Abstract
The accurate assessment of antimicrobial use (AMU) requires relating quantities of active ingredients (AAIs) with population denominators. These data can be used to prioritize potential sources of selective pressure for antimicrobial resistance and to establish reduction targets. Here, we estimated AMU in Vietnam (human population 93.4 M in 2015), and compared it with European Union (EU) data (population 511.5 M in 2014). We extrapolated AMU data on each key animal species and humans from different published sources to calculate overall AMU (in tonnes) in Vietnam. We then compared these data with published statistics on AMU in the European Union (EU). A total of 3838 t of antimicrobials were used in Vietnam, of which 2751 (71.7%) corresponded to animal use, and the remainder (1086 t; 28.3%) to human AMU. This equates to 261.7 mg and 247.3 mg per kg of human and animal biomass, compared with 122.0 mg and 151.5 mg in the EU. The greatest quantities of antimicrobials (in decreasing order) were used in pigs (41.7% of total use), humans (28.3%), aquaculture (21.9%) and chickens (4.8%). Combined AMU in other species accounted for < 1.5%. These results are approximate and highlight the need to conduct targeted surveys to improve country-level estimates of AMU.
Keywords: Antimicrobial use, Surveillance, Human medicine, Veterinary medicine, Vietnam, European Union
Main text
Antimicrobial resistance (AMR) in bacterial pathogens is now firmly recognized as major global health problem [1]. AMR arises as a direct consequence of antimicrobial usage (AMU) in humans and animals and resistant organisms and AMR-encoding genes are capable of crossing species barriers [2]. Therefore, the emergence and transfer of AMR means that control solutions need to be conducted from a ‘One Health’ perspective [3]. However, if we are to reduce AMR we need accurate estimates of where the majority of AMU occurs. Sustained surveillance and monitoring of AMU are widely acknowledged as critical components of the fight against AMR and one of the strategic priorities of the AMR Global Action Plan (GAP) [4].
There is considerable uncertainty regarding AMU in different animal species and humans in most countries. This knowledge gap is due to the absence of reliable AMU data in humans and animals and ill-defined animal population denominators. Many higher income countries, such as those within the European Union (EU), regularly publish their data on AMU in humans and animals, and relate these values to denominator populations in terms of biomass [5]. Conversely, the majority of low- and middle-income countries (LMICs) do not regularly collect and report equivalent AMU statistics.
Recently, the World Organization for Animal Health (OIE) estimated that worldwide, on average 168.7 mg of antimicrobial active ingredients (AAIs) were used to raise 1 kg of animal biomass [6]. Although the report does not include between-country- or species-specific data, it shows however considerable differences between different OIE regions. However, this report did not indicate which animal production sectors are responsible for the largest degree of AMU. Such data are essential for estimating where AMR is most likely to be generated and maintained and pivotal for policy makers to set reduction targets. Here, by integrating various data sources, we aimed to estimate AMU in humans and different animal populations in Vietnam. These data were compared against available human and animal AMU statistics from the EU.
Human biomass in Vietnam was calculated using 2015 population data stratified by age [7]. Adult (> 18 years-old) body weight was taken from published Figs. (58.4 kg males; 50.8 kg females) [8]. For non-adult age-gender strata, we assigned bodyweights to US populations [9], after adjusting for the difference in body mass between populations in the two countries. This was achieved by applying the correction factors of 0.642 and 0.651, which represent, respectively, the ratios of weights of adult males and adult females in the two countries. The total biomass of terrestrial animals in Vietnam was calculated from official statistics [10] following the approach used by the OIE [6] that combined data on the number of slaughtered animals and standing populations. For aquaculture (farmed fish and shellfish), production data broken down by type of market (domestic, export) (2016) were used [11].
Data on human AMU in Vietnam were extracted from a multi-country survey in hospitals and the community [12]. The reported number of Defined Daily Doses (DDD) (per 1000) were converted to weight of antimicrobial active ingredient (AAI) using the four most common administered antimicrobials (ceftriaxone, ampicillin, azithromycin and levofloxacin). The daily consumption data was extrapolated for a whole year (365 days).
