ML, Chmurny GN and Keefer LK, J. Org. Chem, 2005, 70, 7647–7653. [DOI] [PubMed] [Google Scholar]
- 3.Hrabie JA, Arnold EV, Citro ML, George C and Keefer LK, J. Org. Chem, 2000, 65, 5745–5751. [DOI] [PubMed] [Google Scholar]
- 4.The Chemistry of Amidines and Imidates, ed. Perrin CL, Patai S and Rappoport Z, John Wiley and Sons, Ltd., Chichester, 1991, vol. 2, pp. 147–229. [Google Scholar]
- 5.Hrabie JA, Klose JR, Wink DA and Keefer LK, J. Org. Chem, 1993, 58, 1472–1476. [Google Scholar]
- 6.Saavedra JE, Dunams TM, Flippen-Anderson JL and Keefer LK, J. Org. Chem, 1992, 57, 6134–6138. [Google Scholar]
- 7.Hrabie JA, Saavedra JE, Roller PP, Southan GJ and Keefer LK, Bioconjugate Chem, 1999, 10, 838–842. [DOI] [PubMed] [Google Scholar]
- 8.(a) Chakrapani H, Goodblatt MM, Udupi V, Malaviya S, Shami PJ, Keefer LK and Saavedra JE, Bioorg. Med. Chem. Lett, 2008, 18, 950–953; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chakrapani H, Kalathur RC, Maciag AE, Citro ML, Ji X, Keefer LK and Saavedra JE, Bioorg. Med. Chem, 2008, 16, 9764–9771; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nandurdikar RS, Maciag AE, Citro ML, Shami PJ, Keefer LK, Saavedra JE and Chakrapani H, Bioorg. Med. Chem. Lett, 2009, 19, 2760–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Saavedra JE, Srinivasan A, Bonifant CL, Chu J, Shanklin AP, Flippen-Anderson JL, Rice WG, Turpin JA, Davies KM and Keefer LK, J. Org. Chem, 2001, 66, 3090–3098; [DOI] [PubMed] [Google Scholar]; (b) Maciag AE, Saavedra JE and Chakrapani H, Anticancer Agents in Med. Chem., 2009, 9, 798–803. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wu X, Tang X, Xian M and Wang PG, Tetrahedron Lett, 2001, 42, 3779–3782; [Google Scholar]; (b) Showalter BM, Reynolds MM, Valdez CA, Saavedra JE, Davies KM, Klose JR, Chmurny GN, Citro ML, Barchi JJ Jr., Merz SI, Meyerhoff ME and Keefer LK, J. Am. Chem. Soc, 2005, 127, 14188–14189; [DOI] [PubMed] [Google Scholar]; (c) Cai TB, Lu D, Landerholm M and Wang PG, Org. Lett, 2004, 6, 4203–4205; [DOI] [PubMed] [Google Scholar]; (d) Wu X, Xian M and Wang PG, Tetrahedron Lett, 2001, 42, 3779–3782. [Google Scholar]
- 11.(a) Valdez CA, Saavedra JE, Showalter BM, Davies KM, Wilde TC, Citro ML, Barchi JJ Jr., Deschamps JR, Parrish D, El-Gayer S, Schleicher U, Bogdan C and Keefer LK, J. Med. Chem, 2008, 51, 3961–3970; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Nandurdikar RS, Maciag AE, Hong SY, Chakrapani H, Citro ML, Keefer LK and Saavedra JE, Org. Lett, 2009, 12, 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.