Abstract
Background: Acute kidney injury (AKI) is a well-established complication of extra-corporal membrane oxygenation (ECMO) in the adult population. The data in the pediatric and neonatal population is still limited. Moreover, the mortality risk of AKI among pediatric patients requiring ECMO remains unclear. Thus, this meta-analysis aims to assess the incidence of AKI, AKI requiring renal replacement therapy and AKI associated mortality in pediatric/neonatal patients requiring ECMO. Methods: A literature search was performed utilizing MEDLINE, EMBASE, and the Cochrane Database from inception through June 2019. We included studies that evaluated the incidence of AKI, severe AKI requiring renal replacement therapy (RRT) and the risk of mortality among pediatric patients on ECMO with AKI. Random-effects meta-analysis was used to calculate the pooled incidence of AKI and the odds ratios (OR) for mortality. Results: 13 studies with 3523 pediatric patients on ECMO were identified. Pooled incidence of AKI and AKI requiring RRT were 61.9% (95% confidence interval (CI): 39.0–80.4%) and 40.9% (95%CI: 31.2–51.4%), respectively. A meta-analysis limited to studies with standard AKI definitions showed a pooled estimated AKI incidence of 69.2% (95%CI: 59.7–77.3%). Compared with patients without AKI, those with AKI and AKI requiring RRT while on ECMO were associated with increased hospital mortality ORs of 1.70 (95% CI, 1.38–2.10) and 3.64 (95% CI: 2.02–6.55), respectively. Conclusions: The estimated incidence of AKI and severe AKI requiring RRT in pediatric patients receiving ECMO are high at 61.9% and 40.9%, respectively. AKI among pediatric patients on ECMO is significantly associated with reduced patient survival.
Keywords: AKI, ECMO, extracorporeal membrane oxygenation, incidence, mortality
1. Introduction
For the past 20 years, extra-corporal membrane oxygenation (ECMO) use has remarkably increased, as it has become an essential rescue therapy for severe refractory cardiac and pulmonary dysfunction in both adults and children [1]. In general, ECMO provides temporary support for patients who have a predicted mortality rate of 80% and above [2]. Indications for ECMO can be categorized into: (1) Respiratory Support and (2) Cardiac Support. Based on the indication for ECMO, the modality of ECMO is adapted for the patient. Veno-venous (VV) ECMO provides only respiratory support, and requires patients to have stable hemodynamics, whereas veno-arterial (VA) ECMO provides dual cardiac and respiratory support [1,2,3,4,5,6,7]. In VA ECMO, the blood bypasses both the heart and lungs. Generally, there is minimal vascular pulsatility in VA ECMO, while there is considerable pulsatility in VV ECMO. Pulsatility has been suggested to be essential in maintaining optimal perfusion to the vital organs, including the brain, heart and kidneys [8]. For instance, Pappalardo et al. [9] has shown that the incidence of acute ischemic stroke in VV ECMO is 1.4% compared to 3.8% in VA ECMO [10].
A recent meta-analysis has demonstrated that the pooled incidence of AKI and severe AKI requiring renal replacement therapy (RRT) among adult patients on ECMO was 63% and 45%, respectively. Furthermore, adult ECMO patients with AKI requiring RRT have a significantly increased mortality with a pooled odds ratio (OR) of 3.73 [11]. Although the incidence and associated mortality of AKI in adults on ECMO are widely reported, comparable data in the pediatric population are limited. While one study has reported a mortality rate of 27.4 and 41.6%, respectively, for neonatal and pediatric patients who received ECMO support for non-cardiac indications [12], the impact of AKI and its severity on outcomes is less clear. Furthermore, the need for RRT during ECMO is considered an independent risk factor for the failure to wean from ECMO [13]. Therefore, we performed this meta-analysis to assess the incidence rate of AKI and its associated mortality in pediatric patients receiving ECMO for various indications. The results of our study will help define the burden of AKI and its clinical impact on pediatric patients receiving ECMO, and allow comparisons to the adult counterpart.
2. Method
2.1. Literature Review and Search Strategy
We conducted a systematic literature review of Ovid MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews until June 2019 to assess the incidence and mortality risk of AKI in pediatric patients on ECMO. Independent reviewers (C.T. and P.L.) conducted a systematic literature search using a search strategy that incorporated the search terms “extracorporeal membrane oxygenation” OR “ECMO” AND “acute kidney injury” OR “acute renal failure”, as shown in Supplementary Data S1. A manual search for potentially relevant studies utilizing the references of the initial included articles was subsequently performed. There was no language limitation. This systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) [14].
