Abstract
Neuroimaging studies in adults showed that cortical midline regions including medial prefrontal cortex (mPFC) and posterior parietal cortex (PPC) are important in self-evaluations. The goals of this study were to investigate the contribution of these regions to self-evaluations in late childhood, adolescence, and early adulthood, and to examine whether these differed per domain (academic, physical and prosocial) and valence (positive versus negative). Also, we tested whether this activation changes across adolescence. For this purpose, participants between ages 11–21-years (N = 150) evaluated themselves on trait sentences in an fMRI session. Behaviorally, adolescents rated their academic traits less positively than children and young adults. The neural analyses showed that evaluating self-traits versus a control condition was associated with increased activity in mPFC (domain-general effect), and positive traits were associated with increased activity in ventral mPFC (valence effect). Self-related mPFC activation increased linearly with age, but only for evaluating physical traits. Furthermore, an adolescent-specific decrease in striatum activation for positive self traits was found. Finally, we found domain-specific neural activity for evaluating traits in physical (dorsolateral PFC, dorsal mPFC) and academic (PPC) domains. Together, these results highlight the importance of domain distinctions when studying self-concept development in late childhood, adolescence, and early adulthood.
Keywords: Self, fMRI, Adolescence, Development, Medial prefrontal cortex, Self-concept
1. Introduction
Adolescence is a life period during which the self-concept undergoes significant changes. For example, adolescents form increasingly abstract self-descriptions and they develop a more differentiated self-concept that varies across domains and different social contexts (Harter, 2012). It is thought that these changes are triggered by the development of cognitive abilities, by taking on new social roles, and by changes in the environment of adolescents (Brown, 2004, Harter, 2012). Importantly, these developmental changes become increasingly domain-specific, with, for example, more differentiated self-evaluations for social, physical and academic domains (Marsh and Ayotte, 2003). These domain-specific self-evaluations may be dependent on contextual factors such as school environment and social relations (Harter, 2012). Additionally, although the positivity bias (the overestimation of own abilities, and unrealistically positive self-views) is thought to decline from childhood to adolescence (Harter, 2012, Trzesniewski et al., 2003), the exact development of the valence of self-evaluations in adolescence is still debated (Steiger et al., 2014). It has been hypothesized that the development of the valence of self-concept also differs per domain (Cole et al., 2001, Shapka and Keating, 2005).
Concurrent with changes in self-evaluations, adolescents show large functional and structural changes in brain structures that are implicated in self-referential processing such as the medial prefrontal cortex (mPFC) and posterior parietal cortex (PCC) (Mills et al., 2014, Pfeifer and Peake, 2012, Somerville et al., 2013). However, to date neuroimaging studies have not yet examined domain- and valence-specificity of self-evaluations in adolescence. The current study set out to test domain and valence differences in self-evaluation in adolescence using functional neuroimaging methods.
1.1. Self-related brain regions
The role of the mPFC in self-evaluations has been well studied in adults. In these studies, participants evaluated whether, and to what extent, certain traits were descriptive of the self. Elevated activation in the ventral and rostral mPFC has consistently been found for self-evaluations relative to other-evaluations or baseline activation (for a review and meta-analyses, see Amodio and Frith, 2006, Denny et al., 2012, Murray et al., 2012). Interestingly, some studies reported stronger ventral mPFC activation for evaluating positive traits than for evaluating negative traits (Moran et al., 2006, van der Cruijsen et al., 2017) and this region has previously been linked to positive valuation processes (Kringelbach and Rolls, 2004, Peters and Büchel, 2010). This suggests that the ventral part of the mPFC is especially involved in affective evaluation of self-traits (D’Argembeau, 2013).
In addition to studies focusing on brain regions for general self-evaluations, several studies reported that a broader network of brain regions is involved when comparing self-evaluations across different domains. Evaluating physical traits has been associated with activation in dorsolateral prefrontal cortex (DLPFC), whereas character evaluations have been associated with posterior cingulate cortex (PCC) activation (Moran et al., 2010, Pfeifer et al., 2013, van der Cruijsen et al., 2017).
Recently, several studies investigated the neural activations underlying self-evaluations in childhood and adolescence. A study focusing on 14-to-16-year-old adolescents revealed stronger ventral mPFC, dorsal mPFC and medial posterior parietal cortex (mPPC) activation for evaluations of self compared to evaluations of others (Romund et al., 2017). Other studies reported increased rostral mPFC activation in children (9–10-years) compared to adults (23–31-years) for self-evaluations relative to evaluations of others (Pfeifer et al., 2009, Pfeifer et al., 2007). However, other studies reported similar cortical midline activation for direct self-evaluations for early adolescents (11–14-years) and adults (22–31-years) (Jankowski et al., 2014), and showed that ventral mPFC activation for self-evaluations increased with age and pubertal development in the social but not the academic domain from age 10–13 years (Pfeifer et al., 2013). Similarly, a prior study that found rostral ACC activation during memory encoding for traits of self versus mother, showed that this activation increased from age 7–13 years (Ray et al., 2009). A similar study revealed that activation in this region for self versus a distant other increased across adolescence from age 13–19 (Dégeilh et al., 2015).
Together these studies suggest that mid to late childhood/early adolescence (7–13 years) may be an important period for the development of brain regions underlying self-evaluations and provide initial evidence that these changes may be domain-specific. That is, the development of self-related brain activation might differ for evaluating the self in different domains. However, prior studies included adolescents in narrow age ranges and varying age groups. Consequently, these studies mostly compared specific age groups (children and/or adolescents) with adults. A developmental pattern of self-related brain activation across childhood, adolescence, and early adulthood has not yet been tested. Moreover, it remains to be determined whether adolescents also show neural activations in distinct regions for evaluations in different domains, similar to what has previously been found in adults (Moran et al., 2010, van der Cruijsen et al., 2017).
1.2. Current study
In the current study, we aimed to test domain- and valence-specificity of self-concept development in adolescence, by including a large sample (N = 150) of participants across a broad age range from (11–21-years). For this purpose, participants evaluated themselves on descriptions of positive and negative traits in three domains (academic, physical, prosocial). Our specific aims were 1) to investigate whether ventral/rostral mPFC was more active for self-evaluations compared to a baseline condition in adolescents (Amodio and Frith, 2006, Denny et al., 2012, Murray et al., 2012, Pfeifer et al., 2013, Pfeifer et al., 2007), 2) to unravel domain-specific neural activation with a focus on DLPFC for physical self-evaluations, and PCC for character (academic and prosocial) self evaluations (Moran et al., 2010, van der Cruijsen et al., 2017), 3) to test whether ventral mPFC is more active for evaluating positive than negative self-traits in adolescence, similar to what has previously been reported in adults (Moran et al., 2006, van der Cruijsen et al., 2017), and 4) to explore whether activation in these brain regions would show age-related changes across adolescence (Dégeilh et al., 2015, Jankowski et al., 2014, Pfeifer et al., 2013, Pfeifer et al., 2009, Pfeifer et al., 2007, Ray et al., 2009).
We tested for linear changes with age (both positive and negative) based on studies that compared children/early adolescents with adults (Jankowski et al., 2014, Pfeifer et al., 2009). Even though no prior studies examined changes in self-evaluations from mid to late adolescence, a prior study that examined self-consciousness showed stronger mPFC activation in mid adolescents compared to children and adults when participants believed they were being observed by others (Somerville et al., 2013). Therefore, we also tested whether the activation in self-related brain regions would show a quadratic change.
2. Method
2.1. Participants
This study was part of a larger study (the Leiden Self-Concept study). Participants were 160 right-handed children, adolescents, and young adults, of whom 10 were excluded due to the following reasons: excessive head movements during the fMRI scans (more than 3 mm across the full run, n = 8), did not complete scan (n = 1), and technical error (n = 1). Consequently, a total of 150 healthy participants (80 female) aged between 11 and 21 years old (mean age = 15.7, SD = 2.9) were included in the analyses. Motion correlated negatively with age, indicating that older participants moved less during the scan than younger participants (r = −0.314, p < 0.001). We added motion parameters to all the analyses to control for these differences (see below).