For pigs, chickens, and aquaculture (all aquatic species combined) data on AMU were obtained from quantitative published surveys [13–16]. Data on on AMU through consumption of commercial feed (i.e. antimicrobial growth promoters) were extrapolated from a survey of 1462 pig and chicken commercial feeds in Vietnam [17]. Antimicrobial consumption in aquaculture was extrapolated from a previous study [18], assuming that, on average, antimicrobial products have a 20% strength (weight of AAI related to total weight of product) based on the same study. For ruminants (bovines, buffaloes, sheep, goats) data on AMU in Japan (a high-income country in Asia) for 2010 were used [19]. For non-chicken poultry species (ducks, Muscovy ducks, geese and quails) the authors could not find any published data. AMU was, therefore, conservatively estimated as 50% of that reported in chickens, based on the authors’ field experience. We excluded companion animals and equines since no AMU data are available. Best and worst-case AMU scenarios (i.e. lowest and highest AMU) were calculated for all species: for poultry species, upper and lower limits were calculated based on ±25% of the final AMU estimate. For ruminants, the lower limit of AMU was taken from Japanese cattle AMU statistics [19]. The upper limit was set at 50% higher than this estimate; for our summary estimations we used the intermediate value between these two limits. We compared the resulting AMU data with those published in the second ECDC/EFSA/EMA Joint Report on AMU (data for 2014), corresponding with AMU data in relation to the total biomass of terrestrial animal species in 28 EU countries [5] as well as with the Third World Organization for Animal Health (OIE) Report [6] (data for 2015).
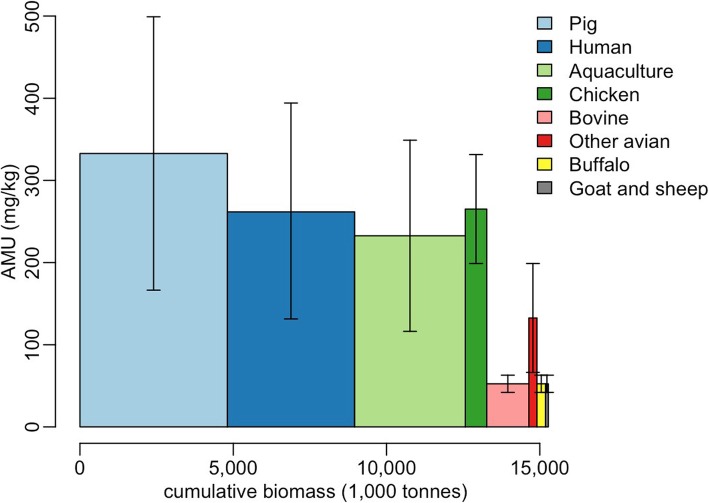
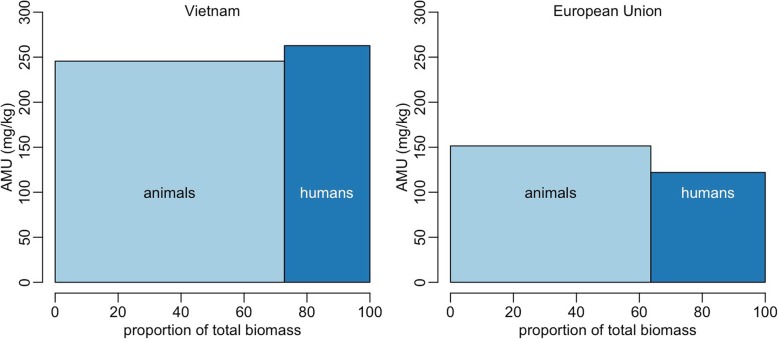
Our estimates of human and animal biomass in Vietnam from the above calculations are 4153 and 11,125 thousand tonnes, respectively (Table 2 in Appendix 1 and Table 1). Estimates of AMU showed that in 2015, a total of 3842 t of antimicrobials were used in Vietnam, of which 2751 (71.7%) was associated with animal use, and the remainder (1086 t; 28.3%) corresponded to human AMU. The greatest quantities of antimicrobials (in decreasing order) were used in pigs (41.7% of total use), humans (28.3%), aquaculture (21.9%) and chickens (4.8%). Combined AMU in other species accounted for < 1.5% (Table 1 and Fig. 1). We estimate that, in total, 261.7 mg (131.4–394.3 mg) of AAI were administered per 1 kg of human and 247.3 mg (130.3–364.3 mg) per 1 kg of animal in Vietnam. The corresponding figures from the EU were 122.0 mg/kg and 151.1 mg/kg in humans and animals, respectively (Fig. 2).