2.2. Selection Criteria
Inclusion criteria consisted of observational studies or clinical trials that evaluated both the incidence of acute kidney injury (AKI) and/or severe AKI requiring RRT and mortality risk of AKI in pediatric patients (aged younger than 18 years old) on ECMO. Retrieved articles were individually assessed for study eligibility by the two investigators previously noted. Any discrepancies were resolved by mutual consensus.
2.3. Data Abstraction
A data collecting form was utilized to derive the following information from the individual studies: Title, study year, publication year, name of authors, country where the study was performed, ECMO type, definition of AKI, incidence rate of AKI, incidence rate of AKI requiring RRT and risk of mortality in pediatric patients on ECMO with AKI.
2.4. Statistical Analysis
Analyses were performed using the Comprehensive Meta-Analysis 3.3 software (version 3; Biostat Inc, Englewood, NJ, USA). Pooled AKI incidence and mortality risk of included studies were incorporated by the generic inverse variance method of DerSimonian-Laird, which indicated the weight of each study depending on its variance [15]. Because of the likelihood of inter-observation variance, we utilized a random-effects model for meta-analyses of the incidence and mortality risk of AKI among pediatric patients receiving ECMO. Statistical heterogeneity of studies was evaluated by the Cochran’s Q test (statistically significant as p < 0.05) and the I2 statistic (≤25% represents insignificant heterogeneity, 26% to 50% represents low heterogeneity, 51% to 75% represents moderate heterogeneity, and ≥75% represents high heterogeneity) [16]. Publication bias was evaluated via the Egger test [17].
3. Results
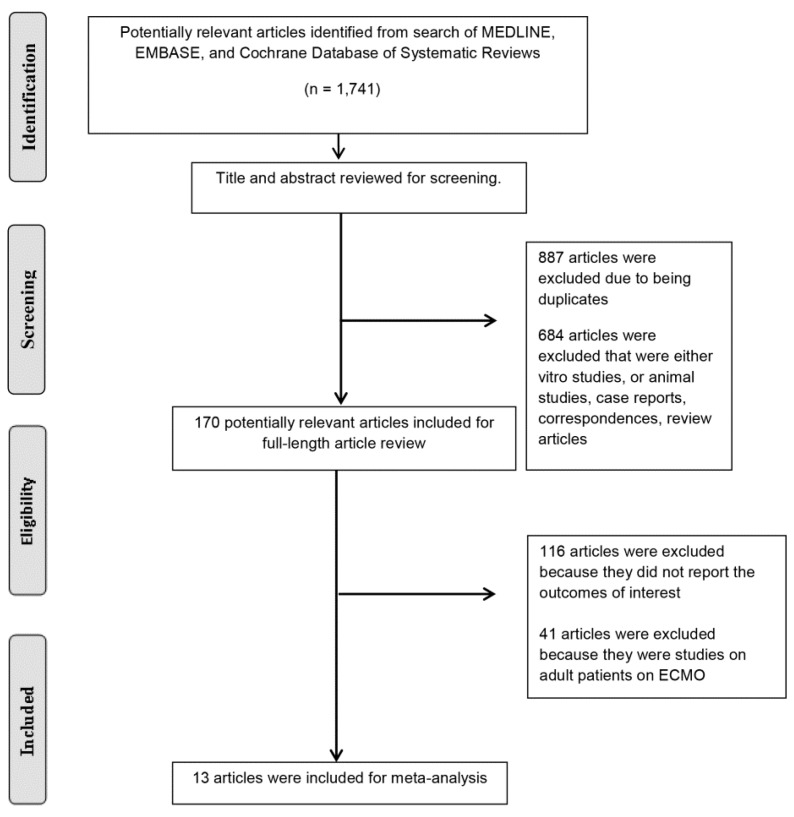
Our search strategy produced a total of 1741 potentially eligible articles. 887 articles were excluded because they were duplicated. and 684 articles because they were case reports, correspondences, review articles, in vitro studies, or animal studies. 170 articles remained for full-length review. Forty one articles were subsequently excluded because the study cohort was an adult patient population, and 116 studies were excluded due to a lack of data on the outcomes of interest. Consequently, 13 cohort studies [12,18,19,20,21,22,23,24,25,26,27,28,29] comprised of 3523 pediatric patients on ECMO were identified in this systematic review. The systematic review process is presented in Figure 1. The characteristics of the included studies are demonstrated in Table 1. The kappa coefficient (0.87) indicated that agreement between the authors was acceptable.
Figure 1.
Flow diagram of our search strategy.
Table 1.
