Abstract
Background
Racial disparities are common in healthcare. Venous thromboembolism (VTE) is a leading cause of preventable harm, and disparities observed in prevention practices. We examined the impact of a patient-centered VTE education bundle on the non-administration of preventive prophylaxis by race.
Methods
A post-hoc, subset analysis (stratified by race) of a larger nonrandomized trial. Pre-post comparisons analysis were conducted on 16 inpatient units; study periods were October 2014 through March 2015 (baseline) and April through December 2015 (post-intervention). Patients on 4 intervention units received the patient-centered, nurse educator-led intervention if the electronic health record alerted a non-administered dose of VTE prophylaxis. Patients on 12 control units received no intervention. We compared the conditional odds of non-administered doses of VTE prophylaxis when patient refusal was a reason for non-administration, stratified by race.
Results
Of 272 patient interventions, 123 (45.2%) were white, 126 (46.3%) were black, and 23 (8.5%) were other races. A significant reduction was observed in the odds of non-administration of prophylaxis on intervention units compared to control units among patients who were black (OR 0.61; 95% CI, 0.46–0.81, p<0.001), white (OR 0.57; 95% CI, 0.44–0.75, p<0.001), and other races (OR 0.50; 95% CI, 0.29–0.88, p = 0.015).
Conclusion
Our finding suggests that the patient education materials, developed collaboratively with a diverse group of patients, improved patient’s understanding and the importance of VTE prevention through prophylaxis. Quality improvement interventions should examine any differential effects by patient characteristics to ensure disparities are addressed and all patients experience the same benefits.
Introduction
Health disparities occur across many dimensions and result from a range of social determinants.[1] Racial disparities are common among hospitalized patients, which limits the impact of quality improvement strategies for all patient populations, potentially resulting in health disparities and undue costs.[2,3] Large nationally representative studies demonstrate differences in health care and outcomes among white, black, and other minority races.[4] Patient information in electronic health record (EHR) systems allows an analysis of health-related outcomes by different socioeconomic strata and racial/ethnic groups.[2,3] Health disparities are multifaceted and may be the result of inequitable (meaning unjust) care, inequalities in socioeconomic, behavioral or other factors. Health equity stands on the principle that each person’s full health potential is not restrained by any form of social injustice or inequality. Conversely, health care inequality connotes variability and disproportionate healthcare access and utilization and stems from the concept that access to healthcare should be the same for everyone.[5,6] A systematic examination of inequalities proposes nine modern theories to explain the differences in health for different groups. [7] Some authors point to specific factors such as institutional or large-scale societal biases (e.g., rural vs. urban location, academic vs. non-academic hospital), and other factors such as provider factors (e.g., age, sex, race/ethnicity), whether explicit or implicit bias, are integral contributors to existing disparities.[1,8]
Venous thromboembolism (VTE) is a leading cause of preventable in-hospital morbidity and mortality.[9,10] Consequently, universal risk assessment and prescription of risk-appropriate prophylaxis for VTE is recommended for all hospitalized patients.[11] Efforts have improved provider compliance with prescribing risk-appropriate VTE prophylaxis and increased patient acceptance of doses.[12,13] Yet some disparities in VTE development are due to the genetic makeup of different races.[4,14–16] However, socioeconomic risk factors also play a role.[17] There are no differences in recommended prophylaxis regimens by race, even though evidence suggests that rates of VTE vary among race/ethnic groups.[18,19] Over a decade, a multidisciplinary VTE Collaborative implemented a variety of interventions to optimize the prescription and delivery of risk-appropriate VTE prophylaxis.[20] They demonstrated that implementation of a computer clinical decision support tool eliminated race and sex-based disparities in VTE prophylaxis prescription,[21,22] and was an unintended consequence, or “halo effect,”[23] of applying quality improvement strategies to optimize care for all patients.
While these interventions improved prescription of risk-appropriate VTE prophylaxis,[21,24,25] they discovered that prescribed doses were not reliably administered to patients,[26,27] contributing to the development of potentially preventable VTE events.[28,29] Furthermore, they discovered that administration of VTE prophylaxis differed significantly by race; the leading cause of non-administration was patient refusal.[27] To reduce the frequency of non-administered doses and potentially address this issue in patients of all races, this group involved a racially diverse group of patients in developing a patient education bundle about VTE and prevention and published the handout in 8 languages.[27,30] The education was designed to improve health literacy as part of a strategy to improve patient-centered care and health promotion.[30] The bundle is coupled with a real-time alert built into the EHR system to identify and target hospitalized patients who miss doses and has been associated with decreases in non-administration.[10] Observation from studies that have looked at the outcomes of medical innovations and inequalities indicates that before the beneficial effects of the implemented change there is usually a disproportionate effect observed in different groups.[31,32] We hypothesized that use of this education bundle would provide equal care to all patients regardless of race, and concomitantly reduce disparities in the administration of VTE prophylaxis. The purpose of this study was to examine the impact of the patient-centered VTE education bundle on the non-administration of preventive prophylaxis by race.
Methods
Study setting and design
This project is a post-hoc, subset analysis (stratified by race) of a larger prospective study that examined the overall impact of the bundle on improving administration of prescribed pharmacologic VTE prophylaxis for hospitalized patients, which has been previously described.[33] The current analysis used a controlled pretest-posttest parallel experimental design to evaluate the impact of the bundle on prophylaxis administration by race; study period was April 1, 2015, to December 31, 2015.[10] The study included 16 adult medical and surgical nursing units, excluding intensive care, at The Johns Hopkins Hospital. Using a convenience sample, we assigned 4 units to receive the intervention: 2 surgical and 2 medical. The remaining 12 units (6 surgical and 7 medical) served as controls. io:dx.doi.org/10.17504/protocols.io.9u3h6yn.
We performed a power calculation and, based on the number of patients and the very large number of doses historically prescribed, found we would have sufficient power for this study.[26] Our blinded biostatistician team (JW, GY) was not involved in the outcome determination and analyses were conducted from June 2016 through November 2017 and followed the TREND guidelines for nonrandomized controlled trials.[34]
Intervention
The intervention included a real-time alert, triggered when a patient missed a dose of their pharmacologic VTE prophylaxis, and patient education. The alert was built into our hospital EHR system and used the unit name field to identify participating units. Our trained health educator carried a pager and the EHR system paged and e-mailed only the educator with the name and unit location of the patient whom missed the prophylaxis dose. The health educator then visited the floor to engage the bedside nurse and determine the cause of the missed dose. If the patient had refused the dose, the patient was provided with the education bundle. If the dose was missed for other reasons, the educator explained the importance of prophylaxis for VTE prevention to the documenting nurse and tried to resolve it. No intervention was provided to patients and nurses on control units. Although the health educator was not consistently present in the hospital, all missed doses required documentation by the bedside nurse as administered or nonadministered, and the reason for nonadministration.