All participants reported normal or corrected-to-normal vision, and an absence of neurological or psychiatric impairments. Participants completed two subtests of the WISC-III or WAIS-III (Similarities and Block Design). Estimated IQ scores fell between 80.0 and 137.5 (M = 110.30, SD = 11.06), and IQ did not correlate with age (r(148) = 0.007, p = 0.934). Pubertal status was assessed using the Pubertal Development Scale (PDS; Petersen et al., 1988). Pubertal status scores ranged from 5 to 20 in girls (mean = 15.3, SD = 3.5), and from 5 to 20 in boys (mean = 14.3, SD = 4.2). This corresponds to an average PDS stage of 4.15 (range 1–5) for girls, and 3.57 (range 1–5) for boys.
All participants and both parents of minors signed informed consent before inclusion in the study. The study was approved by the University Medical Ethical Committee. Prior to the scan session, participants were screened for MRI contra-indications and self-reported psychiatric diagnoses or psychotropic medication. All scans were viewed by a radiologist and no clinically relevant findings were observed.
2.2. Task description
All participants completed an fMRI task in which they were presented with short sentences describing either positively or negatively valenced traits in the academic, physical or prosocial domain (Fig. 1, Appendix A). In the self-condition, participants were asked to indicate to what extent the trait sentences applied to them on a scale of 1–4. Participants responded to 60 trait sentences (e.g. ‘I am smart’, ‘I am unattractive’) by pressing buttons from 1 (‘not at all’) to 4 (‘completely’) with the index to little finger of their right hand. Twenty trait sentences were shown for each domain; ten with a positive valence and ten with a negative valence. In the baseline condition, all response demands were the same, except that in this condition participants were asked to categorize other trait sentences according to four categories: (1) school, (2) social, (3) appearance, or (4) I don’t know. Twenty trait sentences were shown in this block; ten with a positive valence and ten with a negative valence. The two conditions appeared in separate runs and the order of conditions was counterbalanced across participants. All stimuli and the average number of words per sentence in each condition can be found in Appendix A. Analyses on the sentence length revealed a domain x valence interaction effect (F(2, 18) = 4.92 p = 0.020). Post-hoc tests showed that positive sentences were comparable in length for all domains (F(2, 18) = 0.02, p = 0.986), whereas negative prosocial sentences consisted of more words compared to negative sentences in the other domains (F(2, 18) = 9.32, p = 0.002). Moreover, academic positive sentences consisted of more words than academic negative sentences (F(1, 9) = 6.44, p = 0.032).
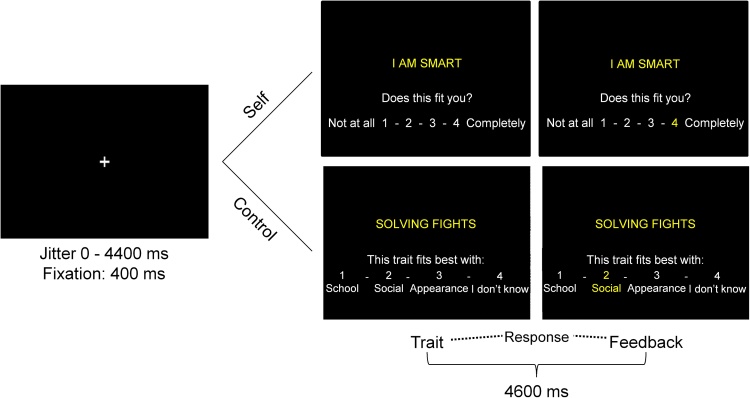
Fig. 1.
Example of a trial in the Self and the Control block. Each trial started with a black screen with a jittered duration between 0 and 4400 ms. Subsequently, a fixation cross was shown for 400 ms after which the stimulus appeared. In the Self block, participants rated on a scale of 1–4 to what extent the traits fit themselves. In the Control block, participants categorized the trait sentences into one of four options. The stimulus was shown for 4600 ms. If participants responded within this timeframe, the number of their choice would turn yellow. If participants failed to respond within this timeframe, a screen with the phrase ‘Too Late!’ was shown for an additional 100 ms after which the next trial would start.
Participants completed the trials in both conditions in a pseudorandomized order. Each trial began with a 400 ms fixation cross. Subsequently, the stimulus was presented for 4600 ms, which consisted of the trait sentence and the response options (1–4). Within this timeframe, participants could respond to the sentence. To assure participants that their choice had been registered, the number they chose turned yellow for the remaining stimulus time. If the participant failed to respond within the 4600 ms, they were shown the phrase ‘Too late!’ for 1000 ms. These trials were modeled separately and were not included in the analysis. Too late responses occurred on 1.1% of the trials in the Self block and on 0.7% of trials in the control condition. The order of the trials was optimized using Optseq (Dale, 1999). Additionally, OptSeq was used to add jittered intertrial intervals, that varied between 0 and 4.4 s.
The fMRI task was validated by correlating the self-evaluations in the three domains to subscales of the Dutch version of the Self Perception Profile for Adolescents (SPPA) (Harter, 1988) (Table 2). We also included the subscale “prosocial” from the Strengths and Difficulties Questionnaire (SDQ) as an additional index for prosocial self-evaluations. Scores on the positive academic and negative academic domain, correlated significantly with the subscales scholastic competence (positive academic: r(148) = 0.57, p < 0.001; negative academic: r(148) = −0.45, p < 0.001) and behavioral conduct (positive academic: r(148) = 0.50, p < 0.001; negative academic: r(148) = −0.47, p < 0.001). Scores on the physical domain correlated with the subscales physical appearance (positive physical: r(148) = 0.60, p < 0.001; negative physical: r(148) = −0.61, p < 0.001), social competence (positive physical: r(148) = 0.25, p = 0.002; negative physical: r(148) = −0.25, p = 0.003) and athletic competence (negative physical: r(148) = −0.21, p = 0.009). Scores on the prosocial domain correlated with the subscale close friendship (positive prosocial: r(148) = 0.61, p = 0.002; negative prosocial: r(148) = −0.20, p = 0.013) and with the SDQ prosocial subscale (positive prosocial: r(148) = 0.61, p < 0.001; negative prosocial: r(148) = −0.42, p < 0.001). All other correlations are presented in Table 1.
Table 2.
Regions activated during the domain contrasts.
| Region | BA | Coordinates | Cluster Size | T | |||
|---|---|---|---|---|---|---|---|
| (A) Physical > Academic & Prosocial (FDRc < 0.001 = 97) | |||||||
| Frontal cortex/Subcortical | R Superior Medial Frontal (dmPFC) | 3 | 41 | 34 | 1626 | 6.50 | |
| L Superior Medial Frontal | 10 | −3 | 56 | 13 | 6.26 | ||
| R Superior Medial Frontal | 9 | 0 | 53 | 31 | 6.10 | ||
| R Middle Frontal (VLPFC) | 46 | 51 | 41 | 13 | 1042 | 8.66 | |
| R Inferior Frontal orb | 47 | 27 | 32 | −14 | 7.10 | ||
| R Insula | 13 | 30 | 20 | −17 | 6.86 | ||
| L Inferior Frontal (VLPFC) | 46 | −42 | 38 | 10 | 809 | 8.32 | |
| L Middle Frontal | 47 | −27 | 38 | −11 | 7.82 | ||
| L Insula | 13 | −27 | 14 | −17 | 6.89 | ||
| Parietal cortex | R Inferior Parietal (IPL) | 39 | 42 | −55 | 46 | 361 | 6.18 |
| R Angular | 39 | 39 | −67 | 46 | 5.40 | ||
| R Inferior Parietal | 40 | 57 | −40 | 49 | 3.77 | ||
| L Inferior Parietal | 39 | −48 | −55 | 49 | 354 | 4.98 | |
| L Inferior Parietal | 39 | −33 | −67 | 43 | 4.94 | ||
| L Inferior Parietal | 39 | −39 | −55 | 43 | 4.93 | ||
| R Posterior Cingulum (PC/PCC) | 0 | −34 | 28 | 229 | 5.89 | ||
| R Middle Cingulum | 24 | 0 | −7 | 34 | 5.57 | ||
| R Precuneus (PC/PCC) | 7 | 12 | −67 | 40 | 173 | 5.48 | |
| L Precuneus (PC/PCC) | 7 | −9 | −73 | 40 | 3.96 | ||
| Temporal cortex | L Inferior Temporal (ITG) | 37 | −54 | −52 | −17 | 97 | 5.92 |
| R Inferior Temporal (ITG) | 21 | 60 | −43 | −11 | 154 | 4.98 | |
| R Middle Temporal | 21 | 66 | −34 | −8 | 4.83 | ||
| (B) Academic > Physical & Prosocial (FDRc < .001 = 58) | |||||||
| Frontal cortex/Subcortical | L Middle Frontal (DLPFC) | 8 | −21 | 29 | 49 | 85 | 4.46 |
| L Superior Frontal | 8 | 12 | 44 | 46 | 3.68 | ||
| Parietal cortex | L Precuneus (PC/PCC) | 23 | −3 | −58 | 16 | 294 | 6.93 |
| L Mid Cingulum | 23 | −3 | −43 | 34 | 4.59 | ||
| L Angular (IPC) | 39 | −48 | −73 | 34 | 58 | 4.93 | |
| (C) Prosocial > Academic & Physical (FDRc.001 = 190) | |||||||
| Occipital cortex | R Lingual | 18 | 9 | −79 | −2 | 190 | 4.18 |
Names were based on the Automatic Anatomical Labeling (AAL) atlas.