Included studies in this systematic review of acute kidney injury (AKI) incidence and mortality in pediatric patients requiring ECMO.
| Study | Year | Country | Patient Population | Number | Definition of AKI | Incidence of AKI | Mortality |
|---|---|---|---|---|---|---|---|
| Kolovos et al. [18] | 2003 | USA | Patients on ECMO within seven days after cardiac surgery | 74 | RRT | RRT 26/74 (35%) |
Hospital mortality RRT 20/26 (77%) |
| Hoover et al. [19] | 2008 | USA | Patients aged 1 month to 18 years with respiratory failure requiring ECMO | 86 | CRRT | CRRT 26/86 (30%) | Hospital mortality RRT 7/26 (27%) |
| Gbadegesin et al. [20] | 2009 | USA | Patients aged < 3 years requiring ECMO after cardiac surgery | 104 | CRRT | CRRT 42/104 (40%) | Hospital mortality RRT 35/42 (83%) |
| Gadepalli et al. [21] | 2010 | USA | Congenital diaphragmatic hernia patients requiring ECMO |
68 | AKI: RIFLE | AKI 48/68 (71%) RIFLE—failure AKI 33/68 (49%) CRRT 11/68 = 16% |
Mortality RIFLE—failure AKI 24/33 (73%) |
| Goto et al. [22] | 2011 | USA | Patients aged 19 days to 20 years with respiratory failure and/or heart failure requiring ECMO | 14 | RRT | RRT 7/14 (50%) | Hospital mortality RRT 2/7 (29%) |
| Askenazi et al. [12] | 2011 | USA | All non-cardiac patients requiring ECMO | 9903 Neonates -7941 Children -1962 |
SCr > 1.5 mg/dL or ICD-9 for acute renal failure | Neonates AKI—638/7941 (8%) RRT—1786/7941 (22%) Children AKI—402/1962 (20%) RRT—840/1962 (43%) |
Neonates Hospital mortality AKI—413/638 (65%) RRT—863/1786 (48%) Children Hospital mortality AKI—264/402 (66%) RRT—487/840 (58%) |
| Ricci et al. [23] | 2012 | Italy | Patients aged 13 days to 13 years on VA ECMO after cardiac surgery | 10 | CRRT | CRRT 3/10 (30%) | Hospital mortality RRT 2/3 (66%) |
| Hoffman et al. [24] | 2013 | USA | Patients with persistent hypoxia or cardiovascular instability requiring ECMO | 10 | AKI; (1) urine output < 1 ml/kg/h with SCr > 1 mg/dL for 24 hours, (2) SCr of > 1.5 mg/dL, (3) failure to improve creatinine clearance by > 50% |
AKI 5/10 (50%) |
N/A |
| Zwiers et al. [25] | 2013 | Netherlands | Neonates aged < 28 days requiring ECMO | 242 | AKI: RIFLE | AKI 153/242 (63%) |
Hospital mortality AKI 43/153 (28%) |
| Fleming et al. [26] | 2016 | USA | Pediatric patients aged < 18 requiring ECMO | 832 | AKI: KDIGO | AKI by SCr—502/832 (60%) AKI by SCr + renal support therapy—615/832 (74%) |
N/A |
| Yang et al. [27] | 2016 | China | Patients aged 1 to 13 years with refractory cardiopulmonary failure requiring ECMO | 12 | CRRT | CRRT 1/12 (8%) | Hospital mortality RRT 0/1 (0%) |
| Elella et al. [28] | 2017 | Saudi Arabia | Pediatric patients requiring VA-ECMO after cardiac surgery | 59 | AKI pRIFLE | AKI 53/59 (90%) RRT 29/59 (49%) |
Hospital mortality AKI 33/53 (62%) |
| Borasino et al. [29] | 2018 | USA | Pediatric cardiac patient on ECMO in CICU | 50 | AKI: increase in SCr of 200% from baseline | AKI 35/50 (70%) RRT 26/50 (52%) |
Hospital mortality RRT 16/26 (61%) |
Abbreviations: AKIN, Acute Kidney Injury Network; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, Pediatric Risk, Injury, Failure, Loss of kidney function; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; RRT, renal replacement therapy; SCr, serum creatinine; ECMO, extra-corporal membrane oxygenation.
