Abstract
Biological invasions are largely considered to be a “numbers game”, wherein the larger the introduction effort, the greater the probability that an introduced population will become established. However, conditions during transport – an early stage of the invasion – can be particularly harsh, thereby greatly reducing the size of a population available to establish in a new region. Some successful non-indigenous species are more tolerant of environmental and anthropogenic stressors than related native species, possibly stemming from selection (ie survival of only pre-adapted individuals for particular environmental conditions) during the invasion process. By reviewing current literature concerning population genetics and consequences of selection on population fitness, we propose that selection acting on transported populations can facilitate local adaptation, which may result in a greater likelihood of invasion than predicted by propagule pressure alone. Specifically, we suggest that detailed surveys should be conducted to determine interactions between molecular mechanisms and demographic factors, given that current management strategies may underestimate invasion risk.
Biological invasions are largely considered to be a “numbers game”, in that the probability that a population will become established increases with the extent of the introduction effort (defined as both the number of individuals and the number of introduction events) (Simberloff 2009). This pattern occurs mainly because small populations are vulnerable and more prone to extinction than are larger ones due to demographic instability (eg Allee effects; Bock et al. 2015) and environmental stochasticity (Simberloff 2009). There has been little recognition, however, that the invasion process itself, and in particular the transport of propagules, can drive selection of pre-adapted genotypes. Pre-adapted populations that are small in size may have a much greater probability of successful establishment than the classic “numbers game” theory would predict, and may even have a probability of success similar to that of large, unselected populations.
Biological invasions are typically multistage processes consisting of transport, introduction, establishment, and spread, with invasion success or failure determined by multiple factors at each stage (Blackburn et al. 2011). The transport stage of this process can have especially severe consequences for potential invasive species (eg Seiden et al. 2011; Sobek et al. 2011). Consequently, the number of individuals released into the recipient habitat might be much smaller than the number initially loaded and transported (Briski et al. 2013, 2014; Chan et al. 2015). Similar to the development of bacterial antibiotic resistance associated with overuse of clinical drugs (Sandegren 2014), a population subjected to harsh conditions during transport may contain adapted, or highly fit, survivors from the original complement. Thus, in contrast to the classical view, which stressed the importance of maintaining genetic variation in small populations (Dobzhansky 1970), transport of a non-indigenous species (NIS) to a new habitat may select for few but well-adapted individuals (genotypes) with a high probability of establishment. Rapid adaptation is recognized as an important component of successful invasions (Colautti and Lau 2015), but invasion-related adaptations have generally been observed only after successful establishment and spread, and rarely have evolutionary processes been attributed to earlier, pre-introduction stages of invasion. Although selection may occur at any stage of the invasion process, overlooking the importance of evolution and adaptation during the first stage – transport – may result in underestimates of invasion risk and inefficient management strategies. To illustrate our case, we review current literature concerning population genetics of biological invasions, describe the consequence of selection during transport on population fitness, construct a conceptual model of “human-mediated selection” using the stage-based invasion framework (Blackburn et al. 2011), and support our model by referring to current studies.
Is colonization simply a numbers game?
Williamson (1996) coined the term “propagule pressure” to predict species invasiveness, and this concept has since become common in the invasion ecology literature (cited in 1271 papers as of October 2017, based on a Web of Science search). Meta-analyses have confirmed propagule pressure as a strong determinant of invasion success (Hayes and Barry 2008). Its popularity stems from its multidimensional nature, the main components of which include the number of individuals introduced (ie propagule size) and the number of introduction events (ie propagule number) (Lockwood et al. 2005). The likelihood of overcoming random fluctuations in population growth rate, where mortality exceeds reproduction, increases with larger population size; at the same time, a larger number of introduction events may allow demographic rescue of previously introduced individuals and provide multiple colonization opportunities in the face of fluctuating environmental conditions in the receiving habitat (Lockwood et al. 2005; Simberloff 2009).
Lockwood et al. (2009) used a simulated community with a log-series species–abundance distribution to demonstrate that mean propagule pressure (ie the average abundance of any single species) rises as the total number of individuals released into a habitat increases. This finding, while not unexpected, holds important implications for management because it implies that invasion risk can be diminished simply by reducing the abundance of propagules introduced to any ecosystem. The finding also has parallels to genetic diversity of introduced populations, in that larger inocula (ie number of individuals released into a habitat) are more likely to incorporate more of the allelic diversity in the source population than are smaller ones, possibly enhancing establishment success (Dlugosch and Parker 2008; Bock et al. 2015).
Traditional perspectives consider high genetic variation to be crucial for selection and rapid adaptation to novel environments during invasions, as the loss of genetic variation can lead to many disadvantages in introduced populations, such as inbreeding depression, increased fixation of deleterious alleles through genetic drift, and reduced evolutionary potential to respond to novel pressures (Schrieber and Lachmuth 2017). Reductions in propagule pressure are thus expected to be associated with founder effects and subsequent loss of genetic diversity. Indeed, multiple lines of evidence have demonstrated that many traits of introduced populations can be determined by genetic variation and can rapidly evolve in response to selection pressures experienced during the invasion process (Dlugosch et al. 2015). As a result, selection acting under changing environmental conditions during invasion can lead to traits facilitating local adaptation among populations when these traits are heritable and/or affect fitness (Shimada et al. 2011). Such selection based on genetic variation is widely considered to be one of the major mechanisms responsible for invasion success (Bock et al. 2015; Colautti and Lau 2015; Dlugosch et al. 2015).