Patient education bundle
Patients could choose to receive one or more components from the patient education bundle. The education bundle included: 1) a one-on-one dialogue with the nurse educator, 2) a 2-page, paper handout (in English or one of seven other languages), and 3) a 10-minute video (bit.ly/bloodclots) viewed on a handheld tablet. We developed this education bundle using a modified Delphi method to build consensus on the content and modes of delivery of VTE prevention information to hospitalized patients.[30] We had input from over 400 stakeholders from three national organizations and our local hospital Patient and Family Advisory Council. A detailed description of the education bundle is published elsewhere.[10,30,35]
Statistical analysis
Our primary outcome of interest was the proportion of doses missed due to patient refusal or for other documented reasons (holds on orders for a procedure, patient away from bed) stratified by racial group. Thus, we reported patient visits (admissions/encounters) rather than unique patients.[10] We compared the change in the rates of VTE prophylaxis administration for all included patient visits before the intervention (October 1, 2014 –March 31, 2015) to after the intervention (April 1 –December 31, 2015).[10] We also hypothesized a differential effect on medicine and surgery units and thus, performed a pre-specified stratified analysis by floor type. Patient-level and nurse-level demographic characteristics for the pre-intervention period were delineated by arm (Table 1). Two-sample t-tests with equal variance were used to compare age. Chi-square tests were used to compare gender, race and floor type. The non-parametric Wilcoxon rank-sum tests were used to compare the number of dosages and length of stay.
Table 1. Demographic characteristics of patient visits by race in the intervention and control groups.
| Intervention pre-period | Intervention post-period | Control pre-period | Control post-period | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | Others | p | White | Black | Others | p | White | Black | Others | p | White | Black | Others | p | |
| Unique Visits | 1,088 | 941 | 193 | 1,533 | 1308 | 270 | 2,850 | 2307 | 500 | 4,432 | 3469 | 761 | ||||
| Unique Patients | 922 | 762 | 170 | 1,279 | 1039 | 221 | 2,428 | 1860 | 431 | 3,669 | 2635 | 648 | ||||
| Unique Nurses | 121 | 100 | 27 | 131 | 114 | 29 | 409 | 268 | 67 | 421 | 335 | 79 | ||||
| Mean Age (SD), yearsa | 56.1 (17.3) | 51.8 (17.4) | 51.7 (16.4) | < .001 | 57.7 (16.6) | 51.8 (18.1) | 50.6 (17.6) | < .001 | 58.5 (16.7) | 54.3 (16.7) | 53.3 (16.7) | < .001 | 58.3 (16.5) | 53.2 (16.8) | 53.5 (17.6) | < .001 |
| Sex, n (%)b | 0.97 | 0.10 | < .001 | < .001 | ||||||||||||
| Male | 526 (48.3%) | 449 (47.8%) | 93 (48.2%) | 738 (89.1%) | 637 (48.7%) | 149 (55.2%) | 1599 (56.1%) | 1083 (46.9%) | 288 (57.6%) | 2438 (55.0%) | 1712 (49.4%) | 435 (57.2%) | ||||
| Female | 562 (51.7%) | 491 (52.2%) | 100 (51.8%) | 795 (51.9%) | 671 (51.3%) | 121 (44.8%) | 1251 (43.9%) | 1224 (53.1%) | 212 (42.4%) | 1994 (45.0%) | 1755 (50.6%) | 328 (42.8%) | ||||
| Floor Type, n (%)b | < .001 | < .001 | < .001 | < .001 | ||||||||||||
| Surgery Floors | 717 (65.9%) | 319 (33.9%) | 119 (61.7%) | 1012 (66.0%) | 482 (36.9%) | 153 (56.7%) | 1732 (60.8%) | 528 (22.9%) | 270 (54.0%) | 2736 (61.7%) | 907 (26.1%) | 433 (56.9%) | ||||
| Medicine Floors | 371 (34.1%) | 622 (66.1%) | 74 (38.3%) | 521 (34.0%) | 826 (63.1%) | 117 (43.3%) | 1118 (39.2%) | 1779 (77.1%) | 230 (46.0%) | 1696 (38.3%) | 2562 (73.9%) | 328 (43.1%) | ||||
| Median Number of Prescribed Doses per Patient visit (Q1, Q3) | 7 (3–14) | 6 (3–11) | 6 (3–14) | < .001 | 8 (4–14) | 6 (3–12) | 6.5 (3–13) | < .001 | 8 (4–15) | 7 (3–13) | 8 (4–14) | < .001 | 8 (4–15) | 7 (3–14) | 8 (4–15) | < .001 |
| Mean (SD)c | 10.5 (11.3) | 8.5 (8.6) | 11.3 (14.0) | 11.2 (11.9) | 9.72 (15.5) | 10.4 (11.0) | 12.0 (14.1) | 10.3 (10.9) | 10.8 (10.9) | 11.7 (12.1) | 10.8 (12.5) | 12.7 (17.9) | ||||
| Median Length of Stay, days (Q1-Q3) | 4 (2–7) | 3 (2–6) | 4 (2–8) | < .001 | 5 (3–8) | 4 (2–7) | 4 (2–7) | < .001 | 5 (2–8) | 4 (2–7) | 5 (2–8) | < .001 | 5 (3–8) | 4 (2–8) | 5 (2–9) | < .001 |
| Mean (SD)c | 6.0 (5.8) | 5.2 (6.4) | 7.6 (11.4) | 6.5 (6.7) | 6.0 (9.3) | 6.3 (7.9) | 7.3 (9.4) | 6.0 (8.0) | 7.2 (8.8) | 7.5 (10.4) | 6.5 (8.7) | 8.3 (12.4) | ||||
a The p values were calculated using two-sample t-tests with equal variances.
b The p values were calculated using chi-square tests.
c The p values were calculated using Wilcoxon rank-sum tests.
For race, specialty, and time comparisons we used generalized linear mixed-effects models to account for correlation within floor and nurse, and multiple outputations to account for multiple VTE doses per patient across nurses or units. This method selected one VTE prophylaxis dose per patient and reiterated the procedure 1000 times to bootstrap the P values and corresponding 95% confidence intervals (CI) for the comparisons.[36]
The models included group (intervention vs. control) and time (pre- vs. post-intervention) stratified by race as the primary predictors. To estimate the conditional odds ratios (OR) and 95% CI, we used the binomial family with the logit link command and the Poisson family with the log link for the conditional proportions. Stratified analyses were performed by unit type (medicine and surgery) using the same models.
All comparisons were performed at < 0.05 level of statistical significance. We performed manual medical record chart review to determine missing patient sex. Statistical analyses were performed using Stata version 14.1 MP—Parallel Edition (College Station, Texas 77845). The primary study “Education Bundle to Decrease Patient Refusal of VTE Prophylaxis” ClinicalTrials.gov NCT02402881.
Study approval
The research application was approved by the Johns Hopkins University Institutional Review Board. The requirement for written informed consent was waived for participants before inclusion in this study. However, participants provided verbal consent to receive the education and the intervention was administered if they were willing to be engaged by the nurse educator. Patient-level data was analyzed after approval by the IRB for its use without consent.
Results
Overall, 19 652 patient visits in which at least 1 dose of VTE prophylaxis medication was prescribed during their patient’s hospitalization were included. Table 1 (and S1 Table) delineate the clinical and demographic characteristics of patient visits by race. By race, the proportion of patients was similar for the intervention and control units and in the pre- and post-intervention periods (Table 1). Mean age was significantly different by arm and intervention period between race categories (p<0.001). Males accounted for a higher proportion of patients on control vs. intervention units. The median number of prescribed VTE doses per hospitalization was significantly different in the pre- and post-intervention periods on both control and intervention units (Table 1).
