Abstract
Background:
Elevated blood lead levels (EBLL, ≥5 µg/dL) are more prevalent among refugee children resettled in the United States than the general US population and contribute to permanent health and neurodevelopmental problems. Therefore, CDC recommends routine screening of refugee children aged 6 months to 16 years upon arrival in the United States, and retesting those aged 6 months-<6 years between 3 and 6 months post-arrival.
Methods:
We analyzed EBLL prevalence among refugee children resettling to the U.S. by country of pre-departure examination and among those rescreened 3 to 6 months after initial testing. We assessed the prevalence of EBLL among refugee children 6 mos–16 yrs) screened during the domestic refugee medical examination between January 1, 2010, and September 30, 2014 and changes in EBLL during follow-up screening.
Results:
Twelve sites provided data on 27,284 children representing nearly 25% of refugee children resettling during the time period of this analysis. The prevalence of EBLL during initial testing was 19.3%. EBLL was associated with younger age, male sex, and country of overseas examination. Among 1,121 children from five sites with available follow-up test results, EBLL prevalence was 22.7%; higher follow-up BLLs were associated with younger age and country of pre-departure examination.
Conclusions:
EBLL decreased over the time period of our analysis in this population of refugee children. Refugee children may be exposed to lead before and after resettlement to the United States. Efforts to identify incoming refugee populations at high risk for EBLL can inform prevention efforts both domestically and overseas.
Table of Contents Summary
Examination of blood lead levels among a cohort of refugee children living in the United States by country of overseas medical examination and other variables.
Introduction
Nearly three million refugees from around the world have resettled to the United States since 1980, with 85,000 arriving between October 2015 and September 2016.1 Among these refugees, 40.1% were children under 16 years old.2 These children may be at increased risk for elevated blood lead levels (EBLL) related to exposures before and after arrival in the United States.
Lead, a neurotoxicant, has no physiologic role in the human body; any level is potentially harmful. Exposure can cause neurological and neurodevelopmental problems, anemia, and, at higher levels, severe brain and kidney damage leading to death.3 Children are especially at risk for lead exposure due to behaviors such as playing on the floor--increasing contact with dust and dirt potentially containing lead--and mouthing of potentially contaminated objects. Children’s bodies also absorb more lead by surface area than adult’s bodies.3 Micronutrient deficiencies (for example, iron and calcium) also can increase the body’s absorption of lead.3
Blood lead levels (BLL) among children in the United States have declined in recent decades.4 In 2012, CDC lowered the reference level for EBLL from 10 µg/dL to 5 µg/dL based on the Advisory Committee on Childhood Lead Poisoning Prevention’s5 recommendations. This value (5 µg/dL) represents the 97.5th percentile of the distribution of BLLs measured on children age 1–5 years in the 2007–2010 National Health and Nutrition Examination Survey (NHANES) and is used by clinical and public health care providers to identify children requiring public health action.6
Previous investigations have found a higher EBLL prevalence among refugee children than in the general population of children in the United States.7–14 Investigators identified associations between EBLL and overseas lead exposures, nutritional deficits, imported products containing lead (e.g., food, cosmetics, toys), and resettlement in older housing in the United States.7–12,15
CDC refugee screening guidelines recommend checking blood lead levels for all refugee children aged 6 months to 16 years16 at the time of arrival in the United States; rescreening refugees aged 6 months to 6 years 3–6 months post-resettlement regardless of initial BLL result; and providing all children aged 6 months to 6 years with daily pediatric multivitamins with iron.16
Although previous analyses have examined EBLL prevalence among refugee children living in individual states,7–11,14 larger analyses across multiple states have not been performed. We sought to determine the prevalence over time of EBLL among newly resettled refugee children across multiple states, stratified by country of pre-departure examination, and to determine changes in BLL among children rescreened within 3–6 months post-resettlement.
Methods
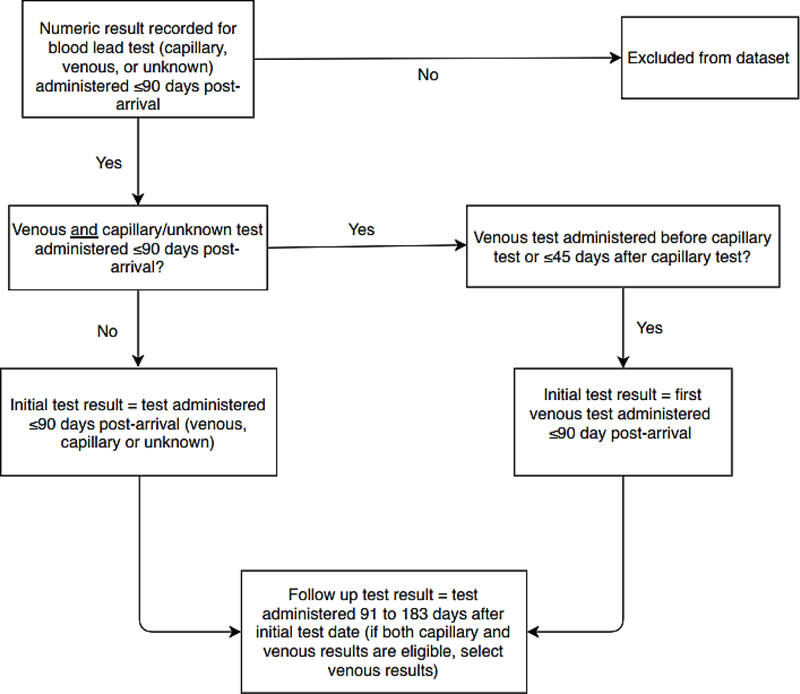
State and local refugee health programs provided CDC with BLL test results and demographic information routinely collected during the domestic refugee health examinations of children aged 6 months to 16 years resettled to the United States from January 1, 2010, to September 30, 2014. Some participating partners received funding through CDC’s CK12–1205 Strengthening Surveillance for Diseases among Newly Arrived Immigrants and Refugees cooperative agreement. We preferentially included venous BLL testing results—because capillary BLLs may be subject to contamination from lead traces on fingers17—but accepted capillary or undocumented specimen results if venous results were unavailable. We defined a valid initial BLL test as a quantitative blood lead value from testing conducted ≤90 days after arrival. If a child had both capillary and venous BLL testing during the first 90 days, we preferentially selected the venous BLL, if the date of collection was either before or ≤45 days after the capillary test (Figure 1). Tests administered 91–183 days (3–6 months) after the initial selected test were designated valid follow-up tests. Qualitative test results (i.e., reported without a numeric value) and results of specimens collected outside the defined time frames were excluded. We were unable to collect data on potential lead exposures.
