Abstract
Background
A standard approach to treating resectable esophageal adenocarcinoma is chemoradiotherapy (CRT) followed by surgery; however, recurrence is common. To improve this, we designed a single‐arm, phase II trial that added an epidermal growth factor receptor (EGFR) inhibitor, cetuximab (C), to CRT, with the hypothesis that EGFR inhibition would improve pathologic complete response (pCR) rate.
Materials and Methods
We aimed to increase the pCR rate from 25% to 45%. A Simon two‐stage design (α and β of 0.10) required pCR/enrolled 5/18 for stage 1 and 14/40 total. CRT: oxaliplatin 85 mg/m2 days 1, 15, and 29; infusional 5‐fluorouracil 180 mg/m2/24 hours × 35 days; C 400 mg/m2 day 1 then 250 mg/m2 days 8, 15, 22, and 29 and radiation (intensity modulated radiotherapy [IMRT] allowed) 180 cGy/day × 25 fractions (Monday through Friday). Following esophagectomy, adjuvant chemotherapy (CT): weekly docetaxel 35 mg/m2 and C 250 mg/m2 5 out of 6 weeks for two cycles.
Results
Of 21 eligible patients enrolled, 17 had surgery; 4 died before operation (due to pulmonary embolism 4 days after CRT, G3 diarrhea, progressive disease during CRT, sepsis/hypoxia during CRT, and acute respiratory distress syndrome [ARDS]). pCR = 7/17. Three postoperative deaths due to ARDS resulted in seven total study‐related deaths. Of the 14 remaining patients, 12 started and completed adjuvant CT. Two of seven patients with pCR died, both of ARDS. Out of the 21 eligible subjects in this study, 13 have died and 8 remain alive. The use of IMRT did not correlate with ARDS.
Conclusion
This regimen demonstrated promising activity. Toxicity was significant, with seven study‐related deaths leading to closure after stage 1. All postoperative deaths were due to ARDS. This regimen is not recommended.
Implications for Practice
Esophageal cancer is a disease with a high death rate. The current treatment involves giving chemotherapy plus radiation followed by surgery, but this cures only a quarter of patients. In order to improve survival, better treatments are needed. This trial evaluated the addition of a novel drug, cetuximab, to chemotherapy plus radiation. Unfortunately, the side effects were too great and the study was stopped early.
Keywords: Esophageal adenocarcinoma, Chemoradiotherapy, Cetuximab
Short abstract
The results of a phase II study evaluating the addition of cetuximab to chemotherapy plus radiation in the treatment of esophageal cancer are presented. Although the regimen demonstrated promising activity, the toxicity was significant, leading to early termination of the study.
Introduction
Esophageal adenocarcinoma is an epithelial tumor in which the epidermal growth factor receptor (EGFR) is overexpressed in approximately 30%–70% of cases and for which improved prognostic methods and therapies are needed 1, 2. Although it makes up a small percentage (1.5%) and low incidence (14,000) of total cancer cases in the U.S., mortality remains high 3. Locally advanced, nonmetastatic disease is curable in up to 40%–45% of patients when multimodality therapy is used 4.
Preoperative chemoradiotherapy (CRT) increases survival versus surgery alone in patients with adenocarcinoma. The CALGB 9780 study randomized 56 patients with esophageal or gastroesophageal junction adenocarcinoma to either surgery alone or surgery after CRT with cisplatin and 5‐fluorouracil (5‐FU). Five‐year survival was 39% (95% confidence interval [CI], 21%–57%) versus 16% (95% CI, 5%–33%) in favor of preoperative CRT 5. The more recently reported CROSS trial randomized 400 patients with gastroesophageal cancer (75% adenocarcinoma) to preoperative CRT with carboplatin and paclitaxel versus surgery alone 6. In the long‐term results after a minimum follow‐up of 5 years, survival in the cohort with adenocarcinoma was significantly better in the combined modality arm (43.2 months [24.9–61.4] vs. 27.1 months [13.0–41.2]; hazard ratio [HR], 0.73 [95% CI, 0.55–0.98]; log‐rank p = .038]) 7. However, neither trial included adjuvant chemotherapy or targeted therapy, and overall survival (OS) did not exceed 50%. Additional safe and effective CRT combinations have been studied in phase II trials, in particular one that substitutes oxaliplatin for cisplatin 8.
Another validated approach for treatment of locally advanced, resectable gastric and gastroesophageal junction (GEJ) adenocarcinoma was reported in the FLOT4‐AIO study. These data apply to our study given that 41% of these patients also had GEJ adenocarcinoma. In summary, perioperative treatment with Arbeitsgemeinschaft Internistische Onkologie (AIO) versus epirubicin, cisplatin, 5‐FU (ECF) resulted in a significantly greater fraction of pathologic responders 9, further supporting the benefit of neo‐adjuvant treatment in this population. Differences in treatment compared with this study included the absence of preoperative radiation and much higher fraction of patients receiving adjuvant chemotherapy.
