Abstract
Background:
Despite an increase in the number of therapies available to treat patients with immune thrombocytopenia (ITP), there are minimal data from randomized trials to assist physicians with the management of patients.
Objective:
These evidence-based guidelines of the American Society of Hematology (ASH) are intended to support patients, clinicians, and other health care professionals in their decisions about the management of ITP.
Methods:
In 2015, ASH formed a multidisciplinary guideline panel that included 8 adult clinical experts, 5 pediatric clinical experts, 2 methodologists with expertise in ITP, and 2 patient representatives. The panel was balanced to minimize potential bias from conflicts of interest. The panel reviewed the ASH 2011 guideline recommendations and prioritized questions. The panel used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, including evidence-to-decision frameworks, to appraise evidence (up to May 2017) and formulate recommendations.
Results:
The panel agreed on 21 recommendations covering management of ITP in adults and children with newly diagnosed, persistent, and chronic disease refractory to first-line therapy who have non–life-threatening bleeding. Management approaches included: observation, corticosteroids, IV immunoglobulin, anti-D immunoglobulin, rituximab, splenectomy, and thrombopoietin receptor agonists.
Conclusions:
There was a lack of evidence to support strong recommendations for various management approaches. In general, strategies that avoided medication side effects were favored. A large focus was placed on shared decision-making, especially with regard to second-line therapy. Future research should apply standard corticosteroid-dosing regimens, report patient-reported outcomes, and include cost-analysis evaluations.
Summary of recommendations
Background
These guidelines are based on updated and original systematic reviews of evidence conducted under the direction of the University of Oklahoma Health Sciences Center (OUHSC). The guideline panel followed best practice for guideline development recommended by the Institute of Medicine and the Guidelines International Network (GIN).1-4 The panel used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach5-10 to assess the certainty in the evidence and formulate recommendations.
These guidelines focus on the management of immune thrombocytopenia (ITP). ITP is an acquired autoimmune disorder characterized by a low platelet count resulting from platelet destruction and impaired platelet production. The incidence of ITP is estimated to be 2 to 5 per 100 000 persons in the general population.11-15 Large randomized trials on the management of ITP are lacking, resulting in significant controversy and variation in practice. We summarize available evidence and recommendations regarding first- and second-line management of adults and children with ITP.
Interpretation of strong and conditional recommendations
The strength of a recommendation is expressed as either strong (“the guideline panel recommends…”) or conditional (“the guideline panel suggests…”) and has the following interpretation:
Strong recommendation
For patients: Most individuals in this situation would want the recommended course of action, and only a small proportion would not.
For clinicians: Most individuals should follow the recommended course of action. Formal decision aids are not likely to be needed to help individual patients make decisions consistent with their values and preferences.
For policy makers: The recommendation can be adopted as policy in most situations. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator.
For researchers: The recommendation is supported by credible research or other convincing judgments that make additional research unlikely to alter the recommendation. On occasion, a strong recommendation is based on low or very low certainty in the evidence. In such instances, further research may provide important information that alters the recommendations.
Conditional recommendation
For patients: The majority of individuals in this situation would want the suggested course of action, but many would not. Decision aids may be useful in helping patients to make decisions consistent with their individual risks, values, and preferences.
For clinicians: Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with the patient’s values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their individual risks, values, and preferences.
For policy makers: Policy-making will require substantial debate and involvement of various stakeholders. Performance measures about the suggested course of action should focus on whether an appropriate decision-making process is duly documented.
For researchers: This recommendation is likely to be strengthened (for future updates or adaptation) by additional research. An evaluation of the conditions and criteria (and the related judgments, research evidence, and additional considerations) that determined the conditional (rather than strong) recommendation will help identify possible research gaps.
Interpretation of good practice statements
As described by the GRADE Guidance Group, good practice statements endorse interventions or practices that the guideline panel agreed have unequivocal net benefit yet may not be widely recognized or used.16 Good practice statements in these guidelines are not based on a systematic review of available evidence. Nevertheless, they may be interpreted as strong recommendations.
Recommendations
Management of adult patients with newly diagnosed ITP
Corticosteroids vs observation.
Recommendation 1a.
In adults with newly diagnosed ITP and a platelet count of <30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the American Society of Hematology (ASH) guideline panel suggests corticosteroids rather than management with observation (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: There may be a subset of patients within this group for whom observation might be appropriate. This should include consideration of the severity of thrombocytopenia, additional comorbidities, use of anticoagulant or antiplatelet medications, need for upcoming procedures, and age of the patient.
Recommendation 1b.
In adults with newly diagnosed ITP and a platelet count of ≥30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel recommends against corticosteroids and in favor of management with observation (strong recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: For patients with a platelet count at the lower end of this threshold, for those with additional comorbidities, anticoagulant or antiplatelet medications, or upcoming procedures, and for elderly patients (>60 years old), treatment with corticosteroids may be appropriate.
Good practice statement.
The treating physician should ensure that the patient is adequately monitored for potential corticosteroid side effects regardless of the duration or type of corticosteroid selected. This includes close monitoring for hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis. Given the potential impact of corticosteroids on mental health, the treating physician should conduct an assessment of health-related quality of life (HRQoL) (depression, fatigue, mental status, etc) while patients are receiving corticosteroids.
Inpatient vs outpatient management.
Recommendation 2a.
In adults with newly diagnosed ITP and a platelet count of <20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests admission to the hospital rather than management as an outpatient (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). In adults with an established diagnosis of ITP and a platelet count of <20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests outpatient management rather than hospital admission (conditional recommendation based on very low certainty in the evidence ⊕◯◯◯). Remark: Patients who are refractory to treatment, those with social concerns, uncertainty about the diagnosis, significant comorbidities with risk of bleeding, and more significant mucosal bleeding may benefit from admission to the hospital. Patients not admitted to the hospital should receive education and expedited follow-up with a hematologist. The need for admission is also variable across the range of platelet counts represented here (0 to 20 × 109/L).
Recommendation 2b.
In adults with a platelet count of ≥20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests management as an outpatient rather than hospital admission (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: Patients who are refractory to treatment, with social concerns, uncertainty about the diagnosis, significant comorbidities with risk of bleeding, and more significant mucosal bleeding may benefit from admission to the hospital. Patients not admitted to the hospital should receive education and expedited follow-up with a hematologist. The need for admission is also variable across the range of platelet counts represented here (20 × 109/L to 150 × 109/L).
Good practice statement.
The referring physician should ensure that the patient has follow-up with a hematologist within 24 to 72 hours of the diagnosis or disease relapse.
Duration and type of corticosteroids.
Recommendation 3.
In adults with newly diagnosed ITP, the ASH guideline panel recommends against a prolonged course (>6 weeks including treatment and taper) of prednisone and in favor of a short course (≤6 weeks) (strong recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Good practice statement.
The treating physician should ensure that the patient is adequately monitored for potential corticosteroid side effects regardless of duration or type of corticosteroid selected. This includes close monitoring for hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis. Given the impact of corticosteroids on mental health, the treating physician should conduct an assessment of HRQoL (depression, fatigue, mental status, etc) while patients are receiving corticosteroids.
Recommendation 4.
In adults with newly diagnosed ITP, the ASH guideline panel suggests either prednisone (0.5-2.0 mg/kg per day) or dexamethasone (40 mg per day for 4 days) as the type of corticosteroid for initial therapy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: If a high value is placed on rapidity of platelet count response, an initial course of dexamethasone may be preferred over prednisone, given that dexamethasone showed increased desirable effects with regard to response at 7 days.
Good practice statement.
The treating physician should ensure that the patient is adequately monitored for potential corticosteroid side effects regardless of the duration or type of corticosteroid selected. This includes close monitoring for hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis. Given the impact of corticosteroids on mental health, the treating physician should assess HRQoL (depression, fatigue, mental status, etc) while patients are receiving corticosteroids.
Rituximab as initial treatment.
Recommendation 5.
In adults with newly diagnosed ITP, the ASH guideline panel suggests corticosteroids alone rather than rituximab and corticosteroids for initial therapy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: If high value is placed on the possibility for remission over concerns for potential side effects of rituximab, then an initial course of corticosteroids with rituximab may be preferred.
Management of adults with ITP who are corticosteroid-dependent or do not have a response to corticosteroids
Eltrombopag vs romiplostim.
Recommendation 6.
In adults with ITP for ≥3 months who are corticosteroid-dependent or unresponsive to corticosteroids and are going to be treated with a thrombopoietin receptor agonist (TPO-RA), the ASH guideline panel suggests either eltrombopag or romiplostim (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: Individual patient preference may place a higher value on the use of a daily oral medication or weekly subcutaneous injections.
Second-line therapies: splenectomy, TPO-RA, and rituximab compared 1 against the other.
Recommendation 7.
In adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids, the ASH guideline panel suggests either splenectomy or a TPO-RA (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 8.
In adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids, the ASH guideline panel suggests rituximab rather than splenectomy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 9.
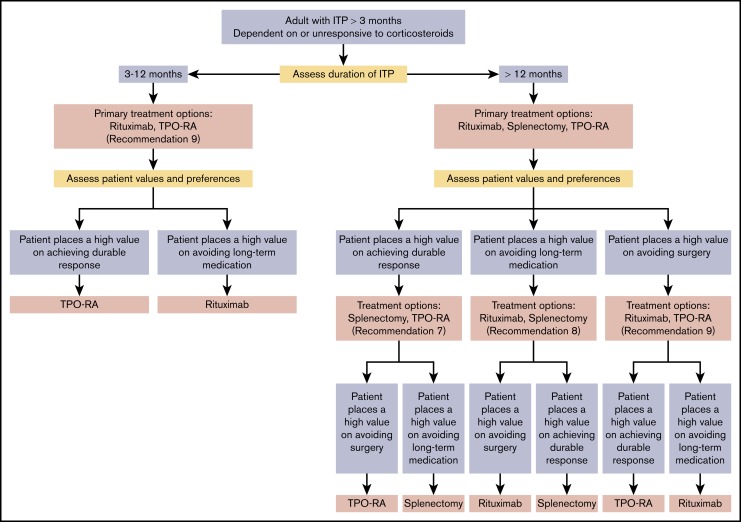
In adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids, the ASH guideline panel suggests a TPO-RA rather than rituximab (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: These recommendations are the result of dichotomous evaluation of treatments that are often being considered simultaneously. Each of these second-line treatments may be effective therapy and therefore the choice of treatment should be individualized based on duration of ITP, frequency of bleeding episodes requiring hospitalization or rescue medication, comorbidities, age of the patient, medication adherence, medical and social support networks, patient values and preferences, cost, and availability. Patient education and shared decision-making are encouraged. If possible, splenectomy should be delayed for at least 1 year after diagnosis because of the potential for spontaneous remission in the first year. Patients who value avoidance of long-term medication may prefer splenectomy or rituximab. Patients who wish to avoid surgery may prefer a TPO-RA or rituximab. Patients who place a high value on achieving a durable response may prefer splenectomy or TPO-RAs.
Good practice statement.
The treating physician should ensure that patients have appropriate immunizations prior to splenectomy and that they receive counseling regarding antibiotic prophylaxis following splenectomy. The treating physician should also educate the patient on prompt recognition and management of fever and refer to current recommendations on pre- and postsplenectomy care.
Management of children with newly diagnosed ITP
Outpatient vs inpatient management.
Recommendation 10a.
In children with newly diagnosed ITP and a platelet count of <20 × 109/L who have no or mild bleeding (skin manifestations) only, the ASH guideline panel suggests against admission to the hospital and in favor of management as an outpatient (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: For patients with uncertainty about the diagnosis, those with social concerns, those who live far from the hospital, and those for whom follow-up cannot be guaranteed, admission to the hospital may be preferable.
Recommendation 10b.
In children with newly diagnosed ITP and a platelet count of ≥20 × 109/L who have no or mild bleeding (skin manifestations) only, the ASH guideline panel suggests against admission to the hospital and in favor of management as an outpatient (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: For patients with uncertainty about the diagnosis, those with social concerns, those who live far from the hospital, or those for whom follow-up cannot be guaranteed, admission to the hospital may be preferable.
Good practice statement.
The referring physician should ensure that the patient has follow-up with a hematologist within 24 to 72 hours of diagnosis.
Treatment vs observation.
Recommendation 11.
In children with newly diagnosed ITP who have no or minor bleeding, the ASH guideline panel suggests observation rather than corticosteroids (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 12.
In children with newly diagnosed ITP who have no or minor bleeding, the ASH guideline panel recommends observation rather than IV immunoglobulin (IVIG) (strong recommendation based on moderate certainty in the evidence of effects ⊕⊕⊕◯).
Recommendation 13.
In children with newly diagnosed ITP who have no or minor bleeding, the ASH guideline panel recommends observation rather than anti-D immunoglobulin (strong recommendation based on moderate certainty in the evidence of effects ⊕⊕⊕◯).
Corticosteroid duration and type.
Recommendation 14.
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel recommends against courses of corticosteroids longer than 7 days and in favor of courses 7 days or shorter (strong recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 15.
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests prednisone (2-4 mg/kg per day; maximum, 120 mg daily, for 5-7 days) rather than dexamethasone (0.6 mg/kg per day; maximum, 40 mg per day for 4 days) (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Treatment of children with non–life-threatening bleeding and/or diminished HRQoL.
Recommendation 16.
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests corticosteroids rather than anti-D immunoglobulin (conditional recommendation based on low certainty in the evidence of effects ⊕⊕◯◯). Remark: This recommendation assumes corticosteroid dosing as outlined recommendations 14 and 15. This recommendation is reserved only for children with nonmajor mucosal bleeding.
Recommendation 17.
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests either anti-D immunoglobulin or IVIG (conditional recommendation based on low certainty in the evidence of effects ⊕⊕◯◯). Remark: This recommendation is reserved only for children with nonmajor mucosal bleeding.
Recommendation 18.
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests corticosteroids rather than IVIG (conditional recommendation based on low certainty in the evidence of effects ⊕⊕◯◯). Remark: This recommendation assumes that a short course of corticosteroids is being used for treatment as recommended in recommendation 14. This recommendation is reserved only for children with nonmajor mucosal bleeding.
Management of children with ITP who do not have a response to first-line treatment
Second-line therapies: splenectomy, TPO-RA, and rituximab compared 1 against the other.
Recommendation 19.
In children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment, the ASH guideline panel suggests the use of TPO-RAs rather than rituximab (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 20.
In children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment, the ASH guideline panel suggests TPO-RAs rather than splenectomy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 21.
In children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment, the ASH guideline panel suggests rituximab rather than splenectomy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Good practice statement.
The treating physician should ensure that the patient has appropriate immunizations prior to splenectomy and that they receive counseling regarding antibiotic prophylaxis following splenectomy. The treating physician should educate the patient on prompt recognition and management of fever and refer to current recommendations on pre- and postsplenectomy care.
Introduction
Aim of these guidelines and specific objectives
The purpose of these guidelines is to provide evidence-based recommendations for the management of adults and children with ITP. The primary goals of these guidelines are to review, critically appraise, and implement evidence-based recommendations that describe the impact of treatments, platelet count response, adverse events, and patient-reported outcomes. These guidelines specifically focus on the management of adults and children with ITP and non–life-threatening bleeding. Recommendations 1 to 5 address adults with newly diagnosed ITP whereas 6 to 9 relate to second-line therapies. Recommendations 10 to 21 concern pediatric patients (10-18 newly diagnosed, 19-21 second-line therapy). They do not address emergency management of ITP, pregnancy, or treatments that were introduced after 2017.
Through improved provider and patient education of the available evidence and evidence-based recommendations, this guideline aims to provide clinical decision-making support for different treatment pathways. The inclusion of patient-reported outcomes also helps to ensure that the information provided in this guideline relates closely to benefits that matter most to patients.
The target audience includes patients, hematologists, general practitioners, emergency room physicians, and other clinicians and decision-makers. Policy-makers interested in these guidelines include those involved in developing local, national, or international plans with the goal to implement best practice, reduce cost, and improve patient outcomes. This document may also be adapted by local, regional, or national guideline panels.
Description of the health problem
ITP is an acquired autoimmune disorder characterized by a low platelet count resulting from platelet destruction and impaired platelet production. ITP has an incidence of 2 to 5 per 100 00011-15 and can be an isolated primary condition or it may be secondary to other conditions. ITP is a heterogeneous disorder with variable clinical symptoms and remains a diagnosis of exclusion of other causes of thrombocytopenia.17 The likelihood of a spontaneous remission from ITP is age related, with 1-year remission rates of 74% in children <1 year of age, 67% in those between 1 and 6 years of age, and 62% in those 10 to 20 years of age.18,19 Natural history data in adults are less well studied, with reports of 20% to 45% of patients achieving complete remission by 6 months; identifying spontaneous remissions beyond 6 months is more difficult secondary to the use of disease-modifying therapies.20-23 In both adult and pediatric studies, defining remission is often based on a single point in time and, therefore, patients may be misclassified as in remission and relapse at a later time depending on the criteria applied to define remission status. The clinical course of ITP may also be different depending on whether it is primary ITP (not associated with any other conditions), occurs in the setting of additional autoimmune cytopenias (Evans syndrome), is the manifestation of a primary immunodeficiency, or is associated with an underlying autoimmune condition or infection (secondary ITP). In the latter, the treatment of ITP is often directed at management of the underlying condition.
Bleeding events are often unpredictable, and patients with ITP, even in the setting of severe thrombocytopenia, may not exhibit bleeding beyond bruising and petechiae.24-26 However, more serious mucosal bleeding may occur, including menorrhagia, epistaxis, gastrointestinal hemorrhage, hematuria, or, rarely, intracranial hemorrhage (ICH).25,27 ICH has been reported in 1.4% of adults and 0.1% to 0.4% of children with ITP.24,25 Severe bleeding is reported in 9.5% (95% confidence interval [CI], 4.1-17.1) of adults and 20.2% (10.0-32.9) of children.25 Adults with ITP have a 1.3- to 2.2-fold higher mortality than the general population due to cardiovascular disease, infection, and bleeding.28
In addition to bleeding, ITP has a significant impact on HRQoL, particularly in the first year after diagnosis, related to restrictions on activities, anxiety due to the risk of bleeding, and the burden of treatment and monitoring.29,30 Fatigue is common and reported in 22% to 45% of patients with ITP.31-33 Reported studies show that the effect of treatment on HRQoL and fatigue may vary by treatment, but this area requires further study.34
The decision as to whether a patient can be observed or requires further intervention is highly complex and varies based on comorbidities, medications, and age, which all impact the risk of bleeding.19,35,36 In addition, management approaches may vary based on disease duration, access to care, quality-of-life implications, and patient and provider preferences, among other factors. Given the considerable interpatient variability in the pathophysiology of the immune dysregulation and the lack of validated predictors of response to treatments, once the decision to treat has been made, the choice of appropriate therapy varies greatly among practitioners.37 Although the list of available treatment options continues to expand, few randomized studies have compared the outcomes of different approaches, making decision-making challenging for both clinicians and patients. In these guidelines, clinical questions were prioritized, and then published evidence was gathered, rigorously evaluated, and used by an expert panel to provide recommendations regarding the management of children and adults with ITP.
Methods
The guideline panel developed and graded the recommendations and assessed the certainty of the supporting evidence following the GRADE approach.5-10 The overall guideline development process, including funding of the work, panel formation, management of conflicts of interest, internal and external review, and organizational approval was guided by ASH policies and procedures derived from the GIN-McMaster Guideline Development Checklist (http://cebgrade.mcmaster.ca/guidecheck.html) and was intended to meet recommendations for trustworthy guidelines by the Institute of Medicine and the GIN.1-4
Organization, panel composition, planning, and coordination
The work of this panel was coordinated by ASH and the OUHSC (funded by ASH under a paid agreement). Project oversight was provided by the ASH Committee on Quality. ASH vetted and appointed individuals to the guideline panel. OUHSC vetted and retained researchers to conduct systematic reviews of evidence and coordinate the guideline development process including the use of the GRADE approach. The membership of the panel and the OUHSC team is described in supplemental File 1.
The panel included 8 adult hematologists and 5 pediatric hematologists, all of whom had clinical and research expertise on the guideline topic, 2 methodologists with expertise in ITP, and 2 patient representatives. The panel chair was a content expert; the vice chair was a methodologist with experience in guideline development processes.
In addition to systematically synthesizing evidence, the OUHSC team supported the guideline development process, including determining methods, preparing meeting materials, and facilitating panel discussions. The panel’s work was done using web-based tools (www.gradepro.org) and face-to-face and online meetings.
Guideline funding and management of conflicts of interest
Development of these guidelines was wholly funded by ASH, a nonprofit medical specialty society that represents hematologists. Most members of the guideline panel were members of ASH. ASH staff supported panel appointments and coordinated meetings but had no role in choosing the guideline questions or determining the recommendations.
Members of the guideline panel received travel reimbursement for attendance at in-person meetings, and the patient representatives received an honorarium of $200 each. The panelists received no other payments. Through the OUHSC, some researchers who contributed to the systematic evidence reviews received salary or grant support. Other researchers participated to fulfill requirements of an academic degree or program.
Conflicts of interest of all participants were managed through disclosure, panel composition, and recusal, according to recommendations of the Institute of Medicine38 and the GIN.4 Participants disclosed all financial and nonfinancial interests relevant to the guideline topic. ASH staff and the ASH Committee on Quality reviewed the disclosures and composed the guideline panel to include a diversity of expertise and perspectives. Greatest attention was given to direct financial conflicts with for-profit companies that could be directly affected by the guidelines. A majority of the panel, including the chair and vice chair, had no such conflicts. None of the OUHSC-affiliated researchers who contributed to the systematic evidence reviews or who supported the guideline development process had any financial interest in a commercial entity with any product that could be affected by the guidelines.
Recusal was also used to manage conflicts of interest. During deliberations about recommendations, any panel member with a current, direct financial interest in a commercial entity that marketed any product that could be affected by a specific recommendation participated in discussion about the evidence and clinical context but was recused from making judgments or voting about individual domains (eg, magnitude of desirable consequences) and the direction and strength of the recommendation.4,39-41 The evidence-to-decision (EtD) framework for each recommendation describes which individuals were recused from making judgments about each recommendation.
In 2019, after the guideline panel had agreed on recommendations, it was discovered that 1 panelist had a direct financial conflict with an affected company (a meal in 2016) that had not been previously reported. Members of the Guideline Oversight Subcommittee reviewed the guidelines in relation to this last disclosure and agreed that this conflict was unlikely to have influenced any of the recommendations.
Supplemental File 2 provides the complete “Disclosure of Interests” forms of all panel members. In part A of the forms, individuals disclosed direct and indirect financial interests for 2 years prior to appointment; and, in part B, interests that were not mainly financial were disclosed. Part C summarizes ASH decisions about which interests were judged to be conflicts and how they were managed. Part D describes new interests disclosed by individuals after appointment.
Supplemental File 3 provides the complete disclosure-of-interest forms of researchers who contributed to these guidelines.