Figure 1.
Selection of test results for inclusion in an analysis of blood lead levels among refugee children resettled in the United States
Demographic and Clinical Information
Refugee health program partners provided demographic data, including sex, age at time of BLL testing, US arrival date, and overseas examination country. When available, height, weight, and hemoglobin results from the initial domestic medical examination were also provided. If sites could not provide complete demographic information but provided a unique identification number, we used this number to extract relevant data from CDC’s Electronic Disease Notification system.18 We categorized participants by examination country (location of the overseas immigration medical screening exam and typically where a refugee lived during the 3 to 6 months before US arrival), because our data on nationality were incomplete and may not reflect where a refugee was exposed to lead. Our reference country was Malaysia, because it had the lowest EBLL prevalence among the five examination countries with the largest arrival volumes.
Data Analysis
We defined EBLL as a blood lead level of ≥5 µg/dL.6 The comparison group for all analyses was children with BLL values <5 µg/dL. We used the month of testing, grouped by quarter (e.g. January-March), to evaluate the potential association between time of year and BLL. We defined moderate to severe anemia as hemoglobin <10 g/dL, regardless of age or sex.19 We used height and weight measurements to calculate anthropometric z-scores using the World Health Organization’s Child Growth Standards SAS igrowup macro package (SAS version 9.4). Stunting was defined as <−2 standard deviations (SD) from median height-for-age z-score (HAZ) for reference population, and wasting as <−2 SD from median weight-for-height (WHZ) or body mass index (BMI) z-score for reference population.20
We assessed the relationship between EBLL on the initial screening test and demographic characteristics and other covariates in our data set by calculating prevalence ratios and 95% confidence intervals (CI) using generalized estimating equations to account for state-level clustering, and both with and without age stratification (comparing children <7 years with children 7–16 years).We included variables in the adjusted model if they were significantly associated with EBLL in the bivariate model at the 0.05 alpha level. In our age-stratified analysis, sex was the only variable with significantly different BLLs so we included an interaction term for age and sex in our model. We also analyzed the association between nutritional status (i.e. stunting, wasting or severe anemia) and EBLL using the same modeling approach on the subset of our population with available nutritional indicator data. We used the Cochran-Armitage test to assess for trends in EBLL prevalence over the time period covered by our data set. To evaluate changes in BLL after arrival, we calculated the prevalence of a ≥2 µg/dL increase in BLL using a generalized estimating equation, and used the Sign test to compare the change in median BLL from the initial and follow-up tests among children who had EBLL on both tests. We evaluated the relationship between EBLL on the follow-up test and multiple covariates using the same modeling approach described for the initial testing. All data analysis was performed using SAS 9.4 software (SAS Institute Inc.; Cary, NC). This analysis was determined to be non-research surveillance by a CDC Human Subjects Advisor; IRB review was not required.
Results
We received valid BLL results for 27,284 resettled refugee children 6 months to 16 years old at the time of initial domestic medical examination from 12 sites: 11 states (Colorado, Idaho, Illinois, Kentucky, Massachusetts, Minnesota, North Carolina, New York [excluding New York City], Texas, Utah, and Washington), and one county health department (Marion County Public Health Department of Indiana); these data represent nearly a quarter (24.0%) of all refugee arrivals <17 years old to the United States over the time period.20 Female children comprised 49% (n = 13,355) of our data set, and the mean age was 95.6 months (8 years; median: 92 months, interquartile range (IQR): 46–142 months). The top five overseas examination countries by arrivals were Thailand (n = 5,574, 20.7%), Nepal (n = 4,117, 15.2%), Malaysia (n = 3,431, 12.8%), Iraq (n = 2,360, 8.8%), and Kenya (n = 1,962, 7.3%). Demographics of children in our data set did not differ from those of the overall population of refugee child arrivals to the United States during the same period by age, sex, arrival year, or country of exam.20
Overall, 5,275 (19.3%) children had EBLL on initial testing; children <7 years old had the highest prevalence (n = 2,836, 22.8%). Children ≥7 years old had an EBLL prevalence of 16.5%. There were 579 (2.1%) children with BLL over 10 µg/dL, and 6 (0.02%) children with BLL ≥45µg/dL. The mean time elapsed between arrival in the United States and the initial BLL test was 29.8 days (SD = 20.2 days) and 72.1% of initial tests were venous (Table 1).
