Abstract
The gut microbiota has emerged as an important regulator of host physiology, with recent data suggesting a role in modulating cardiovascular health. The present study determined if gut microbial signatures could transfer cardiovascular risk phenotypes between lean and obese mice using cecal microbiota transplantation (CMT). Pooled cecal contents collected from obese leptin-deficient (Ob) mice or C57Bl/6j control (Con) mice were transplanted by oral gavage into cohorts of recipient Ob and Con mice maintained on identical low-fat diets for 8 wk (n = 9–11/group). Cardiovascular pathology was assessed as the degree of arterial stiffness (aortic pulse wave velocity) and myocardial infarct size following a 45/120 min ex vivo global cardiac ischemia-reperfusion protocol. Gut microbiota was characterized by 16S rDNA sequencing, along with measures of intestinal barrier function and cecal short-chain fatty acid (SCFA) composition. Following CMT, the gut microbiota of recipient mice was altered to resemble that of the donors. Ob CMT to Con mice increased arterial stiffness, left ventricular (LV) mass, and myocardial infarct size, which were associated with greater gut permeability and reduced cecal SCFA concentrations. Conversely, Con CMT to Ob mice increased cecal SCFA, reduced LV mass, and attenuated myocardial infarct size, with no effects on gut permeability or arterial stiffness. Collectively, these data demonstrate that obesity-related changes in the gut microbiota, independent of dietary manipulation, regulate hallmark measures of cardiovascular pathology in mice and highlight the potential of microbiota-targeted therapeutics for reducing cardiovascular pathology and risk in obesity.
NEW & NOTEWORTHY These data are the first to demonstrate that cecal microbiota transplantation (CMT) can alter cardiovascular pathology in lean and obese mice independent from alterations in dietary intake. Myocardial infarct size was reduced in obese mice receiving lean CMT and worsened in lean mice receiving obese CMT. Lean mice receiving obese CMT also displayed increased aortic stiffness. These changes were accompanied by alterations in short-chain fatty acids and gut permeability.
Keywords: arterial stiffness, cecal transplant, ischemia-reperfusion, microbiota, obesity
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the United States and other developed nations, accounting for nearly one-third of all deaths worldwide (22). CVD encompasses a broad spectrum of disorders, the vast majority of which are characterized by dysfunction of the cardiac and peripheral arteries. Narrowing and dysfunction of the coronary arteries, for example, can result in ischemic heart disease that commonly manifests as myocardial infarction (MI) (37). Dysfunction of peripheral arteries, most notably stiffening of large elastic arteries such as the aorta, can result in increased systolic blood pressure, cardiac hypertrophy, and end-organ damage (10). Aortic stiffening is highly predictive of future cardiovascular events and mortality (58), and the size of MI correlates inversely with both short-term (40) and long-term survival (24) following an ischemic event.
More than one-third of the world’s population is considered overweight or obese, and this number is projected to increase to ~50% by 2030 (21, 43). Obese individuals are nearly two times more likely to die of CVD than nonobese individuals (28). This increased mortality risk is due, at least in part, to pathogenic changes in the heart and peripheral arteries that manifest as greater aortic stiffness (45, 50) and reduced myocardial tolerance to ischemia (1). Therefore, identifying the underlying causes of these changes is important for developing new potential therapies for managing CVD in obese individuals.
The gut microbiota has emerged as an important regulator of human physiology, and potentially deleterious alterations to the microbiota, commonly termed dysbiosis, are associated with numerous adverse physiological outcomes (25). Obesity is commonly characterized by microbial dysbiosis, and an increasing number of studies have implicated dysbiosis in the pathology of obesity-related disorders, including cardiovascular dysfunction (5, 6, 53, 57). Study of the gut microbiota has been hastened by the innovative use of three experimental models: broad spectrum antibiotic administration, germ-free mice, and microbiota transplantations (29). Despite its labor intensiveness and technical challenges, microbiota transplantation has the unique advantage of allowing examination of both the pathogenic and therapeutic potential of the microbiota by exchanging microbial samples between healthy and diseased subjects. Unlike antibiotic administration, microbiota transplantation also allows for microbiota analysis to examine specific microbial patterns that may underlie observed physiological changes (2).
In the present study, microbiota transplantation was employed to examine the link between obesity-related cardiovascular dysfunction and the gut microbiota by exchanging the microbiota between healthy mice and genetically obese (B6.Cg-Lepob/J) mice (Ob). Given that Ob mice develop obesity on a low-fat control diet, their use avoids the confounding effects of altering dietary composition (e.g., high-fat feeding) on the gut microbiota. Previous studies have shown that Ob mice display a microbiota that is distinct from lean mice (9), but it is unclear whether these differences contribute to greater cardiovascular pathology observed in these mice. We hypothesized that recipient mice would acquire the microbial signature and cardiovascular pathology phenotype of the donor mice, such that gut dysbiosis, aortic stiffness, and myocardial infarct size would be worsened in lean (Con) mice receiving microbiota from obese animals and alleviated in Ob mice receiving the Con mouse microbiota.
MATERIALS AND METHODS
Experimental design.
All animal procedures were reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee. Male leptin-deficient mice homozygous for the obese spontaneous mutation B6.Cg.Lepob (Ob) and C57BL/6J (Con) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and acclimated for 2 wk in a temperature- and humidity-controlled environment on a 12:12-h light-dark cycle. Mouse cages contained standard bedding (7090 Teklad Sani-Chips; Envigo Teklad Diets, Madison, WI). Mice were housed in pairs with ad libitum access to a purified maintenance diet (TD.08485; Envigo Teklad Diets) consisting of 13.0% fat, 67.9% carbohydrate, and 19.1% protein calories for the duration of the study. Following acclimatization, aortic stiffness and gut permeability were determined in donor Ob and Con mice (n = 6/group) using methods described below to ensure the expected phenotypes were present. Donor mice were then euthanized, and cecal contents were collected, diluted 1:20 in reduced PBS containing 30% glycerol to create homogenous donor samples, aliquoted, and stored at −80°C until needed for subsequent transplants. All remaining mice were used as transplant recipients, resulting in a 2 (genotype) × 2 (microbiota) experimental design with four groups: 1) control mice receiving control microbiota (Con + Con; n = 9); 2) control mice receiving obese microbiota (Con + Ob; n = 11); 3) obese mice receiving control microbiota (Ob + Con; n = 10); and 4) obese mice receiving obese microbiota (Ob + Ob; n = 10). Mice were ~10 wk of age at the start of the study.
Cecal microbiota transplantation.
To suppress the endogenous microbiota in recipient mice before CMT, a broad-spectrum antibiotic cocktail was administered via daily oral gavage (10 mL/kg) for 2 wk (consisting of vancomycin (5 mg/mL, no. 00315; Chem-Impex International, Wood Dale, IL), neomycin sulfate (10 mg/mL, no. 14287; Cayman Chemical, Ann Arbor MI), metronidazole (10 mg/mL, no. 443-48-1; Research Products International, Mount Prospect, IL), and ampicillin (10 mg/mL, no. 14417; Cayman Chemical). This protocol has been used previously to suppress the gut microbiota in mice and avoids the issue of taste aversion-related weight loss that occurs when antibiotics are administered via drinking water (48). Ampicillin, which has broad-spectrum activity without associated taste aversion, was also added to the drinking water (1 g/L). Importantly, this duration of antibiotic administration does not affect metabolic or cardiovascular parameters and serves only to suppress the commensal microbes to enhance colonization of donor microbiota during transplantation. Following microbial suppression, Con and Ob recipient mice were randomized to receive donor cecal material via serial oral gavage (100 µL once daily for 5 days, followed by biweekly booster inoculations), and body weight and food intake were recorded weekly during the transplantation period.
Determination of antibiotic effectiveness.
