ABSTRACT
Emerging evidence reveals the importance of long non-coding RNAs (lncRNAs) in the development and progression of keloid formation, whereas the underlying mechanisms are not well understood. In the present study, we investigated the biological effects and molecular mechanisms of lncRNA HOXA11-AS in keloid formation. First, the expression levels of HOXA11-AS, miR-124-3p, and transforming growth factor β receptor type I (TGFβR1) were measured in both keloid tissues and human keloid fibroblasts (HKFs) using qRT-PCR and western blot analysis, respectively. Next, we adopted both gain- and loss-of-function strategies to explore the significance of HOXA11-AS. TUNEL, flow cytometry, DNA ladder, and tube formation assays were performed to measure cell apoptosis and angiogenesis, respectively. Besides, the potential binding relationship between HOXA11-AS and miR-124-3p, as well as miR-124-3p and TGFβR1 was identified using bioinformatic screening and verified by luciferase reporter assay. Furthermore, we explored the importance of miR-124-3p in HOXA11-AS-induced phenotypes and regulations on TGFβ signaling or PI3K/Akt signaling. We found that HOXA11-AS and TGFβR1 were significantly up-regulated, while miR-124-3p was down-regulated both in keloid tissues or fibroblasts than in normal skin tissues or fibroblasts. Functionally, high expression of HOXA11-AS essentially inhibited cell apoptosis and promoted fibroblast-induced angiogenesis. Mechanistically, miR-124-3p was identified as a downstream effector to be involved in HOXA11-AS-mediated phenotypes through directly targeting TGFβR1, thus modulating PI3K/Akt signaling pathway. Taken together, our findings revealed that HOXA11-AS inhibits cell apoptosis and promotes angiogenesis through miR-124-3p/TGFβR1 axis, contributing to the progression of keloid formation, which might provide a novel target for keloid therapy.
KEYWORDS: Keloid formation, lncRNA HOXA11-AS, miR-124-3p, TGFβ receptor type I
Introduction
Keloids are benign proliferative skin lesions arising from abnormal wound healing, growing beyond and above the boundaries of original injuries, and pathologically characterized by the presence of excessively produced, thickened, and disorganized collagen bundles together with capillary proliferation [1,2]. The incidence of keloids varies among populations with different ethnic backgrounds and keloids are more prevalent among people with darker skin (such as African descendants) than those with lighter skin, suggesting genetic basis as a risk factor for the development [3]. The molecular and cellular pathogenic mechanisms of keloids are not well understood, although hyper-proliferative fibroblasts and a plethora of up-regulated growth factors and pro-inflammatory cytokines, such as transforming growth factor β (TGFβ), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor (IGF-I), interleukin (IL)-1β, IL-4, IL-10, and tumor necrosis factor α (TNFα), are strongly believed to play an essential role in keloid formation [1,4]. Thus, advancing the understanding on mechanisms underlying keloid formation is critical for developing effective therapies.
Transforming growth factor β (TGFβ) is well demonstrated as an inducer of keloid formation [5]. TGFβ signaling mainly involves two transmembrane TGFβ receptors, TGFβR1 and TGFβR2, which activates downstream Smad-dependent and Smad-independent signaling pathways, and stimulates the production of extracellular matrix (ECM) components, pro-angiogenic factors, and tissue fibrosis [5,6]. The importance of TGFβ signaling in keloid formation is corroborated by the increased expression of TGFβ, TGFβR1 and TGFβR2 in keloid fibroblasts than that in normal skin fibroblasts [7]. Therefore, targeting TGFβ signaling is being intensively explored as a potential therapy for keloids.
In addition to the TGFβ signaling, other pathways have been demonstrated to contribute to keloid pathogenesis. For example, the PI3K/Akt/mTOR signaling is shown to stimulate the overproduction of collagen and ECM [8,9], to desensitize keloid fibroblasts to ionizing radiation [10], and to impact phenotypes of keloid fibroblasts in response to different stimuli [11,12].
Long non-coding RNAs (lncRNAs) are a heterogenous population of RNA molecules of >200 nucleotides in length and encoding no protein products [13]. Although the molecular mechanisms of lncRNAs are not completely understood, cumulative evidence indicates that lncRNA may serve as a source of microRNA (miRNA), function as a negative regulator of miRNA, directly target mRNA for degradation, or regulate gene transcription by recruiting chromatin modifiers [13]. Functionally, lncRNAs play a critical role in normal physiology as well as the pathological development of various human diseases. A recent study using pathway-focused lncRNA microarray revealed four keloid-related lncRNA biomarkers, CACNA1G-AS1, HOXA11-AS, LINC00312 and RP11-91I11 [14]. Since previous studies on HOXA11-AS primarily focused on different types of cancer, demonstrating its importance in cancer development, and keloids are considered benign proliferative skin tumors [15], herein, we propose that HOXA11-AS may alter multiple biological processes and significantly impact keloid formation. Earlier studies showed that sponging miRNA is a key mechanism by which HOXA11-AS functions and miR-124-3p (later named as miR-124-3p) is a target for HOXA11-AS in multiple cancer paradigms [16–21]. Besides, HOXA11-AS, by sponging miR-124-3p, inhibited the proliferation and promoted the apoptosis of osteoblasts, thus affecting fracture healing. However, the potential molecular mechanisms by which HOXA11-AS regulates keloid progression remain to be further explored.
In this study, we investigated the potential role of HOXA11-AS in keloid formation. Our findings showed that HOXA11-AS, a highly expressed lncRNA, suppressed cell apoptosis and promoted angiogenesis through regulating miR-124-3p/TGFβR1 axis, thus accelerating the development and progression of keloid formation. Therefore, the present study supports that HOXA11-AS may become a novel target for keloid treatment.
Materials and methods
Human samples
A total of 20 patients diagnosed with earlobe keloids who underwent surgical excision in our hospital were enrolled into this study. Patients with the same diagnosis but have received any previous treatments (either surgical or medicinal) were excluded from this study. The clinicopathological features of all patients were summarized in Table 1. Keloid samples were collected from the earlobes of patients. As controls, normal skin tissues were obtained from abdomens of healthy volunteers undergoing plastic surgery. A fraction of each tissue was snap frozen in liquid nitrogen and the remaining fixed in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) till further use. The study procedures on clinical specimens were approved by the Ethics Committee of Henan People’s Hospital (Henan, China) and written consents were acquired from all participants.
Table 1.