Table 1.
Characteristics of refugee children, aged 6 months through 16 years, in 12 US sites by initial blood lead level (BLL) resultsa—January 1, 2010–September 30, 2014
| Characteristic | Total | Initial BLL | |||
|---|---|---|---|---|---|
| <5 µg/dL | 5–9.9 µg/dL | ≥10 µg/dL | |||
| N (column %) | N (row %) | N (row %) | N (row %) | χ2 | |
| Total | 27284 (100) | 22009 (80.7) | 4693 (17.2) | 582 (2.1) | |
| Age at US arrival (years) | <0.001 | ||||
| Mean ±SD | 8.0 ±4.7 | 8.2 ±4.7 | 7.2 ±4.4 | 6.8 ±4.4 | |
| <2 | 3019 (11.1) | 2342 (77.6) | 580 (19.2) | 97 (3.2) | |
| 2–6 | 9446 (34.6) | 7287 (77.1) | 1935 (20.5) | 224 (2.4) | |
| 7–11 | 9608 (35.2) | 7897 (82.2) | 1515 (15.8) | 196 (2) | |
| 12–16 | 5211 (19.1) | 4483 3 (86) | 663 (12.7) | 65 (1.2) | |
| Sex | <0.001 | ||||
| Female | 13372 (49.0) | 11272 (84.3) | 1871 (14) | 229 (1.7) | |
| Male | 13907 (51.0) | 10732 (77.2) | 2822 (20.3) | 353 (2.5) | |
| Year of US arrival | <0.001 | ||||
| 2010 | 6005 (22.0) | 4533 (75.5) | 1311 (21.8) | 161 (2.7) | |
| 2011 | 4896 (17.9) | 3738 (76.3) | 1041 (21.3) | 117 (2.4) | |
| 2012 | 5692 (20.9) | 4625 (81.3) | 950 (16.7) | 117 (2.1) | |
| 2013 | 6094 (22.3) | 5179 (85.0) | 813 (13.3) | 102 (1.7) | |
| 2014 | 4597 (16.8) | 3934 (85.6) | 578 (12.6) | 85 (1.8) | |
| Overseas exam country | <0.001 | ||||
| Iraq | 2362 (8.7) | 1873 (79.3) | 428 (18.1) | 61 (2.6) | |
| Kenya | 1972 (7.2) | 1590 (80.6) | 314 (15.9) | 68 (3.4) | |
| Malaysia | 3434 (12.6) | 2915 (84.9) | 493 (14.4) | 26 (0.8) | |
| Nepal | 4100 (15) | 2972 (72.5) | 1036 (25.3) | 92 (2.2) | |
| Thailand | 5627 (20.6) | 4446 (79) | 1079 (19.2) | 102 (1.8) | |
| Otherb | 9789 (35.9) | 8213 (83.9) | 1343 (13.7) | 233 (2.4) | |
| Duration from US arrival to initial test (days) | <0.001 | ||||
| Mean ±SD | 29.8 (20.2) | 30.3 (20.4) | 27.6 (19.0) | 29.5 (19.7) | |
| <30 | 16107 (59) | 12802 (79.5) | 2964 (18.4) | 341 (2.1) | |
| ≥30 | 11177 (41) | 9207 (82.4) | 1729 (15.5) | 241 (2.2) | |
| Month of initial test | <0.001 | ||||
| January-March | 6141 (22.5) | 5086 (82.8) | 951 (15.5) | 104 (1.7) | |
| April-June | 6707 (24.6) | 5427 (80.9) | 1141 (17) | 139 (2.1) | |
| July-September | 8298 (30.4) | 6534 (78.7) | 1551 (18.7) | 213 (2.6) | |
| October-December | 6138 (22.5) | 4962 (80.8) | 1050 (17.1) | 126 (2.1) | |
| Test type | <0.001 | ||||
| Venous | 19668 (72.1) | 16010 (81.4) | 3235 (16.4) | 423 (2.2) | |
| Capillary | 3304 (12.1) | 2692 (81.5) | 530 (16) | 82 (2.5) | |
| Unknown | 4312 (15.8) | 3307 (76.7) | 928 (21.5) | 77 (1.8) | |
| Nutritional status totalc | 8951 (100) | 7254 (81) | 1541 (17.2) | 156 (1.7) | |
| Anemia (hemoglobin<10g/dL) | 0.02 | ||||
| Yes | 327 (3.7) | 245 (74.9) | 68 (20.8) | 14 (4.3) | |
| No | 8624 (96.3) | 7009 (81.3) | 1473 (17.1) | 142 (1.6) | |
| Wastingd | 0.05 | ||||
| Yes | 423 (4.7) | 329 (77.8) | 90 (21.3) | 4 (0.9) | |
| No | 8528 (95.3) | 6925 (81.2) | 1451 (17) | 152 (1.8) | |
| Stuntinge | <0.001 | ||||
| Yes | 1865 (20.8) | 1460 (78.3) | 361 (19.4) | 44 (2.4) | |
| No | 7086 (79.2) | 5794 (81.8) | 1180 (16.7) | 111 (1.6) | |
Notes. BLL=blood lead level; SD=standard deviation; BMI=body mass index.
Initial BLL testing was performed within 90 days after arrival.
Other higher volume (>300 arrivals over time period) overseas examination countries include Ethiopia, Jordan, Turkey, Syria, Uganda, Tanzania, Egypt, Cuba, South Africa, and Rwanda.