Not all evidence supports the view that reduced genetic diversity necessarily impairs invasion success; many studies have found very limited or even no genetic variation in established populations of NIS when neutral genomic regions were explored (Roman and Darling 2007), and have often failed to detect evidence of strong selection derived from recent invasions (Dlugosch and Parker 2008). Therefore, detailed surveys should be conducted to confirm the lack of genetic variation using state-of-the-art techniques, such as high-throughput sequencing, particularly for functional genes that respond to key environmental factors (eg Pu and Zhan 2017). At present, all evidence suggests that the mechanisms of invasion can be complex and that selection based on genetic variation is not the only determinant of invasion success (eg Huang et al. 2017).
Recent studies have also revealed that epigenetic variation (see description below) may be more important than genetic diversity within populations, particularly for traits expressed under changing environments (Hu and Barrett 2017; Huang et al. 2017; Pu and Zhan 2017). Epigenetic variation involves differential gene expression, allowing individual phenotypes to vary in response to environmental stress (see Hu and Barrett 2017); for example, successful invasion of diverse habitats was correlated with epigenetic differentiation in response to new and dynamic microclimate conditions (Richards et al. 2012). Epigenetic variation may function in populations in the absence of genetic diversity, and could compensate for low genetic diversity during invasions (Ardura et al. 2017).
As such, there are reasons to believe that propagule composition, including genetic variation and epigenetic potential, may be as important as the numbers game in determining invasion success. Given that the mechanisms of invasion success are extremely complex, genetic and epigenetic variation, as well as their interactions, may enable organisms to adjust their phenotypes to survive novel and/or rapidly changing environments by providing the necessary material for natural selection and adaptation, in many cases despite low propagule pressure (Huang et al. 2017; Lin et al. 2017). These considerations do not, however, exhaust the possible mechanisms by which invasive species may escape the assumed negative consequences of reduced propagule size. Indeed, introduced populations may retain high invasive potential despite low propagule pressure and low genetic diversity.
The stage-based invasion framework: “human-mediated selection”
The null model of the transport stage of invasion assumes reductions in propagule pressure associated with both entrainment in the transport vector (ie a random subsampling of the native population) and decline in population size due to random mortality during transport (Figure 1). The outcome of this model is a population (represented by population “1C” in Figure 1) poorly suited for invasion success, given that both propagule pressure and genetic diversity are low.
Figure 1.
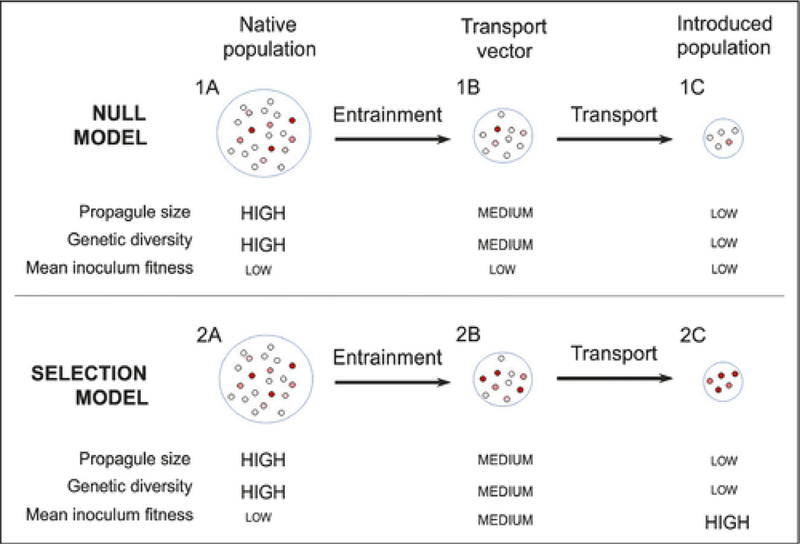
A simplified, stage-based framework of selection during the transport stage of the invasion process. Small circles represent individual genotypes; red color indicates high fitness for the transport stage and recipient environment.
An alternative model, referred to here as the selection model, assumes similar reductions in population size upon entrainment and transport, and similar associated reductions in genetic diversity. However, this model makes two key assumptions that differ from the null model: (1) that population reduction during entrainment and transport are non-random with respect to genotype (ie that they are under selection) and (2) that the selective pressures driving those population reductions are similar to the selective pressures that introduced populations will experience in the recipient environment (Figure 1). Under the selection model, strong selection results in non-random survival of genotypes in transport; propagule size sharply declines, but the surviving population has elevated fitness. The introduced population of the null model (“1C” in Figure 1) has a very small probability of establishment owing to low propagule size, with few if any released individuals pre-adapted to the new habitat. Conversely, the population introduced in the selection model (“2C” in Figure 1) has high probability of establishment success owing to selection for genotypes pre-adapted to the recipient environment. In other words, if the assumptions of the selection model hold, then one would expect the risk of invasion posed by introduced populations (“2C”) to be considerably higher than those posed under the null model (“1C”), even though propagule pressure and overall genetic diversity are similar in both cases.