Table 1.
Correlations of scores on all conditions of the Self block with all SPPA subscales and the SDQ prosocial subscale.
| Academic Positive | Academic Negative | Physical Positive | Physical Negative | Prosocial Positive | Prosocial Negative | ||
|---|---|---|---|---|---|---|---|
| Scholastic Competence | R | 0.566** | −0.447** | 0.187* | −0.063 | 0.020 | 0.086 |
| p-value | 0.000 | 0.000 | 0.022 | 0.446 | 0.811 | 0.294 | |
| Behavioral Conduct | R | 0.500** | −0.466** | −0.039 | −0.055 | 0.071 | 0.117 |
| p-value | 0.000 | 0.000 | 0.635 | 0.506 | 0.389 | 0.153 | |
| Social Competence | R | −0.025 | −0.042 | 0.250** | −0.245** | 0.027 | −0.109 |
| p-value | 0.758 | 0.608 | 0.002 | 0.003 | 0.740 | 0.184 | |
| Athletic competence | R | −0.074 | −0.038 | 0.151 | −0.214** | −0.082 | 0.009 |
| p-value | 0.369 | 0.643 | 0.066 | 0.009 | 0.316 | 0.910 | |
| Physical Appearance | R | 0.136 | −0.114 | 0.596** | −0.606** | −0.108 | 0.013 |
| p-value | 0.097 | 0.165 | 0.000 | 0.000 | 0.190 | 0.873 | |
| Close friendship | R | 0.137 | −0.036 | 0.242** | −0.108 | 0.249** | −0.203* |
| p-value | 0.095 | 0.660 | 0.003 | 0.188 | 0.002 | 0.013 | |
| Global self-worth | R | 0.234** | −0.246** | 0.431** | −0.465** | −0.068 | 0.042 |
| p-value | 0.004 | 0.002 | 0.000 | 0.000 | 0.406 | 0.608 | |
| SDQ_Prosocial | R | 0.201* | −0.059 | 0.009 | 0.068 | 0.605** | −0.420** |
| p-value | 0.013 | 0.470 | 0.909 | 0.407 | 0.000 | 0.000 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
2.3. fMRI data acquisition
MRI scans were acquired on a Philips 3T MRI scanner, using a standard whole-head coil. Functional scans were acquired in two runs with T2*-weighted echo-planar imaging (EPI) sequence (TR = 2200 msec, TE = 30 msec, sequential acquisition, 37 slices of 2.75 mm, FOV = 220 × 220 × 111.65 mm). The first two volumes were discarded to account for T1 saturation. After the functional scans, a high-resolution 3D T1-FFE scan for anatomical reference was obtained (TR = shortest msec, TE = 4.6 msec, 140 slices, voxel size = 0.875 mm, FOV = 224 × 178.5 × 168 mm). Sentences were projected on a screen behind the scanner and could be seen by the participant via a mirror attached to the head coil. Head movement was restricted by placing foam inserts inside the coil.
2.4. fMRI preprocessing and statistical analysis
All data were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London). The functional scans were corrected for slice-timing acquisition and differences in rigid body movement. All structural and functional volumes were spatially normalized to T1 templates. The normalization algorithm used a 12-parameter affine transformation together with a nonlinear transformation involving cosine basis functions. The algorithm resampled the volumes to 3 mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997). Functional volumes were spatially smoothed with a 6 mm FWHM isotropic Gaussian kernel.
Task effects for each participant were estimated using the general linear model in SPM8. The fMRI time series were modelled as a series of zero duration events convolved with the hemodynamic response function (HRF). Modelled events of interest for the self task were “Academic-Positive”, “Academic-Negative”, “Physical-Positive”, “Physical-Negative”, “Prosocial-Positive” and “Prosocial-Negative”. For the control task, only one event of interest was modelled: “Control” (collapsed across domains and valences). Trials in which participants failed to respond were modelled as events of no interest. The events were used as covariates in a general linear model, along with a basic set of cosine functions that high-pass filtered the data. Six motion regressors were added to the model. The resulting contrast images, computed on a subject-by-subject basis, were submitted to group analyses.
To investigate our aims, we performed two separate analyses. In the first analysis, all self-condition trials (collapsed across domains and valences) were compared to the control trials using a one sample t-test for the contrast Self > Control. The goal of this analysis was to reveal regions that were more engaged during self-evaluations. In the second analysis, we tested for domain- and valence-specificity using trials in the self-condition. For this analysis a 3 (domain: academic, physical, prosocial) x 2 (valence: positive, negative) whole-brain ANOVA was computed. For all analyses, we applied FDR cluster level correction (p < 0.05) at an initial uncorrected threshold of p < 0.001, as implemented in SPM8.
Next, whole-brain analyses were performed to investigate possible linear and quadratic age effects, using age as a linear or quadratic regressor in all the contrasts (positive and negative). Finally, we used the Marsbar ROI toolbox to perform follow-up analyses on 5 ROIs from the Self > Control contrast. The results were corrected for multiple comparisons using a Bonferroni method adjusting for correlated variables (http://www.quantitativeskills.com/sisa/calculations/bonfer.htm) (Perneger, 1998, Sankoh et al., 1997). The average correlation between variables (5 ROIs) was r = 0.30, which resulted in an adjusted significance level (2-sided adjusted) of α = 0.016. Greenhouse–Geisser corrected p-values were reported when appropriate.
3. Results
3.1. Behavioral results
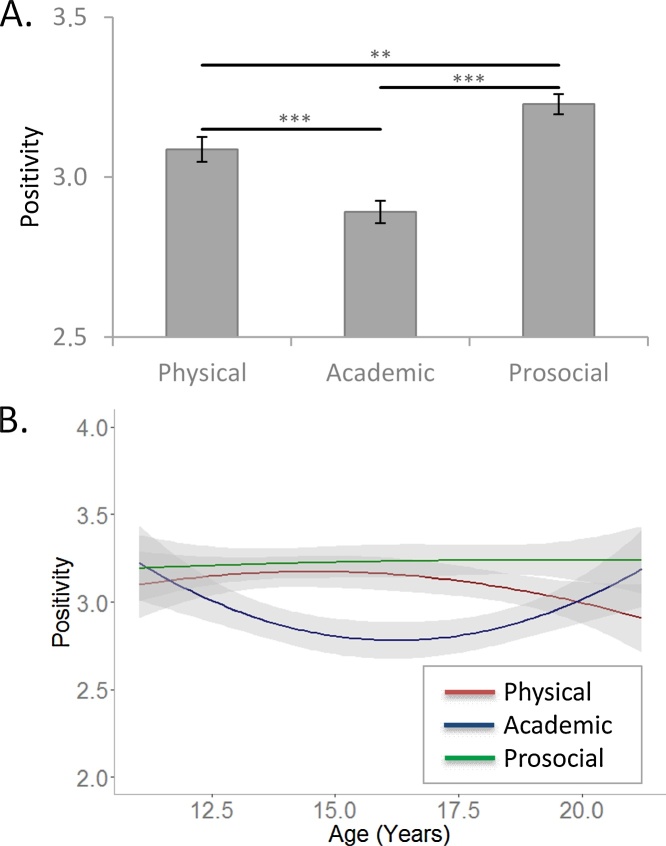
To investigate how participants evaluated themselves on trait sentences in different domains we performed a repeated measures ANOVA with domain (physical, academic, prosocial) as a within subjects factor. Negative self-evaluations were recoded and the combined scores of positive and negative evaluations resulted in an applicability score, such that higher applicability scores indicated a more positive evaluation of the self. The results showed a main effect of domain (F(2, 298) = 27.73, p < 0.001, ƞƿ2 = 0.16). This effect indicated that participants were most positive about their prosocial traits (prosocial versus physical: p = 0.002, prosocial versus academic: p < 0.001), and least positive about their academic traits (academic versus physical: p < 0.001), whereas physical traits were rated in the middle (Fig. 2a). To test how these evaluations differed with age, age was added as a covariate to these analyses, first as a linear factor, and second as a quadratic age factor. There were no main-effects or interaction effects with age as a linear factor, and there was no main-effect of age as a quadratic factor (all p-values >0.47). However, a significant domain x quadratic age interaction effect was found (F(2, 296) = 6.83, p = 0.001, ƞƿ2 = 0.04). Follow-up analyses showed a negative quadratic effect of age in the academic domain (F(1, 148) = 9.86, p = 0.002, ƞƿ2 = 0.06), but not in the physical (F(1, 148) = 1.16, p = 0.28, ƞƿ2 = 0.01) or prosocial (F(1, 148) = 0.037, p = 0.85, ƞƿ2 = 0.00) domain. As can be seen in Fig. 2b, academic traits were rated less positive in mid adolescence compared to childhood and early adulthood.