Basic characteristics of 20 keloid patients.
| Characteristic | Number of lesion site (n = 20) |
|---|---|
| Age (y) | |
| Range | 25– 58 |
| Mean | 31.5 |
| Sex | |
| Male | 4 |
| Female | 16 |
| Site of lesions | |
| Ear lobe | 20 |
| Causative factors | |
| Piercing | 16 |
| Trauma | 3 |
| Surgical scar | 1 |
| Family history | |
| Positive | 12 |
| Maternal hereditary tendency | 10 |
| Paternal hereditary tendency | 2 |
| Negative | 8 |
Cell culture
The normal human skin fibroblasts (HSFs) and human keloid fibroblasts (HKFs) were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s-modified Eagle medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 U/ml penicillin (Gibco), and 100 μg/ml streptomycin (Gibco) at 37°C in an atmosphere of 95% air and 5% CO2. The human umbilical vein endothelial cells (HUVECs) were purchased from Sigma-Aldrich and cultured in endothelial cell growth medium (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions.
Cell transfection
The pLenti-EF1a-EGFP-F2A-puro plasmid (Obio, Shanghai, China) was used to construct lentiviral vector expressing HOXA11-AS (plv-HOXA11-AS) or short-hairpin RNA (shRNA) specifically targeting HOXA11-AS (sh-HOXA11-AS). As the negative control, empty vector (plv-ctrl) or that expressing control shRNA (shNC) was prepared in parallel. The lentiviral constructs, miR-124-3p mimic, mimic-NC, miR-124-3p inhibitor, or inhibitor-NC (all from GenePharma. Shanghai, China) were transfected into target cells using Lipofectamine 2000 (Invitrogen, USA), according to the manufacturer’s instructions.
Histological examinations and immunofluorescence (IF) staining
For histological analysis, tissues were fixed in 4% paraformaldehyde overnight and prepared into 4-µm paraffin-embedded sections. Hematoxylin and eosin (HE) staining was performed using HE staining kit (Vector Labs, Burlingame, CA, USA) according to the manufacturer’s instructions.
For immunohistochemistry (IHC) staining, tissue sections were de-paraffinized in xylene and rehydrated in a series of diluted alcohol. Antigen retrieval was performed in boiling 10 mM citrate buffer (pH 6.0) for 10 min. After incubating with primary anti-TGFβR1 antibody (Abcam, Cambridge, MA, USA, 1:100) overnight, the sections were incubated with biotinylated secondary antibody at room temperature for 30 min, followed by Vectastain ABC-HRP solution (Vector Labs) at room temperature for 30 min, according to the manufacturer’s instructions. The signal of a target protein was developed using Diaminobenzidine (DAB) substrate (Vector Labs).
For immunofluorescence (IF) staining, tissues were stained with primary anti-TGFβR1 antibody (Abcam, 1:100) overnight, followed by PE-conjugated secondary antibody (Abcam, 1:1000). The tissues were then mounted with VECTASHIELD antifade mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA), imaged with a Leica DM300 microscope (Leica Microsystems, Bannockburn, IL, USA).
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from frozen tissues or cultured cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. cDNA was synthesized using Takara reverse transcription system (Dalian, China). qRT-PCR analysis was performed on ABI-7500 using iQTM SYBR® Green Supermix (Bio Rad, Hercules, CA) using the following primers: GAPDH (internal control) forward primer 5ʹ- AGGTCGGTGTGAACGGATTTG-3ʹ, reverse primer 5ʹ- GGGGTCGTTGATGGCAACA-3ʹ; HOXA11-AS forward primer 5ʹ-CGGCTAACAAGGAGATTTGG-3ʹ, reverse primer 5ʹ-AGGCTCAGGGATGGTAGTCC-3ʹ; miR-124-3p forward primer 5ʹ-TAAGGCACGCGGTGAATGCC-3ʹ, reverse primer 5ʹ-GATTGAATCGAGCACCAGTTAC-3ʹ; TGFβR1 forward primer 5ʹ- TCAGCTCTGGTTGGTGTCAG-3ʹ, reverse primer 5ʹ- ATGTGAAGATGGGCAAGACC-3ʹ. U6 and GAPDH were used as endogenous controls for the expression of lncRNAs and miRNAs. The relative expression of a target gene to internal control was calculated using the 2−ΔΔCt method [22].
Western blot
Total proteins were extracted from human tissues or cells using RIPA buffer (Thermo Fisher Scientific, Waltman, MA, USA) containing a protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were measured using BCA kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Equal amounts of total proteins from different samples were separated on SDS-PAGE gel. Following the transfer of separated proteins onto a polyvinylidene difluoride membrane, the membrane was blocked with 5% nonfat milk in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, 0.5% Tween 20) at room temperature for 1 h, washed three times in TBST, and incubated with the primary antibodies against TGFβR1 (1:1000), Bax (1:1000), Bcl-2 (1:1000), p-PI3K (1:500), PI3K (1:1000), p-Akt (1:500), Akt (1:1000), or GAPDH (1:2000) (all from Santa Cruz Biotech. Santa Cruz, CA, USA) at 4°C overnight. After three washes with TBST, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 2 h. The blots were detected via the ECL detection system (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. GAPDH was used as endogenous control [23].
Terminal deoxynucleotidyl transferase dutp nick end labeling (TUNEL) assay
TUNEL assay was performed using TUNEL assay kit (ab66110; Abcam) according to the manufacturer’s protocol.
Apoptosis analysis
Flow cytometry was performed for cell apoptosis analysis. Briefly, cells were incubated with Annexin V-FITC and PI (both from Thermo Fisher Scientific) at room temperature for 15 min and the apoptotic cells were examined by using the FACScan Flow Cytometer (BD Biosciences, San Jose, CA, USA).
DNA fragmentation using agarose gel electrophoresis
DNA fragmentation was analyzed using agarose gel electrophoresis as described previously [24]. Briefly, cells were lysed in TSE lysis buffer containing 10 mM Tris-HCl, 0.2% Triton X-100, and 10 mM EDTA, treated with RNase A (Sigma-Aldrich) at 37ºC for 1 h followed by proteinase K (Sigma-Aldrich) at 50ºC for 2 h. Then, DNA samples were separated by agarose gel electrophoresis and photographed under a UV light.
Tube formation assay
To examine the impact of HSFs or HKFs on the tube formation of HUVECs, we adopted a Transwell co-culture system where HSFs or HKFs were separated from HUVECs by a Transwell insert. Briefly, the 24-well plate was coated with Matrigel (BD Biosciences, San Jose, CA, USA). Upon gel solidification, 1 × 104 HUVECs were plated onto the pre-coated Matrigel. Then, a Transwell insert (pore size 0.4 µM, Corning, NY, USA) was added and 1 × 104 HSFs or HKFs were seeded on top of the insert. Upon co-culturing HUVECs and HSFs or HKFs at 37°C for indicated times, the insert was removed, and the capillary-like structures of HUVECs were photographed with a microscope and analyzed using Image J software with the Angiogenesis Analyzer plugin.