Formulating specific clinical questions and determining outcomes of interest
The panel met in person and via conference calls to generate possible questions to address. To do so, the panel reviewed all questions from the previous 2011 guidelines and introduced new clinical questions. Each panel member then anonymously ranked the questions, and this was followed by discussion until consensus was reached and the final questions described in Table 1 were carried forward for systematic review.
Table 1.
Recommendation questions
| Recommendation questions |
|---|
| 1a. Should adults with newly diagnosed ITP and a platelet count of <30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding be treated with corticosteroids or observation? |
| 1b. Should adults with newly diagnosed ITP and a platelet count of ≥30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding be treated with corticosteroids or observation? |
| 2a. Should adults with ITP and a platelet count <20 × 109/L who are asymptomatic or have mild mucocutaneous bleeding be treated as an outpatient or be admitted to the hospital? |
| 2b. Should adults with ITP and a platelet count ≥20 × 109/L who are asymptomatic or have mild mucocutaneous bleeding be treated as an outpatient or be admitted to the hospital? |
| 3. Should adults with newly diagnosed ITP be treated with a short course (≤6 wk) or a prolonged course (>6 wk including treatment and taper) of prednisone as initial treatment? |
| 4. Should adults with newly diagnosed ITP be treated with prednisone (0.5-2 mg/kg/d) or dexamethasone (40 mg/d × 4 d) as the type of corticosteroid for initial therapy? |
| 5. Should adults with newly diagnosed ITP be treated with rituximab with corticosteroids or corticosteroids alone for initial therapy? |
| 6. Should adults with ITP for ≥3 mo who are corticosteroid-dependent or have no response to corticosteroids and are going to be treated with a TPO-RA receive eltrombopag or romiplostim? |
| 7. Should adults with ITP lasting ≥3 mo who are corticosteroid-dependent or have no response to corticosteroids undergo splenectomy or be treated with a TPO-RA? |
| 8. Should adults with ITP lasting ≥3 mo who are corticosteroid-dependent or have no response to corticosteroids undergo splenectomy or be treated with rituximab? |
| 9. Should adults with ITP lasting ≥3 mo who are corticosteroid-dependent or have no response to corticosteroids be treated with rituximab or a TPO-RA? |
| 10a. Should children with newly diagnosed ITP and a platelet count of <20 × 109/L who have no or mild bleeding (skin manifestations) be treated as outpatients or admitted to the hospital? |
| 10b. Should children with newly diagnosed ITP and a platelet count ≥20 × 109/L who have no or mild bleeding (skin manifestations) be treated as outpatients or admitted to the hospital? |
| 11. Should children with newly diagnosed ITP who have no or minor bleeding be treated with observation or corticosteroids for initial therapy? |
| 12. Should children with newly diagnosed ITP who have no or minor bleeding be treated with observation or IVIG? |
| 13. Should children with newly diagnosed ITP who have no or minor bleeding be treated with observation or anti-D immunoglobulin for initial therapy? |
| 14. Should children with newly diagnosed ITP who have non–life-threatening bleeding and/or diminished HRQoL receive a course of corticosteroids longer than 7 d vs 7 d or shorter? |
| 15. Should children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL receive dexamethasone (0.6 mg/kg/d; maximum, 40 mg/d × 4 d) or prednisone (2-4 mg/kg/d for 5-7 d; maximum, 120 mg daily, for 5-7 d)? |
| 16. Should children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL be treated with anti-D immunoglobulin or corticosteroids for initial therapy? |
| 17. Should children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL be treated with IVIG or anti-D immunoglobulin for initial therapy? |
| 18. Should children with newly diagnosed ITP who have non–life-threatening-mucosal bleeding and/or diminished HRQoL be treated with IVIG or corticosteroids? |
| 19. Should children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment be treated with TPO-RAs or rituximab? |
| 20. Should children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment be treated with TPO-RAs or splenectomy? |
| 21. Should children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment be treated with rituximab or splenectomy? |
Recommendations from the 2011 ASH guidelines that were not prioritized to be addressed by these guidelines are presented in Table 2. Supporting evidence was not reviewed, and the recommendations were not discussed or updated by the guideline panel. They are presented here for reader context and convenience.
Table 2.
Recommendations from 2011 ASH guideline for ITP that are not addressed in the 2019 ASH guideline on ITP
| ITP in adults |
| Newly diagnosed ITP in adults |
| Initial diagnosis of ITP |
| 4.1.A. We recommend: |
| • Testing patients for HCV and HIV (grade 1B*) |
| 4.1.B. We suggest: |
| • Further investigations if there are abnormalities (other than thrombocytopenia and perhaps findings of iron deficiency) in the blood count or smear (grade 2C) |
| • A bone marrow examination is not necessary irrespective of age for patients presenting with typical ITP (grade 2C) |
| First-line treatment of adult ITP |
| 4.3.A. We suggest: |
| • IVIG be used with corticosteroids when a more rapid increase in platelet count is required (grade 2B) |
| • Either IVIG or anti-D (in appropriate patients) be used as a first-line treatment if corticosteroids are contraindicated (grade 2C) |
| • If IVIG is used, the dose should initially be 1 g/kg as a 1-time dose; this dosage may be repeated if necessary (grade 2B) |
| Laparoscopic vs open splenectomy and vaccination prior to splenectomy |
| 4.5.A. We recommend: |
| • That for medically suitable patients, both laparoscopic and open splenectomy offer similar efficacy (grade 1C) |
| Treatment of ITP in pregnancy |
| Management of ITP during pregnancy |
| 6.1.A. We recommend: |
| • Pregnant patients requiring treatment receive either corticosteroids or IVIG (grade 1C) |
| Treatment of ITP during labor and delivery |
| 6.2.A. We suggest: |
| • For pregnant women with ITP, the mode of delivery should be based on obstetric indications (grade 2C) |
| Treatment of specific forms of secondary ITP |
| Management of secondary ITP, HCV-associated |
| 7.1.A. We suggest: |
| • For patients with secondary ITP due to HCV infection, antiviral therapy should be considered in the absence of contraindications (grade 2C); however, the platelet count should be closely monitored due to a risk of worsening thrombocytopenia attributable to interferon |
| • ITP is required, the initial treatment should be IVIG (grade 2C) |
| Management of secondary ITP, HIV-associated |
| 7.2.A. We recommend: |
| • For patients with secondary ITP due to HIV, treatment of the HIV infection with antiviral therapy should be considered before other treatment options unless the patient has clinically significant bleeding complications (grade 1A) |
| • If treatment of ITP is required, initial treatment should consist of corticosteroids, IVIG, or anti-D (grade 2C) and splenectomy in preference to other agents in symptomatic patients who fail corticosteroids, IVIG, or anti-D (grade 2C) |
| Management of secondary ITP, H pylori–associated |
| 7.3.A. We recommend: |
| • That eradication therapy be administered for patients who are found to have H pylori infection (based on urea breath tests, stool antigen tests, or endoscopic biopsies) (grade 1B) |
| 7.3.B. We suggest: |
| • Screening for H pylori be considered for patients with ITP in whom eradication therapy would be used if testing is positive (grade 2C) |
| ITP in children |
| Newly diagnosed ITP in children |
| Diagnosis of ITP |
| 1.1.A. We recommend: |
| • Bone marrow examination is unnecessary in children and adolescents with the typical features of ITP (grade 1B) |
| • Bone marrow examination is not necessary in children who fail IVIG therapy (grade 1B) |
| 1.1.B. We suggest: |
| • Bone marrow examination is also not necessary in similar patients prior to initiation of treatment with corticosteroids or before splenectomy (grade 2C) |
| • Testing for antinuclear antibodies is not necessary in the evaluation of children and adolescents with suspected ITP (grade 2C) |
| Children who are treatment nonresponders |
| H pylori testing in children with persistent or chronic ITP |
| 2.3.A. We recommend: |
| • Against routine testing for H pylori in children with chronic ITP (grade 1B) |
| Management of MMR-associated ITP |
| 3.1.A. We recommend: |
| • Children with a history of ITP who are unimmunized receive their scheduled first MMR vaccine (grade 1B) |
| • In children with either nonvaccine or vaccine-related ITP who have already received their first dose of MMR vaccine, vaccine titers can be checked; if the child displays full immunity (90% to 95% of children), then no further MMR vaccine should be given; if the child does not have adequate immunity, then the child should be reimmunized with MMR vaccine at the recommended age (grade 1B) |
H pylori, Helicobacter pylori; HCV, hepatitis C virus; MMR, measles, mumps, and rubella.
Evidence grades: The number value indicates the strength of the recommendation. A value of 1 indicates a high degree of confidence that the desirable outcomes of an intervention exceed the undesirable outcomes effects (or vice versa) in most patient populations. A value of 2 indicates a lower degree of confidence that the desirable outcomes outweigh undesirable outcomes (or vice versa). The letter score indicates the quality of the underlying evidence. “A” indicates that the recommendation is supported by consistent evidence from RCTs or exceptionally strong observational studies. “B” indicates that the recommendation is supported by RCTs with important limitations or strong evidence from observational studies. “C” indicates evidence derived from RCTs with serious flaws, weaker observational studies, or indirect evidence.42
The panel selected outcomes of interest for each question a priori, following the approach described in detail elsewhere.43 In brief, the panel first brainstormed all possible outcomes before rating their relative importance for decision-making following the GRADE approach.43 While acknowledging considerable variation in the impact on patient outcomes, the panel considered the outcomes outlined in supplemental File 4 as critical for clinical decision-making for each of the prioritized questions.
During this rating process, the panel used definitions of the outcomes that were consistent with published terminology.44 The list of definitions is available in Table 3.
Table 3.
Definition of terms in 2019 ASH guideline on ITP
| Terms and definitions |
|---|
| Corticosteroid-dependent: Ongoing need for continuous prednisone >5 mg/d (or corticosteroid equivalent) or frequent courses of corticosteroids to maintain a platelet count ≥30 × 109/L and/or to avoid bleeding |
| Durable response: Platelet count ≥30 × 109/L and at least doubling of the baseline count at 6 mo |
| Early response: Platelet count ≥30 × 109/L and at least doubling baseline at 1 wk |
| Initial response: Platelet count ≥30 × 109/L and at least doubling baseline at 1 mo |
| Major bleeding: (1) WHO grade 3 or 4 bleeding, (2) Buchanan severe grade, (3) Bolton-Maggs and Moon “major bleeding,” (4) IBLS grade 2 or higher, or (5) life-threatening or intracerebral hemorrhage bleeding |
| Minor bleeding: Any bleeding not meeting the criteria for “major bleeding” |
| Newly diagnosed ITP: ITP duration of <3 mo |
| Persistent ITP: ITP duration of 3-12 mo |
| Chronic ITP: ITP duration of >12 mo |
| Remission: Platelet count >100 × 109/L at 12 mo |
IBLS, ITP Bleeding Scale; WHO, World Health Organization.
Evidence review and development of recommendations
For each guideline question, the OUHSC prepared a GRADE EtD framework, using the GRADEpro Guideline Development Tool (www.gradepro.org).5,10 The EtD table summarized the results of systematic reviews of the literature that were updated or performed for these guidelines. The EtD table addressed effects of interventions, resource utilization (cost-effectiveness), values and preferences (relative importance of outcomes), equity, acceptability, and feasibility. The guideline panel reviewed draft EtD tables before, during, and after the guideline panel meeting and made suggestions for corrections and identified missing evidence. To ensure that recent studies were not missed, searches (presented in supplemental File 5) were updated in May of 2017, and panel members were asked to suggest any studies that may have been considered missed and fulfilled the inclusion criteria for the individual questions.
Under the direction of the OUHSC, researchers followed the general methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (handbook.cochrane.org) for conducting updated or new systematic reviews of intervention effects. Risk of bias was assessed at the health-outcome level using the Cochrane Collaboration’s risk-of-bias tool for randomized trials or nonrandomized studies. In addition to conducting systematic reviews of intervention effects, the researchers searched for evidence related to baseline risks, values, preferences, and costs, and summarized findings within the EtD frameworks.5,10 Subsequently, the certainty of the body of evidence (also known as quality of the evidence or confidence in the estimated effects) was assessed for each effect estimate of the outcomes of interest following the GRADE approach based on the following domains: risk of bias, precision, consistency and magnitude of the estimates of effects, directness of the evidence, risk of publication bias, presence of large effects, dose-response relationship, and an assessment of the effect of residual, opposing confounding. The certainty was categorized into 4 levels ranging from very low to high.6-8
During 2 in-person 2-day meetings followed by online communication and conference calls, the panel developed clinical recommendations based on the evidence summarized in the EtD tables. For each recommendation, the panel took a population perspective and came to consensus on the following: the certainty in the evidence, the balance of benefits and harms of the compared management options, and the assumptions about the values and preferences associated with the decision. The guideline panel also explicitly took into account the extent of resource use associated with alternative management options. Cost was estimated using a Lexicomp (http://online.lexi.com/action/home) calculation for a 1-month supply (as of August 2017) for a 30-kg/1-m2 child or a 70-kg/2-m2 adult for therapies that are indefinite. For therapy with a set number of doses, the number of doses is noted in the EtD. The panel agreed on the recommendations (including direction and strength), remarks, and qualifications by consensus, or, in rare instances, by voting (an 80% majority was required for a strong recommendation), based on the balance of all desirable and undesirable consequences. The final guidelines, including recommendations, were reviewed and approved for publication by all members of the panel.
Interpretation of strong and conditional recommendations
The recommendations are labeled as either “strong” or “conditional” according to the GRADE approach. The words “the guideline panel recommends” are used for strong recommendations, and “the guideline panel suggests” for conditional recommendations. Table 4 provides GRADE’s interpretation of strong and conditional recommendations by patients, clinicians, health care policy-makers, and researchers.
Table 4.
Interpretation of strong and conditional recommendations
| Implications for | Strong recommendation | Conditional recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not | The majority of individuals in this situation would want the suggested course of action, but many would not; decision aids may be useful in helping patients to make decisions consistent with their individual risks, values, and preferences |
| Clinicians | Most individuals should follow the recommended course of action; formal decision aids are not likely to be needed to help individual patients make decisions consistent with their values and preferences | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with the patient’s values and preferences; decision aids may be useful in helping individuals to make decisions consistent with their individual risks, values, and preferences |
| Policy-makers | The recommendation can be adopted as policy in most situations; adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator | Policy-making will require substantial debate and involvement of various stakeholders; performance measures should assess whether decision-making is appropriate |
| Researchers | The recommendation is supported by credible research or other convincing judgments that make additional research unlikely to alter the recommendation; on occasion, a strong recommendation is based on low or very low certainty in the evidence; in such instances, further research may provide important information that alters the recommendations | The recommendation is likely to be strengthened (for future updates or adaptation) by additional research; an evaluation of the conditions and criteria (and the related judgments, research evidence, and additional considerations) that determined the conditional (rather than strong) recommendation will help identify possible research gaps |
Interpretation of good practice statements
As described by the GRADE Guidance Group, good practice statements endorse interventions or practices that the guideline panel agreed have unequivocal net benefit yet may not be widely recognized or used.16 Good practice statements in these guidelines are not based on a systematic review of available evidence. Nevertheless, they may be interpreted as strong recommendations.
Document review
Draft recommendations were reviewed by all members of the panel, revised, and then made available online on 20 November 2018 for external review by stakeholders including allied organizations, other medical professionals, patients, and the public. Twenty-seven individuals or organizations submitted comments. The document was revised to address pertinent comments, but no changes were made to the direction or strength of the recommendations. The guidelines were reviewed by the ASH Guideline Oversight Subcommittee on 26 August 2019. On 6 September 2019, the ASH Committee on Quality approved that the defined guideline development process was followed, and, on 13 September 2019, the officers of the ASH Executive Committee approved submission of the guidelines for publication under the imprimatur of ASH. The guidelines were then subjected to peer review by Blood Advances.
How to use these guidelines
ASH guidelines are primarily intended to help clinicians make decisions about diagnostic and treatment alternatives. Other purposes are to inform policy, to promote education and advocacy, and to state future research needs. They may also be used by patients. These guidelines are not intended to serve, or be construed, as a standard of care. Clinicians must make decisions on the basis of the clinical presentation of each individual patient, ideally through a shared decision-making process that considers the patient’s values and preferences with respect to the anticipated outcomes of the chosen option. Decisions may be constrained by the realities of a specific clinical setting and local resources, including but not limited to institutional policies, time limitations, or availability of treatments. These guidelines may not include all appropriate methods of care for the clinical scenarios described. As science advances and new evidence becomes available, recommendations may become outdated. Following these guidelines cannot guarantee successful outcomes. ASH does not warrant or guarantee any products described in these guidelines.
Statements about the underlying values and preferences as well as qualifying remarks accompanying each recommendation are its integral parts and serve to facilitate more accurate interpretation. They should never be omitted when quoting or translating recommendations from these guidelines. The use of these guidelines is also facilitated by the links to the EtD frameworks and interactive summary-of-findings tables in each section.
Recommendations
Management of adult patients with newly diagnosed ITP
Corticosteroids vs observation
Question: Should adults with newly diagnosed ITP and a platelet count of <30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding be treated with corticosteroids or observation?
Recommendation 1a
In adults with newly diagnosed ITP and a platelet count of <30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests corticosteroids rather than management with observation (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: There may be a subset of patients within this group for whom observation might be appropriate. This should include consideration of the severity of thrombocytopenia, additional comorbidities, use of anticoagulant or antiplatelet medications, need for upcoming procedures, and age of the patient.
Summary of evidence.
We included all randomized controlled trials (RCTs) and observational studies that had internal comparators. Due to the scarcity of RCTs for this question, we also included all single-arm prospective studies of ≥50 adults with newly diagnosed ITP treated with corticosteroids or managed with observation with a platelet count <30 × 109/L. For management with observation, we also included retrospective studies of ≥50 adults with newly diagnosed ITP due to a lack of prospective studies. We found no studies that directly compared corticosteroids vs management with observation in adult patients with ITP with a platelet count of <30 × 109/L. We found 5 RCTs46-50 (corticosteroids vs comparator other than observation) and 2 prospective studies51,52 reporting outcomes of interest for patients receiving corticosteroids but not management with observation. Four studies reported data on response within 7 days,46-48,51 6 studies reported on remission,46,48-52 3 studies reported on major bleeding,47,51,52 4 studies reported on mortality,49-52 and 0 studies reported on overall HRQoL for patients receiving corticosteroids. There were no studies in ITP patients with platelet counts of <30 × 109/L who were managed with observation alone. The EtD framework is shown online at https://guidelines.gradepro.org/profile/5F9D4FEE-B20A-B114-A337-FD0697FCFAB9.
Benefits.
The relative effects were not estimable from the data because of a lack of direct comparisons. The panel acknowledged that there was a lack of observational data on patients managed with observation. They had moderate confidence in platelet count response at 7 days (55.8%) with corticosteroids; however, remission rates remain low (30.2%). There were no data on HRQoL for either corticosteroids or observation to comment on a benefit.
Harms and burden.
The relative effects were not estimable from the data because of a lack of direct comparisons. The panel agreed that there were possible moderate undesirable effects associated with observation in this setting given that thrombocytopenia is a surrogate for potential future bleeding events in the adult population. Bleeding events (3.3%) and mortality (5.7%) were reported only for corticosteroid-treated patients. The panel also recognized the known side effects associated with corticosteroid treatment.
Other EtD criteria and considerations.
The panel agreed that there might be considerable variability in patient preferences depending on the degree of concern patients have over potential for bleeding compared with a desire to avoid side effects associated with corticosteroids. The panel did not think that there were any acceptability or feasibility considerations that would impair implementation of this recommendation. Corticosteroids are universally available. The cost of corticosteroid therapy is negligible.
Conclusions and research needs for this recommendation.
The guideline panel determined that there was very-low-certainty evidence for treatment with corticosteroids in this population. Given the body of evidence available, this recommendation was based primarily on the benefit of an early 7-day response in platelet count demonstrated with corticosteroids and unknown data about the incidence and progression of bleeding in the absence of treatment. The panel acknowledged that the exact platelet count threshold at which the risk of bleeding increases is not known. The benefit of treatment is less certain in younger patients and those with higher platelet counts within this range.
The panel identified the following research needs:
Natural history studies of adults with newly diagnosed ITP and a platelet count of <30 × 109/L managed with observation.
Question: Should adults with newly diagnosed ITP and a platelet count of ≥30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding be treated with corticosteroids or observation?
Recommendation 1b
In adults with newly diagnosed ITP and a platelet count of ≥30 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel recommends against corticosteroids and in favor of management with observation (strong recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: For patients with a platelet count at the lower end of this threshold, for those with additional comorbidities, anticoagulant or antiplatelet medications, or upcoming procedures, and for elderly patients (>60 years old), treatment with corticosteroids may be appropriate.
Good practice statement
The treating physician should ensure that the patient is adequately monitored for potential corticosteroid side effects regardless of duration or type of corticosteroid selected. This includes close monitoring for hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis. Given the potential impact of corticosteroids on mental health, the treating physician should assess HRQoL (depression, fatigue, mental status etc) while patients are receiving corticosteroids.
Summary of evidence.
We included all RCTs and observational studies that had internal comparators. Due to the scarcity of RCTs for this question, we also included all single-arm prospective studies of ≥50 adults with newly diagnosed ITP treated with corticosteroids or managed with observation with a platelet count of ≥30 × 109/L. For management with observation, we also included retrospective studies of ≥50 adults with newly diagnosed ITP due to a lack of prospective studies. We found no studies that directly compared corticosteroids vs observation in this population; thus, corticosteroids and management with observation represented different populations. We found 2 RCTs53,54 (prednisone vs a comparator other than observation) and 1 prospective study55 reporting outcomes of interest for patients receiving corticosteroids. We found 2 retrospective studies56,57 reporting outcomes of interest for patients who were managed with observation. Two studies reported data on response within 7 days (both corticosteroid arm53,54), 4 studies reported on remission (2 corticosteroid arm,53,55 2 observational arm56,57), 4 studies reported on major bleeding (2 corticosteroid arm,53,55 2 observational arm56,57), 1 study reported on mortality (1 observational arm56), and 0 studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/9BCF9E58-DF2C-081A-952B-877E5318FDD9.
Benefits.
The relative effects were not estimable from the data because of a lack of direct comparisons. There were no data on response at 7 days for patients with a platelet count of ≥30 × 109/L managed with observation. There was a remission of 71.7% with observation compared with only 23.9% with corticosteroids; however, the panel had very low confidence in this estimate with regard to the observation arm because the mean platelet count was over 80 × 109/L in the 2 studies. Therefore, remission was not considered in assessment of benefits. There was no difference with major bleeding events, which were low with both approaches (0.9% for corticosteroids and 0% for observation). There were no data on HRQoL. Overall, the panel judged the potential benefits to be unknown.
Harms and burden.
The panel did not prioritize any harms a priori. Indirect evidence supported that the side effects from corticosteroids are not trivial, and, therefore, the undesirable effects of corticosteroids were considered to be moderate by the panel with high-quality evidence when using indirect data.
Other EtD criteria and considerations.