Intervention delivery
Of 726 patient visits eligible for an intervention, 364 (50.1%) were white, 307 (42.3%) were black, and 55 (7.6%) were other races. Interventions were implemented with 272/726 (37.5%) unique patients (Table 2). The proportion of patients who received the intervention by race were, 123/364 (33.7%) white, 126/307 (41.0%) black, and 23/55 (41.8%) other races. Significantly more non-white patients received an intervention compared to white patients (41.0% vs. 33.7%, p = 0.040, respectively).
Table 2. Proportion of interventions delivered compared by floor type and race.
| Variables | Total | White | Black | Other | Black+ Other | P Valuea |
|---|---|---|---|---|---|---|
| Total eligible for intervention, n | 726 | 364 | 307 | 55 | 362 | 0.040 |
| Total received intervention, n (%) | 272 (37.5%) | 123 (33.7%) | 126 (41.0%) | 23 (41.8%) | 149 (41.0%) | |
| Surgery | ||||||
| Total eligible for intervention, n | 289 | 171 | 97 | 21 | 118 | 0.022 |
| Total received intervention, n(%) | 66 (22.8%) | 31 (18.1%) | 29 (29.9%) | 6 (28.6%) | 35 (30.0%) | |
| Medicine | ||||||
| Total eligible for intervention, n | 437 | 193 | 210 | 34 | 244 | 0.844 |
| Total received intervention, n(%) | 206 (47.1%) | 92 (47.7%) | 97 (46.2%) | 17 (50.0%) | 114 (46.7%) | |
a The p value compares the proportion of interventions completed for eligible white patients versus non-white (black + other) patients
VTE prophylaxis medication administration by race
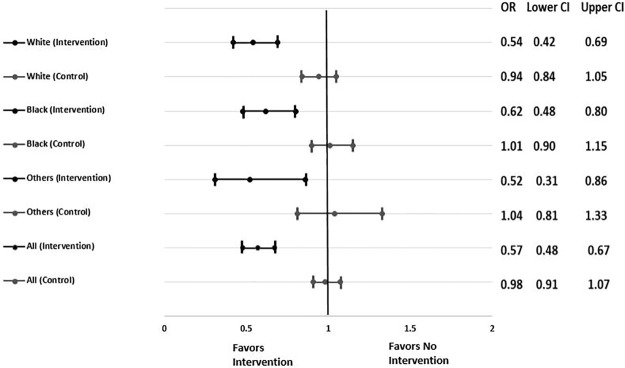
The odds of nonadministration of VTE prophylaxis declined on intervention units by 38% (OR 0.62, 95% CI, 0.48 to 0.80) for black patients, by 46% (OR 0.54, 95% CI, 0.42 to 0.69) for white patients, and by 48% (OR 0.52, 95% CI 0.31 to 0.86) for other races (Fig 1). No change in odds of nonadministration of VTE prophylaxis was observed on control units for any race group. Upon testing the interaction between the odds of nonadministration on intervention units compared to control units, a significant decline was observed among patients who were black (OR 0.61; 95% CI, 0.46 to 0.81), white (OR 0.57; 95% CI, 0.44 to 0.75), and other races (OR 0.50; 95% CI, 0.29 to 0.88, Fig 1).
Fig 1. Effect of the patient-centered education bundle, comparing the conditional odds ratios for the intervention and control arms stratified by race.
Fig 1 shows the effect of the patient-centered education bundle by comparing the conditional odds ratios for the intervention and control arms stratified by race. Odds ratios (OR) and 95% confidence intervals (CI) are reported.
Reason for VTE prophylaxis nonadministration by race
The odds of dose refusal on intervention units decreased pre-post by 43% (OR 0.57; 95% CI; 0.42 to 0.78) among black patients, by 48% (OR 0.52; 95% CI, 0.38 to 0.70) among white patients, and by 58% (OR 0.42; 95% CI, 0.21 to 0.82) among other races (Table 3). The odds of dose refusal remained unchanged on control units. The decline in refused doses on intervention units was statistically significantly different from control units for patients who were black (OR 0.57; 95%, 0.41 to 0.79), white (OR 0.55; 95%, 0.39 to 0.77), and other races (OR 0.41; 95%, CI 0.20 to 0.84). No significant differences were observed in the decline of non-administered doses for other reasons between intervention and control units (Table 3).
Table 3. Proportion of doses missed stratified by race for all reasons, refusal and other reasons: Comparisons between pre- vs. post-intervention by treatment group.
| Race/Period | Intervention | Control | OR (95% CI) p valuea | |
|---|---|---|---|---|
| Any Missed Dose | ||||
| Black | Pre-Intervention % (95% CI) | 7.4% (4.0%, 13.7%) | 12.3% (8.6%, 17.5%) | 0.57 (0.25, 1.28) p = 0.173 |
| Post-Intervention % (95% CI) | 4.9% (2.6%, 9.0%) | 12.3% (8.7%, 17.5%) | 0.35 (0.15, 0.78) p = 0.011 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.62 (0.48, 0.80) p<0.001 | 1.01 (0.90, 1.15) p = 0.820 | 0.61 (0.46, 0.81) p<0.001b | |
| White | Pre-Intervention % (95% CI) | 10.6% (5.8%, 19.5%) | 14.6% (10.3%, 20.8%) | 0.69 (0.31, 1.54) p = 0.366 |
| Post-Intervention % (95% CI) | 6.2% (3.4%, 11.5%) | 13.9% (9.8%, 19.6%) | 0.39 (0.18, 0.89) p = 0.024 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.54 (0.42, 0.69) p<0.001 | 0.94 (0.84, 1.05) p = 0.246 | 0.57 (0.44, 0.75) p<0.001b | |
| Other | Pre-Intervention % (95% CI) | 8.8% (4.4%, 17.4%) | 13.9% (9.5%, 20.5%) | 0.59 (0.25, 1.39) p = 0.229 |
| Post-Intervention % (95% CI) | 4.9% (2.4%, 10.2%) | 14.3% (9.8%, 20.8%) | 0.30 (0.12, 0.74) p = 0.009 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.52 (0.31, 0.86) p = 0.011 | 1.04 (0.81, 1.33) p = 0.773 | 0.50 (0.29, 0.88) p = 0.015b | |
| Dose Refused by Patient | ||||
| Black | Pre-Intervention % (95% CI) | 4.9% (2.0%, 11.7%) | 7.9% (4.8%, 13.1%) | 0.59 (0.19, 1.80) p = 0.355 |
| Post-Intervention % (95% CI) | 3.0% (1.2%, 7.2%) | 8.0% (4.8%, 13.2%) | 0.34 (0.11, 1.02) p = 0.055 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.57 (0.42, 0.78) p<0.001 | 1.01 (0.88, 1.17) p = 0.868 | 0.57 (0.41, 0.79) p = 0.001b | |
| White | Pre-Intervention % (95% CI) | 6.7% (2.8%, 16.0%) | 9.3% (5.7%, 15.4%) | 0.70 (0.23, 2.12) p = 0.527 |
| Post-Intervention % (95% CI) | 3.7% (1.6%, 9.0%) | 8.8% (5.4%, 14.5%) | 0.38 (0.13, 1.17) p = 0.092 | |