Luciferase reporter assay
We used miRanda software (http://www.microrna.org/microrna/getGeneForm.do) and predicted the potential binding sites between miR-124-3p and HOXA11-AS or between miR-124-3p and the 3ʹ-untranslated region (3ʹ-UTR) of TGFβR1. Accordingly, mutations were generated either within HOXA11-AS sequence or within TGFβR1 3ʹ-UTR sequence to disrupt the potential interaction. Then, either wild-type (WT) HOXA11-AS or TGFβR1 sequence containing the potential binding site for miR-124-3p or the sequence containing mutations (MUT) was cloned into pGL-CMV luciferase reporter plasmid, which was co-transfected into HKFs with miR-124-3p mimic or control mimic using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. At 48 h following the transfection, luciferase activity was detected using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Statistical analysis
All data were analyzed by SPSS 17.0 software (SPSS, Chicago, USA) and presented as mean ± standard deviation (SD) from at least three independent experiments. Two-tailed Student’s t test was used to compare the mean values between two groups. One-way analysis of variance (ANOVA) was used for multi-group comparisons. A P value of less than 0.05 was considered statistically significant.
Results
HOXA11-AS and TGFΒR1 are up-regulated, while miR-124-3p is decreased in keloid tissues or fibroblasts
To understand the potential interactions among HOXA11-AS, miR-124-3p, and TGFβR1, we first profiled their expressions between normal human skin tissues and keloid tissues. By HE staining, we observed significantly thickened and hyalinized collagen bundles in keloid tissues, but not in normal skin tissues. While the collagen fibers were parallel to the surface of the normal skin, they were arranged in a haphazard pattern in keloid tissues (Figure 1(a)). Both IHC and IF staining showed abundant expression of TGFβR1 in keloid tissues, but not in normal skin tissues (Figure 1(a)). Quantitative analysis on the levels of HOSA11-AS, miR-124-3p, and TGFβR1 revealed that HOXA11-AS and TGFβR1 were significantly up-regulated, while miR-124-3p was down-regulated in keloid tissues, compared with normal skin tissues (Figure 1(b,c)). Since normal or keloid skin tissues contain a variety of cell types, to understand whether the alterations on these three molecules exist in fibroblasts, we explored their relative expressions in HSFs vs. HKFs. Consistent with the findings of skin tissues, the levels of HOXA11-AS and TGFβR1 were also significantly higher, yet that of miR-124-3p was drastically lower in HKFs when compared with HSFs (Figure 1(d,e)). Taken together, the data revealed an inverse regulation between miR-124-3p and HOXA11-AS or TGFβR1.
Figure 1.
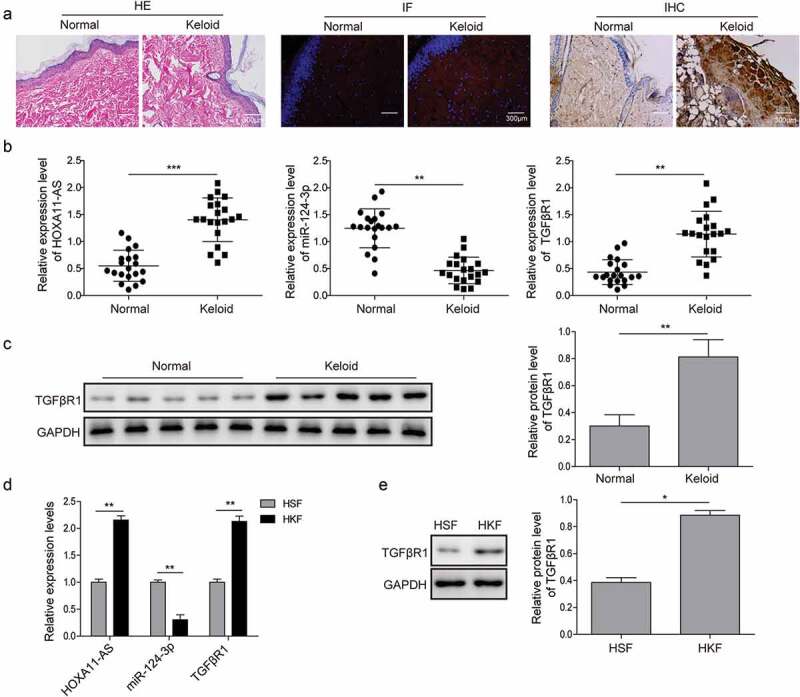
HOXA11-AS and TGFβR1 are up-regulated, while miR-124-3p is down-regulated in keloid tissues or fibroblasts.
(a) A total of 20 keloid tissues and 20 normal skin tissues were acquired from patients or healthy individuals. HE staining (left panels), IHC (middle panels), and IF staining of TGFβR1 (right panels) were performed. Scale bar, 300 μm. (b) and (c) In keloid tissues and matching normal skin tissues, the expression patterns of HOXA11-AS, miR-124-3p and TGFβR1 were measured by qRT-PCR and western blot analysis, respectively. (d) and (e) qRT-PCR and western blot analysis were subjected to detect the expression of HOXA11-AS, miR-124-3p and TGFβR1 in HKFs vs. HSFs. *P < 0.05, **P < 0.01, ***P < 0.001.
HOXA11-AS inhibits cell apoptosis and promotes fibroblast-induced angiogenesis
Current knowledge on HOXA11-AS is mostly acquired from studies in different types of human cancers and little is known on its biological effects in fibroblasts. Therefore, we applied the gain-of-function approach in HSFs showing low expression of endogenous HOXA11-AS and the loss-of-function approach in HKFs presenting high expression of endogenous HOXA11-AS. Transfecting cells with plv-HOXA11-AS significantly elevated its level in HSFs, while with shHOXA11-AS potently reduced its level in HKFs (Figure 2(a)). For functional significance, we focused on apoptosis of fibroblasts and fibroblast-mediated angiogenesis, two biological phenotypes important for keloid formation. As shown in Figure 2(b–d), overexpression of HOXA11-AS significantly reduced the apoptosis of HSFs, while knockdown of HOXA11-AS potently boosted the apoptosis of HKFs. Furthermore, HSFs overexpressing HOXA11-AS also markedly promoted the angiogenesis of HUVECs, while the tube formation of HUVECs was repressed in HKFs silencing HOXA11-AS (Figure 2(e)). These data suggest that HOXA11-AS might be a vital mediator in the progression of keloid formation.
Figure 2.