The panel did not think that there were acceptability or feasibility considerations that would impair implementation of this recommendation. Observation was judged to be acceptable as long as adequate follow-up could be ensured. Furthermore, there was judged to be no difference between the treatments with respect to health equity, and the cost of corticosteroids was judged to be negligible. The panel did recognize that for patients with a platelet count at the lower end of this threshold, for those with additional comorbidities that predispose to bleeding, those taking anticoagulant or antiplatelet medications in preparation for upcoming procedures, and for elderly patients (>60 years old), treatment with corticosteroids may be appropriate.
Conclusions and research needs for this question.
The guideline panel determined that there was very-low-certainty evidence that there is benefit or harm in treatment with corticosteroids in this patient population with ITP. The GRADE Handbook outlines paradigmatic situations in which a strong recommendation may be used despite low confidence in the effects; 1 such situation is when low-quality evidence suggests benefit and high-quality evidence suggests harm or a very high cost. Therefore, despite very low certainty in the evidence of benefit, the panel decided that there was high-quality evidence in other patient populations that suggest potential for harm and the panel opted for a strong recommendation secondary to the moderate potential for harm with inappropriate corticosteroid exposure at doses used in adult ITP.
The panel prioritized the following research needs:
Better delineation of risks of bleeding in elderly patients and those treated with anticoagulant and antiplatelet drugs;
Determination of platelet thresholds for procedures.
Inpatient vs outpatient management
Question: Should adults with ITP and a platelet count of <20 × 109/L who are asymptomatic or have mild mucocutaneous bleeding be treated as an outpatient or admitted to the hospital?
Question: Should adults with ITP and a platelet count of ≥20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding be treated as an outpatient or admitted to the hospital?
Recommendation 2a
In adults with newly diagnosed ITP and a platelet count of <20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests admission to the hospital rather than management as an outpatient (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). In adults with an established diagnosis of ITP and a platelet count of <20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests outpatient management rather than hospital admission (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: Patients with social concerns, uncertainty about the diagnosis, significant comorbidities with risk of bleeding, and more significant mucosal bleeding may benefit from admission to the hospital. Patients not admitted to the hospital should receive education and expedited follow-up with a hematologist. The need for admission is also highly variable across the range of platelet counts represented (0 to 20 × 109/L).
Recommendation 2b
In adults with a platelet count of ≥20 × 109/L who are asymptomatic or have minor mucocutaneous bleeding, the ASH guideline panel suggests outpatient management rather than hospital admission (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: Patients with social concerns, uncertainty about the diagnosis, significant comorbidities with risk of bleeding, and more significant mucosal bleeding may benefit from admission to the hospital. Patients not admitted to the hospital should receive education and expedited follow-up with a hematologist. The need for admission is also highly variable across the range of platelet counts represented (20 × 109/L to 150 × 109/L).
Good practice statement
The referring physician should ensure that the patient has follow-up with a hematologist within 24 to 72 hours of the diagnosis.
Summary of evidence.
We included all systematic reviews, RCTs, and observational studies that had internal comparators that compared hospitalized vs nonhospitalized patients with ITP. No studies were found that addressed the question of interest. Surveys were administered to the adult hematologists on the panel. However, limitations of the survey included: recall bias, difficulty determining individual provider management practice compared with center-wide practice, and the fact that hematologists may be consulted only after the decision for admission has been made by another provider. Results of the survey data are reflected in the EtD frameworks. The EtD framework for adults with newly diagnosed ITP and a platelet count of <20 × 109/L is shown online at https://guidelines.gradepro.org/profile/C09C5039-7839-8C91-9708-9CC0BE37FE82. The EtD framework for adults with an established diagnosis of ITP and a platelet count of <20 × 109/L is shown online at https://guidelines.gradepro.org/profile/99E5F4DA-474A-CD59-BF3B-0F1D318A80E0. The EtD framework for adults with a platelet count of ≥20 × 109/L is shown online at https://guidelines.gradepro.org/profile/7222BB36-DBE4-0B0A-A296-48005D72BCE4.
Benefits.
On initial presentation, the panel judged there to be moderate desirable effects of admission for patients with a platelet count of <20 × 109/L, mostly allowing confirmation of the diagnosis of ITP, establishment of care, determination of platelet count trend and responsiveness to therapy, and assessment of additional bleeding risk. Furthermore, in this group, if serious bleeding were to ensue this would allow for prompt management. For established patients, the benefits were trivial because these care components have already been documented. For patients with a platelet count of ≥20 × 109/L, the clinical experience of the panel was that significant bleeding was less likely in this population in the absence of confounding features such as those listed above in the remarks, and that many patients can undergo a diagnostic workup as outpatients so long as there is assurance of follow-up. The patient representatives on the panel expressed that following the diagnosis of ITP, patients may experience an increase in HRQoL by being able to be managed as an outpatient rather than having to be admitted to the hospital.
Harms and burden.
The undesirable effects were determined to be small in all scenarios. The primary burden was reflected in missed time at work and other obligations with hospital admission. In addition, the panel members considered the small risk of hospital-acquired infections.
Other EtD criteria and considerations.
The panel determined that the decision to admit to the hospital was different depending on duration of ITP and platelet count. The panel recognized that there might be important differences in patient values that would affect the decision to admit and acknowledged that there was a large cost associated with management as an inpatient compared with those for outpatients. The panel did not think that there were feasibility considerations that would impair implementation of this recommendation. The recommendations may have variable acceptability to stakeholders, particularly given the wide range of platelet counts considered in this recommendation as well as the number of providers involved in decision-making. Drug treatment should be provided as outlined in recommendations 1a and 1b regardless of the decision to admit or not.
Conclusions and research needs for these recommendations.
The guideline panel determined that there is very-low-certainty evidence for a net health benefit from admission to the hospital for patients presenting initially with a diagnosis of ITP and a platelet count of <20 × 109/L. This is based on the small undesirable effects and moderate desirable effects of hospitalization. Hospitalization should be used to confirm the diagnosis of ITP, establish care, determine platelet count trend and responsiveness to therapy, assess additional bleeding risk, confirm the diagnosis of ITP, establish hematology care, initiate platelet-raising therapy, and determine the patient’s bleeding risk before recovery of the platelet count. The panel determined that there is very-low-certainty evidence for a net health benefit for outpatient management once a patient has established ongoing care with a hematologist, has had proper education about the disease and manifestations to watch for, and once the response to therapy is known. The desirable effects of hospitalization are diminished at this time, and outpatient management may increase HRQoL and reduce the burden of the disease on the patient. The panel determined that there is very-low-certainty evidence for a net health harm of inpatient management for patients with a platelet count of >20 × 109/L.
Duration and type of corticosteroids
Question: Should adults with newly diagnosed ITP be treated with a short course (≤6 weeks) or a prolonged course (>6 weeks including treatment and taper) of prednisone as initial treatment?
Recommendation 3
In adults with newly diagnosed ITP, the ASH guideline panel recommends against a prolonged course (>6 weeks including treatment and taper) of prednisone and in favor of a short course (≤6 weeks) (strong recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Good practice statement
The treating physician should ensure that the patient is adequately monitored for potential corticosteroid side effects regardless of duration or type of corticosteroid selected. This includes close monitoring for hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis. Given the impact of corticosteroids on mental health, the treating physician should assess HRQoL (depression, fatigue, mental status, etc) while patients are receiving corticosteroids.
Summary of evidence.
We included all systematic reviews, RCTs, or observational studies that had internal comparators that compared adult ITP patients treated with a short (≤6 weeks) vs a prolonged course of prednisone. We also searched for all single-arm prospective studies of ≥50 adults treated with either a short or prolonged course of steroids. No studies were found that had data on short courses of prednisone; therefore, no data from patients with ITP from the literature were used to evaluate this question. A survey was administered to the adult hematologists on the panel; however, limitations of the survey included recall bias and difficulty determining individual provider management practice compared with center-wide practice. Results of the survey data are reflected in the EtD framework shown online at https://guidelines.gradepro.org/profile/1133D039-1124-7059-85DD-148FC5439B2A. Articles reporting data about the side effects of corticosteroids in any patient group were used to assess the harm.
Benefits.
Based on clinical experience, the panel agreed that there was likely trivial benefit in continuing corticosteroids beyond 6 weeks. For the majority of patients, a trial of 6 weeks of corticosteroids should determine whether a patient is going to enter remission or will require additional therapy. For patients who require additional therapy, consideration of alternative therapy is preferred over ongoing exposure to corticosteroids.
Harms and burden.
The panel agreed that based on indirect evidence, the risk of harm and likelihood of adverse events were large with the use of courses of corticosteroids for >6 weeks. Side effects taken into consideration included hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis.45,58-60
Other EtD criteria and considerations.
There is no significant cost difference with longer courses of corticosteroids. Shorter courses of corticosteroids were thought to be feasible. The panel agreed that a longer course of steroids would likely not be acceptable to patients given the impact of corticosteroids on mood, sleep, weight gain, and other side effects. The panel acknowledged that a duration of 6 weeks is not evidence based; however, this represents a reasonable duration to provide a standard maximum 21 days of treatment plus additional time for the taper.
Conclusions and research needs for these recommendations.
The guideline panel determined with very low certainty in the evidence that there is benefit or harm in longer courses of corticosteroids. The GRADE Handbook outlines paradigmatic situations for when a strong recommendation may be used despite low confidence in the effects; 1 such situation is when low-quality evidence suggests benefit and high-quality evidence suggests harm or a very high cost. Despite very low levels of evidence, there was moderate certainty among the panel in their clinical observations, survey data, and indirect evidence. In the absence of demonstrated increased benefits with longer courses of corticosteroids and the known complications and side effects associated with prolonged corticosteroid exposure, the panel thought that the balance of effects favored a shorter course of corticosteroids (≤6 weeks) over longer courses. The panel provided a strong recommendation based on indirect evidence for risk exposure over time with corticosteroid use.
Question: Should adults with newly diagnosed ITP be treated with prednisone (0.5-2.0 mg/kg per day) or dexamethasone (40 mg per day for 4 days) as the type of corticosteroids for initial therapy?
Recommendation 4
In adults with newly diagnosed ITP, the ASH guideline panel suggests either prednisone (0.5-2.0 mg/kg per day) or dexamethasone (40 mg per day for 4 days) as the type of corticosteroid for initial therapy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: If a high value is placed on rapidity of platelet count response, an initial course of dexamethasone over prednisone may be preferred, given that dexamethasone showed increased desirable effects with regards to response at 7 days.
Good practice statement
The treating physician should ensure that the patient is adequately monitored for potential corticosteroid side effects regardless of the duration or type of corticosteroid selected. This includes close monitoring for hypertension, hyperglycemia, sleep and mood disturbances, gastric irritation or ulcer formation, glaucoma, myopathy, and osteoporosis. Given impact of corticosteroids on mental health, the treating physician should assess HRQoL (depression, fatigue, mental status, etc) while patients are receiving corticosteroids.
Summary of evidence.
We included all systematic reviews and RCTs comparing dexamethasone and prednisone in adults with newly diagnosed ITP. We found 1 systematic review61 and 6 RCTs46-48,50,53,62 that compared dexamethasone and prednisone. Three studies reported data on response within 7 days,46-48 3 studies reported on response within 1 month,46,53,62 5 studies reported on durable response,46,48,50,53,62 4 studies reported on remission,46,48,50,53 3 studies reported on major bleeding,47,50,53 and 0 studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/2EDB76B0-69D0-ACCC-9ED4-6F1E3DA3C1FF .
Benefits.
Randomized trial data show an increased platelet count response at 7 days with dexamethasone (relative risk [RR], 1.31; 95% CI, 1.11-1.54) with high certainty in the evidence. Remission was higher among the dexamethasone-treated patients (RR, 2.96; 95% CI, 1.03-8.45); however, the panel had low confidence in the evidence secondary to indirectness of the definition of remission applied by the trials, as well as heterogeneity in corticosteroid dosing regimens used. There was no clear benefit with regard to response at 1 month, durable response, or major bleeding. There were no available data on HRQoL.
Value was placed on the stability of the platelet count seen with prednisone compared with dexamethasone, and given that the duration of initial response following a cycle of dexamethasone is highly variable, the panel recommended that the platelet count be monitored closely around this time.
Harms and burden.
No outcomes related to harm were prioritized. Although there is a lack of direct evidence, the panel agreed that the risk of adverse effects in clinical practice varies based on the dose and duration of corticosteroid therapy, patient comorbidities, and age of the patient. Specifically, the panel expressed concerns about the use of dexamethasone for patients with underlying diabetes and in the elderly (>60 years old).
Other EtD criteria and considerations.
There was no important uncertainty about patient values. The panel did not think that there were acceptability, equity, or feasibility issues that would impair implementation of this recommendation. The cost difference was negligible. Optimal dosing regimens for both prednisone and dexamethasone have not been determined.
Conclusions and research needs for these recommendations.
The guideline panel determined that there is very low certainty evidence for use of either dexamethasone or prednisone. Based on the body of available evidence, it is likely that there is no difference between the 2 treatment regimens addressed here. There is also an absence of adequate reporting of important patient-reported outcomes such as HRQoL and lack of detailed reporting of side effects.
The panel identified the following research needs:
Properly designed studies with controlled dosing regimens that report total patient corticosteroid exposure during the study period;
Assessment of differences in platelet count variability during treatment with dexamethasone compared with prednisone and need for rescue therapy;
Assessment of the magnitude and impact of adverse effects associated with corticosteroid use;
Application of prioritized outcomes such as HRQoL in RCTs and use of standardized outcomes with regard to platelet count outcomes;
Understanding difference in management with regard to elderly patients (>60 years old).
Rituximab as initial treatment
Question: Should adults with newly diagnosed ITP be treated with rituximab and corticosteroids or corticosteroids alone for initial therapy?
Recommendation 5
In adults with newly diagnosed ITP, the ASH guideline panel suggests corticosteroids alone rather than rituximab and corticosteroids for initial therapy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: If high value is placed on possibility for remission over concerns for potential side effects of rituximab, then an initial course of corticosteroids with rituximab may be preferred.
Summary of evidence.
We included all RCTs comparing rituximab and corticosteroids to corticosteroids alone for initial therapy in adults with newly diagnosed ITP. We found 3 RCTs63-65 that compared rituximab and corticosteroids vs corticosteroids alone. Two studies reported data on response within 1 month,63,64 3 studies reported on durable response,63-65 1 study reported on remission,63 3 studies reported on major bleeding,63-65 2 studies reported on mortality,64,65 and 0 studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/6F40A44F-AA8B-CD36-A276-064B225F9F9B.
Benefits.
Moderate desirable effects were seen with concomitant use of rituximab and corticosteroids, particularly with regard to higher durable response (RR, 1.70; 95% CI, 1.34-2.16) and remission (RR, 1.58; 95% CI, 1.00-2.52). There was no difference with regard to impact on 1-month response, prevention of major bleeding or mortality, and no data regarding HRQoL. The panel thought there was very low certainty in the evidence for benefits, due to missing HRQoL data, unknown and nonstandardized dose of corticosteroid for comparison, and a lack of longer-term follow-up. The panel also acknowledged that there were 2 RCTs that were not included,66,67 as they did not specifically address newly diagnosed/treatment-naive patients.
Harms and burden.
There was no difference in the prioritized outcome of infection; however, there was a large CI (RR, 3.18; 95% CI, 0.13-76.25). The panel had very low certainty in the evidence for harms. Further prioritization of outcomes related to harm may be more informative.
Other EtD criteria and considerations.
Possible uncertainty or variability in how much individuals value the main outcomes may exist. If high value is placed on remission, then perhaps there is a benefit of rituximab; however, this must be weighed against the potential for increased adverse events. Addition of rituximab increases treatment costs by $31 266 for 4 weekly doses and further clinical costs related to the infusion. In the absence of cost analysis data, it is unknown whether these additional upfront costs are offset by avoidance of later expenses. The panel also thought that the concomitant initial rituximab may not be acceptable to all stakeholders and could result in reduced health equity if access to rituximab is limited. Lastly, the addition of rituximab may not be universally feasible due to need for infusion centers, insurance coverage, and drug availability.
Conclusions and research needs for these recommendations.
The guideline panel determined that there is very-low-certainty evidence for use of corticosteroids alone rather than in combination with rituximab in newly diagnosed patients. The balance of desirable and undesirable effects probably favored the concomitant use of corticosteroids and rituximab; however, in the setting of very-low-quality evidence weighed against substantial increased cost and implementation considerations, the panel favored corticosteroids alone until more robust data are available. The use of rituximab for patients who fail corticosteroids is addressed in recommendations 8 and 9.
The panel identified the following research needs:
Properly designed studies with controlled dosing regimens, longer-term follow-up, and adequate reporting of adverse effects;
Studies assessing total corticosteroid exposure as an outcome;
Inclusion of prioritized outcomes such as HRQoL in RCTs;
Detailed cost-effectiveness analysis.
Management of adults with ITP who are corticosteroid-dependent or unresponsive to corticosteroids
Eltrombopag vs romiplostim
Question: Should adults with ITP for ≥3 months who are corticosteroid-dependent or have no response to corticosteroids and are going to be treated with a TPO-RA receive eltrombopag or romiplostim?
Recommendation 6
In adults with ITP for ≥3 months who are corticosteroid-dependent or unresponsive to corticosteroids and are going to be treated with a TPO-RA, the ASH guideline panel suggests either eltrombopag or romiplostim (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: Individual patient preference may place higher value on use of a daily oral medication or weekly subcutaneous injection.
Summary of evidence.
We included all systematic reviews and RCTs comparing eltrombopag and romiplostim in adults with ITP. We found no studies that directly compared eltrombopag and romiplostim in this population; thus, eltrombopag and romiplostim represent different populations. We found 1 systematic review (and update) that indirectly compared eltrombopag and romiplostim; this review reported on durable response.68,69 A second systematic review70 compared romiplostim vs placebo and eltrombopag vs placebo for the outcomes of major bleeding and reduction or discontinuation of corticosteroids. No studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/D6D75FC4-6FBA-93B5-AFD3-8C23FC90D98E.
Benefits.
Durable response between eltrombopag and romiplostim was indirectly compared (odds ratio = 0.20; 95% CI, 0.01-2.13). The relative effects for major bleeding and discontinuation or reduction of corticosteroids were not estimable from the data because of a lack of comparisons. The difference in desirable effects was determined to be minimal. No difference was detected in the outcomes of durable response, bleeding rates, and rates of corticosteroid discontinuation or reduction. There were no included data on HRQoL, remission, or mortality.
Harms and burden.
No outcomes related to harms were prioritized by the panel a priori. The difference in undesirable effects was determined to be trivial. Transaminitis associated with eltrombopag, while not a prioritized outcome, was acknowledged by the panel but considered to be mild and reversible in the majority of cases and therefore did not affect the balance of undesirable effects.
Other EtD criteria and considerations.
Cost is balanced between the 2 agents; however, there is a wider range of cost associated with romiplostim based on Lexicomp calculations secondary to larger dose range (1-10 μg/kg). Since the completion of the literature review, cost analysis studies have been conducted.71,72 In a US analysis of primary trial results for each agent,73,74 the total cost of eltrombopag was estimated at $66 560 and that of romiplostim at $91 039, inclusive of care for adverse and bleeding events over 26 weeks.72 Eltrombopag was superior to romiplostim in the model as being less expensive and more effective in terms of bleeding events avoided.72 In a model from the United Kingdom, eltrombopag again was superior to romiplostim; however, in this case, it was found to be equal in efficacy but less costly.71 The dietary restrictions for food and polyvalent cations, such as calcium, with eltrombopag may be found burdensome for patients and may therefore affect its use and adherence.
Conclusions and research needs for these recommendations.
The guideline panel determined with very low certainty in the evidence that there is no net health benefit or harm difference using either eltrombopag or romiplostim. Based on the body of available evidence, it is likely there is no difference between the 2 treatments. There is also an absence of adequate reporting of certain important patient-reported outcomes, such as HRQoL. Patient preference for route of administration, that is, oral daily medication compared with weekly subcutaneous injection, will likely drive decision-making.
The panel identified the following research needs:
Second-line therapies: splenectomy, TPO-RA, and rituximab compared 1 against the other
Question: Should adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids undergo splenectomy or be treated with a TPO-RA?
Question: Should adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids undergo splenectomy or be treated with rituximab?
Question: Should adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids be treated with a TPO-RA or rituximab?
Recommendation 7
In adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids, the ASH guideline panel suggests either splenectomy or a TPO-RA (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 8
In adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids, the ASH guideline panel suggests rituximab rather than splenectomy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Recommendation 9
In adults with ITP lasting ≥3 months who are corticosteroid-dependent or have no response to corticosteroids, the ASH guideline panel suggests a TPO-RA rather than rituximab (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: These recommendations are the result of dichotomous evaluation of treatments that are often being considered simultaneously. Each of these second-line treatments may be effective therapy and therefore the choice of treatment should be individualized based on duration of ITP, frequency of bleeding episodes requiring hospitalization or rescue medication, comorbidities, age of the patient, medication adherence, medical and social support networks, patient values and preferences, cost, and availability. Patient education and shared decision-making are encouraged. If possible, splenectomy should be delayed for at least 1 year after diagnosis because of the potential for spontaneous remission in the first year. Patients who value avoidance of long-term medication may prefer splenectomy or rituximab. Patients who wish to avoid surgery may prefer a TPO-RA or rituximab. Patients who place a high value on achieving a durable response may prefer splenectomy or TPO-RAs.
Good practice statement
The treating physician should ensure that patients have appropriate immunizations prior to splenectomy and that they receive counseling regarding antibiotic prophylaxis following splenectomy. The treating physician should educate the patient on prompt recognition and management of fever and refer to current recommendations on pre- and postsplenectomy care.
Summary of evidence.