Postoperative chemotherapy is another approach, although infrequently studied, to increase survival versus surgery alone. The phase II E8296 trial evaluated adjuvant cisplatin (75 mg/m2) and paclitaxel (175 mg/m2 over 3 hours) every 3 weeks for four courses in patients with completely resected, node‐positive adenocarcinoma of the esophagus, GEJ, and gastric cardia. After a median follow‐up of 2.9 years (minimum follow‐up of 2 years), the actuarial 2‐year survival rate was 60% 10. The Japan Clinical Oncology Group evaluated postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus, but this was limited to squamous cell cancer 11.
Current knowledge about the molecular mechanisms of cancer‐related pathways involved in cellular signaling, cell cycle regulation, cell death, and angiogenesis is yielding effective therapies directed at specific components of these pathways. The EGFR is a target of several drugs, including the small molecules gefitinib and erlotinib as well as the monoclonal antibodies cetuximab and panitumumab. The EGFR is a prognostic factor and a target for therapy with anti‐EGFR monoclonal antibodies in a number of epithelial malignancies including head and neck squamous cell cancer and colorectal cancer 12, 13. In addition, overexpression of the EGFR in patients with esophageal adenocarcinoma (EAC) treated with preoperative CRT correlates with worse outcome 2.
With the goal of improving efficacy without increasing toxicity in this patient population, we incorporated the anti‐EGFR monoclonal antibody, cetuximab, into preoperative CRT for patients with locally advanced, resectable EAC. An oxaliplatin/5‐fluorouracil chemotherapy backbone was chosen based on the regimen developed by Khushulani and colleagues, which has been further evaluated in more contemporary studies 8, 14. In addition, we evaluated the safety and tolerability of the combination of cetuximab and docetaxel given weekly postoperatively to this group of patients already pretreated with CRT and surgery. Docetaxel was chosen based upon its activity against EAC as well as a potential or inhibiting angiogenesis 15, 16, 17. Secondary objectives also included exploratory studies to determine if this regimen's activity correlates with EGFR‐related genetic and pathway activation markers and circulating endothelial and tumor cells—to be reported elsewhere.
Materials and Methods
Eligibility Criteria
Eligible patients >18 years of age must have had newly diagnosed biopsy‐proven adenocarcinoma of the esophagus stages (American Joint Committee on Cancer 6th edition) T2N0M0, T3N0M0, T1‐3N+M0, or T1‐3N0‐1M1a as determined by imaging studies (positron emission tomography/computed tomography, endoscopic ultrasound) performed less than 4 weeks prior to registration, with no greater than 2 cm extension into the cardia. Patient tumors must be considered surgically resectable (not T4). Patients with a history of a curatively treated malignancy must have been disease‐free for at least 2 years and have a survival prognosis that is greater than 5 years. Performance status by Eastern Cooperative Oncology Group (ECOG) 0–1, normal laboratory and organ functions, and no acute intercurrent illness were required. Women of childbearing potential were excluded.
The trial was reviewed and approved by the institutional review board at each participating institution.
Treatment
Preoperative therapy consisted of the following: oxaliplatin 85 mg/m2 over 120 minutes intravenously (IV) days 1, 15, 29; cetuximab initial dose 400 mg/m2 over 120 minutes day 1 and subsequent doses 250 mg/m2 over 60 minutes IV days 8, 15, 22, 29; and 5‐FU 180 mg/m2 continuous infusion IV over 24 hours days 1–35.
Radiation was as follows: computed tomography‐based planning was required. External beam radiotherapy (RT) with megavoltage linear accelerator was given 5 days per week at 180 cGy per day to a total dose of 45 Gy. All fields were treated each day, and portal films were obtained of at least two fields per week or more often if needed. Treatment was given with a combination of anterior/posterior, posterior oblique, or lateral fields, such that the dose to the target volume met the required uniformity criteria. Parallel opposed oblique fields could not be used for the entire course. If a three‐field technique was used, all three fields were treated daily. The patient treatment position was either supine or prone (which may allow a shift in the esophagus in cases in which sparing of the spinal cord is difficult). Simulation on a diagnostic quality RT simulator or computed tomography simulator was required. In accordance with current guidelines for use of IMRT in clinical trials (see http://www.qarc.org), IMRT was used only if the degree of tumor motion was assessed and could be limited to 1.0 cm. If required to achieve this goal, techniques for managing or suppressing tumor motion were applied.
Surgery occurred between 28 and 56 days after completion of preoperative CRT. Patients underwent a history, physical exam, and computed tomography of the chest and upper abdomen within 2 weeks prior to surgery to rule out evidence of distant disease or unresectability. Choice of type of resection (Ivor‐Lewis, transhiatal, etc.) was left to the operating surgeon. One field lymph node dissection was required. A microscopic proximal or distal margin of <1 mm was considered positive. Proximal and distal margins were at least 2 cm beyond gross tumor as measured in the operating room after removal of the esophagus but prior to fixation of the specimen. Frozen sections were obtained to ensure microscopically negative proximal and distal margins.
Postoperative therapy was administered only to patients who had an R0 or R1 resection. It was started after recovery from surgery (28–56 days) and no later than day 56 postoperatively. Docetaxel 35 mg/m2 was given over 60 minutes IV once per week for 5 weeks out of 6 along with cetuximab initial dose 400 mg/m2 over 120 minutes day 1 and subsequent doses 250 mg/m2 over 60 minutes IV weekly for a total of 11 doses. Two cycles of 6 weeks each were given.
