Abstract
Background
Oncology research increasingly involves biospecimen collection and data sharing. Ethical challenges emerge when researchers seek to use archived biospecimens for purposes that were not well defined in the original informed consent document (ICD). We sought to inform ongoing policy debates by assessing patient views on these issues.
Materials and Methods
We administered a cross‐sectional self‐administered survey to patients with cancer at an academic medical center. Survey questions addressed attitudes toward cancer research, willingness to donate biospecimens, expectations regarding use of biospecimens, and preferences regarding specific ethical dilemmas.
Results
Among 240 participants (response rate 69%), virtually all (94%) indicated willingness to donate tissue for research. Most participants (86%) expected that donated tissue would be used for any research deemed scientifically important, and virtually all (94%) expected that the privacy of their health information would be protected. Broad use of stored biospecimens and data sharing with other researchers increased willingness to donate tissue. For three scenarios in which specific consent for proposed biobank research was unclear within the ICD, a majority of patient's favored allowing the research to proceed: 76% to study a different cancer, 88% to study both inherited (germline) and tumor specific (somatic) mutations, and 70% to permit data sharing. A substantial minority believed that research using stored biospecimens should only proceed with specific consent.
Conclusion
When debates arise over appropriate use of archived biospecimens, the interests of the research participants in seeing productive use of their blood or tissue should be considered, in addition to addressing concerns about potential risks and lack of specific consent.
Implications for Practice
This survey evaluated views of patients with cancer regarding the permissible use of stored biospecimens from cancer trials when modern scientific methods are not well described in the original informed consent document. The vast majority of patients support translational research and expect that any biospecimens they donate will be used to advance knowledge. When researchers, policy makers, and those charged with research oversight debate use of stored biospecimens, it is important to recognize that research participants have an interest in productive use of their blood, tissue, or data, in addition to considerations of risks and the adequacy of documented consent.
Keywords: Biobank, Research ethics, Cancer trials, Patient survey, Informed consent
Short abstract
Ethical challenges emerge when researchers seek to use archived biospecimens for purposes that were not well defined in the original informed consent document. This article assesses the patient's perspective on the subject.
Introduction
Translational research, in which blood or tissue from patients (termed “biospecimens”) is used to understand the molecular basis of disease or response to treatment, is an essential component of cancer clinical trials 1. Patients participating in trials are routinely asked to donate biospecimens for purely scientific purposes and often have their biospecimens and data stored for future research. There are now many biobanks built from tissues contributed by participants in completed cancer clinical trials that represent a valuable scientific resource. However, although informed consent is required at the time of donation, both the nature of research with biospecimens and ethical concerns over their use continue to evolve over time. The details of informed consent documents (ICD) signed by patients at the time of donation can fail to describe important aspects of the science or potential risks to participants that would routinely be included in modern ICD, or understandably fail to imagine the science that might be possible in the future. This leads to an ethical dilemma: can identifiable biospecimens donated by cancer clinical trial participants for future use only be used for research within the scope of the original ICD, or, with appropriate scientific and ethical oversight, may they be used for research that was not anticipated when the biospecimens were collected? Which choice is most consistent with the interests and preferences of the patients who donate tissue? Under what conditions could this be considered?
These are not purely academic questions. There are many examples of cancer trials in which patients agreed to donate biospecimens on the basis of ICD that do not cover details of modern cancer research. For example, older consent forms may discuss only the patient's specific cancer type, failing to anticipate the modern understanding of molecular changes that transcend tissues of origin. Some older ICD discuss only tumor specific “somatic” genetics and not inherited “germline” genetics, raising questions about whether researchers can use modern next‐generation sequencing that evaluates all genetic changes. Additionally, older ICD often state that access to participants’ data will be restricted to a specific institution or collaborative group, failing to anticipate the modern world of data sharing.
This study seeks to address pragmatic questions that many investigators and ethical oversight bodies wrestle with daily: under what circumstances, if any, can archived biospecimens be ethically used when the proposed research is outside the scope described in the ICD? Ongoing efforts to prospectively improve ICD are largely silent on the question of how to manage existing biobanks 2, 3, 4. The answers to these questions have implications for how we use the vast collections of stored biospecimens in modern research and how we can best respect the interests of clinical research participants 5.
Although studies have documented strong public support for biobank participation, there is little empirical literature to guide deliberations over use of archived biospecimens when the adequacy of informed consent is uncertain 6, 7, 8. This challenge can be expected to persist as biobanks proliferate and both scientific methods and standards for informed consent evolve 4, 9. No prior empirical work has addressed the views of patients regarding permissibility of biobank research when historical informed consent appears inadequate. We sought to address this gap in the literature and identify factors that impact patient perspectives in this area.
Subjects, Materials, and Methods
We conducted a self‐administered anonymous paper survey among patients receiving care at the Massachusetts General Hospital Cancer Center (MGH) between January and July 2017. Eligible patients were English‐speaking adults presenting for routine cancer care. Patients were offered a paper survey to return in clinic or by mail. The study specific survey was developed by a multidisciplinary team with expertise in oncology, research oversight, bioethics, and patient advocacy. Questions were based on prior literature, focus groups, and expert opinion 10. Survey domains consisted of sociodemographics, clinical characteristics, attitudes toward clinical research, knowledge of genetics, expectations regarding use of biospecimens, and expectations regarding protection of research participants. Three scenarios were presented based on real ethical dilemmas involving proposed use of archived biospecimens in cancer research. The survey included a glossary of terms, piloted and refined based on five patient focus groups, and defined technical issues and terms prior to the scenario questions 10.