| Odds Ratio Post/Pre (95% CI) | 0.52 (0.38, 0.70) p<0.001 | 0.94 (0.82, 1.08) p = 0.366 | 0.55 (0.39, 0.77) p<0.001b | |
| Other | Pre-Intervention % (95% CI) | 6.1% (2.4%, 15.8%) | 8.6% (5.0%, 14.8%) | 0.70 (0.22, 2.25) p = 0.547 |
| Post-Intervention % (95% CI) | 2.8% (1.0%, 7.9%) | 8.9% (5.2%, 15.1%) | 0.28 (0.08, 0.98) p = 0.047 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.42 (0.21, 0.82) p = 0.011 | 1.03 (0.76, 1.40) p = 0.831 | 0.41 (0.20, 0.84) p = 0.015b | |
| Other Reason for Missed Dose (not patient refused) | ||||
| Black | Pre-Intervention % (95% CI) | 1.7% (1.0%, 2.8%) | 2.9% (2.2%, 3.8%) | 0.57 (0.33, 01.00) p = 0.052 |
| Post-Intervention % (95% CI) | 1.5% (0.9%, 2.3%) | 2.9% (2.3%, 3.8%) | 0.49 (0.28, 0.84) p = 0.011 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.86 (0.58, 1.29) p = 0.464 | 1.01 (0.84, 1.23) p = 0.891 | 0.85 (0.55, 1.32) p = 0.465b | |
| White | Pre-Intervention % (95% CI) | 2.8% (1.8%, 4.4%) | 3.8% (2.9%, 4.8%) | 0.74 (0.43, 1.25) p = 0.255 |
| Post-Intervention % (95% CI) | 1.8% (1.21%, 2.9%) | 3.6% (2.8%, 4.6%) | 0.50 (0.29, 0.86) p = 0.0134 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.64 (0.45, 0.91) p = 0.014 | 0.94 (0.81, 1.11) p = 0.478 | 0.68 (0.46, 1.01) p = 0.0584b | |
| Other | Pre-Intervention % (95% CI) | 0.9% (0.0%, -) | 3.7% (2.6%, 5.23%) | 0.40 (0.18, 0.90) p = 0.038 |
| Post-Intervention % (95% CI) | 1.5% (0.7%, 3.2%) | 3.8% (2.7%, 5.4%) | 0.38 (0.17, 0.86) p = 0.0281 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.98 (0.39, 2.43) p = 0.964 | 1.04 (0.71, 1.51) p = 0.842 | 0.94 (0.36, 2.49) p = 0.905b | |
OR, odds ratio; CI, confidence interval.
a P values for the odds ratios were calculated using multiple outputation of the generalized linear mixed-effects models with the binomial family and a logit link.
b Two way interactions were performed including pre vs. post time period, and control vs. intervention units
Stratified analysis by floor type (surgery and medicine) by race
The pre-specified analysis by floor type revealed a decline in the odds of nonadministration of VTE prophylaxis for all race groups (Table 4). On surgery intervention units, nonadministration significantly decreased pre-post among patients who were black (OR 0.53; 95% CI, 0.33 to 0.85), white (OR 0.57; 95% CI, 0.40 to 0.82), and other races (OR 0.30; 95% CI, 0.12 to 0.75). When comparing the odds of nonadministered doses on surgery intervention and control units, there was a significant difference for patients of other races (OR 0.33; 95% CI 0.12–0.91), but no significant difference for white or black groups.
Table 4. Subgroup analysis of floor type (surgery and medicine) effect on proportion of prescribed venous thromboembolism prophylaxis doses by race.
| Surgery | ||||
|---|---|---|---|---|
| Period | Intervention | Control | p valuea | |
| Any Missed Dose | ||||
| Black | Pre-Intervention, % (95% CI) | 5.2% (2.9%, 9.4%) | 9.7% (6.9%, 13.7%) | 0.50 (0.23, 1.06) p = 0.072 |
| Post-Intervention, % (95% CI) | 2.9% (1.6%, 5.2%) | 7.9% (5.6%, 11.0%) | 0.34 (0.16, 0.71) p = 0.004 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.53 (0.33, 0.85) p = 0.008 | 0.78 (0.62, 0.99) p = 0.039 | 0.68 (0.41, 1.14) p = 0.145b | |
| White | Pre-Intervention, % (95% CI) | 4.8% (2.8%, 8.1%) | 9.2% (6.7%, 12.6%) | 0.48 (0.24, 0.97) p = 0.042 |
| Post-Intervention, % (95% CI) | 2.8% (1.7%, 4.8%) | 7.9% (5.8%, 10.8%) | 0.33 (0.16, 0.67) p = 0.002 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.57 (0.40, 0.82) p = 0.002 | 0.84 (0.73, 0.98) p = 0.028 | 0.68 (0.46, 1.01) p = 0.054b | |
| Other | Pre-Intervention, % (95% CI) | 5.7% (3.1%, 10.3%) | 9.2% (6.1%, 13.7%) | 0.58 (0.27, 1.28) p = 0.178 |
| Post-Intervention, % (95% CI) | 1.8% (0.7%, 4.9%) | 8.3% (5.7%, 12.1%) | 0.19 (0.06, 0.59) p = 0.004 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.30 (0.12, 0.75) p = 0.010 | 0.89 (0.62, 1.28) p = 0.541 | 0.33 (0.12, 0.91) p = 0.031b | |
| Patient Refused Dose | ||||
| Black | Pre-Intervention, % (95% CI) | 2.7% (1.2%, 5.9%) | 4.7% (3.0%, 7.4%) | 0.54 (0.20, 1.44) p = 0.219 |
| Post-Intervention, % (95% CI) | 1.4% (0.6%, 3.1%) | 3.9% (2.5%, 6.1%) | 0.34 (0.12, 0.92) p = 0.033 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.50 (0.24, 1.03) p = 0.061 | 0.80 (0.59, 1.09) p = 0.162 | 0.62 (0.29, 1.35) p = 0.228b | |
| White | Pre-Intervention, % (95% CI) | 2.2% (1.1%, 4.4%) | 4.4% (2.9%, 6.7%) | 0.47 (0.19, 1.16) p = 0.100 |
| Post-Intervention, % (95% CI) | 1.3% (0.6%, 2.8%) | 3.5% (2.3%, 5.3%) | 0.36 (0.14, 0.91) p = 0.031 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.60 (0.34, 1.06) p = 0.079 | 0.79 (0.64, 0.97) p = 0.024 | 0.77 (0.42, 1.41) p = 0.393b | |
| Other | Pre-Intervention, % (95% CI) | 3.8% (-, -)c | 5.1% (3.0%, 8.7%) | 0.72 (0.26, 1.96) p = 0.521 |
| Post-Intervention, % (95% CI) | 0.8% (0.1%, 4.9%) | 3.9% (2.4%, 6.5%) | 0.19 (0.03, 1.31) p = 0.093 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.20 (0.03, 1.16) p = 0.072 | 0.74 (0.47, 1.19) p = 0.214 | 0.27 (0.04, 1.66) p = 0.158b | |
| Other Reason for Missed Dose (not patient refused) | ||||
| Black | Pre-Intervention, % (95% CI) | 2.2% (1.1%, 4.5%) | 4.0% (2.7%, 6.2%) | 0.53 (0.23, 1.23) p = 0.140 |