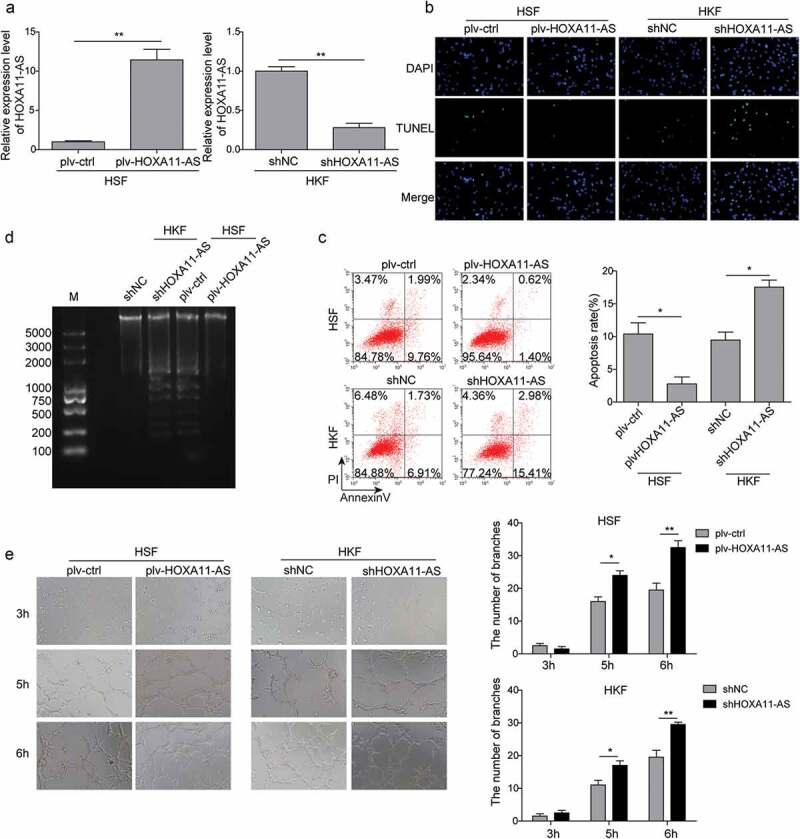
HOXA11-AS suppresses apoptosis of fibroblasts and promotes fibroblast-mediated angiogenesis.
(a) qRT-PCR assay was performed to examine the expression of HOXA11-AS, demonstrating the efficacy of overexpressing or knocking down HOXA11-AS. (b–d) Cell apoptosis was examined using TUNEL assay (b), flow cytometry (c), and DNA fragmentation (d). (e) HUVECs were seeded onto Matrigel and co-cultured with HSFs or HKFs separated by a transwell membrane. The tube formation of HUVECs was imaged after 3, 5, and 6 h, respectively. *P < 0.05, **P < 0.01.
HOXA11-AS directly targets miR-124-3p
Previous studies suggest that HOXA11-AS may sponge miR-124-3p and release the suppression of the latter on its target genes [17,19]. To examine whether there is a direct interaction between HOXA11-AS and miR-124-3p, bioinformatics analysis was performed. We identified the complementary binding sites on both molecules and introduced mutation into the binding sequence (Figure 3(a)). The luciferase reporter assay showed that miR-124-3p mimic specifically reduced the luciferase activity driven by WT HOXA11-AS vector, but not by MUT HOXA11-AS sequence (Figure 3(b)). Furthermore, qRT-PCR analysis also verified that HOXA11-AS could negatively regulate miR-124 expression (Figure 3(c)). However, in return, treating HSFs with miR-124-3p inhibitor or HKFs with miR-124-3p mimic did not significantly altered the level of HOXA11-AS (Figure 3(d)), suggesting that the unidirectional regulation of HOXA11-AS on miR-124-3p.
Figure 3.
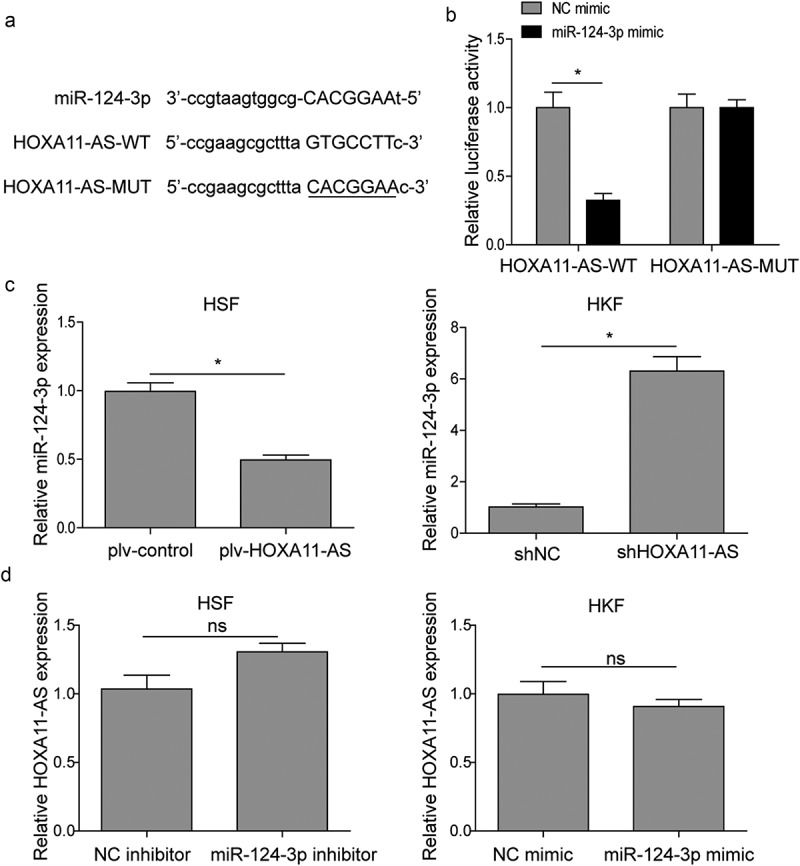
HOXA11-AS directly binds to and targets miR-124-3p.
(a) Sequence alignment showing the binding site between miR-124-3p and wildtype HOXA11-AS (HOXA11-AS-WT), as well as the mutations made in HOXA11-AS (HOXA11-AS-MUT). (b) Cells were transfected with luciferase reporter gene driven by either HOXA11-AS-WT or HOXA11-AS-MUT. The luciferase activity was measured and compared between cells treated with miR-124-3p mimic or NC mimic. *P < 0.05. (c) and (d) qRT-PCR analysis was performed to determine the expression of HOXA11-AS and miR-124-3p. *P < 0.05, **P < 0.01.
miR-124-3p mediates the effects of HOXA11-AS on apoptosis and fibroblast-induced angiogenesis
To determine the role of miR-124-3p in HOXA11-AS-mediated phenotypes, we treated HSFs overexpressing HOXA11-AS with either NC mimic or miR-124-3p mimic and HKFs silencing HOXA11-AS with either NC inhibitor or miR-124-3p inhibitor. As shown in Figure 4(a), HOXA11-AS overexpression caused the notable decrease of miR-124-3p expression, while the effect was dramatically reversed by miR-124-3p mimic. Similarly, increased miR-124-3p induced by silencing HOXA11-AS was abolished by miR-124-3p inhibitor. Besides, we found that the anti-apoptotic effect of plv-HOXA11-AS in HSFs was completely abolished by miR-124-3p mimic, as detected by TUNEL and flow cytometry assays (Figure 4(b,c)). In contrast, miR-124-3p inhibitor reversed the apoptosis-inducing activity of shHOXA11-AS in HKFs (Figure 4(b,c)). For fibroblast-induced angiogenesis, treating plv-HOXA11-AS cells with miR-124-3p mimic disrupted the tube formation of HUVECs (Figure 4(d)). In contrast, miR-124-3p inhibitor released the suppression of shHOXA11-AS on tube formation (Figure 4(d)). Taken together, these data suggest that miR-124-3p is an essential effector in antagonizing the effects of HOXA11-AS on multiple phenotypes of skin fibroblasts.