Total bacterial loads were quantified using qPCR with Universal bacteria primers (forward: 5′-AAACTCAAAKGAATTGACGG-3′; reverse: 5′-CTCACRRCACGAGCTGA-3′) (3). Cycling conditions using the Biorad CFX96 thermal cycler were optimized as follows: 95°C for 3 min and then 40 cycles of 95°C for 15 s, 61°C for 15 s, 72°C for 10 s, and 85°C for 5 s followed by florescence detection. Standard curves were prepared from Bifidobacterium lactis genomic DNA, and copy number was determined based on a product size of 136 bp with the following equation: copy number = (ng × number/mol)/(bp × ng/g × g/mol of bp).
Aortic stiffness.
Arterial stiffness was determined by aortic pulse wave velocity (aPWV) as previously described by our laboratory (4, 5). Briefly, mice were anaesthetized with 2% isoflurane and oxygen at 2 L/min, placed supine on a heating board with legs secured to ECG electrodes, and maintained at a target heart rate of ~450 beats/min by adjusting isoflurane concentration. Doppler probes (20 MHz; Mouse Doppler Data Acquisition System; Indus Instruments, Houston, TX) were placed on the transverse aortic arch and abdominal aorta, and the distance between the probes was determined simultaneously with precision calipers. Five consecutive 2-s recordings were obtained for each mouse and used to determine the time between the R wave of the ECG and the foot of the Doppler signal for each probe site (Δtime). aPWV (in cm/s) was calculated as aPWV = (distance between the two probes)/(Δtimeabdominal − Δtimetransverse).
Gut permeability.
Mice were water fasted for 12 h overnight before oral gavage of 600 mg/mL FITC-dextran (4,000 molecular weight) diluted in PBS. After 4 h, during which time food was also withheld, blood was collected via tail bleed for determination of plasma FITC-dextran concentration. Plasma samples were diluted 1:2 in PBS, and fluorescence was measured on a spectrophotometrer at 485/20 (excitation) and 528/20 (emission). Concentrations were calculated based on a standard curve of known FITC-dextran concentrations prepared in control plasma from untreated mice and reported in micrograms per milliliters.
Animal termination and tissue collection.
Animals were placed under deep anesthesia with intraperitoneal injection of sodium pentobarbital (50 mg/kg) solution before being euthanized by midline thoracotomy and rapid excision of the heart. Blood was collected with a syringe and immediately centrifuged at 2,000 rcf for 10 min at 4°C to obtain serum. The spleen, cecum, and adipose tissue depots (subcutaneous, epididymal, and mesenteric) were isolated and weighed. Serum and tissues were then frozen (−80°) for subsequent analyses.
Myocardial ischemia-reperfusion injury protocol.
Immediately following confirmation of deep anesthesia, hearts were excised, trimmed free of excess lung or adipose tissue, and immediately placed in ice-cold Krebs-Henseleit buffer containing the following (in mM): 117.4 NaCl, 4.7 KCl, 1.9 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 5 pyruvate, 11 glucose, 0.5 EDTA, and25 NaHCO3 at pH 7.4 with 95%O2-5%CO2. Whole hearts were weighed and cannulated via the ascending aorta for retrograde perfusion in Langendorff-mode at a constant pressure of 80 mmHg. All hearts underwent 20 min of stabilization before no-flow global ischemia for 45 min. Following ischemia, hearts were reperfused for 120 min, with coronary flow rate monitored at 1, 5, and 120 min after reperfusion by collection of coronary effluent.
Immediately following the 120-min reperfusion period, hearts were removed from the cannula, dried by blotting, and dissected to isolate the left ventricle (LV). The LV was then weighed before placing into a matrix and sliced into five, 1-mm transverse sections. Each slice was weighed before incubation in 0.1% triphenyltetrazolium chloride (TTC) solution for 10 min in a shaking incubator at 37°C. Slices were then fixed in 10% Formalin for 1 h before imaging with a camera attached to a dissecting scope (Ken-A-Vision). TTC stains living tissue brick-red, while infarcted tissue appears white/yellow. NIH ImageJ software was used to calculate the areas of living to dead tissue. Infarct size (percentage of total LV) was calculated by averaging the ratio of white/red on each side of the slice and then weighting that average by the percent that slice made up of the total LV: {[(%infarct side A) + (%infarct side B)]/2} × [(mass of slice/total LV mass) × 100], and then, each slice was added together to get total infarct of the LV.
Microbiota characterization.
Fecal material was collected from the colon at termination, and DNA was extracted using the PureLink Microbiome DNA Purification Kit (A29790; Invitrogen, Carlsbad, CA). Paired-end sequencing libraries were constructed by following the Earth Microbiome protocol (http://press.igsb.anl.gov/earthmicrobiome/protocols-and-standards/16s/), which includes amplification of the V4 regions of the 16S rRNA gene, purification of amplicons using AmPure beads, quantification, denaturation and library pooling, and sequencing on an Illumina MiSeq. Paired-end sequence reads were concatenated, and combined 16S sequences were filtered, trimmed, and processed with the DADA2 (8) implementation included in the open source bioinformatics tool myPhyloDB version 1.2.1 (https://omictools.com/myphylodb-tool) (38). Briefly, all primers were removed from each sequence using the open source Python program Cutadapt48, and amplicon sequence variants (ASVs) were inferred using the default pipeline in DADA2. Each sequence variant identified in DADA2 was classified to the closest reference sequence contained in the Green Genes reference database (Vers. 13_5_99) using the usearch_global option (minimum identity of 97%) contained in the open source program VSEARCH49 (49). After processing, data were normalized using Laplace smoothing followed by subsampling with replacement. Using 100 independent iterations, a minimum sample size of 23,850 reads was obtained. Further analyses were conducted in MyPhyloDB and MicrobiomeAnalyst (15). Unique reads and those that were not present in at least 20% of the samples were excluded. Core metrics included measures of alpha (Chao1 estimates at species level, Shannon diversity index at genus level) and beta diversity (Bray-Curtis distances at species level) as well as determination of differentially abundant ASVs between transplant groups using LEfSe (52). Data files are publicly available on Qiita (https://qiita.ucsd.edu/) (23) under project ID 12650.
Aortic collagen content.
Transverse (7 um) sections of the TA were obtained using a Microm HM550 cyostat (Thermo Scientific, Waltham, MA) and mounted on glass slides. Sections were stained for detection of collagen fibers using Picro-Sirius Red (ScyTek Laboratories, Logan, UT). Stained aorta sections were coverslipped with Permount (Fisher Scientific, Pittsburgh, PA) and imaged using polarized light microscopy (Olympus Intelligent Microscope Model BX63, Tokyo, Japan). Four independent researchers established the manual threshold levels, and collagen content was determined by percent cross-sectional area using CellSens imaging software (Olympus) as described previously (34).
Plasma lipopolysaccharide binding protein.
Because of well-established difficulties obtaining reliable circulating LPS levels, as well as limitations of commercially available chromogenic assays (7, 41), LPS signaling was determined by circulating levels of lipopolysaccharide binding protein (LBP), a commonly used surrogate. Plasma LBP (ALX-850-304/1; Enzo Life Sciences, Farmingdale, NY) was measured via ELISA per manufacturer’s instructions using 1:800 dilution of plasma.
Cecal short-chain fatty acids.
Short-chain fatty acids (SCFA) were extracted from frozen cecal contents and analyzed as previously described (20). Cecal contents were weighed and extracted using acidified water (pH 2.5) containing an internal standard of 5 mM ethylbutyric acid. Following homogenization and sonication, samples were centrifuged two times at 10,0000 rpm for 10 min to remove particulate matter. Collected supernatant was analyzed on a Gas Chromatograph with Flame Ionization Detection (GC-FID; Agilent 6890 Plus GC Series, Agilent 7683 Injector Series, GC Column: TG-WAXMS A 30 m × 0.25 mm × 0.25 μm). Peak areas were normalized to the internal standard and quantified using standard curves from dilutions of commercial stocks.
Statistical analyses.