Dose Modifications
All toxicities were graded according to the Common Terminology Criteria for Adverse Events (version 3.0).
If treatment was interrupted for 4 consecutive weeks, protocol treatment was discontinued.
In addition to treatment delays, all drugs could be dose reduced as follows. Oxaliplatin could be dose reduced two levels (65 mg/m2 and 50 mg/m2) based on extent of neurologic, pulmonary, and hematologic events. Fluorouracil could be dose reduced two levels (135 mg/m2 and 90 mg/m2) for mainly gastrointestinal, oral‐mucosal, and palmar‐plantar toxicities. The continuous 5‐FU was not interrupted. Cetuximab could be reduced two levels (200 mg/m2 and 150 mg/m2) for skin and pulmonary toxicities. Infusion reactions were managed per package insert guidelines. Docetaxel could be reduced two levels (28 mg/m2 and 22 mg/m2) mainly for hematologic, neurologic, stomatitis, and elevation of bilirubin toxicities.
There were to be no radiation dose modifications. Radiotherapy could be interrupted for grade >3 radiotherapy‐related toxicity except for grade 3 esophagitis and skin reaction, which were managed with supportive care. Treatment resumed when toxicity resolved to grade 2. For grade 4 toxicity requiring hospitalization (even if unrelated to radiotherapy), the treatment could be interrupted at the discretion of the treating physician. If radiation therapy was interrupted, 5‐FU infusion continued, and if radiation extended past 35 days, the 5‐FU infusion continued until radiation was completed.
Response Criteria
A pathologic complete response (pCR) was the absence of any histopathologic evidence of tumor in the resected esophageal and nodal tissues.
A pathologic incomplete response was defined as the presence of histopathologic evidence of any tumor in the resected esophageal and/or nodal tissues.
Overall survival was measured from the date of registration to the date of death. Patients lost to follow‐up were censored for survival analysis.
Study Design and Statistical Methods
This study had a two‐stage design 18. At the initial stage, 19 patients would be entered, with an assumption that 18 of those patients would be eligible. If at least five responses were observed among the first 18 eligible patients, 23 additional patients would then be entered (with the assumption that 22 would be eligible). Eligible patients would then total to 40. If at least 14 responses were observed among the first 40 eligible patients, the treatment would be considered promising. Estimated accrual was at 30 patients per year.
A complete response rate of 45% or more was defined as evidence of activity. The study had at least 90% power against the null of 25% complete response rate with a one‐sided significance level of 0.10.
Toxicity was a secondary endpoint. The design had a 34% chance of at least one grade 3 or higher toxicity for all 42 patients accrued if the true complication rate was 1%, and an 88% chance of at least one complication if the true complication rate was 5%. With 42 patients total, a 90% confidence interval for the true but unknown rate of complication would be no wider than 27%.
Therapy was to be discontinued for any of the following reasons: treatment interruption for 4 consecutive weeks, extraordinary medical circumstances, progressive disease, disease recurrence, R2 resection, unacceptable toxicity, or patient choice.
All patients (including those who discontinued early from protocol therapy) were followed until progression and/or death, for 2 years from date of registration at every 3 months, and then another 3–5 years at every 6 months.
Data lock occurred November 10, 2011.
Results
This study was activated on June 10, 2008, suspended on April 10, 2009, and terminated on January 8, 2010, with a final accrual of 22 subjects (1 ineligible). The ineligible subject had an initial pathologic diagnosis of esophageal adenocarcinoma; however, after resection, the final pathology was gastric (fundus) adenocarcinoma with superior extension into the cardia.
Patient Characteristics
Table 1 displays patient demographics at baseline of the 21 eligible patients. The median age was 62 years (range 45–79 years). All 21 patients were white, and 19 (90%) were male. Esophagus primary site was distributed as follows: one subject (4.8%) mid‐thoracic, nine (42.9%) lower thoracic, nine (42.9%) GEJ, and two (9.5%) esophagus not otherwise specified.
Table 1.