3.1. Incidence of AKI among Pediatric Patients on ECMO
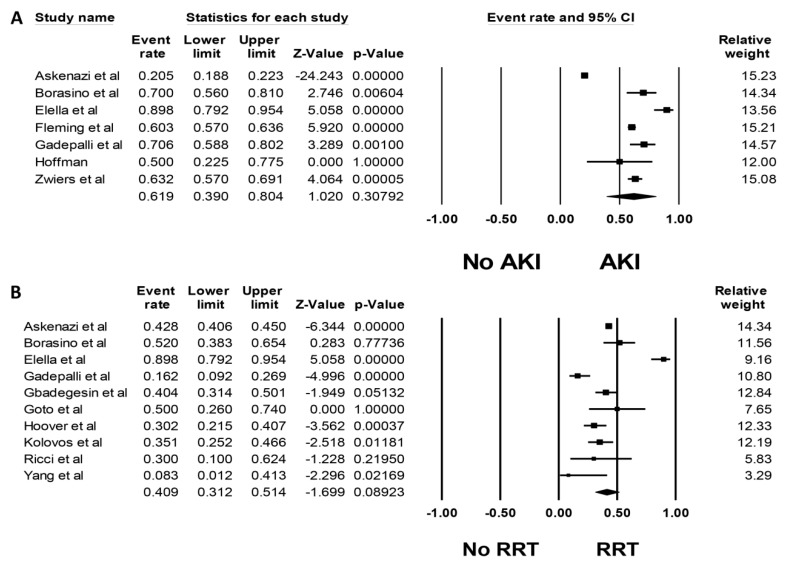
Pooled estimated incidence of AKI and severe AKI requiring RRT were 61.9% (95%CI: 39.0–80.4%, I2 = 98%, Figure 2A) and 40.9% (95%CI: 31.2–51.4%, I2 = 86%, Figure 2B), respectively. We additionally performed a meta-analysis limited to studies using standard AKI definitions; this showed a pooled estimated AKI incidence of 69.2% (95%CI: 59.7–77.3%, I2 = 83%, Figure S1).
Figure 2.
Forest plots of included studies on pediatric patients requiring extra-corporal membrane oxygenation (ECMO) that evaluated (A) incidence of AKI and (B) incidence of AKI requiring renal replacement therapy (RRT).
Subgroup analysis based on patient population (cardiac surgery vs. non-cardiac surgery) was performed. Pooled incidence of AKI was 81.4% (95%CI: 54.4–94.2%, I2 = 84%, Figure S2) among patients after cardiac surgery and 52.3% (95%CI: 27.3–76.2%, I2 = 99%, Figure S2) among non-cardiac surgery patients. Pooled incidence of AKI requiring RRT was 52.2% (95%CI: 32.5–71.2%, I2 = 88%, Figure S3) among patients after cardiac surgery and 30.2% (95%CI: 18.7–44.9%, I2 = 84%, Figure S3) among non-cardiac surgery patients.
3.2. Mortality in Pediatric ECMO Patients with AKI
Table 1 and Table 2 demonstrate the AKI-associated mortality rate and risk in pediatric patients on ECMO, respectively. Pooled estimated hospital or 90-day mortality rates of pediatric ECMO patients with AKI and AKI requiring RRT were 51.7% (95%CI: 26.5–76.0%, I2 = 96%, Figure S4) and 61.8% (95%CI: 48.8–73.4%, I2 = 74%, Figure S5), respectively.
Table 2.
Mortality Risk of AKI among pediatric patients requiring ECMO.
| Study | Mortality Rate | OR for Mortality |
|---|---|---|
| Kolovos et al. [18] | Hospital mortality RRT 20/26 (77%) |
Hospital mortality RRT: OR 5.6 (1.8–17.9) |
| Hoover et al. [19] | Hospital mortality RRT 7/26 (27%) |
Hospital mortality CRRT: 1.55 (0.42–5.70) |
| Gbadegesin et al. [20] | Hospital mortality RRT 35/42 (83%) |
Hospital mortality CRRT: 9.8 (3.7–25.7) |
| Gadepalli et al. [21] | Mortality RIFLE—failure AKI 24/33 (73%) |
Hospital mortality RIFLE—Failure AKI: 10.67 (3.45–32.96) |
| Goto et al. [22] | Hospital mortality RRT 2/7 (29%) |
Hospital mortality RRT: 0.53 (0.06–4.91) |
| Askenazi et al. [12] | Neonates Hospital mortality AKI—413/638 (65%) RRT—863/1786 (48%) Children Hospital mortality AKI—264/402 (66%) RRT—487/840 (58%) |
Hospital mortality Neonates AKI: 3.2 (2.6–4.0) RRT: 1.9 (1.6–2.2) Children AKI: 1.7 (1.3–2.3) RRT: 2.5 (1.9–3.2) |
| Ricci et al. [23] | Hospital mortality RRT 2/3 (66%) |
Hospital mortality CRRT: 2.67 (0.16–45.14) |
| Zwiers et al. [25] | Hospital mortality AKI 43/153 (28%) |
Hospital mortality AKI: 1.35 (0.73–2.48) |
| Fleming et al. [26] | N/A | Hospital mortality AKI-SCr: 1.77 (1.22–2.55) AKI-SCr+RST: 2.50 (1.61–3.90) |
| Yang et al. [27] | Hospital mortality RRT 0/1 (0%) |
Hospital mortality CRRT: 0/1 vs. 4/11 |
| Elella et al. [28] | Hospital mortality AKI 33/53 (62%) |
Hospital mortality AKI: 8.25 (0.90–75.79) |
| Borasino et al. [29] | Hospital mortality RRT 16/26 (61%) |
Hospital morality RRT: 2.67 (0.85–8.37) |
Abbreviations: AKIN, Acute Kidney Injury Network; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; KDIGO, Kidney Disease Improving Global Outcomes; pRIFLE, Pediatric Risk, Injury, Failure, Loss of kidney function; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; RRT, renal replacement therapy; RST, renal support therapy; SCr, serum creatinine.
