Abstract
Ischemic stroke is a devastating brain injury resulting in high mortality and substantial loss of function. Understanding the pathophysiology of ischemic stroke risk, mortality, and functional loss is critical to the development of new therapies. Age and sex have a complex and interactive effect on ischemic stroke risk and pathophysiology. Aging is the strongest nonmodifiable risk factor for ischemic stroke, and aged stroke patients have higher mortality and morbidity and poorer functional recovery than their young counterparts. Importantly, patient age modifies the influence of patient sex in ischemic stroke. Early in life, the burden of ischemic stroke is higher in men, but stroke becomes more common and debilitating for women in elderly populations. The profound effects of sex and age on clinical ischemic stroke are mirrored in the results of experimental in vivo and in vitro studies. Here, we review current knowledge on the influence of age and sex in the incidence, mortality, and functional outcome of ischemic stroke in clinical populations. We also discuss the experimental evidence for sex and age differences in stroke pathophysiology and how a better understanding of these biological variables can improve clinical care and enhance development of novel therapies.
This mini-review examines the interactive effects of age and sex on ischemic stroke pathology.
Stroke is a deadly and debilitating disease, affecting >15 million people worldwide each year. Stroke is a disease of aging—most strokes occur in people >65 years (1, 2). Aged patients have higher mortality and poorer quality of life after stroke compared with younger patients (3–8). Sex also affects stroke incidence and outcome; although men have a higher incidence of stroke throughout most of the lifespan, women have higher stroke prevalence overall because of the increase in stroke risk with aging and a longer average lifespan in females (9).
Ischemic strokes, caused by a loss of blood flow to the brain, account for 87% of all strokes in the United States (2). Age and sex influence ischemic stroke epidemiology, pathophysiology, and treatment efficacy. Fascinatingly, one recent clinical study found that minocycline, an inhibitor of cell death, improves functional outcome after ischemic stroke in male patients only, with no benefit to female patients (10). Echoing this, a male-specific benefit of minocycline was also seen in an experimental mouse model of ischemic stroke, illustrating the importance of assessing sex as a variable in both clinical and experimental research (11).
Unfortunately, the consideration of sex as a variable remains critically underassessed in many clinical trials. Notably, the Systolic Blood Pressure Improvement Trail (SPRINT) study reported that aggressive control of blood pressure improves composite cardiovascular risk, including the risk for stroke. Unfortunately, only 36% of the patients enrolled in SPRINT were female, and the study was terminated because of improved outcomes in men [hazard ratio (HR), 0.72; 95% CI, 0.59 to 0.88] before statistical significance was reached in females (HR, 0.84; 95% CI, 0.62 to 1.14) (12, 13). It remains unclear whether the SPRINT trial findings are generalizable to female patients without further study (14). In cases such as this, enrollment and/or follow-up in women should continue until the study is adequately powered to definitively assess the effects of the intervention in both sexes.
Effect of Sex and Age on Clinical Ischemic Stroke
Age-dependent sex differences in stroke incidence, mortality, and morbidity
Sex differences in ischemic stroke epidemiology depend on patient age because the influence of sex on stroke risk and outcome changes across the lifespan. In childhood and early adulthood, males have a higher incidence of ischemic stroke and poorer functional outcomes than do females (9, 15). In middle age, the rates of ischemic stroke begin to increase in women, concomitant with the onset of menopause and loss of female sex hormones (16). After middle age, stroke rates continue to increase in women, with some reports of higher stroke incidence in elderly women (age >85 years) compared with elderly men (9, 15).
Mortality risk in ischemic stroke is high, with estimated 30-day case fatality ranging from 16% to 32% (1, 17). The complex relationship between age and sex complicates the assessment of sex differences in stroke-related mortality. Numerous studies have reported higher mortality in women with ischemic stroke, yet sex differences in age of stroke patients represent major confounders (9, 18, 19). After adjustment for age and prestroke function, no independent effect of sex on stroke mortality risk was observed (19–23). Although female sex may not independently predict mortality, it is important to recognize that the average female stroke patient will be at higher risk for death than will her male counterparts. Therefore, sex stratification of clinical trial results is critical to determining whether the risk/benefit ratio is sex-dependent (as in the minocycline trial) (10, 24).
Because most ischemic stroke patients survive their initial injury, functional outcome in stroke survivors is a major determinant of overall disease burden. There are ~ 6 million stroke survivors living in the United States (3.8 million female and 2.2 million male), and this number is projected to increase to 10 million by 2030 as the aging population expands (2). The economic cost of stroke survivor care increases with age because elderly stroke survivors (age ≥65 years) are more likely to have severe deficits and require greater care. Sex disparities in functional outcome after ischemic stroke are well described, with overwhelming evidence for poorer functional outcomes and a reduced quality of life in female patients (25–31). Importantly, these sex differences persist after adjustment for confounders, indicating that female sex may be an independent risk factor for poor functional outcome and impaired quality of life after ischemic stroke. In addition, elderly women tend to have poorer baseline functional status before stroke, resulting in their frequent exclusion from clinical trials and increasing the likelihood of underestimation in therapeutic benefit (19, 23).
These sex differences in stroke epidemiology are likely due to both socioeconomic and biologic differences. Several socioeconomic risk factors for poor stroke outcomes are significantly more common in women. Poststroke depression occurs in 33% of stroke survivors and is associated with poor functional recovery and increased mortality (32–34). Most studies included in a large meta-analysis found higher rates of poststroke depression in women (35). Female stroke patients are also more likely to live alone than are male patients, which may contribute to a delay in hospital arrival and poor social support during recovery (36). Other factors, including sex differences in recognition of stroke symptoms and clinical care, have been comprehensively reviewed elsewhere (37, 38). In this review, we focus on sex differences in the biology of ischemic stroke, including sex differences in the incidence, timing, and magnitude of risk factors for ischemic stroke.
Age and sex differences in ischemic stroke risk factors
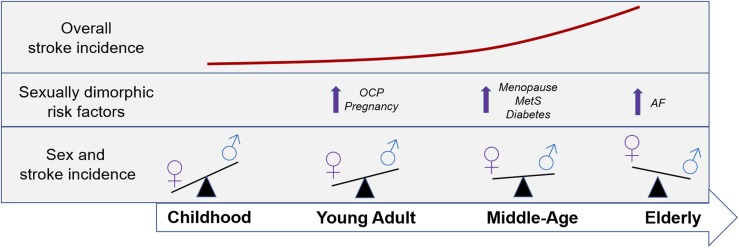
Stroke risk factors are important determinants of the incidence and pathophysiology of ischemic stroke (39). Age itself is a significant, nonmodifiable risk factor for ischemic stroke. Because women tend to be older than men at stroke onset, sex differences in stroke incidence and stroke outcomes may be partially explained by age-dependent differences in the modifiable risk factor profile of male and female patients (19, 25, 40). Some of these modifiable risk factors (Table 1) are sex-specific, whereas others occur in both men and women but have a higher incidence or confer a higher overall stroke risk in one sex (69). Interestingly, sexually dimorphic risk factors change throughout the lifespan, contributing to the shifting sex differences in stroke incidence, as depicted in Fig. 1.