Table 1.
Calculation of total annual AMU in each animal production type
| Category | Sub-category | No. of animals | Type of dataa | Weight unit (kg) | Annual bodymass (kg) | AMUb (mg per kg) | AGPs in commercial feed (mg per kg) | Total AMU (mg per kg) |
Total AMU (tonnes) |
|---|---|---|---|---|---|---|---|---|---|
| Swine | Breeding pigs | 4,128,032 | Census | 240 | 990,727,726 | 46.11 | 286.62 | 332.7 | 329.6 |
| Slaughter pigs (except breeders) | 48,567,582 | Production | 78.6 | 3,817,411,914 | 46.11 | 286.62 | 332.7 | 1270.1 | |
| Poultry | Chickens | 88,777,000 | Production | 1.8 | 699,798,600 | 187.73, 4 | 77.42 | 265.1 | 185.5 |
| Ducks | 101,931,884 | Production | 2 | 203,863,767 | 93.95 | 38.75 | 132.6 | 27.0 | |
| Muscovies | 17,652,638 | Production | 3.2 | 56,488,440 | 93.95 | 38.75 | 132.6 | 7.5 | |
| Geese | 641,212 | Production | 3.2 | 2,051,877 | 93.95 | 38.75 | 132.6 | 0.3 | |
| Quails | 13,526,147 | Production | 0.13 | 1,758,399 | 93.95 | 38.75 | 132.6 | 0.2 | |
| Bovine | Breeding bovines | 3,472,891 | Census | 325 | 1,128,330,008 | 52.46 | 0.0 | 52.4 | 59.1 |
| Slaughter bovines (except breeding animals) | 1,220,131 | Production | 200 | 244,026,240 | 52.46 | 0.0 | 52.4 | 12.8 | |
| Buffalo | Breeding buffaloes | 378,549 | Population | 500 | 189,274,500 | 52.46 | 0.0 | 52.4 | 9.9 |
| Slaughter buffaloes | 297,216 | Production | 300 | 89,164,711 | 52.46 | 0.0 | 52.4 | 4.7 | |
| Sheep | Breeding animals (est.) | 26,901 | Census | 75 | 2,017,556 | 52.46 | 0.0 | 52.4 | 0.1 |
| Number slaughtered (except breeders) | 64,368 | Production | 75 | 4,827,600 | 52.46 | 0.0 | 52.4 | 0.3 | |
| Goats | Breeding animals (est.) | 444,411 | Census | 75 | 33,330,833 | 52.46 | 0.0 | 52.4 | 1.7 |
| Number slaughtered (except breeders) | 699,515 | Production | 75 | 52,463,597 | 52.46 | 0.0 | 52.4 | 2.7 | |
| Aquaculture | All species (domestic) | – | Production | 835,000,000 | 477.17 | – | 477.17 | 398.5 | |
| All species (export) | – | Production | 2,775,000,000 | 159.18 | – | 159.18 | 441.4 | ||
| All animals | 11,125,535,768 | 2751.4 | |||||||
AMU Antimicrobial use, AGPs Antimicrobial growth promoters (in commercial feed)
aData derived from official country statistics [10, 11]. ‘Census’ refers to ‘No. standing animals’, ‘Production’ refers to ‘No. of slaughtered animals’, except for aquaculture, where it refers to ‘No. of kg produced’
bExcluding antimicrobial growth promoters in commercial feed; 1 Nguyen et al. (2016) [15]; 2 Van Cuong et al. (2016) [17]; 3,4 Average of two studies: Carrique-Mas et al. (2014) [13] and Cuong et al. (2019) [14]; 5 Based on 50% of quantities used in chicken production; 6 Hosoi et al. (2014) [19]; 7 Pham et al. (2015) [16]; 8 Assuming that AMU for export production is 1/3 of the magnitude of AMU for domestic production
Fig. 1.