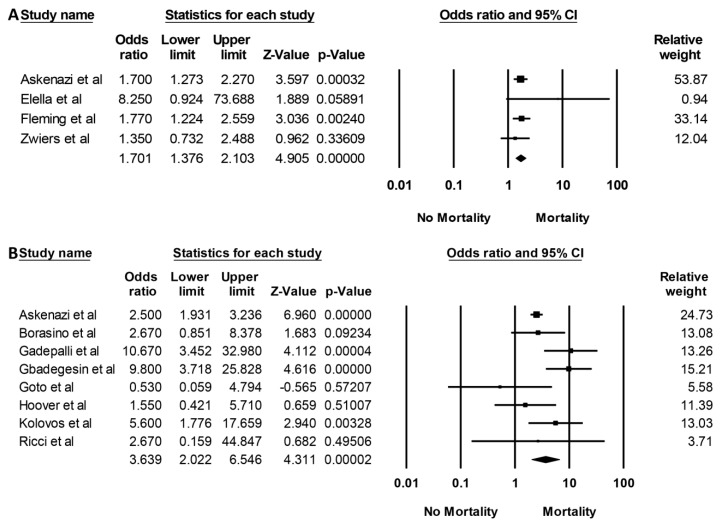
The hospital mortality pooled OR in pediatric ECMO patients with AKI and severe AKI requiring RRT were 1.70 (95% CI: 1.38–2.10, I2 = 0%, Figure 3A) and 3.64 (95% CI, 2.02–6.55, I2 = 58%, Figure 3B), respectively. Meta-regression analysis was additionally performed, and it demonstrated that year of study was not correlated with either mortality risk associated with AKI (p = 0.65) or AKI requiring RRT (p = 0.46).
Figure 3.
Forest plots of included studies on pediatric patients requiring ECMO that evaluated (A) hospital mortality with AKI and (B) hospital mortality with AKI necessitating RRT.
3.3. Assessment for Publication Bias
Funnel plots (Figures S6 and S7) and Egger’s regression asymmetry tests were used to evaluate for publication bias. We found no significant publication bias for the incidence of AKI and severe AKI requiring RRT (p = 0.16 and p = 0.74, respectively).
4. Discussion
In this study, we have shown that the incidence of AKI and severe AKI in the pediatric population requiring ECMO is high (62% and 41%, respectively), especially in cardiac surgery patients requiring ECMO. AKI is associated with high mortality (OR 3.64) and AKI-associated mortality did not change over time. Similar to the adult population, our study in pediatric patients concurs that ECMO is associated with a higher risk of AKI and hospital mortality [11].
The mechanism by which ECMO support predisposes patients to AKI is not well established. Certainly, patients requiring ECMO often possess many known comorbidities and risk factors for AKI, such as low cardiac output, septic shock, anemia, diabetes, exposure to nephrotoxic drugs, etc. [30,31]. In general, there are fewer comorbidities among pediatric patients. Conversely, their renal reserve may not have fully developed [12].
However, it is proposed that ECMO itself is capable of inducing AKI via detrimental (1) hemodynamic alterations; (2) hormonal imbalances; and (3) systemic inflammatory cytokines [11,30,31]. Hemodynamic alterations may include the potential loss of pulsatility with VA ECMO versus the maintenance of pulsatility with VV ECMO. Evidence is supportive of the vital impact of pulsatility on maintaining renal cortical perfusion, thus helping to prevent acute tubular necrosis [8]. Slight hemodynamic changes could potentiate a significant reduction in renal perfusion. Current data on ECMO-associated hormonal changes show conflicting evidence. Saito et al. hypothesized that upregulation of plasma renin activity may be an adaptive response to the loss of pulsatile renal perfusion [32]. However, Semmekrot et al. observed a reduction of atrial natriuretic peptide (ANP) level, plasma renin activity (PRA) and angiotensin II levels in neonates requiring VA ECMO [33]. Further clinical investigation is needed to better understand the impact and interaction of different hormones on renal perfusion. Finally, studies have demonstrated that the continuous exposure of blood to the non-endothelialized/non-biological ECMO interface results in an activation of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL (interleukin)-1β, IL-6, IL-8 [34,35].