Fig. 2.
Applicability scores in the Self block. Higher scores indicate more positive evaluations about the self. A. Participants rate themselves most positive on prosocial traits and least positive on academic traits. B. Academic traits are rated less positively in mid adolescence compared to in childhood and young adulthood.
Next, we examined reaction time differences between conditions. We conducted a repeated measures ANOVA with domain (physical, academic, prosocial) as a within subjects factor. The analysis resulted in a main effect of domain (F(2, 298) = 122.11, p < 0.001, ƞƿ2 = 0.45), indicating the slowest reaction times for prosocial sentences (prosocial versus academic and physical: p < 0.001), and the fastest reaction times for physical sentences (physical versus academic and prosocial: p < 0.001). With age added as linear or quadratic covariate, we only found a main effect of linear age (F(1, 148) = 16.89, p < 0.001, ƞƿ2 = 0.10), showing a decrease in reaction times with increasing age.
As we were interested in differences for positive and negative self-evaluations, we conducted a 3 (domain) x 2 (valence) post-hoc repeated-measures ANOVA to test for a main effect of valence. The analysis showed that participants responded faster to positive compared to negative trait sentences (F(1, 149) = 17.66, p < 0.001, ƞƿ2 = 0.11). With age added as linear or quadratic covariate, we did not find any interaction effects of valence with age.
3.2. fMRI results
3.2.1. Domain-general self evaluations
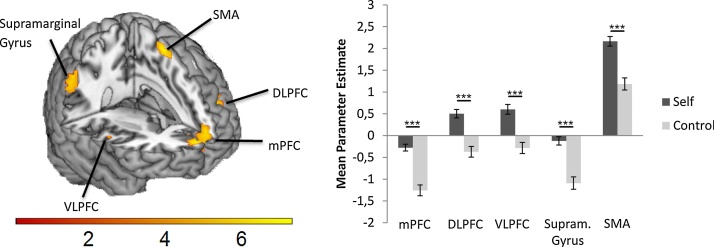
To detect brain regions that were generally involved in self-evaluations, we conducted a whole-brain one-sample t-test for Self > Control. This analysis revealed significantly stronger activation for Self relative to Control trials in mPFC, right ventrolateral prefrontal cortex (VLFPC), left dorsolateral prefrontal cortex (DLPFC), right supramarginal gyrus and left supplementary motor area (SMA) (Fig. 3).
Fig. 3.
Brain activation in the Self > Control contrast. A whole-brain t-test (FDR-cluster corrected at p < 0.001) revealed significantly stronger activation for Self relative to Control trials in mPFC, left DLPFC, right VLPFC, right Supramarginal gyrus and left Supplementary Motor Area (SMA). *** = p<0.001.
3.2.2. Domain- and valence-specific self evaluations
Next, we tested whether self-evaluations in the three domains and for positive and negative valence showed distinct activation patterns. Valence was added as additional factor based on prior studies showing that positively valenced traits are processed differently at a neural level compared to negatively valenced traits.
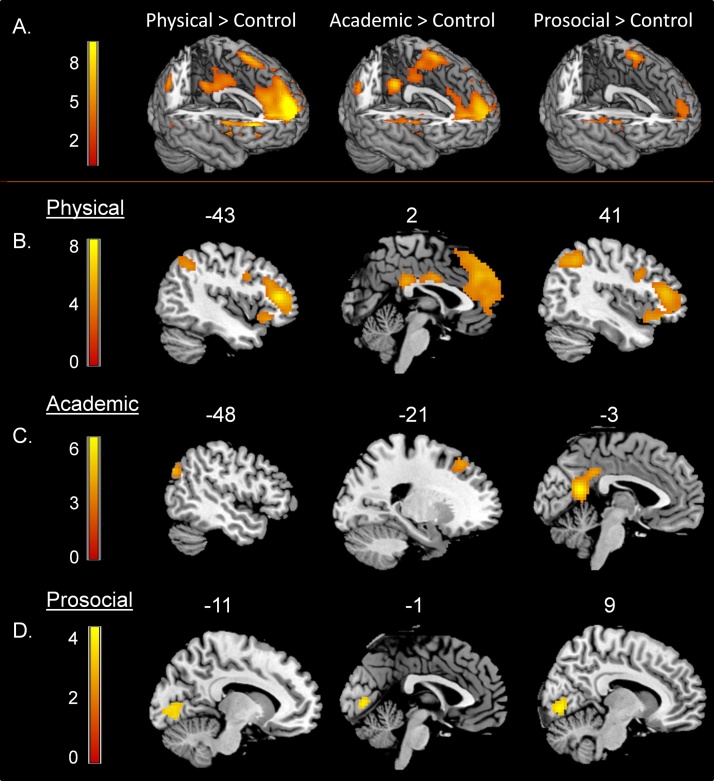
Fig. 4a illustrates activations for each domain relative to the control condition. To investigate domain- and valence-specific activation patterns, trials from the self-condition were included in a 3 (domain) x 2 (valence) whole-brain full factorial ANOVA. This analysis revealed a main effect of domain in bilateral ventrolateral prefrontal cortex (VLPFC), bilateral inferior parietal lobule (IPL), bilateral inferior temporal gyrus (ITG) and precuneus/posterior cingulate cortex (PC/PCC). In addition, the main effect of valence showed activation in the vmPFC (mid orbital gyrus) and in bilateral lingual gyrus.
Fig. 4.
A. Activity for physical > control, academic > control, and prosocial > control. B. Specific activation for evaluating physical trait sentences in bilateral VLPFC, bilateral IPL, bilateral ITG, PC/PCC and dmPFC. C. Specific activation for evaluating academic trait sentences in PC/PCC, left IPC and left DLPFC. D. Specific activation for evaluating prosocial trait sentences in right Lingual gyrus. All regions survived FDR-cluster correction at p < 0.001.
To inspect these main effects for domain in more detail, we compared activity for the contrasts physical > academic & prosocial (Table 2a), academic > physical & prosocial (Table 2b), and prosocial > academic & physical (Table 2c). Specific activation for evaluating physical trait sentences appeared in bilateral VLPFC, bilateral IPL, bilateral ITG, PC/PCC, and dorsal mPFC (Fig. 4b). Specific activation for evaluating academic trait sentences was found in PC/PCC, left inferior parietal cortex (IPC), and in left dorsolateral prefrontal cortex (DLPFC) (Fig. 4c). Specific activation for evaluating prosocial trait sentences was only found in right lingual gyrus (Fig. 4d).
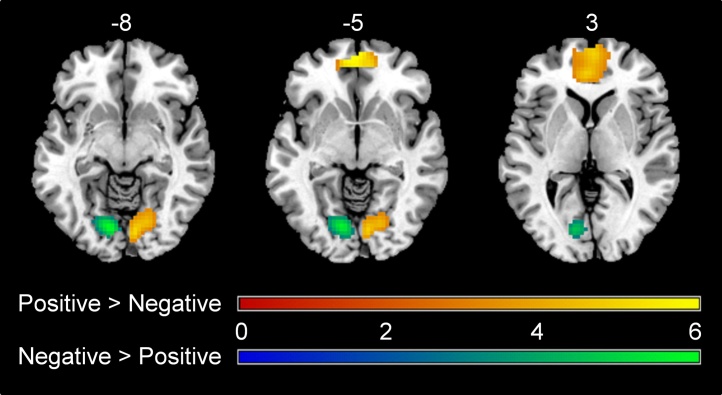
The main effect of valence was followed up by direct comparisons. The one-sample t-tests for positive > negative (Table 3a) showed specific activation for evaluating positive trait sentences in vmPFC and right lingual gyrus. The negative > positive contrast revealed activation for evaluating negative sentences in left lingual gyrus only (Table 3b; Fig. 5).