All data are expressed as means ± SE. Student t tests were used to determine differences in gut permeability and aortic stiffness between control (Con) and obese (Ob) donor mice, as well as total bacteria count pre- and postantibiotic administration. Differences among the four treatment groups (Con + Con; Con + Ob; Ob + Ob; and Ob + Con) were determined using two-way ANOVA (genotype × transplant) with Tukey’s post hoc test to determine specific pairwise differences (SPSS for Windows, release 11.5.0; SPSS, Chicago, IL). P < 0.05 was considered statistically significant. Bivariate relations between variables of interest were determined using the Pearson correlation coefficient (GraphPad Prism, La Jolla, CA). Separation of groups based on Bray-Curtis distances was statistically determined by PERMANOVA with 1,000 iterations. Differences in taxa abundance were assessed by analysis of covariance and LEfSe, which utilize nonparametric Kruskal-Wallis tests to identify differences between groups, and LEfSe further adds a Wilcoxon rank sum and linear discriminant analysis to identify biologically important features that differ between groups.
RESULTS
Before transplantations, we first sought to confirm that the Con and Ob donor mice displayed differences in aortic stiffness and gut permeability. In vivo assessment revealed greater gut permeability in Ob mice compared with Con (Fig. 1A). As hypothesized, Ob mice also displayed higher aortic pulse wave velocity (aPWV) (Fig. 1B). We confirmed the gut microbiota was suppressed in antibiotic-treated animals. Using qPCR with universal 16S primers, we observed that microbial populations in antibiotic-treated animals were decreased by two orders of magnitude compared with their preantibiotic bacterial loads (Fig. 1C).
Fig. 1.
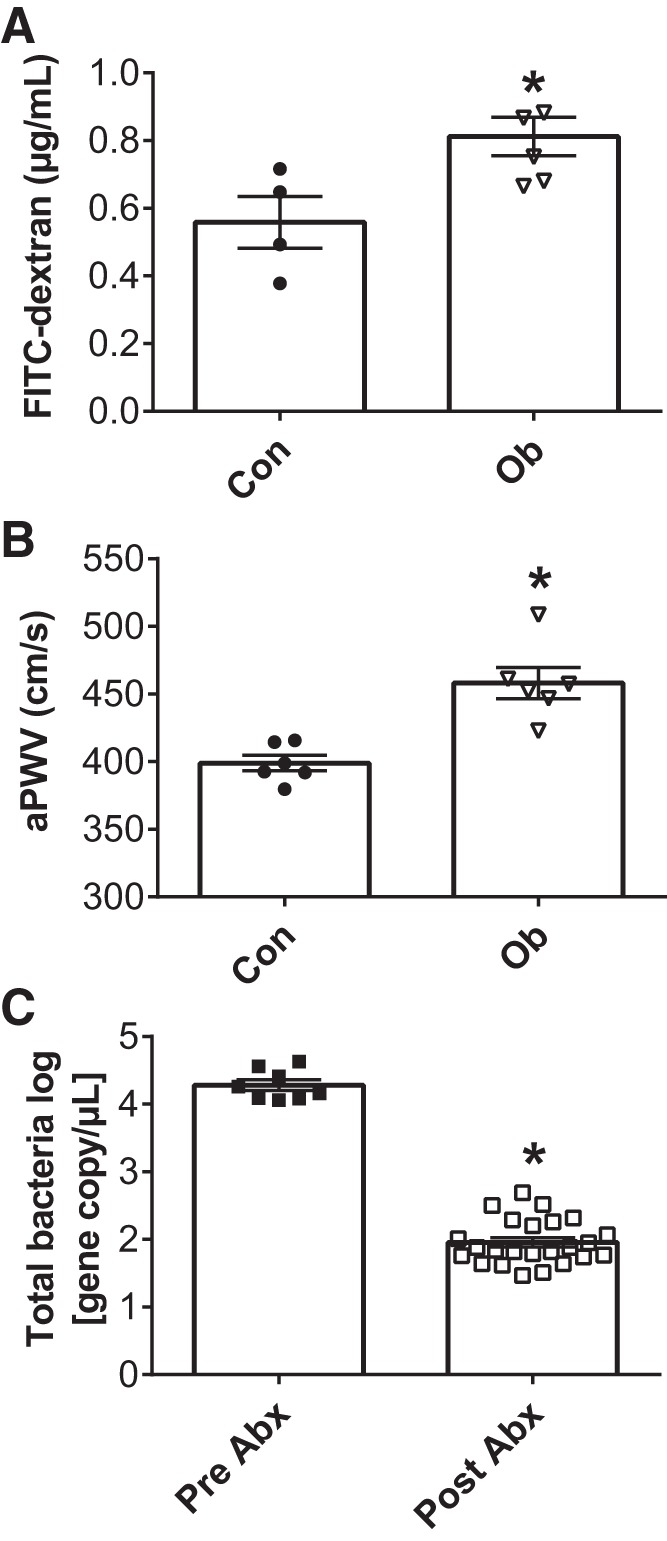
Gut permeability and aortic stiffness in donor mice and the effects of antibiotic administration on microbial suppression. Gut permeability was assessed as plasma FITC-dextran concentration 4 h after oral administration (A). Arterial stiffness was measured by in vivo aortic pulse wave velocity (aPWV; B). The effects of an antibiotic cocktail before transplantation on total gut bacteria (C). Con, C57 control mice; Ob, ob/ob mice; Abx, antibiotics; Data were analyzed via t tests and are expressed as means ± SE; n = 5–6/group; *P < 0.05 vs. Con or Pre-Abx.
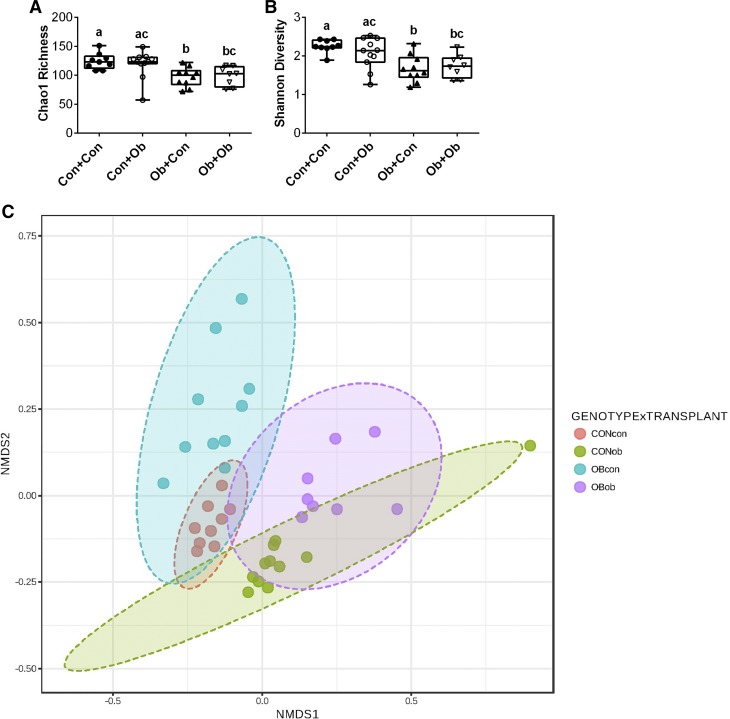
Following microbiota transplantations, we first examined whether recipient genotype and microbiota transplantations differentially affected indexes of alpha and beta diversity. Chao1 richness, a bootstrap-based estimate of the number of organisms present in a sample, was significantly lower in the two Ob groups compared with Con + Con, whereas the Con + Ob group was not significantly different from either Con + Con or Ob + Ob (Fig. 2A). The same pattern was observed for Shannon diversity (Fig. 2B). Principle coordinates analysis using nonmetric dimensional scaling of Bray-Curtis distances revealed that Ob + Ob and Con + Con mice displayed distinct microbiota signatures (genotype × transplant PERMANOVA with 1,000 iterations, P = 0.001), confirming previous data that the microbiota of ob/ob mice differed from lean mice (Fig. 2C). Interestingly, following transplantation, mice began to acquire microbiota characteristics of their donor groups, such that Ob + Con mice began to cluster towards the Con + Con mice, whereas Con + Ob mice began to cluster toward Ob + Ob mice (Fig. 2C); this indicates that our transplantation paradigm was successful in altering the microbiota, although the alterations were generally intermediate between genotype and microbiota transplantation groups.