We categorized participants’ attitudes toward medical research as an independent variable based on response to four survey questions developed from focus group participant's statements about research (see Fig. 1), including two positive and two negative statements. Participants were characterized as possessing positive views (agreement with both positive statements and rejection of both negative statements), negative views (agreement with both negative statements), or mixed views (any other combination). We also characterized participants’ genetics knowledge using two questions adapted from a study of genetic literacy (“genes are inside of a cell” and “cancer genes cannot change over time,” with response options of “true,” “false,” or “not sure” for each statement), rating participants as more knowledgeable (both questions correct) or less knowledgeable (all other responses) 11.
Figure 1.
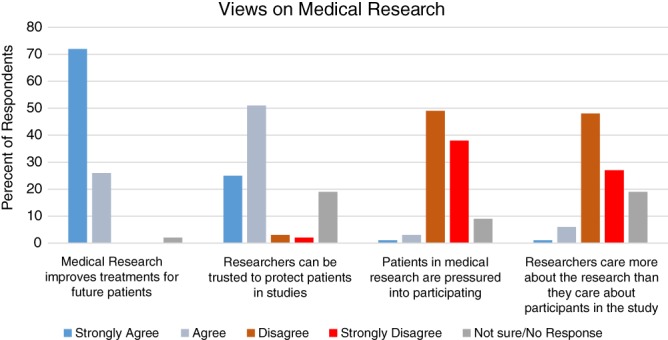
General views of medical research among patients with cancer.
All data were double entered into REDCap, a web‐based, password‐protected electronic database. Frequency distributions summarized descriptive data and predictors of responses were evaluated using Fisher's exact test with a two‐sided significance threshold of p < .05. Logistic regression was used to assess whether univariate associations with p < .20 remained significant after adjustment for other covariates in a multivariate model; however, for all three scenarios, multiple variables were never simultaneously associated with an affirmative scenario response using p < .05; thus only univariate results are presented. The study was approved by the Partners HealthCare institutional review board.
Results
Among 348 patients approached, 240 completed surveys (69% response rate). Demographics are presented in Table 1. Median age of participants was 59 (range, 24–91), 88% were white, 5% were Hispanic, 3% were Asian, 2% were black, 79% were women, and 81% were college educated. Fifty percent of participants reported household annual household income over $100,000, 25% reported $50,000–$100,000, and 15% reported annual income under $50,000. The majority of patients had breast cancer, but 13 cancer types were represented. Thirty percent of participants had metastatic disease, 27% had donated tissue for research, and 37% had participated in some form of clinical research.
Table 1.
Survey respondent characteristics (n = 240)
| Characteristic | n (%) |
|---|---|
| Median age (range), yr | 59 (24–91) |
| Gender | |
| Female | 189 (79) |
| Male | 49 (20) |
| Race and ethnicity | |
| White | 210 (88) |
| Black | 5 (2) |
| Asian | 8 (3) |
| Hispanic | 12 (5) |
| Other | 4 (2) |
| Education | |
| <High school | 44 (18) |
| College | 94 (39) |
| Graduate degree | 100 (42) |
| Household income | |
| <$50,000 | 36 (15) |
| $51–100,000 | 59 (25) |
| >$100,000 | 120 (50) |
| Don't know; no answer | 25 (10) |
| Cancer type | |
| Breast | 160 (67) |
| Lung | 18 (8) |
| GI cancer | 16 (7) |
| GU cancer | 8 (3) |
| Melanoma | 7 (3) |
| Lymphoma | 6 (3) |
| Multiple myeloma | 6 (3) |
| Other | 22 (9) |
| Stage IV, metastatic | 71 (30) |
| Participated in a clinical trial | 73 (30) |
| Donated tissue for research | 65 (27) |
| Willingness to donate tissue for research | 225 (93) |
| Family members with history of cancer | 172 (72) |
| Experience working in medicine or research | 22 (9) |
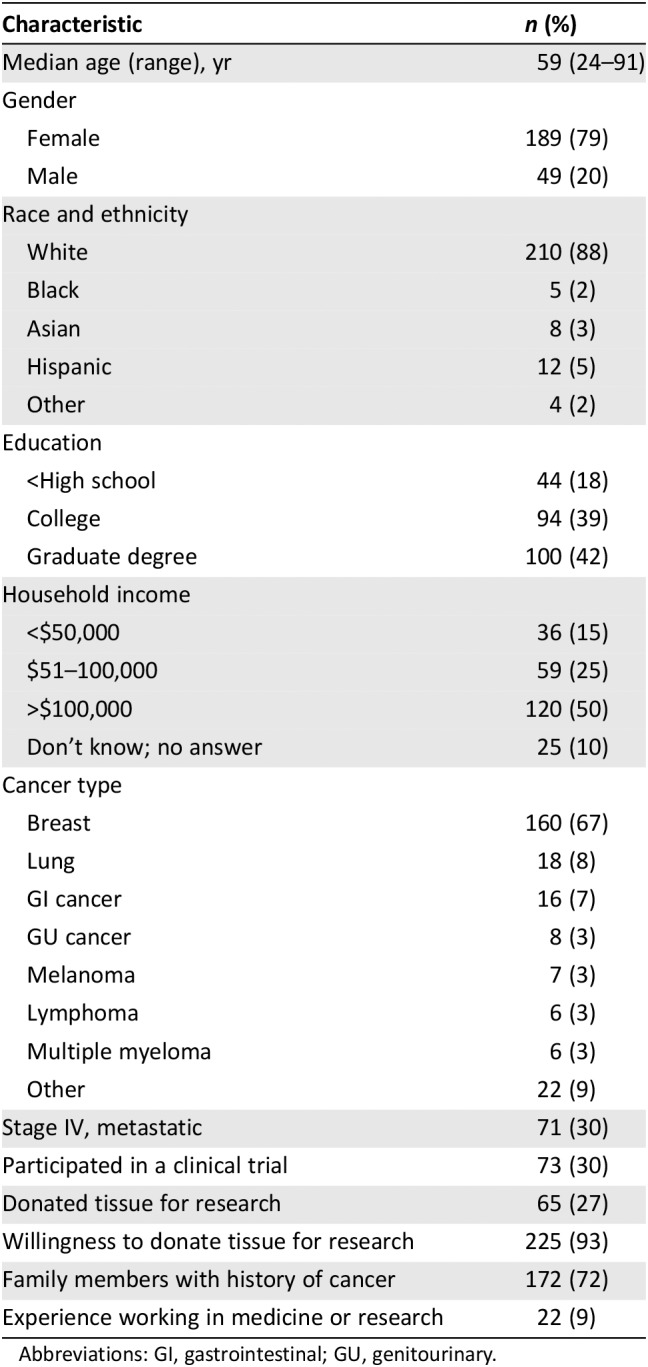
Abbreviations: GI, gastrointestinal; GU, genitourinary.
Attitudes Toward Biomedical Research