Figure 4.
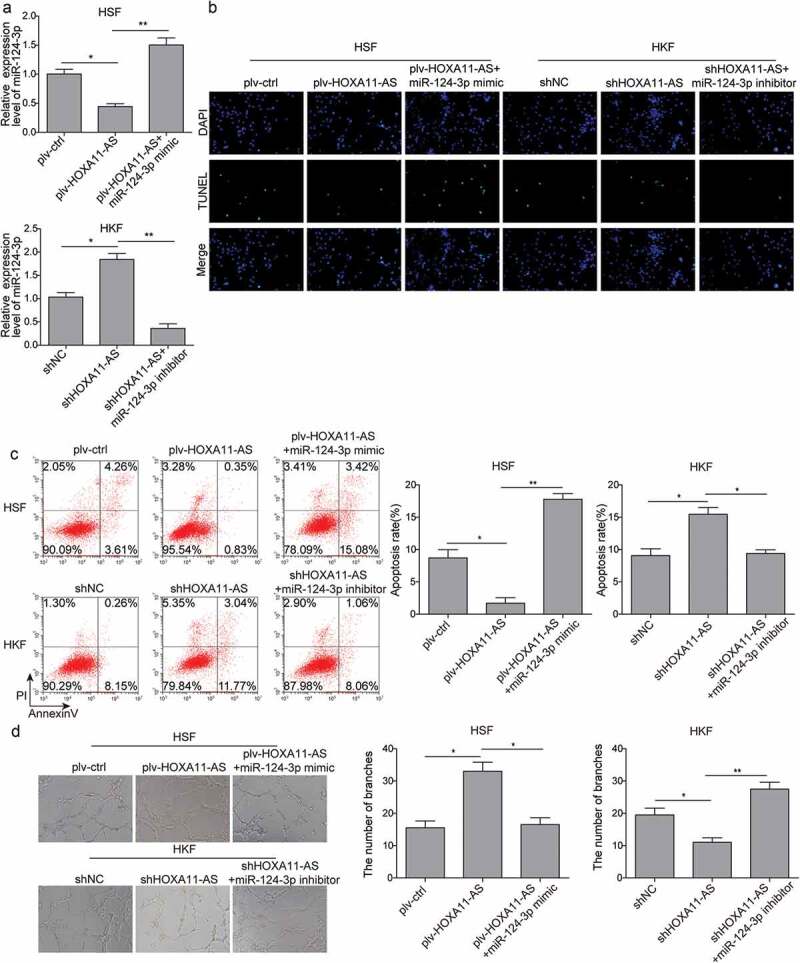
Effects of miR-124-3p on HOXA11-AS-regulated apoptosis and fibroblast-induced angiogenesis.
HSFs were transfected with plv-ctrl, plv-HOXA11-AS+mimic NC, or plv-HOXA11-AS+miR-124-3p mimic; HKFs were transfected with shNC, shHOXA11-AS+inhibitor NC, or shHOXA11-AS+miR-124-3p inhibitor. (a) qRT-PCR assay was performed to examine the expression of miR-124-3p. (b and c) Cell apoptosis was examined by TUNEL assay (b), flow cytometry (c). (d) HUVECs were seeded onto Matrigel and co-cultured with HSFs or HKFs separated by a transwell membrane. The tube formation of HUVECs was detected. *P < 0.05, **P < 0.01.
TGFΒR1 is a direct target of miR-124-3p
To explore the underlying downstream targets of miR-124-3p, the online bioinformatics software miRanda was performed. We found the complementary binding sites between miR-124-3p and TGFβR1 (Figure 5(a)). Subsequently, we generated mutations within the 3ʹ-untranslated region (UTR) of TGFβR1, and then cloned either TGFβR1-WT or TGFβR1-MUT sequence into the luciferase reporter construct. HEK293T cells were co-transfected with the luciferase reporter construct and miR-124-3p mimic or NC mimic. The results showed that miR-124-3p could directly interact with TGFβR1 since the reporter activity driven by WT but not MUT TGFβR1 sequence was specifically reduced by miR-124-3p mimic (Figure 5(b)). Moreover, the mRNA and protein levels of TGFβR1 were also down-regulated by miR-124-3p mimic, while up-regulated by miR-124-3p inhibitor (Figure 5(c,d)). Since TGFβ signaling plays a vital role in keloid formation [5], these findings indicate that HOXA11-AS may act as a sponge of miR-124-3p to activate TGFβR1 signaling, thus contributing to keloid formation.
Figure 5.
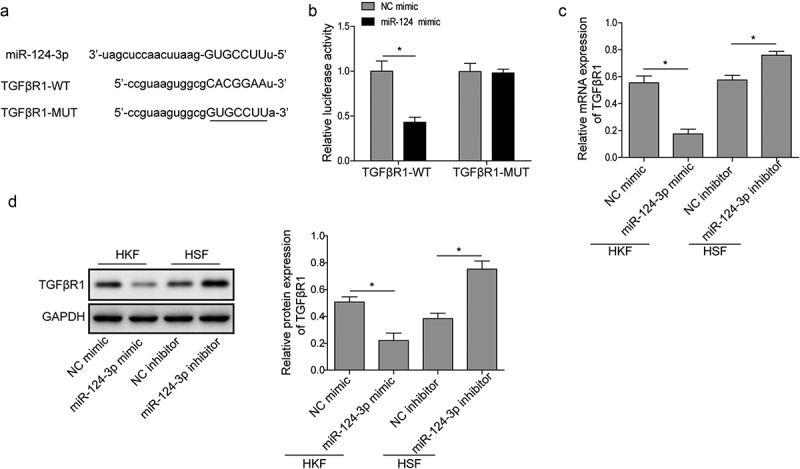
TGFβR1 is a direct target of miR-124-3p.
(a) Sequence alignment showing the binding site between miR-124-3p and wild-type TGFβR1 (TGFβR1-WT), as well as the mutations made in TGFβR1 (TGFβR1-MUT). (b) Cells were transfected with luciferase reporter gene driven by either TGFβR1-WT or TGFβR1-MUT. The luciferase activity was measured and compared between cells treated with miR-124-3p mimic or NC mimic. (c) and (d) qRT-PCR and western blot assays were performed to detect the expression of TGFβR1. *P < 0.05, **P < 0.01.