Fig. 2.
Effects of microbiota transplantation on measures of microbial diversity. Alpha diversity was determined by Chao1 richness (A) and Shannon diversity (B). Nonmetric dimensional scaling (NMSD) biplot of mouse fecal microbial communities colored by recipient group. Distance measurements between samples (beta diversity) was determined at the species level by Bray-Curtis index (C). Con + Con, control mice receiving control microbiota; Con + Ob = control mice receiving obese microbiota; Ob + Con, obese mice receiving control microbiota; Ob + Ob, obese mice receiving obese microbiota. Data are expressed as means ± SE; n = 9–11/group. a,b,cP < 0.05, data with different superscript letters are significantly different.
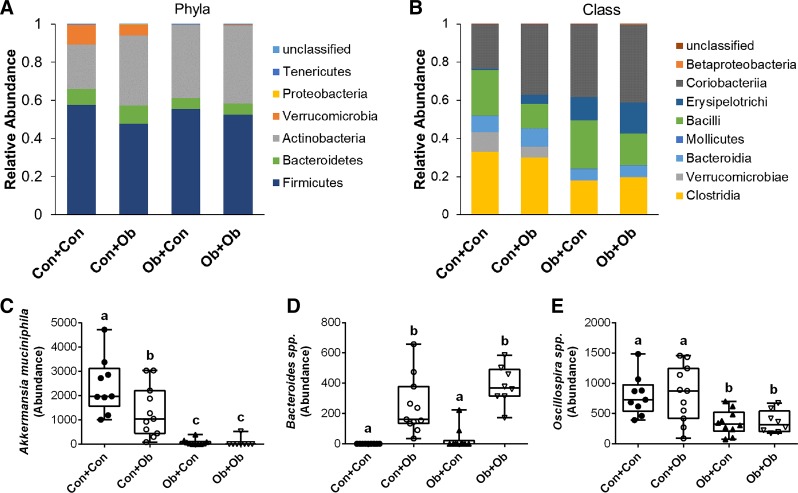
We next determined the effects of microbiota transplantation on fecal microbiota composition. At the phyla level, significant differences were observed in relative abundance of Verrucomicrobia, which was reduced in all groups compared with Con + Con (Fig. 3A). The relative abundance decreased from 10.2% in Con + Con to 5.7% in Con + Ob (P = 0.028) and was nearly absent in Ob + Con (0.3%, P < 0.001 vs. Con + Con) and Ob + Ob (0.3%, P < 0.001 vs. Con + Con). Although the abundance of Bacteriodetes was lower in Ob mice (main effect of genotype, P = 0.011), there was no significant effect of transplantation and no impact on Firmicutes:Bacteroidetes ratio. A similar effect of genotype was observed at the genus level; that is, Ob mice displayed reduced abundance of Clostridia and Oscillospira spp. (main effect of genotype, P = 0.001), with no significant effect of transplantation (Fig. 3B). At the species level, bacteria were differentially affected by both genotype and transplantation, as determined using LEfSe (Table 1). Of note, the abundance of Akkermansia muciniphila, the primary gut-associated taxa within the phyla Verrucomicrobia, was significantly reduced in Con + Ob mice compared with Con + Con (Fig. 3C) and was further reduced in each of the Ob groups compared with the two Con groups. Levels of Bacteroides sp. were also strongly affected by transplantation, with significantly higher levels present in Con + Ob and Ob + Ob mice compared with Con + Con and Ob + Con mice (Fig. 3D). Lastly, Oscillospira spp. was not significantly altered by transplant type and showed reduced expression in both Ob groups compared with Con mice (Fig. 3E).
Fig. 3.
Effects of microbiota transplantation on bacterial abundance at the phyla, class, and species level. Relative abundance of fecal bacteria at the phyla (A) and class (B) levels. Relative abundance of fecal Akkermansia muciniphila (C), Bacteroides spp. (D), and Oscillospira spp. (E). Con + Con, control mice receiving control microbiota; Con + Ob, control mice receiving obese microbiota; Ob + Con, obese mice receiving control microbiota; Ob + Ob, obese mice receiving obese microbiota. Data are expressed as means ± SE; n = 9–11/group. a,b,cP < 0.05, data with different superscript letters are significantly different.
Table 1.
Species-level linear discriminate analysis in recipient mice
| P Value | FDR | LDA Score | |
|---|---|---|---|
| Lactobacillus_unclassified | 0.015478 | 0.02902 | 3.16 |
| Akkermansia_muciniphila | 3.69E-06 | 3.69E-05 | 3.06 |
| Lactococcus_unclassified | 0.083016 | 0.1132 | 2.62 |
| Oscillospira_unclassified | 0.0025017 | 0.0093812 | 2.37 |
| Bacteroides_unclassified | 1.85E-06 | 3.69E-05 | 2.28 |
| Coprococcus_unclassified | 0.00075037 | 0.004032 | 2.09 |
FDR, false discovery rate; LDA, linear discriminant analysis.
Physical characteristics of mice at termination are shown in Table 2. In general, Ob mice were significantly heavier with larger organ masses, and transplantation had no effect on these parameters in either obese or control mice. We next examined the effect of transplantation on cardiovascular function. Arterial stiffness was significantly increased in Con + Ob mice compared with Con + Con (Fig. 4A); in contrast, transplantation was not sufficient to alter PWV in Ob + Con. There was a strong inverse relation between fecal A. muciniphila abundance and aPWV across all recipient mice (Fig. 4B). The increase in PWV in Con + Ob was accompanied by an increase in aortic collagen content, whereas no change in collagen was observed in Ob + Con (Fig. 4C).
Table 2.
Characteristics of recipient mice following microbiota transplant
| Con + Con | Con + Ob | Ob + Con | Ob + Ob | |
|---|---|---|---|---|
| Body weight, g | 29.9 ± 1.0a | 29.7 ± 0.6a | 56.4 ± 1.0b | 62.1 ± 1.8c |
| Change in body weight, g | 3.1 ± 0.4a | 2.0 ± 0.5a | 7.7 ± 0.7b | 8.8 ± 0.8b |
| Epididymal fat mass, mg | 835.5 ± 99.0a | 730.4 ± 77.4a | 1,928.2 ± 127.7b | 2,302.7 ± 211.1b |
| Subcutaneous fat mass, mg | 428.9 ± 60.1a | 464.6 ± 49.1a | 5,063.2 ± 281.2b | 6,033.2 ± 536.7b |
| Mesenteric fat mass, mg | 344.9 ± 43.2a | 348.3 ± 36.1a | 1,149.1 ± 57.9b | 1,038.0 ± 142.1b |
| PVAT mass, mg | 23.3 ± 2.7a | 29.4 ± 3.2a | 140.5 ± 12.1b | 164.3 ± 10.4b |
| Spleen mass, mg | 101.7 ± 8.6a | 92.4 ± 6.0a | 187.5 ± 32.8b | 212.2 ± 14.4b |
| Cecum mass, mg | 298.4 ± 13.9a | 328.3 ± 19.4a,b | 403.0 ± 36.1b | 377.2 ± 35.1a,b |
| Colon length, cm | 6.4 ± 0.3a | 6.5 ± 0.1a | 8.1 ± 0.3b | 8.4 ± 0.3b |
Values are means ± SE; n = 7–11/group. PVAT, perivascular adipose tissue; Con + Con, control mice receiving control microbiota; Con + Ob, control mice receiving obese microbiota; Ob + Con, obese mice receiving control microbiota; Ob + Ob, obese mice receiving obese microbiota.
P < 0.05, data with different superscript letters are significantly different.
Fig. 4.