Table 1.
Summary of Current Knowledge Regarding Sex Differences in Risk Factors for Ischemic Stroke
| Risk Factor | Type | Risk Magnitude and Modifiers (Reference) |
|---|---|---|
| Pregnancy | Sex-specific (female) | Low overall (34:100,000 deliveries) (41) |
| Highest risk postpartum (up to 12 wk) (42) | ||
| Elevated risk in preeclampsia and gestational hypertension (41, 43) | ||
| OCP | Sex-specific (female) | Increased risk (1.4-fold to 2-fold) in women (44) |
| Higher risk correlates to higher dose of estrogens (45) | ||
| Synergistic risk in women who have migraines with aura (46–48) | ||
| Postmenopausal HRT | Sex-specific (female) | Late HRT shows no benefit or increases stroke risk (49–52) |
| Evidence for benefit of early HRT (<6 y from menopause onset) in cardiovascular disease, stroke risk still in follow-up (53) | ||
| AF | Shared | Ischemic stroke risk conferred by AF increases with age (54, 55) |
| Prevalence of AF and cardioembolic stroke higher in women; women tend to be older at onset (56–60) | ||
| AF confers stronger stroke risk in women (61) | ||
| Metabolic syndrome | Shared | Metabolic syndrome confers stronger stroke risk in women (62) |
| Risk factor profile of women with metabolic syndrome dominated by abdominal obesity, which is suggested to confer greater stroke risk in women (63) | ||
| Diabetes | Shared | Type 2 diabetes confers greater risk for stroke in women (64) |
| Risk for fatal stroke higher in diabetic female patients than male patients (65) | ||
| Hypertension | Shared | Hypertension is more common in women and more likely to be poorly controlled (66) |
| Stroke risk conferred by hypertension equivalent between men and women (67) | ||
| Coronary artery disease | Shared | Coronary artery disease is more prevalent in men (16, 68) |
Sexually dimorphic risk factors are identified as sex-specific (pregnancy, OCP use, postmenopausal HRT) or shared (metabolic syndrome, diabetes, hypertension, coronary artery disease). Current evidence for the overall risk, any risk modifiers or synergistic relationships, and any sex differences in the risk magnitude are described with pertinent citations given.
Abbreviations: AF, atrial fibrillation; HRT, hormone replacement therapy; OCP, oral contraceptive pill.
Figure 1.
Schematic illustration of the relationship between age- and sex-related risk factors across the lifespan. An approximation of the overall increase in stroke incidence with aging is given, in addition to sex-specific or sexually dimorphic stroke risk factors at each stage of life. Sex differences in stroke incidence throughout the lifespan are also depicted. AF, atrial fibrillation; MetS, metabolic syndrome; OCP, oral contraceptive pill.
Women have several unique stroke risks, including oral contraceptive pill (OCP) use, pregnancy, menopause, and hormone replacement therapy (HRT). Although stroke incidence is low in women of reproductive age, OCP use significantly increases stroke risk, with the highest risk conferred by high-estrogen OCPs (44). Estrogens have many positive cardiovascular effects (as discussed later), but they also enhance coagulation and may elevate the risk for clotting in women taking estrogen-containing OCP (41, 70). Interestingly, there is a synergistic stroke risk in women taking OCPs with a history of migraines, particularly in patients who experience migraine auras (42, 43).
Stroke incidence in women also increases during pregnancy, with particularly high risk during the final trimester and early postpartum period (45–48). Stroke risk is further increased in women who develop gestational hypertension and preeclampsia during pregnancy (49, 50). Rates of preeclampsia are higher with advanced maternal age, with risk increasing from 6.4% to 9.4% in primiparous pregnant women >35 years (51). Importantly, although preeclampsia is often thought to be a disorder of primiparity, several studies have shown that advanced maternal age increases the risk for preeclampsia even in multiparous women (52–56). Although the short-term consequences of preeclampsia, including acute stroke and mortality, are well known, data on the long-term complications of preeclampsia are also beginning to emerge (46, 55, 56). Women with a history of preeclampsia have a greater risk for future cerebrovascular disease, with more frequent and severe white matter lesions in the brain out to 18 years after pregnancy (57). A meta-analysis of 6.4 million women confirmed that preeclampsia is significantly associated with long-term cardiovascular disease risk, including a twofold increase in stroke risk later in life (58). Fascinatingly, evidence also supports long-term effects in children exposed to preeclampsia in utero, who experience higher blood pressure and increased body mass index later in life (59–62). Although studies of long-term outcomes are rare, Kajantie et al. (63) reported an increased risk for stroke in children of mothers with both gestational hypertension (HR, 1.4; 95% CI, 1.0 to 1.8) and preeclampsia (HR, 1.9; 95% CI, 1.2 to 3.0). The mechanism behind this increased risk remains unknown, but preeclampsia alters endothelial function in both mother and her offspring, potentially via alterations in miRNA, aberrant vessel development, and preeclampsia-induced changes in epigenetic gene regulation (64–66).
Although men have higher ischemic stroke rates throughout most of adulthood, ischemic stroke incidence increases in middle-aged women secondary to menopause and the resulting decline in female sex hormones. The onset of early menopause in women (<42 years) is associated with a twofold increased risk for ischemic stroke, even after adjustment for age and other cardiovascular risk factors (67, 68, 71). Several randomized clinical trials were designed to examine whether HRT could reduce the incidence or severity of ischemic stroke in postmenopausal women. Unfortunately, two of these trials found no benefit of HRT after menopause on ischemic stroke rates (72, 73). Two additional trials found that postmenopausal women receiving HRT were at increased risk for ischemic stroke and experienced more severe strokes compared with women in the placebo arm (74, 75).
One potential reason for the paradoxical failure of these HRT clinical trials was the timing of hormone replacement—many patients did not begin receiving HRT until well after the onset of menopause (76). Subsequently, the Early Versus Late Intervention Trial with Estradiol trial was designed to examine the importance of timing in HRT therapy by comparing outcomes in women receiving early HRT (initiated <6 years from the onset of menopause) with those among women receiving late HRT (initiated ≥10 years from menopause onset) (77). Although the follow-up period is still insufficient to assess the effects of HRT timing on stroke risk, early results from the 5-year follow-up study indicate that early, but not late, HRT significantly reduced the progression of cardiovascular disease (77). In support of this, a recent experimental study found that the timing of HRT in a mouse model of stroke significantly affected stroke outcome; aged female mice receiving early 17β-estradiol replacement had significantly improved stroke outcomes compared with females that received delayed HRT (78). These results demonstrate that patient age at menopause and the timing of HRT replacement therapy after menopause may play a critical role in ischemic stroke risk and the efficacy of HRT replacement.
In addition to sex-specific risk factors, sexual dimorphisms exist in shared ischemic stroke risk factors (79). Increased ischemic stroke incidence in the aging population is largely driven by an increased prevalence of these risk factors, including atrial fibrillation, obesity, type 2 diabetes, hypertension, hypercholesterolemia, and coronary artery disease (39). Interestingly, certain common risk factors are more likely to give rise to the different major subtypes of ischemic stroke (large-vessel disease, small-vessel disease, and cardioembolic stroke). Small- and large-vessel strokes result from narrowing or thrombus formation within the cerebral arteries themselves, whereas cardioembolic strokes result from clots formed in the heart (80). As a result, clots of cardioembolic origin tend to occlude the larger cerebral arteries, resulting in greater tissue damage and poorer outcomes (29).