HOXA11-AS promotes keloid-related phenotypes through mir-124-3p/TGFΒR1-induced activation of PI3K/Akt signaling
To further verify the HOXA11-AS/miR-124-3p/TGFβR1 regulatory network in keloid formation, the alteration of TGFβR1 expression was performed. Firstly, the transduction efficiency of TGFβR1 was determined using qRT-PCR (Figure 6(a)). Subsequently, the data of fibroblast apoptosis and fibroblast-induced angiogenesis of HUVECs implied that TGFβR1 could reverse miR-124-3p-mediated biological phenotypes: TGFβR1 overexpression significantly attenuated cell apoptosis and promoted tube formation of HUVECs in HKFs pretreated with miR-124-3p mimic, while knockdown of TGFβR1 displayed the opposite effects (Figure 6(b–e)). Furthermore, we found that the expression levels of TGFβR1, Bcl-2, p-PI3K, and p-Akt were significantly up-regulated, while that of Bax was decreased in HSFs overexpressing HOXA11-AS. However, the expression patterns of these molecules were dramatically reversed by miR-124-3p mimic. In contrast, opposite results were observed in HKFs (figure 6(f)). Collectively, these data indicated that HOXA11-AS may contribute to keloid-related phenotypes through miR-124-3p/TGFβR1-induced activation of PI3K/Akt signaling.
Figure 6.
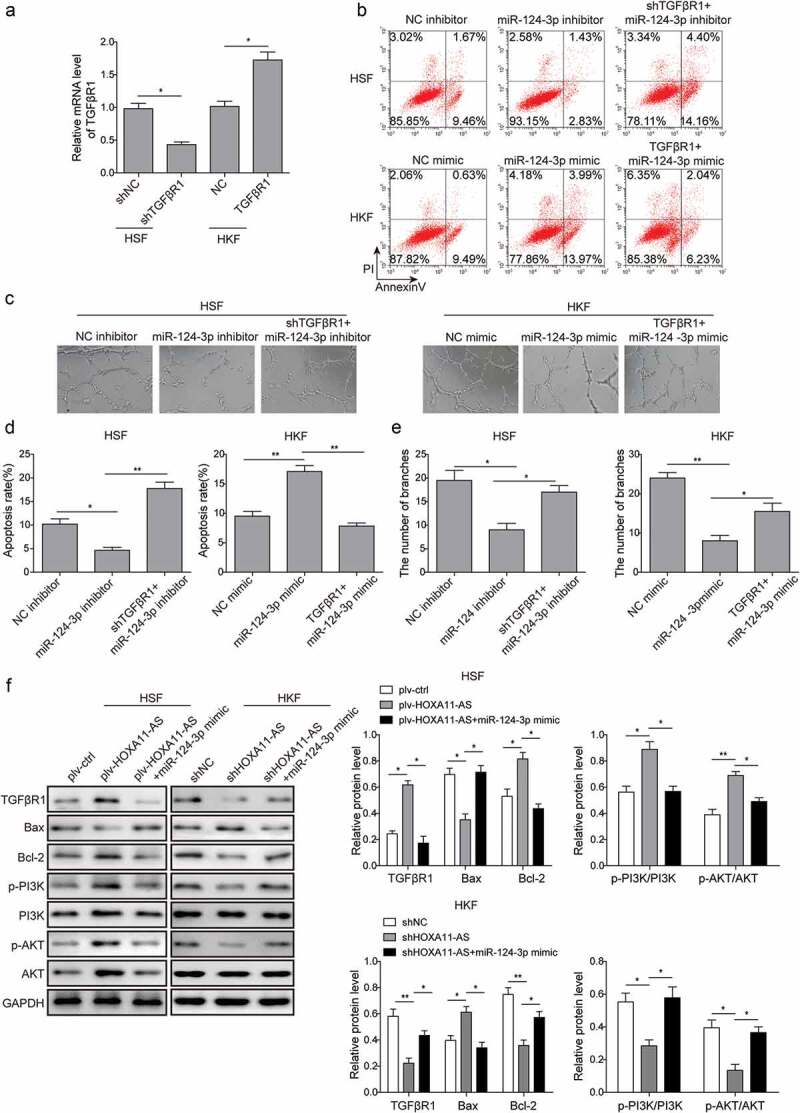
HOXA11-AS promotes keloid-related phenotypes via miR-124-3p/TGFβR1-induced activation of PI3K/Akt signaling.
(a) qRT-PCR assay was performed to examine the expression of TGFβR1. (b) and (d) Cells apoptosis was examined by flow cytometry. (c) and (e) The angiogenesis of HUVECs were detected by tube formation assay. (f) Western blot analysis of the expression of TGFβR1, Bax, Bcl-2, p-PI3K, PI3K, p-Akt, Akt. *P < 0.05, **P < 0.01.
Discussion
An earlier study identified HOXA11-AS as a keloid-specific biomarker lncRNA [14]. In this study, we showed that high expression of HOXA11-AS was required to inhibit the apoptosis of keloid fibroblasts and to enable fibroblast-induced angiogenesis. In normal skin fibroblasts, overexpressing HOXA-11AS alone was sufficient to reduce cell apoptosis and promote fibroblast-mediated angiogenesis. Mechanistically, HOXA11-AS functioned as an important mediator via sponging miR-124-3p, releasing the suppression of miR-124-3p on TGFβR1, activating PI3K/Akt signaling, and thus promoting the development and progression of keloid formation.
HOXA11-AS was first identified as an up-regulated lncRNA in human uterine cervix carcinoma from a lncRNA microarray analysis [25]. Since then, the clinicopathological significance and the biological functions of HOXA11-AS have been intensively investigated in different types of human cancers, where HOXA11-AS presents a plethora of oncogenic activities and correlates with higher malignancy of tumors [18–21,26-28]. The oncogenic phenotypes of HOXA11-AS include promoting cell proliferation, migration/invasion, epithelial–mesenchymal transition (EMT), cancer stemness [18–21,27,29]. So far, few studies have examined the status or activities of HOXA11-AS in non-cancerous diseases [30]. Following up on an earlier finding that HOXA11-AS is a keloid-specific lncRNA, here we showed that not only the expression of HOXA11-AS was significantly elevated in keloid tissues and HKFs, when compared to normal skin tissues and HSFs, respectively, but also was it functionally essential for inhibiting apoptosis of HKFs and stimulating HKF-induced angiogenesis. Furthermore, overexpressing HOXA11-AS in HSFs reduced the apoptosis and promoted HSF-mediated angiogenesis, both phenotypes critical for keloid formation [2]. This is the first study showing that altering HOXA11-AS level in one cell type (HSFs or HKFs) may act in trans and impact the biological behavior of a second cell type (in vitro tube formation of HUVECs in this study).