Table 3.
Regions activated during the valence contrasts.
| Region | BA | Coordinates | Cluster Size | T | |||
|---|---|---|---|---|---|---|---|
| (A) Positive > Negative (FDRc < 0.001 = 170) | |||||||
| Frontal cortex | L Medial Frontal orb (vmPFC) | 10 | −3 | 56 | −5 | 393 | 6.05 |
| L Anterior Cingulum | 32 | −3 | 44 | −2 | 5.47 | ||
| Occipital cortex | R Lingual | 18 | 9 | −82 | −8 | 170 | 5.11 |
| R Lingual | 18 | 18 | −73 | −8 | 4.57 | ||
| (B) Negative > Positive (FDRc < 0.001 = 202) | |||||||
| Occipital cortex | L Lingual | 18 | −12 | −76 | −8 | 202 | 6.63 |
| L Calcarine | 17 | −12 | −79 | 4 | 5.04 | ||
Names were based on the Automatic Anatomical Labeling (AAL) atlas.
Fig. 5.
Specific activation for evaluating positive trait sentences in vmPFC and right lingual gyrus. Specific activation for evaluating negative trait sentences in left lingual gyrus. All regions survived FDR-cluster correction at p < 0.001.
3.2.3. Whole-brain linear and quadratic age effects
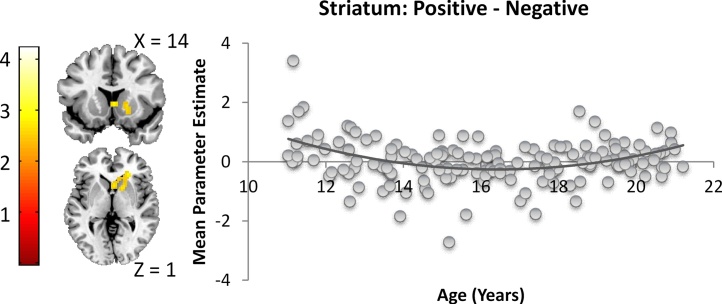
To test for possible linear and quadratic developmental patterns in the contrasts specified above, whole-brain regressions were performed for all contrasts (self > control, academic > physical & prosocial, physical > academic & prosocial, prosocial > academic & physical, positive > negative and negative > positive) using age as a linear and quadratic covariate. Only one region survived FDR-cluster correction at p < .001 (with RT correction, this result survives FWE-cluster correction, but not FDR-cluster correction): in the positive > negative contrast, there was a quadratic age effect in the striatum (x = 24, y = 32, z = 1). As can be seen in Fig. 6, striatum activation for positive compared to negative self-evaluations were attenuated in mid- to late-adolescents compared to their younger and older peers.
Fig. 6.
In the positive > negative contrast, there was a quadratic age effect in the striatum (x = 24, y = 32, z = 1), showing that mid- and late-adolescents involve less striatum activation for positive compared to negative self-evaluations, compared to children and young adults.
3.2.4. Linear and quadratic age effects in domain-general self-evaluation regions
The whole-brain analyses reported above only showed a significant age effect for the contrast positive versus negative valence traits. To explore possible linear and quadratic changes within domain-general self-evaluation regions, we performed exploratory age analyses on specific ROIs from the Self > Control contrast. Because these regions were interconnected and spanned several brain regions, we used a more stringent voxel level FWE-correction (p < 0.05) to separate the regions into separate ROIs. All coordinates for this analysis are reported in Table 4a. To test for developmental differences in the neural correlates of self-evaluations across valences and domains, we performed 2 separate 3 (domain) x 2 (valence) ANOVAs with 1) linear age and 2) quadratic age as a covariate on the ROIs. Only effects including the age factor are reported.
Table 4.
Regions activated during the Self > Control and reversed contrast.
| Region | BA | Coordinates | Cluster Size | T | |||
|---|---|---|---|---|---|---|---|
| (A) Self > Control (FWE < 0.05) | |||||||
| Frontal cortex | R Superior Medial Frontal (mPFC) | 10 | 6 | 62 | 13 | 298 | 7.41 |
| L Anterior Cingulum | 32 | −6 | 44 | 1 | 6.43 | ||
| L Superior Medial Frontal | 10 | −6 | 56 | 13 | 6.35 | ||
| L Suppl. Motor Area | 6 | −6 | 2 | 67 | 41 | 6.95 | |
| R Inferior Frontal oper. | 44 | 57 | 11 | 22 | 34 | 6.53 | |
| L Middle Frontal Gyrus | 10 | −27 | 47 | 31 | 27 | 5.70 | |
| Parietal cortex | L Supramarginal Gyrus | 40 | 60 | −28 | 46 | 59 | 6.64 |
| (B) Control > Self (FWE < 0.05) | |||||||
| Frontal cortex | L Precentral | 8 | −42 | 8 | 31 | 259 | 8.44 |
| L Inferior Frontal Tri | 44 | −45 | 20 | 25 | 7.95 | ||
| L Inferior Frontal Tri | 46 | −51 | 29 | 19 | 7.66 | ||
| L Middle Frontal | 6 | −27 | 14 | 52 | 53 | 7.30 | |
| Parietal cortex | L Precuneus | 7 | −6 | −67 | 46 | 61 | 5.87 |
| L Precuneus | 7 | −3 | −58 | 49 | 5.83 | ||
| Occipital cortex | L Lingual | 18 | −12 | −85 | −11 | 1950 | 11.63 |
| R Fusiform | 19 | 30 | −79 | −14 | 10.59 | ||
| L Middle Occipital | 19 | −30 | −88 | 13 | 10.58 | ||
Names were based on the Automatic Anatomical Labeling (AAL) atlas.
3.2.4.1. Age x domain effects
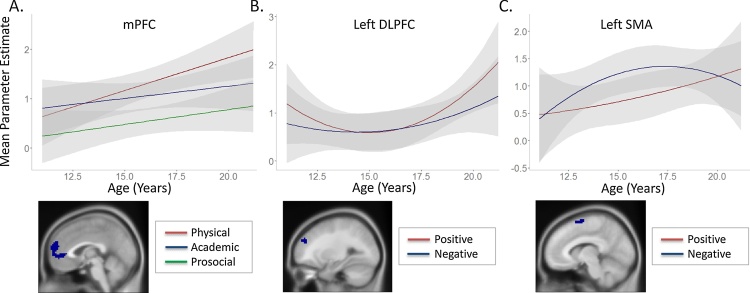
In mPFC, we found an interaction effect between linearly increasing age and domain (F(2, 296) = 4.39, p = 0.013 (with RT-correction p > 0.05)) (Fig. 7a). Post hoc tests reveal that in mPFC, activation in response to sentences in the physical domain increased linearly with age (p = 0.008 (with RT-correction p = 0.013)). No age effects were observed for the academic and prosocial domains.
Fig. 7.
Age effects within Self > Control derived ROIs. A. mPFC activation for physical trait evaluations increased linearly with age. B. Less left DLPFC activation for positive trait evaluations in mid-adolescents. C. Stronger left SMA activation for negative trait evaluations in mid- to late-adolescents.
3.2.4.2. Age x valence effects
In left DLPFC and left SMA we found an interaction effect of valence and quadratic age (DLPFC: F(1, 148) = 5.87, p = 0.017 (note that p > 0.016) (with RT-correction p = 0.019); SMA: F(1, 148) = 7.43, p = 0.007) (Fig. 7b/c). For left DLPFC, positively valenced traits resulted in less pronounced activation in mid-adolescents relative to children and young adults. For SMA, negatively valenced trials resulted in more activation in mid- to late-adolescents relative to children and young adults.
