Abstract
Elevated serum uric acid (SUA) is associated with a variety of medical conditions, such as hypertension, diabetes and obesity. Analyses investigating uric acid and obesity were primarily conducted using anthropometric measures like BMI and waist circumference. However, different adipose tissue depots might be differentially affected in uric acid metabolism. We analyzed the relation of SUA with visceral, subcutaneous and hepatic fat as quantified by Magnetic Resonance Imaging in N = 371 individuals from a cross-sectional sample of a population-based cohort. Associations of SUA and fat depots were calculated by regressions adjusted for potential confounders. We found that SUA was correlated with all fat measures (e.g. Pearson’s r between SUA and hepatic fat: 0.50, 95%-CI: 0.42, 0.57). Associations with visceral and hepatic fat, but not with subcutaneous fat, remained evident after adjustment for anthropometric measures (e.g. visceral fat: β = 0.51 l, 95%-CI: 0.30 l, 0.72 l). In conclusion, these results show how different adipose tissue compartments are affected by SUA to varying degrees, thus emphasizing the different physiological roles of these adipose tissues in uric acid metabolism.
Subject terms: Diagnostic markers, Epidemiology
Introduction
Serum Uric Acid (SUA) originates from the metabolic breakdown of purine nucleotides. When SUA exceeds the normal range of homeostasis, it is deposited in articulations and soft tissues in the form of monosodium urate crystals. Thus, elevated SUA levels (hyperuricemia) are the major etiologic factor for developing gout1. However, hyperuricemia has also been linked to several metabolic disorders such as chronic kidney disease2,3, fatty liver4, metabolic syndrome5–7 and its components, including hypertension8–10, diabetes11–13 and adiposity14,15.
Prevalence of adiposity and fatty liver – and thus also the prevalence of related comorbidities – is rising16,17. It is therefore important to investigate biomarkers such as SUA that might help to understand pathways and mechanisms of adipose tissue metabolism and examine their potential causal role in the development of adiposity.
However, the investigation of the association of SUA with adiposity and fatty liver is often hampered by insufficient methodology to quantify adipose tissue content. Adiposity is usually determined by anthropometric measures such as waist circumference (WC) and Body Mass Index (BMI); but these crude measures do not give a complete picture of the fat distribution in the body and cannot quantify the amount of metabolically active adipose tissue. Adipose tissue is a heterogeneous entity and different compartments might be affected by SUA in different ways. A recent Mendelian Randomization study found that genetically predicted BMI was associated with the risk of gout, whereas increased waist circumference was not, indicating the differential involvement of adipose tissue in uric acid metabolism18. Therefore, accurate and differential quantification of body adipose tissue is needed.
There have already been efforts to use medical imaging such as computed tomography (CT) to determine adipose tissue in the context of its association with SUA14,15,19. However, magnetic resonance imaging (MRI) has emerged as the gold standard for the quantification of adipose tissue, due to its high sensitivity and high resolution. Strong evidence of a correlation of SUA with MRI-derived abdominal adipose tissue and hepatic fat in a population-based sample is still lacking.
We therefore aim to analyze the association of SUA as an exposure variable with outcomes of MRI-derived visceral, subcutaneous and hepatic fat fraction (HFF) in a population-based sample.
Materials and Methods
Study population
The KORA-MRI study includes a cross-sectional sample of 400 participants from a population-based cohort of The Cooperative Health Research in the Augsburg Region (KORA) in Southern Germany. The general design and setup of the KORA studies has been described previously20. Briefly, the baseline survey (S4) from 1999/2001 included 4261 participants who were randomly sampled from the city of Augsburg and two adjacent counties. The first follow-up (F4) took place in 2006/2008 and included 3080 of the original participants. The second follow-up (FF4) was conducted in 2013/2014 with 2279 participants. Among the participants of the second follow-up, a whole body MRI was performed on 400 individuals who met the inclusion criteria, as detailed in Fig. 1. The main setup of the KORA-MRI study has been described previously21.
Figure 1.
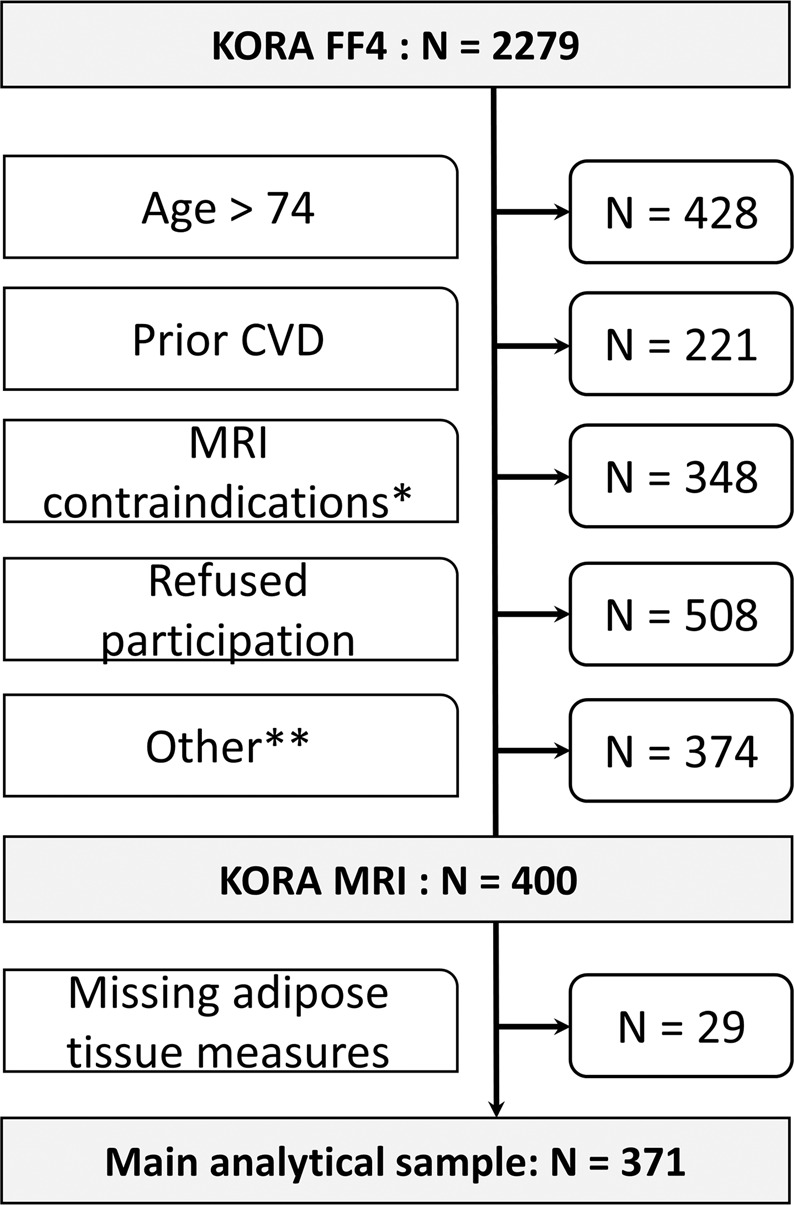
Flowchart of participants. CVD: cardiovascular disease defined as myocardial infarction, stroke or revascularization; * non-removable metal parts in the body, such as pacemakers or stents, renal insufficiency, known gadolinium allergy, claustrophobia, inability to lay down and/or hold breath, pregnancy or breast-feeding; ** unreachable by phone call (n = 39), scheduling problems (n = 8) and not included for matching (n = 327).
