Abstract
Insulin resistance has wide-ranging effects on metabolism, but there are knowledge gaps regarding the tissue origins of systemic metabolite patterns and how patterns are altered by fitness and metabolic health. To address these questions, plasma metabolite patterns were determined every 5 min during exercise (30 min, ∼45% of V̇o2peak, ∼63 W) and recovery in overnight-fasted sedentary, obese, insulin-resistant women under controlled conditions of diet and physical activity. We hypothesized that improved fitness and insulin sensitivity following a ∼14-wk training and weight loss intervention would lead to fixed workload plasma metabolomics signatures reflective of metabolic health and muscle metabolism. Pattern analysis over the first 15 min of exercise, regardless of pre- versus postintervention status, highlighted anticipated increases in fatty acid tissue uptake and oxidation (e.g., reduced long-chain fatty acids), diminution of nonoxidative fates of glucose [e.g., lowered sorbitol-pathway metabolites and glycerol-3-galactoside (possible glycerolipid synthesis metabolite)], and enhanced tissue amino acid use (e.g., drops in amino acids; modest increase in urea). A novel observation was that exercise significantly increased several xenometabolites (“non-self” molecules, from microbes or foods), including benzoic acid-salicylic acid-salicylaldehyde, hexadecanol-octadecanol-dodecanol, and chlorogenic acid. In addition, many nonannotated metabolites changed with exercise. Although exercise itself strongly impacted the global metabolome, there were surprisingly few intervention-associated differences despite marked improvements in insulin sensitivity, fitness, and adiposity. These results and previously reported plasma acylcarnitine profiles support the principle that most metabolic changes during submaximal aerobic exercise are closely tethered to absolute ATP turnover rate (workload), regardless of fitness or metabolic health status.
Keywords: cystine, galactose, 2-hydroxybutyrate, muscle fatigue, succinate, taurine, xenobiotic
INTRODUCTION
Insulin resistance and type 2 diabetes mellitus (T2DM) are associated with perturbations in metabolism of sugars, long-chain fatty acids (LCFAs), and amino acids. For instance, with insulin resistance or T2DM whole body markers of LCFA metabolism are altered (e.g., increased indices of incomplete β-oxidation; see Refs. 2 and 52), and there is impaired metabolic flexibility (flexibility defined by a shift away from LCFA oxidation toward carbohydrate metabolism in response to increased insulin: see, e.g., Refs. 33, 34, 49, and 74). Altered fatty acid oxidation with insulin resistance is reflected in part by a mismatch between fatty acid availability to mitochondria and fatty acid oxidation (FAO) in muscle and other tissues (“mitochondrial overload;” see Ref. 37). Incomplete LCFA β-oxidation is more evident in cultured myotubes isolated from obese, insulin-resistant individuals compared with normal-weight, insulin-sensitive persons (3) and in insulin-resistant rat myocytes (37). Based on metabolite profiling, we have hypothesized (1, 2, 21) that incomplete LCFA β-oxidation may stem in part from imbalanced mitochondrial anaplerosis/cataplerosis due to suboptimal anaplerosis [e.g., from branched-chain amino acids (BCAAs)] and increased cataplerosis that is coincident with enhanced fatty acid oxidation. That impaired BCAA-driven anaplerosis takes place in insulin-resistant human muscle is supported by studies comparing metabolism in cultured myotubes from healthy and T2DM persons (44). Notably, cataplerosis was enhanced in rat heart preparations (13) or isolated mouse muscle mitochondria (70) during active LCFA oxidation.
Despite the well-established role for muscle in whole body energy homeostasis, identifying metabolites that specifically reflect muscle metabolism or LCFA β-oxidation dynamics is a challenge, especially those that are modified by metabolic health status. Circulating metabolites responsive to an acute, submaximal exercise bout are potentially reflective of muscle metabolism and overall metabolic health, with the important caveat that interrogation of the blood pool without cross-tissue flux determinations cannot fully discern tissue-specific metabolism. We recently described exercise-associated plasma patterns of short-, medium-, and long-chain acylcarnitines in a cohort of insulin-resistant, sedentary, obese women before and after a ∼14-wk fitness and weight loss intervention that significantly improved insulin sensitivity (82). Markers of incomplete β-oxidation rose rapidly with exercise and then dropped with exercise cessation, similar to observations by others (28, 42, 79). Lehmann et al. (42) and Xu et al. (79) confirmed that exercising muscle is a net exporter of medium-chain acylcarnitines. Because improved fitness and insulin sensitivity did not alter the exercise-associated changes in these markers in our cohort when pre- versus postintervention periods were compared at the same workload (82), we concluded that under the conditions tested, β-oxidation in muscle is closely tethered to ATP turnover in vivo, regardless of fitness or insulin resistance status. Such a scenario is consistent with the idea that inefficient β-oxidation (and coincident mitochondrial export of chain-shortened acylcarnitines) is a normal physiological mechanism to support rapid rates of fat oxidation while sustaining the finite intramitochondrial CoASH pool.
In the current paper, we extend our previous findings from the cohort (9, 82) to explore how global exercise-related blood metabolite patterns are impacted by improved insulin sensitivity and fitness. In an attempt to focus on muscle metabolism, the acute exercise duration (30 min) and moderate intensity (∼40–45% V̇o2peak) used for exercise metabolomics studies were designed to rapidly activate muscle work and robust fatty acid oxidation (7, 68) while minimizing increases in liver β-oxidation (19, 20). We aimed to describe how acute submaximal exercise impacts the human blood metabolome and hypothesized that fitness and weight loss intervention would alter select metabolites from pathways responsive to changes in metabolic health and insulin sensitivity.
MATERIALS AND METHODS
Ethical Approval and Human Subject Information
Extensive details regarding diet, recruitment, Test Week protocols, and other intervention-associated aspects for this cohort are provided in our reports of oral glucose tolerance test metabolomics (9) and acute exercise acylcarnitine profiling (82). A succinct summary is provided here. All protocols were approved by the University of California at Davis Institutional Review Board, in alignment with the Declaration of Helsinki, and all subjects provided informed, written consent. The study is listed in ClinicalTrials.gov (NCT01494025). Women who were 30–50 yr of age, obese, and modestly hyperinsulinemic were recruited from the greater Davis and Sacramento, CA, communities. All participants were eumenorrheic, nonsmoking, and sedentary (typical planned exercise <30 min/wk). Body mass index (BMI) was between 30 and 37.5 kg/m2, and participants were weight stable over the previous 6 mo (from self-report). Insulin resistance at the time of screening was identified through overnight-fasted blood glucose and insulin and an abbreviated oral glucose tolerance test. Insulin resistance was defined as follows: 1) as per the American Diabetes Association guidelines for prediabetes, fasting glucose ≥100 and <126 mg/dL or 2-h OGTT glucose ≥140 and <199 mg/dL; and/or 2) a target Quantitative Insulin Sensitivity Check Index (QUICKI) score of <0.315, homeostasis model assessment (HOMA) of >3.67, or logHOMA of >0.085. Exclusion criteria included any clinical signs of infection, chronic disease, personal history of cardiovascular disease, elevated blood pressure (>130/85 mmHg), diabetes, regular medications other than oral contraceptives, and pregnancy or lactation. Sixteen participated through the first phase of the study; one subject was not adherent to the prescribed diet provided during either Test Week and, therefore, was excluded. Three subjects dropped following Test Week 1, before or during weight loss/exercise intervention, leaving 12 of 15 adherent subjects available for re-examination in Test Week 2.
Pre- and Postintervention Test Week Protocol
Participants completed testing before (Test Week 1) and after (Test Week 2) an exercise and weight loss intervention (14–17 wk; variable due to disparate menstrual cycle patterns, stages were matched pre- and postintervention) (see Ref. 9). During each Test Week, subjects refrained from exercise and were weight stable at the time of testing.
Test Week diet.
To minimize variability in metabolomics that could be influenced by differences in diet composition, during Test Week 1 and Test Week 2 the participants were provided lot-matched foods such that they ate identical diets for the Test Weeks (details in Ref. 9); body mass was determined daily and changes in calories were made to maintain body mass within 5%. During Test Weeks, participants were instructed to eat and drink only what was provided to them, to eat meals on-site when possible, and self-reported adherence was determined with daily food diaries.
Peak exercise test.
On day 4 or 5 of each Test Week, a graded cycle ergometer test (SRM Ergometer, Colorado Springs, CO) was performed to determine peak oxygen consumption (V̇o2peak). Participants arrived at the University of California (UC) Davis Sports Medicine Clinic after consuming a standard breakfast (the 2nd menu; Ref. 9) 2–3 h before exercise. During Test Week 1, the participants received a resting ECG, a spirometry test, and a medical clearance exam by a UC Davis Sports Medicine Clinic physician to ensure that there were no health issues precluding exercise. For the fitness test, participants completed a 5-min warmup, followed by a graded exercise test to exhaustion (initial workload of 50 W, increased by 20 W every 2 min until volitional fatigue). V̇o2peak was determined as the highest V̇o2 (mL·kg−1·min−1) over a 30-s period. The V̇o2peak measurement was replicated during Test Week 2.
Plasma metabolite profiling during submaximal exercise test.
Forty-eight hours after the peak exercise test (also described in Ref. 82), on day 7 or 8 (depending on subject) of the Test Weeks, participants reported to the Western Human Nutrition Research Center (WHNRC) Physiology Support Laboratory in the morning following a 12-h overnight fast and having refrained from any moderate to vigorous physical activity since the V̇o2peak test. Subjects were fitted with a heart rate (HR) monitor (Polar Vantage NV model no. 1901001; Polar Electro Inc., Port Washington, NY). An intravenous catheter was placed in an arm vein (typically antecubital). The first blood sample was taken 5 min after the catheter was placed and before exercise. After a brief 5-min warmup involving pedaling without workload, subjects were fitted with headgear and nose clip for metabolic cart measurements. During Test Week 1, the tension on the cycle ergometer (Monark 828E) was set to elicit an individualized workload at 45% V̇o2peak (average: 64 W) determined from the peak exercise test. Participants pedaled at the appropriate cadence of 50–60 rpm for 30 min. Between 0 and 5 min of exercise, indirect calorimetry values were checked, and workload was slightly adjusted to ensure the subject was working at an intensity of 45% V̇o2peak. The apparatus was then removed until a second calorimetry measurement between 15 and 20 min. Blood was sampled every 5 min throughout the entire protocol, as was HR. After 30 min, the workload was reduced to zero watts for a 5 min “cool down” during which subjects continued to pedal at a slow pace. After the “cool down” period between 30 and 35 min, participants moved to a chair where they rested for 15 min (between 35 and 50 min of the protocol). They continued to have blood drawn every 5 min. Blood was collected into EDTA Vacutainers (Becton-Dickinson), placed in ice, and centrifuged for plasma that was transferred to −80°C. During Test Week 2, the same regimen was applied, but with the workload on the cycle ergometer matching the individual’s Test Week 1 workload to ensure equal muscle work at each of the time periods.
Weight Loss and Fitness Regimen
Subjects were prescribed a self-selected, calorie-restricted diet (∼500–600 kcal/day reduction) based on the 2005 Dietary Guidelines for Americans and targeting a 10% body mass loss over 14 wk (9). A physical activity questionnaire (5) was administered to assess self-reported physical activity level, with a score of 5 for the lowest activity and 15 for the highest activity related to work, sport/exercise, and nonsport leisure categories; a score of 7 was used to calculate maintenance calories. Participants recorded daily food intake in diaries and received weekly counseling from a registered dietitian. Subjects were provided with a daily nutritional supplement (Bayer One-a-Day for Women) to assure adequate intake of essential vitamins and minerals. Body mass was measured weekly.