Two-dimensional diagram representing the estimated annual amounts (areas of bars) of antimicrobials used in each of species (including humans) in Vietnam, and whether these quantities are more affected by the total biomass (width of bars) or the intensity of AMU (height of bars). Bars are sorted from higher to lower overall AMU. AMU = Antimicrobial use. The vertical lines represent the range between best- and worst-case scenarios. ‘Other avian’ includes ducks, muscovies, geese and quails
Fig. 2.
Two-dimensional diagram showing the relative annual amounts of AMU in the European Union (2014) and in Vietnam. In order to render comparison between European Union and Vietnam possible, the biomasses of animals and humans on the x-axis have been scaled to proportions. AMU = Antimicrobial use
Here, using a combination of available statistics alongside published AMU survey and extrapolation data, we estimated AMU related to biomass in humans and animal production in Vietnam. Our results suggest that in this country pig production and aquaculture should be the main target if the country aims to reduce its AMU footprint in animal production. AMU in humans in Vietnam (32.0 DDD per 1000 inhabitants per day) ranks higher than in most countries in the EU. These human data were generated using limited retail surveys [12]. However, EU countries such as Romania, Greece, France, Spain, and Ireland featured a higher magnitude of AMU (in terms of DDD related to population) than Vietnam. A recent report from Thailand, a LMIC country which is more comparable to Vietnam, estimated that in 2017 a total of 53.0 DDD per 1000 inhabitants per day were used in 2017 [20]. The Thai study used surveillance data on declared quantities of antimicrobials, which is a compulsory requirement for companies trading with antimicrobials in that country.
Whilst these are the first specific calculations for AMU in Vietnam, there is a considerable uncertainty around these estimates due to the lack of reliable data. For example, AMU data in humans, pigs, and aquaculture originate from single studies, all conducted prior to 2015. Furthermore, there are no data whatsoever on AMU in non-chicken poultry species and ruminants. The situation is likely to be even worse in other LMICs where there are practically no AMU data in any production sector.
Since different animal types are raised over variable periods, the same magnitude of AMU related to body mass may have different implications for the development and maintenance of AMR For example, in Vietnam chickens are raised over a period ranging from 1 to 5 months, compared with 5–8 months for pigs. The implications of this need to be further investigated.
Because of its relative simplicity, we propose to regularly (i.e. annually) estimate/update quantities of antimicrobials used in relation to body mass as a first step to develop a fully-fledged AMU surveillance system. These estimates could be fine-tuned by conducting targeted surveys tailored to different production types (i.e. meat chickens, layers, breeders, fattening pigs, etc.). It may also be necessary to differentiate the extent of AMU by level of intensification of the production system (i.e. backyard, small-scale, large-scale, industrial), as different systems require variable quantities of antimicrobials. It has been shown that in the Mekong Delta of Vietnam smaller chicken farms tend to use more antimicrobials [13]. Lastly, it would be desirable to incorporate detailed information regarding the classes and formulations of antimicrobials used, since there is a great variability regarding the strength of different antimicrobial products and their impact on development of AMR.
In conclusion, in the absence of reliable statistics on sales of AAIs, the challenges of monitoring AMU in animal production in LMICs such as Vietnam can be overcome by the use of innovative approaches that maximize the use of existing animal population statistics and AMU data. These estimates should help elucidate secular changes in AMU and help refine policies and interventions aimed at reducing AMU at country level.
Acknowledgements
Not applicable.
Appendix 1
Table 2
Table 2.