Participants expressed generally positive attitudes toward biomedical research. As shown in Figure 1, 98% agreed that “medical research improves treatments for future patients.” Similarly, 76% agreed that “researchers can be trusted to protect patients in studies,” only 5% disagreed, and 19% were unsure. Four percent of respondents agreed that “patients are pressured into participating in research,” and 7% agreed that “researchers care more about the research than they care about study participants.” In total, when characterized as described above, 68% of respondents had positive views of research, 21% held mixed views, and 10% had negative views.
Willingness to Donate and Expectations Regarding Use of Tissue
We asked a series of questions regarding participants’ willingness to donate biospecimens for cancer research and expectations regarding use of their samples if they did donate. Virtually all participants reported willingness to donate blood (94%) or cancer tissue (94%) for research. Most (94%) expected that if they donated blood or tissue, “It will be used to help as many patients as possible.” Furthermore, 86% expected that their biospecimens would be used for “any research viewed as important by researchers, provided there was no risk to participants.” However, 31% also expected that their biospecimens would be used only for research they had specifically approved. Additionally, 94% expected that the privacy of their personal health information would be “carefully protected.” There were mixed expectations regarding data sharing, with 46% expecting that their health information would not be shared with other researchers (30% disagreed; 23% were unsure).
Factors Influencing Willingness to Donate Tissue for Research
To better understand the motivation of potential biospecimen donors, we asked participants how selected factors would impact their willingness to donate tissue for research (Fig. 2). The majority of respondents indicated that they would be more likely to donate if their tissue was used for broad purposes, supporting use for other cancers (78%) and other diseases (70%). Genetics research was viewed positively by most respondents, with 70% reporting increased willingness to donate.
Figure 2.
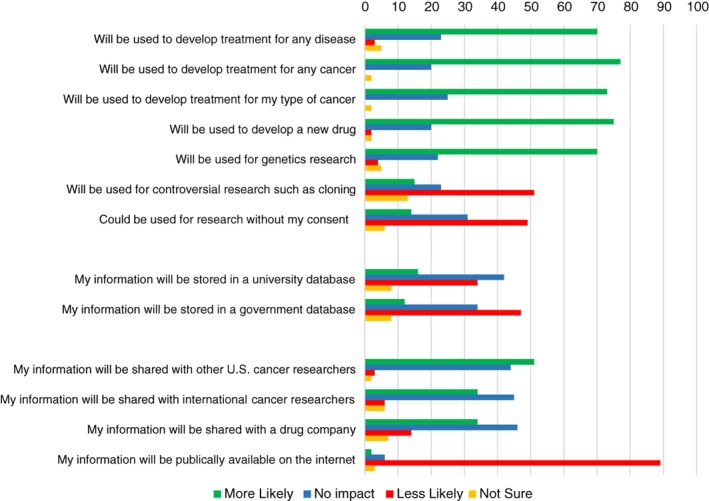
Factors impacting willingness to donate tissue for research.
Potential use of their tissue without any consent (as might be done with deidentified residual tissue from surgery) had a negative impact on willingness to donate among 49% of patients; 31% reported no impact. Potential use of tissue for “controversial research such as cloning” (specified as controversial in the survey) negatively impacted willingness to donate for 49% of respondents, had no impact for 23%, and had a positive impact for 15%. Participants generally favored data sharing with both U.S. and international cancer researchers, with the prospect of data sharing making them more likely to donate tissue. Despite support for data sharing, some privacy concerns did negatively impact willingness to donate. Data storage within a government database (as opposed to a university research database) decreased willingness to participate among 47% of respondents, and, testing what we viewed as an extreme, 89% reported that availability of their health information on the internet would make them less likely to donate. With regard to views on governance of biobanks, 35% of participants reported that community involvement in decisions over appropriate use of their tissue would increase their willingness to donate, whereas 49% reported no impact and 8% viewed this negatively.