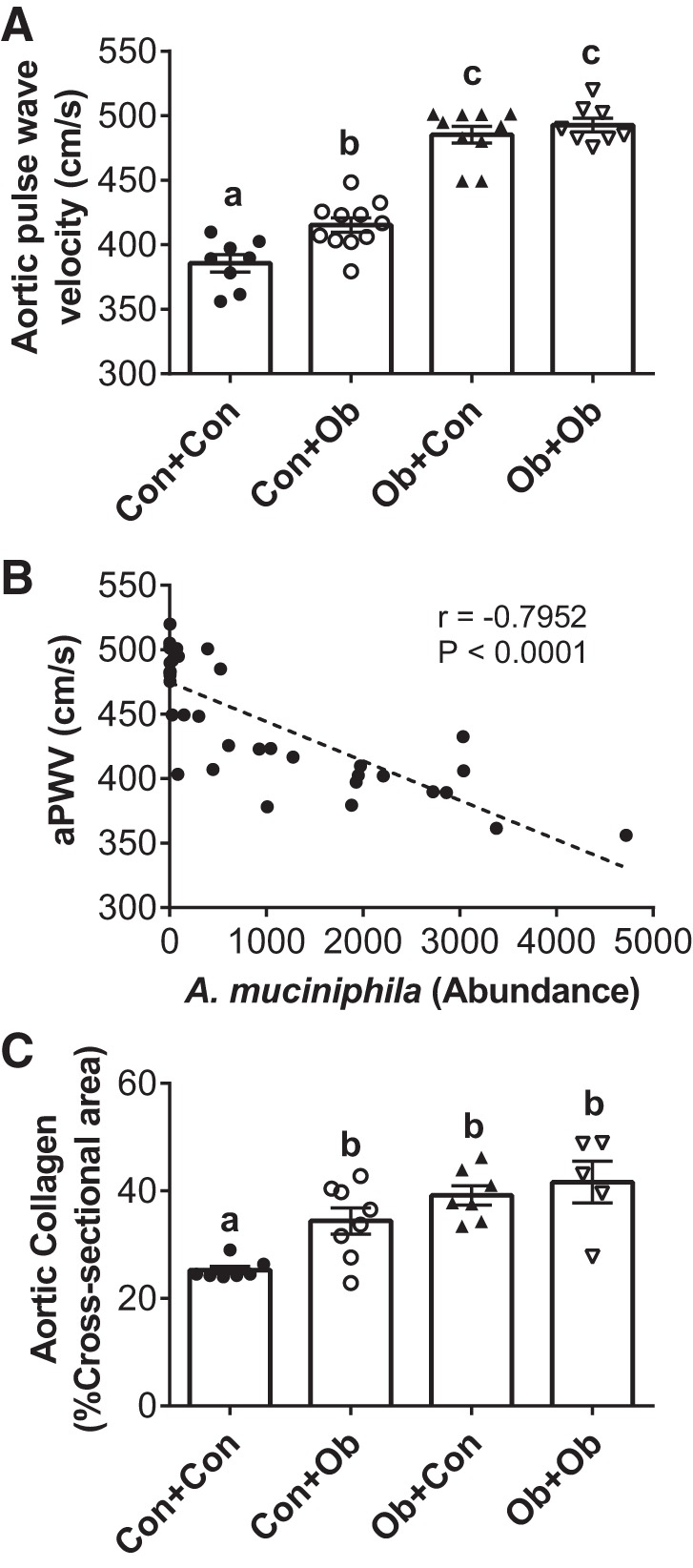
Effects of microbiota transplantation on aortic pulse wave velocity (aPWV). The effects of microbiota transplantation on arterial stiffness as determined by aPWV (A), association of aPWV with Akkermansia muciniphila (B), and aortic collagen content following transplantation (C). Con + Con, control mice receiving control microbiota; Con + Ob, control mice receiving obese microbiota; Ob + Con, obese mice receiving control microbiota; Ob + Ob, obese mice receiving obese microbiota. Data in A and B were analyzed by two-way ANOVA and are expressed as means ± SE; data in B were determined using the Pearson correlation coefficient (B); n = 9–11/group. a,b,cP < 0.05, data with different superscript letters are significantly different.
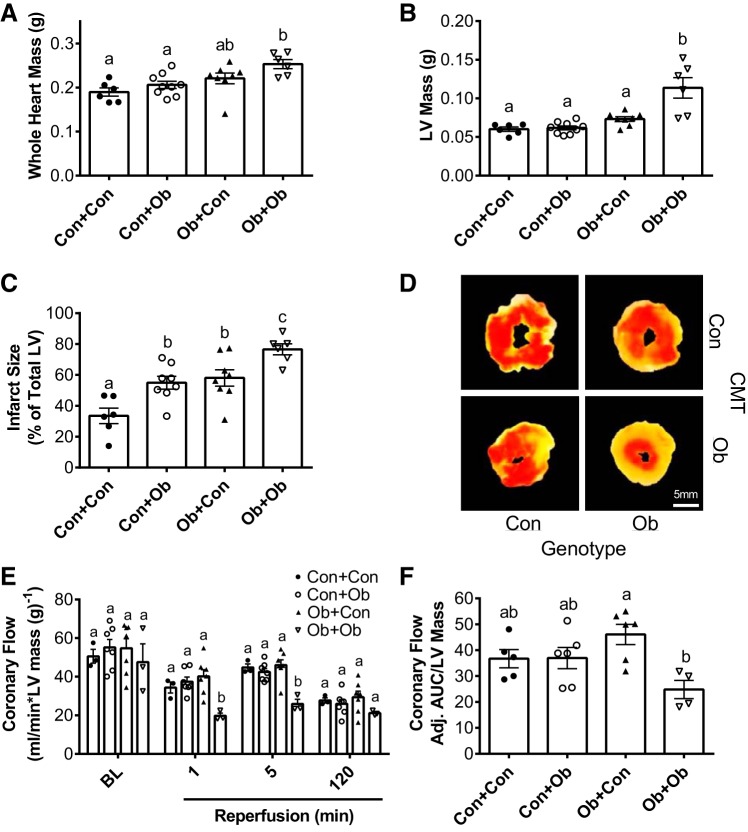
Whole heart mass was higher in Ob compared with Con groups (genotype main effect; P < 0.05), with no significant influence of transplantation within groups (Fig. 5A). LV mass was higher in Ob + Ob than all other groups (Fig. 5B), suggesting a suppression of obesity-related LV hypertrophy with colonization by Con microbiota (Ob + Con group; Fig. 5B). Myocardial infarct size was ~20% greater in Con + Ob vs. Con + Con (55 ± 4 vs. 34 ± 5%; P < 0.05; Fig. 5, C and D), indicating a significant reduction in myocardial ischemic tolerance in Con mice receiving Ob microbiota. Ob + Ob infarct size (77 ± 4%) was significantly higher than all other groups, including Ob + Con (58 ± 5%), indicating a protective effect of Con microbiota in Ob mice. Postischemic coronary flow rates were similar across all groups except Ob + Ob, which was significantly lower than all groups at every time point (Fig. 5, E and F).
Fig. 5.
Effect of microbiota transplantation on myocardial mass and ischemic tolerance. The effects of microbiota transplantation on whole heart mass (A), left ventricular (LV) mass (B), infarct size (C and D), and coronary flow (E and F). CMT, cecal microbiota transplantation; Adj. AUC, adjusted area under the curve; Con + Con, control mice receiving control microbiota; Con + Ob, control mice receiving obese microbiota; Ob + Con, obese mice receiving control microbiota; Ob + Ob, obese mice receiving obese microbiota. Data were analyzed by two-way ANOVA and are expressed as means ± SE; n = 9–11/group. a,b,cP < 0.05, data with different superscript letters are significantly different.
To gain mechanistic insight into what factors may have driven the observed alterations in vascular stiffness and infarct responses, we assessed plasma levels of LBP, a marker of LPS signaling that is associated with cardiovascular outcomes (33). LBP was significantly elevated in both Ob groups, irrespective of CMT (Fig. 6A), suggesting that changes in LPS signaling did not mediate alterations in cardiovascular function induced by transplantations. We next examined the effect of transplantation on microbially produced SCFAs and gut permeability. Mice receiving control microbiota displayed significantly higher levels of all three SCFAs: acetate (main effect of transplant, P = 0.008), propionate (main effect of transplant, P < 0.001), and butyrate (main effect of transplant, P < 0.001). Specifically, the concentration of propionate and butyrate were significantly reduced in Con + Ob compared with Con + Con, while levels of these SCFA were significantly higher in Ob + Con compared with Ob + Ob (Fig. 6B). Gut permeability followed a similar pattern as aPWV, being higher in Con + Ob compared with Con + Con mice but not altered by CMT in Ob mice (Fig. 6C).