Ethics approval and consent to participate
The KORA FF4 study was approved by the ethics committee of the Bavarian Chamber of Physicians, Munich; the MRI sub-study was approved by the institutional review board of the Ludwig-Maximilians-University Munich. The investigations were carried out in accordance with the Declaration of Helsinki, including written informed consent of all participants.
Outcome assessment
All MRI scans were done on a 3 Tesla Magnetom Skyra (Siemens Healthineers, Erlangen, Germany), as detailedly described elsewhere21. For the assessment of adipose tissue, a volume-interpolated three-dimensional in/opposed-phase VIBE-Dixon sequence was used and adipose tissue was quantified semiautomatically using a Matlab-based (MathWorks Inc., Natick, Massachusetts) inhouse algorithm. Visceral Fat (VAT) was calculated from the femoral head to the diaphragm and subcutaneous fat (SAT) from the femoral head to the cardiac apex. VAT and SAT are summed up to total abdominal adipose tissue. All measurements are indicated in liter (l)22,23.
Multi-echo single voxel proton magnetic resonance spectroscopy, based on a high-speed T2-corrected multi-echo technique (HISTO), was used to quantify HFF in the right and left lobe of the liver. The arithmetic mean of left and right lobe HFF was used for further analysis (HFF_HISTO). Additionally, a multiecho Dixon-sequence was utilized to quantify HFF at the level of portal vein (HFF_dixon)24. HFF is given in %.
Exposure and covariate assessment
Venous blood samples were taken without stasis as a part of the standardized examination procedure at the study center. SUA was measured using an enzymatic colorimetric test based on uric acid cleavage by uricase forming allantoin and hydrogen peroxide (Cobas C, Roche). Hyperuricemia was defined as SUA levels >6 mg/dL in women and >7 mg/dL in men25. Standard enzymatic and immunonephelometric assays were used to quantify serum cholesterol and albumin levels, respectively.
Weight and height of participants were measured by Seca’s digital scales (Seca GmbH & Co, KG, Hamburg, Germany), determined to the closest 0.1 kg and 0.1 cm, respectively. BMI was computed as weight in kg divided by height in m squared. WC was measured with an inelastic measuring tape between the iliac crest and lower rib margin.
Systolic and diastolic blood pressure were measured with an automated oscillometric device (Omron HEM-705CP) while participants were seated. Three measurements with three-minute intervals in between were obtained and the mean of the second and third measurement was used as the final value.
Participants’ glycemic status was categorized as either type 2 diabetes (established type 2 diabetes or newly detected type 2 diabetes by Oral Glucose Tolerance Test (OGTT) with 2-h glucose ≥200 mg/dL and/or fasting glucose ≥125 mg/dL according to WHO criteria), prediabetes (2-h glucose between 140 and 200 mg/dL and/or fasting glucose between 110 and 125 mg/dL according to WHO criteria) or normoglycemic.
Smoking history, alcohol consumption and medication intake were assessed by self-report during the standardized interview.
Medication was considered antihypertensive if it contained antihypertensive agents according to the most current guidelines of the German Hypertension Association and if individuals were aware of having hypertension. Diuretics, lipid-lowering medication and gout medication were defined according to the Anatomical Therapeutic Chemical (ATC) classification as C03, C10 and M04, respectively.
Statistical methods
Descriptive characteristics of the study population, stratified by presence of hyperuricemia, are presented as arithmetic mean and SD for continuous covariables and as counts and percentages for categorical covariables. Differences were evaluated by t-test or χ2-Test, where applicable.
Correlation between SUA and measures of adipose tissue were assessed graphically by scatterplots and quantitatively by Pearson’s correlation coefficient with respective 95% confidence intervals (CI). Differences in adipose tissue measures according to presence of hyperuricemia were assessed graphically by boxplots and quantitatively by t-test.
To assess the association between SUA and adipose tissue, we used a multiple linear regression model adjusted for age, sex, systolic blood pressure, total serum cholesterol, serum albumin, alcohol consumption, gout medication, diuretic medication, antihypertensive medication, lipid-lowering medication, antidiabetic medication and glycemic status. SUA served as the exposure variable and adipose tissue (VAT, SAT, hepatic fat fraction) as the outcome. Continuous exposure and adjustment variables were standardized (mean = 0, standard deviation = 1) before analysis. The outcome hepatic fat fraction (HFF_dixon and HFF_HISTO) was log-transformed and resulting estimates therefore denote the percent change in mean HFF. Goodness-of-fit of the models was estimated by the R2 metric which denotes the percentage of variance in the outcome that is explained by the model. In a second step, the model was additionally adjusted for either BMI or WC. We calculated all models for continuous exposure SUA and dichotomized exposure hyperuricemia; for the whole sample as well as in a sex-stratified fashion. In a sensitivity analysis, all individuals taking anti-gout medication were excluded. In another sensitivity analysis, fasting serum glucose instead of glycemic status was used for adjustment.
R version 3.4.4 was used for all analyses.