Responses to Specific Ethical Dilemmas Regarding Use of Stored Biospecimens
The survey presented three scenarios, based on the types of real cases that regularly confront investigators and those involved in research oversight. In each case, permissibility of research with archived biospecimens was questioned based on a potential conflict between research activities and the ICD (Fig. 3). All scenarios involved stored tissue collected from breast cancer trial participants who could not be contacted for reconsent.
Figure 3.
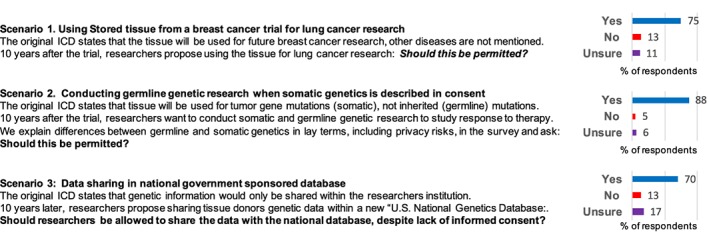
Patient views on use archived biospecimens when proposed research conflicts with the consent document.
Abbreviation: ICD, informed concent document.
Scenario 1: Use of Breast Cancer Tissue for Lung Cancer Research
The first scenario involved the question of using tissue for lung cancer research when the trial ICD stated that tissue would be used for future breast cancer research. Despite failure of the ICD to mention other types of cancer research, most participants (76%) supported allowing use for lung cancer research to proceed, 13% were opposed, and 11% were unsure.
To better understand the basis for responses to this scenario, we asked the more general question of whether stored tissue should only be used for research specifically described in the ICD. Overall, 36% agreed with this statement, 51% disagreed, and 13% were unsure. Support for specific consent was shared by virtually all (97%) who opposed permitting lung cancer research in this scenario. However, it is notable that 23% of participants who endorsed specific consent as a general principle still approved of the lung cancer research proposed in this scenario. This was particularly salient given that the majority of participants had breast cancer and thus did not feel scientific use should be restricted to their type of cancer if deemed useful for another disease, regardless of limitations in the ICD. In univariate analysis, responses to this scenario were not associated with gender, race and ethnicity, education, income, disease stage, or experience with research. However, younger patients (<50) were somewhat more likely to support use of biospecimens from the breast cancer trials for lung cancer research than older patients (89% vs 73%, p = .02).
Scenario 2: Use of Tissue for Germline Genetic Research Without Specific Consent
The second scenario involved a proposal to use a new scientific technique (next‐generation sequencing) that would study both inherited genetic differences between patients (germline mutations) and noninherited genetic differences in tumors (somatic mutations). Participants were informed that the ICD stated that only somatic mutations would be studied and did not discuss risks of germline research. The scenario text also explained that the novel research technique did add risks to privacy for participants and their family members. As above, a glossary provided at the front of the survey and just prior this scenario defined germline and somatic mutations as well as genetics and genes in lay terms.
Despite lack of documented consent for germline research based on this ICD, the vast majority of participants (88%) favored allowing next‐generation sequencing that included investigation of germline mutations to proceed. In univariate analysis, participants characterized as “more knowledgeable” versus “less knowledgeable” about genetics appeared at least as likely to support allowing germline research to proceed (93% vs 84%, p = .08). As with the first scenario, younger participants were more likely to support the proposed research than older participants (98% vs. 87%, p = .03), although it was supported by a sizeable majority among both groups. Other sociodemographic and clinical characteristics of participants were not associated with responses.
Scenario 3: Data Sharing in a National Government‐Sponsored Database
The final scenario involved the question of whether genetic information from donated tissue could be shared with international researchers within a new “National Genetics Database” despite a statement in the ICD that stored data would only be shared with researchers within the cancer center. Although by smaller margins than for the prior scenarios, the majority of respondents (70%) favored sharing stored genetic data despite the limitation described in the ICD. Interestingly, participants with a medical or research background appeared somewhat less likely to support data sharing in this scenario (55% vs. 75%, p = .07). Otherwise, no sociodemographic or clinical characteristics were associated with response to this scenario in univariate analysis. Respondents with a positive view of research were more likely to support data sharing (76% vs. 57%, p = .02).
One of the issues often cited as an ethical concern related to genetics research is the theoretical impossibility of completely deidentifying genetic data 12, 13. We sought to understand if concerns over limits of deidentification of genetic data affected participants’ views, but only 25% reported this issue as a concern. Among those concerned with this risk, 42% still favored allowing data sharing, compared with 86% who were not concerned (p < .0001).