Participants engaged in a prescribed exercise regimen a minimum of 4 times/wk for the duration of the intervention, as supervised by WHNRC exercise physiologists. Over the first 4 wk of intervention, participants exercised aerobically 4 days/wk for 30 min each (treadmill or cycle ergometer) at an intensity of 60–70% of their maximal HR, as determined in the V̇o2peak test. During intervention weeks 5–8, exercise sessions were increased to 40 min/session, 4 days/wk, and during intervention week 9 and onward the intensity was increased to a HR of 75% of maximal. Participants wore HR monitors during all exercise sessions to ensure that they were exercising at the appropriate intensity for the prescribed amount of time.
Metabolomics Analysis
The details of sample handling, metabolite detection, and analysis have been presented in detail elsewhere (21, 22). In brief, EDTA plasma aliquots (15 µL) were extracted, and a set of 13 C8–C30 fatty acid methyl ester internal standards were added, followed by methoximation/trimethylsilylation derivatization with 10 µL of methoxyamine hydrochloride in pyridine, followed by 90 µL of N-methyl-N- trimethylsilyltrifluoroacetamide. Analytes in a 0.5-µL sample injection were separated using an Agilent 6890 gas chromatograph (Santa Clara, CA) equipped with a 30 m × 0.25 mm id Rtx5Sil‐MS column with 0.25 µm 5% diphenyl film and a 10-m integrated guard column (Restek, Bellefonte, PA). Chromatography was performed with constant flow of 1 mL/min while ramping the oven temperature from 50 to 330°C with 22 min of total run time. Mass spectra were acquired on a Leco Pegasus IV time of flight mass spectrometer (St. Joseph, MI) with a 280°C transfer line, a 250°C ion source, and −70 eV of electron ionization impact. Mass spectra were acquired from m/z 85–500 at 17 spectra/s and 1,850 V of detector voltage. Result files were exported to servers and processed by the Fiehn Laboratory Metabolomics BinBase database. Database entries in BinBase were matched against the Fiehn mass spectral library of 1,200 authentic metabolite spectra using retention index and mass spectrum information or the NIST17 commercial library. Identified metabolites were reported if present in ≥50% of the samples. Peak heights of quantifier ions for each reported metabolite were normalized to the sum intensities of all annotated (known) metabolites, and these normalized abundances were used for statistical investigation. External five‐point calibration curves established with mixtures of 30 metabolites allowed for the routine assessment of instrument sensitivity and analyte intensity with respect to the instrument dynamic range. Each chromatogram was further controlled with respect to the total number of identified metabolites and total peak intensities to ensure that outliers did not confound the statistical analysis. Final metabolite values are reported as normalized peak heights for quantifier ions specific to each metabolite.
Statistics and Analysis
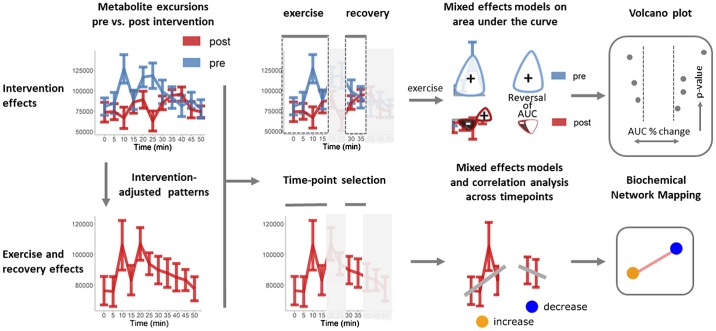
Analyses were conducted using the R programming language (version 3.4.0). Data pretreatment and analysis workflows were implemented to identify 1) exercise and recovery and 2) intervention-associated effects in metabolite excursion patterns (Fig. 1). Analysis of exercise and recovery time course data was limited to 0–15 and 30–35 min, respectively. Principal components analysis (PCA) was used in a data quality overview of each individual’s pre- and postintervention scores and identified no outliers. Mixed-effects models were calculated to identify intervention- and time-dependent changes in metabolite concentrations with subjects as the random term. False discovery rate-adjusted P values were calculated according to Benjamini and Hochberg (6). A combination of biochemical network mapping and volcano plots were used to visualize the mixed-effect model results for exercise and recovery (time dependent) and intervention effects, respectively. The magnitude and direction of the time-dependent effects were summarized based on Spearman correlations between metabolite peak intensities and intervention-adjusted data. Intervention effects were summarized based on area under the curve (AUC; baseline adjusted by time 0 min and calculated using trapezoidal rule; metabolites that were decreased were also calculated in this manner as area over the curve, but for clarity this is referred here as AUC) for exercise data and expressed as the difference between 35 and 30 min for the immediate recovery period.
Fig. 1.
Visual representation of the data analysis workflow. Data pretreatment and analysis workflows were implemented to identify 1) exercise and recovery and 2) intervention-associated effects in metabolite excursion patterns. The magnitude and direction of the time-dependent effects were summarized based on Spearman correlations between metabolite peak intensities and time for intervention-adjusted data. Intervention effects were summarized based on area under the curve (AUC) for exercise data and change over 30–35 min for recovery.
Volcano plots.
Volcano plots were used to visualize the significance (mixed-effects P value, P < 0.05) and magnitude (AUC %change) of differences in metabolite patterns between pre- and postintervention measurements. AUC percent change was calculated based on (pre-post)/pre × 100 AUC values. The direction (sign) of the postintervention AUC and difference in the sign compared with pre (reversal in AUC) is also highlighted in the visualization. For example, if one starts with +2 and ends with a −1 AUC, the change is +2 – (−1) = 3 total units of change. These three units are normalized by the starting amount = 3/2 to get 1.5 or −150% change.
Network analysis.
Biochemical (KEGG) and chemical similarity (Tanimoto similarity >0.7 for PubChem CID) networks were calculated using metaMapR (25) to visualize time-dependent exercise- and recovery-associated changes in plasma metabolite concentrations. Significant time effects were annotated based on mixed-effect P values of ≤ 0.05. Magnitude and directionality of the time-associated trends were summarized based on the sign and magnitude of the Spearman correlations for residuals from linear model for intervention (intervention adjusted) over the measured time points.
Predictive modeling.
Random forest (RF) models (47) were developed to identify optimal metabolite discriminants between intervention groups. Models were optimized on two-thirds of the 15 sample pairs (training data) and tested on the one-third left-out data (test data). Recursive feature elimination (38) was used to identify optimal model discriminants based on maximization of model area under the receiver operator characteristic curve (ROC) based on sevenfold cross-validation repeated three times on the training data. The final models were validated on the held-out data.
RESULTS
Body Mass Loss and Improvements in Metabolic Health Indices Following a Fitness and Dieting Intervention
We previously reported that the intervention led to significant reductions in body weight and adiposity, with significant improvements of fitness (VO2peak) and insulin sensitivity (9, 82). Overnight-fasted and same-day post-glucose tolerance test metabolomics patterns also have been reported from this cohort (9).
Intervention-Independent, Acute Exercise-Associated Plasma Metabolite Patterns
A combination of statistical analysis results and network mapping was used to visualize intervention-independent time effects on annotated metabolites during 0–15 min of exercise and recovery phases within a biochemical context (Figs. 2 and 3, respectively). This time frame for exercise was chosen considering the substantial temporal and directional shifts in several metabolites that were apparent at the ∼20-min point of exercise upon inspection of individual metabolite patterns (Supplemental Figs. S1 and S2; Supplemental Material for this article can be found online at https://doi.org/10.15482/USDA.ADC/1504422). The shift was most apparent for just a subset of metabolites (e.g., adenosine-5-phosphate, cystine and cysteine-glycine, γ-glutamyl-valine, glycerol-3-galactoside, and others); thus, such a phenomenon cannot be explained by a time-specific gross sample handling issue or GC-TOF analysis artefact. These nonlinear effects reduced the quality of calculated AUCs as summary metrics for metabolic responses and were omitted from the AUC analyses. Although we cannot discount the possibility that the observed phenomenon is a normal physiological response to exercise, we speculate that there was some sort of stress around the 20-min time frame (e.g., removal of the calorimetry mask coupled to a higher blood draw volume), which may have triggered responses impacting select metabolic pathways. Regardless, trends captured in the acute exercise network from 0 to 15 min clearly reflect changes that take place with acute engagement of muscle work, and this time frame is the focus of this paper.
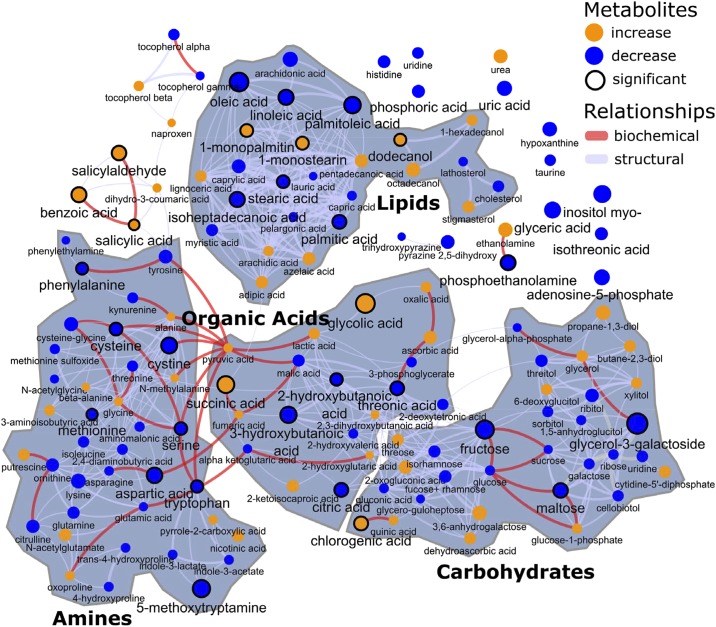
Fig. 2.
Patterns of plasma metabolites in overnight-fasted women during an acute exercise bout, using combined data from before and after a weight loss and training intervention. Results are illustrated as metabolite network clusters representing structural and biochemical similarity classes, with blue symbols indicating an exercise-associated reduction and orange symbols indicating an increased concentration during the first 15 min of exercise. Only annotated (known) metabolites are depicted. Circles outlined with a black line indicate metabolites with concentration changes that were statistically significant (mixed-effects model, P < 0.05), and node sizes convey the magnitude of change. Individual metabolite excursions may be found in Supplemental Figs. S1, A and B, and S2.
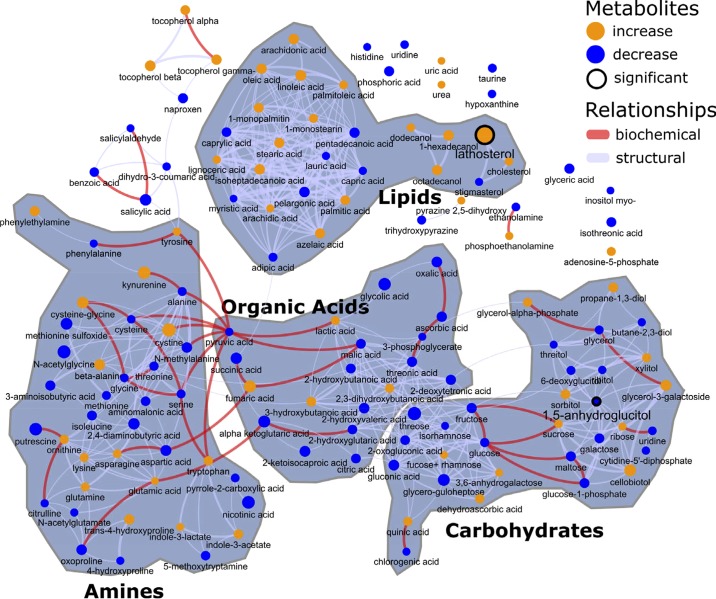
Fig. 3.
