LETTER
We read with much interest the article by Maseda et al. entitled “Nonsteroidal Anti-inflammatory Drugs Alter the Microbiota and Exacerbate Clostridium difficile Colitis while Dysregulating the Inflammatory Response” (1). Today, C. difficile infection (CDI) is one the most prevalent nosocomial infections and a major concern for public health globally (2). Several risk factors, including antibiotic therapy, prolonged hospitalization, a weakened immune system, diet, advanced age, and an imbalanced gut microbiota, have emerged as modulators of CDI severity and risk (3, 4). Recent epidemiological data have demonstrated an association between the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and CDI, but the mechanisms to explain this have not been elucidated (5).
As noted by the authors, treatment of mice with the NSAID indomethacin altered the proinflammatory profile and disrupted the intestinal barrier by perturbing epithelial cell junctions. They also showed that these effects were paralleled by specific alterations in the gut microbiota, which dramatically increased mortality and the intestinal pathology associated with CDI in mice. Interestingly, they observed that indomethacin pretreatment prevented downregulation of the Ptgs1 and Ptgs2 genes, encoding COX-1 and COX-2, respectively, and induced the expression of the Ptges gene and paradoxically increased the prostaglandin E2 (PGE2) concentrations upon CDI. The authors partly justified this paradox by stating that indomethacin reduced expression of the gene encoding the PGE2-inactivating enzyme called 15-hydroxyprostaglandin dehydrogenase (Hpgd, 15-PGDH) and suggested a “rebound” effect following temporary exposure to indomethacin prior to CDI. In contrast, several previous studies have strongly shown the stimulatory effects of NSAIDs, particularly indomethacin, on transcriptional and protein expression of 15-PGDH and its activity in different cell lines (6–9). In line with these findings, Munoz-Miralles et al. observed a significant reduction of PGE2 levels in cecal tissues of mice infected with C. difficile upon administration of indomethacin (10).
PGE2 is a metabolite of arachidonic acid and is synthesized by the cyclooxygenase (COX) enzymes. To date, three COX isoforms have been described: COX-1, COX-2, and COX-3. COX-1 is considered a housekeeping enzyme and is constitutively expressed in crypt epithelial cells, while COX-2 can be induced in a variety of cell types, including epithelial cells, macrophages, and fibroblasts. The COX-3 isoform is a splice variant of COX-1; however, there is much debate on the function of this enzyme. Notably, COX-2 can be rapidly induced upon exposure to various stimuli, such as proinflammatory cytokines, lipopolysaccharide (LPS), injury, and infectious agents (11). PGE2 mediates a variety of cellular processes and is able to exert pleiotropic effects in colorectal tumors, promoting proliferation, survival, angiogenesis, migration, and invasion mainly via the COX-2/PGE2 signaling pathway (12).
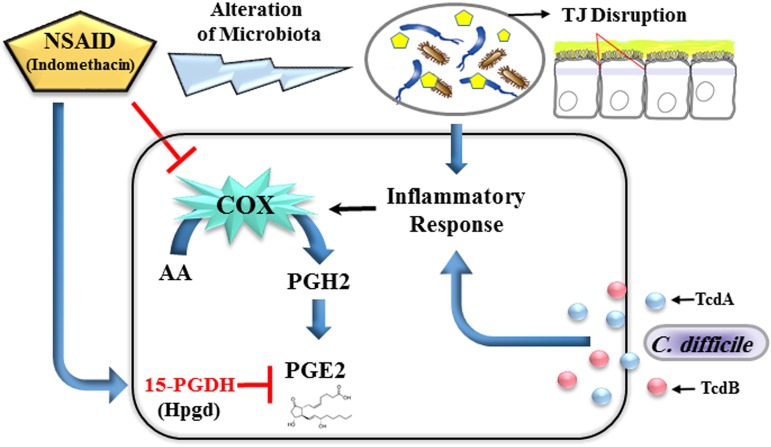
Previous studies using animal models and humans suggest that C. difficile and its toxins induce the production of PGE2 (13–16). Kim et al. have shown that TcdA toxin induces COX-2 expression and releases PGE2 in a dose- and time-dependent manner (14). Moreover, Meyer et al. reported that TcdB also can directly stimulate human mast cells to synthesize PGE2/PGD2 in a p38 mitogen-activated protein kinase (MAPK)-dependent pathway (16). Taken together, we respectfully suggest, in disagreement with the conclusions of the Maseda et al. (1), that the NSAID indomethacin is able to induce 15-PGDH expression rather than its suppression, which may lead to elevated PGE2 production. Furthermore, we hypothesize that indomethacin-induced gut microbiota dysbiosis in association with a CDI-related proinflammatory profile can trigger the induction of inducible COX-2, which in turn enhances the production of PGE-2 (Fig. 1).
FIG 1.