Estimation of human bodymass of the Vietnamese population from population pyramid, adult bodyweight and age-gender specific bodyweights
| Age (years) | Total population | Average weight (US) (kg) | Average weight (Vietnam)a (kg) | Estimated bodymass (kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Total | Males | Females | Males | Females | Males | Females | Total | |
| > 18 | 32,448,992 | 32,448,992 | 64,897,984 | 58.4 | 50.8 | 1,895,021,133 | 1,648,408,794 | 3,543,429,926 | ||
| 15 to 18 | 3,582,536 | 3,390,322 | 6,972,858 | 64.4 | 54.4 | 41.4 | 35.4 | 148,234,592 | 120,066,219 | 268,300,812 |
| 10 to 14 | 3,406,698 | 3,207,976 | 6,614,674 | 39.9 | 41.5 | 25.6 | 27.0 | 87,333,258 | 86,668,284 | 174,001,542 |
| 5 to 9 | 3,774,596 | 3,446,644 | 7,221,240 | 22.9 | 22.4 | 14.7 | 14.6 | 55,536,575 | 50,260,341 | 105,796,916 |
| 0 to 4 | 4,078,564 | 3,662,281 | 7,740,845 | 12.5 | 12.0 | 8.0 | 7.8 | 32,755,967 | 28,609,739 | 61,365,706 |
| 93,447,601 | 2,218,881,525 | 1,934,013,377 | 4,152,894,902 | |||||||
aEstimated from US data after applying a correction factor 0.642 (males) and 0.651 (females)
Appendix 2
Table 3.
Table 3.
Estimation of weight of antimicrobial active ingredient from antimicrobials consumed by humans
| Antimicrobial class | Antimicrobial | No. DDDs (per 1000 inhabitants per day) | Dose in g (for a typical inhabitant) | Daily amount of AAI (per 1000 inhabitants) (g) |
|---|---|---|---|---|
| Cephalosporin | Ceftriaxone | 8 | 1.5 | 12 |
| Broad-spectrum beta lactam | Ampicillin | 8 | 1.5 | 12 |
| Macrolide | Azithromycin | 8 | 0.5 | 4 |
| Oral fluoroquinolone | Levofloxacin | 8 | 0.5 | 4 |
| All | 32 |
Authors’ contributions
SB and JC conceived the study and wrote the first draft of manuscript. MC and NC contributed to data analysis and drawing the figures. GT contributed to the discussion. All authors contributed to final version. All authors read and approved the final manuscript.
DDDs Defined Daily Doses, AAI Antimicrobial active ingredient
Funding
This work was funded by the Wellcome Trust through and Intermediate Clinical Fellowship awarded to Juan J Carrique-Mas (Grant Ref. No. 110085/Z/15/Z).
Availability of data and materials
The data presented and analysed here have all been extracted from publicly available data sets and publications cited in the body text.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.O'Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. [Google Scholar]
- 2.da Costa Paulo, Loureiro Luís, Matos Augusto. Transfer of Multidrug-Resistant Bacteria Between Intermingled Ecological Niches: The Interface Between Humans, Animals and the Environment. International Journal of Environmental Research and Public Health. 2013;10(1):278–294. doi: 10.3390/ijerph10010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collignon Peter, McEwen Scott. One Health—Its Importance in Helping to Better Control Antimicrobial Resistance. Tropical Medicine and Infectious Disease. 2019;4(1):22. doi: 10.3390/tropicalmed4010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Global action plan on antimicrobial resistance. 2015. [DOI] [PubMed] [Google Scholar]
- 5.EFSA. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. 2017. 10.2903/j.efsa.2017.4872. [DOI] [PMC free article] [PubMed]
- 6.Anon. OIE annual report on antimicrobial agents intended for use in animals. Paris: International Organisation of Animal Health; 2018. [Google Scholar]
- 7.Anon. Population pyramid of Vietnam, 2015. 2019. [Google Scholar]
- 8.Anon . Average sizes of men and women. 2019. [Google Scholar]
- 9.Anon . Weight and height: babies to teenagers. 2019. [Google Scholar]
- 10.Anon . Animal farming statistics (Vietnam) 2018. [Google Scholar]
- 11.Food and Agriculture Organization. 2018. The state of world fisheries and aquaculture. Available at: http://www.fao.org/3/ca0191en/ca0191en.pdf. Accessed 4 Oct 2019.