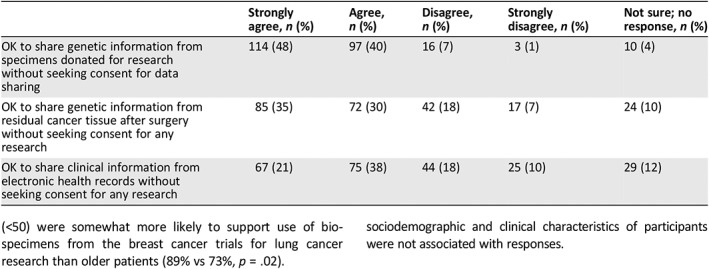
To better understand attitudes toward privacy and data sharing, we asked participants if they would be comfortable having their personal data shared under several conditions relevant to research today. As shown in Table 2, participants were most comfortable with sharing data derived from biospecimens collected with informed consent (88%) versus sharing data derived from residual blood or tissue from clinical procedures without research consent (65%) versus sharing clinical information from an electronic health record without research consent (59%).
Table 2.
Support for sharing data for research without specific consent among patients with cancer
| Strongly agree, n (%) | Agree, n (%) | Disagree, n (%) | Strongly disagree, n (%) | Not sure; no response, n (%) | |
|---|---|---|---|---|---|
| OK to share genetic information from specimens donated for research without seeking consent for data sharing | 114 (48) | 97 (40) | 16 (7) | 3 (1) | 10 (4) |
| OK to share genetic information from residual cancer tissue after surgery without seeking consent for any research | 85 (35) | 72 (30) | 42 (18) | 17 (7) | 24 (10) |
| OK to share clinical information from electronic health records without seeking consent for any research | 67 (21) | 75 (38) | 44 (18) | 25 (10) | 29 (12) |
Discussion
This study was motivated by real scenarios in which the permissibility of using archived biospecimens based on details of the ICD was in question. On the surface, such cases seem to balance potential for scientific progress against our obligation to respect the autonomy of research participants as expressed through informed consent. However, as is well documented, the informed consent process is an imperfect expression of both understanding and intentions of research participants 14, 15, 16. It is therefore not clear that when historical ICDs fail to address a modern scientific proposal that the ethical default position should be to prohibit research with stored biospecimens. There is a need to consider the magnitude of potential scientific benefits, the potential risks of the research, and the intentions and interests of those who donated the blood or tissue. This study highlights the potential for donors to have an interest in seeing their blood or tissue used productively to advance science.
Participants demonstrated very high levels of support for translational research (94% willing to donate tissue), and the vast majority (86%) expected that if they donated, their tissue or data would be used to address any important scientific question. The fact that 31% also expected to be asked to consent to such research could be taken as a contradictory response. However, in light of the responses to specific scenario questions (with 65% of this group supporting the lung cancer research, 88% germline genetics research, and 57% supporting data sharing, respectively) this may be an indication that although many patients expect to provide broad consent upfront or that they will be recontacted and reconsented as needed, a majority may still support use of biospecimens for research when consent is uncertain. These data suggest that investigators should seek broad consent and plan for recontact at the time of tissue collection when possible 4.
In total, for each of the three scenarios we proposed, a sizeable majority of respondents favored proceeding with biospecimen research despite the fact that some aspect of the study was not described in the ICD used at the time of tissue collection. A minority (less than 15% in all cases) took an opposing view. How investigators and those charged with research oversight should respond to this important but minority view remains an open question. This study demonstrates that issues of respecting the interests and intentions of participants and sustaining trust in science cut both ways. The questions on factors that impact willingness to donate as related to questions of broad versus narrow use, governance, and data storage can all be taken as a proxy for support for research and trust in science. For many patients, willingness to participate, and perhaps trust in science (not directly addressed), seems to increase if broad questions are pursued, regardless of specific consent. We do not suggest that these data demonstrate that the concerns of the minority should be overlooked, but rather that the specific factors of each case must be considered, with appreciation of the interests of research participants on both sides of the debate. Table 3 lists ethical considerations and key questions that can guide deliberations over use of archived biospecimens on a case‐by‐case basis.
Table 3.
Ethical considerations for use of archived biospecimens without specific consent
| Ethical issues | Key questions |
|---|---|
| Scientific value | Is the proposed research feasible? Is it important? What is its potential impact? |
| Risks to participants | What is the likelihood and nature of the harm to biospecimen donors if the research is approved? |
| Risks to family and groups | In cases involving germline genetic research or data sharing, is there potential for harm to the participant's family or racial or ethnic group? |
| Potential conflict with nonwelfare interests of participants | Is the proposed research on a subject that could raise religious, political, or cultural objections among participants? |
| Language in the ICD regarding the research in question | To what extent does the ICD directly prohibit the research practice in question? Is the proposed research prohibited or simply omitted? |
| Language in the ICD regarding future research | If the ICD does not directly address the proposed research, is broad use and/or data sharing otherwise stated or implied? |
| Potential to recontact participants | Can biospecimen donors be recontacted to seek consent for the research? |
| Unique Biobank or dataset | How novel and important is the biobank? Can a similar biobank be developed from new donors to support the research question, and what would this require? |
| Potential benefits to participants | Are their anticipated or potential direct benefits to participants if the research proceeds? |
| Potential benefits to relatives and groups | Are their anticipated or potential benefits to relatives of participants or those with shared genetic traits from the research? |
| Distribution of potential benefits from the science | Will results of successful research (new drug or biomarker development) be accessible to members of the participants community? |

Abbreviation: ICD, informed consent document.