Atrial fibrillation (AF) is a major risk factor for ischemic stroke, accounting for 45% of all cardioembolic-subtype strokes (79). Furthermore, age is a major risk factor for AF, and the stroke risk conferred by AF increases with age (81, 82). The prevalence of AF and cardioembolic ischemic stroke are significantly higher in women than men, likely driven in part by the fact that women tend to be older at the time of stroke onset (83–87). Having AF confers a greater stroke risk in women than in men, and women with AF tend to experience more severe strokes than their male counterparts (88, 89). Because cardioembolic strokes have been linked to larger strokes, higher mortality, and poorer functional outcomes, the increased incidence of AF and cardioembolic strokes in women may contribute to sex differences in mortality and functional outcome after ischemic stroke, particularly in elderly populations.
In recent years, studies have reported a link between ischemic stroke incidence and a common constellation of risk factors known as the metabolic syndrome (90–92). Clinical metabolic syndrome is characterized by the presence of hyperglycemia, central adiposity, hypertension, increased triglyceride levels, and low high-density lipoprotein cholesterol (93). A large meta-analysis recently found that the magnitude of stroke risk associated with metabolic syndrome is significantly higher in female patients than male patients (92). Although men tend to have a more heterogeneous combination of metabolic syndrome risk factors, the risk factor profile of women with metabolic syndrome tends to be dominated by abdominal obesity, as defined by waist circumference ≥88 cm (men) or ≥102 cm (women) (94). Abdominal obesity is a combined measure of both increased subcutaneous fat as well as increased visceral (intra-abdominal) fat. Rates of overall abdominal obesity, particularly visceral adiposity, increase significantly around the time of menopause (95–100). Interestingly, whereas women tend to have lower visceral fat mass and higher subcutaneous fat mass than men in younger groups, levels of visceral adiposity in women increase to equalize with those of men in older populations (100, 101)
Several studies suggest that overall abdominal obesity confers greater stroke risk in female patients compared with male patients (16, 102, 103). Recent work has shown that adipose-resident CD8 T cells, which contribute to adipose tissue inflammation and obesity-induced insulin resistance, are more prevalent and exhibit more activated proinflammatory phenotypes in middle-aged women than in men (104–106). Loss of estrogens in middle-aged women and underlying chromosomal sex (XX) are both believed to drive this increase in abdominal adiposity, adipose inflammation, and insulin resistance (107, 108). Furthermore, increased abdominal obesity in women at menopause has also been linked to an elevated risk for insulin resistance and type 2 diabetes (109).
Diabetes is also a strong risk factor for stroke incidence, recurrence, and stroke-related mortality (110–114). Importantly, a recent large-scale meta-analysis demonstrated that type 2 diabetes confers greater risk for stroke in women (relative risk, 2.28; 95% CI, 1.93 to 2.69) than in men (relative risk, 1.83; 95% CI, 1.60 to 2.08) (115). The risk for fatal stroke is higher in female diabetic patients than in male patients, and a recent study reported that diabetic women are at significantly higher risk for 5-year mortality after stroke than nondiabetic women, a risk that relationship that was not seen in men (116, 117). Overall, diabetes represents a stronger risk factor for stroke in women than in men, and female diabetic patients have poorer outcomes after stroke than their male counterparts. The driving factors behind this sex disparity remain largely unknown, although both epidemiological (delayed diagnosis, undertreatment) and biological (exacerbated endothelial dysfunction, elevated proinflammatory signaling) mechanisms have been suggested (116).
In conclusion, age and sex have an interactive effect on ischemic stroke risk, incidence, and outcome. Studying the mechanisms and consequences of this interaction are critical to the development of safe and effective treatments for stroke patients of both sexes. In the next section, we discuss the current experimental evidence regarding the effect of sex and age on ischemic stroke pathophysiology and therapeutic response.
Age and Sex Differences in the Pathophysiology of Experimental Ischemic Stroke
With the exception of thrombolytic drugs, no pharmacologic therapies have been approved for ischemic stroke (118). Although countless compounds have shown promise in preclinical studies, these protective effects have failed to translate into clinical efficacy for patients (119). This failure may be due, in part, to the use of young male animals in most preclinical studies (119, 120). Although studies comparing ischemic stroke in male and female animals are limited, it is clear that sex-specific responses to cerebral ischemia can be replicated in vivo and in vitro. Furthermore, these results highlight the critical importance of considering age and sex into account when determining therapeutic efficacy in both preclinical studies and clinical trials.
A wealth of evidence has shown that estrogens play protective roles throughout many tissues, including the brain, adipose tissue, heart, and vasculature (121, 122). In the context of ischemic stroke, estrogens are highly neuroprotective at multiple levels, including suppression of stroke risk factor pathology via antiatherogenic effects in the vasculature and the regulation of adipogenesis. Estrogens also ameliorate stroke pathology itself via vasodilation of the coronary arteries and direct neuroprotection of brain and glial cells during ischemia (123). Preclinical studies have also indicated a role for progestins (specifically progesterone) in neuroprotection after ischemic stroke, as reviewed comprehensively elsewhere (124, 125). Interestingly, stroke incidence and severity are higher in males before the onset of puberty, indicating that biological (chromosomal) sex may also be an important determinant of ischemic stroke risk and pathophysiology (126, 127). In this section, we highlight basic science findings that indicate a role for both hormonal and chromosomal sex in the biology of ischemic stroke.
Evidence for sex and age differences in experimental models of ischemic stroke
In vitro stroke modeling is performed by using oxygen glucose deprivation (OGD), in which neuronal, glial, or mixed cells are cultured without glucose in deoxygenated conditions, mimicking the acute energy failure after loss of cerebral blood flow (128). OGD causes greater damage to male-derived neurons, endothelial cells, and astrocytes compared with those from females (129–132). Small in vivo rodent models are also used to model ischemic stroke and to test potential therapeutic strategies. Studies of sex differences in experimental rodent models show that female animals are protected against cerebral ischemia compared with males (133). Importantly, this advantage disappears after ovariectomy, indicating that estrogens are critical to the ischemic resistance seen in females (134–137).
As in clinical ischemic stroke, sexual dimorphism in ischemic stroke is highly dependent on age. In middle-aged mice (age 16 months), the ischemic protection phenotype actually reverses, resulting in larger infarcts and poorer outcomes in female animals compared with males (138). Because this age coincides with the onset of reproductive senescence in mice, increased ischemic sensitivity in middle-aged females may be driven by the recent loss of estrogens, mirroring the effects seen in ovariectomized young females (133, 134). Supporting this theory, and echoing results in patients, early replacement of 17β-estradiol in middle-aged animals has been found to reduce stroke size in mice of both sexes (138). In elderly male and female mice, which have similar sex hormone levels, stroke size equalizes (139). These results show that sex and age also have an interactive effect on experimental stroke pathophysiology, in addition to their influence on clinical stroke.
