Abstract
Affective touch has been associated with affiliative behavior during early stages of infant development; however, its underlying brain mechanisms are still poorly understood. This study used fNIRS (functional near-infrared spectroscopy) to examine both affective and discriminative touch in 7- month-old infants (n=35). Infants were provided affective stimuli on the forearm for 10 sec followed by a 20 sec rest period. The protocol was repeated for discriminative touch, and both affective and discriminative stimuli were given in a counterbalanced order. Brain activation (oxy-hemoglobin and deoxy-hemoglobin levels) in the somatosensory and temporal regions was registered during administration of the stimuli. There was an increase in oxy-hemoglobin and decrease in deoxy-hemoglobin only in the somatosensory region in response to both affective and discriminative touch. No other activations were found. Seven-month-old infants’ brain activation in the somatosensory cortex was similar for both discriminative and affective touch, but the stimuli did not elicit any activation in the temporal region/ pSTS. Our study is the first to suggest that 7-month-old infants do not yet recruit socio-emotional brain areas in response to affective touch.
Keywords: Somatosensory processing, Brain development, fNIRS, Infancy
1. Introduction
Skin is the largest human body sensory system, while touch is the first sensory system to develop in utero (Field, 2001, Montagu, 1986). These two systems interact in major development processes, and skin touch represents the major communication channel between a mother and her newborn (Barnett, 2005, Field, 2001, Field, 2010), playing a core role in attachment processes (Hertenstein et al., 2006).
The critical role of touch in developmental processes is well illustrated in previous studies examining touch deprivation. These studies have found that infants from depressed mothers, who reported decreased contact interactions with their child (i.e., less touching), exhibit more self-touch behaviors as compared to infants whose mothers reported higher levels of touching (Herrera et al., 2004). Another study found that infants who received less touch from their mothers also exhibit a greater prevalence of touching behaviors such as grabbing, patting, or pulling when exposed to stressful situations (Moszkowski et al., 2009). Only a few studies have examined the benefits of touching during early development stages. These studies also showed benefits of touch or physical contact with caregivers including: reduced weight/height, reduced rates of infection or hypothermia, and a reduction in the risk of premature mortality (Conde-Agudelo and Diaz-Rossello, 2016). Interestingly, touch has also been shown to positively impact important neurodevelopmental outcomes, such as better sleep-wake cycles, arousal modulation, and sustained exploration, during the post-partum period (Feldman et al., 2014; Feldman et al., 2002). A study by Feldman et al. also found long term benefits in the infant́s stress response, organized sleep, and cognitive control (Feldman et al., 2014).
To date, research on touch has mostly focused on a class of receptors responsible for transducing information regarding pressure/vibration, temperature, itch, and pain. These low-threshold mechanoreceptors (LTMs) present in the skin and joints are innervated by myelinated Aβ fibers that conduct high-speed impulses (50 m/s) and subserve discriminative functions (e.g., handling objects, exploring surfaces) (McGlone et al., 2007, McGlone et al., 2014). The discriminative system responds quickly and is responsible for detecting and discriminating external stimuli, needed for human survival. However, findings that mammals, such as cats and monkeys, present a more primitive system constituted by unmyelinated low-threshold mechanoreceptors (C tactile – CT afferents) existent in hairy skin led some researchers to test this mechanism in humans (Olausson et al., 2010). Studies with adults showed that CT fibers respond poorly in the processing of discriminative components of touch (linked instead to myelinated Aβ fibers) such as vibration and force/pressure (Olausson et al., 2002, Olausson et al., 2008a, Olausson et al., 2008b). CT fibers are, however, quite effective coding low-velocity/force stroking movements, with maximum firing at velocities between 1 and 10 cm−1, a speed consistent with that of the human caress (Essick et al., 2010, Loken et al., 2009, Olausson et al., 2010). Considering these properties, it has been hypothesized that CT fibers might be tuned to affiliative behaviors or affective touch present in mammals, including mother and infant.
Neuroimaging studies conducted with adults have shown that CT afferents activate a particular network of brain regions including the posterior insula, posterior superior temporal cortex (pSTS), medial prefrontal cortex, and dorsal anterior cingulate cortex (dACC) (Gordon et al., 2013, Olausson et al., 2002, Olausson et al., 2008a, Olausson et al., 2008b, Voos et al., 2013). According to recent studies, this network is already in place during childhood, with research showing that five year-olds have similar pSTS activations in response to affective touch when compared to both adolescents and adults (Bjornsdotter et al., 2014, Van de Winckel et al., 2013).
Although touch plays an important role early in infancy, the brain response to touch in infants is still poorly understood. Limited research in this area is partially justified by the lack of feasible neuroimaging techniques available for this population (Lloyd-Fox et al., 2010). The emergence of functional near-infrared spectroscopy (fNIRS) has, however, opened the opportunity to address this gap (Lloyd-Fox et al., 2010, Lloyd-Fox et al., 2016; Papademetriou et al., 2014; Lloyd-Fox et al., 2015), but, to our knowledge, only one study has looked into the effects of discriminative and affective touch among infants (i.e., 3-, 6- and 10 months of age) (Kida and Shinohara, 2013b), reporting that 10-month-old-infants, but not younger infants, present activations in the anterior prefrontal cortex in response to affective touch. This finding suggests that the period between 6 and 10 months might be critical for the development of the social-network involved in the processing of affective touch. However, in Kida and Shinoharás study, the administered stimuli (both discriminative and affective) were targeted at the palm of the infant’s hand (glabrous skin), a region where CT afferents are less prevalent and, therefore, brain responses to affective touch on the CT targeted areas are rather limited. The study also examined a single region of interest (anterior prefrontal cortex), which limits the ability to extrapolate findings to other regions likely to be recruited at these ages.