These questions should be further evaluated among diverse patient populations and settings 17. Although we achieved a high response rate, suggesting that the results are generalizable within our cancer center, this survey was conducted at a single academic medical center with a predominantly white, affluent, and well‐educated patient population. Although the majority of participants had breast cancer, 80 patients representing 12 additional distinct cancer populations were also included. Furthermore, we did not detect differences in responses based on most sociodemographic or clinical factors, including stage or type of cancer. Younger patients seemed generally more supportive of broad use of biospecimens without specific consent than older patients.
Whereas our work confronts the novel question of what to do with existing biobanks as both scientific techniques and ethical standards evolve, much of the prior work in this area has focused on how to prospectively reduce issues related to future use through improvements in the informed consent process and biobanking governance as new samples are collected 3, 18, 19. This work has informed the revised Common Rule that now includes support for broad generalized consent language 20, 21. Prior work demonstrates strong public support for biobanking and the acceptability of broad generalized consent when tissue is collected 18, 22, 23, 24. A systematic literature review of attitudes toward biobanking among bioethicists, patients, and the general public found that those facing illness are typically more supportive of broad‐based consent for use of their tissue and less concerned with potential risks of reidentification and data sharing compared with other groups 25.
Prior work has also identified two concerns regarding broad consent for biobanking in the general population: commercialization and the nonwelfare interests of participants. With regard to commercialization, multiple studies have demonstrated concerns over commercialization of biospecimens among the public and potential for this to decrease willingness to contribute to biobanks and to erode public support for biobank research 26, 27. The literature demonstrates that disclosure of potential for commercial use and transparency can mitigate these concerns, and public support for commercialization can be fostered by independent governance of biobanks and reinvestment of financial support in future research 27. In our study, participants indicated that use of their biospecimen to develop a new drug or sharing of information with a drug company would generally increase willingness to donate, with less than 20% holding a negative view. These limited data suggest that patients with cancer might be more open to commercialization of their biospecimens then members of the general public.
We did not substantially explore concerns over nonwelfare interests (interests related not to direct benefit or harm but to the religious, political, or cultural views of participants). These issues have been shown to impact willingness to participate in biobanks in the general public 28. Participants in our study supported broad use of their biospecimens for other cancers, other diseases, or using techniques or data sharing not specified in the ICD, but with the exception of one question about “controversial research” and cloning (which most did not support), we did not touch upon issues that might raise nonwelfare concerns. The focus group research that informed the development of this survey offers further exploration of the potential rationale motivating the views expressed in the survey 10.
Our study demonstrates support for biobanking and broad consent among a population with cancer. Previous work focused on questions of how to prospectively plan biobank research in oncology. Braun et al. conducted structured interviews with 30 patients with cancer in Hawaii and found strong interest in donating tissue for biobanking and acceptance of broad consent 29. In a survey involving 224 patients with cancer, Bryant et al. found that 84% were willing to have leftover tissue used for research and majorities favored both broad one‐time consent for future research (71%) and linkage between their biospecimens and clinical data (62%) 30. Similar to our findings, a minority (21%) preferred specific consent for any proposed use of their biospecimens. Pentz et al. explored potential racial and ethnic differences in views of biobanking among 315 patients with cancer and reported that 95% supported biobanking and most favored broad consent without recontact; similarly, the authors found that views did not differ based on race, gender, education, or clinical characteristics 31.
To our knowledge, this is the first empirical study to address patient views on how dilemmas involving novel scientific techniques and questions of adequate informed consent with stored biospecimens should be resolved. Several conceptual papers frame these issues as a conflict between scientific progress and the protection of research participants 32, 33. Others argue that research participants may have an interest in seeing productive scientific use of their tissue, regardless of consent 7, 8, 9. Our study provides empirical evidence documenting the validity of this interest.
Patient preference is not the only factor to consider when determining the permissibility of research with stored biospecimens. Importantly, even in this relatively homogenous sample of predominantly affluent white women with breast cancer, there is diversity of opinion on virtually every topic, including a need for specific consent for each scenario. However, the fact that in all cases, a large majority of participants favored research advancing even without specific consent should give pause to policies that call for blanket restriction of research with stored biospecimens to the scope of older consent forms. The scientific value of some collected samples from prior clinical trials may be difficult to replicate, and it is not clear that we serve the interests of the research participants, trust in science, or any other ethical principle by restricting all research that was not adequately described by modern standards.
Patients signing informed consent are not typically given a wide range of options to indicate their preferences for future use of their blood, tissue, or data. Nor does signing consent imply a clear understanding of the way their biospecimens will or will not be used 34, 35. As demonstrated by Beskow and colleagues, even with extensive efforts to improve disclosure and informed consent, many potential biobank participants lack understanding of future research and potential risks 16. In this context, the data presented here suggest that by restricting research with stored biospecimens we may be violating the interests of many participants, just as we fear we are violating the interests of some if we permit research to move forward without specific consent.
In addition to limitations noted above, we acknowledge that respondents to hypothetical scenarios in a survey are an imperfect proxy for the actual research participants in similar cases. Furthermore, although most participants in this survey favored allowing the proposed research to proceed, this does not diminish the interests of the minority who oppose research without clear and specific consent. This study documented potential risks to trust in science and future participation in research that may arise from either strict or more permissive interpretations of historical ICD for archived biospecimens. There will be a persistent need to consider the specifics of each case as dilemmas over use of biospecimens arise in the future.