Patterns of plasma metabolites in overnight-fasted women during the first 5 min of recovery following a 30-min exercise bout, using combined data from before and after a weight loss and training intervention. Results are illustrated as metabolite network clusters representing structural and biochemical similarity classes, with blue symbols indicating an exercise-associated reduction and orange symbols indicating an increased concentration. Only annotated (known) metabolites are depicted, and node sizes convey magnitude of change. The change in lathosterol was the only statistically significant metabolite change (mixed-effects model, P < 0.05). Individual metabolite excursions may be found in Supplemental Figs. S1, A and B, and S2.
Significant time effects are highlighted based on mixed-model effects (P < 0.05), and the direction and magnitude of the trend in metabolites over time were denoted based on Spearman correlations (Fig. 2). Unlike AUC statistics, which summarize all changes across individual time points into a single value, the described network approach highlights linear trends in metabolite patterns across the indicated time interval and without regard for the pre- versus postintervention status. Individual patterns of metabolites are provided in Supplemental Fig. S1, and as-yet unidentified, nonannotated metabolite excursions are shown in Supplemental Fig. S2. The following blood metabolite patterns were generally shared, regardless of pre- or post-weight loss and fitness intervention status. Note that to place certain metabolites into better pathway context, some discussion in this section also focuses on significant correlations of metabolites across the entire experimental period (all time points from exercise + recovery), which were considered relevant only if the correlations were robust (r ≥ 0.5 and P < 0.05 both pre- and postintervention, from data provided in Supplemental Table S3, tabs 1 and 2).
Lipid metabolism.
Generally speaking, LCFA concentrations dropped in the acute phase of exercise, with the notable exceptions of 1-monopalmitin and 1-monostearin; the latter fatty acids were increased (Fig. 2, top middle cluster), suggestive of alterations in desaturation pathways. The increases in 1-mono-fatty acids appeared to be more robust for the postintervention samples (Supplemental Figs. S1). No clear patterns emerged for cholesterol or lathosterol (cholesterol precursor) or for the products of peroxisomal ω-oxidation adipic and azelaic acids. Notably, with the exception of the 1-mono-LCFAs, the individual LCFA patterns were highly and significantly correlated with one another when considered over the entire exercise plus recovery periods (Supplemental Table S3, tab 1). The pattern of 1-monopalmitin was significantly but modestly correlated with that of 1-monostearin over the entire exercise plus recovery period (r = 0.30 and r = 0.51 in pre- and postintervention, respectively), yet this relationship was not unique in that the patterns for 1-mono-LCFAs appeared to be correlated with metabolites from several different metabolite classes (Supplemental Table S3, tab 1). The main end product of ketogenesis, 3-hydroxybutanoic acid (β-hydroxybutyrate), was significantly reduced with exercise, and concentration patterns generally tracked those of LCFA over the entire course of the exercise plus recovery experiment (see Supplemental Fig. S1, and Supplemental Table S3, tab 1), including oleic (r > 0.7), arachidonic (r = ∼0.6), linoleic (r = ∼0.6–0.7), palmitic (r = ∼0.6–0.7), and palmitoleic (r = ∼0.5).
Amino acid metabolism; nitrogenous metabolites.
Plasma amino acids were reduced, as a biochemical class, by acute exercise (Fig. 2, bottom left cluster). The reductions in concentrations of methionine, cysteine, cystine, serine, tryptophan, phenylalanine, serine, and aspartic acid were statistically significant. When considering the entire experimental period of exercise and then recovery, it was clear that patterns for virtually all amino acids were tightly correlated with one another throughout (generally, r ≥ 0.5 and P < 0.05 both pre- and postintervention, respectively; Supplemental Table S3, tab 1). A tryptophan derivative involved in the melatonin/serotonin biochemical pathway, 5-methoxytryptamine (5-MT), was significantly lowered in the acute phase of the exercise bout. As for 5-MT patterns, there were no robust correlations (i.e., r > 0.5) with other metabolites that were consistent in the pre- and postintervention states (Supplemental Table S3, tab 1). Taurine, a cysteine derivative, was acutely reduced with exercise, as was uric acid, but these changes were not statistically significant. Urea displayed a steady rise during the exercise bout. The metabolite 2-hydroxybutanoic acid (α-hydroxybutyrate) was significantly lowered by acute exercise; this metabolite is likely the product of a reduction reaction upon the methionine or threonine catabolic derivative α-ketobutyrate. Although there were many statistically significant correlations with other metabolites considering the entire exercise and rest periods, none were robust (r ≥ 0.5 and P < 0.05, both pre- and postintervention, respectively; Supplemental Table S3, tab 1).
Carbohydrate metabolism.
Plasma concentrations of carbohydrates and sugar derivatives mostly dropped with acute exercise (Fig. 2, bottom right cluster). The small change in blood glucose per se did not change significantly, but large relative reductions were observed in the minor sugars fructose and maltose. When considering the entire experiment of exercise and recovery, the fructose patterns were most clearly correlated with those of citric acid, myo-inositol, glyceric acid, glucose, threonic acid, and α-ketoglutaric acid. The glycerol- and galactose-linked metabolite glycerol-3-galactoside (galactosylglycerol) displayed a significant reduction in concentration. Over the exercise plus recovery periods, this metabolite appeared to be correlated with phenylalanine and cholesterol, but notably, not glycerol per se (Supplemental Table S3, tab 1). Indeed, inspection of the glycerol pattern over the entire period demonstrated a steady rise as the experiment progressed (Fig. 2 and Supplemental Fig. S1). Over the entire experimental period and considering pre- and postintervention periods, glycerol was most correlated to the LCFAs and ethanolamine (Supplemental Table S3, tab 1).
Organic acids, other metabolites, and xenometabolites.
Organic acids arising from multiple fuel sources displayed disparate patterns during acute exercise (Fig. 2, bottom middle cluster). For instance, citric acid was significantly reduced and malic acid lowered (not significant), whereas succinic acid was increased significantly. There was no change in fumaric acid concentration (note outlier at time zero). For citric acid, considering all of the exercise and recovery periods, patterns were most robustly correlated to those of α-ketoglutaric acid (r > 0.7), malic acid, fructose, glucose, threonic acid, and phosphoric acid. For succinic acid, although there were many statistically significant correlations to other metabolites, none were robust (r ≥ 0.5 and P < 0.05, both pre- and postintervention, respectively; Supplemental Table S3, tab 1).
Glycolic acid was significantly increased; this metabolite may arise from multistep conversion of citrate (via cis-aconitate and isocitrate, leading to production of succinate plus glyoxylate) with glyoxylate conversion to glycolic acid (glycolate). Although there were many statistically significant correlations, none were robust (r ≥ 0.5 and P < 0.05, both pre- and postintervention, respectively).
Phosphoethanolamine concentration was decreased significantly, and ethanolamine concentrations increased (not significant) with exercise. Although no robust correlations were identified for phosphoethanolamine concentration when considering the entire exercise and recovery periods, ethanolamine was significantly correlated with glycerol at r > 0.5 (Supplemental Table S3, tab 1).
Blood concentrations of several xenometabolites and derivatives were impacted by acute exercise. 1-Dodecanol was significantly increased, and there were trends for increases in the related molecules 1-hexadecanol and 1-octadecanol (Fig. 2 and Supplemental Fig. S1); 1-dodecanol was significantly correlated with 1-hexadecanol and 1-octadecanol, but the correlation was modest (generally, r < 0.5). Concentrations of chlorogenic acid (a food-derived quinic acid-caffeic acid ester abundant in berries and coffee) were higher with acute exercise. Although there were many statistically significant correlations, none were robust (r ≥ 0.5 and P < 0.05 both pre- and postintervention, respectively; see Supplemental Table S3, tab 1). A family of related benzoic acid pathway metabolites [benzoic acid, salicylic acid (alternative name, 2-hydroxybenzoic acid), salicylaldehyde (alternative name, 2-hydroxybenzaldehyde)] were all increased significantly. Over the entire span of the exercise and recovery, the latter molecules were weakly (r = ∼0.3–0.4) but significantly correlated, with no other robust correlations present (as defined by (r ≥ 0.5 and P < 0.05 both pre- and postintervention, respectively; see Supplemental Table S3, tab 1).
Blood concentrations of nonannotated (as-yet unidentified) metabolites were impacted by acute exercise. Concentrations of a variety of as-yet unidentified metabolites displayed exercise-associated shifts (see Supplemental Fig. S2). These are not included in the network analysis of Fig. 2 since their biochemical similarity cannot be ascertained at this time. Some initial information with respect to chemical class or pathways for unknown metabolites of interest may be deduced by examination of correlations to known metabolites (see Supplemental Table S3, tab 2) or by investigation of occurrence and abundances across species and organs using BinVestigate (39). However, a deeper analysis of the nature of these metabolites is beyond the scope of this paper.
Intervention-Independent Plasma Metabolite Patterns in the Period Immediately Following Cessation of Exercise
To assess intervention-independent trends in broad classes of plasma metabolites that change over the immediate “recovery” phase (participants remained on the cycle, but with no resistance and light pedaling), a chemical similarity network of annotated (“known”) metabolites that depicts directional changes over the first 5 min of exercise cessation was constructed (Fig. 3). This time frame was chosen considering the substantial temporal and directional shifts in several metabolites that were apparent at the ∼30- to 50-min points (30 min being the final exercise blood draw) upon inspection of individual metabolite patterns (Supplemental Figs. S1 and S2), thus invalidating calculation of a meaningful AUC encompassing the 30- to 50-min period. With these caveats in mind, the following metabolite patterns from the exercise-to-rest transition were noted.
Lipid metabolism.
There were no statistically significant changes in concentrations of specific LCFA in the first 5 min of recovery (Fig. 3, top center cluster); however, upward trends in most LCFA contrast with the reductions seen in the first 15 min of exercise. In addition, a postexercise rise in LCFA concentrations is quite apparent when looking over the entire resting period; the 1-mono-fatty acids (1-monostearin and 1-monopalmitin) displayed disparate patterns (Supplemental Fig. S1). The exercise-associated drop in 3-hydroxybutanoic acid (β-hydroxybutyrate) remained intact between 30 and 35 min; however, concentrations rose substantially after that, in concert with LCFA concentrations (see Supplemental Fig. S1 and Supplemental Table S3). Lathosterol, which had little change or a slight reduction in the acute exercise phase, was significantly increased immediately following exercise (Fig. 3, top right). However, the latter was due to a low concentration specifically observed at the 30-min time point for this metabolite and hence, may be some type of artefact (Supplemental Fig. S1).
Amino acid metabolism: nitrogenous metabolites.