Empirical research suggests that affective touch, in both children and adults, activates key-nodes of the social brain, namely, the posterior insula, pSTS, medial prefrontal cortex, and dACC (Bjornsdotter et al., 2014, Gordon et al., 2013, Olausson et al., 2008a, Olausson et al., 2008b, Olausson et al., 2002, Voos et al., 2013). In summary, a review of relevant literature shows that research examining affective touch in infants is still quite scarce, despite advances in neuroimaging techniques, as we were only able to find one study involving infants as young as 10 months of age. Therefore, the present study examines the brain mechanisms associated with the processing of both affective and discriminative touch in 7-month-old infants using fNIRS. We used a paradigm that targets both the CT afferents (affective touch) and the Aβ fibers (discriminative touch), while examining two distinct regions of interest: the pSTS and the somatosensory cortex. We chose 7-month old infants to not only fill the population gap in the literature, but also because it is around this age that core social behaviors emerge, like discrimination of emotional displays (Leppanen and Nelson, 2006, Leppanen and Nelson, 2009) and attachment (Zeanah et al., 2011). Regarding the ROI, we chose to investigate the somatosensory region as the primary sensory region and the temporal region as the “social” region. Brain activity in the somatosensory region has been recorded previously following tactile stimulation in infants (Verriotis et al., 2016) and the temporal region, in particular the pSTS, has shown to be activated following affective touch in adults (Bennett et al., 2014). We hypothesized that 7-month-old infants would elicit a significant haemodynamic response in the somatosensory region in response to both affective and discriminative stimuli. In addition, we also hypothesized that the hemodynamic response to affective touch would also occur in adult-like areas, specifically the pSTS.
2. Methods
2.1. Participants
This study included 35 7-month-old infants (mean age 228.77 ± 89.19, range 214–244; 14 females) born full term (mean time in weeks 39.07 ± 1.22, range 36.6–41) with a normal birth weight (>2500 g, there was one infant who weighted 2350 g), and who had no reported hearing problems or neurological conditions. Fourteen additional infants were tested, but then excluded due to fussiness (n = 2) or not having at least 3 valid trials (n = 12). The total attrition rate was 28.6%. Infant demographics and developmental data are shown in Table 1.
Table 1.
Sample demographics.
| Characterization of the sample (n = 35) |
|---|
| Age at birth (weeks):39.074 ± 1.220 (36.6–41) |
| Age at study (days): −228.770 ± 9.197 (214–244) |
| Female infants: 14 |
| Weight at birth (g): 3384.235 ± 459.592 (2350–4390) |
| Height at birth (cm): 48.9 ± 2.64 (43–54) |
| Cesarean deliveries: 7 (20%) |
| Apgar 1: 9.33 ± 1.10 (5–10) |
| Apgar 10: 9.96 ± 0.19 (9–10) |
Infants were recruited from early parenting classes, social networks, and daycare centers. Mothers signed an informed consent prior to the start of the experiment and the experimental protocol was approved by the University of Minho Ethics Committee.
2.2. Stimuli
The study protocol included two types of stimuli: affective and discriminative touch. The administration of affective stimuli consisted of touching the infants with a 7 cm wide watercolor brush (Bennett et al., 2014, Kaiser et al., 2016) while the discriminative stimuli was administered using a squared-shape piece of wood 2 × 2 cm (Kida and Shinohara, 2013a). The affective stimulus was administered at a slow stroke speed (8 cm/s) and from a proximal-distal direction to the infant’s forearm. This velocity has been used in previous experiments and shown to adequately elicit CT fibers (Loken et al., 2009). Our selected protocol for manual administration of the affective stimuli has also been previously validated against automatic stimulation by a robot (Triscoli et al., 2013). The discriminative stimuli consisted of applying pressure on the forearm at 3 different points and from the proximal to the distal part of the arm for the same period of time (resulting in 21–24 stimuli per trial). The discriminative stimuli did not include any stroking movement, assuring that the fibers stimulated were the Aβ fibers. Both discriminative and affective stimuli were delivered to the right dorsal forearm of the infant (bare arm) and by one trained staff member.
Our protocol used a within-subject block design procedure. More specifically, there were two alternating blocks of each experimental condition (affective and discriminative), and each was administered 8 times (i.e., 8 trials) (Bennett et al., 2014). Blocks were counterbalanced between the subjects. One full trial consisted of 10 s of stimulation followed by a baseline period of 20 s of rest. Baseline stimuli (rest) consisted of the infant watching a silent movie (Czech cartoon Krtecek) (Fairhurst et al., 2014) that played continuously throughout the session. See Fig. 1 for schematic representation of the paradigm.
Fig. 1.
Conceptual scheme of the experimental design. Each complete block was done 8 times (i.e., 8 trials) and consisted of 1) tactile stimulus delivered for 10 s, and 2) 20 s of rest. The complete set of 8 trials was applied twice to each affective and discriminative conditions (2 affective + 2 discriminative) in a counterbalanced order. The order of the experimental conditions was randomized across participants.
2.3. NIRS recording
Hemodynamic responses were recorded using the UCL – fNIRS topography system (Everdell et al., 2005) with 12 sources and 6 detectors. This system uses 2 continuous wavelengths of source light at 780 and 850 nm to make spectroscopic measurements. Data were sampled every 100 ms (10 Hz) (for a detailed description of fNIRS methodology see Lloyd-Fox et al., 2010).