We included all RCTs and all observational studies that had internal comparators. Due to the scarcity of RCTs for these questions, we also included all single-arm prospective studies of ≥50 adults with ITP who were treated with splenectomy, TPO-RAs, or rituximab. For splenectomy only, we included a systematic review published in 2004 and all retrospective studies of ≥100 patients published after 2004 due to the lack of prospective studies. We did not identify any RCTs directly comparing splenectomy, TPO-RAs, or rituximab with 1 another; thus, splenectomy, TPO-RAs, and rituximab arms represent different populations. Two retrospective cohort studies compared rituximab with splenectomy.77,78 Regarding splenectomy, we identified 1 systematic review,79 10 additional retrospective studies,80-89 and 1 prospective study.90 Ten studies reported data on response at 1 month,79-88 1 study reported on durable response,80 6 studies reported on remission,79,83,85,86,89,90 8 reported on major bleeding,80-87 8 studies reported on infection,80-87 8 studies reported on thrombosis,80-87 9 studies reported on operative complications,79,81-83,85-87,89,91,92 and 0 studies reported on overall HRQoL for patients receiving splenectomy. Two additional retrospective comparisons of splenectomy with rituximab also provided data on remission.77,78 With respect to TPO-RAs, we identified 9 RCTs73,74,93-99 (TPO-RA vs a comparator other than rituximab or splenectomy). All 9 studies reported data on response within 1 month,73,74,93-99 3 studies reported on durable response,73,74,99 0 studies reported on remission, 7 studies reported on major bleeding,73,74,93-96,99 3 studies reported on infection,73,94,95 8 studies reported on thrombosis,73,74,93,95-99 and 3 reported on overall HRQoL74,95,99 for patients receiving TPO-RAs. Regarding rituximab, we identified 2 RCTs67,100 (rituximab vs comparator besides splenectomy or TPO-RA), 2 single-arm phase 2 studies,101,102 1 prospective study,103 and 1 prospective registry study.104 Five studies reported data on response within 1 month,67,100,102-104 3 studies reported on durable response,100,102,103 5 studies reported on remission,100-104 6 studies reported on infection,67,100-104 4 studies reported on major bleeding,67,100,102,104 2 studies reported on thrombosis,67,100 and 0 studies reported data on overall HRQoL for patients receiving rituximab. Two additional retrospective comparisons of splenectomy with rituximab also provided data on remission.77,78 The EtD framework for splenectomy compared with TPO-RAs is shown online at https://guidelines.gradepro.org/profile/6647F4D9-028E-C88F-9AF2-7697D58AB301. The EtD framework for splenectomy compared with rituximab is shown online at https://guidelines.gradepro.org/profile/6ED06816-4D2A-3FA9-8A34-EC148BC0F509. The EtD framework for rituximab compared with TPO-RAs is shown online at https://guidelines.gradepro.org/profile/F6795F46-991E-E43A-99FA-95F588C70354.
Benefits.
Response rates at 1 month for splenectomy, TPO-RAs, and rituximab were 86.7%, 65.7%, and 62.1%, respectively. Compared with TPO-RAs, rituximab was associated with lower durable response (63.2% vs 39.4%). Compared with rituximab, splenectomy was associated with higher durable response (53.0% vs 39.4%) and higher rate of remission (68.8% vs 23.5%). No difference in major bleeding was observed for patients treated with splenectomy, TPO-RAs, and rituximab (4.6%, 3.5%, and 2.2% respectively). Based on the greater durability of response observed with splenectomy and TPO-RAs, the panel determined that these treatment modalities had moderate desirable effects compared with rituximab. Overall, the certainty of these estimated effects is very low owing to a lack of randomized comparisons between treatments, imprecision of the estimates, and the need for ongoing treatment with TPO-RAs to achieve a durable response.
Harms and burden.
The panel prioritized the following adverse effects: infection, thrombosis, and operative complications. Infection occurred in 10.0%, 6.9%, and 3.7% of patients treated with splenectomy, TPO-RAs and rituximab, respectively. Thrombosis was observed in a similar percentage of patients with each treatment modality (2.4% with splenectomy, 2.5% with TPO-RAs, 2.2% with rituximab). The panel recognized that duration of follow-up for these potential side effects varied by study. Operative complications were reported in 12.8% of patients undergoing splenectomy. The panel determined that splenectomy had moderate undesirable effects compared with rituximab, largely because of the potential for operative complications with splenectomy. The panel noted that there is significant difference in comparing the possibility of a patient encountering side effects when comparing TPO-RAs (which require ongoing drug exposure), rituximab (which has a more limited exposure), and splenectomy (which imparts a life-long risk of complications that may decline some over time). Therefore, the panel did not make a judgment on the undesirable effects of TPO-RAs compared with splenectomy or rituximab. Overall, the certainty of these estimated effects is very low owing to a lack of randomized comparisons between treatments and imprecision of the estimates.
Other EtD criteria and considerations.
The panel determined that TPO-RAs are more expensive than splenectomy or rituximab, whereas the difference in cost between splenectomy and rituximab is negligible. The panel also noted that equity and feasibility may be reduced with TPO-RAs and rituximab because these agents are not available or have limited availability in some jurisdictions and because they may not be covered by all payors. The panel further noted that there are differences in the administration of the TPO-RAs, eltrombopag, and romiplostim, which may influence feasibility and acceptability. Romiplostim requires weekly subcutaneous injection that currently needs to occur in a health care facility, whereas eltrombopag is a daily oral medication that must be taken several hours removed from food and polyvalent cations such as calcium. The panel emphasized that patient values and preferences play a key role in selecting among treatment options. Patients who place a high value on avoiding surgery may opt for medical therapy with TPO-RAs or rituximab. Patients who wish to avoid long-term medication may prefer splenectomy or rituximab. Patients who place a high value on achieving durable response may prefer splenectomy or TPO-RAs.
Furthermore, the duration of ITP may influence decision-making. The recommendation questions posed by the panel included a range in ITP status, both persistent and chronic. It is possible that TPO-RAs and rituximab may be more favorable earlier in the course of the disease; however, this may change later in the course of the disease, when remission is less likely and/or the duration of medication exposure becomes important. For this reason, it is critical to reassess decision-making and patient preference regularly.
Conclusions and research needs for this recommendation.
We treated each of these comparisons as individual dichotomous decisions, however, we recognize that in clinical practice these options are often all being considered simultaneously. Based on these considerations, the panel acknowledged that there is no single second-line treatment that is optimal for all adult patients with ITP. Rather, treatment should be individualized based on duration of ITP, frequency of bleeding episodes requiring hospitalization or rescue medication, comorbidities, age of the patient, medication adherence, medical and social support networks, patient values and preferences, cost, and availability.
For patients with ITP of <12 months’ duration, it may be preferable to delay splenectomy when possible because of the potential for spontaneous remission in the first year. For such patients where the primary options are TPO-RAs and rituximab, the panel made a conditional recommendation in favor of TPO-RAs, largely because of the greater durability of response seen with ongoing use of these agents. However, rituximab might be preferable to a patient who places a high value on avoiding long-term treatment or who cannot afford TPO-RAs.
For adults who have had ITP for >12 months, the panel considered splenectomy, TPO-RAs, and rituximab to all be viable options. For patients who place a high value on avoiding long-term therapy, splenectomy and rituximab are the primary options. Even though splenectomy is associated with greater durable response and remission rates than rituximab, the panel made a conditional recommendation in favor of rituximab over splenectomy because of the operative risks and irreversible nature of splenectomy with the attendant long-term risks of infection and thrombosis. For patients who prefer medical therapy and place a high value on avoiding surgery, the primary options are TPO-RAs and rituximab. The panel made a conditional recommendation in favor of TPO-RAs over rituximab, largely because of the greater durability of response with this class of agents. Finally, for patients who place a high value on achieving a durable response, the primary options are splenectomy and TPO-RAs. The panel made a conditional recommendation for either TPO-RAs or splenectomy in this scenario, recognizing that issues of cost, accessibility, desire to avoid surgery, and desire to avoid long-term medication are likely to vary substantially among individual patients.
An individualized approach to selection of second-line therapy based on duration of ITP and patient values and preferences is summarized in Figure 1.
Figure 1.
Algorithm for the selection of second-line therapy in adults with ITP. Selection of second-line therapy in adults with ITP should be individualized based on duration of disease and patient values and preferences. Other factors that may influence treatment decisions include frequency of bleeding sufficient to require hospitalization or rescue medication, comorbidities, compliance, medical and social support networks, cost, and availability of treatments. Patient education and shared decision-making is encouraged. Patient characteristics are shown in blue boxes, actions in yellow boxes, and treatment options in red boxes. Numbered recommendations corresponding to each treatment option are provided.
The panel identified the following research priorities:
Obtaining data to determine whether patients are able to achieve and maintain an acceptable platelet count off treatment with TPO-RAs. Preliminary data from TPO-RAs clinical trial suggests that approximately one-third of patients (32%) are able to maintain a platelet count of >50 × 109/L for 24 consecutive weeks off treatment105;
Defining predictors of durable response to rituximab;
Establishing research models on how to understand, assess, and support patient values and preferences in shared decision-making;
Comparison and increased data on additional novel agents such as fostamatinib, a splenic tyrosine kinase inhibitor that was recently approved by the FDA for chronic ITP and has been studied primarily in the third-line setting but whose role as a second-line agent has not been established106,107;
Ongoing comparative effectiveness research of the different TPO-RAs, inclusive of newer agents such as avatrombopag, which is now approved by the FDA for chronic ITP.75,76
Management of children with newly diagnosed ITP
Outpatient vs inpatient management
Question: Should children with newly diagnosed ITP and a platelet count of <20 × 109/L who have no or mild bleeding (skin manifestations) be treated as an outpatient or be admitted to the hospital?
Question: Should children with newly diagnosed ITP and a platelet count of ≥20 × 109/L who have no or mild bleeding (skin manifestations) be treated as an outpatient or be admitted to the hospital?
Recommendation 10a
In children with newly diagnosed ITP and a platelet count of <20 × 109/L who have no or mild bleeding (skin manifestations) only, the ASH guideline panel suggests against admission to the hospital rather than outpatient treatment (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: For patients with uncertainty about the diagnosis, those with social concerns, those who live far from the hospital, and those for whom follow-up cannot be guaranteed, admission to the hospital may be preferable.
Recommendation 10b
In children with newly diagnosed ITP and a platelet count of ≥20 × 109/L who have no or mild bleeding (skin manifestations) only, the ASH guideline panel suggests against admission to the hospital rather than management as an outpatient (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯). Remark: For patients with uncertainty about the diagnosis, those with social concerns, those who live far from the hospital, or those for whom follow-up cannot be guaranteed, admission to the hospital may be preferable.
Good practice statement
The referring physician should ensure that the patient has follow-up with a hematologist within 24 to 72 hours of diagnosis.
Summary of evidence.
We included all systematic reviews, RCTs, and observational studies that assessed hospitalized vs nonhospitalized children with ITP. No studies were found that addressed the question of interest. Surveys were administered to the pediatric providers; however, they were limited by recall bias, determination of individual provider practice compared with center-wide practice, and the fact that all panel members practice at large tertiary care centers. Results of the survey data are reflected in the EtD frameworks. The EtD framework for children with a platelet count <20 × 109/L is shown online at https://guidelines.gradepro.org/profile/96F8E8CC-E08B-DFB0-AB8E-03D975B4A1F1. The EtD framework for children with a platelet count ≥20 × 109/L is shown online at https://guidelines.gradepro.org/profile/5F52E87D-5650-B3EB-8C8F-B02F469CFF69.
Benefits.
The panel agreed that the benefits of admission were trivial for both populations so long as the patient is able to have prompt (within 24 to 72 hours) follow-up with a pediatric hematologist. The primary goal is to ensure the correct diagnosis of ITP based on careful assessment of the peripheral blood smear as well as to provide proper education to the parents and depending on age, the child as well.
Harms and burden.
The undesirable effects of hospitalization were determined to be moderate. The primary burden was reflected in missed time at work, school, and other obligations with hospitalization. In addition, the panel members considered the small risk of hospital-acquired infections.
Other EtD criteria and considerations.
The panel recognized that there might be important differences in patient values that would affect the decision to admit. The decision should include assessment of any uncertainty about the diagnosis, social factors, distance from the care center, and likelihood of follow-up. There are large cost savings with management as an outpatient compared with inpatient care. The panel did not think that there were acceptability or feasibility considerations that would impair implementation of this recommendation.
Conclusions and research needs for these recommendations.
The guideline panel determined that there is very-low-certainty evidence for net health harm from admission rather than outpatient care for children with a diagnosis of ITP and no or mild bleeding only regardless of the platelet count. In any setting, patients with social concerns, uncertainty about the diagnosis, and concerns about follow-up may benefit from admission to the hospital.
The panel prioritized the following research needs:
Understanding the impact of pathway of care and types of encounters on short- and long-term patient outcomes such as HRQoL, patient experience, disease perception, and bleeding;
Determination of impact of initial outpatient management on patient outcomes, family comfort with disease diagnosis, and HRQoL.
Treatment vs observation
Question: Should children with newly diagnosed ITP who have no or minor bleeding be treated with observation or corticosteroids for initial therapy?
Recommendation 11
In children with newly diagnosed ITP who have no or minor bleeding, the ASH guideline panel suggests observation rather than corticosteroids (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Summary of evidence.
We included all RCTs and all observational studies that had internal comparators that compared corticosteroids and management with observation in children with newly diagnosed ITP. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP. For management with observation, we also included retrospective studies of ≥25 children with ITP due to a lack of prospective studies. We found 4 RCTs108-111 that compared corticosteroids and management with observation in children with newly diagnosed ITP. We found 4 RCTs54,109,112,113 (prednisone vs a comparator besides observation), 1 prospective study,114 and 1 observational study with a comparator (IVIG plus prednisone)115 that reported data on children receiving prednisone but not management with observation. We found 5 prospective studies109,114,116-118 and 4 retrospective studies119-122 that reported data on children receiving management with observation but not prednisone. Among the RCTs that directly compared prednisone and management with observation, 2 studies reported on durable response,108,109 2 studies reported on response within 7 days110,111 and 1 study reported on major bleeding.109 There were no RCTs that reported data on remission, mortality, or overall HRQoL. For the studies that reported only 1 arm of data, 12 studies reported on durable response (6 prednisone arm,54,109,112-115 6 observation arm109,114,117,118,121,122), 5 studies reported on remission (1 prednisone arm,123 4 observation arm116,117,119,121), 6 studies reported on major bleeding (2 prednisone arm,109,112 4 observation arm109,117-119), 6 studies reported on mortality (1 prednisone arm,123 5 observation arm109,117,119,120,122), and 0 studies reported overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/90AC9459-1960-FF50-A0D0-54AEE19FBF7E.
Benefits.
The data available did not show any substantial desirable effects of corticosteroids with regard to response at 1 month (RCT data: RR, 1.28; 95% CI, 0.54-3.02) or durable platelet response (RCT data: RR, 0.96; 95% CI, 0.74-1.25; observational data: 78.5% with corticosteroids and 87.3% with observation).There was no perceived benefit with regard to remission (observational data: 76.6% with corticosteroids and 63.6% with observation) or reduction in major bleeding (0% for both treatments for RCT and observational data). There were no reported deaths in the observational studies. There were no data on HRQoL. The panel thought that overall, any benefit of corticosteroids was trivial.
Harms and burden.
The panel did not prioritize any harms a priori. Clinically, the panel agreed that there were small undesirable effects associated with corticosteroids in this setting. There are recognized side effects of corticosteroids; however, the panel agreed that these may be less pronounced with the short courses used to treat children. The harm of corticosteroids will be magnified if longer courses of corticosteroids are given. The panel also appreciated that there may be a negative impact on HRQoL with observation (such as increased anxiety); however, there were no data to support this.
Other EtD criteria and considerations.
The panel agreed that there might be considerable variability in patient values depending on the degree of concerns patients have over potential for bleeding compared with a desire to avoid side effects associated with corticosteroids. The panel did not think that there were acceptability or feasibility considerations that would impair implementation of this recommendation. There was no significant cost difference between observation and corticosteroid treatment.
Conclusions and research needs for this question.
The guideline panel determined that there is very low certainty in evidence for treatment with observation rather than corticosteroids. Based on the available body of evidence, it is likely that the risk of bleeding without treatment is low and there is a lack of any large desirable effects to be gained with corticosteroids as well as exposure to side effects. The quality of the evidence was very low and the recommendation does not meet the paradigmatic situations in which a strong recommendation may be warranted despite low or very low confidence in the effect estimates, secondary to the low risk associated with a short course (<7 days) of corticosteroids. The panel acknowledges that important information on the impact of observation on patient-reported outcomes is lacking; however, a lack of evidence does not imply that there is no effect on these outcomes.
The panel identified the following research needs:
Better classification of bleeding and identification of factors that influence bleeding to identify children at risk of bleeding who would benefit from treatment;
Determination of biologic markers that may predict response to treatment;
Application of prioritized outcomes such as HRQoL in RCTs;
Detailed cost-effectiveness analysis.
Question: Should children with newly diagnosed ITP who have no or minor bleeding be treated with observation or IVIG?
Recommendation 12
In children with newly diagnosed ITP who have no or minor bleeding, the ASH guideline panel recommends observation rather than IVIG (strong recommendation based on moderate certainty in the evidence effects ⊕⊕⊕◯).
Summary of evidence.
We included all RCTs, and all observational studies that compared IVIG and management with observation in children with newly diagnosed ITP. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP. For management with observation we also included retrospective studies of ≥25 children with ITP due to lack of prospective studies. We found 1 RCT108 that compared IVIG and management with observation in children with newly diagnosed ITP. We found 10 RCTs109,113,123-130 (9 RCTS of IVIG vs comparators other than observation; 1 RCT of IVIG doses of 0.3 g vs 1 g/kg per day for 2 days) that reported data on children receiving IVIG but not management with observation. We found 1 RCT,109 5 prospective studies,109,114,116-118 and 4 retrospective studies119-122 that reported data on children receiving management with observation but not IVIG. The RCT108 that directly compared IVIG and management with observation evaluated durable response but did not report data on remission, major bleeding, mortality, hemolysis, or overall HRQoL. For the studies that reported only 1 arm of data, 15 studies reported on durable response (8 IVIG arm,109,113,123-126,129,130 7 observation arm109,114,117,118,120,122 [6 publications]), 7 studies reported on remission (3 IVIG arm,123,128,129 4 observation arm116,117,119,121), 10 studies reported on major bleeding (5 observation arm109,117-119 [4 publications]), 3 studies reported on hemolysis (yes/no) (3 IVIG arm113,126,127), 7 studies reported on mortality (1 IVIG arm,123 6 observation arm109,117,119,120,122 [5 publications]), and 0 studies reported overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/02A4142E-6737-F07D-8186-E66B0CF581D4.
Benefits.
The available data did not support any substantial desirable effects of IVIG with regard to durable response (RCT: RR, 1.09; 95% CI, 0.82-1.46, observational data 81.1% with IVIG and 86.6% with observation). This was consistent with the panel’s clinical experience even in the absence of significant data. There were no randomized data available at the time of the literature search on remission (observational data: 78.0% with IVIG and 63.6% with observation); however, the panel acknowledged results for a randomized trial of IVIG vs observation that showed no difference in outcomes at 12 months.131 The incidence of bleeding and mortality based on observational data was similar between the 2 groups (0.6% with IVIG and 0% with observation for bleeding; 1.8% with IVIG and 0% with observation for mortality). There were no data on the impact of either intervention on HRQoL.
Harms and burden.
The only harm that was prioritized a priori was hemolysis; however, the panel recognized that this is less of a concern with IVIG than with anti-D immunoglobulin. The percentage with hemolysis was 6.3% based on observational data. The panel agreed that there were moderate undesirable effects associated with IVIG. There is a black box warning for IVIG related to thrombosis and acute renal failure. Although it was not prioritized a priori, the panel stated that headache associated with IVIG, although not life threatening, can be significant and lead to additional medical interventions such as computerized tomography of the brain evaluating for ICH. The panel also appreciated that there may be a negative impact on HRQoL with observation (such as increased anxiety); however, there were no data to support this.
Other EtD criteria and considerations.
The panel agreed that the balance of effects favored observation rather than IVIG. There is significant cost associated with IVIG compared with observation. In addition, IVIG infusion may require an inpatient admission, and it can be difficult to obtain insurance approval for outpatient administration. IVIG may also not be acceptable to some patient populations, such as Jehovah Witnesses, who will not receive blood products. The panel did not think that there were feasibility considerations that would impair implementation of this recommendation.
Since the literature search was completed, a randomized trial comparing IVIG and observation in children with newly diagnosed ITP has been published.131 This trial was not included in the EtD framework. Children ages 3 months to 16 years with a platelet count of <20 × 109/L and mild to moderate bleeding (grade 1-3 on the Buchanan and Adix score) were randomized upfront to treatment with a single dose of 0.8 mg/kg IVIG or observation. With regard to the prioritized outcomes specific to this recommendation, there was no difference in the development of chronic disease (a platelet count of <150 × 109/L at 6 months) or ongoing disease at 12 months. There were no reported deaths in either group. Grade 4 to 5 bleeding (using the Buchanan and Adix bleeding score) occurred in 9% of the observation group and 1% of the IVIG group. It is important to note, however, that because this trial enrolled children with moderate bleeding, the results do not directly apply to the patient population in this recommendation, which involves no or minor bleeding only. There was no reported information on HRQoL.
Conclusions and research needs for this question.
The guideline panel determined that there is very-low-certainty evidence for treatment with observation rather than IVIG. The GRADE Handbook outlines paradigmatic situations when a strong recommendation may be used despite low confidence in the effects; 1 such situation is when low-quality evidence suggests benefit and high-quality evidence suggests harm or a very high cost. Based on the available body of evidence, it is likely that the risk of major bleeding without treatment is low and there is a lack of any large desirable effects with exposure to potential side effects associated with IVIG. This a strong recommendation despite very low certainty based on high-quality evidence of less harm for observation compared with IVIG based on the black box warning associated with IVIG thrombosis and acute renal failure. The panel acknowledges that important information on the impact of observation on patient-reported outcomes is lacking; however, a lack of evidence does not imply that there is no effect on these outcomes.
The panel identified the following research needs:
Adequate assessment of the side effects associated with IVIG use;
Better classification of bleeding and identification of factors that influence bleeding to identify children at risk of bleeding who would benefit from treatment;
Determination of biologic markers that may predict response to treatment;
Application of prioritized outcomes such as HRQoL in RCTs;
Detailed cost-effectiveness analysis.
Question: Should children with newly diagnosed ITP and no or minor bleeding be treated with observation or anti-D immunoglobulin for initial therapy?
Recommendation 13
In children with newly diagnosed ITP who have no or minor bleeding, the ASH guideline panel recommends observation rather than anti-D immunoglobulin (strong recommendation based on moderate certainty in the evidence of effects ⊕⊕⊕◯).
Summary of evidence.
We included all RCTs and all observational studies that had internal comparators that compared anti-D immunoglobulin and management with observation in children with newly diagnosed ITP. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP. For management with observation, we also included retrospective studies of ≥25 children with ITP. We found no RCTs that compared anti-D immunoglobulin and management with observation of children with newly diagnosed ITP; thus, anti-D immunoglobulin and management with observation arms represent different populations. We found 8 RCTs113,127-129,132-135 (anti-D immunoglobulin vs comparators other than observation) and 1 prospective study136 that reported data on children receiving anti-D immunoglobulin but not management with observation. We found 2 RCTs108,109 (observation vs comparators other than anti-D immunoglobulin), 5 prospective studies,109,114,116-118 and 4 retrospective studies119-122 that reported data on children receiving management with observation but not anti-D immunoglobulin. For the studies that reported only 1 arm of data, 12 studies reported on durable response (4 anti-D immunoglobulin arm,113,129,135,136 8 observation arm108,109,114,117,118,120,122 [7 publications]), 6 studies reported on remission (2 anti-D immunoglobulin arm,128,129 4 observation arm116,117,119,121), 6 studies reported on major bleeding (6 observation arm108,109,117-119 [5 publications]), 3 studies reported on hemolysis (yes/no) (3 anti-D immunoglobulin arm108,135,136), 7 studies reported on mortality (1 anti-D immunoglobulin arm,135 6 observation arm109,117,119,120,122 [5 publications]), and 0 studies reported overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/B8325DAB-B19B-F804-8C42-95E850E3C615.