Total (N=8,951) represents the available data set for nutritional assessment.
Wasting is <−2 SD from median weight-for-height or BMI z-score for reference population based on World Health Organization’s AnthroPlus SAS Macro anthropometric calculator.
Stunting is <−2 SD from median height-for-age z-score for reference population based on World Health Organization’s AnthroPlus SAS Macro anthropometric calculator.
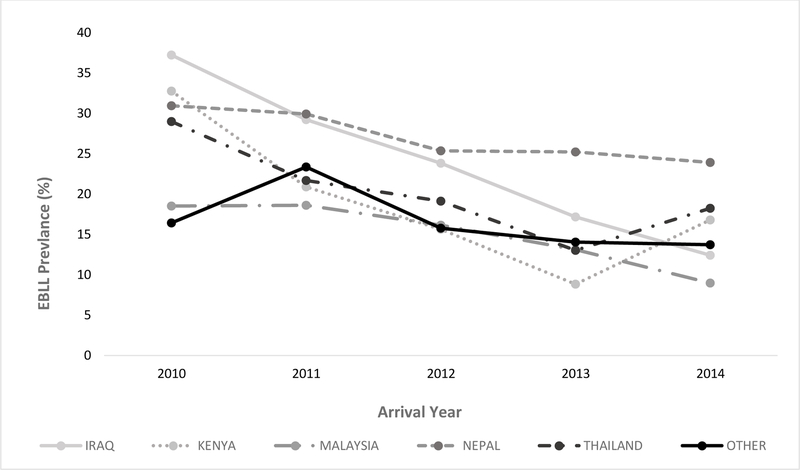
Of the five exam countries with the largest volume of arrivals, children from Nepal had the highest EBLL prevalence (n = 1,128, 27.5%), followed by Thailand (n = 1,181, 21.0%) and Iraq (n = 489, 20.7%); Kenya had the highest prevalence of BLL ≥10 µg/dL (3.4%) (Table 1). Independent of arrival volume, EBLL prevalence was highest among refugees from India (57.9%) and Afghanistan (55.1%) (Table 2); the exam country with the highest prevalence of BLL over 10 µg/dL was Afghanistan (16.7%). Males had a higher prevalence of EBLL (22.8%) than females (15.7%, p <0.001) (Table 1), but the geometric mean EBLL did not differ between sexes (6.5 µg/dL [95% CI = 6.4–6.6] for both). EBLL prevalence did not differ between sexes for children aged <2 years; in all other age groups, females had a significantly lower prevalence of EBLL than males, and the sex disparity increased with age. EBLL was significantly associated with time of year during initial testing (highest during July through September, 21.2%, p <0.001). EBLL prevalence declined by arrival year, dropping from 24.4% of arrivals in 2010 to 14.4% of arrivals in 2014 (p <0.001, Figure 2).
Table 2.
Prevalence of initial blood lead levels (BLL) among refugee children in 12 US sites by country of overseas exam—January 1, 2010–September 30, 2014
| Initial BLL |
|||||
|---|---|---|---|---|---|
| <5 µg/dL | 5–9.9 µg/dL | ≥5 µg/dL | ≥10 µg/dL | ||
| Country of Exam | n (%) | n (%) | n (%) | n (%) | Total |
| Thailand | 4446 (79) | 1079 (19.2) | 1181 (21) | 102 (1.8) | 5627 |
| Nepal | 2972 (72.5) | 1036 (25.3) | 1128 (27.5) | 92 (2.2) | 4100 |
| Malaysia | 2915 (84.9) | 493 (14.4) | 519 (15.1) | 26 (0.8) | 3434 |
| Iraq | 1873 (79.3) | 428 (18.1) | 489 (20.7) | 61 (2.6) | 2362 |
| Kenya | 1590 (80.6) | 314 (15.9) | 382 (19.4) | 68 (3.4) | 1972 |
| Ethiopia | 1155 (85.9) | 166 (12.3) | 190 (14.1) | 24 (1.8) | 1345 |
| Jordan | 1002 (93.5) | 62 (5.8) | 70 (6.5) | 8 (0.8) | 1072 |
| Turkey | 771 (93.1) | 51 (6.2) | 57 (6.9) | 6 (0.7) | 828 |
| Syria | 556 (77.3) | 153 (21.3) | 163 (22.7) | 10 (1.4) | 719 |
| Uganda | 469 (78.7) | 107 (18) | 127 (21.3) | 20 (3.4) | 596 |
| Tanzania | 494 (92.9) | 33 (6.2) | 38 (7.1) | 5 (0.9) | 532 |
| Egypt | 380 (84.6) | 62 (13.8) | 69 (15.4) | 7 (1.6) | 449 |
| Cuba | 320 (84.2) | 56 (14.7) | 60 (15.8) | 4 (1.1) | 380 |
| South Africa | 353 (93.9) | 22 (5.9) | 23 (6.1) | 1 (0.3) | 376 |
| Rwanda | 349 (96.7) | 12 (3.3) | 12 (3.3) | - | 361 |
| Burma | 177 (62.8) | 89 (31.6) | 105 (37.2) | 16 (5.7) | 282 |
| Djibouti | 144 (81.8) | 26 (14.8) | 32 (18.2) | 6 (3.4) | 176 |
| Zambia | 151 (86.8) | 19 (10.9) | 23 (13.2) | 4 (2.3) | 174 |
| Lebanon | 126 (89.4) | 14 (9.9) | 15 (10.6) | 1 (0.7) | 141 |
| Afghanistan | 62 (44.9) | 53 (38.4) | 76 (55.1) | 23 (16.7) | 138 |
| India | 56 (42.1) | 67 (50.4) | 77 (57.9) | 10 (7.5) | 133 |
| Austria | 109 (92.4) | 9 (7.6) | 9 (7.6) | - | 118 |
| Ukraine | 99 (99) | 1 (1) | 1 (1) | - | 100 |
| Othera | 1440 (77.1) | 341 (18.2) | 429 (22.9) | 88 (4.7) | 1869 |
| Total | 22009 (80.7) | 4693 (17.2) | 5275 (19.3) | 582 (2.1) | 27284 |