| Post-Intervention, % (95% CI) | 1.3% (0.0%, -0.0%) | 3.2% (2.2%, 4.7%) | 0.40 (0.19, 0.81) p = 0.011 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.59 (0.34, 1.01) p = 0.052 | 0.78 (0.55, 1.11) p = 0.167 | 0.75 (0.40, 1.42) p = 0.374b | |
| White | Pre-Intervention, % (95% CI) | 2.3% (1.3%, 4.2%) | 3.9% (2.8%, 5.6%) | 0.58 (0.28, 1.18) p = 0.133 |
| Post-Intervention, % (95% CI) | 1.3% (0.7%, 2.4%) | 3.6% (2.6%, 5.1%) | 0.35 (0.17, 0.73) p = 0.005 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.56 (0.37, 0.84) p = 0.006 | 0.91 (0.76, 1.10) p = 0.334 | 0.62 (0.39, 0.98) p = 0.042b | |
| Other | Pre-Intervention, % (95% CI) | 0.2% (0-, -)c | 3.1% (1.9%, 5.1%) | 0.47 (0.11, 1.99) p = 0.307 |
| Post-Intervention, % (95% CI) | 0.04% (0-, -)c | 3.6% (2.3%, 5.6%) | 0.32 (0.07, 1.54) p = 0.155 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.78 (0.14, 4.38) p = 0.781 | 1.15 (0.70, 1.89) p = 0.571 | 0.68 (0.12, 3.97) p = 0.667b | |
| Medicine | ||||
| Any Missed Dose | ||||
| Black | Pre-Intervention, % (95% CI) | 13.3% (8.0%, 22.1%) | 16.6% (12.7%, 21.7%) | 0.77 (0.39, 1.49) p = 0.432 |
| Post-Intervention, % (95% CI) | 9.1% (5.5%, 15.2%) | 17.4% (13.4%, 22.7%) | 0.46 (0.24, 0.90) p = 0.022 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.65 (0.48, 0.88) p = 0.005 | 1.08 (0.93, 1.24) p = 0.314 | 0.60 (0.43, 0.84) p = 0.003b | |
| White | Pre-Intervention, % (95% CI) | 22.3% (13.4%, 37.2%) | 20.6% (15.6%, 27.1%) | 1.10 (0.56, 2.16) p = 0.781 |
| Post-Intervention, % (95% CI) | 12.9% (7.7%, 21.6%) | 20.9% (16.0%, 27.3%) | 0.54 (0.28, 1.06) p = 0.072 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.51 (0.36, 0.70) p<0.001 | 1.03 (0.87, 1.21) p = 0.743 | 0.49 (0.34, 0.71) p<0.001b | |
| Other | Pre-Intervention, % (95% CI) | 14.4% (7.3%, 28.6%) | 19.0% (13.3%, 27.1%) | 0.71 (0.31, 1.64) p = 0.426 |
| Post-Intervention, % (95% CI) | 10.5% (5.2%, 21.1%) | 21.1% (15.2%, 29.4%) | 0.42 (0.18, 0.99) p = 0.048 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.69 (0.33, 1.49) p = 0.321 | 1.16 (0.82, 1.64) p = 0.409 | 0.60 (0.27, 1.33) p = 0.207b | |
| Patient Refused Dose | ||||
| Black | Pre-Intervention, % (95% CI) | 11.6% (6.1%, 22.0%) | 13.5% (9.6%, 19.0%) | 0.84 (0.37, 1.89) p = 0.669 |
| Post-Intervention, % (95% CI) | 7.2% (3.8%, 13.8%) | 14.0% (10.0%, 19.5%) | 0.47 (0.21, 1.06) p = 0.069 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.59 (0.42, 0.82) p = 0.002 | 1.05 (0.90, 1.23) p = 0.542 | 0.56 (0.39, 0.81) p = 0.002b | |
| White | Pre-Intervention, % (95% CI) | 18.2% (9.6%, 34.6%) | 16.2% (11.4%, 22.9%) | 1.14 (0.50, 2.60) p = 0.748 |
| Post-Intervention, % (95% CI) | 9.8% (5.1%, 18.9%) | 16.5% (11.7%, 23.1%) | 0.53 (0.23, 1.22) p = 0.135 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.48 (0.33, 0.69) p<0.001 | 1.03 (0.86, 1.23) p = 0.772 | 0.47 (0.31, 0.70) p<0.001b | |
| Other | Pre-Intervention, % (95% CI) | 12.3% (5.4%, 2.8%) | 13.6% (8.8%, 21.0%) | 0.89 (0.33, 2.41) p = 0.813 |
| Post-Intervention, % (95% CI) | 7.6% (3.3%, 17.8%) | 15.9% (10.6%, 23.9%) | 0.42 (0.15, 1.17) p = 0.098 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.58 (0.25, 1.34) p = 0.205 | 1.22 (0.81, 1.81) p = 0.340 | 0.48 (0.19, 1.20) p = 0.116b | |
| Other Reason for Missed Dose (not patient refused) | ||||
| Black | Pre-Intervention, % (95% CI) | 1.4% (0.7%, 2.5%) | 2.4% (1.7%, 3.4%) | 0.56 (0.27, 1.13) p = 0.104 |
| Post-Intervention, % (95% CI) | 1.6% (0.8%, 3.1%) | 2.8% (2.0%, 3.8%) | 0.57 (0.27, 1.20) p = 0.139 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 1.17 (0.68, 2.02) p = 0.571 | 1.15 (0.91, 1.46) p = 0.204 | 1.02 (0.57, 1.80) p = 0.958b | |
| White | Pre-Intervention, % (95% CI) | 3.5% (1.9%, 6.6%) | 3.6% (2.6%, 5.2%) | 0.96 (0.46, 2.02) p = 0.914 |
| Post-Intervention, % (95% CI) | 2.7% (1.4%, 5.2%) | 3.7% (2.6%, 5.1%) | 0.72 (0.33, 1.56) p = 0.399 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 0.75 (0.42, 1.37) p = 0.352 | 1.01 (0.76, 1.34) p = 0.940 | 0.75 (0.38, 1.46) p = 0.393b | |
| Other | Pre-Intervention, % (95% CI) | 0.02% (0-, -)c | 4.5% (2.7%, 7.4%) | 0.50 (0.09, 2.89) p = 0.439 |
| Post-Intervention, % (95% CI) | 2.3% (0.7%, 7.2%) | 4.3% (2.6%, 7.0%) | 0.52 (0.15, 1.83) p = 0.305 | |
| Odds Ratio Post/Pre (95% CI) p valuea | 1.00 (0.16, 6.08) p = 1.000 | 0.979 (0.54, 1.76) p = 0.920 | 1.03 (0.17, 6.35) p = 0.974b | |
OR, odds ratio; CI, confidence interval.
a P values for the odds ratios were calculated using multiple outputation of the generalized linear mixed-effects models with the binomial family and a logit link.
b Two way interactions were performed including pre vs. post time period, and control vs. intervention units for Surgery
c Not provided by models, due to small numbers
On medicine intervention units, nonadministered doses significantly decreased pre-post among patients who were black (OR 0.65; 95% CI, 0.48 to 0.88) and white (OR 0.51; 95% CI, 0.36 to 0.70; Table 4). No significant difference was observed among patients of other races (OR 0.69 95% CI 0.33–1.49). When comparing the odds of nonadministration on medicine intervention and control units, there was a significant decline among patients who were black (OR 0.60; 95% CI, 0.43 to 0.84) and white (OR 0.49; 95% CI, 0.34 to 0.71), but not other races (OR 0.60; 95% CI, 0.27 to 1.33). The proportion of refused doses decreased significantly for both white and black patients on medical units.