Despite this ongoing uncertainty, we believe that these results are helpful to the field on several levels. First, although our focus was previously collected specimens, these results strongly support current efforts to develop improved biobank participation and consent prospectively. Broad consent, as opposed to specific consent, is not only acceptable but preferred by most participants and will mitigate some questions regarding ICD adequacy in the future 25. Our study suggests that explaining potential for broad future use in the consent process is likely to increase willingness to donate tissue. Second, our results indicate a need for caution in assuming that the interests of research participants are best protected by restricting research to areas and methods specified in historical ICD. We do not suggest that when there is a clear contradiction between the consent document and proposed research that this be ignored, but for issues such as the extent of data sharing permitted or changes in scientific technique (such as next‐generation sequencing) that may not be described but introduce minimal additional risk, caution should be taken before assuming that a potentially valuable scientific resource should not be used on ethical grounds 8. We feel that the factors outlined in Table 3 should be considered in future cases to guide case‐by‐case decisions. Finally, given the apparent diversity of patient views, there is a need for increased transparency, community involvement in deliberations, and consideration of documentation and publication of regulatory decisions regarding biobank governance to allow for public comment, consistency, and accountability 8, 27, 36.
Conclusion
Oncology translational research using archived biospecimens relies on a partnership between researchers and patients who donate blood or tissue. Our scientific efforts must have a strong ethical foundation of informed consent and respect for the interests and autonomy of research participants. This includes the participant's interest in maximizing the scientific value of donated tissue.
Author Contributions
Conception/design: Jeffrey Peppercorn, Eric Campbell, Steve Isakoff, Nora K. Horick, Deborah Collyar, Fay Hlubocky, Debra Mathews
Provision of study material or patients: Jeffrey Peppercorn, Steve Isakoff, Lecia V. Sequist, Aditya Bardia
Collection and/or assembly of data: Jeffrey Peppercorn, Nora K. Horick, Julia Rabin, Katharine Quain, Debra Mathews
Data analysis and interpretation: Jeffrey Peppercorn, Eric Campbell, Steve Isakoff, Nora K. Horick, Julia Rabin, Katharine Quain, Fay Hlubocky, Debra Mathews
Manuscript writing: Jeffrey Peppercorn, Eric Campbell, Steve Isakoff, Nora K. Horick, Julia Rabin, Katharine Quain, Lecia V. Sequist, Aditya Bardia, Deborah Collyar, Fay Hlubocky, Debra Mathews
Final approval of manuscript: Jeffrey Peppercorn, Eric Campbell, Steve Isakoff, Nora K. Horick, Julia Rabin, Katharine Quain, Lecia V. Sequist, Aditya Bardia, Deborah Collyar, Fay Hlubocky, Debra Mathews
Disclosures
Lecia V. Sequist: AZ, Merrimack, Genentech, Blueprint Medicines, Janssen (C/A), AZ, Novartis, BI, Genentech, Blueprint Medicines, LOXO, Merrimack (RF), AZ (H); Aditya Bardia: Genentech/Roche, Immunomedics, Novartis, Pfizer, Merck, Radius Health, Spectrum Pharma, Taiho Pharm, Diiachi, Sanofi (C/A), Biotheranostics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was funded by the Greenwall Foundation for Bioethics.
Disclosures of potential conflicts of interest may be found at the end of this article.
Footnotes
For Further Reading: Sonia Yip, Jennifer Fleming, Heather L. Shepherd et al. “As Long as You Ask”: A Qualitative Study of Biobanking Consent—Oncology Patients’ and Health Care Professionals’ Attitudes, Motivations, and Experiences—the B‐PPAE Study. The Oncologist 2019;24:844–856.
Implications for Practice: Patients and health care professionals (HCPs) who experienced cancer biobanking consent were overwhelmingly supportive of biobanking. The motivations and approaches to seeking consent were largely mirrored between the groups. The findings of this study support the opt‐in model of biobanking favored by patients; however, HCPs preferred an opt‐out model. Both groups recognize the importance of making the request for biobanking at an appropriate time, preferably with emotional or family support, and respecting the timing of the request and privacy of the patient. Biobanking success can be promoted by hospital departments with a research focus by identifying an institutional biobanking champion and ensuring local infrastructure is available.