4. Discussion
In this study, we aimed to investigate the neural correlates of domain- and valence-specific self-evaluations from late childhood, across adolescence, into early adulthood. Furthermore, we tested for linear and quadratic developmental patterns. The neuroimaging results revealed three main findings. First, we replicated prior research showing self-relevant neural activity in mPFC. Second, partly overlapping and partly distinct neural networks were involved for evaluating traits in the academic, physical and prosocial domains. Third, age analyses revealed an age-related increase in mPFC for physical self traits, and showed decreased striatum activation for positive self-evaluations in mid- to late-adolescence, with activation in two general self-related brain regions (left DLPFC and SMA) mirroring this pattern. In the discussion we first summarize the behavioral results. Subsequently the discussion is organized along the lines of the main neuroimaging findings.
The behavioral results showed that participants were most positive about their prosocial traits and least positive about their academic traits. Additionally, mid- to late-adolescents were relatively more negative about their academic traits compared to children and young adults. This finding extends previous studies indicating a decrease in academic self-concept in mid- to late-adolescents (Shapka and Keating, 2005), and shows that young adults recover from this decrease. Overall, the behavioral findings suggest that the differentiation of self-evaluations according to specific domains that arises in childhood (Jacobs et al., 2002), persists into adolescence (Harter, 2012). The finding that children, adolescents, and young adults rate themselves higher on prosocial compared to other domains possibly indicates that prosocial traits are important in the process of social reorientation (Nelson et al., 2005).
4.1. Self-, domain- and valence-specific neural activation
The primary goal of this study was to investigate neural activation patterns for general self-evaluations spanning a broad range from childhood to young adulthood (11–21-years) and to test for possible domain- and valence-specific activation patterns. Consistent with our expectations, we found increased mPFC activation for evaluating self-traits compared to a baseline task (Denny et al., 2012, Murray et al., 2012, Pfeifer et al., 2013, Pfeifer et al., 2007, Romund et al., 2017). This has been consistently reported in studies with adult participants (for a meta-analysis, see Denny et al., 2012), and the current study adds to this literature by showing that adolescents engage this region as well for self-evaluations (see also Pfeifer and Peake, 2012, Romund et al., 2017).
An important goal of this study was to test if there are domain-specific brain regions that are involved in processing self-traits in different domains. In line with previous research investigating self-concept in different domains in adults (Moran et al., 2010, van der Cruijsen et al., 2017), for evaluating physical trait sentences, we found activation in regions involved in autobiographical memory retrieval (PC/PCC: Fink et al., 1996, Northoff and Bermpohl, 2004, van der Meer et al., 2010), empathy, mentalizing and perspective taking (IPL: David et al., 2006, Ruby and Decety, 2003, Vogeley et al., 2004), and cognitive control and action monitoring (VLPFC, dmPFC: Crone and Steinbeis, 2017). Interestingly, the dorsal mPFC and VLPFC are also often involved in self- versus other-referential evaluations and self-monitoring (Denny et al., 2012, Murray et al., 2012, Ochsner et al., 2005, Schmitz et al., 2004). More specifically, dorsal mPFC has been linked to less relevant self-evaluations or evaluations of dissimilar others (D’Argembeau et al., 2007, Denny et al., 2012, Mitchell et al., 2006, Murray et al., 2012). Moreover, this region has been found to be activated when making mental state attributions and in the formation of impressions (Mitchell et al., 2005a, Mitchell et al., 2005b, Mitchell et al., 2006), in mental imagery including familiar people (Hassabis et al., 2013, Szpunar et al., 2014), and in social rejection (Achterberg et al., 2016). Therefore, one possibility is that when evaluating one’s physical traits, adolescents (11–21 years) might be more focused on (the opinions of) others compared to when evaluating one’s academic or prosocial traits.
For evaluating academic trait sentences, we found increased activation in the PC/PCC, the left IPC and the left DLPFC. These findings fit with prior studies in adults which showed more activation in the PC/PCC for character or academic evaluations (Moran et al., 2010, van der Cruijsen et al., 2017), and suggest processes of autobiographical (PC/PCC: Fink et al., 1996, Northoff and Bermpohl, 2004, van der Meer et al., 2010) and semantic (DLPFC: Badre and Wagner, 2007, Martinelli et al., 2012, Thompson-Schill et al., 2005) memory retrieval in evaluating one’s academic traits. These regions (PC/PCC, DLPFC, IPC) were activated for evaluating both physical versus academic and prosocial traits, and academic versus physical and prosocial traits, suggesting that these regions are especially less involved in evaluating prosocial traits. Moreover, when evaluating prosocial traits, adolescents did not engage the social brain regions that were engaged in a previous study in adults (van der Cruijsen et al., 2017). Possibly, prosocial self-evaluations in children, adolescents and young adults rely mainly on a general self-evaluations network and do not engage additional regions outside of this network, but future studies should test these developmental differences in more detail.
Interestingly, when we distinguished between positively and negatively valenced traits, it was observed that positive trait sentences elicited more activation in vmPFC (located in the anatomical medial orbital gyrus) than negative trait sentences, consistent with prior research in adults (Moran et al., 2006, van der Cruijsen et al., 2017). The medial part of the orbital gyrus has previously been linked to positive valuation processes in adults (Kringelbach and Rolls, 2004, Peters and Büchel, 2010). It should be noted that positively valenced traits were also rated as more applicable to self, so it is possible that this region represents self-relevance (D’Argembeau, 2013). Another notable finding was the clear hemispheric difference in lingual gyrus activation for positive (right lingual gyrus) and negative (left lingual gyrus) trait evaluations. As we are not aware of any other study reporting such hemispheric differences, future research is required to confirm and possibly explain this result.
4.2. Age effects
An additional aim that was addressed in this study was to test whether self-evaluation regions showed developmental changes between ages 11 and 21 years. Previous behavioural studies have demonstrated pronounced changes in self-evaluation during this age range (Harter, 2012). In this study, we found that mPFC activation increased with age for evaluating physical trait sentences. These findings are consistent with other studies showing increased self-related mPFC activation across adolescence, although this was specifically observed in the social domain in a prior study (Pfeifer et al., 2013). Other studies using a self-reference effect paradigm also showed increases in self-related mPFC activation from childhood to early adolescence, and from early to late adolescence (Dégeilh et al., 2015, Ray et al., 2009). However, previous studies contrasting adolescent with adult samples showed mixed results, with some studies indicating stronger self-related mPFC activation in children compared to adults, suggesting a decrease across adolescence (Pfeifer et al., 2009; Pfeifer, Lieberman, & Dapretto, 2007), and one study reporting similar levels of activation for early adolescents and adults (Jankowski et al., 2014). This study adds to the literature by showing a linear increase in self-related mPFC activation from late childhood into early adulthood that is domain specific for physical traits. An interesting question for future studies is to test in more detail at which ages mPFC activity is more pronounced. The current findings show that these patterns are possibly depending on domain.
Finally, the whole-brain results further showed that positive relative to negative self traits elicited increased activity in the striatum in children and adults, and a dip in mid to late adolescence. Striatum activation has been implicated in self-relevance, self-relatedness, intrinsic value (de Greck et al., 2008, Enzi et al., 2009, Phan et al., 2004, Rameson et al., 2010, Schmitz and Johnson, 2007, Zink et al., 2004, Zink et al., 2003), and in salience and valuation associated with reward (Knutson, 2005, McClure et al., 2004). This suggests that positive versus negative self-descriptions are less self-relevant or salient for mid- to late-adolescents compared to children and young adults. The results relate to a possible decrease in self-evaluation, as also observed in behavioural scores in the academic domain. The dip for positive traits in mid-adolescence was also observed in left DLPFC, whereas in left SMA, there was a peak in activation for evaluating negative traits in mid- to late-adolescence. Together, these findings are important in the context of adolescent-emergent depression (Giedd et al., 2008). For example, a recent study found attenuated striatum activation in response to positive self-descriptions in depressed adolescents, suggesting that this attenuation might heighten the risk for depression (Quevedo et al., 2017). Future longitudinal research is necessary to test these relations in more detail.
4.3. Limitations and future directions
This study had several strengths including a focus on different domains and testing children, adolescents and young adults across the range of 11–21-years. However, several limitations also deserve attention. First, a limitation in this study is related to differences between the stimuli. The negative prosocial sentences consisted of more words compared to negative sentences in the other conditions, and academic positive sentences consisted of more words than academic negative sentences. These differences can possibly account for the different activation patterns in the lingual gyrus. Second, as it was important that participants in the control condition would read trait sentences, think about the sentences, but not think about themselves, the sentences in this condition are slightly different in structure compared to the sentences in the self-evaluation condition (see Appendix A). This might be a confound in the results. Third, even though the sample size is large compared to prior studies, this study did not include a large group of early adolescents. In this period, pubertal development is most varied. Hence, our design was not optimized to test for puberty-specific effects. Moreover, there was not enough power to distinguish between age and puberty effects, as these are highly correlated. These are questions that can be best-captured in future longitudinal designs.