There were no consistent patterns observed for plasma amino acids immediately postexercise, and unlike the acute exercise period there were no significantly different shifts in any specific metabolite in this class (Fig. 3, bottom left cluster). Several amino acids displayed different directional changes in this period when contrasted to the acute exercise period (e.g., tryptophan, cystine; compare Figs. 2 and 3). 5-Methoxytryptamine, which dropped significantly with exercise, remained low with resting postexercise (Supplemental Fig. S1). The exercise-associated reduction in taurine was maintained, and the increase in urea appeared to level off in the recovery period (Supplemental Fig. S1). 2-Hydroxybutanoic acid (α-hydroxybutyrate) was significantly lowered by acute exercise but appeared to recover by the end of exercise, and hence, no differences in concentration were seen immediately postexercise (Supplemental Fig. S1). The pattern for uric acid (reduced by acute exercise) suggested a recovery almost to baseline by the end of the exercise and recovery periods.
Carbohydrate metabolism.
Sugars and sugar metabolites (Fig. 3, bottom right cluster) that displayed significant concentration differences with exercise showed no significant shifts in the immediate postexercise period (e.g., fructose, maltose, glycerol-3-galactoside; compare Figs. 1 and 2). The exercise-associated drop in fructose, maltose, and glycerol-3-galactoside concentrations remained in effect throughout the resting period (Supplemental Fig. S1).
Organic acids, other metabolites, and xenometabolites.
Metabolites from this group that had significantly altered concentrations with exercise (see above) did not display significant alterations in the immediate post-exercise phase (compare Figs. 2 and 3). Discussion of unknown metabolite plasma concentration patterns may be found above in Intervention-Independent, Acute Exercise-Associated Plasma Metabolite Patterns. As for annotated metabolites, 1) concentrations of organic acids arising from multiple fuel sources were not significantly changed in the immediate postexercise phase (Fig. 3, bottom middle cluster); reduced citric acid concentration remained so, and exercise-increased succinic acid remained higher (Supplemental Fig. S1); 2) glycolic acid was significantly increased by exercise, and this higher concentration appeared to be unchanged with the immediate resting period (Supplemental Fig. S1); 3) phosphoethanolamine concentration, which was decreased significantly by exercise, appeared to have recovered by the end of the exercise challenge (Supplemental Fig. S1); and 4) regarding immediate postexercise blood plasma concentrations of several xenometabolites and derivatives that were impacted by immediate recovery from acute exercise, a) the exercise-associated rise in 1-dodecanol and the trends for higher 1-hexadecanol and 1-octadecanol remained by the end of exercise (this also was true for chlorogenic acid; see Supplemental Fig. S1), and b) an inspection of the individual metabolite concentration excursions in Supplemental Fig. S1 reveals that the benzoic acid pathway metabolites [benzoic acid, salicylic acid (alternative name, 2-hydroxybenzoic acid), salicylaldehyde (alternative name, 2-hydroxybenzaldehyde)] displayed complex patterns at the exercise-to-rest transition. For benzoic acid, the exercise-associated rise in concentration appeared to remain intact, whereas salicylic acid dropped (albeit not significantly, and transiently with high variability). Salicylaldehyde remained high in preintervention subjects but returned to baseline and did not change in the 30- to 35-min period in postintervention subjects.
Intervention-Dependent Changes During Exercise and Immediate Postexercise Recovery
Our initial intent was to leverage the exercise AUC (0- to 15-min exercise period) or short-term change (immediate recovery, 30–35 min) metrics for all detected metabolites to generate a multivariate statistical model that is predictive of the pre- versus post-weight loss and fitness intervention states, vis-à-vis metabolite pattern “signatures” reflective of these states. However, the random forest models failed to reach meaningful discrimination in this small data set. Supplemental Table S4, tab 1, displays the number of differentially abundant plasma metabolites at P values of <0.05 for each comparison. Supplemental Table S4, tab 1, also displays RF model performance for the held-out validation data and the number of optimally selected metabolites for each comparison. The developed RF models exhibited low accuracy and kappa statistics for prediction of intervention effects. This suggests that there were few robust intervention-associated metabolite shifts that occurred following intervention in this cohort under our conditions. With this in mind, we instead present a set of exercise bout metabolites with plasma concentration changes that were statistically significantly different when comparing the pre- (obese, insulin-resistant, sedentary) versus postintervention (lower weight, insulin-sensitive, physically fit) periods (Table 1 for annotated metabolites; statistics for all metabolites are provided in Supplemental Table S4, tabs 2 and 3). Volcano plots were also developed to compare and contrast intervention-associated changes in metabolite AUCs between exercise and recovery phases (Figs. 4 and 5).
Table 1.
Annotated plasma metabolites with concentration changes that differed significantly during a fixed workload exercise bout (0–15 min) or the immediate (first 5 min) postexercise recovery period in women before and after a fitness and weight loss intervention
| Preintervention | Postintervention | P Value* | |
|---|---|---|---|
| Exercise (0–15 min AUC) | |||
| Quinic acid | −194 ± 594 | 2,730 ± 1,155 | 0.0169 |
| Aminomalonic acid | −1,710 ± 5,680 | −25,600 ± 5,774 | 0.0037 |
| Tocopherol-β | −174 ± 465 | 821 ± 433 | 0.0423 |
| 1-Monostearin | 295 ± 310 | 1,310 ± 375 | 0.0053 |
| Methionine sulfoxide | −1,220 ± 465 | 2,650 ± 1,732 | 0.0178 |
| 1-Hexadecanol | 451 ± 491 | 1,850 ± 606 | 0.0427 |
| Threose | −339 ± 258 | 399 ± 234 | 0.0421 |
| 1-Monopalmitin | 1,150 ± 516 | 2,410 ± 346 | 0.0334 |
| Nicotinic acid | 27,500 ± 4,906 | 4,380 ± 9,815 | 0.0271 |
| Recovery (30–35 min of change) | |||
| Phosphoethanolamine | 130 ± 46 | −161 ± 92 | 0.0001 |
| 1-Monopalmitin | −113 ± 41 | 176 ± 64 | 0.0005 |
| Glycerol-α-phosphate | −59.9 ± 46 | 226 ± 78 | 0.0037 |
| Lathosterol | 20 ± 19 | 89.2 ± 13 | 0.0071 |
| Mannose | −2,300 ± 1,652 | −9,730 ± 1,992 | 0.0085 |
| Stearic acid | −5,520 ± 10,844 | 35,300 ± 11,258 | 0.0173 |
| Oxamic acid | 1,370 ± 955 | −2,250 ± 1,068 | 0.0215 |
| Benzoic acid | −3,410 ± 2,272 | 3,530 ± 1,617 | 0.0230 |
| 2-Hydroxybutanoic acid | 3,000 ± 1,291 | −1,730 ± 1,472 | 0.0290 |
| Hydroxycarbamate | 47.1 ± 57 | −153 ± 87 | 0.0304 |
| Shikimic acid | 21.5 ± 127 | −800 ± 375 | 0.0421 |
| Aconitic acid | −55 ± 24 | 15.5 ± 20 | 0.0479 |
Values are summarized as means ± SE; values are calculated on metabolite quantifier ion peak heights. Prediet and exercise intervention, n = 15; postdiet and exercise intervention, n = 12; Changes in body weight and other metabolic variables have been presented previously (9). AUC, area under the curve.
Comparisons using mixed-effects models for intervention across the time interval with subjects as the random term.
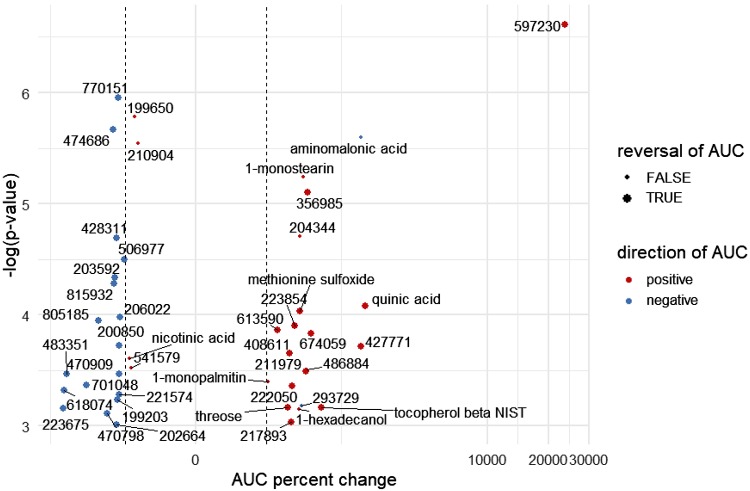
Fig. 4.
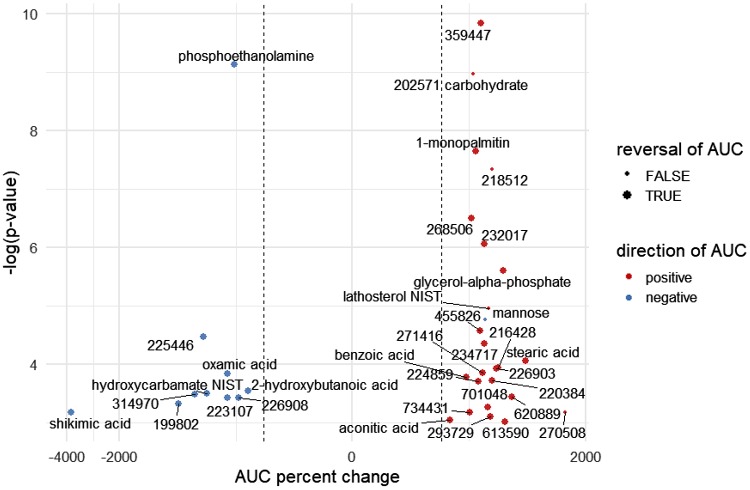
Volcano plot depicting plasma metabolites with acute exercise concentration excursions [area under the curve (AUC)] that were significantly altered by a weight loss and fitness intervention in women. Data represent magnitudinal changes in AUC as a percent (x-axis) when comparing the postintervention exercise test versus the preintervention test, with lowest to highest negative log P values (mixed-effects P values) also represented (y-axis). Any known annotated metabolites are fully labeled by chemical name, whereas unidentified (nonannotated) metabolites bear a “BinBase number” that is assigned as part of the Fiehn Laboratory metabolomics workflow. Point colors denote the direction of the postintervention AUC (red, positive; blue, negative), and the dashed vertical lines identify 100% changes in AUC. Larger point size denotes reversal of AUC trends from positive to negative or vice versa between pre- and postintervention. The mixed-effects approach is helpful for reducing the variation in pre- and postintervention comparisons by accounting for within-subject and across-group variation. Note: aminomalonate had a negative AUC that became more pronounced postintervention.
Fig. 5.
Volcano plot depicting plasma metabolites with immediate postexercise recovery (1st 5 min) concentration excursions that were significantly altered by a weight loss and fitness intervention in women. Data represent magnitudinal changes in the first 5-min recovery concentration delta (x-axis) when the postintervention exercise test versus the preintervention test was compared, with lowest to highest negative log P values (mixed-effects P values) also represented (y-axis). Any known annotated metabolites are fully labeled by chemical name, whereas unidentified (nonannotated) metabolites bear a “BinBase number” that is assigned as part of the Fiehn Laboratory metabolomics workflow. Point colors denote the direction of the postintervention first 5-min recovery concentration delta (red, positive; blue, negative), and the dashed vertical lines identify 100% changes in the first 5-min recovery concentration delta. Larger point size denotes reversal of area under the curve (AUC) trends from positive to negative or vice versa between pre- and postintervention. The mixed-effects approach is helpful for reducing the variation in pre- and postintervention comparisons by accounting for within-subject and across-group variation.
Acute exercise.