Other includes Zimbabwe, Russia, Yemen, Chad, Moldova, United Arab Emirates, Sudan, Cameroon, Burundi, Tunisia, Malawi, Somalia, Mozambique, Malta, Pakistan, Ghana, Vietnam, Gabon, Belarus, Botswana, Guinea, Senegal, Slovak Republic, Philippines, Democratic Republic of Congo, Kuwait, Central African Republic, Ecuador, Côte d’Ivoire, Sri Lanka, Kyrgyzstan, Nigeria, China, Azerbaijan, Republic of Congo, Indonesia, Mali, Romania, Georgia, Bahrain, The Gambia, Kazakhstan, Namibia, Oman, Iran, Libya, Republic Of Moldova, Bangladesh, Bhutan, Colombia, Saudi Arabia, Tajikistan, Canada, Liberia, Eritrea, Slovakia, Algeria, Benin, Cambodia, Costa Rica, Macau, Australia, France, Israel, Kiribati, Morocco, S.Georgia/S.Sandwich Island, Sierra Leone, Tonga, Unknown, Uruguay, Uzbekistan, Western Sahara
Figure 2.
EBLL prevalence by arrival year and country of exam for Iraq, Kenya, Malaysia, Nepal, Thailand, and all other countries combined, 2010–2014
The nutritional analysis subset included 8,951 children from 8 sites with complete hemoglobin results and height (or length) and weight information; 1,697 (19.0%) of these children had EBLL. In unadjusted analyses, EBLL was associated with moderate to severe anemia (PR = 1.4, 95% CI 1.2, 1.7; p = 0.001) and stunting (PR = 1.2, 95% CI 1.1, 1.3; p = 0.001), but not wasting (PR = 1.2, 95% CI 1.0, 1.4); p = 0.09). However, none of these variables remained associated with EBLL after adjustment for age, sex, and time of year.
In the adjusted model (Table 3), prevalence of EBLL among males and females was not significantly different in children under 2 years of age. In children age 2 years and older, males were more likely to have EBLL than females of the same age, and the disparity increased with age. No other covariates differed by age in age-stratified analysis. Children examined in Nepal, Iraq, Kenya, and Thailand were more likely to have EBLL on follow-up testing than children examined in Malaysia.
Table 3.
Adjusted prevalence ratios for elevated blood lead levels (EBLL) results on initial testing among refugee children 6 months to 16 years screened in 12 US sites—January 1, 2010–September 30, 2014
| Characteristic | aPRa (95% CI) |
|---|---|
| Age/sex at US arrival | |
| <2 years | |
| M | 1.1 (1.0, 1.2) |
| F | Ref |
| 2–6 years | |
| M | 1.2 (1.2, 1.3) |
| F | Ref |
| 7–11 years | |
| M | 1.5 (1.4, 1.7) |
| F | Ref |
| 12–16 years | |
| M | 3.0 (2.5, 3.7) |
| F | Ref |
| Year of arrival | |
| 2014 | Ref |
| 2013 | 1.0 (0.9, 1.2) |
| 2012 | 1.3 (1.1, 1.5) |
| 2011 | 1.6 (1.2, 2.1) |
| 2010 | 1.7 (1.5, 2.0) |
| Overseas exam country | |
| Malaysia | Ref |
| Nepal | 1.8 (1.6, 2.1) |
| Iraq | 1.5 (1.3, 1.6) |
| Kenya | 1.3 (1.1, 1.6) |
| Thailand | 1.4 (1.1, 1.7) |
| Other | 1.1 (1.0, 1.3) |
| Duration from US arrival to initial test (days) | |
| <30 | 1.2 (1.0, 1.3) |
| ≥30 | Ref |
| Month of initial test | |
| January-March | Ref |
| April-June | 1.1 (1.0, 1.3) |
| July-September | 1.2 (1.1, 1.3) |
| October-December | 1.1 (1.0, 1.1) |
Adjusted for variables in the table as well as site reporting data
Follow-up BLL Testing
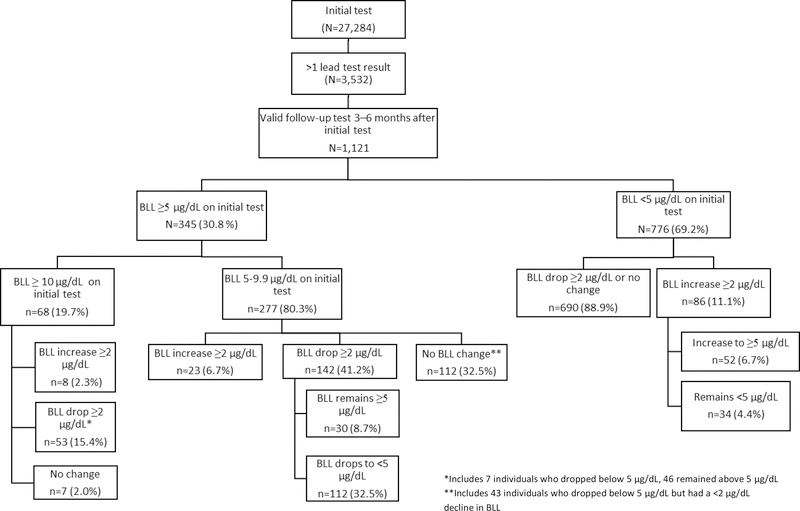
Five sites (CO, IL, IN, MN, NY) provided follow-up testing results on 3,532 (13.0%) children. Among children with multiple BLL results, 1,121 (31.7%) had valid follow-up tests (Figure 2), of which 76.3% were venous tests. The proportion of children with a valid follow up test was 5.0% among children younger than 7 years old (22.8% EBLL prevalence) and 3.4% among children ≥7 years old (16.5% EBLL prevalence).