Benefits.
The relative effects were not estimable from the data because of a lack of direct comparisons. The panel found only trivial benefit from the use of anti-D immunoglobulin. No difference was appreciated from the observational data on durable response (71.9% with anti-D immunoglobulin and 86.3% with observation) or remission (66.7% with anti-D immunoglobulin and 63.6% with observation). There were no observational data on major bleeding with anti-D immunoglobulin, and no major bleeding was reported with observation; therefore, the panel could not comment on the prevention of major bleeding with use of anti-D immunoglobulin. There were no reported deaths in either group. There were no data on the impact of either intervention on HRQoL.
Harms and burden.
The relative effects were not estimable from the data because of a lack of direct comparisons. The panel determined there were moderate undesirable effects associated with anti-D immunoglobulin. There is a black box warning for anti-D immunoglobulin related to intravascular hemolysis. Despite severe and fatal cases of intravascular hemolysis being reported, the incidence included in the EtD framework (15.2%) did not include information regarding severity of hemolysis. Therefore, the full magnitude of harm associated with anti-D immunoglobulin associated hemolysis is difficult to assess.
Other EtD criteria and considerations.
The panel determined that the balance of effects favored observation rather than anti-D immunoglobulin. There is significant cost associated with anti-D immunoglobulin compared with observation. Anti-D immunoglobulin availability is also limited in many places and requires that a patient be Rh+ with an intact spleen to be effective. Anti-D immunoglobulin may also not be acceptable to some patient populations, such as Jehovah's Witnesses, who will not receive blood products. The panel did not think that there were feasibility considerations that would impair implementation of this recommendation.
Conclusions and research needs for this question.
The guideline panel determined that there is very-low-certainty evidence for treatment with observation rather than anti-D immunoglobulin. The GRADE Handbook outlines paradigmatic situations when a strong recommendation may be used in spite of low confidence in the effects; 1 such situation is when low-quality evidence suggests benefit and high-quality evidence suggests harm or a very high cost. Based on the available body of evidence, it is likely that the risk of major bleeding without treatment is low and there is a lack of any large desirable effects with potential side effects with anti-D immunoglobulin. This is a strong recommendation despite very low-quality evidence based on high-quality evidence of less harm for observation than anti-D immunoglobulin, based on the black box warning associated with anti-D immunoglobulin-associated intravascular hemolysis. The panel acknowledges that important information on the impact of observation on patient-reported outcomes is lacking; however, a lack of evidence does not imply that there is no effect on these outcomes.
The panel identified the following research needs:
Adequate assessment of side effects associated with anti-D immunoglobulin use;
Better classification of bleeding and identification of factors that influence bleeding to identify children at risk of bleeding who would benefit from treatment;
Determination of biologic markers that may predict response to treatment;
Application of prioritized outcomes such as HRQoL in RCTs;
Detailed cost-effectiveness analysis.
Corticosteroid duration and type
Question: Should children with newly diagnosed ITP who have non–life-threatening bleeding and/or diminished HRQoL receive a course of corticosteroids longer than 7 days vs 7 days or shorter?
Recommendation 14
In children with newly diagnosed ITP who have non–life-threatening bleeding and/or diminished HRQoL, the ASH guideline panel recommends against courses of corticosteroids longer than 7 days rather than courses 7 days or shorter (strong recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Summary of evidence.
We included all systematic reviews, RCTs, or observational studies that compared children with ITP treated with a short (≤7 days) vs a longer course of prednisone. We also searched for all single-arm prospective studies of ≥25 children treated with either a short or longer course of steroids. No studies were found that had data on short courses of prednisone; therefore, no data from patients with ITP from the literature were used to evaluate this question. Articles reporting data about the side effects of corticosteroids in any patient group were used to assess harm. A survey was administered to the pediatric providers; however, it was limited by recall bias and by individual provider practice compared with center-wide practice. Results of the survey data are reflected in the EtD framework online at https://guidelines.gradepro.org/profile/70992151-AA06-05D8-877E-8435125779C8.
Benefits.
The panel agreed that there was likely trivial benefit in continuing corticosteroids beyond 7 days. This was based on the low risk of bleeding, high rates of spontaneous remission in children, and lack of evidence for benefit with long-term corticosteroids.
Harms and burden.
The panel agreed that based on indirect evidence the risk of harm and likelihood of adverse events were large with the use of courses of corticosteroids for >7 days.
Other EtD criteria and considerations.
There was no cost difference. The panel did not think that there were patient value or feasibility considerations that would impair implementation of this recommendation. The panel agreed that a longer course of steroids would likely not be acceptable to patients and might result in poor adherence.
Conclusions and research needs for these recommendations.
The guideline panel determined that there is very-low-certainty evidence for net health benefit for longer courses of corticosteroids (>7 days) rather than a shorter course. The GRADE Handbook outlines paradigmatic situations when a strong recommendation may be used in spite of low confidence in the effects; 1 such situation is when low-quality evidence suggests benefit and high-quality evidence suggests harm or a very high cost. Despite very low levels of evidence, there was moderate certainty by the panel in their clinical observations, survey data, and indirect evidence.45,58-60 In the absence of demonstrated increased benefits with longer courses of corticosteroids and the known complications and side effects associated with prolonged corticosteroid exposure, the panel agreed that the balance of effects favored a shorter course of corticosteroids (≤7 days) over longer courses. The panel provided a strong recommendation based on indirect evidence for increased risk with longer exposure to corticosteroids.
The panel identified the following research needs:
Properly designed studies applying more modern short-course dosing regimens;
Assessment of the magnitude and impact of adverse effects associated with corticosteroid use;
Application of prioritized outcomes such as HRQoL in RCTs.
Question: Should children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL receive dexamethasone (0.6 mg/kg per day; maximum, 40 mg per day for 4 days) or prednisone (2-4 mg/kg per day for 5-7 days; maximum, 120 mg daily, for 5-7 days)?
Recommendation 15
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests prednisone (2-4 mg/kg per day; maximum, 120 mg daily, for 5-7 days) rather than dexamethasone (0.6 mg/kg per day; maximum, 40 mg per day for 4 days) (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Summary of evidence.
We included all RCTs and all observational studies that compared dexamethasone and prednisone in children with newly diagnosed ITP. We also included prospective studies of ≥25 children with ITP. We found no studies that directly compared dexamethasone and prednisone in children and no single-arm studies of ≥25 children who received dexamethasone. Therefore, we used data on adults with ITP, taking into account the fact that this is an indirect population. For adults, we found 6 RCTs46-48,50,53,62 that compared dexamethasone and prednisone. Three studies reported data on response within 7 days,46-48 3 studies reported on response within 1 month,46,53,62 5 studies reported on durable response,46,48,50,53,62 4 studies reported on remission,46,48,50,53 3 studies reported on major bleeding,47,50,53 and 0 studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/024DC9C4-9E84-730D-AAD5-E02D0D6C8CB1.
Benefits.
There was no evidence from pediatric trials to confirm a desirable effect of treatment with dexamethasone compared with prednisone. Given the low rates of bleeding, high rates of remission, and overall low morbidity of pediatric ITP, the desired effects of increased response at 7 days identified in the adult trials were thought to be trivial in the pediatric population.
Harms and burden.
The panel recognized that in adult trials, repeated courses of dexamethasone were given and compared with longer courses of prednisone than what is preferred in pediatrics. For this reason, the overall corticosteroid exposure would be increased with use of repeated dexamethasone courses. In addition, the higher corticosteroid dose of dexamethasone was deemed to be potentially intolerable by some pediatric patients with regard to short-term side effects.
Other EtD criteria and considerations.
The panel did not think that there were acceptability, patient value, equity, or feasibility considerations that would impair implementation of this recommendation. The cost difference was negligible.
Conclusions and research needs for these recommendations.
The guideline panel determined that there is very-low-certainty evidence for a net health benefit for prednisone rather than dexamethasone. In the absence of data, the panel agreed that there was no evidence to suggest that dexamethasone is superior to prednisone; however, the side-effect profile for dexamethasone is assumed to be higher based on panel experience. The panel members thought that extrapolation from adult studies was not reasonable.
The panel identified the following research needs:
Determination of an age or clinical scenario when children may benefit from adult guidelines;
Randomized trials with patient-reported outcomes such as tolerability, side effects, and potential effect on platelet pathophysiology of the 2 drugs.
Treatment of children with non–life-threatening bleeding and/or diminished HRQoL
Question: Should children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL be treated with anti-D immunoglobulin or corticosteroids for initial therapy?
Recommendation 16
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests corticosteroids rather than anti-D immunoglobulin (conditional recommendation based on low certainty in the evidence of effects ⊕⊕◯◯). Remark: This recommendation assumes corticosteroid dosing as outlined in recommendations 14 and 15. This recommendation is reserved only for children with nonmajor mucosal bleeding.
Summary of evidence.
We included all RCTs, and all observational studies that compared corticosteroids and anti-D immunoglobulin in children with newly diagnosed ITP. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP treated with either corticosteroids or anti-D immunoglobulin. We found 1 RCT113 that compared corticosteroids to anti-D immunoglobulin in children with newly diagnosed ITP. We found 4 RCTs54,109,112,113 (prednisone vs a comparator other than anti-D immunoglobulin), 1 prospective study,114 and 1 observational study with a comparator (IVIG plus prednisone)115 that reported data on children receiving prednisone but not anti-D immunoglobulin. We found 2 RCTs128,129 (anti-D immunoglobulin vs comparators other than prednisone), 1 RCT135 (75 vs 50 µg/kg anti-D immunoglobulin), and 1 prospective study136 that reported data on children receiving anti-D immunoglobulin but not prednisone. The RCT113 that directly compared prednisone and anti-D immunoglobulin evaluated durable response and hemolysis but did not report data on remission, major bleeding, mortality, or overall HRQoL. For the studies that reported only 1 arm of data, 10 studies reported on durable response (7 prednisone arm54,108,109,112,114,115 [6 publications], 3 anti-D immunoglobulin arm129,135,136), 3 studies reported on remission (1 prednisone arm,123 2 anti-D immunoglobulin arm128,129), 4 studies reported on major bleeding (4 prednisone arm108,109,123 [3 publications]), 2 studies reported on hemolysis (yes/no) (2 anti-D immunoglobulin arm135,136), 2 studies reported on mortality (1 prednisone arm,123 1 anti-D immunoglobulin arm135), and 0 studies reported overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/E2E38C37-B301-5F72-9AFE-1051BFBD8990.
Benefits.
The benefits were determined to be unknown by the panel, as the available data were not comprehensive enough. There was benefit to treatment with anti-D immunoglobulin with regard to durable response (1 RCT: RR, 0.72; 95% CI, 0.54-0.97; observational data 76.6% with corticosteroids and 73.4% with anti-D immunoglobulin). Observational data showed little or no difference in remission (76.6% with corticosteroids and 66.7% with anti-D immunoglobulin). No data were available on major bleeding with anti-D immunoglobulin, and the incidence was 0 with corticosteroids, making it difficult to show a benefit in major bleeding reduction with anti-D immunoglobulin. There were no reported deaths for either group. There were no data on HRQoL.
Harms and burden.
The panel agreed that there were small undesirable effects associated with anti-D immunoglobulin. Anti-D immunoglobulin was associated with hemolysis compared with corticosteroids (RR for no hemolysis, 0.72; 95% CI, 0.64-0.92). Although there is a black box warning for anti-D immunoglobulin related to fatal intravascular hemolysis, this is a rare event. The majority of patients will experience at least some minor side effects while on treatment. The panel tried to balance the rare but serious side effects of anti-D immunoglobulin against the common but milder side effects associated with a short course of corticosteroids. Because of the lack of data and the combined difference in magnitude of undesirable effects, the balance of effects was decided to be unknown.
Other EtD criteria and considerations.
The use of corticosteroids is substantially less expensive than anti-D immunoglobulin. Anti-D immunoglobulin availability is also limited in many places and requires that a patient be Rh+ with an intact spleen to be effective. Anti-D immunoglobulin may also not be acceptable to some patient populations, such as Jehovah's Witnesses, who will not receive blood products. It is therefore not an appropriate treatment of all patients. The panel did not think that there were feasibility considerations that would impair implementation of this recommendation.
Conclusions and research needs for this question.
The guideline panel determined that there is low certainty evidence for a net health benefit from treatment with corticosteroids rather than anti-D immunoglobulin. Based on the available body of evidence, there was significant cost associated with anti-D immunoglobulin and the potential for serious side effects compared with corticosteroids.
The panel identified the following research needs:
Comparative effectiveness trials of first-line agents that account not only for efficacy but also for cost, side effects, and patient-reported outcomes;
Determination of upfront treatment selection on long-term outcomes;
Biologic studies to predict treatment response;
Assessment of other treatments (eg, TPO-RAs) for use in newly diagnosed patients to minimize side effects and potentially modify disease.
Question: Should children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL be treated with IVIG or anti-D immunoglobulin for initial therapy?
Recommendation 17
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests either anti-D immunoglobulin or IVIG (conditional recommendation based on low certainty in the evidence of effects ⊕⊕◯◯). Remark: This recommendation is reserved only for children with nonmajor mucosal bleeding.
Summary of evidence.
We included all RCTs and all observational studies that had internal comparators that compared IVIG and anti-D immunoglobulin in children with newly diagnosed ITP. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP treated with either IVIG or anti-D immunoglobulin. We found 7 RCTs113,127-129,132-134 that compared IVIG to anti-D immunoglobulin in children with newly diagnosed ITP. We found 1 RCT135 (75 vs 50 µg/kg anti-D immunoglobulin) and 1 prospective study136 that reported data on children receiving anti-D immunoglobulin but not IVIG. We found 6 RCTs108,109,123-126 (IVIG vs comparators other than anti-D immunoglobulin) and 1 RCT130 comparing IVIG doses (0.3 g/kg per day for 2 days vs 1 g/kg per day for 2 days) that reported data on children receiving IVIG but not anti-D immunoglobulin. Among the RCTs that directly compared IVIG and anti-D immunoglobulin, 2 studies reported on durable response,113,129 3 studies reported on remission,113,128,129 4 studies reported on response within 7 days,127,129,133,134 and 6 studies reported on hemolysis (1 study reported hemolysis [yes/no]113 and 5 studies reported hemolysis by mean decrease in hemoglobin127-129,133,134). There were no RCTs that reported data on mortality, major bleeding, or overall HRQoL. For the studies that reported only 1 arm of data, 9 studies reported on durable response (7 IVIG arm,108,109,123-126,130 2 anti-D immunoglobulin arm135,136), 1 study reported on remission (1 IVIG arm123), 3 studies reported on hemolysis (yes/no) (1 IVIG arm,126 2 anti-D immunoglobulin arm135,136), 2 studies reported on mortality (1 IVIG arm,123 1 anti-D immunoglobulin arm135), 5 studies reported on major bleeding (5 IVIG arm108,109,123,126,130), and 0 studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/8810F037-714A-05B5-A135-A20932FAC54F.
Benefits.
There is trivial benefit of IVIG over anti-D immunoglobulin; however, there was significant heterogeneity in dosing across studies that is not reflective of current practice and made comparison difficult. Based on 2 included randomized trials, there was no difference in 7-day platelet count response (RR, 1.01; 95% CI, 0.9-1.15), durable response (RR, 0.8; 95% CI, 0.63-1.51), or remission (RR, 0.85; 95% CI, 0.69-1.06). Major bleeding was not reported with anti-D immunoglobulin but was only 0.6% with IVIG, making demonstration of a benefit in reduced bleeding with anti-D immunoglobulin unlikely. Mortality was 0% with anti-D immunoglobulin and 1.8% with IVIG. HRQoL data were not reported.
Harms and burden.
Although the panel prioritized the outcome of hemolysis, this was recognized to be a greater concern with anti-D immunoglobulin. The panel agreed the balance of undesirable outcomes was unknown because the side effect of IVIG-associated headache was not prioritized a priori. The panel stated that this side effect associated with IVIG can be significant and lead to additional medical interventions, such as computed tomography scans of the brain evaluating for ICH. Both treatments are also associated with black box warnings regarding anti-D immunoglobulin–associated intravascular hemolysis and IVIG-related thrombosis and acute renal failure. There is a black box warning regarding hemolysis with anti-D immunoglobulin; the incidence of hemolysis was higher for anti-D immunoglobulin as compared with IVIG (RR, 2.72; 95% CI, 1.05-7.07). The severity and degree of hemolysis with either treatment is not known.
Other EtD criteria and considerations.
The 2 treatment approaches were equal with regard to cost. There was a recognized difference in the administration with anti-D immunoglobulin being a faster infusion than IVIG. Anti-D immunoglobulin availability is limited in many places and requires that a patient be Rh+ with an intact spleen to be effective. The panel did not think that there were acceptability or feasibility considerations that would impair implementation of this recommendation.
Conclusions and research needs for this question.
The guideline panel determined that there is low-certainty evidence for treatment with either anti-D immunoglobulin or IVIG. Based on the body of evidence available, both agents were thought to have similar benefits. In consideration of harms, both treatments are associated with FDA black box warnings for rare but potentially serious events and require careful monitoring during use.
The panel identified the following research needs:
Comparative effectiveness trials of first-line agents that account not only for efficacy but also for cost, side effects, and patient-reported outcomes;
Determination of upfront treatment selection on long-term outcomes;
Biologic studies to predict treatment response;
Assessment of other treatments (eg, TPO-RAs) for use in newly diagnosed patients to minimize side effects and potentially modify disease.
Question: Should children with newly diagnosed ITP who have non–life-threatening-mucosal bleeding and/or diminished HRQoL be treated with IVIG or corticosteroids?
Recommendation 18
In children with newly diagnosed ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL, the ASH guideline panel suggests corticosteroids rather than IVIG (conditional recommendation based on low certainty in the evidence of effects ⊕⊕◯◯). Remark: This recommendation assumes that a short course of corticosteroids is being used for treatment as recommended in recommendation 14. This recommendation is reserved only for children with nonmajor mucosal bleeding.
Summary of evidence.
We included all RCTs and all observational studies that compared IVIG and prednisone in children with newly diagnosed ITP. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP treated with either IVIG or prednisone. We found 4 RCTs108,109,113,123 that compared IVIG to prednisone in children with newly diagnosed ITP. We found 5 RCTs124-126,128,129 (IVIG vs comparators other than prednisone) and 1 RCT130 comparing IVIG doses (0.3 g/kg per day for 2 days vs 1 g/kg per day for 2 days) that reported data on children receiving IVIG but not prednisone. We found 2 RCTs52,109 (prednisone vs comparator besides IVIG), 1 prospective study,114 and 1 observational study with a comparator (IVIG plus prednisone)115 that reported data on children receiving prednisone but not IVIG. Among the RCTs that directly compared IVIG and prednisone, 4 studies reported on durable response,108,109,113,123 1 study reported on remission,123 1 study reported data on mortality,123 3 studies reported on major bleeding,108,109,123 and 0 reported on overall HRQoL. Of the studies that reported only 1 arm of data, 9 studies reported on durable response (5 IVIG arm,124-126,129,130 4 prednisone arm54,109,114,115 [6 publications]), 2 studies reported on remission (1 IVIG arm128,129), 4 studies reported on major bleeding (3 IVIG arm,126,128,130 1 prednisone arm109), and 0 studies reported on overall HRQoL. The EtD framework is shown online at https://guidelines.gradepro.org/profile/7c39219f-87a9-46c8-a11c-915f9177faae.
Benefits.
Trivial benefits were seen with the use of IVIG compared with corticosteroids. Based on the RCT data, there was no observed desirable effect with the prioritized outcomes of durable response (RR, 1.08; 95% CI, 0.95-1.23), remission (RR, 1.08; 95% CI, 0.88-1.33), or prevention of bleeding events (RR, 0.99; 95% CI, 0.95-1.03) and mortality (RR, 0.98; 95% CI, 0.93-1.03). The observational data were similar. There was no reported data on the important patient-reported outcome of HRQoL.
Harms and burden.
The panel did not prioritize any harms a priori, and therefore, the undesirable effects were determined to be unknown. The panel assumed that a short course of corticosteroids was to be given, which would be associated with some mild side effects in the majority of patients. IVIG, however, is associated with a black box warning for associated thrombosis and renal failure. Furthermore, the side effect of IVIG-associated headache was not prioritized a priori. The panel stated that the particular side effect of headache associated with IVIG can be significant and lead to additional medical interventions, such as computed tomography scans of the brain evaluating for ICH. This resulted in the balance of effects being unknown based on the evidence provided.
Other EtD criteria and considerations.
IVIG has high costs compared with a short course of corticosteroids. In many centers, administration of IVIG would also require inpatient admission, and it often requires insurance approval, especially for outpatient administration. IVIG may also not be widely available, whereas corticosteroids represent a universally available treatment that should be acceptable to most stakeholders. IVIG may not be acceptable to some patient populations, such as Jehovah's Witnesses, who will not receive blood products.
Conclusions and research needs for this question.
The guideline panel determined that there is low-certainty evidence for treatment with corticosteroids rather than IVIG. Based on the available body of evidence, there was significant cost associated with IVIG and the potential for serious side effects compared with corticosteroids. In addition, the need for IV access, possible admission, and donor exposure with IVIG compared with corticosteroids were all considered. This recommendation is reserved only for children with nonmajor mucosal bleeding.
The panel identified the following research needs:
Comparative effectiveness trials of first-line agents that account not only for efficacy but also for cost, side effects, and patient-reported outcomes;
Determination of upfront treatment selection on long-term outcomes;
Biologic studies to predict treatment response;
Assessment of other treatments (eg, TPO-RAs) for use in newly diagnosed patients to minimize side effects and potentially modify disease.
Management of children with ITP who do not have a response to first-line treatment
Question: Should children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment be treated with TPO-RAs or rituximab?
Recommendation 19
In children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment, the ASH guideline panel suggests the use of TPO-RAs rather than rituximab (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Summary of evidence.
We included all RCTs and all observational studies that compared TPO-RAs and rituximab in children with ITP who are unresponsive to first-line treatment. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP treated with either TPO-RAs or rituximab. We found no RCTs that directly compared TPO-RAs and rituximab; thus, TPO-RAs and rituximab arms represent different populations. We found 5 RCTs (TPO-RA vs placebo)137-141 and 1 open-label extension of an RCT140 reporting outcomes of interest in pediatric patients receiving TPO-RA. We found 1 systematic review,142 1 RCT (rituximab vs vincristine),143 1 prospective phase 1/2 study,144 and 1 longitudinal observational cohort study145 reporting outcomes of interest in pediatric patients receiving rituximab. Six studies reported data on response within 1 month (4 TPO-RA arm,137-140 2 rituximab arm142,143), 5 studies reported on durable response (2 TPO-RA arm,140,146 3 rituximab arm143-145), 1 study reported on remission (1 rituximab arm144), 6 studies reported on major bleeding (5 TPO-RA arm,137-141 1 rituximab arm144), 7 studies reported on infections (4 TPO-RA arm,137,138,140,141 3 rituximab arm142-144), 4 studies reported on thrombosis (4 TPO-RA arm137,139,140,141), and 2 studies reported on discontinuation of corticosteroids (2 TPO-RA arm140,141). The EtD framework is shown online at https://guidelines.gradepro.org/profile/E06726A7-144B-0A75-A88F-2ECD81A91494.