Our study shows that the incidence of AKI, severe AKI requiring RRT and AKI-associated hospital mortality between adults and pediatric patients are similar [11]. This further supports ECMO as a potential independent predictor for AKI. However, we found a significant higher incidence of AKI and RRT in pediatric patients that the indication for ECMO was after cardiac surgery, than those who required ECMO for non-cardiac surgery related conditions. Despite improved knowledge of the indications for ECMO, patient comorbidities, and associated risk factors that may contribute to ECMO-associated AKI, ECMO as an independent risk factor for AKI cannot be determined from this meta-analysis alone. A pooled meta-analysis of both adult and pediatric patients assessing the incidence of AKI and mortality following ECMO support would be useful. Furthermore, a subgroup analysis comparing adult and pediatric patients would further elaborate whether ECMO is associated with AKI and increased mortality.
There were a few limitations in this study. Most of the included studies defined AKI based on serum creatinine levels, while studies utilizing the more sensitive urine output criteria or use of novel AKI biomarkers were limited [36]. Moreover, our meta-analysis is primarily based on observational studies, making it impossible to establish a causal relationship. Future data from population-based studies or prospective clinical trials focusing on AKI prevention in ECMO patients would be beneficial.
5. Conclusions
Our study demonstrates that AKI is common following ECMO in pediatric patients with an incidence of 68%. Approximately 40% of pediatric patients on ECMO develop severe AKI requiring RRT. We have also shown that presence of AKI is associated with a higher risk of hospital mortality. There is no difference in mortality between recent and remote studies. Clinical trials focusing on preventive measures for AKI with ECMO therapy is also encouraged.
Supplementary Materials
The following are available online at https://www.mdpi.com/2305-6320/6/4/109/s1, Data S1: Search terms for systematic review, Figure S1: Forest plots of the included studies assessing incidence of AKI limited to studies with standard AKI definitions was performed and demonstrated the pooled estimated incidence of AKI, Figure S2: Forest plots of the included studies assessing incidence of AKI based on cardiac surgery status, Figure S3: Forest plots of the included studies assessing incidence of severe AKI requiring RRT based on cardiac surgery status, Figure S4: Forest plots of the included studies assessing hospital and/or 90-day mortality rates of pediatric patients on ECMO with AKI, Figure S5: Forest plots of the included studies assessing hospital and/or 90-day mortality rates of pediatric patients on ECMO with severe AKI requiring RRT, Figure S6: Funnel plot evaluating for publication bias evaluating incidence of AKI while on ECMO, Figure S7: Funnel plot evaluating for publication bias evaluating incidence of severe AKI requiring RRT while on ECMO.
Author Contributions
Conceptualization, P.H., C.T., W.C., S.A.S., A.C., N.S., M.A.M., P.U., K.W. (Karn Wijarnpreecha), W.K. and T.B.; Data curation, C.T.; Formal analysis, T.B.; Funding acquisition, T.B.; Investigation, P.H., P.L., C.T. and T.B.; Methodology, P.H., P.L., C.T., W.C., W.K. and T.B.; Project administration, P.H., W.K. and T.B.; Resources, P.L., K.W. (Kanramon Watthanasuntorn) and T.B.; Software, K.W. (Kanramon Watthanasuntorn) and T.B.; Supervision, W.C., N.R.A., S.A.S., A.C., N.S., M.A.M., P.U., K.W. (Karn Wijarnpreecha), W.K. and T.B.; Validation, P.L., C.T., W.C. and T.B.; Visualization, P.L., S.A.S., A.C. and N.S.; Writing—original draft, P.H.; Writing—review & editing, P.H., P.L., C.T., W.C., N.R.A., S.A.S., A.C., K.W. (Kanramon Watthanasuntorn), N.S., M.A.M., P.U., K.W. (Karn Wijarnpreecha), W.K. and T.B.