Previous research has indicated that school transitions may play a role in the development of self-concept in adolescents (Alfeld-Liro and Sigelman, 1998, Chung et al., 2014, Wigfield et al., 1991, Zanobini and Usai, 2002). Future studies could use an optimized design to test for nonlinear and nonquadratic age effects in order to examine the effect of school transitions on the neural signature of self-evaluations.
As peers become increasingly important during adolescence, and adolescents attach more value to the opinions of others (Harter, 2012, Sebastian et al., 2008, Steinberg and Morris, 2001), future studies could focus on the development of reflected self-evaluations as well. A prior study comparing self-evaluations in early adolescents (11–14 years) with self-evaluations in young adults (23–30 years), showed stronger mPFC activation for evaluating social traits from the perspective of friends, but for evaluating academic traits from the perspective of mothers (Pfeifer et al., 2009). Therefore, studies investigating reflected self-concept across adolescence will be important to examine domain-specific sensitivities in more detail.
4.4. Conclusions
We investigated developmental changes in the neural mechanisms of self-concept across late childhood, adolescence, and early adulthood, and we explored distinct underlying processes of self-evaluations in different domains. Previous research has mainly focused on self-related brain regions in adults (Denny et al., 2012, Murray et al., 2012), and few studies have looked at differences between adolescents and adults (Jankowski et al., 2014, Pfeifer et al., 2007). In this study, we showed an increase in self-related mPFC activation in individuals aged between 11 and 21 years, for rating sentences in the physical domain. Moreover, mid- to late-adolescents show less striatum activation for positive self traits compared to their older and younger peers, suggesting that positive self-descriptions might be less salient for these adolescents. Together, these results highlight the importance of domain distinctions when studying self-concept development in late childhood, adolescence, and early adulthood.
Conflict of interest
None.
Acknowledgments
We thank all participants for their collaboration and everyone involved with data collection for the Leiden Self-Concept study. This work was supported by a grant from The Netherlands Organization for Scientific Research (NWO-VICI 453-14-001 E.A.C.).
Contributor Information
R. van der Cruijsen, Email: l.w.p.van.der.cruijsen@fsw.leidenuniv.nl.
S. Peters, Email: s.peters@fsw.leidenuniv.nl.
L.P.E. van der Aar, Email: l.p.e.van.der.aar@fsw.leidenuniv.nl.
E.A. Crone, Email: ecrone@fsw.leidenuniv.nl.
Appendix A. Stimuli as used in the self condition and in the control condition.
Stimuli as used in the self condition and in the control condition.
| Self task | Control task |
|---|---|
| Academic Positive | Mooie ogen hebben |
| Ik ben gemotiveerd op school | Lage cijfers halen |
| Ik ben goed in het maken van toetsen | Gemeen zijn tegen anderen |
| Ik leer graag | Mollig zijn |
| Ik leer snel | Spelfouten maken |
| Ik ben slim | Anderen buitensluiten |
| Ik doe het goed op school | Een gezond gewicht hebben |
| Ik heb mijn werk altijd op tijd klaar | Netjes werken |
| Ik ben een harde werker | Anderen pesten |
| Ik haal goede cijfers | Ruzie maken |
| Ik werk zelfstandig | Anderen vergeven |
| Academic Negative | Spullen delen met anderen |
| Ik stel dingen uit | Er moe uit zien |
| Ik heb veel hulp nodig op school | Langzaam lezen |
| Ik ben lui | Puistjes hebben |
| Ik werk sloom | Ruzies oplossen |
| Ik ben onverstandig | Tevreden zijn met je uiterlijk |
| Ik werk chaotisch | Een goed geheugen hebben |
| Ik vind school moeilijk | Een doorzetter zijn |
| Ik ben snel afgeleid | Goed zijn in rekenen |
| Ik werk slordig | |
| Ik ben dom | |
| Physical Positive | |
| Ik ben mooi | |
| Ik zie er stralend uit | |
| Ik ben knap | |
| Ik heb een goed lichaam | |
| Ik heb een mooie lach | |
| Ik heb een goede kledingstijl | |
| Ik zie er aantrekkelijk uit | |
| Ik mag blij zijn met mijn lichaam | |
| Ik zie er goed uit | |
| Ik heb een mooi figuur | |
| Physical Negative | |
| Ik ben te zwaar | |
| Ik ben lelijk | |
| Ik heb een slechte huid | |
| Ik zweet veel | |
| Ik ben dik | |
| Ik zie er suf uit | |
| Ik ben onaantrekkelijk | |
| Ik heb overgewicht | |
| Ik zie er onverzorgd uit | |
| Ik heb lelijke tanden | |
| Prosocial Positive | |
| Ik leef met anderen mee | |
| Ik troost anderen | |
| Ik houd rekening met anderen | |
| Ik help anderen | |
| Ik voel met anderen mee | |
| Ik geef om anderen | |
| Ik kom voor anderen op | |
| Ik zorg graag voor anderen | |
| Ik doe graag iets voor een ander | |
| Ik deel graag met anderen | |
| Prosocial Negative | |
| Ik kies altijd voor mezelf | |
| Ik laat anderen hun problemen zelf oplossen | |
| Ik leen mijn spullen niet graag uit | |
| Ik houd alleen rekening met mezelf | |
| Ik denk vooral aan mezelf | |
| Ik help anderen alleen als ik er iets voor terug krijg | |
| Ik zorg alleen voor mezelf | |
| Ik negeer andermans problemen | |
| Ik help nooit een vreemde | |
| Ik houd alles voor mezelf |
Average number of words per sentence
| Self task | #Words |
|---|---|
| Academic Positive | 4.80 |
| Academic Negative | 3.70 |
| Physical Positive | 4.80 |
| Physical Negative | 3.70 |
| Prosocial Positive | 4.70 |
| Prosocial Negative | 6.00 |
| Control task | |
| Control condition | 2.90 |
References
- Achterberg M., van Duijvenvoorde A.C.K., Bakermans-Kranenburg M.J., Crone E.A. Control your anger! The neural basis of aggression regulation in response to negative social feedback. Soc. Cogn. Affect. Neurosci. 2016;11(5):712–720. doi: 10.1093/scan/nsv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfeld-Liro C., Sigelman C.K. Sex differences in self-concept and symptoms of depression during the transition to college. J. Youth Adolesc. 1998;27(2):219–244. [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268––277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D., Wagner A.D. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Brown B.B. Adolescents’ relationships with peers. In: Lerner R.M., Steinberg L., editors. Handbook of Adolescent Psychology. 2nd ed. John Wiley & Sons; 2004. pp. 363–394. [Google Scholar]
- Chung J.M., Robins R.W., Trzesniewski K.H., Noftle E.E., Roberts B.W., Widaman K.F. Continuity and change in self-esteem during emerging adulthood. J. Pers. Soc. Psychol. 2014;106(3):469–483. doi: 10.1037/a0035135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco C.A., Kollokian C., Kwan R.K.S., Evans A.C. Online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:S425. [Google Scholar]
- Cole D.A., Maxwell S.E., Martin J.M., Peeke L.G., Seroczynski A.D., Tram J.M., Hoffman K.B., Ruiz M.D., Jacquez F., Maschman T. The development of multiple domains of child and adolescent self-concept: a cohort sequential longitudinal design. Child Dev. 2001;72(6):1723–1746. doi: 10.1111/1467-8624.00375. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Steinbeis N. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn. Sci. 2017;21(3):205–215. doi: 10.1016/j.tics.2017.01.003. [DOI] [PubMed] [Google Scholar]
- de Greck M., Rotte M., Paus R., Moritz D., Thiemann R., Proesch U., Bruer U., Moerth S., Tempelmann C., Bogerts B., Northoff G. Is our self based on reward? Self-relatedness recruits neural activity in the reward system. Neuroimage. 2008;39(4):2066–2075. doi: 10.1016/j.neuroimage.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Dégeilh F., Guillery-Girard B., Dayan J., Gaubert M., Chételat G., Egler P.J., Baleyte J.M., Eustache F., Viard A. Neural correlates of self and its interaction with memory in healthy adolescents. Child Dev. 2015;86(6):1966–1983. doi: 10.1111/cdev.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A., Ruby P., Collette F., Degueldre C., Balteau E., Luxen A., Maquet P., Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J. Cogn. Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front. Hum. Neurosci. 2013;7:1–13. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David N., Bewernick B.H., Cohen M.X., Newen A., Lux S., Fink G.R., Shah N.J., Vogeley K. Neural representations of self versus other: visual-spatial perspective taking and agency in a virtual ball-tossing game. J. Cogn. Neurosci. 2006;18(6):898–910. doi: 10.1162/jocn.2006.18.6.898. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. A meta-analysis of functional neuroimaging studies of self and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J. Cogn. Neurosci. 2012;24(8):1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi B., de Greck M., Prösch U., Tempelmann C., Northoff G. Is our self nothing but reward? Neuronal overlap and distinction between reward and personal relevance and its relation to human personality. PLoS One. 2009;4(12) doi: 10.1371/journal.pone.0008429. e8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G.R., Markowitsch H.J., Reinkemeier M., Bruckbauer T., Kessler J., Heiss W.D. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J. Neurosci. 1996;16(13):4275––4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. http://www.ncbi.nlm.nih.gov/pubmed/8753888 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Keshavan M., Paus T. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S. University of Denver; 1988. Self-perception Profile for Adolescents. [Google Scholar]