Results
Characteristics of the study sample
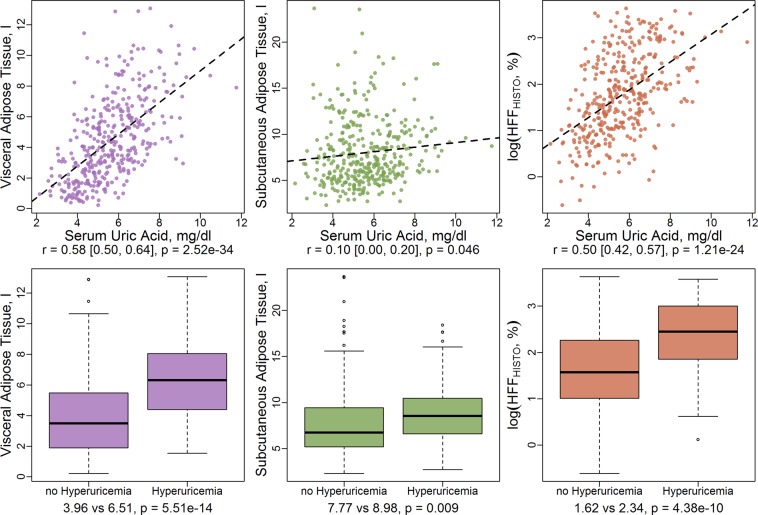
Baseline characteristics of the study sample, stratified by presence of hyperuricemia, are given in Tables 1 and 2. Sex-stratified results are provided in Supplementary Table S1. The sample comprised 371 middle-aged individuals (mean age 56.1 ± 9.1 years) with a mean SUA level of 5.6 ± 1.5 mg/dL. Prevalence of hyperuricemia was almost 20%, with a higher prevalence in men (26.3%) than in women (10.4%). Participants with hyperuricemia were on average older, had higher BMI and WC, higher blood pressure and generally a more unfavorable risk profile (compare Table 1). The amount of VAT, SAT and HFF was evidently higher in participants with hyperuricemia compared to those without (compare Table 2 and boxplots in Fig. 2). Continuous SUA was correlated to VAT (r = 0.58, p = 2.52E-24) and HFF (r = 0.50, p = 1.21E-24) and to a lesser extent to SAT (r = 0.10, p = 0.04, compare scatterplots in Fig. 2).
Table 1.
Baseline characteristics of the sample.
| Whole sample | no Hyperuricemia | Hyperuricemia | pvalue | |
|---|---|---|---|---|
| N = 371 | N = 298 (80.3%) | N = 73 (19.7%) | ||
| Age, years | 56.1 ± 9.1 | 55.6 ± 9.1 | 57.9 ± 8.8 | 0.054 |
| Men | 217 (58.5%) | 160 (53.7%) | 57 (78.1%) | 2.54E-04 |
| BMI, kg/m2 | 27.8 ± 4.7 | 27.3 ± 4.6 | 29.8 ± 4.3 | 4.19E-05 |
| Waist circumference, cm | 97.9 ± 13.7 | 95.9 ± 13.6 | 106.1 ± 10.7 | 4.79E-09 |
| Systolic Blood Pressure, mmHg | 120.7 ± 16.5 | 118.8 ± 15.6 | 128.1 ± 18.1 | 1.31E-05 |
| Diastolic Blood Pressure, mmHg | 75.4 ± 9.9 | 74.9 ± 9.6 | 77.4 ± 10.7 | 0.052 |
| Albumin, mg/dL | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.4 ± 0.3 | 0.033 |
| Total cholesterol, mg/dL | 217.6 ± 36.4 | 216.9 ± 35.4 | 220.3 ± 40.4 | 0.482 |
| HDL cholesterol, mg/dL | 61.9 ± 17.8 | 63.4 ± 17.6 | 55.8 ± 17.2 | 9.86E-04 |
| LDL cholesterol, mg/dL | 139.3 ± 32.9 | 139.1 ± 32.6 | 140.3 ± 34.1 | 0.788 |
| Triglycerides, mg/dL | 131.7 ± 86.1 | 119.9 ± 69.4 | 180.1 ± 123.4 | 4.98E-08 |
| Creatinine, mg/dL | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.1 | 5.44E-07 |
| Uric acid, mg/dL | 5.6 ± 1.5 | 5.1 ± 1.1 | 7.7 ± 1.0 | 4.12E-57 |
| Antihypertensive medication | 90 (24.3%) | 66 (22.1%) | 24 (32.9%) | 0.078 |
| Lipid-lowering medication | 39 (10.5%) | 32 (10.7%) | 7 (9.6%) | 0.941 |
| Gout medication | 9 (2.4%) | 8 (2.7%) | 1 (1.4%) | 1 |
| Diuretic medication | 45 (12.1%) | 28 (9.4%) | 17 (23.3%) | 0.002 |
| Antidiabetic medication | 29 (7.8%) | 22 (7.4%) | 7 (9.6%) | 0.699 |
| Smoking | ||||
| never-smoker | 134 (36.1%) | 107 (35.9%) | 27 (37.0%) | 0.060 |
| ex-smoker | 161 (43.4%) | 123 (41.3%) | 38 (52.1%) | |
| smoker | 76 (20.5%) | 68 (22.8%) | 8 (11.0%) | |
| Alcohol consumption, g/day | 18.9 ± 24.2 | 17.0 ± 23.0 | 26.4 ± 27.3 | 0.003 |
| thereof beer | 11.6 ± 18.9 | 9.3 ± 16.4 | 20.7 ± 24.8 | 2.75E-06 |
| thereof wine | 6.8 ± 14.1 | 7.2 ± 14.3 | 5.1 ± 12.9 | 0.246 |
| thereof spirits | 0.5 ± 1.6 | 0.5 ± 1.6 | 0.6 ± 1.4 | 0.642 |
| Fasting serum glucose, mg/dL | 104.0 ± 22.7 | 103.4 ± 24.2 | 106.6 ± 15.1 | 0.271 |
| Glycemic Status | ||||
| normoglycemic | 231 (62.3%) | 195 (65.4%) | 36 (49.3%) | 0.039 |
| prediabetes | 90 (24.3%) | 66 (22.1%) | 24 (32.9%) | |
| diabetes | 50 (13.5%) | 37 (12.4%) | 13 (17.8%) | |
Continuous variables are presented as mean ± standard deviation with p-values from t-test. Categorical variables are presented as counts and percentage with p-values from Wilcoxon rank-sum test. Hyperuricemia was defined as serum uric acid levels >6 mg/dL in women and >7 mg/dL in men. P-values are exploratory and not corrected for multiple testing.
Table 2.