Funding
This research received no external funding.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- 1.Makdisi G., Wang I.W. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 2015;7:166–176. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askenazi D.J., Selewski D.T., Paden M.L., Cooper D.S., Bridges B.C., Zappitelli M. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin. J. Am. Soc. Nephrol. 2012;7:1328–1336. doi: 10.2215/CJN.12731211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jesus-Brugman N., Hobson M.J., Herrmann J.L., Friedman M.L., Cordes T., Mastropietro C.W. Improved outcomes in neonates who require venoarterial extracorporeal membrane oxygenation after the Norwood procedure. Int. J. Artif. Organs. 2019 doi: 10.1177/0391398819882020. [DOI] [PubMed] [Google Scholar]
- 4.Constantinescu A.R., Adler J.L., Watkins E., Negroni-Balasquide X.L., Laufenberg D., Scholl F.G., Lavandosky G.J. Aquapheresis (AQ) in Tandem with Extracorporeal Membrane Oxygenation (ECMO) in Pediatric Patients. J. Extra Corpor. Technol. 2019;51:163–168. [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Herce J., Casado E., Diez M., Sanchez A., Fernandez S.N., Bellon J.M., Santiago M.J. Renal function in children assisted with extracorporeal membrane oxygenation. Int. J. Artif. Organs. 2019 doi: 10.1177/0391398819876294. [DOI] [PubMed] [Google Scholar]
- 6.Mallory P.P., Selewski D.T., Askenazi D.J., Cooper D.S., Fleming G.M., Paden M.L., Murphy L., Sahay R., King E., Zappitelli M., et al. Acute Kidney Injury, Fluid Overload, and Outcomes in Children Supported With Extracorporeal Membrane Oxygenation for a Respiratory Indication. ASAIO J. 2019 doi: 10.1097/MAT.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 7.Chen S.W., Lu Y.A., Lee C.C., Chou A.H., Wu V.C., Chang S.W., Fan P.C., Tian Y.C., Tsai F.C., Chang C.H. Long-term outcomes after extracorporeal membrane oxygenation in patients with dialysis-requiring acute kidney injury: A cohort study. PLoS ONE. 2019;14:0212352. doi: 10.1371/journal.pone.0212352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemoto M. Experimental evaluation of the influence of complete artificial circulation on renal circulation and tissue metabolism -comparative study of pulsatile vs nonpulsatile circulation. Ann. Thorac. Cardiovasc. Surg. 2003;9:355–364. [PubMed] [Google Scholar]
- 9.Pappalardo F., Montisci A. Neurologic complications during V-V extracorporeal membrane oxygenation: Still counting. J. Thorac. Dis. 2017;9:2774–2776. doi: 10.21037/jtd.2017.08.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiagarajan R.R., Barbaro R.P., Rycus P.T., McMullan D.M., Conrad S.A., Fortenberry J.D., Paden M.L. Extracorporeal Life Support Organization Registry International Report 2016. Asaio J. 2017;63:60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 11.Thongprayoon C., Cheungpasitporn W., Lertjitbanjong P., Aeddula N.R., Bathini T., Watthanasuntorn K., Srivali N., Mao M.A., Kashani K. Incidence and Impact of Acute Kidney Injury in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. J. Clin. Med. 2019;8:981. doi: 10.3390/jcm8070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askenazi D.J., Ambalavanan N., Hamilton K., Cutter G., Laney D., Kaslow R., Keith G., Douglas C.B., Reed A.D. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. 2011;12:1–6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 13.Wu M.Y., Lin P.J., Tsai F.C., Haung Y.K., Liu K.S., Tsai F.C. Impact of preexisting organ dysfunction on extracorporeal life support for non-postcardiotomy cardiopulmonary failure. Resuscitation. 2008;79:54–60. doi: 10.1016/j.resuscitation.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS ONE Med. 2009;6:1000097. [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 18.Kolovos N.S., Bratton S.L., Moler F.W., Bove E.L., Ohye R.G., Bartlett R.H., Kulik T.J. Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann. Thorac. Surg. 2003;76:1435–1441. doi: 10.1016/S0003-4975(03)00898-1. [DOI] [PubMed] [Google Scholar]
- 19.Hoover N.G., Heard M., Reid C., Wagoner S., Rogers K., Foland J., Paden M.L., Fortenberry J.D. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34:2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 20.Gbadegesin R., Zhao S., Charpie J., Brophy P.D., Smoyer W.E., Lin J.J. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr. Nephrol. 2009;24:589–595. doi: 10.1007/s00467-008-1047-z. [DOI] [PubMed] [Google Scholar]