Table 1 provides a list of the eight annotated metabolites whose blood excursions during the 0-to 15-min exercise bout significantly differed when the pre- and postintervention periods were compared (Supplemental Table S4, tab 2, provides information on the entire list of annotated and nonannotated metabolites). Pattern changes include 1) metabolites with positive or flat exercise AUCs that became more positive following intervention, 2) metabolites with negative exercise AUCs that “flipped” and had positive AUCs following intervention, 3) metabolites with positive exercise AUCs that became less positive following intervention, and 4) metabolites with flat or negative exercise AUCs that became more negative following intervention. A volcano plot provides an overview of the intervention-related changes in all annotated and nonannotated metabolites that were significant (Fig. 4). Individual plots of pre- and postintervention plasma concentration changes can be seen in Supplemental Figs. S1 and S2; comments on specific annotated metabolites follow. The overnight-fasted concentration of quinic acid was lower, and exercise led to a higher increase postintervention, and this also was the case for β-tocopherol and 1-monostearin. In contrast, in the postintervention condition, aminomalonic acid was higher in the overnight-fasted state, and exercise led to a greater drop when compared with the preintervention condition. Although statistically significant when testing pre- versus postintervention, the pattern differences for methionine sulfoxide, 1-hexadecanol, threose, 1-monopalmitin, and nicotinic acid were less convincing over the 0- to 15-min exercise period.
Immediate postexercise recovery.
Table 1 provides a list of the 12 annotated metabolites whose blood excursions during the 30- to 35-min immediate exercise recovery period significantly differed when the pre- and postintervention periods were compared (Supplemental Table S4, tab 3, provides the entire list of annotated and nonannotated metabolites). A volcano plot displays percent changes of change and identifies the four major patterns of change described above for all significantly altered metabolites during the recovery period (Fig. 5). Individual plots of pre- and postintervention plasma concentration changes can be seen in Supplemental Figs. S1 and S2; comments on some specific annotated metabolites follow. The immediate exercise recovery change in concentration for 1-monopalmitin was profoundly different when preintervention (reduction in the first 5 min recovery) and postintervention (increase) were compared. The immediate postexercise rise in lathosterol concentration was lower postintervention, but the pre- and post- concentrations became similar by the end of the entire recovery period. In contrast, mannose concentration dropped less robustly immediately following exercise in the postintervention condition (although concentrations continued to fall and match those of preintervention by the end of recovery). The immediate recovery stearic acid pattern differed in that an immediate rise was observed in the pre-intervention condition compared with a drop and then increase for the postintervention phase. 2-Hydroxybutanoic acid, in contrast, showed an immediate recovery rise in the postintervention condition compared with a drop or stable change during recovery in the preintervention condition. Hydroxycarbamate appeared stable with recovery in the postintervention condition but dropped in the initial recovery period in the preintervention condition. Although statistically significant when testing pre- versus postintervention, the pattern differences for phosphoethanolamine, glycerol-α-phosphate, oxamic acid, benzoic acid, shikimic acid, and aconitic acid were less convincing during the initial exercise recovery period.
DISCUSSION
Studies of acute exercise blood metabolomics have enabled a broader view of the metabolic landscape of physical activity in humans at various exertions and at times ranging between ∼10 and 60 min (e.g., see Refs. 8, 11, and 45) (14, 28, 54, 56–58, 60, 80). Notably, most results have been derived from males, and to our knowledge, there are have not been evaluations of shifts in the exercise metabolome that are associated with improved metabolic health and fitness measured in the same cohort. In the current report, we fill key knowledge gaps by focusing on women and determining changes in the exercise metabolome when comparing a less healthy state (obesity, insulin resistance, and sedentary lifestyle) with a significantly improved health status (substantially increased insulin sensitivity and fitness that followed a multi-week weight loss and exercise training intervention) (9). We hypothesized that improved fitness, whole body and muscle insulin sensitivity, and weight loss would lead to significant and manifold differences in the metabolic responses to a fixed-workload exercise bout, as reflected in the blood metabolome.
One major take-home message from our results, and contrary to our initial hypothesis, is that despite major improvements in metabolic health status following weight loss and fitness intervention (9), the global metabolome patterns in response to acute submaximal fixed-workload exercise and recovery were largely the same pre- versus postintervention. This is best illustrated by the paucity of significantly different blood concentration patterns of annotated metabolites that we observed when comparing the pre- and postintervention phases. This is further highlighted by surveying the individual metabolite patterns (see Supplemental Figs. S1 and S2). Qualitatively, these results are consistent with our previous report of blood acylcarnitine patterns in exercise, which were largely unchanged by intervention in the same cohort (82). We proposed that shifts in exercise-associated fatty acid and amino acid catabolic pathways (as reflected in blood acylcarnitines) are largely tethered to ATP turnover, regardless of insulin sensitivity status and improved metabolic health. The current results bolster this assertion. Thus, our findings using targeted and untargeted metabolomics to date speak to what seems to be a fundamental principle of human submaximal aerobic exercise physiology, that engagement or repression of disparate biochemical pathways is driven primarily by the energy requirement to meet the workload, with little effect on these processes by metabolic health status.
Chemoinformatics algorithms that relate structures, pathways, and metabolite temporal correlations were coupled to biochemical deduction and evaluation of the historic literature to discern several key aspects of specific metabolic systems regulation by acute exercise. Patterns reflect the anticipated engagement of macronutrient oxidation and mobilization of fatty acids, carbohydrates, and amino acids that take place with acute modest exercise (77) and reveal novel aspects of metabolic physiology, as described below.
Fatty Acid and Ketone Body Metabolism and Contrasts in Metabolic Regulation Between Muscle and Liver During Exercise
With exercise progression the plasma glycerol concentration increased, whereas LCFAs dipped, similar to what has been reported by others, depending on the workload (e.g., see Refs. 28 and 68). Such a pattern is consistent with exercise-associated lipolysis driven by sympathetic nervous system activation coupled with accelerated muscle (and heart) LCFA uptake and combustion (7, 68). Interestingly, in preliminary studies we found that LCFA concentrations in the muscle interstitium were also lower in adults during a moderate exercise bout (81). In the immediate exercise recovery period, concentrations of LCFAs rose quite dramatically at least through 15 min of recovery (also see, e.g., Refs. 11, 28, and 68), which we interpret to be reflective of continued activation of sympathetic tone and lipolysis concurrent with reduced fatty acid oxidation when muscle work ceased. The rapidity of the drop in fatty acid oxidation following 30 min of submaximal exercise is apparent when looking at the postexercise fall in acylcarnitine markers of mitochondrial fatty acid oxidation in the current cohort (82). However, the dynamics of postexercise fat oxidation may be different, depending on exercise duration and intensity, since Nieman et al. (56) reported sustained increases in LCFA and some acylcarnitines at 1.5 h of recovery in male athletes who had cycled 75 km.
In the current study, plasma 3-hydroxybutanoic acid concentration patterns correlated with those of the LCFAs. Lewis et al. (45) also reported lower ketone body concentration (acetoacetate) at the 10-min mark of a strenuous exercise bout. 3-Hydroxybutanoic acid concentration reflects the net balance of primarily hepatic β-oxidation and tissue consumption of ketone bodies for energy. Our experiment was at a workload and over a short period of time designed to minimize activation of hepatic ketogenesis (19, 20). With this in mind, we interpret the 3-hydroxybutanoic acid patterns to reflect 1) even or reduced hepatic ketogenesis during exercise, concurrent with increased muscle and heart ketone body oxidation, and 2) a recovery-associated rise in ketogenesis as LCFA concentrations increased. We conclude from our metabolomics results and the observation of close temporal tracking between plasma medium-chain acylcarnitines and exercise (up) or recovery (rapid fall) (82) that there are exquisitely sensitive and rapid mechanisms in muscle that titrate the intramitochondrial flow of LCFA and β-oxidation in direct response to acute changes in workload. Ketogenesis is an effective means to manage excess LCFA-derived reducing equivalents and to help maintain the finite intramitochondrial CoASH pool, but unlike liver the muscle lacks this “relief valve” pathway. Therefore, in muscle, the workload-sensitive LCFA catabolism must be explained in large part by the coupling of fuel-derived reducing equivalent pools and electron flow to ATP turnover (53); acute regulation at the level of carnitine palmitoyltransferase 1 (CPT1) may also play a role.
Amino Acids and Nitrogenous Metabolites
Modest aerobic exercise increases oxidation of amino acids, with the caveat that fatty acids and glucose remain primary fuel sources (67). This could have the net effect to reduce circulating amino acid concentrations. Consistent with these principles, blood plasma amino acids were almost uniformly reduced by exercise in the current study, patterns of most amino acids were tightly correlated with one another, and blood urea (a by-product of amino acid catabolism) rose with exercise. In our recent report of muscle interstitial fluid metabolomics, amino acids tended to be increased by moderate exercise (81), supporting the idea of enhanced uptake from the blood. The observed pattern of a drop in many blood amino acids with acute exercise is consistent with results of Hansen et al. (28). However, this global amino acid reduction pattern was not always apparent in other exercise metabolomics studies (e.g see Refs. 45, 60, and 80) or in experiments in which targeted amino acid determinations were made at 10 or 40 min of mild to moderate exercise (18) or 25 or 50 min of exercise at ∼65% of V̇o2max (48, 64). One aspect that seems consistent across several studies, including our own, is that acute exercise is associated with a modest to significant rise in blood alanine (8, 18, 28, 45, 48, 54, 64, 80). The work of Felig and Wahren (18) using cross-muscle arteriovenous methods showed that, during exercise, the muscle is a net producer of alanine. The rest-to-exercise blood alanine and pyruvate were closely correlated (18), and our results concur with that observation (r = 0.44, P < 0.0001). This is believed to reflect the interrelationship of alanine and pyruvate in the alanine-glucose cycle, in which muscle production of alanine (from transamination of pyruvate) provides a gluconeogenic substrate for liver (17).
As for amino acid derivatives during the exercise and recovery periods, the acute exercise-induced drop in the tryptophan derivative 5-methoxytryptamine (5-MT) has not been described previously. This shift in 5-MT might reflect changes in serotonin or melatonin conversions to 5-MT, should such reactions take place in mammals in an exercise-regulated manner. The metabolite 2-hydroxybutanoic acid (2-HB; α-hydroxybutyrate) was reduced with acute exercise, but its origins are not fully established. 2-HB is likely produced via a reduction of the methionine or threonine catabolic derivative α-ketobutyrate (1), and it is interesting to note that higher blood 2-HB has been associated with insulin resistance and type 2 diabetes phenotypes (21, 23). The metabolite aminomalonate displayed a higher concentration and larger drop with exercise following weight loss and fitness intervention. The origins of this metabolite are not clear, but we previously noted that blood aminomalonate drops with progression of the type 2 diabetes-like phenotype in a rat model (61). Altogether, our results suggest that a higher aminomalonate blood status is associated with better metabolic health.