We acknowledge that the simplified model presented in Figure 1 does not encompass all possible scenarios; for instance, multiple introductions from the same and/or distinct regions that might increase establishment probability – due to increased genetic variability and/or admixture among different populations or closely related species – are not included in the model. In addition, we assume that there is no in-situ reproduction during transport. We also recognize that the basic assumptions of the selection model may not hold true in many cases. Any invasion stage that changes the mean inoculum fitness for the respective environment functionally becomes a selective filter, and sequential selective filters may interact with one another, structuring the gene pool available for later stages. Contrary to our example, environmental mismatch of selective filters at different stages may impair invasion success. The transport stage may create a unique selective environment compared to later invasion stages. Due to spatial constraints, physical conditions during the transport stage may be less patchy as compared to the conditions of the recipient environment along various gradients, offering less diverse refugia from stressors. Furthermore, biotic interactions, like predation, competition, or facilitation, may differ between the transport stage and the later stages of invasion due to distinct species assemblages. Moreover, short duration of the transport stage may favor survival, whereas a longer duration of the transport stage and/or the application of management practices to decrease invasion risk may create especially harsh and selective environments (eg Seiden et al. 2011; Sobek et al. 2011; Chan et al. 2015). High epigenetic potential of the inoculum may mitigate the strength of natural selection during the invasion process (eg Huang et al. 2017), which might be particularly important for harsh and abrupt environmental changes and enable propagules to survive otherwise potentially lethal stress levels (Huang et al. 2017). At present we lack knowledge about both genetic and epigenetic processes occurring between the uptake and release of propagules; nevertheless, we believe that under certain circumstances our simplified model reasonably approximates transport dynamics, and that both assumptions of the selection model are likely to hold true.
Evidence for selection during transport
Entrainment (association with a transport vector, such as ships’ ballast tanks) is the first step in the translocation of introduced species and the first opportunity for selection to drive populations toward increased invasiveness. In the case of the intentional transfer of organisms, individuals are usually artificially selected for introduction; for example, historical acclimatization societies, in the course of importing species from their home countries to colonies, introduced healthy and strong individuals (WebTable 1; Lever 1992). Agriculture, aquaculture, horticulture, and pet trade introductions predominantly include populations and strains of species that have already been adapted to human-altered habitats for decades or even centuries (WebTable 1). However, introductions of intentionally selected organisms are not the focus of our selection model, which focuses instead on unintentional introductions resulting in unintentional selection. The unintentional sampling of a native population via entrainment in a transport vector may be more common for native populations that have been pre-adapted to human-altered habitats, and are thus more likely to succeed in similarly altered recipient habitats. Such “anthropogenically induced adaptation to invade” (AIAI) has been cited as an important phenomenon in driving global invasions (Hufbauer et al. 2012). Generally, AIAI is likely operative in many intentional and accidental introductions, despite a paucity of direct empirical evidence; for instance, little fire ant (Wasmannia auropunctata) and Asian green mussel (Perna viridis) populations inhabiting human-altered habitats are more tolerant of hot/dry conditions and low-oxygen environments, respectively, than those from natural habitats, and non-indigenous birds prefer urbanized habitats (WebTable 2; Foucaud et al. 2013; Huhn et al. 2016; Sol et al. 2017). Of greater relevance to our model is the more specific phenomenon of an entrainment event selectively sampling individuals from the native population that are likely to exhibit higher capacity to survive transport or establish upon arrival. Although we are not aware of direct evidence for this phenomenon, plausible mechanisms do exist. For example, limited residence times of vessels in harbors predict that individuals with a propensity to early successional fouling are more likely to be transported and subsequently better adapted to take advantage of open niches in recipient habitats. The frequency with which early successional fouling species are found as components of non-indigenous biota suggests selective pressures on hull fouling taxa that may be operative at the population level as well (Berntsson and Jonsson 2003; Chapman et al. 2013). Similar non-random entrainment of invertebrate taxa in the ballast tanks of ships has also been observed (Briski et al. 2012). In the terrestrial context, the importance of seed contaminants as vectors of accidental plant introductions suggests that selection for traits likely to enhance entrainment (eg weediness in managed crop fields) may result in the transport of individuals with a propensity to invade (Lehan et al. 2013).
Evidence indicates that transport can impose selective pressures on entrained populations that may be similar to those experienced in the recipient habitat. Unfortunately, much of this evidence is circumstantial or anecdotal, suggesting that considerably more research is required to assess the importance of this phenomenon in determining invasion success. In one case, an extensive body of research indicates that selective pressures imposed during transport by vessels (eg those imposed by heavy metal coatings aimed at reducing fouling) not only result in adaptation of some fouling species but may also contribute to the dominance of those taxa in impaired recipient environments as well (Figure 2). In other cases, despite a lack of direct evidence for selection during transport, stresses associated with transport clearly offer opportunities for selection to act in ways relevant to invasion success (WebTable 2). Examples range broadly across both aquatic and terrestrial vectors of introduction, suggesting that the potential for this phenomenon to impact invasion risk may be considerable. Of particular importance is the fact that some of the stressors potentially associated with selection during transport are imposed intentionally for the purposes of reducing risk of invasion.
Figure 2.