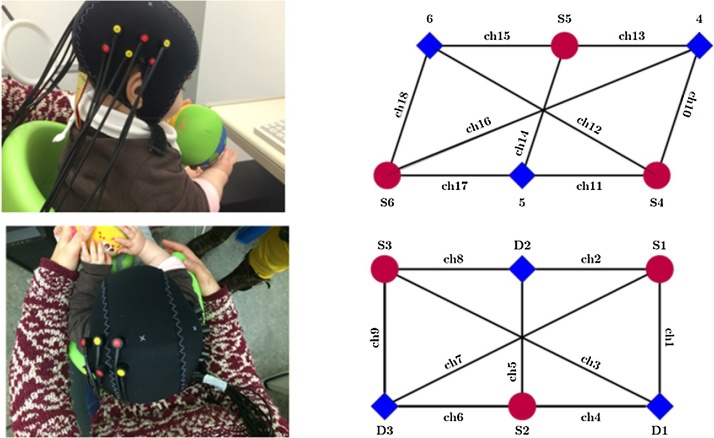
NIRS data were recorded from 18 channels. Nine channels were placed over the left somatosensory region, while the remaining 9 were placed over the right temporal region (See Fig. 2). This asymmetric sensor array was placed in a position so as to cover the left somatosensory cortex, as the contralateral primary somatosensory cortex (SI) is a well-established target region for processing discriminative touch (Aβ fibers). To target affective touch (CT fibers), we chose the right temporal region, because it has shown to be activated following affective touch administered to the right arm (Bennett et al., 2014, Gordon et al., 2013, Voos et al., 2013). The sensory array accounted for the limited number of channels and distinct somatosensory pathways for Aβ and CT fibers. The NIRS probe was customized for this experiment using an elastic cap (Easy Cap; reference 10–5 system) (See Fig. 2b) (Jurcak et al., 2007). The inter-optode distance was placed at 22 mm from the temporal region (except for the two longest channels that crossed the middle of the array, around 45 mm) and between 20 and 25 mm in the somatosensory region (except for the two longest channels that crossed the middle of the array, around 45 mm). Before the experiment, measurements of head circumference (44.16 cm ± 1.17) and nasion-inion (28.84 ± 2.74 cm) were taken to align the headgear with the 10–5 system. The cap was adjusted for head circumference and was placed centrally in the top of Cz, with channel 11 (correspondent to TP8) placed above the peri-auricular point.
Fig. 2.
NIRS data were recorded from 18 channels, 9 placed over the right temporal region (top left panel) and 9 placed over the left somatosensory region (bottom left panel). Red circles represent the sources and blue squares represent the detectors. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.4. Procedure
Infants were first familiarized with the setting for about 10 min, while the mother completed the consent form. Staff then performed head measurements of the infants and fitted the cap accordingly. The infant sat on a baby seat throughout the experiment in order to avoid physical contact with the mother.
The infant was seated approximately 70 cm away from the computer screen (screen size: 53*30). The experimenter sat in the right back hand side of the infant and parent, administered the stimuli, and re-directed the infant’s attention to the screen when needed. No visual contact took place between the experimenter and the infant during the course of the entire experiment. Parents were instructed to avoid any interaction with the infant unless he/she became fussy. The light of the room where the experiment took place was also adjusted (i.e., dimmed light) to avoid light interference with the screen. Breaks were introduced when needed to keep the infant engaged throughout the experiment. The experiment ended when the infant completed the 4 blocks (16 min) or when he/she became fussy. All the sessions were videotaped for data processing.
2.5. Data processing
All experimental sessions were videotaped and coded offline by a trained observer. Participants were only included if they completed at least 3 good quality trials out of the 16 total as included in our protocol (Lloyd-Fox et al., 2015). More specifically, we considered a trial of good quality when the following conditions were met: 1) the infant did not move the arm in any direction while the stimulus was being administered; 2) the infant was not looking at the experimenter or the mother while the stimulus was being administered; 3) the infant did not touch the experimenter or the mother while the stimulus was being delivered. Infants completed an average of 6.7 ± 3.1 affective trials (range 3–14) and 7.3 ± 2.8 (3–17) discriminative trials. There was no statistical difference in the number of trials completed (x2 = 119.79, p = 0.09).
Concentration changes in oxy-hemoglobin (HbO2), deoxy-hemoglobin (Hbb), and total hemoglobin (HbT) (μmol) were used as indicators of hemodynamic activity. Hemodynamic activity data were processed using HOMER2 (MGH – Martinos Center for Biomedical Imaging, Boston, MA, USA), a MATLAB (The MathWorks, Inc., Natick, MA, USA) software package. The attenuated light intensities measured by the detecting optodes were converted to optical density units and assessed for movement artifact using Principal Component Analysis (PCA) set at 0.9. No artifact correction was applied. We also chose not to perform artifact corrections and instead rejected trials as previously described, a method that has been considered more preferable than correction approaches (Cooper et al., 2012). Data were then low-pass filtered at 0.5 Hz (Lloyd-Fox et al., 2015) and used to calculate the change in concentration of the hemoglobin chromophore according to the modified Beer-Lambert Law (Delpy et al., 1988) and assuming a pathlenght factor of 5 (Duncan et al., 1995). Traces were segmented into 30 s epochs, starting 2 s prior to each stimulus. Similar to previous studies, the baseline was set as the mean concentration recorded between −2 to 0 s. (Ravicz et al., 2015). Following visual inspection of the data, we identified abnormal patterns in the 4 long channels (s-d distance 40 and 45 mm, channels 3, 7, 12 and 16) and therefore, these channels were not considered for analysis. No additional trials were rejected.