- Harter S. 1st ed. Guilford Press; New York, NY: 2012. The Construction of the Self. [Google Scholar]
- Hassabis D., Spreng R.N., Rusu A.A., Robbins C.A., Mar R.A., Schacter D.L. Imagine all the people: how the brain creates and uses personality models to predict behavior. Cereb. Cortex. 2013;24(8):1979–1987. doi: 10.1093/cercor/bht042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.E., Lanza S., Osgood D.W., Eccles J.S., Wigfield A. Changes in children’s self-competence and values: gender and domain differences across grades one through twelve. Child Dev. 2002;73(2):509–527. doi: 10.1111/1467-8624.00421. [DOI] [PubMed] [Google Scholar]
- Jankowski K.F., Moore W.E., Merchant J.S., Kahn L.E., Pfeifer J.H. But do you think I’m cool?: Developmental differences in striatal recruitment during direct and reflected social self-evaluations. Dev. Cognit. Neurosci. 2014;8:40–54. doi: 10.1016/j.dcn.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B. Distributed neural representation of expected value. J. Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., Rolls E.T. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Marsh H.W., Ayotte V. Do multiple dimensions of self-concept become more differentiated with age? The differential distinctiveness hypothesis. J. Educ. Psychol. 2003;95(4):687–706. [Google Scholar]
- Martinelli P., Sperduti M., Piolino P. Neural substrates of the self-memory system: new insights from a meta-analysis. Hum. Brain Mapp. 2012;34(7):1515–1529. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., York M.K., Montague P.R. The neural substrates of reward processing in humans: the modern role of fMRI. Neuroscientist. 2004;10(3):260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S. Developmental changes in the structure of the social brain in late childhood and adolescence. SCAN. 2014;9:123–131. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Banaji M.R., Macrae C.N.1. The link between social cognition and self-referential thought in the medial prefrontal cortex. J. Cogn. Neurosci. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. Forming impressions of people versus inanimate objects: social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26(1):251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran J.M., Macrae C.N., Heatherton T.F., Wyland C.L., Kelley W.M. Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci. 2006;18(9):1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Moran J.M., Lee S.M., Gabrieli J.D.E. Dissociable neural systems supporting knowledge about human character and appearance in ourselves and others. J. Cogn. Neurosci. 2010;23(9):2222–2230. doi: 10.1162/jocn.2010.21580. [DOI] [PubMed] [Google Scholar]
- Murray R.J., Schaer M., Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci. Biobehav. Rev. 2012;36(3):1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(April):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Northoff G., Bermpohl F. Cortical midline structures and the self. Trends Cogn. Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Beer J.S., Robertson E.R., Cooper J.C., Gabrieli J.D.E., Kihsltrom J.F., D’Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Perneger T.V. What’s wrong with Bonferroni adjustments. Br. Med. J. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Büchel C. Neural representations of subjective reward value. Behav. Brain Res. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Peake S.J. Self-development: integrating cognitive, socioemotional, and neuroimaging perspectives. Dev. Cognit. Neurosci. 2012;2(1):55–69. doi: 10.1016/j.dcn.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Lieberman M.D., Dapretto M.1. I know you are but what am I?!: Neural bases of self- and social knowledge retrieval in children and adults. J. Cogn. Neurosci. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80(4):1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Kahn L.E., Merchant J.S., Peake S.J., Veroude K., Masten C.L. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. J. Neurosci. 2013;33(17):7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Taylor S.F., Welsh R.C., Ho S.H., Britton J.C., Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Quevedo K.M., Ng R., Scott H., Smyda G., Pfeifer J.H., Malone S. The neurobiology of self-processing in abused depressed adolescents. Dev. Psychopathol. 2017;29:1057–1073. doi: 10.1017/S0954579416001024. [DOI] [PubMed] [Google Scholar]
- Rameson L.T., Satpute A.B., Lieberman M.D. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50(2):701–708. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Ray R.D., Shelton A.L., Hollon N.G., Michel B.D., Frankel C.B., Gross J.J., Gabrieli J.D.E. Cognitive and neural development of individuated self-representation in children. Child Dev. 2009;80(4):1232–1242. doi: 10.1111/j.1467-8624.2009.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romund L., Golde S., Lorenz R.C., Raufelder D., Pelz P., Gleich T. Neural correlates of the self-concept in adolescence—a focus on the significance of friends. Hum. Brain Mapp. 2017;996(March):987–996. doi: 10.1002/hbm.23433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P., Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur. J. Neurosci. 2003;17(11):2475–2480. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Sankoh A.J., Huque M.F., Dubey S.D. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schmitz T.W., Johnson S.C. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neurosci. Biobehav. Rev. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz T.W., Kawahara-Baccus T.N., Johnson S.C. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., Burnett S., Blakemore S. Development of the self-concept during adolescent. Trends Cogn. Sci. 2008;12(11):441–446. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Shapka J.D., Keating D.P. Structure and change in self-concept during adolescence. Can. J. Behav. Sci. 2005;37(2):83–96. [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A.E., Allemand M., Robins R.W., Fend H. a. Low and decreasing self-esteem during adolescence predict adult depression two decades later. J. Pers. Social Psychol. 2014;106(2):325–338. doi: 10.1037/a0035133. [DOI] [PubMed] [Google Scholar]
- Steinberg L., Morris A.S. Adolescent development. Annu. Rev. Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Szpunar K.K., Jacques P.L.S., Robbins C.A., Wig G.S., Schacter D.L.1. Repetition-related reductions in neural activity reveal component processes of mental simulation. Soc. Cogn. Affect. Neurosci. 2014;9(5):712–722. doi: 10.1093/scan/nst035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill S.L., Bedny M., Goldberg R.F. The frontal lobes and the regulation of mental activity. Curr. Opin. Neurobiol. 2005;15(2):219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Trzesniewski K.H., Robins R.W., Roberts B.W., Caspi A. Personality and self-esteem development across the life span. Adv. Cell Aging Gerontol. 2003;15:163–185. [Google Scholar]
- van der Cruijsen R., Peters S., Crone E.A. Neural correlates of evaluating self and close-other in physical, academic and prosocial domains. Brain Cogn. 2017;118(June):45–53. doi: 10.1016/j.bandc.2017.07.008. [DOI] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Vogeley K., May M., Ritzl A., Falkai P., Zilles K., Fink G.R. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 2004;16(5):817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Wigfield A., Eccles J.S., Mac Iver D., Reuman D.A., Midgley C. Transitions during early adolescence: changes in children’s domain-specific self-perceptions and general self-esteem across the transition to junior high school. Dev. Psychol. 1991;27(4):552–565. [Google Scholar]
- Zanobini M., Usai M.C. Domain-specific self-concept and achievement motivation in the transition from primary to low middle school. Educ. Psychol. 2002;22(2):203–217. [Google Scholar]
- Zink C.F., Pagnoni G., Martin M.E., Dhamala M., Berns G.S. Human striatal response to salient nonrewarding stimuli. J. Neurosci. 2003;23(22):8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. 0270-6474/03/238092-06.00/0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink C.F., Pagnoni G., Martin-Skurski M.E., Chappelow J.C., Berns G.S. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]