Measures of adipose tissue, as derived by MRI.
| Whole sample | no Hyperuricemia | Hyperuricemia | pvalue | |
|---|---|---|---|---|
| N = 371 | N = 298 (80.3%) | N = 73 (19.7%) | ||
| Total abdominal adipose tissue, l | 12.5 ± 5.3 | 11.7 ± 5.2 | 15.5 ± 4.7 | 3.68E-08 |
| Visceral adipose tissue, l | 4.5 ± 2.7 | 4.0 ± 2.5 | 6.5 ± 2.5 | 5.51E-14 |
| Subcutaneous adipose tissue, l | 8.0 ± 3.6 | 7.8 ± 3.6 | 9.0 ± 3.3 | 0.009 |
| HFF_HISTO, % | 8.6 ± 7.8 | 7.4 ± 7.2 | 13.3 ± 8.5 | 5.52E-09 |
| HFF_right lobe, % | 9.4 ± 8.5 | 8.1 ± 7.6 | 14.8 ± 9.9 | 6.52E-10 |
| HFF_left lobe, % | 7.8 ± 7.6 | 6.8 ± 7.2 | 11.8 ± 8.0 | 3.15E-07 |
| log(HFF_HISTO, %) | 1.8 ± 0.9 | 1.6 ± 0.9 | 2.3 ± 0.8 | 4.38E-10 |
| HFF_dixon, % | 8.3 ± 8.3 | 7.1 ± 7.7 | 13.0 ± 9.0 | 2.62E-08 |
| log(HFF_dixon, %) | 1.7 ± 0.9 | 1.5 ± 0.9 | 2.3 ± 0.8 | 3.28E-10 |
Data are presented as arithmetic means and standard deviations. HFF_HISTO: hepatic fat fraction as measured by high-speed T2-corrected multi-echo technique (HISTO). HFF_dixon: hepatic fat fraction, as measured by multiecho Dixon-sequence. Hyperuricemia was defined as serum uric acid levels >6 mg/dL in women and >7 mg/dL in men. P-values are exploratory and not corrected for multiple testing.
Figure 2.
Correlation of serum uric acid and hyperuricemia with MRI-derived adipose tissue compartments.
Cross-sectional association of SUA/hyperuricemia and adipose tissue
Results of the adjusted linear regression models describing the cross-sectional association of SUA or hyperuricemia with the different adipose tissue outcomes are presented in Table 3. A change of one standard deviation of SUA was associated with an increase of 0.82 l in VAT. The association was attenuated after adjustment for BMI and even further attenuated after adjustment for WC, but was still highly evident. Effect size estimates for the dichotomous variable hyperuricemia were even more pronounced.
Table 3.
Association of SUA and Hyperuricemia with MRI-derived adipose tissue content.
| VAT | SAT | HFF_HISTO | HFF_dixon | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95%-CI | p-value | R2 | β | 95%-CI | p-value | R2 | Estimate | 95%-CI | p-value | R2 | Estimate | 95%-CI | p-value | R2 | |
| Exposure SUA | ||||||||||||||||
| age + sex adjusted | 1.03 | [0.77, 1.29] | 4.8E-14 | 0.43 | 1.19 | [0.76, 1.62] | 9.6E-08 | 0.10 | 1.46 | [1.32, 1.62] | 1.3E-13 | 0.28 | 1.48 | [1.34, 1.63] | 3.8E-13 | 0.27 |
| men | 0.84 | [0.53, 1.15] | 2.79E-07 | 0.18 | 0.89 | [0.48, 1.31] | 3.34E-05 | 0.08 | 1.30 | [1.16, 1.45] | 4.47E-06 | 0.12 | 1.28 | [1.15, 1.43] | 1.20E-05 | 0.11 |
| women | 0.86 | [0.60, 1.11] | 1.08E-09 | 0.36 | 1.06 | [0.44, 1.68] | 8.69E-04 | 0.08 | 1.49 | [1.32, 1.67] | 1.79E-10 | 0.34 | 1.54 | [1.36, 1.73] | 1.68E-10 | 0.32 |
| fully adjusted | 0.82 | [0.58, 1.07] | 2.20E-10 | 0.56 | 0.81 | [0.39, 1.24] | 1.87E-04 | 0.27 | 1.32 | [1.20, 1.45] | 1.07E-08 | 0.44 | 1.31 | [1.20, 1.45] | 4.56E-08 | 0.45 |
| men | 0.66 | [0.36, 0.96] | 2.19E-05 | 0.34 | 0.65 | [0.25, 1.04] | 0.002 | 0.26 | 1.19 | [1.07, 1.31] | 6.70E-04 | 0.38 | 1.16 | [1.05, 1.28] | 0.003 | 0.38 |
| women | 0.55 | [0.30, 0.79] | 2.18E-05 | 0.54 | 0.5 | [−0.11, 1.12] | 0.110 | 0.26 | 1.36 | [1.2, 1.54] | 1.72E-06 | 0.40 | 1.38 | [1.21, 1.57] | 1.99E-06 | 0.42 |
| fully adjusted + WC | 0.39 | [0.20, 0.58] | 8.06E-05 | 0.76 | −0.15 | [−0.37, 0.08] | 0.198 | 0.81 | 1.19 | [1.09, 1.30] | 1.22E-04 | 0.54 | 1.17 | [1.07, 1.28] | 3.96E-04 | 0.55 |
| men | 0.23 | [0.01, 0.45] | 0.038 | 0.67 | −0.04 | [−0.25, 0.18] | 0.725 | 0.80 | 1.08 | [0.99, 1.19] | 0.082 | 0.51 | 1.07 | [0.97, 1.17] | 0.170 | 0.49 |
| women | 0.33 | [0.15, 0.51] | 4.83E-04 | 0.76 | −0.18 | [−0.50, 0.14] | 0.272 | 0.81 | 1.27 | [1.14, 1.43] | 4.00E-05 | 0.49 | 1.27 | [1.14, 1.43] | 5.31E-05 | 0.54 |
| fully adjusted + BMI | 0.51 | [0.30, 0.72] | 2.08E-06 | 0.70 | 0 | [−0.21, 0.22] | 0.976 | 0.82 | 1.23 | [1.13, 1.35] | 8.28E-06 | 0.51 | 1.22 | [1.12, 1.34] | 3.06E-05 | 0.52 |
| men | 0.34 | [0.09, 0.58] | 0.007 | 0.59 | 0.05 | [−0.16, 0.27] | 0.633 | 0.80 | 1.11 | [1.01, 1.21] | 0.029 | 0.47 | 1.09 | [0.99, 1.2] | 0.069 | 0.45 |
| women | 0.38 | [0.19, 0.56] | 1.13E-04 | 0.74 | −0.07 | [−0.36, 0.23] | 0.659 | 0.84 | 1.31 | [1.16, 1.46] | 1.41E-05 | 0.46 | 1.31 | [1.16, 1.48] | 1.87E-05 | 0.51 |
| Exposure Hyperuricemia | ||||||||||||||||
| age + sex adjusted | 1.76 | [1.22, 2.31] | 7.53E-10 | 0.40 | 1.62 | [0.71, 2.53] | 5.33E-04 | 0.22 | 1.72 | [1.39, 2.12] | 7.28E-07 | 0.22 | 1.77 | [1.42, 2.20] | 4.92E-07 | 0.21 |
| men | 1.55 | [0.83, 2.27] | 3.13E-05 | 0.15 | 1.30 | [0.34, 2.26] | 8.23E-03 | 0.03 | 1.55 | [1.21, 1.99] | 5.61E-04 | 0.08 | 1.60 | [1.25, 2.05] | 3.25E-04 | 0.08 |