Fig. 6.
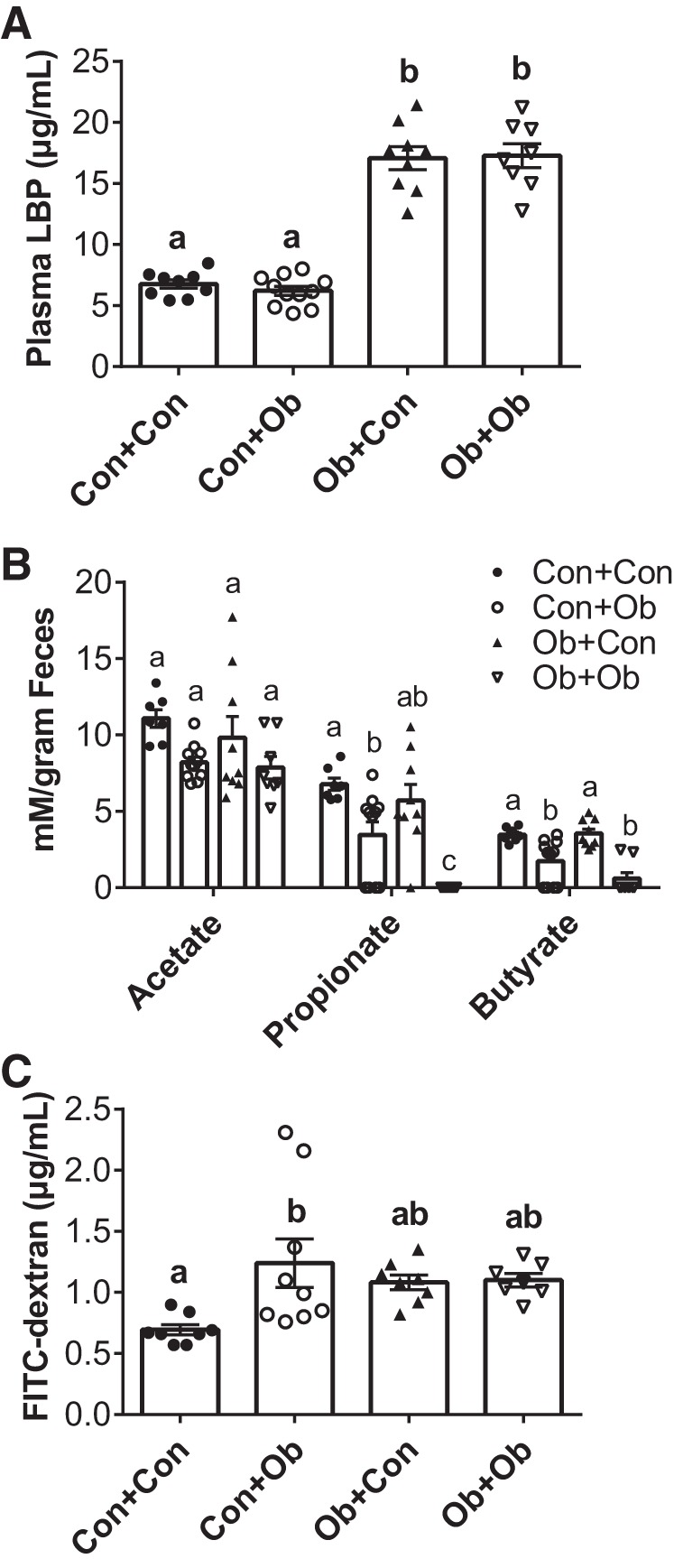
Effects of microbiota transplantation on LPS-binding protein (LBP), short-chain fatty acid (SCFA) production and gut permeability. The effects of microbiota transplantation on plasma LBP (A), cecal SCFA concentrations (B), and gut permeability as assessed by plasma FITC-dextran (C). Con + Con, control mice receiving control microbiota; Con + Ob, control mice receiving obese microbiota; Ob + Con, obese mice receiving control microbiota; Ob + Ob, obese mice receiving obese microbiota. Data were analyzed by 2-way ANOVA and are expressed as means ± SE; n = 9–11/group. a,b,cP < 0.05, data with different superscript letters are significantly different.
DISCUSSION
The present study tested the hypothesis that changes to the gut microbiota contribute to the greater degree of cardiovascular risk and pathology associated with obesity. The validity of our experimental model system was demonstrated by distinct microbiota profiles between genetically obese and lean mice fed identical diets and a shift in microbial composition following CMT in both lean and obese mice toward that of the donor groups. Results indicate that myocardial susceptibility to injury following ischemia is strongly influenced by gut microbiota composition, with Con CMT attenuating infarct size in Ob mice and Ob CMT exacerbating infarction in Con mice. Aortic stiffness was also significantly influenced by gut microbiota composition, as evidenced by higher PWV in Con mice following Ob CMT, although Con CMT did not significantly reduce PWV in Ob mice. These cardiovascular responses were accompanied by significant alterations in gut permeability and SCFA content, providing some mechanistic insight regarding how changes to the gut microbiota might regulate cardiovascular pathology.
To our knowledge, this is the first study to examine the effects of microbiota transplantation on cardiac susceptibility to ischemia-reperfusion injury. Baker and colleagues reported that broad-spectrum antibiotic administration reduced infarct size in Dahl salt-sensitive rat hearts (30, 31), providing the first evidence that the host microbiota may contribute to cardiac injury following ischemia. However, Tang et al. (55) found that antibiotic depletion of the gut microbiota significantly increased mortality after MI in vivo, implicating a protective role of gut microbiota-derived SCFAs in post-MI repair and remodeling mediated by CX3CR1+ monocyte infiltration. The present study in isolated perfused hearts demonstrates that changes in the gut microbiota influence cardiac ischemic tolerance by mechanisms intrinsic to the heart itself. Moreover, compositional patterns associated with obesity exacerbated injury in both lean and obese mice, implicating mechanisms that at least partially transcend the genetic background and metabolic status of the host. Improvements in ischemic tolerance of Ob hearts conferred by Con CMT might be partially related to better matching of oxygen supply and demand during reperfusion, reflected by greater postischemic flow and reduced LV mass. This suggests a potential link between CMT-mediated signals that regulate tissue remodeling and myocardial stress resistance. However, Ob CMT reduced ischemic tolerance in lean mice without altering LV mass or postischemic flow, indicating that distinct mechanisms intrinsic to cardiomyocytes might also be involved. Elucidating these mechanisms will require further investigation, but evidence from the present study suggests a possible influence of gut permeability and microbial SCFA production on vascular homeostasis, discussed further below.