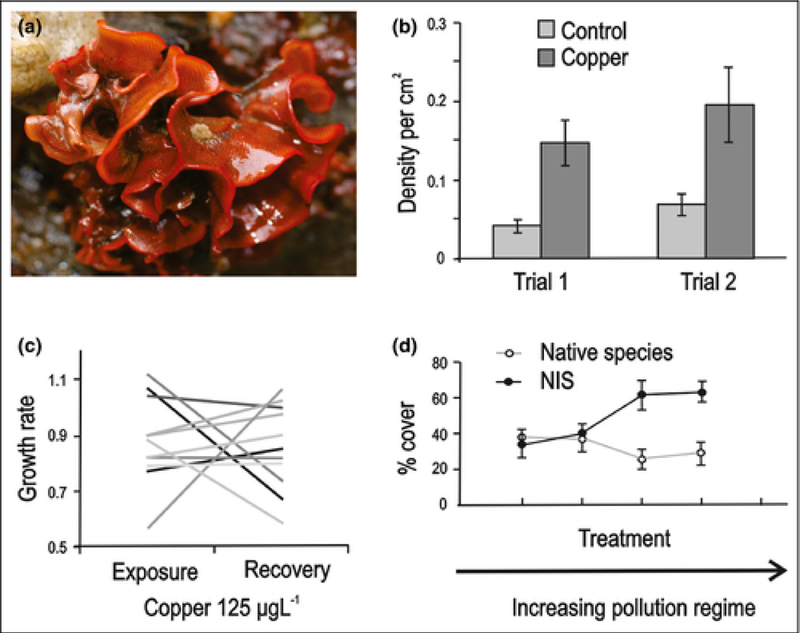
(a) Watersipora subtorquata, a global invader spread by hull fouling. (b) W subtorquata settles at high density on copper-treated plates. (c) Reaction norms (each line represents a different W subtorquata genotype) indicating heritability of response to copper stress. (d) Non-indigenous species (NIS) are more dominant in more polluted recipient environments. Error bars in (b) and (d) represent standard errors. Adapted from McKenzie et al. (2012a,b) and Piola and Johnston (2008) with permission from John Wiley & Sons and LA Solórzano. LA Solórzano.
Substantial reductions in the propagule size of plankton often occur in ships’ ballast water during transport (Figure 3; WebTable 2; Briski et al. 2014; Chan et al. 2015), suggesting that species may be exposed to high selection pressures as a consequence of changes in temperature, salinity, and dissolved oxygen levels, and exposure to epoxies, rust, and metal-based paints, among other factors (eg Seiden et al. 2011). Such declines in propagule size are therefore probably non-random, with transport potentially selecting for individuals tolerant of harsh conditions, and these individuals may have a higher chance of establishment once they are discharged into human-altered habitats, including harbors. Likewise, pre-exposure to elevated temperatures or slow heating during phytosanitary heat treatment of wood products can induce heat shock responses in wood-boring insects, such as the highly invasive emerald ash borer (Agrilus planipennis), allowing individuals to survive otherwise lethal temperatures (Figure 3; WebTable 2; Sobek et al. 2011). Overland transport of fanwort (Cabomba caroliniana) via boat trailers may impose selection pressures for desiccation tolerance on transported populations (Figure 3; Barnes et al. 2013; Bickel 2014). Hydrodynamic conditions experienced by fouling organisms on the hulls of ships may favor individuals with high attachment strength and/or a low drag coefficient, both of which promote the introduction of non-indigenous populations into novel ecosystems (WebTable 2; Clarke Murray et al. 2012).
Figure 3.
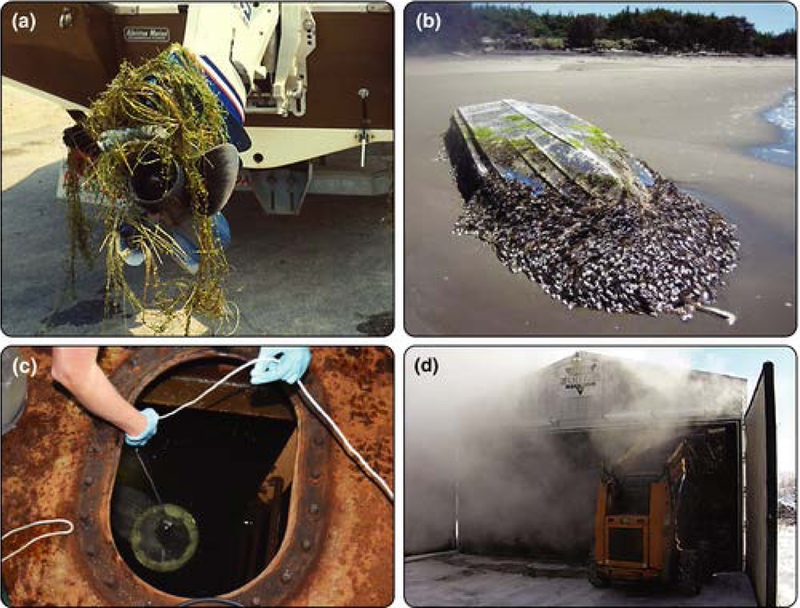
Examples of stressors associated with transport that could potentially drive selection for invasive traits. (a) Aquatic weeds are frequently transported long distances overland, and selection for desiccation tolerance could result in populations more prone to survive future spread. (b) Distance-rafting on long-lived anthropogenic materials (eg this fiberglass boat washed ashore in North America years after being swept out to sea from Japan in the 2011 tsunami) may select for tolerance to food scarcity, acute changes in salinity, exposure to ultraviolet light, or other stressors. (c) Water in ballast tanks may be subjected to extreme changes in temperature, salinity, and dissolved oxygen levels, and may be exposed to metal-based paints, epoxies, and rust (among other factors) – all of which have been shown to exert strong selective pressures at the species level. Even stressors intentionally applied to prevent invasions (ie physical or chemical treatments) could paradoxically lead to adaptations favoring invasion success. (d) Phytosanitary heat treatment of wood pallets can lead to selection for heat shock tolerance in introduced insects (see main text for more details). K Brown and U of Florida (IFAS) A Pleus and WDFW Fisheries and Oceans Canada USDA Forest Service Forest Products Laboratory
Thus, we argue that sufficient evidence exists that the conditions required to satisfy the selection model do occur under certain circumstances, and we propose that in such cases introduced populations possibly exhibit far greater risk of establishment and invasion success than would be predicted by the null model. Furthermore, the fact that successful NIS tend to be more tolerant of environmental and anthropogenic stressors than related native species could be a consequence of selection during the invasion process (Dafforn et al. 2011). The case of the selection model is therefore of special concern.