2.6. Statistical analysis
Statistical analysis was performed using SAS (Statistical Analysis Software) 9.4 v. For each channel, the maximum change in HbO2 and/or HHb was first assessed relative to the baseline using a linear mixed model. A significant increase in HbO2 and significant decrease in Hbb have been considered as indicators of cortical activation in NIRS studies with infants (Lloyd-Fox et al., 2010), but similar to previous research, we decided to limit our analysis in the changes observed only in HbO2 (Lloyd-Fox et al., 2014, Lloyd-Fox et al., 2016). After visual inspection of the grand mean concentration changes of each chromophore for each condition, we realized that the discriminative stimuli resulted in earlier hemodynamic responses when compared to the affective response. Based on this observation and to include the maximum signal changes of both stimuli, 3 time windows were determined for our second set of analyses: t1 = 10 to 15 s; t2 = 15–25 s, and t3 = 25 to 28 s. We computed linear mixed-models separately for each channel to test the differences between condition (2 levels) and time (3 levels), on HbO2 activity. The variable time was dummy coded to capture non-linear relations between HbO2 concentration and stimuli over time, and reported p values were not adjusted for type-I error rates.
3. Results
3.1. Discriminative touch. The analyses revealed significant hemodynamic increases in HbO2 centered over the somatosensory cortex for channel 1 at time 1 (t (102) = 3.86, p < 0.001) and time 2 (t (102) = 2.45, p = 0.016); for channel 2 at time 1 (t (102) = 3.04, p = 0.003) and time 2 (t (102) = 1.97, p = 0.051); and for channel 5 at time 1 (t (102) = 2.18, p = 0.032). There was also a significant hemodynamic increase in HbO2 over the temporal region/area correspondent to the STS, at time 1 for channel 13 (t (102) = 2.60, p = 0.010) (Table 2). Hemodynamic response function for discriminative touch in channel 1 is illustrated in Fig. 3.
Table 2.
Contrasts between discriminative and affective stimuli deemed statistically significant against baseline for HbO2.
| Discriminative > Baseline |
Affective > Baseline |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel | Time Window | β | SE | p value | Channel | Time Window | β | SE | p value |
| 1 | 1 | 0.034 | 0.009 | <0.001 | 1 | 2 | 0.021 | 0.011 | 0.051 |
| 2 | 0.021 | 0.009 | 0.016 | ||||||
| 2 | 1 | 0.049 | 0.016 | 0.003 | |||||
| 2 | 0.031 | 0.016 | 0.051 | Discriminative > Affective | |||||
| 5 | 1 | 0.026 | 0.012 | 0.031 | 2 | 1 | 0.064 | 0.024 | 0.009 |
| 2 | 0.055 | 0.024 | 0.026 | ||||||
| 13 | 1 | 0.035 | 0.014 | 0.010 | 5 | 1 | 0.033 | 0.014 | 0.019 |
time window 1: 10–15 seg; time window 2: 15–25 seg; channels 1, 2, and 5 are located in somatosensory region; channel 13 is located in temporal region. β value refers to relative hemodynamic change; SE refers to standard error.
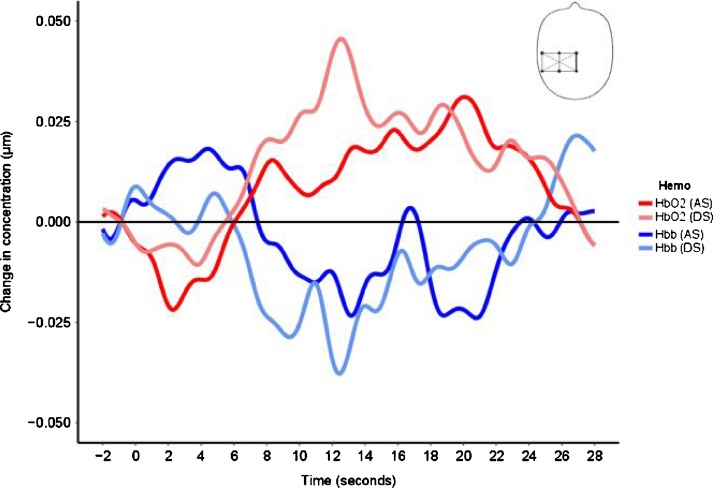
Fig. 3.
Hemodynamic response function in a channel placed in the somatosensory region (channel 1) for discriminative (DS) and affective stimuli (AS).
3.1. Affective touch.
Analysis of all the individual channels revealed a significant hemodynamic increase in HbO2 over the somatosensory cortex for channel 1 at time 2 (t (102) = 1.97, p = 0.051). There was no significant hemodynamic response over the temporal region/area correspondent to the STS (Table 2). Fig. 3 shows the hemodynamic response function for affective touch in channel 1.