| women | 2.41 | [1.54, 3.27] | 1.74E-07 | 0.31 | 2.39 | [0.35, 4.42] | 2.17E-02 | 0.04 | 2.20 | [1.46, 3.29] | 1.67E-04 | 0.21 | 2.32 | [1.49, 3.56] | 2.27E-04 | 0.19 |
| fully adjusted | 1.48 | [0.99, 1.98] | 9.50E-09 | 0.55 | 1.10 | [0.25, 1.94] | 0.011 | 0.25 | 1.48 | [1.22, 1.79] | 7.59E-05 | 0.41 | 1.49 | [1.22, 1.80] | 6.62E-05 | 0.43 |
| men | 1.33 | [0.68, 1.98] | 7.82E-05 | 0.34 | 1.02 | [0.15, 1.89] | 0.022 | 0.24 | 1.39 | [1.12, 1.72] | 0.003 | 0.37 | 1.39 | [1.12, 1.72] | 0.003 | 0.38 |
| women | 1.75 | [0.95, 2.55] | 2.82E-05 | 0.54 | 1.09 | [−0.93, 3.10] | 0.288 | 0.25 | 1.73 | [1.14, 2.64] | 0.010 | 0.33 | 1.70 | [1.09, 2.64] | 0.019 | 0.34 |
| fully adjusted + WC | 0.87 | [0.50, 1.24] | 5.49E-06 | 0.76 | −0.24 | [−0.67, 0.20] | 0.284 | 0.81 | 1.26 | [1.06, 1.49] | 9.01E-03 | 0.53 | 1.27 | [1.06, 1.51] | 0.008 | 0.54 |
| men | 0.62 | [0.16, 1.08] | 0.009 | 0.68 | −0.11 | [−0.56, 0.35] | 0.638 | 0.80 | 1.19 | [0.98, 1.43] | 0.075 | 0.51 | 1.21 | [0.99, 1.48] | 0.060 | 0.49 |
| women | 1.32 | [0.75, 1.88] | 9.94E-06 | 0.77 | −0.24 | [−1.27, 0.78] | 0.643 | 0.81 | 1.52 | [1.03, 2.23] | 0.033 | 0.45 | 1.43 | [0.97, 2.12] | 0.067 | 0.49 |
| fully adjusted + BMI | 1.06 | [0.66, 1.47] | 3.58E-07 | 0.71 | 0.05 | [−0.37, 0.46] | 0.827 | 0.82 | 1.34 | [1.12, 1.58] | 1.72E-03 | 0.49 | 1.34 | [1.12, 1.62] | 0.002 | 0.51 |
| men | 0.89 | [0.37, 1.40] | 7.74E-04 | 0.60 | 0.22 | [−0.24, 0.67] | 0.352 | 0.80 | 1.26 | [1.04, 1.54] | 0.021 | 0.47 | 1.27 | [1.04, 1.55] | 0.018 | 0.46 |
| women | 1.24 | [0.63, 1.84] | 9.14E-05 | 0.74 | −0.64 | [−1.59, 0.30] | 0.182 | 0.84 | 1.52 | [1.02, 2.27] | 0.040 | 0.40 | 1.42 | [0.94, 2.14] | 0.089 | 0.45 |
Results from a linear regression model with outcome adipose tissue for the whole study sample, as well as sex-stratified. Exposure was either the continuous variable SUA or the dichotomous variable Hyperuricemia, defined as serum uric acid levels >6 mg/dL in women and >7 mg/dL in men. The fully adjusted model was adjusted for age, sex, systolic blood pressure, total serum cholesterol, serum albumin, alcohol consumption, gout medication, diuretic medication, antihypertensive medication, lipid-lowering medication, antidiabetic medication and glycemic status. Outcome hepatic fat fraction (HFF) was log-transformed and resulting estimates therefore denote the percent change in mean HFF. Continuous exposure and adjustment variables were standardized (mean = 0, standard deviation = 1) before analysis; therefore estimates for models with exposure SUA denote the change in adipose tissue per one standard deviation of SUA. Note that for sex-stratified models, standardization was also performed in a stratified fashion. R2 denotes the percentage of variance in the outcome that is explained by the model. P-values are exploratory and not corrected for multiple testing. VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; HFF_HISTO: hepatic fat fraction as measured by high-speed T2-corrected multi-echo technique (HISTO); HFF_dixon: hepatic fat fraction, as measured by multiecho Dixon-sequence.
A change of one standard deviation of SUA was associated with an increase of 0.81 l in SAT, but this model could only explain 27% of the variation in SAT. After adjustment for BMI or WC, more than 80% of the variation in SAT could be explained, but the association of SUA was attenuated (compare Table 3).
Results for HFF were similar for the average HFF of the right and left liver lobe (HFF_HISTO) and HFF measured at the level of the portal vein (HFF_dixon). A change in one standard deviation of SUA was associated with an increase of 32% in HFF_HISTO and an increase of 31% in HFF_dixon. Comparable to the results for VAT, the association of SUA with HFF was attenuated after adjustment for BMI or WC, but was still evident; and the effect sizes for the dichotomous variable hyperuricemia were larger in size.
In a sensitivity analysis excluding all participants taking anti-gout medication, similar results were obtained (Supplementary Table S2). Furthermore, in another sensitivity analysis adjusting for serum fasting glucose instead of glycemic status, similar results were obtained (Supplementary Table S3).
Results for the male and female subsample were similar. However, the association of SUA and hyperuricemia with HFF was more pronounced in women.
Discussion
In this population-based sample, SUA and hyperuricemia were strongly related to increased storage of VAT and hepatic fat, independently of additional confounders and independently of anthropometric measures of overall adiposity. In contrast, the association of SUA and SAT was not independent of anthropometric measures.