Among 1,121 children with valid follow-up BLLs, 183 (16.3%) had EBLL on both initial and follow-up tests, and 71 (6.3%) did not have EBLL initially but had EBLL at follow-up (Figure 3). In total, 117 (10.4%) children experienced a ≥2 µg/dL increase in BLL on the follow-up test. Increases in BLL were most common among children <2 years (20.8% of retested children <2 years) but 5.2% of children 7 years and older experienced a ≥2 µg/dL increase in BLL. Overall, the median BLL declined significantly between initial and follow-up testing (8.0 µg/dL, 95% CI = 8.0, 8.7, and 7.0 µg/dL, 95% CI = 6.2, 7.1, respectively; Sign test p<0.0001) for children with EBLL on both tests.
Figure 3.
Comparison of initial and follow-up blood lead levels (BLL) 3–6 months after initial testing among N= 1,121 refugee children aged 6 months to 16 years in 5 US sites—January 1, 2010–September 30, 2014
A ≥2 µg/dL rise in BLL on the follow-up test was associated with younger age, particularly <2 years old; testing time of year (April through June); and exam country (adjusted model, Table 4). An EBLL result on the follow-up test was associated with younger age (<7 years), earlier arrival years (2010–2011), country of exam (Iraq and Kenya), and time of year (July through September) (adjusted model, data not shown).
Table 4.
Prevalence and adjusted prevalence ratio of a ≥2 µg/dL blood lead level (BLL) increase 3–6 months after initial testing among refugee children aged 6 months to 16 years arriving in 5 US sites—January 1, 2010–September 30, 2014 (N=1,121)
| Characteristic | % with a > 2 µg/dL increase in BLL | aPRa (95% CI) |
|---|---|---|
| Age | ||
| <2 years | 20.8 | 4.5 (2.6, 7.6) |
| 2–6 | 11.6 | 2.4 (2.0, 2.9) |
| 7–11 | 5.6 | 1.2 (1.1, 1.4) |
| 12+ | 4.3 | Ref |
| Male | 11.0 | 1.1 (0.8, 1.5) |
| Female | 9.9 | Ref |
| Year of arrival | ||
| 2014 | 8.0 | Ref |
| 2013 | 10.2 | 1.3 (1.1, 1.5) |
| 2012 | 5.8 | 0.7 (0.5, 0.8) |
| 2011 | 12.3 | 1.3 (0.9, 1.9) |
| 2010 | 14.7 | 1.5 (1.2, 1.9) |
| Country of exam | ||
| Iraq | 6.1 | 1.2 (0.5, 2.5) |
| Kenya | 13.9 | 2.3 (1.2, 4.5) |
| Malaysia | 9.5 | Ref |
| Nepal | 8.8 | 1.6 (0.7, 3.7) |
| Thailand | 6.3 | 2.6 (1.3, 5.3) |
| Other | 16.0 | 1.2 (0.6, 2.23) |
| Month of follow-up test | ||
| January-March | 13.8 | Ref |
| April-June | 10.2 | 1.6 (1.1, 2.3) |
| July-September | 11.6 | 1.4 (0.9, 2.0) |
| October-December | 5.9 | 1.4 (1.0, 2.0) |
Adjusted for variables reported in table as well as site reporting data
Discussion
This report expands our knowledge of the prevalence of EBLL among resettled refugee children, using data from a representative sample of children resettled to multiple states. The EBLL prevalence in this population of recently arrived refugee children (23.7% among 1–5-year-olds) was tenfold higher than the NHANES-estimated prevalence of 2.3% among all 1–5–year-old children in the United States from 1999 to 2010.21 Further, up to 10% of children had increases in BLL 3–6 months after the initial test, including 5% of children who were >6 years old.
Initial EBLL results likely indicate overseas lead exposures, because testing was conducted within 3 months of arrival. Among five overseas sites with the largest arrival volume, EBLL prevalence was highest among children with overseas medical examinations in Nepal (generally children of Bhutanese origin/nationality), Thailand (Burma origin), and Iraq (Iraq origin). Children examined in India, Afghanistan, Burma, and Nepal had the highest overall prevalence of EBLL, and children examined in Afghanistan had the highest prevalence of BLL ≥10 µg/dL, although the relatively small numbers of refugee arrivals from these countries limited our ability to draw definitive conclusions, and EBLL risk may vary by sub-national factors such as city or refugee camp of residence, which we were unable to analyze. Our findings may warrant further investigation of EBLL as a common health concern for children from these countries.