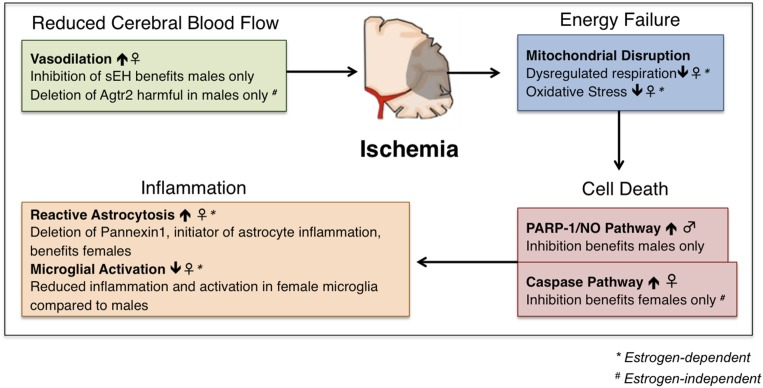
During ischemic stroke, loss of blood flow to the brain results in two stages of damage. The primary stage is characterized by mitochondrial disruption, energy failure, and ionic imbalances, causing rapid and irreversible cell death (140, 141). Neuronal and glial cell death results in the extracellular release of excitatory neurotransmitters, reactive species, and inflammatory factors that contribute to secondary damage pathways, including excitatory neurotoxicity, oxidative damage, and inflammation (142–144). A schematic overview of the current knowledge regarding sex differences in experimental stroke pathophysiology and treatment efficacy is given in Fig. 2.
Figure 2.
Schematic representation of sex differences in stroke pathology and therapeutic efficacy. Sex differences in mitochondrial disruption exist; experimental stroke models in both mice and rats have shown that female animals experience less oxidative stress and dysregulated respiration after ischemia than males, an advantage that is eliminated by ovariectomy or aging. Sex differences in vasodilation have also been described, in which experimental mouse models have shown that females experience enhanced vasodilation at baseline compared with males, and male animals show benefit only when vasodilation is enhanced [inhibition of soluble epoxide hydrolase (sEH), selection of angiotensin II type 2 receptor (Agtr2)]. Studies in mice have shown enhanced reactive astrocytosis in females after ischemic, which can be ameliorated by the deletion of the proinflammatory protein Pannexin 1. Conversely, female mice show reduced microglial activation compared with male animals in experimental mouse models of ischemic stroke. In vivo and in vitro studies in mice and mouse-derived neuronal cells have demonstrated that the PARP-1/nitric oxide (NO) pathway of cell death predominates in males, whereas the caspase pathway of cell death is dominant in females. In line with this, inhibition of each of these pathways benefits only males, or only females, respectively.
Sex differences in neuronal dysfunction and cell death
Primary ischemic damage includes the disruption of neural cell mitochondria, which are critical to maintaining cellular energy and regulating oxidative stress (141). Both estrogens and progestins enhance mitochondrial respiration and augment antioxidative processes in neurons (145). Female animals have more efficient oxidative respiration than males overall, even after ischemic stroke (146, 147). Ovariectomy in females impairs mitochondrial respiration and antioxidant capabilities, which can be reversed by the addition of exogenous 17β-estradiol (146, 148–153).
Mitochondrial disruption and energy failure will ultimately result in the activation of cell death pathways. Striking differences exist in ischemic-induced cell death between males and females. Male cell death is dominated by the formation of nitric oxide and activation of poly-ADP ribose polymerase 1 (PARP-1), causing mitochondrial depolarization and cell death (154). Inhibition of PARP-1 and nitric oxide in experimental ischemic stroke is protective only in male animals, with no benefit to females (11, 133). Conversely, caspase-inhibiting therapies are preferentially protective in female animals after experimental stroke, as cell death in females largely depends on ischemia-induced activation of caspases (155–157). This sexual dimorphism in cell death pathways persists in females after ovariectomy, indicating that sex hormones are not solely responsible for the sexual dimorphism seen in cell death (155). Chromosomal sex may contribute to this sex difference, as female neurons (XX chromosomes) exhibit greater activation of the caspase pathway compared with male neurons (XY) after OGD in culture (131).
Sex differences in endothelial and glial responses to ischemia
In addition to findings in neuronal cells, sexually dimorphic responses are also seen in other central nervous system cell populations after ischemia, including cerebral endothelial cells, astrocytes, and microglia.
Endothelial cells are major regulators of cerebral blood flow and play a critical role in ischemic injury. Ischemic protection in young females is mediated in part via sex differences in endothelial cell function. As in neurons, endothelial cells derived from male rodent brains exhibit greater sensitivity to OGD than female endothelial cells (132). Males have higher levels of brain soluble epoxide hydrolase (sEH), a suppressor of vasodilation, compared with females (158). Pharmacologic inhibition of sEH reduces ischemic sensitivity in male endothelial cells, and targeted in vivo deletion of sEH improves cerebral blood flow and infarct size in male animals only, with no effects in females (132, 158). Deletion of another vasodilatory mediator, angiotensin II type 2 receptor (AGTR2), exacerbates infarct size and worsens cerebral blood flow in male mice, but not in females (159). Ovariectomy did not worsen female outcomes, suggesting that the sex difference in AGTR2, which is expressed on the X chromosome, may be mediated by chromosomal sex differences (159).
Astrocytes are critical to the health of the cerebrovascular system, assisting in restoration of the blood-brain barrier and scar formation after brain injury (160). Female astrocytes are resistant to OGD-induced ischemic damage, a protective effect that depends on estrogens (161). Sex differences have also been described in glial fibrillary acidic protein, a protein critical to scar tissue formation that is highly expressed in astrocytes (160). After ischemia, female mice have an augmented upregulation of astrocytic glial fibrillary acidic protein compared with that in males (162). Knockout or inhibition of another astrocytic protein known as Pannexin 1, an initiator of astrocytic inflammation, is uniquely protective in middle-aged female animals after ischemic stroke, with no effect in males (163).
Cerebral ischemia also induces massive activation of microglia, the resident immune cell of the central nervous system (164, 165). Immune activation after ischemic is critical to both secondary tissue damage and tissue repair and regeneration (142). Sex differences in ischemia-induced inflammation have been reported at both the hormonal and the chromosomal level (78, 166–168). Early in life, at the neonatal and young adult stages, male animals have higher ischemia-induced microglial activation than do female animals (169, 170). This anti-inflammatory phenotype in females may be driven by female sex hormones, as in vitro studies show that estrogens and progestins can suppress the release of inflammatory mediators in microglia and reduce neuronal cell death (171–173).