Even short-term selection matters
Due to severe population bottlenecks during invasion events, some genetic variation is typically lost (Dlugosch and Parker 2008), which may lead to inbreeding depression, loss of evolutionary potential and, ultimately, colonization failure (Keller and Waller 2002). However, in the population of our selection model (“2C” in Figure 1), reductions in genetic variation are a consequence of adaptation, and may in fact have positive outcomes in terms of invasiveness. In cases of strong selection, the genetic composition of the introduced population deviates from the population initially taken up by the transport vector (Figure 4; Falconer and MacKay 1996). Subsequent random mating would occur between individuals with higher mean fitness than those in the pre-transport population, as low fitness individuals have been previously lost due to selection. If the environment in the recipient ecosystem is similar to that experienced during transport, the mean fitness of offspring (ie the F1 generation) will be greater than that of the parental generation due to directional selection (for a description, see Figure 4; Falconer and MacKay 1996). The implication is that population “2C” in Figure 1 may not only possess higher capacity to invade than population “1C”, but in certain cases may even represent higher risk than population “2A”, directly contradicting the presumed importance of propagule pressure under the assumptions of the null model. Given the dynamics of transport, selection is likely to be imposed on a single or very few generations. However, previous studies have shown that even single-generation selection can have pronounced evolutionary effects in strongly selective environments (Christie et al. 2016), lending further support for the potential importance of the selection model in promoting invasion risk. This is particularly noteworthy given that reproduction during transport, though possible (eg bacteria in ballast tanks; Seiden et al. 2011), is likely rare for most vectors and taxa.
Figure 4.
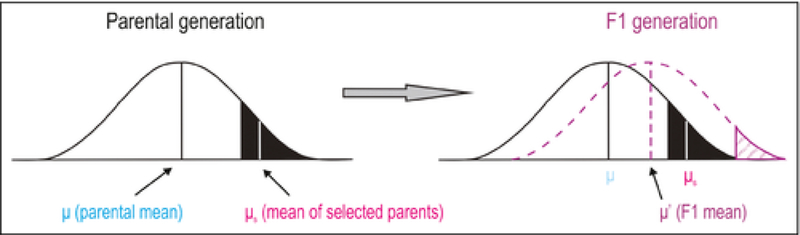
Directional selection on quantitative traits. The left side of the panel illustrates the impact of selection within the parental generation, with the parental mean trait value shifting to the right (from μ to μs). Those selected parents will produce offspring with a mean trait value (μ’) shifted right relative to that of the offspring of unselected parents. The first generation (F1) is represented by the dashed line in the right side of the panel; the dotted area at the far-right tail denotes “new” phenotypic variability available as a result of early selection. Adapted from Falconer and MacKay (1996).
This phenomenon may also be driven by interactions between molecular mechanisms and demographic factors. Such interactions can be critical in determining colonization success, and their importance may depend on the degree to which environmental conditions in recipient habitats impose adaptive challenges on introduced populations (Szucs et al. 2014). Although strong population bottlenecks created during the transport stage may result in loss of genetic diversity, if such loss is the consequence of responses to natural selection, then that loss may not impair an introduced population’s ability to successfully colonize new habitats. Estoup et al. (2016) recognized this scenario as a case of “spurious genetic paradox”, in which true loss of genetic diversity nevertheless fails to predict reductions in invasion success.
If selective pressures in the transport and recipient environments are similar, selection during transport may result in populations with relatively high fitness once released into the recipient system, thus providing a genetic background against which subsequent demographic factors may act to facilitate successful establishment and spread. Drake (2006) has demonstrated that fitness advantages during early stages of post-establishment population growth can have a “catapult effect” on the demographic trajectory of colonizing populations. In effect, early-stage fitness can buffer populations against the risks of immediate extinction associated with demographic stochasticity. Drake (2006) explored this phenomenon in the context of heterosis (“hybrid vigor”), revealing that hybridization between multiple parental strains increases the likelihood of establishment by providing an ephemeral fitness increase and conferring greater likelihood of populations persisting through periods of small population size. Populations pre-adapted to recipient environments through selection during transport would similarly be expected to exhibit early safeguards against premature extinction.