Finally, we recently reported novel results that moderate exercise in rats (∼60% of estimated V̇o2max) and humans (65% of predicted maximal heart rate) increased several indices of the AMP catabolic pathway [AMP → inosine monophosphate (IMP) → inosine → hypoxanthine → xanthine → uric acid → allantoic acid] in muscle bed interstitial fluid (81). Allantoic acid also was reported to increase (and uric acid decrease) in muscle biopsy and blood immediately following short-term (∼4–5 min) exhaustive exercise in men (29). Pathways for AMP conversion to IMP are proposed to be key in the management of muscle adenine nucleotide pools and ATP/ADP ratios, which are critical to the continued function of muscle during times of energy demand and accelerated ATP turnover (see Ref. 26). The concerted activities of adenylate kinase (AK; 2 ADP ↔ ATP + AMP) and AMP deaminase (AMPD; AMP + H2O → IMP + NH3) regulate IMP production and would thus impact downstream metabolite concentrations. Although speculative, the exercise-associated rise in plasma glycolic acid in the current study could hypothetically be linked to purine metabolism; in sequential reactions of allantoicase and ureidoglycolate lyase, increased allantoic acid could drive glyoxylate production, which could in theory be converted to glycolic acid by glyoxylate reductase. Glyoxylate reductase mRNA expression is high in tissues such as muscle, kidney, and liver; however, allantoicase expression appears limited to germ cells (http://biogps.org; see Ref. 78), and a human ureidoglycolate lyase is not established. Thus, more research is needed to better understand how exercise duration, intensity, and site specificity impact the reactions that regulate the ATP/AMP/ADP/IMP pools and to determine whether these pathways impact glyoxylate and glycolic acid metabolism. An alternate view is that glycolic acid is derived from the collagen component hydroxyproline; e.g., humans can derive a significant amount of glyoxylate (and hence glycolate) from dietary collagen (36). If the latter is the primary source, then the exercise-associated rise in blood glycolic acid observed herein illustrates an example of how exercise impacts the xenometabolome.
Carbohydrates and Sugar Derivatives
Plasma concentrations of most monosaccharides and sugar derivatives were reduced with exercise despite little change in blood glucose and a small rise in lactate and pyruvate concentrations. We speculate that most of the effects on sugar derivatives during exercise stem from glucose fuel being directed toward oxidative fates (and away from anabolic pathways) to help meet ATP demands in muscle and other tissues. From the rested state, glucose oxidation rises severalfold at the modest exercise level used herein (68). Thus, flow toward nonoxidative pathways for glucose, such as the polyol pathway that generates fructose, would be reduced and hence, reflected in blood concentrations of these and other sugar derivatives. In preliminary results from participants exercising at 65% of estimated maximal heart rate (81), muscle interstitial fluid fructose dropped by ∼50% compared with rest, suggesting that the polyol pathway is reduced in this tissue with exercise.
A novel observation was that blood concentrations of glycerol-3-galactoside (galactosylglycerol) were reduced significantly by acute exercise, and the minor sugar galactose also was lowered modestly (albeit not statistically significant). Should lower blood concentrations of these metabolites be reflective of changes in their tissue pools, and under the assumption that these metabolites are not food derived, at least two speculative biochemical pathways may be considered. One scenario involves the well-established inhibition of glycogen synthesis that accompanies exercise (when glycogenolysis is activated), which would reduce tissue pools of UDP-glucose. UDP-glucose could be a driver of d-galactose production via a multi-enzyme process (see KEGG map00520: “amino sugar and nucleotide sugar metabolism”), should this pathway exist in mammalian biochemistry; thus, under this scenario, if glycogen synthesis slows, it would lower net galactose synthesis. Galactosidase (α- or β-galactosidase) is thought to convert glycerol-3-galactoside to galactose and glycerol plus water (46); hence, if these somewhat ubiquitous enzymes are reversible, then net glycerol-3-galactoside production would slow, concurrent with reduced galactose production during exercise. A second theoretical model, which we favor, is that glycerol-3-galactoside production slows during exercise due to inhibition of glycerolipid metabolism from glycolysis precursors. Because glucose carbon is shunted toward oxidation and away from anabolic pathways, there is a reduced pathway flux of 2-phosphoglycerate (a glycolysis intermediate) toward glyceric acid (D-glycerate), an initial step in glycerolipid production (see KEGG map00561: “glycerolipid metabolism”). This would attenuate production of the downstream intermediate 1,2-diacyl-sn-glycerol (DAG); the latter can be converted to 1,2-diacyl-3-β-d-galactosyl-sn-glycerol, which in turn is converted to glycerol-3-galactoside (3-β-d-galactosyl-sn-glycerol) via galactolipase. Support for this glycerolipid pathway model stems from the exercise-associated reduction in plasma glyceric acid observed in our study. Future experiments that utilize metabolite tracer kinetics are needed to test these working models and determine whether the pathways exist in mammals. Despite these caveats, the overall metabolite patterns for glucose and sugar derivatives reported herein support the idea that, during exercise, glucose flux is primarily toward oxidation to energy and less toward nonoxidative fates, at least in some tissues.
TCA Cycle Intermediates, Threonic Acid
A common finding among human exercise metabolomics studies is that acute aerobic exercise bouts tend to raise one or more blood (8, 45, 54, 60, 80) or muscle interstitial fluid (81) organic acids associated with the mitochondrial tricarboxylic acid cycle and other nonmitochondrial metabolic pathways central to energy management, e.g., citrate, isocitrate, α-ketoglutarate, succinate, fumarate, or malate. Our results are consistent with these observations in that plasma succinic acid was significantly increased by short-term exercise, although citric acid was reduced. To date, no experiments have been designed to fully address the basis for generally higher blood or interstitium TCA intermediates. We have highlighted in previous papers (21, 81) that one driver could be the underappreciated interplay between enhanced fatty acid oxidation and mitochondrial cataplerosis (export of TCA cycle intermediates). Our studies in isolated mouse muscle mitochondria (70) and studies by others using a perfused rat heart model (76) support the idea that signals related to oxidation of LCFA promote cataplerosis. If true, this could help explain in part the incomplete β-oxidation that is inherent to exercise (28, 42, 82). Because several of the organic acids can originate from non-TCA cytosolic pools, such hypotheses must be tempered by this fact.
Threonic acid was another exercise-altered metabolite that correlated with a wide variety of important metabolic markers when considering the patterns over the entire study period, e.g., glucose, myo-inositol, ribitol, pseudouridine, threitol, citric acid, isothreonic acid, LCFAs, and others. This suggests that the metabolism of threonic acid is somehow closely linked to overall metabolic flux, but its specific role and why it would be reduced in the blood with exercise remains to be established. Purportedly, it is a product of the oxidation of the ascorbic acid (vitamin C) derivative dehydroascorbate (71). Perhaps changes in these metabolites with exercise report on higher oxidative stress and ascorbic acid metabolism that accompany the increased oxidative metabolism of muscle.
Xenometabolites (“Non-Self” Metabolites) and Exercise
We have previously highlighted that xenometabolite patterns in blood from this cohort were altered when comparing the pre- versus postintervention phases either following a glucose tolerance test (e.g., tricarballylic acid; see Ref. 9) or during the exercise regimen described herein (γ-butyrobetaine and the xenolipid derivative cis-3,4-methyleneheptanoylcarnitine; see Ref. 82). These changes occurred despite a highly controlled diet that was equivalent in the pre- and postintervention phases in the days leading up to sample collection and instructions to arrive for the experiments in the overnight-fasted state, with liquid intake restricted to just water (no coffee, tea, or other beverages; confirmed by self-report the morning of the studies). In a rat model of spontaneous onset of a type 2 diabetes-like phenotype, a phenomenon of cecal xenometabolite changes with progression of disease was observed despite dietary and other variables controlled (62). Taken together, the results have led us to hypothesize (9, 62, 82) that the metabolic health of the host independent of diet or other factors known to modify the gut microbiome can profoundly impact the metabolism and ecosystem of the microbiota. Such a concept is bolstered by recent results in metabolic syndrome (55). The details of host-microbiome communication that drive these associations remain to be established. Even less is known about how an exercise bout per se influences patterns of circulating xenometabolites.
Several “non-self” molecules displayed increases in blood concentrations in response to exercise. For instance, plasma 1-dodecanol rose significantly coincident with upward trends in closely related molecules 1-hexadecanol and 1-octadecanol. These molecules are known products of microbial metabolism (certain yeasts) and may serve as “quorum sensing” metabolites (e.g., see Refs. 31 and 50). That they are microbe produced is bolstered by the fact that metabolomics of fresh postexercise sweat failed to detect decanol-based metabolites, whereas incubated sweat containing microbial growth was enriched in these molecules (51). Another metabolite with raised blood concentration with exercise is chlorogenic acid (5-O-caffeoylquinic acid), a phytomolecule prominent in coffee and berries (12). Another diet-derived molecule is maltose, which was significantly reduced by in plasma by the exercise bout. Interestingly, maltose concentration was increased by ∼90% alongside higher glucose in muscle interstitial fluid of exercised humans when compared with the rested state in a preliminary study (blood analysis not available) (81). Taken together, these data suggest active uptake of simple sugars such as maltose from the blood into the interstitium during exercise. Finally, there was an interesting cluster of structurally related metabolites, i.e., benzoic acid, salicylic acid (2-hydroxybenozoic acid), and salicylaldehyde (2-hydroxybenzaldehyde), that were increased in blood with exercise. The origins of these patterns are elusive, but a survey of the literature indicates that the reactions underlying interconversion of the metabolites can occur in plants (e.g., see Refs. 41, 43, and 69a), and salicylic acid is a known component of certain plant-derived foods. These reports indicate that benzoic acid 2-hydroxylase can catalyze the biosynthesis of salicylic acid from benzoic acid (benzoic acid + NADPH + H+ → salicylic acid + NADP+ + H2O); benzoic acid is potentially a derivative of trans-cinnamic acid conversion. One can envision conversion of salicylic acid to produce salicylaldehyde via a reverse aldehyde dehydrogenase reaction (salicylic acid + NADH+ + H+ → salicylaldehyde + NAD+ + H2O). Interestingly, salicylic acid is a known activator of AMP kinase (35), thus highlighting an intriguing link between the exercise metabolome and a molecular pathway central to metabolic regulation.
The observation of exercise-modifiable blood xenometabolite increases is novel and raises the question, “How can such patterns emerge if humans do not synthesize these molecules and if they are seen in the overnight-fasted state?” At least four possibilities must be considered. First, it is possible that some biochemical reactions that are historically attributed to only plants and microbes can take place in mammals, especially under challenged conditions such as exercise (and detectable by the highly sensitive analytical methods used herein). Taking benzoic acid as an example, the NADPH- and NADH-dependent reactions that generate salicylic and salicylaldehyde, respectively, might conceivably be driven by hydroxylases and aldehyde dehydrogenases, especially when reducing equivalents become more abundant during the active macronutrient catabolism and fatty acid oxidation of exercise. Second, because lipolysis and blood flow are activated with exercise, some xenometabolites could be released from storage pools in tissues such as adipose. We have hypothesized that this is the case for some long-chain xenolipids, for instance, that give rise to medium-chain acylcarnitine derivatives that track exercise (82). Third, we cannot discount the possibility that reductions in certain metabolites’ renal excretion lead to increases in blood pool concentrations during exercise. Supporting this possibility is that renal blood flow drops with increments of exertion (32), and although glomerular filtration rate reductions seem to lag this somewhat, overall renal function dampens with exercise, as blood is shunted more toward working muscle (reviewed in Refs. 10, 63, and 73). Fourth, should exercise increase xenometabolite uptake from the gastrointestinal (GI) tract, via enhanced splanchnic blood flow, increased gut permeability, and/or more rapid intestinal motility, it could help explain exercise-associated changes in the blood xenometabolome. However, in humans the hepatosplanchnic blood flow during submaximal exercise does not change or is reduced (15, 16, 27, 66, 69). Furthermore, moderate and short-term exercise (i.e., at ≤70% of V̇o2max) did not change markers of gut permeability (40, 59, 75); permeability increases only with very prolonged and strenuous exercise (see Refs. 4 and 24). As for gut motility, moderate exercise either does not change this parameter or slightly reduces it (65, 72, 75). Thus, the rise in blood concentrations of xenometabolites during the modest exercise regimen used in the current study cannot be explained solely by invoking increased splanchnic blood flow, higher GI tract permeability, or enhanced GI transit time. Whether or not gut-draining lymphatic pools of xenometabolites or other molecules are the source of exercise-associated changes in the blood metabolome or whether storage sites are mobilized with exercise remain open questions.