Studies on different human cancers reveal three major mechanisms by which HOXA11-AS functions. In gastric cancers, Liu et al. showed that by interacting with Staufen 1, HOXA11-AS targeted Krüppel-like Factor 2 mRNA to degradation [31]. By recruiting the chromatin modifier enhancer of zeste homolog 2, HOXA11-AS repressed the transcription of p21 in gastric cancer [31] and Uveal Melanoma [19], and that of Dual specificity protein phosphatase 5 in hepatocellular carcinoma [32]. Third, in most other studies, HOCA11-AS acted by sponging miRNAs, including miR-642b-3p [33], miR-124-3p [17,19,21,30], miR-125a-5p [16], miR-140-5p [18], miR-214-3p [20], miR-200b [34], and miR-1297 [35]. In this study, we showed that HOXA11-AS directly bound to miR-124-3p, suggesting HOXA11-AS may function as a sponge for miR-124-3p. Furthermore, we found that HOXA11-AS both necessarily and sufficiently controlled the endogenous miR-124-3p expression, while overexpressing or knocking down miR-124-3p failed to alter HOXA11-AS level in skin fibroblasts, suggesting that HOXA11-AS acts upstream and regulates the transcription of miR-124-3p. In addition to being the most abundant miRNA in the brain, studies have established the importance of miR-124-3p not only in the central nervous system [36], but also in the immune system, immune disorders [37], as well as in cancers [38,39]. In contrast, little is known on miR-124-3p in keloid formation. Our study showed that the level of miR-124-3p was down-regulated in keloid tissues or HKFs. Moreover, miR-124-3p was found to reverse cell apoptosis and fibroblast-induced angiogenesis mediated by HOXA11-AS, suggesting that miR-124-3p may be a key downstream target in HOXA11-AS-mediated keloid formation.
With minimal knowledge on miR-124-3p in keloid formation, we resorted to bioinformatic approach to identify potential targets of miR-124-3p that may convey the essential control on keloid formation; we identified and chose to focus on TGFβR1. The etiology and pathogenesis of keloid formation are not well understood. However, TGFβ has been considered as a key growth factor/cytokine for keloid formation. There are three TGFβ isoforms in mammals, TGFβ1 to 3. The signaling of all TGFβ isoforms starts with the binding of ligand to TGFβR2, followed by the recruitment and phosphorylation of TGFβR1, and the activation of downstream Smad-dependent and Smad-independent signaling cascades [40], the latter including different branches of PI3K/Akt pathways, Rho GTPase pathways, and mitogen-activated protein kinase (MAPK) pathways [41]. Altogether, TGFβ harness an intricate signaling network to carry out its biological activities in the context of different physiological or pathological paradigms. During wound healing, TGFβ signaling essentially controls fibroblast proliferation, mobility, production and deposition of ECM proteins, and angiogenesis [5,23]. Since TGFβR1 is required for mediating the signaling of all three isoforms of TGFβ, targeting its expression will have a significant impact on TGFβ signaling. In this study, we showed that miR-124-3p not only directly bound to 3ʹ UTR of TGFβR1 but also was necessary and sufficient to inhibit endogenous TGFβR1. HOXA11-AS, by down-regulating miR-124-3p, up-regulated TGFβR1, reduced the expression of pro-apoptotic Bax, increased the level of anti-apoptotic Bcl-2, and activated PI3K/Akt pathway, consistent with the anti-apoptosis phenotype observed in HOXA11-AS-overexpressing HSFs. In contrast, miR-124-3p abolished the changes on the signaling molecules or phenotypes induced by HOXA11-AS. Previous studies showed that miR-124-3p targeted Smad4 and Ras homology Growth-related in TGFβ signaling [42,43]. To our knowledge, this is the first study showing that TGFβR1 is a direct target of miR-124-3p. Considering the essential role of TGFβ signaling in keloid formation and its potential as therapeutic target, boosting miR-124-3p may provide a promising strategy to achieve the inactivation of TGFβ signaling and benefit the treatment of keloid disease. A potential caveat for this study is that the sample size (n = 20) for both normal skin tissues and keloid tissues was relatively small, and these samples were not well matched for the age or gender of individuals. Therefore, it is difficult to validate the correlations among HOXA11-AS, miR-124-3p, and TGFβR1, or to analyze whether the findings would remain significant among different age groups or patients of opposite genders. Future studies that include matching normal and keloid tissues from the same individuals or contain a much larger sample size will help to address these questions.
In summary, we identified the HOXA11-AS/miR-124-3p/TGFβR1 axis as a novel mechanism promoting keloid formation in skin fibroblasts. By targeting miR-124-3p, HOXA11-AS up-regulated TGFβR1, activated downstream Smad-independent PI3K/Akt signaling, inhibited the apoptosis of keloid fibroblasts, and stimulated fibroblast-induced angiogenesis. These findings support that targeting HOXA11-AS or resuming miR-124-3p may provide therapeutic benefits for keloid disease. Further studies should explore the effects of elevating miR-124-3p in in vivo keloid models and pre-clinical trials.
Author contributions
JJ, Conceptualization; ZHJ, Data curation; XHL, Formal analysis; JJ, Funding acquisition; HFZ, Investigation; JJ, Methodology; HFZ, Project administration; ZHJ, Resources; XHL, Software; HFZ, Supervision; JJ, Validation; ZHJ, Visualization; HFZ, Roles/Writing - original draft; JJ, Writing - review & editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Ethics statement
The study procedures on clinical specimens were approved by the Ethics Committee of Plastic surgery of Henan People’s Hospital (Henan, China) and written consents were acquired from all participants.