Patient characteristics (n = 22)
| Characteristics | n (%) |
|---|---|
| Sex | |
| Male | 20 (90.9) |
| Female | 2 (9.1) |
| Age, years | |
| ≤65 | 15 (68.2) |
| >65 | 7 (31.8) |
| Race | |
| White | 22 (100.0) |
| Ethnicity | |
| Missing/unknown | 1 (4.5) |
| Non‐Hispanic | 21 (95.5) |
| PS | |
| 0 | 17 (77.3) |
| 1 | 5 (22.7) |
| Stage | |
| T2N0M0 or T3N0M0 | 6 (27.3) |
| T1‐3N+M0 or T1‐3N0‐1M1A | 16 (72.7) |
| Site | |
| Esophagus | 12 (54.5) |
| GEJ | 10 (45.5) |
| Differentiation | |
| Moderate, grade 2 | 4 (18.2) |
| Poor, grade 3 | 18 (81.8) |
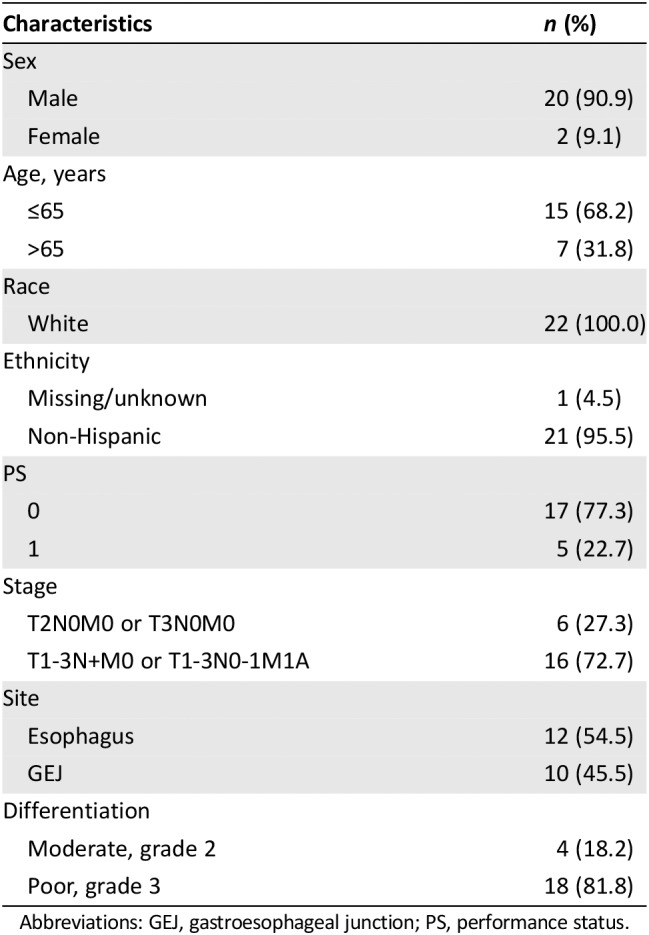
Abbreviations: GEJ, gastroesophageal junction; PS, performance status.
Treatment Received
Preoperative Chemotherapy
All 21 eligible subjects started on preoperative treatment with oxaliplatin, 5‐FU, cetuximab, and radiation; however, 1 patient did not receive oxaliplatin, 5‐FU, or radiation. Multiple subjects had dose modifications or missed doses during the preoperative treatment: oxaliplatin (n = 12), 5‐FU (n = 1), and cetuximab (n = 21). For cetuximab, all subjects experienced a dose modification from day 1 of treatment (mean of 791.4 mg daily) to day 8 (mean of 515.9 mg daily). Reasons for dose modifications included hematologic, diarrhea, hypotension, hypomagnesemia, fatigue, hypotension, and the fatal events listed below.
Four patients did not go to surgery because of cetuximab reaction, death from pulmonary embolism 4 days after CRT, G3 diarrhea during CRT, and death from sepsis/hypoxia during CRT. Two deaths occurred during the preoperative treatment.
Radiation
Twelve patients received conventional (standard radiation), and eight received conformal (IMRT). Median dose was 4,500 cGy. Interruptions to radiation were experienced by eight subjects. There was no correlation between pulmonary toxicity (acute respiratory distress syndrome [ARDS]) and radiation modality. The small numbers of patients with ARDS preclude a robust comparison of this event with radiation dose parameters, but descriptive results do not suggest an association. The median proportion of volume of lung receiving 5 Gy and 20 Gy for those with and without ARDS was 60% (46%–72%) versus 50% (17%–78%), and for volume receiving 20 Gy, the proportion was 17% (17%–19%) versus 11% (5%–30%).
Surgery
Perioperative events that occurred in the 17 patients who underwent resection included pneumonia (two instances), arrhythmia (two instances), and vocal cord palsy (three instances—all three documented by laryngoscopy). In addition, there were three postoperative deaths from ARDS.
During surgery, tumor extent (extent of invasion) was assessed for five different areas: trachea, pericardium, diaphragm, major blood vessel, and pleura/lung. Among the 17 subjects who underwent surgery, none of these areas were involved. All 17 subjects had negative proximal and distal margins. Two had unknown lateral/deep margins, whereas 16 were negative. Adventitial margins were unknown in four and negative in the remainder.
The pathologic completed response rate was 7/17 (41%).patients who underwent surgery and 7/21 (33%) for the intention‐to‐treat population.
Postoperative Chemotherapy
Of the 14 patients remaining alive after surgery, 12 started and completed postoperative treatment. Docetaxel was generally well tolerated, with only a few weekly doses held. For cetuximab, a large adjustment in dosing occurred between weeks 1 and 2, lowering the average dose from 786.92 mg in week 1 to 490.85 mg in week 2. Once the dose stabilized, few more were missed. No subjects reported nonprotocol therapy, in either cycle 1 or cycle 2.
Recurrence and Survival
As of November 2011 analysis of 21 eligible subjects, 9 recurred (of 14 alive after surgery): 5 distant only, 2 local only, and 2 both. Of these nine, six received postoperative treatment. Causes of death were as follows: treatment complications (see toxicity) and disease recurrence and unknown. Out of the 21 eligible subjects in this study, 13 have died and 8 remain alive.