The relation between SUA and anthropometric measures of overall adiposity is well established. Several studies found cross-sectional associations between BMI or WC with SUA or hyperuricemia26–28. Moreover, SUA levels have predictive ability with respect to weight gain. In normotensive, non-obese men, baseline SUA predicted 5-year changes in BMI29. A more recent study found that hyperuricemia in individuals without any other comorbidities predicted an increased 5-year risk of developing obesity, independent of other confounders30.
Without the appropriate methodology, VAT and SAT cannot be quantified separately. Therefore, analyses of their specific effects are constrained to studies using medical imaging; however those studies have mainly focused on total abdominal fat without drawing any distinction of VAT and SAT. In this regard, our results are in line with previous studies. In a cross-sectional study of 801 healthy Japanese men, Yamada et al. found an independent association between both hepatic and visceral fat tissue quantified by CT with hyperuricemia19. Another study using CT for quantification of visceral adiposity found that elevated SUA levels were associated with visceral fat accumulation31. Yet to our knowledge epidemiological studies assessing the relationship between subcutaneous adipose tissue and SUA are scarce.
Adipose tissue is a central endocrine organ and both VAT and SAT have key metabolic functions in energy homeostasis and glucose regulation. However, they have different cellular properties and exhibit major functional differences. Recent preliminary ontogenetic studies suggest that VAT and SAT cells originate from different embryonic mesodermal regions32.
The role of SUA on a cellular level is not quite clear. Mouse models have shown that adipose tissues can produce and secrete uric acid and secretion is enhanced in obesity33. Uric acid is also involved in the production of key pro-inflammatory adipokines in adipose tissue34. Especially VAT secretes pro-inflammatory adipocytokines and cytokine‐like factors such as tumor necrosis factor α and interleukin-6, thereby contributing to chronic low-grade inflammation. This might be mainly due to accelerated hypertrophic lipogenesis without appropriate angiogenesis, thus resulting in hypoxia, apoptotic and necrotic adipocytes, inflammation and an overbalance of unfavorably polarized M1-macrophages35. Our results demonstrate a strong cross-sectional association of SUA with VAT, beyond BMI and WC.
SAT, on the other hand, has a higher secretion of adiponectin, which has anti-inflammatory and insulin-sensitizing properties. Uric acid inhibits adiponectin production in adipocytes34,36 Adiponectin levels are negatively correlated with uric acid37 and adiponectin production is lower in obese individuals. In our analysis, we found higher amounts of SAT in individuals with hyperuricemia, but this association was attenuated after adjustment for anthropometric measures.
In the context of hepatic fat, cross-sectional associations of elevated SUA and NAFLD have been reported from smaller clinical and larger epidemiological studies38–41. Moreover, a predictive role of SUA has been suggested. In a 5-year retrospective cohort study of 4954 healthy Korean participants, whose intrahepatic fat was quantified using abdominal ultrasonography, Lee et al. found an association between hyperuricemia and an increased risk of developing NAFLD42. Jensen et al. used data from 8025 participants of a retrospective Japanese cohort study and found that accelerated SUA increase over a period of five years was associated with a higher risk of developing NAFLD from a healthy liver, independently of other metabolic confounders43.
Anatomy suggests a close interrelation of VAT and hepatic fat, as inflammation-enhancing adipokines and free fatty acids that are secreted from VAT can be directly deposited in the liver through the portal vein. In line with this, our results relating SUA to hepatic fat were similar to those relating SUA to VAT, as both showed strong cross-sectional associations which pertained after adjustment for BMI or WC. Several pathways potentially link hyperuricemia to hepatic lipogenesis: First, increased intra-cellular uric acid levels affect hepatocyte mitochondrial function. Thus, they promote an increased accumulation of reactive oxygen species and induce stress on the endoplasmatic reticulum which results in lipogenesis44,45. Second, increased uric acid levels upregulate fructokinase, an enzyme responsible for dietary fructose metabolism. Overexpression of fructokinase modulates the lipogenic effects of fructose by inducing increased triglyceride accumulation in hepatocytes46. Third, uric acid leads to downregulation of adenosine monophosphate activated kinase (AMPK). Lower expression of AMPK leads to reduced lipolysis, i.e. lower depletion of hepatocytes, lower fat oxidation and higher lipogenesis47. However, the directionality of this effect is not straightforward, as fatty liver has also been found to be a predictor of incident hyperuricemia48,49.
In sex-stratified analyses, we found similar tendencies for men and women. However, estimates of the association of SUA with hepatic fat were substantially higher in women. This is in line with two other population-based studies that found stronger associations of uric acid with NAFLD in women compared to men50,51. However, as other studies report stronger associations in men52,53, findings remain inconclusive. Genetic and hormonal sex differences in the development and pathophysiology of NAFLD have been established (recently reviewed by Lonardo et al.54) but the exact mechanism that would influence the differential association of SUA to hepatic fat is not yet clear.
Our study only comprises cross-sectional observational data and thus cannot establish the direction of the relation between SUA and adipose tissue nor its causality. Further research on larger, longitudinal cohort studies is needed to investigate the issue. Another option is the use of Mendelian Randomization analyses to investigate causality. In this respect, a recent meta-GWAS of serum urate identified 147 previously unknown SNPs which could be used to build an informative genetic instrument for such a Mendelian Randomization analysis55. On the other hand, data on the association of these SNPs with VAT, SAT and hepatic fat would be needed as well. Although GWAS of VAT, SAT and non-alcoholic fatty liver disease (NAFLD) have been conducted56–58, genome-wide summary statistics - i.e. results for every analyzed SNP and not only the significant ones - are not publicly available. In theory, these associations could also be estimated from our sample at hand, but given the limited size of the study sample, and given the fact that genetic effects are usually extremely small, such an estimation is severely statistically underpowered. Therefore, these analyses constitute interesting future research, when more GWAS data and increased sample sizes become available.
Besides these limitations, a major strength of our study is the use of a population-based sample from a well-characterized, prospective cohort study and the use of MRI, which allows a precise quantification of VAT, SAT and hepatic fat. Furthermore, we can rule out confounding by cardiovascular disease or renal impairment, as these were global exclusion criteria for the study sample.
In conclusion, our findings show how VAT, SAT and hepatic fat are differentially affected by serum uric acid, thus highlighting the different physiological roles of these fat compartments in uric acid metabolism.