Discussion
In this study, we conducted a post-hoc analysis by race to determine the impact of a patient-centered education bundle on disparities related to the administration of prescribed doses of VTE prophylaxis. We found that the intervention was equitable and effective for all patients regardless of race. The odds of a patient refusing a prophylaxis dose significantly decreased on intervention units for all race groups after the education bundle was implemented, with approximately the same effect size. We hypothesized a differential effect between medicine and surgery units because of the heterogeneity in service types. However, the odds of nonadministered prophylaxis doses significantly declined for both clinical services. These findings suggest that the patient education materials, which were developed collaboratively with a diverse group of patients, improved the patient’s understanding of VTE and the importance of prevention through prophylaxis. This concept of health literacy may positively impact the quality of care for all patients and the potential to reduce disparities in health outcomes.
Our patient education bundle included an interactive conversation between the nurse educator and patient and was most likely associated with rate declines in missed VTE prophylaxis doses regardless of patient race. One likely reason was the patient-centered, inclusive nature of the education. We originally translated the handout into 8 languages (Arabic, Chinese, English, Korean, Nepalese, Portuguese, Russian and Spanish) to ensure we reached the racial distribution of patients at The Johns Hopkins Hospital. Some patients responded favorably when given material in their native language. For the patient education video, we consciously recruited patients from a variety of racial backgrounds, and of varying ages and sexes (bit.ly/bloodclots). Another likely reason for rate declines was the interactive approach we used, which is consistent with prior research of health literacy.[21,30,35] Health literacy depends on effective communication in which information is explained clearly to make the topic easily understood. When we surveyed patients and families before developing the bundle, face-to-face conversations with a health care provider was the preferred method of receiving information about VTE.[30] Our patient-centered approach ensured that patients participated in their care pathway and made well-informed decisions. Thus, we demonstrated that real-time delivery of a patient-centered education intervention can improve health literacy and concomitantly reduce patient refusal and non-administration of pharmacologic VTE prophylaxis. It is important to note that providers must seek out patient preferences for receiving education.[37]
Despite the remarkable progress observed, there remained missed doses of VTE prophylaxis for reasons other than patient refusal. The available data does not allow additional exploration to determine the reason for this trend. However, we speculate that nursing factors, unit culture, or variation in practice relative to contraindications could have influenced decision-making; our intervention may have indirectly affected these issues. The direct effect of our intervention demonstrates that the bundle was not restricted to any specific patient populations and can be implemented regardless of clinical service. Similar declines in the odds of non-administration on both medical and surgical intervention units for all race groups supported the portability of the education bundle.
The present work extends previous initiatives designed and implemented to reduce disparities in health care delivery.[38–40] Strategies to identify and address inequalities in the quality of care that minority patients receive is emerging and one goal of Healthy People 2020.[41] Our overall goal was to improve the quality of care for all patients. However, non-differential improvement of doses accepted by patients was always a secondary goal of our project. Our approach to ensure health care equity was to target the subjective culture, comprising perceptions, attitudes, beliefs, and stereotypes surrounding the silent but debilitating and deadly nature of VTE. We engaged a representative group of local and national patient stakeholders to understand what they wanted to know about VTE and how they preferred to learn. The purpose was to develop education that targeted their preferences while improving their health literacy about VTE and appropriate prevention and their ability to make informed care decisions.[30]
Previous research has shown that racial and ethnic minorities tend to receive lower quality of care than non-minorities related to patient, process, and structural factors.[42–44] Consistent with best practices of patient-centered care delivery,[45] our study highlighted the relevance of leveraging health information technology as a quality improvement strategy to improve care through adherence to administered doses of pharmacologic VTE prophylaxis. Our approach also points to policies that support the advancement of risk-appropriate VTE prophylaxis through a risk assessment tool built into our EHR that follows best practice recommendations for VTE.[25] We concurrently attempted to break the gaps between prescription and administration practices by improving health literacy, which by its nature can be distributed equally across all race subpopulations.
Quality improvement efforts and initiatives have skyrocketed nationwide for well over a decade. While we used quality improvement to explore and eliminate disparities,[22] we did not find other groups who examined the differential effect of race on their study findings. Moving forward, we suggest that all quality improvement initiatives be rigorously examined to ensure there is no differential effect by race, and other demographics such as age, sex, gender, and ethnicity, as appropriate.[22] While some may say that this is a negative study, we still see benefit in objectively studying and publishing these types of data to ensure quality improvement efforts do not inadvertently worsen health disparities.
Our study has limitations. First, our analyses did not examine the effect of patient-provider race concordance. The difficulty in making definitive assertions about the role of race concordance for the missed doses due to other reasons points to the value of additional research. Second, our findings reflect a single medical institution and our patient demographics may not be representative of other medical facilities. Third, as we looked at smaller and smaller strata, we may have lost power to detect statistically significant differences. For example, in the strata by race for the outcome of refused doses, the point estimates for the effect sizes were similar to the overall numbers (in the vicinity of about a 40% to 50% improvement), yet the confidence intervals were wide and p values above 0.05. While we cannot convincingly claim statistical significance, we did find that the estimates were in the same direction and of similar magnitude. Fourth, unknown patient engagement interventions by physicians regarding VTE prevention may have impacted our results, but these likely would have been the same between pre- and post-periods and between floors.
Projections from the 2010 US Census estimate that racial and ethnic minority populations will substantially increase and eventually be the majority by 2050.[41,46] It is crucial to implement effective health education interventions that incorporate an understanding of the cultural values, perceptions, and attitudes of a diverse, multiethnic patient population and involve this audience as stakeholders in the processes from inception to implementation, placing greater emphasis on improving the quality of care and ensuring equity. Quality improvement efforts should consciously target all patient populations equally, regardless of race, ethnicity, sex, gender, or age.
Supporting information
(DOCX)
(PDF)
(PDF)
(TIF)
Data Availability
The data set underlying this study contains protected health information, which by nature of the study cannot be de-identified. For the purpose of this study, information included patient identifiers, nurse identifiers, dates of hospitalization, dates/times of medication administration, and hospital location, which are all required components for the analysis that would potentially enable individuals to identify specific patients and nurses similar to our nurse education trial (Lau PLOS ONE 2017). The Johns Hopkins University Institutional Review Board approved the research application, and data were analyzed following IRB approval. Since our application fell under the umbrella of quality improvement, we did not explicitly state that the raw data from this study would be shared. Therefore, for ethical, legal, and HIPAA privacy reasons, we cannot make a data set publicly available. For related data access queries please contact the director of the Institute for Clinical and Translational Research and the vice dean for clinical investigation at the Johns Hopkins School of Medicine, Daniel E Ford, via email: dford@jhmi.edu.