Toxicity
Seven subjects died on treatment, either preoperatively (n = 4) or shortly after surgery (n = 3; Table 2), of ARDS, pulmonary embolism, pneumonia, and sepsis.
Table 2.
Perioperative deaths
| Patient | Cause of death |
|---|---|
| 22001 | Died after resection of ARDS |
| 22005 | Died after resection of ARDS |
| 22009 | Died 4 days after completing CRT of pulmonary embolus |
| 22010 | Died of pneumonia/disease progression preoperatively, after only completing 18 days of CRT |
| 22012 | Died of cardiac arrest 34 days after completion of CRT. Had grade 4 ARDS before death |
| 22013 | Died of ARDS. Did not receive full preoperative chemotherapy but received full‐course RT |
| 22019 | Died prior to completing CRT of sepsis and hypoxia |
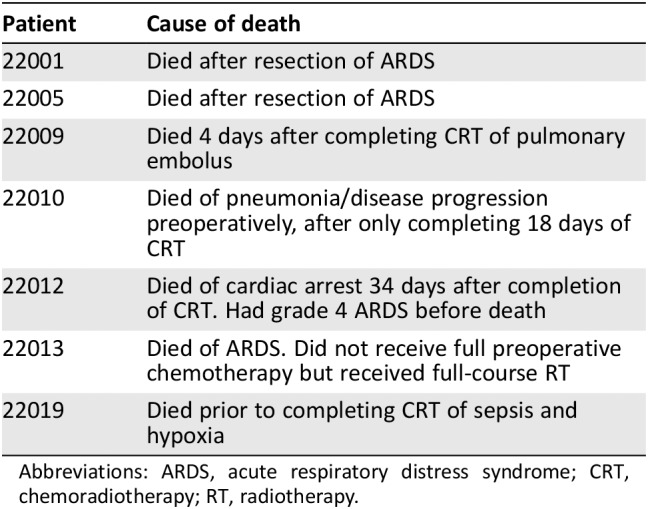
Abbreviations: ARDS, acute respiratory distress syndrome; CRT, chemoradiotherapy; RT, radiotherapy.
A summary of grade 3 or higher treatment‐related adverse events is given in Table 3 (counts, out of all 22 treated patients). Six patients (22001, 22005, 22009, 22012, 22013, and 22019) were reported as having grade 5 events (three ARDS, one infection, one thrombosis/embolism, and one multiorgan failure). Of note, in addition to other reported grade 4 events, there was one ARDS reported (case 22012).
Table 3.
Toxicity summary
| Toxicity type | Treatment arm A (n = 22), n | ||
|---|---|---|---|
| Grade | |||
| 3 | 4 | 5 | |
| Allergic reaction | — | 1 | — |
| Hemoglobin | 4 | — | — |
| Leukocytes | 2 | — | — |
| Lymphopenia | 3 | 2 | — |
| Neutrophils | 1 | — | — |
| Platelets | — | 2 | — |
| Heart block asystole | — | 1 | — |
| Atrial fibrillation | — | 1 | — |
| Cardiac troponin I | 1 | — | — |
| Hypotension | 1 | 1 | — |
| Fatigue | 4 | 1 | — |
| Weight loss | 1 | — | — |
| PTT | 1 | — | — |
| Thrombotic microangiopathy | — | 1 | — |
| Coagulation—other | 1 | 1 | — |
| Alopecia | 1 | — | — |
| Rash/desquamation | 1 | — | — |
| Rash: acne/acneiform | 1 | — | — |
| Radiation dermatitis | 1 | — | — |
| Death—multiorgan failure | — | — | 1 |
| Anorexia | 5 | — | — |
| Constipation | 1 | — | — |
| Dehydration | 8 | — | — |
| Diarrhea without prior colostomy | 4 | — | — |
| Distention/bloating, abdominal | 1 | — | — |
| Dysphagia | 3 | — | — |
| Esophagitis | 5 | — | — |
| Malabsorption | 1 | — | — |
| Mucositis/stomatitis (symptom) esophagus | 3 | — | — |
| Nausea | 5 | — | — |
| Necrosis, small bowel NOS | — | 1 | — |
| Perforation, colon | 1 | — | — |
| Stenosis (including anastomotic) esophagus | 1 | — | — |
| Vomiting | 2 | — | — |
| Surgical hemorrhage | — | 1 | — |
| Infection grade 0–2 neutropenia, brain | — | 1 | — |
| Infection grade 0–2 neutropenia, colon | 1 | — | — |
| Infection grade 0–2 neutropenia, lung | — | 1 | — |
| Infection grade 0–2 neutropenia, small bowel | — | 1 | — |
| Infection with unknown ANC wound | 2 | — | — |
| Infection grade 0–2 neutropenia, blood | — | 1 | 1 |
| Hypoalbuminemia | 3 | — | — |
| Alkaline phosphatase | 1 | — | — |
| Hypocalcemia | 4 | — | — |
| Hyperglycemia | 1 | — | — |
| Hypomagnesemia | — | 1 | — |
| Hypophosphatemia | 1 | — | — |
| Hypokalemia | 2 | — | — |
| Hyponatremia | 2 | — | — |
| Nonneuropathic generalized weakness | 1 | — | — |
| Laryngeal nerve dysfunction | 1 | — | — |
| Neuropathy‐motor | — | 1 | — |
| Depressed level of consciousness | — | 1 | — |
| Syncope | 1 | — | — |