Conclusion
Sex and age have a complex and substantial influence on ischemic stroke risk, outcome, and pathology. The high burden of stroke in men throughout early life reverses in middle-age, with increased stroke risk, mortality, and poor functional outcome seen in elderly women. Clear sex differences exist in stroke risk factor profiles, particularly in the incidence and risk conferred by atrial fibrillation and the metabolic syndrome. Although many of these differences are mediated by age-related changes in sex hormones, evidence from experimental studies suggests that chromosomal sex also affects ischemic stroke pathophysiology. Most importantly, this review highlights the critical importance of considering sex and age as biological variables. This can be achieved by (1) designing future clinical research to ensure that studies are adequately powered to assess the effects of sex and age on therapeutic outcome and (2) the inclusion of young and aged animals of both sexes in studies of ischemic stroke pathophysiology and in studies using novel therapeutics.
Acknowledgments
Financial Support: This work is supported by National Institute of Neurological Diseases and Stroke (NINDS) Grant F30NS098628 (to M.R.-O.) and NINDS Grants R01NS055215 and R37 NSO96493 (to L.D.M.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AF
atrial fibrillation
- Agtr2
angiotensin II type 2 receptor
- HR
hazard ratio
- HRT
hormone replacement therapy
- OCP
oral contraceptive pill
- OGD
oxygen glucose deprivation
- PARP-1
poly-ADP ribose polymerase 1
- sEH
soluble epoxide hydrolase
- SPRINT
Systolic Blood Pressure Improvement Trail
References
- 1. Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2017 update: a report from the American Heart Association [published correction appears in Circulation. 2017;135(10):e646 and 2017;136(10):e196] Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arboix A, García-Eroles L, Massons J, Oliveres M, Targa C. Acute stroke in very old people: clinical features and predictors of in-hospital mortality. J Am Geriatr Soc. 2000;48(1):36–41. [DOI] [PubMed] [Google Scholar]
- 4. Dennis MS, Burn JP, Sandercock PA, Bamford JM, Wade DT, Warlow CP. Long-term survival after first-ever stroke: the Oxfordshire Community Stroke Project. Stroke. 1993;24(6):796–800. [DOI] [PubMed] [Google Scholar]
- 5. Di Carlo A, Lamassa M, Pracucci G, Basile AM, Trefoloni G, Vanni P, Wolfe CD, Tilling K, Ebrahim S, Inzitari D; European BIOMED Study of Stroke Care Group . Stroke in the very old : clinical presentation and determinants of 3-month functional outcome: a European perspective. Stroke. 1999;30(11):2313–2319. [DOI] [PubMed] [Google Scholar]
- 6. Kammersgaard LP, Jørgensen HS, Reith J, Nakayama H, Pedersen PM, Olsen TS; Copenhagen Stroke Study . Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing. 2004;33(2):149–154. [DOI] [PubMed] [Google Scholar]
- 7. Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Comparison of stroke features and disability in daily life in patients with ischemic stroke aged 55 to 70 and 71 to 85 years. Stroke. 1997;28(4):729–735. [DOI] [PubMed] [Google Scholar]
- 8. Rojas JI, Zurrú MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old--risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. Eur J Neurol. 2007;14(8):895–899. [DOI] [PubMed] [Google Scholar]
- 9. Appelros P, Nydevik I, Viitanen M. Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke. 2003;34(1):122–126. [DOI] [PubMed] [Google Scholar]
- 10. Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, Rezaei Y. An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand. 2015;131(1):45–50. [DOI] [PubMed] [Google Scholar]
- 11. Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29(4):670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenger NK, Ferdinand KC, Bairey Merz CN, Walsh MN, Gulati M, Pepine CJ; American College of Cardiology Cardiovascular Disease in Women Committee . Women, hypertension, and the Systolic Blood Pressure Intervention Trial. Am J Med. 2016;129(10):1030–1036. [DOI] [PubMed] [Google Scholar]
- 13. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension. 2016;68(6):1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2(2):e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69(20):1898–1904. [DOI] [PubMed] [Google Scholar]
- 17. Grysiewicz RA, Thomas K, Pandey DK. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26(4):871–895, vii. [DOI] [PubMed] [Google Scholar]
- 18. Heron M. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1–96. [PubMed] [Google Scholar]
- 19. Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac D, Sturm J, Heeley E, Otahal P, Konstantinos V, Anderson C, Parmar P, Krishnamurthi R, Barker-Collo S, Feigin V, Bejot Y, Cabral NL, Carolei A, Sacco S, Chausson N, Olindo S, Rothwell P, Silva C, Correia M, Magalhães R, Appelros P, Kõrv J, Vibo R, Minelli C, Gall S. Sex differences in long-term mortality after stroke in the INSTRUCT (INternational STRoke oUtComes sTudy): a meta-analysis of individual participant data. Circ Cardiovasc Qual Outcomes. 2017;10(2):10. [DOI] [PubMed] [Google Scholar]
- 20. Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4(7):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gall SL, Donnan G, Dewey HM, Macdonell R, Sturm J, Gilligan A, Srikanth V, Thrift AG. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology. 2010;74(12):975–981. [DOI] [PubMed] [Google Scholar]
- 22. Reid JM, Dai D, Gubitz GJ, Kapral MK, Christian C, Phillips SJ. Gender differences in stroke examined in a 10-year cohort of patients admitted to a Canadian teaching hospital. Stroke. 2008;39(4):1090–1095. [DOI] [PubMed] [Google Scholar]
- 23. Renoux C, Coulombe J, Li L, Ganesh A, Silver L, Rothwell PM; Oxford Vascular Study . Confounding by pre-morbid functional status in studies of apparent sex differences in severity and outcome of stroke. Stroke. 2017;48(10):2731–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazure CM, Jones DP. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health. 2015;15(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, Olson D, Peterson E. Sex differences in quality of life after ischemic stroke. Neurology. 2014;82(11):922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D; European BIOMED Study of Stroke Care Group . Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34(5):1114–1119. [DOI] [PubMed] [Google Scholar]
- 27. Gargano JW, Reeves MJ; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators . Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke. 2007;38(9):2541–2548. [DOI] [PubMed] [Google Scholar]
- 28. Glader EL, Stegmayr B, Norrving B, Terént A, Hulter-Asberg K, Wester PO, Asplund K; Riks-Stroke Collaboration . Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34(8):1970–1975. [DOI] [PubMed] [Google Scholar]
- 29. Gray LJ, Sprigg N, Bath PM, Boysen G, De Deyn PP, Leys D, O’Neill D, Ringelstein EB; TAIST Investigators . Sex differences in quality of life in stroke survivors: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST). Stroke. 2007;38(11):2960–2964. [DOI] [PubMed] [Google Scholar]
- 30. Sturm JW, Donnan GA, Dewey HM, Macdonell RA, Gilligan AK, Srikanth V, Thrift AG. Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2004;35(10):2340–2345. [DOI] [PubMed] [Google Scholar]
- 31. Willers C, Lekander I, Ekstrand E, Lilja M, Pessah-Rasmussen H, Sunnerhagen KS, von Euler M. Sex as predictor for achieved health outcomes and received care in ischemic stroke and intracerebral hemorrhage: a register-based study. Biol Sex Differ. 2018;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. [DOI] [PubMed] [Google Scholar]