An additional benefit of this interplay of genetic and demographic factors could be prevention of further erosion of genetic diversity. Loss of genetic variation associated with founder effects depends not only on the severity of a population bottleneck (reflected in effective population size) but also on the duration of the bottleneck. Populations that rapidly pass through periods of small population size and subsequently experience rapid population growth are likely to maintain substantial proportions of their original variation (Swaegers et al. 2013). Pre-adaptation to selective regimes imposed early after introduction could therefore set a population on a path not only toward early establishment success, but also toward future capacity to adapt to novel environments during range expansion.
Recommendations for future in-situ studies and management
Knowledge about the relationships between population size, genetic variation, and fitness is important for understanding invasion success. Studies that explore in-situ selection during transport, as well as laboratory selection experiments, are therefore needed to explore whether loss of genetic diversity of introduced populations is non-random and whether the resulting population is better suited or less suited to its new environment. Transport vectors, such as ballast water, are ideal systems for characterizing this relationship, as they typically contain abundant and diverse taxa that experience different population dynamics during transport. In addition, recent tsunami-driven rafting objects would be of particular interest; the long-distance transport of almost 300 different species over several years from Japan to Hawaii and the west coast of North America following the 2011 Tōhoku earthquake off the northeastern coast of Japan (Figure 3; Carlton et al. 2017) has likely resulted in numerous opportunities for selection to act on introduced populations. The most direct evidence for selection during transport would come from molecular studies of populations of species in different taxonomic groups that are collected at both the beginning and end of a transport event, which would be an extremely challenging undertaking in most contexts. However, laboratory selection experiments that assess adaptation potential of diverse taxa (such as that described in Panel 1) could also be particularly valuable (Lee and Petersen 2003; Krause et al. 2017). All such studies will be greatly aided by the increasing accessibility of state-of-the-art techniques for studying genomic architecture, such as high-throughput sequencing to identify restriction-site-associated DNA (RAD) that can be used to determine changes in diversity in neutral and non-neutral genes.
Panel 1. Demonstrating selection during transport: the case of hull fouling
A clear demonstration of selection during transport requires multiple lines of evidence: an introduced population must exhibit increased tolerance to a stressor associated with transport, that tolerance must reflect an adaptive response (ie it must be heritable), and must ultimately contribute to increased success of the introduced population in the recipient environment. To the best of our knowledge, these criteria have yet to be fully met in any system, but the case of hull fouling organisms comes very close. Specifically, the encrusting bryozoan Watersipora subtorquata (Figure 2a) shows evidence of being able to adapt to the copper-based paints used to prevent fouling on ocean-going vessels. W subtorquata is one of several fouling organisms that have achieved widespread invasive status, stimulating research to understand the basis of its success. Experimental settling studies have shown that invasive W subtorquata settles at high densities on plates treated with copper (Figure 2b), revealing a strong positive effect of heavy metal contamination on recruitment (McKenzie et al. 2012a). Additional experiments revealed substantial interactions between W subtorquata genotypes and copper-contaminated environments (Figure 2c), with considerable variation in copper tolerance and recovery after copper exposure, suggesting great potential for adaptation to heavy metal stress (McKenzie et al. 2012b). Broader studies have demonstrated strong correlations between the dominance of non-indigenous species (NIS) like W subtorquata and pollution regimes (Figure 2d), suggesting that selection for tolerance to heavy metals and other pollutants could play an important role in determining the success of introduced species and in shaping biotic communities in human-impacted environments (Piola and Johnston 2008).
Preventative actions like import health standards, quarantine periods, and cleaning measures have been identified as the most cost-effective and productive ways of managing biological invasions (Hewitt and Campbell 2007). Although the idea that “fewer is better” is generally sound, selection during transport as described above, and particularly if further demonstrated by experimental and empirical studies, indicates that current management strategies may be improved by considering more than just the numbers game. As such, when developing or evaluating different management strategies, it will be important to examine not just the number of viable individuals but also the composition of the propagules (including fitness). A single-minded focus on the numbers game may result in an underestimation of invasion risk. In this context it may also be critical to assess the effectiveness of methods used to reduce propagule pressure during transport. While these measures are undoubtedly valuable in reducing overall invasion risk, the possibility of adaptive responses by some populations in transport may be cause for concern. In at least two of the cases discussed above, procedures adopted to prevent invasion have been shown (even if indirectly) to select for individuals with traits that potentially enhance invasiveness (McKenzie et al. 2011, 2012b; Sobek et al. 2011). In evaluating the efficacy of such procedures (anti-fouling coatings, ballast water treatment, phytosanitary approaches, etc), managers should consider the hidden risks posed by selection during transport; this will provide opportunities to conduct further research to elucidate the potential importance of this phenomenon in determining the likelihood of invasion.
Supplementary Material
In a nutshell:
The movement of species as a result of anthropogenic activity (biological invasions) is one of the main threats to biodiversity, and is largely the result of globalization and rapid increases in global trade and travel
Current management strategies for the prevention of new invasions focus primarily on reducing the number of individuals introduced into new habitats and ignore the structure of the introduced populations
We propose that selection (survival of only pre-adapted individuals for particular environmental conditions) during transport can facilitate local adaptation, which may result in greater likelihood of invasion success than predicted solely based on the number of introduced individuals
We argue that selection during transport requires further exploration and possible consideration in management strategies
Acknowledgements
Financial support was provided by: the Alexander von Humboldt Sofja Kovalevskaja Award (to EB), which financed the June 2016 workshop “Selection during transport and introduction stages of the invasion process”; Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery and Canada Research Chair grants (to HJM); an NSERC Discovery Grant (to SAB); an NSERC Visiting Fellowship at Fisheries and Oceans Canada (to FTC); the Estonian Ministry of Education and Research (IUT02-20) (to VL); and the National Natural Science Foundation of China (31622011) (to AZ). The US Environmental Protection Agency, through its Office of Research and Development, supported the work described here. Though it has been subjected to Agency administrative review and approved for publication, its content does not necessarily reflect official Agency policy.