Consistent with previous investigations in mice and humans, PWV was higher in Ob compared with Con mice in the present study, emphasizing the effect of obesity on this classic marker of arterial stiffness (11, 50). We show for the first time that transplanting an obese microbiota to lean mice increases aPWV, suggesting a link between gut microbiota composition and vascular stiffness in obesity. Previous data from our laboratory showed that suppression of the gut microbiota via antibiotics reduces PWV in a mouse model of diet-induced obesity (5). The current results extend these findings by demonstrating that obesity-induced changes in microbiota composition regulate large artery stiffness in the absence of nutrient variations (e.g., fat content) in the host diet. Interestingly, despite the bidirectionality of CMT effects on myocardial infarct size in lean and obese mice, Con CMT did not significantly reduce PWV in obese mice. This might reflect a degree of structural remodeling in the Ob mice vasculature that requires a longer-term intervention to reverse. Indeed, the increase in PWV in control mice was paralleled by an increase in aortic collagen content, whereas no change was observed in Ob + Con. Furthermore, Con CMT was able to preserve postischemic coronary flow early during reperfusion in obese mice despite no differences in preischemic flow rate, reflecting improvements in vascular reactivity rather than reversal of structural remodeling. Alternatively, these discrepant findings could indicate that shifts in specific gut bacterial populations influence myocardial ischemic tolerance differently from those that influence aortic stiffness. In this regard, we found that changes in A. muciniphila followed a similar pattern as that observed for aortic stiffness, whereby Con CMT did not change A. muciniphila levels in obese mice, but Ob CMT significantly reduced levels in lean mice. Furthermore, within the entire experimental cohort, A. muciniphila abundance was inversely related to PWV. The dramatically lower abundance of A. muciniphila in obese mice is in agreement with previous work (14, 16) and is consistent with the notion that A. muciniphila is protective against cardiometabolic dysfunction associated with obesity (13, 36). A. muciniphila utilizes intestinal mucins as a sole carbon source, and its protective effects may be due to its ability to stimulate mucus turnover, thereby maintaining the gut barrier and reducing gut permeability (17, 36). Indeed, in Con mice receiving Ob microbiota, reduced A. muciniphila populations were accompanied by an increase in gut permeability. The reason for a lack of increase in A. muciniphila in Ob mice following transplantation is unclear, although luminal leptin has been shown to stimulate intestinal mucin-secretion by goblet cells (47); thus the lack of leptin in Ob mice may have resulted in insufficient mucin to sustain A. muciniphila colonization. Alternatively, given its anaerobic nature, A. muciniphila may have been unable to survive the gavage preparation. In any case, the results collectively suggest a sequence of events whereby transplantation of the obese microbiota to lean mice caused a decrease in A. muciniphila, which subsequently led to an increase in gut permeability and aortic stiffness.
To begin to explore factors that could mediate the alterations in cardiovascular pathology downstream of the microbiota, we first assessed plasma levels of LBP, a marker of LPS signaling associated with cardiovascular risk (33). Circulating LBP was significantly increased in obese mice compared with lean but was not affected by transplantation in either lean or obese animals. Thus, although the increased LBP levels in obese mice are consistent with a chronic proinflammatory state, the lack of change following transplantation suggests that LPS signaling is not essential for changes associated with obesity-related cardiovascular pathology in this model. We next examined cecal levels of SCFAs, a major product of microbial fermentation of dietary carbohydrate (26). Butyrate and propionate were significantly lower in Ob + Ob than Con + Con, and transplantation caused a significant increase in Ob + Con and decrease in Con + Ob. These changes are consistent with accumulating evidence that SCFAs protect against intestinal and cardiovascular dysfunction (6, 42). For example, butyrate serves as the preferred fuel source for colonic epithelial cells and promotes epithelial barrier function (27). Natarajan et al. (42) reported that mice with dysregulated SCFA signaling display isolated systolic hypertension and elevated aortic pulse wave velocity. More recently, Tang et al. (55) found that SCFA supplementation improved survival of mice following MI. The beneficial effects of SCFA may be elicited via direct action on the vasculature or indirectly by affecting the secretion of other gut-derived signals. For example, SCFAs increase glucagon-like peptide 1 (GLP-1) secretion from the intestinal L cells (12, 59). GLP-1 stimulation, in turn, has been shown to improve aortic stiffness and ischemic injury in clinical and experimental models (32, 51, 56). Thus the changes in SCFA levels in the present study may have contributed to alterations the gut permeability, aortic stiffness, and infarct size by mechanisms that merit further investigation.
Ob/ob mice have been used extensively to examine the underlying causes of vascular dysfunction in the obese state (11, 46, 54, 60), as well as the role of dysbiosis in obesity-related metabolic disturbances (9, 18, 19, 35). Ob/ob mice were chosen in the current study to directly examine the contribution of the obese microbiota to cardiovascular dysfunction, independent of diet. However, given the monogenetic basis of obesity in these mice, it is possible that the gut microbiota plays a smaller role in the development of cardiometabolic disturbances in these animals than in generalized obesity. Therefore, it is reasonable to speculate that the cardiovascular effects of transplantation may be even more pronounced in a diet-induced model of obesity. It is important to note that only male mice were used in the current study. Previous work has indicated that sex is a determinant of microbiota composition and may also influence the response to microbiota-targeted interventions (39, 44); thus our results should not be extrapolated beyond male mice, and future studies should examine the possibility of sexual dimorphic responses to transplantation.
In conclusion, the present work corroborates and expands previous data linking the gut microbiota to alterations in cardiovascular function. Moreover, we provide new evidence that modifications to the gut microbiota composition in obesity, independent of changes in diet and body weight, are sufficient to alter two powerful indexes of cardiovascular pathology and risk in the peripheral vasculature and heart. These data lend further support for the development of microbiome-targeted therapies in the treatment and prevention of obesity-related cardiovascular disease.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-144611-01 and American Heart Association Grant 18TPAA34170585 (to C. L. Gentile and T. L. Weir).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.C., T.L.W., and C.L.G. conceived and designed research; M.L.B., D.M.L., L.C.L.P., K.E.E., K.N.T., H.P.F., A.J.C., and C.L.G. performed experiments; M.L.B., L.C.L.P., H.P.F., A.J.C., T.L.W., and C.L.G. analyzed data; D.M.L., L.C.L.P., H.P.F., A.J.C., T.L.W., and C.L.G. interpreted results of experiments; M.L.B., L.C.L.P., H.P.F., A.J.C., T.L.W., and C.L.G. prepared figures; M.L.B., T.L.W., and C.L.G. drafted manuscript; H.P.F., A.J.C., and T.L.W. edited and revised manuscript; T.L.W. and C.L.G. approved final version of manuscript.