Funding Statement
This work was supported by contracts from the Patient Centered Outcomes Research Institute (PCORI) entitled “Preventing Venous Thromboembolism: Empowering Patients and Enabling Patient-Centered Care via Health Information Technology” (CE-12-11-4489) and “Preventing Venous Thromboembolism (VTE): Engaging Patients to Reduce Preventable Harm from Missed/Refused Doses of VTE Prophylaxis” (DI-1603-34596). The funders had no role in the conduct of this study. We thank our key stakeholder organizations- the National Blood Clot Alliance (NBCA), the North American Thrombosis Forum (NATF), Clot Care, and The Johns Hopkins Hospital Patient and Family Advisory Council (PFAC).”
References
- 1.Singh GK, Daus GP, Allender M, Ramey CT, Martin EK, Perry C, et al. Social Determinants of Health in the United States: Addressing Major Health Inequality Trends for the Nation, 1935–2016. Int J MCH AIDS 2017;6(2):139–164. 10.21106/ijma.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Lainz A, McDonald M, Fonseca-Ford M, Penman-Aguilar A, Waterman SH, Truman BI, et al. Collection of Data on Race, Ethnicity, Language, and Nativity by US Public Health Surveillance and Monitoring Systems: Gaps and Opportunities. Public Health Rep 2018. Jan-Feb;133(1):45–54. 10.1177/0033354917745503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filice CE, Joynt KE. Examining Race and Ethnicity Information in Medicare Administrative Data. Med Care 2017. December;55(12):e170–e176. 10.1097/MLR.0000000000000608 [DOI] [PubMed] [Google Scholar]
- 4.Narayan MC, Scafide KN. Systematic Review of Racial/Ethnic Outcome Disparities in Home Health Care. J Transcult Nurs 2017. March 1:1043659617700710. [DOI] [PubMed] [Google Scholar]
- 5.Kawachi I, Subramanian SV, Almeida-Filho N. A glossary for health inequalities. J Epidemiol Community Health 2002. September;56(9):647–652. 10.1136/jech.56.9.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braveman P, Gruskin S. Defining equity in health. J Epidemiol Community Health 2003. April;57(4):254–258. 10.1136/jech.57.4.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackenbach JP. The persistence of health inequalities in modern welfare states: the explanation of a paradox. Soc Sci Med 2012. August;75(4):761–769. 10.1016/j.socscimed.2012.02.031 [DOI] [PubMed] [Google Scholar]
- 8.Haider AH, Schneider EB, Sriram N, Dossick DS, Scott VK, Swoboda SM, et al. Unconscious race and class bias: Its association with decision making by trauma and acute care surgeons. J Trauma Acute Care Surg 2014. September;77(3):409–416. 10.1097/TA.0000000000000392 [DOI] [PubMed] [Google Scholar]
- 9.Streiff MB, Lau BD. Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program 2012;2012:631–637. [DOI] [PubMed] [Google Scholar]
- 10.Haut ER, Aboagye JK, Shaffer DL, Wang J, Hobson DB, Yenokyan G, et al. Effect of Real-Time Patient-Centered Education Bundle on Venous Thromboembolism Prevention in Hospitalized Patients. JAMA Netw Open 2018;1(7):e184741 10.1001/jamanetworkopen.2018.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau BD, Streiff MB, Pronovost PJ, Haut ER. VTE quality measures fail to accurately measure quality. Circulation 2018;137(12):1278–1284. 10.1161/CIRCULATIONAHA.116.026897 [DOI] [PubMed] [Google Scholar]
- 12.Lau BD, Arnaoutakis GJ, Streiff MB, Howley IW, Poruk KE, Beaulieu R, et al. Individualized Performance Feedback to Surgical Residents Improves Appropriate Venous Thromboembolism Prophylaxis Prescription and Reduces Potentially Preventable VTE: A Prospective Cohort Study. Ann Surg 2016. December;264(6):1181–1187. 10.1097/SLA.0000000000001512 [DOI] [PubMed] [Google Scholar]
- 13.Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf 2014. March;23(3):187–195. 10.1136/bmjqs-2012-001782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muellner SK, Haut ER, Streiff MB, Holcomb JB, Cotton BA. ABO blood group as a potential risk factor for venous thromboembolism in acutely injured patients. Thromb Haemost 2011. January 3;105(1):5–13. 10.1160/TH10-08-0504 [DOI] [PubMed] [Google Scholar]
- 15.Margaglione M, Grandone E. Population genetics of venous thromboembolism. A narrative review. Thromb Haemost 2011. February;105(2):221–231. 10.1160/TH10-08-0510 [DOI] [PubMed] [Google Scholar]
- 16.Key NS, Reiner AP. Genetic basis of ethnic disparities in VTE risk. Blood 2016. April 14;127(15):1844–1845. 10.1182/blood-2016-03-701698 [DOI] [PubMed] [Google Scholar]
- 17.Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA 2000. May 17;283(19):2579–2584. 10.1001/jama.283.19.2579 [DOI] [PubMed] [Google Scholar]
- 18.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res 2009;123 Suppl 4:S11–7. [DOI] [PubMed] [Google Scholar]
- 19.Owodunni OP, Weiss MJ, Haut ER. Is Venous Thromboembolism in Asian Patients Undergoing Gastrectomy Different From Venous Thromboembolism in Their Western Counterparts? JAMA Surg 2018. July 18. [DOI] [PubMed] [Google Scholar]
- 20.Streiff MB, Lau BD, Hobson DB, Kraus PS, Shermock KM, Shaffer DL, et al. The Johns Hopkins Venous Thromboembolism Collaborative: Multidisciplinary team approach to achieve perfect prophylaxis. J Hosp Med 2016. December;11 Suppl 2:S8–S14. [DOI] [PubMed] [Google Scholar]
- 21.Streiff MB, Carolan H, Hobson DB, Kraus PS, Holzmueller C, Demski R, et al. Lessons from the Johns Hopkins Multi-Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ 2012;344:e3935 10.1136/bmj.e3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau BD, Haider AH, Streiff MB, Lehmann CU, Kraus PS, Hobson DB, et al. Eliminating Health Care Disparities With Mandatory Clinical Decision Support: The Venous Thromboembolism (VTE) Example. Med Care 2015. January;53(1):18–24. 10.1097/MLR.0000000000000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau BD, Streiff MB, Hobson DB, Kraus PS, Shaffer DL, Popoola VO, et al. Beneficial "halo effects" of surgical resident performance feedback. J Surg Res 2016. September;205(1):179–185. 10.1016/j.jss.2016.06.024 [DOI] [PubMed] [Google Scholar]
- 24.Zeidan AM, Streiff MB, Lau BD, Ahmed SR, Kraus PS, Hobson DB, et al. Impact of a venous thromboembolism prophylaxis "smart order set": Improved compliance, fewer events. Am J Hematol 2013. July;88(7):545–549. 10.1002/ajh.23450 [DOI] [PubMed] [Google Scholar]
- 25.Haut ER, Lau BD, Kraenzlin FS, Hobson DB, Kraus PS, Carolan HT, et al. Improved Prophylaxis and Decreased Preventable Harm with a Mandatory Computerized Clinical Decision Support Tool for Venous Thromboembolism (VTE) Prophylaxis in Trauma Patients. Arch Surg 2012;10(147):901–907. [DOI] [PubMed] [Google Scholar]