References
- Ardura A, Zaiko A, Morán P, et al. 2017. Epigenetic signatures of invasive status in populations of marine invertebrates. Sci Rep-UK 7: 42193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MA, Jerde CL, Keller D, et al. 2013. Viability of aquatic plant fragments following desiccation. Invas Plant Sci Mana 6: 320–25. [Google Scholar]
- Berntsson KM and Jonsson PR. 2003. Temporal and spatial patterns in recruitment and succession of a temperate marine fouling assemblage: a comparison of static panels and boat hulls during the boating season. Biofouling 19: 187–95. [DOI] [PubMed] [Google Scholar]
- Bickel TO. 2014. A boat hitchhiker’s guide to survival: Cabomba caroliniana desiccation resistance and survival ability. Hydrobiologia 746: 123–34. [Google Scholar]
- Blackburn TM, Pyšek P, Bacher S, et al. 2011. A proposed unified framework for biological invasions. Trends Ecol Evol 26: 333–39. [DOI] [PubMed] [Google Scholar]
- Bock D, Caseys C, Cousens RD, et al. 2015. What we still do not know about invasion genetics. Mol Ecol 24: 2277–97. [DOI] [PubMed] [Google Scholar]
- Briski E, Bailey SA, Casas-Monroy O, et al. 2012. Relationship between propagule pressure and colonization pressure in invasion ecology: a test with ships’ ballast. P Roy Soc B-Biol Sci 279: 2990–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski E, Bailey SA, Casas-Monroy O, et al. 2013. Taxon- and vector- specific variation in species richness and abundance during the transport stage of biological invasions. Limnol Oceanogr 58: 1361–72. [Google Scholar]
- Briski E, Chan F, MacIsaac HJ, and Bailey SA. 2014. A conceptual model of community dynamics during the transport stage of the invasion process: a case study of ships’ ballast. Divers Distrib 20: 236–44. [Google Scholar]
- Carlton JT, Chapman JW, Geller JB, et al. 2017. Tsunami-driven rafting: transoceanic species dispersal and implications for marine biogeography. Science 357: 1402–06. [DOI] [PubMed] [Google Scholar]
- Chan FT, Bradie J, Briski E, et al. 2015. Assessing introduction risk using species’ rank-abundance distributions. P Roy Soc B-Biol Sci 282: 20141517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Breitenstein R, and Carlton J. 2013. Port-by-port accumulations and dispersal of hull fouling invertebrates between the Mediterranean Sea, the Atlantic Ocean and the Pacific Ocean. Aquat Invasions 8: 249–60. [Google Scholar]
- Christie MR, Marine ML, Fox SE, et al. 2016. A single generation of domestication heritably alters the expression of hundreds of genes. Nat Commun 7: 10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke Murray C, Therriault TW, and Martone PT. 2012. Adapted for invasion? Comparing attachment, drag and dislodgment of native and nonindigenous hull fouling species. Biol Invasions 14: 1651–63. [Google Scholar]
- Colautti RI and Lau JA. 2015. Contemporary evolution during invasion: evidence for differentiation, natural selection, and local adaptation. Mol Ecol 24: 1999–2017. [DOI] [PubMed] [Google Scholar]
- Dafforn KA, Lewis JA, and Johnstone EL. 2011. Antifouling strategies: history and regulation, ecological impacts and mitigation. Mar Pollut Bul 62: 453–65. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM and Parker IM. 2008. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17: 431–49. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Anderson SR, Braasch J, et al. 2015. The devil is in the details: genetic variation in introduced populations and its contributions to invasion. Mol Ecol 24: 2095–111. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T 1970. Genetics of the evolutionary process New York, NY: Columbia University Press. [Google Scholar]
- Drake JM. 2006. Heterosis, the catapult effect and establishment success of a colonizing bird. Biol Lett 2: 304–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estoup A, Ravigné V, Hufbauer R, et al. 2016. Is there a genetic para- dox of biological invasion? Annu Rev Ecol Evol S 47: 51–72. [Google Scholar]
- Falconer DS and MacKay TFC. 1996. Introduction to quantitative genetics. London, UK: Pearson. [Google Scholar]
- Foucaud J, Rey O, Robert S, et al. 2013. Thermotolerance adaptation to human-modified habitats occurs in the native range of the invasive ant Wasmannia auropunctata before long-distance dispersal. Evol Appl 6: 721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KR and Barry SC. 2008. Are there any consistent predictors of invasion success? Biol Invasions 10: 483–506. [Google Scholar]
- Hewitt CL and Campbell ML. 2007. Mechanisms for the prevention of marine bioinvasions for better biosecurity. Mar Pollut Bull 55: 395–401. [DOI] [PubMed] [Google Scholar]
- Hu J and Barrett RDH. 2017. Epigenetics in natural animal populations. J Evol Biol 30: 1612–32. [DOI] [PubMed] [Google Scholar]
- Huang X, Li S, Ni P, et al. 2017. Rapid response to changing environ- ments during biological invasions: DNA methylation perspectives. Mol Ecol; 10.1111/mec.14382. [DOI] [PubMed] [Google Scholar]