REFERENCES
- 1.Apaijai N, Chattipakorn SC, Chattipakorn N. Roles of obese-insulin resistance and anti-diabetic drugs on the heart with ischemia-reperfusion injury. Cardiovasc Drugs Ther 28: 549–562, 2014. doi: 10.1007/s10557-014-6553-6. [DOI] [PubMed] [Google Scholar]
- 2.Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol 29: 79–84, 2013. doi: 10.1097/MOG.0b013e32835a4b3e. [DOI] [PubMed] [Google Scholar]
- 3.Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods 86: 351–356, 2011. doi: 10.1016/j.mimet.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Battson ML, Lee DM, Jarrell DK, Hou S, Ecton KE, Phan AB, Gentile CL. Tauroursodeoxycholic acid reduces arterial stiffness and improves endothelial dysfunction in type 2 diabetic mice. J Vasc Res 54: 280–287, 2017. doi: 10.1159/000479967. [DOI] [PubMed] [Google Scholar]
- 5.Battson ML, Lee DM, Jarrell DK, Hou S, Ecton KE, Weir TL, Gentile CL. Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am J Physiol Endocrinol Metab 314: E468–E477, 2018. doi: 10.1152/ajpendo.00187.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battson ML, Lee DM, Weir TL, Gentile CL. The gut microbiota as a novel regulator of cardiovascular function and disease. J Nutr Biochem 56: 1–15, 2018. doi: 10.1016/j.jnutbio.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 124: 11–20, 2016. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583, 2016. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 10.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 57: 1511–1522, 2011. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Chen JY, Tsai PJ, Tai HC, Tsai RL, Chang YT, Wang MC, Chiou YW, Yeh ML, Tang MJ, Lam CF, Shiesh SC, Li YH, Tsai WC, Chou CH, Lin LJ, Wu HL, Tsai YS. Increased aortic stiffness and attenuated lysyl oxidase activity in obesity. Arterioscler Thromb Vasc Biol 33: 839–846, 2013. doi: 10.1161/ATVBAHA.112.300036. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen CB, Gabe MB, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 315: G53–G65, 2018. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 13.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K, Clement K; MICRO-Obes Consortium . Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436, 2016. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 14.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 40: 54–62, 2017. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 15.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res 45, W1: W180–W188, 2017. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellekilde M, Krych L, Hansen CH, Hufeldt MR, Dahl K, Hansen LH, Sørensen SJ, Vogensen FK, Nielsen DS, Hansen AK. Characterization of the gut microbiota in leptin deficient obese mice—correlation to inflammatory and diabetic parameters. Res Vet Sci 96: 241–250, 2014. doi: 10.1016/j.rvsc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everard A, Cani PD. Diabetes, obesity and gut microbiota. Best Pract Res Clin Gastroenterol 27: 73–83, 2013. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, Delzenne NM, Schrenzel J, Cani PD. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60: 2775–2786, 2011. [Erratum in Diabetes 60: 3307, 2011.] doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Febvre HP, Rao S, Gindin M, Goodwin ND, Finer E, Vivanco JS, Lu S, Manter DK, Wallace TC, Weir TL. PHAGE study: effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients 11: 666, 2019. doi: 10.3390/nu11030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med 42: 563–570, 2012. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Fuster V. Global burden of cardiovascular disease: time to implement feasible strategies and to monitor results. J Am Coll Cardiol 64: 520–522, 2014. doi: 10.1016/j.jacc.2014.06.1151. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez A, Navas-Molina JA, Kosciolek T, McDonald D, Vázquez-Baeza Y, Ackermann G, DeReus J, Janssen S, Swafford AD, Orchanian SB, Sanders JG, Shorenstein J, Holste H, Petrus S, Robbins-Pianka A, Brislawn CJ, Wang M, Rideout JR, Bolyen E, Dillon M, Caporaso JG, Dorrestein PC, Knight R. Qiita: rapid, web-enabled microbiome meta-analysis. Nat Methods 15: 796–798, 2018. doi: 10.1038/s41592-018-0141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herlitz J, Bengtson A, Hjalmarson A, Karlson BW. Morbidity during five years after myocardial infarction and its relation to infarct size. Clin Cardiol 11: 672–677, 1988. doi: 10.1002/clc.4960111004. [DOI] [PubMed] [Google Scholar]
- 25.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214, 2012. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7: 2839–2849, 2015. doi: 10.3390/nu7042839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671, 2015. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krauss RM, Winston M, Fletcher BJ, Grundy SM. Obesity: impact on cardiovascular disease. Circulation 98: 1472–1476, 1998. doi: 10.1161/01.CIR.98.14.1472. [DOI] [PubMed] [Google Scholar]
- 29.Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, Knight R. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet 13: 47–58, 2012. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS One 11: e0160840, 2016. doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 26: 1727–1735, 2012. doi: 10.1096/fj.11-197921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambadiari V, Pavlidis G, Kousathana F, Varoudi M, Vlastos D, Maratou E, Georgiou D, Andreadou I, Parissis J, Triantafyllidi H, Lekakis J, Iliodromitis E, Dimitriadis G, Ikonomidis I. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol 17: 8, 2018. doi: 10.1186/s12933-017-0646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laugerette F, Alligier M, Bastard JP, Drai J, Chanséaume E, Lambert-Porcheron S, Laville M, Morio B, Vidal H, Michalski MC. Overfeeding increases postprandial endotoxemia in men: inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol Nutr Food Res 58: 1513–1518, 2014. doi: 10.1002/mnfr.201400044. [DOI] [PubMed] [Google Scholar]
- 34.Lee DM, Battson ML, Jarrell DK, Cox-York K, Foster MT, Weir TL, Gentile CL. Fuzhuan tea reverses arterial stiffening after modest weight gain in mice. Nutrition 33: 266–270, 2017. doi: 10.1016/j.nut.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070–11075, 2005. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation 133: 2434–2446, 2016. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 37.Manfroi WC, Peukert C, Berti CB, Noer C, Gutierres DA, Silva FT. Acute myocardial infarction: the first manifestation of ischemic heart disease and relation to risk factors. Arq Bras Cardiol 78: 392–395, 2002. doi: 10.1590/S0066-782X2002000400006. [DOI] [PubMed] [Google Scholar]
- 38.Manter DK, Korsa M, Tebbe C, Delgado JA. myPhyloDB: a local web server for the storage and analysis of metagenomic data. Database (Oxford) 2016: baw037, 2016. doi: 10.1093/database/baw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339: 1084–1088, 2013. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 40.Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation 92: 334–341, 1995. doi: 10.1161/01.CIR.92.3.334. [DOI] [PubMed] [Google Scholar]
- 41.Munford RS. Endotoxemia-menace, marker, or mistake? J Leukoc Biol 100: 687–698, 2016. doi: 10.1189/jlb.3RU0316-151R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48: 826–834, 2016. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 766–781, 2014. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, Lusis AJ. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 7: 313–322, 2016. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orr JS, Gentile CL, Davy BM, Davy KP. Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension 51: 1519–1524, 2008. doi: 10.1161/HYPERTENSIONAHA.108.112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paneni F, Costantino S, Cosentino F. p66(Shc)-induced redox changes drive endothelial insulin resistance. Atherosclerosis 236: 426–429, 2014. doi: 10.1016/j.atherosclerosis.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Plaisancie P, Ducroc R, El Homsi M, Tsocas A, Guilmeau S, Zoghbi S, Thibaudeau O, Bado A. Luminal leptin activates mucin-secreting goblet cells in the large bowel. Am J Physiol Gastrointest Liver Physiol 290: G805–G812, 2006. doi: 10.1152/ajpgi.00433.2005. [DOI] [PubMed] [Google Scholar]
- 48.Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One 6: e17996, 2011. doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ 4: e2584, 2016. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Safar ME, Czernichow S, Blacher J. Obesity, arterial stiffness, and cardiovascular risk. J Am Soc Nephrol 17, Suppl 2: S109–S111, 2006. doi: 10.1681/ASN.2005121321. [DOI] [PubMed] [Google Scholar]
- 51.Scalzo RL, Moreau KL, Ozemek C, Herlache L, McMillin S, Gilligan S, Huebschmann AG, Bauer TA, Dorosz J, Reusch JE, Regensteiner JG. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complications 31: 449–455, 2017. doi: 10.1016/j.jdiacomp.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60, 2011. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serino M, Blasco-Baque V, Nicolas S, Burcelin R. Far from the eyes, close to the heart: dysbiosis of gut microbiota and cardiovascular consequences. Curr Cardiol Rep 16: 540, 2014. doi: 10.1007/s11886-014-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taguchi K, Kobayashi T, Matsumoto T, Kamata K. Dysfunction of endothelium-dependent relaxation to insulin via PKC-mediated GRK2/Akt activation in aortas of ob/ob mice. Am J Physiol Heart Circ Physiol 301: H571–H583, 2011. doi: 10.1152/ajpheart.01189.2010. [DOI] [PubMed] [Google Scholar]
- 55.Tang TW, Chen HC, Chen CY, Yen CY, Lin CJ, Prajnamitra RP, Chen LL, Ruan SC, Lin JH, Lin PJ, Lu HH, Kuo CW, Chang CM, Hall AD, Vivas EI, Shui JW, Chen P, Hacker TA, Rey FE, Kamp TJ, Hsieh PC. Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 139: 647–659, 2019. doi: 10.1161/CIRCULATIONAHA.118.035235. [DOI] [PubMed] [Google Scholar]
- 56.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 53: 501–510, 2009. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Toral M, Robles-Vera I, de la Visitación N, Romero M, Sánchez M, Gómez-Guzmán M, Rodriguez-Nogales A, Yang T, Jiménez R, Algieri F, Gálvez J, Raizada MK, Duarte J. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiol (Oxf) 227: e13285, 2019. doi: 10.1111/apha.13285. [DOI] [PubMed] [Google Scholar]
- 58.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 59.Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 288: 25088–25097, 2013. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang R, Sikka G, Larson J, Watts VL, Niu X, Ellis CL, Miller KL, Camara A, Reinke C, Savransky V, Polotsky VY, O’Donnell CP, Berkowitz DE, Barouch LA. Restoring leptin signaling reduces hyperlipidemia and improves vascular stiffness induced by chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 300: H1467–H1476, 2011. doi: 10.1152/ajpheart.00604.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]