- 33. Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. Am J Psychiatry. 1993;150(1):124–129. [DOI] [PubMed] [Google Scholar]
- 34. Schubert DS, Taylor C, Lee S, Mentari A, Tamaklo W. Physical consequences of depression in the stroke patient. Gen Hosp Psychiatry. 1992;14(1):69–76. [DOI] [PubMed] [Google Scholar]
- 35. Poynter B, Shuman M, Diaz-Granados N, Kapral M, Grace SL, Stewart DE. Sex differences in the prevalence of post-stroke depression: a systematic review. Psychosomatics. 2009;50(6):563–569. [DOI] [PubMed] [Google Scholar]
- 36. Gall S, Phan H, Madsen TE, Reeves M, Rist P, Jimenez M, Lichtman J, Dong L, Lisabeth LD. Focused update of sex differences in patient reported outcome measures after stroke. Stroke. 2018;49(3):531–535. [DOI] [PubMed] [Google Scholar]
- 37. Berglund A, Schenck-Gustafsson K, von Euler M. Sex differences in the presentation of stroke. Maturitas. 2017;99:47–50. [DOI] [PubMed] [Google Scholar]
- 38. Falsetti L, Viticchi G, Buratti L, Balucani C, Marra AM, Silvestrini M. From head to toe: Sex and gender differences in the treatment of ischemic cerebral disease. Pharmacol Res. 2017;121:240–250. [DOI] [PubMed] [Google Scholar]
- 39. Sacco RL. Risk factors and outcomes for ischemic stroke. Neurology. 1995;45(2Suppl 1)S10–S14. [PubMed] [Google Scholar]
- 40. Gattringer T, Ferrari J, Knoflach M, Seyfang L, Horner S, Niederkorn K, Culea V, Beitzke M, Lang W, Enzinger C, Fazekas F. Sex-related differences of acute stroke unit care: results from the Austrian stroke unit registry. Stroke. 2014;45(6):1632–1638. [DOI] [PubMed] [Google Scholar]
- 41. Roy-O’Reilly M, McCullough LD. Sex differences in stroke: the contribution of coagulation. Exp Neurol. 2014;259:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sacco S, Merki-Feld GS, Ægidius KL, Bitzer J, Canonico M, Kurth T, Lampl C, Lidegaard Ø, Anne MacGregor E, MaassenVanDenBrink A, Mitsikostas DD, Nappi RE, Ntaios G, Sandset PM, Martelletti P; European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC) . Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. 2017;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sheikh HU, Pavlovic J, Loder E, Burch R. Risk of stroke associated with use of estrogen containing contraceptives in women with migraine: a systematic review. Headache. 2018;58(1):5–21. [DOI] [PubMed] [Google Scholar]
- 44. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284(1):72–78. [DOI] [PubMed] [Google Scholar]
- 45. Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MS. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370(14):1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scott CA, Bewley S, Rudd A, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence, risk factors, management, and outcomes of stroke in pregnancy. Obstet Gynecol. 2012;120(2 Pt 1):318–324. [DOI] [PubMed] [Google Scholar]
- 47. Miller EC, Gallo M, Kulick ER, Friedman AM, Elkind MSV, Boehme AK. Infections and risk of peripartum stroke during delivery admissions. Stroke. 2018;49(5):1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Elkind MS, Willey JZ. Risk of pregnancy-associated stroke across age groups in New York State. JAMA Neurol. 2016;73(12):1461–1467. [DOI] [PubMed] [Google Scholar]
- 49. James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106(3):509–516. [DOI] [PubMed] [Google Scholar]
- 50. McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018;49(3):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lamminpää R, Vehviläinen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997-2008. BMC Pregnancy Childbirth. 2012;12(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jahromi BN, Husseini Z. Pregnancy outcome at maternal age 40 and older. Taiwan J Obstet Gynecol. 2008;47(3):318–321. [DOI] [PubMed] [Google Scholar]
- 53. Ngowa JD, Ngassam AN, Dohbit JS, Nzedjom C, Kasia JM. Pregnancy outcome at advanced maternal age in a group of African women in two teaching hospitals in Yaounde, Cameroon. Pan Afr Med J. 2013;14:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ogawa K, Urayama KY, Tanigaki S, Sago H, Sato S, Saito S, Morisaki N. Association between very advanced maternal age and adverse pregnancy outcomes: a cross sectional Japanese study. BMC Pregnancy Childbirth. 2017;17(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol. 2001;97(4):533–538. [DOI] [PubMed] [Google Scholar]
- 56. Sidorov EV, Feng W, Caplan LR. Stroke in pregnant and postpartum women. Expert Rev Cardiovasc Ther. 2011;9(9):1235–1247. [DOI] [PubMed] [Google Scholar]
- 57. Aukes AM, De Groot JC, Wiegman MJ, Aarnoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG. 2012;119(9):1117–1122. [DOI] [PubMed] [Google Scholar]
- 58. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, Mamas MA. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):10. [DOI] [PubMed] [Google Scholar]
- 59. Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–e1561. [DOI] [PubMed] [Google Scholar]
- 60. Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension. 2011;58(1):63–69. [DOI] [PubMed] [Google Scholar]
- 61. Lawlor DA, Macdonald-Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, Sattar N, Deanfield J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33(3):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mamun AA, Kinarivala MK, O’Callaghan M, Williams G, Najman J, Callaway L. Does hypertensive disorder of pregnancy predict offspring blood pressure at 21 years? Evidence from a birth cohort study. J Hum Hypertens. 2012;26(5):288–294. [DOI] [PubMed] [Google Scholar]
- 63. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–1180. [DOI] [PubMed] [Google Scholar]
- 64. Brodowski L, Burlakov J, Hass S, von Kaisenberg C, von Versen-Höynck F. Impaired functional capacity of fetal endothelial cells in preeclampsia. PLoS One. 2017;12(5):e0178340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martin E, Ray PD, Smeester L, Grace MR, Boggess K, Fry RC. Epigenetics and preeclampsia: defining functional epimutations in the preeclamptic placenta related to the TGF-β pathway. PLoS One. 2015;10(10):e0141294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou C, Zou QY, Li H, Wang RF, Liu AX, Magness RR, Zheng J. Preeclampsia downregulates microRNAs in fetal endothelial cells: roles of miR-29a/c-3p in endothelial function. J Clin Endocrinol Metab. 2017;102(9):3470–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17(3):506–510. [DOI] [PubMed] [Google Scholar]
- 68. Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750. [DOI] [PubMed] [Google Scholar]
- 69. Bushnell C, McCullough L. Stroke prevention in women: synopsis of the 2014 American Heart Association/American Stroke Association guideline. Ann Intern Med. 2014;160(12):853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kemmeren JM, Tanis BC, van den Bosch MA, Bollen EL, Helmerhorst FM, van der Graaf Y, Rosendaal FR, Algra A. Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) study: oral contraceptives and the risk of ischemic stroke. Stroke. 2002;33(5):1202–1208. [DOI] [PubMed] [Google Scholar]
- 71. Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40(4):1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N; HERS Research Group . Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288(1):49–57. [DOI] [PubMed] [Google Scholar]
- 73. Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, Barrett-Connor E, Hulley SB. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen-progestin Replacement Study (HERS). Circulation. 2001;103(5):638–642. [DOI] [PubMed] [Google Scholar]