Younger age and male sex (regardless of age) were also associated with EBLL at arrival. While the oldest children (age 12–16 years) had a lower EBLL prevalence (14%) than children aged 2–4 (24%), they had a higher prevalence of EBLL than has been reported among the general US adolescent population,22,23 supporting CDC’s current recommendations for testing refugee children through age 16. As previously noted,9,12 initial EBLL prevalence was highest among children tested between July and September.
EBLL prevalence among refugee arrivals declined over the period of our analysis, which could have resulted from many factors, including improved conditions overseas, such as the introduction of electricity in some refugee camps,24 phasing out of leaded gasoline in multiple countries in the years leading up to this analysis;25 and changes in the countries of origin of resettlement populations. For example, refugees resettling from Thailand may have been exposed to lead poisoning prevention messaging as part of an overseas educational campaign on lead early in the analysis period, although our analysis was unable to evaluate the effect of this education. While EBLL declined overall, we noted upward trends in Thailand and Kenya in 2014; further investigation would be needed to identify contributors.
We identified an overall decline in median BLL among children who received a follow-up test, although a minority experienced post-resettlement BLL increases, which may indicate domestic lead exposures. We had no information on specific lead exposures, but other investigations of EBLL among refugee children have identified associations between post-resettlement increases in BLL and anemia, parasitic infections, pre-1950’s housing,9 and imported infant remedies and cosmetics,7 for example.
There were limitations to this analysis. First, reporting differences between sites lead to the exclusion of some data from analysis (e.g., qualitative results). Some sites did not provide height, weight, and hemoglobin data. Most sites were unable to provide laboratory limit of detection data, so we could not calculate mean BLLs for children with results <5 µg/dL. Most sites were unable to provide follow-up BLL data, usually because refugee health programs only have access to initial post-arrival screening data and follow-up testing is performed outside the refugee health program. Accordingly, children in our follow-up dataset had a higher initial prevalence of EBLL (30.8%) than children in our overall dataset (19.3%), which may explain why some children older than 6 years were retested. A majority (89.4%) of our follow-up test results were collected from two sites; therefore, related findings cannot be generalized to the broader data set. We were unable to ascertain whether follow-up tests had taken place after settlement in permanent housing; therefore we chose to use a narrow definition for follow-up testing, which excluded a number (2,411) of follow-up tests not meeting the re-testing time period we specified. Finally, we were not always able to determine which BLL testing method was used in each site. Based on a May 2017 FDA advisory, use of LeadCare® analyzers for venous BLL testing may produce falsely low BLL results.26 If participating sites used this method, it could have underestimated EBLL prevalence.
Efforts to reduce EBLL among foreign-born children—including refugee children—are an important part of the Healthy People 2020 strategy of achieving BLLs <5.2 µg/dL for ≥97.5 percent of the population aged 1–5 years.27 While EBLL prevalence in resettled refugee children declined over the period of this analysis, it remains far higher than the general U.S. prevalence. EBLL prevalence varied by exam country, and refugee children remain susceptible to both overseas and domestic exposures, as illustrated by post-arrival increases in BLL for some children. Refugee children with potential lead exposure can arrive in any state, so it remains important to improve linkages between federal, state and local lead and refugee health programs to facilitate collaboration, ensure appropriate screening and follow-up for refugee children, and share best practices. We encourage states to notify CDC of specific lead exposures or refugee populations with unusually high EBLL prevalence rates, which can help facilitate overseas and domestic interventions. Finally, we suggest that states develop and share translated resources for lead education, and encourage lead poisoning prevention education for refugee families early and often. As refugee children are a subset of all immigrant children, who may have similar exposures, such resources may also benefit other children migrating to the United States.
Conclusion
Refugee children remain at risk for EBLL, despite declines in prevalence over time. Our findings underscore the importance of screening all arriving refugee children aged 6 months to 16 years for EBLL, followed by rescreening of refugee children aged 6 months to 6 years 3-to-6 months post-resettlement. Although domestic BLL increases were more likely to occur in younger children, our data suggest that older children and adolescents also experience increases in BLL after arrival. Since our follow-up data were limited to five sites, further work is necessary to determine whether this pattern is consistent across sites and its implications for the recommended age limits for follow-up testing.
Supplementary Material
What’s Known on This Subject
Refugee children have a higher risk of elevated blood lead levels (EBLL) than children in the general US population. State-specific reports have linked EBLL to overseas exposures, resettlement in older housing, and culturally specific exposures (e.g., traditional remedies or cosmetics).
What This Study Adds
Analysis of a multistate, multiyear data set permitted assessment of EBLL among refugee children by country of examination to identify populations at higher risk of EBLL. Increases in BLL after arrival may indicate US-based lead exposures among refugee children.
Acknowledgments:
The authors thank Collin Elias (ID), Kenneth Mulanya (Marion County, IN), Shandy Dearth (Marion County, IN), P. Joseph Gibson (Marion County, IN), and Amelia Self (UT) for the use of their data and their assistance. The authors also thank Adrienne Ettinger (Centers for Disease Control and Prevention/Division of Environmental Health Science and Practice) for providing lead subject matter expertise and Yecai Liu, Christina Phares, and Emily Jentes (Centers for Disease Control and Prevention/Division of Global Migration and Quarantine) for their guidance and support during data analysis and manuscript preparation.
Funding Source: Nine sites (CO; IL; Marion County, IN; MA; Catholic Charities KY; MN; NY; TX; and Thomas Jefferson University) were supported by the CK12-1205 Strengthening Surveillance for Diseases among Newly Arrived Immigrants and Refugees federally-funded cooperative agreement from CDC.