- 21.Gadepalli S.K., Selewski D.T., Drongowski R.A., Mychaliska G.B. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: An insidious problem. J. Pediatr. Surg. 2011;46:630–635. doi: 10.1016/j.jpedsurg.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Goto T., Suzuki Y., Suzuki Y., Osanai A., Aoki K., Yamazaki A., Daitoku K., Fukuda I. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with respiratory and heart failure: Review of our experience. Artif. Organs. 2011;35:1002–1009. doi: 10.1111/j.1525-1594.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- 23.Ricci Z., Morelli S., Favia I., Garisto C., Brancaccio G., Picardo S. Neutrophil gelatinase-associated lipocalin levels during extracorporeal membrane oxygenation in critically ill children with congenital heart disease: Preliminary experience. Pediatr. Crit. Care Med. 2012;13:51–54. doi: 10.1097/PCC.0b013e3181fe4717. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman S.B., Massaro A.N., Soler-Garcia A.A., Perazzo S., Ray P.E. A novel urinary biomarker profile to identify acute kidney injury (AKI) in critically ill neonates: A pilot study. Pediatr. Nephrol. 2013;28:2179–2188. doi: 10.1007/s00467-013-2524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwiers A.J., de Wildt S.N., Hop W.C., Dorresteijn E.M., Gischler S.J., Tibboel D., Cransberg K. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: A 14-year cohort study. Crit. Care. 2013;17:R151. doi: 10.1186/cc12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming G.M., Sahay R., Zappitelli M., King E., Askenazi D.J., Bridges B.C., Paden M.L., Selewski D.T., Cooper D.S. The Incidence of Acute Kidney Injury and Its Effect on Neonatal and Pediatric Extracorporeal Membrane Oxygenation Outcomes: A Multicenter Report From the Kidney Intervention During Extracorporeal Membrane Oxygenation Study Group. Pediatr. Crit. Care Med. 2016;17:1157–1169. doi: 10.1097/PCC.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z.H., Ning B.T., Zhang C.M., Lin R., Ye S., Liu T. Clinical application of extracorporeal membrane oxygenation in children with refractory cardiopulmonary failure. World J. Pediatr. 2016;12:364–367. doi: 10.1007/s12519-016-0030-1. [DOI] [PubMed] [Google Scholar]
- 28.Elella R.A., Habib E., Mokrusova P., Joseph P., Aldalaty H., Ahmadi M.A., Al Halees Z. Incidence and outcome of acute kidney injury by the pRIFLE criteria for children receiving extracorporeal membrane oxygenation after heart surgery. Ann. Saudi Med. 2017;37:201–206. doi: 10.5144/0256-4947.2017.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borasino S., Kalra Y., Elam A.R., Carlisle O’Meara L., Timpa J.G., Goldberg K.G., Leslie Collins Gaddis J., Alten J.A. Impact of Hemolysis on Acute Kidney Injury and Mortality in Children Supported with Cardiac Extracorporeal Membrane Oxygenation. J. Extra Corpor. Technol. 2018;50:217–224. [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C.Y., Chen Y.C., Tsai F.C., Tian Y.C., Jenq C.C., Fang J.T., Yang C.W. RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol. Dial. Transplant. 2006;21:2867–2873. doi: 10.1093/ndt/gfl326. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia I., Ho K.C., Rocha B.A., Yam N., Lun K.S., Yung T.C., Au W.K.T. Pediatric ventricular assist device therapy for advanced heart failure-Hong Kong experience. J. Artif. Organs. 2019:1–7. doi: 10.1007/s10047-019-01140-4. [DOI] [PubMed] [Google Scholar]
- 32.Saito S., Westaby S., Piggot D., Dudnikov S., Robson D., Catarino P.A., Clelland C., Nojiri C. End-organ function during chronic nonpulsatile circulation. Ann. Thorac. Surg. 2002;74:1080–1085. doi: 10.1016/S0003-4975(02)03846-8. [DOI] [PubMed] [Google Scholar]
- 33.Semmekrot B.A., Pesman G.J., Span P.N., Sweep C.G., van Heijst A.F., Monnens L.A., van de Bor M., Tanke R.B., van der Staak F.H.J.M. Serial plasma concentrations of atrial natriuretic peptide, plasma renin activity, aldosterone, and antidiuretic hormone in neonates on extracorporeal membrane oxygenation. Asaio J. 2002;48:26–33. doi: 10.1097/00002480-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y., Yu W., Shi J., Chen Q., Shen J., Lin Z., He C., Li N., Li J. Effects of continuous renal replacement therapy on renal inflammatory cytokines during extracorporeal membrane oxygenation in a porcine model. J. Cardiothorac. Surg. 2013;8:113. doi: 10.1186/1749-8090-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McI R.B., Timpa J.G., Kurundkar A.R., Holt D.W., Kelly D.R., Hartman Y.E., Neel M.L., Karnatak R.K., Schelonka R.L., Anantharamaiah G.M., et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab. Investig. 2010;90:128–139. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashani K., Cheungpasitporn W., Ronco C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017;55:1074–1089. doi: 10.1515/cclm-2016-0973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.