- 74. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 75. Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249. [DOI] [PubMed] [Google Scholar]
- 76. Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD Jr. Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19(3):272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N, Stanczyk FZ, Selzer RH, Azen SP; ELITE Research Group . Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int. 2012;61(8):1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Piña IL, Reeves MJ, Rexrode KM, Saposnik G, Singh V, Towfighi A, Vaccarino V, Walters MR; American Heart Association Stroke Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council for High Blood Pressure Research . Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2014 May;45(5):e95 and 2014 Oct;45(10);e214] Stroke. 2014;45(5):1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31(5):1062–1068. [DOI] [PubMed] [Google Scholar]
- 81. Holmegard HN, Nordestgaard BG, Jensen GB, Tybjærg-Hansen A, Benn M. Sex hormones and ischemic stroke: a prospective cohort study and meta-analyses. J Clin Endocrinol Metab. 2016;101(1):69–78. [DOI] [PubMed] [Google Scholar]
- 82. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92(1):17–40, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev. 2010;6(3):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Arboix A, Oliveres M, García-Eroles L, Maragall C, Massons J, Targa C. Acute cerebrovascular disease in women. Eur Neurol. 2001;45(4):199–205. [DOI] [PubMed] [Google Scholar]
- 85. Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32(12):2735–2740. [DOI] [PubMed] [Google Scholar]
- 86. Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34(7):1581–1585. [DOI] [PubMed] [Google Scholar]
- 87. Stuart-Shor EM, Wellenius GA, DelloIacono DM, Mittleman MA. Gender differences in presenting and prodromal stroke symptoms. Stroke. 2009;40(4):1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344(May 30 3):e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lang C, Seyfang L, Ferrari J, Gattringer T, Greisenegger S, Willeit K, Toell T, Krebs S, Brainin M, Kiechl S, Willeit J, Lang W, Knoflach M; Austrian Stroke Registry Collaborators . Do women with atrial fibrillation experience more severe strokes? Results From the Austrian Stroke Unit Registry. Stroke. 2017;48(3):778–780. [DOI] [PubMed] [Google Scholar]
- 90. Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MV, Wright C, Giardina EG, DiTullio MR, Homma S, Paik MC. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39(1):30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chei CL, Yamagishi K, Tanigawa T, Kitamura A, Imano H, Kiyama M, Sato S, Iso H. Metabolic syndrome and the risk of ischemic heart disease and stroke among middle-aged Japanese. Hypertens Res. 2008;31(10):1887–1894. [DOI] [PubMed] [Google Scholar]
- 92. Li X, Li X, Lin H, Fu X, Lin W, Li M, Zeng X, Gao Q. Metabolic syndrome and stroke: A meta-analysis of prospective cohort studies. J Clin Neurosci. 2017;40:34–38. [DOI] [PubMed] [Google Scholar]
- 93. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association;World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 94. Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care. 2010;33(11):2457–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Davis SR, Castelo-Branco C, Chedraui P, Lumsden MA, Nappi RE, Shah D, Villaseca P; Writing Group of the International Menopause Society for World Menopause Day 2012 . Understanding weight gain at menopause. Climacteric. 2012;15(5):419–429. [DOI] [PubMed] [Google Scholar]
- 96. Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27(10):2444–2449. [DOI] [PubMed] [Google Scholar]
- 97. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–1437. [DOI] [PubMed] [Google Scholar]
- 98. Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, Chandler WL, Boyko EJ, Brunzell JD. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. 2009;94(4):1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, Ravussin E, Ryan DH, Smith SR, Katzmarzyk PT. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity (Silver Spring). 2011;19(2):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rodríguez-Campello A, Jiménez-Conde J, Ois Á, Cuadrado-Godia E, Giralt-Steinhauer E, Vivanco RM, Soriano-Tárraga C, Subirana I, Muñoz D, Gómez-González A, Puig-Pijoan A, Roquer J. Sex-related differences in abdominal obesity impact on ischemic stroke risk. Eur J Neurol. 2017;24(2):397–403. [DOI] [PubMed] [Google Scholar]
- 103. Zahn K, Linseisen J, Heier M, Peters A, Thorand B, Nairz F, Meisinger C. Body fat distribution and risk of incident ischemic stroke in men and women aged 50 to 74 years from the general population. The KORA Augsburg cohort study. PLoS One. 2018;13(2):e0191630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ahnstedt H, Roy-O’Reilly M, Spychala MS, Mobley AS, Bravo-Alegria J, Chauhan A, Aronowski J, Marrelli SP, McCullough LD. Sex differences in adipose tissue CD8+ T cells and regulatory T cells in middle-aged mice. Front Immunol. 2018;9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. [DOI] [PubMed] [Google Scholar]
- 106. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32(3):451–463. [DOI] [PubMed] [Google Scholar]
- 107. Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP, Reue K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Choi EK, Kim WK, Sul OJ, Park YK, Kim ES, Suh JH, Yu R, Choi HS. TNFRSF14 deficiency protects against ovariectomy-induced adipose tissue inflammation. J Endocrinol. 2013;220(1):25–33. [DOI] [PubMed] [Google Scholar]
- 109. Garaulet M, Pérez-Llamas F, Baraza JC, Garcia-Prieto MD, Fardy PS, Tébar FJ, Zamora S. Body fat distribution in pre-and post-menopausal women: metabolic and anthropometric variables. J Nutr Health Aging. 2002;6(2):123–126. [PubMed] [Google Scholar]
- 110. Idris I, Thomson GA, Sharma JC. Diabetes mellitus and stroke. Int J Clin Pract. 2006;60(1):48–56. [DOI] [PubMed] [Google Scholar]
- 111. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998;50(1):208–216. [DOI] [PubMed] [Google Scholar]
- 112. Tuomilehto J, Rastenyte D, Jousilahti P, Sarti C, Vartiainen E. Diabetes mellitus as a risk factor for death from stroke. Prospective study of the middle-aged Finnish population. Stroke. 1996;27(2):210–215. [DOI] [PubMed] [Google Scholar]
- 113. Banerjee C, Moon YP, Paik MC, Rundek T, Mora-McLaughlin C, Vieira JR, Sacco RL, Elkind MS. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2012;43(5):1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Eriksson M, Carlberg B, Eliasson M. The disparity in long-term survival after a first stroke in patients with and without diabetes persists: the Northern Sweden MONICA study. Cerebrovasc Dis. 2012;34(2):153–160. [DOI] [PubMed] [Google Scholar]
- 115. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383(9933):1973–1980. [DOI] [PubMed] [Google Scholar]
- 116. Soriano-Reixach MM, Vivanco-Hidalgo RM, Ois A, Rodríguez-Campello A, Roquer J. Interaction of sex and diabetes on outcome after ischemic stroke. Front Neurol. 2018;9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care. 2004;27(1):201–207. [DOI] [PubMed] [Google Scholar]
- 118. Chacon MR, Jensen MB, Sattin JA, Zivin JA. Neuroprotection in cerebral ischemia: emphasis on the SAINT trial. Curr Cardiol Rep. 2008;10(1):37–42. [DOI] [PubMed] [Google Scholar]
- 119. Sohrabji F, Park MJ, Mahnke AH. Sex differences in stroke therapies. J Neurosci Res. 2017;95(1-2):681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. van der Worp HB, de Haan P, Morrema E, Kalkman CJ. Methodological quality of animal studies on neuroprotection in focal cerebral ischaemia. J Neurol. 2005;252(9):1108–1114. [DOI] [PubMed] [Google Scholar]