Summary and Primary Conclusions
By leveraging the powerful tool of metabolomics, our study confirmed well-established pathway changes while revealing novel metabolite patterns associated with exercise in adults, and through correlation analysis the studies highlight coordinated patterns among disparate metabolite classes during the exercise and recovery periods. Strengths of the study included careful monitoring of dietary intakes and efforts to exactly match pre- versus postintervention diet components (even lot numbers were matched on the nonperishable food menu), keeping the individuals’ menstrual cycle phases consistent before blood collections, ensuring within-week weight stability during the pre- and post-Test Weeks and ensuring that exercise metabolomics were examined at equal workloads pre- and postintervention (9, 82). Thus, variances in metabolomics outcomes due to differences in these factors were minimized. The experiment has several limitations. Because participants were not exercised for several days beforehand, this may have hindered our ability to detect pre- versus postintervention differences in acute exercise metabolite patterns. Significant interindividual variability in nontargeted metabolomics patterns was observed, and this limited sensitivity in identifying exercise- or intervention-associated differences in some metabolites and pathways. We also acknowledge that our findings are confined to nondiabetic women without any clinical manifestations of heart disease or other ailments; additional age groups, male cohorts, and persons with or without disease are needed to test whether fixed-workload exercise metabolomics patterns are impacted by different phenotypes throughout the lifespan.
In summary, the results from the study revealed two heretofore underappreciated aspects of metabolic physiology in humans. First, despite marked improvements in metabolic health, adiposity, fitness, and insulin sensitivity following a weight loss and training intervention in sedentary, obese, insulin-resistant individuals, the exercise-associated metabolite patterns at a fixed workload changed very little. This finding concurs with previously reported blood acylcarnitine exercise-associated patterns showing no intervention-related change in patterns of markers of incomplete β-oxidation (82). These outcomes highlight that changes in most metabolic pathways during aerobic submaximal exercise are closely tethered to ATP turnover, regardless of fitness or insulin sensitivity status. Second, exercise drives acute changes in blood xenometabolite and food-derived molecule patterns with as-yet undefined origins and implications. Future studies should address the tissue-specific origins of exercise-related metabolite changes, how the metabolome responds across the entire spectrum of low- to modest- to high-intensity exercise, which pathways and metabolites link to exertion and fatigue signaling, and the potential interrelationships between exercise, fitness, and the xenometabolome.
GRANTS
This work was supported in part by USDA-ARS Projects 5306-51530-016-00D, 5306-51530-019-00, and 6026-51000-010-05S and National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK078328 (S. H. Adams, C. L. Hoppel, O. Fiehn, N. L. Keim, J. W. Newman, W. T. Garvey, G. R. Hunter, J. R. Fernandez, M. E. Harper) and U24-DK-097154 for the West Coast Metabolomics Center (OF, J. W. Newman). The USDA is an equal opportunity provider and employer.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.F., C.C., C.J.C., D.J.B., E.C.S., G.A.C., N.L.K., J.W.N., G.R.H., J.R.F., W.T.G., C.L.H., M.-E.H., and S.H.A. conceived and designed research; O.F., C.C., C.J.C., D.J.B., E.C.S., G.A.C., and S.H.A. performed experiments; D.G., O.F., C.C., C.J.C., D.J.B., E.C.S., G.A.C., and S.H.A. analyzed data; D.G., O.F., C.C., C.J.C., E.C.S., G.A.C., and S.H.A. interpreted results of experiments; D.G. and S.H.A. prepared figures; D.G. and S.H.A. drafted manuscript; D.G., O.F., C.C., C.J.C., D.J.B., E.C.S., G.A.C., N.L.K., J.W.N., G.R.H., J.R.F., W.T.G., C.L.H., M.-E.H., and S.H.A. edited and revised manuscript; D.G., O.F., C.C., C.J.C., D.J.B., E.C.S., G.A.C., N.L.K., J.W.N., G.R.H., J.R.F., W.T.G., C.L.H., M.-E.H., and S.H.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mary Gustafson, Ellen Bonnel, and the WHNRC Human Studies Unit plus the Analytical Support Laboratory staff of the USDA-ARS WHNRC for helping support screening recruitment efforts and blood collection and processing.
REFERENCES
- 1.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2: 445–456, 2011. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, Dent R, Hwang DH, Adams SH, Harper ME. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J 29: 336–345, 2015. doi: 10.1096/fj.14-255901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansley L, Bonini M, Delgado L, Del Giacco S, Du Toit G, Khaitov M, Kurowski M, Hull JH, Moreira A, Robson-Ansley PJ. Pathophysiological mechanisms of exercise-induced anaphylaxis: an EAACI position statement. Allergy 70: 1212–1221, 2015. doi: 10.1111/all.12677. [DOI] [PubMed] [Google Scholar]
- 5.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36: 936–942, 1982. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 7.Brooks GA. Importance of the ‘crossover’ concept in exercise metabolism. Clin Exp Pharmacol Physiol 24: 889–895, 1997. doi: 10.1111/j.1440-1681.1997.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 8.Brugnara L, Vinaixa M, Murillo S, Samino S, Rodriguez MA, Beltran A, Lerin C, Davison G, Correig X, Novials A. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS One 7: e40600, 2012. doi: 10.1371/journal.pone.0040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell C, Grapov D, Fiehn O, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Gustafson MB, Keim NL, Newman JW, Hunter GR, Fernandez JR, Garvey WT, Harper ME, Hoppel CL, Meissen JK, Take K, Adams SH. Improved metabolic health alters host metabolism in parallel with changes in systemic xeno-metabolites of gut origin. PLoS One 9: e84260, 2014. [Correction in: PLoS One 9: e91258, 2014.] doi: 10.1371/journal.pone.0084260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castenfors J. Renal function during prolonged exercise. Ann N Y Acad Sci 301: 151–159, 1977. doi: 10.1111/j.1749-6632.1977.tb38194.x. [DOI] [PubMed] [Google Scholar]
- 11.Chorell E, Moritz T, Branth S, Antti H, Svensson MB. Predictive metabolomics evaluation of nutrition-modulated metabolic stress responses in human blood serum during the early recovery phase of strenuous physical exercise. J Proteome Res 8: 2966–2977, 2009. doi: 10.1021/pr900081q. [DOI] [PubMed] [Google Scholar]
- 12.Clifford MN, Jaganath IB, Ludwig IA, Crozier A. Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Nat Prod Rep 34: 1391–1421, 2017. doi: 10.1039/C7NP00030H. [DOI] [PubMed] [Google Scholar]
- 13.Corkey BE, Martin-Requero A, Walajtys-Rode E, Williams RJ, Williamson JR. Regulation of the branched chain alpha-ketoacid pathway in liver. J Biol Chem 257: 9668–9676, 1982. [PubMed] [Google Scholar]
- 14.Davison G, Vinaixa M, McGovern R, Beltran A, Novials A, Correig X, McClean C. Metabolomic Response to Acute Hypoxic Exercise and Recovery in Adult Males. Front Physiol 9: 1682, 2018. doi: 10.3389/fphys.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J Physiol 544: 957–962, 2002. doi: 10.1113/jphysiol.2002.025148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Febbraio MA, Ott P, Nielsen HB, Steensberg A, Keller C, Krustrup P, Secher NH, Pedersen BK. Hepatosplanchnic clearance of interleukin-6 in humans during exercise. Am J Physiol Endocrinol Metab 285: E397–E402, 2003. doi: 10.1152/ajpendo.00134.2003. [DOI] [PubMed] [Google Scholar]
- 17.Felig P. The glucose-alanine cycle. Metabolism 22: 179–207, 1973. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- 18.Felig P, Wahren J. Amino acid metabolism in exercising man. J Clin Invest 50: 2703–2714, 1971. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Féry F, Balasse EO. Ketone body turnover during and after exercise in overnight-fasted and starved humans. Am J Physiol 245: E318–E325, 1983. doi: 10.1152/ajpendo.1983.245.4.E318. [DOI] [PubMed] [Google Scholar]
- 20.Féry F, Balasse EO. Response of ketone body metabolism to exercise during transition from postabsorptive to fasted state. Am J Physiol 250: E495–E501, 1986. doi: 10.1152/ajpendo.1986.250.5.E495. [DOI] [PubMed] [Google Scholar]
- 21.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 5: e15234, 2010. doi: 10.1371/journal.pone.0015234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiehn O, Kind T. Metabolite profiling in blood plasma. Methods Mol Biol 358: 3–17, 2007. doi: 10.1007/978-1-59745-244-1_1. [DOI] [PubMed] [Google Scholar]
- 23.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E; RISC Study Group . alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5: e10883, 2010. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannetti MP. Exercise-induced anaphylaxis: literature review and recent updates. Curr Allergy Asthma Rep 18: 72, 2018. doi: 10.1007/s11882-018-0830-6. [DOI] [PubMed] [Google Scholar]
- 25.Grapov D, Wanichthanarak K, Fiehn O. MetaMapR: pathway independent metabolomic network analysis incorporating unknowns. Bioinformatics 31: 2757–2760, 2015. doi: 10.1093/bioinformatics/btv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock CR, Brault JJ, Terjung RL. Protecting the cellular energy state during contractions: role of AMP deaminase. J Physiol Pharmacol 57, Suppl 10: 17–29, 2006. [PubMed] [Google Scholar]
- 27.Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, Pedersen BK, Plomgaard P. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab 4: 551–560, 2015. doi: 10.1016/j.molmet.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen JS, Zhao X, Irmler M, Liu X, Hoene M, Scheler M, Li Y, Beckers J, Hrabĕ de Angelis M, Häring HU, Pedersen BK, Lehmann R, Xu G, Plomgaard P, Weigert C. Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia 58: 1845–1854, 2015. doi: 10.1007/s00125-015-3584-x. [DOI] [PubMed] [Google Scholar]
- 29.Hellsten Y, Tullson PC, Richter EA, Bangsbo J. Oxidation of urate in human skeletal muscle during exercise. Free Radic Biol Med 22: 169–174, 1997. doi: 10.1016/S0891-5849(96)00286-9. [DOI] [PubMed] [Google Scholar]
- 31.Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol 54: 1212–1223, 2004. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawakami S, Yasuno T, Matsuda T, Fujimi K, Ito A, Yoshimura S, Uehara Y, Tanaka H, Saito T, Higaki Y. Association between exercise intensity and renal blood flow evaluated using ultrasound echo. Clin Exp Nephrol 22: 1061–1068, 2018. doi: 10.1007/s10157-018-1559-1. [DOI] [PubMed] [Google Scholar]
- 33.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest 115: 1699–1702, 2005. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277: E1130–E1141, 1999. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med 48: e224, 2016. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight J, Jiang J, Assimos DG, Holmes RP. Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int 70: 1929–1934, 2006. doi: 10.1038/sj.ki.5001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn M, Wing J, Weston S, Williams A, Keefer C, Engelhardt A, Cooper T, Mayer Z, Kenkel B; The R Core Team, Benesty M, Lescarbeau R, Ziem A, Scrucca L, Tang Y, Candan C, Hunt T. Caret (Classification and Regression Training) R Package that Contains Misc Functions for Training and Plotting Classification and Regression Models (Online) https://github.com/topepo/caret/ [16 Oct 2019].