Supplementary information
Acknowledgements
The authors wish to thank all participants of the KORA-MRI study and all involved radiologists, technicians and data managers for their contribution. This project has been financed in part through HGF Future Topic AMPro. The KORA study was initiated and financed by the Helmholtz Zentrum München – German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research (BMBF) and by the State of Bavaria. Furthermore, KORA research was supported within the Munich Center of Health Sciences (MC-Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ. The KORA-MRI sub-study received funding by the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft, BA 4233/4-1, http://www.dfg.de). The KORA-MRI sub-study was supported by an unrestricted grant from Siemens Healthcare (https://www.healthcare.siemens.de). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Conceptualization: S.R.; Methodology: S.R.; Statistical Analyses: S.R., D.D.; Evaluation and Interpretation: S.R., D.D., K.M.P.; Original Paper Draft: S.R., D.D.; Revision and Editing: S.R., D.D., K.M.P., K.S., F.B., A.P.; Main Study Design: A.P., F.B., K.S.; Data Curation and Quality Assurance: A.P., F.B., K.S.; Supervision: A.P., F.B. All authors have read and approved the final manuscript.
Data availability
Informed consent given by KORA study participants does not cover data posting in public databases. However, data are available upon request from KORA-gen (http://epi.helmholtz-muenchen.de/kora-gen/) by means of a project agreement. Requests should be sent to kora.passt@helmholtz-muenchen.de and are subject to approval by the KORA Board.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57459-z.
References
- 1.Doherty M. New insights into the epidemiology of gout. Rheumatology. 2009;48:ii2–ii8. doi: 10.1093/rheumatology/kep086. [DOI] [PubMed] [Google Scholar]
- 2.Obermayr RP, et al. Elevated Uric Acid Increases the Risk for Kidney Disease. Journal of the American Society of Nephrology. 2008;19:2407–2413. doi: 10.1681/asn.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tseng W-C, et al. Hyperuricemia Predicts an Early Decline in Renal Function among Older People: A Community-Based Cohort Study. Scientific reports. 2019;9:980. doi: 10.1038/s41598-018-37529-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clinical Biochemistry. 2016;49:636–642. doi: 10.1016/j.clinbiochem.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Bombelli M, et al. Uric acid and risk of new-onset metabolic syndrome, impaired fasting glucose and diabetes mellitus in a general Italian population: data from the Pressioni Arteriose Monitorate E Loro Associazioni study. Journal of hypertension. 2018;36:1492–1498. doi: 10.1097/hjh.0000000000001721. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/circulationaha.106.657627. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa, T. et al. A causal role for uric acid in fructose-induced metabolic syndrome. American Journal of Physiology-Renal Physiology (2006). [DOI] [PubMed]
- 8.Jossa F, et al. Serum uric acid and hypertension: the Olivetti heart study. J. Hum. Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 9.Kuwabara M, et al. Uric Acid Is a Strong Risk Marker for Developing Hypertension From Prehypertension. Hypertension (Dallas, Tex.: 1979) 2018;71:78–86. doi: 10.1161/HYPERTENSIONAHA.117.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, et al. Plasma Uric Acid and Hypertension in a Chinese Community: Prospective Study and Metaanalysis. Clinical Chemistry. 2009;55:2026–2034. doi: 10.1373/clinchem.2009.124891. [DOI] [PubMed] [Google Scholar]
- 11.Bhole V, Choi JWJ, Woo Kim S, de Vera M, Choi H. Serum Uric Acid Levels and the Risk of Type 2 Diabetes: A Prospective Study. The American Journal of Medicine. 2010;123:957–961. doi: 10.1016/j.amjmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehghan A, van Hoek M, Sijbrands EJG, Hofman A, Witteman JCM. High Serum Uric Acid as a Novel Risk Factor for Type 2 Diabetes. Diabetes Care. 2008;31:361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 13.Kodama S, et al. Association Between Serum Uric Acid and Development of Type 2 Diabetes. Diabetes Care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyngdoh T, et al. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PloS one. 2012;7:e39321. doi: 10.1371/journal.pone.0039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi S, et al. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism: clinical and experimental. 1997;46:1162–1165. doi: 10.1016/S0026-0495(97)90210-9. [DOI] [PubMed] [Google Scholar]
- 16.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism - Clinical and Experimental. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Younossi Z, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology &Amp; Hepatology. 2017;15:11. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 18.Larsson SC, Burgess S, Michaelsson K. Genetic association between adiposity and gout: a Mendelian randomization study. Rheumatology (Oxford) 2018;57:2145–2148. doi: 10.1093/rheumatology/key229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada A, et al. Association of Visceral Fat and Liver Fat With Hyperuricemia. Arthritis care & research. 2016;68:553–561. doi: 10.1002/acr.22729. [DOI] [PubMed] [Google Scholar]
- 20.Holle R, Happich M, Lowel H, Wichmann HE. KORA–a research platform for population based health research. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)) 2005;67(Suppl 1):S19–25. doi: 10.1055/s-2005-858235. [DOI] [PubMed] [Google Scholar]
- 21.Bamberg F, et al. Subclinical Disease Burden as Assessed by Whole-Body MRI in Subjects With Prediabetes, Subjects With Diabetes, and Normal Control Subjects From the General Population: The KORA-MRI Study. Diabetes. 2017;66:158–169. doi: 10.2337/db16-0630. [DOI] [PubMed] [Google Scholar]
- 22.Lorbeer R, et al. Correlation of MRI-derived adipose tissue measurements and anthropometric markers with prevalent hypertension in the community. Journal of hypertension. 2018;36:1555–1562. doi: 10.1097/hjh.0000000000001741. [DOI] [PubMed] [Google Scholar]
- 23.Storz, C. et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. The British journal of radiology, 20170808, 10.1259/bjr.20170808 (2018). [DOI] [PMC free article] [PubMed]
- 24.Lorbeer R, et al. Association between MRI-derived hepatic fat fraction and blood pressure in participants without history of cardiovascular disease. Journal of hypertension. 2017;35:737–744. doi: 10.1097/hjh.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira EP, Burini RC. High plasma uric acid concentration: causes and consequences. Diabetology & metabolic syndrome. 2012;4:12. doi: 10.1186/1758-5996-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Kang J, Kim G-T. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clinical rheumatology. 2018;37:2529–2538. doi: 10.1007/s10067-018-4130-2. [DOI] [PubMed] [Google Scholar]