- 121. Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72(5):381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Moolman JA. Unravelling the cardioprotective mechanism of action of estrogens. Cardiovasc Res. 2006;69(4):777–780. [DOI] [PubMed] [Google Scholar]
- 123. Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29(2):209–215. [DOI] [PubMed] [Google Scholar]
- 124. Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131(Pt 2):318–328. [DOI] [PubMed] [Google Scholar]
- 125. Wong R, Renton C, Gibson CL, Murphy SJ, Kendall DA, Bath PM; Progesterone Pre-Clinical Stroke Pooling Project Collaboration . Progesterone treatment for experimental stroke: an individual animal meta-analysis. J Cereb Blood Flow Metab. 2013;33(9):1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61(2):189–194. [DOI] [PubMed] [Google Scholar]
- 127. Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G; International Pediatric Stroke Study Group . Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40(1):52–57. [DOI] [PubMed] [Google Scholar]
- 128. Tasca CI, Dal-Cim T, Cimarosti H. In vitro oxygen-glucose deprivation to study ischemic cell death. Methods Mol Biol. 2015;1254:197–210. [DOI] [PubMed] [Google Scholar]
- 129. Fairbanks SL, Young JM, Nelson JW, Davis CM, Koerner IP, Alkayed NJ. Mechanism of the sex difference in neuronal ischemic cell death. Neuroscience. 2012;219:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. J Neurosci Methods. 2008;171(2):214–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sharma J, Nelluru G, Wilson MA, Johnston MV, Hossain MA. Sex-specific activation of cell death signalling pathways in cerebellar granule neurons exposed to oxygen glucose deprivation followed by reoxygenation. ASN Neuro. 2011;3(2):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arterioscler Thromb Vasc Biol. 2012;32(8):1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Herson PS, Palmateer J, Hurn PD. Biological sex and mechanisms of ischemic brain injury. Transl Stroke Res. 2013;4(4):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29(1):159–165, discussion 166. [DOI] [PubMed] [Google Scholar]
- 135. Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. [DOI] [PubMed] [Google Scholar]
- 136. Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4(5):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Carswell HV, Dominiczak AF, Macrae IM. Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2000;278(1):H290–H294. [DOI] [PubMed] [Google Scholar]
- 138. Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol. 2013;249:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J Bioenerg Biomembr. 2015;47(1-2):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gaignard P, Liere P, Thérond P, Schumacher M, Slama A, Guennoun R. Role of sex hormones on brain mitochondrial function, with special reference to aging and neurodegenerative diseases. Front Aging Neurosci. 2017;9:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. [DOI] [PubMed] [Google Scholar]
- 144. Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497–500. [DOI] [PubMed] [Google Scholar]
- 145. Yang SH, Sarkar SN, Liu R, Perez EJ, Wang X, Wen Y, Yan LJ, Simpkins JW. Estrogen receptor beta as a mitochondrial vulnerability factor. J Biol Chem. 2009;284(14):9540–9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gaignard P, Savouroux S, Liere P, Pianos A, Thérond P, Schumacher M, Slama A, Guennoun R. Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology. 2015;156(8):2893–2904. [DOI] [PubMed] [Google Scholar]
- 147. Srinivasan S, Spear J, Chandran K, Joseph J, Kalyanaraman B, Avadhani NG. Oxidative stress induced mitochondrial protein kinase A mediates cytochrome c oxidase dysfunction. PLoS One. 2013;8(10):e77129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Feng Z, Zhang JT. Long-term melatonin or 17beta-estradiol supplementation alleviates oxidative stress in ovariectomized adult rats. Free Radic Biol Med. 2005;39(2):195–204. [DOI] [PubMed] [Google Scholar]
- 149. Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol. 2012;24(1):236–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sandhir R, Sethi N, Aggarwal A, Khera A. Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem Int. 2014;74:16–23. [DOI] [PubMed] [Google Scholar]
- 152. Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial β-amyloid. Neurobiol Aging. 2012;33(8):1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Yao Y, Li H, Gu Y, Davidson NE, Zhou Q. Inhibition of SIRT1 deacetylase suppresses estrogen receptor signaling. Carcinogenesis. 2010;31(3):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30(8):2967–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke. 2011;42(4):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ying W, Xiong ZG. Oxidative stress and NAD+ in ischemic brain injury: current advances and future perspectives. Curr Med Chem. 2010;17(20):2152–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang W, Iliff JJ, Campbell CJ, Wang RK, Hurn PD, Alkayed NJ. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. J Cereb Blood Flow Metab. 2009;29(8):1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Fujita T, Iwai M, Ito M, Horiuchi M. Sex-different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res. 2009;1300:14–23. [DOI] [PubMed] [Google Scholar]
- 160. Magaki SD, Williams CK, Vinters HV. Glial function (and dysfunction) in the normal & ischemic brain. Neuropharmacology. 2018;134(Pt B):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27(1):135–141. [DOI] [PubMed] [Google Scholar]
- 162. Cordeau P Jr, Lalancette-Hébert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke. 2008;39(3):935–942. [DOI] [PubMed] [Google Scholar]
- 163. Freitas-Andrade M, Bechberger JF, MacVicar BA, Viau V, Naus CC. Pannexin1 knockout and blockade reduces ischemic stroke injury in female, but not in male mice. Oncotarget. 2017;8(23):36973–36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. [DOI] [PubMed] [Google Scholar]
- 165. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. [DOI] [PubMed] [Google Scholar]
- 166. Manwani B, Bentivegna K, Benashski SE, Venna VR, Xu Y, Arnold AP, McCullough LD. Sex differences in ischemic stroke sensitivity are influenced by gonadal hormones, not by sex chromosome complement. J Cereb Blood Flow Metab. 2015;35(2):221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. McCullough LD, Mirza MA, Xu Y, Bentivegna K, Steffens EB, Ritzel R, Liu F. Stroke sensitivity in the aged: sex chromosome complement vs. gonadal hormones. Aging (Albany NY). 2016;8(7):1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, Ander BP, Verro P, Patel V, Pevec WC, Hedayati N, Dawson DL, Jauch EC, Pancioli A, Broderick JP, Sharp FR. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke. 2012;43(2):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol. 2015;278:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Mirza MA, Ritzel R, Xu Y, McCullough LD, Liu F. Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. J Neuroinflammation. 2015;12(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–5032. [DOI] [PubMed] [Google Scholar]
- 172. Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111(1-2):77–85. [DOI] [PubMed] [Google Scholar]
- 173. Müller E, Kerschbaum HH. Progesterone and its metabolites 5-dihydroprogesterone and 5-3-tetrahydroprogesterone decrease LPS-induced NO release in the murine microglial cell line, BV-2. Neuroendocrinol Lett. 2006;27(5):675–678. [PubMed] [Google Scholar]