Benefits.
The relative effects were not estimable from the data because of a lack of comparisons. There is a moderate benefit of TPO-RAs over rituximab. The 2 treatments appear similar with regard to 1-month response (57.7% with TPO-RAs and 64.8% with rituximab) and durable response (46.8% for TPO-RAs and 47.0% for rituximab). Although remission and long-term data are lacking for the TPO-RAs, the panel recognized the low reported remission (20%) seen with rituximab. The TPO-RAs also seemed to reduce bleeding events compared with rituximab (3% compared with 6.7%), although a relative effect could not be estimated. TPO-RA use resulted in a reduction or discontinuation of corticosteroids in 6.5% of children; there are no data available on this outcome for rituximab. The panel recognized that the benefits of TPO-RAs require ongoing drug treatment.
Harms and burden.
The relative effects were not estimable from the data because of a lack of comparisons. The undesirable effects were thought to be small. There were reported episodes of infection with the TPO-RAs (4.8%); however, these may be unrelated to the drug, compared with 1.4% seen with rituximab, which are more likely directly attributed to drug use. Panel members also expressed concern about the development of persistent hypogammaglobulinemia seen following use of rituximab in the pediatric population. This complication was thought to be underreported given that only small clinical trials of rituximab have been conducted in children. Thrombosis, which has occurred in adults receiving TPO-RAs, was not seen in children.
Other EtD criteria and considerations.
A single course of rituximab is similar in cost to 1 month of low-dose TPO-RA use; however, given the need for ongoing TPO-RAs over time rituximab, will cost less. TPO-RAs were thought to be acceptable to stakeholders; however, there may be high patient variability in terms of goals and feasibility. If a patient places high value on short-term treatment, then rituximab may be favorable. Furthermore, for some patients, the need for daily oral medication with dietary restrictions (eltrombopag) or weekly injections at the physician’s office (romiplostim) may not fit with the patient’s lifestyle. At the time of the guideline panel meetings, an additional limitation was the lack of a liquid preparation for eltrombopag, which has since become available.
Conclusions and research needs for this question.
The guideline panel determined that there is low-certainty evidence for TPO-RAs rather than rituximab in children with ITP who are unresponsive to first-line treatment. Based on the body of evidence, the risks associated with TPO-RAs were thought to be low and the potential benefits high. The panel also placed high value on avoiding immunosuppression in the pediatric population, especially given that many children are likely to undergo spontaneous remission.
The panel identified the following research needs:
Assessment of impact of treatments on patient-reported outcomes such as fatigue, HRQoL, and bleeding;
Cost analysis of second-line therapies;
Determination of patient and parent preferences that influence treatment selection;
Biologic studies to predict treatment response and investigate the effect of agents on immunomodulation;
Randomized trial or observational trials to assess long-term outcomes;
Additional studies of novel second-line agents in children.
Question: Should children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment be treated with TPO-RAs or splenectomy?
Recommendation 20
In children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment, the ASH guideline panel suggests TPO-RAs rather than splenectomy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Summary of evidence.
We included all RCTs and all observational studies that compared TPO-RAs and splenectomy in children with ITP who are unresponsive to first-line treatment. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP treated with either TPO-RAs or splenectomy. For splenectomy only, we also included retrospective studies of ≥25 children with ITP due to a lack of prospective studies. We found no RCTs that directly compared TPO-RA and splenectomy; thus, TPO-RA and splenectomy arms represent different populations. We found 5 RCTs (TPO-RA vs placebo)137-141 and 1 open-label extension of an RCT140 reporting outcomes of interest in pediatric patients receiving TPO-RA. We found 1 prospective registry study147 and 17 retrospective studies84,148-162 reporting outcomes of interest for patients who received a splenectomy. Eleven studies reported data on response within 1 month (4 TPO-RA arm,137-140 7 splenectomy arm84,147-151,162), 12 studies reported on durable response (2 TPO-RA arm,140,146 10 splenectomy arm84,151-159), 6 studies reported on remission (6 splenectomy arm147,150,151,155,160,161), 12 studies reported on major bleeding (5 TPO-RA arm,137-141 7 splenectomy arm149-151,154,156,158,159), 13 studies reported on infections (4 TPO-RA arm,137,138,140,141 9 splenectomy arm84,147,150-152,154-156,162), 4 studies reported on thrombosis (4 TPO-RA arm137,138,140,141), 2 studies reported on discontinuation of corticosteroids (2 TPO-RA arm140,141), and 3 studies reported on operative complications (3 splenectomy arm147,151,154). The EtD framework is shown online at https://guidelines.gradepro.org/profile/48EBDD57-1185-3054-9ADF-00ABE6EC1D18.
Benefits.
The relative effects were not estimable from the data because of a lack of comparisons. There was a moderate benefit of splenectomy over TPO-RAs, given the high reported remission rates with splenectomy (68.5%). There are no data available for comparison with TPO-RAs; however, based on clinical experience, TPO-RAs can provide a stable long-term platelet response. Splenectomy also seemed to demonstrate superior 1-month (91.1% with splenectomy and 57.7% with TPO-RAs) and durable (76.7% with splenectomy and 46.8% with TPO-RAs) remission. The TPO-RAs seem to reduce bleeding events compared with splenectomy (3.0% compared with 6.3%), although a relative effect could not be estimated. TPO-RA use resulted in a reduction or discontinuation of corticosteroids in 6.5% of children; there are no data available on this outcome for splenectomy. The panel recognized that the benefits of TPO-RAs require ongoing drug treatment.
Harms and burden.
The relative effects were not estimable from the data because of a lack of comparisons. The undesirable effects were moderate for splenectomy. These moderate undesirable effects are magnified in the younger patient population, given the ongoing lifelong risks following splenectomy. There were reported episodes of infection with the TPO-RAs (4.8%); however, these may be unrelated to the drug, compared with 3.8% that can likely be directly attributed to splenectomy. Additional operative complications associated with splenectomy were identified in 5.9% of children. Thrombosis, which has occurred in adults receiving TPO-RAs, was not seen in children. Although splenectomy is also associated with thrombosis, this outcome was not reported in the pediatric trials.
Other EtD criteria and considerations.
Despite a high 1-time cost for surgery, there is likely a higher cost associated with the ongoing use of TPO-RAs. TPO-RAs were thought to be acceptable; however, patients are often reluctant to undergo splenectomy. There may be high patient variability in terms of goals with both approaches, and TPO-RAs may not be universally available. If a patient places high value on a short-term procedure with a chance for long-term remission, splenectomy may be preferable. Furthermore, for some patients, the need for daily oral medication with dietary restrictions (eltrombopag) or weekly injections at the physician’s office (romiplostim) may not fit with the patient’s lifestyle. At the time of the panel meetings, an additional limitation was the lack of a liquid preparation for eltrombopag, which has since become available.
Conclusions and research needs for this question.
The guideline panel determined that there is low-certainty evidence for TPO-RAs rather than splenectomy in children with ITP who are unresponsive to first-line treatment. Based on the available body of evidence, the risks associated with TPO-RAs were thought to be low and the potential benefits high. The panel also placed high value on avoiding splenectomy in the pediatric population, especially given that many children are likely to undergo spontaneous remission.
The panel identified the following research needs:
Assessment of impact of treatments on patient-reported outcomes such as fatigue, HRQoL, and bleeding;
Cost analysis of second-line therapies;
Determination of patient and parent preferences that influence treatment selection;
Biologic studies to predict treatment response and investigate the effect of agents on immunomodulation;
Randomized trials or observational trials to assess long-term outcomes;
Additional studies of novel second-line agents in children.
Question: Should children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment be treated with rituximab or splenectomy?
Recommendation 21
In children with ITP who have non–life-threatening mucosal bleeding and/or diminished HRQoL and do not respond to first-line treatment, the ASH guideline panel suggests rituximab rather than splenectomy (conditional recommendation based on very low certainty in the evidence of effects ⊕◯◯◯).
Good practice statement
The treating physician should ensure that the patient has appropriate immunizations prior to splenectomy and that they receive counseling regarding antibiotic prophylaxis following splenectomy. The treating physician should educate the patient on prompt recognition and management of fever and refer to current recommendations on pre- and postsplenectomy care.
Summary of evidence.
We included all RCTs and all observational studies that compared rituximab and splenectomy in children with ITP who are unresponsive to first-line treatment. Due to the scarcity of RCTs for this question, we also included all prospective studies of ≥25 children with ITP treated with either rituximab or splenectomy. For splenectomy only, we also included retrospective studies of ≥25 children with ITP due to a lack of prospective studies. We found no RCTs that directly compared rituximab and splenectomy; thus, rituximab and splenectomy arms represent different populations. We found 1 systematic review,142 1 RCT (rituximab vs vincristine),143 1 prospective phase 1/2 study,144 and 1 longitudinal observational cohort study145 reporting outcomes of interest in pediatric patients receiving rituximab. We found 1 prospective registry study147 and 17 retrospective studies82,148-162 reporting outcomes of interest for patients who received a splenectomy. Nine studies reported data on response within 1 month (2 rituximab arm,142,143 7 splenectomy arm82,147-151,162), 13 studies reported on durable response (3 rituximab arm,143-145 10 splenectomy arm82,151-159), 7 studies reported on remission (1 rituximab arm,144 6 splenectomy arm147,150,151,155,160,161), 8 studies reported on major bleeding (1 rituximab arm,144 7 splenectomy arm149-151,154,156,158,159), 12 studies reported on infections (3 rituximab arm,142-144 9 splenectomy arm84,147,150-152,154-156,162), 3 studies reported on operative complications (3 splenectomy arm147,151,154), and 0 studies reported data on thrombosis or reduction or discontinuation of steroids. The EtD framework is shown online at https://guidelines.gradepro.org/profile/25C16C9D-58EB-B0BA-9129-3D4FF5207F08.
Benefits.
The relative effects were not estimable from the data because of a lack of direct comparisons. There was a moderate benefit of splenectomy over rituximab. Given the high reported remission rates with splenectomy (68.5%) compared with rituximab (20%), the panel thought there was a large desirable effect of splenectomy. Splenectomy also showed some benefit over rituximab with durable remission, 76.7% compared with 47.0%. Major bleeding was similar between the 2 treatments (6.7% with rituximab and 6.3% with splenectomy). There were no reported data on reduction or discontinuation of corticosteroids with either treatment.
Harms and burden.
The relative effects were not estimable from the data because of a lack of direct comparisons. The undesirable effects were also moderate for splenectomy. These moderate undesirable effects were magnified in the younger patient population, given the ongoing lifelong risks following splenectomy. Overall infection rates were lower for rituximab than splenectomy (1.4% vs 3.8%). Fatal sepsis has also been reported with splenectomy, and therefore, although the numerical difference in rates may be small, the overall impact was viewed as moderate. Panel members also expressed concern about the development of persistent hypogammaglobulinemia seen following use of rituximab in the pediatric population. This complication was thought to be underreported given that only small clinical trials of rituximab have been conducted in children. Additional operative complications associated with splenectomy were identified in 5.9% of children.
Other EtD criteria and considerations.
The high 1-time cost for surgery is greater than the direct drug costs associated with a course of rituximab. Rituximab was thought to be acceptable to stakeholders; however, patients are often reluctant to undergo splenectomy. There may be high patient variability in terms of goals with regard to both treatments, and rituximab may not be available. If a patient places high value on a chance for long-term remission, splenectomy may be preferable. Rituximab may not be universally available, whereas splenectomy was thought to be feasible regardless of geography.
Conclusions and research needs for this question.
The guideline panel determined that there is low-certainty evidence for treatment with rituximab rather than splenectomy. Based on the available body of evidence, the risks associated with rituximab were thought to be less than with splenectomy, but not negligible. The panel also placed high value on avoiding splenectomy in the pediatric population, especially given that many children are likely to undergo spontaneous remission.
The panel identified the following research needs:
Assessment of impact of treatments on patient-reported outcomes such as fatigue, HRQoL, and bleeding;
Cost analysis of second-line therapies;
Determination of patient and parent preferences that influence treatment selection;
Biologic studies to predict treatment response and investigate the effect of agents on immunomodulation;
Randomized trials or observational trials to assess long-term outcomes;
Additional studies of novel second-line agents in children.
Other treatments for adults and children with ITP
There are many treatment options for ITP that were not formally evaluated by a systemic review and panel discussion of the EtD framework. The guidelines address prioritized questions that were posed by the panel for which assessment based on comparisons of outcomes could be conducted. The panel also prioritized the use of third-line agents for children and adults with ITP. However, given the large number of agents in the category, a lack of data, variable outcomes chosen, and no manner by which these options could be compared directly, the panel was unable to assess their use within the EtD framework. The panel did, however, recognize the importance of this question for ITP patients who have failed the treatment options reviewed herein or for whom cost or availability precludes their use. Therefore, a systematic review of published outcomes was conducted, and summaries of other treatment options are presented in alphabetical order with summary efficacy, safety, and cost data provided in supplemental File 6. It is important to note that the data are derived from studies with relatively small and heterogeneous groups of patients with regard to disease duration, severity, and prior treatments, which may differ in important ways from the use of these drugs in current practice. No recommendations are made with regard to these medications.
Azathioprine is an immunosuppressive drug that has been used since 1957 in the prevention of solid-organ transplant rejection and in the treatment of autoimmune disorders. It is rapidly converted from the prodrug to the active form, 6-mercaptopurine, which inhibits purine synthesis and subsequently DNA synthesis, especially in immune cells. It is usually administered at an oral dose of 50 to 200 mg per day in adults and is sometimes administered with danazol but with little data to support a higher response for the combination. It often takes several months for its full effect in ITP and durable response reported in 2 trials is 51.2% and 64.2%. It is important to note that approximately one-half of the patients with a durable response required ongoing therapy to maintain a response. Azathioprine is 1 of the few ITP medications deemed “safe” in pregnancy, with no increased rate of fetal malformation, and safe during lactation. Its major adverse effects are nausea, infection (9.9%), liver function abnormalities, neutropenia, and anemia. The active drug is degraded by the enzyme thiopurine methyltransferase; up to 0.25% of the population lacks this enzyme which may result in serious cytopenias. Patients displaying cytopenias should therefore be tested for thiopurine methyltransferase deficiency.
Cyclophosphamide is a chemotherapy drug related to nitrogen mustard that has been used since 1959 to treat malignant disease (at high doses) and as an immunosuppressive drug to treat autoimmune disorders (at lower doses). Cyclophosphamide is converted to its active metabolite, phosphoramide mustard, which then forms DNA cross-links leading to apoptosis of cells. However, phosphoramide mustard is rapidly inactivated by the enzyme aldehyde dehydrogenase. Because aldehyde dehydrogenase is present at high levels in many tissues (eg, bone marrow), cyclophosphamide is active only in tissues with low levels of this enzyme (eg, lymphocytes). The usual oral dose for adults is 50 to 200 mg per day and 1.5 to 3 mg/kg per day for children. Because it has a slow onset of effect there is no anticipated response at 7 days in ITP. Response at 1 month is highly variable with 2 studies reporting 10% and 70%. Durable response was ∼60% in the 2 studies that reported this outcome, with 22% of patients being off therapy in the study by Pizzuto and Ambriz.88,163-165 Its major adverse effects are bone marrow suppression, infection (9.9%), infertility, secondary malignancies, and hemorrhagic cystitis. Its use is contraindicated in pregnancy and in lactation. There have been no reported studies of this agent in ITP since 2005.
Cyclosporine A is a natural product (cyclic peptide of 11 amino acids) initially identified in the fungus Tolypocladium inflatum, which has been used since 1983 as a potent immunosuppressive agent. It reduces T-cell activity and is widely used as an immunosuppressant therapy in organ transplantation and autoimmune disorders. Cyclosporine A binds to the protein cyclophilin, and the complex inhibits calcineurin, a protein vital for T-cell activation. The dose varies and is adjusted with monitoring of trough drug levels; however, the usual starting dose is 3 to 6 mg/kg per day with a maximum of 200 mg for both adults and children. Response at 1 month ranges from 37.8% to 56.7%; durable response ranges from 23.3% to 44%. The lower response of 23.3% represents durable response for patients off therapy, whereas with the higher response, all patients were on therapy at the time of response assessment.166-170 Major adverse effects of cyclosporine A include gingival hyperplasia (6.6%), hypertension (11.6%), nephrotoxicity (6.7%), and nausea. It is not for use during pregnancy or lactation.
Danazol is a modified steroid molecule that has been used since 1971 for the treatment of endometriosis, angioedema, and ITP. Danazol binds to many steroid receptors, including the androgen and glucocorticoid receptors, with a modest effect on increasing androgenic and glucocorticoid effects, the latter resulting in immune inhibition. In addition, it has been shown to decrease monocyte-binding sites for Fc receptors. The usual oral dose is 200 to 800 mg per day for adults and 400 to 600 mg per day or 15 mg/kg per day in children. In clinical studies in ITP, danazol response ranges from 23.8% to 57.9% at 1 month. There is a large range of reported durable responses, ranging from 9.5% to a high as 96%. Much of this variability may be accounted for by differences in response criteria, timing of assessment, and whether patients were assessed on or off therapy. Of note, in 1 pediatric trial of 20 patients, no patients demonstrated a response.88,171-178 Its androgenic effects are its major adverse property (especially in women), with elevated liver function tests (16.5%), weight gain (8.4%), acne (4.2%), rash, and mood changes also being common. Amenorrhea (10.6%) and virilization (3%) have also been reported. Liver function tests should be done at least monthly. It is contraindicated in pregnancy and during lactation. It has sometimes been combined with azathioprine but with little evidence to support an added benefit of the combination.
Dapsone is an antibiotic that has been used to treat infections (eg, leprosy), skin conditions, and autoimmune diseases since 1937 (having been first synthesized in 1908). Like sulfonamides, it inhibits bacterial dihydrofolic acid and kills bacteria. Its inhibition of neutrophil myeloperoxidase may account for its anti-inflammatory action. It is a potent oxidant and causes methemoglobinemia and red blood cell hemolysis by overcoming the reductive capacity of glucose-6-phosphate dehydrogenase (G6PD); presumably, the hemolyzed red blood cells then saturate the phagocytic potential of macrophages, thereby sparing platelets from destruction. It is administered orally at a dose of 50 to 100 mg daily for both adults and children. Response in ITP is highly variable, a response of 36% to 63% has been reported at 1 month, with durable response ranging from 0% to 55%.179-183 Treatment is well tolerated, with nausea/vomiting occurring in 11% of patients. Mild hemolysis occurs in most patients, with significant hemolysis being less common. Patients with G6PD deficiency are particularly at risk, and all patients at risk for G6PD deficiency (eg, males of African, Italian, and/or Greek ancestry) should be screened before being treated. Patients should also be monitored for the potential development of methemoglobin; in 1 trial, 5.7% reported having developed methemoglobinuria.
Mycophenolate mofetil is a product of Penicillium fungi that was discovered in 1893 based upon its antibacterial properties but since 1995 has been used primarily as an immunosuppressant drug to prevent rejection in solid-organ transplants and to treat some autoimmune disorders (eg, systemic lupus erythematosus and ITP). Mycophenolate mofetil is converted to its active form, mycophenolic acid, which then inhibits inosine monophosphate dehydrogenase, an enzyme necessary for purine synthesis primarily in lymphocytes, and thereby inhibits DNA synthesis of T and B cells. It is administered orally at a dose of 500 to 2000 mg per day in adults and doses of 1300 mg/m2 per day (maximum of 2000 mg) in children. Its effect in ITP is relatively slow, with responses of ∼15% at 1 week but with a response by 1 month in roughly one-half of the treated patients, with durable response of 56.7% to 61.9%.184-187 Diarrhea is a common side effect (6.8%), more so than with azathioprine. Other significant side effects include neutropenia, anemia, and viral infections; with prolonged use, there is a small increased risk of malignancy and progressive multifocal leukoencephalopathy. It is also associated with pure red aplasia. It is a teratogen and should not be used during pregnancy or lactation.
The vinca alkaloids are derived from the rose periwinkle plant (Catharanthus roseus), whose medicinal properties have been known for centuries; its 2 best-defined members, vincristine and vinblastine, have been used since 1993, primarily in the treatment of leukemia and lymphoma. The vinca alkaloids avidly bind tubulin, prevent microtubule formation, and inhibit mitosis, thereby leading to apoptosis. Vinca alkaloids affect all dividing cells but have found a role in treatment of several lymphoid malignancies. Their effect in immune disorders may also be mediated by lymphocyte inhibition, and their primary mechanism in ITP is probably their ability to inhibit macrophage function and thus reduce platelet phagocytosis.188 Weekly IV doses of vincristine (1-2 mg per dose for 2-4 weeks in adults and 1.5 mg/m2 per dose or 1 mg per dose) weekly in children) or vinblastine (10 mg per dose for 1-3 weeks in adults and 6 mg/m2 per day in children) can be associated with rapid responses at 7 days. Reported response at 1 month is highly variable but can be as low as 18%; durable response ranges from 0% to 42%.164,188-198 Unfortunately, these drugs are accompanied by significant toxicity. Almost all patients experience side effects, with vincristine neuropathy (27.8%), vinblastine-associated bone marrow suppression, constipation (3.5%), hyponatremia, and infusion site vesication (10.5%) being most common; these effects worsen with repeated dosing. It has been reported that these adverse effects are reduced when vinca alkaloids are given by a prolonged infusion188,191 or loaded into platelets.199 Platelets contain abundant tubulin and bind large amounts of vinca alkaloid after ex vivo incubation; infusion of vinca-loaded platelets into ITP patients targets macrophages and reduces platelet phagocytosis.197 Vinca alkaloids are contraindicated during pregnancy and lactation.
What others are saying and what is new in these guidelines?
In 2018, recommendations from a joint working group (JWG) of several European hematology societies (Germany, Austria, and Switzerland) were published.200 Similarly to these guidelines, the JWG recommend corticosteroids for adults with ITP who have no or mild bleeding (World Health Organization [WHO] 0-II) and platelet counts below 20 × 109/L to 30 × 109/L, and observation for patients with platelet count values above 20 × 109/L to 30 × 109/L. Although no specific recommendation was made with regards to corticosteroid duration, prolonged corticosteroid use is discouraged and there was no preference stated for the type of corticosteroid. The JWG guidelines did not discuss the addition of rituximab to corticosteroid treatment in newly diagnosed adults.
A primary difference is with regard to second-line management. The JWG guidelines place a priority on TPO-RAs as second-line treatment with rituximab being considered a third-line agent reserved for patients who have failed a TPO-RA. Additionally, splenectomy is not directly compared with either treatment but rather is primarily reserved for patients with ITP for >12 months’ durations or major bleeding (WHO III, IV). The ASH guidelines herein also include a remark that splenectomy should be delayed until 12 months when possible.