| Abdomen, pain | 1 | — | — |
| Esophagus, pain | 1 | — | — |
| ARDS | — | 1 | 4 |
| Dyspnea | 2 | — | — |
| Hiccoughs | 1 | — | — |
| Hypoxia | — | 4 | — |
| Pleural effusion (nonmalignant) | 1 | 1 | — |
| Pneumonitis/pulmonary infiltrates | 1 | 1 | — |
| Pulmonary/upper respiratory—other | 1 | — | — |
| Vascular access, thrombosis/embolism | — | 1 | — |
| Thrombosis/thrombus/embolism | — | 2 | 1 |
| Worst degree | 7 | 6 | 6 |
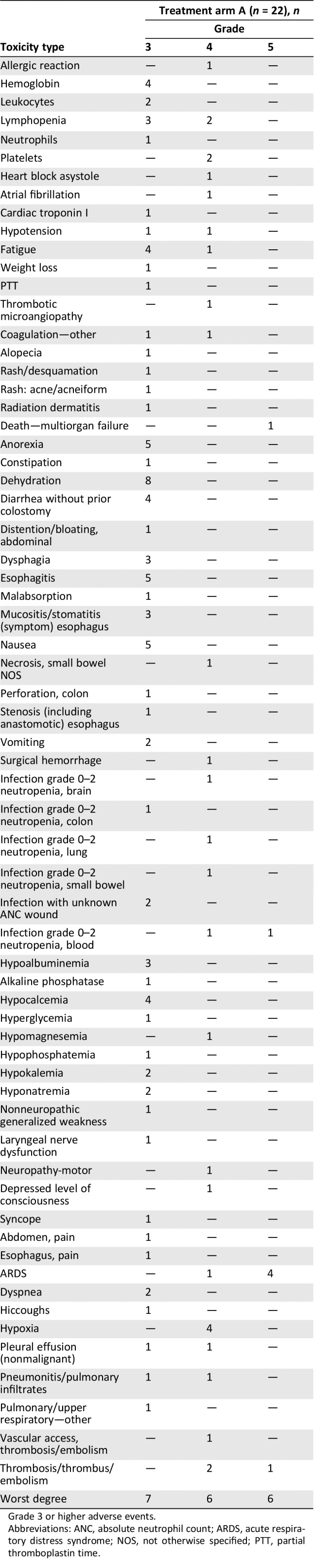
Grade 3 or higher adverse events.
Abbreviations: ANC, absolute neutrophil count; ARDS, acute respiratory distress syndrome; NOS, not otherwise specified; PTT, partial thromboplastin time.
Discussion
We designed this trial in an attempt to improve upon the modest survival results achieved with preoperative CRT using cytotoxic drugs. At the time that this study was designed, the most commonly used drugs were cisplatin and 5‐fluorouracil. Concurrent CRT in the Radiation Therapy Oncology Group (RTOG) 85‐01 19 and Intergroup 0123 trials 20 demonstrated the superiority of the CRT versus surgery alone as definitive therapy for locally advanced disease. The benefit of radiosensitization was studied preoperatively with the goal of downstaging disease, facilitating surgery, and increasing survival. An update of these approaches substituted cisplatin/5‐FU with FOLFOX 8. In this study by Khushulani and colleagues, oxaliplatin with continuous‐infusion 5‐FU achieved similar efficacy with lower toxicity.
In order to improve the survival for patients with resectable esophageal adenocarcinoma, one must improve both local and distant control. To achieve this goal, we designed a single‐arm, phase II study of the addition of cetuximab to preoperative CRT and postoperative chemotherapy in patients with locally advanced, resectable esophageal adenocarcinoma. The primary efficacy endpoint was pathologic response, with a two‐stage design to account for early assessment of safety and toxicity. Although the study nearly met the endpoint of a pCR of 45%, toxicity was the major take‐home message. A total of 9 of the 15 patients who underwent surgery recurred, 4 of these locally. This is in contrast to the experience reported in the SAKK trial, in which there were fewer local recurrences 21.
Unexpectedly, significant pulmonary toxicity occurred. Of 21 patients entered and treated on the study, 7 died during the pre‐ or immediately postoperative period. Of particular concern were the four deaths from ARDS. The study was stopped early because of these grade 5 events, the cause of which remains unknown. Several etiologies have been proposed. One concern was the interaction between IMRT and the preoperative regimen. However, we reviewed the QARQ data extensively and did not find a correlation between patients receiving IMRT (n = 8) and conventional (n = 12) approaches. In addition, in the 86‐patient predecessor trial, ECOG 1201, that gave neoadjuvant chemoradiotherapy along with paclitaxel/cisplatin or irinotecan/cisplatin chemotherapy, no ARDS cases occurred 22. Remarkably, for the 15 patients in E2205 who received radiation that met the dosing criteria of E1201, there were 3 cases of ARDS. With the possibility of radiation delivery anomalies as a causative factor being less likely, we turned to the theory that addition of cetuximab to CRT was the culprit.