3.2. Effect of condition and time
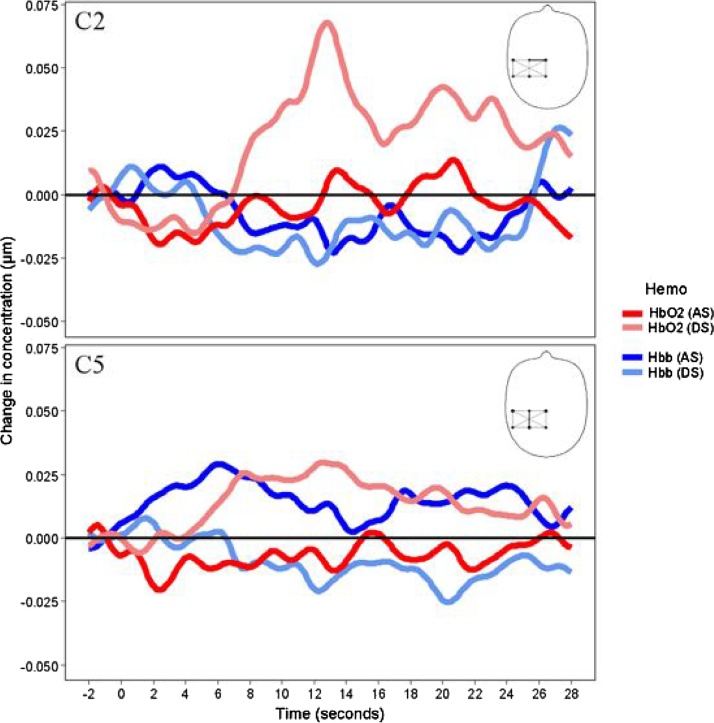
These analyses were limited to channels previously identified as capturing a statistically significant hemodynamic response against the baseline. For the discriminative > affective contrast in channels placed over the somatosensory region, channel 2 showed greater activation for discriminative stimuli compared to affective stimuli at time 1 (mean = 0.064, standard error = 0.024; t (170) = 2.64, p = 0.009), time 2 (mean = 0.055, standard error = 0.024; t (170) = 2.25, p = 0.026), and channel 5 at time 1 (mean= 0.033, standard error = 0.014; t (172) = 2.36, p = 0.019) (mean = 0.064, standard error = 0.024; t (170) = 2.64, p = 0.009) and time 2 (mean = 0.055, standard error = 0.024; t (170) = 2.25, p = 0.026); and channel 5 at time 1 (mean= 0.033, standard error = 0.014; t (172) = 2.36, p = 0.019). There were no statistically significant differences for channels placed over the temporal region. Hemodynamic response function for the channels 2 and 5 is illustrated in fig. 4.
Fig. 4.
Hemodynamic response function in channels 2 (C2) and 5 (C5) placed over the somatosensory region for discriminative (DS) and affective stimuli (AS).
4. Discussion
This study examined brain responses elicited by affective and discriminative touch in 7-month-old infants. As hypothesized, results showed that 7-month-old infants process both affective and discriminative components of touch in the somatosensory cortex. However, results did not support our second hypothesis that affective touch would further activate the right temporal region in infants, specifically the posterior STS, a critical region associated with social cognition (Bennett et al., 2014, Bjornsdotter et al., 2014). Both the activation of somatosensory region and lack of activation of the temporal region suggest that there are unique patterns in this age group and that activation in the brain as a result of affective touch follows a specific developmental trajectory.
Interestingly, no hemodynamic response function was found in any of the channels that have an approximate location to pSTS in response to the affective stimuli. These findings are similar to those of Kida and Shinohara (2013a), who found no activation in other social-related brain regions (e.g., anterior prefrontal cortex) when 3 and 6-month old infants were subject to a pleasant stimulus. Instead, this study found that activation at the anterior prefrontal cortex activation only occurred among 10-month old infants (Kida and Shinohara, 2013a). To the best of our knowledge no other study has looked at cortical response trajectories for affective touch during infancy; however, the combined findings of our study with Kida and Shinohara, (2013a) suggest that activation in response to affective touch in the temporal region does not occur until after the age of 7 months. One additional study that has looked at physiological responses to affective touch in 9-month-olds confirmed that infants are sensitive to affective touch (Fairhurst et al., 2014). Fairhurst et al. (2014) replicated findings from adult experiments (Loken et al., 2009) by showing that infants presented a lower heart rate when exposed a stimuli at a medium velocity and when compared to slower or faster velocities of touch. These results indicate that the medium velocity (CT optimal velocity) can lead to an increased parasympathetic activity and, consequently, a decrease in arousal. In this study, we used brush stroking to elicit stimulation of CT afferents, as used by other researchers (Bennett et al., 2014, Bjornsdotter et al., 2014, Olausson et al., 2002). Yet, it could be that in young ages, the stimulation needs to occur at human arm skin temperature (32°) (Ackerley et al., 2014), a condition that has been found to best activate the CT system. The temperature could represent the necessary condition to promote the sense of interpersonal touch and affiliative behavior. These findings might also be explained by social-cognitive developmental patterns that occur at these ages. Although infants are tuned to stimuli with social relevance from the time they are born (Johnson, 2005, Johnson et al., 1991), it is not until 9 months that they start presenting a group of core social behaviors like joint attention, social referencing, and implicit mental state attribution (Carpenter et al., 1998, Striano and Reid, 2006). See Happe and Frith (2014) for a review on the neurodevelopmental trajectory of social cognition (Happe and Frith, 2014).
Another important finding of this study was that both affective and discriminative components of touch elicited activation on contralateral somatosensory cortex. This finding goes along with other fNIRS studies reporting activations in contralateral somatosensory region in response to non-noxious tactile stimuli in infants aged 0–19 days (Verriotis et al., 2016). The cortical response to touch with this type of stimuli has been reported in infants as young as 28 weeks gestation (Bartocci et al., 2006). Our study is the first to report activation at the somatosensory cortex resultant from both discriminative and affective stimuli in infants. This work demonstrates that infants (i.e., 7-month-olds), similar to children and adolescents (Bjornsdotter et al., 2014), and adults (Gordon et al., 2013, Kaiser et al., 2016, Morrison, 2016), already process affective touch in the contralateral primary somatosensory cortex (SI).