The JWG guidelines are more limited in their discussion about management of children. The JWG guidelines recommend that a low platelet count alone is not sufficient to start treatment in children with newly diagnosed ITP, and that the majority of children with no or only mild bleeding do not require treatment. The JWG acknowledge that there is no standard treatment of chronic ITP and referral to a specialist center is recommended. They do suggest that splenectomy be avoided in children and reserved as a last option in therapy.
Limitations of these guidelines
The limitations of these guidelines are inherent in the low or very low certainty in the evidence identified for many of the questions. The contribution of indirect evidence is specified for each recommendation. For some recommendations, which related to relatively common clinical questions, there was very little or no published direct or relevant indirect evidence, and the guideline panel was surveyed to provide unpublished collective data on which decisions could then be based. However, interpretation of the survey data was limited by recall bias, determination of individual provider practice compared with center-wide practice, and the fact that hematologists are generally consulted only after the decision for admission has been made by another provider. This process is explicitly identified for relevant recommendations. Additionally, these guidelines focus on the clinical and management aspects of ITP and do not address the pathophysiologic aspects of the disease. These guidelines are also limited by not being inclusive of all possible clinical scenarios. The panel prioritized questions for which there is clinical uncertainty or where it was felt there might be new information to guide decision-making. Identification of the specific population then informed the literature search. For these reasons, these guidelines do not address the diagnosis of ITP, management of patients with severe or life-threatening bleeding, ITP in pregnancy, and treatments available after 2017. Some of these clinical scenarios were addressed in the 2011 guidelines and have been carried forward. Furthermore, the guidelines relied on dichotomous comparisons that may not reflect clinical practice in which >2 treatment options may be considered at a given time.
Revision or adaptation of the guidelines
Plans for updating these guidelines
After publication of these guidelines, ASH will maintain them through surveillance for new evidence, ongoing review by experts, and regular revisions.
Updating or adapting recommendations locally
Adaptation of these guidelines will be necessary in many circumstances. These adaptations should be based on the associated EtD frameworks.201
Priorities for research
Specific suggestions for research are detailed with each recommendation. Overall, the panel was able to make a strong recommendation for approximately one-fourth (5/21) of the prioritized questions. However, the panel noted that, although the need for RCTs in ITP is not debated, the conduct of these trials is challenging secondary to the significant difference among some of the treatment options with regard to administration, duration of therapy, and even surgical considerations. The panel recommends that collaborative cohort studies (retrospective and prospective), registries, and other observational studies addressing these issues could contribute much to improve the current levels of evidence and are likely more feasible than RCTs. These studies should apply standard dosing regimens and definitions, consistently report on patient-reported outcomes including HRQoL and side effects, and report long-term follow-up data. This would allow for greater comparison of approaches in the absence of randomized trials. The panel also recommends ongoing collaborative engagement of patients to best understand how to apply these guidelines within the context of shared decision-making. Lastly, the panel recognizes that many of the agents covered in these recommendations are unavailable in certain countries, therefore global cost-effective strategies should also be assessed.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge members of the systematic review team (Evaren Page, Jessica Reese, and Lauren Quick) who contributed evidence summaries to the guidelines. The authors acknowledge Kendall Alexander for support of the guideline panel, management of conflicts of interest, and assistance with the written guideline template.
D.R.T. was supported by a career development award from the National Institutes of Health, National Heart, Lung, and Blood Institute, award number 1K01HL135466.
The National Heart, Lung, and Blood Institute did not have any involvement in the study design, data collection, analysis, interpretation of the data, writing of the report, or the decision to submit the report for publication.
Authorship
Contribution: C.N., D.R.T., and S.K.V. wrote the first draft of this manuscript and revised the manuscript based on authors’ suggestions; guideline panel members (D.M.A., G.B., D.B.C., N.C., A.C., J.M.D., J.N.G., R.F.G., T.K., D.J.K., W.L., K.R.M., B.P., and H.S.) critically reviewed the manuscript and provided suggestions for improvement; all authors approved of the content; and C.N. and S.K.V. chaired the panel and led the panel meetings.
Conflict-of-interest disclosure: All authors were members of the guideline panel or members of the systematic review team or both. As such, they completed disclosure-of-interest forms, which were reviewed by ASH and are available as supplemental Files 2 and 3.
Correspondence: Cindy Neunert, Department of Pediatrics, Columbia University Medical Center, 3959 Broadway, CHN 10-03, New York, NY 10032; e-mail: cn2401@cumc.columbia.edu.
References
- 1.Schünemann HJ, Wiercioch W, Etxeandia I, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186(3):E123-E142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, eds. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156(7):525-531. [DOI] [PubMed] [Google Scholar]
- 4.Schünemann HJ, Al-Ansary LA, Forland F, et al. ; Board of Trustees of the Guidelines International Network. Guidelines International Network: principles for disclosure of interests and management of conflicts in guidelines. Ann Intern Med. 2015;163(7):548-553. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016;353:i2089. [DOI] [PubMed] [Google Scholar]
- 6.Atkins D, Eccles M, Flottorp S, et al. ; GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res. 2004;4(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schunemann HJ, Best D, Vist G, Oxman AD; GRADE Working Group. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. CMAJ. 2003;169(7):677-680. [PMC free article] [PubMed] [Google Scholar]
- 10.Schünemann HJ, Mustafa R, Brozek J, et al. ; GRADE Working Group. GRADE guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76:89-98. [DOI] [PubMed] [Google Scholar]
- 11.Abrahamson PE, Hall SA, Feudjo-Tepie M, Mitrani-Gold FS, Logie J. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 2009;83(2):83-89. [DOI] [PubMed] [Google Scholar]
- 12.Schoonen WM, Kucera G, Coalson J, et al. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 2009;145(2):235-244. [DOI] [PubMed] [Google Scholar]
- 13.Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 2006;4(11):2377-2383. [DOI] [PubMed] [Google Scholar]
- 14.Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 2010;85(3):174-180. [DOI] [PubMed] [Google Scholar]
- 15.Yong M, Schoonen WM, Li L, et al. Epidemiology of paediatric immune thrombocytopenia in the General Practice Research Database. Br J Haematol. 2010;149(6):855-864. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt GH, Alonso-Coello P, Schünemann HJ, et al. Guideline panels should seldom make good practice statements: guidance from the GRADE Working Group. J Clin Epidemiol. 2016;80:3-7. [DOI] [PubMed] [Google Scholar]
- 17.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett CM, Neunert C, Grace RF, et al. Predictors of remission in children with newly diagnosed immune thrombocytopenia: data from the Intercontinental Cooperative ITP Study Group Registry II participants. Pediatr Blood Cancer. 2018;65(1). [DOI] [PubMed] [Google Scholar]
- 19.Imbach P, Kühne T, Müller D, et al. Childhood ITP: 12 months follow-up data from the prospective registry I of the Intercontinental Childhood ITP Study Group (ICIS). Pediatr Blood Cancer. 2006;46(3):351-356. [DOI] [PubMed] [Google Scholar]
- 20.Sailer T, Lechner K, Panzer S, Kyrle PA, Pabinger I. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica. 2006;91(8):1041-1045. [PubMed] [Google Scholar]
- 21.George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3-40. [PubMed] [Google Scholar]
- 22.Stasi R, Stipa E, Masi M, et al. Long-term observation of 208 adults with chronic idiopathic thrombocytopenic purpura. Am J Med. 1995;98(5):436-442. [DOI] [PubMed] [Google Scholar]
- 23.Schifferli A, Holbro A, Chitlur M, et al. ; Intercontinental Cooperative ITP Study Group (ICIS). A comparative prospective observational study of children and adults with immune thrombocytopenia: 2-year follow-up. Am J Hematol. 2018;93(6):751-759. [DOI] [PubMed] [Google Scholar]
- 24.Neunert CE, Buchanan GR, Blanchette V, et al. Relationships among bleeding severity, health-related quality of life, and platelet count in children with immune thrombocytopenic purpura. Pediatr Blood Cancer. 2009;53(4):652-654. [DOI] [PubMed] [Google Scholar]
- 25.Neunert C, Noroozi N, Norman G, et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 2015;13(3):457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neunert CE, Buchanan GR, Imbach P, et al. ; Intercontinental Childhood ITP Study Group Registry II Participants. Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 2008;112(10):4003-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood. 2009;114(23):4777-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frederiksen H, Maegbaek ML, Nørgaard M. Twenty-year mortality of adult patients with primary immune thrombocytopenia: a Danish population-based cohort study. Br J Haematol. 2014;166(2):260-267. [DOI] [PubMed] [Google Scholar]
- 29.Kuter DJ, Mathias SD, Rummel M, et al. Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 2012;87(5):558-561. [DOI] [PubMed] [Google Scholar]
- 30.Snyder CF, Mathias SD, Cella D, Isitt JJ, Wu AW, Young J. Health-related quality of life of immune thrombocytopenic purpura patients: results from a web-based survey. Curr Med Res Opin. 2008;24(10):2767-2776. [DOI] [PubMed] [Google Scholar]
- 31.Newton JL, Reese JA, Watson SI, et al. Fatigue in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2011;86(5):420-429. [DOI] [PubMed] [Google Scholar]
- 32.Hill QA, Newland AC. Fatigue in immune thrombocytopenia. Br J Haematol. 2015;170(2):141-149. [DOI] [PubMed] [Google Scholar]
- 33.Blatt J, Weston B, Gold S. Fatigue as marker of thrombocytopenia in childhood idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 2010;27(1):65-67. [DOI] [PubMed] [Google Scholar]
- 34.Trotter P, Hill QA. Immune thrombocytopenia: improving quality of life and patient outcomes. Patient Relat Outcome Meas. 2018;9:369-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kühne T, Buchanan GR, Zimmerman S, et al. ; Intercontinental Childhood ITP Study Group. A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 2003;143(5):605-608. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Molony JT, Cetin K, Wasser JS, Altomare I. Rate of bleeding-related episodes in elderly patients with primary immune thrombocytopenia: a retrospective cohort study. Curr Med Res Opin. 2018;34(2):209-216. [DOI] [PubMed] [Google Scholar]
- 37.Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):620-632. [DOI] [PubMed] [Google Scholar]
- 38.Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice. Conflict of Interest in Medical Research, Education, and Practice. Lo B, Fields MJ, eds. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 39.Akl EA, El-Hachem P, Abou-Haidar H, Neumann I, Schünemann HJ, Guyatt GH. Considering intellectual, in addition to financial, conflicts of interest proved important in a clinical practice guideline: a descriptive study. J Clin Epidemiol. 2014;67(11):1222-1228. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt G, Akl EA, Hirsh J, et al. The vexing problem of guidelines and conflict of interest: a potential solution. Ann Intern Med. 2010;152(11):738-741. [DOI] [PubMed] [Google Scholar]
- 41.Schünemann HJ, Osborne M, Moss J, et al. ; ATS Ethics and Conflict of Interest Committee and the Documents Development and Implementation Committee. An official American Thoracic Society Policy statement: managing conflict of interest in professional societies. Am J Respir Crit Care Med. 2009;180(6):564-580. [DOI] [PubMed] [Google Scholar]
- 42.Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190-4207. [DOI] [PubMed] [Google Scholar]
- 43.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395-400. [DOI] [PubMed] [Google Scholar]
- 44.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. [DOI] [PubMed] [Google Scholar]
- 45.Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J Am Acad Dermatol. 2017;76(1):1-9. [DOI] [PubMed] [Google Scholar]
- 46.Din B, Wang X, Shi Y, Li Y. Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 2015;133(1):124-128. [DOI] [PubMed] [Google Scholar]
- 47.Praituan W, Rojnuckarin P. Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 2009;7(6):1036-1038. [DOI] [PubMed] [Google Scholar]
- 48.Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E. Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 2012;20(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs P, Wood L, Novitzky N. Intravenous gammaglobulin has no advantages over oral corticosteroids as primary therapy for adults with immune thrombocytopenia: a prospective randomized clinical trial. Am J Med. 1994;97(1):55-59. [DOI] [PubMed] [Google Scholar]
- 50.Wei Y, Ji XB, Wang YW, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296-302, quiz 370. [DOI] [PubMed] [Google Scholar]
- 51.DiFino SM, Lachant NA, Kirshner JJ, Gottlieb AJ. Adult idiopathic thrombocytopenic purpura. Clinical findings and response to therapy. Am J Med. 1980;69(3):430-442. [DOI] [PubMed] [Google Scholar]
- 52.Houwerzijl EJ, Louwes H, Sluiter WJ, Smit JW, Vellenga E, de Wolf JT. Platelet production rate predicts the response to prednisone therapy in patients with idiopathic thrombocytopenic purpura. Ann Hematol. 2008;87(12):975-983. [DOI] [PubMed] [Google Scholar]
- 53.Matschke J, Müller-Beissenhirtz H, Novotny J, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 Study. Acta Haematol. 2016;136(2):101-107. [DOI] [PubMed] [Google Scholar]
- 54.Mazzucconi MG, Francesconi M, Fidani P, et al. Treatment of idiopathic thrombocytopenic purpura (ITP): results of a multicentric protocol. Haematologica. 1985;70(4):329-336. [PubMed] [Google Scholar]
- 55.Centurioni R, Braianzoni F, Olivieri A, et al. Treatment of autoimmune thrombocytopenic purpura. Acta Haematol Pol. 1990;21(2):139-143. [PubMed] [Google Scholar]
- 56.Zimmer J, Andrès E, Noel E, Koumarianou A, Blicklé JF, Maloisel F. Current management of adult idiopathic thrombocytopenic purpura in practice: a cohort study of 201 patients from a single center. Clin Lab Haematol. 2004;26(2):137-142. [DOI] [PubMed] [Google Scholar]
- 57.Bizzoni L, Mazzucconi MG, Gentile M, et al. Idiopathic thrombocytopenic purpura (ITP) in the elderly: clinical course in 178 patients. Eur J Haematol. 2006;76(3):210-216. [DOI] [PubMed] [Google Scholar]
- 58.Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457-465. [DOI] [PubMed] [Google Scholar]
- 59.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711-1723. [DOI] [PubMed] [Google Scholar]
- 60.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96(1):23-43. [DOI] [PubMed] [Google Scholar]
- 61.Mithoowani S, Gregory-Miller K, Goy J, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 2016;3(10):e489-e496. [DOI] [PubMed] [Google Scholar]
- 62.Bae SH, Ryoo H-M, Lee WS, et al. High dose dexamethasone vs. conventional dose prednisolone for adults with immune thrombocytopenia: a prospective multicenter phase III trial [abstract]. Blood. 2010;116(21). Abstract 3687. [Google Scholar]
- 63.Li Z, Mou W, Lu G, et al. Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J .Hematol. 2011;93(1):91-98. [DOI] [PubMed] [Google Scholar]
- 64.Zaja F, Baccarani M, Mazza P, et al. Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 2010;115(14):2755-2762. [DOI] [PubMed] [Google Scholar]
- 65.Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 2013;121(11):1976-1981. [DOI] [PubMed] [Google Scholar]
- 66.Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356-1362. [DOI] [PubMed] [Google Scholar]
- 67.Ghanima W, Khelif A, Waage A, et al. ; RITP Study Group. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9978):1653-1661. [DOI] [PubMed] [Google Scholar]
- 68.Cooper K, Matcham J, Helme K, Akehurst R. Update on romiplostim and eltrombopag indirect comparison. Int J Technol Assess Health Care. 2014;30(1):129-130. [DOI] [PubMed] [Google Scholar]
- 69.Cooper KL, Fitzgerald P, Dillingham K, Helme K, Akehurst R. Romiplostim and eltrombopag for immune thrombocytopenia: methods for indirect comparison. Int J Technol Assess Health Care. 2012;28(3):249-258. [DOI] [PubMed] [Google Scholar]
- 70.Wang L, Gao Z, Chen XP, et al. Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: a systematic review and meta-analysis. Sci Rep. 2016;6:39003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen R, Bryden P, Grotzinger KM, Stapelkamp C, Woods B. Cost-effectiveness of eltrombopag versus romiplostim for the treatment of chronic immune thrombocytopenia in England and Wales. Value Health. 2016;19(5):614-622. [DOI] [PubMed] [Google Scholar]
- 72.Tremblay G, Dolph M, Bhor M, Said Q, Elliott B, Briggs A. Cost-consequence model comparing eltrombopag versus romiplostim for adult patients with chronic immune thrombocytopenia. Clinicoecon Outcomes Res. 2018;10:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395-403. [DOI] [PubMed] [Google Scholar]
- 74.Cheng G, Saleh MN, Marcher C, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study [published correction appears in Lancet. 2011;377(9763):382]. Lancet. 2011;377(9763):393-402. [DOI] [PubMed] [Google Scholar]
- 75.Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bussel JB, Kuter DJ, Aledort LM, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. Blood. 2014;123(25):3887-3894. [DOI] [PubMed] [Google Scholar]
- 77.Moulis G, Sailler L, Sommet A, Lapeyre-Mestre M, Derumeaux H, Adoue D. Rituximab versus splenectomy in persistent or chronic adult primary immune thrombocytopenia: an adjusted comparison of mortality and morbidity. Am J Hematol. 2014;89(1):41-46. [DOI] [PubMed] [Google Scholar]
- 78.Chater C, Terriou L, Duhamel A, et al. Reemergence of splenectomy for ITP second-line treatment? Ann Surg. 2016;264(5):772-777. [DOI] [PubMed] [Google Scholar]
- 79.Kojouri K, Vesely SK, Terrell DR, George JN. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104(9):2623-2634. [DOI] [PubMed] [Google Scholar]
- 80.Wang T, Xu M, Ji L, Han ZC, Yang R. Splenectomy for adult chronic idiopathic thrombocytopenic purpura: experience from a single center in China. Eur J Haematol. 2005;75(5):424-429. [DOI] [PubMed] [Google Scholar]
- 81.Vianelli N, Galli M, de Vivo A, et al. ; Gruppo Italiano per lo Studio delle Malattie Ematologiche dell’Adulto. Efficacy and safety of splenectomy in immune thrombocytopenic purpura: long-term results of 402 cases. Haematologica. 2005;90(1):72-77. [PubMed] [Google Scholar]
- 82.Sampath S, Meneghetti AT, MacFarlane JK, Nguyen NH, Benny WB, Panton ON. An 18-year review of open and laparoscopic splenectomy for idiopathic thrombocytopenic purpura. Am J Surg. 2007;193(5):580-583, discussion 583-584. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez-Porras JR, Escalante F, Pardal E, et al. ; Grupo de Trombosis y Hemostasia de Castilla y León. Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 2013;91(3):236-241. [DOI] [PubMed] [Google Scholar]
- 84.Ahmed R, Devasia AJ, Viswabandya A, et al. Long-term outcome following splenectomy for chronic and persistent immune thrombocytopenia (ITP) in adults and children: splenectomy in ITP. Ann Hematol. 2016;95(9):1429-1434. [DOI] [PubMed] [Google Scholar]
- 85.Zheng CX, Zheng D, Chen LH, Yu JF, Wu ZM. Laparoscopic splenectomy for immune thrombocytopenic purpura at a teaching institution. Chin Med J (Engl). 2011;124(8):1175-1180. [PubMed] [Google Scholar]
- 86.Guan Y, Wang S, Xue F, et al. Long-term results of splenectomy in adult chronic immune thrombocytopenia. Eur J Haematol. 2017;98(3):235-241. [DOI] [PubMed] [Google Scholar]
- 87.Park YH, Yi HG, Kim CS, et al. ; Gyeonggi/Incheon Branch, The Korean Society of Hematology. Clinical outcome and predictive factors in the response to splenectomy in elderly patients with primary immune thrombocytopenia: a multicenter retrospective study. Acta Haematol. 2016;135(3):162-171. [DOI] [PubMed] [Google Scholar]
- 88.Li HQ, Zhang L, Zhao H, Ji LX, Yang RC. Chronic idiopathic thrombocytopenic purpura in adult Chinese patients: a retrospective single-centered analysis of 1791 cases. Chin Med J (Engl). 2005;118(1):34-37. [PubMed] [Google Scholar]
- 89.Montalvo J, Velazquez D, Pantoja JP, Sierra M, López-Karpovitch X, Herrera MF. Laparoscopic splenectomy for primary immune thrombocytopenia: clinical outcome and prognostic factors. J Laparoendosc Adv Surg Tech A. 2014;24(7):466-470. [DOI] [PubMed] [Google Scholar]
- 90.Balagué C, Vela S, Targarona EM, et al. Predictive factors for successful laparoscopic splenectomy in immune thrombocytopenic purpura: study of clinical and laboratory data. Surg Endosc. 2006;20(8):1208-1213. [DOI] [PubMed] [Google Scholar]
- 91.Wang T, Zhao H, Ren H, et al. Type 1 and type 2 T-cell profiles in idiopathic thrombocytopenic purpura. Haematologica. 2005;90(7):914-923. [PubMed] [Google Scholar]
- 92.Zheng D, Huang CS, Huang SB, Zheng CX. Laparoscopic splenectomy for primary immune thrombocytopenia: current status and challenges. World J Gastrointest Endosc. 2016;8(17):610-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672-1681. [DOI] [PubMed] [Google Scholar]
- 94.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237-2247. [DOI] [PubMed] [Google Scholar]
- 95.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641-648. [DOI] [PubMed] [Google Scholar]