References
- [1].Mari W, Alsabri SGTabal N, et al. Novel insights on understanding of keloid scar: article review. J Am Coll Clin Wound Spec. 2016;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mogili NS, Krishnaswamy VR, Jayaraman M, et al. Altered angiogenic balance in keloids: a key to therapeutic intervention. Transl Res. 2012;159:182–189. [DOI] [PubMed] [Google Scholar]
- [3].Halim AS, Emami A, Salahshourifar I, et al. Keloid scarring: understanding the genetic basis, advances, and prospects. Arch Plast Surg. 2012;39:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jagadeesan J, Bayat A.. Transforming growth factor beta (TGFbeta) and keloid disease. Int J Surg. 2007;5:278–285. [DOI] [PubMed] [Google Scholar]
- [6].Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- [7].Unahabhokha T, Sucontphunt A, Nimmannit U, et al. Molecular signalings in keloid disease and current therapeutic approaches from natural based compounds. Pharm Biol. 2015;53:457–463. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Q, Kelly AP, Wang L, et al. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol. 2006;126:2607–2613. [DOI] [PubMed] [Google Scholar]
- [9].Bujor AM, Pannu J, Bu S, et al. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol. 2008;128:1906–1914. [DOI] [PubMed] [Google Scholar]
- [10].Si LB, Zhang MZ, Han Q, et al. Sensitization of keloid fibroblasts by quercetin through the PI3K/Akt pathway is dependent on regulation of HIF-1alpha. Am J Transl Res. 2018;10:4223–4234. [PMC free article] [PubMed] [Google Scholar]
- [11].An G, Liang S, Sheng C, et al. Upregulation of microRNA-205 suppresses vascular endothelial growth factor expression-mediated PI3K/Akt signaling transduction in human keloid fibroblasts. Exp Biol Med (Maywood). 2017;242:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [12].Zhu W, Wu X, Yang B, et al. miR-188-5p regulates proliferation and invasion via PI3K/Akt/MMP-2/9 signaling in keloids. Acta Biochim Biophys Sin (Shanghai). 2019;51:185–196. [DOI] [PubMed] [Google Scholar]
- [13].Dykes IM, Emanueli C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genomics Proteomics Bioinformatics. 2017;15:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sun XJ, Wang Q, Guo B, et al. Identification of skin-related lncRNAs as potential biomarkers that involved in Wnt pathways in keloids. Oncotarget. 2017;8:34236–34244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Andrews JP, Marttala J, Macarak E, et al. Keloids: the paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix Biol. 2016;51:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen D, Sun Q, Zhang L, et al. The lncRNA HOXA11-AS functions as a competing endogenous RNA to regulate PADI2 expression by sponging miR-125a-5p in liver metastasis of colorectal cancer. Oncotarget. 2017;8:70642–70652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cui M, Wang J, Li Q, et al. Long non-coding RNA HOXA11-AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p in osteosarcoma. Biomed Pharmacother. 2017;92:437–444. [DOI] [PubMed] [Google Scholar]
- [18].Cui Y, Yi L, Zhao JZ, et al. Long noncoding RNA HOXA11-AS functions as miRNA sponge to promote the glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol. 2017;36:822–828. [DOI] [PubMed] [Google Scholar]
- [19].Lu Q, Zhao N, Zha G, et al. LncRNA HOXA11-AS exerts oncogenic functions by repressing p21 and miR-124 in Uveal Melanoma. DNA Cell Biol. 2017;36:837–844. [DOI] [PubMed] [Google Scholar]
- [20].Xu C, He T, Li Z, et al. Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth, migration and invasion of glioma cells. Biomed Pharmacother. 2017;95:1504–1513. [DOI] [PubMed] [Google Scholar]
- [21].Yu W, Peng W, Jiang H, et al. LncRNA HOXA11-AS promotes proliferation and invasion by targeting miR-124 in human non-small cell lung cancer cells. Tumour Biol. 2017;39:1010428317721440. [DOI] [PubMed] [Google Scholar]
- [22].Rao X, Huang X, Zhou Z, et al. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- [23].Tang M, Bian W, Cheng L, et al. Ginsenoside Rg3 inhibits keloid fibroblast proliferation, angiogenesis and collagen synthesis in vitro via the TGFbeta/Smad and ERK signaling pathways. Int J Mol Med. 2018;41:1487–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kasibhatla S, Amarante-Mendes GP, Finucane D, et al. Analysis of DNA fragmentation using agarose gel electrophoresis. CSH Protoc. 2006;2006. [DOI] [PubMed] [Google Scholar]
- [25].Chen J, Fu Z, Ji C, et al. Systematic gene microarray analysis of the lncRNA expression profiles in human uterine cervix carcinoma. Biomed Pharmacother. 2015;72:83–90. [DOI] [PubMed] [Google Scholar]
- [26].Li T, Xu C, Cai B, et al. Expression and clinicopathological significance of the lncRNA HOXA11-AS in colorectal cancer. Oncol Lett. 2016;12:4155–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li W, Jia G, Qu Y, et al. Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer invasion and metastasis by regulating epithelial-mesenchymal transition. Med Sci Monit. 2017;23:3393–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang Q, Zhang J, Liu Y, et al. A novel cell cycle-associated lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett. 2016;373:251–259. [DOI] [PubMed] [Google Scholar]
- [29].Kim HJ, Eoh KJ, Kim LK, et al. The long noncoding RNA HOXA11 antisense induces tumor progression and stemness maintenance in cervical cancer. Oncotarget. 2016;7:83001–83016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang XN, Zhang LH, Cui XD, et al. lncRNA HOXA11-AS is involved in fracture healing through regulating mir-124-3p. Eur Rev Med Pharmacol Sci. 2017;21:4771–4776. [PubMed] [Google Scholar]
- [31].Liu Z, Chen Z, Fan R, et al. Over-expressed long noncoding RNA HOXA11-AS promotes cell cycle progression and metastasis in gastric cancer. Mol Cancer. 2017;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu B, Li J, Liu X, et al. Long non-coding RNA HOXA11-AS promotes the proliferation HCC cells by epigenetically silencing DUSP5. Oncotarget. 2017;8:109509–109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Y, Luo J, Wang X, et al. A comprehensive analysis of the predicted targets of miR-642b-3p associated with the long non-coding RNA HOXA11-AS in NSCLC cells. Oncol Lett. 2018;15:6147–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chen JH, Zhou LY, Xu S, et al. Overexpression of lncRNA HOXA11-AS promotes cell epithelial-mesenchymal transition by repressing miR-200b in non-small cell lung cancer. Cancer Cell Int. 2017;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun M, Nie F, Wang Y, et al. LncRNA HOXA11-AS promotes proliferation and invasion of gastric cancer by scaffolding the chromatin modification factors PRC2, LSD1, and DNMT1. Cancer Res. 2016;76:6299–6310. [DOI] [PubMed] [Google Scholar]
- [36].Sun Y, Luo ZM, Guo XM, et al. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci. 2015;9:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Qin Z, Wang PY, Su DF, et al. miRNA-124 in immune system and immune disorders. Front Immunol. 2016;7:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].He RQ, Yang X, Liang L, et al. MicroRNA-124-3p expression and its prospective functional pathways in hepatocellular carcinoma: A quantitative polymerase chain reaction, gene expression omnibus and bioinformatics study. Oncol Lett. 2018;15:5517–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang G, Chen L, Khan AA, et al. miRNA-124-3p/neuropilin-1(NRP-1) axis plays an important role in mediating glioblastoma growth and angiogenesis. Int J Cancer. 2018;143:635–644. [DOI] [PubMed] [Google Scholar]
- [40].Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang YE. Non-smad signaling pathways of the TGF-beta family. Cold Spring Harb Perspect Biol. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jun JH, Joo CK. MicroRNA-124 controls transforming growth factor beta1-induced epithelial-mesenchymal transition in the retinal pigment epithelium by targeting RHOG. Invest Ophthalmol Vis Sci. 2016;57:12–22. [DOI] [PubMed] [Google Scholar]
- [43].Zu L, Xue Y, Wang J, et al. The feedback loop between miR-124 and TGF-beta pathway plays a significant role in non-small cell lung cancer metastasis. Carcinogenesis. 2016;37:333–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