Pulmonary toxicity of cetuximab is rare (package insert, 23), occurring in <0.5% of patients treated with cetuximab alone or with chemotherapy. When given concurrently with radiation, in general, there is no increase in pulmonary adverse events, such as pneumonitis or ARDS. The RTOG 0617 trial, for example, evaluated standard‐dose versus high‐dose conformal radiotherapy with concurrent chemotherapy and the addition of cetuximab to concurrent chemoradiation for patients with inoperable stage III non‐small cell lung cancer 24. There was no difference in pulmonary toxicity between arms.
RTOG 0436 used a similar approach for the treatment of locally advanced squamous cell and adenocarcinoma of the esophagus 25. Cisplatin and paclitaxel with or without cetuximab yielded no difference in pulmonary toxicity. The SAKK trial also studied the addition of cetuximab to preoperative CRT (cisplatin and docetaxel). There were no treatment‐related deaths in the cetuximab arm, and OS with cetuximab versus control was 5.1 years (95% CI, 3.7 to not reached) versus 3.0 years (95% CI, 2.2–4.2) for cetuximab and control, respectively (HR, 0.73; 95% CI, 0.52–1.01; p = .055) 21. Other agents used concurrently with CRT and known to cause pulmonary toxicity include gemcitabine, taxanes, anthracyclines, bleomycin, tyrosine kinase inhibitors, and immune checkpoint inhibitors 26.
Regarding the use of epidermal growth factor receptor antagonists in esophageal cancer, many studies have been done but do not show benefit, with the exception of the SAKK trial. RTOG 0436, which was definitive treatment without surgery, was a negative trial for efficacy. In SCOPE1, also a nonsurgical trial, 258 patients were randomized to receive CRT alone or CRT with cetuximab, and the cetuximab arm did worse 27.
In the setting of advanced/metastatic disease, the results are similar. The REAL‐3 trial evaluated the addition of panitumumab to epirubicin, oxaliplatin, and capecitabine for first‐line treatment of esophagogastric cancer and reported no difference in survival 28. The EXPAND trial added cetuximab to cisplatin and capecitabine, with similar results 29. Several studies evaluating the addition of anti‐EGFR tyrosine kinase inhibitors also did not show benefit.
Conclusion
This study evaluated the addition of cetuximab to chemoradiation followed by surgery for locally advanced esophageal adenocarcinoma. It was stopped early because of significant and severe pulmonary toxicity of an unknown cause. Given this toxicity as well as other data showing lack of efficacy of epidermal growth factor receptor inhibition in this disease, cetuximab cannot be recommended in this setting.
Author Contributions
Conception/design: Michael K. Gibson, Paul Catalano, Lawrence R. Kleinberg, Charles A. Staley, III, Al B. Benson, III
Collection and/or assembly of data: Paul Catalano
Data analysis and interpretation: Michael K. Gibson, Paul Catalano, Lawrence R. Kleinberg, Charles A. Staley, III, Elizabeth A. Montgomery, Antonio Jimeno, Wei (Frank) Song, Mary F. Mulcahy, Lawrence P. Leichman, Al B. Benson, III
Manuscript writing: Michael K. Gibson, Paul Catalano, Lawrence R. Kleinberg, Charles A. Staley, III, Elizabeth A. Montgomery, Antonio Jimeno, Wei (Frank) Song, Mary F. Mulcahy, Lawrence P. Leichman, Al B. Benson, III
Final approval of manuscript: Michael K. Gibson, Paul Catalano, Lawrence R. Kleinberg, Charles A. Staley, III, Elizabeth A. Montgomery, Antonio Jimeno, Wei (Frank) Song, Mary F. Mulcahy, Lawrence P. Leichman, Al B. Benson, III
Disclosures
Paul Catalano: Eli Lilly and Company (C/A); Al B. Benson, III: Bristol‐Myers Squibb, Guardant Health, Eli Lilly and Company, Exelixis, Purdue Pharma, inVentive Health Inc., Axio, Genentech, Bayer, Merck, Rafael Pharmaceuticals, Astellas, Terumo, Taiho, Thera Bionic, LSK, Axio (C/A), Acerta, Celegene, Advanced Accelerator Applications, Novartis, Infinity Pharmaceuticals, Merck Sharp & Dohme, Taiho Pharmaceutical, Bristol‐Myers Squibb, Medimmune/AstraZeneca, Xencor, PreECOG, Astellas, Amgen, ECOG‐ACRIN (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was coordinated by the ECOG‐ACRIN Cancer Research Group (Peter J. O'Dwyer, M.D., and Mitchell D. Schnall, M.D., Ph.D., Group Co‐Chairs) and supported in part by Public Health Service Grants CA180794, CA180820, CA180853, CA180844, CA180802, CA180864, CA180834, and CA180888 and by the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute. Research funding and study drugs were also provided by Bristol‐Myers Squibb and Sanofi‐Aventis. Lawrence P. Leichman is currently affiliated with UC San Diego Health ‐ La Jolla, Moores Cancer Center.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. al‐Kasspooles M, Moore JH, Orringer MB et al. Amplification and over‐expression of the EGFR and erbB‐2 genes in human esophageal adenocarcinomas. Int J Cancer 1993;54:213–219. [DOI] [PubMed] [Google Scholar]
- 2. Gibson MK, Abraham SC, Wu TT et al. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res 2003;9:6461–6468. [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–249. [DOI] [PubMed] [Google Scholar]