- 96.Shirasugi Y, Ando K, Miyazaki K, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized phase III clinical trial. Int J Hematol. 2011;94(1):71-80. [DOI] [PubMed] [Google Scholar]
- 97.Tomiyama Y, Miyakawa Y, Okamoto S, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 2012;10(5):799-806. [DOI] [PubMed] [Google Scholar]
- 98.Yang R, Li J, Jin J, et al. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br J Haematol. 2017;176(1):101-110. [DOI] [PubMed] [Google Scholar]
- 99.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889-1899. [DOI] [PubMed] [Google Scholar]
- 100.Zhou H, Xu M, Qin P, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Godeau B, Porcher R, Fain O, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter phase 2 study. Blood. 2008;112(4):999-1004. [DOI] [PubMed] [Google Scholar]
- 102.Tran H, Brighton T, Grigg A, et al. A multi-centre, single-arm, open-label study evaluating the safety and efficacy of fixed dose rituximab in patients with refractory, relapsed or chronic idiopathic thrombocytopenic purpura (R-ITP1000 study). Br J Haematol. 2014;167(2):243-251. [DOI] [PubMed] [Google Scholar]
- 103.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232-239. [DOI] [PubMed] [Google Scholar]
- 104.Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood. 2014;124(22):3228-3236. [DOI] [PubMed] [Google Scholar]
- 105.Newland A, Godeau B, Priego V, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172(2):262-273. [DOI] [PubMed] [Google Scholar]
- 106.Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bussel JB, Arnold DM, Boxer MA, et al. Long-term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blanchette VS, Luke B, Andrew M, et al. A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenic purpura. J Pediatr. 1993;123(6):989-995. [DOI] [PubMed] [Google Scholar]
- 109.Fujisawa K, Iyori H, Ohkawa H, et al. ; Japanese Study Group on Childhood ITP. A prospective, randomized trial of conventional, dose-accelerated corticosteroids and intravenous immunoglobulin in children with newly diagnosed idiopathic thrombocytopenic purpura. Int J Hematol. 2000;72(3):376-383. [PubMed] [Google Scholar]
- 110.Sartorius JA. Steroid treatment of idiopathic thrombocytopenic purpura in children. Preliminary results of a randomized cooperative study. Am J Pediatr Hematol Oncol. 1984;6(2):165-169. [DOI] [PubMed] [Google Scholar]
- 111.Buchanan GR, Holtkamp CA. Prednisone therapy for children with newly diagnosed idiopathic thrombocytopenic purpura. A randomized clinical trial. Am J Pediatr Hematol Oncol. 1984;6(4):355-361. [DOI] [PubMed] [Google Scholar]
- 112.Imbach P. A multicenter European trial of intravenous immune globulin in immune thrombocytopenic purpura in childhood. Vox Sang. 1985;49(suppl 1):25-31. [DOI] [PubMed] [Google Scholar]
- 113.Blanchette V, Imbach P, Andrew M, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 1994;344(8924):703-707. [DOI] [PubMed] [Google Scholar]
- 114.Ozsoylu S, Irken G, Karabent A. High-dose intravenous methylprednisolone for acute childhood idiopathic thrombocytopenic purpura. Eur J Haematol. 1989;42(5):431-435. [DOI] [PubMed] [Google Scholar]
- 115.Ou CY, Hsieh KS, Chiou YH, Chang YH, Ger LP. A comparative study of initial use of intravenous immunoglobulin and prednisolone treatments in childhood idiopathic thrombocytopenic purpur. Acta Paediatr Taiwan. 2006;47(5):226-231. [PubMed] [Google Scholar]
- 116.Mori PG, Lanza T, Mancuso G, et al. Treatment of acute idiopathic thrombocytopenic purpura (AITP): cooperative Italian study group results. Pediatr Hematol Oncol. 1988;5(3):169-178. [DOI] [PubMed] [Google Scholar]
- 117.Dickerhoff R, von Ruecker A. The clinical course of immune thrombocytopenic purpura in children who did not receive intravenous immunoglobulins or sustained prednisone treatment. J Pediatr. 2000;137(5):629-632. [DOI] [PubMed] [Google Scholar]
- 118.Duru F, Fisgin T, Yarali N, Kara A. Clinical course of children with immune thrombocytopenic purpura treated with intravenous immunoglobulin G or megadose methylprednisolone or observed without therapy. Pediatr Hematol Oncol. 2002;19(4):219-225. [DOI] [PubMed] [Google Scholar]
- 119.Baronci C, Petrone A, Miano C, et al. Treatment of acute idiopathic thrombocytopenic purpura in children. A retrospective evaluation of 120 cases. Ann Ist Super Sanita. 1998;34(4):457-461. [PubMed] [Google Scholar]
- 120.Kumar M, Vik TA, Johnson CS, Southwood ME, Croop JM. Treatment, outcome, and cost of care in children with idiopathic thrombocytopenic purpura. Am J Hematol. 2005;78(3):181-187. [DOI] [PubMed] [Google Scholar]
- 121.Evim MS, Baytan B, Gunes AM. Childhood immune thrombocytopenia: long-term follow-up data evaluated by the criteria of the international working group on immune thrombocytopenic purpura. Turk J Haematol. 2014;31(1):32-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yildiz I, Ozdemir N, Celkan T, et al. Initial management of childhood acute immune thrombocytopenia: single-center experience of 32 years. Pediatr Hematol Oncol. 2015;32(6):406-414. [DOI] [PubMed] [Google Scholar]
- 123.Imbach P, Wagner HP, Berchtold W, et al. Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet. 1985;2(8453):464-468. [DOI] [PubMed] [Google Scholar]
- 124.Ozsoylu S, Sayli TR, Oztürk G. Oral megadose methylprednisolone versus intravenous immunoglobulin for acute childhood idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 1993;10(4):317-321. [DOI] [PubMed] [Google Scholar]
- 125.Rosthøj S, Nielsen S, Pedersen FK; Danish I.T.P. Study Group. Randomized trial comparing intravenous immunoglobulin with methylprednisolone pulse therapy in acute idiopathic thrombocytopenic purpura. Acta Paediatr. 1996;85(8):910-915. [DOI] [PubMed] [Google Scholar]
- 126.Erduran E, Aslan Y, Gedik Y, Orhan F. A randomized and comparative study of intravenous immunoglobulin and mega dose methylprednisolone treatments in children with acute idiopathic thrombocytopenic purpura. Turk J Pediatr. 2003;45(4):295-300. [PubMed] [Google Scholar]
- 127.Tarantino MD, Young G, Bertolone SJ, et al. ; Acute ITP Study Group. Single dose of anti-D immune globulin at 75 microg/kg is as effective as intravenous immune globulin at rapidly raising the platelet count in newly diagnosed immune thrombocytopenic purpura in children. J Pediatr. 2006;148(4):489-494. [DOI] [PubMed] [Google Scholar]
- 128.Papagianni A, Economou M, Tragiannidis A, et al. Standard-dose intravenous anti-D immunoglobulin versus intravenous immunoglobulin in the treatment of newly diagnosed childhood primary immune thrombocytopenia. J Pediatr Hematol Oncol. 2011;33(4):265-269. [DOI] [PubMed] [Google Scholar]
- 129.Celik M, Bulbul A, Aydogan G, et al. Comparison of anti-D immunoglobulin, methylprednisolone, or intravenous immunoglobulin therapy in newly diagnosed pediatric immune thrombocytopenic purpura. J Thromb Thrombolysis. 2013;35(2):228-233. [DOI] [PubMed] [Google Scholar]
- 130.Benesch M, Kerbl R, Lackner H, et al. Low-dose versus high-dose immunoglobulin for primary treatment of acute immune thrombocytopenic purpura in children: results of a prospective, randomized single-center trial. J Pediatr Hematol Oncol. 2003;25(10):797-800. [DOI] [PubMed] [Google Scholar]
- 131.Heitink-Pollé KMJ, Uiterwaal CSPM, Porcelijn L, et al. ; TIKI Investigators. Intravenous immunoglobulin vs observation in childhood immune thrombocytopenia: a randomized controlled trial. Blood. 2018;132(9):883-891. [DOI] [PubMed] [Google Scholar]
- 132.Shahgholi E, Vosough P, Sotoudeh K, et al. Intravenous immune globulin versus intravenous anti-D immune globulin for the treatment of acute immune thrombocytopenic purpura. Indian J Pediatr. 2008;75(12):1231-1235. [DOI] [PubMed] [Google Scholar]
- 133.Farahmandinia Z, Naderi A, Sabzevari F, Parvaresh S. Comparison of intravenous immunoglobulin (IVIG) and intravenous anti-D for treatment of acute idiopathic thrombocytopenic purpura. Int J Hematol Oncol Stem Cell Res. 2010;4(4):10-13. [Google Scholar]
- 134.Son DW, Jeon IS, Yang SW, Cho SH. A single dose of anti-D immunoglobulin raises platelet count as efficiently as intravenous immunoglobulin in newly diagnosed immune thrombocytopenic purpura in Korean children. J Pediatr Hematol Oncol. 2008;30(8):598-601. [DOI] [PubMed] [Google Scholar]
- 135.Swain TR, Jena RK, Swain KP. High dose intravenous anti-D immune globulin is more effective and safe in Indian paediatric patients of immune thrombocytopenic purpura. J Clin Diagn Res. 2016;10(12):FC12-FC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moser AM, Shalev H, Kapelushnik J. Anti-D exerts a very early response in childhood acute idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 2002;19(6):407-411. [DOI] [PubMed] [Google Scholar]
- 137.Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118(1):28-36. [DOI] [PubMed] [Google Scholar]
- 138.Grainger JD, Locatelli F, Chotsampancharoen T, et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial [published correction appears in Lancet. 2015;386(10004):1630]. Lancet. 2015;386(10004):1649-1658. [DOI] [PubMed] [Google Scholar]
- 139.Elalfy MS, Abdelmaksoud AA, Eltonbary KY. Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 2011;90(11):1341-1344. [DOI] [PubMed] [Google Scholar]
- 140.Bussel JB, de Miguel PG, Despotovic JM, et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): a randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015;2(8):e315-e325. [DOI] [PubMed] [Google Scholar]
- 141.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomized, double-blind, placebo-controlled study. Lancet. 2016;388(10039):45-54. [DOI] [PubMed] [Google Scholar]
- 142.Liang Y, Zhang L, Gao J, Hu D, Ai Y. Rituximab for children with immune thrombocytopenia: a systematic review. PLoS One. 2012;7(5):e36698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dai WJ, Zhang RR, Yang XC, Yuan YF. Efficacy of standard dose rituximab for refractory idiopathic thrombocytopenic purpura in children. Eur Rev Med Pharmacol Sci. 2015;19(13):2379-2383. [PubMed] [Google Scholar]
- 144.Bennett CM, Rogers ZR, Kinnamon DD, et al. Prospective phase 1/2 study of rituximab in childhood and adolescent chronic immune thrombocytopenic purpura. Blood. 2006;107(7):2639-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grace RF, Bennett CM, Ritchey AK, et al. Response to steroids predicts response to rituximab in pediatric chronic immune thrombocytopenia. Pediatr Blood Cancer. 2012;58(2):221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tarantino MD, Bussel JB, Blanchette VS, et al. Romiplostim in children with immune thrombocytopenia: a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388(10039):45-54. [DOI] [PubMed] [Google Scholar]
- 147.Kühne T, Blanchette V, Buchanan GR, et al. ; Intercontinental Childhood ITP Study Group. Splenectomy in children with idiopathic thrombocytopenic purpura: a prospective study of 134 children from the Intercontinental Childhood ITP Study Group. Pediatr Blood Cancer. 2007;49(6):829-834. [DOI] [PubMed] [Google Scholar]
- 148.Holt D, Brown J, Terrill K, et al. Response to intravenous immunoglobulin predicts splenectomy response in children with immune thrombocytopenic purpura [published correction appears in Pediatrics. 2004;113(1):184]. Pediatrics. 2003;111(1):87-90. [PubMed] [Google Scholar]
- 149.den Ottolander GJ, Gratama JW, de Koning J, Brand A. Long-term follow-up study of 168 patients with immune thrombocytopenia. Implications for therapy. Scand J Haematol. 1984;32(1):101-110. [DOI] [PubMed] [Google Scholar]
- 150.El-Alfy MS, El-Tawil MM, Shahein N. 5- to 16-year follow-up following splenectomy in chronic immune thrombocytopenic purpura in children. Acta Haematol. 2003;110(1):20-24. [DOI] [PubMed] [Google Scholar]
- 151.Wang T, Xu M, Ji L, Yang R. Splenectomy for chronic idiopathic thrombocytopenic purpura in children: a single center study in China. Acta Haematol. 2006;115(1-2):39-45. [DOI] [PubMed] [Google Scholar]
- 152.Aronis S, Platokouki H, Avgeri M, Pergantou H, Keramidas D. Retrospective evaluation of long-term efficacy and safety of splenectomy in chronic idiopathic thrombocytopenic purpura in children. Acta Paediatr. 2004;93(5):638-642. [PubMed] [Google Scholar]
- 153.Pawelski S, Konopka L, Zdziechowska H. Recurrence of thrombocytopenia in patients splenectomized for idiopathic thrombocytopenic purpura. Blut. 1981;43(6):355-360. [DOI] [PubMed] [Google Scholar]
- 154.Davis PW, Williams DA, Shamberger RC. Immune thrombocytopenia: surgical therapy and predictors of response. J Pediatr Surg. 1991;26(4):407-412, discussion 412-413. [DOI] [PubMed] [Google Scholar]
- 155.Walker RW, Walker W. Idiopathic thrombocytopenia, initial illness and long term follow up. Arch Dis Child. 1984;59(4):316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mantadakis E, Buchanan GR. Elective splenectomy in children with idiopathic thrombocytopenic purpura. J Pediatr Hematol Oncol. 2000;22(2):148-153. [DOI] [PubMed] [Google Scholar]
- 157.Ben-Yehuda D, Gillis S, Eldor A; Israeli ITP Study Group. Clinical and therapeutic experience in 712 Israeli patients with idiopathic thrombocytopenic purpura. Acta Haematol. 1994;91(1):1-6. [DOI] [PubMed] [Google Scholar]
- 158.Belletrutti M, Ali K, Barnard D, et al. ; Canadian Pediatric Thrombosis and Hemostasis Network. Chronic immune thrombocytopenic purpura in children: a survey of the Canadian experience. J Pediatr Hematol Oncol. 2007;29(2):95-100. [DOI] [PubMed] [Google Scholar]
- 159.Hollander LL, Leys CM, Weil BR, Rescorla FJ. Predictive value of response to steroid therapy on response to splenectomy in children with immune thrombocytopenic purpura. Surgery. 2011;150(4):643-648. [DOI] [PubMed] [Google Scholar]
- 160.Ramenghi U, Amendola G, Farinasso L, et al. Splenectomy in children with chronic ITP: long-term efficacy and relation between its outcome and responses to previous treatments. Pediatr Blood Cancer. 2006;47(suppl 5):742-745. [DOI] [PubMed] [Google Scholar]
- 161.Durakbasa CU, Timur C, Sehiralti V, Mutus M, Tosyali N, Yoruk A. Pediatric splenectomy for hematological diseases: outcome analysis. Pediatr Surg Int. 2006;22(8):635-639. [DOI] [PubMed] [Google Scholar]
- 162.Watts RG. Idiopathic thrombocytopenic purpura: a 10-year natural history study at the Childrens Hospital of Alabama. Clin Pediatr (Phila). 2004;43(8):691-702. [DOI] [PubMed] [Google Scholar]
- 163.Verlin M, Laros RK Jr, Penner JA. Treatment of refractory thrombocytopenic purpura with cyclophosphamine. Am J Hematol. 1976;1(1):97-104. [DOI] [PubMed] [Google Scholar]
- 164.Pizzuto J, Ambriz R. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: multicentric trial of the Cooperative Latin American Group on Hemostasis and Thrombosis. Blood. 1984;64(6):1179-1183. [PubMed] [Google Scholar]
- 165.Joseph A, Evans DI. Immunosuppressive treatment of idiopathic thrombocytopenic purpura in children. Acta Paediatr Scand. 1982;71(3):467-469. [DOI] [PubMed] [Google Scholar]
- 166.Choudhary DR, Naithani R, Mahapatra M, Kumar R, Mishra P, Saxena R. Efficacy of cyclosporine as a single agent therapy in chronic idiopathic thrombocytopenic purpura. Haematologica. 2008;93(10):e61-e62, discussion e63. [DOI] [PubMed] [Google Scholar]
- 167.Kappers-Klunne MC, van’t Veer MB. Cyclosporin A for the treatment of patients with chronic idiopathic thrombocytopenic purpura refractory to corticosteroids or splenectomy. Br J Haematol. 2001;114(1):121-125. [DOI] [PubMed] [Google Scholar]
- 168.Liu AP, Cheuk DK, Lee AH, et al. Cyclosporin A for persistent or chronic immune thrombocytopenia in children. Ann Hematol. 2016;95(11):1881-1886. [DOI] [PubMed] [Google Scholar]
- 169.Perrotta S, Amendola G, Locatelli F, et al. Treatment with short-term, high-dose cyclosporin A in children with refractory chronic idiopathic thrombocytopenic purpura. Br J Haematol. 2003;121(1):143-147. [DOI] [PubMed] [Google Scholar]
- 170.Li J, Wang Z, Dai L, et al. Effects of rapamycin combined with low dose prednisone in patients with chronic immune thrombocytopenia. Clin Dev Immunol. 2013;2013:548085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ahn YS, Fernandez LF, Kim CI, et al. Danazol therapy renders red cells resistant to osmotic lysis. FASEB J. 1989;3(2):157-162. [DOI] [PubMed] [Google Scholar]
- 172.Ambríz R, Pizzuto J, Morales M, Chávez G, Guillén C, Avilés A. Therapeutic effect of danazol on metrorrhagia in patients with idiopathic thrombocytopenic purpura (ITP). Nouv Rev Fr Hematol. 1986;28(5):275-279. [PubMed] [Google Scholar]
- 173.Fenaux P, Quiquandon I, Huart JJ, Caulier MT, Bauters F. The role of danazol in the treatment of refractory idiopathic thrombocytopenic purpura. A report of 22 cases. Nouv Rev Fr Hematol. 1990;32(2):143-146. [PubMed] [Google Scholar]
- 174.Liu W, Gu X, Fu R, et al. The effect of danazol in primary immune thrombocytopenia: an analysis of a large cohort from a single center in China. Clin Appl Thromb Hemost. 2016;22(8):727-733. [DOI] [PubMed] [Google Scholar]
- 175.Maloisel F, Andrès E, Zimmer J, et al. Danazol therapy in patients with chronic idiopathic thrombocytopenic purpura: long-term results. Am J Med. 2004;116(9):590-594. [DOI] [PubMed] [Google Scholar]
- 176.Sundar S, Moorleedursingh GS, Kumar K, Dube B, Singh VP. Danazol therapy in chronic idiopathic thrombocytopenic purpura. J Assoc Physicians India. 1992;40(5):350-351. [PubMed] [Google Scholar]
- 177.Wang S, Yang R, Zou P, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96(2):222-228. [DOI] [PubMed] [Google Scholar]
- 178.Kim SW, Rice L, McCarthy JJ. Efficacy of danazol with autoimmune thrombocytopenia. Clin Appl Thromb Hemost. 1997;3(4):251-255. [Google Scholar]
- 179.Damodar S, Viswabandya A, George B, Mathews V, Chandy M, Srivastava A. Dapsone for chronic idiopathic thrombocytopenic purpura in children and adults--a report on 90 patients. Eur J Haematol. 2005;75(4):328-331. [DOI] [PubMed] [Google Scholar]
- 180.Godeau B, Durand JM, Roudot-Thoraval F, et al. Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol. 1997;97(2):336-339. [DOI] [PubMed] [Google Scholar]
- 181.Patel AP, Patil AS. Dapsone for immune thrombocytopenic purpura in children and adults. Platelets. 2015;26(2):164-167. [DOI] [PubMed] [Google Scholar]
- 182.Vancine-Califani SM, De Paula EV, Ozelo MC, Orsi FL, Fabri DR, Annichino-Bizzacchi JM. Efficacy and safety of dapsone as a second-line treatment in non-splenectomized adults with immune thrombocytopenic purpura. Platelets. 2008;19(7):489-495. [DOI] [PubMed] [Google Scholar]
- 183.Zaja F, Marin L, Chiozzotto M, Puglisi S, Volpetti S, Fanin R. Dapsone salvage therapy for adult patients with immune thrombocytopenia relapsed or refractory to steroid and rituximab. Am J Hematol. 2012;87(3):321-323. [DOI] [PubMed] [Google Scholar]
- 184.Hou M, Peng J, Shi Y, et al. Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;70(6):353-357. [DOI] [PubMed] [Google Scholar]
- 185.Miano M, Ramenghi U, Russo G, et al. Mycophenolate mofetil for the treatment of children with immune thrombocytopenia and Evans syndrome. A retrospective data review from the Italian association of paediatric haematology/oncology. Br J Haematol. 2016;175(3):490-495. [DOI] [PubMed] [Google Scholar]
- 186.Taylor A, Neave L, Solanki S, et al. Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol. 2015;171(4):625-630. [DOI] [PubMed] [Google Scholar]
- 187.Zhang WG, Ji L, Cao XM, et al. Mycophenolate mofetil as a treatment for refractory idiopathic thrombocytopenic purpura. Acta Pharmacol Sin. 2005;26(5):598-602. [DOI] [PubMed] [Google Scholar]
- 188.Ahn YS, Harrington WJ, Mylvaganam R, Allen LM, Pall LM. Slow infusion of vinca alkaloids in the treatment of idiopathic thrombocytopenic purpura. Ann Intern Med. 1984;100(2):192-196. [DOI] [PubMed] [Google Scholar]
- 189.Ahn YS, Harrington WJ, Seelman RC, Eytel CS. Vincristine therapy of idiopathic and secondary thrombocytopenias. N Engl J Med. 1974;291(8):376-380. [DOI] [PubMed] [Google Scholar]
- 190.Facon T, Caulier MT, Wattel E, Jouet JP, Bauters F, Fenaux P. A randomized trial comparing vinblastine in slow infusion and by bolus i.v. injection in idiopathic thrombocytopenic purpura: a report on 42 patients. Br J Haematol. 1994;86(3):678-680. [DOI] [PubMed] [Google Scholar]
- 191.Fenaux P, Quiquandon I, Caulier MT, Simon M, Walter MP, Bauters F. Slow infusions of vinblastine in the treatment of adult idiopathic thrombocytopenic purpura: a report on 43 cases. Blut. 1990;60(4):238-241. [DOI] [PubMed] [Google Scholar]
- 192.Fresneau B, Petit A, Courcoux MF, et al. Vinblastine in the treatment of children and adolescents with refractory immune thrombocytopenia. Am J Hematol. 2011;86(9):785-787. [DOI] [PubMed] [Google Scholar]
- 193.Manoharan A. Targeted-immunosuppression with vincristine infusion in the treatment of immune thrombocytopenia. Aust N Z J Med. 1991;21(4):405-407. [DOI] [PubMed] [Google Scholar]
- 194.Massimo L, Genova R, Marchi A, Masera G, Massolo I, Mori PG. More on vincristine in treatment of ITP in children. N Engl J Med. 1977;297(7):397-398. [DOI] [PubMed] [Google Scholar]
- 195.Nomura T, Maekawa T, Uchino H, et al. Clinical usefulness of vinca alkaloid slow infusion in the treatment of chronic refractory idiopathic thrombocytopenic purpura: a multicenter cooperative study. Nippon Ketsueki Gakkai Zasshi. 1990;53(1):98-104. [PubMed] [Google Scholar]
- 196.Park YH, Yi HG, Lee MH, Kim CS, Lim JH. Clinical efficacy and tolerability of vincristine in splenectomized patients with refractory or relapsed immune thrombocytopenia: a retrospective single-center study. Int J Hematol. 2016;103(2):180-188. [DOI] [PubMed] [Google Scholar]
- 197.Sikorska A, Słomkowski M, Marlanka K, Konopka L, Górski T. The use of vinca alkaloids in adult patients with refractory chronic idiopathic thrombocytopenia. Clin Lab Haematol. 2004;26(6):407-411. [DOI] [PubMed] [Google Scholar]
- 198.Stirnemann J, Vigan M, Hamroun D, et al. The French Gaucher’s disease registry: clinical characteristics, complications and treatment of 562 patients. Orphanet J Rare Dis. 2012;7(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ahn YS, Byrnes JJ, Harrington WJ, et al. The treatment of idiopathic thrombocytopenia with vinblastine-loaded platelets. N Engl J Med. 1978;298(20):1101-1107. [DOI] [PubMed] [Google Scholar]
- 200.Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia - current diagnostics and therapy: recommendations of a joint working group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(suppl 5):1-30. [DOI] [PubMed] [Google Scholar]
- 201.Schünemann HJ, Wiercioch W, Brozek J, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81:101-110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.