Dr. Brown has received consultant fees from Meridian Bioscience Inc.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Abbreviations:
- BLL
blood lead level
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EBLL
elevated blood lead level
- HAZ
height-for-age z score
- IQR
interquartile range
- NHANES
National Health and Nutrition Examination Survey
- SD
standard deviation
- SE
standard error
- WHZ
weight-for-height z score
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: The remaining authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.United States Citizenship and Immigration Services. Table 13. Refugee Arrivals: Fiscal Years 1980 to 2016. Washington, DC: Department of Homeland Security;2018. [Google Scholar]
- 2.United States Citizenship and Immigration Services. Table 15. Refugee Arrivals By Relationship To Principal Applicant And Sex, Age, And Marital Status: Fiscal Year 2016. Washington, DC: Department of Homeland Security;2018. [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry. Toxicological Profile for Lead. Atlanta, GA: ATSDR;2007. [PubMed] [Google Scholar]
- 4.Jones RL, Homa DM, Meyer PA, et al. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics. 2009;123(3):e376–385. [DOI] [PubMed] [Google Scholar]
- 5.Raymond J, Brown MJ. Childhood Blood Lead Levels in Children Aged <5 Years - United States, 2009–2014. Morbidity and mortality weekly report Surveillance summaries. 2017;66(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Advisory Committee on Childhood Lead Poisoning Prevention. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention. Atlanta: CDC;2012. [Google Scholar]
- 7.Ritchey MD, Scalia Sucosky M, Jefferies T, et al. Lead poisoning among Burmese refugee children--Indiana, 2009. Clinical pediatrics. 2011;50(7):648–656. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Elevated blood lead levels in refugee children--New Hampshire, 2003–2004. MMWR Morbidity and mortality weekly report. 2005;54(2):42–46. [PubMed] [Google Scholar]
- 9.Eisenberg KW, van Wijngaarden E, Fisher SG, et al. Blood lead levels of refugee children resettled in Massachusetts, 2000 to 2007. American journal of public health. 2011;101(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geltman PL, Brown MJ, Cochran J. Lead poisoning among refugee children resettled in Massachusetts, 1995 to 1999. Pediatrics. 2001;108(1):158–162. [DOI] [PubMed] [Google Scholar]
- 11.Raymond JS, Kennedy C, Brown MJ. Blood lead level analysis among refugee children resettled in New Hampshire and Rhode Island. Public Health Nurs. 2013;30(1):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotinsky RN, Straetemans M, Wong LY, et al. Risk factors for elevated blood lead levels among African refugee children in New Hampshire, 2004. Environmental research. 2008;108(3):404–412. [DOI] [PubMed] [Google Scholar]
- 13.Hebbar S, Vanderslice R, Simon P, Vallejo ML. Blood levels in refugee children in Rhode Island. Med Health R I. 2010;93(8):254–255. [PubMed] [Google Scholar]
- 14.Yun K, Matheson J, Payton C, et al. Health Profiles of Newly Arrived Refugee Children in the United States, 2006–2012. American journal of public health. 2016;106(1):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Fatal pediatric lead poisoning--New Hamphshire, 2000. MMWR Morbidity and mortality weekly report. 2001;50(22):457–459. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Screening for Lead during the Domestic Medical Examination for Newly Arrived Refugees. 2012; https://www.cdc.gov/immigrantrefugeehealth/guidelines/lead-guidelines.html. Accessed 9/5/2017.
- 17.Caldwell KL, Cheng PY, Jarrett JM, et al. Measurement Challenges at Low Blood Lead Levels. Pediatrics. 2017;140(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D, Philen R, Wang Z, et al. Disease surveillance among newly arriving refugees and immigrants--Electronic Disease Notification System, United States, 2009. Morbidity and mortality weekly report Surveillance summaries. 2013;62(7):1–20. [PubMed] [Google Scholar]
- 19.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization;2011. [Google Scholar]
- 20.World Health Organization. Nutrition Landscape Information System (NLIS) Country Profile Indicators Interpretation Guide. World Health Organization;2010. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Blood lead levels in children aged 1–5 years - United States, 1999–2010. MMWR Morbidity and mortality weekly report. 2013;62(13):245–248. [PMC free article] [PubMed] [Google Scholar]
- 22.Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public health reports. 2000;115(6):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain RB. Trends and variability in blood lead concentrations among US children and adolescents. Environ Sci Pollut Res Int. 2016;23(8):7880–7889. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell T, Jentes E, Ortega L, et al. Lead poisoning in United States-bound refugee children: Thailand-Burma border, 2009. Pediatrics. 2012;129(2):e392–399. [DOI] [PubMed] [Google Scholar]
- 25.Meyer P, Brown M, Falk H. Global approach to reducing lead exposure and poisoning. Mutation research - Reviews in mutation research. 2008;659(1–2):166–175. [DOI] [PubMed] [Google Scholar]
- 26.FDA warns against using Magellan Diagnostis LeadCare testing systems with blood obtained from a vein [press release]. Silver Springs, MD: 2017. [Google Scholar]
- 27.Office of Disease Prevention and Health Promotion. Environmental Health: EH-8.1 Reduce blood levels in children. Healthy People 2020 2014; https://www.healthypeople.gov/2020/data-search/Search-the-Data#objid=4356. Accessed 2/01/2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.