We also found that at the somatosensory region, the discriminative stimuli resulted in an increased hemodynamic response and in a greater number of activated channels when compared to the affective stimuli. These patterns were unexpected, because affective and discriminative touch share a common pathway (the lemniscal pathway) responsible for carrying information from low-threshold mechanoreceptors with large myelinated (Aβ) afferents that conduct impulses relative to stimuli discrimination/detection. The majority of neuroimaging studies with adults have examined specifically both affective and discriminative stimuli separately and, therefore, do not allow for direct comparison between these two stimuli. However, a recent study by Morrison (2016) used an activation likelihood estimate (ALE) meta-analysis to compare both stimuli and concluded that although the affective and discriminative touch activations overlap in primary and secondary cortices, the activation likelihood between the two is distinct. Morrison and colleagues specifically found that primary somatosensory cortices (SI) are more likely to be activated for discriminative touch while the posterior insula is more likely to be activated in response to affective touch (Morrison, 2016). These results showed that although there might be a dissociation for affective and discriminative touch in some brain regions, the two kinds of touch share somatosensory co-activations.
Our study also found that there was some variability associated with the timing of the activation pattern for affective and discriminative touch. The peak of the activation of oxy-hemoglobin in the somatosensory cortex that was associated with discriminative touch occurred between 10 and 15 s, while the activation associated with affective touch occurred later, between 15 and 25 s. No other study has looked at this region for affective touch, and the study that examined affective touch in infants did not report peak activation (Kida and Shinohara, 2013a). Studies with adults have shown that affective touch in the pSTS peaks between 8.4 and 9.7 s for a stimulus presented for 6 s (Bennett et al., 2014). The differences in peak latency between our study and that of Bennett, can be explained by the differences in the paradigm used. Another possible explanation for the differences in latency is that the developmental trajectory for hemodynamic response to visual, auditory, and tactile (pain) seems to become more rapid with age (Lloyd-Fox et al., 2016, Slater et al., 2006). For discriminative touch, most activations occurred earlier, between 10 and 15 s. This replicates other studies using an exclusively tactile paradigm (Verriotis et al., 2016) or sensorimotor paradigm combining touch and movement (Kusaka et al., 2011). Furthermore, our differential temporal responses for discriminative and affective touch support the hypothesis that these two kinds of touch are subserved by distinct fibers: 1) myelinated fibers that are fast and responsible for the discriminative aspects of touch, and 2) unmyelinated fibers that are slow and have a smaller preponderance in human survival. It is likely that the timing of processing at the cortical level occurs faster for the discriminative touch (Loken et al., 2009, McGlone et al., 2014, Morrison et al., 2010).
An unexpected result was the hemodynamic response to discriminative touch in an optode placed over the temporal region (channel 13) that showed activation between 10 and 15 s. Although our aim was to target the STS, the channels placed over the temporal region cover a broader area that goes beyond the STS (Lloyd-Fox et al., 2014). This might explain the activation in channel 13, the location of which is close to the temporo-parietal region (Lloyd-Fox et al., 2014), where the secondary somatosensory cortex is located; it is likely that this region is also sensitive to discriminative touch (Morrison, 2016). Other discriminative stimuli, namely vibrotactile stimuli, are known to activate the temporal lobe (Beauchamp et al., 2008, Davidovic et al., 2016), which suggests that the region is not only sensitive to affective touch.
In addition, there was also a time and channel interaction worth mentioning. For the one channel placed over the somatosensory cortex, no differences were found between the two types of stimuli, confirming that the primary somatosensory cortex processes both kinds of stimuli (Morrison, 2016). However, for the two other channels placed in the same region, discriminative touch resulted in greater activation compared with affective touch between 10 and 25 s, which was probably related to a deactivation observed for the affective touch. Finally, the lack of differences between stimuli in the channels placed over the temporal region reinforces that at the age of 7 months, it is possible that tactile stimuli are mainly processed in primary sensory regions.
To the best of our knowledge, this is the first study examining the differential effects of discriminative and affective touch in infants. However, there were a few methodological constraints worth mentioning. The use of different types of stimulation (brush and wood block) might have led to different volumes of touch. Still, we defined the same touch contact time per stimuli for both brush and wood block. An important limitation of the study was the use of an asymmetric sensor pad, which limits the interpretation regarding the recruitment of pSTS for the processing of affective touch. Future studies should target both hemispheres to fully understand the role of this brain region in touch processing mechanisms. Another limitation of the study was that we selected a baseline stimulus (i.e., silent video) that had previously been used in a touch paradigm with infants (Fairhurst et al., 2014). Thus, this might not induce a true baseline state, though we found this to be an effective approach to capture the infant’s attention and improve the signal-to-noise-ratio. Future studies should test the utility of different baseline paradigms (e.g., sound movies instead of silent movies for example) and identify how these interfere with the hemodynamic responses. Finally, although our design minimized exclusion and non-compliance with the protocol, there was still a small number of participants that were not included in the final sample due to fussiness or for not enduring the minimum number of good trials. Future research needs to address brain activation profiles that are likely to differ between non-compliant and compliant infants.
In conclusion, the role of touch for mammals’ survival is undeniable. Touch is a critical modality for affiliative behaviors and dyadic reciprocity between the caregiver and the offspring. However, little is known about what happens at the brain level when infants are subject to different kinds of tactile stimulation. Our study contributes to a better understanding of the brain mechanisms underlying the processing of affective and discriminative touch among 7-month-olds. Future research should examine these mechanisms among infants of various age groups and using longitudinal designs to account for parallel developmental processes that take place as infants age. Researchers should also consider including observational measures to understand how brain responses to affective touch relate to individual infant differences and maternal care in infancy. Such studies can contribute to an enhanced understanding of how affective touch is linked to social-emotional development in infancy and childhood, and ultimately contribute to a heightened awareness of sensory processes in some neurodevelopmental disorders, namely on autism spectrum disorder.