- 26.Lau BD, Streiff MB, Kraus PS, Hobson DB, Shaffer DL, Aboagye JK, et al. Missed Doses of Venous Thromboembolism (VTE) Prophylaxis at Community Hospitals: Cause for Alarm. J Gen Intern Med 2018. January;33(1):19–20. 10.1007/s11606-017-4203-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shermock KM, Lau BD, Haut ER, Hobson DB, Ganetsky VS, Kraus PS, et al. Patterns of Non-Administration of Ordered Doses of Venous Thromboembolism Prophylaxis: Implications for Novel Intervention Strategies. PLoS One 2013. June 14;8(6):e66311 10.1371/journal.pone.0066311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrow NE, Lau BD, JohnBull EA, Hobson DB, Kraus PS, Taffe ER, et al. Is the Meaningful Use Venous Thromboembolism VTE-6 Measure Meaningful? A retrospective analysis of one hospital’s VTE-6 cases. Jt Comm J Qual Patient Saf 2016;42(9):410–416. 10.1016/s1553-7250(16)42082-9 [DOI] [PubMed] [Google Scholar]
- 29.Haut ER, Lau BD, Kraus PS, Hobson DB, Maheshwari B, Pronovost PJ, et al. Preventability of Hospital-Acquired Venous Thromboembolism. JAMA Surg 2015. July 29;150(9):912–925. 10.1001/jamasurg.2015.1340 [DOI] [PubMed] [Google Scholar]
- 30.Popoola VO, Lau BD, Shihab HM, Farrow NE, Shaffer DL, Hobson DB, et al. Patient Preferences for Receiving Education on Venous Thromboembolism Prevention—A Survey of Stakeholder Organizations. PLoS One 2016. March 31;11(3):e0152084 10.1371/journal.pone.0152084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss D, Rydland HT, Oversveen E, Jensen MR, Solhaug S, Krokstad S. Innovative technologies and social inequalities in health: A scoping review of the literature. PLoS One 2018. April 3;13(4):e0195447 10.1371/journal.pone.0195447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang VW, Lauderdale DS. Fundamental cause theory, technological innovation, and health disparities: the case of cholesterol in the era of statins. J Health Soc Behav 2009. September;50(3):245–260. 10.1177/002214650905000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patient Centered Outcomes Research Institute. Research & Results. https://www.pcori.org/research-results-home Accessed May 29, 2019.
- 34.Des Jarlais DC, Lyles C, Crepaz N, TREND Group. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004. March;94(3):361–366. 10.2105/ajph.94.3.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau BD, Shaffer DL, Hobson DB, Yenokyan G, Wang J, Sugar EA, et al. Effectiveness of two distinct web-based education tools for bedside nurses on medication administration practice for venous thromboembolism prevention: A randomized clinical trial. PLoS One 2017. August 16;12(8):e0181664 10.1371/journal.pone.0181664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Follmann D, Proschan M, Leifer E. Multiple outputation: inference for complex clustered data by averaging analyses from independent data. Biometrics 2003. June;59(2):420–429. 10.1111/1541-0420.00049 [DOI] [PubMed] [Google Scholar]
- 37.Wong A, Kraus PS, Lau BD, Streiff MB, Haut ER, Hobson DB, et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med 2015;10(2):108–111. 10.1002/jhm.2282 [DOI] [PubMed] [Google Scholar]
- 38.Shekelle PG, Pronovost PJ, Wachter RM, McDonald KM, Schoelles K, Dy SM, et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med 2013. March 5;158(5 Pt 2):365–368. 10.7326/0003-4819-158-5-201303051-00001 [DOI] [PubMed] [Google Scholar]
- 39.Shekelle PG, Wachter RM, Pronovost PJ, Schoelles K, McDonald KM, Dy SM, et al. Making Health Care Safer II: An Updated Critical Analysis of the Evidence for Patient Safety Practices. Evid Rep Technol Assess (Full Rep) 2013;211:1–945. [PMC free article] [PubMed] [Google Scholar]
- 40.Weaver SJ, Lubomksi LH, Wilson RF, Pfoh ER, Martinez KA, Dy SM. Promoting a culture of safety as a patient safety strategy: a systematic review. Ann Intern Med 2013. March 5;158(5 Pt 2):369–374. 10.7326/0003-4819-158-5-201303051-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020 [Internet]. (https://www.healthypeople.gov/2020) Accessed May 29, 2019.
- 42.Zaitoun A, Al-Najafi S, Musa T, Szpunar S, Light D, Lalonde T, et al. The association of race with quality of health in peripheral artery disease following peripheral vascular intervention: The Q-PAD Study. Vasc Med 2017. December;22(6):498–504. 10.1177/1358863X17733065 [DOI] [PubMed] [Google Scholar]
- 43.Haider AH, Ong'uti S, Efron DT, Oyetunji TA, Crandall ML, Scott VK, et al. Association between hospitals caring for a disproportionately high percentage of minority trauma patients and increased mortality: a nationwide analysis of 434 hospitals. Arch Surg 2012. January;147(1):63–70. 10.1001/archsurg.2011.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen MG, Fonarow GC, Peterson ED, Moscucci M, Dai D, Hernandez AF, et al. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation 2010. June 1;121(21):2294–2301. 10.1161/CIRCULATIONAHA.109.922286 [DOI] [PubMed] [Google Scholar]
- 45.Finkelstein J, Knight A, Marinopoulos S, Gibbons MC, Berger Z, Aboumatar H, et al. Enabling Patient-Centered Care Through Health Information Technology. 2012;206. [PMC free article] [PubMed] [Google Scholar]
- 46.United States Census Bureau. 2010 Census U.S. Census Bureau. 2013; http://ftp2.census.gov/. Accessed Oct 18, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(PDF)
(TIF)
Data Availability Statement
The data set underlying this study contains protected health information, which by nature of the study cannot be de-identified. For the purpose of this study, information included patient identifiers, nurse identifiers, dates of hospitalization, dates/times of medication administration, and hospital location, which are all required components for the analysis that would potentially enable individuals to identify specific patients and nurses similar to our nurse education trial (Lau PLOS ONE 2017). The Johns Hopkins University Institutional Review Board approved the research application, and data were analyzed following IRB approval. Since our application fell under the umbrella of quality improvement, we did not explicitly state that the raw data from this study would be shared. Therefore, for ethical, legal, and HIPAA privacy reasons, we cannot make a data set publicly available. For related data access queries please contact the director of the Institute for Clinical and Translational Research and the vice dean for clinical investigation at the Johns Hopkins School of Medicine, Daniel E Ford, via email: dford@jhmi.edu.