References
- 1. Vaught J, Rogers J, Myers K et al. An NCI perspective on creating sustainable biospecimen resources. J Natl Cancer Inst Monogr 2011;2011:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Beskow LM, Lin L, Dombeck CB et al. Improving biobank consent comprehension: A national randomized survey to assess the effect of a simplified form and review/retest intervention. Genet Med 2017;19:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strech D, Bein S, Brumhard M et al. A template for broad consent in biobank research. Results and explanation of an evidence and consensus‐based development process. Eur J Med Genet 2016;59:295–309. [DOI] [PubMed] [Google Scholar]
- 4. Grady C, Eckstein L, Berkman B et al. Broad consent for research with biological samples: Workshop conclusions. Am J Bioeth 2015;15:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caulfield T, Murdoch B. Genes, cells, and biobanks: Yes, there's still a consent problem. PLoS Biol 2017;15:e2002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bathe OF, McGuire AL. The ethical use of existing samples for genome research. Genet Med 2009;11:712–715. [DOI] [PubMed] [Google Scholar]
- 7. Helgesson G, Dillner J, Carlson J et al. Ethical framework for previously collected biobank samples. Nat Biotechnol 2007;25:973–976. [DOI] [PubMed] [Google Scholar]
- 8. Peppercorn J, Shapira I, Deshields T et al. Ethical aspects of participation in the database of genotypes and phenotypes of the National Center for Biotechnology Information: The Cancer and Leukemia Group B Experience. Cancer 2012;118:5060–5068. [DOI] [PubMed] [Google Scholar]
- 9. Petrini C. “Broad” consent, exceptions to consent and the question of using biological samples for research purposes different from the initial collection purpose. Soc Sci Med 2010;70:217–220. [DOI] [PubMed] [Google Scholar]
- 10. Mathews DJH, Rabin JT, Quain K et al. Secondary use of patient tissue in cancer biobanks; The Oncologist: 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haga SB, Barry WT, Mills R et al. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomarkers 2013;17:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Homer N, Szelinger S, Redman M et al. Resolving individuals contributing trace amounts of DNA to highly complex mixtures using high‐density SNP genotyping microarrays. PLoS Genet 2008;4:e1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jamal L, Sapp JC, Lewis K et al. Research participants' attitudes towards the confidentiality of genomic sequence information. Eur J Hum Genet 2014;22:964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schumacher A, Sikov WM, Quesenberry MI et al. Informed consent in oncology clinical trials: A Brown University Oncology Research Group prospective cross‐sectional pilot study. PLoS One 2017;12:e0172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. del Carmen MG, Joffe S. Informed consent for medical treatment and research: A review. The Oncologist 2005;10:636–641. [DOI] [PubMed] [Google Scholar]
- 16. Beskow LM, Weinfurt KP. Exploring understanding of “understanding”: The paradigm case of biobank consent comprehension. Am J Bioeth 2019;19:6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bussey‐Jones J, Garrett J, Henderson G et al. The role of race and trust in tissue/blood donation for genetic research. Genet Med 2010;12:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joly Y, Dalpé G, So D et al. Fair shares and sharing fairly: A survey of public views on open science, informed consent and participatory research in biobanking. PLoS One 2015;10:e0129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D'Abramo F, Schildmann J, Vollmann J. Research participants’ perceptions and views on consent for biobank research: A review of empirical data and ethical analysis. BMC Med Ethics 2015;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hudson KL, Collins FS. Bringing the common rule into the 21st century. N Engl J Med 2015;373:2293–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subpart A of 45 CFR Part 46: Basic HHS Policy for Protection of Human Subjects. Rockville, MD: Office for Human Research Protections, 2018.
- 22. Valle‐Mansilla JI, Ruiz‐Canela M, Sulmasy DP. Patients’ attitudes to informed consent for genomic research with donated samples. Cancer Invest 2010;28:726–734. [DOI] [PubMed] [Google Scholar]
- 23. Garrison NA, Sathe NA, Antommaria AH et al. A systematic literature review of individuals’ perspectives on broad consent and data sharing in the United States. Genet Med 2016;18:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pulley JM, Brace MM, Bernard GR et al. Attitudes and perceptions of patients towards methods of establishing a DNA biobank. Cell Tissue Bank 2008;9:55–65. [DOI] [PubMed] [Google Scholar]
- 25. Husedzinovic A, Ose D, Schickhardt C et al. Stakeholders’ perspectives on biobank‐based genomic research: Systematic review of the literature. Eur J Hum Genet 2015;23:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicol D, Critchley C, McWhirter R et al. Understanding public reactions to commercialization of biobanks and use of biobank resources. Soc Sci Med 2016;162:79–87. [DOI] [PubMed] [Google Scholar]
- 27. Spector‐Bagdady K, De Vries RG, Gornick MG et al. Encouraging participation and transparency in biobank research. Health Aff (Millwood) 2018;37:1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Vries RG, Tomlinson T, Kim HM et al. The moral concerns of biobank donors: The effect of non‐welfare interests on willingness to donate. Life Sci Soc Policy 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braun KL, Tsark JU, Powers A et al. Cancer patient perceptions about biobanking and preferred timing of consent. Biopreserv Biobank 2014;12:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bryant J, Sanson‐Fisher R, Fradgley E et al. Oncology patients overwhelmingly support tissue banking. BMC Cancer 2015;15:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pentz RD, Billot L, Wendler D. Research on stored biological samples: Views of African American and White American cancer patients. Am J Med Genet A 2006;140:733–739. [DOI] [PubMed] [Google Scholar]
- 32. Caenazzo L, Tozzo P, Pegoraro R. Biobanking research on oncological residual material: A framework between the rights of the individual and the interest of society. BMC Med Ethics 2013;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Doherty KC, Burgess MM, Edwards K et al. From consent to institutions: Designing adaptive governance for genomic biobanks. Soc Sci Med 2011;73:367–374. [DOI] [PubMed] [Google Scholar]
- 34. Joffe S, Cook EF, Cleary PD et al. Quality of informed consent in cancer clinical trials: A cross‐sectional survey. Lancet 2001;358:1772–177. [DOI] [PubMed] [Google Scholar]
- 35. Koyfman SA, Reddy CA, Hizlan S et al; Phase I Informed Consent (POIC) Research Team . Informed consent conversations and documents: A quantitative comparison. Cancer 2016;122:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyhch HF. Opening closed doors: Promoting IRB transparency. J Law Med Ethics 2018;46:145–58. [Google Scholar]