Author contributions
HM conceived of the study, participated in its design, collected data, performed data processing and analysis, and drafted the manuscript. ICL collected data and participated in data processing; OG and AS conceived of the study, participated in the study design, and helped draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
None.
Contributor Information
Helga O. Miguel, Email: helga.miguel@gmail.com.
Isabel C. Lisboa, Email: isabel.lisbo@gmail.com.
Óscar F. Gonçalves, Email: goncalves@psi.uminho.pt.
Adriana Sampaio, Email: adriana.sampaio@psi.uminho.pt.
References
- Ackerley R., Backlund Wasling H., Liljencrantz J., Olausson H., Johnson R.D., Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J. Neurosci. 2014;34(8):2879–2883. doi: 10.1523/JNEUROSCI.2847-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett L. Keep in touch: the importance of touch in infant development. Infant Obs. 2005;8(2):115–123. [Google Scholar]
- Bartocci M., Bergqvist L.L., Lagercrantz H., Anand K.J. Pain activates cortical areas in the preterm newborn brain. Pain. 2006;122(1–2):109–117. doi: 10.1016/j.pain.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S., Yasar N.E., Frye R.E., Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 2008;41(3):1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R.H., Bolling D.Z., Anderson L.C., Pelphrey K.A., Kaiser M.D. fNIRS detects temporal lobe response to affective touch. Soc. Cogn. Affect. Neurosci. 2014;9(4):470–476. doi: 10.1093/scan/nst008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter M., Gordon I., Pelphrey K.A., Olausson H., Kaiser M.D. Development of brain mechanisms for processing affective touch. Front. Behav. Neurosci. 2014;8(24) doi: 10.3389/fnbeh.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M., Nagell K., Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr. Soc. Res. Child Dev. 1998;63(4):1–143. i–vi. [PubMed] [Google Scholar]
- Conde-Agudelo A., Diaz-Rossello J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2016;8 doi: 10.1002/14651858.CD002771.pub4. CD002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.J., Selb J., Gagnon L., Phillip D., Schytz H.W., Iversen H.K., Boas D.A. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 2012;6:147. doi: 10.3389/fnins.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovic M., Jonsson E.H., Olausson H., Bjornsdotter M. Posterior superior temporal sulcus responses predict perceived pleasantness of skin stroking. Front. Hum. Neurosci. 2016;10:432. doi: 10.3389/fnhum.2016.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D.T., Cope M., van der Zee P., Arridge S., Wray S., Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988;33(12):1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- Duncan A., Meek J.H., Clemence M., Elwell C.E., Tyszczuk L., Cope M., Delpy D.T. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol. 1995;40(2):295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- Essick G.K., McGlone F., Dancer C., Fabricant D., Ragin Y., Phillips N., Guest S. Quantitative assessment of pleasant touch. Neurosci. Biobehav. Rev. 2010;34(2):192–203. doi: 10.1016/j.neubiorev.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Everdell N.L., Gibson A.P., Tullis I.D.C., Vaithianathan T., Hebden J.C., Delpy D.T. A frequency multiplexed near-infrared topography system for imaging functional activation in the brain. Rev. Sci. Instrum. 2005;76:093705. [Google Scholar]
- Fairhurst M.T., Loken L., Grossmann T. Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychol. Sci. 2014;25(5):1124–1131. doi: 10.1177/0956797614527114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Weller A., Sirota L., Eidelman A.I. Skin-to-Skin contact (Kangaroo care) promotes self-regulation in premature infants: sleep-wake cyclicity, arousal modulation, and sustained exploration. Developmental Psychology. 2002;38(2):194–207. doi: 10.1037//0012-1649.38.2.194. [DOI] [PubMed] [Google Scholar]
- Feldman R., Rosenthal Z., Eidelman A.I. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry. 2014;75(1):56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Field T. 2001. Touch. Cambridge, MA. [Google Scholar]
- Field T. Touch for socioemotional and physical well-being: a review. Dev. Rev. 2010;30(4):367–383. [Google Scholar]
- Gordon I., Voos A.C., Bennett R.H., Bolling D.Z., Pelphrey K.A., Kaiser M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapp. 2013;34(4):914–922. doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F., Frith U. Annual research review: towards a developmental neuroscience of atypical social cognition. [Review] J. Child Psychol. Psychiatry. 2014;55(6):553–557. doi: 10.1111/jcpp.12162. [DOI] [PubMed] [Google Scholar]
- Herrera E., Reissland N., Shepherd J. Maternal touch and maternal child-directed speech: effects of depressed mood in the postnatal period. J. Affect. Disord. 2004;81(1):29–39. doi: 10.1016/j.jad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hertenstein M.J., Verkamp J.M., Kerestes A.M., Holmes R.M. The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 2006;132(1):5–94. doi: 10.3200/mono.132.1.5-94. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Dziurawiec S., Ellis H., Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1–2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Subcortical face processing. Nat. Rev. Neurosci. 2005;6(10):766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage. 2007;34(4):1600–1611. doi: 10.1016/j.neuroimage.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Yang D.Y., Voos A.C., Bennett R.H., Gordon I., Pretzsch C., Pelphrey K.A. Brain mechanisms for processing affective (and nonaffective) touch are atypical in autism. Cereb. Cortex. 2016;26(6):2705–2714. doi: 10.1093/cercor/bhv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida T., Shinohara K. Gentle touch activates the anterior prefrontal cortex: an NIRS study. Neurosci. Res. 2013;76(1–2):76–82. doi: 10.1016/j.neures.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Kida T., Shinohara K. Gentle touch activates the prefrontal cortex in infancy: an NIRS study. Neurosci. Lett. 2013;541:63–66. doi: 10.1016/j.neulet.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Kusaka T., Isobe K., Miki T., Ueno M., Koyano K., Nakamura S., Itoh S. Functional lateralization of sensorimotor cortex in infants measured using multichannel near-infrared spectroscopy. Pediatr. Res. 2011;69:430–435. doi: 10.1203/PDR.0b013e3182125cbd. 5 Pt. 1. [DOI] [PubMed] [Google Scholar]