- 39.Lai Z, Tsugawa H, Wohlgemuth G, Mehta S, Mueller M, Zheng Y, Ogiwara A, Meissen J, Showalter M, Takeuchi K, Kind T, Beal P, Arita M, Fiehn O. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat Methods 15: 53–56, 2018. doi: 10.1038/nmeth.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambert GP, Lang J, Bull A, Pfeifer PC, Eckerson J, Moore G, Lanspa S, O’Brien J. Fluid restriction during running increases GI permeability. Int J Sports Med 29: 194–198, 2008. doi: 10.1055/s-2007-965163. [DOI] [PubMed] [Google Scholar]
- 41.Lee HI, León J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA 92: 4076–4079, 1995. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehmann R, Zhao X, Weigert C, Simon P, Fehrenbach E, Fritsche J, Machann J, Schick F, Wang J, Hoene M, Schleicher ED, Häring HU, Xu G, Niess AM. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS One 5: e11519, 2010. doi: 10.1371/journal.pone.0011519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.León J, Shulaev V, Yalpani N, Lawton MA, Raskin I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci USA 92: 10413–10417, 1995. doi: 10.1073/pnas.92.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerin C, Goldfine AB, Boes T, Liu M, Kasif S, Dreyfuss JM, De Sousa-Coelho AL, Daher G, Manoli I, Sysol JR, Isganaitis E, Jessen N, Goodyear LJ, Beebe K, Gall W, Venditti CP, Patti ME. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol Metab 5: 926–936, 2016. doi: 10.1016/j.molmet.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, Gerszten RE. Metabolic signatures of exercise in human plasma. Sci Transl Med 2: 33ra37, 2010. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Liu S, Wang C, Li K, Shan YJ, Wang XJ, Sun CH. Novel biomarkers of 3-chloro-1,2-propanediol exposure by ultra performance liquid chromatography/mass spectrometry based metabonomic analysis of rat urine. Chem Res Toxicol 23: 1012–1017, 2010. doi: 10.1021/tx900400p. [DOI] [PubMed] [Google Scholar]
- 47.Liaw A, Wiener M. Classification and regression by randomForest. R News 2: 18–22, 2002. [Google Scholar]
- 48.MacLaren DP, Nevill AM, Thake CD, Campbell IT, Cheetham E, Keegan MA, Lane C, Roberts NB. Human erythrocyte and plasma amino acid concentrations during exercise. Med Sci Sports Exerc 32: 1244–1249, 2000. doi: 10.1097/00005768-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 49.Mandarino LJ, Consoli A, Jain A, Kelley DE. Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol 270: E463–E470, 1996. doi: 10.1152/ajpendo.1996.270.3.E463. [DOI] [PubMed] [Google Scholar]
- 50.Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell 6: 2429–2436, 2007. doi: 10.1128/EC.00252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meijerink J, Braks MAH, Brack AA, Adam W, Dekker T, Posthumus MA, Van Beek TA, Van Loon JJA. Identification of olfactory stimulants for anopheles gambiae from human sweat samples. J Chem Ecol 26: 1367–1382, 2000. doi: 10.1023/A:1005475422978. [DOI] [Google Scholar]
- 52.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 18: 1695–1700, 2010. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148, 1961. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 54.Mueller-Hennessen M, Sigl J, Fuhrmann JC, Witt H, Reszka R, Schmitz O, Kastler J, Fischer JJ, Müller OJ, Giannitsis E, Weis T, Frey N, Katus HA. Metabolic profiles in heart failure due to non-ischemic cardiomyopathy at rest and under exercise. ESC Heart Fail 4: 178–189, 2017. doi: 10.1002/ehf2.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, Michel ML, Chong-Nguyen C, Roussel R, Straube M, Jegou S, McQuitty C, Le Gall M, da Costa G, Lecornet E, Michaudel C, Modoux M, Glodt J, Bridonneau C, Sovran B, Dupraz L, Bado A, Richard ML, Langella P, Hansel B, Launay JM, Xavier RJ, Duboc H, Sokol H. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab 28: 737–749.e4, 2018. doi: 10.1016/j.cmet.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Nieman DC, Gillitt ND, Sha W, Meaney MP, John C, Pappan KL, Kinchen JM. Metabolomics-Based Analysis of Banana and Pear Ingestion on Exercise Performance and Recovery. J Proteome Res 14: 5367–5377, 2015. doi: 10.1021/acs.jproteome.5b00909. [DOI] [PubMed] [Google Scholar]
- 57.Nieman DC, Scherr J, Luo B, Meaney MP, Dréau D, Sha W, Dew DA, Henson DA, Pappan KL. Influence of pistachios on performance and exercise-induced inflammation, oxidative stress, immune dysfunction, and metabolite shifts in cyclists: a randomized, crossover trial. PLoS One 9: e113725, 2014. doi: 10.1371/journal.pone.0113725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nieman DC, Sha W, Pappan KL. IL-6 Linkage to Exercise-Induced Shifts in Lipid-Related Metabolites: A Metabolomics-Based Analysis. J Proteome Res 16: 970–977, 2017. doi: 10.1021/acs.jproteome.6b00892. [DOI] [PubMed] [Google Scholar]
- 59.Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol (1985) 82: 571–576, 1997. doi: 10.1152/jappl.1997.82.2.571. [DOI] [PubMed] [Google Scholar]
- 60.Peake JM, Tan SJ, Markworth JF, Broadbent JA, Skinner TL, Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am J Physiol Endocrinol Metab 307: E539–E552, 2014. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 61.Piccolo BD, Graham JL, Stanhope KL, Fiehn O, Havel PJ, Adams SH. Plasma amino acid and metabolite signatures tracking diabetes progression in the UCD-T2DM rat model. Am J Physiol Endocrinol Metab 310: E958–E969, 2016. doi: 10.1152/ajpendo.00052.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piccolo BD, Graham JL, Stanhope KL, Nookaew I, Mercer KE, Chintapalli SV, Wankhade UD, Shankar K, Havel PJ, Adams SH. Diabetes-associated alterations in the cecal microbiome and metabolome are independent of diet or environment in the UC Davis Type 2 Diabetes Mellitus Rat model. Am J Physiol Endocrinol Metab 315: E961–E972, 2018. doi: 10.1152/ajpendo.00203.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poortmans JR. Exercise and renal function. Sports Med 1: 125–153, 1984. doi: 10.2165/00007256-198401020-00003. [DOI] [PubMed] [Google Scholar]
- 64.Poortmans JR, Siest G, Galteau MM, Houot O. Distribution of plasma amino acids in humans during submaximal prolonged exercise. Eur J Appl Physiol Occup Physiol 32: 143–147, 1974. doi: 10.1007/BF00421572. [DOI] [PubMed] [Google Scholar]
- 65.Rao KA, Yazaki E, Evans DF, Carbon R. Objective evaluation of small bowel and colonic transit time using pH telemetry in athletes with gastrointestinal symptoms. Br J Sports Med 38: 482–487, 2004. doi: 10.1136/bjsm.2003.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rehrer NJ, Smets A, Reynaert H, Goes E, De Meirleir K. Effect of exercise on portal vein blood flow in man. Med Sci Sports Exerc 33: 1533–1537, 2001. doi: 10.1097/00005768-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 67.Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr 20: 457–483, 2000. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- 68.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol 265: E380–E391, 1993. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 69.Rowell LB, Blackmon JR, Bruce RA. Indocyanine Green Clearance and Estimated Hepatic Blood Flow during Mild to Maximal Exercise in Upright Man. J Clin Invest 43: 1677–1690, 1964. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69a.Sawada H, Shim IS, Usui K. Induction of benzoic acid 2-hydroxylase and salicylic acid biosynthesis—Modulation by salt stress in rice seedlings. Plant Sci 171: 263–270, 2006. doi: 10.1016/j.plantsci.2006.03.020. [DOI] [Google Scholar]
- 70.Seifert EL, Fiehn O, Bezaire V, Bickel DR, Wohlgemuth G, Adams SH, Harper ME. Long-chain fatty acid combustion rate is associated with unique metabolite profiles in skeletal muscle mitochondria. PLoS One 5: e9834, 2010. doi: 10.1371/journal.pone.0009834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simpson GL, Ortwerth BJ. The non-oxidative degradation of ascorbic acid at physiological conditions. Biochim Biophys Acta 1501: 12–24, 2000. doi: 10.1016/S0925-4439(00)00009-0. [DOI] [PubMed] [Google Scholar]
- 72.Soffer EE, Summers RW, Gisolfi C. Effect of exercise on intestinal motility and transit in trained athletes. Am J Physiol 260: G698–G702, 1991. doi: 10.1152/ajpgi.1991.260.5.G698. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki M. Physical exercise and renal function. J Phys Fit Sports Med 4: 17–29, 2015. doi: 10.7600/jpfsm.4.17. [DOI] [Google Scholar]
- 74.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115: 1934–1941, 2005. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Nieuwenhoven MA, Brouns F, Brummer RJ. Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol 91: 429–434, 2004. doi: 10.1007/s00421-003-1007-z. [DOI] [PubMed] [Google Scholar]
- 76.Vincent G, Comte B, Poirier M, Rosiers CD. Citrate release by perfused rat hearts: a window on mitochondrial cataplerosis. Am J Physiol Endocrinol Metab 278: E846–E856, 2000. doi: 10.1152/ajpendo.2000.278.5.E846. [DOI] [PubMed] [Google Scholar]
- 77.Wolfe RR. Metabolic interactions between glucose and fatty acids in humans. Am J Clin Nutr 67, Suppl: 519S–526S, 1998. doi: 10.1093/ajcn/67.3.519S. [DOI] [PubMed] [Google Scholar]
- 78.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW III, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu G, Hansen JS, Zhao XJ, Chen S, Hoene M, Wang XL, Clemmesen JO, Secher NH, Häring HU, Pedersen BK, Lehmann R, Weigert C, Plomgaard P. Liver and muscle contribute differently to the plasma acylcarnitine pool during fasting and exercise in humans. J Clin Endocrinol Metab 101: 5044–5052, 2016. doi: 10.1210/jc.2016-1859. [DOI] [PubMed] [Google Scholar]
- 80.Zafeiridis A, Chatziioannou AC, Sarivasiliou H, Kyparos A, Nikolaidis MG, Vrabas IS, Pechlivanis A, Zoumpoulakis P, Baskakis C, Dipla K, Theodoridis GA. Global Metabolic Stress of Isoeffort Continuous and High Intensity Interval Aerobic Exercise: A Comparative 1H NMR Metabonomic Study. J Proteome Res 15: 4452–4463, 2016. doi: 10.1021/acs.jproteome.6b00545. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, Bhattacharyya S, Hickner RC, Light AR, Lambert CJ, Gale BK, Fiehn O, Adams SH. Skeletal muscle interstitial fluid metabolomics at rest and associated with an exercise bout: application in rats and humans. Am J Physiol Endocrinol Metab 316: E43–E53, 2019. doi: 10.1152/ajpendo.00156.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J, Light AR, Hoppel CL, Campbell C, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Hughen RW, Keim NL, Newman JW, Hunter GR, Fernandez JR, Garvey WT, Harper ME, Fiehn O, Adams SH. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp Physiol 102: 48–69, 2017. doi: 10.1113/EP086019. [DOI] [PMC free article] [PubMed] [Google Scholar]