- 12.Klein Eili Y., Van Boeckel Thomas P., Martinez Elena M., Pant Suraj, Gandra Sumanth, Levin Simon A., Goossens Herman, Laxminarayan Ramanan. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proceedings of the National Academy of Sciences. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrique-Mas Juan J, Trung Nguyen V., Hoa Ngo T., Mai Ho Huynh, Thanh Tuyen H., Campbell James I., Wagenaar Jaap A., Hardon Anita, Hieu Thai Quoc, Schultsz Constance. Antimicrobial Usage in Chicken Production in the Mekong Delta of Vietnam. Zoonoses and Public Health. 2014;62:70–78. doi: 10.1111/zph.12165. [DOI] [PubMed] [Google Scholar]
- 14.Cuong NV, Phu DH, Van NTB, Dinh Truong B, Kiet BT, Hien BV, Thu HTV, Choisy M, Padungtod P, Thwaites G, Carrique-Mas J. High-resolution monitoring of antimicrobial consumption in Vietnamese small-scale chicken farms highlights discrepancies between study metrics. Front Vet Sci. 2019. 10.3389/fvets.2019.00174. [DOI] [PMC free article] [PubMed]
- 15.Nguyen Nhung T., Nguyen Hoa M., Nguyen Cuong V., Nguyen Trung V., Nguyen Men T., Thai Hieu Q., Ho Mai H., Thwaites Guy, Ngo Hoa T., Baker Stephen, Carrique-Mas Juan. Use of Colistin and Other Critical Antimicrobials on Pig and Chicken Farms in Southern Vietnam and Its Association with Resistance in Commensal Escherichia coli Bacteria. Applied and Environmental Microbiology. 2016;82(13):3727–3735. doi: 10.1128/AEM.00337-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham Dang Kim, Chu Jacqueline, Do Nga Thuy, Brose François, Degand Guy, Delahaut Philippe, De Pauw Edwin, Douny Caroline, Van Nguyen Kinh, Vu Ton Dinh, Scippo Marie-Louise, Wertheim Heiman F. L. Monitoring Antibiotic Use and Residue in Freshwater Aquaculture for Domestic Use in Vietnam. EcoHealth. 2015;12(3):480–489. doi: 10.1007/s10393-014-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cuong Nguyen, Nhung Nguyen Thi, Nghia Nguyen Huu, Mai Hoa Nguyen Thi, Trung Nguyen Vinh, Thwaites Guy, Carrique-Mas Juan. Antimicrobial Consumption in Medicated Feeds in Vietnamese Pig and Poultry Production. EcoHealth. 2016;13(3):490–498. doi: 10.1007/s10393-016-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phu Tran Minh, Phuong Nguyen Thanh, Scippo Marie-Louise, Dalsgaard Anders. Quality of Antimicrobial Products Used in Striped Catfish (Pangasianodon hypophthalmus) Aquaculture in Vietnam. PLOS ONE. 2015;10(4):e0124267. doi: 10.1371/journal.pone.0124267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HOSOI Y., ASAI T., KOIKE R., TSUYUKI M., SUGIURA K. Sales of veterinary antimicrobial agents for therapeutic use in food-producing animal species in Japan between 2005 and 2010. Revue Scientifique et Technique de l'OIE. 2014;33(3):1007–1015. doi: 10.20506/rst.33.3.2337. [DOI] [PubMed] [Google Scholar]
- 20.Anon . Consumption of antimicrobial agents in Thailand in 2017. Thailand: Food and Drug Administration, and International Health Policy Program, Ministry of Public Health; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented and analysed here have all been extracted from publicly available data sets and publications cited in the body text.