- Leppanen J.M., Nelson C.A. The development and neural bases of facial emotion recognition. Adv. Child Dev. Behav. 2006;34:207–246. doi: 10.1016/s0065-2407(06)80008-x. [DOI] [PubMed] [Google Scholar]
- Leppanen J.M., Nelson C.A. Tuning the developing brain to social signals of emotions. Nat. Rev. Neurosci. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Richards J.E., Blasi A., Murphy D.G., Elwell C.E., Johnson M.H. Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics. 2014;1(2):025006. doi: 10.1117/1.NPh.1.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Szeplaki-Kollod B., Yin J., Csibra G. Are you talking to me? Neural activations in 6-month-old infants in response to being addressed during natural interactions. Cortex. 2015;70:35–48. doi: 10.1016/j.cortex.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Begus K., Halliday D., Pirazzoli L., Blasi A., Papademetriou M., Elwell C.E. Cortical specialisation to social stimuli from the first days to the second year of life: a rural Gambian cohort. Dev. Cogn. Neurosci. 2016 doi: 10.1016/j.dcn.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12(5):547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- McGlone F., Vallbo A.B., Olausson H., Loken L., Wessberg J. Discriminative touch and emotional touch. Can. J. Exp. Psychol. 2007;61(3):173–183. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- McGlone F., Wessberg J., Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82(4):737–755. doi: 10.1016/j.neuron.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Montagu A. Harper & Row; New York: 1986. Touching: the Human Significance of the Skin. [Google Scholar]
- Morrison I., Loken L.S., Olausson H. The skin as a social organ. [Review] Exp. Brain Res. 2010;204(3):305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- Morrison I. ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch. Hum. Brain Mapp. 2016;37(4):1308–1320. doi: 10.1002/hbm.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszkowski R.J., Stack D.M., Girouard N., Field T.M., Hernandez-Reif M., Diego M. Touching behaviors of infants of depressed mothers during normal and perturbed interactions. Infant Behav. Dev. 2009;32(2):183–194. doi: 10.1016/j.infbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., Morin C., Wallin B.G., Starck G., Bushnell M.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5(9):900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson H., Cole J., Rylander K., McGlone F., Lamarre Y., Wallin B.G., Vallbo A. Functional role of unmyelinated tactile afferents in human hairy skin: sympathetic response and perceptual localization. Exp. Brain Res. 2008;184(1):135–140. doi: 10.1007/s00221-007-1175-x. [DOI] [PubMed] [Google Scholar]
- Olausson H.W., Cole J., Vallbo A., McGlone F., Elam M., Kramer H.H., Bushnell M.C. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci. Lett. 2008;436(2):128–132. doi: 10.1016/j.neulet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010;34(2):185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Ravicz M.M., Perdue K.L., Westerlund A., Vanderwert R.E., Nelson C.A. Infants’ neural responses to facial emotion in the prefrontal cortex are correlated with temperament: a functional near-infrared spectroscopy study. Front. Psychol. 2015;6(922) doi: 10.3389/fpsyg.2015.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater R., Cantarella A., Gallella S., Worley A., Boyd S., Meek J., Fitzgerald M. Cortical pain responses in human infants. J. Neurosci. 2006;26(14):3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striano T., Reid V.M. Social cognition in the first year. [Research support, non-U. S. gov’t] Trends Cogn. Sci. 2006;10(10):471–476. doi: 10.1016/j.tics.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Triscoli C., Olausson H., Sailer U., Ignell H., Croy I. CT-optimized skin stroking delivered by hand or robot is comparable. Front. Behav. Neurosci. 2013;7(208) doi: 10.3389/fnbeh.2013.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Winckel A., Klingels K., Bruyninckx F., Wenderoth N., Peeters R., Sunaert S., Feys H. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res. Dev. Disabil. 2013;34(1):183–197. doi: 10.1016/j.ridd.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Verriotis M., Fabrizi L., Lee A., Cooper R.J., Fitzgerald M., Meek J. Mapping cortical responses to somatosensory stimuli in human infants with simultaneous near-Infrared spectroscopy and event-Related potential recording. eNeuro. 2016;3(2) doi: 10.1523/ENEURO.0026-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos A.C., Pelphrey K.A., Kaiser M.D. Autistic traits are associated with diminished neural response to affective touch. Soc. Cogn. Affect. Neurosci. 2013;8(4):378–386. doi: 10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C.H., Berlin L.J., Boris N.W. Practitioner review: clinical applications of attachment theory and research for infants and young children. J. Child Psychol. Psychiatry. 2011;52(8):819–833. doi: 10.1111/j.1469-7610.2011.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]