- 4. Pennathur A, Gibson MK, Jobe BA et al. Oesophageal carcinoma. Lancet 2013;381:400–412. [DOI] [PubMed] [Google Scholar]
- 5. Tepper J, Krasna MJ, Niedzwiecki D et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 7. Shapiro J, van Lanschot JJB, Hulshof M et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long‐term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 8. Khushalani NI, Leichman CG, Proulx G et al. Oxaliplatin in combination with protracted‐infusion fluorouracil and radiation: Report of a clinical trial for patients with esophageal cancer. J Clin Oncol 2002;20:2844–2850. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Batran SE, Hofheinz RD, Pauligk C et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4‐AIO): Results from the phase 2 part of a multicentre, open‐label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697–1708. [DOI] [PubMed] [Google Scholar]
- 10. Armanios M, Xu R, Forastiere A et al. Adjuvant chemotherapy for resected adenocarcinoma of the esophagus, gastro‐esophageal junction, and cardia: Phase II trial (E8296) of the Eastern Cooperative Oncology Group. J Clin Oncol 2004;22:4495–4499. [DOI] [PubMed] [Google Scholar]
- 11. Ando N, Iizuka T, Ide H et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: A Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol 2003;21:4592–4596. [DOI] [PubMed] [Google Scholar]
- 12. Ang KK, Berkey BA, Tu X et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002;62:7350–7356. [PubMed] [Google Scholar]
- 13. Moroni M, Veronese S, Benvenuti S et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: A cohort study. Lancet Oncol 2005;6:279–286. [DOI] [PubMed] [Google Scholar]
- 14. Mukherjee S, Hurt CN, Gwynne S et al. NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre‐operative chemoradiation for resectable oesophageal adenocarcinoma. Eur J Cancer 2017;74:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller KD, Sweeney CJ, Sledge GW Jr. Redefining the target: Chemotherapeutics as antiangiogenics. J Clin Oncol 2001;19:1195–1206. [DOI] [PubMed] [Google Scholar]
- 16. Ajani JA, Moiseyenko VM, Tjulandin S et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: The V‐325 Study Group. J Clin Oncol 2007;25:3205–3209. [DOI] [PubMed] [Google Scholar]
- 17. Tsuburaya A, Yoshida K, Kobayashi M et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S‐1 versus UFT or S‐1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886–893. [DOI] [PubMed] [Google Scholar]
- 18. Simon R. Optimal two‐stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 19. Herskovic A, Martz K, al‐Sarraf M et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–1598. [DOI] [PubMed] [Google Scholar]
- 20. Minsky BD, Pajak TF, Ginsberg RJ et al. INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002;20:1167–1174. [DOI] [PubMed] [Google Scholar]
- 21. Ruhstaller T, Thuss‐Patience P, Hayoz S et al. Neoadjuvant chemotherapy followed by chemoradiation and surgery with and without cetuximab in patients with resectable esophageal cancer: A randomized, open‐label, phase III trial (SAKK 75/08). Ann Oncol 2018;29:1386–1393. [DOI] [PubMed] [Google Scholar]
- 22. Kleinberg LR, Catalano PJ, Forastiere AA et al. Eastern Cooperative Oncology Group and American College of Radiology Imaging Network randomized phase 2 trial of neoadjuvant preoperative paclitaxel/cisplatin/radiation therapy (RT) or irinotecan/cisplatin/RT in esophageal adenocarcinoma: Long‐term outcome and implications for trial design. Int J Radiat Oncol Biol Phys 2016;94:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbitux [package insert]. Branchburg, NJ: ImClone LLC. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125084s0228lbl.pdf. Accessed June 17, 2019.
- 24. Bradley JD, Paulus R, Komaki R et al. Standard‐dose versus high‐dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non‐small‐cell lung cancer (RTOG 0617): A randomised, two‐by‐two factorial phase 3 study. Lancet Oncol 2015;16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suntharalingam M, Winter K, Ilson D et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: The NRG oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol 2017;3:1520–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kroeze SG, Fritz C, Hoyer M et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: A systematic review. Cancer Treat Rev 2017;53:25–37. [DOI] [PubMed] [Google Scholar]
- 27. Crosby T, Hurt CN, Falk S et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised trial. Lancet Oncol 2013;14:627–637. [DOI] [PubMed] [Google Scholar]
- 28. Waddell T, Chau I, Cunningham D et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open‐label phase 3 trial. Lancet Oncol 2013;14:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lordick F, Kang YK, Chung HC et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open‐label phase 3 trial. Lancet Oncol 2013;14:490–499. [DOI] [PubMed] [Google Scholar]