- 27.Liu Dong-mei, Jiang Lin-di, Gan Lu, Su Yang, Li Fei. ASSOCIATION BETWEEN SERUM URIC ACID LEVEL AND BODY MASS INDEX IN SEX- AND AGE-SPECIFIC GROUPS IN SOUTHWESTERN CHINA. Endocrine Practice. 2019;25(5):438–445. doi: 10.4158/EP-2018-0426. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, et al. The relationship between body mass index and uric acid: a study on Japanese adult twins. Environmental Health and Preventive Medicine. 2015;20:347–353. doi: 10.1007/s12199-015-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum Uric Acid and Plasma Norepinephrine Concentrations Predict Subsequent Weight Gain and Blood Pressure Elevation. Hypertension (Dallas, Tex.: 1979) 2003;42:474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 30.Kuwabara Masanari, Niwa Koichiro, Hisatome Ichiro, Nakagawa Takahiko, Roncal-Jimenez Carlos A., Andres-Hernando Ana, Bjornstad Petter, Jensen Thomas, Sato Yuka, Milagres Tamara, Garcia Gabriela, Ohno Minoru, Lanaspa Miguel A., Johnson Richard J. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases. Hypertension. 2017;69(6):1036–1044. doi: 10.1161/HYPERTENSIONAHA.116.08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hikita M, et al. Relationship between hyperuricemia and body fat distribution. Internal medicine (Tokyo, Japan) 2007;46:1353–1358. doi: 10.2169/internalmedicine.46.0045. [DOI] [PubMed] [Google Scholar]
- 32.Chau Y-Y, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nature cell biology. 2014;16:367. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsushima Y, et al. Uric acid secretion from adipose tissue and its increase in obesity. The Journal of biological chemistry. 2013;288:27138–27149. doi: 10.1074/jbc.M113.485094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldwin W, et al. Hyperuricemia as a Mediator of the Proinflammatory Endocrine Imbalance in the Adipose Tissue in a Murine Model of the Metabolic Syndrome. Diabetes. 2011;60:1258–1269. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 36.Buemann B, et al. Possible role of adiponectin and insulin sensitivity in mediating the favorable effects of lower body fat mass on blood lipids. The Journal of clinical endocrinology and metabolism. 2006;91:1698–1704. doi: 10.1210/jc.2005-1062. [DOI] [PubMed] [Google Scholar]
- 37.Brzeska, A., Sołtysiak, M., Ziemak, J., Miazgowski, T. & Widecka, K. Plasma adiponectin in hypertensive patients with and without metabolic syndrome. Vol. 22 (2018).
- 38.Hu X-Y, et al. Risk factors and biomarkers of non-alcoholic fatty liver disease: an observational cross-sectional population survey. BMJ Open. 2018;8:e019974. doi: 10.1136/bmjopen-2017-019974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, et al. Relationship of serum uric acid level with non-alcoholic fatty liver disease and its inflammation progression in non-obese adults. Hepatology Research. 2017;47:E104–E112. doi: 10.1111/hepr.12734. [DOI] [PubMed] [Google Scholar]
- 40.Sertoglu E, et al. The relationship of serum uric acid with non-alcoholic fatty liver disease. Clinical Biochemistry. 2014;47:383–388. doi: 10.1016/j.clinbiochem.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Sirota JC, et al. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: Liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism: clinical and experimental. 2013;62:392–399. doi: 10.1016/j.metabol.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JW, et al. Serum uric Acid as a predictor for the development of nonalcoholic Fatty liver disease in apparently healthy subjects: a 5-year retrospective cohort study. Gut and liver. 2010;4:378–383. doi: 10.5009/gnl.2010.4.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen T, et al. Increased Serum Uric Acid over five years is a Risk Factor for Developing Fatty Liver. Scientific reports. 2018;8:11735. doi: 10.1038/s41598-018-30267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi Y-J, et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Laboratory Investigation. 2014;94:1114. doi: 10.1038/labinvest.2014.98. [DOI] [PubMed] [Google Scholar]
- 45.Yang, Y. et al. Effect of uric acid on mitochondrial function and oxidative stress in hepatocytes. Genet Mol Res15 (2016). [DOI] [PubMed]
- 46.Lanaspa MA, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PloS one. 2012;7:e47948. doi: 10.1371/journal.pone.0047948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanaspa MA, et al. Counteracting Roles of AMP Deaminase and AMP Kinase in the Development of Fatty Liver. PloS one. 2012;7:e48801. doi: 10.1371/journal.pone.0048801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryu S, et al. A Cohort Study of Hyperuricemia in Middle-aged South Korean Men. American Journal of Epidemiology. 2011;175:133–143. doi: 10.1093/aje/kwr291. [DOI] [PubMed] [Google Scholar]
- 49.Xu C, et al. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: One stone hits two birds. Journal of hepatology. 2015;62:1412–1419. doi: 10.1016/j.jhep.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 50.Wu SJ, et al. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults: a large population-based study. Medicine. 2015;94:e802. doi: 10.1097/MD.0000000000000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu L, et al. Association between serum uric acid and nonalcoholic fatty liver disease in community patients with type 2 diabetes mellitus. PeerJ. 2019;7:e7563. doi: 10.7717/peerj.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu K, et al. Gender effect of hyperuricemia on the development of nonalcoholic fatty liver disease (NAFLD): A clinical analysis and mechanistic study. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2019;117:109158. doi: 10.1016/j.biopha.2019.109158. [DOI] [PubMed] [Google Scholar]
- 53.Yu XL, Shu L, Shen XM, Zhang XY, Zheng PF. Gender difference on the relationship between hyperuricemia and nonalcoholic fatty liver disease among Chinese: An observational study. Medicine. 2017;96:e8164. doi: 10.1097/MD.0000000000008164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lonardo, A. et al. Sex differences in NAFLD: state of the art and identification of research gaps. Hepatology. [DOI] [PMC free article] [PubMed]
- 55.Tin A, et al. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet. 2019 doi: 10.1038/s41588-019-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu AY, et al. Multiethnic genome-wide meta-analysis of ectopic fat depots identifies loci associated with adipocyte development and differentiation. Nat Genet. 2017;49:125–130. doi: 10.1038/ng.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox CS, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet. 2012;8:e1002695. doi: 10.1371/journal.pgen.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Speliotes EK, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Informed consent given by KORA study participants does not cover data posting in public databases. However, data are available upon request from KORA-gen (http://epi.helmholtz-muenchen.de/kora-gen/) by means of a project agreement. Requests should be sent to kora.passt@helmholtz-muenchen.de and are subject to approval by the KORA Board.