- Hufbauer RA, Facon B, Ravigné V, et al. 2012. Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote inva- sions. Evol Appl 5: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn M, Hattich GS, Zamani NP, et al. 2016. Tolerance to stress dif- fers between Asian green mussels Perna viridis from the impacted Jakarta Bay and from natural habitats along the coast of West Java. Mar Pollut Bull 110: 757–66. [DOI] [PubMed] [Google Scholar]
- Keller LF and Waller DM. 2002. Inbreeding effects in wild popula- tions. Trends Ecol Evol 17: 230–41. [Google Scholar]
- Krause KE, Dinh KV, and Nielsen TG. 2017. Increased tolerance to oil exposure by the cosmopolitan marine copepod Acartia tonsa. Sci Total Environ 607/608: 87–94. [DOI] [PubMed] [Google Scholar]
- Lee CE and Petersen CH. 2003. Effects of developmental acclimation on adult salinity tolerance in the freshwater-invading copepod Eurytemora affinis. Physiol Biochem Zool 76: 296–301. [DOI] [PubMed] [Google Scholar]
- Lehan NE, Murphy JR, Thorburn LP, and Bradley BA. 2013. Accidental introductions are an important source of invasive plants in the continental United States. Am J Bot 100: 1287–93. [DOI] [PubMed] [Google Scholar]
- Lever C 1992. They dined on eland: the story of acclimatization socie- ties. London, UK: Quiller Press. [Google Scholar]
- Lin Y, Chen Y, Yi C, et al. 2017. Genetic signatures of natural selection in a model invasive ascidian. Sci Rep-UK 7: 44080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood JL, Cassey P, and Blackburn TM. 2005. The role of prop- agule pressure in explaining species invasions. Trends Ecol Evol 20: 223–28. [DOI] [PubMed] [Google Scholar]
- Lockwood JL, Cassey P, and Blackburn TM. 2009. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib 15: 904–10. [Google Scholar]
- McKenzie L, Brooks R, and Johnston EL. 2011. Heritable pollution tolerance in a marine invader. Environ Res 111: 926–32. [DOI] [PubMed] [Google Scholar]
- McKenzie LA, Johnston EL, and Brooks R. 2012a. Using clones and copper to resolve the genetic architecture of metal tolerance in a marine invader. Ecol Evol 2: 1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie LA, Brooks RC, and Johnston EL. 2012b. A widespread contaminant enhances invasion success of a marine invader. J Appl Ecol 49: 767–33. [Google Scholar]
- Piola RF and Johnston EL. 2008. Pollution reduces native diversity and increases invader dominance in marine hard-substrate com- munities. Divers Distrib 14: 329–42. [Google Scholar]
- Pu C and Zhan A. 2017. Epigenetic divergence of key genes associated with water temperature and salinity in a highly invasive model ascidian. Biol Invasions 19: 2015–28. [Google Scholar]
- Richards CL, Schrey AW, and Pigliucci M. 2012. Invasion of diverse habitats by few Japanese knotweed genotypes is correlated with epigenetic differentiation. Ecol Lett 15: 1016–25. [DOI] [PubMed] [Google Scholar]
- Roman J and Darling JA. 2007. Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22: 454–64. [DOI] [PubMed] [Google Scholar]
- Sandegren L 2014. Selection of antibiotic resistance at very low anti- biotic concentrations. Upsala J Med Sci 119: 103–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrieber K and Lachmuth S. 2017. The genetic paradox of invasions revisited: the potential role of inbreeding × environment interactions in invasion success. Biol Rev 92: 939–52. [DOI] [PubMed] [Google Scholar]
- Seiden JM, Way CJ, and Rivkin RB. 2011. Bacterial dynamics in ballast water during trans-oceanic voyages of bulk carriers: environ- mental controls. Mar Ecol-Prog Ser 436: 145–59. [Google Scholar]
- Shimada Y, Shikano T, and Merilä J. 2011. A high incidence of selection on physiologically important genes in the threespined stickleback, Gasterosteus aculeatus. Mol Biol Evol 28: 181–93. [DOI] [PubMed] [Google Scholar]
- Simberloff D 2009. The role of propagule pressure in biological inva- sions. Annu Rev Ecol Evol S 40: 81–102. [Google Scholar]
- Sobek S, Rajamohan A, Dillon D, et al. 2011. High temperature tolerance and thermal plasticity in emerald ash borer Agrilus planipen- nis. Agric Forest Entomol 13: 333–40. [Google Scholar]
- Sol D, González-Lagos C, Lapiedra O, et al. 2017. Why are exotic birds so successful in urbanized environments? In: Murgui E and Hedblom M (Eds). Ecology and conservation of birds in urban environments. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Swaegers J, Mergeay J, Therry L, et al. 2013. Rapid range expansion increases genetic differentiation while causing limited reduction in genetic diversity in a damselfly. Heredity 111: 422–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs M, Melbourne BA, Tuff T, and Hufbauer RA. 2014. The roles of demography and genetics in the early stages of colonization. P Roy Soc B-Biol Sci 281: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M 1996. Biological invasions. London, UK: Chapman and Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