Possible scenario for impacts of the NSAID indomethacin on the up-modulation of 15-hydroxyprostaglandin dehydrogenase (Hpgd, 15-PGDH) expression and alteration of the gut microbiota, which disrupt the tight junctions (TJs) of intestinal epithelial cells to allow the translocation of bacteria and their metabolites into the bloodstream. In parallel, Clostridium difficile and its TcdA and TcdB toxins can induce the proinflammatory response that triggers the induction of inducible cyclooxygenase 2 (COX-2), leading to enhancement of prostaglandin E2 (PGE2) production.
Footnotes
Citation Noori M, Yadegar A, Zali MR. 2020. A complex scenario of nonsteroidal anti-inflammatory drugs induced prostaglandin E2 production and gut microbiota alteration in Clostridium difficile-infected mice. mBio 11:e02596-19. https://doi.org/10.1128/mBio.02596-19.
REFERENCES
- 1.Maseda D, Zackular JP, Trindade B, Kirk L, Roxas JL, Rogers LM, Washington MK, Du L, Koyama T, Viswanathan VK, Vedantam G, Schloss PD, Crofford LJ, Skaar EP, Aronoff DM, Maseda D, Zackular JP, Trindade B, Kirk L, Roxas JL, Rogers LM, Washington MK, Du L, Koyama T, Viswanathan VK, Vedantam G, Schloss PD, Crofford LJ, Skaar EP, Aronoff DM. 2019. Nonsteroidal anti-inflammatory drugs alter the microbiota and exacerbate Clostridium difficile colitis while dysregulating the inflammatory response. mBio 10:e02282-18. doi: 10.1128/mBio.02282-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin JSH, Monaghan TM, Wilcox MH. 2016. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol 13:206–216. doi: 10.1038/nrgastro.2016.25. [DOI] [PubMed] [Google Scholar]
- 3.Bauer MP, Notermans DW, van Benthem BHB, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ. 2011. Clostridium difficile infection in Europe: a hospital-based survey. Lancet 377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 4.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MRM, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. 2015. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Permpalung N, Upala S, Sanguankeo A, Sornprom S. 2016. Association between NSAIDs and Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Can J Gastroenterol Hepatol 2016:7431838–7431838. doi: 10.1155/2016/7431838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai HH, Chi X, Tong M. 2011. Regulation of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by non-steroidal anti-inflammatory drugs (NSAIDs). Prostaglandins Other Lipid Mediat 96:37–40. doi: 10.1016/j.prostaglandins.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Frenkian M, Segond N, Pidoux E, Cohen R, Jullienne A. 2001. Indomethacin, a cox inhibitor, enhances 15-PGDH and decreases human tumoral C cells proliferation. Prostaglandins Other Lipid Mediat 65:11–20. doi: 10.1016/s0090-6980(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 8.Frenkian M, Pidoux E, Baudoin C, Segond N, Jullienne A. 2001. Indomethacin increases 15-PGDH mRNA expression in HL60 cells differentiated by PMA. Prostaglandins Leukot Essent Fatty Acids 64:87–93. doi: 10.1054/plef.2001.0246. [DOI] [PubMed] [Google Scholar]
- 9.Chi X, Freeman BM, Tong M, Zhao Y, Tai H-H. 2009. 15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is up-regulated by flurbiprofen and other non-steroidal anti-inflammatory drugs in human colon cancer HT29 cells. Arch Biochem Biophys 487:139–145. doi: 10.1016/j.abb.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Miralles J, Trindade BC, Castro-Córdova P, Bergin IL, Kirk LA, Gil F, Aronoff DM, Paredes-Sabja D. 2018. Indomethacin increases severity of Clostridium difficile infection in mouse model. Future Microbiol 13:1271–1281. doi: 10.2217/fmb-2017-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caron MMJ, Emans PJ, Sanen K, Surtel DAM, Cremers A, Ophelders D, van Rhijn LW, Welting TJM. 2016. The role of prostaglandins and COX-enzymes in chondrogenic differentiation of ATDC5 progenitor cells. PLoS One 11:e0153162. doi: 10.1371/journal.pone.0153162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhough A, Smartt HJM, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. 2009. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Rhee SH, Pothoulakis C, Lamont JT. 2007. Inflammation and apoptosis in Clostridium difficile enteritis is mediated by PGE2 up-regulation of Fas ligand. Gastroenterology 133:875–886. doi: 10.1053/j.gastro.2007.06.063. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Rhee SH, Kokkotou E, Na X, Savidge T, Moyer MP, Pothoulakis C, LaMont JT. 2005. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J Biol Chem 280:21237–21245. doi: 10.1074/jbc.M413842200. [DOI] [PubMed] [Google Scholar]
- 15.Alcantara C, Stenson WF, Steiner TS, Guerrant RL. 2001. Role of inducible cyclooxygenase and prostaglandins in Clostridium difficile toxin A-induced secretion and inflammation in an animal model. J Infect Dis 184:648–652. doi: 10.1086/322799. [DOI] [PubMed] [Google Scholar]
- 16.Meyer GKA, Neetz A, Brandes G, Tsikas D, Butterfield JH, Just I, Gerhard R. 2007. Clostridium difficile toxins A and B directly stimulate human mast cells. Infect Immun 75:3868–3876. doi: 10.1128/IAI.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]